Abstract
The past decade has seen enormous progress in cancer immunotherapy. Checkpoint inhibitors are a class of immunotherapy that act to recruit endogenous T cells of a patient’s immune system against cancer-associated peptide-MHC antigens. In this process, mutated antigenic peptides referred to as neoantigens often serve as the target on cancer cells that are recognized by the T cell receptor (TCR) on endogenous T cells. Another successful immunotherapy has involved adoptive T cell therapy, where therapeutic doses of T cells expressing a gene for an anti-cancer receptor are delivered to a patient. This approach has been used primarily against hematopoietic cancers using synthetic receptors called chimeric antigen receptors (CARs). CARs typically contain an antibody fragment (single-chain Fv, scFv) against a cancer cell surface antigen such as the B cell molecule CD19. While therapeutic CARs (and full antibodies) target antigens expressed on cell surfaces, TCRs can target a much larger array of intracellular proteins by binding to any cellular peptide associated with an MHC product. These cancer targets include self-peptides from aberrantly expressed/overexpressed proteins or neoantigens. In this review, we discuss the use of TCRs in adoptive T cell therapy and their target antigens. We focus on two properties that impact sensitivity, potency, and possible toxic cross-reactivity of TCR-mediated therapy: (1) the affinity of the TCR for the target antigen, and (2) the density of the target antigen. Finally, we provide a comprehensive listing of the current clinical trials that involve TCRs in adoptive T cell cancer therapy.
Keywords: T cell receptor, cancer, adoptive T cell therapy, clinical trials
I. INTRODUCTION
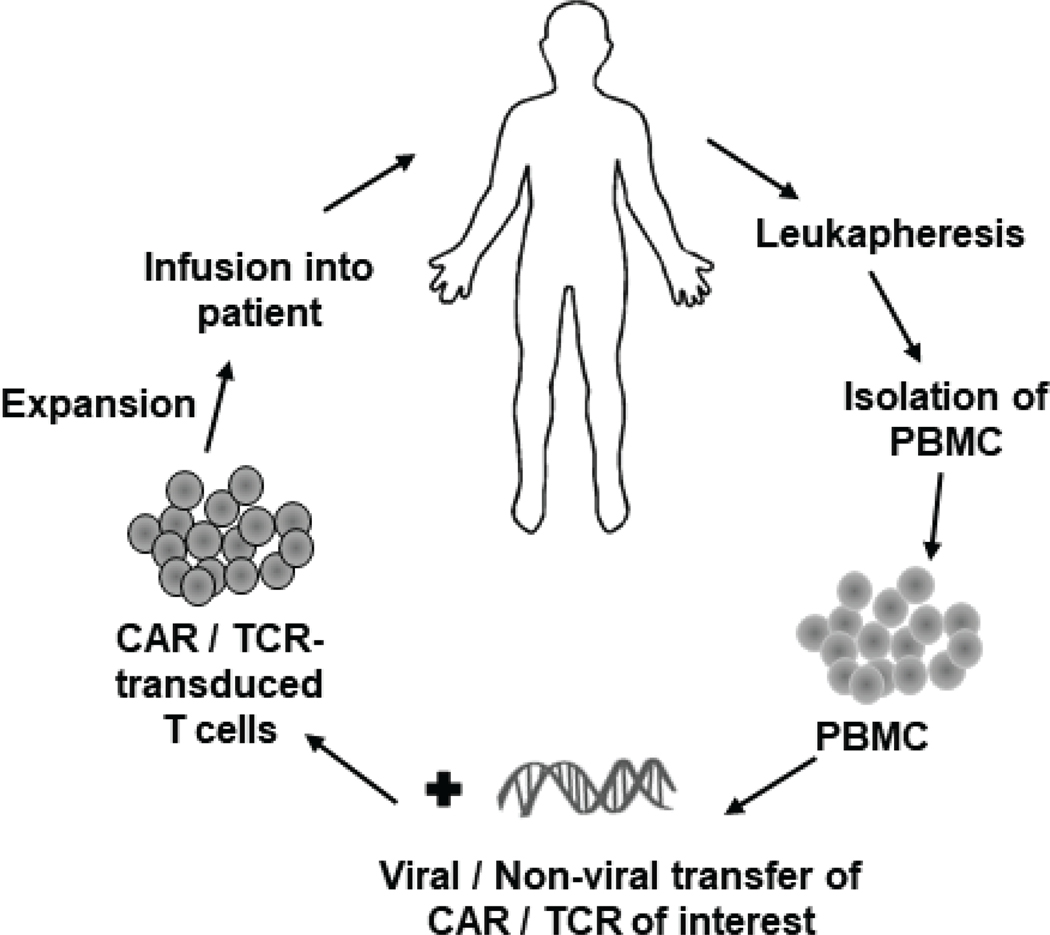
Cancer immunotherapy offers the potential for greater efficacy with fewer side effects than conventional chemotherapies. The hallmark of immunotherapies, in line with the era of precision medicine, is the targeting of cancer-associated antigens that are not expressed on normal cells. In some forms, ongoing immunotherapeutic approaches are extensions of therapies with monoclonal antibodies in which a cancer-associated cell surface antigen is targeted with an antibody (typically an IgG), leading to either direct effects on the cancer cell or recruitment of immune cells through Fc-mediated effects. For example, use of antibody fragments (single-chain Fv, scFv) as components of synthetic chimeric antigen receptors (CARs) are used to directly mediate T cell activity against cancer cells.1–3 This treatment requires personalized treatment: ex vivo expansion of peripheral blood T cells, followed by gene transfer of the CAR, and reinfusion of the T cell product; this process is termed adoptive T cell therapy (ACT) (Fig. 1).
FIG. 1:
Schematic of ACT using genetically modified (CAR- or TCR-) transduced T cells.
The class of immunotherapies known as checkpoint inhibitors operate quite distinctly by enhancing the activity of a patient’s own T cells against potentially many different antigens (often mutated peptides, called neoantigens), presented as complexes of a cancer peptide bound to a major histocompatibility complex (MHC) product, or pepMHC.4 While checkpoint inhibitors offer great promise in some cancer types, they have been less successful in cancers with fewer mutations and in cases where the tumor microenvironment is immunosuppressive (i.e., noninflamed, or “cold”).5 Ex vivo expansion of tumor-infiltrating lymphocytes (TILs) provide yet another alternative immunotherapy that attempts to harness the power of therapeutic doses of T cells and the potential for targeting multiple cancer antigens as pepMHC products.6,7 However, TILs are difficult to isolate from most patients, and their expansion can be time consuming.
Combining the potency of T cells with the vast array of possible cancer antigens as pepMHC complexes is a form of adoptive T cell therapy in which T cells are endowed with cancer-antigen specific T cell receptors (TCRs) (Fig. 1). In this review, we focus on ACT with such TCR-transduced T cells. By way of background, T cells express an αβ-TCR that recognizes peptides only when they are bound to a product of the MHC complex.8 The recognition of self-peptide/MHC antigens by T cells plays an important role during thymic development. TCRs mediate negative selection (deletion of the T cell) when they bind to a self-peptide/MHC with too high an affinity. This process is termed central tolerance and it is key to avoiding autoimmune reactivities.9 However, TCRs also must bind to self-peptide/MHC with some minimal affinity in order to drive positive selection, whereby T cells and the TCR are required to recognize peptides only when they are bound (“restricted”) by the MHC. This intricate process positions TCRs to drive T cell activity when a foreign peptide, as an MHC complex, binds with even a small increase in binding affinity. However, this narrow affinity window underlies the critical nature of identifying TCRs that are optimally active against a cancer antigen but not cross-reactive with self-peptides.
Nevertheless, because TCRs can recognize potentially any peptide antigen bound to MHC, they can target virtually any peptide arising from protein degradation inside the cancer cell. These antigens include peptides arising from viral proteins, mutated proteins, or aberrantly expressed self-proteins that are associated with cancer. Over 400 cancer-associated peptide antigens have been described in the cancer antigenic peptide database.10 Hence, TCR-mediated adoptive T cell therapy remains an attractive area but so far has not had significant success compared to its counterpart, CAR-mediated therapy. However, given their exquisite potency, a number of pharmaceutical companies and academic labs have TCR campaigns to determine the appropriate parameters for effective use of TCRs in therapeutic settings.
Although many cancer-associated antigens have been identified over the past several decades, selection of an antigen that is truly cancer-specific and that is not expressed on normal tissue remains a challenge in the field of TCR-mediated ACT.11,12 Although there is excitement in targeting cancer neoantigens as pepMHC because of their cancer specificity, these antigens typically differ from patient to patient, requiring personalized treatment strategies.6,13 On the other hand, cancer-associated self-antigens that are either aberrantly expressed or highly overexpressed in cancerous (compared to normal) tissue offer an advantage, as these are shared among patient populations. These include differentiation antigens (e.g., melanoma antigens: MART-1, gp100, tyrosinase), overexpressed antigens [e.g., Wilms’ tumor antigen (WT1)], and cancer testis antigens (e.g., NY-ESO-1, the MAGE family of antigens) that can be overexpressed in cancer, but are expressed normally in restricted and sometimes dispensable tissues. Antigens from these categories have been studied in TCR ACT clinical trials over the past 15 years (Table 1), and modest responses have been obtained with low-affinity TCRs (high micromolar affinities) used to target shared or overexpressed antigens. On the other hand, targeting antigens like NY-ESO-1 with an engineered, higher-affinity TCR (affinity in the low micromolar to high nanomolar range) appears to show more promise. However, targeting overexpressed antigens with higher-affinity TCRs has been challenging because of recognition of lower-density antigens on normal tissue or because of recognition of structure-related antigen(s). Overall, studies with TCRs have shown significant potential in cancer immunotherapy, but they have also taught important lessons about harnessing their power in an “optimal therapeutic window.” Here, we discuss the potential targets for TCRs in ACT and two parameters that must be considered in identifying this optimal window for ACT with TCRs: the density of the pepMHC antigen complex on cancer cells and the affinity of the TCR for the pepMHC antigen. We end with a review of ACT clinical trials to date that involve TCR transfer.
TABLE 1:
Selected clinical trials using TCR ACT for cancera
| Target, sequenceb | HLA | TCR; affinity-matured (yes/no/not specified); KD (if known) | Cancer(s)c | No. of patientsd | Trial phase | Responsee; trial status | Sponsor,f country | Trial ID, start date | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | p53:264–272; LLGRNSFEV | HLA-A*02:01 | p53 TCR; no74,75 | Metastatic melanoma and other metastatic cancers | 12 | II | No information | NCI, USA | NCT00393029, Oct 2006 |
| 2 | p53 | HLA-A*02:01 | p53 TCR; not specified | Progressive or recurrent metastatic cancer | 3 | II | Terminated (withdrawal of collaborators’ support) | NCI, USA | NCT00704938, June 2008 |
| 3 | MART-1, AAGIGILTV | HLA-A*02:01 | DMF4; no; KD = 170 μM63 | Metastatic melanoma | 15 | — | 13% OR (2/15) | NCI, USA | See ref. 62 |
| 4 | MART-1, AAGIGILTV | HLA-A*02:01 | DMF5; yes; KD = 40 μM63 | Metastatic melanoma | 20 | II | 30% OR (6/20); 55% uveitis (11/20); 50% hearing loss (10/20) | NCI, USA | NCI-07-C-0175, NCT00509288, June 200743 |
| 5 | MART-1, AAGIGILTV | HLA-A*02:01 | DMF5; yes; KD = 40 μM63 | Metastatic melanoma | 1 | I, II | Terminated (low accrual) | NCI, USA | NCT00924001, Aug 2007 |
| 6 | MART-1, AAGIGILTV | HLA-A*02:01 | DMF5; yes; KD = 40 μM63 | Metastatic melanoma | 4 | II | Terminated (low accrual) | NCI, USA | NCT00612222, Jan 2008 |
| 7 | MART-1 | HLA-A*02:01 | DMF5; yes; KD = 40 μM63 | Melanoma | 50 | II | Terminated (low enrollment) | NCI, USA | NCT00706992, June 2008 |
| 8 | MART-1 | HLA-A*02:01 | DMF5; yes; KD = 40 μM63 | Metastatic melanoma | 13 | II | 69% tumor regression (9/13); 38% progressive disease (5/13); 54% stable disease (7/13) | JCCC (UCLA), USA | NCT00910650, Oct 200976,77 |
| 9 | MART-1:26–35, EAAGIGILTV | HLA-A*02:01 | 1D3 HM CysTCR; no78 | Stage IV skin melanoma, eye melanoma | 12 | I, II | Active, not recruiting, no results posted | Netherlands Cancer Institute, Netherlands | NCT02654821, Mar 201279 |
| 10 | gp100, KTWGQYWQV | HLA-A*02:01 | gp100154 mouse TCR; no | Metastatic melanoma | 16 | II | 19% OR (3/16); 25% uveitis (4/16); 31% mild hearing loss (5/16); Terminated | NCI, USA | NCI-07-C-0174, NCT00509496, June 200743 |
| 11 | NY-ESO-1: 157–165, SLLMWITQC | HLA-A*02:01 | 1G4-α95:LY; yes; KD = 730 nM80 | Metastatic SCS, metastatic melanoma, metastatic SCS, metastatic melanoma | Metastatic SCS: 6; metastatic melanoma: 11; metastatic SCS: 18; metastatic melanoma: 20 | II | Metastatic SCS: 66% OR (4/6); metastatic melanoma: 16% PR (1/6); metastatic SCS: 45% OR (5/11); metastatic melanoma: 18% CR (2/11), 61% OR (11/18), 55% OR (11/20) | NCI, USA | NCT00670748, April 200864; NCT00670748, April 200865 |
| 12 | NY-ESO-1/LAGE-1: SLLMWITQC | HLA-A*02:01 | NY-ESO-1c259; yes; KD = 730 nM81 | Multiple Myeloma | 20 | I, II | 70% nCR/CR (14/20); 10% VGPR (2/20); 10% PR (2/20); active, not recruiting | GSK, USA | NCT01352286, May 201166 |
| 13 | NY-ESO-1 | HLA-A*02:01 | NY-ESO-1c259; yes; KD = 730 nM81 | Melanoma | 4 | I, II | Terminated (lack of enrollment) | Adaptimmune, USA | NCT01350401, June 2011 |
| 14 | NY-ESO-1/LAGE-1,SLLMWITQC | HLA-A*02:01, HLA-A*02:05, and/or HLA-A*02:06 | NY-ESO-1c259; yes; KD = 730 nM81 | Metastatic SCS | 12 | I | 50% OR (6/12); 42% PR (5/12); 8% CR (1/12); Recruiting | GSK, USA | NCT01343043, Sep 201282,83 |
| 15 | NY-ESO-1:157–165, SLLMWITQC | HLA-A*02:01 | NY-ESO-1 TCR; not specified | Malignant neoplasm | 22 (est.) | II | Recruiting | JCCC (UCLA), USA | NCT01697527, Nov 2012 |
| 16 | NY-ESO-1:157–165, SLLMWITQC | HLA A*02:01, HLA-A*02:05, and/or HLA-A*02:06 | NY-ESO-1c259; yes; KD = 730 nM81 | Ovarian | 6 | II | 0% response (not recruiting) | Adaptimmune, USA | NCT01567891, Jul 2013 |
| 17 | NY-ESO-1 and/or LAGE-1 | HLA-A*02:01 | NY-ESO-1c259; yes; KD = 730 nM81 | Multiple myeloma | 6 | I, II | Terminated (sponsor decision) | Adaptimmune, USA | NCT01892293, Oct 2013 |
| 18 | NY-ESO-1 | HLA-A*02:01 | NY-ESO-1 TCR with murine chains; no84 | Melanoma, meningioma, breast cancer, NSCLC, HCC | 43 | II | Recruiting | NCI, USA | NCT01967823, Oct 2013 |
| 19 | NY-ESO-1 | HLA-A*02:01 | NY-ESO-1 TCR; not specified | Metastatic melanoma | 2 | II | Terminated (low accrual) | NCI, USA | NCT02062359, Feb 2014 |
| 20 | NY-ESO-1:157–165 | HLA-A*02:01 | NY-ESO-1 TCR; not specified | Solid cancers | 4 | I | Terminated (low accrual) | JCCC (UCLA), USA | NCT02070406, Jul 2014 |
| 21 | NY-ESO-1 | HLA-A*02:01 or HLA-A*02:06 | NY-ESO-1 TCR (TBI-1301); not specified | Solid cancers | 9 | I | Active, not recruiting | Mie University, Japan | NCT02366546, Mar 2015 |
| 22 | NY-ESO-1 | HLA-A* 02 | NY-ESO-1 TCR; not specified | Solid cancers | 36 (est.) | 1 | Recruiting | Shenzhen Second People’s Hospital, China | NCT02457650, Apr 2015 |
| 23 | NY-ESO-1 | HLA-A*02:01, HLA-A*02:05, and/or HLA-A*02:06 | NY-ESO-1c259; yes; KD = 730 nM81 | Advanced (stage IIIb or IV) NSCLC | 10 (est.) | I | Recruiting | GSK, USA | NCT02588612, Feb 201685 |
| 24 | NY-ESO-1 | HLA-A*02:01 | NY-ESO-1 TCR with murine chains; no84 | Metastatic cancers | 10 (est.) | — | Recruiting | Albert Einstein College of Medicine, USA | NCT02774291, Aug 2016 |
| 25 | NY-ESO-1 | Not specified | NY-ESO-1 TCR; not specified | Advanced malignant solid tumors | 15 (est) | — | Recruitment status unknown | Fudan University, China | NCT03047811, Aug 2016 |
| 26 | NY-ESO-1 | HLA-A*02:01, HLA-A*02:06 | NY-ESO-1 TCR (TBI-1301); not specified | Solid cancers | 15 (est.) | I | Recruiting | University Health Network, Canada | NCT02869217, Sep 2016 |
| 27 | NY-ESO-1 | HLA-A*02:01, HLA-A*02:05, or HLA-A*02:06 | NY-ESO-1c259; yes; KD = 730 nM81 | Advanced MRCLS | 10 (est.) | II | Recruiting | GSK, USA | NCT02992743, Dec 2016 |
| 28 | NY-ESO-1:157–165 | HLA-A*02:01 | NY-ESO-1 TCR; not specified | Stage IV or locally advanced solid tumors | 12 (est.) | I | Recruiting | JCCC (UCLA), USA | NCT02775292, Jan 2017 |
| 29 | NY-ESO-1 | HLA-A2*02:01 | NY-ESO-1 TCR TAEST16001; yes | Advanced NSCLC | 20 (est.) | I | Recruiting | Guangzhou Institute of Respiratory Disease, China | NCT03029273, Mar 2017 |
| 30 | NY-ESO-1 | HLA-A2*02:01 | NY-ESO-1 TCR TAEST16001; yes | Solid tumors | 20 (est.) | I | Recruiting | Zhujiang Hospital, China | NCT03159585, Apr 2017 |
| 31 | NY-ESO-1 | HLA-A*02:01 | NY-ESO-1 TCR; not specified | Stage IV or locally advanced unresectable cancers | 12 (est.) | I | Recruiting | JCCC (UCLA), USA | NCT03240861, July 2017 |
| 32 | NY-ESO-1 /LAGE-1 | HLA-A*02:01, HLA-A*02:05, and/or HLA-A*02:06 | NY-ESO-1c259; yes; KD = 730 nM81 | Multiple myeloma | 24 (est) | II | Recruiting | GSK, USA | NCT03168438,Aug 2017 (Follow-up; see ref. 83) |
| 33 | NY-ESO-1:157–165, SLLMWITQC | HLA-A*02:01 and HLA-A*02:06 | NY-ESO-1 TCR; not specified | SCS | 8 (est.) | I, II | Recruiting | Takara Bio Inc., Japan | NCT03250325, Sep 2017 |
| 34 | NY-ESO-1 | HLA-A*02:01 | NY-ESO-1 TCR with murine chains; no84 | Recurrent or refractory ovarian, primary peritoneal, or fallopian tube carcinoma | 12 (est.) | I | Recruiting | Roswell Park Cancer Institute, USA | NCT03017131, Dec 2017 |
| 35 | NY-ESO-1 | Not specified | NY-ESO-1c259(GSK3377794); yes; KD = 730 nM81 | — | 200 (est.) | I | Recruiting | GSK, USA | NCT03391778, Apr 2018 (long-term follow-up of subjects exposed to NY-ESO-1c259 T cells) |
| 36 | NY-ESO-1 | HLA-A2*02:01 | NY-ESO-1 TCR TAEST; yes | Sarcoma | 20 (est.) | I | Recruiting | Sun Yat-sen University, China | NCT03462316, May 2018 |
| 37 | NY-ESO-1 | HLA-A*02:01 | NY-ESO-1 TCR (NYCE T cells); not specified | Multiple myeloma, melanoma, SCS, MRCLS | 18 (est.) | I | Recruiting | UPenn, USA | NCT03399448, Sep 2018 |
| 38 | NY-ESO-1/LAGE-la | HLA-A*02:01, HLA-A*02:05, and/or HLA-A*02:06 | NY-ESO-1c259(GSK3377794); yes; KD = 730 nM81 | NSCLC | 44 (est.) | II | Recruiting | MSD, USA | NCT03709706, Dec 2018 |
| 39 | NY-ESO-1 | HLA-A*02:01 and HLA-DP*04 | NY-ESO-1 TCR; not specified | Recurrent or refractory ovarian, fallopian tube, or primary peritoneal cancers | 15 (est.) | I | Recruiting | Roswell Park Cancer Institute, USA | NCT03691376, Jan 2019 |
| 40 | CEA, IMIGVLVGV | HLA-A*02:01 | PG13-CEA mouse TCR; yes86 | Metastatic CRC | 3 | I | 33% OR (1/3); 100% colitis (3/3) (CEA on normal colon mucosa); Terminated | NCI, USA | NCT00923806, Dec 200844 |
| 41 | TRAIL/DR4 | HLA-independent | 2G-1 TCR; no87 | Metastatic renal cancer | 5 | I, II | Terminated | NCI, USA | NCT00923390, Mar 2009 |
| 42 | MAGE-A3, KVAELVHFL, MAGE-A12, KMVELVHFL | HLA-A*02:01 | PG13-MAGE-A3 TCR9W11; yes88 | Metastatic melanoma, SCS, esophageal cancer | 9 | I, II | 11% CR (1/9); 44% PR (4/9); 22% neurotoxicity (2/9) (MAGEA12 in brain); Terminated | NCI, USA | NCT01273181, Dec 201055 |
| 43 | MAGE-A3, EVDPIGHLY | HLA-A*01 | a3a TCR; yes; KD = 2.3 μM57 | Melanoma, high-risk or relapsed myeloma | 2 | III, IV | 2/2 deaths (cardiovascular toxicity to titin peptide: ESDPIVAQY in heart); Terminated | UPenn, Adaptimmune, USA | Dec 2011 (see refs. 56,57) |
| 44 | MAGE-A3 | HLA-A*01 | MAGE-A3 TCR; not specified | Breast, cervical, renal, bladder cancers; melanoma | 3 | I, II | Terminated | NCI, USA | NCT02153905, Jul 2014 |
| 45 | MAGE-A3:248–258, QHFVQENYLEY | HLA-DP0401 | MAGE-A3-DP4 TCR; no89 | Cervical, renal, urothelial, breast cancers; melanoma | 107 (est.) | I, II | Recruiting | NCI, USA | NCT02111850, Feb 2014 |
| 46 | MAGE-A3 and/or MAGE-A6 | HLA-DPB1*04:01 | MAGE-A3/A6 TCR (KITE-718); not specified | Solid tumors | 75 (est.) | I | Recruiting | Kite (Gilead), USA | NCT03139370, May 201790 |
| 47 | MAGE-A4:143–151, NYKRCFPVI | HLA-A*24:02 | MAGE-A4 TCR (TBI-1201); no91 | Solid cancers | 12 (est.) | I | Persistence of TCR-transduced T cells in 50% of patients (5/10); Recruiting | Mie University, Japan | UMIN00000239, NCT02096614, Apr 201492 |
| 48 | MAGE-A4:230–239, GVYDGREHTV | HLA-A*02 | MAGE-A4c1032 TCR; yes; not specified | Urinary bladder, head and neck, ovarian, esophageal, gastric cancers; SCS, NSCLC, MRCLS, melanoma | 42 (est.) | I | Recruiting | Adaptimmune, USA, Canada | NCT03132922, May 2017 |
| 49 | MAGE A10:254–262, GLYDGMEHL | HLA-A*02:01 and/or HLA-A*02:06 | MAGEA10c796; yes; KD = 370 nM93 | Advanced NSCLC | 28 (est.) | I | Recruiting | Adaptimmune, USA, Canada, Spain, LTK | NCT02592577, Nov 201585 |
| 50 | MAGE-A10 | HLA-A*02:01 and/or HLA-A*02:06 | MAGEA10c796; yes; KD = 370 nM93 | Urothelial carcinoma, bladder urothelial carcinoma, head and neck cancer; melanoma | 22 (est.) | I | Recruiting | Adaptimmune, USA, Canada, Spain | NCT02989064, Oct 201694 |
| 51 | MAGE-A4:230–239 GVYDGREHTV, MAGE-A10:254–262, GLYDGMEHL | HLA-A*02, HLA-A*02:01, HLA-A*02:06 | MAGE-A4c1032; yes; not specified; MAGEA10c796; yes; KD = 370 nM93 | Solid and hematological malignancies | 300 (est.) | — | Enrolling by invitation | Adaptimmune, USA, Canada | NCT03391791, Feb 2018 (long-term follow-up of subjects exposed to genetically engineered TCRs) |
| 52 | WT1: 126–134 RMFPNAPYL | HLA-A*02:01 | Cys1 WT1 TCR; no95 | AML, CML | 7 | I, II | No results posted | Cell Medica Ltd., UK | NCT01621724, April 2012 |
| 53 | WT1: 126–134 RMFPNAPYL | HLA-A*02:01 | WT1 TCRc4; no11 | High-risk AML, MDS, CML | 45 | I, II | Active, not recruiting, no results posted | Fred Hutch, USA | NCT01640301, Jul 2012 |
| 54 | WT1:126–134 RMFPNAPYL | HLA-A*02:01 | WT1 TCRc4 (JTCR016: Celgene); no11 | Stage III–IV NSCLC or mesothelioma | 20 (est.) | I, II | Active, not recruiting, no results posted | Fred Hutch, USA | NCT02408016, May 2015 |
| 55 | WT1 | HLA-A*02:01 | WT1 TCR (CMD-602); not specified | MDS, AML | 3 | I, II | No results posted | Cell Medica Ltd., Belgium, Germany, UK | NCT02550535, Sep 2015 |
| 56 | WT1:126–134 RMFPNAPYL | HLA-A*02:01 | WT1 TCRc4; no11 | AML | 9 | I, II | Active, not recruiting | Fred Hutch, USA | NCT02770820, Nov 2017 |
| 57 | Tyrosinase:368–376, 370D:YMDGTMSQV, 370N:YMNGTMSQV | HLA-A*02:01 | 1383I TCR; no; KD = 10 μM96,97 | Melanoma | 14 | I | 33% tumor shrinkage (1/3); 66% vitiligo (2/3) | Loyola University, USA | NCT01586403, July 201297 |
| 58 | Tyrosinase 368–376 370D:YMDGTMSQV, 370N:YMNGTMSQV | HLA-A*02:01 | 1383I TCR; no; KD = 10 μM96,97 | Melanoma | 18 (est.) | I | Recruiting | NCI, USA | NCT02870244, Feb 2015 |
| 59 | E6:29–38 TIHDIILECV | HLA-A*02:01 | HPV-16 E6 TCR; no98 | HPV-associated cancers | 12 | I, II | 1 CR; 1 PR | NCI, USA | NCT02280811, Oct 201499 |
| 60 | E6:29–38 TIHDIILECV |
HLA-A*02:01 | HPV-16 E6 TCR; no98 | High-grade squamous intraepithelial lesion | 200 (est.) | I | Recruiting | NCI, USA | NCT03197025, Jan 2018 |
| 61 | E7:11–19 epitope of HPV E7 protein | HLA-A*02:01 | E7 TCR; not specified100 | HPV-associated cancers | 180 (est.) | I, II | Recruiting | NCI, USA | NCT02858310, Jan 2017 |
| 62 | HBV antigen | Not specified | HBV antigen-specific TCR; not specified | HCC | 10 (est.) | I | Recruiting | Lion TCR Pte. Ltd., China | NCT02686372, Dec 2015 |
| 63 | HERV-E–derived antigen: ATWLGSKTWK | HLA-A* 11:01 | HERV-E TCR; not specified | Metastatic ccRCC | 24 (est.) | I | Recruiting | NHLBI, USA | NCT03354390, July 2018 |
| 64 | Human thyroglobulin (hTG) | HLA-A*02:01 | hTG mouse TCR; not specified | Metastatic thyroid cancer | 0 | I, II | Withdrawn | NCI, USA | NCT02390739, Mar 2015 |
| 65 | PRAME | HLA-A*02:01 | PRAME TCR (BPX-701); not specified101 | AML, MDS, uveal melanoma | 116 (est.) | I, II | Recruiting | Bellicum Pharmaceuticals, USA | NCT02743611, Apr 2017 |
| 66 | PRAME | HLA-A2*02:01 | MDG1011; not specified | High-risk myeloid and lymphoid neoplasms | 92 (est.) | I, II | Recruiting | Medigene AG, Germany | NCT03503968, Mar 2018 |
| 67 | AFP:158–166 FMNKFIYEI |
HLA-A*02:01 or HLA-A*02:642 | AFPc332 TCR; yes; KD = 10.6 μM102 | HCC | 24 (est.) | I | Recruiting | Adaptimmune, USA, Spain, UK | NCT03132792, May 2017 |
| 68 | KRAS G12V: (V) VVGAVGVGK, NRAS G12V, HRAS G12V | HLA-A*11:01 | KRAS G12V mouse TCR; no103 | Pancreatic, gastric, gastrointestinal, colon, rectal cancers | 110 (est.) | I, II | Recruiting | NCI, USA | NCT03190941, Sep 2017 |
| 69 | G12D variant of mutated RAS | HLA-A*11:01 | KRAS G12D mouse TCR; no103 | Gastrointestinal, pancreatic, gastric, colon, rectal cancers | 70 (est.) | I, II | Recruiting | NCI, USA | NCT03745326, Apr 2019 |
| 70 | Not specified | HLA-A*02:01 | IMA201; no | Solid tumors; HNSCC, NSCLC | 16 (est.) | I | Recruiting | Immatics US, Inc., USA | NCT03247309, Sep 2017 |
| 71 | Not specified | Not specified | IMA202; no | Solid tumors, including NSCLC and HCC | 16 (est.) | I | Recruiting | Immatics US, Inc., USA | NCT03441100, Apr 2019 |
| 72 | Not specified | Not specified | IMA203; no | Refractory/recurrent solid tumors | 16 (est.) | I | Recruiting | Immatics US, Inc., USA | NCT03686124, Mar 2019 |
| 73 | HA-1: VLHDDLLEA | HLA-A*02:01 | HA-1 TCR; no104 | Relapsed or refractory acute Leukemia | 24 (est.) | I | Recruiting | Fred Hutch, USA | NCT03326921, Feb 2018 |
| 74 | TGFβII | HLA-A*02 | Not specified; no | CRC | 5 (est.) | I, II | Recruiting | Oslo University Hospital, Norway | NCT03431311, Mar 2018 |
Previous or combination treatments are not listed.
AFP: Alpha-fetoprotein; CEA: carcinoembryonic antigen; HA-1: minor histocompatibility (H) antigen; HBV: hepatitis B virus; HPV: human papilloma virus; HERV-E–derived antigen: human endogenous retrovirus–derived antigen; MART-1: melanoma antigen recognized by T cells 1; NY-ESO-1: New York esophageal squamous cell carcinoma 1 [LAGE-1: cancer testis antigen homologous to NY-ESO-1 containing 157–165 peptide (SLLMWITQC)]; PRAME: preferentially expressed antigen in melanoma; TGFβII: transforming growth factor beta receptor type II; TRAIL/DR4: TNF-related apoptosis–inducing ligand bound to its receptor DR4; WT1: Wilms’ tumor antigen
AML: acute myeloid leukemia; ccRCC: clear cell renal cell carcinoma; CML: chronic myeloid leukemia; CRC: colorectal cancer; HCC: hepatocellular cancer; HNSCC: head and neck squamous cell carcinoma; MDS: myelodysplastic syndrome; MRCLS: myxoid/round cell liposarcoma; NSCLC: non-small-cell lung cancer; SCS: synovial cell sarcoma.
est.: estimated patient enrollment
CR: complete regression; nCR: near complete response; OR: objective regression; PR: partial response; VGPR: very good partial response
Fred Hutch: Fred Hutchinson Cancer Research Center; GSK: GlaxoSmithKline; JCCC (UCLA): Jonsson Comprehensive Cancer Center at the University of California, Los Angeles; MSD: Merck Sharp and Dohme Corp.; NCI: National Cancer Institute; NHLBI: National Heart, Lung, and Blood Institute; UPenn: University of Pennsylvania
II. CANCER-ASSOCIATED ANTIGENS AS TARGETS FOR TCR-MEDIATED ADOPTIVE T CELL THERAPY
Just over ten years ago, the National Cancer Institute (NCI) sponsored a workshop of experts who generated a prioritization list of 75 cancer-associated peptides that could potentially serve as targets for vaccines or T cell therapies.14 That report described the properties of peptides that could be considered in their “targetability” as complexes with MHC products. Here, rather than focusing on specific peptides, we discuss the advantages and disadvantages of targeting such self-antigens in comparison with targeting neoantigens, a rapidly emerging class of interest with significant potential. A recent study discussed some aspects of this topic.15 From a mechanistic standpoint, self-peptides and neoantigenic peptides share some features. Peptides from upregulated proteins are expressed at higher levels as a pepMHC complex than at the normal levels that operate during tolerance induction. Similarly, a mutation in a neoantigen that increases the binding of the peptide to MHC is also present at higher levels than the normal (wild-type) pepMHC. So long as the mutation does not also alter the structure of the peptide “seen” by the TCR, this scenario yields the same outcome for the upregulated pepMHC and the mutated pepMHC: a higher level of specific pepMHC on the tumor than on normal cells. Accordingly, what really matters from a quantitative perspective in this comparison is the extent of upregulation (e.g., 10-fold), or the increase in affinity of the neoantigenic peptide for the MHC. Because some mutations can yield a 100-fold or greater increase in MHC binding (e.g., determined as stability or affinity),16 it can be difficult to achieve a comparable increase in upregulation of protein levels. Despite this argument, upregulated proteins have the distinct advantage that they are often shared among cancers of many different patients, whereas individual neoantigens are typically unique and thus require personalized TCR identification for each patient. However, there are recent examples of several shared neoantigens which may provide opportunities.17–20 In addition, it could be argued that with new and more rapid TCR discovery platforms, it will ultimately be possible to deploy neoantigen-specific TCRs on a personalized basis.13,21–24
Another scenario for neoantigens is mutations that could impact binding to the TCR, either because they are in exposed residues or they alter the conformation of the peptide or MHC in regions that contact the TCR.25 Here, the neoantigen peptide might be viewed as an advantage over self-peptides as there could be neoantigen-reactive T cells that have not undergone tolerance against the wild-type peptide. However, it is also possible that T cells against self-peptide/MHC expressed at higher levels, as on cancer cells, have not been deleted through negative selection.26–28 At issue in all of these scenarios is identifying TCRs that mediate activity with the level of the pepMHC on the cancer cell but not with the level of the self-peptide MHC on normal cells.
The window that exists to achieve therapeutic effects without side effects due to reaction with normal tissue is key to the success of a TCR. This window must consider the density of the cancer pepMHC complex on the cancer cell versus normal cells, and it must consider the affinity of the TCR and the thresholds for mediating CD4 and CD8 activity.
III. DENSITY OF ANTIGENIC pepMHC COMPLEXES
The density of antigenic pepMHC complexes refers to the number of antigenic pepMHC complexes expressed on a target cell surface. Immune responses to a pepMHC cancer antigen depends on the surface density of the antigen,29 and a minimum threshold is required for T cell activation. As described below, the coreceptors CD4 and CD8 act to synergize with the TCR, lowering the number of required pepMHC complexes to one or just a few.30–33 The affinity of the TCR also impacts this density threshold.34 Accordingly, pepMHC complexes from upregulated self-antigens could activate T cells if their overexpression exceeded the threshold at which TCRs are “tolerized” during selection in the thymus. As described above, neoantigens with mutations that yield enhanced binding to MHC could activate T cells because the density of the pepMHC may greatly exceed this threshold.
The density of a specific pepMHC complex is dependent on various factors, including the level of the intracellular protein, the efficiency with which the peptide is processed from the protein, and the binding affinity of the peptide for the MHC product.35 The antigen-processing and presentation pathway has several steps, and hence each participant of the pathway can potentially impact peptide loading and hence pepMHC density on the cell surface. It is hence not surprising that cancer cells can hijack the cellular machinery to downregulate pepMHC expression to “hide” from naturally existing low-affinity T cells.36,37 For example, genes encoding the MHC heavy-chain or beta-2 microglobulin can be downregulated. Similarly, proteins involved in generation of peptides (i.e., components of the immunoproteasome), peptide loading, and folding and transport of MHC molecules (e.g., TAP, calnexin, calreticulin, tapasin) can be downregulated by cancer cells to directly impact pepMHC density. In such scenarios, T cells transduced with affinity-enhanced TCRs can enable recognition of the low-density cancer antigen but often require an optimal affinity window to ensure a cancer-specific response without reactivity to self-antigens (explained below).
In addition to the antigen presentation pathway, the intrinsic ability of a peptide to bind to the peptide-binding groove of the MHC also directly impacts the number of pepMHC complexes exported to the cell surface. Therefore, peptides with optimal anchor residues are expected to be present at higher densities as pepMHC complexes compared to those with suboptimal anchors.38 Accordingly, neoantigens that arise because of mutations in anchor residues leading to improved MHC binding are expressed at higher levels, similar to aberrantly upregulated cancer-associated self-antigens.39 On the other hand, mutations that destabilize peptide–MHC interaction limit stable expression of such pepMHC complexes on the cell surface and result in reduced T cell responses.40 In a neoantigen trial for melanoma, peptides were prioritized for vaccination based on mutations that resulted in anchor-residue changes (among other criteria that resulted in class I MHC binding epitopes), indicating the importance of pepMHC stability and density in initiating immune response.41 This approach led to the induction of T cell responses in all patients, with 4/6 patients showing no recurrence of disease after 25 months. Other studies have also indicated that the presence of neoantigens that have higher binding affinity for class I MHC (compared to wild-type antigens) correlate with survival in certain cancer types.42
While TCR-mediated recognition of neoantigens results in cancer-specific responses, targeting upregulated cancer-associated antigens with TCRs is more challenging because of their normal levels of expression on non-cancerous tissues. In several clinical trials, targeting an upregulated (i.e., higher-density) cancer-associated self-antigen resulted in activity against their normal (i.e., lower-density) expression on normal tissues.43,44 Accordingly, such “shared” cancer-associated antigens need to be carefully targeted with TCRs, especially when using higher-affinity receptors because of their lower threshold requirements (see below). Recent observations from clinical trials have suggested thorough examination not only of target antigen expression profiles in normal and cancer tissues but also of TCR reactivity to panels of normal human cell lines and tissues prior to adoptive T cell therapy in humans.
IV. TCR AFFINITY REQUIRED FOR CD4 AND CD8 T CELL RESPONSES
TCR affinity for pepMHC is known to determine the sensitivity of the T cell. In the context used here, sensitivity refers to how many specific pepMHC complexes per target cell are required to induce T cell signaling. Remarkably, while the affinity of many TCRs for “foreign” peptides in complex with an MHC molecule is low (micromolar), especially compared to most antibodies (nanomolar), these TCRs are able to mediate activity, as noted above, when induced by only a few pepMHC molecules per target cell.30–33 This exquisite sensitivity comes in part from the TCR/CD3 machinery itself and in part from synergy with the coreceptors CD4 and CD8.45, 46 The coreceptors facilitate T cell activity through binding of the ligands as the TCR and class I and class II MHC (although binding of class I by CD8 appears to be more effective than class II binding by CD4).32,34 Sensitivity is also enhanced by signaling mechanisms achieved through recruitment of the coreceptor-associated kinase Lck.47
While CD8-dependent signaling through the TCR enables such sensitivity, it also impacts potential cross-reactivity with noncognate self-peptides because of the low-affinity threshold required. TCR affinities against cancer self-peptides are generally lower than TCR affinities against foreign pepMHC,48 probably because of negative selection. However, it is possible to use various screening or engineering approaches to raise the affinity of these TCRs.49 This strategy can yield greater TCR sensitivity (i.e., recognition of lower levels of the specific pepMHC) and can even obviate the requirement for CD8.34,50 TCRs with higher affinity (e.g., KD values of ≤ 1 μM) can thus drive activity of CD4 T cells,51 a feature that is especially valuable in elimination of cancers through direct lytic action of CD4 T cells and through recruitment of other immune cells through CD4 T cell polyfunctional activities.52,53
The risk of using higher-affinity TCRs against cancer-associated pepMHC antigens is that they have not been through a stringent negative selection process and so they may cross-react with structurally similar self-peptides.54 This has in fact led to two different clinical trials with lethal toxicities.55,56 The use of non-natural TCRs can be mitigated to some extent by careful screening of normal tissues and by in silico screens of possible MHC-binding structurally similar self-peptides.57–59 It is possible to use natural TCRs isolated against neoantigen pepMHC complexes in autologous T cell transfers, but this process requires personalized workup of the antigens and the TCRs for each individual.13,15,22,60,61 Regardless of the preclinical workup and safety screens done for human TCR gene therapies, clinical trials are required to fully ascertain possible detrimental cross-reactivity and safety issues.
V. CLINICAL TRIALS WITH TCR GENE TRANSFER
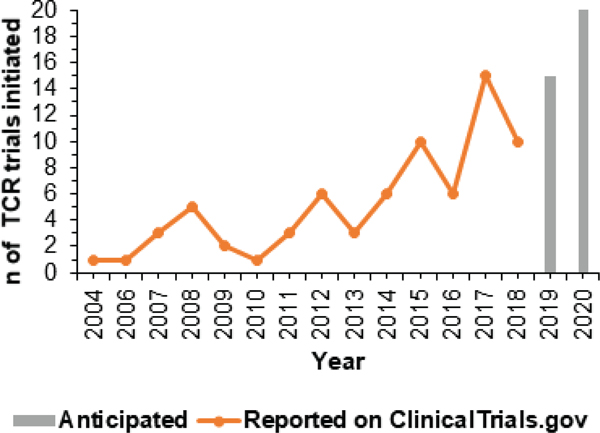
TCRs used clinically in an ACT format have been identified by isolation of a T cell clone that recognizes a specific cancer-associated pepMHC complex. These TCRs are subjected to thorough in vitro analysis to understand sensitivity and specificity prior to use in autologous T cells isolated from patients (Fig. 1). In 2004, Rosenberg and colleagues at the NCI enrolled metastatic melanoma patients for treatment by adoptive transfer of autologous lymphocytes that were genetically modified to express the TCR called DMF4 against the melanoma antigen MART-1/HLA-A2 complex (Table 1). The results of their “first in human” trial demonstrated the therapeutic potential of using TCRs to genetically engineer cells for cancer.62 While they noted objective regression of melanoma lesions in only 2 out of 15 patients, their study provided the groundwork for further efforts on the optimization of TCRs and other parameters. Since then the number of TCR trials initiated worldwide for cancer treatment has been increasing (Fig. 2).
FIG. 2:
Number of cancer clinical trials in the ClinicalTrials.gov database that use TCR-transduced T cells for ACT. The database was searched for TCR trials on January 9, 2019. The search was delimited by “T cell receptors” and “Cancer” as key words.
As DMF4 had a lower affinity to MART-1 (KD = 170 μM), the efficacy of an affinity-enhanced TCR, DMF5 (KD = 40 μM),63 was subsequently examined in melanoma patients to determine if higher-affinity TCRs could mediate higher antitumor reactivity owing to recognition of lower amounts of antigen.43 While the objective responses increased to 30% in this trial, patients also experienced uveitis and hearing loss due to recognition of normal cells expressing MART-1 in the eye and ear. Similarly, targeting carcinoembryonic antigen (CEA) in metastatic colorectal cancer patients with an affinity-enhanced TCR resulted in 33% objective response but also in development of colitis in all patients due to recognition of normal levels of CEA on the colon mucosa.44 Results from these trials demonstrate that, while higher-affinity TCRs can yield improved efficacy, the enhanced sensitivity may also elicit on-target reactivity with normal tissues that are normally nonreactive with lower-affinity TCRs. These results also prompted pursuit of alternative targets such as cancer testis antigens that can be more exclusively associated with expression in cancerous tissue (e.g., NY-ESO-1, LAGE-1, MAGE family of antigens).
Results from NY-ESO-1 clinical trials have been promising, with objective responses ranging from 45 to 70% (Table 1).64–66 It is therefore not surprising that TCR trials for a variety of cancers are targeting this antigen with an affinity-enhanced TCR, NY-ESO-1c259 (KD = 730 nM).66 In contrast, two TCRs that each targeted a different MAGE antigen resulted in patient fatalities due to unexpected off-target cross-reactivities. In one case, targeting MAGE-A3/HLA-A2 antigen with an affinity-enhanced TCR resulted in neurotoxicity due to unexpected expression of a related antigen, MAGE-A12, in the brain.55 In the second case, targeting the MAGE-A3 antigen (HLA-A1–restricted) with an affinity-enhanced TCR (a3a, KD = 2.3 μM) resulted in cardiotoxicity due to unexpected cross-reactivity with the cardiac peptide from the titin protein that shared 5 out of 9 residues with the targeted antigen.56,57 Following these reports of lethal off-target cross-reactivity, safety screens with TCRs now include reactivity with (1) all variants of the targeted peptide, (2) structurally similar self-peptides identified by in silico screens of the proteome,59 and (3) panels of normal human cell lines and tissues in preclinical assays.67 With these key lessons, the use of TCRs in ACT is expanding to safely pursue additional cancer-associated antigens.
Trials are now underway for targeting MAGE-A4, A6, A10, WT-1, Tyrosinase, PRAME, AFP, and KRAS antigens among many others (Table 1). Based on our analysis, there are currently 74 clinical trials that involve either affinity-enhanced TCRs or wild-type TCRs in ACT. For example, Adaptimmmune’s panel of engineered TCRs for ACT have enhanced affinity [these are termed specific peptide-enhanced affinity receptor (SPEAR) T cells] and have been assessed for optimal affinity and cross-reactivity. In contrast, Immatics conducts high-throughput screening of natural human T cell repertoires to isolate therapeutic TCRs with optimal affinity.
Although not addressed in detail here, mispairing of exogenous TCRs with endogenous TCRs can present a challenge in ACT by impacting TCR transduction efficiencies or possibly creating unknown specificities. The addition of cysteines in the constant domains68 or the use of murine constant domains69 has allowed preferential assembly of exogenous TCRs. With the advent of CRISPR/Cas9, engineered T cells can have their endogenous TCR α and β loci disrupted.70,71 TCRs against the NY-ESO-1 antigen with CRISPR-disrupted endogenous TCR chains (NYCE) and/or PD-1 are now in clinical trials for multiple indications (NCT03399448).
Since tumor microenvironment is often immunosuppressive,9,72 combination treatments with checkpoint inhibitors are being assessed in clinical trials—for example, to prevent engineered T cells from inhibitory interactions with PD-L-1 on cancer cells among other cell types (e.g., NCT03709706, NCT03168438, NCT02070406). In addition, in order to achieve durable responses in patients, there is also significant interest in TCR engineering of memory subsets of T cells to achieve durable anticancer response (e.g., NCT02408016, NCT0277082073).
VI. CONCLUDING REMARKS
TCR gene transfer into T cells has tremendous potential as an effective cancer therapeutic because of the potency of T cells and the opportunities to identify novel targets (pepMHC). Continued understanding of T cell and cancer biology, in addition to the discovery of unique targets matched with specific T cell receptors, will allow safer targeting of diverse types of cancers. The field has realized the importance of affinity thresholds of TCRs, in both CD4 and CD8 T cells, when treating patients with genetically modified T cells. In addition, the basic principles of dependence of T cell activation not only on TCR affinity but also on ligand density, coreceptors, CD3 subunits, costimulatory or inhibitory molecules, and downstream signaling mechanisms have guided the expanding array of clinical studies in progress.
ACKNOWLEDGMENTS
We thank past and present members of the Kranz lab for their contributions over the years. This work was supported by NIH grant R01 CA178844 (D.M.K).
ABBREVIATIONS:
- ACT
adoptive T cell therapy
- CAR
chimeric antigen receptor
- HLA
human leukocyte antigen (refers to human MHC alleles)
- KD
dissociation constant
- MHC
major histocompatibility complex
- pepMHC
peptide-major histocompatibility complex antigen
- TCR
T cell receptor
REFERENCES
- 1.Eshhar Z, Waks T, Gross G, Schindler DG. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc Natl Acad Sci U S A. 1993;90(2):720–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.June CH, Riddell SR, Schumacher TN. Adoptive cellular therapy: a race to the finish line. Sci Transl Med. 2015. Mar 25;7(280):280–7. [DOI] [PubMed] [Google Scholar]
- 3.Sadelain M. Chimeric antigen receptors: driving immunology towards synthetic biology. Curr Opin Immunol. 2016. Jun 30;41:68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015. Apr 3;348(6230):56–61. [DOI] [PubMed] [Google Scholar]
- 5.Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018. Mar 23;359(6382):1350–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nature Rev Immunol. 2012. Apr;12(4):269–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stroncek DF, Berger C, Cheever MA, Childs RW, Dudley ME, Flynn P, Gattinoni L, Heath JR, Kalos M, Marincola FM, Miller JS, Mostoslavsky G, Powell DJ Jr., Rao M, Restifo NP, Rosenberg SA, O’Shea J, Melief CJ. New directions in cellular therapy of cancer: a summary of the summit on cellular therapy for cancer. J Trans Med. 2012. Mar 15;10(1):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rudolph MG, Stanfield RL, Wilson IA. How TCRs bind MHCs, peptides, and coreceptors. Annu Rev Immunol. 2006;24:419–66. [DOI] [PubMed] [Google Scholar]
- 9.Schietinger A, Greenberg PD. Tolerance and exhaustion: defining mechanisms of T cell dysfunction. Trends Immunol. 2014. Feb;35(2):51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vigneron N, Stroobant V, Van den Eynde BJ, van der Bruggen P. Database of T cell-defined human tumor antigens: the 2013 update. Cancer Immunity. 2013;13:15. [PMC free article] [PubMed] [Google Scholar]
- 11.Stromnes IM, Schmitt TM, Chapuis AG, Hingorani SR, Greenberg PD. Re-adapting T cells for cancer therapy: from mouse models to clinical trials. Immunol Rev. 2014. Jan;257(1):145–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schreiber H, Greenberg P. Editorial overview: tumour immunology. Curr Opin Immunol. 2015. Apr;33:ix–xi. [DOI] [PubMed] [Google Scholar]
- 13.Tran E, Robbins PF, Rosenberg SA. “Final common pathway” of human cancer immunotherapy: targeting random somatic mutations. Nature Immunol. 2017. Feb 15;18(3):255–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheever MA, Allison JP, Ferris AS, Finn OJ, Hastings BM, Hecht TT, Mellman I, Prindiville SA, Viner JL, Weiner LM, Matrisian LM. The prioritization of cancer antigens: a National Cancer Institute pilot project for the acceleration of translational research. Clin Cancer Res. 2009. Sep 1;15(17):5323–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garber K. Driving T-cell immunotherapy to solid tumors. Nature Biotechnol. 2018. Mar 6;36(3):215–9. [DOI] [PubMed] [Google Scholar]
- 16.Blaha DT, Anderson SD, Yoakum DM, Hager MV, Zha Y, Gajewski TF, Kranz DM. High-throughput stability screening of neoantigen/HLA complexes improves immunogenicity predictions. Cancer Immunol Res. 2019. Jan;7(1):50–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chheda ZS, Kohanbash G, Okada K, Jahan N, Sidney J, Pecoraro M, Yang X, Carrera DA, Downey KM, Shrivastav S, Liu S, Lin Y, Lagisetti C, Chuntova P, Watchmaker PB, Mueller S, Pollack IF, Rajalingam R, Carcaboso AM, Mann M, Sette A, Garcia KC, Hou Y, Okada H. Novel and shared neoantigen derived from histone 3 variant H3.3K27M mutation for glioma T cell therapy. J Exp Med. 2018. Jan 2;215(1):141–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klebanoff CA, Wolchok JD. Shared cancer neoantigens: making private matters public. J Exp Med. 2018. Jan 2;215(1):5–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van der Lee DI, Reijmers RM, Honders MW, Hagedoorn RS, de Jong RC, Kester MG, van der Steen DM, de Ru AH, Kweekel C, Bijen HM, Jedema I, Veelken H, van Veelen PA, Heemskerk MH, Falkenburg JHF, Griffioen M. Mutated nucleophosmin 1 as immunotherapy target in acute myeloid leukemia. J Clin Invest. 2019. Feb 1;129(2):774–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lo W, Parkhurst M, Robbins PF, Tran E, Lu YC, Jia L, Gartner JJ, Pasetto A, Deniger D, Malekzadeh P, Shelton TE, Prickett T, Ray S, Kivitz S, Paria BC, Kriley I, Schrump DS, Rosenberg SA. Immunologic recognition of a shared p53 mutated neoantigen in a patient with metastatic colorectal cancer. Cancer Immunol Res. 2019. Apr;7(4):534–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blankenstein T, Leisegang M, Uckert W, Schreiber H. Targeting cancer-specific mutations by T cell receptor gene therapy. Curr Opin Immunol. 2015. Apr;33:112–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stronen E, Toebes M, Kelderman S, van Buuren MM, Yang W, van Rooij N, Donia M, Boschen ML, Lund-Johansen F, Olweus J, Schumacher TN. Targeting of cancer neoantigens with donor-derived T cell receptor repertoires. Science. 2016. Jun 10;352(6291):1337–41. [DOI] [PubMed] [Google Scholar]
- 23.Smith SN, Wang Y, Baylon JL, Singh NK, Baker BM, Tajkhorshid E, Kranz DM. Changing the peptide specificity of a human T-cell receptor by directed evolution. Nature Commun. 2014;5:5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsuda T, Leisegang M, Park JH, Ren L, Kato T, Ikeda Y, Harada M, Kiyotani K, Lengyel E, Fleming GF, Nakamura Y. Induction of neoantigen-specific cytotoxic T cells and construction of T-cell receptor-engineered T cells for ovarian cancer. Clin Cancer Res. 2018. Nov 1;24(21):5357–67. [DOI] [PubMed] [Google Scholar]
- 25.Riley TP, Hellman LM, Gee MH, Mendoza JL, Alonso JA, Foley KC, Nishimura MI, Vander Kooi CW, Garcia KC, Baker BM. T cell receptor cross-reactivity expanded by dramatic peptide-MHC adaptability. Nat Chem Biol. 2018. Oct;14(10):934–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cook JR, Wormstall E-M, Hornell T, Russell J, Connolly JM, Hansen TH. Quantitation of the cell surface level of Ld resulting in positive versus negative selection of the 2C transgenic T cell receptor in vivo. Immunity. 1997;7:233–41. [DOI] [PubMed] [Google Scholar]
- 27.Delaney JR, Sykulev Y, Eisen HN, Tonegawa S. Differences in the level of expression of class I major histocompatibility complex proteins on thymic epithelial and dendritic cells influence the decision of immature thymocytes between positive and negative selection. Proc Natl Acad Sci U S A. 1998;95(9):5235–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bouneaud C, Kourilsky P, Bousso P. Impact of negative selection on the T cell repertoire reactive to a self-peptide: a large fraction of T cell clones escapes clonal deletion. Immunity. 2000. Dec;13(6):829–40. [DOI] [PubMed] [Google Scholar]
- 29.Bullock TN, Colella TA, Engelhard VH. The density of peptides displayed by dendritic cells affects immune responses to human tyrosinase and gp100 in HLA-A2 transgenic mice. J Immunol. 2000. Mar 1;164(5):2354–61. [DOI] [PubMed] [Google Scholar]
- 30.Sykulev Y, Joo M, Vturina I, Tsomides TJ, Eisen HN. Evidence that a single peptide-MHC complex on a target cell can elicit a cytolytic T cell response. Immunity. 1996;4:565–71. [DOI] [PubMed] [Google Scholar]
- 31.Irvine DJ, Purbhoo MA, Krogsgaard M, Davis MM. Direct observation of ligand recognition by T cells. Nature. 2002. Oct 24;419(6909):845–9. [DOI] [PubMed] [Google Scholar]
- 32.Li QJ, Dinner AR, Qi S, Irvine DJ, Huppa JB, Davis MM, Chakraborty AK. CD4 enhances T cell sensitivity to antigen by coordinating Lck accumulation at the immunological synapse. Nature Immunol. 2004. Aug;5(8):791–9. [DOI] [PubMed] [Google Scholar]
- 33.Huang J, Brameshuber M, Zeng X, Xie J, Li QJ, Chien YH, Valitutti S, Davis MM. A single peptide-major histocompatibility complex ligand triggers digital cytokine secretion in CD4(+) T cells. Immunity. 2013. Nov 14;39(5):846–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holler PD, Kranz DM. Quantitative analysis of the contribution of TCR/pepMHC affinity and CD8 to T cell activation. Immunity. 2003;18:255–64. [DOI] [PubMed] [Google Scholar]
- 35.Blum JS, Wearsch PA, Cresswell P. Pathways of antigen processing. Annu Rev Immunol. 2013. Mar 21;31:443–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leone P, Shin EC, Perosa F, Vacca A, Dammacco F, Racanelli V. MHC class I antigen processing and presenting machinery: organization, function, and defects in tumor cells. J Natl Cancer Institute. 2013. Aug 21;105(16):1172–87. [DOI] [PubMed] [Google Scholar]
- 37.Reeves E, James E. Antigen processing and immune regulation in the response to tumours. Immunology. 2017. Jan;150(1):16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rammensee H, Bachmann J, Emmerich NP, Bachor OA, Stevanovic S. SYFPEITHI: database for MHC ligands and peptide motifs. Immunogenetics. 1999. Nov;50(3–4): 213–9. [DOI] [PubMed] [Google Scholar]
- 39.Sharma P, Kranz DM. Recent advances in T-cell engineering for use in immunotherapy. F1000 Res. 2016;5. DOI: 10.12688/f1000research.9073.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Engels B, Engelhard VH, Sidney J, Sette A, Binder DC, Liu RB, Kranz DM, Meredith SC, Rowley DA, Schreiber H. Relapse or eradication of cancer is predicted by peptide-major histocompatibility complex affinity. Cancer Cell. 2013. Apr 15;23(4):516–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ott PA, Hu Z, Keskin DB, Shukla SA, Sun J, Bozym DJ, Zhang W, Luoma A, Giobbie-Hurder A, Peter L, Chen C, Olive O, Carter TA, Li S, Lieb DJ, Eisenhaure T, Gjini E, Stevens J, Lane WJ, Javeri I, Nellaiappan K, Salazar AM, Daley H, Seaman M, Buchbinder EI, Yoon CH, Harden M, Lennon N, Gabriel S, Rodig SJ, Barouch DH, Aster JC, Getz G, Wucherpfennig K, Neuberg D, Ritz J, Lander ES, Fritsch EF, Hacohen N, Wu CJ. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature. 2017. Jul 13;547(7662):217–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ghorani E, Rosenthal R, McGranahan N, Reading JL, Lynch M, Peggs KS, Swanton C, Quezada SA. Differential binding affinity of mutated peptides for MHC class I is a predictor of survival in advanced lung cancer and melanoma. Ann Oncol. 2018. Jan 1;29(1):271–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johnson LA, Morgan RA, Dudley ME, Cassard L, Yang JC, Hughes MS, Kammula US, Royal RE, Sherry RM, Wunderlich JR, Lee CC, Restifo NP, Schwarz SL, Cogdill AP, Bishop RJ, Kim H, Brewer CC, Rudy SF, VanWaes C, Davis JL, Mathur A, Ripley RT, Nathan DA, Laurencot CM, Rosenberg SA. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood. 2009. Jul 16;114(3):535–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parkhurst MR, Yang JC, Langan RC, Dudley ME, Nathan DA, Feldman SA, Davis JL, Morgan RA, Merino MJ, Sherry RM, Hughes MS, Kammula US, Phan GQ, Lim RM, Wank SA, Restifo NP, Robbins PF, Laurencot CM, Rosenberg SA. T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol Ther. Mar;19(3):620–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harris DT, Hager MV, Smith SN, Cai Q, Stone JD, Kruger P, Lever M, Dushek O, Schmitt TM, Greenberg PD, Kranz DM. Comparison of T cell activities mediated by human TCRs and CARs that use the same recognition domains. J Immunol. 2018. Feb 1;200(3):1088–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krogsgaard M, Davis MM. How T cells “see” antigen. Nature Immunol. 2005. Mar;6(3):239–45. [DOI] [PubMed] [Google Scholar]
- 47.Artyomov MN, Lis M, Devadas S, Davis MM, Chakraborty AK. CD4 and CD8 binding to MHC molecules primarily acts to enhance Lck delivery. Proc Natl Acad Sci U S A. 2010. Sep 28;107(39):16916–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aleksic M, Liddy N, Molloy PE, Pumphrey N, Vuidepot A, Chang KM, Jakobsen BK. Different affinity windows for virus and cancer-specific T-cell receptors: implications for therapeutic strategies. Eur J Immunol. 2012. Dec;42(12):3174–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Richman SA, Kranz DM. Display, engineering, and applications of antigen-specific T cell receptors. Biomol Eng. 2007. Mar 12;24:361–73. [DOI] [PubMed] [Google Scholar]
- 50.Zhao Y, Bennett AD, Zheng Z, Wang QJ, Robbins PF, Yu LY, Li Y, Molloy PE, Dunn SM, Jakobsen BK, Rosenberg SA, Morgan RA. High-affinity TCRs generated by phage display provide CD4+ T cells with the ability to recognize and kill tumor cell lines. J Immunol. 2007. Nov 1;179(9):5845–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stone JD, Chervin AS, Kranz DM. T-cell receptor binding affinities and kinetics: impact on T-cell activity and specificity. Immunology. 2009. Feb;126(2):165–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Engels B, Chervin AS, Sant AJ, Kranz DM, Schreiber H. Long-term persistence of CD4(+) but rapid disappearance of CD8(+) T cells expressing an MHC class I-restricted TCR of nanomolar affinity. Mol Ther. 2012. Mar;20(3):652–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Soto CM, Stone JD, Chervin AS, Engels B, Schreiber H, Roy EJ, Kranz DM. MHC-class I-restricted CD4 T cells: a nanomolar affinity TCR has improved anti-tumor efficacy in vivo compared to the micromolar wild-type TCR. Cancer Immunol Immunother. 2013. Aug 25;62(2):359–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Holler PD, Chlewicki LK, Kranz DM. TCRs with high affinity for foreign pMHC show self-reactivity. Nature Immunol. 2003. Jan;4(1):55–62. [DOI] [PubMed] [Google Scholar]
- 55.Morgan RA, Chinnasamy N, Abate-Daga D, Gros A, Robbins PF, Zheng Z, Dudley ME, Feldman SA, Yang JC, Sherry RM, Phan GQ, Hughes MS, Kammula US, Miller AD, Hessman CJ, Stewart AA, Restifo NP, Quezado MM, Alimchandani M, Rosenberg AZ, Nath A, Wang T, Bielekova B, Wuest SC, Akula N, McMahon FJ, Wilde S, Mosetter B, Schendel DJ, Laurencot CM, Rosenberg SA. Cancer regression and neurological toxicity following Anti-MAGE-A3 TCR gene therapy. J Immunother. 2013. Feb;36(2):133–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Linette GP, Stadtmauer EA, Maus MV, Rapoport AP, Levine BL, Emery L, Litzky L, Bagg A, Carreno BM, Cimino PJ, Binder-Scholl GK, Smethurst DP, Gerry AB, Pumphrey NJ, Bennett AD, Brewer JE, Dukes J, Harper J, Tayton-Martin HK, Jakobsen BK, Hassan NJ, Kalos M, June CH. Cardiovascular toxicity and titin cross-reactivity of affinity-enhanced T cells in myeloma and melanoma. Blood. 2013. Aug 8;122(6):863–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cameron BJ, Gerry AB, Dukes J, Harper JV, Kannan V, Bianchi FC, Grand F, Brewer JE, Gupta M, Plesa G, Bossi G, Vuidepot A, Powlesland AS, Legg A, Adams KJ, Bennett AD, Pumphrey NJ, Williams DD, Binder-Scholl G, Kulikovskaya I, Levine BL, Riley JL, Varela-Rohena A, Stadtmauer EA, Rapoport AP, Linette GP, June CH, Hassan NJ, Kalos M, Jakobsen BK. Identification of a titin-derived HLA-A1-presented peptide as a cross-reactive target for engineered MAGE A3-directed T cells. Sci Transl Med. 2013. Aug 7;5(197):197ra03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stone JD, Kranz DM. Role of T cell receptor affinity in the efficacy and specificity of adoptive T cell therapies. Front Immunol. 2013;4:244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stone JD, Harris DT, Kranz DM. TCR affinity for p/MHC formed by tumor antigens that are self-proteins: impact on efficacy and toxicity. Curr Opin Immunol. 2015. Apr;33:16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015. Apr 3;348(6230):69–74. [DOI] [PubMed] [Google Scholar]
- 61.Leisegang M, Engels B, Schreiber K, Yew PY, Kiyotani K, Idel C, Arina A, Duraiswamy J, Weichselbaum RR, Uckert W, Nakamura Y, Schreiber H. Eradication of large solid tumors by gene therapy with a T-cell receptor targeting a single cancer-specific point mutation. Clin Cancer Res. 2016. Jun 1;22(11):2734–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, Royal RE, Topalian SL, Kammula US, Restifo NP, Zheng Z, Nahvi A, de Vries CR, Rogers-Freezer LJ, Mavroukakis SA, Rosenberg SA. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006. Oct 6;314(5796):126–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Borbulevych OY, Santhanagopolan SM, Hossain M, Baker BM. TCRs used in cancer gene therapy cross-react with MART–1/Melan-A tumor antigens via distinct mechanisms. J Immunol. 2011. Sep 1;187(5):2453–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Robbins PF, Morgan RA, Feldman SA, Yang JC, Sherry RM, Dudley ME, Wunderlich JR, Nahvi AV, Helman LJ, Mackall CL, Kammula US, Hughes MS, Restifo NP, Raffeld M, Lee CC, Levy CL, Li YF, El-Gamil M, Schwarz SL, Laurencot C, Rosenberg SA. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J Clin Oncol. 2011. Mar 1;29(7):917–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Robbins PF, Kassim SH, Tran TL, Crystal JS, Morgan RA, Feldman SA, Yang JC, Dudley ME, Wunderlich JR, Sherry RM, Kammula US, Hughes MS, Restifo NP, Raffeld M, Lee CC, Li YF, El-Gamil M, Rosenberg SA. A pilot trial using lymphocytes genetically engineered with an NY-ESO-1-reactive T-cell receptor: long-term follow-up and correlates with response. Clin Cancer Res. 2015. Mar 1;21(5):1019–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rapoport AP, Stadtmauer EA, Binder-Scholl GK, Goloubeva O, Vogl DT, Lacey SF, Badros AZ, Garfall A, Weiss B, Finklestein J, Kulikovskaya I, Sinha SK, Kronsberg S, Gupta M, Bond S, Melchiori L, Brewer JE, Bennett AD, Gerry AB, Pumphrey NJ, Williams D, Tayton-Martin HK, Ribeiro L, Holdich T, Yanovich S, Hardy N, Yared J, Kerr N, Philip S, Westphal S, Siegel DL, Levine BL, Jakobsen BK, Kalos M, June CH. NY-ESO-1-specific TCR-engineered T cells mediate sustained antigen-specific antitumor effects in myeloma. Nat Med. 2015. Aug;21(8):914–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hickman ES, Lomax ME, Jakobsen BK. Antigen selection for enhanced affinity T-cell receptor-based cancer therapies. J Biomol Screening. 2016. Sep;21(8):769–85. [DOI] [PubMed] [Google Scholar]
- 68.Kuball J, Dossett ML, Wolfl M, Ho WY, Voss RH, Fowler C, Greenberg PD. Facilitating matched pairing and expression of TCR chains introduced into human T cells. Blood. 2007. Mar 15;109(6):2331–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cohen CJ, Zhao Y, Zheng Z, Rosenberg SA, Morgan RA. Enhanced antitumor activity of murine-human hybrid T-cell receptor (TCR) in human lymphocytes is associated with improved pairing and TCR/CD3 stability. Cancer Res. 2006. Sep 1;66(17):8878–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Legut M, Dolton G, Mian AA, Ottmann OG, Sewell AK. CRISPR-mediated TCR replacement generates superior anticancer transgenic T cells. Blood. 2018. Jan 18;131(3):311–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Roth TL, Puig-Saus C, Yu R, Shifrut E, Carnevale J, Li PJ, Hiatt J, Saco J, Krystofinski P, Li H, Tobin V, Nguyen DN, Lee MR, Putnam AL, Ferris AL, Chen JW, Schickel JN, Pellerin L, Carmody D, Alkorta-Aranburu G, Del Gaudio D, Matsumoto H, Morell M, Mao Y, Cho M, Quadros RM, Gurumurthy CB, Smith B, Haugwitz M, Hughes SH, Weissman JS, Schumann K, Esensten JH, May AP, Ashworth A, Kupfer GM, Greeley SAW, Bacchetta R, Meffre E, Roncarolo MG, Romberg N, Herold KC, Ribas A, Leonetti MD, Marson A. Reprogramming human T cell function and specificity with non-viral genome targeting. Nature. 2018. Jul;559(7714):405–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schietinger A, Philip M, Krisnawan VE, Chiu EY, Delrow JJ, Basom RS, Lauer P, Brockstedt DG, Knoblaugh SE, Hammerling GJ, Schell TD, Garbi N, Greenberg PD. Tumor-specific T cell dysfunction is a dynamic antigen-driven differentiation program initiated early during tumorigenesis. Immunity. 2016. Aug 16;45(2):389–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kondo T, Imura Y, Chikuma S, Hibino S, Omata-Mise S, Ando M, Akanuma T, Iizuka M, Sakai R, Morita R, Yoshimura A. Generation and application of human induced-stem cell memory T cells for adoptive immunotherapy. Cancer Sci. 2018. Jul;109(7):2130–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cohen CJ, Zheng Z, Bray R, Zhao Y, Sherman LA, Rosenberg SA, Morgan RA. Recognition of fresh human tumor by human peripheral blood lymphocytes transduced with a bicistronic retroviral vector encoding a murine anti-p53 TCR. J Immunol. 2005. Nov 1;175(9):5799–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Davis JL, Theoret MR, Zheng Z, Lamers CH, Rosenberg SA, Morgan RA. Development of human anti-murine T-cell receptor antibodies in both responding and nonresponding patients enrolled in TCR gene therapy trials. Clin Cancer Res. 2010. Dec 1;16(23):5852–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chodon T, Comin-Anduix B, Chmielowski B, Koya RC, Wu Z, Auerbach M, Ng C, Avramis E, Seja E, Villanueva A, McCannel TA, Ishiyama A, Czernin J, Radu CG, Wang X, Gjertson DW, Cochran AJ, Cornetta K, Wong DJ, Kaplan-Lefko P, Hamid O, Samlowski W, Cohen PA, Daniels GA, Mukherji B, Yang L, Zack JA, Kohn DB, Heath JR, Glaspy JA, Witte ON, Baltimore D, Economou JS, Ribas A. Adoptive transfer of MART-1 T-cell receptor transgenic lymphocytes and dendritic cell vaccination in patients with metastatic melanoma. Clin Cancer Res. 2014. May 1;20(9):2457–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ma C, Cheung AF, Chodon T, Koya RC, Wu Z, Ng C, Avramis E, Cochran AJ, Witte ON, Baltimore D, Chmielowski B, Economou JS, Comin-Anduix B, Ribas A, Heath JR. Multifunctional T-cell analyses to study response and progression in adoptive cell transfer immunotherapy. Cancer Discov. 2013. Apr;3(4):418–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dietrich PY, Le Gal FA, Dutoit V, Pittet MJ, Trautman L, Zippelius A, Cognet I, Widmer V, Walker PR, Michielin O, Guillaume P, Connerotte T, Jotereau F, Coulie PG, Romero P, Cerottini JC, Bonneville M, Valmori D. Prevalent role of TCR alpha-chain in the selection of the preimmune repertoire specific for a human tumor-associated self-antigen. J Immunol. 2003. May 15;170(10):5103–9. [DOI] [PubMed] [Google Scholar]
- 79.Rohaan MW, Berg Jvd, Gomez-Eerland R, Mv Zon, Rd Boer, Bakker EAM Pronk LM, Wiel BVD, Nuijen B, Foppen MG, Thienen JVV, Blank CU, Haanen JBAG. Multicenter phase I/IIa study using T cell receptor gene therapy in metastatic melanoma. J Clin Oncol. 2018;36(15_Suppl):TPS9602-TPS. [Google Scholar]
- 80.Robbins PF, Li YF, El-Gamil M, Zhao Y, Wargo JA, Zheng Z, Xu H, Morgan RA, Feldman SA, Johnson LA, Bennett AD, Dunn SM, Mahon TM, Jakobsen BK, Rosenberg SA. Single and dual amino acid substitutions in TCR CDRs can enhance antigen-specific T cell functions. J Immunol. 2008. May 1;180(9):6116–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tan MP, Gerry AB, Brewer JE, Melchiori L, Bridgeman JS, Bennett AD, Pumphrey NJ, Jakobsen BK, Price DA, Ladell K, Sewell AK. T cell receptor binding affinity governs the functional profile of cancer-specific CD8+ T cells. Clin Exper Immunol. 2015. May;180(2):255–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mackall C, Tap WD, Glod J, Druta M, Chow WA, Araujo DM, Grupp SA, Tine BAV, Chagin K, Winkle EV, Kari G, Trivedi T, Norry E, Holdich T, Bartlett-Pandite AN, Amado RG, D’Angelo SP. Open label, non-randomized, multi-cohort pilot study of genetically engineered NY-ESO-1 specific NY-ESO-1c259t in HLA-A2+ patients with synovial sarcoma (NCT01343043). J Clin Oncol. 2017;35(15_suppl):3000. [Google Scholar]
- 83.D’Angelo SP, Melchiori L, Merchant MS, Bernstein D, Glod J, Kaplan R, Grupp S, Tap WD, Chagin K, Binder GK, Basu S, Lowther DE, Wang R, Bath N, Tipping A, Betts G, Ramachandran I, Navenot JM, Zhang H, Wells DK, Van Winkle E, Kari G, Trivedi T, Holdich T, Pandite L, Amado R, Mackall CL. Antitumor activity associated with prolonged persistence of adoptively transferred NY-ESO-1 (c259) T cells in synovial sarcoma. Cancer Discov. 2018. Aug;8(8):944–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rosati SF, Parkhurst MR, Hong Y, Zheng Z, Feldman SA, Rao M, Abate-Daga D, Beard RE, Xu H, Black MA, Robbins PF, Schrump DA, Rosenberg SA, Morgan RA. A novel murine T-cell receptor targeting NY-ESO-1. J Immunother. 2014. Apr;37(3):135–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Creelan BC, Gainor JF, Govindan R, Hardy NM, Heymach J, Mudad R, Reckamp KL, Bardwell W, Holdich T, Bartlett-Pandite AN, Amado RG. Two phase I/II open label clinical trials evaluating the safety and efficacy of autologous T cells expressing enhanced TCRs specific for NY-ESO-1 or MAGE-A10 in subjects with stage IIIb or stage IV non-small cell lung cancer (NCT02588612/NCT02592577). J Clin Oncol. 2017;35(15_Suppl):TPS3096-TPS. [Google Scholar]
- 86.Parkhurst MR, Joo J, Riley JP, Yu Z, Li Y, Robbins PF, Rosenberg SA. Characterization of genetically modified T-cell receptors that recognize the CEA:691–699 peptide in the context of HLA-A2.1 on human colorectal cancer cells. Clin Cancer Res. 2009. Jan 1;15(1):169–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang QJ, Hanada K, Yang JC. Characterization of a novel nonclassical T cell clone with broad reactivity against human renal cell carcinomas. J Immunol. 2008. Sep 15;181(6):3769–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chinnasamy N, Wargo JA, Yu Z, Rao M, Frankel TL, Riley JP, Hong JJ, Parkhurst MR, Feldman SA, Schrump DS, Restifo NP, Robbins PF, Rosenberg SA, Morgan RA. A TCR targeting the HLA-A*0201-restricted epitope of MAGE-A3 recognizes multiple epitopes of the MAGE-A antigen superfamily in several types of cancer. J Immunol. 2011. Jan 15;186(2):685–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yao X, Lu YC, Parker LL, Li YF, El-Gamil M, Black MA, Xu H, Feldman SA, van der Bruggen P, Rosenberg SA, Robbins PF. Isolation and characterization of an HLA-DPB1*04: 01-restricted MAGE-A3 T-cell receptor for cancer immunotherapy. J Immunother. 2016. Jun;39(5):191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kebriaei P, Klebanoff CA, Creelan BC, Hong DS, Blumenschein GR, Drakaki A, Tewari A, Thomrongsith L, Jiang Y, Jain RK. A phase 1 multicenter study evaluating the safety and efficacy of MHC class II-restricted MAGE-A3/A6 T-cell receptor engineered T cells (KITE–718) in patients with advanced cancers. J Clin Oncol. 2018;36(15_suppl):TPS3104-TPS. [Google Scholar]
- 91.Miyahara Y, Naota H, Wang L, Hiasa A, Goto M, Watanabe M, Kitano S, Okumura S, Takemitsu T, Yuta A, Majima Y, Lemonnier FA, Boon T, Shiku H. Determination of cellularly processed HLA-A2402-restricted novel CTL epitopes derived from two cancer germ line genes, MAGE-A4 and SAGE. Clin Cancer Res. 2005. Aug 1;11(15):5581–9. [DOI] [PubMed] [Google Scholar]
- 92.Kageyama S, Ikeda H, Miyahara Y, Imai N, Ishihara M, Saito K, Sugino S, Ueda S, Ishikawa T, Kokura S, Naota H, Ohishi K, Shiraishi T, Inoue N, Tanabe M, Kidokoro T, Yoshioka H, Tomura D, Nukaya I, Mineno J, Takesako K, Katayama N, Shiku H. Adoptive transfer of MAGE-A4 T-cell receptor gene-transduced lymphocytes in patients with recurrent esophageal cancer. Clin Cancer Res. 2015. May 15;21(10):2268–77. [DOI] [PubMed] [Google Scholar]
- 93.Border EC, Sanderson JP, Weissensteiner T, Gerry AB, Pumphrey NJ. Affinity-enhanced T-cell receptors for adoptive T-cell therapy targeting MAGE-A10: strategy for selection of an optimal candidate. Oncoimmunology. 2019;8(2):e1532759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hong DS, Butler MO, Sullivan RJ, Erickson-Miller CL, Trivedi T, Chagin K, Bartlett-Pandite AN, Amado RG. A phase I single arm, open label clinical trial evaluating safety of MAGE-A10c796T in subjects with advanced or metastatic head and neck, melanoma, or urothelial tumors (NCT02989064). J Clin Oncol. 2017;35(15_Suppl):TPS3098-TPS. [Google Scholar]
- 95.Thomas S, Xue SA, Cesco-Gaspere M, San Jose E, Hart DP, Wong V, Debets R, Alarcon B, Morris E, Stauss HJ. Targeting the Wilms tumor antigen 1 by TCR gene transfer: TCR variants improve tetramer binding but not the function of gene modified human T cells. J Immunol. 2007. Nov 1;179(9):5803–10. [DOI] [PubMed] [Google Scholar]
- 96.Nishimura MI, Avichezer D, Custer MC, Lee CS, Chen C, Parkhurst MR, Diamond RA, Robbins PF, Schwartzentruber DJ, Rosenberg SA. MHC class I-restricted recognition of a melanoma antigen by a human CD4+ tumor infiltrating lymphocyte. Cancer Res. 1999. Dec 15;59(24):6230–8. [PubMed] [Google Scholar]
- 97.Moore T, Wagner CR, Scurti GM, Hutchens KA, Godellas C, Clark AL, Kolawole EM, Hellman LM, Singh NK, Huyke FA, Wang SY, Calabrese KM, Embree HD, Orentas R, Shirai K, Dellacecca E, Garrett-Mayer E, Li M, Eby JM, Stiff PJ, Evavold BD, Baker BM, Le Poole IC, Dropulic B, Clark JI, Nishimura MI. Clinical and immunologic evaluation of three metastatic melanoma patients treated with autologous melanoma-reactive TCR-transduced T cells. Cancer Immunol Immunother. 2018. Feb;67(2):311–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Draper LM, Kwong ML, Gros A, Stevanovic S, Tran E, Kerkar S, Raffeld M, Rosenberg SA, Hinrichs CS. Targeting of HPV-16+ epithelial cancer cells by TCR gene engineered T cells directed against E6. Clin Cancer Res. 2015. Oct 1;21(19):4431–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hinrichs CS, Doran SL, Stevanovic S, Adhikary S, Mojadidi M, Kwong ML, Faquin WC, Feldman S, Somerville R, Sherry RM, Yang JC, Rosenberg SA. A phase I/II clinical trial of E6 T-cell receptor gene therapy for human papillomavirus (HPV)-associated epithelial cancers. J Clin Oncol. 2017;35(15_Suppl):3009. [Google Scholar]
- 100.Jin BY, Campbell TE, Draper LM, Stevanovic S, Weissbrich B, Yu Z, Restifo NP, Rosenberg SA, Trimble CL, Hinrichs CS. Engineered T cells targeting E7 mediate regression of human papillomavirus cancers in a murine model. JCI Insight. 2018. Apr 19;3(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Amir AL, van der Steen DM, van Loenen MM, Hagedoorn RS, de Boer R, Kester MD, de Ru AH, Lugthart GJ, van Kooten C, Hiemstra PS, Jedema I, Griffioen M, van Veelen PA, Falkenburg JH, Heemskerk MH. PRAME-specific Allo-HLA-restricted T cells with potent antitumor reactivity useful for therapeutic T-cell receptor gene transfer. Clin Cancer Res. 2011. Sep 1;17(17):5615–25. [DOI] [PubMed] [Google Scholar]
- 102.Docta RY, Ferronha T, Sanderson JP, Weissensteiner T, Pope GR, Bennett AD, Pumphrey NJ, Ferjentsik Z, Quinn LL, Wiedermann GE, Anderson VE, Saini M, Maroto M, Norry E, Gerry AB. Tuning T-cell receptor affinity to optimize clinical risk-benefit when targeting alpha-fetoprotein-positive liver cancer. Hepatology (Baltimore, MD). 2019. May;69(5):2061–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang QJ, Yu Z, Griffith K, Hanada K, Restifo NP, Yang JC. Identification of T-cell receptors targeting KRAS-mutated human tumors. Cancer Immunol Res. 2016. Mar;4(3):204–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dossa RG, Cunningham T, Sommermeyer D, Medina-Rodriguez I, Biernacki MA, Foster K, Bleakley M. Development of T-cell immunotherapy for hematopoietic stem cell transplantation recipients at risk of leukemia relapse. Blood. 2018. Jan 4;131(1):108–20. [DOI] [PMC free article] [PubMed] [Google Scholar]