Abstract
Circular RNAs (circRNAs) are cardinal players in numerous physiological and pathological processes. CircRNAs play dual roles as tumor suppressors and oncogenes in different oncological contexts, including hepatocellular carcinoma (HCC). Their roles significantly impact the disease at all stages, including initiation, development, progression, invasion, and metastasis, in addition to the response to treatment. In this review, we discuss the biogenesis and regulatory functional roles of circRNAs, as well as circRNA–protein–mRNA ternary complex formation, elucidating the intricate pathways tuned by circRNAs to modulate gene expression and cellular processes through a comprehensive literature search, in silico search, and bioinformatics analysis. With a particular focus on the interplay between circRNAs, epigenetics, and HCC pathology, the article sets the stage for further exploration of circRNAs as novel investigational theranostic agents in the dynamic realm of HCC.
Keywords: circRNAs, HCC, initiation, progression, metastasis, epigenetics, theranostics, ncRNA, in silico, bioinformatics
1. Introduction
Circular RNAs (circRNAs) have recently been in the spotlight, yet their origins date back over four decades [1,2]. The exploration of circRNAs goes back to observations in murine retroviruses and plant pathogenic viruses, namely viroids. In 1979, the circular structure of circRNAs was confirmed through the electron microscope analysis of eukaryotic cells. Subsequently, in 1986, circRNAs were identified in the hepatitis delta virus, marking their initial detection in humans [1,3]. These early breakthroughs established the basis for elaborating on the diverse functions of circRNAs in biological processes [4,5,6,7,8].
2. CircRNA Nomenclature
The dynamic landscape of circRNA research and the lack of standardized nomenclature pose a major challenge [4,9,10]. Existing databases such as circBase 0.1 http://www.circbase.org/ [11] (accessed on 17 May 2023) use arbitrary numbering and have limited knowledge of the host gene and chromosomal location of some circRNAs. To solve this problem, a circRNA nomenclature system was developed based on the host gene and precise start/end positions within the hosting gene. This innovative approach was implemented in the newly developed Circbank Database, http://www.circbank.cn/ (accessed on 17 May 2023) which contains 140,790 human circRNAs. This database not only comprehensively organizes circRNA data but also provides valuable information, including microRNA (miRNA) binding sites, conservation, m6A modifications, circRNA mutations, protein-coding potential, and predicted internal ribosome entry sites (IRESs), providing a basis for further development of circRNA nomenclature and functions [12].
According to Bagheri Moghaddam et al. [13], the CircBank Database recently introduced a new nomenclature system for circRNAs based on the host genome and the specific location of the circRNA within that gene. Specifically, the upstream circRNA is assigned the starting number. Regarding intergenic circRNAs, the naming convention follows the format “hsa-circChrom#_#”, where the chromosomal number denotes the first number, and the second number follows the same rules as circRNAs derived from coding genes.
3. CircRNA Classification
The classification of circRNAs is based on their origin. The exonic circRNAs (EcircRNAs) originate from the coding regions of genes and play a crucial role in controlling genes [14] after they have been transcribed [14,15]. They act like conductors in a symphony, specifically by sequestering miRNAs [16,17]. In simpler terms, they function as a control center, fine-tuning the orchestra of genes in our cells after the transcription of genetic information [18]. In contrast, circular intronic RNAs (ciRNAs), originating from intronic regions of genes, are primarily localized within the nucleus, where they intricately coordinate transcriptional dynamics. Last but not least, a unique composition of exon–intron circRNAs (EIcircRNAs) are involved in complex interactions with RNA polymerase II, a key enzyme involved in the transcription of genes [10]. This interplay with RNA polymerase II underscores the multifaceted role of EIcircRNAs in modulating gene expression processes within the cellular nucleus [19].
CircRNAs play vital roles in physiology and pathology, acting as sponges for miRNA, regulating gene transcription, controlling RNA-binding proteins, and producing functional peptides [6,20]. Interestingly, almost 25% of eukaryotic genes that code for proteins in the mammalian brain are encoded by circRNAs. For example, circAcbd6 has a role in transforming neural stem cells into cholinergic neurons. This is achieved by inhibiting the function of miR-320-5p, thereby affecting the expression of Osbpl2, hence providing valuable insights into the mechanisms by which circRNAs promote or inhibit neurogenesis [21].
4. CircRNA Biogenesis
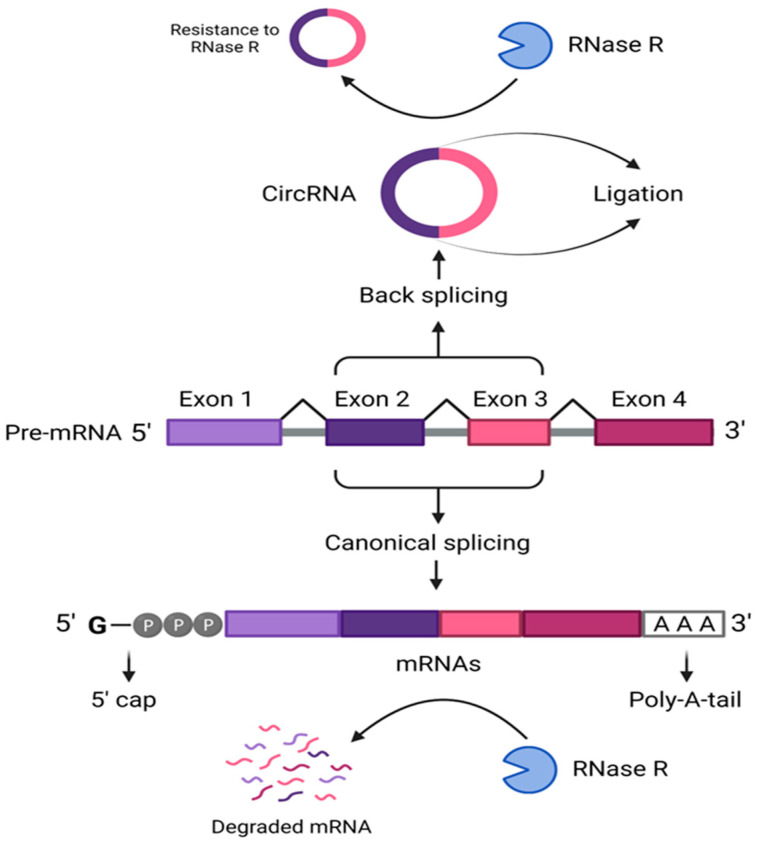
CircRNAs are endogenously synthesized from exons by the “Exons Back Splicing” method, a form of non-canonical messenger RNA splicing [5,22]. CircRNAs are distinguished by their single-strand closed structure, produced via ligating the 5′-3′-splice, and donor–acceptor sites [23,24,25]. This contrasts the normal splicing of the pre-mRNAs finished with a 5′ cap and 3′ polyadenylated tails, as shown in Figure 1 [26,27]. Difference points between circRNA and mRNA are summarized in Table 1, indicating the direction of the splicing, the pre-mRNA, the ligation sites, the structure, the parent genetic material, and susceptibility to RNase R [9,28].
Figure 1.
Splicing of circRNAs versus mRNAs. This figure demonstrates the non-canonical back splicing of the circRNAs from the pre-messenger RNA, showing its single-strand closed-loop structure, which is resistant to RNase, versus the canonical splicing of the mRNA with its polyadenylated 3′ tail. [CircRNA: circular RNA; mRNA: messenger RNA, Poly-A-tail: polyadenylated tail].
Table 1.
Differences between circRNA and mRNA during and after the RNA transcript maturation process.
| Difference | CircRNA | Linear mRNA |
|---|---|---|
| Splicing | Back | Normal |
| Pre-mRNA | Non-canonical | Canonical pre-mRNA |
| Production | By ligation | With a free 5′-cap and 3′-tail |
| Structure | No free cap and tail | With a free cap and tail |
| Final structure | Covalent closed-loop structure; circular | Linear |
| Formed from | Exons located in the cytoplasm or the nucleus increase nuclear protein retention, and circRNAs within introns remain in the nucleus | Pre-mRNA from a DNA template in the cell nucleus |
| Resistant to RNase R | Yes | No |
5. Mechanisms of CircRNA Biogenesis
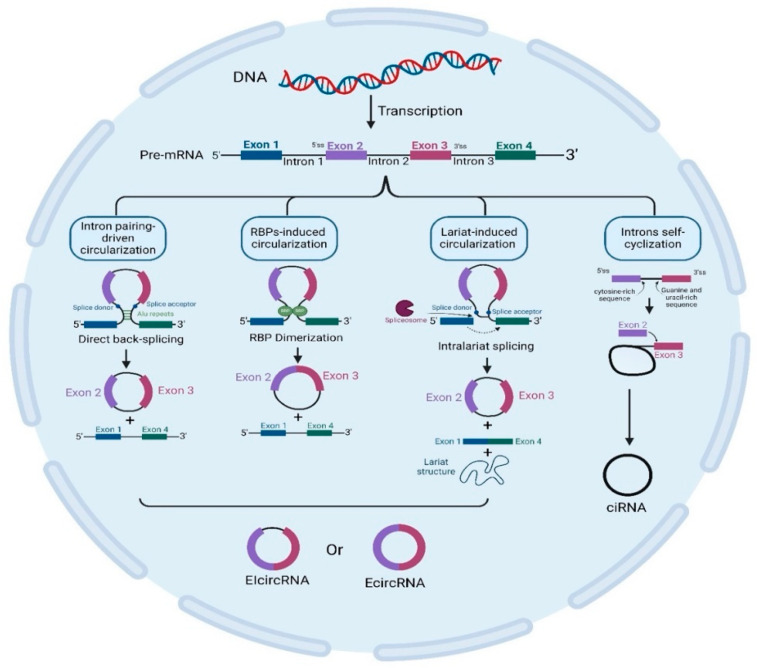
As mentioned earlier, circRNAs are sub-classified into three categories, EcircRNA, ciRNA, and EIcircRNA, as illustrated in Figure 2 [29,30]. CircRNAs are generated via various mechanisms. Table 2 demonstrates the different mechanisms involved in circRNA circularization, including intron pairing-driven circularization, RBP-induced circularization, lariat-induced circularization, and intro self-cyclization. Following their biogenesis, circRNAs are regulated by the associated miRNA levels in their producing cells and then transferred to body fluids through exosomes [28].
Figure 2.
CircRNA biogenesis. This figure illustrates how circularization can be induced by intron pairing, RBPs, and lariat, which is triggered by spliceosomes and results in the synthesis of EIcircRNA or EcircRNA. It also depicts introns self-cyclization producing ciRNA. [CircRNA: circular RNA; ciRNA: intron circRNA; EIcircRNA: exon–intron circular RNA; Ecirc: exonic circular RNA; RBPs: RNA-binding proteins].
Table 2.
Molecular circularization of circRNAs.
| CircRNA Biogenesis | CircRNA Product | Biogenesis Mechanism | Refs. |
|---|---|---|---|
| Intron pairing-driven circularization | EcircRNAs or ElciRNAs |
The method by which EcircRNA and EIcircRNA cyclize is known as “direct back splicing” or intron pairing-driven cyclization; pre-mRNA containing ALU repeats is sheared to form EcircRNA following reverse-base complementary pairing. EIciRNAs are produced if introns are kept in between exons. | [19,31] |
| RBP-induced circularization | RBPs (trans-acting factors) are Quaking, Muscleblind, and Fused-in Sarcoma. Circularization is facilitated by bridging comparable intronic regions. RBP dimerization links the 3′ and 5′ ends of circularized exons. | [32,33] | |
| Lariat-induced circularization driven by spliceosomes | Exon circularization is spliceosome-dependent and is collected at the back-splicing site to help join the 5′-3′ donor–acceptor sites. Within lariat, internal splicing releases EcircRNAs or EIcircRNAs. | [34,35,36,37] | |
| Intron self-cyclization | ciRNA | Intron self-cyclization is brought about by the 7 nucleotides of the G/U-rich sequence located near 1 exon and the 11 nucleotides of the C-rich sequence located near another exon in pre-mRNA. Three distinct kinds of circRNAs are produced: ciRNAs, EIcircRNAs, and EcircRNAs. A closed RNA loop (covalently EcircRNA) is formed when the 3′ end of an exon (5’ss) is joined to the 5′ end of either the same exon (single-exon circRNA) or an upstream exon (multiple-exon circRNA). |
[22,38,39,40] |
[CircRNA: circular RNA; ciRNA: intronic circRNA; EIcircRNA: exon–intron circular RNA; Ecirc: exonic circular RNA; RBP: RNA-binding protein].
6. CircRNAs and Cancer Pathology
In the context of cancer pathology, circRNAs are critical players with significant implications across diverse cancer types, spanning from brain cancer to myeloma. The expression patterns of circRNAs exhibit associations with crucial stages in cancer progression, impacting immune response, cellular differentiation, pluripotency, apoptosis, and angiogenesis. Investigating the specific types of circRNAs and their precise chromosomal locations in distinct cancer types provides valuable insights into their roles and actions [41]. CircRNAs have been shown to possess significant implications in HCC development and advancement. They contribute to cell proliferation, tumor metastasis, evasion of immune responses, and resistance to drugs [42,43,44,45,46,47].
7. HCC Prevalence and Etiology
The most prevalent type of primary liver cancer and the third leading cause of cancer death globally is hepatocellular carcinoma (HCC). Regarding frequency, HCC ranks ninth in women and fifth in men. Its incidence rates vary across different regions worldwide [48,49,50,51]. According to Ferlay et al., one million individuals will be affected annually by HCC in one way or another by 2025 [52].
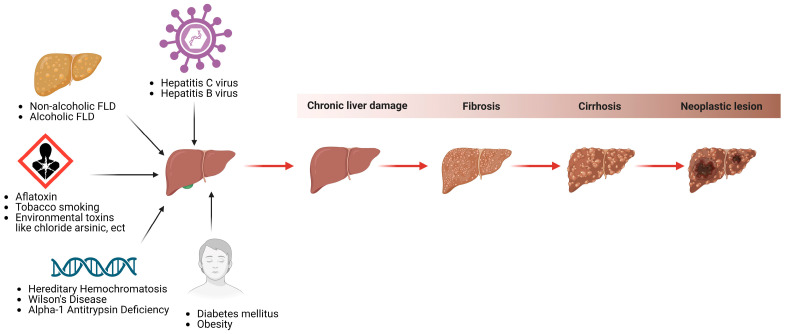
The etiology of HCC is multifactorial, involving interactions between various causative agents [53,54,55,56]. HCC can result from long-term viral infections such as chronic hepatitis B virus (HBV) and hepatitis C virus (HCV) [57,58,59]. Metabolic problems like obesity and diabetes, especially in women [57,59,60,61,62,63], and inherited conditions like hemochromatosis and Wilson’s disease can also cause HCC, as shown in Figure 3. [52,64,65,66]. Biological and molecular mechanisms in HCC involve either tumor suppressor genes or oncogenes [51,67], interleukins [2,68,69], immunoglobulin-like receptors [70,71,72], and/or various cytokines and their polymorphisms [57,58,73].
Figure 3.
Some HCC predisposing factors. This figure depicts some hereditary diseases (hemochromatosis, Wilson’s, alpha-1 antitrypsin deficiency), acquired conditions (hepatitis C and B viruses, non-alcoholic FLD and alcoholic FLD), metabolic abnormalities (diabetes mellitus and obesity), and environmental risk factors (aflatoxins, tobacco, and others) that predispose people to chronic liver damage and hepatic cancers. [HCC: hepatocellular carcinoma, FLD: fatty liver disease].
Some hereditary conditions become more significant as we age, contributing to HCC risk. It is worth mentioning that around 10–20% of HCC cases occur in individuals without liver cirrhosis. Non-alcoholic fatty liver disease (NAFLD) represents an independent risk factor for HCC and is often linked to obesity due to the increased consumption of fatty diets [57,74]. Other risk factors include exposure to aflatoxins, excess iron in the body, and even smoking. Most of these risk factors promote the development of cirrhosis, which is present in more than 80–90% of HCC cases [75], as depicted in Figure 3.
The term poor prognosis usually accompanies HCC because HCC lacks symptoms in its early stages [76]. It is worth mentioning that survival rates are directly linked to HCC early diagnosis and, hence, a better prognosis. Another reason that may contribute to HCC’s bad prognosis is the fact that HCC represents the end stage of liver disease, so there is minimal reserving capacity at this stage [77]. Further, HCC itself, being an aggressive cancer that is highly metastasized, contributes as well to the disease’s poor prognosis [78]. Hence, even if HCC is appropriately diagnosed, it is still difficult to control [79].
8. HCC Molecular Heterogeneity
The underlying pathogenic condition(s) affect(s) the molecular pathways involved in the etiology of HCC. HCV-mediated hepatocarcinogenesis primarily occurs through host–viral protein interactions, particularly involving the core, non-structural proteins NS3, NS4A, and NS5A [80,81].
Abundant and enduring RNA molecules, such as miRNAs or long non-coding RNAs (lncRNAs), showcase a manifold of abilities in physiological and pathological contexts. They function as repositories, regulatory elements, catalysts of translation, identifiers, and healthy tumor suppressors. Hence, their significance was demonstrated across multiple cancer types like colon, liver, and breast [82]. LncRNAs can regulate gene expression in three ways: epigenetic, transcriptional, and post-transcriptional [83,84]. LncRNAs have been well investigated in terms of their role in the regulation of cancer. For example, in HCV, lncRNA-ATB is highly associated with fibrosis and may also be involved in developing HCC [4,85,86,87,88,89,90,91,92]. In this aspect, lncRNA-ATB was reported to promote tumor metastasis via the induction of epithelial–mesenchymal transition (EMT) [93]. On the other hand, miRNAs function as post-transcriptional regulators by binding mRNA and inhibiting its translation into a protein. Several studies have identified an association between dysregulated miRs and the development of HCC [94,95].
9. Role of CircRNAs in HCC
The functions of circRNAs in HCC are complicated, as they can act either as good or evil, being tumor suppressors or oncogenes, respectively [96,97,98,99]. CircRNAs play various functional roles in tuning the initiation, development, progression, and metastasis of HCC.
10. CircRNAs Act as miRNA Sponges or Decoys
CircTRIM33-12 sponging miR-191 upregulates the expression of tet methylcytosine dioxygenase 1 (TET1), which lowers the levels of 5-hydroxymethylcytosine in HCC cells [100]. By acting as a decoy for miR-9, CircMTO1 inhibits cell proliferation in HCC and functions as a tumor suppressor by upregulating p21 [101]. Similarly, circHIPK3 promotes HCC proliferation by sponging some miRs, including miR-124 [97] and miR-29b [102], as illustrated in Figure 4, leading to the release of their target genes responsible for cell growth regulation. Other studies demonstrate the oncogenic behavior of HIPK3 through sponging miR-338-3p [103]. By sponging miR-3619-5p, increasing catenin beta 1 (CTNNB1) expression, and triggering Wnt/β-catenin signaling, CircZFR controls cell proliferation, the epithelial–mesenchymal transition, and the Wnt/β-catenin pathway [104]. Another circRNA, circFBLIM1, acts as a competing endogenous RNA (ceRNA) that enhances HCC progression via sponging miR-346 [105]. CircMAT2B promotes glycolysis and HCC malignancy by sponging miR-338-3p to activate the pyruvate kinase M2 (PKM2) axis under hypoxia [43]. CircTP63 increases ZBTB18 expression by sponging miR-155-5p, which advances HCC. The latter was reported to positively correlate with mortality rates in HCC patients [106]. However, by upregulating tissue inhibitor of metalloproteinase 3 (TIMP3), a well-known tumor suppressor that functions by sponging miR-17-3p and miR-181b-5p, hsa_circ_0001445 (cSMARCA5) suppresses the migration and proliferation of HCC cells [46].
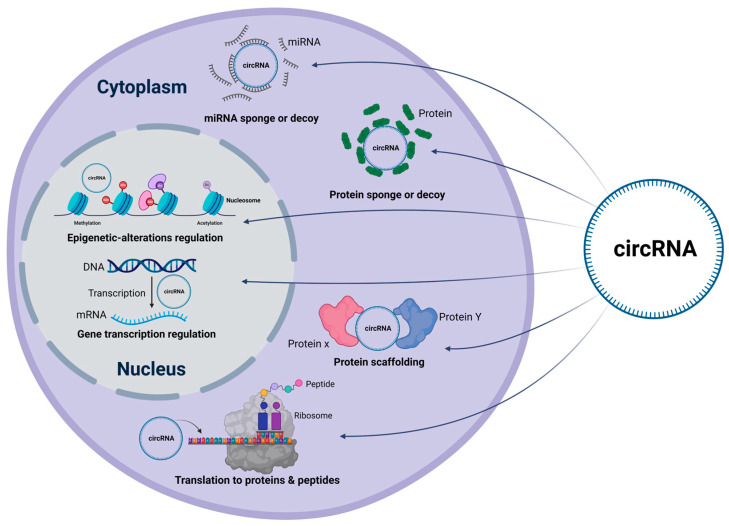
Figure 4.
Regulatory mechanisms of circRNAsin HCC. This figure summarizes the different mechanisms by which circRNAs contribute to HCC. CircRNAs function as miRs or protein sponges. They can also scaffold cellular proteins and regulate the latter’s translation in the cytoplasm. Further, circRNAs can alter epigenetic regulations and modulate gene transcription within the nucleus. [CircRNA: circular RNA; miRNA: microRNA; mRNA: messenger RNA].
A recent study proposed that circ_0001806 expedites HCC advancement by upregulating matrix metalloproteinase (MMP)-16 expression by inhibiting miR-193a-5p [107]. Another preliminary investigation, yet to be approved, revealed that circYTHDF3 fosters liver carcinogenesis via the miR-136-5p/chromobox 4 (CBX4)/vascular endothelial growth factor (VEGF) pathway [108]. Furthermore, earlier research reveals that circCFH stimulates HCC by modulating cellular functions via the miRNA 377-3p/RNF38 axis, including proliferation, apoptosis, migration, invasion, and glycolysis [109]. Lastly, circRNA CDR1as affects HCC progression by interacting with markers and miR-1287 bands within the Raf1 pathways [110].
In patients with HCC, Hsa_circ_0085616 (circASAP1) induced pulmonary metastases by stimulating the proliferation of cells, in vitro colony formation, migration, and invasion [42]. CircASAP1 was reported to operate as a ceRNA for the endogenous colony-stimulating factor (CSF) and mitogen-activated protein kinase (MAPK) suppressors miR-326 and miR-532-5p. MAPK and CSF are known to mediate tumor-associated macrophage infiltration, which is also linked to cell invasion and proliferation [42].
11. CircRNAs Function as Protein Sponges or Decoys
CircBACH1 interacts with human antigen R (HuR), an RBP, leading to the downregulation of p27 expression, as shown in Figure 4. Through the interferon-responsive sequence motif in the p27 5′-untranslated region, this interaction prevents translation. HuR transport and accumulation in the cytoplasm are similarly facilitated by CircBACH1 [111]. By competitively binding to fragile X mental retardation protein (FMRP), on the other hand, CircZKSCAN1 functions as a tumor suppressor by influencing the translation of cell division cycle and apoptosis regulator protein 1 (CCAR1) mRNA and blocking the Wnt signaling pathway (Table 3) [112].
Table 3.
List of circRNAs, their functional roles, and mechanisms of action in HCC.
| Functional Role | CircRNAs | Mechanism | Refs. |
|---|---|---|---|
| MiR sponge or decoy | CircTRIM33-12 | increases the production of TET1 by the sponging of miR-191, lowering the levels of 5-hydroxymethylcytosine in HCC cells | [100] |
| CircMTO1 | downregulates p21 level by sponging oncogenic miR-9 to inhibit HCC progression. | [101] | |
| CircHIPK3 | regulates AQP3 expression, sponges miR-124, alters cell proliferation and HCC migration | [97] | |
| CircZFR | regulates cell proliferation, epithelial–mesenchymal transition, Wnt/β-catenin via quenching miR-3619-5p, enhancing CTNNB1 expression and activating Wnt/β-catenin signaling | [104] | |
| CircFBLIM1 | ceRNA that enhances HCC progression via sponging miR-346 | [105] | |
| CircMAT2B | encourages HCC malignancy, glycolysis, and miR-338-3p quenching to activate the PKM2 axis under hypoxic conditions | [43] | |
| CircTP63 | sponges miR-155-5p and thus increases ZBTB18 expression, which is positively correlated with mortality rates in HCC patients | [106] | |
| CircSMARCA5 | TIMP3 expression via sponging miR-17-3p and miR-181b-5p | [46] | |
| Circ_0001806 | expedites HCC advancement by upregulating MMP-16 expression through the inhibition of miR-193a-5p | [107] | |
| CircYTHDF3 | fosters HCC via miR-136-5p/CBX4/VEGF pathway | [108] | |
| CircCFH | promotes HCC by influencing cellular proliferation, apoptosis, migration, invasion and glycolysis via miRNA 377-3p/RNF38 axis | [109] | |
| CDR1as | interacts with markers and miR-1287 bands within the Raf1 pathways to modulate HCC progression | [110] | |
| CircASAP1 | ceRNA for miR-326 and miR-532-5p regulates the expression of MAPK1 and CSF-1 targets, facilitating invasion, HCC cell proliferation and infiltration of tumor-associated macrophages | [42] | |
| CircSORE | induces sorafenib resistance by competitively activating the Wnt/β-catenin pathway through miR-103a-2-5p and miR-660-3p | [45] | |
| Protein sponge or decoy | CircBACH1 | interacts with HuR; RBP downregulates p27 expression, blocks translation in the p27 5′-untranslated region by an interferon-responsive sequence element, encourages HuR translocation and cytoplasmic accumulation | [111] |
| CircZKSCAN1 | competitively binding FMRP to modulate the translation of CCAR1 mRNA and inhibiting the Wnt signaling pathway | [112] | |
| Protein scaffold | CircAMOTL1 | combines with c-myc, STAT3, PDK1, and AKT1 to promote their translocation to the nucleus, modulating the expression of their target genes. | [113,114,115] |
| CircRHOT1 | recruits TIP60 to NR2F6, initiating NR2F6 transcription and HCC progression | [116] | |
| CircADD3 | protein scaffold inhibits HCC metastasis via CDK1-mediated EZH2 ubiquitination | [117] | |
| CircPABPC1 | a tumor suppressor, directly delivering ITGβ1 to the proteasome for HCC ubiquitin-independent destruction | [118] | |
| CircSORE | causes sorafenib resistance by binding oncogenic YBX1 and blocking its nuclear interaction with E3 ubiquitin ligase PRP19 | [44] | |
| Gene transcription regulation | CircIPO11 | binds TOP1 to trigger GLI1 transcription, with Hedgehog signaling activation. | [119] |
| Translation to proteins and peptides | CircCTNNB1 | creates 370 amino acid β-catenin isoform, uses circularization to block translation at a new stop codon, uses Wnt to stimulate HCC cell development | [120] |
| Epigenetic alterations’ regulation | CircSOD2 | induces epigenetic alteration to drive HCC progression by activating JAK2/STAT3 signaling. | [121] |
[AKT1: AKT serine/threonine kinase 1; AQP3: Aquaporin 3; CBX4: chromobox 4; CCAR1: cell division cycle and apoptosis regulator protein 1; CDK1: cyclin-dependent kinase 1; CTNNB1: catenin beta 1; CSF: colony-stimulating factor 1; EZH2: enhancer of zeste homolog 2; FMRP: fragile X mental retardation protein; GLI1: GLI family zinc finger 1; HCC: hepatocellular carcinoma; ITGβ1: integrin β1; MAPK: mitogen-activated protein kinase; miR: microRNA; Hur: human antigen R; JAK2: Janus kinase 2; MMP: matrix metalloproteinase; NR2F6: nuclear receptor subfamily 2 group F member 6; PDK1: 3-phosphoinositide-dependent kinase 1; PKM2: pyruvate kinase M2; RBP: RNA-binding protein; STAT3: signal transducer and activator of transcription 3; TIMP3: metalloproteinase 3; VEGF: vascular endothelial growth factor; YBX1: Y-box binding protein 1.]
12. CircRNAs Can also Serve as Scaffolding for Proteins
CircAMOTL1 facilitates the translocation of c-myc, 3-phosphoinositide-dependent kinase 1 (PDK1), AKT serine/threonine kinase 1 (AKT1), and signal transducer and activator of transcription 3 (STAT3) to the nucleus. Their target genes’ expression is modulated by this activity [113,114,115]. NR2F6 transcription and the advancement of HCC are triggered by CircRHOT1, which recruits TIP60 to the nuclear receptor subfamily 2 group F member (6NR2F6) promoter [116]. Another downregulated circRNA, hsa_circ_0020007 (circADD3), has been linked to vascular invasion and distant and intrahepatic metastasis of HCC, as summarized in Table 3. Mechanistically, circADD3 promotes the ubiquitination of EZH2 and the subsequent degradation of that protein. CircADD3 boosts the interaction between EZH2 and cyclin-dependent kinase 1 (CDK1) to accomplish this activity. The expression of several anti-metastatic genes, including dampening circADD3 itself, is increased when EZH2 is downregulated. This is achieved by lowering the histone tri-methylation marker H3K27me3 on the promoter regions of the anti-metastatic genes [117].
In HCC, CircPABPC1, another circRNA, directly feeds ITGβ1 to the proteasome for ubiquitin-independent degradation, demonstrating tumor-suppressive activity [118].
CircRNAs are also involved in regulating gene transcription. CircIPO11, for example, binds topoisomerase I (TOP1), which triggers GLI family zinc finger 1 (GLI1) transcription. This interaction leads to activating the Hedgehog signaling pathway [119]. It has also been demonstrated that circRNAs play a role in translating proteins or peptides. circCTNNB1 produces a new 370-amino-acid β-catenin isoform. This isoform is generated through the circularization process, which leads to translation termination at a new stop codon. This mechanism promotes HCC cell growth through the Wnt signaling pathway [120].
Last but not least, circRNAs were shown to regulate epigenetic alterations. For example, circSOD2 induces an epigenetic alteration that drives HCC progression by activating the JAK2/STAT3 signaling pathway [121]. In summary, Table 3 classifies the different circRNAs involved in HCC according to their functional roles while describing the corresponding mechanisms.
13. CircRNA–Protein–mRNA Ternary Complexes
Ternary circRNA–protein–mRNA complexes play crucial roles in regulating mRNA stability and translation. These complexes involve circRNA interactions with RBPs and mRNAs simultaneously. For instance, circNSUN2 can assemble into a complex with high mobility group A (HMGA2) mRNA and insulin-like growth factor 2 mRNA-binding protein 2 (IGF2BP2) to stabilize mRNA, triggering EMT and enhancing the aggressiveness of colorectal cancer (CRC) [122].
Moreover, circPOK functions differently than its linear counterpart, Pokemon, a tumor suppressor gene. CircPOK promotes the stability of VEGF mRNA and interleukin 6 (IL6) via interacting with the interleukin enhancer binding factor 2/3 (ILF2/3) complex. Additionally, it strengthens ILF2/3’s binding to the IL6 promoter. CircPOK regulates the tumor cell secretome both transcriptionally and post-transcriptionally [123]. Similarly, the circFNDC3B-IGF2BP3-CD44 mRNA ternary complex supports CD44 overexpression and mRNA stability [124].
In contrast, some circRNAs act as brakes in translation. With the aid of 11 complementary nucleotides and IRES, a tumor suppressor mRNA, a three-part complex is formed between circMALAT1 with paired box 5 (PAX5) and ribosome, causing mRNA breakdown. It additionally initiates the JAK2/STAT3 signaling pathway and functions as a sponge for miR-6887-3p [125].
Another circRNA was discovered to interfere with the translation initiation process. CircYap, which is known to interact with Yap mRNA, was found to interact with poly(A)-binding protein (PABP) and eukaryotic initiation factor 4 gamma (eIF4G), which bind to the 3′-tail and 5′-cap of the mRNA, respectively. This complex prohibits PABP and eIF4G interaction, thereby hindering Yap translation initiation [126].
CircRNAs play a role in the EMT, which is linked to drug resistance in HCC. Clear examples of dysregulated circRNAs involved in HCC drug resistance include higher expression of CircFoxo3 in adriamycin-resistant tissues, potentially contributing to resistance through the miR-199a-5p/ABCC1 pathway [127]. Furthermore, HCC cells’ release of CircUHRF1 wears down natural killer cells and increases their resistance to anti-programmed cell death protein 1 (PD1) immunotherapy [47]. On the other hand, reduced levels of circ_0003418 promote cisplatin resistance along with activation of the Wnt/β-catenin signaling cascade [128,129].
To sum up, circRNAs are viewed as regulatory ncRNA molecules that exert their effect directly by regulating the transcription and splicing of genes or indirectly by altering other regulators, including proteins and miRNAs (Table 3 and Figure 1). Accordingly, it is clear that the regulatory role circRNAs play in HCC remains a topic of ongoing research and needs further investigation.
14. Are circRNAs Involved in Therapeutic Resistance Experienced by HCC Patients?
In the context of resistance, circRNAs act as molecular sponges, preventing specific miRNA inhibitory effects on critical genes linked to resistance, which result in a loss of control [130,131]. So, surprisingly, the answer to the question is yes. CircRNAs influence crucial signaling pathways, affecting how HCC cells respond to therapeutic agents [132]. Their modulation extends to apoptosis and cell survival pathways, strengthening HCC cells against treatment-induced cell death and promoting resistance. Interestingly, interactions with RNA-binding proteins add a level of complexity to cellular responses following therapeutic interventions [133]. Sorafenib-induced resistance arose from circSORE that competitively activated the Wnt/β-catenin pathway by sponging miR-103a-2-5p and miR-660-3p [45]. Another proposed mechanism by which circSORE promotes sorafenib resistance involves binding to the oncogene Y-box binding protein 1 (YBX1). By blocking YBX1’s nuclear connection with the E3 ubiquitin ligase PRP19, this association inhibits the enzyme’s breakdown and increases the resistance to sorafenib [44].
15. Could circRNAs Act as Theranostic Agents for HCC Patients?
As mentioned earlier, HCC diagnosis is challenging. The main reason is the lack of exclusive, specific biomarkers for HCC. Moreover, there are not enough appropriate blood molecular markers for surveillance and early HCC diagnosis. The current biomarkers have low sensitivity and inconsistent specificity despite having different cut-off values [134].
Biomarkers like alpha-fetoprotein (AFP) are elevated in HCC and other pathological conditions such as chronic liver diseases. Further, it has also been demonstrated that around 40% of HCC patients present with normal AFP levels [135,136]. Des-γ-carboxy-prothrombin (DCP) has been studied as a promising biomarker for HCC [137]. Research is still ongoing in this area, and whole genome-wide sequences and DNA microarray analysis have identified markers of early HCC [138,139] that still need to be validated.
The concept of theranostics is linked to having a personalized health compass that guides treatment decisions and illuminates the unique individual molecular landscape [140]. This dynamic approach merges therapy and diagnostics, ensuring that medical interventions are tailored to the patient’s needs. In HCC, circRNAs act as molecular sensors, possessing traits ideal for early cancer detection and identifying subtle clues in tissues and body fluids [141,142]. These circRNAs, while still in the early stages of their therapeutic paths, have shown promising potential in preclinical trials [142]. For instance, in experiments involving circRNAs like circMYLK and circMAST1, the introduction of small interfering RNA (siRNA) demonstrated an ability to suppress tumor formation [141]. This is a precision strike against cancer cells guided by these circRNA navigators. Moreover, developing a plasma circRNA panel for diagnosing HCC is akin to having a sophisticated diagnostic tool, providing accuracy surpassing traditional markers. In this narrative of theranostics, circRNA emerges as a molecular marker and an active player, potentially transforming how we approach the personalized treatment landscape in HCC and beyond.
16. Exosomal circRNA Is a New Hot Area of Research
Exosomal hsa_circ_0051443, frequently downregulated in HCC cells, has been demonstrated to have anti-proliferative and pro-apoptotic properties in cells. This is facilitated by upregulating BRI1-associated kinase 1 as a consequence of miR-331-3p binding [143]. Exo_circ_79050 (also named hsa_circ_0009024) is a circRNA that is exosomal, originating from a “pseudogene” being upregulated in HCC as retrieved in silico from exoRBase v2.0 [144] (http://www.exorbase.org/exoRBaseV2/detail/detailInfo?id=exo_circ_79050&kind=circRNA&tab=profile, accessed on 18 May 2023). Its genomic position is chrY:19587210-19587507, with the positive strand upregulated in HCC. Moreover, four protein-coding exosomal circRNAs are upregulated in HCC, as shown in Table 4 (retrieved from exoRBase v2.0, http://www.exorbase.org/exoRBaseV2/browse/toIndex?kind=circRNA, accessed on 18 May 2023).
Table 4.
Exosomal circRNAs upregulated in HCC urine or blood samples retrieved from exoRBase v2.0.
| circID | circBase ID | Genomic Position | Strand | Gene Symbol |
|---|---|---|---|---|
| exo_circ_11335 | NA | chr12:94169153-94186473 | + | PLXNC1 |
| exo_circ_23574 | hsa_circ_0041462 | chr17:3814322-3816270 | − | NCBP3 |
| exo_circ_71780 | hsa_circ_0006320 | chr8:22474954-22498112 | + | PPP3CC |
| exo_circ_79066 | hsa_circ_0001953 | chrY:2953909-2961646 | + | ZFY |
http://www.exorbase.org/exoRBaseV2/browse/toIndex?kind=circRNA, accessed on 18 May 2023.
17. CircRNAs in HCC: Bioinformatics Analysis
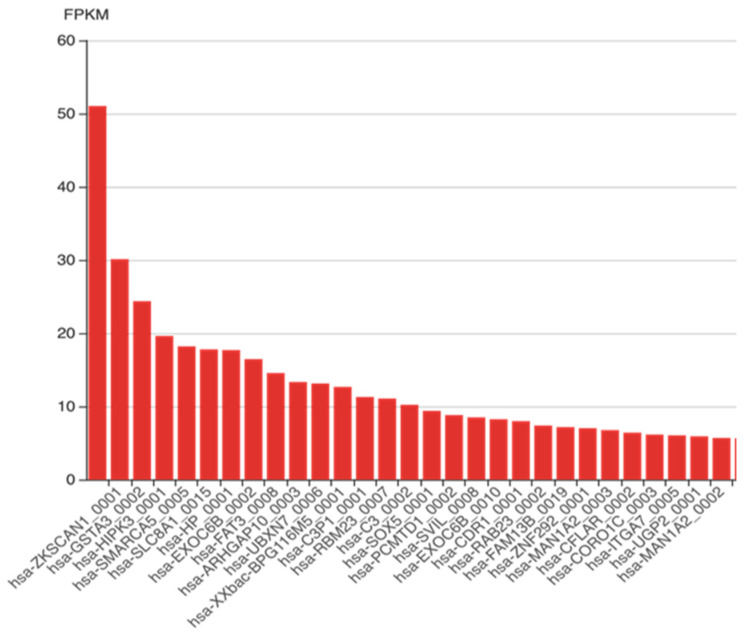
We accessed circAtlas 2.0 (http://circatlas.biols.ac.cn/) on 17 May 2023, for the liver’s most highly expressed human circRNAs, as shown in Figure 5.
Figure 5.
Liver-specific high expression of circRNAs. Figure 5 lists the most prevalent circRNA in the human liver in descending order of highest expression, as retrieved from circATlas 2.0, http://circatlas.biols.ac.cn/, accessed on 17 May 2023. [FPKM: fragments per kilobase of transcript per million mapped reads].
18. CircRNAs in Different Liver Diseases
The upcoming chapter demonstrates the association between circRNAs and liver disease. In particular, the upregulated or downregulated circRNA expression patterns, circRNA-associated genes, sponged miRNAs, biological functions (e.g., proliferation, migration, and invasion), and molecular mechanisms (e.g., ceRNA, PI3K-AKT, FOXO, SIRT1, PPAR-a signaling pathways) of circRNA in various liver diseases are discussed. Data in Table 5 are retrieved from the circRNADisease v2.0 database (last updated January 2023 [145]), http://cgga.org.cn:9091/circRNADisease/, accessed on 17 May 2023.
Table 5.
CircRNAs in different liver diseases retrieved from circRNADisease v2.0 bioinformatics database search.
| Downregulated circRNAs | |||
| CircRNAs |
Hepatic Disease/ Biological Function |
Mechanism | Molecular Mechanism/Associated miR (Sponged miR) |
| circRNA_0046366 | Hepatocellular steatosis | - | circRNA_0046366/miR-34a/PPAR-a signaling |
| hsa_circ_0070963, hsa_circ_0061893 and hsa_circ_0013255 | Liver fibrosis | - | - |
| circRNAs_100395 | Liver cancer | inhibits cell proliferation, induces apoptosis | miR-1228 |
| circScd1 | NAFLD | encourages the JAK2/STAT5 pathway, which causes fatty liver disease | - |
| circCDK13 | Liver cancer | suppresses progression via JAK/STAT and PI3K/Akt signaling | - |
| circRNA_101764 | HBV-related HCC | - | hsa-miR-181 |
| circ_03848, circ_08236, circ_13398 and circ_15013 |
Liver regeneration | - | - |
| circRNA-4099 | Hepatitis | unknown/triggers keap1/Nrf2 and p38MAPK | miR-706 aggravating H2O2-induced injury |
| Upregulated circRNAs | |||
| CircRNAs |
Hepatic Disease/ Biological Function |
Mechanism |
Molecular Mechanism/ Associated miR (Sponged miR) |
| hsa_circRNA_0000657, hsa_circRNA_0000659, hsa_circRNA_0003247, hsa_circRNA_0001535 |
Hepatotoxicity | - | - |
| hsa_circ_0072765, hsa_circ_0071410, hsa_circ_0054345 | Liver fibrosis | - | - miR-9-5p - |
| circZFR, circFUT8 circIPO11 |
Liver cancer | - | - |
| circMEG3 | Liver cancer | inhibits telomerase activity, shortens telomere lifespan, reduces Cbf5 | - |
| circRNA-0067835 | Liver fibrosis | promotes cell proliferation, inhibits apoptosis | miR-155 to promote FOXO3a |
| circ_0091579 | Liver cancer | promotes proliferative and metastasis | miR-490-3p |
| hsa_circ_0003056 hsa_circ_0067127 |
Carcinoma | - | - |
| circRNA-1984 | HSCs-related to fibrosis | - | miR-146b |
| circ_0015756 | Hepatoblastoma | - | - |
| hsa_circ_0000594 | Hepatoblastoma | - | mir-217/SIRT1 regulatory axis |
| circFBLIM1 | Hepatoblastoma | Promotes cell viability, proliferation, invasion | miR-346-ceRNA to regulate FBLIM1 expression |
| circHMGCS1 | Hepatoblastoma | Regulates proliferation, apoptosis and glutaminolysis | miR-503-5p/IGF/PI3K/AKT axis; regulates IGF2 and IGF1R expression |
| circ-PWWP2A | Fibrogenesis | Downstream reactor of TGF-ß and LPS | miR-203 and miR-223 |
http://cgga.org.cn:9091/circRNADisease/ accessed on 17 May 2023. Table 5 demonstrates the upregulated and downregulated circRNAs in the different hepatic diseases while denoting the involved molecular mechanism and/or associated miRNA as retrieved from circRNADisease v2.0 bioinformatics database, http://cgga.org.cn:9091/circRNADisease/, accessed on 17 May 2023. [AKT: AKT serine/threonine kinase; FBLIM1: Filamin-binding LIM protein 1; FOXO3a: Forkhead box O3; IGF: insulin-like growth factor; IGF1R: insulin-like growth factor 1 receptor; JAK2: Janus kinase 2; Keap 1: Kelch-like erythroid cell-derived protein with CNC homology [ECH]-associated protein 1; HSCs: hepatic stellate cells; LPS: Lipopolysaccharide; NAFLD: non-alcoholic fatty liver disease; Nrf2: nuclear factor erythroid 2 [NF-E2]-related factor 2; PI3K: phosphoinositide 3-kinase; PPAR: Peroxisome proliferator-activated receptor; p38MAPK: p38 mitogen-activated protein kinase; SIRT1: Sirtuin 1; STAT5: signal transducer and activator of transcription 5; TGF-ß: transforming growth factor beta].
Several circRNAs are downregulated in various liver diseases, each with its own specific biological function and molecular mechanism. In hepatocellular steatosis, circRNA_0046366 is downregulated and is associated with the miR-34a/PPAR-a signaling pathway, although the exact mechanism remains unknown. For liver fibrosis, hsa_circ_0070963, hsa_circ_0061893, and hsa_circ_0013255 are also downregulated. CircRNA_100395 in liver cancer suppresses growth and triggers apoptosis, potentially via controlling miR-1228. The JAK2/STAT5 pathway links the downregulation of CircScd1 in non-alcoholic fatty liver disease (NAFLD) to the advancement of fatty liver disease. In liver cancer, CircCDK13 inhibits the JAK/STAT and PI3K/Akt signaling pathways to prevent the disease from progressing. CircRNA_101764 is downregulated and linked to hsa-miR-181 in HBV-related HCC. Although their precise functions are uncertain, circ_03848, circ_08236, circ_13398, and circ_15013 are downregulated in liver regeneration. CircRNA-4099 in hepatitis triggers the keap1/Nrf2 and p38MAPK pathways and is associated with the aggravation of H2O2-induced injury through the regulation of miR-706, although the specific mechanism remains unclear.
On the other hand, several circRNAs are upregulated in various hepatic diseases and biological functions. In hepatotoxicity, hsa_circRNA_0000657, hsa_circRNA_0000659, hsa_circRNA_0003247, and hsa_circRNA_0001535 are upregulated, although the specific mechanism and associated miRNAs are not yet identified. For liver fibrosis, hsa_circ_0072765, hsa_circ_0071410, and hsa_circ_0054345 are upregulated, but their mechanisms remain unknown. In liver cancer, circZFR, circFUT8, and circIPO11 are also upregulated. CircMEG3 is also upregulated and has been shown to inhibit telomerase activity, shortening telomere lifespan and reducing Cbf5. Through the miR-155/FOXO3a pathway, circRNA-0067835 stimulates cell division and suppresses apoptosis in liver fibrosis. Circ_0091579 in liver cancer promotes proliferation and metastasis via miR-490-3p. For other hepatic diseases, hsa_circ_0003056 and hsa_circ_0067127 are upregulated in cancer, while mmu_circRNA_005186 is upregulated in ischemia/reperfusion injury, acting through the miR-124-3p/Epha2 pathway. CircRNA-1984 in hepatic stellate cells (HSCs) is related to fibrosis, possibly through the miR-146b pathway. Circ_0015756 and hsa_circ_0000594 are upregulated in hepatoblastoma, with hsa_circ_0000594 potentially acting through the mir-217/SIRT1 regulatory axis. CircFBLIM1 in hepatoblastoma promotes cell viability, proliferation, and invasion through the miR-346-ceRNA mechanism. CircHMGCS1 in hepatoblastoma regulates proliferation, apoptosis, and glutaminolysis, possibly through the miR-503-5p/IGF/PI3K/AKT axis and by regulating IGF2 and IGF1R expression. Circ-PWWP2A is upregulated in fibrogenesis and acts downstream of TGF-ß and LPS, possibly through the miR-203 and miR-223 pathways.
19. Expert Authors’ Opinions, Recommendations, and Future Perspective
To our knowledge, there are no clinical reports of circRNAs having a positive or negative impact on HCC by modifying an individual’s (epi)genes or polymorphism(s). CircRNAs have been linked to liver metastasis from CRC and HBV-mediated HCC [104,146]. Restrictive limitations on applying circRNAs as molecular markers in the HCC clinical field are related to inadequate clinical information about circRNA-potential axes and various HCC hallmarks.
Nevertheless, several known hsa-circRNA-miR downstream signaling targets were found, analyzed, and validated for HCC and/or liver disorders. To demonstrate their efficacy, these targets could be further investigated for other cancer types, such as BC or neurodegenerative diseases (NDDs).
Developing ncRNA precision therapeutic regimens can be achieved by targeting the hsa-circRNA-miR downstream signaling cascades through drug repurposing using molecular docking, followed by experimental validation of the selected drug’s efficacy.
20. Conclusions
Utilizing in silico databases, bioinformatics analysis (Supplementary File), and literature exploration, we emphasized in the current review the link between circRNAs and liver illnesses, particularly HCC. The significance of circRNAs as one of the epigenetic ncRNAs was highlighted. We compiled comprehensive background information regarding circRNA-related liver diseases with a particular emphasis on HCC. Specifically, we discussed the biological roles of circRNA in liver disorders, the molecular mechanisms by which they contribute to HCC as cancer molecular markers, the miRs they target to sponge, and the ultimate downstream signaling cascade. Nonetheless, the authors have shed light on a promising clinical implementation for circRNAs: their suitability as theranostic agents for HCC and their involvement in chemotherapeutic resistance experienced by some HCC patients. Yet these areas still need further investigation by the scientific community. Notably, circRNAs serve as promising targets for therapeutic interventions. CircRNAs are expected to have a novel function in tumor immunotherapy and/or controlling the tumor immune microenvironment in HCC. Ultimately, circRNAs have the potential to serve as an effective molecular tool in combating multi-drug resistance (MDR).
While emerging evidence suggests that circRNAs may play important roles in the pathogenesis and progression of HCC, it is important to note that the definitive establishment of their crucial role requires further investigation. Studies have shown that circRNAs are differentially expressed in HCC tissues, may regulate oncogenes and tumor suppressors, and can impact cellular processes such as proliferation, apoptosis, and metastasis. However, additional research is needed to fully elucidate their significance and mechanisms in HCC.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cells13151245/s1, Figure S1: Genomic distribution of human circRNAs; number of circRNAs per chromosome retrieved from circRNADb; Figure S2: CircRNA distribution in different tissues, including the liver; Figure S3: Number of circRNAs in different liver cell lines retrieved from CIRCpedia v2; Table S1: Experiments browsed for circRNAs in liver cancer.
Author Contributions
Conceptualization, R.A.Y., N.M.H. and M.A.A.; formal analysis, N.M.H.; investigation, R.A.Y., H.A.H., T.A., A.A.H., H.M.E.M., M.A., J.V., M.T., M.G., N.M.H. and M.A.A.; resources, M.G.; data curation, N.M.H.; writing—original draft preparation, R.A.Y., H.A.H., T.A., A.A.H., H.M.E.M. and N.M.H.; writing—review and editing, R.A.Y., M.A., J.V., M.T., M.G., N.M.H. and M.A.A.; software—in silico and bioinformatics analysis, N.M.H.; visualization, R.A.Y., H.A.H., T.A., M.A., N.M.H. and M.A.A.; supervision, R.A.Y. and N.M.H.; project administration, N.M.H. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data obtained and/or analyzed during the current study are available within the manuscript or the bioinformatics databases.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
No funding was provided for this work.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Pisignano G., Michael D.C., Visal T.H., Pirlog R., Ladomery M., Calin G.A. Going circular: History, present, and future of circRNAs in cancer. Oncogene. 2023;42:2783–2800. doi: 10.1038/s41388-023-02780-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elemam N.M., Mekky R.Y., Rashid G., Braoudaki M., Youness R.A. Pharmacogenomic and epigenomic approaches to untangle the enigma of IL-10 blockade in oncology. Expert. Rev. Mol. Med. 2024;26:e1. doi: 10.1017/erm.2023.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilusz J.E. A 360 view of circular RNAs: From biogenesis to functions. Wiley Interdiscip. Rev. RNA. 2018;9:e1478. doi: 10.1002/wrna.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.El-Aziz M.K.A., Dawoud A., Kiriacos C.J., Fahmy S.A., Hamdy N.M., Youness R.A. Decoding hepatocarcinogenesis from a noncoding RNAs perspective. J. Cell Physiol. 2023;238:1982–2009. doi: 10.1002/jcp.31076. [DOI] [PubMed] [Google Scholar]
- 5.Dawoud A., Ihab Zakaria Z., Hisham Rashwan H., Braoudaki M., Youness R.A. Circular RNAs: New layer of complexity evading breast cancer heterogeneity. Noncoding RNA Res. 2023;8:60–74. doi: 10.1016/j.ncrna.2022.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abaza T., El-Aziz M.K.A., Daniel K.A., Karousi P., Papatsirou M., Fahmy S.A., Hamdy N.M., Kontos C.K., Youness R.A. Emerging Role of Circular RNAs in Hepatocellular Carcinoma Immunotherapy. Int. J. Mol. Sci. 2023;24:16484. doi: 10.3390/ijms242216484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fahmy S.A., Dawoud A., Zeinelabdeen Y.A., Kiriacos C.J., Daniel K.A., Eltahtawy O., Abdelhalim M.M., Braoudaki M., Youness R.A. Molecular Engines, Therapeutic Targets, and Challenges in Pediatric Brain Tumors: A Special Emphasis on Hydrogen Sulfide and RNA-Based Nano-Delivery. Cancers. 2022;14:5244. doi: 10.3390/cancers14215244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El-Daly S.M., Talaat R.M., Braoudaki M., Youness R.A., Cho W.C. Editorial: Recent breakthroughs in the decoding of circulating nucleic acids and their applications to human diseases. Front. Mol. Biosci. 2023;10:1203495. doi: 10.3389/fmolb.2023.1203495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.ZeinElAbdeen Y.A., AbdAlSeed A., Youness R.A. Decoding Insulin-Like Growth Factor Signaling Pathway From a Non-coding RNAs Perspective: A Step Towards Precision Oncology in Breast Cancer. J. Mammary Gland. Biol. Neoplasia. 2022;27:79–99. doi: 10.1007/s10911-022-09511-z. [DOI] [PubMed] [Google Scholar]
- 10.Dawoud A., Elmasri R.A., Mohamed A.H., Mahmoud A., Rostom M.M., Youness R.A. Involvement of CircRNAs in regulating The “New Generation of Cancer Hallmarks”: A Special Depiction on Hepatocellular Carcinoma. Crit. Rev. Oncol. Hematol. 2024;196:104312. doi: 10.1016/j.critrevonc.2024.104312. [DOI] [PubMed] [Google Scholar]
- 11.Glažar P., Papavasileiou P., Rajewsky N. circBase: A database for circular RNAs. RNA. 2014;20:1666–1670. doi: 10.1261/rna.043687.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu M., Wang Q., Shen J., Yang B.B., Ding X. Circbank: A comprehensive database for circRNA with standard nomenclature. RNA Biol. 2019;16:899–905. doi: 10.1080/15476286.2019.1600395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bagheri Moghaddam M., Maleki M., Oveisee M., Bagheri Moghaddam M., Arabian M., Malakootian M. Circular RNAs: New Players in Cardiomyopathy. Genes. 2022;13:1537. doi: 10.3390/genes13091537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jeck W.R., Sharpless N.E. Detecting and characterizing circular RNAs. Nat. Biotechnol. 2014;32:453–461. doi: 10.1038/nbt.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chu Q., Zhang X., Zhu X., Liu C., Mao L., Ye C., Zhu Q.-H., Fan L. PlantcircBase: A database for plant circular RNAs. Mol. Plant. 2017;10:1126–1128. doi: 10.1016/j.molp.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 16.Rashid G., Khan N.A., Elsori D., Youness R.A., Hassan H., Siwan D., Seth N., Kamal M.A., Rizvi S., Babker A.M., et al. miRNA expression in PCOS: Unveiling a paradigm shift toward biomarker discovery. Arch. Gynecol. Obs. 2024;309:1707–1723. doi: 10.1007/s00404-024-07379-4. [DOI] [PubMed] [Google Scholar]
- 17.Youness R.A., Habashy D.A., Khater N., Elsayed K., Dawoud A., Hakim S., Nafea H., Bourquin C., Abdel-Kader R.M., Gad M.Z. Role of Hydrogen Sulfide in Oncological and Non-Oncological Disorders and Its Regulation by Non-Coding RNAs: A Comprehensive Review. Noncoding RNA. 2024;10:7. doi: 10.3390/ncrna10010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu K.-S., Pan F., Mao X.-D., Liu C., Chen Y.-J. Biological functions of circular RNAs and their roles in occurrence of reproduction and gynecological diseases. Am. J. Transl. Res. 2019;11:1. [PMC free article] [PubMed] [Google Scholar]
- 19.Li Z., Huang C., Bao C., Chen L., Lin M., Wang X., Zhong G., Yu B., Hu W., Dai L., et al. Exon-intron circular RNAs regulate transcription in the nucleus. Nat. Struct. Mol. Biol. 2015;22:256–264. doi: 10.1038/nsmb.2959. [DOI] [PubMed] [Google Scholar]
- 20.Chen L., Huang C., Shan G. Circular RNAs in physiology and non-immunological diseases. Trends Biochem. Sci. 2022;47:250–264. doi: 10.1016/j.tibs.2021.11.004. [DOI] [PubMed] [Google Scholar]
- 21.Zhang J., Wang C., Jia C., Zhang Y., Qing X., Zhang Y., Liu J., Xu S., Pan Z. The role of circular rnas in the physiology and pathology of the mammalian ovary. Int. J. Mol. Sci. 2022;23:15204. doi: 10.3390/ijms232315204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y., Zhang X.O., Chen T., Xiang J.F., Yin Q.F., Xing Y.H., Zhu S., Yang L., Chen L.L. Circular intronic long noncoding RNAs. Mol. Cell. 2013;51:792–806. doi: 10.1016/j.molcel.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 23.Zeinelabdeen Y., Abaza T., Yasser M.B., Elemam N.M., Youness R.A. MIAT LncRNA: A multifunctional key player in non-oncological pathological conditions. Noncoding RNA Res. 2024;9:447–462. doi: 10.1016/j.ncrna.2024.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rashwan H.H., Taher A.M., Hassan H.A., Awaji A.A., Kiriacos C.J., Assal R.A., Youness R.A. Harnessing the supremacy of MEG3 LncRNA to defeat gastrointestinal malignancies. Pathol. Res. Pr. 2024;256:155223. doi: 10.1016/j.prp.2024.155223. [DOI] [PubMed] [Google Scholar]
- 25.Elmasri R.A., Rashwan A.A., Gaber S.H., Rostom M.M., Karousi P., Yasser M.B., Kontos C.K., Youness R.A. Puzzling out the role of MIAT LncRNA in hepatocellular carcinoma. Noncoding RNA Res. 2024;9:547–559. doi: 10.1016/j.ncrna.2024.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geng X., Jia Y., Zhang Y., Shi L., Li Q., Zang A., Wang H. Circular RNA: Biogenesis, degradation, functions and potential roles in mediating resistance to anticarcinogens. Epigenomics. 2020;12:267–283. doi: 10.2217/epi-2019-0295. [DOI] [PubMed] [Google Scholar]
- 27.Zhang X.O., Dong R., Zhang Y., Zhang J.L., Luo Z., Zhang J., Chen L.L., Yang L. Diverse alternative back-splicing and alternative splicing landscape of circular RNAs. Genome Res. 2016;26:1277–1287. doi: 10.1101/gr.202895.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou W.-Y., Cai Z.-R., Liu J., Wang D.-S., Ju H.-Q., Xu R.-H. Circular RNA: Metabolism, functions and interactions with proteins. Mol. Cancer. 2020;19:172. doi: 10.1186/s12943-020-01286-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang M., Yu F., Li P.J.C. Circular RNAs: Characteristics, function and clinical significance in hepatocellular carcinoma. Cancers. 2018;10:258. doi: 10.3390/cancers10080258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bolha L., Ravnik-Glavač M., Glavač D. Circular RNAs: Biogenesis, Function, and a Role as Possible Cancer Biomarkers. Int. J. Genom. 2017;2017:6218353. doi: 10.1155/2017/6218353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jeck W.R., Sorrentino J.A., Wang K., Slevin M.K., Burd C.E., Liu J., Marzluff W.F., Sharpless N.E. Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA. 2013;19:141–157. doi: 10.1261/rna.035667.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Conn S.J., Pillman K.A., Toubia J., Conn V.M., Salmanidis M., Phillips C.A., Roslan S., Schreiber A.W., Gregory P.A., Goodall G.J. The RNA binding protein quaking regulates formation of circRNAs. Cell. 2015;160:1125–1134. doi: 10.1016/j.cell.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 33.Errichelli L., Dini Modigliani S., Laneve P., Colantoni A., Legnini I., Capauto D., Rosa A., De Santis R., Scarfò R., Peruzzi G., et al. FUS affects circular RNA expression in murine embryonic stem cell-derived motor neurons. Nat. Commun. 2017;8:14741. doi: 10.1038/ncomms14741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ashwal-Fluss R., Meyer M., Pamudurti N.R., Ivanov A., Bartok O., Hanan M., Evantal N., Memczak S., Rajewsky N., Kadener S. circRNA biogenesis competes with pre-mRNA splicing. Mol. Cell. 2014;56:55–66. doi: 10.1016/j.molcel.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 35.Kelly S., Greenman C., Cook P.R., Papantonis A. Exon Skipping Is Correlated with Exon Circularization. J. Mol. Biol. 2015;427:2414–2417. doi: 10.1016/j.jmb.2015.02.018. [DOI] [PubMed] [Google Scholar]
- 36.Starke S., Jost I., Rossbach O., Schneider T., Schreiner S., Hung L.H., Bindereif A. Exon circularization requires canonical splice signals. Cell Rep. 2015;10:103–111. doi: 10.1016/j.celrep.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 37.Liang D., Tatomer D.C., Luo Z., Wu H., Yang L., Chen L.L., Cherry S., Wilusz J.E. The Output of Protein-Coding Genes Shifts to Circular RNAs When the Pre-mRNA Processing Machinery Is Limiting. Mol. Cell. 2017;68:940–954.e3. doi: 10.1016/j.molcel.2017.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ragan C., Goodall G.J., Shirokikh N.E., Preiss T. Insights into the biogenesis and potential functions of exonic circular RNA. Sci. Rep. 2019;9:2048. doi: 10.1038/s41598-018-37037-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang X.-O., Wang H.-B., Zhang Y., Lu X., Chen L.-L., Yang L. Complementary sequence-mediated exon circularization. Cell. 2014;159:134–147. doi: 10.1016/j.cell.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 40.Yu C.-Y., Kuo H.-C. The emerging roles and functions of circular RNAs and their generation. J. Biomed. Sci. 2019;26:29. doi: 10.1186/s12929-019-0523-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ng W.L., Mohd Mohidin T.B., Shukla K. Functional role of circular RNAs in cancer development and progression. RNA Biol. 2018;15:995–1005. doi: 10.1080/15476286.2018.1486659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hu Z.Q., Zhou S.L., Li J., Zhou Z.J., Wang P.C., Xin H.Y., Mao L., Luo C.B., Yu S.Y., Huang X.W. Circular RNA sequencing identifies CircASAP1 as a key regulator in hepatocellular carcinoma metastasis. Hepatology. 2020;72:906–922. doi: 10.1002/hep.31068. [DOI] [PubMed] [Google Scholar]
- 43.Li Q., Pan X., Zhu D., Deng Z., Jiang R., Wang X. Circular RNA MAT2B promotes glycolysis and malignancy of hepatocellular carcinoma through the miR-338-3p/PKM2 axis under hypoxic stress. Hepatology. 2019;70:1298–1316. doi: 10.1002/hep.30671. [DOI] [PubMed] [Google Scholar]
- 44.Xu J., Ji L., Liang Y., Wan Z., Zheng W., Song X., Gorshkov K., Sun Q., Lin H., Zheng X. CircRNA-SORE mediates sorafenib resistance in hepatocellular carcinoma by stabilizing YBX1. Signal Transduct. Target. Ther. 2020;5:298. doi: 10.1038/s41392-020-00375-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu J., Wan Z., Tang M., Lin Z., Jiang S., Ji L., Gorshkov K., Mao Q., Xia S., Cen D. N6-methyladenosine-modified CircRNA-SORE sustains sorafenib resistance in hepatocellular carcinoma by regulating β-catenin signaling. Mol. Cancer. 2020;19:163. doi: 10.1186/s12943-020-01281-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu J., Xu Q.-g., Wang Z.-g., Yang Y., Zhang L., Ma J.-z., Sun S.-h., Yang F., Zhou W.-p. Circular RNA cSMARCA5 inhibits growth and metastasis in hepatocellular carcinoma. J. Hepatol. 2018;68:1214–1227. doi: 10.1016/j.jhep.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 47.Zhang P.-F., Gao C., Huang X.-Y., Lu J.-C., Guo X.-J., Shi G.-M., Cai J.-B., Ke A.-W. Cancer cell-derived exosomal circUHRF1 induces natural killer cell exhaustion and may cause resistance to anti-PD1 therapy in hepatocellular carcinoma. Mol. Cancer. 2020;19:110. doi: 10.1186/s12943-020-01222-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu Y., Liu L. Changes in the epidemiology of hepatocellular carcinoma in Asia. Cancers. 2022;14:4473. doi: 10.3390/cancers14184473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang C.h., Cheng Y., Zhang S., Fan J., Gao Q. Changing epidemiology of hepatocellular carcinoma in Asia. Liver Int. 2022;42:2029–2041. doi: 10.1111/liv.15251. [DOI] [PubMed] [Google Scholar]
- 50.Serraino D., Fratino L., Piselli P. Hepatocellular Carcinoma. Springer International Publishing; Cham, Switaerland: 2022. Epidemiological Aspects of Hepatocellular Carcinoma; pp. 3–9. [Google Scholar]
- 51.Shaalan Y.M., Handoussa H., Youness R.A., Assal R.A., El-Khatib A.H., Linscheid M.W., El Tayebi H.M., Abdelaziz A.I. Destabilizing the interplay between miR-1275 and IGF2BPs by Tamarix articulata and quercetin in hepatocellular carcinoma. Nat. Prod. Res. 2018;32:2217–2220. doi: 10.1080/14786419.2017.1366478. [DOI] [PubMed] [Google Scholar]
- 52.Ferlay J., Soerjomataram I., Dikshit R., Eser S., Mathers C., Rebelo M., Parkin D.M., Forman D., Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 53.El-Mesallamy H.O., Hamdy N.M., Sallam A.A. Effect of obesity and glycemic control on serum lipocalins and insulin-like growth factor axis in type 2 diabetic patients. Acta Diabetol. 2013;50:679–685. doi: 10.1007/s00592-012-0373-6. [DOI] [PubMed] [Google Scholar]
- 54.Khella M.S., Hamdy N.M., Amin A.I., El-Mesallamy H.O. The (FTO) gene polymorphism is associated with metabolic syndrome risk in Egyptian females: A case- control study. BMC Med. Genet. 2017;18:101. doi: 10.1186/s12881-017-0461-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Youness R.A., Mohamed A.H., Efthimiadou E.K., Mekky R.Y., Braoudaki M., Fahmy S.A. A Snapshot of Photoresponsive Liposomes in Cancer Chemotherapy and Immunotherapy: Opportunities and Challenges. ACS Omega. 2023;8:44424–44436. doi: 10.1021/acsomega.3c04134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kiriacos C.J., Khedr M.R., Tadros M., Youness R.A. Prospective Medicinal Plants and Their Phytochemicals Shielding Autoimmune and Cancer Patients Against the SARS-CoV-2 Pandemic: A Special Focus on Matcha. Front. Oncol. 2022;12:837408. doi: 10.3389/fonc.2022.837408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Youssef S.S., Abbas E., Youness R.A., Elemeery M.N., Nasr A.S., Seif S. PNPLA3 and IL 28B signature for predicting susceptibility to chronic hepatitis C infection and fibrosis progression. Arch. Physiol. Biochem. 2022;128:483–489. doi: 10.1080/13813455.2019.1694039. [DOI] [PubMed] [Google Scholar]
- 58.Youssef S.S., Youness R.A., Abbas E.A.E., Osman N.M., ELFiky A., El-Kassas M. miR-516a-3P, a potential circulating biomarker in hepatocellular carcinoma, correlated with rs738409 polymorphism in PNPLA3. Per. Med. 2022;19:483–493. doi: 10.2217/pme-2022-0005. [DOI] [PubMed] [Google Scholar]
- 59.Mekky R.Y., El-Ekiaby N., El Sobky S.A., Elemam N.M., Youness R.A., El-Sayed M., Hamza M.T., Esmat G., Abdelaziz A.I. Epigallocatechin gallate (EGCG) and miR-548m reduce HCV entry through repression of CD81 receptor in HCV cell models. Arch. Virol. 2019;164:1587–1595. doi: 10.1007/s00705-019-04232-x. [DOI] [PubMed] [Google Scholar]
- 60.Aboouf M.A., Hamdy N.M., Amin A.I., El-Mesallamy H.O. Genotype screening of APLN rs3115757 variant in Egyptian women population reveals an association with obesity and insulin resistance. Diabetes Res. Clin. Pract. 2015;109:40–47. doi: 10.1016/j.diabres.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 61.El Mesallamy H.O., Hamdy N.M., Mostafa D.M., Amin A.I. The serine protease granzyme B as an inflammatory marker, in relation to the insulin receptor cleavage in human obesity and type 2 diabetes mellitus. J. Interferon Cytokine Res. 2014;34:179–186. doi: 10.1089/jir.2013.0059. [DOI] [PubMed] [Google Scholar]
- 62.Mekky R.Y., Elemam N.M., Eltahtawy O., Zeinelabdeen Y., Youness R.A. Evaluating Risk: Benefit Ratio of Fat-Soluble Vitamin Supplementation to SARS-CoV-2-Infected Autoimmune and Cancer Patients: Do Vitamin-Drug Interactions Exist? Life. 2022;12:1654. doi: 10.3390/life12101654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Youness R.A., Dawoud A., ElTahtawy O., Farag M.A. Fat-soluble vitamins: Updated review of their role and orchestration in human nutrition throughout life cycle with sex differences. Nutr. Metab. 2022;19:60. doi: 10.1186/s12986-022-00696-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ito T., Nguyen M.H. Perspectives on the underlying etiology of HCC and its effects on treatment outcomes. J. Hepatocell. Carcinoma. 2023;10:413–428. doi: 10.2147/JHC.S347959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rahmoon M.A., Youness R.A., Gomaa A.I., Hamza M.T., Waked I., El Tayebi H.M., Abdelaziz A.I. MiR-615-5p depresses natural killer cells cytotoxicity through repressing IGF-1R in hepatocellular carcinoma patients. Growth Factors. 2017;35:76–87. doi: 10.1080/08977194.2017.1354859. [DOI] [PubMed] [Google Scholar]
- 66.Ahmed Youness R., Amr Assal R., Mohamed Ezzat S., Zakaria Gad M., Abdel Motaal A. A methoxylated quercetin glycoside harnesses HCC tumor progression in a TP53/miR-15/miR-16 dependent manner. Nat. Prod. Res. 2020;34:1475–1480. doi: 10.1080/14786419.2018.1509326. [DOI] [PubMed] [Google Scholar]
- 67.Ali N.A., Hamdy N.M., Gibriel A.A., El Mesallamy H.O. Investigation of the relationship between CTLA4 and the tumor suppressor RASSF1A and the possible mediating role of STAT4 in a cohort of Egyptian patients infected with hepatitis C virus with and without hepatocellular carcinoma. Arch. Virol. 2021;166:1643–1651. doi: 10.1007/s00705-021-04981-8. [DOI] [PubMed] [Google Scholar]
- 68.El-Derany M.O., Hamdy N.M., Al-Ansari N.L., El-Mesallamy H.O. Integrative role of vitamin D related and Interleukin-28B genes polymorphism in predicting treatment outcomes of Chronic Hepatitis C. BMC Gastroenterol. 2016;16:19. doi: 10.1186/s12876-016-0440-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rizk H.H., Hamdy N.M., Al-Ansari N.L., El-Mesallamy H.O. Pretreatment Predictors of Response to PegIFN-RBV Therapy in Egyptian Patients with HCV Genotype 4. PLoS ONE. 2016;11:e0153895. doi: 10.1371/journal.pone.0153895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.El-Mesallamy H.O., Hamdy N.M., Rizk H.H., El-Zayadi A.R. Apelin serum level in Egyptian patients with chronic hepatitis C. Mediat. Inflamm. 2011;2011:703031. doi: 10.1155/2011/703031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.El-Mesallamy H.O., Hamdy N.M., Zaghloul A.S., Sallam A.M. Serum retinol binding protein-4 and neutrophil gelatinase-associated lipocalin are interrelated in pancreatic cancer patients. Scand. J. Clin. Lab. Invest. 2012;72:602–607. doi: 10.3109/00365513.2012.723135. [DOI] [PubMed] [Google Scholar]
- 72.El-Mesallamy H.O., Hamdy N.M., Zaghloul A.S., Sallam A.M. Clinical value of circulating lipocalins and insulin-like growth factor axis in pancreatic cancer diagnosis. Pancreas. 2013;42:149–154. doi: 10.1097/MPA.0b013e3182550d9d. [DOI] [PubMed] [Google Scholar]
- 73.Youssef S.S., Hamdy N.M. SOCS1 and pattern recognition receptors: TLR9 and RIG-I; novel haplotype associations in Egyptian fibrotic/cirrhotic patients with HCV genotype 4. Arch. Virol. 2017;162:3347–3354. doi: 10.1007/s00705-017-3498-7. [DOI] [PubMed] [Google Scholar]
- 74.Ueno M., Takeda H., Takai A., Seno H. Risk factors and diagnostic biomarkers for nonalcoholic fatty liver disease-associated hepatocellular carcinoma: Current evidence and future perspectives. World J. Gastroenterol. 2022;28:3410–3421. doi: 10.3748/wjg.v28.i27.3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kew M.C. Hepatocellular carcinoma: Epidemiology and risk factors. J. Hepatocell. Carcinoma. 2014;1:115–125. doi: 10.2147/JHC.S44381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Assal R.A., Elemam N.M., Mekky R.Y., Attia A.A., Soliman A.H., Gomaa A.I., Efthimiadou E.K., Braoudaki M., Fahmy S.A., Youness R.A. A Novel Epigenetic Strategy to Concurrently Block Immune Checkpoints PD-1/PD-L1 and CD155/TIGIT in Hepatocellular Carcinoma. Transl. Oncol. 2024;45:101961. doi: 10.1016/j.tranon.2024.101961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tsuchiya N., Sawada Y., Endo I., Saito K., Uemura Y., Nakatsura T. Biomarkers for the early diagnosis of hepatocellular carcinoma. World J. Gastroenterol. WJG. 2015;21:10573. doi: 10.3748/wjg.v21.i37.10573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lin Y.L., Li Y. Study on the hepatocellular carcinoma model with metastasis. Genes. Dis. 2020;7:336–350. doi: 10.1016/j.gendis.2019.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Youness R.A., Gohar A., Kiriacos C.J., El-Shazly M. Heat Shock Proteins: Central Players in Oncological and Immuno-Oncological Tracks. Adv. Exp. Med. Biol. 2023;1409:193–203. doi: 10.1007/5584_2022_736. [DOI] [PubMed] [Google Scholar]
- 80.Sharma S.D. Hepatitis C virus 1b viral factors (core, NS3, and NS5A) and increased risk of hepatocellular carcinoma. Hepatology. 2013;58:491–493. doi: 10.1002/hep.26362. [DOI] [PubMed] [Google Scholar]
- 81.Zhou Y., Zhao Y., Gao Y., Hu W., Qu Y., Lou N., Zhu Y., Zhang X., Yang H. Hepatitis C virus NS3 protein enhances hepatocellular carcinoma cell invasion by promoting PPM1A ubiquitination and degradation. J. Exp. Clin. Cancer Res. 2017;36:1–13. doi: 10.1186/s13046-017-0510-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mahmoud M.M., Sanad E.F., Hamdy N.M. MicroRNAs’ role in the environment-related non-communicable diseases and link to multidrug resistance, regulation, or alteration. Environ. Sci. Pollut. Res. Int. 2021;28:36984–37000. doi: 10.1007/s11356-021-14550-w. [DOI] [PubMed] [Google Scholar]
- 83.Dykes I.M., Emanueli C. Transcriptional and post-transcriptional gene regulation by long non-coding RNA. Genom. Proteom. Bioinform. 2017;15:177–186. doi: 10.1016/j.gpb.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang C., Wang L., Ding Y., Lu X., Zhang G., Yang J., Zheng H., Wang H., Jiang Y., Xu L. LncRNA structural characteristics in epigenetic regulation. Int. J. Mol. Sci. 2017;18:2659. doi: 10.3390/ijms18122659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang C.-Z., Yan G.-X., Dong D.-S., Xin H., Liu Z.-Y. LncRNA-ATB promotes autophagy by activating Yes-associated protein and inducing autophagy-related protein 5 expression in hepatocellular carcinoma. World J. Gastroenterol. 2019;25:5310. doi: 10.3748/wjg.v25.i35.5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Emam O., Wasfey E.F., Hamdy N.M. Notch-associated lncRNAs profiling circuiting epigenetic modification in colorectal cancer. Cancer Cell Int. 2022;22:316. doi: 10.1186/s12935-022-02736-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Abd El Fattah Y.K., Abulsoud A.I., AbdelHamid S.G., AbdelHalim S., Hamdy N.M. CCDC144NL-AS1/hsa-miR-143-3p/HMGA2 interaction: In-silico and clinically implicated in CRC progression, correlated to tumor stage and size in case-controlled study; step toward ncRNA precision. Int. J. Biol. Macromol. 2023;253:126739. doi: 10.1016/j.ijbiomac.2023.126739. [DOI] [PubMed] [Google Scholar]
- 88.Eldash S., Sanad E.F., Nada D., Hamdy N.M. The Intergenic Type LncRNA (LINC RNA) Faces in Cancer with In Silico Scope and a Directed Lens to LINC00511: A Step toward ncRNA Precision. Non-Coding RNA. 2023;9:58. doi: 10.3390/ncrna9050058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Eldosoky M.A., Hammad R., Elmadbouly A.A., Aglan R.B., Abdel-Hamid S.G., Alboraie M., Hassan D.A., Shaheen M.A., Rushdi A., Ahmed R.M., et al. Diagnostic Significance of hsa-miR-21-5p, hsa-miR-192-5p, hsa-miR-155-5p, hsa-miR-199a-5p Panel and Ratios in Hepatocellular Carcinoma on Top of Liver Cirrhosis in HCV-Infected Patients. Int. J. Mol. Sci. 2023;24:3157. doi: 10.3390/ijms24043157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sokolov D., Sharda N., Banerjee A., Denisenko K., Basalious E.B., Shukla H., Waddell J., Hamdy N.M., Banerjee A. Differential Signaling Pathways in Medulloblastoma: Nano-biomedicine Targeting Non-coding Epigenetics to Improve Current and Future Therapeutics. Curr. Pharm. Des. 2024;30:31–47. doi: 10.2174/0113816128277350231219062154. [DOI] [PubMed] [Google Scholar]
- 91.Mahmoud M.M., Sanad E.F., Elshimy R.A.A., Hamdy N.M. Competitive Endogenous Role of the LINC00511/miR-185-3p Axis and miR-301a-3p From Liquid Biopsy as Molecular Markers for Breast Cancer Diagnosis. Front. Oncol. 2021;11:749753. doi: 10.3389/fonc.2021.749753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hammad R., Eldosoky M.A., Elmadbouly A.A., Aglan R.B., AbdelHamid S.G., Zaky S., Ali E., Abd El Hakam F.E., Mosaad A.M., Abdelmageed N.A., et al. Monocytes subsets altered distribution and dysregulated plasma hsa-miR-21-5p and hsa-miR-155-5p in HCV-linked liver cirrhosis progression to hepatocellular carcinoma. J. Cancer Res. Clin. Oncol. 2023;149:15349–15364. doi: 10.1007/s00432-023-05313-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fu N., Niu X., Wang Y., Du H., Wang B., Du J., Li Y., Wang R., Zhang Y., Zhao S. Role of LncRNA-activated by transforming growth factor beta in the progression of hepatitis C virus-related liver fibrosis. Discov. Med. 2016;22:29–42. [PubMed] [Google Scholar]
- 94.Plissonnier M.-L., Herzog K., Levrero M., Zeisel M.B. Non-coding RNAs and hepatitis C virus-induced hepatocellular carcinoma. Viruses. 2018;10:591. doi: 10.3390/v10110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li J., Bao H., Huang Z., Liang Z., Wang M., Lin N., Ni C., Xu Y. Little things with significant impact: miRNAs in hepatocellular carcinoma. Front. Oncol. 2023;13:1191070. doi: 10.3389/fonc.2023.1191070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li D., Chen B., Zeng Y., Wang H. UHRF1 Could Be a Prognostic Biomarker and Correlated with Immune Cell Infiltration in Hepatocellular Carcinoma. Int. J. Gen. Med. 2021;14:6769–6776. doi: 10.2147/IJGM.S335016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen G., Shi Y., Liu M., Sun J. circHIPK3 regulates cell proliferation and migration by sponging miR-124 and regulating AQP3 expression in hepatocellular carcinoma. Cell Death Dis. 2018;9:175. doi: 10.1038/s41419-017-0204-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kim G., Yoon J.S., Jang S.Y., Park S.Y., Tak W.Y., Kweon Y.-O., Hur K. Clinical significance of noncoding circHIPK3 RNA in human hepatocellular carcinoma. Cancer Res. 2018;78:2471. doi: 10.1158/1538-7445.AM2018-2471. [DOI] [Google Scholar]
- 99.Dong X., Zhang P., Liu L., Li H., Cheng S., Li S., Wang Y., Zheng C., Dong J., Zhang L. The Circ_0001367/miR-545-3p/LUZP1 axis regulates cell proliferation, migration and invasion in glioma cells. Front. Oncol. 2021;11:781471. doi: 10.3389/fonc.2021.781471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang P.-F., Wei C.-Y., Huang X.-Y., Peng R., Yang X., Lu J.-C., Zhang C., Gao C., Cai J.-B., Gao P.-T. Circular RNA circTRIM33–12 acts as the sponge of MicroRNA-191 to suppress hepatocellular carcinoma progression. Mol. Cancer. 2019;18:105. doi: 10.1186/s12943-019-1031-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Han D., Li J., Wang H., Su X., Hou J., Gu Y., Qian C., Lin Y., Liu X., Huang M. Circular RNA circMTO1 acts as the sponge of microRNA-9 to suppress hepatocellular carcinoma progression. Hepatology. 2017;66:1151–1164. doi: 10.1002/hep.29270. [DOI] [PubMed] [Google Scholar]
- 102.Zheng Q., Bao C., Guo W., Li S., Chen J., Chen B., Luo Y., Lyu D., Li Y., Shi G., et al. Circular RNA profiling reveals an abundant circHIPK3 that regulates cell growth by sponging multiple miRNAs. Nat. Commun. 2016;7:11215. doi: 10.1038/ncomms11215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li W., Xue H., Li Y., Li P., Ma F., Liu M., Kong S. HIPK3 circular RNA promotes metastases of HCC through sponging miR-338-3p to induce ZEB2 expression. Dig. Dis. Sci. 2021;66:3439–3447. doi: 10.1007/s10620-020-06688-3. [DOI] [PubMed] [Google Scholar]
- 104.Tan A., Li Q., Chen L. CircZFR promotes hepatocellular carcinoma progression through regulating miR-3619–5p/CTNNB1 axis and activating Wnt/β-catenin pathway. Arch. Biochem. Biophys. 2019;661:196–202. doi: 10.1016/j.abb.2018.11.020. [DOI] [PubMed] [Google Scholar]
- 105.Bai N., Peng E., Qiu X., Lyu N., Zhang Z., Tao Y., Li X., Wang Z. circFBLIM1 act as a ceRNA to promote hepatocellular cancer progression by sponging miR-346. J. Exp. Clin. Cancer Res. 2018;37:172. doi: 10.1186/s13046-018-0838-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang J., Che J. CircTP63 promotes hepatocellular carcinoma progression by sponging miR-155-5p and upregulating ZBTB18. Cancer Cell Int. 2021;21:156. doi: 10.1186/s12935-021-01753-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhou H., Chen Y. CircRNA has_circ_0001806 promotes hepatocellular carcinoma progression via the miR-193a-5p/MMP16 pathway. Braz. J. Med. Biol. Res. Rev. Bras. Pesqui. Medicas E Biol. 2021;54:e11459. doi: 10.1590/1414-431x2021e11459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nie G., Peng D., Wen N., Wang Y., Li B., Lu J. CircYTHDF3 Promotes Hepatocellular Carcinoma Progression through Modulating miR-136-5p/CBX4/VEGF Axis. 2023. [(accessed on 10 July 2024)]. Available online: https://susy.mdpi.com/user/assigned/production_form/eb3b73aef73bf6716a290b888d514329.
- 109.Chen Z., Du J., Yang C., Si G., Chen Y. circ-CFH promotes the development of HCC by regulating cell proliferation, apoptosis, migration, invasion, and glycolysis through the miR-377-3p/RNF38 axis. Open Life Sci. 2022;17:248–260. doi: 10.1515/biol-2022-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhang B., Li F., Zhu Z., Ding A., Luo J. CircRNA CDR1as/miR-1287/Raf1 axis modulates hepatocellular carcinoma progression through MEK/ERK pathway. Cancer Manag. Res. 2020;12:8951–8964. doi: 10.2147/CMAR.S252679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liu B., Yang G., Wang X., Liu J., Lu Z., Wang Q., Xu B., Liu Z., Li J. CircBACH1 (hsa_circ_0061395) promotes hepatocellular carcinoma growth by regulating p27 repression via HuR. J. Cell. Physiol. 2020;235:6929–6941. doi: 10.1002/jcp.29589. [DOI] [PubMed] [Google Scholar]
- 112.Zhu Y.-J., Zheng B., Luo G.-J., Ma X.-K., Lu X.-Y., Lin X.-M., Yang S., Zhao Q., Wu T., Li Z.-X. Circular RNAs negatively regulate cancer stem cells by physically binding FMRP against CCAR1 complex in hepatocellular carcinoma. Theranostics. 2019;9:3526. doi: 10.7150/thno.32796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yang Z.-G., Awan F.M., Du W.W., Zeng Y., Lyu J., Wu D., Gupta S., Yang W., Yang B.B. The circular RNA interacts with STAT3, increasing its nuclear translocation and wound repair by modulating Dnmt3a and miR-17 function. Mol. Ther. 2017;25:2062–2074. doi: 10.1016/j.ymthe.2017.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zeng Y., Du W.W., Wu Y., Yang Z., Awan F.M., Li X., Yang W., Zhang C., Yang Q., Yee A. A circular RNA binds to and activates AKT phosphorylation and nuclear localization reducing apoptosis and enhancing cardiac repair. Theranostics. 2017;7:3842. doi: 10.7150/thno.19764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yang Q., Du W.W., Wu N., Yang W., Awan F.M., Fang L., Ma J., Li X., Zeng Y., Yang Z. A circular RNA promotes tumorigenesis by inducing c-myc nuclear translocation. Cell Death Differ. 2017;24:1609–1620. doi: 10.1038/cdd.2017.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang L., Long H., Zheng Q., Bo X., Xiao X., Li B. Circular RNA circRHOT1 promotes hepatocellular carcinoma progression by initiation of NR2F6 expression. Mol. Cancer. 2019;18:119. doi: 10.1186/s12943-019-1046-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sun S., Wang W., Luo X., Li Y., Liu B., Li X., Zhang B., Han S., Li X. Circular RNA circ-ADD3 inhibits hepatocellular carcinoma metastasis through facilitating EZH2 degradation via CDK1-mediated ubiquitination. Am. J. Cancer Res. 2019;9:1695. [PMC free article] [PubMed] [Google Scholar]
- 118.Shi L., Liu B., Shen D.-d., Yan P., Zhang Y., Tian Y., Hou L., Jiang G., Zhu Y., Liang Y. A tumor-suppressive circular RNA mediates uncanonical integrin degradation by the proteasome in liver cancer. Sci. Adv. 2021;7:eabe5043. doi: 10.1126/sciadv.abe5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gu Y., Wang Y., He L., Zhang J., Zhu X., Liu N., Wang J., Lu T., He L., Tian Y. Circular RNA circIPO11 drives self-renewal of liver cancer initiating cells via Hedgehog signaling. Mol. Cancer. 2021;20:132. doi: 10.1186/s12943-021-01435-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Liang W.-C., Wong C.-W., Liang P.-P., Shi M., Cao Y., Rao S.-T., Tsui S.K.-W., Waye M.M.-Y., Zhang Q., Fu W.-M. Translation of the circular RNA circβ-catenin promotes liver cancer cell growth through activation of the Wnt pathway. Genome Biol. 2019;20:84. doi: 10.1186/s13059-019-1685-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhao Z., Song J., Tang B., Fang S., Zhang D., Zheng L., Wu F., Gao Y., Chen C., Hu X. CircSOD2 induced epigenetic alteration drives hepatocellular carcinoma progression through activating JAK2/STAT3 signaling pathway. J. Exp. Clin. Cancer Res. 2020;39:259. doi: 10.1186/s13046-020-01769-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chen R.-X., Chen X., Xia L.-P., Zhang J.-X., Pan Z.-Z., Ma X.-D., Han K., Chen J.-W., Judde J.-G., Deas O. N 6-methyladenosine modification of circNSUN2 facilitates cytoplasmic export and stabilizes HMGA2 to promote colorectal liver metastasis. Nat. Commun. 2019;10:4695. doi: 10.1038/s41467-019-12651-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Guarnerio J., Zhang Y., Cheloni G., Panella R., Mae Katon J., Simpson M., Matsumoto A., Papa A., Loretelli C., Petri A. Intragenic antagonistic roles of protein and circRNA in tumorigenesis. Cell Res. 2019;29:628–640. doi: 10.1038/s41422-019-0192-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hong Y., Qin H., Li Y., Zhang Y., Zhuang X., Liu L., Lu K., Li L., Deng X., Liu F. FNDC3B circular RNA promotes the migration and invasion of gastric cancer cells via the regulation of E-cadherin and CD44 expression. J. Cell. Physiol. 2019;234:19895–19910. doi: 10.1002/jcp.28588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chen L., Kong R., Wu C., Wang S., Liu Z., Liu S., Li S., Chen T., Mao C., Liu S. Circ-MALAT1 functions as both an mRNA translation brake and a microRNA sponge to promote self-renewal of hepatocellular cancer stem cells. Adv. Sci. 2020;7:1900949. doi: 10.1002/advs.201900949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wu N., Yuan Z., Du K.Y., Fang L., Lyu J., Zhang C., He A., Eshaghi E., Zeng K., Ma J. Translation of yes-associated protein (YAP) was antagonized by its circular RNA via suppressing the assembly of the translation initiation machinery. Cell Death Differ. 2019;26:2758–2773. doi: 10.1038/s41418-019-0337-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Huang W., Huang F., Feng C. CircFoxo3 Promotes Adriamycin Resistance Through Regulation of miR-199a-5p/ATP Binding Cassette Subfamily C Member 1 Axis in Hepatocellular Carcinoma. OncoTargets Ther. 2020;13:5113–5122. doi: 10.2147/OTT.S243571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chen H., Liu S., Li M., Huang P., Li X. circ_0003418 Inhibits Tumorigenesis And Cisplatin Chemoresistance Through Wnt/β-Catenin Pathway In Hepatocellular Carcinoma. OncoTargets Ther. 2019;12:9539–9549. doi: 10.2147/OTT.S229507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Papatsirou M., Artemaki P.I., Scorilas A., Kontos C.K. The role of circular RNAs in therapy resistance of patients with solid tumors. Pers. Med. 2020;17:469–490. doi: 10.2217/pme-2020-0103. [DOI] [PubMed] [Google Scholar]
- 130.Rao D., Yu C., Sheng J., Lv E., Huang W. The emerging roles of circFOXO3 in cancer. Front. Cell Dev. Biol. 2021;9:659417. doi: 10.3389/fcell.2021.659417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Xiong D., He R., Dang Y., Wu H., Feng Z., Chen G. The latest overview of circRNA in the progression, diagnosis, prognosis, treatment, and drug resistance of hepatocellular carcinoma. Front. Oncol. 2021;10:608257. doi: 10.3389/fonc.2020.608257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Liao R., Liu L., Zhou J., Wei X., Huang P. Current molecular biology and therapeutic strategy status and prospects for circRNAs in HBV-associated hepatocellular carcinoma. Front. Oncol. 2021;11:697747. doi: 10.3389/fonc.2021.697747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jiang M.-P., Xu W.-X., Hou J.-C., Xu Q., Wang D.-D., Tang J.-H. The emerging role of the interactions between circular RNAs and RNA-binding proteins in common human cancers. J. Cancer. 2021;12:5206. doi: 10.7150/jca.58182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Piñero F., Dirchwolf M., Pessôa M.G. Biomarkers in Hepatocellular Carcinoma: Diagnosis, Prognosis and Treatment Response Assessment. Cells. 2020;9:1370. doi: 10.3390/cells9061370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Janevska D., Chaloska-Ivanova V., Janevski V. Hepatocellular carcinoma: Risk factors, diagnosis and treatment. Open Access Maced. J. Med. Sci. 2015;3:732. doi: 10.3889/oamjms.2015.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lee C.W., Tsai H.I., Lee W.C., Huang S.W., Lin C.Y., Hsieh Y.C., Kuo T., Chen C.W., Yu M.C. Normal Alpha-Fetoprotein Hepatocellular Carcinoma: Are They Really Normal? J. Clin. Med. 2019;8:1736. doi: 10.3390/jcm8101736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhang Y.S., Chu J.H., Cui S.X., Song Z.Y., Qu X.J. Des-γ-carboxy prothrombin (DCP) as a potential autologous growth factor for the development of hepatocellular carcinoma. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2014;34:903–915. doi: 10.1159/000366308. [DOI] [PubMed] [Google Scholar]
- 138.Aparna G.M., Tetala K.K.R. Recent Progress in Development and Application of DNA, Protein, Peptide, Glycan, Antibody, and Aptamer Microarrays. Biomolecules. 2023;13:602. doi: 10.3390/biom13040602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Dabbish A.M., Abdelzaher H.M., Abohawya M., Shamma S., Mahmoud Y.H., Maged A., Manaa M., Hassany M., Kobeissy F., Bazgir O., et al. Prognostic MicroRNA Panel for HCV-Associated HCC: Integrating Computational Biology and Clinical Validation. Cancers. 2022;14:3036. doi: 10.3390/cancers14133036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bonferoni M.C., Gavini E., Rassu G., Maestri M., Giunchedi P. Chitosan nanoparticles for therapy and theranostics of hepatocellular carcinoma (HCC) and liver-targeting. Nanomaterials. 2020;10:870. doi: 10.3390/nano10050870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wang M., Wu M., Xie T., Chen J. Circular RNAs Sparkle in the Diagnosis and Theranostics of Hepatocellular Carcinoma. Front. Genet. 2021;11:628655. doi: 10.3389/fgene.2020.628655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhang S., Zhou Y., Wang Y., Wang Z., Xiao Q., Zhang Y., Lou Y., Qiu Y., Zhu F. The mechanistic, diagnostic and therapeutic novel nucleic acids for hepatocellular carcinoma emerging in past score years. Brief. Bioinform. 2021;22:1860–1883. doi: 10.1093/bib/bbaa023. [DOI] [PubMed] [Google Scholar]
- 143.Chen W., Quan Y., Fan S., Wang H., Liang J., Huang L., Chen L., Liu Q., He P., Ye Y. Exosome-transmitted circular RNA hsa_circ_0051443 suppresses hepatocellular carcinoma progression. Cancer Lett. 2020;475:119–128. doi: 10.1016/j.canlet.2020.01.022. [DOI] [PubMed] [Google Scholar]
- 144.Lai H., Li Y., Zhang H., Hu J., Liao J., Su Y., Li Q., Chen B., Li C., Wang Z. exoRBase 2.0: An atlas of mRNA, lncRNA and circRNA in extracellular vesicles from human biofluids. Nucleic Acids Res. 2022;50:D118–D128. doi: 10.1093/nar/gkab1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhao Z., Wang K., Wu F., Wang W., Zhang K., Hu H., Liu Y., Jiang T. circRNA disease: A manually curated database of experimentally supported circRNA-disease associations. Cell Death Dis. 2018;9:475. doi: 10.1038/s41419-018-0503-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wu C., Deng L., Zhuo H., Chen X., Tan Z., Han S., Tang J., Qian X., Yao A. Circulating circRNA predicting the occurrence of hepatocellular carcinoma in patients with HBV infection. J. Cell Mol. Med. 2020;24:10216–10222. doi: 10.1111/jcmm.15635. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the data obtained and/or analyzed during the current study are available within the manuscript or the bioinformatics databases.