Abstract
Background
Previous meta-analyses have investigated the efficacy of lipid-lowering therapies for atherosclerotic cardiovascular disease; however, few have focused on patients with acute coronary syndrome (ACS). This meta-analysis aimed to compare the benefits of intensive lipid-lowering therapy with those of background statin therapy in patients with ACS.
Methods
Searches were performed on PubMed, Embase, the Cochrane Central Register of Controlled Trials, and ClinicalTrials.gov databases for articles published until April 13, 2023. Randomized controlled trials that compared intensive lipid-lowering therapies and background statin therapies in patients with prior ACS and recorded the outcome of three-point major cardiovascular events (MACE) were included. The risk ratio (RR) with 95% confidence interval (CI) was used as a measure of primary and secondary outcomes.
Results
Nine trials involving 38,640 patients with ACS were identified. Pooled results suggested that intensive lipid-lowering therapies are associated with a reduction in the risk of three-point MACE (RR, 0.88; 95% CI, 0.83–0.94; p < 0.001), recurrent ACS (RR, 0.82; 95% CI, 0.71–0.96; p = 0.013), nonfatal myocardial infarction (MI) (RR, 0.87; 95% CI, 0.81–0.93; p < 0.001), stroke (RR, 0.83; 95% CI, 0.73–0.94; p = 0.003), and unstable angina-related hospitalization (RR, 0.57; 95% CI, 0.33–0.99; p = 0.046), but not all-cause mortality (RR, 0.94; 95% CI, 0.82–1.07; p = 0.329), cardiovascular disease-related mortality (RR, 0.96; 95% CI, 0.88–1.06; p = 0.457) or coronary revascularization (RR, 0.89; 95% CI, 0.79–1.00; p = 0.057).
Conclusions
Intensive lipid-lowering therapies may reduce the risk of three-point MACE, recurrent ACS, nonfatal MI, stroke, and hospitalization for unstable angina in patients with ACS undergoing background statin therapy. These results may assist in clinical decision-making for the secondary prevention of cardiovascular events to initiate intensive lipid-lowering therapies immediately after ACS.
Keywords: Intensive lipid-lowering, acute coronary syndrome, ezetimibe, PCSK9 inhibitor, cardiovascular risk
Background
The application of intensive statin therapy is associated with progressive reductions in low-density lipoprotein cholesterol (LDL-C) levels and decreased risk of adverse cardiovascular outcomes [1, 2]. Patients with acute coronary syndrome (ACS) continue to face higher odds of experiencing recurrent cardiovascular events, particularly at early time points after the index event [3–7]. This residual risk is in part attributable to difficulties achieving sufficiently low LDL-C levels, with many patients with atherosclerotic cardiovascular disease (ASCVD) struggling to achieve the lipid targets recommended by established guidelines through treatment with statins alone [8, 9]. Therefore, there is growing interest in combining statins with non-statin lipid-modifying treatment regimens [10–12].
In patients with high-to-very high cardiovascular risk, treatment with a combination of ezetimibe or proprotein convertase subtilisin/kexin 9 (PCSK9) inhibitors has been recommended as a means of achieving LDL-C targets [13], based primarily on a dyslipidemia treatment model that seeks to maximize lipid reduction. Accordingly, current evidence-based therapeutic guidelines recommend the prevention of cardiovascular diseases using intensive lipid-lowering therapies, including statins combined with ezetimibe and/or PCSK9 inhibitors. A meta-analysis of published randomized controlled trials (RCTs) found that combined ezetimibe plus statin treatment was associated with a notable reduction in both major adverse cardiovascular events (MACE) and nonfatal myocardial infarction (MI) risk among patients with ASCVD [14]. In a separate network meta-analysis, comparable stroke and nonfatal MI rates were observed in patients with high or very high cardiovascular risk who were already undergoing maximum-tolerated statin treatment combined with either ezetimibe or PCSK9 inhibitors [15]. Moreover, the RACING trial demonstrated that combination therapy using moderate-intensity statins and ezetimibe reduced statin intolerance and was more effective in controlling LDL-C concentrations together with non-inferior long-term clinical outcomes than high-intensity statin monotherapy [16]. However, although this therapy is supported by substantial evidence and recommended by the guidelines, the results of the SANTORINI study showed that only a low proportion of patients with high and very high cardiovascular risks underwent intensive lipid-lowering therapy [17].
Additionally, despite several studies on the effects of ezetimibe and PCSK9 inhibitors on the secondary prevention of cardiovascular disease, most of these studies focused on patients with ASCVD rather than on patients with ACS, who tend to have a higher residual cardiovascular risk [14, 15]. Therefore, there is an urgent need to systematically evaluate the cardiovascular benefits of ezetimibe and/or PCSK9 inhibitors for secondary prevention in patients with ACS [18]. To assess the necessity for the immediate initiation of intensive lipid-lowering treatment following ACS, we conducted a meta-analysis to compare the benefits of intensive lipid-lowering therapies with those of background statin therapy in patients with ACS.
Methods
The present meta-analysis was conducted in accordance with the PRISMA guidelines when abstracting data and assessing validity [19], and the protocol for this study was registered in INPLASY (https://inplasy.com/) under the record number INPLASY202340040.
Search strategy
Searches were performed on PubMed, Embase, the Cochrane Central Register of Controlled Trials, and ClinicalTrials.gov trial registry databases for all relevant RCTs published until April 13, 2023, without any language restrictions. The search terms used for this study included ‘PCSK9 inhibitors’, ‘ezetimibe’, and ‘ACS’, with the most recent update to the search being completed on July 15, 2023. For full details regarding the search terms utilized, see Supplementary Material. The references of prior articles were manually reviewed, and additional trials were identified by searching two grey literature databases (Open Gray and the National Technical Information Service).
Eligibility criteria
To be eligible for inclusion, studies needed to: (1) include either all participants or an identifiable subset of participants with a history of ACS, including episodes of unstable angina or acute ST-/non-ST-segment elevation MI; (2) assess the benefits of different intensive lipid-lowering treatment regimens (e.g. PCSK9 inhibitors and/or ezetimibe plus statin vs. statin; PCSK9 inhibitors and ezetimibe plus statin vs. PCSK9 inhibitors or ezetimibe plus statin); (3) report three-point MACE (a composite of cardiovascular death, nonfatal stroke, and nonfatal MI); (4) have an RCT design. When trials exhibited overlapping datasets, the study with the largest population and the most comprehensive details was included in this meta-analysis.
Data extraction
After eliminating duplicate articles, the titles and abstracts of the remaining studies were screened for eligibility, followed by a full-text review when appropriate. Relevant data were extracted from the RCTs using a standardized data extraction form, and disagreements were resolved by consensus. The extracted data included participant and trial characteristics (first author name, publication year, age, sex, sample size, follow-up duration, treatment arms with dosages, and control arms), number of events, mean baseline LDL-C levels, and differences in LDL-C levels between the groups following treatment. The authors of the articles lacking pertinent information were contacted.
Risk of bias and certainty of evidence analyses
The risk of bias for the included studies was independently evaluated by two reviewers (Xian-Dan Wu and Yue-Lin) using the Cochrane risk of bias tool for randomized trials (ROB2) [20, 21]. Assessments of risk were categorized as low risk, some concerns, or high risk.
The quality of evidence for each outcome was assessed according to the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) framework [22]. Two reviewers (Xian Lin and Yan-Yan Li) independently assigned each outcome a grade of very low, low, moderate, or high. A third reviewer (Xuan-Yan Liu) resolved all disagreements.
Statistical analysis
The primary endpoint of this study was three-point MACE, defined as a composite of cardiovascular disease-related mortality, nonfatal MI, and nonfatal stroke. Recurrent ACS (including recurrent unstable angina or MI), nonfatal MI, stroke, all-cause mortality, coronary revascularization, and unstable angina-related hospitalization were considered as secondary outcomes. A Dersimonian–Laird estimator was implemented when at least two RCTs included sufficient data to permit pooled analyses of a given outcome. Risk ratios (RRs) and 95% confidence intervals (CIs) were used to compare the relative efficacies of intensive lipid-lowering regimens and background statin therapy for the outcomes of interest by calculating the number needed to treat (NNT). A two-sided P-value < 0.05 was considered statistically significant. Heterogeneity levels were assessed based on χ2 calculations and the I2 statistic, with respective I2 values of 0–29%, 30–49%, 50–74%, and 75–100% classified as not important, moderate, substantial, and considerable inconsistency, respectively [23].
Subgroup analyses were conducted for patients stratified based upon several parameters including age (<65 vs. ≥65 years), baseline LDL-C (<100 vs. ≥ 100 mg/dL), degree of LDL-C reduction (<39 vs. ≥ 39 mg/dL), percent reduction in LDL-C levels (<30% vs. ≥30%), study duration (<1 vs. ≥1 year), sample size (<200 vs. 200–1000 vs. >1000 patients), and the percentage of patients that underwent percutaneous coronary intervention (<50% vs. 50%–100% vs. 100%). Microsoft Excel was used to construct forest plots for subgroup analyses [24].
Funnel plot asymmetry attributable to publication bias was identified and corrected using the Trim-and-Fill method [25], while small-study effects were detected using Egger’s test. Additionally, a leave-one-out sensitivity analysis was conducted to identify any RCTs that may have had a disproportional effect. While background statin treatment was administered to all participating patients, the precise statins prescribed were not consistent across studies, and the relative benefits of different statin types and doses, when applied in combination with ezetimibe and/or PCSK9 inhibitors, are uncertain. Sensitivity analyses were also performed wherein only patients prescribed atorvastatin were included in the control group. According to the guidelines established by the American College of Cardiology/American Heart Association, high-intensity statin therapy is a reasonable approach to reducing LDL-C levels and the associated risk [26], An additional sensitivity analysis was performed in which only RCTs with patients on high-intensity background statin regimens were included. Stata v 17.0 (StataCorp, TX, USA) was used for all analyses.
Results
Study selection
An initial literature review identified 80 potentially relevant studies, of which 9 were RCTs that met the inclusion criteria (Supplemental Figure S1) [27–35]. The characteristics of the trials are summarized in Table 1. In total, these trials enrolled 38,640 patients with ACS (9,894 female [25.6%]; mean age, 61.1 [10.1] years), and the average follow-up duration was 4.7 years (range: 8 weeks − 7 years). While no limitations were imposed on baseline patient LDL-C levels in this meta-analysis, the majority of the included studies imposed restrictions on patient entry [27–30, 32, 34]. All trials compared ezetimibe or PCSK9 inhibitors plus statin treatment with statin treatment alone, including five studies on ezetimibe [27, 29, 31, 33, 35] and three on PCSK9 inhibitors [30, 32, 34], with the exception of one study that compared PCSK9 inhibitors plus ezetimibe and statins with ezetimibe plus statins [28].
Table 1.
Characteristics of included studies.
| Study | Population | Intervention treatment | Comparative treatment | Sample size (women) | Age, y | Study duration | Baseline LDL-C, mg/dL | LDL-C difference between groups after treatment, mg/dL | LDL-C after treatment (intervention, mg/dL) | LDL-C after treatment (control, mg/dL) |
|---|---|---|---|---|---|---|---|---|---|---|
| Cannon, 2015 [27] | ACS patients with LDL-C levels ≥ 50 mg/dL | Ezetimibe 10 mg, plus simvastatin 40 mg | Simvastatin 40 mg | 18144 (25.4%) | 63.6 | 7 y | 94 | 17 (18.1%) | 53.2 | 69.9 |
| Japaridze, 2017 [29] | ACS patients with LDL-C levels ≥ 70 mg/dL | Ezetimibe 10 mg, plus atorvastatin 20 mg | Atorvastatin 40 mg | 292 (46.2%) | 62.4 | 12 w | 108 | 12 (11.1%) | 61.9 | 73.9 |
| Liu, 2017 [31] | ACS patients | Ezetimibe 10 mg, plus atorvastatin 10 mg | Atorvastatin 20 mg | 230 (48.3%) | 84 | 1 y | 87 | 8 (9.2%) | 46.4 | 54.1 |
| Ran, 2017 [33] | Non-ST segment elevation ACS patients underwent urgent PCI | Ezetimibe 10 mg, plus rosuvastatin 10 mg | Rosuvastatin 10 mg or 20 mg | 125 (25.6%) | 60.5 | 12 w | 141 | 25 (17.7%) | 46..0 | 64.0 |
| Schwartz, 2018 [34] | ACS patients with LDL-C levels ≥ 70 mg/dL, non–HDL-C ≥ 100 mg/dL, or apolipoprotein B ≥ 80 mg/dL | Alirocumab 75 mg, plus atorvastatin 40 or 80 mg or rosuvastatin 20 or 40 mg | Atorvastatin 40 or 80 mg or rosuvastatin 20 or 40 mg | 18924 (25.2%) | 58.6 | 2.8 y | 92 | 37 (40.2%) | 66.0 | 101.0 |
| Koskinas, 2019 [30] | ACS patients with LDL-C levels ≥ 70 mg/dL (high-intensity statin); ≥90 mg/dL (low or moderate intensity statin); or ≥ 125 mg/dL (without stable statin) | Evolocumab 420 mg, plus atorvastatin 40 mg | Atorvastatin 40 mg | 308 (18.5%) | 60.7 | 8 w | 135 | 55 (40.7%) | 36.7 | 80.0 |
| Tan, 2021 [35] | ACS patients | Ezetimibe 10 mg, plus, atorvastatin 10 mg | Atorvastatin 40 mg | 183 (44.8%) | 49.0 | 24 m | 128 | 35 (27.3%) | 58.0 | 106.3 |
| Räber, 2022 [32] | Patients underwent urgent PCI with LDL-C levels ≥ 70 mg/dL (receiving stable statin) or ≥ 125 mg/dL (without stable statin treatment) | Alirocumab 150 mg, plus rosuvastatin 20 mg | Rosuvastatin 20 mg | 298 (18.8%) | 58.5 | 52 w | 153 | 55 (35.9%) | 23.6 | 74.4 |
| Yan, 2022 [28] | Patients underwent urgent PCI with LDL-C levels ≥ 116 mg/dL | Evolocumab 140 mg, plus atorvastatin 40 mg and ezetimibe 10 mg | Atorvastatin 40 mg and ezetimibe 10 mg | 136 (31.6%) | 62.2 | 3 m | 137 | 27 (19.7%) | 22.4 | 49.1 |
Abbreviations: ACS, acute coronary syndrome; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; PCI, percutaneous coronary intervention.
The ROB2 assessment results of these RCTs are shown in Figure S2. Four of these trials exhibited a low risk of bias [27, 30, 32, 34], whereas five showed a high risk of bias [28, 29, 31, 33, 35].
Three-point MACE incidence
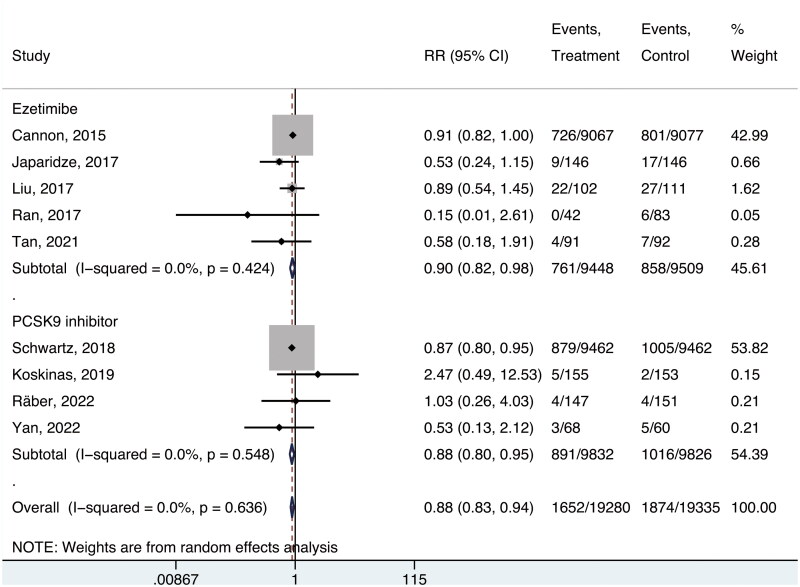
Pooled analyses of the nine studies [27–35] conducted with a random-effects model indicated that intensive lipid-lowering therapies were associated with a reduction in three-point MACE risk relative to background statin treatment (absolute risk, 8.6% and 9.7%, respectively; RR, 0.88; 95% CI, 0.83–0.94; p < 0.001; I2 = 0%; NNT in 4.7 years, 89). No significant differences in benefit were observed when comparing regimens including ezetimibe to those including PCSK9 inhibitors (RR, 0.90; 95% CI, 0.82–0.98; and RR; 0.88, 95% CI, 0.80–0.95, respectively; p = 0.72, Figure 1).
Figure 1.
Risk of three-point MACE.
Recurrent ACS incidence
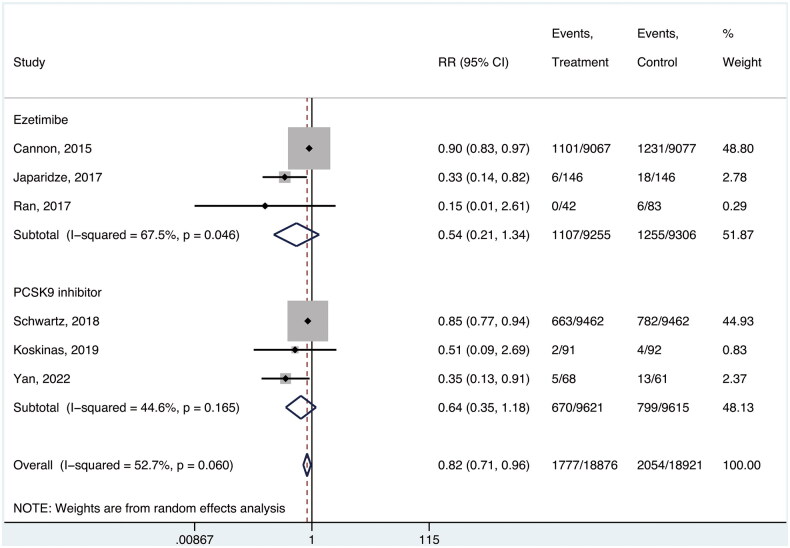
Six studies enrolling 47,797 participants [27–30, 33, 34] reported the incidence of recurrent ACS or all components thereof. Relative to background statin treatment, intensive lipid-lowering therapy was associated with a significant reduction in ACS recurrence, although this endpoint was subject to substantial heterogeneity (absolute risk, 9.4% and 10.9%, respectively; RR, 0.82; 95% CI, 0.71–0.96; p = 0.013; I2 = 52.7%; NNT, 69). No significant differences were observed between the ezetimibe and PCSK9 inhibitors subgroups (RR, 0.54; 95% CI, 0.21–1.34; and RR; 0.64, 95% CI, 0.35–1.18, respectively; p = 0.52, Figure 2).
Figure 2.
Risk of recurrent ACS.
All-cause mortality
Pooled analyses of four trials enrolling 37,656 patients [27, 30, 32, 34] revealed no significant differences in all-cause mortality rates as a function of treatment regimen (absolute risk, 8.2% and 8.6%, respectively; RR, 0.94; 95% CI, 0.82–1.07; p = 0.329; I2 = 38.2%; NNT, 263). This risk also did not vary when comparing the two different intensive lipid-lowering strategies (RR, 0.99; 95% CI, 0.92–1.06 in the ezetimibe group; and RR; 0.84, 95% CI, 0.74–0.99 in PCSK9 inhibitors group; p = 0.92).
Cardiovascular disease-related mortality, nonfatal MI, and stroke incidence
Cardiovascular disease-related mortality rates were reported in eight studies that enrolled 38,497 patients [27–32, 34, 35]. Pooled analyses revealed no significant reductions in these rates when comparing intensive lipid-lowering regimens to statin monotherapy (absolute risk, 4.1% and 4.3%, respectively; RR, 0.96; 95% CI, 0.88–1.06; p = 0.457; I2 = 0%; NNT, 673). Additionally, no differences were noted between the ezetimibe and PCSK9 inhibitors subgroups (RR, 1.00; 95% CI, 0.89–1.12; and RR; 0.90, 95% CI, 0.76–1.07, respectively; p = 0.32). All nine trials reported on nonfatal MI rates among patients with ACS, revealing a significant reduction in such morbidity for patients administered intensive lipid-lowering regimens (absolute risk, 8.3% and 9.5%, respectively; RR, 0.87; 95% CI, 0.81–0.93; p < 0.001; I2 = 0%; NNT, 80), with comparable benefit levels in the ezetimibe and PCSK9 inhibitor subgroups (RR, 0.87; 95% CI, 0.80–0.95; and RR; 0.86, 95% CI, 0.78–0.96, respectively; p = 0.88). Seven RCTs that enrolled 38,314 patients [27–32, 34] indicated that intensive lipid-lowering regimens conferred a significant reduction in stroke risk (absolute risk, 2.2% and 2.7%, respectively; RR, 0.83; 95% CI, 0.73–0.94; p = 0.003; I2 = 0%; NNT, 211), with no significant differences between the ezetimibe and PCSK9 inhibitor subgroups (RR, 0.87; 95% CI, 0.75–1.01 in ezetimibe group; and RR, 0.73, 95% CI, 0.57–0.92 in PCSK9 inhibitors group; p = 0.20).
Coronary revascularization
Seven studies that enrolled 38,364 patients [27, 29–32, 34, 35] reported on coronary revascularization rates, which did not differ significantly between the intensive lipid-lowering treatment and background statin treatment groups, with a moderate heterogeneity (absolute risk, 12.9% and 14.1%, respectively; RR, 0.89; 95% CI, 0.79–1.00; p = 0.057; I2 = 44.7%; NNT, 64). Coronary revascularization rates were also comparable in the ezetimibe and PCSK9 inhibitor subgroups (RR, 0.95, 95% CI, 0.60–1.50; and RR; 0.78, 95% CI, 0.57–1.05, respectively; p = 0.57).
Unstable angina-related hospitalization rates
Pooled analyses of six studies that enrolled 37,922 patients [27–30, 33, 34] revealed a significant difference in the rates of unstable angina-related hospitalization between groups (absolute risk, 1.1% and 1.3%, respectively; RR, 0.57; 95% CI, 0.33–0.99; p = 0.046; I2 = 64.0%; NNT, 534), with no significant differences between the ezetimibe and PCSK9 inhibitor subgroups (RR, 0.53, 95% CI, 0.16–1.73; and RR; 0.56, 95% CI, 0.38–0.83, respectively; p = 0.12).
Sensitivity tests
No differences in the pooled results were observed for the three-point MACE and ACS recurrence rates when sensitivity analyses were conducted using a leave-one-out approach. However, the results for the pooled analysis of unstable angina-related hospitalization rates contradicted those in the abovementioned pooled analysis when the study by Cannon et al. [27] was omitted (Figure S3). When restricting these analyses to patients prescribed atorvastatin, relative to background statin treatment, the administration of intensive lipid-lowering therapies was associated with a significant reduction in the risk of three-point MACE incidence [28–31, 34, 35], ACS recurrence [28–30, 34], nonfatal MI [28–31, 34, 35], stroke [28–31, 34], and unstable angina-related hospitalization [28–30, 34], whereas no differences were observed in the rates of all-cause mortality [30, 34], cardiovascular disease-related death [28–31, 34, 35], or coronary revascularization [29–31, 34, 35]. Sensitivity analyses specifically focused on RCTs in which patients in the control group underwent high-intensity statin treatment revealed that intensive lipid-lowering therapies were associated with significant reductions in rates of three-point MACE incidence [28–30, 32–35], ACS recurrence [28–30, 33, 34], all-cause mortality [30, 32, 34], nonfatal MI [28–30, 32–35], stroke [28–30, 32, 34], and unstable angina-related hospitalization [28–30, 33, 34], whereas no differences were observed with respect to rates of cardiovascular disease-related death [28–30, 32, 34, 35] or coronary revascularization [29, 30, 32, 34, 35]. The effects of intensive lipid-lowering therapy versus background statin treatment on the primary and secondary study outcomes in patients with ACS are summarized in Table 2.
Table 2.
Association of aggressive lipid-lowering vs background statin therapy with primary and secondary outcomes among patients with ACS.
| Endpoint | LDL-lowering, No./Total. (%) |
RR (95%CI) | NNT | |
|---|---|---|---|---|
| Aggressive lipid-lowering | Background statin therapy | |||
| All eligible trials | ||||
| Three-point MACE | 1652/19280 (8.6) | 1874/19335 (9.7) | 0.88 (0.83, 0.9) | 89 |
| Recurrent ACS | 1777/18876 (9.4) | 2054/18921 (10.9) | 0.82 (0.71, 0.96) | 69 |
| All-cause mortality | 1553/18831 (8.2) | 1624/18825 (8.6) | 0.94 (0.82, 1.07) | 263 |
| Cardiovascular disease-related mortality | 794/19244 (4.1) | 823/19253 (4.3) | 0.96 (0.88, 1.06) | 673 |
| Nonfatal MI | 1592/19222 (8.3) | 1836/19275 (9.5) | 0.87 (0.81, 0.93) | 80 |
| Stroke | 422/19153 (2.2) | 513/19161 (2.7) | 0.83 (0.73, 0.94) | 211 |
| Coronary revascularization | 2480/19172 (12.9) | 2704/19192 (14.1) | 0.89 (0.79, 1.00) | 64 |
| Unstable angina-related hospitalization | 199/18940 (1.1) | 235/18982 (1.3) | 0.57 (0.33, 0.99) | 534 |
| Analysis restricted to patients with atorvastatin | ||||
| Three-point MACE | 922/10027 (9.2) | 1063/10024 (10.6) | 0.87 (0.80, 0.94) | 71 |
| Recurrent ACS | 676/9767 (6.9) | 817/9761 (8.4) | 0.49 (0.25, 0.99) | 69 |
| All-cause mortality | 336/9617 (3.5) | 392/9597 (4.1) | 1.04 (0.35, 3.13) | 169 |
| Cardiovascular disease-related mortality | 255/10030 (2.5) | 285/10025 (2.8) | 0.89 (0.75, 1.05) | 333 |
| Nonfatal MI | 645/9966 (6.5) | 749/9964 (7.5) | 0.86 (0.78, 0.95) | 96 |
| Stroke | 126/9939 (1.3) | 167/9933 (1.7) | 0.76 (0.61, 0.96) | 242 |
| Coronary revascularization | 778/9958 (7.8) | 883/9964 (8.9) | 0.88 (0.77, 1.02) | 95 |
| Unstable angina-related hospitalization | 43/9831 (0.4) | 82/9822 (0.8) | 0.53 (0.37, 0.77) | 252 |
| Analysis restricted to patients with high-intense statins | ||||
| Three-point MACE | 904/10111 (8.9) | 1046/10147 (10.3) | 0.87 (0.80, 0.94) | 73 |
| Recurrent ACS | 676/9809 (6.9) | 823/9844 (8.4) | 0.50 (0.27, 0.93) | 68 |
| All-cause mortality | 338/9764 (3.5) | 393/9748 (4.0) | 0.86 (0.74, 0.99) | 175 |
| Cardiovascular disease-related mortality | 252/10069 (2.5) | 282/10065 (2.8) | 0.89 (0.75, 1.05) | 334 |
| Nonfatal MI | 637/10047 (6.3) | 742/10087 (7.4) | 0.86 (0.78, 0.95) | 98 |
| Stroke | 113/9978 (1.1) | 157/9973 (1.6) | 0.72 (0.57, 0.92) | 226 |
| Coronary revascularization | 780/9997 (7.8) | 905/10004 (9.0) | 0.75 (0.56, 1.01) | 80 |
| Unstable angina-related hospitalization | 43/9873 (0.4) | 87/9905 (0.9) | 0.52 (0.36, 0.75) | 226 |
Abbreviations: ACS, acute coronary syndrome; LDL-C, low-density lipoprotein cholesterol; MACE, major adverse cardiovascular event; MI, myocardial infarction; RR, risk ratio; NNT, number needed to treat.
Subgroup analyses
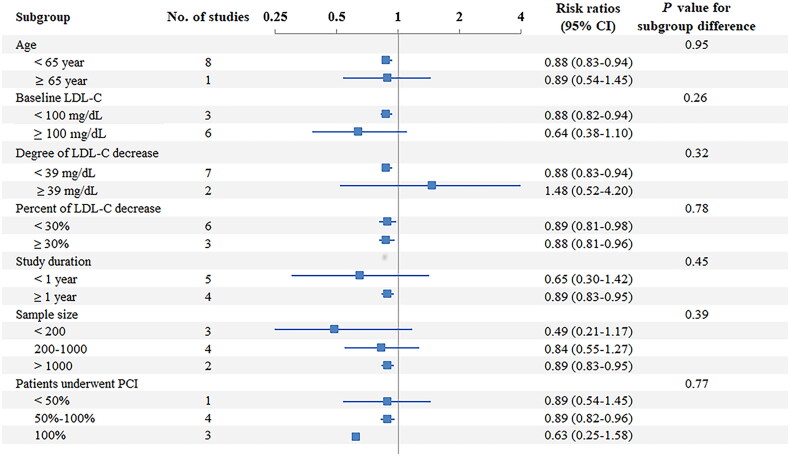
No substantial heterogeneity was observed in any subgroup analysis, and the differences between the groups were not significant (Figure 3).
Figure 3.
Subgroup analysis of the primary outcome.
Publication bias
No apparent publication bias was observed via the Trim-and-Fill method when imputing two studies on the right side, with an RR of 0.88 (95% CI: 0.82–0.94) (Figure S4). No small-study effects were detected using Egger’s test (p = 0.342).
GRADE assessment
Quality levels for four outcomes (three-point MACE, cardiovascular disease-related death, nonfatal MI, and stroke) among the eight pooled analyses were classified as moderate, while three outcomes (ACS recurrence, coronary revascularization, and unstable angina-related hospitalization) were of very low quality, and all-cause mortality results were of low quality (Table S1).
Discussion
This meta-analysis focused on nine RCTs that enrolled 38,640 patients post-ACS. The results revealed that the initiation of immediate intensive lipid-lowering therapy could improve cardiovascular prognosis after ACS. In the present analysis, intensive lipid-lowering treatment regimens were associated with a 12% reduction in the three-point MACE risk and an 18% reduction in the risk of ACS recurrence compared with background statin therapy, together with a 13% reduction in nonfatal MI incidence. The NNT to prevent a three-point MACE incidence in 4.7 years was 89, while the NNTs to prevent ACS recurrence and nonfatal MI incidence were 69 and 80, respectively. The administration of ezetimibe or PCSK9 inhibitors plus statins was also associated with lower rates of stroke and unstable angina-related hospitalization than statin monotherapy, although no differences in all-cause mortality, cardiovascular disease-related mortality, or coronary revascularization rates were observed.
While the current European Society of Cardiology guidelines for the management of ACS recommend the initiation of high-dose statin treatment early after ACS, combination therapy comprising statins and ezetimibe or a PCSK9 inhibitor is usually only initiated in patients with unsatisfactory LDL-C levels despite the prescription of a high-intensity statin [36]. However, in the present study, when the pooled analyses were restricted to studies in which high-intensity statins were administered to patients in the control group, a significant reduction in all-cause mortality risk was observed and the effect size was reduced. We considered that the initiation of intensive lipid-lowering treatment as soon as possible after ACS could benefit patients more than the use of a high-intensity statin without the consideration of costs. This was confirmed by the findings of the FOURIER-OLE trial that early intensive lipid-lowering therapy is associated with better cardiovascular outcomes [37]. However, caution is warranted when interpreting the results of analysis restricted to studies on high-intensity statins in the present study, considering that up to 95% CI approached the null value.
In a previous meta-analysis, a reduction in MACE risk proportional to the magnitude of LDL-C reduction for statin-based therapies was observed in individuals with established atherosclerotic cardiovascular disease when used for secondary prevention [38], although this finding was not confirmed in the subgroup analyses herein. The primary determinant of this difference may be that only two RCTs with 606 patients were included in the subgroup that exhibited greater lipid-lowering effects. One meta-analysis found that higher baseline LDL-C levels were associated with greater cardiovascular protection and that significant differences in interactions were detectable among subgroups when comparing baseline LDL-C levels of LDL-C < 100 mg/dL to those ≥ 100 mg/dL [39]. However, in the subgroup analysis conducted according to baseline LDL-C levels, intensive lipid-lowering therapies were associated with a significant reduction in the three-point MACE risk among patients with lower baseline levels of LDL-C (< 100 mg/dL). No significant subgroup differences were found between baseline LDL-C levels. Notably, in the present analysis, although the reduction in lipid levels was greater in patients with higher baseline LDL-C levels, the final LDL-C levels for these patients varied. This suggests that achieving target LDL-C levels is more important than the percentage of LDL-C reduction. This finding was confirmed by the Treat Stroke to Target Trial [40] in which groups with lower and higher targets were compared and showed a reduced risk of MACE in patients in the lower-target group. The IMPROVE-IT trial also revealed that LDL-C levels lower than the previous targets could provide additional benefit [27]. Moreover, the PRECISE-IVUS trial reported an association between low LDL-C levels and atheroma volume regression [41]. Based on this clinical evidence, the most recent guidelines (published after 2019) have adjusted the goal of LDL-C treatment from less than 70 mg/dL to less than 55 mg/dL. Unfortunately, even with a goal of < 70 mg/dL, real-world data showed that only 20%—26% of high-risk patients prescribed long-term statin monotherapy achieved the recommended target [42]. These poor LDL-C control rates warrant the development of more intensive lipid-lowering therapies, such as the combination of statins with ezetimibe or PCSK9 inhibitors.
Additionally, it has been hypothesized that PCSK9 inhibitors could improve cardiovascular prognosis through mechanisms other than those directly related to LDL-C regulation. PCSK9 inhibitors can purportedly influence lipoprotein (a) biogenesis and clearance [43], which has been established as an independent risk factor associated with coronary, cerebrovascular, and peripheral artery diseases owing to its atherogenic, proinflammatory, and prothrombotic characteristics [44, 45]. Moreover, most RCTs in which the patients’ baseline LDL-C levels were high were designed with a focus on lipid modification rather than on clinical outcomes, such that the mean follow-up duration for these studies (4 months) was relatively short. The Cholesterol Treatment Trialists Collaboration meta-analysis of trials focusing on PCSK9 inhibitors and statin therapy found that PCSK9 inhibitors were associated with a non-significant reduction in cardiovascular risk over a two-year period compared with statin therapy [46].
When administered in combination with statins, ezetimibe lowers LDL-C levels by an additional 23–24% on average, whereas PCSK9 inhibitors are associated with a 62% reduction in LDL-C levels [47, 48]. However, no significant differences were observed between the ezetimibe and PCSK9 inhibitor regimens for any endpoint in the present meta-analysis, despite greater reductions in LDL-C levels. This is consistent with the results of a previous meta-analysis that focused on patients with high or very high cardiovascular risk who underwent high-dose statin treatment [15]. Heterogeneity with respect to baseline patient LDL-C levels may explain these discrepancies, and the relatively short follow-up durations of the enrolled studies additionally restricted the acquisition of sufficient events when attempting to reliably compare the differences between ezetimibe and PCSK9 inhibitors administered in combination with statins.
Limitations
This study has some limitations. First, different trials varied with respect to the statin types and doses utilized, with additional variability regarding whether patients had undergone stable statin treatment before recruitment. Consequently, these different RCTs exhibited inconsistent baseline LDL-C inclusion levels, potentially affecting primary outcome consistency. However, no significant heterogeneity was observed when subgroup analyses were conducted based on study characteristics in the present meta-analysis, thus partially mitigating this risk. Second, the risk of MACEs varies among patients with ACS based on comorbidities, demographic characteristics, and other parameters. Many current guidelines recommend the administration of ezetimibe and/or PCSK9 inhibitors in individuals with ASCVD at high-or very high-risk levels, although there are some differences among guidelines with respect to how these risk levels are defined [1, 49, 50]. No risk stratification-based analyses of recurrent cardiovascular event incidence in patients with ACS were conducted in this meta-analysis because of the absence of sufficient information pertaining to the high-risk factors required for such stratification. Third, direct evidence regarding the relative benefits of ezetimibe versus PCSK9 inhibitors remains limited because most of these comparisons were indirect, with no studies directly comparing these treatment approaches for all primary and secondary outcomes. Fourth, we did not evaluate the cost-effectiveness of intensive lipid-lowering therapies because of insufficient information on quality-adjusted life years, incremental cost-effectiveness ratios, or other data to calculate these results. Fifth, caution should be exercised when generalizing these conclusions to clinical practice, as most of the included studies imposed patient enrolment restrictions. Real-world studies without additional entry restrictions could generate more robust evidence.
Conclusion
In summary, the pooled analyses conducted herein suggest that among patients with prior ACS undergoing background statin treatment, the additional administration of ezetimibe or PCSK9 inhibitors may contribute to a reduction in the three-point MACE incidence and ACS recurrence rates, although the degree of these reductions in patients with particular cardiovascular risks requires further clarification. The results of the present meta-analysis may assist in clinical decision-making for the secondary prevention of cardiovascular events to initiate intensive lipid-lowering therapies immediately after ACS.
Supplementary Material
Acknowledgment
We acknowledge the Language Editing Service of Taylor & Francis Editing Services.
Funding Statement
No funding was received.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Authors contributions
Xian-Dan Wu, Xuan-Yan Liu and Jing-Chao Sun contributed to the study design, the data acquisition, analysis, interpretation, the drafting, and revision of the manuscript and agreed to be accountable for all aspects of the work. Yan-Yan Li contributed to the study conceive, the supervision, and data interpretation. Xian Lin, Yue Lin and Jing-Chao Sun contributed to the study conceive, design, data analysis, and interpretation. Xin-Yue Ye and Bin-Hua Ye contributed to revised the manuscript. All authors read and approved the final manuscript and agree to be accountable for all aspects of the work.
Disclosure statement
No potential competing interest was reported by the authors.
Availability of data and materials
All data generated or analysed during this study are included in this published article and its supplementary information files. Raw data (in excel sheet) used in the statistical analysis will be made available on request through the corresponding author (Jing-Chao Sun).
References
- 1.Grundy SM, Stone NJ, Bailey AL, et al. . 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary: a Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139(25):e1046–e1081. doi: 10.1161/CIR.0000000000000699. [DOI] [PubMed] [Google Scholar]
- 2.Cannon CP, Steinberg BA, Murphy SA, et al. . Meta-analysis of cardiovascular outcomes trials comparing intensive versus moderate statin therapy. J Am Coll Cardiol. 2006;48(3):438–445. doi: 10.1016/j.jacc.2006.04.070. [DOI] [PubMed] [Google Scholar]
- 3.Collet J-P, Thiele H, Barbato E, et al. . 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2021;42(14):1289–1367. doi: 10.1093/eurheartj/ehaa575. [DOI] [PubMed] [Google Scholar]
- 4.Vogel B, Claessen BE, Arnold SV, et al. . ST-segment elevation myocardial infarction. Nat Rev Dis Primers. 2019;5(1):39. doi: 10.1038/s41572-019-0090-3. [DOI] [PubMed] [Google Scholar]
- 5.Silverio A, Cancro FP, Esposito L, et al. . Secondary cardiovascular prevention after acute coronary syndrome: emerging risk factors and novel therapeutic targets. J Clin Med. 2023;12(6):2161. doi: 10.3390/jcm12062161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hess CN, Clare RM, Neely ML, et al. . Differential occurrence, profile, and impact of first recurrent cardiovascular events after an acute coronary syndrome. Am Heart J. 2017;187:194–203. doi: 10.1016/j.ahj.2017.01.016. [DOI] [PubMed] [Google Scholar]
- 7.Kaasenbrood L, Boekholdt SM, van der Graaf Y, et al. . Distribution of estimated 10-year risk of recurrent vascular events and residual risk in a secondary prevention population. Circulation. 2016;134(19):1419–1429. doi: 10.1161/CIRCULATIONAHA.116.021314. [DOI] [PubMed] [Google Scholar]
- 8.Gao Y, Shah LM, Ding J, et al. . US trends in cholesterol screening, lipid levels, and lipid-lowering medication use in US adults, 1999 to 2018. J Am Heart Assoc. 2023;12(3):e028205. doi: 10.1161/JAHA.122.028205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee S, Cannon CP.. Combination lipid-lowering therapies for the prevention of recurrent cardiovascular events. Curr Cardiol Rep. 2018;20(7):55. doi: 10.1007/s11886-018-0997-4. [DOI] [PubMed] [Google Scholar]
- 10.Sabatine MS. PCSK9 inhibitors: clinical evidence and implementation. Nat Rev Cardiol. 2019;16(3):155–165. doi: 10.1038/s41569-018-0107-8. [DOI] [PubMed] [Google Scholar]
- 11.Gunta SP, O’Keefe JH, O’Keefe EL, et al. . PCSK9 inhibitor, ezetimibe, and bempedoic acid: evidence-based therapies for statin-intolerant patients. Prog Cardiovasc Dis. 2023;79:12–18. doi: 10.1016/j.pcad.2023.02.007. [DOI] [PubMed] [Google Scholar]
- 12.Gallego-Colon E, Daum A, Yosefy C.. Statins and PCSK9 inhibitors: a new lipid-lowering therapy. Eur J Pharmacol. 2020;878:173114. doi: 10.1016/j.ejphar.2020.173114. [DOI] [PubMed] [Google Scholar]
- 13.Virani SS, Newby LK, Arnold SV, et al. . 2023 AHA/ACC/ACCP/ASPC/NLA/PCNA Guideline for the Management of Patients With Chronic Coronary Disease: a Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation. 2023;148(9):e9–e119. doi: 10.1161/CIR.0000000000001168. [DOI] [PubMed] [Google Scholar]
- 14.Zhan S, Tang M, Liu F, et al. . Ezetimibe for the prevention of cardiovascular disease and all-cause mortality events. Cochrane Database Syst Rev. 2018;11(11):CD012502. doi: 10.1002/14651858.CD012502.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khan SU, Yedlapati SH, Lone AN, et al. . PCSK9 inhibitors and ezetimibe with or without statin therapy for cardiovascular risk reduction: a systematic review and network meta-analysis. BMJ. 2022;377:e069116. doi: 10.1136/bmj-2021-069116. [DOI] [PubMed] [Google Scholar]
- 16.Kim BK, Hong SJ, Lee YJ, et al. . Long-term efficacy and safety of moderate-intensity statin with ezetimibe combination therapy versus high-intensity statin monotherapy in patients with atherosclerotic cardiovascular disease (RACING): a randomised, open-label, non-inferiority trial. Lancet. 2022; Jul 30400(10349):380–390. doi: 10.1016/S0140-6736(22)00916-3. [DOI] [PubMed] [Google Scholar]
- 17.Ray KK, Haq I, Bilitou A, et al. . Treatment gaps in the implementation of LDL cholesterol control among high- and very high-risk patients in Europe between 2020 and 2021: the multinational observational SANTORINI study. Lancet Reg Health Eur. 2023; Apr 529:100624. doi: 10.1016/j.lanepe.2023.100624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hao Q, Aertgeerts B, Guyatt G, et al. . PCSK9 inhibitors and ezetimibe for the reduction of cardiovascular events: a clinical practice guideline with risk-stratified recommendations. BMJ. 2022;377:e069066. doi: 10.1136/bmj-2021-069066. [DOI] [PubMed] [Google Scholar]
- 19.Page MJ, McKenzie JE, Bossuyt PM, et al. . The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sterne JAC, Savović J, Page MJ, et al. . RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 21.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated August 2023). Cochrane, 2023. Available from https://www.training.cochrane.org/handbook. [Google Scholar]
- 22.Guyatt GH, Oxman AD, Vist GE, et al. . GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Higgins JPT, Thompson SG, Deeks JJ, et al. . Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neyeloff JL, Fuchs SC, Moreira LB.. Meta-analyses and Forest plots using a Microsoft excel spreadsheet: step-by-step guide focusing on descriptive data analysis. BMC Res Notes. 2012;5(1):52. doi: 10.1186/1756-0500-5-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi L, Lin L.. The trim-and-fill method for publication bias: practical guidelines and recommendations based on a large database of meta-analyses. Medicine (Baltimore). 2019;98(23):e15987. doi: 10.1097/MD.0000000000015987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arnett DK, Blumenthal RS, Albert MA, et al. . 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: a Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e596–e646. doi: 10.1161/CIR.0000000000000725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cannon CP, Blazing MA, Giugliano RP, et al. . Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372(25):2387–2397. doi: 10.1056/NEJMoa1410489. [DOI] [PubMed] [Google Scholar]
- 28.Hao Y, Yang YL, Wang YC, et al. . Effect of the early application of evolocumab on blood lipid profile and cardiovascular prognosis in patients with extremely high-risk acute coronary syndrome. Int Heart J. 2022;63(4):669–677. doi: 10.1536/ihj.22-052. [DOI] [PubMed] [Google Scholar]
- 29.Japaridze L, Sadunishvili M.. The short-term effect of atorvastatin plus ezetimibe therapy versus atorvastatin monotherapy on clinical outcome in acute coronary syndrome patients by gender. Kardiol Pol. 2017;75(8):770–778. doi: 10.5603/KP.a2017.0074. [DOI] [PubMed] [Google Scholar]
- 30.Koskinas KC, Windecker S, Pedrazzini G, et al. . Evolocumab for early reduction of ldl cholesterol levels in patients with acute coronary syndromes (EVOPACS). J Am Coll Cardiol. 2019;74(20):2452–2462. doi: 10.1016/j.jacc.2019.08.010. [DOI] [PubMed] [Google Scholar]
- 31.Liu Z, Hao H, Yin C, et al. . Therapeutic effects of atorvastatin and ezetimibe compared with double-dose atorvastatin in very elderly patients with acute coronary syndrome. Oncotarget. 2017;8(25):41582–41589. doi: 10.18632/oncotarget.15078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Räber L, Ueki Y, Otsuka T, et al. . Effect of alirocumab added to high-intensity statin therapy on coronary atherosclerosis in patients with acute myocardial infarction: the PACMAN-AMI randomized clinical trial. Jama. 2022;327(18):1771–1781. doi: 10.1001/jama.2022.5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ran D, Nie HJ, Gao YL, et al. . A randomized, controlled comparison of different intensive lipid-lowering therapies in Chinese patients with non-ST-elevation acute coronary syndrome (NSTE-ACS): Ezetimibe and rosuvastatin versus high-dose rosuvastatin. Int J Cardiol. 2017;235:49–55. doi: 10.1016/j.ijcard.2017.02.099. [DOI] [PubMed] [Google Scholar]
- 34.Schwartz GG, Steg PG, Szarek M, et al. . Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379(22):2097–2107. doi: 10.1056/NEJMoa1801174. [DOI] [PubMed] [Google Scholar]
- 35.Tan H, Liu L, Zheng Q, et al. . Effects of combined lipid-lowering therapy on low-density lipoprotein cholesterol variability and cardiovascular adverse events in patients with acute coronary syndrome. Adv Ther. 2021;38(6):3389–3398. doi: 10.1007/s12325-021-01741-7. [DOI] [PubMed] [Google Scholar]
- 36.Byrne RA, Rossello X, Coughlan JJ, et al. . 2023 ESC Guidelines for the management of acute coronary syndromes. Eur Heart J. 2023; Oct 1244(38):3720–3826. doi: 10.1093/eurheartj/ehad191. [DOI] [PubMed] [Google Scholar]
- 37.O’Donoghue ML, Giugliano RP, Wiviott SD, et al. . Long-term evolocumab in patients with established atherosclerotic cardiovascular disease. Circulation. 2022; Oct 11146(15):1109–1119. doi: 10.1161/CIRCULATIONAHA.122.061620. [DOI] [PubMed] [Google Scholar]
- 38.Koskinas KC, Siontis GCM, Piccolo R, et al. . Effect of statins and non-statin LDL-lowering medications on cardiovascular outcomes in secondary prevention: a meta-analysis of randomized trials. Eur Heart J. 2018;39(14):1172–1180. doi: 10.1093/eurheartj/ehx566. [DOI] [PubMed] [Google Scholar]
- 39.Navarese EP, Robinson JG, Kowalewski M, et al. . Association between baseline LDL-C level and total and cardiovascular mortality after LDL-C lowering: a systematic review and meta-analysis. JAMA. 2018;319(15):1566–1579. doi: 10.1001/jama.2018.2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Amarenco P, Kim JS, Labreuche J, et al. . Yield of dual therapy with statin and ezetimibe in the treat stroke to target trial. Stroke. 2022;53(11):3260–3267. doi: 10.1161/STROKEAHA.122.039728. [DOI] [PubMed] [Google Scholar]
- 41.Tsujita K, Sugiyama S, Sumida H, et al. . Impact of dual lipid-lowering strategy with ezetimibe and atorvastatin on coronary plaque regression in patients with percutaneous coronary intervention: the multicenter randomized controlled PRECISE-IVUS trial. J Am Coll Cardiol. 2015; Aug 466(5):495–507. doi: 10.1016/j.jacc.2015.05.065. [DOI] [PubMed] [Google Scholar]
- 42.Hagiwara N, Kawada-Watanabe E, Koyanagi R, et al. . Low-density lipoprotein cholesterol targeting with pitavastatin + ezetimibe for patients with acute coronary syndrome and dyslipidaemia: the HIJ-PROPER study, a prospective, open-label, randomized trial. Eur Heart J. 2017; Aug 138(29):2264–2276. PMID: 28430910; PMCID: PMC5837267. doi: 10.1093/eurheartj/ehx162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Watts GF, Chan DC, Pang J, et al. . PCSK9 Inhibition with alirocumab increases the catabolism of lipoprotein(a) particles in statin-treated patients with elevated lipoprotein(a). Metabolism. 2020;107:154221. doi: 10.1016/j.metabol.2020.154221. [DOI] [PubMed] [Google Scholar]
- 44.O’Donoghue ML, Fazio S, Giugliano RP, et al. . Lipoprotein(a), PCSK9 Inhibition, and Cardiovascular Risk. Circulation. 2019;139(12):1483–1492. doi: 10.1161/CIRCULATIONAHA.118.037184. [DOI] [PubMed] [Google Scholar]
- 45.Schwartz GG, Giugliano RP.. Proprotein convertase subtilisin/kexin type 9 inhibition after acute coronary syndrome or prior myocardial infarction. Curr Opin Lipidol. 2022;33(3):147–159. doi: 10.1097/MOL.0000000000000830. [DOI] [PubMed] [Google Scholar]
- 46.Ference BA, Cannon CP, Landmesser U, et al. . Reduction of low density lipoprotein-cholesterol and cardiovascular events with proprotein convertase subtilisin-kexin type 9 (PCSK9) inhibitors and statins: an analysis of FOURIER, SPIRE, and the Cholesterol Treatment Trialists Collaboration. Eur Heart J. 2018;39(27):2540–2545. doi: 10.1093/eurheartj/ehx450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morrone D, Weintraub WS, Toth PP, et al. . Lipid-altering efficacy of ezetimibe plus statin and statin monotherapy and identification of factors associated with treatment response: a pooled analysis of over 21,000 subjects from 27 clinical trials. Atherosclerosis. 2012;223(2):251–261. doi: 10.1016/j.atherosclerosis.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 48.Robinson JG, Farnier M, Krempf M, et al. . Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372(16):1489–1499. doi: 10.1056/NEJMoa1501031. [DOI] [PubMed] [Google Scholar]
- 49.Jellinger PS, Handelsman Y, Rosenblit PD, et al. . American Association of Clinical Endocrinologists and American College of Endocrinology guidelines for management of dyslipidemia and prevention of cardiovascular disease. Endocr Pract. 2017;23(Suppl 2):1–87. doi: 10.4158/EP171764.APPGL. [DOI] [PubMed] [Google Scholar]
- 50.Editorial Board of Chinese Journal of Cardiology . [Chinese expert consensus on lipid management of very high-risk atherosclerotic cardiovascular disease patients]. Zhonghua Xin Xue Guan Bing Za Zhi. 2020;48(4):280–286. doi: 10.3760/cma.j.cn112148-20200121-00036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article and its supplementary information files. Raw data (in excel sheet) used in the statistical analysis will be made available on request through the corresponding author (Jing-Chao Sun).