Abstract
Introduction
Armodafinil is a psychostimulant that promotes alertness, and it has been shown to improve attention, memory, and fatigue in healthy adults and adults with neurodevelopmental conditions that share symptoms with Attention Deficit Hyperactivity Disorder (ADHD). It is generally well tolerated and safe, and most of the adverse events reported are considered not serious. However, the available evidence on the efficacy of armodafinil for the treatment of ADHD in adults is scarce.
Objective
The present review aims to perform a systematized search of the available evidence on the possible therapeutic benefit of armodafinil treatment in adult patients with ADHD.
Methods
A literature review using PubMed was conducted to compile and summarize the available clinical and scientific evidence on the possible use of armodafinil as a pharmacological treatment in adult patients with ADHD.
Results
From the 86 articles reviewed, the available evidence showed that both acute and chronic treatment with armodafinil can improve wakefulness, memory, impulse control, and executive functions in adults with sleep disorders and other conditions. In addition, evidence of improvement in cognitive functions and mood alterations in other neuropsychiatric conditions was shown.
Conclusion
Armodafinil could be useful for the treatment of ADHD in adults, according to the review of the literature from both pre-clinical and clinical studies.
Keywords: Attention deficit hyperactivity disorder, circadian rhythms, sleep problems, cognitive disturbances, psychostimulant drugs, armodafinil
1. INTRODUCTION
Attention deficit hyperactivity disorder (ADHD) is the most common neurodevelopmental condition during pediatric age. The symptomatology is observed from infancy, and it is estimated that up to 60% of patients continue to present symptoms and significant impairment during adulthood and childhood. This condition presents as a major clinical condition characterized by the presence of impairment of executive function, cognitive problems such as attention deficit, and a considerable increase in hyperactivity and impulsivity [1-3].
Prevalence estimates of ADHD vary according to age groups and diagnostic criteria. In the United States of America, it is estimated that about 9.4% of patients between 2 and 17 years of age are diagnosed with ADHD [4, 5], while in adulthood, there is an estimated 2.5% in the general population [6, 7], more frequently reported in men than in women in a ratio of 2:1 [8]. Previously, ADHD was thought to be a childhood condition; however, recent evidence indicates that significant symptoms continue to occur in adulthood, which are often difficult to distinguish from the symptoms of other neuropsychiatric conditions, making it more complex to adequately address and treat the condition.
Nowadays, ADHD symptomatology in adults is poorly studied and misunderstood by health professionals. Consequently, it is estimated that less than 20% of adults with ADHD are currently being diagnosed and treated by a psychiatric specialist, and those who seek care usually do so to treat some other comorbid condition, such as the presence of sleep disturbances or mood disorders [9]. Diagnosis and treatment are limited, and they are usually focused on modulating symptomatology related to the main comorbidities that patients present with, such as affective disorders, anxiety, personality, substance abuse, and eating disorders [9-11].
The first line of pharmacological treatment includes the use of psychostimulant drugs such as methylphenidate and amphetamine formulations such as lisdexamfetamine, which have shown high efficacy in patients of all ages [12, 13]. However, nearly 20 to 30% of patients report excessive side effects or do not respond to this line of treatment [14]. Non-psychostimulant drugs include atomoxetine, guanfacine, and clonidine, which are considered second-line treatments because they show a lower response and effect sizes and are usually reserved for patients who respond poorly or have significant side effects to first-line treatment [12, 13].
The lack of an effective, safe, not only in the short term but also in the long term, low addictive and long-lasting treatment highlights the current problem for the management of this condition and the reason why it is necessary to propose new therapeutic strategies that are safe and accessible to the population.
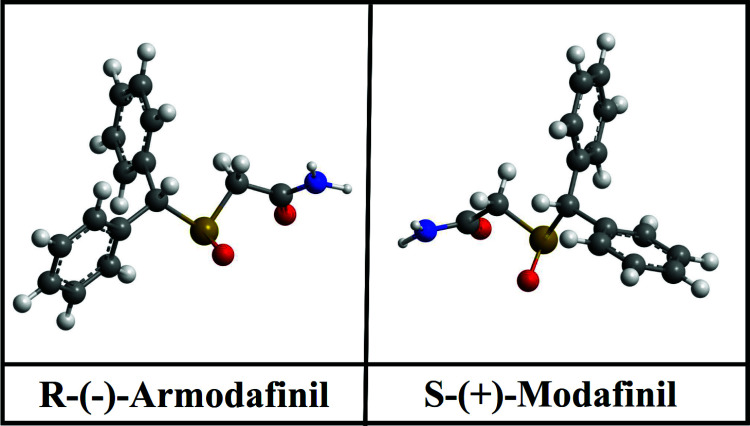
Armodafinil and its enantiomer S (modafinil) (Fig. 1) have been described as psychostimulants with a different pharmacological profile, chemical structure, and mechanism of action than amphetamines, which have drawn attention in their use as an alternative treatment for the symptoms observed in ADHD, and this possibility could provide an answer to the current concern about the risk of dependence that has been reported with the abuse of psychostimulants [15-18]. There is evidence to suggest that these drugs improve function in several cognitive domains, including working memory and episodic memory, and other processes that depend on the prefrontal cortex and cognitive control in adults, as well as being well tolerated with a reduced rate of adverse events and risk of dependence [19]. This review aims to compile and summarize the available information on the efficacy of armodafinil treatment and the known neurobiological mechanisms involved in adult patients with ADHD.
Fig. (1).
The R enantiomer (armodafinil) and S enantiomer (modafinil) chemical structure.
2. METHODS OF SEARCH FOR THE INFORMATION
This review aims to compile and summarize the available evidence suggesting the potential use of armodafinil for the treatment of ADHD in adults. Clinical trial results and original studies in adult patients were included. The search for information was conducted using the National Library of Medicine, National Center for Biotechnology Information (NIH) PubMed. Logical functions and operators such as “or”, “and”, and “not” were included in the search using the following keywords: Attention-Deficit/Hyperactivity Disorder (ADHD), epidemiology of ADHD, sleep disorders and ADHD, attention, hyperactivity, psychostimulants, and ADHD, modafinil and ADHD, armodafinil and cognition, armodafinil and memory, armodafinil and sleep disorders. Articles written in languages other than English were excluded from the review. Selected studies that met the inclusion and exclusion criteria (86 articles) were analyzed and discussed in this review.
3. OVERVIEW OF ATTENTION DEFICIT/HYPER-ACTIVITY DISORDER (ADHD)
3.1. ADHD in Adults
ADHD is one of the most complex and prevalent neurodevelopmental conditions worldwide. It is a debilitating condition that generates important changes in behavioral patterns in adults [20]. Although the pathophysiological mechanisms underlying this disorder are not yet fully understood, available evidence suggests that there are several morphological alterations in the prefrontal, frontal, parietal, temporal, and entorhinal cortexes [21] and an imbalance of the noradrenergic and dopaminergic systems, especially in the frontal cortex [22].
Adult patients with ADHD show greater dysfunction in attention and higher executive functions, such as inhibitory control, emotional regulation, and cognitive flexibility, compared to the pediatric population, where hyperactivity and impulsivity generally predominate [23]. Therefore, the manifestations observed in adults with ADHD consist of a greater difficulty in maintaining focus and attention, leading to frequent forgetfulness and errors that cause frustration and significant discomfort, difficulties in planning, organizing, and executing tasks, an unpleasant physical restlessness that is reflected in the constant movement of the limbs, but also, greater difficulty in regulating emotions adequately [23, 24]. Likewise, a study published in 2006 indicates that adults with ADHD have a high rate of failure in school performance, work performance, and maintaining emotional bonds [25]. In support of the above, it has been observed that the clinical manifestations in adults with this condition have a direct impact on their functioning, as well as on the adequate development of interpersonal relationships, also hindering their social functioning [26]. On the other hand, major comorbid disorders such as substance abuse, especially those associated with earlier onset and more severe development, and various affective disorders appear to arise to some extent from a later onset as a consequence of primary ADHD, especially when the patient does not receive treatment promptly, which may contribute to misdiagnosis and delay in the patient's treatment and recovery [6, 26, 27].
ADHD is generally diagnosed using the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) criteria [28]. The diagnostic criteria for ADHD focus on inattention and hyperactivity/impulsivity and can be classified into three different subtypes: inattentive (20-30% of cases), hyperactive-impulsive (15% of cases), and combined (50-75% of cases) [29]. However, currently, the symptom profile of adults with ADHD does not consider for its diagnosis the deficit of executive functions and emotional dysregulation [23].
Psychostimulant drugs such as methylphenidate and amphetamines are considered the first-line treatments, as they have a good tolerance profile [30, 31] and have shown to be effective in ADHD by inhibiting the reuptake of norepinephrine and dopamine [32]. These medications have an approximate duration of no more than 12 to 13 hours per dose, considering the extended-release formulations; thus, some patients require a combination of an extended-release and an immediate-release formulation to ensure that the effect lasts throughout the day [30, 33]. However, the most common side effects are appetite suppression, insomnia, dry mouth, and nausea. In addition, long-term treatment has been associated with alterations in growth trajectory, specifically in relation to height and weight [13]. For those patients who do not tolerate the first-line treatment of choice, non-stimulant therapy is used, including tricyclic antidepressants, α-agonists, clonidine, and guanfacine, among others [12, 34-36]. For this reason, stimulating agents are needed to treat those patients who do not respond satisfactorily to standard pharmacological treatment.
3.2. Circadian Rhythms and Sleep Disorders in Adult Patients with Attention-deficit/Hyperactivity Disorder (ADHD)
One of the main manifestations in adults with ADHD is sleep and circadian rhythm disturbances, which are associated with simple sleep-related movement disorders such as restless legs syndrome/periodic limb movements [37-39]. Sleep quality disturbances, i.e., insomnia and daytime sleepiness, are associated with inattention and hyperactivity in adults [40, 41]. This situation takes on special importance because untreated adults with ADHD have greater difficulty falling asleep and, therefore, a considerable increase in daytime attention problems [42]. The circadian system is hierarchically organized with a central pacemaker in the suprachiasmatic nucleus of the hypothalamus of the brain and is responsible for the generation of behavioral and physiological rhythms on a nearly 24-period basis and plays a key role in determining the rhythm of the sleep/wake cycle [43]. Previous studies indicate that in ADHD patients, circadian rhythm disturbance is closely related to dysfunction of melatonin and cortisol rhythms with subsequent decreased sleep duration and quality [44]. Melatonin and cortisol are key indicators in the sleep/wake cycle and are believed to be responsible for some of the altered behaviors characteristic of ADHD [45]. Likewise, it is known that deep circadian control and the alteration of dopaminergic neurotransmission are related; for this reason, the therapeutic approach to ADHD through the use of psychostimulants represents one of the first treatment alternatives [46]. Numerous studies have highlighted the importance of the use of psychostimulants for the treatment of ADHD in adults to decrease central symptomatology and behavioral problems.
3.3. Armodafinil
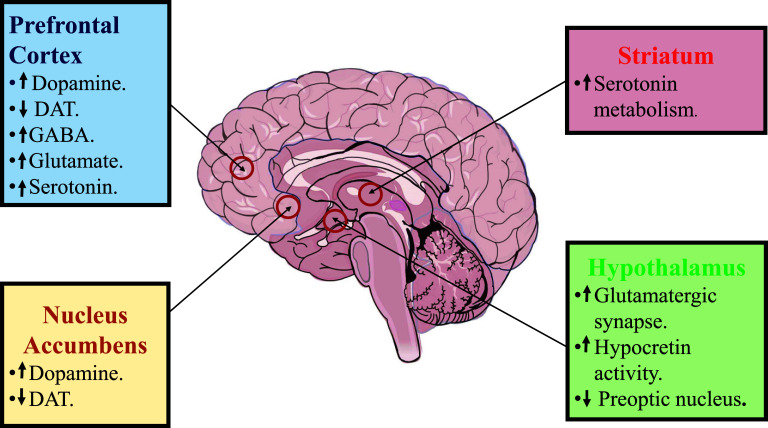
The R enantiomer armodafinil is a non-amphetamine central nervous system stimulant that promotes wakefulness in adults and reaches highest plasma concentrations between 6 and 14 h after administration, with an associated longer duration of wakefulness-promoting activity in healthy adults [19]. It has a half-life of 10 to 14 h compared to 3-4 h induced by that of the S enantiomer (modafinil) [47]. The mechanism of action of armodafinil is not yet fully explained. However, its effects are attributed to an increase in the concentration of dopamine in the prefrontal cortex and nucleus accumbens as it binds to the dopamine transporter (DAT), improving executive functions such as attention, impulsivity, memory, and impulse control [48]. On the other hand, it has been suggested that armodafinil can promote glutamatergic synapses in hypocretin/orexin neurons in the lateral hypothalamus that regulate wakefulness effects [49]. In support of the above aspects, the available evidence focuses on the use of modafinil; consequently, this information indicates that the R enantiomer (armodafinil) may be a potential treatment for this condition (Fig. 2).
Fig. (2).
Mechanism of action of armodafinil: Prefrontal cortex, increase the concentration of dopamine (DA) through inhibition of dopamine transporter (DAT) and can promote GABAergic, glutamatergic and serotonergic activity. Nucleus accumbens, increase the concentration DA through inhibition of DAT. Striatum, increased serotonin metabolism. Hypothalamus, promotes glutamatergic synapse in hypocretin/orexin neurons and is capable of inhibiting activity of the preoptic nucleus.
3.3.1. Modafinil as a Treatment for ADHD in Adults
Modafinil (2-[(diphenylmethyl) sulfinyl] acetamide) is an attention-promoting pharmacological agent that could be an effective and safe treatment option for ADHD. It is known to act on multiple areas of the attention and ascending arousal systems to increase frontal cortical activity [50]. The effects of commonly prescribed stimulant drugs are designed to mitigate ADHD symptoms during waking hours and have been shown to mitigate persistent problems related to planning, time awareness, and task prioritization.
Modafinil (S enantiomer) is a psychostimulant that exhibits potential as a cognitive enhancer in healthy adults and patients with ADHD. In healthy adult patients receiving a single dose of placebo or either 100 or 200 mg doses of modafinil, treated patients significantly improved their visual pattern recognition memory, spatial planning, and decreased impulsivity [51]. With the same aim, a double-blind, randomized, placebo-controlled crossover study using a single 200 mg dose of modafinil produced a significant pattern of improvement in short-term memory and visual memory ability in adult patients diagnosed with ADHD [14]. Another randomized, double-blind, placebo-controlled study reported that treatment with modafinil (206.8 mg/day) improved ADHD symptoms with results similar to those obtained with dextroamphetamine treatment (21.8 mg/day) [52]. In addition, modafinil has been reported to be well tolerated in children and adolescents and significantly improved ADHD symptomatology [53, 54]. In general, modafinil is well tolerated, and the adverse reactions reported were insomnia, headache, and decreased appetite [55, 56]. This evidence supports the idea that armodafinil may be beneficial in the treatment of ADHD; however, it highlights the importance of conducting preclinical and clinical trials to support it as a therapeutic alternative.
3.3.2. Armodafinil as a Possible Treatment for Adult ADHD
In addition to the core symptoms that are commonly present in adults diagnosed with ADHD, such as attention difficulties and hyperactivity, there are those related to sleep disturbances and circadian rhythm. The changes caused by circadian rhythms generate complications associated with cognitive and executive function [57]. Previous reports indicate that armodafinil is safe and effective for the treatment of excessive sleepiness induced by shift work disorders or narcolepsy [58, 59]. With the same objective, several studies in healthy adult men indicated that treatment with armodafinil (250 mg) improved alertness in sleep-deprived patients compared to the group of patients who received only placebo treatment [60, 61]. Rosenberg et al. reported that armodafinil was generally well tolerated at a dosage of 150 mg/day and increases wakefulness after eastbound travel through 6 time zones in patients with a history of jet lag symptoms [62].
In addition, reports indicate that prolonged treatment with armodafinil (150 mg) for 12 weeks is well tolerated and is associated with significant improvement in memory, attention, and fatigue in patients aged 18 to 65 years with narcolepsy [63]. On the other hand, it has been observed that prolonged treatment with armodafinil (150 mg/day) for 12 weeks significantly improved long-term memory, patient-estimated wakefulness, and reduced fatigue compared to placebo in patients with obstructive sleep apnea/hypopnea syndrome [47], while in patients with obstructive sleep apnea, it significantly improved wakefulness, long-term memory and patients' ability to participate in activities of daily living [64]. Another study showed that treatment with armodafinil (150 mg/day) reduced chronic fatigue in patients undergoing radiotherapy [65]. A double-blind, placebo-controlled, crossover study demonstrated that treatment with armodafinil (250 mg) significantly improved verbal memory and learning in patients with multiple sclerosis [66].
Another study indicated that armodafinil (150 mg) significantly improved depressive symptoms compared to placebo in patients with a major depressive episode associated with bipolar I disorder [67]. Safety data for treatment with armodafinil (150 mg) showed that it was well tolerated, and the most common adverse reactions that occurred were headache, insomnia, nausea, and diarrhea [62, 67, 68] (Table 1) and several reports indicate that treatment with armodafinil shows a pharmacological profile similar to that observed with the use of conventional psychostimulants used as first-line treatment for ADHD, as it has proven to be a cognitive enhancer in diverse pathologies characterized by inducing cognitive impairment about executive function, fatigue and sleep disorders [70-75] (Table 2).
Table 1.
Safety and effectiveness of the different psychostimulants.
| Treatment | References | Study Type | Evaluation Method | Main Side Effects | Main Finding |
|---|---|---|---|---|---|
| Armodafinil | [68] | Multicenter, prospective, randomized, double-blind, placebo-controlled | CANTAB Vital parameters (heart rate, systolic and diastolic blood pressure) | Headache (19%), nasopharyngitis (14%), and diarrhea (10%) | Armodafinil was generally well tolerated but with 200 mg/day for 2 weeks did not improve work memory in 21 patients with obstructive sleep apnea (OSA) and excessive sleepiness |
| [62] | Double-blind, randomized, parallel-group, multicenter study | Multiple Sleep Latency Test, CGI, Karolinska Sleepiness Scale and Nocturnal Polysomnography | Headache (27%), nausea (13%), diarrhea (5%), circadian rhythm sleep disorder (5%), and palpitations (5%) | Armodafinil was generally well tolerated and at a dosage of 150 mg/day for three days increases wakefulness in 427 patients with a history of symptoms of jet lag | |
| Modafinil | [55] | A Double-Blind, Randomized, Placebo-Controlled Study | CANTAB | Headache, increased anxiety, drowsiness, sleep disturbance | Modafinil was well tolerated and report improvements on test of episodic memory and working memory but with a single dose of 200 mg did not improve the attention and the executive functions in 30 patients with remitted depression |
| [14] | Double-blind, randomized, placebo-controlled crossover study | CANTAB | Patients reported feeling of excited | Modafinil was well tolerated and improvements on tests of short-term memory span, visual memory, spatial planning, and stop-signal motor inhibition with a single dose of 200 mg in 20 adults with ADHD | |
| Methylphenidate (MPH) | [69] | Pilot study randomized placebo-controlled | Reverse Digit Span Test Trail making test Bochum matrices-advanced test |
Patients reported sleep-onset and sleep maintenance insomnia, headache and restlessness | MPH Improved in fatigue and declarative memory with two sessions separated by approximately one week of 20 mg immediate-release methylphenidate in 48 healthy volunteers |
| [70] | Large, prospective, randomized multi-center clinical trial | Vital parameters (heart rate, systolic and diastolic blood pressure, body weight) EKG |
Decreased appetite (22%) dry mouth (15%), palpitations (13%), gastrointestinal infection (11%), agitation (11%), restlessness (10%), hyperhidrosis, tachycardia, weight decrease (6.3%) | MPH was safe and well-tolerated with a dose of 10mg for long term (52 weeks) treatment in 205 adults with ADHD | |
| Lisdexamfetamine (LDX) | [71] | Open label multicenter, Double-blind placebo-controlled trial | Vital parameters (heart rate, systolic and diastolic blood pressure) EKG ADHD-RS CGI |
87.7% presented any TAESs: Upper respiratory tract infection 21.8%, insomnia 19.5%, headache 17.2%, dry mouth 16.6%, decrease appetitive 14.3%, irritability 11.2%, anxiety 8.3% | LDX was generally well tolerated and efficacious with a dose of 30 mg to 70 mg/day for long term (12 months) treatment in 349 (191 completed the trial) adults with ADHD |
| [72] | Open label multicenter, randomized, double-blind, placebo-controlled | Vital parameters (heart rate, systolic and diastolic blood pressure, height and weight) EKG ADHD-RS, CGI, YQOL-R |
86.7% presented (TAESs) 10% presented common TEAEs: decreased appetite (21.1%), headache (20.8%), insomnia (12.1%), and dizziness (5.3%) 4.9% experienced 18 severe TEAEs included dizziness, headache, migraine, aggression, agitation, dermatitis contact |
LDX was generally safety and effectiveness with a dose of 30 mg, 50 mg and 70 mg/day For long term (12 months) treatment in 198 (119 completed the trial) adolescents with ADHD |
Abbreviations: CANTAB = Cambridge neuropsychological test automated battery, CGI = The clinical global impression, EKG = electrocardiogram, ADHD-RS = ADHD Rating Scale-IV, YQOL-R = The Youth Quality of Life-Research Version, TAESs = treatment emergent adverse events.
Table 2.
Effects of treatment with armodafinil on behaviors associated with ADHD.
| Study Type | Treatment | Pathology | Effect | References |
|---|---|---|---|---|
| Double-blind, placebo-controlled, crossover study | Armodafinil unique dose (250 mg) | Multiple Sclerosis | Improves delayed verbal recall in 17 patients with Multiple Sclerosis | [66] |
| Randomized, double-blind, placebo-controlled trial | Armodafinil 6 weeks (250 mg/day) | Schizophrenia or schizoaffective disorder | Improved anhedonia-asociality in 60 patients with schizophrenia or schizoaffective disorder | [73] |
| Pilot trial | Armodafinil 12 weeks (125-250 mg/day) | Dementia with Lewy bodies | Improvements in hypersomnia and wakefulness in 20 patients with Dementia with Lewy Bodies | [74] |
| Randomized, placebo-controlled, double-blind trial followed by open-label extension. | Armodafinil 12 weeks (50-250 mg/day) | Mild to moderate closed traumatic brain injury | Significantly improved sleep latency in 88 patients with excessive sleepiness associated with mild to moderate traumatic brain injury | [75] |
4. ARMODAFINIL IN EXPERIMENTAL ANIMAL MODELS
4.1. Armodafinil as a Promoter of Wakefulness and Cognitive Enhancement in Animals
Clinical studies have reported that armodafinil is a useful neurostimulant for improving cognitive impairment and sleep disorders [76]. Wisor et al. reported that the intraperitoneal administration of 100 mg/kg of armodafinil to rats significantly increased the time of wakefulness and locomotor activity evaluated by electroencephalographic signals when compared with those of the control group that did not receive the treatment. It was also observed that this effect was similar to that obtained with D-methamphetamine administered at a dose of 1 mg/kg, ip, a classic neurostimulant usually used for treating excessive sleepiness [77]. This effect is significant because sleep deprivation decreases neurogenesis and reduces the size of the hippocampus, leading to cognitive impairment, mainly affecting learning and memory [78]. On the other hand, a more up-to-date study by Zhu et al. observed that intranasal administration of armodafinil (30 mg/kg) in sleep-deprived rats presented a shorter escape latency and longer transfer times in the Morris water maze, as well as an increase in the central distance and vertical position of the animals in the open field test and increased expression of brain-derived neurotrophic factor (BDNF) in the hippocampus when compared to sleep-deprived rats that did not receive armodafinil as treatment; these data demonstrate that treatment with armodafinil could improve cognition and wakefulness in these animals [79].
While, in a study by Fiocchi et al. reported that treatment with armodafinil (100 mg/kg, i.p.) increases wakefulness in rats and is associated with an increased number of neurons labeled with the transcription factor c-Fos, a marker of functional activity, both in the striatum and the anterior cingulate cortex, regions closely related to the activation of the dopaminergic system during waking states. This finding is relevant, as it has been reported that neurons of the ventrolateral preoptic nucleus (VLPO) of rats increase c-Fos activity after sleep, promoting GABAergic innervation of the main monoamine excitatory systems so that VLPO lesions are related to the onset of insomnia in animals [81]. Likewise, Vetrivelan et al. concluded that armodafinil administered intraperitoneally at a dose of 200 mg/kg promotes long-lasting wakefulness states in rats with VLPO lesions [82].
Due to a high content of polyunsaturated fatty acids, brain tissue is very sensitive to neurodegeneration processes after oxidative stress. The ability of cells to counteract cellular stress is regulated by protective genes known as vitagenes, capable of activating specific pro-survival cellular pathways [83]. During the last decades, special interest has been shown in Nuclear Factor Erythroid 2 (NRF-2) because it constitutes a key transcription factor for the regulation of redox homeostasis as well as in the activation of multiple mechanisms that protect against neuroinflammation and promotes neuronal plasticity [84]. This adaptive process is regulated by mechanisms like the hormetic effect in which a dose response or concentration may be induced for a mild stress response and cause a positive adaptive response, while higher concentrations evoke dysfunctional or toxic effects [85].
Armodafinil has been observed to have a neuroprotective effect by preventing memory loss after administration of scopolamine (3 mg/kg) as a model of Alzheimer's disease. That was observed by Katta et al. in a model of Alzheimer's disease induced with scopolamine (3 mg/kg) showed that treatment with armodafinil at an oral administration dose of 30 mg/kg once a day for 15 days decreased cognitive impairment, and increased spatial memory compared to the group of animals that did not receive the treatment. In addition, they reported that the treatment with armodafinil improves oxidative stress to decreased lipid peroxidation and increased glutamate concentration, additionally increased acetylcholinesterase levels, and effects associated with an improvement in learning and memory [86]. All those findings support the hypothesis relating oxidative stress and the pathophysiology of neurodegenerative and neurodevelopmental disorders such as ADHD.
CONCLUSION
The main findings of this review indicate that impairments in the dopaminergic system and circadian cycles play a central role in the pathophysiology of ADHD; for this reason, the use of psychostimulant drugs remains one of the main therapies for the management of symptomatology. However, amphetamine drugs and methylphenidate offer temporary relief of symptomatology and represent a risk of abuse for the population. Currently, there is increased interest in the use of drugs to help mitigate ADHD symptoms that are safe, effective, and well-accepted by patients. Following this review, we found numerous studies suggesting that armodafinil may be a good candidate for long-term treatment in adults with ADHD, as it has been shown to have several therapeutic benefits on cognition and alertness in several pathologies that share similarities with ADHD. However, the use of this psychostimulant to alleviate symptoms such as fatigue, drowsiness and inactivity is inconclusive. The evidence collected suggests that armodafinil is well tolerated at doses ranging from 50 to 250 mg, with mostly adverse events such as insomnia, headache, and decreased appetite. Armodafinil may have advantages over current therapies, including amphetamines and methylphenidate, because it has a neurobiological profile capable of modulating alertness and wakefulness. Armodafinil could potentially be an emerging treatment option for ADHD; however, the lack of comparative and long-term clinical studies with current treatments demonstrating its therapeutic effectiveness hinders its use in patients with this condition. The information contained in this review article highlights the importance of the correct identification of the symptomatology to generate new long-term therapeutic strategies; likewise, the management of sleep problems in ADHD patients with armodafinil could represent an alternative that allows adult patients to substantially improve their quality of life.
ACKNOWLEDGEMENTS
We thank Alonso Romero-Sanchez for the design of the images.
LIST OF ABBREVIATIONS
- ADHD
Attention Deficit Hyperactivity Disorder
- BDNF
Brain-derived Neurotrophic Factor
- DAT
Dopamine Transporter
- NRF-2
Nuclear Factor Erythroid 2
- VLPO
Ventrolateral Preoptic Nucleus
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Salvi V., Migliarese G., Venturi V., Rossi F., Torriero S., Viganò V., Cerveri G., Mencacci C. ADHD in adults: Clinical subtypes and associated characteristics. Riv. Psichiatr. 2019;54(2):84–89. doi: 10.1708/3142.31249. [DOI] [PubMed] [Google Scholar]
- 2.Memarmoghaddam M., Torbati H.T., Sohrabi M., Mashhadi A., Kashi A. Effects of a selected exercise programon executive function of children with attention deficit hyperactivity disorder. J. Med. Life. 2016;9(4):373–379. doi: 10.22336/jml.2016.0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spera V., Maiello M., Pallucchini A., Novi M., Elefante C., De Dominicis F., Palagini L., Biederman J., Perugi G. Adult attention-deficit hyperactivity disorder and clinical correlates of delayed sleep phase disorder. Psychiatry Res. 2020;291:113162. doi: 10.1016/j.psychres.2020.113162. [DOI] [PubMed] [Google Scholar]
- 4.Wolraich M.L., Hagan J.F., Jr, Allan C., Chan E., Davison D., Earls M., Evans S.W., Flinn S.K., Froehlich T., Frost J., Holbrook J.R., Lehmann C.U., Lessin H.R., Okechukwu K., Pierce K.L., Winner J.D., Zurhellen W. Clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/] hyperactivity disorder in children and adolescents. Pediatrics. 2019;144(4):e20192528. doi: 10.1542/peds.2019-2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kazda L., McGeechan K., Bell K., Thomas R., Barratt A. Association of attention-deficit/hyperactivity disorder diagnosis with adolescent quality of life. JAMA Netw. Open. 2022;5(10):e2236364. doi: 10.1001/jamanetworkopen.2022.36364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katzman M.A., Bilkey T.S., Chokka P.R., Fallu A., Klassen L.J. Adult ADHD and comorbid disorders: Clinical implications of a dimensional approach. BMC Psychiatry. 2017;17(1):302. doi: 10.1186/s12888-017-1463-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gift T.E., Reimherr F.W., Marchant B.K., Steans T.A., Wender P.H. Personality disorder in adult attention-deficit/hyperactivity disorder. J. Nerv. Ment. Dis. 2016;204(5):355–363. doi: 10.1097/NMD.0000000000000470. [DOI] [PubMed] [Google Scholar]
- 8.Salvi V., Ribuoli E., Servasi M., Orsolini L., Volpe U. ADHD and bipolar disorder in adulthood: Clinical and treatment implications. Medicina. 2021;57(5):466. doi: 10.3390/medicina57050466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ginsberg Y., Quintero J., Anand E., Casillas M., Upadhyaya H.P. Underdiagnosis of attention-deficit/hyperactivity disorder in adult patients: A review of the literature. Prim. Care Comp. CNS Disord. 2014;16(3):PCC.13r01600. doi: 10.4088/PCC.13r01600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coelho R.M., Drummond C., Mota N.B., Erthal P., Bernardes G., Lima G., Molina R., Sudo F.K., Tannock R., Mattos P. Network analysis of narrative discourse and attention-deficit hyperactivity symptoms in adults. PLoS One. 2021;16(4):e0245113. doi: 10.1371/journal.pone.0245113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nelson J.M., Liebel S.W. Anxiety and depression among college students with attention-deficit/hyperactivity disorder (ADHD): Cross-informant, sex, and subtype differences. J. Am. Coll. Health. 2018;66(2):123–132. doi: 10.1080/07448481.2017.1382499. [DOI] [PubMed] [Google Scholar]
- 12.Posner J., Polanczyk G.V., Sonuga-Barke E. Attention-deficit hyperactivity disorder. Lancet. 2020;395(10222):450–462. doi: 10.1016/S0140-6736(19)33004-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Young J.L., Goodman D.W. Adult attention-deficit/hyperactivity disorder diagnosis, management, and treatment in the DSM-5 era. Prim. Care Companion CNS Disord. 2016;18(6):10.4088/PCC.16r02000. doi: 10.4088/PCC.16r02000. [DOI] [PubMed] [Google Scholar]
- 14.Turner D.C., Clark L., Dowson J., Robbins T.W., Sahakian B.J. Modafinil improves cognition and response inhibition in adult attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2004;55(10):1031–1040. doi: 10.1016/j.biopsych.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 15.Loland C.J., Mereu M., Okunola O.M., Cao J., Prisinzano T.E., Mazier S., Kopajtic T., Shi L., Katz J.L., Tanda G., Newman A.H. R-modafinil (armodafinil): A unique dopamine uptake inhibitor and potential medication for psychostimulant abuse. Biol. Psychiatry. 2012;72(5):405–413. doi: 10.1016/j.biopsych.2012.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boellner S.W., Earl C.Q., Arora S. Modafinil in children and adolescents with attention-deficit/hyperactivity disorder: A preliminary 8-week, open-label study. Curr. Med. Res. Opin. 2006;22(12):2457–2465. doi: 10.1185/030079906X148300. [DOI] [PubMed] [Google Scholar]
- 17.Swanson J.M., Greenhill L.L., Lopez F.A., Sedillo A., Earl C.Q., Jiang J.G., Biederman J. Modafinil film-coated tablets in children and adolescents with attention-deficit/hyperactivity disorder: Results of a randomized, double-blind, placebo-controlled, fixed-dose study followed by abrupt discontinuation. J. Clin. Psychiatry. 2006;67(1):137–147. doi: 10.4088/JCP.v67n0120. [DOI] [PubMed] [Google Scholar]
- 18.Gao W-J., Urban K.R. Psychostimulants as cognitive enhancers in adolescents: More risk than reward? Front. Public Health. 2017;5:260. doi: 10.3389/fpubh.2017.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Minzenberg M.J., Carter C.S. Modafinil: A review of neurochemical actions and effects on cognition. Neuropsychopharmacology. 2008;33(7):1477–1502. doi: 10.1038/sj.npp.1301534. [DOI] [PubMed] [Google Scholar]
- 20.Del-Ponte B., Anselmi L., Assunção M.C.F., Tovo-Rodrigues L., Munhoz T.N., Matijasevich A., Rohde L.A., Santos I.S. Sugar consumption and attention-deficit/hyperactivity disorder (ADHD): A birth cohort study. J. Affect. Disord. 2019;243:290–296. doi: 10.1016/j.jad.2018.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kerekes N., Sanchéz-Pérez A.M., Landry M. Neuroinflammation as a possible link between attention-deficit/hyperactivity disorder (ADHD) and pain. Med. Hypotheses. 2021;157:110717. doi: 10.1016/j.mehy.2021.110717. [DOI] [PubMed] [Google Scholar]
- 22.Sharma A., Couture J. A review of the pathophysiology, etiology, and treatment of attention-deficit hyperactivity disorder (ADHD). Ann. Pharmacother. 2014;48(2):209–225. doi: 10.1177/1060028013510699. [DOI] [PubMed] [Google Scholar]
- 23.Adler L.A., Faraone S.V., Spencer T.J., Berglund P., Alperin S., Kessler R.C. The structure of adult ADHD. Int. J. Methods Psychiatr. Res. 2017;26(1):e1555. doi: 10.1002/mpr.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Das D., Cherbuin N., Butterworth P., Anstey K.J., Easteal S. A population-based study of attention deficit/hyperactivity disorder symptoms and associated impairment in middle-aged adults. PLoS One. 2012;7(2):e31500. doi: 10.1371/journal.pone.0031500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Biederman J., Faraone S.V., Spencer T.J., Mick E., Monuteaux M.C., Aleardi M. Functional impairments in adults with self-reports of diagnosed ADHD: A controlled study of 1001 adults in the community. J. Clin. Psychiatry. 2006;67(4):524–540. doi: 10.4088/JCP.v67n0403. [DOI] [PubMed] [Google Scholar]
- 26.Fayyad J., De Graaf R., Kessler R., Alonso J., Angermeyer M., Demyttenaere K., De Girolamo G., Haro J.M., Karam E.G., Lara C., Lépine J.P., Ormel J., Posada-Villa J., Zaslavsky A.M., Jin R. Cross-national prevalence and correlates of adult attention-deficit hyperactivity disorder. Br. J. Psychiatry. 2007;190(5):402–409. doi: 10.1192/bjp.bp.106.034389. [DOI] [PubMed] [Google Scholar]
- 27.Crunelle C.L., van den Brink W., Moggi F., Konstenius M., Franck J., Levin F.R., van de Glind G., Demetrovics Z., Coetzee C., Luderer M., Schellekens A., Matthys F. International consensus statement on screening, diagnosis and treatment of substance use disorder patients with comorbid attention deficit/hyperactivity disorder. Eur. Addict. Res. 2018;24(1):43–51. doi: 10.1159/000487767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Castells X., Blanco-Silvente L., Cunill R. Amphetamines for attention deficit hyperactivity disorder (ADHD) in adults. Cochrane Libr. 2018;2018(8):CD007813. doi: 10.1002/14651858.CD007813.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson J., Morris S., George S. Attention deficit hyperactivity disorder in adults: What the non-specialist needs to know. Br. J. Hosp. Med. 2020;81(3):1–11. doi: 10.12968/hmed.2019.0188. [DOI] [PubMed] [Google Scholar]
- 30.Jaeschke R.R., Sujkowska E., Sowa-Kućma M. Methylphenidate for attention-deficit/hyperactivity disorder in adults: A narrative review. Psychopharmacology. 2021;238(10):2667–2691. doi: 10.1007/s00213-021-05946-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cortese S., Adamo N., Del Giovane C., Mohr-Jensen C., Hayes A.J., Carucci S., Atkinson L.Z., Tessari L., Banaschewski T., Coghill D., Hollis C., Simonoff E., Zuddas A., Barbui C., Purgato M., Steinhausen H.C., Shokraneh F., Xia J., Cipriani A. Comparative efficacy and tolerability of medications for attention-deficit hyperactivity disorder in children, adolescents, and adults: A systematic review and network meta-analysis. Lancet Psychiatry. 2018;5(9):727–738. doi: 10.1016/S2215-0366(18)30269-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greenhill L.L., Pliszka S., Dulcan M.K., Bernet W., Arnold V., Beitchman J., Benson R.S., Bukstein O., Kinlan J., McClellan J., Rue D., Shaw J.A., Stock S. Practice parameter for the use of stimulant medications in the treatment of children, adolescents, and adults. J. Am. Acad. Child Adolesc. Psychiatry. 2002;41(2):26S–49S. doi: 10.1097/00004583-200202001-00003. [DOI] [PubMed] [Google Scholar]
- 33.Pozzi M., Bertella S., Gatti E., Peeters G.G.A.M., Carnovale C., Zambrano S., Nobile M. Emerging drugs for the treatment of attention-deficit hyperactivity disorder (ADHD). Expert Opin. Emerg. Drugs. 2020;25(4):395–407. doi: 10.1080/14728214.2020.1820481. [DOI] [PubMed] [Google Scholar]
- 34.Rugino T. A review of modafinil film-coated tablets for attention-deficit/hyperactivity disorder in children and adolescents. Neuropsychiatr. Dis. Treat. 2007;3(3):293–301. [PMC free article] [PubMed] [Google Scholar]
- 35.Biederman J., Faraone S.V. Attention-deficit hyperactivity disorder. Lancet. 2005;366(9481):237–248. doi: 10.1016/S0140-6736(05)66915-2. [DOI] [PubMed] [Google Scholar]
- 36.Adler L.A., Liebowitz M., Kronenberger W., Qiao M., Rubin R., Hollandbeck M., Deldar A., Schuh K., Durell T. Atomoxetine treatment in adults with attention-deficit/hyperactivity disorder and comorbid social anxiety disorder. Depress. Anxiety. 2009;26(3):212–221. doi: 10.1002/da.20549. [DOI] [PubMed] [Google Scholar]
- 37.Walters A.S., Silvestri R., Zucconi M., Chandrashekariah R., Konofal E. Review of the possible relationship and hypothetical links between attention deficit hyperactivity disorder (ADHD) and the simple sleep related movement disorders, parasomnias, hypersomnias, and circadian rhythm disorders. J. Clin. Sleep Med. 2008;4(6):591–600. doi: 10.5664/jcsm.27356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Andel E., Bijlenga D., Vogel S.W.N., Beekman A.T.F., Kooij J.J.S. Attention-deficit/hyperactivity disorder and delayed sleep phase syndrome in adults: A randomized clinical trial on the effects of chronotherapy on sleep. J. Biol. Rhythms. 2022;37(6):673–689. doi: 10.1177/07487304221124659. [DOI] [PubMed] [Google Scholar]
- 39.Gamble K.L., May R.S., Besing R.C., Tankersly A.P., Fargason R.E. Delayed sleep timing and symptoms in adults with attention-deficit/hyperactivity disorder: A controlled actigraphy study. Chronobiol. Int. 2013;30(4):598–606. doi: 10.3109/07420528.2012.754454. [DOI] [PubMed] [Google Scholar]
- 40.Gau S.S.F., Kessler R.C., Tseng W.L., Wu Y.Y., Chiu Y.N., Yeh C.B., Hwu H.G. Association between sleep problems and symptoms of attention-deficit/hyperactivity disorder in young adults. Sleep. 2007;30(2):195–201. doi: 10.1093/sleep/30.2.195. [DOI] [PubMed] [Google Scholar]
- 41.Mahajan N., Hong N., Wigal T.L., Gehricke J.G. Hyperactive-impulsive symptoms associated with self-reported sleep quality in nonmedicated adults with ADHD. J. Atten. Disord. 2010;14(2):132–137. doi: 10.1177/1087054709347170. [DOI] [PubMed] [Google Scholar]
- 42.Kooij J.J.S., Bijlenga D. The circadian rhythm in adult attention-deficit/hyperactivity disorder: current state of affairs. Expert Rev. Neurother. 2013;13(10):1107–1116. doi: 10.1586/14737175.2013.836301. [DOI] [PubMed] [Google Scholar]
- 43.Zisapel N. New perspectives on the role of melatonin in human sleep, circadian rhythms and their regulation. Br. J. Pharmacol. 2018;175(16):3190–3199. doi: 10.1111/bph.14116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baird A.L., Coogan A.N., Siddiqui A., Donev R.M., Thome J. Adult attention-deficit hyperactivity disorder is associated with alterations in circadian rhythms at the behavioural, endocrine and molecular levels. Mol. Psychiatry. 2012;17:988–995. doi: 10.1038/mp.2011.149. [DOI] [PubMed] [Google Scholar]
- 45.Coogan A.N., Baird A.L., Popa-Wagner A., Thome J. Circadian rhythms and attention deficit hyperactivity disorder: The what, the when and the why. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2016;67:74–81. doi: 10.1016/j.pnpbp.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 46.Parekh P.K., Ozburn A.R., McClung C.A. Circadian clock genes: Effects on dopamine, reward and addiction. Alcohol. 2015;49(4):341–349. doi: 10.1016/j.alcohol.2014.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hirshkowitz M., Black J.E., Wesnes K., Niebler G., Arora S., Roth T. Adjunct armodafinil improves wakefulness and memory in obstructive sleep apnea/hypopnea syndrome. Respir. Med. 2007;101(3):616–627. doi: 10.1016/j.rmed.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 48.Sousa A., Dinis-Oliveira R.J. Pharmacokinetic and pharmacodynamic of the cognitive enhancer modafinil: Relevant clinical and forensic aspects. Subst. Abus. 2020;41(2):155–173. doi: 10.1080/08897077.2019.1700584. [DOI] [PubMed] [Google Scholar]
- 49.Schwartz J., Roth T., Drake C. Armodafinil in the treatment of sleep/wake disorders. Neuropsychiatr. Dis. Treat. 2010;6:417–427. doi: 10.2147/NDT.S3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang S.M., Han C., Lee S.J., Jun T.Y., Patkar A.A., Masand P.S., Pae C.U. Modafinil for the treatment of attention-deficit/hyperactivity disorder: A meta-analysis. J. Psychiatr. Res. 2017;84:292–300. doi: 10.1016/j.jpsychires.2016.09.034. [DOI] [PubMed] [Google Scholar]
- 51.Turner D.C., Robbins T.W., Clark L., Aron A.R., Dowson J., Sahakian B.J. Cognitive enhancing effects of modafinil in healthy volunteers. Psychopharmacology. 2003;165(3):260–269. doi: 10.1007/s00213-002-1250-8. [DOI] [PubMed] [Google Scholar]
- 52.Taylor F.B., Russo J. Efficacy of modafinil compared to dextroamphetamine for the treatment of attention deficit hyperactivity disorder in adults. J. Child Adolesc. Psychopharmacol. 2000;10(4):311–320. doi: 10.1089/cap.2000.10.311. [DOI] [PubMed] [Google Scholar]
- 53.Amiri S., Mohammadi M.R., Mohammadi M., Nouroozinejad G.H., Kahbazi M., Akhondzadeh S. Modafinil as a treatment for Attention-Deficit/Hyperactivity Disorder in children and adolescents: A double blind, randomized clinical trial. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2008;32(1):145–149. doi: 10.1016/j.pnpbp.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 54.Rugino T.A., Copley T.C. Effects of modafinil in children with attention-deficit/hyperactivity disorder: an open-label study. J. Am. Acad. Child Adolesc. Psychiatry. 2001;40(2):230–235. doi: 10.1097/00004583-200102000-00018. [DOI] [PubMed] [Google Scholar]
- 55.Kaser M., Deakin J.B., Michael A., Zapata C., Bansal R., Ryan D., Cormack F., Rowe J.B., Sahakian B.J. Modafinil improves episodic memory and working memory cognition in patients with remitted depression: A double-blind, randomized, placebo-controlled study. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 2017;2(2):115–122. doi: 10.1016/j.bpsc.2016.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Turner D. A review of the use of modafinil for attention-deficit hyperactivity disorder. Expert Rev. Neurother. 2006;6(4):455–468. doi: 10.1586/14737175.6.4.455. [DOI] [PubMed] [Google Scholar]
- 57.Coogan A.N., Schenk M., Palm D., Uzoni A., Grube J., Tsang A.H., Kolbe I., McGowan N.M., Wandschneider R., Colla M., Oster H., Thome J., Faltraco F. Impact of adult attention deficit hyperactivity disorder and medication status on sleep/wake behavior and molecular circadian rhythms. Neuropsychopharmacology. 2019;44(7):1198–1206. doi: 10.1038/s41386-019-0327-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Czeisler C.A., Walsh J.K., Wesnes K.A., Roth T., Arora S. Armodafinil for treatment of excessive sleepiness associated with shift work disorder: A randomized controlled study. Mayo Clin. Proc. 2009;84(11):958–972. doi: 10.1016/S0025-6196(11)60666-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schwartz J.R.L., Khan A., McCall W.V., Weintraub J., Tiller J. Tolerability and efficacy of armodafinil in naïve patients with excessive sleepiness associated with obstructive sleep apnea, shift work disorder, or narcolepsy: A 12-month, open-label, flexible-dose study with an extension period. J. Clin. Sleep Med. 2010;6(5):450–457. doi: 10.5664/jcsm.27934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gasior M., Freeman J., Zammit G., Donnelly P., Gao J., Ferreira-Cornwell M.C., Roth T. Maintenance of wakefulness with lisdexamfetamine dimesylate, compared with placebo and armodafinil in healthy adult males undergoing acute sleep loss. J. Clin. Psychopharmacol. 2014;34(6):690–696. doi: 10.1097/JCP.0000000000000202. [DOI] [PubMed] [Google Scholar]
- 61.Dinges D.F., Arora S., Darwish M., Niebler G.E. Pharmacodynamic effects on alertness of single doses of armodafinil in healthy subjects during a nocturnal period of acute sleep loss. Curr. Med. Res. Opin. 2006;22(1):159–167. doi: 10.1185/030079906X80378. [DOI] [PubMed] [Google Scholar]
- 62.Rosenberg R.P., Bogan R.K., Tiller J.M., Yang R., Youakim J.M., Earl C.Q., Roth T. A phase 3, double-blind, randomized, placebo-controlled study of armodafinil for excessive sleepiness associated with jet lag disorder. Mayo Clin. Proc. 2010;85(7):630–638. doi: 10.4065/mcp.2009.0778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Harsh J.R., Hayduk R., Rosenberg R., Wesnes K.A., Walsh J.K., Arora S., Niebler G.E., Roth T. The efficacy and safety of armodafinil as treatment for adults with excessive sleepiness associated with narcolepsy. Curr. Med. Res. Opin. 2006;22(4):761–774. doi: 10.1185/030079906X100050. [DOI] [PubMed] [Google Scholar]
- 64.Roth T., Rippon G.A., Arora S. Armodafinil improves wakefulness and long-term episodic memory in nCPAP-adherent patients with excessive sleepiness associated with obstructive sleep apnea. Sleep Breath. 2008;12(1):53–62. doi: 10.1007/s11325-007-0137-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Page B.R., Shaw E.G., Lu L., Bryant D., Grisell D., Lesser G.J., Monitto D.C., Naughton M.J., Rapp S.R., Savona S.R., Shah S., Case D., Chan M.D. Phase II double-blind placebo-controlled randomized study of armodafinil for brain radiation-induced fatigue. Neuro-oncol. 2015;17(10):1393–1401. doi: 10.1093/neuonc/nov084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bruce J., Hancock L., Roberg B., Brown A., Henkelman E., Lynch S. Impact of armodafinil on cognition in multiple sclerosis: A randomized, double-blind crossover pilot study. Cogn. Behav. Neurol. 2012;25(3):107–114. doi: 10.1097/WNN.0b013e31826df7fd. [DOI] [PubMed] [Google Scholar]
- 67.Frye M.A., Ketter T.A., Yang R., Calabrese J.R. A double-blind, placebo-controlled, multicenter trial of adjunctive armodafinil for the treatment of major depression associated with bipolar I disorder. Value Health. 2013;16(3):A55–A56. doi: 10.1016/j.jval.2013.03.1552. [DOI] [Google Scholar]
- 68.Greve D.N., Duntley S.P., Larson-Prior L., Krystal A.D., Diaz M.T., Drummond S.P.A., Thein S.G., Kushida C.A., Yang R., Thomas R.J. Effect of armodafinil on cortical activity and working memory in patients with residual excessive sleepiness associated with CPAP-Treated OSA: A multicenter fMRI study. J. Clin. Sleep Med. 2014;10(2):143–153. doi: 10.5664/jcsm.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Repantis D., Bovy L., Ohla K., Kühn S., Dresler M. Cognitive enhancement effects of stimulants: A randomized controlled trial testing methylphenidate, modafinil, and caffeine. Psychopharmacology. 2021;238(2):441–451. doi: 10.1007/s00213-020-05691-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kis B., Lücke C., van Elst L.T., Müller H.H.O., Philipsen A., Abdel-Hamid M., Heßmann P., Graf E., Berger M., Matthies S., Borel P., Sobanski E., Alm B., Rösler M., Retz W., Jacob C., Colla M., Huss M., Jans T. Safety profile of methylphenidate under long-term treatment in adult ADHD patients - results of the COMPAS study. Pharmacopsychiatry. 2020;53(6):263–271. doi: 10.1055/a-1207-9851. [DOI] [PubMed] [Google Scholar]
- 71.Weisler R., Young J., Mattingly G., Gao J., Squires L., Adler L. Long-term safety and effectiveness of lisdexamfetamine dimesylate in adults with attention-deficit/hyperactivity disorder. CNS Spectr. 2009;14(10):573–586. doi: 10.1017/S1092852900024056. [DOI] [PubMed] [Google Scholar]
- 72.Findling R.L., Cutler A.J., Saylor K., Gasior M., Hamdani M., Ferreira-Cornwell M.C., Childress A.C. A long-term open-label safety and effectiveness trial of lisdexamfetamine dimesylate in adolescents with attention-deficit/hyperactivity disorder. J. Child Adolesc. Psychopharmacol. 2013;23(1):11–21. doi: 10.1089/cap.2011.0088. [DOI] [PubMed] [Google Scholar]
- 73.Bobo W.V., Woodward N.D., Sim M.Y., Jayathilake K., Meltzer H.Y. The effect of adjunctive armodafinil on cognitive performance and psychopathology in antipsychotic-treated patients with schizophrenia/schizoaffective disorder: A randomized, double-blind, placebo-controlled trial. Schizophr. Res. 2011;130(1-3):106–113. doi: 10.1016/j.schres.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 74.Lapid M.I., Kuntz K.M., Mason S.S., Aakre J.A., Lundt E.S., Kremers W., Allen L.A., Drubach D.A., Boeve B.F. Efficacy, safety, and tolerability of armodafinil therapy for hypersomnia associated with dementia with lewy bodies: A pilot study. Dement. Geriatr. Cogn. Disord. 2017;43(5-6):269–280. doi: 10.1159/000471507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Menn S.J., Yang R., Lankford A. Armodafinil for the treatment of excessive sleepiness associated with mild or moderate closed traumatic brain injury: A 12-week, randomized, double-blind study followed by a 12-month open-label extension. J. Clin. Sleep Med. 2014;10(11):1181–1191. doi: 10.5664/jcsm.4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kuan Y-C., Wu D., Huang K-W., Chi N-F., Hu C-J., Chung C-C., Tam K-W., Huang Y-H. Effects of modafinil and armodafinil in patients with obstructive sleep apnea: A meta-analysis of randomized controlled trials. Clin. Ther. 2016;38(4):874–888. doi: 10.1016/j.clinthera.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 77.Wisor J., Dement W., Aimone L., Williams M., Bozyczkocoyne D. Armodafinil, the R-enantiomer of modafinil: Wake-promoting effects and pharmacokinetic profile in the rat. Pharmacol. Biochem. Behav. 2006;85(3):492–499. doi: 10.1016/j.pbb.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 78.Kreutzmann J.C., Havekes R., Abel T., Meerlo P. Sleep deprivation and hippocampal vulnerability: Changes in neuronal plasticity, neurogenesis and cognitive function. Neuroscience. 2015;309:173–190. doi: 10.1016/j.neuroscience.2015.04.053. [DOI] [PubMed] [Google Scholar]
- 79.Zhu S., Zhang S., Pang L., Ou G., Zhu L., Ma J., Li R., Liu Y., Wang L., Wang L., Du L., Jin Y. Effects of armodafinil nanocrystal nasal hydrogel on recovery of cognitive function in sleep-deprived rats. Int. J. Pharm. 2021;597:120343. doi: 10.1016/j.ijpharm.2021.120343. [DOI] [PubMed] [Google Scholar]
- 80.Fiocchi E.M., Lin Y.G., Aimone L., Gruner J.A., Flood D.G. Armodafinil promotes wakefulness and activates Fos in rat brain. Pharmacol. Biochem. Behav. 2009;92(3):549–557. doi: 10.1016/j.pbb.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 81.Lu J., Greco M.A., Shiromani P., Saper C.B. Effect of lesions of the ventrolateral preoptic nucleus on NREM and REM sleep. J. Neurosci. 2000;20(10):3830–3842. doi: 10.1523/JNEUROSCI.20-10-03830.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fuller P., Vetrivelan R., Saper C. Armodafinil-induced wakefulness in animals with ventrolateral preoptic lesions. Nat. Sci. Sleep. 2014;6:57–63. doi: 10.2147/NSS.S53132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Concetta Scuto M., Mancuso C., Tomasello B., Laura O.M., Cavallaro A., Frasca F., Maiolino L., Trovato S.A., Calabrese E.J., Calabrese V. Curcumin, hormesis and the nervous system. Nutrients. 2019;11(10):2417. doi: 10.3390/nu11102417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Calabrese V., Cornelius C., Dinkova-Kostova A.T., Calabrese E.J., Mattson M.P. Cellular stress responses, the hormesis paradigm, and vitagenes: Novel targets for therapeutic intervention in neurodegenerative disorders. Antioxid. Redox Signal. 2010;13(11):1763–1811. doi: 10.1089/ars.2009.3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Calabrese V., Giordano J., Signorile A., Laura Ontario M., Castorina S., De Pasquale C., Eckert G., Calabrese E.J. Major pathogenic mechanisms in vascular dementia: Roles of cellular stress response and hormesis in neuroprotection. J. Neurosci. Res. 2016;94(12):1588–1603. doi: 10.1002/jnr.23925. [DOI] [PubMed] [Google Scholar]
- 86.Sai K.S., Akkiraju S., Chinni S., Kanala S.R. Evaluation of armodafinil’s anti-amnestic activity in scopolamine-induced amnesia in wistar rats. J. Young Pharm. 2023;15(1):83–91. doi: 10.5530/097515050389. [DOI] [Google Scholar]