Abstract
Background/Aims
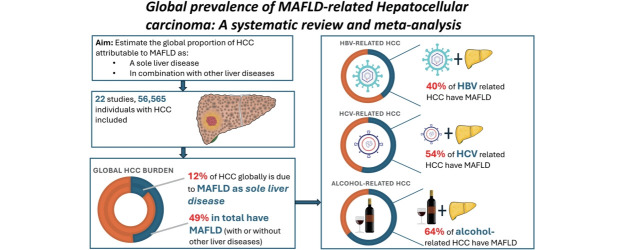
The global proportion of hepatocellular carcinoma (HCC) attributable to metabolic dysfunction-associated fatty liver disease (MAFLD) is unclear. The MAFLD diagnostic criteria allows objective diagnosis in the presence of steatosis plus defined markers of metabolic dysfunction, irrespective of concurrent liver disease. We aimed to determine the total global prevalence of MAFLD in HCC cohorts (total-MAFLD), including the proportion with MAFLD as their sole liver disease (single-MAFLD), and the proportion of those with concurrent liver disease where MAFLD was a contributary factor (mixed-MAFLD).
Methods
This systematic review and meta-analysis included studies systematically ascertaining MAFLD in HCC cohorts, defined using international expert panel criteria including ethnicity-specific BMI cut-offs. A comparison of clinical and tumour characteristics was performed between single-MAFLD, mixed-MAFLD, and non-MAFLD HCC.
Results
22 studies (56,565 individuals with HCC) were included. Total and single-MAFLD HCC prevalence was 48.7% (95% confidence interval [CI] 34.5–63.0%) and 12.4% (95% CI 8.3–17.3%), respectively. In HCC due to chronic hepatitis B, C, and alcohol-related liver disease, mixed-MAFLD prevalence was 40.0% (95% CI 30.2–50.3%), 54.1% (95% CI 40.4–67.6%) and 64.3% (95% CI 52.7–75.0%), respectively. Mixed-MAFLD HCC had significantly higher likelihood of cirrhosis and lower likelihood of metastatic spread compared to single-MAFLD HCC, and a higher platelet count and lower likelihood of macrovascular invasion compared to non-MAFLD HCC.
Conclusions
MAFLD is common as a sole aetiology, but more so as a co-factor in mixed-aetiology HCC, supporting the use of positive diagnostic criteria.
Keywords: Fatty liver, Hepatocellular carcinoma, Epidemiology, Prevalence, Metabolic syndrome
Graphical Abstract
INTRODUCTION
Hepatocellular carcinoma (HCC) is a leading cause of cancer-related mortality, with annual deaths predicted to rise substantially in coming decades from 800,000 in 2020 to 1,300,000 by 2040 [1]. A major reason for this is the global rise in fatty liver disease driven by epidemics of obesity, diabetes, and metabolic dysfunction [2]. Metabolic (dysfunction)-associated fatty liver disease (MAFLD) is an increasingly important contributor to global HCC incidence, yet the true scale of its contribution remains unknown. In part, this is due to the previous non-alcoholic fatty liver disease/non-alcoholic steatohepatitis (NAFLD/NASH) diagnostic criteria which relied on exclusion of other causes of hepatic steatosis. Thus, in HCC cohorts, NAFLD/NASH-related HCC is often diagnosed and reported only when it is the sole identifiable liver disease. The MAFLD definition proposed in 2019 [3,4] allows a positive diagnosis to be made in the presence of hepatic steatosis plus one of either (1) overweight or obese as defined by body mass index (BMI), (2) type 2 diabetes mellitus (T2DM), or (3) lean or normal weight with at least two markers of metabolic dysregulation (Supplementary Fig. 1), irrespective of concurrent liver disease. Additionally, standard convention in prevalence and prospective interventional studies is to categorise HCC by a single or “dominant” aetiology [5]. Thus, while numerous studies have reported prevalence of fatty liver disease in HCC (using either NAFLD or MAFLD definitions) [6], these may underestimate the true impact of MAFLD by excluding individuals with multiple interacting aetiologies. Data are therefore needed to define the role of MAFLD in HCC regardless of the presence or absence of other liver diseases.
There is growing recognition that accurately classifying underlying liver disease(s) is important. Not only do patient demographics and comorbidities differ between individuals of different liver disease aetiologies [7], but so do the underlying aetiological mechanisms of HCC development, tumour immune microenvironments [8], biological behaviour and perhaps responses to therapy [9-13]. In this regard, liver disease aetiology has garnered interest as a potential stratification tool to guide therapy [14].
The aim of this systematic review and meta-analysis is thus to estimate the proportion of HCC attributable to MAFLD either as a sole liver disease aetiology or in combination with another liver disease. The secondary aims were to assess variation in single or combined aetiology MAFLD-HCC by geographical region, secondary liver disease aetiology, and MAFLD sub-phenotype (lean, overweight/obese, diabetic), and to compare the clinical characteristics of single-aetiology MAFLD, combined-aetiology MAFLD, and non-MAFLD HCC.
MATERIALS AND METHODS
Search strategy and inclusion criteria
The review was synthesised in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement (Supplementary Table 1) [15]. A search was conducted on 16th April 2023 using Medline, Embase, PubMed, and Web of Science from database inception to April 2023 for publications that contained information on MAFLD-related HCC. The search terms were [“metabol* adj2 associa* fatty liver disease” OR “mafld” OR “mash” OR “metabol* adj2 steatohepatitis”] AND [“hcc” OR “liver cell carcinoma/” OR “hepatocellular carcinoma” OR “hepatoma” OR “liver cancer”] on the search of title and abstracts. The references were compiled on Endnote and duplicates were removed.
Eligibility and selection criteria
Two reviewers (H.C. and C.G.) independently reviewed titles and abstracts to screen for eligible studies. Full texts of any potentially relevant studies were obtained for further evaluation. Inclusion criteria were as follows: an original article or abstract published in a peer-reviewed journal; the study reported on the prevalence of MAFLD in a cohort of individuals with HCC. HCC cohorts were defined as any group of consecutive HCC cases of any aetiology (apart from MAFLD-only cohorts). MAFLD was defined as the presence of hepatic steatosis with metabolic dysregulation as defined by the international expert consensus panel criteria [4]. Steatosis could be defined by any method including radiology, histology or blood-based investigations such as fatty liver index. Overweight/obese was defined as BMI ≥23 kg/m2 in Asian cohorts and ≥25 kg/m2 in Western cohorts, and individuals under these respective cut-offs were defined as “lean”. We excluded studies which did not reference the criteria used to diagnose MAFLD or used a definition inconsistent with the internationally accepted criteria (including region-specific BMI and waist circumference cut-offs) unless authors were able to provide the appropriate clarifications on their MAFLD diagnostic criteria. Studies were also excluded if there was ambiguity as to whether their MAFLD prevalence data referred to single-MAFLD or total-MAFLD prevalence within their cohort unless clarifications were provided by authors. In the case of multiple studies reporting on overlapping cohorts, the most recently published study was included in the analysis. However, if a non-overlapping subgroup could be extracted from another study, then that subgroup was eligible for inclusion in the overall analysis.
Three prevalence estimates were made:
· Single-MAFLD HCC, defined as the proportion of HCC whereby MAFLD is the sole identifiable liver disease.
· Mixed-MAFLD HCC, defined as the prevalence of MAFLD amongst individuals with HCC with another (non-MAFLD) liver disease aetiology.
· Total-MAFLD HCC, defined as the total prevalence of MAFLD in an HCC cohort (i.e. the total sum of single-MAFLD and mixed-MAFLD HCC).
A comparison of the clinical and tumour characteristics was performed between three groups: single-MAFLD HCC, mixed-MAFLD HCC, and non-MAFLD HCC (defined by HCC in the absence of MAFLD).
Statistical analysis
For MAFLD prevalence estimates, single proportions were transformed using the Freeman-Tukey double arcsine transformation method and weighted by inverse variance for pooling [16]. A random-effects model was used for all analyses with between-study variance estimated using a DerSimonian and Laird model [17]. Statistical heterogeneity was assessed by I² and Cochran's Q test values. Prespecified subgroup analysis by geographic region, and in the case of mixed-MAFLD prevalence by other liver disease aetiology (HBV, HCV or ALD) was performed. A post-hoc meta-regression analysis was performed to investigate study level factors influencing MAFLD prevalence heterogeneity. Covariates used for the analysis were median cohort age, gender, geographical setting (Asia vs. outside Asia), duration of study enrolment, whether the cohort was limited to early-stage (resectable) HCC, and proportion of mixed-MAFLD cohort with HBV.
To compare clinical and tumour characteristics between groups, comparative meta-analysis of odds ratios (OR) for binary variables, and mean difference (MD) for continuous variables were performed. When mean and standard deviation were not reported, they were estimated using the method described by Wan et al. [18]. Publication bias was assessed by the Egger’s test and by assessing for asymmetry on funnel plots, and if present, was adjusted using the trim-and-fill method [19]. A P-value was considered statistically significant if ≤0.05. All analyses were conducted in RStudio (R version 4.3.1).
Quality assessment
Quality assessment for included articles was done using the critical appraisal tool proposed by Hoy et al. [20] for assessing bias risk in prevalence studies. The tool consists of 10 items addressing four domains plus a summary risk of bias assessment, with scores of 0–3 considered to reflect a low overall risk of bias, scores of 4–6 representing moderate risk of bias, and 7–10 representing high risk of bias.
RESULTS
Summary of included studies
The initial search from Medline, Embase, PubMed, and Web of Science yielded 713 results (Fig. 1). After duplicate removal, 317 articles were screened for inclusion. Three studies [21-23] were excluded due to overlapping study populations; only a non-overlapping subgroup of another study [24] was included in the primary prevalence analysis. One additional study was excluded due to a BMI cutoff not in line with accepted ethnicity-specific cutoffs [25]. 11 authors were contacted to provide further data or clarifications on definitions used for MAFLD diagnosis. 22 studies, comprising a total of 56,565 HCC cases, were included in the final analysis. Eleven studies reported prevalence in Asian cohorts, four from Europe, three from Australia, one from North America, one from Central America, one reporting prevalence in a mixed cohort from Europe and Asia, and one in a mixed cohort from North America and Europe. All included studies used ethnicity-appropriate BMI cut-offs and T2DM status in their MAFLD diagnostic criteria. The method of hepatic steatosis assessment was variable between studies and is described in Supplementary Table 2.
Figure 1.
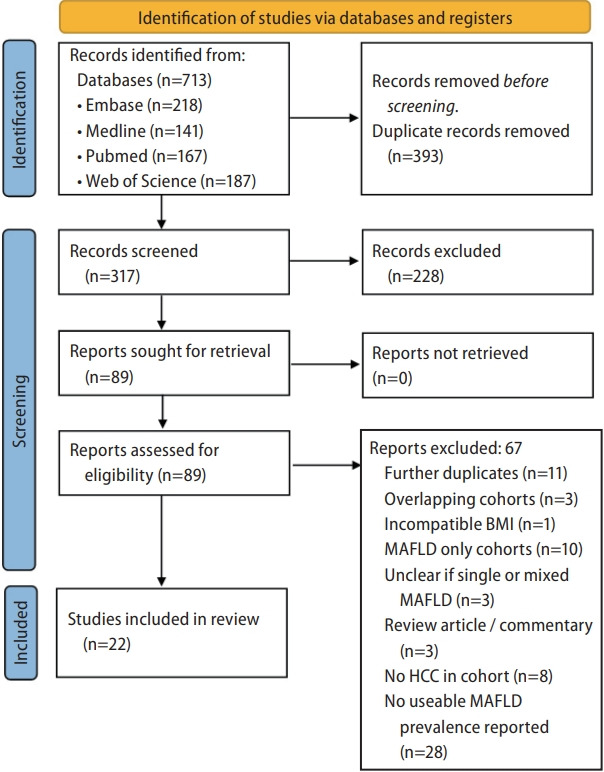
PRISMA flow diagram. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-analyses; MAFLD, metabolic dysfunction- associated fatty liver disease; HCC, hepatocellular carcinoma; BMI, body mass index.
The risk of bias for each of the included studies in the meta-analysis is shown in Supplementary Table 3. Each of the included studies had an overall low risk of study bias.
Single-MAFLD HCC
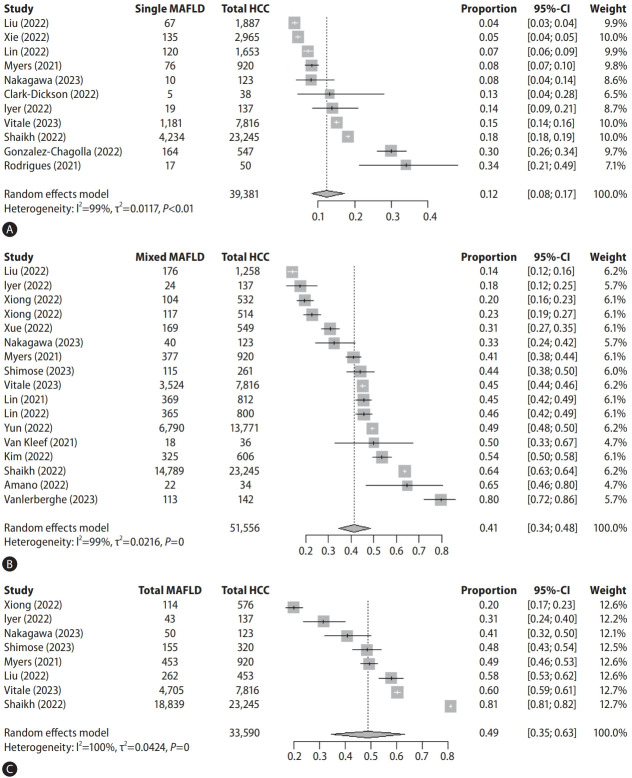
Eleven studies (comprising 39,381 HCC cases) reported the prevalence of single-aetiology MAFLD HCC with a pooled overall prevalence of 12.4% (95% confidence interval [CI] 8.3–17.3%) (Fig. 2A). There was high heterogeneity between study estimates (I2=99%, Cochran’s Q <0.01). Individual single-MAFLD prevalence estimates varied between cohorts from 4–34%. Subgroup analysis based on geographical region showed highest prevalence in Central America (30%), followed by Australia (19%, 95% CI 12–28%), North America (18%), Europe (12%, 95% CI 6–18%), and Asia (6%, 95% CI 3–9%) (Table 1). There was no evidence of publication bias.
Figure 2.
Forrest plots and pooled prevalence estimates for single (A), mixed (B), and total (C) MAFLD-HCC. MAFLD, metabolic dysfunctionassociated fatty liver disease; HCC, hepatocellular carcinoma; CI, confidence interval.
Table 1.
MAFLD HCC prevalence
| Patient group | Sample size (number of studies) | MAFLD prevalence (%) (95% CI) | I2 (%) | Cochran’s Q |
|---|---|---|---|---|
| Single-MAFLD HCC | ||||
| Overall prevalence | 39,381 (11) | 12.4 (8.3–17.3) | 99.1 | <0.01 |
| Geographical Region | ||||
| Central America | 547 (1) | 30.0 (26.2–33.9) | N/A | N/A |
| Australia | 225 (3) | 19.4 (8.5–33.0) | 78 | 0.01 |
| North America | 23,245 (1) | 18.2 (17.7–18.7) | N/A | N/A |
| Europe | 8,736 (2) | 11.5 (5.7–19.0) | 97.3 | <0.01 |
| Asia | 6,628 (4) | 5.3 (3.6–7.4) | 89.3 | <0.01 |
| Clinical phenotype | ||||
| MAFLD with T2DM | 5,691 (5) | 52.1 (30.1–73.7) | 97.7 | <0.01 |
| Lean MAFLD | 5,690 (5) | 31.4 (24.9–38.4) | 84.1 | <0.01 |
| Mixed-MAFLD HCC | ||||
| Overall prevalence | 51556 (17) | 41.3 (34.3–48.5) | 99.5 | <0.01 |
| Geographic region | ||||
| North America | 23,245 (1) | 63.6 (63.0–64.2) | N/A | N/A |
| Europe | 8,914 (4) | 53.8 (43.2–64.3) | 96.3 | <0.01 |
| Asia | 19,260 (11) | 37.2 (27.6–47.4) | 99.1 | <0.01 |
| Australia | 137 (1) | 17.5 (11.6–24.4) | N/A | N/A |
| Primary liver disease | ||||
| HBV | 20,166 (12) | 40.0 (30.2–50.3) | 99.2 | <0.01 |
| HCV | 13,090 (5) | 54.2 (40.4–67.6) | 99.3 | <0.01 |
| ALD | 6,841 (5) | 64.3 (52.7–75.0) | 97.7 | <0.01 |
| Clinical phenotype | ||||
| MAFLD with T2DM | 26,548 (9) | 35.6 (26.3–45.4) | 97.4 | <0.01 |
| Lean-MAFLD | 25,934 (7) | 21.2 (9.8–35.5) | 99.9 | <0.01 |
| Total-MAFLD HCC | ||||
| Overall prevalence | 33,590 (8) | 48.7 (34.6–63.0) | 99.7 | <0.01 |
| Total-MAFLD phenotype | ||||
| MAFLD with T2DM | 32,169 (11) | 34.7 (25.9–44.0) | 98.6 | <0.01 |
| Lean-MAFLD | 31,373 (9) | 21.7 (12.6–32.6) | 99.8 | <0.01 |
MAFLD, metabolic dysfunction-associated fatty liver disease; HCC, hepatocellular carcinoma; CI, confidence interval; T2DM, type 2 diabetes mellitus; HBV, hepatitis B virus; HCV, hepatitis C virus; ALD, alcohol-related liver disease.
Mixed-MAFLD HCC
Seventeen studies (comprising 51,556 HCC cases) reported the prevalence of mixed-MAFLD with a pooled prevalence estimate of 41.3% (95% CI 34.3–48.5%) (Fig. 2B). There was significant heterogeneity between studies (I2=99%, Cochran’s Q <0.01) with individual prevalence estimates ranging from 14% to 80%. A subgroup analysis by geographic region showed highest prevalence in North America (64%), followed by Europe (54%, 95% CI 37–71%), Asia (37%, 95% CI 28–47%), and Australia (18%). Notably, there was evidence of funnel plot asymmetry (P=0.032), and after adjustment for publication bias, the adjusted prevalence estimate was 55.1% (95% CI 47.9–62.2%).
Mixed-MAFLD HCC was stratified by other liver disease aetiology. Twelve studies (comprising 20,166 HCC cases) reported the prevalence of MAFLD in individuals with HBV-related HCC cohorts with a pooled total prevalence of 40.0% (95% CI, 30.1–50.3%). Five studies (comprising 13,090 HCC cases) reported the prevalence of MAFLD in individuals with HCV-related HCC with a pooled total prevalence of 54.1% (95% CI 40.4–67.6%). Five studies (comprising 6,841 HCC cases) reported the prevalence of MAFLD in ALD-related HCC cohorts with a pooled total prevalence of 64.3% (95% CI 52.7–75.0%) (Table 1).
Total-MAFLD HCC
Eight studies (comprising 33,590 HCC cases) reported the total-MAFLD prevalence in HCC cohorts with a pooled prevalence estimate of 48.7% (95% CI 34.5–63.0%) (Fig. 2C). Heterogeneity was high (I2=99.7%, Cochran’s Q <0.0-1) with individual studies reporting total-MAFLD prevalence from 20% to 81%. There was significant funnel plot asymmetry (P=0.037), and after adjustment for publication bias, the adjusted prevalence estimate was 74.1% (95% CI 62.1–84.5%).
MAFLD HCC phenotypes
MAFLD HCC with T2DM
11 studies (comprising 32,169 MAFLD-related HCC cases) reported the prevalence of T2DM in individuals with MAFLD-related HCC (as a single or mixed aetiology), with a total pooled prevalence of 35% (95% CI 26–44%). Four studies (comprising 5,624 HCC cases) reported the prevalence of T2DM in single-aetiology MAFLD HCC cohorts, with a pooled prevalence of 58% (95% CI 35–80%). Nine studies (26,548 HCC cases) reported T2DM prevalence in mixed-aetiology MAFLD HCC cohorts with a pooled prevalence of 36% (95% CI 26–45%) (Supplementary Fig. 2).
Lean MAFLD
Nine studies (comprising 31,373 HCC cases) reported the proportion of individuals with a “lean” phenotype in MAFLD HCC cohorts (single or mixed aetiology), with a total pooled prevalence of 22% (95% CI 13–32%). Four studies (5,623 HCC cases) reported lean phenotype in individuals with single-aetiology MAFLD HCC with pooled prevalence of 30% (95% CI 23–38%). Seven studies (25,934 HCC cases) reported lean phenotype in mixed-aetiology MAFLD HCC with a pooled prevalence of 21% (95% CI 10–35%) (Supplementary Fig. 3).
Clinical characteristics of single-MAFLD, mixed-MAFLD and non-MAFLD HCC
Compared to individuals with non-MAFLD HCC, single-aetiology MAFLD HCC were older (MD 6.64 years, 95% CI 1.87–11.41), more likely to be female (OR 1.21, 95% CI 1.02–1.43) and less likely to have cirrhosis (OR 0.27, 95% CI 0.15–0.51). Single-aetiology MAFLD HCC was associated with lower AFP level (MD –166.34, 95% CI –265.15 to –67.52), a lower likelihood of macrovascular invasion (OR 0.78, 95% CI 0.62–0.97) and higher likelihood of metastatic disease (OR 2.03, 95% CI 1.48–2.79).
Single-MAFLD HCCs were also distinct from mixed-MAFLD HCCs in that they were less likely to have cirrhosis (OR 0.24, 95% CI 0.10–0.58), with higher likelihood of metastatic disease (OR 1.71, 95% CI 1.30–2.25).
Mixed-MAFLD was compared to non-MAFLD HCC. Mixed-MAFLD had higher platelet count (MD 5.74, 95% CI 0.44–11.05), with lower likelihood of macrovascular invasion (OR 0.63, 95% CI 0.45–0.87). There was a trend towards mixed-MAFLD having lower AFP levels than non-MAFLD that did not reach significance (–120.44 CI –240.98 to 0.10, P=0.0502). Data is shown in Table 2.
Table 2.
Comparison of clinical characteristics between single-MAFLD, mixed-MAFLD, and non-MAFLD HCC
| Variable | Single-MAFLD vs. mixed-MAFLD |
Mixed-MAFLD vs. non-MAFLD |
Single-MAFLD vs. non-MAFLD |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample size (number of studies) | Effect size (95% CI) | P-value | I² | Sample size (number of studies) | Effect size (95% CI) | P-value | I² | Sample size (number of studies) | Effect size (95% CI) | P-value | I² | |
| Age | 5,401 (3) | MD 5.82 (–0.64 to 12.28) | 0.077 | 97.7% | 12,606 (9) | MD 0.32 (–0.78 to 1.42) | 0.57 | 80.3% | 5,050 (3) | MD 6.64 (1.87 to 11.40) | 0.006* | 95.6% |
| Female (%) | 5,401 (3) | OR 1.22 (0.47 to 3.16) | 0.68 | 93.5% | 12,606 (9) | 1.12 (0.67 to 1.87) | 0.67 | 96.9% | 5,050 (3) | OR 1.21 (1.02 to 1.43) | 0.028* | 0.0% |
| AFP | 5,158 (2) | MD –3.95 (–73.25 to 65.35) | 0.91 | 68.5% | 7,955 (5) | MD –120.44 (–240.98 to 0.10) | 0.050 | 94.1% | 3,901 (2) | MD –166.34 (–265.15 to –67.52) | 0.001* | 77.5% |
| Platelets | 696 (2) | MD 48.06 (–6.51 to 102.63) | 0.084 | 88.1% | 3,192 (4) | MD 5.74 (0.44 to 11.05) | 0.034* | 0.0% | 1,692 (2) | MD 51.27 (–11.42 to 113.97) | 0.11 | 91.4% |
| Cirrhosis | 5,327 (3) | OR 0.24 (0.10 to 0.58) | 0.002* | 84.3% | 10,008 (7) | OR 1.05 (0.90 to 1.23) | 0.5377 | 21.0% | 4,860 (3) | OR 0.27 (0.15 to 0.51) | <0.001* | 75.6% |
| Largest HCC diameter | 4,948 (2) | MD 0.08 (–0.99 to 1.15) | 0.88 | 80.1% | 8,861 (5) | MD –0.17 (–0.36 to 0.01) | 0.0630 | 58.7% | 4,507 (2) | MD –0.46 (–2.31 to 1.38) | 0.62 | 94.8% |
| Macrovascular invasion | 4,948 (2) | OR 1.06 (0.88 to 1.28) | 0.56 | 0.0% | 7,279 (3) | OR 0.63 (0.45 to 0.87) | 0.005* | 55.5% | 4,507 (2) | OR 0.78 (0.62 to 0.97) | 0.029* | 4.1% |
| Metastatic spread | 4,705 (1) | OR 1.71 (1.30 to 2.25) | <0.001* | N/A | 6,021 (2) | OR 0.84 (0.41 to 1.73) | 0.64 | 86.2% | 3,358 (1) | OR 2.03 (1.48 to 2.79) | <0.001* | N/A |
MAFLD, metabolic dysfunction-associated fatty liver disease; HCC, hepatocellular carcinoma; CI, confidence interval; AFP, alpha fetoprotein; OR, odds ratio; MD, mean difference.
Statistical significance, P<0.05.
Sensitivity analysis and meta-regression
Prevalence estimates were repeated after excluding two studies in which investigators did not confirm the presence of hepatic steatosis. Updated prevalence estimates were 11.6% (95% CI 7.0–17.0%), 39.6% (95% CI 31.3–48.1%) and 40.9% (95% CI 28.2–54.3%) for single-MAFLD, mixed-MAFLD, and total-MAFLD HCC, respectively.
Meta-regressions were performed to identify study level factors which might explain the high between-study heterogeneity (Supplementary Figs. 4–6). For singleMAFLD HCC, studies conducted within Asia, early-stage HCC cohorts, younger cohorts, and cohorts with a higher proportion of HBV were associated with lower prevalence of single-MAFLD HCC. For mixed-MAFLD HCC, earlystage HCC cohorts, shorter duration of study enrolment, and younger cohort age were associated with lower mixedMAFLD prevalence. For total-MAFLD HCC, higher proportion of HBV was associated with lower prevalence of MAFLD. Due to the relatively small number of studies in the sample size, multivariable meta-regressions were not performed. Residual heterogeneity remained high (I2 >95%) in all single meta-regressions. Bubble plots are shown in the supplemental material.
DISCUSSION
Researchers typically report aetiology in HCC cohorts by the single disease thought to represent the most likely cause of liver dysfunction. Dichotomising aetiology into distinct groups has made sense from a pragmatic viewpoint; however, with the increasing global prevalence of MAFLD, there is now the reality that a significant proportion of HCC occurs in the setting of multiple interacting liver diseases. Our understanding regarding MAFLD-related liver disease and HCC continues to evolve [14,26], yet little is known about the prevalence, distinct mechanisms, outcomes, and responses to treatment in these mixed-MAFLD tumours. Thus, in this study, we provide the best global estimate for the total proportion of HCCs attributable to MAFLD, as both a single and mixed aetiology. We find that approximately half of patients with HCC have MAFLD; however, HCC in individuals who have MAFLD as the sole cause of liver disease accounts for only 12% (or 1 in 8 cases). MAFLD is a common HCC cofactor in individuals with all other liver disease aetiologies. Indeed, with advancements in screening and treatment for viral hepatitis, offset by the rising prevalence of obesity and metabolic syndrome, MAFLD may in fact be dominant driver of HCC in many cases which have previously been attributed to viral aetiologies [27]. We have also described the prevalence of different MAFLD-HCC phenotypes including MAFLD with T2DM and lean MAFLD. Lean-MAFLD in particular is purported to have distinct pathophysiological mechanisms and possibly worse outcomes in a non-HCC setting [28], although data is limited in HCC cohorts. Notably, the proportion of MAFLD-HCC classified as “lean” that we have reported is similar to the 19.2% reported in a nonHCC setting by a recent large meta-analysis [29].
To our knowledge, there exists no previous systematic review and meta-analysis estimating the global proportion of HCC attributable to MAFLD, in which systematic ascertainment for MAFLD (using MAFLD diagnostic criteria) has taken place. Numerous cohort studies have reported the proportion of HCC attributable to NAFLD, as recently summarised in the review by Huang et al. [6]. In addition to geographic variability, limiting generalisability is the substantial heterogeneity in how NAFLD has been defined, with estimated global proportions ranging from 1% to 38% [30,31]. Several large HCC cohorts have reported NAFLD prevalence using ICD coding [32] or transplant listing diagnosis [33,34], but such cohorts typically lack systematic evaluation of NAFLD. NAFLD is also known to be underdiagnosed more broadly [35] and thus NAFLD burden in these cohorts is likely under-reported. Smaller studies which utilise radiologic or histologic criteria to diagnose NAFLD, with or without variable components of the metabolic syndrome, still rely on exclusion of other causes of liver disease and estimation of alcohol intake which is known to be unreliable [36]. Significant variability also exists in classification of cryptogenic cirrhosis between studies [6]; thus, using the NAFLD framework to estimate its total burden in HCC cohorts has been problematic. This study is the first systematic review and meta-analysis which has used “positive” diagnostic criteria to estimate the burden of MAFLD. In addition to providing a more robust framework for capturing those with fatty liver disease, those with MAFLD generally represent a more metabolically unhealthy population compared to those with NAFLD. MAFLD is associated with worse markers of liver damage and fibrosis [37], factors associated with HCC risk. MAFLD is also associated with higher risk of extrahepatic events such as atherosclerotic cardiovascular disease [38], reflux oesophagitis [39], chronic obstructive pulmonary disease [40], oesophageal squamous cell carcinoma [41], and Helicobacter pylori negative gastric cancer [42], highlighting that patient populations are not identical.
We have also provided a subgroup analysis of MAFLD prevalence by geographic region. Interestingly, the proportion of HCC attributable to MAFLD was lower in Asia compared to Western cohorts. This contrasts with studies reporting a high prevalence of MAFLD in Asia (estimated to be 36% in a recent meta-analysis [43]), as well as a relatively higher incidence of liver complications due to MAFLD in Asia compared to global incidence rates [44]. This seemingly contradictory finding may be partly explained by the competing risk of viral hepatitis which remains the predominant risk factor for HCC in Asia, although studies have shown a progressive shift towards MAFLD [45,46].
A number of studies have examined differences in clinical characteristics and outcomes of HCC between different aetiologies of liver disease [47-49]. A recent meta-analysis by Tan et al. [7] compared NAFLD HCC (as defined by imaging, histology or ICD coding in the absence of significant alcohol consumption or other causes of chronic liver disease) to other causes of HCC. This study reported larger and more frequently uninodular tumours in NAFLD-HCC, but no difference in AFP, tumour stage, treatment allocation or overall survival. However, as we have shown in our meta-analysis, there is significant overlap between MAFLD and other causes of HCC which may make interpretation of this comparison difficult. Thus, in order to further explore this mixed-MAFLD phenotype, we performed a three-way comparison between single-MAFLD, mixed-MAFLD, and non-MAFLD. We report a number of phenotypic differences between the study subgroups. Consistent with Tan et al. [7], single-MAFLD HCC tended to occur in subjects who were older with noncirrhotic liver disease compared to non-MAFLD HCC. We also report findings to suggest differences in tumour biology and behaviour, including a lower AFP level, higher likelihood of metastatic spread, and lower likelihood of macrovascular invasion.
Importantly, we also sought to find differences between non-MAFLD and mixed-MAFLD HCC, in order to determine whether the addition of MAFLD as a co-factor to another liver disease might influence clinical phenotype or tumour biology. One might hypothesise that our finding of a higher platelet count in mixed-MAFLD compared to non-MAFLD HCC may reflect a propensity for the addition of MAFLD to cause HCC at an earlier stage of liver disease. Likewise, the lower likelihood of macrovascular invasion and trend towards lower AFP suggests these mixed-aetiology tumours may have different molecular and biological behaviour compared to “pure” non-MAFLD HCC.
The “mixed-MAFLD” category used in this analysis itself represents a heterogenous group comprised of various primary liver disease aetiologies, each with their own distinct pathological mechanisms. Nevertheless, the idea that the addition of MAFLD to another liver disease aetiology might alter tumour biology is intriguing, and at the very least, adds to the impetus to accurately diagnose and report MAFLD in future research studies so that the interaction between liver diseases can be better understood.
This study has several strengths. We analysed data from 22 studies and over 56,000 HCC cases in order to provide the best current global estimate for MAFLD prevalence in individuals with HCC. We included only studies which diagnosed MAFLD using the international expert panel criteria including use of ethnicity-specific BMI cut-offs. The objective diagnostic criteria eliminate subjectivity from diagnosis (such as estimating alcohol intake) and theoretically produce a more replicable prevalence estimate.
Nevertheless, our study has limitations. There were relatively few studies reporting MAFLD prevalence from certain geographic regions (most notably North America); thus these prevalence estimates require further validation and should be interpreted with caution. Secondly, few (if any) studies published data on all metabolic variables which make up the MAFLD diagnostic criteria. We included studies which defined metabolic dysfunction using a minimum of BMI and T2DM data. Of note, data from a recent large Korean MAFLD cohort suggested the non-overweight, non-diabetic phenotype accounted for only 5% of individuals with MAFLD [50], and thus our inclusion criteria likely capture the majority of individuals with MAFLD but may still underestimate the true prevalence. Thirdly, the method of assessing hepatic steatosis was variable across studies which may account for some heterogeneity in MAFLD prevalence estimates. In particular, two large multicentre cohorts (Italian ITA.LI.CA. HCC registry and U.S. United Network for Organ Sharing [UNOS] liver transplant registry) defined MAFLD by metabolic dysfunction alone without confirmation of present or historical hepatic steatosis. It is notable that most mixed-MAFLD HCC cases in these large Western cohorts (87% and 94%, respectively) were metabolically unhealthy individuals in the setting of either ALD or HCV infection, in whom the vast majority of individuals would be expected to have current or historical evidence of hepatic steatosis; nevertheless, these studies may have overestimated MAFLD prevalence. We have reported a sensitivity analysis excluding these studies. Data is also still lacking on HCC outcomes between these groups and is an area that requires further research.
In conclusion, MAFLD is common among individuals with HCC, both as the sole cause of liver disease and as a cofactor with other liver diseases. This study supports the benefit of systematically ascertaining for metabolic dysfunction using positive diagnostic criteria, irrespective of alcohol use or other liver disease, so that the interaction between concurrent liver diseases can be better understood.
Acknowledgments
The authors would like to acknowledge the NSW Government through the Cancer Institute NSW for grant funding that supported this work (2021/ATRG2028). JG is supported by the Robert W. Storr Bequest to the Sydney Medical Foundation, University of Sydney; a National Health and Medical Research Council of Australia (NHMRC) Program Grant (APP1053206), Project, Ideas and Investigator grants (APP2001692, APP1107178, APP1108422, APP1196492) and a Cancer Institute, NSW grant (2021/ATRG2028).
Abbreviations
- MAFLD
metabolic dysfunction-associated fatty liver disease
- HCC
hepatocellular carcinoma
- CI
confidence interval
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- ALD
alcohol-related liver disease
- NAFLD/NASH
non-alcoholic fatty liver disease/non-alcoholic steatohepatitis
- BMI
body mass index
- T2DM
type 2 diabetes mellitus
- FLI
fatty liver index
- OR
odds ratio
- MD
mean difference
- AFP
alpha fetoprotein
- ICD
international classification of diseases
Study Highlights
• The proportion of HCC for which MAFLD is the aetiological cause or contributing factor is unknown.
• In this global systematic review and meta-analysis of over 56,000 individuals with HCC, we define the prevalence of MAFLD in the presence and absence of other liver diseases.
• 49% of all individuals with HCC have MAFLD; however, MAFLD as a sole liver disease accounts for 12% of HCC.
• Individuals with MAFLD plus another liver disease appeared phenotypically distinct from both “pure” MAFLD and non-MAFLD HCC.
• This supports the use of positive diagnostic criteria and systematically ascertaining for MAFLD in all individuals with HCC.
Footnotes
Authors’ contribution
Study concept and supervision: HC, GE, JG. Study design: HC, GE, JG. Data analysis: HC, GE. Data collection: All authors. Drafting of manuscript: HC, JG. Data interpretation, review, and revision of manuscript: All authors.
Conflicts of Interest
JG is on Advisory Boards and has received honoraria for talks from Novo Nordisk, Astra Zeneca, Roche, BMS, Pfizer, Cincera, Pharmaxis, Boehringer Mannheim.
SUPPLEMENTAL MATERIAL
Supplementary material is available at Clinical and Molecular Hepatology website (http://www.e-cmh.org).
PRISMA checklist
Characteristics of studies included in meta-analysis
Bias assessment
MAFLD diagnostic criteria (adapted with permission from 1). MAFLD, metabolic dysfunction-associated fatty liver disease; BMI, body mass index; HDL, High-density lipoprotein.
Forrest plots and pooled prevalence of T2DM in all individuals with MAFLD-HCC (A), single-aetiology MAFLDHCC (B), and mixed-aetiology MAFLD HCC (C). T2DM, type 2 diabetes mellitus; MAFLD, metabolic dysfunction-associated fatty liver disease; HCC, hepatocellular carcinoma; CI, confidence interval.
Forrest plots and pooled prevalence of “lean MAFLD” in all individuals with MAFLD-HCC (A), single-aetiology MAFLD-HCC (B), and mixed-aetiology MAFLD HCC (C). MAFLD, metabolic dysfunction-associated fatty liver disease; HCC, hepatocellular carcinoma; CI, confidence interval.
Meta-regression analysis as depicted by bubble plots for single-MAFLD HCC. Transformed prevalence depicted on y-axis. Sources of heterogeneity assessed are whether study conducted within Asia (A), whether study limited to early stage HCC cohorts (B), enrolment duration (C), median cohort age (D), proportion of HCC cohort with HBV (E), and proportion of cohort female (F). MAFLD, metabolic dysfunction-associated fatty liver disease; HCC, hepatocellular carcinoma; HBV, hepatitis B virus.
Mixed-MAFLD HCC meta-regression bubble plots showing whether study conducted within Asia (A), whether study limited to early stage HCC cohorts (B), enrolment duration (C), median cohort age (D), proportion of HCC cohort with HBV (E), proportion of cohort female (F). MAFLD, metabolic dysfunction-associated fatty liver disease; HCC, hepatocellular carcinoma; HBV, hepatitis B virus.
Total-MAFLD HCC meta-regression bubble plots showing whether study conducted within Asia (A), whether study limited to early stage HCC cohorts (B), enrolment duration (C), median cohort age (D), proportion of HCC cohort with HBV (E), and proportion of cohort female (F). MAFLD, metabolic dysfunction-associated fatty liver disease; HCC, hepatocellular carcinoma; HBV, hepatitis B virus.
REFERENCES
- 1.Rumgay H, Arnold M, Ferlay J, Lesi O, Cabasag CJ, Vignat J, et al. Global burden of primary liver cancer in 2020 and predictions to 2040. J Hepatol. 2022;77:1598–1606. doi: 10.1016/j.jhep.2022.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Petrick JL, McGlynn KA. The changing epidemiology of primary liver cancer. Curr Epidemiol Rep. 2019;6:104–111. doi: 10.1007/s40471-019-00188-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J Hepatol. 2020;73:202–209. doi: 10.1016/j.jhep.2020.03.039. [DOI] [PubMed] [Google Scholar]
- 4.Eslam M, Sanyal AJ, George J, International Consensus Panel MAFLD: A consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. 2020;158:1999–2014.e1. doi: 10.1053/j.gastro.2019.11.312. [DOI] [PubMed] [Google Scholar]
- 5.Kawaguchi T, Tsutsumi T, Nakano D, Eslam M, George J, Torimura T. MAFLD enhances clinical practice for liver disease in the Asia-Pacific region. Clin Mol Hepatol. 2022;28:150–163. doi: 10.3350/cmh.2021.0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang DQ, El-Serag HB, Loomba R. Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2021;18:223–238. doi: 10.1038/s41575-020-00381-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tan DJH, Ng CH, Lin SY, Pan XH, Tay P, Lim WH, et al. Clinical characteristics, surveillance, treatment allocation, and outcomes of non-alcoholic fatty liver disease-related hepatocellular carcinoma: a systematic review and meta-analysis. Lancet Oncol. 2022;23:521–530. doi: 10.1016/S1470-2045(22)00078-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dudek M, Pfister D, Donakonda S, Filpe P, Schneider A, Laschinger M, et al. Auto-aggressive CXCR6+ CD8 T cells cause liver immune pathology in NASH. Nature. 2021;592:444–449. doi: 10.1038/s41586-021-03233-8. [DOI] [PubMed] [Google Scholar]
- 9.Pfister D, Núñez NG, Pinyol R, Govaere O, Pinter M, Szydlowska M, et al. NASH limits anti-tumour surveillance in immunotherapy-treated HCC. Nature. 2021;592:450–456. doi: 10.1038/s41586-021-03362-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rimini M, Rimassa L, Ueshima K, Burgio V, Shigeo S, Tada T, et al. Atezolizumab plus bevacizumab versus lenvatinib or sorafenib in non-viral unresectable hepatocellular carcinoma: an international propensity score matching analysis. ESMO Open. 2022;7:100591. doi: 10.1016/j.esmoop.2022.100591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shimose S, Hiraoka A, Casadei-Gardini A, Tsutsumi T, Nakano D, Iwamoto H, et al. The beneficial impact of metabolic dysfunction-associated fatty liver disease on lenvatinib treatment in patients with non-viral hepatocellular carcinoma. Hepatol Res. 2023;53:104–115. doi: 10.1111/hepr.13843. [DOI] [PubMed] [Google Scholar]
- 12.Crane H, Gofton C, Sharma A, George J. MAFLD: an optimal framework for understanding liver cancer phenotypes. J Gastroenterol. 2023;58:947–964. doi: 10.1007/s00535-023-02021-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao F, Chen G, Byrne CD, Targher G, Cheung TT, Zheng MH. Metabolic dysfunction-associated fatty liver disease and hepatocellular carcinoma: present and future. Hepatobiliary Surg Nutr. 2023;12:945–948. doi: 10.21037/hbsn-23-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Llovet JM, Willoughby CE, Singal AG, Greten TF, Heikenwälder M, El-Serag HB, et al. Nonalcoholic steatohepatitisrelated hepatocellular carcinoma: pathogenesis and treatment. Nat Rev Gastroenterol Hepatol. 2023;20:487–503. doi: 10.1038/s41575-023-00754-7. [DOI] [PubMed] [Google Scholar]
- 15.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and metaanalyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freeman MF, Tukey JW. Transformations related to the angular and the square root. The Annals of Mathematical Statistics. 1950;21:607–611. [Google Scholar]
- 17.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 18.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duval S, Tweedie R. A nonparametric “Trim and Fill” method of accounting for publication bias in meta-analysis. J Am Stat Assoc. 2000;95:89–98. [Google Scholar]
- 20.Hoy D, Brooks P, Woolf A, Blyth F, March L, Bain C, et al. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol. 2012;65:934–939. doi: 10.1016/j.jclinepi.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 21.Yun B, Ahn SH, Oh J, Yoon JH, Kim BK. Prognostic impact of MAFLD following surgical resection of hepatitis B virus-related hepatocellular carcinoma: A nationwide cohort study. Cancers (Basel) 2022;14:5002. doi: 10.3390/cancers14205002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reggidori N, Bucci L, Santi V, Stefanini B, Lani L, Rampoldi D, et al. Lack of substantial improvements in the landscape of alcohol-related hepatocellular carcinoma in the last 15 years: The need to improve cancer prevention and surveillance. Dig Liver Dis. 2023;55(Suppl 1):S16–S17. [Google Scholar]
- 23.Conci S, Cipriani F, Donadon M, Marchitelli I, Ardito F, Famularo S, et al. Hepatectomy for Metabolic Associated Fatty Liver Disease (MAFLD) related HCC: Propensity case-matched analysis with viral- and alcohol-related HCC. Eur J Surg Oncol. 2022;48:103–112. doi: 10.1016/j.ejso.2021.07.015. [DOI] [PubMed] [Google Scholar]
- 24.Kim MN, Han K, Yoo J, Hwang SG, Ahn SH. Metabolic dysfunction-associated fatty liver disease subgroups and risk of hepatocellular carcinoma and mortality in patients with chronic viral hepatitis. J Hepatol. 2022;77:S100. [Google Scholar]
- 25.Yu MW, Lin CL, Liu CJ, Wu WJ, Hu JT, Huang YW. Metabolicassociated fatty liver disease, hepatitis B surface antigen seroclearance, and long-term risk of hepatocellular carcinoma in chronic hepatitis B. Cancers (Basel) 2022;14:6012. doi: 10.3390/cancers14236012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feng G, Valenti L, Wong VW, Fouad YM, Yilmaz Y, Kim W, et al. Recompensation in cirrhosis: unravelling the evolving natural history of nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 2024;21:46–56. doi: 10.1038/s41575-023-00846-4. [DOI] [PubMed] [Google Scholar]
- 27.van der Spek DPC, Katwaroe WK, van Kleef LA, Brakenhoff S, de Man RA, de Knegt RJ, et al. Time-trends in disease characteristics and comorbidities in patients with chronic hepatitis B in the period 1980-2020. Eur J Intern Med. 2023;107:86–92. doi: 10.1016/j.ejim.2022.11.012. [DOI] [PubMed] [Google Scholar]
- 28.Eslam M, El-Serag HB, Francque S, Sarin SK, Wei L, Bugianesi E, et al. Metabolic (dysfunction)-associated fatty liver disease in individuals of normal weight. Nat Rev Gastroenterol Hepatol. 2022;19:638–651. doi: 10.1038/s41575-022-00635-5. [DOI] [PubMed] [Google Scholar]
- 29.Ye Q, Zou B, Yeo YH, Li J, Huang DQ, Wu Y, et al. Global prevalence, incidence, and outcomes of non-obese or lean non-alcoholic fatty liver disease: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5:739–752. doi: 10.1016/S2468-1253(20)30077-7. [DOI] [PubMed] [Google Scholar]
- 30.Park JW, Chen M, Colombo M, Roberts LR, Schwartz M, Chen PJ, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int. 2015;35:2155–2166. doi: 10.1111/liv.12818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patkar S, Parray A, Mahendra B, Kurunkar S, Goel M. Performance of Hong Kong Liver Cancer staging system in patients of hepatocellular carcinoma treated with surgical resection: An Indian validation study. J Surg Oncol. 2019;120:1119–1125. doi: 10.1002/jso.25704. [DOI] [PubMed] [Google Scholar]
- 32.Younossi ZM, Otgonsuren M, Henry L, Venkatesan C, Mishra A, Erario M, et al. Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004 to 2009. Hepatology. 2015;62:1723–1730. doi: 10.1002/hep.28123. [DOI] [PubMed] [Google Scholar]
- 33.Haldar D, Kern B, Hodson J, Armstrong MJ, Adam R, Berlakovich G, et al. Outcomes of liver transplantation for non-alcoholic steatohepatitis: A European Liver Transplant Registry study. J Hepatol. 2019;71:313–322. doi: 10.1016/j.jhep.2019.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Younossi ZM, Stepanova M, Ong J, Trimble G, AlQahtani S, Younossi I, et al. Nonalcoholic steatohepatitis is the most rapidly increasing indication for liver transplantation in the United States. Clin Gastroenterol Hepatol. 2021;19:580–589. doi: 10.1016/j.cgh.2020.05.064. e5. [DOI] [PubMed] [Google Scholar]
- 35.Alexander M, Loomis AK, Fairburn-Beech J, van der Lei J, Duarte-Salles T, Prieto-Alhambra D, et al. Real-world data reveal a diagnostic gap in non-alcoholic fatty liver disease. BMC Med. 2018;16:130. doi: 10.1186/s12916-018-1103-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Staufer K, Huber-Schönauer U, Strebinger G, Pimingstorfer P, Suesse S, Scherzer TM, et al. Ethyl glucuronide in hair detects a high rate of harmful alcohol consumption in presumed nonalcoholic fatty liver disease. J Hepatol. 2022;77:918–930. doi: 10.1016/j.jhep.2022.04.040. [DOI] [PubMed] [Google Scholar]
- 37.van Kleef LA, Ayada I, Alferink LJM, Pan Q, de Knegt RJ. Metabolic dysfunction-associated fatty liver disease improves detection of high liver stiffness: The Rotterdam Study. Hepatology. 2022;75:419–429. doi: 10.1002/hep.32131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsutsumi T, Eslam M, Kawaguchi T, Yamamura S, Kawaguchi A, Nakano D, et al. MAFLD better predicts the progression of atherosclerotic cardiovascular risk than NAFLD: Generalized estimating equation approach. Hepatol Res. 2021;51:1115–1128. doi: 10.1111/hepr.13685. [DOI] [PubMed] [Google Scholar]
- 39.Fukunaga S, Nakano D, Tsutsumi T, Kawaguchi T, Eslam M, Yoshinaga S, et al. Lean/normal-weight metabolic dysfunction-associated fatty liver disease is a risk factor for reflux esophagitis. Hepatol Res. 2022;52:699–711. doi: 10.1111/hepr.13795. [DOI] [PubMed] [Google Scholar]
- 40.Tsutsumi T, Nakano D, Kawaguchi M, Hashida R, Yoshinaga S, Takahashi H, et al. MAFLD associated with COPD via systemic inflammation independent of aging and smoking in men. Diabetol Metab Syndr. 2022;14:115. doi: 10.1186/s13098-022-00887-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fukunaga S, Mukasa M, Nakane T, Nakano D, Tsutsumi T, Chou T, et al. Impact of non-obese metabolic dysfunctionassociated fatty liver disease on risk factors for the recurrence of esophageal squamous cell carcinoma treated with endoscopic submucosal dissection: A multicenter study. Hepatol Res. 2024;54:201–212. doi: 10.1111/hepr.13973. [DOI] [PubMed] [Google Scholar]
- 42.Nakane T, Fukunaga S, Nakano D, Tsutsumi T, Tanaka H, Chou T, et al. Impact of metabolic dysfunction-associated fatty liver disease on the incidence of Helicobacter pylori-negative gastric cancer. Hepatol Res. 2024;54:540–550. doi: 10.1111/hepr.14010. [DOI] [PubMed] [Google Scholar]
- 43.Chan KE, Koh TJL, Tang ASP, Quek J, Yong JN, Tay P, et al. Global prevalence and clinical characteristics of metabolicassociated fatty liver disease: A meta-analysis and systematic review of 10 739 607 Individuals. J Clin Endocrinol Metab. 2022;107:2691–2700. doi: 10.1210/clinem/dgac321. [DOI] [PubMed] [Google Scholar]
- 44.Golabi P, Paik JM, AlQahtani S, Younossi Y, Tuncer G, Younossi ZM. Burden of non-alcoholic fatty liver disease in Asia, the Middle East and North Africa: Data from Global Burden of Disease 2009-2019. J Hepatol. 2021;75:795–809. doi: 10.1016/j.jhep.2021.05.022. [DOI] [PubMed] [Google Scholar]
- 45.Zhang CH, Cheng Y, Zhang S, Fan J, Gao Q. Changing epidemiology of hepatocellular carcinoma in Asia. Liver Int. 2022;42:2029–2041. doi: 10.1111/liv.15251. [DOI] [PubMed] [Google Scholar]
- 46.Enomoto H, Ueno Y, Hiasa Y, Nishikawa H, Hige S, Takikawa Y, et al. The transition in the etiologies of hepatocellular carcinoma-complicated liver cirrhosis in a nationwide survey of Japan. J Gastroenterol. 2021;56:158–167. doi: 10.1007/s00535-020-01748-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kumar R, Goh BG, Kam JW, Chang PE, Tan CK. Comparisons between non-alcoholic steatohepatitis and alcohol-related hepatocellular carcinoma. Clin Mol Hepatol. 2020;26:196–208. doi: 10.3350/cmh.2019.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tokushige K, Hashimoto E, Yatsuji S, Tobari M, Taniai M, Torii N, et al. Prospective study of hepatocellular carcinoma in nonalcoholic steatohepatitis in comparison with hepatocellular carcinoma caused by chronic hepatitis C. J Gastroenterol. 2010;45:960–967. doi: 10.1007/s00535-010-0237-1. [DOI] [PubMed] [Google Scholar]
- 49.Piscaglia F, Svegliati-Baroni G, Barchetti A, Pecorelli A, Marinelli S, Tiribelli C, et al. Clinical patterns of hepatocellular carcinoma in nonalcoholic fatty liver disease: A multicenter prospective study. Hepatology. 2016;63:827–838. doi: 10.1002/hep.28368. [DOI] [PubMed] [Google Scholar]
- 50.Chung GE, Yu SJ, Yoo JJ, Cho Y, Lee KN, Shin DW, et al. Lean or diabetic subtypes predict increased all-cause and disease-specific mortality in metabolic-associated fatty liver disease. BMC Med. 2023;21:4. doi: 10.1186/s12916-022-02716-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA checklist
Characteristics of studies included in meta-analysis
Bias assessment
MAFLD diagnostic criteria (adapted with permission from 1). MAFLD, metabolic dysfunction-associated fatty liver disease; BMI, body mass index; HDL, High-density lipoprotein.
Forrest plots and pooled prevalence of T2DM in all individuals with MAFLD-HCC (A), single-aetiology MAFLDHCC (B), and mixed-aetiology MAFLD HCC (C). T2DM, type 2 diabetes mellitus; MAFLD, metabolic dysfunction-associated fatty liver disease; HCC, hepatocellular carcinoma; CI, confidence interval.
Forrest plots and pooled prevalence of “lean MAFLD” in all individuals with MAFLD-HCC (A), single-aetiology MAFLD-HCC (B), and mixed-aetiology MAFLD HCC (C). MAFLD, metabolic dysfunction-associated fatty liver disease; HCC, hepatocellular carcinoma; CI, confidence interval.
Meta-regression analysis as depicted by bubble plots for single-MAFLD HCC. Transformed prevalence depicted on y-axis. Sources of heterogeneity assessed are whether study conducted within Asia (A), whether study limited to early stage HCC cohorts (B), enrolment duration (C), median cohort age (D), proportion of HCC cohort with HBV (E), and proportion of cohort female (F). MAFLD, metabolic dysfunction-associated fatty liver disease; HCC, hepatocellular carcinoma; HBV, hepatitis B virus.
Mixed-MAFLD HCC meta-regression bubble plots showing whether study conducted within Asia (A), whether study limited to early stage HCC cohorts (B), enrolment duration (C), median cohort age (D), proportion of HCC cohort with HBV (E), proportion of cohort female (F). MAFLD, metabolic dysfunction-associated fatty liver disease; HCC, hepatocellular carcinoma; HBV, hepatitis B virus.
Total-MAFLD HCC meta-regression bubble plots showing whether study conducted within Asia (A), whether study limited to early stage HCC cohorts (B), enrolment duration (C), median cohort age (D), proportion of HCC cohort with HBV (E), and proportion of cohort female (F). MAFLD, metabolic dysfunction-associated fatty liver disease; HCC, hepatocellular carcinoma; HBV, hepatitis B virus.