Abstract
The last decades have witnessed an increase in the global population and movements of companion animals, contributing to changes in density and distribution of pet parasites. Control of companion animal parasites (CAPs) becomes increasingly relevant because of the intensifying human-animal bond. Parasites impact on the health of humans and their pets, but also of wildlife and the environment. We conducted a qualitative review on the current advancements, gaps and priorities for the monitoring and treatment of CAPs with a focus on securing public health. There is a need to raise awareness, coordinate global surveillance schemes and better quantify the impact of companion animal parasites on One Health.
Keywords: Companion animals, Parasites, One health, Research gaps, Control measures
Highlights
-
•
Dogs, cats and their parasites have a poorly quantified burden on One Health.
-
•
Parasite-borne illnesses in humans may be transmitted by companion animals. Control measures should involve companion animals to increase their effectiveness.
-
•
Use of parasiticides remains today's cornerstone to mitigate and control the animal and human health threats posed by companion animal parasites.
1. Companion animal parasites: a One Health issue
The last few decades have witnessed an increase in the global population of dogs and cats. This trend has notably increased during the COVID-crisis, with people valuing more and more pet companionship, as people searched for animal closeness in times of reduced social contacts [1]. In 2022, more than half of the global population housed a pet at home, and the budget spent on animal health care followed parallel increases [2]. Animal health companies thus have expanded their investments in research and development of companion animal (CA) products, enhancing innovation in the development of novel vaccines, parasiticides, diagnostics and digital technologies [3]. Parasiticides represent the second largest segment of the global animal health market, after biologicals, and account for € 7 billion in sales (23% of the market share) [4]. In this regard, the sales of medicines for pets represent 47% of the total share [5]. The market of dog and cat parasiticides has been growing over the last decade from 31% to 34% of the product portfolio and is expected to grow further by 6% from 2022 to 2027 [5]. Undeniably, pets play an important role in companionship, entertainment, and emotional support to their owners [6]. Indeed, dog or cat ownership has been linked to reduction in cardiovascular disease risk, shorter hospital stays and positive health and welfare effects in patients affected by cancers or autism [6]. However, the closer human-animal bond also leads to more frequent violation of hygiene principles such as keeping pets in the bed(room), animals licking face and wounds as well as bite and scratch accidents. This, in turn, leads to an increased risk of exposure to parasitic diseases [7], whose importance varies depending on the context in which there are evaluated.
Never before as in the last decades, the subtle connection between animal, human and environmental health has reached the attention of the public, with the concept of “One Medicine” imprinting the direction of future health policies [6]. According to this approach, the health of people, either physical or mental, is closely connected to the health of animals and the shared environment. Although the main themes of discussion influencing the global politics of decision-making bodies tend to linger on the matters of antimicrobial resistance, food safety, or environmental health, the prevention of zoonotic diseases is also a source of major concern [7]. Within this theme, CA and their parasites have an undeniable, yet poorly quantified, burden on One Health.
In this review we analyse different drivers for increased transmission risk of companion animal parasites (CAPs) to humans and contextualise them in the frame of One Health approaches, which may vary depending on the priority given to specific diseases in different geographical settings. Via exemplary parasitic diseases in CA, we aim to illustrate the challenges when imprinting the direction of future health policies for CAP control.
2. Relevance for pet health
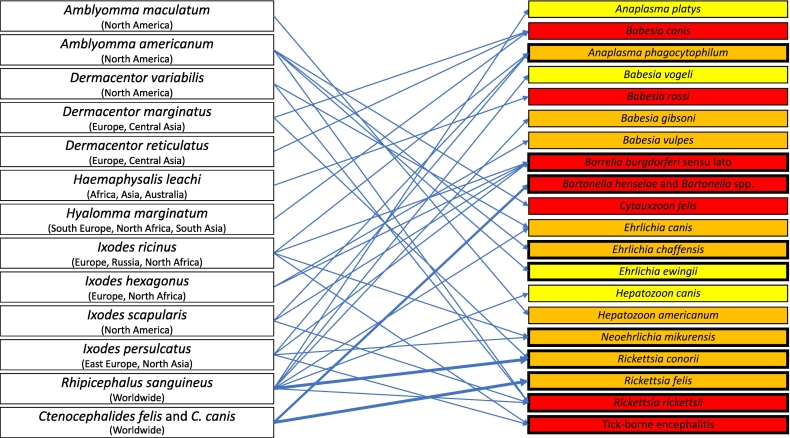
The spectrum of endo- and ectoparasites affecting dogs and cats is large [8,9]. Protozoa and helminths are a significant cause of diarrhoea, respiratory issues, vomiting or weight loss in CAs (Table 1). Similarly, ectoparasites (arthropods) are a common problem to dogs and cats, causing general discomfort, anaemia, and allergic reactions or skin lesions, and potentially transmitting viruses, bacteria, protozoa, and helminths. Ticks, fleas, mosquitoes, and sand flies are all involved in the spread of the so called “vector borne diseases” (VBDs) (Fig. 1) [10,11].
Table 1.
Main endo- and ectoparasites of dogs and cats, along with data on their role as reservoir for humans, geographical distribution and relevance for public health. Classification into minor or major zoonotic diseases is based on the inclusion of the parasite species in WHO control programs or its classification as a Neglected Tropical Disease.
| Parasite species infecting dogs (D) and/or cats (C) |
Degree of pathogenicity in D/Cδ | Zoonotic relevance for humansγ | VBDα? | Usual mode of transmission | Geographical distribution | Reference |
|---|---|---|---|---|---|---|
| Protozoa | ||||||
| Leishmania infantum (D, C) | High | High (a) (NTDβ, in WHO roadmap) |
Yes | Bite of infected phlebotomine sand flies, bites, wounds, blood transfusions, venereal or prenatal transmission | Mediterranean, Middle East, Central Asia, Latin America | [12] |
| Trypanosoma cruzi (D, C) | High | High (a) (NTD, in WHO roadmap) |
Yes | Contact via small skin lesions or mucous membranes, ingestion and crushing infected bug vectors or eating an infected host | South America | [79] |
| Giardia duodenalis (D, C) | Moderate | Low | No | Ingestion of the cysts in undercooked, contaminated meat, accidental ingestion of oocysts in cat faeces, congenital, rarely via organ donation or blood transfusion | Worldwide | [80] |
| Toxoplasma gondii (C) | Moderate | Moderate | No | Direct contact with meat and viscera of infected animals; through food with parasitised meat and viscera or food contaminated with oocysts | Worldwide | [14] |
|
Cystoisospora felis, C. rivolta (C) C. canis, C. ohioensis, C. burrowsi (D) |
Low | No | No | Direct ingestion of sporulated oocysts or ingestion of paratenic hosts or undercooked meat | Worldwide | [81] |
| Neospora caninum (D) | Low (b) | No | No | Prenatal infection of puppies, ingestion of tissues cysts | Worldwide | [82] |
| Helminths | ||||||
| Opisthorchis felineus (D, C) | High | High (a) (NTD, in WHO roadmap) |
No | Ingestion of raw or undercooked fish | East Europe, Russia, Asia | [83] |
| Paragonimus westermani (D, C) | Moderate | High (a) (NTD, in WHO roadmap) |
No | Ingestion of raw or undercooked crab or crayfish | Asia | [84] |
| Paragonimus kellicotti (D, C) | Moderate | High (a) (NTD, in WHO roadmap) |
No | Ingestion of raw or undercooked crab or crayfish | North America | [84] |
| Echinococcus. granulosus sensu lato (D) | Low | High (NTD, in WHOroadmap) |
No | Accidental ingestion of eggs | Worldwide, except Northern Europe and North America | [31] |
| Echinococcus multilocularis (D) | Low | High (NTD, in WHO roadmap) | No | Accidental ingestion of eggs | Northern hemisphere | [33] |
| Ancylostoma ceylanicum,(D, C) | Moderate | High (NTD, in WHO roadmap) |
No | Ingestion of infective stages, percutaneous infection is possible | South East Asia, South Africa, Australia | [18] |
| Dirofilaria immitis (D, C) | High | Low | Yes | Bite of infected mosquitoes | Worldwide, except Scandinavia, Central and Northeastern Europe | [19] |
| Dirofilaria repens (D, C) | Low | Moderate | Yes | Bite of infected mosquitoes | Europe, Asia, Africa | [85] |
| Onchocerca lupi (D, C) | Moderate | Moderate | Unknown | Probably via a dipteran vector | Europe, United States, Middle East | [86] |
| Thelazia callipaeda (D, C) | Low | Low | Yes | Transmission via fruit flies | Europe, Far East | [87] |
| Toxocara cati (C), T. canis (D) | Low | Moderate | No | Ingestion of infective stages, somatic migration (prenatal) in pregnant bitches, and lactogenic transmission | Worldwide | [15] |
| Ancylostoma caninum, Uncinaria. stenocephala (D), Ancylostoma tubaeforme (C) | Low (c) | Moderate | No | Ingestion of infective stages, percutaneous infection is possible. A. caninum is also transmitted via lactogenic route | Worldwide, mainly warmer climates | [18] |
| Dipylidium caninum (D, C) | Low | Low | Yes | Ingestion of fleas | Worldwide | [88] |
| Aelurostrongylus abstrusus (C) | Moderate | No | No | Ingestion of infective stages, snails or small animals during predation | Worldwide | [89] |
| Angiostrongylus vasorum (D) | High | No | No | Ingestion of infective larvae via gastropods | Europe, certain areas of Africa and America. | [90] |
|
Taenia hydatigena, T. pisiformis, T. ovis, T. multiceps (D) T. taeniaeformis (C) |
Low | No | No | Ingestion of raw organs | Worldwide | [91] |
| Ectoparasites | ||||||
| Ticks See Fig. 1 |
Variable | See Fig. 1 | – | Environment | See Fig. 1 | [[92], [93], [94], [95], [96], [97]] |
| Fleas Ctenocephalides felis and C. canis Pulex irritans |
Variable | See Fig. 1 | – | Environment | Worldwide | [13] |
| Lice Trichodectes, Linognathus, Felicola |
Low to moderate | No | – | Close contact with infested animals, contaminated environment | Worldwide | [98] |
| Mites Demodex, Notoedres, Otodectes, Sarcoptes, Cheyletiella, Neotrombicula |
Low to High | Low | – | Close contact with infested animals | Worldwide | [99] |
Pathogen transmitted by an arthropod vector.
Neglected Tropical Disease; (a) In the presence of the vector, otherwise in non-endemic countries the zoonotic relevance to humans is very low.
Based on the severity of the symptoms, risk for contracting the infection, or a combination of these elements.
(b) can be high in puppies and/or in immune suppressed animals; (c) can be high in puppies.
Fig. 1.
Main tick and flea species infesting dogs and cats and their vectored pathogens. Pathogen colours refer to the degree of pathogenicity in pets (yellow = mild, orange = moderate, red = high); marked boxes indicate pathogens that can also infect humans and deep blue arrows indicate the confirmed role of dogs and cats as reservoir for human infection. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Of all CAPs that affect humans, some can be considered of higher importance than other. However, their impact depends on the context of the assessment and can vary according to geographical distribution of the parasite, its abundance, its pathogenicity in animals or humans as well as the transmission mode between pets and humans (Table 1). There are different ways in which CAPs impact on human health, depending if the main reservoir for human infections is (i) the CA population (e.g., Leishmania infantum or fleas) [12,13]; (ii) the environment which was contaminated by CAs (e.g., Toxoplasma gondii or Toxocara spp.) [14,15]; (iii) wildlife (such as in the case of Echinococcus multilocularis or Borrelia burgdorferi sensu lato) [16,17]); or (iv) when there is lack of a clear parasite reservoir with pathogens that thrive equally in animals and in human hosts, with life cycles that can be maintained by transmission between humans only, such as for Ancylostoma ceylanicum [18]. The impact can also largely differ depending on whether animal or human health is considered first and foremost. For example, heartworm disease caused by Dirofilaria immitis is a severe disease in dogs with a limited public health importance [19]. In contrast, Echinococcus granulosus causes no disease in dogs but is a significant burden on global public health [20].
3. Relevance for wildlife health
Pathogens of human and domestic animal origin may infect wildlife, or vice versa, potentially resulting in outbreaks of diseases [21]. A key example includes the transmission of E. multilocularis from the wildlife reservoir (foxes) to dogs through common habitats with infected intermediate hosts. Dogs, in turn, represent a potential risk for alveolar echinococcosis in humans, especially in (peri) urban recreational environments [[22], [100]]. The progressive reduction of green areas and forests and the expansion of suburban areas brings wild animals, humans, and domestic animals in close contact with each other, with increasing risks for transmission of parasites, as in the case of Lyme disease [23]. This phenomenon was considered as a rare event in the past but is likely to become more frequent in the future [22,23]. Encroachment of human settlements and conversion of forests into rural areas increases the likelihood of encounters with ticks, known as the “crossroads effect” [23].
In addition, some CAPs may directly or indirectly affect the fitness of wildlife, potentially causing devastating impacts on biodiversity and species conservation [21]. Toxoplasma gondii may be a consistent danger for wildlife species which have not co-evolved with the parasite. Outbreaks of hyperacute toxoplasmosis have been reported worldwide in zoo animals and wildlife, most likely among kangaroos, wallabies, squirrel monkeys and colonies of captive lemurs [24]. Similarly, T. gondii causes mortality in several species of marine mammals, including threatened Southern sea otters and endangered Hawaiian monk seals [25]. The future of many of threatened species increasingly hinges on our ability to control toxoplasmosis in feral cats.
4. Relevance for human health
Current evaluations indicate that approximately six out of every ten known infectious diseases in humans can be spread by animals and, out of all new human pathogens detected in the last three decades, three pathogens out of four were originating from animals [26]. Neglected tropical diseases (NTDs), as defined by the World Health Organization (WHO), include a list of parasitic, bacterial and viral diseases that cause substantial illness for more than one billion people globally. At least eight of the 14 parasitic NTDs identified by the WHO (Chagas disease, leishmaniasis, echinococcosis, zoonotic hookworms, food-borne trematodes, human African trypanosomiasis, zoonotic scabies and other ectoparasites) may involve dogs or cats as potential reservoir for human infection (Table 1). Therefore, the control programmes should also involve epidemiological surveillance in CAs to increase their effectiveness. Several parasitic infections of pets have a collateral impact on human health, due to the direct or indirect transmission of the infection to humans (and vice versa) [27]. The geographical areas and socio-economic conditions where these parasitic diseases thrive differ considerably and require appropriate control measures in dogs and cats are essential to reduce the impact in humans. We shortly outline the situation of three emblematic CAPs in the following sections.
4.1. Zoonotic visceral leishmaniasis: lessons from Brazil
Leishmaniasis (or leishmaniosis in the context of infection in dogs) is a complex mammalian diseases caused by protozoa of the genus Leishmania, whose transmission to humans relies on the availability of reservoir animals and phlebotomine sand fly vectors [27]. The incidence of canine leishmaniosis (CL) varies according to the endemicity of the area, ranging from 5% to 30%, and as high as 70% in some spots [28]. Human Visceral leishmaniasis is a life-threatening disease that affects ≈200,000–400,000 persons annually and causes an estimated ≈20,000–40,000 deaths per year [29]. Zoonotic visceral leishmaniasis (ZVL) caused by Leishmania infantum is an important disease of humans and dogs in an area that stretches from the Mediterranean basin and Middle East to northern China, and across Central and Latin America, including Brazil [28,29]. Distribution of human cases is correlated with the occurrence of seropositive animals (dogs and other potential reservoirs) [30], which suggests that prevention of leishmaniosis in dogs has an impact on the reduction of transmission risk to humans. However, control based on culling infected dogs only has been demonstrated to be ineffective in reducing the risk for visceral leishmaniasis [[29], [30]]. This calls for integrated control methods that both (i) prevent the feeding activity of sand flies on infected hosts (e.g., through insecticide impregnated collars), and (ii) reduce parasite loads in the main reservoir host (e.g., through treatment) to scale down the incidence of ZVL. However, besides the need for vaccines with higher efficacy, the role out of effective control programmes is often hindered by socio-economic conditions and innovative control campaigns are needed to reduce Leishmania transmission to animals and humans.
4.2. Cystic echinococcosis: a remaining global problem
The genus Echinococcus includes several species and genotypes of zoonotic tapeworms [31]. The adult stages mostly occur in the intestine of dogs, and occasionally in cats, for both without clinical relevance. The larval stages develop in tissues of various organs of a variety of mammalian intermediate hosts, including man, for which alveolar (E. multilocularis) and cystic echinococcosis (E. granulosus) can be lethal. Echinococcus granulosus causing cystic echinococcosis is principally maintained within a dog-sheep cycle in pastoral regions [22]. Cystic echinococcosis is globally distributed and found in every continent except Antarctica. In endemic regions, human incidence rates can reach more than 50 per 100,000 person-years, and prevalence levels of 5%–10% may occur in parts of Argentina, Peru, East Africa, Central Asia and China [22]. The global burden of cystic echinococcosis has been estimated at 19,300 deaths and 184,000 disability-adjusted life-years (DALYs) each year, with potential underestimation of the global impact of the disease [32]. Integrated control programmes based on deworming of dogs and vaccination of lambs are known to be efficacious and can eliminate the disease in transmission zones [22,32,33], but they depend on the availability of effective implementation tools, which include control of stray dogs, slaughter supervision, public education campaigns, and routine anthelmintic treatment of dogs.
4.3. Fleas, households and pets
Among ectoparasites, fleas are accounted as the most frequent external parasites of CAs worldwide [34]. Due to a low degree of host-specificity, fleas of dogs and cats may have significant wildlife reservoirs. These ectoparasites have also adapted well to living in artificially heated homes being today perceived as a year-round pest [14]. The cat flea Ctenocephalides felis is the most abundant ectoparasite of CAs and represents the great majority of fleas in human homes. Fleas may transmit pathogens causing hemoplasmosis, rickettsiosis, dypilidiosis and bartonellosis (causing cat-scratch disease in children and immunocompromised people) [35]. However, insidious attacks by fleas on people and domestic animals causing irritation, blood loss and severe discomfort are equally important, but remain unquantified [35]. The control of a flea infestation requires a comprehensive approach on both the hosts and the environment, considering the parasites spotted on the surface of the animals represent the smallest part of the overall population, whereas the bulk of the population has typically spread to the surrounding household [14]. This means treating a flea infestation requires eliminating the parasite both on the animal and in the surrounding house.
5. Changing environment affecting CAP control
5.1. Climate change shifting parasite boundaries
Most parasites require either a suitable environment and/or intermediate hosts to complete their life cycle [36]. This makes them sensitive to environmental modifications and their distribution patterns susceptible to change [[37], [38], [39]]. Data collected over the last decade demonstrates that the epidemiology of parasitic diseases is subject to changes and will undoubtedly continue to do so in the future, with both expansions and reductions of the distribution range [40,41]. Frequently, increases in temperature enable both the parasite and their vectors to multiply faster, eventually leading to growth of the parasite infected vector population and higher disease transmission risks to humans and CAs [42].
The European Centre for Diseases Prevention and Control (ECDC), for instance, reported in 2021 new areas of presence for the mosquito species Aedes albopictus, A. japonicus and A. koreicus [43] which act as (new) competent vectors for a range of VBDs, including the canine heartworm D. immitis. Already in 2005, and using a climate model, a study predicted that D. immitis would spread into previously unaffected areas in Europe [44]. Although the prevalence has been declining throughout the world from 10.8% in 1965–1998 to 7.6% after 2016 [45], D. immitis is currently spreading with autochthonous cases in Central, Northeastern Europe and Siberia [46].
On the other hand, predictive models using future climate scenarios also indicate that parasite species with a limited capacity to adapt to novel environmental conditions will most likely show reduction in their distribution range. Amblyomma ticks, for instance, are expected to have a range reduction in Brazil due to climate warming and limitation of suitable conditions for their survival [47]. Complementary, a net decrease of areas suitable for malaria vectors has been anticipated in Africa [48]. In both cases, such contraction and expansion scenarios should not be strictly interpreted as a reduction of VBDs transmission risk but as a shifting of parasite boundaries, either at regional or global level.
5.2. Increased pet travel
A strengthening of the human-animal bond materialises into an increase in pets joining their owners during holidays abroad [49]. Indeed, with a valid passport, microchipping and rabies vaccination, the application of the Pet Travel Scheme (PETS) allows dogs, cats and ferrets to travel within the27 EU countries, Norway, Switzerland, or other non-European territories of the EU [49]. While on the one hand this legislative flexibility is simplifying the mobility of animals between countries, it may favour the introduction of parasites into previously non-endemic areas [50,56]. Indeed, with a few exceptions, such as the required treatment of dogs against E. multilocularis before entry into certified free countries, other parasites like Leishmania or ticks are prone to introduction in new areas and are not regulated or routinely monitored [50,51]. Infection with tick borne pathogens has also increased substantially in recent decades due in part to the rise in dog and cat travel [51]. In the UK, in 2005–2016, R. sanguineus ticks were the most common species found on animals with a history of travel mainly to Southern Europe, the USA, and the United Arab Emirates [51]. The importation of ticks to non-endemic areas due to pet travel has considerable importance for public health, as exemplified by R. sanguineus, atick vector and/or potential reservoir of numerous zoonotic pathogens, including Rickettsia species (Rickettsia conorii complex) (Fig. 1) [51]. The enactment of infectious diseases in previously non-endemic areas relies on the complex interaction between abiotic and biotic factors. Accordingly, pet owners need to be informed about the epidemiological situation in the area to be visited and apply adequate preventive treatments based on a risk-benefit assessment principle. Furthermore, continuous surveillance on CAs returning from abroad home should be implemented, including the search for infection with unusual parasites.
5.3. Changing societies
The importance of globalisation through its many facets cannot be denied in the modern era. Today, more than half of the world population lives in urban areas and this trend is expected to continue, with the urban population predicted to double its current size by 2050 [52]. Congested and overcrowded urban areas may provide a conducive environment for the dissemination of gastrointestinal parasites and ectoparasites, whereas deforestation followed by land use change alter the development opportunities of arthropod vectors [53]. Infectious disease transmission is also a function of underlying vulnerabilities of modern society. Unsustainable land use, poverty, and political instability may have measurable consequences on the dynamics of certain parasitic diseases [54]. In veterinary medicine, as an example, the relocation of sheltered dogs and cats following adoptions mediated by animal charities has a direct impact on the distribution of pathogens that may find suitable conditions enabling their survival. Data from North America provide some key examples on this phenomenon. The prevalence of canine dirofilariosis in Colorado rose from 0.5% in 2013 to 0.84% in 2017, an increase of 67.5% probably following the introduction of dogs adopted from states with a higher heartworm prevalence [55]. In Europe, 38% of dogs imported or travelling back from Southern (Spain, Italy, Greece, Turkey, France, Malta, Portugal) to Central (Germany, Switzerland, Austria) Europe were serologically Leishmania positive [56]. Similarly, the relocation and homing of unowned cats, while reducing animal suffering and social problems, may be linked to a higher risk of zoonotic pathogens transmission, such as Bartonella spp. or Rickettsia spp. [57]. Therefore, higher surveillance, stricter legislation as well as informed awareness of veterinarians, animal welfare organisations and animal owners importing pets from abroad along with the periodic administration of effective parasiticide treatments and adherence to basic hygiene principles are key to prevent parasites to establishing in previously unaffected areas.
6. Pet parasite control
6.1. Parasiticides
The use of parasiticides is today's cornerstone to mitigate and control the animal and human health threats posed by CAPs. Current endoparasiticides comprise benzimidazoles (e.g., fenbendazole), imidazothiazoles (i.e., levamisole), octadepsipeptides (e.g., emodepside), tetrahydropyrimidines (e.g., pyrantel) and pyrazinoisoquinolines (e.g., praziquantel). Ectoparasiticides include pyrethrins and synthetic pyrethroids, organophosphates, carbamates, formamidines, pyrazoles, neonicotinoids, spinosyns, semicarbazones, isoxazolines, and insect growth regulators. Endectocides include the macrocyclic lactones ivermectin, moxidectin, eprinomectin and milbemycin oxime [4]. The release of new parasiticides is driven by customer demands and aims to introduce formulations providing long-lasting activity, acting against both ecto- and endoparasites and with a user friendly administration route. Recent studies have also shown that anthelmintic resistance in pets should not be ignored and needs to be monitored [58,59].
Antiparasitic resistance is the genetic ability of parasites to survive treatment with an antiparasitic drug that was generally effective against those parasites in the past [59]. After an animal is treated with an antiparasitic active ingredient, the susceptible parasites are eliminated, and the resistant individuals survive to pass on resistance genes to their offspring. Increasing levels of drug resistance are documented for some human parasites, like Leishmania and soil-transmitted nematodes, as well as for livestock parasites like helminths [60] and Trypanosoma. There is no known important transfer of resistance genes from animal to human parasites or vice-versa.
In CAs, antiparasitic resistance is a known problem for heartworm D. immitis prevention in dogs, with macrocyclic lactone preventives showing progressively reduced efficacies since 2005 [61]. For other parasite species of dogs and cats, resistance is uncommon. However, recently the spread of multiple anthelmintic resistance in Ancylostoma caninum and single cases of Dipylidium caninum resistance against praziquantel have been shown in the US [59,62,63]. The current understanding of resistance in CAPs must be followed up with the aim to limit further onset and spread. Moreover, lessons can be learned from anthelmintic resistance research in livestock and CAs for the control of soil-transmitted helminths in humans according a One Health approach [63].
6.2. Diagnostics
Diagnostics provide the foundation for parasite surveillance, helping track prevalence and parasite displacement across regions, while also enabling to detect infections or evaluate the efficacy of treatments in individual animals. Although microscopy remains the cornerstone of parasitological diagnostics [64], increased availability of point-of-care tests and molecular assays in the modern era allow for more rapid and accurate diagnosis and increased sensitivity in the identification of parasitic infections. Assays detecting antigens, antibodies or DNA of parasites are now routinely used for the diagnostics of multiple pathogens, including D. immitis, A. vasorum, tick-borne pathogens and Giardia [65], although not easily accessible in low-income countries [66].
Computer-based algorithms to identify parasites in microscopy-based faecal examinations have recently been developed and demonstrate a similar qualitative performance to the parasitologists' eye with conventional faecal flotation techniques [67,68]. Smartphone apps and electronic maps with data on parasite distribution are used not only by research groups but also by end users [69,70]. Risk maps developed by scientific councils or private diagnostic companies serve as a general representation of the parasite activity and risk for given areas. Data are designed to show the proportion of pets positive for a given infection, allowing to better estimate the exposure to a parasite threat and design better CAP prevention advices during travel. Similarly, citizen science apps and project such as Tekenscanner in The Netherlands [70], or ZanzaMapp in Italy allow to record, identify ticks and test them for pathogens [71] and to assess citizens' perception of mosquito abundance and nuisance to feed spatial analyses for monitoring and control by local administrations [71]. Such tools will increasingly support veterinarians for faster and more reliable diagnosis, and scientists to investigate new emerging parasites and epidemiological trends.
6.3. Best practices and veterinary advice
Best practice regarding the prevention and treatment of parasites in pets are promoted through guidelines released by specific scientific councils which operate in different regions of the world, such as the European Scientific Counsel Companion Animal Parasites (ESCCAP) [72], the Companion Animal Parasite Council (CAPC) in the USA [73], and the Tropical Council for Companion Animal Parasites (TroCCAP) [74]. The guidelines, derived from solid investigations and applied field research, are continually revised based on the evolution of parasite epidemiology and applicable legislation. All together, these procedures support veterinary professionals and animal owners, with a relevant/proven impact on the control of canine and feline parasites [75].
Considering that both management plans and treatment regimens depend on local legislation, availability of registered medicinal products at national level and the epidemiological situation of given parasitic infections, lifelong control of common parasites of dogs and cats is generally encouraged by parasite councils as only a few infections are strictly age-related [[72], [73], [74]]. The principle to administer an effective parasiticide applies to all pets, being refined based on individual assessments and calibrated risk-based approach, i.e. the lifestyle and behaviour as well as the geographical location of the animal [[72], [73], [74]]. For instance, owned dogs with no outdoors contact with other animals, parks, sandpits, playgrounds, or gastropods are allocated to a low-risk category for round- and tapeworms, thus reducing the diagnostic and treatment practice to a periodicity of 1–2 times a year [72] (Fig. 2).
Fig. 2.
Best veterinary practice regarding prevention and treatment in companion animals. Information is based on Guidelines developed by ESCCAP, with a focus on the general recommendations and on management of endo- and ectoparasites.
Despite the existence of these recommendations, surveys on the perception of these messages by owners often outline a non-compliance with the proposed indications [76,77]. An European study showed that only 2% of dogs and no cats were being dewormed 4 times a year, despite 93% of dogs and 54% of cats falling into the highest risk group requiring at least this frequency [76]. In addition, animal owners may not fully perceive the risk posed by some potentially life-threatening parasites (such as D. immitis or A. vasorum in dogs), which together with the zoonotic Toxocara sp. and Echinococcus sp. are considered the key pet parasites of major concern [76,77]. The owner choice of a parasiticide is mainly driven by the selling price of the product, spectrum of action and treatment regime. Preference for a spot on, tablet or collar also plays a role as the mode of application can affect the confidence and willingness of an owner to apply it [76,77]. The understanding of pet owners towards parasite risks may also vary based on sociodemographic factors, as demonstrated in Australia where female respondents scored greater that males; or according to duration of animal ownership, which is generally positively linked to an improved appreciation about zoonoses [78]. Regardless of stats and figures, an increased frequency in visiting veterinary clinics is positively associated with likelihood of owners performing antiparasitic treatments, proper faecal disposal, and cooking meat before feeding to animals [78]. Engagement of pet owners with veterinary clinics is also beneficial in reinforcing the One Health role of scientific councils and veterinary professionals in the era of the information technology revolution.
7. Concluding remarks
Looking at CAPs through a One Health lens allowed to identify the existence of numerous unmet needs associated to their control. Current trends suggest that multimodal approaches for a responsible control of CAPs deserve dedicated attention. This should prompt end-users, industry, and governmental bodies to prioritise the discussion of action plans for integrated control tools across the veterinary, medical and environmental disciplines. In particular:
-
(1)
Climate change and globalisation are altering the health and welfare conditions of pet animals and their owners. There is a need for improving surveillance, prediction and awareness systems supported by insights from basic research on how global changes are altering parasite epidemiology and transmission between pets and humans.
-
(2)
There are hardly any quantitative indicators assessing the impact of CAP control to the benefit of human, animal and environmental health. We need more cost-benefit and benefit-risk assessments at the local and societal level.
-
(3)
Recommendations regarding responsible pet ownership, including importance of hygienic practices, are key for mitigating (re-)emerging parasite infections. We need better knowledge of pet owner perceptions to develop adapted communications strategies.
-
(4)
Future control approaches should adopt new possibilities in diagnostics and risk prediction to prepare for the changing challenges in pet parasite control.
Funding
This study received financial support from HealthforAnimals.
Declaration of competing interest
Alessio Giannelli is employed at Inovet (Belgium). All other authors declare that they have no competing interests.
Acknowledgements
Authors thank Alexander Rinkus for proofreading the manuscript.
Data availability
No data was used for the research described in the article.
References
- 1.Krouzecky C., Aden J., Hametner K., Klaps A., Kovacovsky Z., Stetina B.U. Fantastic beasts and why it is necessary to understand our relationship-animal companionship under challenging circumstances using the example of long-Covid. Animals (Basel) 2022;12:1892. doi: 10.3390/ani12151892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GFK Man’s Best Friend: Global Pet Ownership and Feeding Trends. 2016. https://www.gfk.com/insights/mans-best-friend-global-pet-ownership-and-feeding-trends
- 3.Health For Animals Global Trends in the Animal Health Sector. 2022. https://www.healthforanimals.org/reports/global-trends-in-the-animal-health-sector/
- 4.Selzer P.M., Epe C. Antiparasitics in animal health: quo Vadis? Trends Parasitol. 2021;37:77–89. doi: 10.1016/j.pt.2020.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Animal Health Europe Key Figures. 2022. https://animalhealtheurope.eu/about-us/annual-reports/2022-2/key-figures-2022 (accessed 01 October 2022)
- 6.Takashima G.K., Day M.J. Setting the one health agenda and the human-companion animal bond. Int. J. Environ. Res. Public Health. 2014;11:11110–11120. doi: 10.3390/ijerph111111110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Overgaauw P.A.M., Vinke C.M., Hagen M.A.E.V., Lipman L.J.A. A one health perspective on the human-companion animal relationship with emphasis on zoonotic aspects. Int. J. Environ. Res. Public Health. 2020;17:3789. doi: 10.3390/ijerph17113789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taylor M.A., Coop R.L., Wall R.L. In: Veterinary Parasitology. 4th edition. Taylor M.A., Coop R.L., Wall R.L., editors. Wiley-Blackwell; Oxford: 2015. Parasites of dogs and cats; pp. 599–677. [Google Scholar]
- 9.Deplazes P., Eckert J., Mathis A., von Samson-Himmelstjerna G., Zahner H. Wageningen Academic Publishers; Wegeningen: 2016. Parasitology in Veterinary Medicine. [Google Scholar]
- 10.Bowman D., Fogarty E., Barr S.C. Teton NewMedia; New York: 2002. Parasitology Diagnosis and Treament of Common Parasitisms in Dogs and Cats. [Google Scholar]
- 11.Otranto D. Arthropod-borne pathogens of dogs and cats: from pathways and times of transmission to disease control. Vet. Parasitol. 2018;251:68–77. doi: 10.1016/j.vetpar.2017.12.021. [DOI] [PubMed] [Google Scholar]
- 12.Baneth G., Solano-Gallego L. Leishmaniasis. Vet. Clin. North Am. Small Anim. Pract. 2022;52:1359–1375. doi: 10.1016/j.cvsm.2022.06.012. [DOI] [PubMed] [Google Scholar]
- 13.Iannino F., Sulli N., Maitino A., Pascucci I., Pampiglione G., Salucci S. Fleas of dog and cat: species, biology and flea-borne diseases. Vet. Ital. 2017;53:277–288. doi: 10.12834/VetIt.109.303.3. [DOI] [PubMed] [Google Scholar]
- 14.Huertas-López A., Álvarez-García G., Sánchez-Sánchez R., Cantos-Barreda A., Ibáñez-López F.J., Martínez-Subiela S., Cerón J.J., Martínez-Carrasco C. A systematic review and meta-analysis of the serological diagnosis of toxoplasma gondii infection highlight the lack of a one health integrative research. Res. Vet. Sci. 2023;155:137–149. doi: 10.1016/j.rvsc.2023.01.005. [DOI] [PubMed] [Google Scholar]
- 15.Carlin E.P., Tyungu D.L. Toxocara: protecting pets and improving the lives of people. Adv. Parasitol. 2020;109:3–16. doi: 10.1016/bs.apar.2020.01.001. [DOI] [PubMed] [Google Scholar]
- 16.Carmena D., Cardona G.A. Echinococcosis in wild carnivorous species: epidemiology, genotypic diversity, and implications for veterinary public health. Vet. Parasitol. 2020;2014:69–94. doi: 10.1016/j.vetpar.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 17.Couret J., Schofield S., Narasimhan S. The environment, the tick, and the pathogen - it is an ensemble. Front. Cell. Infect. Microbiol. 2022;12:1049646. doi: 10.3389/fcimb.2022.1049646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Colella V., Bradbury R., Traub R. Ancylostoma ceylanicum. Trends Parasitol. 2021;37:844–845. doi: 10.1016/j.pt.2021.04.013. [DOI] [PubMed] [Google Scholar]
- 19.Dantas-Torres F., Ketzis J., Pérez Tort G., Mihalca A.D., Baneth G., Otranto D., Watanabe M., Linh B.K., Inpankaew T., Borrás P., Arumugam S., Penzhorn B.L., Ybañez A.P., Irwin P., Traub R.J. Heartworm adulticide treatment: a tropical perspective. Parasit. Vectors. 2023;16:48. doi: 10.1186/s13071-023-05690-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kachani M., Heath D. Dog population management for the control of human echinococcosis. Acta Trop. 2014;139:99–108. doi: 10.1016/j.actatropica.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 21.Thompson R.C. Parasite zoonoses and wildlife: one health, spillover and human activity. Int. J. Parasitol. 2013;43:1079–1088. doi: 10.1016/j.ijpara.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deplazes P., van Knapen F., Schweiger A., Overgaauw P.A. Role of pet dogs and cats in the transmission of helminthic zoonoses in Europe, with a focus on echinococcosis and toxocarosis. Vet. Parasitol. 2011;182:41–53. doi: 10.1016/j.vetpar.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 23.Tsao J.I., Hamer S.A., Han S., Sidge J.L., Hickling G.J. The contribution of wildlife hosts to the rise of ticks and tick-borne diseases in North America. J. Med. Entomol. 2021;58:1565–1587. doi: 10.1093/jme/tjab047. [DOI] [PubMed] [Google Scholar]
- 24.Denk D., De Neck S., Khaliq S., Stidworthy M.F. Toxoplasmosis in zoo animals: a retrospective pathology review of 126 cases. Animals (Basel). 2022;12:619. doi: 10.3390/ani12050619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dubey J.P., Murata F.H.A., Cerqueira-Cézar C.K., Kwok O.C.H., Grigg M.E. Recent epidemiologic and clinical importance of toxoplasma gondii infections in marine mammals: 2009-2020. Vet. Parasitol. 2020;288 doi: 10.1016/j.vetpar.2020.109296. [DOI] [PubMed] [Google Scholar]
- 26.Di Bari C., Venkateswaran N., Fastl C., Gabriël S., Grace D., Havelaar A.H., Huntington B., Patterson G.T., Rushton J., Speybroeck N., Torgerson P., Pigott D.M., Devleesschauwer B. The global burden of neglected zoonotic diseases: current state of evidence. One Health. 2023;17 doi: 10.1016/j.onehlt.2023.100595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baneth G., Thamsborg S.M., Otranto D., Guillot J., Blaga R., Deplazes P., Solano-Gallego L. Major parasitic Zoonoses associated with dogs and cats in Europe. J. Comp. Pathol. 2016;155:54–74. doi: 10.1016/j.jcpa.2015.10.179. [DOI] [PubMed] [Google Scholar]
- 28.Maia C., Conceição C., Pereira A., Rocha R., Ortuño M., Muñoz C., Jumakanova Z., Pérez-Cutillas P., Özbel Y., Töz S., Baneth G., Monge-Maillo B., Gasimov E., Van der Stede Y., Torres G., Gossner C.M., Berriatua E. The estimated distribution of autochthonous leishmaniasis by Leishmania infantum in Europe in 2005-2020. PLoS Negl. Trop. Dis. 2023;17 doi: 10.1371/journal.pntd.0011497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dantas-Torres F., Miró G., Baneth G., Bourdeau P., Breitschwerdt E., Capelli G., Cardoso L., Day M.J., Dobler G., Ferrer L., Irwin P., Jongejan F., Kempf V.A.J., Kohn B., Lappin M., Little S., Madder M., Maggi R., Maia C., Marcondes M., Naucke T., Oliva G., Pennisi M.G., Penzhorn B.L., Peregrine A., Pfeffer M., Roura X., Sainz A., Shin S., Solano-Gallego L., Straubinger R.K., Tasker S., Traub R., Wright I., Bowman D.D., Gradoni L., Otranto D. Canine Leishmaniasis control in the context of one health. Emerg. Infect. Dis. 2019;25:1–4. doi: 10.3201/eid2512.190164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martín-Sánchez J., Rodríguez-Granger J., Morillas-Márquez F., Merino-Espinosa G., Sampedro A., Aliaga L., Corpas-López V., Tercedor-Sánchez J., Aneiros-Fernández J., Acedo-Sánchez C., Porcel-Rodríguez L., Díaz-Sáez V. Leishmaniasis due to Leishmania infantum: integration of human, animal and environmental data through a one health approach. Transbound. Emerg. Dis. 2020;67:2423–2434. doi: 10.1111/tbed.13580. [DOI] [PubMed] [Google Scholar]
- 31.Woolsey I.D., Miller A.L. Echinococcus granulosus sensu lato and Echinococcus multilocularis: a review. Res. Vet. Sci. 2021;135:517–522. doi: 10.1016/j.rvsc.2020.11.010. [DOI] [PubMed] [Google Scholar]
- 32.Budke C.M., Casulli A., Kern P., Vuitton D.A. Cystic and alveolar echinococcosis: successes and continuing challenges. PLoS Negl. Trop. Dis. 2017;11 doi: 10.1371/journal.pntd.0005477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Romig T., Deplazes P., Jenkins D., Giraudoux P., Massolo A., Craig P.S., Wassermann M., Takahashi K., de la Rue M. Ecology and life cycle patterns of Echinococcus species. Adv. Parasitol. 2017;95:213–314. doi: 10.1016/bs.apar.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 34.Abdullah S., Helps C., Tasker S., Newbury H., Wall R. Pathogens in fleas collected from cats and dogs: distribution and prevalence in the UK. Parasit. Vectors. 2019;12:71. doi: 10.1186/s13071-019-3326-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bitam I., Dittmar K., Parola P., Whiting M.F., Raoult D. Fleas and flea-borne diseases. Int. J. Infect. Dis. 2010;14:e667–e676. doi: 10.1016/j.ijid.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 36.Morgan E.R. Risks from emerging parasitic zoonoses in companion animals. Comp. Animal. 2016;21 doi: 10.12968/coan.2016.21.4.218. [DOI] [Google Scholar]
- 37.Charlier J., Barkema H.W., Becher P., De Benedictis P., Hansson I., Hennig-Pauka I., La Ragione R., Larsen L.E., Madoroba E., Maes D., Marín C.M., Mutinelli F., Nisbet A.J., Podgórska K., Vercruysse J., Vitale F., Williams D.J.L., Zadoks R.N. Disease control tools to secure animal and public health in a densely populated world. Lancet Planet Health. 2022;6:e812–e824. doi: 10.1016/S2542-5196(22)00147-4. [DOI] [PubMed] [Google Scholar]
- 38.Short E.E., Caminade C., Thomas B.N. Climate change contribution to the emergence or re-emergence of parasitic diseases. Infect. Dis. (Auckl). 2017;10 doi: 10.1177/1178633617732296. 1178633617732296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pozio E. How globalization and climate change could affect foodborne parasites. Exp. Parasitol. 2020;208 doi: 10.1016/j.exppara.2019.107807. [DOI] [PubMed] [Google Scholar]
- 40.Mordecai E.A., Caldwell J.M., Grossman M.K., Lippi C.A., Johnson L.R., Rohr M. Neira M.J.R., Ryan S.J., Savage V., Shocket M.S., Sippy R., Ibarra A.M. Stewart, Thomas M.B., Villena O. Thermal biology of mosquito-borne disease. Ecol. Lett. 2019;22:1690–1708. doi: 10.1111/ele.13335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Semenza J.C., Rocklöv J., Ebi K.L. Climate change and cascading risks from infectious disease. Infect. Dis. Ther. 2022;11:1371–1390. doi: 10.1007/s40121-022-00647-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Protopopova A., Ly L.H., Eagan B.H., Brown K.M. Climate change and companion animals: identifying links and opportunities for mitigation and adaptation strategies. Integr. Comp. Biol. 2021;61:166–181. doi: 10.1093/icb/icab025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.European Centre for Disease Prevention and Control, Mosquito Maps. 2023 https://www.ecdc.europa.eu/en/disease-vectors/surveillance-and-disease-data/mosquito-maps (accessed 15 August 2022).
- 44.Genchi C., Rinaldi L., Mortarino M., Genchi M., Cringoli G. Climate and Dirofilaria infection in Europe. Vet. Parasitol. 2009;163:286–292. doi: 10.1016/j.vetpar.2009.03.026. [DOI] [PubMed] [Google Scholar]
- 45.Anvari D., Narouei E., Daryani A., Sarvi S., Moosazadeh M., Ziaei Hezarjaribi H., Narouei M.R., Gholami S. The global status of Dirofilaria immitis in dogs: a systematic review and meta-analysis based on published articles. Res. Vet. Sci. 2020;131:104–116. doi: 10.1016/j.rvsc.2020.04.002. [DOI] [PubMed] [Google Scholar]
- 46.Genchi C., Kramer L.H. The prevalence of Dirofilaria immitis and D. Repens in the Old World. Vet. Parasitol. 2020;280 doi: 10.1016/j.vetpar.2019.108995. [DOI] [PubMed] [Google Scholar]
- 47.Oliveira S.V., Romero-Alvarez D., Martins T.F., Santos J.P.D., Labruna M.B., Gazeta G.S., Escobar L.E., Gurgel-Gonçalves R. Amblyomma ticks and future climate: range contraction due to climate warming. Acta Trop. 2017;176:340–348. doi: 10.1016/j.actatropica.2017.07.033. [DOI] [PubMed] [Google Scholar]
- 48.Ryan S.J., McNally A., Johnson L.R., Mordecai E.A., Ben-Horin T., Paaijmans K., Lafferty K.D. Mapping physiological suitability limits for malaria in Africa under climate change. Vect. Borne Zoonot. Dis. 2015;15:718–725. doi: 10.1089/vbz.2015.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Loeb J. New pet travel rules ‘a good thing’ for cats. Vet. Rec. 2021;188:405. doi: 10.1002/vetr.591. [DOI] [PubMed] [Google Scholar]
- 50.Wright I., Jongejan F., Marcondes M., Peregrine A., Baneth G., Bourdeau P., Bowman D.D., Breitschwerdt E.B., Capelli G., Cardoso L., Dantas-Torres F., Day M.J., Dobler G., Ferrer L., Gradoni L., Irwin P., Kempf V.A.J., Kohn B., Krämer F., Lappin M., Madder M., Maggi R.G., Maia C., Miró G., Naucke T., Oliva G., Otranto D., Pennisi M.G., Penzhorn B.L., Pfeffer M., Roura X., Sainz A., Shin S., Solano-Gallego L., Straubinger R.K., Tasker S., Traub R., Little S. Parasites and vector-borne diseases disseminated by rehomed dogs. Parasit. Vectors. 2020;13:546. doi: 10.1186/s13071-020-04407-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Buczek A., Buczek W. Importation of ticks on companion animals and the risk of spread of tick-borne diseases to non-endemic regions in Europe. Animals (Basel). 2020;11:6. doi: 10.3390/ani11010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gu D., Andreev K., Dupre M.E. Major trends in population growth around the world. China CDC Wkly. 2021;3:604–613. doi: 10.46234/ccdcw2021.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dantas-Torres F. Climate change, biodiversity, ticks and tick-borne diseases: the butterfly effect. Int. J. Parasitol. Parasit. Wildl. 2015;4:452–461. doi: 10.1016/j.ijppaw.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hotez P. Communicating science and protecting scientists in a time of political instability. Trends Mol. Med. 2022;28:173–175. doi: 10.1016/j.molmed.2022.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Drake J., Parrish R.S. Dog importation and changes in heartworm prevalence in Colorado 2013-2017. Parasit. Vectors. 2019;12:207. doi: 10.1186/s13071-019-3473-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mettler M., Grimm F., Naucke T.J., Maasjost C., Deplazes P. Canine leishmaniosis in Central Europe: retrospective survey and serological study of imported and travelling dogs. Berl. Munch. Tierarztl. Wochenschr. 2005;118:37–44. [PubMed] [Google Scholar]
- 57.Maggi R.G., Halls V., Krämer F., Lappin M., Pennisi M.G., Peregrine A.S., Roura X., Schunack B., Scorza V., Tasker S., Baneth G., Bourdeau P., Bowman D.D., Breitschwerdt E.B., Capelli G., Cardoso L., Dantas-Torres F., Dobler G., Ferrer L., Gradoni L., Irwin P., Jongejan F., Kempf V.A.J., Kohn B., Little S., Madder M., Maia C., Marcondes M., Miró G., Naucke T., Oliva G., Otranto D., Penzhorn B.L., Pfeffer M., Sainz A., Shin S., Solano-Gallego L., Straubinger R.K., Traub R., Wright I. Vector-borne and other pathogens of potential relevance disseminated by relocated cats. Parasit. Vectors. 2022;15:415. doi: 10.1186/s13071-022-05553-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Noack S., Harrington J., Carithers D.S., Kaminsky R., Selzer P.M. Heartworm disease - overview, intervention, and industry perspective. Int. J. Parasitol. Drugs Drug Resist. 2021;16:65–89. doi: 10.1016/j.ijpddr.2021.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.von Samson-Himmelstjerna G., Thompson R.A., Krücken J., Grant W., Bowman D.D., Schnyder M., Deplazes P. Spread of anthelmintic resistance in intestinal helminths of dogs and cats is currently less pronounced than in ruminants and horses - yet it is of major concern. Int. J. Parasitol. Drugs Drug Resist. 2021;17:36–45. doi: 10.1016/j.ijpddr.2021.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Charlier J., Bartley D.J., Sotiraki S., Martinez-Valladares M., Claerebout E., von Samson-Himmelstjerna G., Thamsborg S.M., Hoste H., Morgan E.R., Rinaldi L. Anthelmintic resistance in ruminants: challenges and solutions. Adv. Parasitol. 2022;115:171–227. doi: 10.1016/bs.apar.2021.12.002. [DOI] [PubMed] [Google Scholar]
- 61.Prichard R.K. Macrocyclic lactone resistance in Dirofilaria immitis: risks for prevention of heartworm disease. Int. J. Parasitol. 2021;51:1121–1132. doi: 10.1016/j.ijpara.2021.08.006. [DOI] [PubMed] [Google Scholar]
- 62.Marsh A.E., Lakritz J. Reflecting on the past and fast forwarding to present day anthelmintic resistant Ancylostoma caninum-a critical issue we neglected to forecast. Int. J. Parasitol. Drugs Drug Resist. 2023;22:36–43. doi: 10.1016/j.ijpddr.2023.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Venkatesan A., Jimenez Castro P.D., Morosetti A., Horvath H., Chen R., Redman E., Dunn K., Collins J.B., Fraser J.S., Andersen E.C., Kaplan R.M., Gilleard J.S. Molecular evidence of widespread benzimidazole drug resistance in Ancylostoma caninum from domestic dogs throughout the USA and discovery of a novel β-tubulin benzimidazole resistance mutation. PLoS Pathog. 2023;19 doi: 10.1371/journal.ppat.1011146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Khurana S., Singh S., Mewara A. Diagnostic techniques for soil-transmitted helminths - recent advances. Res. Rep. Trop. Med. 2021;12:181–196. doi: 10.2147/RRTM.S278140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Momčilović S., Cantacessi C., Arsić-Arsenijević V., Otranto D., Tasić-Otašević S. Rapid diagnosis of parasitic diseases: current scenario and future needs. Clin. Microbiol. Infect. 2019;25:290–309. doi: 10.1016/j.cmi.2018.04.028. [DOI] [PubMed] [Google Scholar]
- 66.Lorusso V. Parasitology and one health-perspectives on Africa and beyond. Pathogens. 2021;10:1437. doi: 10.3390/pathogens10111437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nagamori Y., Sedlak R.H., DeRosa A., Pullins A., Cree T., Loenser M., Larson B.S., Smith R.B., Penn C., Goldstein R. Further evaluation and validation of the VETSCAN IMAGYST: in-clinic feline and canine fecal parasite detection system integrated with a deep learning algorithm. Parasit. Vectors. 2021;14:89. doi: 10.1186/s13071-021-04591-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rinaldi L., Krücken J., Martinez-Valladares M., Pepe P., Maurelli M.P., de Queiroz C., de Agüero V. Castilla Gómez, Wang T., Cringoli G., Charlier J., Gilleard J.S., von Samson-Himmelstjerna G. Advances in diagnosis of gastrointestinal nematodes in livestock and companion animals. Adv. Parasitol. 2022;118:85–176. doi: 10.1016/bs.apar.2022.07.002. [DOI] [PubMed] [Google Scholar]
- 69.Braks M., Schaffner F., Medlock J.M., Berriatua E., Balenghien T., Mihalca A.D., Hendrickx G., Marsboom C., Van Bortel W., Smallegange R.C., Sprong H., Gossner C.M., Czwienczek E., Dhollander S., Briët O., Wint W. VectorNet: putting vectors on the map. Front. Public Health. 2022;10 doi: 10.3389/fpubh.2022.809763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kooyman F.N.J., Zweerus H., Nijsse E.R., Jongejan F., Wagenaar J.A., Broens E.M. Monitoring of ticks and their pathogens from companion animals obtained by the “tekenscanner” application in the Netherlands. Parasitol. Res. 2022;121:1887–1893. doi: 10.1007/s00436-022-07518-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Caputo B., Manica M., Filipponi F., Blangiardo M., Cobre P., Delucchi L., De Marco C.M., Iesu L., Morano P., Petrella V., Salvemini M., Bianchi C., Torre A. Della. ZanzaMapp: a scalable citizen science tool to monitor perception of mosquito abundance and nuisance in Italy and beyond. Int. J. Environ. Res. Public Health. 2020;17:7872. doi: 10.3390/ijerph17217872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.European Scientific Counsel Companion Animal Parasites (ESCCAP) Guidelines. 2024. https://www.esccap.org/guidelines/ (accessed 15 September 2022)
- 73.Companion Animal Parasite Council (CAPC) Guidelines. 2024. https://capcvet.org/guidelines/ (accessed 15 September 2022)
- 74.Dantas-Torres F., Ketzis J., Mihalca A.D., Baneth G., Otranto D., Tort G.P., Watanabe M., Linh B.K., Inpankaew T., Jimenez Castro P.D., Borrás P., Arumugam S., Penzhorn B.L., Ybañez A.P., Irwin P., Traub R.J. TroCCAP recommendations for the diagnosis, prevention and treatment of parasitic infections in dogs and cats in the tropics. Vet. Parasitol. 2020;283 doi: 10.1016/j.vetpar.2020.109167. [DOI] [PubMed] [Google Scholar]
- 75.Vrhovec M.G., Alnassan A.A., Pantchev N., Bauer C. Is there any change in the prevalence of intestinal or cardiopulmonary parasite infections in companion animals (dogs and cats) in Germany between 2004-2006 and 2015-2017? An assessment of the impact of the first ESCCAP guidelines. Vet. Parasitol. 2022;312 doi: 10.1016/j.vetpar.2022.109836. [DOI] [PubMed] [Google Scholar]
- 76.McNamara J., Drake J., Wiseman S., Wright I. Survey of European pet owners quantifying endoparasitic infection risk and implications for deworming recommendations. Parasit. Vectors. 2018;11:571. doi: 10.1186/s13071-018-3149-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pennelegion C., Drake J., Wiseman S., Wright I. Survey of UK pet owners quantifying internal parasite infection risk and deworming recommendation implications. Parasit. Vectors. 2020;13:218. doi: 10.1186/s13071-020-04086-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bebrysz M., Wright A., Greaves M., Rathwell Deault D., Hopkins G., Gildea E., Aballéa S. How pet owners choose antiparasitic treatments for their dogs: a discrete choice experiment. Prev. Vet. Med. 2021;196 doi: 10.1016/j.prevetmed.2021.105493. [DOI] [PubMed] [Google Scholar]
- 79.Moretti N.S., Mortara R.A., Schenkman S. Trypanosoma cruzi. Trends Parasitol. 2020;36:404–405. doi: 10.1016/j.pt.2019.10.002. [DOI] [PubMed] [Google Scholar]
- 80.Dixon B.R. Giardia duodenalis in humans and animals - transmission and disease. Res. Vet. Sci. 2021;135:283–289. doi: 10.1016/j.rvsc.2020.09.034. [DOI] [PubMed] [Google Scholar]
- 81.Dubey J.P. 1st ed. CRC Press; 2019. Coccidiosis in Livestock, Poultry, Companion Animals, and Humans. [DOI] [Google Scholar]
- 82.Campero L.M., Basso W., Moré G., Fiorani F., Hecker Y.P., Echaide I., Cantón G.J., Cirone K.M., Campero C.M., Venturini M.C., Moore D.P. Neosporosis in Argentina: past, present and future perspectives. Vet. Parasit. Reg. Stud. Report. 2023;41 doi: 10.1016/j.vprsr.2023.100882. [DOI] [PubMed] [Google Scholar]
- 83.Chai J.Y., Jung B.K. General overview of the current status of human foodborne trematodiasis. Parasitology. 2022;149:1262–1285. doi: 10.1017/S0031182022000725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Blair D. Lung flukes of the genus Paragonimus: ancient and re-emerging pathogens. Parasitology. 2022;149:1286–1295. doi: 10.1017/S0031182022000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Capelli G., Genchi C., Baneth B., Bourdeau P., Brianti E., Cardoso L., Danesi P., Fuehrer H.P., Giannelli A., Ionică A.M., Maia C., Modrý D., Montarsi F., Krücken J., Papadopoulos E., Petrić D., Pfeffer M., Savić S., Otranto D., Poppert S., Silaghi C. Recent advances on Dirofilaria repens in dogs and humans in Europe. Parasit. Vectors. 2018;11:663. doi: 10.1186/s13071-018-3205-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cambra-Pellejà M., Gandasegui J., Balaña-Fouce R., Muñoz J., Martínez-Valladares M. Zoonotic implications of Onchocerca species on human health. Pathogens. 2020;9:761. doi: 10.3390/pathogens9090761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Otranto D., Mendoza-Roldan J.A., Dantas-Torres F. Thelazia callipaeda. Trends Parasitol. 2021;37:263–264. doi: 10.1016/j.pt.2020.04.013. [DOI] [PubMed] [Google Scholar]
- 88.Rousseau J., Castro A., Novo T., Maia C. Dipylidium caninum in the twenty-first century: epidemiological studies and reported cases in companion animals and humans. Parasit. Vectors. 2022;15:131. doi: 10.1186/s13071-022-05243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Morelli S., Diakou A., Colombo M., Di Cesare A., Barlaam A., Dimzas D., Traversa D. Cat respiratory nematodes: current knowledge, novel data and warranted studies on clinical features, treatment and control. Pathogens. 2021;10:454. doi: 10.3390/pathogens10040454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schnyder M., Bilbrough G., Hafner C., Schaper R. Angiostrongylus vasorum, “the French heartworm”: a serological survey in dogs from France introduced by a brief historical review. Parasitol. Res. 2017;116:31–40. doi: 10.1007/s00436-017-5489-8. [DOI] [PubMed] [Google Scholar]
- 91.Mendoza Roldan J.A., Otranto D. Zoonotic parasites associated with predation by dogs and cats. Parasit. Vectors. 2023;16:55. doi: 10.1186/s13071-023-05670-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Skotarczak B. The role of companion animals in the environmental circulation of tick-borne bacterial pathogens. Ann. Agric. Environ. Med. 2018;25:473–480. doi: 10.26444/aaem/93381. [DOI] [PubMed] [Google Scholar]
- 93.Baneth G. Tick-borne infections of animals and humans: a common ground. Int. J. Parasitol. 2014;44:591–596. doi: 10.1016/j.ijpara.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 94.Heylen D., Day M., Schunack B., Fourie J., Labuschange M., Johnson S., Githigia S.M., Akande F.A., Nzalawahe J.S., Tayebwa D.S., Aschenborn O., Marcondes M., Madder M. A community approach of pathogens and their arthropod vectors (ticks and fleas) in dogs of African sub-Sahara. Parasit. Vectors. 2021;14:576. doi: 10.1186/s13071-021-05014-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Madder M., Day M., Schunack B., Fourie J., Labuschange M., van der Westhuizen W., Johnson S., Githigia S.M., Akande F.A., Nzalawahe J.S., Tayebwa D.S., Aschenborn O., Marcondes M., Heylen D. A community approach for pathogens and their arthropod vectors (ticks and fleas) in cats of sub-Saharan Africa. Parasit. Vectors. 2022;15:321. doi: 10.1186/s13071-022-05436-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Maggi R.G., Krämer F. A review on the occurrence of companion vector-borne diseases in pet animals in Latin America. Parasit. Vectors. 2019;12:145. doi: 10.1186/s13071-019-3407-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Irwin P.J., Jefferies R. Arthropod-transmitted diseases of companion animals in Southeast Asia. Trends Parasitol. 2004;20:27–34. doi: 10.1016/j.pt.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 98.Benelli G., Caselli A., Di Giuseppe G., Canale A. Control of biting lice, Mallophaga - a review. Acta Trop. 2018;177:211–219. doi: 10.1016/j.actatropica.2017.05.031. [DOI] [PubMed] [Google Scholar]
- 99.Moroni B., Rossi L., Bernigaud C., Guillot J. Zoonotic episodes of Scabies: a global overview. Pathogens. 2022;11:213. doi: 10.3390/pathogens11020213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Deplazes P., Hegglin D., Gloor S., Romig T. Wilderness in the city: the urbanization of Echinococcus multilocularis. Trends Parasitol. 2004;20:77–84. doi: 10.1016/j.pt.2003.11.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.