Abstract
Objective
Sustained hyperlipidemia contributes to fatty liver and liver cirrhosis. Red yeast rice (RYR) effectively improved the lipid profile; however, the effects of RYR on the risk of incident liver cirrhosis remain to be elucidated. We aimed to evaluate the beneficial effects of RYR use on the risk and outcome of liver cirrhosis.
Patients and methods
We identified 156,587 adults who had newly diagnosed hyperlipidemia in 2010–2016 from health insurance data in this retrospective cohort study. Using propensity score matching, we selected 34,367 patients who used RYR and 34,367 patients who used lovastatin. Events of incident liver cirrhosis that occurred in the two cohorts during the follow-up period of 2010–2019 were identified. We calculated adjusted hazard ratios (HRs) and 95% confidence intervals (Cis) for liver cirrhosis risk associated with RYR use in the multiple Cox proportional hazard model.
Results
Compared with patients who used lovastatin, patients who used RYR had a decreased risk of liver cirrhosis (HR 0.60, 95% CI 0.57–0.63), and this association was significant in various subgroups. A biological gradient relationship between the frequency of RYR use and decreased liver cirrhosis was observed (p for trend < 0.0001). Reduced postcirrhosis jaundice (HR 0.56, 95% CI 0.43–0.72), ascites (HR 0.37, 95% CI 0.28–0.50), hepatic coma (HR 0.36, 95% CI 0.26–0.50), and mortality (HR 0.48, 95% CI 0.38–0.61) were also associated with RYR use.
Conclusion
We demonstrated the beneficial effects of RYR use on the risk and outcome of liver cirrhosis; however, the lack of compliance data should be considered. However, our study did not infer causality or claim the superiority of RYR over lovastatin.
Keywords: hyperlipidemia, liver cirrhosis, lovastatin, outcome, red yeast rice, risk
Introduction
Liver cirrhosis is prevalent and results in various complications and mortality in low-income, middle-income, and high-income countries.1 Globally, liver cirrhosis remains one of the leading causes of death, as there were more than 1.32 million deaths in 2017 compared with less than 899,000 deaths in 1990.2 It is already known that liver cirrhosis is the end stage of progressive liver fibrosis, and the most common causes are alcohol-related liver disease, chronic viral hepatitis B and C, and nonalcoholic fatty liver disease.1,2
Non-high-density lipoprotein cholesterol independently predicts new onset of nonalcoholic fatty liver disease.3 Remnant cholesterol was independently associated with the risk of metabolic dysfunction-associated fatty liver disease and predicted all-cause, cardiovascular, and cancer-related mortalities in patients with metabolic dysfunction-associated fatty liver disease.4 Individuals with nonalcoholic fatty liver disease showed significantly higher risks for cirrhosis and hepatocellular carcinoma.5 Serum cholesterol is also a significant and independent predictor of poor outcome and mortality in patients with liver cirrhosis.6,7 However, more than half of patients with dyslipidemia have no awareness of dyslipidemia, and most of them do not use medication to control their condition.8
Statins are commonly used to control non-high-density lipoprotein cholesterol in the clinical setting of Western medicine.9 In the clinical setting of traditional Chinese medicine, physicians have used scientifically processed red yeast rice (RYR, also known as Monascus purpureus Went rice, contains monacolin K [lovastatin]) to control the status of hyperlipidemia, such as Xuezhikang®, HypoCol®, and LipoCol Forte®.10–14 The clinical trial in Taiwan indicated that the 8-week treatment with RYR (patients received a twice-daily dose of 600 mg for 8 weeks) showed significantly greater reduction than the placebo treatment in low-density lipoprotein cholesterol levels, total cholesterol/high-density lipoprotein cholesterol, low-density lipoprotein cholesterol/high-density lipoprotein cholesterol and apolipoprotein B/apolipoprotein A-I ratios.15
The effects of statin use on the reduced risk of developing liver cirrhosis were investigated in previous studies.14–19 Some studies also suggested that reduced complications and mortality were found in patients with liver cirrhosis who underwent statin treatment.20–22 Because reliable references suggested that RYR is beneficial in lowering lipid profiles and reducing the risks of stroke, diabetes, and postoperative adverse events,10–15 we considered that use of RYR is probably also associated with reduced liver cirrhosis. However, little was known regarding the association between the use of RYR and the risk of liver cirrhosis.
A triple-blind randomized clinical trial considered that RYR is safe to add to statins medications significantly decreases total cholesterol.23 There were only few case reports that remind the potential side effects of RYR, such as hepatotoxicity and symptomatic myopathy, and these effects being partially similar to the effects of statins.24,25 Many studies also suggested that the intake of RYR improves lipid profiles may be a treatment option for dyslipidemic patients who cannot tolerate statin therapy.26,27
Based on the above evidences and suggestions, we used real-world data to evaluate the risk of liver cirrhosis in patients with hyperlipidemia who underwent RYR treatment in this study. However, our purpose is not to infer causality or claim the superiority of RYR over lovastatin.
Methods
Source of Data
We collected patient information from the academic research database of the public medical insurance, which was maintained by the government in Taiwan. Details of this research database were described and evaluated previously.10–12,28 According to the regulation of the Ministry of Health and Welfare in Taiwan, informed consent from the study participants is not required because patient identification was decoded and scrambled in data. Our study was evaluated by the Institutional Review Board of Taipei Medical University (TMU-JIRB-202303013; TMU-JIRB-201905042; TMUJIRB-201902053).
Study Design
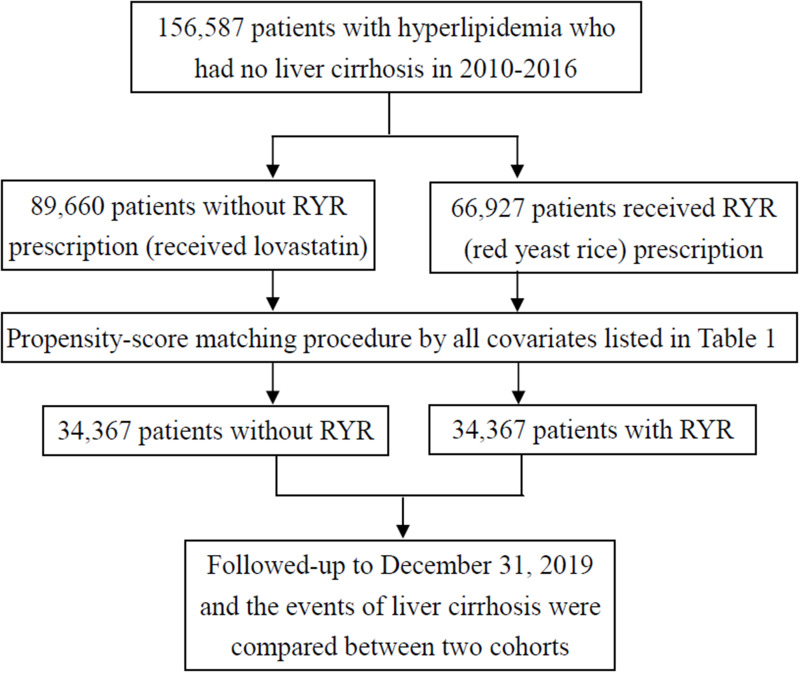
Among nearly 23.4 million people covered in government health insurance in Taiwan, we identified 156,587 patients aged years and older who were first diagnosed with hyperlipidemia with the use of RYR (n = 66927) and lovastatin (n = 89660) or in 2010–2016 in this study. We considered patients who used lovastatin as the control group (without the use of RYR). Patients who sought treatment for hyperlipidemia within the washout period of 2 years were excluded, as this study aimed to investigate patients with newly diagnosed hyperlipidemia. Both cohorts had medical records of liver cirrhosis before physician’s diagnosis of hyperlipidemia or used lipid-lowering medications (RYR or lovastatin). Between cohorts with and without the use of RYR, we conducted propensity score matching to obtain similar baseline characteristics. After the matching procedure, there were 34,367 patients in the RYR cohort and 34,367 patients in the non-RYR cohort in this study. Both cohorts were followed up to December 31, 2019. The events of newly diagnosed liver cirrhosis that occurred during the follow-up period were considered as outcomes between the RYR and non-RYR cohorts in this study. There was no immortal time bias in this study because the follow-up started from the time of the use of medication (lovastatin or RYR) or the index date and lasted until the occurrence of liver cirrhosis, censoring due to death, migration, or loss of follow-up by the end of 2019. We evaluated the risk of incident liver cirrhosis between the RYR cohort and the non-RYR cohort (use of lovastatin) during the follow-up period (Figure 1).
Figure 1.
The selection process of adequate study subjects.
Criteria and Definitions
We defined patients with hyperlipidemia as those who had at least one visit for outpatient care with a physician’s diagnosis. Cirrhosis and other medical conditions were also identified by the records of medical visits in the database. Details of these diagnosis codes are listed in Table S1. To strictly identify the RYR cohort under the coverage of Taiwan’s Health Insurance Program, we defined people who visited TCM clinics and received a prescription for RYR from a physician. The criteria and definition were verified and the corresponding details of RYR prescription could be found in our previous studies.10–12
Patients with liver cirrhosis were defined as having at least two visits for medical care with a physician’s primary diagnosis of liver cirrhosis. The criteria were used and verified in our previous study.28 We identified patients with low-income status as those who qualified for waived medical copayment, and this status was verified by the local and central government. Renal dialysis was considered one of the medical conditions that were defined by administration code (D8, D9).
Statistical Analysis
To reduce confounding bias, we used a propensity score-matched pair procedure to balance the covariates between the RYR and non-RYR cohorts. By using a nonparsimonious multivariable logistic regression model with a greedy matching algorithm (without replacement). We matched RYR patients to non-RYR patients and the clinical significance guided the initial choices of covariates (listed in Table 1) were included in this multivariable logistic regression model. This method could remove a majority of bias from measured covariates.
Table 1.
Baseline Characteristics Between Cohorts with and without Use of Red Yeast Rice Prescription
| No RYR N=34367 | RYR prescription N=34367 | p-value | |||
|---|---|---|---|---|---|
| n | (%) | n | (%) | ||
| Sex | 1.0000 | ||||
| Female | 19741 | (57.4) | 19,741 | (57.4) | |
| Male | 14626 | (42.6) | 14,626 | (42.6) | |
| Age, years | 1.0000 | ||||
| 20–29 | 407 | (1.2) | 407 | (1.2) | |
| 30–39 | 1920 | (5.6) | 1920 | (5.6) | |
| 40–49 | 6582 | (19.2) | 6582 | (19.2) | |
| 50–59 | 13,417 | (39.0) | 13,417 | (39.0) | |
| 60–69 | 8548 | (24.9) | 8548 | (24.9) | |
| 70–79 | 3016 | (8.8) | 3016 | (8.8) | |
| ≥80 | 477 | (1.4) | 477 | (1.4) | |
| Low income | 1.0000 | ||||
| No | 33854 | (98.5) | 33,854 | (98.5) | |
| Yes | 513 | (1.5) | 513 | (1.5) | |
| Number of hospitalizations | 1.0000 | ||||
| 0 | 18,338 | (53.4) | 18,338 | (53.4) | |
| 1 | 7062 | (20.6) | 7062 | (20.6) | |
| 2 | 3130 | (9.1) | 3130 | (9.1) | |
| ≥3 | 5837 | (17.0) | 5837 | (17.0) | |
| Number of emergency visits | 1.0000 | ||||
| 0 | 12,111 | (35.2) | 12,111 | (35.2) | |
| 1 | 7216 | (21.0) | 7216 | (21.0) | |
| 2 | 4259 | (12.4) | 4259 | (12.4) | |
| ≥3 | 10,781 | (31.4) | 10,781 | (31.4) | |
| Medical conditions | |||||
| Hypertension | 17814 | (51.8) | 17,814 | (51.8) | 1.0000 |
| Diabetes | 11844 | (34.5) | 11,844 | (34.5) | 1.0000 |
| Mental disorders | 13805 | (40.2) | 13,805 | (40.2) | 1.0000 |
| COPD | 2025 | (5.9) | 2025 | (5.9) | 1.0000 |
| Ischemic heart disease | 5904 | (17.2) | 5904 | (17.2) | 1.0000 |
| Heart failure | 618 | (1.8) | 618 | (1.8) | 1.0000 |
| Renal dialysis | 217 | (0.6) | 217 | (0.6) | 1.0000 |
| CCI, score | 1.0000 | ||||
| 0 | 7055 | (20.5) | 7055 | (20.5) | |
| 1 | 7260 | (21.1) | 7260 | (21.1) | |
| 2 | 4942 | (14.4) | 4942 | (14.4) | |
| ≥3 | 15,110 | (44.0) | 15,110 | (44.0) | |
| Anti-hypertension drug use | 1.0000 | ||||
| No | 22290 | (64.9) | 22,290 | (64.9) | |
| Yes | 12077 | (35.1) | 12,077 | (35.1) | |
| Anticoagulant drug use | 1.0000 | ||||
| No | 33925 | (98.7) | 33,925 | (98.7) | |
| Yes | 442 | (1.3) | 442 | (1.3) | |
| NASH | 0.0007 | ||||
| No | 29507 | (85.9) | 29,195 | (85.0) | |
| Yes | 4860 | (14.1) | 5172 | (15.0) | |
| Obesity | <0.0001 | ||||
| No | 33502 | (97.5) | 33,254 | (96.8) | |
| Yes | 865 | (2.5) | 1113 | (3.2) | |
Abbreviations: CCI, Charlson comorbidity index; NASH, non-alcoholic steatohepatitis; RYR, red yeast rice.
Chi-square tests were used to present the balance of covariates between patients with and without RYR. We then used multivariate Cox proportional hazard models to calculate the adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) of liver cirrhosis between the RYR and non-RYR cohorts during the follow-up period. Stratified analyses by age, sex, low income, emergency room visits, hospitalizations, and the Charlson Comorbidity Index were performed to examine the association between RYR use and the risk of developing liver cirrhosis in these subgroups. In the sensitivity analysis, we excluded the initial events of liver cirrhosis during the start of the follow-up period (the first 1, 2, 3, 4, 5, and 6 months) to evaluate the adjusted HRs and 95% CIs of liver cirrhosis associated with RYR. To correct for the competing risk of mortality, we performed a sensitivity analysis and excluded the deaths that occurred during the follow-up period. The cumulative use of RYR was estimated for the calculated adjusted HRs and 95% CIs of liver cirrhosis associated with the frequency of RYR use. Kaplan–Meier survival analysis was used to test the cirrhosis-free curve during the follow-up period between RYR and non-RYR cohorts.
Results
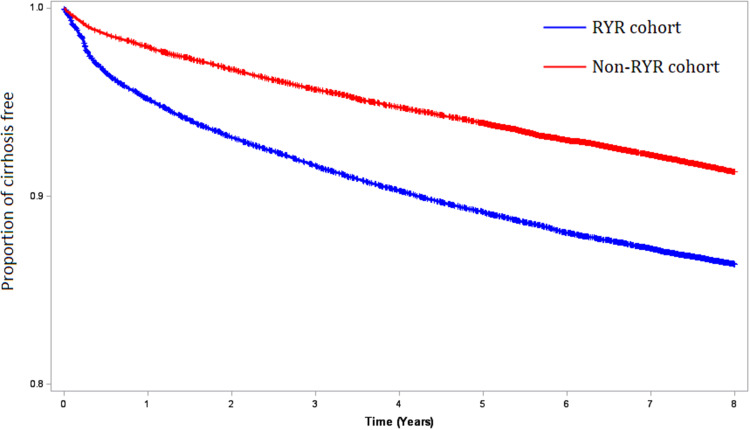
After propensity score matching among 68734 patients with hyperlipidemia (Table 1), there was no significant difference in baseline characteristics between the RYR (n = 34367) and non-RYR (n = 34367) cohorts. Because non-alcoholic steatohepatitis and obesity were not included in the matching, patients with RYR had higher proportions of non-alcoholic steatohepatitis (p = 0.0007) and obesity (<0.0001) compared with cohort without RYR. A total of 8215 patients developed liver cirrhosis during the follow-up period (Table 2). After correcting for immortal time bias, patients who used RYR had a reduced risk of developing liver cirrhosis compared with patients who used lovastatin (HR 0.61, 95% CI 0.58–0.64). In Figure 2, the Kaplan‒Meier analysis showed that the RYR cohort had a lower incidence of liver cirrhosis than the lovastatin cohort during the follow-up period (p < 0.0001).
Table 2.
The Adjusted Risk of Incident Liver Cirrhosis Between People with and without Use of Red Yeast Rice Prescription During the Follow-Up Period
| Incident liver cirrhosis | |||||||
|---|---|---|---|---|---|---|---|
| n | PYs | Events | Incidence† | HR | (95% CI)‡ | ||
| All | No RYR | 34367 | 321,051 | 5172 | 16.1 | 1.00 | (reference) |
| RYR | 34367 | 270,278 | 3043 | 11.3 | 0.60 | (0.57–0.63) | |
| Women | No RYR | 19741 | 186,572 | 2830 | 15.2 | 1.00 | (reference) |
| RYR | 19741 | 155,816 | 1629 | 10.5 | 0.60 | (0.56–0.64) | |
| Men | No RYR | 14626 | 134,479 | 2342 | 17.4 | 1.00 | (reference) |
| RYR | 14626 | 114,461 | 1414 | 12.4 | 0.60 | (0.56–0.64) | |
| Age, 20–39 years | No RYR | 2327 | 21,510 | 400 | 18.6 | 1.00 | (reference) |
| RYR | 2327 | 19,126 | 209 | 10.9 | 0.52 | (0.44–0.62) | |
| Age, 40–49 years | No RYR | 6582 | 61,380 | 1130 | 18.4 | 1.00 | (reference) |
| RYR | 6582 | 53,443 | 631 | 11.8 | 0.58 | (0.52–0.64) | |
| Age, 50–59 years | No RYR | 13417 | 126,621 | 2035 | 16.1 | 1.00 | (reference) |
| RYR | 13417 | 106,317 | 1268 | 11.9 | 0.65 | (0.60–0.69) | |
| Age, 60–69 years | No RYR | 8548 | 79,775 | 1207 | 15.1 | 1.00 | (reference) |
| RYR | 8548 | 65,362 | 690 | 10.6 | 0.58 | (0.52–0.64) | |
| Age, ≥70 years | No RYR | 3493 | 31,765 | 400 | 12.6 | 1.00 | (reference) |
| RYR | 3493 | 26,030 | 245 | 9.41 | 0.58 | (0.49–0.68) | |
| No low income | No RYR | 33854 | 316,644 | 5070 | 16.0 | 1.00 | (reference) |
| RYR | 33854 | 266,383 | 2973 | 11.2 | 0.60 | (0.57–0.63) | |
| Low income | No RYR | 513 | 4407 | 102 | 23.1 | 1.00 | (reference) |
| RYR | 513 | 3895 | 70 | 18.0 | 0.56 | (0.41–0.77) | |
| Emergency visits, 0 | No RYR | 12111 | 97,548 | 3405 | 34.9 | 1.00 | (reference) |
| RYR | 12111 | 86,551 | 1777 | 20.5 | 0.47 | (0.45–0.50) | |
| Emergency visits, 1 | No RYR | 7216 | 69,830 | 854 | 12.2 | 1.00 | (reference) |
| RYR | 7216 | 57,053 | 534 | 9.36 | 0.72 | (0.65–0.81) | |
| Emergency visits, 2 | No RYR | 4259 | 42,773 | 350 | 8.18 | 1.00 | (reference) |
| RYR | 4259 | 34,776 | 250 | 7.19 | 0.89 | (0.75–1.05) | |
| Emergency visits, ≥3 | No RYR | 10781 | 110,901 | 563 | 5.08 | 1.00 | (reference) |
| RYR | 10781 | 91,898 | 482 | 5.24 | 1.11 | (0.98–1.26) | |
| Hospitalizations, 0 | No RYR | 18338 | 159,652 | 4127 | 25.8 | 1.00 | (reference) |
| RYR | 18338 | 136,959 | 2251 | 16.4 | 0.52 | (0.49–0.55) | |
| Hospitalizations, 1 | No RYR | 7062 | 70,464 | 648 | 9.20 | 1.00 | (reference) |
| RYR | 7062 | 57,475 | 460 | 8.00 | 0.87 | (0.77–0.99) | |
| Hospitalizations, 2 | No RYR | 3130 | 32,110 | 182 | 5.67 | 1.00 | (reference) |
| RYR | 3130 | 26,332 | 151 | 5.73 | 1.03 | (0.82–1.29) | |
| Hospitalizations, ≥3 | No RYR | 5837 | 58,824 | 215 | 3.65 | 1.00 | (reference) |
| RYR | 5837 | 49,512 | 181 | 3.66 | 1.06 | (0.87–1.30) | |
| CCI score, 0 | No RYR | 7055 | 56,365 | 1925 | 34.2 | 1.00 | (reference) |
| RYR | 7055 | 50,176 | 985 | 19.6 | 0.45 | (0.42–0.49) | |
| CCI score, 1 | No RYR | 7260 | 63,619 | 1558 | 24.5 | 1.00 | (reference) |
| RYR | 7260 | 54,968 | 845 | 15.4 | 0.52 | (0.48–0.57) | |
| CCI score, 2 | No RYR | 4942 | 47,756 | 630 | 13.2 | 1.00 | (reference) |
| RYR | 4942 | 39,311 | 392 | 9.97 | 0.71 | (0.63–0.81) | |
| CCI score, ≥3 | No RYR | 15110 | 153,310 | 1059 | 6.91 | 1.00 | (reference) |
| RYR | 15110 | 125,823 | 821 | 6.53 | 0.98 | (0.89–1.08) | |
Notes: †Per 1000 person-years. ‡Adjusted for all covariates listed in Table 1.
Abbreviations: CI, confidence interval; CCI, Charlson comorbidity index; HR, hazard ratio; PYs, person-years; RYR, red yeast rice.
Figure 2.
Kaplan-Meier model for measuring the cirrhosis-free probability in hyperlipidemia patients with and without RYR prescription (log rank test, P < 0.0001).
In Table 2, the use of RYR was associated with a reduced risk of developing liver cirrhosis in women (HR 0.60, 95% CI 0.56–0.64), men (HR 0.60, 95% CI 0.56–0.64), and people aged 20–39 years (HR 0.52, 95% CI 0.44–0.62), 40–49 years (HR 0.58, 95% CI 0.52–0.64), 50–59 years (HR 0.65, 95% CI 0.60–0.69), 60–69 years (HR 0.58, 95% CI 0.52–0.64), and ≥70 years (HR 0.58, 95% CI 0.49–0.68). Among people with (HR 0.56, 95% CI 0.41–0.77) and without low income (HR 0.60, 95% CI 0.57–0.63), reduced liver cirrhosis was found in people who used RYR. The association between RYR use and a reduced risk of developing liver cirrhosis was significant in people with no emergency room visits (HR 0.47, 95% CI 0.45–0.50), one emergency room visit (HR 0.72, 95% CI 0.65–0.81), no hospitalizations (HR 0.52, 95% CI 0.49–0.55), and one hospitalization (HR 0.87, 95% CI 0.77–0.99). We also found that the RYR cohort had reduced liver cirrhosis compared with the lovastatin cohort in people with Charlson Comorbidity Index scores of 0 (HR 0.45, 95% CI 0.42–0.49), 1 (HR 0.52, 95% CI 0.48–0.57), and 2 (HR 0.71, 95% CI 0.63–0.81).
In Table 3, the sensitivity analysis showed that the adjusted HRs of liver cirrhosis associated with RYR use for excluding cirrhosis events in the initial 1 month, 2 months, 3 months, 4 months, 5 months and 6 months of the following period were 0.62 (95% CI 0.59–0.65), 0.62 (95% CI 0.60–0.65), 0.64 (95% CI 0.61–0.67), 0.66 (95% CI 0.63–0.70), 0.68 (95% CI 0.64–0.71), and 0.69 (95% CI 0.65–0.72), respectively. After excluding the deaths that occurred in the follow-up period, surviving patients who used RYR had a lower risk of liver cirrhosis than surviving patients who did not use RYR (HR 0.62, 95% CI 0.59–0.65).
Table 3.
Sensitivity Analysis for the Risk of Liver Cirrhosis Associated with Red Yeast Rice Prescription After Excluding the Initial Incident Events
| After excluding the incident liver cirrhosis cases during initial | Incident liver cirrhosis | ||||||
|---|---|---|---|---|---|---|---|
| N | PYs | Events | Incidence* | HR | (95% CI)† | ||
| One month | No RYR | 34126 | 321,039 | 4931 | 15.4 | 1.00 | (reference) |
| RYR | 34251 | 270,273 | 2927 | 10.8 | 0.62 | (0.59–0.65) | |
| Two months | No RYR | 33936 | 321,015 | 4741 | 14.8 | 1.00 | (reference) |
| RYR | 34158 | 270,261 | 2834 | 10.5 | 0.62 | (0.60–0.65) | |
| Three months | No RYR | 33681 | 320,960 | 4486 | 14.0 | 1.00 | (reference) |
| RYR | 34068 | 270,242 | 2744 | 10.2 | 0.64 | (0.61–0.67) | |
| Four month | No RYR | 33451 | 320,896 | 4256 | 13.3 | 1.00 | (reference) |
| RYR | 33994 | 270,221 | 2670 | 9.88 | 0.66 | (0.63–0.70) | |
| Five months | No RYR | 33322 | 320,848 | 4127 | 12.9 | 1.00 | (reference) |
| RYR | 33948 | 270,204 | 2624 | 9.71 | 0.68 | (0.64–0.71) | |
| Six months | No RYR | 33198 | 320,791 | 4003 | 12.5 | 1.00 | (reference) |
| RYR | 33897 | 270,181 | 2573 | 9.52 | 0.69 | (0.65–0.72) | |
| Excluded deaths | No RYR | 32704 | 311,720 | 4909 | 15.7 | 1.00 | (reference) |
| RYR | 33332 | 264,844 | 2945 | 11.1 | 0.62 | (0.59–0.65) | |
Notes: *Per 1000 person-years. †Adjusted for all covariates listed in Table 1.
Abbreviations: CI, confidence interval; HR, hazard ratio; PYs, person-years; RYR, red yeast rice.
Compared with non-RYR use (Table 4), the frequency of RYR use was associated with a reduced risk of developing liver cirrhosis (≥5 prescriptions: HR 0.59, 95% CI 0.55–0.63), and there was a biological gradient relationship (p < 0.0001). Reduced risks of cirrhosis-related complications and mortality were found in people who used RYR (Table 5), such as jaundice (HR 0.56, 95% CI 0.43–0.72), ascites (HR 0.37, 95% CI 0.28–0.50), hepatic coma (HR 0.36, 95% CI 0.26–0.50), and mortality (HR 0.48, 95% CI 0.38–0.61).
Table 4.
Risk of Liver Cirrhosis in People with Use Frequency of Red Yeast Rice Prescriptions
| Incident liver cirrhosis | ||||||
|---|---|---|---|---|---|---|
| n | PYs | Events | Incidence* | HR | (95% CI)† | |
| No-RYR cohort (used lovastatin) | 34367 | 321,051 | 5172 | 16.1 | 1.00 | (reference) |
| RYR cohort, frequency of RYR use | ||||||
| 1 | 9998 | 76,790 | 873 | 11.4 | 0.63 | (0.59–0.68) |
| 2 | 4789 | 36,763 | 433 | 11.8 | 0.63 | (0.57–0.70) |
| 3 | 3249 | 25,002 | 291 | 11.6 | 0.61 | (0.54–0.69) |
| 4 | 2468 | 19,011 | 218 | 11.5 | 0.59 | (0.51–0.67) |
| ≥5 | 13,863 | 112,713 | 1228 | 10.9 | 0.59 | (0.55–0.63) |
Notes: *Per 1000 person-years. †Adjusted for all covariates listed in Table 1.
Abbreviations: CI, confidence interval; HR, hazard ratio; PYs, person-years; RYR, red yeast rice.
Table 5.
Complications and Mortality After Liver Cirrhosis in People with and without Use of Red Yeast Rice Prescription
| No RYR (N=34,367) | RYR (N=34,367) | |||||||
|---|---|---|---|---|---|---|---|---|
| Events | Incidence* | HR | (95% CI)† | Events | Incidence* | HR | (95% CI)† | |
| Jaundice | 164 | 0.54 | 1.00 | (reference) | 101 | 0.39 | 0.56 | (0.43–0.72) |
| Ascites | 154 | 0.51 | 1.00 | (reference) | 69 | 0.27 | 0.37 | (0.28–0.50) |
| Hepatic coma | 130 | 0.43 | 1.00 | (reference) | 55 | 0.21 | 0.36 | (0.26–0.50) |
| Mortality | 263 | 0.86 | 1.00 | (reference) | 98 | 0.38 | 0.48 | (0.38–0.61) |
Notes: *Per 1000 person-years. †Adjusted for all covariates listed in Table 1.
Abbreviations: CI, confidence interval; HR, hazard ratio; RYR, red yeast rice.
The stratified analysis by medical conditions and medication for the association between the risk of liver cirrhosis and RYR is presented in Table S2. In Table S3, the adjusted HRs for alcoholic cirrhosis, nonalcoholic cirrhosis, and unspecified cirrhosis among people who used RYR were 0.37 (95% CI 0.18–0.73), 0.64 (95% CI 0.51–0.81), and 0.61 (95% CI 0.58–0.64), respectively.
Table S4 shows the baseline characteristics between the RYR and non-RYR cohorts (before propensity score matching). In Table S5, the analysis before propensity score matching showed that RYR use was associated with a reduced risk of developing liver cirrhosis during the follow-up period (HR 0.61, 95% CI 0.58–0.64). Compared with RYR cohort, patients received pravastatin (HR 1.73, 95% CI 1.63–1.83) and simvastatin (HR 1.76, 95% CI 1.68–1.84) had increased risk of liver cirrhosis. However, patients received rosuvastatin (HR 0.72, 95% CI 0.69–0.75) had reduced risk of liver cirrhosis than those received RYR (Table S6).
Discussion
To our knowledge, this study was the first to document the beneficial effects of the use of RYR on the risk of liver cirrhosis among patients with hyperlipidemia. In this retrospective cohort study based on real-world insurance data, we also found a dose–response relationship between the use of RYR and the risk of cirrhosis development after controlling for potential confounding factors. These findings were also observed among various subgroups of age, sex, or medical conditions, and the sensitivity analysis strengthened the association between the use of RYR and a decreased risk of liver cirrhosis.
Based on the Taiwan insurance database, the effects of statin use on the reduced risk of developing liver cirrhosis have been investigated in several studies.16–19,21 A previous study suggested that statin use was associated with a reduced risk of developing cirrhosis in a dose-dependent manner among patients with HCV infection.16 Another study showed that a cumulative does–response association occurred between statin use and a reduced risk of developing decompensated liver cirrhosis among patients with alcohol use disorder.17 The effectiveness of statins in reducing the risk of developing decompensated liver cirrhosis in patients with diabetes is dose-dependent.18 Patients with chronic hepatitis B who underwent statin therapy experienced a dose-dependent reduction in the risk of cirrhosis and its decompensation.19 Statin use decreases the decompensation rate in both hepatitis B virus- and hepatitis C virus-related cirrhosis.21 Although a study investigated the beneficial effects of RYR on liver cancer, no information has revealed the effects of RYR on reducing the risk of liver cirrhosis. In this study, we raised the possibility that patients with hyperlipidemia who received treatment with RYR had a relatively low risk and fewer adverse outcomes of liver cirrhosis.
Some explanations may explain the relationship between RYR and liver cirrhosis in the present study. First, total cholesterol and low-density lipoprotein cholesterol were reported to independently predict new onset of nonalcoholic fatty liver disease.3,4 Individuals with nonalcoholic fatty liver disease and increased liver enzyme levels showed significantly higher risks for cirrhosis.5 Several studies have suggested that lovastatin or other types of statins effectively reduce the level of total cholesterol or low-density lipoprotein cholesterol (LDL-C) and provide reliable evidence of benefits on liver cirrhosis risk.16–19,21 Phytomedicine RYR contains monacolin K (lovastatin), which can reduce the levels of total cholesterol, LDL-C, and triglycerides.13,15,26 Therefore, we speculated that the use of RYR is beneficial in reducing fatty liver and subsequent liver cirrhosis.
Second, sustained hyperlipidemia is considered a type of inflammation that is also a risk factor for liver cirrhosis.29–31 RYR has potential anti-inflammatory effects.32–34 This molecular study provides theoretical support for the wide application of RYR as an antioxidant dietary supplement that reduces oxidative stress-related inflammation and improves intestinal microbiota.33 An animal study suggested that RYR was effective in combatting inflammation, insulin resistance, and nonalcoholic fatty liver diseases in mice, irrespective of monacolin K levels.32 RYR was also suggested to protect against nonalcoholic fatty liver disease by inhibiting lipid synthesis and mediating hepatic inflammation in mice.34
Third, diabetes is considered a significant risk factor for the development of liver cirrhosis among Chinese people.35 In a population-based study in China, individuals with diabetes had a higher risk of cirrhosis than those without diabetes.36 Our previous reports suggested that a decreased risk of incident diabetes was found in people who used RYR.11 Because of the above potential evidence and findings of this study, we hypothesized that the reduction in the risk of diabetes is beneficial for the prevention of liver cirrhosis.
Fourth, licensed physicians provided medical services for traditional Chinese medicine, which was considered the second medical opinion (western medicine, also called biochemical medicine, was the first choice) and is commonly used in Taiwan and other Asian countries.37,38 Previous studies have suggested that people who use traditional Chinese medicine have better health-related lifestyles.37,38 In this study, we hypothesized that patients with hyperlipidemia who were treated with RYR (as prescribed by physicians with specialties in traditional Chinese medicine) may have better knowledge, attitudes, and practices regarding disease prevention and health promotion. It is also possible that better knowledge, attitudes, and practices may also contribute to the decreased incidence of liver cirrhosis in patients who used RYR.
Jaundice, ascites, and hepatic coma are common cirrhosis-related complications,1 while cirrhosis-related mortality increases the global burden of health.2 Serum cholesterol predicts poor outcomes and mortality in patients with liver cirrhosis,6,7 and some studies have suggested that statin treatment reduces complications and mortality in patients with liver cirrhosis.20–22 Therefore, in this study, we considered it reasonable that RYR is beneficial in reducing complications and mortality after liver cirrhosis.
Before prescribing RYR for patients, physicians need to consider the safety of RYR use.39 Although some case reports have indicated side effects from the consumption of RYR, including myopathy, hepatotoxicity, and erectile dysfunction,40–42 several clinical trials have suggested that RYR has a good safety profile and could be considered for patients who cannot tolerate statin drugs.26,39,43,44 As Xuezhikang® and HypoCol® are scientific Chinese medicines,13,14 we evaluated RYR (LipoCol Forte®), which was prescribed by physicians in this study, and it had good manufacturing practices and was relatively stable and safe. Nevertheless, continuous monitoring by clinicians for muscular and hepatic safety is important, and we suggest comprehensive review by policy-makers to harmonize the regulatory status of these treatments. The side effects of RYR need to be cautioned and more assessments of the potential side effects of RYR are needed.
People with high total cholesterol/high-density lipoprotein ratio or triglycerides//high-density lipoprotein ratio, or both, have a greater risk for nonalcoholic fatty liver disease.45–47 The levels of triglycerides is also highly associated with non-alcoholic fatty liver disease.48,49 Many studies have suggested that RYR could significantly reduce the level of triglycerides.50–53 Compared to patients receiving statins therapy, adding RYR to statin medications significantly decreases the serum level of total cholesterol in patients with dyslipidemia.23 A previous study performed the animal experiment and found that RYR ameliorated non-alcoholic fatty liver disease through inhibiting lipid synthesis and NF-κB/NLRP3 inflammasome-mediated hepatic inflammation in mice. The above finding helps us to clarify the role of RYR in reducing the risk of liver cirrhosis in this study.34 In summary, of the above previous findings, we believed that RYR has additional effects to reduce lipid profile, risk of fatty liver, and the subsequent liver cirrhosis. We may expect future results of the clinical trial that was conducted to compare the beneficial effects on reducing lipid profile between RYR and statins.54
Study Limitations
The first limitation of our study is that we have no clinical examination data, such as levels of total cholesterol, GOT, and GPT; ultrasound of fatty liver; and the liver cirrhosis severity. We could not evaluate the severity of hypercholesterolemia for the relationship between RYR use and the risk of developing liver cirrhosis. Second, this retrospective study has limitations in that we could not determine whether compliance with RYR or lovastatin was optimal. However, we hypothesized that the compliance rate may be distributed equally in patients who used RYR and patients who used lovastatin. Because this is not a clinical trial, our observational study could not provide real compliance of use of medications (included RYR and lovastatin). Third, knowledge, attitudes, and practices regarding health care, lifestyle (such as smoking and alcohol drinking), and family support were also unavailable in this study. Thus, we could not control for these factors in multiple regression, and we could not perform subgroup or sensitivity analyses. In addition, we admit that the real mechanism of the association between RYR and the risk of liver cirrhosis remains unclear, and we could not prove it in this study. Finally, sociodemographic information, medical conditions, use of medical care, CCI, and medications were considered in our study, which could not cover all potential confounding factors. The possibility of residual confounding could not be excluded.
Conclusion
In conclusion, this study revealed beneficial effects of RYR use on the risk and outcome of liver cirrhosis among patients with hyperlipidemia. However, caution is needed because of the study limitations regarding the causal inference of retrospective cohort studies and patient compliance with medications. Our study did not infer causality or claim the superiority of RYR over lovastatin. We suggest that this study should be followed by a randomized controlled trial to examine the effects of both statins and RYR on reducing LDL-C and triglycerides and preventing cirrhosis caused by metabolic-associated steatotic liver disease, non-alcoholic steatohepatitis, or alcohol.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work. Chuen-Chau Chang and Ta-Liang Chen are co-first authors for this study.
Funding Statement
This study was supported in part by the National Science and Technology Council, Taiwan (NSCT113-2629-B-532-001; NSTC112-2314-B-038-141; MOST111-2320-B-532-001-MY3; MOST110-2314-B-038-108-MY2).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Ginès P, Krag A, Abraldes JG, Solà E, Fabrellas N, Kamath PS. Liver cirrhosis. Lancet. 2021;398(10308):1359–1376. doi: 10.1016/S0140-6736(21)01374-X [DOI] [PubMed] [Google Scholar]
- 2.Sepanlou SG, Safiri S, Bisignano C, GBD. Cirrhosis collaborators. the global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet Gastroenterol Hepatol. 2020;5(3):245–266. doi: 10.1016/S2468-1253(19)30349-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zelber-Sagi S, Salomone F, Yeshua H, et al. Non-high-density lipoprotein cholesterol independently predicts new onset of non-alcoholic fatty liver disease. Liver Int. 2014;34(6):e128–35. doi: 10.1111/liv.12318 [DOI] [PubMed] [Google Scholar]
- 4.Huang H, Guo Y, Liu Z, Zeng Y, Chen Y, Xu C. Remnant cholesterol predicts long-term mortality of patients with metabolic dysfunction-associated fatty liver disease. J Clin Endocrinol Metab. 2022;107(8):e3295–e3303. doi: 10.1210/clinem/dgac283 [DOI] [PubMed] [Google Scholar]
- 5.Huang YH, Chan C, Lee HW, et al. Influence of nonalcoholic fatty liver disease with increased liver enzyme levels on the risk of cirrhosis and hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2023;21(4):960–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Janičko M, Veselíny E, Leško D, Jarčuška P. Serum cholesterol is a significant and independent mortality predictor in liver cirrhosis patients. Ann Hepatol. 2013;12(4):581–587. doi: 10.1016/S1665-2681(19)31342-0 [DOI] [PubMed] [Google Scholar]
- 7.He X, Liu X, Peng S, Han Z, Shen J, Cai M. Association of low high-density lipoprotein cholesterol levels with poor outcomes in hepatitis B-associated decompensated cirrhosis patients. Biomed Res Int. 2021;2021:9927330. doi: 10.1155/2021/9927330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martone AM, Landi F, Petricca L, et al. Prevalence of dyslipidemia and hypercholesterolemia awareness: results from the Lookup 7+ online project. Eur J Public Health. 2022;32(3):402–407. doi: 10.1093/eurpub/ckab224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mangione CM, Barry MJ, Nicholson WK, et al. Statin use for the primary prevention of cardiovascular disease in adults: US preventive services task force recommendation statement. JAMA. 2022;328(8):746–753. doi: 10.1001/jama.2022.13044 [DOI] [PubMed] [Google Scholar]
- 10.Chen TL, Yeh CC, Lin CS, Shih CC, Liao CC. Effects of red yeast rice prescription (LipoCol Forte) on adverse outcomes of surgery. QJM. 2019;112(4):253–259. doi: 10.1093/qjmed/hcy278 [DOI] [PubMed] [Google Scholar]
- 11.Chen TL, Lin CS, Lin JA, et al. Evaluating risk of incident diabetes between patients who used lovastatin and red yeast rice prescriptions (LipoCol Forte): a retrospective cohort study based on a real-world database. Diabetes Metab Syndr Obes. 2020;13:89–98. doi: 10.2147/DMSO.S223833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang CC, Sun MF, Chou YC, et al. Decreased risk of stroke in people using red yeast rice prescriptions (LipoCol Forte®): a total population-based retrospective cohort study. Evid Based Complement Alternat Med. 2022;2022:8160425. doi: 10.1155/2022/8160425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bogsrud MP, Ose L, Langslet G, et al. HypoCol (red yeast rice) lowers plasma cholesterol - A randomized placebo controlled study. Scand Cardiovasc J. 2010;44(4):197–200. doi: 10.3109/14017431003624123 [DOI] [PubMed] [Google Scholar]
- 14.Moriarty PM, Roth EM, Karns A, et al. Effects of Xuezhikang in patients with dyslipidemia: a multicenter, randomized, placebo-controlled study. J Clin Lipidol. 2014;8(6):568–575. doi: 10.1016/j.jacl.2014.09.002 [DOI] [PubMed] [Google Scholar]
- 15.Huang CF, Li TC, Lin CC, Liu CS, Shih HC, Lai MM. Efficacy of Monascus purpureus went rice on lowering lipid ratios in hypercholesterolemic patients. Eur J Cardiovasc Prev Rehabil. 2007;14(3):438–440. doi: 10.1097/HJR.0b013e32801da137 [DOI] [PubMed] [Google Scholar]
- 16.Yang YH, Chen WC, Tsan YT, et al. Statin use and the risk of cirrhosis development in patients with hepatitis C virus infection. J Hepatol. 2015;63(5):1111–1117. doi: 10.1016/j.jhep.2015.07.006 [DOI] [PubMed] [Google Scholar]
- 17.Chiu WC, Shan JC, Yang YH, Chen VC, Chen PC. Statins and the risks of decompensated liver cirrhosis and hepatocellular carcinoma determined in patients with alcohol use disorder. Drug Alcohol Depend. 2021;228:109096. doi: 10.1016/j.drugalcdep.2021.109096 [DOI] [PubMed] [Google Scholar]
- 18.Wu SY, Chen WM, Chiang MF, et al. Protective effects of statins on the incidence of NAFLD-related decompensated cirrhosis in T2DM. Liver Int. 2023;43(10):2232–2244. doi: 10.1111/liv.15656 [DOI] [PubMed] [Google Scholar]
- 19.Huang YW, Lee CL, Yang SS, et al. Statins reduce the risk of cirrhosis and its decompensation in chronic hepatitis B patients: a nationwide cohort study. Am J Gastroenterol. 2016;111(7):976–985. doi: 10.1038/ajg.2016.179 [DOI] [PubMed] [Google Scholar]
- 20.Mohanty A, Tate JP, Garcia-Tsao G. Statins are associated with a decreased risk of decompensation and death in veterans with hepatitis C-related compensated cirrhosis. Gastroenterology. 2016;150(2):430–440. doi: 10.1053/j.gastro.2015.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang FM, Wang YP, Lang HC, et al. Statins decrease the risk of decompensation in hepatitis B virus- and hepatitis C virus-related cirrhosis: a population-based study. Hepatology. 2017;66(3):896–907. doi: 10.1002/hep.29172 [DOI] [PubMed] [Google Scholar]
- 22.Bang UC, Benfield T, Bendtsen F. Reduced risk of decompensation and death associated with use of statins in patients with alcoholic cirrhosis: a nationwide case-cohort study. Aliment Pharmacol Ther. 2017;46(7):673–680. doi: 10.1111/apt.14243 [DOI] [PubMed] [Google Scholar]
- 23.Tavan A, Noroozi S, Zamiri B, et al. Evaluation the effects of red yeast rice in combination with statin on lipid profile and inflammatory indices; a randomized clinical trial. BMC Nutr. 2022;8(1):138. doi: 10.1186/s40795-022-00639-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loubser L, Weider KI, Drake SM. Acute liver injury induced by red yeast rice supplement. BMJ Case Rep. 2019;12(3):e227961. doi: 10.1136/bcr-2018-227961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mueller PS. Symptomatic myopathy due to red yeast rice. Ann Intern Med. 2006;145(6):474–475. doi: 10.7326/0003-4819-145-6-200609190-00021 [DOI] [PubMed] [Google Scholar]
- 26.Becker DJ, Gordon RY, Halbert SC, French B, Morris PB, Rader DJ. Red yeast rice for dyslipidemia in statin-intolerant patients: a randomized trial. Ann Intern Med. 2009;150(12):830–839. doi: 10.7326/0003-4819-150-12-200906160-00006 [DOI] [PubMed] [Google Scholar]
- 27.Banach M, Patti AM, Giglio RV, et al. The role of nutraceuticals in statin intolerant patients. J Am Coll Cardiol. 2018;72(1):96–118. doi: 10.1016/j.jacc.2018.04.040 [DOI] [PubMed] [Google Scholar]
- 28.Wu HY, Lin CS, Yeh CC, et al. Cirrhosis patients’ stroke risks and adverse outcomes: two nationwide studies. Atherosclerosis. 2017;263:29–35. doi: 10.1016/j.atherosclerosis.2017.05.030 [DOI] [PubMed] [Google Scholar]
- 29.Xie Y, He C, Wang W. A potential novel inflammation biomarker for predicting the prognosis of decompensated liver cirrhosis. Ann Med. 2022;54(1):3201–3210. doi: 10.1080/07853890.2022.2142277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tarao K, Miyakawa K, Miyagi Y, et al. Severe inflammation in the background liver cirrhosis correlates with the development of poorly differentiated HCC in HCV-associated liver cirrhosis. Intern Med. 2012;51(18):2495–2501. doi: 10.2169/internalmedicine.51.7744 [DOI] [PubMed] [Google Scholar]
- 31.Mahemuti N, Jing X, Zhang N, et al. Association between systemic immunity-inflammation index and hyperlipidemia: a population-based study from the NHANES (2015-2020). Nutrients. 2023;15(5):1177. doi: 10.3390/nu15051177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fujimoto M, Tsuneyama K, Chen SY, et al. Study of the effects of monacolin k and other constituents of red yeast rice on obesity, insulin-resistance, hyperlipidemia, and nonalcoholic steatohepatitis using a mouse model of metabolic syndrome. Evid Based Complement Alternat Med. 2012;2012:892697. doi: 10.1155/2012/892697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang Y, Li P, Li Z, et al. Red yeast rice dietary intervention reduces oxidative stress-related inflammation and improves intestinal microbiota. Food Funct. 2022;13(12):6583–6595. doi: 10.1039/D1FO03776E [DOI] [PubMed] [Google Scholar]
- 34.Zou J, Yan C, Wan JB. Red yeast rice ameliorates non-alcoholic fatty liver disease through inhibiting lipid synthesis and NF-κB/NLRP3 inflammasome-mediated hepatic inflammation in mice. Chin Med. 2022;17(1):17. doi: 10.1186/s13020-022-00573-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li X, Gao Y, Xu H, Hou J, Gao P. Diabetes mellitus is a significant risk factor for the development of liver cirrhosis in chronic hepatitis C patients. Sci Rep. 2017;7(1):9087. doi: 10.1038/s41598-017-09825-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pang Y, Kartsonaki C, Turnbull I, et al. Diabetes, plasma glucose, and incidence of fatty liver, cirrhosis, and liver cancer: a prospective study of 0.5 million people. Hepatology. 2018;68(4):1308–1318. doi: 10.1002/hep.30083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liao CC, Lin JG, Tsai CC, et al. An investigation of the use of traditional Chinese medicine in stroke patients in Taiwan. Evid Based Complement Alternat Med. 2012;2012:387164. doi: 10.1155/2012/387164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shih CC, Su YC, Liao CC, Lin JG. Patterns of medical pluralism among adults: results from the 2001 national health interview survey in Taiwan. BMC Health Serv Res. 2010;10(1):191. doi: 10.1186/1472-6963-10-191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fogacci F, Banach M, Mikhailidis DP, et al. Safety of red yeast rice supplementation: a systematic review and meta-analysis of randomized controlled trials. Pharmacol Res. 2019;143:1–16. doi: 10.1016/j.phrs.2019.02.028 [DOI] [PubMed] [Google Scholar]
- 40.Roselle H, Ekatan A, Tzeng J, Sapienza M, Kocher J. Symptomatic hepatitis associated with the use of herbal red yeast rice. Ann Intern Med. 2008;149(7):516–517. doi: 10.7326/0003-4819-149-7-200810070-00021 [DOI] [PubMed] [Google Scholar]
- 41.Liu Z, Chen P. A case of erectile dysfunction induced by red yeast rice in lipid-lowering therapy. Phytother Res. 2018;32(5):953–954. doi: 10.1002/ptr.6025 [DOI] [PubMed] [Google Scholar]
- 42.Raschi E, Girardi A, Poluzzi E, et al. Adverse events to food supplements containing red yeast rice: comparative analysis of FAERS and CAERS reporting systems. Drug Saf. 2018;41(8):745–752. doi: 10.1007/s40264-018-0661-3 [DOI] [PubMed] [Google Scholar]
- 43.Nafrialdi N, Hudyono J, Suyatna FD, Setiawati A. Safety and efficacy of NC120 for improving lipid profile: a double blind randomized controlled trial. Acta Med Indones. 2019;51(1):19–25. [PubMed] [Google Scholar]
- 44.Halbert SC, French B, Gordon RY, et al. Tolerability of red yeast rice (2,400 mg twice daily) versus pravastatin (20 mg twice daily) in patients with previous statin intolerance. Am J Cardiol. 2010;105(2):198–204. doi: 10.1016/j.amjcard.2009.08.672 [DOI] [PubMed] [Google Scholar]
- 45.Wu KT, Kuo PL, Su SB, et al. Nonalcoholic fatty liver disease severity is associated with the ratios of total cholesterol and triglycerides to high-density lipoprotein cholesterol. J Clin Lipidol. 2016;10(2):420–5.e1. doi: 10.1016/j.jacl.2015.12.026 [DOI] [PubMed] [Google Scholar]
- 46.Ren XY, Shi D, Ding J, et al. Total cholesterol to high-density lipoprotein cholesterol ratio is a significant predictor of nonalcoholic fatty liver: jinchang cohort study. Lipids Health Dis. 2019;18(1):47. doi: 10.1186/s12944-019-0984-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou YG, Tian N, Xie WN. Total cholesterol to high-density lipoprotein ratio and nonalcoholic fatty liver disease in a population with chronic hepatitis B. World J Hepatol. 2022;14(4):791–801. doi: 10.4254/wjh.v14.i4.791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rodríguez-Hernández H, Simental-Mendía LE. The triglycerides and glucose index is highly associated with non-alcoholic fatty liver disease in overweight and obese women. Ir J Med Sci. 2023;192(6):2741–2746. doi: 10.1007/s11845-023-03335-4 [DOI] [PubMed] [Google Scholar]
- 49.Cheng Y, Zhang Q, Li H, et al. Remnant cholesterol, stronger than triglycerides, is associated with incident non-alcoholic fatty liver disease. Front Endocrinol. 2023;14:1098078. doi: 10.3389/fendo.2023.1098078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang TJ, Lien AS, Chen JL, Lin CH, Yang YS, Yang SH. A randomized clinical efficacy trial of red yeast rice (Monascus pilosus) against hyperlipidemia. Am J Chin Med. 2019;47(2):323–335. doi: 10.1142/S0192415X19500150 [DOI] [PubMed] [Google Scholar]
- 51.Ruscica M, Pavanello C, Gandini S, et al. Nutraceutical approach for the management of cardiovascular risk - A combination containing the probiotic Bifidobacterium longum BB536 and red yeast rice extract: results from a randomized, double-blind, placebo-controlled study. Nutr J. 2019;18(1):13. doi: 10.1186/s12937-019-0438-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cimaglia P, Vieceli Dalla Sega F, Vitali F, et al. Effectiveness of a novel nutraceutical compound containing red yeast rice, polymethoxyflavones and antioxidants in the modulation of cholesterol levels in subjects with hypercholesterolemia and low-moderate cardiovascular risk: the NIRVANA study. Front Physiol. 2019;10:217. doi: 10.3389/fphys.2019.00217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhao SP, Liu L, Cheng YC, Li YL. Effect of xuezhikang, a cholestin extract, on reflecting postprandial triglyceridemia after a high-fat meal in patients with coronary heart disease. Atherosclerosis. 2003;168(2):375–380. doi: 10.1016/S0021-9150(03)00142-4 [DOI] [PubMed] [Google Scholar]
- 54.Xu J, Zhu L, Xie Y, et al. Effects of Xuezhikang versus pravastatin on triglyceride level in patients with T2DM and dyslipidemia: study protocol for a multicenter randomized controlled trial. Curr Vasc Pharmacol. 2023;21(3):211–217. doi: 10.2174/1570161121666230328110215 [DOI] [PMC free article] [PubMed] [Google Scholar]