Abstract
PURPOSE
Treatment options are limited for patients with previously treated metastatic colorectal cancer (mCRC). In the LEAP-017 study, we evaluate whether lenvatinib in combination with pembrolizumab improves outcomes compared with standard of care (SOC) in previously treated mismatch repair proficient or not microsatellite instability high (pMMR or not MSI-H) mCRC.
METHODS
In this international, multicenter, randomized, controlled, open-label, phase III study, eligible patients age 18 years and older with unresectable, pMMR or not MSI-H mCRC, that had progressed on or after, or could not tolerate, standard treatment, were randomly assigned 1:1 to lenvatinib 20 mg orally once daily plus pembrolizumab 400 mg intravenously once every 6 weeks or investigator's choice of regorafenib or trifluridine/tipiracil (SOC). Randomization was stratified by presence or absence of liver metastases. The primary end point was overall survival (OS). LEAP-017 is registered at ClinicalTrials.gov (NCT04776148), and has completed recruitment.
RESULTS
Between April 8, 2021, and December 21, 2021, 480 patients were randomly assigned to lenvatinib plus pembrolizumab (n = 241) or SOC (n = 239). At final analysis (median follow-up of 18.6 months [IQR, 3.9]), median OS with lenvatinib plus pembrolizumab versus SOC was 9.8 versus 9.3 months (hazard ratio [HR], 0.83 [95% CI, 0.68 to 1.02]; P = .0379; prespecified threshold P = .0214). Grade ≥3 treatment-related adverse events occurred in 58.4% (lenvatinib plus pembrolizumab) versus 42.1% (SOC) of patients. Two participants died due to treatment-related adverse events, both in the lenvatinib plus pembrolizumab arm.
CONCLUSION
In patients with pMMR or not MSI-H mCRC that had progressed on previous therapy, there was no statistically significant improvement in OS after lenvatinib plus pembrolizumab treatment versus SOC. No new safety signals were observed.
INTRODUCTION
Despite advances in treatment and earlier detection, the prognosis remains poor for patients with recurrent or metastatic colorectal cancer (mCRC) that is mismatch repair proficient or not microsatellite instability high (pMMR or not MSI-H), with a 5-year survival of approximately 15%.1-3 For patients with pMMR or not MSI-H mCRC that progresses after treatment with fluoropyrimidine-based regimens with or without targeted therapies such as anti–vascular endothelial growth factor (VEGF) or epidermal growth factor receptor (EGFR) monoclonal antibodies, the most commonly accepted standard of care (SOC) includes regorafenib, trifluridine/tipiracil, and, more recently, trifluridine/tipiracil plus bevacizumab.4-7
CONTEXT
Key Objective
Does the multikinase inhibitor lenvatinib (len) plus PD-1 inhibitor pembrolizumab (pembro) improve efficacy and safety versus standard of care (regorafenib or trifluridine/tipiracil) in patients with mismatch repair proficient or not microsatellite instability high (pMMR or not MSI-H) metastatic colorectal cancer (mCRC)?
Knowledge Generated
This study did not meet its primary end point—there was no significant difference in overall survival between treatment arms—and the significance of secondary end points (progression-free survival and overall response rate) was not tested per the prespecified statistical analysis plan. No new safety signals were observed; treatment exposure was longer in the len plus pembro group.
Relevance (A.H. Ko)
The negative results of this oral tyrosine kinase inhibitor plus immune checkpoint inhibitor strategy highlight the need to develop alternative immune-based combinatorial approaches for treating microsatellite stable colorectal cancer.*
*Relevance section written by JCO Associate Editor Andrew H. Ko, MD, FASCO.
In patients with mCRC, regorafenib and trifluridine/tipiracil have limited clinical activity, with median overall survival (OS) of approximately 7.0 months.8,9 Data from the phase III SUNLIGHT study showed that the addition of bevacizumab to trifluridine/tipiracil significantly improved median OS (10.8 v 7.5 months) versus trifluridine/tipiracil alone.7 Recently, fruquintinib was approved for patients with refractory mCRC on the basis of prolonged OS versus placebo in the FRESCO and FRESCO-2 trials.10,11 However, the overall prognosis remains poor for this population, with a median OS under 1 year with existing therapies. As such, development of novel therapeutic options for these patients remains an area of high unmet need.
Previous studies suggest that combining tyrosine kinase inhibitors (TKIs) with immunotherapies might be efficacious in this population. The TKI regorafenib plus anti–PD-1 inhibitor nivolumab showed some antitumor activity (overall response rate [ORR] of 7%-33%) in patients with microsatellite stable (MSS) mCRC12,13; and in REGOMUNE, regorafenib plus anti–PD-L1 avelumab increased immune cell infiltration in MSS mCRC, although ORR was 0%.14 The TKI lenvatinib in combination with the anti–PD-1 therapy pembrolizumab is approved by the Food and Drug Administration for the first-line treatment of renal cell carcinoma and previously treated pMMR or not MSI-H advanced endometrial cancer.15,16 These approvals were based in part on data from the KEYNOTE-581 and KEYNOTE-775 studies that showed significantly longer progression-free survival (PFS) and OS with lenvatinib plus pembrolizumab compared with the control arms. Initial results from the multicohort phase II LEAP-005 study showed that lenvatinib plus pembrolizumab provided promising antitumor activity in patients with previously treated pMMR or not MSI-H mCRC, including an ORR of 21.9% and disease control rate of 47% at the first interim analysis, with a manageable safety profile.17
We report results of the phase III, randomized LEAP-017 study of lenvatinib plus pembrolizumab versus investigator's choice of standard treatment with regorafenib or trifluridine/tipiracil in patients with previously treated pMMR or not MSI-H mCRC.
METHODS
Study Design and Participants
Eligible patients were age 18 years and older with histologically or cytologically confirmed diagnosis of unresectable and metastatic colorectal adenocarcinoma (stage IV A-C as defined by American Joint Committee on Cancer 2017 classification, 8th edition) that was pMMR or not MSI-H by local testing, with Eastern Cooperative Oncology Group performance status of 0 to 1, and measurable disease per RECIST v1.1 as assessed by the investigator. Patients must have received previous treatment and have disease that progressed per RECIST v1.1 on or after, or that could not tolerate, standard treatment. Standard treatment was defined as receiving fluoropyrimidine, irinotecan, and oxaliplatin, with or without an anti-VEGF monoclonal antibody (eg, bevacizumab), with cetuximab or panitumumab (KRAS-/NRAS-wildtype tumors), and BRAF inhibitor (in combination with cetuximab with or without binimetinib) for BRAFV600E-mutant tumors, if those treatments were approved or available locally. Patients with brain metastases; a gastrointestinal condition that may affect study drug absorption; and who received previous treatment with a combination of anti–PD-1, anti–PD-L1, or anti–PD-L2 with anti-VEGF monoclonal antibodies or inhibitors, or had previously received regorafenib or trifluridine/tipiracil, were excluded.
Trial Design and Treatment
In this phase III, randomized, controlled, open-label study, patients were randomly assigned centrally 1:1 to receive lenvatinib plus pembrolizumab (Arm A) or investigator's choice of regorafenib or trifluridine/tipiracil (Arm B). The choice of regorafenib or trifluridine/tipiracil was determined before random assignment and reasons for selection documented. Randomization was stratified by the presence or absence (yes/no) of liver metastases. Crossover was not allowed.
Patients received either 20 mg lenvatinib orally once daily plus 400 mg pembrolizumab once every 6 weeks intravenously or SOC treatment with 160 mg regorafenib (once daily on days 1-21, no dose on days 22-28; in cycle one, regorafenib may be administered per local or institutional guidelines as 80 mg once daily on days 1-7, the 120 mg once daily on days 8-14, followed by 160 mg once daily on days 15-21, and 160 mg once daily on subsequent cycles [days 1-21])18 or 35 mg/m2 trifluridine/tipiracil (twice daily on days 1-5 and 8-12, no dose on days 6-7 or 13-28) orally once every 4 weeks. Pembrolizumab dosing is allowed for up to 18 administrations (approximately 2 years). Patients could continue to receive lenvatinib after completing pembrolizumab (≥25 cycles of lenvatinib) until reaching a discontinuation criterion (eg, disease progression or intolerance). Treatment continued until disease progression, unacceptable toxicity, intercurrent illness preventing administration of treatment, pregnancy, noncompliance, or withdrawal of consent. Complete details are in the protocol and a summary of its amendments (Appendix 1, online only).
End Points
The primary end point was OS (time from random assignment to death from any cause). Secondary end points included PFS (time from random assignment to first disease progression per RECIST v1.1 by blinded independent central review [BICR] or death from any cause), ORR (proportion of patients with complete or partial response), and duration of response (DOR; time from first complete or partial response until first disease progression) per RECIST v1.1 by BICR, health-related quality of life (HRQOL) as assessed by the EORTC QLQ-C30 and EORTC QLQ-CR29 questionnaires, safety, and tolerability. Protocol-specified exploratory end points include HRQOL as assessed by the EQ-5D-5L questionnaire. Data from HRQOL assessments will be reported in future publications.
Statistical Analysis
Efficacy was assessed in the intention-to-treat population of all randomly assigned patients. Safety was assessed in the as-treated population of randomly assigned patients who received at least one dose of study treatment. The Kaplan-Meier method was used to estimate OS, PFS, and DOR. Between-group differences in OS and PFS were assessed using a stratified log-rank test. Differences in response rate were assessed with the stratified Miettinen and Nurminen method. A stratified Cox proportional hazards model with Efron's method of tie handling was used to estimate the hazard ratios (HRs) and associated 95% CIs. More details can be found in Appendix 1.
RESULTS
Patients and Treatment
From April 8, 2021, to December 21, 2021, 633 patients were screened from 91 medical centers globally and 480 were randomly assigned to receive lenvatinib plus pembrolizumab (N = 241) or investigator's choice of regorafenib or trifluridine/tipiracil (N = 239) in the intention-to-treat population. Baseline characteristics were generally well balanced between groups. Patients had a median age of 58 years (IQR, 17), 336 (70%) had presence of liver metastasis, 181 (38%) had PD-L1 combined positive score (CPS) ≥1 tumors, 267 (56%) had RAS-mutant status, 219 (46%) had ≥3 previous therapies, and 242 (50%) versus 238 (50%) were assigned to receive regorafenib versus trifluridine/tipiracil, respectively, by investigator's choice before random assignment (Table 1). Enrollment across regions was consistent between the treatment groups. All patients had pMMR or not MSI-H status confirmed by local testing. At the data cutoff date of February 20, 2023, the median follow-up time was 18.6 months (IQR, 3.9).
TABLE 1.
Demographic and Patient Characteristics at Baseline
| Characteristic | Lenvatinib + Pembrolizumab (n = 241) | Standard of Care (n = 239) |
|---|---|---|
| Age, years, median (IQR) | 58.0 (16) | 58.0 (18) |
| ≥65 | 79 (33) | 76 (32) |
| Male, No. (%) | 136 (56) | 142 (59) |
| ECOG PS 0, No. (%) | 129 (54) | 132 (55) |
| Region, No. (%) | ||
| Asia | 77 (32) | 77 (32) |
| Western Europe/North America | 90 (37) | 83 (35) |
| Rest of the world | 74 (31) | 79 (33) |
| Primary tumor location, No. (%) | ||
| Left tumor | 176 (73) | 177 (74) |
| Right tumor | 64 (27) | 58 (24) |
| Other/missing tumor site | 1 (<1) | 4 (2) |
| MSI status, No. (%) | ||
| pMMR only | 127 (53) | 130 (54) |
| Non–MSI-H only | 72 (30) | 73 (31) |
| pMMR and non–MSI-H | 42 (17) | 36 (15) |
| Presence of liver metastasis, No. (%) | ||
| Yes | 168 (70) | 168 (70) |
| No | 73 (30) | 71 (30) |
| No. of previous lines of systemic therapy, No. (%) | ||
| 1 | 10 (4) | 5 (2) |
| 2 | 126 (52) | 120 (50) |
| ≥3 | 105 (44) | 114 (48) |
| Previous treatmenta, No. (%) | ||
| Fluoropyrimidine | 241 (100) | 239 (100) |
| Oxaliplatin | 241 (100) | 239 (100) |
| Irinotecan | 241 (100) | 239 (100) |
| Anti-EGFR | 96 (40) | 109 (46) |
| Anti-VEGF/VEGFR | 203 (84) | 207 (87) |
| Previous neoadjuvant/adjuvant therapy, No. (%) | ||
| Adjuvant | 47 (20) | 31 (13) |
| Neoadjuvant ± adjuvant | 6 (2) | 11 (5) |
| None | 188 (78) | 197 (82) |
| PD-L1 status, No. (%) | ||
| CPS ≥1 | 85 (35) | 96 (40) |
| CPS <1 | 126 (52) | 113 (47) |
| Missing | 30 (12) | 30 (13) |
| Mutation status, No. (%) | ||
| BRAF-wildtype | 217 (90) | 212 (89) |
| BRAF-mutant | 5 (2) | 13 (5) |
| RAS-wildtype | 99 (41) | 111 (46) |
| RAS-mutant | 139 (58) | 128 (54) |
| BRAF, RAS unknown, or other | 22 (9) | 14 (6) |
| Chemotherapy choice before random assignment, No. (%) | ||
| Regorafenib | 119 (49) | 123 (51) |
| Trifluridine/tipiracil | 122 (51) | 116 (49) |
NOTE. Data are shown for the intention-to-treat population. Percentages may not total 100 because of rounding.
Abbreviations: CPS, combined positive score; ECOG PS, Eastern Cooperative Oncology Group performance status; EGFR, epidermal growth factor receptor; pMMR, mismatch repair proficient; MSI-H, microsatellite instability high; VEGF, vascular endothelial growth factor.
Fluoropyrimidine includes fluorouracil, capecitabine, or S1 (tegafur, gimeracil, oteracil); anti-EGFR includes cetuximab or panitumumab; and anti-VEGF/VEGFR includes bevacizumab, aflibercept, or ramucirumab.
A total of 238 patients in the lenvatinib plus pembrolizumab group and 235 in the SOC group received at least one dose of treatment, with a mean (standard deviation) of 6.0 (5.3) and 3.3 (3.1) months on therapy, respectively. Study treatment exposure of at least 6 months occurred in 88 (37%) patients in the lenvatinib plus pembrolizumab group and 33 (14%) patients in the SOC group. Of 238 patients who started lenvatinib plus pembrolizumab, 216 (91%) discontinued treatment, including 171 (72%) because of progressive disease, 16 (7%) because of clinical progression, and 19 (8%) because of an adverse event. All 235 patients who started SOC discontinued, including 195 (83%) because of progressive disease and 19 (8%) because of clinical progression (Appendix Fig A1). At data cutoff, 22 (9%) patients in the lenvatinib plus pembrolizumab group, and none in the SOC group, were ongoing on study treatment (Appendix Fig A1).
Overall Survival
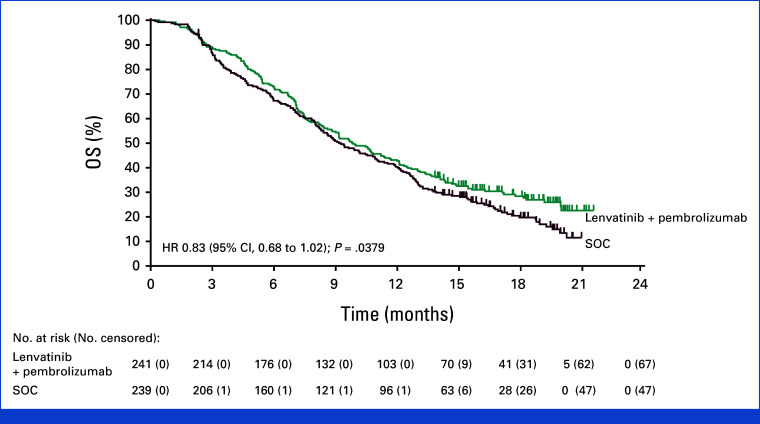
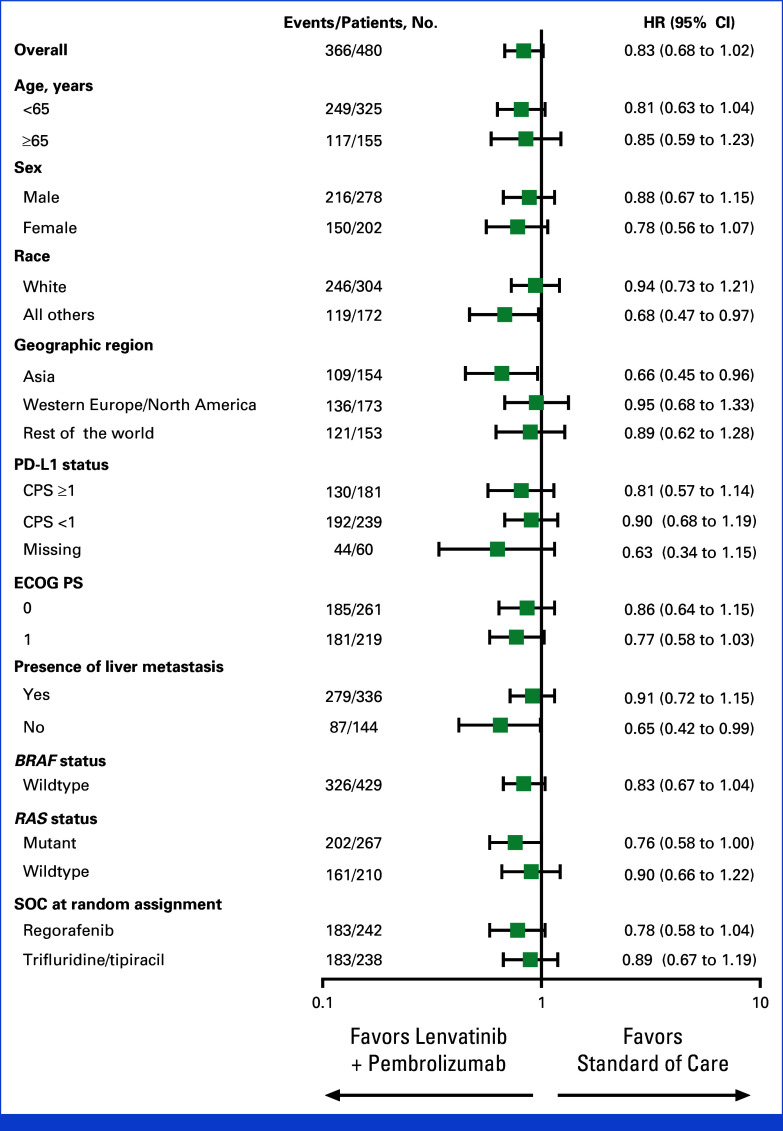
At final analysis, OS with lenvatinib plus pembrolizumab (median 9.8 months, 95% CI, 8.4 to 11.6) versus SOC (median 9.3 months [95% CI, 8.2 to 10.9]) did not meet the prespecified one-sided boundary of P = .0214 required for superiority (HR, 0.83 [95% CI, 0.68 to 1.02]; P = .0379; Fig 1). A total of 366 patients died, 174 in the lenvatinib plus pembrolizumab group and 192 in the SOC group. The 12-month OS rates were 42.7% (95% CI, 36.4 to 48.9) and 40.3% (95% CI, 34.1 to 46.5), respectively, with 18-month OS rates of 28.4% (95% CI, 22.6 to 34.4) and 19.8% (95% CI, 14.6 to 25.5), respectively. OS was generally consistent across most prespecified subgroups (Fig 2). Favorable OS with lenvatinib plus pembrolizumab was observed in patients from Asia (HR, 0.66 [95% CI, 0.45 to 0.96]) and those without liver metastases (HR, 0.65 [95% CI, 0.42 to 0.99]), although no conclusions can be drawn because these were subgroup analyses.
FIG 1.
OS in patients with unresectable, pMMR or not MSI-H metastatic colorectal cancer at final analysis. Kaplan-Meier estimates of OS in the intention-to-treat population. HR, hazard ratio; MSI-H, microsatellite instability high; OS, overall survival; pMMR, mismatch repair proficient; SOC, standard of care.
FIG 2.
Forest plot of overall survival across prespecified subgroups of patients with unresectable, pMMR or not MSI-H metastatic colorectal cancer. The Cox proportional hazard model with Efron's method of tie handling was used to assess the magnitude of the treatment difference between arms. CPS, combined positive score; ECOG PS, Eastern Cooperative Oncology Group performance status; HR, hazard ratio; MSI-H, microsatellite instability high; pMMR, mismatch repair proficient; SOC, standard of care.
In this study, 111 (46%) patients in the lenvatinib plus pembrolizumab group and 142 (59%) patients in the SOC group received subsequent anticancer therapy. This included 41% versus 49% of patients in the lenvatinib plus pembrolizumab versus SOC groups, respectively, who received chemotherapy, and 10% versus 15% of patients, respectively, who received targeted therapies (Appendix Table A2).
Progression-Free Survival
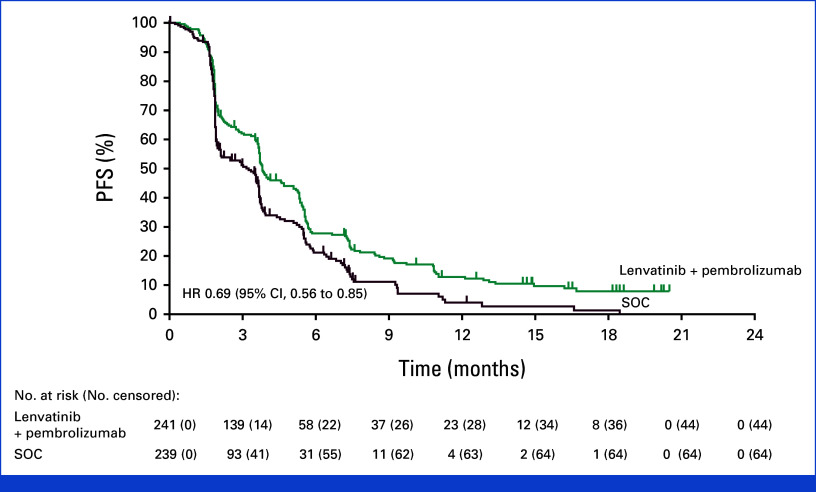
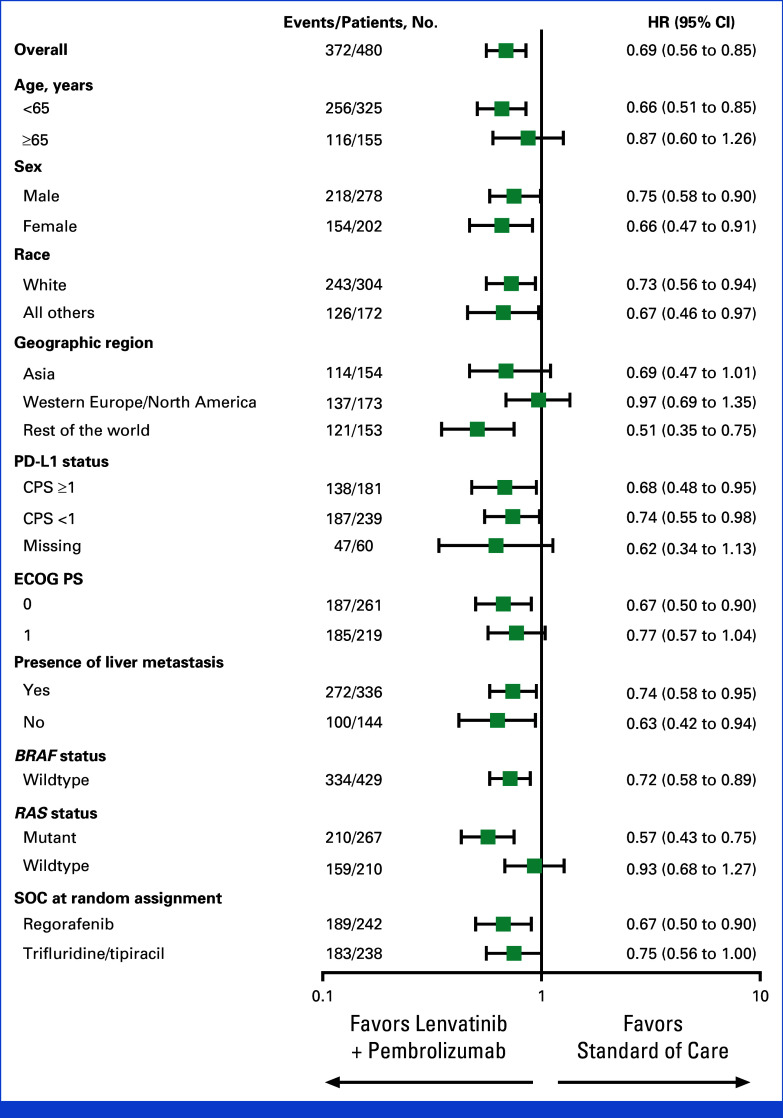
Median PFS was 3.8 months with lenvatinib plus pembrolizumab (95% CI, 3.7 to 5.1) versus 3.3 months for SOC (95% CI, 2.0 to 3.7) at final analysis (HR, 0.69 [95% CI, 0.56 to 0.85]). The PFS was not formally tested for statistical significance per the prespecified multiplicity strategy as the OS null hypothesis was not rejected (Fig 3). The 12-month PFS rates were 12.8% (95% CI, 8.6 to 17.8) for lenvatinib plus pembrolizumab versus 4.4% (95% CI, 1.6 to 9.7) for SOC; 18-month PFS rates were 7.9% (95% CI, 4.4 to 12.7) and 1.5% (95% CI, 0.1 to 6.6), respectively. PFS with lenvatinib plus pembrolizumab versus SOC was generally consistent across the prespecified subgroups, with overlapping confidence intervals (Appendix Fig A2).
FIG 3.
PFS in patients with unresectable, pMMR or not MSI-H metastatic colorectal cancer. Kaplan-Meier estimates of progression-free survival in the intention-to-treat population. Progression-free survival was assessed per the RECIST version 1.1 by blinded, independent central review. HR, hazard ratio; MSI-H, microsatellite instability high; PFS, progression-free survival; pMMR, mismatch repair proficient; SOC, standard of care.
Antitumor Response
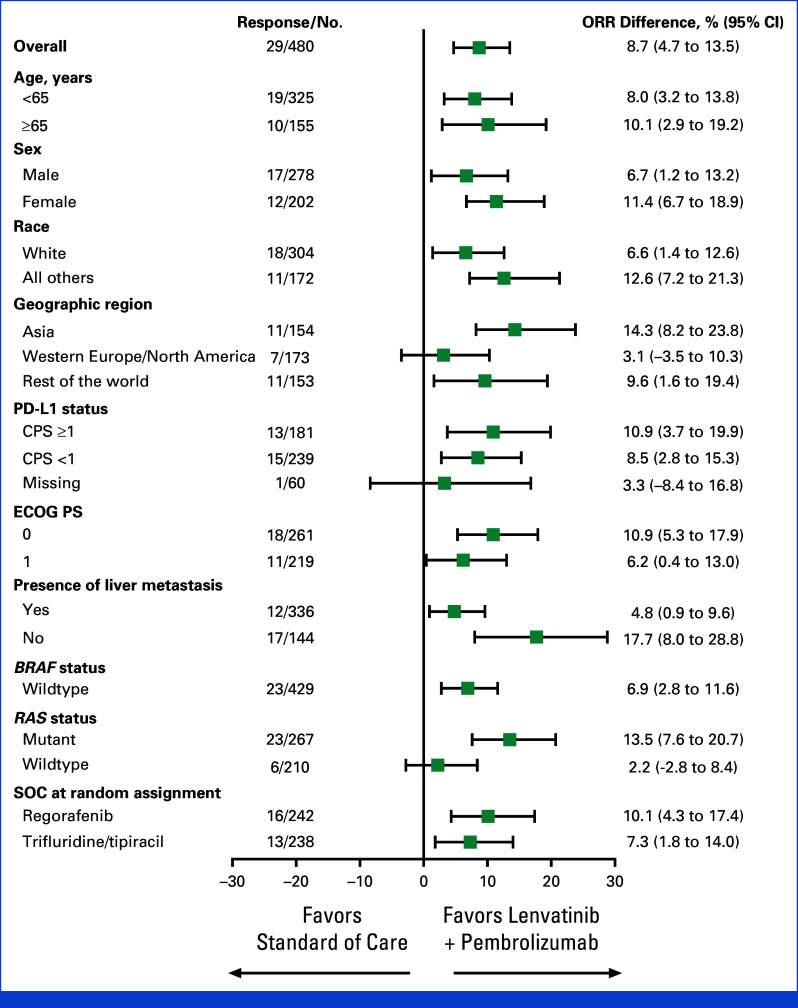
At final analysis, the ORR was 10.4% (25 of 241 [95% CI, 6.8 to 14.9]) in the lenvatinib plus pembrolizumab group and 1.7% (four of 239 [95% CI, 0.5 to 4.2]) in the SOC group, with a difference of 8.7% (95% CI, 4.7 to 13.5); this difference was not formally tested for statistical significance per the prespecified multiplicity strategy as the OS null hypothesis was not rejected. The median DOR was 11.1 months (IQR, 5.7) in the lenvatinib plus pembrolizumab group versus 7.6 months (IQR, 2.1) in the SOC group. The difference in objective response was consistent across the prespecified subgroups (Appendix Fig A3).
Safety
The median duration of treatment exposure was 4.2 months (range, 0.1-21.6) versus 2.1 months (range, 0.0-20.0) for patients in the lenvatinib plus pembrolizumab versus SOC groups, respectively. Adverse events of any cause occurred in 237 (100%) patients in the lenvatinib plus pembrolizumab and 230 (98%) patients in the SOC group (Table 2). The most common (≥30% in any group) were hypertension (58% v 24%), proteinuria (45% v 12%), diarrhea (42% v 25%), hypothyroidism (38% v 7%), decreased appetite (30% v 26%), fatigue (30% v 25%), and nausea (26% v 30%). Events of grade 3 or greater occurred in 183 (77%) versus 138 (59%) patients, respectively, most commonly hypertension (28% v 9%), proteinuria (11% v 1%), and diarrhea (9% v 4%). There were two treatment-related deaths in the lenvatinib plus pembrolizumab group, one due to cerebral hemorrhage and one due to pneumonitis (Table 2).
TABLE 2.
Summary of Adverse Events in all Treated Patients
| Event | Lenvatinib + Pembrolizumab (n = 238) | Standard of Care (n = 235) | ||
|---|---|---|---|---|
| Any adverse event, No. (%) | 237 (100) | 230 (98) | ||
| Treatment-related events, No. (%) | 226 (95) | 201 (86) | ||
| Grade 3 to 4 | 138 (58) | 99 (42) | ||
| Led to discontinuation of any drug | 30 (13) | 5 (2) | ||
| Led to deatha | 2 (1) | 0 | ||
| Adverse events of interest, No. (%) | 115 (48) | 23 (10) | ||
| Treatment-related events ≥10% in any armb, No. (%) | Any | Grade ≥3 | Any | Grade ≥3 |
| Hypertension | 117 (49) | 58 (24) | 34 (14) | 14 (6) |
| Proteinuria | 101 (42) | 26 (11) | 19 (8) | 1 (<1) |
| Hypothyroidism | 85 (36) | 1 (<1) | 12 (5) | 0 |
| Diarrhea | 84 (35) | 16 (7) | 45 (19) | 8 (3) |
| Fatigue | 55 (23) | 5 (2) | 42 (18) | 4 (2) |
| Decreased appetite | 52 (22) | 1 (<1) | 43 (18) | 4 (2) |
| Palmar-plantar erythrodysesthesia syndrome | 45 (19) | 7 (3) | 45 (19) | 8 (3) |
| Dysphonia | 43 (18) | 0 | 19 (8) | 1 (<1) |
| Aspartate aminotransferase increased | 38 (16) | 5 (2) | 16 (7) | 1 (<1) |
| Asthenia | 36 (15) | 6 (3) | 27 (11) | 2 (1) |
| Nausea | 36 (15) | 1 (<1) | 52 (22) | 3 (1) |
| Platelet count decreased | 36 (15) | 5 (2) | 20 (9) | 2 (1) |
| Vomiting | 36 (15) | 2 (1) | 26 (11) | 2 (1) |
| Alanine aminotransferase increased | 32 (13) | 4 (2) | 12 (5) | 2 (1) |
| Rash | 26 (11) | 3 (1) | 10 (4) | 2 (1) |
| Arthralgia | 25 (11) | 3 (1) | 2 (1) | 0 |
| Stomatitis | 23 (10) | 2 (1) | 10 (4) | 1 (<1) |
| Anemia | 14 (6) | 3 (1) | 30 (13) | 7 (3) |
| Neutropeniac | 8 (3) | 4 (2) | 31 (13) | 23 (10) |
| Neutrophil count decreased | 6 (3) | 1 (<1) | 30 (13) | 23 (10) |
| Adverse events of interestd, No. (%) | ||||
| Hypothyroidism | 90 (38) | 1 (<1) | 16 (7) | 0 |
| Hyperthyroidism | 11 (5) | 0 | 2 (1) | 0 |
| Colitis | 5 (2) | 3 (1) | 0 | 0 |
| Adrenal insufficiency | 4 (2) | 0 | 1 (<1) | 0 |
| Hepatitis | 3 (1) | 1 (<1) | 0 | 0 |
| Infusion reactions | 3 (1) | 1 (<1) | 0 | 0 |
| Myositis | 2 (1) | 0 | 0 | 0 |
| Nephritis | 1 (<1) | 1 (<1) | 0 | 0 |
| Pancreatitis | 3 (1) | 3 (1) | 1 (<1) | 1 (<1) |
| Pneumonitis | 5 (2) | 4 (2) | 1 (<1) | 1 (<1) |
| Severe skin reactions | 7 (3) | 5 (2) | 3 (1) | 3 (1) |
| Thyroiditis | 5 (2) | 0 | 0 | 0 |
| Type 1 diabetes mellitus | 1 (<1) | 1 (<1) | 0 | 0 |
NOTE. The as-treated population included all patients who were randomly assigned and received at least one study treatment. Percentages may not total 100 because of rounding.
Grade 5 treatment-related events occurred in two patients in the lenvatinib plus pembrolizumab arm because of pneumonitis and cerebral hemorrhage in one patient each.
Reported are treatment-related adverse events that occurred in at least 10% of patients in any group. Grade 3 or greater events among these events are reported.
Neutropenia is the clinical diagnosis resulting from decreased neutrophil count. Both are reported here separately.
Adverse events of interest (immune-mediated adverse events and infusion reactions) were based on a list of terms specified by the sponsor, regardless of attribution to any study treatment by investigators. All adverse events of interest are reported.
As expected, there were more potentially immune-mediated adverse events and infusion reactions, which occurred in 115 (48%) patients in the lenvatinib plus pembrolizumab group and 23 (10%) patients in the SOC group, the most common of which were hypothyroidism (38% v 7%) and hyperthyroidism (5% v 1%). Grade 3 or greater events occurred in 18 (8%) and five (2%) patients, respectively. One grade 5 event because of pneumonitis occurred with lenvatinib plus pembrolizumab (Table 2).
When adjusted for exposure duration, the rates of adverse events were similar between both treatment groups (Appendix Table A3), including for grade 3 or greater adverse events (26.63 events per 100 person-months in the lenvatinib plus pembrolizumab group v 31.56 in the SOC group) and for grade 3 or greater drug-related adverse events (16.59 v 17.89).
DISCUSSION
In this randomized, open-label, phase III study, the difference between lenvatinib plus pembrolizumab and standard of care with regorafenib or trifluridine/tipiracil did not meet prespecified significance for improved OS in patients with previously treated pMMR or not MSI-H mCRC. As a result, per the prespecified statistical analysis plan, the key secondary end points of PFS and ORR were not tested for statistical significance. The safety profile was consistent with previous reports for the therapeutic combination in other solid tumors.15,16,19
Significant advances have been made in the treatment of certain subgroups of mCRC. In patients with dMMR or MSI-H mCRC, anti–PD-1 therapy is highly efficacious and has been approved as first-line monotherapy20; by contrast, pMMR or not MSI-H tumors are poorly immunogenic.21 Accordingly, studies evaluating immune checkpoint inhibitors in an unselected population of pMMR or not MSI-H mCRC patients, either as monotherapy or in combination, have not consistently demonstrated clinical benefit, nor have randomized studies of immunotherapy in this population shown statistical superiority over SOC.22-24
Despite the lack of statistical significance for OS in LEAP-017, outcomes from other clinically relevant end points did show numerical improvement for patients treated with lenvatinib plus pembrolizumab. For example, DOR was longer in patients treated with lenvatinib plus pembrolizumab compared with SOC (median 11.1 months v 7.6 months). Furthermore, the ORR of 10.4% reported with lenvatinib plus pembrolizumab in LEAP-017 is numerically higher than that of available therapies in this population, including the SUNLIGHT regimen, which had an ORR of 6.1%.7,17
Data in the current study suggest that some subgroups may benefit more from lenvatinib plus pembrolizumab, including in patients without liver metastases and patients from Asia (Fig 2). Descriptive subgroup analyses in this study should be interpreted with caution because they are not adjusted for in the multiplicity strategy and the study was not powered to compare specific subgroups. Nevertheless, these results highlight the need to refine the selection of subpopulations of pMMR or not MSI-H mCRC that may maximally benefit from immunotherapy-based combination therapy.
The reduced benefit of immunotherapy in this study for patients with liver metastases was also observed in early clinical studies of regorafenib plus anti–PD-1 therapy, nivolumab, regorafenib plus nivolumab and ipilimumab, and botensilimab plus bastilimab.12,13,25,26 Several ongoing phase III studies (ClinicalTrials.gov identifiers: NCT05425940, NCT05064059, and NCT05328908) will investigate the impact of liver metastases and other biomarkers on the efficacy of immunotherapy in patients with pMMR or not MSI-H mCRC.
Although tumor PD-L1 status has been predictive of patient benefit after checkpoint inhibitor therapy in other indications, there was no difference in efficacy outcomes in LEAP-017 on the basis of PD-L1 status (CPS <1 or CPS ≥1 tumors). Anti–PD-1 monotherapy has demonstrated limited activity in pMMR or not MSI-H colorectal cancer, including in PD-L1–expressing tumors.23 Furthermore, evaluations of efficacy by PD-L1 tumor expression in combinations of checkpoint inhibitors plus TKIs have produced inconsistent results. Although no association between PD-L1 expression and tumor response was observed in REGOMUNE14 or the initial REGONIVO study,12 an association between PD-L1 expression and PFS (P = .0027) was observed in a subsequent phase II of regorafenib plus nivolumab study.13 In the study of the RIN regimen (regorafenib, ipilimumab, and nivolumab) of the patients with available PD-L1 expression data, the only responder had a score of 0, suggesting that PD-L1 expression is not required for effective combinations of VEGF TKIs and checkpoint inhibitors.25 In the IMblaze370 randomized trial combining cobimetinib, a TKI targeting MEK, and the PD-L1 inhibitor atezolizumab, no clinically meaningful differences in efficacy were observed on the basis of PD-L1 expression.22 Overall, these data suggest limited utility of tumor PD-L1 expression as a predictive biomarker for anti–PD-1/PD-L1 inhibitors in combination with TKIs in pMMR or not MSI-H mCRC.
In LEAP-017, patients in the SOC arm treated with regorafenib or trifluridine/tipiracil had a median OS of 9.3 months, which was longer than the protocol assumption of 7 months on the basis of the CORRECT and RECOURSE studies.8,9 One potential explanation is that patients in this study are less heavily pretreated compared with other studies—44% of patients in the experimental arm received ≥3 lines of previous therapy compared with 74% in CORRECT,8 73% in FRESCO-2,11 and 82% in RECOURSE9 (SUNLIGHT had only 2% of patients with ≥3 lines of previous therapy7). Another explanation is a relatively higher rate of subsequent anticancer therapies in this study compared with other studies—59% of patients in the control arm in LEAP-017 received subsequent therapy (Appendix Table A2) versus 42% in RECOURSE9 and 26% in CORRECT.8 The SUNLIGHT regimen is less likely to have contributed to the difference, as a higher proportion of patients in the lenvatinib plus pembrolizumab arm received this regimen compared with those in the SOC arm.
The safety profile in this study was consistent with the safety profiles observed with lenvatinib and pembrolizumab as monotherapies or in combination across solid tumors.15,16,19,27 No new safety concerns were observed. Of note, adverse events associated with lenvatinib plus pembrolizumab were likely to have been influenced by the longer treatment duration in this group than in the SOC group; when adjusted for exposure, the safety profiles between the two arms were generally similar (Appendix Table A3). A potential limitation of this study is the open-label design, which may have influenced adherence to study medication and biased patient management. Additionally, this study did not include an experimental arm investigating the impact of lenvatinib alone in this population, although the limited antitumor activity of lenvatinib monotherapy was well characterized in the phase II LEMON study.27
In conclusion, in the final analysis of the phase III LEAP-017 study, lenvatinib plus pembrolizumab did not meet prespecified significance for improved OS versus SOC in patients with pMMR or not MSI-H mCRC. No new safety signals were observed; treatment exposure was longer in the lenvatinib plus pembrolizumab group. Novel therapeutic options for patients with previously treated pMMR or not MSI-H mCRC are needed; future studies should further identify subgroups of patients who could benefit from novel immunotherapeutic approaches.
ACKNOWLEDGMENT
The authors thank the patients and their families and caregivers for participating in the study, all primary investigators and their site personnel, Nageatte Ibrahim and Abby Brena Siegel (M.S.D.) for critical review of the manuscript, and David K. Edwards V, PhD, CMPP, and Luana Atherly-Henderson, PhD, CMPP (both M.S.D.), for medical writing assistance. A complete list of investigators who participated in the LEAP-017 study is provided in Appendix Table A1.
APPENDIX 1. SUPPLEMENTAL METHODS
Assessments
Disease assessment by computed tomography or magnetic resonance imaging was performed at screening within 28 days of random assignment, at 8 weeks from the date of random assignment, and then every 8 weeks, or as clinically indicated until confirmed disease progression. Treatment beyond centrally verified progression per RECIST v1.1 may be permitted in Arm A at the discretion of the investigator after consultation with the sponsor and receiving documented informed consent. For patients who received surgery with curative intent, imaging assessments were performed at least 4 weeks after surgery and no more than 8 weeks before the next treatment cycle. Survival assessments were performed every 12 weeks until consent withdrawal, lost to follow-up or death, or end of study. Adverse events were collected throughout the study and up to 30 days (90 days for serious events) after treatment discontinuation and graded according to the NCI Common Terminology Criteria for Adverse Events, version 4.0.
Trial Oversight
The protocol and all amendments were approved by the relevant institutional review board or independent ethics committee at each study center. The study was conducted in accordance with the protocol, Good Clinical Practice guidelines, and the Declaration of Helsinki. All patients provided written informed consent. The study was designed by academic investigators and employees of the sponsor (Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc, Rahway, NJ). An external data safety monitoring committee reviewed interim study results to ensure patient safety and to assess efficacy at a prespecified interim analysis. All authors had access to the data, were involved in reviewing and editing the manuscript, and approved the submitted draft, and vouch for the accuracy of the data reported.
Statistical Analysis
The protocol specified one interim analysis and one final analysis. Approximately 434 patients were planned to be enrolled. The protocol-specified final analysis of overall survival (OS) was performed after approximately 336 deaths occurred and 7 months after the interim analysis. This would allow comparison of the superiority of lenvatinib plus pembrolizumab versus standard of care for OS to have approximately 90% power to detect a hazard ratio of 0.7 at the initially allocated one-sided alpha of 2.5%. The progression-free survival (PFS) and objective response hypotheses were only tested if the OS null hypothesis was rejected.
The overall type I error was strongly controlled at a one-sided alpha of 2.5% using the graphical method of Maurer and Bretz, with 0.025 initially allocated to OS, 0 to PFS, and 0 to overall response rate. If the OS null hypothesis was rejected, the corresponding alpha could be reallocated equally to PFS and objective response. The Lan-De-Mets O’Brien-Fleming alpha spending function was used to construct sequential boundaries to control the type 1 error rate. Statistical analyses were performed using SAS (v9.4). Sample size and power calculations were performed using the gsDesign package in R. Complete study and treatment details are provided in the study protocol.
FIG A1.
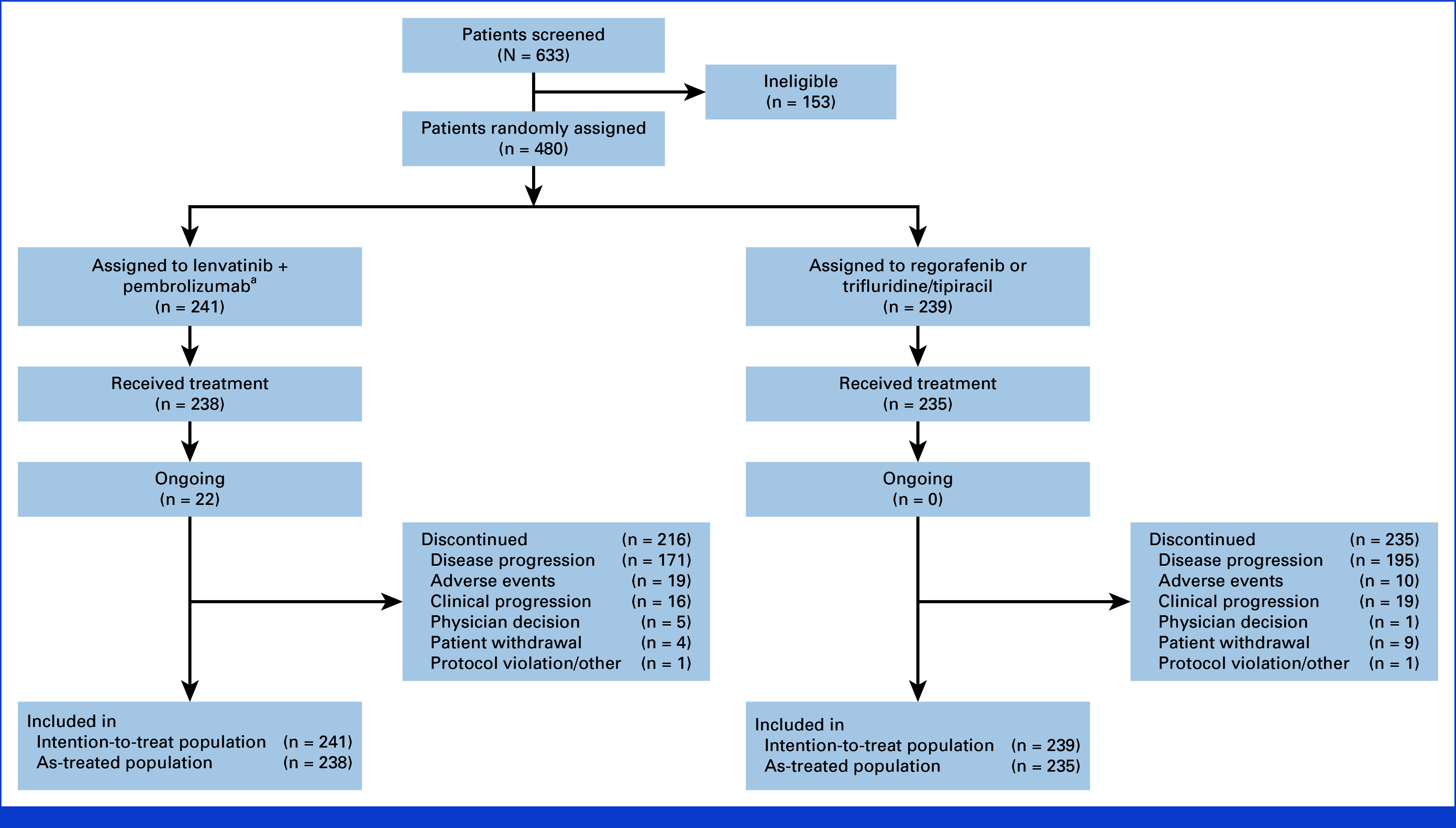
Study disposition at final analysis. aTwo patients assigned to lenvatinib + pembrolizumab received regorafenib or trifluridine/tipiracil instead and were moved to the standard-of-care treatment arm.
FIG A2.
Forest plot of progression-free survival across prespecified subgroups. CPS, combined positive score; ECOG PS, Eastern Cooperative Oncology Group performance status; SOC, standard of care.
FIG A3.
Forest plot of difference in overall response rate across prespecified subgroups. CPS, combined positive score; ECOG PS, Eastern Cooperative Oncology Group performance status; SOC, standard of care.
TABLE A1.
LEAP-017 Primary Investigators Who Screened ≥1 Participant for Enrollment
| Country/Region | Primary Investigator | Site Name |
|---|---|---|
| Argentina | Cundom, Juan | IDIM—Instituto de Diagnostico e Investigaciones Metabolicas |
| Slutsky, Ezequiel | Fundacion Favaloro Para la Docencia e Investigacion Medica-Oncologia | |
| Grasselli, Julieta | Centro de Educacion Medica e Investigaciones Clinicas (CEMIC)-Medical Oncology | |
| Fein, Luis | Instituto de Oncologia de Rosario | |
| Bella Quero, Luciana | Hospital Britanico de Buenos Aires-Oncology | |
| Australia | Joubert, Warren | Gallipoli Medical Research Foundation-GMRF CTU |
| Gibbs, Peter | Western Health-Sunshine Hospital | |
| Price, Timothy | The Queen Elizabeth Hospital | |
| Burge, Matthew | Royal Brisbane and Women's Hospital-Medical Oncology Clinical Trials Unit, Cancer Care Services | |
| Ananda, Sumitra | Epworth Freemason | |
| Khattak, Muhammad | Hollywood Private Hospital-Medical Oncology | |
| Canada | Colwell, Bruce | NSHA-QEII Health Sciences Centre-Dickson Bldg-Dept. of Medical Oncology |
| Couture, Felix | Centre Integre de Cancerologie du CHU de Quebec Universite Laval, Hopital de l'Enfant-Jesus | |
| Meyers, Brandon | Hamilton Health Sciences-Juravinski Cancer Center | |
| Towns, Kathryn | North York General Hospital | |
| Sawyer, Michael | Cross Cancer Institute-Department of Medical Oncology | |
| Sideris, Lucas | CIUSSS de l'Est-de-l'Ile-de-Montreal | |
| China | Xu, Ruihua | Sun Yat-Sen University Cancer Center |
| Wang, Wei | The First People's Hospital of Foshan-Gastrointestinal oncology | |
| Pan, Hongming | Sir Run Run Shaw Hospital-Medical Oncology | |
| Denmark | Pfeiffer, Per | Odense Universitetshospital |
| Jensen, Lars Henrik | Vejle Sygehus-Department of Oncology | |
| Qvortrup, Camilla | Rigshospitalet | |
| Germany | Stintzing, Sebastian | Charite Universitaetsmedizin Berlin—Campus Mitte |
| Arnold, Dirk | Asklepios Altona-Oncology | |
| Lorenzen, Sylvie | Klinikum rechts der isar der technischen universitat munchen-Klinik und Poliklinik fur Innere Mediz | |
| Kubicka, Stefan | Klinikum am Steinenberg-Kreiskliniken Reutlingen GmbH | |
| Depenbusch, Reinhard | Onkodok GmbH | |
| Israel | Passhak, Maria | Rambam Health Care Campus-Oncology |
| Geva, Ravit | Sourasky Medical Center-Oncology | |
| Hubert, Ayala | Hadassah Medical Center-Oncology | |
| Shacham-Shmueli, Einat | Sheba Medical Center | |
| Kornev, Gleb | Shaare Zedek Medical Center-Oncology | |
| Japan | Kawazoe, Akihito | National Cancer Center Hospital East |
| Masuishi, Toshiki | Aichi Cancer Center Hospital | |
| Takashima, Atsuo | National Cancer Center Hospital | |
| Hara, Hiroki | Saitama Prefectural Cancer Center | |
| Kawakami, Hisato | Kindai University Hospital—Osakasayama Campus-Medical Oncology | |
| Machida, Nozomu | Kanagawa Cancer Center | |
| Yamazaki, Kentaro | Shizuoka Cancer Center | |
| Yasui, Hisateru | Kobe City Medical Center General Hospital | |
| Tsuji, Akihito | Kagawa University Hospital | |
| Esaki, Taito | National Hospital Organization Kyushu Cancer Center | |
| Yamaguchi, Kensei | Japanese Foundation for Cancer Research-GI Oncology | |
| Korea, Republic of | Kim, Tae-You | Seoul National University Hospital-Internal Medicine |
| Ahn, Joong Bae | Severance Hospital, Yonsei University Health System-Medical Oncology | |
| Lee, Myung Ah | The Catholic Univ. of Korea Seoul St Mary's Hospital | |
| Kim, Tae Won | Asan Medical Center | |
| Park, Joon Oh | Samsung Medical Center-Division of Hematology/Oncology | |
| Lee, Soohyeon | Korea University Anam Hospital | |
| Russia | Orlova, Rashida | Saint-Petersburg City Clinical Oncology Dispensary-Department of Chemotherapy |
| Sarzhevskiy, Vladislav | The National Medico-Surgical Center N.I. Pirogov | |
| Sekacheva, Marina | First Moscow State Medical University I.M. Sechenov-Interhospital Institution Health Management | |
| Tjulandin, Sergey | Fed State Budgetary Inst N.N. Blokhin Med Center of Oncology MHRF | |
| Shirokova, Oksana | Sverdlovsk Regional Oncology Dispensary | |
| Iskhakova, Alsu | GBUZ Republican Clinical Oncological Dispensary-Antitumor Drug Therapy Department | |
| Lebedinets, Andrey | SHBI Leningrad Regional Clinical Oncology Dispensary-Clinical Trials Department | |
| Spain | Jimenez Fonseca, Paula | Hospital Universitario Central de Asturias-Digestive |
| Rivera Herrero, Fernando | Hospital Universitario Marques de Valdecilla | |
| Elez Fernandez, Elena | Hospital Universitari Vall d'Hebron-Oncology | |
| Garcia Alfonso, Pilar | Hospital General Universitario Gregorio Marañón | |
| Gomez Reina, Maria Jose | Hospital Universitario Virgen de Valme-Departamento de Oncologia | |
| Taiwan | Yeh, Kun-Huei | National Taiwan University Hospital |
| Teng, Hao-Wei | Taipei Veterans General Hospital-Oncology | |
| Yang, Tsai Sheng | Chang Gung Medical Foundation. Linkou Branch | |
| Wang, Hwei-Ming | China Medical University Hospital-Surgical Department | |
| Yeh, Yu-Min | National Cheng-Kung Uni. Hosp. | |
| Turkey | Ozguroglu, Mustafa | Istanbul Universitesi Cerrahpasa-Medical Oncology |
| Gumus, Mahmut | TC Saglik Bakanligi Goztepe Prof Dr Suleyman Yalcin Sehir Hastanesi-Oncology | |
| Yalcin, Suayib | Hacettepe Universitesi-Oncology Hospital | |
| Erdogan, Bulent | Trakya University-Oncology | |
| Demirci, Umut | Memorial Ankara Hastanesi-Medical Oncology | |
| Gursoy, Pinar | Ege University Medicine of Faculty-Medical Oncology | |
| Harputluoglu, Hakan | İnönü Üniversitesi Turgut Özal Tıp Merkezi | |
| Demir, Atakan | Acibadem Maslak Hastanesi | |
| United Kingdom | Shiu, Kai-Keen | UCLH-Cancer Clinical Trials Unit |
| Brown, Ewan | Western General Hospital | |
| Ross, Paul | Guy's and St Thomas' NHS Foundation Trust | |
| Smyth, Elizabeth | Addenbrooke's Hospital | |
| Chau, Ian | The Royal Marsden NHS Foundation Trust | |
| Saunders, Mark | The Christie-Medical Oncology | |
| United States | Vaccaro, Gina | Providence Portland Medical Center |
| McCune, Steven | Northwest Georgia Oncology Centers, a Service of Wellstar Cobb Hospital | |
| Wadlow, Raymond | Inova Schar Cancer Institute | |
| Khan, Gazala | Henry Ford Hospital | |
| Bashir, Babar | Thomas Jefferson University—Clinical Trials Office | |
| Koontz, Michael | Pacific Cancer Care | |
| Martin, Ludmila | Northwest Medical Specialties, PLLC | |
| Shergill, Ardaman | University of Chicago Medical Center-Medicine—Section of Hematology/Oncology—Gastrointestinal P | |
| Cobb, Patrick | St Vincent Frontier Cancer Center | |
| Kochenderfer, Mark | Blue Ridge Cancer Care |
TABLE A2.
Subsequent Anticancer Therapies
| Subsequent Therapy | Lenvatinib + Pembrolizumab (n = 241) | Standard of Care (n = 239) |
|---|---|---|
| Patients who received subsequent anticancer therapy, No. (%) | 111 (46.1) | 142 (59.4) |
| Chemotherapy, No. (%) | 98 (40.7) | 116 (48.5) |
| Fluoropyrimidine ± targeted therapy | 18 (7.5) | 25 (10.5) |
| FOLFIRI ± targeted therapy | 15 (6.2) | 33 (13.8) |
| FOLFOX ± targeted therapy | 33 (13.7) | 33 (13.8) |
| FOLFOXIRI ± targeted therapy | 3 (1.2) | 7 (2.9) |
| Irinotecan ± targeted therapy | 2 (0.8) | 8 (3.3) |
| Trifluridine/tipiracil | 31 (12.9) | 26 (10.9) |
| Trifluridine/tipiracil + bevacizumab | 25 (10.4) | 14 (5.9) |
| Targeted therapy, No. (%) | 24 (10.0) | 37 (15.5) |
| Anti–PD-1/PD-L1 | 4 (1.7) | 18 (7.5) |
| Regorafenib | 18 (7.5) | 20 (8.4) |
| Other TKI | 2 (0.8) | 2 (0.8) |
| Other, No. (%) | 10 (4.1) | 26 (10.9) |
Abbreviations: FOLFIRI, fluorouracil, leucovorin, and irinotecan; FOLFOX, infusional fluorouracil, leucovorin, and oxaliplatin; FOLFOXIRI, leucovorin, fluorouracil, oxaliplatin, and irinotecan; TKI, tyrosine kinase inhibitors.
TABLE A3.
Summary of Exposure-Adjusted AEs
| Event Count and Rate (events/100 person-months)a | Lenvatinib + Pembrolizumab (n = 238) | Standard of Care (n = 235) |
|---|---|---|
| Total exposure in person-monthsb | 1,633.65 | 1,017.23 |
| Total events (rate) | ||
| AEs | 3,329 (203.78) | 2,087 (205.17) |
| Drug-related AEs | 1,948 (119.24) | 1,133 (111.38) |
| Grade ≥3 AEs | 435 (26.63) | 321 (31.56) |
| Grade ≥3 drug-related AEs | 271 (16.59) | 182 (17.89) |
| Serious AEs | 175 (10.71) | 97 (9.54) |
| Serious drug-related AEs | 71 (4.35) | 25 (2.46) |
| AEs leading to death | 3 (0.18) | 3 (0.29) |
| Drug-related AEs leading to death | 2 (0.12) | 0 |
| AEs leading to drug discontinuation | 42 (2.57) | 11 (1.08) |
| Drug-related AEs leading to drug discontinuation | 35 (2.14) | 6 (0.59) |
| Serious AEs leading to drug discontinuation | 25 (1.53) | 6 (0.59) |
| Serious drug-related AEs leading to drug discontinuation | 20 (1.22) | 1 (0.10) |
Abbreviation: AEs, adverse events.
Event rate per 100 person-months of exposure = event count × 100/person-months of exposure.
Drug exposure defined as the between the first dose date + 1 day and the earlier of the last dose date + 30 or the database cutoff date.
Akihito Kawazoe
Honoraria: Ono Pharmaceutical, Taiho Pharmaceutical, Bristol Myers Squibb, Daiichi Sankyo/UCB Japan, Lilly
Consulting or Advisory Role: Zymeworks, Revolution Medicines, MSD
Speakers' Bureau: Taiho Pharmaceutical, Ono Pharmaceutical, Bristol Myers Squibb, Daiichi Sankyo/UCB Japan, Lilly
Research Funding: Ono Pharmaceutical (Inst), Taiho Pharmaceutical (Inst), AstraZenec (Inst), MSD (Inst)
Rui-Hua Xu
Consulting or Advisory Role: Astellas Pharma, MSD, AstraZeneca, Merck Serono, Roche, Hutchison MediPharma, BeiGene, Innovent Biologics, QiLu Pharmaceutical, Junshi Pharmaceuticals, Hengrui Pharm, Keymed Biosience, CPPC
Research Funding: Merck Sharp & Dohme LLC (Inst)
Pilar García-Alfonso
Consulting or Advisory Role: Amgen, Merck Serono, SERVIER, Pierre Fabre, MSD Oncology
Speakers' Bureau: Merck Serono, Amgen, Sanofi, SERVIER, MSD Oncology, BMS
Research Funding: Merck Sharp & Dohme LLC (Inst)
Maria Passhak
Honoraria: Roche, Merck Serono
Research Funding: MSD Oncology (Inst)
Hao-Wei Teng
Consulting or Advisory Role: MSD
Speakers' Bureau: MSD
Research Funding: Merck Sharp & Dohme LLC (Inst)
Ardaman Shergill
Honoraria: Curio Science, OncLive/MJH Life Sciences, Oklahoma Society of Clinical Oncology, Cholangiocarcinoma Foundation, Cholangiocarcinoma Summit, Saint Joseph Hospital Chicago, ASCO, Colon Cancer Alliance
Consulting or Advisory Role: Triptych Health Partners, Catalyst Pharmaceuticals, KLJ Associates, Pfizer, Guardant Health, Pfizer
Research Funding: TP Therapeutics (Inst), Hutchison MediPharma (Inst), Seattle Genetics/Astellas (Inst), Verastem (Inst), Pfizer (Inst), Gritstone Bio (Inst), Gossamer Bio (Inst), Astellas Pharma (Inst), BMS (Inst), Daiichi Sankyo (Inst), Oncologie (Inst), MacroGenics (Inst), Clovis Oncology (Inst), Merck (Inst), Takeda (Inst), Merck Sharp & Dohme LLC (Inst)
Travel, Accommodations, Expenses: Colon Cancer Alliance
Mahmut Gumus
Honoraria: MSD Oncology (Inst), Pfizer (Inst), GlaxoSmithKline (Inst), Novartis (Inst)
Consulting or Advisory Role: Roche, Lilly (Inst), Amgen (Inst), Gen (Inst), Novartis (Inst), Takeda (Inst), Gilead Sciences (Inst)
Speakers' Bureau: Roche (Inst), MSD Oncology (Inst), Novartis (Inst), Polipharma (Inst), Amgen (Inst)
Research Funding: Amgen (Inst), Pfizer (Inst), Takeda (Inst), MSD Oncology (Inst)
Travel, Accommodations, Expenses: Pfizer
Camilla Qvortrup
Consulting or Advisory Role: Merck KGaA, Pierre Fabre
Research Funding: Roche (Inst), MSD Oncology (Inst), SERVIER (Inst), Pfizer (Inst), Miratis (Inst), Pierre Fabre (Inst)
Travel, Accommodations, Expenses: Roche (Inst), SERVIER (Inst), Merck KGaA (Inst), Pierre Fabre (Inst)
Sebastian Stintzing
Honoraria: Merck KGaA, Roche, Amgen, SERVIER, MSD, Pfizer, Pierre Fabre, Bristol Myers Squibb GmbH, Nordic Bioscience, AstraZeneca, Daiichi Sankyo Europe GmbH
Consulting or Advisory Role: Merck KGaA, Roche, Amgen, Pierre Fabre, MSD, AstraZeneca, SERVIER, GlaxoSmithKline, TERUMO, Nordic Bioscience, Seagen, Daiichi Sankyo Europe GmbH, CV6 Therapeutics, Isofol Medical
Research Funding: Pierre Fabre (Inst), Roche Molecular Diagnostics (Inst), Merck Serono (Inst), Amgen (Inst), MSD (Inst)
Travel, Accommodations, Expenses: Merck KGaA, Roche, Sanofi, Bayer, Sirtex Medical, Amgen, Lilly, Takeda, Pierre Fabre, AstraZeneca
Kathryn Towns
Research Funding: Merck (Inst), Merck Sharp & Dohme LLC (Inst)
Tae Won Kim
Employment: ASAN Medical Center
Research Funding: Roche/Genentech (Inst), Genome Insight (Inst), Merck Sharp & Dohme LLC (Inst)
Kai Keen Shiu
Honoraria: Merck Serono, MSD Oncology, SERVIER
Consulting or Advisory Role: MSD Oncology, Roche, Mirati Therapeutics, Bayer, Seagen
Research Funding: MSD Oncology (Inst), Roche (Inst)
Uncompensated Relationships: MSD Oncology
Juan Cundom
Consulting or Advisory Role: AstraZeneca
Speakers' Bureau: Takeda
Research Funding: Merck Sharp & Dohme LLC (Inst)
Sumitra Ananda
Honoraria: MSD
Research Funding: Merck Sharp & Dohme LLC (Inst)
Andrey Lebedinets
Research Funding: MSD (Inst)
Rong Fu
Employment: MSD RD China
Stock and Other Ownership Interests: MSD RD China
Rishi Jain
Employment: Merck Sharp & Dohme LLC
Stock and Other Ownership Interests: Merck Sharp & Dohme LLC
David Adelberg
Employment: Merck Sharp & Dohme LLC
Stock and Other Ownership Interests: Merck Sharp & Dohme LLC
Travel, Accommodations, Expenses: Merck Sharp & Dohme LLC
Volker Heinemann
Stock and Other Ownership Interests: BioNTech SE
Honoraria: Roche, Amgen, Sanofi, Merck, SERVIER, Pfizer, Pierre Fabre, AstraZeneca, MSD, Seagen, Novartis, Boehringer Ingelheim, Celgene, Sirtex Medical, GlaxoSmithKline
Consulting or Advisory Role: Merck, Amgen, Roche, MSD, Bristol Myers Squibb, Novartis, Pierre Fabre, TERUMO, GlaxoSmithKline, Servier/Pfizer, AstraZeneca, Oncosil, Nordic Bioscience, Sirtex Medical, Halozyme, Janssen
Research Funding: Merck (Inst), Amgen (Inst), Roche (Inst)
Travel, Accommodations, Expenses: Merck, AstraZeneca, Amgen, MSD, Nordic Bioscience
Takayuki Yoshino
Honoraria: Chugai Pharma, MSD K.K, Takeda, Merck
Consulting or Advisory Role: Sumitomo Corp
Research Funding: MSD (Inst), DAIICHI SANKYO COMPANY, LIMITED (Inst), Ono Pharmaceutical (Inst), Taiho Pharmaceutical (Inst), Amgen (Inst), Sanofi (Inst), Pfizer (Inst), Sysmex (Inst), Chugai Pharma (Inst), Eisai (Inst), Molecular Health (Inst), Roche (Inst), FALCO biosystems Ltd (Inst), Merus (Inst), Bristol Myers Squibb Japan (Inst), Medical & Biological Laboratories Co., Ltd (Inst), Takeda (Inst), Merck Sharp & Dohme LLC (Inst)
Elena Elez
Honoraria: Bristol Myers Squibb, SERVIER, Amgen, Merck Serono, Array BioPharma, Sanofi/Aventis, Merck, Novartis, Seagan, Takeda, Pfizer, Bayer, Boehringer Ingelheim, Cure Teq AG, Roche, Janssen, Lilly, Medscape, MSD, Pierre Fabre, Repare Therapeutics, RIN Institute Inc
Consulting or Advisory Role: Amgen, Roche, Merck Serono, Sanofi, SERVIER, Bayer, Bristol Myers Squibb, Array BioPharma, Pierre Fabre, MSD, Bayer, Boehringer Ingelheim, Cure Teq AG, Roche, Janssen, Novartis, Pfizer, Repare Therapeutics Inc, RIN Institute Inc, Seagen, Takeda
Research Funding: Roche (Inst), Bristol Myers Squibb (Inst), SERVIER (Inst), Amgen (Inst), Array BioPharma (Inst), MedImmune (Inst), Pierre Fabre (Inst), Sanofi (Inst), Merck (Inst), BeiGene (Inst), Celgene (Inst), Debiopharm Group (Inst), Genentech (Inst), HalioDx (Inst), Hutchison MediPharma (Inst), Janssen-Cilag SA (Inst), Menarini (Inst), Merck Sharp&Dohme de España SA (Inst), Merus NV (Inst), Mirati Therapeutics (Inst), Novartis (Inst), Pfizer (Inst), PharmaMar (Inst), Taiho Pharmaceutical (Inst), AstraZeneca (Inst), Boehringer Ingelheim (Inst), AbbVie (Inst), Bayer (Inst), Bioncotech (Inst), BioNTech RNA Pharmaceuticals GMBH (Inst), Biontech Small Molecules GMBH (Inst), Boehringer Ingelheim Spain (Inst), Daiichi Sankyo Inc (Inst), Gercor (Inst), Hoffmann-La-Roche Ltd (Inst), Hutchison MediPharma (Inst), Iovance Biotherapeutics (Inst), Janssen Research & Development (Inst), Menarini (Inst), Nouscom SRL (Inst), Novartis FarmacÃutica SA (Inst), PledPharma (Inst), Redx Pharma (Inst), Scandion Oncology (Inst), Seagen (Inst), Sotio (Inst), Taiho Pharma USA (Inst), WntResearch (Inst)
Travel, Accommodations, Expenses: Roche, Merck Serono, Sanofi, Amgen, Array BioPharma, SERVIER, Bristol Myers Squibb
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented at the European Society for Medical Oncology World Congress on Gastrointestinal Cancer, Barcelona, Spain, June 28-July 1, 2023.
SUPPORT
Supported by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc, Rahway, NJ (M.S.D.).
CLINICAL TRIAL INFORMATION
NCT04776148 (LEAP-017)
DATA SHARING STATEMENT
A data sharing statement provided by the authors is available with this article at DOI https://doi.org/10.1200/JCO.23.02736.
AUTHOR CONTRIBUTIONS
Conception and design: Akihito Kawazoe, Rui-Hua Xu, Sebastian Stintzing, David Adelberg, Volker Heinemann, Takayuki Yoshino
Provision of study materials or patients: Akihito Kawazoe, Rui-Hua Xu, Pilar García-Alfonso, Maria Passhak, Hao-Wei Teng, Ardaman Shergill, Mahmut Gumus, Camilla Qvortrup, Sebastian Stintzing, Kathryn Towns, Tae Won Kim, Kai Keen Shiu, Juan Cundom, Sumitra Ananda, Andrey Lebedinets, Takayuki Yoshino, Elena Elez
Collection and assembly of data: Rui-Hua Xu, Pilar García-Alfonso, Maria Passhak, Hao-Wei Teng, Mahmut Gumus, Camilla Qvortrup, Kathryn Towns, Tae Won Kim, Kai Keen Shiu, Sumitra Ananda, Andrey Lebedinets, Rishi Jain, Takayuki Yoshino, Elena Elez, David Adelberg, Rong Fu
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Lenvatinib Plus Pembrolizumab Versus Standard of Care for Previously Treated Metastatic Colorectal Cancer: Final Analysis of the Randomized, Open-Label, Phase III LEAP-017 Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Akihito Kawazoe
Honoraria: Ono Pharmaceutical, Taiho Pharmaceutical, Bristol Myers Squibb, Daiichi Sankyo/UCB Japan, Lilly
Consulting or Advisory Role: Zymeworks, Revolution Medicines, MSD
Speakers' Bureau: Taiho Pharmaceutical, Ono Pharmaceutical, Bristol Myers Squibb, Daiichi Sankyo/UCB Japan, Lilly
Research Funding: Ono Pharmaceutical (Inst), Taiho Pharmaceutical (Inst), AstraZenec (Inst), MSD (Inst)
Rui-Hua Xu
Consulting or Advisory Role: Astellas Pharma, MSD, AstraZeneca, Merck Serono, Roche, Hutchison MediPharma, BeiGene, Innovent Biologics, QiLu Pharmaceutical, Junshi Pharmaceuticals, Hengrui Pharm, Keymed Biosience, CPPC
Research Funding: Merck Sharp & Dohme LLC (Inst)
Pilar García-Alfonso
Consulting or Advisory Role: Amgen, Merck Serono, SERVIER, Pierre Fabre, MSD Oncology
Speakers' Bureau: Merck Serono, Amgen, Sanofi, SERVIER, MSD Oncology, BMS
Research Funding: Merck Sharp & Dohme LLC (Inst)
Maria Passhak
Honoraria: Roche, Merck Serono
Research Funding: MSD Oncology (Inst)
Hao-Wei Teng
Consulting or Advisory Role: MSD
Speakers' Bureau: MSD
Research Funding: Merck Sharp & Dohme LLC (Inst)
Ardaman Shergill
Honoraria: Curio Science, OncLive/MJH Life Sciences, Oklahoma Society of Clinical Oncology, Cholangiocarcinoma Foundation, Cholangiocarcinoma Summit, Saint Joseph Hospital Chicago, ASCO, Colon Cancer Alliance
Consulting or Advisory Role: Triptych Health Partners, Catalyst Pharmaceuticals, KLJ Associates, Pfizer, Guardant Health, Pfizer
Research Funding: TP Therapeutics (Inst), Hutchison MediPharma (Inst), Seattle Genetics/Astellas (Inst), Verastem (Inst), Pfizer (Inst), Gritstone Bio (Inst), Gossamer Bio (Inst), Astellas Pharma (Inst), BMS (Inst), Daiichi Sankyo (Inst), Oncologie (Inst), MacroGenics (Inst), Clovis Oncology (Inst), Merck (Inst), Takeda (Inst), Merck Sharp & Dohme LLC (Inst)
Travel, Accommodations, Expenses: Colon Cancer Alliance
Mahmut Gumus
Honoraria: MSD Oncology (Inst), Pfizer (Inst), GlaxoSmithKline (Inst), Novartis (Inst)
Consulting or Advisory Role: Roche, Lilly (Inst), Amgen (Inst), Gen (Inst), Novartis (Inst), Takeda (Inst), Gilead Sciences (Inst)
Speakers' Bureau: Roche (Inst), MSD Oncology (Inst), Novartis (Inst), Polipharma (Inst), Amgen (Inst)
Research Funding: Amgen (Inst), Pfizer (Inst), Takeda (Inst), MSD Oncology (Inst)
Travel, Accommodations, Expenses: Pfizer
Camilla Qvortrup
Consulting or Advisory Role: Merck KGaA, Pierre Fabre
Research Funding: Roche (Inst), MSD Oncology (Inst), SERVIER (Inst), Pfizer (Inst), Miratis (Inst), Pierre Fabre (Inst)
Travel, Accommodations, Expenses: Roche (Inst), SERVIER (Inst), Merck KGaA (Inst), Pierre Fabre (Inst)
Sebastian Stintzing
Honoraria: Merck KGaA, Roche, Amgen, SERVIER, MSD, Pfizer, Pierre Fabre, Bristol Myers Squibb GmbH, Nordic Bioscience, AstraZeneca, Daiichi Sankyo Europe GmbH
Consulting or Advisory Role: Merck KGaA, Roche, Amgen, Pierre Fabre, MSD, AstraZeneca, SERVIER, GlaxoSmithKline, TERUMO, Nordic Bioscience, Seagen, Daiichi Sankyo Europe GmbH, CV6 Therapeutics, Isofol Medical
Research Funding: Pierre Fabre (Inst), Roche Molecular Diagnostics (Inst), Merck Serono (Inst), Amgen (Inst), MSD (Inst)
Travel, Accommodations, Expenses: Merck KGaA, Roche, Sanofi, Bayer, Sirtex Medical, Amgen, Lilly, Takeda, Pierre Fabre, AstraZeneca
Kathryn Towns
Research Funding: Merck (Inst), Merck Sharp & Dohme LLC (Inst)
Tae Won Kim
Employment: ASAN Medical Center
Research Funding: Roche/Genentech (Inst), Genome Insight (Inst), Merck Sharp & Dohme LLC (Inst)
Kai Keen Shiu
Honoraria: Merck Serono, MSD Oncology, SERVIER
Consulting or Advisory Role: MSD Oncology, Roche, Mirati Therapeutics, Bayer, Seagen
Research Funding: MSD Oncology (Inst), Roche (Inst)
Uncompensated Relationships: MSD Oncology
Juan Cundom
Consulting or Advisory Role: AstraZeneca
Speakers' Bureau: Takeda
Research Funding: Merck Sharp & Dohme LLC (Inst)
Sumitra Ananda
Honoraria: MSD
Research Funding: Merck Sharp & Dohme LLC (Inst)
Andrey Lebedinets
Research Funding: MSD (Inst)
Rong Fu
Employment: MSD RD China
Stock and Other Ownership Interests: MSD RD China
Rishi Jain
Employment: Merck Sharp & Dohme LLC
Stock and Other Ownership Interests: Merck Sharp & Dohme LLC
David Adelberg
Employment: Merck Sharp & Dohme LLC
Stock and Other Ownership Interests: Merck Sharp & Dohme LLC
Travel, Accommodations, Expenses: Merck Sharp & Dohme LLC
Volker Heinemann
Stock and Other Ownership Interests: BioNTech SE
Honoraria: Roche, Amgen, Sanofi, Merck, SERVIER, Pfizer, Pierre Fabre, AstraZeneca, MSD, Seagen, Novartis, Boehringer Ingelheim, Celgene, Sirtex Medical, GlaxoSmithKline
Consulting or Advisory Role: Merck, Amgen, Roche, MSD, Bristol Myers Squibb, Novartis, Pierre Fabre, TERUMO, GlaxoSmithKline, Servier/Pfizer, AstraZeneca, Oncosil, Nordic Bioscience, Sirtex Medical, Halozyme, Janssen
Research Funding: Merck (Inst), Amgen (Inst), Roche (Inst)
Travel, Accommodations, Expenses: Merck, AstraZeneca, Amgen, MSD, Nordic Bioscience
Takayuki Yoshino
Honoraria: Chugai Pharma, MSD K.K, Takeda, Merck
Consulting or Advisory Role: Sumitomo Corp
Research Funding: MSD (Inst), DAIICHI SANKYO COMPANY, LIMITED (Inst), Ono Pharmaceutical (Inst), Taiho Pharmaceutical (Inst), Amgen (Inst), Sanofi (Inst), Pfizer (Inst), Sysmex (Inst), Chugai Pharma (Inst), Eisai (Inst), Molecular Health (Inst), Roche (Inst), FALCO biosystems Ltd (Inst), Merus (Inst), Bristol Myers Squibb Japan (Inst), Medical & Biological Laboratories Co., Ltd (Inst), Takeda (Inst), Merck Sharp & Dohme LLC (Inst)
Elena Elez
Honoraria: Bristol Myers Squibb, SERVIER, Amgen, Merck Serono, Array BioPharma, Sanofi/Aventis, Merck, Novartis, Seagan, Takeda, Pfizer, Bayer, Boehringer Ingelheim, Cure Teq AG, Roche, Janssen, Lilly, Medscape, MSD, Pierre Fabre, Repare Therapeutics, RIN Institute Inc
Consulting or Advisory Role: Amgen, Roche, Merck Serono, Sanofi, SERVIER, Bayer, Bristol Myers Squibb, Array BioPharma, Pierre Fabre, MSD, Bayer, Boehringer Ingelheim, Cure Teq AG, Roche, Janssen, Novartis, Pfizer, Repare Therapeutics Inc, RIN Institute Inc, Seagen, Takeda
Research Funding: Roche (Inst), Bristol Myers Squibb (Inst), SERVIER (Inst), Amgen (Inst), Array BioPharma (Inst), MedImmune (Inst), Pierre Fabre (Inst), Sanofi (Inst), Merck (Inst), BeiGene (Inst), Celgene (Inst), Debiopharm Group (Inst), Genentech (Inst), HalioDx (Inst), Hutchison MediPharma (Inst), Janssen-Cilag SA (Inst), Menarini (Inst), Merck Sharp&Dohme de España SA (Inst), Merus NV (Inst), Mirati Therapeutics (Inst), Novartis (Inst), Pfizer (Inst), PharmaMar (Inst), Taiho Pharmaceutical (Inst), AstraZeneca (Inst), Boehringer Ingelheim (Inst), AbbVie (Inst), Bayer (Inst), Bioncotech (Inst), BioNTech RNA Pharmaceuticals GMBH (Inst), Biontech Small Molecules GMBH (Inst), Boehringer Ingelheim Spain (Inst), Daiichi Sankyo Inc (Inst), Gercor (Inst), Hoffmann-La-Roche Ltd (Inst), Hutchison MediPharma (Inst), Iovance Biotherapeutics (Inst), Janssen Research & Development (Inst), Menarini (Inst), Nouscom SRL (Inst), Novartis FarmacÃutica SA (Inst), PledPharma (Inst), Redx Pharma (Inst), Scandion Oncology (Inst), Seagen (Inst), Sotio (Inst), Taiho Pharma USA (Inst), WntResearch (Inst)
Travel, Accommodations, Expenses: Roche, Merck Serono, Sanofi, Amgen, Array BioPharma, SERVIER, Bristol Myers Squibb
No other potential conflicts of interest were reported.
REFERENCES
- 1.Le DT, Durham JN, Smith KN, et al. : Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 357:409-413, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Le DT, Uram JN, Wang H, et al. : PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 372:2509-2520, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Surveillance, Epidemiology, and End Results (SEER): All Cancer Sites Combined Recent Trends in SEER Age-Adjusted Incidence Rates, 2000-2021. https://seer.cancer.gov/statistics-network/explorer/ [Google Scholar]
- 4.National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) : Colon Cancer. Version 3. 2022. 2022. https://NCCN.org [Google Scholar]
- 5.Cervantes A, Adam R, Roselló S, et al. : Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol 34:10-32, 2023 [DOI] [PubMed] [Google Scholar]
- 6.Hashiguchi Y, Muro K, Saito Y, et al. : Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int J Clin Oncol 25:1-42, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prager GW, Taieb J, Fakih M, et al. : Trifluridine-tipiracil and bevacizumab in refractory metastatic colorectal cancer. N Engl J Med 388:1657-1667, 2023 [DOI] [PubMed] [Google Scholar]
- 8.Grothey A, Van Cutsem E, Sobrero A, et al. : Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): An International, Multicentre, Randomised, Placebo-Controlled, phase 3 trial. Lancet 381:303-312, 2013 [DOI] [PubMed] [Google Scholar]
- 9.Mayer RJ, Van Cutsem E, Falcone A, et al. : Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med 372:1909-1919, 2015 [DOI] [PubMed] [Google Scholar]
- 10.Li J, Qin S, Xu RH, et al. : Effect of fruquintinib vs placebo on overall survival in patients with previously treated metastatic colorectal cancer: The FRESCO randomized clinical trial. JAMA 319:2486-2496, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dasari A, Lonardi S, Garcia-Carbonero R, et al. : Fruquintinib versus placebo in patients with refractory metastatic colorectal cancer (FRESCO-2): An International, Multicentre, Randomised, Double-Blind, phase 3 study. Lancet 402:41-53, 2023 [DOI] [PubMed] [Google Scholar]
- 12.Fukuoka S, Hara H, Takahashi N, et al. : Regorafenib plus nivolumab in patients with advanced gastric or colorectal cancer: An open-label, dose-escalation, and dose-expansion phase Ib trial (REGONIVO, EPOC1603). J Clin Oncol 38:2053-2061, 2020 [DOI] [PubMed] [Google Scholar]
- 13.Fakih M, Raghav KPS, Chang DZ, et al. : Regorafenib plus nivolumab in patients with mismatch repair-proficient/microsatellite stable metastatic colorectal cancer: A single-arm, open-label, multicentre phase 2 study. EClinicalMedicine 58:101917, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cousin S, Cantarel C, Guegan JP, et al. : Regorafenib-avelumab combination in patients with microsatellite stable colorectal cancer (REGOMUNE): A single-arm, open-label, phase II trial. Clin Cancer Res 27:2139-2147, 2021 [DOI] [PubMed] [Google Scholar]
- 15.Makker V, Colombo N, Casado Herráez A, et al. : Lenvatinib plus pembrolizumab for advanced endometrial cancer. N Engl J Med 386:437-448, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Motzer R, Alekseev B, Rha SY, et al. : Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med 384:1289-1300, 2021 [DOI] [PubMed] [Google Scholar]
- 17.Lwin Z, Gomez-Roca C, Saada-Bouzid E, et al. : LBA41 LEAP-005: Phase II study of lenvatinib (len) plus pembrolizumab (pembro) in patients (pts) with previously treated advanced solid tumours. Ann Oncol 31:S1170, 2020 [Google Scholar]
- 18.Bekaii-Saab TS, Ou F-S, Anderson DM, et al. : Regorafenib dose optimization study (ReDOS): Randomized phase II trial to evaluate dosing strategies for regorafenib in refractory metastatic colorectal cancer (mCRC): An ACCRU Network study. J Clin Oncol 36, 2018. (4_suppl; abstr 611) [Google Scholar]
- 19.Gomez-Roca C, Yanez E, Im S-A, et al. : LEAP-005: A phase II multicohort study of lenvatinib plus pembrolizumab in patients with previously treated selected solid tumors—Results from the colorectal cancer cohort. J Clin Oncol 39, 2021. (3_suppl; abstr 94) [Google Scholar]
- 20.Diaz LA Jr., Shiu KK, Kim TW, et al. : Pembrolizumab versus chemotherapy for microsatellite instability-high or mismatch repair-deficient metastatic colorectal cancer (KEYNOTE-177): Final analysis of a randomised, open-label, phase 3 study. Lancet Oncol 23:659-670, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lizardo DY, Kuang C, Hao S, et al. : Immunotherapy efficacy on mismatch repair-deficient colorectal cancer: From bench to bedside. Biochim Biophys Acta Rev Cancer 1874:188447, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eng C, Kim TW, Bendell J, et al. : Atezolizumab with or without cobimetinib versus regorafenib in previously treated metastatic colorectal cancer (IMblaze370): A multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol 20:849-861, 2019 [DOI] [PubMed] [Google Scholar]
- 23.O'Neil BH, Wallmark JM, Lorente D, et al. : Safety and antitumor activity of the anti-PD-1 antibody pembrolizumab in patients with advanced colorectal carcinoma. PLoS One 12:e0189848, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lenz H-J, Parikh AR, Spigel DR, et al. : Nivolumab (NIVO) + 5-fluorouracil/leucovorin/oxaliplatin (mFOLFOX6)/bevacizumab (BEV) versus mFOLFOX6/BEV for first-line (1L) treatment of metastatic colorectal cancer (mCRC): Phase 2 results from CheckMate 9X8. J Clin Oncol 40, 2022. (4_suppl; abstr 8) [Google Scholar]
- 25.Fakih M, Sandhu J, Lim D, et al. : Regorafenib, ipilimumab, and nivolumab for patients with microsatellite stable colorectal cancer and disease progression with prior chemotherapy: A phase 1 nonrandomized clinical trial. JAMA Oncol 9:627-634, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bullock A, Fakih M, Gordon M, et al. : LBA-4 results from an expanded phase 1 trial of botensilimab (BOT), a multifunctional anti-CTLA-4, plus balstilimab (BAL; anti-PD-1) for metastatic heavily pretreated microsatellite stable colorectal cancer (MSS CRC). Ann Oncol 34:S178-S179, 2023 [Google Scholar]
- 27.Iwasa S, Okita N, Kuchiba A, et al. : Phase II study of lenvatinib for metastatic colorectal cancer refractory to standard chemotherapy: The LEMON study (NCCH1503). ESMO Open 5:e000776, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
A data sharing statement provided by the authors is available with this article at DOI https://doi.org/10.1200/JCO.23.02736.