Abstract
Background
Heart failure is a chronic and progressive disease where the heart muscle is unable to pump enough blood and oxygen to meet the body’s needs. Oxidative stress and inflammation are key elements in the development and progression of heart failure. Astaxanthin, a carotenoid, has strong anti-inflammatory and antioxidant effects that may protect the cardiovascular system. A study will evaluate the effect of astaxanthin supplementation on inflammatory status, oxidative stress, lipid profile, uric acid levels, endothelial function, quality of life, and disease symptoms in people with heart failure.
Methods
The current study is a double-blind controlled randomized clinical trial for 8 weeks, in which people with heart failure were randomly assigned to two groups: intervention (one capsule containing 20 mg of astaxanthin per day, n = 40) and placebo (one capsule containing 20 mg of maltodextrin per day, n = 40) will be divided. At the beginning and end of the intervention, uric acid, lipid profile, oxidative stress indices, inflammatory markers, blood pressure, nitric oxide, and anthropometric factors will be measured, and questionnaires measuring quality of life, fatigue intensity, shortness of breath, and appetite will be completed. SPSS version 22 software will be used for statistical analysis.
Discussion
There is a growing global interest in natural and functional food products. This RCT contributes to the expanding body of research on the potential benefits of astaxanthin in heart failure patients, including its antioxidant, lipid-lowering, and anti-inflammatory effects.
Trial registration
Iranian Registry of Clinical Trials IRCT20200429047235N3. Registered on 26 March 2024.
Keywords: Heart failure, Astaxanthin, Inflammation, Antioxidant, Lipid, Blood pressure
Administrative information
Note: the numbers in curly brackets in this protocol refer to SPIRIT checklist item numbers. The order of the items has been modified to group similar items
| Title {1} | The effect of astaxanthin supplementation on inflammatory markers, oxidative stress indices, lipid profile, uric acid level, blood pressure, endothelial function, quality of life, and disease symptoms in heart failure subjects |
|---|---|
| Trial registration {2a and 2b}. | IRCT20200429047235N3; 26 March 2024. |
| Protocol version {3} | Version 1, April 2024. |
| Funding {4} |
Isfahan university of medical sciences. Grant number:3402807 |
| Author details {5a} |
Shirin Ghotboddin Mohammadi1, Awat Feizi2, Mohammad Bagherniya3, Davood Shafie4, Ali-Reza Ahmadi5, Marziyeh kafeshani6 1Department of Clinical Nutrition, School of Nutrition & Food Sciences, Isfahan University of Medical Sciences, Isfahan, Iran. 2Epidemiology and Biostatistics Department, Health School, Isfahan University of Medical Sciences, Isfahan, Iran 3Department of Community Nutrition, Food Security Research Center, School of Nutrition and Food Science Isfahan University of Medical Sciences Isfahan Iran. 4Heart Failure Research Center, Cardiovascular Research Institute, Isfahan University of Medical Sciences, Isfahan, Iran. 5Department of Biomedical Sciences, Women Research Center, Alzahra University, Tehran, Iran 6Nutrition and Food Security Research Center and Department of Clinical Nutrition, School of Nutrition and Food Science, Isfahan University of Medical Sciences, Isfahan, Iran |
| Name and contact information for the trial sponsor {5b} |
Isfahan University of Medical Sciences Postal Code: 81,746–73,461 Tell: (+ 98)-31–3668–0048 |
| Role of sponsor {5c} | Financial support and supervision |
Introduction
Background and rationale {6a}
In recent years, the rapid increase in non-communicable diseases has posed a significant health challenge, threatening the well-being and economic progress of societies. Chronic non-communicable diseases such as cardiovascular diseases, cancer, respiratory diseases, and diabetes mellitus are the leading causes of death globally [1].
Heart failure, for example, is a progressive condition where the heart muscle is unable to adequately pump blood to meet the body’s needs [2].
The symptoms and complications of these diseases can significantly impact patients’ quality of life, increasing the risk of hospitalization and mortality [3]. The American Heart Association (AHA), 2014 reports that approximately 7.3% of all deaths from cardiovascular diseases are due to heart failure. It is predicted that by 2030, more than 23.3 million individuals will lose their lives annually due to cardiovascular diseases, and the prevalence of heart failure is expected to increase by 25% [4].
The prevalence of heart failure in Iran has been reported as 8%, which is higher than in other Asian countries, including Japan (0.8%), China (3.5%), and India (0.3%) [5]. Common symptoms of heart failure include fatigue, loss of appetite, shortness of breath, ankle swelling, and exercise intolerance [6].
In recent years, oxidative stress and inflammation have gained significant attention as key pathophysiological elements of the heart failure syndrome and potential factors in the development of heart failure. The dysfunction of cardiac mitochondria is a symptom of heart failure and a major contributor to oxidative stress, which in turn has damaging effects on cellular components, including the mitochondria themselves, leading to the creation of a defective cycle. Oxidative stress leads to damage and inflammation of the myocardial tissue, ultimately contributing to the development and progression of heart failure. Some markers of oxidative stress and inflammation are increased in chronic heart failure and have prognostic importance. Uric acid is a biomarker of oxidative stress in heart failure patients, and high uric acid levels are a risk factor for adverse outcomes, including mortality. The prevalence of hyperuricemia in patients with heart failure has been reported to range from 30 to 60% [7].
Moreover, increased oxidative stress leads to a reduction in the bioavailability of nitric oxide. In this condition, nitric oxide (NO) is deactivated by superoxide anions, and this reaction can potentially generate peroxynitrite, which is a toxic substance. Numerous studies have indicated that patients with heart failure experience a decrease in the body’s nitric oxide levels, potentially leading to elevated blood pressure and inflammation in the heart vessel walls [8, 9].
Monocyte chemotactic protein 1 (MCP-1) is a cytokine with pro-inflammatory properties, which is generated in reaction to injury or exposure to other cytokines. Its concentration rises in individuals with heart failure, potentially leading to a significant inflammatory response and the development of atherosclerosis [10, 11].
Blood lipid levels play a crucial role as risk factors for heart failure. Atherogenic index of plasma (AIP) is a strong predictor for assessing the risk of atherosclerosis and coronary heart disease, calculated using the formula (log) TG/HDL-C [12, 13]. The amount of AIP is notably higher in patients with heart failure compared to healthy individuals and is inversely related to left ventricular ejection fraction (LVEF) [14, 15].
Due to these reasons, antioxidants or anti-inflammatory medications may yield beneficial results in managing patients with heart failure [16–18].
Astaxanthin, a carotenoid similar to carotene and lycopene, is commonly found in nature, particularly in naturally occurring deep red seafood such as salmon, shrimp, and crab [19]. As humans are unable to produce carotenoids, astaxanthin must be acquired through dietary sources [20]. As per reports, astaxanthin exhibits a potent antioxidant effect that is more than 100 times stronger than alpha-tocopherol [21]. Astaxanthin also possesses various properties including anti-inflammatory, anti-oxidative stress, anti-cancer, immune system modulation, and blood pressure reduction [22–24]. In 1999, the US Food and Drug Administration approved astaxanthin for use as a dietary supplement [25]. Astaxanthin, derived from microalgae such as Haematococcus pluvialis, is commercially available as a food supplement in many countries [26]. Previous studies have demonstrated the effectiveness of astaxanthin in reducing inflammatory biomarkers and cytokines, as well as improving lipid profile levels [27, 28].
In a meta-analysis study conducted in 2015, Ersonia et al. investigated the impact of astaxanthin supplementation on lipid profile and glycemic markers. Seven studies involving 280 adult participants were evaluated, using a dosage of 4 to 20 mg over a study period of 4 weeks to 3 months. The results indicated that the supplementation did not affect the lipid profile, but did lead to a slight decrease in blood glucose levels [29].
In a meta-analysis conducted in 2020 by Xia et al., the impact of astaxanthin supplementation on obesity, blood pressure, lipid profile, and glycemic biomarkers was investigated. The study revealed that astaxanthin consumption led to an increase in HDL-C and a decrease in CRP (at doses higher than 12 mg), with no significant effect on other factors [30].
In a 2021 systematic review and meta-analysis conducted by Mokhtari et al., the impact of astaxanthin supplementation on blood pressure was investigated. The study evaluated 10 clinical trials with a total of 493 adult participants. The findings indicated that astaxanthin supplementation may lead to a reduction in diastolic blood pressure, particularly when administered at a dosage exceeding 12 mg per day in Asian individuals [22].
Another meta-analysis conducted in 2022 investigated the impact of astaxanthin supplementation on the health outcomes of adults at risk of metabolic syndrome. Seven studies involving 321 participants were assessed, and the results indicated that astaxanthin supplementation had marginal effects on reducing total cholesterol and systolic blood pressure, as well as significant effects on reducing LDL levels [31].
Based on a study conducted by Qarai et al. in 2022 involving 60 women with polycystic ovary syndrome, it was found that the administration of 8 mg of astaxanthin per day for 40 days resulted in an increase in the total antioxidant capacity compared to the placebo group. However, it did not have a significant effect on the levels of malondialdehyde and superoxide dismutase [32].
In a study by Rostami et al. in 2023, 50 infertile women with endometriosis were administered a daily dose of 6 mg of astaxanthin for a period of 12 weeks. The study’s findings revealed a significant increase in the total antioxidant capacity and superoxide dismutase, along with a significant decrease in malondialdehyde, interleukin-6, and TNF-α levels as a result of the supplement intake [33].
Hence, taking into account the positive impact of astaxanthin in decreasing oxidative stress and inflammation, and the limited research on the impact of astaxanthin supplementation on individuals with heart failure, we aim to examine the effects of astaxanthin supplementation on inflammatory markers, oxidative stress levels, lipid profile, uric acid levels, blood pressure, and endothelial function, as well as the quality of life and symptoms of heart failure in a double-blind randomized controlled clinical trial.
Objectives {7}
Main aim: To assess the impact of astaxanthin supplementation on inflammatory markers, oxidative stress indices, lipid profile, uric acid level, blood pressure, endothelial function, quality of life, and disease symptoms in individuals with heart failure.
Primary objective: To evaluate the effect of 8-week astaxanthin consumption on levels of total antioxidant capacity (TAC) in individuals with heart failure.
Secondary objective: To evaluate the effect of 8-week astaxanthin consumption on variation in lipid profile (cholesterol, LDL-C, HDL-C, TG, AIP), oxidative stress levels (SOD, MDA, TAC), inflammatory markers (CRP, MCP-1, TNFα), uric acid levels, endothelial function (nitric oxide), quality of life and symptoms of heart failure (fatigue, loss of appetite, shortness of breath). Also to determine the impact of astaxanthin supplementation on the average alterations in anthropometric measurements (such as weight, BMI, and waist circumference) in individuals with heart failure pre- and post-intervention.
Trial design {8}
This is an 8-week, prospective, double-blinded, parallel assigned, randomized controlled clinical trial (RCT) in which subjects with heart failure will be randomized to an interventional (astaxanthin) or placebo group.
Methods: participants, interventions, and outcomes
Study setting {9}
In this clinical trial, 80 adults suffering from stage C and D heart failure according to AHA criteria [34] and diagnosed by cardiologists, will be recruited from Chamran Heart Hospital in Isfahan, Iran, if they meet the eligibility criteria.
Eligibility criteria {10}
The inclusion criteria of subjects include (1) willingness to participate in the study and completion of the consent form, (2) age 18 years and above, (3) known heart failure in stages C and D based on AHA criteria and cardiologist diagnosis. Exclusion criteria include (1) unwillingness to continue participation; (2) pregnancy and breastfeeding; (3) consumption of alcoholic beverages; (4) drug addiction; (5) suffering from uncontrolled diabetes; (6) use of herbal and medicinal supplements especially antioxidant supplements 3 months before starting the study; (7) warfarin drug use; (8) suffering from chronic liver, kidney, and lung diseases; (9) following a special diet or exercise program; (10) suffering from cancer; (11) suffering from chronic inflammatory diseases such as Crohn’s and ulcerative colitis; and (12) suffering from acute coronary heart syndrome or heart surgery in the last 4 weeks.
Dropout criteria included any side effects, consumption of less than 80% of the total astaxanthin supplement, change in the patient’s medication plan, and patient’s unwillingness to continue the study. Participants are allowed to withdraw from the study at any time.
Diagnosis criteria for heart failure
The diagnosis of heart failure is based on the AHA criteria. The American Heart Association categorizes heart failure based on the stages of the disease, with the first two stages being asymptomatic and the second two stages accompanied by symptoms.
Stage A: Individuals are at risk for heart failure, with no symptoms, structural heart disease, or evidence of elevated cardiac biomarkers, but they have present risk factors. Risk factors include high blood pressure, diabetes, metabolic syndrome, cardiotoxic medications, or genetic cardiomyopathy.
Stage B: Prior to the onset of heart failure, patients exhibit no signs or symptoms of the condition, but there is dysfunction in the heart.
Stage C: With underlying structural heart disease and a present or previous history of symptoms related to heart failure.
Stage D: Severe heart failure, symptoms that do not respond to treatment and disrupt daily activities, or frequent need for hospitalization [34].
Who will take informed consent? {26a}
All subjects will be asked for their consent before any information is collected. Once they have reviewed the consent forms and had any questions answered by the study staff, those who wish to participate will be requested to sign the forms. Participants will receive a copy of the consent form, and the study staff will retain all original signed consent forms.
Additional consent provisions for collection and use of participant data and biological specimens {26b}
All information pertaining to the participants will be kept confidential, and only the general and group findings of this research will be published without disclosing the names and specific characteristics of the individuals.
Additionally, individuals will have the option to receive their own individual review results.
Interventions
Explanation for the choice of comparators {6b}
At first, the diagnosis of people with stage C and D heart failure based on the criteria of the American Heart Association (AHA) [34] is performed by a cardiologist from those who refer to Shahid Chamran Hospital. Finally, after checking other inclusion and exclusion criteria and if they have all the necessary criteria and obtain a written consent, the eligible people will be included in the study. In this study, the sample size required for each group is 40 people, and a total of 80 people will be studied. They will be randomly divided into two intervention and placebo groups and will be studied for 8 weeks.
Intervention description {11a}
The intervention group took one daily capsule of astaxanthin (containing 20 mg) made by the company “Taravat Zendeh Biotechnology” located in Al-Zahra University and the control group took one daily placebo capsule (containing 20 mg of maltodextrin) with the Foodchem brand, prepared from Osina Chemi in a packaged form and covered and having the same appearance (same taste, smell and color) will receive astaxanthin supplement (blinding) for 60 consecutive days.
Both astaxanthin supplement capsules and placebo were coded by someone outside the study (codes A and B) until the end of the study. The codes used will remain hidden from the people involved in the implementation of the study plan (concealment). Both groups should eat 1 capsule containing astaxanthin or maltodextrin with a specified main meal (for example, with lunch). Patients will be seen again in the mid-term of the intervention (week 4) in order to complete the food record and physical activity questionnaire.
Previous research has utilized doses of 0.16 to 40 mg per day and intervention durations ranging from 3 weeks to 3 months for astaxanthin [31]. Even in the dose of 45 mg per day, no adverse effect was observed [35]. Most studies have found a dosage higher than 12 mg to be effective [30, 36]. Therefore, based on a review of relevant articles and the safe amount of astaxanthin consumption, the appropriate dosage for astaxanthin supplementation will be 20 mg per day in the form of oral capsules [35, 37]. In order to reduce the effect of confounding factors, patients will be asked not to change their diet and physical activities during the study.
Criteria for discontinuing or modifying allocated interventions {11b}
If the participant does not want to continue the study, observe any side effects, change in the patient’s medication plan, and unstable clinical condition, the intervention will be stopped.
Strategies to improve adherence to interventions {11c}
In order to ensure that the participants in the study are taking the capsules, they will be contacted weekly by phone or SMS to remind them to take the capsules. Additionally, a daily supplement consumption checklist will be provided for participants to track their consumption, and at the end of the study, supplemental cans will be provided.
The project manager will cover the cost of all supplements and tests, and the patient will not be required to pay any fees.
Relevant concomitant care permitted or prohibited during the trial {11d}
Throughout the study, all patients (intervention and control groups) will receive the standard treatment method. They will continue to receive their routine medications. Participation in this project is voluntary and informed, and individuals are not obligated to take part in this research. Additionally, if individuals choose not to participate in the study, they will still have access to regular diagnostic and therapeutic care, and their relationship with the treatment center and healthcare provider will not be affected.
Provisions for post-trial care {30}
Based on previous reports, there are no known complications or potential risks for individuals participating in this study. If there is an allergy to plant materials such as astaxanthin, taking the supplement may cause side effects. In case of sensitivity, one should not participate in this project. If any complications are observed, the treatment method of stopping the intervention is used to treat unpleasant side effects. If during and after the research, any physical or mental problem occurs to the person due to participating in this research, the responsibility for treatment, costs, and compensation will lie with the organizer.
Outcomes {12}
Primary outcomes
The primary outcome is the significant difference in the mean changes of total antioxidant capacity (TAC) before and after the intervention.
Secondary outcomes
The significant difference in the mean lipid profile changes (total cholesterol level, LDL-cholesterol, HDL-cholesterol, TG) and plasma atherogenic index before and after the intervention
The significant difference in the mean changes of oxidative stress indices (MDA and SOD) before and after the intervention
The significant difference in the average changes of inflammatory markers (TNFα, CRP, MCP1) before and after the intervention
The significant difference in changes in endothelial function (nitric oxide level) before and after the intervention
The significant difference in the average changes in uric acid levels before and after the intervention
The significant difference in the mean changes of systolic and diastolic blood pressure before and after the intervention
The significant difference in the average changes of the total score of the quality of life of people with heart failure before and after the intervention
The significant difference in the average changes of the total score of fatigue intensity of people with heart failure before and after the intervention
The significant difference in the average changes of the total score of the severity of shortness of breath of people with heart failure before and after the intervention
The significant difference in the average changes of the total appetite score of people with heart failure before and after the intervention
The significant difference in the average change s of anthropometric indices (weight, BMI, and waist circumference) of people with heart failure before and after the intervention
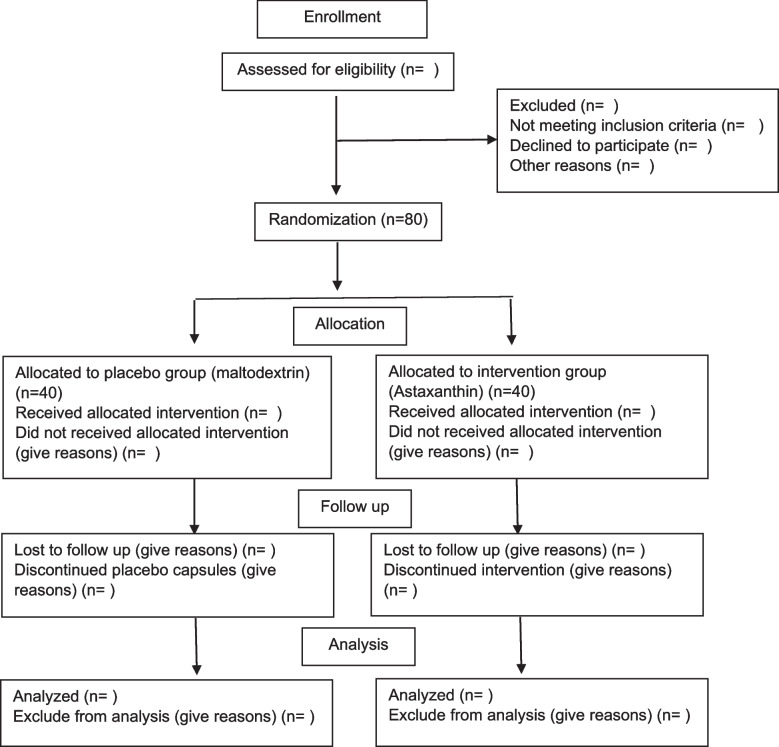
Participant timeline {13}
The study flow diagram is according to Table 1 and Fig. 1.
Table 1.
Timeline and applied tests
| Timepoint | Baseline | After 8 weeks | |
|---|---|---|---|
| Enrollment | ✓ | × | |
| Eligibility screening | ✓ | × | |
| Inform consent | ✓ | × | |
| Randomization | ✓ | × | |
| Allocation | ✓ | × | |
| Blood sample | ✓ | ✓ | |
| Blood pressure measurement | ✓ | ✓ | |
| Anthropometric measurement | ✓ | ✓ | |
| Complete questionnaires | ✓ | ✓ | |
| Intervention | Treatment group: astaxanthin capsules (20 mg/day for 8 weeks) | ✓ | × |
| Control group: placebo capsules (20 mg/day maltodextrin for 8 weeks) | |||
Fig. 1.
Participant flow diagram
Sample size {14}
The sample size is determined based on the following formula, taking into consideration a significance level of 5% (Z = 1.96) and a statistical power of 80% (Z = 0.84) to detect the standardized effect size of Δ = 0.7 for total antioxidant capacity (TAC) [37], and considering the same number of samples in each group φ = 1, the number of 33 people in each group was determined. In order to account for the potential loss, an additional 20% of the total sample size will be included, meaning that 40 individuals will be examined in each group.
Recruitment {15}
We are planning to enroll a total of 80 participants, with 40 in each group, who have heart failure stage Cand D in Isfahan, Iran for our clinical trial. Eligible volunteers, who are patients at Chamran Heart Hospital and receive regular hospital clinic visits every 1 month, will be recruited. Moreover, the study will also include other volunteer individuals who are referred to the hospital after seeing an advertisement and meeting the eligibility criteria. To ensure sufficient participant enrollment, the hospital’s clinic staff will be notified about the study, and patients will be engaged in informational discussions.
Assignment of interventions: allocation
Sequence generation {16a}
The participants will be randomly assigned to either the intervention or placebo groups using permuted block randomization with a block size of 4 generated by statistical software or Sealed Envelope website.
Concealment mechanism {16b}
Both the astaxanthin supplement capsules and the placebo will be coded by someone outside the study as codes A and B, respectively. The codes will remain undisclosed to the individuals involved in the study’s implementation until the end of the study to ensure concealment.
Implementation {16c}
Randomization will be conducted using a sequence generated by statistical software or Sealed Envelope website. The participants will be randomly assigned to either the intervention or placebo groups using permuted block randomization with a block size of 4.
Assignment of interventions: blinding
Who will be blinded {17a}
This study employs a double-blind approach, where patients and the principal investigator are unaware of the type of product being administered. Patients receive either the intervention or placebo in indistinguishable packaging (same taste, smell, color, and appearance).
Procedure for unblinding if needed {17b}
The primary consideration in making such decisions should always be the safety of people. In the case of a medical emergency, the investigator will determine the necessity of unblinding in consultation with a medical specialist. If unblinding is deemed necessary, detailed records of the date and reason for unblinding will be documented.
Data collection and management
Plans for assessment and collection of outcomes {18a}
Main outcomes assessments
Biochemical evaluation
In order to determine the concentration of blood factors, the subjects will be requested to refer to the Chamran hospital laboratory after fasting for 10–12 h at the beginning and end of the study. In the laboratory, 10 cc of venous blood samples will be taken from patients. In the next step, the blood samples will be centrifuged at 3500 revolutions per minute. Then the separated serum is poured into microtubes and kept at − 80 °C until use. Cholesterol-serum, LDL-C, HDL-C, and TG, are measured by enzymatic colorimetric method. CRP will be measured by turbidometry. The level of uric acid will be assessed using the uricase method and the Pars Peyvand kit. To measure antioxidant indices (SOD, TAC, MDA) and nitric oxide will be used from Navand Salamat kits. To measure inflammatory indicators (MCP-1, TNF-α), Karmania Parsgen company kits will be used. AIP based on the formula, (logTG/HDL-C) will be calculated.
Evaluation of the quality of life
In order to measure the quality of life of heart failure patients, the Minnesota Questionnaire will be used. The Minnesota Heart Failure Quality of Life Questionnaire (MLHFQ) includes 21 items that measure the physical, psychological, and socioeconomic limitations caused by the symptoms of heart failure in the last month. This questionnaire is scored on a 6-point Likert scale. Each question has 6 criteria and is scored from 0 to 5, where 0 indicates no restriction and 5 indicates a maximum restriction. The total score is between 0 and 105 and in fact, whatever If the scores obtained from this tool are higher, the patient will have a poorer quality of life [38]. The MLHFQ questionnaire is the most common questionnaire used in heart failure patients and the validity and reliability of the Persian version has been confirmed in the research of Eskandari et al. [39].
Evaluation of fatigue severity
Assessment of fatigue severity will be conducted using the Persian version of the Fatigue Severity Questionnaire (FSS). This questionnaire consists of 9 statements that patients will rate based on their level of agreement, with each item scored from zero to seven (0 = completely agree, 7 = completely disagree). Finally, each individual will receive a score ranging from 0 to 63 [40]. An individual scoring 0–21 is categorized as experiencing mild fatigue, while a score of 22–43 suggests moderate fatigue, and a score of 43–63 indicates severe fatigue [41]. The qualitative content validity of the FSS has been confirmed in previous studies conducted in Iran, and its test–retest reliability has been calculated to be between 0.78 and 0.95 [40].
Assessment of the severity of dyspnea
The severity of dyspnea during physical activity will be evaluated using the Modified Medical Research Council (MMRC) five-item dyspnea scale. This scale grades the severity of dyspnea from zero (no dyspnea during normal activities or vigorous exercise) to four (dyspnea during normal activities). Patients will be asked to read descriptive statements on the scale and choose a number that best represents their level of dyspnea [42]. The scale’s reliability has been established in previous research using the intraclass correlation coefficient, with a reported value of 0.82 both at the initial assessment and during the follow-up period [43]. The reliability of the MMRC scale in Iran was assessed using the qualitative content validity method, incorporating feedback from experts in heart, lung, and nursing fields, and its reliability was determined to be 0.8 through the intraclass correlation coefficient [44].
Assessment of appetite
The study will assess the participants’ appetite using the Simplified Nutritional Appetite Questionnaire (SNAQ), which consists of four questions. The score will range from 4 to 20, and a score of ≤ 14 indicates the risk of unintentional loss of at least 5% of body weight in 6 months [45].
Secondary outcomes assessments
Anthropometric measures
Anthropometric measurements, including height, waist circumference, and weight, are measured at the beginning of the study and 8 weeks after the intervention. Body weight is measured with approximately 0.1 kg in the morning, fasting, without shoes, and with minimal clothes, using a digital scale. Height is measured to the nearest 0.1 cm in a standing position without shoes by an inflexible meter. BMI is calculated by dividing the weight (kilograms) by the square of the height (meters). The waist circumference is also approximately 0.1 cm from the area of the smallest diameter between the last rib or the lower edge of the chest and the crown of the upper anterior rib with a non-elastic measuring tape while the person is standing, will be measured and recorded.
Confounding variable assessments
Demographic characteristics
Demographic variables (including age, gender, marital status, menopausal status, smoking, history of illness and medical history, level of education, occupation, economic and social status, consumption of supplements and drugs) will be collected from all participants by completing a general information questionnaire.
Assessment of dietary intake
To assess the usual dietary intake of patients, individuals will be requested to complete 3-day food records (2 regular days and 1 holiday day) at the beginning, middle, and end of the study. The data obtained from food records will be calculated and analyzed using the Nutritionist IV software and SPSS version 22.
Evaluation of physical activity
The participants’ level of physical activity at the start, middle, and end of the study will be documented using the International Physical Activity Questionnaire (IPAQ). The metabolic equivalent (MET) will be calculated for the specified physical activity. The metabolic equivalent will be 8 for intense activity, 4 for moderate activity, and 3.3 for walking. Then, these values will be multiplied by the duration and frequency of the physical activity [46].
Plans to promote participant retention and complete follow-up {18b}
To encourage individuals to take part in the research, we will provide an explanation of the impact of astaxanthin supplementation on heart failure. Additionally, participants will be informed that all testing costs during the study are covered. In order to ensure that the participants in the study are taking the capsules, they will be contacted weekly by phone or SMS to remind them to take the capsules. Additionally, a daily supplement consumption checklist will be provided for participants to track their consumption, and at the end of the study, supplemental cans will be provided.
Data management {19}
Participants will receive a unique code number ranging from 1 to 80, which will be securely stored in the master randomization list. After the intervention period, the collected data will be entered into SPSS software (SPSS Inc., Chicago, IL, USA). Some variables will be recoded after input, based on the defined cut-off point. Interim analysis will not be conducted on primary and secondary outcomes, but will be conducted on diet and physical activity.
The trial will undergo internal monitoring, with the study leader overseeing the overall progress. Regular meetings between the principal investigator and study leader will be conducted to address concerns and review progress. Any information regarding serious adverse events believed to be related to the treatment will be provided to the study cardiologist. If there is suspicion of harm, a review of discontinuing the trial will be conducted.
Confidentiality {27}
All information pertaining to the participants will be kept confidential, and only the general and group findings of this research will be published without disclosing the names and specific characteristics of the individuals.
Additionally, individuals will have the option to receive their own individual test results.
Plans for collection, laboratory evaluation, and storage of biological specimens for genetic or molecular analysis in this trial/future use {33}
Not applicable. The blood samples obtained from individuals will solely be utilized for assessing the findings of this research, and will not be employed for genetic and molecular analyses.
Statistical methods
Statistical methods for primary and secondary outcomes {20a}
This research will present quantitative variables as mean with standard deviation and qualitative variables as numbers with percentages. We will report the results of both the intention-to-treat approach and per protocol analysis, taking into account any missing data. In order to assess the normality of data distribution, the Kolmogorov–Smirnov test and Q-Q plot will be employed. Comparison of basic variables of patients (quantitative and qualitative) will be done using independent t-test and chi-square. The changes within the group will be analyzed in terms of quantitative variables using paired t-test for normal variables and Wilcoxon signed rank test for abnormal variables. For nominal qualitative variables (such as appetite), McNemar’s test will be used, and for ranked qualitative variables (such as the severity of shortness of breath and intensity of fatigue), the Friedman test will be used. We will use covariance analysis to compare the changes in quantitative variables and generalized estimating equation (GEE) to compare the changes in qualitative variables between the two groups. The data analysis will be conducted using SPSS version 22 software, with a significance level set at P < 0.05.
Interim analyses {21b}
An interim analysis will not be conducted for primary and secondary outcomes, but will be conducted for diet and physical activity to ensure that there are no significant changes during the intervention period.
Methods for additional analyses (e.g., subgroup analyses) {20b}
Potential analyses will be performed by gender and menopausal status.
Methods in analysis to handle protocol non-adherence and any statistical methods to handle missing data {20c}
We will report the results of both the intention-to-treat approach and per protocol analysis, taking into account any missing data.
Plans to give access to the full protocol, participant-level data and statistical code {31c}
The corresponding author can provide the full protocol information and the relevant data analyzed during the development of this study upon reasonable request.
Oversight and monitoring
Composition of the coordinating center and trial steering committee {5d}
The researchers’ performance and all phases of the study will be closely monitored by the Ethics Committee and the Vice Chancellor of Isfahan University of Medical Sciences. Although this study does not have an independent steering committee, based on our university’s rules, the ethics committee appoints two independent supervisors for each ongoing trial and the duties of these people include oversight of the practical aspects of the study as well as ensuring that the study continues to be run in a way which is both safe for the patients and provides appropriate safety and efficacy data to the ethics committee and investigators. Any violation of ethical principles, endangerment of patient health, or disregard for human values will result in necessary corrections or termination of the study.
The PhD thesis in Nutrition Sciences includes this study, and the student (S.G.M.) is primarily responsible for developing the protocol, implementing the study, identifying and recruiting participants, collecting data, carrying out the intervention, following up with study participants, and publishing study reports.
The research team consists of the students, supervisors, and advisors. The project supervisor and advisors are tasked with reviewing the protocol, ensuring the project is executed properly from both scientific and executive perspectives, monitoring the study’s progress, approving protocol changes if needed, and verifying the accuracy of results for publication. The core research team will meet at the conclusion of each trial phase and as necessary. The rest of the team will maintain communication throughout the research process and will meet as required at each stage.
Composition of the data monitoring committee, its role and reporting structure {21a}
The academic committee of Isfahan University of Medical Sciences will conduct impartial and continuous monitoring of the data for scientific validity, safety, and general integrity of trials.
Adverse event reporting and harms {22}
All adverse events will be documented and reported.
Frequency and plans for auditing trial conduct {23}
The Ethics Committee of Isfahan University of Medical Sciences will oversee the current study. The study will undergo at least two unexpected inspections to ensure accuracy, correctness, and proper conduct by the researchers.
Unscheduled evaluations will be conducted to assess the data quality and trial progress. Auditing involves reviewing the trial dataset and conducting site visits as needed. Auditors are separate from the trial investigators.
Plans for communicating important protocol amendments to relevant parties (e.g. trial participants, ethical committees) {25}
The authors will seek agreement on any significant alterations to the project implementation method that could impact the potential benefit, safety, and physical or mental well-being of the participants. Participants will also be informed of any modifications, and additional consent will be obtained. These changes will be communicated to the Ethical Committee of Isfahan University of Medical Sciences. All adjustments will be thoroughly documented on https://irct.ir/.
Dissemination plans {31a}
The findings from this clinical trial will be accessible to the general public. The act of making information known to the public can include publishing an article in a scientific journal, presenting an abstract with a poster or oral presentation at a scientific meeting, or revealing the information through other methods. The study participants will receive reports of their individual results. In every instance, the results of the trial are reported in an objective, accurate, balanced, and comprehensive manner, including a discussion of the strengths and limitations. Authors are given access to statistical tables, figures, and reports that are essential for assessing the intended publication.
Discussion
Astaxanthin, a carotenoid found widely in nature, particularly in marine products, possesses various properties including anti-inflammatory, antioxidant, immune system modulation, blood pressure reduction, and improvement of lipid profile [18, 47, 48]. Yoshida et al.’s [36] study involved supplementing 61 individuals with mild hyperlipidemia with doses of 6, 12, and 18 mg of astaxanthin per day for 12 weeks. The study’s findings revealed that HDL levels increased with 6 and 12 mg doses, while triglyceride levels decreased and adiponectin levels increased with 12 and 18 mg doses. Additionally, BMI and LDL levels remained unchanged across all doses [36]. In a 2011 study by Choi et al., conducted on 23 subjects with a BMI over 25 for 3 weeks, astaxanthin treatment decreased MDA and isoprostane and increased total antioxidant capacity and superoxide dismutase [49]. Another study by the same author, which was conducted on 27 people with a BMI above 25 for 12 weeks, showed that treatment with astaxanthin reduced LDL and Apo B, but no significant changes occurred in other lipid biomarkers [37]. Therefore, in general, astaxanthin supplementation improved the lipid profile and oxidative stress. In 2018, Shukri et al. conducted a study where 44 individuals with type 2 diabetes were given a daily dose of 8 mg of astaxanthin for 8 weeks. The results indicated a significant decrease in visceral fat, triglyceride levels, and systolic blood pressure, along with an increase in adiponectin levels due to the supplementation [50]. Yurakaz et al. conducted a study in 2021 where 53 prediabetic subjects were given a daily dose of 12 mg of astaxanthin for 12 weeks. The levels of MDA, LDL, HbA1c, Apo E, and 2hrBG decreased. Insulin resistance showed improvement, but there were no changes in the levels of HDL, TG, and total cholesterol [51].
Indeed, several studies reported that astaxanthin inhibits oxidative stress and systemic inflammatory responses. Since increased oxidative stress and systemic inflammation are associated with cardiovascular disorders and can lead to cardiovascular disease, astaxanthin may have protective effects on the cardiovascular system [18, 47, 48].
Strengths
This study will be the first of its kind to explore the impact of astaxanthin supplementation on heart failure patients. It will also be the initial clinical trial to investigate the effects of astaxanthin on nitric oxide, mcp1, and heart failure symptoms such as shortness of breath, fatigue, and decreased appetite. Additionally, the study includes features designed to contribute to the current knowledge on the effects of astaxanthin on fasting blood lipids, blood pressure, anthropometric indices, inflammatory markers, and oxidative stress.
Limitations
The study involves individuals who are undergoing medical treatment for heart failure. This may potentially limit the generalizability of the results to the whole population. Nonetheless, a significant portion of adults who are at risk for cardiovascular disease rely on at least one prescribed medication to manage their cardiovascular risk factors. Therefore, even though a placebo control will be utilized in our study, we will only be able to assess the impact of astaxanthin supplementation, as the medications are the standard treatment for patients with heart failure. Another constraint of the research is the absence of examination of blood markers for astaxanthin. Assessing blood astaxanthin levels undoubtedly offers valuable insights into astaxanthin consumption and absorption.
There has been a growing global interest in natural and functional food products. Reliable evidence is necessary to support health claims associated with functional foods, and this can be attained through randomized, placebo-controlled, interventional trials involving human subjects. In this regard, it would be interesting to conduct research aimed at evaluating the effectiveness of astaxanthin for use in health promotion and disease prevention and management. This RCT adds to the growing literature on the potential antioxidant, antihypertensive, lipid-lowering, and anti-inflammatory effects of astaxanthin in humans.
Trial status
The existing protocol is version 1, established on 20 April 2024, and any modifications to the protocol will be shared with all relevant parties, including participants. The recruitment process commenced on 15th August 2024 and is expected to continue for a duration of 5 months.
Acknowledgements
The research is supported by the School of Nutrition and Food Sciences at Isfahan University of Medical Sciences in Isfahan, Iran. We extend our gratitude to the staff of Shahid Chamran Hospital, as well as to the volunteers who took part in the study.
Abbreviations
- AHA
American Heart Association
- AIP
Atherogenic index of plasma
- BMI
Body mass index
- CRP
C-reactive protein
- DBP
Diastolic blood pressure
- GEE
Generalized estimating equation
- HDL-C
High-density lipoprotein cholesterol
- IL-6
Interleukin-6
- LDL-C
Low-density lipoprotein cholesterol
- LVEF
Left ventricular ejection fraction
- MCP-1
Monocyte chemoattractant protein 1
- MDA
Malondealdehyde
- MMRC
Modified Medical Research Council
- NO
Nitric oxide
- RCT
Randomized controlled clinical trial
- SBP
Systolic blood pressure
- SOD
Super oxidase dysmotase
- TAC
Total antioxidant capacity
- TG
Triglyceride
- TNF-α
Tumor necrosis factor-alpha
Authors’ contributions {31b}
M.K. took on the role of Principal Investigator, leading the study, and supervising the development of the proposal and protocol. S.G.M. contributed to the study design and proposal development, with additional input from M.B. and A.F., while D.S. and A.A. helped in writing the manuscript. The manuscript has been reviewed and approved by all authors.
Funding {4}
The research is supported by the School of Nutrition and Food Sciences at Isfahan University of Medical Sciences in Isfahan, Iran. The funding party is not actively involved in the study design, data collection, analysis, interpretation, or manuscript writing.
Availability of data and materials {29}
The data produced and/or analyzed in the present study will be accessible upon reasonable request from the corresponding author.
Declarations
Ethics approval and consent to participate {24}
The Ethical Research Committee of Pharmacy and Nutrition Faculties at Isfahan University of Medical Sciences approved this plan (approval code: IR.MUI.MED.REC.1402.099) on 29 March 2024.
This trial has been registered in the Iranian Clinical Trials Registry (ID: IRCT20200429047235N3; registered on 26 March 2024). Initially, (S.G.M.) will explain the purpose of the trial to the legal guardian and obtain their written informed consent after enrollment.
Consent for publication {32}
All authors have provided their approval for publication. Permission has been obtained from the patients to publish non-identifiable information about them.
Competing interests {28}
The authors declared that there is no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Baeradeh N, Ghoddusi Johari M, Moftakhar L, Rezaeianzadeh R, Hosseini SV, Rezaianzadeh A. The prevalence and predictors of cardiovascular diseases in Kherameh cohort study: a population-based study on 10,663 people in southern Iran. BMC Cardiovasc Disord. 2022;22(1):244. 10.1186/s12872-022-02683-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ravangard R, Jalali FS, Hajahmadi M, Jafari A. Cost-utility analysis of valsartan, enalapril, and candesartan in patients with heart failure in Iran. Heal Econ Rev. 2023;13(1):44. 10.1186/s13561-023-00457-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zeighami Mohammadi S, Farmani P, Shakoor M, Fahidy F, Fallah Taherpazir E, Mohseni B. Correlation between type D personality and quality of life in heart failure patients. International Journal of Biomedicine and Public Health. 2018;1(2):76–81. [Google Scholar]
- 4.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al. Executive summary: heart disease and stroke statistics–2014 update: a report from the American Heart Association. Circulation. 2014;129(3):399–410. 10.1161/01.cir.0000442015.53336.12 [DOI] [PubMed] [Google Scholar]
- 5.Groenewegen A, Rutten FH, Mosterd A, Hoes AW. Epidemiology of heart failure. Eur J Heart Fail. 2020;22(8):1342–56. 10.1002/ejhf.1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watson RD, Gibbs CR, Lip GY. ABC of heart failure. Clinical features and complications BMJ (Clinical research ed). 2000;320(7229):236–9. 10.1136/bmj.320.7229.236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Packer M. Uric Acid Is a Biomarker of Oxidative Stress in the Failing Heart: Lessons Learned from Trials With Allopurinol and SGLT2 Inhibitors. J Cardiac Fail. 2020;26(11):977–84. 10.1016/j.cardfail.2020.08.015 [DOI] [PubMed] [Google Scholar]
- 8.Searles CD. The nitric oxide pathway and oxidative stress in heart failure. Congest Heart Fail (Greenwich, Conn). 2002;8(3):142–7, 55. 10.1111/j.1527-5299.2002.00715.x [DOI] [PubMed] [Google Scholar]
- 9.Cotton JM, Kearney MT, Shah AM. Nitric oxide and myocardial function in heart failure: friend or foe? Heart (British Cardiac Society). 2002;88(6):564–6. 10.1136/heart.88.6.564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Niu J, Kolattukudy PE. Role of MCP-1 in cardiovascular disease: molecular mechanisms and clinical implications. Clin Sci. (London, England : 1979). 2009;117(3):95–109. 10.1042/CS20080581 [DOI] [PubMed] [Google Scholar]
- 11.Makarewicz-Wujec M, Kozlowska-Wojciechowska M. Nutrient intake and serum level of gamma-glutamyltransferase, MCP-1 and homocysteine in early stages of heart failure. Clinical nutrition (Edinburgh, Scotland). 2011;30(1):73–8. 10.1016/j.clnu.2010.07.008 [DOI] [PubMed] [Google Scholar]
- 12.Dobiasˇova M. Atherogenic index of plasma [log (triglycerides/HDL-cholesterol)]: theoretical and practical implications. Oxford University Press; 2004. p. 1113–5. [DOI] [PubMed] [Google Scholar]
- 13.Niroumand S, Khajedaluee M, Khadem-Rezaiyan M, Abrishami M, Juya M, Khodaee G, et al. Atherogenic Index of Plasma (AIP): A marker of cardiovascular disease. Med J Islam Repub Iran. 2015;29:240. [PMC free article] [PubMed] [Google Scholar]
- 14.Karadağ MK, Yıldırım E. Relationship of atherogenic index of plasma and mean platelet volume with ejection fraction in ischemic and nonischemic heart failure. Biomark Med. 2019;13(3):175–83. 10.2217/bmm-2018-0196 [DOI] [PubMed] [Google Scholar]
- 15.Mondal S, Mondal H, Samantaray R, Das D, Biri S, Naskar A, et al. Atherogenic index of plasma and left ventricular ejection fraction in newly diagnosed type 2 diabetes mellitus patients. Research in Cardiovascular Medicine. 2021;10:73–8. 10.4103/rcm.rcm_16_21 [DOI] [Google Scholar]
- 16.Aimo A, Castiglione V, Borrelli C, Saccaro LF, Franzini M, Masi S, et al. Oxidative stress and inflammation in the evolution of heart failure: From pathophysiology to therapeutic strategies. Eur J Prev Cardiol. 2020;27(5):494–510. 10.1177/2047487319870344 [DOI] [PubMed] [Google Scholar]
- 17.Tsutsui H, Kinugawa S, Matsushima S. Oxidative stress and heart failure. Am J Physiol Heart Circ Physiol. 2011;301(6):H2181–90. 10.1152/ajpheart.00554.2011 [DOI] [PubMed] [Google Scholar]
- 18.van der Pol A, van Gilst WH, Voors AA, van der Meer P. Treating oxidative stress in heart failure: past, present and future. Eur J Heart Fail. 2019;21(4):425–35. 10.1002/ejhf.1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yuan JP, Peng J, Yin K, Wang JH. Potential health-promoting effects of astaxanthin: a high-value carotenoid mostly from microalgae. Mol Nutr Food Res. 2011;55(1):150–65. 10.1002/mnfr.201000414 [DOI] [PubMed] [Google Scholar]
- 20.Hussein G, Sankawa U, Goto H, Matsumoto K, Watanabe H. Astaxanthin, a carotenoid with potential in human health and nutrition. J Nat Prod. 2006;69(3):443–9. 10.1021/np050354+ [DOI] [PubMed] [Google Scholar]
- 21.Okada Y, Ishikura M, Maoka T. Bioavailability of astaxanthin in Haematococcus algal extract: the effects of timing of diet and smoking habits. Biosci Biotechnol Biochem. 2009;73(9):1928–32. 10.1271/bbb.90078 [DOI] [PubMed] [Google Scholar]
- 22.Mokhtari E, Rafiei S, Shokri-Mashhadi N, Saneei P. Impact of astaxanthin supplementation on blood pressure: A systematic review and meta-analysis of randomized controlled trials. Journal of Functional Foods. 2021;87:104860. 10.1016/j.jff.2021.104860 [DOI] [Google Scholar]
- 23.Shokri-Mashhadi N, Tahmasebi M, Mohammadi-Asl J, Zakerkish M, Mohammadshahi M. The antioxidant and anti-inflammatory effects of astaxanthin supplementation on the expression of miR-146a and miR-126 in patients with type 2 diabetes mellitus: A randomised, double-blind, placebo-controlled clinical trial. Int J Clin Pract. 2021;75(5):e14022. 10.1111/ijcp.14022 [DOI] [PubMed] [Google Scholar]
- 24.Chang JJ, Wang YC, Yang SH, Wu JY, Chang MW, Wang HD. Pioneering Astaxanthin-Tumor Cell Membrane Nanoparticles for Innovative Targeted Drug Delivery on Melanoma. Int J Nanomedicine. 2024;19:2395–407. 10.2147/IJN.S439476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guerin M, Huntley ME, Olaizola M. Haematococcus astaxanthin: applications for human health and nutrition. Trends Biotechnol. 2003;21(5):210–6. 10.1016/S0167-7799(03)00078-7 [DOI] [PubMed] [Google Scholar]
- 26.Ambati RR, Phang SM, Ravi S, Aswathanarayana RG. Astaxanthin: sources, extraction, stability, biological activities and its commercial applications–a review. Mar Drugs. 2014;12(1):128–52. 10.3390/md12010128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li H, Li J, Hou C, Li J, Peng H, Wang Q. The effect of astaxanthin on inflammation in hyperosmolarity of experimental dry eye model in vitro and in vivo. Exp Eye Res. 2020;197:108113. 10.1016/j.exer.2020.108113 [DOI] [PubMed] [Google Scholar]
- 28.Visioli F, Artaria C. Astaxanthin in cardiovascular health and disease: mechanisms of action, therapeutic merits, and knowledge gaps. Food Funct. 2017;8(1):39–63. 10.1039/C6FO01721E [DOI] [PubMed] [Google Scholar]
- 29.Ursoniu S, Sahebkar A, Serban MC, Banach M. Lipid profile and glucose changes after supplementation with astaxanthin: a systematic review and meta-analysis of randomized controlled trials. Archives of medical science : AMS. 2015;11(2):253–66. 10.5114/aoms.2015.50960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xia W, Tang N, Kord-Varkaneh H, Low TY, Tan SC, Wu X, et al. The effects of astaxanthin supplementation on obesity, blood pressure, CRP, glycemic biomarkers, and lipid profile: A meta-analysis of randomized controlled trials. Pharmacol Res. 2020;161:105113. 10.1016/j.phrs.2020.105113 [DOI] [PubMed] [Google Scholar]
- 31.Leung LY, Chan SM, Tam HL, Wong ES. Astaxanthin influence on health outcomes of adults at risk of metabolic syndrome: a systematic review and meta-analysis. Nutrients. 2022;14(10):2050. 10.3390/nu14102050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gharaei R, Alyasin A, Mahdavinezhad F, Samadian E, Ashrafnezhad Z, Amidi F. Randomized controlled trial of astaxanthin impacts on antioxidant status and assisted reproductive technology outcomes in women with polycystic ovarian syndrome. J Assist Reprod Genet. 2022;39(4):995–1008. 10.1007/s10815-022-02432-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rostami S, Alyasin A, Saedi M, Nekoonam S, Khodarahmian M, Moeini A, et al. Astaxanthin ameliorates inflammation, oxidative stress, and reproductive outcomes in endometriosis patients undergoing assisted reproduction: A randomized, triple-blind placebo-controlled clinical trial. Front Endocrinol. 2023;14:1144323. 10.3389/fendo.2023.1144323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145(18):e895–1032. [DOI] [PubMed] [Google Scholar]
- 35.Brendler T, Williamson EM. Astaxanthin: How much is too much? A safety review. Phytotherapy research : PTR. 2019;33(12):3090–111. 10.1002/ptr.6514 [DOI] [PubMed] [Google Scholar]
- 36.Yoshida H, Yanai H, Ito K, Tomono Y, Koikeda T, Tsukahara H, et al. Administration of natural astaxanthin increases serum HDL-cholesterol and adiponectin in subjects with mild hyperlipidemia. Atherosclerosis. 2010;209(2):520–3. 10.1016/j.atherosclerosis.2009.10.012 [DOI] [PubMed] [Google Scholar]
- 37.Choi HD, Youn YK, Shin WG. Positive effects of astaxanthin on lipid profiles and oxidative stress in overweight subjects. Plant foods for human nutrition (Dordrecht, Netherlands). 2011;66(4):363–9. 10.1007/s11130-011-0258-9 [DOI] [PubMed] [Google Scholar]
- 38.Rector T. Patient’s self-assessment of their congestive heart failure: content, reliability, validity of a new measure, the Minnesota Living with Heart Failure questionnaire. Heart failure. 1987;3:198–209. [Google Scholar]
- 39.Eskandari S, Heravi-Karimooi M, Rejeh N, Ebadi A, Montazeri A. Translation and validation study of the Iranian version of Minnesota living with heart failure questionnaire. Payesh (Health Monitor). 2015;14(4):475–84. [Google Scholar]
- 40.Ghotbi N, Ansari NN, Fetrosi S, Shamili A, Choobsaz H, Montazeri H. Fatigue in Iranian patients with neurological conditions: An assessment with Persian Fatigue Severity Scale. Health Sci J. 2013;7(4):395. [Google Scholar]
- 41.Krupp LB, Alvarez LA, LaRocca NG, Scheinberg LC. Fatigue in multiple sclerosis. Arch Neurol. 1988;45(4):435–7. 10.1001/archneur.1988.00520280085020 [DOI] [PubMed] [Google Scholar]
- 42.Bosnak-Guclu M, Arikan H, Savci S, Inal-Ince D, Tulumen E, Aytemir K, et al. Effects of inspiratory muscle training in patients with heart failure. Respir Med. 2011;105(11):1671–81. 10.1016/j.rmed.2011.05.001 [DOI] [PubMed] [Google Scholar]
- 43.Mahler DA, Ward J, Waterman LA, McCusker C, ZuWallack R, Baird JC. Patient-reported dyspnea in COPD reliability and association with stage of disease. Chest. 2009;136(6):1473–9. 10.1378/chest.09-0934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hossein Pour AH, Gholami M, Saki M, Birjandi M. The effect of inspiratory muscle training on fatigue and dyspnea in patients with heart failure: A randomized, controlled trial. Jpn J Nurs Sci. 2020;17(2):e12290. 10.1111/jjns.12290 [DOI] [PubMed] [Google Scholar]
- 45.Hezaveh ZS, Golafrouz H, Piran A, Vafa M. Validity and reliability of the Persian version of the council on nutrition appetite questionnaire and its simplified version in Iranian community-dwelling older adults. J Nutrition Food Sec. 2023;8(2):163–71. [Google Scholar]
- 46.Moghaddam MB, Aghdam FB, Jafarabadi MA, Allahverdipour H, Nikookheslat SD, Safarpour S. The Iranian Version of International Physical Activity Questionnaire (IPAQ) in Iran: content and construct validity, factor structure, internal consistency and stability. World Appl Sci J. 2012;18(8):1073–80. [Google Scholar]
- 47.Kato T, Kasai T, Sato A, Ishiwata S, Yatsu S, Matsumoto H, et al. Effects of 3-month astaxanthin supplementation on cardiac function in heart failure patients with left ventricular systolic dysfunction-a pilot study. Nutrients. 2020;12(6):1896. 10.3390/nu12061896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pashkow FJ, Watumull DG, Campbell CL. Astaxanthin: a novel potential treatment for oxidative stress and inflammation in cardiovascular disease. Am J Cardiol. 2008;101(10a):58d–68d. 10.1016/j.amjcard.2008.02.010 [DOI] [PubMed] [Google Scholar]
- 49.Choi HD, Kim JH, Chang MJ, Kyu-Youn Y, Shin WG. Effects of astaxanthin on oxidative stress in overweight and obese adults. Phytotherapy research : PTR. 2011;25(12):1813–8. 10.1002/ptr.3494 [DOI] [PubMed] [Google Scholar]
- 50.Mashhadi NS, Zakerkish M, Mohammadiasl J, Zarei M, Mohammadshahi M, Haghighizadeh MH. Astaxanthin improves glucose metabolism and reduces blood pressure in patients with type 2 diabetes mellitus. Asia Pac J Clin Nutr. 2018;27(2):341–6. [DOI] [PubMed] [Google Scholar]
- 51.Urakaze M, Kobashi C, Satou Y, Shigeta K, Toshima M, Takagi M, et al. The beneficial effects of astaxanthin on glucose metabolism and modified low-density lipoprotein in healthy volunteers and subjects with prediabetes. Nutrients. 2021;13(12):4381. 10.3390/nu13124381 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data produced and/or analyzed in the present study will be accessible upon reasonable request from the corresponding author.