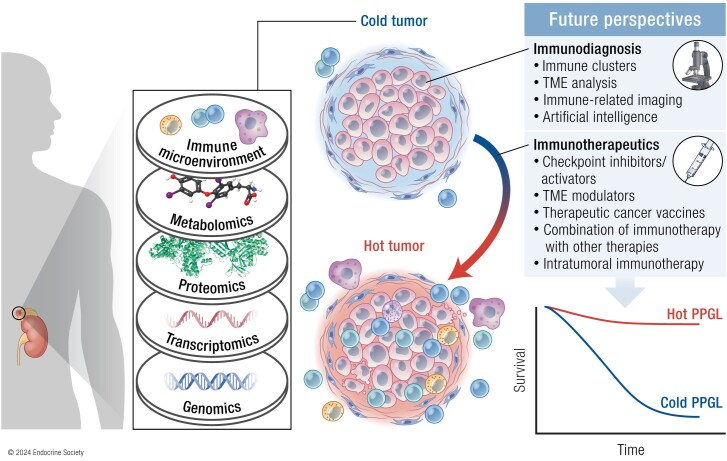
Abstract
Pheochromocytomas and paragangliomas (PPGLs) are rare neuroendocrine tumors derived from neural crest cells from adrenal medullary chromaffin tissues and extra-adrenal paraganglia, respectively. Although the current treatment for PPGLs is surgery, optimal treatment options for advanced and metastatic cases have been limited. Hence, understanding the role of the immune system in PPGL tumorigenesis can provide essential knowledge for the development of better therapeutic and tumor management strategies, especially for those with advanced and metastatic PPGLs. The first part of this review outlines the fundamental principles of the immune system and tumor microenvironment, and their role in cancer immunoediting, particularly emphasizing PPGLs. We focus on how the unique pathophysiology of PPGLs, such as their high molecular, biochemical, and imaging heterogeneity and production of several oncometabolites, creates a tumor-specific microenvironment and immunologically “cold” tumors. Thereafter, we discuss recently published studies related to the reclustering of PPGLs based on their immune signature. The second part of this review discusses future perspectives in PPGL management, including immunodiagnostic and promising immunotherapeutic approaches for converting “cold” tumors into immunologically active or “hot” tumors known for their better immunotherapy response and patient outcomes. Special emphasis is placed on potent immune-related imaging strategies and immune signatures that could be used for the reclassification, prognostication, and management of these tumors to improve patient care and prognosis. Furthermore, we introduce currently available immunotherapies and their possible combinations with other available therapies as an emerging treatment for PPGLs that targets hostile tumor environments.
Keywords: pheochromocytoma, paraganglioma, neuroendocrine tumors, cancer immunotherapy, perspectives, immune system
Graphical Abstract
Graphical Abstract.
Essential Points.
Pheochromocytomas and paragangliomas (PPGLs) have been previously considered as immunologically “cold” tumors due to their overall low mutational burden, their lack of tumor antigens, and, therefore, the decreased presence of immune cells in their microenvironment
The recent genetic reclustering of these tumors and advancements in techniques and bioinformatics tools focusing on the assessment of the tumor microenvironment have led to the uncovering of immunogenomic signatures as new promising avenues for the reclassification, prognostication, and management of PPGLs in the near future
According to the newest trends in cancer immunotherapies, research, and clinical studies/trials related to immunotherapy for PPGLs should focus on approaches activating both adaptive and innate immune systems to efficiently attract immune cells in these tumors and convert them from “cold” to “hot,” effectively making them highly vulnerable to subsequent immunotherapies
Combining various immunotherapeutic approaches with other supporting and therapeutic modalities, which further enhances immune system targeting of PPGLs and promotes long-term immunological memory, are promising approaches to targeting metastatic or advanced PPGLs or even preventing their development
Background and Relevance
Pheochromocytomas and paragangliomas (PPGLs) are neural crest tumors that arise from chromaffin cells of the adrenal gland or extra-adrenal tissues, respectively (1). Metastatic variants of these tumors are notoriously difficult to treat and often only stabilize (without regression) upon treatment with various agents. In a small fraction of patients, these tumors can be very aggressive and associated with rapid growth of organ metastatic lesions that ultimately cause either organ failure or severe catecholamine-related cardiovascular and other events, leading to morbidity and mortality (2). Thus, identifying new therapeutic options for treating metastatic or advanced PPGLs is urgently needed.
Over the last few years, considerable interest has been placed on systemic radiotherapies based on the concept of theranostics, a “treat what has been imaged approach” (3, 4). Although promising and valuable for many patients with metastatic PPGLs, these treatments most often promote disease stabilization. Thus, the majority of the guidelines recommend avoiding such therapies in patients with rapidly progressive tumors who are often preferably treated with chemotherapies (eg, a combination of cyclophosphamide/vincristine/dacarbazine, tyrosine-kinase inhibitors, and temozolomide) (2, 5, 6). Furthermore, less common therapeutic models that may be conceptually efficacious are practically nonexistent. These would include neoadjuvant therapies, combined therapies (eg, combining chemotherapy with radiotherapy or immunotherapy with radiotherapy), or the use of dual targeted radionuclide therapies (7, 8). Currently, most clinical trials still solely focus on discrete/solitary approaches, such as chemotherapies, radiotherapies, or treatments altering signaling pathways rather than combined management strategies.
By extension, these suboptimal therapeutic options for PPGLs are a natural consequence of the inherent (and remarkable) developmental, molecular, biochemical, and imaging heterogeneity of these tumors, consequently hindering a “one size fits all” approach.
Currently, PPGLs are classified into 3 consensus genomic subtypes, which are strongly linked with 3 different classes of known driver genes (9, 10). Specifically, the pseudohypoxic subtype, classically known as Clusters C1A and C1B, includes tumors carrying pathogenic variants in Krebs cycle genes (SDHB, SDHA, SDHC, SDHD, SDHAF2, FH, MDH2, and IDH1) and pathogenic variants in genes involved in the regulation of hypoxia transcription factors hypoxia-inducible factor (HIF)-1α and/or HIF-2α (VHL, EPAS1, EGLN1/2, and DLST). This subtype shows a transcriptomic profile that reflects the cellular response to hypoxia (“pseudohypoxic” signature) and predominantly the noradrenergic phenotype (11). The kinase-signaling subtype, classically defined as Cluster C2, comprises pathogenic variants in RET, NF1, TMEM127, HRAS, MAX, and FGFR1-mutated tumors and exhibits an activated kinase receptor profile and increased protein translation, in addition to producing epinephrine and norepinephrine. The third group comprises tumors with activated Wnt signaling that contain a MAML3 fusion gene or CSDE1 pathogenic variants, with The Cancer Genome Atlas (TCGA) study having been the first to report the presence of both alterations in PPGLs (9, 12).
Besides the transcriptomic subtypes, different “omics” profiling techniques, such as copy number alteration profiling, miRNomics, DNA methylomics, metabolomics, and reverse-phase protein arrays, have provided additional information that could facilitate the clear classification of PPGLs according to their genetic background (13, 14). Nevertheless, despite the significant and continuous progress and countless important discoveries in the genomics of these tumors, current therapeutic options for PPGLs have remained relatively stagnant, with only select strategies having been implemented thus far.
New trends in promising therapies, however, have recently emerged with pioneering discoveries in the role of the tumor microenvironment (TME), particularly the immune system and its inhibitory and stimulatory checkpoint mechanisms (15). The inclusion of the immune system in the revised hallmarks of cancer, increased use of immunomics, launch of a consensus guideline (ie, iRECIST) and expert recommendations for human intratumoral immunotherapy, machine learning (ML), and other methods for identifying the role of the immune system in the metastatic behavior of cancer (including PPGLs) represent a few new frontiers in the therapeutic modification of the immune system to fight cancer (16-21). PPGLs, although rare, have not been overlooked, as evident from the various studies and a recent review describing how the components of the PPGL microenvironment could be used to better understand their pathogenesis, progression, and therapies (22). A few original studies have focused on programmed cell death protein 1 (PD-1) and programmed cell death ligand (PD-L)1/2 (receptor–ligand) expression, with their findings suggesting that some PPGLs could benefit from PD-L1/2-targeted therapies more than others (21, 23-25), although this has yet to be verified in clinical studies. Recently, a phase 2 clinical trial using pembrolizumab in patients with metastatic PPGL showed that around 40% of patients exhibited no disease progression, with an overall response rate of 9% (26). This clearly shows that the presence of immune checkpoint mechanisms may not sufficiently predict responses to immunotherapy. Indeed, a recent commentary related to the immune landscape of PPGLs and some experimental studies from Hong et al (27, 28) have revealed that some metastatic cancers may require treatment that involves both arms of the immune system, namely innate and adaptive immunity. This is even more important for tumors that lack immune cells, otherwise known as “cold” tumors, which include PPGLs. Thus, aside from the previously described new initiatives, which introduce and assess the role of the immune system in cancer, current studies have shown that an immunoscore or immune-based classification of tumors is tightly linked to cancer behavior and patient outcomes (29, 30). In fact, much effort has been invested toward converting PPGLs into immunologically active lesions (otherwise known as “hot” tumors), thereby ushering in an era of new and potentially promising treatment options.
This review outlines the fundamental principles of the immune system and its consequent role in cancer pathogenesis, progression, and metastasis, with a particular emphasis on PPGLs. Additionally, we highlight recently published studies related to the immune signatures of PPGLs and how these signatures could be used for the reclassification, prognostication, and management of these tumors in near future. Given that PPGLs are inherently heterogenous tumors, isolated conventional treatments (systemic radiotherapy or a particular chemotherapy regimen) may have limited impact and success. In contrast, immunotherapies engender a unique response from each specific tumor, thereby facilitating a personalized approach to the treatment of PPGLs and other rare tumors in this burgeoning era of personalized medicine.
Cancer Immunoediting: The Role of the Immune System in Tumor Development
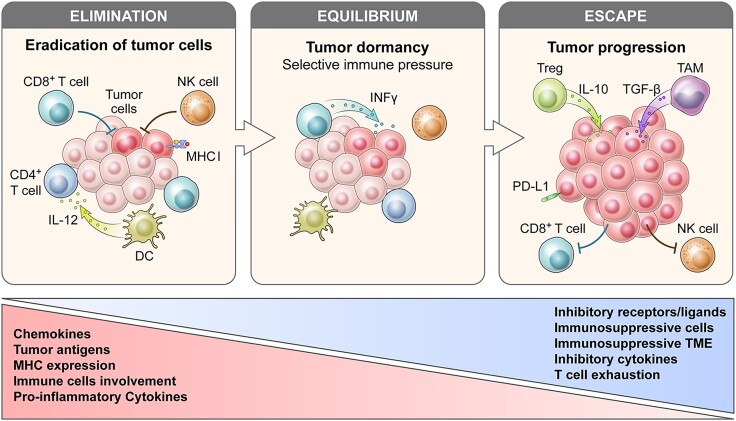
Understanding the relationship between cancer development and the immune system has been one of the most challenging undertakings in immunology. Although the initial concept had been proposed by Paul Ehrlich in the early 1900s (31), this hypothesis had ultimately been abandoned given the limited knowledge of the immune system at that time. However, progress in the understanding of the composition and function of the immune system, together with conformation of the existence of tumor antigens over following 50 years, had culminated in the postulation of the main hypothesis called cancer immunosurveillance by Lewis Thomas and Frank Macfarlane Burnet (32). They speculated that adaptive immunity could prevent cancer development before disease manifestation. Key findings, such as the importance of interferon-gamma (IFN-γ) in tumor cell rejection or the increased susceptibility of immunodeficient murine models to tumor development (33, 34), had driven the subsequent extension of this hypothesis into 3 phases, “Elimination,” “Equilibrium,” and “Escape,” collectively called cancer immunoediting (35, 36). Each phase describes the role of the immune system in the entire process, from the successful eradication of tumor cells and selective immune pressure to the final tumor progression and associated clinical manifestations. The key principle of the cancer immunoediting process is the ability to recognize tumor antigens (37, 38). Tumor antigens stimulate or activate the immune system. Once activated, the immune system recognizes these antigens as foreign or abnormal and initiates an immune response aimed at damaged or tumor cells carrying these antigens.
The immune system comprises 2 main parts, the innate and adaptive immune systems that work synergistically. The innate system acts nonspecifically, using various cells and proteins like the complement system to mark and eliminate pathogens, damaged, and abnormal cells. Innate immune cells, such as macrophages, dendritic cells (DCs), granulocytes, mast cells, and natural killer (NK) cells, recognize these opsonized pathogens and abnormal cells and eliminate them. Additionally, the innate immune system produces cytokines and chemokines involved in direct cytotoxicity or trafficking other immune cells into site of inflammation, crucial for shaping the adaptive response and maintaining immune balance. The adaptive immune system, composed of B and T cells, generates specific responses against antigens. B cells mainly produce specific antibodies whereas T cells play a critical role in cell-mediated immunity by recognizing and killing infected or abnormal cells. The adaptive immune system is also capable of immune memory, which allows for a more rapid and effective response to repeated antigen exposure. The coordinated actions between the innate and adaptive immune systems are essential for effective immune responses and maintaining immune homeostasis.
In the first phase of cancer immunoediting (Fig. 1), called the elimination phase, newly arising and immunogenic tumor cells can be recognized and controlled by the immune system and subsequently eliminated from the organism before tumors become clinically detectable. Tumor-infiltrating effector immune cells, such as NK, NKT, and T cells, can recognize tumor neoantigens and other surface molecules on tumor cells, such as cell stress ligands and apoptosis-inducing molecules, and eliminate the tumor cells. Effector cells produce interleukin (IL)-12, interferons, tumor necrosis factor, perforins, and other cytokines to eliminate tumor cells. Stressed and dying cells, including apoptotic and necrotic cells, release alarmins, which are also called damage-associated molecular patterns (DAMPs) (39). Examples of well-studied DAMPs include high mobility group box 1 protein, heat shock proteins, adenosine triphosphate, uracil acid, or DNA (comprehensively reviewed in (35, 36)). These molecules are recognized by pattern recognition receptors (PRRs) expressed mainly on innate immune cells, such as DCs and macrophages (37, 38). The ligation of PRRs by DAMPs may contribute to the immune-mediated eradication of tumor cells via the promotion of DC activation and subsequent increase in antigen presentation to T cells.
Figure 1.
Simplified cancer immunoediting process. Cancer immunoediting proceeds through the phases of elimination, equilibrium, and escape. During the elimination phase the innate and adaptive immune system can recognize MHC expression and antigens of tumor cells and eliminate them. If cancer cells survive the elimination phase, they progress into equilibrium phase where immune pressure select low-immunogenic tumor cell clones. Subsequently, the edited tumor cells can escape from immune surveillance. In this escape phase, tumors are clinically detectable. Tumor cells promote an immunosuppressive microenvironment by recruiting immune regulatory cells, altering immune cell trafficking, dysregulating the secretion of signaling molecules and metabolites, and upregulating surface molecules. Abbreviations: DC, dendritic cell; IL, interleukin; INF, interferon; MHC, major histocompatibility complex; NK cells, natural killer cells; PD-L1, programmed death-ligand 1; TAM, tumor-associated macrophage; TME, tumor microenvironment; TGF, tumor growth factor; Treg, T regulatory cells.
In the second phase, called the equilibrium phase, the immune system and tumor cells are in balance and the immune system recognizes and eliminates immunogenic tumor cells through the aforementioned mechanism. This selective immune pressure may promote the production of resistant and often nonimmunogenic clones of tumor cells, resulting in the escape phase.
During the last phase, called the escape phase, tumor cells accumulate additional immunosuppressive changes that allow them to escape from immunosurveillance. One of the main immune escape mechanisms common for many tumor types is the loss of tumor antigens. Tumor cells may either decrease antigenicity or lose major histocompatibility complex (MHC) molecules required for the presentation of neoantigens to T cells and their subsequent activation, leading to reduced immune recognition.
Other escape mechanisms may result from the development of an immunosuppressive TME unique to each tumor. Tumor cells often promote an immunosuppressive microenvironment by recruiting immune regulatory cells, altering immune-cell trafficking, dysregulating the secretion of signaling molecules and metabolites, and upregulating surface molecules. Immunosuppressive cytokines, such as vascular endothelial growth factor (VEGF), transforming growth factor beta (TGF-β), or IL-10 may dysregulate immune-cell function and recruit regulatory immune cells (40). In response to the immunosuppressive cytokines, 3 main immunosuppressive subpopulation of leukocytes, namely regulatory T cells (Tregs), myeloid-delivered suppressor cells (MDSCs), and tumor-associated macrophages (TAMs), infiltrate the tumor and further dysregulate antitumor responses via different mechanisms (reviewed in (41)). Tumor cells can also regulate the production of metabolites, such as adenosine, tryptophan, or lactate that further impair leukocyte functions (42-44). The last immune escape mechanism is the upregulation of surface molecules called checkpoint ligands on tumor cells, such as PD-L1/PD-L2, CD47 (which acts as a “do not eat me” signal), and others (45, 46).
A better understanding of each phase throughout the cancer immunoediting process, especially in development of PPGLs, may provide new insights into improving the immunotherapy of these rare tumors.
Evidence for the Immune Escape of PPGLs
The TME is a complex ecosystem consisting of tumor cells, stromal cells, and other cellular and noncellular components that may have an impact on immune-cell presence in TME. The diversity of the tumor-infiltrating immune cells may be associated with tumor growth, prognosis, prediction, and response to various immunotherapies. Tumors can be divided into 3 groups according to their TME: “cold,” “altered,” and “hot” tumors (29). “Cold” (noninflamed) tumors have no tumor-infiltrating lymphocytes (TILs) and low mutation burden. “Altered” tumors are characterized by the upregulation of inhibitory mediators and presence of immunosuppressive cells, resulting in T cell infiltration impairment. “Hot” (inflamed) tumors are characterized by TIL infiltration and accumulation of proinflammatory cytokines and genomic instability. Several studies have shown that “hot” tumors exhibit higher response rates to immunotherapy than do “cold” tumors. PPGLs can be considered as “cold” or “altered” tumors given their low mutational burden and absence of TILs, suggesting immune escape. Next, we comprehensively discuss current evidence for the immune escape of PPGLs.
Lack of Tumor Antigens
Tumor mutational burden (TMB) represents the number of genetic mutations in tumor cells. Such mutations may lead to neoantigen peptides and subsequently be presented by antigen presenting cells (APCs) to T cells in the MHC complex. Previous reports have shown that tumors with a high TMB had greater TIL presence than did those with a low TMB (47). With the increasing number of neoantigens, TILs have better chances to recognize and successfully eliminate tumor cells. Data also show that TMB can serve as a biomarker of beneficial clinical response to immune checkpoint inhibitors (ICIs) in various cancers (reviewed in (48)).
Unlike other cancers, such as melanomas or lung squamous cell carcinomas, which have a high TMB, PPGLs have a low TMB (9, 13, 21, 25, 49-52). In fact, PPGLs have one of the lowest median neoantigen burden among all other cancers across TCGA (21), which may reduce and prevent TIL infiltration. Indeed, most PPGLs are lymphocyte-depleted tumors, the details of which will be discussed later. Focusing on the PPGL genetic landscape, a recent study by Calsina et al showed that primary metastatic PPGLs and metastases have a higher TMB than do nonmetastatic primary tumors (21). The same authors also observed that increased TMB and microsatellite instability scores were associated with decreased time to progression. Significant differences in TMB were also observed between genomic subtypes, with the Wnt-altered subtype having the highest values, regardless of tumor behavior. Among all other PPGL genotypes, MAML3 tumors (Wnt signaling cluster) showed the highest neoantigen load (21). However, Tamborero et al described that the TMB of PPGLs were not correlated with their cytotoxic immunophenotypes that represent immune infiltration in tumors and clustering of cytotoxic cell based on gene signature (53).
Taken together, current evidence indicates that TMB by itself may not be a reliable predictive marker for the cytotoxic immune-cell composition of PPGLs and, in turn, their responses to ICIs. Indeed, another group previously reported that TMB should not be used in isolation but should rather be considered along with multiple other factors, such as neoantigen clonality and host immunological environment, which impact the balance of protumor and antitumor cytokines and TIL infiltration (54).
Absence of Tumor-Infiltrating T Cells
Tumor-infiltrating T cells, which include naïve, effector, memory, and Tregs, play a key role in tumor development and progression. Upon initial stimulation of naïve helper CD4+ or cytotoxic CD8+ T cells by an antigen, a cell-intrinsic program guides the differentiation of the cells into their effector phenotypes. CD4+ effector T cells differentiate into 2 major subtypes based on their response to several cytokines: (1) type 1 T helper (Th1) cells generated in response to IFN-γ and increase the cell-mediated response through macrophage and cytotoxic CD8+ T cell activation and (2) type 2 T helper (Th2) cells associated with IL-4, IL-5, and IL-10, which inhibit macrophage activation and promote humoral immune response through B cells. After the expansion of effector CD4+ or CD8+ T cells and clearance of specific antigens, most T cells die, except for a small subpopulation of memory T cells that provide long-term memory and protection against a second exposure to the same antigen. However, chronic exposure of T cells to the same antigen may deregulate their cell activation and differentiation, resulting in their dysfunction and exhaustion, which is one of the main escape mechanisms during cancer immunosurveillance. Tregs are a subpopulation of T cells that act as suppressor cells and therefore maintain immune homeostasis and self-tolerance within the body. However, some tumor cells can hijack Tregs in TME, giving them increased protection against the antitumor mechanisms of the immune system.
As stated earlier, TIL infiltration indicates the immunosurveillance status of the tumors and serves as a marker of their response to ICIs. Based on TCGA data on 29 tumor types, PPGLs have been listed under the category of tumors with the lowest expression of CD45, a cell surface marker of all nucleated hematopoietic cells (10, 55), indicating the limited presence of immune cells in the PPGL microenvironment. Despite exhibiting higher lymphocyte infiltration than the non-neoplastic adrenal medulla, PPGLs have still been considered to be strongly lymphocyte-depleted tumors compared with other cancer types (53). The amount of T cells present within the PPGL microenvironment usually represents only a small fraction of immune cells, with a higher proportion of CD4+ than CD8+ T cells (10, 56). Immunohistochemical studies focusing on the localization of PPGL T cells showed that these cells were homogenously distributed within the TME, without T cell–excluded areas (57). However, each cohort and method showed different results regarding T cell subpopulation infiltration, especially in metastatic PPGLs (summarized in Table 1). An immunohistochemistry (IHC) study by Gao et al showed that samples with a Pheochromocytoma of the Adrenal Gland Scaled Score (PASS) of <4 exhibited a greater presence of CD8+ T cells than did those with a PASS of ≥4 and that well differentiated PPGLs (based on the Grading System for Adrenal Pheochromocytoma and Paraganglioma) had a greater presence of CD8+ T cells than did moderately differentiated ones (58). Another IHC study by Jin et al showed no difference in CD4+ and CD8+ T cells between primary and metastatic tumors (25). Notably, an IHC study by Celada et al showed no difference in CD8+ T cells between nonmetastatic and metastatic PPGLs but did find higher CD8+ T cell infiltration in metastatic primary PPGLs than in metastases (59). Calsina et al revealed that metastatic primary tumors had a lower proportion of CD8+ (RNA-Seq study) than did nonmetastatic tumors (validated by IHC) (21). Yu et al showed that tumors expressing PD-L1 had a lower proportion of CD8+ and CD4+ T cells among CD3+ TILs than did those not expressing PD-L1 (60). Two other studies showed that Treg infiltration was associated with PPGL progression (20, 21) but not with any somatic pathogenic variant of tested tumor samples (61). Focusing on PPGL clusters, studies have shown that SDHB-negative tumors have fewer CD8+ T cells than do SDHB-positive tumors (56, 59), with MAML3 tumors having the highest number CD8+ T cells among all other genotypes. Within the pseudohypoxia cluster, evidence suggests that CD4+ but not CD8+ T cell infiltration was higher in VHL-mutated tumors than in SDHB-mutated tumors.
Table 1.
Studies focused on tumor-infiltrating T cells in PPGLs
| Marker | Method and number of samples | Results | References |
|---|---|---|---|
| Identification of immune cell subsets | RNA-Seq: n = 179 (TCGA) | PPGLs in lymphocyte depleted subtype. | Thorsson et al (55) |
| ↓Th1 response. | |||
| CD45 | IHC: n = 17 | Tumor-infiltrating T cells. | Guadagno et al (57) |
| CD3 | No correlation with any clinic-pathological variables. | ||
| ↓immunoscore in metastatic cases. | |||
| CD4 | IHC: n = 39 | ↑CD8+ T cells in PASS < 4 than PASS ≥ 4. | Gao et al (58) |
| CD8 | ↑CD8+ T cells in well differentiated than moderately differentiated PPGL (based on GAPP score). | ||
| CD8+ T cells inversely correlated with tumor size. | |||
| Identification of immune cell subsets | RNA-Seq: n = 179 (TCGA) | ↑CD4+ memory T cells in older patients (>45 year). | Batchu (61) |
| Proportion of CD4+ memory T cells and Tregs did not associate with any somatic mutations. | |||
| Identification of immune cell subsets | (sn)RNA-Seq: n = 30 + 2 NAM | Lymphocytes in smaller fraction of all immune cells with predominant subpopulation of CD4+ T cells and minor subpopulations of CD8+ T cells, Tregs, and NK cells. | Zethoven et al (10) |
| bulk-tissue RNA-Seq: n = 735 | |||
| CD3 | IHC: n = 65 + 20 NAM | ↑proportion of immune cells in tumors compared with NAM. | Tufton et al (56) |
| CD4 | ↑proportion of CD4+ T cells than CD8+ T cells. | ||
| CD8 | ↑proportion of CD4+ T cells in aggressive tumors. | ||
| ↑level of CD4+ T cells in VHL- compared with SDHB-related tumors. | |||
| ↓CD4+ and CD8+ T cells in SDHB-negative tumors. | |||
| ↓ratio of CD8+/CD4+ T cell compared with NAM. | |||
| CD4 | IHC: n = 63 | 49.2% of samples positive for intratumoral and 22.2% for stromal CD8+ T cells (61.3% of these samples had percentage of intratumoral CD8+ T cells around 1%). | Jin et al (25) |
| CD8 | 2.7% of samples positive for intratumoral and 3.1% for stromal CD4+ T cells. | ||
| No difference between CD4+ and CD8+ T cells and primary and metastatic tumors. | |||
| Identification of immune cell subsets | RNA-Seq: n = 177 (TCGA) | Five immune cluster introduced: 1, M2 macrophages; 2, monocytes; 3, activated NK cells; 4, Tregs and M0 macrophages; 5, CD8+ and CD4+ T cells. | Ghosal et al (20) |
| RT-PCR + NanoString: n = 48 (validation cohort) | ↑proportion of resting CD4+ T cells and resting NK cells had a negative effect on better clinical outcomes. | ||
| CD8 | IHC: n = 102 | CD8+ T cells detected in 19.7% samples. | Celada et al (59) |
| 60% of tumors with levels of infiltrating CD8+ T cells also displayed ↑levels of tumor cells with PD-L1. | |||
| ↑CD8+ T cells in metastatic primary PPGLs than metastases. | |||
| No difference in CD8+ T cells between nonmetastatic and metastatic PPGLs. | |||
| ↑CD8+ T cells more frequent in SDHB-positive than negative tumors. | |||
| ↑CD8+ T cells higher among tumors with negative HIF2alfa IHC. | |||
| No associations were found between CD8+ T cells and overall survival. | |||
| Identification of immune cell subsets | WES: n = 261 | ↑resting, ↓activated CD4+ T cells, ↓proportion of CD8+ and ↑resting and ↓activated NK cells in metastatic primary compared with nonmetastatic tumors (CD8+ T cell proportions validated by IHC). | Calsina et al (21) |
| RNA-Seq: n = 265 | ↓Th1 gene signature in primary metastatic tumors compared with nonmetastatic primary tumors. | ||
| IHC: n = 39 | ↑Tregs associated with shorter time to progression. | ||
| (CNIO, TCGA) | ↑number of CD8+ T cells in MAML3 tumors compared with other genotypes. | ||
| Tumor-infiltrating T cells | FACS: n = 7 | Proportion of CD8+ and CD4+ T cells among CD3+ TILs of PD-L1+ tumors was ↓ than PD-L1− tumors. | Yu et al (60) |
| qPCR: n = 16 | Expression of perforin and granzyme-B was ↓ in the PD-L1+ tumors. | ||
| mIF: n = 6 | Proportion of effector memory T cells in the PD-L1+ tumors was significantly ↑than that in the PD-L1− tumors. |
Abbreviations: CD3, cluster of differentiation—T cells; CD4, cluster of differentiation—helper T cells; CD8, cluster of differentiation—cytotoxic T cells; CD45, cluster of differentiation—leukocytes; CNIO, Centro Nacional de Investigaciones Oncológicas; FACS, fluorescence-activated cell sorting; GAPP, Grading System for Adrenal Pheochromocytoma and Paraganglioma; IHC, immunohistochemistry; mIF, multiplex immunofluorescence; NAM, normal adrenal medulla; NK, natural killer; PASS, Pheochromocytoma of the Adrenal Gland Scaled Score; PPGL, pheochromocytoma/paraganglioma; qPCR, quantitative real-time polymerase chain reaction; RNA-seq, RNA sequencing; (sn)RNA-seq, single nucleus RNA sequencing; SDHB, succinate dehydrogenase subunit B; TCGA, The Cancer Genome Atlas database; Tregs, regulatory T cells; WES, whole exome sequencing; PD-L1, programmed cell death ligand 1.
In summary, PPGLs are mostly T cell-depleted tumors in which the proportion of helper CD4+ cells usually exceed that of cytotoxic CD8+ T cells, suggesting strong immune escape mechanisms preventing sufficient T cell infiltration. Clinically unfavorable outcomes have been observed in PPGLs infiltrated with Tregs, confirming their suppressive roles.
Higher Presence of Tumor-Associated Macrophages
Macrophages, typically derived from monocytes, are professional phagocytes with a broad spectrum of functions, including digestion of pathogens and cellular debris, presentation of digested antigens, shaping of immune response through cytokine production, presentation of digested antigens, and facilitation of wound healing (62). Cells of the monocyte–macrophage lineage are widely present throughout all bodily tissues and polarized according to the tissue microenvironment in their respective subpopulations. TAMs are usually divided into the binary M1–M2 macrophage polarization system. M1 macrophages are defined as proinflammatory cells capable of producing major inflammatory cytokines, such as IL-6, tumor necrosis factor (TNF)-α, or IL-12, whereas M2 macrophages are anti-inflammatory cells known for their production of IL-10 or TGF-β (63). The increased presence of TAMs in many solid cancers, such as glioblastomas or breast, bladder, and prostate cancers, has been associated with tumor progression and invasive behavior due to their immunosuppressive abilities (64).
Several studies have reported the increased presence of monocytes (precursors of macrophages) and macrophages in PPGLs (summarized in Table 2). Thorsson et al, who focused on the immune subtypes in TCGA samples, revealed that PPGLs, together with adrenocortical carcinomas, hepatocellular carcinomas, and gliomas, displayed a more prominent macrophage signature with a high anti-inflammatory M2 response and the least favorable outcome compared with other tumors (55). Farhat et al also described the population of cells immunohistochemically positive for monocyte–macrophage lineage markers CD163 and CD68 (65). A recent publication based on single-nuclei RNA-seq (snRNA-seq) and bulk-tissue gene expression analysis showed that myeloid cells were the predominant leukocytes in PPGLs, confirming previous results (10). These myeloid cells mostly consisted of macrophages (94%), with a few monocytes (4.4%), dendritic (2.9%), and mast cells (3%) (10). A study by Calsina et al showed a higher presence of M2 macrophages in metastatic than in nonmetastatic PPGLs (21). Another study also found an increased presence of M2 macrophages in aggressive PPGLs (56). The bulk-tissue gene expression data revealed that the pseudohypoxia cluster, specifically SDHx head and neck and VHL PPGLs, had a higher expression of macrophage markers (ie, CD68, CD86, CD163, and MRC1) than did the other subtypes. The same authors also showed that the number of CD163+ and CD206+ (M2 macrophage marker) cells was higher in VHL-mutated PPGLs than in the other subtypes (10). After immunoprofiling PPGLs, Ghosal et al identified 5 immune clusters, 3 of which were related to macrophages (specifically M2 macrophages), monocytes, and M0 macrophages (20). Notably, the same study showed that tumors within immune clusters that exhibited increased M0 and M2 macrophage infiltration were associated with aggressive, metastatic, or advanced-stage disease, supporting previous results from Thorsson et al (55). Interestingly, SDHx-mutated pseudohypoxic tumors, which often display metastatic behavior, had an increased proportion of immune cluster associated with M2 macrophages, which also supports previous results (55).
Table 2.
Studies focused on tumor associated macrophages in PPGLs
| Marker | Method and number of samples | Results | References |
|---|---|---|---|
| Identification of immune cell subsets | RNA-Seq: n = 179 (TCGA) | More prominent macrophage signature with ↑M2 response and the least favorable outcome (together with adrenocortical carcinoma, hepatocellular carcinoma, glioma) compared with other tumors. | Thorsson et al (55) |
| CD68 | IHC: n = 62 | Dense population of CD68+, CD163+ cells in PPGLs. | Farhat et al (65) |
| CD163 | No significant differences detectable in relation to patients’ gender, age, or mutational status. | ||
| CD68 | IHC: n = 39 | ↑CD68+ cells in PPGL with regular and normal histological pattern compared with those with abnormal patterns: inversely correlate with tumor size (based on GAPP score). | Gao et al (58) |
| CD163 | CD163+ cells correlated with CD8+ T cells. | ||
| Identification of immune cell subsets | RNA-Seq: n = 179 (TCGA) | ↓M2 in tumors from older patient (>45 years). | Batchu (61) |
| Missense mutational burden in genes FRG1B and RET positively correlated with M2 macrophages. | |||
| Identification of immune cell subsets | (sn)RNA-Seq: n = 30 + 2 NAM | Myeloid cells as dominant leukocyte subpopulation, consisting mostly of macrophages (94%) with a minor population of monocytes (4.4%). | Zethoven et al (10) |
| CD68, CD163, and CD206 markers | bulk-tissue RNA-Seq: n = 735 | ↑expression of the macrophage markers in SDHx-head and neck and VHL subtypes compared with other subtypes. | |
| IHC n = 12 (validation cohort) | ↑CD163+ and CD206+ cells in VHL PPGLs compared with other subtypes. | ||
| CD68 | IHC: n = 65 + 20 NAM | ↑proportion of M2:M1 in aggressive tumors (together with CD4+ T cells). | Tufton et al (56) |
| CD163 | ↑higher proportion of M2 infiltration with higher M2:M1 in aggressive SDHB-mutated PPGLs. | ||
| Identification of immune cell subsets | RNA-Seq: n = 177 (TCGA) | Five immune clusters introduced 1, M2 macrophages; 2, monocytes; 3, activated NK cells, 4, Tregs and M0 macrophages; 5, CD8+ and CD4+ T cells. | Ghosal et al (20) |
| RT-PCR + NanoString: n = 48 (validation cohort) | Immune clusters consisted of higher proportion of M0 and M2 macrophages were associated with aggressive, metastatic, or advanced-stage tumors. | ||
| ↑proportion of the immune cluster associated with M2 macrophages in SDHx-mutated pseudohypoxic tumors. | |||
| Identification of immune cell subsets | WES: n = 261 | ↑M2 macrophages in metastatic primary tumors compared with nonmetastatic. | Calsina et al (21) |
| RNA-Seq: n = 265 (CNIO, TCGA) |
Abbreviations: CD68, cluster of differentiation—marker of monocyte/macrophage lineage; CD163, cluster of differentiation—marker of monocyte/macrophage lineage; CD206, cluster of differentiation—M2 macrophages; CNIO, Centro Nacional de Investigaciones Oncológicas; FRG1B, FSHD Region Gene 1 Family Member B, Pseudogene; IHC, immunohistochemistry; NAM, normal adrenal medulla; PPGL, pheochromocytoma/paraganglioma; qPCR, quantitative real-time polymerase chain reaction; RET, proto-oncogene, receptor tyrosine kinase; RNA-seq, RNA sequencing; (sn)RNA-seq, single nucleus RNA sequencing; SDHB, succinate dehydrogenase subunit B; TCGA, The Cancer Genome Atlas database; WES, whole exome sequencing.
Overall, macrophages are the dominant leukocyte subpopulation in PPGLs, with an increased proportion of M2 macrophages being associated with immunosuppressive and aggressive/metastatic behavior. This suggests that targeting of these cells could lead to better outcomes in PPGLs.
Immune Checkpoint Molecules
Immune checkpoint proteins are regulatory receptors that can be found, along with their ligands, in various cell types. Under physiological conditions, immune checkpoint molecules regulate the immune system, diminishing the immune response after the mitigation of an infection. However, immune checkpoint interactions may also be involved in cancer development and growth, representing a particularly major mechanism underlying tumor immune escape (66-69). Among the most studied checkpoint molecules, cytotoxic T lymphocyte-associated protein 4 (CTLA-4) and PD-1 and its ligands (PD-L1 and PD-L2) are currently considered the most relevant immune system inhibitory molecules, interrupting the costimulatory signals or interfering with TCR signaling, respectively (70). Although CTLA-4 (CD152) is expressed almost exclusively on T cells, limited expression had also been found on B cells. PD-1 (CD279) is expressed on the surface of T cells, B cells, professional APCs, NK cells, and some tumor cells. The signal is activated through 2 specific ligands, namely PD-L1 (CD274) and PD-L2 (CD273). PD-L1 is expressed on T and B cells, DCs, macrophages, endothelial cells, and some tumor cells. PD-L2 is restricted to activated DCs, macrophages, bone marrow–derived mast cells, and tumor cells (71-74). Recently, other inhibitory receptors/mechanisms limiting immune-cell function in cancer have been described, such as T cell immunoglobulin and mucin-domain containing-3 (TIM-3), lymphocyte activation gene 3 (LAG-3), and T cell immunoreceptor with Ig and ITIM domains (TIGIT), and further research and clinical studies related to this inhibitory signaling are underway (70, 75-77). Apart from T cells, other immune cells also express immune checkpoints, but the function of these checkpoints and their manipulation have been much less explored (78).
In the first study on PD-L1/2 and PPGLs published in 2017, Pinato et al detected tumor PD-L1 immunopositivity in 40% (4/10) and 15% (14/90) of metastatic and nonmetastatic cases, respectively, with such immunopositivity not being correlated with germline pathogenic variants, tumor location, tumor size, and patient survival (23). Meanwhile, PD-L2 immunopositivity was found in 50% of metastatic cases (5/10) but in only 12% of nonmetastatic specimens (11/90). The proportion of PD-L2 positivity was higher in PPGLs with vascular invasion and was also correlated with a high expression of genes associated with hypoxia and immune-cell exhaustion (23). A subsequent study that included 77 PPGLs showed PD-L1 positivity in 59.7% of the tumors but found no correlation between PD-L1 presence and relapse/distant metastasis, tumor size, capsular invasion, tumor necrosis, and specific catecholamine production/secretion (79). Nevertheless, a significant correlation was observed between Ki-67, a proliferation marker for tumor cells, and hypertension (79). Although these preliminary studies showed that a considerable number of PPGLs had high PD-L1 expression, later studies revealed much lower positivity in the cohorts. For all clinically advanced PPGLs, PD-L1 immunostaining of tumor cells revealed a positivity rate of less than 10% (52). In fact, a recent study showed a PD-L1 positivity rate of only 7.9% measured in primary and metastatic tumors, in a cohort of 53 patients with PPGL (25). After IHC staining of whole-tumor sections, Hsu et al found that 26.1% of cases expressed PD-L1 in ≥1% of tumor cells, but only 8.7% of the cases expressed PD-L1 in approximately 10% of tumor cells (80). The proportion of PD-L1-positive cells is important information given its association with different therapeutic outcomes in various cancers (81-83). Batchu et al, who analyzed TCGA mRNA expression data, showed that the increased expression of PD-L1 and PD-L2 in the pseudohypoxic cluster was located in the stromal compartments, suggesting that the expression originated from non-PPGL cells in TME and that further studies on the TME of PPGLs are necessary to evaluate immune checkpoint therapies (84). Furthermore, sympathetic PPGLs exhibited higher PD-L1 expression than did parasympathetic PPGLs (59). All studies are summarized in Table 3.
Table 3.
Studies focused on immune checkpoint proteins in PPGLs
| Marker | Method and number of samples | Results | References |
|---|---|---|---|
| PD-L1 | IHC: n = 10 metastatic, n = 90 nonmetastatic | PD-L1 positive 40% metastatic and 15% nonmetastatic—no correlation with germline mutations, tumor location, tumor size, or survival. | Pinato et al (23) |
| PD-L2 | PD-L2 positive 50% metastatic and 12% nonmetastatic—correlation with hypoxia and immune-exhaustion. | ||
| PD-L1 | IHC: n = 77 | PD-L1 positivity 59.7%. | Guo et al (79) |
| qPCR: n = 20 | No correlation to age, sex, tumor size, capsular invasion, tumor necrosis, relapse/distant metastasis, and secretion of catecholamines. | ||
| Correlation to Ki-67 and hypertension. | |||
| PD-L1 | IHC: n = 24 | PD-L1 positivity ≥10% of tumor cells in 8.7% tumors. | Hsu et al (80) |
| PD-L1 positivity in ≥1% of tumor cells in 26.1% tumors. | |||
| PD-L1 | IHC: n = 128 | PD-L1 positivity <10%. | Bratslavsky et al (52) |
| Gene amplification: n = 128 | No CD274 gene amplification. | ||
| PD-L1 | IHC: n = 53 | PD-L1 positivity 7.9%. | Jin et al (25) |
| No correlation to metastatic disease. | |||
| PD-L1 | qPCR: n = 48 | PD-L1 mRNA ↑in PPGL vs NAM, ↓in pseudohypoxic cluster vs sporadic cases and kinase signaling cluster. | Hadrava Vanova et al (24) |
| PD-L2 | validation: n = 72 (TCGA) | PD-L2 mRNA no difference. | |
| No correlation to metastatic status and Ki-67. | |||
| PD-L1 | RNA-Seq—gene expression deconvolution: n = 175 (TCGA) | ↑PD-L1, PD-L2 mRNA in the stromal compartments in pseudohypoxic and Wnt-altered cluster. | Batchu et al (84) |
| PD-L2 | |||
| CTLA-4 | IHC: n = 48 | Higher CTLA-4 positive cells compared with other tumors. | Dum et al (85) |
| PD-L1 | IHC: n = 94 primary, n = 8 metastases | PD-L1 positivity 16.7%, 12.7% in primary samples and 62.5% metastatic samples, ↑metastatic, ↓SDHB-negative tumors. | Celada et al (59) |
| qPCR: n = 184 (TCGA) | ↓pseudohypoxic cluster from the TCGA. | ||
| PD-L1 | IHC: n = 44 | PD-L1 positivity 34%, ↓pseudohypoxic tumors, ↑Wnt-altered subtype. Highly expressed in MAML3-related tumors; <25% of kinase/pseudohypoxic tumors positive. | Calsina et al (21) |
| TIGIT | RNA-Seq: n = 264 (CNIO + TCGA) | No association to clinical behavior. | |
| LAG-3 | No significant changes in TIGIT and LAG-3 expression between metastatic and nonmetastatic or between clusters. | ||
| PD-L1 | IHC: n = 60 | PD-L1 positivity 33.3%. ↑PD-L1 mRNA to NAM. | Yu et al (60) |
| PD-1 | RNA-Seq: n = 7 | ↑PD-L1, PD-1, TIM3, LAG-3 mRNA in IHC PD-L1+ samples; no significant change in TIGIT between IHC PD-L1+ and IHC PD-L1- samples. | |
| TIM3 | qPCR: n = 16 | ↑PD-1, TIM3, LAG-3, TIGIT protein in T cells, correlation with PD-L1 expression. | |
| LAG-3 | FACS: n = 7 | ||
| TIGIT |
Abbreviations: IHC, immunohistochemistry; NAM, normal adrenal medulla; PPGL, pheochromocytoma/paraganglioma; qPCR, quantitative real-time polymerase chain reaction; RNA-seq, RNA sequencing; SDHB, succinate dehydrogenase subunit B; TCGA, The Cancer Genome Atlas database; WES, whole exome sequencing; PD-1, programmed cell death protein 1; PD-L1/2, programmed cell death ligand 1/2; CTLA-4, cytotoxic T-lymphocyte associated protein 4; TIGIT, T-Cell immunoreceptor with Ig And ITIM domains; LAG-3, lymphocyte-activation gene 3; TIM3, T-cell immunoglobulin and mucin-domain containing-3; FACS, fluorescence-activated cell sorting.
Some studies have suggested that metastatic PPGLs more frequently express PD-L1 than do nonmetastatic PPGLs (23, 59), whereas no correlation had been found in other studies (24, 25). The variety of results from different cohorts currently prevents researchers from definitively concluding whether metastatic PPGLs express more checkpoint molecules than do nonmetastatic PPGLs.
Several recent studies with varying cohorts and analysis of TCGA data have shown that PD-L1 expression was significantly lower in pseudohypoxic PPGLs, which are often metastatic except from VHL and EPAS1 ones, than in sporadic cases and PPGLs from the kinase-signaling subtype cluster (21, 24, 59). This suggests that PD-1/PD-L1-targeted immunotherapy may have limited outcomes in the pseudohypoxic cluster. Recently, the Wnt-altered subtype had been shown to have the highest PD-L1 expression among the other subtypes, including all metastatic MAML3-tumors in this cohort. However, no association was observed between these tumors and clinical behavior (21).
To date, most studies have focused exclusively on PD-L1/PD-L2 and that of other immune checkpoints's expression, and their relevance to tumor behavior is now being explored (eg, CTLA-4 (85)). A recent study by Yu et al found that the increased PD-L1 expression in PPGL samples was correlated with T cell exhaustion markers, including T-cell immunoglobulin and mucin-domain containing-3, LAG-3, and TIGIT, suggesting that TILs in PPGLs showed the function–failure phenotype and may be a potential target for some patients with PPGL (60). Nevertheless, studies with heterogeneous cohorts and possibly different methodologies, and those focusing mainly on PD-1 and its ligands expression, are still limited. Hence, it is difficult to conclude whether immune checkpoint proteins’ expression in PPGLs has predictive significance in therapy. Given the rarity of these tumors, multi-institutional collaborative efforts could help increase the number of patients and provide more robust and clinically relevant studies.
Dysregulation of Signaling Pathways and Metabolites
Besides immune system components, the TME contains many nonimmune elements that can affect immune signaling and immune-cell invasion. Specifically, the secretion and presence of various growth factors and (onco)metabolites have protumorigenic effects that play important roles in tumor maintenance and progression with subsequent metastasis occurrence. Here, we focus on TME elements that potentially contribute to the immune escape of PPGLs.
Vascular Endothelial Growth Factor
VEGF is one of the most well-described angiogenic factors involved in new blood vessel development, tumor growth, tumor cell invasiveness, and immune-cell regulation. Studies have shown that VEGF can mediate macrophages and T cell chemotaxis, inhibit dendritic and T cell differentiation and function, and promote the accumulation of Tregs in various tumors, thereby having profound protumorigenic effects (86-88).
PPGLs are considered to be highly vascularized tumors (1). Notably, studies have shown that metastatic PPGLs had higher VEGF transcripts than did nonmetastatic PPGLs (89-92), suggesting an association between VEGF-mediated angiogenesis and tumor progression. Vascularization and expression of key angiogenic molecules have also been shown to be upregulated in pseudohypoxic PPGLs, regardless of their metastatic status, possibly due to activation of hypoxia-driven angiogenic pathways in this PPGL cluster (93, 94). Focusing on the connection between VEGF and the immune system in PPGLs, 1 study found that VEGF-A overexpression was correlated with microvessel density, which further correlated with the number of intratumoral macrophages (95). This reflects 1 of the known effects of VEGF on the immune system; however, no further studies have been conducted on the role of VEGF on the immune microenvironment in well-defined PPGL molecular clusters.
Currently, several completed and ongoing clinical trials have focused on VEGF inhibitors/tyrosine-kinase inhibitors (TKIs) in patients with PPGL (Anlotinib: NCT05133349, NCT05883085, NCT04860700; Axitinib: NCT03839498, NCT01967576; Sunitinib: NCT00843037, NCT01371201; Lenvatinib: NCT03008369; Cabozantinib: NCT02302833, NCT04400474; Fostamatinib: NCT00923481; Dovitinib: NCT01635907; Temsirolimus: NCT01155258; Vatalanib: NCT00655655). Given the rarity of these tumors, the number of clinical trials indicates the expected efficacy in patients with PPGLs, especially those with advanced/metastatic cases. Sunitinib has shown therapeutic efficacy in patients with pheochromocytomas, resulting in partial or near-complete tumor regression (96). Furthermore, a recent systematic review and meta-analysis including 7 studies demonstrated that metastatic PPGLs can benefit from TKI therapy. According to this meta-analysis, more than 30% patients can achieve partial response, whereas more than 80% of patients can achieve disease control (97).
Unfortunately, no study has focused on the impact of these inhibitors on the function and recruitment of immune cells in PPGLs. Given the higher expression of VEGF in metastatic and pseudohypoxic PPGLs, targeting the VEGF pathway to modulate the TME for concurrent immunotherapy may be promising.
Catecholamines
Catecholamines, namely epinephrine, norepinephrine (NE), and dopamine (DA), are hormones that are synthesized and secreted by chromaffin cells in the medulla of the adrenal gland. Additionally, NE is synthesized and released locally by postganglionic sympathetic neurons (98), whereas DA further originates from dopaminergic neurons (99). Owing to the variety of receptor isoforms for catecholamine binding, they can trigger different and even opposite effects, such as vasoconstriction, vasodilatation, increased heart rate, and smooth muscle relaxation (98, 100). Adrenoceptors were found to be present on both cancer cells and immune cells (101, 102). Thus, catecholamines may play a role in cancer development and progression by either directly affecting cancer cell proliferation and genomic instability or affecting the immune cells in the TME (98, 99). Catecholamine release under chronic stress has been shown to suppress different aspects of immune function, such as antigen presentation, T cell proliferation, and humoral and cellular immunity (103, 104). Catecholamines have been shown to inhibit the generation of antitumor cytotoxic T cells through β-adrenoceptor signaling in vivo (105-107) and NK cell cytotoxicity and cytokine production through β-adrenoceptors and dopamine receptors (108-110). In a preclinical models of several cancers, β-adrenoceptor signaling decreased the efficacy of CD8+ T cell-targeting immunotherapies (107, 111), whereas β-adrenoceptor antagonists increased the sensitivity of tumors to immunotherapy (107, 112, 113).
PPGLs can be characterized by their high production rates of catecholamines and metanephrine (114). Although several clinical trials have investigated the use of α-adrenoceptor blockade to prevent catecholamine crisis during surgery for PPGLs, they generally do not focus on the potential of therapy to delay tumor growth. Moreover, the potential of concurrent targeting of adrenoceptors to increase immune-cell infiltration into PPGLs has not been studied. Recent study has suggested that epinephrine levels were correlated with neutrophil infiltration and that NE levels were correlated with macrophage infiltration in PPGLs, possibly suggesting that catecholamines had differing effects and that immune infiltration could be correlated with the functional status of tumors (56). Yet, this has to be validated in bigger PPGL cohort. Estrada et al showed that NE treatment of human CD8+ T cells decreased IFN-γ and TNF-α secretion and suppressed CD8+ T cell cytolytic capacity in response to TCR activation, which could be specifically reversed by β2-adrenoceptor antagonists (115). Moreover, preclinical model of aggressive renal carcinoma suggested that faulty IFN signaling could possibly increase the risk of metastatic and aggressive tumor behavior (116). Decreased IFN-γ levels have already been shown in SDH-deficient tumors, which generally have a higher risk for metastasis (117). Blocking catecholamine synthesis through metyrosine (α-methyl-paratyrosine), which has been shown to reduce catecholamine biosynthesis by 35% to 80%, in patients with catecholamine-secreting PPGLs (118) had been found to be effective in reducing pulmonary metastasis (119). Furthermore, DA in plasma, or specifically its metabolite 3-methoxytyramine, is a validated prognostic marker for metastatic PPGLs (120). DA has been shown to suppress proliferation of isolated NK cells and subsequent IFN-γ synthesis (109), which could suggest specific targeting for NK- and IFN-depleted tumors, such as SDHx-mutated PPGLs. A small molecule antagonist of dopamine receptor DRD2, ONC201, has already been shown effective in phase II for PPGLs (NCT03034200) (121). ONC201 can initiate intratumoral NK cell infiltration as confirmed in preclinical studies on colorectal and breast human tumor xenografts and patient biopsies (122, 123).
In conclusion, the effects of catecholamines on immune system response and tumorigenic pathways have already been described in preclinical models of some cancers and high concentrations of catecholamines in PPGLs could contribute to the immunologically “cold” behavior of PPGLs.
Succinate
Succinate, a Krebs cycle metabolite, is considered an oncometabolite that can accumulate in the TME and initiate or promote tumor growth through several pathways, such as DNA hypermethylation, HIF stabilization, and succinate receptor 1 (SUCNR1) activation (124-128). Besides these well-documented pro-oncogenic effects of succinate, several studies have focused on its role in the immune system. While mitochondrial succinate accumulation in macrophages acts as an inflammatory modulator (129), extracellular succinate has been shown to induce macrophage polarization into M2 and promote tumorigenic signaling (127, 130, 131). Tumor-associated succinate concentrations have been shown to exert a direct suppressive effect on human T cells in vitro, resulting in lower IFN-γ and TNF-α secretion (117).
Succinate accumulation is mainly characteristic of SDH- and FH-mutated PPGLs (132, 133). A recent study revealed that SDH-deficient tumors had decreased expression of IFN-γ-induced genes, suggesting suppressed T cell function as described above (117). In individuals with SDHB pathogenic variant, isolated neutrophils showed increased succinate accumulation and enhanced survival (134), suggesting the possible mechanism of neutrophil elevation in SDHB carriers. Reports have shown that elevated circulating neutrophils are involved in the immunosuppressive response, thereby supporting tumor cell growth and metastasis (135). Indeed, increased neutrophil–lymphocyte ratio has recently been linked to decreased overall survival among patients with PPGL (136). Apart from the mentioned studies, no other research has investigated the direct outcomes of succinate accumulation in PPGLs and on the immune response, despite the described role of succinate in immune signaling and tumorigenesis. Given the immunosuppressive environment highlighted recently for pseudohypoxic PPGLs, especially SDHB-mutated PPGLs (21, 59), and the possible effects of succinate on T cell function and cytokine secretion in SDH-deficient tumors (117), one can hypothesize that succinate modulates the immune microenvironment in PPGLs to favor tumor growth.
Kynurenine
Abnormal activation of the kynurenine pathway has been repeatedly observed in numerous cancer types. Its functions are hijacked to promote tumor growth and cancer cell dissemination through multiple mechanisms (137, 138). Kynurenine is produced from tryptophan by indoleamine 2,3-dioxygenase (IDO), the rate-limiting enzyme of tryptophan metabolism, which can help create tolerogenic TME (139). IDO exerted immunosuppressive effects by directly inhibiting CD8+ T cell activity and inducing Treg cell differentiation in preclinical models (reviewed in (139, 140)). Moreover, its pathway metabolites can polarize APCs to exhibit an immunotolerant phenotype in mice (138, 141).
Research has shown that PPGLs exhibit increased expression of IDO2 (142), IDO1, and of transporters for tryptophan uptake (143). Moreover, kynurenine metabolic pathway enzymes, such as kynurenine 3-monooxygenase and kynureninase, are significantly downregulated, suggesting the possibility of kynurenine accumulation in PPGLs (143). Indeed, evidence has shown that pseudohypoxic PPGLs had fewer kynurenine pathway metabolites than did the kinase-signaling PPGL cluster, which could be associated with the shorter metastasis-free survival of patients with pseudohypoxic PPGLs (144).
Taken together, these results suggest that the kynurenine pathway may be affected in PPGLs, especially the pseudohypoxic PPGL cluster, and that IDO inhibitors may serve as an immunometabolic adjuvant to enhance systemic immune responses.
Polyamines
Polyamines, such as putrescine, spermine, and spermidine, are organic polycationic alkylamines synthesized from L-ornithine, an intermediate generated from L-arginine (145). They play essential roles in several fundamental processes including protein and nucleic acid metabolism, cell signaling, functioning of cytoskeleton, and cell death regulation (145, 146). Moreover, studies have documented its role in the immune response. In the TME, excessive L-arginine catabolism (leading to polyamine production) by suppressive myeloid and tumor cells dampens cytotoxic T cell function, suggesting a link between polyamines and T cell suppression (145, 147, 148). Indeed, polyamine blockade therapy relieved polyamine-mediated immunosuppression in TME and allowed for T cell activation in preclinical melanoma model (149). One study found that polyamines and their metabolites were elevated in various tumors, such as breast, lung, colorectal, ovarian, prostate, and pancreatic cancers (150). Several polyamine pathway inhibitors have been evaluated in preclinical and clinical studies with some encouraging outcomes (150).
Rai et al found that SDHx-mutated PPGLs associated with more aggressive or metastatic behavior had increased expression of several polyamines (146), suggesting another immunometabolic target in SDHx-mutated PPGLs that could help “cold” tumors into “hot” tumors.
Hypoxia Signaling
Hypoxia is a common feature of most solid tumors. The low oxygen concentration and consequent metabolic outcomes promote metabolic stress and tumor metastases, favor tumor immune evasion, and affect immune-cell infiltration through the production of chemokines and cytokines (151-153). Hypoxia triggers several pathways that induce immunosuppression; reduction in CD8+ T cells, NK cells, and M1 macrophages; and enhanced tumor-tolerant populations, such as M2 macrophages, MDSCs, and Treg cells (154-157).
Endocrine cancers frequently exhibit major hypoxic areas, and high expression of hypoxia signaling genes has been associated with endocrine tumor development (158). The role of (pseudo)hypoxia in the development of PPGLs, particularly pseudohypoxic PPGLs (159, 160), as well as its effects on the immune system of patients with PPGL (20, 21, 56, 59, 60), has been documented. Moreover, hypoxia has been shown to further promote catecholamine production in mice (161, 162), whose role in immunosuppression we had already been discussed earlier. In PPGLs, HIF-2α was suggested to drive mesenchymal transition that promotes a pro-metastatic phenotype (163-165). In general, hypoxia is believed to contribute to immunotherapy resistance (154, 156, 157). Thus, targeting of hypoxia alongside immunotherapy and other therapies for PPGLs may increase treatment efficacy.
Telomerase
A striking characteristic feature of metastatic PPGLs is its frequent association with telomerase activation either by gene fusion (166) or TERT promoter hotspot mutation (167). Indeed, telomerase activation is an independent adverse prognostic characteristic for overall survival (167), which has been recognized in many cancers (168). Several clinical studies with telomerase inhibitors or vaccines to target TERT peptides have been accomplished with promising results in pancreatic cancer (169), squamous anal cell carcinoma (170), and prostate and renal cancer (171).
Recently, a study demonstrated an association between TERT and immunosuppressive signatures (eg, Th2 cell, Tregs, NK CD56dim cells, MDSCs) across 24 cancer types derived from TCGA; in experimental models, TERT activation was shown to activate endogenous retroviruses and trigger interferon signaling leading to infiltration of suppressive T cells (Th2 cells and Tregs) in tumors (172). Whether or not this action of TERT is relevant to the “cold” immune state of PPGLs requires further study.
Cancer-Associated Fibroblasts in the PPGL Microenvironment
The TME consists of not only tumor and immune cells but also endothelial cells and fibroblasts (58). Endothelial cells play a crucial role in angiogenesis and nutrient delivery. Cancer-associated fibroblasts (CAFs) are a major component of tumor stroma involved in tumor proliferation, aggressiveness, angiogenesis, and immunosuppression through the recruitment and function of various immune cells (173). CAFs usually arise from tumor resident fibroblasts under the influence of cytokines released from cancer cells and are often associated with poor prognosis in many cancer types (173). Several studies have confirmed the connection between fibroblasts and human or murine PPGL cells in vitro (174-176). One in vitro study showed mutual metabolic changes between the primary fibroblast and SDHB-silenced neuroblastoma cells and their increased proliferation compared with their monoculture counterparts (174). Other in vitro studies have shown that SDHB-silenced murine pheochromocytoma cell (MTT cells) spheroids cocultured with CAFs had increased migration/invasion (177, 178) and that CAF metabolism directly affects the invasion process of tumor cells (179). Recent studies have reported that fibroblasts mediate angiogenesis of pheochromocytomas by increasing mitochondrial cytochrome C oxidase subunit 4 subtype 2 (177, 178). Furthermore, insulin-like growth factor 1 (IGF1) and its receptor (IGF1R) have been associated with murine pheochromocytoma development. Specifically, IGF1R-deficient murine fibroblasts exhibited a negative impact on tumors, promoting a lower incidence and slower tumor proliferation rate (180).
In humans, pericytes, fibroblast-like cells known for their role in vessel formation, are the dominant fibroblasts in PPGLs and account for up to 24% of all cells (10). Myofibroblasts, fibroblasts with a matrix-producing contractile phenotype associated with α-smooth muscle actin expression (181), are detected in up to 1.5% of cells in PPGLs, but are absent in normal adrenal medulla (10). Pericytes are also emerging regulators of cancer progression and development: as in tumor angiogenesis, premetastatic niche formation, sustained tumor growth, and evasion of immune response (182). Myofibroblasts contribute to an immunosuppressive TME in several ways, including paracrine and extracellular matrix remodeling. For instance, in pancreatic cancer, myofibroblasts suppress immune surveillance by increasing Tregs in the tumors (183).
While current studies have mainly focused on the metabolic role of fibroblast and/or CAFs in preclinical models of PPGLs, future research may reveal their immunosuppressive potential in murine and human PPGLs.
Immune Signature Classifications: The Current Status and Road to Consensus
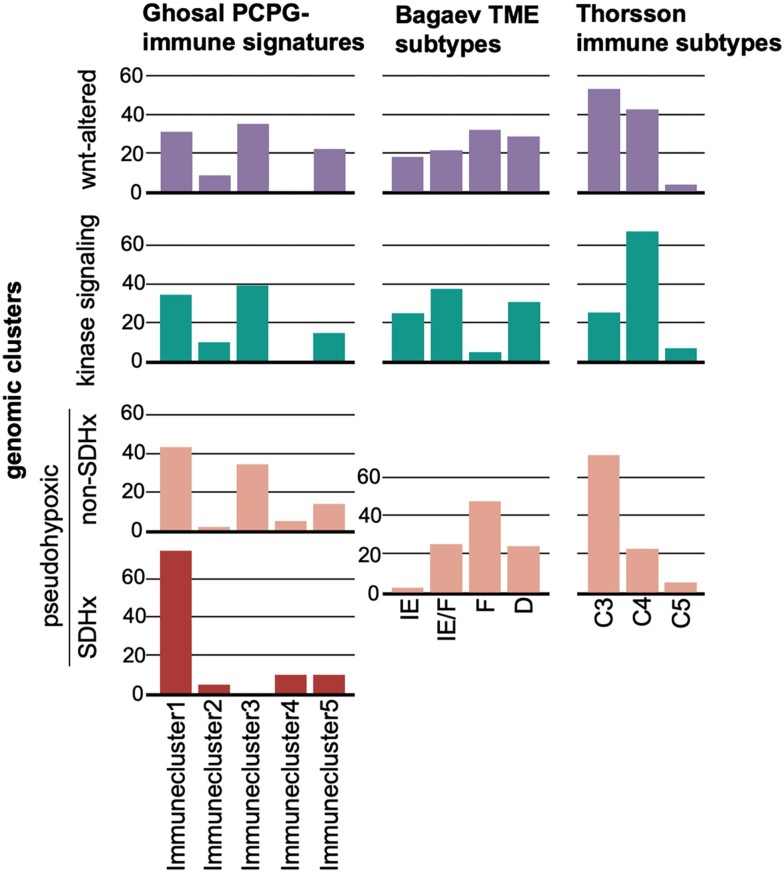
As technology advances, so has the understanding of the complexity and diversity of the immune context of the TME. In fact, recent advancements have allowed for the identification of different subclasses within the immune environment that may influence patient prognosis and potential response to therapy. To date, some reports have partially defined the PPGL TME based on low-resolution methodologies, such as immunohistochemistry. Additionally, other studies have used bulk RNA-Seq and applied techniques such as CIBERSORT to estimate the abundance of the immune infiltrate (20, 21) or more complex gene expression signatures that help characterize other stromal compartments, such as fibroblasts or blood vessels (21, 55, 184). Ghosal et al defined 5 PPGL-specific immune signatures based on the abundance of immune-cell types with high variance in their infiltration scores (immune clusters 1-5: 1, M2 macrophages; 2, monocytes; 3, activated NK cells; 4, Tregs and M0 macrophages; 5, CD8+ and CD4+ T cells (Fig. 2)) (20). Meanwhile, Thorsson et al defined 6 immune subtypes (C1-C6) based on scores obtained from 5 immune expression signatures (macrophages/monocytes, total lymphocyte infiltration, TGF-β response, IFN-γ response, and wound healing) (55). Bagaev et al established 4 additional subtypes (immune-enriched, fibrotic [IE/F], immune-enriched, nonfibrotic [IE], fibrotic [F], and immune-depleted [D]) from 29 knowledge-based functional gene expression signatures representing the major functional components of the TME (184). Both classification methodologies were developed using pancancer data obtained from TCGA project. Recently, Calsina et al applied both methodologies to a large PPGL data set (21) and classified PPGLs into Thorsson's C3 to C5 subtypes and Bagaev's 4 subtypes.
Figure 2.
Classification of immune subtypes within genomic cluster. The proportion (%) of samples of each immune subtype per genomic cluster is shown (x-axis). Data have been extracted from Ghosal et al (20) (first graphs column: Immunecluster1, M2 macrophages; Immunecluster2, monocytes; Immunecluster3, activated NK cells; Immunecluster4, Tregs and M0 macrophages; Immunecluster5, CD8+ and CD4+ T cells) and Calsina et al (21) (second and third graph column: D, depleted; F, fibrotic; IE, immune-enriched, nonfibrotic; IE/F, immune-enriched, fibrotic; C3, inflammatory; C4, lymphocyte depleted; C5, immunologically quiet).
Although the patient cohorts are not large enough to set robust assumptions, some conclusions related to PPGLs and TME can still be established. Pseudohypoxic PPGL tumors are most represented in immune cluster 4 (20) and subtype F (184), which are characterized by their lack of leukocyte/lymphocyte infiltration and TGF-β-driven immune suppression, high M0 macrophage infiltration, low cell proliferation, low levels of overall somatic copy number alterations and tumor mutational burden, and high vascularity and fibrosis (presence of more CAFs). These TME/immune subtypes have been associated with worse prognosis, with TME subtype F being particularly correlated with low response rates to immunotherapy in patients with skin cutaneous melanoma, bladder, lung, or gastric cancer (184). Moreover, the authors compared not only response to the immune checkpoint inhibitors, but other immune-based therapies such as therapeutic vaccination and adoptive cell transfer, suggesting that this classification can be applied to other immunotherapies as a biomarker of response. On the other hand, PPGLs within the kinase-signaling subtype are more represented in immune cluster 3 from Ghosal et al (20) and TME subtype IE and IE/F from Bagaev et al (184), which have been described as having high levels of immune infiltrate, significantly increased cytolytic score, and high T cell activity and T and B cell diversity. They have been associated with a better prognosis, with the TME subtypes IE and IE/F exhibiting the highest percentage of responders to immunotherapies in cohorts of patients with skin cutaneous melanoma, bladder, lung, or gastric cancer. However, the immune infiltrate in some of the cases broadly included in this group could be associated with the increased presence of Th2 cells and Tregs (184). Moreover, the Wnt-altered subtype, described by Fishbein et al in 2017, had been less represented in the PPGL cohorts whose TME had been attempted to be characterized (9). Nevertheless, the recent study from Calsina et al described positivity for PD-L1, which had the highest tumor mutational and neoantigen load, and CD8+ T cell infiltration specifically for MAML3-related tumors, which are part of the Wnt-related subtype, making them the most popular PPGLs in terms of future immunotherapeutic approaches (21). Even though PPGLs are considered to be tumors with the limited presence of immune cells in the microenvironment compared with other tumors and, therefore, response to immunotherapy may be limited, based on the TME clustering and response to immunotherapy in 4 above-described cancer types, we may assume that some PPGL subtypes such as kinase signaling may response better to immunotherapy than other subtypes.
Current Immunotherapy in PPGLs
Cancer immunotherapy research has experienced rapid progress in recent years, resulting in diverse immunotherapy approaches for treating various cancer types. The very first widely known immunotherapy used in humans involved direct stimulation of the immune system within the TME via intratumoral injection of whole bacteria, known as Coley's toxin. William Coley, an American surgeon, reported that 80% of patients with malignant tumors, which at that time had no treatment, survived for more than 5 years (185, 186). However, limited understanding of the immune system and better-defined radiology repressed the use of this approach in clinical practice. With the growing interest in immunology in the 20th century, cancer immunotherapy has become more focused on individual mechanisms and pathways of the immune system. For instance, we have witnessed the approval of tumor-specific monoclonal antibodies, interferons, IL-2, and checkpoint inhibitors for the treatment of specific types of tumors. Blocking immune system regulators, such as PD-1, its ligands, and CTLA-4 have improved outcomes for a broad spectrum of cancers (187-190).
Although specific antibodies against CTLA-4 and PD-1/PD-L1 have shown to be beneficial for the treatment of several types of cancers, including neuroendocrine tumors (NETs) (191-196), data focusing on PPGLs have still been very limited. For instance, 11 patients with metastatic PPGLs were included in a clinical trial (NCT02721732) with pembrolizumab (anti-PD-1). One patient experienced toxicity before week 27 and was therefore excluded from the primary outcome analysis. Among the remaining 10 patients, 4 survived, with no evidence of progression, at 27 weeks. The clinical benefit rate and nonprogression rate at 27 weeks were 72% (95% CI 39-94%) and 40% (95% CI 12-74%), respectively (26). Interestingly, this fascinating and pioneering clinical study also uncovered that IHC staining of tumor PD-L1 did not predict therapy response (26). In 2020, Economides et al published a case of a patient with metastatic disease that did not respond to cabozantinib (tyrosine-kinase inhibitor) and nivolumab (anti-PD-1 antibody) when administered separately but exhibited major tumor response for at least 22 months when administered in combination (197). Similarly, the treatment approach involving the combination of nivolumab and ipilimumab (anti-CTLA-4 antibody) showed benefit in another case of metastatic pheochromocytoma (198). However, the 2 patients with metastatic PGL recruited for a clinical trial (NCT03333616) on ipilimumab/nivolumab exhibited no response, although these numbers are too small to make any useful conclusions regarding efficacy of this therapeutic approach (199). To date, most of the clinical studies on PPGLs are focusing on ICIs (summarized in Table 4). On top of ICIs, a phase I/II study (NCT04187404) is evaluating the effects of vaccine EO2401, which is based on 3 microbiome-derived CD8+ epitopes mimicking parts of tumor-associated antigens, in combination with checkpoint blockade (nivolumab) in patients with metastatic PPGL. Moreover, a recently completed phase I study with VSV-IFNβ-NIS (NCT02923466) virally encoded IFN-β for oncolytic virotherapy. In conclusion, clinical studies for PPGL immunotherapy are still limited and better understanding of immune signatures of PPGL clusters may help to define further development of clinical trials.
Table 4.
Registered clinical trials for immunotherapy of PPGLs
| Clinical trial # | Study phase | Trial name | Condition/Disease | Treatment | Statusa |
|---|---|---|---|---|---|
| NCT05885399 | Phase II | The efficacy and safety of Penpulimab in the treatment of metastatic patients with PPGL who fail to other systemic treatment | Metastatic PPGL | Penpulimab | Recruiting |
| NCT04895748 | Phase I | DFF332 as a single agent and in combination with Everolimus and immuno-oncology agents in advanced/relapsed renal cancer and other malignancies | Renal cell carcinoma | DFF332 | Recruiting |
| Hereditary PPGL | RAD001 | ||||
| PDR001 (Spartalizumab) | |||||
| NIR178 | |||||
| NCT03333616 | Phase II | Nivolumab combined with Ipilimumab for patients with advanced rare genitourinary tumors | Rare genitourinary malignancies | Ipilimumab | Active, not recruiting |
| Adrenal tumors including paraganglioma | Nivolumab | ||||
| NCT04187404 | Phase I/Phase II | A novel therapeutic vaccine (EO2401) in metastatic adrenocortical carcinoma, or malignant pheochromocytoma/paraganglioma | Adrenocortical carcinomaPPGL | EO2401Nivolumab | Recruiting |
| NCT02332668 | Phase I/Phase II | A study of Pembrolizumab (MK-3475) in pediatric participants with an advanced solid tumor or lymphoma | Lymphoma | Pembrolizumab | Recruitingb |
| Advanced solid tumors including paraganglioma | |||||
| NCT02721732 | Phase II | Pembrolizumab in treating patients with rare tumors that cannot be removed by surgery or ere metastatic | Several neoplasms including metastatic PPGL, unresectable PPGL | Pembrolizumab | Active, not recruitingc |
| NCT02834013 | Phase II | Nivolumab and Ipilimumab in treating patients with rare tumors | Several solid tumors including PPGL | Ipilimumab Nivolumab | Active, not recruiting |
| NCT04400474 | Phase II | Trial of Cabozantinib plus Atezolizumab in advanced and progressive neoplasms of the endocrine system. | Neuroendocrine tumorAnaplastic thyroid cancer | CabozantinibAtezolizumab | Active, not recruiting |
| Adenocarcinoma | |||||
| PPGL | |||||
| NCT02923466 | Phase I | Ph1 Administration of VSV-IFNβ-NIS monotherapy and in combination with Avelumab in patients with refractory solid tumors | Solid tumors including PPGL | VSV-IFNβ-NISAvelumab | Completed (April, 2022) |
| NCT05944237 | Phase I/II | HTL0039732 in participants with advanced solid tumors | Solid tumors including PPGL | HTL0039732atezolizumab | Recruiting |
Perspectives
Beyond Conservative Treatments
PPGLs are considered rare neoplasms with significant heterogeneity in their molecular features, clinical behavior, and treatment response. They are one of the most hereditary human neoplasms and are associated with more than 20 susceptible genes described so far (9, 12, 200-209). The genetic landscape of PPGLs undoubtedly affects their TME, responses to various treatment options, and prognosis. While we can now well stratify these tumors into molecular clusters based on their transcriptional profiles, their vast heterogeneity, further supported by their different catecholamine phenotypes, origin, and location, has so far hindered efforts to uncover near optimal and somewhat general treatments, especially for metastatic, locally aggressive, and inoperable PPGLs. According to the World Health Organization classification, all PPGLs are considered to have metastatic potential, replacing the previous term “malignant” (1, 210-212). Thus, there is a continuous need for identifying novel potential treatments beyond surgery, which is currently the only option for cure in some patients. Given the high number of germline pathogenic variants, presence of activation or inhibition of many metabolic pathways with various oncometabolite levels, adrenal and extra-adrenal (including head and neck) locations, and unique molecular signatures, establishing a universal treatment that could target most of these tumors seems impossible, especially when they present with metastatic lesions. While some individual treatments are costly and time-consuming, the main focus in the era of precision medicine is to tailor therapies to specific molecular and metabolic features of tumors (213, 214). Thus, we hypothesize that aside from the current or evolving treatment options, targeting the immune system, along with other therapeutic options further boosted with outcome data from ML modeling approaches that will also include immune signatures (27, 215), will open a new era for the successful treatment of PPGLs.
Prediction of Tumor Behavior and Prognosis
Ongoing clinical and basic investigations on PPGLs have provided much relevant information that has improved our understanding of tumor biology, which can aid in the establishment of patient-tailored disease management and treatment approaches (212, 213). Besides clinical evaluation, the presence of PPGL-specific cell membrane transporters allows the use of many radiolabeled analogs for functional characterization, localization, and highly specific visualization of these tumors even prior to their anatomic assessment (212). Furthermore, studies have recognized the importance of developing and applying immunoscores to classify cancers according to their immune-cell infiltration (216). An immune-based classification of tumors has now been accepted as a better approach for stratifying tumor behavior and patient outcomes over a molecular-based one (216). As discussed earlier in this review, studies have reported immunoscores and immune signatures in PPGLs, leading to a new potential classification for their prognostication and treatment stratification, although confirmation from larger and well-designed studies are still awaited (20, 21, 56).
Recent reports on immune signatures in PPGLs have added another piece into the puzzle that is the pathophysiology of PPGLs, with the implementation of such methodology into routine clinical practice being only a matter of time. Meanwhile, there is a need to uncover additional immune markers that could be measured easily and economically in clinical practice, could well predict positive responses to immunotherapy and other therapies, and could further contribute to the comprehensive understanding of immune signatures in the clinical behavior of these tumors. Recently, Zhong et al had identified a new potential prognostic marker based on the presence of inflammatory markers in the blood of patients with PPGL (136). Notably, the aforementioned study, which included 728 patients with PPGL, showed that an elevated neutrophil–lymphocyte ratio and elevated platelet–lymphocyte ratio was associated with decreased survival, whereas an elevated platelet–lymphocyte ratio was associated with decreased metastasis-free survival. Although such findings remain to be validated in other cohorts, they may become valuable in clinical practice, especially in combination with other methods and approaches for predicting PPGL behavior. Regardless, ongoing studies are seeking to discover additional tumor-relevant inflammatory biomarkers, which would allow for more accurate tumor prognostication and treatment.
The role of the microbiome and its alterations in tumor development have already been recognized as a new hallmark of cancer (217, 218). Microbiota and their bioactive metabolites possess immunomodulatory functions that are linked to the immune system's role in solid tumors (218-220). The variability of the patients’ microbiome and its subsequent manipulation has already proven important for the immunotherapy and other therapies of some cancers (221-223). Evidence suggests that the analysis of the patients’ microbiome has significance in predicting immunotherapy outcomes in melanoma, nonsmall-cell lung cancer, and renal cell carcinoma (224-226). Furthermore, the manipulation of the microbiota can improve the response to immunotherapy in several cancers (227, 228) or even reinduced sensitivity to immunotherapy in several cases of anti-PD-1-refractory metastatic melanoma (221, 222). A preclinical study showed that the crosstalk between intestinal microbiota and adrenal medulla promoted an epinephrine surge during acute stress, indicating a link between the microbiota and adrenal function (229). Variations in the microbiome and their possible link to treatment outcomes have yet to be studied in patients with PPGL. Nonetheless, we personally believe that this could be an additional important step toward successful (immuno)therapy of these tumors.
In conclusion, the combination of immune classification and precise histopathological, molecular, and imaging classifications will undoubtedly improve the unique categorization of PPGLs into clusters, which will be used to provide personalized treatment options that we predict will further improve the outcomes and survival of these patients, especially those with metastatic disease. The addition of various tools, including artificial intelligence (AI)/deep learning (as described below), will undoubtedly be used to monitor the immune TME and further modify treatment in hopes of achieving the best possible outcomes for patients with PPGL while being able to detect and predict the metastatic behavior of these tumors and their responses to therapies in near future.
Imaging Strategies for PPGL Immunophenotyping
Nuclear medicine covers the spectrum of diagnostic and therapeutic procedures, relying on the versatility of radiopharmaceuticals that can be linked to a diverse array of radionuclides. Whether for diagnostics or treatment, its effectiveness in targeting tumors, especially PPGLs, is grounded in its ability to specifically bind tumor cells or their surrounding microenvironment through molecular targeting, unlike external beam radiotherapy. Thus, nuclear medicine plays a pivotal role in the era of precision medicine. The so-called theranostics approach encapsulates the integration of diagnostic and therapeutic functions within the same pharmaceutical platform (a theranostics pair), which is uniquely positioned for PPGLs. For instance, results derived from PPGL-specific 68Ga-DOTATATE positron emission tomography (PET)/computed tomography (CT) or 123I-metaiodobenzylguanidine (MIBG) scintigraphy can determine whether an individual patient is likely to benefit from a specific treatment using the same related compound labeled with a therapeutic radionuclide, here 177Lu or 131I, respectively.
Significant progress has been made in the field of imaging techniques to predict and assess the effectiveness of immunotherapy (230). In this context, nuclear medicine holds vast potential with 4 main objectives: (1) provide prognostic information, (2) predict and monitor responses to immunotherapy and assess potential immune-related adverse events, (3) improve the efficacy of immunotherapy via combination therapies, and (4) characterize and develop therapeutic strategies targeting the TME.
Given that glucose is a crucial metabolite that fuels cancer cells and is widely available across tissues, 18F-FDG (a radiolabeled glucose analog) plays a vital role in detecting and monitoring cancer patients and providing some prognostic information. As previously stated, there is a strong association between PPGL driver genes and tumor transcriptional programs (10), with some imaging features in 18F-FDG uptake typical to pseudohypoxia and kinase-signaling PPGL clusters (231). In metastatic PPGLs, 18F-FDG PET response follows serum succinate levels (133). Despite the lack of specificity, 18F-FDG PET/CT together with genetics, tumor localization, biochemical phenotype, all of which are closely interconnected, offer valuable indicators for prognostic assessment. When translated to immunotherapy, 18F-FDG PET/CT can assist in predicting and assessing response to immunotherapy using ICIs (232) with specific scoring systems (eg, iPERCIST and imPERCIST5) (233-235). 18F-FDG PET/CT can also detect immune-related adverse events before the onset of clinical symptoms (eg, thyroiditis, pancreatitis, hypophysitis, pneumonia, and enteritis), which can help with a treatment adjustment.
Beyond 18F-FDG, newly developed PET molecular probes targeting the composition of the TME could be potentially applied in the context of PPGL immunophenotyping and used as a predictor of response to immunotherapy (Fig. 3). Molecular imaging provides the opportunity to directly target various receptors, co-receptors, or other costimulatory molecules expressed by T cells (eg, TCR and CD8 coreceptor) (236, 237), immune checkpoint molecules (eg, PD-1, CTLA-4, LAG-3, and TIGIT) (238-240), or PD-L1 expressed by cancer cells.
Figure 3.
Examples of targets of immune system and TME for PET imaging. Abbreviations: Ara-G, arabinosylguanine; CTLA-4, cytotoxic T-lymphocyte-associated protein 4; FAP, fibroblast activation protein; IL2-R, interleukin-2 receptor; LAG-3, lymphocyte activation gene 3; PD-1, programmed cell death protein 1; PD-L1, programmed death-ligand 1; TIGIT, T cell immunoreceptor with Ig and ITIM domains; TSPO, translocator protein.
The more advanced approaches are based on the use of radiolabeled antibodies, minibodies, and diabodies (also called immune PET). Radiolabeling is well established for 89Zr, 64Cu, and 124I PET isotopes. However, many challenges related to the long physical half-life of the isotope and high effective dose compared with 18F-FDG still need to be addressed. Therefore, image acquisition is usually performed at delayed phases (over 5-7 days for 89Zr) and require highly sensitive PET cameras. CD8 coreceptor targeting is particularly attractive given that responders to immunotherapy have increased CD8+ T cells within the tumor. The first human trial of 89Zr-Df-IAB22M2C (humanized minibody) that targets CD8 had proven its safety and validity for predicting early response to immunotherapy in patients with solid malignancies (236, 237). The future deployment of highly sensitive, ultra-extended field of view PET scanners (ie, total body) will allow the capturing of images despite using very small tracer quantities with the potential for late imaging. Another approach involves targeting PD-1 with the 89Zr-anti-PD-1 antibody. Beyond antibodies, adnectin-based PET tracers have been developed to target PD-L1 (241). Two anti-PD-L1 adnectins have been generated (FN3hPD-L1-01 and BMS-986192). Studies have shown that 18F-BMS986192 and 89Zr-Df-nivolumab uptake is higher in melanoma lesions with PD-L1 >50% and is therefore correlated with more favorable responses to immunotherapy (239). 68Ga-BMS986192 has been developed and evaluated in preclinical studies (242, 243). However, a challenging issue called timing T exhaustion had been encountered. This means that activated CD8+ T cells can become dysfunctional or “exhausted” within hours of encountering a tumor (244). Therefore, imaging of T cell functionality could be an attractive alternative approach. Several targeting molecules have been developed for imaging and are currently at the early developmental phase, such as imaging of granzyme B (245, 246), IL-2 receptor (IL2-R) (247), OX-40 (CD134) (247), or T cell metabolism with 18F-arabinosyl guanine (248).
CAFs present in the TME of various cancers have been recognized as important regulators of the metastatic tumor behavior. CAFs expressing fibroblast activation protein (FAP) can be selectively addressed by targeted molecular imaging using FAP inhibitors (FAPIs). FAPIs display a remarkable ability to accumulate within tumors with low normal tissue uptake and rapid clearance, leading to an excellent tumor to background uptake ratio. Unlike 18F-FDG PET, FAPI imaging does not require dietary preparation, and images can be captured earlier. In some indications, FAPI imaging can complement or even surpass 18F-FDG PET when used for head/neck, gastrointestinal, pancreatic, liver, gynecological, and brain malignancies (249). For neuroendocrine tumors, FAPI PET/CT can offer some prognostic information and new opportunities for targeted radionuclide therapy (250). FAPI targeting and molecular imaging of hypoxia or angiogenesis deserve specific evaluations into their ability to prognosticate and predict responses to immunotherapy. As stated above, proangiogenic cell types and macrophages, as well as (pseudo)hypoxia in some inherited tumors, had been found to dominate the PPGL TME. Therefore, CD206 expressed by TAMs (251) or translocator protein expressed by various inflammatory cells (macrophages, neutrophils, and lymphocytes) could represent new TME targets for imaging.
In the context of PPGLs, the tandem use of 18F-FDG PET and immune PET, combined with other factors like CT radiomics (252), genetics, and genomics, could effectively guide physicians in selecting candidates for immunotherapy. Data from single-nuclei and bulk-tissue gene-expression analysis can also help to identify novel PET agents to target and evaluate the TME in PPGLs (10). Regarding conversion of “cold” into “hot” tumors (see below), radiopharmaceutical therapy holds potential for fostering an immunogenic TME as has been achieved with external beam radiation therapy (so-called RadScopal approach) (253).
The effectiveness of mutagenic methods in preparing the immune system hinges on not only their rate but also the types of mutations they generate and their capacity to trigger protein misfolding. When considering radiation-based approaches, physicians should account for constrained count of induced mutations, which might hinder the immune-activating impact. These mutations could be randomly dispersed and might impact noncoding regions or RNA molecules, potentially reducing their immunogenic potential. A compelling approach to substantiate the rationale behind these combined interventions is to position them at the neoadjuvant stage, creating a research “platform” to assess the treatment's impact on genomic, peptidome, and immune microenvironmental signatures and other parameters extracted from the resected tumors. Molecular imaging has the potential to facilitate live tracking of the shift from “cold” to “hot” tumors, which aids in identifying the optimal timing for implementing specific immunotherapies.
Overall, orchestrating combination trials is also challenging due to the important potential combinations and sequencing possibilities. Moreover, these investigations necessitate significant patient enrollment, a challenge compounded by the rarity of conditions like PPGLs. Hence, an advanced program tailored to deliver combined therapeutic strategies, encompassing a diverse array of combinations, including targeted radionuclide therapies to patients with PPGLs warrants initiation at the global level. The integration of AI and ML into predictive modeling offers promising results in anticipating responses at both the individual and study levels (eg, survival curves, hazard ratios), thereby expediting the cessation of unproductive therapeutic strategies. It is, however, not feasible to incorporate all imaging modalities into trials given the risk of increasing complexity and limiting therapy access.
Overall, technological breakthroughs and development of new tracers that could potentially assess antitumor response and TME dynamics in vivo will continue to secure the position of nuclear medicine among the significant disciplines facilitating precision medicine. To effectively advance these initiatives, it is crucial that nuclear physicians receive training in theranostics and immunotherapy.
The Role of Artificial Intelligence in Prediction, Immunoscores, and Prognostic Reclassification
AI refers to complex computer algorithms that mimic human cognitive functions, including learning and problem-solving. ML is a branch of AI that further focuses on enabling machines to learn from data and improve their performance without being explicitly programmed (254). With the continued technological advancements, deep learning has emerged as a class of ML that allows for computational models composed of multiple processing layers to learn representations of data with multiple levels of abstraction (255). These methods have dramatically improved the state of the art in several domains, including the diagnosis and prognosis of many cancers (256-259). AI can assist with multiple levels of diagnostic and predictive processes in cancer, such as the evaluation of imaging methods (260, 261), detection of biomarkers (256, 262), or detection of circulating tumor cells to predict metastatic behavior (263). Moreover, studies have shown that AI can help with predicting the response of several cancers to a variety of treatments, including immunotherapy (260, 264-268).
This rapidly developing approach in diagnosing and predicting tumor behavior has been recently applied to PPGLs. AI/ML has been introduced for the evaluation of PPGL metastatic behavior, with the researchers incorporating 9 clinical and biochemical features into the ML model (215). The external validation cohort displayed a sensitivity of 83% and specificity of 92% for the final model, providing a noninvasive preoperative approach for predicting metastases and guiding individualized patient management and follow-up (215). This first incorporation of AI/ML in the diagnosis and prognosis of PPGLs has shown promising results. Together with emerging information on immunoscores and the existing role of genomics in predicting tumor behavior and its treatment targets, we believe that AI-assisted immunogenomics will extend beyond the traditional clustering of PPGLs with a promise to uncover their new treatment options. To train the machines, however, vast amounts of data from high-quality training sets will be needed. For instance, AI has been recently used to automatically evaluate IHC results for CTLA-4 in 90 different tumors; however, 1 of the antibodies demonstrated nonspecific staining for pheochromocytoma and would lead to an incorrect evaluation when used (85).
Nevertheless, the availability of high-quality and diverse datasets for rare disease, such as PPGLs, will be further secured only through large and very well-designed multicenter and preferably global collaboration. Furthermore, health care professionals will need to learn how to understand the basic underlying algorithms and interpret the results accurately to avoid errors and overcome some ethical issues related to AI-based technologies. This indicates the need for a new generation of health care professionals molded, for instance, by new education courses, seminars, and lectures, and with new curricula for medical and other students.
Perspective Immunotherapy Approaches
Several different immunotherapy approaches are currently being studied for various cancers, including immunomodulators, targeting cytokines and checkpoint inhibitors, adoptive cellular therapy, genetically modified or antigen-specific T cells, or cancer vaccines. All these approaches have their advantages and disadvantages. In the following paragraphs, we further discuss the potential treatment options, promising combinations, and future directions for PPGLs with respect to immunotherapies.
Immune Checkpoint Inhibitors
The discovery of immune checkpoints and the possibility of blocking their functions using ICIs to enhance immune system response to tumor cells can be considered a breakthrough in the treatment of many cancers. However, several concerns need to be addressed. The response rate to ICIs, and the rate of immune-related adverse events following such treatments, seems to be variable even among the same type of cancer (71, 269). To improve response to therapy, combination strategies have been utilized. Although the use of anti-CTLA-4 agents in conjunction with anti-PD-1/PD-L1 therapies (270, 271) have improved the response rate, the incidence and severity of toxicities have been lamentable (71, 272, 273). Given the low PD-L1 expression in many PPGL cases, disrupting other T cell exhaustion pathways may enhance the effectivity of ICIs. In fact, a clinical trial that recruited 2 patients with metastatic PGL who received anti-CTLA-4 and anti-PD-1 agents (NCT03333616) found no response to treatment (199). Although a cohort of 2 patients is an extremely small sample to conclude any reliable efficacy, this finding could redirect the focus from currently used ICIs to other newer ICIs. LAG-3, which is expressed on activated human T and NK cells, can act as a marker of T cell exhaustion in a chronic inflammatory state, such as that in a tumor (274-276). Recently, the combination of spartalizumab (anti-PD-1) and LAG525 (LAG-3 antibody) showed promising activity in NETs (NCT03365791) (277) and could be an interesting option for PPGLs.
Adoptive Cellular Therapy
Another promising immunotherapeutic approach is based on the adoptive transfer of immune cells that are manipulated and expanded ex vivo. By taking advantage of their ability to eliminate cancer cells, T cells can be used in cellular therapies through 2 main strategies. The first strategy involves the isolation and in vitro expansion of antigen-specific TILs, which have already infiltrated the TME, whereas the second involves isolation of T cells from the patient's blood with consequent in vitro genetic modification of their receptor (called CAR T cells from chimeric antigen receptor T cells) to improve their antigen recognition and biological activity. Research on CAR T cell has continued to progress to the point that the current generation (fourth) contains additional costimulatory signals and transcription factors that activate innate immune response through the production and secretion of IL-12 or other proinflammatory cytokines (278). While T cell immunotherapy using CAR T cells has shown encouraging results in the treatment of NETs (278), no clinical trial has included any patient with PPGL. TIL therapy is another adoptive cellular therapy studied in a clinical trial (NCT01174121) that included metastatic NETs where CD8+-enriched TILs isolated from the tumor after its resection were administered to the patient alongside conditioning chemotherapy and IL-2 cytokine to further stimulate T cell proliferation and maturation. Currently, it remains uncertain whether patients with PPGL could benefit from TIL therapy given that most PPGLs lack tumor T cell infiltration. CAR T cells are personalized for each patient and may be combined with other therapeutic approaches to target the hostile TME. However, given the limited number of general PPGL neoantigens, the development of CAR T cell therapies or other therapeutic vaccines against PPGLs may be limited (see below). Nonetheless, proteins involved in catecholamine syntheses, such as tyrosine hydroxylase, dopamine β-hydroxylase, and phenylethanolamine N-methyltransferase may be considered. Unfortunately, such personalized therapy is time-consuming for in vitro preparation and is often associated with safety limitations as manifested by its most common side effect called cytokine release syndrome (279). This can, of course change, with new information and techniques that would allow generalized development and safer applications of CAR T cell therapies to patients.
Intratumoral Immunotherapies
Despite the clinical success of immunotherapies in different tumors, especially checkpoint inhibitors, several limitations still need to be addressed. These 1-way oriented approaches, which target only isolated pathways in cancer immunoediting process, could be ineffective against many types of solid tumors, especially those with strong or multiple immune escape mechanisms, such as PPGLs. Additionally, the immunotherapies are often administered intravenously followed by systemic distribution to the tumors through the circulatory system, which may result in decreased drug penetration into the tumors, higher drug dosages to achieve the required effect, and increased off-target systemic toxicity (Fig. 4A) (280, 281). These limitations can be overcome through the use of prodrugs with selective accumulation in the tumors or intratumoral applications that enable the direct delivery of a therapy into a tumor lesion, lower drug dose, and increased local concentration, which consequently decrease the rate of systemic toxicities (280, 282). Such direct application of immunostimulatory agents in a tumor may promote trafficking of immune cells into the tumor and local priming of the antitumor immune responses even without tumor antigens, which can be very beneficial, especially in immunologically “cold” tumors such as PPGLs. Moreover, the local priming may generate a systemic immune response even against noninjected distal metastases. Furthermore, preclinical studies in mice have also shown the potential of ICI delivery to the draining lymph nodes, resulting in improved ICI efficacy and safety profiles for local and distant metastases in mice (283, 284).
Figure 4.
Intratumoral immunotherapy. (A) Advantages and disadvantages of intratumoral and systemic administration of immunotherapy. (B) Mechanism of MBTA immunotherapy (preclinical study). (1) MBTA therapy is consisted of mannan-BAM, 3 TLR ligands, and anti-CD40 antibody. After intratumoral application of MBTA therapy, mannan-BAM is nonspecifically anchored to lipid bilayer of tumor cells. (2) TLR ligands recruit the immune cells into the tumor. Opsonized tumor cells are then recognized by infiltrated innate immune cells and killed, resulting in (3) the release of antigens which can be present in lymphatic nodes and activate adaptive immune cells. The anti-CD40 antibody binds to CD40 receptor, which mainly expressed on APCs and initiates their activation. (4) Adaptive immune cells, mostly CD4+ and CD8+ T cells, then infiltrate the tumor and kill tumor cells. Abbreviations: DCs, dendritic cells; NK cells, natural killer cells; TLR, toll-like receptor.
One of the main concerns for intratumoral immunotherapies is feasibility. Recent studies have revived this approach, which dates back to early 20th century, and have proven its feasibility in patients with various cancer types and tumor locations (285-288), including neuroendocrine tumors and adrenal glands (289). Another apparent concern for using intratumoral applications or core needle biopsy in PPGLs are catecholamine-related complications. A systematic review and individual patient meta-analysis from Zhang et al evaluated the risk of complications after core needle biopsy in patients with primary or metastatic PPGL (290). Notably, the study found zero deaths and a minority of patients with PPGL with catecholamine-related complications after the biopsy. However, given the lack of scientific evidence, no firm conclusion can be reached. The authors state that core needle biopsy is a justifiable approach that should be conducted with thorough consideration, adequate preparation, and close follow-up, confirming the feasibility and potential use of intratumoral injection in future clinical trials on patients with PPGL.
Several intratumoral immunostimulatory agents are already in clinical trials, whereas some promising agents are in preclinical evaluation, including oncolytic and nononcolytic viruses, PRRs agonists such as toll-like receptors (TLRs), mRNA encoding agents, cytokines, and immune cells. Currently, the most used intratumoral immunotherapy in clinical trials are oncolytic viruses, which induce immunogenic death and promote antitumor immunity after intratumoral administration and subsequent infection of tumor cells (291). The oncolytic herpes simplex virus type 1 Talimogene Laherparepvec, better known as T-VEC or Imlygic, has currently been approved for unresectable stage III melanoma in several states worldwide (292).
The second most studied intratumoral immunostimulatory agent in clinical trials are agonists for TLRs, 1 of the 5 families of PRRs that are crucial for activating the innate immune system. They are membrane-bound receptors expressed mainly on APCs that are able to recognize pathogen-associated molecular patterns, such as nucleic acids, bacterial polysaccharides, or flagellin. Currently, 10 TLRs are known to be present in humans. Ligation of TLRs causes the activation of the cells and subsequent secretion of cytokines and chemokines involved in the chemoattraction of other immune cells or direct cytotoxic effects. Therefore, TLRs can be targeted to traffic immune cells into the tumor and convert their immunologically “cold” microenvironment into a “hot” one. However, it should be mentioned that TLRs have been implicated in tumor suppression and progression (293, 294). Several TLR ligands are currently being evaluated in clinical studies as reported in a detailed reviewed elsewhere (295).
We predict that intratumoral immunotherapy would become the best approach for effective cancer immunotherapy given its ability to activate innate and adaptive immune system, resulting in the activation of multiple pathways and, therefore, potentially successful antitumor responses. Therefore, a preclinical intratumoral immunotherapy, called MBTA therapy, has been developed. MBTA therapy is based on the combination of phagocytosis ligand, TLR ligands, and an immunostimulatory CD40 antibody (296) (Fig. 4B). One of the main advantages of this therapy is its independence from specific tumor antigens or the immunosuppressive TME. After intratumoral injection of MBTA, mannan-BAM, a phagocytosis ligand connected to the biocompatible anchor for cell membrane, is artificially anchored into the tumor cell membrane, resulting in the activation of the complement system and subsequent opsonization of tumor cells by the complement proteins. Simultaneously, TLR ligands recruit the immune cells into the tumor. Opsonized tumor cells are then recognized by infiltrated innate immune cells and killed, resulting in the release of antigens. Anti-CD40 antibodies bind to the CD40 receptors expressed mainly on APCs and initiate their activation. APCs can further process the tumor antigens and activate adaptive immune system by presenting the antigens to T cells in the lymphatic nodes.
The efficacy of the described MBTA therapy was tested in various murine tumor models, including pheochromocytoma (297), pancreatic adenocarcinoma, melanoma (297), or colon carcinoma (298), with similar outcomes having been observed. In a murine model of pheochromocytoma, MBTA therapy promoted complete elimination of tumors or significant prolongation of survival and reduction of distal metastases with no age-dependent differences, including a strong immune-cell infiltration into treated tumors and distant nontreated metastases (299-301). Moreover, MBTA therapy was able to switch the phenotype of macrophages from M2 to M1 not only in injected tumors but also systemically in distal metastases. Our preclinical data support further investigation regarding the use of intratumoral MBTA therapy in patients with nonmetastatic or metastatic PPGLs. Given that MBTA therapy targets more immune pathways and does not require specific tumor antigens, we believe that numerous patients with PPGL would benefit from this therapy independent of their genetic background or TME in the future.
Therapeutic Cancer Vaccines
The main goal of therapeutic cancer vaccines is to induce antitumor response, establish long-lasting immune memory, and avoid off-target toxicities. Cancer vaccines typically involve exogenous administration of tumor antigens that activate DCs. Current sources of tumor antigen delivery include DNA, RNA, and synthetic peptide vaccines. Another approach is based on direct in vitro delivery of monocyte-derived DCs loaded with antigen peptides (302). The success of therapeutic cancer vaccines is heavily dependent on specific tumor antigens. As discussed earlier in the chapter focused on CAR T cells, specific tumor neoantigens for PPGLs can be limiting. However, 1 preclinical study on murine models of pheochromocytoma (MPC) showed that chromogranin A, which is elevated in most neuroendocrine tumors including PPGLs, can be used as a specific target molecule for DC vaccination (303). The same authors demonstrated that CgA peptide-based immunotherapy induced cytotoxic immune response as evidenced by the high intratumoral CD8+ T cell infiltration and MHC I-restricted lysis toward pheochromocytoma cells. Future research should focus more on identifying general PPGL tumor antigens that could be used for not only DC-based therapeutic vaccines but also CAR T cells. Currently recruiting phase I/II first in human clinical trial will test safety and efficacy of EO2401 vaccine together with nivolumab (NCT04187404). EO2401 is cancer peptides therapeutic vaccine based on delivery of microbiome-derived peptides with mimicking the tumor-associated antigens (IL13Rα2, BIRC5, and FOXM1), which are upregulated in adrenocortical carcinoma and metastatic PPGL to stimulate targeted T cells mediated tumor killing (304).
Another approach is the use of whole-tumor cell lysate, which enables the use of complete tumor cell antigens instead of only 1 specific antigen. Such an approach could be more suitable for PPGLs. In fact, 2 preclinical studies showed that subcutaneous whole-tumor cell vaccines consisting of irradiated tumor cells in vitro pulsed with the MBTA therapy described earlier inhibited tumor growth and prevented metastases in murine models of aggressive breast cancer, melanoma, and colon carcinoma (298, 305). Although this approach has not been attempted in murine models of pheochromocytoma, there is high possibility that whole-tumor cell MBTA vaccines could also work in this model given that the intratumoral application of MBTA therapy had positive effects on pheochromocytoma reduction. Moreover, this approach may preclude the need for intratumoral injection and increase the availability of this therapy for more patients with less accessible metastases, for instance, those in the bones. However, the source of the antigens remains an important question. For allogeneic vaccines, the material is sourced from another member of the same species, such as a cancer cell line. Autologous vaccines use material derived directly from a patient's tumor to create personalized treatment. Given that each source has its advantages and disadvantages, future research should determine which source would be most appropriate for PPGLs.
Combination of Therapies to Outsmart Resistant or Metastatic PPGLs
As stated earlier, the significant rate of failure associated with immunotherapy is a critical aspect that should be addressed. Focusing particularly on ICIs, the response rate to single-agent blockade of checkpoint inhibitor ranges from 10% to 70%, depending on cancer type (306). The Immunotherapy Resistance Taskforce of the Society for Immunotherapy of Cancer established 3 definitions of resistance to ICIs that are observed in clinic: (1) primary resistance, where a cancer does not respond to an immunotherapy, (2) secondary or acquired resistance where a cancer initially responded to immunotherapy but later followed by disease progression, and (3) progression after discontinuation of treatment (307). Schoenfeld and Hellmann discussed in their review that unlike primary resistance, secondary resistance to an immunotherapy has not been routinely reported (306). They attempted to infer the rates of acquired resistance during ICIs based on available data among different types of tumors and showed variances, suggesting disease-specific characteristics of acquired resistance. As described in detail earlier in the chapter focusing on immunoediting, several tumor cell intrinsic factors, such as lack of immunogenicity, and TME-associated extrinsic factors, such as the presence of immunosuppressive cells, may lead to immunotherapy resistance and tumor progression. Since PPGLs have unique TME with many of these factors, the rate of resistance to immunotherapy may increase. Additionally, the resistance can be observed in case of metastatic diseases where only partial response to immunotherapy is often noted. However, such resistance can be overcome using combining therapeutic strategies. Treatment combinations that target the “cancer–immunity cycle,” thereby boosting the presence antitumor immune cells and other tumor inhibitory pathways while inhibiting tumor immunosuppressive mechanisms, with other available therapeutic approaches may improve the resistance in patients with PPGL (308) (Fig. 5). Understanding the complexities behind these resistances will become paramount when the duration of patient response can be examined, the underlying mechanisms that contribute to resistance can be uncovered, and this resistance can be overcome and identified not only in PPGLs but also in other cancers.
Figure 5.
Enhancing the antitumor efficacy of immunotherapy with immune-supportive therapies. Variety of immunotherapies can be effective in targeting PPGL. To maximize the benefits of immunotherapy, combination of more immunotherapies, different delivery routes (eg, systemic vs intratumoral), and supportive therapies that target hostile TME may be selected to enhance the effective elimination of tumor cells by immune system. #Currently in clinical trials or clinical use for cancer treatment (status to date December 1, 2023, clinicaltrials.gov). *Therapies in the clinical use or trial for PPGL. Abbreviations: IL, interleukin; IFN, interferon; poly(ICLC), derivate of polyinosinic:polycytidylic acid; CpG, cytosine-phosphorothioate-guanine oligodeoxynucleotides; LTA, lipoteichoic acid; DC, dendritic cell; TLR, Toll-like receptor; CAR-T cell, chimeric antigen receptor T cells; PD-1, programmed cell death protein 1; PD-L1, programmed death-ligand 1; CTLA-4, cytotoxic T-lymphocyte-associated protein 4; LAG-3, lymphocyte-activation gene 3; TIGIT, T-Cell immunoreceptor with Ig and ITIM domains; HIF, hypoxia-inducible factor; TKI, tyrosine-kinase inhibitors; VEGF, vascular endothelial growth factor; SUCNR1, succinate receptor 1; IDO, indoleamine 2,3-dioxygenase; MIBG, metaiodobenzylguanidine.
Bridging the Innate and Adoptive Immune System
The immunosuppressive TME and concurrently low expression levels of immune checkpoints in some PPGLs suggest that combining immunotherapeutic approaches to engage innate immunity may be superior for developing effective antitumor immunity. Such an approach is currently being utilized in ongoing clinical trials in solid tumors including some NETs. An active phase I study (NCT03891953) on NETs is combining spartalizumab (anti-PD1) with DKY709 for immune enhancement. DKY709 reduced the suppressive activity of human Treg cells and rescues cytokine production in exhausted effector T cells in experimental studies (309). Another phase I study (NCT04261439) is combining spartalizumab (anti-PD1) or tislelizumab (anti-PD1) with NIZ985, which promotes cytotoxic lymphocyte proliferation, killing function, and organ/tumor infiltration, thereby promoting anticancer effects (preliminary results (310)). FT500, an iPSC-derived NK cell product (NCT03841110), is another promising tool that engages the adaptive arm of the immune system while synergizing with ICIs. In preclinical studies, FT500 mediated both direct tumor lysis and T cell activation and recruitment, which can augment checkpoint inhibitor therapies (311).
Given the general PPGL immune phenotype of excessive M2 infiltration and lack of T cell infiltration, we propose that innate immune system stimulation will navigate the perspective immunotherapy approaches for PPGLs. TLR agonists have been shown to reprogram immune-suppressive M2 macrophages (312, 313), which is a major immune-cell subtype for PPGLs. Moreover, TLR ligands seem to have potential to synergize with ICI treatment in preclinical models of several cancers (313-316). Currently, ICIs (the CTLA-4 antibody remelimumab and PD-L1 antibody durvalumab) have been combined with poly(ICLC) in a clinical trial including NETs (NCT02643303). Poly(ICLC) is a potent TLR3 ligand that activates DCs and repolarizes M2 macrophages and TAMs, promoting the secretion of IFN and proinflammatory cytokines in glioblastoma (317). It can also attract and stimulate T cells, supporting its combination with ICIs (317). CD40 agonists are another tool to immunologically convert the TME (318, 319). For the last 2 decades, new agonistic CD40 monoclonal antibodies have been developed and shown to reduce tumor fibrosis, decrease M2-like TAMs, increase maturity of intratumoral DCs and cytokines, and activate T cells in human pancreatic cancer (320). Currently, the combination of ICIs and CD40 agonistic antibodies have been tested in various cancers based on the promising results of clinical and preclinical studies (NCT03214250, NCT05231122, NCT04635995, NCT02706353, NCT03502330) (321-323). Interestingly, low expression of CD40 and TLR4 was identified in metastatic PPGLs (21), which supports the potential for stimulating CD40 and TLR4. The advantage of repurposing drugs/approaches from ongoing trials is the knowledge of their safety and efficacy for other NETs, which may facilitate clinical trials for patients with PPGL.
Fighting the Hostile TME: Immunotherapy and TME Molecular Manipulation
Although immune-cell concentrations and tumor cell recognition can be effectively increased, immunosuppressive TME can repel effective and persistent immune attacks. Therefore, targeting pro-oncogenic metabolic waste accumulation or limiting the access to necessary substances required for proper cell function and proliferation to overcome resistance to cancer immunotherapy may be an essential component of a successful immunotherapy. Targeting the pro-angiogenic signaling, hypoxic TME, accumulation of succinate and other metabolites, and/or adrenoceptor signaling, which are the main features of PPGL tumorigenesis, can help to modify the hostile TME and enhance immunotherapy efficacy.
Immunotherapy and Blocking of the VEGF Pathway
VEGF inhibitors were shown to decrease macrophage recruitment in preclinical model (88). Given that PPGLs are often infiltrated by TAMs, patients with PPGL may benefit from treatment beyond the effect on tumor vascularization. However, it seems that PPGLs escape from the effects of VEGFR/TKI over time and that most cases simply exhibit a period of tumor stabilization (96). Thus, combination with other therapeutic approaches may be beneficial for patients. Currently, none of the studies in patients with PPGL have combined immunotherapy with VEGFR inhibitors. This combination has already been introduced in studies on other immunologically “cold” tumors including NETs (NCT0500029). The combination of ICIs and VEGF inhibitors seem to be effective for advanced and metastatic renal cell carcinomas (324-327), hepatocellular carcinomas (328), advanced gastric and colorectal cancers (328), and advanced endometrial cancers (329). These results seem promising for patients with advanced and metastatic cancers and could improve the treatment of immunologically “cold” or resistant PPGLs.
Immunotherapy and Hypoxia Signaling
Hypoxia is considered a critical driver of cancer pathogenesis; thus, several hypoxia-inducing factor (HIF) inhibitors have been developed and are currently being tested in clinical trials. Currently available data on hypoxia signaling in PPGLs and ongoing clinical trials have been reviewed extensively elsewhere (330). A previous case study on 1 patient with Pacak–Zhuang syndrome indicated that the selective HIF-2α inhibitor belzutifan had great potential (331). An active phase II study (NCT04924075) on belzutifan may highlight the potential of hypoxia suppression in a larger PPGL cohort. Moreover, a phase I study on DFF33 (NCT04895748), another selective HIF-2α inhibitor, which will enroll patients with VHL, FH, and SDHx pathogenic variants and EPAS1 mutations, will be evaluating the safety and efficacy of the inhibitor itself or in combination with spartalizumab, a PD-1 antibody. Another phase I study on renal carcinoma has been launched to measure the safety and efficacy of combining HIF-2α inhibition with ICIs, suggesting possible future directions for hypoxia inhibitors (NCT05030506).
Besides the direct targeting of HIFs, several drugs can affect (pseudo)hypoxia to suppress the hostile TME in preclinical models (157, 332). However, no clinical studies in patients with PPGL have tested nonselective HIF-targeting drugs (332). Recently, metformin has been shown to rescue murine and human CD8+ T cells from hypoxia-induced apoptosis and increase their proliferation and cytokine production while blunting the upregulation of PD-1 and LAG-3 (333), inhibiting the M2 polarization (334), and increasing M1 macrophages in murine cancer models (335). Indeed, several preclinical studies in PPGL models (MTT, PC-12, PTJ64i, and PTJ86i cells) have also shown that metformin possesses antiproliferative effects in vitro (179, 336, 337); however, it failed to affect murine PHEO tumor growth (MPC cells) in vivo (338). While metformin as a single agent did not produce sufficient effects, it may indeed decrease HIF1A in PPGL cell models (PC-12 cells) (336), affecting the hostile TME to increase efficacy of immunotherapies. Moreover, the immune effects of metformin appeared to result from a reduced production of reactive oxygen species (333), making the treatment especially appealing for SDHx-mutated PPGLs that are characterized with high reactive oxygen species production (339).
Immunotherapy and Catecholamine Metabolism
As discussed earlier, β2-adrenoceptor antagonists seem to be a reasonable cotarget for immunotherapy, especially for pseudohypoxic PPGLs. Some β-adrenoceptor antagonists have been associated with the improved clinical outcomes of immunotherapy in melanomas (340). Labetalol is a noncardioselective β-adrenoceptor and selective α-adrenoceptor blocker that has been proven effective in controlling PPGL-associated hypertension, indicating its safety. Other noncardioselective β-adrenoceptor blockers, such as propranolol and nadolol, and cardioselective agents, such as atenolol and metoprolol, could also be used in combination with immunotherapeutic approaches. Furthermore, DA receptor antagonist, ONC201, has already proven effective in a phase II study on PPGL (NCT03034200) (121). Preclinical results have indicated the potential use of DA receptor inhibitor, ONC201, in combination with ICIs in advanced solid tumors (123). Moreover, given that catecholamines have been shown to upregulate VEGF to enhance tumor growth in human melanoma tumor cell lines (341), we hypothesize that combining β-adrenoceptor blockers, VEGF inhibitors, and immunotherapy may improve PPGL treatment, especially for those with advanced and metastatic cases that rely on nutrients and immunosuppressive TME.
Other Potentially Targetable Pathways From Preclinical Studies for SDHx-Mutated Tumors
The treatment and prognosis evaluation of SDHx-mutated tumors appear to be challenging. Given the multiple pathways affected by SDHx protein loss, selecting multiple targets may be necessary to overcome the hostile TME. SDHx-, especially SDHB-mutated tumors, account for the majority of metastatic and aggressive tumors (212). Aside from the targets for synergizing immunotherapy mentioned elsewhere in the current review, several more need to be considered for SDHx pathogenic variant carriers. Matlac et al described that succinate mediates its effects mainly via the SUCNR1 receptor in pheochromocytoma cells and that inhibition of SUNCR1, especially expressed on tumor cells, may decrease tumor immunosuppression (126). Furthermore, increased IDO expression and tryptophan depletion may contribute to this immunosuppressive TME in PPGLs. Although IDO inhibitors are available and have been actively tested in several clinical trials, they have instead been used as an adjunct to other treatments in order to overcome resistance to the original treatment and/or enhance efficacy (342). Several currently ongoing trials have focused on further combining IDO inhibitors with immunotherapies, especially ICIs (342), although none of these studies include patients with PPGL. Polyamine inhibition decreases myeloid suppressor cells and increases CD8+ T cell infiltration into the preclinical models of tumor (149, 343). Rai et al had already shown that polyamine inhibitors suppress the growth of SDHx-mutated pheochromocytoma tumor cells in mice (146) and that targeting this pathway in PPGL may increase the efficacy of other treatments by changing the unhostile TME, especially in SDHx-mutated tumors.
Somatostatin Receptor for Navigating Immunotherapies
Targeting PPGL-specific biological pathways and receptors with radionucleotide scintigraphy and radiotherapeutics has become an important part in customized treatment (344), as compressively discussed earlier. The norepinephrine transporter system binding the radioemitter 123/131I-MIBG and somatostatin receptor (SSTR)–binding agent 90Y/68Ga/177Lu-DOTA-SSA are promising radiopharmaceuticals for PPGL theranostics and have been successfully adopted in clinical practice (344). Given the high SSTR expression regardless of tumor location (345, 346) and association between SSTR2 and SDHx-mutated PPGLs, metastatic disease, and head and neck PGLs, independent of SDHx pathogenic variant status (347), co-biding with SSTR agonists/antagonists could be used as delivery systems for the direct delivery of ICIs or other therapies into the tumor. Indeed, a similar approach had been utilized in a clinical study on NETs and gastrointestinal tumors (NCT03411915) combining SSTR2 and anti-CD3–bispecific antibodies (Tidutamab) that direct T cell-mediated cytotoxicity to cells with SSTR2 positivity. Moreover, radiation delivered to the tumor site induces cancer cell damage, tumor-specific antigen exposure to immune cells, and TME modulation, which facilitates the recruitment and infiltration of immune cells (348, 349). Given the positive outcomes of radiotheranostics in PPGLs, radioimmunotherapy seems to be next phase.
Surgery and Neoadjuvant Immunotherapies
Surgery still remains the primary treatment option for PPGL patients. However, the risk for primary tumor recurrence or distal metastases can be more than 16% after the surgery (350-353). Several preclinical and clinical studies focusing on the combination of surgery and immunotherapy have recently shown better efficacy and broader immune responses in various tumors with preoperative or neoadjuvant application of immunotherapies than with adjuvant application of immunotherapy alone (354-356). Postoperative immunosuppression enables cancer cells to proliferate and dormant cancer cells to awake, resulting in rapid recurrences and/or metastases. One of the main advantages of neoadjuvant immunotherapy is the easier interaction between the immune system and TME due to the undamaged structure of the lymphatic system. In other words, the tumor itself can be used as a source for a wider variety of tumor antigens, resulting in the activation of T cell clones, improved antitumor responses, and elimination of dormant tumor cells or distant micrometastases after surgery (357).
In the future, PPGL patients could receive 1 of the suggested immunotherapies in the neoadjuvant setting, which could promote primary tumor shrinkage, less invasive tumor resection, and minimal recurrence after surgery. Indeed, the aforementioned intratumoral MBTA immunotherapy had been tested in the neoadjuvant setting followed by resection in murine pheochromocytoma, with the results showing better prevention against the development of distal metastases with this combination than with the control condition of resection only, even in large (advanced) tumors (unpublished results). However, such results must be confirmed in clinical studies. It remains unclear whether patients would benefit from other neoadjuvant immunotherapy approaches, such as blockade of the PD-1/PD-L1 axis currently widely used in clinic practice. To the best of our knowledge, no clinical study has focused on checkpoint inhibitors/activators in the neoadjuvant setting for PPGL patients. Another important concern for futures studies is whether neoadjuvant immunotherapies could be used for patients with metastatic PGGLs in whom surgery is still limited.
Concluding Remarks
We herein reviewed currently available information regarding the immune system's role in PPGL development and progression, current immunotherapy outcomes, and perspectives on the use of the immunotherapeutic approach to improve PPGL treatment. We have largely focused on the characterization of immune escape mechanisms, considering the role of T cells, macrophages, immune checkpoints, and a complex unhostile TME resulting from high genomic and biochemical PPGL heterogeneity. Understanding the immune functions and escape mechanisms will be crucial for the development of new approaches and continuous improvement of immunotherapeutic strategies for PPGLs. Nonetheless, several questions need to be addressed first, particularly those regarding the immunomodulatory actions of some metabolites and signaling pathways and their effects on immunotherapy outcomes or the safety of combination therapies for patients. Clinical trials and further investigations should soon provide us with more information to help improve our understanding of the underlying biology, which will likely reveal additional potent treatments in the future. We hypothesize that combined therapies, utilizing 1 or more immunotherapies and TME manipulation, would likely become the standard of care for PPGLs in the near future.
Abbreviations
- AI
artificial intelligence
- APC
antigen-presenting cell
- CAF
cancer-associated fibroblast
- CAR
chimeric antigen receptor
- CT
computed tomography
- CTLA-4
cytotoxic T-lymphocyte–associated protein 4
- DA
dopamine
- DAMP
damage-associated molecular pattern
- DC
dendritic cell
- FH
fumarate hydratase
- HIF
hypoxia-inducible factor
- ICI
immune checkpoint inhibitor
- IDO
indoleamine 2,3-dioxygenase
- IHC
immunohistochemistry
- IFN
interferon
- IL
interleukin
- LAG-3
lymphocyte-activation gene-3
- MHC
major histocompatibility complex
- MIBG
metaiodobenzylguanidine
- ML
machine learning
- NE
norepinephrine
- NET
neuroendocrine tumor
- NK
natural killer
- PASS
Pheochromocytoma of the Adrenal Gland Scaled Score
- PD-1
programmed cell death protein 1
- PD-L1
programmed cell death ligand 1
- PD-L2
programmed cell death ligand 2
- PET
positron emission tomography
- poly(ICLC)
derivate of polyinosinic:polycytidylic acid
- PPGL
pheochromocytoma/paraganglioma
- PPR
pattern recognition receptor
- RNA-seq
RNA sequencing
- SDHB
succinate dehydrogenase subunit B
- SSTR
somatostatin receptor
- TAM
tumor-associated macrophage
- TCGA
The Cancer Genome Atlas
- TGF-β
tumor growth factor beta
- TIGIT
T-cell immunoreceptor with Ig and ITIM domains
- TIL
tumor-infiltrating lymphocyte
- TKI
tyrosine kinase inhibitor
- TLR
toll-like receptor
- TMB
tumor mutational burden
- TME
tumor microenvironment
- TNF
tumor necrosis factor
- Treg
regulatory T cell
- VEGF
vascular endothelial growth factor
- VHL
Von Hippel-Lindau gene
Contributor Information
Ondrej Uher, Section of Medical Neuroendocrinology, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD 20892-1109, USA.
Katerina Hadrava Vanova, Section of Medical Neuroendocrinology, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD 20892-1109, USA.
David Taïeb, Department of Nuclear Medicine, CHU de La Timone, Marseille 13005, France.
Bruna Calsina, Hereditary Endocrine Cancer Group, Human Cancer Genetics Program, Spanish National Cancer Research Centre (CNIO), Madrid 28029, Spain; Familiar Cancer Clinical Unit, Human Cancer Genetics Program, Spanish National Cancer Research Centre (CNIO), Madrid 28029, Spain.
Mercedes Robledo, Hereditary Endocrine Cancer Group, Human Cancer Genetics Program, Spanish National Cancer Research Centre (CNIO), Madrid 28029, Spain; Centro de Investigación Biomédica en Red de Enfermedades Raras (CIBERER), Institute of Health Carlos III (ISCIII), Madrid 28029, Spain.
Roderick Clifton-Bligh, Department of Endocrinology, Royal North Shore Hospital, Sydney 2065, NSW, Australia; Cancer Genetics Laboratory, Kolling Institute, University of Sydney, Sydney 2065, NSW, Australia.
Karel Pacak, Section of Medical Neuroendocrinology, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD 20892-1109, USA.
Funding
This study was funded by the National Institutes of Health, MD, USA (grant number Z1AHD008735) awarded to Karel Pacak. This work was supported, by the Intramural Research Program of the National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Disclosures
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- 1. Mete O, Asa SL, Gill AJ, Kimura N, de Krijger RR, Tischler A. Overview of the 2022 WHO classification of paragangliomas and pheochromocytomas. Endocr Pathol. 2022;33(1):90‐114. [DOI] [PubMed] [Google Scholar]
- 2. Nolting S, Bechmann N, Taieb D, et al. Personalized management of pheochromocytoma and paraganglioma. Endocr Rev. 2022;43(2):199‐239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Taïeb D, Jha A, Treglia G, Pacak K. Molecular imaging and radionuclide therapy of paraganglioma and pheochromocytoma. Endocr Relat Cancer. 2019;26(11):R627‐R652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Navalkissoor S, Gnanasegaran G, Grossman A. Optimisation of radioligand therapy in neuroendocrine tumours: current and evolving evidence. J Neuroendocrinol. 2022;34(11):e13208. [DOI] [PubMed] [Google Scholar]
- 5. Jimenez C, Xu G, Varghese J, Graham PH, Campbell MT, Lu Y. New directions in treatment of metastatic or advanced pheochromocytomas and sympathetic paragangliomas: an American, contemporary, pragmatic approach. Curr Oncol Rep. 2022;24(1):89‐98. [DOI] [PubMed] [Google Scholar]
- 6. Fishbein L, Del Rivero J, Else T, et al. The North American Neuroendocrine Tumor Society consensus guidelines for surveillance and management of metastatic and/or unresectable pheochromocytoma and paraganglioma. Pancreas. 2021;50(4):469‐493. [DOI] [PubMed] [Google Scholar]
- 7. Jimenez C, Armaiz-Pena G, Dahia PLM, et al. Endocrine and neuroendocrine tumors special issue-checkpoint inhibitors for adrenocortical carcinoma and metastatic pheochromocytoma and paraganglioma: do they work? Cancers (Basel). 2022;14(3):467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mak IYF, Hayes AR, Khoo B, Grossman A. Peptide receptor radionuclide therapy as a novel treatment for metastatic and invasive phaeochromocytoma and paraganglioma. Neuroendocrinology. 2019;109(4):287‐298. [DOI] [PubMed] [Google Scholar]
- 9. Fishbein L, Leshchiner I, Walter V, et al. Comprehensive molecular characterization of pheochromocytoma and paraganglioma. Cancer Cell. 2017;31(2):181‐193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zethoven M, Martelotto L, Pattison A, et al. Single-nuclei and bulk-tissue gene-expression analysis of pheochromocytoma and paraganglioma links disease subtypes with tumor microenvironment. Nat Commun. 2022;13(1):6262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cascón A, Remacha L, Calsina B, Robledo M. Pheochromocytomas and paragangliomas: bypassing cellular respiration. Cancers (Basel). 2019;11(5):683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gimenez-Roqueplo AP, Robledo M, Dahia PLM. Update on the genetics of paragangliomas. Endocr Relat Cancer. 2023;30(4):e220373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Castro-Vega LJ, Letouze E, Burnichon N, et al. Multi-omics analysis defines core genomic alterations in pheochromocytomas and paragangliomas. Nat Commun. 2015;6:6044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. de Cubas AA, Leandro-García LJ, Schiavi F, et al. Integrative analysis of miRNA and mRNA expression profiles in pheochromocytoma and paraganglioma identifies genotype-specific markers and potentially regulated pathways. Endocr Relat Cancer. 2013;20(4):477‐493. [DOI] [PubMed] [Google Scholar]
- 15. Sharma P, Allison JP. Immune checkpoint therapy: forging ahead. Sci Transl Med. 2022;14(670):eadf2947. [DOI] [PubMed] [Google Scholar]
- 16. Bonaguro L, Schulte-Schrepping J, Ulas T, Aschenbrenner AC, Beyer M, Schultze JL. A guide to systems-level immunomics. Nat Immunol. 2022;23(10):1412‐1423. [DOI] [PubMed] [Google Scholar]
- 17. Marabelle A, Andtbacka R, Harrington K, et al. Starting the fight in the tumor: expert recommendations for the development of human intratumoral immunotherapy (HIT-IT). Ann Oncol. 2018;29(11):2163‐2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Garralda E, Laurie SA, Seymour L, de Vries EGE. Towards evidence-based response criteria for cancer immunotherapy. Nat Commun. 2023;14(1):3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Champiat S, Tselikas L, Farhane S, et al. Intratumoral immunotherapy: from trial design to clinical practice. Clin Cancer Res. 2021;27(3):665‐679. [DOI] [PubMed] [Google Scholar]
- 20. Ghosal S, Vanova KH, Uher O, et al. Immune signature of pheochromocytoma and paraganglioma in context of neuroendocrine neoplasms associated with prognosis. Endocrine. 2023;79(1):171‐179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Calsina B, Pineiro-Yanez E, Martinez-Montes AM, et al. Genomic and immune landscape of metastatic pheochromocytoma and paraganglioma. Nat Commun. 2023;14(1):1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Martinelli S, Amore F, Canu L, Maggi M, Rapizzi E. Tumour microenvironment in pheochromocytoma and paraganglioma. Front Endocrinol (Lausanne). 2023;14:1137456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pinato DJ, Black JR, Trousil S, et al. Programmed cell death ligands expression in phaeochromocytomas and paragangliomas: relationship with the hypoxic response, immune evasion and malignant behavior. Oncoimmunology. 2017;6(11):e1358332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vanova K H, Uher O, Meuter L, et al. PD-L1 expression and association with genetic background in pheochromocytoma and paraganglioma. Front Oncol. 2022;12:1045517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jin B, Han W, Guo J, et al. Initial characterization of immune microenvironment in pheochromocytoma and paraganglioma. Front Genet. 2022;13:1022131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jimenez C, Subbiah V, Stephen B, et al. Phase II clinical trial of pembrolizumab in patients with progressive metastatic pheochromocytomas and paragangliomas. Cancers (Basel). 2020;12(8):2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pacak K, Nazari MA, Taieb D. Immune landscape of pheochromocytoma and paraganglioma: a potentially novel avenue for prognostic reclassification? J Clin Endocrinol Metab. 2023;108(11):e1456‐e1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hong WX, Haebe S, Lee AS, et al. Intratumoral immunotherapy for early-stage solid tumors. Clin Cancer Res. 2020;26(13):3091‐3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Galon J, Bruni D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat Rev Drug Discov. 2019;18(3):197‐218. [DOI] [PubMed] [Google Scholar]
- 30. Galon J, Mlecnik B, Bindea G, et al. Towards the introduction of the ‘Immunoscore’ in the classification of malignant tumours. J Pathol. 2014;232(2):199‐209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ehrlich P. Ueber den jetzigen Stand der Karzinomforschung. Ned Tijdschr Geneeskd. 1909;53:273‐290. [Google Scholar]
- 32. Burnet M. Cancer: a biological approach. III. Viruses associated with neoplastic conditions. IV. Practical applications. Br Med J. 1957;1(5023):841‐847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kaplan DH, Shankaran V, Dighe AS, et al. Demonstration of an interferon gamma-dependent tumor surveillance system in immunocompetent mice. Proc Natl Acad Sci U S A. 1998;95(13):7556‐7561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shankaran V, Ikeda H, Bruce AT, et al. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410(6832):1107‐1111. [DOI] [PubMed] [Google Scholar]
- 35. Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3(11):991‐998. [DOI] [PubMed] [Google Scholar]
- 36. Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331(6024):1565‐1570. [DOI] [PubMed] [Google Scholar]
- 37. Pishesha N, Harmand TJ, Ploegh HL. A guide to antigen processing and presentation. Nat Rev Immunol. 2022;22(12):751‐764. [DOI] [PubMed] [Google Scholar]
- 38. Jhunjhunwala S, Hammer C, Delamarre L. Antigen presentation in cancer: insights into tumour immunogenicity and immune evasion. Nat Rev Cancer. 2021;21(5):298‐312. [DOI] [PubMed] [Google Scholar]
- 39. Zitvogel L, Kepp O, Kroemer G. Decoding cell death signals in inflammation and immunity. Cell. 2010;140(6):798‐804. [DOI] [PubMed] [Google Scholar]
- 40. Berraondo P, Sanmamed MF, Ochoa MC, et al. Cytokines in clinical cancer immunotherapy. Br J Cancer. 2019;120(1):6‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Labani-Motlagh A, Ashja-Mahdavi M, Loskog A. The tumor microenvironment: a milieu hindering and obstructing antitumor immune responses. Front Immunol. 2020;11:940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vijayan D, Young A, Teng MWL, Smyth MJ. Targeting immunosuppressive adenosine in cancer. Nat Rev Cancer. 2017;17(12):709‐724. [DOI] [PubMed] [Google Scholar]
- 43. Moon YW, Hajjar J, Hwu P, Naing A. Targeting the indoleamine 2,3-dioxygenase pathway in cancer. J Immunother Cancer. 2015;3:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. de la Cruz-Lopez KG, Castro-Munoz LJ, Reyes-Hernandez DO, Garcia-Carranca A, Manzo-Merino J. Lactate in the regulation of tumor microenvironment and therapeutic approaches. Front Oncol. 2019;9:1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jiang X, Wang J, Deng X, et al. Role of the tumor microenvironment in PD-L1/PD-1-mediated tumor immune escape. Mol Cancer. 2019;18(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhang W, Huang Q, Xiao W, et al. Advances in anti-tumor treatments targeting the CD47/SIRPalpha axis. Front Immunol. 2020;11(18). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Brown SD, Warren RL, Gibb EA, et al. Neo-antigens predicted by tumor genome meta-analysis correlate with increased patient survival. Genome Res. 2014;24(5):743‐750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wood MA, Paralkar M, Paralkar MP, et al. Population-level distribution and putative immunogenicity of cancer neoepitopes. BMC Cancer. 2018;18(1):414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wang X, Li M. Correlate tumor mutation burden with immune signatures in human cancers. BMC Immunol. 2019;20(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lawrence MS, Stojanov P, Polak P, et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 2013;499(7457):214‐218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Fishbein L, Khare S, Wubbenhorst B, et al. Whole-exome sequencing identifies somatic ATRX mutations in pheochromocytomas and paragangliomas. Nat Commun. 2015;6:6140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bratslavsky G, Sokol ES, Daneshvar M, et al. Clinically advanced pheochromocytomas and paragangliomas: a comprehensive genomic profiling study. Cancers (Basel). 2021;13(13):3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tamborero D, Rubio-Perez C, Muinos F, et al. A pan-cancer landscape of interactions between solid tumors and infiltrating immune cell populations. Clin Cancer Res. 2018;24(15):3717‐3728. [DOI] [PubMed] [Google Scholar]
- 54. Jardim DL, Goodman A, de Melo Gagliato D, Kurzrock R. The challenges of tumor mutational burden as an immunotherapy biomarker. Cancer Cell. 2021;39(2):154‐173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Thorsson V, Gibbs DL, Brown SD, et al. The immune landscape of cancer. Immunity. 2018;48(4):812‐830.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tufton N, Hearnden RJ, Berney DM, et al. The immune cell infiltrate in the tumour microenvironment of phaeochromocytomas and paragangliomas. Endocr Relat Cancer. 2022;29(11):589‐598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Guadagno E, Russo D, Pignatiello S, Del Basso De Caro M. . Inflammation in the neoplasms of the adrenal gland: is there a prognostic role? An immunohistochemical study. Pathol Res Pract. 2020;216(9):153070. [DOI] [PubMed] [Google Scholar]
- 58. Gao X, Yamazaki Y, Pecori A, et al. Histopathological analysis of tumor microenvironment and angiogenesis in pheochromocytoma. Front Endocrinol (Lausanne). 2020;11:587779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Celada L, Cubiella T, San-Juan-Guardado J, et al. Pseudohypoxia in paraganglioma and pheochromocytoma is associated with an immunosuppressive phenotype. J Pathol. 2023;259(1):103‐114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yu A, Xu X, Pang Y, et al. PD-L1 expression is linked to tumor-infiltrating T cell exhaustion and adverse pathological behavior in pheochromocytoma/paraganglioma. Laboratory Investigation. 2023;103(9):100210. [DOI] [PubMed] [Google Scholar]
- 61. Batchu S. Age-related differences of immune infiltrates in pheochromocytomas and paragangliomas. J Endocrinol Invest. 2021;44(7):1543‐1546. [DOI] [PubMed] [Google Scholar]
- 62. Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496(7446):445‐455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122(3):787‐795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Qiu SQ, Waaijer SJH, Zwager MC, de Vries EGE, van der Vegt B, Schroder CP. Tumor-associated macrophages in breast cancer: innocent bystander or important player? Cancer Treat Rev. 2018;70:178‐189. [DOI] [PubMed] [Google Scholar]
- 65. Farhat NA, Powers JF, Shepard-Barry A, Dahia P, Pacak K, Tischler AS. A previously unrecognized monocytic component of pheochromocytoma and paraganglioma. Endocr Pathol. 2019;30(2):90‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Morad G, Helmink BA, Sharma P, Wargo JA. Hallmarks of response, resistance, and toxicity to immune checkpoint blockade. Cell. 2021;184(21):5309‐5337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015;161(2):205‐214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Bour-Jordan H, Esensten JH, Martinez-Llordella M, Penaranda C, Stumpf M, Bluestone JA. Intrinsic and extrinsic control of peripheral T-cell tolerance by costimulatory molecules of the CD28/ B7 family. Immunol Rev. 2011;241(1):180‐205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Papaioannou NE, Beniata OV, Vitsos P, Tsitsilonis O, Samara P. Harnessing the immune system to improve cancer therapy. Ann Transl Med. 2016;4(14):261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Sharma P, Goswami S, Raychaudhuri D, et al. Immune checkpoint therapy—current perspectives and future directions. Cell. 2023;186(8):1652‐1669. [DOI] [PubMed] [Google Scholar]
- 71. Wei SC, Duffy CR, Allison JP. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 2018;8(9):1069‐1086. [DOI] [PubMed] [Google Scholar]
- 72. Seidel JA, Otsuka A, Kabashima K. Anti-PD-1 and anti-CTLA-4 therapies in cancer: mechanisms of action, efficacy, and limitations. Front Oncol. 2018;8:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wang X, Yang X, Zhang C, et al. Tumor cell-intrinsic PD-1 receptor is a tumor suppressor and mediates resistance to PD-1 blockade therapy. Proc Natl Acad Sci USA. 2020;117(12):6640‐6650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Wang Y, Du J, Gao Z, et al. Evolving landscape of PD-L2: bring new light to checkpoint immunotherapy. Br J Cancer. 2023;128(7):1196‐1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Parry RV, Chemnitz JM, Frauwirth KA, et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005;25(21):9543‐9553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Blackburn SD, Shin H, Haining WN, et al. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10(1):29‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Anderson AC, Joller N, Kuchroo VK. Lag-3, Tim-3, and TIGIT: co-inhibitory receptors with specialized functions in immune regulation. Immunity. 2016;44(5):989‐1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Guo Z, Zhang R, Yang AG, Zheng G. Diversity of immune checkpoints in cancer immunotherapy. Front Immunol. 2023;14:1121285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Guo D, Zhao X, Wang A, Xie Q, Xu X, Sun J. PD-L1 expression and association with malignant behavior in pheochromocytomas/paragangliomas. Hum Pathol. 2019;86:155‐162. [DOI] [PubMed] [Google Scholar]
- 80. Hsu YR, Torres-Mora J, Kipp BR, et al. Clinicopathological, immunophenotypic and genetic studies of mediastinal paragangliomas†. Eur J Cardiothorac Surg. 2019;56(5):867‐875. [DOI] [PubMed] [Google Scholar]
- 81. Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823‐1833. [DOI] [PubMed] [Google Scholar]
- 82. Zhu AX, Finn RS, Edeline J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19(7):940‐952. [DOI] [PubMed] [Google Scholar]
- 83. Balar AV, Castellano D, O'Donnell PH, et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol. 2017;18(11):1483‐1492. [DOI] [PubMed] [Google Scholar]
- 84. Batchu S, Hakim A, Henry OS, et al. Transcriptome-guided resolution of tumor microenvironment interactions in pheochromocytoma and paraganglioma subtypes. J Endocrinol Invest. 2022;45(5):989‐998. [DOI] [PubMed] [Google Scholar]
- 85. Dum D, Henke TLC, Mandelkow T, et al. Semi-automated validation and quantification of CTLA-4 in 90 different tumor entities using multiple antibodies and artificial intelligence. Lab Invest. 2022;102(6):650‐657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Bourhis M, Palle J, Galy-Fauroux I, Terme M. Direct and indirect modulation of T cells by VEGF-A counteracted by anti-angiogenic treatment. Front Immunol. 2021;12:616837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Li YL, Zhao H, Ren XB. Relationship of VEGF/VEGFR with immune and cancer cells: staggering or forward? Cancer Biol Med. 2016;13(2):206‐214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Giraudo E, Inoue M, Hanahan D. An amino-bisphosphonate targets MMP-9-expressing macrophages and angiogenesis to impair cervical carcinogenesis. J Clin Invest. 2004;114(5):623‐633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Favier J, Plouin PF, Corvol P, Gasc JM. Angiogenesis and vascular architecture in pheochromocytomas: distinctive traits in malignant tumors. Am J Pathol. 2002;161(4):1235‐1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Salmenkivi K, Heikkila P, Liu J, Haglund C, Arola J. VEGF in 105 pheochromocytomas: enhanced expression correlates with malignant outcome. APMIS. 2003;111(4):458‐464. [DOI] [PubMed] [Google Scholar]
- 91. Zielke A, Middeke M, Hoffmann S, et al. VEGF-mediated angiogenesis of human pheochromocytomas is associated to malignancy and inhibited by anti-VEGF antibodies in experimental tumors. Surgery. 2002;132(6):1056‐1063; discussion 1063. [DOI] [PubMed] [Google Scholar]
- 92. Ferreira CV, Siqueira DR, Romitti M, et al. Role of VEGF-A and its receptors in sporadic and MEN2-associated pheochromocytoma. Int J Mol Sci. 2014;15(4):5323‐5336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Favier J, Igaz P, Burnichon N, et al. Rationale for anti-angiogenic therapy in pheochromocytoma and paraganglioma. Endocr Pathol. 2012;23(1):34‐42. [DOI] [PubMed] [Google Scholar]
- 94. Eisenhofer G, Huynh TT, Pacak K, et al. Distinct gene expression profiles in norepinephrine- and epinephrine-producing hereditary and sporadic pheochromocytomas: activation of hypoxia-driven angiogenic pathways in von Hippel-Lindau syndrome. Endocr Relat Cancer. 2004;11(4):897‐911. [DOI] [PubMed] [Google Scholar]
- 95. Białas M, Dyduch G, Dudała J, et al. Study of microvessel density and the expression of vascular endothelial growth factors in adrenal gland pheochromocytomas. Int J Endocrinol. 2014;2014:104129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Nölting S, Grossman AB. Signaling pathways in pheochromocytomas and paragangliomas: prospects for future therapies. Endocr Pathol. 2012;23(1):21‐33. [DOI] [PubMed] [Google Scholar]
- 97. Zhou Y, Cui Y, Zhang D, Tong A. Efficacy and safety of tyrosine kinase inhibitors in patients with metastatic pheochromocytomas/paragangliomas. J Clin Endocrinol Metab. 2023;108(3):755‐766. [DOI] [PubMed] [Google Scholar]
- 98. Wackerhage H, Christensen JF, Ilmer M, von Luettichau I, Renz BW, Schönfelder M. Cancer catecholamine conundrum. Trends Cancer. 2022;8(2):110‐122. [DOI] [PubMed] [Google Scholar]
- 99. Matt SM, Gaskill PJ. Where is dopamine and how do immune cells see it?: Dopamine-mediated immune cell function in health and disease. J Neuroimmune Pharmacol. 2020;15(1):114‐164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Hein L, Kobilka BK. Adrenergic receptor signal transduction and regulation. Neuropharmacology. 1995;34(4):357‐366. [DOI] [PubMed] [Google Scholar]
- 101. Sharma D, Farrar JD. Adrenergic regulation of immune cell function and inflammation. Semin Immunopathol. 2020;42(6):709‐717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Cole SW, Sood AK. Molecular pathways: beta-adrenergic signaling in cancer. Clin Cancer Res. 2012;18(5):1201‐1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Antoni MH, Lutgendorf SK, Cole SW, et al. The influence of bio-behavioural factors on tumour biology: pathways and mechanisms. Nat Rev Cancer. 2006;6(3):240‐248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Chen Y, Qian Y, Huang W, et al. Chronic stress promotes tumor immune evasion via the suppression of MHC-I expression and the upregulation of PD-L1. Am J Cancer Res. 2022;12(11):5286‐5299. [PMC free article] [PubMed] [Google Scholar]
- 105. Kalinichenko VV, Mokyr MB, Graf LH Jr, Cohen RL, Chambers DA. Norepinephrine-mediated inhibition of antitumor cytotoxic T lymphocyte generation involves a beta-adrenergic receptor mechanism and decreased TNF-alpha gene expression. J Immunol. 1999;163(5):2492‐2499. [PubMed] [Google Scholar]
- 106. Cook-Mills JM, Cohen RL, Perlman RL, Chambers DA. Inhibition of lymphocyte activation by catecholamines: evidence for a non-classical mechanism of catecholamine action. Immunology. 1995;85(4):544‐549. [PMC free article] [PubMed] [Google Scholar]
- 107. Globig A-M, Zhao S, Roginsky J, et al. The β1-adrenergic receptor links sympathetic nerves to T cell exhaustion. Nature. 2023;622(7982):383‐392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Sun Z, Hou D, Liu S, Fu W, Wang J, Liang Z. Norepinephrine inhibits the cytotoxicity of NK92-MI cells via the β2-adrenoceptor/cAMP/PKA/p-CREB signaling pathway. Mol Med Rep. 2018;17(6):8530‐8535. [DOI] [PubMed] [Google Scholar]
- 109. Mikulak J, Bozzo L, Roberto A, et al. Dopamine inhibits the effector functions of activated NK cells via the upregulation of the D5 receptor. J Immunol. 2014;193(6):2792‐2800. [DOI] [PubMed] [Google Scholar]
- 110. Capellino S, Claus M, Watzl C. Regulation of natural killer cell activity by glucocorticoids, serotonin, dopamine, and epinephrine. Cell Mol Immunol. 2020;17(7):705‐711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Nissen MD, Sloan EK, Mattarollo SR. β-Adrenergic signaling impairs antitumor CD8+ T-cell responses to B-cell lymphoma immunotherapy. Cancer Immunol Res. 2018;6(1):98‐109. [DOI] [PubMed] [Google Scholar]
- 112. Fjæstad KY, Rømer AMA, Goitea V, et al. Blockade of beta-adrenergic receptors reduces cancer growth and enhances the response to anti-CTLA4 therapy by modulating the tumor microenvironment. Oncogene. 2022;41(9):1364‐1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Oh MS, Guzner A, Wainwright DA, et al. The impact of Beta blockers on survival outcomes in patients with non–small-cell lung cancer treated with immune checkpoint inhibitors. Clin Lung Cancer. 2021;22(1):e57-–e62.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Eisenhofer G, Pamporaki C, Lenders JWM. Biochemical assessment of pheochromocytoma and paraganglioma. Endocr Rev. 2023;44(5):862‐909. [DOI] [PubMed] [Google Scholar]
- 115. Estrada LD, Ağaç D, Farrar JD. Sympathetic neural signaling via the β2-adrenergic receptor suppresses T-cell receptor-mediated human and mouse CD8(+) T-cell effector function. Eur J Immunol. 2016;46(8):1948‐1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Perelli L, Carbone F, Zhang L, et al. Interferon signaling promotes tolerance to chromosomal instability during metastatic evolution in renal cancer. Nature Cancer. 2023;4(7):984‐1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Gudgeon N, Munford H, Bishop EL, et al. Succinate uptake by T cells suppresses their effector function via inhibition of mitochondrial glucose oxidation. Cell Rep. 2022;40(7):111193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Gruber LM, Jasim S, Ducharme-Smith A, Weingarten T, Young WF, Bancos I. The role for metyrosine in the treatment of patients with pheochromocytoma and paraganglioma. J Clin Endocrinol Metab. 2021;106(6):e2393‐e2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Serri O, Comtois R, Bettez P, Dubuc G, Buu NT, Kuchel O. Reduction in the size of a pheochromocytoma pulmonary metastasis by metyrosine therapy. N Engl J Med. 1984;310(19):1264‐1265. [DOI] [PubMed] [Google Scholar]
- 120. Eisenhofer G, Lenders JW, Siegert G, et al. Plasma methoxytyramine: a novel biomarker of metastatic pheochromocytoma and paraganglioma in relation to established risk factors of tumour size, location and SDHB mutation status. Eur J Cancer. 2012;48(11):1739‐1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Anderson PM, Trucco MM, Tarapore RS, et al. Phase II study of ONC201 in neuroendocrine tumors including pheochromocytoma-paraganglioma and desmoplastic small round cell tumor. Clin Cancer Res. 2022;28(9):1773‐1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Wagner J, Kline CL, Zhou L, et al. Dose intensification of TRAIL-inducing ONC201 inhibits metastasis and promotes intratumoral NK cell recruitment. J Clin Invest. 2018;128(6):2325‐2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Stein MN, Malhotra J, Tarapore RS, et al. Safety and enhanced immunostimulatory activity of the DRD2 antagonist ONC201 in advanced solid tumor patients with weekly oral administration. J Immunother Cancer. 2019;7(1):136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Letouzé E, Martinelli C, Loriot C, et al. SDH mutations establish a hypermethylator phenotype in paraganglioma. Cancer Cell. 2013;23(6):739‐752. [DOI] [PubMed] [Google Scholar]
- 125. Selak MA, Armour SM, MacKenzie ED, et al. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer Cell. 2005;7(1):77‐85. [DOI] [PubMed] [Google Scholar]
- 126. Matlac DM, Hadrava Vanova K, Bechmann N, et al. Succinate mediates tumorigenic effects via succinate receptor 1: potential for new targeted treatment strategies in succinate dehydrogenase deficient paragangliomas. Front Endocrinol (Lausanne). 2021;12:589451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Wu JY, Huang TW, Hsieh YT, et al. Cancer-derived succinate promotes macrophage polarization and cancer metastasis via succinate receptor. Mol Cell. 2020;77(2):213‐227.e5. [DOI] [PubMed] [Google Scholar]
- 128. Mu X, Zhao T, Xu C, et al. Oncometabolite succinate promotes angiogenesis by upregulating VEGF expression through GPR91-mediated STAT3 and ERK activation. Oncotarget. 2017;8(8):13174‐13185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Mills E, O’Neill LAJ. Succinate: a metabolic signal in inflammation. Trends Cell Biol. 2014;24(5):313‐320. [DOI] [PubMed] [Google Scholar]
- 130. Trauelsen M, Hiron TK, Lin D, et al. Extracellular succinate hyperpolarizes M2 macrophages through SUCNR1/GPR91-mediated Gq signaling. Cell Rep. 2021;35(11):109246. [DOI] [PubMed] [Google Scholar]
- 131. Kes MMG, Van den Bossche J, Griffioen AW, Huijbers EJM. Oncometabolites lactate and succinate drive pro-angiogenic macrophage response in tumors. Biochim Biophys Acta Rev Cancer. 2020;1874(2):188427. [DOI] [PubMed] [Google Scholar]
- 132. Richter S, Peitzsch M, Rapizzi E, et al. Krebs cycle metabolite profiling for identification and stratification of pheochromocytomas/paragangliomas due to succinate dehydrogenase deficiency. J Clin Endocrinol Metab. 2014;99(10):3903‐3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Lamy C, Tissot H, Faron M, et al. Succinate: a Serum biomarker of SDHB-mutated paragangliomas and pheochromocytomas. J Clin Endocrinol Metab. 2022;107(10):2801‐2810. [DOI] [PubMed] [Google Scholar]
- 134. Jones R, McDonald KE, Willson JA, et al. Mutations in succinate dehydrogenase B (SDHB) enhance neutrophil survival independent of HIF-1α expression. Blood. 2016;127(21):2641‐2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Mahmud Z, Rahman A, Mishu ID, Kabir Y. Mechanistic insights into the interplays between neutrophils and other immune cells in cancer development and progression. Cancer Metastasis Rev. 2022;41(2):405‐432. [DOI] [PubMed] [Google Scholar]
- 136. Zhong X, Su T, Yang Y, et al. Platelet-lymphocyte and neutrophil-lymphocyte ratio are prognostic markers for pheochromocytomas and paragangliomas. J Clin Endocrinol Metab. 2023;108(9):2230‐2239. [DOI] [PubMed] [Google Scholar]
- 137. Gouasmi R, Ferraro-Peyret C, Nancey S, et al. The kynurenine pathway and cancer: why keep it simple when you can make it complicated. Cancers (Basel). 2022;14(11):2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Stone TW, Williams RO. Interactions of IDO and the kynurenine pathway with cell transduction systems and metabolism at the inflammation-cancer interface. Cancers (Basel). 2023;15(11):2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Munn DH, Mellor AL. Indoleamine 2,3-dioxygenase and tumor-induced tolerance. J Clin Invest. 2007;117(5):1147‐1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Munn DH, Mellor AL. IDO in the tumor microenvironment: inflammation, counter-regulation, and tolerance. Trends Immunol. 2016;37(3):193‐207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Ravishankar B, Liu H, Shinde R, et al. Tolerance to apoptotic cells is regulated by indoleamine 2,3-dioxygenase. Proc Natl Acad Sci U S A. 2012;109(10):3909‐3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Vázquez Cervantes GI, Navarro Cossio J, Pérez de la Cruz G, Salazar A, Pérez de la Cruz V, Pineda B. Bioinformatic analysis of kynurenine pathway enzymes and their relationship with glioma hallmarks. Metabolites. 2022;12(11):1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Perez-Castro L, Garcia R, Venkateswaran N, Barnes S, Conacci-Sorrell M. Tryptophan and its metabolites in normal physiology and cancer etiology. Febs J. 2023;290(1):7‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Murakami M, Sun N, Greunke C, et al. Mass spectrometry imaging identifies metabolic patterns associated with malignant potential in pheochromocytoma and paraganglioma. Eur J Endocrinol. 2021;185(1):179‐191. [DOI] [PubMed] [Google Scholar]
- 145. Sagar NA, Tarafdar S, Agarwal S, Tarafdar A, Sharma S. Polyamines: functions, metabolism, and role in human disease management. Med Sci (Basel). 2021;9(2):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Rai SK, Bril F, Hatch HM, et al. Targeting pheochromocytoma/paraganglioma with polyamine inhibitors. Metab Clin Exp. 2020;110:154297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Hesterberg RS, Cleveland JL, Epling-Burnette PK. Role of polyamines in immune cell functions. Med Sci (Basel). 2018;6(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9(3):162‐174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Alexander ET, Minton A, Peters MC, Phanstiel OT, Gilmour SK. A novel polyamine blockade therapy activates an anti-tumor immune response. Oncotarget. 2017;8(48):84140‐84152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Casero RA, Murray Stewart T, Pegg AE. Polyamine metabolism and cancer: treatments, challenges and opportunities. Nature Reviews Cancer. 2018;18(11):681‐695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Casazza A, Di Conza G, Wenes M, Finisguerra V, Deschoemaeker S, Mazzone M. Tumor stroma: a complexity dictated by the hypoxic tumor microenvironment. Oncogene. 2014;33(14):1743‐1754. [DOI] [PubMed] [Google Scholar]
- 152. Riera-Domingo C, Audigé A, Granja S, et al. Immunity, hypoxia, and metabolism-the mnage à trois of cancer: implications for immunotherapy. Physiol Rev. 2020;100(1):1‐102. [DOI] [PubMed] [Google Scholar]
- 153. Sethumadhavan S, Silva M, Philbrook P, et al. Hypoxia and hypoxia-inducible factor (HIF) downregulate antigen-presenting MHC class I molecules limiting tumor cell recognition by T cells. PLoS One. 2017;12(11):e0187314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Kopecka J, Salaroglio IC, Perez-Ruiz E, et al. Hypoxia as a driver of resistance to immunotherapy. Drug Resist Updat. 2021;59:100787. [DOI] [PubMed] [Google Scholar]
- 155. McDonald PC, Chafe SC, Dedhar S. Overcoming hypoxia-mediated tumor progression: combinatorial approaches targeting pH regulation, angiogenesis and immune dysfunction. Front Cell Dev Biol. 2016;4:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Mpekris F, Voutouri C, Baish JW, et al. Combining microenvironment normalization strategies to improve cancer immunotherapy. Proc Natl Acad Sci USA. 2020;117(7):3728‐3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Wang B, Zhao Q, Zhang Y, et al. Targeting hypoxia in the tumor microenvironment: a potential strategy to improve cancer immunotherapy. J Exp Clin Cancer Res. 2021;40(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Watts D, Jaykar MT, Bechmann N, Wielockx B. Hypoxia signaling pathway: a central mediator in endocrine tumors. Front Endocrinol (Lausanne). 2023;13:1103075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Jochmanová I, Yang C, Zhuang Z, Pacak K. Hypoxia-inducible factor signaling in pheochromocytoma: turning the rudder in the right direction. J Natl Cancer Inst. 2013;105(17):1270‐1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Kluckova K, Tennant DA. Metabolic implications of hypoxia and pseudohypoxia in pheochromocytoma and paraganglioma. Cell Tissue Res. 2018;372(2):367‐378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Bechmann N, Poser I, Seifert V, et al. Impact of extrinsic and intrinsic hypoxia on catecholamine biosynthesis in absence or presence of Hif2α in pheochromocytoma cells. Cancers (Basel). 2019;11(5):594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Watts D, Bechmann N, Meneses A, et al. HIF2α regulates the synthesis and release of epinephrine in the adrenal medulla. J Mol Med (Berl). 2021;99(11):1655‐1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Loriot C, Burnichon N, Gadessaud N, et al. Epithelial to mesenchymal transition is activated in metastatic pheochromocytomas and paragangliomas caused by SDHB gene mutations. J Clin Endocrinol Metab. 2012;97(6):E954‐E962. [DOI] [PubMed] [Google Scholar]
- 164. Bechmann N, Moskopp ML, Ullrich M, et al. HIF2α supports pro-metastatic behavior in pheochromocytomas/paragangliomas. Endocr Relat Cancer. 2020;27(11):625‐640. [DOI] [PubMed] [Google Scholar]
- 165. Bechmann N, Eisenhofer G. Hypoxia-inducible factor 2α: a key player in tumorigenesis and metastasis of pheochromocytoma and paraganglioma? Exp Clin Endocrinol Diabetes. 2022;130(5):282‐289. [DOI] [PubMed] [Google Scholar]
- 166. Dwight T, Flynn A, Amarasinghe K, et al. TERT structural rearrangements in metastatic pheochromocytomas. Endocr Relat Cancer. 2018;25(1):1‐9. [DOI] [PubMed] [Google Scholar]
- 167. Job S, Draskovic I, Burnichon N, et al. Telomerase activation and ATRX mutations are independent risk factors for metastatic pheochromocytoma and paraganglioma. Clin Cancer Res. 2019;25(2):760‐770. [DOI] [PubMed] [Google Scholar]
- 168. Shay JW, Bacchetti S. A survey of telomerase activity in human cancer. Eur J Cancer. 1997;33(5):787‐791. [DOI] [PubMed] [Google Scholar]
- 169. Vonderheide RH, Kraynyak KA, Shields AF, et al. Phase 1 study of safety, tolerability and immunogenicity of the human telomerase (hTERT)-encoded DNA plasmids INO-1400 and INO-1401 with or without IL-12 DNA plasmid INO-9012 in adult patients with solid tumors. J Immunother Cancer. 2021;9(7):e003019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Rebucci-Peixoto M, Vienot A, Adotevi O, et al. A phase II study evaluating the interest to combine UCPVax, a telomerase CD4 T(H)1-inducer cancer vaccine, and atezolizumab for the treatment of HPV positive cancers: volATIL study. Front Oncol. 2022;12:957580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Fenoglio D, Traverso P, Parodi A, et al. A multi-peptide, dual-adjuvant telomerase vaccine (GX301) is highly immunogenic in patients with prostate and renal cancer. Cancer Immunol Immunother. 2013;62(6):1041‐1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Mao J, Zhang Q, Wang Y, et al. TERT activates endogenous retroviruses to promote an immunosuppressive tumour microenvironment. EMBO Rep. 2022;23(4):e52984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Gupta S, Roy A, Dwarakanath BS. Metabolic cooperation and competition in the tumor microenvironment: implications for therapy. Front Oncol. 2017;7:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Rapizzi E, Fucci R, Giannoni E, et al. Role of microenvironment on neuroblastoma SK-N-AS SDHB-silenced cell metabolism and function. Endocr Relat Cancer. 2015;22(3):409‐417. [DOI] [PubMed] [Google Scholar]
- 175. D'Antongiovanni V, Martinelli S, Richter S, et al. The microenvironment induces collective migration in SDHB-silenced mouse pheochromocytoma spheroids. Endocr Relat Cancer. 2017;24(10):555‐564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Martinelli S, Riverso M, Mello T, et al. SDHB and SDHD silenced pheochromocytoma spheroids respond differently to tumour microenvironment and their aggressiveness is inhibited by impairing stroma metabolism. Mol Cell Endocrinol. 2022;547:111594. [DOI] [PubMed] [Google Scholar]
- 177. Mao Y, Zhuo R, Ma W, et al. Fibroblasts mediate the angiogenesis of pheochromocytoma by increasing COX4I2 expression. Front Oncol. 2022;12:938123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Sun F, Zhuo R, Ma W, et al. From clinic to mechanism: proteomics-based assessment of angiogenesis in adrenal pheochromocytoma. J Cell Physiol. 2019;234(12):22057‐22070. [DOI] [PubMed] [Google Scholar]
- 179. Martinelli S, Amore F, Mello T, Mannelli M, Maggi M, Rapizzi E. Metformin treatment induces different response in pheochromocytoma/paraganglioma tumour cells and in primary fibroblasts. Cancers (Basel). 2022;14(14):3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Martin A, Venara M, Matho C, Olea FD, Fernandez MC, Pennisi PA. Fibroblast deficiency of insulin-like growth factor 1 receptor type 1 (IGF1R) impairs initial steps of murine pheochromocytoma development. Biochimie. 2019;163:108‐116. [DOI] [PubMed] [Google Scholar]
- 181. Sahai E, Astsaturov I, Cukierman E, et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat Rev Cancer. 2020;20(3):174‐186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Ribeiro AL, Okamoto OK. Combined effects of pericytes in the tumor microenvironment. Stem Cells Int. 2015;2015:868475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Liu T, Han C, Wang S, et al. Cancer-associated fibroblasts: an emerging target of anti-cancer immunotherapy. J Hematol Oncol. 2019;12(1):86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Bagaev A, Kotlov N, Nomie K, et al. Conserved pan-cancer microenvironment subtypes predict response to immunotherapy. Cancer Cell. 2021;39(6):845‐865.e7. [DOI] [PubMed] [Google Scholar]
- 185. Coley WB. The treatment of malignant tumors by repeated inoculations of erysipelas: with a report of ten original cases. 1. The American Journal of the Medical Sciences (1827–1924). 1893;105(6):487. [PubMed] [Google Scholar]
- 186. Coley WB. Late results of the treatment of inoperable sarcoma by the mixed toxins of erysipelas and Bacillus prodigiosus. Trans Southern Surg Gynecol Ass. 1906;18:197. [Google Scholar]
- 187. Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711‐723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Weber JS, O'Day S, Urba W, et al. Phase I/II study of ipilimumab for patients with metastatic melanoma. J Clin Oncol. 2008;26(36):5950‐5956. [DOI] [PubMed] [Google Scholar]
- 189. Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255‐265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378(14):1277‐1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Capdevila J, Hernando J, Teule A, et al. Durvalumab plus tremelimumab for the treatment of advanced neuroendocrine neoplasms of gastroenteropancreatic and lung origin. Nat Commun. 2023;14(1):2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Weber MM, Fottner C. Immune checkpoint inhibitors in the treatment of patients with neuroendocrine neoplasia. Oncol Res Treat. 2018;41(5):306‐312. [DOI] [PubMed] [Google Scholar]
- 193. Xu G, Wang Y, Zhang H, She X, Yang J. Immunotherapy and potential predictive biomarkers in the treatment of neuroendocrine neoplasia. Future Oncol. 2021;17(9):1069‐1081. [DOI] [PubMed] [Google Scholar]
- 194. Naing A, Meric-Bernstam F, Stephen B, et al. Phase 2 study of pembrolizumab in patients with advanced rare cancers. J Immunother Cancer. 2020;8(1):e000347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Mehnert JM, Bergsland E, O'Neil BH, et al. Pembrolizumab for the treatment of programmed death-ligand 1-positive advanced carcinoid or pancreatic neuroendocrine tumors: results from the KEYNOTE-028 study. Cancer. 2020;126(13):3021‐3030. [DOI] [PubMed] [Google Scholar]
- 196. Geoerger B, Kang HJ, Yalon-Oren M, et al. Pembrolizumab in paediatric patients with advanced melanoma or a PD-L1-positive, advanced, relapsed, or refractory solid tumour or lymphoma (KEYNOTE-051): interim analysis of an open-label, single-arm, phase 1-2 trial. Lancet Oncol. 2020;21(1):121‐133. [DOI] [PubMed] [Google Scholar]
- 197. Economides MP, Shah AY, Jimenez C, Habra MA, Desai M, Campbell MT. A durable response with the combination of nivolumab and cabozantinib in a patient with metastatic paraganglioma: a case report and review of the current literature. Front Endocrinol (Lausanne). 2020;11:594264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Rodriguez RR, Rizwan S, Alhamad K, Finley GG. The use of immunotherapy treatment in malignant pheochromocytomas/paragangliomas: a case report. J Med Case Rep. 2021;15(1):172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. McGregor BA, Campbell MT, Xie W, et al. Results of a multicenter, phase 2 study of nivolumab and ipilimumab for patients with advanced rare genitourinary malignancies. Cancer. 2021;127(6):840‐849. [DOI] [PubMed] [Google Scholar]
- 200. Neumann HP, Bausch B, McWhinney SR, et al. Germ-line mutations in nonsyndromic pheochromocytoma. N Engl J Med. 2002;346(19):1459‐1466. [DOI] [PubMed] [Google Scholar]
- 201. Dahia PL. Pheochromocytoma and paraganglioma pathogenesis: learning from genetic heterogeneity. Nat Rev Cancer. 2014;14(2):108‐119. [DOI] [PubMed] [Google Scholar]
- 202. Favier J, Amar L, Gimenez-Roqueplo AP. Paraganglioma and phaeochromocytoma: from genetics to personalized medicine. Nat Rev Endocrinol. 2015;11(2):101‐111. [DOI] [PubMed] [Google Scholar]
- 203. Crona J, Lamarca A, Ghosal S, Welin S, Skogseid B, Pacak K. Genotype-phenotype correlations in pheochromocytoma and paraganglioma: a systematic review and individual patient meta-analysis. Endocr Relat Cancer. 2019;26(5):539‐550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Cascón A, Calsina B, Monteagudo M, et al. Genetic bases of pheochromocytoma and paraganglioma. J Mol Endocrinol. 2023;70(3):e220167. [DOI] [PubMed] [Google Scholar]
- 205. Remacha L, Currás-Freixes M, Torres-Ruiz R, et al. Gain-of-function mutations in DNMT3A in patients with paraganglioma. Genet Med. 2018;20(12):1644‐1651. [DOI] [PubMed] [Google Scholar]
- 206. Remacha L, Pirman D, Mahoney CE, et al. Recurrent germline DLST mutations in individuals with multiple pheochromocytomas and paragangliomas. Am J Hum Genet. 2019;104(5):1008‐1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Buffet A, Zhang J, Rebel H, et al. Germline DLST variants promote epigenetic modifications in pheochromocytoma-paraganglioma. J Clin Endocrinol Metab. 2021;106(2):459‐471. [DOI] [PubMed] [Google Scholar]
- 208. Hadrava Vanova K, Pang Y, Krobova L, et al. Germline SUCLG2 variants in patients with pheochromocytoma and paraganglioma. J Natl Cancer Inst. 2022;114(1):130‐138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Javaid S, Patton A, Tinoco G, Oghumu S, Iwenofu OH. Metastatic sporadic paraganglioma with EWSR1::CREM gene fusion: a unique molecular profile that expands the phenotypic diversity of the molecular landscape of the EWSR1::CREM gene fusion positive tumors. Genes Chromosomes Cancer. 2023;62(2):85‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Lam AK. Update on adrenal tumours in 2017 World Health Organization (WHO) of endocrine tumours. Endocr Pathol. 2017;28(3):213‐227. [DOI] [PubMed] [Google Scholar]
- 211. Li M, Prodanov T, Meuter L, et al. Recurrent disease in patients with sporadic pheochromocytoma and paraganglioma. J Clin Endocrinol Metab. 2023;108(2):397‐404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Pacak K. New biology of pheochromocytoma and paraganglioma. Endocr Pract. 2022;28(12):1253‐1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Nölting S, Bechmann N, Taieb D, et al. Personalized management of pheochromocytoma and paraganglioma. Endocr Rev. 2022;43(2):199‐239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Crona J, Taïeb D, Pacak K. New perspectives on pheochromocytoma and paraganglioma: toward a molecular classification. Endocr Rev. 2017;38(6):489‐515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Pamporaki C, Berends AMA, Filippatos A, et al. Prediction of metastatic pheochromocytoma and paraganglioma: a machine learning modelling study using data from a cross-sectional cohort. Lancet Digit Health. 2023;5(9):e551‐e559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Angell H, Galon J. From the immune contexture to the Immunoscore: the role of prognostic and predictive immune markers in cancer. Curr Opin Immunol. 2013;25(2):261‐267. [DOI] [PubMed] [Google Scholar]
- 217. Senga SS, Grose RP. Hallmarks of cancer-the New Testament. Open Biol. 2021;11(1):200358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Hanahan D. Hallmarks of cancer: new dimensions. Cancer Discov. 2022;12(1):31‐46. [DOI] [PubMed] [Google Scholar]
- 219. Fessler J, Matson V, Gajewski TF. Exploring the emerging role of the microbiome in cancer immunotherapy. J Immunother Cancer. 2019;7(1):108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Sepich-Poore GD, Zitvogel L, Straussman R, Hasty J, Wargo JA, Knight R. The microbiome and human cancer. Science. 2021;371(6536):eabc4552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Baruch EN, Youngster I, Ben-Betzalel G, et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science. 2021;371(6529):602‐609. [DOI] [PubMed] [Google Scholar]
- 222. Davar D, Dzutsev AK, McCulloch JA, et al. Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science. 2021;371(6529):595‐602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Gopalakrishnan V, Helmink BA, Spencer CN, Reuben A, Wargo JA. The influence of the gut microbiome on cancer, immunity, and cancer immunotherapy. Cancer Cell. 2018;33(4):570‐580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Gopalakrishnan V, Spencer CN, Nezi L, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018;359(6371):97‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Matson V, Fessler J, Bao R, et al. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science. 2018;359(6371):104‐108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Routy B, Le Chatelier E, Derosa L, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359(6371):91‐97. [DOI] [PubMed] [Google Scholar]
- 227. Francisco-Anderson L, Shariffudin S, Gardner H, et al. Abstract PS11-27: a phase I/II clinical trial of EDP1503 with pembrolizumab for triple-negative breast cancer. Cancer Res. 2021;81(4_Supplement):PS11-27‐PS11-27. [Google Scholar]
- 228. Matson V, Chervin CS, Gajewski TF. Cancer and the microbiome-influence of the commensal microbiota on cancer, immune responses, and immunotherapy. Gastroenterology. 2021;160(2):600‐613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Xiang C, Chen P, Zhang Q, et al. Intestinal microbiota modulates adrenomedullary response through Nod1 sensing in chromaffin cells. iScience. 2021;24(8):102849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Shankar LK, Schoder H, Sharon E, et al. Harnessing imaging tools to guide immunotherapy trials: summary from the National Cancer Institute Cancer Imaging Steering Committee workshop. Lancet Oncol. 2023;24(3):e133‐e143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Taïeb D, Pacak K. New insights into the nuclear imaging phenotypes of cluster 1 pheochromocytoma and paraganglioma. Trends Endocrinol Metab. 2017;28(11):807‐817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Seol HY, Kim YS, Kim SJ. Diagnostic test accuracy of (18)F-FDG PET/CT for prediction of programmed death ligand 1 (PD-L1) expression in solid tumours: a meta-analysis. Clin Radiol. 2021;76(11):863.e19‐863.e25. [DOI] [PubMed] [Google Scholar]
- 233. Aide N, Hicks RJ, Le Tourneau C, Lheureux S, Fanti S, Lopci E. FDG PET/CT for assessing tumour response to immunotherapy: report on the EANM symposium on immune modulation and recent review of the literature. Eur J Nucl Med Mol Imaging. 2019;46(1):238‐250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Goldfarb L, Duchemann B, Chouahnia K, Zelek L, Soussan M. Monitoring anti-PD-1-based immunotherapy in non-small cell lung cancer with FDG PET: introduction of iPERCIST. EJNMMI Res. 2019;9(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Ito K, Teng R, Schoder H, et al. (18)F-FDG PET/CT for monitoring of ipilimumab therapy in patients with metastatic melanoma. J Nucl Med. 2019;60(3):335‐341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Pandit-Taskar N, Postow MA, Hellmann MD, et al. First-in-humans imaging with (89)Zr-Df-IAB22M2C anti-CD8 minibody in patients with solid malignancies: preliminary pharmacokinetics, biodistribution, and lesion targeting. J Nucl Med. 2020;61(4):512‐519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Farwell MD, Gamache RF, Babazada H, et al. CD8-targeted PET imaging of tumor-infiltrating T cells in patients with cancer: a phase I first-in-humans study of (89)Zr-df-IAB22M2C, a radiolabeled anti-CD8 minibody. J Nucl Med. 2022;63(5):720‐726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Miedema IHC, Huisman MC, Zwezerijnen GJC, et al. (89)Zr-immuno-PET using the anti-LAG-3 tracer [(89)Zr]Zr-BI 754111: demonstrating target specific binding in NSCLC and HNSCC. Eur J Nucl Med Mol Imaging. 2023;50(7):2068‐2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Nienhuis PH, Antunes IF, Glaudemans A, et al. (18)F-BMS986192 PET imaging of PD-L1 in metastatic melanoma patients with brain metastases treated with immune checkpoint inhibitors: a pilot study. J Nucl Med. 2022;63(6):899‐905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Wang X, Zhou M, Chen B, et al. Preclinical and exploratory human studies of novel (68)Ga-labeled D-peptide antagonist for PET imaging of TIGIT expression in cancers. Eur J Nucl Med Mol Imaging. 2022;49(8):2584‐2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Bouleau A, Nozach H, Dubois S, et al. Optimizing immuno-PET imaging of tumor PD-L1 expression: pharmacokinetic, biodistribution, and dosimetric comparisons of (89)Zr-labeled anti-PD-L1 antibody formats. J Nucl Med. 2022;63(8):1259‐1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Zhou H, Bao G, Wang Z, et al. PET imaging of an optimized anti-PD-L1 probe (68)Ga-NODAGA-BMS986192 in immunocompetent mice and non-human primates. EJNMMI Res. 2022;12(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Robu S, Richter A, Gosmann D, et al. Synthesis and preclinical evaluation of a (68)Ga-labeled adnectin, (68)Ga-BMS-986192, as a PET agent for imaging PD-L1 expression. J Nucl Med. 2021;62(9):1228‐1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Rudloff MW, Zumbo P, Favret NR, et al. Hallmarks of CD8(+) T cell dysfunction are established within hours of tumor antigen encounter before cell division. Nat Immunol. 2023;24(9):1527‐1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Larimer BM, Wehrenberg-Klee E, Dubois F, et al. Granzyme B PET imaging as a predictive biomarker of immunotherapy response. Cancer Res. 2017;77(9):2318‐2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Iravani A, Hicks RJ. Imaging the cancer immune environment and its response to pharmacologic intervention, part 2: the role of novel PET agents. J Nucl Med. 2020;61(11):1553‐1559. [DOI] [PubMed] [Google Scholar]
- 247. Alam IS, Simonetta F, Scheller L, et al. Visualization of activated T cells by OX40-ImmunoPET as a strategy for diagnosis of acute graft-versus-host disease. Cancer Res. 2020;80(21):4780‐4790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Levi J, Goth S, Huynh L, et al. (18)F-AraG PET for CD8 profiling of tumors and assessment of immunomodulation by chemotherapy. J Nucl Med. 2021;62(6):802‐807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Mori Y, Dendl K, Cardinale J, Kratochwil C, Giesel FL, Haberkorn U. FAPI PET: fibroblast activation protein inhibitor use in oncologic and nononcologic disease. Radiology. 2023;306(2):e220749. [DOI] [PubMed] [Google Scholar]
- 250. Kosmala A, Serfling SE, Schlotelburg W, et al. Impact of 68 Ga-FAPI-04 PET/CT on staging and therapeutic management in patients with digestive system tumors. Clin Nucl Med. 2023;48(1):35‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Gondry O, Xavier C, Raes L, et al. Phase I study of [(68)Ga]Ga-anti-CD206-sdAb for PET/CT assessment of protumorigenic macrophage presence in solid tumors (MMR phase I). J Nucl Med. 2023;64(9):1378‐1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Sun R, Lerousseau M, Briend-Diop J, et al. Radiomics to evaluate interlesion heterogeneity and to predict lesion response and patient outcomes using a validated signature of CD8 cells in advanced melanoma patients treated with anti-PD1 immunotherapy. J Immunother Cancer. 2022;10(10):e004867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Patel RR, He K, Barsoumian HB, et al. High-dose irradiation in combination with non-ablative low-dose radiation to treat metastatic disease after progression on immunotherapy: results of a phase II trial. Radiother Oncol. 2021;162:60‐67. [DOI] [PubMed] [Google Scholar]
- 254. Le Berre C, Sandborn WJ, Aridhi S, et al. Application of artificial intelligence to gastroenterology and hepatology. Gastroenterology. 2020;158(1):76‐94.e2. [DOI] [PubMed] [Google Scholar]
- 255. LeCun Y, Bengio Y, Hinton G. Deep learning. Nature. 2015;521(7553):436‐444. [DOI] [PubMed] [Google Scholar]
- 256. Luan Y, Zhong G, Li S, et al. A panel of seven protein tumour markers for effective and affordable multi-cancer early detection by artificial intelligence: a large-scale and multicentre case-control study. EClinicalMedicine. 2023;61:102041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Tokutake K, Morelos-Gomez A, Hoshi KI, Katouda M, Tejima S, Endo M. Artificial intelligence for the prevention and prediction of colorectal neoplasms. J Transl Med. 2023;21(1):431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Prasoppokakorn T, Tiyarattanachai T, Chaiteerakij R, et al. Application of artificial intelligence for diagnosis of pancreatic ductal adenocarcinoma by EUS: a systematic review and meta-analysis. Endosc Ultrasound. 2022;11(1):17‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Kourou K, Exarchos TP, Exarchos KP, Karamouzis MV, Fotiadis DI. Machine learning applications in cancer prognosis and prediction. Comput Struct Biotechnol J. 2015;13:8‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Yu FH, Miao SM, Li CY, et al. Pretreatment ultrasound-based deep learning radiomics model for the early prediction of pathologic response to neoadjuvant chemotherapy in breast cancer. Eur Radiol. 2023;33(8):5634‐5644. [DOI] [PubMed] [Google Scholar]
- 261. Zhu J, Chen X, Liu Y, et al. Improving accelerated 3D imaging in MRI-guided radiotherapy for prostate cancer using a deep learning method. Radiat Oncol. 2023;18(1):108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Yerukala Sathipati S, Tsai MJ, Shukla SK, Ho SY. Artificial intelligence-driven pan-cancer analysis reveals miRNA signatures for cancer stage prediction. HGG Adv. 2023;4(3):100190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Akashi T, Okumura T, Terabayashi K, et al. The use of an artificial intelligence algorithm for circulating tumor cell detection in patients with esophageal cancer. Oncol Lett. 2023;26(1):320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Dong W, Ji Y, Pi S, Chen QF. Noninvasive imaging-based machine learning algorithm to identify progressive disease in advanced hepatocellular carcinoma receiving second-line systemic therapy. Sci Rep. 2023;13(1):10690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Franco D, Granata V, Fusco R, et al. Artificial intelligence and radiation effects on brain tissue in glioblastoma patient: preliminary data using a quantitative tool. Radiol Med. 2023;128(7):813‐827. [DOI] [PubMed] [Google Scholar]
- 266. Saad MB, Hong L, Aminu M, et al. Predicting benefit from immune checkpoint inhibitors in patients with non-small-cell lung cancer by CT-based ensemble deep learning: a retrospective study. Lancet Digit Health. 2023;5(7):e404‐e420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Xu B, Lu M, Yan L, et al. A pan-cancer analysis of predictive methylation signatures of response to cancer immunotherapy. Front Immunol. 2021;12:796647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Polano M, Chierici M, Dal Bo M, et al. A pan-cancer approach to predict responsiveness to immune checkpoint inhibitors by machine learning. Cancers (Basel). 2019;11(10):1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Postow MA, Sidlow R, Hellmann MD. Immune-Related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378(2):158‐168. [DOI] [PubMed] [Google Scholar]
- 270. Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369(2):122‐133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Johnson DB, Balko JM, Compton ML, et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med. 2016;375(18):1749‐1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Moslehi JJ, Salem JE, Sosman JA, Lebrun-Vignes B, Johnson DB. Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis. Lancet. 2018;391(10124):933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Andrews LP, Marciscano AE, Drake CG, Vignali DA. LAG3 (CD223) as a cancer immunotherapy target. Immunol Rev. 2017;276(1):80‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. Triebel F, Jitsukawa S, Baixeras E, et al. LAG-3, a novel lymphocyte activation gene closely related to CD4. J Exp Med. 1990;171(5):1393‐1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Marin-Acevedo JA, Kimbrough EO, Lou Y. Next generation of immune checkpoint inhibitors and beyond. J Hematol Oncol. 2021;14(1):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Uboha NV, Milhem MM, Kovacs C, et al. Phase II study of spartalizumab (PDR001) and LAG525 in advanced solid tumors and hematologic malignancies. J Clin Oncol. 2019;37(15_suppl):2553‐2553. [Google Scholar]
- 278. Fanciulli G, Modica R, La Salvia A, et al. Immunotherapy of neuroendocrine neoplasms: any role for the chimeric antigen receptor T cells? Cancers (Basel). 2022;14(16):3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Brudno JN, Kochenderfer JN. Toxicities of chimeric antigen receptor T cells: recognition and management. Blood. 2016;127(26):3321‐3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280. Melero I, Castanon E, Alvarez M, Champiat S, Marabelle A. Intratumoural administration and tumour tissue targeting of cancer immunotherapies. Nat Rev Clin Oncol. 2021;18(9):558‐576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Ascierto PA, Del Vecchio M, Robert C, et al. Ipilimumab 10 mg/kg versus ipilimumab 3 mg/kg in patients with unresectable or metastatic melanoma: a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2017;18(5):611‐622. [DOI] [PubMed] [Google Scholar]
- 282. Marabelle A, Tselikas L, de Baere T, Houot R. Intratumoral immunotherapy: using the tumor as the remedy. Ann Oncol. 2017;28(suppl_12):xii33‐xii43. [DOI] [PubMed] [Google Scholar]
- 283. Kreiter S, Selmi A, Diken M, et al. Intranodal vaccination with naked antigen-encoding RNA elicits potent prophylactic and therapeutic antitumoral immunity. Cancer Res. 2010;70(22):9031‐9040. [DOI] [PubMed] [Google Scholar]
- 284. Mishra R, Sukhbaatar A, Mori S, Kodama T. Metastatic lymph node targeted CTLA4 blockade: a potent intervention for local and distant metastases with minimal ICI-induced pneumonia. J Exp Clin Cancer Res. 2023;42(1):132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Shahrouki P, Lee JM, Barclay J, et al. Technical feasibility and safety of repeated computed tomography-guided transthoracic intratumoral injection of gene-modified cellular immunotherapy in metastatic NSCLC. JTO Clin Res Rep. 2021;2(11):100242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. Suh RD, Goldin JG, Wallace AB, et al. Metastatic renal cell carcinoma: CT-guided immunotherapy as a technically feasible and safe approach to delivery of gene therapy for treatment. Radiology. 2004;231(2):359‐364. [DOI] [PubMed] [Google Scholar]
- 287. Tselikas L, Dardenne A, de Baere T, et al. Feasibility, safety and efficacy of human intra-tumoral immuno-therapy. Gustave Roussy's initial experience with its first 100 patients. Eur J Cancer. 2022;172:1‐12. [DOI] [PubMed] [Google Scholar]
- 288. Waddill W 3rd, Wright W Jr, Unger E, et al. Human gene therapy for melanoma: CT-guided interstitial injection. AJR Am J Roentgenol. 1997;169(1):63‐67. [DOI] [PubMed] [Google Scholar]
- 289. Sheth RA, Murthy R, Hong DS, et al. Assessment of image-guided intratumoral delivery of immunotherapeutics in patients with cancer. JAMA Netw Open. 2020;3(7):e207911. [DOI] [PubMed] [Google Scholar]
- 290. Zhang L, Åkerström T, Mollazadegan K, et al. Risk of complications after core needle biopsy in pheochromocytoma/paraganglioma. Endocr Relat Cancer. 2023;30(7):e220354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291. Shalhout SZ, Miller DM, Emerick KS, Kaufman HL. Therapy with oncolytic viruses: progress and challenges. Nat Rev Clin Oncol. 2023;20(3):160‐177. [DOI] [PubMed] [Google Scholar]
- 292. Rehman H, Silk AW, Kane MP, Kaufman HL. Into the clinic: Talimogene laherparepvec (T-VEC), a first-in-class intratumoral oncolytic viral therapy. J Immunother Cancer. 2016;4:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Huang B, Zhao J, Unkeless JC, Feng ZH, Xiong H. TLR signaling by tumor and immune cells: a double-edged sword. Oncogene. 2008;27(2):218‐224. [DOI] [PubMed] [Google Scholar]
- 294. Shi M, Chen X, Ye K, Yao Y, Li Y. Application potential of toll-like receptors in cancer immunotherapy: systematic review. Medicine (Baltimore). 2016;95(25):e3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295. Rolfo C, Giovannetti E, Martinez P, McCue S, Naing A. Applications and clinical trial landscape using Toll-like receptor agonists to reduce the toll of cancer. NPJ Precis Oncol. 2023;7(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296. Uher O, Caisova V, Hansen P, et al. Coley's immunotherapy revived: innate immunity as a link in priming cancer cells for an attack by adaptive immunity. Semin Oncol. 2019;46(4-5):385‐392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297. Caisová V, Uher O, Nedbalová P, et al. Effective cancer immunotherapy based on combination of TLR agonists with stimulation of phagocytosis. Int Immunopharmacol. 2018;59:86‐96. [DOI] [PubMed] [Google Scholar]
- 298. Medina R, Wang H, Caisová V, et al. Induction of immune response against metastatic tumors via vaccination of mannan-BAM, TLR ligands and anti-CD40 antibody (MBTA). Adv Ther (Weinh). 2020;3(9):2000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Uher O, Huynh TT, Zhu B, et al. Identification of immune cell infiltration in murine pheochromocytoma during combined mannan-BAM, TLR ligand, and anti-CD40 antibody-based immunotherapy. Cancers (Basel). 2021;13(16):3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300. Uher O, Hadrava Vanova K, Lencova R, et al. Intratumoral immunotherapy of murine pheochromocytoma shows no age-dependent differences in its efficacy. Front Endocrinol (Lausanne). 2023;14:1030412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301. Caisova V, Li L, Gupta G, et al. The significant reduction or complete eradication of subcutaneous and metastatic lesions in a pheochromocytoma mouse model after immunotherapy using mannan-BAM, TLR ligands, and anti-CD40. Cancers (Basel). 2019;11(5):654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302. Yu J, Sun H, Cao W, Song Y, Jiang Z. Research progress on dendritic cell vaccines in cancer immunotherapy. Exp Hematol Oncol. 2022;11(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303. Papewalis C, Kouatchoua C, Ehlers M, et al. Chromogranin A as potential target for immunotherapy of malignant pheochromocytoma. Mol Cell Endocrinol. 2011;335(1):69‐77. [DOI] [PubMed] [Google Scholar]
- 304. Baudin E, Grisanti S, Fassnacht M, et al. 2MO EO2401 (EO) therapeutic vaccine for patients (pts) with adrenocortical carcinoma (ACC) and malignant pheochromocytoma/paraganglioma (MPP): phase I/II SPENCER study. Ann Oncol. 2022;33:S545‐S546. [Google Scholar]
- 305. Ye J, Wang H, Medina R, et al. rWTC-MBTA: autologous vaccine prevents metastases via antitumor immune responses. J Exp Clin Cancer Res. 2023;42(1):163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306. Schoenfeld AJ, Hellmann MD. Acquired resistance to immune checkpoint inhibitors. Cancer Cell. 2020;37(4):443‐455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307. Bedognetti D, Ceccarelli M, Galluzzi L, et al. Toward a comprehensive view of cancer immune responsiveness: a synopsis from the SITC workshop. J Immunother Cancer. 2019;7(1):131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308. Daniel S C, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39(1):1‐10. [DOI] [PubMed] [Google Scholar]
- 309. Bonazzi S, d'Hennezel E, Beckwith REJ, et al. Discovery and characterization of a selective IKZF2 glue degrader for cancer immunotherapy. Cell Chem Biol. 2023;30(3):235‐247.e12. [DOI] [PubMed] [Google Scholar]
- 310. Conlon K, Watson DC, Waldmann TA, et al. Phase I study of single agent NIZ985, a recombinant heterodimeric IL-15 agonist, in adult patients with metastatic or unresectable solid tumors. J Immunother Cancer. 2021;9(11):e003388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311. Cichocki F, Bjordahl R, Gaidarova S, et al. iPSC-derived NK cells maintain high cytotoxicity and enhance in vivo tumor control in concert with T cells and anti-PD-1 therapy. Sci Transl Med. 2020;12(568):eaaz5618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312. van Dalen FJ, van Stevendaal M, Fennemann FL, Verdoes M, Ilina O. Molecular repolarisation of tumour-associated macrophages. Molecules. 2019;24(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313. Rodell CB, Arlauckas SP, Cuccarese MF, et al. TLR7/8-agonist-loaded nanoparticles promote the polarization of tumour-associated macrophages to enhance cancer immunotherapy. Nat Biomed Eng. 2018;2(8):578‐588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314. Sharma N, Vacher J, Allison JP. TLR1/2 ligand enhances antitumor efficacy of CTLA-4 blockade by increasing intratumoral treg depletion. Proc Natl Acad Sci U S A. 2019;116(21):10453‐10462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315. Ota Y, Nagai Y, Hirose Y, et al. DSP-0509, a systemically available TLR7 agonist, exhibits combination effect with immune checkpoint blockade by activating anti-tumor immune effects. Front Immunol. 2023;14:1055671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316. Takeda Y, Kataoka K, Yamagishi J, Ogawa S, Seya T, Matsumoto M. A TLR3-specific adjuvant relieves innate resistance to PD-L1 blockade without cytokine toxicity in tumor vaccine immunotherapy. Cell Rep. 2017;19(9):1874‐1887. [DOI] [PubMed] [Google Scholar]
- 317. De Waele J, Verhezen T, van der Heijden S, et al. A systematic review on poly(I:C) and poly-ICLC in glioblastoma: adjuvants coordinating the unlocking of immunotherapy. J Exp Clin Cancer Res. 2021;40(1):213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318. Vonderheide RH. CD40 agonist antibodies in cancer immunotherapy. Annu Rev Med. 2020;71:47‐58. [DOI] [PubMed] [Google Scholar]
- 319. Vonderheide RH, Dutcher JP, Anderson JE, et al. Phase I study of recombinant human CD40 ligand in cancer patients. J Clin Oncol. 2001;19(13):3280‐3287. [DOI] [PubMed] [Google Scholar]
- 320. Byrne KT, Betts CB, Mick R, et al. Neoadjuvant selicrelumab, an agonist CD40 antibody, induces changes in the tumor microenvironment in patients with resectable pancreatic cancer. Clin Cancer Res. 2021;27(16):4574‐4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321. Padrón LJ, Maurer DM, O'Hara MH, et al. Sotigalimab and/or nivolumab with chemotherapy in first-line metastatic pancreatic cancer: clinical and immunologic analyses from the randomized phase 2 PRINCE trial. Nat Med. 2022;28(6):1167‐1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322. Morrison AH, Diamond MS, Hay CA, Byrne KT, Vonderheide RH. Sufficiency of CD40 activation and immune checkpoint blockade for T cell priming and tumor immunity. Proc Natl Acad Sci U S A. 2020;117(14):8022‐8031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323. Bajor DL, Mick R, Riese MJ, et al. Long-term outcomes of a phase I study of agonist CD40 antibody and CTLA-4 blockade in patients with metastatic melanoma. Oncoimmunology. 2018;7(10):e1468956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324. Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1116‐1127. [DOI] [PubMed] [Google Scholar]
- 325. Choueiri TK, Powles T, Burotto M, et al. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2021;384(9):829‐841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326. Motzer R, Alekseev B, Rha SY, et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med. 2021;384(14):1289‐1300. [DOI] [PubMed] [Google Scholar]
- 327. Albiges L, Barthélémy P, Gross-Goupil M, Negrier S, Needle MN, Escudier B. Tinivo: safety and efficacy of tivozanib-nivolumab combination therapy in patients with metastatic renal cell carcinoma. Ann Oncol. 2021;32(1):97‐102. [DOI] [PubMed] [Google Scholar]
- 328. Fukuoka S, Hara H, Takahashi N, et al. Regorafenib plus nivolumab in patients with advanced gastric or colorectal cancer: an open-label, dose-escalation, and dose-expansion phase ib trial (REGONIVO, EPOC1603). J Clin Oncol. 2020;38(18):2053‐2061. [DOI] [PubMed] [Google Scholar]
- 329. Makker V, Rasco D, Vogelzang NJ, et al. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer: an interim analysis of a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2019;20(5):711‐718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330. Toledo RA, Jimenez C, Armaiz-Pena G, Arenillas C, Capdevila J, Dahia PLM. Hypoxia-Inducible factor 2 alpha (HIF2α) inhibitors: targeting genetically driven tumor hypoxia. Endocr Rev. 2023;44(2):312‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331. Kamihara J, Hamilton KV, Pollard JA, et al. Belzutifan, a potent HIF2α inhibitor, in the Pacak-Zhuang syndrome. N Engl J Med. 2021;385(22):2059‐2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332. Peng S, Zhang J, Tan X, et al. The VHL/HIF axis in the development and treatment of pheochromocytoma/paraganglioma. Front Endocrinol (Lausanne). 2020;11:586857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333. Finisguerra V, Dvorakova T, Formenti M, et al. Metformin improves cancer immunotherapy by directly rescuing tumor-infiltrating CD8 T lymphocytes from hypoxia-induced immunosuppression. J Immunother Cancer. 2023;11(5):e005719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334. Ding L, Liang G, Yao Z, et al. Metformin prevents cancer metastasis by inhibiting M2-like polarization of tumor associated macrophages. Oncotarget. 2015;6(34):36441‐36455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335. Uehara T, Eikawa S, Nishida M, et al. Metformin induces CD11b+-cell-mediated growth inhibition of an osteosarcoma: implications for metabolic reprogramming of myeloid cells and anti-tumor effects. Int Immunol. 2019;31(4):187‐198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336. Meireles CG, Lourenço de Lima C, Martins de Paula Oliveira M, et al. Antiproliferative effects of metformin in cellular models of pheochromocytoma. Mol Cell Endocrinol. 2022;539:111484. [DOI] [PubMed] [Google Scholar]
- 337. Thakur S, Daley B, Klubo-Gwiezdzinska J. The role of an anti-diabetic drug metformin in the treatment of endocrine tumors. J Mol Endocrinol. 2019;63(2):R17‐R35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338. Ghayee HK, Vanova KH, Pacak K, Uher O. PSAT089 lack of effect of metformin in murine pheochromocytoma model. Journal of the Endocrine Society. 2022;6(Supplement_1):A116‐A116. [Google Scholar]
- 339. Hadrava Vanova K, Yang C, Meuter L, Neuzil J, Pacak K. Reactive oxygen Species: a promising therapeutic target for SDHx-mutated pheochromocytoma and paraganglioma. Cancers (Basel). 2021;13(15):3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340. Kokolus KM, Zhang Y, Sivik JM, et al. Beta blocker use correlates with better overall survival in metastatic melanoma patients and improves the efficacy of immunotherapies in mice. Oncoimmunology. 2018;7(3):e1405205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341. Yang EV, Kim SJ, Donovan EL, et al. Norepinephrine upregulates VEGF, IL-8, and IL-6 expression in human melanoma tumor cell lines: implications for stress-related enhancement of tumor progression. Brain Behav Immun. 2009;23(2):267‐275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342. Le Naour J, Galluzzi L, Zitvogel L, Kroemer G, Vacchelli E. Trial watch: IDO inhibitors in cancer therapy. Oncoimmunology. 2020;9(1):1777625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343. Hayes CS, Shicora AC, Keough MP, Snook AE, Burns MR, Gilmour SK. Polyamine-blocking therapy reverses immunosuppression in the tumor microenvironment. Cancer Immunol Res. 2014;2(3):274‐285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344. Taïeb D, Pacak K. Molecular imaging and theranostic approaches in pheochromocytoma and paraganglioma. Cell Tissue Res. 2018;372(2):393‐401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345. Patel M, Tena I, Jha A, Taieb D, Pacak K. Somatostatin receptors and analogs in pheochromocytoma and paraganglioma: old players in a new precision medicine world. Front Endocrinol (Lausanne). 2021;12:625312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346. Reubi JC, Waser B, Khosla S, et al. In vitro and in vivo detection of somatostatin receptors in pheochromocytomas and paragangliomas. J Clin Endocrinol Metab. 1992;74(5):1082‐1089. [DOI] [PubMed] [Google Scholar]
- 347. Fischer A, Kloos S, Maccio U, et al. Metastatic pheochromocytoma and paraganglioma: somatostatin receptor 2 expression, genetics and therapeutic responses. J Clin Endocrinol Metab. 2023;108(10):2676‐2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348. Wang Y, Deng W, Li N, et al. Combining immunotherapy and radiotherapy for cancer treatment: current challenges and future directions. Front Pharmacol. 2018;9:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349. Zhang Z, Liu X, Chen D, Yu J. Radiotherapy combined with immunotherapy: the Dawn of cancer treatment. Signal Transduction and Targeted Therapy. 2022;7(1):258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350. Holscher I, van den Berg TJ, Dreijerink KMA, Engelsman AF, Nieveen van Dijkum EJM. Recurrence rate of sporadic pheochromocytomas after curative adrenalectomy: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2021;106(2):588‐597. [DOI] [PubMed] [Google Scholar]
- 351. Johnston PC, Mullan KR, Atkinson AB, et al. Recurrence of phaeochromocytoma and abdominal paraganglioma after initial surgical intervention. Ulster Med J. 2015;84(2):102‐106. [PMC free article] [PubMed] [Google Scholar]
- 352. Parisien-La Salle S, Chbat J, Lacroix A, et al. Postoperative recurrences in patients operated for pheochromocytomas and paragangliomas: new data supporting lifelong surveillance. Cancers (Basel). 2022;14(12):2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353. Van Slycke S, Caiazzo R, Pigny P, et al. Local-regional recurrence of sporadic or syndromic abdominal extra-adrenal paraganglioma: incidence, characteristics, and outcome. Surgery. 2009;146(6):986‐992. [DOI] [PubMed] [Google Scholar]
- 354. Hong WX, Sagiv-Barfi I, Czerwinski DK, Sallets A, Levy R. Neoadjuvant intratumoral immunotherapy with TLR9 activation and anti-OX40 antibody eradicates metastatic cancer. Cancer Res. 2022;82(7):1396‐1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355. Huang AC, Orlowski RJ, Xu X, et al. A single dose of neoadjuvant PD-1 blockade predicts clinical outcomes in resectable melanoma. Nat Med. 2019;25(3):454‐461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356. Powles T, Kockx M, Rodriguez-Vida A, et al. Clinical efficacy and biomarker analysis of neoadjuvant atezolizumab in operable urothelial carcinoma in the ABACUS trial. Nat Med. 2019;25(11):1706‐1714. [DOI] [PubMed] [Google Scholar]
- 357. Krishnamoorthy M, Lenehan JG, Maleki Vareki S. Neoadjuvant immunotherapy for high-risk, resectable malignancies: scientific rationale and clinical challenges. J Natl Cancer Inst. 2021;113(7):823‐832. [DOI] [PMC free article] [PubMed] [Google Scholar]