SUMMARY
The metabolic conditions that prevail during bacterial growth have evolved with the faithful operation of repair systems that recognize and eliminate DNA lesions caused by intracellular and exogenous agents. This idea is supported by the low rate of spontaneous mutations (10−9) that occur in replicating cells, maintaining genome integrity. In contrast, when growth and/or replication cease, bacteria frequently process DNA lesions in an error-prone manner. DNA repairs provide cells with the tools needed for maintaining homeostasis during stressful conditions and depend on the developmental context in which repair events occur. Thus, different physiological scenarios can be anticipated. In nutritionally stressed bacteria, different components of the base excision repair pathway may process damaged DNA in an error-prone approach, promoting genetic variability. Interestingly, suppressing the mismatch repair machinery and activating specific DNA glycosylases promote stationary-phase mutations. Current evidence also suggests that in resting cells, coupling repair processes to actively transcribed genes may promote multiple genetic transactions that are advantageous for stressed cells. DNA repair during sporulation is of interest as a model to understand how transcriptional processes influence the formation of mutations in conditions where replication is halted. Current reports indicate that transcriptional coupling repair-dependent and -independent processes operate in differentiating cells to process spontaneous and induced DNA damage and that error-prone synthesis of DNA is involved in these events. These and other noncanonical ways of DNA repair that contribute to mutagenesis, survival, and evolution are reviewed in this manuscript.
KEYWORDS: B. subtilis, stationary phase-associated mutagenesis (SPM), DNA repair, sporulation, germination/outgrowth
INTRODUCTION
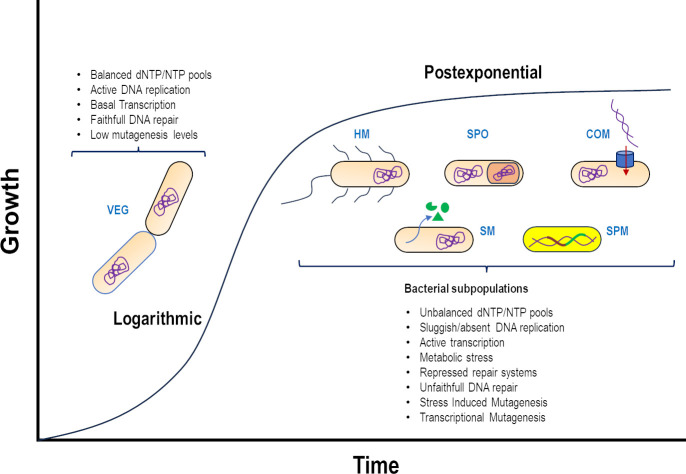
Bacteria constantly face changing environments and display specific adaptive responses that preserve or modify their genomic sequences, thereby having a direct effect on their survival and evolution (1, 2). The gram-positive bacterium Bacillus subtilis has been used to study morpho-physiological events associated with the post-exponential phase of growth, including sporulation, competence, hypermotility, and stationary phase- or stress-associated mutagenesis (SPM/SAM) (3–5) (Fig. 1). These processes that occur under nutritional stress in subpopulations of nonreplicating cells are driven by transcriptional factors that regulate, in time and space, programs of differential expression or evolvability (6, 7) (Fig. 1). The correct operation of a multiplicity of preventive and repair pathways (and supported by a favorable metabolic environment) maintains the genomic fidelity of bacterial cells during exponential growth (8) (Fig. 1). In B. subtilis, base and nucleotide incision/excision repair mechanisms operate in sync with high-fidelity replicating machinery and mismatch repair (MMR) systems to keep the mutation rate of exponentially growing cells in the order of 10−9 to 10−10 (9, 10). Genes-coding proteins of the nucleotide excision repair (NER) system (namely UvrA, UvrB, and UvrC) are found in the chromosome of this microorganism (9–11). The coding gene uvrC is independently expressed, while uvrB and uvrA are arranged in a bicistronic operon (11–13). In E. coli, uvrA and uvrB (but not uvrC) are part of the SOS response. In contrast, the uvrA, B, and C genes are induced by DNA-damaging factors such as ultraviolet (UV)-C light and mitomycin-C (MC) in B. subtilis; therefore, they are part of the transcriptional SOS response, which is under the control of DinR (a homolog of E. coli LexA) and RecA (12, 14–18). However, a significant number of reports have shown that RecA plays a wider role in B. subtilis, as the SOS response can be activated during competence, sporulation, and spore germination/outgrowth (19–22).
Fig 1.
Logarithmic growth. Favorable metabolic conditions during exponential growth support vegetative growth and faithful DNA repair. Post-exponential growth. High cell density and nutrient exhaustion push B. subtilis to establish cell subpopulations with well-differentiated morpho-physiological properties to survive growth-limiting conditions, including sporulation (SPO), hypermotility (HM), competence (COM), production of secondary metabolites (SM), and stationary-phase mutagenesis. Adverse metabolic conditions during post-exponential growth promote low-fidelity DNA repair and elicit SPM.
A canonical base excision repair (BER) pathway is also present in B. subtilis. In addition to enzymes that specifically recognize and excise deaminated and alkylates bases, including Ung, AlkA, and Aag, B. subtilis contains MutY and MutM—DNA glycosylases that repair oxidized guanine (the guanine oxidized; GO system) (10, 23–30). In B. subtilis, the Ung coding gene is expressed during the exponential and stationary phases of growth (27), similar patterns of expression were found for mutM and mutY (30, 31). In contrast, alkA is induced as part of the adaptative (ada) response together with the adaA and adaB genes, which encode methylphosphotriester DNA methyltransferase (AdaA protein) and O6-methylguanine DNA methyltransferase (AdaB protein) (32). An additional pathway of excision repair that operates through noncanonical modes has also been described in B. subtilis. This pathway uses YwqL, an EndoV homolog whose coding gene is expressed in growing and stationary-phase cells (27), together with the apurinic/apyrimidinic (AP) endonuclease ExoA and the polymerase PolA to repair deaminated DNA bases (33). Recently, a second, alternative excision pathway composed of the UV endonuclease YwjD, the exonuclease YpcP, and low-fidelity (LF) DNA polymerases to repair distinct DNA photodimers was reported to operate in sporulating B. subtilis cells (34). Dormant spores are highly resistant to several DNA-damaging factors and can persist in the environment for undefined periods of time (35, 36). However, during its return to vegetative growth, the reviving spore must process the genetic damage suffered during dormancy and generated during germination/outgrowth. Current evidence has found that excision repair pathways, the recombination system, and checkpoint events counteract genetic damage elicited by reactive oxygen species (ROS) for a spore’s efficient return to vegetative growth (22, 37–40). Interestingly, under growth-limiting conditions, e.g., during nutritional stress, repair pathways in B. subtilis are suppressed or operate with LF polymerases to produce genetic diversity and increase the likelihood of escape from growth-limiting conditions (3–5, 41). Thus, while error-prone repair events associated with SPM are RecA independent, they are influenced by competence, coordinated by transcription factors such as mutation frequency-decline protein (Mfd) and GreA, and regulated by the second messenger, cyclic diadenosine-monophosphate (c-di-AMP), which divergently modulates growth and SPM in this bacterium (42–46). The biochemical, structural, and functional aspects of the DNA repair pathways operating in B. subtilis, which are beyond the scope of this manuscript, have been deeply revised by Lenhart et al. (10) and Wozniak and Simons (47), respectively (10, 47). In this manuscript, we review the experimental evidence collected for more than two decades describing the genetic and physiological aspects underlying SPM and repair events occurring during cell differentiation in B. subtilis.
B. SUBTILIS STATIONARY PHASE-ASSOCIATED MUTAGENESIS
SPM (or SAM) is defined as a process that increases the frequency of mutations in nonproliferating cells subjected to a nonlethal selective pressure that allows these cells to grow, divide, and escape from the stressful condition (4–6). SPM has been studied in E. coli, Pseudomonas putida, and the eukaryotic yeast Saccharomyces cerevisiae (6, 48, 49). These mutations and their underlying mechanisms are highly relevant as they propagate genetic diversity across all life domains and affect cell survival and evolution.
In B. subtilis, several cellular processes activated by stress, including sporulation, competence, production of secondary metabolites, and the acquisition of resistance to environmental stressors, occur during post-exponential growth and are established in differentiated cell populations displaying distinctive morpho-physiological characteristics (3, 4, 50) (Fig. 1). In this context, Sung and Yasbin (3) demonstrated the existence of SPM in B. subtilis and implicated the development of the K-state (natural competence—the ability of a cell to take up DNA from its environment and incorporate it into the genome) in this process. Recent evidence has advanced this concept by showing that K-cells are more prone to produce stationary-phase mutations, particularly under oxidative stress conditions, than non-K cells. This supports the idea that a cell subpopulation that develops during the post-exponential growth phase is tasked with producing genetic diversity through RecA-dependent and -independent pathways (3, 51).
Approaches to study SPM in B. subtilis
Gain-of-function (reversion) systems
The SPM process and the mechanisms involved have been addressed in B. subtilis and other microorganisms employing a diversity of gain-of-function and loss-of-function genetic assays (3, 6, 48, 49, 52), the most relevant are revised below. Previous experiments conducted at the Francis Ryan laboratory may constitute the first evidence of bacterial SPM (53, 54). Those experiments demonstrated the appearance of bacterial colonies with a His+ phenotype after long periods of starvation of an E. coli strain auxotrophic for histidine. Cell growth remained undetectable, and DNA replication slowed over time (53, 54). However, the E. coli FC40 system that uses an F´ lac frameshift reversion construct to determine the production of colonies capable of growing in lactose as the only carbon source has, thus far, been the most widely employed system to study SPM in laboratories (55–58). Later evidence has found that Lac+-revertant colonies generated during the stationary phase are the product of point mutations or gene amplification triggered by stress and the SOS response (59, 60). A plasmid-based system was also developed to study SPM in P. putida. In this system, the codon 22 (Leu) of the phenol monooxygenase gene pheA has been replaced with a premature stop codon permitting to monitoring base substitution mutations that restore the functional gene and enable P. putida to utilize phenol as a sole growth substrate (Phe+ phenotype) (48). P. putida cells carrying plasmid-encoded promoter-less pheAB genes cannot grow on phenol, but Phe+ revertants capable of degrading phenol, activating a silent pheA gene on a plasmid under carbon-starved conditions, accumulate for 10 days (48). Employment of this and additional gain-of-function approaches to explore mutagenic events in the chromosome of P. putida have allowed to unravel the mechanisms responsible for the appearance of mutations in stationary-phase populations of this microorganism (61–63).
The gain-of-function SPM in B. subtilis employs the YB955 strain carrying the chromosomal auxotrophies hisC952, metB5, and leuC427. The first two auxotrophies originate from nonsense amber (Gln→stop) and ochre (Glu→stop) mutations, respectively, while the last one corresponds to a missense mutation that changes a Gly to an Arg (3, 4) (Table 1). Cells of this strain, cultured to a late stationary phase of growth, are independently plated in minimum media lacking His, Met, or Leu (Fig. 2). The number of prototrophic His+, Met+, or Leu+ colonies accumulated during 10 days of incubation (referred to as the initial cell titer) is used to calculate the reversion frequencies to the hisC952, metB5, and leuC427 mutant alleles. In these experiments, the amino-acid-revertant colonies generated during the first 2 days are not considered true SP mutants as they can be slow-growing revertants generated during active growth (3, 41) (Fig. 3). Experiments have also established that colonies growing in the absence of the amino acids under selection are true revertants, and their ability to grow compared to the parent is unaffected (3, 41). This mutagenesis system has been successfully employed over 20 years to unravel the mechanisms underlying SPM in B. subtilis.
TABLE 1.
Base substitutions of amino-acid-mutated genes in the B. subtilis YB955 strain
| Targeted gene | Position of substitution | Type of substitution | Change in DNA | Effect of mutation | Enzyme affected |
|---|---|---|---|---|---|
| hisC952 | 952 | Transition |
CAG C→T |
Gln → stop (amber, UAG) | Histidinol phosphate aminotransferase |
| metB5 | 346 | Transversion |
GAA G→T |
Glu → stop (ocre, UAA) | Homoserine O-succinyltransferase |
| leuC427 | 427 | Transition |
GGA G→A |
Gly→ Arg (missense, AGA) | Isopropylmalate isomerase |
Fig 2.
(A) Mutation events giving rise to His+, Met+, or Leu+ reversions in the B. subtilis YB955 strain. This strain carries chromosomal nonsense hisc952, metB5, and missense leuC427 mutations, which generate auxotrophies to His, Met, and Leu in B. subtilis. Both hisc952 and metB5 can be reverted by true or suppressor mutations. (B) A schematic exemplifying how suppressor mutations can generate His+ prototrophs. Left, Gln is inserted at the -CAG- codon of WT hisC. Middle, translation stops at the UAG stop codon, which generates an incomplete HisC protein that releasing factor (RF) releases. Right, mutant tRNA (Sup-tRNA) inserts an unpredicted amino acid at a stop codon, generating a complete HisC protein. Sup, suppressors.
Fig 3.
Stationary-phase mutagenesis protocol determines the reversion of chromosomal hisC, metB, and leuC mutant genes in the B. subtilis YB955 strain. This strain is propagated in a rich medium 90 minutes past T0 (the culture time when logarithmic and stationary phases intersect). Cells are collected, washed, and resuspended in a minimal medium (MM). Cells are independently plated in MM lacking His, Met, or Leu. Viable counts are determined in MM plates supplemented with His, Met, and Leu.
Furthermore, B. subtilis strains carrying missense and nonsense mutations in the gfp and argF genes (and transcriptionally controlled by an IPTG-inducible Pspac promoter) have been successfully used to study the influence of DNA base lesions and secondary structures in B. subtilis SPM (50, 64). Ambriz-Aviña et al. (50) developed a flow cytometry-based approach to study transcription-associated mutations (TAM) to directly detect in bacterial cultures, at single-cell level, the population of cells that gained or lost a fluorescent phenotype owing to mutational events occurring in a gfp reporter gene. Two-point mutated gfp alleles encoding nonfunctional GFP proteins were used; the first one gfpns, where the third base of the 57th TGG codon was replaced for adenine, resulting in a TGA stop codon. In the second one, the 66th tyrosine codon essential for GFP fluorescence was mutated to a histidine codon, generating a full-sized nonfluorescent product, named gfpTyr-His (50). Using this gain-of-function system, it was found that albeit transcriptional derepression promoted mutations that elevated the number of cells with a functional GFP phenotype, these events were more frequent in stationary-phase cultures of the WT strain bearing the gfpns allele (50). It was also found that the genetic inactivation of the base deamination (27) and guanine-oxidized excision repair pathways (29) enhanced the population of fluorescent cells in stationary-phase cultures that overexpressed the missense and nonsense gfp alleles. From this evidence, it was postulated that in nongrowing B. subtilis cells, the accumulation of spontaneous genetic lesions saturates the capacity of DNA repair systems promoting transcriptional-associated mutagenesis (50).
Misalignments during replication can lead to DNA rearrangements such as deletions or duplications of different lengths ranging from a few nucleotides to complete genes. This process, also termed replication slippage (65), gives rise to genome deletions between short duplications and takes place in eukaryotes and bacteria (66–70). Primer-template misalignments can promote mutagenic events in runs of direct or inverted sequence repeats in replicating bacteria (71–73). In lactose-starved, stationary-phase E. coli cells, the point mutations giving rise to Lac+ reversions in the FC40 system are mainly −1 deletions; in contrast, Lac+ revertants generated during growth are associated to –1 s, +2 s, and larger insertions and deletions (55, 74). These adaptive mutations were further postulated to be generated by the low-fidelity polymerase PolIV at mononucleotide repeats (75).
The contribution of tandem repeats (TR) to mutagenesis and its impact in protein evolution was recently addressed in B. subtilis employing a genetic reversion reporter system (76). While the system allows to detect growth-associated mutations at single-cell level, it has the potential to investigate if slippage-induced mutations occur in response to nutrient deprivation (76). In this method, the excision of a repeated TR in the ORF of cryptic transcriptional regulator (tfcr) activates its ability to bind a promoter that regulates positively the expression of the gfp-gdh operon. Therefore, Gdh-deficient B. subtilis cells harboring a WT Tf can generate fluorescence and grow upon transcriptional activation of the gfp-gdh operon (77, 78). Overall, the system allows to discriminate among nonmutated (nonfluorescent) and mutated (fluorescent) cells and study the effect of the growth condition on the appearance of the mutation (76).
DNA slippages can generate stable alternative DNA structures, including hairpins, triplex, and quadruplex. These non-B DNA sequences promote genomic instability (79, 80), which led to disruption of transcription and replication processes (81, 82). To circumvent these events, eukaryotes recruit repair factors like the human homolog Mfd and error-prone polymerases (83, 84). Non-B DNA hairpin sequences promote genetic instability through slippage events which can result in insertions or deletions (indels) during replication (85). In growing E. coli cells, the presence of a guanine tetraplex (G4) DNA sequence in an actively transcribed reporter gene strongly increased mutation rates; the evidence showed that the transcriptional regulator Hfq impacted this process by binding and stabilizing the G4 DNA structure (86). The impact of G4 and hairpin DNA structures on B. subtilis SPM was recently addressed. To this end, Ermy et al. (64) generated gene constructs carrying IPTG-inducible argF alleles differing in their ability to form hairpin and G4 DNA structures, and such constructs were recombined into an ectopic locus. It was found that the strains containing argF alleles with sequences predicted to form the non-B DNA structures tested accumulated more ArgF+ prototrophs than those argF alleles less likely to form such structures. These experiments also revealed a positive impact of Mfd on the G4 and hairpin DNA structures-promoted argF mutagenic events (64).
Different auxotrophy-based gain-of-function systems have been implemented to study SPM in S. cerevisiae, including: (i) starvation for an essential amino acid, either histidine (87–89), homoserine (90, 91), lysine (49, 90–98), or tryptophane (88, 92, 93, 99); (ii) starvation for the essential nucleobase adenine (99–102); and (iii) glucose starvation (103). However, most studies on SPM in this microorganism have used the frameshift allele lys2ΔBglII that causes lysine auxotrophy (104). Regardless of the selection system, the most widely experimental setup consists of growing subcultures of the strains in liquid complete medium to obtain the required cell numbers, collecting and transferring these cells to selective solid medium (resulting in a starvation-induced cell cycle arrest), and incubation for at least 10 days. Finally, the appearance of revertant colonies or papillae is checked and scored daily to determine the frequency of reversion of the mutant gene under selection.
Loss-of-function (forward) systems
While SPM have been mostly studied in B. subtilis strains containing point mutations in chromosomal amino acid genes, experiments conducted in distinct microorganisms have revealed that loss-of-function or modification-of-function mutational events are by far more frequent than gain-of-function mutational events (105). A forward loss-of-function system, which determines mutations that inactivate the thymidylate synthase (TMS) gene and confer a trimethoprim-resistant phenotype, was developed in B. subtilis (TmpR) (52). Thymidine synthesis performs an important role in DNA metabolism. Thymidylate synthase transforms dUMP to dTMP using N5N10-methylenetetrahydrofolate as cofactor (106). thyA and thyB genes encode TMSs in B. subtilis (107, 108). As thymine auxotrophs incorporate this metabolite more proficiently than the wild-type strain and are able to grow in the presence of trimethoprim, the loss of TMS function allows selection of TmpR mutants that requires exogenous thymine for growth (106, 109). The strain described by Villegas-Negrete et al. (52) carries deficiencies in thyA, thyB, and an IPTG-inducible Pspac-thyA cassette. The generation of TmpR colonies can be determined in nutritionally stressed bacteria under repressed/derepressed conditions. Evidence revealed that the generation of transcription-associated TmpR mutants was dependent on Mfd and GreA, two proteins known to process RNA polymerase (RNAP) pausing, suggesting that under nutritional stress RNAP backtracking and/or RNAP pausing promotes mutagenesis in non-growing B. subtilis cells. These assays have shown that, in addition to base substitutions, a broad mutational spectrum, including insertion/deletions (indels), occurs in SP and produces TmpR cells (52).
Forward loss-of-function mutation events have been associated with the acquisition of resistance to antifungal drugs in the yeasts S. cerevisiae and Candida albicans (110). SAM experiments conducted in response to 5-fluorocytosine or flucytosine (5-FC), and caspofungin (CSP) showed that adaptive mutation frequencies giving rise to 5-FC and CSP resistance increased in both yeasts (110). While the targeted genes involved in adaptive drug resistance were not identified, it was shown that the spectrum of adaptive mutations was different than those generated in replicating yeast cells (110). Therefore, different molecular mechanisms can be involved in generating spontaneous and adaptive drug resistance in these eukaryotic systems.
Overall, the versatility of the tools implemented to study SPM has advanced our knowledge regarding the mechanisms deployed by microorganisms to modify their genomes, adapt to challenging environments, and escape from growth-limiting conditions.
FACTORS THAT INFLUENCE B. SUBTILIS SPM
The role of DNA repair in B. subtilis SPM
The mismatch repair system
Mutation in nutritionally stressed B. subtilis, where chromosomal replication is sluggish or absent, may rely on repair mechanisms for genome modification. In B. subtilis, SPM may increase mutation frequencies by suppressing DNA repair systems or activating mechanisms that introduce DNA changes in the genome. The MMR system fixes a range of potentially mutagenic events, including mismatched bases, insertions, and deletions that escape proofreading during replication. It contributes to genome fidelity by avoiding illegitimate recombination and the mutagenic effects promoted by base deamination (111–114). While the MMR system in E. coli integrates three proteins, MutS, MutL, and MutH, as well as a methylation signaling system (112–114), most bacteria—including B. subtilis—only possess MutS, MutL, and an undefined mechanism to discriminate between old and nascent DNA strands (10, 114). In B. subtilis, the MMR system is encoded by the mutSL operon (10, 11, 114, 115), and its genetic disruption increases the frequency of prototrophic revertants (the hisC, metB, and leuC system) during the stationary phase (116). Furthermore, overexpression of the entire mutSL operon or the mutS gene (but not mutL) significantly diminishes the production of stationary-phase-associated mutations (116). From these results, it has been postulated that the generation of stationary-phase mutants in B. subtilis occurs through the loss or decrease of MutS activity during the stationary phase of growth (116). The MMR system also influences SPM in P. putida and S. cerevisiae. Accordingly, the frequency of stationary-phase mutations increased in carbon-starved cells of P. putida, lacking a functional MMR system (62, 117). A similar result was found in the yeast S. cerevisiae, as deletion of the MutS homolog, MSH2, increased the frequency of the lys2ΔBgl frameshift mutation during the stationary phase of growth (49). Based on its demonstrated biochemical function in E. coli and B. subtilis, MutS participates as a sensor during the recognition of mispaired bases (118, 119). MutL plays a similar role by coordinating the formation of the MutSL complex, which is necessary to process DNA mispairs in both bacteria (118, 119). Mismatches generated by replication in cells experiencing depressed levels of MutS may produce SP mutations via the saturation of the DNA repair capacity of the MMR system (116). Equally plausible is the possibility that the activity of this repair pathway or accessory proteins is reduced or inactivated in a subpopulation of the culture, leading to an accumulation of mutations. Interestingly, in E. coli, adaptive mutagenesis is affected by MutL but not MutS (120). The genetic assays in B. subtilis YB955 are different from the F´ Lac mutagenesis system of E. coli, but the suppression or downregulation of the MMR system is an important factor for promoting genetic diversity in both bacteria. One interesting aspect of SPM in B. subtilis is that the bacterium lacks a methylation signaling mechanism to discriminate DNA strands during MMR (114–116, 118, 119). A recent report showed that in mutSL-deficient B. subtilis, both the sequence context and the replicating DNA strand influenced the repair of base mismatches generated by base deamination (114). Therefore, characterizing the specific involvement of the MMR proteins in different microbial models should help in understanding the overall importance of the MMR mechanism in mutagenesis.
The base excision repair system
Genetic analyses of bacteria deficient in preventive/repair mechanisms of oxidative DNA damage suggest that a multiplicity of base lesions and their repair intermediates, including 8-OxoG, AP sites, single-strand breaks (SSBs), and deaminated bases, propel genetic diversity in nutritionally stressed bacteria (10, 29–31, 43, 121–125). Much work has been dedicated to understand how bacteria under growth-limiting conditions process damaged DNA bases and the effect of such processing in SPM (29, 43, 123, 125). Oxidative stress is a significant producer of 8-Oxo-dGTP, and this oxidized precursor is frequently incorporated opposite adenine in DNA (126). However, direct oxidation of DNA also generates 8-OxoG, which elicits G:C→T:A and A:T→C:G transversions during replication that can promote cytotoxic and genotoxic effects in cells (127). The simultaneous action of three components of the GO system, namely MutY, MutM, and YtkD (MutTA), prevents the harmful effects of this lesion in B. subtilis (128). This task is accomplished through different mechanisms: MutM hydrolyzes 8-OxoG from DNA, and MutY catalyzes the elimination of adenine incorrectly paired with oxidized guanine (A-GO) (128) and other mismatches, e.g., A-C and/or A-G, which have been shown to promote mutagenesis (31). In contrast, YtkD avoids the incorporation of 8-OxoG in replicating DNA (129, 130). Genetic and biochemical evidence has uncovered a complete GO system in B. subtilis (28–30, 131, 132). Furthermore, other studies that have examined the effect of the GO system in B. subtilis (28–30, 131) have shown that disabling this system generates hypermutagenic B. subtilis cells that accumulate high levels of genomic 8-OxoG (29, 133).
In the context of SPM, it was found that B. subtilis cells of the YB955 strain lacking a GO system had a dramatic propensity to increase the number of His+ and Met+ revertants during the stationary phase (29). Notably, no significant differences were found between the ∆GO strain and parental strains in the generation of SP Leu+ prototrophs (29). A further study has shown that, in starved YB955 cells and under conditions that saturate or inactivate the MMR system, MutY promotes a generation of mutations that increase the frequency of the leu reversion (31) (Fig. 4). Further reports have supported these observations, as genetic disabling of MutM and MutY in the YB955 strain increases the reversion frequencies of the hisC and metB mutant genes but decreases the production of colonies with a Leu+ phenotype. These observations have supported the notion that ROS-promoted synthesis of 8-OxoG in amino-acid-starved bacteria is involved in hisC and metB reversions. However, most of the His and Met prototrophs generated in cells with disruptions of GO system components were produced by suppressor mutations. In marked contrast, the leuC reversions dependent on MutY were derived mainly from intragenic and true reversions (31). These observations indicate that the generation and processing of an 8-OxoG lesion through the components of the GO system are an expanded mechanism that promotes genetic diversity and allows bacteria to escape growth-limited conditions, as demonstrated in E. coli and P. putida as well (29–31, 43, 121–125).
Fig 4.
Putative mechanism of Mfd-RNAP-MutY interaction to promote adaptive reversions in the leuC allele of the B. subtilis YB955 strain (1). A:C mismatch (red letters) at the -AGA- codon of the leuC427 mutant gene is left unrepaired by the mismatch repair system and reaches the stationary phase (2, 3). MutY binding to A:C mismatch removes adenine, generates an AP site, and establishes an intermediate repair MutY-AP complex (4, 5). RNA polymerase stalls at the MutY-AP site complex to activate an Mfd-dependent transcription-coupled repair event that dislodges RNAP and disassembles the MutY-AP complex, exposing the apurinic/apyrimidinic site (6–8). The AP site is processed by Nfo, ExoA, or Nth to generate a 5′-OH end that can be extended by PolX and complete repairs by a DNA ligase event.
Additional lesions affecting the chemical structure of nucleobases (as those derived from adenine, guanine, and cytosine deamination) also affect SPM in B. subtilis. Deamination of DNA is a process that can be indirectly promoted by oxidative stress (112). The processing of xanthine (X), hypoxanthine (HX), and uracil (U) by the uracil DNA glycosylase and the alternative excision repair (AER) endonuclease YwqL occurs in an error-prone manner and elicits SPM in the B. subtilis YB955 (27). AP sites spontaneously generated or produced via the hydrolysis of damaged DNA bases by specific DNA glycosylases such as Ung, MutY, and MutM are among the most prominent lesions in DNA (112, 134, 135). However, MutM not only removes an oxidized base but also possesses AP lyase activity and can catalyze the breakdown of the deoxyribose sugar, thereby generating an SSB (112, 135). AP sites and their derived SSBs are potentially mutagenic and toxic for cells by affecting DNA replication and transcription. Moreover, AP sites are processed by AP endonucleases, which are conserved among the organisms of the three kingdoms of life (112, 135). In addition to Nfo and ExoA, B. subtilis possesses a third AP endonuclease termed Nth (136). It has been reported that amino-acid-starved B. subtilis cells, deficient for these three AP endonucleases, significantly increase the reversion frequencies in the hisC, metB, and leuC mutant genes of the B. subtilis YB955 strain. In addition, disruption of polX, which encodes an LF DNA polymerase, ablates the production of His+, Met+, and Leu+ prototrophs in the Nfo/ExoA/Nth-deficient strain (136). These observations support a model in which the processing of AP sites via LF repair in stressed B. subtilis cultures, or in a subpopulation of cells, produces mutations with a highly adaptive value (136) (Fig. 4).
The transcription-coupled repair
The transcription process remains active in slow-growing or nondividing bacterial DNA, where replication is dramatically decreased or absent. While proper gene expression is highly dependent on RNA polymerase fidelity, under stressful conditions, the absence of high-fidelity repair can promote the formation of mutations (137–140). Thus, a combination of transcriptional derepression and error-prone repair events in stressed bacteria can elicit mutations in highly transcribed regions of DNA. The resulting genetic variants are selected for an adaptive advantage, thus facilitating an escape from arrested growth (44, 50, 138–140).
The effect of transcription on mutagenic processes can be considered from two perspectives: the consequences of derepression and the consequences of transcriptional bypass (when the elongation complex generates an altered mRNA) (140, 141). During transcription, single-stranded DNA may supercoil and generate secondary stem-loop structures. This leaves DNA bases vulnerable to spontaneous and ROS-promoted damage such as deamination and oxidation, which result in the production of several lesions including U, X, HX, thymine glycol, 8-oxoG, AP sites, SSB- and double-strand breaks (DSBs), among others (4, 50, 141).
Deaminated bases including, U, X, HX, and some oxidized bases, such as thymine glycol, may not induce significant distortions in the DNA, leading to a low impact on the elongation process of the RNAP (112, 135). However, these lesions can lead to the incorrect incorporation of a nucleotide into the mRNA through a process known as transcription-associated mutagenesis and generate mutant proteins that result in the appearance of pseudo-prototrophs in nondividing bacteria (135, 137, 142). Recent evidence has found that, in nongrowing B. subtilis cells, AP sites, SSBs, and 8-OxoG can be a source of mutations mediated by the transcriptional process (29–31, 43). In this context, the number of beneficial mutations in B. subtilis increases dramatically in cells lacking a functional GO system (29, 30). This reinforces the notion that oxidative stress is a mechanism that generates genetic diversity in starved B. subtilis cells (29–31, 43).
The efficiency of the elongation and termination processes during transcription is highly dependent on the interaction of the RNAP with distinct transcriptional factors. B. subtilis possesses seven transcription factors called NusA, NusB, NusE, NusG, GreA, Mfd, and Rho (143), and robust scientific evidence supports the idea that some of these transcription factors mediate mutagenic processes that increase the likelihood of surviving stress in this microorganism (44, 45, 140, 144).
Mfd is among the best-studied transcription factors (141, 145, 146). This evolutionarily conserved bacterial protein mediates one type of transcription-coupled repair (TCR) (145). This factor, initially described by Evelyn Witkin (145), directs NER-dependent repair to transcriptionally active genes when RNAP is stalled by pyrimidine dimers and other distorting DNA lesions. The mechanistic details and the role of Mfd in TCR have been thoroughly elucidated in E. coli and appear to operate similarly in several bacterial species, including B. subtilis (145–150). However, the physiological role of Mfd in B. subtilis is much broader, ranging from conferring protection against protein oxidation to a mediator of carbon catabolite repression and affecting SPM (42, 44, 151, 152).
In starved cells of the strain B. subtilis YB955, Mfd is required to promote mutations that result in the generation of amino acid reversions in the hisC, metB, and leuC mutant genes. This effect is more pronounced in leuC, as the absence of this protein almost eliminated the appearance of Leu+ prototrophs (44). A further study revealed that these effects were exerted through transcription, as the reversion rates of the leuC gene (subjected to derepression through an S-box riboswitch) were significantly diminished following the disruption of mfd (144). Furthermore, reports have also indicated that Mfd combines with the error-prone repair of DNA in transcriptionally active genes, which requires the contribution of NER or BER (43, 50).
One recent report found that Mfd, beyond its mutagenic and repair functions, plays a role in protecting B. subtilis against protein oxidation. A deficiency of this protein elicits the expression of OhrR, a repressor that controls the response to organic peroxide exposure (42). Remarkably, results from a RNAseq study applied to an Mfd-deficient strain and its parental strain revealed that Mfd can affect the physiology of B. subtilis through global gene regulation. Thus, the loss of Mfd affects the expression of hundreds of genes involved in motility, endospore formation, protection against protein oxidation, and sensitivity to UV light (153).
In addition to Mfd, experiments studying the effect of the elongation RNAP factor GreA on mutagenic processes and DNA repair in B. subtilis have shown that this factor prevents mutagenesis during growth but promotes it in nutritionally stressed cells (45). Bacterial Gre factors associate with RNAP and stimulate intrinsic cleavage of the nascent transcript at the active site of the RNAP complex (154). Gre factors consist of an extended N-terminal coiled-coil domain and a C-terminal globular domain (155, 156). B. subtilis possesses one Gre factor, designated GreA. This associates with core RNAPs during transcriptional initiation and elongation and resolves stalling at the promoter or promoter-proximal regions, resulting in an even distribution of the RNAPs throughout the transcribed regions in B. subtilis cells (157). The role of GreA in B. subtilis TAM was initially tested using a mutagenesis system based on the acquisition of the Tmpr phenotype. By employing this system, it was found that Mfd and GreA, under conditions in which transcription was derepressed, promoted the appearance of SPMs in this bacterium (52). Interestingly, the disruption of the mfd or greA genes nullified the increased mutagenesis observed in the absence of a GO system or the main AP endonucleases Nfo, ExoA, and Nth (45). These results suggest that in nutritionally stressed B. subtilis cells, spontaneous nonbulky DNA lesions are processed in an error-prone manner with the participation of Mfd and GreA (45). Based on this evidence, it was postulated that Mfd can modulate mutagenic events promoted by RNAP-GreA complexes elicited by ROS-promoted DNA lesions (45) (Fig. 5 and 6).
Fig 5.
Postulated role of GreA in stationary-phase mutagenesis events promoted by RNA polymerase stalled at an apurinic/apyrimidinic site. Following pausing at an AP site, GreA (i) pushes back RNAP and (ii) elicits hydrolysis of misaligned RNA. Once it correctly aligns the mRNA, GreA promotes RNAP elongation and generates mutant mRNA, which can generate His+, Met+, or Leu+ prototrophs through retromutagenesis.
Fig 6.
Postulated role of Mfd in stationary-phase mutagenesis events promoted by the RNA polymerase-GreA complex stalled at an apurinic/apyrimidinic site. Following pausing at an AP site, GreA pushes back RNAP. The paused RNAP-GreA complex elicits an Mfd-dependent transcription-coupled-repair event to dislodge RNAP and the GreA complex and release the incomplete mRNA. Mfd directly or indirectly recruits AP endonucleases (APE) to process the AP lesion and low-fidelity polymerases (LFPs) to generate a mutant gene, giving rise to His+, Met+, or Leu+ prototrophs.
The transcription factor NusA, which fulfills essential functions during the elongation and termination steps of RNAP, plays a role in E. coli adaptive mutagenesis (158). In nutritionally stressed E. coli cells harboring the F´ lac frameshift reversion system, the production of Lac+ colonies requires a functional NusA protein (158). The mechanism underlying this type of mutation has been attributed to an Mfd-independent TCR pathway that requires NusA and the LF polymerase DinB (159). Interestingly, neither NusA nor DinB appears to play a relevant role in B. subtilis SPM. However, recent evidence indicates that NusA and NusG interact to regulate functions in RNAP beyond transcriptional pausing, including the coupling of transcription/translation and efficient transcription termination (160–162). Therefore, future experiments should explore the single and combined roles of NusG and NusA in the formation of mutations in nutritionally stressed cells of B. subtilis.
Impact of signal transduction in B. subtilis SPM
Role of c-di-AMP and (p)ppGpp in B. subtilis SPM
Microorganisms use signal transduction pathways to respond to constantly changing environmental conditions (163). Acting as second messengers, nucleotide signaling molecules play key roles in activating morphophysiological pathways that allow bacteria to adjust their metabolism and promote genetic diversity when cells experience growth-limiting conditions (163, 164).
The second messenger, guanosine tetra/penta-phosphate [(p)ppGpp; herein, GT/PP], can modulate metabolism throughout all stages of bacterial growth. During exponential growth, bacteria keep low levels of GT/PP that contribute to maintain a balanced metabolism (165). However, during transition from exponential to stationary phase of growth or when facing nutrient starvation, heat shock, or antibiotic stress, (p)ppGpp levels are suddenly increased to trigger a cellular stress response called the “stringent response” (166–171). The synthesis of the tetra- and pentaphosphate guanosine alarmones is carried out by RelA/SpoT homolog proteins, which transfer two phosphates from ATP to GDP or GTP, resulting in the production of guanosine tetraphosphate (ppGpp) and guanosine pentaphosphate (pppGpp), respectively (172, 173). These alarmones can directly modulate gene transcription or indirectly target proteins that play distinct cellular roles, thus inducing widespread genetic and physiological changes in cellular metabolism (169). In E. coli, the synthesis and breakdown of GT/PP can be managed by RelA and the GPP phosphohydrolase, respectively (172, 174). However, this bacterium also utilizes SpoT, a bifunctional enzyme that has weak GT/PP synthetic activity but a stronger capacity to hydrolyze these alarmones (172, 175). In B. subtilis, GT/PP is synthesized by the dual-function enzyme RelA that has hydrolase activity in addition to synthase, as well as the RelP and RelQ proteins that are only synthases (176).
The influence of GT/PP and the stringent response on mutation rates were first reported in E. coli K12 (177), and it was shown that increases in the reversion of a mutant leuB gene correlated with higher levels of GT/PP during exponential growth (177, 178). Furthermore, higher levels of leuB mRNA were produced in relA-proficient than in relA-deficient E. coli cells, leading to the hypothesis that the genetic derepression induced by amino acid starvation and GT/PP accumulation can enhance mutation rates (179, 180). This transcription-enhanced mutation hypothesis was further tested in B. subtilis by Rudner et al. (181) who compared the reversion rates of distinct auxotrophies on B. subtilis differing in their relA proficiency. Whereas mutation rates of the lys-3, hisH2, and leuB1 auxotrophies were significantly lower in strains deficient for relA, an opposite effect was observed following overexpression of relA, suggesting a positive role for GT/PP in B. subtilis mutagenesis (181). These studies suggested that mutation rates depend, at least in part, on the rates of transcription of the selection alleles, on the protein RelA as well as on the levels of the signal molecule GT/PP (177, 181). During stationary phase, when bacteria experience a decreased availability of amino acids and accumulation of uncharged tRNAs, RelA-dependent synthesis of GT/PP is activated, and the accumulation of the alarmone triggers the stringent response and decreases the GTP pools (182–184). In this growth stage, B. subtilis produces GT/PP, which halts DNA replication through inhibition of the DNA primase and represses the synthesis of proteins (185–189). Biosynthesis of GT/PP indirectly contributes to the depletion of the GTP pools; altogether, GT/PP and GTP depletion have a positive impact on the synthesis of amino acids (190, 191). In E. coli, the GT/PP alarmone has been involved in stress-associated mutagenic processes; accordingly, stationary-phase cells of this bacterium accumulate a higher number of arg and leu adaptive mutants in relA-proficient strains than in relA-deficient strains, and such increase correlates with the accumulation of transcript levels of the alleles under selection (192). However, aside from the genetic studies with relA− strains (181), there are no current reports providing a clear link between GT/PP and SPM in B. subtilis.
Under conditions of nutritional stress, cells of the strain B. subtilis YB955 deficient for NrdR, the repressor of the ribonucleotide reductase (RNR), exhibit a hypermutagenic phenotype associated with the overexpression of RNR and have a strong propensity to generate amino acid reversions in SPM assays (193). Results from a high-throughput LC-MS/MS proteomic study showed that amino-acid-starved cells of this hypermutagenic strain possess an altered content of proteins involved in the stringent response; furthermore, a decreased content in the transcriptional repressor CodY was also detected (194). In summary, 30 proteins of the stringent response and from the CodY-regulon involved in carbon catabolism, synthesis of amino acids, and secondary metabolites of the stationary phase of growth (195, 196) were found dysregulated in content in the RNR-overexpressing strain (194). A transient drop in GTP levels during transition from the exponential to the stationary phase of growth inactivates the repressor functions of CodY; therefore, the synthesis of GT/PP which decreases the levels of this nucleotide can indirectly derepress the CodY regulon, impacting the ability of bacteria to adapt to starving conditions (183, 197).
Besides dysregulation in the content of proteins associated with the stringent response, the proteome of the RNR-overexpressing B. subtilis strain revealed alterations in proteins related to the metabolism of the second messenger, c-di-AMP (194). During the stationary phase, B. subtilis cells overproducing RNR downregulate the content of the c-di-AMP synthase DisA; in contrast, the levels of CdaR, the negative regulator of the c-di-AMP synthase CdaA, the phosphatase YfkN that degrades c-di-AMP (198, 199), and the c-di-AMP-interacting protein DarA (200) were found to be increased (194). Results from a chemical proteomics approach in Listeria monocytogenes identified several c-di-AMP-interacting proteins, including the enzyme pyruvate carboxylase and the transcriptional repressor NrdR (201). In B. subtilis, RNR which converts ribonucleotides to 2'-deoxyribonucleotides to provide precursors for DNA synthesis and repair (202), was found to be necessary for SPM (193). Furthermore, disruption of nrdR, which leads to overexpression of nrdEF, promotes growth-dependent mutagenesis and SPM (193). Altogether, these observations unveil a role of the c-di-AMP signaling pathway in regulating RNR-dependent B. subtilis SPM.
In B. subtilis, the diadenylate cyclases (DACs), DisA, CdaA, and CdaS synthesize c-di-AMP, and three phosphodiesterases, GdpP, PgpH, and YfkN are involved in its degradation (199, 200). In gram-positive bacteria, this second messenger has been implicated in distinct biological processes, including potassium transport, osmotic balance, cell wall metabolism, and DNA repair (203). SPM experiments conducted in the strain B. subtilis YB955 bearing genetic disruptions in cdaA and disA revealed a dramatic decline in the reversion frequencies of the hisC, metB, and leuC mutant alleles compared to the parental cdaA/disA-proficient strain, suggesting that c-di-AMP promotes genetic diversity under nutrient limitation (46). However, whereas CdaA and DisA had only a mild impact on growth-associated spontaneous mutagenesis, both DACS play important roles in counteracting mutagenesis induced by compounds that induce oxidation and alkylation of DNA (46). To the best of our knowledge, this is the only study so far that reports a relationship between c-di-AMP and SPM in B. subtilis. However, a previous report in Mycobacterium smegmatis revealed that disruption of the phosphodiesterase-encoding gene pde, and accumulation of c-di-AMP, promoted ciprofloxacin and rifampicin resistance in the exponential, early stationary and late stationary phases of growth of this bacterium (204). While this evidence supports a role for c-di-AMP in regulating mutagenesis in bacteria, the mechanistic aspect involved remains rather unclear and will require to be further studied.
The c-di-AMP pathway and the stringent response converge to influence SPM
Bacteria frequently possess overlapping regulatory systems, e.g., in firmicutes, the signaling networks of GT/PP and c-di-AMP intersect, working together to synchronize bacterial viability and stress responses (205, 206). The alarmone GT/PP can target the membranal GdpP and PgpH c-di-AMP phosphodiesterases to regulate its hydrolytic activity, thus impacting the intracellular levels of c-di-AMP (207, 208). Interestingly, the regulation can also work in the other way, c-di-AMP can control the intracellular levels of GT/PP through its interaction with DarB (209). In B. subtilis and L. monocytogenes, under cell stress and low levels of c-di-AMP, DarB, a target of this cyclic dinucleotide, can bind RelA to activate the Rel-dependent stringent response, which stimulates the synthesis and accumulation of GT/PP (209, 210). However, when levels of c-di-AMP are high, DarB forms a homodimer that binds two molecules of this cyclic dinucleotide, thus hindering DarB interaction with RelA and thus limiting the intracellular concentration of GT/PP (211). Thus, c-di-AMP and GT/PP autoregulate its concentration in a signaling loop. Furthermore, levels of GT/PP directly influence intracellular nucleotide pools as accumulation of this alarmone during the stringent response leads to a decrease in the intracellular GTP pool, which subsequently deactivates the CodY repressor, resulting in the derepression of its regulon (197). CodY represses transcription by binding to promoter nearby regions of its target genes, thus blocking the access of the RNA polymerase (77). Deactivation of CodY during the transition to the stationary phase of growth activates the expression of genes implicated in adaptation to nutrient limitation (197). Presumably, the transcriptional derepression of the amino acid biosynthetic genes resulting from CodY inactivation can promote B. subtilis SPM (46, 193, 194).
The existence of a signaling loop between GT/PP and c-di-AMP is further supported by results showing that dacA mutants of L. monocytogenes, completely devoid of c-di-AMP, exhibited defects in growth and survival due to the toxic accumulation of GT/PP (176). The subsequent altered concentrations of GTP led to the appearance of suppressor mutants that inactivated CodY and these mutants deficient for CodY, no longer required the c-di-AMP synthase dacA for growth and survival (176). Additionally, high levels of c-di-AMP can lead to alterations in peptidoglycan synthesis with a negative impact on cell wall integrity (200). This connection between c-di-AMP and cell wall homeostasis is another aspect of the indirect involvement of this signaling molecule in the stringent response, as cell wall remodeling is often associated with activation of bacterial stress responses (200).
The c-di-AMP pathway and the stringent response represent a complex interplay between two essential signaling systems in bacteria to regulate mechanisms that impact biosynthetic processes, activate stress-related genes, and alter cellular metabolism to enhance bacterial survival under stress conditions, such as nutrient deprivation or amino acid starvation (190, 212). The interplay between c-di-AMP and the stringent response is thought to be a coordinated effort to balance the need for genetic diversity through mutagenesis with the preservation of genomic integrity. The potential mechanisms underlying this interplay could involve c-di-AMP modulating the stringent response or vice versa. For example, c-di-AMP might influence the magnitude or duration of the stringent response, while the stringent response, through downstream effectors like GT/PP, could impact c-di-AMP levels or its signaling functions (209).
Understanding how the c-di-AMP pathway and the stringent response converge to influence nucleotide pool balance and SPM is crucial at unraveling the intricate regulatory networks that bacteria employ during stationary-phase adaptation. This knowledge will widen our comprehension of bacterial stress responses and adaptive strategies, with implications for fields such as antimicrobial resistance and bacterial evolution.
DEVELOPMENTAL DNA DAMAGE PREVENTION AND REPAIR IN B. SUBTILIS
Processing genetic damage during B. subtilis sporulation
Bacteria deploy multiple strategies, including sporulation, to survive adverse environmental conditions (213, 214). Sporulation in B. subtilis occurs under growth-limiting conditions in two unequal-sized compartments in a single sporulating cell, the mother cell and the forespore, each containing an identical chromosomal copy (213, 214). Once these two-cell compartments are established, the chromosomes within them are no longer replicated but remain transcriptionally active to orchestrate a differential program of gene expression regulated in time and space to complete the synthesis of the endospore (213, 214). Hence, spontaneous or environmental factors that damage DNA can potentially compromise the sporulation gene expression program and the efficient formation of spores. To cope with these events, B. subtilis activates checkpoint mechanisms that ensure the integrity of the chromosomes and arrest the differentiation process until the genetic damage is eliminated (215–219). These mechanisms are discussed below.
DNA-damage checkpoints that regulate the initial stages of sporulation
To initiate sporulation, B. subtilis relies on a signal transduction system known as phosphorelay (220–222). In this process, a set of histidine kinase sensors recognize the signals generated by the phosphorylation cascade and transduce them to influence the expression of genes whose coding products are required for sporogenesis (223–226). Pathways controlled by histidine kinase signals are widely distributed in bacteria to sense transient metabolic changes, characteristics of the surrounding environment, and cell cycle progression (227, 228).
In B. subtilis, the sporulation process is triggered by activation of the histidine kinase sensors KinA, KinB, and KinC, which activate a phosphorylation cascade whose main target is Spo0A, the transcription factor and master regulator of sporulation (229–232). Thus, the activation of Spo0A through phosphorylation, directly and indirectly, activates the transcription of at least 121 genes necessary for the synthesis of a mature spore, including those that promote asymmetric division in the sporangium and specific sporulation sigma factors (195, 232). Spo0A~P binds multiple sites at the OriC of B. subtilis, and such cis-acting sequences overlap with binding regions for the replication initiation protein DnaA (233, 234). These Spo0A~P actions interfere with the binding of DnaA to OriC, a prerequisite for the recruitment of proteins needed to load the replicative helicase DnaC (234). The binding of Spo0A-P to its DNA boxes at the OriC blocks new rounds of chromosomal replication (234). Phosphorylated Spo0A also positively regulates the transcription of sirA, which encodes SirA (sporulation inhibitor of replication). SirA is part of a checkpoint mechanism that guarantees the existence of a single chromosome in the cell compartments of sporulating B. subtilis cells (195, 232, 234). Functionally, SirA directly interacts with DnaA to displace it from the OriC (235, 236). Also, the protein Sda (suppressor of DnaA) is a second checkpoint that prevents sporulation by inhibiting autophosphorylation of KinA, thus preventing activation of Spo0A (217, 237). The transcription of sda is upregulated by DNA damage and factors that promote replication stress and activate the SOS response. Accordingly, the expression of sda is under the control of RecA and the LexA repressor (18). In summary, Sda works to prevent entry into sporulation in the presence of DNA damage by blocking replication. This process ensures the high-fidelity duplication of the chromosome during the onset of sporulation (217, 237).
Processing genetic damage during the progression of sporulation
Once the sporulating cell has progressed to the differentiation stage t2, the chromosomal copies in the sporangium compartments will not replicate during further sporulation stages. However, DNA integrity is required for the transcription process to remain active and for efficient sporulation. Therefore, protecting the integrity of the sporangium chromosomal copies ensures proper gene expression of the developmental program in both cross-talking compartments. It has been postulated that genetic damage during sporulation must be preferentially directed to transcriptionally active genes (20, 21, 238). In support of this hypothesis, evidence has shown that the Mfd-mediated TCR pathway is active during sporogenesis and must counteract the cytotoxic and genotoxic effects elicited by physical and chemical factors (238). Thus, mfd expression primarily takes place in the forespore compartment, under the control of the σF factor. Its disruption affects sporulation and generates sporangia that are sensitized by the helix-distorting agents UV-C and MC (238). Further results have shown that, during spore morphogenesis, Mfd mainly operates over transcriptionally active genes damaged by the physical factor UV-C (238).
During the t2 stage of the sporulation process, the DNA integrity scanning protein DisA plays an important role in the checkpoint process to measure chromosomal damage of the copy to be translocated from the mother cell to the forespore (239). Experimental evidence has indicated that DisA scans the chromosome in the mother cell in search of lesions in an ATP-dependent manner. DisA senses damage promoted by nalidixic acid (NAL) or MC, stops chromosome movement, delays sporulation, and then activates repair until the integrity of the chromosome has been restored (239). These activities are affected by the capacity of DisA to produce the second messenger, c-di-AMP, while scanning DNA (240). Upon stalling at a DNA lesion, DisA-dependent c-di-AMP synthesis ceases, and decreased levels of this molecule act as a cue for the recruitment of repair systems to process lesions (239, 240). The importance of this checkpoint is supported by results showing that disruption of disA leads to sporulation with compromised chromosome integrity. DisA also regulates sporulation by controlling the activation of Spo0A when DNA damage promoted by NAL is detected, resulting in delayed entry into sporulation (239, 240).
To ensure spore resistance to factors that can damage DNA, B. subtilis uses the multifunctional protein RecA. Evidence from genetic studies has shown that the viability of spores deficient for recA is compromised following its exposure to DNA-damaging factors, including heat, UV irradiation, hydrogen peroxide, ultra-high vacuum desiccation, and ionizing radiation (a factor that generates SSBs or DSBs) (37, 38, 241–243). However, while these effects are measured by the ability of treated spores to generate viable colonies, the function of RecA is also required during spore morphogenesis. Experimental evidence has demonstrated that the RecA-dependent SOS response is active during this differentiation stage and necessary to protect the sporulating cells from DNA damage (21). Importantly, RecA-deficient sporangia exhibit morphological defects, including abnormal nucleoid condensation and delay of the sporulation process by ~2 h, suggesting that a RecA-dependent checkpoint occurs in cells committed to sporulation (21, 244). In support of this idea, the YabT-dependent phosphorylation of RecA, which induces the synthesis of RecA filaments in response to DNA damage, has a negative effect on spore morphogenesis. Of note, the expression of yabT coding a serine/threonine kinase occurs in the forespore compartment under the control of SigF (245). Therefore, phosphorylation of RecA by YabT may represent a developmental checkpoint event that delays or prevents spore development when DNA is damaged (245). While components of the SOS response along with Mfd and additional repair pathways can largely meet the need to ensure a damage-free chromosome during sporulation, experimental evidence suggests that some lesions can remain unprocessed in the mature spore and that further damage accumulates during the dormancy process (35, 37, 242). These lesions are suppressed or processed during spore germination to ensure a healthy return to vegetative growth, a task in which RecA participates (35, 37, 40, 242).
Processing of 8-OxoG through Mfd activates a RecA/Sda-dependent sporulation checkpoint
DNA lesions in B. subtilis cells committed to sporulation activate the checkpoint function of Sda in a RecA-dependent manner (18). This activation has been studied using exogenous sources of DNA damage; however, determining which cellular physiological processes promote the formation of genetic lesions that activate this checkpoint has remained elusive. The GO system, which is integrated by MutM, MutY, and YtkD, influences B. subtilis SPM (29, 30, 43). A recent report found that the accumulation of the oxidized base 8-OxoG and its processing through Mfd can generate repair intermediates that activate a checkpoint at the onset of sporulation (20). Those experiments showed a dramatic decline in sporulation efficiency in B. subtilis cells deficient in Mfd and the repair/prevention GO system (20). Of note, these cells exhibit defects at the early and late stages of sporulation, and microscopy analyses have indicated a failure to generate dormant spores (20). Furthermore, results from genetic epistasis analyses have found that this checkpoint requires RecA and a functional Sda protein (20). Based on these observations, it is reasonable to postulate that Mfd couples the repair of 8-OxoG and replication/transcription stress to activate the SOS response and delay the initiation and progression of sporulation (Fig. 7).
Fig 7.
Mfd-dependent mechanism of transcription-coupled repair activated by transcription/replication stress during 8-OxoG repair. 8-OxoG or its repair intermediates activate the checkpoint function of DisA and the RecA-dependent SOS response. High levels of Sda and stalled DisA interfere with KinA/KinB-mediated phosphorylation of SpoA, thus blocking the progression of B. subtilis sporulation. In contrast, high levels of Spo0A~P activate the Spo0A regulon and increase SirA levels, which interfere with DnaA binding to the replication origin (OriC). Together, these events lead to asymmetric cell division and sporulation progression.
DNA repair mechanisms operating at the onset and progression of sporulation
The cellular processes that enable entry, progress, and completion of the sporulation developmental program require a fine degree of coordination, and checkpoint mechanisms exist to ensure the genomes of the cells undergoing this process are protected. Different enzymes and DNA repair pathways have been described in B. subtilis that cope with potential threats and damage to genetic material. While most are usually active during vegetative growth, some are transcriptionally regulated to exert their function during spore development (21, 37, 238). In-line with this notion, genes coding different enzymes of the BER system are regulated during sporangium development either in the mother cell, the forespore, or in both compartments, including nfo, exoA, and aag (246–248). The AP endonucleases Nfo and ExoA are important repair factors for spores to efficiently return to vegetative growth during germination and outgrowth (38, 40, 248). The expression of aag is driven by σG inside the forespore compartment, although expression of this gene is also under the control of the general stress factor σB (24). Recent results have found that Aag possesses HX glycosylase activity but also removes alkylated bases from DNA. Furthermore, Aag protects sporulating cells from the cytotoxic and mutagenic effects promoted by base deamination and alkylation (24).
Additional repair transactions occur in developing spores, including TCR, carried out by Mfd in concert with UvrA to counteract damage that can distort DNA and compromise the developmental program of sporulation (238). Interestingly, B. subtilis sporangia deficient for Mfd and RecA exhibit an exacerbated sensitivity to agents that induce bulky DNA lesions, such as MC and UV-C light, which emphasizes the importance of DNA repair during the sporulation process of B. subtilis (21).
In addition to typical NER and BER systems, the AER pathway has been found to operate during the development of B. subtilis spores. This AER pathway is initiated by the UV endonuclease YwjD, whose coding gene is expressed during sporulation under the control of the forespore-specific factor σF and protects sporulating cells from the harmful effects of UV-C light (249). Biochemical studies have demonstrated that YwjD can operate over three types of UV-induced photolesions, namely cyclobutane pyrimidine dimers (CPDs), 6–4 photoproducts (6–4 PDs), and Dewar isomers (DWIs) (34). Mechanistic details indicate that YwjD catalyzes the hydrolysis of the phosphodiester immediately located at the 5´-end of ds-DNA containing any of these photolesions (Fig. 8). Of note, the LF polymerase YqjW processes the YwjD repair intermediates of 6–4 PDs and DWIs but not those of CPDs. YwjD-dependent repair of CPD lesions requires the simultaneous actions of the putative flap endonuclease YpcP and the LF polymerase YqjH (34) (Fig. 8). These results support previous observations, indicating that YqjH and YqjW are active in sporulating cells to contend with the genotoxic effects of UV-C and MC, and, together with Mfd, they promote the formation of mutations induced by these DNA-damaging agents (250). An AER pathway, dependent on the EndoV homolog YwqL, has been found to protect B. subtilis sporangia from the cytotoxic and genotoxic effects of base deamination (33). This repair protein catalyzes a double hydrolytic reaction over flanking phosphodiester bonds of HX, and X, U, and AP sites are processed through a single incision event taking place at the adjacent 3´-phosphodiester bond of these lesions (27, 33). Downstream repair stages of deaminated bases initiated by YwqL are completed by ExoA and PolA (33).
Fig 8.
Postulated YwjD-dependent mechanism of incision/excision repair of CPD (cyclobutane pyrimidine dimers; left), 6–4 PP (photoproducts 6–4; middle), and DWI (Dewar isomers; right) in sporulating B. subtilis cells. In the first step, YwjD catalyzes a hydrolytic event on the 5´phosphodiester bond before any of the three types of PDs on dsDNA. The following steps depend on the type of PD in the repair products. Intermediaries of 6–4 PDs and DWIs (middle and right reactions) can be directly extended by YqjH. CPDs (left reaction) require the previous action of YpcP before YqjW-dependent synthesis. The three pathways require a ligase to complete the repair events.
Spore-specific repair and preventive factors that contribute to B. subtilis spore resistance
B. subtilis spores are metabolically inactive and can persist in the environment for unpredicted periods of time; however, they are constantly exposed to chemical and physical factors that can potentially damage the structure of its chromosome (37, 251–253). As noted above, during the synthesis of the spore B. subtilis deploys preventive and repair mechanisms to eliminate genetic damage that can interfere with this process of cell differentiation (10, 37). Interestingly, some genes coding repair proteins are expressed inside of the forespores, and their products are packaged into the mature spores (21, 24, 39, 238, 246, 249, 254–257). Therefore, the successful return of spores to life is highly dependent on the ability of packaged repair proteins to eliminate the DNA lesions accumulated during spore dormancy (258–261). The arsenal of repair and preventive proteins operating through this mode of action includes SplB, UvrA, RecA, Nfo, ExoA, KatX, SodA, YkoUV, YwjD, Mfd, and Aag (21, 24, 39, 238, 246, 249, 254–257). It must be pointed that these repair factors are not active until the spore achieves partial or total core hydration during the germination-outgrowth stage (35).
As noted above, in their dormant state, B. subtilis spores are highly resistant to different types of environmental stresses, such as toxic chemicals, desiccation, pressure, extreme temperatures, and UV light (242). Such resistance is in great part due to the own spore structure, which is constituted by thick layers of cross-linked proteins and the spore cortex (262). Other factors contributing to spore’s resistance to genotoxic agents include the low content of water, the high levels of dipicolinic acid, and a group of acid-soluble proteins (α/β SASP) as DNA protectants (37). α/β SASP proteins, which are synthesized during sporulation in the forespore compartment, saturate DNA, change its conformation from a B-type to an A-type structure, and impact its photo response to UV-light (252, 263). Hence, while 254 nm UV light generates in vegetative cells, cyclobutane dimers (CPDs), and 6–4 adducts between adjacent pyrimidines (6–4 PP), in dormant spores, UV light generates mainly a thyminyl-thymine adduct called spore photoproduct (SP) (251, 252). Several lines of evidence have revealed that these mutagenic UV lesions are rapidly removed during spore germination/outgrowth with participation of the SP lyase (SplB) and the NER system (264, 265). α/β SASP proteins also protect spores DNA from the damage inflicted by hydrogen peroxide (H2O2) but not from that produced by nitrous acid, formaldehyde, and alkylating agents such as ethyl-methane-sulfonate (242). Indeed, most oxidizing agents damage the outer coatings and components of the inner membrane causing spores to rupture during spore germination/outgrowth (242, 266, 267).
B. subtilis spores are more resistant than vegetative cells to ionizing radiation (γ) (35), while the precise spectrum of DNA lesions in γ-irradiated dormant spores is not known (268), enzymes involved in the repair of double- and single-strand breaks as well as AP sites are key factors in such resistance. Presumably, the nonhomologous recombination (NHEJ) enzymes YkoU and YkoV, and the AP endonucleases Nfo and ExoA eliminate such lesions during spores return to vegetative growth (269, 270). These enzymes together with RecA have also been implicated in repairing dry heat injury to DNA, including strand breaks and AP sites (39, 248, 271, 272).
Processing DNA damage during B. subtilis spore germination-outgrowth
The repertoire of spore-specific repair/preventive proteins undoubtedly contributes to the ability of the spores to survive from the chromosomal damage inflicted by several chemical and physical factors. However, results from a transcriptome analysis have revealed the expression of DNA repair genes, including nth, ung, yqjW, and ypcP, during spore’s return to vegetative growth (259). Therefore, as described in this section, these de novo expressed factors and those synthesized during sporulation and packaged in the dormant spore are extremely important for an efficient germination-outgrowth process.
Oxidative DNA damage under conditions of spore germination-outgrowth
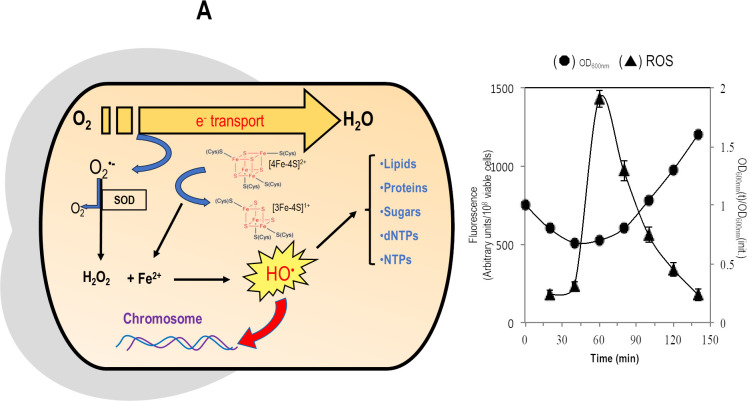
When spore membrane receptors sense favorable environmental conditions, the spores can return to vegetative growth through a two-phase process termed germination and outgrowth (260, 261). It has been hypothesized that during spore germination, the entrance of water and the activation of aerobic metabolism can promote oxidative stress with the consequent production of ROS (38, 40) (Fig. 9). The superoxide anion (O2.−), hydrogen peroxide (H2O2), and the hydroxyl radical (OH.−) (273) react with and damage proteins, lipids, and DNA (274). Oxidative species cause various DNA lesions; the latter include the AP sites, oxidation, and other chemical alterations of purine and pyrimidine bases, damage to deoxyribose, and SSBs or DSBs (112, 135). These toxics and premutagenic lesions must be rapidly processed and eliminated before activation of transcription and replication during spore outgrowth (38, 40, 275). The latent spore’s low (or absent) metabolic activity precludes the repair of damaged DNA, necessitating active repair during spore germination/outgrowth when aerobic metabolism is reactivated (261). Furthermore, if this repair is insufficient, the return of spores to vegetative growth is compromised (37, 251, 253, 261). Another measure of how spores are resilient and resistant to extreme environments is the speed and precision with which the accumulated damage to their DNA is repaired during germination/outgrowth (35).
Fig 9.
(A) Activation of aerobic metabolism during spore outgrowth can be a source of reactive oxygen species-promoted genetic damage. Superoxide radicals (O2.−) generated during oxygen (O2) reduction in the electron transport chain can attack iron/sulfur proteins, releasing partially oxidized iron (Fe2+). Superoxide can also be processed by superoxide dismutase (SOD), which generates O2 and hydrogen peroxide (H2O2). Fe2+ and H2O2 can react to produce the highly reactive hydroxyl radical (HO.), which may target DNA and other biomolecules. (B) A burst of ROS production occurred during B. subtilis spore outgrowth. Samples of germinated/outgrown spores collected at different times after the onset of germination were disrupted, and the cell-free extract was processed to determine the content of ROS (133).
DNA repair and checkpoints operate during spore germination-outgrowth
The AP endonucleases Nfo and ExoA are part of the BER system and, together with RecA and the NER system, play an important role in eliminating DNA damage induced by H2O2 during germination/outgrowth (38). The enzymes Nfo, ExoA, YkoU, YkoV, and the RecA-mediated SOS response are factors implicated in repairing dry-heat-induced injuries to DNA such as strand breaks and AP sites (24, 39, 271, 272).
B. subtilis spores lacking Nfo and ExoA have been reported to exhibit delayed germination/outgrowth (38, 40). This lag during outgrowth is attributed to unrepaired DNA, as spores lacking these AP endonucleases have a significantly higher mutation frequency than wild-type spores (38). Thus, it is reasonable to speculate that the AP endonucleases Nfo and ExoA process oxidative DNA damage produced during germination/outgrowth (38). RecA, whose coding gene is expressed during outgrowth (37), also protects B. subtilis during this developmental stage from spontaneous and ROS-promoted mutagenesis and DNA damage (38). In addition to RecA, the NER system is also involved in repairing DNA damage promoted by hydrogen peroxide during spore germination and outgrowth (38). Remarkably, ROS-promoted lesions activate a DisA-dependent checkpoint during spore outgrowth. During germination/outgrowth, B. subtilis spores lacking the AP endonucleases Nfo and ExoA accumulate 8-OxoGs and AP sites, which delays replication and their return to vegetative growth (40). Notably, disruption of disA suppresses the spore germination/outgrowth delay phenotype of nfo exoA spores (40), suggesting that DisA functions as part of a checkpoint during this developmental stage (40). Mutagenesis experiments support this claim, showing that spores deficient in DisA and treated with hydrogen peroxide during outgrowth are more likely to generate Rifr colonies than wild-type spores (40). Evidence has further uncovered that, in addition to DSBs (276, 277), 8-OxoG and/or AP sites activate the checkpoint function of DisA during spore germination and outgrowth (40). In support of this, processing of 8-OxoG by the glycosylase/lyase activity of MutM can generate SSBs and DSBs that can interfere with replication and activate DisA (40, 112, 135). In addition to Nfo and ExoA, B. subtilis uses Nth, an AP endonuclease that also possesses DNA glycosylase activity capable of operating over 8-OxoG DNA lesions (278, 279). Disruption of the nth gene increases the susceptibility of B. subtilis cells deficient for Nfo and ExoA to oxidants, demonstrating that Nth also participates in eliminating genetic damage promoted by oxidative stress (136).
As noted above, in vegetative and sporulating B. subtilis cells, the damage to transcriptionally active genes in the template strand is preferentially processed through TCR (151, 238). Mfd plays a central role during this process. While additional roles have been attributed to Mfd during recombination and SPM (14, 151), a recent report has found that chromosomal replication during spore germination/outgrowth can be severely affected by disrupting Mfd or the NER system in B. subtilis spores lacking DisA (22). These effects were attributed to unrepaired lesions affecting transcription and/or replication during outgrowth. Accordingly, the outgrowth of Mfd (TCR)- or UvrA (NER)-deficient spores is affected by 4-NQO, an agent that interferes with DNA replication (22). Therefore, the TCR and NER pathways are crucial during sporulation (238) and spore germination/outgrowth (22). Moreover, outgrowing spores lacking DisA and UvrA exhibit a delay in the first round of chromosomal replication and increased levels of spontaneous Rifr mutagenesis (22). Therefore, DisA and UvrA act in a common pathway to prevent spontaneous genetic lesions that can block replication (22). This suggests that Mfd and NER play an antimutagenic role during spore germination and outgrowth. In contrast, disruption of DisA decreases the spontaneous mutation frequency to Rifr in outgrown spores lacking mfd (22). Thus, Mfd is, in coordination with DisA, involved in processing ROS-promoted DNA lesions spontaneously generated by the metabolic conditions operating during spore germination/outgrowth (22) (Fig. 10).
Fig 10.
Mfd and DisA coordinate faithful or error-prone excision repair events elicited by reactive oxygen species-promoted lesions (8-OxoG, AP sites, and strand breaks) that can interfere with spore outgrowth. Left, ROS-promoted lesions interfering with transcription activate an Mfd-dependent transcription-coupled repair event that promotes (i) nucleotide excision repair-dependent accurate repair or (ii) a transcriptional bypass leading to error-prone repair. Right, base excision repair and/or BER intermediaries of oxidative DNA lesions stall RNA polymerase. DisA binds at a forked stalled replisome. These events activate a DisA-dependent error-prone repair pathway.
In addition, DisA is a factor involved in spore resistance to ionizing radiation (x-rays) and ultra-high vacuum desiccation, two conditions that generate SSBs and DSBs in spore chromosomes. Specifically, DisA loss decreases the survival of spores deficient for RadA, RecU, RuvAB, or the LF polymerases YqjH/YqjW following exposure to these artificial DNA-damaging agents (277).
Additional factors affecting spore germination and outgrowth
B. subtilis can also be exposed to damaging agents generated by nitric oxide oxidation or reduction, including reactive nitrogen species. These species are important as they cause base deamination, generating, among others, the HX analog base (277, 280). In B. subtilis, the endonuclease YwqL, a homolog of EndoV, removes the HX analog base from DNA (27, 33). However, B. subtilis also possesses aag, which encodes a DNA glycosylase that operates over HX and alkylated adenine derivatives (24). Previous reports have demonstrated that aag follows a forespore-specific pattern of gene expression; however, the synthesis of its coding product also takes place during spore germination and outgrowth (24). B. subtilis Δaag sporangia show a slight but significantly increased susceptibility to HNO2. However, YwqL-endonuclease-deficient sporangia were more severely sensitized to this genotoxic chemical (24). Notably, outgrown wild-type Rifr spores have a high proportion of spontaneous rpoB substitutions derived from base deamination (22). Therefore, while YwqL and aag appear to work together to protect sporulating B. subtilis cells from base deamination’s genotoxic, cytotoxic, and mutagenic effects (24), its role in protecting the spores from base deamination and alkylation during germination and outgrowth remains to be established.
CONCLUDING REMARKS
The existence of SPM in B. subtilis was demonstrated more than two decades ago in the laboratory of Ronald Yasbin (3). Further evidence has found that this cellular process occurs during the post-exponential growth phase in cell subpopulations with suppressed repair and error-prone DNA synthesis. Through this process, B. subtilis promotes genetic diversity to escape growth-limiting conditions. Therefore, SPM must be enlisted, with sporulation, competence, and biofilm formation as survival strategies with evolutionary implications. While the former three SP-associated processes are regulated by the quorum-sensing ComQXPA and RapA-PhrH systems (281), SPM is regulated by the second messenger, ci-di-AMP (46). However, the mechanistic aspects of the influence that c-di-AMP exerts on B. subtilis SPM remain unclear. Therefore, future studies should (i) elucidate how the c-di-AMP effector NrdR (an RNR repressor) and other yet-to-be-identified effectors of this cyclic dinucleotide modulate adaptive mutations and (ii) define how the c-di-AMP pathway talks with the stringent response [modulated by the alarmone, (p)ppGpp] to affect B. subtilis SPM.
Oxidative stress promotes several genetic lesions, among which 8-OxoG stands out. In B. subtilis, this lesion influences post-exponential cellular processes, including SPM and sporulation (20, 29). Deficiencies in proteins that counteract the cytotoxic and genotoxic effects of 8-OxoG increase the production of amino acid reversions by ~5–6 logs (29). While these effects are regulated by the transcriptional factors Mfd and GreA (45), the detailed mechanism(s) involved in potentiating mutations that suppress amber hisC952 and ochre metB5 nonsense mutations demands further investigation.
A novel role of 8-OxoG during B. subtilis sporulation was recently uncovered, and evidence has suggested that the Mfd-dependent processing of repair intermediates of this oxidative lesion activates a RecA/Sda-dependent checkpoint that negatively affects the onset of sporulation (20). Therefore, transcription/replication stress generated through 8-OxoG repair may be the signal that activates RecA and additional DNA-damage-dependent checkpoint proteins such as Sda, SirA, and DisA. However, it remains to be clarified which factor(s) increase (in a temporal fashion) the levels of 8-OxoG and how this lesion and/or its repair intermediates avoid sporulation and elicit vegetative growth during the SP of growth in B. subtilis.
Dormant spores of B. subtilis spontaneously accumulate genetic lesions of an oxidative nature (including AP sites but mostly 8-OxoGs), and such lesions are removed during germination/outgrowth with the participation of the checkpoint protein DisA (40). Accumulation of AP sites in spores deficient for the AP endonucleases Nfo and ExoA activates the checkpoint function of DisA, presumably for the accumulation of 3´-blocking SSBs generated by DNA glycosylases during the removal of 8-OxoGs, which can generate DSBs during replication. Interestingly, the inactivation of a third AP endonuclease, Nth, appears to activate a DisA-independent checkpoint as Nfo/ExoA/DisA/Nth-deficient spores exhibit a severe delay in returning to vegetative growth; the factor(s) involved in this alternative checkpoint are currently unknown. These genetic lesions and/or repair proteins may represent a barrier that disrupts the chromosomal displacement of DisA. Stalled DisA triggers a cellular response that culminates in a temporary blockage of replication, slowing outgrowth and cell division until the lesions are removed from the genome (22, 40, 277). The mechanism employed by DisA to stop replication and recruit repair proteins during spore outgrowth is currently unknown. The genetic and biochemical aspects governing these cellular processes should be investigated.
ACKNOWLEDGMENTS
This work was supported by the National Council of Humanities, Sciences and Technologies (CONAHCYT; grants 221231 and A1-S-27116) of México, NIH (grant GM131410), and the University of Guanajuato (grant CIIC 023/2023). K.A.-Y., L.E.M., and A.R.-M. were supported by scholarships from CONAHCYT. R.C.B.-O. was supported by a postdoctoral scholarship from CONAHCYT.
Biographies

Mario Pedraza-Reyes is a professor expert in Prokaryotic Mutagenesis & DNA Repair. He obtained a Doctorate in Sciences (Biology) at the University of Guanajuato and made postdoctoral stays at the Universities of Arizona (Tucson) and Texas Dallas (UTD), holding a full bright fellowship. His laboratory is interested in understanding how soil bacteria handles DNA damage under conditions of nutritional stress and the impact of these processes in cell differentiation, mutagenesis, survival, and evolution. He is a current fellow of the National System of Investigators (SNI, level III), is a member of the Mexican Academy of Sciences and an editor of the Journals PloS One and Front. Microbiol.

Karen Abundiz-Yañez is a doctoral student and research associate in the laboratory of Dr. Mario Pedraza-Reyes at the University of Guanajuato, where she studies the role of cyclic di-AMP in Bacillus subtilis mutagenesis and DNA repair. Karen is interested in how second messenger-based signaling pathways regulate DNA repair and promote mutations that allow bacteria to escape stressful conditions throughout B. subtilis life cycle. Karen received her Master in Sciences degree at University of Guanajuato in the laboratory of Dr. Mario Pedraza-Reyes.

Alejandra Rangel-Mendoza is an associate doctoral student in the laboratory of Dr. Mario Pedraza-Reyes at the University of Guanajuato, Mexico, where she studies the germination/outgrowth process of Bacillus subtilis spores. Alejandra is currently characterizing DNA damage checkpoints and repair proteins during B. subtilis spore/germination outgrowth. Alejandra has a bachelor's degree in Biological Pharmaceutical Chemistry and obtained her MSc degree at the University of Guanajuato, Mexico in the laboratory of Dr. Pedraza-Reyes.

Lissett E. Martínez is an associate doctoral student in the laboratory of Dr. Mario Pedraza-Reyes at the University of Guanajuato, Mexico. She studies how microorganisms implement genetic and biochemical strategies to deal with stressful conditions that limit their growth, including antibiotic resistance. She is currently elucidating how the oxidative lesion 8-OxoG impacts B. subtilis stress survival, mutagenesis and DNA repair. Lissett has a bachelor’s degree in Biological Pharmaceutical Chemistry and obtained her MSc degree at the University of Guanajuato, Mexico in the laboratory of Dr. Pedraza-Reyes.

Rocío C. Barajas-Ornelas is a postdoctoral research associate in the laboratory of Dr. Mario Pedraza-Reyes at the Division of Natural and Exact Sciences at the University of Guanajuato, where she studies how the processing of the oxidative DNA damage impact on mechanisms that regulate the germination/outgrowth of Bacillus subtilis spores. Rocío received her Ph.D. at the University of Guanajuato in the laboratory of Dr. Mario Pedraza-Reyes, where she studied the role of the Base Excision Repair (BER) at the Stationary Phase Mutagenesis phenomenon at Bacillus subtilis.

Mayra Cuéllar-Cruz holds a MSc in Biochemistry granted by the Center for Research and Advanced Studies of the National Polytechnic Institute; CINVESTAV) and a PhD in Molecular Biology from the Potosi Institute of Scientific and Technological, México (IPICyT). She is a Professor at the University of Guanajuato, México. Her scientific interests are focused on the biomineralization, biophysics, biochemistry, molecular biology and the chemical origin of life. She is also an expert in the development of vaccines, in the identification “moon light” proteins, and in the formation of nanocrystals of various species of Candida. She is a pioneer in the synthesis and identification of biomorphs (silicacarbonates of alkaline-earth metals) with nucleic acids. Prof. Cuéllar-Cruz is the founder and current President of the Mexican Society of Synchrotron Light, A.C and a Topic Editor of the journal CRYSTALS (MDPI).

Hilda C. Leyva-Sánchez is a postdoctoral researcher in the laboratory of Dr. Eduardo Robleto at the University of Nevada, Las Vegas (UNLV). She is studying the mechanistic aspects employed by the transcription-coupled repair factor (Mfd) in regulating cellular processes, including microbial pathogenesis, transcriptional regulation, modulation of cell differentiation, and promotion of genetic diversity. Hilda received her M.Sc. and Ph.D. degrees at the University of Guanajuato, Mexico in the laboratory of Dr. Mario Pedraza-Reyes, where she investigated mechanisms of transcription factors involved in adaptive mutagenesis in Bacillus subtilis.

Víctor M. Ayala-García is a full-time professor of the Faculty of Chemical Sciences at Juárez University of Durango State in Mexico. He received the degrees of M.S. and Ph.D. in Biology from the University of Guanajuato studying deaminated-DNA repair pathways of B. subtilis in the laboratory of Dr. Mario Pedraza-Reyes and completed a postdoctoral fellowship at the National Laboratory of Genomics for Biodiversity (LANGEBIO) of CINVESTAV-IPN, Mexico. His lines of research are focused on the study and use of metabolic pathways and bacterial cell signaling for biotechnological purposes with impact on health, agriculture, and the environment.

Luz I. Valenzuela-García is a postdoctoral Fellow at the Advanced Materials Research Center. She received her PhD in the University of Guanajuato, Mexico in the laboratory of Dr. Mario Pedraza Reyes, where studied mechanisms of DNA repair and checkpoint during the spore germination-outgrowth. Her current research focuses on studying the genetic responses of microorganisms to environmental contaminants for the design of whole-cell biosensors.

Eduardo A. Robleto is a Latino professor at the University of Nevada, Las Vegas School of Life Sciences. Dr. Robleto is a native of Nicaragua and got his BSc in 1986 from the Escuela Agricola Panamericana in Honduras. He earned his M.Sc. in 1994 and Ph.D. in 1998 from the University of Wisconsin-Madison. Also, he was a Postdoctoral fellow at UW Madison and Tufts School of Medicine during 1998-2002. Dr. Robleto is the Program Coordinator of Nevada-INBRE, which is designed to improve the biomedical research infrastructure and diversify the STEM workforce in Nevada. The Robleto lab studies DNA repair, mutagenesis, and gene expression in cells experiencing stress. Dr. Robleto believes that strong and committed mentoring empowers students to meet their potential in research and academic development. Providing role models for diverse students fosters a learning and inclusive environment that promotes innovation and creativity at UNLV and consequently improves the nation's workforce.
Contributor Information
Mario Pedraza-Reyes, Email: pedrama@ugto.mx.
Corrella S. Detweiler, University of Colorado Boulder, Boulder, Colorado, USA
REFERENCES
- 1. Galhardo RS, Hastings PJ, Rosenberg SM. 2007. Mutation as a stress response and the regulation of evolvability. Crit Rev Biochem Mol Biol 42:399–435. doi: 10.1080/10409230701648502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cairns J, Overbaugh J, Miller S. 1988. The origin of mutants. Nature 335:142–145. doi: 10.1038/335142a0 [DOI] [PubMed] [Google Scholar]
- 3. Sung HM, Yasbin RE. 2002. Adaptive, or stationary-phase, mutagenesis, a component of bacterial differentiation in Bacillus subtilis. J Bacteriol 184:5641–5653. doi: 10.1128/JB.184.20.5641-5653.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Robleto EA, Yasbin R, Ross C, Pedraza-Reyes M. 2007. Stationary phase mutagenesis in B. subtilis: a paradigm to study genetic diversity programs in cells under stress. Crit Rev Biochem Mol Biol 42:327–339. doi: 10.1080/10409230701597717 [DOI] [PubMed] [Google Scholar]
- 5. Yashin RE, Pedraza-Reyes M. 2004. Stationary-phase-induced mutagenesis: is directed mutagenesis alive and well within Neo-Darwinian theory? In Microbial evolution. [Google Scholar]
- 6. Foster PL. 1999. Mechanisms of stationary phase mutation: a decade of adaptive mutation. Annu Rev Genet 33:57–88. doi: 10.1146/annurev.genet.33.1.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wright BE. 2004. Stress-directed adaptive mutations and evolution. Mol Microbiol 52:643–650. doi: 10.1111/j.1365-2958.2004.04012.x [DOI] [PubMed] [Google Scholar]
- 8. Battista JR, Earl AM. 2014. Mutagenesis and DNA repair: the consequences of error and mechanisms for remaining the same. In Microbial evolution. [Google Scholar]
- 9. Yasbin RE, Cheo D, Bol D. 1993. DNA repair systems, p 529–537. In Sonenshein AL, Hoch JA, Losick R (ed), Bacillus subtilis and other Gram-positive bacteria: biochemistry, physiology, and molecular genetics. American Society for Microbiology, Washington, D.C. [Google Scholar]
- 10. Lenhart JS, Schroeder JW, Walsh BW, Simmons LA. 2012. DNA repair and genome maintenance in Bacillus subtilis. Microbiol Mol Biol Rev 76:530–564. doi: 10.1128/MMBR.05020-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pedreira T, Elfmann C, Stülke J. 2022. The current state of SubtiWiki, the database for the model organism Bacillus subtilis. Nucleic Acids Res 50:D875–D882. doi: 10.1093/nar/gkab943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Love PE, Yasbin RE. 1986. Induction of the Bacillus subtilis SOS-like response by Escherichia coli RecA protein. Proc Natl Acad Sci USA 83:5204–5208. doi: 10.1073/pnas.83.14.5204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Love PE, Yasbin RE. 1984. Genetic characterization of the inducible SOS-like system of Bacillus subtilis. J Bacteriol 160:910–920. doi: 10.1128/jb.160.3.910-920.1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Marrero R, Yasbin RE. 1988. Cloning of the Bacillus subtilis recE+ gene and functional expression of recE+ in B. subtilis. J Bacteriol 170:335–344. doi: 10.1128/jb.170.1.335-344.1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Miller MC, Resnick JB, Smith BT, Lovett CM. 1996. The Bacillus subtilis dinR gene codes for the analogue of Escherichia coli LexA: purification and characterization of the DinR protein. J Biol Chem 271:33502–33508. doi: 10.1074/jbc.271.52.33502 [DOI] [PubMed] [Google Scholar]
- 16. Winterling KW, Levine AS, Yasbin RE, Woodgate R. 1997. Characterization of DinR, the Bacillus subtilis SOS repressor. J Bacteriol 179:1698–1703. doi: 10.1128/jb.179.5.1698-1703.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Winterling KW, Chafin D, Hayes JJ, Sun JI, Levine AS, Yasbin RE, Woodgate R. 1998. The Bacillus subtilis DinR binding site: redefinition of the consensus sequence. J Bacteriol 180:2201–2211. doi: 10.1128/JB.180.8.2201-2211.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Au N, Kuester-Schoeck E, Mandava V, Bothwell LE, Canny SP, Chachu K, Colavito SA, Fuller SN, Groban ES, Hensley LA, O’Brien TC, Shah A, Tierney JT, Tomm LL, O’Gara TM, Goranov AI, Grossman AD, Lovett CM. 2005. Genetic composition of the Bacillus subtilis SOS system. J Bacteriol 187:7655–7666. doi: 10.1128/JB.187.22.7655-7666.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lovett CM, Love PE, Yasbin RE. 1989. Competence-specific induction of the Bacillus subtilis RecA protein analog: evidence for dual regulation of a recombination protein. J Bacteriol 171:2318–2322. doi: 10.1128/jb.171.5.2318-2322.1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Suárez VP, Martínez LE, Leyva-Sánchez HC, Valenzuela-García LI, Lara-Martínez R, Jiménez-García LF, Ramírez-Ramírez N, Obregon-Herrera A, Cuéllar-Cruz M, Robleto EA, Pedraza-Reyes M. 2021. Transcriptional coupling and repair of 8-OxoG activate a RecA-dependent checkpoint that controls the onset of sporulation in Bacillus subtilis. Sci Rep 11:2513. doi: 10.1038/s41598-021-82247-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ramírez-Guadiana FH, Barajas-Ornelas RDC, Corona-Bautista SU, Setlow P, Pedraza-Reyes M. 2016. The RecA-dependent SOS response is active and required for processing of DNA damage during Bacillus subtilis sporulation. PLoS One 11:e0150348. doi: 10.1371/journal.pone.0150348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Valenzuela-García LI, Ayala-García VM, Regalado-García AG, Setlow P, Pedraza-Reyes M. 2018. Transcriptional coupling (Mfd) and DNA damage scanning (DisA) coordinate excision repair events for efficient Bacillus subtilis spore outgrowth. Microbiologyopen 7:e00593. doi: 10.1002/mbo3.593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Aamodt RM, Falnes PØ, Johansen RF, Seeberg E, Bjørås M. 2004. The Bacillus subtilis counterpart of the mammalian 3-methyladenine DNA glycosylase has hypoxanthine and 1,N6-ethenoadenine as preferred substrates. J Biol Chem 279:13601–13606. doi: 10.1074/jbc.M314277200 [DOI] [PubMed] [Google Scholar]
- 24. Ayala-García VM, Valenzuela-García LI, Setlow P, Pedraza-Reyes M. 2016. Aag hypoxanthine-DNA glycosylase is synthesized in the forespore compartment and involved in counteracting the genotoxic and mutagenic effects of hypoxanthine and alkylated bases in DNA during Bacillus subtilis sporulation. J Bacteriol 198:3345–3354. doi: 10.1128/JB.00625-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pérez-Lago L, Serrano-Heras G, Baños B, Lázaro JM, Alcorlo M, Villar L, Salas M. 2011. Characterization of Bacillus subtilis uracil-DNA glycosylase and its inhibition by phage φ29 protein p56. Mol Microbiol 80:1657–1666. doi: 10.1111/j.1365-2958.2011.07675.x [DOI] [PubMed] [Google Scholar]
- 26. Baños-Sanz JI, Mojardín L, Sanz-Aparicio J, Lázaro JM, Villar L, Serrano-Heras G, González B, Salas M. 2013. Crystal structure and functional insights into uracil-DNA glycosylase inhibition by phage Φ29 DNA mimic protein p56. Nucleic Acids Res 41:6761–6773. doi: 10.1093/nar/gkt395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. López-Olmos K, Hernández MP, Contreras-Garduño JA, Robleto EA, Setlow P, Yasbin RE, Pedraza-Reyes M. 2012. Roles of endonuclease V, uracil-DNA glycosylase, and mismatch repair in Bacillus subtilis DNA base-deamination-induced mutagenesis. J Bacteriol 194:243–252. doi: 10.1128/JB.06082-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sasaki M, Yonemura Y, Kurusu Y. 2000. Genetic analysis of Bacillus subtilis mutator genes. J Gen Appl Microbiol 46:183–187. doi: 10.2323/jgam.46.183 [DOI] [PubMed] [Google Scholar]
- 29. Vidales LE, Cárdenas LC, Robleto E, Yasbin RE, Pedraza-Reyes M. 2009. Defects in the error prevention oxidized guanine system potentiate stationary-phase mutagenesis in Bacillus subtilis. J Bacteriol 191:506–513. doi: 10.1128/JB.01210-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gómez-Marroquín M, Vidales LE, Debora BN, Santos-Escobar F, Obregón-Herrera A, Robleto EA, Pedraza-Reyes M. 2015. Role of Bacillus subtilis DNA glycosylase MutM in counteracting oxidatively induced DNA damage and in stationary-phase-associated mutagenesis. J Bacteriol 197:1963–1971. doi: 10.1128/JB.00147-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Debora BN, Vidales LE, Ramírez R, Ramírez M, Robleto EA, Yasbin RE, Pedraza-Reyes M. 2011. Mismatch repair modulation of MutY activity drives Bacillus subtilis stationary-phase mutagenesis. J Bacteriol 193:236–245. doi: 10.1128/JB.00940-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Morohoshi F, Hayashi K, Munkata N. 1993. Bacillus subtilis alkA gene encoding inducible 3-methyladenine DNA glycosylase is adjacent to the ada operon. J Bacteriol 175:6010–6017. doi: 10.1128/jb.175.18.6010-6017.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Patlán AG, Ayala-García VM, Valenzuela-García LI, Meneses-Plascencia J, Vargas-Arias PL, Barraza-Salas M, Setlow P, Brieba LG, Pedraza-Reyes M. 2019. YwqL (EndoV), ExoA and PolA act in a novel alternative excision pathway to repair deaminated DNA bases in Bacillus subtilis. PLoS One 14:e0211653. doi: 10.1371/journal.pone.0211653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Patlán AG, Corona SU, Ayala-García VM, Pedraza-Reyes M. 2018. Non-canonical processing of DNA photodimers with Bacillus subtilis UV-endonuclease YwjD, 5′→3′ exonuclease YpcP and low-fidelity DNA polymerases YqjH and YqjW. DNA Repair (Amst) 70:1–9. doi: 10.1016/j.dnarep.2018.07.007 [DOI] [PubMed] [Google Scholar]
- 35. Nicholson WL, Munakata N, Horneck G, Melosh HJ, Setlow P. 2000. Resistance of Bacillus endospores to extreme terrestrial and extraterrestrial environments. Microbiol Mol Biol Rev 64:548–572. doi: 10.1128/MMBR.64.3.548-572.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pedraza-Reyes M, Ramírez-Ramírez N, Vidales-Rodríguez LE, Robleto EA. 2012. Mechanisms of bacterial spore survival. In Bacterial spores. Current Research and Applications. [Google Scholar]
- 37. Setlow B, Setlow P. 1996. Role of DNA repair in Bacillus subtilis spore resistance. J Bacteriol 178:3486–3495. doi: 10.1128/jb.178.12.3486-3495.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ibarra JR, Orozco AD, Rojas JA, López K, Setlow P, Yasbin RE, Pedraza-Reyes M. 2008. Role of the Nfo and ExoA apurinic/apyrimidinic endonucleases in repair of DNA damage during outgrowth of Bacillus subtilis spores. J Bacteriol 190:2031–2038. doi: 10.1128/JB.01625-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang ST, Setlow B, Conlon EM, Lyon JL, Imamura D, Sato T, Setlow P, Losick R, Eichenberger P. 2006. The forespore line of gene expression in Bacillus subtilis. J Mol Biol 358:16–37. doi: 10.1016/j.jmb.2006.01.059 [DOI] [PubMed] [Google Scholar]
- 40. Campos SS, Ibarra-Rodriguez JR, Barajas-Ornelas RC, Ramírez-Guadiana FH, Obregón-Herrera A, Setlow P, Pedraza-Reyes M. 2014. Interaction of apurinic/apyrimidinic endonucleases Nfo and ExoA with the DNA integrity scanning protein DisA in the processing of oxidative DNA damage during Bacillus subtilis spore outgrowth. J Bacteriol 196:568–578. doi: 10.1128/JB.01259-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sung HM, Yeamans G, Ross CA, Yasbin RE. 2003. Roles of YqjH and YqjW, homologs of the Escherichia coli UmuC/DinB or Y superfamily of DNA polymerases, in stationary-phase mutagenesis and UV-induced mutagenesis of Bacillus subtilis. J Bacteriol 185:2153–2160. doi: 10.1128/JB.185.7.2153-2160.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Martin HA, Porter KE, Vallin C, Ermi T, Contreras N, Pedraza-Reyes M, Robleto EA. 2019. Mfd protects against oxidative stress in Bacillus subtilis independently of its canonical function in DNA repair. BMC Microbiol 19:26. doi: 10.1186/s12866-019-1394-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gómez-Marroquín M, Martin HA, Pepper A, Girard ME, Kidman AA, Vallin C, Yasbin RE, Pedraza-Reyes M, Robleto EA. 2016. Stationary-phase mutagenesis in stressed Bacillus subtilis cells operates by Mfd-dependent mutagenic pathways. Genes (Basel) 7:33. doi: 10.3390/genes7070033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pybus C, Pedraza-Reyes M, Ross CA, Martin H, Ona K, Yasbin RE, Robleto E. 2010. Transcription-associated mutation in Bacillus subtilis cells under stress. J Bacteriol 192:3321–3328. doi: 10.1128/JB.00354-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Leyva-Sánchez HC, Villegas-Negrete N, Abundiz-Yañez K, Yasbin RE, Robleto EA, Pedraza-Reyes M. 2020. Role of Mfd and GreA in Bacillus subtilis base excision repair-dependent stationary-phase mutagenesis. J Bacteriol 202:e00807-19. doi: 10.1128/JB.00807-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Abundiz-Yañez K, Leyva-Sánchez HC, Robleto EA, Pedraza-Reyes M. 2022. Stress-associated and growth-dependent mutagenesis are divergently regulated by c-di-AMP levels in Bacillus subtilis. Int J Mol Sci 24:455. doi: 10.3390/ijms24010455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wozniak KJ, Simmons LA. 2022. Bacterial DNA excision repair pathways. Nat Rev Microbiol 20:465–477. doi: 10.1038/s41579-022-00694-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kasak L, Hõrak R, Kivisaar M. 1997. Promoter-creating mutations in Pseudomonas putida: a model system for the study of mutation in starving bacteria. Proc Natl Acad Sci USA 94:3134–3139. doi: 10.1073/pnas.94.7.3134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hałas A, Baranowska H, Policińska Z. 2002. The influence of the mismatch-repair system on stationary-phase mutagenesis in the yeast Saccharomyces cerevisiae. Curr Genet 42:140–146. doi: 10.1007/s00294-002-0334-7 [DOI] [PubMed] [Google Scholar]
- 50. Ambriz-Aviña V, Yasbin RE, Robleto EA, Pedraza-Reyes M. 2016. Role of base excision repair (BER) in transcription-associated mutagenesis of nutritionally stressed nongrowing Bacillus subtilis cell subpopulations. Curr Microbiol 73:721–726. doi: 10.1007/s00284-016-1122-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Martin HA, Kidman AA, Socea J, Vallin C, Pedraza-Reyes M, Robleto EA. 2020. The Bacillus subtilis k-state promotes stationary- phase mutagenesis via oxidative damage. Genes (Basel) 11:190. doi: 10.3390/genes11020190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Villegas-Negrete N, Robleto EA, Obregón-Herrera A, Yasbin RE, Pedraza-Reyes M. 2017. Implementation of a loss-of-function system to determine growth and stress-associated mutagenesis in Bacillus subtilis. PLoS One 12:e0179625. doi: 10.1371/journal.pone.0179625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nakada D, Ryan FJ. 1961. Replication of deoxyribonucleic acid in non-dividing bacteria. Nature 189:398–399. doi: 10.1038/189398a0 [DOI] [PubMed] [Google Scholar]
- 54. Ryan FJ. 1955. Spontaneous mutation in non-dividing bacteria. Genetics 40:726–738. doi: 10.1093/genetics/40.5.726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rosenberg SM, Thulin C, Harris RS. 1998. Transient and heritable mutators in adaptive evolution in the lab and in nature. Genetics 148:1559–1566. doi: 10.1093/genetics/148.4.1559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Harris RS, Longerich S, Rosenberg SM. 1994. Recombination in adaptive mutation. Science 264:258–260. doi: 10.1126/science.8146657 [DOI] [PubMed] [Google Scholar]
- 57. McKenzie GJ, Harris RS, Lee PL, Rosenberg SM. 2000. The SOS response regulates adaptive mutation. Proc Natl Acad Sci USA 97:6646–6651. doi: 10.1073/pnas.120161797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. McKenzie GJ, Rosenberg SM. 2001. Adaptive mutations, mutator DNA polymerases and genetic change strategies of pathogens. Curr Opin Microbiol 4:586–594. doi: 10.1016/s1369-5274(00)00255-1 [DOI] [PubMed] [Google Scholar]
- 59. Hendrickson H, Slechta ES, Bergthorsson U, Andersson DI, Roth JR. 2002. Amplification-mutagenesis: evidence that “directed” adaptive mutation and general hypermutability result from growth with a selected gene amplification. Proc Natl Acad Sci USA 99:2164–2169. doi: 10.1073/pnas.032680899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Slechta ES, Bunny KL, Kugelberg E, Kofoid E, Andersson DI, Roth JR. 2003. Adaptive mutation: general mutagenesis is not a programmed response to stress but results from rare coamplification of dinB with lac. Proc Natl Acad Sci USA 100:12847–12852. doi: 10.1073/pnas.1735464100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kivisaar M. 2010. Mechanisms of stationary-phase mutagenesis in bacteria: mutational processes in pseudomonads. FEMS Microbiol Lett 312:1–14. doi: 10.1111/j.1574-6968.2010.02027.x [DOI] [PubMed] [Google Scholar]
- 62. Ukkivi K, Kivisaar M. 2018. Involvement of transcription-coupled repair factor Mfd and DNA helicase UvrD in mutational processes in Pseudomonas putida. DNA Repair (Amst) 72:18–27. doi: 10.1016/j.dnarep.2018.09.011 [DOI] [PubMed] [Google Scholar]
- 63. Slater JH, Weightman AJ, Hall BG. 1985. Dehalogenase genes of Pseudomonas putida PP3 on chromosomally located transposable elements. Mol Biol Evol 2:557–567. doi: 10.1093/oxfordjournals.molbev.a040366 [DOI] [PubMed] [Google Scholar]
- 64. Ermi T, Vallin C, García AGR, Bravo M, Cordero IF, Martin HA, Pedraza-Reyes M, Robleto E. 2021. Non-b DNA-forming motifs promote mfd-dependent stationary-phase mutagenesis in Bacillus subtilis. Microorganisms 9:1284. doi: 10.3390/microorganisms9061284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Canceill D, Viguera E, Ehrlich SD. 1999. Replication slippage of different DNA polymerases is inversely related to their strand displacement efficiency. J Biol Chem 274:27481–27490. doi: 10.1074/jbc.274.39.27481 [DOI] [PubMed] [Google Scholar]
- 66. Madsen CS, Ghivizzani SC, Hauswirth WW. 1993. In vivo and in vitro evidence for slipped mispairing in mammalian mitochondria. Proc Natl Acad Sci USA 90:7671–7675. doi: 10.1073/pnas.90.16.7671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tran HT, Degtyareva NP, Koloteva NN, Sugino A, Masumoto H, Gordenin DA, Resnick MA. 1995. Replication slippage between distant short repeats in Saccharomyces cerevisiae depends on the direction of replication and the RAD50 and RAD52 genes. Mol Cell Biol 15:5607–5617. doi: 10.1128/MCB.15.10.5607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Trinh TQ, Sinden RR. 1993. The influence of primary and secondary DNA structure in deletion and duplication between direct repeats in Escherichia coli. Genetics 134:409–422. doi: 10.1093/genetics/134.2.409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Trinh TQ, Sinden RR. 1991. Preferential DNA secondary structure mutagenesis in the lagging strand of replication in E. coli. Nature 352:544–547. doi: 10.1038/352544a0 [DOI] [PubMed] [Google Scholar]
- 70. Rosche WA, Trinh TQ, Sinden RR. 1995. Differential DNA secondary structure-mediated deletion mutation in the leading and lagging strands. J Bacteriol 177:4385–4391. doi: 10.1128/jb.177.15.4385-4391.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Rosche WA, Ripley LS, Sinden RR. 1998. Primer-template misalignments during leading strand DNA synthesis account for the most frequent spontaneous mutations in a quasipalindromic region in Escherichia coli. J Mol Biol 284:633–646. doi: 10.1006/jmbi.1998.2193 [DOI] [PubMed] [Google Scholar]
- 72. Sinden RR, Hashem VI, Rosche WA. 1999. DNA‐directed mutations: leading and lagging strand specificity. Ann N Y Acad Sci 870:173–189. doi: 10.1111/j.1749-6632.1999.tb08878.x [DOI] [PubMed] [Google Scholar]
- 73. Kroutil LC, Kunkel TA. 1999. Deletion errors generated during replication of CAG repeats. Nucleic Acids Res 27:3481–3486. doi: 10.1093/nar/27.17.3481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Foster PL, Trimarchi JM. 1994. Adaptive reversion of a frameshift mutation in Escherichia coli by simple base deletions in homopolymeric runs. Science 265:407–409. doi: 10.1126/science.8023164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. McKenzie GJ, Lee PL, Lombardo M-J, Hastings PJ, Rosenberg SM. 2001. SOS mutator DNA polymerase IV functions in adaptive mutation and not adaptive amplification. Mol Cell 7:571–579. doi: 10.1016/s1097-2765(01)00204-0 [DOI] [PubMed] [Google Scholar]
- 76. Dormeyer M, Lentes S, Ballin P, Wilkens M, Klumpp S, Kohlheyer D, Stannek L, Grünberger A, Commichau FM. 2018. Visualization of tandem repeat mutagenesis in Bacillus subtilis. DNA Repair (Amst) 63:10–15. doi: 10.1016/j.dnarep.2018.01.006 [DOI] [PubMed] [Google Scholar]
- 77. Belitsky BR, Sonenshein AL. 1998. Role and regulation of Bacillus subtilis glutamate dehydrogenase genes. J Bacteriol 180:6298–6305. doi: 10.1128/JB.180.23.6298-6305.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Gunka K, Tholen S, Gerwig J, Herzberg C, Stülke J, Commichau FM. 2012. A high-frequency mutation in Bacillus subtilis: requirements for the decryptification of the gudB glutamate dehydrogenase gene. J Bacteriol 194:1036–1044. doi: 10.1128/JB.06470-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Du X, Gertz EM, Wojtowicz D, Zhabinskaya D, Levens D, Benham CJ, Schäffer AA, Przytycka TM. 2014. Potential non-B DNA regions in the human genome are associated with higher rates of nucleotide mutation and expression variation. Nucleic Acids Res 42:12367–12379. doi: 10.1093/nar/gku921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Piazza A, Adrian M, Samazan F, Heddi B, Hamon F, Serero A, Lopes J, Teulade-Fichou M-P, Phan AT, Nicolas A. 2015. Short loop length and high thermal stability determine genomic instability induced by G‐quadruplex‐forming minisatellites. EMBO J 34:1718–1734. doi: 10.15252/embj.201490702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Szlachta K, Thys RG, Atkin ND, Pierce LCT, Bekiranov S, Wang Y-H. 2018. Alternative DNA secondary structure formation affects RNA polymerase II promoter-proximal pausing in human. Genome Biol 19:89. doi: 10.1186/s13059-018-1463-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Teng F-Y, Hou X-M, Fan S-H, Rety S, Dou S-X, Xi X-G. 2017. Escherichia coli DNA polymerase I can disrupt G‐quadruplex structures during DNA replication. FEBS J 284:4051–4065. doi: 10.1111/febs.14290 [DOI] [PubMed] [Google Scholar]
- 83. Northam MR, Moore EA, Mertz TM, Binz SK, Stith CM, Stepchenkova EI, Wendt KL, Burgers PMJ, Shcherbakova PV. 2014. DNA polymerases ζ and Rev1 mediate error-prone bypass of non-B DNA structures. Nucleic Acids Res 42:290–306. doi: 10.1093/nar/gkt830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Scheibye-Knudsen M, Tseng A, Borch Jensen M, Scheibye-Alsing K, Fang EF, Iyama T, Bharti SK, Marosi K, Froetscher L, Kassahun H, Eckley DM, Maul RW, Bastian P, De S, Ghosh S, Nilsen H, Goldberg IG, Mattson MP, Wilson DM, Brosh RM, Gorospe M, Bohr VA. 2016. Cockayne syndrome group A and B proteins converge on transcription-linked resolution of non-B DNA. Proc Natl Acad Sci USA 113:12502–12507. doi: 10.1073/pnas.1610198113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Sinden RR, Zheng GX, Brankamp RG, Allen KN. 1991. On the deletion of inverted repeated DNA in Escherichia coli: effects of length, thermal stability, and cruciform formation in vivo. Genetics 129:991–1005. doi: 10.1093/genetics/129.4.991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Parekh VJ, Niccum BA, Shah R, Rivera MA, Novak MJ, Geinguenaud F, Wien F, Arluison V, Sinden RR. 2019. Role of Hfq in genome evolution: instability of G-quadruplex sequences in E. coli. Microorganisms 8:28. doi: 10.3390/microorganisms8010028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Hall BG. 1992. Selection-induced mutations occur in yeast. Proc Natl Acad Sci USA 89:4300–4303. doi: 10.1073/pnas.89.10.4300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Marini A, Matmati N, Morpurgo G. 1999. Starvation in yeast increases non-adaptive mutation. Curr Genet 35:77–81. doi: 10.1007/s002940050435 [DOI] [PubMed] [Google Scholar]
- 89. Babudri N, Pavlov YI, Matmati N, Ludovisi C, Achilli A. 2001. Stationary-phase mutations in proofreading exonuclease-deficient strains of the yeast Saccharomyces cerevisiae. Mol Genet Genomics 265:362–366. doi: 10.1007/s004380000424 [DOI] [PubMed] [Google Scholar]
- 90. Heidenreich E, Wintersberger U. 1997. Starvation for a specific amino acid induces high frequencies of rho − mutants in Saccharomyces cerevisiae. Curr Genet 31:408–413. doi: 10.1007/s002940050223 [DOI] [PubMed] [Google Scholar]
- 91. Heidenreich E, Novotny R, Kneidinger B, Holzmann V, Wintersberger U. 2003. Non-homologous end joining as an important mutagenic process in cell cycle-arrested cells. EMBO J 22:2274–2283. doi: 10.1093/emboj/cdg203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Storchová Z, Vondrejs V. 1999. Starvation-associated mutagenesis in yeast Saccharomyces cerevisiae is affected by Ras2/cAMP signaling pathway. Mutat Res 431:59–67. doi: 10.1016/s0027-5107(99)00157-8 [DOI] [PubMed] [Google Scholar]
- 93. Steele DF, Jinks-Robertson S. 1992. An examination of adaptive reversion in Saccharomyces cerevisiae. Genetics 132:9–21. doi: 10.1093/genetics/132.1.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Greene CN, Jinks-Robertson S. 1999. Comparison of spontaneous and adaptive mutation spectra in yeast. J Genet 78:51–55. doi: 10.1007/BF02994703 [DOI] [Google Scholar]
- 95. Heidenreich E, Wintersberger U. 2001. Adaptive reversions of a frameshift mutation in arrested Saccharomyces cerevisiae cells by simple deletions in mononucleotide repeats. Mutat Res 473:101–107. doi: 10.1016/s0027-5107(00)00141-x [DOI] [PubMed] [Google Scholar]
- 96. Heidenreich E, Holzmann V, Eisler H. 2004. Polymerase ζ dependency of increased adaptive mutation frequencies in nucleotide excision repair-deficient yeast strains. DNA Repair (Amst) 3:395–402. doi: 10.1016/j.dnarep.2003.12.004 [DOI] [PubMed] [Google Scholar]
- 97. Heidenreich E, Eisler H, Steinboeck F. 2006. Epistatic participation of REV1 and REV3 in the formation of UV-induced frameshift mutations in cell cycle-arrested yeast cells. Mutat Res 593:187–195. doi: 10.1016/j.mrfmmm.2005.07.012 [DOI] [PubMed] [Google Scholar]
- 98. Baranowska H, Policińska Z, Jachymczyk WJ. 1995. Effects of the CDC2 gene on adaptive mutation in the yeast Saccharomyces cerevisiae. Curr Genet 28:521–525. doi: 10.1007/BF00518164 [DOI] [PubMed] [Google Scholar]
- 99. Storchová Z, Rojas Gil AP, Janderová B, Vondrejs V. 1998. The involvement of the RAD6 gene in starvation-induced reverse mutation in Saccharomyces cerevisiae. Mol Gen Genet 258:546–552. doi: 10.1007/s004380050766 [DOI] [PubMed] [Google Scholar]
- 100. Storchová Z, Gil AP, Janderová B, Vlasák J, Vondrejs V. 1997. Accumulation of Ade+ reversions in isoauxotrophic stains of Saccharomyces cerevisiae allelic in RAD6 during adenine starvation. Folia Microbiol (Praha) 42:47–51. doi: 10.1007/BF02898645 [DOI] [PubMed] [Google Scholar]
- 101. Rojas Gil AP, Vondrejs V. 1999. Development of papillae on colonies of two isopoly-auxotrophic strains of Saccharomyces cerevisiae allelic inRAD6 during adenine starvation. Folia Microbiol (Praha) 44:299–305. doi: 10.1007/BF02818551 [DOI] [PubMed] [Google Scholar]
- 102. Cejka P, Vondrejs V, Storchová Z. 2001. Dissection of the functions of the Saccharomyces cerevisiae RAD6 postreplicative repair group in mutagenesis and UV sensitivity. Genetics 159:953–963. doi: 10.1093/genetics/159.3.953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Heidenreich E, Steinboeck F. 2017. Glucose starvation as a selective tool for the study of adaptive mutations in Saccharomyces cerevisiae. J Microbiol Methods 132:4–8. doi: 10.1016/j.mimet.2016.11.007 [DOI] [PubMed] [Google Scholar]
- 104. Heidenreich E. 2007. Adaptive mutation in Saccharomyces cerevisiae. Crit Rev Biochem Mol Biol 42:285–311. doi: 10.1080/10409230701507773 [DOI] [PubMed] [Google Scholar]
- 105. Behe MJ. 2010. Experimental evolution, loss-of-function mutations, and “the first rule of adaptive evolution.”. Q Rev Biol 85:419–445. doi: 10.1086/656902 [DOI] [PubMed] [Google Scholar]
- 106. Wilson MC, Farmer JL, Rothman F. 1966. Thymidylate synthesis and aminopterin resistance in Bacillus subtilis. J Bacteriol 92:186–196. doi: 10.1128/jb.92.1.186-196.1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Fox KM, Maley F, Garibian A, Changchien LM, Van Roey P. 1999. Crystal structure of thymidylate synthase A from Bacillus subtilis. Protein Sci 8:538–544. doi: 10.1110/ps.8.3.538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Neuhard J, Price AR, Schack L, Thomassen E. 1978. Two thymidylate synthetases in Bacillus subtilis. Proc Natl Acad Sci USA 75:1194–1198. doi: 10.1073/pnas.75.3.1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Hawser S, Lociuro S, Islam K. 2006. Dihydrofolate reductase inhibitors as antibacterial agents. Biochem Pharmacol 71:941–948. doi: 10.1016/j.bcp.2005.10.052 [DOI] [PubMed] [Google Scholar]
- 110. Quinto-Alemany D, Canerina-Amaro A, Hernández-Abad LG, Machín F, Romesberg FE, Gil-Lamaignere C. 2012. Yeasts acquire resistance secondary to antifungal drug treatment by adaptive mutagenesis. PLoS ONE 7:e42279. doi: 10.1371/journal.pone.0042279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Kunkel TA, Erie DA. 2005. DNA mismatch repair. Annu Rev Biochem 74:681–710. doi: 10.1146/annurev.biochem.74.082803.133243 [DOI] [PubMed] [Google Scholar]
- 112. Friedberg EC, Walker GC, Siede W, Wood RD, Schultz RA, Ellenberger T. 2005. DNA repair and mutagenesis. 2nd ed. American Society for Microbiology, Washington, DC. [Google Scholar]
- 113. Fukui K. 2010. DNA mismatch repair in eukaryotes and bacteria. J Nucleic Acids 2010:260512. doi: 10.4061/2010/260512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Patlán-Vázquez AG, Ayala-García VM, Vallin C, Cortés J, Vásquez-Morales SG, Robleto EA, Nudler E, Pedraza-Reyes M. 2022. Dynamics of mismatch and alternative excision-dependent repair in replicating Bacillus subtilis DNA examined under conditions of neutral selection. Front Microbiol 13:866089. doi: 10.3389/fmicb.2022.866089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Ginetti F, Perego M, Albertini AM, Galizzi A. 1996. Bacillus subtilis mutS mutL operon: identification, nucleotide sequence and mutagenesis. Microbiology (Reading) 142:2021–2029. doi: 10.1099/13500872-142-8-2021 [DOI] [PubMed] [Google Scholar]
- 116. Pedraza-Reyes M, Yasbin RE. 2004. Contribution of the mismatch DNA repair system to the generation of stationary-phase-induced mutants of Bacillus subtilis. J Bacteriol 186:6485–6491. doi: 10.1128/JB.186.19.6485-6491.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Saumaa S, Tarassova K, Tark M, Tover A, Tegova R, Kivisaar M. 2006. Involvement of DNA mismatch repair in stationary-phase mutagenesis during prolonged starvation of Pseudomonas putida. DNA Repair (Amst) 5:505–514. doi: 10.1016/j.dnarep.2005.12.003 [DOI] [PubMed] [Google Scholar]
- 118. Simmons LA, Davies BW, Grossman AD, Walker GC. 2008. β-Clamp directs localization of mismatch repair in Bacillus subtilis. Mol Cell 29:291–301. doi: 10.1016/j.molcel.2007.10.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Lenhart JS, Pillon MC, Guarné A, Biteen JS, Simmons LA. 2016. Mismatch repair in Gram-positive bacteria. Res Microbiol 167:4–12. doi: 10.1016/j.resmic.2015.08.006 [DOI] [PubMed] [Google Scholar]
- 120. Harris RS, Feng G, Ross KJ, Sidhu R, Thulin C, Longerich S, Szigety SK, Winkler ME, Rosenberg SM. 1997. Mismatch repair protein MutL becomes limiting during stationary-phase mutation. Genes Dev 11:2426–2437. doi: 10.1101/gad.11.18.2426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Bridges BA. 1996. Elevated mutation rate in mutT bacteria during starvation: evidence for DNA turnover? J Bacteriol 178:2709–2711. doi: 10.1128/jb.178.9.2709-2711.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Bridges BA, Sekiguchi M, Tajiri T. 1996. Effect of mutY and mutM/fpg-1 mutations on starvation-associated mutation in Escherichia coli: implications for the role of 7,8-dihydro-8-oxoguanine. Mol Gen Genet 251:352–357. doi: 10.1007/BF02172526 [DOI] [PubMed] [Google Scholar]
- 123. Bridges BA. 1998. The role of DNA damage in stationary phase ('adaptive’) mutation. Mutat Res 408:1–9. doi: 10.1016/s0921-8777(98)00008-1 [DOI] [PubMed] [Google Scholar]
- 124. Bridges BA, Ereira S. 1998. DNA synthesis and viability of a mutT derivative of Escherichia coli WP2 under conditions of amino acid starvation and relation to stationary-phase (adaptive) mutation. J Bacteriol 180:2906–2910. doi: 10.1128/JB.180.11.2906-2910.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Saumaa S, Tover A, Tark M, Tegova R, Kivisaar M. 2007. Oxidative DNA damage defense systems in avoidance of stationary-phase mutagenesis in Pseudomonas putida. J Bacteriol 189:5504–5514. doi: 10.1128/JB.00518-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Shibutani S, Takeshita M, Grollman AP. 1991. Insertion of specific bases during DNA synthesis past the oxidation-damaged base 8-oxodG. Nature 349:431–434. doi: 10.1038/349431a0 [DOI] [PubMed] [Google Scholar]
- 127. Tajiri T, Maki H, Sekiguchi M. 1995. Functional cooperation of MutT, MutM and MutY proteins in preventing mutations caused by spontaneous oxidation of guanine nucleotide in Escherichia coli. Mutat Res 336:257–267. doi: 10.1016/0921-8777(94)00062-b [DOI] [PubMed] [Google Scholar]
- 128. Michaels ML, Miller JH. 1992. The GO system protects organisms from the mutagenic effect of the spontaneous lesion 8-hydroxyguanine (7,8-dihydro-8-oxoguanine). J Bacteriol 174:6321–6325. doi: 10.1128/jb.174.20.6321-6325.1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Maki H, Sekiguchi M. 1992. MutT protein specifically hydrolyses a potent mutagenic substrate for DNA synthesis. Nature 355:273–275. doi: 10.1038/355273a0 [DOI] [PubMed] [Google Scholar]
- 130. Taddei F, Hayakawa H, Bouton MF, Cirinesi AM, Matic I, Sekiguchi M, Radman M. 1997. Counteraction by MutT protein of transcriptional errors caused by oxidative damage. Science 278:128–130. doi: 10.1126/science.278.5335.128 [DOI] [PubMed] [Google Scholar]
- 131. Ramírez MI, Castellanos-Juárez FX, Yasbin RE, Pedraza-Reyes M. 2004. The ytkD (mutTA) gene of Bacillus subtilis encodes a functional antimutator 8-Oxo-(dGTP/GTP)ase and is under dual control of sigma A and sigma F RNA polymerases. J Bacteriol 186:1050–1059. doi: 10.1128/JB.186.4.1050-1059.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Castellanos-Juárez FX, Alvarez-Alvarez C, Yasbin RE, Setlow B, Setlow P, Pedraza-Reyes M. 2006. YtkD and MutT protect vegetative cells but not spores of Bacillus subtilis from oxidative stress. J Bacteriol 188:2285–2289. doi: 10.1128/JB.188.6.2285-2289.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Santos-Escobar F, Gutiérrez-Corona JF, Pedraza-Reyes M. 2014. Role of Bacillus subtilis error prevention oxidized guanine system in counteracting hexavalent chromium-promoted oxidative DNA damage. Appl Environ Microbiol 80:5493–5502. doi: 10.1128/AEM.01665-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Hosfield DJ, Guan Y, Haas BJ, Cunningham RP, Tainer JA. 1999. Structure of the DNA repair enzyme endonuclease IV and its DNA complex: double-nucleotide flipping at abasic sites and three-metal-ion catalysis. Cell 98:397–408. doi: 10.1016/s0092-8674(00)81968-6 [DOI] [PubMed] [Google Scholar]
- 135. Chatterjee N, Walker GC. 2017. Mechanisms of DNA damage, repair, and mutagenesis. Environ Mol Mutagen 58:235–263. doi: 10.1002/em.22087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Barajas-Ornelas R del C, Ramírez-Guadiana FH, Juárez-Godínez R, Ayala-García VM, Robleto EA, Yasbin RE, Pedraza-Reyes M. 2014. Error-prone processing of apurinic/apyrimidinic (AP) sites by polX underlies a novel mechanism that promotes adaptive mutagenesis in Bacillus subtilis. J Bacteriol 196:3012–3022. doi: 10.1128/JB.01681-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Holmquist GP. 2002. Cell-selfish modes of evolution and mutations directed after transcriptional bypass. Mutat Res 510:141–152. doi: 10.1016/s0027-5107(02)00259-2 [DOI] [PubMed] [Google Scholar]
- 138. Jinks-Robertson S, Bhagwat AS. 2014. Transcription-associated mutagenesis. Annu Rev Genet 48:341–359. doi: 10.1146/annurev-genet-120213-092015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Morreall JF, Petrova L, Doetsch PW. 2013. Transcriptional mutagenesis and its potential roles in the etiology of cancer and bacterial antibiotic resistance. J Cell Physiol 228:2257–2261. doi: 10.1002/jcp.24400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Robleto E, Martin HA, Vallin C, Pedraza-Reyes M, Yasbin R. 2013. Transcription-mediated mutagenic processes. In Mittelman D (ed), Stress-induced mutagenesis. Springer, New York. [Google Scholar]
- 141. Saxowsky TT, Doetsch PW. 2006. RNA polymerase encounters with DNA damage: transcription-coupled repair or transcriptional mutagenesis? Chem Rev 106:474–488. doi: 10.1021/cr040466q [DOI] [PubMed] [Google Scholar]
- 142. Bridges BA. 1997. DNA turnover and mutation in resting cells. Bioessays 19:347–352. doi: 10.1002/bies.950190412 [DOI] [PubMed] [Google Scholar]
- 143. Doherty GP, Meredith DH, Lewis PJ. 2006. Subcellular partitioning of transcription factors in Bacillus subtilis. J Bacteriol 188:4101–4110. doi: 10.1128/JB.01934-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Martin HA, Pedraza-Reyes M, Yasbin RE, Robleto EA. 2011. Transcriptional de-repression and Mfd are mutagenic in stressed Bacillus subtilis cells. J Mol Microbiol Biotechnol 21:45–58. doi: 10.1159/000332751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Witkin EM. 1966. Radiation-induced mutations and their repair. Science 152:1345–1353. doi: 10.1126/science.152.3727.1345 [DOI] [PubMed] [Google Scholar]
- 146. Selby CP, Sancar A. 1993. Molecular mechanism of transcription-repair coupling. Science 260:53–58. doi: 10.1126/science.8465200 [DOI] [PubMed] [Google Scholar]
- 147. Savery NJ. 2007. The molecular mechanism of transcription-coupled DNA repair. Trends Microbiol 15:326–333. doi: 10.1016/j.tim.2007.05.005 [DOI] [PubMed] [Google Scholar]
- 148. Hanawalt PC, Spivak G. 2008. Transcription-coupled DNA repair: two decades of progress and surprises. Nat Rev Mol Cell Biol 9:958–970. doi: 10.1038/nrm2549 [DOI] [PubMed] [Google Scholar]
- 149. Deaconescu AM, Chambers AL, Smith AJ, Nickels BE, Hochschild A, Savery NJ, Darst SA. 2006. Structural basis for bacterial transcription-coupled DNA repair. Cell 124:507–520. doi: 10.1016/j.cell.2005.11.045 [DOI] [PubMed] [Google Scholar]
- 150. Pani B, Nudler E. 2017. Mechanistic insights into transcription coupled DNA repair. DNA Repair (Amst) 56:42–50. doi: 10.1016/j.dnarep.2017.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Ayora S, Rojo F, Ogasawara N, Nakai S, Alonso JC. 1996. The Mfd protein of Bacillus subtilis 168 is involved in both transcription-coupled DNA repair and DNA recombination. J Mol Biol 256:301–318. doi: 10.1006/jmbi.1996.0087 [DOI] [PubMed] [Google Scholar]
- 152. Zalieckas JM, Wray LV, Ferson AE, Fisher SH. 1998. Transcription-repair coupling factor is involved in carbon catabolite repression of the Bacillus subtilis hut and gnt operons. Mol Microbiol 27:1031–1038. doi: 10.1046/j.1365-2958.1998.00751.x [DOI] [PubMed] [Google Scholar]
- 153. Martin HA, Sundararajan A, Ermi TS, Heron R, Gonzales J, Lee K, Anguiano-Mendez D, Schilkey F, Pedraza-Reyes M, Robleto EA. 2021. Mfd affects global transcription and the physiology of stressed Bacillus subtilis cells. Front Microbiol 12:625705. doi: 10.3389/fmicb.2021.625705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Hochschild A. 2007. Gene-specific regulation by a transcript cleavage factor: facilitating promoter escape. J Bacteriol 189:8769–8771. doi: 10.1128/JB.01611-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Koulich D, Nikiforov V, Borukhov S. 1998. Distinct functions of N and C-terminal domains of GreA, an Escherichia coli transcript cleavage factor. J Mol Biol 276:379–389. doi: 10.1006/jmbi.1997.1545 [DOI] [PubMed] [Google Scholar]
- 156. Stebbins CE, Borukhov S, Orlova M, Polyakov A, Goldfarb A, Darst SA. 1995. Crystal structure of the GreA transcript cleavage factor from Escherichia coli. Nature 373:636–640. doi: 10.1038/373636a0 [DOI] [PubMed] [Google Scholar]
- 157. Kusuya Y, Kurokawa K, Ishikawa S, Ogasawara N, Oshima T. 2011. Transcription factor GreA contributes to resolving promoter-proximal pausing of RNA polymerase in Bacillus subtilis cells. J Bacteriol 193:3090–3099. doi: 10.1128/JB.00086-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Cohen SE, Walker GC. 2010. The transcription elongation factor NusA is required for stress-induced mutagenesis in Escherichia coli. Curr Biol 20:80–85. doi: 10.1016/j.cub.2009.11.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Cohen SE, Godoy VG, Walker GC. 2009. Transcriptional modulator nusA interacts with translesion DNA polymerases in Escherichia coli. J Bacteriol 191:665–672. doi: 10.1128/JB.00941-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Mandell ZF, Oshiro RT, Yakhnin AV, Vishwakarma R, Kashlev M, Kearns DB, Babitzke P. 2021. Nusg is an intrinsic transcription termination factor that stimulates motility and coordinates gene expression with nusa. Elife 10:e61880. doi: 10.7554/eLife.61880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Strauß M, Vitiello C, Schweimer K, Gottesman M, Rösch P, Knauer SH. 2016. Transcription is regulated by NusA:NusG interaction. Nucleic Acids Res 44:5971–5982. doi: 10.1093/nar/gkw423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Jayasinghe OT, Mandell ZF, Yakhnin AV, Kashlev M, Babitzke P. 2022. Transcriptome-wide effects of NusA on RNA polymerase pausing in Bacillus subtilis. J Bacteriol 204:e0053421. doi: 10.1128/jb.00534-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Bonilla CY. 2020. Generally stressed out bacteria: environmental stress response mechanisms in Gram-positive bacteria. Integr Comp Biol 60:126–133. doi: 10.1093/icb/icaa002 [DOI] [PubMed] [Google Scholar]
- 164. Camilli A, Bassler BL. 2006. Bacterial small-molecule signaling pathways. Science 311:1113–1116. doi: 10.1126/science.1121357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Kundra S, Colomer-Winter C, Lemos JA. 2020. Survival of the fittest: the relationship of (p)ppGpp with bacterial virulence. Front Microbiol 11:601417. doi: 10.3389/fmicb.2020.601417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Gallant J, Palmer L, Pao CC. 1977. Anomalous synthesis of ppGpp in growing cells. Cell 11:181–185. doi: 10.1016/0092-8674(77)90329-4 [DOI] [PubMed] [Google Scholar]
- 167. Glass TL, Holmes WM, Hylemon PB, Stellwag EJ. 1979. Synthesis of guanosine tetra- and pentaphosphates by the obligately anaerobic bacterium Bacteroides thetaiotaomicron in response to molecular oxygen. J Bacteriol 137:956–962. doi: 10.1128/jb.137.2.956-962.1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Wells DH, Gaynor EC. 2006. Helicobacter pylori initiates the stringent response upon nutrient and pH downshift. J Bacteriol 188:3726–3729. doi: 10.1128/JB.188.10.3726-3729.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Kanjee U, Ogata K, Houry WA. 2012. Direct binding targets of the stringent response alarmone (p)ppGpp. Mol Microbiol 85:1029–1043. doi: 10.1111/j.1365-2958.2012.08177.x [DOI] [PubMed] [Google Scholar]
- 170. Hobbs JK, Boraston AB. 2019. (P)ppGpp and the stringent response: an emerging threat to antibiotic therapy. ACS Infect Dis 5:1505–1517. doi: 10.1021/acsinfecdis.9b00204 [DOI] [PubMed] [Google Scholar]
- 171. Schäfer H, Beckert B, Frese CK, Steinchen W, Nuss AM, Beckstette M, Hantke I, Driller K, Sudzinová P, Krásný L, Kaever V, Dersch P, Bange G, Wilson DN, Turgay K. 2020. The alarmones (p)ppGpp are part of the heat shock response of Bacillus subtilis. PLoS Genet 16:e1008275. doi: 10.1371/journal.pgen.1008275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Atkinson GC, Tenson T, Hauryliuk V. 2011. The RelA/SpoT homolog (RSH) superfamily: distribution and functional evolution of ppGpp synthetases and hydrolases across the tree of life. PLoS ONE 6:e23479. doi: 10.1371/journal.pone.0023479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Mittenhuber G. 2001. Comparative genomics and evolution of genes encoding bacterial (p)ppGpp synthetases/hydrolases (the Rel, RelA and SpoT proteins). J Mol Microbiol Biotechnol 3:585–600. [PubMed] [Google Scholar]
- 174. Keasling JD, Bertsch L, Kornberg A. 1993. Guanosine pentaphosphate phosphohydrolase of Escherichia coli is a long-chain exopolyphosphatase. Proc Natl Acad Sci USA 90:7029–7033. doi: 10.1073/pnas.90.15.7029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Xiao H, Kalman M, Ikehara K, Zemel S, Glaser G, Cashel M. 1991. Residual guanosine 3’,5’-bispyrophosphate synthetic activity of relA null mutants can be eliminated by spoT null mutations. J Biol Chem 266:5980–5990. doi: 10.1016/S0021-9258(19)67694-5 [DOI] [PubMed] [Google Scholar]
- 176. Whiteley AT, Pollock AJ, Portnoy DA. 2015. The PAMP c-di-AMP is essential for Listeria monocytogenes growth in rich but not minimal media due to a toxic increase in (p)ppGpp. Cell Host Microbe 17:788–798. doi: 10.1016/j.chom.2015.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Wright BE. 1996. The effect of the stringent response on mutation rates in Escherichia coli K‐12. Mol Microbiol 19:213–219. doi: 10.1046/j.1365-2958.1996.367892.x [DOI] [PubMed] [Google Scholar]
- 178. Wright BE, Minnick MF. 1997. Reversion rates in a leuB auxotroph of Escherichia coli K-12 correlate with ppGpp levels during exponential growth. Microbiology (Reading) 143:847–854. doi: 10.1099/00221287-143-3-847 [DOI] [PubMed] [Google Scholar]
- 179. Wright BE, Longacre A, Reimers JM. 1999. Hypermutation in derepressed operons of Escherichia coli K12. Proc Natl Acad Sci USA 96:5089–5094. doi: 10.1073/pnas.96.9.5089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Wright BE. 1997. Does selective gene activation direct evolution? FEBS Lett 402:4–8. doi: 10.1016/s0014-5793(96)01479-2 [DOI] [PubMed] [Google Scholar]
- 181. Rudner R, Murray A, Huda N. 1999. Is there a link between mutation rates and the stringent response in Bacillus subtilis? Ann N Y Acad Sci 870:418–422. doi: 10.1111/j.1749-6632.1999.tb08917.x [DOI] [PubMed] [Google Scholar]
- 182. Barker MM, Gaal T, Josaitis CA, Gourse RL. 2001. Mechanism of regulation of transcription initiation by ppGpp. I. Effects of ppGpp on transcription initiation in vivo and in vitro. J Mol Biol 305:673–688. doi: 10.1006/jmbi.2000.4327 [DOI] [PubMed] [Google Scholar]
- 183. Handke LD, Shivers RP, Sonenshein AL. 2008. Interaction of Bacillus subtilis CodY with GTP. J Bacteriol 190:798–806. doi: 10.1128/JB.01115-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Kriel A, Bittner AN, Kim SH, Liu K, Tehranchi AK, Zou WY, Rendon S, Chen R, Tu BP, Wang JD. 2012. Direct regulation of GTP homeostasis by (p)ppGpp: a critical component of viability and stress resistance. Mol Cell 48:231–241. doi: 10.1016/j.molcel.2012.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Gourse RL, Keck JL. 2007. Magic spots cast a spell on DNA primase. Cell 128:823–824. doi: 10.1016/j.cell.2007.02.020 [DOI] [PubMed] [Google Scholar]
- 186. Krásný L, Gourse RL. 2004. An alternative strategy for bacterial ribosome synthesis: Bacillus subtilis rRNA transcription regulation. EMBO J 23:4473–4483. doi: 10.1038/sj.emboj.7600423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Wang JD, Sanders GM, Grossman AD. 2007. Nutritional control of elongation of DNA replication by (p)ppGpp. Cell 128:865–875. doi: 10.1016/j.cell.2006.12.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Chatterji D, Ojha AK. 2001. Revisiting the stringent response, ppGpp and starvation signaling. Curr Opin Microbiol 4:160–165. doi: 10.1016/s1369-5274(00)00182-x [DOI] [PubMed] [Google Scholar]
- 189. Jain V, Kumar M, Chatterji D. 2006. ppGpp: stringent response and survival. J Microbiol 44:1–10. [PubMed] [Google Scholar]
- 190. Eymann C, Homuth G, Scharf C, Hecker M. 2002. Bacillus subtilis functional genomics: global characterization of the stringent response by proteome and transcriptome analysis. J Bacteriol 184:2500–2520. doi: 10.1128/JB.184.9.2500-2520.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Kuroda A. 2006. A polyphosphate-lon protease complex in the adaptation of Escherichia coli to amino acid starvation. Biosci Biotechnol Biochem 70:325–331. doi: 10.1271/bbb.70.325 [DOI] [PubMed] [Google Scholar]
- 192. Reimers JM, Schmidt KH, Longacre A, Reschke DK, Wright BE. 2004. Increased transcription rates correlate with increased reversion rates in leuB and argH Escherichia coli auxotrophs. Microbiology (Reading) 150:1457–1466. doi: 10.1099/mic.0.26954-0 [DOI] [PubMed] [Google Scholar]
- 193. Castro-Cerritos KV, Yasbin RE, Robleto EA, Pedraza-Reyes M. 2017. Role of ribonucleotide reductase in Bacillus subtilis stress-associated mutagenesis. J Bacteriol 199:e00715-16. doi: 10.1128/JB.00715-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Castro-Cerritos KV, Lopez-Torres A, Obregón-Herrera A, Wrobel K, Wrobel K, Pedraza-Reyes M. 2018. LC–MS/MS proteomic analysis of starved Bacillus subtilis cells overexpressing ribonucleotide reductase (nrdEF): implications in stress-associated mutagenesis. Curr Genet 64:215–222. doi: 10.1007/s00294-017-0722-7 [DOI] [PubMed] [Google Scholar]
- 195. Molle V, Fujita M, Jensen ST, Eichenberger P, González-Pastor JE, Liu JS, Losick R. 2003. The Spo0A regulon of Bacillus subtilis. Mol Microbiol 50:1683–1701. doi: 10.1046/j.1365-2958.2003.03818.x [DOI] [PubMed] [Google Scholar]
- 196. Ratnayake-Lecamwasam M, Serror P, Wong K-W, Sonenshein AL. 2001. Bacillus subtilis CodY represses early-stationary-phase genes by sensing GTP levels. Genes Dev 15:1093–1103. doi: 10.1101/gad.874201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Geiger T, Wolz C. 2014. Intersection of the stringent response and the CodY regulon in low GC Gram-positive bacteria. Int J Med Microbiol 304:150–155. doi: 10.1016/j.ijmm.2013.11.013 [DOI] [PubMed] [Google Scholar]
- 198. Chambert R, Pereira Y, Petit-Glatron M-F. 2003. Purification and characterization of YfkN, a trifunctional nucleotide phosphoesterase secreted by Bacillus subtilis. J Biochem 134:655–660. doi: 10.1093/jb/mvg189 [DOI] [PubMed] [Google Scholar]
- 199. Mehne FMP, Gunka K, Eilers H, Herzberg C, Kaever V, Stülke J. 2013. Cyclic di-AMP homeostasis in Bacillus subtilis. J Biol Chem 288:2004–2017. doi: 10.1074/jbc.M112.395491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Gundlach J, Mehne FMP, Herzberg C, Kampf J, Valerius O, Kaever V, Stülke J. 2015. An essential poison: synthesis and degradation of cyclic di-AMP in Bacillus subtilis. J Bacteriol 197:3265–3274. doi: 10.1128/JB.00564-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Sureka K, Choi PH, Precit M, Delince M, Pensinger DA, Huynh TAN, Jurado AR, Goo YA, Sadilek M, Iavarone AT, Sauer JD, Tong L, Woodward JJ. 2014. The cyclic dinucleotide c-di-AMP is an allosteric regulator of metabolic enzyme function. Cell 158:1389–1401. doi: 10.1016/j.cell.2014.07.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Kolberg M, Strand KR, Graff P, Kristoffer Andersson K. 2004. Structure, function, and mechanism of ribonucleotide reductases. Biochim Biophys Acta Proteins Proteom 1699:1–34. doi: 10.1016/S1570-9639(04)00054-8 [DOI] [PubMed] [Google Scholar]
- 203. Mudgal S, Manikandan K, Mukherjee A, Krishnan A, Sinha KM. 2021. Cyclic di-AMP: small molecule with big roles in bacteria. Microb Pathog 161:105264. doi: 10.1016/j.micpath.2021.105264 [DOI] [PubMed] [Google Scholar]
- 204. Pal AK, Ghosh A. 2022. c-di-AMP signaling plays important role in determining antibiotic tolerance phenotypes of Mycobacterium smegmatis. Sci Rep 12:13127. doi: 10.1038/s41598-022-17051-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Potrykus K, Cashel M. 2008. (p)ppGpp: still magical? Annu Rev Microbiol 62:35–51. doi: 10.1146/annurev.micro.62.081307.162903 [DOI] [PubMed] [Google Scholar]
- 206. Geiger T, Goerke C, Fritz M, Schäfer T, Ohlsen K, Liebeke M, Lalk M, Wolz C. 2010. Role of the (p)ppGpp synthase RSH, a RelA/SpoT homolog, in stringent response and virulence of Staphylococcus aureus. Infect Immun 78:1873–1883. doi: 10.1128/IAI.01439-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Huynh TN, Luo S, Pensinger D, Sauer J-D, Tong L, Woodward JJ. 2015. An HD-domain phosphodiesterase mediates cooperative hydrolysis of c-di-AMP to affect bacterial growth and virulence. Proc Natl Acad Sci USA 112:E747–E756. doi: 10.1073/pnas.1416485112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Corrigan RM, Bowman L, Willis AR, Kaever V, Gründling A. 2015. Cross-talk between two nucleotide-signaling pathways in Staphylococcus aureus. J Biol Chem 290:5826–5839. doi: 10.1074/jbc.M114.598300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Krüger L, Herzberg C, Wicke D, Bähre H, Heidemann JL, Dickmanns A, Schmitt K, Ficner R, Stülke J. 2021. A meet-up of two second messengers: the c-di-AMP receptor DarB controls (p)ppGpp synthesis in Bacillus subtilis. Nat Commun 12:1210. doi: 10.1038/s41467-021-21306-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Peterson BN, Young MKM, Luo S, Wang J, Whiteley AT, Woodward JJ, Tong L, Wang JD, Portnoy DA. 2020. (p)ppGpp and c-di-AMP homeostasis is controlled by CbpB in Listeria monocytogenes. mBio 11:e01625-20. doi: 10.1128/mBio.01625-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Heidemann JL, Neumann P, Krüger L, Wicke D, Vinhoven L, Linden A, Dickmanns A, Stülke J, Urlaub H, Ficner R. 2022. Structural basis for c-di-AMP–dependent regulation of the bacterial stringent response by receptor protein DarB. J Biol Chem 298:102144. doi: 10.1016/j.jbc.2022.102144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Nanamiya H, Kasai K, Nozawa A, Yun C-S, Narisawa T, Murakami K, Natori Y, Kawamura F, Tozawa Y. 2008. Identification and functional analysis of novel (p)ppGpp synthetase genes in Bacillus subtilis. Mol Microbiol 67:291–304. doi: 10.1111/j.1365-2958.2007.06018.x [DOI] [PubMed] [Google Scholar]
- 213. Errington J. 2003. Regulation of endospore formation in Bacillus subtilis. Nat Rev Microbiol 1:117–126. doi: 10.1038/nrmicro750 [DOI] [PubMed] [Google Scholar]
- 214. Piggot PJ, Hilbert DW. 2004. Sporulation of Bacillus subtilis. Curr Opin Microbiol 7:579–586. doi: 10.1016/j.mib.2004.10.001 [DOI] [PubMed] [Google Scholar]
- 215. Mandelstam J, Higgs SA. 1974. Induction of sporulation during synchronized chromosome replication in Bacillus subtilis. J Bacteriol 120:38–42. doi: 10.1128/jb.120.1.38-42.1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Ireton K, Grossman AD. 1994. A developmental checkpoint couples the initiation of sporulation to DNA replication in Bacillus subtilis. EMBO J 13:1566–1573. doi: 10.1002/j.1460-2075.1994.tb06419.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Burkholder WF, Kurtser I, Grossman AD. 2001. Replication initiation proteins regulate a developmental checkpoint in Bacillus subtilis. Cell 104:269–279. doi: 10.1016/s0092-8674(01)00211-2 [DOI] [PubMed] [Google Scholar]
- 218. Michael WM. 2001. Cell cycle: connecting DNA replication to sporulation in Bacillus. Curr Biol 11:R443–R445. doi: 10.1016/s0960-9822(01)00256-1 [DOI] [PubMed] [Google Scholar]
- 219. Veening JW, Murray H, Errington J. 2009. A mechanism for cell cycle regulation of sporulation initiation in Bacillus subtilis. Genes Dev 23:1959–1970. doi: 10.1101/gad.528209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Hoch JA. 2014. Control of cellular development in sporulating bacteria by the phosphorelay two-component signal transduction system. In Two-component signal transduction [Google Scholar]
- 221. Hoch JA. 2000. Two-component and phosphorelay signal transduction. Curr Opin Microbiol 3:165–170. doi: 10.1016/s1369-5274(00)00070-9 [DOI] [PubMed] [Google Scholar]
- 222. de Jong IG, Veening J-W, Kuipers OP. 2010. Heterochronic phosphorelay gene expression as a source of heterogeneity in Bacillus subtilis spore formation. J Bacteriol 192:2053–2067. doi: 10.1128/JB.01484-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. LeDeaux JR, Yu N, Grossman AD. 1995. Different roles for KinA, KinB, and KinC in the initiation of sporulation in Bacillus subtilis. J Bacteriol 177:861–863. doi: 10.1128/jb.177.3.861-863.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Kobayashi K, Shoji K, Shimizu T, Nakano K, Sato T, Kobayashi Y. 1995. Analysis of a suppressor mutation ssb (kinC) of sur0B20 (spo0A) mutation in Bacillus subtilis reveals that kinC encodes a histidine protein kinase. J Bacteriol 177:176–182. doi: 10.1128/jb.177.1.176-182.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. LeDeaux JR, Grossman AD. 1995. Isolation and characterization of kinC, a gene that encodes a sensor kinase homologous to the sporulation sensor kinases KinA and KinB in Bacillus subtilis. J Bacteriol 177:166–175. doi: 10.1128/jb.177.1.166-175.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Jiang M, Shao W, Perego M, Hoch JA. 2000. Multiple histidine kinases regulate entry into stationary phase and sporulation in Bacillus subtilis. Mol Microbiol 38:535–542. doi: 10.1046/j.1365-2958.2000.02148.x [DOI] [PubMed] [Google Scholar]
- 227. Dutta R, Qin L, Inouye M. 1999. Histidine kinases: diversity of domain organization. Mol Microbiol 34:633–640. doi: 10.1046/j.1365-2958.1999.01646.x [DOI] [PubMed] [Google Scholar]
- 228. Robinson VL, Buckler DR, Stock AM. 2000. A tale of two components: a novel kinase and a regulatory switch. Nat Struct Biol 7:626–633. doi: 10.1038/77915 [DOI] [PubMed] [Google Scholar]
- 229. Burbulys D, Trach KA, Hoch JA. 1991. Initiation of sporulation in B. subtilis is controlled by a multicomponent phosphorelay. Cell 64:545–552. doi: 10.1016/0092-8674(91)90238-t [DOI] [PubMed] [Google Scholar]
- 230. Burkholder WF, Grossman AD. 2014. Regulation of the initiation of endospore formation in Bacillus subtilis. In Prokaryotic development [Google Scholar]
- 231. Sonenshein AL. 2000. Control of sporulation initiation in Bacillus subtilis. Curr Opin Microbiol 3:561–566. doi: 10.1016/s1369-5274(00)00141-7 [DOI] [PubMed] [Google Scholar]
- 232. Fujita M, González-Pastor JE, Losick R. 2005. High- and low-threshold genes in the Spo0A regulon of Bacillus subtilis. J Bacteriol 187:1357–1368. doi: 10.1128/JB.187.4.1357-1368.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Castilla-Llorente V, Muñoz-Espín D, Villar L, Salas M, Meijer WJJ. 2006. Spo0A, the key transcriptional regulator for entrance into sporulation, is an inhibitor of DNA replication. EMBO J 25:3890–3899. doi: 10.1038/sj.emboj.7601266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Boonstra M, de Jong IG, Scholefield G, Murray H, Kuipers OP, Veening J-W. 2013. Spo0A regulates chromosome copy number during sporulation by directly binding to the origin of replication in Bacillus subtilis. Mol Microbiol 87:925–938. doi: 10.1111/mmi.12141 [DOI] [PubMed] [Google Scholar]
- 235. Wagner JK, Marquis KA, Rudner DZ. 2009. SirA enforces diploidy by inhibiting the replication initiator DnaA during spore formation in Bacillus subtilis. Mol Microbiol 73:963–974. doi: 10.1111/j.1365-2958.2009.06825.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Rahn-Lee L, Gorbatyuk B, Skovgaard O, Losick R. 2009. The conserved sporulation protein YneE inhibits DNA replication in Bacillus subtilis. J Bacteriol 191:3736–3739. doi: 10.1128/JB.00216-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Rowland SL, Burkholder WF, Cunningham KA, Maciejewski MW, Grossman AD, King GF. 2004. Structure and mechanism of action of Sda, an inhibitor of the histidine kinases that regulate initiation of sporulation in Bacillus subtilis. Mol Cell 13:689–701. doi: 10.1016/s1097-2765(04)00084-x [DOI] [PubMed] [Google Scholar]
- 238. Ramírez-Guadiana FH, Del Carmen Barajas-Ornelas R, Ayala-García VM, Yasbin RE, Robleto E, Pedraza-Reyes M. 2013. Transcriptional coupling of DNA repair in sporulating Bacillus subtilis cells. Mol Microbiol 90:1088–1099. doi: 10.1111/mmi.12417 [DOI] [PubMed] [Google Scholar]
- 239. Bejerano-Sagie M, Oppenheimer-Shaanan Y, Berlatzky I, Rouvinski A, Meyerovich M, Ben-Yehuda S. 2006. A checkpoint protein that scans the chromosome for damage at the start of sporulation in Bacillus subtilis. Cell 125:679–690. doi: 10.1016/j.cell.2006.03.039 [DOI] [PubMed] [Google Scholar]
- 240. Oppenheimer-Shaanan Y, Wexselblatt E, Katzhendler J, Yavin E, Ben-Yehuda S. 2011. C-di-AMP reports DNA integrity during sporulation in Bacillus subtilis. EMBO Rep 12:594–601. doi: 10.1038/embor.2011.77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Hanlin JH, Lombardi SJ, Slepecky RA. 1985. Heat and UV light resistance of vegetative cells and spores of Bacillus subtilis Rec- mutants. J Bacteriol 163:774–777. doi: 10.1128/jb.163.2.774-777.1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Setlow P. 2006. Spores of Bacillus subtilis: their resistance to and killing by radiation, heat and chemicals. J Appl Microbiol 101:514–525. doi: 10.1111/j.1365-2672.2005.02736.x [DOI] [PubMed] [Google Scholar]
- 243. Vlašić I, Mertens R, Seco EM, Carrasco B, Ayora S, Reitz G, Commichau FM, Alonso JC, Moeller R. 2014. Bacillus subtilis RecA and its accessory factors, RecF, RecO, RecR and RecX, are required for spore resistance to DNA double-strand break. Nucleic Acids Res 42:2295–2307. doi: 10.1093/nar/gkt1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Sciochetti SA, Blakely GW, Piggot PJ. 2001. Growth phase variation in cell and nucleoid morphology in a Bacillus subtilis recA mutant. J Bacteriol 183:2963–2968. doi: 10.1128/JB.183.9.2963-2968.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Bidnenko V, Shi L, Kobir A, Ventroux M, Pigeonneau N, Henry C, Trubuil A, Noirot-Gros MF, Mijakovic I. 2013. Bacillus subtilis serine/threonine protein kinase YabT is involved in spore development via phosphorylation of a bacterial recombinase. Mol Microbiol 88:921–935. doi: 10.1111/mmi.12233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Urtiz-Estrada N, Salas-Pacheco JM, Yasbin RE, Pedraza-Reyes M. 2003. Forespore-specific expression of Bacillus subtilis yqfS, which encodes type IV apurinic/apyrimidinic endonuclease, a component of the base excision repair pathway. J Bacteriol 185:340–348. doi: 10.1128/JB.185.1.340-348.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Salas-Pacheco JM, Urtiz-Estrada N, Martínez-Cadena G, Yasbin RE, Pedraza-Reyes M. 2003. YqfS from Bacillus subtilis is a spore protein and a new functional member of the type IV apurinic/apyrimidinic-endonuclease family. J Bacteriol 185:5380–5390. doi: 10.1128/JB.185.18.5380-5390.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Salas-Pacheco JM, Setlow B, Setlow P, Pedraza-Reyes M. 2005. Role of the Nfo (YqfS) and ExoA apurinic/apyrimidinic endonucleases in protecting Bacillus subtilis spores from DNA damage. J Bacteriol 187:7374–7381. doi: 10.1128/JB.187.21.7374-7381.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Ramírez-Guadiana FH, Barraza-Salas M, Ramírez-Ramírez N, Ortiz-Cortés M, Setlow P, Pedraza-Reyes M. 2012. Alternative excision repair of ultraviolet B- and C-Induced DNA damage in dormant and developing spores of Bacillus subtilis. J Bacteriol 194:6096–6104. doi: 10.1128/JB.01340-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Rivas-Castillo AM, Yasbin RE, Robleto E, Nicholson WL, Pedraza-Reyes M. 2010. Role of the y-family DNA polymerases YqjH and YqjW in protecting sporulating Bacillus subtilis cells from DNA damage. Curr Microbiol 60:263–267. doi: 10.1007/s00284-009-9535-3 [DOI] [PubMed] [Google Scholar]
- 251. Setlow P. 2007. I will survive: DNA protection in bacterial spores. Trends Microbiol 15:172–180. doi: 10.1016/j.tim.2007.02.004 [DOI] [PubMed] [Google Scholar]
- 252. Setlow P. 2014. Spore resistance properties. Microbiol Spectr 2. doi: 10.1128/microbiolspec.TBS-0003-2012 [DOI] [PubMed] [Google Scholar]
- 253. Setlow P. 1995. Mechanisms for the prevention of damage to DNA in spores of Bacillus species. Annu Rev Microbiol 49:29–54. doi: 10.1146/annurev.mi.49.100195.000333 [DOI] [PubMed] [Google Scholar]
- 254. Bagyan I, Casillas-Martinez L, Setlow P. 1998. The katX gene, which codes for the catalase in spores of Bacillus subtilis, is a forespore-specific gene controlled by σF, and katX is essential for hydrogen peroxide resistance of the germinating spore. J Bacteriol 180:2057–2062. doi: 10.1128/JB.180.8.2057-2062.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Inaoka T, Matsumura Y, Tsuchido T. 1999. SodA and manganese are essential for resistance to oxidative stress in growing and sporulating cells of Bacillus subtilis. J Bacteriol 181:1939–1943. doi: 10.1128/JB.181.6.1939-1943.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Kuwana R, Kasahara Y, Fujibayashi M, Takamatsu H, Ogasawara N, Watabe K. 2002. Proteomics characterization of novel spore proteins of Bacillus subtilis. Microbiology (Reading) 148:3971–3982. doi: 10.1099/00221287-148-12-3971 [DOI] [PubMed] [Google Scholar]
- 257. Pedraza-Reyes M, Gutiérrez-Corona F, Nicholson WL. 1997. Spore photoproduct lyase operon (splAB) regulation during Bacillus subtilis sporulation: modulation of splB-lacZ fusion expression by P1 promoter mutations and by an in-frame deletion of splA. Curr Microbiol 34:133–137. doi: 10.1007/s002849900157 [DOI] [PubMed] [Google Scholar]
- 258. Moir A, Cooper G. 2015. Spore germination. Microbiol Spectr 3. doi: 10.1128/microbiolspec.TBS-0014-2012 [DOI] [PubMed] [Google Scholar]
- 259. Keijser BJF, Ter Beek A, Rauwerda H, Schuren F, Montijn R, van der Spek H, Brul S. 2007. Analysis of temporal gene expression during Bacillus subtilis spore germination and outgrowth. J Bacteriol 189:3624–3634. doi: 10.1128/JB.01736-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Setlow P, Wang S, Li YQ. 2017. Germination of spores of the orders Bacillales and Clostridiales. Annu Rev Microbiol 71:459–477. doi: 10.1146/annurev-micro-090816-093558 [DOI] [PubMed] [Google Scholar]
- 261. Setlow P. 2003. Spore germination. Curr Opin Microbiol 6:550–556. doi: 10.1016/j.mib.2003.10.001 [DOI] [PubMed] [Google Scholar]
- 262. Fajardo-Cavazos P, Nicholson WL. 1995. Molecular dissection of mutations in the Bacillus subtilis spore photoproduct lyase gene which affect repair of spore DNA damage caused by UV radiation. J Bacteriol 177:4402–4409. doi: 10.1128/jb.177.15.4402-4409.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Nicholson WL, Setlow B, Setlow P. 1990. Binding of DNA in vitro by a small, acid-soluble spore protein from Bacillus subtilis and the effect of this binding on DNA topology. J Bacteriol 172:6900–6906. doi: 10.1128/jb.172.12.6900-6906.1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Nicholson WL, Setlow B, Setlow P. 1991. Ultraviolet irradiation of DNA complexed with α/β-type small, acid-soluble proteins from spores of Bacillus or Clostridium species makes spore photoproduct but not thymine dimers. Proc Natl Acad Sci U S A 88:8288–8292. doi: 10.1073/pnas.88.19.8288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Pedraza-Reyes M, Gutiérrez-Corona F, Nicholson WL. 1994. Temporal regulation and forespore-specific expression of the spore photoproduct lyase gene by sigma-G RNA polymerase during Bacillus subtilis sporulation. J Bacteriol 176:3983–3991. doi: 10.1128/jb.176.13.3983-3991.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Cortezzo DE, Koziol-Dube K, Setlow B, Setlow P. 2004. Treatment with oxidizing agents damages the inner membrane of spores of Bacillus subtilis and sensitizes spores to subsequent stress. J Appl Microbiol 97:838–852. doi: 10.1111/j.1365-2672.2004.02370.x [DOI] [PubMed] [Google Scholar]
- 267. Setlow B, Tautvydas KJ, Setlow P. 1998. Small, acid-soluble spore proteins of the α/β type do not protect the DNA in Bacillus subtilis spores against base alkylation. Appl Environ Microbiol 64:1958–1962. doi: 10.1128/AEM.64.5.1958-1962.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Setlow P, Christie G. 2021. What’s new and notable in bacterial spore killing! World J Microbiol Biotechnol 37:144. doi: 10.1007/s11274-021-03108-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Moeller R, Setlow P, Pedraza-Reyes M, Okayasu R, Reitz G, Nicholson WL. 2011. Role of the Nfo and ExoA apurinic/apyrimidinic endonucleases in radiation resistance and radiation-induced mutagenesis of Bacillus subtilis spores. J Bacteriol 193:2875–2879. doi: 10.1128/JB.00134-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Moeller R, Raguse M, Reitz G, Okayasu R, Li Z, Klein S, Setlow P, Nicholson WL. 2014. Resistance of Bacillus subtilis spore DNA to lethal ionizing radiation damage relies primarily on spore core components and DNA repair, with minor effects of oxygen radical detoxification. Appl Environ Microbiol 80:104–109. doi: 10.1128/AEM.03136-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Moeller R, Stackebrandt E, Reitz G, Berger T, Rettberg P, Doherty AJ, Horneck G, Nicholson WL. 2007. Role of DNA repair by nonhomologous-end joining in Bacillus subtilis spore resistance to extreme dryness, mono- and polychromatic UV, and ionizing radiation. J Bacteriol 189:3306–3311. doi: 10.1128/JB.00018-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Setlow B, Setlow P. 1995. Small, acid-soluble proteins bound to DNA protect Bacillus subtilis spores from killing by dry heat. Appl Environ Microbiol 61:2787–2790. doi: 10.1128/aem.61.7.2787-2790.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Storz G, Imlay JA. 1999. Oxidative stress. Curr Opin Microbiol 2:188–194. doi: 10.1016/s1369-5274(99)80033-2 [DOI] [PubMed] [Google Scholar]
- 274. Riley PA. 1994. Free radicals in biology: oxidative stress and the effects of ionizing radiation. Int J Radiat Biol 65:27–33. doi: 10.1080/09553009414550041 [DOI] [PubMed] [Google Scholar]
- 275. Loeb LA, Preston BD. 1986. Mutagenesis by apurinic/ apyrimidinic sites. Annu Rev Genet 20:201–230. doi: 10.1146/annurev.ge.20.120186.001221 [DOI] [PubMed] [Google Scholar]
- 276. Witte G, Hartung S, Büttner K, Hopfner KP. 2008. Structural biochemistry of a bacterial checkpoint protein reveals diadenylate cyclase activity regulated by DNA recombination intermediates. Mol Cell 30:167–178. doi: 10.1016/j.molcel.2008.02.020 [DOI] [PubMed] [Google Scholar]
- 277. Raguse M, Torres R, Seco EM, Gándara C, Ayora S, Moeller R, Alonso JC. 2017. Bacillus subtilis DisA helps to circumvent replicative stress during spore revival. DNA Repair (Amst) 59:57–68. doi: 10.1016/j.dnarep.2017.09.006 [DOI] [PubMed] [Google Scholar]
- 278. Collier C, Machón C, Briggs GS, Smits WK, Soultanas P. 2012. Untwisting of the DNA helix stimulates the endonuclease activity of Bacillus subtilis Nth at AP sites. Nucleic Acids Res 40:739–750. doi: 10.1093/nar/gkr785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Matsumoto Y, Zhang QM, Takao M, Yasui A, Yonei S. 2001. Escherichia coli Nth and human hNTH1 DNA glycosylases are involved in removal of 8-oxoguanine from 8-oxoguanine/guanine mispairs in DNA. Nucleic Acids Res 29:1975–1981. doi: 10.1093/nar/29.9.1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280. Shapiro R, Pohl SH. 1968. The reaction of ribonucleosides with nitrous acid. Side products and kinetics. Biochemistry 7. doi: 10.1021/bi00841a057 [DOI] [PubMed] [Google Scholar]
- 281. Kalamara M, Spacapan M, Mandic-Mulec I, Stanley-Wall NR. 2018. Social behaviours by Bacillus subtilis: quorum sensing, kin discrimination and beyond. Mol Microbiol 110:863–878. doi: 10.1111/mmi.14127 [DOI] [PMC free article] [PubMed] [Google Scholar]