Abstract
Developing functional organs from stem cells remains a challenging goal in regenerative medicine. Existing methodologies, such as tissue engineering, bioprinting and organoids, only offer partial solutions. This Perspective focuses on two emerging approaches promising for engineering human organs from stem cells: stem cell-based embryo models and interspecies organogenesis. Both approaches exploit the premise of guiding stem cells to mimic natural development. We begin by summarizing what is known about early human development, as a blueprint for recapitulating organogenesis in both embryo models and interspecies chimeras. The latest advances in both fields are discussed, before highlighting the technological and knowledge gaps to be addressed before the goal of developing human organs could be achieved using the two approaches. We conclude by discussing challenges facing embryo modeling and interspecies organogenesis and outline future prospects for advancing both fields towards the generation of human tissues and organs for basic research and translational applications.
eTOC
Developing organs fit for transplantation remains a challenging goal in regenerative medicine. In this Perspective, what is known about early human development is summarized as a blueprint for recapitulating organogenesis and stem cell-based embryo models and interspecies organogenesis are proposed as promising strategies for generating human tissues and organs.
Introduction
For a wide range of life-threatening diseases such as end-stage kidney, liver, heart and lung failure, whole organ transplantation often stands as the only viable treatment option. However, a global shortage of donor organs has exacerbated the issue. Notably, in the United States alone, more than 100,000 individuals find themselves on the national transplant waiting list at any given time, with 17 people dying daily as they wait for an organ transplant (https://www.organdonor.gov/learn/organ-donation-statistics). The scarcity of donor organs has prompted physicians and scientists to look for alternative solutions, including xenotransplantation of animal organs with close anatomical / physiological similartities to human ones. However, even though there is notable progress in using genetically modified organs from animals, such as those of pigs, for xenotransplantation therapies,1 it remains uncertain whether animal organs are suitable for long-term human transplants.
In addition to xenotransplantation, there are other strategies available for organ engineering based on cultured human cells, including tissue engineering, bioprinting, and organoid technologies. Decades of tissue engineering studies have led to successful applications of various engineered tissue constructs, including most recent ones for skin and corneal tissue grafting to treat skin disease and restore vision, respectively.2,3 Another emerging tissue engineering approach utilizes decellularized extracellular matrix (ECM) from different organs, such as the heart, liver, kidney and lung, to provide biomimetic scaffolds that support the generation of bioartificial organs.4 Bioprinting allows precise deposition of bioinks (often containing cells) and support structures to create three-dimensional (3D) tissue architectures with unparalleled topological complexities.5,6 The advent of organoids, 3D structures derived from self-organizing tissue-specific stem cells and progenitors, has shown considerable promise in modeling human organ development and disease.7,8 There are notable recent progresses in enhancing the complexity of organoids, including incorporating vascular networks within organoids and assembling multi-tissue organoids to study intra- and inter-organ communication.9 Although organ engineering studies based on tissue engineering, bioprinting and organoid technologies are becoming increasingly sophisticated, they still offer imperfect solutions. For instance they fall short in their ability to recapitulate essential functional elements, including vasculature, innervation, lymphatics, and the accurate number, diversity and organization of functional and supporting cell types from different germ layer lineages within solid organs.10
The focus of this Perspective is two cutting-edge strategies based on human stem cells that are promising for addressing the challenges of organ engineering: stem cell-based embryo models and interspecies organogenesis (Figure 1). Both approaches are grounded in a unified conceptual framework that emphasizes the replication of natural processes of germ layer lineage development and organization and microenvironments essential for organ formation. Mimicking the trajectory of natural embryonic development, formation of human organ primordia using both approaches would follow the canonical developmental blueprint, progressing from gastrulation to organogenesis. In vivo, the foundational cells for gastrulation and organogenesis are the pluripotent epiblast (EPI) cells. They differentiate and self-organize into patterned embryonic germ layers during gastrulation, setting the stage for subsequent tissue development and interaction. This, combined with tissue-level morphogenetic processes, leads to the formation of early organ structures.
Figure 1. A schematic overview of two innovative strategies for creating human organs from pluripotent stem cells (PSCs).
(A) In vitro generation of organ via stem cell-derived embryo models. Such models, mimicking the initial stages of embryonic development, could potentially be advanced through cultivation in bioreactors and other ex vivo methods to nurture the growth of organ primordia into sizable, functional organs. (B) In vivo generation of organ via interspecies chimeras. Chimera competent human PSCs can be injected into animal embryos that lack essential genes for organ formation. This process facilitates the production of human organs in animal within the animal host as it undergoes its natural developmental processes.
Contrary to tissue engineering and bioprinting approaches that add layers of complexity in increments to primitive tissues composed of scaffolds and cultured cells, embryo models and interspecies organogenesis initiate organ development with inherent structural complexity through self-organization and -construction of embryonic germ layers guided by genetic programs. Both approaches, in theory, could enable human organs to develop in an environment that closely mimic their natural growth conditions, whether provided by embryo models themselves or in an interspecies host, as opposed to taking organ development out of its natural context as in tissue engineering or bioprinting. Similarly, despite their promise for modeling organ development, organoids are often generated in culture conditions distintly different from natural embryonic environments, such as missing supporting cell types from different embryonic germ layers. As such, organoids tend to lack the complex structural organization and tissue architecture of different germ layer lineages found in native organs that are essential for their physiological functions.
The rapid emergence of stem cell-based embryo models takes advantage of the recent knowledge that pluripotent stem cells (PSCs) largely follow the natural developmental programs of the epiblast cells when differentiated in vitro and their progenies possess remarkable self-organizing properties, giving rise to organized multicellular structures that mimic embryonic tissues.7,11 In addition, the ever increasing knowledge of early post-implantation human development, leading to organogenesis, derived from studies on cultured and aborted human embryos,12-19 provide critical information for guilding human embryo model development as well as for their authentication and benchmarking. The exciting prospect of using embryo models for organ engineering has been further elevated by recent advancements of mouse embryo models, which recapitulate certain aspects of the germ layer lineage diversification and organization during gastrulation and early organogenesis, albeit with low efficiencies and developed organ primordia showing notable defects.20-22 Similar progress in human embryo models, although currently lagging, would, in principle, lead to useful experimental systems for dissecting the molecular and cellular events driving human gastrulation and early organogenesis. Furthermore, such advanced human embryo models would contain most, if not all, foundational embryonic cell types essential for complex solid organ formation. We speculate that with proper spatial organization and interactions between embryonic germ layer lineages, like inherent programmed organogenesis in vivo, these embryo models would have the self-organizing potential to form different organ primordia, thereby opening up exciting new frontiers for organ engineering and related applications.
Human-animal chimeras and blastocyst complementation represent another promising route for generating transplantable human tissues and organs. Nature’s intricate system for embryonic development creates functional tissues and organs through a dynamic interplay between genetic programming and extrinsic developmental niche. This guides embryonic cells in their differentiation and complex tissue formation. Advances in gene-targeting technologies and PSCs have greatly enhanced our understanding of how genetic and epigenetic factors drive embryonic development. Disruptions in these factors can lead to developmental defects, including missing cell lineages or organs, in embryos. This creates "empty" developmental niches that may be filled using donor PSCs, a strategy known as blastocyst complementation.23 Initially demonstrated with mouse embryonic stem cells (ESCs) in 1993,24 this technique was later adapted to interspecies contexts, successfully producing functional rat pancreas in mice.25 These groundbreaking studies have continuously motivated interspecies organogenesis research towards the goal of growing human tissues and organs in other species.
Though numerous challenges remain, it is now techanically conceivable for the formation of human organ primordia in stem cell-derived embryo models or within animal hosts. It is the belief of the authors of this Perspective that with further optimizations, organ rudiments in embryo models or animal hosts could, in principle, develop and grow into fully functional organs, supported by in vivo-like developmental niches and nurtured by blood circulation systems provided by embryo models or through advanced culture systems, such as artificial placentas, or animal hosts.
Even though functional human organs have yet to be generated through embryo modeling and interspecies organogenesis, this Perspective aims to encapsulate recent advances in both fields and speculate about their promise for regenerative medicine. We start by briefly summarizing human pluripotent and extraembryonic stem cells, which constitute the starting cell populations for both approaches. We then discuss the natural development program up to early organogenesis, side by side with the latest advancements in embryo modeling and interspecies organogenesis. We emphasize the importance of closely mimicking natural developmental processes to ensure proper germ layer diversification, interactions and organization, which are fundamental for tissue lineage specification and morphogenesis, ultimately leading to organ formation. We then elaborate on the challenges and expectations and conclude by addressing the future prospects and ethical considerations in embryo modeling and interspecies organogenesis. Given the numerous technical and ethical hurdles facing the two fields, it is our hope that this Perspective will provide a useful framework for guiding both fields towards one of the main goals of regenerative medicine: the generation of functional human tissues and organs for fundamental and translational applications.
Embryonic and Extra-embryonic Stem Cells
Different types of human PSCs (hPSCs) and/or extraembryonic stem cells have been utilized as starting cell populations in embryo modeling (Table 1) and interspecies organogenesis (Table 2). Stem cells of the three foundational lineages of early mouse embryos - epiblast, trophectoderm, and primitive endoderm - have all been well established in vitro as ESCs, trophoblast stem cells (TSCs), and extraembryonic endoderm stem cells (XENs), respectively.26-29 Human TSCs (hTSCs) have only been derived recently from cytotrophoblasts, blastocysts or naïve hPSCs.30-33 Although human stem cells analogous to mouse XENs have not yet been fully established, XEN-like cells have been reported through differentiation from naïve or intermediate hPSCs.34-36
Table 1: Summary of available embryo models generated using pluripotent stem cells from different species.
Pluripotent stem cells (including both embryonic and induced pluripotent stem cells): PSCs; Extended / expanded pluripotent stem cells: EPSCs; Epiblast stem cells: EpiSCs; Trophoblast stem cells: TSCs; Extraembryonic endoderm stem cells: XENs; Inducible XEN cells (naïve PSCs transiently expressing Gata4/6 or SOX17): iXENs; Inducible TSCs (naïve PSCs transiently expressing CDX2 or TFAP2C): iTSCs; Totipotent blastomere-like cells: TBLCs; Trophectoderm: TE; Primitive endoderm: PE; Hypoblast: HYP; Extraembryonic cells: xEMs.
| Human embryo models | |||||
|---|---|---|---|---|---|
| Starting cells | Culture condition |
Additional cells |
Developmental stages to model |
Model name | References |
| Naïve PSCs | Aggregation of single cell type | N/A | Pre-implantation development | Blastoid | 65,66,69,72 |
| EPSCs | Aggregation of single cell type | N/A | Pre-implantation development | Blastoid | 68 |
| EPSCs | Aggregation of single cell type | TE-like cells | Pre-implantation development | EPS-blastoid | 67 |
| Somatic reprogramming intermediates | Aggregation of reprogramming intermediates | N/A | Pre-implantation development | iBlastoid | 79 |
| Primed-to-naïve intermediates | Aggregation during primed-to-naïve-state conversion | N/A | Pre-implantation development | Blastoid | 80 |
| Naïve PSCs | Aggregation of single cell type | N/A | Pre- and post-implantation development up to early gastrulation | Blastoid | 71 |
| Primed PSCs | Aggregation of single cell type | N/A | Early post-implantation development up to early gastrulation | Post-implantation amniotic sac embryoid | 104,105 |
| Primed PSCs | Aggregation of single cell type | N/A | Early post-implantation development up to early gastrulation | Epiblast model | 106 |
| Primed PSCs | Aggregation of different cell types | xEMs | Early post-implantation development up to early gastrulation | Post-attached embryo model | 107 |
| Naïve PSCs | Aggregation of different cell types | TSCs | Early post-implantation development up to early gastrulation | E-assembloid | 60 |
| Naïve PSCs | Aggregation of different cell types | PE/ExEM-like cells, TE-like cells | Early post-implantation development up to early gastrulation | Post-implantation stem-cell-based embryo model | 108 |
| PSCs with intermediate pluripotency | Aggregation of single cell type | N/A | Early post-implantation development up to early gastrulation | Extra-embryoid | 109 |
| Naïve PSCs | Aggregation of different cell types | iTSCs, iXENs | Early post-implantation development up to early gastrulation | Inducible embryoid | 110 |
| Naïve PSCs | Aggregation of different cell types | HYP-like cells, TE-like cells | Early post-implantation development up to early gastrulation | Bilaminoid | 111 |
| EPSCs | Aggregation of single cell type | N/A | Early post-implantation development up to early organogenesis | Peri-gastruloid | 113 |
| Primed PSCs | Co-culture of different cell types | HYP-like cells | Early post-implantation development up to early gastrulation and haematopoiesis | heX-embryoid | 112 |
| Primed PSCs | Patterned 2D cell colonies | N/A | Gastrulation | N/A | 143-145 |
| Primed PSCs | Aggregation of single cell type | N/A | Gastrulation | Gastruloid | 160 |
| Primed PSCs | Aggregation of single cell type | N/A | Gastrulation and early organogenesis | Elongating multi-lineage organized gastruloid | 161 |
| Primed PSCs | Aggregation of single cell type | N/A | Spinal cord and somite development in the trunk | Trunk-like structure | 163,164 |
| Primed PSCs | Aggregation of single cell type | N/A | Somitogenesis | Somitoid, segmentoid | 165 |
| Primed PSCs | Aggregation of single cell type | N/A | Somitogenesis | Somitoid | 166 |
| Primed PSCs | Aggregation of single cell type | N/A | Somitogenesis | Axioloid | 167 |
| Primed PSCs | Patterned 2D cell colonies | N/A | Neuroectoderm patterning | N/A | 173 |
| Primed PSCs | Patterned 2D cell colonies | N/A | Ectoderm patterning and neurulation | Neuruloid | 175 |
| Primed PSCs | Patterned 2D cell colonies | N/A | Germ layer patterning and neurulation | N/A | 177 |
| Primed PSCs | Patterned 2D cell colonies | N/A | Ectoderm patterning and neurulation | N/A | 176 |
| Primed PSCs | Aggregation of single cell type | N/A | Patterned spinal cord development | N/A | 170 |
| Primed PSCs | Aggregation of single cell type | N/A | Patterned spinal cord development | N/A | 171 |
| Primed PSCs | Patterned 2D cell colonies | N/A | Patterned neural tube development | N/A | 180 |
| Mouse embryo models | |||||
|---|---|---|---|---|---|
| Starting cells | Culture condition |
Additional cells |
Developmental stages to model |
Model name | References |
| Naïve PSCs | Aggregation of different cell types | TSCs | Pre-implantation development | Blastoid | 73 |
| Primed-to-naïve intermediates | Aggregation during primed-to-naïve-state conversion | N/A | Pre-implantation development | Blastocyst-like cyst | 76 |
| TBLCs | Aggregation of single cell type | N/A | Pre-implantation development | TBLC-blastoid | 77 |
| EPSCs | Aggregation of different cell types | TSCs | Pre- and early post-implantation development | EPS-blastoid | 74 |
| EPSCs | Aggregation of single cell type | N/A | Pre- and early post-implantation development | EPS-blastoid | 75 |
| Naïve PSCs | Assembly of two cell aggregates | TSCs | Early post-implantation development up to early gastrulation | ETS embryoid | 156 |
| Naïve PSCs | Aggregation of different cell types | TSCs, XENs | Early post-implantation development up to early gastrulation | ETX embryoid | 157,158 |
| Naïve PSCs | Aggregation of different cell types | TSCs, iXENs | Early post-implantation development up to early gastrulation | iETX embryoid | 159 |
| Naïve PSCs | Aggregation of different cell types | TSCs, iXENs | Post-implantation development up to early organogenesis | ETiX embryoid | 20 |
| Naïve PSCs | Aggregation of different cell types | iTSCs, iXENs | Post-implantation development up to early organogenesis | sEmbryo | 21 |
| Naïve PSCs | Aggregation of different cell types | iTSCs, iXENs | Post-implantation development up to early organogenesis | EiTiX embryoid | 22 |
| Naïve PSCs | Aggregation of single cell type | N/A | Gastrulation | Gastruloid | 146-148,150-152 |
| Naïve PSCs | Aggregation of single cell type | N/A | Gastrulation and early organogenesis | Trunk-like structure | 149 |
| Naïve PSCs | Assembly of two cell aggregates | N/A | Gastrulation and early organogenesis | Embryoid | 153 |
| Naïve PSCs | Assembly of two cell aggregates | TSCs | Gastrulation and early organogenesis | EpiTS embryoid | 154 |
| Naïve PSCs | Aggregation of different cell types | XENs | Gastrulation and early organogenesis | XEN enhanced gastruloid | 155 |
| Naïve PSCs | Single cell clonal assay | N/A | Patterned spinal cord development | N/A | 168 |
| Embryo models of other species | |||||
|---|---|---|---|---|---|
| Starting cells | Culture condition |
Additional cells |
Developmental stages to model |
Model name | References |
| Monkey naïve PSCs | Aggregation of single cell type | N/A | Pre- and post-implantation development up to early gastrulation | Blastoid | 84 |
| Bovine EPSCs | Aggregation of different cell types | TSCs | Pre-implantation development | Blastoid | 85 |
Table 2:
A summary of human-animal interspecies chimera studies with different types of human pluripotent stem cells.
| Types of human PSCs | Host species | Level of chimerism |
|---|---|---|
| 8CLCs | Mice | ~1% (mice, E10.5)42 |
| Extended/Expanded potental | Mice | ~1% (mice, E10.5)49 |
| Monkeys | ~7% (monkeys, E15, ex vivo)114 | |
| Naïve (2iLDOX, 5iLA and PXGL) |
Mice, pigs, monkeys | Little to no chimerism90-92 |
| Naïve (HENSM) | Mice | ~1-2% (mice, E9.5-10.5)43 |
| Naïve-like/Intermediate | Mice | ~0.1-4% (mice, E17.5)45 |
| unknown (mice, 10.5)44,89 | ||
| Pigs | ~0.001-0.01% (pigs, E28)90 | |
| Naïve (HENSM, apoptosis inhibited) | Mice | ~1-20% (mice, E9.5-10.5)43 |
| Naïve (4CL, apoptosis inhibited) | Pigs | unknown (pigs, E25 and E28)183 |
| Primed | Mice, pigs, monkeys | Little to no chimerism |
| Primed (apoptosis inhibited) | Mice | ~1% (mice, E10.5)94,95,189,190 |
| Pigs | ~0.05 (pigs, E17)182 | |
| 0.001-0.1% (pigs, E20 and E27)181 |
The in vivo human pluripotency continuum has been recapitulated in vitro with various types of hPSCs representing distinct pluripotency states.37 These hPSCs in different pluripotency states are believed to be suitable for modeling the behaviors of pluripotent epiblast cells at different stages of early human development. Conventional hPSCs represent post-implantation (or embryonic day or E10-12) rather than blastocyst stage (E6-7) human epiblast cells and reside in the primed pluripotency state that show limited potential in differentiation towards extraembryonic cell lineages.38 The unrestricted developmental potential of naïve mouse PSCs has inspired intensive efforts to derive naïve hPSCs. Assessment of naïve pluripotency in hPSCs, due to ethical challenges associated with stringent functional tests like germline transmission in chimeras and tetraploid complementation,39 relies exclusively on molecular benchmarking. hPSCs cultured under various conditions, such as 5i/L/A,40 t2iLGöY,41 4CL42 and HENSM,43 have met most, if not all, of the established molecular criteria. These naïve hPSCs exhibit transcriptomic profiles similar to pre-implantation E6-7 human epiblast cells, a result that supports multiple culture conditions to stabilize naïve pluripotency in humans. In contrast, some hPSCs initially thought of as naïve or naïve-like display transcriptomic features more similar to post-implantation E8-9 human epiblast cells, suggesting that these cells reside in intermediate states between naïve and primed pluripotency.44-46 Formative pluripotency, one such intermediate state, has recently garnered some attention.47 This state represents a developmental window when naïve pluripotency is reconfigured to prepare for multilineage competency, including germ cell specification. Although hPSCs with formative pluripotency have recently been reported,38,48 currently there is a lack of well accepted criteria for authenticating the human formative pluripotency state.
Recent studies have identified mouse PSCs that display some characteristics consistent with totipotency; herein, these cells are collectively referred to as totipotent-like pluripotent stem cells (TPSCs). So far, reported mouse TPSCs include extended and expanded potential stem cells (EPSCs),49,50 totipotent blastomere-like cells (TBLCs),51 chemically induced totipotent stem cells (ciTotiSCs),52 totipotent-like stem cells (TLSCs),53 and totipotent potential stem (TPS) cells.54 Although the establishment of stable human TPSCs remains elusive, several recent studies have successfully identified metastable human eight cell-like cells (8CLCs) under naïve hPSC cultures that activate a range of zygotic genome activation (ZGA) genes.42,55,56 These putative human totipotent-like cells, whether transient or stable, provide a starting cell population likely useful for modeling pre-implantation human developmental events, ranging from early blastomere development to blastocyst formation. They also hold potential for applications in interspecies organogenesis.
Recapitulating Embryonic Development Leading to Organogenesis
Embryonic development serves as a blueprint for embryo modeling and interspecies organogenesis to form functional organs through spatiotemporally dynamic intercellular interactions and organizations. Organ formation in vivo necessitates stereotypical developmental progression, from the implantation, gastrulation to organogenesis (Figure 2A). Pre-implantation human development has been well characterized, thanks to in vitro culture conditions developed for fertilized embryos used in assisted human reproduction.57 Human development from implantation to early organogenesis, however, is much less clear, due to both technical and ethical difficulties associated with intrauterine development after implantation. Our knowledge of post-implantation human development primarily comes from descriptive analyses of historical human embryo collections,58 recent research on primary human embryonic samples,17,18 and studies on cultured human embryos.12-14,16,59,60 In this section, we discuss the current understanding of early human development, focusing on lineage development, morphogenetic events and dynamic tissue organizations that culminate in the formation of organ primordia. Alongside the exploration into the current understanding of early human development, we discuss what has been achieved in embryo modeling and interspecies organogenesis. For detailed discussions on human development from blastocyst formation to gastrulation, we direct readers to some recent comprehensive reviews.61,62 It is important, however, to acknowledge that our discussion, especially regarding peri- and post-implantation development, is based on knowledge that may be incomplete or subject to revision by future research. Current understanding of the molecular and cellular mechanisms of human development should be best considered as evolving hypotheses rather than established facts. To serve this goal, we try to highlight issues of particular uncertainty or controversy and to indicate the limits of our knowledge.
Figure 2. A summary of human stem cell derived embryo models and the developmental stages in vivo they represent.
(A) During early human development, the embryo develops from a zygote and proceeds through specific recognizable stages of (i) pre-implantation, (ii) peri-implantation, and (iii) organogenesis. During this process, cells in the human embryo differentiate and diversify while acting in a coordinated fashion to enact tissue morphogenesis and patterning programs to shape the body plan. (B) PSC-derived human embryo models are generated to mimic various in vivo developmental stages. (C) Chimera competent human PSCs are introduced into pre-implantation blastocysts or early post-implantation embryos of host animals. This process is designed to produce human-animal chimeras, along with tissues and organs enriched with humanb cells.
Pre-implantation development
Pre-implantation human development displays notable autonomy and self-organization. Human embryos, from fertilization to implantation, progress through an ordered series of cell-fate decisions and symmetry-breaking events. This developmental sequence results in the formation of a blastocyst, composed oftrophectodermsurrounding the blastocoel and an inner cell mass (ICM) (Figure 2A[i]). Post-implantation, thetrophectodermcontributes to placental development. Within the blastocyst, the ICM differentiates into two distinct cell lineages: epiblast, forming the embryo, and hypoblast (HYP), or called primititve endodermin mice. Before implantation the epiblast and hypoblast compartments are separated by a basal lamina, with hypoblast cells forming a polarized cuboidal epithelium lining the blastocoelic cavity (Figure 2A[i]).
Human blastocyst implantation initiates notable tissue reorganization and lineage development.61-64 However, knowledge about dynamic cell lineage specification and differentiation, fate patterning, morphogenetic tissue organization, and underlying molecular and cellular mechanisms during early post-implantation human development remains limited. Histochemical analyses of early post-implantation human embryos reveal that invasive trophoblast cells at the embryonic pole of implanting blastocysts proliferate and establish connections with the maternal uterine tissue. Some of these cells lose plasma membranes, forming syncytiotrophoblasts, which grow and enclose the implanting blastocyst. Remaining trophoblast cells along the blastocyst wall maintain their membranes and constitute the cytotrophoblast.
Modeling blastocyst development
Recent years have witnessed significant advancements in the development of human blastocyst models, known as ‘blastoids’65-72 (Table 1). These models encompass all the founding cell lineages of the fetus and its supporting tissues and as such are considered as integrated embryo models (Figure 2B[i]). The generation of human blastoids was inspired by initial success in mouse blastoid formation.73-75 Mouse blastoids are created by combining mTSCs with mESCs73 or mEPSCs74 in confining microwells, or by differentiating mEPSCs into EPI-, TE-, and PE-like cells in microwells75 (Table 1). Additionally, mouse blastoids have been developed through chemical reprogramming of primed mouse epiblast stem cells (mEpiSCs) to form induced blastocyst-like precursors that subsequently self-organize into blastoids76 (Table 1). Recently, mouse blastoids have also been derived from mouse TPSCs53,54,77 (Table 1).
Providing mouse stem cells with geometric confinements is a critical step to promote cell-cell interactions and self-organization. This yields mouse blastoids with morphological features, lineage compositions and organization, and gene expression patterns showing different levels of similarities to mouse blastocysts.53,54,73-77 Even though mouse blastoids transferred into the uteri of pseudopregnant mice could initiate an implantation-like process and induce decidualization, they exhibit very limited growth or development before resorption.73-76 This suggests that mouse blastoids do not have the same developmental potential as mouse blastocysts. Optimizing mouse blastoids to progress through implantation, gastrulation and organogenesis, ultimately leading to the formation of functional organs in surrogate mouse uteri or in vitro, remains an unrealized goal in embryo modeling. If achieved, this would represent a significant milestone in the use of mammalian embryo models for organ engineering.
Current methods for generating human blastoids are similar to those used for mouse blastoids generated from a single starting cell type.53,54,75,77 Given their developmental potential for both embryonic- and extraembryonic-like cells,31,32,34,49,78 naïve hPSCs and hEPSCs have been the cells of choice to create human blastoids (Table 1). These cells, sometimes with their derivatives, are placed in microwells and subjected to chemical inductions, promoting differentiation and self-organization into segregated EPI-, TE-, and HYP-like compartments. This process results in blastoids with different levels of similarities in morphology, global gene expression, and lineage composition to human blastocysts.65-72 Human blastoids have also been generated with chemically reprogrammed hPSCs that resemble eight-cell stage blastomeres (i.e. 8CLCs)42,55 (Table 1). It remains unclear whether human 8CLCs have the intrinsic capacity to differentiate and self-organize into blastoids without the influence of external factors. Alternatively, human blastoids have been generated from transitioning intermediates of somatic cell reprograming (iBlastoids)79 and primed-to-naïve conversion80 (Table 1). However, comparative transcriptome studies suggest that TE-like cells in iBlastoids may actually represent post-implantation amniotic ectoderm cells.81,82 As an integrated embryo model, human blastoid research warrents careful scientific and ethical oversight processes.83 Ethical constraints prohibit in vivo implantation studies of human blastoids.83
Besides mouse and human blastoids, researchers have recently created monkey84 and bovine blastoids85 using naïve-like monkey ESCs and through assembling bovine TSCs86 and EPSCs,87 respectively. Prolonged culture of monkey blastoids shows cellular features and molecular markers consistent with peri-gastrulation primate development.84 Monkey and bovine blastoids, when transferred into surrogate uteruses, appear capable of establishing early pregnancy based on ultrasound observations and/or hormone level detections.84,85 These in vivo transplantation assays provide the most stringent test of blastoid developmental potential. However, it is yet to be shown whether implanted monkey or bovine blastoids can exhibit stereotypical tissue organization and lineage diversification consistent with post-implantation development.
Interspecies chimeric contributions to blastocyst formation
Generation of human organs in animals via blastocyst complementation requires donor hPSCs to effectively contribute to the ICM of host blastocysts. Studies in mice reveal that ICM incorporation and chimera competency positively correlates with the developmental potential of donor PSCs: mouse TPSCs exhibit the highest capacity to form chimeras in embryonic tissues,49,51,52 followed by naïve and intermediate/formative mouse PSCs, while primed mouse EpiSCs rarely contribute to mouse blastocyst ICMs.39,88 Likewise, 8CLCs, naïve, naïve-like and intermediate hPSCs have shown robust colonization into ICMs of mouse, pig, rabbit and monkey blastocysts42-45,89-93 (Figure 2C[i] and Table 2). In contrast, primed hPSCs were inefficiently incorporated into mouse, rabbit, cow or pig ICMs, and the cells undergo apoptosis following blastocyst injection90,94,95 (Table 2).
Peri-implantation development
During peri-implantation human development, hypoblast proliferates, extending beyond the epiblast, differentiating into visceral and parietal endoderm. The visceral endoderm lies beneath the epiblast, forming a continuous, polarized cuboidal epithelium, while peripheral hypoblast cells transform into spindle-shaped parietal endoderm, creating the inner lining for the cytotrophoblast. The parietal endoderm expands gradually to line the entire inner cavity of the cytotrophoblast, leading to primary yolk sac formation (Figure 2A[ii]).
During primary yolk sac formation, extraembryonic mesoderm arises as spindle-shaped cells situated between parietal endoderm and cytotrophoblast. The origin of early extraembryonic mesoderm in humans is a subject of debate. One hypothesis suggests it may derive from either visceral or parietal endoderm. Another theory proposes that it could originate from peri-implantation epiblast cells while they form the amniotic cavity and undergo symmetry breaking to generate the amniotic ectoderm.96,97 As human peri-implantation development progresses, extraembryonic mesoderm expands, enveloping the epiblast compartment and primary yolk sac, thereby separating them from the cytotrophoblast (Figure 2A[ii]).
During human peri-implantation development, the epiblast compartment forms the amniotic cavity through lumenogenesis59 (Figure 2A[ii]). This lumenal epiblast sac gradually resolves into a bipolar structure, with epiblast cells neighboring invading cytotrophoblast cells becoming squamous amniotic ectoderm and remaining epiblast cells on the opposite pole maintaining pluripotency and forming a discoid embryonic disc. At this stage, the epiblast and visceral endoderm form the bilaminar embryonic disc, positioned between the amniotic cavity (dorsally) and the primary yolk sac cavity (ventrally) (Figure 2A[ii]). Prior to gastrulation, a chorionic cavity forms in the extraembryonic mesoderm by dividing it into two layers. At this stage, the yolk sac structure beneath the bilaminar embryonic disc transitions from the primary to definitive yolk sac. How the definitive yolk sac forms to replace the primary yolk sac remains elusive. One theory suggests that the definitive yolk sac takes shape by visceral endoderm expansion, giving rise to a new membrane that pushes the primary yolk sac forward. It eventually pinches off from the primary yolk sac, with the primary yolk sac tissue degenerating into vesicles at the abembryonic end of the chorionic cavity. Simultaneously, the chorionic cavity expands, separating the human embryo with its attached amnion and yolk sac from the blastocyst's outer wall (now called chorion), suspended solely by a thick stalk of tissue, the connecting stalk. The cellular composition of human connecting stalk remains to be fully characterized and likely contains mainly extraembryonic mesoderm.
There are many fundamental questions unanswered about peri-implantation human development. Compared to mice, human amniotic ectoderm and extraembryonic mesoderm emerge earlier. Even though differentiations of amniotic ectoderm- and extraembryonic mesoderm-like cells from cultured hPSCs have been demonstrated,98,99 the origins of these two lineages, molecular mechanisms underlying their specifications, and their roles in human peri-implantation development remain to be elucidated. Recent studies using cultured human embryos support the role of ECM signaling in the lumenogenesis and formation of the amniotic cavity in the epiblast during peri-implantation human development.13,59 Another in vitro study suggests a role of ECM rigidity-dependent BMP signaling in regulating amniotic differentiation of primed hPSCs.98 How ECM and developmental signaling, tissue mechanics and morphogenetic events, and lineage fate decisions are interconnected during peri-implantation human development remains elusive. It also remains to be clarified the molecular and cellular mechanisms underlying the primary and definitive yolk sac formation in humans. During human peri-implantation development, thetrophectodermderivatives become physically separated from the bilaminar embryonic disc by the amnion, a distinction from the pre-gastrulation structure of the mouse egg cylinder (Figure 2A[ii]).
Modeling peri-implantation development
Notable differences exist between mouse and human peri-implantation development.61-64,100 Mouse models of peri-implantation development, made using embryonic and extraembryonic stem cells, have successfully mimicked tissue organization and lineage segregation seen in early post-implantation mouse embryos (Table 1). More recently, improvements of a rotating bottle culture system initially pioneered by Dennis New101 have allowed for prolonged ex utero mouse embryo culture.102 Importantly, this rotating bottle culture system has also enabled stem cell-derived mouse peri-implantation embryo models to progress beyond gastrulation, initiating early organogenesis, albeit with a very low efficiency and organ primordia exhibiting notable defects (Table 1).20-22 Specifically, these mouse embryo models develop structures mimicking headfolds with brain subdivisions, a heart, a trunk structure with a neural tube and somites, a tail bud containing neuromesodermal progenitors (NMPs), and a gut tube.20-22 These mouse studies showcase the exciting promise of stem cell-based embryo models for generating organ primordia through progressive development from the gastrulation to early organogenesis.
In extended 3D cultures, human blastoids show features of early post-implantation development, including amniotic cavity and primary yolk sac formation, growth and differentiation of thetrophectodermlineage, and the emergence of gastrulating cells.71,80 However, the low efficiency of human blastoids exhibiting these developmental events limits their applications for studying peri- and post-implantation human development. Advancements in this area may be facilitated by ongoing research aimed at improving prolonged human blastocyst cultures in vitro, along with efforts in developing models of implantation and placentation using human endometrial cells.69,70
Besides prolonged 3D cultures of human blastoids,71,80 there are other embryo models developed for studying human peri-implantation development. Early studies showed lumen formation as an intrinsic property of primed hPSCs, supporting their use for modeling amniotic cavity formation.103 Additional studies revealed that naïve hPSCs could not readily form lumens, and the epiblast compartment of in vitro cultured human blastocysts only forms the amniotic cavity after epiblast cells exit the naïve pluripotency.59 Recently, a study demonstrated that clusters of primed hPSCs in a 3D culture underwent lumenogenesis before evolving into a bipolar structure mimicking post-implantation amnion-EPI patterning (Table 1).104 Progressive development of this structure showed delamination of gastrulating cells from the EPI-like pole, a feature consistent with the onset of gastrulation. More recently, a microfluidic amniotic sac model was developed, allowing for controlled formation of primed hPSC clusters. This was followed by asymmetrical chemical stimulations of hPSC clusters in the microfluidic device, which improved the efficiency and controllability of the amniotic sac model (Table 1).105 This model also demonstrated features consistent with induction of human primordial germ cells (PGCs) during peri-gastrulation human development.105 This microfluidic amniotic sac model highlights the promising applications of bioengineering tools in controlling tissue geometry, as well as biochemical and biophysical conditions, for embryo modeling to boost their efficiency and controllability.
Another 3D peri-implantation human development model was also developed using primed hPSCs to model anterior (A)-posterior (P) symmetry breaking of the epiblast at the onset of gastrulation (Table 1).106 A follow-up study utilized an assembloid approach to combine primed hPSCs and extraembryonic-like cells to examine the role of embryonic-extraembryonic interactions during the same developmental event (Table 1).107
Very recently, several new human embryo models have been reported, utilizing either naïve hPSCs or hEPSCs, and sometimes with their derivatives, to simulate human peri-implantation development up to the gastrulation60,108-112 or early organogenesis113 (Figure 2B[ii] and Table 1). Some of these embryo models exhibit complex cellular developments and organizations consistent with the development of nearly all known lineages and structures of peri-implantation human embryos. These structures include bilaminar disc formation, epiblast lumenogenesis for amniotic cavity formation, patterned amniogenesis, A-P symmetry breaking in the epiblast, human PGC specification, primary yolk sac formation, extraembryonic mesoderm and chorionic cavity development, and atrophectodermlineage-surrounding compartment. Although one such model reports signs of a trilaminar disc-like structure and primary neurulation,113 it remains to be fully validated whether these most recent peri-implantation human development models can faithfully emulate the multifaceted human gastrulation process and even reach the early organogenesis stage.60,108-112 Since the development of these embryo models relies on spontaneous aggregation and differentiation of naïve hPSCs or hEPSCs, they often exhibit suboptimal efficiency and/or disorganized cellular structures. Even though it remains to be seen how these models will be utilized as experimental tools to advance fundamental knowledge of peri-implantation human development, they represent the most recent advances of human embryo modeling.
Interspecies chimeric contributions to peri-implantation development
Despite exhibiting robust colonization of host blastocyst ICM, naïve hPSCs, such as those cultured in 5i/L/A and PXGL conditions, surprisingly show limited chimeric contribution in mouse, pig, and monkey peri-implantation or early post-implantation embryos90-92 (Table 2). In comparison, hPSCs in intermediate states show improved contributions in early post-implantation chimera formation in pig (E20-E28),90 mouse (E9.5-10.5)43-45,89 and monkey (E15)114 embryos (Figure 2C[iii] and Table 1). These findings indicate that intermediate or naïve-like hPSCs might be more effective as donor cells for interspecies blastocyst complementation, or that the culture condtions for naïve hPSCs need refinement for optimal use in interspecies chimera applications. Supporting this idea, naïve hPSCs cultured under 5i/L/A and PXGL conditions have been found to exhibit genomic instabilities and a loss of DNA methylation at primary imprints.115,116
Studies of mouse TPSCs suggest that, if successfully developed, human TPSCs could be valuable for interspecies chimera formation and blastocyst complementation. Several recent studies support this hypothesis (Table 2), with human EPSCs demonstrating increased chimera competency in both mouse and monkey embryos.49,114,117 In addition, human cells were readily detected in E10.5 mouse embryos following blastocyst injection of human 8CLCs.42
Although primed hPSCs undergo apoptosis and cannot contribute to chimera formation following blastocyst injection, they can effectively engraft the posterior epiblast compartment in gastrula-stage mouse embryos and differentiate into cell lineages from all the three germ layers118,119 (Figure 2C[ii]). Thus, utilizing primed hPSCs for interspecies organogenesis via an "EPI complementation" in gastrula-stage mouse embryos appears as an attractive alternative strategy.88 To achieve this goal, a prolonged ex utero embryo culture system, like the one recently reported,102 will be needed for prolonged culture of mouse gastrula, due to a lack of effective methods for transferring gastrula-stage embryos into a surrogate uterus. By grafting primed hPSCs into the posterior epiblast of an organogenesis-disabled, pre-gastrulation mouse embryo, it might be possible to generate human organ primordia through prolonged ex utero culture of these chimeric embryos.
Gastrulation and organogenesis
Gastrulation in vivo involves the formation of the primitive streak in the epiblast and differentiating epiblast cells moving through the PS, intercalating with underlying visceral endoderm cells, and eventually replacing them with embryonic endoderm cells. The transition of visceral endoderm to definitive endoderm and the role of definitive endoderm in definitive yolk sac development in humans are yet to be clarified. In mice, a fraction of visceral endoderm cells persists at least until the formation of the early gut tube.120 Other gastrulating cells migrate bilaterally from the PS and then cranially or laterally between the endoderm and epiblast, coalescing to form the embryonic mesoderm. epiblast cells that do not ingress through the PS are fated to become the embryonic ectoderm. Thus, through gastrulation, the epiblast in human embryos transforms into a trilaminar germ disc structure.
Current molecular understanding of mammalian gastrulation is primarily derived from mouse studies, emphasizing how interactions between the epiblast and surrounding extraembryonic tissues lead to gene expression patterns that initiate symmetry breaking and body axis formation.121 In mouse embryos, signals from the anterior visceral endoderm inhibit epiblast differentiation. Developmental signaling, involving BMP, WNT and NODAL pathways, at the posterior epiblast prompts epithelial-mesenchymal transition (EMT) and cell ingression through the PS, acquiring mesendoderm identities. The precise molecular mechanisms for symmetry breaking in human epiblast at the onset of gastrulation remain unclear. Recent studies on monkey and human embryos reveal a population of putative visceral endoderm cells at the anterior end expressing WNT and NODAL antagonists,122-124 akin to the mouse anterior visceral endoderm, suggesting shared mechanisms in mammalian species for epiblast symmetry breaking during gastrulation.
During mouse gastrulation, PGCs develop from somatic gastrulating epiblast cells due to BMP signals from adjacent extraembryonic tissues.125-127 Knowledge about early PGC development in primate embryos is limited.123,128 Unlike mice, cynomolgus monkey PGCs seem to emerge in the nascent amniotic ectoderm compartment before gastrulation.123 Observations from in vitro cultured human embryos, in vivo post-implantation human and monkey embryos, and human embryo models also support the emergence of human PGCs firstly in nascent amniotic ectoderm prior to the gastrulation.105,128,129 This observation requires further confirmation using other peri-gastrulation human and monkey embryonic tissues. It remains to be elucidated the molecular and cellular differences between human PGCs originated in the amniotic ectoderm compartment vs. those from somatic gastrulating epiblast cells.
Human gastrulation remains a profound mystery.130 Prior to gastrulation, the human epiblast compartment is surrounded by two extraembryonic tissues, dorsal amniotic ectoderm and ventral visceral endoderm. Data from human embryo models support a possible inductive role of posterior amniotic ectoderm in triggering the onset of gastrulation in the posterior epiblast compartment.105 How the amniotic ectoderm and visceral endoderm coordinate to mediate symmetry breaking, body axis formation, and PS development in human gastrula remains an important question to address in the future. Additionally, the mechanisms governing how gastrulating human cells segregate and give rise to organized germ layer lineages, as well as the development of human PGCs – including their origin and underlying genetic and molecular mechanisms - during human gastrulation, remain largely unresolved. These fundamental questions have profound implications for reproductive and regenerative medicine.
During gastrulation, germ layer subpopulations in the trilaminar embryonic disc come together, facilitating interactions that shape tissue layers, specify cell types, and initiate organ rudiment development (Figure 2A[iii]). A critical event in embryonic ectoderm is neural induction,131 where it divides into the neuroectoderm (central) and surface ectoderm (lateral, future epidermis). The neuroectoderm forms the neural plate, which subsequently folds into the neural tube, covered by the surface ectoderm through the process of primary neurulation.132 The rostral neural tube,from the brain to the rostral part of the spinal cord up to its mid-thoracic region is formed through primary neurulation. Caudal spinal cord, in contrast, is developed during the elongation of the embryo, through a less characterized secondary neurulation process. It is hypothesized that during gastrulation, caudal epiblast cells first ingress to give rise to a part of the tail bud mesenchyme, which contains a population of bipotent NMPs that give rise to both caudal spinal cord and paraxial mesoderm derivatives during the elongation of the embryo.133 This tail bud mesenchyme subsequently epithelializes and undergoes cavitation, leading to the formation of one or several lumens (i.e., secondary neurulation). Both primary and secondary neurulation have been observed in human embryos;134,135 however, their exact contribution to human neural tube formation is still a matter of debate.
During neural tube formation, neural crest cells arise from the neural plate’s edges.136 These cells delaminate from the closing neural tube and migrate to various locations to generate diverse cell types. The identity of neural crest derivatives correlates with their position along the rostral-caudal body axis, with cranial neural crest cells preferentially generating mesenchymal derivatives in the head, and trunk neural crest cells giving rise to sympathoadrenal cells. It remains poorly understood how mammalian neural crest cells are regionalized with different differentiation potentials along the rostral-caudal axis.137 Within the neural tube,cells differentiate into distinct classes of neuronal progenitors at defined positions along both the rostral-caudal and dorsal-ventral body axes under the influence of inductive factors emanating from adjacent tissues, including two organizer regions that extend along the dorsal and ventral midlines of the embryo: dorsal surface ectoderm and ventral notochord.
Gastrulation organizes embryonic mesodermal cells into various regions, including cardiogenic mesoderm, axial mesoderm of the prechordal plate and notochord, paraxial mesoderm, intermediate mesoderm and lateral plate mesoderm. Each of these mesodermal regions undergoes some form of segmentation. The most notable segmentation occurs in the trunk and tail paraxial mesoderm, leading to somite formation, which contributes to skeletal muscles, axial skeleton, and dermis. This process, known as somitogenesis, is accompanied by a molecular oscillator called the segmentation clock.138,139 The interaction of the segmentation clock with a signal wave traveling in the paraxial mesoderm along the cranial-caudal axis (the clock-and-wavefront model) is generally believed to control somite number, size, and axial identity in developing embryos.140-142
After gastrulation, the trilaminar embryonic disc undergoes folding due to differential growth rates. As a result, the cranial, lateral, and caudal edges of the embryonic disc converge along the ventral midline. The endodermal, mesodermal, and ectodermal layers fuse to their corresponding layers on the opposite side, creating the basic tube-within-a-tube body plan. This process transforms the flat embryonic endoderm into a primitive gut tube surrounded by mesoderm. Initially, the gut tube consists of foregut and hindgut separated by the midgut, which remains open to the definitive yolk sac. As the lateral edges of embryonic disc layers continue to join along the ventral midline, the midgut progressively transforms into a tube, and the definitive yolk sac neck narrows into a slender vitelline duct. Reciprocal interactions with mesoderm lead to regionalization of the gut tube along the rostral-caudal and dorsal-ventral body axes and the budding of endodermal organ domains. These organ buds develop as outgrowths of endodermal epithelium that intermingle with surrounding mesenchyme, and together they grow, branch, and eventually form functional endodermal organs. The foregut gives rise to the esophagus, trachea, stomach, lungs, thyroid, liver, biliary system, and pancreas; the midgut forms the small intestine, while the hindgut forms the large intestine.
After gastrulation, the cardiogenic mesoderm forms a cardiac crescent at the cranial end of the embryo, giving rise to a pair of lateral endocardial tubes through vasculogenesis. These tubes later fuse along the ventral midline in the future thoracic region to form a single heart tube, which consists of a single endocardial tube with adjacent mesoderm differentiating into contractile cardiomyocytes. The primary heart tube undergoes morphogenetic processes, like looping, remodeling, realignment, and septation, eventually leading to the development of a four-chamber heart, facilitating the separation of pulmonary and systemic circulations.
Modeling gastrulation and early organogenesis
The first human gastrulation model was created based on micropatterned two-dimensional (2D) colonies of primed hPSCs (Table 1), displaying a thickened PS-like ring structure and concentric regions of ectodermal, mesodermal, and endodermal tissues, surrounded by extraembryonic domains at colony boundaries.143 The precision, reproducibility, and compatibility with high-resolution imaging of this model facilitate mechanistic investigations of molecular and cellular events involved in human gastrulation. Given its 2D topology, this model has been integrated with bioengineering tools, such as hydrogel substrates with tunable mechanical stiffnesses144 and microfluidic gradient devices,145 to study the roles of biophysical and biochemical signals in the gastrulation and aixal patterning of germ layer lineages (Table 1).
3D models of gastrulation and early organogenesis have been most successfully demonstrated using mouse stem cells (Table 1). In one such model, termed gastruloids, aggregated mESCs are embedded in culture medium containing diluted natural ECM molecules and are stimulated with exogeneous signals, typically WNT molecules, to induce cell differentiation and tissue patterning (Table 1).146,147 Early mouse gastruloids were shown to model trunk development, exhibiting symmetry breaking, axial elongation, spinal cord-like structure and bilateral somite formation, a gut tube-like structure, a tail bud-like structure containing NMPs, and development of PGC-like cells (PGCLCs).146-149 Recent mouse gastruloids showed features associated with cardiogenesis150 and hematopoietic precursor- and erythroid-like cells spatially localized to a vascular-like structure,151 mimicking in vivo blood cell development (Table 1). To promote development of anterior neural tissues, surrounding hydrogel signals in mouse gastruloids were modulated, together with WNT inhibition instead of WNT activation (Table 1).152 When mESC aggregates were assembled with another mESC aggregate pre-treated with exogenous BMP4, resulting mouse gastruloids developed organ primordia similar to those in neurula-stage mouse embryos, including patterned neural tube- and gut tube-like structures, somitic and intermediate mesodermal tissues, cardiac tissues, and a vasculature network (Table 1).153 Moreover, including mTSCs or XEN cells in mouse gastruloids facilitated the development of neuroepithelial structures, such as regions resembling the anterior brain (Table 1).154,155
3D models of mouse gastrulation have also been developed by assembling mESCs and mTSCs (Table 1).156 These models replicated morphogenetic events in embryonic and extraembryonic tissues during the mouse egg cylinder development. They also successfully induced the formation of definitive mesoderm and PGCLCs.156 Further incorporation of XEN cells in these models resulted in the development of tissue structures resembling those in mouse gastrula.20,21,157-159 Additionally, as previously discussed, ex utero culture of co-aggregated mESCs and mESC-derived TE- and extraembryonic endoderm-like cells in an improved rotating bottle culture system yielded advanced 3D mouse embryo models that could progress into early stages of organogenesis, albeit with a very low efficiency and organ primordia showing notable abnomalities.20-22
Significant progress has also been achieved in developing human 3D gastruloids (Figure 2B[iii] and Table 1). Using culture protocols similar to those for mouse gastruloids, free-floating aggregates of primed hPSCs under uniform chemical treatments break symmetry and form an A-P axis.160,161 Human gastruloids undergo axial elongation with spatial cellular organizations of the three definitive germ layer lineages.160,161 Under shaking cultures, human gastruloids demonstrate more organized trunk-like development, featuring spinal cord-like and gut tube-like structures integrated with peripheral neurons derived from neural crest cells.161
Interestingly, axial progenitor-like cells derived from primed hPSCs, which likely contain NMPs, could self-organize and exhibit in vivo-like co-morphogenesis of multiple tissues and their topographic organization in the trunk region, including spinal cord and bilateral somites (Table 1).162-164 Furthermore, recent research further utilized primed hPSCs to specifically model somitogenesis165-167 (Figure 2B[iii] and Table 1).
There are other human embryo models created to recapitulate early neural developmental events, such as the formation of the neural plate and neural fold, closure of neural folds, and neural tube regional patterning (Table 1). Following an early work using mESCs168, dorsal-ventral neural patterning was imitated using hPSC-derived lumenal neural cysts in 3D cultures under caudalizing and ventralizing chemical environments.169-172 Many of the human neural developmental models were achieved using micropatterned 2D colonies of primed hPSCs subjected to chemical induction of ectodermal lineage development.173-177 In one of these models, a self-organized ectodermal structure or “neuruloid” was generated, featuring a central lumenal neural epithelial structure overlaid by neural crest cells, with the entire structure covered with a layer of a prospective epidermis.175 Thus, the tissue morphology and spatial cellular organization of the neuruloid is reminiscent of the ectodermal organization observed in vivo at the neurulation stage. Another neuruloid study further recapitulated the morphogenetic cellular events during the folding and closure of the neural plate in neurulation.176 In addition, microfluidic gradient generation devices have been successfully utilized to superimpose exogenous patterning signals on hPSC-derived neural tissues to achieve their regional patterning.178 In one pioneering study, patterned by microfluidic WNT signal gradients, hPSC-derived, planar neural tissues were generated that exhibit progressive caudalization from forebrain to midbrain to hindbrain, including formation of isthmic organizer characteristics.179 Very recently, using two orthogonal and independently controllable microfluidic gradients, an hPSC-based, microfluidic neural tube-like structure (or μNTLS) was demonstrated, whose development recapitulates some critical aspects of neural patterning in both brain and spinal cord regions and along both rostral-caudal and dorsal-ventral axes180 (Figure 2B[iii]). Studying neuronal lineage development using μNTLS revealed pre-patterning of axial identities of neural crest progenitors and functional roles of NMPs in spinal cord and trunk neural crest development.180 The μNTLS approach is promising for studying interregional and long-range cellular interactions in neural development that are critical for complex network functions.
Interspecies chimeric contributions to early organogenesis
While there have been considerable advancements in intraspecies organogenesis via blastocyst complementation, success in the interspecies context remains limited even among closely related species like rats and mice, largely due to alleged xenogeneic barriers (discussed below). The challenges are even more pronounced in the realm of human-animal blastocyst complementation, where only a handful of attempts have been made, yielding variable outcomes.
Despite these obstacles, recent progress in human-animal interspecies organogenesis is encouraging23 (Figure 2C[iii] and Table 2). The initial successful attempt at generating human tissues in animals via blastocyst complementation came from a study by Garry and colleagues. This team successfully generated human endothelium in E17-E18 ETV2-null pig embryos from injected hPSCs.181 Following this pioneering work, a subsequent study from the same group created human skeletal muscle tissue in MYF5/MYOD/MYF6-null pig embryos (E20 and E27) using hiPSCs.182 In a major step forward, Lai and colleagues utilized multiple technologies to improve human chimerism in animal embryos and early-stage organs, leading to the production of a humanized pig mesonephros, comprising 40% to 60% human cells, within 3-4 weeks old pig fetuses.183 These advances, in large part, can be attributed to continuous efforts in understanding and overcomeing the xenogeneic barriers that exist between donor hPSCs and animal embryo hosts.23
Challenges and Expectations
Challenges for organ generation using stem cell-based embryo models
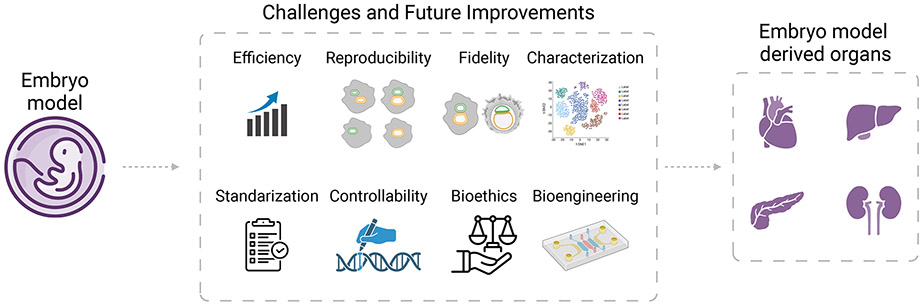
There remains numerous challenges in stem cell-based embryo modeling for organ engineering. Addressing these challenges will require concerted and dedicated efforts in optimizing human stem cell cultures, standardizing protocols, and improving characterization methods and controllability of embryo modeling (Figure 3).
Figure 3. Challenges and future improvements in utilizing stem cell based embryo models for organ engineering.
Efficiency, reproducibility and standardization.
Despite significant strides in embryo modeling, achieving models with high fidelity, efficiency, controllability, and in vivo-like cellular organization and tissue architecture remains a substantial challenge. This difficulty is primarily attributed to the inherent variabilities in the self-organization and differentiation capabilities of human stem cells and their derivatives within the uncontrolled culture environments typical of most current embryo modeling efforts. As a result, embryo models are often influenced by transcriptional and epigenetic noise as well as unpredicatable cellular interactions within their local culture microenvironment. Moreover, the use of various stem cell types as starting populations for embryo modeling, each requiring different culture conditions to encourage differentiation and self-organization, introduces additional complexity. The establishment of cultures that accurately represent different human embryonic and extraembryonic cells are still in progress. The inherent variability and poorly understood characteristics of human stem cells further complicate the robust development of embryo models, thereby limiting their utility. Additionally, the absence of standardized protocols for embryo modeling exacerbates variability in culture conditions and experimental results across different research laboratories, obstructing reproducibility and the ability to compare findings from embryo modeling studies effectively.
To overcome the challenges of limited efficiency and reproducibility in embryo modeling, it is imperative to harness advanced bioengineering tools capable of precisely managing tissue topological boundaries and dynamic chemical and mechanical signals in culture environments. These tools will be instrumental in creating high-fidelity, high-efficiency embryo models.9,184 Recent advancements have yielded bioengineered human embryo models featured by enhanced precision, reproducibility, and compatibility with high-resolution imaging techniques, facilitating detailed mechanistic studies of the molecular and cellular processes underlying human development.105,143,145,162,175,176,179,180 Looking ahead, the field of embryo modeling stands to gain substantially from integrative efforts that apply bioengineering strategies, including micropatterning, microfluidics, 3D bioprinting, and synthetic biology techniques like optogenetics, as well as cell-instructive biomaterials. These approaches aim to meticulously direct pattern formation, morphogenesis, and cell differentiation, thereby achieving more accurate control over the development of embryo models. This will enhance their efficiency, reproducibility, controllability, complexity, and in vivo relevance. Parallel efforts in establishing and thoroughly characterizing various human stem cell lines, especially those representing genuine human extraembryonic stem cells, will further advance these endeavors, making it possible to generate more accurate and useful models of human development.30-36,78
Recaptulating gastrulation and organogenesis in embryo models.
Organ primordia in current embryo models often show notable structural defects and variations, are small in size, and lack organ-specific functionalities. In vivo, organogenesis occurs after gastrulation, a process where embryonic germ layers and their subpopulations within the trilaminar embryonic disc structure come together, promoting tissue-tissue interactions to specify cell types, drive morphogenetic events and initiate organ rudiment development. Thus, the most important outcome of gastrulation is the emergence of a recognizable structure containing organized germ layer lineages with spatially distinct identities in a fully-defined coordinate system.130 Current 3D human embryo models fall short of replicating the intricate structural organization of embryonic germ layer lineages during peri-gastrulation development, posing a significant obstacle in accurately modeling organogenesis. There are certain human embryo models containing axial progenitor-like cells that exhibit organized development of trunk regions, featuring the formation of structures such as the primitive gut tube, spinal cord, and bilateral somites.162-164 These studies highlight the importance of future embryo modeling in promoting proper differentiation and spatial organization of embryonic germ layer lineages and their subpopulations, which will facilitate autonomous cellular interactions and provide an effective morphogenetic environment for organ formation.
The ongoing efforts in deriving bona fide human extraembryonic stem cells30-36,78 and in developing in vitro implantation models69,70,185 using endometrial cells will promote future development of more advanced human embryo models containing embryonic, extraembryonic and/or maternal components. The extraembryonic and maternal tissues will likely be pivotal in providing structural stability, managing topological boundaries, and facilitating endogenous, multidirectional tissue interactions. Together, they create a conductive morphogenetic environment that fosters cell differentiation and organization reminiscent of the gastrulation process. Continuous developments and refinements, particularly those incorporating bioengineering tools and cell-instructive biomaterial systems to precisely modulate dynamic biophysical and biochemical niche signals, will lead to more sophisticated human embryo models exhibiting proper organogenesis processes, with improved efficiency and controllability. Additionally, harnessing advanced bioreactor systems, including artificial placentas, is crucial for long-term culture of human embryo models. These systems, equipped with a continuous medium supply, automated sampling, real-time sensing, and meticulous control over culture conditions—including physiological and mechanical forces—might enable the growth of organ primordia into sizable, functional organs in embryo models.
Challenges for interspecies organogenesis
While successful in closely related rodent species like rats and mice, applying blastocyst complementation to humans remains challenging.181,182 Key steps for successful human-animal blastocyst complementation include generating hPSCs that can robustly contribute to interspecies chimeras and overcoming developmental barriers between species to fully unlock this technique’s potential for growing human organs in animals.
Despite various attempts using different hPSC types and host species, the chimeric contribution of human cells in interspecies chimeras remains markedly low. Furthermore, there are inconsistent results about the efficiency and the extent to which hPSCs can integrate into embryos of evolutionarily distant host species. This uncertainty largely stems from the technical challenges in detecting and analyzing low levels of chimerism, especially in later stages of embryo and fetal development. To tackle this challenge, developing more effective quantification methods for low chimerism is crucial. Additionally, exploring how interspecies differences in early development contribute to the limited human chimerism observed in animal embryos, an issue often referred to as ‘xenogeneic barriers’,23,186 is essential. A better understanding of xenogeneic barriers will be the key in addressing the challenge of low human chimerism, thereby advancing the use of interspecies blastocyst complementation for human organ generation in animals.
It necessitates a deeper understanding of the molecular and cellular events triggered by interspecies cell mixing in early development, in order to overcome xenogeneic barriers and translate the success of rat-mouse to human-animal blastocyst complementation. In contrast to chimera formation within the same species or between closely related species, numerous factors can differ significantly between humans and host animals of distant evolutionary origin, hindering efficient and extensive chimerism. Here, we discuss several key barriers that limit successful chimeric formation, including cell competition, incompatibility in cell adhesion, heterochrony, and ligand-receptor mismatches (Figure 4).
Figure 4. Xenogeneic barriers.
(A) A notable competitive interaction was identified between primed PSCs from evolutionarily distant species (e.g., human-mouse, human-cow, human-rat) based on interspecies PSC co-culture experiments. The elimination of the “loser” cells (e.g., human PSCs when co-cultured with mouse epiblast stem cells [EpiSCs]) is governed by the NF-κB signaling pathway. Disabling the P65 gene (also known as RELA) or an upstream regulator (MYD88) of the NF-κB complex in human cells can overcome this competition, thus enhancing the survival and chimerism of human cells within early mouse embryos. In “winner” cells (e.g., mouse EpiSCs), the retinoic acid-inducible gene I (RIG-I)-like receptor (RLR) signaling pathway, an RNA sensor, appears to play an important role in determining the outcome of competitive interactions between co-cultured mouse and human PSCs. (B) Incompatibilities in cell adhesion, particularly among primed PSCs from different species, present a significant xenogeneic barrier. Employing 3D interspecies PSC co-cultures offers a valuable in vitro method to investigate this barrier. A notable approach to overcoming this issue involves engineering synthetic cell adhesion. This can potentially be achieved by leveraging membrane-anchored nanobody-antigen interactions to facilitate cell adhesion compatibility between PSCs from different species. (C) Heterochrony represents another xenogeneic barrier. Matching developmental timing of the donor PSCs with host embryos is an important consideration for the successful generation of intra- and inter-species chimeras. (D) Genomic evolution leading to mismatched ligand-receptor pairs poses another xenogeneic challenge.
Cell competition.
Cell competition describes a vital cell-cell interaction essential for multicellular life. It was initially studied in Drosophila melanogaster during wing disc development within genetic mosaics, where cells carrying a heterozygous Minute mutation are eliminated through apoptosis by surrounding wild-type cells.187 More recently, cell competition has been observed in various mammalian tissues, supporting that this process is conserved.188 During early mammalian development, epiblast cells undergo drastic changes in proliferation rate and reorganization of transcriptional, epigenetic, metabolic, and signaling networks. The complexity of these changes raises the likelihood of aberrant cells emerging, requiring intrinsic cellular mechanisms to detect and eliminate such cells to ensure normal development. In the context of interspecies chimera formation, xenogeneic hPSCs might be perceived as unfit or aberrant cells by neighboring host cells and targeted for elimination through cell competition. In aggreement, strategies to suppress hPSCs apoptosis improved human chimerism in mouse and pig embryos.43,94,95,181,182,189
To model cell competition in interspecies chimeras, researchers utilized an interspecies PSC co-culture strategy to uncover a previously unknown competive interaction between primed, but not naïve, PSCs from evolutionarily distant species (e.g., humans vs. mice; humans vs. cows)190 (Figure 4A). Comparative transcriptomic analysis of hPSCs in co-cultures vs. separate cultures revealed that genes related to the NF-κB signaling pathway, among others, were upregulated in "loser" hPSCs.190 Genetic perturbation of the NF-κB signaling pathway by knocking out a core component of NF-κB complex, P65 (also known as RELA), and an upstream adaptor MyD88 in hPSCs prevented their apoptosis during co-culture with mEpiSCs and furthermore, improved their survival and chimerism in early mouse embryos190 (Figure 4A). MyD88 is one of the primary adaptors for most mammalian Toll-like receptors (TLRs). The TLRs/MyD88/RELA-dependent loser cell apoptosis observed in human-mouse primed PSC competition is strikingly similar to the role Toll-related receptors (TRRs)-NF-κB played during cell competition in Drosophila wing disc development,191 suggesting that the innate immunity pathway acts as a conserved gatekeeper to ensure normal development.
In contrast to loser cells, little is known regarding what enacts the winner status during interspecies PSC competition. A recent preprint study suggests that RNA sensing and innate immunity operates in "winner" cells during interspecies PSC competition.192 By suppressing the retinoic acid-inducible gene I (RIG-I)-like receptor (RLR) pathway in mouse embryos, researchers observed improved survival and chimerism from unmodified donor human PSCs (Figure 4A). This study suggests an alternative approach to promote interspecies chimerism of donor hPSCs by modifying host embryos.
Cell adhesion.
Interspecies incompatibility may also result from mismatches in cell adhesion molecules (CAMs) between different species. During development, cell adhesion is crucial for the assembly of individual cells into 3D tissues, and differential cell adhesion is important for cell sorting and tissue boundary formation. For interspecies chimera formation, differential cell adhesion may impede donor hPSCs from effectively integrating with host counterparts and contributing to host development (Figure 4B). Mismatches of CAMs can result from structural and sequence differences between homologous adhesion proteins or from varying expression patterns and levels of adhesion molecules in embryos of different species. For donor hPSCs not expressing CAMs compatible with host embryos, they might not participate effectively in the development of the epiblast lineage, ultimately leading to their expulsion from the embryo. To address this issue, strategies to modify key components of CAMs in hPSCs to render them more compatible with corresponding proteins from host species can be explored. For instance, the first extracellular loop of CLAUDIN, a tight junction (TJ) protein, plays a significant role in recognizing other CLAUDINs on neighboring cells. Thus, any divergence in its sequence may impair CLAUDIN binding and TJ formation. Consequently, it might serve as a useful strategy to replace this part of human CLAUDIN in hPSCs with the sequence from host species, thus allowing hPSCs to form proper TJs with host epiblast cells for more effective interspecies chimera formation.
The prospect of modifying each CAM involved in cell-cell adhesion incompatibility between species at different developmental stages can be very challenging. An alternative approach can employ synthetic biology to regulate adhesive interactions between cells through membrane-localized nanobody-antigen interactions193,194 (Figure 4B). Nanobodies, which are single monomeric domain antibody fragments derived from camelid heavy chain IgG antibodies, offer several advantages, such as the ability to bind small antigens and robust expression in various model systems.195 Recent studies have successfully utilized nanobody-antigen pairs to induce artificial cell adhesion in bacterial systems.193 A recent study further expanded on this strategy for mammalian systems by developing synthetic CAMs (synCAMs) that combine orthogonal (nanobody-antigen) extracellular interactions with intracellular domains of native adhesion molecules.194 This orthogonal system does not interfere with natural adhesion processes in mammalian cells and can be easily modified using multiple nanobody-antigen pairs or by altering the nanobody sequence to adjust adhesion strength. It will be intriguing to explore whether synCAMs can be utilized to enhance cell-cell adhesion between species, thus improving human cell chimerism in animal embryos.
Heterochrony.
First proposed by Ernst Haeckel in 1875, heterochrony is a concept that encompasses any genetically regulated variations in the timing, rate, or duration of the developmental process in an organism, in comparison to its ancestral lineage or other species. Heterochrony can present a potential xenogeneic barrier to interspecies chimerism, as discrepancies in the developmental timing, rate, and duration between donor and host species may obstruct donor cells from effectively responding to environmental cues for proliferation and differentiation in synchronization with host cells, thus hindering the harmonious integration of donor and host cells (Figure 4C).
Consistent with the concept of heterochrony, mammals exhibit considerable variation in the rate of embryonic development, which is often correlated with differences in body shape and size, age of sexual maturity, and lifespan. Interestingly, species-specific pace of development is often corroborated by directed differentiation of PSCs of various species outside the uterus. For example, one study showed that, using the same neural differentiation protocol, hPSCs took significantly longer to generate target neuronal cell types compared to mouse PSCs.196 Intriguingly, human-specific neural differentiation rate could even persist in teratomas generated from hPSCs in a mouse host, suggesting that external host factors could not accelerate the developmental clock of donor human cells.197 In addition to sequential gene regulation mediating developmental timing, oscillators, such as the "segmentation clock", can serve as timers controlling the tempo of morphogenesis and tissue formation. Recent studies show that the periodicity of the segmentation clock during somitogenesis in utero is retained in somite precursors derived from PSCs in vitro, adhering to the species-specific tempo.198-201 These findings support that developmental timing requires a significant degree of cell autonomy, likely involving species-specific biochemical reaction speeds196,198 and/or mitochondria metabolism.202
Despite inherent developmental timing differences among species, there are studies showing that some xenogeneic donor cells could adopt the developmental pace of host species when injected into preimplantation blastocysts. Successful generation of several human-animal chimeric embryos, as mentioned earlier, implies that a small portion of hPSCs accelerate their developmental rate to match that of their embryonic host species.43,45,90 Supporting this notion, another study shows that PSCs from horses, which have a significantly longer gestation period (~11-12 months) compared to mice (~20 days), could contribute to chimera formation in early mouse embryos.38 Adding to this evidence, a recent paper demonstrates that co-differentiation with the presence of mouse PSCs could accelerate the differentiation speed of hPSCs.203 Additionally, two very recent preprint studies reveal that rat neurons could adjust to the developmental pace of their mouse hosts following blastocyst injection of rat PSCs into mouse blastocysts.204,205 Together, these studies support that given their inherent plasticity, PSCs may be more adaptive in terms of differentiation pace than initially believed. Furthermore, non-cell-autonomous mechanisms may exist to regulate developmental timing of both donor and host cells during embryogenesis. This highlights the need for future studies to improve foundamental understanding of how developmental tempo is enacted during interspcies chimera formation.
A recent study conducted a comparative analysis of chimera formation success rates following injection of primary neural crest cells (NCCs) into blastocysts or ESCs into E8.5 mouse embryos (heterochronic injection), versus injecting ESCs into blastocysts or NCCs into E8.5 mouse embryos (isochronic injection).206 Efficient chimera formation was observed under isochronic injection conditions, and conversely, no functional chimeric contribution was detected in heterochronic injections. Notably, human NCCs contribute to coat pigmentation in postnatal mice chimeras after in utero injection into gastrulating mouse embryos, albeit at an very low efficiency.206 In agreement with this, primed hPSCs seldom contribute to chimera formation following injection into mouse blastocysts but could successfully integrate and differentiate after grafting into the epiblast of gastrulating mouse embryos118,119 (Figure 4C). These findings support that isochronic injection could improve successful engraftment of human cells into animal embryos.
Ligand-receptor incompatibility.
Another potential barrier is the interspecies incompatibility between ligands and receptors, stemming from genetic diversification (Figure 4D). This often results from ligand-receptor co-evolution aimed at refining binding affinity and specificity. Consequently, ligands from one species might either fail to recognize or manifest reduced potency in activating receptors from another species. For instance, while stem cell factor (SCF) across diverse mammalian species shares over 75% sequence similarity, there's a marked difference in their receptor activation across species. Specifically, human SCF displays restricted potency in activating the mouse KIT, yet the efficacy of rodent SCF in engaging and activating the human KIT nearly parallels that of human SCF.207 It is a daunting task to identify and optimize all mismatched ligand-receptor pairs across species. A strategic approach could be to pinpoint critical signaling pathways hindered by such incompatibilities and the use of genetic replacement or modification of pivotal receptors to help further improve interspecies chimerism.
Current developments and future persectives in interspecies organogenesis.
As of now, interspecies chimerism and blastocyst complementation remain inefficient. Even in experiments between closely related species like rats and mice, chimeric efficiency is still notably lower than intraspecies chimeras, despite a lack of PSC competition190 and their closely aligned developmental timing - differing by just 1-2 days in gestation period. Notably, a high degree of rat chimerism in mice can lead to embryonic lethality due to developmental incompatibilities.208 These observations underscore the inherent challenges of cross-species chimerism, even among evolutionary neighbors. Consequently, when considering chimerism and blastocyst complementation between more distantly related species, such as humans and mice or humans and pigs, expectations should be adjusted accordingly. Despite the substantial challenges, the vision of generating human organs in animals - to mitigate the global organ donor shortage - persists with renewed hope. A recent study achieved a significant advancement by successfully generating a humanized mesonephros within pig fetuses.183 This feat was accomplished by improving multiple aspects of interspecies organogenesis, including an optimized human PSC culture, enhancing the survival and competitiveness of human donor cells, and utilizing a genetically emptied host developmental organ niche.183
It should be noted that interspecies organogenesis through the generation of chimeras is different from the xenotransplantation approach, aiming to produce organs in pigs that are predominantly human-cell derived. Future studies stand to benefit by merging these two strategies: enriching genetically modified pig organs with human cells through blastocyst complementation. This combination could further diminish immune barriers and render the organs more analogous to human ones.
Conclusion and Future Outlook
In the past 25 years, we have made great strides since first capturing human embryonic pluripotency in culture. Human PSCs have revolutionized regenerative medicine, paving the way for fundamental discoveries and translations. Recently, the identification of a variety of human pluripotency states has provided exciting opportunities to explore fresh, intriguing aspects of human development and organ engineering. Human PSCs are notable for their ability to proliferate indefinitely in vitro, coupled with their inherent developmental potential and exceptional capacity for self-organization. These properties of hPSCs have granted us access to an extensive array of human embryo models, some of which showing promising potential of generating different organ primordia, such as the heart, gut tube, neural tube and somites. There is little doubt now that stem cell-based embryo models have become useful experimental tools for advancing molecular and cellular understanding of human development. Additionally, when used in interspecies chimeras and paired with gene-editing technologies and methods to overcome early interspecies developmental barriers, hPSCs could be used for the generation of human organ primordia within animal hosts. Given the rapid progress in embryo modeling and interspecies organogenesis, it is the authors’ prediction that successful creations of human organ primordia, either in vitro using stem cell-derived embryo models or in vivo within interspecies chimeras, will be achieved in the near future.
Compared to embryo models and interspecies chimeras, tissue engineering, bioprinting, and organoid technologies are more established approaches for organ engineering. In this Perspective, we suggest that embryo models and interspecies chimeras offer alternative strategies promising for human organ engineering. Nonetheless, organ engineering remains a distant goal for both fields that requires careful strategic and integrative efforts to address the remaining numerous technical and ethical hurdles. Some of the technical difficulties have been discussed in previous sections. There is another critical challenge about how to grow hPSC-derived organ primordia from embryo models or animal hosts into fully functional and sizeable organs suitable for human transplants. Unfortunately, there is no direct solution currently available for this signifcant difficulty. We envision addressing this challenge will require parallel developments of related emerging technologies, such as advanced bioreactor-based culture systems or artificial placentas that can effectively connect with the vasculature of growing organs or embryo models to supply oxygen and nutrients while removing carbon dioxide and waste products. Such technological innovations are needed for prolonging the development of hPSC-derived organ primordia into fully functional organs. Another potential solution involves ectopical transplantation of hPSC-derived organ primordia, for example, into the kidney capsule or omentum of animal hosts, to integrate human organ primordia with the animal host’s blood circulation for oxygen and nutrient supplies. Regarding interspecies organogenesis, the success observed in generating fully functional organs between mice and rats supports that the production of human organs in animals that are evolutionarily closer to humans could be technically more achievable. Needless to say, these proposed technological developments and chimera approaches themselves are technically challenging and ethically sensitive. Nonetheless, they hold the key for advancing embryo modeling and interspecies organogenesis towards the goal of creating complex, functional solid human organs in the laboratory.
Besides technical difficulties, there are abundant ethical challenges facing both embryo models and interspecies chimeras for human organ engineering. This is especially true when certain human tissues develop in embryo models and interspecies chimeras, particularly those involving neural cells in the central nervous system and germ cells - a situation often referred to as "moral humanization". To navigate these ethical considerations, precise genome engineering technologies such as CRISPR-Cas9 can be utilized to selectively deactivate genes necessary for neural development and germ cell specification. This way, hPSCs could be genetically modified to only differentiate into endodermal and mesodermal lineages - those responsible for the production of desired organs - thereby eliminating the risk of producing human neural cells derived from the ectodermal lineage or germ cells. In addition, there are several recent reviews and commentaries on current ethical considerations surrounding embryo modeling and interspecies organogenesis.83,209 Readers are encouraged to consult these references to understand the complex ethical considerations and landscapes. Crucially, continuous and proactive ethical discussions involving scientists, bioethicists, policymakers, and the public are essential to establish, maintain and update ethical guidelines. These ethical guidelines should be in place before research on embryo models and interspecies organogenesis can proceed with due caution to prevent ethical dilemmas. Such ethical guidelines should be regularly updated, and in some cases, anticipate scientific and technological advances to ensure responsible research conduct.
Acknowledgements
J.W. is a New York Stem Cell Foundation – Robertson Investigator and Virginia Murchison Linthicum Scholar in Medical Research. Research in the Wu laboratory is supported by National Institutes of Health (UM1HG011996, R01HD103627, R01GM138565 and R21HD105349), NYSCF, ARSM, and the Welch Foundation (I-2088). Research in the Fu laboratory is supported by the Michigan-Cambridge Research Initiative, University of Michigan Mid-career Biosciences Faculty Achievement Recognition Award, National Institutes of Health (R21NS113518, R21HD100931, R21HD105126, R21HD105192, R21HD109635, R21NS127983, R01GM143297 and R01NS129850), National Science Foundation (CMMI1917304, CBET1901718 and PFI2213845), and the 21st Century Jobs Trust Fund received through the Michigan Strategic Fund from the State of Michigan (CASE315037). The authors apologize to colleagues whose work they could not cite owing to space restrictions.
Footnotes
Declaration of Interests
The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anand RP, Layer JV, Heja D, Hirose T, Lassiter G, Firl DJ, Paragas VB, Akkad A, Chhangawala S, Colvin RB, et al. (2023). Design and testing of a humanized porcine donor for xenotransplantation. Nature 622, 393–401. 10.1038/s41586-023-06594-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rafat M, Jabbarvand M, Sharma N, Xeroudaki M, Tabe S, Omrani R, Thangavelu M, Mukwaya A, Fagerholm P, Lennikov A, et al. (2023). Bioengineered corneal tissue for minimally invasive vision restoration in advanced keratoconus in two clinical cohorts. Nat Biotechnol 41, 70–81. 10.1038/s41587-022-01408-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hirsch T, Rothoeft T, Teig N, Bauer JW, Pellegrini G, De Rosa L, Scaglione D, Reichelt J, Klausegger A, Kneisz D, et al. (2017). Regeneration of the entire human epidermis using transgenic stem cells. Nature 551, 327–332. 10.1038/nature24487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, Netoff TI, and Taylor DA (2008). Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart. Nat Med 14, 213–221. 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 5.Lee A, Hudson AR, Shiwarski DJ, Tashman JW, Hinton TJ, Yerneni S, Bliley JM, Campbell PG, and Feinberg AW (2019). 3D bioprinting of collagen to rebuild components of the human heart. Science 365, 482–487. 10.1126/science.aav9051. [DOI] [PubMed] [Google Scholar]
- 6.Grigoryan B, Paulsen SJ, Corbett DC, Sazer DW, Fortin CL, Zaita AJ, Greenfield PT, Calafat NJ, Gounley JP, Ta AH, et al. (2019). Multivascular networks and functional intravascular topologies within biocompatible hydrogels. Science 364, 458–464. 10.1126/science.aav9750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clevers H (2016). Modeling Development and Disease with Organoids. Cell 165, 1586–1597. 10.1016/j.cell.2016.05.082. [DOI] [PubMed] [Google Scholar]
- 8.Kim J, Koo BK, and Knoblich JA (2020). Human organoids: model systems for human biology and medicine. Nat Rev Mol Cell Biol 21, 571–584. 10.1038/s41580-020-0259-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shao Y, and Fu J (2022). Engineering multiscale structural orders for high-fidelity embryoids and organoids. Cell Stem Cell 29, 722–743. 10.1016/j.stem.2022.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Das S, Gordian-Velez WJ, Ledebur HC, Mourkioti F, Rompolas P, Chen HI, Serruya MD, and Cullen DK (2020). Innervation: the missing link for biofabricated tissues and organs. NPJ Regen Med 5, 11. 10.1038/s41536-020-0096-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sasai Y (2013). Cytosystems dynamics in self-organization of tissue architecture. Nature 493, 318–326. 10.1038/nature11859. [DOI] [PubMed] [Google Scholar]
- 12.Deglincerti A, Croft GF, Pietila LN, Zernicka-Goetz M, Siggia ED, and Brivanlou AH (2016). Self-organization of the in vitro attached human embryo. Nature 533, 251–254. 10.1038/nature17948. [DOI] [PubMed] [Google Scholar]
- 13.Shahbazi MN, Jedrusik A, Vuoristo S, Recher G, Hupalowska A, Bolton V, Fogarty NNM, Campbell A, Devito L, Ilic D, et al. (2016). Self-organization of the human embryo in the absence of maternal tissues. Nat Cell Biol 18, 700–708. 10.1038/ncb3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiang L, Yin Y, Zheng Y, Ma Y, Li Y, Zhao Z, Guo J, Ai Z, Niu Y, Duan K, et al. (2020). A developmental landscape of 3D-cultured human pre-gastrulation embryos. Nature 577, 537–542. 10.1038/s41586-019-1875-y. [DOI] [PubMed] [Google Scholar]
- 15.Petropoulos S, Edsgard D, Reinius B, Deng Q, Panula SP, Codeluppi S, Plaza Reyes A, Linnarsson S, Sandberg R, and Lanner F (2016). Single-Cell RNA-Seq Reveals Lineage and X Chromosome Dynamics in Human Preimplantation Embryos. Cell 165, 1012–1026. 10.1016/j.cell.2016.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou F, Wang R, Yuan P, Ren Y, Mao Y, Li R, Lian Y, Li J, Wen L, Yan L, et al. (2019). Reconstituting the transcriptome and DNA methylome landscapes of human implantation. Nature 572, 660–664. 10.1038/s41586-019-1500-0. [DOI] [PubMed] [Google Scholar]
- 17.Tyser RCV, Mahammadov E, Nakanoh S, Vallier L, Scialdone A, and Srinivas S (2021). Single-cell transcriptomic characterization of a gastrulating human embryo. Nature 600, 285–289. 10.1038/s41586-021-04158-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu Y, Zhang T, Zhou Q, Hu M, Qi Y, Xue Y, Nie Y, Wang L, Bao Z, and Shi W (2023). A single-cell transcriptome atlas profiles early organogenesis in human embryos. Nat Cell Biol 25, 604–615. 10.1038/s41556-023-01108-w. [DOI] [PubMed] [Google Scholar]
- 19.Xiao Z, Cui L, Yuan Y, He N, Xie X, Lin S, Yang X, Zhang X, Shi P, Wei Z, et al. (2024). 3D reconstruction of a gastrulating human embryo. Cell. 10.1016/j.cell.2024.03.041. [DOI] [PubMed] [Google Scholar]
- 20.Amadei G, Handford CE, Qiu C, De Jonghe J, Greenfeld H, Tran M, Martin BK, Chen DY, Aguilera-Castrejon A, Hanna JH, et al. (2022). Embryo model completes gastrulation to neurulation and organogenesis. Nature 610, 143–153. 10.1038/s41586-022-05246-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tarazi S, Aguilera-Castrejon A, Joubran C, Ghanem N, Ashouokhi S, Roncato F, Wildschutz E, Haddad M, Oldak B, Gomez-Cesar E, et al. (2022). Post-gastrulation synthetic embryos generated ex utero from mouse naive ESCs. Cell 185, 3290–3306 e3225. 10.1016/j.cell.2022.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lau KYC, Rubinstein H, Gantner CW, Hadas R, Amadei G, Stelzer Y, and Zernicka-Goetz M (2022). Mouse embryo model derived exclusively from embryonic stem cells undergoes neurulation and heart development. Cell Stem Cell 29, 1445–1458 e1448. 10.1016/j.stem.2022.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng C, Ballard EB, and Wu J (2021). The road to generating transplantable organs: from blastocyst complementation to interspecies chimeras. Development 148. 10.1242/dev.195792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen J, Lansford R, Stewart V, Young F, and Alt FW (1993). RAG-2-deficient blastocyst complementation: an assay of gene function in lymphocyte development. Proc Natl Acad Sci U S A 90, 4528–4532. 10.1073/pnas.90.10.4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kobayashi T, Yamaguchi T, Hamanaka S, Kato-Itoh M, Yamazaki Y, Ibata M, Sato H, Lee YS, Usui J, Knisely AS, et al. (2010). Generation of rat pancreas in mouse by interspecific blastocyst injection of pluripotent stem cells. Cell 142, 787–799. 10.1016/j.cell.2010.07.039. [DOI] [PubMed] [Google Scholar]
- 26.Tanaka S, Kunath T, Hadjantonakis AK, Nagy A, and Rossant J (1998). Promotion of trophoblast stem cell proliferation by FGF4. Science 282, 2072–2075. 10.1126/science.282.5396.2072. [DOI] [PubMed] [Google Scholar]
- 27.Evans MJ, and Kaufman MH (1981). Establishment in culture of pluripotential cells from mouse embryos. Nature 292, 154–156. 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 28.Martin GR (1981). Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci U S A 78, 7634–7638. 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kunath T, Arnaud D, Uy GD, Okamoto I, Chureau C, Yamanaka Y, Heard E, Gardner RL, Avner P, and Rossant J (2005). Imprinted X-inactivation in extra-embryonic endoderm cell lines from mouse blastocysts. Development 132, 1649–1661. 10.1242/dev.01715. [DOI] [PubMed] [Google Scholar]
- 30.Okae H, Toh H, Sato T, Hiura H, Takahashi S, Shirane K, Kabayama Y, Suyama M, Sasaki H, and Arima T (2018). Derivation of Human Trophoblast Stem Cells. Cell Stem Cell 22, 50–63 e56. 10.1016/j.stem.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 31.Dong C, Beltcheva M, Gontarz P, Zhang B, Popli P, Fischer LA, Khan SA, Park KM, Yoon EJ, Xing X, et al. (2020). Derivation of trophoblast stem cells from naive human pluripotent stem cells. Elife 9. 10.7554/eLife.52504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guo G, Stirparo GG, Strawbridge SE, Spindlow D, Yang J, Clarke J, Dattani A, Yanagida A, Li MA, Myers S, et al. (2021). Human naive epiblast cells possess unrestricted lineage potential. Cell Stem Cell 28, 1040–1056 e1046. 10.1016/j.stem.2021.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Io S, Kabata M, Iemura Y, Semi K, Morone N, Minagawa A, Wang B, Okamoto I, Nakamura T, Kojima Y, et al. (2021). Capturing human trophoblast development with naive pluripotent stem cells in vitro. Cell Stem Cell 28, 1023–1039 e1013. 10.1016/j.stem.2021.03.013. [DOI] [PubMed] [Google Scholar]
- 34.Linneberg-Agerholm M, Wong YF, Romero Herrera JA, Monteiro RS, Anderson KGV, and Brickman JM (2019). Naive human pluripotent stem cells respond to Wnt, Nodal and LIF signalling to produce expandable naive extra-embryonic endoderm. Development 146. 10.1242/dev.180620. [DOI] [PubMed] [Google Scholar]
- 35.Mackinlay KM, Weatherbee BA, Souza Rosa V, Handford CE, Hudson G, Coorens T, Pereira LV, Behjati S, Vallier L, Shahbazi MN, and Zernicka-Goetz M (2021). An in vitro stem cell model of human epiblast and yolk sac interaction. Elife 10. 10.7554/eLife.63930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wei Y, Zhang E, Yu L, Ci B, Sakurai M, Guo L, Zhang X, Lin S, Takii S, Liu L, et al. (2023). Dissecting embryonic and extraembryonic lineage crosstalk with stem cell co-culture. Cell 186, 5859–5875 e5824. 10.1016/j.cell.2023.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Du P, and Wu J (2024). Hallmarks of totipotent and pluripotent stem cell states. Cell Stem Cell 31, 312–333. 10.1016/j.stem.2024.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu L, Wei Y, Sun HX, Mahdi AK, Pinzon Arteaga CA, Sakurai M, Schmitz DA, Zheng C, Ballard ED, Li J, et al. (2021). Derivation of Intermediate Pluripotent Stem Cells Amenable to Primordial Germ Cell Specification. Cell Stem Cell 28, 550–567 e512. 10.1016/j.stem.2020.11.003. [DOI] [PubMed] [Google Scholar]
- 39.De Los Angeles A, Ferrari F, Xi R, Fujiwara Y, Benvenisty N, Deng H, Hochedlinger K, Jaenisch R, Lee S, Leitch HG, et al. (2015). Hallmarks of pluripotency. Nature 525, 469–478. 10.1038/nature15515. [DOI] [PubMed] [Google Scholar]
- 40.Theunissen TW, Powell BE, Wang H, Mitalipova M, Faddah DA, Reddy J, Fan ZP, Maetzel D, Ganz K, Shi L, et al. (2014). Systematic identification of culture conditions for induction and maintenance of naive human pluripotency. Cell Stem Cell 15, 471–487. 10.1016/j.stem.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guo G, von Meyenn F, Santos F, Chen Y, Reik W, Bertone P, Smith A, and Nichols J (2016). Naive Pluripotent Stem Cells Derived Directly from Isolated Cells of the Human Inner Cell Mass. Stem Cell Reports 6, 437–446. 10.1016/j.stemcr.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mazid MA, Ward C, Luo Z, Liu C, Li Y, Lai Y, Wu L, Li J, Jia W, Jiang Y, et al. (2022). Rolling back human pluripotent stem cells to an eight-cell embryo-like stage. Nature 605, 315–324. 10.1038/s41586-022-04625-0. [DOI] [PubMed] [Google Scholar]
- 43.Bayerl J, Ayyash M, Shani T, Manor YS, Gafni O, Massarwa R, Kalma Y, Aguilera-Castrejon A, Zerbib M, Amir H, et al. (2021). Principles of signaling pathway modulation for enhancing human naive pluripotency induction. Cell Stem Cell 28, 1549–1565 e1512. 10.1016/j.stem.2021.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gafni O, Weinberger L, Mansour AA, Manor YS, Chomsky E, Ben-Yosef D, Kalma Y, Viukov S, Maza I, Zviran A, et al. (2013). Derivation of novel human ground state naive pluripotent stem cells. Nature 504, 282–286. 10.1038/nature12745. [DOI] [PubMed] [Google Scholar]
- 45.Hu Z, Li H, Jiang H, Ren Y, Yu X, Qiu J, Stablewski AB, Zhang B, Buck MJ, and Feng J (2020). Transient inhibition of mTOR in human pluripotent stem cells enables robust formation of mouse-human chimeric embryos. Sci Adv 6, eaaz0298. 10.1126/sciadv.aaz0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ware CB, Nelson AM, Mecham B, Hesson J, Zhou W, Jonlin EC, Jimenez-Caliani AJ, Deng X, Cavanaugh C, Cook S, et al. (2014). Derivation of naive human embryonic stem cells. Proc Natl Acad Sci U S A 111, 4484–4489. 10.1073/pnas.1319738111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith A (2017). Formative pluripotency: the executive phase in a developmental continuum. Development 144, 365–373. 10.1242/dev.142679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kinoshita M, Barber M, Mansfield W, Cui Y, Spindlow D, Stirparo GG, Dietmann S, Nichols J, and Smith A (2021). Capture of Mouse and Human Stem Cells with Features of Formative Pluripotency. Cell Stem Cell 28, 453–471 e458. 10.1016/j.stem.2020.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang Y, Liu B, Xu J, Wang J, Wu J, Shi C, Xu Y, Dong J, Wang C, Lai W, et al. (2017). Derivation of Pluripotent Stem Cells with In Vivo Embryonic and Extraembryonic Potency. Cell 169, 243–257 e225. 10.1016/j.cell.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang J, Ryan DJ, Wang W, Tsang JC, Lan G, Masaki H, Gao X, Antunes L, Yu Y, Zhu Z, et al. (2017). Establishment of mouse expanded potential stem cells. Nature 550, 393–397. 10.1038/nature24052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shen H, Yang M, Li S, Zhang J, Peng B, Wang C, Chang Z, Ong J, and Du P (2021). Mouse totipotent stem cells captured and maintained through spliceosomal repression. Cell 184, 2843–2859 e2820. 10.1016/j.cell.2021.04.020. [DOI] [PubMed] [Google Scholar]
- 52.Hu Y, Yang Y, Tan P, Zhang Y, Han M, Yu J, Zhang X, Jia Z, Wang D, Yao K, et al. (2023). Induction of mouse totipotent stem cells by a defined chemical cocktail. Nature 617, 792–797. 10.1038/s41586-022-04967-9. [DOI] [PubMed] [Google Scholar]
- 53.Yang M, Yu H, Yu X, Liang S, Hu Y, Luo Y, Izsvak Z, Sun C, and Wang J (2022). Chemical-induced chromatin remodeling reprograms mouse ESCs to totipotent-like stem cells. Cell Stem Cell 29, 400–418 e413. 10.1016/j.stem.2022.01.010. [DOI] [PubMed] [Google Scholar]
- 54.Xu Y, Zhao J, Ren Y, Wang X, Lyu Y, Xie B, Sun Y, Yuan X, Liu H, Yang W, et al. (2022). Derivation of totipotent-like stem cells with blastocyst-like structure forming potential. Cell Res 32, 513–529. 10.1038/s41422-022-00668-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yu X, Liang S, Chen M, Yu H, Li R, Qu Y, Kong X, Guo R, Zheng R, Izsvak Z, et al. (2022). Recapitulating early human development with 8C-like cells. Cell Rep 39, 110994. 10.1016/j.celrep.2022.110994. [DOI] [PubMed] [Google Scholar]
- 56.Taubenschmid-Stowers J, Rostovskaya M, Santos F, Ljung S, Argelaguet R, Krueger F, Nichols J, and Reik W (2022). 8C-like cells capture the human zygotic genome activation program in vitro. Cell Stem Cell 29, 449–459 e446. 10.1016/j.stem.2022.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Johnson M (2019). Human in vitro fertilisation and developmental biology: a mutually influential history. Development 146, dev183145. 10.1242/dev.183145. [DOI] [PubMed] [Google Scholar]
- 58.O'Rahilly R, and Muller F (2010). Developmental stages in human embryos: revised and new measurements. Cells Tissues Organs 192, 73–84. 10.1159/000289817. [DOI] [PubMed] [Google Scholar]
- 59.Shahbazi MN, Scialdone A, Skorupska N, Weberling A, Recher G, Zhu M, Jedrusik A, Devito LG, Noli L, Macaulay IC, et al. (2017). Pluripotent state transitions coordinate morphogenesis in mouse and human embryos. Nature 552, 239–243. 10.1038/nature24675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ai Z, Niu B, Yin Y, Xiang L, Shi G, Duan K, Wang S, Hu Y, Zhang C, Zhang C, et al. (2023). Dissecting peri-implantation development using cultured human embryos and embryo-like assembloids. Cell Res 33, 661–678. 10.1038/s41422-023-00846-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shahbazi MN (2020). Mechanisms of human embryo development: from cell fate to tissue shape and back. Development 147. 10.1242/dev.190629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rossant J, and Tam PPL (2022). Early human embryonic development: Blastocyst formation to gastrulation. Dev Cell 57, 152–165. 10.1016/j.devcel.2021.12.022. [DOI] [PubMed] [Google Scholar]
- 63.Rossant J, and Tam PPL (2017). New Insights into Early Human Development: Lessons for Stem Cell Derivation and Differentiation. Cell Stem Cell 20, 18–28. 10.1016/j.stem.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 64.Shahbazi MN, and Zernicka-Goetz M (2018). Deconstructing and reconstructing the mouse and human early embryo. Nat Cell Biol 20, 878–887. 10.1038/s41556-018-0144-x. [DOI] [PubMed] [Google Scholar]
- 65.Yu L, Wei Y, Duan J, Schmitz DA, Sakurai M, Wang L, Wang K, Zhao S, Hon GC, and Wu J (2021). Blastocyst-like structures generated from human pluripotent stem cells. Nature 591, 620–626. 10.1038/s41586-021-03356-y. [DOI] [PubMed] [Google Scholar]
- 66.Yanagida A, Spindlow D, Nichols J, Dattani A, Smith A, and Guo G (2021). Naive stem cell blastocyst model captures human embryo lineage segregation. Cell Stem Cell 28, 1016–1022 e1014. 10.1016/j.stem.2021.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fan Y, Min Z, Alsolami S, Ma Z, Zhang E, Chen W, Zhong K, Pei W, Kang X, Zhang P, et al. (2021). Generation of human blastocyst-like structures from pluripotent stem cells. Cell Discov 7, 81. 10.1038/s41421-021-00316-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sozen B, Jorgensen V, Weatherbee BAT, Chen S, Zhu M, and Zernicka-Goetz M (2021). Reconstructing aspects of human embryogenesis with pluripotent stem cells. Nat Commun 12, 5550. 10.1038/s41467-021-25853-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kagawa H, Javali A, Khoei HH, Sommer TM, Sestini G, Novatchkova M, Scholte Op Reimer Y, Castel G, Bruneau A, Maenhoudt N, et al. (2022). Human blastoids model blastocyst development and implantation. Nature 601, 600–605. 10.1038/s41586-021-04267-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yu L, Logsdon D, Pinzon-Arteaga CA, Duan J, Ezashi T, Wei Y, Ribeiro Orsi AE, Oura S, Liu L, Wang L, et al. (2023). Large-scale production of human blastoids amenable to modeling blastocyst development and maternal-fetal cross talk. Cell Stem Cell 30, 1246–1261 e1249. 10.1016/j.stem.2023.08.002. [DOI] [PubMed] [Google Scholar]
- 71.Karvas RM, Zemke JE, Ali SS, Upton E, Sane E, Fischer LA, Dong C, Park KM, Wang F, Park K, et al. (2023). 3D-cultured blastoids model human embryogenesis from pre-implantation to early gastrulation stages. Cell Stem Cell 30, 1148–1165 e1147. 10.1016/j.stem.2023.08.005. [DOI] [PubMed] [Google Scholar]
- 72.Guo M, Wu J, Chen C, Wang X, Gong A, Guan W, Karvas RM, Wang K, Min M, Wang Y, et al. (2024). Self-renewing human naive pluripotent stem cells dedifferentiate in 3D culture and form blastoids spontaneously. Nat Commun 15, 668. 10.1038/s41467-024-44969-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rivron NC, Frias-Aldeguer J, Vrij EJ, Boisset JC, Korving J, Vivie J, Truckenmuller RK, van Oudenaarden A, van Blitterswijk CA, and Geijsen N (2018). Blastocyst-like structures generated solely from stem cells. Nature 557, 106–111. 10.1038/s41586-018-0051-0. [DOI] [PubMed] [Google Scholar]
- 74.Sozen B, Cox AL, De Jonghe J, Bao M, Hollfelder F, Glover DM, and Zernicka-Goetz M (2019). Self-Organization of Mouse Stem Cells into an Extended Potential Blastoid. Dev Cell 51, 698–712 e698. 10.1016/j.devcel.2019.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li R, Zhong C, Yu Y, Liu H, Sakurai M, Yu L, Min Z, Shi L, Wei Y, Takahashi Y, et al. (2019). Generation of Blastocyst-like Structures from Mouse Embryonic and Adult Cell Cultures. Cell 179, 687–702 e618. 10.1016/j.cell.2019.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kime C, Kiyonari H, Ohtsuka S, Kohbayashi E, Asahi M, Yamanaka S, Takahashi M, and Tomoda K (2019). Induced 2C Expression and Implantation-Competent Blastocyst-like Cysts from Primed Pluripotent Stem Cells. Stem Cell Reports 13, 485–498. 10.1016/j.stemcr.2019.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang P, Zhai X, Huang B, Sun S, Wang W, and Zhang M (2022). Highly efficient generation of blastocyst-like structures from mouse totipotent blastomere-like cells. Cold Spring Harbor Laboratory. [DOI] [PubMed] [Google Scholar]
- 78.Gao X, Nowak-Imialek M, Chen X, Chen D, Herrmann D, Ruan D, Chen ACH, Eckersley-Maslin MA, Ahmad S, Lee YL, et al. (2019). Establishment of porcine and human expanded potential stem cells. Nat Cell Biol 21, 687–699. 10.1038/s41556-019-0333-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu X, Tan JP, Schroder J, Aberkane A, Ouyang JF, Mohenska M, Lim SM, Sun YBY, Chen J, Sun G, et al. (2021). Modelling human blastocysts by reprogramming fibroblasts into iBlastoids. Nature 591, 627–632. 10.1038/s41586-021-03372-y. [DOI] [PubMed] [Google Scholar]
- 80.Tu Z, Bi Y, Zhu X, Liu W, Hu J, Wu L, Mao T, Zhou J, Wang H, Wang H, et al. (2023). Modeling human pregastrulation development by 3D culture of blastoids generated from primed-to-naive transitioning intermediates. Protein Cell 14, 337–349. 10.1093/procel/pwac041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhao C, Reyes AP, Schell JP, Weltner J, Ortega NM, Zheng Y, Björklund ÅK, Rossant J, Fu J, Petropoulos S, and Lanner F (2021). Reprogrammed blastoids contain amnion-like cells but not trophectoderm. Cold Spring Harbor Laboratory. [Google Scholar]
- 82.Zheng Y, Yan RZ, Sun S, Kobayashi M, Xiang L, Yang R, Goedel A, Kang Y, Xue X, Esfahani SN, et al. (2022). Single-cell analysis of embryoids reveals lineage diversification roadmaps of early human development. Cell Stem Cell 29, 1402–1419 e1408. 10.1016/j.stem.2022.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Clark AT, Brivanlou A, Fu J, Kato K, Mathews D, Niakan KK, Rivron N, Saitou M, Surani A, Tang F, and Rossant J (2021). Human embryo research, stem cell-derived embryo models and in vitro gametogenesis: Considerations leading to the revised ISSCR guidelines. Stem Cell Reports 16, 1416–1424. 10.1016/j.stemcr.2021.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li J, Zhu Q, Cao J, Liu Y, Lu Y, Sun Y, Li Q, Huang Y, Shang S, Bian X, et al. (2023). Cynomolgus monkey embryo model captures gastrulation and early pregnancy. Cell Stem Cell 30, 362–377 e367. 10.1016/j.stem.2023.03.009. [DOI] [PubMed] [Google Scholar]
- 85.Pinzon-Arteaga CA, Wang Y, Wei Y, Ribeiro Orsi AE, Li L, Scatolin G, Liu L, Sakurai M, Ye J, Hao M, et al. (2023). Bovine blastocyst-like structures derived from stem cell cultures. Cell Stem Cell 30, 611–616 e617. 10.1016/j.stem.2023.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang Y, Ming H, Yu L, Li J, Zhu L, Sun HX, Pinzon-Arteaga CA, Wu J, and Jiang Z (2023). Establishment of bovine trophoblast stem cells. Cell Rep 42, 112439. 10.1016/j.celrep.2023.112439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhao L, Gao X, Zheng Y, Wang Z, Zhao G, Ren J, Zhang J, Wu J, Wu B, Chen Y, et al. (2021). Establishment of bovine expanded potential stem cells. Proc Natl Acad Sci U S A 118. 10.1073/pnas.2018505118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wu J, and Izpisua Belmonte JC (2015). Dynamic Pluripotent Stem Cell States and Their Applications. Cell Stem Cell 17, 509–525. 10.1016/j.stem.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 89.Taei A, Kiani T, Taghizadeh Z, Moradi S, Samadian A, Mollamohammadi S, Sharifi-Zarchi A, Guenther S, Akhlaghpour A, Asgari Abibeiglou B, et al. (2020). Temporal activation of LRH-1 and RAR-gamma in human pluripotent stem cells induces a functional naive-like state. EMBO Rep 21, e47533. 10.15252/embr.201847533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wu J, Platero-Luengo A, Sakurai M, Sugawara A, Gil MA, Yamauchi T, Suzuki K, Bogliotti YS, Cuello C, Morales Valencia M, et al. (2017). Interspecies Chimerism with Mammalian Pluripotent Stem Cells. Cell 168, 473–486 e415. 10.1016/j.cell.2016.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Aksoy I, Rognard C, Moulin A, Marcy G, Masfaraud E, Wianny F, Cortay V, Bellemin-Menard A, Doerflinger N, Dirheimer M, et al. (2021). Apoptosis, G1 Phase Stall, and Premature Differentiation Account for Low Chimeric Competence of Human and Rhesus Monkey Naive Pluripotent Stem Cells. Stem Cell Reports 16, 56–74. 10.1016/j.stemcr.2020.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Theunissen TW, Friedli M, He Y, Planet E, O'Neil RC, Markoulaki S, Pontis J, Wang H, Iouranova A, Imbeault M, et al. (2016). Molecular Criteria for Defining the Naive Human Pluripotent State. Cell Stem Cell 19, 502–515. 10.1016/j.stem.2016.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Takashima Y, Guo G, Loos R, Nichols J, Ficz G, Krueger F, Oxley D, Santos F, Clarke J, Mansfield W, et al. (2014). Resetting transcription factor control circuitry toward ground-state pluripotency in human. Cell 158, 1254–1269. 10.1016/j.cell.2014.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Huang K, Zhu Y, Ma Y, Zhao B, Fan N, Li Y, Song H, Chu S, Ouyang Z, Zhang Q, et al. (2018). BMI1 enables interspecies chimerism with human pluripotent stem cells. Nat Commun 9, 4649. 10.1038/s41467-018-07098-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang X, Li T, Cui T, Yu D, Liu C, Jiang L, Feng G, Wang L, Fu R, Zhang X, et al. (2018). Human embryonic stem cells contribute to embryonic and extraembryonic lineages in mouse embryos upon inhibition of apoptosis. Cell Res 28, 126–129. 10.1038/cr.2017.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ross C, and Boroviak TE (2020). Origin and function of the yolk sac in primate embryogenesis. Nat Commun 11, 3760. 10.1038/s41467-020-17575-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Luckett WP (1978). Origin and differentiation of the yolk sac and extraembryonic mesoderm in presomite human and rhesus monkey embryos. Am J Anat 152, 59–97. 10.1002/aja.1001520106. [DOI] [PubMed] [Google Scholar]
- 98.Shao Y, Taniguchi K, Gurdziel K, Townshend RF, Xue X, Yong KMA, Sang J, Spence JR, Gumucio DL, and Fu J (2017). Self-organized amniogenesis by human pluripotent stem cells in a biomimetic implantation-like niche. Nat Mater 16, 419–425. 10.1038/nmat4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pham TXA, Panda A, Kagawa H, To SK, Ertekin C, Georgolopoulos G, van Knippenberg S, Allsop RN, Bruneau A, Chui JS, et al. (2022). Modeling human extraembryonic mesoderm cells using naive pluripotent stem cells. Cell Stem Cell 29, 1346–1365 e1310. 10.1016/j.stem.2022.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gerri C, Menchero S, Mahadevaiah SK, Turner JMA, and Niakan KK (2020). Human Embryogenesis: A Comparative Perspective. Annu Rev Cell Dev Biol 36, 411–440. 10.1146/annurev-cellbio-022020-024900. [DOI] [PubMed] [Google Scholar]
- 101.New DA, Coppola PT, and Terry S (1973). Culture of explanted rat embryos in rotating tubes. J Reprod Fertil 35, 135–138. 10.1530/jrf.0.0350135. [DOI] [PubMed] [Google Scholar]
- 102.Aguilera-Castrejon A, Oldak B, Shani T, Ghanem N, Itzkovich C, Slomovich S, Tarazi S, Bayerl J, Chugaeva V, Ayyash M, et al. (2021). Ex utero mouse embryogenesis from pre-gastrulation to late organogenesis. Nature 593, 119–124. 10.1038/s41586-021-03416-3. [DOI] [PubMed] [Google Scholar]
- 103.Taniguchi K, Shao Y, Townshend RF, Tsai YH, DeLong CJ, Lopez SA, Gayen S, Freddo AM, Chue DJ, Thomas DJ, et al. (2015). Lumen Formation Is an Intrinsic Property of Isolated Human Pluripotent Stem Cells. Stem Cell Reports 5, 954–962. 10.1016/j.stemcr.2015.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shao Y, Taniguchi K, Townshend RF, Miki T, Gumucio DL, and Fu J (2017). A pluripotent stem cell-based model for post-implantation human amniotic sac development. Nat Commun 8, 208. 10.1038/s41467-017-00236-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zheng Y, Xue X, Shao Y, Wang S, Esfahani SN, Li Z, Muncie JM, Lakins JN, Weaver VM, Gumucio DL, and Fu J (2019). Controlled modelling of human epiblast and amnion development using stem cells. Nature 573, 421–425. 10.1038/s41586-019-1535-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Simunovic M, Metzger JJ, Etoc F, Yoney A, Ruzo A, Martyn I, Croft G, You DS, Brivanlou AH, and Siggia ED (2019). A 3D model of a human epiblast reveals BMP4-driven symmetry breaking. Nat Cell Biol 21, 900–910. 10.1038/s41556-019-0349-7. [DOI] [PubMed] [Google Scholar]
- 107.Simunovic M, Siggia ED, and Brivanlou AH (2022). In vitro attachment and symmetry breaking of a human embryo model assembled from primed embryonic stem cells. Cell Stem Cell 29, 962–972 e964. 10.1016/j.stem.2022.05.001. [DOI] [PubMed] [Google Scholar]
- 108.Oldak B, Wildschutz E, Bondarenko V, Comar MY, Zhao C, Aguilera-Castrejon A, Tarazi S, Viukov S, Pham TXA, Ashouokhi S, et al. (2023). Complete human day 14 post-implantation embryo models from naive ES cells. Nature 622, 562–573. 10.1038/s41586-023-06604-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pedroza M, Gassaloglu SI, Dias N, Zhong L, Hou TJ, Kretzmer H, Smith ZD, and Sozen B (2023). Self-patterning of human stem cells into post-implantation lineages. Nature 622, 574–583. 10.1038/s41586-023-06354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Weatherbee BAT, Gantner CW, Iwamoto-Stohl LK, Daza RM, Hamazaki N, Shendure J, and Zernicka-Goetz M (2023). Pluripotent stem cell-derived model of the post-implantation human embryo. Nature 622, 584–593. 10.1038/s41586-023-06368-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Okubo T, Rivron N, Kabata M, Masaki H, Kishimoto K, Semi K, Nakajima-Koyama M, Kunitomi H, Kaswandy B, Sato H, et al. (2023). Hypoblast from human pluripotent stem cells regulates epiblast development. Nature. 10.1038/s41586-023-06871-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hislop J, Song Q, Keshavarz FK, Alavi A, Schoenberger R, LeGraw R, Velazquez JJ, Mokhtari T, Taheri MN, Rytel M, et al. (2023). Modelling post-implantation human development to yolk sac blood emergence. Nature. 10.1038/s41586-023-06914-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liu L, Oura S, Markham Z, Hamilton JN, Skory RM, Li L, Sakurai M, Wang L, Pinzon-Arteaga CA, Plachta N, et al. (2023). Modeling post-implantation stages of human development into early organogenesis with stem-cell-derived peri-gastruloids. Cell 186, 3776–3792 e3716. 10.1016/j.cell.2023.07.018. [DOI] [PubMed] [Google Scholar]
- 114.Tan T, Wu J, Si C, Dai S, Zhang Y, Sun N, Zhang E, Shao H, Si W, Yang P, et al. (2021). Chimeric contribution of human extended pluripotent stem cells to monkey embryos ex vivo. Cell 184, 2020–2032 e2014. 10.1016/j.cell.2021.03.020. [DOI] [PubMed] [Google Scholar]
- 115.Pastor WA, Chen D, Liu W, Kim R, Sahakyan A, Lukianchikov A, Plath K, Jacobsen SE, and Clark AT (2016). Naive Human Pluripotent Cells Feature a Methylation Landscape Devoid of Blastocyst or Germline Memory. Cell Stem Cell 18, 323–329. 10.1016/j.stem.2016.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liu X, Nefzger CM, Rossello FJ, Chen J, Knaupp AS, Firas J, Ford E, Pflueger J, Paynter JM, Chy HS, et al. (2017). Comprehensive characterization of distinct states of human naive pluripotency generated by reprogramming. Nat Methods 14, 1055–1062. 10.1038/nmeth.4436. [DOI] [PubMed] [Google Scholar]
- 117.Liu B, Chen S, Xu Y, Lyu Y, Wang J, Du Y, Sun Y, Liu H, Zhou H, Lai W, et al. (2021). Chemically defined and xeno-free culture condition for human extended pluripotent stem cells. Nat Commun 12, 3017. 10.1038/s41467-021-23320-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wu J, Okamura D, Li M, Suzuki K, Luo C, Ma L, He Y, Li Z, Benner C, Tamura I, et al. (2015). An alternative pluripotent state confers interspecies chimaeric competency. Nature 521, 316–321. 10.1038/nature14413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mascetti VL, and Pedersen RA (2016). Human-Mouse Chimerism Validates Human Stem Cell Pluripotency. Cell Stem Cell 18, 67–72. 10.1016/j.stem.2015.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kwon GS, Viotti M, and Hadjantonakis AK (2008). The endoderm of the mouse embryo arises by dynamic widespread intercalation of embryonic and extraembryonic lineages. Dev Cell 15, 509–520. 10.1016/j.devcel.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Takaoka K, and Hamada H (2012). Cell fate decisions and axis determination in the early mouse embryo. Development 139, 3–14. 10.1242/dev.060095. [DOI] [PubMed] [Google Scholar]
- 122.Nakamura T, Okamoto I, Sasaki K, Yabuta Y, Iwatani C, Tsuchiya H, Seita Y, Nakamura S, Yamamoto T, and Saitou M (2016). A developmental coordinate of pluripotency among mice, monkeys and humans. Nature 537, 57–62. 10.1038/nature19096. [DOI] [PubMed] [Google Scholar]
- 123.Sasaki K, Nakamura T, Okamoto I, Yabuta Y, Iwatani C, Tsuchiya H, Seita Y, Nakamura S, Shiraki N, Takakuwa T, et al. (2016). The Germ Cell Fate of Cynomolgus Monkeys Is Specified in the Nascent Amnion. Dev Cell 39, 169–185. 10.1016/j.devcel.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 124.Mole MA, Coorens THH, Shahbazi MN, Weberling A, Weatherbee BAT, Gantner CW, Sancho-Serra C, Richardson L, Drinkwater A, Syed N, et al. (2021). A single cell characterisation of human embryogenesis identifies pluripotency transitions and putative anterior hypoblast centre. Nat Commun 12, 3679. 10.1038/s41467-021-23758-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ohinata Y, Payer B, O'Carroll D, Ancelin K, Ono Y, Sano M, Barton SC, Obukhanych T, Nussenzweig M, Tarakhovsky A, et al. (2005). Blimp1 is a critical determinant of the germ cell lineage in mice. Nature 436, 207–213. 10.1038/nature03813. [DOI] [PubMed] [Google Scholar]
- 126.Saitou M, Barton SC, and Surani MA (2002). A molecular programme for the specification of germ cell fate in mice. Nature 418, 293–300. 10.1038/nature00927. [DOI] [PubMed] [Google Scholar]
- 127.Lawson KA, Dunn NR, Roelen BA, Zeinstra LM, Davis AM, Wright CV, Korving JP, and Hogan BL (1999). Bmp4 is required for the generation of primordial germ cells in the mouse embryo. Genes Dev 13, 424–436. 10.1101/gad.13.4.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chen D, Sun N, Hou L, Kim R, Faith J, Aslanyan M, Tao Y, Zheng Y, Fu J, Liu W, et al. (2019). Human Primordial Germ Cells Are Specified from Lineage-Primed Progenitors. Cell Rep 29, 4568–4582 e4565. 10.1016/j.celrep.2019.11.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Castillo-Venzor A, Penfold CA, Morgan MD, Tang WW, Kobayashi T, Wong FC, Bergmann S, Slatery E, Boroviak TE, Marioni JC, and Surani MA (2023). Origin and segregation of the human germline. Life Sci Alliance 6. 10.26508/lsa.202201706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sheng G, Martinez Arias A, and Sutherland A (2021). The primitive streak and cellular principles of building an amniote body through gastrulation. Science 374, abg1727. 10.1126/science.abg1727. [DOI] [PubMed] [Google Scholar]
- 131.Munoz-Sanjuan I, and Brivanlou AH (2002). Neural induction, the default model and embryonic stem cells. Nat Rev Neurosci 3, 271–280. 10.1038/nrn786. [DOI] [PubMed] [Google Scholar]
- 132.Colas JF, and Schoenwolf GC (2001). Towards a cellular and molecular understanding of neurulation. Dev Dyn 221, 117–145. 10.1002/dvdy.1144. [DOI] [PubMed] [Google Scholar]
- 133.Wymeersch FJ, Wilson V, and Tsakiridis A (2021). Understanding axial progenitor biology in vivo and in vitro. Development 148. 10.1242/dev.180612. [DOI] [PubMed] [Google Scholar]
- 134.Santos C, Murray A, Marshall AR, Metcalfe K, Narayan P, de Castro SCP, Maniou E, Greene NDE, Galea GL, and Copp AJ (2023). Spinal neural tube formation and regression in human embryos. eLife. 10.7554/eLife.88584.1. [DOI] [Google Scholar]
- 135.Saitsu H, and Shiota K (2008). Involvement of the axially condensed tail bud mesenchyme in normal and abnormal human posterior neural tube development. Congenit Anom (Kyoto) 48, 1–6. 10.1111/j.1741-4520.2007.00178.x. [DOI] [PubMed] [Google Scholar]
- 136.Simoes-Costa M, and Bronner ME (2015). Establishing neural crest identity: a gene regulatory recipe. Development 142, 242–257. 10.1242/dev.105445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rocha M, Beiriger A, Kushkowski EE, Miyashita T, Singh N, Venkataraman V, and Prince VE (2020). From head to tail: regionalization of the neural crest. Development 147. 10.1242/dev.193888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Dubrulle J, McGrew MJ, and Pourquie O (2001). FGF signaling controls somite boundary position and regulates segmentation clock control of spatiotemporal Hox gene activation. Cell 106, 219–232. 10.1016/s0092-8674(01)00437-8. [DOI] [PubMed] [Google Scholar]
- 139.Dubrulle J, and Pourquie O (2004). fgf8 mRNA decay establishes a gradient that couples axial elongation to patterning in the vertebrate embryo. Nature 427, 419–422. 10.1038/nature02216. [DOI] [PubMed] [Google Scholar]
- 140.Cooke J, and Zeeman EC (1976). A clock and wavefront model for control of the number of repeated structures during animal morphogenesis. J Theor Biol 58, 455–476. 10.1016/s0022-5193(76)80131-2. [DOI] [PubMed] [Google Scholar]
- 141.Harima Y, Takashima Y, Ueda Y, Ohtsuka T, and Kageyama R (2013). Accelerating the tempo of the segmentation clock by reducing the number of introns in the Hes7 gene. Cell Rep 3, 1–7. 10.1016/j.celrep.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 142.Schroter C, and Oates AC (2010). Segment number and axial identity in a segmentation clock period mutant. Curr Biol 20, 1254–1258. 10.1016/j.cub.2010.05.071. [DOI] [PubMed] [Google Scholar]
- 143.Warmflash A, Sorre B, Etoc F, Siggia ED, and Brivanlou AH (2014). A method to recapitulate early embryonic spatial patterning in human embryonic stem cells. Nat Methods 11, 847–854. 10.1038/nmeth.3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Muncie JM, Ayad NME, Lakins JN, Xue X, Fu J, and Weaver VM (2020). Mechanical Tension Promotes Formation of Gastrulation-like Nodes and Patterns Mesoderm Specification in Human Embryonic Stem Cells. Dev Cell 55, 679–694 e611. 10.1016/j.devcel.2020.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Manfrin A, Tabata Y, Paquet ER, Vuaridel AR, Rivest FR, Naef F, and Lutolf MP (2019). Engineered signaling centers for the spatially controlled patterning of human pluripotent stem cells. Nat Methods 16, 640–648. 10.1038/s41592-019-0455-2. [DOI] [PubMed] [Google Scholar]
- 146.van den Brink SC, Baillie-Johnson P, Balayo T, Hadjantonakis AK, Nowotschin S, Turner DA, and Martinez Arias A (2014). Symmetry breaking, germ layer specification and axial organisation in aggregates of mouse embryonic stem cells. Development 141, 4231–4242. 10.1242/dev.113001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Beccari L, Moris N, Girgin M, Turner DA, Baillie-Johnson P, Cossy AC, Lutolf MP, Duboule D, and Arias AM (2018). Multi-axial self-organization properties of mouse embryonic stem cells into gastruloids. Nature 562, 272–276. 10.1038/s41586-018-0578-0. [DOI] [PubMed] [Google Scholar]
- 148.van den Brink SC, Alemany A, van Batenburg V, Moris N, Blotenburg M, Vivie J, Baillie-Johnson P, Nichols J, Sonnen KF, Martinez Arias A, and van Oudenaarden A (2020). Single-cell and spatial transcriptomics reveal somitogenesis in gastruloids. Nature 582, 405–409. 10.1038/s41586-020-2024-3. [DOI] [PubMed] [Google Scholar]
- 149.Veenvliet JV, Bolondi A, Kretzmer H, Haut L, Scholze-Wittler M, Schifferl D, Koch F, Guignard L, Kumar AS, Pustet M, et al. (2020). Mouse embryonic stem cells self-organize into trunk-like structures with neural tube and somites. Science 370. 10.1126/science.aba4937. [DOI] [PubMed] [Google Scholar]
- 150.Rossi G, Broguiere N, Miyamoto M, Boni A, Guiet R, Girgin M, Kelly RG, Kwon C, and Lutolf MP (2021). Capturing Cardiogenesis in Gastruloids. Cell Stem Cell 28, 230–240 e236. 10.1016/j.stem.2020.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Rossi G, Giger S, Hübscher T, and Lutolf MP (2021). Gastruloids asin vitromodels of embryonic blood development with spatial and temporal resolution. Cold Spring Harbor Laboratory. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Girgin MU, Broguiere N, Mattolini L, and Lutolf MP (2021). Gastruloids generated without exogenous Wnt activation develop anterior neural tissues. Stem Cell Reports 16, 1143–1155. 10.1016/j.stemcr.2021.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Xu PF, Borges RM, Fillatre J, de Oliveira-Melo M, Cheng T, Thisse B, and Thisse C (2021). Construction of a mammalian embryo model from stem cells organized by a morphogen signalling centre. Nat Commun 12, 3277. 10.1038/s41467-021-23653-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Girgin MU, Broguiere N, Hoehnel S, Brandenberg N, Mercier B, Arias AM, and Lutolf MP (2021). Bioengineered embryoids mimic post-implantation development in vitro. Nat Commun 12, 5140. 10.1038/s41467-021-25237-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bérenger-Currias NMLP, Mircea M, Adegeest E, Van Den Berg PR, Feliksik M, Hochane M, Idema T, Tans SJ, and Semrau S (2020). Extraembryonic endoderm cells induce neuroepithelial tissue in gastruloids. Cold Spring Harbor Laboratory. [Google Scholar]
- 156.Harrison SE, Sozen B, Christodoulou N, Kyprianou C, and Zernicka-Goetz M (2017). Assembly of embryonic and extraembryonic stem cells to mimic embryogenesis in vitro. Science 356, eaal1810. 10.1126/science.aal1810. [DOI] [PubMed] [Google Scholar]
- 157.Sozen B, Amadei G, Cox A, Wang R, Na E, Czukiewska S, Chappell L, Voet T, Michel G, Jing N, et al. (2018). Self-assembly of embryonic and two extra-embryonic stem cell types into gastrulating embryo-like structures. Nat Cell Biol 20, 979–989. 10.1038/s41556-018-0147-7. [DOI] [PubMed] [Google Scholar]
- 158.Zhang S, Chen T, Chen N, Gao D, Shi B, Kong S, West RC, Yuan Y, Zhi M, Wei Q, et al. (2019). Implantation initiation of self-assembled embryo-like structures generated using three types of mouse blastocyst-derived stem cells. Nat Commun 10, 496. 10.1038/s41467-019-08378-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Amadei G, Lau KYC, De Jonghe J, Gantner CW, Sozen B, Chan C, Zhu M, Kyprianou C, Hollfelder F, and Zernicka-Goetz M (2021). Inducible Stem-Cell-Derived Embryos Capture Mouse Morphogenetic Events In Vitro. Dev Cell 56, 366–382 e369. 10.1016/j.devcel.2020.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Moris N, Anlas K, van den Brink SC, Alemany A, Schroder J, Ghimire S, Balayo T, van Oudenaarden A, and Martinez Arias A (2020). An in vitro model of early anteroposterior organization during human development. Nature 582, 410–415. 10.1038/s41586-020-2383-9. [DOI] [PubMed] [Google Scholar]
- 161.Olmsted ZT, and Paluh JL (2021). Co-development of central and peripheral neurons with trunk mesendoderm in human elongating multi-lineage organized gastruloids. Nat Commun 12, 3020. 10.1038/s41467-021-23294-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Yaman YI, Huang R, and Ramanathan S (2022). Coupled organoids reveal that signaling gradients drive traveling segmentation clock waves during human axial morphogenesis. Cold Spring Harbor Laboratory. [Google Scholar]
- 163.Yaman YI, and Ramanathan S (2023). Controlling human organoid symmetry breaking reveals signaling gradients drive segmentation clock waves. Cell 186, 513–527 e519. 10.1016/j.cell.2022.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Gribaudo S, Robert R, van Sambeek B, Mirdass C, Lyubimova A, Bouhali K, Ferent J, Morin X, van Oudenaarden A, and Nedelec S (2023). Self-organizing models of human trunk organogenesis recapitulate spinal cord and spine co-morphogenesis. Nat Biotechnol. 10.1038/s41587-023-01956-9. [DOI] [PubMed] [Google Scholar]
- 165.Miao Y, Djeffal Y, De Simone A, Zhu K, Silberfeld A, Lee JG, Rao J, Tarazona OA, Mongera A, Rigoni P, et al. (2022). Reconstruction and deconstruction of human somitogenesis in vitro. Cold Spring Harbor Laboratory. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sanaki-Matsumiya M, Matsuda M, Gritti N, Nakaki F, Sharpe J, Trivedi V, and Ebisuya M (2022). Periodic formation of epithelial somites from human pluripotent stem cells. Nat Commun 13, 2325. 10.1038/s41467-022-29967-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Yamanaka Y, Hamidi S, Yoshioka-Kobayashi K, Munira S, Sunadome K, Zhang Y, Kurokawa Y, Ericsson R, Mieda A, Thompson JL, et al. (2023). Reconstituting human somitogenesis in vitro. Nature 614, 509–520. 10.1038/s41586-022-05649-2. [DOI] [PubMed] [Google Scholar]
- 168.Meinhardt A, Eberle D, Tazaki A, Ranga A, Niesche M, Wilsch-Brauninger M, Stec A, Schackert G, Lutolf M, and Tanaka EM (2014). 3D reconstitution of the patterned neural tube from embryonic stem cells. Stem Cell Reports 3, 987–999. 10.1016/j.stemcr.2014.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Duval N, Vaslin C, Barata TC, Frarma Y, Contremoulins V, Baudin X, Nedelec S, and Ribes VC (2019). BMP4 patterns Smad activity and generates stereotyped cell fate organization in spinal organoids. Development 146, dev175430. 10.1242/dev.175430. [DOI] [PubMed] [Google Scholar]
- 170.Ogura T, Sakaguchi H, Miyamoto S, and Takahashi J (2018). Three-dimensional induction of dorsal, intermediate and ventral spinal cord tissues from human pluripotent stem cells. Development 145, dev162214. 10.1242/dev.162214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zheng Y, Xue X, Resto-Irizarry AM, Li Z, Shao Y, Zheng Y, Zhao G, and Fu J (2019). Dorsal-ventral patterned neural cyst from human pluripotent stem cells in a neurogenic niche. Sci Adv 5, eaax5933. 10.1126/sciadv.aax5933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Abdel Fattah AR, Daza B, Rustandi G, Berrocal-Rubio MA, Gorissen B, Poovathingal S, Davie K, Barrasa-Fano J, Condor M, Cao X, et al. (2021). Actuation enhances patterning in human neural tube organoids. Nat Commun 12, 3192. 10.1038/s41467-021-22952-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Xue X, Sun Y, Resto-Irizarry AM, Yuan Y, Aw Yong KM, Zheng Y, Weng S, Shao Y, Chai Y, Studer L, and Fu J (2018). Mechanics-guided embryonic patterning of neuroectoderm tissue from human pluripotent stem cells. Nat Mater 17, 633–641. 10.1038/s41563-018-0082-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Britton G, Heemskerk I, Hodge R, Qutub AA, and Warmflash A (2019). A novel self-organizing embryonic stem cell system reveals signaling logic underlying the patterning of human ectoderm. Development 146, dev179093. 10.1242/dev.179093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Haremaki T, Metzger JJ, Rito T, Ozair MZ, Etoc F, and Brivanlou AH (2019). Self-organizing neuruloids model developmental aspects of Huntington's disease in the ectodermal compartment. Nat Biotechnol 37, 1198–1208. 10.1038/s41587-019-0237-5. [DOI] [PubMed] [Google Scholar]
- 176.Karzbrun E, Khankhel AH, Megale HC, Glasauer SMK, Wyle Y, Britton G, Warmflash A, Kosik KS, Siggia ED, Shraiman BI, and Streichan SJ (2021). Human neural tube morphogenesis in vitro by geometric constraints. Nature 599, 268–272. 10.1038/s41586-021-04026-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Sahni G, Chang SY, Meng JTC, Tan JZY, Fatien JJC, Bonnard C, Utami KH, Chan PW, Tan TT, Altunoglu U, et al. (2021). A Micropatterned Human-Specific Neuroepithelial Tissue for Modeling Gene and Drug-Induced Neurodevelopmental Defects. Adv Sci (Weinh) 8, 2001100. 10.1002/advs.202001100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Sun S, Xue X, and Fu J (2023). Modeling development using microfluidics: bridging gaps to foster fundamental and translational research. Curr Opin Genet Dev 82, 102097. 10.1016/j.gde.2023.102097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Rifes P, Isaksson M, Rathore GS, Aldrin-Kirk P, Møller OK, Barzaghi G, Lee J, Egerod KL, Rausch DM, Parmar M, et al. (2020). Modeling neural tube development by differentiation of human embryonic stem cells in a microfluidic WNT gradient. Nat Biotechnol 38, 1265–1273. 10.1038/s41587-020-0525-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Xue X, Kim YS, Ponce-Arias AI, O'Laughlin R, Yan RZ, Kobayashi N, Tshuva RY, Tsai YH, Sun S, Zheng Y, et al. (2024). A Patterned Human Neural Tube Model Using Microfluidic Gradients. Nature. 10.1038/s41586-024-07204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Das S, Koyano-Nakagawa N, Gafni O, Maeng G, Singh BN, Rasmussen T, Pan X, Choi KD, Mickelson D, Gong W, et al. (2020). Generation of human endothelium in pig embryos deficient in ETV2. Nat Biotechnol 38, 297–302. 10.1038/s41587-019-0373-y. [DOI] [PubMed] [Google Scholar]
- 182.Maeng G, Das S, Greising SM, Gong W, Singh BN, Kren S, Mickelson D, Skie E, Gafni O, Sorensen JR, et al. (2021). Humanized skeletal muscle in MYF5/MYOD/MYF6-null pig embryos. Nat Biomed Eng 5, 805–814. 10.1038/s41551-021-00693-1. [DOI] [PubMed] [Google Scholar]
- 183.Wang J, Xie W, Li N, Li W, Zhang Z, Fan N, Ouyang Z, Zhao Y, Lai C, Li H, et al. (2023). Generation of a humanized mesonephros in pigs from induced pluripotent stem cells via embryo complementation. Cell Stem Cell 30, 1235–1245 e1236. 10.1016/j.stem.2023.08.003. [DOI] [PubMed] [Google Scholar]
- 184.Fu J, Warmflash A, and Lutolf MP (2021). Stem-cell-based embryo models for fundamental research and translation. Nat Mater 20, 132–144. 10.1038/s41563-020-00829-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Shibata S, Endo S, Nagai LAE, E HK, Oike A, Kobayashi N, Kitamura A, Hori T, Nashimoto Y, Nakato R, et al. (2024). Modeling embryo-endometrial interface recapitulating human embryo implantation. Sci Adv 10, eadi4819. 10.1126/sciadv.adi4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wu J, Greely HT, Jaenisch R, Nakauchi H, Rossant J, and Belmonte JC (2016). Stem cells and interspecies chimaeras. Nature 540, 51–59. 10.1038/nature20573. [DOI] [PubMed] [Google Scholar]
- 187.Morata G, and Ripoll P (1975). Minutes: mutants of drosophila autonomously affecting cell division rate. Dev Biol 42, 211–221. 10.1016/0012-1606(75)90330-9. [DOI] [PubMed] [Google Scholar]
- 188.Baker NE (2020). Emerging mechanisms of cell competition. Nat Rev Genet 21, 683–697. 10.1038/s41576-020-0262-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Zhu Y, Zhang Z, Fan N, Huang K, Li H, Gu J, Zhang Q, Ouyang Z, Zhang T, Tang J, et al. (2022). Generating functional cells through enhanced interspecies chimerism with human pluripotent stem cells. Stem Cell Reports 17, 1059–1069. 10.1016/j.stemcr.2022.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Zheng C, Hu Y, Sakurai M, Pinzon-Arteaga CA, Li J, Wei Y, Okamura D, Ravaux B, Barlow HR, Yu L, et al. (2021). Cell competition constitutes a barrier for interspecies chimerism. Nature 592, 272–276. 10.1038/s41586-021-03273-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Meyer SN, Amoyel M, Bergantinos C, de la Cova C, Schertel C, Basler K, and Johnston LA (2014). An ancient defense system eliminates unfit cells from developing tissues during cell competition. Science 346, 1258236. 10.1126/science.1258236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Hu Y, Sun HX, Sakurai M, Jones AE, Liu L, Cheng T, Zheng C, Li J, Ravaux B, Luo Z, et al. (2023). RNA Sensing and Innate Immunity Constitutes a Barrier for Interspecies Chimerism. bioRxiv, 2023.2003.2007.531624. 10.1101/2023.03.07.531624. [DOI] [Google Scholar]
- 193.Glass DS, and Riedel-Kruse IH (2018). A Synthetic Bacterial Cell-Cell Adhesion Toolbox for Programming Multicellular Morphologies and Patterns. Cell 174, 649–658 e616. 10.1016/j.cell.2018.06.041. [DOI] [PubMed] [Google Scholar]
- 194.Stevens AJ, Harris AR, Gerdts J, Kim KH, Trentesaux C, Ramirez JT, McKeithan WL, Fattahi F, Klein OD, Fletcher DA, and Lim WA (2023). Programming multicellular assembly with synthetic cell adhesion molecules. Nature 614, 144–152. 10.1038/s41586-022-05622-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Beghein E, and Gettemans J (2017). Nanobody Technology: A Versatile Toolkit for Microscopic Imaging, Protein-Protein Interaction Analysis, and Protein Function Exploration. Front Immunol 8, 771. 10.3389/fimmu.2017.00771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Rayon T, Stamataki D, Perez-Carrasco R, Garcia-Perez L, Barrington C, Melchionda M, Exelby K, Lazaro J, Tybulewicz VLJ, Fisher EMC, and Briscoe J (2020). Species-specific pace of development is associated with differences in protein stability. Science 369. 10.1126/science.aba7667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Barry C, Schmitz MT, Jiang P, Schwartz MP, Duffin BM, Swanson S, Bacher R, Bolin JM, Elwell AL, McIntosh BE, et al. (2017). Species-specific developmental timing is maintained by pluripotent stem cells ex utero. Dev Biol 423, 101–110. 10.1016/j.ydbio.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Matsuda M, Yamanaka Y, Uemura M, Osawa M, Saito MK, Nagahashi A, Nishio M, Guo L, Ikegawa S, Sakurai S, et al. (2020). Recapitulating the human segmentation clock with pluripotent stem cells. Nature 580, 124–129. 10.1038/s41586-020-2144-9. [DOI] [PubMed] [Google Scholar]
- 199.Diaz-Cuadros M, Wagner DE, Budjan C, Hubaud A, Tarazona OA, Donelly S, Michaut A, Al Tanoury Z, Yoshioka-Kobayashi K, Niino Y, et al. (2020). In vitro characterization of the human segmentation clock. Nature 580, 113–118. 10.1038/s41586-019-1885-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Chu LF, Mamott D, Ni Z, Bacher R, Liu C, Swanson S, Kendziorski C, Stewart R, and Thomson JA (2019). An In Vitro Human Segmentation Clock Model Derived from Embryonic Stem Cells. Cell Rep 28, 2247–2255 e2245. 10.1016/j.celrep.2019.07.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Lazaro J, Costañzo M, Sanaki-Matsumiya M, Girardot C, Hayashi M, Hayashi K, Diecke S, Hildebrandt TB, Lazzari G, Wu J, et al. (2023). A stem cell zoo uncovers intracellular scaling of developmental tempo across mammals. Cell Stem Cell 30, 938–949 e937. 10.1016/j.stem.2023.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Iwata R, Casimir P, Erkol E, Boubakar L, Planque M, Gallego Lopez IM, Ditkowska M, Gaspariunaite V, Beckers S, Remans D, et al. (2023). Mitochondria metabolism sets the species-specific tempo of neuronal development. Science 379, eabn4705. 10.1126/science.abn4705. [DOI] [PubMed] [Google Scholar]
- 203.Brown J, Barry C, Schmitz MT, Argus C, Bolin JM, Schwartz MP, Van Aartsen A, Steill J, Swanson S, Stewart R, et al. (2021). Interspecies chimeric conditions affect the developmental rate of human pluripotent stem cells. PLoS Comput Biol 17, e1008778. 10.1371/journal.pcbi.1008778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Huang J, He B, Yang X, Long X, Wei Y, Li L, Tang M, Gao Y, Fang Y, Ying W, et al. (2024). Generation of rat forebrain tissues in mice. Cell 187, 2129–2142 e2117. 10.1016/j.cell.2024.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Throesch BT, Khadeesh bin Imtiaz M, Muñoz-Castaneda R, Sakurai M, Hartzell AL, James KN, Rodriguez AR, Martin G, Lippi G, Kupriyanov S, et al. (2023). Building functional circuits in multispecies brains. bioRxiv, 2023.2004.2013.536815. 10.1101/2023.04.13.536815. [DOI] [Google Scholar]
- 206.Cohen MA, Wert KJ, Goldmann J, Markoulaki S, Buganim Y, Fu D, and Jaenisch R (2016). Human neural crest cells contribute to coat pigmentation in interspecies chimeras after in utero injection into mouse embryos. Proc Natl Acad Sci U S A 113, 1570–1575. 10.1073/pnas.1525518113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Jiang X, Gurel O, Mendiaz EA, Stearns GW, Clogston CL, Lu HS, Osslund TD, Syed RS, Langley KE, and Hendrickson WA (2000). Structure of the active core of human stem cell factor and analysis of binding to its receptor kit. EMBO J 19, 3192–3203. 10.1093/emboj/19.13.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Yamaguchi T, Sato H, Kobayashi T, Kato-Itoh M, Goto T, Hara H, Mizuno N, Yanagida A, Umino A, Hamanaka S, et al. (2018). An interspecies barrier to tetraploid complementation and chimera formation. Sci Rep 8, 15289. 10.1038/s41598-018-33690-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Hyun I, Clayton EW, Cong Y, Fujita M, Goldman SA, Hill LR, Monserrat N, Nakauchi H, Pedersen RA, Rooke HM, et al. (2021). ISSCR guidelines for the transfer of human pluripotent stem cells and their direct derivatives into animal hosts. Stem Cell Reports 16, 1409–1415. 10.1016/j.stemcr.2021.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]