Abstract
Background
Most mental disorders have their onset in adolescence. Preventive interventions during this period are important; however, help-seeking behavior is generally poor in this age group resulting in low treatment rates. Internet interventions are expected to be an effective, low-threshold, and scalable approach to overcome barriers to help-seeking, particularly for individuals experiencing subclinical symptoms. Internet-delivered indicated prevention seems promising as it targets individuals with minimal symptoms of mental disorders who might need care but are not help-seeking yet. Previous indicated prevention-approaches have mainly targeted specific risk-syndromes. However, this contradicts the increasing recognition of emerging psychopathology as a complex system characterized by co-occurrence and rapid shifts of subclinical symptoms cutting across diagnostic categories. Therefore, this study will investigate the efficacy, mediators, moderators, and core symptomatic changes of a transdiagnostic Internet-delivered indicated prevention program (EMPATIA program) for adolescents.
Methods
This randomized controlled trial (RCT) will be conducted in a general population sample (planned n = 152) of adolescents aged 12–18 years with subclinical symptoms but without any current or past mental disorder. Participants will be randomly assigned to the EMPATIA program or a care as usual (CAU) control condition. The 8-week guided EMPATIA program encompasses 8 modules targeting the following transdiagnostic mechanisms: repetitive negative thinking, self-perfectionism, emotion regulation, intolerance of uncertainty, rejection sensitivity, and behavioral avoidance. Participants will be asked to answer online self-report questionnaires at baseline, after 8 weeks, and at 6-, 9-, and 12-month follow-up. Diagnostic telephone interviews will be conducted at baseline and at 12-month follow-up. Additionally, intervention-specific constructs (motivation, alliance, negative effects, satisfaction, adherence) will be assessed during and after the EMPATIA program. The level of self-reported general psychopathology post-intervention is the primary outcome.
Discussion
Results will be discussed considering the potential of Internet interventions as a scalable, low-threshold option for indicated prevention in adolescents experiencing subclinical symptoms. The EMPATIA program introduces a novel Internet prevention program targeting six transdiagnostic mechanisms associated with various mental health outcomes. Thereby, this trial pursues a very timely and important topic because it may contribute to narrow the current care gap for adolescents, to prevent mental health problems and related negative consequences, and to promote mental health in the long-term.
Trial registration
The trial was approved by Swissmedic (Registration Number: 10001035, 08/22/2022) and the Ethics Committee of Bern (Registration Number: 2022-D0036, 08/22/2022). The trial was registered at ClinicalTrials.gov NCT05934019 on 07–03-2023.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13063-024-08241-3.
Keywords: Prevention, Internet intervention, Adolescents, Mental health, Transdiagnostic, Subclinical, Digital, Youth
Administrative information
| Title {1} | Efficacy of a Transdiagnostic Internet Prevention Approach in Adolescents (EMPATIA-Study): Study Protocol of a Randomized Controlled Trial |
| Trial registration {2a and 2b} |
ClinicalTrials.gov NCT05934019. Registered on 07–03-2023 Trial code/protocol number: 10001035 Swissmedic number: 102676067. Registered on 08–22-2022 BASEC number: 2022-D0036. Registered on 08–22-2022 |
| Protocol version {3} | Version 1, 03–29-2024 |
| Funding {4} | This study is funded by the Swiss National Science Foundation (SNSF) as an Eccellenza Grant (Project number: PCEGP1_186913; «Understanding emerging psychopathology in adolescence: Towards a transdiagnostic indicated prevention approach»; Principal Investigator (PI): Prof. Dr. phil. Stefanie Schmidt) |
| Author details {5a} |
Anja Hirsig1, Xenia A. Häfeli.1, Stefanie J. Schmidt1 1Division of Clinical Child and Adolescent Psychology, University of Bern, Bern, Switzerland All authors contributed to the refinement of the study protocol and approved the final manuscript |
| Name and contact information for the trial sponsor {5b} |
Trial Sponsor: University of Bern Contact name: Prof. Dr. Stefanie Schmidt Address: Division of Clinical Child and Adolescent Psychology, Institute of Psychology, Fabrikstrasse 8, 3012 Bern Telephone: + 41 31 684 82 63 Email: stefanie.schmidt@unibe.ch |
| Role of sponsor {5c} | The Sponsor-Investigator (i.e., the PI) is responsible for the study design and will take responsibility for data collection, management, and steps to submit the report for publication. The funder, i.e., the Swiss National Science Foundation (SNSF), had no impact on study design, collection, management, analysis, interpretation of data; writing the report, and the decision to submit the report for publication |
Background and rationale {6a}
Mental disorders are the major health problem experienced by adolescents [1–3]. More than 34% of all lifetime mental disorders have their onset before the age of 14 and over 60% before the age of 25 [4]. They negatively affect educational and occupational attainments, social relationships, and physical health, both in the short and long term [5–8]. Furthermore, they impose a huge burden on family and friends [9].
Notably, not only mental disorders but also subclinical symptoms (i.e., psychopathology before diagnostic criteria are fully met) cause considerable functional impairment [10] and lead to high healthcare costs and reduced economic productivity [11, 12]. Subclinical symptoms are highly prevalent in adolescence [13, 14], associated with neurobiological and cognitive abnormalities [15–18], and enhance the risk of developing a mental disorder later on [19–21]. Therefore, adolescence is currently regarded as one of the most promising periods for preventive interventions [22–24]. This is also reflected by the current agenda of various global health organizations [3, 25] highlighting the promotion of mental health and the prevention of mental disorders in adolescence as a key priority and a global public health challenge.
While universal and targeted prevention should be conceptualized as complementary approaches [22, 26], indicated prevention targeting individuals with minimal signs or symptoms of a mental disorder below the diagnostic threshold [22, 27] seems especially beneficial. Indicated prevention approaches have shown the potential to reduce subclinical symptoms or even prevent the onset of a mental disorder in help-seeking and community samples [28–31] with a relatively good benefit–cost ratio and with high participant satisfaction by allocating resources to those reporting a need for it [26, 32].
Despite the potential for prevention and the huge need for care, adolescents access and use mental health services less frequently than any other age group due to under-detection, a lack of available psychotherapeutic services, and poor help-seeking [24, 33]. Barriers to help-seeking in adolescence include a lack of confidence in mental health professionals, a preference for self-reliance, difficulties with physical and financial access to care, and feelings of embarrassment and stigma [34–37]. This results in both low treatment rates of 38% for any mental disorder in this age group [38] and many adolescents in need of care who nevertheless remain untreated (known as the “care gap”) [24, 39, 40].
Those adolescents who seek help at mental health services often already experience functional impairments and comorbid mental health problems [41, 42]. Given the evidence for risk enrichment for help-seeking samples compared to general population samples [42], it seems important to detect and to intervene as early as possible. Therefore, a prevention approach targeted to those individuals in the general population, who experience subclinical symptoms but who are not necessarily help-seeking yet, seems promising [22, 43–45]. This may also result in more representative samples (e.g., in terms of socio-economic background) than recruitment based on self-selection [46, 47].
One possible solution to overcome help-seeking barriers and to better reach non-help-seeking individuals in need of care is Internet interventions (i.e., evidence-based interventions delivered online). They are easily scalable and offer help at a low threshold and with high confidentiality as well as privacy [2, 39]. Further, Internet interventions meet the preference of adolescents for self-reliance because they can use them at their own pace, at low costs, and independent of time and place [38, 48]. Accordingly, adolescents use the Internet frequently when searching for mental health topics [35, 49] and are intrigued by Internet interventions on mental health promotion [50, 51], especially when subclinical symptoms are present [52].
Internet interventions developed for adults have shown beneficial effects compared to wait-list controls for various mental disorders in clinical as well as general population samples [53–55]. Furthermore, they tend to be as effective as face-to-face interventions [53, 56, 57]. Regarding Internet interventions for adolescents, substantially fewer and methodologically less rigorous randomized controlled trials (RCTs) have been conducted [47, 58]. Recent reviews and meta-analyses on adolescents found that studies widely differ in the included age range, the applied definition of digital or Internet interventions, and the targeted mental health problem. Most of them report small but significant pooled effect sizes in favor of Internet interventions compared to predominantly non-active control groups (i.e., waitlist or placebo) [47, 59, 60]. However, evidence is still mixed as a recent meta-analysis focusing on young people (≤ 18 years) and Internet-delivered (vs. technology-delivered) interventions only found a significant reduction in anxiety and functioning but not in depressive symptoms [59].
Meta-analyses with a specific focus on Internet-delivered prevention reported small significant effects on subclinical symptoms [45, 61–63], which were generally smaller or even insignificant for adolescents compared to adults [62]. Some studies found larger effects indicated than for universal or selective Internet-delivered prevention approaches for adolescents and adults [62, 64]. However, it remains unclear whether such interventions have positive effects not only on subclinical symptoms but also on the onset of a mental disorder [65]. Furthermore, RCTs on Internet-delivered interventions for a broad range of subclinical symptoms are needed as most studies in adolescents so far have focused on subthreshold depression and could not show any significant reductions in anxiety and stress [45].
Indicated prevention approaches focusing on specific risk syndromes, such as depression or psychosis, contradict the increasing recognition of emerging psychopathology as a complex system characterized by rapid shifts of and interactions between subclinical symptoms cutting across diagnostic categories [66–68]. These earliest expressions of psychopathology have high rates of co-occurrence, change from one clinical picture to another, and only gradually differentiate into more distinct mental disorders [67, 69, 70]. Ways to tackle this high rate of co-occurring subclinical symptoms and heterogeneous mental health needs are personalized transdiagnostic interventions [22, 71–73]. Meta-analytic results in adult samples suggest that transdiagnostic Internet interventions targeting co-occurring subclinical symptoms of anxiety and depression can be as effective as diagnosis-specific interventions in improving the primary clinical diagnosis and comorbidity [74–76]. However, less consistent results have been found for such interventions in samples of younger adults, for example university students, often due to difficulties with recruitment [33]. With regard to adolescent samples, transdiagnostic Internet interventions have often focused on psychological mechanisms common to several mental disorders instead of co-occurring, disorder-specific symptoms [77, 78]. Several of such transdiagnostic mechanisms have already been derived theoretically and empirically. Among these are behavioral avoidance [79–82], repetitive negative thinking [83–85], intolerance of uncertainty [86], emotion regulation [87, 88], self-perfectionism [89–91], and rejection sensitivity [92, 93]. Indicated prevention programs in adolescents focusing on these mechanisms have found significant positive effects on subclinical depressive and/or anxiety symptoms in adolescents compared to no and to (active) wait-list control groups when delivered via the Internet [78, 94–98]. However, several limitations need to be mentioned hampering firm conclusions, such as lack of control groups, short follow-up periods, and small sample sizes. Moreover, there is still a lack of Internet-delivered indicated prevention that targets multiple transdiagnostic mechanisms within one program and that aims to improve a broad range of subclinical symptoms beyond anxiety and depression. Another limitation refers to the high dropout rates not specific to transdiagnostic approaches but generally found in Internet interventions for adolescents [99]. Although it seems necessary to better address individual and dynamic needs of participants by personalizing the intervention, less is known if this can be achieved by providing individualized e-guidance [47, 62, 99]. Further, it remains largely unclear what the most important symptoms and transdiagnostic mechanisms are that should be targeted in such a diverse sample of adolescents spanning a large age range and development peculiarities [71, 100].
This is one of the first transdiagnostic Internet-delivered indicated prevention approaches for adolescents focusing on several established transdiagnostic mechanisms and examining its effect on both a broad range of subclinical symptoms typically present in adolescence and on the onset of mental disorders.
Objectives {7}
The overall objective of this study is to develop and evaluate an Internet-delivered indicated prevention program (i.e., EMPATIA program) for adolescents with subclinical symptoms designed to target transdiagnostic mechanisms. The EMPATIA program will be evaluated in a RCT compared to care as usual (CAU). The primary aim is to investigate the efficacy of the EMPATIA program compared to CAU on general psychopathology after the intervention. It is hypothesized that the EMPATIA program will produce significantly larger effects on the primary outcome measure than CAU. The secondary aims are (1) to investigate the effects of the EMPATIA program compared to CAU on general psychopathology at follow-up assessments and on secondary outcomes including service usage, acceptability, and safety after the intervention as well as the level of functioning, subclinical symptoms and onset of mental disorder(s) at all follow-up assessments; (2) to investigate if intervention effects are moderated by adherence, therapeutic alliance, and therapy motivation; (3) to assess if intervention effects are mediated by the targeted transdiagnostic mechanisms; and (4) to evaluate if the intervention leads to larger effects in those subclinical symptoms that are most central in symptom-networks of emerging psychopathology in adolescents [100]. It is hypothesized that the intervention produces significantly larger effects on secondary outcomes than CAU after the intervention and at all follow-up assessment points. Intervention effects are hypothesized to be moderated by adherence, therapeutic alliance, and level of motivation as well as mediated by the targeted transdiagnostic mechanisms. Finally, the intervention is hypothesized to lead to larger effects in the most central symptoms [100].
Trial design {8}
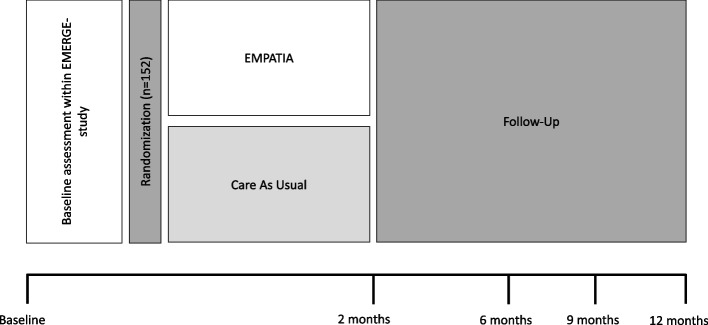
The EMPATIA trial is a randomized, controlled, single-blinded, monocentric, superiority trial with two parallel groups. Participants will be randomly allocated to start the 8-week EMPATIA intervention immediately or to receive access to the intervention after the 12-month follow-up. The trial arms thus are (1) EMPATIA program and (2) CAU (see Fig. 1).
Fig. 1.
Trial design
Methods: participants, interventions, and outcomes
Study setting {9}
This single-center RCT intends to recruit a sample of 152 adolescents from the Swiss general population. All assessments are carried out online or via phone, and the EMPATIA program is delivered via the Internet. The study site is listed at ClinicalTrials.gov (NCT05934019). This study protocol follows the SPIRIT reporting guidelines [101].
Eligibility criteria {10}
The EMPATIA study is part of a larger research project consisting of two studies combining basic and intervention research: (1) a prospective, naturalistic 1-year follow-up study with five assessment points every 3 months to examine the course, pattern, and critical warning signs of subclinical symptoms and transdiagnostic mechanisms in emerging psychopathology (i.e., EMERGE study, [100]) and (2) based thereupon this intervention study (i.e., the EMPATIA study). Both studies are linked to each other in that the follow-up assessment of the EMERGE study corresponds to the baseline assessment of the EMPATIA study (see Fig. 1).
To be included in the EMPATIA study, subjects need to (1) be between 12 and 18 years old at baseline assessment of the EMPATIA study; (2) read and speak German fluently so that they take part in the interview and fill in the questionnaires; (3) have their main residency in a German-speaking canton of Switzerland; (4) have access to the Internet; (5) experience at least mild (subclinical), self-reported symptoms at baseline-assessment as indicated by scoring above a pre-defined cutoff in at least one of the respective screening-instruments (see Additional file 1); and (6) provide informed consent (IC). Exclusion criteria are (1) a current or lifetime diagnosis of a mental disorder according to the Diagnostic Interview for Mental Disorders for Children and Adolescents (Kinder-DIPS [102, 103]) or a known developmental disorder (DSM-5 [104]) at baseline assessment as this study evaluates an indicated prevention program and (2) acute suicidality at baseline-assessment as indicated by a score of “3” on the suicide item of the Patient Health Questionnaire-9 for Adolescents (PHQ-A [105]) and/or by active suicidal plans reported in the Kinder-DIPS [102, 103]. Notably, adolescents with a past and/or present diagnosis of a specific phobia will not be excluded as specific phobias are highly prevalent in adolescence but only a minority of them are clinically relevant and require treatment [106, 107]. Further, specific phobias are currently discussed as a potential marker and predictor of the onset of mental disorder(s), making them a valuable factor in characterizing preventive stages [108].
Who will take informed consent? {26a}
After the last follow-up assessment of the EMERGE study participants will be asked if they agree to be recontacted if they are eligible for the EMPATIA study. If participants agree to be recontacted and if they fulfill all inclusion criteria, they will receive the participant information (for themselves and their parent(s)/legal guardian) and IC via e-mail and postal mail. In addition to the written information, potential participants will be informed about the study orally by a phone call from the research team within 14 days after receiving the written information. This also serves to answer all remaining questions about the study. Afterwards, participants will be asked to provide oral as well as written IC and to return the signed form in a prepaid return envelope. Adolescents aged 14 years or older will provide oral and signed IC by themselves. For adolescents younger than 14 years, a parent/legal guardian will provide oral consent and signed IC while the adolescents will give oral assent.
Additional consent provisions for collection and use of participant data and biological specimens {26b}
By signing the IC for the EMPATIA study, participants agree that their data from the 12-month follow-up assessment of the EMERGE study can be used for the EMPATIA study. Participants give permission to share relevant data with the concerned ethical authorities. This trial does not involve the collection of biological data.
Interventions
Explanation for the choice of comparators {6b}
CAU was chosen as a comparator to the EMPATIA program. Participants of the CAU group will be allowed to use all types of mental health services during their study participation. This comparator was selected as it would be unethical to assign adolescents reporting subclinical symptoms to a waitlist control-condition spanning 12 months with no access to care thereby potentially causing negative long-term effects.
Intervention description {11a}
EMPATIA program
The intervention group will receive the EMPATIA program as the experimental condition. The EMPATIA program is set up on the self-help server of the University of Bern and can be accessed from regular Internet browsers through a secure website and by a personalized link on every laptop, smartphone, or tablet. Each participant has a password-protected account.
To enhance engagement with the EMPATIA program, a co-design approach was applied before and during the development of the program. Single and focus-group interviews have been carried out with young people from the general population to improve understandability as well as usability and to find the best way to implement features known to facilitate participation in Internet interventions, such as personalization and confidentiality [109].
Participants will be guided through the intervention by e-coaches who are trained and supervised master students in Clinical Child and Adolescent Psychology. The e-coaches monitor the progress of the participants and contact them via the chat function in the program at least once a week to provide personalized feedback, encourage further engagement, and answer any questions. E-guidance will be used to monitor progress and to discuss any other issues related to the program.
The EMPATIA program comprises a total of eight sessions (modules) of around 60 min each and one booster module 3 weeks after the last session (refresh module) (see Table 1). Based on previous studies [58, 110], participants are recommended to complete one module every week, to work at their own pace and time availability, to select the modules based on their own preferences, and to make use of additional examples and exercises for everyday life as much as possible. While there is a prototypical sequence, modules are organized in a modular way so that participants can select them independent from each other. To reduce the number of text-based parts, interventions are mainly introduced and practiced through short video-clips, audios, images, and interactive elements.
Table 1.
Content of the EMPATIA program
| Number module | Name module |
Transdiagnostic mechanism(s) | Key aims and interventions |
|---|---|---|---|
| 1 | Introductory module |
• Introduction of the EMPATIA program (aims, characteristics, content, usability) • Identify own challenges related to mental health, top problem assessment • Identify personal values and setting specific goals based on perceived discrepancies between values and current behavior using motivational enhancement techniques • Link personal goals to the EMPATIA program, set SMART goals |
|
| 2 | Worries and confidence about the future l | RNT |
• Introduction to the main characteristics of RNT (including rumination and worrying; additional topic: co-rumination) • Generate a personal RNT model by identifying triggers and consequences of RNT • Reduce RNT by modifying the reaction to triggers • Practice if–then-plans (concrete thinking style, problem-solving, experiences of flow and mindfulness) • Modify biased meta-cognitive beliefs about RNT |
| 3 | Worries and confidence about the future ll | IoU, RNT, BA |
• Introduction to the main characteristics of IoU and its relevance for everyday life • Identify own dysfunctional beliefs about uncertainty • Identify avoidance and safety behaviors when facing uncertainty • Modify dysfunctional beliefs about uncertainty through behavioral experiments • Develop a flexible thinking style by challenging cognitive distortions related to uncertainty |
| 4 | Self-perfectionism and self-confidence | SP, BA |
• Introduction to self-criticism, self-perfectionism, and self-compassion (additional topic: other-oriented and socially prescribed perfectionism) • Identify difficulties associated with being overly perfectionistic • Get to know how self-criticism and perfectionism develop • Develop a flexible thinking style by challenging inflexible standards and cognitive distortions related to perfectionism • Differentiate achievable goals from unachievable goals • Practice exercises to increase self-compassion |
| 5 | Stress and emotions l | ER, BA |
• Get to know the three parts of an emotion (cognitive, behavioral, and physiological) and the functionality of emotions • Identify and change triggers and consequences of maladaptive emotion regulation strategies • Improve emotion regulation skills by applying different relaxation strategies (mindfulness, imagination, acceptance-based exercises) |
| 6 | Stress and emotions ll | ER, BA |
• Improve emotion regulation skills by planning positive activities and enhancing positive emotions (behavioral activation) • Get to know and practice the concept of opposite action and acceptance of emotions • Enhance tolerance to distress associated with intense emotions by emotion-focused experiments • Reduce maladaptive emotional behaviors that reinforce the intensity of emotional distress |
| 7 | Interpersonal relationships | RS, BA |
• Identify own and other’s reactions to social rejection in ambiguous situations • Develop a flexible thinking style by identifying cognitive distortions as well as avoidance behavior and by developing alternative explanations for ambiguous social situations • Challenge own reactions with exposure to situations of rejection using behavioral experiments • Practice strategies to deal with criticism and to give critical feedback • Practice positive communication strategies to enhance self-efficacy • Additional topic: Bullying |
| 8 | Closing module | All mechanisms |
• Review of the program contents • Review of own goals for the EMPATIA program • Identify favorite or most appropriate strategies depending on the context • Practice transfer of strategies to everyday life |
| 9 | Refresh module | Integration |
• Evaluate favorite strategies and identify successful strategies • Optimize existing strategies based on contextual characteristics • Plan the future implementation of strategies by dealing with potential barriers |
RNT Repetitive negative thinking, IoU Intolerance of uncertainty, BA Behavioral avoidance, SP Self-perfectionism, ER Emotion regulation, RS Rejection sensitivity
The modules of the EMPATIA program are dedicated to six transdiagnostic mechanisms. To target these transdiagnostic mechanisms, cognitive-behavioral interventions are applied that have already proven to be effective in adolescents or young adults (repetitive negative thinking [98, 111–114], intolerance of uncertainty [115–118], behavioral avoidance [81, 82, 111, 119], self-perfectionism [120–123], emotion regulation [82, 94, 124], rejection sensitivity [125–127]). Additional information is provided on topics of special interest in adolescence, such as sleep, sexuality, or bullying.
Each module follows the same structure. It begins with a review including a prompt to summarize the last module the respective participant has completed. Furthermore, participants are asked to re-assess the severity of the top personal problems [128] that were identified at the first session so they can perceive their own progress throughout the EMPATIA program. Afterwards, an overview of the respective module is provided including the contents, aims, and the expected total duration. Based thereupon, psychoeducation is used to define the core constructs of each module and their relevance for everyday life. This is also supported by a self-observation exercise. Within the main part of each module, strategies to improve the respective transdiagnostic mechanism are introduced and practiced throughout the module by exercises. Additional examples are provided on how to transfer strategies to everyday life, and participants are asked to choose one or more of these strategies to practice in the upcoming week (“challenge of the week”). At the end of each module, a summary and a short quiz to review and consolidate learning contents is offered.
To better take the diversity and heterogeneity of the participants into account, a broad array of age- and gender-specific topics, examples, and main characters is offered. Personalization is realized by self-evaluation questions at the beginning of every module to clarify the relevance of each topic for the respective participant. Moreover, each participant can individualize one’s own profile. With regard to the content, further personalization can be achieved by choosing the most relevant examples (e.g., dealing with anger or sadness; friendship or romantic relationships) and by saving one’s favorite strategies in an e-journal.
CAU condition
The control group will receive CAU. In line with previous RCTs on Internet interventions [129, 130], participants in the control condition will not receive any intervention or support from the research team. They will receive full access to the EMPATIA program but without the e-guidance after the 12-month follow-up.
Criteria for discontinuing or modifying allocated interventions {11b}
There are no criteria for discontinuing or modifying the allocated intervention due to the intention-to-treat principle and the fact that this intervention is not expected to be associated with any severe harm or severe worsening of mental health problems. All participants are allowed to withdraw their informed consent at any timepoint without reason. Their data collected up to this point will be used for further analysis.
Strategies to improve adherence to interventions {11b}
In the first module of the EMPATIA program (see Table 1), techniques of motivational interviewing are applied to improve adherence to the EMPATIA program. Further, e-guidance is provided for the intervention group to answer open questions regarding the content of the program and/or technical issues, send summaries of achievements and performed activities, and remind participants on a weekly basis if they have not accessed the intervention at all or if they have not started with specific modules. If participants are inactive for three consecutive weeks, the research team will send them a personalized reminder via e-mail.
Relevant concomitant care permitted or prohibited during the trial {11d}
All participants are allowed to make use of any form of concomitant care. As additional service use can affect the efficacy of both groups, it will be assessed at the 12-month follow-up and controlled for in the analysis.
Outcomes {12}
Self-report outcomes will be assessed at five measurement timepoints: before randomization (i.e., baseline), 2 months after randomization (i.e., post-intervention), and 6, 9, and 12 months after randomization (i.e., follow-up). Intervention-specific outcomes are assessed every 2 weeks (see Table 2). Diagnostic interviews will be conducted at baseline and 12-month follow-up. During the baseline telephone interviews, the following data will be assessed: demographic information (gender, age, nationality), native language, and living as well as family situation (current place of residence, number of siblings, number of persons currently living together, and relations to them). The Family Affluence Scale (FAS [131]) is a 6-item questionnaire and used as an indicator for socioeconomic status. The FAS has demonstrated good psychometric properties to detect socioeconomic differences in adolescents in Western European countries [131, 132]. The experience of critical life events will be assessed by the List of Threatening Experiences (LTE [133]).
Table 2.
Participant timeline
| Study periods | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Form | Baselinea | Enrolment | Allocation | Intervention (8 weeks) | Follow-up | |||||||
| Timepoint | − T1 | T0 | T1 | T2 | T3 | T4 | T5 | T6 | T7 | |||
| Time (month, weeks) | - max. 4 (w) | 0 (w) | 0 (w) | 2 (w) | 4 (w) | 6 (w) | 8 (w)/ 2 (m) | 6 (m) | 9 (m) | 12 (m) | ||
| Patient information and informed consent | IC | x | ||||||||||
| Allocation | x | |||||||||||
| Demographics | TI | x | ||||||||||
| In-/exclusion criteria | TI | x | ||||||||||
| Lifetime and current diagnosis | TI | x | x | |||||||||
| Social and role functioning | TI | x | x | |||||||||
| Service use | TI | x | ||||||||||
| Life events | TI | x | x | |||||||||
| General psychopathology | OS | x | x | x | x | x | ||||||
| Suicidality | OS | x | x | x | x | x | x | x | x | x | ||
| Subclinical symptoms | OS | x | x | x | x | x | ||||||
| Transdiagnostic mechanisms | OS | x | x | x | x | x | ||||||
| Optional qualitative interview | TI | x | ||||||||||
| Satisfaction with the intervention | OS | x | ||||||||||
| Adherence | x | |||||||||||
| Negative intervention effects | OS | x | ||||||||||
| Working alliance | OS | x | x | x | x | |||||||
| Therapy motivation | OS | x | x | x | x | x | ||||||
| Adverse events and device deficiencies | OS/TI | x | x | x | x | x | x | x | x | |||
TI Telephone interview, OS Online survey, w Weeks, m Months
aBaseline assessments have been approved and conducted within the EMERGE study [100]
Primary outcome
The primary outcome will be the change in the sum score of self-reported general psychopathology from baseline to post-intervention as assessed by the German version of the Strength and Difficulties Questionnaire, self-report (SDQ-s [134]). The SDQ-s rates 25 items on a 3-point scale from Not true (0) to Certainly true (2). The factorial structure of the five subscales was largely confirmed [135]. The SDQ-s has moderate to good psychometric properties [134].
Secondary outcomes (self-report)
For the assessment timepoints of secondary outcomes, see Table 2. Secondary outcomes will be the change from baseline in sum scores or means of the following assessments measuring subclinical symptoms: (1) the Patient Health Questionnaire-9 for Adolescents (PHQ-A [105]), a 9-item questionnaire assessing the frequency of depressive symptoms over the last 2 weeks on a 4-point Likert scale from Not at all (0) to Nearly every day (3). Item 9 will additionally be used to assess acute suicidality. The PHQ-A has good psychometric properties to detect depression in adolescents [136]; (2) the Generalized Anxiety Disorder Screener (GAD-7 [137]), a 7-item questionnaire assessing general anxiety symptoms on a 4-point Likert scale from Not at all (0) to Nearly every day (3). The GAD-7 produced good psychometric properties in adolescents [138]; (3) the Altman Self-Rating Mania Scale (ASRM [139]), a 5-item questionnaire assessing hypomania/bipolar symptoms by rating different statements that indicate the severity of the respective symptom on a 5-point Likert scale from Not present (0) to Present to severe degree (4). The ASRM revealed good psychometric properties in clinical and healthy adult samples [140]; (4) the Short Obsessive–Compulsive Disorder Screener (SOCS [141]), a 6-item questionnaire assessing obsessive–compulsive symptoms. It is considered a well-established instrument to assess OCD in adolescent clinical samples with comparable psychometric properties to other OCD self-report tools [141]; (5) the Community Assessment of Psychic Experiences—Positive (CAPE-P15 [142]), a 15-item questionnaire measuring psychotic-like experiences on a 4-point Likert scale from Never (1) to Very often (4). The CAPE-P15 has been shown to accurately classify and differentiate psychotic-like experiences in an adolescent general population sample [143]; (6) the Car, Relax, Alone, Forget, Family/Friends, and Trouble scale (CRAFFTd [144]), a 6-item questionnaire screening for problematic substance consumption in adolescents. The CRAFFT-d is a valid screening instrument for problematic alcohol consumption in adolescents [144, 145]; (7) the Child Eating Disorder Examination-Questionnaire (ChEDE-Q8 [146]), an 8-item questionnaire measuring specific eating disorder psychopathology in children and adolescents over the past 28 days. The ChEDE-Q8 is suitable for nonclinical as well as clinical populations and has good psychometric properties [146]; (8) the Strengths and Difficulties Questionnaire, self-report (SDQ-s [134]) with its subscale “conduct disorder” (five items) will be used to assess subclinical conduct problems on a 3-point scale from Not true (0) to Certainly true (2). The SDQ-s has moderate to good psychometric properties [134]; (9) the Somatic Symptom Scale (SSS-8 [147]), an 8-item questionnaire assessing somatic symptoms over the past 7 days on a 5-point scale from Not at all (0) to Very strongly (4). The German version of the SSS-8 has good psychometric properties in adolescents [148]; (10) the Perceived Stress Scale (PSS-4 [149]), a 4-item questionnaire measuring perceived stress on a 5-point scale from Never (1) to Very often (5). The PSS-4 has reliable psychometric properties in online and offline formats [149, 150] and proved to be a useful instrument in the general population in different European countries [151]; (11) the Work and Social Adjustment Scale for Youth Self-Report Version (WSAS-Y [151]), a 5-item questionnaire measuring adjustment to daily life on a 9-point Likert scale from Not at all (0) to Severely impaired (8). The WSAS-Y is a simple, reliable, valid, and sensitive-to-change measure of functional impairment [152]; (12) to assess the presence of non-suicidal self-injurious behaviors the additional module of the Kinder-DIPS will be used [102, 103, 153]. This is based on seven items according to the DSM-5 criteria [103] to examine if and how often participants have applied non-suicidal self-injurious behaviors and which expectations, mental states, and interpersonal difficulties have been associated with it.
Further secondary outcomes include sum scores on the following assessments measuring transdiagnostic mechanisms: (1) the Checklist of Avoidance Strategy Engagement for Adolescents (CEASE-A; adapted from [154]), a 29-item checklist assessing behavioral avoidance on a 5-point Likert scale from Never do to deal with feelings (0) to Always do to deal with feelings (4). The CEASE-A has good psychometric properties in adolescents [155]; (2) the Frost Multi-Dimensional Perfectionism Scale-Brief (F-MPS-B [156]), an 8-item questionnaire measuring perfectionism on a 5-point Likert-type scale from Not at all true (1) to Very true (5). The two subscales (“Evaluative Concerns” and “Striving”) showed strong internal consistency in community and clinical samples [157]. The F-MPS-B was reported to be a promising measurement for adolescents [158]; (3) the Repetitive Thinking Questionnaire (RTQ-10 [84]), a 10-item questionnaire measuring repetitive negative thinking on a 5-point Likert-type scale from Not true at all (1) to Very true (5). The RTQ-10 has been demonstrated to be a reliable and valid transdiagnostic measure of RNT in adolescents [86]; (4) the Difficulties in Emotion Regulation Scale (DERS-16 [159]), a 16-item questionnaire measuring emotion regulation on a 5-point Likert-type scale from Almost never (1) to Almost always (5). The DERS-16 has good psychometric properties in adolescents [159] and has been used in adolescent clinical as well as in nonclinical samples before [160, 161]; (5) the Intolerance of Uncertainty Scale (IUS-12 [162]), a 12-item questionnaire measuring reactions to uncertainty, ambiguous situations, and the future on a 5-point Likert scale from Not at all characteristic of me (1) to Entirely characteristic of me (5). The IUS-12 demonstrated very good psychometric properties [163, 164] which have been replicated in clinical as well as nonclinical samples [164–166]; (6) the Children’s Rejection Sensitivity Questionnaire (C-RSQ [167, 168]) assesses rejection sensitivity through six hypothetical interpersonal situations with ambiguous outcomes. Participants indicate on a 6-point Likert scale whether they would be nervous about the situation, how mad they would be about the situation, and what their outcome expectancy is. The measurement was developed and validated in a sample of fifth to seventh graders [167].
Secondary outcomes (diagnostic interviews)
The Global Functioning Social and Role Scale (GFs/GFr [169]) will be carried out. The two subscales allow for an assessment of the level of functioning (concurrently and highest/lowest in the past year) on a scale ranging from 1 to 10 with 10 representing the highest level. The Kinder-DIPS [102, 103] will be used to assess the presence of current and past mental disorders and the time until the onset of a mental disorder. It is a structured diagnostic interview for adolescents with very good psychometric properties [170]. The Client Socio-Demographic and Service Receipt Inventory-European Version (CSSRI-EU [171, 172]) is a standardized interview to assess the utilization of help and help-seeking behavior. The applied German version has already been used in adolescents [130, 172].
Therapeutic and safety measures (self-report)
Therapeutic and safety measures will be assessed in the intervention group only. These measures include the following: (1) the Motivation for Youth’s Treatment Scale (MYTS [173]), an 8-item questionnaire measuring adolescent’s motivation for therapy on a 5-point Likert scale from Strongly disagree (1) to Strongly agree (5); (2) the Inventory for Assessment of Negative Effects in Psychotherapy (INEP [174, 175]), a 12-item questionnaire assessing negative effects of the intervention. The INEP, which was developed and validated in German and has good psychometric properties [174], was slightly adapted for its use with Internet interventions and for adolescents in previous studies [130]; (3) the ZUF-8 [176] is an 8-item questionnaire for global, unidimensional assessment of patient satisfaction by rating the different statements on a 4-point Likert scale from 1 to 4 (e.g., Very satisfied). The ZUF-8 has good psychometric properties [177] and was slightly adapted for its use with Internet interventions in previous studies [130]; (4) the Working Alliance Inventory for Children and Adolescents (WAI-CA [178]), a 12-item questionnaire measuring therapeutic alliance adapted to Internet interventions [130] with therapeutic support on a 5-point Likert scale from Rarely (1) to Always (5). The Reliable Change Index [179] will be used to assess symptom deterioration according to the primary outcome (i.e., SDQ-s). Deterioration, a possible adverse effect, is defined as the clinically significant increase of baseline scores at any follow-up assessment timepoint. Adherence will be operationalized through the extent to which the Internet intervention is used. The number of finished modules, the number of completed exercises, and the time spent in the online intervention are recorded automatically. As in other studies of Internet interventions, adherence will be calculated with respect to each of these variables and with respect to a composite measure [180].
Participant timeline {13}
Table 2 provides an overview of the participant timeline. Participants will be screened for subclinical symptoms after the 12-month follow-up assessment of the EMERGE study [100] if they have orally agreed to take part in the EMPATIA study. If they screen above a certain pre-defined cutoff score [see Additional file 1], all remaining eligibility criteria will be checked. All eligible participants will receive a personalized written participant information for themselves and their legal guardians via postal mail and e-mail and will give informed consent. After having received the written IC, participants will be randomized according to the procedure described under sequence generation and will be informed about their allocation results via e-mail. The randomization timestamp marks the beginning of study participation. The participants of the intervention group will additionally receive their personalized link to the EMPATIA program. They will be assigned to an e-coach after their first login into the program, which corresponds to the beginning of the e-guidance provided for 8 weeks.
The main assessments for both groups will take place 2 months after randomization and at a follow-up of 6, 9, and 12 months. For every main assessment, participants will receive a personalized link to the online survey via e-mail with up to four reminders every 5 days. A final diagnostic interview will be administered 12 months after randomization. After the last assessment point, all participants will be paid an expense allowance in the form of vouchers for each completed online survey and diagnostic interview. During the intervention, participants of the EMPATIA group will additionally receive a personalized link via e-mail for the intervention-specific assessments at weeks 0, 2, 4, 6, and 8 after randomization with up to three reminders every 2 days. After 8 weeks of intervention, the e-guides will ask their participants if they are willing to answer questions about their satisfaction with the EMPATIA program. If so, they will be contacted via phone for the optional qualitative interview (see Table 2).
Sample size {14}
To be included in this study, participants must report at least mild subclinical symptoms in any one of the assessed mental disorders (see Additional file 1). Based on previous empirical findings on Internet interventions targeting adolescent samples with subclinical symptoms [59] or adolescent and young adult samples with elevated levels of repetitive negative thinking as a transdiagnostic mechanism [98], we expect that the EMPATIA program will produce small effects on general psychopathology after the intervention compared to CAU. To detect at least a small effect size (f = 0.10) for the interaction between time (five assessment points) and group (intervention vs. CAU), an a priori power analysis with G*Power [181] using repeated measures analyses of variance (ANOVA, within-between-interaction), an assumed power of 0.80, an alpha-level of 0.05, and an expected drop-out rate of 20% [98], revealed a required sample size of 152 participants. Based on an expected participation rate of 60% [98], at least 254 participants will have to be contacted who are eligible for the EMPATIA study.
Recruitment {15}
The larger project (SNSF; PCEGP1-186913), in which the EMPATIA study is embedded, consists of two studies. The studies are linked to each other in that the follow-up assessment of the EMERGE study corresponds to the baseline assessment of the EMPATIA study. For the EMERGE study, the Federal Statistical Office will draw a random general population sample of 6900 individuals aged 11 to 17 years. The provided contact details included names, addresses, and—if available—phone numbers of adolescents living in the German-speaking cantons of Switzerland. The sample will be stratified for age, gender, and degree of regionalization (as defined by the Federal Statistical Office: rural area–intermediate area–urban area). All participants who will be eligible at 12-month follow-up constitute the population of potential participants for the EMPATIA study. Thereof, the required 152 participants, now aged 12–18, will be recruited.
Assignment of interventions: allocation
Sequence generation {16a}
Participants who fulfill the inclusion criteria and do not meet the exclusion criteria will be randomized with equal probability to one of both conditions, i.e., the EMPATIA program or CAU. The random allocation will be done by means of a pre-generated computerized randomization table applying block randomization which will be unknown to the research team. The randomization table will be uploaded to the web-based Research Electronic Data Capture (REDCap) [182, 183] by a REDCap administrator from the Clinical Trials Unit. Afterwards, it will become inaccessible for the external researcher who generated the randomization table and remain inaccessible for the research team.
Concealment mechanism {16b}
Randomization will be conducted by the inbuilt randomization module of REDCap [182, 183]. Allocation concealment will be ensured as the allocation code will not be visible to the research team before a participant has been assigned to one of the treatment conditions.
Implementation {16c}
The randomization table providing the basis for the computerized allocation in REDCap [182, 183] will be generated by an external researcher, who will be the only one with access to the original randomization table. Enrolment and the generation of the allocation code will be automatized via REDCap [182, 183] and hence cannot be influenced by any member of the research team.
Assignment of interventions: Blinding
Who will be blinded {17a}
Study participants and e-coaches cannot be blinded regarding the intervention condition as they will be informed about group allocation. Members of the research team who are conducting the diagnostic interviews do not have any other contact with the participants and will thus be blinded regarding group allocation. Participants will be advised to avoid talking about group allocation in the diagnostic interview. At the 12-month follow-up interview, it will be assessed and documented whether participants have unblinded their group allocation in any form.
Procedure for unblinding if needed {17b}
No unexpected unblinding during the trial is to be expected. Therefore, there is no planned procedure for this event.
Data collection and management
Plans for assessment and collection of outcomes {18a}
All self-report assessments will be collected online via the survey platform REDCap [182, 183]. Diagnostic interviews will be conducted via phone and answers will be saved through REDCap. Interviews will be conducted by advanced master and PhD students specialized in Clinical Child and Adolescent Psychology, who will receive a 2-day training on carrying out and documenting the diagnostic interviews. Additionally, they will receive weekly supervision. Only instruments with sufficient reliability and validity as described under outcomes will be used to measure the outcomes of this trial.
Plans to promote participant retention and complete follow-up {18b}
All participants will receive access to the EMPATIA program (immediately vs. after 12 months) and will receive a voucher as a reward. The value of the voucher will be based on how many assessments they finish (with a maximum of 60 Swiss francs). Even if participants skip one assessment, they will be encouraged to complete the next one. At every main assessment, participants will receive up to four reminders via e-mail. Afterwards, they will receive a personalized e-mail once and finally they will be contacted via phone. Additionally, the intervention group will be provided e-guidance to promote motivation during participation in the EMPATIA program and will be reminded with up to three reminders every 2 days for the additional, intervention-specific online surveys.
Data management {19}
Access to the databases is possible only with a valid personal user identification (username and password). Access to the databases is granular to the principle of “need to know”. REDCap [182, 183] will be used for data collection (interviews, online surveys). It provides user-level access control as it is only accessible to authorized personnel. To ensure traceability of relevant processing operations, changes with username, date, time, and modification are logged (audit trail) and can be controlled at any time. The responsibility for hosting the system lies with the Clinical Trials Unit of the University of Bern. The PI and the research team will export data directly from REDCap using the data export tool page which includes advanced data export features to implement data de-identification methods. All involved researchers are thus advised to remove any identifying data before the export. For each data extraction, quality checks are carried out, and the data is adjusted and made securely available in a coded form. Access to the Internet intervention and the data contained on the self-help server of the University of Bern is treated with utmost discretion and is only accessible to authorized persons who need the data to fulfill their tasks within the EMPATIA study. All study personnel must sign a confidentiality agreement, which guarantees the secure handling of all sensitive data.
Confidentiality {27}
All data and personal communications collected in the context of this study are subject to professional discretion and will be treated confidentially. All data collected during the diagnostic interview and the online surveys via REDCap [182, 183] will be coded. Participants will state their real name only in the IC, which will be stored separately from the data provided by the Federal Statistical Office and the data collected via REDCap [182, 183]. Participants will be assigned an unambiguous code and all data collected via REDCap will be directly assigned to this code. The identification of the participants only by the code is not possible. Therefore, they can only be tracked back to the identity of the participant using the case allocation form. The case allocation form will be saved in an encrypted file on the Firewall-protected Network Attached Storage Unit server of the Institute of Psychology of the University of Bern. After the completion of the study, this form will be stored encrypted for at least 10 years. Access to the EMPATIA program is password protected and data transmission via the Internet is encrypted. Further, no names or data that could make the participant identifiable are stored in or by the self-help program. Notably, participants are advised to generate an e-mail address with a pseudonym for the study. Direct access to the source documents will be permitted solely for the purpose of monitoring and inspections. For the analysis of the data, only the data without any personal characteristics will be used. At final analyses, data files will be extracted from the database to be analyzed using statistical packages. The output tables will be stored on the protected server of the University of Bern. The names of the participants will never appear in any reports, talks, or publications.
Statistical methods
Statistical methods for primary and secondary outcomes {20a}
For the primary outcome, statistical analyses will be performed based on the intention-to-treat principle using mixed models for repeated measures (MMRM; main effects for group and time, interaction effects time × group). Age and gender will be included as covariates and will be controlled for in the analyses. Moreover, between-group effect sizes (Cohen’s d) will be calculated to evaluate group differences. The same analyses will be applied to the level of functioning and subclinical symptoms as secondary outcomes. The secondary outcome, time to onset of a mental disorder (treated as a binary variable), will be analyzed by Cox regression analysis with main effects for groups and baseline symptom severity as covariate. Effect sizes for group differences will be quantified using hazard ratios. Longitudinal Structural Equation Modeling (SEM) will be used to test the hypothesized moderation and mediation effects [184]. The significance of the indirect effect for mediation will be tested by calculating bootstrapped, bias-corrected confidence intervals. Symptom-specific efficacy will be investigated using mixed models for repeated measures with core symptoms as the dependent variable. Cohen’s d will be calculated for each comparison separately and effects will be compared between core symptoms and symptoms with low centrality. The analyses will be performed after all participants finished their participation (last participant out).
Methods in analysis to handle protocol non-adherence and any statistical methods to handle missing data {20c}
The pattern of missing data will be investigated. If data are missing at random or completely at random, they will be replaced by multiple imputations. If they are not missing at random full models with full information, maximum likelihood estimator will be carried out.
Plans to give access to the full protocol, participant-level data, and statistical code {31c}
The PI has all rights for all data-related activities. As stated in the data management plan, the copyright and the intellectual property of the generated data will remain with the researcher. Quantitative aggregated data arising from the project will be made openly available for the scientific community in an anonymized form upon request by the authors if no personality rights of the participants are violated.
Oversight and monitoring
Composition of the coordinating center and trial steering committee {5d}
SJS and AH are responsible for the conduct of the study, for preparing the protocol, revisions, investigators brochure, and electronic Case Report Forms as well as for the publication of study reports (including Annual Safety Report (ASR) and Serious Adverse Events (SAE) reporting) and providing support via e-mail to the participants. Several trained and supervised master-level students of Psychology will contribute either as e-coaches or interviewers or will support digital content creation. General administrative and technical support is provided by the Institute of Psychology and the Faculty of Human Sciences of the University of Bern.
Composition of the data monitoring committee, its role, and reporting structure {21a}
Data monitoring will be carried out by an external person in the research management of the Faculty of Human Sciences of the University of Bern. This institution and its staff will not otherwise be involved in the study. One person in the research management will check the electronic Case Report Forms for completeness, the quality of the data, and the correctness of the procedures. The first quality check will be done initially 1 month after the beginning of the data collection and thereafter every third month until the end of data collection.
Adverse event reporting and harms {22}
No negative long-term effects of study participation, i.e., taking part in diagnostic interviews, online surveys, and the EMPATIA program, are expected. Nevertheless, negative effects of the EMPATIA program will be assessed by the INEP [174] every 2 weeks during program participation. If any negative intervention effect is reported by the participants, this will be documented in the electronic Case Report Forms and reported to the PI. Additionally, there is the possibility for participants to leave a comment at the end of every online survey. Regarding adverse events (AEs), subclinical symptoms will be assessed at baseline, post-intervention (2 months), and follow-up after 6, 9, and 12 months after randomization. Symptom deterioration according to the primary outcome (i.e., SDQ-s) will also be assessed as a possible negative effect at baseline, post-intervention, and 6, 9, and 12 months. Suicidality will be screened at baseline, during the intervention (week 2, 4, 6), post-intervention (week 8), and at all follow-up assessments (month 2, 6, 9, and 12). This allows the documentation of AEs and SAEs over a period of 12 months. All AEs and SAEs will be documented in the electronic Case Report Forms. Given that the EMPATIA intervention is a self-help Internet-delivered program, any potential device deficiency (DD) will not affect the safety of the participants. However, the participants can report any DD or malfunction either to their e-coach, in the comment box at the end of each assessment, or via e-mail to the study team or PI directly. In case of unavailability of the web application, audios/videos, modules, or input fields, the participants will not be able to access the software until it is back online. In such a case, an e-mail will be sent to the participants once it is back online. Errors in the web application will be saved automatically and will be reviewed daily to fix them.
All health hazards, AEs, SAEs, and DD will be reported to the PI within 24 h of becoming aware of the event. Any SAE that has a causal relation to the intervention will be reported to the Competent Ethics Committee and to the Competent Authority. Furthermore, an ASR will be submitted to the Competent Ethics Committee and to the Competent Authority. The ASR contains a list of all Serious Adverse Device Effects, DDs, and a report on their degree of seriousness, causal relationship with the intervention and procedure, and participants’ safety.
Frequency and plans for auditing trial conduct {23}
The quality of the data (e.g., data protection control), the correct implementation of the procedures (e.g., contacting participants after completion of the baseline survey), and the quality of the intervention (e.g., answering participants' questions) will be monitored by AH and SJS, who is also responsible for proper training of all involved study personnel. The research management of the Faculty of Human Sciences at the University of Bern will do regular quality checks, especially regarding the electronic Case Report Forms. Also, for quality assurance the Ethics Committee may visit the research site.
Plans for communicating important protocol amendments to relevant parties (e.g., trial participants, ethical committees) {25}
Substantial amendments will only be implemented after approval by the ethical committees. All non-substantial amendments will be communicated to the Competent Ethics Committee and to the Competent Authority together with the ASR. All protocol deviations to protect the rights, safety, and well-being of the participants may proceed without prior approval yet will be communicated to all relevant authorities (including to all participants if necessary) within 2 days.
Dissemination plans {31a}
Results of the study will be published in scientific journals with open access, newspapers, and online articles for all relevant stakeholders (e.g., youth mental health services and institutions, child and youth policies, school services). Any observed gender effects will be published in the final study report. Additionally, results will be published on our website and participants will be sent an e-mail with the final results of the study as soon as the project is finished. All research personnel contributing to the study will be eligible as authors and co-authors on scientific publications. There is no conflict of interest. The final dataset used will be made available as described above.
Discussion
Internet interventions are perceived as a low-threshold, low-cost, high-autonomy, and high-confidentiality option for reaching adolescents with mental health problems who otherwise rarely seek help. Moreover, adolescents with subclinical symptoms seem to prefer Internet interventions over face-to-face interventions [52, 185] making them a promising tool for prevention. Nevertheless, evidence based on previous empirical studies is still inconclusive whether such Internet interventions can reduce subclinical symptoms beyond depression and anxiety and whether they can actually prevent the onset of a mental disorder [59]. Furthermore, while many previous indicated prevention programs have focused on risk syndromes of specific mental disorders in help-seeking individuals, transdiagnostic approaches have the potential to improve a broad range of emerging psychopathology by targeting the underlying mechanisms. This may help to better tackle the diversity and complexity of mental health needs of young people that are often fluctuating across time and diagnostic boundaries [73, 186, 187]. As help-seeking is known to be associated with a self-selection bias, there is an urgent need to offer help at very early stages even before individuals actively seek help while keeping the advantages of targeted prevention [26, 32]. Therefore, the aim of this study is to evaluate the efficacy of an Internet-delivered indicated prevention program targeting several transdiagnostic mechanisms in a general population sample of adolescents with heightened subclinical levels of psychopathology.
Despite the advantages of this study, some potential limitations should be considered. First, there is still no consensus on the most relevant transdiagnostic mechanisms in psychological interventions in adolescents [188]. Therefore, the EMPATIA program targets transdiagnostic mechanisms that have repeatedly been found to be associated with a broad range of mental health problems in longitudinal studies and that can be treated by evidence-based interventions. However, this may have led to other important transdiagnostic mechanisms being neglected (e.g., social, neuropsychological, and physiological factors). Second, while participants are recruited based on screening of a broad range subclinical symptoms, it is still not clear how to best characterize and measure these early stages in non-help-seeking individuals in terms of progression and extension beyond the traditional symptom sets that only distinguish subthreshold from full-threshold disorders [73]. Third, while the EMPATIA program allows for some personalization in terms of content, feedback, and usage, future studies are necessary to investigate what are the most relevant features for personalization depending on factors such as the developmental stage [189, 190].
Taken together, this trial is expected to contribute to a better understanding of the preventative potential of Internet interventions by targeting transdiagnostic mechanisms. Thereby, this trial pursues a very timely and important topic and may contribute to narrowing the current care gap for mental health of adolescents, to prevent mental health problems and associated negative consequences, and to promote mental health in the long-term.
Trial status
Protocol version nr. 3, 10–30-2023. Recruitment for this study began on 06–09-2023 (first participant in: 06–19-2023) and was completed on 03–07-2024. Due to the unexpected additional effort required to obtain both ethical approval from the ethics committee and the competent authority for medical devices as well as the additional challenge and effort to coordinate the recruitment with the EMERGE study, which serves as the baseline assessment, this study protocol could not be submitted earlier. Data collection is expected to be completed on 03–07-2025.
Supplementary Information
Additional file 1. Table of pre-defined cutoffs in subclinical symptoms.
Additional file 2. Ethical approval document.
Additional file 3. Copy of the original funding documentation.
Additional file 4. Model informed consent forms.
Acknowledgements
The authors would like to thank the following persons for their support during the study: Aline Banz, Alina Hunkeler, Irina Lory, Livia Ruckli, Maxine Schmidt, Momo Käser, Nadja Hornburg, Nadja Mauerhofer, Nika Saxer, Pelin Koyuncu, Samira Zurbrügg, Sarah Oberli, Sarah Schneiter, Stella Ludwig, Stéfanie Mayer, Sarah Wüthrich, Taina Thees, Vera Bächler and Wenja Käch (University of Bern) as well as Robinson Aschoff and the research management of the Faculty of Human Sciences (University of Bern). Moreover, we would like to thank Prof. Michel J. Dugas and his team (University of Québec) for sharing the behavioral experiments for Intolerance of Uncertainty, Prof. Shannon Sauer-Zavala and her team (University of Kentucky) for sharing their concept of a brief online intervention.
Abbreviations
- AE
Adverse event
- ASRM
Altman Self-Rating Mania Scale
- ASR
Annual Safety Report
- BA
Behavioral avoidance
- CAU
Care as usual
- CAPE-P15
Community Assessment of Psychic Experiences—Positive
- CEASE-A
Checklist of Avoidance Strategy Engagement for Adolescents
- ChEDE-Q8
Child Eating Disorder Examination-Questionnaire
- CI
Confidence interval
- CRAFFTd
Car, Relax, Alone, Forget, Family/Friends, and Trouble scale
- CSSRI-EU
Client Socio-Demographic and Service Receipt Inventory-European Version
- DERS-16
Difficulties in Emotion Regulation Scale
- DD
Device deficiency
- ER
Emotion regulation
- FAS
Family Affluence Scale
- F-MPS-B
Frost Multi-Dimensional Perfectionism Scale-Brief
- GAD-7
Generalized Anxiety Disorder Screener
- GFs/GFr
Global Functioning Social and Role Scale
- IC
Informed consent
- INEP
Inventory for assessment of negative effects in psychotherapy
- IoU
Intolerance of uncertainty
- IUS-12
Intolerance of Uncertainty Scale
- Kinder-DIPS
Diagnostic Interview for Mental Disorders for Children and Adolescents
- LTE
List of Threatening Experiences
- MYTS
Motivation for Youth’s Treatment Scale
- OS
Online survey
- PSS-4
Perceived Stress Scale
- PHQ-A
Patient Health Questionnaire-9 for Adolescents
- PI
Primary Investigator
- RCT
Randomized controlled trial
- REDCap
Research Electronic Data Capture
- RNT
Repetitive negative thinking
- RS
Rejection sensitivity
- RTQ-10
Repetitive Thinking Questionnaire
- SAE
Serious adverse event
- SDQ-s
Strength and Difficulties Questionnaire, self-report
- SEM
Longitudinal Structural Equation Modeling
- SNSF
Swiss National Science Foundation
- SOCS
Short Obsessive–Compulsive Disorder Screener
- SSS-8
Somatic Symptom Scale
- TI
Telephone interview
- WAI-CA
Working Alliance Inventory for Children and Adolescents
- WSAS-Y
Work and Social Adjustment Scale for Youth Self-Report Version
Authors’ contributions {31b}
Funding for this study was obtained by SJS (PI), who also designed the study. AH (study coordinator) and SJS developed the content of the intervention. AH is responsible for the study coordination, participant recruitment, and data management during the study. She wrote the first draft of the manuscript and critically revised it. XAH is involved in the recruitment process of the participants and contributed substantially to the paper by providing critical revision of the manuscript. All authors critically reviewed the manuscript and approved the final version of the manuscript.
Availability of data and materials {29}
All authors of this paper will have access to the final data set. Anonymized datasets arising from this trial as well as statistical codes will be made available upon request by the authors.
Declarations
Ethics approval and consent to participate {24}
Ethical and professional guidelines will be followed at all times, in line with Good Clinical Practice guidelines. The trial has been approved by the Competent Ethics Committee (Kantonale Ethikkommission Bern, approved 08–22-2022) and the Competent Authority (Swissmedic, approved 08–22-2022). The trial amendment (i.e., if more than 1 month has passed since the last follow-up assessment of the EMERGE study, the follow-up assessment will be repeated as the baseline assessment of the EMPATIA study to re-evaluate the in- and exclusion criteria for the EMPATIA study. No further changes are made to the recruitment method.) was approved by both committees (approved, 10–26-2023). All participants (or their legal guardians when participant is younger than 14 years old) will provide oral and written informed consent before participating in the trial.
Consent for publication {32}
For the study model informed consent forms, see Additional file 4. As we have not included any details, images, or videos relating to an individual person, no consent for publication of the protocol had to be obtained.
Competing interests {28}
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Armocida B, Monasta L, Sawyer S, Bustreo F, Segafredo G, Castelpietra G, et al. Burden of non-communicable diseases among adolescents aged 10–24 years in the EU, 1990–2019: a systematic analysis of the Global Burden of Diseases Study 2019. Lancet Child Adolesc Health. 2022;6(6):367–83. 10.1016/S2352-4642(22)00073-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McGorry PD, Mei C, Chanen A, Hodges C, Alvarez-Jimenez M, Killackey E. Designing and scaling up integrated youth mental health care. World Psychiatry. 2022;1:61–76. 10.1002/wps.20938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.UNICEF, editor. On my mind: promoting, protecting and caring for children’s mental health. New York, NY: UNICEF; 2021.
- 4.Solmi M, Radua J, Olivola M, Croce E, Soardo L, De SalazarPablo G, et al. Age at onset of mental disorders worldwide: large-scale meta-analysis of 192 epidemiological studies. Mol Psychiatry. 2022;27(1):281–95. 10.1038/s41380-021-01161-7. 10.1038/s41380-021-01161-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alonso J, Mortier P, Auerbach RP, Bruffaerts R, Vilagut G, Cuijpers P, et al. Severe role impairment associated with mental disorders: Results of the WHO World Mental Health Surveys International College Student Project. Depress Anxiety. 2018;35(9):802–14. 10.1002/da.22778. 10.1002/da.22778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mojtabai R, Stuart EA, Hwang I, Eaton WW, Sampson N, Kessler RC. Long-term effects of mental disorders on educational attainment in the National Comorbidity Survey ten-year follow-up. Soc Psychiatry Psychiatr Epidemiol. 2015;50(10):1577–91. 10.1007/s00127-015-1083-5. 10.1007/s00127-015-1083-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Obradović J, Hipwell A. Psychopathology and social competence during the transition to adolescence: the role of family adversity and pubertal development. Dev Psychopathol. 2010;22(3):621–34. 10.1017/S0954579410000325. 10.1017/S0954579410000325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Power E, Hughes S, Cotter D, Cannon M. Youth mental health in the time of COVID-19. Ir J Psychol Med. 2020;37(4):301–5. 10.1017/ipm.2020.84. 10.1017/ipm.2020.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dahl RE, Allen NB, Wilbrecht L, Suleiman AB. Importance of investing in adolescence from a developmental science perspective. Nature. 2018;554(7693):441–50. 10.1038/nature25770. 10.1038/nature25770 [DOI] [PubMed] [Google Scholar]
- 10.Roberts RE, Fisher PW, Blake Turner J, Tang M. Estimating the burden of psychiatric disorders in adolescence: the impact of subthreshold disorders. Soc Psychiatry Psychiatr Epidemiol. 2015;50(3):397–406. 10.1007/s00127-014-0972-3. 10.1007/s00127-014-0972-3 [DOI] [PubMed] [Google Scholar]
- 11.Fatori D, Salum G, Itria A, Pan P, Alvarenga P, Rohde LA, et al. The economic impact of subthreshold and clinical childhood mental disorders. J Ment Health. 2018;27(6):588–94. 10.1080/09638237.2018.1466041. 10.1080/09638237.2018.1466041 [DOI] [PubMed] [Google Scholar]
- 12.GBD 2019 Mental Disorders Collaborators. Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry. 2022;9(2):137–50. 10.1016/S2215-0366(21)00395-3. 10.1016/S2215-0366(21)00395-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Polanczyk GV, Salum GA, Sugaya LS, Caye A, Rohde LA. Annual Research Review: a meta-analysis of the worldwide prevalence of mental disorders in children and adolescents. J Child Psychol Psychiatry. 2015;56(3):345–65. 10.1007/s10488-012-0408-x. 10.1007/s10488-012-0408-x [DOI] [PubMed] [Google Scholar]
- 14.Werner-Seidler A, Maston K, Calear A, Batterham P, Larsen M, Torok M, et al. The Future Proofing Study: design, methods and baseline characteristics of a prospective cohort study of the mental health of Australian adolescents. Int J Methods Psychiatr Res. 2022;28(32): e1954. 10.1002/mpr.1954. 10.1002/mpr.1954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Besteher B, Gaser C, Nenadić I. Brain structure and subclinical symptoms: a dimensional perspective of psychopathology in the depression and anxiety spectrum. Neuropsychobiology. 2019;79(4–5):270–83. 10.1159/000501024. 10.1159/000501024 [DOI] [PubMed] [Google Scholar]
- 16.Catalan A, de SalazarPablo G, Aymerich C, Damiani S, Sordi V, Radua J, et al. Neurocognitive functioning in individuals at clinical high risk for psychosis: a systematic review and meta-analysis. JAMA Psychiatry. 2021;78(8):859–67. 10.1001/jamapsychiatry.2021.1290. 10.1001/jamapsychiatry.2021.1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dotson VM, McClintock SM, Verhaeghen P, Kim JU, Draheim AA, Syzmkowicz SM, et al. Depression and cognitive control across the lifespan: a systematic review and meta-analysis. Neuropsychol Rev. 2020;30(4):461–76. 10.1007/s11065-020-09436-6. 10.1007/s11065-020-09436-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang X, Cheng X, Chen J, Zhang B, Wu Q, Deng W, et al. Association of subthreshold manic symptoms and cognitive impairments in euthymic patients with bipolar disorder I. Psychiatry Res. 2019;278:303–8. 10.1016/j.psychres.2019.06.032. 10.1016/j.psychres.2019.06.032 [DOI] [PubMed] [Google Scholar]
- 19.Bertha EA, Balázs J. Subthreshold depression in adolescence: a systematic review. Eur Child Adolesc Psychiatry. 2013;22(10):589–603. 10.1007/s00787-013-0411-0. 10.1007/s00787-013-0411-0 [DOI] [PubMed] [Google Scholar]
- 20.Iorfino F, Scott EM, Carpenter JS, Cross SP, Hermens DF, Killedar M, et al. Clinical stage transitions in persons aged 12 to 25 years presenting to early intervention mental health services with anxiety, mood, and psychotic disorders. JAMA Psychiat. 2019;76(11):1167. 10.1001/jamapsychiatry.2019.2360. 10.1001/jamapsychiatry.2019.2360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mennigen E, Bearden CE. Psychosis risk and development: what do we know from population-based studies? Biol Psychiatry. 2020;88(4):315–25. 10.1016/j.biopsych.2019.12.014. 10.1016/j.biopsych.2019.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fusar-Poli P, Correll CU, Arango C, Berk M, Patel V, Ioannidis JPA. Preventive psychiatry: a blueprint for improving the mental health of young people. World Psychiatry. 2021;20(2):200–21. 10.1002/wps.20869. 10.1002/wps.20869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malla A, Shah J, Iyer S, Boksa P, Joober R, Andersson N, et al. Youth mental health should be a top priority for health care in Canada. Can J Psychiatry. 2018;63(4):216–22. 10.1177/0706743718758968. 10.1177/0706743718758968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patel V, Saxena S, Lund C, Thornicroft G, Baingana F, Bolton P, et al. The Lancet Commission on global mental health and sustainable development. The Lancet. 2018;392(10157):1553–98. 10.1016/S0140-6736(18)31612-X. 10.1016/S0140-6736(18)31612-X [DOI] [PubMed] [Google Scholar]
- 25.WHO. Mental health of adolescents; 2021. Available from: https://www.who.int/news-room/fact-sheets/detail/adolescent-mental-health. Cited 2023 Nov 19.
- 26.Dodge KA. Annual Research Review: Universal and targeted strategies for assigning interventions to achieve population impact. J Child Psychol Psychiatry. 2020;61(3):255–67. 10.1111/jcpp.13141. 10.1111/jcpp.13141 [DOI] [PubMed] [Google Scholar]
- 27.Institute of Medicine (US) Committee on Prevention of Mental Disorders. Reducing risks for mental disorders: frontiers for preventive intervention research. Mrazek PJ, Haggerty RJ, editors. Washington (DC): National Academies Press (US); 1994. Available from: http://www.ncbi.nlm.nih.gov/books/NBK236319/. Cited 2024 Mar 20. [PubMed]
- 28.Cuijpers P, Pineda BS, Quero S, Karyotaki E, Struijs SY, Figueroa CA, et al. Psychological interventions to prevent the onset of depressive disorders: a meta-analysis of randomized controlled trials. Clin Psychol Rev. 2021;83: 101955. 10.1016/j.cpr.2020.101955. 10.1016/j.cpr.2020.101955 [DOI] [PubMed] [Google Scholar]
- 29.Moreno-Peral P, Conejo-Cerón S, Rubio-Valera M, Fernández A, Navas-Campaña D, Rodríguez-Morejón A, et al. Effectiveness of psychological and/or educational interventions in the prevention of anxiety: a systematic review, meta-analysis, and meta-regression. JAMA Psychiat. 2017;74(10):1021–9. 10.1001/jamapsychiatry.2017.2509. 10.1001/jamapsychiatry.2017.2509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmidt SJ, Schultze-Lutter F, Schimmelmann BG, Maric NP, Salokangas RKR, Riecher-Rössler A, et al. EPA guidance on the early intervention in clinical high risk states of psychoses. Eur Psychiatry. 2015;30(3):388–404. 10.1016/j.eurpsy.2015.01.013. 10.1016/j.eurpsy.2015.01.013 [DOI] [PubMed] [Google Scholar]
- 31.Skinner A, Occhipinti JA, Song YJC, Hickie IB. Population-level effectiveness of alternative approaches to preventing mental disorders in adolescents and young adults. Sci Rep. 2023;13(1):19982. 10.1038/s41598-023-47322-2. 10.1038/s41598-023-47322-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rapee RM, Wignall A, Sheffield J, Kowalenko N, Davis A, McLoone J, et al. Adolescents’ reactions to universal and indicated prevention programs for depression: perceived stigma and consumer satisfaction. Prev Sci. 2006;7(2):167–77. 10.1007/s11121-006-0035-4. 10.1007/s11121-006-0035-4 [DOI] [PubMed] [Google Scholar]
- 33.Bolinski F, Kleiboer A, Neijenhuijs K, Karyotaki E, Wiers R, De Koning L, et al. Challenges in recruiting university students for web-based indicated prevention of depression and anxiety: results from a randomized controlled trial (ICare Prevent). J Med Internet Res. 2022;24(12):e40892. 10.2196/40892. 10.2196/40892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aguirre Velasco A, Cruz ISS, Billings J, Jimenez M, Rowe S. What are the barriers, facilitators and interventions targeting help-seeking behaviours for common mental health problems in adolescents? A systematic review. BMC Psychiatry. 2020;20(1):293. 10.1186/s12888-020-02659-0. 10.1186/s12888-020-02659-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ebert DD, Mortier P, Kaehlke F, Bruffaerts R, Baumeister H, Auerbach RP, et al. Barriers of mental health treatment utilization among first-year college students: first cross-national results from the WHO World Mental Health International College Student Initiative. Int J Methods Psychiatr Res. 2019;28(2):e1782. 10.1002/mpr.1782. 10.1002/mpr.1782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Radez J, Tessa Reardon, Cathy Creswell, Peter J. Lawrence, Evdoka-Burton G, Polly Waite. Why do children and adolescents (not) seek and access professional help for their mental health problems? A systematic review of quantitative and qualitative studies. Adolesc Psychiatry. 2021. 10.1007/s00787-019-01469-4 [DOI] [PMC free article] [PubMed]
- 37.Westberg KH, Nyholm M, Nygren JM, Svedberg P. Mental health problems among young people—a scoping review of help-seeking. Int J Environ Res Public Health. 2022;19(3):1430. 10.3390/ijerph19031430. 10.3390/ijerph19031430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang S, Li Q, Lu J, Ran H, Che Y, Fang D, et al. Treatment rates for mental disorders among children and adolescents: a systematic review and meta-analysis. JAMA Netw Open. 2023;6(10):e2338174. 10.1001/jamanetworkopen.2023.38174. 10.1001/jamanetworkopen.2023.38174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kazdin AE. Annual Research Review: Expanding mental health services through novel models of intervention delivery. J Child Psychol Psychiatry. 2019;60(4):455–72. 10.1111/jcpp.12937. 10.1111/jcpp.12937 [DOI] [PubMed] [Google Scholar]
- 40.Kazdin AE. Addressing the treatment gap: expanding the scalability and reach of treatment. J Consult Clin Psychol. 2023;91(1):3–5. 10.1016/j.brat.2016.06.004. 10.1016/j.brat.2016.06.004 [DOI] [PubMed] [Google Scholar]
- 41.Falkenberg I, Valmaggia L, Byrnes M, Frascarelli M, Jones C, Rocchetti M, et al. Why are help-seeking subjects at ultra-high risk for psychosis help-seeking? Psychiatry Res. 2015;228(3):808–15. 10.1016/j.psychres.2015.05.018. 10.1016/j.psychres.2015.05.018 [DOI] [PubMed] [Google Scholar]
- 42.Fusar-Poli P, Rocchetti M, Sardella A, Avila A, Brandizzi M, Caverzasi E, et al. Disorder, not just state of risk: meta-analysis of functioning and quality of life in people at high risk of psychosis. Br J Psychiatry. 2015;207(3):198–206. 10.1192/bjp.bp.114.157115. 10.1192/bjp.bp.114.157115 [DOI] [PubMed] [Google Scholar]
- 43.Ajnakina O, David AS, Murray RM. ‘At risk mental state’ clinics for psychosis – an idea whose time has come – and gone! Psychol Med. 2019;49(4):529–34. 10.1017/S0033291718003859. 10.1017/S0033291718003859 [DOI] [PubMed] [Google Scholar]
- 44.Murray RM, David AS, Ajnakina O. Prevention of psychosis: moving on from the at-risk mental state to universal primary prevention. Psychol Med. 2021;51(2):223–7. 10.1017/S003329172000313X. 10.1017/S003329172000313X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Noh D, Kim H. Effectiveness of online interventions for the universal and selective prevention of mental health problems among adolescents: a systematic review and meta-analysis. Prev Sci. 2023;24(2):353–64. 10.1007/s11121-022-01443-8. 10.1007/s11121-022-01443-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Walder N, Frey AT, Berger T, Schmidt SJ. Digital mental health interventions for prevention and treatment of social anxiety disorder in children, adolescents and young adults. Systematic review and meta-analysis of randomized controlled trials. Under review.
- 47.Lehtimaki S, Martic J, Wahl B, Foster KT, Schwalbe N. Evidence on digital mental health interventions for adolescents and young people: systematic overview. JMIR Ment Health. 2021;8(4):e25847. 10.2196/25847. 10.2196/25847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee YY, Barendregt JJ, Stockings EA, Ferrari AJ, Whiteford HA, Patton GA, et al. The population cost-effectiveness of delivering universal and indicated school-based interventions to prevent the onset of major depression among youth in Australia. Epidemiol Psychiatr Sci. 2017;26(5):545–64. 10.1017/S2045796016000469. 10.1017/S2045796016000469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Freeman JL, Caldwell PHY, Bennett PA, Scott KM. How adolescents search for and appraise online health information: a systematic review. J Pediatr. 2018;195:244-255.e1. 10.1016/j.jpeds.2017.11.031. 10.1016/j.jpeds.2017.11.031 [DOI] [PubMed] [Google Scholar]
- 50.Cohen KA. Traditional and nontraditional mental healthcare services: usage and preferences among adolescents and younger adults. 2021;537–553. 10.1007/s11414-020-09746-w. [DOI] [PMC free article] [PubMed]
- 51.Cross S, Nicholas J, Mangelsdorf S, Valentine L, Baker S, McGorry P, et al. Developing a theory of change for a digital youth mental health service (moderated online social therapy): mixed methods knowledge synthesis study. JMIR Form Res. 2023;7:e49846. 10.2196/49846. 10.2196/49846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marko M, Fogel J, Mykerezi E, Voorhees BWV. Adolescent Internet depression prevention: preferences for intervention and predictors of intentions and adherence. J Cyber Ther Rehabil. 2010;3:9. [PMC free article] [PubMed] [Google Scholar]
- 53.Andersson G, Carlbring P, Titov N, Lindefors N. Internet interventions for adults with anxiety and mood disorders: a narrative umbrella review of recent meta-analyses. Can J Psychiatry. 2019;64(7):465–70. 10.1177/0706743719839381. 10.1177/0706743719839381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Karyotaki E, Efthimiou O, Miguel C, Maas genanntBermpohl F, Furukawa TA, Cuijpers P, et al. Internet-based cognitive behavioral therapy for depression: a systematic review and individual patient data network meta-analysis. JAMA Psychiatry. 2021;78(4):361–71. 10.1001/jamapsychiatry.2020.4364. 10.1001/jamapsychiatry.2020.4364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sander L, Rausch L, Baumeister H. Effectiveness of Internet-based interventions for the prevention of mental disorders: a systematic review and meta-analysis. JMIR Ment Health. 2016;3(3):e38. 10.2196/mental.6061. 10.2196/mental.6061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Carlbring P, Andersson G, Cuijpers P, Riper H, Hedman-Lagerlöf E. Internet-based vs. face-to-face cognitive behavior therapy for psychiatric and somatic disorders: an updated systematic review and meta-analysis. Cogn Behav Ther. 2018;47(1):1–18. 10.1080/16506073.2017.1401115. 10.1080/16506073.2017.1401115 [DOI] [PubMed] [Google Scholar]
- 57.Catarino A, Harper S, Malcolm R, Stainthorpe A, Warren G, Margoum M, et al. Economic evaluation of 27,540 patients with mood and anxiety disorders and the importance of waiting time and clinical effectiveness in mental healthcare. Nat Ment Health. 2023;1(9):667–78. 10.1038/s44220-023-00106-z. 10.1038/s44220-023-00106-z [DOI] [Google Scholar]
- 58.Van Doorn M, Nijhuis LA, Egeler MD, Daams JG, Popma A, Van Amelsvoort T, et al. Online indicated preventive mental health interventions for youth: a scoping review. Front Psychiatry. 2021;12:580843. 10.3389/fpsyt.2021.580843. 10.3389/fpsyt.2021.580843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eilert N, Wogan R, Leen A, Richards D. Internet-delivered interventions for depression and anxiety symptoms in children and young people: systematic review and meta-analysis. JMIR Pediatr Parent. 2022;5(2):e33551. 10.2196/33551. 10.2196/33551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Linardon J, Shatte A, Messer M, Firth J, Fuller-Tyszkiewicz M. E-mental health interventions for the treatment and prevention of eating disorders: an updated systematic review and meta-analysis. J Consult Clin Psychol. 2020;88(11):994–1007. 10.1037/ccp0000575. 10.1037/ccp0000575 [DOI] [PubMed] [Google Scholar]
- 61.Cuijpers P, Pineda BS, Ng MY, Weisz JR, Muñoz RF, Gentili C, et al. A meta-analytic review: psychological treatment of subthreshold depression in children and adolescents. J Am Acad Child Adolesc Psychiatry. 2021;60(9):1072–84. 10.1016/j.jaac.2020.11.024. 10.1016/j.jaac.2020.11.024 [DOI] [PubMed] [Google Scholar]
- 62.Rigabert A, Motrico E, Moreno-Peral P, Resurrección DM, Conejo-Cerón S, Cuijpers P, et al. Effectiveness of online psychological and psychoeducational interventions to prevent depression: systematic review and meta-analysis of randomized controlled trials. Clin Psychol Rev. 2020;82:101931. 10.1016/j.cpr.2020.101931. 10.1016/j.cpr.2020.101931 [DOI] [PubMed] [Google Scholar]
- 63.Torok M, Han J, Baker S, Werner-Seidler A, Wong I, Larsen ME, et al. Suicide prevention using self-guided digital interventions: a systematic review and meta-analysis of randomised controlled trials. Lancet Digit Health. 2020;2(1):e25-36. 10.1016/S2589-7500(19)30199-2. 10.1016/S2589-7500(19)30199-2 [DOI] [PubMed] [Google Scholar]
- 64.Conley CS, Durlak JA, Shapiro JB, Kirsch AC, Zahniser E. A meta-analysis of the impact of universal and indicated preventive technology-delivered interventions for higher education students. Prev Sci. 2016;17(6):659–78. 10.1007/s11121-016-0662-3. 10.1007/s11121-016-0662-3 [DOI] [PubMed] [Google Scholar]
- 65.Cuijpers P, Miguel C, Ciharova M, Ebert D, Harrer M, Karyotaki E. Transdiagnostic treatment of depression and anxiety: a meta-analysis. Psychol Med. 2023;1–12. 10.1017/S0033291722003841. [DOI] [PMC free article] [PubMed]
- 66.Hartmann JA, McGorry PD, Destree L, Amminger GP, Chanen AM, Davey CG, et al. Pluripotential risk and clinical staging: theoretical considerations and preliminary data from a transdiagnostic risk identification approach. Front Psychiatry. 2021;11:553578. 10.3389/fpsyt.2020.553578. 10.3389/fpsyt.2020.553578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McGorry PD, Hartmann JA, Spooner R, Nelson B. Beyond the “at risk mental state” concept: transitioning to transdiagnostic psychiatry. World Psychiatry. 2018;17:133–42. 10.1002/wps.20514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Van Os J. The dynamics of subthreshold psychopathology: implications for diagnosis and treatment. Am J Psychiatry. 2013;170(7):695–8. 10.1176/appi.ajp.2013.13040474. Am Psychiatric Assoc. 10.1176/appi.ajp.2013.13040474 [DOI] [PubMed] [Google Scholar]
- 69.Crouse JJ, Ho N, Scott J, Parker R, Park SH, Couvy-Duchesne B, et al. Dynamic networks of psychological symptoms, impairment, substance use, and social support: The evolution of psychopathology among emerging adults. Eur Psychiatry. 2022;65(1):e32. 10.1192/j.eurpsy.2022.23. 10.1192/j.eurpsy.2022.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Scott J, Crouse JJ, Ho N, Iorfino F, Martin N, Parker R, et al. Early expressions of psychopathology and risk associated with trans-diagnostic transition to mood and psychotic disorders in adolescents and young adults. Guloksuz S, editor. PLOS One. 2021;16(6):e0252550. 10.1371/journal.pone.0252550. 10.1371/journal.pone.0252550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Eaton NR, Bringmann LF, Elmer T, Fried EI, Forbes MK, Greene AL, et al. A review of approaches and models in psychopathology conceptualization research. Nat Rev Psychol. 2023;2(10):622–36. 10.1038/s44159-023-00218-4. 10.1038/s44159-023-00218-4 [DOI] [Google Scholar]
- 72.Ratheesh A, Hammond D, Gao C, Marwaha S, Thompson A, Hartmann J, et al. Empirically driven transdiagnostic stages in the development of mood, anxiety and psychotic symptoms in a cohort of youth followed from birth. Transl Psychiatry. 2023;13(1):103. 10.1038/s41398-023-02396-4. 10.1038/s41398-023-02396-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shah JL, Scott J, McGorry PD, Cross SPM, Keshavan MS, Nelson B, et al. Transdiagnostic clinical staging in youth mental health: a first international consensus statement. World Psychiatry. 2020;19(2):233–42. 10.1002/wps.20745. 10.1002/wps.20745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Newby JM, Twomey C, Yuan Li SS, Andrews G. Transdiagnostic computerised cognitive behavioural therapy for depression and anxiety: a systematic review and meta-analysis. J Affect Disord. 2016;199:30–41. 10.1016/j.jad.2016.03.018. 10.1016/j.jad.2016.03.018 [DOI] [PubMed] [Google Scholar]
- 75.Păsărelu CR, Andersson G, Bergman Nordgren L, Dobrean A. Internet-delivered transdiagnostic and tailored cognitive behavioral therapy for anxiety and depression: a systematic review and meta-analysis of randomized controlled trials. Cogn Behav Ther. 2017;46(1):1–28. 10.1080/16506073.2016.1231219. 10.1080/16506073.2016.1231219 [DOI] [PubMed] [Google Scholar]
- 76.Schaeuffele C, Meine LE, Schulz A, Weber MC, Moser A, Paersch C, et al. A systematic review and meta-analysis of transdiagnostic cognitive behavioural therapies for emotional disorders. Nat Hum Behav. 2024. 10.1038/s41562-023-01787-3. 10.1038/s41562-023-01787-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sauer-Zavala S, Gutner CA, Farchione TJ, Boettcher HT, Bullis JR, Barlow DH. Current definitions of “transdiagnostic” in treatment development: a search for consensus. Behav Ther. 2017;48(1):128–38. 10.1016/j.beth.2016.09.004. 10.1016/j.beth.2016.09.004 [DOI] [PubMed] [Google Scholar]
- 78.Shapiro MO, Allan NP, Raines AM, Schmidt NB. A randomized control trial examining the initial efficacy of an intolerance of uncertainty focused psychoeducation intervention. J Psychopathol Behav Assess. 2022. 10.1007/s10862-022-10002-y. 10.1007/s10862-022-10002-y [DOI] [Google Scholar]
- 79.Gros DF, Saulnier KG, Allan NP. Exploring trajectories in transdiagnostic behavior therapy. J Trauma Stress. 2023;36(4):668–81. 10.1002/jts.22963. 10.1002/jts.22963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Malik K, Ibrahim M, Bernstein A, Venkatesh RK, Rai T, Chorpita B, et al. Behavioral Activation as an ‘active ingredient’ of interventions addressing depression and anxiety among young people: a systematic review and evidence synthesis. BMC Psychol. 2021;9(1):150. 10.1186/s40359-021-00655-x. 10.1186/s40359-021-00655-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Martin F, Oliver T. Behavioral activation for children and adolescents: a systematic review of progress and promise. Eur Child Adolesc Psychiatry. 2019;28(4):427–41. 10.1007/s00787-018-1126-z. 10.1007/s00787-018-1126-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ehrenreich-May J, Kennedy SM, Sherman JA, Bilek EL, Buzzella BA, Bennett SM, et al. Unified protocols for transdiagnostic treatment of emotional disorders in children and adolescents: Therapist guide. New York: Oxford University Press; 2017.
- 83.Bell I, Marx W, Nguyen K, Grace S, Gleeson J, Alvarez-Jimenez M. The effect of psychological treatment on repetitive negative thinking in youth depression and anxiety: a meta-analysis and meta-regression. Psychol Med. 2023;53(1):6–16. 10.1017/S0033291722003373. 10.1017/S0033291722003373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McEvoy PM, Salmon K, Hyett MP, Jose PE, Gutenbrunner C, Bryson K, et al. Repetitive negative thinking as a transdiagnostic predictor of depression and anxiety symptoms in adolescents. Assessment. 2019;26(2):324–35. 10.1177/1073191117693923 [DOI] [PubMed] [Google Scholar]
- 85.McEvoy PM, Hyett MP, Ehring T, Johnson SL, Samtani S, Anderson R, et al. Transdiagnostic assessment of repetitive negative thinking and responses to positive affect: Structure and predictive utility for depression, anxiety, and mania symptoms. J Affect Disord. 2018;232:375–84. 10.1016/j.jad.2018.02.072. 10.1016/j.jad.2018.02.072 [DOI] [PubMed] [Google Scholar]
- 86.McEvoy PM, Hyett MP, Shihata S, Price JE, Strachan L. The impact of methodological and measurement factors on transdiagnostic associations with intolerance of uncertainty: A meta-analysis. Clin Psychol Rev. 2019;73:101778. 10.1016/j.cpr.2019.101778. 10.1016/j.cpr.2019.101778 [DOI] [PubMed] [Google Scholar]
- 87.Hernández-Posadas A, Lommen MJJ, de la Rosa GA, Bouman TK, Mancilla-Díaz JM, del Palacio GA. Transdiagnostic factors in symptoms of depression and post-traumatic stress: a systematic review. Curr Psychol. 2024;43(7):5933–48. 10.1007/s12144-023-04792-x. 10.1007/s12144-023-04792-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Klein RJ, Nguyen ND, Gyorda JA, Jacobson NC. Adolescent emotion regulation and future psychopathology: a prospective transdiagnostic analysis. J Res Adolesc. 2022;32(4):1592–611. 10.1111/jora.12743. 10.1111/jora.12743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Levine SL, Tabri N, Milyavskaya M. Trajectories of depression and anxiety symptoms over time in the transition to university: Their co-occurrence and the role of self-critical perfectionism. Dev Psychopathol. 2023;35(1):345–56. 10.1017/S0954579421000626. 10.1017/S0954579421000626 [DOI] [PubMed] [Google Scholar]
- 90.Williams BM, Levinson CA. A model of self-criticism as a transdiagnostic mechanism of eating disorder comorbidity: a review. New Ideas Psychol. 2022;66:100949. 10.1016/j.newideapsych.2022.100949. 10.1016/j.newideapsych.2022.100949 [DOI] [Google Scholar]
- 91.Lunn J, Greene D, Callaghan T, Egan SJ. Associations between perfectionism and symptoms of anxiety, obsessive-compulsive disorder and depression in young people: a meta-analysis. Cogn Behav Ther. 2023;52(5):460–87. 10.1080/16506073.2023.2211736. 10.1080/16506073.2023.2211736 [DOI] [PubMed] [Google Scholar]
- 92.Foxhall M, Hamilton-Giachritsis C, Button K. The link between rejection sensitivity and borderline personality disorder: a systematic review and meta-analysis. Br J Clin Psychol. 2019;58(3):289–326. 10.1111/bjc.12216. 10.1111/bjc.12216 [DOI] [PubMed] [Google Scholar]
- 93.Gao S, Assink M, Cipriani A, Lin K. Associations between rejection sensitivity and mental health outcomes: a meta-analytic review. Clin Psychol Rev. 2017;57:59–74. 10.1016/j.cpr.2017.08.007. 10.1016/j.cpr.2017.08.007 [DOI] [PubMed] [Google Scholar]
- 94.Garcia-Lopez LJ, Jimenez-Vazquez D, Muela-Martinez JA, Piqueras JA, Espinosa-Fernandez L, Canals-Sans J, et al. Effectiveness of a transdiagnostic indicated preventive intervention for adolescents at high risk for anxiety and depressive disorders. Curr Psychol. 2023. 10.1007/s12144-023-05421-3. 10.1007/s12144-023-05421-3 [DOI] [Google Scholar]
- 95.Sandín B, García-Escalera J, Valiente RM, Espinosa V, Chorot P. Clinical utility of an Internet-delivered version of the Unified Protocol for Transdiagnostic Treatment of Emotional Disorders in Adolescents (iUP-A): a pilot open trial. Int J Environ Res Public Health. 2020;17(22):8306. 10.3390/ijerph17228306. 10.3390/ijerph17228306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schmitt JC, Valiente RM, García-Escalera J, Arnáez S, Espinosa V, Sandín B, et al. Prevention of depression and anxiety in subclinical adolescents: effects of a transdiagnostic Internet-delivered CBT program. Int J Env Res Public Health. 2022;19:5365. 10.3390/ijerph19095365. 10.3390/ijerph19095365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shu CY, Watson HJ, Anderson RA, Wade TD, Kane RT, Egan SJ. A randomized controlled trial of unguided internet cognitive behaviour therapy for perfectionism in adolescents: Impact on risk for eating disorders. Behav Res Ther. 2019;120:103429. 10.1016/j.brat.2019.103429. 10.1016/j.brat.2019.103429 [DOI] [PubMed] [Google Scholar]
- 98.Topper M, Emmelkamp PMG, Watkins E, Ehring T. Prevention of anxiety disorders and depression by targeting excessive worry and rumination in adolescents and young adults: a randomized controlled trial. Behav Res Ther. 2017;90:123–36. 10.1016/j.brat.2016.12.015. 10.1016/j.brat.2016.12.015 [DOI] [PubMed] [Google Scholar]
- 99.Ciharova M, Cuijpers P, Amanvermez Y, Riper H, Klein AM, Bolinski F, et al. Use of tailoring features and reasons for dropout in a guided internet-based transdiagnostic individually-tailored cognitive behavioral therapy for symptoms of depression and/or anxiety in college students. Internet Interv. 2023;34:100646. 10.1016/j.invent.2023.100646. 10.1016/j.invent.2023.100646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Häfeli XA, Hirsig A, Schmidt SJ. Understanding the transdiagnostic mechanisms underlying emerging psychopathology in adolescence: study protocol of a 1-year prospective epidemiological (EMERGE) study. Under Review.
- 101.Chan A-W, Tetzlaff JM, Gøtzsche PC, Altman DG, Mann H, Berlin J, Dickersin K, Hróbjartsson A, Schulz KF, Parulekar WR, Krleža-Jerić K, Laupacis A, Moher D. SPIRIT 2013 Explanation and Elaboration: Guidance for protocols of clinical trials. BMJ. 2013;346:e7586. 10.1136/bmj.e7586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Margraf J, Cwik JC, Pflug V, Schneider S. Strukturierte klinische Interviews zur Erfassung psychischer Störungen über die Lebensspanne. Z Für Klin Psychol Psychother. 2017;46(3):176–86. 10.1026/1616-3443/a000430 [DOI] [Google Scholar]
- 103.Schneider S, Pflug V, Margraf J, In-Albon T. Kinder-DIPS: Open Access: Diagnostisches Interview bei psychischen Störungen im Kindes- und Jugendalter. Bochum: Forschungs- und Behandlungszentrum für psychische Gesundheit, Ruhr-Universität Bochum. 2017. 10.13154/rub.101.90
- 104.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th. 2013. [Google Scholar]
- 105.Johnson JG, Harris ES, Spitzer RL, Williams JBW. The patient health questionnaire for adolescents: validation of an instrument for the assessment of mental disorders among adolescent primary care patients. J Adolesc Health Off Publ Soc Adolesc Med. 2002;30(3):196–204. 10.1016/S1054-139X(01)00333-0. 10.1016/S1054-139X(01)00333-0 [DOI] [PubMed] [Google Scholar]
- 106.Wardenaar KJ, Lim CCW, Al-Hamzawi AO, Alonso J, Andrade LH, Benjet C, et al. The cross-national epidemiology of specific phobia in the World Mental Health Surveys. Psychol Med. 2017;47(10):1744–60. 10.1017/S0033291717000174. 10.1017/S0033291717000174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Benjet C, Borges G, Stein DJ, Méndez E, Medina-Mora ME. Epidemiology of fears and specific phobia in adolescence: results from the Mexican Adolescent Mental Health Survey. J Clin Psychiatry. 2012;73(2):152–8. 10.4088/JCP.11m07442. 10.4088/JCP.11m07442 [DOI] [PubMed] [Google Scholar]
- 108.de Vries YA, Al-Hamzawi A, Alonso J, Borges G, Bruffaerts R, Bunting B, et al. Childhood generalized specific phobia as an early marker of internalizing psychopathology across the lifespan: results from the World Mental Health Surveys. BMC Med. 2019;17(1):101. 10.1186/s12916-019-1328-3. 10.1186/s12916-019-1328-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Liverpool S, Mota CP, Sales CMD, Čuš A, Carletto S, Hancheva C, et al. Engaging children and young people in digital mental health interventions: systematic review of modes of delivery, facilitators, and barriers. J Med Internet Res. 2020;22(6):e16317. 10.2196/16317. 10.2196/16317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Reis S, Matthews EL, Grenyer BFS. Characteristics of effective online interventions: implications for adolescents with personality disorder during a global pandemic. Res Psychother Psychopathol Process Outcome. 2021;23(3):256–78. 10.4081/ripppo.2020.488. 10.4081/ripppo.2020.488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Feldhaus CG, Jacobs RH, Watkins ER, Peters AT, Bessette KL, Langenecker SA. Rumination-focused cognitive behavioral therapy decreases anxiety and increases behavioral activation among remitted adolescents. J Child Fam Stud. 2020;29(7):1982–91. 10.1007/s10826-020-01711-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Teismann T, Ehring T. Pathologisches Grübeln. Göttingen: Hogrefe Verlag GmbH & Company KG; 2019.
- 113.Watkins ER. Rumination-focused cognitive-behavioral therapy for depression. New York: Guilford Press; 2016.
- 114.Newby JM, Williams AD, Andrews G. Reductions in negative repetitive thinking and metacognitive beliefs during transdiagnostic internet cognitive behavioural therapy (iCBT) for mixed anxiety and depression. Behav Res Ther. 2014;59:52–60. 10.1016/j.brat.2014.05.009. 10.1016/j.brat.2014.05.009 [DOI] [PubMed] [Google Scholar]
- 115.Dugas MJ, Sexton KA, Hebert EA, Bouchard S, Gouin JP, Shafran R. Behavioral experiments for intolerance of uncertainty: a randomized clinical trial for adults with generalized anxiety disorder. Behav Ther. 2022;53(6):1147–60. 10.1016/j.beth.2022.05.003. 10.1016/j.beth.2022.05.003 [DOI] [PubMed] [Google Scholar]
- 116.Hebert EA, Dugas MJ. Behavioral experiments for intolerance of uncertainty: challenging the unknown in the treatment of generalized anxiety disorder. Cogn Behav Pract. 2019;26(2):421–36. 10.1016/j.cbpra.2018.07.007. 10.1016/j.cbpra.2018.07.007 [DOI] [Google Scholar]
- 117.Robichaud M. Cognitive behavior therapy targeting intolerance of uncertainty: application to a clinical case of generalized anxiety disorder. Cogn Behav Pract. 2013;20(3):251–63. 10.1016/j.cbpra.2012.09.001. 10.1016/j.cbpra.2012.09.001 [DOI] [Google Scholar]
- 118.Wahlund T, Andersson E, Jolstedt M, Perrin S, Vigerland S, Serlachius E. Intolerance of uncertainty–focused treatment for adolescents with excessive worry: a pilot feasibility study. Cogn Behav Pract. 2020;27(2):215–30. 10.1016/j.cbpra.2019.06.002. 10.1016/j.cbpra.2019.06.002 [DOI] [Google Scholar]
- 119.McCauley E, Schloredt KA, Gudmundsen GR, Martell CR, Dimidjian S. Behavioral activation with adolescents: a clinician’s guide. New York: Guilford Publications; 2016.
- 120.Adams V, Howell J, Egan SJ. Self-compassion as a moderator between clinical perfectionism and psychological distress. Aust Psychol. 2023;58(1):31–40. 10.1080/00050067.2022.2125281. 10.1080/00050067.2022.2125281 [DOI] [Google Scholar]
- 121.Fursland A, Raykos B, Steele A. Perfectionism in perspective. Perth West Aust Cent Clin Interv. 2009.
- 122.Shafran R, Egan S, Wade T. Overcoming Perfectionism: a self-help guide using scientifically supported cognitive behavioural techniques. London: Little Brown Book Group; 2018. [Google Scholar]
- 123.Wade TD, Kay E, de Valle MK, Egan SJ, Andersson G, Carlbring P, et al. Internet-based cognitive behaviour therapy for perfectionism: more is better but no need to be prescriptive. Clin Psychol. 2019;23(3):196–205. 10.1111/cp.12193. 10.1111/cp.12193 [DOI] [Google Scholar]
- 124.Sauer-Zavala S, Tirpak JW, Eustis EH, Woods BK, Russell K. Unified Protocol for the Transdiagnostic Prevention of Emotional Disorders: evaluation of a brief, online course for college freshmen. Behav Ther. 2021;52(1):64–76. 10.1016/j.beth.2020.01.010. 10.1016/j.beth.2020.01.010 [DOI] [PubMed] [Google Scholar]
- 125.Chen R, Zheng J, Li T, Zhang Q, Li C, Cui L. Cognitive bias modification of interpretation training for Chinese undergraduates with depressive symptoms. Curr Psychol. 2022;41(9):6024–37. 10.1007/s12144-020-01094-4. 10.1007/s12144-020-01094-4 [DOI] [Google Scholar]
- 126.Dresbach E, Döpfner M. Gleichaltrigenprobleme im Jugendalter: SELBST–Therapieprogramm für Jugendliche mit Selbstwert-, Leistungs-und Beziehungsstörungen, Band 3. Vol. 3. Göttingen: Hogrefe Verlag GmbH & Company KG; 2020. 10.1026/02234-000.
- 127.LeBeau RT. The rejection sensitivity framework’s promise as a guiding force for the development of sexual and gender minority mental health interventions. Arch Sex Behav. 2020;49(7):2275–9. 10.1007/s10508-019-01613-w. 10.1007/s10508-019-01613-w [DOI] [PubMed] [Google Scholar]
- 128.Weisz JR, Chorpita BF, Frye A, Ng MY, Lau N, Bearman SK, et al. Youth top problems: Using idiographic, consumer-guided assessment to identify treatment needs and to track change during psychotherapy. J Consult Clin Psychol. 2011;79(3):369–80. 10.1037/a0023307. 10.1037/a0023307 [DOI] [PubMed] [Google Scholar]
- 129.Bolinski F, Kleiboer A, Karyotaki E, Bosmans JE, Zarski AC, Weisel KK, et al. Effectiveness of a transdiagnostic individually tailored Internet-based and mobile-supported intervention for the indicated prevention of depression and anxiety (ICare Prevent) in Dutch college students: study protocol for a randomised controlled trial. Trials. 2018;19(1):118. 10.1186/s13063-018-2477-y. 10.1186/s13063-018-2477-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Walder N, Berger T, Schmidt SJ. Prevention and treatment of social anxiety disorder in adolescents: protocol for a randomized controlled trial of the online guided self-help intervention SOPHIE. JMIR Res Protoc. 2023;12:e44346. 10.2196/44346. 10.2196/44346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Torsheim T, Cavallo F, Levin KA, Schnohr C, Mazur J, Niclasen B, et al. Psychometric validation of the revised family affluence scale: a latent variable approach. Child Indic Res. 2016;9(3):771–84. 10.1007/s12187-015-9339-x. 10.1007/s12187-015-9339-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hobza V, Hamrik Z, Bucksch J, De Clercq B. The Family Affluence Scale as an indicator for socioeconomic status: validation on regional income differences in the Czech Republic. Int J Environ Res Public Health. 2017;14(12):1540. 10.3390/ijerph14121540. 10.3390/ijerph14121540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rosmalen JGM, Bos EH, de Jonge P. Validation of the Long-term Difficulties Inventory (LDI) and the List of Threatening Experiences (LTE) as measures of stress in epidemiological population-based cohort studies. Psychol Med. 2012;42(12):2599–608. 10.1017/S0033291712000608. 10.1017/S0033291712000608 [DOI] [PubMed] [Google Scholar]
- 134.Becker A, Wang B, Kunze B, Otto C, Schlack R, Hölling H, et al. Normative data of the self-report version of the German Strengths and Difficulties Questionnaire in an epidemiological setting. Z Für Kinder- Jugendpsychiatrie Psychother. 2018;46(6):523–33. 10.1024/1422-4917/a000589. 10.1024/1422-4917/a000589 [DOI] [PubMed] [Google Scholar]
- 135.Schneider S, Adornetto C. Diagnostisches Vorgehen. In: Schneider S, Margraf J, editors. Lehrbuch der Verhaltenstherapie, Band 3: Psychologische Therapie bei Indikationen im Kindes- und Jugendalter. Berlin: Heidelberg: Springer; 2019. p. 121–43. 10.1007/978-3-662-57369-3_8. [Google Scholar]
- 136.Richardson LP, McCauley E, Grossman DC, McCarty CA, Richards J, Russo JE, et al. Evaluation of the Patient Health Questionnaire-9 Item for detecting major depression among adolescents. Pediatrics. 2010;126(6):1117–23. 10.1542/peds.2010-0852. 10.1542/peds.2010-0852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Löwe B, Decker O, Müller S, Brähler E, Schellberg D, Herzog W, et al. Validation and Standardization of the Generalized Anxiety Disorder Screener (GAD-7) in the General Population. Med Care. 2008;46(3):266–74. 10.1097/MLR.0b013e318160d093. 10.1097/MLR.0b013e318160d093 [DOI] [PubMed] [Google Scholar]
- 138.Tiirikainen K, Haravuori H, Ranta K, Kaltiala-Heino R, Marttunen M. Psychometric properties of the 7-item Generalized Anxiety Disorder Scale (GAD-7) in a large representative sample of Finnish adolescents. Psychiatry Res. 2019;272:30–5. 10.1016/j.psychres.2018.12.004. 10.1016/j.psychres.2018.12.004 [DOI] [PubMed] [Google Scholar]
- 139.Altman E, Hedeker D, Peterson JL, Davis JM. A comparative evaluation of three self-rating scales for acute mania. Biol Psychiatry. 2001;50(6):468–71. 10.1016/S0006-3223(01)01065-4. 10.1016/S0006-3223(01)01065-4 [DOI] [PubMed] [Google Scholar]
- 140.Meyer TD, Crist N, La Rosa N, Ye B, Soares JC, Bauer IE. Are existing self-ratings of acute manic symptoms in adults reliable and valid?—A systematic review. Bipolar Disord. 2020;22(6):558–68. 10.1111/bdi.12906. 10.1111/bdi.12906 [DOI] [PubMed] [Google Scholar]
- 141.Piqueras JA, Rodríguez-Jiménez T, Ortiz AG, Moreno E, Lázaro L, Godoy A. Validation of the Short Obsessive-Compulsive Disorder Screener (SOCS) in children and adolescents. BJPsych Open. 2015;1(1):21–6. 10.1192/bjpo.bp.115.000695. 10.1192/bjpo.bp.115.000695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Capra C, Kavanagh DJ, Hides L, Scott J. Brief screening for psychosis-like experiences. Schizophr Res. 2013;149(1–3):104–7. 10.1016/j.schres.2013.05.020. 10.1016/j.schres.2013.05.020 [DOI] [PubMed] [Google Scholar]
- 143.Núñez D, Godoy MI, Gaete J, Faúndez MJ, Campos S, Fresno A, Spencer R. The Community Assessment of Psychic Experiences-Positive scale (CAPE-P15) accurately classifies and differentiates psychotic experience levels in adolescents from the general population. PLoS One. 2021;16(8):e0256686. 10.1371/journal.pone.0256686. 10.1371/journal.pone.0256686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tossmann P, Kasten L, Lang P, Strüber E. Bestimmung der konkurrenten Validität des CRAFFT-d: Ein Screeninginstrument für problematischen Alkoholkonsum bei Jugendlichen. Z Für Kinder- Jugendpsychiatrie Psychother. 2009;37(5):451–9. 10.1024/1422-4917.37.5.451. 10.1024/1422-4917.37.5.451 [DOI] [PubMed] [Google Scholar]
- 145.Wartberg L, Kriston L, Diestelkamp S, Arnaud N, Thomasius R. Psychometric properties of the German version of the CRAFFT. Addict Behav. 2016;59:42–7. 10.1016/j.addbeh.2016.03.020. 10.1016/j.addbeh.2016.03.020 [DOI] [PubMed] [Google Scholar]
- 146.Kliem S, Schmidt R, Vogel M, Hiemisch A, Kiess W, Hilbert A. An 8-item short form of the Eating Disorder Examination-Questionnaire adapted for children (ChEDE-Q8). Int J Eat Disord. 2017;50(6):679–86. 10.1002/eat.22658. 10.1002/eat.22658 [DOI] [PubMed] [Google Scholar]
- 147.Gierk B, Kohlmann S, Kroenke K, Spangenberg L, Zenger M, Brähler E, et al. The Somatic Symptom Scale–8 (SSS-8): a brief measure of somatic symptom burden. JAMA Intern Med. 2014;174(3):399. 10.1001/jamainternmed.2013.12179. 10.1001/jamainternmed.2013.12179 [DOI] [PubMed] [Google Scholar]
- 148.Kliem S, Krieg Y, Beller J, Brähler E, Baier D. Psychometric properties of the Somatic Symptom Scale 8 (SSS-8) in a representative sample of German adolescents. J Psychosom Res. 2021;149:110593. 10.1016/j.jpsychores.2021.110593. 10.1016/j.jpsychores.2021.110593 [DOI] [PubMed] [Google Scholar]
- 149.Herrero J, Meneses J. Short Web-based versions of the perceived stress (PSS) and Center for Epidemiological Studies-Depression (CESD) Scales: a comparison to pencil and paper responses among Internet users. Comput Hum Behav. 2006;22(5):830–46. 10.1016/j.chb.2004.03.007. 10.1016/j.chb.2004.03.007 [DOI] [Google Scholar]
- 150.Cohen S, Williamson G. Perceived stress in a probability sample of the United States. In: Spacapam S, Oskamp S, editors. The social psychology of health: Claremont Symposium on applied social psychology. Newbury Park: Sage; 1988. [Google Scholar]
- 151.Vallejo MA, Vallejo-Slocker L, Fernández-Abascal EG, Mañanes G. Determining Factors for Stress Perception Assessed with the Perceived Stress Scale (PSS-4) in Spanish and Other European Samples. Front Psychol. 2018;9:39. 10.3389/fpsyg.2018.00037. 10.3389/fpsyg.2018.00037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Jassi A, Lenhard F, Krebs G, Gumpert M, Jolstedt M, Andrén P, et al. The Work and Social Adjustment Scale, Youth and Parent Versions: Psychometric Evaluation of a Brief Measure of Functional Impairment in Young People. Child Psychiatry Hum Dev. 2020;51(3):453–60. 10.1007/s10578-020-00956-z. 10.1007/s10578-020-00956-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.In-Albon T, Kraus L. Nichtsuizidale Selbstverletzungen [Non-suicidal self-injury]. Klinische Psychologie und Psychotherapie des Kindes- und Jugendalters: Universität Koblenz-Landau; 2017. [Google Scholar]
- 154.Kamphuis JH, Telch MJ. Assessment of strategies to manage or avoid perceived threats among panic disorder patients: the Texas Safety Maneuver Scale (TSMS). Clin Psychol Psychother. 1998;5(3):177–86. [DOI] [Google Scholar]
- 155.Kennedy S. Assessing behavioral and emotional avoidance in adolescents: a psychometric validation study. https://scholarship.miami.edu/discovery/fulldisplay/alma991031448067902976/01UOML_INST:ResearchRepository
- 156.Burgess AM, Frost RO, DiBartolo PM. Development and validation of the Frost Multidimensional Perfectionism Scale-Brief. J Psychoeduc Assess. 2016;34(7):620–33. 10.1177/0734282916651359. 10.1177/0734282916651359 [DOI] [Google Scholar]
- 157.Burgess A, Frost RO, Marani C, Gabrielson I. Imperfection, indecision, and hoarding. Curr Psychol. 2018;37(2):445–53. 10.1007/s12144-017-9695-4. 10.1007/s12144-017-9695-4 [DOI] [Google Scholar]
- 158.Leone EM, Wade TD. Measuring perfectionism in children: a systematic review of the mental health literature. Eur Child Adolesc Psychiatry. 2018;27(5):553–67. 10.1007/s00787-017-1078-8. 10.1007/s00787-017-1078-8 [DOI] [PubMed] [Google Scholar]
- 159.Bjureberg J, Ljótsson B, Tull MT, Hedman E, Sahlin H, Lundh LG, et al. Development and validation of a brief version of the Difficulties in Emotion Regulation Scale: The DERS-16. J Psychopathol Behav Assess. 2016;38(2):284–96. 10.1007/s10862-015-9514-x. 10.1007/s10862-015-9514-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Charak R, Byllesby BM, Fowler JC, Sharp C, Elhai JD, Frueh BC. Assessment of the revised Difficulties in Emotion Regulation Scales among adolescents and adults with severe mental illness. Psychiatry Res. 2019;279:278–83. 10.1016/j.psychres.2019.04.010. 10.1016/j.psychres.2019.04.010 [DOI] [PubMed] [Google Scholar]
- 161.Neumann A, van Lier PAC, Gratz KL, Koot HM. Multidimensional assessment of emotion regulation difficulties in adolescents using the Difficulties in Emotion Regulation Scale. Assessment. 2010;17(1):138–49. 10.1177/1073191109349579. 10.1177/1073191109349579 [DOI] [PubMed] [Google Scholar]
- 162.Carleton RN, Mulvogue MK, Thibodeau MA, McCabe RE, Antony MM, Asmundson GJG. Increasingly certain about uncertainty: intolerance of uncertainty across anxiety and depression. J Anxiety Disord. 2012;26(3):468–79. 10.1016/j.janxdis.2012.01.011. 10.1016/j.janxdis.2012.01.011 [DOI] [PubMed] [Google Scholar]
- 163.Carleton RN, Norton MAPJ, Asmundson GJG. Fearing the unknown: a short version of the Intolerance of Uncertainty Scale. J Anxiety Disord. 2007;21(1):105–17. 10.1016/j.janxdis.2006.03.014. 10.1016/j.janxdis.2006.03.014 [DOI] [PubMed] [Google Scholar]
- 164.Carleton RN, Sharpe D, Asmundson GJG. Anxiety sensitivity and intolerance of uncertainty: requisites of the fundamental fears? Behav Res Ther. 2007;45(10):2307–16. 10.1016/j.brat.2007.04.006. 10.1016/j.brat.2007.04.006 [DOI] [PubMed] [Google Scholar]
- 165.McEvoy PM, Mahoney AEJ. Achieving certainty about the structure of intolerance of uncertainty in a treatment-seeking sample with anxiety and depression. J Anxiety Disord. 2011;25(1):112–22. 10.1016/j.janxdis.2010.08.010. 10.1016/j.janxdis.2010.08.010 [DOI] [PubMed] [Google Scholar]
- 166.Khawaja NG, Yu LNH. A comparison of the 27-item and 12-item intolerance of uncertainty scales. Clin Psychol. 2010;14(3):97–106. 10.1080/13284207.2010.502542. 10.1080/13284207.2010.502542 [DOI] [Google Scholar]
- 167.Downey G, Lebolt A, Rincón C, Freitas AL. Rejection Sensitivity and Children’s Interpersonal Difficulties. Child Dev. 1998;69(4):1074–91. 10.1111/j.1467-8624.1998.tb06161.x. 10.1111/j.1467-8624.1998.tb06161.x [DOI] [PubMed] [Google Scholar]
- 168.Downey G, Lebolt A, Rincón C, Freitas AL. Children’s Rejection Sensitivity Questionnaire (CRSQ). 2013. https://socialrelationslab.psychology.columbia.edu/sites/default/files/content/Children-RSQ_6_Item.pdf Accessed 27 Mar 2024.
- 169.Cornblatt BA, Auther AM, Niendam T, Smith CW, Zinberg J, Bearden CE, et al. Preliminary findings for two new measures of social and role functioning in the prodromal phase of schizophrenia. Schizophr Bull. 2007;33(3):688–702. 10.1093/schbul/sbm029. 10.1093/schbul/sbm029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Neuschwander M, In-Albon T, Adornetto C, Roth B, Schneider S. Interrater reliability of the «Diagnostic Interview bei psychischen Störungen im Kindes- und Jugendalter (Kinder-DIPS). Z Kinder Jugendpsychiatr Psychother. 2013;41(5):319–34. 10.1024/1422-4917//a000247. 10.1024/1422-4917//a000247 [DOI] [PubMed] [Google Scholar]
- 171.Chisholm D, Knapp MRJ, Knudsen HC, Amaddeo F, Gaite L, van Wijngaarden B, et al. Client Socio-Demographic and Service Receipt Inventory – European Version: development of an instrument for international research: EPSILON Study 5. Br J Psychiatry. 2000;177(S39):s28-33. 10.1192/bjp.177.39.s28. 10.1192/bjp.177.39.s28 [DOI] [PubMed] [Google Scholar]
- 172.Roick C, Kilian R, Matschinger H, Bernert S, Mory C, Angermeyer MC. Die deutsche version des client sociodemographic and service receipt inventory. Psychiatr Prax. 2001;28(Sup. 2):84–90. 10.1055/s-2001-17790 [DOI] [PubMed] [Google Scholar]
- 173.Breda CS, Riemer M. Motivation for Youth’s Treatment Scale (MYTS): a new tool for measuring motivation among youths and their caregivers. Adm Policy Ment Health Ment Health Serv Res. 2012;39(1–2):118–32. 10.1007/s10488-012-0408-x. 10.1007/s10488-012-0408-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ladwig I, Rief W, Nestoriuc Y. Welche Risiken und Nebenwirkungen hat Psychotherapie? - Entwicklung des Inventars zur Erfassung Negativer Effekte von Psychotherapie (INEP). Verhaltenstherapie. 2014;24(4):252–63. 10.1159/000367928 [DOI] [Google Scholar]
- 175.Bieda A, Pflug V, Scholten S, Lippert M, Ladwig I, Nestoriuc Y, et al. Unerwünschte Nebenwirkungen in der Kinder- und Jugendlichenpsychotherapie – Eine Einführung und Empfehlungen. PPmP - Psychother - Psychosom - Med Psychol. 2018;68(09/10):383–90. 10.1055/s-0044-102291. 10.1055/s-0044-102291 [DOI] [PubMed] [Google Scholar]
- 176.Schmidt J, Lamprecht F, Wittmann W. Satisfaction with inpatient management. Development of a questionnaire and initial validity studies. Psychother Psychosom Med Psychol. 1989;39(7):248–55. [PubMed] [Google Scholar]
- 177.Kriz D, Nübling R, Steffanowski A, Wittmann WW, Schmidt J. Patientenzufriedenheit in der stationären Rehabilitation: Psychometrische Reanalyse des ZUF-8 auf der Basis multizentrischer Stichproben verschiedener Indikation. Z Für Med Psychol. 2008;17(2–3):67–79. [Google Scholar]
- 178.Figueiredo B, Dias P, Lima VS, Lamela D. Working Alliance Inventory for Children and Adolescents (WAI-CA). Eur J Psychol Assess. 2016;35(1):22–8. 10.1027/1015-5759/a000364. 10.1027/1015-5759/a000364 [DOI] [Google Scholar]
- 179.Jacobson NS, Truax P. Clinical significance: a statistical approach to defining meaningful change in psychotherapy research. In: Methodological issues & strategies in clinical research. Washington, DC: American Psychological Association; 1992. p. 631–48. [Google Scholar]
- 180.Beatty L, Binnion C. A systematic review of predictors of, and reasons for, adherence to online psychological interventions. Int J Behav Med. 2016;23(6):776–94. 10.1007/s12529-016-9556-9. 10.1007/s12529-016-9556-9 [DOI] [PubMed] [Google Scholar]
- 181.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175–91. 10.3758/BF03193146. 10.3758/BF03193146 [DOI] [PubMed] [Google Scholar]
- 182.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81. 10.1016/j.jbi.2008.08.010. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform. 2019;95:103208. 10.1016/j.jbi.2019.103208. 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Little TD. Longitudinal structural equation modeling. 2nd ed. New York: Guilford Publications; 2023.
- 185.Ranney ML, Choo EK, Spirito A, Mello MJ. Adolescents’ preference for technology-based emergency department behavioral interventions: does it depend on risky behaviors? Pediatr Emerg Care. 2013;29(4):475. 10.1097/PEC.0b013e31828a322f. 10.1097/PEC.0b013e31828a322f [DOI] [PubMed] [Google Scholar]
- 186.Mei C, Fitzsimons J, Allen N, Alvarez-Jimenez M, Amminger GP, Browne V, et al. Global research priorities for youth mental health. Early Interv Psychiatry. 2020;14(1):3–13. 10.1111/eip.12878. 10.1111/eip.12878 [DOI] [PubMed] [Google Scholar]
- 187.Shah JL, Jones N, van Os J, McGorry PD, Gülöksüz S. Early intervention service systems for youth mental health: integrating pluripotentiality, clinical staging, and transdiagnostic lessons from early psychosis. Lancet Psychiatry. 2022;9(5):413–22. 10.1016/S2215-0366(21)00467-3. 10.1016/S2215-0366(21)00467-3 [DOI] [PubMed] [Google Scholar]
- 188.Taubner S, Ioannou Y, Saliba A, Sales CMD, Volkert J, Protić S, et al. Mediators of outcome in adolescent psychotherapy and their implications for theories and mechanisms of change: a systematic review. Eur Child Adolesc Psychiatry. 2023. 10.1007/s00787-023-02186-9. 10.1007/s00787-023-02186-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Holmes EA, Ghaderi A, Harmer CJ, Ramchandani PG, Cuijpers P, Morrison AP, et al. The Lancet Psychiatry Commission on psychological treatments research in tomorrow’s science. Lancet Psychiatry. 2018;5(3):237–86. 10.1016/S2215-0366(17)30513-8. 10.1016/S2215-0366(17)30513-8 [DOI] [PubMed] [Google Scholar]
- 190.Huibers MJH, Lorenzo-Luaces L, Cuijpers P, Kazantzis N. On the road to personalized psychotherapy: a research agenda based on cognitive behavior therapy for depression. Front Psychiatry. 2021;11:607508. 10.3389/fpsyt.2020.607508/full. 10.3389/fpsyt.2020.607508/full [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Table of pre-defined cutoffs in subclinical symptoms.
Additional file 2. Ethical approval document.
Additional file 3. Copy of the original funding documentation.
Additional file 4. Model informed consent forms.
Data Availability Statement
All authors of this paper will have access to the final data set. Anonymized datasets arising from this trial as well as statistical codes will be made available upon request by the authors.