Abstract
Background
Fish contains high-quality omega-3 fatty acids, protein, vitamins, and minerals and due to this it is termed as an essential component of a balanced diet. But there have been concerns raised about the risks of consuming fish that is contaminated with toxins such as methylmercury, polychlorinated biphenyls (PCBs), dioxins, pesticides, and plastic waste. Consumption of contaminated fish containing these pollutants is raising global mortality and morbidity rates.
Scope and approaches
The review examines the current research outputs on the health benefits and potential health risks of fish consumption. The review also discusses various approaches to mitigating the health problems caused by fish consumption, highlights the roles of balancing the risks and benefits when consuming fish.
Key findings and conclusion
Different findings indicated that contaminants cause cancer, kidney failure, adverse neurological effect, cardiovascular diseases, and so on to vulnerable groups such as pregnant, child breast-feeding and children. In conclusion, there is a need to get more tangible evidence about the advantages and disadvantages of fish consumption to safeguard the wellbeing of the society.
Keywords: Fish, Omega-3 fatty acids, Methyl mercury, PCBs, Health benefits, Health risks
1. Introduction
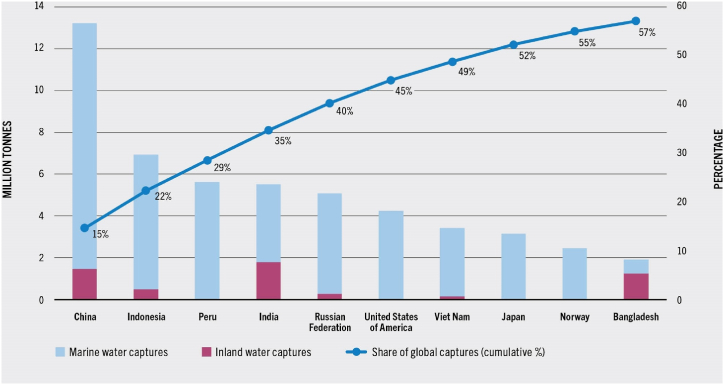
Fish is considered as a staple food in many cultures and has long been known to be rich in essential nutrients such as protein, omega-3 fatty acids [1], and sources of minerals (iodine, selenium), vitamin-D, and amino acids (taurine, carnitine, melatonin, tryptophan, and polyamines) [2]. All these make the fish an important and delicious part of a healthy diet. Due to such reasons, the production and consumption of fish has been increasing across the globe. As indicated in Fig. 1, in 2020, China constituted about 15 % of the global captures, surpassing the combined totals of the second- and third-placed nations. Nearly 49 % of the world's total capture production was produced by the top seven producers (China, Indonesia, Peru, India, Russian Federation, United States of America, and Vietnam).
Fig. 1.
Top ten global fish production in 2020 [3]. (Source: Food and Agriculture Organization of the United Nations. Reproduced with permission.)
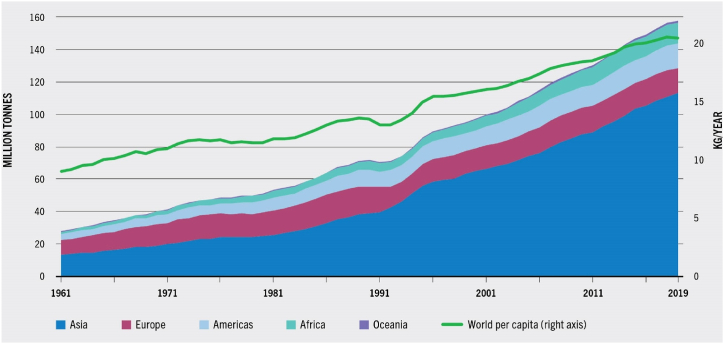
In the last 20 years, from 1998 to 2018, the average annual global intake of fish increased from 15.6 to 20.4 kg/year on a live weight basis and from 11.5 to 15.1 kg/year in edible weight and by 2050, it is expected to increase from 93.6 to 152 million tons [4]. The primary determinant of fish consumption across nations is their income, as illustrated in Fig. 2. Asians are the biggest users, followed by Africans and Oceanians as the lowest.
Fig. 2.
Aquatic food consumption by continent, 1961–2019 [3]. (Source: Food and Agriculture Organization of the United Nations. Reproduced with permission.)
Consumption of fish has various health benefits, including a lower risk of heart disease and stroke, fetal development as well as increased cognitive performance [5,6]. However, due to the presence of environmental contaminants and other potential hazards, consumption of fish poses potential human health risks. Methyl mercury, polychlorinated biphenyls (PCBs), dioxins, and pesticides [7] and also currently plastic wastes are common contaminates [8,9]. In addition, arsenic, cadmium, lead, selenium, polycyclic aromatic hydrocarbons (PAHs) and chlorinated hydrocarbon pesticides are some other contaminants to fish [10]. Generally, the sources of fish contaminants can be natural or anthropogenic. Natural sources include metals like mercury and arsenic from the earth's crust, while anthropogenic sources include pollutants from human activities like industrial chemicals and pesticides. Industrial chemicals and pesticides enter the environment through manufacturing, waste disposal, agricultural practices, and PCBs, which were once widely used in industrial applications [11]. Moreover, the degree of contamination depends on the fish species and growing areas (rural and urbanization). These contaminants can accumulate in fish tissue and, when consumed in excess amounts, can have adverse health effects on humans. Pregnant women child breast-feeding women and young children are highly likely affected by contaminants via fish consumption [12]. In addition, consumption of contaminated fish also poses adverse health effects on adults. Therefore, the exposure of consumers to methylmercury, PCBs, dioxins and pesticides via contaminated fish consumption induces adverse health effects such as neurological damage, developmental delays in children and risk of cancer [13]. Fish species that are highly contaminated with methyl mercury are shark, swordfish and king mackerel, and vulnerable groups should avoid consuming them, but they can consume salmon, tuna, tilapia, cod, and catfish which are likely lower in methylmercury content.
Currently, plastic wastes also get great attention as a fish contaminant. This contaminant causes adverse health effects to consumers such as, inflammation, oxidative stress, genotoxicity, tissue damage, cytotoxicity, neurotoxicity, immune system disruption, and carcinogenesis [9]. So, fish consumption has been a topic of interest in the field of human nutrition for many years [14]. Due to conflicting information available on the risks and benefits of consuming fish, it is important to have a comprehensive understanding of current scientific evidence to make informed decisions about fish consumption. There is a need to better understand the complex relationship between fish consumption and human health, and how it can be optimized to maximize benefits while minimizing negative impacts. Thus, this review summarizes the current state of scientific knowledge on the risks and benefits of fish consumption and provides recommendations and mitigation approaches for safe and healthy consumption patterns. This information could have a significant contribution for future researchers, policy makers, concerned bodies like World Health Organization (WHO), Food and Agriculture Organization (FAO) and United States Environmental Protection Agency (US EPA), consumers and fish processing industries.
2. Health benefits of fish consumption
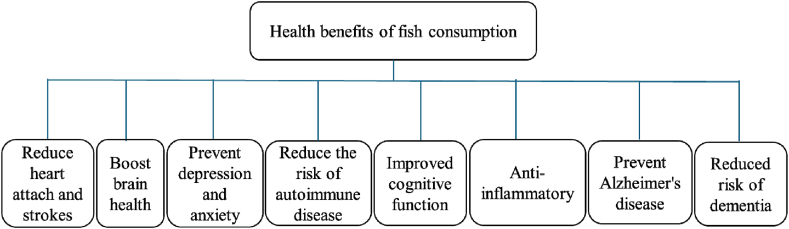
Due to its high nutritional value, fish is a nutrient-dense food, and its consumption has long been associated with several health benefits as indicated in Fig. 3. Fish consumption helps to prevent heart disease, stroke, and other cardiovascular diseases due to its omega-3 fatty acids [15]. Consuming fish twice a week can lower the risk of heart disease and stroke due to its high content of omega-3 fatty acids [16]. Omega-3 fatty acids also have been linked to reduced inflammation, improved blood lipid levels, and better vascular function [17]. Additionally, fish is rich in protein, vitamins, and minerals that are essential for maintaining good health. For example, salmon is a good source of vitamin-D and selenium, while tuna is high in vitamin B12 and potassium [18]. Therefore, it is important to include a variety of fish in a diet to promote optimal nutrition and overall well-being.
Fig. 3.
Some health benefits of fish consumption [[16], [17], [18], [19], [20], [21], [22], [23]].
In addition to its cardiovascular benefits, fish consumption has been associated with improved cognitive function [5,19,20] and reduced risk of dementia [21], which is common and affect older adults. Dementia, a general term for impaired memory, thinking, and decision-making, is a common form of Alzheimer's disease, characterized by initial memory impairment and cognitive decline [22]. Though dementia mostly affects older adults, it is not a part of normal aging of human being [23]. Additionally, research suggests that regular fish consumption may be associated with reduced symptoms of depression and anxiety, but this regular consumption should be in line with Food and Drug Authority (FDA)'s and WHO's daily intake recommendations [14,24]. Table 1 shows the general consumption of fish recommended by WHO/FDA for different target groups. Other studies have shown that fish oils omega-3 fatty acids may enhance neuronal growth and function which play a role in brain health [25].
Table 1.
WHO Recommendations of fish consumption.
However, the recommended fish consumption may depend on the income of the countries. Mostly, poor nations, especially Africa and Asia, may not consume the above recommended amounts of fish since they may not be able to afford it.
Consumption of a long-chain omega-3 fatty acids, in particular docosahexaenoic acid (DHA), is crucial for brain and eye development and as well as for mental health in early childhood and adulthood [28]. Consumption of omega-3 fatty acids also improves learning, memory, cognitive well-being, and blood flow in the brain [29]. Moreover, some research suggests that regular fish intake may reduce the risk of age-related cognitive decline and dementia [30,31].
Another benefit of fish consumption is its potential to promote weight loss and weight management [32] since fish is a low-calorie, high-protein food that can help people feel satiated and satisfied after meals [2]. In fact, some studies have found that incorporating fish into a weight loss diet can lead to greater fat loss compared to diets that exclude fish or other animal proteins [33]. Additionally, choosing fish as a protein source may be beneficial for individuals with type 2 diabetes, as it has been shown to improve glycemic control and insulin sensitivity [34,35].
Finally, there is evidence to suggest that regular fish consumption may have anti-inflammatory [36], analgesic (anti-nociceptive) [37] and immunoregulatory [2] properties. This is due to the presence of certain bioactive compounds found in fish, such as astaxanthin and omega-3 fatty acids, which can help reduce inflammation throughout the body. As inflammation has been linked to a number of chronic conditions, including arthritis and cancer, this anti-inflammatory effect may have significant health benefits. Fish is high in immunoregulatory substances such as omega-3 fatty acids, melatonin, tryptophan, taurine, and polyamines [2]. Furthermore, regular fish consumption promotes the growth of beneficial gut microbiota, such as short-chain fatty acid-producing bacteria [38,39]. However, due to the increasing amount of fish contamination, regular consumption leads to health risks.
To avoid health risks, it is important to select fish with low contaminates and follow safe recommendations from concerned organizations. Certain fish, such as salmon, trout, and sardines, are low in contaminants, making them a safer option for breastfeeding mothers, and ⁓114 g servings of these fish not more than two times per week have little mercury and optimal levels of omega-3 fatty acids for pregnant or nursing women and people with heart disease [40]. Kolipinski et al. (2020) [40] reported that a pregnant woman of average weight might take ⁓114 g servings per week providing at least 25 % of the weekly required omega-3 consumption leads to less ingestion of contaminants. Whereas adults would have to eat 142–680 g portions of shrimp, catfish, tilapia, clams, and scallops to meet the omega-3 recommendation for pregnant women and people with heart disease [40]. In fact, consuming fish during lactation has been associated with positive outcomes such as improved infant cognitive development, visual acuity, and immune function [41,42].
3. Sources of contaminants
Even though fish is a major source of protein and other nutrients it can also be a source of contaminants, such as heavy metals, polychlorinated biphenyls (PCBs), dioxins, and furans which can accumulate in fish tissues and can pose a health risk to consumers. According to Akhtar et al., (2020) [43], the sources of fish contaminants can be divided into two main categories: natural and anthropogenic. Natural sources of contaminants include metals that are released from the earth's crust, such as mercury and arsenic. Mercury is a naturally occurring metal that is found in the earth's crust. It can be released into the environment from volcanic activity, mining, and the burning of fossil fuels. Mercury can also be found in fish that live in contaminated waters. Arsenic is another naturally occurring metal that is found in the earth's crust. It can be released into the environment from mining, smelting, and the burning of fossil fuels. Arsenic can also be found in fish that live in contaminated waters.
Anthropogenic sources of contaminants include pollutants that are released into the environment from human activities, such as industrial chemicals and pesticides. Industrial chemicals and pesticides are two major sources of anthropogenic contaminants in fish. Industrial chemicals can enter the environment through industrial processes, such as manufacturing and waste disposal. Pesticides can enter the environment through agricultural practices, such as spraying and runoff. PCBs are a group of synthetic chemicals that were once widely used in industrial applications. PCBs can enter the environment through industrial processes and waste disposal. PCBs can also be found in fish that live in contaminated waters. Dioxins and furans are a group of toxic chemicals that are formed as byproducts of industrial processes, such as incineration and smelting. Dioxins and furans can enter the environment through air pollution and can be deposited on land and water. Dioxins and furans can also be found in fish that live in contaminated waters.
4. Types of contaminants in fish
It is known that fish can contain contaminants including methylmercury, PCBs, dioxins, pesticides and more recently plastic wastes that can cause restrictions to fish consumption [44,45]. In addition, arsenic, cadmium, lead, selenium, and chlorinated hydrocarbon pesticides are some other contaminants to fish [10,46]. These contaminants are often the result of human activities such as industrial pollution, runoff from agricultural practices, and atmospheric deposition from sources such as coal-fired power plants. These contaminants can find their way into the aquatic food chain through industrial waste, agriculture, or other human activities. They can accumulate over time in fish as they are absorbed from water sources and food, potentially leading to adverse health effects in humans who consume them. Therefore, fish contaminants, ways to enter the food chain, and their potential health risks to consumers are discussed in detail in the following sections.
4.1. Mercury and its derivative (methylmercury)
Mercury is a heavy metal and well-known toxic environmental contaminant that can accumulate in fish tissue and pose risks to human health when consumed in excess. It is a naturally occurring element that can be found in rocks, soil, and water [47] and in marine ecosystem [48]. In nature, mercury exists in three forms, elemental, inorganic and organic [40]. Elemental mercury can be found in the atmosphere, while inorganic and organic mercury can be predominantly found in soil, water and plants [49]. It has also become a prevalent pollutant and is rising in the atmosphere as a result of human activities [50].
Inorganic mercury can be converted to methyl mercury by the action of anaerobic bacteria [51], and microorganisms in soil and water. Mercury is transformed into methylmercury by the metabolic activities of algae and bacteria, which subsequently bioaccumulates up through the trophic levels [40]. In addition, when mercury is in contact with aquatic sediments, it can be converted to methylmercury [47]. Therefore, methylmercury is the main type of contaminant that exist in fish [[52], [53], [54]]. It enters the human body through the food chain via infected fish, shellfish, and wildlife that have been polluted by poisonous microbes. After being absorbed into the human body, methylmercury enters the circulation and causes a variety of neurological disorders [55], neurotoxicity [56,57], central nervous system (brain and spinal cord) damage, kidney damage [58], minamata disease [59]. Certain fish species, such as shark, swordfish, king mackerel, and tilefish, are known to contain higher levels of methylmercury and are considered to be risky for consumption, particularly for pregnant women and young children [60].
4.1.1. How mercury enters into fish flesh?
Mercury enters the marine from a range of natural and artificial sources, ultimately accumulating in fish and other aquatic species' tissues [52]. Mercury enters the aquatic food chain through a variety of pathways. One important pathway is atmospheric deposition, where mercury that has been released from natural sources or human activities settles onto land or water surfaces [61,62]. Atmospheric deposition is one of the major ways that mercury enters to oceans and lakes [54]. Mercury is released into the atmosphere through natural processes, such as volcanic eruptions, as well as anthropogenic activities, including coal-fired power plants and mining operations [47]. The released mercury eventually settles in water bodies, where it is converted to methylmercury, which is absorbed by small aquatic organisms and eventually consumed by larger predatory fish. Mercury can also enter aquatic systems through runoff or erosion of contaminated soil, as well as through industrial processes such as coal combustion [43,63]. Another important pathway is direct discharge of mercury-containing waste into waterways from factories and mines [64]. For example, gold mining operations often use mercury to extract gold from ore, leading to significant environmental contamination [65]. These human activities have led to widespread contamination of fish worldwide, with some species containing levels of methylmercury that exceed safe consumption guidelines [66]. The other way is through diet. Fish can ingest mercury directly from contaminated water or from prey that have ingested mercury [67]. The diet of fish plays a critical role in mercury bioaccumulation. Benthic organisms that live in sediment, such as worms, may concentrate mercury in their bodies and serve as a food source for bottom-feeding fish. Similarly, zooplankton, which are consumed by small fish, can also contain elevated levels of methylmercury [68]. As these smaller fish are consumed by larger predatory species, the concentrations of mercury can increase considerably up the food chain. The process of biomagnification occurs when organisms at higher trophic levels consume prey that contains lower amounts of methylmercury. This bioaccumulation occurs up the food chain until it reaches predatory species such as sharks, swordfish, and tuna [69]. These predatory fish can accumulate very high concentrations of methylmercury and are therefore of concern to human health. Mercury can also enter fish flesh through their gills or skin [[70], [71], [72]]. When fish breathe, they take in water that contains mercury and then it can be absorbed through the gills or skin. Another key pathway for mercury exposure in fish is through contaminated water [52]. This can happen due to both natural and manmade causes. Runoff from agricultural areas and city garbage can pollute rivers and lakes significantly. Runoff from agricultural fields and municipal waste can introduce significant contaminants to rivers and lakes [73]. Additionally, mercury may be present in sediments and soil, which can leach into the water and accumulate in fish tissue [74].
The amount of methylmercury that a fish accumulates rely on a various of aspects, such as the concentration of methylmercury in the environment, the fish's diet, and the fish's size [52,75]. Fish that live in areas with high concentrations of mercury, such as near industrial facilities or in polluted waters, and fish that eat other fish that have accumulated methylmercury are more likely to have high levels of methylmercury in their flesh. Finally, larger fish could have much amount of methylmercury in their flesh than smaller fish.
4.2. PCBs
PCBs are another group of contaminants that can be present in fish [76,77]. PCBs are a group of synthetic organic chemicals that were widely used in electrical equipment [78,79] and other industrial applications [78] until they were banned in the 1970s due to their toxicity and persistence and harmful effects on human health and the environment [7]. Despite the ban, PCBs persist in the environment and food chain, including in fish and seafood. PCBs bioaccumulate and biomagnify in the food web, meaning that as fish consume smaller organisms, such as plankton and smaller fish, PCB levels increase in their tissues over time. PCBs have been linked to cancer [80], immune system dysfunction including thymicatrophy and suppressed immune responses and cardiovascular diseases, such as stroke and hypertension [7], and neurodevelopmental problems in children [81]. PCB concentrations in fish tissue were strongly correlated with industrialization and urbanization [82]. Thus, PCB contaminants can accumulate in fish tissue through both natural processes and human activities [83]. As a result, certain fish species such as trout, salmon, and catfish may contain higher levels of PCBs than others [84]. The bioaccumulation of PCBs in fish can also vary depending on factors such as age, sex, and feeding habits. For example, larger and older fish tend to have higher levels of PCBs than smaller and younger ones due to their longer exposure time and greater accumulation. Fish that feed on other fish or are high up in the food chain may also have higher levels of PCBs due to biomagnification [85].
4.2.1. How PCBs enter to the fish?
PCBs enter fish through various pathways. One of the primary ways is through contaminated waterways [86,87]. PCBs can be released into water from industrial discharges, waste disposal sites, and urban runoff. Once released into water, PCBs can adhere to sediment and accumulate in the benthic food chain [88,89]. Fish that feed on bottom-dwelling organisms can therefore become contaminated with PCBs from the sediment. Additionally, PCBs can travel long distances through water currents and rivers, spreading contamination to other regions.
Another route of PCB contamination in fish is through atmospheric deposition [90]. PCBs can be transported by air from their original sources to remote areas where they can then be deposited onto land or water surfaces. Once deposited, PCBs can be taken up by aquatic plants or creatures that are eaten by fish [91]. Additionally, high levels of PCB contamination in fish have been found in areas close to urban centers and industrial sites, indicating that atmospheric deposition may contribute to a significant amount of PCB contamination [7].
Furthermore, there is evidence that PCBs can be absorbed through fish gills [92]. Fish have a unique respiratory system that allows them to extract oxygen from water by passing it over their gills. However, this also means that pollutants, including PCBs, can be taken up by the gills and enter the bloodstream. This can result in direct transfer of PCBs from water to fish and can lead to high levels of contamination in fish that are exposed to heavily polluted waterways.
Once these chemicals enter into waterways, they can be taken up by organisms at the base of the food chain such as phytoplankton and zooplankton. PCBs can accumulate and magnify in these organisms over time due to their lipophilic nature, meaning they have an affinity for fats.
Moreover, it has been found that some fish species preferentially accumulate more PCBs than others. For example, studies have shown that some species of large predatory fish, such as swordfish and shark, tend to have higher levels of PCB contamination than other fish [93]. This is likely due to the fact that these fish are at the top of the food chain and feed on other fish that have already accumulated PCBs, leading to biomagnification in their tissues.
4.3. Dioxins
Dioxins are highly toxic chemicals that can be present in the fatty tissues of some fish species [85]. These compounds are formed as byproducts of industrial processes such as waste incineration and chemical manufacturing [94]. They are persistent in the environment and can accumulate in the food chain, posing a risk to human health. Fish are particularly vulnerable to dioxin contamination, as they can absorb dioxins through their food and water. Dioxins can affect fish at all stages of their life cycle, from eggs to adults. In fish eggs, dioxins can cause developmental defects, such as deformed spines and fins [95]. In some fish species, dioxins can cause stunted growth and delay sexual maturation, liver damage, and reproductive problems [96]. Dioxins' reproductive toxicity is primarily mediated by an aryl hydrocarbon receptor (AhR) [97]. Dioxins can also pose a risk to human health if they are consumed in fish. Exposure to high levels of dioxins has been linked to cancer [98], reproductive, immune, nervous, and endocrine system disorders [99], coronary artery disease, diabetes, affect metabolism, reduced testosterone and thyroid hormones, porphyria, endometriosis, skin, tooth, and nail abnormalities, early menopause, and altered growth factor signaling [100]. Due to this, the USEPA has set a limit for dioxins in fish of 0.00000003 parts per million (ppm). This limit is based on the amount of dioxins that is known to cause adverse health effects in animals. The risk of dioxin exposure from fish consumption depends on a number of factors, including the type of fish, the amount of fish consumed, and the level of dioxin contamination in the fish [100]. Fish that are high in fat, such as salmon, tuna, and mackerel, are more likely to contain dioxins than fish that are low in fat. The level of dioxin contamination in fish can vary depending on the location of the fish and the type of water it lives in.
4.4. Pesticides
Pesticides including herbicides, insecticides, and fungicides are a major source of water pollution, and they can contaminate fish and other aquatic organisms. Pesticides can enter marine environment by subsurface drainage, leaching, runoff, and spray drift. Fish can then be exposed to pesticides through their food, their water, or by direct contact. There are three main ways that pesticides are introduced to fish and aquatic creatures. Orally, through drinking or consuming contaminated water, dermal absorption, and inhalation through gill uptake during respiration. Pesticides, including dichlorodiphenyltrichloroethane (DDT) and its metabolites, organophosphate, carbamates, organochlorine, pyrethroids, and necotenoides have also been found in fish [101]. DDT usage has been banned but the chemical persists in soil and water and can still be detected in some fish species. The health effects of exposure to pesticides in fish are not well established, but it affects the production of reactive oxygen species (ROS) and free radicals, leads to oxidative stress damage and significantly destructs the normal cellular structure of different tissues, and a variety of histopathologies in different fish tissues [102]. Pesticides also have a variety of harmful effects on fish, including neurotoxicity, reproductive toxicity, immunotoxicity and carcinogenicity [102]. Due to reproductive toxicity caused by pesticides, fertility, hatching success and larval survival can be decreased [103]. This results in a scarcity of fish for human consumption. In addition to the direct effects of pesticides on fish, they can also have indirect effects on the ecosystem. Pesticides can kill off beneficial insects and other organisms, which can lead to a decline in the food supply for fish. Pesticides can also alter the physical and chemical properties of water, which can make it less suitable for fish habitat. The presence of pesticides in fish tissue also poses health risks to consumers. Pesticides can accumulate in the tissues of fish, and they can be passed on to humans who eat them. Studies have shown that exposure to pesticides can increase the risk of cancer, reproductive problems, depression, neurological deficits, diabetes, respiratory diseases, fetal death, spontaneous abortion, and genetic diseases to consumers [104]. This showed that the pesticide residues pose a significant threat to human health and the environment, prompting increased interest in eliminating them. It is difficult to understand global trends in pesticide concentrations in streams, fish, and humans, as well as pesticide removal procedures using various adsorbents. To mitigate this problem, various methods, including adsorption, advanced oxidation, membrane filtration, phytoremediation, bioremediation, and activated sludge, are used [105]. Recently research focus on phytoremediation and Nanotechnology. Phytoremediation is a cost-effective and environmentally friendly method for removing methyl parathion pesticides from water and soil. Natural and cost-effective adsorbents like waste jute fiber carbon, Rhizopus oryzae biomass, and Typha australis leaf powder have been used for this purpose. Aquatic macrophytes, such as Eichornia crassipes and Pistia strateotes, have been found to be effective in removing pyrethroid pesticides. Mangroves, which are exclusive to coastlines, have been found to be more effective in reducing contaminant levels than non-mangrove systems. However, phytoremediation has limitations such as selecting suitable plant species for specific pesticide removal, coexisting with other ions and organics, determining soil and pH effectiveness, and disposing of biomass waste Nano materials like nanoparticles, nanotubes, and nanocomposites offer advantages in pesticide removal due to their easy fabrication, quick results, and high efficiency.
4.5. Histamine
Even though the consumption of fish has numerous nutritional benefits, the presence of environmental contaminants and biogenic amines like histamine is a global concern. Histamine is a naturally occurring compound in some types of fish, and its presence can be increased when fish is improperly stored or processed. Histamine is formed by bacteria when fish is improperly stored, handled, or cooked and during spoilage and fermentation of fish and fish products [106,107]. Scombrotoxin poisoning, also known as histamine fish poisoning, is caused by the ingestion of high levels of histamine in certain types of fish, such as tuna and mackerel that is characterized by symptoms such as flushing, headache, rapid or irregular heartbeat, sweating, and diarrhea [108]. These symptoms can occur within 20–30 min and lasts for 4–48 h after consuming the contaminated fish, and they can be severe in some cases [109]. The symptoms severity is influenced by individual's sensitivity and the extent of histamine consumed. To reduce the risk of scombroid poisoning, it is important to follow guidelines for safe fish consumption including proper storage and cooking [107]. This includes keeping it at the appropriate temperature (4 °C) during transport and storage and cooking it thoroughly before consumption. The level of safe fish consumption varies depending on the species of fish and individual characteristics such as age and pregnancy status. Scombrotoxin poisoning mainly affects pregnant women and young children and they should take extra care when consuming fish [107,110]. To mitigate the aforementioned problems, further research is needed to better understand the risks associated with histamine in fish and to establish appropriate safe limits for its consumption [106].
4.6. Bioremediation of fish contaminants
Toxicants in the environment have been successfully reduced recently by the application of several approaches, including adsorption, physio-biochemical, molecular, and phytoremediation mechanisms [111]. Bioremediation is a crucial process for reducing pollutants like plastics, heavy metals, PCB, and agrochemicals in the environment. It involves removing, breaking down, detoxifying, and immobilizing pollutants. Microorganisms, especially several bacterial species, aerobes and anaerobes, play a key role in this bioremediation process [112]. Phytoremediation is a bioremediation technique involving plants and microbes to reduce pollutants in aquatic environments. Microbial enzymes convert toxic contaminants to safe ones. Some Lactobacillus spp. effectively remediate heavy metals through acidification, biosorption, and bonding. Microorganisms like bacteria, fungi, and algal species detoxify heavy metals for environmental cleanliness [111,113].
5. Health risks of fish consumption
Fish, rich in omega-3 fatty acids, can reduce heart disease risk, triglyceride levels, plaque growth, blood pressure, and benefit developing babies [17]. However, fish contamination by heavy metals like methylmercury, pollutants like PCBs, and dioxins [114], harmful bacteria like vibrio species [115] and plastic wastes can negatively impact human health. Fish can take in these contaminants from the water and the food they eat. Different groups of fish consumers are affected by consuming contaminated fish. But, the most vulnerable groups of consumers that can be highly likely affected by consuming fish are pregnant women, child breast-feeding women and children [116]. Adults can also be affected by fish contaminants. Therefore, types of fish contaminants with their health risks for each vulnerable group are discussed in detail.
5.1. Health risks of fish consumption for pregnant woman
Pregnant women are often advised to consume fish due to its nutritional benefits, including omega-3 fatty acids that are important for fetal brain development [117]. However, fish also contains a variety of contaminants that can pose a risk to both the mother and developing fetus [118].
Fish contains harmful levels of environmental contaminants such as methylmercury, PCBs, dioxins [119], and brominated flame retardants (BFRs) [120], which can pose significant risks to human health, especially for pregnant women and developing fetuses [12]. Exposure to these chemicals has been associated with adverse neurological, developmental, endocrine, reproductive, and immune effects in offspring, as well as increased risk of preterm birth, low birth weight, and cognitive deficits [121].
According to Misser et al. (2022) [122], prenatal exposure to methylmercury has been linked to adverse developmental outcomes such as impaired cognitive function and behavioral problems. High levels of methylmercury in fish can also lead to fetal growth restriction and preterm birth [118].
In addition to methylmercury, PCBs, dioxins, and other persistent organic pollutants have been linked to negative effects on reproductive health and immune function, as well as increased cancer risk [7]. Pregnant women are therefore advised to avoid consuming predatory fish species such as shark, swordfish, tilefish, and king mackerel, which tend to have higher levels of these contaminants [123].
Infections induced by fish consumption during pregnancy can harm a developing fetus. These infections can be fatal to the mother, fetus, or newborn when it is untreated. Furthermore, it can lead to viral infections, potentially causing spontaneous abortions or organ diseases [124]. However, pregnancy exposure to methylmercury is unlikely to be a significant risk factor for low neurodevelopmental functioning [57,125,126] especially in terms of cognitive performance [126] in early childhood.
Therefore, it is recommended that pregnant women consume a variety of low-mercury fish that provide essential nutrients without exposing them to excessive levels of contaminants. The US FDA and EPA advise a maximum intake of 8–12 ounces (2–3 servings) per week of cooked fish low in mercury, such as shrimp, salmon, canned light tuna, tilapia, and catfish [127]. According to FDA recommendation, pregnant women, women of childbearing age, and young children should also avoid high-mercury fish such as shark, swordfish, king mackerel, and tilefish [26] which can accumulate more toxins in their tissues due to their longer lifespan and higher trophic level in the food chain. By following these guidelines and taking appropriate safety measures when handling and preparing fish, pregnant women can enjoy the benefits of fish consumption while minimizing the risks to their health and that of their offspring.
In addition to contaminants, some species of microbs such as E. coli, Aeromonas hydrophila, Yersinia spp., Brucella spp., Shigella spp., Salmonella spp., Streptococcus iniae, Clostridium botulinum, Klebsiella spp., and Edwardsiella tarda are already isolated from fish [128]. Therefore, some fish species may harbor infectious agents such as Listeria monocytogenes, Vibrio parahaemolyticus, and Salmonella spp., which can cause foodborne illnesses in pregnant women [129]. These pathogens can cross the placenta and infect the fetus, leading to miscarriage, stillbirth, or severe neonatal infection. To minimize the risk of foodborne illness, pregnant women should avoid eating raw or undercooked fish, refrigerated smoked fish, and sushi made with raw fish.
5.2. Health risks on breastfeeding women
Consumption of fish contaminated with methylmercury, PCBs, dioxins and pesticides is associated with numerous health risks to pregnant and breastfeeding women. These contaminants accumulate in fatty tissues, and are present in higher amounts in larger predatory fish species [114]. Breastfeeding women are particularly vulnerable to these contaminants since they can be transferred from the mother's body to the infant via breast milk [130,131]. The study conducted by Mahaffey et al. (2009) [132] found that PCB levels were positively associated with fish consumption in breastfeeding women. To prove these outcomes, additional studies should be conducted.
Methyl mercury accumulation in the body can lead to neurological and developmental problems in infants whose mothers consume large amounts of mercury-contaminated fish during pregnancy and breastfeeding [12]. This is of particular concern for breastfeeding women and therefore, pregnant, and breastfeeding woman must take care during consumption of these fish species.
PCBs and dioxins have also been associated to a variety of negative health consequences in breastfeeding women, including cognitive and neurobehavioral impairments, dementia, immune system dysfunctions, cardiovascular disease, and cancer [7]. Particularly, PCBs showed reduced birth weight, smaller head circumference, shortened gestational age, and altered neurodevelopment in infants when consumed during pregnancy [133].
To minimize the possible risks linked with fish intake during breastfeeding, there are safe consumption guidelines that breastfeeding women should follow. The American College of Obstetricians and Gynecologists (ACOG) advises breastfeeding women to consume up to 340 g of fish per week. Breastfeeding women should choose low-mercury fish such as salmon, trout, and herring, which are rich in omega-3 fatty acids [134]. WHO recommends that breastfeeding women limit their consumption of fish known to be high in PCBs and dioxins. Therefore, breastfeeding women should follow safe consumption guidelines and choose low-polluted fish to ensure optimal health benefits while minimizing potential risks.
5.3. Health risks of fish consumption for children
Like pregnant and breast-feeding woman, consumption of contaminated fish in general also can pose risks to children's health. According to the Al-Saleh et al. (2020) [57], high methylmercury exposure in children can cause developmental delays, cognitive deficits, and other health issues. Children are highly susceptible to methylmercury's toxic effects, particularly during their early brain development stages, which increases their risk of neurologic impairment [135]. Children who eat fish frequently, particularly high-mercury fish, may be at risk of these impacts. Moreover, children are more vulnerable to the harmful effects of mercury because their brains and bodies are still developing. A study conducted by Ref. [136] found that children who consumed higher amounts of fish with high levels of mercury were at an increased risk for cognitive deficits and developmental delays. The same study also found that children whose mothers had higher mercury levels during pregnancy had decreased cognitive ability as well. Another potential risk of consuming contaminated fish for children is reproductive harm. A study published in Environmental Health Perspectives found that prenatal exposure to PCBs was associated with lower IQ scores in school-aged children [137]. Moreover, some research has suggested that exposure to mercury from contaminated fish may increase the risk for autoimmune disorders such as diabetes mellitus in children [138].
To minimize the risks of fish consumption for children, the EPA recommends that parents limit their intake of high-mercury fish such as shark, swordfish, king mackerel, and tilefish. Instead, they should choose low-mercury fish such as salmon, trout, and haddock. To reduce the risk of foodborne illness in children advises them to avoid raw or undercooked fish and shellfish. By following these recommendations, parents can ensure that their children get the benefits of fish with limited exposure to excessive levels of contaminants. Therefore, pregnant women and young children should avoid consuming certain types of fish with high levels of mercury such as swordfish, shark, king mackerel and tilefish.
5.4. Health risks of fish consumption for adults
The consumption of contaminated fish can expose adults to various health risks, such as heavy metal toxicity (e.g., mercury, lead, cadmium) and accumulation of POPs [114,139]. High levels of methylmercury exposure can damage the nervous system, leading to tremors, depression, memory problems [140,141], neurological damage, kidney damage, and reproductive problems in adults [48]. However, Downer et al. (2017) [142] stated that there is rare information that frequent fish consumption raises the risk of cardiovascular disease in a community. But a recent study by Ref. [143] showed that even low-level mercury exposure from fish consumption may increase the risk of cardiovascular disease for adults. Therefore, like other vulnerable groups, it is important for adults to choose fish with lower levels of contaminants and limit their intake of high-mercury fish for optimal health.
In addition, other heavy metals such as lead and cadmium are also found in fish and cause harmful effects on the kidneys, bones, and nervous system to adults [107,144]. POPs may accumulate in the fatty tissues of fish and affect the immune system, reproductive organs, and hormonal balance of adults [95,133].
PCBs can be linked to cancer and other health problems in adults [145]. Higher levels of PCBs in blood were associated with an increased risk of prostate cancer in men [146,147] and breast cancer in women [148]. According to Ref. [149], exposure to PCB can cause the risk of cancer which is 20 % higher in men than women.
To reduce the risks associated with fish consumption, it is recommended to follow safe consumption guidelines. For instance, the WHO advises limiting the intake of predatory fish species and consuming smaller fish that are low in contaminants [107]. Individuals with health conditions such as liver or kidney diseases should also consult a healthcare provider before consuming fish. It is also recommended to vary the types of fish consumed to avoid overexposure to one particular contaminant. By doing so, adults can benefit from the nutritional advantages of fish while minimizing the potential risks associated with its consumption.
6. Effect of plastic wastes in fish
The global issue of plastic waste has become a significant environmental concern, with the potential to harm marine life, including fish. Plastic waste has become a major problem in our oceans and water bodies. As a result, many marine animals, including fish, are negatively impacted by the presence of plastic waste in their environment [150].
Because of their polymeric nature and the existence of various plasticizers, plastics are composed of H, C, N, Cl, and other components that contribute to their longevity and make them low/non-biodegradable and its natural breakdown could take thousands of years [104]. But, plastic degradation is primarily caused by mechanical abrasion, resulting in the formation of micro- and nano-plastics due to prolonged UV radiation exposure (due to photooxidation) and weathering processes (high temperature and humidity, decomposition or loss of plasticizers) [104,151,152].
Fish is crucial for freshwater ecosystems, providing food and livelihoods for millions, and contributing to the economy through exporting, tourism, and recreation in developing countries [153]. When fish ingest plastic waste, the waste can accumulate in their digestive systems, leading to blockages and other physical harm [154] leading to starvation. Additionally, plastic waste can adsorb and concentrate pollutants in the water, such as pesticides, heavy metals (e.g., mercury lowered fish swimming velocity and resistance time considerably) and persistent organic pollutants (POPs) [105,155]. When fish consume these pollutants that are attached to plastic waste, they can become contaminated with these harmful toxins.
Fish can uptake microplastics from seawater through passive (by gill water filtration) and active means (via prey confusion), consuming contaminated prey, and using nets for capture [156]. Microplastics are characterized as small pieces of plastic <5 mm in size with no lower limit established and found in aquatic system, formed from larger debris fragmentation or introduced into water and sediments as micro- or nano-sized particles [8,157]. These include fishing gear fragments, vehicle tyres, packages and drink bottles, paints, synthetic textiles, cosmetics and personal care products, and electrical equipment. According to the report of [156] microplastics were identified in 49 % of 150 examined fish, with 35 % in the gastrointestinal tract, 36 % in the gills, and 32 % in the dorsal muscle. Also Khan et al. (2024) [153] stated that, in the Rio de la Plata Estuary, microplastics were discovered in the stomach contents of coastal freshwater fish. The presence of microplastics was found in all of the studied fish (100 %) and the concentrations in stomach contents were much higher near sewage outflow. Moreover, a study conducted by Savoca et al. (2021) [158] found that, of 171,774 marine and estuarine fish species revealed that over two-thirds had ingested plastic, while only one-quarter were well-studied and commonly ingested. Plastic waste contaminants can cause various health risks in fish, including mortality, reduced feeding rate, reduced growth, behavioral changes, decreased predatory performance, neurotoxicity, and intestinal damage due to acetycholinesterase inhibition and oxidative stress [159,160] and several other adverse effects such as reduced growth rates, altered reproductive behavior, weakened immune systems and even death [9,161]. It is important for individuals and organizations to take action to reduce plastic waste to minismize its impact on fish and other marine life. This could be achieved through initiatives such as reducing plastic consumption, proper disposal of plastic waste and supporting policies that aim to minimize plastic pollution.
6.1. Effect of plastic wastes on human health through fish consumption
Aquatic fish may absorb plastic wastes actively by feeding in the water column, or inadvertently by devouring seafood-like species and/or by consuming prey that has previously ingested plastic wastes [9]. Microplastics may be absorbed by fish intake after digestion. As with nanoplastics, the cellular uptake of microplastics may be greatly impacted by their interactions with surrounding biological components like proteins, phospholipids, or carbohydrates [156,[162], [163], [164]]. According to Barceló et al. (2023) [165] human body can absorb microplastics smaller than 150 μm. Prominently, microplastics with particle sizes of smaller than 2.5 μm can enter the circulatory system by gastrointestinal adsorption and direct inhalation which cause inflammation and dose-dependent accumulation in the human body [154,[166], [167], [168]]. Fish consumption, along with air, water, and other food sources, exposes the human body to microplastics through physical and chemical pathways like endocytosis and persorption; causes to the human health such as inflammation, oxidative stress, genotoxicity, tissue damage, cytotoxicity, neurotoxicity, immune system disruption, and carcinogenesis [9].
As mentioned above, plastic wastes have shown a significant adverse effect on living things. Thus, further research on microplastics, pollutants, and health effects in fish and seafood should be conducted in collaboration with governmental and non-governmental sectors to formulate effective plastic waste management systems.
7. Mortality rate due to fish consumption
Some studies indicated that the impact of contaminated fish consumption has increased mortality and morbidity rates over the world as indicated in Table 2. According to the report of Jayedi et al. (2018) [169], from 71,384 total participants’ 21,194 (all-cause deaths: 16,295 and cardiovascular disease (CVD) deaths: 4899) were died within 17 year follow-up with 20 g/day serving. From this, the total percentage death was 29.69 %. From this, the total percentage death was 29.69 %. As Bakre et al. (2022) [170] reviewed, from total participants of 4165 [1387 once a week or less serving 110 (33.43 %) were died, 1129 > Once a week and < daily serving 78 (23.71 %) were died, 631 once a day serving 48 (14.59 %) were died, 274> Daily serving 19 (5.78 %) were died] through fish consumption. As Philibert et al. (2022) [171] studied, 239 [113 (47.28 %) females and 136 (56.9 %) males] were died from the total participants of 633 (308 females and 325 males) with mercury≥ 15 μg/g at least once serving. Donat-Vargas et al. (2020) [172] reported that 16779 (24.13 %) females and males were died from the total participants of 69497 by a nutritional-toxicological aspect of fish consumption. According to the finding of Zhou et al. (2023) [173], 75574 (17.53 %) of 383248 oily fish consumption and 70092 (16.26 %) of 410499 non-oily fish consumption were died with ≥2serving/week. Wallin et al. (2018) [174] reported, 794(15.56 %) of 5103 female participants were died by coronary heart disease (CHD) and type-2 diabetes case with fish consumption 1–3 servings/month. In summary, as we understood from different findings, consuming food substances with more than the dosage could associated with many risks of human health (e.g. CVD and cancer) cause for death. Therefore, taking with the baseline of the recommended dose could be implemented and well-practiced worldwide.
Table 2.
Mortality rate due to fish consumption.
| Research studies | Serving | No of participant | Mortality |
Reference | |||
|---|---|---|---|---|---|---|---|
| Total | (%) | ||||||
| Fish consumption and risk of all-cause and cardiovascular mortality: | 17 years (follow-up year) 20 g/d | 71,384 | All-cause deaths: 6295 CVD deaths: 4899 | 29.69 | [169] | ||
| Impact of fish consumption on all-cause mortality in older people with and without dementia: a community-based cohort study | Once a week or less | 1387 | N = 4165 | 110 | n = 329 | 33.43 | [170] |
| > Once a week and < daily | 1129 | 78 | 23.71 | ||||
| Once a day | 631 | 48 | 14.59 | ||||
| > Daily | 274 | 19 | 5.78 | ||||
| Mercury exposure and premature mortality in the Grassy Narrows First Nation community: a retrospective longitudinal study |
Mercury≥ 15 μg/g at least once | Female | 308 | 113 | 47.28 | [171] | |
| Male | 325 | 136 | 56.9 | ||||
| Cardiovascular and cancer mortality in relation to dietary polychlorinated biphenyls and marine polyunsaturated fatty acids: a nutritional-toxicological aspect of fish consumption |
1 year (follow-up year) 165 and 231 ng/day for female and male respectively |
Female | 32952 | 16776 (6338 by CVD and 5421 by cancer) | 24.13 | [172] | |
| Male | 36545 | ||||||
| Association of oily fish and non-oily fish intakes with all-cause mortality and cause-specific mortality: a large population-based prospective study |
≥2serving/week) | Oily fish consumption | 383,248 | 75,574 | 17.53 | [173] | |
| Non-oily fish consumption | 410,499 | 70,092 | 16.26 | ||||
| Fish consumption in relation to myocardial infarction, stroke and mortality among women and men with type 2 diabetes: A prospective cohort study | 1-3 servings/month | Female | 5103 | 326 CHD and 468 type 2 diabetes case) | 15.56 | [174] | |
8. Various approaches to mitigating the risks associated with fish consumption
There are several approaches that can be taken to mitigate the risks associated with fish consumption. These include:
-
•
Control the environmental contamination: Replacement of products and processes containing environmental contaminants with products and processes without those contaminants may be one of the most powerful preventive measures for influencing the entire flow of fish contaminants through the aquatic environment.
-
•
Regulation and maximum allowable limits: Implement advises from concerned organizations. The FDA advises pregnant women, breast-feeding mothers, and young children to avoid eating mercury-rich fish such as shark, swordfish, king mackerel, and tilefish than mercury-free fish include salmon, shrimp, pollock, and catfish. The FDA recommends that adults eat no more than ⁓340 g of fish per week, and that children eat no more than ⁓170 g per week. Therefore, strictly follow the WHO, FDA and USEPA fish consumption guidelines. In general, avoid fish that have been caught in polluted waters. Fish that have been caught in polluted waters may be more likely to contain contaminants.
-
•
Culinary treatments: Numerous factors influence the metal concentration in cooked and raw seafood, such as metal speciation, the cooking method, cooking time, temperature and the presence of different food additives. For example, cooking treatment lowers the bio-accessibility of Hg, i.e., it is reduced by 16 % for Hg (II) and 19 % for MeHg [113]. Cooking fish properly at 63 °C also used to kill harmful microorganisms.
-
•
Applying bioremediation strategies: Bioremediation is a convenient and ecofriendly option that can be used to restore the contaminated environment by removing toxic metals from the environment. Bioremediation of toxicants can be done by adsorption, physio-biochemical mechanisms, and molecular mechanisms.
9. Conclusion
Fish consumption offers numerous health benefits, including reducing cardiovascular disease risk, improving cognitive function, and promoting weight loss. However, it also poses potential health risks due to environmental contaminants like mercury, PCBs, dioxins, pesticides, and plastic waste. Pregnant, breastfeeding, and children are most vulnerable, with adverse neurological, developmental, and immune effects in offspring. In addition, high levels of methyl mercury can lead to developmental delays, cognitive deficits, and lower IQ scores in children. Mitigating these risks can be achieved through choosing limiting consumption of contaminated fish and follow proper cooking. In developing countries, the problem is more likely high. Therefore, further research is needed to better understand the risks associated with fish contaminants. The results of the research could be an important input to future researchers, policy makers and consumers.
Ethics approval
Not Applicable.
Funding
There is no funding resource that could be reported for this publication.
Consent for publication
All the authors have given approval for the publication of this manuscript.
Data availability
No data was used for the research described in the article.
CRediT authorship contribution statement
Biresaw Demelash Abera: Writing – review & editing, Writing – original draft, Visualization, Conceptualization. Mekuannt Alefe Adimas: Writing – review & editing, Writing – original draft, Visualization, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Hosomi R., Yoshida M., Fukunaga K. Seafood consumption and components for health. Global J. Health Sci. 2012;4(3):72–86. doi: 10.5539/gjhs.v4n3p72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mendivil C.O. Fish consumption: a review of its effects on metabolic and hormonal health. Nutr. Metab. Insights. 2021;14 doi: 10.1177/11786388211022378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.FAO . FAO; Rome: 2022. The State of World Fisheries and Aquaculture 2022. Towards Blue Transformation. [DOI] [Google Scholar]
- 4.Naylor R.L., et al. Blue food demand across geographic and temporal scales. Nat. Commun. 2021;12(1) doi: 10.1038/s41467-021-25516-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lichtenstein A.H., et al. 2021 dietary guidance to improve cardiovascular health: a scientific statement from the American heart association. Circulation. 2021;144(23):E472–E487. doi: 10.1161/CIR.0000000000001031. [DOI] [PubMed] [Google Scholar]
- 6.Tsoupras A., Brummell C., Kealy C., Vitkaitis K., Redfern S., Zabetakis I. Cardio‐Protective properties and health benefits of fish lipid bioactives; the effects of thermal processing. Mar. Drugs. 2022;20(3):1–46. doi: 10.3390/md20030187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Montano L., et al. Polychlorinated biphenyls (PCBs) in the environment: occupational and exposure events, effects on human health and fertility. Toxics. 2022;10(7) doi: 10.3390/toxics10070365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alberghini L., Truant A., Santonicola S., Colavita G., Giaccone V. Microplastics in fish and fishery products and risks for human health: a review. Int. J. Environ. Res. Publ. Health. 2023;20(1) doi: 10.3390/ijerph20010789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhuyan S. Effects of microplastics on fish and in human health. 2022;10(March):1–17. doi: 10.3389/fenvs.2022.827289. [DOI] [Google Scholar]
- 10.Soerensen A.L., Faxneld S., Pettersson M., Sköld M. Science of the Total Environment Fish tissue conversion factors for mercury , cadmium , lead and nine per- and poly fl uoroalkyl substances for use within contaminant monitoring. 2023;858(June 2022) doi: 10.1016/j.scitotenv.2022.159740. [DOI] [PubMed] [Google Scholar]
- 11.Jaishankar M., Tseten T., Anbalagan N., Mathew B.B., Beeregowda K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscipl. Toxicol. 2014;7(2):60–72. doi: 10.2478/intox-2014-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen B., Dong S. Mercury contamination in fish and its effects on the health of pregnant women and their fetuses, and guidance for fish consumption—a narrative review. Int. J. Environ. Res. Publ. Health. 2022;19(23) doi: 10.3390/ijerph192315929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hauptman M., Woolf A.D. Childhood ingestions of environmental toxins: what are the risks? Pediatr. Ann. 2017;46(12):e466–e471. doi: 10.3928/19382359-20171116-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maulu S., Nawanzi K., Abdel-Tawwab M., Khalil H.S. Fish nutritional value as an approach to children's nutrition. Front. Nutr. 2021;8(December):1–10. doi: 10.3389/fnut.2021.780844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li J.J., et al. Role of omega-3 fatty acids in the prevention and treatment of cardiovascular Diseases: a consensus statement from the Experts' Committee of National Society of Cardiometabolic Medicine. Front. Pharmacol. 2022;13(December):1–19. doi: 10.3389/fphar.2022.1069992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kris-Etherton P.M., Harris W.S., Appel L.J. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002;106(21):2747–2757. doi: 10.1161/01.CIR.0000038493.65177.94. [DOI] [PubMed] [Google Scholar]
- 17.Djuricic I., Calder P.C. Beneficial outcomes of omega-6 and omega-3 polyunsaturated fatty acids on human health: an update for 2021. Nutrients. 2021;13(7) doi: 10.3390/nu13072421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hustad K.S., Ottestad I., Olsen T., Sæther T., Ulven S.M., Holven K.B. Salmon fish protein supplement increases serum vitamin B12 and selenium concentrations: secondary analysis of a randomised controlled trial. Eur. J. Nutr. 2022;61(6):3085–3093. doi: 10.1007/s00394-022-02857-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Damsgaard C.T., et al. Effects of oily fish intake on cardiovascular risk markers, cognitive function, and behavior in school-aged children: study protocol for a randomized controlled trial. Trials. 2016;17(1):1–8. doi: 10.1186/s13063-016-1647-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kokubun K., Nemoto K., Yamakawa Y. Fish intake may affect brain structure and improve cognitive ability in healthy people. Front. Aging Neurosci. 2020;12(March):1–9. doi: 10.3389/fnagi.2020.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sarris J., et al. Nutritional medicine as mainstream in psychiatry. Lancet Psychiatr. 2015;2(3):271–274. doi: 10.1016/S2215-0366(14)00051-0. [DOI] [PubMed] [Google Scholar]
- 22.Jellinger K.A. The neuropathological diagnosis of Alzheimer disease. J. Neural. Transm. Suppl. 1998;5(53):97–118. doi: 10.1007/978-3-7091-6467-9_9. [DOI] [PubMed] [Google Scholar]
- 23.Irwin K., Sexton C., Daniel T., Lawlor B., Naci L. Healthy aging and dementia: two roads diverging in midlife? Front. Aging Neurosci. 2018;10(SEP):1–12. doi: 10.3389/fnagi.2018.00275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morales-Suárez-Varela M., et al. Prevalence of depression and fish consumption among first year Spanish university students: UniHcos project. Nutrients. 2023;15(12) doi: 10.3390/nu15122757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gómez-Pinilla F. Brain foods: the effects of nutrients on brain function. Nat. Rev. Neurosci. 2008;9(7):568. doi: 10.1038/nrn2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Evans E.C. The FDA recommendations on fish intake during pregnancy. J. Obstet. Gynecol. Neonatal Nurs. 2002;31(6):715–720. doi: 10.1177/0884217502239205. [DOI] [PubMed] [Google Scholar]
- 27.Wenstrom K.D. The FDA's new advice on fish: it's complicated. Am. J. Obstet. Gynecol. 2014;211(5):475–478.e1. doi: 10.1016/j.ajog.2014.07.048. [DOI] [PubMed] [Google Scholar]
- 28.Dinicolantonio J.J., O’keefe J.H. The importance of marine OMEGA-3S for brain development and the prevention and treatment of behavior, mood, and other brain disorders. Nutrients. 2020;12(8):1–15. doi: 10.3390/nu12082333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dighriri I.M., et al. Effects of omega-3 polyunsaturated fatty acids on brain functions: a systematic review. Cureus. 2022;14(10) doi: 10.7759/cureus.30091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takeuchi H., Kawashima R. Diet and dementia: a prospective study. Nutrients. 2021;13(12):1–12. doi: 10.3390/nu13124500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ylilauri M.P.T., Hantunen S., Lönnroos E., Salonen J.T., Tuomainen T.P., Virtanen J.K. Associations of dairy, meat, and fish intakes with risk of incident dementia and with cognitive performance: the Kuopio Ischaemic Heart Disease Risk Factor Study (KIHD) Eur. J. Nutr. 2022;61(5):2531–2542. doi: 10.1007/s00394-022-02834-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tørris C., Molin M., Smastuen M.C. Fish consumption and its possible preventive role on the development and prevalence of metabolic syndrome-a systematic review. Diabetol. Metab. Syndrome. 2014;6(1) doi: 10.1186/1758-5996-6-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frestedt J.L., Zenk J.L., Kuskowski M.A., Ward L.S., Bastian E.D. A whey-protein supplement increases fat loss and spares lean muscle in obese subjects: a randomized human clinical study. Nutr. Metab. 2008;5(1):1–7. doi: 10.1186/1743-7075-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barrea L., et al. Comprehensive approach to medical nutrition therapy in patients with type 2 diabetes mellitus: from diet to bioactive compounds. Antioxidants. 2023;12(4) doi: 10.3390/antiox12040904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muntis F.R., et al. A high protein diet is associated with improved glycemic control following exercise among adolescents with type 1 diabetes. Nutrients. 2023;15(8):1–17. doi: 10.3390/nu15081981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Costabile G., et al. An oily fish diet improves subclinical inflammation in people at high cardiovascular risk: a randomized controlled study. Molecules. 2021;26(11):1–11. doi: 10.3390/molecules26113369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carballo-Casla A., García-Esquinas E., Banegas J.R., Rodríguez-Artalejo F., Ortolá R. Fish consumption, omega-3 fatty acid intake, and risk of pain: the Seniors-ENRICA-1 cohort. Clin. Nutr. 2022;41(11):2587–2595. doi: 10.1016/j.clnu.2022.09.007. [DOI] [PubMed] [Google Scholar]
- 38.Fusco W., et al. Short-chain fatty-acid-producing bacteria: key components of the human gut microbiota. Nutrients. 2023;15(9) doi: 10.3390/nu15092211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Holmes Z.C., et al. Microbiota responses to different prebiotics are conserved within individuals and associated with habitual fiber intake. Microbiome. 2022;10(1):1–16. doi: 10.1186/s40168-022-01307-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kolipinski M., Subramanian M., Kristen K., Borish S., Ditta S. Sources and toxicity of mercury in the San Francisco bay area, spanning California and beyond. J. Environ. Public Health. 2020;2020 doi: 10.1155/2020/8184614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bragg M.G., Prado E.L., Stewart C.P. Choline and docosahexaenoic acid during the first 1000 days and children's health and development in low- and middle-income countries. Nutr. Rev. 2022;80(4):656–676. doi: 10.1093/nutrit/nuab050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koletzko B., et al. Nutrition during pregnancy, lactation and early childhood and its implications for maternal and long-term child health: the early nutrition project recommendations. Ann. Nutr. Metab. 2019;74(2):93–106. doi: 10.1159/000496471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Akhtar N., Syakir Ishak M.I., Bhawani S.A., Umar K. Various natural and anthropogenic factors responsible for water quality degradation: a review. Water (Switzerland) 2021;13(19) doi: 10.3390/w13192660. [DOI] [Google Scholar]
- 44.Gandhi N., Drouillard K.G., Arhonditsis G.B., Gewurtz S.B., Bhavsar S.P. Are fish consumption advisories for the great lakes adequately protective against chemical mixtures? Environ. Health Perspect. 2017;125(4):586–593. doi: 10.1289/EHP104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Uzomah A., Lundebye A.K., Kjellevold M., Chuku F.A., Stephen O.A. A review of chemical contaminants in marine and fresh water fish in Nigeria. Foods. 2021;10(9):1–18. doi: 10.3390/foods10092013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Soerensen A.L., Faxneld S., Pettersson M., Sköld M. Fish tissue conversion factors for mercury, cadmium, lead and nine per- and polyfluoroalkyl substances for use within contaminant monitoring. Sci. Total Environ. 2023;858(November 2022) doi: 10.1016/j.scitotenv.2022.159740. [DOI] [PubMed] [Google Scholar]
- 47.Gworek B., Dmuchowski W., Baczewska-Dąbrowska A.H. Mercury in the terrestrial environment: a review. Environ. Sci. Eur. 2020;32(1) doi: 10.1186/s12302-020-00401-x. [DOI] [Google Scholar]
- 48.Kaur M., Sharma A., Aditya A review on heavy metal accumulation and toxicity in biotic and abiotic components. IOP Conf. Ser. Earth Environ. Sci. 2021;889(1) doi: 10.1088/1755-1315/889/1/012062. [DOI] [Google Scholar]
- 49.Kim M., Zoh K. 2012. Fate and Transport of Mercury in Environmental Media and Human Exposure; pp. 335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mitra S., et al. Impact of heavy metals on the environment and human health: novel therapeutic insights to counter the toxicity. J. King Saud Univ. Sci. 2022;34(3) doi: 10.1016/j.jksus.2022.101865. [DOI] [Google Scholar]
- 51.Wang Y., Roth S., Schaefer J.K., Reinfelder J.R., Yee N. Production of methylmercury by methanogens in mercury contaminated estuarine sediments. FEMS Microbiol. Lett. 2020;367(23):1–7. doi: 10.1093/femsle/fnaa196. [DOI] [PubMed] [Google Scholar]
- 52.Al-Sulaiti M.M., Soubra L., Al-Ghouti M.A. The causes and effects of mercury and methylmercury contamination in the marine environment: a review. Curr. Pollut. Reports. 2022;8(3):249–272. doi: 10.1007/s40726-022-00226-7. [DOI] [Google Scholar]
- 53.Hong Y.S., Kim Y.M., Lee K.E. Methylmercury exposure and health effects. J. Prev. Med. Public Heal. 2012;45(6):353–363. doi: 10.3961/jpmph.2012.45.6.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Silbernagel S.M., et al. Recognizing and preventing overexposure to methylmercury from fish and seafood consumption: information for physicians. J. Toxicol. 2011;2011 doi: 10.1155/2011/983072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rice K.M., Walker E.M., Wu M., Gillette C., Blough E.R. Environmental mercury and its toxic effects. J. Prev. Med. Public Heal. 2014;47(2):74–83. doi: 10.3961/jpmph.2014.47.2.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Creed J.H., et al. Methylmercury exposure, genetic variation in metabolic enzymes, and the risk of glioma. Sci. Rep. 2019;9(1):1–7. doi: 10.1038/s41598-019-47284-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Al-Saleh I., et al. Effects of early and recent mercury and lead exposure on the neurodevelopment of children with elevated mercury and/or developmental delays during lactation: a follow-up study. Int. J. Hyg Environ. Health. 2020;230(April) doi: 10.1016/j.ijheh.2020.113629. [DOI] [PubMed] [Google Scholar]
- 58.Lee J.Y., Hwang G.W., Naganuma A., Satoh M. Methylmercury toxic mechanism related to protein degradation and chemokine transcription. Environ. Health Prev. Med. 2020;25(1):3–7. doi: 10.1186/s12199-020-00868-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yorifuji T., Kadowaki T., Yasuda M., Kado Y. Neurological and neurocognitive impairments in adults with a history of prenatal methylmercury poisoning: minamata disease. Int. J. Environ. Res. Publ. Health. 2023;20(12) doi: 10.3390/ijerph20126173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cusack L.K., Smit E., Kile M.L., Harding A.K. Regional and temporal trends in blood mercury concentrations and fish consumption in women of child bearing Age in the United States using NHANES data from 1999-2010. Environ. Heal. A Glob. Access Sci. Source. 2017;16(1):1–11. doi: 10.1186/s12940-017-0218-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen L., et al. Release of legacy mercury and effect of aquaculture on mercury biogeochemical cycling in highly polluted Ya-Er Lake, China. Chemosphere. 2021;275 doi: 10.1016/j.chemosphere.2021.130011. [DOI] [PubMed] [Google Scholar]
- 62.Chen X., et al. Mercury in sediment reflecting the intensive coal mining activities: evidence from stable mercury isotopes and Bayesian mixing model analysis. Ecotoxicol. Environ. Saf. 2022;234(March) doi: 10.1016/j.ecoenv.2022.113392. [DOI] [PubMed] [Google Scholar]
- 63.Hsu-Kim H., et al. Challenges and opportunities for managing aquatic mercury pollution in altered landscapes. Ambio. 2018;47(2):141–169. doi: 10.1007/s13280-017-1006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Khan E.A., Abbas Z. A scoping review of sources of mercury and its health effects among Pakistan's most vulnerable population. Rev. Environ. Health. 2021;36(1):39–45. doi: 10.1515/reveh-2019-0099. [DOI] [PubMed] [Google Scholar]
- 65.Keane S., et al. Mercury and artisanal and small-scale gold mining: review of global use estimates and considerations for promoting mercury-free alternatives. Ambio. 2023;52(5):833–852. doi: 10.1007/s13280-023-01843-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Issifu I., Alava J.J., Lam V.W.Y., Sumaila U.R. Impact of ocean warming, overfishing and mercury on European fisheries: a risk assessment and policy solution framework. Front. Mar. Sci. 2022;8(February):1–13. doi: 10.3389/fmars.2021.770805. [DOI] [Google Scholar]
- 67.de Vasconcellos A.C.S., Ferreira S.R.B., de Sousa C.C., de Oliveira M.W., de Oliveira Lima M., Basta P.C. Health risk assessment attributed to consumption of fish contaminated with mercury in the Rio branco basin, roraima, amazon, Brazil. Toxics. 2022;10(9):1–18. doi: 10.3390/toxics10090516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Harris R., et al. Mercury in the gulf of Mexico: sources to receptors. Environ. Res. 2012;119:42–52. doi: 10.1016/j.envres.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 69.Riesgo L., Sanpera C., García-Barcelona S., Sánchez-Fortún M., Coll M., Navarro J. Understanding the role of ecological factors affecting mercury concentrations in the blue shark (Prionace glauca) Chemosphere. 2023;313(December 2022) doi: 10.1016/j.chemosphere.2022.137642. [DOI] [PubMed] [Google Scholar]
- 70.Latif M., et al. Bioaccumulation of lead in different organs of Ctenopharyngodon Idella (grass fish) and Tor putitora (Mahseer) fish. Braz. J. Biol. 2024;84:1–7. doi: 10.1590/1519-6984.260355. [DOI] [PubMed] [Google Scholar]
- 71.Łuczyńska J., Łuczyński M.J., Nowosad J., Kowalska-Góralska M., Senze M. Total mercury and fatty acids in selected fish species on the polish market: a risk to human health. Int. J. Environ. Res. Publ. Health. 2022;19(16) doi: 10.3390/ijerph191610092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhou H.Y., Wong M.H. Mercury accumulation in freshwater fish with emphasis on the dietary influence. Water Res. 2000;34(17):4234–4242. doi: 10.1016/S0043-1354(00)00176-7. [DOI] [Google Scholar]
- 73.Lencha S.M., Tränckner J., Dananto M. Assessing the water quality of lake hawassa Ethiopia—trophic state and suitability for anthropogenic uses—applying common water quality indices. Int. J. Environ. Res. Publ. Health. 2021;18(17) doi: 10.3390/ijerph18178904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Clarkson T.W., Magos L. The toxicology of mercury and its chemical compounds. Crit. Rev. Toxicol. 2006;36(8):609–662. doi: 10.1080/10408440600845619. [DOI] [PubMed] [Google Scholar]
- 75.Dezfouli A.B., Salar-Amoli J., Ali-Esfahani T., Hosseini H., Ghanati K. Evaluating total mercury and methyl mercury contents in canned tuna fish of the Persian gulf. Iran. J. Pharm. Res. (IJPR) 2018;17(2):585–592. [PMC free article] [PubMed] [Google Scholar]
- 76.Huang T., et al. Human exposure to polychlorinated biphenyls embodied in global fish trade. Nat. Food. 2020;1(5):292–300. doi: 10.1038/s43016-020-0066-1. [DOI] [Google Scholar]
- 77.Polak-Juszczak L., Waszak I., Szlinder-Richert J., Wójcik I. Levels, time trends, and distribution of dioxins and polychlorinated biphenyls in fishes from the Baltic Sea. Chemosphere. 2022;306(June) doi: 10.1016/j.chemosphere.2022.135614. [DOI] [PubMed] [Google Scholar]
- 78.Erickson M.D., Kaley R.G. Applications of polychlorinated biphenyls. Environ. Sci. Pollut. Res. 2011;18(2):135–151. doi: 10.1007/s11356-010-0392-1. [DOI] [PubMed] [Google Scholar]
- 79.Markowitz G., Rosner D. vol. 39. Palgrave Macmillan UK; 2018. (Monsanto, PCBs, and the Creation of a “World-wide Ecological Problem,”). no. 4. [DOI] [PubMed] [Google Scholar]
- 80.Ghosh S., et al. PCB exposure and potential future cancer incidence in Slovak children: an assessment from molecular finger printing by Ingenuity Pathway Analysis (IPA®) derived from experimental and epidemiological investigations. Environ. Sci. Pollut. Res. 2018;25(17):16493–16507. doi: 10.1007/s11356-017-0149-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kennedy C.L., Spiegelhoff A., Lavery T., Wang K., Manuel R.S., Wang Z., Wildermuth H., Keil Stietz K.P. Developmental polychlorinated biphenyl (PCB) exposure alters voiding physiology in young adult male and female mice. Am. J. Clin. Exp. Urol. 2022;10(2):82–97. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9077147/ [PMC free article] [PubMed] [Google Scholar]
- 82.Batang Z.B., et al. Congener-specific levels and patterns of polychlorinated biphenyls in edible fish tissue from the central Red Sea coast of Saudi Arabia. Sci. Total Environ. 2016;572:915–925. doi: 10.1016/j.scitotenv.2016.07.207. [DOI] [PubMed] [Google Scholar]
- 83.Zhang H., et al. Ecosystem impact and dietary exposure of polychlorinated biphenyls (PCBs) and heavy metals in Chinese mitten crabs (Eriocheir sinensis) and their farming areas in Jiangsu, China. Ecotoxicol. Environ. Saf. 2021;227 doi: 10.1016/j.ecoenv.2021.112936. [DOI] [PubMed] [Google Scholar]
- 84.Saktrakulkla Panithi, Lan Tuo, Hua Jason, Marek Rachel F., Thorne Peter S., Hornbuckle Keri C. Polychlorinated biphenyls in food. Environ. Sci. Technol. 2020;54:11443–11452. doi: 10.1021/acs.est.0c03632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Banout J., Urban O., Musil V., Szakova J., Balik J. Agent orange footprint still visible in rural areas of central Vietnam. J. Environ. Public Health. 2014;2014 doi: 10.1155/2014/528965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cui X., et al. Polychlorinated biphenyls in the drinking water source of the Yangtze River: characteristics and risk assessment. Environ. Sci. Eur. 2020;32(1) doi: 10.1186/s12302-020-00309-6. [DOI] [Google Scholar]
- 87.Megahed A.M., et al. Polychlorinated biphenyls water pollution along the River Nile, Egypt. Sci. World J. 2015;2015 doi: 10.1155/2015/389213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Agarwal S., Al-Abed S.R., Dionysiou D.D. In situ technologies for reclamation of PCB-contaminated sediments: current challenges and research thrust areas. J. Environ. Eng. 2007;133(12):1075–1078. doi: 10.1061/(asce)0733-9372(2007)133:12(1075). [DOI] [Google Scholar]
- 89.Prince K.D., et al. Mussels drive polychlorinated biphenyl (PCB) biomagnification in a coastal food web. Sci. Rep. 2021;11(1):1–11. doi: 10.1038/s41598-021-88684-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Obanya H.E., Ntor C., Okoroafor C.U., Nwanze R. Occurrence of a polychlorinated biphenyl (PCB) congener in surface water, sediments and blackchin Tilapia (Sarotherodon melanotheron) from ologe lagoon, Nigeria. J. Appl. Sci. Environ. Manag. 2019;23(10):1805–1811. doi: 10.4314/jasem.v23i10.6. [DOI] [Google Scholar]
- 91.Ngoubeyou P.S.K., Wolkersdorfer C., Ndibewu P.P., Augustyn W. Toxicity of polychlorinated biphenyls in aquatic environments – a review. Aquat. Toxicol. 2022;251(August) doi: 10.1016/j.aquatox.2022.106284. [DOI] [PubMed] [Google Scholar]
- 92.Bo B. Human health risk of polychlorinated biphenyls in some brackish and marine fish species from Ondo South-West Nigeria. Biomed. J. Sci. Tech. Res. 2020;30(2):23258–23263. doi: 10.26717/bjstr.2020.30.004928. [DOI] [Google Scholar]
- 93.Lanphear B.P., Wright R.O., Dietrich K.N. Environmental neurotoxins. Pediatr. Rev. 2005;26(6):191–198. doi: 10.1542/pir.26-6-191. [DOI] [PubMed] [Google Scholar]
- 94.Themba N., Sibali L.L., Chokwe T.B. A review on the formation and remediations of polychlorinated dibenzo p-dioxins and dibenzo-furans (PCDD/Fs) during thermal processes with a focus on MSW process. Air Qual. Atmos. Heal. 2023;16(10):2115–2132. doi: 10.1007/s11869-023-01394-1. [DOI] [Google Scholar]
- 95.Kumar M., et al. Environmental endocrine-disrupting chemical exposure: role in non-communicable diseases. Front. Public Health. 2020;8(September):1–28. doi: 10.3389/fpubh.2020.553850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pilsner J.R., Parker M., Sergeyev O., Suvorov A. Spermatogenesis disruption by dioxins: epigenetic reprograming and windows of susceptibility. Reprod. Toxicol. 2017;69:221–229. doi: 10.1016/j.reprotox.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Faiad W., Soukkarieh C., Murphy D.J., Hanano A. Effects of dioxins on animal spermatogenesis: a state-of-the-art review. Front. Reprod. Heal. 2022;4(October):1–15. doi: 10.3389/frph.2022.1009090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Danjou A.M.N., et al. Long-term airborne dioxin exposure and breast cancer risk in a case-control study nested within the French E3N prospective cohort. Environ. Int. 2019;124(July 2018):236–248. doi: 10.1016/j.envint.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 99.Peivasteh-roudsari L., et al. Origin, dietary exposure, and toxicity of endocrine-disrupting food chemical contaminants: a comprehensive review. Heliyon. 2023;9(7) doi: 10.1016/j.heliyon.2023.e18140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nevalainen L., Tuomisto J., Haapasaari P., Lehikoinen A. Spatial aspects of the dioxin risk formation in the Baltic Sea: a systematic review. Sci. Total Environ. 2021;753 doi: 10.1016/j.scitotenv.2020.142185. [DOI] [PubMed] [Google Scholar]
- 101.Jürgens M.D., Crosse J., Hamilton P.B., Johnson A.C., Jones K.C. The long shadow of our chemical past – high DDT concentrations in fish near a former agrochemicals factory in England. Chemosphere. 2016;162:333–344. doi: 10.1016/j.chemosphere.2016.07.078. [DOI] [PubMed] [Google Scholar]
- 102.Rohani M.F. Pesticides toxicity in fish: histopathological and hemato-biochemical aspects – a review. Emerging Contam. 2023;9(3) doi: 10.1016/j.emcon.2023.100234. [DOI] [Google Scholar]
- 103.Fernanda K., et al. 2017. Accepted Manuscript. [DOI] [Google Scholar]
- 104.Fernanda C., Batista D., Pradhan A. 2022. Plastic Interactions with Pollutants and Consequences to Aquatic Ecosystems : what We Know and what We Do Not Know. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chakraborty P., et al. Interlinkage between persistent organic pollutants and plastic in the waste management system of India: an overview. Bull. Environ. Contam. Toxicol. 2022;109(6):927–936. doi: 10.1007/s00128-022-03466-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.FAO and WHO . 2012. Joint FAO/WHO Expert Meeting on the Public Health Risks of Histamine and Other Biogenic Amines from Fish and Fishery Products 23 – 27 July 2012 FAO Headquarters , Rome , Italy Table of Contents; pp. 1–112. no. July. [Google Scholar]
- 107.Naqvi S.A.R., Idrees F., Sherazi T.A., Shahzad S.A., Hassan S.U.L., Ashraf N. Toxicology of heavy metals used in cosmetics. J. Chil. Chem. Soc. 2022;67(3):5615–5622. doi: 10.4067/S0717-97072022000305615. [DOI] [Google Scholar]
- 108.Karti S.S., et al. Non-cardiogenic pulmonary oedema in the course of verapamil intoxication. Emerg. Med. J. 2002;19(5):458–459. doi: 10.1136/emj.19.5.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Taylor R., Burg J., Opara N. The Sea was angry that day my friends: an inland case of acute scombroid poisoning with a twist. Cureus. 2021;13(9) doi: 10.7759/cureus.18394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mih H., Lacherai A. Evaluation of histamine contents during the fish meal production process. Ribar. Croat. J. Fish. 2021;78(4):203–209. doi: 10.2478/cjf-2020-0020. [DOI] [Google Scholar]
- 111.Jamil Emon F., et al. Bioaccumulation and bioremediation of heavy metals in fishes—a review. Toxics. 2023;11(6):1–28. doi: 10.3390/toxics11060510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bala S., et al. Recent strategies for bioremediation of emerging pollutants: a review for a green and sustainable environment. Toxics. 2022;10(8):1–24. doi: 10.3390/toxics10080484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jinadasa B.K.K.K., Jayasinghe G.D.T.M., Pohl P., Fowler S.W. Mitigating the impact of mercury contaminants in fish and other seafood—a review. Mar. Pollut. Bull. 2021;171(June) doi: 10.1016/j.marpolbul.2021.112710. [DOI] [PubMed] [Google Scholar]
- 114.Elvevoll E.O., James D., Toppe J., Gamarro E.G., Jensen I.J. Food safety risks posed by heavy metals and persistent organic pollutants (POPs) related to consumption of sea cucumbers. Foods. 2022;11(24) doi: 10.3390/foods11243992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Manchanayake T., Salleh A., Amal M.N.A., Yasin I.S.M., Zamri-Saad M. Pathology and pathogenesis of Vibrio infection in fish: a review. Aquac. Reports. 2023;28(April 2022) doi: 10.1016/j.aqrep.2022.101459. [DOI] [Google Scholar]
- 116.Lokossou Y.U.A., Tambe A.B., Azandjèmè C., Mbhenyane X. Socio-cultural beliefs influence feeding practices of mothers and their children in Grand Popo, Benin. J. Health Popul. Nutr. 2021;40(1):1–12. doi: 10.1186/s41043-021-00258-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Starling P., Charlton K., McMahon A.T., Lucas C. Fish intake during pregnancy and foetal neurodevelopment-A systematic review of the evidence. Nutrients. 2015;7(3):2001–2014. doi: 10.3390/nu7032001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Papadopoulou E., et al. Maternal seafood intake during pregnancy, prenatal mercury exposure and child body mass index trajectories up to 8 years. Int. J. Epidemiol. 2021;50(4):1134–1146. doi: 10.1093/ije/dyab035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nøstbakken O.J., et al. Levels of omega 3 fatty acids, vitamin D, dioxins and dioxin-like PCBs in oily fish; a new perspective on the reporting of nutrient and contaminant data for risk–benefit assessments of oily seafood. Environ. Int. 2021;147 doi: 10.1016/j.envint.2020.106322. [DOI] [PubMed] [Google Scholar]
- 120.Lyche J.L., Rosseland C., Berge G., Polder A. Human health risk associated with brominated flame-retardants (BFRs) Environ. Int. 2015;74:170–180. doi: 10.1016/j.envint.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 121.Bottinger E.P. Foundations, promises and uncertainties of personalized medicine. MSJM (Mt. Sinai J. Med.) 2007;74(1):15–21. doi: 10.1002/msj.20005. New York. [DOI] [PubMed] [Google Scholar]
- 122.Sewberath Misser V.H.D.A., Shankar A., Wickliffe J.K., Lichtveld M.Y., Mans D.R. Prenatal exposure to mercury, manganese, and lead and adverse birth outcomes in Suriname: a population-based birth cohort study. Toxics. 2022;10(8):464. doi: 10.3390/toxics10080464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Taylor C.M., Emmett P.M., Emond A.M., Golding J. Europe PMC funders group europe PMC funders author manuscripts A review of guidance on fish consumption in pregnancy : is it fit for purpose. 2018;21(11):2149–2159. doi: 10.1017/S1368980018000599.A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Auriti C., et al. Pregnancy and viral infections: mechanisms of fetal damage, diagnosis and prevention of neonatal adverse outcomes from cytomegalovirus to SARS-CoV-2 and Zika virus. Biochim. Biophys. Acta, Mol. Basis Dis. 2021;1867(10) doi: 10.1016/j.bbadis.2021.166198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dack K., Fell M., Taylor C.M., Havdahl A., Lewis S.J. Prenatal mercury exposure and neurodevelopment up to the age of 5 Years: a systematic review. Int. J. Environ. Res. Publ. Health. 2022;19(4) doi: 10.3390/ijerph19041976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hibbeln J.R., et al. Maternal seafood consumption in pregnancy and neurodevelopmental outcomes in childhood (ALSPAC study): an observational cohort study. Obstet. Gynecol. Surv. 2007;62(7):437–439. doi: 10.1097/01.ogx.0000268661.03197.57. [DOI] [PubMed] [Google Scholar]
- 127.Zhang Z., Fulgoni V.L., Kris-Etherton P.M., Mitmesser S.H. Dietary intakes of EPA and DHA omega-3 fatty acids among US childbearing-age and pregnant women: an analysis of NHANES 2001–2014. Nutrients. 2018;10(4) doi: 10.3390/nu10040416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rahman M.T., et al. Zoonotic diseases: etiology, impact, and control. Microorganisms. 2020;8(9):1–34. doi: 10.3390/microorganisms8091405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sheng L., Wang L. The microbial safety of fish and fish products: recent advances in understanding its significance, contamination sources, and control strategies. Compr. Rev. Food Sci. Food Saf. 2021;20(1):738–786. doi: 10.1111/1541-4337.12671. [DOI] [PubMed] [Google Scholar]
- 130.Bernasconi S., Street M.E., Iughetti L., Predieri B. Chemical contaminants in breast milk: a brief critical overview. Glob. Pediatr. 2022;2(May) doi: 10.1016/j.gpeds.2022.100017. [DOI] [Google Scholar]
- 131.Pajewska-Szmyt M., Sinkiewicz-Darol E., Gadzała-Kopciuch R. The impact of environmental pollution on the quality of mother's milk. Environ. Sci. Pollut. Res. 2019;26(8):7405–7427. doi: 10.1007/s11356-019-04141-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mahaffey K.R., Clickner R.P., Jeffries R.A. Adult women's blood mercury concentrations vary regionally in the United States: association with patterns of fish consumption (NHANES 1999-2004) Environ. Health Perspect. 2009;117(1):47–53. doi: 10.1289/ehp.11674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wahlang B. Exposure to persistent organic pollutants: impact on women's health. Rev. Environ. Health. 2018;33(4):331–348. doi: 10.1515/reveh-2018-0018. [DOI] [PubMed] [Google Scholar]
- 134.Davies G.A.L., et al. Obesity in pregnancy. J. Obstet. Gynaecol. Can. 2010;32(2):165–173. doi: 10.1016/S1701-2163(16)34432-2. [DOI] [PubMed] [Google Scholar]
- 135.Barone G., et al. Levels of mercury, methylmercury and selenium in fish: insights into children food safety. Toxics. 2021;9(2):1–14. doi: 10.3390/toxics9020039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Oken E., et al. Maternal fish intake during pregnancy, blood mercury levels, and child cognition at age 3 years in a US cohort. Am. J. Epidemiol. 2008;167(10):1171–1181. doi: 10.1093/aje/kwn034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Stewart P.W., Lonky E., Reihman J., Pagano J., Gump B.B., Darvill T. The relationship between prenatal PCB exposure and intelligence (IQ) in 9-year-old children. Environ. Health Perspect. 2008;116(10):1416–1422. doi: 10.1289/ehp.11058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ghorbani Nejad Behnam, Raeisi Tahereh, Janmohammadi Parisa, Mehravar Fatemeh, Zarei Mahtab, Dehghani Azadeh, Bahrampour Niki, Darijani Mohammad Hosein, Ahmadipour Fatemeh, Mohajeri Mohammad, Alizadeh Shahab. Mercury exposure and risk of type 2 diabetes: a systematic review and meta-analysis. Int. J. Clin. Pract. 2022;2022 doi: 10.1155/2022/7640227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bayen S., Koroleva E., Lee H.K., Obbard J.P. Persistent organic pollutants and heavy metals in typical seafoods consumed in Singapore. J. Toxicol. Environ. Health, Part A. 2005;68(3):151–166. doi: 10.1080/15287390590890437. [DOI] [PubMed] [Google Scholar]
- 140.Azeh Engwa G., Udoka Ferdinand P., Nweke Nwalo F., Unachukwu M.N. Mechanism and health effects of heavy metal toxicity in humans. Poisoning Mod. World - New Tricks an Old Dog? 2019 doi: 10.5772/intechopen.82511. [DOI] [Google Scholar]
- 141.Gençpinar P., Büyüktahtakin B., Ibişoʇlu Z., Genç Ş., Yilmaz A., Mihçi E. Mercury poisoning as a cause of intracranial hypertension. J. Child Neurol. 2015;30(6):760–763. doi: 10.1177/0883073814538503. [DOI] [PubMed] [Google Scholar]
- 142.Downer M.K., et al. Mercury exposure and risk of cardiovascular disease: a nested case-control study in the PREDIMED (PREvention with MEDiterranean Diet) study. BMC Cardiovasc. Disord. 2017;17(1):1–11. doi: 10.1186/s12872-016-0435-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hu X.F., Lowe M., Chan H.M. Mercury exposure, cardiovascular disease, and mortality: a systematic review and dose-response meta-analysis. Environ. Res. 2021;193(December 2020) doi: 10.1016/j.envres.2020.110538. [DOI] [PubMed] [Google Scholar]
- 144.Balali-Mood M., Naseri K., Tahergorabi Z., Khazdair M.R., Sadeghi M. Toxic mechanisms of five heavy metals: mercury, lead, chromium, cadmium, and arsenic. Front. Pharmacol. 2021;12(April):1–19. doi: 10.3389/fphar.2021.643972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Donato F., et al. Polychlorinated biphenyls and risk of hepatocellular carcinoma in the population living in a highly polluted area in Italy. Sci. Rep. 2021;11(1):1–9. doi: 10.1038/s41598-021-82657-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ali I., et al. Exposure to polychlorinated biphenyls and prostate cancer: population-based prospective cohort and experimental studies. Carcinogenesis. 2016;37(12):1144–1151. doi: 10.1093/carcin/bgw105. [DOI] [PubMed] [Google Scholar]
- 147.eun Lim J., Nam C., Yang J., Rha K.H., Lim K.M., Jee S.H. Serum persistent organic pollutants (POPs) and prostate cancer risk: a case-cohort study. Int. J. Hyg Environ. Health. 2017;220(5):849–856. doi: 10.1016/j.ijheh.2017.03.014. [DOI] [PubMed] [Google Scholar]
- 148.Parada H., et al. Plasma levels of polychlorinated biphenyls (PCBs) and breast cancer mortality: the Carolina Breast Cancer Study. Int. J. Hyg Environ. Health. 2020;227(2020) doi: 10.1016/j.ijheh.2020.113522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Othman N., Ismail Z., Selamat M.I., Sheikh Abdul Kadir S.H., Shibraumalisi N.A. A review of polychlorinated biphenyls (PCBs) pollution in the air: where and how much are we exposed to? Int. J. Environ. Res. Publ. Health. 2022;19(21) doi: 10.3390/ijerph192113923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Thushari G.G.N., Senevirathna J.D.M. Plastic pollution in the marine environment. Heliyon. 2020;6(8) doi: 10.1016/j.heliyon.2020.e04709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Anand U., et al. Biotechnological methods to remove microplastics: a review. Environ. Chem. Lett. 2023;21(3):1787–1810. doi: 10.1007/s10311-022-01552-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Dube E., Okuthe G.E. Plastics and micro/nano-plastics (MNPs) in the environment: occurrence, impact, and toxicity. Int. J. Environ. Res. Publ. Health. 2023;20(17) doi: 10.3390/ijerph20176667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Khan M.L., et al. vol. 84. 2024. pp. 1–16. (Effects of Microplastics in Freshwater Fishes Health and the Implications for Human Health). [DOI] [PubMed] [Google Scholar]
- 154.Ghosh S., Sinha J.K., Ghosh S., Vashisth K., Han S., Bhaskar R. Microplastics as an emerging threat to the global environment and human health. Sustain. Times. 2023;15(14) doi: 10.3390/su151410821. [DOI] [Google Scholar]
- 155.Menéndez-Pedriza A., Jaumot J. Interaction of environmental pollutants with microplastics: a critical review of sorption factors, bioaccumulation and ecotoxicological effects. Toxics. 2020;8(2) doi: 10.3390/TOXICS8020040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Barboza L.G.A., et al. Microplastics in wild fish from North East Atlantic Ocean and its potential for causing neurotoxic effects, lipid oxidative damage, and human health risks associated with ingestion exposure. Sci. Total Environ. 2020;717 doi: 10.1016/j.scitotenv.2019.134625. [DOI] [PubMed] [Google Scholar]
- 157.Kye H., Kim J., Ju S., Lee J., Lim C., Yoon Y. Microplastics in water systems: a review of their impacts on the environment and their potential hazards. Heliyon. 2023;9(3) doi: 10.1016/j.heliyon.2023.e14359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Savoca M.S., Mcinturf A.G., Hazen E.L. 2021. Plastic Ingestion by Marine Fish Is Widespread and Increasing; pp. 2188–2199. no. January. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Avignon G.D., Wang D., Reid H.B., Gregory-eaves I., Ricciardi A. 2023. Effects of Elevated Temperature and Microplastic Exposure on Growth and Predatory Performance of a Freshwater Fi Sh; pp. 2245–2260. [DOI] [Google Scholar]
- 160.Onolame J., Igwaran A. Microplastics in seafood : implications for food security , safety , and human health. J. Sea Res. 2023;194(January) doi: 10.1016/j.seares.2023.102410. [DOI] [Google Scholar]
- 161.Zolotova N., Kosyreva A., Dzhalilova D. 2022. Harmful Effects of the Microplastic Pollution on Animal Health : a Literature Review; pp. 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lehner R., Weder C., Petri-Fink A., Rothen-Rutishauser B. Emergence of nanoplastic in the environment and possible impact on human health. Environ. Sci. Technol. 2019;53(4):1748–1765. doi: 10.1021/acs.est.8b05512. [DOI] [PubMed] [Google Scholar]
- 163.Subaramaniyam U., et al. Effects of microplastics, pesticides and nano-materials on fish health, oxidative stress and antioxidant defense mechanism. Front. Physiol. 2023;14(June):1–24. doi: 10.3389/fphys.2023.1217666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Yee M.S.L., et al. Impact of microplastics and nanoplastics on human health. Nanomaterials. 2021;11(2):1–23. doi: 10.3390/nano11020496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Barceló D., Picó Y., Alfarhan A.H. Microplastics: detection in human samples, cell line studies, and health impacts. Environ. Toxicol. Pharmacol. 2023;101(March) doi: 10.1016/j.etap.2023.104204. [DOI] [PubMed] [Google Scholar]
- 166.Campanale C., Massarelli C., Savino I., Locaputo V., Uricchio V.F. A detailed review study on potential effects of microplastics and additives of concern on human health. Int. J. Environ. Res. Publ. Health. 2020;17(4) doi: 10.3390/ijerph17041212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kannan K., Vimalkumar K. A review of human exposure to microplastics and insights into microplastics as obesogens. Front. Endocrinol. 2021;12(August):1–19. doi: 10.3389/fendo.2021.724989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Osman A.I., et al. Microplastic sources, formation, toxicity and remediation: a review. Springer International Publishing. 2023;21(4) doi: 10.1007/s10311-023-01593-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Jayedi A., Shab-Bidar S., Eimeri S., Djafarian K. Fish consumption and risk of all-cause and cardiovascular mortality: a dose-response meta-analysis of prospective observational studies. Publ. Health Nutr. 2018;21(7):1297–1306. doi: 10.1017/S1368980017003834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bakre A.T., et al. Impact of fish consumption on all-cause mortality in older people with and without dementia: a community-based cohort study. Eur. J. Nutr. 2022;61(7):3785–3794. doi: 10.1007/s00394-022-02887-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Philibert A., Fillion M., Mergler D. Mercury exposure and premature mortality in the Grassy Narrows First Nation community: a retrospective longitudinal study. Lancet Planet. Health. 2020;4(4):e141–e148. doi: 10.1016/S2542-5196(20)30057-7. [DOI] [PubMed] [Google Scholar]
- 172.Donat-Vargas C., Bellavia A., Berglund M., Glynn A., Wolk A., Åkesson A. Cardiovascular and cancer mortality in relation to dietary polychlorinated biphenyls and marine polyunsaturated fatty acids: a nutritional-toxicological aspect of fish consumption. J. Intern. Med. 2020;287(2):197–209. doi: 10.1111/joim.12995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zhou J., et al. Association of oily fish and nonoily fish intakes with all-cause mortality and cause-specific mortality: a large population-based prospective study. J. Transl. Med. 2023;21(1):1–10. doi: 10.1186/s12967-023-04097-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wallin A., Orsini N., Forouhi N.G., Wolk A. Fish consumption in relation to myocardial infarction, stroke and mortality among women and men with type 2 diabetes: a prospective cohort study. Clin. Nutr. 2018;37(2):590–596. doi: 10.1016/j.clnu.2017.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.