Abstract
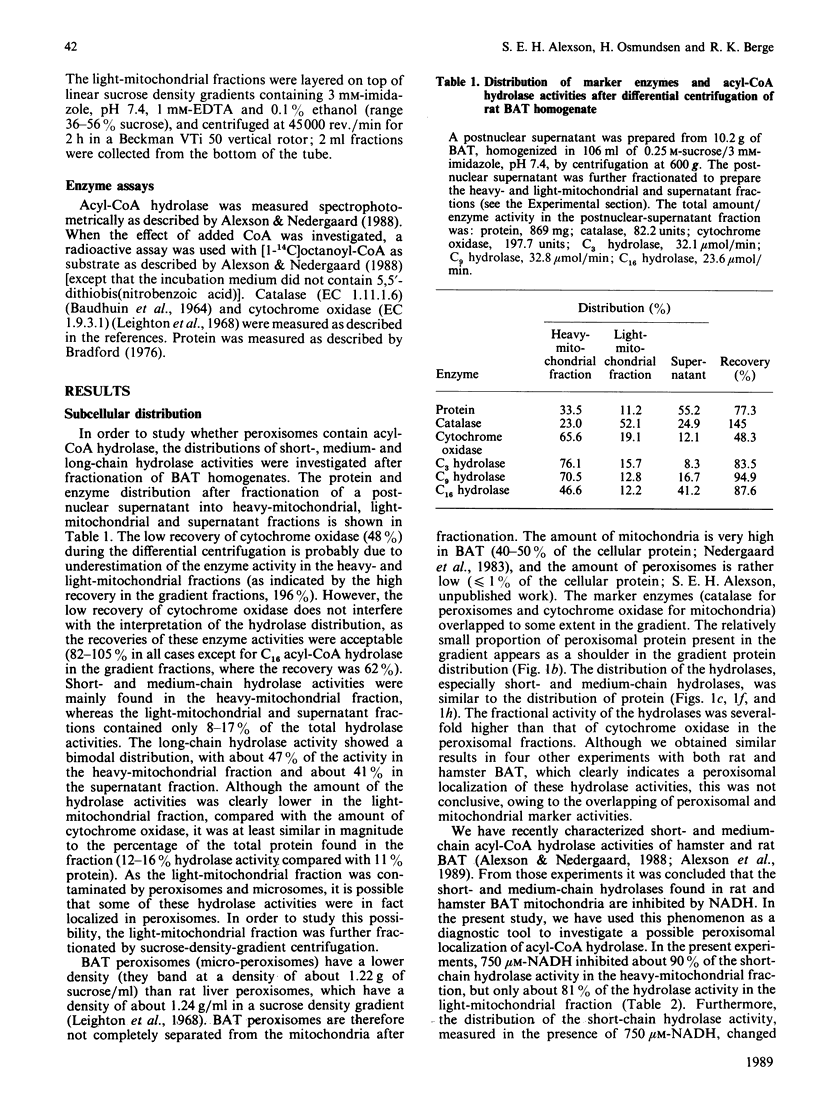
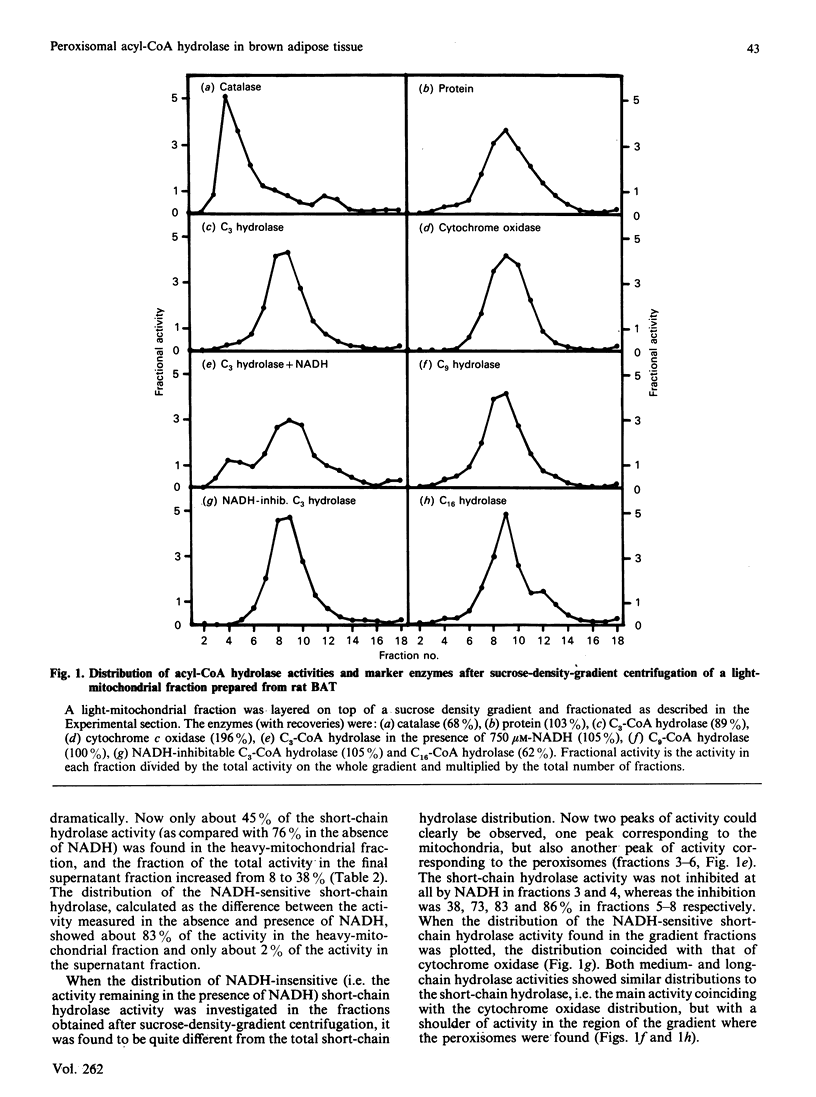
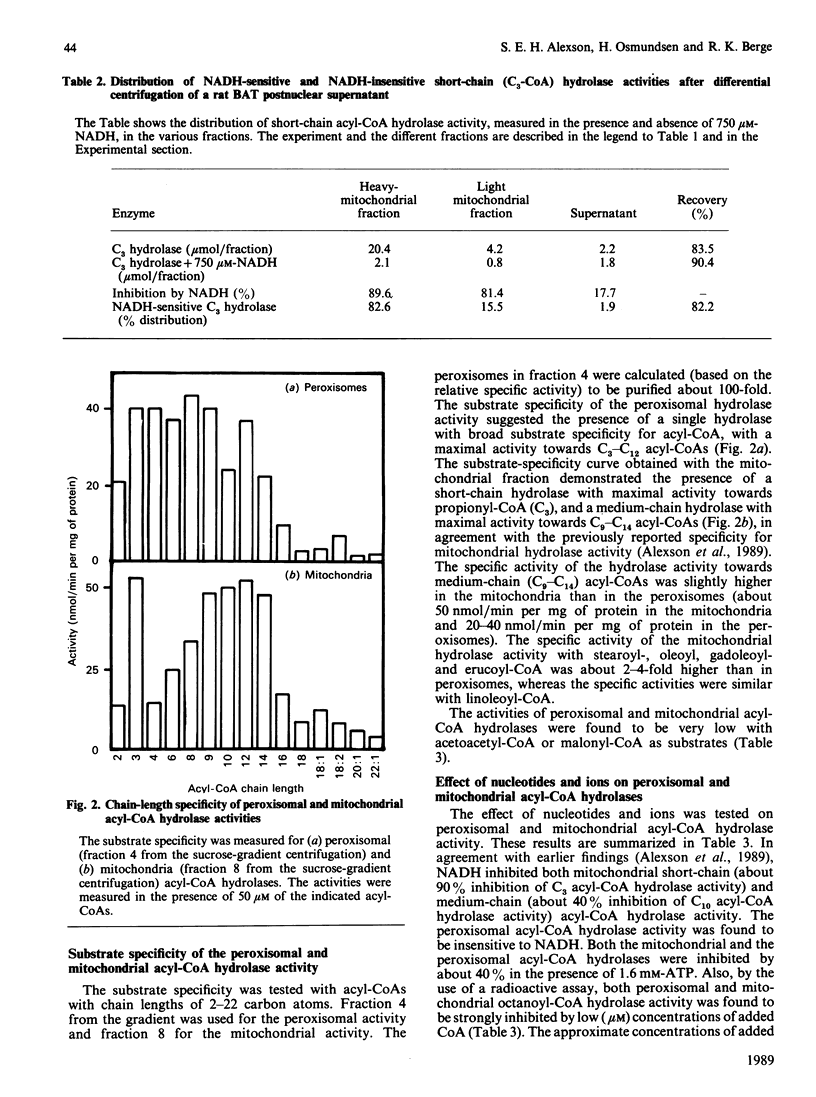
The subcellular distribution of acyl-CoA hydrolase was studied in rat brown adipose tissue, with special emphasis on possible peroxisomal localization. Subcellular fractionation by sucrose-density-gradient centrifugation, followed by measurement of short-chain (propionyl-CoA) acyl-CoA hydrolase in the presence of NADH, resulted in two peaks of activity in the gradient: one peak corresponded to the distribution of cytochrome oxidase (mitochondrial marker enzyme), and another peak of activity coincided with the peroxisomal marker enzyme catalase. The distribution of the NADH-inhibited short-chain hydrolase activity fully resembled that of cytochrome oxidase. The substrate-specificity curve of the peroxisomal acyl-CoA hydrolase activity indicated the presence of a single enzyme exhibiting a broad substrate specificity, with maximal activity towards fatty acids with chain lengths of 3-12 carbon atoms. The mitochondrial acyl-CoA hydrolase substrate specificity, in contrast, indicated the presence of at least two acyl-CoA hydrolases (of short- and medium-chain-length specificity). The peroxisomal acyl-CoA hydrolase activity was inhibited by CoA at low (microM) concentrations and by ATP at high concentrations (greater than 0.8 mM). In contrast with the mitochondrial short-chain hydrolase, the peroxisomal acyl-CoA hydrolase activity was not inhibited by NADH.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexson S. E., Cannon B. A direct comparison between peroxisomal and mitochondrial preferences for fatty-acyl beta-oxidation predicts channelling of medium-chain and very-long-chain unsaturated fatty acids to peroxisomes. Biochim Biophys Acta. 1984 Oct 24;796(1):1–10. doi: 10.1016/0005-2760(84)90231-5. [DOI] [PubMed] [Google Scholar]
- Alexson S. E., Nedergaard J. A novel type of short- and medium-chain acyl-CoA hydrolases in brown adipose tissue mitochondria. J Biol Chem. 1988 Sep 25;263(27):13564–13571. [PubMed] [Google Scholar]
- Bartlett K., Watmough N. J., Causey A. G. Intermediates of beta-oxidation. Biochem Soc Trans. 1988 Jun;16(3):410–416. doi: 10.1042/bst0160410a. [DOI] [PubMed] [Google Scholar]
- Baudhuin P., Beaufay H., Rahman-Li Y., Sellinger O. Z., Wattiaux R., Jacques P., De Duve C. Tissue fractionation studies. 17. Intracellular distribution of monoamine oxidase, aspartate aminotransferase, alanine aminotransferase, D-amino acid oxidase and catalase in rat-liver tissue. Biochem J. 1964 Jul;92(1):179–184. doi: 10.1042/bj0920179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berge R. K., Aarsland A. Correlation between the cellular level of long-chain acyl-CoA, peroxisomal beta-oxidation, and palmitoyl-CoA hydrolase activity in rat liver. Are the two enzyme systems regulated by a substrate-induced mechanism? Biochim Biophys Acta. 1985 Nov 14;837(2):141–151. doi: 10.1016/0005-2760(85)90237-1. [DOI] [PubMed] [Google Scholar]
- Berge R. K., Døssland B. Differences between microsomal and mitochondrial-matrix palmitoyl-coenzyme A hydrolase, and palmitoyl-L-carnitine hydrolase from rat liver. Biochem J. 1979 Jul 1;181(1):119–125. doi: 10.1042/bj1810119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berge R. K., Farstad M. Dual localization of long-chain acyl-CoA hydrolase in rat liver: one in the microsomes and one in the mitochondrial matrix. Eur J Biochem. 1979 Mar 15;95(1):89–97. doi: 10.1111/j.1432-1033.1979.tb12942.x. [DOI] [PubMed] [Google Scholar]
- Berge R. K., Flatmark T., Osmundsen H. Enhancement of long-chain acyl-CoA hydrolase activity in peroxisomes and mitochondria of rat liver by peroxisomal proliferators. Eur J Biochem. 1984 Jun 15;141(3):637–644. doi: 10.1111/j.1432-1033.1984.tb08239.x. [DOI] [PubMed] [Google Scholar]
- Berge R. K., Hosøy L. H., Farstad M. N. Influence of dietary status on liver palmitoyl-CoA hydrolase, peroxisomal enzymes, CoASH and long-chain acyl-CoA in rats. Int J Biochem. 1984;16(4):403–410. doi: 10.1016/0020-711x(84)90139-3. [DOI] [PubMed] [Google Scholar]
- Berge R. K., Skrede S., Farstad M. Effects of clofibrate on the intracellular localization of palmitoyl-CoA hydrolase and palmitoyl-L-carnitine hydrolase in rat liver. FEBS Lett. 1981 Feb 9;124(1):43–47. doi: 10.1016/0014-5793(81)80050-6. [DOI] [PubMed] [Google Scholar]
- Berge R. K., Slinde E., Farstad M. Intracellular localization of long-chain acyl-coenzyme A hydrolase and acyl-L-carnitine hydrolase in brown adipose tissue from guinea pigs. Biochem J. 1979 Aug 15;182(2):347–351. doi: 10.1042/bj1820347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernson S. M. Acetyl-CoA hydrolase; activity, regulation and physiological significance of the enzyme in brown adipose tissue from hamster. Eur J Biochem. 1976 Aug 16;67(2):403–410. doi: 10.1111/j.1432-1033.1976.tb10705.x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Diczfalusy U., Alexson S. E., Pedersen J. I. Chain-shortening of prostaglandin F2 alpha by rat liver peroxisomes. Biochem Biophys Res Commun. 1987 May 14;144(3):1206–1213. doi: 10.1016/0006-291x(87)91439-2. [DOI] [PubMed] [Google Scholar]
- Diczfalusy U., Alexson S. E. Peroxisomal chain-shortening of prostaglandin F2 alpha. J Lipid Res. 1988 Dec;29(12):1629–1636. [PubMed] [Google Scholar]
- Grigat K. P., Koppe K., Seufert C. D., Söling H. D. Acetyl-coenzyme A deacylase activity in liver is not an artifact. Subcellular distribution and substrate specificity of acetyl-coenzyme A deacylase activities in rat liver. Biochem J. 1979 Jan 1;177(1):71–79. doi: 10.1042/bj1770071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiltunen J. K., Kärki T., Hassinen I. E., Osmundsen H. beta-Oxidation of polyunsaturated fatty acids by rat liver peroxisomes. A role for 2,4-dienoyl-coenzyme A reductase in peroxisomal beta-oxidation. J Biol Chem. 1986 Dec 15;261(35):16484–16493. [PubMed] [Google Scholar]
- Hovik R., Osmundsen H. Peroxisomal beta-oxidation of long-chain fatty acids possessing different extents of unsaturation. Biochem J. 1987 Nov 1;247(3):531–535. doi: 10.1042/bj2470531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarow P. B., De Duve C. A fatty acyl-CoA oxidizing system in rat liver peroxisomes; enhancement by clofibrate, a hypolipidemic drug. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2043–2046. doi: 10.1073/pnas.73.6.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. Y., Schulz H. Isolation, properties, and regulation of a mitochondrial acyl coenzyme A thioesterase from pig heart. J Biol Chem. 1979 Jun 10;254(11):4516–4523. [PubMed] [Google Scholar]
- Leighton F., Poole B., Beaufay H., Baudhuin P., Coffey J. W., Fowler S., De Duve C. The large-scale separation of peroxisomes, mitochondria, and lysosomes from the livers of rats injected with triton WR-1339. Improved isolation procedures, automated analysis, biochemical and morphological properties of fractions. J Cell Biol. 1968 May;37(2):482–513. doi: 10.1083/jcb.37.2.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannaerts G. P., Van Veldhoven P., Van Broekhoven A., Vandebroek G., Debeer L. J. Evidence that peroxisomal acyl-CoA synthetase is located at the cytoplasmic side of the peroxisomal membrane. Biochem J. 1982 Apr 15;204(1):17–23. doi: 10.1042/bj2040017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazawa S., Furuta S., Hashimoto T. Induction of a novel long-chain acyl-CoA hydrolase in rat liver by administration of peroxisome proliferators. Eur J Biochem. 1981 Jul;117(2):425–430. doi: 10.1111/j.1432-1033.1981.tb06356.x. [DOI] [PubMed] [Google Scholar]
- Nedergaard J., Alexson S., Cannon B. Cold adaptation in the rat: increased brown fat peroxisomal beta-oxidation relative to maximal mitochondrial oxidative capacity. Am J Physiol. 1980 Nov;239(5):C208–C216. doi: 10.1152/ajpcell.1980.239.5.C208. [DOI] [PubMed] [Google Scholar]
- Nedergaard J., Becker W., Cannon B. Effects of dietary essential fatty acids on active thermogenin content in rat brown adipose tissue. J Nutr. 1983 Sep;113(9):1717–1724. doi: 10.1093/jn/113.9.1717. [DOI] [PubMed] [Google Scholar]
- Osmundsen H., Neat C. E., Borrebaek B. Fatty acid products of peroxisomal beta-oxidation. Int J Biochem. 1980;12(4):625–630. doi: 10.1016/0020-711x(80)90015-4. [DOI] [PubMed] [Google Scholar]
- Prass R. L., Isohashi F., Utter M. F. Purification and characterization of an extramitochondrial acetyl coenzyme A hydrolase from rat liver. J Biol Chem. 1980 Jun 10;255(11):5215–5223. [PubMed] [Google Scholar]
- Schepers L., Casteels M., Vamecq J., Parmentier G., Van Veldhoven P. P., Mannaerts G. P. Beta-oxidation of the carboxyl side chain of prostaglandin E2 in rat liver peroxisomes and mitochondria. J Biol Chem. 1988 Feb 25;263(6):2724–2731. [PubMed] [Google Scholar]
- Snoswell A. M., Tubbs P. K. Deacylation of acetyl-coenzyme A and acetylcarnitine by liver preparations. Biochem J. 1978 May 1;171(2):299–303. doi: 10.1042/bj1710299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söling H. D., Rescher C. On the regulation of cold-labile cytosolic and of mitochondrial acetyl-CoA hydrolase in rat liver. Eur J Biochem. 1985 Feb 15;147(1):111–117. doi: 10.1111/j.1432-1033.1985.tb08726.x. [DOI] [PubMed] [Google Scholar]
- Van Broekhoven A., Peeters M. C., Debeer L. J., Mannaerts G. P. Subcellular distribution of coenzyme A: evidence for a separate coenzyme A pool in peroxisomes. Biochem Biophys Res Commun. 1981 May 15;100(1):305–312. doi: 10.1016/s0006-291x(81)80097-6. [DOI] [PubMed] [Google Scholar]
- Van Veldhoven P. P., Just W. W., Mannaerts G. P. Permeability of the peroxisomal membrane to cofactors of beta-oxidation. Evidence for the presence of a pore-forming protein. J Biol Chem. 1987 Mar 25;262(9):4310–4318. [PubMed] [Google Scholar]


