Abstract
Retroviral vectors are transcriptionally silenced in hematopoietic stem cells, and this phenomenon must be overcome for effective gene therapy of blood diseases. The murine stem cell virus (MSCV) vector completely silences β-globin reporter genes regulated by locus control region (LCR) elements 5′HS2 to 5′HS4 in seven of eight transgenic mice. Here, we show that no single known MSCV silencer element is sufficient for complete LCR β-globin transgene silencing. However, partial silencing of high-copy transgenes is conveyed by the MSCV direct repeat and promoter elements. The CpG methylation pattern of silenced and expressed MSCV promoter transgenes is virtually identical, demonstrating that silencing does not absolutely correlate with methylation status. Combined mutations in all four MSCV silencer elements leads to expression of β-globin in 6 of 10 transgenic mice. The same mutations incorporated into the HSC1 retrovirus vector direct neo gene expression in 71% of transduced F9 embryonic carcinoma cells. These studies demonstrate that combined mutation of four retroviral silencer elements relieves complete silencing in most transgenic mice and transduced F9 cells and suggests that novel silencer elements remain. Enhanced expression of the HSC1 vector in primitive stem cells is well suited for blood gene therapy applications.
Transcriptional silencing of retrovirus vectors in primitive stem cells is a major obstacle to current gene therapy approaches to the treatment of blood diseases (5, 50, 52). Due to the difficulty in purifying hematopoietic stem cells and infecting them with retrovirus vectors (26), the most widely used models for studying such silencing are preimplantation mouse embryos and embryonic carcinoma (EC) cell lines. Studies by Jaenisch and colleagues demonstrated that silencing of retroviral gene expression in EC cells correlated with de novo methylation of proviral sequences (23, 47), and this silencing could be partially rescued with a methylation inhibitor (47). Taken together with recent studies on embryonic stem (ES) cells that are null for the dnmt1 methyltransferase (28, 49, 51), these findings suggest the presence of an embryonic-cell-specific de novo methylase activity that is responsible for shutting off retroviral gene expression (22, 56). Recently, such a family of embryonic-cell-specific de novo methylases has been identified (35). However, other reports compared the timing of the establishment of silencing to that of methylation in EC cells and showed that while silencing of retroviral gene expression occurs within 48 h of infection, methylation is not established until 10 to 15 days postinfection (17, 25, 34). This suggest that other mechanisms are responsible for silencing and that methylation is a consequence or a secondary step of these mechanisms. Indeed, silencing of adeno-associated virus vectors can be relieved with histone deacetylase inhibitors but not with methylation inhibitors (7), suggesting that chromatin modification is involved in the establishment or maintenance of silencing. The mechanism of silencing therefore remains controversial.
There is more agreement on the cis-acting elements that participate in silencing. These silencers include the negative control region (NCR), the direct repeat (DR) element, and the primer-binding site (PBS) (see Fig. 1A) (3, 9, 14, 21). Molecular characterization of trans-acting factor-binding sites in these regions has shown that the NCR is bound by the multifunctional YY-1 factor (13) that interacts with RPD3 histone deacetylase (54), the DR is bound by at least six trans-acting factors (46), and the PBS is bound by factor A (38). In addition, the retrovirus long terminal repeat (LTR) promoter fragment contains a cluster of 13 CpG sites in 100 bp that become highly methylated in silenced cell types and can therefore be considered a fourth potential silencer element. Combination of mutations in some of these silencer elements in retrovirus vectors leads to higher levels of viral gene expression in EC cells, demonstrating that their effects are additive (6, 18, 20, 41). However, silencing is not completely relieved with any of these vectors. Recently, the MND retrovirus, which contains alterations in the NCR, DR, and PBS and expresses transgenes in approximately 43% of infected F9 embryonic cells (6), was found to evade silencing in over 50% of secondary spleen CFU in mice that were serially transplanted with transduced marrow (40). These findings suggest that either all four elements must be simultaneously removed to completely escape silencing or other unidentified silencing elements exist.
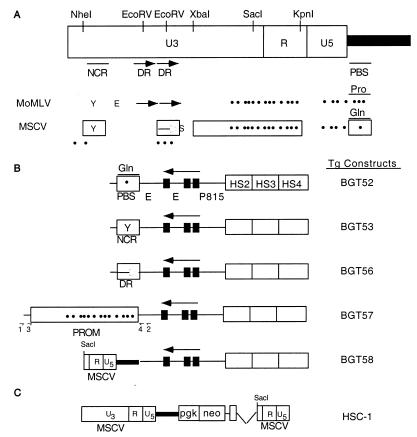
FIG. 1.
Schematic diagram of retroviral sequences and transgene (Tg) constructs. (A) Locations of the NCR, DR, and PBS elements in the MoMLV 5′ LTR. The drawing is not to scale. The differences between MoMLV and MSCV are shown at the bottom. The boxed elements within MSCV indicate elements used in transgenic constructs. Y, YY-1-binding site; E, embryonal LTR-binding protein binding site; S, Sp1-binding site; Pro, wild-type proline tRNA-binding site; Gln, mutant glutamine tRNA-binding site. Dots represent all of the CpG dinucleotides present in the LTR. (B) Schematic diagram of transgenic constructs containing retroviral silencer elements linked to the BGT14 LCR β-globin transgene. Black boxes, β-globin gene introns; P815, 815-bp human β-globin gene promoter (PROM); E, human β-globin gene enhancer; 1, primer LTRPCRF; 2, primer LTRPCRR; 3, primer LTRSEQF; 4, primer LTRSEQR. (C) Schematic diagram of the HSC1 retrovirus construct.
Examination of retroviral silencing in the above-described experiments is limited by the requirement to produce infectious virus. This restriction can be avoided by performing silencing assays with transgenic mice, where silencing also spreads into adjacent sequences to silence linked mammalian promoters in the transgene (30). It is important to utilize a strong internal reporter gene that is expressed reproducibly in the absence of retroviral sequences. To identify specific retroviral sequences sufficient to establish silencing, we have combined such a strong reporter gene with the use of transgenic mice. Human β-globin transgenes regulated by the locus control region (LCR) are ideal for this purpose.
The β-globin LCR is composed of four DNase I-hypersensitive sites (15, 48). In transgenic mice, the LCR directs copy number-dependent, position effect-independent expression (19, 42). All four DNase I-hypersensitive sites (5′HS1 to 5′HS4) are required for full LCR activity (37); however, only 5′HS3 is capable of reproducibly expressing β-globin at every single-copy integration site in transgenic mice (12). A 5′HS2 β-globin transgene is completely silenced when linked to the 5′ LTR and gag sequence of Moloney murine leukemia virus (MoMLV) (30). Indeed, our own findings confirm that a 5′HS2-to-5′HS4 β-globin transgene is completely silenced when linked to MoMLV and show that it is silenced by MSCV to undetectable levels in seven of eight animals (36). To dissect the silencing activities within retrovirus vectors, we have used the BGT14 reporter cassette that contains a 3.0-kb LCR (5′HS2 to 5′HS4) linked to the human β-globin gene and directs 16 to 71% expression at all integration sites of transgenic mice (11). This approach permits us to assess the activity of each silencer element and to investigate the possible mechanisms that participate in silencing. Furthermore, by removing components of all known silencing elements from the retrovirus, we have generated the BGT58 transgene and its equivalent HSC1 retrovirus vector that directs expression in about 60% of transgenic mice and infected F9 embryonic cells.
MATERIALS AND METHODS
Plasmid construction.
BGT52, BGT53, and BGT56 were made by ligation of double-stranded oligonucleotides with NdeI-compatible ends spanning the MSCV PBS (38 bp), NCR (74 bp), and DR (86 bp) elements, respectively, into the NdeI site 3′ of the β-globin gene within BGT14 (11). BGT57 was constructed by addition of NdeI linkers (New England Biolabs) to the 187-bp XbaI-KpnI MSCV LTR promoter fragment from MSCVneoEB (20) and ligation into the NdeI site of BGT14. BGT58 contains the 1.1-kb SacI-HpaI 5′ LTR fragment of MSCVneoEB cloned by using NdeI linkers into the BGT14 NdeI site. HSC1 was constructed by ligation of the 4.1-kb ScaI-ClaI 5′ LTR MSCVneoEB fragment to a double-stranded ClaI-SacI HSC5NCR oligonucleotide and the 2.0-kb SacI-ScaI 3′ LTR MSCVneoEB fragment.
Oligonucleotides.
The sequences of oligonucleotides used in this study were as follows: PBSs, 5′-TATGAGCTCGGAGGTTCCACCGAGATTTGGAGACCCCA-3′; PBSa, 5′-TATGGGGTCTCCAAATCTCGGTGGAACCTCCGAGCTCA-3′; NCRs, 5′-TATGAGCTCAGCTAGCTTAAGTAACGCCATTTTGCAAGGCATGGAAAATACATAACTGAGAATAGAGAAGTTCA-3′; NCRa, 5′-TATGAACTTCTCTATTCTCAGTTATGTATTTTCCATGCCTTGCAAA ATGGCGTTACTTAAGCTAGCTGAGCTCA-3′; DRs, 5′-TATGAGCTCGA CAGCAGAATATGGGCCAAACAGGATATCTGTGGTAAGCAGTTCCTG CCCCGGCTCAGGGCCAAGAACAGATGGCA-3′; DRa, 5′-TATGCCATCTGT TC T TGGCCC TGAGCCGGGGCAGGAAC TGC T TACCACAGATATCCTGTTTGGCCCATAT TC TGC TG TCGAGC TCA-3′; HSC5NCRs, 5′-CGA TCATATGGATAAAATAAAAGATTTTATTTAGTCTCCAGAAAAAGGG GGGAATGAAAGACCCCACCTGTAGG T T TGGCAAGC TAGGAGCT-3′; HSC5NCRa, 5′-CCTAGCTTGCCAAACCTACAGGTGGGGTCTTTCATTC CCCCCTTTTTCTGGAGACTAAATAAAATCTTTTATTTTATCCATATGA T-3′; LTRPCRF, 5′-TTTGAGTAATAGTTTTTTGATTTT-3′; LTRPCRR, 5′-TCCTACCATTTATAC(A/G)AAAATTAATAAACC-3′; LTRSEQF, 5′-ATATTTTTATTGTTTGTTTTTATGAGAGTG-3′; LTRSEQR, 5′-AAAATAAACCTAACACAAAAAAACAAATAC-3′.
Transgenic mice.
Transgenic mice were produced in FVB fertilized eggs as previously described (11). BGT52, BGT53, BGT57, and BGT58 were purified as 7.2-kb EcoRV fragments, and BGT56 was purified as a 7.2-kb XmnI-NotI fragment. Day 15.5 postinjection fetal mice were dissected, and DNA was extracted from head tissue while the fetal liver was saved frozen in halves for future analyses. Transgenic fetuses were identified by slot blot hybridization of fetal head DNA with the βivs2 probe using standard techniques.
DNA analysis.
Transgene copy number was determined by digesting transgenic fetal head DNA with either EcoRI or BamHI, which both cut once within the transgene. Southern blots were probed with an EcoRI-BamHI 0.9-kb β-globin intron 2 fragment by standard procedures. Copy number determination was performed by using a Molecular Dynamics PhosphorImager. Transgene mosaicism was determined by digesting fetal liver DNA with AccI, which cuts twice within the human β-globin gene, and comparison of the band intensity to that of the single-copy B26 transgenic bred line (11). Percent transgenicity was determined by using a Molecular Dynamics PhosphorImager and the formula (Tg Hβ/Tg mThy-1)/(B26 Hβ × copy number/B26 mThy-1), where Tg is transgenic, Hβ is human β-globin, m-Thy-1 is mouse Thy-1, and B26 is one-copy bred line B26.
RNA analysis.
Day 15.5 fetal liver RNA was extracted by using Trizol Reagent (Gibco BRL). RNA (1 μg) was hybridized to [γ-32P]ATP-labeled double-stranded DNA probes, digested with 75 U of S1 nuclease (Boehringer Mannheim), and run on a 6% sequencing gel as previously described (1). The protected 153-nucleotide Huβ and 95-nucleotide Moβ bands were quantitated on a Molecular Dynamics PhosphorImager, and percent expression was calculated according to the formula (Huβ/2Moβ) × 100 to account for the specific activity differences. Percent expression per transgene copy was calculated as (2 Moβ genes/transgene copy number) × (% expression/% transgenicity) × 100.
Electrophoretic mobility shift assays.
Methylcytosine binding protein (MeCP) competition studies were performed as previously described (45). In brief, the M-CG11 probe was incubated with HeLa cell nuclear extracts, and MeCP complexes competed with methylated and unmethylated LTR promoter fragments. The competitor fragment was prepared by digestion of MSCVneoEB with XbaI and KpnI and gel purification. Half of this fragment was then SssI methylase treated (New England Biolabs) and digested with the methylation-sensitive enzyme HhaI. The methylated fragment that was uncut by HhaI was gel purified and used in competition assays along with the unmethylated fragment.
Bisulfite sequencing for determination of DNA methylation.
Bisulfite sequencing was performed as previously described (45). Briefly, genomic fetal liver DNA was treated with bisulfite, which converts deoxycytosine but not 5-methylcytosine residues into uracil. The LTR promoter was then PCR amplified from the bisulfite-treated DNA by using primers LTRPCRF and LTRPCRR. The PCR-amplified products were amplified and sequenced by using nested primers LTRSEQF and LTRSEQR.
Cell lines.
PA317, NIH3T3, and F9 cells were obtained from the American Type Culture Collection. N2/PA317#14 producer cells were obtained from J. Dick. F9 cells were cultured in Dulbecco’s modified Eagle’s medium with 15% fetal bovine serum and grown on plates coated with 0.1% gelatin. PA317 and NIH 3T3 cells were grown in Dulbecco’s modified Eagle’s medium with 10% fetal bovine serum. HSC1 and MSCVneoEB PA317 cell producer populations were prepared by transfection using Lipofectamine and Optimem (Gibco-BRL) and selection in G418 (Gibco-BRL) at 500 μg/ml.
Viral titer determination in F9 and 3T3 cells.
Viral supernatants harvested from confluent PA317 cells producing HSC1, MSCVneoEB, and N2 virus (2) were spun briefly to remove intact cells. The viral supernatants were serially diluted, and 2 ml (plus Polybrene at 8 μg/ml) was added to F9 and NIH 3T3 cells plated at 105/100-mm2 plate the previous evening. After 2 h, 8 ml of growth medium was added. Twenty-four hours later, G418 (500 μg/ml)-containing medium was added and cells were cultured for 10 days, after which time no colonies were seen in uninfected cultures. G418-resistant colonies were revealed by staining with 0.33% methylene blue–0.11% basic fuchsin in methanol. Titers are reported as percentages of F9 cell colonies relative to NIH 3T3 cell colonies. Average relative titrations are reported with standard errors.
Viral titer determination by RNA dot blot assay.
Individual PA317 colonies transfected with HSC1 virus were picked, and high-titer producer clones were identified by viral RNA dot blot assay as previously described (32). Briefly, viral RNA was extracted from 0.4 ml of viral supernatant by using Trizol LS (Gibco-BRL) and transferred to a nitrocellulose membrane. The membrane was hybridized with a 1-kb neo probe in Express Hyb solution (Clontech, Palo Alto, Calif.). As a titration control, 0.4 ml of isolated N2 viral RNA was blotted neat and at 1/10 and 1/100 dilutions.
RESULTS
Murine stem cell virus (MSCV) is a variant form of MoMLV that contains a mutation within the PBS, lacks one of two DR elements (Fig. 1A), and was designed for optimal expression in hematopoietic and ES cells (20). However, MSCV also contains the wild-type NCR and LTR promoter elements. Between the NCR and DR, MSCV has no embryonal LTR-binding protein site and contains a novel Sp1 activator-binding site (20). We have shown that the MSCV 5′ LTR and flanking gag sequence are sufficient to completely silence the BGT14 transgene in seven of eight transgenic animals (36). It is not clear whether any single retroviral silencer element is sufficient to establish this transgene silencing, and this information is important for construction of nonsilenced retroviral vectors.
Individual silencer elements in transgenic mice.
To determine whether the individual silencer elements in MSCV are sufficient to silence the β-globin LCR, we linked oligonucleotides spanning the PBS (38 bp), NCR (74 bp), and DR (86 bp) regions of MSCV to the antisense BGT14 transgene to create transgene constructs BGT52, BGT53, and BGT56, respectively (Fig. 1B). BGT14 is expressed at 16 to 71% per transgene copy and at all integration sites (11) and therefore is an excellent reporter for silencing activity. The antisense orientation is preferred in β-globin retrovirus vectors in order to preserve the introns during retrovirus replication (27, 43). In addition to the PBS, NCR, and DR silencing elements, the LTR promoter region contains multiple CpG sites which are potential sites of methylation. We therefore linked the 187-bp XbaI-KpnI LTR promoter to the BGT14 transgene to generate transgene BGT57. Transgenic mice were generated as previously described (11). Embryonic day 15.5 founder animals for these individual silencer constructs were analyzed for transgene copy number and intactness by Southern blotting. Transgene mosaicism in the fetal livers of these founder animals was calculated by comparison of their signal intensity with that of the single-copy B26 bred line. Analysis of founder animals excludes passage of the transgene through the germ line and is an accurate model of somatic gene therapy, since patients will also be mosaic for the transgene.
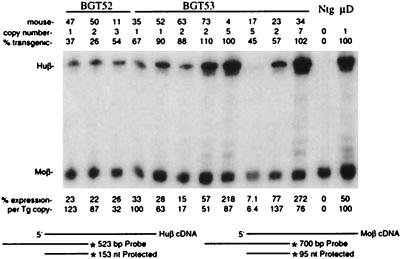
Transgene expression was analyzed by S1 nuclease assays on fetal liver RNA, and expression levels were calculated to take into account the level of transgene mosaicism and copy number. Mouse β-globin transgenes alone, in the absence of the LCR, express up to 2% of the endogenous mouse β major globin gene per copy (39, 42). This level of expression indicates that the LCR is not functional and hence that partial silencing has occurred. Complete silencing is defined as absence of detectable transgene expression. Expression of human β-globin mRNA from BGT52 transgenes ranged from 32 to 123% per copy, suggesting that the mutant PBS in MSCV is not sufficient to convey transgene silencing at a low copy number (Fig. 2). The BGT53 transgene also expressed human β-globin in all transgenic mice at levels between 6 and 137% per copy (Fig. 2). Therefore, the wild-type NCR present in MSCV is not sufficient to convey transgene silencing at copy numbers of seven or lower.
FIG. 2.
Expression of globin mRNA in transgenic mice containing the BGT52 and -53 transgenes. S1 nuclease analysis of globin expression in RNA of 15.5-day fetal livers showing that the mutant PBS and NCR (respectively) of the MSCV retrovirus are not sufficient to silence LCR β-globin transgenes in mice. Transgene (Tg) copy number and percent transgenicity in the liver are shown at the top, and transgene expression is shown at the bottom. Huβ, human β-globin protected probe fragment; Moβ, mouse β major protected probe fragment; Ntg, nontransgenic; μD; one-copy μD14 microlocus transgenic line. The sizes of the protected fragments for the Huβ and Moβ probes are shown below. nt, nucleotides.
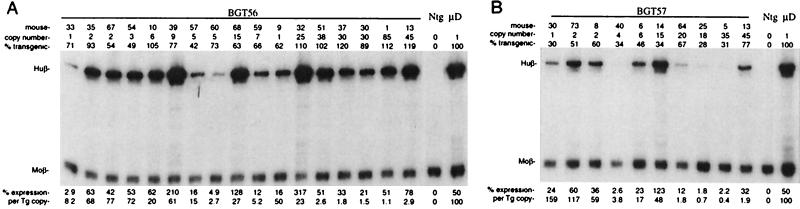
In contrast to the above-described PBS and NCR constructs, very high copy numbers were obtained with the BGT56 and BGT57 transgenes. Full expression from such high-copy transgenes would be expected to cause fatal thalassemia, and therefore it was likely that these constructs were silenced. S1 analysis of BGT56 fetal liver RNA showed that transgene copy numbers of greater than 25 result in significant human β-globin expression, but per-copy expression is extremely low, consistent with a partial-silencing phenotype (Fig. 3A). At lower copy numbers, BGT56 was expressed at 3 to 77% per copy. Similarly, the BGT57 transgene was partially silenced at copy numbers of greater than 15 (Fig. 3B). Although the range of expression at low copy numbers is greater than expected, these data show that at higher copy numbers the DR and LTR promoter from MSCV are sufficient to convey partial transgene silencing and suggest that their removal from the vector would improve expression in primitive cell types.
FIG. 3.
Expression of globin mRNA in transgenic mice containing transgene (Tg) BGT56 and transgene BGT57. (A) S1 nuclease analysis of globin expression in RNA of 15.5-day fetal livers showing that the DR element in high-copy BGT56 transgenic animals partially silences LCR β-globin transgenes. (B) S1 nuclease analysis of globin expression in RNA of 15.5-day fetal livers showing that the LTR promoter element in high-copy BGT57 transgenic animals partially silences LCR β-globin transgenes. Abbreviations are as in Fig. 2.
Methylation studies on the LTR promoter fragment.
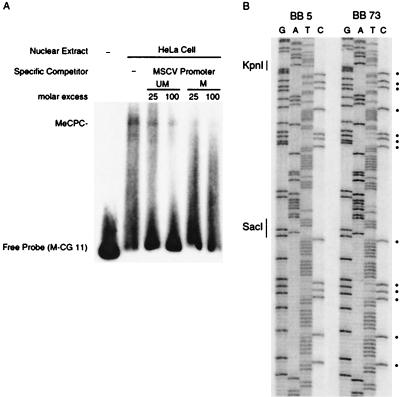
Silencing by the DR is likely to be established by the trans-acting factors that bind to it, but the presence of a cluster of 13 CpG sites in the LTR promoter suggests that methylation is involved in silencing mediated by this region. For example, if these CpG sites were methylated, they could be bound by MeCP1 or MeCP2 (10, 29, 53). To test whether a methylated LTR promoter fragment can bind an MeCP-like protein, we performed gel retardation competition assays (45). In the assay shown in Fig. 4A, the M-CG11 probe is bound by an MeCP complex present in HeLa extracts and the MeCP complex is competed with the highest efficiency by in vitro-methylated XbaI-KpnI LTR promoter fragments. These data suggest that a methylated LTR promoter could bind MeCPs in vivo.
FIG. 4.
Methylation analysis of the LTR promoter. (A) Competition for MeCP complexes by an in vitro-methylated MSCV LTR promoter fragment. This mobility shift assay for MeCP complexes binding to methylated probe M-CG 11 shows that a methylated XbaI-KpnI MSCV LTR promoter fragment competes for binding better than an unmethylated LTR fragment. UM, unmethylated; M, methylated; MeCPC, methylcytosine-binding protein complex. (B) Methylation of CpG sites within the MSCV LTR. Bisulfite sequencing of the LTR promoter region from an animal not expressing BGT57 (BB5) and an animal expressing BGT57 (BB73) is shown. Unmethylated cytosines have been converted to thymidines. Restriction enzyme sites are indicated. Dots represent CpG dinucleotides.
As MeCP factors bind only to methylated CpG sites, the methylation status of the LTR promoter may be critical for silencing. To determine whether there is any correlation between methylation of the LTR promoter and transgene silencing, we bisulfite sequenced this region from two BGT57 animals; founder 73 had two concatemeric transgene copies and expressed the transgene highly, whereas founder 5 had 35 transgene copies and expression was silenced to only 0.4% per copy (Fig. 3B). Figure 4B shows that both founders 5 and 73 are strongly methylated throughout the LTR promoter, indicating that methylation of the LTR promoter does not absolutely correlate with silencing of the linked β-globin promoter.
Partial relief of silencing by multiple mutations.
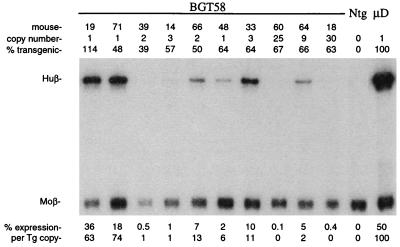
The finding that no single known silencer element tested in MSCV was sufficient to silence the reporter transgene at all integration sites suggests that multiple silencers cooperate to impose complete silencing on linked genes. Therefore, we wished to test whether removal of multiple silencing elements could alleviate transgene silencing. We truncated the LTR sequence 5′ of the SacI site in MSCV to remove the NCR, the DR element, and approximately half of the CpG sites within the LTR promoter. The resulting fragment contains the mutant PBS and was linked to the antisense BGT14 transgene to create the BGT58 construct (Fig. 1B). Six of 10 BGT58 transgenic animals expressed the transgene at 2 to 74% per copy (Fig. 5), showing that the truncated LTR construct can escape complete silencing at many integration sites and is superior to the MSCV vector in this regard. Moreover, these data confirm that removal of multiple elements alleviates silencing most effectively (6, 18, 20, 41) and also show that silencers remain in BGT58.
FIG. 5.
Expression of globin mRNA in transgenic mice containing the BGT58 transgene. S1 nuclease analysis of globin expression in RNA of 15.5-day fetal livers shows that 60% of the transgenic animals escaped complete silencing. Abbreviations are as in Fig. 2.
HSC1 retrovirus vector generation.
Finally, we determined whether the truncated LTR in BGT58 could be used to generate an infectious retroviral vector. Sequences between the NheI and SacI sites were removed from the 3′ LTR of the MSCVneoEB vector (Fig. 1C). The resulting HSC1 vector was transfected into PA317 packaging cells to produce retrovirus stocks for titration on F9 EC cells and NIH 3T3 fibroblasts (Table 1). F9 cells silence MoMLV-based retrovirus vectors, as shown by the 0.4% ± 0.1% relative titer of N2 virus (2) on F9 cells compared to NIH 3T3 cells. The relative titer of MSCV was 43% ± 14%, whereas HSC1 expressed neo in 71% ± 15% of infected F9 cells. These findings demonstrate that the HSC1 retrovirus is infectious, efficiently transfers genes into mammalian cells, and effectively escapes silencing in most transgenic mice and infected F9 cells.
TABLE 1.
Retrovirus titers
| Vector | No. of Neor CFU/ml [F9, 3T3 cells (F9/3T3, %)] in:
|
Avg F9/3T3 (%) ± SE | ||||
|---|---|---|---|---|---|---|
| Expt 1 | Expt 2 | Expt 3 | Expt 4 | Expt 5 | ||
| N2 | 1 × 104, 3 × 106 (0.3) | 4 × 103, 1 × 106 (0.4) | 1 × 104, 4 × 106 (0.25) | 8 × 103, 1 × 106 (0.8) | 0.4 ± 0.1 | |
| MSCV | 3 × 104, 2 × 105 (15) | 2 × 104, 8 × 104 (25) | 9 × 104, 1.5 × 105 (60) | 1 × 105, 3.5 × 105 (29) | 4 × 104, 4.5 × 104 (89) | 43 ± 14 |
| HSC1 | 3 × 104, 1 × 105 (30) | 6 × 104, 7 × 104 (86) | 5 × 104, 5 × 104 (100) | 1 × 105, 2.5 × 105 (40) | 1.5 × 105, 1.5 × 105 (100) | 71 ± 15 |
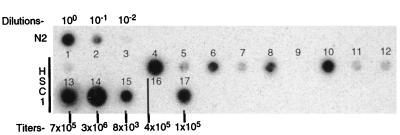
The HSC1 titer from the producer populations is too low for efficient gene transfer into bone marrow. To identify a high-titer clone, HSC1 producer colonies were isolated from freshly transfected PA317 cells and its titer was determined by a viral RNA dot blot assay (32). As shown in Fig. 6, this assay rapidly identified five potentially high-titer producer clones. Virus from these clones was subjected to biological titration on NIH 3T3 cells and found to have titers of up to 3 × 106 G418r CFU/ml that directly correlated with the RNA dot blot signal intensity. Such high titers and improved expression levels from HSC1 vectors are well suited for gene therapy of stem cells.
FIG. 6.
Determination of the virus titers of individual clones of HSC1-producing PA317 packaging cells. Shown is a viral RNA dot blot assay with a neo probe done to identify high-titer HSC1 producer clones. The biological titers of five highly producing clones in NIH 3T3 cells are shown at the bottom. Serial dilutions of N2 virus are shown in the top row.
DISCUSSION
Successful gene therapy of blood diseases in hematopoietic stem cells will require the development of nonsilenced retrovirus vectors (5, 50, 52). To this end, we found that no single known element in the MSCV vector is sufficient for complete silencing in transgenic mice, that the DR and LTR promoter elements partially silence high-copy transgenes, and that methylation of the LTR promoter does not correlate with silencing. Finally, we mutated all four silencer elements to create the HSC1 retrovirus vector, which escapes silencing in about 60% of transgenic mice and transduced F9 cells.
Individual PBS and NCR elements fail to silence transgenes.
The BGT52 construct expresses human β-globin in all transgenic mice, showing that the 38-bp mutant PBS element is not sufficient for silencing. This is not surprising, since the mutant PBS element is unable to bind factor A, which has been implicated in silencing (38). The 74-bp NCR element in BGT53 is wild type and binds the multifunctional YY-1 factor but is also unable to silence expression in our transgenic assay, suggesting that recruitment of the RPD3 histone deacetylase by YY-1 (54) is not sufficient to establish silencing. If such putative RPD3 recruitment occurs in vivo and is important for silencing, then it must also require additional retrovirus sequences for this function. As such, the NCR behaves in a manner similar to the Drosophila engrailed silencer element, which contains a consensus YY-1 site that is bound by the YY-1 homolog pleiohomeotic (PHO), a member of the polycomb group of factors (4). In Drosophila spp., multiple copies of engrailed silencer YY-1 sites are not sufficient for polycomb-dependent silencing, indicating that surrounding sequences are also important in this system. We have examined a series of other potential YY-1 consensus sites (55) in MSCV by using gel retardation assays but found that only the NCR site bound the YY-1 factor in vitro (data not shown).
DR and LTR promoter elements partially silence high-copy transgenes.
The BGT56 construct is able to partially silence the LCR β-globin reporter at greater than 25 transgene copies, but low-copy transgenes were expressed. These data demonstrate that the 86-bp DR element in BGT56 is sufficient for partial silencing at a high copy number, suggesting that DR elements interact with each other for this function. Similar silencing at high copy numbers has been observed with lacZ reporter transgenes (16). At a lower copy number, the DR has little effect on transgene expression but may functionally interact with other silencer elements in an intact single-copy retrovirus vector. Given this concern, the DR element should be removed from gene therapy vectors. Multiple trans-acting factors bind through the DR region (46), and it is not clear from our analysis which sequences are involved in silencing. However, the DR elements have previously been deleted in self-inactivating retrovirus vectors and therefore are dispensable (57).
The LTR promoter element present in BGT57 is also sufficient for partial silencing at greater than 15 transgene copies, suggesting an interaction between multiple promoter elements. The specific sequences responsible for silencing have not been precisely fine mapped, but the 13 CpG sites clustered within the fragment where methylation occurs are good candidates for mediators of silencing activity. Examination of methylation by bisulfite genomic sequencing revealed no major difference between expressed low-copy and silenced high-copy BGT57 transgenes. This indicates that methylation does not absolutely correlate with silencing of C-type retroviruses in mammals, although a threshold of greater than 15 methylated elements may be sufficient for partial silencing. This finding agrees with recent data suggesting that methylation does not correlate with silencing of retrotransposons in the invertebrate chordate Ciona intestinalis (44) but contrasts with data supporting a role for methylation in the silencing of murine IAP-type retroviruses (51).
A methylated LTR promoter fragment was able to compete in binding assays for MeCP complexes. These experiments suggest that MeCP complex binding in vivo distinguishes the silenced from the expressed transgenes and functions by recruiting mSin3a corepressor-histone deactylase complexes, as has recently been described for MeCP2 (24, 33). In this scenario, MeCP complex binding would not be entirely dependent on the presence of methylation alone and may require a threshold number of methylated elements. Since MeCP complexes are barely detectable in F9 cells (31), the probability of MeCP binding in embryonic cells would be greater when multiple methylated LTR promoter elements are present. Regardless of the specific mechanism, prudence suggests that the LTR promoter be removed from the vector in a way that permits retrovirus replication.
Multiple elements influence silencing.
It has previously been shown that combined mutations in some of the known retrovirus silencers lead to improved gene expression in primitive ES cells (6, 18, 20, 41). To examine whether combined mutations in all four elements relieve silencing in transgenic mice, we created the BGT58 construct. This transgene resulted from a truncation of sequences 5′ of the SacI site in the middle of the LTR promoter that also removed the NCR and DR elements. The remaining sequences in the LTR promoter include the TATA box, and this is coupled to the mutant PBS and gag sequences of MSCV. An MoMLV vector backbone containing the equivalent deletion and the wild-type PBS completely silences a 5′HS2 β-globin transgene in mice (30). However, 6 of 10 transgenic mice generated with the BGT58 construct expressed the transgene at between 2 and 74% per copy. These data show that combined mutations of all four of the silencer elements relieve complete silencing in most transgenic mice and are a large improvement over the undetectable expression from intact MSCV sequences. However, 4 of 10 BGT58 transgenic mice were completely silenced, indicating that functional silencer elements remain in the construct. Given that no individual MSCV silencer element is sufficient for complete silencing in transgenic mice, we propose that silencing may require multiple pathways which act on different elements.
HSC1 virus generation.
The BGT58 construct escapes complete silencing in most transgenic mice, but it is unclear whether infectious retrovirus stocks could be made with a similarly large deletion from the NheI site 5′ of the NCR to the SacI site near the TATA box in the LTR promoter. A precedent does exist for deletion of the NCR (6, 41) and deletion from the DR to the SacI site (57). Therefore, we created the HSC1 deletion in the 3′ LTR of MSCV and generated retrovirus stocks from PA317 packaging cell populations. Infection of F9 and NIH 3T3 cells demonstrated that 71% of the F9 cells expressed this neo vector, in contrast to the 43% relative titer of the parental MSCV vector and the 0.4% of the MoMLV-based N2 vector (2). These results show that expression by HSC1 is superior to expression by MSCV in F9 cells. Furthermore, individual HSC1 clones could be isolated from packaging cell populations that have infectious virus titers of up to 3 × 106 G418r colonies/ml.
Future vector development.
The HSC1 vector is a clear improvement on the MSCV vector and is the only retrovirus vector that contains mutations in all four known silencer elements. It also has the added safety of being self-inactivating, which reduces the likelihood of a recombination event that could generate a replication-competent retrovirus (58). It may therefore prove to be suitable for use in blood gene therapy applications. Nevertheless, there is room for further improvement, as 30 to 40% of the BGT58 transgenic mice and HSC1-transduced F9 cells failed to express the transgene. We conclude that either the remaining half of the LTR promoter contains silencing activity or novel silencer elements exist in the U5 and gag sequences. These sequences can be tested individually in the transgenic assay or can be mutated within the context of the BGT58 construct.
Alternatively, the residual silencing may be due to a promoter interference effect in which transcription from the LTR prevents expression of the internal β-globin promoter. Reverse transcription-PCR experiments with fetal liver RNA from MSCV-silenced transgenic mice failed to provide any evidence of such LTR expression across the junction from gag to the 3′ β-globin sequences (data not shown), and the HSC1 promoter would be predicted to be exceptionally weak in nonsilenced cell types, as it merely contains a TATA box. Efforts are in progress to overcome the residual silencing activity through the development of more powerful LCR β-globin transgenes that are routinely expressed at near endogenous levels in single-copy animals (37) and by testing of the ability of insulator elements (8) to limit the spread of silencing from the retrovirus vector sequences into the internally located β-globin promoter. A better understanding of the mechanism of retrovirus silencing will also facilitate efforts to develop pharmacological inhibitors of silencing for use in conjunction with gene therapy vectors.
ACKNOWLEDGMENTS
This work was supported by grants from the Bayer/CRCS Research and Development Fund and the Medical Research Council of Canada to J.E.; NIH grant R37-DK29902 and a grant from the Masonic Cancer Center Fund, Inc., to G.D.G.; and an Ontario Thalassemia Foundation Fellowship to C.S.O.
We thank P. Leboulch for the p141 plasmid, R. Hawley for the MSCVneoEB plasmid, and J. Dick for N2/PA317#14 cells. We are indebted to P.B. Robbins for helpful discussions, R. Hawley and J. Dick for reading the manuscript, D. Tran for technical assistance, and F. Posteraro for secretarial assistance.
REFERENCES
- 1.Antoniou M, deBoer E, Habets G, Grosveld F. The human beta-globin gene contains multiple regulatory regions: identification of one promoter and two downstream enhancers. EMBO J. 1988;7:377–384. doi: 10.1002/j.1460-2075.1988.tb02824.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armentano D, Yu S F, Kantoff P W, von Ruden T, Anderson W F, Gilboa E. Effect of internal viral sequences on the utility of retroviral vectors. J Virol. 1987;61:1647–1650. doi: 10.1128/jvi.61.5.1647-1650.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barklis E, Mulligan R C, Jaenisch R. Chromosomal position or virus mutation permits retrovirus expression in embryonal carcinoma cells. Cell. 1986;47:391–399. doi: 10.1016/0092-8674(86)90596-9. [DOI] [PubMed] [Google Scholar]
- 4.Brown J L, Mucci D, Whiteley M, Dirksen M-L, Kassis J A. The Drosophila polycomb group gene pleiohomeotic encodes a DNA binding protein with homology to the transcription factor YY1. Mol Cell. 1998;1:1057–1064. doi: 10.1016/s1097-2765(00)80106-9. [DOI] [PubMed] [Google Scholar]
- 5.Challita P M, Kohn D B. Lack of expression from a retroviral vector after transduction of murine hematopoietic stem cells is associated with methylation in vivo. Proc Natl Acad Sci USA. 1994;91:2567–2571. doi: 10.1073/pnas.91.7.2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Challita P-M, Skelton D, el-Khoueiry A, Yu X-J, Weinberg K, Kohn D B. Multiple modifications in cis elements of the long terminal repeat of retroviral vectors lead to increased expression and decreased DNA methylation in embryonic carcinoma cells. J Virol. 1995;69:748–755. doi: 10.1128/jvi.69.2.748-755.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen W Y, Bailey E C, McCune S L, Dong J Y, Townes T M. Reactivation of silenced, virally transduced genes by inhibitors of histone deacetylase. Proc Natl Acad Sci USA. 1997;94:5798–5803. doi: 10.1073/pnas.94.11.5798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung J H, Whiteley M, Felsenfeld G. A 5′ element of the chicken beta-globin domain serves as an insulator in human erythroid cells and protects against position effect in Drosophila. Cell. 1993;74:505–514. doi: 10.1016/0092-8674(93)80052-g. [DOI] [PubMed] [Google Scholar]
- 9.Colicelli J, Goff S P. Isolation of a recombinant murine leukemia virus utilizing a new primer tRNA. J Virol. 1986;57:37–45. doi: 10.1128/jvi.57.1.37-45.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cross S H, Meehan R R, Nan X, Bird A. A component of the transcriptional repressor MeCP1 shares a motif with DNA methyltransferase and HRX proteins. Nat Genet. 1997;16:256–259. doi: 10.1038/ng0797-256. [DOI] [PubMed] [Google Scholar]
- 11.Ellis J, Pasceri P, Tan-Un K C, Wu X, Harper A, Fraser P, Grosveld F. Evaluation of beta-globin gene therapy constructs in single copy transgenic mice. Nucleic Acids Res. 1997;25:1296–1302. doi: 10.1093/nar/25.6.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ellis J, Tan-Un K C, Harper A, Michalovich D, Yannoutsos N, Philipsen S, Grosveld F. A dominant chromatin-opening activity in 5′ hypersensitive site 3 of the human beta-globin locus control region. EMBO J. 1996;15:562–568. [PMC free article] [PubMed] [Google Scholar]
- 13.Flanagan J R, Becker K G, Ennist D L, Gleason S L, Driggers P H, Levi B Z, Appella E, Ozato K. Cloning of a negative transcription factor that binds to the upstream conserved region of Moloney murine leukemia virus. Mol Cell Biol. 1992;12:38–44. doi: 10.1128/mcb.12.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flanagan J R, Krieg A M, Max E E, Khan A S. Negative control region at the 5′ end of murine leukemia virus long terminal repeats. Mol Cell Biol. 1989;9:739–746. doi: 10.1128/mcb.9.2.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forrester W C, Thompson C, Elder J T, Groudine M. A developmentally stable chromatin structure in the human beta-globin gene cluster. Proc Natl Acad Sci USA. 1986;83:1359–1363. doi: 10.1073/pnas.83.5.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garrick D, Fiering S, Martin D I, Whitelaw E. Repeat-induced gene silencing in mammals. Nat Genet. 1998;18:56–59. doi: 10.1038/ng0198-56. [DOI] [PubMed] [Google Scholar]
- 17.Gautsch J W, Wilson M C. Delayed de novo methylation in teratocarcinoma suggests additional tissue-specific mechanisms for controlling gene expression. Nature. 1983;301:32–37. doi: 10.1038/301032a0. [DOI] [PubMed] [Google Scholar]
- 18.Grez M, Akgun E, Hilberg F, Ostertag W. Embryonic stem cell virus, a recombinant murine retrovirus with expression in embryonic stem cells. Proc Natl Acad Sci USA. 1990;87:9202–9206. doi: 10.1073/pnas.87.23.9202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grosveld F, van Assendelft G B, Greaves D R, Kollias G. Position-independent, high-level expression of the human beta-globin gene in transgenic mice. Cell. 1987;51:975–985. doi: 10.1016/0092-8674(87)90584-8. [DOI] [PubMed] [Google Scholar]
- 20.Hawley R G, Lieu F H, Fong A Z, Hawley T S. Versatile retroviral vectors for potential use in gene therapy. Gene Ther. 1994;1:136–138. [PubMed] [Google Scholar]
- 21.Hilberg F, Stocking C, Ostertag W, Grez M. Functional analysis of a retroviral host-range mutant: altered long terminal repeat sequences allow expression in embryonal carcinoma cells. Proc Natl Acad Sci USA. 1987;84:5232–5236. doi: 10.1073/pnas.84.15.5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jaenisch R. DNA methylation and imprinting: why bother? Trends Genet. 1997;13:323–329. doi: 10.1016/s0168-9525(97)01180-3. [DOI] [PubMed] [Google Scholar]
- 23.Jahner D, Stuhlmann H, Stewart C L, Harbers K, Lohler J, Simon I, Jaenisch R. De novo methylation and expression of retroviral genomes during mouse embryogenesis. Nature. 1982;298:623–628. doi: 10.1038/298623a0. [DOI] [PubMed] [Google Scholar]
- 24.Jones P L, Veenstra G J C, Wade P A, Vermaak D, Kass S U, Landsberger N, Strouboulis J, Wolffe A P. Methylated DNA and MeCP2 recruit histone deacetylase to repress transcription. Nat Genet. 1998;19:188–191. doi: 10.1038/561. [DOI] [PubMed] [Google Scholar]
- 25.Kempler G, Freitag B, Berwin B, Nanassy O, Barklis E. Characterization of the Moloney murine leukemia virus stem cell-specific repressor binding site. Virology. 1993;193:690–699. doi: 10.1006/viro.1993.1177. [DOI] [PubMed] [Google Scholar]
- 26.Larochelle A, Vormoor J, Hanenberg H, Wang J C, Bhatia M, Lapidot T, Moritz T, Murdoch B, Xiao X L, Kato I, Williams D A, Dick J E. Identification of primitive human hematopoietic cells capable of repopulating NOD/SCID mouse bone marrow: implications for gene therapy. Nat Med. 1996;2:1329–1337. doi: 10.1038/nm1296-1329. [DOI] [PubMed] [Google Scholar]
- 27.Leboulch P, Huang G M, Humphries R K, Oh Y H, Eaves C J, Tuan D Y, London I M. Mutagenesis of retroviral vectors transducing human beta-globin gene and beta-globin locus control region derivatives results in stable transmission of an active transcriptional structure. EMBO J. 1994;13:3065–3076. doi: 10.1002/j.1460-2075.1994.tb06605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lei H, Oh S P, Okano M, Juttermann R, Goss K A, Jaenisch R, Li E. De novo DNA cytosine methyltransferase activities in mouse embryonic stem cells. Development. 1996;122:3195–3205. doi: 10.1242/dev.122.10.3195. [DOI] [PubMed] [Google Scholar]
- 29.Lewis J D, Meehan R R, Henzel W J, Maurer-Fogy I, Jeppesen P, Klein F, Bird A. Purification, sequence, and cellular localization of a novel chromosomal protein that binds to methylated DNA. Cell. 1992;69:905–914. doi: 10.1016/0092-8674(92)90610-o. [DOI] [PubMed] [Google Scholar]
- 30.McCune S L, Townes T M. Retroviral vector sequences inhibit human beta-globin gene expression in transgenic mice. Nucleic Acids Res. 1994;22:4477–4481. doi: 10.1093/nar/22.21.4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meehan R R, Lewis J D, McKay S, Kleiner E L, Bird A P. Identification of a mammalian protein that binds specifically to DNA containing methylated CpGs. Cell. 1989;58:499–507. doi: 10.1016/0092-8674(89)90430-3. [DOI] [PubMed] [Google Scholar]
- 32.Murdoch B, Pereira D S, Wu X, Dick J E, Ellis J. A rapid screening procedure for the identification of high-titer retrovirus packaging clones. Gene Ther. 1997;4:744–749. doi: 10.1038/sj.gt.3300448. [DOI] [PubMed] [Google Scholar]
- 33.Nan X, Ng H-H, Johnson C A, Laherty C D, Turner B M, Eisenman R N, Bird A. Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature. 1998;393:386–389. doi: 10.1038/30764. [DOI] [PubMed] [Google Scholar]
- 34.Niwa O, Yokota Y, Ishida H, Sugahara T. Independent mechanisms involved in suppression of the Moloney leukemia virus genome during differentiation of murine teratocarcinoma cells. Cell. 1983;32:1105–1113. doi: 10.1016/0092-8674(83)90294-5. [DOI] [PubMed] [Google Scholar]
- 35.Okano M, Xie S, Li E. Cloning and characterization of a family of novel mammalian DNA (cytosine-5) methyltransferases. Nat Genet. 1998;19:219–220. doi: 10.1038/890. [DOI] [PubMed] [Google Scholar]
- 36.Pannell, D., C. S. Osborne, P. Pasceri, A. Karaiskakis, H. D. Lipshitz, and J. Ellis. Submitted for publication.
- 37.Pasceri P, Pannell D, Wu X, Ellis J. Full activity from human beta-globin locus control region transgenes requires 5′HS1, distal beta-globin promoter and 3′ beta-globin sequences. Blood. 1998;92:653–663. [PubMed] [Google Scholar]
- 38.Petersen R, Kempler G, Barklis E. A stem cell-specific silencer in the primer-binding site of a retrovirus. Mol Cell Biol. 1991;11:1214–21. doi: 10.1128/mcb.11.3.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Philipsen S, Pruzina S, Grosveld F. The minimal requirements for activity in transgenic mice of hypersensitive site 3 of the beta globin locus control region. EMBO J. 1993;12:1077–1085. doi: 10.1002/j.1460-2075.1993.tb05749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Robbins P B, Skelton D C, Yu X J, Halene S, Leonard E H, Kohn D B. Consistent, persistent expression from modified retroviral vectors in murine hematopoietic stem cells. Proc Natl Acad Sci USA. 1998;95:10182–10187. doi: 10.1073/pnas.95.17.10182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robbins P B, Yu X J, Skelton D M, Pepper K A, Wasserman R M, Zhu L, Kohn D B. Increased probability of expression from modified retroviral vectors in embryonal stem cells and embryonal carcinoma cells. J Virol. 1997;71:9466–9474. doi: 10.1128/jvi.71.12.9466-9474.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ryan T M, Behringer R R, Martin N C, Townes T M, Palmiter R D, Brinster R L. A single erythroid-specific DNase I super-hypersensitive site activates high levels of human beta-globin gene expression in transgenic mice. Genes Dev. 1989;3:314–323. doi: 10.1101/gad.3.3.314. [DOI] [PubMed] [Google Scholar]
- 43.Sadelain M, Wang C H, Antoniou M, Grosveld F, Mulligan R C. Generation of a high-titer retroviral vector capable of expressing high levels of the human beta-globin gene. Proc Natl Acad Sci USA. 1995;92:6728–6732. doi: 10.1073/pnas.92.15.6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simmen M W, Leitgeb S, Charlton J, Jones S J, Harris B R, Clark V H, Bird A. Nonmethylated transposable elements and methylated genes in a chordate genome. Science. 1999;283:1164–1167. doi: 10.1126/science.283.5405.1164. [DOI] [PubMed] [Google Scholar]
- 45.Singal R, Ferris R, Little J A, Wang S Z, Ginder G D. Methylation of the minimal promoter of an embryonic globin gene silences transcription in primary erythroid cells. Proc Natl Acad Sci USA. 1997;94:13724–13729. doi: 10.1073/pnas.94.25.13724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Speck N A, Baltimore D. Six distinct nuclear factors interact with the 75-base-pair repeat of the Moloney murine leukemia virus enhancer. Mol Cell Biol. 1987;7:1101–1110. doi: 10.1128/mcb.7.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stewart C L, Stuhlmann H, Jahner D, Jaenisch R. De novo methylation, expression, and infectivity of retroviral genomes introduced into embryonal carcinoma cells. Proc Natl Acad Sci USA. 1982;79:4098–4102. doi: 10.1073/pnas.79.13.4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tuan D, Solomon W, Li Q, London I M. The “beta-like-globin” gene domain in human erythroid cells. Proc Natl Acad Sci USA. 1985;82:6384–6388. doi: 10.1073/pnas.82.19.6384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tucker K L, Beard C, Dausmann J, Jackson-Grusby L, Laird P W, Lei H, Li E, Jaenisch R. Germ-line passage is required for establishment of methylation and expression patterns of imprinted but not of nonimprinted genes. Genes Dev. 1996;10:1008–1020. doi: 10.1101/gad.10.8.1008. [DOI] [PubMed] [Google Scholar]
- 50.Verma I M, Somia N. Gene therapy—promises, problems and prospects. Nature. 1997;389:239–242. doi: 10.1038/38410. [DOI] [PubMed] [Google Scholar]
- 51.Walsh C P, Bestor T H. Cytosine methylation and mammalian development. Genes Dev. 1999;13:112–124. doi: 10.1101/gad.13.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weinberg K I, Kohn D B. Gene therapy for congenital lymphoid immunodeficiency diseases. Semin Hematol. 1998;35:354–366. [PubMed] [Google Scholar]
- 53.Weitzel J M, Buhrmester H, Stratling W H. Chicken MAR-binding protein ARBP is homologous to rat methyl-CpG-binding protein MeCP2. Mol Cell Biol. 1997;17:5656–5666. doi: 10.1128/mcb.17.9.5656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang W M, Inouye C, Zeng Y, Bearss D, Seto E. Transcriptional repression by YY1 is mediated by interaction with a mammalian homolog of the yeast global regulator RPD3. Proc Natl Acad Sci USA. 1996;93:12845–50. doi: 10.1073/pnas.93.23.12845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yant S R, Zhu W, Millinoff D, Slightom J L, Goodman M, Gumucio D L. High affinity YY1 binding motifs: identification of two core types (ACAT and CCAT) and distribution of potential binding sites within the human beta globin cluster. Nucleic Acids Res. 1995;23:4353–4362. doi: 10.1093/nar/23.21.4353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yoder J A, Walsh C P, Bestor T H. Cytosine methylation and the ecology of intragenomic parasites. Trends Genet. 1997;13:335–340. doi: 10.1016/s0168-9525(97)01181-5. [DOI] [PubMed] [Google Scholar]
- 57.Yu S F, von Ruden T, Kantoff P W, Garber C, Seiberg M, Ruther U, Anderson W F, Wagner E F, Gilboa E. Self-inactivating retroviral vectors designed for transfer of whole genes into mammalian cells. Proc Natl Acad Sci USA. 1986;83:3194–8. doi: 10.1073/pnas.83.10.3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zufferey R, Dull T, Mandel R J, Bukovsky A, Quiroz D, Naldini L, Trono D. Self-inactivating lentivirus vector for safe and efficient in vivo gene delivery. J Virol. 1998;72:9873–9880. doi: 10.1128/jvi.72.12.9873-9880.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]