Abstract
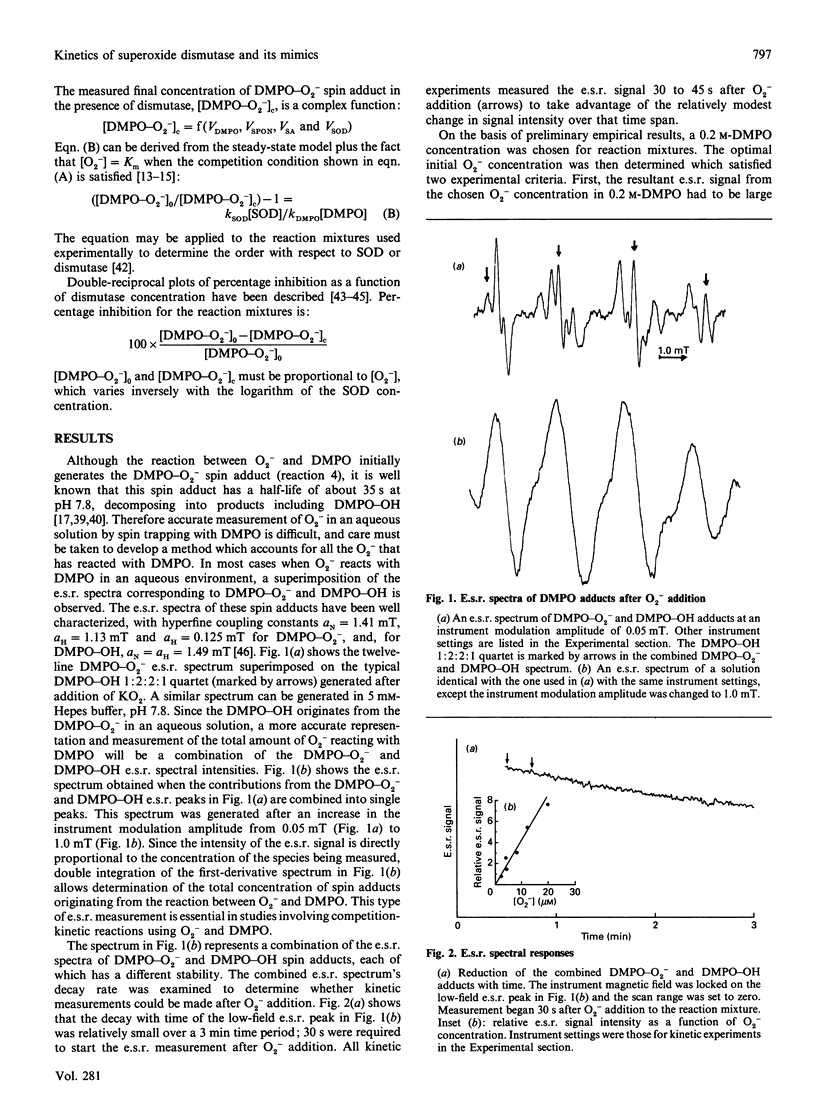
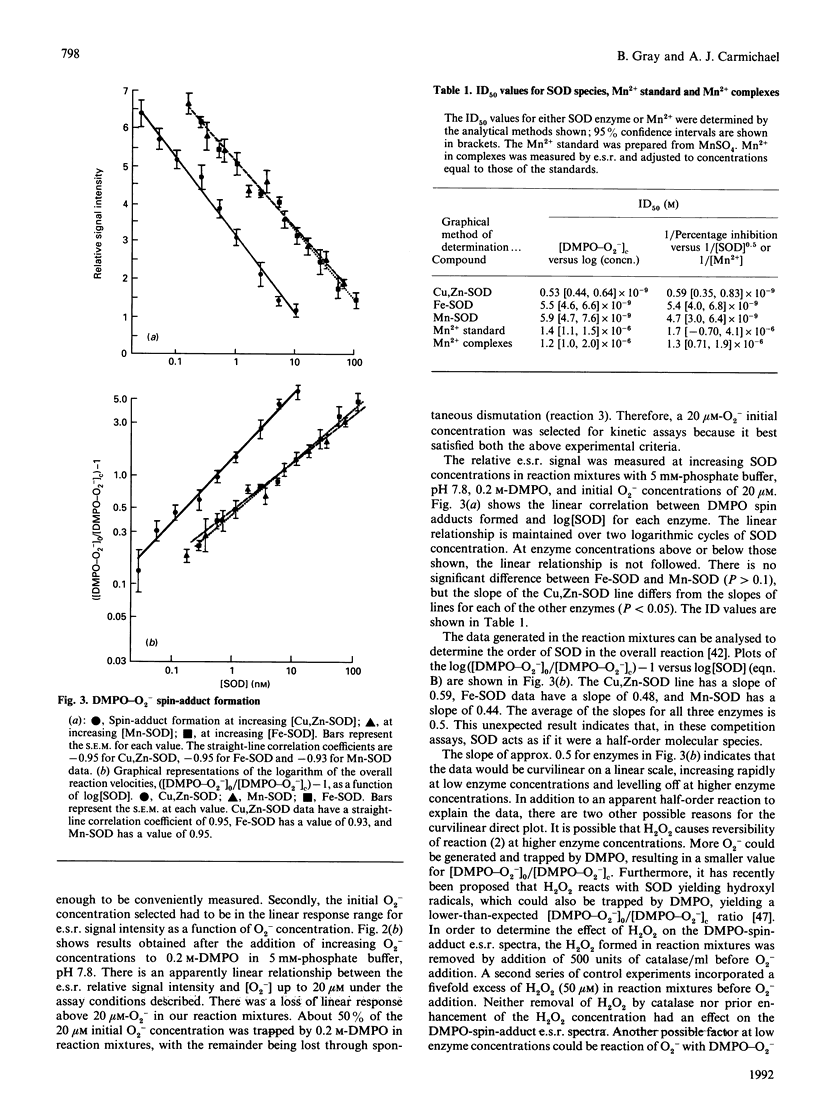
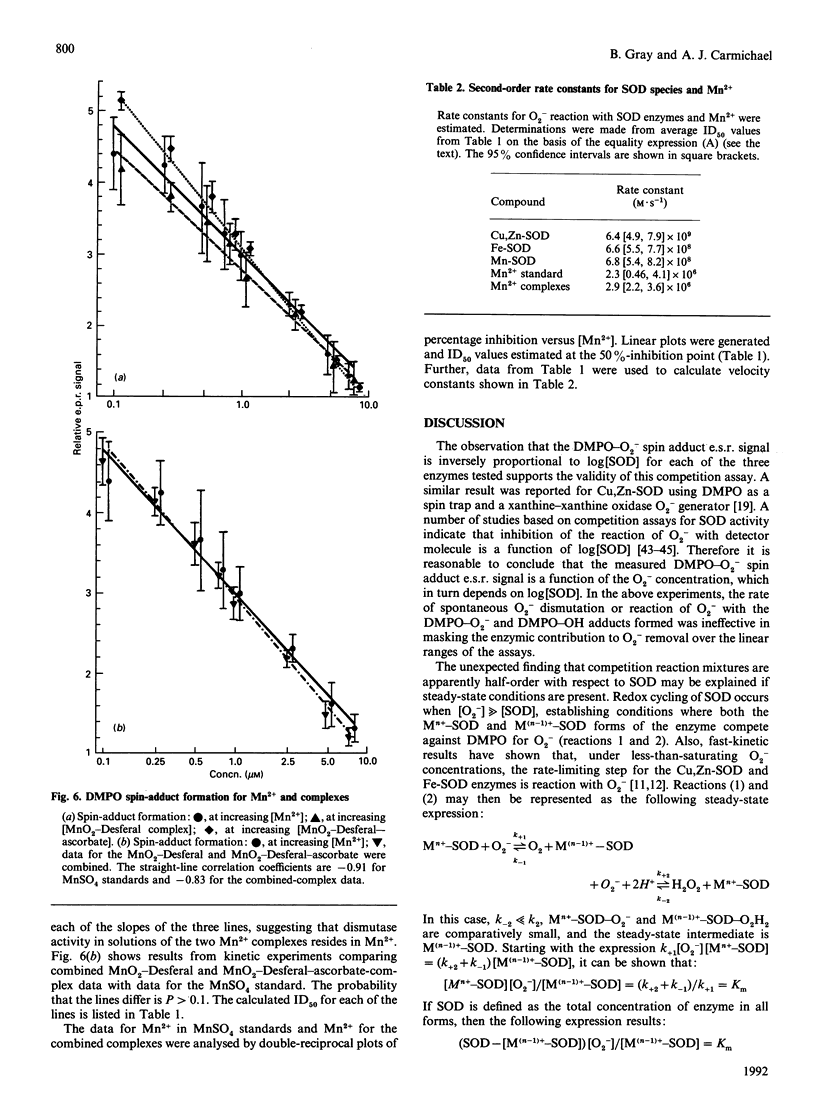
This study presents an e.s.r. assay for superoxide dismutase (SOD). Enzymic reactions were studied in which Cu,Zn-SOD, Mn-SOD and Fe-SOD each competed with the spin trap 5,5-dimethyl-1-pyrroline 1-oxide (DMPO) for superoxide anion (O2-) at pH 7.8 O2- from dissolved KO2 (potassium superoxide) in dimethyl sulphoxide was added directly to the enzyme solutions containing DMPO. The results show that, in this competition reaction system, the kinetics of the reactions between the enzymes and O2- follow a function y = f[( SOD]0.5). The rate constant, kSOD = 6.4 x 10(9) M-1. S-1, determined for Cu,Zn-SOD is approximately an order of magnitude larger than those for Mn-SOD and Fe-SOD. A comparative study of reported SOD mimics, including Mn2+, MnO2-desferrioxamine mesylate (Desferal) and MnO2-Desferal-ascorbate, was done. The results show that solutions of these complexes are approximately three orders of magnitude less active than Cu,Zn-SOD and approximately two orders of magnitude less active than Mn-SOD or Fe-SOD. The results also suggest that the reactivity toward O2- in solutions of these complexes originates from the Mn2+ present and not from the MnO2-Desferal complexes.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archibald F. S., Fridovich I. The scavenging of superoxide radical by manganous complexes: in vitro. Arch Biochem Biophys. 1982 Apr 1;214(2):452–463. doi: 10.1016/0003-9861(82)90049-2. [DOI] [PubMed] [Google Scholar]
- Beyer W. F., Jr, Fridovich I. Characterization of a superoxide dismutase mimic prepared from desferrioxamine and MnO2. Arch Biochem Biophys. 1989 May 15;271(1):149–156. doi: 10.1016/0003-9861(89)90265-8. [DOI] [PubMed] [Google Scholar]
- Briggs G. E., Haldane J. B. A Note on the Kinetics of Enzyme Action. Biochem J. 1925;19(2):338–339. doi: 10.1042/bj0190338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buettner G. R., Oberley L. W. Considerations in the spin trapping of superoxide and hydroxyl radical in aqueous systems using 5,5-dimethyl-1-pyrroline-1-oxide. Biochem Biophys Res Commun. 1978 Jul 14;83(1):69–74. doi: 10.1016/0006-291x(78)90398-4. [DOI] [PubMed] [Google Scholar]
- Carmichael A. J., Samuni A., Riesz P. Photogeneration of superoxide and decarboxylated peptide radicals by carboquone, mitomycin C and streptonigrin. An electron spin resonance and spin trapping study. Photochem Photobiol. 1985 Jun;41(6):635–642. doi: 10.1111/j.1751-1097.1985.tb03616.x. [DOI] [PubMed] [Google Scholar]
- Darr D., Zarilla K. A., Fridovich I. A mimic of superoxide dismutase activity based upon desferrioxamine B and manganese(IV). Arch Biochem Biophys. 1987 Nov 1;258(2):351–355. doi: 10.1016/0003-9861(87)90354-7. [DOI] [PubMed] [Google Scholar]
- Dougherty H. W., Sadowski S. J., Baker E. E. A new iron-containing superoxide dismutase from Escherichia coli. J Biol Chem. 1978 Jul 25;253(14):5220–5223. [PubMed] [Google Scholar]
- Eldred G. E., Hoffert J. R. A test for endogenous interference in superoxide dismutase assays. Anal Biochem. 1981 Jan 1;110(1):137–143. doi: 10.1016/0003-2697(81)90124-x. [DOI] [PubMed] [Google Scholar]
- Fee J. A., Bull C. Steady-state kinetic studies of superoxide dismutases. Saturative behavior of the copper- and zinc-containing protein. J Biol Chem. 1986 Oct 5;261(28):13000–13005. [PubMed] [Google Scholar]
- Finkelstein E., Rosen G. M., Rauckman E. J., Paxton J. Spin trapping of superoxide. Mol Pharmacol. 1979 Sep;16(2):676–685. [PubMed] [Google Scholar]
- Forman H. J., Fridovich I. Superoxide dismutase: a comparison of rate constants. Arch Biochem Biophys. 1973 Sep;158(1):396–400. doi: 10.1016/0003-9861(73)90636-x. [DOI] [PubMed] [Google Scholar]
- Fridovich I. Superoxide dismutases. Adv Enzymol Relat Areas Mol Biol. 1986;58:61–97. doi: 10.1002/9780470123041.ch2. [DOI] [PubMed] [Google Scholar]
- Goldstein S., Michel C., Bors W., Saran M., Czapski G. A critical reevaluation of some assay methods for superoxide dismutase activity. Free Radic Biol Med. 1988;4(5):295–303. doi: 10.1016/0891-5849(88)90050-0. [DOI] [PubMed] [Google Scholar]
- Ilan Y., Ilan Y. A., Czapski G. Do copper ions influence the reduction of ferricytochrome C by O-2? Biochim Biophys Acta. 1978 Aug 8;503(2):399–401. doi: 10.1016/0005-2728(78)90197-4. [DOI] [PubMed] [Google Scholar]
- Keele B. B., Jr, McCord J. M., Fridovich I. Superoxide dismutase from escherichia coli B. A new manganese-containing enzyme. J Biol Chem. 1970 Nov 25;245(22):6176–6181. [PubMed] [Google Scholar]
- Klug D., Rabani J., Fridovich I. A direct demonstration of the catalytic action of superoxide dismutase through the use of pulse radiolysis. J Biol Chem. 1972 Aug 10;247(15):4839–4842. [PubMed] [Google Scholar]
- Kobayashi Y., Okahata S., Tanabe K., Usui T. Use of logit paper in determination of superoxide dismutase activity in human blood cells. J Immunol Methods. 1978;24(1-2):75–78. doi: 10.1016/0022-1759(78)90088-1. [DOI] [PubMed] [Google Scholar]
- Koppenol W. H., Levine F., Hatmaker T. L., Epp J., Rush J. D. Catalysis of superoxide dismutation by manganese aminopolycarboxylate complexes. Arch Biochem Biophys. 1986 Dec;251(2):594–599. doi: 10.1016/0003-9861(86)90368-1. [DOI] [PubMed] [Google Scholar]
- Kubota S., Yang J. T. Bis[cyclo(histidylhistidine)]copper(II) complex that mimicks the active center of superoxide dismutase has its catalytic activity. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3283–3286. doi: 10.1073/pnas.81.11.3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavelle F., McAdam M. E., Fielden E. M., Roberts P. B. A pulse-radiolysis study of the catalytic mechanism of the iron-containing superoxide dismutase from Photobacterium leiognathi. Biochem J. 1977 Jan 1;161(1):3–11. doi: 10.1042/bj1610003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengfelder E., Weser U. Superoxide dismutation by low molecular weight Cu-complexes. Bull Eur Physiopathol Respir. 1981;17 (Suppl):73–80. [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Pick M., Rabani J., Yost F., Fridovich I. The catalytic mechanism of the manganese-containing superoxide dismutase of Escherichia coli studied by pulse radiolysis. J Am Chem Soc. 1974 Nov 13;96(23):7329–7333. doi: 10.1021/ja00830a026. [DOI] [PubMed] [Google Scholar]
- Rabinowitch H. D., Privalle C. T., Fridovich I. Effects of paraquat on the green alga Dunaliella salina: protection by the mimic of superoxide dismutase, Desferal-Mn(IV). Free Radic Biol Med. 1987;3(2):125–131. doi: 10.1016/s0891-5849(87)80007-2. [DOI] [PubMed] [Google Scholar]
- Rabinowitch H. D., Rosen G. M., Fridovich I. A mimic of superoxide dismutase activity protects Chlorella sorokiniana against the toxicity of sulfite. Free Radic Biol Med. 1989;6(1):45–48. doi: 10.1016/0891-5849(89)90158-5. [DOI] [PubMed] [Google Scholar]
- Samuni A., Krishna C. M., Riesz P., Finkelstein E., Russo A. A novel metal-free low molecular weight superoxide dismutase mimic. J Biol Chem. 1988 Dec 5;263(34):17921–17924. [PubMed] [Google Scholar]
- Samuni A., Krishna C. M., Riesz P., Finkelstein E., Russo A. Superoxide reaction with nitroxide spin-adducts. Free Radic Biol Med. 1989;6(2):141–148. doi: 10.1016/0891-5849(89)90111-1. [DOI] [PubMed] [Google Scholar]
- Sun Y., Oberley L. W., Li Y. A simple method for clinical assay of superoxide dismutase. Clin Chem. 1988 Mar;34(3):497–500. [PubMed] [Google Scholar]
- Yamazaki I., Piette L. H., Grover T. A. Kinetic studies on spin trapping of superoxide and hydroxyl radicals generated in NADPH-cytochrome P-450 reductase-paraquat systems. Effect of iron chelates. J Biol Chem. 1990 Jan 15;265(2):652–659. [PubMed] [Google Scholar]
- Yim M. B., Chock P. B., Stadtman E. R. Copper, zinc superoxide dismutase catalyzes hydroxyl radical production from hydrogen peroxide. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5006–5010. doi: 10.1073/pnas.87.13.5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yost F. J., Jr, Fridovich I. An iron-containing superoxide dismutase from Escherichia coli. J Biol Chem. 1973 Jul 25;248(14):4905–4908. [PubMed] [Google Scholar]


