Abstract
Vibrio parahaemolyticus, Vibrio vulnificus and non‐O1/non‐O139 Vibrio cholerae are the Vibrio spp. of highest relevance for public health in the EU through seafood consumption. Infection with V. parahaemolyticus is associated with the haemolysins thermostable direct haemolysin (TDH) and TDH‐related haemolysin (TRH) and mainly leads to acute gastroenteritis. V. vulnificus infections can lead to sepsis and death in susceptible individuals. V. cholerae non‐O1/non‐O139 can cause mild gastroenteritis or lead to severe infections, including sepsis, in susceptible individuals. The pooled prevalence estimate in seafood is 19.6% (95% CI 13.7–27.4), 6.1% (95% CI 3.0–11.8) and 4.1% (95% CI 2.4–6.9) for V. parahaemolyticus, V. vulnificus and non‐choleragenic V. cholerae, respectively. Approximately one out of five V. parahaemolyticus‐positive samples contain pathogenic strains. A large spectrum of antimicrobial resistances, some of which are intrinsic, has been found in vibrios isolated from seafood or food‐borne infections in Europe. Genes conferring resistance to medically important antimicrobials and associated with mobile genetic elements are increasingly detected in vibrios. Temperature and salinity are the most relevant drivers for Vibrio abundance in the aquatic environment. It is anticipated that the occurrence and levels of the relevant Vibrio spp. in seafood will increase in response to coastal warming and extreme weather events, especially in low‐salinity/brackish waters. While some measures, like high‐pressure processing, irradiation or depuration reduce the levels of Vibrio spp. in seafood, maintaining the cold chain is important to prevent their growth. Available risk assessments addressed V. parahaemolyticus in various types of seafood and V. vulnificus in raw oysters and octopus. A quantitative microbiological risk assessment relevant in an EU context would be V. parahaemolyticus in bivalve molluscs (oysters), evaluating the effect of mitigations, especially in a climate change scenario. Knowledge gaps related to Vibrio spp. in seafood and aquatic environments are identified and future research needs are prioritised.
Keywords: analytical methods, antimicrobial resistance, climate change, interventions, public health, risk assessment modelling, virulence
SUMMARY
The European Food Safety Authority (EFSA) asked the Panel on Biological Hazards (BIOHAZ) to deliver a scientific opinion on the public health (PH) aspects of Vibrio spp. related to the consumption of seafood in the EU. Infections deriving from environmental, recreational and/or occupational exposure to vibrios were not considered. The assessment had to cover Vibrio parahaemolyticus, Vibrio vulnificus and non‐O1/non‐O139 Vibrio cholerae; other species had to be considered as well, when relevant.
In ToR1, EFSA was requested to review, for the relevant Vibrio spp., the existing information on occurrence and concentration in seafood, available analytical methods, pathogenicity to humans and virulence factors, as well as antimicrobial resistance (AMR) and persistence mechanisms in different environments. It was decided that the relevant Vibrio spp. to be considered in the current opinion are V. parahaemolyticus, V. vulnificus and non‐O1/non‐O139 V. cholerae. Other species as Vibrio alginolyticus, Vibrio fluvialis and Vibrio mimicus may occasionally lead, particularly in individuals with underlying health conditions, to seafood‐associated infection, but their PH relevance is minor compared to those three species.
Based on the available data on seafood placed or intended to be placed on the EU market, it was concluded that, across seafood categories, the V. parahaemolyticus pooled prevalence estimate was 19.6% (95% CI 13.7–27.4), with the highest estimates for bivalve molluscs (27.8%; 95% CI 18.6–39.5) and gastropods (28.8%; 95% CI 10.5–58.3). About one out of five (18.4%; 95% CI 11.1–29.01) of V. parahaemolyticus‐positive samples contained pathogenic (i.e. tdh+ and/or trh+) strains. V. parahaemolyticus pooled mean concentration in bivalve molluscs was 1.91 log10 CFU/g or MPN/g (95% CI 0.68–3.14), while in crustaceans, was 3.33 log10 CFU/g (single study). The V. vulnificus pooled prevalence estimate was 6.1% (95% CI 3.0–11.8) and was highest for bivalve molluscs with 9.9% (95% CI 4.0–22.60); with a pooled mean concentration of 1.84 log10 CFU/g or MPN/g (95% CI –2.33 to 6.01). Non‐choleragenic V. cholerae pooled prevalence estimate was 4.1% (95% CI 2.4–6.9) and the mean concentration was 1.34 MPN/g (single study). No data were retrieved in either tunicates or echinoderms.
Standardised microbiological methods are available for the detection of the relevant Vibrio spp. in seafood and for the enumeration of V. parahaemolyticus and V. vulnificus. These methods rely on culturing and include molecular tests for species identification and/or for the detection of genes associated with pathogenicity. Alternative approaches are available for the detection, enumeration and identification (e.g. loop‐mediated isothermal amplification (LAMP)‐PCR, quantitative, digital and viability PCR, matrix‐assisted laser desorption ionisation–time of flight mass spectroscopy or MALDI–TOF MS). For characterisation, serotyping and pulsed‐field gel electrophoresis (PFGE) have been progressively replaced by sequencing technologies [e.g. (core genome) multi‐locus sequence typing or (cg)MLST schemes]. Whole genome sequencing (WGS) is progressively more applied for full characterisation of clinical Vibrio isolates and in outbreak investigation but currently has limited use in official food control activities in the EU.
Pathogenic Vibrio species possess a wide array of virulence factors (VFs) that allow the colonisation and spread in the hosts. Their virulence is multifactorial; although with different impact, some VFs may be present in all species (i.e. capsule, flagellar mobility and pili), while other VFs are specific to certain species or strains within one species. The outcome of an infection with Vibrio species is determined both by bacterial and host factors. V. parahaemolyticus mainly leads to acute gastroenteritis (GE), also in healthy individuals. Its pathogenicity is significantly associated with the haemolysins TDH (thermostable direct haemolysin) and TRH (TDH‐related haemolysin), which are well‐established pathogenicity markers. The type 3 secretion system 2 (T3SS2) has been also associated with pathogenic strains. V. vulnificus infections are relatively rare and affect mainly individuals with underlying health conditions, possibly leading to sepsis and death. This species possesses several VFs but, as reliable discrimination of pathogenic from non‐pathogenic strains is lacking, to date all V. vulnificus strains are considered potentially pathogenic. V. cholerae non‐O1/non‐O139 is generally associated with self‐limited GE or mild extraintestinal symptoms. However, in susceptible individuals, strains can cause more severe infections, sepsis and death. For this species, no single pathogenicity marker has been identified so far.
A large spectrum of AMRs, of which some are known as intrinsic resistances, has been reported in the few studies on Vibrio isolates from seafood or food‐borne infections in Europe. The AMRs most frequently detected were ampicillin (70%–100%; seven studies) and streptomycin (30%–70%; six studies) for V. parahaemolyticus, and colistin (87%–100%; four studies), ampicillin (4%–75%; five studies) and streptomycin (11%–68%; four studies) for non‐O1/non‐O139 V. cholerae. Antimicrobial resistance genes (ARGs) associated with mobile genetic elements, conferring resistance to various β‐lactam types, quinolones, sulfonamides, aminoglycosides, tetracyclines, folate pathway inhibitors and phenicols, have been detected in Vibrio spp. Resistances against medically important antimicrobials (MIAs) like carbapenems (authorised only for humans) and 3rd/4th‐generation cephalosporins (categorised as highly important antimicrobials) associated with mobile elements are increasingly found in the relevant Vibrio spp., and detection in imported seafood isolates has been reported.
The main persistence mechanisms of the relevant Vibrio species in the aquatic environment include the VBNC state, biofilm formation on biotic and abiotic surfaces, persister cells, anti‐grazing strategies to avoid protozoan predation and association with other aquatic organisms acting as reservoirs.
In ToR2, EFSA was requested to identify the factors in the aquatic environments and in food that influence occurrence and growth of the relevant Vibrio spp. and affect transmission of their virulence and resistance determinants. It was concluded that the impacting factors are temperature, (sea)water salinity, solar and UV radiation (extrinsic factors); pH, water activity of food, nutrient content (intrinsic factors) and predation, parasitism and commensalism (implicit factors). Temperature is the most relevant driver for Vibrio abundance in the aquatic environment and in food, followed, in the environment, by salinity. Complex interactions among environmental factors make region‐specific environmental models and validations required. Transmission of virulence or resistance determinants is affected by the presence of chitin (triggering natural competence) and phages (acting as reservoirs of these genetic determinants), and contaminants of the aquatic ecosystem such as antimicrobials and heavy metals.
In ToR3, EFSA was requested to review the evidence on the impact of climate change on the occurrence and levels of the relevant Vibrio spp. in water environments and seafood. Climate change induces a shift towards conditions in the aquatic environment suitable and more conducive for Vibrio growth and persistence. It affects: (i) the geographical distribution of the coastal areas suitable for Vibrio spp., with areas characterised by brackish/low‐salinity waters (e.g. Baltic Sea, transitional waters of the Baltic and the North Sea, the Black Sea) and coastal areas with large riverine inputs at higher risk; (ii) the temporal distribution of conditions suitable for Vibrio spp.; (iii) the frequency, distribution and potentially intensity of extreme weather events which may provide conditions favourable to Vibrio spp. Climate change may also affect the structure (i.e. the composition) of the Vibrio populations in the aquatic environments or speed up the evolution or selection of new Vibrio variants. It is anticipated that the occurrence and levels of the relevant Vibrio spp. in seafood will increase both globally and in Europe in response to coastal warming and extreme weather events as heatwaves, especially in low‐salinity/brackish waters.
In ToR4, EFSA was requested to list and review prevention and control measures along the food chain for the relevant Vibrio spp. It was concluded that maintaining the cold chain is important to prevent vibrios' growth in seafood. A mild thermal treatment of oysters in water at 50°C with or without thermal shock, (flash) freezing followed by long‐term frozen storage, high‐pressure processing using industrially feasible conditions or irradiation reduce vibrios in seafood. Depuration under controlled conditions, although with variable reductions, is a post‐harvest processing treatments for the segment of the market preferring consumption of live oysters.
In ToR5, EFSA was requested to review risk assessment modelling options for Vibrio spp. in seafood and to identify the knowledge gaps and data needed to perform a risk assessment on the public health impact of the relevant Vibrio spp. in seafood at the EU level. The identified QMRA addressed V. parahaemolyticus in various types of seafood, and V. vulnificus in raw oysters and octopus, and no risk assessment addressed non‐O1/non‐O139 V. cholerae. The majority of QMRAs had a national or regional scope and two covered conditions in Europe. Most rely heavily on the first US FDA assessment of V. parahaemolyticus in raw oysters, which was also applied to V. vulnificus. Their scope ranged from harvest to consumption. Changes in numbers of Vibrio spp. in seafood are estimated from time–temperature at different stages in the food chain considering growth and sometimes inactivation and removal/washing. The beta‐Poisson dose–response (DR) models for V. parahaemolyticus (based on tdh+ strains) and V. vulnificus have limitations and the applicability to conditions in EU is unknown. The endpoints are the probability of illness for the general or susceptible populations and range from GE to severe illness for V. parahaemolyticus and septicaemia for V. vulnificus. Other endpoints or populations are evaluated by multiplying with the proportions of illness cases affected by these outcomes or the proportion of the population of interest to the whole population based on epidemiological and census data. Consumption data, dietary habits, food origin and food types are very specific to the country or region of interest and were based on several types of consumption surveys and/or additional consumer related data to estimate exposure. Mitigations during harvest or directly after included practices motivated by sea surface temperature (SST) – considering the effects of seasons and climate change – and by pathogen occurrence, such as time to refrigeration and depuration. In later stages, practices evaluated were time–temperature during storage and cooking/heating, the effect of setting different pathogen target levels on the number of illnesses averted and the subsequent percentage of non‐compliant harvest lost (harvest above the target levels), as well as more specific mitigations (e.g. adding vinegars, washing of fish cavities). The impact of climate change and different mitigations was only evaluated for V. parahaemolyticus in bivalve molluscs, most notably oysters. A QMRA relevant in an EU context would be V. parahaemolyticus in bivalve molluscs (oysters), evaluating the baseline risk and the effect of mitigations, especially in a climate change scenario.
In ToR6, EFSA was requested to recommend areas for future research on Vibrio spp. in seafood and aquatic environments. A key priority for future research is to establish an EU‐wide baseline survey for the relevant Vibrio spp. in relevant seafood products, including at the primary production and retail stages. This survey will support: (i) gathering of representative and harmonised data on the relevant Vibrio spp.; (ii) definition of sentinel sites at primary production level to investigate temporal trends in Vibrio spp. occurrence; (iii) obtaining a representative collection of food isolates for characterisation (detection of VFs and of AMR); (iv) establishing a baseline for future reference in relation to the assessment of the effect of climate change. Other research needs include to improve analytical methods (for detection, quantification and AMR testing) and their comparability, to improve understanding of Vibrio VFs and discrimination of pathogenic strains, to gather data on Vibrio abundance during specific climatic events, to reassess DR models, to develop/validate models on the occurrence of Vibrio spp. (particularly V. parahaemolyticus) and environmental factors in the EU context, and to develop an exploratory QMRA to identify the most important gaps and guide research.
It is recommended to develop a case definition for human ‘vibriosis’ at EU level and to consider vibriosis for compulsory reporting. It is also recommended to systematically report data on Vibrio spp. occurrence in seafood collected in national monitoring programmes in the EU monitoring and collection of information on zoonoses (Zoonoses Directive 2003/99/EC), to systematically characterise V. parahaemolyticus isolates of clinical and food origin for pathogenicity (tdh and trh genes) and V. cholerae isolates of clinical and food origin for serotype and/or presence of cholera toxin genetic determinants and to characterise by WGS a selection of isolates of Vibrio spp. of PH relevance of clinical, food and environmental origin to allow the implementation of genomic surveillance. Further, it is recommended to arrange computational resources to sustain the development and long‐term operability of EU‐wide Vibrio suitability maps operating with high‐resolution data of SST and sea surface salinity. Finally, it is recommended to survey resistances against MIAs associated with mobile elements in Vibrio spp. isolates from seafood.
1. INTRODUCTION
1.1. Background and Terms of Reference as provided by the requestor
Vibrio spp. are a group of common, Gram‐negative, rod‐shaped bacteria that are part of the natural microbiome of freshwater, estuarine and marine environments. A dozen of the over 160 recognised species 1> of Vibrio are potentially pathogenic to humans. Vibrio cholerae (O1 or O139 serogroups) is the aetiological agent of cholera while other pathogenic Vibrio species include V. parahaemolyticus, V. vulnificus, and non‐O1/non‐O139 V. cholerae. Vibrios grow in temperate and warm waters with moderate salinity [5–25 ppt (part per thousand)]. Under non‐favourable conditions vibrios may enter the viable but non‐culturable (VBNC) state, a state of reduced metabolic activity characterised by higher resistance to environmental stressors (Li et al., 2014).
Non‐cholera Vibrio spp. cause vibriosis — infections normally acquired through exposure to sea water or consumption of raw/undercooked seafood. They may be occasionally detected also in other commodities, such as fermented foods, raw vegetables or ready‐to‐eat foods, but the factors influencing their transmission in the food chain is still a matter of discussion (Valero et al., 2021). The accumulation of vibrios in seafood, and in particular bivalves, followed by consumption of those products either raw or not fully cooked is an established route of human exposure. Other factors affecting exposure include storage and transportation at inappropriate temperatures, contamination by an infected food handler, or cross‐contamination through contact with contaminated seafood or seawater (FAO and WHO, 2020). Further to this, V. parahaemolyticus displays the ability to form biofilms (Wang et al., 2022), underlying the need for cleaning and disinfection in seafood handling environments. Clinical manifestations are most commonly mild, self‐limiting gastroenteritis (GE), except for V. vulnificus, an opportunistic pathogen whose infection in individuals with predisposing health conditions (e.g., chronic liver diseases, hemochromatosis, immunocompromised) can rapidly lead to fatal septicaemia (Baker‐Austin et al., 2018). Several other Vibrio species and some strains of V. parahaemolyticus and V. vulnificus can lead to vibriosis in aquatic animals and negatively impact animal production (de Souza Valente & Wan, 2021).
Current predictions show that the warming of marine waters as a result of global climatic change may have an impact on the transmission of these pathogens. In fact, it has been argued that this group of pathogens represents an important and tangible barometer of climate change in marine systems (Baker‐Austin et al., 2016). Indeed, the association between the increase of sea surface temperatures (SST) in the Baltic area between 1982 and 2010 and the emergence of Vibrio infections clustered around the Baltic Sea area was observed (Baker‐Austin et al., 2013) and was confirmed in correspondence with an extreme heat wave in northern Scandinavia during summer 2014 (Baker‐Austin et al., 2016). As a consequence, the European Centre for Disease Prevention and Control (ECDC) started to monitor Vibrio growth in the Baltic Sea during summer through a near real‐time model (i.e., the Vibrio suitability tool in a Vibrio map viewer 2 ) that uses daily updated remote sensing data to examine environmental conditions such as SST and salinity. Alongside, climate change as a driver of emerging risk of Vibrio infections through food has been raised by EFSA in the CLEFSA (CLimate change and Emerging risks for Food Safety) project. Vibrio spp., especially V. parahaemolyticus and V. vulnificus, were indicated among the ‘biological hazards to human health’ issues with a very high likelihood of emerging in Europe under a near‐future climate scenario (EFSA, 2020a).
Odeyemi (2016) reviewed the occurrence of V. parahaemolyticus in seafood based on studies reported in the 2003–2015 period; European studies reported prevalences from 7.83% for mussels (Normanno et al., 2006) to 77.8% for shrimps (Copin et al., 2012). Concentrations were also variable depending on studies and methods: in Italy, an average concentration of 77 CFU/g (range 3–1.8 × 103) was reported in bivalve shellfish (Suffredini et al., 2014) and 400 most probable number (MPN)/g (range 3–1.6 × 105) in crustacea (Caburlotto et al., 2016). Outbreaks of V. parahaemolyticus infections following consumption of contaminated raw or undercooked seafood have been reported around the world. In the EU, seven strong‐evidence foodborne outbreaks (FBOs) caused by V. parahaemolyticus with ‘Crustaceans, shellfish, molluscs and products thereof’ as the suspected vehicle have been reported (2010–2020 period), causing 127 cases, of which 50 were hospitalised. Only a minority of V. parahaemolyticus environmental isolates are pathogenic to humans, pathogenicity being mainly associated with the production of haemolysins (thermostable direct haemolysin, TDH, or TDH‐related haemolysin, TRH). Recent studies, however, demonstrated the absence of the genes codifying for TDH and TRH in approximately 10% of clinical isolates (Raghunath, 2015), highlighting the relevance of other possible virulence factors (VFs), such as type 3 and type 6 secretion systems, adhesion and iron uptake systems.
Vibrio vulnificus is the leading cause of seafood‐related deaths in the United States and displays the highest fatality rate of any foodborne pathogen (Scallan et al., 2011); 51.6% of cases reported to the Food and Drug Administration (FDA) between 1992 and 2007 died (Jones & Oliver, 2009). V. vulnificus optimum salinity lays between 10 and 18 ppt; it is rarely isolated from waters with salinities > 25 ppt, so occurrence and human infections are infrequent in such environments (e.g., the Mediterranean Sea). Although survival of V. vulnificus in cold water (< 10°C) is achieved by entering the VBNC state, it is rarely isolated when water temperatures are lower than 13°C, so that most cases occur in the warmer summer months. Attempts to identify a single genetic marker for V. vulnificus virulence have failed. Clinical and environmental strains are often divided according to polymorphisms of pilF (pilus type IV), vcg gene, 16S RNA gene, or capsular polysaccharide, however no single target has proved completely reliable (Roig et al., 2018).
Non‐O1/non‐O139 V. cholerae strains do not generally produce the cholera toxin typical of the O1/O139 serogroups but may be responsible for GE cases varying from mild to serious in severity in association with the production of a heat‐stable enterotoxin or the expression of other VFs (e.g., the El Tor haemolysin or the RtxA cytotoxin). Similar pathogenicity may be expressed by non‐cholera‐toxin‐producing V. cholerae O1 and O139 strains. Occurrence of non‐O1/non‐O139 V. cholerae in seafood is well documented, with a prevalence of 5.6% in different products collected in Italy (Ottaviani et al., 2009), 11% to 16% in mussels from German production areas (Huehn et al., 2014), and with a sporadic detection in water from shellfish‐growing areas in France (Cantet et al., 2013).
The issue of the public health (PH) risk associated with Vibrio spp. in seafood was considered, to different levels, in several documents. In 2001 the Scientific Committee on Veterinary Measures relating to Public Health published an opinion on V. vulnificus and V. parahaemolyticus in raw and undercooked seafood, concluding that the incidence of infections by these two species in Europe could not be quantified. Concern was raised, however, that the trends in international trade, consumption of raw seafood and number of susceptible persons may lead to an increase of infections (SCVM, 2001). Following this, risk assessments were developed by FAO/WHO on V. vulnificus in raw oysters (FAO and WHO, 2005b), V. cholerae O1 and O139 in warm water shrimp (FAO and WHO, 2005a), and V. parahaemolyticus in seafood (FAO and WHO, 2011). However, as modelling in these risk assessments was mainly based on data gathered in the United States, their application in areas where initial Vibrio concentrations, environmental conditions, and harvesting/post‐harvesting practices may differ significantly, should be considered with caution. With regard to the EU area, in 2022, the German Federal Institute for Risk Assessment published a health risk assessment of the occurrence of Vibrio spp. in food, which remarked that food investigation should concentrate on V. parahaemolyticus, V. vulnificus and V. cholerae, and that the consumption of raw or insufficiently cooked food products is one of the most important factors for their transmission (BfR, 2022).
Risk assessments focused on the EU situation have not taken place since the previously mentioned opinion of the SCVM relating to Public Health published in 2001. This, together with the presence of pathogenic Vibrio spp. in European marine and freshwater and in seafood, the underreporting of human cases due to the lack of surveillance of non‐cholerae Vibrio infections in the EU, the evidence of outbreaks of Vibrio infections following consumption of contaminated raw or undercooked seafood also in the EU, the absence of food safety criteria of Vibrio spp. in seafood, and the warming pattern of the SST in particular areas, underpin the need to propose a self‐task mandate by the BIOHAZ panel on the PH aspects of Vibrio spp. related to the consumption of seafood in the EU.
Terms of reference
The BIOHAZ Panel is asked to issue a scientific opinion on the PH aspects of Vibrio parahaemolyticus, Vibrio vulnificus and non‐O1/non‐O139 Vibrio cholerae (and other species whenever relevant) related to the consumption of seafood for the EU population. More specifically, EFSA is requested to address the following terms of reference (ToRs):
ToR1. To review, for the relevant Vibrio spp., the existing information on occurrence and concentration in seafood, available analytical methods, pathogenicity to humans and virulence factors, as well as antimicrobial resistance and persistence mechanisms in different environments.
ToR2. To identify the factors in the aquatic environments and in food (including during production and processing) that influence occurrence and growth of the relevant Vibrio spp., and affect transmission of their virulence and resistance determinants.
ToR3. To review the evidence on the impact of climate change on the occurrence and levels of the relevant Vibrio spp. in water environments and seafood.
ToR4. To list and review prevention and control measures along the food chain for the relevant Vibrio spp.
ToR5. To review risk assessment modelling options for Vibrio spp. in seafood and to identify the knowledge gaps and data needed to perform a risk assessment on the PH impact of the relevant Vibrio spp. in seafood at the EU level.
ToR6. To recommend areas for future research on Vibrio spp. in seafood and aquatic environments.
1.2. Interpretation of the Terms of Reference
Seafood, for this assessment, was understood to be food of marine and animal origin. It encompasses a variety of food products, including finfish, crustaceans, molluscs (with the classes cephalopods, bivalve molluscs and gastropods), tunicates and echinoderms (e.g. sea urchins and holoturidae). The seafood groups characterised by an exoskeleton are often referred to as shellfish. A representation of these groups can be found in Figure 1. Food products from inland aquaculture settings reproducing the marine environment were included. Marine food of non‐animal origin (e.g. seaweed) were not considered.
FIGURE 1.
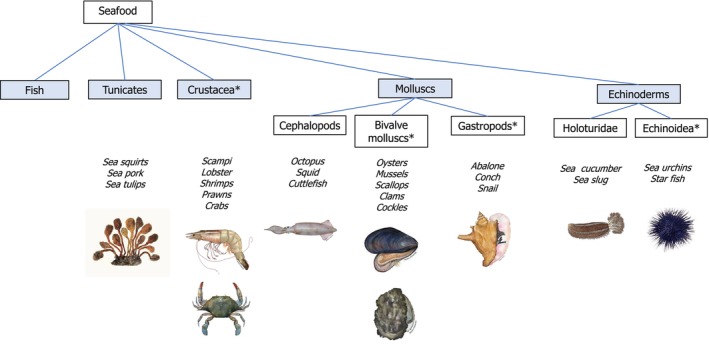
Classification of seafood as used in this assessment. Images are used from the fish and invertebrate photo gallery of the Alaska Fisheries Science Center, National Oceanic and Atmospheric Administration (NOAA) Fisheries. *Referred to as shellfish.
The assessment deals with Vibrio food‐borne transmission through the consumption, by the EU population, of seafood as placed or intended to be placed on the EU market (thus including global trade). Hence, infections deriving from environmental, recreational and/or occupational exposure to vibrios were not considered. The consumer's part in the risk assessment [e.g. storage, cross‐contamination or (under)cooking during food preparation] were addressed in ToR4 and 5.
The assessment covers V. parahaemolyticus, V. vulnificus and non‐O1/non‐O139 V. cholerae. Other species were to be covered as well when they were as relevant as these three species considering their presence in seafood placed or intended to be placed on the EU market and their reliable identification as the cause of illness in humans in the EU through seafood consumption.
Specifically, for ToR1, the occurrence and concentration of the relevant Vibrio spp. in seafood covered both the detection/enumeration of the aforementioned species and, where achievable, the specific detection/enumeration of their pathogenic strains or of strains with characteristic features associated with pathogenicity or virulence. Analytical methods covered those used for the detection and enumeration of the different relevant Vibrio spp. including, where available, methods targeting pathogenic strains within a species. Methods addressing the identification and characterisation of virulence profiles and closely related strains of the different relevant Vibrio spp. were also considered. Strengths and limitations of the described methods were addressed.
Pathogenicity was defined as the ability of the relevant Vibrio spp. (or of a subset of strains within the species) to cause disease in a human host and virulence as the degree to which a pathogen causes damage to the host. For the review of the pathogenicity and VFs, features differentiating Vibrio strains with pandemic potential were addressed. Antimicrobial resistance (AMR) was understood as the evidence of resistance to antimicrobial compounds in isolates of relevant Vibrio spp. taken from seafood or in clinical isolates associated with FBOs or cases. Clinical isolates with no clear link with food‐borne transmission were excluded. Both phenotypic expression of resistance as well as genotypic information were considered. Emerging AMRs that are regarded as a possible threat for the public were also addressed. Persistence is understood as the ability of a given organism to establish itself and remain within a given environment for a long term (long‐term survival in the specific environment).
For ToR2, factors referred to extrinsic and intrinsic factors that affect the presence or concentration (due to growth or inactivation), or that affect horizontal or vertical transmission of pathogenicity, virulence or resistance determinants in the relevant Vibrio spp. in aquatic environments and in food. Implicit factors affecting occurrence, growth and transmission of virulence/resistance determinants were also addressed. The aquatic environments were considered in their whole and not limited to areas associated with seafood production (aquaculture or fishing). Furthermore, both seawaters and brackish waters were considered. Seafood was covered in all its production stages, including the aquaculture process, harvesting/fishing, processing and transport up to retail level. Domestic handling and consumption practices were out of the scope.
For ToR3, the evidence on the impact of climate change was interpreted as the reporting of empirical observations or of modelling studies assessing the changes of the occurrence and/or concentration of the relevant Vibrio spp. (in water environments and/or in seafood) in correlation with one or more of the changes of the marine environment associated with global climate change (e.g. increase of SST associated with warming trends or extreme weather events).
For ToR4, control measures for vibrios in seafood that are already in place (either locally or globally), as well as those experimentally tested for possible implementation were included. The advantages and disadvantages of the prevention and control measures were listed and included, when relevant and feasible, a qualitative evaluation of their efficacy (effect on prevalence/concentration of the relevant Vibrio spp. in seafood). Economic or environmental impacts or user acceptance were not considered.
For ToR5, the risk assessment modelling options for Vibrio spp. in seafood were reviewed based on existing risk assessments, models and approaches and covered identifying the data needed to perform a risk assessment on the PH impact of the relevant Vibrio spp. in seafood at the EU level. The characteristics of the available models considered the methods, assumptions, data, model limitations, etc. Performing a risk assessment itself was not envisaged.
Each ToR were translated into an assessment question (AQ). These read as follows:
- AQ1/For the relevant Vibrio spp., what is their occurrence and concentration in seafood, available analytical methods, pathogenicity to humans and virulence factors, as well as antimicrobial resistance and persistence mechanisms in different environments?
-
○SQ1.1/What are the Vibrio spp. of highest relevance for PH in the EU through consumption of seafood beyond V. parahaemolyticus, V. vulnificus and non‐O1/non‐O139 V. cholerae?
-
○SQ1.2/Considering the relevant Vibrio spp. (from SQ1.1), what is their prevalence and concentration in seafood placed or intended to be placed on the EU market?
-
○SQ1.3/Considering the relevant Vibrio spp. (from SQ1.1), what are the available analytical methods for the detection, enumeration and characterisation in seafood?
-
○SQ1.4/Considering the available analytical methods (from SQ1.3), what are their characteristics and how do they contribute to risk assessment?
-
○SQ1.5/Considering the relevant Vibrio spp. (from SQ1.1), what is their pathogenicity to humans and which are their virulence factors?
-
○SQ1.6/Which are the most frequently detected antimicrobial resistances in the relevant Vibrio spp. (from SQ1.1) isolated from seafood and from seafood‐borne infections?
-
○SQ1.7/Which of the detected antimicrobial resistances (SQ1.6) are of concern due to their possible horizontal transmission to other bacteria or for their emerging in the EU?
-
○SQ1.8/Considering the relevant Vibrio spp. (from SQ1.1), what are their persistence mechanisms in different environments?
-
○
- AQ2/What are the factors in the aquatic environments and in seafood that influence occurrence and growth of the relevant Vibrio spp., and affect transmission of their virulence and resistance determinants?
-
○SQ2.1/What are the factors in the aquatic environments and in food, including during production and processing that affect the presence or concentration of the relevant Vibrio spp. (from SQ1.1)?
-
○SQ2.2/What factors in the aquatic environments and in food are considered to affect transmission of Vibrio virulence and resistance determinants?
-
○
- AQ3/What is the impact of climate change on the occurrence and levels of the relevant Vibrio spp. in water environments and seafood?
-
○SQ3.1/What data sources, tools and models are available or are under development to evaluate the impact of climate change on the relevant Vibrio spp. in water environments and seafood and what are their limitations?
-
○SQ3.2/How is climate change affecting the occurrence and levels of the relevant Vibrio spp. in the aquatic environment at a global level and in Europe?
-
○SQ3.3/How is climate change affecting the occurrence and levels of the relevant Vibrio spp. in seafood at global level and in Europe?
-
○
AQ4/What are the prevention and control measures along the seafood chain for the relevant Vibrio spp.?
- AQ5/What are the risk assessment modelling options for Vibrio spp. in seafood and which are the knowledge gaps and data needed to perform a risk assessment on the PH impact of the relevant Vibrio spp. in seafood at the EU level?
-
○SQ5.1/What are the available (semi‐) quantitative risk assessments (QMRA) for the relevant Vibrio spp. (SQ1.1) in seafood or in specific seafood types/products?
-
○SQ5.2/What are the characteristics of the available risk assessment models (listed in SQ5.1)?
-
○SQ5.3/Which are the knowledge gaps and data needed to perform assessments addressing the PH impact of relevant Vibrio spp. in different types of seafood at the EU level?
-
○
AQ6/What are areas for future research on Vibrio spp. in seafood and aquatic environments?
1.3. Additional information
Regulation (EC) No 853/2004 sets out definitions and hygiene requirements for bivalve molluscs and fishery products. Bivalve molluscs are filter‐feeding lamellibranch molluscs. Typical products in an EU context include mussels (Mytilus edulis), oysters (Crassostrea gigas) and scallops (Pectenus maximus). Fishery products mean all seawater or freshwater animals (except for live bivalve molluscs, live echinoderms, live tunicates and live marine gastropods, and all mammals, reptiles and frogs) whether wild or farmed and including all edible forms, parts and products of such animals.
Implementing Regulation (EU) 2019/627 classifies production areas of live bivalve molluscs according to the levels of faecal indicators (Escherichia coli) in the mollusc flesh and intravalvular liquid in one of three categories, Classes A, B and C. According to Regulation (EU) 853/2004, Class A molluscs may be placed on the market directly for human consumption, while molluscs from Class B areas must be treated in a purification centre or relayed, while those from Class C areas must be relayed over a long period (at least 2 months); after treatment they must comply with the Class A health standards prior to being placed on the market. Molluscs from Class B and Class C areas may also be heat‐treated using one of the legally prescribed methods. Bivalve molluscs should not be harvested from non‐classified areas, i.e. areas with no data or exceeding the limit of the faecal indicator. These requirements also apply to echinoderms, tunicates and marine gastropods.
2. DATA AND METHODOLOGIES
The approach to answer the ToRs was defined in advance and is described in the protocol (Annex A). It covers both the problem formulation (i.e. what the assessment aims to address) and which methods will be used for addressing the problem. The problem formulation (‘what’) includes the clarification of the mandate (see further refined in Section 1.2) and consists of the steps (1) translation of the mandate into scientifically answerable AQs, (2) definition of the sub‐questions (SQs) of each AQ, if needed, and their relationship (conceptual model) and (3) the selection of the approach for the assessment. The planning of the methods for conducting the assessment (‘how’) consists of (1) specifying the evidence needs and the methods for answering each AQ/SQ, including uncertainty analysis and (2) the methods for integrating evidence across AQs/SQs and addressing the remaining and overall uncertainty. Protocol development followed the draft framework for protocol development for EFSA's scientific assessments (EFSA, 2020b).
2.1. Data
2.1.1. Data on food‐borne outbreaks at EU/EEA level
Data on ‘strong and weak evidence’ FBOs with Vibrio spp. as causative agent were extracted from the EFSA zoonoses database on 2 February 2023. The datafile was filtered considering time (from 2010 to 2021) and relevant food vehicles [i.e. the food(stuff) suspected of causing human cases] considered as seafood or with evidence that it contained seafood. The available evidence was summarised by Vibrio spp., retrieving information on the number of outbreaks, number of cases, number of hospitalised cases and number of deaths. Reporting per year and per country was described. Further information from other free data fields, such as food vehicle information and contributory factors, was consulted and included, when relevant. More information about the reporting of FBOs can be found in (EFSA, 2022).
2.1.2. Data of the Rapid Alert System for Food and Feed (RASFF)
Commission Regulation (EU) No 16/2011 3 lays down the implementing measures for the requirements of Regulation (EC) No 178/2002 around the RASFF. 4 This RASFF system facilitates the notification of food and feed safety alerts among the competent authorities of Member States. It is not an epidemiological surveillance system but is primarily a communication facility enabling food safety risks to be averted before they could be harmful to European consumers. RASFF data were used to investigate the Vibrio spp. found in seafood since 2010. Data were extracted from the RASFF database on 2 February 2023 for the notification type ‘food’ and the hazard category ‘pathogenic microorganisms’ (more specific the hazard Vibrio). The extracted data were filtered considering time (from 2010 onwards) and for the product categories ‘bivalve molluscs and products thereof’, ‘cephalopods and products thereof’, ‘crustaceans and products thereof’, ‘farmed crustaceans and products thereof’, ‘fish and fish products’, ‘molluscs and products thereof’ and ‘wild caught crustaceans and products thereof’. The information was summarised using alert/notification per product category, Vibrio spp. and year. The occurrence of notification types associated with human disease (‘food poisoning – alert’ or ‘consumer complaint – information’) was checked specifically in notifications related to samples containing exclusively species other than V. parahaemolyticus, V. cholerae and V. vulnificus. Information on detection of Vibrio spp. with specific VFs was checked.
2.1.3. Zoonoses monitoring data
Reporting of monitoring data on zoonoses in animals, food and feed is mandatory for the EU Member States, in compliance with Directive 2003/99/EC. 5 Data must be reported on a mandatory basis for eight zoonotic agents and based on the epidemiological situations in the Member State, and also on the agents and zoonoses spelled out in Annex I of that Directive. On the food‐animal side there is no coordinated monitoring at EU level for Vibrio spp. Data on the occurrence of Vibrio spp. on food were extracted on 14 February 2023 from the zoonoses monitoring database without time restrictions (until 2021). The extracted data were filtered for matrix considering seafood. Data were summarised considering the units (single units and batches), stage, context (e.g. surveillance, monitoring) and strategy of sampling (e.g. ‘census’, ‘convenience’ and ‘objective’ vs. ‘suspect’, ‘selective’).
2.1.4. Food Standards Australia New Zealand information request
Following Australia's largest V. parahaemolyticus outbreak in raw oysters, 6 an information request from the Food Standards Australia New Zealand (FSANZ) was shared with the EFSA Microbiological Risk Assessment (MRA) Network and international partners in 2022. Replies to the following questions were received from Croatia, Cyprus, Estonia, France, Germany, Ireland, Norway, Sweden (through MRA representatives) and US Food and Drug Administration (US FDA), Canadian Food Inspection Agency (Canada), New Zealand NZ Ministry for Primary Industries (MPI, New Zealand), Food Safety Commission of Japan (Japan) and FSANZ (Australia) (through international collaboration) and can be found in Annex B.
Is V. parahaemolyticus and/or V. vulnificus notifiable human diseases in your country? Have you witnessed an increase in Vibrio species illness over the past 3–5 years? If so, do you consider that this is climate change related? Is there any substantive correlative evidence for this?
What Vibrio/environmental monitoring (e.g. sea water temperature, salinity) in shellfish‐growing areas, if any, do you or your colleagues undertake? Has changes in domestic requirements in your country changed requirements for equivalence testing of imported seafood?
What phenotypic methodologies (quantitative and presence/absence) are currently being used for routine identification and testing of vibrios in shellfish? Noting we've witnessed challenges with non‐culturable Vibrio and issues with MPN methods.
Are environmental or human isolates routinely sequenced? Are there specific genes (other than tdh and trh for V. parahaemolyticus) that are of interest from a risk perspective? Are sequences on a public or internal database and what metadata is uploaded associated with those sequences?
2.2. Methodologies
2.2.1. Vibrio spp. of highest PH relevance in the EU through consumption of seafood (SQ1.1)
A general literature search was conducted to provide a cross‐sectoral data source of review papers, books or book chapters to be used for the various AQs and SQs; a study was considered eligible when the record included information on V. parahaemolyticus, V. vulnificus, non‐O1/non‐O139 V. cholerae or other relevant species with regards to the topics covered under the different ToRs. The search strategy and study selection can be found in the protocol (Annex A).
Specifically, for SQ1.1, a study was eligible when it informed on any Vibrio spp. of PH relevance in the EU through consumption of seafood. Also, recent reports, articles and reviews were consulted related to Vibrio FBOs/cases in the EU. Information on seafood transmission of Vibrio species other than V. parahaemolyticus, V. vulnificus or non‐O1/non‐O139 V. cholerae in the EU was retrieved through a literature search in Eurosurveillance 7 (on 26 February 2023 with the keyword ‘Vibrio’ in all fields) and in Web of Science™ Core Collection 8 (on 19 June 2023).
The archive of ProMED records 9 was screened on 12 March 2023 using the keyword ‘Vibrio’ in posts' title or text; no time restrictions were applied (data from inception to 2023). All retrieved posts were screened for evidence of food‐borne vibriosis occurring in the EU. In addition, the recent risk assessments undertaken by EU member states and international bodies on the topic were collected and screened for evidence of reported food‐borne vibriosis. Furthermore, the results of the FSANZ information request (Section 2.1.4) were considered. This information was supplemented by the knowledge/expertise of the Working Group (WG) and Panel members.
The data retrieved from the FBOs (Section 2.1.1), RASFF (Section 2.1.2) and EFSA zoonoses monitoring data (Section 2.1.3) were summarised as described above.
A list of Vibrio spp. that are found to be associated with seafood and with human disease in EU was prepared, obtained from the various sources of evidence, and the most relevant Vibrio species were identified based on the available data and expert judgement. These are referred to as relevant Vibrio species in the text.
2.2.2. Occurrence of the relevant Vibrio spp. in seafood placed or intended to be placed on the EU market (SQ1.2)
A systematic review 10 was used to identify and extract data on the occurrence (i.e. prevalence and concentration 11 ) of the relevant Vibrio spp. in seafood produced and/or commercialised in Europe. The search covered studies published between January 2010 and September 2023. The search strategy, study selection and data extraction can be found in the protocol. 12 Data were extracted for EU member states, countries that were EU Members in the period considered in the extensive literature search (ELS) (i.e. UK), EU accession candidates and members of the European single market. The evidence retrieved was synthesised by listing the data on the occurrence of the relevant Vibrio spp. in seafood on the EU market according to seafood category and type (animal species). The synthesis includes country of the study, year(s) of sampling, the sampling point (stage of the food chain), sampling size and number of positive units, concentration measure (for quantitative analysis), the tested amount, a reference to the method used. Where reported, data related to pathogenic strains (occurrence) or to the identification of features associated with pathogenicity/virulence (e.g. serotype, sequence type, toxin genes) were captured. For V. cholerae, due to the scarcity of data explicitly addressing non‐O1/non‐O139 V. cholerae, data were included in the synthesis when dealing with non‐choleragenic V. cholerae, i.e. when strains were tested using either serological (sero‐agglutination) tests (i.e. non‐O1, non‐O139 or both) or molecular tests (i.e. no detection of cholera toxin‐associated genes, ctx, ctxA and/or ctxB). Reporting pathogenic V. parahaemolyticus was highly heterogeneous among studies 13 and results were harmonised to express proportion of samples containing tdh+ and/or trh+ V. parahaemolyticus upon V. parahaemolyticus‐positive samples.
The literature search identified 234, 436, 404 and 15 bibliographic references in PubMed, Web of Science Core Collection, Scopus and SciELO, respectively. A total of 463 out of 1089 primary research studies were removed after deduplication. From the remaining 626 publications (primary research studies), 462 were discarded after screening of titles and abstracts. At the end of full text screening of 164 publications, 49 publications were considered eligible. Two publications concerned results already contained and extracted from other records and, therefore, the data from 47 primary studies were extracted using a standardised spreadsheet. Most of these publications included data on more than one Vibrio species relevant for PH, seafood category or species, sampling area or sampling periods, etc. Therefore, finally, a total of 389 data entries (sample size ≥ 3) were associated with the 47 primary research publications. One paper with detailed yearly analysis was redundant with a pluriannual synthesis paper and then removed for further analysis. Removing 14 duplicated information, the final number of data entries is N = 375 for 46 primary research publications. Excluding entries not dealing with non‐choleragenic V. cholerae (N = 5) reduced the database to 370 entries and 46 primary research publications. Extracted data are available at https://zenodo.org/records/11640367 and will be incorporated in the ‘Pathogens in Food (PIF) database’ 14 accessible through a web application (https://pif.esa.ipb.pt/) (Faria et al., 2022). A random‐effect meta‐analysis model was carried out, considering study as random effect and a logit transformation of the prevalence data (Gonzales‐Barron et al., 2021; Xavier et al., 2014), using metafor package (v3.8‐1) (Viechtbauer, 2010) environment of RStudio (v 2023.03.0). To estimate parameters and their confidence interval the restricted maximum likelihood estimator (RMLE) procedure was used. The I 2 statistics or intraclass correlation estimates the proportion of between study variance from the total variance. If I 2 is higher than 50%, the heterogeneity between studies is notable (Higgins & Thompson, 2002). Between‐study variability can be considered as significant when it represents at least 25% of the total variability in the outcome measure (Gonçalves‐Tenório et al., 2018; Higgins & Thompson, 2002). Samples sizes below 3 units were not considered in statistical analysis. Quantification data for Vibrio were scarce and heterogeneous (reporting being either based on positive or on positive and negative units). Only quantified data with available means and standard deviation, after standardisation in log10 units, (positive or above LOQ), were used to estimate a pool mean and its confidence interval by meta‐analysis using weights assigned to each sample based on the inverse of the overall error variance (i.e. 1/variance) with study as random effect.
2.2.3. Available analytical methods for the detection, enumeration and characterisation of the relevant Vibrio spp. in seafood and their contribution to risk assessment (SQ1.3–1.4)
International Organization for Standardization (ISO)/European Committee for Standardization (CEN) catalogues were consulted as well as other methods from national bodies (e.g. FDA BAM, Ministry for Primary Industries NZ, Health Products and Food Branch Canada). Also, information shared by international bodies were consulted. Food and Agriculture Organization (FAO)/World Health Organization (WHO) reviews of methods were considered in the discussion of the characteristics of the available methods. The recent reviews collected through the general literature search (Section 2.2.1) as well as the results of the FSANZ information request (Section 2.1.4) were used. Finally, primary research studies were considered using the data collected through the ELS as described in Section 2.2.2, in which the method of detection (and also quantification) were extracted.
Data from included studies were extracted in relation to the general structure of the method, principle, scope, laboratory technique adopted, breadth of application, availability of performance characteristics [limit of detection (LOD), limit of quantification (LOQ), accuracy, specificity, sensitivity, etc.] and framework of their collection (in‐house, ILS). Priority was given to official control and standardised methods; analytical methods only experimentally tested were only briefly summarised.
For SQ1.3, the evidence retrieved was synthesised by listing the methods used for the detection, enumeration and characterisation of the relevant Vibrio species, and describing their use on different seafood types and/or in different contexts and summarising (when available) their performance characteristics. For SQ1.4, the characteristics of the analytical methods identified in SQ1.3, grouped per types, were addressed, and summarised through a Strengths, Weaknesses, Opportunities, and Threats (SWOT) analysis.
2.2.4. Pathogenicity to humans and virulence factors of the relevant Vibrio spp. (SQ1.5)
The virulence factor database (VFDB) 15 was consulted to list the possible virulence genes of the most relevant Vibrio spp. in seafood. This list was used as a basis for bibliographic searches. Recent reviews collected through the general literature search (Section 2.2.1) were also used to gather information about the relevant Vibrio species with regards to their pathogenicity to humans and VFs. Furthermore, the literature searches performed on PubMed (Section 2.2.1) were screened to identify relevant studies to complete the data obtained previously. Comprehensive lists were created, including VFs important for all pathogenic vibrios and the specific determinants for each species.
2.2.5. Antimicrobial resistances in the relevant Vibrio spp. isolated from seafood and from seafood‐borne infections (SQ1.6) and those of concern due to their possible horizontal transmission to other bacteria or for their emerging in the EU (SQ1.7)
The AMR group of the Zoonoses Network was contacted to share available data, or the information on where to find them (e.g. National Reports, public databases, outbreak investigation) for the relevant Vibrio species, that stem from isolates from seafood (environmental samples, retail or production, e.g. primary production). Additionally, the recent reviews collected through the general literature search (Section 2.2.1) were used as well as the results of the FSANZ information request (Section 2.1.4). Furthermore, specific literature searches were performed on 10 January 2023 in PubMed using ‘Vibrio AND Antimicrobial resistance’ alone or combined with ‘shellfish’ as search terms. Records were screened to identify studies from Europe in which food isolates were examined.
Data were extracted and presented in a first table summarising phenotypic AMR data from the European consortium ASK, 16 French data (CNR Vibrio et Choléra) and data extracted from primary research papers. As most papers do not contain information on the coding AMR genes, information on AMR genes is depicted in another table, presenting examples of the AMR determinants in food strains.
Dr Jens‐Andre Hammerl, Head of the Consultant Laboratory for Vibrio spp. in Food of the German Federal Institute for Risk Assessment (BfR) attended the WG meeting on 10 January 2024 as hearing expert to summarise the BfR Opinion ‘Bacterial foodborne Vibrio infections: health risk assessment of the occurrence of Vibrio spp. (non‐cholera vibrios) in food’ (BfR, 2022). Further, he gave some examples of studies on Vibrio of the BfR related to (i) Vibrio spp. occurrence in German aquaculture systems, (ii) Vibrio spp. ecological cut off values for different species, and (iii) emergence of multidrug‐resistant V. parahaemolyticus in imported seafood – genetic basis and spread of resistance plasmids.
The distribution of AMR genes for the EU and EFTA countries data was obtained from the global V. parahaemolyticus genomes based on a collection of nearly 10,000 genomes. Some of the strains are from the collection of prof. Jaime Martinez‐Urtaza (Department of Genetics and Microbiology, Universitat Autònoma de Barcelona) or are part of ongoing studies to expand the genome collection; other genomes were retrieved from the European Nucleotide Archive (ENA) 17 and the National Center for Biotechnology Information (NCBI). 18 For detection of AMR genes, a resistance genes detection pipeline based on one of the standard databases (CARD database 19 ) was used. The phylogenetic tree was prepared and includes the reference genome from Japan ‘Osaka’ as reference. The RIMD 2210633 strain has been added as the global reference strain which has been historically used for all the phylogenetic analysis of V. parahaemolyticus. The metadata includes the source of the strain, i.e. country, origin (clinical, environmental or unclear), date of isolation and subtype. The antibiotic‐resistant genes are shown as present, absent or not applicable. To build the ARGs European V. parahaemolyticus tree, the Parsnp tool, a fast core‐genome multi‐aligner and SNP detector, from the Harvest suite was used (Treangen et al., 2014). Parsnp calculates the MUMi distances between the reference genome (RIMD_2210633) and each one of the 152 genomes used in this study. The resulting Newick formatted core genome SNP tree was then uploaded onto the webtool I‐Tol (Letunic & Bork, 2021), midpoint rooted and the metadata of the samples was incorporated.
2.2.6. Persistence mechanisms in different environments of the relevant Vibrio spp. (SQ1.8)
Information on the different strategies that the relevant Vibrio spp. use to persist in the environment, including the aquatic environment and environments associated with food production/processing/transport was mainly retrieved from review articles, books and book chapters as collected from the search strategy described in Section 2.2.1 and from reports. The primary research studies were collected through non‐extensive searches and snowballing (or citation searching). The evidence retrieved was synthesised in a narrative way by describing all the bacterial mechanisms that allow the bacteria to persist in the different environments.
2.2.7. Factors in the aquatic environments and in food, including during production and processing that affect the presence or concentration of the relevant Vibrio spp. (SQ2.1)
Information on the factors in the aquatic environments and in food, including during production and processing that influence presence or concentration (growth or inactivation) of the relevant Vibrio spp. was mainly retrieved from review articles, books and book chapters as collected from the search strategy described in Section 2.2.1 and from reports. The primary research studies were collected through snowballing through non‐extensive searches in PubMed and Scopus using, as search terms, the three species relevant for PH, and the selected identified factors (temperature, salinity, pH, etc.). The evidence retrieved was synthesised in a narrative way by listing the factors under the various categories (extrinsic, intrinsic or implicit factors). Data on available growth or inactivation models within selected temperature ranges were extracted and summarised in tables.
2.2.8. Factors in the aquatic environments and in food that are considered to affect transmission of Vibrio virulence and resistance determinants (SQ2.2)
Information on the different factors that affect transmission of VFs and/or resistance determinants of the relevant Vibrio spp. in the aquatic environment or in the food was mainly retrieved from review articles, books and book chapters as collected from the search strategy described in Section 2.2.1 and from reports. The primary research studies were collected through snowballing through non‐extensive searches and snowballing. The evidence retrieved was synthesised in a narrative way by listing the factors that promote the transmission of VFs and resistance determinants in the different environments.
2.2.9. Impact of climate change on the occurrence and levels of the relevant Vibrio spp. in seafood (AQ3)
At first, general information on the effect of climate change on Vibrio spp. was retrieved from review articles, books and book chapters as collected from the search strategy described in Section 2.2.1. Following, technical reports from international organisations [e.g. European Environmental Agency, Intergovernmental Panel on Climate Change (IPCC), etc.] and primary research papers on the topic were collected based on the knowledge of the WG members, through snowballing from the reviews, book chapters and reports retrieved in the cross‐sectoral literature search, or through specific literature searches in the Web of Science™ Core Collection or in NCBI. Primary research studies specifically addressing seafood‐associated Vibrio outbreaks with a strong climatic component of the event were collected through non‐extensive searches and snowballing.
Available data sources [oceanographic data sets and websites, e.g. Copernicus, National Oceanic and Atmospheric Administration (NOAA), ECDC] and predictive models (mechanistic models, suitability models, etc.) were compiled based on technical reports and on experts' knowledge.
The evidence retrieved was synthesised visually, using Vibrio suitability projections for Europe (e.g. figures showing mapping of areas likely to sustain vibrios occurrence and growth in the future under different climate emission scenarios), and in a narrative way. In addition, specific case studies of past Vibrio outbreaks in Europe and throughout the world, either published or provided by the experts, were used to outline climatic/environmental conditions likely to drive risk.
2.2.10. Prevention and control measures along the seafood chain for the relevant Vibrio spp. (AQ4)
Information on prevention and control measures along the seafood chain for the relevant Vibrio spp. focused on those measures already in place while methods that have only been tested experimentally were summarised briefly. Reviews, books and book chapters were first considered and will be collected from the search described in Section 2.2.1. In addition, recent risk assessments undertaken by EU member states and international bodies were considered. More recent papers describing primary research studies were collected, where needed, through non‐extensive searches and snowballing. The measures were first listed based on the available information and a definition for each of the measures was provided. The evidence retrieved was synthesised in tables giving first an overview of the measures already in place and those measures that have only been tested experimentally. For those measures already in place, their advantages and disadvantages were provided and, if relevant and possible, a qualitative evaluation of their efficacy was provided.
2.2.11. Risk assessment modelling options for Vibrio spp. in seafood and knowledge gaps and data needs (AQ5)
First, the available QMRAs covering the relevant Vibrio spp. in seafood or in specific seafood types/products were identified and listed based on non‐extensive literature searches, snowballing, and based on the knowledge and expertise of the WG and Panel members. Further, the characteristics of each of the steps (hazard identification, exposure assessment, hazard characterisation and risk characterisation) in the identified QMRAs were summarised and evaluated. The evaluation addressed objectives, approaches, methods, assumptions, data and limitations. The outcome of the evaluation together with the information and conclusions presented in response to the other ToRs was the basis for an analysis to identify the data and knowledge needed to perform assessments addressing the PH impact of relevant Vibrio spp. in relevant types of seafood at the EU level. The analysis considered different factors impacting on the relevance and scope of such assessments (e.g. the number of food‐borne cases, frequency of consumption, production volumes).
2.2.12. Areas for future research on Vibrio spp. in seafood and aquatic environments (AQ6)
Based on the knowledge gaps identified through the outcome of the approach followed as described in previous sections, research needs were identified based on expert knowledge (WG and BIOHAZ Panel members) and prioritised based on expert group judgement.
2.2.13. Uncertainty analysis
As recommended by the EFSA guidance and related principles and methods on uncertainty analysis in scientific assessments (EFSA Scientific Committee, 2018a, 2018b), an uncertainty analysis was implemented. Given the nature and context of the ToRs of the mandate, the uncertainty analysis was restricted to an overview of the uncertainty sources affecting the different AQs (Table A.1 in Appendix A). They describe the strengths and weaknesses of the collected evidence and served as a source of information for the discussion on knowledge gaps and research needs.
3. ASSESSMENT
3.1. Relevant Vibrio spp. in seafood (SQ1.1)
3.1.1. Introduction
The 2001 opinion of the Scientific Committee on Veterinary Measures relating to Public Health on V. vulnificus and V. parahaemolyticus in raw and undercooked seafood stated that three Vibrio species represent a serious and growing PH hazard: V. cholerae (serogroups O1 and O139, causing cholera), V. parahaemolyticus and V. vulnificus. Furthermore, the opinion underlined that non‐O1/non‐O139 V. cholerae strains, and strains of V. fluvialis, V. hollisae and V. mimicus had been associated with infections (mostly GE) arising from seafood consumption, and that they should in future be considered (SCVM, 2001).
Except for cholera, vibriosis are not included among the food‐borne and waterborne diseases monitored within the European Food‐ and Waterborne Diseases and Zoonoses Network (FWD‐Net) managed by ECDC. Therefore, no harmonised surveillance of Vibrio infections is currently available in the EU.
Data from the US Centers for Disease Control and Prevention (CDC) Foodborne Diseases Active Surveillance Network (FoodNet) report an incidence of Vibrio infections of 0.99 per 100,000 inhabitants (223 cases) for 2022 and 5527 infections during 1996–2022. The largest part of these infections is represented by V. parahaemolyticus (52%), followed by V. alginolyticus, V. vulnificus and V. cholerae (12%, 11% and 8%, resp.) considering culture‐confirmed infections. 20 Significantly, despite the availability in the US of data from long‐standing active (FoodNet 21 ) and passive (COVIS, Cholera and Other Vibrio Illness Surveillance 22 ) surveillance systems, overall underreporting (underdiagnosis plus underreporting stricto sensu) was estimated to exceed a factor 1.8 for V. vulnificus, 36 for toxigenic V. cholerae and 150 for V. parahaemolyticus and the other species. This leads to a yearly estimated number of food‐borne vibriosis in the US of 52,408, with 278 hospitalisations and 48 deaths (Scallan et al., 2011). Most illnesses (70.8%–73.6%) are attributed to molluscs' consumption, followed by crustaceans (13.5%–18.9%) and fish (3.7%–7.5%) (Painter et al., 2013). It can be presumed that in the EU, in the absence of a harmonised surveillance system, an overall higher underestimation of incidence (including underdiagnosis and underreporting) occurs for vibriosis.
3.1.2. Food‐borne outbreaks at EU/EEA level
During 2010–2021, 32 strong and weak evidence FBOs associated with Vibrio species in seafood were reported. V. parahaemolyticus in the ‘Crustaceans, shellfish, molluscs and products thereof’ or ‘Fish and fish products’ food categories was responsible for 30 of the 32 FBOs, causing 221 cases and 57 hospitalisations. One outbreak (12 cases) was caused by an unspecified Vibrio spp. in the ‘Crustaceans, shellfish, molluscs and products thereof’ category and another one (47 cases in a residential institution) was reported for non‐toxigenic V. cholerae in mixed food (Table 1), possibly in relation to cross‐contamination. Outbreaks have been mainly reported by France (N = 23), Spain (N = 8) and Portugal (N = 1). A yearly average of 2.7 FBOs were reported, with a maximum of 7 and 5 in 2016 and 2018, respectively.
TABLE 1.
Summary of the food vehicles in the ‘strong and weak evidence’ FBO associated with Vibrio spp. in the EU/EEA as reported in EFSA's zoonoses database (2010–2021).
| FBO agent | FBO vehicle | No of FBO | Cases | Hospitalisations |
|---|---|---|---|---|
| V. parahaemolyticus | Total | 30 | 221 | 57 |
| Crustaceans, shellfish, molluscs and products thereof a | 27 b | 212 | 52 | |
| Fish and fish products | 3 | 9 | 5 | |
| Non‐toxigenic V. cholerae | Total | 1 | 47 | 1 |
| Mixed food c | 1 | 47 | 1 | |
| Vibrio spp., unspecified | Total | 1 | 12 | 0 |
| Crustaceans, shellfish, molluscs and products thereof a | 1 | 12 | 0 | |
| All | Total | 32 | 280 | 58 |
Abbreviation: FBO, food‐borne outbreaks.
This group includes crustaceans, molluscs, tunicates and echinoderms.
FBO vehicle information: cooked crustaceans (1), unavailable (26).
The residents ate puree meals; one of the puree menus included hake; information provided by Mrs Carmen Varela Martínez by e‐mail on 2 June 2023 (Varela Martínez, 2023).
3.1.3. RASFF notifications
Considering the relevant food categories, 75 RASFF notifications related to Vibrio spp. were reported between 2010 and January 2023. Only four notifications were associated with food of EU origin. Most (66/75) notifications were for crustaceans and products thereof, five were for bivalve molluscs and products thereof, two for fish and fish products, and two for cephalopods and products thereof.
The Vibrio spp. reported in the RASFF notifications are summarised in Table 2. In five notifications, more than one Vibrio spp. was detected. V. parahaemolyticus was present, alone or in combination with other species, in about one third of notifications (27%/75%–36%), followed by V. vulnificus (21%/75%–28%) and non‐O1/non‐O139 V. cholerae (6%/75%–8%), with further 28 notifications related to V. cholerae non‐O1 or serologically uncharacterised V. cholerae (for a total of 34%/75%–45% notification associated with V. cholerae). Additionally, Vibrio alginolyticus (4 notifications) and Vibrio mimicus (one notification) were also notified, mostly in association with other Vibrio spp. Since official controls customarily rely on standardised or otherwise recognised analytical methods, species included in such methods – as V. parahaemolyticus, V. vulnificus and V. cholerae – are expected to be more readily reported.
TABLE 2.
Number of RASFF notifications related to single or multiple Vibrio spp. detection in various seafood categories (2010–February 2023).
| Vibrio species | No of notifications | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| V. parahaemolyticus | V. vulnificus | V. cholerae non‐O1/non‐O139 | V. cholerae non‐O1 | V. cholerae | V. alginolyticus | V. mimicus | Vibrio unspecified | Total | From outside Europe | From EU | |
| Crustaceans and products thereof | 66 | 66 | 0 | ||||||||
| √ | 19 | 19 | 0 | ||||||||
| √ | 17 | 17 | 0 | ||||||||
| √ | 15 | 15 | 0 | ||||||||
| √ | 5 | 5 | 0 | ||||||||
| √ | √ | 4 | 4 | 0 | |||||||
| √ | √ | 1 | 1 | 0 | |||||||
| √ | 1 | 1 | 0 | ||||||||
| √ | 1 | 1 | 0 | ||||||||
| √ | √ | √ | 1 | 1 | 0 | ||||||
| √ | √ | √ | 1 | 1 | 0 | ||||||
| √ | 1 | 1 | 0 | ||||||||
| Bivalve molluscs and products thereof | 5 | 1 | 4 | ||||||||
| √ | 2 | 0 | 2 | ||||||||
| √ | 1 | 0 | 1 | ||||||||
| √ | √ | 1 | 1 | 0 | |||||||
| √ | √ | √ | 1 a | 0 | 1 | ||||||
| Cephalopods and products thereof | 2 | 2 | 0 | ||||||||
| √ | 1 | 1 | 0 | ||||||||
| √ | 1 | 1 | 0 | ||||||||
| Fish and fish products | 2 | 2 | 0 | ||||||||
| √ | √ | 1 | 1 | 0 | |||||||
| √ | 1 | 1 | 0 | ||||||||
| Total | 27 | 21 | 6 | 1 | 27 | 4 | 1 | 1 | 75 | 71 | 4 |
Notification stemming from food poisoning or consumer complaints.
Five notifications between 2018 and 2021 related to detection of V. parahaemolyticus in imported products included information on the presence of genes associated with pathogenicity (two notifications for tdh+, one for trh+ and two for tdh+/trh+ isolates; see Section 3.4 for information on Vibrio pathogenicity and virulence markers).
Only one notification was reported as stemming from food poisoning or consumer complaints and was associated with the occurrence of multiple species (non‐O1/non‐O139 V. cholerae, V. alginolyticus, V. mimicus).
3.1.4. Zoonoses monitoring data
The EU monitoring data for Vibrio spp. in seafood included data for the 2018–2021 period, mostly in the distribution stage, from three EU countries only. In total, 111 and 110 samples were found positive for V. parahaemolyticus and serologically uncharacterised V. cholerae, respectively. V. vulnificus was found in 9 samples (Table 3). As for RASFF notifications, also monitoring programmes mostly rely on recognised analytical methods and, therefore, it can be assumed that species covered by such methods are more easily detected.
TABLE 3.
Number of seafood samples tested in various seafood categories for Vibrio spp. for monitoring purposes in the period 2018–2021 in the EU. a
| Species | Total units positive | Total units tested | |
|---|---|---|---|
| Crustaceans | V. parahaemolyticus | 106 | 299 |
| V. cholerae | 103 | 256 | |
| V. vulnificus | 9 | 20 | |
| Vibrio unspecified | 0 | 9 | |
| Fish | V. parahaemolyticus | 5 | 205 |
| V. cholerae | 7 | 577 | |
| Fishery products | V. parahaemolyticus | 0 | 3 |
| Vibrio unspecified | 0 | 6 | |
| Grand total | 230 | 1375 |
Single units (759 units) and batches (616 units) were sampled. The sampling context was mainly monitoring (1351 units) and for few samples surveillance (24 units). The sampling strategy consisted mainly of objective sampling (1361 units), the remainder (14 units) using selective sampling.
3.1.5. Literature search
A total of 25 recent reviews dealing with Vibrio cases or outbreaks were retrieved through the general literature. Only one record (Ahmed et al., 2022) reported a case in an EU country (The Netherlands) attributed to a species other than V. parahaemolyticus, V. vulnificus or non‐O1/non‐O139 V. cholerae. The case was a sepsis episode in a 50‐year‐old man with a history of tuberculosis, chronic obstructive pulmonary disease and alcohol abuse. Species attribution was controversial due to the reporting of the isolate as Vibrio albensis in the review, as opposed to its identification as non‐O1/non‐O139 V. cholerae in the primary study (Engel et al., 2016). Both seawater contact and seafood consumption (ready‐made tuna salad) were reported as possible source of infection, but no Vibrio spp. were detected in the suspected seafood product.
Among the 1046 records captured through the literature searches, five records reported human infections with Vibrio species other than V. parahaemolyticus, V. vulnificus or non‐O1/non‐O139 V. cholerae in EU countries with an identified or suspected food‐borne origin. Amato et al. (2022), in a multi‐country study on vibriosis in Norway, Sweden, Denmark, Finland, Poland and Estonia in 2018, reported 152 V. alginolyticus infections. However, information on exposure was available for a minority of cases (20/152), and only two infections were attributed to consumption of contaminated food or water. Similarly, Hoefler et al. (2022) reported 23 V. alginolyticus infections in Bay of Biscay (France) between 2001 and 2019, but only one characterised by GE, the others being associated with otitis, osteitis or pneumonia. Brehm et al. (2021), in a retrospective study on vibriosis in Germany (2018–2019) reported one GE case due to V. fluvialis lacking conclusive information on seafood consumption. Finally, two GE cases following seafood consumption (oysters and cockles) due to Vibrio hollisae were reported by (Edouard et al., 2009; Gras‐Rouzet et al., 1996). Due to the reclassification of this species in the distinct Grimontia genus (Thompson et al., 2003), these cases were not further considered.
In comparison, a considerable number of V. parahaemolyticus and non‐O1/non‐O139 V. cholerae cases associated with seafood consumption were described in Europe since the 1990s (summarised in Annex C). These include large V. parahaemolyticus outbreaks or sporadic cases reported in four EU member states, and several non‐O1/non‐O139 V. cholerae sporadic cases in many countries. Two reports on seafood‐borne V. vulnificus infections were retrieved for cases in Spain and French overseas territories, but several investigations of V. vulnificus septicaemia cases were inconclusive due to critical patients’ conditions.
3.1.6. ProMED records
In total, 13 of the 408 retrieved ProMED records reported non‐cholera Vibrio cases in EU countries between 1997 and 2021. The cases were mostly related to wound infections or bacteraemia/septicaemia associated with non‐O1/non‐O139 V. cholerae, V. vulnificus and V. alginolyticus, following exposure to seawater. No report of food‐borne infections with species different than V. parahaemolyticus, V. vulnificus or non‐O1/non‐O139 V. cholerae was retrieved.
3.1.7. FSANZ data request
The FSANZ data request (Annex B) illustrated that human disease caused by V. parahaemolyticus and/or V. vulnificus are not notifiable in Croatia, Cyprus, Estonia, France, Ireland, but is notifiable in Sweden since 2004, in Norway since 2019 and in Germany since 2020.
There was no reporting of an increase of human illness caused by Vibrio species over the past 3–5 years in Croatia, Cyprus, Estonia, Germany, Ireland and Norway.
In Sweden, there was a slight increase in numbers over the last 10‐year period; however, this may be due to an increased awareness among physicians. The maximum number of reported cases in Sweden (178) was in 2018, which was a warm summer. Most cases are swimming related (e.g. ear infections in children, wound and blood infections). The epidemiology of vibriosis cases in countries bordering the North Sea and Baltic Sea area has been fully described in Amato et al. (2022).
The number of non‐cholera Vibrio infections is increasing in France (an average of 10 cases per year between 1995 and 2016, 25 cases in 2017, 59 in 2018, 67 in 2019, 47 in 2020, 46 in 2021). V. parahaemolyticus was the most frequently reported species since 2018, mainly in association with moderate forms of GE following seafood consumption, followed by V. cholerae non‐O1/non‐O139, but with which displays a significant number of imported cases. The emergence of V. fluvialis associated with GE in the last 5 years was also reported. Details are provided by the reports of the French National Reference Centre for Vibrio and cholera related to 2017–2020 23 : excluding travel‐associated cases, five V. fluvialis cases (three in 2018, two in 2019) and one V. mimicus case (2018) associated with seafood consumption were reported. In some of these cases, predisposing health conditions were documented. In comparison, in the same period 2017–2020, the reported number of V. parahaemolyticus cases associated with seafood consumption was 75, and the number of seafood‐borne non‐O1/non‐O139 V. cholerae cases was 14.
3.1.8. Previous risk assessments in EU countries
The 2012 ANSES risk assessment summarised the non‐cholerae Vibrio spp. leading to human infections in the 1995–2009 period. In total, 15 cases of GE (potentially of food origin and linked to seafood consumption) were due to non‐O1/non‐O139 V. cholerae (2 cases contracted in France, 7 abroad) and V. parahaemolyticus (65 cases contracted in France, 1 abroad) (ANSES, 2012).
The 2022 BfR risk assessment reported that the annual number of infections with non‐cholera vibrios in coastal areas in Germany ranged from 0 to 20 between 2002 and 2019. The majority involved wound and ear infections and only occasionally GE cases. BfR concluded that investigation of foods for potentially pathogenic Vibrio species should concentrate on V. parahaemolyticus, V. vulnificus and V. cholerae, as these species are worldwide in the aquatic ecosystems, and can therefore be present in international trade and locally produced seafood. The hazard identification also considered V. alginolyticus and V. metschnikovii, as these species had often been detected in seafood samples in Germany between 2017 and 2020. Other Vibrio species (V. mimicus, V. fluvialis, V. furnissii and V. harveyi) are detected only occasionally in food products in Germany and were not further addressed (BfR, 2022).
3.1.9. Concluding remarks
No harmonised surveillance system for non‐cholera vibriosis is currently available in the EU, and human disease by V. parahaemolyticus or V. vulnificus is notifiable in a few EU Member States. Based on this, it should be expected that the underestimation of non‐cholera vibriosis in the EU is considerable.
Vibrio infections acquired during travels abroad or through seawater exposure and leisure activities are often reported in the EU. Therefore, proper case description and investigation are crucial to define if infections are domestically acquired and if they should be attributed to food‐borne transmission.
V. parahaemolyticus is currently the species most frequently associated with human disease through seafood consumption in the EU, followed by non‐O1/non‐O139 V. cholerae. Food‐borne transmission of V. vulnificus is rarely documented in the EU.
Other Vibrio species as V. alginolyticus, V. fluvialis and V. mimicus may occasionally lead, particularly in individuals with underlying health conditions, to seafood‐associated infections, but their PH relevance is minor compared to V. parahaemolyticus, V. vulnificus or non‐O1/non‐O139 V. cholerae.
According to RASFF notifications, which are mostly associated with seafood imported to the EU, the species V. parahaemolyticus, non‐O1/non‐O139 V. cholerae and V. vulnificus are more often reported in seafood products placed or intended to be placed on the EU market compared to other Vibrio species. Other species reported are V. alginolyticus and V. mimicus.
The Vibrio spp. considered in the current opinion are V. parahaemolyticus, V. vulnificus and non‐O1/non‐O139 V. cholerae as, other species are less frequently found in seafood placed or intended to be placed on the EU market and, to date, have been rarely identified as the cause of illness in humans in the EU through seafood consumption.
3.2. Occurrence of the relevant Vibrio spp. in seafood placed or intended to be placed on the EU market (SQ1.2)
Overall, the 46 primary research publications retrieved thorough ELS covered 370 data entries on the occurrence (i.e. prevalence and/or concentration) of the relevant Vibrio spp. in seafood produced and/or commercialised in Europe. Of these, 224 entries concerned V. parahaemolyticus, 77 entries non‐choleragenic V. cholerae and 69 entries V. vulnificus, with studies undertaken in 13 EU countries. 24 A further 9 studies were carried out in non‐EU countries. 25 Publications concerned V. parahaemolyticus (N = 42), non‐choleragenic V. cholerae (N = 21) and V. vulnificus (N = 19). The overall number of samples tested for detection (sometimes associated with enumeration) and for enumeration only was, respectively, 10,614 and 64 for V. parahaemolyticus, 2907 and 64 for V. vulnificus, and 2613 and 7 for non‐choleragenic V. cholerae.
The studies encompassed samplings performed between 2000 and 2022 lasting from 2 to 120 months (median: 16 months), for 16 studies being shorter than 1 year. Moreover, the sampling period could be restricted to some months; most of the time, the summer period was covered by studies conducting sampling at primary production stage. Less than one third of the publications summarised studies that began in the last decade (i.e. initiated in or after 2014). In 11 publications, it was not possible to ascertain the year of sampling.
The largely uneven distribution of the studies across countries is reflected in the FAO areas and subareas of origin of the products. Seafood products originated mostly from the Mediterranean and Black Sea 26 and, secondly, from the Northeast Atlantic, 27 while testing of products from the Pacific Ocean 28 was reported in a minority of cases.
Sampling at the primary production stage and at the distribution stage were roughly equally represented in the data set (154 and 189 entries, 27 and 19 publications, respectively), while sampling at the manufacturing stage was present in 27 entries and 4 publications. However, while entries dealing with the distribution or manufacturing stage addressed the different seafood categories (58 entries for molluscs, 53 for crustaceans, 85 for fish meat and 20 for other seafood types, like sushi or surimi), the data related to primary production were almost exclusively associated with molluscs (145 of 154 entries). Significantly, despite the high number of publications related to bivalves at primary production (21 publications), only two (Suffredini et al., 2014) reported information on the classification 29 , 30 of the corresponding harvesting areas with separate results for each seafood category.
Based on the extracted data, the prevalence of Vibrio species of PH relevance in studies performed on seafood products in the European market is summarised in Figure 2. The result of random‐effect meta‐analysis of the prevalence data is included.
FIGURE 2.
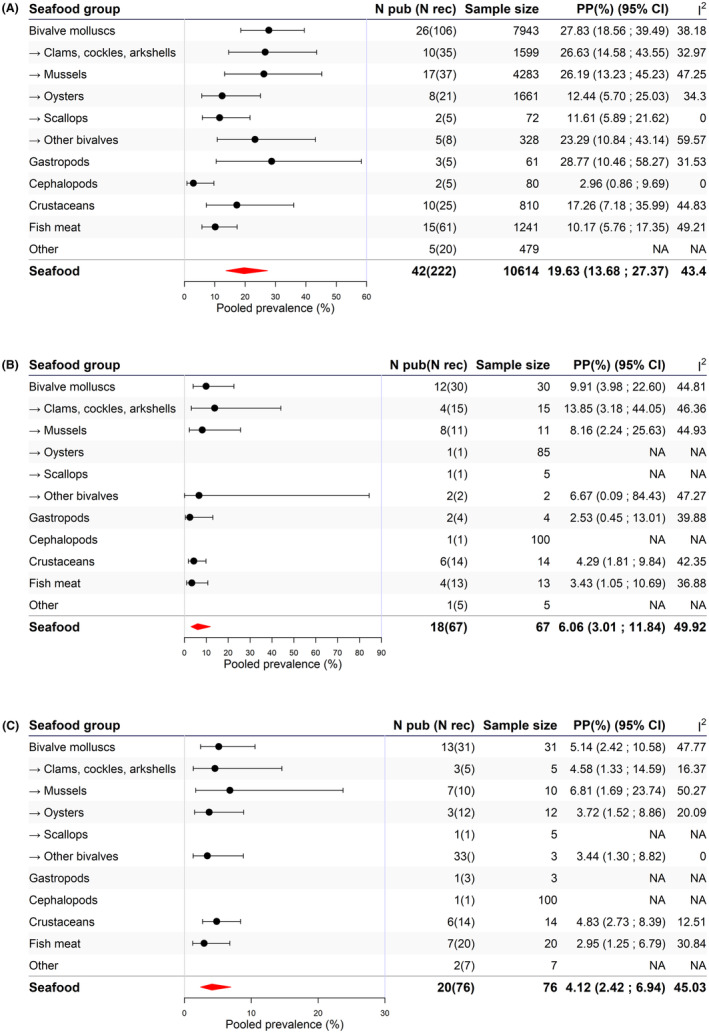
Pooled prevalence of V. parahaemolyticus (A), V. vulnificus (B) and non‐choleragenic V. cholerae (C) in the different seafood groups based on the meta‐analysis. I 2, intraclass correlation estimates the proportion of variance, from the total variance, explained by differences between the studies. Whenever the number of studies is small and the variability inside studies is high, the I 2 can be uncertain; NA, not applicable. Non‐choleragenic V. cholerae includes samples that were tested using either sero‐agglutination tests (non‐O1, non‐O139 or both) or molecular tests (no detection of cholera toxin‐associated genes, ctx, ctxA and/or ctxB). Meta‐analysis results are not presented for those food groups with only a single data entry and for the category ‘other’.
Most studies (n = 42) and entries (n = 224) reported data on the prevalence or concentration of V. parahaemolyticus (222 entries reported prevalence data). The type of seafood considered is given in Figure 2A. The entries dealing with bivalve molluscs mostly reported data on clams (e.g. Ruditapes, Ensis, Meretrix, Chamelea), mussels (Mytilus spp.), oysters (Crassostrea spp.) and scallops. The pooled prevalence estimate of V. parahaemolyticus was 19.63% (95% CI 13.68–27.37). The highest prevalence was found in bivalve molluscs (27.83%; 95% CI 18.56–39.49) and gastropods (abalones, winkles, conchs) (28.77%; 95% CI 10.46–58.27). Within the bivalve molluscs category, oysters and scallops had the lowest prevalence. The pooled prevalence estimates for mussels harvested in the Northeast Atlantic area was 55.89% (95% CI 23.64–83.84) (I 2 = 45.99), while for those harvested from the Mediterranean and Black Sea it was 23.17% (95% CI 8.40–49.79) (I 2 = 51.79). However, using random meta‐regression, this difference is not significant by Moderator test QM test (p > 0.05). Other factors such as the period of sampling, the species of mussels and environmental factors could interfere with the direct comparison. Least, the representativity of each fishing area for the comparison is not guaranteed. Overall, for most seafood categories, the heterogeneity between studies was found to be significant (I 2 > 25%) (Gonçalves‐Tenório et al., 2018), indicating that variability between studies remains non negligible; this variability could be explained by some factors such as different sites, years, period of sampling, or methods of detection between studies.
A significant proportion of the V. parahaemolyticus studies (19/42) included information on the detection of both genetic markers associated with V. parahaemolyticus pathogenicity, namely tdh and trh genes in positive samples (with sample size ≥ 3). The result of random‐effect meta‐analysis of the proportion of samples containing tdh+ and/or trh+ V. parahaemolyticus on V. parahaemolyticus‐positive samples is given in Table 4. Considering all seafood categories, on average about one out of five (18.41%; 95% CI 11.06–29.05) of V. parahaemolyticus‐positive samples contained pathogenic (i.e. tdh+ and/or trh+) V. parahaemolyticus.
TABLE 4.
Proportion of samples with tdh+ and/or trh+ V. parahaemolyticus on V. parahaemolyticus‐positive samples.
| Seafood group | N publications (N entries) | Sample size in the category | Proportion (%) [95% CI] and I 2 |
|---|---|---|---|
| Bivalve molluscs | 16 (36) | 588 | 20.89 [11.65, 34.58] I 2 = 32.61 |
|
5 (12) | 112 | 24.26 [16.73, 33.80] I 2 = 0 |
|
9 (17) | 329 | 19.87 [8.07, 41.20] I 2 = 33.71 |
|
4 (5) | 128 | 27.56 [20.12, 36.50] I 2 = 0 |
|
0 | 0 | NA |
|
2 (2) | 19 | 4.90 [0.68, 27.78] I 2 = 0 |
| Gastropods: abalones, winkles, conchs | 2 (2) | 14 | 10.09 [2.03, 37.77] I 2 = 0 |
| Cephalopods | 0 | 0 | NA |
| Crustaceans | 4 (8) | 121 | 10.93 [6.13, 18.75] I 2 = 0 |
| Fish meat | 1 (3) | 11 | NA |
| Other | 0 | 0 | NA |
| Total | 19 (49) | 734 | 18.41 [11.06, 29.05] I 2 = 34.08 |
Abbreviations: CI, confidence interval; I 2, intraclass correlation estimates the proportion of variance, from the total variance, explained by differences between the studies. Whenever the number of studies is small and the variability inside studies is high, the I 2 can be uncertain; NA, not applicable.
V. vulnificus prevalence was the object of 18 publications and 67 entries, the result of the random‐effect meta‐analysis of prevalence data is given in the Figure 2B. V. vulnificus pooled prevalence was 6.06% (95% CI 3.01–11.84) and was highest for bivalve molluscs (9.91%; 95% CI 3.98–22.60), especially in clams, cockles and arkshells.
The prevalence of non‐choleragenic V. cholerae was the focus of 20 publications and 76 entries (Figure 2C). Most studies tested for cholera toxin‐associated genes ctxA and/or ctxB, and few cases tested for O1/O139 sero‐agglutination. The non‐choleragenic V. cholerae pooled prevalence was 4.12% (95% CI 2.42–6.94).
Quantitative data on the concentration of the three relevant species were reported in only 8 publications, yielding 49 data entries dealing with V. parahaemolyticus (7 studies) in molluscs (23 entries) or crustaceans (10 entries), with non‐choleragenic V. cholerae in bivalve molluscs (3 publications, 5 entries) or with V. vulnificus in bivalve molluscs (3 publications, 11 entries). Considering entries with mean and standard deviation reported (13 entries, 4 publications, mostly concerning bivalves) V. parahaemolyticus pooled mean in bivalve molluscs was 1.91 log10 CFU/g or MPN/g (95% CI 0.68–3.14). The maximum mean concentration found in a survey was 4.99 log10 CFU/g reported in farmed mussels at retail (Stratev et al., 2021). For crustaceans, a mean concentration was estimated in shrimp of 3.33 log10 CFU/g (one entry). The maximum concentration in one sample was greater than 5.2 log10 MPN/g (Caburlotto et al., 2016). For V. vulnificus, the mean and standard deviation were only available in 3 of 11 entries (2 publications) concerning bivalves, with contrasting results. The pooled mean was approximately 1.84 log10 CFU/g or MPN/g (95% CI −2.33 to 6.01). For non‐choleragenic V. cholerae, only one entry quantified a mean of 1.34 MPN/g in cockles (Baron et al., 2017).
Several limitations were identified in the retrieved data, including (i) absence of relevant information on the sampling plan, (ii) absence of sampling period and the year of sampling, (iii) uneven seasonal distribution of sampling, (iv) uneven geographic distribution of sampling, (v) limited information on sample origin/strategy (at retail level) or on sanitary classification of production areas (for bivalve shellfish), (vi) heterogeneous reporting for pathogenicity testing and concentration estimates, (vii) lack of linkage between environmental parameters (temperature, salinity) and sample results in primary production, (viii) mixing of different seafood species or different origins in results, (ix) results presented, in particular for pathogenicity testing, as a mix of isolates from different samples, and (x) small sample size for each species or each type of seafood (N < 30).
3.2.1. Concluding remarks
Data on the prevalence and quantitative levels of the relevant Vibrio spp. in seafood produced and/or commercialised in Europe were extracted from 46 primary studies performed in 16 countries yielding 376 data entries (2000–2022 period). When reported, most studies dealt with samples from the Mediterranean Sea and, to a minor extent, the Northeast Atlantic. Among them, 228 entries concerned V. parahaemolyticus, 78 entries non‐choleragenic V. cholerae and 70 entries V. vulnificus.
- The main aim of the studies was the detection of one or more of the relevant Vibrio species, and quantitative analysis was rarely performed (49 entries). In seafood placed or intended to be placed on the EU market:
-
○Across seafood categories, the V. parahaemolyticus pooled prevalence estimate was 19.6% (95% CI 13.7–27.4). The highest prevalence was estimated for bivalve molluscs (27.8%; 95% CI 18.6–39.5) and gastropods (abalones, winkles, conchs) (28.8%; 95% CI 10.5–58.3). Within the bivalve molluscs category, oysters and scallops had the lowest prevalence.
-
○Considering all seafood categories, about one out of five (18.4%; 95% CI 11.1–29.1) of V. parahaemolyticus‐positive samples contained pathogenic (i.e. tdh+ and/or trh+) V. parahaemolyticus.
-
○The V. vulnificus pooled prevalence was estimated as 6.1% (95% CI 3.0–11.8) and was highest for bivalve molluscs (9.9%; 95% CI 4.0–22.6), especially in clams, cockles and arkshells.
-
○Non‐choleragenic V. cholerae pooled prevalence was estimated as 4.1% (95% CI 2.4–6.9).
-
○For most seafood categories, the heterogeneity between studies was found to be significant which could be explained by factors such as different sites, years, period of sampling, or methods of detection.
-
○Concentration data on the three Vibrio species were rarely reported. V. parahaemolyticus pooled mean concentration in bivalve molluscs was 1.91 log10 CFU/g or MPN/g (95% CI 0.68–3.14), while for crustaceans only the mean from one study was available (3.33 log10 CFU/g). For V. vulnificus the pooled mean concentration in bivalve molluscs was 1.84 log10 CFU/g or MPN/g (95% CI −2.33 to 6.01), and for non‐choleragenic V. cholerae, the mean from one study on cockles was 1.34 MPN/g.
-
○No data were retrieved on detection or quantification of V. parahaemolyticus, non‐O1/non‐O139 V. cholerae or V. vulnificus in tunicates or echinoderms.
-
○
Several limitations were identified in the retrieved data, including the absence of essential sampling descriptors, uneven temporary and geographic distribution, heterogeneity of data reporting, data aggregation among different species, and limited number of tested samples for certain species.
3.3. Analytical methods for the detection, enumeration and characterisation of the relevant Vibrio spp. in seafood and their contribution to risk assessment (SQ1.3–SQ1.4)
3.3.1. Methods for the detection and enumeration
Well‐established and standardised microbiological procedures are being used in routine monitoring of Vibrio spp. International and National Health Organisations and Authorities recommend the use of standard microbiological methods for the detection and enumeration of the relevant Vibrio spp. in seafood (Annex D). These procedures rely on cultural methods and the isolation of bacterial isolates occasionally combined with molecular identification/characterisation.
The available methods for the detection of the relevant Vibrio species rely on direct plating on selective or non‐selective agar media or on conventional selective enrichment of a suspension of seafood homogenate in alkaline peptone water followed by plating onto one or two selective agar media for isolation of presumptive pathogenic Vibrio spp. Up to 10 characteristic colonies are then subjected to identification by biochemical testing, colony hybridisation and/or PCR and further characterisation by serotyping or detection of pathogenicity markers (e.g. TDH for V. parahaemolyticus) [ISO 21872‐1:2017 31 ; FDA‐BAM (2004) 32 ; MFLP‐37 (2019) 33 and MFLP‐102 (2017) 34 ]. Serotyping of V. cholerae O1 and O139 is included in FDA‐BAM (2004) but only suggested, with no technical information on the procedure, in ISO 21872‐1:2017.
The available standardised methods for the enumeration of V. parahaemolyticus and/or V. vulnificus in seafood utilise direct plating of seafood homogenate suspensions on selective agar or on non‐selective agar followed by colony hybridisation [ISO/TS 21872–2:2020, 35 FDA‐BAM (2004) 36 ] or conventional MPN enrichment followed by colony isolation and species identification by biochemical testing, colony hybridisation or PCR [FDA‐BAM (2004) 37 , GB 4789.7‐2013, 38 GB 4789.44‐2020 39 ].
These detection and enumeration methods include the use of selective and/or differential agar media which provide discrimination between Vibrio species and other microbiota and, especially when chromogenic media are adopted, differentiation of Vibrio spp. of PH relevance from other Vibrio spp. Molecular confirmation methods markedly improve identification of Vibrio spp. of PH relevance and discrimination between pathogenic and non‐pathogenic strains, e.g. of V. parahaemolyticus. However, if a species of PH interest or a pathogenic strain within the species is present in much lower concentrations than other Vibrio species or non‐pathogenic strains, the isolation and confirmation of a larger number of colonies, or the application directly on enrichments of PCR methods for species/pathogenicity genes detection, may be required to achieve detection. Despite some limitations (see Section 3.3.3), the ISO methods are commonly used at the international level for the detection of V. parahaemolyticus, V. vulnificus and/or V. cholerae and the enumeration of total and potentially enteropathogenic V. parahaemolyticus in seafood (Hartnell et al., 2019; Suffredini et al., 2014). These methods and the FDA‐BAM methods have been used in most studies reporting data on the occurrence of the relevant Vibrio spp. in seafood as presented in Section 3.2.
Further to the standardised methods, a variety of other methods combining culture‐dependent and molecular methods have been developed and evaluated for optimised and rapid detection and enumeration of Vibrio in environmental and seafood samples. These include qualitative and quantitative (MPN) methods with reduced culture enrichment time combined with PCR detection approaches (Blanco‐Abad et al., 2009; Luan et al., 2008; Machado & Bordalo, 2016; Robert‐Pillot et al., 2014) and/or isolation of strains (Chen et al., 2012). Furthermore, quantitative PCR (qPCR) methods have been reported (Niu et al., 2018; Robert‐Pillot et al., 2010), especially for the enumeration of total and pathogenic V. parahaemolyticus. Aside from already existing methods to confirm identification of vibrios, matrix‐assisted laser desorption ionisation–time of flight (MALDI–TOF) mass spectrometry (MS) with databases built on environmental, clinical and reference strains; e.g. PVBase 40 (Liu et al., 2022) proved to be precise and cost‐effective tools for accurate and rapid identification of a large number of Vibrio spp. Loop‐mediated isothermal amplification (LAMP) assays have also been developed and successfully applied to detect V. parahaemolyticus and V. vulnificus from environmental water and aquatic products (Liu et al., 2017; Tian et al., 2022). Recent FBO investigations involving digital droplet PCR (Hu et al., 2022; Zhao et al., 2022; Zhou et al., 2023) or combining next generation sequencing (NGS) and digital PCR (Li et al., 2019) showed that these methods provide new fast tools for accurate detection and quantification of pathogenic Vibrio species. Finally, viability PCR showed to be a promising approach to overcome the underestimation of microorganisms in the VBNC state by culture‐based methods, and different assays have been developed to detect and quantify VBNC V. parahaemolyticus and V. cholerae, although, to date, their application has been mostly on experimentally contaminated seafood (Liu et al., 2018; Wu et al., 2015).
3.3.2. Methods for characterisation
Classical typing analyses of pathogenic Vibrio species have been based on serotyping, which specific schemes developed for the O‐ antigen of V. cholerae and the O‐ and K‐antigens of V. parahaemolyticus. Serotyping was useful to discriminate strains from outbreaks in the earlier days and was afterwards enhanced by pulsed‐field gel electrophoresis (PFGE), which allowed for a higher discriminatory power. These techniques showed some inconsistences in the serotyping scheme particularly of V. parahaemolyticus, where identical serotypes showed different genetic profiles and extensive serotype diversity was present among isolates representing the same genetic types (Zhang et al., 2022).
With the introduction of sequencing technologies, multi‐locus sequence typing (MLST) schemes based on PCR‐amplification and Sanger sequencing of a limited number of housekeeping genes (from 5 to 10) were developed for non‐O1/non‐O139 V. cholerae (Octavia et al., 2013), V. cholerae O1/O139 (Kanampalliwar & Singh, 2020), V. parahaemolyticus (González‐Escalona et al., 2008) and V. vulnificus (Bisharat et al., 2007) using a dedicated website to analyse and deposit genomic data and profiles. 41
The emergence of genomic epidemiology approaches applying WGS and other NGS approaches, coupled to open access bioinformatic tools, has further changed the techniques applied for characterisation at the strain level. In particular, WGS replaced the PCR‐Sanger approach for MLST with in silico analysis of variants for each genome locus. 42 An extended core genome MLST (cgMLST) scheme using 2254 core genes of V. parahaemolyticus genome was developed by (Gonzalez‐Escalona et al., 2017) and an analogous scheme is available for V. cholerae (Liang et al., 2020), both of which outperform previous methods in terms of resolution. WGS is today routinely applied in the investigation by public health agencies (e.g. CDC, FDA, China CDC, UK‐PHE) of Vibrio FBOs to identify genetic variants of strains with particular epidemic potential, to follow ecological expansion and emergence of highly pathogenic V. parahaemolyticus clones 43 (Abanto et al., 2020), for clinical surveys, and for research purposes, but the use in food control activities (beyond outbreak investigations) has been limited. Most application of NGS in Vibrio studies reported to date have been focused on WGS of isolates obtained by culture. Other omics techniques, such as metabarcoding (based on 16S rRNA gene or other target sequences) (Zampieri et al., 2021) or metagenomics have been applied only in research contexts and rarely for food safety. Shotgun metagenomics, for instance, allowed the identification of markers in the intestinal microbiome of patients with cholera at the time of exposure to V. cholerae (Levade et al., 2021) and rare pathogens such as V. vulnificus in patients with skin and soft tissue infections (Wang et al., 2020). Metagenomic shotgun sequencing have been also applied in environmental investigations to determine the presence of pathogenic vibrios linked to some of extreme weather events, such as hurricanes (Brumfield et al., 2023).
3.3.3. Contribution to risk assessment
The methods currently available for the analysis of Vibrio spp. of PH relevance display a variegate assortment of advantages and disadvantages, as well as notable areas for future development. The SWOT of these methods in regards of their contribution to risk assessment is provided in Table 5.
TABLE 5.
Characteristics of the available analytical methods for the detection and enumeration of potentially enteropathogenic Vibrio spp. in seafood and their contribution to risk assessment.
| Culture‐based detection methods | Culture‐based quantification methods | Conventional typing methods | WGS (from isolates) | Metagenomics (targeted and untargeted) | |
|---|---|---|---|---|---|
| Strengths |
|
|
|
|
|
| Weakness |
|
|
|
|
|
| Opportunities |
|
|
|
|
|
| Threats |
|
|
|
|
|
Abbreviations: DR, dose response; ISO, International Organization for Standardization; PCR, polymerase chain reaction; PH, public health; TDH, thermostable direct haemolysin; TRH, TDH‐related haemolysin; VBNC, viable but non‐culturable; Vc, V. cholerae; Vp, V. parahaemolyticus; Vv, V. vulnificus; WGS, whole genome sequencing.
In particular, conventional cultural methods provide the advantage of standardisation, which allow for an improved comparability of data in surveys on Vibrio occurrence and concentrations, though it should be noted that full harmonisation and intercomparison of Vibrio methods (including evaluation of LOD and LOQ estimates) is still needed for interpreting and aggregating occurrence data. Besides, quantitative approaches for routine application in monitoring are missing for some of the species of PH relevance, limiting the production of quantitative data to integrate in exposure estimates of classical QMRAs. It should also be considered that, while the ability to provide isolates for further characterisation is a relevant feature of cultural methods, the culture and isolation process may disadvantage less common strains, as the pathogenic ones, therefore leading to an underestimation of their risk. On the other side, molecular methods, and in particular WGS and NGS/WGS methods, may provide a significant and variegate contribution to risk assessment (extensively reviewed in EFSA BIOHAZ Panel, 2019). Beside their application in outbreak investigation and source attribution, they can contribute to the hazard characterisation by identifying molecular markers associated with specific phenotypes, or by supporting the definition of strain/virulence‐related DR curves (Pouillot et al., 2024). Furthermore, the omics methodologies can be particularly helpful to investigate the variability of vibrios in relation with virulence/AMR, behaviour in food, in the environment and in animal reservoirs (Rantsiou et al., 2018) and may provide, with metagenomics, a shift from studying microorganisms dynamics as a standalone towards studying them within the dynamics of the microbial communities.
3.3.4. Concluding remarks
Standardised microbiological methods are available for the detection of V. parahaemolyticus, V. cholerae, V. vulnificus in seafood and for the quantification of V. parahaemolyticus and V. vulnificus. These methods rely on microorganisms' culture and include molecular tests for species identification and/or for the detection of genes associated with pathogenicity. Serological tests are included in some cases for the identification of V. cholerae O1 and O139.
Performance characteristics of detection and quantification methods of Vibrio spp. are only reported for ISO methods. Most studies investigating environmental matrices and food samples report FDA‐BAM and ISO methods. However, a comparison of the performance of these methods has not been performed.
To address some of the limitations of the standardised microbiological methods, a variety of different methods have been developed, including alternative approaches for detection, quantification and identification (e.g. LAMP‐PCR, quantitative, digital and viability PCR, MALDI–TOF MS).
Among methods for the characterisation of Vibrio species, serotyping and PFGE, have been progressively replaced by sequencing technologies. MLST schemes are available for all Vibrio spp. of PH relevance while (cg)MLST schemes are available for V. parahaemolyticus, non‐O1/non‐O139 V. cholerae. WGS has been progressively more applied for full characterisation of Vibrio isolates in clinical surveys, research activities and outbreak investigations, but its use in official food control activities has been limited.
- The available analytical methods contribute to different elements of risk assessment:
-
○culture‐based methods provide harmonised data on occurrence and levels of Vibrio spp. in seafood products for exposure estimates, support the isolation of strains for further characterisation and, being easy and affordable, can be extensively applied in widely different contexts;
-
○WGS, in association with other omics as transcriptomic, proteomic and metabolomic, contribute to hazard characterisation through the identification of molecular markers associated with phenotypic traits, the investigation of species variability in relation to virulence/AMR and behaviour of the microorganism in food and in the environment, and by supporting outbreak investigation and source‐attribution studies;
-
○metagenomics provides information on microbial populations (including non‐culturable strains and/or difficult to cultivate species) and may support the study of microorganisms' dynamics within complex microbial communities.
-
○
- The main limitations of the current analytical methods which affect risk assessment are:
-
○for culture‐based methods: the lack of intercomparison of standard methods' performance, the absence of systematic characterisation of pathogenic strains within the methods, the possible underestimation of prevalence and contamination levels and thus, exposure due to underdetection of pathogenic strains and of microorganisms in the VBNC state;
-
○for NGS/WGS approaches: the complexity of data production and metadata sharing, the insufficient standardisation in data analysis which may lead to inconsistencies and, for metagenomics, the absence of isolates for further characterisation, the absence of phenotypic information and the reporting of signals from non‐viable microorganisms.
-
○
3.4. Pathogenicity to humans and virulence factors of the relevant Vibrio spp. (SQ1.5)
V. parahaemolyticus can cause acute GE in humans, including in healthy individuals after consumption of raw or undercooked fish or seafood. The main symptoms include watery diarrhoea (in the most severe cases bloody diarrhoea), abdominal pain, fever, vomiting, nausea, fatigue and headache. In healthy individuals, infection is normally self‐limiting, lasting 1–10 days. In subjects with underlying clinical conditions (e.g. immunocompromised), the infection can occasionally result in septicaemia (Ceccarelli et al., 2019). GE due to V. parahaemolyticus occurs as outbreaks or sporadic cases caused by different serotypes. However, two transcontinental expansions of the pathogen have been reported over the last two decades. The pandemic clonal complex 3 (CC3) [or sequence type 3 (ST3)], which was most often identified as serotype O3:K6, has caused most of the disease globally since 1996. The recent CC36 (or ST36, identified as serotype O4:K12), which was restricted to the Pacific Northwest region of the USA and Canada until its detection in the northwest of Spain in association with a large outbreak in August 2012 (Han et al., 2019; Martinez‐Urtaza et al., 2017).
V. vulnificus can infect a wide variety of hosts, including humans and different fish species, and it is considered zoonotic as it can be transmitted from infected fish to humans. Humans can be infected with V. vulnificus by eating raw seafood or can develop severe wound infections after contact with seawater, both of which can lead to sepsis. The progression of the infection to sepsis depends on the infected patient's health status and gender (Baker‐Austin & Oliver, 2018; Merkel Sandra et al., 2001). In subjects with no predisposing condition, the disease is usually self‐limiting and rarely leads to septicaemia, while the infection can lead to death by sepsis in individuals with underlying conditions (Ceccarelli et al., 2019). The most frequently documented risk factors are chronic liver diseases, haemochromatosis, diabetes mellitus, malignancies and biliary tract diseases (Hernández‐Cabanyero & Amaro, 2020). In the USA, the mortality rate associated with V. vulnificus infection is ~33%, and V. vulnificus causes > 95% of seafood‐related deaths (Walter, 2023). CDC data suggest that, after eating raw oysters, people with liver disease are 80 times more likely to become ill with a V. vulnificus infection and 200 times more likely to die from this infection than people without liver disease (Hlady et al., 1993). The species is highly variable and is divided into 5 phylogenetic lineages plus one pathovar (pv. piscis), which groups all the strains harbouring the fish virulence plasmid (pFv) (Carmona‐Salido et al., 2021). Regardless of lineage, however, a single strain or clone can be virulent or avirulent to human. A subcutaneously inoculated iron‐dextran treated mouse model has been used to evaluate virulence potential (skin infection versus systemic infection and death) in V. vulnificus (Lydon et al., 2021). There have been several unsuccessful attempts to find a genetic marker for pathogenicity by comparing environmental and clinical strains. Analysing the genome of 80 isolates from different sources, nearly 90% of the virulence‐related genes described are found in the core genome of the species. This suggests that all V. vulnificus strains should be considered potentially pathogenic for humans (Roig et al., 2018).
V. cholerae is classified according to its major surface antigen, the O antigen, into ~200 O‐serogroups, however, only 2 serogroups, O1 and O139, are strongly associated with cholera toxin (CTX) and toxin‐co‐regulated pilus (TCP) production, related to pandemic cholera. Unlike O1 and O139 strains, non‐O1/non‐O139 V. cholerae strains are, with few exceptions, generally devoid of CTX and TCP and are not associated with epidemic cholera. Since both CTX and TCP are encoded within the genome of a filamentous bacteriophage, it has been hypothesised that non‐O1/non‐O139 V. cholerae strains can occasionally acquire genes for toxin production by transduction or other horizontal gene transfer (HGT) mechanisms (Vezzulli et al., 2020). Nevertheless, non‐O1/non‐O139 V. cholerae may produce other toxins and are generally associated with self‐limited GE or mild extraintestinal symptoms (Ceccarelli et al., 2019). In susceptible host, non‐O1/non‐O139 V. cholerae members can cause exfoliated wound infections, tissue necrosis or sepsis, and high mortality (Igere et al., 2022).
Pathogenic Vibrio species possess a wide array of VFs that will allow the colonisation and spread in the host. The virulence of these pathogens is multifactorial, i.e. several of the VFs interact to produce damage in the host. Some factors may be present in all species, while others are specific to certain species or strains within one species. The VFDB was consulted (see Section 2.2.4) to extract a list of the main VFs of V. cholerae O395, V. parahaemolyticus RIMD 2210633 and V. vulnificus CMCP6 and YJ016, 44 and then complemented by literature search.
Among the VFs present in all pathogenic species are factors associated with motility, attachment and colonisation, such as the capsule, flagellum and adhesins. The capsule has been described as essential for a successful infection, colonisation of intestine cells or resistance to the phagocytosis (Johnson, 2013; Jones & Oliver, 2009; Letchumanan et al., 2014), and may characterise different impact within the species (Bian et al., 2020; Pettis & Mukerji, 2020). Flagellar motility is a highly regulated processes linked to host colonisation, chemotaxis, adhesion, biofilm formation and virulence in vibrios (Khan et al., 2020). Other types of appendages are the pili, among which a special subfamily of pili, termed Type IV pili, can bind to specific receptors of the epithelium and other types of cell tissue of different mammals, giving them a role in invasion and colonisation. Other factors required for infection are related with the nutrient acquisition as vibrios must produce iron uptake systems in iron limited environment as the human gut (Johnson, 2013; Roig et al., 2018). Numerous studies have been carried out to understand the regulatory systems controlling expression of virulence genes in Vibrio spp. These processes are regulated by well characterised bacterial regulatory systems like quorum sensing (QS), virulence regulators ToxR and ToxT or the ferric uptake regulator (Fur) among others (Hernández‐Cabanyero & Amaro, 2020; Midgett & Kull, 2023).
The outcome of Vibrio infections is determined both by bacterial and host factors. This includes the specific virulence genes of the pathogen, the ability of the host to modulate an adequate immune response, and the ability of the host to eliminate the pathogen. This relationship between pathogen and host is especially important for V. vulnificus or V. cholerae infections since certain predisposing factors of the host are determining factors for the onset of symptoms.
Clinical strains of Vibrio isolated from patients have a variety of VFs. Only for V. parahaemolyticus, some genes are considered well‐established pathogenicity markers. In fact, TDH and TRH are currently the most predictive indicators of pathogenicity, and most V. parahaemolyticus infections are associated with strains that possess these genes, although some papers reported the detection of tdh−/trh– strains among clinical isolates (Baker‐Austin et al., 2020; Jones et al., 2012). However, in these cases, no direct evidence on the pathogenicity of these strains or their role in causing the infections was available. For V. vulnificus, several attempts to find a good genetic virulence marker were unsuccessful. Therefore, the WHO considers all V. vulnificus isolates as potentially pathogenic for humans (FAO and WHO, 2021). Non‐O1/non‐O139 V. cholerae is a very variable group of strains that continues to be very little studied, hence a genetic marker that can be used to determine the pathogenicity of an isolate has not yet been identified.
As mentioned, beyond the previously described VFs common to more than one species (i.e. capsule, flagella, etc.), vibrios have a large repertoire of VFs that include, among others, proteases, cytolysins and different toxins. A summary of the most relevant VFs specific of V. parahaemolyticus, V. vulnificus and V. cholerae non‐O1/non‐O139, their coding genes and mode of action is presented in Table 6.
TABLE 6.
Virulence factors specific of V. parahaemolyticus, V. vulnificus and non‐O1/non‐O139 V. cholerae.
| Virulence factor | Presence | Gene(s) | Mode of action | Reference |
|---|---|---|---|---|
| V. parahaemolyticus | ||||
| Thermostable direct haemolysin (TDH) | Pathogenic strains | tdh |
This protein exhibits enterotoxigenic activity, cytotoxicity, cardiotoxicity and increased vascular permeability. Clinical strains of Vp isolated from ill patients usually produce a clear beta‐type haemolysis on Wagatsuma agar due to production of TDH. This phenomenon is called the Kanagawa phenomenon (KP). Only a small proportion of the environmental strains show a positive KP (< 1%). Therefore, TDH encoded by the tdh gene has been considered a major VF of Vp. Five sequence variants of the tdh gene (tdh1 to tdh5) can be distinguished, which are > 97% identical. Only tdh4 is codified in a plasmid. All KP‐positive Vp strains possess tdh1 and tdh2, and both are encoded in the 80 kb pathogenicity island Vp‐PAI. The expression level of tdh2 is higher than the rest, this protein being the main responsible for the KP. Some KP‐negative isolates are tdh‐positive strains (around 48%) as they possess the chromosomal tdh3 and tdh5 genes, and the plasmid‐borne tdh4 genes. These strains produce TDH toxin at a lower level, so that the KP effect is not appreciated. |
Cai and Zhang (2018), Gavilan et al. (2021) |
| TDH‐related haemolysin (TRH) | Pathogenic strains | trh | Strains carrying the trh gene code for TRH, which is a heat labile toxin, with immunological, haemolytic and toxicity properties similar to TDH. TRH was judged to be associated with reported illnesses and thus considered to be another important VF of Vp. There are two sequence variants of the trh gene (trh1–trh2). | Gavilan et al. (2021) |
| Type III Secretion System (T3SS) | T3SS2, located on Vp‐PAI, pathogenic strains | Gene apparatus including translocon complex genes and effectors genes |
These secretion systems are multicomponent nanomachines that enable Gram‐negative bacteria the delivery of proteins called ‘effector’ into the host cell and manipulate host cellular processes. Unlike T3SS1 which is present in all isolates of the species, the T3SS2 genes are present in pathogenic strains on pathogenicity island Vp‐PAI on chromosome 2. T3SS2 can deliver up to 10 effector proteins that, in this case, are able to control the actin cytoskeleton dynamics and the innate inflammatory responses. Two distinct lineages (T3SS2α and T3SS2β) have been reported, correlating with the presence of tdh and trh genes, respectively. |
Caburlotto et al. (2009), Ceccarelli et al. (2019), Okada et al. (2009) |
| V. vulnificus | ||||
| Haemolysin/cytolysin | All strains | vvhA | VvhA is a pore‐forming toxin conferring strong haemolytic activity to Vv. It triggers apoptosis, necrotic cell death and autophagy by dysregulation of host cell signalling, leading to host tissue damage. Also, injection of purified VvhA induces proinflammatory cytokine and inflammatory chemokines in a mouse model, which presumably results in septic shock. | Kaus et al. (2014), Yuan et al. (2020) |
| Metalloproteinase | All strains | vvp | This protease has been shown to increase vascular permeability and oedema through the generation of bradykinin. Furthermore, activation of prothrombin and fibrinolysis by Vvp results in clotting and protects the bacteria from the host immune defences. Both activities support the dissemination of Vv from the intestines to the blood stream leading to sepsis. Vvp can also facilitate iron uptake by Vv by digesting haem proteins, transferrin and lactoferrin. Vvp was thought to be important in bacterial growth and disease development. Nevertheless, a Vvp‐deficient mutant has been shown to be even more virulent than the wild‐type strain, probably because of overexpression of the cytolysin in the absence of Vvp. | Li and Wang (2020), Shao and Hor (2001) |
| Multifunctional autoprocessing repeats‐in‐toxin (MARTX) toxin | All strains | rtxABCD | Rtxa belongs to the MARTX toxins, which are modular proteins of high molecular weight with conserved external modules that form pores in the eukaryotic membrane, through which the toxin's central module composed by different effector domains is translocated. Seven distinct toxin variants have been identified in Vv according to the repertoire and organisation of the effector domains. The effector content can vary from 2 to 5 effectors selected from a total of nine known effector domains. RtxA has been involved in the programmed necrotic death, changes of cytoskeleton structure, actin aggregation of host cells, production of reactive oxygen species and pore formation in the host cell membrane. In the murine infection model is the primary virulence factor for Vv. | Gavin and Satchell (2015), Kling et al. (2022) |
| Neuraminidase | All strains | Nan locus | Vv can utilise N‐acetylneuraminic acid as a nutrient during infection, adherence to epithelial cells and intestinal colonisation. Mutants in some of the genes of the nan locus have been proved to have an attenuated virulence in murine infection model | Jeong et al. (2009), Kim and Kim (2023) |
| Non‐O1/non‐O139 V. cholerae | ||||
| Type III Secretion System (T3SS) | Unknown/unclear a | Genes encoding the T3SS structural proteins (VcsRTCNS2, VcsVUQ2, VcsJ2 and VspD), the translocated proteins (Vops) and the T3SS transcriptional regulators (VttRA and VttRB) (in strain AM‐19226) | The Vc T3SS is most closely related to the Vp T3SS2. In strain AM‐19226 (Vc O39) T3SS function is important for colonisation. The genome of this strain codifies 12 different effectors proteins, some of them with critical role in colonisation and others that could be essential for adherence to eukaryotic cells. | Chaand et al. (2015), Schirmeister et al. (2014), Shin et al. (2011) |
| Haemagglutinin protease ([HA]/protease) | Unknown/unclear | hapA | [HA]/protease is a versatile metalloprotease that can increase the severity of a cholera infection by multiple mechanisms; produce inflammation and caused morphological changes in the small intestine. It enhances fluid accumulation in the rabbit ileal loop model. When purified protein was injected in this animal model a dose‐dependant haemorrhagic fluid response accompanied with significant tissue damage was observed. | Benitez and Silva (2016) |
| MARTX toxin of Vibrio cholerae (MARTX Vc ) | All strains | rtxABCD | All Vc strains have a rtxA gene except for classical strains. Vc has a MARTX toxin that delivers three effectors by autoprocessing: an actin crosslinking domain that introduces an isopeptide bond between actin molecules, making the actin unable to assemble an actin cytoskeleton; a Rho‐inactivation domain that induces actin depolymerisation through loss of active RhoA; and an alpha‐beta hydrolyse enzyme that has not yet been characterised. The MARTX Vc effector domains simultaneously promote virulence and suppress inflammatory responses. | Dolores and Satchell (2013), Woida and Satchell (2020) |
| Neuraminidase | Unknown/unclear | Nan locus | Catabolism of N‐acetylneuraminic acid. These genes provide the capacity to utilise sialic acid as a carbon and energy source, an advantage in the mucus‐rich environment of the gut. | Ceccarelli et al., 2019) |
| Type VI Secretion System (T6SS) | Unknown/unclear | IAHP (IcmF associated homologous proteins) gene cluster, together with other virulence‐associated secretion (VAS), Hcp and VgrG genes | T6SS is a specialised transport apparatus which is capable of translocating proteins to a target cell that could be eukaryotic or prokaryotic. In bacteria, immunity genes are the best‐characterised defences, protecting against specific cognate effectors. In Vc the immunity to antibacterial T6SS effector function is necessary for host colonisation. Some non‐O1/non‐139 Vc strains possesses a constitutively active T6SS and have been linked to outbreak of GE, although its contribution to disease still needs to be more systematically evaluated. | Ceccarelli et al. (2015), Fu et al. (2013), Ho et al. (2014) |
| Toxin‐coregulated pilus | Unknown/unclear | tcp | TCP is a Type IV bacterial pilus that is required for colonisation of the intestinal tract. It is not associated with epidemic cholera. | Ghosh et al. (1997) |
| Haemolysin/cytolysin A | All strains | hlyA | Haemolysin A (HlyA) is an important virulence factor that belongs to the pore‐forming toxin family. HlyA has vacuolar and cytocidal activity in many cell lines and is associated with rapid mortality in mice. | Gutierrez et al. (2007) |
| Heat‐stable enterotoxin (NAG‐ST) | Unknown/unclear | st (stn/sto) | Heat‐stable enterotoxin is a 17 amino acid peptide. ST acts in a manner similar to the Escherichia coli toxin STa; the toxin will increase in the intracellular concentrations of cyclic guanosine monophosphate. The intracellular increase in cyclic guanosine monophosphate results in a stimulation of chloride secretion and net fluid secretion, resulting in diarrhoea. | Hodges and Hecht (2012) |
Abbreviations: [HA]/protease, haemagglutinin protease; GE, gastroenteritis; IAHP, IcmF‐associated homologous proteins; KP, Kanagawa phenomenon; MARTX, multifunctional autoprocessing repeats‐in‐toxin; NAG‐ST, heat‐stable enterotoxin; PAI, pathogenicity island; PAI, pathogenicity island; RID, Rho‐inactivation domain; TCP, Toxin‐coregulated pilus; TDH, thermostable direct haemolysin; TRH, TDH‐related haemolysin; VAS, virulence‐associated secretion; Vc, V. cholerae; Vp, V. parahaemolyticus; Vv, V. vulnificus.
Comparison of non‐O1/non‐O139 V. cholerae isolates from ear/wound infections and from diarrheal patients in Germany showed the presence of T3SS exclusively in the latter strains (Schirmeister et al., 2014).
3.4.1. Concluding remarks
Pathogenic species of the genus Vibrio possess a wide array of VFs that allow the colonisation and spread in the host. The virulence of these pathogens is multifactorial, i.e. several VFs interact to produce damage in the host. Although with different impact, some of these factors may be present in all species (i.e. the capsule, flagellar mobility and pili), while others are specific to certain species or strains within one species.
The severity of infections with Vibrio species is determined both by bacterial (specific virulence genes) and host factors (predisposing conditions and immune response).
- V. parahaemolyticus mainly leads to acute GE, also in healthy individuals.
-
○The pathogenicity in humans is significantly associated with two haemolysins, TDH and TRH, and these factors are considered well‐established pathogenicity markers, but strains lacking their coding genes (tdh 1–5, trh 1–2) can be occasionally detected in clinical specimens.
-
○The presence of a type 3 secretion system (T3SS2), located on pathogenicity island Vp‐PAI, has been associated with pathogenic strains.
-
○V. parahaemolyticus infections occur as either outbreaks or sporadic cases caused by different serotypes/sequence types. Two transcontinental expansions of specific serotypes/sequence types (ST3 and ST36) of V. parahaemolyticus have been reported from the late 1990s.
-
○
- V. vulnificus infections are relatively rare and affect mainly susceptible individuals with underlying conditions. In those patients the disease can lead to sepsis and possibly death.
-
○This species possesses several VFs, including haemolysins (VvhA), protease (Vvp), multifunctional autoprocessing repeats‐in‐toxin (MARTX) toxin and neuraminidases.
-
○Reliable discrimination of pathogenic from non‐pathogenic strains has not yet been achieved and therefore, to date, all V. vulnificus strains are considered potentially pathogenic.
-
○
- Non‐O1/non‐O139 V. cholerae is generally associated with self‐limited GE or mild extraintestinal symptoms. However, in susceptible individuals, strains can cause more severe infections, sepsis and death.
-
○The most important VFs described for this group of microorganisms are T3SS, MARTX toxin, [HA]/protease and neuraminidase.
-
○Despite the number of studies published for the V. cholerae species, few of them specifically addressed non‐O1/non‐O139 V. cholerae, and no reliable genetic marker associated with pathogenicity has been identified so far.
-
○
3.5. Antimicrobial resistance in the relevant Vibrio spp. isolated from seafood and from seafood‐borne infections (SQ1.6–1.7)
AMR is defined as the inability or reduced ability of an antimicrobial agent to inhibit the growth of a bacterium, which, in the case of a pathogenic organism, could lead to therapy failure (EFSA and ECDC, 2022). Vibrio bacteria are known to acquire AMR by mutations within the bacteria and by uptake of AMR genes through HGT. Various mobile/mobilisable genetic elements were found in vibrios that facilitate the dissemination of AMR genes, e.g. conjugative plasmids, transposons, integrative conjugative elements (ICE), integrons and insertion elements (Das et al., 2020; Dutta et al., 2021; Escudero & Mazel, 2017; Kaushik et al., 2019; Kitaoka et al., 2011).
The presence of antimicrobial‐resistant potentially pathogenic bacteria in food can directly threaten consumers through infections that may be resistant to antibiotic treatment. Additionally, these bacteria may transfer AMR genes to the microbiota of consumers (Brisabois et al., 2019). In a recent EFSA opinion on the role of the food‐producing environment in AMR transmission (EFSA BIOHAZ Panel, 2021), Vibrio were considered as Group 2 bacteria (i.e. environmental or commensal bacteria that could act as donor of resistance genes (e.g. to Gram‐negative pathogenic bacteria) to last‐resort antibiotics). It was noted that the human disease burden resulting from antimicrobial‐resistant indigenous aquatic or fish bacteria has been insufficiently studied. Moreover, insufficient information on the lineages or serotypes able to cause infection and on the AMR profiles of aquatic and fish indigenous bacteria belonging to V. parahaemolyticus and V. vulnificus precludes their current inclusion in Group 1 (bacteria causing infection resistant to antimicrobials of choice for the treatment of serious infections or to last‐resort antibiotics).
As gastrointestinal infections caused by Vibrio spp. are normally self‐limiting (see Section 3.4), treatment with antimicrobials is not recommended (CDC 45 ). Only in cases of severe infections or in individuals with underlying medical conditions antimicrobial treatment should be applied. Long‐term surveillance data from the US (period 1990 to 2010) shows that two thirds of patients with Vibrio spp. illnesses were prescribed antibiotics – in the order quinolones (56.1%), cephalosporins (24.1%), tetracyclines (23.5%) and penicillins (4.3%) (Wong et al., 2015).
Antimicrobials are intensively used in aquaculture systems (Deekshit et al., 2022) and several diseases of farmed animals are caused by Vibrio species, with V. parahaemolyticus and V. vulnificus as important pathogens (Sony et al., 2021). For this reason, Vibrio bacteria isolated from aquaculture systems show considerable AMR patterns (Vaiyapuri et al., 2021). Furthermore, as 70% of the EU demand of aquaculture products is met through import (EUMOFA, 2023), use of antimicrobials in aquaculture in countries exporting seafood to the EU pose a threat for the European consumer (Brisabois et al., 2019). Antimicrobial compounds in aquaculture systems worldwide were used in 11 of the 15 major producing countries between 2008 and 2018, with in total 67 compounds used, and oxytetracycline, sulfadiazine and florfenicol as the most frequently used (Lulijwa et al., 2020). In a meta‐analysis on antibiotic‐resistant bacteria from aquatic food animals of Asia, among the five most detected seafood bacterial species (one of which was Vibrio spp.), resistance was highest to penicillins (60.4%), macrolides (34.2%), sulfonamides (32.9%) and tetracyclines (21.5%). Furthermore, resistance to critically important antimicrobials for human medicine (CIAs 46 ) – now referred to as medically important antimicrobials (MIAs 47 ) (WHO, 2024) – was found in these five species (Schar et al., 2021). Resistance to some compounds in the reserve group of last‐resort antimicrobials for human medicine was also observed and varied by pathogen, e.g. in case of Vibrio spp. polymyxin B resistance was 39.1% (95% CI: 33–44) and colistin resistance was 42.7% (95% CI: 38–47) (Schar et al., 2021). 48
3.5.1. Reported antimicrobial resistances in V. parahaemolyticus, V. vulnificus and non‐O1/non‐O139 V. cholerae isolated from seafood and from seafood‐borne infections in Europe
The EU monitoring of AMR in zoonotic and commensal bacteria from food‐producing animals and food thereof does not include Vibrio spp. (EFSA and ECDC, 2022). EFSA (2019) proposed to perform baseline surveys (BLSs) on AMR in bacteria from aquaculture and/or (imported) seafood, targeting selected bacteria species depending on the matrix and objective, among which Vibrio spp. were listed. As a follow‐up, EFSA proposed and provided technical and scientific support for the development of a BLS on the occurrence of AMR in bacteria isolated from EU/EFTA aquaculture productions that will target for marine aquaculture production: Aeromonas spp. from salmon and seabass; for freshwater aquaculture production: Aeromonas spp. from trout, and for shellfish production, E. coli, ESBL‐producing E. coli, enterococci (E. faecium and E. faecalis) and V. parahaemolyticus and V. alginolyticus from mussels (EFSA, in press).
Annex E provides an overview of recent European studies on the AMR of the relevant Vibrio spp. Nearly all studies, both for clinical and food isolates, used the clinical breakpoints of the Clinical and Laboratory Standards Institute (CLSI). The European Committee on Antimicrobial Susceptibility Testing (EUCAST) has recently developed clinical breakpoints for certain antimicrobials for five Vibrio species, including V. alginolyticus, V. cholerae, V. parahaemolyticus and V. vulnificus (Karatuna et al., 2024). Epidemiological cut‐off (ECOFF), which are used for epidemiological surveillance and monitoring resistance trends in bacterial populations are available, from EUCAST for certain antimicrobials for V. cholerae, 49 whereas they are under development for other Vibrio spp.
The AMR phenotype of Vibrio clinical isolates was investigated by the National Reference Laboratory (NRL) for vibrios in France. In total 72% (N = 72) of the non‐O1/non‐O139 V. cholerae isolates were from gastrointestinal infections, of whom several were travel associated. No V. vulnificus isolates from food‐borne infections were reported, while the overwhelming majority of V. parahaemolyticus isolates (96%; N = 106) stemmed from gastrointestinal infections. In clinical isolates of V. parahaemolyticus and V. cholerae a high prevalence of resistance against polymyxin B (73% in V. parahaemolyticus, 89% in V. cholerae) and colistin (71% in V. parahaemolyticus and 91% in V. cholerae) was observed. Many clinical V. parahaemolyticus isolates also showed resistance to ampicillin (42%) and sulfonamides (31%). Clinical non‐O1/non‐O139 V. cholerae were also resistant to sulfonamides (39%), and sulfamethoxazole/trimethoprim (15%). An Italian study reporting phenotypic data on AMR of nine clinical V. cholerae isolates, showed that all isolates were resistant to colistin and 88% showed intermediate resistance to erythromycin. Resistance to nalidixic acid (22%), some aminoglycosides and β‐lactams was also found (e.g. 55% of strains were resistant/intermediate to ampicillin) (Ottaviani et al., 2018).
Most AMR data on Vibrio isolates from seafood in the EU are on V. parahaemolyticus . In seven studies, the prevalence of ampicillin resistance of V. parahaemolyticus was between 70% and 100%, and resistance against streptomycin was > 30% (up to 68%) in five studies. Colistin resistance of V. parahaemolyticus was only examined in two studies. In one study (Ottaviani et al., 2013), resistance was detected in > 90% of all isolates, whereas in the other (Castello et al., 2022) ~25% of the isolates showed increased tolerance to this compound. A genotypic study of V. parahaemolyticus isolates from retail prawns revealed the presence of resistance genes against aminoglycosides, β‐lactams, folate pathway inhibitors, phenicols, quinolones, sulfonamides, tetracyclines and rifamycins (Janecko et al., 2021), but it remains unclear if the presence of these genes resulted in a reduced susceptibility of the isolates, as other studies reported that AMR phenotype and genotype often do not match (Lepuschitz et al., 2019; Lou et al., 2016; Zhang et al., 2023).
A meta‐analysis comprising worldwide studies assessed the prevalence of AMR phenotypes of V. parahaemolyticus isolates from bivalves (Albini et al., 2022). Whereas for Asia and Africa enough studies were available to estimate the prevalence, for Europe such analysis was not possible. Hence, the genomes of 152 V. parahaemolyticus strains isolated in Europe from clinical, environmental and other sources were used for screening of AMR‐related genes (available in https://doi.org/10.5281/zenodo.12514500). Besides genes present in all European V. parahaemolyticus strains (tet35 and txr, genes encoding proteins of efflux pumps related to tetracycline resistance, parE, the gene encoding a penicillin‐binding‐protein related to PBP3 of Haemophylus influenzae), numerous β‐lactamase encoding genes with varying frequencies were found (most frequently bla CARB‐18 in 55% of all strains, bla CARB‐22 in 18%, bla CARB‐21 in 9%). Resistance genes against other antibiotic classes were present at lower frequencies, e.g. fosX (3%), sul2 (2%), dfrA31 (3%).
AMR data on V. vulnificus seafood isolates were available from only two Italian studies, one showing low levels of resistance to cefazolin (Serratore et al., 2017), while in the other the five isolates were streptomycin resistant (Castello et al., 2022).
Few data on AMR of food‐borne strains of non‐O1/non‐O139 V. cholerae isolated in Europe were reported. The AMR phenotype pattern was similar to that of clinical strains investigated in the study by (Ottaviani et al., 2018). All strains were colistin resistant and showed reduced susceptibility to various aminoglycosides and ß‐lactams. Remarkably many Italian isolates from seafood at retail (92%) displayed an intermediate resistance to the macrolide erythromycin. In another Italian study (Castello et al., 2022), resistance against ß‐lactams and the combination trimethoprim/sulfamethoxazole (42%) was observed. Five non‐O1/non‐O139 V. cholerae isolates from prawns sold in the UK contained resistance genes against aminoglycosides, β‐lactams, phenicols, quinolones and tetracyclines (Janecko et al., 2021). Resistance/intermediate resistance to colistin (87%), imipenem (78%) and ampicillin (11%) was most common in isolates from retail seafood in Germany (Zhang et al., 2022). AMR genes identified through WGS could explain some of the observed in vitro phenotypes, e.g. bla CARB presence and ampicillin resistance, almG presence and colistin resistance, qnrVCs presence and quinolone (ciprofloxacin and nalidixic acid) resistance, varG imipenem resistance and dfrA31 presence and trimethoprim resistance. The presence of genes encoding efflux pumps that may be able to exclude different antibiotics from the bacterial cell were identified in all 63 non‐O1/non‐O139 V. cholerae isolates, however their putative role in conferring resistance must be proven experimentally.
3.5.2. Antimicrobial resistance of concern due to their possible horizontal transmission to other bacteria or for their emerging in the EU
Table 7 shows the genetic localisation of AMR genes observed in the relevant Vibrio spp. isolated from seafood. The emergence of multidrug‐resistant bacteria that possess resistance to more than two different classes of antibiotics is mainly caused by HGT through mobile genetic elements (Das et al., 2020; Dutta et al., 2021). Dissemination of AMR genes (ARGs) through mobile genetic elements (MGEs) like plasmids, ICEs, integrons, transposable elements and insertion sequences has been described for vibrios. Many MGEs that have been found first in the toxigenic V. cholerae strains are also present in environmental and food‐borne non‐O1/non‐O139 V. cholerae strains (Das et al., 2020) and in other Vibrio species. Conjugative Vibrio plasmids were the first identified MGE responsible for the horizontal transfer of ARGs. Plasmids of environmental V. cholerae were shown to be transferable to other Gram‐negative bacteria (intergeneric transfer) or, with higher frequencies, to other strains of the same species (intraspecific transfer) (Amaro et al., 1988). Vibrios also contain integrons which are genetic elements that can capture ARGs and confer AMR to the recipient bacteria. Class 1 integrons that are present in many vibrios are a major player in the dissemination of ARGs across Gram‐negative bacteria (Rapa & Labbate, 2013). In the case of V. cholerae, a large integron termed superintegron is located on the second chromosome. The superintegron carries ARGs but also additional other genes and turned out to be widespread in the genus Vibrio including V. vulnificus and V. parahaemolyticus (Escudero & Mazel, 2017; Hazen et al., 2010). ARGs of Vibrio spp. can be also located in transposons or just adjacent to insertion sequences. These MGEs are able to move themselves (and associated resistance genes) to new locations in the same or in different DNA molecules within a single cell (Partridge et al., 2018). The ICEs are large genomic islands and are self‐transmissible by conjugation. ICEs can excise as a circular form and integrate into an attachment site of another host chromosome (Partridge et al., 2018). The first ICE was discovered in V. cholerae and was termed SXT as it was shown to carry ARGs conferring resistance to sulfamethoxazole, trimethoprim and streptomycin (Waldor et al., 1996). The occurrence of this element and related ICEs (now termed SXT/R391 ICE family) is widespread in Vibrio spp. and elements of this family are found also in other Gram‐negative bacteria (Bioteau et al., 2018; Partridge et al., 2018). In a German study on 63 seafood isolates of non‐O1/non‐O139 V. cholerae, the occurrence of class 1 integrons was observed in 60% of the investigated strains (Zhang et al., 2023). Conserved sequences of the SXT/R391 elements were found in 22% of the strains. These results disclose the potential to exchange ARGs and the frequent presence of these genomic features enhances the genetic plasticity of the species.
TABLE 7.
Reported genetic localisation of AMR genes in Vibrio isolates from seafood.
| Resistance to antimicrobial agents | AMR genes | Genetic localisation | Evidence for HGT | Vibrio species | Food source | Reference |
|---|---|---|---|---|---|---|
| Polymyxins: polymyxin B/colistin | Intrinsic almG | Chromosomal | No | Vv; Vc non‐O1/non‐O139 | Seafood (most isolates) | Henderson et al. (2017), Massad and Oliver (1987) |
| β‐Lactams | Intrinsic bla CARB‐47/48 | Chromosomal | No | Vp | Seafood (all isolates tested, 83 total) | Janecko et al. (2021) |
| Quinolones: nalidixic acid, ciprofloxacin | qnrVC6 | Flanked by IS‐elements, conjugative plasmid | Yes | Vp | Shrimps (China, retail Hong Kong) | Liu et al. (2013) |
| Aminoglycosides | aacA3, aadA1 | Integron on conjugative plasmid | Yes | Vp | Shrimps (China, retail Hong Kong) | Liu et al. (2013) |
| Chloramphenicol | catB2 | |||||
| Trimethoprim | dfrA1 | |||||
| β‐Lactams: carbapenems | bla NDM‐1 | Conjugative plasmid | Yes | Vp | Shrimps (France, imported shrimps from Vietnam) | Briet et al. (2018), Ramírez‐Castillo et al. (2023) |
| Folate pathway inhibitors | ||||||
| Trimethoprim | dfrA16 | |||||
| Sulfonamides | sul1, sul2 | |||||
| Phenicols | floR | |||||
| Aminoglycosides | strA/strB, aad2 | |||||
| Tetracyclines | tet(A) | |||||
| Ciprofloxacin | gyrA, parC alleles | Chromosomal | No | Vp | Shrimps a (China) | Xu, Zheng, et al. (2023) |
| qnrVC variants | Plasmid | Yes | ||||
| Streptomycin | strA/strB | Integrative conjugative element SXT/R391 | Yes | Vp | Shrimps (China) | Bioteau et al. (2018) |
| Tetracycline | tet(A) | |||||
| Folate pathway inhibitors | dfrG, sul2 | |||||
| Polymyxins: Colistin | mcr‐1 | Conjugative plasmid | Yes | Vp | Shrimp, China | Lei et al. (2019) |
| Tetracyclines | tet(B) | Plasmid | Yes | Vp | Shrimp, Mexico | Han et al. (2015) |
| β‐Lactams/β‐Lactamase inhibitors: ampicillin, amoxicillin, clavulanic acid, cefazolin, cefoxitin, meropenem | bla VCC‐1 carbapenemase | Flanked by IS elements | Likely | Vc non‐O1/non‐O139 | Shrimps (Canada, imported from India) | Mangat et al. (2016) |
| Meropenem wild‐type phenotype b | bla NDM‐1 | Conjugative plasmid | Yes | Vp | Shrimps (Germany, imported from Vietnam) | Personal information Hammerl (2024) |
Abbreviations: AMR, antimicrobial resistance; HGT, horizontal gene transfer; Vc, V. cholerae; Vp, V. parahaemolyticus; Vv, V. vulnificus.
Meat products were also sampled, but shrimps represented the vast majority of samples.
Minimum inhibitory concentration (MIC) value for meropenem below the CLSI breakpoint (4 μg/mL), but slightly increased MIC value in comparison to other wild‐type V. parahaemolyticus isolates (0.12 μg/mL).
Monitoring of AMR in vibrios from aquaculture production should aim to analyse ARGs conferring resistance to antimicrobials that are currently used or have been used in the past. As mentioned earlier, in 11 of the major producing countries about 73% of the applied antimicrobials were oxytetracycline, sulfadiazine and florfenicol, and 55% of the countries applied sulfadimethoxine, erythromycin, amoxicillin and enrofloxacin (Lulijwa et al., 2020). Thus, monitoring of AMR should include testing resistance against tetracyclines, sulfonamides, phenicols, macrolides, quinolones and β‐lactams. Another source contributing to the prevalence of AMR in vibrios is the presence of antimicrobial compounds and ARGs in estuarine and coastal waters which originate from riverine runoff, sewage discharge and wastewater treatments plants (Zheng et al., 2021). The spread of ARGs through HGT is of special concern for compounds that are classified as CIA by the WHO. Carbapenem antibiotics are assigned to this group as they are used for treating multidrug‐resistant Enterobacteriaceae for which only limited therapy options are available. Also, they are used for treatment of bacteria transmitted from non‐human sources (Goh et al., 2022). Carbapenems constitutes the class of β‐Lactam antibiotics with the broadest spectrum against Gram‐negative and Gram‐positive bacteria. Isolates of non‐O1/non‐O139 V. cholerae that possessed carbapenemase activity have been detected in seafood from the Adriatic sea (Ottaviani et al., 2018) and isolates of V. parahaemolyticus and non‐O1/non‐O139 V. cholerae that possessed carbapenemase activity have been detected in imported seafood (Briet et al., 2018; Hammerl, 2024; Mangat et al., 2016; Zhang et al., 2023). In the latter cases all carbapenemase genes were associated with mobile genetic elements (plasmids and IS elements), so they were likely acquired by HGT. Other carbapenemase genes were located in a class 1 integron embedded on a plasmid (Aberkane et al., 2015). Among the different mobile genetic elements, plasmids have been identified as the most common way for propagation of carbapenemase genes (Goh et al., 2022). Research has shown that carbapenemase genes are widely distributed in the aquatic environment (e.g. VCC‐1 encoding gene was identified in non‐O1/non‐O139 V. cholerae strains of German coastal waters, Hammerl et al., 2017) and in bacteria from aquatic food animals and are therefore likely to be transferred into the food chain. To date, 10 types of different carbapemases have been found in Vibrio species, with NDM‐1 as the most prevalent type (Goh et al., 2022). Some of the identified carbapenemases, e.g. VCC‐1 (Mangat et al., 2016), are novel types, so far only found in Vibrio species (Goh et al., 2022).
Besides carbapenemases genes also extended‐spectrum‐ß‐lactamases (ESBL)‐producing Vibrio spp. in seafood have been reported. ESBL are a major cause of resistance to 3rd and 4th generation of cephalosporins that belong to the class of CIAs (WHO). In a meta‐analysis of studies on ESBL‐producing Gram‐negative bacteria from different kinds of seafood (Pearce et al., 2023) it was found that Vibrio spp. constituted the second most common group of ESBL producers after Enterobacterales in this type of food.
3.5.3. Concluding remarks
Few European studies on AMR of V. parahaemolyticus, V. vulnificus or non‐O1/non‐O139 V. cholerae isolated from seafood or from food‐borne infections have been conducted. Despite this, a large spectrum of AMR is reported, some of which known to be intrinsic for some species (e.g. colistin, ampicillin).
As regards V. parahaemolyticus, the prevalence of ampicillin resistance/intermediate resistance was between 70% and 100% in seven of nine studies, and resistance/intermediate resistance against streptomycin was higher than 30% (up to 70%) in six of eight studies.
Non‐O1/non‐O139 V. cholerae strains showed colistin resistance (87% to 100%, including intermediate resistance) in four studies. Ampicillin resistance varied between 4% and 75% (five studies) and streptomycin resistance between 11% and 68% in four studies (clinical resistance and intermediate resistance).
The low number of studies and investigated strains does not allow any conclusions to be drawn on AMR of V. vulnificus.
Vibrio bacteria possess several MGEs that contribute to the horizontal transfer of ARGs. Mobile genetic elements of vibrios involved in the dissemination of ARGs are plasmids, integrons, ICEs, transposons and IS‐elements. Almost for all antibiotic classes ARGs that are associated with mobile genetic elements have been detected in Vibrio spp.
ARGs conferring resistance to antimicrobials used in aquaculture systems (e.g. tetracyclines, sulfonamides, phenicols) are of great concern, as they spread quickly within the microbiota of this environment and can enter the food chain. The dissemination of ARGs can be also promoted by the anthropic impact in estuarine and coastal waters.
Resistance against carbapenems and 3rd/4th generation cephalosporins (MIAs categorised as authorised only for humans and highly important antimicrobials, respectively) associated with carbapenemases and ESBLs encoding genes in mobile elements are increasingly found in V. parahaemolyticus, V. vulnificus and non‐O1/non‐O139 V. cholerae and their detection in imported seafood has been reported.
3.6. Persistence mechanisms of the relevant Vibrio spp. in different environments (SQ1.8)
Vibrio species are ubiquitous and widely distributed in aquatic environments worldwide. Their capability to survive is due to multiple strategies that allow the bacteria to deal with stressors as nutrient deprivation, changes in salinity and temperature and to resist predation by heterotrophic protists and bacteriophages. The mechanisms allowing a long‐term survival are summarised below.
3.6.1. Viable but non‐culturable state
The bacteria must survive periods of harsh conditions, such as nutrient limitation or temperature extremes. To survive, the bacteria enter a specialised dormancy state, VBNC (Brumfield et al., 2021; Lutz et al., 2013). Changes in temperature, salinity, pH, oxygen concentration and starvation are all conditions that can induce the VBNC state (Akolkar & Matson, 2023). VBNC state cells have been shown to tolerate typically fatal stressors, including high‐dose antibiotics and are able to resuscitate in vivo, e.g. VBNC V. cholerae O1 (attenuated vaccine strain CVD 101) was shown to convert to a culturable state during passage through human participants (Colwell et al., 1996) and V. cholerae non‐O1/non‐O139 and V. parahaemolyticus VBNC cells converted into the culturable form when co‐cultured with eukaryotic cells such as HT‐29 or Caco‐2 cells (Senoh et al., 2012) indicating the potential for in vivo resuscitation.
3.6.2. Biofilm formation
Bacterial attachment to environmental surfaces and aquatic organisms and the formation of multicellular structures (biofilms) have been shown to be critical for the survival of the genus Vibrio. Biofilms provide a protective environment and stability in a changing environment and seem to provide a substantial survival advantage to aquatic organisms. For instance, extraction of nutrients (e.g. chitin) can be facilitated by attachment to some biotic surfaces. Biofilm formation is a multistep process that begins with surface scanning and initial attachment, followed by the production of extracellular matrix components and the formation of microcolonies, and finally the development of highly organised, three‐dimensional structures (Conner et al., 2016). The ability of vibrios to form biofilms depends upon specific structural genes (flagella, pili and exopolysaccharide biosynthesis) and regulatory processes (two‐component regulators, QS and c‐di‐GMP signalling). The expression of different pili, for example, is related with the range of different surfaces colonised (Aagesen & Häse, 2012; Lutz et al., 2013). Besides, while vibrios share common regulatory proteins and signalling systems, there are several differences that establish the biofilm as something unique for each species, conditioned by the lifestyle to each Vibrio spp., e.g. differences in niche occupation, in the environmental parameters they respond to and even pathogenesis (Yildiz & Visick, 2009).
Of relevance, the human action in the marine environments is increasing the number of different particles that serve as surfaces for biofilm formation. It has been proposed that marine microplastics, that have been proposed as potential reservoirs and vectors for the transport of pathogenic Vibrio spp. (Noorian et al., 2023).
3.6.3. Persister cells
Persister cells are described as slow‐ or non‐growing subpopulations present within a growing culture, which are able to withstand multiple types of stressors, including exposure to antibiotics. In the case of antibiotic resistance, the tolerance of persister cells is non‐genetic, not inheritable and results from a phenotypic switch from the normal, sensitive cell type to the tolerant, persister state (Ayrapetyan et al., 2015). While the relation between persister cells and VBNC state is still under debate (Liu et al., 2023), persister cells are described as a dormancy state in which cultivability is retained and growth on routine media is restored after the inducing stressors are removed. Although most studies of persister cells focus on antibiotic resistance, the persister state can also be induced by stressful environments such as starvation, oxidative stress, DNA damage, stressful pH and antibiotics (Ayrapetyan et al., 2015). At present, persister cells have been identified in almost every bacterial species examined including V. cholerae and V. vulnificus (Ayrapetyan et al., 2015; Van den Bergh et al., 2017).
3.6.4. Anti‐grazing strategies
Vibrio species interact with numerous organisms in their habitats, including heterotrophic protozoa that feed on them. Once in the interior of the protozoa, bacteria cells are processed in the phagosomes where they encounter a harsh environment that includes low pH, antimicrobial peptides, reactive oxygen/nitrogen species, proteolytic enzymes and low iron concentrations (Akolkar & Matson, 2023). To avoid protozoan predation, Vibrio spp. display various anti‐grazing strategies, some of them include the formation of biofilms, production of proteases such as PrtV, secretion of ammonium and pyomelanin, expression of the type VI secretion system (T6SS) of V. cholerae, that kill the model amoeba Dictyostelium discoideum, and the MARTX type III of V. vulnificus, involved in the lysis of a wide range of eukaryotic cells, including amoebae (Espinoza‐Vergara et al., 2020). In V. parahaemolyticus the type III secretion system 2 (T3SS2) mediate cytotoxicity and one of its effectors (VopC) allows the pathogen to escape from the vacuole (de Souza Santos & Orth, 2014). It has been suggested that the resistance of Vibrio spp. to the factors encountered inside of the phagosomes/food vacuoles in heterotrophic protozoa might serve as a pre‐adaptation niche before entering a host, as the passage of bacteria within protozoa might activate specific factors used to resist the strategies that also contribute to the inactivation of bacteria within mammalian hosts (Espinoza‐Vergara et al., 2020).
3.6.5. Association with other aquatic organisms
Vibrios interact with many higher organisms and here we describe organisms that act as reservoirs. Different studies have demonstrated the positive chemotactic responses towards some components of the mucin (GlcNAc and Neu5Ac) present in algae and in the fish skin mucus (Reddi et al., 2018; Valiente et al., 2008). Besides, GlcNAc is also present in the chitin found in the exoskeleton of crustaceans, insects, eggs of arthropods, nematodes and in some diatom algae (Markov et al., 2015). The attraction and adherence to these components allows Vibrio association to zooplankton (primarily copepods), crustaceans, bivalves, fish, aquatic plants, phytoplankton, chironomids (Brumfield et al., 2021), all organisms that can act as potential reservoirs. Chironomid (non‐biting midges) eggs are a natural reservoir for V. cholerae, and non‐O1/non‐O139 V. cholerae have been isolated from chironomid egg masses in several countries, highlighting their potential as an environmental reservoir (Noorian et al., 2023).
3.6.6. Concluding remarks
- Vibrio species are ubiquitous and widely distributed in aquatic environments worldwide. The capability to survive and persist in such environmental niches is due to multiple strategies:
-
○Changes in temperature, salinity, pH, oxygen concentration and starvation can induce the VBNC state in vibrios in which the bacteria are metabolically dormant;
-
○Vibrio can form biofilms on several biotic and abiotic surfaces. In these communities, the bacteria survive several stressors;
-
○A portion of the Vibrio population exist in a slow‐ or non‐growing state (persister cells) that allows the bacterial survive several harsh conditions;
-
○Vibrio spp. display various anti‐grazing strategies to avoid protozoan predation; and
-
○The preferred adhesion of Vibrio to chitin and mucin allows association to other aquatic organisms that can act as reservoirs, such as zooplankton (primarily copepods), crustaceans, bivalves, fishes, aquatic plants, phytoplankton, chironomids.
-
○
3.7. Factors in the aquatic environments and in food that influence occurrence and growth of the relevant Vibrio spp., and affect transmission of their virulence and resistance determinants (AQ2)
3.7.1. Factors in the aquatic environments and in food that influence occurrence and growth of the relevant Vibrio spp. (SQ2.1)
Vibrio species are autochthonous of the aquatic environments (primary marine and brackish waters) and may therefore occur in seafood deriving from such waters. In the aquatic environments as well as in seafood, Vibrio spp. are subjected to a multiplicity of factors that, individually and in combinations, may affect their ability to survive and grow. These factors are schematically presented in Figure 3 and may be divided into:
Extrinsic factors, associated with the external conditions surrounding the microorganism in the aquatic environment or the conditions surrounding the food where Vibrio is present (e.g. temperature, (sea)water salinity, solar and UV radiation);
Intrinsic factors, associated with the inherent properties of the seawater or the food hosting the microorganism and that, for foods, can be natural or related to food processing operations (e.g. pH, water activity of food, nutrient content);
Implicit factors, depending on the interaction of the microorganism with the surrounding microbiota (e.g. microorganisms' interaction mechanisms such as predation, parasitism and commensalism phenomena, production of substances that are inhibitory/lethal to other organisms (antibiotics, bacteriocins, etc.) or that may support their survival or stimulate their growth).
FIGURE 3.
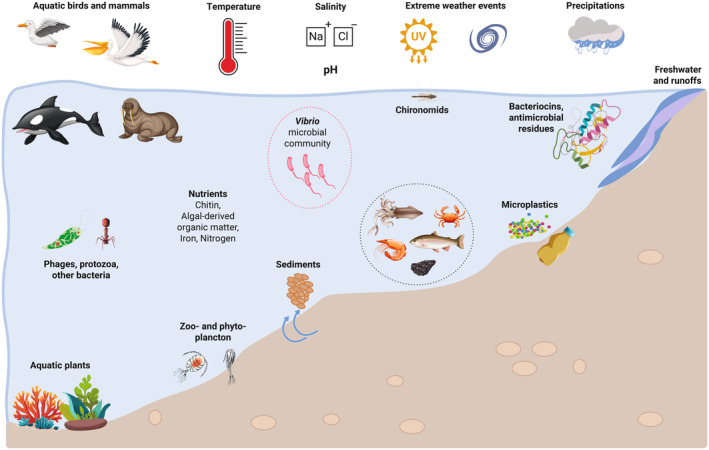
Factors that affect occurrence of Vibrio spp. of relevance in the aquatic environment (adapted from Brumfield et al., 2021).
A meta‐analysis by Takemura et al. (2014) on the dynamics of Vibrionaceae in the environment pointed out that individual Vibrio species are susceptible to different degrees to environmental factors other than temperature. Given the potential short generation times of vibrios (≤ 20 min), rapid fluctuations of Vibrio populations in reaction to sudden and geographically restricted environmental variations should be considered with attention.
For example, factors relevant in one area, e.g. salinity and chlorophyll a of the Gulf Coast, were not found significant in Chesapeake bay on the Atlantic Ocean, where temperature, turbidity and dissolved oxygen were more predictive (Parveen et al., 2008; Zimmerman et al., 2007). Similar conclusions were drawn by Johnson (2015) based on multiple regions (Atlantic Ocean, Pacific Ocean, Gulf Coast). This reflects the complexity of interactions among environmental factors affecting the occurrence of Vibrio species, and the need for region‐specific environmental models and validations.
3.7.1.1. Temperature in the aquatic environment
Water temperature is a major driver for the occurrence and growth of Vibrio species, including those of PH relevance. Vibrios are mesophilic microorganisms and tend to be detected more frequently during warmer seasons in temperate areas, whereas their seasonality is less pronounced in tropical and subtropical waters (Urakawa & Rivera, 2006). In laboratory conditions, Vibrio species grow well > 17°C and up to an optimum at 30–40°C. Vibrio species are sensitive to low temperatures though, and around and below 10°C tend to shift to the VBNC status (see Section 3.6.1). The effect of water temperature on the occurrence of V. parahaemolyticus, V. vulnificus and V. cholerae has been addressed by several studies as reviewed by Johnson (2015) and Sheikh et al. (2022).
V. parahaemolyticus counts in seawater, bivalves (mainly oysters) or sediments have been reported to correlate with temperature in several studies in the USA (Blackwell & Oliver, 2008; DePaola et al., 1990, 2003; Duan & Su, 2005; Johnson et al., 2012; Parveen et al., 2008), in Asian countries (Fukushima & Seki, 2004; Ndraha & Hsiao, 2021; Rehnstam‐Holm et al., 2014; Xu et al., 2020) and in Brazil (Sobrinho et al., 2010). Data from Northern American studies have been largely used to develop the quantitative models applied in the V. parahaemolyticus FAO/WHO risk assessments (FAO and WHO, 2011). In Europe, a French study in sites on the Atlantic coast showed that V. parahaemolyticus presence in seawater correlated with the mean temperature of the precedent week, while in mussels there was also a correlation with chlorophyll a. Also, the occurrence of trh+ V. parahaemolyticus correlated with the mean temperature 2 days before sampling (Deter et al., 2010). In Germany, water temperature was the most important determinant for Vibrio abundance in the central Wadden Sea, although V. parahaemolyticus appeared less susceptible to lower temperatures in the sediments than other species (Böer et al., 2013). The role of temperature on V. parahaemolyticus occurrence and its combined effect with salinity was further confirmed for German North and Baltic Sea coasts (Fleischmann et al., 2022). In Italy, a statistically significant association of V. parahaemolyticus prevalence with the warmer seasons (April–October, average T > 16.45°C) was found in a study on clams harvested in the estuary of the Po River (Northern Adriatic Sea). Interestingly, tdh+ and/or trh+ V. parahaemolyticus did not display significant correlations with any of the environmental parameters (Serratore et al., 2016). A second study performed in Italy, in Sardinian and Northern Adriatic Sea shellfish production areas, showed a correlation between V. parahaemolyticus detection and water temperature, as well as correlation (r = 0.41) between water temperature and V. parahaemolyticus counts (Suffredini et al., 2014). Finally, in studies performed in Galicia (Spain), the occurrence of V. parahaemolyticus in rias 50 was positively and significantly correlated with seawater temperature, but salinity was the dominant factor affecting its probability of detection, while temperature had an effect only in modulating the abundance in periods and areas of lower salinity (Martinez‐Urtaza, Lozano‐Leon, et al., 2008). On the contrary, in offshore areas, where seawater temperature followed a non‐seasonal pattern, the occurrence of V. parahaemolyticus was mostly affected by zooplankton (Martinez‐Urtaza et al., 2012). It should be noted that, while higher water temperatures are considered reliable predictors for greater V. parahaemolyticus abundance, it is not certain that pathogenic strains follow the same pattern, as there are indications that isolation rate of tdh+ and/or trh+ strains is higher in colder months, when the total V. parahaemolyticus counts are lower (DePaola et al., 2003; Williams et al., 2017).
V. vulnificus is a natural inhabitant of waters with a temperature between 10 and 33°C (optimum around 25–28°C). The lower temperature limit for the isolation of viable V. vulnificus from the environment is in the range 12–17°C depending on the studies and specific conditions of the areas (Froelich & Daines, 2020), while at temperatures < 10°C a shift towards the VBNC state (see Section 3.6.1) is reported (Oliver, 2015). Several V. vulnificus studies were undertaken, especially in the USA (Motes et al., 1998; Tamplin, 1994), to address the effect of seawater temperature on occurrence and concentrations. The cited studies (Motes et al., 1998; Tamplin, 1994) were used in the FAO/WHO risk assessment to develop the polynomial regression model describing temperature effects on levels of V. vulnificus in oysters (FAO and WHO, 2005b). Overall, while the importance of temperature on V. vulnificus occurrence and abundance is consistent among studies (Johnson, 2015), the combined effect of temperature and salinity is markedly relevant for this species (see Section 3.7.1.4). Among the few ecological studies performed in Europe dealing with the effect of temperature on V. vulnificus detection (Böer et al., 2013), water temperature was the relevant variable for V. vulnificus in bathing areas in Germany and small temperature variations 51 significantly affected the probability of its detection (Cantet et al., 2013; Fleischmann et al., 2022). Two studies, performed in the Northern Adriatic Sea and in Mediterranean lagoons of the French coast, also showed that V. vulnificus detection was strongly associated with the warmest months of the year (Takemura et al., 2014).
V. cholerae is part of the autochthonous microbiota of temperate aquatic environments and it is often detected in brackish water in the temperature range 15–30°C, with observed greatest abundances around 20°C (Blackwell & Oliver, 2008; DePaola et al., 1983; Singleton et al., 1982). Temperature‐response of V. cholerae has been extensively studied in cholera‐endemic regions of the world, and cholera cases have been positively correlated to temperature globally (see Johnson, 2015). Several studies correlating V. cholerae concentrations (regardless of serogroups) to seawater temperatures have been developed also in other countries, including the USA (Chávez et al., 2005), Japan (Wang & Gu, 2005) and Mexico (Louis et al., 2003). The comprehension of the relation between temperature and V. cholerae, however, is hindered by the complexity of the interactions of this microorganism with other aquatic organisms (see Section 3.6.5), affecting its occurrence and levels (Fleischmann et al., 2022). In Europe, the detection rates of non‐O1/non‐O139 V. cholerae in the aquatic environments display a marked seasonality, with detection only between May and September and concentrations (real‐time PCR) up to 1.8 log10 gene copies/100 mL and 5.0 log10 gene copies/100 mL in samples from the North and the Baltic Sea (Germany) (Cantet et al., 2013) A similar pattern was present in Mediterranean French lagoons, where V. cholerae was detected only during the warm season, with concentrations between 1 and 2 log10 MPN/L of water (Kirschner et al., 2008). Interestingly, data from Austria support the correlation between water temperature and non‐O1/non‐O139 V. cholerae also for inland water bodies. A study from Kirschner et al. (2008) on lake Neusiedler See, a large Austrian alkaline lake, reports a highly significant correlation (ρ = 0.65; p < 0.001) between the detection rate of V. cholerae and temperature, as well as with zooplankton biomass and conductivity. Significantly, detection rate increased from non‐detection in colder months, to 100% in summer (average T = 21.7°C). Thus, the effect of temperature on non‐O1/non‐O139 V. cholerae is evident in studies covering seasonal trends.
3.7.1.2. Temperature in food
According to ICMSF (1996) (Table 8), the optimum growth temperature for the three major Vibrio species is 37°C with growth temperature ranges from 10 to 43°C for V. cholerae, 5–43°C for V. parahaemolyticus and 8–43°C for V. vulnificus. It should be noted, however, that data related to V. cholerae stem from testing of O1 (classic and El Tor) strains and may not fully represent the growth values for non‐O1/non‐O139 isolates.
TABLE 8.
Information on the limits for growth of V. parahaemolyticus, V. vulnificus and V. cholerae (ICMSF, 1996).
| Vibrio species | Temperature (°C) | pH | NaCl (%) | a w | Atmosphere | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Optimum | Range | Optimum | Range | Optimum | Range | Optimum | Range | Optimum | Range | |
| Vibrio cholerae a | 37 | 10–43 | 7.6 | 5.0–9.6 | 0.5 | 0.1–4 | 0.984 | 0.970–0.998 | Aerobic | Aerobic/anaerobic |
| Vibrio parahaemolyticus | 37 | 5–43 | 7.8–8.6 | 4.8–11 | 3.0 | 0.5–10 | 0.981 | 0.940–0.996 | Aerobic | Aerobic/anaerobic |
| Vibrio vulnificus | 37 | 8–43 | 7.8 | 5–10 | 2.5 | 0.5–5.0 | 0.98 | 0.96–0.997 | Aerobic | Aerobic/anaerobic |
These data are referred to V. cholerae O1 strains.
Several studies provide predictive models on the effect of temperature on growth and inactivation of Vibrio in seafood (summarised in Annex F).
Growth/inactivation rates at temperatures ranging from freezing to the maximum growth temperature have been reported for V. parahaemolyticus in different oyster species (C. gigas, C. virginica and Saccostrea glomerata), in some crustaceans such as shrimps, prawns and marinated crabs, and in some finfish species such as salmon, flounder and tilapia. Some of these studies also addressed differences in the growth rates of specific pathogenic V. parahaemolyticus strains (tdh+ and/or trh+, ST36) compared to non‐pathogenic ones. In addition, secondary growth models – mostly based on the square root model – have been developed for oysters by Fernandez‐Piquer et al. (2011), Parveen et al. (2013), Yoon et al. (2008) and Fletcher et al. (2024), for shrimps/prawn by Tang et al. (2015), Chen et al. (2019), Boonyawantang et al. (2012), Wu et al. (2023) and Ma et al. (2023), and for fish [salmon (Oncorhynchus spp.) by Yang et al., 2009; Kim et al., 2012; flounder (Paralichthys spp.) by Kim et al., 2012]. A few secondary models (Arrhenius, or other models), dealing with the survival/inactivation of V. parahaemolyticus in the temperature range between 0°C and the minimum growth temperature, are also available. Compared to V. parahaemolyticus, models on the effect of temperature on V. vulnificus concentration in seafood are limited, covering exclusively oysters (C. gigas, C. virginica), with secondary growth models provided by Kim et al. (2012) and DaSilva et al. (2012). No specific data on growth/inactivation rates of non‐O1/non‐O139 V. cholerae were retrieved, though some studies reported log increase/reduction of non‐O1/non‐O139 V. cholerae strains in prawns/shrimps (Wong et al., 1995).
3.7.1.3. Salinity
Salinity is considered the second most relevant environmental parameter to influence Vibrio abundance. V. parahaemolyticus, V. vulnificus and V. cholerae display significant differences in their halotolerance and salinity requirements. Consequently, salinity shifts in specific areas (e.g. gulfs, estuaries, rias) may affect the proportions of the different species and sustain temporary blooms of the species with the highest fitness for the established conditions.
V. parahaemolyticus, has strict salinity requirements, being unable to grow below 5 ppt NaCl while thriving in water environments at 15–25 ppt NaCl (DePaola et al., 2003). This species displays a noticeable halotolerance and adaptation to osmotic stress (Kalburge et al., 2014), e.g. in experimental settings simulating water systems subjected to salinity increase due to evaporation. Possibly because of the large salinity range supporting its growth, V. parahaemolyticus seems less affected by salinity than other pathogenic vibrios (Randa et al., 2004). This might also explain the observations on the different weight of salinity as a factor influencing V. parahaemolyticus prevalence and levels in temperate and tropical waters (FAO and WHO, 2011): in fact, in tropical areas, where limited oscillation of water temperature is present, salinity emerges as the major driver for V. parahaemolyticus abundance (Deepanjali et al., 2005), while in temperate areas both temperature and salinity are considered important (DePaola et al., 2003).
V. vulnificus is an obligate but moderate halophile, that inhabits marine and estuarine waters with salinities between 2 and 25 ppt (Oliver, 2015), with higher bacterial concentrations occurring in the range 5–20 ppt, and viability enhanced at 5–10 ppt (Parveen & Tamplin, 2013). V. vulnificus has a more complex relationship with the combination of temperature–salinity as, with the increase of salinity, the range of temperatures permissive for its growth tends to narrow. Therefore, at salinities > 30 ppt V. vulnificus occurrence is significantly reduced regardless of the environmental temperature (FAO and WHO, 2005b), at intermediate salinity (> 10 ppt) the permissive temperature is restricted to 22–30°C, and at low salinities (5–10 ppt) the growth range extends from 10 to 32°C (Randa et al., 2004).
Finally, non‐O1/non‐O139 V. cholerae usually colonises low‐salinity and brackish waters (0.5–30 ppt) and its occurrence in freshwater (< 0.5 ppt) has been long recognised in association to pandemic cholera and its transmission through ingestion of contaminated water (Faruque Shah et al., 1998). V. cholerae occurrence and abundance is usually greater in the range 0.5–10 ppt, while higher salinities are less favourable (Takemura et al., 2014).
Few studies evaluated the significance of salinity on Vibrio occurrence in European marine areas. In Germany, for example, a survey dealing with bathing areas of the North and Baltic Sea, highlighted that salinity was a secondary factor to temperature for Vibrio spp. abundance, but it was the main modulator of the composition of the Vibrio community, enhancing occurrence of V. parahaemolyticus in areas with higher salinity, the North Sea, compared to areas as the Baltic Sea, where V. vulnificus was the predominant species (Fleischmann et al., 2022). Studies in France and Spain also underlined how a rapid shift of the salinity in confined areas such as lagoons and rias can lead to a rapid proliferation of a certain species, with V. parahaemolyticus occurrence in Galician rias increasing during periods of lower salinity in correspondence of freshwater discharge points (Martinez‐Urtaza, Lozano‐Leon, et al., 2008). Similarly, the rapid decrease of salinity (from 31–36 ppt to 2–16 ppt within 15 days) in French Mediterranean lagoons following freshwater flash floods, showed the increase of all three species of PH, with V. parahaemolyticus reaching the highest counts at a salinity of 10–20 ppt, V. vulnificus at 10–15 ppt and V. cholerae at 5–12 ppt (Esteves et al., 2015).
3.7.1.4. Temperature and salinity interplay
In consideration of data and statistical analysis limitations, quite often, in environmental studies, either temperature or salinity are considered as sole driving factors for Vibrio occurrence. It has been long suggested, however, that Vibrio abundance in aquatic systems is dependent on both temperature and salinity simultaneously, although the interplay of these two factors may be disguised if studies or samples collection are conducted, for example, in a salinity range too narrow to appreciate correlations (Johnson, 2015; Zimmerman et al., 2007). Some studies specifically addressed the interdependency of temperature and salinity effects, especially for V. vulnificus. (Randa et al., 2004), using experimental and literature data, showed that – when considering the whole set of temperature and salinity values – V. vulnificus abundance is positively correlated to temperature and negatively correlated to salinity (r s = 0.310 and r s = −0.451, respectively). However, splitting the analysis according to salinity ranges, the relationship shows a mixed trend. In the range 5–10 ppt, in fact, V. vulnificus abundance does not correlate with temperature and large variations of concentrations are reported in this salinity range regardless of temperature. At higher salinities (range 20 to 25 ppt), instead, a positive correlation of V. vulnificus abundance to salinity can be established (r s = 0.354).
3.7.1.5. Solar and UV radiation
Direct exposure to solar radiation is a significant inactivation factor in the aquatic environment and higher sensitivity of V. cholerae to solar disinfection compared to other enteric pathogens has been reported (Berney et al., 2006). A prominent role of sunlight exposure on the induction of the lysogenic phage CTXΦ of toxigenic V. cholerae has been documented (Faruque et al., 2000). Furthermore, mechanisms of prophage induction, which enhance UV‐sensitivity, and loss of culturability with induction of the VBNC state in water microcosms exposed to sunlight have been described for V. parahaemolyticus (Deepanjali et al., 2005; Zabala et al., 2009).
UV‐C systems are routinely used for disinfection purposes, e.g. for water treatment in bivalve depuration settings. Based on experimental data (Joux et al., 1999; Mori et al., 2007; Nakahashi et al., 2014; Oguma et al., 2019), UV‐A and UV‐B (wavelength 315–400 nm and 280–315 nm, respectively), inducing DNA damage, cellular membrane damage and growth delay, may interfere with Vibrio spp. survival and replication. A log10 inactivation rate constant k of 0.017 cm2/mJ has been reported, for examples for V. parahaemolyticus subjected to UV‐LED treatment at 300 nm (Oguma et al., 2019).
3.7.1.6. pH
In general, Vibrio species are tolerant to alkaline pH, a feature that is exploited in culture media for their isolation, while they display extreme sensitivity to highly acidic environments (just a few minutes of survival at pH ~2; Koo et al., 2000; Mudoh et al., 2014). Some studies, however, highlighted that V. parahaemolyticus and V. vulnificus may retain viability after long‐term storage (30 days) at pH ranging between 7.0 and 4.0 (Yoon et al., 2017) and that V. vulnificus clinical and environmental strains may produce biofilms in response to acidic environment (pH 5.5), a mechanism that may enhance survival and retention of infectivity in these conditions (Çam & Brinkmeyer, 2020). Overall, even though Vibrio species of PH relevance (and particularly V. parahaemolyticus and V. vulnificus) can adopt strategies to survive mild acidic environments, their optimal pH falls in the neutral/low alkaline area (pH 7.6–7.8) and efficient growth is supported up to highly alkaline conditions (pH ~10) (Mudoh et al., 2014). In general, the pH of aquatic environments is quite stable and, indeed, this factor does not emerge as a significant contributor to the variation of vibrios abundance (Takemura et al., 2014). Raw seafood pH is usually reported in the range between 6.0 and 7.0. Therefore, except for products that may have undergone acidification, this parameter is not relevant for modelling the growth of Vibrio spp. in seafood.
3.7.1.7. Water activity
Vibrio species of PH relevance display optimal growth at a w equal or above 0.98, with a minimum of 0.96 and 0.97 for V. vulnificus and V. cholerae, respectively, and a slightly higher tolerance for V. parahaemolyticus (0.94 according to ICMSF, 1996; 0.936 according to Miles et al., 1997). Considering the characteristics of raw seafood, a w is generally a factor with limited relevance for the survival and growth of these Vibrio spp. while, due to susceptibility of vibrios to dehydration, it may affect processed (e.g. salted, dried) seafood products.
3.7.1.8. Nutrient content
Vibrio spp. occur both as free‐living organisms, using for their metabolism substances dissolved in the aquatic environment, and as attached to biological surfaces that can be used as a nutritive substrate. The most relevant of these substrates is chitin (see also Section 3.7.2.1), which is present in crustaceans, zooplankton (e.g. copepods) and phytoplankton (e.g. diatoms), and that can be used by almost all vibrios as a source of carbon and nitrogen through the production of an extracellular chitinase (Thompson et al., 2004). Further to this, Vibrio spp. can exploit algal‐derived organic matter released following phytoplankton blooms. This was confirmed in experiments on Baltic Sea microcosms supplemented with cyanobacterial‐derived organic matter (Eiler et al., 2007). More recently, the effect of microplastics on vibrios occurrence has been investigated. Vibrio spp., indeed, have been found in high abundances within the plastisphere microbiota (summarised in Bowley et al., 2021); beside the relevance of abiotic surfaces for Vibrio attachment and spread, the ability of some V. parahaemolyticus and V. alginolyticus isolates to biodegrade a plastic polymer (polyvinyl alcohol‐low linear density polyethylene‐blended plastic films) was also reported (Raghul et al., 2014). Further to this, Vibrio occurrence is also modulated by essential micronutrients with limited external supply, such as iron (Fe). Fe concentration in the environment is relevant for proliferation of several Vibrio species, as evidenced in studies on Saharan dust pulses, an important source of iron to tropical marine waters, following which a 5‐ to 30‐fold increase of Vibrio counts was registered (Westrich et al., 2016).
3.7.1.9. Other biotic and abiotic factors
Vibrio proliferation in the aquatic environments is affected by several additional factors, e.g. water turbidity, phyto‐ and zooplankton content, chlorophyll a, dissolved oxygen and organic carbon, chitin, nitrogen, etc. (Brumfield et al., 2021). While all these factors play a role in Vibrio abundance, the meta‐analysis conducted by Takemura et al. (2014) clearly showed that their correlation with Vibrio occurrence is inconsistent and not comparable to the predictive value of temperature and salinity. Further to this, Vibrio spp. occurrence is affected by their dual lifestyle, as either free‐living microorganisms or as particle‐associated bacteria (reviewed in Sampaio et al., 2022), the latter leading to specialised ecological niches with freshwater and estuarine plants, micro‐ and macroalgae, zooplankton and invertebrate animals (including bivalve shellfish and crustaceans) (Herrfurth et al., 2013; Parveen & Tamplin, 2013). Interaction with vertebrate animals may be relevant for Vibrio occurrence: aquatic birds, indeed, feed on plants and animals potentially colonised by Vibrio and their faeces could be a source of Vibrio dissemination to previously non‐colonised water systems, especially in the case of migratory birds (Noorian et al., 2023). Specific interaction mechanisms enhancing survival of Vibrio spp. are addressed in Section 3.6.
3.7.2. Factors in the aquatic environments and in food that affect transmission of their virulence and resistance determinants (SQ2.2)
Vibrio spp. have been shown to evolve continuously by gaining fitness traits through HGT, contributing to the ability of Vibrio spp. to adapt to specific niches (Brumfield et al., 2021). HGT and recombination events may influence the emergence of pandemic clones, such as V. parahaemolyticus O3:K6, which appears to have acquired pathogenic characteristics from various bacterial species (Boyd et al., 2008). Broad distribution of virulence genes among environmental Vibrio spp. is well documented (Hasan et al., 2015; Klein et al., 2014; Nishibuchi et al., 1996; Sechi et al., 2000). Few factors have been described to affect the transmission of virulence or resistance determinants.
3.7.2.1. Presence of chitin
Chitin is used by the different vibrios as a source of energy, carbon and nitrogen. Chitin is a long‐chain polymer of N‐acetylglucosamine and is the second most abundant polysaccharide in nature following the cellulose. Chitin was described to trigger the natural competence of V. cholerae in 2005 (Meibom et al., 2005). The tfoX gene is required for chitin‐dependent natural transformation in V. cholerae and homologues of TfoX were identified in all sequenced Vibrionaceae members suggesting that chitin‐induced natural transformation is a shared trait among the Vibrionaceae (Sun et al., 2013). Given the close association of vibrios to the zooplankton in marine environments, V. cholerae as well as V. parahaemolyticus and V. vulnificus are probably frequently in the state of natural competence enabling them to acquire novel traits providing an advantage in the respective environments (Escudero & Mazel, 2017).
3.7.2.2. Presence of phages
The aquatic environments on earth are estimated to host ∼10 52 phages, where they coexist in a dynamic equilibrium with their bacterial hosts. Phages can exist as free viruses (virulent phages) or integrated within bacterial genomes (temperate phages) as prophage (Molina‐Quiroz & Silva‐Valenzuela, 2023). Factors like salinity, high temperature, nutrient limitation and in vivo conditions such as the intestinal microenvironment induce the lytic stage by activating the SOS response (Nawel et al., 2022). In the aquatic environment, vibriophages can be found in the water column and associated with filter‐feeding animals such as oysters (Richards et al., 2019). A recent study found 5674 prophage‐like elements among 1874 sequenced Vibrio genomes at NCBI (Castillo et al., 2018). The authors reported that these vibrio phages carried virulence genes such as those encoding RTX toxin, collagenases or haemolysins, as well as ARGs, niche adaptation factors with high similarity to heavy metal resistance genes and even genes of the DNA uptake/recombination cascade, as the dprA gene. Vibrio prophages and temperate phages may constitute a major reservoir of virulence and niche adaptation traits in marine systems (Castillo et al., 2018).
3.7.2.3. Aquaculture systems
Aquaculture systems and farms have been designated as ‘genetic reactors’ and ‘hotspots for AMR genes’ where significant genetic exchange and recombination can occur, which can shape the evolution (Watts et al., 2017). The induction of the SOS response that is triggered under stressful conditions and during HGT (Escudero & Mazel, 2017) increases the transfer of ICEs of the SXT/R391 family (Burrus et al., 2006) and drives also conjugative transfer of plasmids. In aquaculture systems, in addition to antimicrobial compounds, biocides and metal‐containing products (e.g. cages) are used. High concentrations of copper, cadmium, lead and mercury have been detected in fish feeds and residues of fertilisers have been found (Watts et al., 2017). The bacterial community is thus exposed to stressful conditions of combinations of heavy metals, antimicrobials and other co‐selecting factors of natural or anthropic origin that are likely to increase the selection of AMR (Hiltunen et al., 2017).
3.7.3. Concluding remarks
- The factors that affect survival and growth of the relevant Vibrio spp. in the aquatic environments and in food are:
-
○factors associated with the external conditions in the aquatic environment or surrounding the food (extrinsic factors) as temperature, (sea)water salinity, solar and UV radiation;
-
○factors associated with the inherent properties of the seawater or the food (intrinsic factors) as pH, water activity of food, nutrient content;
-
○factors depending on the interaction of the microorganism with the surrounding microbiota (implicit factors) as predation, parasitism, commensalism, production of substances that are inhibitory/lethal to other organisms, etc.
-
○
Among the factors affecting occurrence and growth of Vibrio spp., temperature is the most important driver for the abundance of the species of PH interest in both the aquatic environment and food (herein temperature limits for growth ranging from 5, 8 and 10 to 43°C for V. parahaemolyticus, V. vulnificus and V. cholerae), followed by salinity in the environment (optimum range 15–25 ppt for V. parahaemolyticus, 2–20 ppt for V. vulnificus and 0.5–10 ppt for V. cholerae). Other factors as pH and a w may be of relevance in processed seafood products.
The interactions among environmental factors affecting the occurrence of Vibrio species display a high level of complexity and variability and require region‐specific environmental models and validations.
A limited number of ecological studies addressing the effect of environmental factors on the occurrence of Vibrio spp. in seafood production areas in the EU are available.
Growth models have been developed for V. parahaemolyticus in oysters, shrimps/prawns and for some finfish (salmon and flounder) and for V. vulnificus in oysters.
A broad distribution of virulence genes among environmental Vibrio spp. is well documented. The following factors affect the transmission of virulence or resistance determinants: presence of chitin (triggering natural competence), presence of phages (acting as reservoirs of these genetic determinants) and contaminants of the aquatic ecosystem such as antimicrobials and heavy metals.
3.8. Impact of climate change on the occurrence and levels of the relevant Vibrio spp. in water environments and seafood (AQ3)
Human activities, principally through emissions of greenhouse gases, have unequivocally caused global warming, with global average surface temperature reaching, in 2011–2020, 1.1°C above 1850–1900 (IPCC, 2023). In 2023, the world saw the highest global temperatures in over 100,000 years, and heat records were broken in all continents through 2022 (Romanello et al., 2023). In Europe, the climate is changing fast and the extreme events that have taken place over the last years offer a foretaste of what is to come under future global warming. This entails a range of risks to human health, including those associated with changes in water quantity, quality and temperature (EEA, 2024). Climate change has the potential of causing, enhancing or modifying the occurrence and intensity of some food‐borne diseases (EFSA, 2020a), and various physical manifestations of climate change are likely to have direct impacts on diseases, such as those mediated by pathogenic vibrios. These bacterial species grow in warm low‐salinity waters, with their relative abundance in the natural environment tending to mirror environmental conditions (Baker‐Austin et al., 2017). The most recent IPCC report indicates that climate change risks expected in the near term include an increase in food‐borne, waterborne and vector‐borne diseases (high confidence) (IPCC, 2023). With temperatures changing across the globe overall, areas suitable for vibrios will increase (Trinanes & Martinez‐Urtaza, 2021). A recent study looking at projections for coastal warming showed that coastal areas suitable for Vibrio infections could expand significantly by 2100 under the most unfavourable climate warming scenarios (Trinanes & Martinez‐Urtaza, 2021). As V. parahaemolyticus and other Vibrio species have replication times as rapid as 8–9 min (Aiyar et al., 2002; Joseph et al., 1982), this group of pathogens is one of the most responsive to favourable environmental stimuli (Aiyar et al., 2002; Joseph et al., 1982). Although the majority of human pathogenic diseases have been shown to be aggravated by climate change (Mora et al., 2022), the relative simplicity of the relationship between ambient environmental temperature and Vibrio abundance is so clear that they have been deemed a key ‘barometer’ of climate change and climate‐associated impacts (Baker‐Austin et al., 2017).
3.8.1. Data sources, tools and models to evaluate the impact of climate change on the relevant Vibrio spp. in water environments and seafood and their limitations (SQ3.1)
Warming of coastal areas is considered the most pervasive and obvious impact of climate change in aquatic ecosystems worldwide, particularly in light of recent observations demonstrating significant and rapid warming in most of the world's coastlines (Lima & Wethey, 2012). From the late 1970s onwards, global mean SST started to rise rapidly (see Figure 4). Various data sources, tools and models are available to evaluate the impact of climate change on Vibrio spp. in water environments and seafoods. The most recent available data suggests that global SSTs are increasing at truly unprecedented rates, with the warming in 2023 alone record breaking compared to both the recent and long‐term record. In addition to warming trends, various extreme weather events such as heatwaves and storms have increased in the last two decades. Unfortunately, there is currently a lack of high‐quality climatic data indicating how specific climatic events have led to outbreaks and Vibrio infections in Europe.
FIGURE 4.
Climate warming of the global oceanic system. Daily sea surface temperatures for the world (60° S–60° N, 0–360° E) from 1981 to 12.6.2024. Climate Reanalyzer (n.d.) (Daily Sea Surface Temperature). Climate Change Institute, University of Maine. Retrieved (June 14, 2024), from https://climatereanalyzer.org/.
Shared Socioeconomic Pathways (SSPs) are climate change scenarios of projected socioeconomic global changes up to 2100 as defined by the IPCC (2021). They are used to derive greenhouse gas emissions scenarios with different climate policies. The SSPs are part of a new framework that the climate change research community has adopted to facilitate the integrated analysis of future climate impacts, vulnerabilities, adaptation and mitigation (Riahi et al., 2017). Considering the medians under the very low greenhouse gases (GHG) emission scenario SSP1‐2.6 53 and under the very high GHG emission scenario SSP5‐8.5, the ocean temperatures at the surface (Figure 5) are expected to increase by 2100 (as compared to 1991–2010), respectively:
1.3°C (with a 90% spread from 0.2 to 2.5°C, henceforth referred to as a range of SSTs) and 3.5°C (2.2 to 5.8°C) for the Baltic Sea;
1.3°C (0.9 to 2.4°C) and 3.5°C (2.7 to 5.5°C) for the Black Sea;
1.1°C (0.6 to 2.1°C) and 3.4°C (2.6 to 5.0°C) for the Mediterranean Sea;
1.0°C (−0.7 to 1.8°C) and 2.4°C (1.2 to 4.6°C) for the North Sea.
FIGURE 5.
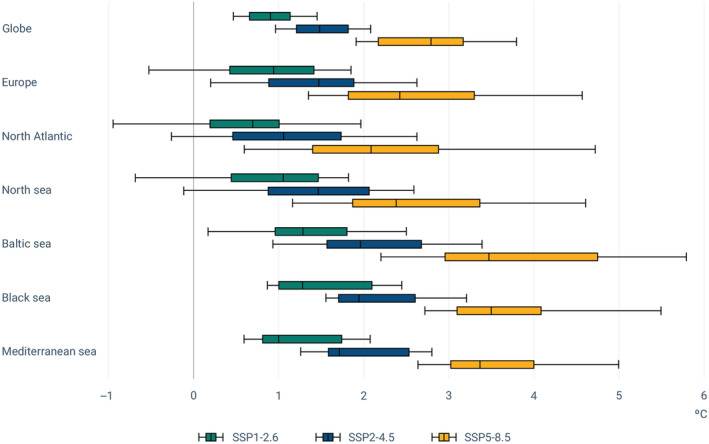
Projected sea surface temperature anomalies by 2100 under three different SSP scenarios (i.e. SSP1–2.6, SSP2–4.5 and SSP5–8.5) for European seas and global ocean as compared to 1991–2010. The boxplot shows the 5th, 25th, 50th, 75th and 95th percentiles (from https://www.eea.europa.eu/en/analysis/indicators/european‐sea‐surface‐temperature).
Marine heatwaves are also projected to increase in frequency, duration, spatial extent and maximum intensity. Such changes could have widespread effects on marine species and cause the reconfiguration of marine ecosystems.
Various data sets have become available over the last two decades that provide compelling evidence regarding the role of changing climate on infectious diseases mediated by pathogenic vibrios. Table 9 describes some key characteristics of the relevant data sources and approaches that have been undertaken to better understand the role of climate and vibriosis, with a focus where relevant in Europe. These include ecological and microbiological studies that show the impact of temperature and extreme weather events on vibrios, a small number of retrospective molecular studies (e.g. that use archived DNA/plankton samples alongside climatic data), epidemiological reports that link climate events to specific Vibrio infections and outbreaks, as well as risk mapping and Vibrio suitability approaches that can be used to infer how variables such as temperature and salinity impact the presence of these bacteria in the environment. There are various advantages as well as limitations to these different data sources that should be noted. However useful, there are few ecological and microbiological studies assessing the direct role of climate on Vibrio bacteria in Europe, although more published studies have emerged in the last decade. Molecular methods provide valuable information on the role of past climatic events on the abundance of Vibrio bacteria in the environment; however, most of the molecular studies that are focussed on analysing the relationship between climatic variables and Vibrio abundance are limited in geographical coverage, with only a small number of published studies using European derived samples (Vezzulli et al., 2012). In Europe, there is a lack of high‐quality epidemiological studies, which has limited the study of climate and disease emergence. Especially, there are only a small number of reported shellfish‐associated outbreaks linked to climatic anomalies from Europe (Baker‐Austin et al., 2010; Martinez‐Urtaza et al., 2013). Further to these studies, some specific Vibrio risk tools and calculators, addressing the risk of V. parahaemolyticus and V. vulnificus infection associated with seafood consumption considering environmental conditions at harvesting, are described under Section 3.10.
TABLE 9.
Data sources of relevance that underpin the association between climate and disease emergence associated with vibrios.
| Data source | Data used in studies | Outputs and geographical scope | Key studies | Applicability and limitations |
|---|---|---|---|---|
| Ecological studies of vibrios in Europe | Microbiological data from water and shellfish matrices | NW Europe, UK, the Netherlands, France | Ford et al. (2020), Schets et al. (2011), Sterk et al. (2015) | Studies are generally based on microbiological data from specific study sites and focusing on a small number of Vibrio species. Some but not all are linked to epidemiological risk and are generally descriptive in nature. |
| Retrospective molecular studies of vibrios using environmental samples | Temperature data coupled to long‐term archived DNA samples such as continuous plankton recorder samples | North Atlantic, NW Europe | Vezzulli et al. (2012), Vezzulli et al. (2013), Vezzulli et al. (2016) | Some studies using molecular analysis of long‐term stored environmental plankton samples provide compelling molecular evidence to suggest vibrios have emerged and proliferated in the last few decades in response to conducive environmental warming trends, such as following warming trends in certain areas (e.g. the North Sea from the 1980s onwards). Key limitation is limited geographical scope of studies and issues with comparing data to various microbiological data sets. |
| Epidemiological studies concerning vibrios and climate events | Various epidemiological data sets as coupled to climatic data | South America, USA, Canada, Europe and specific studies in NW Europe | Amato et al. (2022), Archer et al. (2023), Baker‐Austin et al. (2013) | Studies mainly focus on the role of unusual climatic events such as heatwaves and oceanic anomalies in driving risk, as well as specific FBOs associated with such anomalies. European studies are limited by poor epidemiological metadata associated with human infections. Reporting capabilities may also have changed over time. Paucity of data linking climatic events and human health risk. |
| Vibrio suitability mapping approaches | Salinity, SST, population density and climatic change based on emission pathways a | Uses temperature and salinity to help predict risk of non‐cholera Vibrio infections based on exposure using the model described by (Baker‐Austin et al., 2013). Validated for Baltic Sea, possibly, has global applications. 7‐day forecasting capability. | ECDC E3 Geoportal and NOAA Atlantic OceanWatch ERDDAP Server (Semenza et al., 2017; Trinanes & Martinez‐Urtaza, 2021) | This tool predicts the suitability for Vibrio to be present in the environment using temperature and salinity data. It was developed to facilitate use in recreational (bathing) waters. The output does not provide information on the type of Vibrio spp. that may be involved. Considers only water exposure, though it is assumed that wound exposure and food exposure may result in similar infection rates. The underlying data behind this model is now over 10 years old. More recent studies (Trinanes & Martinez‐Urtaza, 2021) provide useful information that consider projected warming trends and how these may impact future risks. |
| NCCOS Vibrio predictive model | Temperature, salinity and growth rate of Vp and occurrence of Vv in Chesapeake Bay. | Expected Vp levels at harvest and after postharvest handling in Chesapeake Bay, doubling time for Vp in specified regions, Vv occurrence in Chesapeake Bay. | Web‐based product: https://coastalscience.noaa.gov/products/vibrio‐predictive‐models/ | The model predicts Vp levels in certain specific geographical regions in the USA (e.g. Chesapeake Bay, Delaware, Pacific Northwest, etc.) and occurrence of Vv in Chesapeake Bay. Provides shellfish related guidance in specified regions. Needs to be adapted to other regions. The limitation is that relation between risk and abundance may vary in different regions based on strains, attack rates etc. |
Abbreviations: FBO, food‐borne outbreak; NCCOS, National Centers for Coastal Ocean Science; NOAA, National Oceanic and Atmospheric Administration; SST, sea surface temperature; Vp, V. parahaemolyticus; Vv, V. vulnificus.
Various ‘emission pathways’ are used in climate change research. These are based on different emission trajectories for the globe (e.g. predicted reductions, stabilisations or increases in greenhouse gas emissions), generally to the end of this century. Two of the most commonly used are RCP (Representative Concentration Pathway) 4.5 and 8.5. Most of these studies/approaches use an exposure–response function between sea surface temperature and vibriosis cases and project the annual disease incidence to the end of the century under the 4.5 and 8.5 RCP scenarios (8.5 being the ‘worst case scenario’).
3.8.2. Impact of climate change on the occurrence and levels of the relevant Vibrio spp. in the aquatic environment at a global level and in Europe (SQ3.2)
A key aspect related to vibrios of PH relevance such as V. parahaemolyticus, V. vulnificus and non‐O1/non‐O139 V. cholerae is that they tend to grow in warm, low‐salinity waters, and their abundance in the natural environment mirrors ambient environmental temperatures (Baker‐Austin et al., 2016). The close correlation between bacterial counts and environmental temperature makes vibrios an exceptional microbial group to study the interaction between microbiology, climate and infectious diseases (Figure 6). Numerous ecological studies have looked at the impact of temperature on Vibrio concentrations, which is a useful proxy to ascribe changing risks. Average levels of total Vibrio spp. abundance have been recorded in the Delaware and Chesapeake Bays (7.9 × 104 and 5.1 × 103 CFU/100 mL, respectively) (Parveen et al., 2020), where non‐cholera Vibrio infections have recently emerged (King et al., 2019). Total Vibrio concentrations of 1.26 × 105 CFU/100 mL were recorded in the UK during a significant heatwave event (Ford et al., 2020).
FIGURE 6.
Relationship between total Vibrio abundance and ambient environmental temperatures (reproduced from Oliver, 2013).
Recently, non‐cholera Vibrio species have undergone a global expansion, reaching new areas of the world that were previously considered adverse for these organisms (Trinanes & Martinez‐Urtaza, 2021). Notable events in colder/temperate regions includes sporadic wound infections reported in the Baltic Sea (Baker‐Austin et al., 2013), and outbreaks reported in Alaska (McLaughlin et al., 2005), Canada (Taylor, Cheng, et al., 2018), the East coast of the USA (Martinez‐Urtaza et al., 2013) and Chile, the latter linked to the incursion of anomalously warm waters in the region (Martinez‐Urtaza, Huapaya, et al., 2008). In all instances, the outbreaks corresponded with rapid warming of usually colder/temperate areas. There is also evidence of changes in the temporal distribution, e.g. extension in reported infections later into the year as observed in NW Europe into the autumn (Baker‐Austin et al., 2013). There are various other studies, including an observed increase in V. vulnificus infections in the USA during El Nino years (Martinez‐Urtaza et al., 2010), which shows how warming can increase infections during the winter. Although long‐term data on the prevalence of these bacteria is largely lacking, there is compelling molecular evidence to suggest these bacteria have emerged and proliferated in the last few decades in response to conducive environmental warming trends (Vezzulli et al., 2016). Also, extreme weather events, such as heatwaves and storms, have been associated with Vibrio outbreaks and elevated reporting of Vibrio cases. Extreme event attribution aims to elucidate the link between global climate change, extreme weather events and the harms experienced on the ground by people, property and nature. To date, however, there is a lack of long‐term evidence regarding specific extreme climatic events and Vibrio outbreaks. The science in this area, including extreme event attribution (Trinanes & Martinez‐Urtaza, 2021), 54 is rapidly evolving.
Recent modelling studies using projections for global coastal warming indicate that coastal areas suitable for Vibrio infections (defined as areas showing SSTs above 18°C and sea surface salinities below 28 ppt) could cover 38,000 km of new coastal areas by 2100 under the most unfavourable climate warming scenarios. The population at risk in suitable regions almost doubled from 1980 to 2020 (from 610 million to 1100 million under the scenario of medium challenges to mitigation and adaptation, SSP2‐4.5 – Trinanes & Martinez‐Urtaza, 2021). Although this study does not distinguish between food and non‐food‐borne (e.g. recreational) routes of exposure, it is notable that areas conducive for these bacteria will not expand uniformly. Indeed, various temperate coastal areas and marine systems, and in particular regions representative of brackish/low‐salinity systems are at particular risk. Such areas include large bodies of low‐salinity water (e.g. Baltic Sea, transitional waters between the Baltic and the North Sea in Europe, the Black Sea), as well as coastal areas which have large riverine inputs or glacial melt inputs that may undergo rapid warming (Chesapeake Bay, Hudson Bay Canada, various coastal areas of NE USA, coastal areas of China, Alaska, etc.). A recent mapping study using a global‐based coastal projection and different emission scenarios suggests such areas represent ‘hotspots’ of future Vibrio disease emergence (Trinanes & Martinez‐Urtaza, 2021). Figure 7 displays the results of the analysis specifically undertaken for the European continent. It is notable that NE Europe represents a key region undergoing increased risks associated with these bacteria. The combination of rapid warming, low salinity and high population densities (and associated exposures) in regions such as the Baltic Sea are important factors modulating risks.
FIGURE 7.
The map fields display the maximum monthly Vibrio suitability Index (red) and the EMODnet database on shellfish aquaculture in the EU (plus Norway and the UK). Data are missing for Germany, Portugal, Sweden and Croatia. The monthly suitability index is estimated from high‐resolution sea surface temperature and sea surface salinity data within 10 km from the shoreline and it is a measure of the local suitability for Vibrio infections (when sea surface temperatures are above 18°C and sea surface salinities below 28 ppt). Reproduced from Trinanes and Martinez‐Urtaza (2021).
Based on modelling, in 2022, 21 European countries identified Vibrio spp.‐suitable areas, with a cumulative exposure risk of 2188 days, the third highest recorded. The affected coastline extended to 28,263 km, with an average annual increase of 136 km since 1982. Notably, Baltic Sea bordering nations, like Sweden and Finland, saw significant expansions in suitable coastline. Other countries, such as Belgium (207%) and the Netherlands (131%), also experienced expansions of Vibrio spp.‐suitable areas (van Daalen et al., 2024).
It should also be considered that shifts in the environmental conditions may lead to changes in the Vibrio communities, with higher abundance of those variants better adapted to the new conditions. Warming in coastal areas, indeed, introduce a selective pressure on Vibrio populations, selecting for strains with a better fitness. Given the absence of present ecological studies in Europe to assess changes in the structure (i.e. composition) of Vibrio populations occurred in the last decades, anticipating how this selective pressure supplied by climate change will affect the Vibrio populations in Europe is extremely uncertain. It is unknown if these new conditions may promote the presence of virulent strains over the non‐virulent ones or foster the evolution of new variants with virulence traits.
3.8.3. Impact of climate change on the occurrence and levels of the relevant Vibrio spp. in seafood at global level and in Europe (SQ3.3)
There are few data sets that systematically collect relevant information regarding the occurrence and levels of Vibrio species in seafood in Europe or globally, which makes the task of establishing the impact of climate change on seafoods particularly problematic. Numerous studies and modelling efforts (ANSES, 2012; Ndraha et al., 2022) have, however, indicated a clear link between elevated environmental temperatures and seafood‐related Vibrio risks. A recent modelling study indicated that climate change could potentially increase the level of V. parahaemolyticus in oysters in Taiwan by 40%–67% in the near future (Ndraha et al., 2022). Elevated environmental temperature prior to and during the outbreak event tend to be a noticeable environmental signature leading to outbreaks. In 1997, a significant warm water incursion into the region of the Pacific Northwest was associated with an unprecedented V. parahaemolyticus outbreak (Annex G). In this event, 209 infections were reported, all associated with eating seafood harvested from California, Oregon and Washington in the USA and from British Columbia (BC) in Canada and one person died. This outbreak remains one of the largest ever reported in North America and clearly shows the interaction between anomalous seawater temperatures and disease transmission.
Table 10 summarises various Vibrio‐associated seafood outbreaks, all caused by V. parahaemolyticus, that are likely to be linked to climate anomalies over the last 3 decades. Few studies have been published in Europe to date describing shellfish associated outbreaks (Baker‐Austin et al., 2010; Martinez‐Urtaza et al., 2016).
TABLE 10.
Relevant food‐borne Vibrio‐associated outbreaks caused by V. parahaemolyticus.
| Year | Country, region | Epidemiology | Climate‐related evidence |
|---|---|---|---|
| 1997 | USA. Pacific Northwest | 209 infections reported in Oregon and Washington in the USA and from British Columbia (BC). One fatality a | Mean Pacific coastal SSTs were significantly higher (typically 3–5°C) in areas where cases were subsequently reported. Unpublished data (Baker‐Austin and Martinez‐Urtaza). Clear expansion of anomalously warm water evident during outbreak, see Annex G |
| 1999 | Spain, Galicia | 35 infections reported in Galicia, NW Spain | Significant sea surface anomaly reported from August–September 1999 (Baker‐Austin et al., 2010) |
| 2004 | Chile | 3400 cases reported in Puerto Montt, Chile. By the end of March 2005, the total number of cases in Chile was 10,783 | SSTs were ~3°C higher than the average for this time of year (16°C) (Cabello et al., 2007; Fuenzalida et al., 2006) |
| 2005 | USA, Alaska | 62 patients with GE. Vp serotype O6:K18 was isolated from most patients tested and from environmental samples of oysters | Notable anomaly. 2004 was the only year during which mean daily temperatures in July and August at the shellfish harvesting area did not drop below 15.0°C (McLaughlin et al., 2005) |
| 2012 | Spain, Galicia | 100 reported infections in Galicia, NW Spain | Significant sea surface anomaly reported in shellfish harvesting area July and August 2012 (Martinez‐Urtaza et al., 2016). Some evidence based on epidemiological data that post‐harvest contamination of seafood may have played a role. |
| 2012–2013 | USA | During May–July 2012, Vp caused 28 illnesses in nine USA states. 104 persons from 13 states during May–September 2013 | Significant SST anomaly along NE USA coastline from April–June 2012, preceding outbreaks. Unpublished data (Baker‐Austin and Martinez‐Urtaza) |
| 2015 | Canada | 82 cases reported in British Columbia, vast majority during the summer | SSTs were above the historical levels in 2015 (Taylor, Cheng, et al., 2018) |
| 2021 | USA, British Columbia | 52 clinically confirmed cases | Significant heat dome event in NW USA and Canada in July 2021. SSTs ~4°C higher than average in implicated area (Puget sound). Unpublished data (Baker‐Austin and Martinez‐Urtaza) |
Abbreviations: GE, gastroenteritis; SST, sea surface temperature.
Reference: MMWR Morb Mortal Wkly Rep. 1998 Jun 12;47(22):447–62; accessible at https://www.cdc.gov/mmwr/preview/index98.html.
3.8.4. Concluding remarks
- Assessing the impact of climate change on Vibrio spp. in water environments and seafood involves utilising a variety of data sources, tools and models.
-
○Data sources include environmental monitoring data, microbiological surveillance data, climate and oceanographic data as well as epidemiological case studies.
-
○Tools include statistical analysis, risk mapping approaches and predictive modelling tools and retrospective molecular studies.
-
○Models include correlative and ecological models, process‐based models and modelling and mapping approaches that use Vibrio suitability (areas showing SSTs above 18°C and sea surface salinities below 28 ppt) based on different future climate emission scenarios.
-
○
- The main limitations of these approaches are:
-
○challenges with data availability and quality of epidemiological data, especially in Europe;
-
○complexity of the interactions between environmental factors, which makes disentangling the effects of climate change on microorganisms difficult;
-
○uncertainty inherent with current and future climate projections which impact the reliability of predictions;
-
○lack of integration between different types of models, hindering comprehensive assessments.
-
○
-
Climate change affects the occurrence and levels of the relevant Vibrio spp. in the aquatic environment by inducing a shift towards environmental conditions suitable and more conducive for Vibrio growth and persistence. It affects:
-
○
the geographic distribution of the coastal areas suitable for Vibrio spp. (expansion of the areas);
-
○
the temporal distribution of conditions suitable for Vibrio spp. (extension of the period);
-
○
the frequency, distribution and intensity of extreme weather events which may provide – within limited areas and/or time windows – conditions favourable to Vibrio spp. These include heatwaves and storms/flooding events.
-
○
In Europe, warming coastal waters have led to an expansion of areas in which Vibrio spp. can proliferate, potentially resulting in increased risks of infections associated with seafood consumption and recreational activities. It is predicted that these regions will not increase in the future uniformly, and areas with brackish/low‐salinity waters (e.g. Baltic Sea, transitional waters of the Baltic and the North Sea, the Black Sea), and coastal areas with large riverine inputs are at particular risk.
Europe has also experienced an increase in extreme weather events such as heatwaves over the last two decades. Over this time Europe has witnessed a rise in Vibrio infections, including V. vulnificus and V. parahaemolyticus. There are, however, significant gaps in epidemiological data sets, with only a small number of published reports regarding the impact of climate change on seafood‐related outbreaks in Europe.
It is also possible that climate change may affect the structure of the Vibrio populations or speed up the evolution or selection for the emergence of new Vibrio variants, although limited data on this currently exists.
The impact of climate change on the occurrence and levels of the relevant Vibrio spp. in seafood is not specifically addressed by current models. Epidemiological observations, however, placed a clear link between increased environmental temperatures and seafood‐related Vibrio outbreaks.
Based on knowledge on the effects on water environments, it is anticipated that the occurrence and levels of the relevant Vibrio spp. in seafood will increase both globally and in Europe, as they have done in aquatic settings, in response to manifestations of climate change such as coastal warming and extreme weather events as heatwaves, especially in low‐salinity/brackish waters.
3.9. Prevention and control measures along the food chain for the relevant Vibrio spp. (AQ4)
Prevention and control measures along the seafood chain for Vibrio spp. have been addressed in the risk assessments for France dealing with V. parahaemolyticus in seafood (ANSES, 2012) and for Germany dealing with pathogenic vibrios (non‐cholera vibrios) in seafood (BfR, 2022). The AESAN Scientific Committee (Spain) considered measures for the prevention and control of V. cholerae applicable to imported frozen prawns and other fishery products (AESAN, 2024). Also both FAO/WHO risk assessments (FAO and WHO, 2020, 2021) cover post‐harvest treatment technologies for reducing V. parahaemolyticus and V. vulnificus in seafood. In addition, several review papers deal with post‐harvest interventions to reduce vibrios in seafood; some (Ndraha et al., 2020; Spaur et al., 2020) specifically consider V. parahaemolyticus in raw oysters, while others (Ekonomou & Boziaris, 2021; Kontominas et al., 2021; Ronholm et al., 2016) consider non‐thermal processing of seafood. An overview of the measures contained in each of these documents can be found in Annex H. Of note is that some measures only apply to particular seafood groups and that several control measures were especially studied on oysters as RTE and high‐value/price product.
Furthermore, it should be considered that measures to prevent food‐borne vibriosis include consumer education. ANSES (2012) made following recommendation:
to consider that the consumption of raw seafood in summer increases the risk of gastroenteritis (GE) caused by Vibrio.
to place seafood in chilled storage as quickly as possible.
to respect good hygiene practices when food handling and preparation: (i) consume within 2 h of taking out of the refrigerator and (ii) avoid contact between cooked foods and raw seafood to prevent cross‐contamination.
for patients with chronic liver disease, disease exposing to iron overload, or for immunocompromised persons, presenting increased susceptibility to Vibrio infections, to avoid eating raw or undercooked seafood (oysters, mussels, clams, shrimp in particular).
3.9.1. Refrigeration storage/maintaining cold chain
An important measure to reduce or even prevent the proliferation of any vibrios that are potentially present is maintaining the cold chain from the catch or harvest to the retail outlet. While this is important for any seafood, it is particularly critical for foods intended to be consumed raw (BfR, 2022).
Temperature abuse that may occur during processing, transportation or storage can allow V. parahaemolyticus to grow to dangerously high concentrations in oysters (Ndraha et al., 2020). The utility of refrigerated storage was reported in many studies. For example, Gooch et al. (2002) showed that V. parahaemolyticus can multiply rapidly in live American oysters (C. virginica) oysters held at 26°C, showing an increase of 1.7 logs and 2.9 logs after 10 and 24 h, respectively. Mudoh et al. (2014) reported that V. parahaemolyticus concentrations in shellstock Eastern oysters (C. virginica) increased ~3 logs during a 10‐day storage at 20°C, while the counts remained at the initial level or below at 5 and 10°C. Love et al. (2020) estimated that 75% of oysters in cold chains for US farmed oysters had a net decrease in V. parahaemolyticus from harvest to food retail/restaurant. The authors combined a predictive growth model (Parveen et al., 2013) with the tracked internal temperature of oysters. It averaged 4.4 ± 2.7°C but there were spikes in temperature in some shipments (18% of shipments experienced oyster temperatures > 10°C for 1 h or more).
3.9.2. Icing
The icing method involves placing seafood in crushed ice or an ice slurry to chill the seafood as quickly as possible to lower their internal temperature, particularly after harvest, to prevent the growth of food‐borne pathogens.
The use of ice slurries was shown to rapidly cool oysters (from 23.9 to 10°C within 12 min) with no significant changes on Vibrio spp. (Lydon et al., 2015). Also layered ice is an effective means of rapidly cooling oysters, even in warm summer conditions; resulting in 1.1–1.5 and 0.6–2.0 logs lower V. vulnificus and V. parahaemolyticus levels, respectively, in oysters, compared to those that were refrigerated post‐harvest (Jones et al., 2017). Icing either immediately postharvest or just prior to truck loading did not reduce V. vulnificus or V. parahaemolyticus levels in oysters (Melody et al., 2008). This was also the case for V. parahaemolyticus in raw Pacific oysters (C. gigas) over 4 days (Phuvasate et al., 2012).
Of note is that on‐board icing appeared to cause significantly higher oyster gaping, which could lead to economic losses unacceptable to the industry (Melody et al., 2008). Storing oysters in ice slurry for prolonged periods is not recommended as vibrios can multiply in the ice slurry water (Lydon et al., 2015).
3.9.3. Mild heat treatment (with thermal shock)
In mild thermal treatment, oysters are submerged in water at 50°C for a specific processing time while thermal shock involves submerging oysters in water at a given temperature for a specific processing time followed by rapid cooling in ice water (Ndraha et al., 2020).
Submersion of live oysters in water at 50°C for 5 min reduced V. parahaemolyticus and V. vulnificus concentrations from 5 log10 MPN/g to non‐detectable levels (Andrews et al., 2000). In another study, 10 min in water at 50°C were needed to reduce V. vulnificus from 4.8 log10 MPN/g to a non‐detectable level; 5 min exposure led to a reduction to 2.3 log10 MPN/g (Cook & Ruple, 1992). Ye et al. (2012) exposed inoculated live oysters to 40, 45 or 50°C for up to 20 min. A 15 min treatment at 50°C achieved > 7 log10 reduction.
A thermal shock reduced V. parahaemolyticus in oysters from 5 to 6 log10 MPN/g to an undetectable level after being heated to 50°C for 8 to 10 min (Andrews et al., 2003). The authors noted that V. parahaemolyticus O3:K6 was more heat resistant than the non‐pathogenic strains, and recommended a total processing time of at least 22 min at 52°C.
According to Ndraha et al. (2020), inaccurate control of time and temperature (e.g. exposure to > 53°C) affected the sensory quality of oysters (Andrews et al., 2003), but even with strict temperature control, slight changes in colour and smell after treatment of oysters at 50°C for more than 5 min were reported (Ye et al., 2012).
3.9.4. Freezing
Muntada‐Garriga et al. (1995) determined the survival of V. parahaemolyticus in oyster meat homogenates at −18°C and −24°C and various bacterial levels (2–7 log10 CFU/mL). The numbers of V. parahaemolyticus showed a logarithmic decline over time with the time of total inactivation depending on the initial levels and temperature. Freezing of oyster meat homogenates inoculated with the higher bacterial levels (5–7 log10 CFU/g) at −18 and −24°C with storage for 15–28 weeks led to a complete inactivation.
Long‐term storage of half‐shell oysters at −20°C can reduce V. parahaemolyticus and V. vulnificus to non‐detectable levels (Andrews, 2004). However, this does not occur in a reasonable storage time (< 6 months) when the initial levels were > 3–4 log10 CFU/g. Shen et al. (2009) showed that populations of V. parahaemolyticus decreased from 5.46 log10 MPN/g to 1.66 and 0.38 log10 MPN/g, after 75 days at −30°C of shucked and shell stock Zhe oyster (Crassostrea plicatula), respectively. No cells were detected (< 3 log10 MPN/g) in the shucked oysters after 60 days at −18°C. Detrimental effects of freezing were greater at −18°C than at −30°C, consistent with greater bacterial damage at the higher temperatures due to the larger intracellular ice crystal formation.
Flash freezing followed by frozen storage can be used for reducing V. parahaemolyticus contamination in half‐shell Pacific oysters (Liu et al., 2009). Although populations in the oysters declined only slightly (by 0.22 log10) after the freezing process (−95.5°C for 12 min), subsequent storage of frozen oysters resulted in considerable reductions. The decline was 2.45, 1.71 and 1.45 log10 and 4.55, 4.13 and 2.53 log10 after 1 month and 6 months of storage at −10, −20 and −30°C, respectively. Process validation confirmed that flash freezing, followed by storage at −21°C for 5 months, could achieve > 3.52 log10 reductions of V. parahaemolyticus in half‐shell Pacific oysters.
A combination of vacuum packaging and freezing decreased V. vulnificus levels in oysters by ~3 log10 within 7 days post‐freezing, and levels continued to drop throughout frozen storage achieving ~5 log10 reductions after 70 days. Vacuum‐packed freezing was more effective compared to freezing under normal conditions (Parker et al., 1994).
3.9.5. Treatment with sodium metabisulfite
Sodium metabisulfite (E 223) is authorised as a food additive in unprocessed cephalopods and crustaceans according to Regulation (EC) No 1333/2008. It is traditionally used to control a non‐microbiological spoilage symptom of prawns known as melanosis or blackspot (Januário & Dykes, 2005).
According to Januário and Dykes (2005), the exposure of fresh prawns (Penaeus monodon) to seawater with sodium metabisulfite (1%) determined an additional decrease (up to 1.8 log10 units) of the numbers of V. cholerae during chilled storage (1.5°C) for 14 days as compared to chilled untreated prawns (~4 log10 units). Conversely, no significant additional reduction of the numbers of V. cholerae was observed in treated prawns stored at −25°C for 49 days as compared to the untreated ones.
3.9.6. High‐pressure processing
High‐pressure processing (HPP) is a non‐thermal treatment in which foods are subjected to isostatic pressures. Traditionally, this is an in‐batch process that can be applied to solid and liquid prepacked foods. Pressure is transmitted instantaneously and uniformly in an isostatic manner so that all parts of the food are subjected to the same pressure simultaneously. An EFSA scientific opinion on the efficacy and safety of HPP of food has been recently published (EFSA BIOHAZ Panel, 2021). Within the industrial context, pressures of between 400 and 600 MPa are most often applied for microbial inactivation, with common holding times ranging from 1.5 to 6 min. The water used as pressure‐transmitting fluid is often pre‐chilled at 4–8°C.
HPP is used in the seafood industry, while it appears to be effective against the most common pathogenic and spoilage bacteria found on seafood products. Microbiological safety has been reported to be assured for many fish and seafood products with higher added value, such as shrimp, salmon, trout, cod and oysters (Ekonomou & Boziaris, 2021).
According to Roobab et al. (2022), 400–600 MPa inactivates common seafood vegetative pathogens, such as Vibrio and Listeria monocytogenes. HPP using 200–350 MPa is also useful to shuck oysters, lobsters, crabs, mussels, clams and scallops to increase recovery of the edible meat.
V. vulnificus proved to be more pressure sensitive compared to V. parahaemolyticus (Koo et al., 2006; Vu et al., 2018; Ye et al., 2012, 2013). Using inoculated oysters, 276 MPa applied for 5 min reduced V. vulnificus by > 5.4 logs, while less than 4 logs were achieved on V. parahaemolyticus (Koo et al., 2006). A 2‐min treatment at 225 or 250 MPa reduced V. vulnificus in oyster meat by 4.8 and 7.1 logs and V. parahaemolyticus by 1.5 and 3.0 logs, respectively (Ye et al., 2012). In another study, using the same time–pressure conditions (at 20°C), figures were 5.1 and 6.7 logs and 2.6 and 4.1 logs, respectively (Ye et al., 2013). Vu et al. (2018) concluded that V. vulnificus was the most pressure sensitive among the four Vibrio species tested (i.e. V. vulnificus, V. alginolyticus, V. cholerae and V. parahaemolyticus); a > 5 log reduction in mussel homogenates is achieved using (i) 350–450 MPa for ≥ 1 min at 25°C for both V. alginolyticus and V. cholerae, (ii) 250 MPa for ≥ 3 min or 350–450 MPa for ≥ 1 min for V. vulnificus and (iii) 350 MPa for ≥ 3 min or 450 MPa for ≥ 1 min for V. parahaemolyticus. Hu et al. (2005) estimated the D‐values at 276, 310 and 345 MPa as 3.3, 2.7 and 2.0 min using whole oysters inoculated with V. parahaemolyticus (O3:K6).
3.9.7. Irradiation
Different technologies are available and used for the irradiation of food, having to a large extent the same effect on microorganisms. While gamma‐rays are produced from a radioactive source, electron‐beams and X‐rays are produced by specific equipment converting other energy sources, without the involvement of any radioactive substance (EFSA BIOHAZ Panel, 2011). Irradiation methods using UV or LED are emerging technologies (Spaur et al., 2020). In Europe, the Scientific Committee on Food recommended that fish and shellfish could be irradiated at doses up to 3 kGy. A dose of 3 kGy was considered sufficient to effectively reduce non‐sporeforming bacterial pathogens by 2–5 log10 units for most of the fish and fishery products (SCF, 1998).
Irradiation was shown effective to lower the levels of V. parahaemolyticus and V. vulnificus in oysters. Jakabi et al. (2003) evaluated the effect of gamma radiation (0.5 to 3 kGy) on V. parahaemolyticus in oysters (Crassostrea brasiliana). A dose of 1 kGy yielded a 6 log10 reduction in the level of V. parahaemolyticus. In another study, 1 kGy X‐ray resulted in 4.9 and 2 log10 reduction of V. parahaemolyticus in half and whole shell oysters, respectively, while > 6.5 and 3.1 log10 reduction were achieved using 2 kGy (Mahmoud & Burrage, 2009). Similarly, 1 kGy X‐ray resulted in > 6.5 and 3.2 log10 reduction of V. vulnificus in half and whole shell oysters, respectively, while also > 6.5 log10 reductions were achieved using 3 kGy (Mahmoud, 2009). The D‐value was estimated as 0.22 min by Hu et al. (2005) after exposing whole oysters inoculated with V. parahaemolyticus (O3:K6) to gamma irradiation at doses of less than 3 kGy. The radiation decimal reduction doses of V. parahaemolyticus and V. vulnificus in inoculated oyster homogenate using gamma irradiation were estimated as 0.159 and 0.140 kGy, respectively (Thupila et al., 2011). In the study by (Park & Ha, 2018), a 0.5, 1 and 2 kGy treatment using gamma irradiation reduced V. parahaemolyticus in shucked oysters (Grassostrea gigas) by 1.5, 3.2 and 4.2 log10. A dose of 3 kGy did not kill the oysters or affect their sensory attributes (Jakabi et al., 2003).
Ready‐to‐eat (RTE) shrimps inoculated with V. parahaemolyticus were treated with 0.1, 0.2, 0.3, 0.5, 0.75, 1, 2, 3 and 4 kGy X‐ray resulting in a 1.3; 2.4; 2.8; 3.1; 3.6; 4.9 and > 6 log10 reduction of V. parahaemolyticus, respectively. Park and Ha (2018) showed that a 0.5, 1 and 2 kGy gamma irradiation treatment reduced V. parahaemolyticus in clams (Venerupis phillippinarum) by 1.5, 3.1 and 4.1 logs.
3.9.8. Cooking
Live bivalve molluscs from B and C production areas (see Section 1.3) that have not been submitted for purification or relaying may be sent to a processing establishment, where they must undergo treatment to eliminate pathogenic microorganisms. A validated methodology must be used. Procedures based on the HACCP principles must be in place to verify the uniform distribution of heat. The permitted treatment methods, according to Regulation (EU) 853/2004, are:
(a) sterilisation in hermetically sealed containers; and
(b) heat treatments involving:
immersion in boiling water for the period required to raise the internal temperature of the mollusc flesh to not less than 90°C and maintenance of this minimum temperature for a period of not less than 90 s;
cooking for 3–5 min in an enclosed space where the temperature is between 120 and 160°C and the pressure is between 2 and 5 kg/cm2, followed by shelling and freezing of the flesh to a core temperature of –20°C; and
steaming under pressure in an enclosed space satisfying the requirements relating to cooking time and the internal temperature of the mollusc flesh mentioned under (i).
According to BfR (2022), maintaining an interior temperature of 70°C in the food to be consumed for 2 min is a reliable method to ensure the inactivation of vibrios. CDC 55 recommends, for shellfish in the shell, to either boil until the shells open and continue boiling another 3–5 min or to add them to a steamer when the water is already steaming and cook them for another 4–9 min. Shellfish that do not open fully after cooking should not be consumed. Shucked oysters should either be boiled for at least 3 min, fried in oil for at least 3 min at 191°C, broiled 7.5 cm from the heat source for 3 min, or baked at 232°C for 10 min. ANSES (2012) provided the same heat treatments as examples. Also, the New Zealand Food Safety 56 recommends not to eat shellfish raw or undercooked and to cook them thoroughly until they are at least 65°C for 1 min (until they open and are firm to touch). Leftovers should be thoroughly reheated to a minimum core temperature of 75°C. Among other, Health Canada 57 also recommends cooking shellfish, especially oysters, thoroughly before eating and keeping raw and cooked shellfish separate.
3.9.9. Interventions for bivalve molluscs
3.9.9.1. Depuration
Depuration involves the storage of the live molluscs in tanks through which sufficiently clean, typically disinfected, seawater is circulated to facilitate depletion of mollusc microbial load through normal filtration activity of the molluscs (EFSA BIOHAZ Panel, 2015). Regulation (EC) No 853/2004 spells out the requirements for those purification centres.
Depuration thus maintains oyster viability while they filter clean salt water that either continuously flows through a holding tank or is recirculated and replenished periodically (Campbell et al., 2022). It is practised in several countries for a range of bivalve molluscan species (e.g. clams, oysters, mussels, scallops) and this process may be performed in static, flow through or recirculating systems using water treated with ultraviolet (UV) light, chlorine, iodine or ozone (Lee et al., 2008).
While depuration has been reported to be effective in removal of human enteric bacteria endogenous bacteria, including coliforms, the reported efficacy for removal of Vibrio spp. has not been consistent (FAO and WHO, 2020) and has presented challenges (Campbell et al., 2022). Key depuration parameters like depuration processing time, water salinity, water temperature and water flow rate have been tested and the use of processing additives to enhance disinfection in oysters explored (Campbell et al., 2022). According to Spaur et al. (2020), also the feeding status, water to oyster ratio, and type and size of oysters varied in the studies. According to Ndraha et al. (2020), the efficacy of depuration varied between 1.2 and 3.9 log10, depending on the time and temperature of processing, water salinity, the ratio of oyster to seawater, and the additional application of UV light, gamma irradiation or use of NaClO.
When considering temperature, in inoculated (at 104–5 MPN/g) American oyster (C. virginica), 48 h depuration in artificial seawater at 15°C gave higher reductions of V. parahaemolyticus and V. vulnificus (2.1 and 2.9 logs, respectively) compared to 22°C. Extended depuration at 15°C for 96 h provided reductions of 2.6 and 3.3 logs, respectively (Chae et al., 2009). In inoculated (at 104–6 MPN/g) Pacific oysters (C. gigas), depuration in recirculating seawater at 7–15°C reduced V. parahaemolyticus populations by > 3.0 log10 after 5 days, while no significant reductions were obtained at 2 or 3°C (Phuvasate et al., 2012).
The impact of salinity was investigated in Pacific oysters. While V. parahaemolyticus was reduced by 2.1 log10 in UV‐irradiated water with a salinity of 10 ppt (at 12.5°C for 5 days), a reduction > 3.0 logs was achieved using 20–30 ppt (Phuvasate & Su, 2013). The study by (Larsen et al., 2015) using high‐salinity depuration indicated a potential interaction between salinity and temperature. Oysters were held at a salinity of 35 ppt for 10 days at 20, 22.5 or 25°C. There was no significant effect of temperature on the reduction of V. vulnificus and V. parahaemolyticus throughout depuration but there was a tendency for lower temperatures to be less effective than the higher ones.
Ramos et al. (2012) compared depuration using UV light and chlorinated seawater from oysters inoculated with V. parahaemolyticus (at 104–5 MPN/g) and V. vulnificus (at 102–3 MPN/g). The UV light plus chlorine treatment was more efficient for controlling V. parahaemolyticus; after 48 h, the UV plus chlorine treatment reduced counts by 3.1 logs, the UV treatment by 2.4 log10 and the control treatment by 2.0 logs. For V. vulnificus, the UV plus chlorine and the UV treatment were equally efficient (reductions of about 2.5 logs).
In conclusion, and according to Campbell et al. (2022), depuration from 4 to 6 days, low temperature, high salinity and flowing water effectively reduced V. vulnificus and V. parahaemolyticus in live oysters. However, further studies are needed to validate those experiments, in particular with naturally contaminated oysters and in conditions as close as possible to the diverse conditions applied in European shellfish purification centers.
3.9.9.2. Relaying
Relaying involves moving live bivalve molluscs towards the end of the production phase, to designated seawater areas of relatively low microbial content with the explicit purpose of reducing the microbial hazards in the molluscs through the normal filtration processes. The relaying phase lasts at least 2 months (EFSA BIOHAZ Panel, 2015). According to Reg. (EC) No 853/2004, ‘relaying’ means the transfer of live bivalve molluscs to sea, lagoon or estuarine areas (that are subjected to classification and used exclusively for the natural purification of live bivalve molluscs) for the time necessary to reduce contamination to make them fit for human consumption. This does not include transferring bivalve molluscs to areas for further growth or fattening. Food business operators must immerse live bivalve molluscs in seawater at the relaying area for an appropriate period, fixed depending on the water temperature, which period must be of at least 2 months' duration unless the competent authority agrees to a shorter period based on the food business operator's risk analysis.
The potential for reducing levels of V. parahaemolyticus in live oysters by relaying them during warm weather to a site with elevated salinity and consistently low V. parahaemolyticus levels was shown by Taylor, Yu, et al. (2018), achieving a 4.5‐log10 decrease in V. parahaemolyticus levels after 14 days of relay in one occasion. Results suggested that both microbial community and environmental conditions at the relay sites can affect the effectiveness. Parveen et al. (2017) showed that high salinity relaying of oysters – in high‐salinity field sites or recirculating aquaculture systems – was more effective in reducing V. vulnificus than V. parahaemolyticus in oysters (Crassostrea virginica).
3.9.9.3. Other interventions for bivalve molluscs
Harvesting curfews and harvesting cessation are harvesting restriction approaches. Harvesting curfews aim at harvesting oysters under conditions corresponding to low contamination and growth of V. parahaemolyticus. Examples of harvesting curfews include early morning harvests (before the maximum daily temperature) or within specific tidal periods (FAO and WHO, 2021). Harvesting cessation can be used both as a reactive or proactive measure, i.e. as a response to reported illnesses or to prevent them. Proactive measures can be based on temperature or salinity levels in the environment (measured or based on remote sensing models) or can be based on harvesting restrictions during months associated with peaks of illness. The National Shellfish Sanitation Program (NSSP) also developed reactive measures including time to refrigeration and closures based on reported cases (FAO and WHO, 2021).
3.9.9.4. Other technologies
Kontominas et al. (2021) conducted a review of recent studies on innovative seafood processing technologies applied for the preservation and shelf‐life extension of seafood products including HPP, natural preservatives, ozonation, irradiation (summarised in Section 3.9.6), pulse light technology and retort pouch processing. Ekonomou and Boziaris (2021) conducted a review of research studies for the past 20 years on the application of non‐thermal methods for ensuring the microbiological safety and quality of fish and seafood, including apart from HPP (summarised in Section 3.9.5), also ultrasounds, non‐thermal atmospheric plasma, pulsed electric fields and electrolysed water as alternative methods to conventional heat treatments. Phage treatment is covered in the review by Ronholm et al. (2016). Its use is discussed for adding a phage isolated from V. parahaemolyticus in the depuration water and to decontaminate V. parahaemolyticus from oyster meat. Spaur et al. (2020) covered acid treatment but its efficacy on vibrios was only assessed in culture media. These technologies are not covered in this assessment as there is currently only scarce information available on their efficacy on vibrios in seafood.
3.9.10. Concluding remarks
Maintaining the cold chain is required as temperature abuse that may occur during processing, transport or storage can allow vibrios to grow (to dangerously high concentrations) in seafood. This is particularly critical for seafoods intended to be consumed raw. Also icing can retard Vibrio growth in oysters.
(Flash) freezing followed by long‐term frozen storage reduces vibrios in oysters. A mild thermal treatment of oysters in water at 50°C with or without thermal shock reduces V. parahaemolyticus and V. vulnificus to a non‐detectable level but needs to be well controlled to prevent sensorial changes of oysters.
HPP is capable to reduce vibrios in seafood using industrially feasible conditions. Irradiation also proved to lower the levels of V. parahaemolyticus and V. vulnificus in oysters, and V. parahaemolyticus in shrimps and clams.
Other innovative seafood processing technologies could be applied for the preservation and shelf‐life extension of seafood products, such as ozonation, pulse light technology, ultrasounds and pulsed electric fields. There is currently only scarce information available on their efficacy on vibrios in seafood.
Depuration under controlled conditions, although with variable reductions, is a post‐harvest processing treatments for the segment of the market preferring consumption of live oysters.
Furthermore, consumer education can help to prevent food‐borne vibriosis. Food safety authorities have recommended to avoid eating raw or undercooked seafood by susceptible persons and to respect good hygiene practices with food handling and preparation.
3.10. Risk assessment modelling options for the health impact of relevant Vibrio spp. in seafood in the EU (AQ5)
3.10.1. Introduction
To identify the available modelling options, knowledge gaps and data needs to perform a risk assessment on the PH impact of the relevant Vibrio spp. in seafood (from Section 3.1), existing risk assessments were summarised (Section 3.10.2) and further characterised (Section 3.10.3). Several factors can inform the decision on which relevant Vibrio species and seafoods to select for a risk assessment addressing the PH impact in the EU. Data and knowledge gaps for such an assessment are spelled out in Section 3.10.4.
3.10.2. Summary of available QMRAs (SQ5.1)
Table 11 summarises a selection of QMRAs or assessments presenting elements of a QMRA and considered of interest to include. Only two studies covered conditions in Europe, one QMRA (ANSES, 2012) and one qualitative risk assessment (BfR, 2022). V. parahaemolyticus and V. vulnificus but not non‐O1/non‐O139 V. cholerae, have been the subject of the risk assessments. Most studies addressed V. parahaemolyticus, and in seafood such as oysters, bloody clams, mussels, sea squirts, varieties of shrimp and different finfish. V. vulnificus specifically has only been assessed in raw oysters and a type of octopus.
TABLE 11.
A summary of published risk assessments (QMRA and other studies of interest) of Vibrio spp. in various types of seafood.
| Hazard(s) | Food(s) | Title | Reference/source | Comment |
|---|---|---|---|---|
| Vp | Cooked prawns | A semi‐quantitative seafood safety risk assessment | Sumner and Ross (2002) | Hazard/food combinations ranked using Risk Ranger |
| Vc a unspecified | Cooked prawns | |||
| Vv | Oysters | |||
| Pathogenic Vp | Raw oysters (Crassostrea virginica) | Quantitative risk assessment on the public health impact of pathogenic Vibrio parahaemolyticus in raw oysters | US FDA (2005) | The output is adapted and applied in the Vp calculator tool |
| Vv | Raw oysters (C. virginica) | Risk assessment of V. vulnificus in raw oysters | FAO and WHO (2005b) | The output is adapted and applied in the Vv calculator tool |
| Vc a O1 and O139 | Warm water shrimp | Risk assessment of choleragenic Vibrio cholerae O1 and O139 in warm‐water shrimp in international trade | FAO and WHO (2005a) | |
| Vp | Oysters (C. virginica) | An evaluation of the use of remotely sensed parameters for prediction of incidence and risk associated with Vibrio parahaemolyticus in Gulf Coast oysters (Crassostrea virginica) | Phillips et al. (2007) | Remote sensing data integrated into risk assessment |
| Vp | Bloody clams | Quantitative modeling for risk assessment of Vibrio parahaemolyticus in bloody clams in southern Thailand | Yamamoto et al. (2008) | |
| Vp | Raw horse mackerel | Quantitative risk assessment of Vibrio parahaemolyticus in finfish: a model of raw horse mackerel consumption in Japan | Iwahori et al. (2010) | |
| Vp | Raw oysters, bloody clams, raw horse mackerel | Risk assessment of Vibrio parahaemolyticus in seafood | FAO and WHO (2011) | |
| Vp | Mussels and oysters | Quantitative risk assessment of Vibrio parahaemolyticus in seafood (Évaluation du risque lié à Vibrio parahaemolyticus lors de la consommation de coquillages vivants) | ANSES (2012) | |
| Vp | Black tiger shrimps, cooked | The risk assessment of Vibrio parahaemolyticus in cooked black tiger shrimps (Penaeus monodon) in Malaysia | Sani et al. (2013) | |
| Vp | Raw oysters | A quantitative risk assessment model for Vibrio parahaemolyticus in raw oysters in Sao Paulo State, Brazil | Sobrinho et al. (2014) | |
| Vp | Bloody clams | Microbial risk assessment of Vibrio parahaemolyticus in bloody clams in Malaysia: A preliminary model from retail to consumption | Malcolm et al. (2016) | |
| Vp | Raw oysters | The risk of Vibrio parahaemolyticus infections associated with consumption of raw oysters as affected by processing and distribution conditions in Taiwan | Huang et al. (2018) | |
| Vp | Raw oysters | The risk assessment of Vibrio parahaemolyticus in raw oysters in Taiwan under the seasonal variations, time horizons, and climate scenarios | Ndraha and Hsiao (2019b) | |
| Vp | Chilled shrimps | Exposure Assessment and Sensitivity Analysis for Chilled Shrimp During Distribution: A Case Study of Home Delivery Services in Taiwan | Ndraha and Hsiao (2019a) | |
| Vp | Short mackerel | Preliminary quantitative microbial risk assessment of pathogenic Vibrio parahaemolyticus in short mackerel in Malaysia | Tan et al. (2019) | |
| Vp and Vv | Seafood | Risk assessment tools for Vibrio parahaemolyticus and Vibrio vulnificus associated with seafood | FAO and WHO (2020) | Evaluation of existing Vp and Vv risk calculators |
| Vp | Oysters | Influence of climatic factors on the temporal occurrence and distribution of total and pathogenic Vibrio parahaemolyticus in oyster culture environments in Taiwan | Ndraha and Hsiao (2021) | |
| Vp | Sea squirt (Halocynthia roretzi) | Quantitative microbial risk assessment of Vibrio parahaemolyticus food‐borne illness of sea squirt (Halocynthia roretzi) in South Korea | Kang et al. (2021) | |
| Vc a and Vv | Whip‐arm octopus | Risk assessment of vibriosis by Vibrio cholerae and Vibrio vulnificus in whip‐arm octopus consumption in South Korea | Oh et al. (2021) | |
| Vp | Shellfish | Quantitative risk Assessment of Vibrio parahaemolyticus in shellfish from retail to consumption in coastal cities of Eastern China | Ding et al. (2022) | |
| Advances in science and risk assessment tools for Vibrio parahaemolyticus and V. vulnificus associated with seafood | FAO and WHO (2021) | |||
| Vp | Shellfish (oysters and sea urchins) | Prevalence, genomic characterisation, and risk assessment of human pathogenic Vibrio species in seafood | Neetoo et al. (2022) | Only risk associated with oyster consumption only was assessed, using Risk Ranger |
| Vp, Vv and non‐cholera Vc | Seafood (oysters, mussels) | Bacterial food‐borne Vibrio infections: health risk assessment of the occurrence of Vibrio spp. (non‐cholera vibrios) in food | BfR (2022) | Qualitative assessment |
| Vp | Roasted shrimp | Quantitative risk assessment of Vibrio parahaemolyticus toxi infection associated with the consumption of roasted shrimp (Penaeus monodon) | Takoundjou et al. (2022) | |
| Vp | Oysters | Modeling the risk of Vibrio parahaemolyticus in oysters in Taiwan by considering seasonal variations, time periods, climate change scenarios, and post‐harvest interventions | Ndraha et al. (2023) | |
| Vp | Shrimp (with vinegar) | Risk reduction assessment of Vibrio parahaemolyticus on shrimp by a Chinese eating habit | Xu, Liu, et al. (2023) | |
| Vp | Grey mullet (farmed finfish) | Quantitative risk evaluation of antimicrobial‐resistant Vibrio parahaemolyticus isolated from farmed grey mullets in Singapore | Ong et al. (2023) | AMR risk assessment |
Abbreviations: AMR, antimicrobial resistance; QMRA, quantitative microbiological risk assessment; Vc, Vibrio cholerae; Vp, Vibrio parahaemolyticus; Vv, Vibrio vulnificus.
Vibrio cholerae unspecified and O1/O139 are outside the scope of ToR 5 but included for completeness.
Various tools and calculators to support risk assessment are also available as Excel files or on the web and have been described by FAO and WHO (2021). These range from tools to estimate abundance and doubling times of V. parahaemolyticus in oysters at different sites in the USA to the predicted risk of non‐cholera Vibrio infections based on exposure estimated from salinity and SSTs in the Baltic Sea.
3.10.3. Characterisation of available QMRAs (SQ5.2)
3.10.3.1. Objectives/scope
The scope of the QMRAs range from harvest to consumption, but most studies include the harvest or the retail stage only as a starting point with the initial prevalence and concentration of Vibrio bacteria. Mitigations in the harvest or initial post‐harvest processing include practices motivated by SST – considering the effects of seasons and climate change – (e.g. Ndraha & Hsiao, 2019b; US FDA, 2005), depuration (e.g. Ndraha & Hsiao, 2019b; Sobrinho et al., 2014) and washing of fish coming into port (e.g. FAO and WHO, 2011). Almost all studies evaluate the impact of time–temperature during storage at different stages and transport, and several evaluate the effect of cooking or other heating regimes (Annex I). In addition, the potential impact of different pathogen target levels on illnesses averted and percentage of harvest lost has been evaluated (FAO and WHO, 2005b, 2011; US FDA, 2005), as have more specific mitigations such as washing of fish cavities (Iwahori et al., 2010), mixing of food with different vinegars (Xu et al., 2022), different types of markets (Malcolm et al., 2016), different roasting of shrimp protocols (Takoundjou et al., 2022) and inactivation treatments such as thermal treatment, heat shock, irradiation (Ndraha & Hsiao, 2019b), ozone, UV (Sobrinho et al., 2014). The majority of QMRAs have a national or regional scope, also those by FAO and WHO (Annex I). The latter assessments opted for this approach due to data limitations and/or for illustration purposes.
3.10.3.2. Hazard identification
The hazards covered in the QMRAs were pathogenic V. parahaemolyticus, antibiotic‐resistant V. parahaemolyticus, V. cholerae, choleragenic V. cholerae O1 and O139, V. vulnificus and Vibrio spp., but assessments of non‐O1/O139 V. cholerae were lacking (Table 12 and Annex J). These hazards were evaluated in various combinations of food or food groups, i.e. bivalve molluscs (raw oysters, mussels, bloody clam, scallop) and cephalopods (raw whip‐arm octopus), finfish (raw horse mackerel, short mackerel, wild and farmed finfish, farmed grey mullet), crustaceans (warm water shrimp, raw frozen black tiger shrimp, roasted shrimps, shrimps), sea urchins and sea squirts. The general population was considered in most QMRAs, especially for V. parahaemolyticus, although the US FDA (2005) assessment for this hazard also considered the susceptible population. The target populations for V. vulnificus were risk groups and susceptible persons (FAO and WHO, 2005b; Oh et al., 2021). Only a single QMRA addressed production and conditions in Europe, in this case regions in France (ANSES, 2012).
TABLE 12.
Examples of variables and parameters expected to be relevant for a range of potential QMRAs addressing V. parahaemolyticus in oysters. The availability of this information, limitations and comments on the data gaps are also shown. The final variables will depend on the actual processes and mitigations being evaluated.
| Module | Variable | Description | Limitation | Comment |
|---|---|---|---|---|
| QMRA step: Hazard characterisation | ||||
| Dose–response | Dose–response | Beta‐Poisson model | The applicability of the model to EU conditions is not known. It is based on old and limited data, and calibrated to US epidemiology | Existing model can be used to estimate relative effectiveness of mitigations in linear dose‐range |
| QMRA step: Exposure assessment | ||||
| Harvest | Water temperature | Distribution over seasons and climate change scenarios | Data may exist but uncertain if sufficient resolution | Depending on question, the geographic/spatial resolution may be important in relation to the objectives |
| Salinity | Distribution over seasons and climate change scenarios | Data may exist but uncertain if sufficient resolution | The importance of salinity may differ with region and seafood | |
| Levels of Vp at harvest | Relationship with environmental parameters to model this or survey data needed | Quantitative relationship exists for oysters in some regions but not for other bivalves. Survey data largely missing | Relationship with environmental parameters and potential lag between change of environmental parameter and Vp levels, need to be established in European and/or other areas of production | |
| Levels of pathogenic Vp at harvest | Data on the proportion of pathogenic Vp | Limited data available for EU | Make assumptions based on literature data and evaluate uncertainty or perform survey | |
| Post‐harvest | Collection time | Distribution | Need to be collected or based on other QMRAs | Probably available among producers |
| Time out of water | Distribution | Need to be collected or based on other QMRAs | Probably available among producers | |
| Air temperature | Distribution, for seasons and future climate | Need to be collected | Meteorological data and climate models may not have sufficient resolution | |
| Growth model | Predictive model | Broth models available | Need to validate in the seafood | |
| Effect of depuration | Predictive model or log reduction data | Variable effect in the literature indicates impact of local factors | See Section 3.9.9. The effect of depuration needs to be confirmed experimentally on different strains, oysters and environmental conditions | |
| Effect of HPP, irradiation, cooking, etc | Predictive model or log reduction data | Literature data | See Section 3.9. Much data in (Spaur et al., 2020) | |
| Temperature and time transport to retail | Distribution | Need to be collected | Surveys need to cover different types of distribution chains from producer to consumer | |
| Inactivation at retail | Predictive model or log reduction data | Point estimate of die‐off rate or secondary model available | ||
| Time at retail | Distribution | Need to be collected | Literature data or collect from food business operators | |
| Temperature at retail | Distribution | Literature data | Not specifically for oysters | |
| Home preparation | Consumption preference | Proportion eating oysters raw | Probably missing | May be possible to assume based on consumption surveys/literature |
| Temperature of heat treatment | Distribution | Probably missing | May be possible to assume based on consumption surveys/literature | |
| Time of heat treatment | Distribution | Probably missing | May be possible to assume based on consumption surveys/literature | |
| Inactivation during heat treatment | Predictive inactivation model | No model used in QMRAs, but log reduction | ||
| Reduction during heat treatment | Distributions of log reductions | Alternative to inactivation model | Use the same as previous QMRAs | |
| Consumption | Serving size | Distribution | Data in some EU/national databases | Possible to use EU data |
| Frequency of consumption a | Proportion | Depending on survey type may be underestimation | Possible to use EU data | |
| Proportion of consumers a | Proportion | Depending on survey type may be underestimation | Possible to use EU data | |
| Production landing/volume b | Data depending on geographic scope | May or may not involve also imports and reflect actual consumption | ||
| Number of oysters per meal b | Distribution or constant | May not be in EU data bases | Possible to use EU or other data as an assumption | |
| Weight of an oyster b | Distribution or constant | Literature/available among producers | Should be possible to extract from literature or small survey depending on species | |
| The edible fraction b | Distribution or constant | Literature/available among producers | Should be possible to extract from literature or small survey depending on species | |
Abbreviations: QMRA, quantitative microbiological risk assessment; Vp, Vibrio parahaemolyticus.
Not required when only risk per serving is estimated but required when total cases per year or cases per some population is estimated.
Required when no consumption data are available.
3.10.3.3. Hazard characterisation
A range of health endpoints, from GE, severe illness to death have been included in the DR model for V. parahaemolyticus. Of the VFs summarised in Section 3.4, the existing DR models only relate pathogenic strains to the presence of tdh for V. parahaemolyticus while all V. vulnificus strains are considered pathogenic. The most frequently used V. parahaemolyticus DR model was developed by US FDA (2005) based on quite old human clinical feeding studies and a total of 20 subjects. Dose is expressed in pathogenic V. parahaemolyticus, with the pathogenicity characterised as ‘Kanagawa phenomenon positive’ (KP+) (see Section 3.4). This phenomenon is related to the presence of tdh genes, although several QMRAs apply this DR model while defining pathogenic strains as those possessing either tdh or trh, which implicitly assumes the same virulence associated with both VFs. Limitations of the feeding studies were pointed out: the immune status of volunteers prior to the experiment, their gender, age and health status were often not reported. When reported, study subjects were males between 25 and 40 years. Further, the dose was not administered with a food matrix, and antiacids were administered in most cases (US FDA, 2005). The absence of a food matrix is expected to lead to an underestimation of the probability of illness, since no protection from the low gastric pH is provided to the administered microorganisms. In contrast, the use of antacids would contribute to overestimating the probability, given their protective effect on bacteria from low pH. The data were fitted with a beta‐Poisson relationship, as a simplification of the hypergeometric DR model. Parameters α and β were estimated by maximum likelihood (ML), with α = 0.6 and β = 1.31 × 106 giving an ID50 (dose for 50% probability of GE) around 2.8 × 106 (Equation 1). Iwahori et al. (2010) estimated model parameters by extending the data using previously excluded feeding trials which resulted in different parameter values but concluded that the choice of DR parameters was not critical for the evaluation of control measures.
| (1) |
Epidemiological data were used to shift the location of the curve (on the x‐axis), keeping the shape of the previous V. parahaemolyticus beta‐Poisson DR (multiplying β by 27) leading to a new ID50 of 80 × 106 (US FDA, 2005). This was justified because the predictions of the original QMRA model exceeded US epidemiological data. This DR was used for estimating GE cases, both in the general and immunocompromised population. Using other epidemiological data, on culture‐confirmed cases, the probability of septicaemia from V. parahaemolyticus was estimated separately for healthy or immunocompromised individuals and the probability of death following septicaemia was estimated. The occurrence of septicaemia was modelled as an event conditional on the occurrence of illness, but independent of the dose. The proportion of immunocompromised individuals in the US population was estimated from epidemiological data. The probability of septicaemia for the immunocompromised population was estimated at 0.12, and for the healthy population at 0.0165. Probability of death after septicaemia was estimated at 0.2. The uncertainty about α and β parameters was estimated by non‐parametric bootstrap (US FDA, 2005).
Although V. vulnificus may cause mild to severe GE that may progress to septicaemia with a high mortality, the only health endpoint considered in the available DR model is septicaemia in the susceptible population (FAO and WHO, 2005b). This model was developed using US data on the estimated exposure and reported number of septicaemia cases between 1995 and 2001. The monthly mean V. vulnificus concentration in oysters in these years was estimated from sea water temperature. The number of raw oyster servings was estimated from oyster landings data, with an estimate that 50% of oysters were eaten raw and was calculated based on average oyster weight and typical number of oysters per serving. The mean meal size was assumed to be 196 g oyster flesh per serving. Around 7% of the meals were estimated to be eaten by the susceptible population. This percentage was the estimated prevalence of predisposing conditions among the adult population in the US in 1997. The monthly exposure estimate was then attributed to the monthly observed epidemiological data for the same time period to take seasonal variability into account. The level of reporting was not considered. A beta‐Poisson DR model was fitted by ML. The DR assessment assumed that all strains are equally virulent and there are no seasonal or regional changes in virulence. The best parameters in the DR model to estimate the probability of septicaemia for the immunocompromised population were α = 9.3 × 10−6 and β = 110 × 103 (FAO and WHO, 2005b). The ID50 estimate with these parameters is close to infinite values, which suggests more than 50% of the susceptible population is immune, which is unrealistic since the relationship is for the susceptible population and implies that the model cannot fully describe the DR relationship.
There are limitations associated with the available V. parahaemolyticus and V. vulnificus DR models (Figure 8). Both models are to a large extent based on quantification and epidemiological data specific to the USA, and doses estimated based on consumption in the US, and strains, oyster species and conditions around the coast of the US. Neither DR assessment has been validated against independent epidemiological data (FAO and WHO, 2020). Further, some assumptions related to pathogenic strains may not be valid (virulence, proportion of pathogenic strains to the total V. parahaemolyticus count) and strain variability and food matrix effects were not addressed. The assumptions behind the hypergeometric model are in agreement with the classical microbial mechanistic approach for DR (no threshold, single‐hit model) (Haas et al., 1999), but the exact solution may be more appropriate than the beta‐Poisson relationship (Teunis & Havelaar, 2000), in particular at low doses. Since the models are based on US epidemiological data, they reflect the distribution of human hosts, consumption, exposure and VFs in the US which may be similar to other parts of the world or not. Thus, validation on other epidemiological data, is needed. However, FAO and WHO (2020) concluded that if the ranges of expected exposures occur where DR models are linear, most often several logs lower than the ID50, then the risk prediction model is also linear, and the DR models could be applied to estimate the relative effectiveness of control measures.
FIGURE 8.
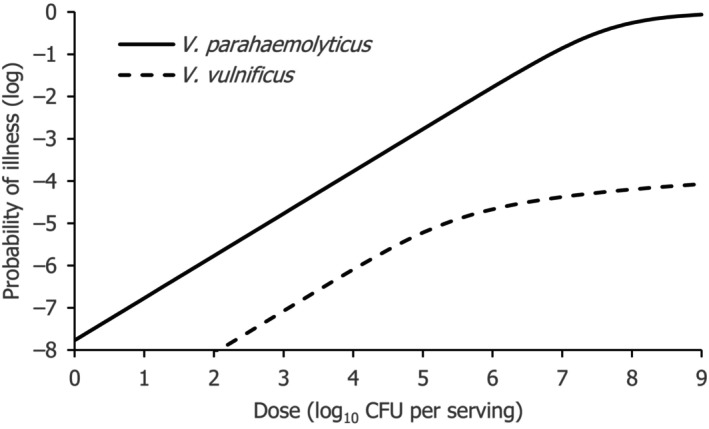
Dose–response models for Vibrio parahaemolyticus for estimating gastroenteritis cases, both in the general and immunocompromised population (US FDA, 2005) and for Vibrio vulnificus septicaemia for the susceptible population (FAO and WHO, 2005b).
3.10.3.4. Exposure assessment
Initial concentration of pathogenic vibrios in seafood
The initial levels of V. parahaemolyticus have been estimated using models either relating environmental parameters, in most cases SST, to the concentration (log10 CFU/g) in shellfish/oysters (ANSES, 2012; Ndraha et al., 2023; Ndraha & Hsiao, 2019b; Sobrinho et al., 2014), or using distributions based on the results of surveys or literature studies of concentration data in bloody clams (FAO and WHO, 2011; Huang et al., 2018; Malcolm et al., 2016; Yamamoto et al., 2008), mackerel (FAO and WHO, 2011; Iwahori et al., 2010; Tan et al., 2019), various sorts of shrimps (Sani et al., 2013; Takoundjou et al., 2022; Xu et al., 2022), sea squirt (Kang et al., 2021), shellfish (Ding et al., 2022) and grey mullets (Ong et al., 2023). For V. parahaemolyticus in oysters the most commonly used model was developed by linear regression (US FDA, 2005):
| (2) |
where and are parameters of the regression and ϵ is a normally distributed error term, .
In most cases, Equation (2) was used directly, but with data on SST from the geographic area of interest. Sobrinho et al. (2014) and Ndraha et al. (2023) developed their own relationships for oysters based on national data. The latter used a model developed using a machine learning approach (Ndraha & Hsiao, 2021), involving many climate factors with the objective to predict SST due to climate change. In a prior study they used the US FDA model with Taiwan SST data and found that the predicted risk was different compared to when using the new model, indicating that it may not be valid to apply a model developed in one geographic area to another even when using local SSTs. An early illustration of an interesting approach to generate current and large‐scale data is the study by Phillips et al. (2007), who used remote sensing to collect environmental data such as SST, turbidity, chlorophyll, later also applied in other studies (e.g. Ndraha and Hsiao (2019b)).
To estimate the dose of pathogenic Vibrio to apply in the DR models, the proportion of pathogenic V. parahaemolyticus was quantified in the US FDA (2005) QMRA based on the studies by DePaola et al. (2003) and Kaufman et al. (2003). These studies utilised a gene probe technique for enumeration of total and pathogenic V. parahaemolyticus considering only tdh but not trh genes which is a limitation. The study by DePaola et al. (2003) represented the Pacific Northwest region, while the study by Kaufman et al. (2003) represented the Mid‐Atlantic and Northeast Atlantic regions. These assumptions were based on the data by Cook et al. (2002) showing that there was no apparent difference in the percentage of tdh+ V. parahaemolyticus in oyster samples among the Gulf Coast, Mid‐Atlantic and Northeast Atlantic regions (US FDA, 2005). The percentage of pathogenic V. parahaemolyticus strains defined as a Kanagawa‐positive (KP+) or thermostable direct haemolysin‐positive (TDH+) was approximated with a Beta distribution with an average of 2.33% for the Pacific Northwest and 0.18% for Atlantic regions (US FDA, 2005). The percentage of V. parahaemolyticus‐positive samples containing pathogenic strains (tdh+ and/or trh+) is highly variable between European studies. For example, in oysters in Catalonia (Lopez‐Joven et al., 2015), between 2008 and 2010, the percentage of pathogenic strains in V. parahaemolyticus‐positive samples, was 29.5%, while in Languedoc shore in oysters, in 2008, it was 5% (Rosec et al., 2012).
An approach applying proportions of pathogenic strains, defined as either KP+/tdh+ or tdh+/trh+, to the estimated total number of V. parahaemolyticus at harvest, was used in all studies addressing pathogenic V. parahaemolyticus. The proportions were based on either the US‐FDA data e.g. in oysters (ANSES, 2012), or data from surveys, e.g. in bloody clams (Yamamoto et al., 2008), in black tiger shrimps (Sani et al., 2013), in raw oysters (Sobrinho et al., 2014).
In the two QMRAs addressing V. vulnificus , Oh et al. (2021) estimated concentrations based on the analyses of whip‐arm octopus samples from a market survey, whereas FAO and WHO (2005b) used a polynomial model, similarly to the V. parahaemolyticus model to estimate the concentration of the pathogen in raw oysters (Equation 3). All V. vulnificus detected were considered pathogenic.
| (3) |
with .
Data – time and temperature
The change of Vibrio populations along the food chain being modelled is to a large extent affected by the time and temperature during harvesting, storage, transportation and processing. The distribution of time and temperatures in the stages covered in the assessments were based on literature data, surveys, observations or targeted studies, sometimes combined with expert opinion. For instance, FAO and WHO (2011), based their estimate on the time period from retail to cooking of bloody clams based on local individual practices and expert opinion. Time and temperatures during harvesting, storage, transportation and processing were data gaps commonly reported as limitations of the assessments.
Estimation of growth
Growth, depending on the rate of cooling at harvest, or the times above growth limiting temperatures during storage further up the chain, has been estimated with predictive growth models or by estimating growth rates from occurrence data along the distribution chain.
Predictive models. The US FDA V. parahaemolyticus QMRA (US FDA, 2005) employed a simple three‐phase log linear model with no lag phase fitted to broth data reported in Miles et al. (1997) and calibrated to growth rates in oysters (Gooch et al., 2002). The calibration data were only representing 26°C and growth rates were four times slower than predicted by the Miles broth model (FAO and WHO, 2020). ANSES (2012) used a model from a more recent publication (Fernandez‐Piquer et al., 2011) covering V. parahaemolyticus growth in live oysters over a greater temperature range. The Miles model showed a strong effect of salinity on the growth rate in broths and the US FDA model assumed a growth rate corresponding to a salinity of 2.7% NaCl, a condition that may vary across different regions. Other limitations or factors that have not been extensively investigated are the effect of V. parahaemolyticus strain and oyster species on growth rates though the limited data suggest systematic differences for both (FAO and WHO, 2020). Iwahori et al. (2010) used the Miles model but with adjustment factors based on V. parahaemolyticus growth in horse mackerel, which were also applied in farmed grey mullets (Ong et al., 2023). Kang et al. (2021) inoculated sea squirt with pathogenic strains and developed primary (Baranyi) and secondary models (square root model) for their QMRA. Growth increase during transport based on mean generation times estimated from experiments simulating transport of shrimps were applied in Takoundjou et al. (2022).
For V. vulnificus, a linear model developed based on growth rate data in Gulf Coast oysters (Cook, 1994, 1997) and minimum growth temperature from Kaspar and Tamplin (1993), was used in FAO and WHO (2005b). This model was evaluated by refitting data to a square root growth model and comparing predictions with observed rates in American and Asian oysters (FAO and WHO, 2020). The evaluation concluded that the original model is appropriate, although tending towards fail‐safe predictions between 15 and 25°C, for predicting growth in at least American oysters (C. virginica) and possibly, only one data set available, also in Asian oysters (C. ariakensis). The applicability of the model to other seafood species is not evaluated. Oh et al. (2021) inoculated a mixture of V. vulnificus and V. cholerae strains, justified by similar growth rates, in whip‐arm octopus and developed primary (Baranyi) and secondary (polynomial) models for growth rate and lag phase duration.
Estimation from count data.
Data of levels of V. parahaemolyticus in bloody clams at harvest and retail indicated that growth occurred in most samples. Based on the assumption that the time from harvest to retail was around 5 h the exponential growth rate was assumed to follow a Normal distribution with mean of 0.236 h−1 and standard deviation of 0.390 h−1 (FAO and WHO, 2011; Yamamoto et al., 2008), which was used in a simple exponential growth model. This distribution implies around a 25% probability of negative growth, i.e. inactivation, which was not commented on or explained in these studies.
Estimation of inactivation
The reported demarcation between inactivation and growth during storage covers a range of storage temperatures and is likely affected by several factors since different cut‐off temperatures have been used (Huang et al., 2018). Inactivation is slow between 5 and 10°C and the definition is further complicated by problems related to detecting VBNC cells. Inactivation of V. parahaemolyticus in raw oysters was considered below a no‐growth temperature of 8–10°C in the US FDA (US FDA, 2005) risk assessment, using a single rate recorded at 3°C based on Gooch et al. (2002), which may have overestimated inactivation. Huang et al. (2018) assumed inactivation to occur below 12°C, and developed secondary models (Arrhenius, square root models) between 4 and 12°C, based on experiments in inoculated raw oysters, while Spaur et al. (2020) considered < 6°C in their systematic review of post‐harvest interventions when estimating log reductions during cold storage. Inactivation of V. vulnificus during storage was assumed at 0.041 log10 per day at normal storage conditions, 3°C (Cook et al., 2002) and was assumed to be the same within the temperatures range 0 to 13°C using the same storage times as in the V. parahaemolyticus in oysters risk assessment (FAO and WHO, 2005b).
In Sobrinho et al. (2014), the effect of depuration combined with UV or ozone treatment was estimated based on few experimental samples using a non‐parametric bootstrap approach, and log reductions described by normal distributions. Cooking or heat inactivation was considered in the consumer stage, and most studies used data on log reduction from the literature. For instance, in shrimps (Sani et al., 2013), grey mullets (Ong et al., 2023), and oysters (Ndraha et al., 2023). Malcolm et al. (2016) evaluated two heating regimes, minimally or moderately cooked bloody clams, with log reductions from the literature, and assuming the proportions of consumers applying them. Yamamoto et al. (2008) used a small survey to develop a beta distribution to describe the proportion of consumers cooking or undercooking bloody clams under the assumption that in the first case none and the second case all V. parahaemolyticus survives in the bloody clams. Tan et al. (2019) combined data from a kitchen study to estimate the maximum log reduction during cooking of short mackerel and took the minimum log reduction from the literature.
Relationships describing the log reduction of V. parahaemolyticus in shrimps treated with three different types of vinegars were developed by fitting distributions to the experimental data (Xu et al., 2022). Further, when evaluating different interventions, Ndraha et al. (2023) used distributions representing the variable log reductions of HPP, irradiation and thermal shock extracted from Spaur et al. (2020).
Estimation of removal and cross‐contamination
Tan et al. (2019) addressed the effect of gilling, gutting and washing the eviscerated cavity on reducing the number of V. parahaemolyticus during consumer preparation and used a lognormal distribution for the reduction by fitting to data from published literature (Watanabe et al., 1994). Ong et al. (2023) used the same data and developed an additional log normal distribution to describe the log reduction due to only gilling and gutting but no washing of farmed grey mullets. The reduction of V. parahaemolyticus from the gills or the surface of fish due to washing of whole fish at landing was modelled in Iwahori et al. (2010) using data in Watanabe et al. (1994). Cross‐contamination during handling was raised as a knowledge gap in several of the QMRAs but this process was not modelled in any of the QMRAs.
Estimation of consumption
Data on consumption, dietary habits, food origin and food types are very specific to the country or region of interest. In the QMRAs consumption data, i.e. serving size and frequency of consumption, were based on general national consumption surveys (e.g. raw horse mackerel – Iwahori et al., 2010, black tiger shrimps – Sani et al., 2013, bloody clams – Malcolm et al., 2016, whip‐arm octopus – Oh et al., 2021, fish and seafood products – BfR, 2022), on literature data combined with general national consumption surveys (e.g. raw oysters – Huang et al., 2018), or small surveys carried out to complement the available information (e.g. bloody clams – Yamamoto et al., 2008, roasted shrimps – Takoundjou et al., 2022, oysters – Ndraha et al., 2023).
To illustrate the approaches and types of data needed in QMRAs related to European conditions we focus on the two European risk assessments in Table 13 to examine the feasibility in European countries. In ANSES (2012), the consumption was established from a combination of data sets. The overall consumption of oysters/mussels was established from landings data, in tons of shellfish (with shell), converted into tons of edible shellfish. The relative contribution, by area of origin (7 areas of production along the coast) to the overall market was estimated from open data available on the business's websites. Imports were neglected. The seasonality by month of consumption was estimated from sales data, and further grouped by season. All oysters were assumed to be consumed raw. The average portion size was obtained from the national consumption survey (INCA1), with a mean portion size around 100 g for oysters and 56 g for mussels.
TABLE 13.
List of knowledge gaps and priorities for future research related to Vibrio spp. in seafood and aquatic environments.
| Knowledge gaps | Priorities for future research |
|---|---|
| Absence of epidemiological data on human non‐cholera vibriosis in the EU | See recommendations under Section 5 |
| Absence of representative and harmonised data on the occurrence (prevalence and level of contamination) of Vibrio spp. of PH in seafood marketed in the EU |
To establish a EU‐wide baseline survey on Vibrio spp. of PH relevance a in the most significant seafood (to be further defined in the technical specifications of this survey). Surveys should adopt harmonised protocols for sampling, analysis and characterisation of Vibrio isolates, and should be undertaken at: (i) retail stage, gathering data on the conditions of transport/storage, and origin (country of imports, FAO fishing area) (ii) primary production level, gathering data on environmental conditions at sampling sites. This survey could also support identification of sentinel sites for long‐term studies investigating Vibrio spp. trends To characterise the isolates collected in the baseline survey with as a prioritisation the determination of the proportion of seafood samples containing pathogenic Vp See also recommendations under Section 5 |
| Absence of baseline data to investigate trends linked to climate change | |
| Absence of robust data on the proportion of Vibrio isolates from seafood marketed in the EU displaying specific genetic traits associated with pathogenicity | |
| Absence of standardised, easy methods for quantitative analysis of the Vibrio spp. of PH relevance | To undertake standardisation of quantitative methods, e.g. based on MPN‐PCR, for the enumeration of Vibrio spp. of PH relevance and of the tdh+ and/or trh+ strains of Vp |
| Lack of full characterisation of the performance of available standardised methods | To produce intercomparison data for ISO and FDA methods and allow the characterisation of methods' performance on VBNC cells |
|
Differentiation of pathogenic strains within a Vibrio species is uncertain and characterisation of VFs of Vibrio spp. of PH relevance is incomplete Absence of systematic WGS typing of Vibrio spp. isolates of food and clinical origin |
To perform characterisation studies of Vibrio isolates of clinical, food and environmental origin, preferably using WGS. See recommendations under Section 5 |
| Identification of genetic traits associated with epidemic/pandemic potential of some V. parahaemolyticus strains is incomplete | To make use of machine learning approaches to identify eco‐evolutionary drivers for pandemic expansion of specific Vp strains |
| Data on AMR of Vibrio spp. in seafood in the EU (EU production and imported seafood) are incomplete | To establish a EU‐wide baseline survey on the prevalence of AMR in Vibrio spp. in seafood. Surveys should include Vibrio isolated from EU/EFTA produced aquaculture animals and imported seafood. Isolates should be phenotypically tested and subjected to WGS |
| ECOFF breakpoints for antimicrobial susceptibility testing of Vibrio spp. are currently missing | To engage in further research efforts and interlaboratory studies to determine, where missing, epidemiological cut‐offs and/or clinical breakpoints for Vibrio spp. of PH relevance, considering also other species of potential PH interest such as V. alginolyticus, V. fluvialis, V. mimicus |
| The role and weight of mobile genetic elements of vibrios in ARGs dissemination is poorly defined | To engage in basic research to define the contribution of ICEs and superintegrons of Vibrio spp. to dissemination of ARGs and to clarify relevance of HGT in aquatic environments (e.g. aquaculture) and in microbiota of the human gut |
| The role of antimicrobials residues/degradation products on AMR development/dissemination in the aquatic environments is unclear | Basic research to investigate how the persistence of low concentrations of antimicrobials and their degradation products in aquaculture contribute to AMR of Vibrio spp. |
| The understanding of persistence mechanisms of Vibrio spp. in the environment is fragmented | Basic research on Vibrio spp. attachment to surfaces and biofilm formation. Ecological studies on the interactions between Vibrio spp. and zoo−/phyto‐ plankton |
| Models correlating environmental factors and growth/survival of the Vibrio spp. of PH relevance lack validation in the EU context | To develop and validate models correlating environmental factors, especially SST and salinity, and the occurrence of Vibrio spp. of PH relevance, with priority to Vp, in EU seafood production areas |
| Lack of specific attribution studies linking role of climate and climate change with human health risk | To gather baseline data on the abundance of Vibrio of PH relevance in the environment over different seasons through a baseline study (see above) and follow‐up studies in sentinel areas to study trends associated with climate change. To gather data on the abundance of Vibrio of PH relevance in the environment during specific climatic events (e.g. heatwaves, floods) through targeted studies |
| Lack of an EU‐wide, real‐time system for prediction of conditions favourable to Vibrio spp. occurrence in seawater/seafood | To set‐up maps of environmental suitability/risk for Vibrio spp. of PH interest in Europe using high‐resolution sea surface salinity and sea surface temperature data, with a priority to coastal regions with shellfish harvesting areas in the EU. Following validation, this predictive tool would allow to direct sampling efforts to periods/areas of highest risk |
| Studies testing effectiveness of interventions on seafood, in particular seafood other than oysters, are scarce | To engage in studies on control measures to better characterise the relationship between the intervention and the reduction in levels of Vibrio spp. of PH relevance in various types of seafood. Such interventions include HPP, relaying and icing |
| Predictive microbiology models (and limitations thereof) | To develop and validate predictive microbiology models for Vibrio spp. of PH interest with priority to Vp growth and inactivation under relevant conditions in the seafood products considered for RA |
| Dose–response models (and limitations thereof) | To assess the applicability to the EU conditions and, if needed, update the current dose–response models with priority to Vp, considering information from outbreak investigations (consumption and abundance), strain virulence, pathogenicity and host susceptibility |
| Prioritisation of data/knowledge gaps | Development of an exploratory QMRA, with the objective to identify the most important gaps affecting risk to guide and allocate research |
Abbreviations: AMR, antimicrobial resistance; ARG, antimicrobial resistance genes; FAO, Food and Agriculture Organization of the United Nations; FDA, Food and Drug Administration (United States of America); HGT, horizontal gene transfer; HPP, high‐pressure processing; ICE, integrating conjugative elements; ISO, International Organization for Standardization; MPN, most probable number; PCR, polymerase chain reaction; PH, public health; QMRA, quantitative microbiological risk assessment; RA, risk assessment; SST, sea surface temperature; VBNC, viable but non‐culturable; VF, virulence factor; Vp, V. parahaemolyticus; WGS, whole genome sequencing.
V. parahaemolyticus, V. vulnificus and non‐O1/non‐O139 V. cholerae.
BfR (2022) established short‐term consumption (relevant for acute risk) at 3.9 g/kg BW per day for marine/freshwater fish, 4.7 g/kg BW per day for shellfish and 2.6 g/kg BW per day for crustacean based on a national nutrition survey II (NVSII) (2005–2006). The frequency of consumers and frequency of consumption was also retrieved from this survey and showed that the majority (75%) of respondents never consume raw fish/seafood products. The frequency of consumption of such products indicated differences by age group and gender. Limitations highlighted were that the consumption data were based on studies conducted 15 years ago, and important changes in population, habits and products can have occurred, and the breakdown by age groups is inconclusive due to too small groups. Surveys in other European countries can be accessed in the EFSA Comprehensive European Food Consumption Database. 58 Dietary surveys results are very dependent on the methodology, e.g. 24‐h dietary recalls underestimate foods that are not regularly consumed, as in the case of raw oysters (Diogène et al., 2023).
3.10.3.5. Risk characterisation
Risk characterisation endpoints were the probability of illness (per serving, per person and year, or per a certain number of servings), or number of cases (per year or per 100,000 population, Annex I). The effects of the evaluated factors and control measures were in some studies reported as the relative change in risk due to the mitigation (e.g. Iwahori et al., 2010; Ndraha et al., 2023). Uncertainties were in most studies not addressed explicitly in quantitative terms but as limitations or data gaps. Since production, preparation and consumption practices vary with the type of seafood, results are presented per seafood group.
Bivalve molluscs (oysters, mussels, bloody clams)
Thee risk assessments addressing V. parahaemolyticus in oysters highlight the importance of the initial total levels at harvest and the proportion of pathogenic strains but other variables that reduce or increase growth also have an impact on the predicted number of illnesses. These variables include shortening the time to refrigeration of oyster after harvest (US FDA, 2005), as well as temperatures during storage and transport (Sobrinho et al., 2014). A common result is the predicted effect of season on the occurrence of V. parahaemolyticus, with higher levels during summer in oysters (ANSES, 2012; Ding et al., 2022; Ndraha et al., 2023; US FDA, 2005) and in mussels (ANSES, 2012). For instance, in France an increased risk to consumers due to a higher temperature in the sea water was predicted for oysters (ANSES, 2012), and a 2°C higher than average temperature during summer increased the risk by a factor of 10. Further, 12 h storage at room temperature increased the risk by another factor of 6. Huang et al. (2018) suggested that maintaining temperatures < 12°C during oyster processing and transport would significantly reduce the annual number of V. parahaemolyticus infections. Thus, the potential effects of higher temperatures due to climate change are relevant and have been investigated in several studies. Ndraha et al. (2023) investigated different climate scenarios defined as different SSPs (see Section 3.8.1) and mitigations. Ndraha et al. (2023) showed that the SSP selected, the future time span considered, as well as different mitigations influenced the predicted risk of illness due to consumption of oysters in South Korea. In the time period 2041–2060 the risk per serving is predicted to increase by 18% to 145% depending on the season and SSP. Thermal processing or HPP were the most efficient mitigations also under the scenario of increasing global temperatures.
V. vulnificus numbers in oysters at harvest depend on SSTs and salinity, and numbers at consumption are influenced by temperature. Different process target levels achieved by post‐harvest mitigation strategies, e.g. mild heat treatment, freezing with extended frozen storage, HPP, were assessed and found to have the capability to reduce risk substantially, from 32 reported cases to one case every 6 year or 8 cases per year depending on the target level, and with a 10‐fold uncertainty of predictions (FAO and WHO, 2005b). The predicted effect of reducing the time oysters were unrefrigerated after harvest had less effect but the magnitudes were dependent on the assumptions made.
In Malaysia, the mean V. parahaemolyticus risk per undercooked meal of bloody clams was estimated as 6.1 × 10−4 (Malcolm et al., 2016). A sensitivity analysis indicated that the initial pathogenic V. parahaemolyticus counts and holding time (retail and home) were the major variable parameters that contributed to the variability in risk of illness per serving. A study in Thailand estimated the risk of illness due to V. parahaemolyticus following consumption of bloody clams to 5.6 × 10−4 per person and year, and the fraction of people that do not boil the clams sufficiently was the primary factor increasing risk (Yamamoto et al., 2008).
Cephalopods and tunicates
Two studies from South Korea addressed this seafood group. The mean probability of illness per person and day due to V. parahaemolyticus following consumption of sea squirt was 4.0 × 10−9, and the impact of time and temperature after harvest was highlighted as important for the magnitude of risk (Kang et al., 2021). The only V. vulnificus assessment in this group considered whip‐arm octopus and concluded that the risk per serving in South Korea was low (9 × 10−15, Oh et al., 2021).
Shrimps
Risk characterisations with V. parahaemolyticus in this group covered cooked or roasted black tiger shrimps or unspecified shrimps from Malaysia, Cameroon and China, respectively. The risk was generally considered as low, with yearly 1.3 cases per 100,000 population (Sani et al., 2013), and < 2 cases per million servings (Takoundjou et al., 2022), not least due to the heat treatments. Treatment of inoculated shrimps for up to 2 min with different recipes of vinegar were found to reduce risk up to a factor of 2.6 (Xu et al., 2022).
Finfish
Different scenarios were evaluated for raw horse mackerel and in the best‐case scenario a contaminated meal contained a mean number of 9 pathogenic V. parahaemolyticus and was associated with a mean risk of 5.6 × 10−6 (Iwahori et al., 2010). This scenario was characterised by washing of whole fish at landing, storage and transportation in clean water, no increase in temperature before preparation, and washing of fish visceral cavities during preparation. The presence of contaminated water had a negligible effect, no wash at landing increased risk by 7%, exposure to higher temperatures before preparation increased risk by 50%, and no wash during preparation by 1400%. For short mackerel, besides cooking, washing was considered the main factor impacting on the magnitude of V. parahaemolyticus risk (Tan et al., 2019).
The main variables influencing the risk outputs following consumption of farmed grey mullets in Singapore was initial concentration of V. parahaemolyticus, and the cooking and washing of the fish cavity both when considering AMR and non‐AMR V. parahaemolyticus (Ong et al., 2023).
3.10.4. Evaluation of data and knowledge gaps (SQ5.3)
The selection of the scope of a risk assessment is a risk management decision and involves considerations of the magnitude of the problem (e.g. the number of food‐borne cases, consumption patterns, production volumes), various management considerations (e.g. evaluation of control measures, preparedness for climate change, need to rank hazards, foods or management options), the feasibility to perform an assessment (i.e. availability of relevant data, unless the purpose for performing the assessment is to define the needed data). Knowledge gaps and data needs for a Vibrio QMRA will in part be general but will also be very specific for the risk management question to be addressed.
QMRAs have been developed for both V. parahaemolyticus and V. vulnificus suggesting that these are possible subjects for an assessment also at the EU level (Annex I). Both species are of interest from food safety and climate change perspectives and the question arises how much new data and knowledge are needed to develop QMRAs relating to the EU. The majority of QMRAs addressed V. parahaemolyticus in oysters, and to a lesser extent in other seafoods. The latter reflecting that the main food source of infection can vary between countries putting different emphasis on different seafoods, e.g. the US (oysters) and Japan (sashimi and sushi). FAO and WHO (2021) concluded that the use of the framework and parameters from the V. parahaemolyticus model was generally successful to achieve a QMRA for V. vulnificus in terms of generating useful insights, e.g. the effect of interventions, and that when additional data were available some aspects of the risk assessment model could be validated (FAO and WHO, 2005b). However, considering the scope and limitations of the original QMRA publications for both V. parahaemolyticus and V. vulnificus, it is necessary to refine the approaches and to use country‐ or region‐specific data to draw conclusions outside the USA.
The general knowledge and data gaps, related to both Vibrio species, include the need to validate DR models using epidemiological data from countries other than the USA, and to update the DR model with new information on virulence, pathogenicity, and host susceptibility. However, the existing models have been deemed sufficient to evaluate the relative effectiveness of mitigations. Further requirements are data on the production of the seafood of interest, i.e. processes, storage conditions, transportation conditions and its relative contribution to total consumption of this seafood in the relevant EU population. In addition, in relation to consumers in the EU, data on consumption frequencies and serving sizes of the seafood of interest, and preparation practices, consumer preferences and storage conditions are needed.
The specific data gaps, i.e. related to assessments of a specific Vibrio species and seafood combination, are data on the occurrence of the relevant pathogenic Vibrio in the water and the seafood of interest and the relation to factors in the environment. Predictive growth and inactivation models that have been validated in the seafoods of interest, as well as the proportion of pathogenic to total V. parahaemolyticus strains in the seafood are also needed.
These are the most important knowledge and data gaps but to what extent they affect the usefulness of a risk assessment is a matter of the intended scope. However, except perhaps for exploratory QMRAs, i.e. screening level risk assessments to identify specific knowledge and data gaps, or to develop estimates of relative risk levels relative to baselines, it is considered that the primary target species for an EU QMRA need realistically be V. parahaemolyticus, the reasons being both the known PH impact of this species and the availability of data. However, even for V. parahaemolyticus, data on the occurrence (prevalence and levels) in seafoods are limited in the EU context. For oysters the relationship describing the levels based on SST can be applied but the validity for areas in Europe are not known. Table 13 summarises the knowledge and data gaps that can be foreseen for a range of assessment questions related to a QMRA topic considered relevant at EU level; V. parahaemolyticus in bivalve molluscs or oysters. The scope of the assessment could be to evaluate the baseline risk and the effect of mitigations, especially in a climate change scenario. In view of the many gaps, it may be worthwhile to attempt an initial, exploratory, QMRA, with the objective to identify the most important gaps where studies are needed and those where proxy data can be used.
3.10.5. Concluding remarks
In total, 28 risk assessments 59 have been identified for V. parahaemolyticus in seafood (e.g. oysters, bloody clams, mussels, sea squirts, varieties of shrimp and different finfish) and V. vulnificus in raw oysters and a type of octopus; no risk assessment was identified for non‐O1/non‐O139 V. cholerae in seafood.
The geographical scope of the majority of QMRAs is national or regional. Two studies covered conditions in Europe. Most QMRAs rely heavily on the first US FDA assessment of V. parahaemolyticus in raw oysters, which early on was applied also to V. vulnificus. These QMRAs apply a stochastic Monte Carlo simulation approach where distributions are used to address mainly variability. Uncertainties were mainly addressed as limitations or data gaps and not explicitly in quantitative terms.
The scope of the QMRAs ranged from harvest – mostly included as a starting point for Vibrio prevalence and concentration – to consumption. For Vibrio in oysters, this initial occurrence is commonly estimated through regression relationship between mean numbers and SST. Changes in numbers of Vibrio spp. in seafood are estimated from time and temperature at different stages in the food chain considering growth (using predictive models or experimental data) and sometimes inactivation and removal/washing (log reductions).
The beta‐Poisson DR models for V. parahaemolyticus and V. vulnificus are developed based on old experimental data and/or epidemiological data from the USA and the applicability of the models to EU conditions are not known. In addition, the V. parahaemolyticus model is only based on tdh+ strains. The health endpoints are for the general or susceptible populations and range from GE to severe illness for V. parahaemolyticus and septicaemia for V. vulnificus. Other endpoints or populations are evaluated by multiplying with the proportions of illness cases affected by these outcomes or the proportion of the population of interest to the whole population based on epidemiological and census data.
Consumption data, dietary habits, food origin and food types are very specific to the country or region of interest and were based on several types of consumption surveys and/or additional consumer related data to estimate exposure.
Risk assessment endpoints were the probability of illness (per serving, per person and year, or per a certain number of servings), or number of cases (per year or per 100,000 population). The effects of mitigations were commonly reported as factors representing the change in risk due to the mitigation (i.e. relative risks).
Mitigations during harvest or directly after included practices motivated by SST – considering the effects of seasons and climate change – and by pathogen occurrence, such as time to refrigeration and depuration. In later stages, practices evaluated were time–temperature during storage and cooking/heating, the effect of setting different pathogen target levels on the number of illnesses averted and the subsequent percentage of non‐compliant harvest lost (harvest above the target levels), as well as more specific mitigations (e.g. adding vinegars, washing of fish cavities).
The impact of climate change scenarios on risk and the effect of different mitigations was only evaluated for V. parahaemolyticus in bivalve molluscs, most notably oysters.
QMRAs have been developed for V. parahaemolyticus and V. vulnificus, and these are possible hazards for an assessment at the EU level, although it is necessary to refine the approaches and to use country‐ or region‐specific data to draw conclusions outside the US. The selection of the scope of a risk assessment in terms of which Vibrio species, seafoods and questions to address, is subject to several considerations and is ultimately a risk management decision.
A QMRA relevant in an EU context would be V. parahaemolyticus in bivalve molluscs (oysters), evaluating the baseline risk and the effect of mitigations, especially in a climate change scenario. The foreseeable specific knowledge and data gaps for a range of risk assessment questions on this topic are presented. An initial, exploratory, QMRA, to identify the most relevant gaps where studies are needed and those where proxy data can be used is suggested.
3.11. Areas for future research on Vibrio spp. in seafood and aquatic environments (AQ6)
The knowledge gaps and the recommendations/priorities for future research related to Vibrio spp. in seafood and aquatic environments are shown in Table 13.
4. CONCLUSIONS
AQ1/For the relevant Vibrio spp., what is their occurrence and concentration in seafood, available analytical methods, pathogenicity to humans and virulence factors, as well as AMR and persistence mechanisms in different environments?
The Vibrio spp. currently of highest public health relevance in the EU through consumption of seafood are Vibrio parahaemolyticus, Vibrio vulnificus and non‐O1/non‐O139 Vibrio cholerae (referred to as the relevant Vibrio spp.). Other species as Vibrio alginolyticus, Vibrio fluvialis and Vibrio mimicus may occasionally lead, particularly in individuals with underlying health conditions, to seafood‐associated infections.
- Based on the available data, prevalence and concentrations of the relevant Vibrio spp. in seafood placed or intended to be placed on the EU market are estimated as follows:
-
○Across seafood categories, the V. parahaemolyticus pooled prevalence estimate is 19.6% (95% CI 13.7–27.4), with the highest estimates for bivalve molluscs (27.8%; 95% CI 18.6–39.5) and gastropods (28.8%; 95% CI 10.5–58.3). About one out of five (18.4%; 95% CI 11.1–29.1) of V. parahaemolyticus‐positive samples contained pathogenic (i.e. tdh+ and/or trh+) strains. V. parahaemolyticus pooled mean concentration in bivalve molluscs is 1.91 log10 CFU/g or MPN/g (95% CI 0.68–3.14), while in crustaceans is 3.33 log10 CFU/g (single study).
-
○The V. vulnificus pooled prevalence estimate is 6.1% (95% CI 3.0–11.8) and is highest for bivalve molluscs with 9.9% (95% CI 4.0–22.6); the pooled mean concentration in bivalve molluscs is 1.84 log10 CFU/g or MPN/g (95% CI 2.33–6.01).
-
○Non‐choleragenic V. cholerae pooled prevalence estimate is 4.1% (95% CI 2.4–6.9) and the mean concentration in cockles is 1.34 MPN/g (single study).
-
○No data were retrieved in either tunicates or echinoderms.
-
○
Standardised microbiological methods are available for the detection of the relevant Vibrio spp. in seafood and for the enumeration of V. parahaemolyticus and V. vulnificus. These methods rely on culturing and include molecular tests for species identification and/or for the detection of genes associated with pathogenicity. Alternative approaches are available for the detection, enumeration and identification (e.g. LAMP‐PCR, quantitative, digital and viability PCR, MALDI–TOF MS). For characterisation, serotyping and PFGE have been replaced by sequencing technologies [e.g. (cg)MLST schemes]. WGS is progressively more applied for full characterisation of clinical Vibrio isolates and in outbreak investigation but currently has limited use in official food control activities in the EU.
-
Pathogenic Vibrio species possess a wide array of virulence factors (VFs) that allow the colonisation and spread in the hosts. Their virulence is multifactorial; although with different impact, some VFs may be present in all species (i.e. capsule, flagellar mobility and pili), while other VFs are specific to certain species or strains within one species. The outcome of an infection with Vibrio species is determined both by bacterial and host factors.
-
○
V. parahaemolyticus mainly leads to acute GE, also in healthy individuals. Its pathogenicity is significantly associated with the haemolysins TDH and TRH, which are well‐established pathogenicity markers. The type 3 secretion system 2 (T3SS2) has been also associated with pathogenic strains.
-
○
V. vulnificus infection are relatively rare and affect mainly individuals with underlying health conditions, possibly leading to sepsis and death. This species possesses several VFs but, as reliable discrimination of pathogenic from non‐pathogenic strains is lacking, to date all V. vulnificus strains are considered potentially pathogenic.
-
○
V. cholerae non‐O1/non‐O139 is generally associated with self‐limited GE or mild extraintestinal symptoms. However, in susceptible individuals, strains can cause more severe infections, sepsis and death. No single pathogenicity marker has been identified so far.
-
○
A large spectrum of AMRs, of which some are known as intrinsic resistances, has been reported in the few studies on Vibrio spp. isolates from seafood or food‐borne infections in Europe. The AMRs most frequently detected were ampicillin (70%–100%; seven studies) and streptomycin (30%–70%; six studies) for V. parahaemolyticus and colistin (87%–100%; four studies), ampicillin (4%–75%; five studies) and streptomycin (11%–68%; four studies) for non‐O1/non‐O139 V. cholerae.
Antimicrobial resistance genes (ARGs) associated with mobile genetic elements, conferring resistance to various ß‐lactam types, quinolones, sulfonamides, aminoglycosides, tetracyclines, folate pathway inhibitors and phenicols, have been detected in Vibrio spp. Resistances against last‐resort antimicrobials as carbapenems and 3rd/4th generation cephalosporins associated with mobile elements are increasingly found in the relevant Vibrio spp., and detection in imported seafood isolates has been reported.
The main persistence mechanisms of the relevant Vibrio species in the aquatic environment include the VBNC state, biofilm formation on biotic and abiotic surfaces, persister cells, anti‐grazing strategies to avoid protozoan predation, and association with other aquatic organisms acting as reservoirs.
AQ2/What are the factors in the aquatic environments and in seafood that influence occurrence and growth of the relevant Vibrio spp., and affect transmission of their virulence and resistance determinants?
Survival and growth of the relevant Vibrio spp. in the aquatic environments and in food are affected by temperature, (sea)water salinity, solar and UV radiation (extrinsic factors); pH, water activity of the food, nutrient content (intrinsic factors) and predation, parasitism and commensalism (implicit factors). Temperature is the most relevant driver for Vibrio abundance in the aquatic environment and in food, followed, in the environment, by salinity. Complex interactions among environmental factors make region‐specific environmental models and validations required.
Transmission of virulence or resistance determinants is affected by the presence of chitin and phages, and by factors of the aquatic ecosystem such as antimicrobials and heavy metals.
AQ3/What is the impact of climate change on the occurrence and levels of the relevant Vibrio spp. in water environments and seafood?
Climate change induces a shift towards conditions in the aquatic environment suitable and more conducive for Vibrio growth and persistence. It affects: (i) the geographic distribution of the coastal areas suitable for Vibrio spp., with areas characterised by brackish/low‐salinity waters (e.g. Baltic Sea, transitional waters of the Baltic and the North Sea, the Black Sea) and coastal areas with large riverine inputs at higher risk; (ii) the temporal distribution of conditions suitable for Vibrio spp.; (iii) the frequency, distribution and potentially intensity of extreme weather events which may provide conditions favourable to Vibrio spp. Climate change may also affect the structure of the Vibrio populations in the aquatic environments or speed up the evolution or selection of new Vibrio variants.
Although not specifically addressed by current models, it is anticipated that the occurrence and levels of the relevant Vibrio spp. in seafood will increase both globally and in Europe in response to coastal warming and extreme weather events as heatwaves, especially in low‐salinity/brackish waters.
AQ4/What are the prevention and control measures along the seafood chain for the relevant Vibrio spp.?
Maintaining the cold chain is important to prevent vibrios' growth in seafood. This is particularly critical for seafoods intended to be consumed raw.
A mild thermal treatment of oysters in water at 50°C with or without thermal shock, (flash) freezing followed by long‐term frozen storage, HPP using industrially feasible conditions or irradiation reduce vibrios in seafood.
Depuration under controlled conditions, although with variable reductions, is a post‐harvest processing treatments for the segment of the market preferring consumption of live oysters.
AQ5/What are the risk assessment modelling options for Vibrio spp. in seafood and which are the knowledge gaps and data needed to perform a risk assessment on the PH impact of the relevant Vibrio spp. in seafood at the EU level?
The identified QMRA addressed V. parahaemolyticus in seafood such as oysters, bloody clams, mussels, sea squirts, shrimp and finfish, and V. vulnificus in raw oysters and a type of octopus. No risk assessment addressed non‐O1/non‐O139 V. cholerae in seafood. The majority of QMRAs had a national or regional scope and two covered conditions in Europe. Most rely heavily on the first US FDA assessment of V. parahaemolyticus in raw oysters, which was also applied to V. vulnificus. Their scope ranged from harvest to consumption. Changes in numbers of Vibrio spp. in seafood are estimated from time–temperature at different stages in the food chain considering growth and sometimes inactivation and removal/washing.
The beta‐Poisson DR models for V. parahaemolyticus (based on tdh+ strains) and V. vulnificus have limitations and the applicability to conditions in EU is unknown. The endpoints are the probability of illness for the general or susceptible populations and range from GE to severe illness for V. parahaemolyticus and septicaemia for V. vulnificus. Other endpoints or populations are evaluated by applying proportions to these outcomes based on epidemiological data.
Consumption data, dietary habits, food origin and food types are very specific to the country or region of interest and were based on several types of consumption surveys and/or additional consumer related data to estimate exposure.
Mitigations during harvest or directly after, included practices motivated by sea surface temperature (SST) – considering the effects of seasons and climate change – and by pathogen occurrence, such as time to refrigeration and depuration. In later stages, practices evaluated were time–temperature during storage and cooking/heating, the effect of setting different pathogen target levels on the number of illnesses averted and the subsequent percentage of non‐compliant harvest lost (harvest above the target levels), as well as more specific mitigations (e.g. adding vinegars, washing of fish cavities). The impact of climate change and different mitigations was only evaluated for V. parahaemolyticus in bivalve molluscs, most notably oysters.
A QMRA relevant in the EU context would be V. parahaemolyticus in bivalve molluscs (oysters), evaluating the baseline risk and the effect of mitigations, especially in a climate change scenario.
AQ6/What are areas for future research on Vibrio spp. in seafood and aquatic environments?
A key research priority is to establish an EU‐wide baseline survey for the relevant Vibrio spp. in relevant seafood products, including at the primary production and retail stages. This survey will support: (i) gathering of representative and harmonised data on the relevant Vibrio spp.; (ii) definition of sentinel sites at primary production level to investigate temporal trends in Vibrio spp. occurrence; (iii) obtaining a representative collection of food isolates for characterisation (detection of VFs and AMR); (iv) establishing a baseline for future reference in relation to the effect of climate change.
Other research needs include improving analytical methods (for detection, quantification and AMR testing) and their comparability, improve understanding of Vibrio VFs and discrimination of pathogenic strains, gathering data on Vibrio abundance during specific climatic events, reassess DR models, develop/validate models on the occurrence of Vibrio spp. (particularly V. parahaemolyticus) and environmental factors in the EU context, and develop an exploratory QMRA to identify the most important gaps and guide research.
5. RECOMMENDATIONS
To develop a case definition for human ‘vibriosis’ at EU level and to consider vibriosis for compulsory reporting.
To systematically report data on Vibrio spp. occurrence in seafood collected in national monitoring programmes to the EU system for the monitoring and collection of information on zoonoses (Zoonoses Directive 2003/99/EC).
To systematically characterise V. parahaemolyticus isolates of clinical and food origin for pathogenicity (tdh and trh genes) and V. cholerae isolates of clinical and food origin for serotype and/or presence of cholera toxin genetic determinants.
To characterise by WGS a selection of isolates of Vibrio spp. of PH relevance of clinical, food and environmental origin to allow the implementation of genomic surveillance.
To arrange computational resources to sustain the development and long‐term operability of EU‐wide Vibrio suitability maps operating with high‐resolution data of sea surface temperature and sea surface salinity.
To survey resistances against MIAs associated with mobile elements in Vibrio spp. isolates from seafood.
Abbreviations
- AMR
antimicrobial resistance
- AQ
assessment question(s)
- ARG
antimicrobial resistance genes
- BIOHAZ
EFSA Panel on Biological Hazards
- CC
clonal complex
- CDC
Centers for Disease Control and Prevention (United States of America)
- CFU
colony forming unit(s)
- cgMLST
core genome multi‐locus sequence type
- CI
confidence interval
- CIA
critically important antimicrobial
- CLSI
Clinical and Laboratory Standards Institute
- CTX
cholera toxin
- DR
dose–response
- ECDC
European Centre for Disease Prevention and Control
- ECOFF
epidemiological cut‐off
- ELS
extensive literature search
- ENA
the European Nucleotide Archive
- ESBL
extended‐spectrum‐ß‐lactamase
- EUCAST
European Committee on Antimicrobial Susceptibility Testing
- FAO
Food and Agriculture Organization of the United Nations
- FBO
food‐borne outbreak(s)
- FDA
Food and Drug Administration (United States of America)
- FSANZ
Food Standards Australia New Zealand
- Fur
ferric uptake regulator
- GE
gastroenteritis
- GHG
greenhouse gases
- [HA]/protease
haemagglutinin protease
- HACCP
hazard analysis and critical control points
- HGT
horizontal gene transfer
- HlyA
haemolysin A
- HPP
high‐pressure processing
- I 2
intraclass correlation
- ICE
integrating conjugative elements
- ID50
dose for 50% probability of gastroenteritis
- IPCC
Intergovernmental Panel on Climate Change
- ISO
International Organization for Standardization
- KP
Kanagawa phenomenon
- LAMP
loop‐mediated isothermal amplification
- LOD
limit of detection
- LOQ
limit of quantification
- MALDI–TOF
matrix‐assisted laser desorption ionisation–time of flight
- MARTX
multifunctional autoprocessing repeats‐in‐toxin
- MGE
mobile genetic elements
- MIA
medically important antimicrobial
- MIC
minimum inhibitory concentration
- ML
maximum likelihood
- MLST
multi‐locus sequence typing
- MLVA
multi‐locus variable number tandem repeat analysis
- MLVST
multi‐virulence‐locus sequence typing
- MPN
most probable number
- MS
mass spectrometry
- NA
not applicable
- NAG‐ST
heat‐stable enterotoxin
- NCBI
the National Center for Biotechnology Information
- NCCOS
National Centers for Coastal Ocean Science
- NGS
next generation sequencing
- NOAA
National Oceanic and Atmospheric Administration
- NRL
National Reference Laboratory
- PAI
pathogenicity island
- PCR
polymerase chain reaction
- PFGE
pulsed‐field gel electrophoresis
- pFv
fish virulence plasmid
- PH
public health
- QMRA
Quantitative Microbiological Risk Assessment
- qPCR
quantitative PCR
- QS
quorum sensing
- RA
risk assessment
- RASFF
Rapid Alert System for Food and Feed
- RID
rho‐inactivation domain
- RMLE
restricted maximum likelihood estimator
- RTE
ready‐to‐eat
- SQ
sub‐question(s)
- SSP
shared socioeconomic pathway
- SST
sea surface temperature
- ST
sequence type
- T3SS
type III Secretion System
- T6SS
type VI Secretion System
- TCP
toxin‐co‐regulated pilus
- TDH
thermostable direct haemolysin
- ToR
Terms of Reference
- TRH
TDH‐related haemolysin
- US FDA
United States Food and Drug Administration
- UV
ultraviolet
- VBNC
viable but non‐culturable
- VF
virulence factor
- VFDB
virulence factor database
- WG
Working Group
- wgMLST
whole genome multi‐locus sequence type
- WGS
whole genome sequencing
- WHO
World Health Organization
CONFLICT OF INTEREST
If you wish to access the declaration of interests of any expert contributing to an EFSA scientific assessment, please contact interestmanagement@efsa.europa.eu.
REQUESTOR
EFSA
QUESTION NUMBER
EFSA‐Q‐2022‐00826
COPYRIGHT FOR NON‐EFSA CONTENT
EFSA may include images or other content for which it does not hold copyright. In such cases, EFSA indicates the copyright holder and users should seek permission to reproduce the content from the original source.
PANEL MEMBERS
Ana Allende, Avelino Alvarez‐Ordóñez, Declan Bolton, Sara Bover‐Cid, Marianne Chemaly, Alessandra De Cesare, Lieve Herman, Friederike Hilbert, Konstantinos Koutsoumanis, Roland Lindqvist, Maarten Nauta, Romolo Nonno, Luisa Peixe, Giuseppe Ru, Marion Simmons, Panagiotis Skandamis and Elisabetta Suffredini.
WAIVER
In accordance with Article 21 of the Decision of the Executive Director on Competing Interest Management a waiver was granted to an expert of the Working Group. Pursuant to Article 21(6) of the aforementioned Decision, the concerned expert was allowed to take part in the discussions and in the drafting of the scientific output but was not allowed to take up the role of chair, vice‐chair or rapporteur within that time frame. Any competing interests are recorded in the respective minutes of the meetings of the working group of the BIOHAZ Panel.
MAP DISCLAIMER
The designations employed and the presentation of material on any maps included in this scientific output do not imply the expression of any opinion whatsoever on the part of the European Food Safety Authority concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries.
Supporting information
Protocol for the assessment of the public health aspects of Vibrio spp. related to the consumption of seafood in the EU
Food Standards Australia New Zealand information request
Vibrio parahaemolyticus, Vibrio vulnificus and non‐O1/non‐O139 Vibrio cholerae human cases associated with seafood consumption in Europe
Analytical methods for the detection and enumeration of potentially enteropathogenic Vibrio spp. in seafood
Antimicrobial resistances of the relevant Vibrio spp. from clinical and seafood associated isolates from Europe as reported in official reports, reviews and primary research papers
Effect of temperature on Vibrio spp. in seafood
Vibrio parahaemolyticus outbreak associated with an increase of seawater temperature (Pacific Northwest, 1997)
Overview of the prevention and control measures along the seafood chain for Vibrio spp.
Characterisation of relevant (quantitative) microbial risk assessment of Vibrio spp. in various types of seafood
ACKNOWLEDGEMENTS
The Panel wishes to thank the following for the support provided to this scientific output: Jens‐Andre Hammerl (hearing expert), Jan Baele (EC DG‐SANTE RASFF), the Pathogens in Food Database consortium (EFSA grant agreement GP/EFSA/BIOHAW/2023/05) and Kenneth Mulligan and Gianluca Rossi (EFSA staff). The Panel wishes to acknowledge all European competent institutions, Member State bodies and other organisations that provided data for this scientific output.
APPENDIX A. Uncertainty analysis
TABLE A.1.
Potential sources of uncertainty linked to specific AQ or SQ in the assessment of the public health aspects of Vibrio spp. related to the consumption of seafood in the EU.
| AQ or SQ | Source or location of the uncertainty | Nature or cause of the uncertainty |
|---|---|---|
| SQ1.1 | Incomplete identification of Vibrio species of public health relevance (beyond Vp, Vv and non‐O1/non‐O139 Vc) |
It is possible that not all available information on human vibriosis cases due to seafood consumption has been retrieved. Multiple data sources (databases, literature, EU member state reports) were used to collect available data and the knowledge of the WG members was consulted. Intrinsic to epidemiological observations, the available information may not contain the complete data due to a systematic underestimation, as human cases may not be further investigated nor reported. Significant underestimation of vibriosis cases in the EU is also expected due to the lack of a specific surveillance system in the EU. Furthermore, the available information may not contain accurate data due to misattribution of cases to different/major species or misattribution of the exposure route (case being attributed to water exposure while being food‐borne). Detection and hence reporting of certain species may be biased by the availability of recognised methods or by their established association with human disease. Finally, climate change may affect the occurrence of Vibrio species in seafood reported as occasional and sporadic cause of disease in individual with predisposing conditions, hence affecting their PH relevance. |
| SQ1.2 | Incompleteness of retrieved information | It is possible that not all available relevant information on Vibrio occurrence in seafood has been retrieved through the literature review. To overcome this, a systematic review was used with sensitive search strings and the retrieved studies were cross‐checked with published review papers. |
| Inaccuracy of retrieved information due to the literature screening and data extraction | Inaccuracy intrinsic to the study methodology and the data sources (i.e. the retrieved publications) may affect several aspects of the data, including key outcomes, as reported prevalence and counts, or descriptors, as sampling period, area, food origin, analytical methods. To limit this source of uncertainty, only studies addressing surveys data were selected (e.g. studies dealing with methods' validation using naturally contaminated samples were excluded) and individual data on tested and positive/enumerated samples were retrieved for each seafood group and type. Bias assessment was performed at data extraction level. | |
| Possible bias of the results |
Representative sampling in terms of production areas and production period at primary production level is not used in the retrieved studies. Due to the possible bias of sampling in high‐risk areas and during the warmer seasons in the studies, it is expected that the prevalence and counts are overestimated. Data at harvest and quantitative data are limited and not all seafood are well covered (in imports, origin, etc). |
|
| Quality of reporting in the studies | The quality of reporting was sometimes limited as stated in Section 3.2 and this impacts the quality and precision of the results. | |
| SQ1.3–1.4 | Incompleteness of information |
It is possible that not all available information on methods used for Vibrio spp. has been retrieved. Multiple data sources (databases, literature, EU member state reports) were used to collect available data and the knowledge of the WG members was consulted. It is possible that not all strengths, weaknesses, opportunities and threats associated with the contribution of analytical methods to RA have been captured. |
| SQ1.5 | Incompleteness of retrieved information | It is possible that not all available information on pathogenicity and VFs has been retrieved. Multiple data sources (databases, literature, EU member state reports) were used to collect available data and the knowledge of the WG members was consulted. |
| Scarcity of data, uncertainty to what extent representative |
Uncertainty intrinsic to the studies are present with regard to the significance of data considering that some VFs and corresponding genetic markers have been evaluated only in few instances on isolates from a restricted number/type of sources (limited variability and data not reproduced independently). Limited amount of data on the specific role of TRH in Vp pathogenicity (independently from TDH) and scarcity of data on genetic markers associated with pathogenicity of non‐O1/non‐O139 Vc |
|
| SQ1.6–1.7 | Incompleteness of information | It is possible that not all of the available information was retrieved concerning European data on AMR of Vp, Vv, and Vc from retail and primary production. Multiple data sources (databases, literature, EU member state reports) were used to collect data and, additionally, the knowledge of the WG members and of hearing experts was included. |
| Inaccuracy of retrieved information due to data reporting | The AMR profiles of clinical strains of Vp and Vc may only partially reflect the AMR of strains causing food‐borne infections as specific information on source of infection are lacking (travel associated cases, seawater contact, and other unknown origin represent confounding factors). | |
| Scarcity of data and limitations of studies | The number of European studies on AMR of vibrios is low, particularly for Vv; relevant phenotypes may not have been identified. Significant differences of reported AMR prevalence between studies may depend on variations of the investigated matrices, but can also be attributed to methodological factors (choice of different antibacterial compounds, application of different antimicrobial susceptibility tests, i.e. disc diffusion/MIC). Furthermore, the interpretative criteria (e.g. CLSI, EUCAST) for resistance or susceptibility may have changed over time. | |
| SQ1.8 | Incompleteness of information | It is possible that not all available information on Vibrio persistence has been retrieved. Multiple data sources (databases, literature, EU member state reports) were used to collect available data and the knowledge of the WG members was consulted. |
| SQ2.1 | Incompleteness of information | It is possible that not all relevant information has been retrieved, which could leave out factors affecting growth and survival of Vibrio in the environment and in food or could underestimate/overestimate the role of certain factors. To reduce this uncertainty, results from the ELS for reviews were complemented by snowballing, non‐systematic search, and expert knowledge. |
| SQ2.2 | Incompleteness of information | It is possible that not all available information on factors affecting transmission of VFs and AMR has been retrieved. Multiple data sources (databases, literature, EU member state reports) were used to collect available data and the knowledge of the WG members was consulted. |
| AQ3 | Incompleteness of data and information regarding climate change |
With regard to data sets and sources of information used to infer the relationship between climate and vibrio risk, in Europe there is a limited availability of high‐quality of data, particularly for epidemiology data sets. The complexity of interactions between environmental factors can make it difficult to isolate the effects of climate change e.g. on growth of these bacteria from other factors. Few attribution studies, which tie particular outcomes (e.g. outbreaks) to specific climate change induced events, are available. Model uncertainty is inherent to the tools adopted for climate change study, and uncertainties associated with future climate projections can impact the reliability of predictions. Additionally, there may be a lack of integration between different types of models, hindering comprehensive assessments. |
| AQ4 | Scarcity of studies testing interventions on seafood, in particular seafood other than oysters | Most studies assessed the impact of interventions on reduction of vibrios in oysters while few studies are available in other seafoods, such as shrimps. Even for oysters, many studies tested the interventions in oyster meat or oyster homogenate. |
| AQ5 | Incompleteness of information | It is possible that not all available relevant information on Vibrio QMRA and seafood has been retrieved which could limit the analysis in terms of missing some useful approaches, models, or data. |
| Scarcity of data, uncertainty to what extent representative for conditions in Europe | The data and knowledge gaps have been listed in Section 3.11 |
Abbreviations: AMR, antimicrobial resistance; AQ, assessment question; CLSI, Clinical and Laboratory Standards Institute; ELS, extensive literature search; EUCAST, the European Committee on Antimicrobial Susceptibility Testing; MIC, minimum inhibitory concentration; PH, public health; QMRA, quantitative microbiological risk assessment; RA, risk assessment; SQ, sub‐question; TDH, thermostable direct haemolysin; TRH, TDH‐related haemolysin; Vc, Vibrio cholerae; VF, virulence factor; Vp, Vibrio parahaemolyticus; Vv, Vibrio vulnificus; WG, Working Group; WGS, whole genome sequencing.
ANNEX A. Protocol for the assessment of the public health aspects of Vibrio spp. related to the consumption of seafood in the EU
A.1.
Annex A is available under the Supporting Information section on the online version of the scientific output.
ANNEX B. Food Standards Australia New Zealand information request
B.1.
Annex B is available under the Supporting Information section on the online version of the scientific output.
ANNEX C. Vibrio parahaemolyticus, Vibrio vulnificus and non‐O1/non‐O139 Vibrio cholerae human cases associated with seafood consumption in Europe
C.1.
Annex C is available under the Supporting Information section on the online version of the scientific output.
ANNEX D. Analytical methods for the detection and enumeration of potentially enteropathogenic Vibrio spp. in seafood.
D.1.
Annex D is available under the Supporting Information section on the online version of the scientific output.
ANNEX E. Antimicrobial resistances of the relevant Vibrio spp. from clinical and seafood associated isolates from Europe as reported in official reports, reviews and primary research papers
E.1.
Annex E is available under the Supporting Information section on the online version of the scientific output.
ANNEX F. Effect of temperature on Vibrio spp. in seafood
F.1.
Annex F is available under the Supporting Information section on the online version of the scientific output.
ANNEX G. Vibrio parahaemolyticus outbreak associated with an increase of seawater temperature (Pacific Northwest, 1997)
G.1.
Annex G is available under the Supporting Information section on the online version of the scientific output.
ANNEX H. Overview of the prevention and control measures along the seafood chain for Vibrio spp.
H.1.
Annex H is available under the Supporting Information section on the online version of the scientific output.
ANNEX I. Characterisation of relevant (quantitative) microbial risk assessment of Vibrio spp. in various types of seafood
I.1.
Annex I is available under the Supporting Information section on the online version of the scientific output.
EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards) , Koutsoumanis, K. , Allende, A. , Alvarez‐Ordóñez, A. , Bolton, D. , Bover‐Cid, S. , Chemaly, M. , De Cesare, A. , Herman, L. , Hilbert, F. , Lindqvist, R. , Nauta, M. , Nonno, R. , Peixe, L. , Ru, G. , Simmons, M. , Skandamis, P. , Baker‐Austin, C. , Hervio‐Heath, D. , … Suffredini, E. (2024). Public health aspects of Vibrio spp. related to the consumption of seafood in the EU. EFSA Journal, 22(7), e8896. 10.2903/j.efsa.2024.8896
Adopted: 12 June 2024
Annexes A‐I are available under the Supporting Information section
Notes
https://geoportal.ecdc.europa.eu/vibriomapviewer/. The model used for the Vibrio viewer has been calibrated to the Baltic Region in Northern Europe and might not apply to other worldwide settings prior to validation.
Commission Regulation (EU) No 16/2011 of 10 January 2011 laying down implementing measures for the Rapid alert system for food and feed. OJ L 6, 11.1.2011, pp. 7–10.
Directive 2003/99/EC of the European Parliament and of the Council of 17 November 2003 on the monitoring of zoonoses and zoonotic agents, amending Council Decision 90/424/EEC and repealing Council Directive 92/117/EEC OJ L 325, 12.12.2003, pp. 31–40.
Including SCI‐Expanded, SSCI, AHCI, CPCI‐P, CPCI‐SSH, ESCI, CCR,Expanded, IC. The search combined the records retrieved using the following two search strings: (a) ALL = (case*) AND TS = (alginolyticus OR fluvialis OR hollisae OR mimicus OR metschnikovii OR moetus OR cincinnatiensis OR furnissi); (b) TS = (vibrio OR vibrios OR Vibrionaceae OR vibriosis OR parahaemolyticus OR vulnificus OR cholerae) AND TS = (case* OR outbreak*) AND [search string #3 from Table A.4 of the protocol] AND [search string #4 from Table A.4 of the protocol].
Conducted under grant agreement GP/EFSA/BIOHAW/2023/05 (Pathogens in foods database: extension with Vibrio and parasites) with as beneficiaries Instituto Politécnico de Bragança (IPB) as coordinator and Agence nationale de sécurité sanitaire de l'alimentation, de l'environnement et du travail (Anses) as co‐beneficiary.
Used as a synonym for cell count or cell density.
Results were expressed as (i) overall prevalence of samples containing tdh/trh‐positive V. parahaemolyticus in all tested samples, (ii) proportion of samples containing tdh/trh‐positive V. parahaemolyticus upon V. parahaemolyticus–positive samples, or (iii) proportion tdh/trh‐positive isolates upon tested isolates of the V. parahaemolyticus species.
Pathogens‐in‐Foods (PIF) is a database of systematically formatted occurrence data of the most important microbiological hazards in foods randomly surveyed in Europe. The database contains data extracted from peer‐reviewed articles published since 2000, retrieved through systematic literature searches and using a publicly available protocol describing the search and screening process. The foodborne pathogens currently covered are: Bacillus cereus, Campylobacter spp., Clostridium perfringens, Listeria monocytogenes, Salmonella spp., Shiga toxin‐producing Escherichia coli, Staphylococcus aureus, Yersinia enterocolitica, Cryptosporidium spp., Giardia spp., Toxoplasma gondii, Hepatitis A virus, Hepatitis E virus and Norovirus. In 2025, PIF database will be populated with data on the occurrence of Vibrio parahaemolyticus, Vibrio vulnificus and non‐O1/non‐O139 Vibrio cholerae in seafood and of fish parasites of public health importance (including Anisakids) in fishery products.
The virulence factor database (VFDB – http://www.mgc.ac.cn/VFs/) is an integrated and comprehensive online resource for curating information about VFs of bacterial pathogens Chen, L., Yang, J., Yu, J., Yao, Z., Sun, L., Shen, Y., & Jin, Q. (2005). VFDB: a reference database for bacterial virulence factors. Nucleic Acids Res, 33(Database issue), D325‐328. https://doi.org/10.1093/nar/gki008.
GenBank – https://www.ncbi.nlm.nih.gov/genbank/; RefSeq – https://www.ncbi.nlm.nih.gov/refseq/; SRA – https://www.ncbi.nlm.nih.gov/sra.
Bulgaria n = 2, Croatia n = 1, Cyprus n = 1, France n = 6, Germany n = 1, Greece n = 1, Italy n = 15, The Netherlands n = 1, Poland n = 2, Portugal n = 2, Slovenia n = 1, Spain n = 3, and Sweden n = 1.
Data from countries that were EU Members in the period (2010–2023) considered in the ELS, EU accession candidates, or members of the European single market were also extracted; Switzerland n = 1, Türkiye n = 7, and the United Kingdom n = 1
FAO area 37, with 26 publications and 160 entries, 115 of which related to the Adriatic Sea, Ionian Sea and Sardinia.
FAO area 27, with 8 publications and 32 entries, 17 of which related to Portuguese waters.
FAO areas 61, 71 and 87, with 5 publications and 9 entries.
Regulation (EU) 2017/625 of the European Parliament and of the Council of 15 March 2017 on official controls and other official activities performed to ensure the application of food and feed law, rules on animal health and welfare, plant health and plant protection products.
Commission Implementing Regulation (EU) 2019/627 of 15 March 2019 laying down uniform practical arrangements for the performance of official controls on products of animal origin intended for human consumption in accordance with Regulation (EU) 2017/625 of the European Parliament and of the Council and amending Commission Regulation (EC) No 2074/2005 as regards official controls.
ISO 21872‐1:2017. Microbiology of the food chain – Horizontal method for the determination of Vibrio spp. Part 1: Detection of potentially enteropathogenic Vibrio parahaemolyticus, Vibrio cholerae and Vibrio vulnificus. International Organization for Standardization, Geneva, Switzerland.
FDA BAM (2004). Bacteriological Analytical Manual Chapter 9: Vibrio. Food & Drug Administration, Bacteriological Analytical Manual, Washington DC. https://www.fda.gov/food/laboratory‐methods‐food/bam‐chapter‐9‐vibrio, accessed on the 14 May 2024.
MFLP‐37 (2019). Detection, isolation, and enumeration of Vibrio parahaemolyticus and/or Vibrio vulnificus in Seafood. Laboratory Procedures for the Microbiological Analysis of Foods. Volume 3. Government of Canada.
MFLP‐102 (2017). Identification of Vibrio parahaemolyticus colonies by real‐time polymerase chain reaction. Laboratory Procedures for the Microbiological Analysis of Foods. Volume 3. Government of Canada.
ISO/TS 21872‐2:2020. Microbiology of the food chain – Horizontal method for the determination of Vibrio spp. Part 2: Enumeration of total and potentially enteropathogenic Vibrio parahaemolyticus in seafood using nucleic acid hybridization. International Organization for Standardization, Geneva, Switzerland.
FDA BAM (2004). Bacteriological Analytical Manual Chapter 9: Vibrio. Food & Drug Administration, Bacteriological Analytical Manual, Washington DC. https://www.fda.gov/food/laboratory‐methods‐food/bam‐chapter‐9‐vibrio, accessed on the 14 May 2024.
FDA BAM (2004). Bacteriological Analytical Manual Chapter 9: Vibrio. Food & Drug Administration, Bacteriological Analytical Manual, Washington DC. https://www.fda.gov/food/laboratory‐methods‐food/bam‐chapter‐9‐vibrio, accessed on the 14 May 2024.
GB 4789.7‐2013. National Food Safety Standard – Microbiological Examination of Food Hygiene – Examination of Vibrio parahaemolyticus. National Standard of the People's Republic of China.
GB 4789.7‐2020. National Food Safety Standard – Microbiological Examination of Food Hygiene – Examination of Vibrio vulnificus. National Standard of the People's Republic of China.
https://pubmlst.org/organisms/vibrio‐parahaemolyticus; https://pubmlst.org/organisms/vibrio‐cholerae.
As of January 2024, publicly available V. parahaemolyticus genomes have reached a total of 10,000 genomes (https://www.ncbi.nlm.nih.gov/assembly/?term=vibrio%20parahaemolyticus; NHI, National Library of Medicine, consulted on 15.1.2024).
Genome sequences available in VFDB, often corresponding to the first genome obtained for V. cholerae O395 (Feng, L., Reeves, P. R., Lan, R., Ren, Y., Gao, C., Zhou, Z., Ren, Y., Cheng, J., Wang, W., Wang, J., Qian, W., Li, D., & Wang, L. (2008). A recalibrated molecular clock and independent origins for the cholera pandemic clones. PLOS ONE, 3(12), e4053. https://doi.org/10.1371/journal.pone.0004053), V. parahaemolyticus RIMD 2210633 (Makino, K., Oshima, K., Kurokawa, K., Yokoyama, K., Uda, T., Tagomori, K., Iijima, Y., Najima, M., Nakano, M., Yamashita, A., Kubota, Y., Kimura, S., Yasunaga, T., Honda, T., Shinagawa, H., Hattori, M., & Iida, T. (2003). Genome sequence of Vibrio parahaemolyticus: a pathogenic mechanism distinct from that of V cholerae. Lancet, 361(9359), 743–749. https://doi.org/10.1016/s0140‐6736(03)12659‐1, ibid.) and V. vulnificus CMCP6 (Kim, Y. R., Lee, S. E., Kim, C. M., Kim, S. Y., Shin, E. K., Shin, D. H., Chung, S. S., Choy, H. E., Progulske‐Fox, A., Hillman, J. D., Handfield, M., & Rhee, J. H. (2003). Characterisation and pathogenic significance of Vibrio vulnificus antigens preferentially expressed in septicemic patients. Infect Immun, 71(10), 5461–5471. https://doi.org/10.1128/iai.71.10.5461‐5471.2003, ibid.) and YJ016 (Chen, C. Y., Wu, K. M., Chang, Y. C., Chang, C. H., Tsai, H. C., Liao, T. L., Liu, Y. M., Chen, H. J., Shen, A. B., Li, J. C., Su, T. L., Shao, C. P., Lee, C. T., Hor, L. I., & Tsai, S. F. (2003). Comparative genome analysis of Vibrio vulnificus, a marine pathogen. Genome Res, 13(12), 2577–2587. https://doi.org/10.1101/gr.1295503).
CIAs, according to WHO. (2019). Critically important antimicrobials for human medicine, 6th rev. https://cdn.who.int/media/docs/default‐source/gcp/who‐mia‐list‐2024‐lv.pdf?sfvrsn=3320dd3d_2, are antimicrobial classes which meet both criteria C1 (the antimicrobial class is the sole, or one of limited available therapies, to treat serious bacterial infections in people) and C2 [the antimicrobial class is used to treat infections in people caused by either (1) bacteria that may be transmitted to humans from non‐human sources or (2) bacteria that may acquire resistance genes from non‐human sources].
Antimicrobial authorised for use in human medicine and therefore listed on the WHO CIA List. Medically important antimicrobials are categorised on the WHO CIA List, according to specific criteria, as either critically important, highly important or important for human medicine.
Resistance to polymyxin B/colistin is intrinsic in V. vulnificus and non‐O1/non‐O139 V. cholerae and has been used in an enrichment medium for these two species adding the two antimicrobials Massad, G., & Oliver, J. D. (1987b). New selective and differential medium for Vibrio cholerae and Vibrio vulnificus. Appl Environ Microbiol, 53(9), 2262–2264. https://doi.org/10.1128/aem.53.9.2262‐2264.1987. The resistance is based on modification of the bacterial LPS decreasing its negatively charged surface and involves the alm operon (almG gene encoding a glycyltransferase, Henderson, J. C., Herrera, C. M., & Trent, M. S. (2017). AlmG, responsible for polymyxin resistance in pandemic Vibrio cholerae, is a glycyltransferase distantly related to lipid A late acyltransferases [Article]. Journal of Biological Chemistry, 292(51), 21205–21215. https://doi.org/10.1074/jbc.RA117.000131) and other LPS modifying genetic determinants Herrera, C. M., Henderson, J. C., Crofts, A. A., & Trent, M. S. (2017). Novel coordination of lipopolysaccharide modifications in Vibrio cholerae promotes CAMP resistance. Mol Microbiol, 106(4), 582–596. https://doi.org/10.1111/mmi.13835. Not all isolates express the resistance.
https://mic.eucast.org/ accessed on 19 April 2024.
A coastal inlet formed by the partial submergence of an unglaciated river valley. It is a drowned river valley that remains open to the sea.
e.g. in a temperature range of 15–20°C, an increment of 1°C in water temperature increased the probability of detecting V. vulnificus by 4%, while temperatures > 20°C, the same increment provided a tenfold increase of the detection probability.
ISO 21872‐1:2017. Microbiology of the food chain – Horizontal method for the determination of Vibrio spp. Part 1: Detection of potentially enteropathogenic Vibrio parahaemolyticus, Vibrio cholerae and Vibrio vulnificus. International Organization for Standardization, Geneva, Switzerland.
The scenarios are referred to as SSPx‐y, where ‘SSPx’ refers to the Shared Socio‐economic Pathway or ‘SSP’ describing the socio‐economic trends underlying the scenario, and ‘y’ refers to the approximate level of radiative forcing (in watts per square metre, or W/m2) resulting from the scenario in the year 2100.
Extreme event attribution aims to elucidate the link between global climate change, extreme weather events, and the harms experienced on the ground by people, property and nature.
QMRA's and assessments presenting elements of a QMRA.
REFERENCES
- Aagesen, A. M. , & Häse, C. C. (2012). Sequence analyses of type IV pili from Vibrio cholerae, Vibrio parahaemolyticus, and Vibrio vulnificus . Microbial Ecology, 64(2), 509–524. 10.1007/s00248-012-0021-2 [DOI] [PubMed] [Google Scholar]
- Abanto, M. , Gavilan, R. G. , Baker‐Austin, C. , Gonzalez‐Escalona, N. , & Martinez‐Urtaza, J. (2020). Global expansion of Pacific Northwest Vibrio parahaemolyticus sequence type 36. Emerging Infectious Diseases, 26(2), 323–326. 10.3201/eid2602.190362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aberkane, S. , Compain, F. , Barraud, O. , Ouédraogo, A. S. , Bouzinbi, N. , Vittecoq, M. , Jean‐Pierre, H. , Decré, D. , & Godreuil, S. (2015). Non‐O1/non‐O139 Vibrio cholerae avian isolate from France cocarrying the bla VIM‐1 and bla VIM‐4 genes. Antimicrobial Agents and Chemotherapy, 59(10), 6594–6596. 10.1128/aac.00400-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- AESAN (Spanish Agency for Food Safety and Nutrition) . (2024). Report of the Scientific Committee of the Spanish Agency for Food Safety and Nutrition (AESAN) on the microbiological criteria for Vibrio cholerae, as additional control measures at border control posts, applicable to imported frozen prawns and other fishery products . Food Risk Assess Europe, AESAN‐2024‐001. 32 pp. 10.2903/fr.efsa.2024.FR-0027 [DOI]
- Ahmed, A. O. E. , Ali, G. A. , Hassen, S. S. , & Goravey, W. (2022). Vibrio albensis bacteremia: A case report and systematic review. IDCases, 29, e01551. 10.1016/j.idcr.2022.e01551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiyar, S. E. , Gaal, T. , & Gourse, R. L. (2002). rRNA promoter activity in the fast‐growing bacterium Vibrio natriegens . Journal of Bacteriology, 184(5), 1349–1358. 10.1128/jb.184.5.1349-1358.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akolkar, J. K. , & Matson, J. S. (2023). Stress responses in pathogenic Vibrios and their role in host and environmental survival. Advances in Experimental Medicine and Biology, 1404, 213–232. 10.1007/978-3-031-22997-8_11 [DOI] [PubMed] [Google Scholar]
- Albini, E. , Orso, M. , Cozzolino, F. , Sacchini, L. , Leoni, F. , & Magistrali, C. F. (2022). A systematic review and meta‐analysis on antimicrobial resistance in marine bivalves. Frontiers in Microbiology, 13, 1040568. 10.3389/fmicb.2022.1040568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaro, C. , Aznar, R. , Garay, E. , & Alcaide, E. (1988). R‐plasmids in environmental Vibrio cholerae non‐O1 strains. Applied and Environmental Microbiology, 54(11), 2771–2776. 10.1128/aem.54.11.2771-2776.1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amato, E. , Riess, M. , Thomas‐Lopez, D. , Linkevicius, M. , Pitkanen, T. , Wolkowicz, T. , Rjabinina, J. , Jernberg, C. , Hjertqvist, M. , MacDonald, E. , Antony‐Samy, J. K. , Bjerre, K. D. , Salmenlinna, S. , Fuursted, K. , Hansen, A. , & Naseer, U. (2022). Epidemiological and microbiological investigation of a large increase in vibriosis, northern Europe, 2018. Eurosurveillance, 27(28), 2101088. 10.2807/1560-7917.Es.2022.27.28.2101088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews, L. (2004). Strategies to control Vibrios in molluscan shellfish. Food Protection Trends, 24(2), 70–76. [Google Scholar]
- Andrews, L. S. , DeBlanc, S. , Veal, C. D. , & Park, D. L. (2003). Response of Vibrio parahaemolyticus 03:K6 to a hot water/cold shock pasteurization process. Food Additives and Contaminants, 20(4), 331–334. 10.1080/0265203031000060896 [DOI] [PubMed] [Google Scholar]
- Andrews, L. S. , Park, D. L. , & Chen, Y. P. (2000). Low temperature pasteurization to reduce the risk of vibrio infections from raw shell‐stock oysters. Food Additives and Contaminants, 17(9), 787–791. 10.1080/026520300415336 [DOI] [PubMed] [Google Scholar]
- ANSES (Agence nationale de sécurité sanitaire de l'alimentation, de l'environnement et du travail) . (2012). Évaluation du risque lié à Vibrio parahaemolyticus lors de la consummation de coquillages vivants. Avis de l'Anses. Saisine n° 2010‐SA‐0301 . https://www.anses.fr/fr/system/files/BIORISK2010sa0301Ra.pdf
- Archer, E. J. , Baker‐Austin, C. , Osborn, T. J. , Jones, N. R. , Martinez‐Urtaza, J. , Trinanes, J. , Oliver, J. D. , González, F. J. C. , & Lake, I. R. (2023). Climate warming and increasing Vibrio vulnificus infections in North America. Scientific Reports, 13(1), 3893. 10.1038/s41598-023-28247-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayrapetyan, M. , Williams, T. C. , Baxter, R. , & Oliver, J. D. (2015). Viable but nonculturable and persister cells coexist stochastically and are induced by human serum. Infection and Immunity, 83(11), 4194–4203. 10.1128/iai.00404-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker‐Austin, C. , Jenkins, C. , Dadzie, J. , Mestanza, O. , Delgado, E. , Powell, A. , Bean, T. , & Martinez‐Urtaza, J. (2020). Genomic epidemiology of domestic and travel‐associated Vibrio parahaemolyticus infections in the UK, 2008–2018. Food Control, 115, 107244. 10.1016/j.foodcont.2020.107244 [DOI] [Google Scholar]
- Baker‐Austin, C. , & Oliver, J. D. (2018). Vibrio vulnificus: New insights into a deadly opportunistic pathogen. Environmental Microbiology, 20(2), 423–430. 10.1111/1462-2920.13955 [DOI] [PubMed] [Google Scholar]
- Baker‐Austin, C. , Oliver, J. D. , Alamo, M. , Ali, A. , Waldor, M. K. , Qadri, F. , & Martinez‐Urtaza, J. (2018). Vibrio spp. infections. Nature Reviews Disease Primers, 4(19), 1–19. 10.1038/s41572-018-0005-8 [DOI] [PubMed] [Google Scholar]
- Baker‐Austin, C. , Stockley, L. , Rangdale, R. , & Martinez‐Urtaza, J. (2010). Environmental occurrence and clinical impact of Vibrio vulnificus and Vibrio parahaemolyticus: A European perspective. Environmental Microbiology Reports, 2(1), 7–18. 10.1111/j.1758-2229.2009.00096.x [DOI] [PubMed] [Google Scholar]
- Baker‐Austin, C. , Trinanes, J. , Gonzalez‐Escalona, N. , & Martinez‐Urtaza, J. (2017). Non‐cholera vibrios: The microbial barometer of climate change. Trends in Microbiology, 25(1), 76–84. 10.1016/j.tim.2016.09.008 [DOI] [PubMed] [Google Scholar]
- Baker‐Austin, C. , Trinanes, J. A. , Salmenlinna, S. , Lofdahl, M. , Siitonen, A. , Taylor, N. G. H. , & Martinez‐Urtaza, J. (2016). Heat wave‐associated Vibriosis, Sweden and Finland, 2014. Emerging Infectious Diseases, 22(7), 1216–1220. 10.32032/eid2207.151996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker‐Austin, C. , Trinanes, J. A. , Taylor, N. G. H. , Hartnell, R. , Siitonen, A. , & Martinez‐Urtaza, J. (2013). Emerging Vibrio risk at high latitudes in response to ocean warming. Nature Climate Change, 3(1), 73–77. 10.1038/nclimate1628 [DOI] [Google Scholar]
- Baron, S. , Larvor, E. , Chevalier, S. , Jouy, E. , Kempf, I. , Granier, S. A. , & Lesne, J. (2017). Antimicrobial susceptibility among urban wastewater and wild shellfish isolates of non‐O1/non‐O139 Vibrio cholerae from La Rance Estuary (Brittany, France). Frontiers in Microbiology, 8, 1637. 10.3389/fmicb.2017.01637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benitez, J. A. , & Silva, A. J. (2016). Vibrio cholerae hemagglutinin (HA)/protease: An extracellular metalloprotease with multiple pathogenic activities. Toxicon, 115, 55–62. 10.1016/j.toxicon.2016.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berney, M. , Weilenmann, H. U. , Simonetti, A. , & Egli, T. (2006). Efficacy of solar disinfection of Escherichia coli, Shigella flexneri, Salmonella Typhimurium and Vibrio cholerae . Journal of Applied Microbiology, 101(4), 828–836. 10.1111/j.1365-2672.2006.02983.x [DOI] [PubMed] [Google Scholar]
- BfR (German Federal Institute for Risk Assessment) . (2022). Bacterial foodborne Vibrio infections: Health risk assessment of the occurrence of Vibrio spp. (non‐cholera vibrios) in food. BfR Opinion No 011/2022 of 2013 April 2022, 37 pp. In: BfR‐Stellungnahmen. Bundesinst. für Risikobewertung. http://www.bfr.bund.de/cm/2349/bacterial‐foodborne‐vibrio‐infections‐health‐risk‐assessment‐of‐the‐occurrence‐of‐vibrio‐spp‐in‐food.pdf
- Bian, S. , Zeng, W. , Li, Q. , Li, Y. , Wong, N. K. , Jiang, M. , Zuo, L. , Hu, Q. , & Li, L. (2020). Genetic structure, function, and evolution of capsule biosynthesis loci in Vibrio parahaemolyticus . Frontiers in Microbiology, 11, 546150. 10.3389/fmicb.2020.546150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bioteau, A. , Huguet, K. , Burrus, V. , & Banerjee, S. (2018). Genome sequence of a Canadian Vibrio parahaemolyticus isolate with unique mobilizing capacity. Genome Announcements, 6(24), e00520‐18. 10.1128/genomeA.00520-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisharat, N. , Cohen, D. I. , Maiden, M. C. , Crook, D. W. , Peto, T. , & Harding, R. M. (2007). The evolution of genetic structure in the marine pathogen, Vibrio vulnificus . Infection, Genetics and Evolution, 7(6), 685–693. 10.1016/j.meegid.2007.07.007 [DOI] [PubMed] [Google Scholar]
- Blackwell, K. D. , & Oliver, J. D. (2008). The ecology of Vibrio vulnificus, Vibrio cholerae, and Vibrio parahaemolyticus in North Carolina estuaries. Journal of Microbiology, 46(2), 146–153. 10.1007/s12275-007-0216-2 [DOI] [PubMed] [Google Scholar]
- Blanco‐Abad, V. , Ansede‐Bermejo, J. , Rodriguez‐Castro, A. , & Martinez‐Urtaza, J. (2009). Evaluation of different procedures for the optimized detection of Vibrio parahaemolyticus in mussels and environmental samples. International Journal of Food Microbiology, 129(3), 229–236. 10.1016/j.ijfoodmicro.2008.11.028 [DOI] [PubMed] [Google Scholar]
- Böer, S. I. , Heinemeyer, E. A. , Luden, K. , Erler, R. , Gerdts, G. , Janssen, F. , & Brennholt, N. (2013). Temporal and spatial distribution patterns of potentially pathogenic Vibrio spp. at recreational beaches of the German North Sea. Microbial Ecology, 65(4), 1052–1067. 10.1007/s00248-013-0221-4 [DOI] [PubMed] [Google Scholar]
- Boonyawantang, A. , Mahakarnchanakul, W. , Rachtanapun, C. , & Boonsupthip, W. (2012). Behavior of pathogenic Vibrio parahaemolyticus in prawn in response to temperature in laboratory and factory. Food Control, 26(2), 479–485. 10.1016/j.foodcont.2012.02.009 [DOI] [Google Scholar]
- Bowley, J. , Baker‐Austin, C. , Porter, A. , Hartnell, R. , & Lewis, C. (2021). Oceanic hitchhikers – Assessing pathogen risks from marine microplastic. Trends in Microbiology, 29(2), 107–116. 10.1016/j.tim.2020.06.011 [DOI] [PubMed] [Google Scholar]
- Boyd, E. F. , Cohen, A. L. V. , Naughton, L. M. , Ussery, D. W. , Binnewies, T. T. , Stine, O. C. , & Parent, M. A. (2008). Molecular analysis of the emergence of pandemic Vibrio parahaemolyticus . BMC Microbiology, 8, 110. 10.1186/1471-2180-8-110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehm, T. T. , Berneking, L. , Martins, M. S. , Dupke, S. , Jacob, D. , Drechsel, O. , Bohnert, J. , Becker, K. , Kramer, A. , Christner, M. , Aepfelbacher, M. , Schmiedel, S. , Rohde, H. , & German Vibrio Study, G . (2021). Heatwave‐associated vibrio infections in Germany, 2018 and 2019. Eurosurveillance, 26(41), 2002041. 10.2807/1560-7917.Es.2021.26.41.2002041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briet, A. , Helsens, N. , Delannoy, S. , Debuiche, S. , Brisabois, A. , Midelet, G. , & Granier, S. A. (2018). NDM‐1‐producing Vibrio parahaemolyticus isolated from imported seafood. Journal of Antimicrobial Chemotherapy, 73(9), 2578–2579. 10.1093/jac/dky200 [DOI] [PubMed] [Google Scholar]
- Brisabois, A. , Svanevik, C. , Price‐Hayward, M. , Bortolaia, V. , Leoni, F. , Granier, S. , Latini, M. , Magistrali, C. , Lunestad, B.‐T. , Peyrat, M.‐B. , Grastilleur, C. , & Sanaa, M. (2019). ASK network for antimicrobial resistance in seafood as common ground for knowledge exchange . https://zenodo.org/records/3592267
- Brumfield, K. D. , Usmani, M. , Chen, K. E. M. , Gangwar, M. , Jutla, A. S. , Huq, A. , & Colwell, R. R. (2021). Environmental parameters associated with incidence and transmission of pathogenic Vibrio spp. Environmental Microbiology, 23(12), 7314–7340. 10.1111/1462-2920.15716 [DOI] [PubMed] [Google Scholar]
- Brumfield, K. D. , Usmani, M. , Santiago, S. , Singh, K. , Gangwar, M. , Hasan, N. A. , Netherland, M., Jr. , Deliz, K. , Angelini, C. , Beatty, N. L. , Huq, A. , Jutla, A. S. , & Colwell, R. R. (2023). Genomic diversity of Vibrio spp. and metagenomic analysis of pathogens in Florida Gulf coastal waters following hurricane Ian. mBio, 14(6), e0147623. 10.1128/mbio.01476-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrus, V. , Marrero, J. , & Waldor, M. K. (2006). The current ICE age: Biology and evolution of SXT‐related integrating conjugative elements. Plasmid, 55(3), 173–183. 10.1016/j.plasmid.2006.01.001 [DOI] [PubMed] [Google Scholar]
- Cabello, F. C. , Espejo, R. T. , Hernandez, M. C. , Rioseco, M. L. , Ulloa, J. , & Vergara, J. A. (2007). Vibrio parahaemolyticus O3:K6 epidemic diarrhea, Chile, 2005. Emerging Infectious Diseases, 13(4), 655–656. 10.3201/eid1304.06-1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caburlotto, G. , Gennari, M. , Ghidini, V. , Tafi, M. , & Lleo, M. M. (2009). Presence of T3SS2 and other virulence‐related genes in tdh‐negative Vibrio parahaemolyticus environmental strains isolated from marine samples in the area of the venetian lagoon, Italy. FEMS Microbiology Ecology, 70(3), 506–514. 10.1111/j.1574-6941.2009.00764.x [DOI] [PubMed] [Google Scholar]
- Caburlotto, G. , Suffredini, E. , Toson, M. , Fasolato, L. , Antonetti, P. , Zambon, M. , & Manfrin, A. (2016). Occurrence and molecular characterisation of Vibrio parahaemolyticus in crustaceans commercialised in Venice area, Italy. International Journal of Food Microbiology, 220, 39–49. 10.1016/j.ijfoodmicro.2015.12.007 [DOI] [PubMed] [Google Scholar]
- Cai, Q. , & Zhang, Y. (2018). Structure, function and regulation of the thermostable direct hemolysin (TDH) in pandemic Vibrio parahaemolyticus . Microbial Pathogenesis, 123, 242–245. 10.1016/j.micpath.2018.07.021 [DOI] [PubMed] [Google Scholar]
- Çam, S. , & Brinkmeyer, R. (2020). The effects of temperature, pH, and iron on biofilm formation by clinical versus environmental strains of Vibrio vulnificus . Folia Microbiologica, 65(3), 557–566. 10.1007/s12223-019-00761-9 [DOI] [PubMed] [Google Scholar]
- Campbell, V. M. , Chouljenko, A. , & Hall, S. G. (2022). Depuration of live oysters to reduce Vibrio parahaemolyticus and Vibrio vulnificus: A review of ecology and processing parameters. Comprehensive Reviews in Food Science and Food Safety, 21(4), 3480–3506. 10.1111/1541-4337.12969 [DOI] [PubMed] [Google Scholar]
- Cantet, F. , Hervio‐Heath, D. , Caro, A. , Le Mennec, C. , Monteil, C. , Quemere, C. , Jolivet‐Gougeon, A. , Colwell, R. R. , & Monfort, P. (2013). Quantification of Vibrio parahaemolyticus, Vibrio vulnificus and vibrio cholerae in French Mediterranean coastal lagoons. Research in Microbiology, 164(8), 867–874. 10.1016/j.resmic.2013.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmona‐Salido, H. , Fouz, B. , Sanjuán, E. , Carda, M. , Delannoy, C. M. J. , García‐González, N. , González‐Candelas, F. , & Amaro, C. (2021). The widespread presence of a family of fish virulence plasmids in Vibrio vulnificus stresses its relevance as a zoonotic pathogen linked to fish farms. Emerging Microbes & Infections, 10(1), 2128–2140. 10.1080/22221751.2021.1999177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castello, A. , Alio, V. , Sciortino, S. , Oliveri, G. , Cardamone, C. , Butera, G. , & Costa, A. (2022). Occurrence and molecular characterization of potentially pathogenic Vibrio spp. in seafood collected in Sicily. Microorganisms, 11(1), 53. 10.3390/microorganisms11010053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo, D. , Kauffman, K. , Hussain, F. , Kalatzis, P. , Rørbo, N. , Polz, M. F. , & Middelboe, M. (2018). Widespread distribution of prophage‐encoded virulence factors in marine Vibrio communities. Scientific Reports, 8(1), 9973. 10.1038/s41598-018-28326-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccarelli, D. , Amaro, C. , Romalde, J. L. , Suffredini, E. , & Vezzulli, L. (2019). Vibrio species. Food Microbiology, 347–388. 10.1128/9781555819972.ch13 [DOI] [Google Scholar]
- Ceccarelli, D. , Chen, A. , Hasan, N. A. , Rashed, S. M. , Huq, A. , & Colwell, R. R. (2015). Non‐O1/non‐O139 Vibrio cholerae carrying multiple virulence factors and V‐cholerae O1 in the Chesapeake Bay, Maryland. Applied and Environmental Microbiology, 81(6), 1909–1918. 10.1128/aem.03540-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaand, M. , Miller, K. A. , Sofia, M. K. , Schlesener, C. , Weaver, J. W. , Sood, V. , & Dziejman, M. (2015). Type 3 secretion system Island encoded proteins required for colonization by non‐O1/non‐O139 Serogroup Vibrio cholerae . Infection and Immunity, 83(7), 2862–2869. 10.1128/iai.03020-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae, M. J. , Cheney, D. , & Su, Y. C. (2009). Temperature effects on the depuration of Vibrio parahaemolyticus and Vibrio vulnificus from the American oyster (Crassostrea virginica). Journal of Food Science, 74(2), M62–M66. 10.1111/j.1750-3841.2008.01031.x [DOI] [PubMed] [Google Scholar]
- Chávez, M. R. C. , Sedas, V. P. , Borunda, E. O. , & Reynoso, F. L. (2005). Influence of water temperature and salinity on seasonal occurrences of Vibrio cholerae and enteric bacteria in oyster‐producing areas of Veracruz, Mexico. Marine Pollution Bulletin, 50(12), 1641–1648. 10.1016/j.marpolbul.2005.06.036 [DOI] [PubMed] [Google Scholar]
- Chen, C. Y. , Wu, K. M. , Chang, Y. C. , Chang, C. H. , Tsai, H. C. , Liao, T. L. , Liu, Y. M. , Chen, H. J. , Shen, A. B. , Li, J. C. , Su, T. L. , Shao, C. P. , Lee, C. T. , Hor, L. I. , & Tsai, S. F. (2003). Comparative genome analysis of Vibrio vulnificus, a marine pathogen. Genome Research, 13(12), 2577–2587. 10.1101/gr.1295503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, L. , Yang, J. , Yu, J. , Yao, Z. , Sun, L. , Shen, Y. , & Jin, Q. (2005). VFDB: A reference database for bacterial virulence factors. Nucleic Acids Research, 33, D325–D328. 10.1093/nar/gki008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, M. , Guo, D. , Wong, H. C. , Zhang, X. , Liu, F. X. , Chen, H. Y. , Chen, M. , Liu, B. , Wang, L. , Wu, F. , & Feng, L. (2012). Development of O‐serogroup specific PCR assay for detection and identification of Vibrio parahaemolyticus . International Journal of Food Microbiology, 159(2), 122–129. 10.1016/j.ijfoodmicro.2012.08.012 [DOI] [PubMed] [Google Scholar]
- Chen, Y.‐R. , Hwang, C.‐A. , Huang, L. , Wu, V. C. H. , & Hsiao, H.‐I. (2019). Kinetic analysis and dynamic prediction of growth of Vibrio parahaemolyticus in raw white shrimp at refrigerated and abuse temperatures. Food Control, 100, 204–211. 10.1016/j.foodcont.2019.01.013 [DOI] [Google Scholar]
- Colwell, R. R. , Brayton, P. , Herrington, D. , Tall, B. , Huq, A. , & Levine, M. M. (1996). Viable but non‐culturable vibrio cholerae O1 revert to a cultivable state in the human intestine. World Journal of Microbiology and Biotechnology, 12(1), 28–31. 10.1007/bf00327795 [DOI] [PubMed] [Google Scholar]
- Conner, J. G. , Teschler, J. K. , Jones, C. J. , & Yildiz, F. H. (2016). Staying alive: Vibrio cholerae's cycle of environmental survival, transmission, and dissemination. Microbiology Spectrum, 4(2). 10.1128/microbiolspec.VMBF-0015-2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook, D. W. (1994). Effect of time and temperature on multiplication of Vibrio vulnificusVibrio vulnificus in postharvest Gulf Coast shellstock oysters. Applied and Environmental Microbiology, 60(9), 3483–3484. 10.1128/aem.60.9.3483-3484.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook, D. W. (1997). Refrigeration of oyster shellstock: Conditions which minimize the outgrowth of Vibrio vulnificus . Journal of Food Protection, 60(4), 349–352. 10.4315/0362-028x-60.4.349 [DOI] [PubMed] [Google Scholar]
- Cook, D. W. , Oleary, P. , Hunsucker, J. C. , Sloan, E. M. , Bowers, J. C. , Blodgett, R. J. , & DePaola, A. (2002). Vibrio vulnificus and Vibrio parahaemolyticus in U.S. retail shell oysters: A national survey from June 1998 to July 1999. Journal of Food Protection, 65(1), 79–87. 10.4315/0362-028x-65.1.79 [DOI] [PubMed] [Google Scholar]
- Cook, D. W. , & Ruple, A. D. (1992). Cold storage and mild heat treatment as processing aids to reduce the numbers of Vibrio vulnificus in raw oysters. Journal of Food Protection, 55(12), 985–989. 10.4315/0362-028X-55.12.985 [DOI] [PubMed] [Google Scholar]
- Copin, S. , Robert‐Pillot, A. , Malle, P. , Quilici, M. L. , & Gay, M. (2012). Evaluation of most‐probable‐number‐PCR method with internal amplification control for the counting of total and pathogenic Vibrio parahaemolyticus in frozen shrimps. Journal of Food Protection, 75(1), 150–153. 10.4315/0362-028x.Jfp-11-165 [DOI] [PubMed] [Google Scholar]
- Das, B. , Verma, J. , Kumar, P. , Ghosh, A. , & Ramamurthy, T. (2020). Antibiotic resistance in Vibrio cholerae: Understanding the ecology of resistance genes and mechanisms. Vaccine, 38, A83–A92. 10.1016/j.vaccine.2019.06.031 [DOI] [PubMed] [Google Scholar]
- DaSilva, L. , Parveen, S. , DePaola, A. , Bowers, J. , Brohawn, K. , & Tamplin, M. L. (2012). Development and validation of a predictive model for the growth of Vibrio vulnificus in postharvest shellstock oysters. Applied and Environmental Microbiology, 78(6), 1675–1681. 10.1128/aem.07304-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza Santos, M. , & Orth, K. (2014). Intracellular Vibrio parahaemolyticus escapes the vacuole and establishes a replicative niche in the cytosol of epithelial cells. mBio, 5(5), e01506‐14. 10.1128/mBio.01506-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza Valente, C. , & Wan, A. H. L. (2021). Vibrio and major commercially important vibriosis diseases in decapod crustaceans. Journal of Invertebrate Pathology, 181(18), 107527. 10.1016/j.jip.2020.107527 [DOI] [PubMed] [Google Scholar]
- Deekshit, V. K. , Maiti, B. , Krishna Kumar, B. , Kotian, A. , Pinto, G. , Bondad‐Reantaso, M. G. , Karunasagar, I. , & Karunasagar, I. (2022). Antimicrobial resistance in fish pathogens and alternative risk mitigation strategies. Reviews in Aquaculture, 15(1), 261–273. 10.1111/raq.12715 [DOI] [Google Scholar]
- Deepanjali, A. , Kumar, H. S. , Karunasagar, I. , & Karunasagar, I. (2005). Seasonal variation in abundance of total and pathogenic Vibrio parahaemolyticus bacteria in oysters along the southwest coast of India. Applied and Environmental Microbiology, 71(7), 3575–3580. 10.1128/aem.71.7.3575-3580.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePaola, A. , Hopkins, L. H. , Peeler, J. T. , Wentz, B. , & McPhearson, R. M. (1990). Incidence of vibrio‐parahaemolyticus in United‐States coastal waters and oysters. Applied and Environmental Microbiology, 56(8), 2299–2302. 10.1128/aem.56.8.2299-2302.1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePaola, A. , Nordstrom, J. L. , Bowers, J. C. , Wells, J. G. , & Cook, D. W. (2003). Seasonal abundance of total and pathogenic Vibrio parahaemolyticus in Alabama oysters. Applied and Environmental Microbiology, 69(3), 1521–1526. 10.1128/aem.69.3.1521-1526.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePaola, A. , Presnell, M. W. , Motes, M. L. , McPhearson, R. M. , Twedt, R. M. , Becker, R. E. , & Zywno, S. (1983). Non‐O1 vibrio‐cholerae in shellfish, sediment and waters of the united‐states gulf‐coasT. Journal of Food Protection, 46(9), 802–806. 10.4315/0362-028x-46.9.802 [DOI] [PubMed] [Google Scholar]
- Deter, J. , Lozach, S. , Veron, A. , Chollet, J. , Derrien, A. , & Hervio‐Heath, D. (2010). Ecology of pathogenic and non‐pathogenic Vibrio parahaemolyticus on the French Atlantic coast. Effects of temperature, salinity, turbidity and chlorophyll a. Environmental Microbiology, 12(4), 929–937. 10.1111/j.1462-2920.2009.02136.x [DOI] [PubMed] [Google Scholar]
- Ding, G. Y. , Zhao, L. I. , Xu, J. , Cheng, J. Y. , Cai, Y. Y. , Du, H. H. , Xiao, G. S. , & Zhao, F. (2022). Quantitative risk assessment of Vibrio parahaemolyticus in shellfish from retail to consumption in coastal cities of eastern China. Journal of Food Protection, 85(9), 1320–1328. 10.4315/jfp-21-238 [DOI] [PubMed] [Google Scholar]
- Diogène, J. , Campàs, M. , Rambla, M. , Rahmani, D. , Reig, L. , Ramirez, M. S. , Poyato, C. , Gil, J. M. , Marques, A. , Costa, P. , Nunes, M. L. , Cardoso, C. , Santos, M. , Maulvault, A. L. , Manganelli, M. , Scardala, S. , Testai, E. , Hung, C. Y. , Minnens, F. , … Thébault, A. (2023). Risk assessment strategies for contaminants in seafood (RASCS). EFSA Supporting Publications, 20(12), 8419E. 10.2903/sp.efsa.2023.EN-8419 [DOI] [Google Scholar]
- Dolores, J. , & Satchell, K. J. (2013). Analysis of Vibrio cholerae genome sequences reveals unique rtxA variants in environmental strains and an rtxA‐null mutation in recent altered El tor isolates. mBio, 4(2), e00624. 10.1128/mBio.00624-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan, J. Y. , & Su, Y. C. (2005). Occurrence of Vibrio parahaemolyticus in two Oregon oyster‐growing bays. Journal of Food Science, 70(1), M58–M63. 10.1111/j.1365-2621.2005.tb09047.x [DOI] [Google Scholar]
- Dutta, D. , Kaushik, A. , Kumar, D. , & Bag, S. (2021). Foodborne pathogenic vibrios: Antimicrobial resistance. Frontiers in Microbiology, 12, 638331. 10.3389/fmicb.2021.638331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edouard, S. , Daumas, A. , Branger, S. , Durand, J. M. , Raoult, D. , & Fournier, P. E. (2009). Grimontia hollisae, a potential agent of gastroenteritis and bacteraemia in the Mediterranean area. European Journal of Clinical Microbiology & Infectious Diseases, 28(6), 705–707. 10.1007/s10096-008-0678-0 [DOI] [PubMed] [Google Scholar]
- EEA (European Environmental Agency) . (2024). Responding to climate change impacts on human health in Europe: Focus on floods, droughts and water quality . EEA report 3/2024. 10.2800/4810 [DOI]
- EFSA (European Food Safety Authority) . (2019). Scientific report on the technical specifications on harmonised monitoring of antimicrobial resistance in zoonotic and indicator bacteria from food‐producing animals and food. EFSA Journal, 17(6), 5709. 10.2903/j.efsa.2019.5709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) . (2020a). Climate change as a driver of emerging risks for food and feed safety, plant, animal health and nutritional quality. EFSA Supporting Publication 2020:EN‐1881. 146 pp. 10.2903/sp.efsa.2020.EN-1881 [DOI]
- EFSA (European Food Safety Authority) . (2020b). Draft framework for protocol development for EFSA's scientific assessments. EFSA Supporting Publication 2020:EN‐1843. 46 pp. 10.2903/sp.efsa.2020.EN-1843 [DOI]
- EFSA (European Food Safety Authority) . (2022). Zoonoses and foodborne outbreaks guidance for reporting 2021 data. EFSA Supporting Publication 2022:EN‐7131. 63 pp. 10.2903/sp.efsa.2022.EN-7131 [DOI]
- EFSA (European Food Safety Authority) . (2024). Technical specifications for a EU‐wide baseline survey of antimicrobial resistance in bacteria from aquaculture animals. EFSA Journal, 22, e8928. 10.2903/j.efsa.2024.8928 [DOI] [Google Scholar]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control) . (2022). The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2020/2021. EFSA Journal, 21(3), 7867. 10.2903/j.efsa.2023.7867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards) . (2011). Scientific opinion on irradiation of food (efficacy and microbiological safety). EFSA Journal, 9(4), 2103. 10.2903/j.efsa.2011.2103 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel . (2015). Scientific opinion on the evaluation of heat treatments, different from those currently established in the EU legislation, that could be applied to live bivalve molluscs from B and C production areas, that have not been submitted to purification or relaying, in order to eliminate pathogenic microorganisms. EFSA Journal, 13(12), 4332. 10.2903/j.efsa.2015.4332 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards) . (2019). Scientific opinion on the whole genome sequencing and metagenomics for outbreak investigation, sourceattribution and risk assessment of food‐borne microorganisms. EFSA Journal, 17(12), 5898. 10.2903/j.efsa.2019.5898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards) . (2021). Scientific opinion on role played by the environment in the emergence and spread of antimicrobial resistance (AMR) through the food chain. EFSA Journal, 19(6), 6651. 10.2903/j.efsa.2022.6651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee . (2018a). Guidance on uncertainty analysis in scientific assessments. EFSA Journal, 16(1), 5123. 10.2903/j.efsa.2018.5123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee . (2018b). Scientific opinion on the principles and methods behind EFSA's guidance on uncertainty analysis in scientific assessment. EFSA Journal, 16(1), 5122. 10.2903/j.efsa.2018.5122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiler, A. , Gonzalez‐Rey, C. , Allen, S. , & Bertilsson, S. (2007). Growth response of Vibrio cholerae and other Vibrio spp. to cyanobacterial dissolved organic matter and temperature in brackish water. FEMS Microbiology Ecology, 60(3), 411–418. 10.1111/j.1574-6941.2007.00303.x [DOI] [PubMed] [Google Scholar]
- Ekonomou, S. I. , & Boziaris, I. S. (2021). Non‐thermal methods for ensuring the microbiological quality and safety of seafood. Applied Sciences, 11(2), 833. 10.3390/app11020833 [DOI] [Google Scholar]
- Engel, M. F. , Muijsken, M. A. , Mooi‐Kokenberg, E. , Kuijper, E. J. , & van Westerloo, D. J. (2016). Vibrio cholerae non‐O1 bacteraemia: Description of three cases in The Netherlands and a literature review. Euro Surveillance, 21(15), 30197. 10.2807/1560-7917.ES.2016.21.15.30197 [DOI] [PubMed] [Google Scholar]
- Escudero, J. A. , & Mazel, D. (2017). Genomic plasticity of Vibrio cholerae . International Microbiology, 20(3), 138–148. 10.2436/20.1501.01.295 [DOI] [PubMed] [Google Scholar]
- Espinoza‐Vergara, G. , Hoque, M. M. , McDougald, D. , & Noorian, P. (2020). The impact of protozoan predation on the pathogenicity of Vibrio cholerae . Frontiers in Microbiology, 11(9), 17. 10.3389/fmicb.2020.00017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteves, K. , Hervio‐Heath, D. , Mosser, T. , Rodier, C. , Tournoud, M. G. , Jumas‐Bilak, E. , Colwell, R. R. , & Monfort, P. (2015). Rapid proliferation of Vibrio parahaemolyticus, Vibrio vulnificus, and Vibrio cholerae during freshwater flash floods in French Mediterranean coastal lagoons. Applied and Environmental Microbiology, 81(21), 7600–7609. 10.1128/aem.01848-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EUMOFA (European Market Observatory for fisheries and aquaculture products) . (2023). The EU fish market . https://eumofa.eu/documents/20124/35668/EFM2023_EN.pdf/95612366‐79d2‐a4d1‐218b‐8089c8e7508c?t=1699541180521
- FAO and WHO (Food and Agriculture Organization of the United Nations & World Health Organization) . (2005a). Risk assessment of choleragenic Vibrio cholerae 01 and 0139 in warm water shrimp for international trade: Interpretative summary and technical report. Microbiological risk assessment series 9. ISBN: 9241563125; 90 pp. https://www.who.int/publications/i/item/9241563125
- FAO and WHO (Food and Agriculture Organization of the United Nations & World Health Organization) . (2005b). Risk assessment of Vibrio vulnificus in raw oysters: Interpretative summary and technical report. Microbiological risk assessment series 8 . ISBN: 9241563109; 114 pp. https://www.who.int/publications/i/item/9241563109
- FAO and WHO (Food and Agriculture Organization of the United Nations & World Health Organization) . (2011). Risk assessment of Vibrio parahaemolyticus in seafood. Interpretative summary and technical report. Microbiological risk assessment series 26 . ISBN: 9789241548175, 200 pp. https://www.who.int/publications/i/item/9789241548175
- FAO and WHO (Food and Agriculture Organization of the United Nations & World Health Organization) . (2020). Risk assessment tools for Vibrio parahaemolyticus and Vibrio vulnificus associated with seafood. Microbiological risk assessment series 20 . ISBN: 9789240000186, 71 pp. https://www.who.int/publications/i/item/9789240000186
- FAO and WHO (Food and Agriculture Organization of the United Nations & World Health Organization) . (2021). Advances in science and risk assessment tools for Vibrio parahaemolyticus and V. vulnificus associated with seafood. Microbiological risk assessment series 35 . https://www.who.int/publications/i/item/9789240024878
- Faria, A. S. , Winter, M. , Thebault, A. , Guillier, L. , Sanaa, M. , Kooh, P. , Cadavez, V. , & Gonzales‐Barron, U. (2022). Pathogens‐in‐foods database: A web application for assessing the occurrence data of microbiological hazards in foods marketed in Europe. Biology and Life Sciences Forum, 18(1), 30. https://www.mdpi.com/2673‐9976/18/1/30 [Google Scholar]
- Faruque, S. M. , Asadulghani, R. M. M. , Waldor, M. K. , & Sack, D. A. (2000). Sunlight‐induced propagation of the lysogenic phage encoding cholera toxin. Infection and Immunity, 68(8), 4795–4801. 10.1128/iai.68.8.4795-4801.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faruque Shah, M. , Albert, M. J. , & Mekalanos John, J. (1998). Epidemiology, genetics, and ecology of toxigenic Vibrio cholerae . Microbiology and Molecular Biology Reviews, 62(4), 1301–1314. 10.1128/mmbr.62.4.1301-1314.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng, L. , Reeves, P. R. , Lan, R. , Ren, Y. , Gao, C. , Zhou, Z. , Ren, Y. , Cheng, J. , Wang, W. , Wang, J. , Qian, W. , Li, D. , & Wang, L. (2008). A recalibrated molecular clock and independent origins for the cholera pandemic clones. PLoS One, 3(12), e4053. 10.1371/journal.pone.0004053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez‐Piquer, J. , Bowman, J. P. , Ross, T. , & Tamplin, M. L. (2011). Predictive models for the effect of storage temperature on Vibrio parahaemolyticus viability and counts of total viable bacteria in Pacific oysters (Crassostrea gigas). Applied and Environmental Microbiology, 77(24), 8687–8695. 10.1128/aem.05568-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischmann, S. , Herrig, I. , Wesp, J. , Stiedl, J. , Reifferscheid, G. , Strauch, E. , Alter, T. , & Brennholt, N. (2022). Prevalence and distribution of potentially human pathogenic Vibrio spp. on German North and Baltic Sea Coasts. Frontiers in Cellular and Infection Microbiology, 12, 846819. 10.3389/fcimb.2022.846819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher, G. C. , Cruz, C. D. , & Hedderley, D. I. (2024). Vibrio parahaemolyticus: Predicting effects of storage temperature on growth in Crassostrea gigas harvested in New Zealand. Aquaculture, 579, 740128. 10.1016/j.aquaculture.2023.740128 [DOI] [Google Scholar]
- Ford, C. L. , Powell, A. , Lau, D. Y. L. , Turner, A. D. , Dhanji‐Rapkova, D. , Martinez‐Urtaza, J. , & Baker‐Austin, C. (2020). Isolation and characterization of potentially pathogenic Vibrio species in a temperate, higher latitude hotspot. Environmental Microbiology Reports, 12(4), 424–434. 10.1111/1758-2229.12858 [DOI] [PubMed] [Google Scholar]
- Froelich, B. A. , & Daines, D. A. (2020). In hot water: Effects of climate change onVibrio−human interactions. Environmental Microbiology, 22(10), 4101–4111. 10.1111/1462-2920.14967 [DOI] [PubMed] [Google Scholar]
- Fu, Y. , Waldor, M. K. , & Mekalanos, J. J. (2013). Tn‐Seq analysis of Vibrio cholerae intestinal colonization reveals a role for T6SS‐mediated antibacterial activity in the host. Cell Host & Microbe, 14(6), 652–663. 10.1016/j.chom.2013.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuenzalida, L. , Hernández, C. , Toro, J. , Rioseco, M. L. , Romero, J. , & Espejo, R. T. (2006). Vibrio parahaemolyticus in shellfish and clinical samples during two large epidemics of diarrhoea in southern Chile. Environmental Microbiology, 8(4), 675–683. 10.1111/j.1462-2920.2005.00946.x [DOI] [PubMed] [Google Scholar]
- Fukushima, H. , & Seki, R. (2004). Ecology of Vibrio vulnificus and Vibrio parahaemolyticus in brackish environments of the Sada River in Shimane Prefecture, Japan. FEMS Microbiology Ecology, 48(2), 221–229. 10.1016/j.femsec.2004.01.009 [DOI] [PubMed] [Google Scholar]
- Gavilan, R. G. , Caro‐Castro, J. , Blondel, C. J. , & Martinez‐Urtaza, J. (2021). Vibrio parahaemolyticus epidemiology and pathogenesis: Novel insights on an emerging foodborne pathogen. In Almagro‐Moreno S. & Pukatzki S. (Eds.), Vibrio spp. infections (Vol. 1404, pp. 233–251). Springer. 10.1007/978-3-031-22997-8 [DOI] [PubMed] [Google Scholar]
- Gavin, H. E. , & Satchell, K. J. F. (2015). MARTX toxins as effector delivery platforms. Pathogens and Disease, 73(9), ftv092. 10.1093/femspd/ftv092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh, C. , Nandy, R. K. , Dasgupta, S. K. , Nair, G. B. , Hall, R. H. , & Ghose, A. C. (1997). A search for cholera toxin (CT), toxin coregulated pilus (TCP), the regulatory element ToxR and other virulence factors in non‐01/non‐0139 Vibrio cholerae . Microbial Pathogenesis, 22(4), 199–208. 10.1006/mpat.1996.0105 [DOI] [PubMed] [Google Scholar]
- Goh, J. X. H. , Tan, L. T. , Law, J. W. , Khaw, K. Y. , Ab Mutalib, N. S. , He, Y. W. , Goh, B. H. , Chan, K. G. , Lee, L. H. , & Letchumanan, V. (2022). Insights into Carbapenem resistance in vibrio species: Current status and future perspectives. International Journal of Molecular Sciences, 23(20), 12486. 10.3390/ijms232012486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves‐Tenório, A. , Silva, B. N. , Rodrigues, V. , Cadavez, V. , & Gonzales‐Barron, U. (2018). Prevalence of pathogens in poultry meat: A meta‐analysis of European published surveys. Food, 7(5), 69. 10.3390/foods7050069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales‐Barron, U. , Thébault, A. , Kooh, P. , Watier, L. , Sanaa, M. , & Cadavez, V. (2021). Strategy for systematic review of observational studies and meta‐analysis modelling of risk factors for sporadic foodborne diseases. Microbial Risk Analysis, 17(11), 100082. 10.1016/j.mran.2019.07.003 [DOI] [Google Scholar]
- Gonzalez‐Escalona, N. , Jolley, K. A. , Reed, E. , & Martinez‐Urtaza, J. (2017). Defining a core genome multilocus sequence typing scheme for the global epidemiology of Vibrio parahaemolyticus . Journal of Clinical Microbiology, 55(6), 1682–1697. 10.1128/jcm.00227-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González‐Escalona, N. , Martinez‐Urtaza, J. , Romero, J. , Espejo, R. T. , Jaykus, L. A. , & DePaola, A. (2008). Determination of molecular phylogenetics of Vibrio parahaemolyticus strains by multilocus sequence typing. Journal of Bacteriology, 190(8), 2831–2840. 10.1128/jb.01808-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooch, J. A. , DePaola, A. , Bowers, J. , & Marshall, D. L. (2002). Growth and survival of Vibrio parahaemolyticus in postharvest American oysters. Journal of Food Protection, 65(6), 970–974. 10.4315/0362-028X-65.6.970 [DOI] [PubMed] [Google Scholar]
- Gras‐Rouzet, S. , Donnio, P. Y. , Juguet, F. , Plessis, P. , Minet, J. , & Avril, J. L. (1996). First European case of gastroenteritis and bacteremia due to vibrio hollisae. European Journal of Clinical Microbiology & Infectious Diseases, 15(11), 864–866. 10.1007/bf01691217 [DOI] [PubMed] [Google Scholar]
- Gutierrez, M. G. , Saka, H. A. , Chinen, I. , Zoppino, F. C. M. , Yoshimori, T. , Bocco, J. L. , & Colombo, M. I. (2007). Protective role of autophagy against Vibrio cholerae cytolysin, a pore‐forming toxin from V‐cholerae. Proceedings of the National Academy of Sciences of the United States of America, 104(6), 1829–1834. 10.1073/pnas.0601437104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas, C. , Rose, J. , & Gerba, C. (1999). Quantitative microbial risk assessment. John Wiley & Sons. [Google Scholar]
- Hammerl, J.‐A. (2024). Dr Jens‐Andre Hammerl, Head of the Consultant Laboratory for Vibrio spp. in Food of the German Federal Institute for Risk Assessment (BfR) attended the WG meeting on 10 January 2024 as hearing expert to provide information .
- Hammerl, J. A. , Jäckel, C. , Bortolaia, V. , Schwartz, K. , Bier, N. , Hendriksen, R. S. , Guerra, B. , & Strauch, E. (2017). Carbapenemase VCC‐1‐producing Vibrio cholerae in coastal waters of Germany. Emerging Infectious Diseases, 23(10), 1735–1737. 10.3201/eid2310.161625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, D. , Yu, F. , Chen, X. , Zhang, R. , & Li, J. (2019). Challenges in Vibrio parahaemolyticus infections caused by the pandemic clone. Future Microbiology, 14(5), 437–450. 10.2217/fmb-2018-0308 [DOI] [PubMed] [Google Scholar]
- Han, J. E. , Mohney, L. L. , Tang, K. F. J. , Pantoja, C. R. , & Lightner, D. V. (2015). Plasmid mediated tetracycline resistance of Vibrio parahaemolyticus associated with acute hepatopancreatic necrosis disease (AHPND) in shrimps. Aquaculture Reports, 2, 17–21. 10.1016/j.aqrep.2015.04.003 [DOI] [Google Scholar]
- Hartnell, R. E. , Stockley, L. , Keay, W. , Rosec, J. P. , Hervio‐Heath, D. , Van den Berg, H. , Leoni, F. , Ottaviani, D. , Henigman, U. , Denayer, S. , Serbruyns, B. , Georgsson, F. , Krumova‐Valcheva, G. , Gyurova, E. , Blanco, C. , Copin, S. , Strauch, E. , Wieczorek, K. , Lopatek, M. , … Baker‐Austin, C. (2019). A pan‐European ring trial to validate an international standard for detection of Vibrio cholerae, Vibrio parahaemolyticus and Vibrio vulnificus in seafoods. International Journal of Food Microbiology, 288, 58–65. 10.1016/j.ijfoodmicro.2018.02.008 [DOI] [PubMed] [Google Scholar]
- Hasan, N. A. , Rezayat, T. , Blatz, P. J. , Choi, S. Y. , Griffitt, K. J. , Rashed, S. M. , Huq, A. , Conger, N. G. , Colwell, R. R. , & Grimes, D. J. (2015). Nontoxigenic Vibrio cholerae non‐O1/O139 isolate from a case of human gastroenteritis in the US Gulf Coast. Journal of Clinical Microbiology, 53(1), 9–14. 10.1128/jcm.02187-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazen, T. H. , Pan, L. , Gu, J. D. , & Sobecky, P. A. (2010). The contribution of mobile genetic elements to the evolution and ecology of Vibrios. FEMS Microbiology Ecology, 74(3), 485–499. 10.1111/j.1574-6941.2010.00937.x [DOI] [PubMed] [Google Scholar]
- Henderson, J. C. , Herrera, C. M. , & Trent, M. S. (2017). AlmG, responsible for polymyxin resistance in pandemic Vibrio cholerae, is a glycyltransferase distantly related to lipid A late acyltransferases. Journal of Biological Chemistry, 292(51), 21205–21215. 10.1074/jbc.RA117.000131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández‐Cabanyero, C. , & Amaro, C. (2020). Phylogeny and life cycle of the zoonotic pathogen Vibrio vulnificus . Environmental Microbiology, 22(10), 4133–4148. 10.1111/1462-2920.15137 [DOI] [PubMed] [Google Scholar]
- Herrera, C. M. , Henderson, J. C. , Crofts, A. A. , & Trent, M. S. (2017). Novel coordination of lipopolysaccharide modifications in Vibrio cholerae promotes CAMP resistance. Molecular Microbiology, 106(4), 582–596. 10.1111/mmi.13835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrfurth, D. , Oeleker, K. , Pund, R. , Strauch, E. , Schwartz, K. , Kleer, J. , Gölz, G. , Alter, T. , & Huehn, S. (2013). Uptake and localization of Vibrio cholerae, Vibrio parahaemolyticus, and Vibrio vulnificus in blue mussels (Mytilus edulis) of the Baltic Sea. Journal of Shellfish Research, 32(3), 855–859. 10.2983/035.032.0329 [DOI] [Google Scholar]
- Higgins, J. P. , & Thompson, S. G. (2002). Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine, 21(11), 1539–1558. 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- Hiltunen, T. , Virta, M. , & Laine, A. L. (2017). Antibiotic resistance in the wild: An eco‐evolutionary perspective. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 372(1712), 20160039. 10.1098/rstb.2016.0039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hlady, W. G. , Mullen, R. C. , & Hopkin, R. S. (1993). Vibrio vulnificus from raw oysters. Leading cause of reported deaths from foodborne illness in Florida. The Journal of the Florida Medical Association, 80(8), 536–538. [PubMed] [Google Scholar]
- Ho, B. T. , Dong, T. G. , & Mekalanos, J. J. (2014). A view to a kill: The bacterial type VI secretion system. Cell Host & Microbe, 15(1), 9–21. 10.1016/j.chom.2013.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges, K. , & Hecht, G. (2012). Chapter 77 – Physiology of host‐pathogen interactions. In Johnson L. R., Ghishan F. K., Kaunitz J. D., Merchant J. L., Said H. M., & Wood J. D. (Eds.), Physiology of the gastrointestinal tract (5th ed., pp. 2047–2073). Academic Press. 10.1016/B978-0-12-382026-6.00077-4 [DOI] [Google Scholar]
- Hoefler, F. , Pouget‐Abadie, X. , Roncato‐Saberan, M. , Lemarie, R. , Takoudju, E. M. , Raffi, F. , Corvec, S. , Le Bras, M. , Cazanave, C. , Lehours, P. , Guimard, T. , & Allix‐Beguec, C. (2022). Clinical and epidemiologic characteristics and therapeutic management of patients with vibrio infections, Bay of Biscay, France, 2001–2019. Emerging Infectious Diseases, 28(12), 2367–2373. 10.3201/eid2812.220748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, L. , Fu, Y. , Zhang, S. , Pan, Z. , Xia, J. , Zhu, P. , & Guo, J. (2022). An assay combining droplet digital PCR with propidium monoazide treatment for the accurate detection of live cells of Vibrio vulnificus in plasma samples. Frontiers in Microbiology, 13, 927285. 10.3389/fmicb.2022.927285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, X. , Mallikarjunan, P. , Koo, J. , Andrews, L. S. , & Jahncke, M. L. (2005). Comparison of kinetic models to describe high pressure and gamma irradiation used to inactivate Vibrio vulnificus and Vibrio parahaemolyticus prepared in buffer solution and in whole oysters. Journal of Food Protection, 68(2), 292–295. 10.4315/0362-028X-68.2.292 [DOI] [PubMed] [Google Scholar]
- Huang, Y. S. , Hwang, C. A. , Huang, L. H. , Wu, V. C. H. , & Hsiao, H. I. (2018). The risk of Vibrio parahaemolyticus infections associated with consumption of raw oysters as affected by processing and distribution conditions in Taiwan. Food Control, 86, 101–109. 10.1016/j.foodcont.2017.10.022 [DOI] [Google Scholar]
- Huehn, S. , Eichhorn, C. , Urmersbach, S. , Breidenbach, J. , Bechlars, S. , Bier, N. , Alter, T. , Bartelt, E. , Frank, C. , Oberheitmann, B. , Gunzer, F. , Brennholt, N. , Boer, S. , Appel, B. , Dieckmann, R. , & Strauch, E. (2014). Pathogenic vibrios in environmental, seafood and clinical sources in Germany. International Journal of Medical Microbiology, 304(7), 843–850. 10.1016/j.ijmm.2014.07.010 [DOI] [PubMed] [Google Scholar]
- ICMSF (International Commission on Microbiological Specifications for Foods) . (1996). Microorganisms in foods 5‐microbiological specifications of food pathogens . 514 pp.
- Igere, B. E. , Okoh, A. I. , & Nwodo, U. U. (2022). Non‐serogroup O1/O139 agglutinable Vibrio cholerae: A phylogenetically and genealogically neglected yet emerging potential pathogen of clinical relevance. Archives of Microbiology, 204(6), 323. 10.1007/s00203-022-02866-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- IPCC (Intergovernmental Panel on Climate Change) . (2021). Summary for policymakers. In Masson‐Delmotte V., Zhai P., Pirani A., Connors S. L., Péan C., Berger S., Caud N., Chen Y., Goldfarb L., Gomis M. I., Huang M., Leitzell K., Lonnoy E., Matthews J. B. R., Maycock T. K., Waterfield T., Yelekçi O., Yu R., & Zhou B. (Eds.), Climate change 2021: The physical science basis. Contribution of working group I to the sixth assessment report of the intergovernmental panel on climate change (pp. 3–32). Cambridge University Press. 10.1017/9781009157896.001 [DOI] [Google Scholar]
- IPCC (Intergovernmental Panel on Climate Change) . (2023). Summary for policymakers. In Core Writing Team , Lee H., & Romero J. (Eds.), Climate change 2023: Synthesis report. Contribution of working groups I, II and III to the sixth assessment report of the intergovernmental panel on climate change (pp. 1–34). IPCC . 10.59327/IPCC/AR6-9789291691647.001 [DOI] [Google Scholar]
- Iwahori, J. , Yamamoto, A. , Suzuki, H. , Yamamoto, T. , Tsutsui, T. , Motoyama, K. , Sawada, M. , Matsushita, T. , Hasegawa, A. , Osaka, K. , Toyofuku, H. , & Kasuga, F. (2010). Quantitative risk assessment of Vibrio parahaemolyticus in finfish: A model of raw horse mackerel consumption in Japan. Risk Analysis, 30(12), 1817–1832. 10.1111/j.1539-6924.2010.01444.x [DOI] [PubMed] [Google Scholar]
- Jakabi, M. , Gelli, D. S. , Torre, J. C. M. D. , Rodas, M. A. B. , Franco, B. D. G. M. , Destro, M. T. , & Landgraf, M. (2003). Inactivation by ionizing radiation of Salmonella enteritidis, Salmonella infantis, and Vibrio parahaemolyticus in oysters (Crassostrea brasiliana). Journal of Food Protection, 66(6), 1025–1029. 10.4315/0362-028X-66.6.1025 [DOI] [PubMed] [Google Scholar]
- Janecko, N. , Bloomfield, S. J. , Palau, R. , & Mather, A. E. (2021). Whole genome sequencing reveals great diversity of vibrio spp in prawns at retail. Microbial Genomics, 7(9), 000647. 10.1099/mgen.0.000647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Januário, F. E. S. , & Dykes, G. A. (2005). Effect of sodium Metabisulphite and storage temperature on the survival of Vibrio cholerae on prawns (Penaeus monodon). World Journal of Microbiology and Biotechnology, 21(6), 1017–1020. 10.1007/s11274-004-7654-3 [DOI] [Google Scholar]
- Jeong, H. G. , Oh, M. H. , Kim, B. S. , Lee, M. Y. , Han, H. J. , & Choi, S. H. (2009). The capability of catabolic utilization of N‐acetylneuraminic acid, a sialic acid, is essential for Vibrio vulnificus pathogenesis. Infection and Immunity, 77(8), 3209–3217. 10.1128/iai.00109-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, C. N. (2013). Fitness factors in vibrios: A mini‐review. Microbial Ecology, 65(4), 826–851. 10.1007/s00248-012-0168-x [DOI] [PubMed] [Google Scholar]
- Johnson, C. N. (2015). Influence of environmental factors on Vibrio spp. in coastal ecosystems. Microbiology Spectrum, 3(3), e‐0008‐2014. 10.1128/microbiolspec.VE-0008-2014 [DOI] [PubMed] [Google Scholar]
- Johnson, C. N. , Bowers, J. C. , Griffitt, K. J. , Molina, V. , Clostio, R. W. , Pei, S. F. , Laws, E. , Paranjpye, R. N. , Strom, M. S. , Chen, A. , Hasan, N. A. , Huq, A. , Noriea, N. F. , Grimes, D. J. , & Colwell, R. R. (2012). Ecology of Vibrio parahaemolyticus and Vibrio vulnificus in the coastal and estuarine waters of Louisiana, Maryland, Mississippi, and Washington (United States). Applied and Environmental Microbiology, 78(20), 7249–7257. 10.1128/aem.01296-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, J. L. , Lüdeke, C. H. , Bowers, J. C. , Garrett, N. , Fischer, M. , Parsons, M. B. , Bopp, C. A. , & DePaola, A. (2012). Biochemical, serological, and virulence characterization of clinical and oyster Vibrio parahaemolyticus isolates. Journal of Clinical Microbiology, 50(7), 2343–2352. 10.1128/jcm.00196-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, J. L. , Lydon, K. A. , Kinsey, T. P. , Friedman, B. , Curtis, M. , Schuster, R. , & Bowers, J. C. (2017). Effects of ambient exposure, refrigeration, and icing on Vibrio vulnificus and Vibrio parahaemolyticus abundances in oysters. International Journal of Food Microbiology, 253, 54–58. 10.1016/j.ijfoodmicro.2017.04.016 [DOI] [PubMed] [Google Scholar]
- Jones, M. K. , & Oliver, J. D. (2009). Vibrio vulnificus: Disease and pathogenesis. Infection and Immunity, 77(5), 1723–1733. 10.1128/iai.01046-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph, S. W. , Colwell, R. R. , & Kaper, J. B. (1982). Vibrio‐parahaemolyticus and related halophilic vibrios. CRC Critical Reviews in Microbiology, 10(1), 77–124. 10.3109/10408418209113506 [DOI] [PubMed] [Google Scholar]
- Joux, F. , Jeffrey, W. H. , Lebaron, P. , & Mitchell, D. L. (1999). Marine bacterial isolates display diverse responses to UV‐B radiation. Applied and Environmental Microbiology, 65(9), 3820–3827. 10.1128/aem.65.9.3820-3827.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalburge, S. S. , Whitaker, W. B. , & Boyd, E. F. (2014). High‐salt preadaptation of Vibrio parahaemolyticus enhances survival in response to lethal environmental stresses. Journal of Food Protection, 77(2), 246–253. 10.4315/0362-028x.Jfp-13-241 [DOI] [PubMed] [Google Scholar]
- Kanampalliwar, A. , & Singh, D. V. (2020). Virulence pattern and genomic diversity of Vibrio cholerae O1 and O139 strains isolated from clinical and environmental sources in India. Frontiers in Microbiology, 11, 1838. 10.3389/fmicb.2020.01838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang, J. , Lee, Y. , Choi, Y. , Kim, S. , Ha, J. , Oh, H. , Kim, Y. , Seo, Y. , Park, E. , Rhee, M. S. , Lee, H. , & Yoon, Y. (2021). Quantitative microbial risk assessment of Vibrio parahaemolyticus foodborne illness of sea squirt (Halocynthia roretzi) in South Korea. Fisheries and Aquatic Sciences, 24(2), 78–88. 10.47853/FAS.2021.e8 [DOI] [Google Scholar]
- Karatuna, O. , Matuschek, E. , Åhman, J. , Caidi, H. , & Kahlmeter, G. (2024). Vibrio species: Development of EUCAST susceptibility testing methods and MIC and zone diameter distributions on which to determine clinical breakpoints. Journal of Antimicrobial Chemotherapy, 79(2), 375–382. 10.1093/jac/dkad391 [DOI] [PubMed] [Google Scholar]
- Kaspar, C. W. , & Tamplin, M. L. (1993). Effects of temperature and salinity on the survival of Vibrio vulnificus in seawater and shellfish. Applied and Environmental Microbiology, 59(8), 2425–2429. 10.1128/aem.59.8.2425-2429.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman, G. E. , Bej, A. K. , Bowers, J. , & DePaola, A. (2003). Oyster‐to‐oyster variability in levels of Vibrio parahaemolyticus . Journal of Food Protection, 66(1), 125–129. 10.4315/0362-028x-66.1.125 [DOI] [PubMed] [Google Scholar]
- Kaus, K. , Lary, J. W. , Cole, J. L. , & Olson, R. (2014). Glycan specificity of the Vibrio vulnificus hemolysin lectin outlines evolutionary history of membrane targeting by a toxin family. Journal of Molecular Biology, 426(15), 2800–2812. 10.1016/j.jmb.2014.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushik, M. , Kumar, S. , Kapoor, R. K. , & Gulati, P. (2019). Integrons and antibiotic resistance genes in water‐borne pathogens: Threat detection and risk assessment. Journal of Medical Microbiology, 68(5), 679–692. 10.1099/jmm.0.000972 [DOI] [PubMed] [Google Scholar]
- Khan, F. , Tabassum, N. , Anand, R. , & Kim, Y. M. (2020). Motility of Vibrio spp.: Regulation and controlling strategies. Applied Microbiology and Biotechnology, 104(19), 8187–8208. 10.1007/s00253-020-10,794-7 [DOI] [PubMed] [Google Scholar]
- Kim, J. , & Kim, B. S. (2023). Bacterial sialic acid catabolism at the host‐microbe Interface. Journal of Microbiology, 9, 369–377. 10.1007/s12275-023-00035-7 [DOI] [PubMed] [Google Scholar]
- Kim, Y. R. , Lee, S. E. , Kim, C. M. , Kim, S. Y. , Shin, E. K. , Shin, D. H. , Chung, S. S. , Choy, H. E. , Progulske‐Fox, A. , Hillman, J. D. , Handfield, M. , & Rhee, J. H. (2003). Characterization and pathogenic significance of Vibrio vulnificus antigens preferentially expressed in septicemic patients. Infection and Immunity, 71(10), 5461–5471. 10.1128/iai.71.10.5461-5471.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, Y. W. , Lee, S. H. , Hwang, I. G. , & Yoon, K. S. (2012). Effect of temperature on growth of Vibrio parahaemolyticus [corrected] and Vibrio vulnificus in flounder, salmon sashimi and oyster meat. International Journal of Environmental Research and Public Health, 9(12), 4662–4675. 10.3390/ijerph9124662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King, M. , Rose, L. , Fraimow, H. , Nagori, M. , Danish, M. , & Doktor, K. (2019). Vibrio vulnificus infections from a previously nonendemic area. Annals of Internal Medicine, 171(7), 520. 10.7326/l19-0133 [DOI] [PubMed] [Google Scholar]
- Kirschner, A. K. T. , Schlesinger, J. , Farnleitner, A. H. , Hornek, R. , Süss, B. , Golda, B. , Herzig, A. , & Reitner, B. (2008). Rapid growth of planktonic Vibrio cholerae non‐O1/non‐O139 strains in a large alkaline lake in Austria: Dependence on temperature and dissolved organic carbon quality. Applied and Environmental Microbiology, 74(7), 2004–2015. 10.1128/aem.01739-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitaoka, M. , Miyata, S. T. , Unterweger, D. , & Pukatzki, S. (2011). Antibiotic resistance mechanisms of Vibrio cholerae . Journal of Medical Microbiology, 60(4), 397–407. 10.1099/jmm.0.023051-0 [DOI] [PubMed] [Google Scholar]
- Klein, S. L. , West, C. K. G. , Mejia, D. M. , & Lovell, C. R. (2014). Genes similar to the Vibrio parahaemolyticus virulence‐related genes tdh, tlh, and scC2 occur in other Vibrionaceae species isolated from a pristine estuary. Applied and Environmental Microbiology, 80(2), 595–602. 10.1128/aem.02895-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kling, K. , Trinh, S. A. , Leyn, S. A. , Rodionov, D. A. , Rodionov, I. D. , Herrera, A. , Cervantes, K. , Pankey, G. , Ashcraft, D. , Ozer, E. A. , Godzik, A. , & Satchell, K. J. F. (2022). Genetic divergence of Vibrio vulnificus clinical isolates with mild to severe outcomes. mBio, 13(5), 11. 10.1128/mbio.01500-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontominas, M. G. , Badeka, A. V. , Kosma, I. S. , & Nathanailides, C. I. (2021). Innovative seafood preservation technologies: Recent developments. Animals, 11(1), 92. 10.3390/ani11010092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo, J. , DePaola, A. , & Marshall, D. L. (2000). Impact of acid on survival of Vibrio vulnificus and Vibrio vulnificus phage. Journal of Food Protection, 63(8), 1049–1052. 10.4315/0362-028x-63.8.1049 [DOI] [PubMed] [Google Scholar]
- Koo, J. , Jahncke, M. L. , Reno, P. W. , Hu, X. , & Mallikarjunan, P. (2006). Inactivation of Vibrio parahaemolyticus and Vibrio vulnificus in phosphate‐buffered saline and in inoculated whole oysters by high‐pressure processing. Journal of Food Protection, 69(3), 596–601. 10.4315/0362-028x-69.3.596 [DOI] [PubMed] [Google Scholar]
- Larsen, A. M. , Rikard, F. S. , Walton, W. C. , & Arias, C. R. (2015). Temperature effect on high salinity depuration of Vibrio vulnificus and V. parahaemolyticus from the eastern oyster (Crassostrea virginica). International Journal of Food Microbiology, 192, 66–71. 10.1016/j.ijfoodmicro.2014.09.025 [DOI] [PubMed] [Google Scholar]
- Lee, R. , Lovatelli, A. , & Ababouch, L. (2008). Bivalve depuration: Fundamental and practical aspects . FAO Fisheries technical paper, no. 511.
- Lei, T. , Zhang, J. M. , Jiang, F. F. , He, M. , Zeng, H. Y. , Chen, M. T. , Wu, S. , Wang, J. , Ding, Y. , & Wu, Q. P. (2019). First detection of the plasmid‐mediated colistin resistance gene mcr‐1 in virulent Vibrio parahaemolyticus . International Journal of Food Microbiology, 308(6), 108290. 10.1016/j.ijfoodmicro.2019.108290 [DOI] [PubMed] [Google Scholar]
- Lepuschitz, S. , Baron, S. , Larvor, E. , Granier, S. A. , Pretzer, C. , Mach, R. L. , Farnleitner, A. H. , Ruppitsch, W. , Pleininger, S. , Indra, A. , & Kirschner, A. K. T. (2019). Phenotypic and genotypic antimicrobial resistance traits of Vibrio cholerae non‐O1/non‐O139 isolated from a large Austrian Lake frequently associated with cases of human infection. Frontiers in Microbiology, 10. 10.3389/fmicb.2019.02600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letchumanan, V. , Chan, K. G. , & Lee, L. H. (2014). Vibrio parahaemolyticus: A review on the pathogenesis, prevalence, and advance molecular identification techniques. Frontiers in Microbiology, 5, 705. 10.3389/fmicb.2014.00705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letunic, I. , & Bork, P. (2021). Interactive Tree Of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Research, 49(W1), W293–w296. 10.1093/nar/gkab301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levade, I. , Saber, M. M. , Midani, F. S. , Chowdhury, F. , Khan, A. I. , Begum, Y. A. , Ryan, E. T. , David, L. A. , Calderwood, S. B. , Harris, J. B. , LaRocque, R. C. , Qadri, F. , Shapiro, B. J. , & Weil, A. A. (2021). Predicting Vibrio cholerae infection and disease severity using metagenomics in a prospective cohort study. Journal of Infectious Diseases, 223(2), 342–351. 10.1093/infdis/jiaa358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, G. , & Wang, M. Y. (2020). The role of Vibrio vulnificus virulence factors and regulators in its infection‐induced sepsis. Folia Microbiologica, 65(2), 265–274. 10.1007/s12223-019-00763-7 [DOI] [PubMed] [Google Scholar]
- Li, L. , Mendis, N. , Trigui, H. , Oliver, J. D. , & Faucher, S. P. (2014). The importance of the viable but non‐culturable state in human bacterial pathogens. Frontiers in Microbiology, 5(20), 258. 10.3389/fmicb.2014.00258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y. , Zhang, S. , Li, J. , Chen, M. , He, M. , Wang, Y. , Zhang, Y. , Jing, H. , Ma, H. , Li, Y. , Zhao, L. , Zhao, H. , Kan, B. , & Pang, B. (2019). Application of digital PCR and next generation sequencing in the etiology investigation of a foodborne disease outbreak caused by Vibrio parahaemolyticus . Food Microbiology, 84(103), 233. 10.1016/j.fm.2019.05.017 [DOI] [PubMed] [Google Scholar]
- Liang, K. Y. H. , Orata, F. D. , Islam, M. T. , Nasreen, T. , Alam, M. , Tarr, C. L. , & Boucher, Y. F. (2020). A Vibrio cholerae core genome multilocus sequence typing scheme to facilitate the epidemiological study of cholera. Journal of Bacteriology, 202(24). 10.1128/jb.00086-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima, F. P. , & Wethey, D. S. (2012). Three decades of high‐resolution coastal sea surface temperatures reveal more than warming. Nature Communications, 3, 704. 10.1038/ncomms1713 [DOI] [PubMed] [Google Scholar]
- Liu, C. , Lu, J. , & Su, Y. C. (2009). Effects of flash freezing, followed by frozen storage, on reducing Vibrio parahaemolyticus in Pacific raw oysters (Crassostrea gigas). Journal of Food Protection, 72(1), 174–177. 10.4315/0362-028x-72.1.174 [DOI] [PubMed] [Google Scholar]
- Liu, J. , Yang, L. , Kjellerup, B. V. , & Xu, Z. (2023). Viable but nonculturable (VBNC) state, an underestimated and controversial microbial survival strategy. Trends in Microbiology, 31(10), 1013–1023. 10.1016/j.tim.2023.04.009 [DOI] [PubMed] [Google Scholar]
- Liu, M. , Wong, M. H. Y. , & Chen, S. (2013). Molecular characterisation of a multidrug resistance conjugative plasmid from Vibrio parahaemolyticus . International Journal of Antimicrobial Agents, 42(6), 575–579. 10.1016/j.ijantimicag.2013.08.014 [DOI] [PubMed] [Google Scholar]
- Liu, N. W. , Zou, D. Y. , Dong, D. R. , Yang, Z. , Ao, D. , Liu, W. , & Huang, L. Y. (2017). Development of a multiplex loopmediated isothermal amplification method for the simultaneous detection of Salmonella spp. and Vibrio parahaemolyticus . Scientific Reports, 7, 45601. 10.1038/srep45601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, T. , Kang, L. , Xu, J. , Wang, J. , Gao, S. , Li, Y. , Li, J. , Yuan, Y. , Yuan, B. , Wang, J. , Zhao, B. , & Xin, W. (2022). PVBase: A MALDI‐TOF MS database for fast identification and characterization of potentially pathogenic vibrio species from multiple regions of China. Frontiers in Microbiology, 13, 872825. 10.3389/fmicb.2022.872825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y. , Zhong, Q. , Wang, J. , & Lei, S. (2018). Enumeration of Vibrio parahaemolyticus in VBNC state by PMA‐combined real‐time quantitative PCR coupled with confirmation of respiratory activity. Food Control, 91, 85–91. 10.1016/j.foodcont.2018.03.037 [DOI] [Google Scholar]
- Lopez‐Joven, C. , De Blas, I. , Furones, M. D. , & Roque, A. (2015). Prevalences of pathogenic and non‐pathogenic Vibrio parahaemolyticus in mollusks from the Spanish Mediterranean coast. Frontiers in Microbiology, 6, 736. 10.3389/fmicb.2015.00736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou, Y. , Liu, H. , Zhang, Z. , Pan, Y. , & Zhao, Y. (2016). Mismatch between antimicrobial resistance phenotype and genotype of pathogenic Vibrio parahaemolyticus isolated from seafood. Food Control, 59, 207–211. 10.1016/j.foodcont.2015.04.039 [DOI] [Google Scholar]
- Louis, V. R. , Russek‐Cohen, E. , Choopun, N. , Rivera, I. N. G. , Gangle, B. , Jiang, S. C. , Rubin, A. , Patz, J. A. , Huq, A. , & Colwell, R. R. (2003). Predictability of Vibrio cholerae in Chesapeake Bay. Applied and Environmental Microbiology, 69(5), 2773–2785. 10.1128/aem.69.5.2773-2785.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love, D. C. , Kuehl, L. M. , Lane, R. M. , Fry, J. P. , Harding, J. , Davis, B. J. K. , Clancy, K. , & Hudson, B. (2020). Performance of cold chains and modeled growth of Vibrio parahaemolyticus for farmed oysters distributed in the United States and internationally. International Journal of Food Microbiology, 313(108), 378. 10.1016/j.ijfoodmicro.2019.108378 [DOI] [PubMed] [Google Scholar]
- Luan, X. Y. , Chen, J. X. , Liu, Y. , Li, Y. , Jia, J. T. , Liu, R. , & Zhang, X. H. (2008). Rapid quantitative detection of Vibrio parahaemolyticus in seafood by MPN‐PCR. Current Microbiology, 57(3), 218–221. 10.1007/s00284-008-9177-x [DOI] [PubMed] [Google Scholar]
- Lulijwa, R. , Rupia, E. J. , & Alfaro, A. C. (2020). Antibiotic use in aquaculture, policies and regulation, health and environmental risks: A review of the top 15 major producers. Reviews in Aquaculture. 12 (2), 640–663. 10.1111/raq.12344 [DOI] [Google Scholar]
- Lutz, C. , Erken, M. , Noorian, P. , Sun, S. Y. , & McDougald, D. (2013). Environmental reservoirs and mechanisms of persistence of Vibrio cholerae . Frontiers in Microbiology, 4, 375. 10.3389/fmicb.2013.00375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lydon, K. , Farrell‐Evans, M. , & Jones, J. (2015). Evaluation of ice slurries as a control for postharvest growth of Vibrio spp. in oysters and potential for filth contamination. Journal of Food Protection, 78(7), 1375–1379. 10.4315/0362-028X.JFP-14-557 [DOI] [PubMed] [Google Scholar]
- Lydon, K. A. , Kinsey, T. , Le, C. , Gulig, P. A. , & Jones, J. L. (2021). Biochemical and virulence characterization of Vibrio vulnificus isolates from clinical and environmental sources. Frontiers in Cellular and Infection Microbiology, 11, 637019. 10.3389/fcimb.2021.637019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, J. Y. , Zhu, X. K. , Hu, R. G. , Qi, Z. Z. , Sun, W. C. , Hao, Z. P. , Cong, W. , & Kang, Y. H. (2023). A systematic review, meta‐analysis and meta‐regression of the global prevalence of foodborne Vibrio spp. infection in fishes: A persistent public health concern. Marine Pollution Bulletin, 187, 114521. 10.1016/j.marpolbul.2022.114521 [DOI] [PubMed] [Google Scholar]
- Machado, A. , & Bordalo, A. A. (2016). Detection and quantification of Vibrio cholerae, Vibrio parahaemolyticus, and Vibrio vulnificus in coastal waters of Guinea‐Bissau (West Africa). EcoHealth, 13(2), 339–349. 10.1007/s10393-016-1104-1 [DOI] [PubMed] [Google Scholar]
- Mahmoud, B. S. (2009). Reduction of Vibrio vulnificus in pure culture, half shell and whole shell oysters (Crassostrea virginica) by X‐ray. International Journal of Food Microbiology, 130(2), 135–139. 10.1016/j.ijfoodmicro.2009.01.023 [DOI] [PubMed] [Google Scholar]
- Mahmoud, B. S. , & Burrage, D. D. (2009). Inactivation of Vibrio parahaemolyticus in pure culture, whole live and half shell oysters (Crassostrea virginica) by X‐ray. Letters in Applied Microbiology, 48(5), 572–578. 10.1111/j.1472-765X.2009.02573.x [DOI] [PubMed] [Google Scholar]
- Makino, K. , Oshima, K. , Kurokawa, K. , Yokoyama, K. , Uda, T. , Tagomori, K. , Iijima, Y. , Najima, M. , Nakano, M. , Yamashita, A. , Kubota, Y. , Kimura, S. , Yasunaga, T. , Honda, T. , Shinagawa, H. , Hattori, M. , & Iida, T. (2003). Genome sequence of Vibrio parahaemolyticus: A pathogenic mechanism distinct from that of V cholerae. The Lancet, 361(9359), 743–749. 10.1016/s0140-6736(03)12659-1 [DOI] [PubMed] [Google Scholar]
- Malcolm, T. T. H. , Cheah, Y. K. , Radzi, C. , Kantilal, H. K. , Martinez‐Urtaza, J. , Nishibuchi, M. , & Son, R. (2016). Microbial risk assessment of Vibrio parahaemolyticus in bloody clams in Malaysia: A preliminary model from retail to consumption. Microbial Risk Analysis, 4, 43–51. 10.1016/j.mran.2016.07.002 [DOI] [Google Scholar]
- Mangat, C. S. , Boyd, D. , Janecko, N. , Martz, S. L. , Desruisseau, A. , Carpenter, M. , Reid‐Smith, R. J. , & Mulvey, M. R. (2016). Characterization of VCC‐1, a novel Ambler class A carbapenemase from Vibrio cholerae isolated from imported retail shrimp sold in Canada. Antimicrobial Agents and Chemotherapy, 60(3), 1819–1825. 10.1128/aac.02812-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markov, E. Y. , Kulikalova, E. S. , Urbanovich, L. Y. , Vishnyakov, V. S. , & Balakhonov, S. V. (2015). Chitin and products of its hydrolysis in Vibrio cholerae ecology. Biochemistry, 80(9), 1109–1116. 10.1134/S0006297915090023 [DOI] [PubMed] [Google Scholar]
- Martinez‐Urtaza, J. , Baker‐Austin, C. , Jones, J. L. , Newton, A. E. , Gonzalez‐Aviles, G. D. , & DePaola, A. (2013). Spread of Pacific Northwest Vibrio parahaemolyticus strain. New England Journal of Medicine, 369(16), 1573–1574. 10.1056/NEJMc1305535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez‐Urtaza, J. , Blanco‐Abad, V. , Rodriguez‐Castro, A. , Ansede‐Bermejo, J. , Miranda, A. , & Rodriguez‐Alvarez, M. X. (2012). Ecological determinants of the occurrence and dynamics of Vibrio parahaemolyticus in offshore areas. ISME Journal, 6(5), 994–1006. 10.1038/ismej.2011.156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez‐Urtaza, J. , Bowers, J. C. , Trinanes, J. , & DePaola, A. (2010). Climate anomalies and the increasing risk of Vibrio parahaemolyticus and Vibrio vulnificus illnesses. Food Research International, 43(7), 1780–1790. 10.1016/j.foodres.2010.04.001 [DOI] [Google Scholar]
- Martinez‐Urtaza, J. , Huapaya, B. , Gavilan, R. G. , Blanco‐Abad, V. , Ansede‐Bermejo, J. , Cadarso‐Suarez, C. , Figueiras, A. , & Trinanes, J. (2008). Emergence of Asiatic vibrio diseases in South America in phase with El Nino. Epidemiology, 19(6), 829–837. 10.1097/EDE.0b013e3181883d43 [DOI] [PubMed] [Google Scholar]
- Martinez‐Urtaza, J. , Lozano‐Leon, A. , Varela‐Pet, J. , Trinanes, J. , Pazos, Y. , & Garcia‐Martin, O. (2008). Environmental determinants of the occurrence and distribution of Vibrio parahaemolyticus in the rias of Galicia, Spain. Applied and Environmental Microbiology, 74(1), 265–274. 10.1128/aem.01307-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez‐Urtaza, J. , Powell, A. , Jansa, J. , Rey, J. L. C. , Montero, O. P. , Campello, M. G. , Lopez, M. J. Z. , Pousa, A. , Valles, M. J. F. , Trinanes, J. , Hervio‐Heath, D. , Keay, W. , Bayley, A. , Hartnell, R. , & Baker‐Austin, C. (2016). Epidemiological investigation of a foodborne outbreak in Spain associated with US West coast genotypes of Vibrio parahaemolyticus . Springerplus, 5, 1–8. 10.1186/s40064-016-1728-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez‐Urtaza, J. , van Aerle, R. , Abanto, M. , Haendiges, J. , Myers, R. A. , Trinanes, J. , Baker‐Austin, C. , & Gonzalez‐Escalona, N. (2017). Genomic variation and evolution of Vibrio parahaemolyticus ST36 over the course of a transcontinental epidemic expansion. mBio, 8(6), e01425‐17. 10.1128/mBio.01425-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massad, G. , & Oliver, J. D. (1987). New selective and differential medium for Vibrio cholerae and Vibrio vulnificus . Applied and Environmental Microbiology, 53(9), 2262–2264. 10.1128/aem.53.9.2262-2264.1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin, J. B. , DePaola, A. , Bopp, C. A. , Martinek, K. A. , Napolilli, N. P. , Allison, C. G. , Murray, S. L. , Thompson, E. C. , Bird, M. M. , & Middaugh, J. P. (2005). Outbreak of Vibrio parahaemolyticus gastroenteritis associated with Alaskan oysters. New England Journal of Medicine, 353(14), 1463–1470. 10.1056/NEJMoa051594 [DOI] [PubMed] [Google Scholar]
- Meibom, K. L. , Blokesch, M. , Dolganov, N. A. , Wu, C. Y. , & Schoolnik, G. K. (2005). Chitin induces natural competence in Vibrio cholerae . Science, 310(5755), 1824–1827. 10.1126/science.1120096 [DOI] [PubMed] [Google Scholar]
- Melody, K. , Senevirathne, R. , Janes, M. , Jaykus, L. A. , & Supan, J. (2008). Effectiveness of icing as a postharvest treatment for control of Vibrio vulnificus and Vibrio parahaemolyticus in the eastern oyster (Crassostrea virginica). Journal of Food Protection, 71(7), 1475–1480. 10.4315/0362-028X-71.7.1475 [DOI] [PubMed] [Google Scholar]
- Merkel Sandra, M. , Alexander, S. , Zufall, E. , Oliver James, D. , & Huet‐Hudson Yvette, M. (2001). Essential role for estrogen in protection againstVibrio vulnificus‐induced endotoxic shock. Infection and Immunity, 69(10), 6119–6122. 10.1128/iai.69.10.6119-6122.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midgett, C. R. , & Kull, F. J. (2023). Structural insights into regulation of Vibrio virulence gene networks. Advances in Experimental Medicine and Biology, 1404, 269–294. 10.1007/978-3-031-22,997-8_14 [DOI] [PubMed] [Google Scholar]
- Miles, D. W. , Ross, T. , Olley, J. , & McMeekin, T. A. (1997). Development and evaluation of a predictive model for the effect of temperature and water activity on the growth rate of Vibrio parahaemolyticus . International Journal of Food Microbiology, 38(2–3), 133–142. 10.1016/s0168-1605(97)00100-1 [DOI] [PubMed] [Google Scholar]
- Molina‐Quiroz, R. C. , & Silva‐Valenzuela, C. A. (2023). Interactions of vibrio phages and their hosts in aquatic environments. Current Opinion in Microbiology, 74, 102308. 10.1016/j.mib.2023.102308 [DOI] [PubMed] [Google Scholar]
- Mora, C. , McKenzie, T. , Gaw, I. M. , Dean, J. M. , Von Hammerstein, H. , Knudson, T. A. , Setter, R. O. , Smith, C. Z. , Webster, K. M. , Patz, J. A. , & Franklin, E. C. (2022). Over half of known human pathogenic diseases can be aggravated by climate change. Nature Climate Change, 12(9), 869. 10.1038/s41558-022-01426-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori, M. , Hamamoto, A. , Takahashi, A. , Nakano, M. , Wakikawa, N. , Tachibana, S. , Ikehara, T. , Nakaya, Y. , Akutagawa, M. , & Kinouchi, Y. (2007). Development of a new water sterilization device with a 365 nm UV‐LED. Medical & Biological Engineering & Computing, 45(12), 1237–1241. 10.1007/s11517-007-0263-1 [DOI] [PubMed] [Google Scholar]
- Motes, M. L. , DePaola, A. , Cook, D. W. , Veazey, J. E. , Hunsucker, J. C. , Garthright, W. E. , Blodgett, R. J. , & Chirtel, S. J. (1998). Influence of water temperature and salinity on Vibrio vulnificus in Northern Gulf and Atlantic Coast oysters (Crassostrea virginica). Applied and Environmental Microbiology, 64(4), 1459–1465. 10.1128/aem.64.4.1459-1465.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudoh, M. F. , Parveen, S. , Schwarz, J. , Rippen, T. , & Chaudhuri, A. (2014). The effects of storage temperature on the growth of Vibrio parahaemolyticus and organoleptic properties in oysters. Frontiers in Public Health, 2, 45. 10.3389/fpubh.2014.00045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muntada‐Garriga, J. M. , Rodriguez‐Jerez, J. J. , Lopez‐Sabater, E. I. , & Mora‐Ventura, M. T. (1995). Effect of chill and freezing temperatures on survival of Vibrio parahaemolyticus inoculated in homogenates of oyster meat. Letters in Applied Microbiology, 20(4), 225–227. 10.1111/j.1472-765x.1995.tb00433.x [DOI] [PubMed] [Google Scholar]
- Nakahashi, M. , Mawatari, K. , Hirata, A. , Maetani, M. , Shimohata, T. , Uebanso, T. , Hamada, Y. , Akutagawa, M. , Kinouchi, Y. , & Takahashi, A. (2014). Simultaneous irradiation with different wavelengths of ultraviolet light has synergistic bactericidal effect on Vibrio parahaemolyticus . Photochemistry and Photobiology, 90(6), 1397–1403. 10.1111/php.12309 [DOI] [PubMed] [Google Scholar]
- Nawel, Z. , Rima, O. , & Amira, B. (2022). An overview on vibrio temperate phages: Integration mechanisms, pathogenicity, and lysogeny regulation. Microbial Pathogenesis, 165, 105490. 10.1016/j.micpath.2022.105490 [DOI] [PubMed] [Google Scholar]
- Ndraha, N. , & Hsiao, H. I. (2019a). Exposure assessment and sensitivity analysis for chilled shrimp during distribution: A case study of home delivery services in Taiwan. Journal of Food Science, 84(4), 859–870. 10.1111/1750-3841.14498 [DOI] [PubMed] [Google Scholar]
- Ndraha, N. , & Hsiao, H. I. (2019b). The risk assessment of Vibrio parahaemolyticus in raw oysters in Taiwan under the seasonal variations, time horizons, and climate scenarios. Food Control, 102, 188–196. 10.1016/j.foodcont.2019.03.020 [DOI] [Google Scholar]
- Ndraha, N. , & Hsiao, H. I. (2021). Influence of climatic factors on the temporal occurrence and distribution of total and pathogenic Vibrio parahaemolyticus in oyster culture environments in Taiwan. Food Microbiology, 98, 103765. 10.1016/j.fm.2021.103765 [DOI] [PubMed] [Google Scholar]
- Ndraha, N. , Huang, L. H. , Wu, V. C. H. , & Hsiao, H. I. (2022). Vibrio parahaemolyticus in seafood: Recent progress in understanding influential factors at harvest and food‐safety intervention approaches. Current Opinion in Food Science, 48, 100927. 10.1016/j.cofs.2022.100927 [DOI] [Google Scholar]
- Ndraha, N. , Lin, H. Y. , Lin, H. J. , & Hsiao, H. (2023). Modeling the risk of Vibrio parahaemolyticus in oysters in Taiwan by considering seasonal variations, time periods, climate change scenarios, and post‐harvest interventions. Microbial Risk Analysis, 25(17), 100275. 10.1016/j.mran.2023.100275 [DOI] [Google Scholar]
- Ndraha, N. , Wong, H. C. , & Hsiao, H. I. (2020). Managing the risk of Vibrio parahaemolyticus infections associated with oyster consumption: A review. Comprehensive Reviews in Food Science and Food Safety, 19(3), 1187–1217. 10.1111/1541-4337.12557 [DOI] [PubMed] [Google Scholar]
- Neetoo, H. , Reega, K. , Manoga, Z. S. , Nazurally, N. , Bhoyroo, V. , Allam, M. , Jaufeerally‐Fakim, Y. , Ghoorah, A. W. , Jaumdally, W. , Hossen, A. M. , Mayghun, F. , Ismail, A. , & Hosenally, M. (2022). Prevalence, genomic characterization, and risk assessment of human pathogenic Vibrio species in seafood. Journal of Food Protection, 85(11), 1553–1565. 10.4315/jfp-22-064 [DOI] [PubMed] [Google Scholar]
- Nishibuchi, M. , Janda, J. M. , & Ezaki, T. (1996). The thermostable direct hemolysin gene (tdh) of vibrio hollisae is dissimilar in prevalence to and phylogenetically distant from the tdh genes of other vibrios: Implications in the horizontal transfer of the tdh gene. Microbiology and Immunology, 40(1), 59–65. 10.1111/j.1348-0421.1996.tb03304.x [DOI] [PubMed] [Google Scholar]
- Niu, B. , Hong, B. , Zhang, Z. , Mu, L. , Malakar, P. K. , Liu, H. , Pan, Y. , & Zhao, Y. (2018). A novel qPCR method for simultaneous detection and quantification of viable pathogenic and non‐pathogenic Vibrio parahaemolyticus (tlh(+), tdh(+), and ureR (+)). Frontiers in Microbiology, 9, 1747. 10.3389/fmicb.2018.01747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noorian, P. , Hoque, M. M. , Espinoza‐Vergara, G. , & McDougald, D. (2023). Environmental reservoirs of pathogenic Vibrio spp. and their role in disease: The list keeps expanding. Advances in Experimental Medicine and Biology, 1404, 99–126. 10.1007/978-3-031-22,997-8_6 [DOI] [PubMed] [Google Scholar]
- Normanno, G. , Parisi, A. , Addante, N. , Quaglia, N. C. , Dambrosio, A. , Montagna, C. , & Chiocco, D. (2006). Vibrio parahaemolyticus, Vibrio vulnificus and microorganisms of fecal origin in mussels (Mytilus galloprovincialis) sold in the Puglia region (Italy). International Journal of Food Microbiology, 106(2), 219–222. 10.1016/j.ijfoodmicro.2005.05.020 [DOI] [PubMed] [Google Scholar]
- Octavia, S. , Salim, A. , Kurniawan, J. , Lam, C. , Leung, Q. , Ahsan, S. , Reeves, P. R. , Nair, G. B. , & Lan, R. (2013). Population structure and evolution of non‐O1/non‐O139 Vibrio cholerae by multilocus sequence typing. PLoS One, 8(6), e65342. 10.1371/journal.pone.0065342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odeyemi, O. A. (2016). Incidence and prevalence of Vibrio parahaemolyticus in seafood: A systematic review and meta‐analysis. Springerplus, 5, 464. 10.1186/s40064-016-2115-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oguma, K. , Rattanakul, S. , & Masaike, M. (2019). Inactivation of health‐related microorganisms in water using UV light‐emitting diodes. Water Supply, 19(5), 1507–1514. 10.2166/ws.2019.022 [DOI] [Google Scholar]
- Oh, H. , Yoon, Y. , Ha, J. , Lee, J. , Shin, I. I. S. , Kim, Y.‐M. , Park, K.‐S. , & Kim, S. (2021). Risk assessment of vibriosis by Vibrio cholerae and Vibrio vulnificus in whip‐arm octopus consumption in South Korea. Fisheries and Aquatic Sciences, 24, 207–218. 10.47853/FAS.2021.e21 [DOI] [Google Scholar]
- Okada, N. , Iida, T. , Park, K. S. , Goto, N. , Yasunaga, T. , Hiyoshi, H. , Matsuda, S. , Kodama, T. , & Honda, T. (2009). Identification and characterization of a novel type III secretion system in trh‐positive Vibrio parahaemolyticus strain TH3996 reveal genetic lineage and diversity of pathogenic machinery beyond the species level. Infection and Immunity, 77(2), 904–913. 10.1128/iai.01184-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver, J. D. (2013). Vibrio vulnificus: Death on the half shell. A personal journey with the pathogen and its ecology. Microbial Ecology, 65(4), 793–799. 10.1007/s00248-012-0140-9 [DOI] [PubMed] [Google Scholar]
- Oliver, J. D. (2015). The biology of Vibrio vulnificus . Microbiology Spectrum, 3(3), e‐0001‐2014. 10.1128/microbiolspec.VE-0001-2014 [DOI] [PubMed] [Google Scholar]
- Ong, H. M. G. , Zhong, Y. , Hu, C. C. , Ong, K. H. , Khor, W. C. , Schlundt, J. , & Aung, K. T. (2023). Quantitative risk evaluation of antimicrobial‐resistant Vibrio parahaemolyticus isolated from farmed Grey mullets in Singapore. Pathogens, 12(1), 93. 10.3390/pathogens12010093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottaviani, D. , Leoni, F. , Rocchegiani, E. , Santarelli, S. , Masini, L. , Di Trani, V. , Canonico, C. , Pianetti, A. , Tega, L. , & Carraturo, A. (2009). Prevalence and virulence properties of non‐O1 non‐O139 Vibrio cholerae strains from seafood and clinical samples collected in Italy. International Journal of Food Microbiology, 132(1), 47–53. 10.1016/j.ijfoodmicro.2009.03.014 [DOI] [PubMed] [Google Scholar]
- Ottaviani, D. , Leoni, F. , Talevi, G. , Masini, L. , Santarelli, S. , Rocchegiani, E. , Susini, F. , Montagna, C. , Monno, R. , D'Annibale, L. , Manso, E. , Oliva, M. , & Pazzani, C. (2013). Extensive investigation of antimicrobial resistance in Vibrio parahaemolyticus from shellfish and clinical sources, Italy. International Journal of Antimicrobial Agents, 42(2), 191–193. 10.1016/j.ijantimicag.2013.05.003 [DOI] [PubMed] [Google Scholar]
- Ottaviani, D. , Medici, L. , Talevi, G. , Napoleoni, M. , Serratore, P. , Zavatta, E. , Bignami, G. , Masini, L. , Chierichetti, S. , Fisichella, S. , & Leoni, F. (2018). Molecular characterization and drug susceptibility of non‐O1/O139 V. Cholerae strains of seafood, environmental and clinical origin, Italy. Food Microbiology, 72, 82–88. 10.1016/j.fm.2017.11.011 [DOI] [PubMed] [Google Scholar]
- Painter, J. A. , Hoekstra, R. M. , Ayers, T. , Tauxe, R. V. , Braden, C. R. , Angulo, F. J. , & Griffin, P. M. (2013). Attribution of foodborne illnesses, hospitalizations, and deaths to food commodities by using Outbreak data, United States, 1998–2008. Emerging Infectious Diseases, 19(3), 407–415. 10.3201/eid1903.111866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, S. Y. , & Ha, S.‐D. (2018). Inactivation of Vibrio parahaemolyticus in shucked raw oyster (Grassostrea gigas) and clam (Venerupis phillippinarum) by using a combination of NaClO and gamma irradiation. Food Science and Technology International, 24(1), 43–52. 10.1177/1082013217726634 [DOI] [PubMed] [Google Scholar]
- Parker, R. W. , Maurer, E. M. , Childers, A. B. , & Lewis, D. H. (1994). Effect of frozen storage and vacuum‐packaging on survival of Vibrio vulnificus in Gulf Coast oysters (Crassostrea virginica). Journal of Food Protection, 57(7), 604–606. 10.4315/0362-028x-57.7.604 [DOI] [PubMed] [Google Scholar]
- Partridge, S. R. , Kwong, S. M. , Firth, N. , & Jensen, S. O. (2018). Mobile genetic elements associated with antimicrobial resistance. Clinical Microbiology Reviews, 31(4), e00088‐17. 10.1128/cmr.00088-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parveen, S. , DaSilva, L. , DePaola, A. , Bowers, J. , White, C. , Munasinghe, K. A. , Brohawn, K. , Mudoh, M. , & Tamplin, M. (2013). Development and validation of a predictive model for the growth of Vibrio parahaemolyticus in post‐harvest shellstock oysters. International Journal of Food Microbiology, 161(1), 1–6. 10.1016/j.ijfoodmicro.2012.11.010 [DOI] [PubMed] [Google Scholar]
- Parveen, S. , Hettiarachchi, K. A. , Bowers, J. C. , Jones, J. L. , Tamplin, M. L. , McKay, R. , Beatty, W. , Brohawn, K. , DaSilva, L. V. , & DePaola, A. (2008). Seasonal distribution of total and pathogenic Vibrio parahaemolyticus in Chesapeake Bay oysters and waters. International Journal of Food Microbiology, 128(2), 354–361. 10.1016/j.ijfoodmicro.2008.09.019 [DOI] [PubMed] [Google Scholar]
- Parveen, S. , Jacobs, J. , Ozbay, G. , Chintapenta, L. K. , Almuhaideb, E. , Meredith, J. , Ossai, S. , Abbott, A. , Grant, A. , Brohawn, K. , Chigbu, P. , & Richards, G. P. (2020). Seasonal and geographical differences in total and pathogenic Vibrio parahaemolyticus and Vibrio vulnificus levels in seawater and oysters from the Delaware and Chesapeake Bays determined using several methods. Applied and Environmental Microbiology, 86(23), e01581‐20. 10.1128/aem.01581-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parveen, S. , Jahncke, M. , Elmahdi, S. , Crocker, H. , Bowers, J. , White, C. , Gray, S. , Morris, A. C. , & Brohawn, K. (2017). High salinity relaying to reduce Vibrio parahaemolyticus and Vibrio vulnificus in Chesapeake Bay oysters (Crassostrea virginica). Journal of Food Science, 82(2), 484–491. 10.1111/1750-3841.13584 [DOI] [PubMed] [Google Scholar]
- Parveen, S. , & Tamplin, M. L. (2013). Vibrio vulnificus, Vibrio parahaemolyticus and Vibrio cholerae . In R. G. Labbé & S. García (Eds.), Guide to foodborne pathogens (pp. 148–176). University of Tasmania. 10.1002/9781118684856.ch9 [DOI] [Google Scholar]
- Pearce, R. , Conrady, B. , & Guardabassi, L. (2023). Prevalence and types of extended‐spectrum β‐lactamase‐producing bacteria in retail seafood. Food, 12(16), 3033. 10.3390/foods12163033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettis, G. S. , & Mukerji, A. S. (2020). Structure, function, and regulation of the essential virulence factor capsular polysaccharide of Vibrio vulnificus . International Journal of Molecular Sciences, 21(9), 3259. 10.3390/ijms21093259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips, A. M. B. , DePaola, A. , Bowers, J. , Ladner, S. , & Grimes, D. J. (2007). An evaluation of the use of remotely sensed parameters for prediction of incidence and risk associated with Vibrio parahaemolyticus in Gulf Coast oysters (Crassostrea virginica). Journal of Food Protection, 70(4), 879–884. 10.4315/0362-028x-70.4.879 [DOI] [PubMed] [Google Scholar]
- Phuvasate, S. , Chen, M.‐H. , & Su, Y.‐C. (2012). Reductions of Vibrio parahaemolyticus in Pacific oysters (Crassostrea gigas) by depuration at various temperatures. Food Microbiology, 31(1), 51–56. 10.1016/j.fm.2012.02.004 [DOI] [PubMed] [Google Scholar]
- Phuvasate, S. , & Su, Y.‐C. (2013). Impact of water salinity and types of oysters on depuration for reducing Vibrio parahaemolyticus in Pacific oysters (Crassostrea gigas). Food Control, 32(2), 569–573. 10.1016/j.foodcont.2013.01.025 [DOI] [Google Scholar]
- Pouillot, R. , Kiermeier, A. , Guillier, L. , Cadavez, V. , & Sanaa, M. (2024). Updated parameters for listeria monocytogenes dose–response model considering pathogen virulence and age and sex of consumer. Food, 13(5), 751. 10.3390/foods13050751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghul, S. S. , Bhat, S. G. , Chandrasekaran, M. , Chandrasekaran, M. , Francis, V. , & Thachil, E. T. (2014). Biodegradation of polyvinyl alcohol‐low linear density polyethylene‐blended plastic film by consortium of marine benthic vibrios. International journal of Environmental Science and Technology, 11, 1827–1834. [Google Scholar]
- Raghunath, P. (2015). Roles of thermostable direct hemolysin (TDH) and TDH‐related hemolysin (TRH) in Vibrio parahaemolyticus . Frontiers in Microbiology, 5(4), 805. 10.3389/fmicb.2014.00805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez‐Castillo, F. Y. , Guerrero‐Barrera, A. L. , & Avelar‐González, F. J. (2023). An overview of carbapenem‐resistant organisms from food‐producing animals, seafood, aquaculture, companion animals, and wildlife. Frontiers in Veterinary Science, 10, 1158588. 10.3389/fvets.2023.1158588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos, R. J. , Miotto, M. , Squella, F. J. , Cirolini, A. , Ferreira, J. F. , & Vieira, C. R. (2012). Depuration of oysters (Crassostrea gigas) contaminated with Vibrio parahaemolyticus and Vibrio vulnificus with UV light and chlorinated seawater. Journal of Food Protection, 75(8), 1501–1506. 10.4315/0362-028x.Jfp-11-467 [DOI] [PubMed] [Google Scholar]
- Randa, M. A. , Polz, M. F. , & Lim, E. (2004). Effects of temperature and salinity on Vibrio vulnificus population dynamics as assessed by quantitative PCR. Applied and Environmental Microbiology, 70(9), 5469–5476. 10.1128/aem.70.9.5469-5476.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rantsiou, K. , Kathariou, S. , Winkler, A. , Skandamis, P. , Saint‐Cyr, M. J. , Rouzeau‐Szynalski, K. , & Amézquita, A. (2018). Next generation microbiological risk assessment: Opportunities of whole genome sequencing (WGS) for foodborne pathogen surveillance, source tracking and risk assessment. International Journal of Food Microbiology, 287, 3–9. 10.1016/j.ijfoodmicro.2017.11.007 [DOI] [PubMed] [Google Scholar]
- Rapa, R. A. , & Labbate, M. (2013). The function of integron‐associated gene cassettes in Vibrio species: The tip of the iceberg. Frontiers in Microbiology, 4(7), 385. 10.3389/fmicb.2013.00385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddi, G. , Pruss, K. , Cottingham, K. L. , Taylor, R. K. , & Almagro‐Moreno, S. (2018). Catabolism of mucus components influences motility of Vibrio cholerae in the presence of environmental reservoirs. PLoS One, 13(7), e0201383. 10.1371/journal.pone.0201383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehnstam‐Holm, A. S. , Atnur, V. , & Godhe, A. (2014). Defining the niche of Vibrio parahaemolyticus during pre‐ and post‐monsoon seasons in the coastal Arabian Sea. Microbial Ecology, 67(1), 57–65. 10.1007/s00248-013-0311-3 [DOI] [PubMed] [Google Scholar]
- Riahi, K. , van Vuuren, D. P. , Kriegler, E. , Edmonds, J. , O'Neill, B. C. , Fujimori, S. , Bauer, N. , Calvin, K. , Dellink, R. , Fricko, O. , Lutz, W. , Popp, A. , Cuaresma, J. C. , Kc, S. , Leimbach, M. , Jiang, L. , Kram, T. , Rao, S. , Emmerling, J. , … Tavoni, M. (2017). The shared socioeconomic pathways and their energy, land use, and greenhouse gas emissions implications: An overview. Global Environmental Change, 42, 153–168. 10.1016/j.gloenvcha.2016.05.009 [DOI] [Google Scholar]
- Richards, G. P. , Chintapenta, L. K. , Watson, M. A. , Abbott, A. G. , Ozbay, G. , Uknalis, J. , Oyelade, A. A. , & Parveen, S. (2019). Bacteriophages against pathogenic Vibrios in Delaware Bay oysters (Crassostrea virginica) during a period of high levels of pathogenic Vibrio parahaemolyticus . Food and Environmental Virology, 11(2), 101–112. 10.1007/s12560-019-09365-5 [DOI] [PubMed] [Google Scholar]
- Robert‐Pillot, A. , Copin, S. , Gay, M. , Malle, P. , & Quilici, M. L. (2010). Total and pathogenic Vibrio parahaemolyticus in shrimp: Fast and reliable quantification by real‐time PCR. International Journal of Food Microbiology, 143(3), 190–197. 10.1016/j.ijfoodmicro.2010.08.016 [DOI] [PubMed] [Google Scholar]
- Robert‐Pillot, A. , Copin, S. , Himber, C. , Gay, M. , & Quilici, M.‐L. (2014). Occurrence of the three major Vibrio species pathogenic for human in seafood products consumed in France using real‐time PCR. International Journal of Food Microbiology, 189, 75–81. 10.1016/j.ijfoodmicro.2014.07.014 [DOI] [PubMed] [Google Scholar]
- Roig, F. J. , Gonzalez‐Candelas, F. , Sanjuan, E. , Fouz, B. , Feil, E. J. , Llorens, C. , Baker‐Austin, C. , Oliver, J. D. , Danin‐Poleg, Y. , Gibas, C. J. , Kashi, Y. , Gulig, P. A. , Morrison, S. S. , & Amaro, C. (2018). Phylogeny of Vibrio vulnificus from the analysis of the core‐genome: Implications for intra‐species taxonomy. Frontiers in Microbiology, 8(13), 2613. 10.3389/fmicb.2017.02613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanello, M. , Napoli, C. D. , Green, C. , Kennard, H. , Lampard, P. , Scamman, D. , Walawender, M. , Ali, Z. , Ameli, N. , Ayeb‐Karlsson, S. , Beggs, P. J. , Belesova, K. , Berrang Ford, L. , Bowen, K. , Cai, W. , Callaghan, M. , Campbell‐Lendrum, D. , Chambers, J. , Cross, T. J. , … Costello, A. (2023). The 2023 report of the lancet countdown on health and climate change: The imperative for a health‐centred response in a world facing irreversible harms. The Lancet, 402(10419), 2346–2394. 10.1016/s0140-6736(23)01859-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronholm, J. , Lau, F. , & Banerjee, S. K. (2016). Emerging seafood preservation techniques to extend freshness and minimize vibrio contamination. Frontiers in Microbiology, 7, 350. 10.3389/fmicb.2016.00350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roobab, U. , Fidalgo, L. G. , Arshad, R. N. , Khan, A. W. , Zeng, X. A. , Bhat, Z. F. , Bekhit, A. E. A. , Batool, Z. , & Aadil, R. M. (2022). High‐pressure processing of fish and shellfish products: Safety, quality, and research prospects. Comprehensive Reviews in Food Science and Food Safety, 21(4), 3297–3325. 10.1111/1541-4337.12977 [DOI] [PubMed] [Google Scholar]
- Rosec, J.‐P. , Causse, V. , Cruz, B. , Rauzier, J. , & Carnat, L. (2012). The international standard ISO/TS 21872‐1 to study the occurence of total and pathogenic Vibrio parahaemolyticus and Vibrio cholerae in seafood: ITS improvement by use of a chromogenic medium and PCR. International Journal of Food Microbiology, 157(2), 189–194. 10.1016/j.ijfoodmicro.2012.04.026 [DOI] [PubMed] [Google Scholar]
- Sampaio, A. , Silva, V. , Poeta, P. , & Aonofriesei, F. (2022). Vibrio spp.: Life strategies, ecology, and risks in a changing environment. Diversity‐Basel, 14(2), 97. 10.3390/d14020097 [DOI] [Google Scholar]
- Sani, N. A. , Ariyawansa, S. , Babji, A. S. , & Hashim, J. K. (2013). The risk assessment of Vibrio parahaemolyticus in cooked black tiger shrimps (Penaeus monodon) in Malaysia. Food Control, 31(2), 546–552. 10.1016/j.foodcont.2012.10.018 [DOI] [Google Scholar]
- Scallan, E. , Hoekstra, R. M. , Angulo, F. J. , Tauxe, R. V. , Widdowson, M. A. , Roy, S. L. , Jones, J. L. , & Griffin, P. M. (2011). Foodborne illness acquired in the United States‐major pathogens. Emerging Infectious Diseases, 17(1), 7–15. 10.3201/eid1701.P11101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCF (Scientific Committee for Food) . (1998). Opinion of the Scientific Committee on Food on the irradiation of eight foodstuffs .
- Schar, D. , Zhao, C. , Wang, Y. , Larsson, D. G. J. , Gilbert, M. , & Van Boeckel, T. P. (2021). Twenty‐year trends in antimicrobial resistance from aquaculture and fisheries in Asia. Nature Communications, 12(1), 5384. 10.1038/s41467-021-25,655-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schets, F. M. , van den Berg, H. , Marchese, A. , Garbom, S. , & Husman, A. M. D. (2011). Potentially human pathogenic vibrios in marine and fresh bathing waters related to environmental conditions and disease outcome. International Journal of Hygiene and Environmental Health, 214(5), 399–406. 10.1016/j.ijheh.2011.05.003 [DOI] [PubMed] [Google Scholar]
- Schirmeister, F. , Dieckmann, R. , Bechlars, S. , Bier, N. , Faruque, S. M. , & Strauch, E. (2014). Genetic and phenotypic analysis of Vibrio cholerae non‐O1, non‐O139 isolated from German and Austrian patients. European Journal of Clinical Microbiology & Infectious Diseases, 33(5), 767–778. 10.1007/s10096-013-2011-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCVM (Scientific Committee on Veterinary Measures relating to Public Health) . (2001). Opinion on Vibrio vulnificus and Vibrio parahaemolyticus in raw and undercooked seafood. 64 pp. https://food.ec.europa.eu/system/files/2020‐12/sci‐com_scv_out45_en.pdf [Google Scholar]
- Sechi, L. A. , Duprè, I. , Deriu, A. , Fadda, G. , & Zanetti, S. (2000). Distribution of Vibrio cholerae virulence genes among different vibrio species isolated in Sardinia, Italy. Journal of Applied Microbiology, 88(3), 475–481. 10.1046/j.1365-2672.2000.00982.x [DOI] [PubMed] [Google Scholar]
- Semenza, J. C. , Trinanes, J. , Lohr, W. , Sudre, B. , Löfdahl, M. , Martinez‐Urtaza, J. , Nichols, G. L. , & Rocklöv, J. (2017). Environmental suitability of Vibrio infections in a warming climate: An early warning system. Environmental Health Perspectives, 125(10), 107004. 10.1289/ehp2198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senoh, M. , Ghosh‐Banerjee, J. , Ramamurthy, T. , Colwell, R. R. , Miyoshi, S. , Nair, G. B. , & Takeda, Y. (2012). Conversion of viable but nonculturable enteric bacteria to culturable by co‐culture with eukaryotic cells. Microbiology and Immunology, 56(5), 342–345. 10.1111/j.1348-0421.2012.00440.x [DOI] [PubMed] [Google Scholar]
- Serratore, P. , Ostanello, F. , Passalacqua, P. L. , Zavatta, E. , Bignami, G. , Serraino, A. , & Giacometti, F. (2016). First multi‐year retrospective study on Vibrio parahaemolyticus and Vibrio vulnificus prevalence in Ruditapes philippinarum harvested in Sacca di Goro, Italy. Italian Journal of Food Safety, 5(4), 214–218. 10.4081/ijfs.2016.6161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serratore, P. , Zavatta, E. , Fiocchi, E. , Serafini, E. , Serraino, A. , Giacometti, F. , & Bignami, G. (2017). Preliminary study on the antimicrobial susceptibility pattern related to the genotype of Vibrio vulnificus strains isolated in the north‐western Adriatic Sea coastal area. Italian Journal of Food Safety, 6(4), 6843. 10.4081/ijfs.2017.6843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao, C. P. , & Hor, L. I. (2001). Regulation of metalloprotease gene expression in Vibrio vulnificus by a Vibrio harveyi LuxR homologue. Journal of Bacteriology, 183(4), 1369–1375. 10.1128/jb.183.4.1369-1375.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh, H. I. , Najiah, M. , Fadhlina, A. , Laith, A. A. , Nor, M. M. , Jalal, K. C. A. , & Kasan, N. A. (2022). Temperature upshift mostly but not always enhances the growth of vibrio species: A systematic review. Frontiers in Marine Science, 9, 959830. 10.3389/fmars.2022.959830 [DOI] [Google Scholar]
- Shen, X. S. , Cai, Y. Q. , Liu, C. C. , Liu, W. W. , Hui, Y. H. , & Su, Y. C. (2009). Effect of temperature on uptake and survival of Vibrio parahaemolyticus in oysters (Crassostrea plicatula). International Journal of Food Microbiology, 136(1), 129–132. 10.1016/j.ijfoodmicro.2009.09.012 [DOI] [PubMed] [Google Scholar]
- Shin, O. S. , Tam, V. C. , Suzuki, M. , Ritchie, J. M. , Bronson, R. T. , Waldor, M. K. , & Mekalanos, J. J. (2011). Type III secretion is essential for the rapidly fatal diarrheal disease caused by non‐O1, non‐O139 Vibrio cholerae . mBio, 2(3), e00106‐11. 10.1128/mBio.00106-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton, F. L. , Attwell, R. , Jangi, S. , & Colwell, R. R. (1982). Effects of temperature and salinity on vibrio‐cholerae growth. Applied and Environmental Microbiology, 44(5), 1047–1058. 10.1128/aem.44.5.1047-1058.1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobrinho, P. D. C. , Destro, M. T. , Franco, B. , & Landgraf, M. (2010). Correlation between environmental factors and prevalence of Vibrio parahaemolyticus in oysters harvested in the southern coastal area of Sao Paulo State, Brazil. Applied and Environmental Microbiology, 76(4), 1290–1293. 10.1128/aem.00861-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobrinho, P. D. C. , Destro, M. T. , Franco, B. , & Landgraf, M. (2014). A quantitative risk assessment model for Vibrio parahaemolyticus in raw oysters in Sao Paulo State, Brazil. International Journal of Food Microbiology, 180, 69–77. 10.1016/j.ijfoodmicro.2014.04.008 [DOI] [PubMed] [Google Scholar]
- Sony, M. , Sumithra, T. G. , Anusree, V. N. , Amala, P. V. , Reshma, K. J. , Alex, S. , & Sanil, N. K. (2021). Antimicrobial resistance and virulence characteristics ofVibrio vulnificus, Vibrio parahaemolyticus and Vibrio harveyi from natural disease outbreaks of marine/estuarine fishes. Aquaculture, 539(14), 736608. 10.1016/j.aquaculture.2021.736608 [DOI] [Google Scholar]
- Spaur, M. , Davis, B. J. K. , Kivitz, S. , DePaola, A. , Bowers, J. C. , Curriero, F. C. , & Nachman, K. E. (2020). A systematic review of post‐harvest interventions for Vibrio parahaemolyticus in raw oysters. Science of the Total Environment, 745(140), 795. 10.1016/j.scitotenv.2020.140795 [DOI] [PubMed] [Google Scholar]
- Sterk, A. , Schets, F. M. , Husman, A. M. D. , de Nijs, T. , & Schijven, J. F. (2015). Effect of climate change on the concentration and associated risks of vibrio spp. in Dutch recreational waters. Risk Analysis, 35(9), 1717–1729. 10.1111/risa.12365 [DOI] [PubMed] [Google Scholar]
- Stratev, D. , Stoyanchev, T. , & Bangieva, D. (2021). Occurrence of Vibrio parahaemolyticus and Staphylococcus aureus in seafood. Italian . Journal of Food Safety, 10(4), 10027. 10.4081/ijfs.2021.10027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suffredini, E. , Mioni, R. , Mazzette, R. , Bordin, P. , Serratore, P. , Fois, F. , Piano, A. , Cozzi, L. , & Croci, L. (2014). Detection and quantification of Vibrio parahaemolyticus in shellfish from Italian production areas. International Journal of Food Microbiology, 184, 14–20. 10.1016/j.ijfoodmicro.2014.04.016 [DOI] [PubMed] [Google Scholar]
- Sumner, J. , & Ross, T. (2002). A semi‐quantitative seafood safety risk assessment. International Journal of Food Microbiology, 77, 55–59. 10.1016/s0168-1605(02)00062-4 [DOI] [PubMed] [Google Scholar]
- Sun, Y. , Bernardy, E. E. , Hammer, B. K. , & Miyashiro, T. (2013). Competence and natural transformation in vibrios. Molecular Microbiology, 89(4), 583–595. 10.1111/mmi.12307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemura, A. E. , Chien, D. M. , & Polz, M. E. (2014). Associations and dynamics of Vibrionaceae in the environment, from the genus to the population level. Frontiers in Microbiology, 5, 38. 10.3389/fmicb.2014.00038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takoundjou, A. C. K. , Bughe, R. N. , Tong, A. N. , Kamdem, S. L. S. , & Essia‐Ngang, J. J. (2022). Quantitative risk assessment of Vibrio parahaemolyticus toxi infection associated with the consumption of roasted shrimp (Penaeus monodon). Journal of Food Quality, 13, 5965151. 10.1155/2022/5965151 [DOI] [Google Scholar]
- Tamplin, M. L. (1994). The seasonal occurrence of Vibrio vulnificus in shellfish, seawater, and sediment in United States coastal waters . Final report to Salstonstall‐Kennedy Grant Program, US Department of Commerce.
- Tan, C. W. , Malcolm, T. T. H. , Premarathne, J. , New, C. Y. , Kuan, C. H. , Thung, T. Y. , Chang, W. S. , Loo, Y. Y. , Rukayadi, Y. , Nakaguchi, Y. , Nishibuchi, M. , & Radu, S. (2019). Preliminary quantitative microbial risk assessment of pathogenic Vibrio parahaemolyticus in short mackerel in Malaysia. Microbial Risk Analysis, 12, 11–19. 10.1016/j.mran.2019.02.001 [DOI] [Google Scholar]
- Tang, X. , Zhao, Y. , Sun, X. , Xie, J. , Pan, Y. , & Malakar, P. K. (2015). Predictive model of Vibrio parahaemolyticus O3:K6 growth on cooked Litopenaeus vannamei. Annals of Microbiology, 65(1), 487–493. 10.1007/s13213-014-0884-1 [DOI] [Google Scholar]
- Taylor, M. , Cheng, J. , Sharma, D. , Bitzikos, O. , Gustafson, R. , Fyfe, M. , Greve, R. , Murti, M. , Stone, J. , Honish, L. , Mah, V. , Punja, N. , Hexemer, A. , McIntyre, L. , Henry, B. , Kendall, P. , Atkinson, R. , Buenaventura, E. , Martinez‐Perez, A. , … Outbreak Invesitigation, T. (2018). Outbreak of Vibrio parahaemolyticus associated with consumption of raw oysters in Canada, 2015. Foodborne Pathogens and Disease, 15(9), 554–559. 10.1089/fpd.2017.2415 [DOI] [PubMed] [Google Scholar]
- Taylor, M. A. , Yu, J. W. , Howell, T. L. , & Jones, S. H. (2018). Varying success of relaying to reduce Vibrio parahaemolyticus levels in oysters (Crassostrea virginica). Journal of Food Protection, 81(4), 659–669. 10.4315/0362-028X.JFP-17-363 [DOI] [PubMed] [Google Scholar]
- Teunis, P. F. M. , & Havelaar, A. H. (2000). The Beta Poisson dose–response model is not a single‐hit model [article]. Risk Analysis, 20(4), 513–520. 10.1111/0272-4332.204048 [DOI] [PubMed] [Google Scholar]
- Thompson, F. L. , Hoste, B. , Vandemeulebroecke, K. , & Swings, J. (2003). Reclassification of Vibrio hollisae as Grimontia hollisae gen. nov., comb. nov. International Journal of Systematic and Evolutionary Microbiology, 53, 1615–1617. 10.1099/ijs.0.02660-0 [DOI] [PubMed] [Google Scholar]
- Thompson, F. L. , Iida, T. , & Swings, J. (2004). Biodiversity of Vibrios. Microbiology and Molecular Biology Reviews, 68(3), 403–431. 10.1128/mmbr.68.3.403-431.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thupila, N. , Ratana‐arporn, P. , & Wilaipun, P. (2011). Radiation resistances and decontamination of common pathogenic bacteria contaminated in white scar oyster (Crassostrea belcheri) in Thailand. Radiation Physics and Chemistry, 80(7), 828–832. 10.1016/j.radphyschem.2011.02.030 [DOI] [Google Scholar]
- Tian, Z. , Yang, L. L. , Qi, X. , Zheng, Q. Y. , Shang, D. J. , & Cao, J. J. (2022). Visual LAMP method for the detection of Vibrio vulnificus in aquatic products and environmental water. BMC Microbiology, 22(1), 256. 10.1186/s12866-022-02656-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treangen, T. J. , Ondov, B. D. , Koren, S. , & Phillippy, A. M. (2014). The harvest suite for rapid core‐genome alignment and visualization of thousands of intraspecific microbial genomes. Genome Biology, 15(11), 524. 10.1186/s13059-014-0524-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinanes, J. , & Martinez‐Urtaza, J. (2021). Future scenarios of risk of vibrio infections in a warming planet: A global mapping study. Lancet Planetary Health, 5(7), E426–E435. 10.1016/S2542-5196(21)00169-8 [DOI] [PubMed] [Google Scholar]
- Urakawa, H. , & Rivera, I. N. G. (2006). In F. L. Thompson, B. Austin, & J. Swings (Eds.), Aquatic environment. (pp. 175–189). Biology of Vibrios. [Google Scholar]
- US FDA (United States of America Food and Drug Administration) . (2005). Quantitative risk assessment on the public health impact of pathogenic Vibrio parahaemolyticus in raw oysters . https://www.fda.gov/food/cfsan‐risk‐safety‐assessments/quantitative‐risk‐assessment‐public‐health‐impact‐pathogenic‐vibrio‐parahaemolyticus‐raw‐oysters
- Vaiyapuri, M. , Pailla, S. , Rao Badireddy, M. , Pillai, D. , Chandragiri Nagarajarao, R. , & Prasad Mothadaka, M. (2021). Antimicrobial resistance in Vibrios of shrimp aquaculture: Incidence, identification schemes, drivers and mitigation measures. Aquaculture Research, 52(7), 2923–2941. 10.1111/are.15142 [DOI] [Google Scholar]
- Valero, A. , Arroyo‐Lopez, F. N. , Cabo, M. L. , Chen, S. , & Perez‐Diaz, I. M. (2021). Editorial: Vibrio species in the food processing chain. Frontiers in Microbiology, 12(2), 796796. 10.3389/fmicb.2021.796796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valiente, E. , Lee, C. T. , Hor, L. I. , Fouz, B. , & Amaro, C. (2008). Role of the metalloprotease Vvp and the virulence plasmid pR99 of Vibrio vulnificus serovar E in surface colonization and fish virulence. Environmental Microbiology, 10(2), 328–338. 10.1111/j.1462-2920.2007.01454.x [DOI] [PubMed] [Google Scholar]
- van Daalen, K. R. , Tonne, C. , Semenza, J. C. , Rocklöv, J. , Markandya, A. , Dasandi, N. , Jankin, S. , Achebak, H. , Ballester, J. , Bechara, H. , Beck, T. M. , Callaghan, M. W. , Carvalho, B. M. , Chambers, J. , Pradas, M. C. , Courtenay, O. , Dasgupta, S. , Eckelman, M. J. , Farooq, Z. , … Lowe, R. (2024). The 2024 Europe report of the lancet countdown on health and climate change: Unprecedented warming demands unprecedented action. The Lancet Public Health, 9(7), E495–E522. 10.1016/s2468-2667(24)00055-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Bergh, B. , Fauvart, M. , & Michiels, J. (2017). Formation, physiology, ecology, evolution and clinical importance of bacterial persisters. FEMS Microbiology Reviews, 41(3), 219–251. 10.1093/femsre/fux001 [DOI] [PubMed] [Google Scholar]
- Varela Martínez, C. (2023). RE: Kind request: Additional detailed information on the FBO caused by non‐toxigenic Vibrio cholerae. Message to zoonoses support.
- Vezzulli, L. , Baker‐Austin, C. , Kirschner, A. , Pruzzo, C. , & Martinez‐Urtaza, J. (2020). Global emergence of environmental non‐O1/O139 Vibrio cholerae infections linked with climate change: A neglected research field? Environmental Microbiology, 22(10), 4342–4355. 10.1111/1462-2920.15040 [DOI] [PubMed] [Google Scholar]
- Vezzulli, L. , Brettar, I. , Pezzati, E. , Reid, P. C. , Colwell, R. R. , Höfle, M. G. , & Pruzzo, C. (2012). Long‐term effects of ocean warming on the prokaryotic community: Evidence from the vibrios. ISME Journal, 6(1), 21–30. 10.1038/ismej.2011.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezzulli, L. , Colwell, R. R. , & Pruzzo, C. (2013). Ocean warming and spread of pathogenic vibrios in the aquatic environment. Microbial Ecology, 65(4), 817–825. 10.1007/s00248-012-0163-2 [DOI] [PubMed] [Google Scholar]
- Vezzulli, L. , Grande, C. , Reid, P. C. , Helaouët, P. , Edwards, M. , Höfle, M. G. , Brettar, I. , Colwell, R. R. , & Pruzzo, C. (2016). Climate influence on Vibrio and associated human diseases during the past half‐century in the coastal North Atlantic. Proceedings of the National Academy of Sciences of the United States of America, 113(34), E5062–E5071. 10.1073/pnas.1609157113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viechtbauer, W. (2010). Conducting meta‐analyses in R with the metafor package. Journal of Statistical Software, 36(3), 1–48. 10.18637/jss.v036.i03 [DOI] [Google Scholar]
- Vu, T. T. T. , Alter, T. , Braun, P. G. , Dittrich, A. J. , & Huehn, S. (2018). Inactivation of Vibrio sp. in pure cultures and mussel homogenates using high hydrostatic pressure. Letters in Applied Microbiology, 67(3), 220–225. 10.1111/lam.13044 [DOI] [PubMed] [Google Scholar]
- Waldor, M. K. , Tschape, H. , & Mekalanos, J. J. (1996). A new type of conjugative transposon encodes resistance to sulfamethoxazole, trimethoprim, and streptomycin in Vibrio cholerae O139. Journal of Bacteriology, 178(14), 4157–4165. 10.1128/jb.178.14.4157-4165.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter, K. (2023). What to know about Vibrio vulnificus . Journal of the American Medical Association, 329(9), 772. 10.1001/jama.2023.0174 [DOI] [PubMed] [Google Scholar]
- Wang, D. , Flint, S. H. , Palmer, J. S. , Gagic, D. , Fletcher, G. C. , & On, S. L. W. (2022). Global expansion of Vibrio parahaemolyticus threatens the seafood industry: Perspective on controlling its biofilm formation. LWT‐Food Science and Technology, 158(9), 113182. 10.1016/j.lwt.2022.113182 [DOI] [Google Scholar]
- Wang, Q. Q. , Miao, Q. , Pan, J. , Jin, W. T. , Ma, Y. Y. , Zhang, Y. , Yao, Y. M. , Su, Y. , Huang, Y. N. , Li, B. , Wang, M. R. , Li, N. , Cai, S. S. , Luo, Y. , Zhou, C. M. , Wu, H. L. , & Hu, B. J. (2020). The clinical value of metagenomic next‐generation sequencing in the microbiological diagnosis of skin and soft tissue infections. International Journal of Infectious Diseases, 100, 414–420. 10.1016/j.ijid.2020.09.007 [DOI] [PubMed] [Google Scholar]
- Wang, Y. L. , & Gu, J. D. (2005). Influence of temperature, salinity and pH on the growth of environmental Aeromonas and Vibrio species isolated from Mai Po and the inner deep bay nature reserve ramsar site of Hong Kong. Journal of Basic Microbiology, 45(1), 83–93. 10.1002/jobm.200410446 [DOI] [PubMed] [Google Scholar]
- Watanabe, T. , Tazawa, T. , Kon, M. , Ohno, S. , Uno, Y. , Terao, M. , & Goto, K. (1994). Growth of Vibrio parahaemolyticus in different methods of cooking fish . Proceeding of the national conference of food safety inspection, Japan, pp. 113–116. 31 oktober 2023 kL. 13:39.
- Watts, J. E. M. , Schreier, H. J. , Lanska, L. , & Hale, M. S. (2017). The rising tide of antimicrobial resistance in aquaculture: Sources, sinks and solutions. Marine Drugs, 15(6), 158. 10.3390/md15060158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westrich, J. R. , Ebling, A. M. , Landing, W. M. , Joyner, J. L. , Kemp, K. M. , Griffin, D. W. , & Lipp, E. K. (2016). Saharan dust nutrients promote Vibrio bloom formation in marine surface waters. Proceedings of the National Academy of Sciences of the United States of America, 113(21), 5964–5969. 10.1073/pnas.1518080113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (World Health Organization) . (2019). Critically important antimicrobials for human medicine, 6th rev. https://cdn.who.int/media/docs/default‐source/gcp/who‐mia‐list‐2024‐lv.pdf?sfvrsn=3320dd3d_2
- WHO (World Health Organization) . (2024). WHO's List of Medically Important Antimicrobials: A risk management tool for mitigating antimicrobial resistance due to non‐human use . https://cdn.who.int/media/docs/default‐source/gcp/who‐mia‐list‐2024‐lv.pdf?sfvrsn=3320dd3d_2
- Williams, T. C. , Froelich, B. A. , Phippen, B. , Fowler, P. , Noble, R. T. , & Oliver, J. D. (2017). Different abundance and correlational patterns exist between total and presumed pathogenic Vibrio vulnificus and V. parahaemolyticus in shellfish and waters along the North Carolina coast. FEMS Microbiology Ecology, 93(6), fix071. 10.1093/femsec/fix071 [DOI] [PubMed] [Google Scholar]
- Woida, P. J. , & Satchell, K. J. F. (2020). The Vibrio cholerae MARTX toxin silences the inflammatory response to cytoskeletal damage before inducing actin cytoskeleton collapse [article]. Science Signaling, 13(614), eaaw9447. 10.1126/scisignal.aaw9447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong, H. C. , Chen, L. L. , & Yu, C. M. (1995). Occurrence of Vibrios in frozen seafoods and survival of psychrotrophic Vibrio cholerae in broth and shrimp homogenate at low temperatures. Journal of Food Protection, 58(3), 263–267. 10.4315/0362-028x-58.3.263 [DOI] [PubMed] [Google Scholar]
- Wong, K. C. , Brown, A. M. , Luscombe, G. M. , Wong, S. J. , & Mendis, K. (2015). Antibiotic use for vibrio infections: Important insights from surveillance data. BMC Infectious Diseases, 15, 226. 10.1186/s12879-015-0959-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, B. , Liang, W. , & Kan, B. (2015). Enumeration of viable non‐culturable Vibrio cholerae using propidium monoazide combined with quantitative PCR. Journal of Microbiological Methods, 115, 147–152. 10.1016/j.mimet.2015.05.016 [DOI] [PubMed] [Google Scholar]
- Wu, Q. , Liu, J. , Malakar, P. K. , Pan, Y. , Zhao, Y. , & Zhang, Z. (2023). Modeling naturally‐occurring Vibrio parahaemolyticus in post‐harvest raw shrimps. Food Research International, 173, 113462. 10.1016/j.foodres.2023.113462 [DOI] [PubMed] [Google Scholar]
- Xavier, C. , Gonzales‐Barron, U. , Paula, V. , Estevinho, L. , & Cadavez, V. (2014). Meta‐analysis of the incidence of foodborne pathogens in Portuguese meats and their products. Food Research International, 55, 311–323. 10.1016/j.foodres.2013.11.024 [DOI] [Google Scholar]
- Xu, H. , Liu, J. , Yuan, M. Q. , Tian, C. F. , Lin, T. , Liu, J. W. , Osaris Caridad, O. C. , Pan, Y. J. , Zhao, Y. , & Zhang, Z. H. (2023). Risk reduction assessment of Vibrio parahaemolyticus on shrimp by a Chinese eating habit. International Journal of Environmental Research and Public Health, 20(1), 317. 10.3390/ijerph20010317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, K. , Wang, Y. , Yang, W. , Cai, H. , Zhang, Y. , & Huang, L. (2022). Strategies for prevention and control of vibriosis in Asian fish culture. Vaccine, 11(1), 98. 10.3390/vaccines11010098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, W. , Gong, L. F. , Yang, S. , Gao, Y. H. , Ma, X. W. , Xu, L. M. , Chen, H. S. , & Luo, Z. H. (2020). Spatiotemporal dynamics of Vibrio communities and abundance in Dongshan Bay, South of China. Frontiers in Microbiology, 11(13), 575287. 10.3389/fmicb.2020.575287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, Y. T. , Zheng, Z. W. , Ye, L. W. , Chan, E. W. C. , & Chen, S. (2023). High prevalence qnrVC variants in Vibrio spp. isolated from food samples in South China. Microbiological Research, 267, 127261. 10.1016/j.micres.2022.127261 [DOI] [PubMed] [Google Scholar]
- Yamamoto, A. , Iwahori, J. , Vuddhakul, V. , Charernjiratragul, W. , Vose, D. , Osaka, K. , Shigematsu, M. , Toyofuku, H. , Yamamoto, S. , Nishibuchi, M. , & Kasuga, F. (2008). Quantitative modeling for risk assessment of Vibrio parahaemolyticus in bloody clams in southern Thailand. International Journal of Food Microbiology, 124(1), 70–78. 10.1016/j.ijfoodmicro.2008.02.021 [DOI] [PubMed] [Google Scholar]
- Yang, Z. Q. , Jiao, X. A. , Li, P. , Pan, Z. M. , Huang, J. L. , Gu, R. X. , Fang, W. M. , & Chao, G. X. (2009). Predictive model of Vibrio parahaemolyticus growth and survival on salmon meat as a function of temperature. Food Microbiology, 26(6), 606–614. 10.1016/j.fm.2009.04.004 [DOI] [PubMed] [Google Scholar]
- Ye, M. , Huang, Y. , & Chen, H. (2012). Inactivation of Vibrio parahaemolyticus and Vibrio vulnificus in oysters by high‐hydrostatic pressure and mild heat. Food Microbiology, 32(1), 179–184. 10.1016/j.fm.2012.05.009 [DOI] [PubMed] [Google Scholar]
- Ye, M. , Huang, Y. , Gurtler, J. B. , Niemira, B. A. , Sites, J. E. , & Chen, H. (2013). Effects of pre‐ or post‐processing storage conditions on high‐hydrostatic pressure inactivation of Vibrio parahaemolyticus and V. vulnificus in oysters. International Journal of Food Microbiology, 163(2–3), 146–152. 10.1016/j.ijfoodmicro.2013.02.019 [DOI] [PubMed] [Google Scholar]
- Yildiz, F. H. , & Visick, K. L. (2009). Vibrio biofilms: So much the same yet so different. Trends in Microbiology, 17(3), 109–118. 10.1016/j.tim.2008.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon, J.‐H. , Bae, Y.‐M. , & Lee, S.‐Y. (2017). Effects of varying concentrations of sodium chloride and acidic conditions on the behavior of Vibrio parahaemolyticus and Vibrio vulnificus cold‐starved in artificial sea water microcosms. Food Science and Biotechnology, 26(3), 829–839. 10.1007/s10068-017-0105-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon, K. S. , Min, K. J. , Jung, Y. J. , Kwon, K. Y. , Lee, J. K. , & Oh, S. W. (2008). A model of the effect of temperature on the growth of pathogenic and nonpathogenic Vibrio parahaemolyticus isolated from oysters in Korea. Food Microbiology, 25(5), 635–641. 10.1016/j.fm.2008.04.007 [DOI] [PubMed] [Google Scholar]
- Yuan, Y. , Feng, Z. , & Wang, J. (2020). Vibrio vulnificus hemolysin: Biological activity, regulation of vvhA expression, and role in pathogenesis. Frontiers in Immunology, 11, 599439. 10.3389/fimmu.2020.599439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabala, B. , García, K. , & Espejo, R. T. (2009). Enhancement of UV light sensitivity of a Vibrio parahaemolyticus O3:K6 pandemic strain due to natural lysogenization by a telomeric phage. Applied and Environmental Microbiology, 75(6), 1697–1702. 10.1128/aem.01995-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zampieri, A. , Babbucci, M. , Carraro, L. , Milan, M. , Fasolato, L. , & Cardazzo, B. (2021). Combining culture‐dependent and culture‐independent methods: New methodology insight on the Vibrio community of Ruditapes philippinarum . Food, 10(6), 1271. 10.3390/foods10061271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, P. , Wu, X. , Yuan, R. , Yan, W. , Xu, D. , Ji, L. , & Chen, L. (2022). Emergence and predominance of a new serotype of Vibrio parahaemolyticus in Huzhou, China. International Journal of Infectious Diseases, 122, 93–98. 10.1016/j.ijid.2022.05.023 [DOI] [PubMed] [Google Scholar]
- Zhang, Q. , Alter, T. , Strauch, E. , Hammerl, J. A. , Schwartz, K. , Borowiak, M. , Deneke, C. , & Fleischmann, S. (2023). Genetic and phenotypic virulence potential of non‐O1/non‐O139 Vibrio cholerae isolated from German retail seafood. Microorganisms, 11(11), 2751. 10.3390/microorganisms11112751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, S. , Lu, X. , Zhang, J. , & Kan, B. (2022). Absolute quantification of viable but nonculturable Vibrio cholerae using droplet digital PCR with oil‐enveloped bacterial cells. Microbiology Spectrum, 10(4), e0070422. 10.1128/spectrum.00704-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, Z. , Ye, L. , Li, R. , & Chen, S. (2021). Whole‐genome sequencing of strains of Vibrio spp. from China reveals different genetic contexts of blaCTX‐M‐14 among diverse lineages. The Journal of Antimicrobial Chemotherapy, 76(4), 950–956. 10.1093/jac/dkaa545 [DOI] [PubMed] [Google Scholar]
- Zhou, H. , Liu, X. , Lu, Z. , Hu, A. , Ma, W. , Shi, C. , Bie, X. , Cheng, Y. , Wu, H. , & Yang, J. (2023). Quantitative detection of Vibrio parahaemolyticus in aquatic products by duplex droplet digital PCR combined with propidium monoazide. Food Control, 144, 109353. 10.1016/j.foodcont.2022.109353 [DOI] [Google Scholar]
- Zimmerman, A. M. , DePaola, A. , Bowers, J. C. , Krantz, J. A. , Nordstrom, J. L. , Johnson, C. N. , & Grimes, D. J. (2007). Variability of total and pathogenic Vibrio parahaemolyticus densities in northern Gulf of Mexico water and oysters. Applied and Environmental Microbiology, 73(23), 7589–7596. 10.1128/aem.01700-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Protocol for the assessment of the public health aspects of Vibrio spp. related to the consumption of seafood in the EU
Food Standards Australia New Zealand information request
Vibrio parahaemolyticus, Vibrio vulnificus and non‐O1/non‐O139 Vibrio cholerae human cases associated with seafood consumption in Europe
Analytical methods for the detection and enumeration of potentially enteropathogenic Vibrio spp. in seafood
Antimicrobial resistances of the relevant Vibrio spp. from clinical and seafood associated isolates from Europe as reported in official reports, reviews and primary research papers
Effect of temperature on Vibrio spp. in seafood
Vibrio parahaemolyticus outbreak associated with an increase of seawater temperature (Pacific Northwest, 1997)
Overview of the prevention and control measures along the seafood chain for Vibrio spp.
Characterisation of relevant (quantitative) microbial risk assessment of Vibrio spp. in various types of seafood


