SUMMARY
A significant increase in the incidence of Candida-mediated infections has been observed in the last decade, mainly due to rising numbers of susceptible individuals. Recently, the World Health Organization published its first fungal pathogen priority list, with Candida species listed in medium, high, and critical priority categories. This review is a synthesis of information and recent advances in our understanding of two of these species—Candida albicans and Candida glabrata. Of these, C. albicans is the most common cause of candidemia around the world and is categorized as a critical priority pathogen. C. glabrata is considered a high-priority pathogen and has become an increasingly important cause of candidemia in recent years. It is now the second most common causative agent of candidemia in many geographical regions. Despite their differences and phylogenetic divergence, they are successful as pathogens and commensals of humans. Both species can cause a broad variety of infections, ranging from superficial to potentially lethal systemic infections. While they share similarities in certain infection strategies, including tissue adhesion and invasion, they differ significantly in key aspects of their biology, interaction with immune cells, host damage strategies, and metabolic adaptations. Here we provide insights on key aspects of their biology, epidemiology, commensal and pathogenic lifestyles, interactions with the immune system, and antifungal resistance.
KEYWORDS: Candida albicans, Candida glabrata, Nakaseomyces glabratus, WHO fungal priority list, epidemiology, pathogenicity, host response, commensalism, antifungal resistance, diagnostics
INTRODUCTION
The World Health Organization (WHO) recently announced its first ranking of priority groups for fungal pathogens based primarily on “concerns over drug resistance and/or treatment management” (https://www.who.int/publications/i/item/9789240060241). This WHO report stresses the threat fungal pathogens pose to public health, especially to immunocompromised patients, with a growing resistance to treatment and a limited number of classes of available antifungal drugs. Of the 19 fungal species in the report, Candida albicans was listed along with Candida auris among the four “critical priority pathogens,” and Candida glabrata was categorized among seven “high-priority pathogens” (along with Candida tropicalis and Candida parapsilosis). C. glabrata is a very distant phylogenetic relative of C. albicans and has been reclassified and renamed within the new Nakaseomyces genus, along with three sister species, and is now called Nakaseomyces glabratus (1). There has been some opposition to reclassifying C. glabrata to N. glabratus on the basis that it may “dilute the importance of Candida as a major human group of pathogens” and that “it engenders uncertainty, difficulties in messaging and hampers advocacy” (2). On the other hand, it has been pointed out that the phylogenetic distance between N. glabratus and C. albicans is double that of humans to snakes. This distance is reflected in divergences in multiple phenotypes including susceptibility to fluconazole and other aspects of pathobiology (summarized in detail in this review). Therefore, it may be better to clearly differentiate these two organisms than confuse them as broadly similar species of yeast within the same genus (1, 3). For the purpose of this review, we will retain the use of C. glabrata to be consistent with the relevant cited literature while recognizing that we are in a period of phylogenetic revision that will see C. glabrata transitioning to a new name that reflects its true phylogeny. Despite the evolutionary distance between C. albicans and C. glabrata, there are some shared characteristics and pathologies, and this review focuses on a comparison of the biology and pathogenesis of these two pathogens.
About 30 species that have previously been assigned within the Candida genus can cause human disease. Of these, C. albicans and C. glabrata, together with C. parapsilosis and C. tropicalis, represent the most common causes of invasive disease. The WHO emphasizes the need for a better understanding of the disease burden and antifungal resistances, and for an improvement of diagnostics and treatments (4).
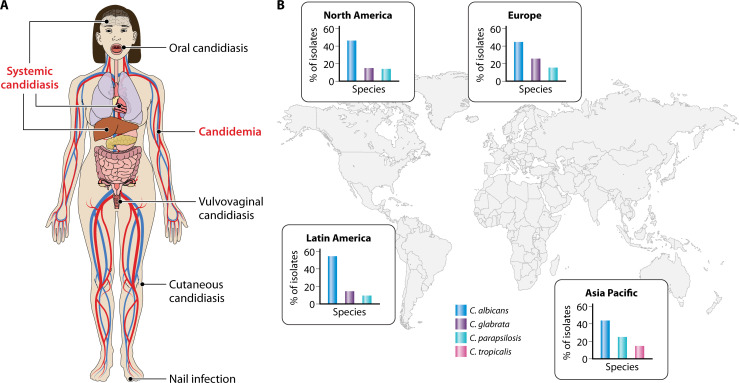
Both C. albicans and C. glabrata cause a range of disease manifestations. Mucosal candidiasis, including vaginitis, is most commonly caused by C. albicans, followed by C. glabrata, and the global burden of recurrent Candida vaginitis (defined as more than four episodes per year) is estimated to be between 103 and 172 million annually (5). The incidence of systemic candidiasis is typically around 2–21 per 100,000 people, with numbers varying considerably, depending on geography and various patient factors (Fig. 1). Candida species normally rank in the top four causes of bloodstream infections, along with Staphylococcus aureus, coagulase-negative staphylococci, and Enterococcus spp. (6, 7). Associated mortality due to invasive candidiasis can be 40%–75% in different healthcare settings, accounting for a total of around 250–700,000 systemic infections and 50–100,000 deaths/year (6–9). Typically, C. albicans accounts for around 40%–80% of Candida isolates recovered from patients in hospitals, while C. glabrata represents only about 5%–30% of such isolates, although these figures vary geographically (10–12). However, more recently, C. glabrata isolation rates have increased in a number of settings in different countries to 2%–28% of Candida isolates, perhaps due to the high number of azole- and echinocandin-resistant strains (13).
Fig 1.
Epidemiology and types of Candida infections. (A) Candida species causing superficial (black text) and systemic (red text) infections. Superficial infections affect the skin or mucosal surfaces of the body and are usually not life threatening. The most common superficial infections include vulvovaginal candidiasis and cutaneous candidiasis. Systemic infections can affect multiple organs including the heart, brain, and kidneys and can potentially lead to septic shock. (B) Epidemiology of Candida species based on SENTRY antimicrobial surveillance program from 2008 to 2009. C. albicans is the most prevalent global species, but variability in the prevalence of non-Candida albicans Candida species exists between different geographical regions. Additionally, the distribution of Candida species can differ in specific patient cohorts between countries.
Candida species have long co-existed with humans as commensals and infectious agents. Hippocrates described oral candidiasis (thrush) as early as 200 BC, but the first scientific studies dealing with C. albicans and C. glabrata took place in the late 20th century (14). A mycotic association for vaginal infection was first shown for C. albicans in 1849 and for C. glabrata in 1917 (15). More recently, climate change has been suggested as a factor in the sudden worldwide appearance of C. auris as a pathogen (16). Vaginal infections with C. albicans are extremely common in otherwise healthy women (11), and C. albicans is responsible for the vast majority of these infections. The incidence of invasive infections with Candida species is higher in individuals with impaired immunity, be it due to treatments required for organ transplants, malignancies, or other immunosuppressive regimens. Indeed, there has been an increase in susceptible individuals in modern times due to the development and widespread use of treatments that lead to immunosuppression (17). Other common predisposing risk factors for systemic candidiasis are the use of antibiotics, chronic kidney disease, presence of central venous catheters, blood transfusions, and extended stays in the intensive care unit (ICU) (18, 19). In summary, C. albicans and C. glabrata represent two major agents of superficial and systemic human diseases of global healthcare concern.
Distant cousins with distinct characteristics
The genus Candida comprises approximately 200 taxonomically diverse species with many different lifestyles and morphologies (14). Most species associated with humans are harmless commensals, but at least 30 can cause human infections (20). Five species are responsible for over 90% of infections: C. albicans, C. glabrata, C. parapsilosis, C. tropicalis, and Candida krusei, ranked from the most common to the least, although regional differences exist (17, 21). The most common, C. albicans and C. glabrata, are frequently isolated as commensals from skin surfaces and mucosal surfaces, in particular the gastrointestinal (GI) tract (22).
Even though they share a similar commensal lifestyle, C. albicans and C. glabrata are distinct in many other aspects, summarized here and described in detail below. They are widely divergent phylogenetically. C. glabrata is taxonomically closer to Saccharomyces cerevisiae (baker’s yeast) than to C. albicans. C. albicans, together with other important Candida spp. such as C. parapsilosis and C. tropicalis, is part of the so-called “CTG clade” in which the CTG codon codes for leucine instead of serine (23). Genetically, C. albicans is a diploid fungus (24), although haploid forms have been generated that are stable enough to create haploid mutant libraries (25). C. glabrata is a haploid organism for which no sexual cycle has been described so far (26) (see below). Phenotypically, C. albicans is polymorphic, being able to transition reversibly between yeast, hyphae, and pseudohyphae, which is a key aspect of its pathogenesis (27, 28). In addition, C. albicans can grow as other distinct phenotypic forms including white, gray, opaque, and gastrointestinally induced transition (GUT) cells (see below) (Fig. 2). In contrast, C. glabrata grows almost exclusively in the yeast form and does not depend on morphological changes to promote infection (29, 30). Both Candida spp. are able to form biofilms, although the mechanisms they use for this differ (31, 32). The two fungi share common adhesion strategies reliant on large families of adhesins, e.g., the Als proteins in C. albicans (33) and Epa proteins in C. glabrata (34, 35).
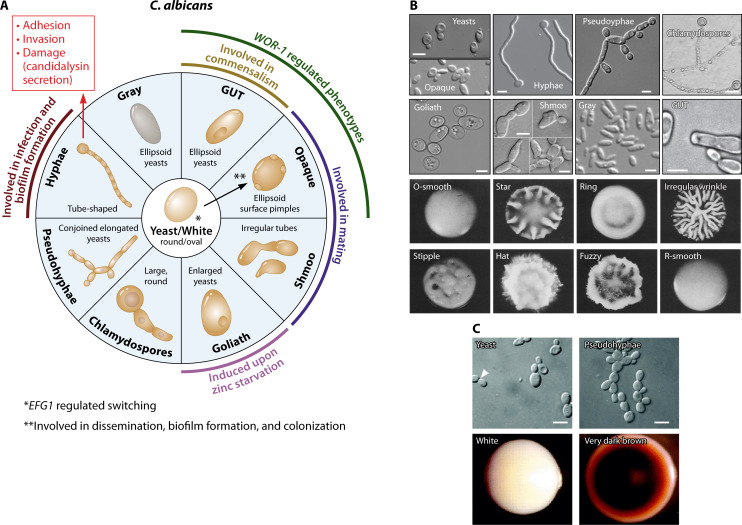
Fig 2.
Morphological plasticity in C. albicans and C. glabrata. (A) Morphological plasticity in C. albicans. Yeast and hyphae are probably the most well-investigated growth forms of C. albicans, with specific roles in commensalism and infection as described in the main text. Pseudohyphae are similarly regularly found in vitro and in vivo, but their role in C. albicans-host interaction remains largely unclear. Opaque and shmoo cells are both involved in mating, while both gray and hyphal cells are associated with different types of infections. Chlamydospores are formed on certain carbohydrate-rich media, and their role in vivo remains unclear. Wor1 and Efg1 are transcriptional regulators of C. albicans morphology, controlling the switch between white (yeast), GUT and opaque cells. (B) Morphotypes of C. albicans. Cell types shown include budding yeast cells, hyphae [reprinted from reference (143) with permission of Springer Nature], elongated yeasts forming pseudohyphae [reprinted from reference (36) with permission of Oxford University Press], chlamydospores formed from suspensor cells [reprinted from reference (37) with permission of John Wiley & Sons], enlarged Goliath cells [reprinted from reference (144) under a Creative Commons license], mating-competent opaque and gray phenotypes [reprinted from reference (179) with permission of Elsevier], elongated chemotactic shmoo-mating projections leading to tetraploid zygote [reprinted from reference (184)], and GUT cells suspected to form in the intestine [reprinted from reference (175) with permission of Springer Nature]. Scale bars represent 5 µm. Colony morphologies of C. albicans, namely, (A) O-smooth, (B) star, (C) ring, (D) irregular wrinkly, (E) stipple, (F) hat, (G) fuzzy, (H) R-smooth [reprinted from reference (166) with permission of AAAS]. (C) Morphotypes of C. glabrata. Cell types include budding yeasts and elongated pseudohyphae-like structures. Different colony phenotypes in the presence of CuSO4 include white and very dark brown. Intermediate variations of brown colonies and wrinkled also exist but are not shown in the above image [reprinted from reference (182) with permission of the Microbiology Society].
During infections, fungi need to acquire nutrients to survive and grow. C. albicans has no known auxotrophies [except biotin (38)], and it is equipped with a broad range of secreted hydrolases and a cytolytic peptide toxin that are able to break down host tissue for nutrients (39–42). In contrast, C. glabrata is auxotrophic for biotin, pyridoxine, nicotinic acid, and thiamine and has only a limited array of secreted proteases (29, 38) but has a range of glycosylphosphatidylinositol (GPI)-anchored cell surface-associated yapsin proteases with a broad range of functions (43–45). Within macrophages, both species can cause a delay in phagosome maturation (46, 47), but only C. albicans forms hyphae that contribute to phagocyte escape (48). C. glabrata appears to multiply inside the phagosome until the high fungal load leads to rupture of the phagocyte (47). In conclusion, within Candida spp., and especially for C. albicans and C. glabrata, the strategies to survive, grow, and cause damage in the host differ significantly. This is discussed in more detail below.
CLINICAL ASPECTS
Epidemiology
Long-term surveillance programs, such as the ARTEMIS DISK epidemiological study, which compiled data from 41 countries over more than 10 years (20), and the SENTRY antimicrobial surveillance program (49), have documented changes in the demographic and geographical incidence and impact of Candida spp. Across these studies, the five major species responsible for most Candida infections are generally found in all geographical region, but with different relative distributions (Fig. 1). In most regions and studies, C. albicans is the most prevalent species (20). However, the two past decades have seen a shift in prevalence from C. albicans to “non-Candida albicans” Candida (NCAC) species, which may in part be due to improved identification methods. For example, in a study about bloodstream infections caused by Candida species in Shanghai, NCAC species outnumbered C. albicans (50). In the SENTRY Antimicrobial Surveillance 2008–2009, C. albicans was the most frequently detected Candida pathogen, but again the frequency of NCAC species differed according to geographical region. C. parapsilosis was found to be the second most common Candida species in the Asia-Pacific area and C. glabrata in other regions (49). Additionally, in another study, C. tropicalis was the main cause of candidemia in Western India, followed by C. parapsilosis (51). In Greece, C. parapsilosis was responsible for most infections in patients with hematological malignancies (52). Thus, distribution of NCAC species can vary greatly not only between different continents but also within regions of the same continent and depending on the patient cohort (13).
C. albicans and C. glabrata can both be found, albeit infrequently, in the environment: C. glabrata has been detected on plants, feces from yellow-legged gulls, and in soil (53, 54). C. albicans is rarely found in the environment but has recently been isolated from soil, the barks of trees, and pigeon droppings (54–57). Zoonotic transmission of Candida spp. is rare, but its potential cannot be ignored. Candida species can be detected and cause disease not only in domesticated animals, including dogs and cats, but also in a very wide range of wild animals and birds (58). Animal risk factors are similar to those in humans—e.g., immunosuppressive disorders—and isolates from humans and animals seem to have no host-specific genotypes or host species-specific lineages (59). This suggests that animals may serve as reservoirs for human infection. In conclusion, Candida spp. are widely distributed and are able to infect both humans and a wide range of other species, and they can occur in natural environments without obligatory associations with animals.
Diagnosis
In general, for clinical treatment and management of Candida and other fungal infections, a late diagnosis equates to a poor prognosis (60). Therefore, accurate and sensitive diagnostics are critical for effective clinical management of invasive disease. C. albicans, C. glabrata, and other Candida yeasts can, however, cause a variety of infections ranging from skin, vaginal, or oral candidiasis to severe chronic forms of granuloma or life-threatening bloodstream infections and invasive candidiasis, and the optimal diagnostic tool reflects the severity and urgency of the infection that is to be treated. The type of disease is linked to a wide number of predisposing factors: pregnancy, diabetes, infancy or old age, hospitalization, catheterization, trauma, transitory, and chronic or genetic immune deficiency. In addition, diet, denture wearing, certain surgical interventions, and other stresses are also implicated in affecting Candida disease prevalence and severity (7, 61). Some of these predisposing factors increase susceptibility to specific Candida infections. For example, denture wearing increases the likelihood of oral candidiasis, and pregnancy increases the likelihood of vaginal candidiasis.
A broad range of options are available to diagnose C. albicans and/or C. glabrata and other yeast infections that differ in their accuracy, speed, specificity, and sensitivity (62). Some of these diagnostic tests have been developed to be performed by non-specialists and are available at “point of care,” while others require the backup of sophisticated high‐technology analytical methods, such as polymerase chain reaction (PCR), DNA‐sequencing‐based approaches, or protein fingerprinting by matrix-assisted laser desorption/ionization time-of-flight (MALDI‐TOF) mass spectrometry. Currently, microscopy and culture from normally sterile or non‐sterile body sites represent the gold standard for diagnostic tools in the detection of yeast infections. Fungal selective or indicator growth media such as Sabouraud agar, CHROMagar, and chocolate or blood agar are used to narrow down the identification of the yeast species. For example, the chromogenic CHROMagar Candida test generates green colonies for C. albicans and mauve colonies for C. glabrata (63). Culturing Candida spp. from the bloodstream or other sites will routinely take 24 h or more but will yield an organism that can then be identified and subjected to specific susceptibility testing. However, more rapid tests are also required for urgent diagnoses. Blood samples can be tested directly via the T2Candida Panel and the T2Dx Instrument (T2Candida) (62). Other tests, such as Platelia Candida Ag Plus EIA (Bio‐Rad, Marnes‐la‐Coquette, Paris, France) and the CandTec latex agglutination test (Ramco Laboratories, Stafford, TX, USA), can quickly detect components (yeast wall and/or metabolites) of fungal cells as biomarkers of infection. However, biomarker tests are normally not able to discriminate between different Candida spp., which may be important in determining the most appropriate treatment. Biomarker tests can be complemented by use of serological assays to detect the host antibody response, including immunodiffusion, counterimmunoelectrophoresis, enzyme‐linked immunosorbent assays, complement fixation, lateral flow assays, radioimmunosorbent assays or agglutination assays, which again will not be species specific. Such tests are, however, normally only available in specialized fungal diagnostic laboratories, and serological tests often lack sensitivity, especially when used for immunocompromised patients. General fungal diagnostics such as those detecting fungal (1,3)-β-D-glucan are useful, rapid, and highly sensitive, but they lack specificity for species or even genus differentiation, essential information for the selection of an appropriate antifungal treatment. In the future, this array of diagnostic formats may be complemented by ultrasensitive laser‐based biophysical biosensors with high fidelity and sensitive detection of novel biomarkers (64).
Types of infection
Candida infections are divided into two broad categories: superficial and systemic (Fig. 1). Superficial infections are those of the skin or mucosal surfaces of the body, e.g., oropharyngeal, esophageal, vulvovaginal, and cutaneous candidiasis. Superficial infections are usually non-life threatening and can mostly be treated with topical antifungals with a high success rate (65). However, even though esophageal candidiasis is a superficial infection, it requires a systemic therapy (66). Vulvovaginal candidiasis affects 80% of women once in their life (67), and cutaneous candidiasis accounts for 7% of all inpatient visits to dermatologists (68). Additionally, recurrent vulvovaginal candidiasis affects 9% of women with severe impact on life quality (69). Chronic mucocutaneous candidiasis is a recurrent superficial infection of mucous membranes, skin, and nails and usually affects immunodeficient patients with a range of defined genetic polymorphisms (68).
Systemic infections are disseminated and can affect nearly all internal organs. Under immunosuppression, systemic Candida infections can originate from the commensals that reside in the GI tract (70) or from external sources, e.g., central venous catheters (71). Systemic Candida infections can affect the heart, brain, kidneys, and many other organs via the bloodstream (candidemia). The mortality rate of such Candida bloodstream infections ranges between 30% and 60% (72, 73). A serious manifestation of systemic infection caused by Candida species is sepsis. Candida spp. are responsible for about 5% of all reported sepsis cases, and when septic shock develops, it is fatal in more than half of the cases (74). This is exacerbated by late diagnosis and delayed antifungal treatment (75). In rare cases, a superficial infection can lead to a secondary systemic infection. Such secondary Candida infections can also occur following bacterial infections or sepsis, and they result in prolonged ICU stays, increased mortality, and considerable healthcare costs (76). In summary, Candida infections can be seen as a broad spectrum of conditions that range from non-life threatening superficial to systemic infection often associated with high mortality.
Candida species also can exacerbate or become exacerbated by other existing diseases. The coronavirus disease 2019 (COVID-19) pandemic has led to an increased incidence of candidemia (77), and COVID-19 patients tend to have a reduced cytokine response to C. albicans (78) and have longer stays in the ICU (79). Human immunodeficiency virus (HIV)-positive patients suffer more commonly from oral candidiasis and/or esophageal candidiasis (in case of low CD4+ counts), but highly active antiretroviral therapy (HAART) has significantly reduced oral and esophageal candidiasis rates and Candida colonization in HIV-positive individuals (80, 81). Recently, it has been shown that patients with severe COVID-19 have a proliferation of C. albicans in the gut. That leads in turn to significantly increased recruitment and NETosis of neutrophils in the lung, thereby exacerbating lung damage (82). This damage was mitigated by antifungal treatment or interleukin (IL)-6 receptor blockade. Patients with diabetes mellitus are more susceptible to oral (83) or vulvovaginal (84) candidiasis. This can be attributed to altered physiological factors in diabetic patients, such as higher concentrations of blood glucose, a weakened immune system, and increased Candida adherence to epithelial cells in this setting (85). In addition, Candida species can promote other diseases. For example, multiple types of gastrointestinal cancers (e.g., stomach and colon cancers) have been linked to the presence of Candida cells in the GI tract, which has also been associated with an increased risk of metastasis (86). C. albicans strains with different capacities to cause damage were also found in the gut of inflammatory bowel disease (IBD) patients, and the high-damaging strains induced pro-inflammatory immunity through the peptide toxin candidalysin, which may contribute to the disease (87). In conclusion, the pathogenic potential of Candida species increases in patients with impaired immune responses and can also contribute to the severity of a range of diseases.
Antifungal treatment
Oral fluconazole, miconazole, or nystatin are commonly used as first-line antifungal agents for oral thrush caused by Candida species. However, many C. glabrata strains have a low susceptibility or genetic resistance to fluconazole and will fail to clear a mucosal infection on a low-dose fluconazole. Serious oral or oropharyngeal infections may be treated with a 2-week course of an echinocandin (caspofungin, micafungin, or anidulafungin), but as intravenous (i.v.) agents, these are not appropriate for managing less invasive disease. Vaginal infections with this yeast are often managed with longer courses of topical antifungals such as miconazole or nystatin or occasionally a 2-week course of oral voriconazole for recalcitrant infections, depending on susceptibility (88, 89). In the future, ibrexafungerp, a triterpene with an action similar to that of echinocandins, but active after oral administration, may prove helpful in these cases (89). For systemic invasive Candida disease, an i.v. administration of an echinocandin is normally recommended (90) as initial therapy, although fluconazole may be an appropriate continuation therapy for susceptible patients. For C. glabrata isolates identified as susceptible-dose-dependent to fluconazole, a high dose (800 mg/day) is normally recommended, although Infectious Diseases Society of America (IDSA) guidelines recommend the use of an echinocandin as a first-line therapy, with fluconazole used only after the patient has responded to an echinocandin. Rezafungin, a new echinocandin that persists longer in the bloodstream and may only require i.v. administration on a weekly basis, could prove to be beneficial in the future (91). Systemic infections due to C. glabrata that are resistant to both azoles and echinocandins can be particularly problematic to treat. These infections may require administration of amphotericin B with or without flucytosine as alternative agents (90).
Antifungal resistance: biological and clinical principles
Both C. albicans and C. glabrata pose clinical challenges due to a range of drug-resistant phenotypes that challenge the efficacy of existing and future generations of antifungal drugs, in particular for treatment of systemic infections (6, 92–95). Increasing resistance to antifungals is normally the consequence of the rise in prevalence of Candida species and strains with intrinsic resistance—such as with fluconazole-resistant C. glabrata strains—but can also be due to de novo induction of resistance in isolates from species that are normally drug susceptible, which is common for C. albicans. Typical surveillance data show that fluconazole resistance exists in approximately 8% of C. albicans strains but as many as 26% of strains of C. glabrata (96).
C. albicans is the most commonly implicated Candida species in candidaemia, although C. glabrata exceeds C. albicans in prevalence in fluconazole-resistant candidaemia cohorts (97). In the clinic, C. glabrata is also increasingly commonly displaying echinocandin resistance, where resistance can vary between 2% and 12% of isolates in different hospitals. Some of these strains may be regarded as multiple drug resistant (MDR) due to co-resistance to fluconazole (92, 98). Approximately 14% of fluconazole-resistant C. glabrata isolates are also resistant to one or more echinocandins. These azole/echinocandin cross-resistant strains are often ERG3 mutants that harbor additional FKS gene mutations (see below). Patients infected with these strains fail to respond to both echinocandin and azole treatments (90, 96, 99).
Newer drugs flowing into the yeast-active antifungal pipeline include rezafungin, isavuconazole, ibrexafungerp, opelconazole, and fosmanogepix. All these novel antifungals have activity against both C. albicans and C. glabrata (100, 101). Rezafungin is a stable echinocandin that only requires once weekly i.v. administration; ibrexafungerp is a new triterpenoid pharmacophore, and fosmanogepix is an inhibitor of the Gwt1 enzyme that is required for GPI anchoring of proteins into the cell wall (100). Olorofim, another new class of antifungal drug that inhibits the enzyme dihydroorotate dehydrogenase, has no activity against either of these two species of Candida.
In recent years, it has become clear that emergent resistance can be distinguished from “heteroresistance” and “tolerance” of a fungus to an antifungal drug (93). Heteroresistance refers to fungal strains where a small number of cells have a much higher minimal inhibitory concentration (MIC) to a specific drug than the significant majority of cells in a given population. Heteroresistance is distinguishable from tolerance (also called “trailing growth” in the clinical literature), which is the ability of a subpopulation of a generally susceptible and isogenic strain to grow slowly in drug concentrations that are well above the MIC (90, 93). Tolerance seems to involve the chaperone Hsp90, the calcineurin pathway, and protein kinase C (93). Both heteroresistance and tolerance are relevant to drug susceptibility of both C. albicans and C. glabrata.
Clinical strategies to mitigate the challenges imposed by drug-resistant and tolerant Candida strains and species in general have to consider existing and new-in-the-pipeline antifungals that have different spectra of activity. Clinical trial data and a range of possible classical mechanisms of resistance, as well as heteroresistance and tolerance mechanisms, also need to be considered for optimal clinical decision making (90, 95). This may require standardized tests to be devised that will allow taking heteroresistance and drug tolerance into account when making clinical decisions about the choice of an antifungal.
Genetic and molecular basis for resistance
antifungal resistance in C. albicans and C. glabrata can involve a wide range of mechanisms. These include reduced drug uptake, overexpression of drug efflux transporters or the targets of azole or echinocandin antifungals, target site mutations, chromosomal aneuploidies, isochromosome formation, loss of heterozygosity, and other changes that collectively affect the drug-resistance profile (93, 98, 102–111). Some of these mechanisms are also important to the resistance profile of Candida nivariensis, and Candida bracarensis, two sibling species in the C. glabrata complex (112, 113). Some antifungal mechanisms also affect or intersect with those affecting virulence attributes such as adhesion, biofilm production, thermotolerance, resistance to immune cells, and the cell wall proteome (107, 108, 114). For example, fluconazole and exposure to macrophages can confer a cross-resistance between antifungals and immune cells via the emergence of “petite” strains of C. glabrata (115–117).
Currently, the key drugs used in the clinic are azoles, which interfere with ergosterol biosynthesis in the cell membrane, and echinocandins, which inhibit cell wall β-1,3-glucan biosynthesis. Resistance to azoles can occur through mutations in the primary azole target, Erg11/Cyp51, which encodes lanosterol 14α-demethylase. This leads, in turn, to changes in the flux through the ergosterol biosynthetic pathway and the accumulation of the toxic sterol intermediate, 14α-methyl-3,6-diol, which is produced by Erg3, a C-5 sterol desaturase. In C. albicans and C. glabrata, loss-of-function mutations in ERG3 can also confer MDR properties (98). Gain-of-function mutations in the ergosterol pathway transcription factor gene UPC2 lead to overexpression of ERG11, and isochromosome formation [i(5L) in C. albicans, which leads to amplification of ERG11 and TAC1 (118)] and other aneuploidies can also increase ERG11 expression by altering the copy number of the ERG11 gene (119). In C. albicans, trisomies in chromosomes 3 and 4 are associated with fluconazole resistance, and an increased expression of CgCDR1 can be associated with aneuploidy in C. glabrata (120, 121). Also, mutations in C. albicans ERG11 commonly confer increased azole resistance, while target site ERG11 mutations are rare in C. glabrata.
Azole resistance can also be due to upregulation of genes encoding azole efflux pumps (CaCDR1, CaCDR2, and CaMDR1) and their transcriptional regulator genes (CaTAC1 for CaCDR1 and CaCDR2, and CaMRR1 for regulation of CaMDR1). In C. glabrata, CgPdr1 regulates the efflux systems encoded by CgCDR1, CgCDR2, and CgSNQ2, and upregulation of CgPDR1 confers azole resistance (93, 98, 103, 105, 108, 110, 111, 122). In C. glabrata, mutations in CgCNE1 and CgEPA13 have also been implicated in drug resistance (123). Gain-of-function mutations in the ergosterol pathway transcription factor gene UPC2 (C. albicans)/UPC2A (C. glabrata) lead to overexpression of ERG11 in both species (124, 125).
The target of echinocandins is the catalytic subunit for β-1,3-glucan biosynthesis (1, 23), β-D-glucan synthase (FKS/GLS), in the cell membrane. Echinocandin-resistant mutants usually involve mutations in the FKS genes that encode this protein. In C. albicans, these mutations occur in two “hot spots” (HS) in the CaFKS1 gene rather than in CaFKS2 and CaFKS3, while in C. glabrata, HS mutations that effect echinocandin MICs occur in both, CgFKS1 and (more commonly) CgFKS2 (99, 126, 127).
In the cell wall of Candida species both chitin and β−1,3-glucan contribute to structural strength. Candida spp. can also upregulate chitin synthesis as a response to damage of β-1,3-glucan, which leads to strengthening of the wall and reduced sensitivity to echinocandins (128–130). This is a reversible process that occurs in vitro and likely in vivo. Because this is a reversible phenotypic adaptation and not a mutation, it may not change the in vitro MIC when the strain is isolated from the patient and grown on non-drug-selective conditions on agar (131). The higher levels of chitin in these echinocandin-adapted strains may affect the immune response to the surviving cell population, potentially rendering them less inflammatory (127, 132). High levels of chitin can explain the “paradoxical growth effect” in some strains, where higher levels of drugs like caspofungin result in higher MIC values (129, 131).
Mutations in the mismatch repair gene MSH2 can generate hypermutator strains with increased frequency of drug resistance to triazole and echinocandin compounds (92, 126). Most of the C. albicans and C. glabrata genes conferring resistance to azoles and echinocandins—e.g., CaERG11, CaERG3, CaTAC1, and CaFKS1/GSC1 in C. albicans, as well as CgERG11, CgPDR1, CgFKS1, and CgFKS2 in C. glabrata—can be rapidly screened for by next-generation sequencing and may increasingly inform clinical decisions (133). However, phenotypic analysis of drug susceptibility will remain key to identifying those isolates with previously unrecognized resistance mutations, those acquiring multiple resistance mechanisms in a stepwise manner, and in those strains where upregulation of normal house-keeping genes causes elevated MICs. It is noted also that the relevance of MICs measured in vitro to the in vivo performance of an antifungal is not always clear.
Continued exposure to a range of antifungals can lead to the stepwise evolution of drug resistance leading to an MDR phenotype that can also involve acquisition of resistance to amphotericin B and flucytosine (134). For example, in C. glabrata, prolonged antifungal treatment of a patient was observed to lead to the selection of mutations in CgFUR1 and CgFKS2, along with the overexpression of CgCDR1 and CgCDR2 (135).
MOLECULAR AND CELLULAR BIOLOGY
Genome biology
The considerable evolutionary distance between C. glabrata and C. albicans is reflected in a number of important differences in the evolution and structural organization of their genomes. C. albicans (but not C. glabrata) is one of at least eight Candida spp. that have a non-canonical CTG codon (the CTG clade). This results in the decoding of the CTG codon as serine instead of leucine (136). This is a fundamental difference in genome biology, reflecting the considerable evolutionary divergence between C. glabrata and C. albicans. This codon reassignment also provides practical constraints in C. albicans molecular genetics; e.g., the expression of heterologous proteins in C. albicans usually requires codon correction and optimization. C. glabrata is a nearer phylogenetic relative to S. cerevisiae than to C. albicans and is part of a group of yeast-like species that have undergone an ancestral whole-genome duplication (WGD) event. The C. glabrata karyotype has 13 chromosomes, while C. albicans has 8 chromosomes with a relatively compact genome that displays relatively short intergenic spacing distances compared to C. glabrata. As a result, the two pathogens display significant differences in gene regulation, expression, clustering, and genome stability. The ancestral WGD event has also shaped the contemporary genome architecture. For example, the 12.3-Mb haploid genome size of C. glabrata is only slightly smaller than the 14.3-Mb diploid C. albicans genome. However, the GC content, average number of genes, and average gene size is comparable in both species (33.5% vs 38.8%; 6,107 genes vs 5,283 genes, and 1,468 bp vs 1,479 bp in C. albicans and C. glabrata, respectively) (23, 137, 138).
C. albicans and C. glabrata have remarkably plastic genomes (138). A major aspect of their extensive genomic diversity is the capacity for aneuploidy, a condition characterized by variability in chromosome number that is relevant, e.g., to the evolution of drug-resistance properties (see above). This phenomenon results from chromosomal mis-segregation during processes such as mating, mitosis, and the response to DNA damage due to environmental stressors. In diploid C. albicans, loss (monosomy) or gain of chromosomes (trisomy or tetrasomy) can occur. Quasi-stable haploid strains of C. albicans have been generated that have promoted new forward genetics strategies for mutant analysis (139, 140). On the other hand, haploid C. glabrata strains can become disomic. While loss of chromosomes in haploid and diploid cells of C. glabrata or C. albicans can potentially be lethal due to the loss of essential genes and potential fitness reduction due to mis-segregation, aneuploidy can also confer advantages under adverse and stressful conditions and may enhance in vivo survival (141, 142). For example, exposure to antifungals can select for aneuploidy variants that have an increased copy number of drug-resistance genes (see above). Aneuploidy’s roles extend beyond resistance, influencing commensal growth. Recent studies revealed that C. albicans can acquire an extra copy of chromosome 7, which alters the dosage of the hyphal repressor gene, NRG1, thereby reducing filamentation and the expression of virulence genes associated with invasive growth in vivo (143). Aneuploidy associated with reduced virulence was reported at a high frequency during exposure of C. albicans to the mouse oral cavity (144). Collectively, these findings suggest that while aneuploidy might pose challenges, it can be well tolerated and even be advantageous.
In addition to aneuploidy, the genomic landscape of C. albicans is also shaped by chromosomal rearrangements, insertions, deletions, point mutations, copy number variations (CNV), short tandem repeats (STRs), and loss of heterozygosity (LOH), all of which can foster adaptability to harsh conditions (141). While STRs are prevalent in C. albicans and confer high mutation rates, large tandem repeats (LTRs, 65–6,499 bp) contribute to CNV, LOH, and chromosomal inversions, further affecting genome structure (145). For example, oropharyngeal infections were found to be associated with an LTR event, causing trisomy of chromosome 6 and a non-virulent phenotype in C. albicans (144). Such tandem repeats in open reading frames are also reported to orchestrate allelic homologous recombination, notably in multigene families encoding enzymes and transporters, thereby influencing pathogenicity (146). In contrast, LOH is not relevant in the haploid C. glabrata genome, which also has fewer STRs, yet this organism displays greater genetic diversity within clades than C. albicans. Extensive CNVs and aneuploidies in C. albicans drive this diversity, resulting in adaptation to antifungals and changes in virulence (147, 148).
Pleomorphism and morphogenesis
Reversible morphological transitions have been identified as important determinants of commensal and pathogenic growth of a range of fungi. Both C. albicans and C. glabrata exhibit a range of cellular and colonial morphologies (Fig. 2). C. albicans can transit from yeasts to parallel-sided, branching hyphae and conjoined elongated synchronously dividing buds called pseudohyphae. Each morphotype displays unique cell properties and interactions with its environment(149). Additionally, C. albicans can also form enlarged yeasts called Goliath cells upon zinc starvation (150) and a range of cell types associated with mating (151, 152). A more limited number of cellular morphotypes exist for C. glabrata; however, emerging evidence suggests that phenotypic switching and mating could influence virulence (147, 153). Recently, some C. glabrata isolates have been found in stable diploid or hyperdiploid (>2 N) states exhibiting different colony morphologies and variations in virulence capacity (154). Similarly, petite phenotypes of C. glabrata influence virulence and antifungal resistance (115, 117). Furthermore, an aggregating phenotype has also been recorded among C. glabrata clinical isolates (155). However, the mechanisms that regulate the transition between these phenotypes are yet to be elucidated.
Hyphal growth and tropisms
Hyphal morphogenesis is critical in C. albicans for invasive infiltration into human tissue and translocation from the gut into the bloodstream (156, 157, 158). Hyphal-associated proteins mediate adhesion and invasion via induced endocytosis (159–161, 162). In addition to induced endocytosis, C. albicans hyphae invade epithelial cells by active penetration (27). Recent microfluidic studies demonstrated that hyphal protrusive forces in the 100-MPa range allow physical penetration of host tissues. However, encounters with stiffer substrates result in Cdc42-independent alteration of cell morphology, suggesting that host cell surface stiffness influences hyphal active penetration (163, 164) and invasion of host membranes by breaching or trans-cellular tunneling (165). One major difference in the physiology of C. albicans and C. glabrata is that C. glabrata does not make filamentous parallel-sided branching hyphae, but it is able to form elongated, conjoined, pseudohyphae under certain conditions (30, 166). C. albicans hyphae display a number of behaviors and growth responses, such as the ability to form helical-shaped cells on hard surfaces and to turn and bend in relation to surface contours on the substratum (thigmotropism) (167–170). These tropisms are calcium-dependent responses (169) and involve regulation of the polarisome complex of proteins in the hyphal apex that marks the site at which cell expansion takes place (168, 170). Furthermore, the Spitzenkörper, a vesicle cluster at the tip of a growing hyphae, has gained attention in recent years in relation to its role in thigmotropism (170–172). It functions synchronously with the polarisome complex to sustain hyphal elongation and directional growth (173). A recent review (174) provides valuable and most current information on effectors and influencers of hyphal growth. It is not yet known to what extent these tropisms confer an advantage to C. albicans in navigating through human tissues.
Phenotypic switching
Phenotypic switching is manifested as a high-frequency reversible transition between different colony types. It is not the result of mutations but rather the consequence of regulation of silent chromatin states in key locations in the genome (175–177). Phenotypic switch variants have changes in physiology that affect virulence and a number of important physiological properties.
Phenotypic switching was first discovered in the C. albicans strain 3153 (175). The white-opaque switching in the C. albicans WO-1 strain was subsequently found to be critical for efficient mating of strains (see below) (178, 179). The more bean-shaped opaque-phase yeast cells were found to be the mating-competent switch variant (180). Switch variants also confer other properties relevant to the organism’s pathology. For example, opaque cells are dominant colonizers of the skin, mediated by the secreted aspartic protease Sap1 (181), and to a lesser extent of the heart and the spleen (182, 183). However, in the mammalian GI tract, C. albicans white cells can also switch to a Wor1-regulated commensal cell type known as the GUT phenotype. GUT cells are distinct from opaque cells and express a transcriptome optimized for the GI tract (184). To add to its phenotypic versatility, C. albicans also displays a “gray” phenotype in a tristable white-gray-opaque switching system. Gray cells differ from white and opaque cells in appearance, mating competency, expression of secreted aspartic proteases (Saps), and virulence (185). In addition, white cells are preferentially phagocytosed over opaque-phase cells, suggesting opaque-phase cells may be better able to escape immune clearance (186). Efg1 and Wor1 are established key regulators of phenotypic switching in C. albicans. More recently, the Cph1 transcription factor was also implicated in phenotypic transition and white cell pheromone response (187). Besides gene expression, gene dosage is also crucial for white-opaque switching, as EFG1 hemizygosity is important for transition to opaque cells and, subsequently, mating. It is therefore not surprising that clinical isolates are often found to have undergone a loss of one functional EFG1 allele via de novo mutation or gene conversion events, particularly in the GI tract (188). However, a recent study reported a Wor1-independent opaque phenotype, suggesting the presence of alternate as-yet unidentified opaque cell regulatory pathways (189). Although some C. albicans phenotypes are extensively studied, limited information is available on the nature of the variability exhibited by other colony phenotypes of C. albicans. For example, the regulatory pathways and cellular features of the originally described smooth, star, irregular-wrinkled, ring, stipple, fuzzy, and revertant and smooth colonies of strain 3513A (190) remain largely unknown. C. glabrata can also exhibit colonial phenotypic switching forming white, light brown, dark brown, and very dark brown colonies that can be distinguished by graded colony coloration on CuSO4-containing agar. These four phenotypes form the core switching system and differ in their expression of MT-II, a metallothionein gene. C. glabrata can also form irregular-wrinkled colonies (191). Although some regulatory mechanisms may remain elusive, various studies have demonstrated that spontaneous phenotypic transitions are crucial for mating, virulence, immune evasion, and adaptation to a range of host environments.
Mating
The recognition of a parasexual cycle as a part of both C. albicans and C. glabrata life cycle has expanded our understanding of Candida phenotypes (153, 192). Mating in C. albicans results in formation of irregular tubular mating projections called “shmoos” (193, 194). Opaque-phase cells of C. albicans that carry both MTLa and MTLα alleles are greatly increased in mating competence. A few clinical isolates have been identified that are MTL-homozygous (a/a or α/α) and facilitate WOR1-mediated white-to-opaque switching to allow mating between a/a and α/α cells (141, 195, 196). Same-sex mating between MTLa cells regulated by the Hsf1-Hsp90 pathway has also been identified (197). Both homothallic (same-sex) and heterothallic (between opposite mating types) mating have been described, with unisexual mating occurring in mutants lacking the Bar1 protease that enables autocrine pheromone signaling (196). Additionally, C. albicans can also undergo switching-independent sexual mating under certain environmental conditions including glucose starvation (178, 198). Although the pathways and functions of the sex genes involved are yet to be elucidated, glucose depletion can result in overexpression of pheromone-sensing and mating-associated genes, and a decreased expression of mating repressor genes. A full sexual cycle for C. albicans has yet to be described, even though most of the genes required for meiosis are known to be present in the genome.
In contrast to C. albicans, C. glabrata is a haploid fungus and contains three mating-type loci: MTL1 (containing a or α information), MTL2 (containing information for a), and MTL3 (containing information for α). MTL1 and MTL2 are transcriptionally active, while MTL3 is subject to subtelomeric silencing (199). In this regard, C. glabrata has adopted a “fluid” MTL identity and can switch its mating type to allow (para)sexual mating (153). At this stage, it is not clear whether C. glabrata can execute all the steps required to complete a full sexual cycle. Phenotypic switching does not seem to be relevant to the mating cycle.
Morphogenesis and biofilms
Regulation of the yeast-to-hypha transition in C. albicans has been studied extensively and is not covered here in detail because it has been frequently reviewed (149, 200–204) and is not relevant to C. glabrata physiology (137). However, the transcriptional machinery that orchestrates morphological transitions involve multiple positive and negative regulatory factors (e.g., Cek1-MAPK, Ras-cAMP, Hog1-MAPK, and Tor1 pathways), some of which also affect other aspects of physiology, such as biofilm formation. Biofilms of C. albicans commonly constitute a profusion of hyphae emanating from a basal layer of yeast cells that colonize a surface. BCR1, EFG1, NDT80, ROB1, TEC1, BRG1, FLO8, GAL4, and RFX2 (205) all play a role in C. albicans biofilms, and TEC1 and STE12 are important for biofilm formation of C. glabrata (206). For successful morphological transitions, these transcriptional circuits rely on co-ordination with chromatin and histone modifier and remodeling complexes (207). For example, the C. albicans SWI/SNF and RSC (Remodels the Structure of Chromatin) complexes and histone deacetylase Sir2 are known to regulate filamentation (208, 209) and influence biofilm formation by extension.
C. albicans and C. glabrata both are capable of forming single or mixed-species biofilm communities in which the fungal cells are encased in an extracellular matrix. This can result in poor penetration of antifungal drugs, encourage antifungal resistance, and also provide protection from immune phagocytes (210). Biofilm formation hinges on the adhesion capacity of the component cells. In C. albicans, the Als family of proteins, especially the hyphal associated proteins Als3, and Hwp1 aid adhesion (33, 161, 211), while Epa proteins serve this role in C. glabrata (212). Many secreted biofilm components of C. albicans, including almost half of all biofilm proteins, are delivered via extracellular vesicles (EVs), and inhibition of EV secretion increases the sensitivity of biofilm cells to fluconazole (213). It is not yet known whether EVs contribute to biofilm formation in C. glabrata. Hyphal-associated Sap proteases are required for proper C. albicans biofilm development in vitro and in vivo (214). While both species form biofilms in vivo, they exhibit stark differences in biofilm structure and composition. C. albicans biofilms typically include a proliferation of filamentous hyphae, whereas C. glabrata biofilms consist of yeast cells with occasional pseudohyphae-like structures reported in vitro (30, 191). Other studies suggest that both species can also form biofilms in which mating takes place (153, 215, 216). In C. albicans, white cells were found to secrete pheromones and create a favorable environment for a small population of opaque cells to mate (217). Furthermore, they can also form mixed-species biofilms with bacteria like Staphylococcus and Streptococcus spp. (218–220). On medical devices, teeth, and other host surfaces, specific biofilms can be formed of unique composition and function, which can alter the host microbiome. These studies collectively demonstrate the phenotypic diversity of Candida biofilms, highlighting their complex nature and the challenges they pose.
Cell wall
The Candida cell wall is a multifunctional organelle and plays a crucial role in physiological processes such as morphogenesis, adherence, biofilm formation, immune recognition and evasion, and antifungal drug targeting (221). It is a complex multilayered structure with a chitin- and β-(1,3)- and β-(1,6)-glucans-rich inner layer and an outer layer composed mainly of highly mannosylated glycoproteins. The cell wall proteins are mostly GPI-anchored via a C-terminal ω-site to β-(1,6)-glucan and thereby to the β-(1,3)-glucan inner skeleton. While the general arrangement of the major polysaccharides in the cell walls of C. albicans and C. glabrata is similar, significant differences exist in the cell wall proteome. Approximately 100 cell wall proteins like adhesins, Saps (C. albicans), yapsins (C. glabrata) and other hydrolases, transglycosidases, deacetylases, and amyloid forming proteins are encoded in the genomes of C. albicans and C. glabrata, of which 10–15 are dominant under any set of environmental conditions (222, 223). A novel class of cell wall proteins with β-helix folds were recently identified in C. glabrata that mediate adhesion in clinical isolates (224). The cell wall can undergo dynamic modifications during morphogenesis and in response to environmental changes. For example, exposure to an echinocandin compromises β-(1,3)-glucan structure, resulting in overproduction of chitin and anchoring of many GPI-proteins to chitin (128, 221, 222). These cell wall compensatory reactions are controlled by multiple signaling pathways including the MKC, HOG, and calcineurin pathways, and a subset of bespoke transcription factors including Rlm1, Sko1, Crz1, and Cas5 (215). The calcineurin pathway was recently found to regulate the cell wall integrity signaling pathway in C. albicans. It modifies chitin synthesis under echinocandin stress and ensures that chitin levels are maintained within fixed boundaries to prevent the wall from becoming too rigid (128). Additionally, transcription factors such as Sfp1 and Czf1 have also been implicated in maintaining cell wall integrity under different environmental conditions (225, 226). Recent reviews (227, 228) provide a comprehensive overview of the cell wall proteome of C. albicans and the diversity of GPI-anchored proteins in fungi, respectively. The role of specific cell wall proteins in commensalism and diseases is discussed below.
INTERACTION BIOLOGY
Immune recognition
The first step in mounting a protective immune response to Candida species is the sensing of the fungus via receptors on host immune cells via recognition of components of pathogens with conserved molecular patterns, termed pathogen-associated molecular patterns (PAMPs). These PAMPs are predominantly fungal cell wall and intracellular components, such as nucleic acids. Cells of the innate immune system recognize these PAMPs directly through membrane-bound and cytoplasmic pattern recognition receptors (PRRs) or indirectly through preopsonization via complement or antibodies. PRRs can be subdivided in several families, including C-type lectin receptors (CLRs), toll-like receptors (TLRs), NOD-like receptors (NLRs), and RIG-like receptors, in which differential expression on various (non-)immune cells leads to tailored activation of protective immune responses (229–231) (Fig. 3). It should be noted that most studies to date of the role of specific PRRs have been carried out only with C. albicans. In addition, limitations in the utility of the mouse model for C. glabrata virulence studies have compromised the ability to assess the consequences of knock-out mutations in the host or fungus on pathogenicity.
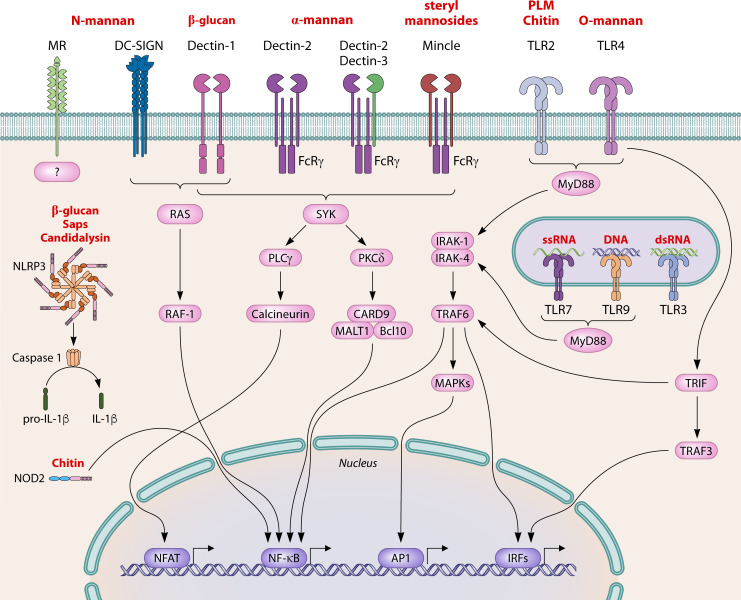
Fig 3.
Overview of selected pattern recognition receptors and their signaling pathways involved in immune recognition of Candida spp. C-type lectin receptors (mannose receptor, DC-SIGN, Dectin-1, Dectin-2, Dectin-3, and Mincle), toll-like receptors (TLR1, TLR2, TLR3, TLR4, TLR6, TLR7, and TLR9), and NOD-like receptors (NOD-2 and NLRP3) recognize conserved molecular patterns, termed pathogen-associated molecular patterns of Candida spp. (including mannan, β-1,3-glucan, chitin, candidalysin, secreted aspartic proteases, RNA, and DNA). Recognition induces downstream signaling via different pathways and transcription factors, such as NF-κB, AP1, IRFs, and NFAT, and activation of the immune response. MR, mannose receptor; DC-SIGN, dendritic cell-specific ICAM3-grabbing non-integrin; Mincle, macrophage-inducible Ca2+-dependent lectin receptor; TLR, Toll-like receptor; FcRγ, Fc receptor γ chain; NOD-2, nucleotide-binding oligomerization domain-containing 2; NLRP3, NLR family pyrin domain-containing 3; PLM, phopholipomannan; Sap, secreted aspartic protease; SYK, spleen tyrosine kinase; PKCδ, protein kinase Cδ; PLCγ, phospholipase C γ; CARD9, caspase activation and recruitment domain-containing 9; MALT1, mucosa-associated lymphoid tissue lymphoma translocation protein 1; Bcl10, B-cell lymphoma/leukemia 10; MyD88, myeloid differentiation primary response 88; IRAK, interleukin-1 receptor-associated kinase; TRAF, TNF receptor associated factor; TRIF, TIR-domain-containing adapter-inducing interferon-β; MAPK, mitogen-activated protein kinase; IL, interleukin; NFAT, nuclear factor of activated T cells; NF-κB, nuclear factor kappa-light-chain enhancer of activated B cells; AP1, activating protein-1; IRF, interferon regulatory factor.
CLRs, alone (e.g., Dectin-1) or via association with Fc receptor γ chain (e.g., Dectin-2, Mincle, and Dectin-3), signal through the Syk/PKCδ/CARD9/Bcl-10/MALT1 or RAF1 pathways. Caspase recruitment domain-containing protein 9 (CARD9) is crucial, as humans and mice with defective CARD9 signaling are more susceptible to invasive Candida infections (232–236). Candida mannans and mannoproteins are recognized by several CLRs including Dectin-2, Dectin-3, Mincle, Mannose receptor, and DC-SIGN. Dectin-2 recognizes high-mannose structures (237, 238), and absence of the receptor reduces innate immune cell recruitment and activation, phagocytosis, NETosis, and induction of Th17-cell responses, rendering mice more susceptible to systemic C. albicans and C. glabrata infection (239–244). In heterodimeric combination with Dectin-2, Dectin-3 recognizes α-mannans, and mice deficient in Dectin-3 are also susceptible to C. albicans infection (245). Recognition of N-linked mannans (238, 246) by mannose receptor induces phagocytosis of C. albicans (247) and production of various pro-inflammatory cytokines (248–250) but is not required for survival in a systemic C. albicans murine infection model (251). DC-SIGN (and murine homolog SIGNR1) also interacts with N-linked mannan (238, 252, 253), and recognition leads to phagocytosis, cytokine and reactive oxygen species (ROS) production, and modulation of TLR signaling via a Raf-1-dependent pathway (254–258). Mincle binds C. albicans steryl mannosides (259, 260) and is involved in modulation of phagocytosis and killing, cytokine responses, and control of kidney fungal burdens (242, 243, 261–263). Candida β-1,3-glucan is recognized by Dectin-1 (264) and mediates phagocytosis, generation of inflammatory cytokines, chemokines and ROS, and Th17-cell differentiation (236, 265). Absence of Dectin-1 in mice was found to be associated with increased mortality, higher fungal burden, and reduced inflammatory cell recruitment after C. albicans or C. glabrata systemic infection (243, 265–267). However, it was noted that the susceptibility of Dectin-1-deficient mice to C. albicans was dependent on the levels of chitin content of the fungal cell wall (132). In humans, a single nucleotide polymorphism (SNP) in CLEC7A (Dectin-1), which affects inflammatory cytokines in response to C. albicans, results in the absence of Dectin-1 from host myeloid cells and increases susceptibility to chronic mucocutaneous candidiasis (268), Candida colonization (269), and recurrent vulvovaginal candidiasis (270).
TLRs recognize Candida spp. via extracellular leucine-rich repeat regions, and signal via an intracellular TIR homology domain leading to the activation of MyD88 or TRIF-dependent pathways. The importance of TLR interaction in Candida recognition is evident from studies using mice that lack MyD88. These animals show increased mortality, fungal burden, and decreased pro-inflammatory cytokine production in systemic C. albicans infections (271). However, humans with MyD88 or IRAK mutations do not present with increased or exaggerated fungal infections (272, 273). TLR2 can form heterodimers in combination with TLR1 and TLR6, and the heterodimeric complex recognizes phospholipomannan (274) and chitin (275, 276), inducing pro- and anti-inflammatory cytokine responses and differentiation of hematopoietic stem cells and T cells (274, 276–280). Mice deficient in TLR2 exhibit increased C. albicans colonization of the gastrointestinal (281) and vaginal tracts (282), whereas in systemic infection, both increased and decreased susceptibility have been reported in a TLR2-deficient background (277, 280). Absence of either TLR1 or TLR6 results in a normal susceptibility in systemic models of C. albicans infection (283). In humans, SNPs in TLR1 and TLR2 have been associated with increased susceptibility to candidemia (284) and recurrent vulvovaginal candidiasis (270), respectively. Candida O-linked mannan (246, 285) recognition by TLR4 induces pro-inflammatory cytokine responses, phagocytosis, and recruitment of immune cells (286–288). Opposing consequences have been described in models for systemic models of C. albicans infection, with TLR4-deficient mice being more susceptible than (286), or not different to (289) wild-type mice. Recognition of Candida DNA by TLR9 induces pro-inflammatory cytokine responses, and absence of the receptor in systemic models of C. albicans infections increased mortality in one study (290) – but showed no effect in another (291). TLR3 and TLR7 both recognize RNA, and while an SNP in TLR3 showed decreased IFN-γ responses to C. albicans and increased susceptibility to cutaneous candidiasis (292), mice lacking TLR7 were more susceptible to systemic C. albicans infection (290).
NLRs are intracellular receptors containing leucine-rich repeats, NACHT, CARD, or PYRIN domains. NOD2 and the inflammasome-activating receptors NLPR3, NLRP10, and NLRC4 are involved in recognition of Candida species. C. albicans chitin induces IL-10 cytokine responses via NOD2 (275), whereas an SNP in NOD2 had no effect on C. albicans-stimulated peripheral blood mononuclear cell (PBMC) cytokine responses, nor was an association with disease in patients with Candida infections observed (293). The NLRP3 inflammasome is activated more strongly by C. albicans hyphae than yeast cells (294). NLRP3 recognition of C. albicans β-glucans, Saps or candidalysin activates caspase-1, or caspase-11, for processing of pro-IL-1β and pro-IL-18 into their biologically active forms (295–298), induces Th17 responses (294), but can also trigger a programmed cell death pathway (pyroptosis) facilitating fungal escape from inside macrophages (299, 300). Mice defective for components of the NLRP3 inflammasome are more susceptible to disseminated C. albicans infection (301–303). In humans, a polymorphism and variable number tandem repeat in the NLRP3 gene are associated with recurrent vulvovaginal candidiasis and decreased IL-1β production in response to C. albicans (304, 305). NLRP3-independent caspase-8 activation by C. albicans β-glucans has also been shown to induce processing of pro-IL-1β and pyroptosis (306, 307). Other inflammasomes, NLRP10 and NLRC4, play a protective role in systemic (308) and mucosal candidiasis (309), respectively, and NLCR4 also regulates NLRP3 inflammasome activity during Candida infection (310).
Other PRRs involved in Candida recognition include Galectin-3 (311, 312), Langerin (256, 313), collectins (MBL, SP-A, and SP-D) (314–316), EphA2 (317, 318), EphB2 (319), CR3 (CD18/CD11b) (320), CD14 (285), CD23 (321), CDw17 (322), LYSMD3 (323), SCARF1 and CD36 (324), NKp46 (325), and MDA5 (326).
Recognition of PAMPs by PRRs leads to activation of innate and adaptive immune responses and effector mechanisms to clear the invading fungus (Fig. 4). Epithelial cells form a physical barrier with the environment and respond to the presence of C. albicans with activation of NF-κB and a biphasic MAPK response (327, 328). Initially, NF-κB and the MAPK c-Jun are activated, independent of cell morphology. Subsequently, a second MAPK phase consists of MKP1 and c-Fos activation via EGFR signaling (40, 329) in the presence of hyphae and the secreted cytolytic eptide, candidalysin. Activation induces secretion of antimicrobial peptides such as cathelicidin (LL-37) and β-defensins, with direct antifungal activity (330–334), and of cytokines, chemokines, and alarmins, resulting in recruitment and activation of innate immune cells, e.g., neutrophils, monocytes, macrophages, and dendritic cells (DCs) (327, 328). These professional phagocytes are crucial for uptake and killing of C. albicans and C. glabrata, and absence of these cells has been associated with increased susceptibility to infection in animal models and in human disease (335–338). Uptake of non-opsonized Candida spp. is initiated by phagocytic PRRs (e.g., Dectin-1, Mannose Receptor, DC-SIGN, Dectin-2, and Mincle), whereas recognition by CR3 and Fc receptors is important for preopsonized Candida spp. (242, 249, 254, 320, 339). C. albicans hyphae are potentially problematic for phagocytic cells to take up (340); however, longer hypha can be folded in order to be engulfed into the phagosome (341). After engulfment, the phagosome undergoes multiple fusion events with endo- and lysosomes to generate an increasingly hostile environment with high acidity, and oxidative and non-oxidative mechanisms to kill Candida spp. Phagocytes produce ROS through the NADPH oxidase complex and myeloperoxidase, while reactive nitrogen species are formed by inducible nitric oxide synthase. Absence of these enzymes has been associated with increased susceptibility to systemic candidiasis in animal models (342, 343), yet in vitro ROS- and NOS-deficient macrophages were not affected in their capacity to kill C. albicans, indicating compensatory roles for other mechanisms (343). These non-oxidative mechanisms include the induction of hydrolases [e.g. lysozyme and chitinases (344, 345) and antimicrobial peptide formation (defensins, cathelicidins, and histatins) (330–334)] with direct anti-Candida activity. Indirect mechanisms such as the restriction of essential nutrients such as metals by calprotectin also contribute to protection (346). In addition to phagocytosis, neutrophils can undergo NETosis, a process of programmed cell death resulting in neutrophil extracellular trap (NET) formation, which consists of a web of DNA and histones, loaded with proteins with antifungal activity (346–348). Other innate-like cells implicated in the anti-Candida immune response include natural killer (NK) cells (349, 350), innate-like lymphocytes (351–353), invariant NK T-cells (354), γδT cells, and natural Th17 cells (355).
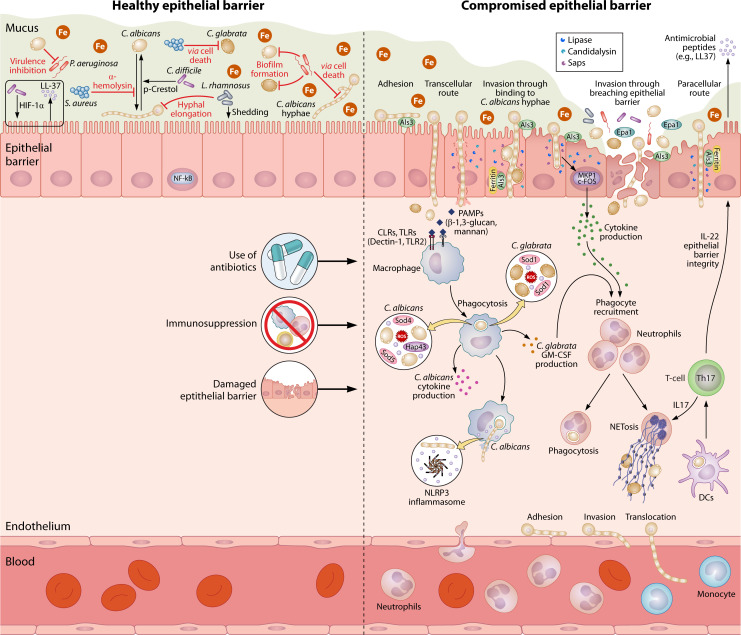
Fig 4.
From commensal to pathogen. C. albicans and C. glabrata can reside in the human body as commensals in balance with the microbiome. C. albicans can be found as both yeast and hyphae on the gut mucosal surfaces, and hyphal-associated genes, e.g., UME6, have been shown to play an important role during commensalism. The iron-rich environment of the gut leads to downregulation of iron acquisition processes to avoid toxicity. During commensalism, the host cells activate the NF-κB pathway, independent of the fungal morphology. Immunosuppression, the use of antibiotics, and physical damage of the epithelial barrier are among the predisposing factors for Candida infections. C. albicans adheres to epithelial cells using adhesins such as Als3, followed by invasion via induced endocytosis (triggered by Als3) or active penetration (by physical forces), leading to either transcellular or paracellular invasion. The transcellular route can cause severe candidalysin-mediated cellular damage. However, moderate damage can be repaired by epithelial cells. In addition to candidalysin, the fungus can secrete an arsenal of hydrolases (e.g., proteases and lipases). C. glabrata invades the epithelial barrier either via damaged barriers or by exploiting invading C. albicans hyphae in co-infections. Epithelial cells invaded by hyphal cells and damaged by candidalysin activate the MKP1/c-FOS pathway, which leads to the production of cytokines and attraction of phagocytes. Once inside the lamina propria, both fungi can get phagocytosed by resident macrophages via recognition of PAMPs (β-1,3-glucan and mannan). Inside the phagosome, fungal cells use superoxide dismutases to detoxify reactive oxygen species. Phagocytosis of C. albicans cells by macrophages triggers the production of high levels of several cytokines, while phagocytosis of C. glabrata causes the secretion of only low levels of granulocyte-macrophage colony-stimulating factor (GM-CSF). Internalized C. albicans cells produce hyphae, induce pyroptosis, and secrete candidalysin, which lead to the activation of the NLRP3 inflammasome and escape from the phagocyte. Cytokine production from both epithelial cells and macrophages recruits further phagocytes (neutrophils, macrophages, and dendritic cells) from the bloodstream. Phagocytosis by dendritic cells activates Th17 immunity and the production of IL-17 and IL-22. IL-17 promotes neutrophil trafficking, and IL-22 contributes to integrity of the epithelial barrier and production of antimicrobial peptides. C. albicans can further adhere to the endothelium and invade and translocate from there to cause bloodstream infections.
DCs not only phagocytose and kill Candida spp. but also link innate to adaptive immunity. Activation of DCs induces upregulation of major histocompatibility complex I and II molecules for the presentation of fungal antigens, and enhances expression of co-stimulatory molecules and release of cytokines and chemokines, which drive CD4+ T-cell responses. Th17 cells, characterized by the production of IL-17 and IL-22, play a pivotal role in anti-Candida immunity. IL-17 promotes neutrophil trafficking and fungicidal activity (356, 357), whereas IL-22 is important for barrier integrity of the epithelium and induction of antimicrobial peptides (358). In mice, deficiency in the IL-17/IL-17R axis and its signaling components is associated with increased susceptibility to mucosal (359, 360), skin (361), and systemic candidiasis (357). Similarly, humans with impairments in Th17 development and IL-17-dependent signaling via mutations in RORC, IL-17RA, IL-17F, ACT1, CARD9, STAT1, or STAT3 show increased development of chronic mucocutaneous candidiasis (232, 362–366). Th1 cells, characterized by the production of IFN-γ, are important for phagocyte maturation and killing of Candida spp. Mice deficient in IL-18, which drives Th1 responses, are more susceptible to disseminated C. albicans infection (367), whereas its supplementation enhances host resistance (368). Similarly, IFN-γ immunotherapy has been shown to improve outcome in humans and mice with systemic candidiasis (369, 370). In contrast, Th2 and T regulatory cell subsets are considered detrimental in Candida infections. Augmented Th2 differentiation in GATA-3-overexpressing mice was associated with increased susceptibility to C. albicans infection (371), whereas blocking IL-4 resulted in increased resistance (372). Tregs were shown to enhance Th17 cell induction, driving pathology (360), and mice deficient in IL-10 were more resistant to systemic candidiasis (372, 373). B cells are characterized by their production of antibodies, but they also phagocytose and present antigens and produce cytokines and chemokines. Their role in the protection against Candida infections is suggested to be modest, as mice lacking B cells were largely unaltered in their susceptibility to C. albicans infection (374–376). However, antibody-independent B-cell responses (377, 378) and exogenous supplementation of antibodies directed against Candida spp. have been shown to be beneficial in the immune response (see below).
Commensal interactions with the host
While the pathogenicity of Candida spp., in particular C. albicans, has been well investigated (379), the commensal lifestyle of these species has only recently come into focus (380–384). Both C. albicans and C. glabrata normally exist as commensals on mucosal surfaces of the human body, and they can frequently be found in the gut and oral or vaginal cavities (385). However, the commensal lifestyle of C. glabrata is not well investigated so far, and further research is needed to better understand the mechanisms and traits that promote the its commensal stage. Most humans in Westernized countries are temporarily or stably colonized by C. albicans (385–387). The ability of C. albicans to grow in different morphologies does not only play a central role in pathogenicity but also seems to be crucial for the commensal colonization of mucosal niches. Until recently, the general consensus was that yeast cells are the predominant form in experimental commensalism in mice (388). However, hypha-associated genes are highly expressed during gut colonization (389, 390), and more recent studies have shown that hyphae are also present during gut colonization in mice (391). The presence of yeast or hyphal cells during commensalism likely depends on the microbiome or the localization in the gut (391). However, the intact murine bacterial microbiota of many mouse strains resists the ability of C. albicans to colonize the gut (392, 393), which has led to colonization models based on antibiotic treatments. Therefore, data obtained from traditional commensal models with antibiotic-treated mice lack the influence of an intact microbiome that may be important for the maintenance of commensalism.
Microevolution experiments in a murine model based on antibiotic treatment led to the selection of C. albicans mutants that had lost their ability to form hyphae (394). Targeted mutants that lack transcriptional regulators of hyphae formation are generally defective in virulence but are often better colonizers of the murine gut than the wild type not only in mouse models based on antibiotic treatment but also in gnotobiotic mice (184, 391, 395). The ability to colonize is, however, not necessarily linked to the morphology per se but seems to be determined by morphology-specific transcriptional programs. A deletion mutant of UME6, coding for a regulator of filamentation under in vitro conditions, colonized better than the wild type but surprisingly still formed hyphae, similar to the wild type, in the murine gut. Its increased ability to colonize mainly stemmed from its lack of expression of the immunogenic secreted aspartic proteinase Sap6 (391). Additionally, overexpressing CRZ2, a filamentation regulator gene (396), enhanced early colonization in a mouse colonization model (397). Another regulator of hyphal morphogenesis, EFG1, has also been found to be crucial for commensalism, and its expression relies on the host’s immune status (398). Efficient colonization therefore seems to require the downregulation of virulence-associated transcription programs in C. albicans.
The gut is generally an iron-rich environment, but its changing abundance can affect the composition of the gut microbiota (399). In order for C. albicans to survive and proliferate under these conditions, it has to regulate its iron acquisition mechanisms. During commensal growth, C. albicans downregulates iron uptake genes through the expression of SFU1, a gene encoding a GATA family transcription factor. Sfu1 inhibits SEF1 expression, which codes for a global regulator of iron uptake (400). C. albicans also has different ferroxidases of different affinities, which were found to have distinct roles in different murine GI niches with different iron availability (401). Moreover, other metabolites such as bile acids can also contribute to the commensal status of the fungus (402, 403). Other factors that affect commensalism of C. albicans include the host’s diet (381, 404) and the physiological conditions of the gut, such as hypoxia (405). Additionally, through the expression of WOR1, C. albicans cells can be transformed to the commensal-specific GUT cell type (184). GUT cells downregulate iron uptake-related genes to prevent iron-mediated toxicity (184), and they have a distinct metabolic profile that promotes commensalism in the lower GI tract. In this short-fatty acid-enriched environment, they benefit from the upregulation of fatty acid catabolism, and they also upregulate catabolism of N-acetylglucosamine, which is beneficial for commensalism (406). Paralleling the findings of the transcription factor mutants, they also downregulate several other genes with functions in virulence (184). No colonization-specific cell types have so far been reported for C. glabrata. However, a remodeling of C. glabrata’s cell wall, specifically the increase of chitin and β-mannans, has been described during colonization in a murine model of induced acute colitis (407).
A possible explanation for the two different lifestyles of C. albicans as a commensal and pathogen could be that these lifestyles are associated with different strains (391, 408). However, a recent study found that commensal isolates from humans retained their ability to cause infection in an invertebrate model and that these isolates are competent to cause infection of humans (409). In fact, phenotypic differences among major C. albicans strain clades are minorhttps://pubmed.ncbi.nlm.nih.gov/19151328/(410). It seems clear that host factors (411, 412) and antagonistic bacteria of the microbiome (413, 414) (see below) are involved in maintaining C. albicans in the commensal phase. However, future research may help to understand the molecular and environmental factors that promote commensal or virulent attributes, and this may open new avenues for suppressing virulence. Because C. albicans cells are predominantly commensal in nature, it is likely that strains are positively adapted for this lifestyle. However, almost all commensal strains have the potential to cause diseases. Thus, the fungus must be exposed to conditions which can “train” the fungus for both commensalism and pathogenicity, a concept that has been proposed as the “commensal virulence school” (415). Antivirulence/avirulence traits in pathogenic fungi and their potential as therapeutic targets have been reviewed extensively in references (416) and (417).
Interactions with bacteria
During their commensal state, Candida spp. constantly interact with many species of bacteria and fungi of the microbiome. These interactions contribute to maintaining Candida commensalism and inhibiting the transition to an infectious state (418, 419). Staphylococcus aureus is a facultative anaerobic bacterium that colonizes the skin and mucosae. In biofilms S. aureus synergizes with C. albicans, and both microbes increase each other’s infectious potential and drug resistance (420). Recent reports suggest that S. aureus can inhibit C. albicans’ transition to the hyphal form and limits its pathogenicity via its toxin, alpha-hemolysin (421). In contrast, S. aureus culture supernatants can induce C. glabrata cell death (422). Cruz and colleagues found that the Gram-positive bacterium, Enterococcus faecalis, and C. albicans impair each other’s virulence in a Caenorhabditis elegans model. E. faecalis excretes a peptide, EntV, that reduces fungal filamentation and virulence (423). Medium conditioned by the growth of the Gram-positive Clostridioides difficile can inhibit hyphal growth, and p-Cresol, a product of the bacterium’s tyrosine metabolism, even promotes the hypha-to-yeast transition in C. albicans. Interestingly, in the presence of C. albicans, C. difficile is able to grow in aerobic conditions, which are normally toxic for the bacterium (424). The interactions of Candida spp. with Pseudomonas aeruginosa are similarly complex: C. albicans inhibits the bacterial virulence during mice colonization via inhibition of pyochelin and pyoverdine expression (425), and conversely P. aeruginosa inhibits in vitro formation of C. albicans and C. glabrata biofilms (426). Interestingly, P. aeruginosa specifically kills hyphae through contact-mediated and soluble factors, but it does not affect yeast cells (427). Indirect interactions via the host can also play a role: clostridial Firmicutes and Bacteroidetes decrease C. albicans colonization by inducing the expression of hypoxia-inducible factor-1α (HIF-1α) in mice, which then leads to the production of the antimicrobial peptide LL-37 (392).
A well-investigated interaction is that between Lactobacillus and Candida spp. Lactobacilli protect against vaginal infections by Candida spp. mainly through the production of lactic acid, which acidifies the vaginal mucosa (428), resulting in enhanced recruitment of neutrophils and cytokine production (429). In an in vitro model, Lactobacillus rhamnosus not only reduced hyphal elongation, but also triggered shedding of epithelial cells that helped to remove hyphae from the epithelial surface and reduced damage (414). C. glabrata’s stress-induced MAP kinase, Hog1, is phosphorylated at lactic acid concentrations that are produced by lactobacilli. By upregulating stress-responsive genes, it allows growth under these conditions and thereby contributes to C. glabrata’s co-colonization with different Lactobacillus spp (430).
Candida spp., the gut microbiota, and the host also interact metabolically with each other. L. rhamnosus has been found to remove carbon, nitrogen, and phosphorus sources, forcing C. albicans metabolic adaptations that compromise pathogenicity (413). Dietary tryptophan is metabolized by Lactobacillus spp. in the gut to indole-3-aldehyde, which, via the host aryl hydrocarbon receptor, leads to IL-22 expression. This IL-22 response promotes resistance against C. albicans colonization and protects the mucosal surface from inflammation (431). In an example for direct metabolic interaction, another study has shown that exposing C. albicans cells to gut metabolome components, specifically metabolites from Bacteroides ovatus, Roseburia faecis, and Roseburia intestinalis, leads to reduced expression of hypha-associated genes such as ECE1, ALS3, and HWP1 and a reduction in epithelial damage (432). The microbiota can also affect C. albicans colonization and growth through the production of short-chain fatty acids (SCFAs). Acetate, butyrate, and propionate have been found to inhibit germ tube and hypha formation and inhibit colonization in mice (392, 433, 434). Butyrate has the most potent effect and is produced by bacteria belonging to Firmicutes (incl. Clostridium spp.) and Bacteroides (435). One study showed that SCFAs lead to increased exposure of fungal β-glucan in the large intestine, which enhances immune recognition of the fungi, leading to decreased colonization in the gut of antibiotic-treated mice (436). While C. albicans’ interactions with other microbes and the effects of them have been well studied in both in vitro and in vivo models, the investigations into these relationships are much less developed for C. glabrata.
A recent discovery demonstrated that Serratia marcescens can predate on Candida cells by injecting novel antifungal effectors into the cytoplasm via the bacterial syringe-like Type VI Secretion System (T6SS) (437). This discovery has expanded the understanding of polymicrobial competitions and is likely to have a broad relevance in Candida biology (438). The T6SS is a complex bacterial contractile system found in numerous Gram-negative bacteria that delivers toxic effector proteins into adjacent cells or its extracellular environment (439). S. marcescens delivers at least two fungal-specific T6SS effector proteins, Tfe1 and Tfe2. Tfe1 triggers plasma membrane depolarization, and Tfe2 disrupts nutrient uptake and induces autophagy resulting in fungal cell death (437). Subsequently, Acinetobacter baumannii has also been found to possess a T6SS, with the TafE antifungal effector protein possessing DNase activity (440).
Early studies on T6SS identified an intriguing anomaly. Certain bacteria such as actinobacteria, cyanobacteria, and some species of proteobacteria were found to possess T6SS that housed a Het-C domain, which in filamentous fungi is important for regulating self/non-self-recognition. The presence of this domain in bacterial T6SS may suggest a role in fungal recognition (441, 442). Because bacteria and fungi coexist in polymicrobial communities, it is possible that antifungal T6SSs are of widespread importance in shaping the mycobiome. Recent reviews provide a more comprehensive and detailed overview of the interactions between Candida spp. and bacteria in health and disease in the GI tract and on other mucosal surfaces (418, 419, 443).
Interactions leading to pathogenicity
Under normal physiological conditions, Candida spp. remain commensals with little evidence of local pathogenesis. Environmental changes such as a shift in the microbial community, disruption of the host’s mucosal surface or weakening of the immune system can result in superficial or systemic infections. Candida spp. have multiple tools at their disposal to effectively infect the host, including adhesion and invasion, damage of the host tissue, immune invasion, and metabolic and nutritional interactions with the host cells (13, 379, 444–446).
Adhesion, invasion, and damage
The first step in a successful infection is the adherence to host cells. Both C. albicans and C. glabrata are equipped with adhesins that allow them to attach to host cells and form biofilms. The best-known family of C. albicans’ adhesins is the Agglutinin-Like Sequences (Als) family, which includes Als1-Als7 and Als9. Especially Als3 is one of the most important and well-studied adhesins. Als3 is expressed during filamentation (447), and its deletion significantly reduces adhesion to epithelial cells (48). Recently, a study found that Als3 and an enolase interact with each other and allow binding to host plasma proteins (448). Another important hypha-associated adhesin is the hyphal wall protein 1, Hwp1 (211). A null mutant had reduced adherence to epithelial cells in vitro (211) and reduced virulence in an in vivo model (449). C. glabrata is similarly equipped with a large repertoire of adhesins, and they are considered to be among its most important pathogenicity traits (450). Its main family of adhesins is the Epa family, which contains at least 17–23 genes depending on the strain (35). Epa1 seems to be mainly responsible for adherence to epithelial cells (35), while other proteins of the family are required for adherence to other cell types, like macrophages and endothelial cells. The C. glabrata-specific GPI-anchored proteins Pwp7 and Aed1 have been described as adhesins required for attachment to umbilical vein endothelial cells in vitro (451). The adhesins of both fungi are also associated with biofilm formation (see above). A C. albicans knockout of Hwp1 results in thin biofilms, and in an in vitro catheter model the mutant was not able to form biofilms (452). Similarly, strains with a higher expression level of ALS3 show a higher biofilm formation rate (453), and an ALS3 deletion mutant is deficient in producing biofilms in vitro (454). The C. glabrata Awp adhesin family is also involved in biofilm formation, together with Epa6 (31, 450).
After adhesion to their surface, the Candida cells need to invade the cells to establish an infection. C. albicans invades host cells via two different routes: (a) induced endocytosis or (b) active penetration via the formation of hyphae. In addition to its function as an adhesin, Als3 can act also as an invasin and induce endocytosis of the fungus by normally non-phagocytic cells. Als3 as well as Ssa1, another invasin, interact with E- and N-cadherins of epithelial and endothelial cells, respectively, to induce endocytosis (162). Als3 can also interact with the heat shock protein gp96 to invade brain endothelial cells (455) and with EphA2 and EGFR to invade oral epithelial cells (456). In a recent paper, it was further shown that E-cadherin is necessary for C. albicans to activate c-Met and EGFR to and lead to endocytosis in oral epithelial cells (457). However, active penetration seems to be the most common and important mechanism of cellular invasion of C. albicans. C. albicans forms hyphae, which can penetrate the host cell membrane. During this process, the fungus excretes a number of hydrolases (proteinases, phospholipases, and lipases) and other factors that may aid in tissue invasion (48). The Saps family comprises ten members (Sap1-Sap10) and is probably the best studied among these hydrolases (458, 459). In addition, C. albicans possesses a hypha-associated toxin called candidalysin, the first (ribosomal) peptide toxin identified in any human fungal pathogen (40, 41, 460). Candidalysin forms pore-like structures in the membrane of host cells resulting in membrane damage (40, 461). Moderate membrane damage levels can be repaired by epithelial cells (462, 463), but sustained levels of damage lead to a series of event that are critical for C. albicans mucosal and systemic infections (464, 465). For example, candidalysin-induced damage activates danger-response and damage protection pathways in host cells (40, 327) (see above) and leads to activation of the epidermal growth factor receptor in epithelial cells and the NLRP3 inflammasome in macrophages (296, 329). It also drives neutrophil recruitment and immunopathology during vaginal infections (466), triggers Type 17 immunity during oral infections (467), and is essential for successful translocation of the fungus through the epithelial barrier (158). In contrast, C. glabrata is not known to produce any toxins.
C. albicans translocates through the epithelial barrier to reach the bloodstream for a disseminated infection. There is proof that translocation occurs through a transcellular route which involves the formation of hyphae (27). Other translocation strategies such as paracellular translocation through the epithelia barrier, and translocation through microfold cells and Peyer’s patches have also been suggested to take place, but have not yet been conclusively shown (157). In contrast C. glabrata invasion of the epithelial barrier does not involve hyphae formation. It may reach the bloodstream through breaches created via trauma, surgery or catheters (19), however, alternative invasion mechanisms have also been suggested. For example, it was shown that C. glabrata can bind to C. albicans hyphae in order to establish colonization or infection in an OPC mice model (468) and may therefore hijack the C. albicans translocation machinery. In another recent study, it was shown that a single human protein, albumin, can dramatically enhance the pathogenic potential of C. glabrata on vaginal epithelial cell by a combination of beneficial effects for the fungus, which includes an increased access to iron, accelerated growth, and increased adhesion (469). Furthermore, it was shown that C. glabrata and other non-hyphae-forming Candida spp. bind to bridging molecules present in human serum to invade the epithelial barrier via bridging molecule-mediated endocytosis (470). In general, C. glabrata’s invasion tactics are not well studied, and more research is needed to better understand how the fungus can take advantage of other microbes or the host itself to achieve invasion. Further details about the adhesion, invasion, and damage potential of Candida spp. have been extensively discussed in past reviews (379, 471–473).
Interaction with host cells
Once invasion occurs, the host’s immune response will be activated (see above). Both C. albicans and C. glabrata can be recognized via PRRs and are phagocytosed by macrophages and other myeloid cells. They are both able to delay phagosome maturation to avoid killing, although the main mechanism of C. albicans to escape detrimental intracellular effect is the formation of hyphae and a fast escape from the macrophages (27, 474, 475). However, escape from macrophages via hyphae formation has only been seen in vitro, and as of yet, there is no validation in a mammalian model. In contrast, C. glabrata, similar to certain bacteria such as Mycobacterium tuberculosis (476), can persist and replicate in the phagosome until the phagocyte bursts (47, 477). Interestingly, a rare non-lytic escape mechanism called vomocytosis from macrophages has also been reported for C. albicans, in which a yeast cell is ejected from the phagocyte without disrupting the phagocyte membrane (478). In a zebrafish infection model, yeast-locked C. albicans spp. have been shown to persist in macrophages up to 40 h and are able to spread in different tissues using the host cells as a Trojan horse (479). Multiple studies have recently shown that C. albicans spp. hijacks the inflammasome and pyroptotic pathway to escape from macrophages using candidalysin to facilitate its exit (296, 480, 481). Other types of cell death, such as the induction of apoptosis (482) and necroptosis (483), have been associated with C. albicans. Additionally, the induction of antiapoptotic signals during C. albicans infection in macrophages has been described (484). However, it is not yet clear whether regulation of these signals serves the host as a mechanism against the pathogen or the fungus as a virulence factor. In contrast, during C. glabrata infection, macrophages show little to no cytokine release (47), and the fungus is not able to trigger pyroptosis (299).
C. glabrata depends on its autophagy to persist inside the phagosome (485), probably to compensate for the lack of nutrients inside this organelle. Damage due to oxidative stress in the phagosome is mitigated by the superoxide dismutase (Sod), Sod1 (486), and to a lesser extent the catalase, Cta1, which is not essential for survival (487). Interestingly, a recently described transcription factor,Tog1,has been described that links oxidative stress responses with metabolic adaptations to macrophage persistence (488). In an ex vivo blood infection model, C. glabrata did not show a significant upregulation of oxidative stress response genes, and while C. albicans upregulated the glyoxylate cycle and fermentative energy production, C. glabrata even downregulated transporters for different nutrients such as amino acids (489). Petite phenotypes of C. glabrata show, in addition to their azole resistance, increased endoplasmatic reticulum stress resistance and survival in phagocytes (115, 117). Similar to C. glabrata, C. albicans uses Sods to protect against oxidative stress by detoxification of ROS (490–492). Mutants of Sod4 and Sod5 showed increased accumulation of ROS and decreased viability inside macrophages and blood cells (490), suggesting killing in a ROS-dependent manner (490, 491).
The Candida cell wall consists of an intricate network of polysaccharides and proteins, and its composition and structural organization are highly dynamic, depending on environment cues and its morphological state (see above). Recognition by immune cells is dependent on PAMP expression, and alterations in the cell wall architecture affect phagocytosis and the release of pro-inflammatory cytokines (493–496). C. albicans modulates the exposure of β-1,3-glucan by actively masking this pro-inflammatory PAMP in response to host signals, such as carbon source, lactate, and other short-chain fatty acids (436, 497), pH (429, 498), hypoxia (499), and iron limitation (500). Avoidance of immune β-1,3-glucan recognition is also achieved by the shaving of cell surface β-1,3-glucan via the secreted glucanases, Xog1 (501) and Eng1 (502). Neutrophils counteract masking by NET-mediated attacks, which trigger active remodeling of the fungal cell wall and enhances immune recognition via β-1,3-glucan in macrophages (503). However, other immune cells, such as monocytes, are trained more on mannans in the outer cell wall than β-1,3-glucan (496), and so expression of the PRR repertoire is immune cell dependent and tailors immune recognition.
Metabolic interactions
In general, C. albicans’ preferred energy source is glucose. However, in specific host niches or inside phagocytes, the fungus can adapt and use alternative energy sources via activating gluconeogenesis and starvation responses. Both C. albicans and C. glabrata are able to use two-carbon compounds, such as acetate derived from fatty acids, for gluconeogenesis (504, 505). This glyoxylate shunt is important for the survival and virulence of both fungi inside the phagosome. In the glucose-poor environment of the phagosome, C. albicans’ proline and arginine catabolism are an important mechanism for filamentation induction (506). During infection by C. albicans, glycolysis, gluconeogenesis, and the glycosylate pathway are required at different times and in different niches. Normal concentrations of glucose repress the glyoxylate and gluconeogenesis pathways in the blood but are activated in phagocytes (507, 508). It is, however, clear that many infected tissues do not behave as a homogenous microenvironment and that microsites may exist where cells of quite different metabolic profiles exist side by side (507). It is also known that physiological concentrations of glucose activate an oxidative stress response that promotes fitness downstream, when Candida cells are engulfed by neutrophils (509). This anticipatory behavior enables the yeast cell to activate and prime its defenses to immune attack before it encounters the toxic environment of the neutrophil phagolysosome.
C. albicans can acquire iron via multiple host sources including hemoglobin, hemin, ferritin, and transferrin (510). When in blood, candidalysin acts as a hemolytic factor for C. albicans (511) and allows utilization of hemoglobin via the Rbt5/Hmx1 system to acquire iron (512), while C. albicans hyphae can also acquire iron via the host iron storage molecule, ferritin, through binding to Als3 (513). C. albicans regulates its iron uptake tightly, depending on environmental iron availability. During iron starvation in the host, e.g., within the blood during bloodstream infections, the fungus upregulates the expression of SEF1 (400). Sef1 activates a large set of genes, including HAP43, to acquire iron from the environment (514). Hap43, a part of the CCAAT-binding complex, upregulates iron uptake genes and downregulates iron-consuming processes. Additionally, Hap43 represses Sfu1, a GATA family transcription factor (515). This contrasts with the regulation to the iron-rich environments that is described above, where Sfu1 represses iron upregulating genes to avoid iron toxicity (400). C. glabrata has a more limited ability to use host iron sources and lacks a high-affinity iron uptake system (516). In iron-poor environments, the Aft1 transcription regulator is activated to upregulate iron uptake and recycling processes (517). At the same time, Cth2 binds to and degrades mRNA involved in iron-consuming processes (517). Interestingly, neither of the two Candida species produce their own siderophores, and both rely on xenosiderophores, e.g., from bacteria or other fungi (516). Nevitt and Thiele identified Sit1, a xenosidephore transporter, which C. glabrata uses to survive in the phagosome (518). However, zinc, another essential metal, can be sequestered by a sophisticated zincophore system by C. albicans (519).
Zinc and copper are transported into the phagosome by macrophages, and both are considered to contribute to ROS production as well as inactivation of many enzymes by mismetallation. C. glabrata counteracts this by upregulation of Cu-binding metallothioneins in the presence of high copper levels (520), while C. albicans pumps copper out using a P-type ATPase (521). When zinc ions are in excess, C. glabrata sequesters zinc to vacuoles via the transporter Zrc1 (522). Both species are auxotrophic for biotin and possess a high-affinity biotin transporter, Vht1, which is required for efficient proliferation inside the phagosome in vitro and for full virulence of C. albicans in a murine systemic infection model (38).
To summarize, both fungi have developed mechanisms to efficiently infect the host and to enable metabolism in a variety of host niches. Both species rely on two-carbon sources and the glyoxylate shunt for survival in the host. They can acquire nutrients such as iron via different mechanisms and can inactivate uptake of non-beneficial nutrients, such as excess copper and zinc, to ensure their survival. The in vivo metabolic adaptations show some similarities but also differ in key elements. Further research is needed to better understand these mechanisms, especially for C. glabrata infections, which are understudied compared to C. albicans.
FUTURE STRATEGIES
A major health goal for the future will be to reduce the number of superficial and life-threatening Candida infections. To this end, it is necessary to establish better, faster, specific, and easily accessible diagnostic tools to detect fungal infections and their drug resistances at an early stage. Techniques such as MALDI-TOF, DNA microarray, and PCR detection have been increasingly used the past years, and these assays are sensitive but not widely available. They also have potential to provide useful additional clinical information such as an understanding of the resistance genes that a Candida strain may harbor.
As yet, there are no traditional or next-generation RNA vaccines against C. albicans or C. glabrata, although a fragment of the C. albicans GPI-anchored cell wall protein Als3 has shown promise in a phase 2 clinical trial as a monovalent vaccine against recurrent vaginitis (523). β-Glucan particles have also been explored as vaccine carriers of fungal antigens (524). It is feasible that polyvalent vaccines will prove to be effective against superficial or systemic disease caused by these two Candida species, and investment is needed to explore the utility of these unexploited therapeutic strategies.
In medical mycology, the use of combinations of antifungal drugs is rare, and most drugs against C. albicans and C. glabrata are used as monotherapies or in sequential monotherapy. This contrasts with the combinatorial approaches taken in other areas of infectious disease therapy (525) and in agriculture, to broaden the spectrum of coverage and/or suppress the emergence of resistant strains. Future strategies should therefore include exploring how optimized drug combinations might be used that are safe and effective and preserve the durability of antifungals by suppressing antifungal drug resistance. For example, chitin synthase and β-1,3-glucan synthase inhibitors would be expected to exhibit synergy as a drug combination, and agents that block cell wall salvage pathways, such as the calcineurin pathway, potentiate the action of inhibitors of cell wall biosynthesis at least in vivo (526). Membrane acting peptides, applied alone and in combination with azoles, have been shown to be effective in disrupting biofilms (527). Another potential way to successfully control Candida infections in the future may be the use of antivirulence drugs. Antivirulence drugs show potential especially against C. albicans infections by inhibiting filamentation and biofilm formation (417).
Also, adjuvants or cell wall components that activate or suppress inflammation may be helpful in treating fungal disease. Purified immunomodulatory components of the cell wall have the potential to promote immune recognition and activate B-cell and T-cell responses that are required for disease suppression. Hyperinflammatory diseases such as Candida vaginitis may be mitigated by blocking the signal cascade that leads to inflammation. Recently, a promising advance has been made showing that the C. albicans zinc-binding protein Pra1 is a natural attractant for neutrophils and thus promotes inflammatory vaginitis (519, 528). A Pra1 homolog does not exists in C. glabrata. Women with recurrent vaginitis often have low zinc levels (529, 530), and exogenous addition of zinc prevented Pra1 production and neutrophil infiltration into the vaginal canal, thus preventing localized inflammatory disease (528). Furthermore, the peptide toxin candidalysin, found in C. albicans but not C. glabrata, has been shown to be a key hypha-associated virulence determinant responsible for the immunopathogenesis of C. albicans vaginitis (466). It was demonstrated that nanobody-mediated neutralization of candidalysin prevents epithelial damage and inflammatory responses that drive the pathogenesis of vulvovaginal candidiasis (531). Future antifungal stewardship strategies may also consider the benefits of combining antifungal drug treatment with immunotherapies.
Empirical, preemptive, and prophylactic therapy is widely used for critically ill patients with high susceptibilities to fungal infections, and the full use of new-generation diagnostics, biomarkers, and colonization indices may lead to further improvements in patient care and survival (532). One possible avenue could be the use of probiotics (live biotherapeutic products) to suppress the transition from commensal to the infectious stage (386). This approach may be especially useful in patients with GI tract-related diseases, such as IBD or colitis. Another way to manipulate the microbiome to prevent possible infections or treat overgrowth is through dietary interventions or the use of fecal microbiota transplantation (FMT) that has been used successfully for the treatment of C. difficile infections. Promising data have shown that FMT can be effective against Candida colonization in the gut (393, 533). Phage therapies have also been suggested as a tool to shape the microbiota and prevent fungal infections. To date, phages have not been found that directly target Candida spp. Their effects on co-habitating bacteria could eliminate fungal pathogens through metabolic interactions either by enhancing bacteria that suppress Candida invasion or by eliminating bacteria that enhance Candida virulence. As an interesting example for direct fungal-phage interactions, Pseudomonas phages can affect in vitro growth of C. albicans, perhaps by sequestering iron and by direct binding to the fungal surface (534). Such microbiota manipulation techniques have only recently been developed, and therefore, many potential side effects and limitations exist that we may not be aware of. Additional research into these therapies may soon elucidate their true potential against Candida infections.
ACKNOWLEDGMENTS
N.A.R.G. acknowledges the support of Wellcome Trust Investigator, Collaborative, Equipment, Strategic and Biomedical Resource awards (101873, 200208, and 215599). N.A.R.G. and M.H.T.S. are further supported by Wellcome Trust Investigator, Collaborative, Equipment, Strategic and Biomedical Resource awards (224323). The University of Exeter provided support to N.A.R.G. and D.M.-J. N.A.R.G. also thanks the MRC (MR/M026663/2) and the MRC Centre for Medical Mycology (MR/N006364/2) for support. This study/research is funded by the National Institute for Health and Care Research (NIHR) Exeter Biomedical Research Centre. The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care. This work was further supported by the Deutsche Forschungsgemeinschaft [DFG, German Research Foundation) Priority Program 2225 Exit Strategies of extracellular pathogens, awarded to S.B. and B.H.; the DFG through the Collaborative Research Center (CRC)/Transregio 124 "FungiNet" project C1 and C2, the DFG within the Cluster of Excellence “Balance of the Microverse” under Germany’s Excellence Strategy–EXC 2051–Project-ID 467 390713860 and the DFG project project Hu 528/20–1, awarded to B.H.]. This study was further funded by the German Federal Ministry of Education and Research within the funding program Photonics Research Germany, Leibniz Center for Photonics in Infection Research, subproject LPI-BT4, contract number 13N15714, awarded to B.H., S.B., and M.K., and the Leibniz Association Campus InfectoOptics SAS-2015-HKI-LWC, awarded to B.H.
Biographies

Myrto Katsipoulaki received her Bachelor’s degree in Biology from Aristotle University of Thessaloniki, Greece, and her M.Sc. degree in Leiden University, Netherlands. She is currently pursuing her Ph.D. at the Department of Microbial Pathogenicity Mechanisms under supervision of Bernhard Hube at the Leibniz-HKI in Jena, Germany. Her current research focuses on studying host-pathogen interactions, specifically between the fungus Candida albicans and its host, via the development of reporters for different cell death pathways.

Dhara Malavia-Jones is a molecular microbiologist interested in clinically and industrially important fungi and other microbes. She trained at the University of Mumbai and then obtained a MRes and PhD at the University of Aberdeen, UK, and then moved to the University of Exeter, MRC Centre for Medical Mycology, in 2018. Her current work focuses on Candida albicans, although she has also worked on C. auris and other fungal species. Her work helped to identify Goliath cells of C. albicans that are generated under conditions of low external zinc concentrations. Her current research is focused on the development of novel molecular tools to identify previously unexplored drugable cell wall targets and the potential to generate new antifungal compounds against these fungal targets.

Sascha Brunke studied biology at the Free University Berlin 1998-2005 and started to focus on pathogenic fungi during his work at the Robert Koch Institute. He obtained his PhD in 2010 for his investigations into the lipases of the pathogenic fungus Malassezia furfur. He then became interested in pathogenic Candida species while working at the Hans-Knöll-Institut (Leibniz-HKI) in Jena and the Jena University Hospital. Since 2013, he is deputy head of the department Microbial Pathogenicity Factors at the Leibniz-HKI. He is author on more than 70 publications, mainly focusing on the evolution of human fungal pathogens, especially Candida glabrata, and their specific adaptations to their host.

Bernhard Hube earned his PhD in Microbiology at the University of Goettingen (1991) and spent his postdoctoral time at the University of Aberdeen (1992 – 1995) and the University of Hamburg (1995 – 2000). In 2000 he became Research Group leader and Head of Division “Mycology” at the Robert Koch Institute, Berlin. In 2006 he was appointed Professor and Chair for Microbial Pathogenicity at the Friedrich Schiller University of Jena, and in 2007 Head of the Department of Microbial Pathogenicity Mechanisms at the Leibniz-HKI in Jena, Germany. He has co-authored >300 publications dealing with the molecular and infection biology of human-pathogenic fungi, in particular Candida species. He discovered one of the first virulence genes, encoding a secreted aspartic proteinase, in C. albicans. His recent research focuses on the first peptide toxin discovered in human-pathogenic fungi, candidalysin.

Neil A. R. Gow trained at the Universities of Edinburgh and Aberdeen, UK, and at the National Jewish Hospital in Denver, USA, before moving to the University of Aberdeen (1984-2018). He moved to the University of Exeter in 2018 as Deputy Vice Chancellor for Research and Impact and is now Professor of Microbiology at the MRC Centre for Medical Mycology at this university. He has served as President of the British Mycological Society, the International Society for Human and Animal Mycology, the Microbiology Society, the British Society for Medical Mycology and from 2023 the European Confederation of Medical Mycology. He has 44 years of experience working on medically important fungi, and his current research investigates the structure and function of the fungal cell wall in relation to morphogenesis and as a target for immune recognition and the development of antifungal drugs.
Contributor Information
Bernhard Hube, Email: bernhard.hube@leibniz-hki.de.
Neil A. R. Gow, Email: n.gow@exeter.ac.uk.
Joseph Heitman, Duke University, Durham, North Carolina, USA.
REFERENCES
- 1. Takashima M, Sugita T. 2022. Taxonomy of pathogenic yeasts Candida, Cryptococcus, Malassezia, and Trichosporon. Med Mycol J 63:119–132. doi: 10.3314/mmj.22.004 [DOI] [PubMed] [Google Scholar]
- 2. Denning DW. 2024. Renaming Candida glabrata—a case of taxonomic purity over clinical and public health pragmatism. PLoS Pathog 20:e1012055. doi: 10.1371/journal.ppat.1012055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Borman AM, Johnson EM. 2023. Changes in fungal taxonomy: mycological rationale and clinical implications. Clin Microbiol Rev 36:e0009922. doi: 10.1128/cmr.00099-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Organization WH . 2022. WHO fungal priority pathogens list to guide research, development and public health action. Available from: https://www.who.int/publications/i/item/9789240060241
- 5. Denning DW, Kneale M, Sobel JD, Rautemaa-Richardson R. 2018. Global burden of recurrent vulvovaginal candidiasis: a systematic review. Lancet Infect Dis 18:e339–e347. doi: 10.1016/S1473-3099(18)30103-8 [DOI] [PubMed] [Google Scholar]
- 6. Kullberg BJ, Arendrup MC. 2015. Invasive candidiasis. N Engl J Med 373:1445–1456. doi: 10.1056/NEJMra1315399 [DOI] [PubMed] [Google Scholar]
- 7. Pappas PG, Lionakis MS, Arendrup MC, Ostrosky-Zeichner L, Kullberg BJ. 2018. Invasive candidiasis. Nat Rev Dis Primers 4:18026. doi: 10.1038/nrdp.2018.26 [DOI] [PubMed] [Google Scholar]
- 8. Bongomin F, Gago S, Oladele RO, Denning DW. 2017. Global and multi-national prevalence of fungal diseases-estimate precision. J Fungi (Basel) 3:57. doi: 10.3390/jof3040057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brown GD, Denning DW, Gow NAR, Levitz SM, Netea MG, White TC. 2012. Hidden killers: human fungal infections. Sci Transl Med 4:165rv13. doi: 10.1126/scitranslmed.3004404 [DOI] [PubMed] [Google Scholar]
- 10. Kotey FC, Dayie NT, Tetteh-Uarcoo PB, Donkor ES. 2021. Candida bloodstream infections: changes in epidemiology and increase in drug resistance. Infect Dis (Auckl) 14:11786337211026927. doi: 10.1177/11786337211026927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fidel Jr PL, Vazquez JA, Sobel JD. 1999. Candida glabrata: review of epidemiology, pathogenesis, and clinical disease with comparison to C. albicans. Clin Microbiol Rev 12:80–96. doi: 10.1128/CMR.12.1.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Andes DR, Safdar N, Baddley JW, Alexander B, Brumble L, Freifeld A, Hadley S, Herwaldt L, Kauffman C, Lyon GM, Morrison V, Patterson T, Perl T, Walker R, Hess T, Chiller T, Pappas PG, TRANSNET Investigators . 2016. The epidemiology and outcomes of invasive Candida infections among organ transplant recipients in the United States: results of the transplant-associated infection surveillance network (TRANSNET). Transpl Infect Dis 18:921–931. doi: 10.1111/tid.12613 [DOI] [PubMed] [Google Scholar]
- 13. Silva S, Negri M, Henriques M, Oliveira R, Williams DW, Azeredo J. 2012. Candida glabrata, Candida parapsilosis and Candida tropicalis: biology, epidemiology, pathogenicity and antifungal resistance. FEMS Microbiol Rev 36:288–305. doi: 10.1111/j.1574-6976.2011.00278.x [DOI] [PubMed] [Google Scholar]
- 14. Odds FC. 1988. Candida and candidosis: a review and bibliography. 2nd ed. Baillièrre Tindall, London. [Google Scholar]
- 15. Anderson HW. 1917. Yeast-like fungi of the human intestinal tract. J Infec Dis 21:341–354. doi: 10.1093/infdis/21.4.341 [DOI] [Google Scholar]
- 16. Casadevall A, Kontoyiannis DP, Robert V. 2019. On the emergence of Candida auris: climate change, azoles, swamps, and birds. mBio 10:e01397-19. doi: 10.1128/mBio.01397-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pfaller MA, Diekema DJ. 2007. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev 20:133–163. doi: 10.1128/CMR.00029-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Spellberg B, Kontoyiannis DP, Fredricks D, Morris MI, Perfect JR, Chin-Hong PV, Ibrahim AS, Brass EP. 2012. Risk factors for mortality in patients with mucormycosis. Med Mycol 50:611–618. doi: 10.3109/13693786.2012.669502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yapar N. 2014. Epidemiology and risk factors for invasive candidiasis. Ther Clin Risk Manag 10:95–105. doi: 10.2147/TCRM.S40160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pfaller MA, Diekema DJ, Gibbs DL, Newell VA, Ellis D, Tullio V, Rodloff A, Fu W, Ling TA, Global Antifungal Surveillance G. 2010. Results from the ARTEMIS DISK global antifungal surveillance study, 1997 to 2007: a 10.5-year analysis of susceptibilities of Candida species to fluconazole and voriconazole as determined by CLSI standardized disk diffusion. J Clin Microbiol 48:1366–1377. doi: 10.1128/JCM.02117-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pfaller MA, Diekema DJ, Rinaldi MG, Barnes R, Hu B, Veselov AV, Tiraboschi N, Nagy E, Gibbs DL. 2005. Results from the ARTEMIS DISK global antifungal surveillance study: a 6.5-year analysis of susceptibilities of Candida and other yeast species to fluconazole and voriconazole by standardized disk diffusion testing. J Clin Microbiol 43:5848–5859. doi: 10.1128/JCM.43.12.5848-5859.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cataldi V, Di Campli E, Fazii P, Traini T, Cellini L, Di Giulio M. 2017. Candida species isolated from different body sites and their antifungal susceptibility pattern: cross-analysis of Candida albicans and Candida glabrata biofilms. Med Mycol 55:624–634. doi: 10.1093/mmy/myw126 [DOI] [PubMed] [Google Scholar]
- 23. Dujon B, Sherman D, Fischer G, Durrens P, Casaregola S, Lafontaine I, De Montigny J, Marck C, Neuvéglise C, Talla E, et al. 2004. Genome evolution in yeasts. Nature 430:35–44. doi: 10.1038/nature02579 [DOI] [PubMed] [Google Scholar]
- 24. Jones T, Federspiel NA, Chibana H, Dungan J, Kalman S, Magee BB, Newport G, Thorstenson YR, Agabian N, Magee PT, Davis RW, Scherer S. 2004. The diploid genome sequence of Candida albicans. Proc Natl Acad Sci U S A 101:7329–7334. doi: 10.1073/pnas.0401648101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hickman MA, Zeng G, Forche A, Hirakawa MP, Abbey D, Harrison BD, Wang Y-M, Su C, Bennett RJ, Wang Y, Berman J. 2013. The 'obligate diploid' Candida albicans forms mating-competent haploids. Nature 494:55–59. doi: 10.1038/nature11865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Muller H, Hennequin C, Gallaud J, Dujon B, Fairhead C. 2008. The asexual yeast Candida glabrata maintains distinct a and alpha haploid mating types. Eukaryot Cell 7:848–858. doi: 10.1128/EC.00456-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dalle F, Wächtler B, L’Ollivier C, Holland G, Bannert N, Wilson D, Labruère C, Bonnin A, Hube B. 2010. Cellular interactions of Candida albicans with human oral epithelial cells and enterocytes. Cell Microbiol 12:248–271. doi: 10.1111/j.1462-5822.2009.01394.x [DOI] [PubMed] [Google Scholar]
- 28. Filler SG, Sheppard DC. 2006. Fungal invasion of normally non-phagocytic host cells. PLoS Pathog 2:e129. doi: 10.1371/journal.ppat.0020129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kaur R, Domergue R, Zupancic ML, Cormack BP. 2005. A yeast by any other name: Candida glabrata and its interaction with the host. Curr Opin Microbiol 8:378–384. doi: 10.1016/j.mib.2005.06.012 [DOI] [PubMed] [Google Scholar]
- 30. Csank C, Haynes K. 2000. Candida glabrata displays pseudohyphal growth. FEMS Microbiol Lett 189:115–120. doi: 10.1111/j.1574-6968.2000.tb09216.x [DOI] [PubMed] [Google Scholar]
- 31. Iraqui I, Garcia-Sanchez S, Aubert S, Dromer F, Ghigo JM, d’Enfert C, Janbon G. 2005. The Yak1p kinase controls expression of adhesins and biofilm formation in Candida glabrata in a Sir4p-dependent pathway. Mol Microbiol 55:1259–1271. doi: 10.1111/j.1365-2958.2004.04475.x [DOI] [PubMed] [Google Scholar]
- 32. Nobile CJ, Mitchell AP. 2006. Genetics and genomics of Candida albicans biofilm formation. Cell Microbiol 8:1382–1391. doi: 10.1111/j.1462-5822.2006.00761.x [DOI] [PubMed] [Google Scholar]
- 33. Hoyer LL, Green CB, Oh SH, Zhao X. 2008. Discovering the secrets of the Candida albicans agglutinin-like sequence (ALS) gene family--a sticky pursuit. Med Mycol 46:1–15. doi: 10.1080/13693780701435317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Timmermans B, De Las Peñas A, Castaño I, Van Dijck P. 2018. Adhesins in Candida glabrata. J Fungi (Basel) 4:60. doi: 10.3390/jof4020060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cormack BP, Ghori N, Falkow S. 1999. An adhesin of the yeast pathogen Candida glabrata mediating adherence to human epithelial cells. Science 285:578–582. doi: 10.1126/science.285.5427.578 [DOI] [PubMed] [Google Scholar]
- 36. Veses V, Gow NAR. 2009. Pseudohypha budding patterns of Candida albicans. Med Mycol 47:268–275. doi: 10.1080/13693780802245474 [DOI] [PubMed] [Google Scholar]
- 37. Staib P, Morschhauser J. 2006. Chlamydospore formation in Candida albicans and Candida dubliniensis– an enigmaticdevelopmental programme. Mycoses 50:1–12. doi: 10.1111/j.1439-0507.2006.01308.x [DOI] [PubMed] [Google Scholar]
- 38. Sprenger M, Hartung TS, Allert S, Wisgott S, Niemiec MJ, Graf K, Jacobsen ID, Kasper L, Hube B. 2020. Fungal biotin homeostasis is essential for immune evasion after macrophage phagocytosis and virulence. Cell Microbiol 22:e13197. doi: 10.1111/cmi.13197 [DOI] [PubMed] [Google Scholar]
- 39. Schaller M, Borelli C, Korting HC, Hube B. 2005. Hydrolytic enzymes as virulence factors of Candida albicans. Mycoses 48:365–377. doi: 10.1111/j.1439-0507.2005.01165.x [DOI] [PubMed] [Google Scholar]
- 40. Moyes DL, Wilson D, Richardson JP, Mogavero S, Tang SX, Wernecke J, Höfs S, Gratacap RL, Robbins J, Runglall M, et al. 2016. Candidalysin is a fungal peptide toxin critical for mucosal infection. Nature 532:64–68. doi: 10.1038/nature17625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wilson D, Naglik JR, Hube B. 2016. The missing link between Candida albicans hyphal morphogenesis and host cell damage. PLoS Pathog 12:e1005867. doi: 10.1371/journal.ppat.1005867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sprague JL, Schille TB, Allert S, Trümper V, Lier A, Großmann P, Priest EL, Tsavou A, Panagiotou G, Naglik JR, Wilson D, Schäuble S, Kasper L, Hube B. 2024. Candida albicans translocation through the intestinal epithelial barrier is promoted by fungal zinc acquisition and limited by NFκB-mediated barrier protection. PLoS Pathog 20:e1012031. doi: 10.1371/journal.ppat.1012031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Askari F, Rasheed M, Kaur R. 2022. The yapsin family of aspartyl proteases regulate glucose homeostasis in Candida glabrata. J Biol Chem 298:101593. doi: 10.1016/j.jbc.2022.101593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bairwa G, Rasheed M, Taigwal R, Sahoo R, Kaur R. 2014. GPI (glycosylphosphatidylinositol)-linked aspartyl proteases regulate vacuole homoeostasis in Candida glabrata. Biochem J 458:323–334. doi: 10.1042/BJ20130757 [DOI] [PubMed] [Google Scholar]
- 45. Rasheed M, Battu A, Kaur R. 2018. Aspartyl proteases in Candida glabrata are required for suppression of the host innate immune response. J Biol Chem 293:6410–6433. doi: 10.1074/jbc.M117.813741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fernández-Arenas E, Bleck CKE, Nombela C, Gil C, Griffiths G, Diez-Orejas R. 2009. Candida albicans actively modulates intracellular membrane trafficking in mouse macrophage phagosomes. Cell Microbiol 11:560–589. doi: 10.1111/j.1462-5822.2008.01274.x [DOI] [PubMed] [Google Scholar]
- 47. Seider K, Brunke S, Schild L, Jablonowski N, Wilson D, Majer O, Barz D, Haas A, Kuchler K, Schaller M, Hube B. 2011. The facultative intracellular pathogen Candida glabrata subverts macrophage cytokine production and phagolysosome maturation. J Immunol 187:3072–3086. doi: 10.4049/jimmunol.1003730 [DOI] [PubMed] [Google Scholar]
- 48. Wächtler B, Citiulo F, Jablonowski N, Förster S, Dalle F, Schaller M, Wilson D, Hube B. 2012. Candida albicans-epithelial interactions: dissecting the roles of active penetration, induced endocytosis and host factors on the infection process. PLoS One 7:e36952. doi: 10.1371/journal.pone.0036952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pfaller MA, Moet GJ, Messer SA, Jones RN, Castanheira M. 2011. Geographic variations in species distribution and echinocandin and azole antifungal resistance rates among Candida bloodstream infection isolates: report from the SENTRY antimicrobial surveillance program (2008 to 2009). J Clin Microbiol 49:396–399. doi: 10.1128/JCM.01398-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yang ZT, Wu L, Liu XY, Zhou M, Li J, Wu JY, Cai Y, Mao EQ, Chen EZ, Lortholary O. 2014. Epidemiology, species distribution and outcome of nosocomial Candida spp. bloodstream infection in Shanghai. BMC Infect Dis 14:241. doi: 10.1186/1471-2334-14-241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rajni E, Chaudhary P, Garg VK, Sharma R, Malik M. 2022. A complete clinico-epidemiological and microbiological profile of candidemia cases in a tertiary-care hospital in Western India. Antimicrob Steward Healthc Epidemiol 2:e37. doi: 10.1017/ash.2021.235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gamaletsou MN, Walsh TJ, Zaoutis T, Pagoni M, Kotsopoulou M, Voulgarelis M, Panayiotidis P, Vassilakopoulos T, Angelopoulou MK, Marangos M, Spyridonidis A, Kofteridis D, Pouli A, Sotiropoulos D, Matsouka P, Argyropoulou A, Perloretzou S, Leckerman K, Manaka A, Oikonomopoulos P, Daikos G, Petrikkos G, Sipsas NV. 2014. A prospective, cohort, multicentre study of candidaemia in hospitalized adult patients with haematological malignancies. Clin Microbiol Infect 20:O50–O57. doi: 10.1111/1469-0691.12312 [DOI] [PubMed] [Google Scholar]
- 53. Al-Yasiri MH, Normand AC, L’Ollivier C, Lachaud L, Bourgeois N, Rebaudet S, Piarroux R, Mauffrey JF, Ranque S. 2016. Opportunistic fungal pathogen Candida glabrata circulates between humans and yellow-legged gulls. Sci Rep 6:36157. doi: 10.1038/srep36157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Opulente DA, Langdon QK, Buh KV, Haase MAB, Sylvester K, Moriarty RV, Jarzyna M, Considine SL, Schneider RM, Hittinger CT. 2019. Pathogenic budding yeasts isolated outside of clinical settings. FEMS Yeast Res 19:foz032. doi: 10.1093/femsyr/foz032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bensasson D, Dicks J, Ludwig JM, Bond CJ, Elliston A, Roberts IN, James SA. 2019. Diverse lineages of Candida albicans live on old oaks. Genetics 211:277–288. doi: 10.1534/genetics.118.301482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Soltani M, Bayat M, Hashemi SJ, Zia M, Pestechian N. 2013. Isolation of Cryptococcus neoformans and other opportunistic fungi from pigeon droppings. J Res Med Sci 18:56–60. [PMC free article] [PubMed] [Google Scholar]
- 57. Sautour M, Lemaître J-P, Ranjard L, Truntzer C, Basmaciyan L, Depret G, Hartmann A, Dalle F. 2021. Detection and survival of Candida albicans in soils. Environmental DNA 3:1093–1101. doi: 10.1002/edn3.230 [DOI] [Google Scholar]
- 58. Seyedmousavi S, Bosco S de MG, de Hoog S, Ebel F, Elad D, Gomes RR, Jacobsen ID, Jensen HE, Martel A, Mignon B, Pasmans F, Piecková E, Rodrigues AM, Singh K, Vicente VA, Wibbelt G, Wiederhold NP, Guillot J. 2018. Fungal infections in animals: a patchwork of different situations. Med Mycol 56:165–187. doi: 10.1093/mmy/myx104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Edelmann A, Krüger M, Schmid J. 2005. Genetic relationship between human and animal isolates of Candida albicans. J Clin Microbiol 43:6164–6166. doi: 10.1128/JCM.43.12.6164-6166.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Morrell M, Fraser VJ, Kollef MH. 2005. Delaying the empiric treatment of Candida bloodstream infection until positive blood culture results are obtained: a potential risk factor for hospital mortality. Antimicrob Agents Chemother 49:3640–3645. doi: 10.1128/AAC.49.9.3640-3645.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Barantsevich N, Barantsevich E. 2022. Diagnosis and treatment of invasive candidiasis. Antibiotics (Basel) 11:718. doi: 10.3390/antibiotics11060718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Arafa SH, Elbanna K, Osman GEH, Abulreesh HH. 2023. Candida diagnostic techniques: a review. J Umm Al-Qura Univ Appll Sci 9:360–377. doi: 10.1007/s43994-023-00049-2 [DOI] [Google Scholar]
- 63. Odds FC, Bernaerts R. 1994. CHROMagar Candida, a new differential isolation medium for presumptive identification of clinically important Candida species. J Clin Microbiol 32:1923–1929. doi: 10.1128/jcm.32.8.1923-1929.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. K Hussain K, Malavia D, M Johnson E, Littlechild J, Winlove CP, Vollmer F, Gow NAR. 2020. Biosensors and diagnostics for fungal detection. J Fungi (Basel) 6:349. doi: 10.3390/jof6040349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hay R. 2018. Therapy of skin, hair and nail fungal infections. J Fungi (Basel) 4:99. doi: 10.3390/jof4030099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Mohamed AA, Lu XL, Mounmin FA. 2019. Diagnosis and treatment of esophageal candidiasis: current updates. Can J Gastroenterol Hepatol 2019:3585136. doi: 10.1155/2019/3585136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Anderson MR, Klink K, Cohrssen A. 2004. Evaluation of vaginal complaints. JAMA 291:1368–1379. doi: 10.1001/jama.291.11.1368 [DOI] [PubMed] [Google Scholar]
- 68. Taudorf EH, Jemec GBE, Hay RJ, Saunte DML. 2019. Cutaneous candidiasis - an evidence-based review of topical and systemic treatments to inform clinical practice. J Eur Acad Dermatol Venereol 33:1863–1873. doi: 10.1111/jdv.15782 [DOI] [PubMed] [Google Scholar]
- 69. Foxman B, Muraglia R, Dietz JP, Sobel JD, Wagner J. 2013. Prevalence of recurrent vulvovaginal candidiasis in 5 European countries and the United States: results from an internet panel survey. J Low Genit Tract Dis 17:340–345. doi: 10.1097/LGT.0b013e318273e8cf [DOI] [PubMed] [Google Scholar]
- 70. Zhai B, Ola M, Rolling T, Tosini NL, Joshowitz S, Littmann ER, Amoretti LA, Fontana E, Wright RJ, Miranda E, Veelken CA, Morjaria SM, Peled JU, van den Brink MRM, Babady NE, Butler G, Taur Y, Hohl TM. 2020. High-resolution mycobiota analysis reveals dynamic intestinal translocation preceding invasive candidiasis. Nat Med 26:59–64. doi: 10.1038/s41591-019-0709-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Nucci M, Anaissie E. 2001. Revisiting the source of candidemia: skin or gut? Clin Infect Dis 33:1959–1967. doi: 10.1086/323759 [DOI] [PubMed] [Google Scholar]
- 72. Pappas PG, Rex JH, Lee J, Hamill RJ, Larsen RA, Powderly W, Kauffman CA, Hyslop N, Mangino JE, Chapman S, Horowitz HW, Edwards JE, Dismukes WE, NIAID Mycoses Study Group . 2003. A prospective observational study of candidemia: epidemiology, therapy, and influences on mortality in hospitalized adult and pediatric patients. Clin Infect Dis 37:634–643. doi: 10.1086/376906 [DOI] [PubMed] [Google Scholar]
- 73. Tortorano AM, Peman J, Bernhardt H, Klingspor L, Kibbler CC, Faure O, Biraghi E, Canton E, Zimmermann K, Seaton S, Grillot R, ECMM Working Group on Candidaemia . 2004. Epidemiology of candidaemia in Europe: results of 28-month European confederation of medical mycology (ECMM) hospital-based surveillance study. Eur J Clin Microbiol Infect Dis 23:317–322. doi: 10.1007/s10096-004-1103-y [DOI] [PubMed] [Google Scholar]
- 74. Delaloye J, Calandra T. 2014. Invasive candidiasis as a cause of sepsis in the critically ill patient. Virulence 5:161–169. doi: 10.4161/viru.26187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Guzman JA, Tchokonte R, Sobel JD. 2011. Septic shock due to candidemia: outcomes and predictors of shock development. J Clin Med Res 3:65–71. doi: 10.4021/jocmr536w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Xie GH, Fang XM, Fang Q, Wu XM, Jin YH, Wang JL, Guo QL, Gu MN, Xu QP, Wang DX, Yao SL, Yuan SY, Du ZH, Sun YB, Wang HH, Wu SJ, Cheng BL. 2008. Impact of invasive fungal infection on outcomes of severe sepsis: a multicenter matched cohort study in critically ill surgical patients. Crit Care 12:R5. doi: 10.1186/cc6766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Machado M, Estévez A, Sánchez-Carrillo C, Guinea J, Escribano P, Alonso R, Valerio M, Padilla B, Bouza E, Muñoz P. 2022. Incidence of candidemia is higher in COVID-19 versus non-COVID-19 patients, but not driven by intrahospital transmission. J Fungi (Basel) 8:305. doi: 10.3390/jof8030305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Moser D, Biere K, Han B, Hoerl M, Schelling G, Choukér A, Woehrle T. 2021. COVID-19 impairs immune response to Candida albicans. Front Immunol 12:640644. doi: 10.3389/fimmu.2021.640644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kayaaslan B, Kaya Kalem A, Asilturk D, Kaplan B, Dönertas G, Hasanoglu I, Eser F, Korkmazer R, Oktay Z, Ozkocak Turan I, Erdem D, Bektas H, Guner R. 2022. Incidence and risk factors for COVID-19 associated candidemia (CAC) in ICU patients. Mycoses 65:508–516. doi: 10.1111/myc.13431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Munro CA, Hube B. 2002. Anti-fungal therapy at the HAART of viral therapy. Trends Microbiol 10:173–177. doi: 10.1016/s0966-842x(02)02330-2 [DOI] [PubMed] [Google Scholar]
- 81. Taverne-Ghadwal L, Kuhns M, Buhl T, Schulze MH, Mbaitolum WJ, Kersch L, Weig M, Bader O, Groß U. 2022. Epidemiology and prevalence of oral candidiasis in HIV patients from chad in the post-HAART era. Front Microbiol 13:844069. doi: 10.3389/fmicb.2022.844069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Kusakabe T, Lin W-Y, Cheong J-G, Singh G, Ravishankar A, Yeung ST, Mesko M, DeCelie MB, Carriche G, Zhao Z, Rand S, Doron I, Putzel GG, Worgall S, Cushing M, Westblade L, Inghirami G, Parkhurst CN, Guo C-J, Schotsaert M, García-Sastre A, Josefowicz SZ, Salvatore M, Iliev ID. 2023. Fungal microbiota sustains lasting immune activation of neutrophils and their progenitors in severe COVID-19. Nat Immunol 24:1879–1889. doi: 10.1038/s41590-023-01637-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Jhugroo C, Divakar DD, Jhugroo P, Al-Amri SAS, Alahmari AD, Vijaykumar S, Parine NR. 2019. Characterization of oral mucosa lesions and prevalence of yeasts in diabetic patients: a comparative study. Microb Pathog 126:363–367. doi: 10.1016/j.micpath.2018.11.028 [DOI] [PubMed] [Google Scholar]
- 84. Gunther LSA, Martins HPR, Gimenes F, Abreu ALP de, Consolaro MEL, Svidzinski TIE. 2014. Prevalence of Candida albicans and non-albicans isolates from vaginal secretions: comparative evaluation of colonization, vaginal candidiasis and recurrent vaginal candidiasis in diabetic and non-diabetic women. Sao Paulo Med J 132:116–120. doi: 10.1590/1516-3180.2014.1322640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Rodrigues CF, Rodrigues ME, Henriques M. 2019. Candida sp. infections in patients with diabetes mellitu. J Clin Med 8:76. doi: 10.3390/jcm8010076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Dohlman AB, Klug J, Mesko M, Gao IH, Lipkin SM, Shen X, Iliev ID. 2022. A pan-cancer mycobiome analysis reveals fungal involvement in gastrointestinal and lung tumors. Cell 185:3807–3822. doi: 10.1016/j.cell.2022.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Li XV, Leonardi I, Putzel GG, Semon A, Fiers WD, Kusakabe T, Lin W-Y, Gao IH, Doron I, Gutierrez-Guerrero A, DeCelie MB, Carriche GM, Mesko M, Yang C, Naglik JR, Hube B, Scherl EJ, Iliev ID. 2022. Immune regulation by fungal strain diversity in inflammatory bowel disease. Nature 603:672–678. doi: 10.1038/s41586-022-04502-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Morris GC, Dean G, Soni S, Sundaram S, Fearnley N, Wilson JD. 2022. Outcomes and experiences of using oral voriconazole with or without concomitant topical agents to treat refractory vulvovaginal yeast infections. Int J STD AIDS 33:1134–1141. doi: 10.1177/09564624221127356 [DOI] [PubMed] [Google Scholar]
- 89. Phillips NA, Rocktashel M, Merjanian L. 2023. Ibrexafungerp for the treatment of vulvovaginal candidiasis: design, development and place in therapy. Drug Des Devel Ther 17:363–367. doi: 10.2147/DDDT.S339349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Murphy SE, Bicanic T. 2021. Drug resistance and novel therapeutic approaches in invasive candidiasis. Front Cell Infect Microbiol 11:759408. doi: 10.3389/fcimb.2021.759408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Zhao Y, Perlin DS. 2020. Review of the novel echinocandin antifungal rezafungin: animal studies and clinical data. J Fungi (Basel) 6:192. doi: 10.3390/jof6040192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Arendrup MC, Patterson TF. 2017. Multidrug-resistant Candida: epidemiology, molecular mechanisms, and treatment. J Infect Dis 216:S445–S451. doi: 10.1093/infdis/jix131 [DOI] [PubMed] [Google Scholar]
- 93. Berman J, Krysan DJ. 2020. Drug resistance and tolerance in fungi. Nat Rev Microbiol 18:319–331. doi: 10.1038/s41579-019-0322-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Fisher MC, Alastruey-Izquierdo A, Berman J, Bicanic T, Bignell EM, Bowyer P, Bromley M, Brüggemann R, Garber G, Cornely OA, Gurr SJ, Harrison TS, Kuijper E, Rhodes J, Sheppard DC, Warris A, White PL, Xu J, Zwaan B, Verweij PE. 2022. Tackling the emerging threat of antifungal resistance to human health. Nat Rev Microbiol 20:557–571. doi: 10.1038/s41579-022-00720-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Gow NAR, Johnson C, Berman J, Coste AT, Cuomo CA, Perlin DS, Bicanic T, Harrison TS, Wiederhold N, Bromley M, Chiller T, Edgar K. 2022. The importance of antimicrobial resistance in medical mycology. Nat Commun 13:5352. doi: 10.1038/s41467-022-32249-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Deravi N, Fathi M, Tabatabaeifar SN, Pooransari P, Ahmadi B, Shokoohi G, Yaghoobpoor S, Vakili K, Lotfali E, Ansari S. 2021. Azole antifungal resistance in Candida albicans and Candida glabrata isolated from vulvovaginal candidiasis patients. Arch Clin Infect Dis 16:e106360. doi: 10.5812/archcid.106360 [DOI] [Google Scholar]
- 97. Aldardeer NF, Albar H, Al-Attas M, Eldali A, Qutub M, Hassanien A, Alraddadi B. 2020. Antifungal resistance in patients with candidaemia: a retrospective cohort study. BMC Infect Dis 20:55. doi: 10.1186/s12879-019-4710-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Robbins N, Cowen LE. 2021. Antifungal drug resistance: deciphering the mechanisms governing multidrug resistance in the fungal pathogen Candida glabrata. Curr Biol 31:R1520–R1523. doi: 10.1016/j.cub.2021.09.071 [DOI] [PubMed] [Google Scholar]
- 99. Alexander BD, Johnson MD, Pfeiffer CD, Jiménez-Ortigosa C, Catania J, Booker R, Castanheira M, Messer SA, Perlin DS, Pfaller MA. 2013. Increasing echinocandin resistance in Candida glabrata: clinical failure correlates with presence of FKS mutations and elevated minimum inhibitory concentrations. Clin Infect Dis 56:1724–1732. doi: 10.1093/cid/cit136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Hoenigl M, Sprute R, Egger M, Arastehfar A, Cornely OA, Krause R, Lass-Flörl C, Prattes J, Spec A, Thompson GR III, Wiederhold N, Jenks JD. 2021. The antifungal pipeline: fosmanogepix, ibrexafungerp, olorofim, opelconazole, and rezafungin. Drugs 81:1703–1729. doi: 10.1007/s40265-021-01611-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Logan A, Wolfe A, Williamson JC. 2022. Antifungal resistance and the role of new therapeutic agents. Curr Infect Dis Rep 24:105–116. doi: 10.1007/s11908-022-00782-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Duggan S, Usher J. 2023. Candida glabrata: a powerhouse of resistance. PLoS Pathog 19:e1011651. doi: 10.1371/journal.ppat.1011651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Bhattacharya S, Sae-Tia S, Fries BC. 2020. Candidiasis and mechanisms of antifungal resistance. Antibiotics (Basel) 9:312. doi: 10.3390/antibiotics9060312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Cowen LE, Anderson JB, Kohn LM. 2002. Evolution of drug resistance in Candida albicans. Annu Rev Microbiol 56:139–165. doi: 10.1146/annurev.micro.56.012302.160907 [DOI] [PubMed] [Google Scholar]
- 105. Denning DW. 2022. Antifungal drug resistance: an update. Eur J Hosp Pharm 29:109–112. doi: 10.1136/ejhpharm-2020-002604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Ford CB, Funt JM, Abbey D, Issi L, Guiducci C, Martinez DA, Delorey T, Li BY, White TC, Cuomo C, Rao RP, Berman J, Thompson DA, Regev A. 2015. The evolution of drug resistance in clinical isolates of Candida albicans. Elife 4:e00662. doi: 10.7554/eLife.00662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Frías-De-León MG, Hernández-Castro R, Conde-Cuevas E, García-Coronel IH, Vázquez-Aceituno VA, Soriano-Ursúa MA, Farfán-García ED, Ocharán-Hernández E, Rodríguez-Cerdeira C, Arenas R, Robledo-Cayetano M, Ramírez-Lozada T, Meza-Meneses P, Pinto-Almazán R, Martínez-Herrera E. 2021. Candida glabrata antifungal resistance and virulence factors, a perfect pathogenic combination. Pharmaceutics 13:1529. doi: 10.3390/pharmaceutics13101529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Hassan Y, Chew SY, Than LTL. 2021. Candida glabrata: pathogenicity and resistance mechanisms for adaptation and survival. J Fungi (Basel) 7:667. doi: 10.3390/jof7080667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Ksiezopolska E, Gabaldón T. 2018. Evolutionary emergence of drug resistance in Candida opportunistic pathogens. Genes (Basel) 9:461. doi: 10.3390/genes9090461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Lotfali E, Fattahi A, Sayyahfar S, Ghasemi R, Rabiei MM, Fathi M, Vakili K, Deravi N, Soheili A, Toreyhi H, Shirvani F. 2021. A review on molecular mechanisms of antifungal resistance in Candida glabrata: update and recent advances. Microb Drug Resist 27:1371–1388. doi: 10.1089/mdr.2020.0235 [DOI] [PubMed] [Google Scholar]
- 111. Whaley SG, Berkow EL, Rybak JM, Nishimoto AT, Barker KS, Rogers PD. 2016. Azole antifungal resistance in Candida albicans and emerging non-albicans Candida species. Front Microbiol 7:2173. doi: 10.3389/fmicb.2016.02173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Borman AM, Petch R, Linton CJ, Palmer MD, Bridge PD, Johnson EM. 2008. Candida nivariensis, an emerging pathogenic fungus with multidrug resistance to antifungal agents. J Clin Microbiol 46:933–938. doi: 10.1128/JCM.02116-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Hou X, Xiao M, Chen SC-A, Wang H, Yu S-Y, Fan X, Kong F, Xu Y-C. 2017. Identification and antifungal susceptibility profiles of Candida nivariensis and Candida bracarensis in a multi-center Chinese collection of yeasts. Front Microbiol 8:5. doi: 10.3389/fmicb.2017.00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Costa-de-Oliveira S, Rodrigues AG. 2020. Candida albicans antifungal resistance and tolerance in bloodstream infections: the triad yeast-host-antifungal. Microorganisms 8:154. doi: 10.3390/microorganisms8020154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Badrane H, Cheng S, Dupont CL, Hao B, Driscoll E, Morder K, Liu G, Newbrough A, Fleres G, Kaul D, Espinoza JL, Clancy CJ, Nguyen MH. 2023. Genotypic diversity and unrecognized antifungal resistance among populations of Candida glabrata from positive blood cultures. Nat Commun 14:5918. doi: 10.1038/s41467-023-41509-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Ferrari S, Sanguinetti M, De Bernardis F, Torelli R, Posteraro B, Vandeputte P, Sanglard D. 2011. Loss of mitochondrial functions associated with azole resistance in Candida glabrata results in enhanced virulence in mice. Antimicrob Agents Chemother 55:1852–1860. doi: 10.1128/AAC.01271-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Siscar-Lewin S, Gabaldón T, Aldejohann AM, Kurzai O, Hube B, Brunke S. 2021. Transient mitochondria dysfunction confers fungal cross-resistance against phagocytic killing and fluconazole. mBio 12:e0112821. doi: 10.1128/mBio.01128-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Selmecki A, Gerami-Nejad M, Paulson C, Forche A, Berman J. 2008. An isochromosome confers drug resistance in vivo by amplification of two genes, ERG11 and TAC1. Mol Microbiol 68:624–641. doi: 10.1111/j.1365-2958.2008.06176.x [DOI] [PubMed] [Google Scholar]
- 119. Silver PM, Oliver BG, White TC. 2004. Role of Candida albicans transcription factor Upc2p in drug resistance and sterol metabolism. Eukaryot Cell 3:1391–1397. doi: 10.1128/EC.3.6.1391-1397.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Anderson MZ, Saha A, Haseeb A, Bennett RJ. 2017. A chromosome 4 trisomy contributes to increased fluconazole resistance in a clinical isolate of Candida albicans. Microbiology (Reading) 163:856–865. doi: 10.1099/mic.0.000478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Shin JH, Chae MJ, Song JW, Jung SI, Cho D, Kee SJ, Kim SH, Shin MG, Suh SP, Ryang DW. 2007. Changes in karyotype and azole susceptibility of sequential bloodstream isolates from patients with Candida glabrata candidemia. J Clin Microbiol 45:2385–2391. doi: 10.1128/JCM.00381-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Torelli R, Posteraro B, Ferrari S, La Sorda M, Fadda G, Sanglard D, Sanguinetti M. 2008. The ATP-binding cassette transporter-encoding gene CgSNQ2 is contributing to the CgPDR1-dependent azole resistance of Candida glabrata. Mol Microbiol 68:186–201. doi: 10.1111/j.1365-2958.2008.06143.x [DOI] [PubMed] [Google Scholar]
- 123. Ksiezopolska E, Schikora-Tamarit MÀ, Beyer R, Nunez-Rodriguez JC, Schüller C, Gabaldón T. 2021. Narrow mutational signatures drive acquisition of multidrug resistance in the fungal pathogen Candida glabrata. Curr Biol 31:5314–5326. doi: 10.1016/j.cub.2021.09.084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Flowers SA, Barker KS, Berkow EL, Toner G, Chadwick SG, Gygax SE, Morschhäuser J, Rogers PD. 2012. Gain-of-function mutations in UPC2 are a frequent cause of ERG11 upregulation in azole-resistant clinical isolates of Candida albicans. Eukaryot Cell 11:1289–1299. doi: 10.1128/EC.00215-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Vu BG, Stamnes MA, Li Y, Rogers PD, Moye-Rowley WS. 2021. The Candida glabrata Upc2A transcription factor is a global regulator of antifungal drug resistance pathways. PLoS Genet 17:e1009582. doi: 10.1371/journal.pgen.1009582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Healey KR, Zhao Y, Perez WB, Lockhart SR, Sobel JD, Farmakiotis D, Kontoyiannis DP, Sanglard D, Taj-Aldeen SJ, Alexander BD, Jimenez-Ortigosa C, Shor E, Perlin DS. 2016. Prevalent mutator genotype identified in fungal pathogen Candida glabrata promotes multi-drug resistance. Nat Commun 7:11128. doi: 10.1038/ncomms11128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Lee KK, Maccallum DM, Jacobsen MD, Walker LA, Odds FC, Gow NAR, Munro CA. 2012. Elevated cell wall chitin in Candida albicans confers echinocandin resistance in vivo. Antimicrob Agents Chemother 56:208–217. doi: 10.1128/AAC.00683-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. da Silva Dantas A, Nogueira F, Lee KK, Walker LA, Edmondson M, Brand AC, Lenardon MD, Gow NAR. 2021. Crosstalk between the calcineurin and cell wall integrity pathways prevents chitin overexpression in Candida albicans. J Cell Sci 134:jcs258889. doi: 10.1242/jcs.258889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Walker LA, Gow NAR, Munro CA. 2013. Elevated chitin content reduces the susceptibility of Candida species to caspofungin. Antimicrob Agents Chemother 57:146–154. doi: 10.1128/AAC.01486-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Walker LA, Munro CA, de Bruijn I, Lenardon MD, McKinnon A, Gow NAR. 2008. Stimulation of chitin synthesis rescues Candida albicans from echinocandins. PLoS Pathog 4:e1000040. doi: 10.1371/journal.ppat.1000040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Walker LA, Gow NAR, Munro CA. 2010. Fungal echinocandin resistance. Fungal Genet Biol 47:117–126. doi: 10.1016/j.fgb.2009.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Marakalala MJ, Vautier S, Potrykus J, Walker LA, Shepardson KM, Hopke A, Mora-Montes HM, Kerrigan A, Netea MG, Murray GI, Maccallum DM, Wheeler R, Munro CA, Gow NAR, Cramer RA, Brown AJP, Brown GD. 2013. Differential adaptation of Candida albicans in vivo modulates immune recognition by dectin-1. PLoS Pathog 9:e1003315. doi: 10.1371/journal.ppat.1003315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Spettel K, Barousch W, Makristathis A, Zeller I, Nehr M, Selitsch B, Lackner M, Rath PM, Steinmann J, Willinger B. 2019. Analysis of antifungal resistance genes in Candida albicans and Candida glabrata using next generation sequencing. PLoS One 14:e0210397. doi: 10.1371/journal.pone.0210397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Jensen RH, Astvad KMT, Silva LV, Sanglard D, Jørgensen R, Nielsen KF, Mathiasen EG, Doroudian G, Perlin DS, Arendrup MC. 2015. Stepwise emergence of azole, echinocandin and amphotericin B multidrug resistance in vivo in Candida albicans orchestrated by multiple genetic alterations. J Antimicrob Chemother 70:2551–2555. doi: 10.1093/jac/dkv140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Chapeland-Leclerc F, Hennequin C, Papon N, Noël T, Girard A, Socié G, Ribaud P, Lacroix C. 2010. Acquisition of flucytosine, azole, and caspofungin resistance in Candida glabrata bloodstream isolates serially obtained from a hematopoietic stem cell transplant recipient. Antimicrob Agents Chemother 54:1360–1362. doi: 10.1128/AAC.01138-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Santos MA, Keith G, Tuite MF. 1993. Non-standard translational events in Candida albicans mediated by an unusual seryl-tRNA with a 5'-CAG-3' (leucine) anticodon. EMBO J 12:607–616. doi: 10.1002/j.1460-2075.1993.tb05693.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Ahmad KM, Kokošar J, Guo X, Gu Z, Ishchuk OP, Piškur J. 2014. Genome structure and dynamics of the yeast pathogen Candida glabrata. FEMS Yeast Res 14:529–535. doi: 10.1111/1567-1364.12145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Butler G, Rasmussen MD, Lin MF, Santos MAS, Sakthikumar S, Munro CA, Rheinbay E, Grabherr M, Forche A, Reedy JL, et al. 2009. Evolution of pathogenicity and sexual reproduction in eight Candida genomes. Nature 459:657–662. doi: 10.1038/nature08064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Gao J, Wang H, Li Z, Wong AH-H, Wang Y-Z, Guo Y, Lin X, Zeng G, Liu H, Wang Y, Wang J. 2018. Candida albicans gains azole resistance by altering sphingolipid composition. Nat Commun 9:4495. doi: 10.1038/s41467-018-06944-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Segal ES, Gritsenko V, Levitan A, Yadav B, Dror N, Steenwyk JL, Silberberg Y, Mielich K, Rokas A, Gow NAR, Kunze R, Sharan R, Berman J. 2018. Gene essentiality analyzed by in vivo transposon mutagenesis and machine learning in a stable haploid isolate of Candida albicans. mBio 9:e02048-18. doi: 10.1128/mBio.02048-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Mba IE, Nweze EI, Eze EA, Anyaegbunam ZKG. 2022. Genome plasticity in Candida albicans: a cutting-edge strategy for evolution, adaptation, and survival. Infect Genet Evol 99:105256. doi: 10.1016/j.meegid.2022.105256 [DOI] [PubMed] [Google Scholar]
- 142. Yang F, Todd RT, Selmecki A, Jiang YY, Cao YB, Berman J. 2021. The fitness costs and benefits of trisomy of each Candida albicans chromosome. Genetics 218:iyab056. doi: 10.1093/genetics/iyab056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Kakade P, Sircaik S, Maufrais C, Ene IV, Bennett RJ. 2023. Aneuploidy and gene dosage regulate filamentation and host colonization by Candida albicans. Proc Natl Acad Sci U S A 120:e2218163120. doi: 10.1073/pnas.2218163120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Forche A, Solis NV, Swidergall M, Thomas R, Guyer A, Beach A, Cromie GA, Le GT, Lowell E, Pavelka N, Berman J, Dudley AM, Selmecki A, Filler SG. 2019. Selection of Candida albicans trisomy during oropharyngeal infection results in a commensal-like phenotype. PLoS Genet 15:e1008137. doi: 10.1371/journal.pgen.1008137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Todd RT, Selmecki A. 2020. Expandable and reversible copy number amplification drives rapid adaptation to antifungal drugs. Elife 9:e58349. doi: 10.7554/eLife.58349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Braun BR, van Het Hoog M, d’Enfert C, Martchenko M, Dungan J, Kuo A, Inglis DO, Uhl MA, Hogues H, Berriman M, et al. 2005. A human-curated annotation of the Candida albicans genome. PLoS Genet 1:36–57. doi: 10.1371/journal.pgen.0010001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Carreté L, Ksiezopolska E, Pegueroles C, Gómez-Molero E, Saus E, Iraola-Guzmán S, Loska D, Bader O, Fairhead C, Gabaldón T. 2022. Patterns of genomic variation in the opportunistic pathogen Candida glabrata suggest the existence of mating and a secondary association with humans. Curr Biol 32:3219. doi: 10.1016/j.cub.2022.06.082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Pais P, Galocha M, Takahashi-Nakaguchi A, Chibana H, Teixeira MC. 2022. Multiple genome analysis of Candida glabrata clinical isolates renders new insights into genetic diversity and drug resistance determinants. Microb Cell 9:174–189. doi: 10.15698/mic2022.11.786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Sudbery PE. 2011. Growth of Candida albicans hyphae. Nat Rev Microbiol 9:737–748. doi: 10.1038/nrmicro2636 [DOI] [PubMed] [Google Scholar]
- 150. Malavia D, Lehtovirta-Morley LE, Alamir O, Weiß E, Gow NAR, Hube B, Wilson D. 2017. Zinc limitation induces a hyper-adherent goliath phenotype in Candida albicans. Front Microbiol 8:2238. doi: 10.3389/fmicb.2017.02238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Gow NAR, Yadav B. 2017. Microbe Profile: Candida albicans: a shape-changing, opportunistic pathogenic fungus of humans. Microbiology (Reading) 163:1145–1147. doi: 10.1099/mic.0.000499 [DOI] [PubMed] [Google Scholar]
- 152. Noble SM, Gianetti BA, Witchley JN. 2017. Candida albicans cell-type switching and functional plasticity in the mammalian host. Nat Rev Microbiol 15:96–108. doi: 10.1038/nrmicro.2016.157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Bedekovic T, Usher J. 2023. Is there a relationship between mating and pathogenesis in two human fungal pathogens, Candida albicans and Candida glabrata? Curr Clin Microbiol Rep 10:47–54. doi: 10.1007/s40588-023-00192-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Zheng Q, Liu J, Qin J, Wang B, Bing J, Du H, Li M, Yu F, Huang G. 2022. Ploidy variation and spontaneous haploid-diploid switching of Candida glabrata clinical isolates. mSphere 7:e0026022. doi: 10.1128/msphere.00260-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Hu C, Fong G, Wurster S, Kontoyiannis DP, Beyda ND. 2022. Clumping morphology influences virulence uncoupled from echinocandin resistance in Candida glabrata. Microbiol Spectr 10:e0183721. doi: 10.1128/spectrum.01837-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Desai JV. 2018. Candida albicans hyphae: from growth initiation to invasion. J Fungi (Basel) 4:10. doi: 10.3390/jof4010010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Sprague JL, Kasper L, Hube B. 2022. From intestinal colonization to systemic infections: Candida albicans translocation and dissemination. Gut Microbes 14:2154548. doi: 10.1080/19490976.2022.2154548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Allert S, Förster TM, Svensson C-M, Richardson JP, Pawlik T, Hebecker B, Rudolphi S, Juraschitz M, Schaller M, Blagojevic M, Morschhäuser J, Figge MT, Jacobsen ID, Naglik JR, Kasper L, Mogavero S, Hube B. 2018. Candida albicans-induced epithelial damage mediates translocation through intestinal barriers. mBio 9:e00915-18. doi: 10.1128/mBio.00915-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Naglik JR, König A, Hube B, Gaffen SL. 2017. Candida albicans-epithelial interactions and induction of mucosal innate immunity. Curr Opin Microbiol 40:104–112. doi: 10.1016/j.mib.2017.10.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Sheppard DC, Filler SG. 2014. Host cell invasion by medically important fungi. Cold Spring Harb Perspect Med 5:a019687. doi: 10.1101/cshperspect.a019687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Wächtler B, Wilson D, Haedicke K, Dalle F, Hube B. 2011. From attachment to damage: defined genes of Candida albicans mediate adhesion, invasion and damage during interaction with oral epithelial cells. PLoS One 6:e17046. doi: 10.1371/journal.pone.0017046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Phan QT, Myers CL, Fu Y, Sheppard DC, Yeaman MR, Welch WH, Ibrahim AS, Edwards Jr JE, Filler SG. 2007. Als3 is a Candida albicans invasin that binds to cadherins and induces endocytosis by host cells. PLoS Biol 5:e64. doi: 10.1371/journal.pbio.0050064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Couttenier E, Bachellier-Bassi S, d’Enfert C, Villard C. 2022. Bending stiffness of Candida albicans hyphae as a proxy of cell wall properties. Lab Chip 22:3898–3909. doi: 10.1039/d2lc00219a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Puerner C, Kukhaleishvili N, Thomson D, Schaub S, Noblin X, Seminara A, Bassilana M, Arkowitz RA. 2020. Mechanical force-induced morphology changes in a human fungal pathogen. BMC Biol 18:122. doi: 10.1186/s12915-020-00833-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Lachat J, Pascault A, Thibaut D, Le Borgne R, Verbavatz JM, Weiner A. 2022. Trans-cellular tunnels induced by the fungal pathogen Candida albicans facilitate invasion through successive epithelial cells without host damage. Nat Commun 13:3781. doi: 10.1038/s41467-022-31237-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Sassani E, Khodavaisy S, Agha Kuchak Afshari S, Darabian S, Aala F, Rezaie S. 2016. Pseudohyphae formation in Candida glabrata due to CO2 exposure. Curr Med Mycol 2:49–53. doi: 10.18869/acadpub.cmm.2.4.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Brand A, Lee K, Veses V, Gow NAR. 2009. Calcium homeostasis is required for contact-dependent helical and sinusoidal tip growth in Candida albicans hyphae. Mol Microbiol 71:1155–1164. doi: 10.1111/j.1365-2958.2008.06592.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Almeida MC, Brand AC. 2017. Thigmo responses: the fungal sense of touch. Microbiol Spectr 5. doi: 10.1128/microbiolspec.FUNK-0040-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Brand A, Shanks S, Duncan VMS, Yang M, Mackenzie K, Gow NAR. 2007. Hyphal orientation of Candida albicans is regulated by a calcium-dependent mechanism. Curr Biol 17:347–352. doi: 10.1016/j.cub.2006.12.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Brand AC, Morrison E, Milne S, Gonia S, Gale CA, Gow NAR. 2014. Cdc42 GTPase dynamics control directional growth responses. Proc Natl Acad Sci U S A 111:811–816. doi: 10.1073/pnas.1307264111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Held M, Kašpar O, Edwards C, Nicolau DV. 2019. Intracellular mechanisms of fungal space searching in microenvironments. Proc Natl Acad Sci U S A 116:13543–13552. doi: 10.1073/pnas.1816423116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Puerner C, Serrano A, Wakade RS, Bassilana M, Arkowitz RA. 2021. A myosin light chain is critical for fungal growth robustness in Candida albicans. mBio 12:e0252821. doi: 10.1128/mBio.02528-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Xie Y, Loh ZY, Xue J, Zhou F, Sun J, Qiao Z, Jin S, Deng Y, Li H, Wang Y, Lu L, Gao Y, Miao Y. 2020. Orchestrated actin nucleation by the Candida albicans polarisome complex enables filamentous growth. J Biol Chem 295:14840–14854. doi: 10.1074/jbc.RA120.013890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Arkowitz RA, Bassilana M. 2019. Recent advances in understanding Candida albicans hyphal growth. F1000Res 8. doi: 10.12688/f1000research.18546.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Slutsky B, Buffo J, Soll DR. 1985. High-frequency switching of colony morphology in Candida albicans. Science 230:666–669. doi: 10.1126/science.3901258 [DOI] [PubMed] [Google Scholar]
- 176. Slutsky B, Staebell M, Anderson J, Risen L, Pfaller M, Soll DR. 1987. "White-opaque transition": a second high-frequency switching system in Candida albicans. J Bacteriol 169:189–197. doi: 10.1128/jb.169.1.189-197.1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Pérez-Martín J, Uría JA, Johnson AD. 1999. Phenotypic switching in Candida albicans is controlled by a SIR2 gene. EMBO J 18:2580–2592. doi: 10.1093/emboj/18.9.2580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Hull CM, Raisner RM, Johnson AD. 2000. Evidence for mating of the "asexual" yeast Candida albicans in a mammalian host. Science 289:307–310. doi: 10.1126/science.289.5477.307 [DOI] [PubMed] [Google Scholar]
- 179. Magee BB, Magee PT. 2000. Induction of mating in Candida albicans by construction of MTLa and MTLalpha strains. Science 289:310–313. doi: 10.1126/science.289.5477.310 [DOI] [PubMed] [Google Scholar]
- 180. Miller MG, Johnson AD. 2002. White-opaque switching in Candida albicans is controlled by mating-type locus homeodomain proteins and allows efficient mating. Cell 110:293–302. doi: 10.1016/s0092-8674(02)00837-1 [DOI] [PubMed] [Google Scholar]
- 181. Kvaal C, Lachke SA, Srikantha T, Daniels K, McCoy J, Soll DR. 1999. Misexpression of the opaque-phase-specific gene PEP1 (SAP1) in the white phase of Candida albicans confers increased virulence in a mouse model of cutaneous infection. Infect Immun 67:6652–6662. doi: 10.1128/IAI.67.12.6652-6662.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Lachke SA, Lockhart SR, Daniels KJ, Soll DR. 2003. Skin facilitates Candida albicans mating. Infect Immun 71:4970–4976. doi: 10.1128/IAI.71.9.4970-4976.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Takagi J, Singh-Babak SD, Lohse MB, Dalal CK, Johnson AD. 2019. Candida albicans white and opaque cells exhibit distinct spectra of organ colonization in mouse models of infection. PLoS One 14:e0218037. doi: 10.1371/journal.pone.0218037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Pande K, Chen C, Noble SM. 2013. Passage through the mammalian gut triggers a phenotypic switch that promotes Candida albicans commensalism. Nat Genet 45:1088–1091. doi: 10.1038/ng.2710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Tao L, Du H, Guan G, Dai Y, Nobile CJ, Liang W, Cao C, Zhang Q, Zhong J, Huang G. 2014. Discovery of a "white-gray-opaque" tristable phenotypic switching system in Candida albicans: roles of non-genetic diversity in host adaptation. PLoS Biol 12:e1001830. doi: 10.1371/journal.pbio.1001830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Lohse MB, Johnson AD. 2008. Differential phagocytosis of white versus opaque Candida albicans by Drosophila and mouse phagocytes. PLoS One 3:e1473. doi: 10.1371/journal.pone.0001473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Deng FS, Lin CH. 2018. Identification and characterization of ORF19.1725, a novel gene contributing to the white cell pheromone response and virulence-associated functions in Candida albicans. Virulence 9:866–878. doi: 10.1080/21505594.2018.1456228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Liang S-H, Anderson MZ, Hirakawa MP, Wang JM, Frazer C, Alaalm LM, Thomson GJ, Ene IV, Bennett RJ. 2019. Hemizygosity enables a mutational transition governing fungal virulence and commensalism. Cell Host Microbe 25:418–431. doi: 10.1016/j.chom.2019.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Park YN, Pujol C, Wessels DJ, Soll DR. 2020. Candida albicans double mutants lacking both EFG1 and WOR1 can still switch to opaque. mSphere 23:e00918-20. doi: 10.1128/mSphere.00918-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Jain N, Hasan F, Fries BC. 2008. Phenotypic switching in fungi. Curr Fungal Infect Rep 2:180–188. doi: 10.1007/s12281-008-0026-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Lachke SA, Joly S, Daniels K, Soll DR. 2002. Phenotypic switching and filamentation in Candida glabrata. Microbiology (Reading) 148:2661–2674. doi: 10.1099/00221287-148-9-2661 [DOI] [PubMed] [Google Scholar]
- 192. Bennett RJ, Johnson AD. 2005. Mating in Candida albicans and the search for a sexual cycle. Annu Rev Microbiol 59:233–255. doi: 10.1146/annurev.micro.59.030804.121310 [DOI] [PubMed] [Google Scholar]
- 193. Lockhart SR, Zhao R, Daniels KJ, Soll DR. 2003. Alpha-pheromone-induced "shmooing" and gene regulation require white-opaque switching during Candida albicans mating. Eukaryot Cell 2:847–855. doi: 10.1128/EC.2.5.847-855.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Johnson A. 2003. The biology of mating in Candida albicans. Nat Rev Microbiol 1:106–116. doi: 10.1038/nrmicro752 [DOI] [PubMed] [Google Scholar]
- 195. Usher J. 2019. The mechanisms of mating in pathogenic fungi-A plastic trait. Genes (Basel) 10:831. doi: 10.3390/genes10100831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Alby K, Schaefer D, Bennett RJ. 2009. Homothallic and heterothallic mating in the opportunistic pathogen Candida albicans. Nature 460:890–893. doi: 10.1038/nature08252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Guan G, Tao L, Yue H, Liang W, Gong J, Bing J, Zheng Q, Veri AO, Fan S, Robbins N, Cowen LE, Huang G. 2019. Environment-induced same-sex mating in the yeast Candida albicans through the Hsf1-Hsp90 pathway. PLoS Biol 17:e2006966. doi: 10.1371/journal.pbio.2006966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Guan G, Tao L, Li C, Xu M, Liu L, Bennett RJ, Huang G. 2023. Glucose depletion enables Candida albicans mating independently of the epigenetic white-opaque switch. Nat Commun 14:2067. doi: 10.1038/s41467-023-37755-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Srikantha T, Lachke SA, Soll DR. 2003. Three mating type-like loci in Candida glabrata. Eukaryot Cell 2:328–340. doi: 10.1128/EC.2.2.328-340.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Brown AJ, Gow NA. 1999. Regulatory networks controlling Candida albicans morphogenesis. Trends Microbiol 7:333–338. doi: 10.1016/s0966-842x(99)01556-5 [DOI] [PubMed] [Google Scholar]
- 201. Jacobsen ID, Wilson D, Wächtler B, Brunke S, Naglik JR, Hube B. 2012. Candida albicans dimorphism as a therapeutic target. Expert Rev Anti Infect Ther 10:85–93. doi: 10.1586/eri.11.152 [DOI] [PubMed] [Google Scholar]
- 202. Chen H, Zhou X, Ren B, Cheng L. 2020. The regulation of hyphae growth in Candida albicans. Virulence 11:337–348. doi: 10.1080/21505594.2020.1748930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Kornitzer D. 2019. Regulation of Candida albicans hyphal morphogenesis by endogenous signals. J Fungi (Basel) 5:21. doi: 10.3390/jof5010021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Villa S, Hamideh M, Weinstock A, Qasim MN, Hazbun TR, Sellam A, Hernday AD, Thangamani S. 2020. Transcriptional control of hyphal morphogenesis in Candida albicans. FEMS Yeast Res 20:foaa005. doi: 10.1093/femsyr/foaa005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Mancera E, Nocedal I, Hammel S, Gulati M, Mitchell KF, Andes DR, Nobile CJ, Butler G, Johnson AD. 2021. Evolution of the complex transcription network controlling biofilm formation in Candida species. Elife 10:e64682. doi: 10.7554/eLife.64682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Purohit D, Gajjar D. 2022. Tec1 and Ste12 transcription factors play a role in adaptation to low pH stress and biofilm formation in the human opportunistic fungal pathogen Candida glabrata. Int Microbiol 25:789–802. doi: 10.1007/s10123-022-00264-7 [DOI] [PubMed] [Google Scholar]
- 207. Iracane E, Vega-Estévez S, Buscaino A. 2021. On and off: epigenetic regulation of C. albicans morphological switches. Pathogens 10:1463. doi: 10.3390/pathogens10111463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Balachandra VK, Verma J, Shankar M, Tucey TM, Traven A, Schittenhelm RB, Ghosh SK. 2020. The RSC (Remodels the Structure of Chromatin) complex of Candida albicans shows compositional divergence with distinct roles in regulating pathogenic traits. PLoS Genet 16:e1009071. doi: 10.1371/journal.pgen.1009071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Zhao G, Rusche LN. 2021. Genetic analysis of sirtuin deacetylases in hyphal growth of Candida albicans. mSphere 6:e00053-21. doi: 10.1128/mSphere.00053-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Roy S, Gow NAR. 2023. The role of the Candida biofilm matrix in drug and immune protection. Cell Surf 10:100111. doi: 10.1016/j.tcsw.2023.100111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Staab JF, Bradway SD, Fidel PL, Sundstrom P. 1999. Adhesive and mammalian transglutaminase substrate properties of Candida albicans Hwp1. Science 283:1535–1538. doi: 10.1126/science.283.5407.1535 [DOI] [PubMed] [Google Scholar]
- 212. d’Enfert C, Janbon G. 2016. Biofilm formation in Candida glabrata: what have we learnt from functional genomics approaches? FEMS Yeast Res 16:fov111. doi: 10.1093/femsyr/fov111 [DOI] [PubMed] [Google Scholar]
- 213. Zarnowski R, Sanchez H, Covelli AS, Dominguez E, Jaromin A, Bernhardt J, Mitchell KF, Heiss C, Azadi P, Mitchell A, Andes DR. 2018. Candida albicans biofilm-induced vesicles confer drug resistance through matrix biogenesis. PLoS Biol 16:e2006872. doi: 10.1371/journal.pbio.2006872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Winter MB, Salcedo EC, Lohse MB, Hartooni N, Gulati M, Sanchez H, Takagi J, Hube B, Andes DR, Johnson AD, Craik CS, Nobile CJ. 2016. Global identification of biofilm-specific proteolysis in Candida albicans. mBio 7:e01514-16. doi: 10.1128/mBio.01514-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Brockert PJ, Lachke SA, Srikantha T, Pujol C, Galask R, Soll DR. 2003. Phenotypic switching and mating type switching of Candida glabrata at sites of colonization. Infect Immun 71:7109–7118. doi: 10.1128/IAI.71.12.7109-7118.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Daniels KJ, Srikantha T, Lockhart SR, Pujol C, Soll DR. 2006. Opaque cells signal white cells to form biofilms in Candida albicans. EMBO J 25:2240–2252. doi: 10.1038/sj.emboj.7601099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Park YN, Daniels KJ, Pujol C, Srikantha T, Soll DR. 2013. Candida albicans forms a specialized "sexual" as well as "pathogenic" biofilm. Eukaryot Cell 12:1120–1131. doi: 10.1128/EC.00112-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Vila T, Kong EF, Montelongo-Jauregui D, Van Dijck P, Shetty AC, McCracken C, Bruno VM, Jabra-Rizk MA. 2021. Therapeutic implications of C. albicans-S. aureus mixed biofilm in a murine subcutaneous catheter model of polymicrobial infection. Virulence 12:835–851. doi: 10.1080/21505594.2021.1894834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Cavalheiro M, Teixeira MC. 2018. Candida biofilms: threats, challenges, and promising strategies. Front Med (Lausanne) 5:28. doi: 10.3389/fmed.2018.00028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Kim D, Sengupta A, Niepa THR, Lee B-H, Weljie A, Freitas-Blanco VS, Murata RM, Stebe KJ, Lee D, Koo H. 2017. Candida albicans stimulates Streptococcus mutans microcolony development via cross-kingdom biofilm-derived metabolites. Sci Rep 7:41332. doi: 10.1038/srep41332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Gow NAR, Lenardon MD. 2023. Architecture of the dynamic fungal cell wall. Nat Rev Microbiol 21:248–259. doi: 10.1038/s41579-022-00796-9 [DOI] [PubMed] [Google Scholar]
- 222. Gow NAR, Latge JP, Munro CA. 2017. The fungal cell wall: structure, biosynthesis, and function. Microbiol Spectr 5. doi: 10.1128/microbiolspec.FUNK-0035-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Netea MG, Brown GD, Kullberg BJ, Gow NAR. 2008. An integrated model of the recognition of Candida albicans by the innate immune system. Nat Rev Microbiol 6:67–78. doi: 10.1038/nrmicro1815 [DOI] [PubMed] [Google Scholar]
- 224. Reithofer V, Fernández-Pereira J, Alvarado M, de Groot P, Essen L-O. 2021. A novel class of Candida glabrata cell wall proteins with beta-helix fold mediates adhesion in clinical isolates. PLoS Pathog 17:e1009980. doi: 10.1371/journal.ppat.1009980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Chang CK, Yang MC, Chen HF, Liao YL, Lan CY. 2022. The role of Sfp1 in Candida albicans cell wall maintenance. J Fungi (Basel) 8:1196. doi: 10.3390/jof8111196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Mottola A, Ramírez-Zavala B, Hünniger K, Kurzai O, Morschhäuser J. 2021. The zinc cluster transcription factor Czf1 regulates cell wall architecture and integrity in Candida albicans. Mol Microbiol 116:483–497. doi: 10.1111/mmi.14727 [DOI] [PubMed] [Google Scholar]
- 227. Essen L-O, Vogt MS, Mösch H-U. 2020. Diversity of GPI-anchored fungal adhesins. Biol Chem 401:1389–1405. doi: 10.1515/hsz-2020-0199 [DOI] [PubMed] [Google Scholar]
- 228. Reyna-Beltran E, Mendez CIB, Iranzo M, Mormeneo S, Luna-Arias JP. 2019. Candida albicans. IntechOpen, Rijeka. [Google Scholar]
- 229. Erwig LP, Gow NAR. 2016. Interactions of fungal pathogens with phagocytes. Nat Rev Microbiol 14:163–176. doi: 10.1038/nrmicro.2015.21 [DOI] [PubMed] [Google Scholar]
- 230. Casadevall A. 2022. Immunity to invasive fungal diseases. Annu Rev Immunol 40:121–141. doi: 10.1146/annurev-immunol-101220-034306 [DOI] [PubMed] [Google Scholar]
- 231. Lionakis MS, Drummond RA, Hohl TM. 2023. Immune responses to human fungal pathogens and therapeutic prospects. Nat Rev Immunol 23:433–452. doi: 10.1038/s41577-022-00826-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Glocker E-O, Hennigs A, Nabavi M, Schäffer AA, Woellner C, Salzer U, Pfeifer D, Veelken H, Warnatz K, Tahami F, Jamal S, Manguiat A, Rezaei N, Amirzargar AA, Plebani A, Hannesschläger N, Gross O, Ruland J, Grimbacher B. 2009. A homozygous CARD9 mutation in a family with susceptibility to fungal infections. N Engl J Med 361:1727–1735. doi: 10.1056/NEJMoa0810719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Lanternier F, Mahdaviani SA, Barbati E, Chaussade H, Koumar Y, Levy R, Denis B, Brunel A-S, Martin S, Loop M, et al. 2015. Inherited CARD9 deficiency in otherwise healthy children and adults with Candida species-induced meningoencephalitis, colitis, or both. J Allergy Clin Immunol 135:1558–1568. doi: 10.1016/j.jaci.2014.12.1930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Gross O, Gewies A, Finger K, Schäfer M, Sparwasser T, Peschel C, Förster I, Ruland J. 2006. Card9 controls a non-TLR signalling pathway for innate anti-fungal immunity. Nature 442:651–656. doi: 10.1038/nature04926 [DOI] [PubMed] [Google Scholar]
- 235. Drewniak A, Gazendam RP, Tool ATJ, van Houdt M, Jansen MH, van Hamme JL, van Leeuwen EMM, Roos D, Scalais E, de Beaufort C, Janssen H, van den Berg TK, Kuijpers TW. 2013. Invasive fungal infection and impaired neutrophil killing in human CARD9 deficiency. Blood 121:2385–2392. doi: 10.1182/blood-2012-08-450551 [DOI] [PubMed] [Google Scholar]
- 236. LeibundGut-Landmann S, Groß O, Robinson MJ, Osorio F, Slack EC, Tsoni SV, Schweighoffer E, Tybulewicz V, Brown GD, Ruland J, Reis e Sousa C. 2007. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat Immunol 8:630–638. doi: 10.1038/ni1460 [DOI] [PubMed] [Google Scholar]
- 237. McGreal EP, Rosas M, Brown GD, Zamze S, Wong SYC, Gordon S, Martinez-Pomares L, Taylor PR. 2006. The carbohydrate-recognition domain of Dectin-2 is a C-type lectin with specificity for high mannose. Glycobiology 16:422–430. doi: 10.1093/glycob/cwj077 [DOI] [PubMed] [Google Scholar]
- 238. Vendele I, Willment JA, Silva LM, Palma AS, Chai W, Liu Y, Feizi T, Spyrou M, Stappers MHT, Brown GD, Gow NAR. 2020. Mannan detecting C-type lectin receptor probes recognise immune epitopes with diverse chemical, spatial and phylogenetic heterogeneity in fungal cell walls. PLoS Pathog 16:e1007927. doi: 10.1371/journal.ppat.1007927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Saijo S, Ikeda S, Yamabe K, Kakuta S, Ishigame H, Akitsu A, Fujikado N, Kusaka T, Kubo S, Chung S, Komatsu R, Miura N, Adachi Y, Ohno N, Shibuya K, Yamamoto N, Kawakami K, Yamasaki S, Saito T, Akira S, Iwakura Y. 2010. Dectin-2 recognition of alpha-mannans and induction of Th17 cell differentiation is essential for host defense against Candida albicans. Immunity 32:681–691. doi: 10.1016/j.immuni.2010.05.001 [DOI] [PubMed] [Google Scholar]
- 240. Ifrim DC, Bain JM, Reid DM, Oosting M, Verschueren I, Gow NAR, van Krieken JH, Brown GD, Kullberg B-J, Joosten LAB, van der Meer JWM, Koentgen F, Erwig LP, Quintin J, Netea MG. 2014. Role of Dectin-2 for host defense against systemic infection with Candida glabrata. Infect Immun 82:1064–1073. doi: 10.1128/IAI.01189-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Ifrim DC, Quintin J, Courjol F, Verschueren I, van Krieken JH, Koentgen F, Fradin C, Gow NAR, Joosten LAB, van der Meer JWM, van de Veerdonk F, Netea MG. 2016. The role of Dectin-2 for host defense against disseminated candidiasis. J Interferon Cytokine Res 36:267–276. doi: 10.1089/jir.2015.0040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Haider M, Dambuza IM, Asamaphan P, Stappers M, Reid D, Yamasaki S, Brown GD, Gow NAR, Erwig LP. 2019. The pattern recognition receptors dectin-2, mincle, and FcRgamma impact the dynamics of phagocytosis of Candida, Saccharomyces, Malassezia, and Mucor species. PLoS One 14:e0220867. doi: 10.1371/journal.pone.0220867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Thompson A, da Fonseca DM, Walker L, Griffiths JS, Taylor PR, Gow NAR, Orr SJ. 2021. Dependence on mincle and Dectin-2 varies with multiple Candida species during systemic infection. Front Microbiol 12:633229. doi: 10.3389/fmicb.2021.633229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Wu SY, Weng CL, Jheng MJ, Kan HW, Hsieh ST, Liu FT, Wu-Hsieh BA. 2019. Candida albicans triggers NADPH oxidase-independent neutrophil extracellular traps through dectin-2. PLoS Pathog 15:e1008096. doi: 10.1371/journal.ppat.1008096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Zhu LL, Zhao XQ, Jiang C, You Y, Chen XP, Jiang YY, Jia XM, Lin X. 2013. C-type lectin receptors Dectin-3 and Dectin-2 form a heterodimeric pattern-recognition receptor for host defense against fungal infection. Immunity 39:324–334. doi: 10.1016/j.immuni.2013.05.017 [DOI] [PubMed] [Google Scholar]
- 246. Netea MG, Gow NAR, Munro CA, Bates S, Collins C, Ferwerda G, Hobson RP, Bertram G, Hughes HB, Jansen T, Jacobs L, Buurman ET, Gijzen K, Williams DL, Torensma R, McKinnon A, MacCallum DM, Odds FC, Van der Meer JWM, Brown AJP, Kullberg BJ. 2006. Immune sensing of Candida albicans requires cooperative recognition of mannans and glucans by lectin and Toll-like receptors. J Clin Invest 116:1642–1650. doi: 10.1172/JCI27114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Ezekowitz RA, Sastry K, Bailly P, Warner A. 1990. Molecular characterization of the human macrophage mannose receptor: demonstration of multiple carbohydrate recognition-like domains and phagocytosis of yeasts in Cos-1 cells. J Exp Med 172:1785–1794. doi: 10.1084/jem.172.6.1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Yamamoto Y, Klein TW, Friedman H. 1997. Involvement of mannose receptor in cytokine interleukin-1beta (IL-1beta), IL-6, and granulocyte-macrophage colony-stimulating factor responses, but not in chemokine macrophage inflammatory protein 1beta (MIP-1beta), MIP-2, and KC responses, caused by attachment of Candida albicans to macrophages. Infect Immun 65:1077–1082. doi: 10.1128/IAI.65.3.1077-1082.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Heinsbroek SEM, Taylor PR, Martinez FO, Martinez-Pomares L, Brown GD, Gordon S. 2008. Stage-specific sampling by pattern recognition receptors during Candida albicans phagocytosis. PLoS Pathog 4:e1000218. doi: 10.1371/journal.ppat.1000218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. van de Veerdonk FL, Marijnissen RJ, Kullberg BJ, Koenen HJPM, Cheng S-C, Joosten I, van den Berg WB, Williams DL, van der Meer JWM, Joosten LAB, Netea MG. 2009. The macrophage mannose receptor induces IL-17 in response to Candida albicans. Cell Host Microbe 5:329–340. doi: 10.1016/j.chom.2009.02.006 [DOI] [PubMed] [Google Scholar]
- 251. Lee SJ, Zheng NY, Clavijo M, Nussenzweig MC. 2003. Normal host defense during systemic candidiasis in mannose receptor-deficient mice. Infect Immun 71:437–445. doi: 10.1128/IAI.71.1.437-445.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Cambi A, Netea MG, Mora-Montes HM, Gow NAR, Hato SV, Lowman DW, Kullberg B-J, Torensma R, Williams DL, Figdor CG. 2008. Dendritic cell interaction with Candida albicans critically depends on N-linked mannan. J Biol Chem 283:20590–20599. doi: 10.1074/jbc.M709334200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Krylov VB, Gómez-Redondo M, Solovev AS, Yashunsky DV, Brown AJP, Stappers MHT, Gow NAR, Ardá A, Jiménez-Barbero J, Nifantiev NE. 2023. Identification of a new DC-SIGN binding pentamannoside epitope within the complex structure of Candida albicans mannan. Cell Surf 10:100109. doi: 10.1016/j.tcsw.2023.100109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Cambi A, Gijzen K, de Vries l JM, Torensma R, Joosten B, Adema GJ, Netea MG, Kullberg B-J, Romani L, Figdor CG. 2003. The C-type lectin DC-SIGN (CD209) is an antigen-uptake receptor for Candida albicans on dendritic cells. Eur J Immunol 33:532–538. doi: 10.1002/immu.200310029 [DOI] [PubMed] [Google Scholar]
- 255. Gringhuis SI, den Dunnen J, Litjens M, van Het Hof B, van Kooyk Y, Geijtenbeek TBH. 2007. C-type lectin DC-SIGN modulates Toll-like receptor signaling via Raf-1 kinase-dependent acetylation of transcription factor NF-kappaB. Immunity 26:605–616. doi: 10.1016/j.immuni.2007.03.012 [DOI] [PubMed] [Google Scholar]
- 256. Takahara K, Yashima Y, Omatsu Y, Yoshida H, Kimura Y, Kang YS, Steinman RM, Park CG, Inaba K. 2004. Functional comparison of the mouse DC-SIGN, SIGNR1, SIGNR3 and Langerin, C-type lectins. Int Immunol 16:819–829. doi: 10.1093/intimm/dxh084 [DOI] [PubMed] [Google Scholar]
- 257. Takahara K, Tokieda S, Nagaoka K, Takeda T, Kimura Y, Inaba K. 2011. C-type lectin SIGNR1 enhances cellular oxidative burst response against C. albicans in cooperation with Dectin-1. Eur J Immunol 41:1435–1444. doi: 10.1002/eji.200940188 [DOI] [PubMed] [Google Scholar]
- 258. Taylor PR, Brown GD, Herre J, Williams DL, Willment JA, Gordon S. 2004. The role of SIGNR1 and the beta-glucan receptor (dectin-1) in the nonopsonic recognition of yeast by specific macrophages. J Immunol 172:1157–1162. doi: 10.4049/jimmunol.172.2.1157 [DOI] [PubMed] [Google Scholar]
- 259. Nguyen T, Hosono Y, Shimizu T, Yamasaki S, Williams SJ. 2020. Candida albicans steryl 6-O-acyl-alpha-d-mannosides agonize signalling through Mincle. Chem Commun (Camb) 56:15060–15063. doi: 10.1039/D0CC06263D [DOI] [PubMed] [Google Scholar]
- 260. Bugarcic A, Hitchens K, Beckhouse AG, Wells CA, Ashman RB, Blanchard H. 2008. Human and mouse macrophage-inducible C-type lectin (Mincle) bind Candida albicans. Glycobiology 18:679–685. doi: 10.1093/glycob/cwn046 [DOI] [PubMed] [Google Scholar]
- 261. Wells CA, Salvage-Jones JA, Li X, Hitchens K, Butcher S, Murray RZ, Beckhouse AG, Lo Y-L-S, Manzanero S, Cobbold C, Schroder K, Ma B, Orr S, Stewart L, Lebus D, Sobieszczuk P, Hume DA, Stow J, Blanchard H, Ashman RB. 2008. The macrophage-inducible C-type lectin, mincle, is an essential component of the innate immune response to Candida albicans. J Immunol 180:7404–7413. doi: 10.4049/jimmunol.180.11.7404 [DOI] [PubMed] [Google Scholar]
- 262. Vijayan D, Radford KJ, Beckhouse AG, Ashman RB, Wells CA. 2012. Mincle polarizes human monocyte and neutrophil responses to Candida albicans. Immunol Cell Biol 90:889–895. doi: 10.1038/icb.2012.24 [DOI] [PubMed] [Google Scholar]
- 263. Thompson A, Davies LC, Liao C-T, da Fonseca DM, Griffiths JS, Andrews R, Jones AV, Clement M, Brown GD, Humphreys IR, Taylor PR, Orr SJ. 2019. The protective effect of inflammatory monocytes during systemic C. albicans infection is dependent on collaboration between C-type lectin-like receptors. PLoS Pathog 15:e1007850. doi: 10.1371/journal.ppat.1007850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Brown GD, Gordon S. 2001. Immune recognition. A new receptor for beta-glucans. Nature 413:36–37. doi: 10.1038/35092620 [DOI] [PubMed] [Google Scholar]
- 265. Taylor PR, Tsoni SV, Willment JA, Dennehy KM, Rosas M, Findon H, Haynes K, Steele C, Botto M, Gordon S, Brown GD. 2007. Dectin-1 is required for beta-glucan recognition and control of fungal infection. Nat Immunol 8:31–38. doi: 10.1038/ni1408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Thompson A, Griffiths JS, Walker L, da Fonseca DM, Lee KK, Taylor PR, Gow NAR, Orr SJ. 2019. Dependence on Dectin-1 varies with multiple Candida species. Front Microbiol 10:1800. doi: 10.3389/fmicb.2019.01800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Chen SM, Shen H, Zhang T, Huang X, Liu XQ, Guo SY, Zhao JJ, Wang CF, Yan L, Xu GT, Jiang YY, An MM. 2017. Dectin-1 plays an important role in host defense against systemic Candida glabrata infection. Virulence 8:1643–1656. doi: 10.1080/21505594.2017.1346756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Ferwerda B, Ferwerda G, Plantinga TS, Willment JA, van Spriel AB, Venselaar H, Elbers CC, Johnson MD, Cambi A, Huysamen C, Jacobs L, Jansen T, Verheijen K, Masthoff L, Morré SA, Vriend G, Williams DL, Perfect JR, Joosten LAB, Wijmenga C, van der Meer JWM, Adema GJ, Kullberg BJ, Brown GD, Netea MG. 2009. Human dectin-1 deficiency and mucocutaneous fungal infections. N Engl J Med 361:1760–1767. doi: 10.1056/NEJMoa0901053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Plantinga TS, van der Velden WJFM, Ferwerda B, van Spriel AB, Adema G, Feuth T, Donnelly JP, Brown GD, Kullberg B-J, Blijlevens NMA, Netea MG. 2009. Early stop polymorphism in human DECTIN-1 is associated with increased Candida colonization in hematopoietic stem cell transplant recipients. Clin Infect Dis 49:724–732. doi: 10.1086/604714 [DOI] [PubMed] [Google Scholar]
- 270. Rosentul DC, Delsing CE, Jaeger M, Plantinga TS, Oosting M, Costantini I, Venselaar H, Joosten LAB, van der Meer JWM, Dupont B, Kullberg B-J, Sobel JD, Netea MG. 2014. Gene polymorphisms in pattern recognition receptors and susceptibility to idiopathic recurrent vulvovaginal candidiasis. Front Microbiol 5:483. doi: 10.3389/fmicb.2014.00483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Villamón E, Gozalbo D, Roig P, Murciano C, O’Connor JE, Fradelizi D, Gil ML. 2004. Myeloid differentiation factor 88 (MyD88) is required for murine resistance to Candida albicans and is critically involved in Candida -induced production of cytokines. Eur Cytokine Netw 15:263–271. [PubMed] [Google Scholar]
- 272. Picard C, Puel A, Bonnet M, Ku CL, Bustamante J, Yang K, Soudais C, Dupuis S, Feinberg J, Fieschi C, et al. 2003. Pyogenic bacterial infections in humans with IRAK-4 deficiency. Science 299:2076–2079. doi: 10.1126/science.1081902 [DOI] [PubMed] [Google Scholar]
- 273. von Bernuth H, Picard C, Jin Z, Pankla R, Xiao H, Ku C-L, Chrabieh M, Mustapha IB, Ghandil P, Camcioglu Y, et al. 2008. Pyogenic bacterial infections in humans with MyD88 deficiency. Science 321:691–696. doi: 10.1126/science.1158298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Jouault T, Ibata-Ombetta S, Takeuchi O, Trinel PA, Sacchetti P, Lefebvre P, Akira S, Poulain D. 2003. Candida albicans phospholipomannan is sensed through toll-like receptors. J Infect Dis 188:165–172. doi: 10.1086/375784 [DOI] [PubMed] [Google Scholar]
- 275. Wagener J, Malireddi RKS, Lenardon MD, Köberle M, Vautier S, MacCallum DM, Biedermann T, Schaller M, Netea MG, Kanneganti T-D, Brown GD, Brown AJP, Gow NAR. 2014. Fungal chitin dampens inflammation through IL-10 induction mediated by NOD2 and TLR9 activation. PLoS Pathog 10:e1004050. doi: 10.1371/journal.ppat.1004050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Fuchs K, Cardona Gloria Y, Wolz O-O, Herster F, Sharma L, Dillen CA, Täumer C, Dickhöfer S, Bittner Z, Dang T-M, et al. 2018. The fungal ligand chitin directly binds TLR2 and triggers inflammation dependent on oligomer size. EMBO Rep 19:e46065. doi: 10.15252/embr.201846065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Netea MG, Sutmuller R, Hermann C, Van der Graaf CAA, Van der Meer JWM, van Krieken JH, Hartung T, Adema G, Kullberg BJ. 2004. Toll-like receptor 2 suppresses immunity against Candida albicans through induction of IL-10 and regulatory T cells. J Immunol 172:3712–3718. doi: 10.4049/jimmunol.172.6.3712 [DOI] [PubMed] [Google Scholar]
- 278. Megías J, Maneu V, Salvador P, Gozalbo D, Gil ML. 2013. Candida albicans stimulates in vivo differentiation of haematopoietic stem and progenitor cells towards macrophages by a TLR2-dependent signalling. Cell Microbiol 15:1143–1153. doi: 10.1111/cmi.12104 [DOI] [PubMed] [Google Scholar]
- 279. Villamón E, Gozalbo D, Roig P, O’Connor JE, Ferrandiz ML, Fradelizi D, Gil ML. 2004. Toll-like receptor 2 is dispensable for acquired host immune resistance to Candida albicans in a murine model of disseminated candidiasis. Microbes Infect 6:542–548. doi: 10.1016/j.micinf.2004.02.015 [DOI] [PubMed] [Google Scholar]
- 280. Villamón E, Gozalbo D, Roig P, O’Connor JE, Fradelizi D, Gil ML. 2004. Toll-like receptor-2 is essential in murine defenses against Candida albicans infections. Microbes Infect 6:1–7. doi: 10.1016/j.micinf.2003.09.020 [DOI] [PubMed] [Google Scholar]
- 281. Prieto D, Carpena N, Maneu V, Gil ML, Pla J, Gozalbo D. 2016. TLR2 modulates gut colonization and dissemination of Candida albicans in a murine model. Microbes Infect 18:656–660. doi: 10.1016/j.micinf.2016.05.005 [DOI] [PubMed] [Google Scholar]
- 282. Miró MS, Rodríguez E, Vigezzi C, Icely PA, García LN, Peinetti N, Maldonado CA, Riera FO, Caeiro JP, Sotomayor CE. 2018. Contribution of TLR2 pathway in the pathogenesis of vulvovaginal candidiasis. Pathog Dis 76. doi: 10.1093/femspd/fty050 [DOI] [PubMed] [Google Scholar]
- 283. Netea MG, van de Veerdonk F, Verschueren I, van der Meer JWM, Kullberg BJ. 2008. Role of TLR1 and TLR6 in the host defense against disseminated candidiasis. FEMS Immunol Med Microbiol 52:118–123. doi: 10.1111/j.1574-695X.2007.00353.x [DOI] [PubMed] [Google Scholar]
- 284. Plantinga TS, Johnson MD, Scott WK, van de Vosse E, Velez Edwards DR, Smith PB, Alexander BD, Yang JC, Kremer D, Laird GM, Oosting M, Joosten LAB, van der Meer JWM, van Dissel JT, Walsh TJ, Perfect JR, Kullberg BJ, Netea MG. 2012. Toll-like receptor 1 polymorphisms increase susceptibility to candidemia. J Infect Dis 205:934–943. doi: 10.1093/infdis/jir867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Tada H, Nemoto E, Shimauchi H, Watanabe T, Mikami T, Matsumoto T, Ohno N, Tamura H, Shibata K, Akashi S, Miyake K, Sugawara S, Takada H. 2002. Saccharomyces cerevisiae- and Candida albicans-derived mannan induced production of tumor necrosis factor alpha by human monocytes in a CD14- and Toll-like receptor 4-dependent manner. Microbiol Immunol 46:503–512. doi: 10.1111/j.1348-0421.2002.tb02727.x [DOI] [PubMed] [Google Scholar]
- 286. Netea MG, Van Der Graaf CAA, Vonk AG, Verschueren I, Van Der Meer JWM, Kullberg BJ. 2002. The role of toll-like receptor (TLR) 2 and TLR4 in the host defense against disseminated candidiasis. J Infect Dis 185:1483–1489. doi: 10.1086/340511 [DOI] [PubMed] [Google Scholar]
- 287. Netea MG, Gow NAR, Joosten LAB, Verschueren I, van der Meer JWM, Kullberg BJ. 2010. Variable recognition of Candida albicans strains by TLR4 and lectin recognition receptors. Med Mycol 48:897–903. doi: 10.3109/13693781003621575 [DOI] [PubMed] [Google Scholar]
- 288. Gasparoto TH, Tessarolli V, Garlet TP, Torres SA, Garlet GP, da Silva JS, Campanelli AP. 2010. Absence of functional TLR4 impairs response of macrophages after Candida albicans infection. Med Mycol 48:1009–1017. doi: 10.3109/13693786.2010.481292 [DOI] [PubMed] [Google Scholar]
- 289. Murciano C, Villamon E, Gozalbo D, Roig P, O’Connor JE, Gil ML. 2006. Toll-like receptor 4 defective mice carrying point or null mutations do not show increased susceptibility to Candida albicans in a model of hematogenously disseminated infection. Med Mycol 44:149–157. doi: 10.1080/13693780500294733 [DOI] [PubMed] [Google Scholar]
- 290. Biondo C, Malara A, Costa A, Signorino G, Cardile F, Midiri A, Galbo R, Papasergi S, Domina M, Pugliese M, Teti G, Mancuso G, Beninati C. 2012. Recognition of fungal RNA by TLR7 has a nonredundant role in host defense against experimental candidiasis. Eur J Immunol 42:2632–2643. doi: 10.1002/eji.201242532 [DOI] [PubMed] [Google Scholar]
- 291. van de Veerdonk FL, Netea MG, Jansen TJ, Jacobs L, Verschueren I, van der Meer JWM, Kullberg BJ. 2008. Redundant role of TLR9 for anti-Candida host defense. Immunobiology 213:613–620. doi: 10.1016/j.imbio.2008.05.002 [DOI] [PubMed] [Google Scholar]
- 292. Nahum A, Dadi H, Bates A, Roifman CM. 2012. The biological significance of TLR3 variant, L412F, in conferring susceptibility to cutaneous candidiasis, CMV and autoimmunity. Autoimmun Rev 11:341–347. doi: 10.1016/j.autrev.2011.10.007 [DOI] [PubMed] [Google Scholar]
- 293. van der Graaf CAA, Netea MG, Franke B, Girardin SE, van der Meer JWM, Kullberg BJ. 2006. Nucleotide oligomerization domain 2 (Nod2) is not involved in the pattern recognition of Candida albicans. Clin Vaccine Immunol 13:423–425. doi: 10.1128/CVI.13.3.423-425.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294. Cheng S-C, van de Veerdonk FL, Lenardon M, Stoffels M, Plantinga T, Smeekens S, Rizzetto L, Mukaremera L, Preechasuth K, Cavalieri D, Kanneganti TD, van der Meer JWM, Kullberg BJ, Joosten LAB, Gow NAR, Netea MG. 2011. The dectin-1/inflammasome pathway is responsible for the induction of protective T-helper 17 responses that discriminate between yeasts and hyphae of Candida albicans. J Leukoc Biol 90:357–366. doi: 10.1189/jlb.1210702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295. Pietrella D, Pandey N, Gabrielli E, Pericolini E, Perito S, Kasper L, Bistoni F, Cassone A, Hube B, Vecchiarelli A. 2013. Secreted aspartic proteases of Candida albicans activate the NLRP3 inflammasome. Eur J Immunol 43:679–692. doi: 10.1002/eji.201242691 [DOI] [PubMed] [Google Scholar]
- 296. Kasper L, König A, Koenig P-A, Gresnigt MS, Westman J, Drummond RA, Lionakis MS, Groß O, Ruland J, Naglik JR, Hube B. 2018. The fungal peptide toxin Candidalysin activates the NLRP3 inflammasome and causes cytolysis in mononuclear phagocytes. Nat Commun 9:4260. doi: 10.1038/s41467-018-06607-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297. Gabrielli E, Pericolini E, Luciano E, Sabbatini S, Roselletti E, Perito S, Kasper L, Hube B, Vecchiarelli A. 2015. Induction of caspase-11 by aspartyl proteinases of Candida albicans and implication in promoting inflammatory response. Infect Immun 83:1940–1948. doi: 10.1128/IAI.02895-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298. Rogiers O, Frising UC, Kucharíková S, Jabra-Rizk MA, van Loo G, Van Dijck P, Wullaert A. 2019. Candidalysin crucially contributes to Nlrp3 inflammasome activation by Candida albicans hyphae. mBio 10:e02221-18. doi: 10.1128/mBio.02221-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Wellington M, Koselny K, Sutterwala FS, Krysan DJ. 2014. Candida albicans triggers NLRP3-mediated pyroptosis in macrophages. Eukaryot Cell 13:329–340. doi: 10.1128/EC.00336-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300. Uwamahoro N, Verma-Gaur J, Shen HH, Qu Y, Lewis R, Lu J, Bambery K, Masters SL, Vince JE, Naderer T, Traven A. 2014. The pathogen Candida albicans hijacks pyroptosis for escape from macrophages. mBio 5:e00003-14. doi: 10.1128/mBio.00003-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301. Gross O, Poeck H, Bscheider M, Dostert C, Hannesschläger N, Endres S, Hartmann G, Tardivel A, Schweighoffer E, Tybulewicz V, Mocsai A, Tschopp J, Ruland J. 2009. Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature 459:433–436. doi: 10.1038/nature07965 [DOI] [PubMed] [Google Scholar]
- 302. Hise AG, Tomalka J, Ganesan S, Patel K, Hall BA, Brown GD, Fitzgerald KA. 2009. An essential role for the NLRP3 inflammasome in host defense against the human fungal pathogen Candida albicans. Cell Host Microbe 5:487–497. doi: 10.1016/j.chom.2009.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303. Joly S, Ma N, Sadler JJ, Soll DR, Cassel SL, Sutterwala FS. 2009. Cutting edge: Candida albicans hyphae formation triggers activation of the Nlrp3 inflammasome. J Immunol 183:3578–3581. doi: 10.4049/jimmunol.0901323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304. Jaeger M, Carvalho A, Cunha C, Plantinga TS, van de Veerdonk F, Puccetti M, Galosi C, Joosten LAB, Dupont B, Kullberg BJ, Sobel JD, Romani L, Netea MG. 2016. Association of a variable number tandem repeat in the NLRP3 gene in women with susceptibility to RVVC. Eur J Clin Microbiol Infect Dis 35:797–801. doi: 10.1007/s10096-016-2600-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305. Lev-Sagie A, Prus D, Linhares IM, Lavy Y, Ledger WJ, Witkin SS. 2009. Polymorphism in a gene coding for the inflammasome component NALP3 and recurrent vulvovaginal candidiasis in women with vulvar vestibulitis syndrome. Am J Obstet Gynecol 200:303. doi: 10.1016/j.ajog.2008.10.039 [DOI] [PubMed] [Google Scholar]
- 306. Gringhuis SI, Kaptein TM, Wevers BA, Theelen B, van der Vlist M, Boekhout T, Geijtenbeek TBH. 2012. Dectin-1 is an extracellular pathogen sensor for the induction and processing of IL-1beta via a noncanonical caspase-8 inflammasome. Nat Immunol 13:246–254. doi: 10.1038/ni.2222 [DOI] [PubMed] [Google Scholar]
- 307. Ganesan S, Rathinam VAK, Bossaller L, Army K, Kaiser WJ, Mocarski ES, Dillon CP, Green DR, Mayadas TN, Levitz SM, Hise AG, Silverman N, Fitzgerald KA. 2014. Caspase-8 modulates dectin-1 and complement receptor 3-driven IL-1beta production in response to beta-glucans and the fungal pathogen, Candida albicans. J Immunol 193:2519–2530. doi: 10.4049/jimmunol.1400276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308. Joly S, Eisenbarth SC, Olivier AK, Williams A, Kaplan DH, Cassel SL, Flavell RA, Sutterwala FS. 2012. Cutting edge: Nlrp10 is essential for protective antifungal adaptive immunity against Candida albicans. J Immunol 189:4713–4717. doi: 10.4049/jimmunol.1201715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. Tomalka J, Ganesan S, Azodi E, Patel K, Majmudar P, Hall BA, Fitzgerald KA, Hise AG. 2011. A novel role for the NLRC4 inflammasome in mucosal defenses against the fungal pathogen Candida albicans. PLoS Pathog 7:e1002379. doi: 10.1371/journal.ppat.1002379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310. Borghi M, De Luca A, Puccetti M, Jaeger M, Mencacci A, Oikonomou V, Pariano M, Garlanda C, Moretti S, Bartoli A, Sobel J, van de Veerdonk FL, Dinarello CA, Netea MG, Romani L. 2015. Pathogenic NLRP3 inflammasome activity during Candida infection is negatively regulated by IL-22 via activation of NLRC4 and IL-1Ra. Cell Host Microbe 18:198–209. doi: 10.1016/j.chom.2015.07.004 [DOI] [PubMed] [Google Scholar]
- 311. Linden JR, De Paepe ME, Laforce-Nesbitt SS, Bliss JM. 2013. Galectin-3 plays an important role in protection against disseminated candidiasis. Med Mycol 51:641–651. doi: 10.3109/13693786.2013.770607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312. Wu SY, Huang JH, Chen WY, Chan YC, Lin CH, Chen YC, Liu FT, Wu-Hsieh BA. 2017. Cell intrinsic galectin-3 attenuates neutrophil ROS-dependent killing of Candida by modulating CR3 downstream Syk activation. Front Immunol 8:48. doi: 10.3389/fimmu.2017.00048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313. de Jong MAWP, Vriend LEM, Theelen B, Taylor ME, Fluitsma D, Boekhout T, Geijtenbeek TBH. 2010. C-type lectin Langerin is a beta-glucan receptor on human Langerhans cells that recognizes opportunistic and pathogenic fungi. Mol Immunol 47:1216–1225. doi: 10.1016/j.molimm.2009.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314. Held K, Thiel S, Loos M, Petry F. 2008. Increased susceptibility of complement factor B/C2 double knockout mice and mannan-binding lectin knockout mice to systemic infection with Candida albicans. Mol Immunol 45:3934–3941. doi: 10.1016/j.molimm.2008.06.021 [DOI] [PubMed] [Google Scholar]
- 315. Rosseau S, Guenther A, Seeger W, Lohmeyer J. 1997. Phagocytosis of viable Candida albicans by alveolar macrophages: lack of opsonin function of surfactant protein A. J Infect Dis 175:421–428. doi: 10.1093/infdis/175.2.421 [DOI] [PubMed] [Google Scholar]
- 316. van Rozendaal BA, van Spriel AB, van De Winkel JG, Haagsman HP. 2000. Role of pulmonary surfactant protein D in innate defense against Candida albicans. J Infect Dis 182:917–922. doi: 10.1086/315799 [DOI] [PubMed] [Google Scholar]
- 317. Swidergall M, Solis NV, Lionakis MS, Filler SG. 2018. EphA2 is an epithelial cell pattern recognition receptor for fungal beta-glucans. Nat Microbiol 3:53–61. doi: 10.1038/s41564-017-0059-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318. Swidergall M, Solis NV, Wang Z, Phan QT, Marshall ME, Lionakis MS, Pearlman E, Filler SG. 2019. EphA2 is a neutrophil receptor for Candida albicans that stimulates antifungal activity during oropharyngeal infection. Cell Rep 28:423–433. doi: 10.1016/j.celrep.2019.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319. Sun W, Wang H, Hu H, Ma X, Zhang H, Chen J, Du Y, He R, Cui Z, Peng Q, Wang C. 2021. Cutting edge: EPHB2 is a coreceptor for fungal recognition and phosphorylation of Syk in the Dectin-1 signaling pathway. J Immunol 206:1419–1423. doi: 10.4049/jimmunol.2001373 [DOI] [PubMed] [Google Scholar]
- 320. Gazendam RP, van Hamme JL, Tool ATJ, van Houdt M, Verkuijlen PJJH, Herbst M, Liese JG, van de Veerdonk FL, Roos D, van den Berg TK, Kuijpers TW. 2014. Two independent killing mechanisms of Candida albicans by human neutrophils: evidence from innate immunity defects. Blood 124:590–597. doi: 10.1182/blood-2014-01-551473 [DOI] [PubMed] [Google Scholar]
- 321. Guo Y, Chang Q, Cheng L, Xiong S, Jia X, Lin X, Zhao X. 2018. C-type lectin receptor CD23 is required for host defense against Candida albicans and Aspergillus fumigatus infection. J Immunol 201:2427–2440. doi: 10.4049/jimmunol.1800620 [DOI] [PubMed] [Google Scholar]
- 322. Li L, Dongari-Bagtzoglou A. 2009. Epithelial GM-CSF induction by Candida glabrata. J Dent Res 88:746–751. doi: 10.1177/0022034509341266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323. He X, Howard BA, Liu Y, Neumann AK, Li L, Menon N, Roach T, Kale SD, Samuels DC, Li H, Kite T, Kita H, Hu TY, Luo M, Jones CN, Okaa UJ, Squillace DL, Klein BS, Lawrence CB. 2021. LYSMD3: a mammalian pattern recognition receptor for chitin. Cell Rep 36:109392. doi: 10.1016/j.celrep.2021.109392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324. Means TK, Mylonakis E, Tampakakis E, Colvin RA, Seung E, Puckett L, Tai MF, Stewart CR, Pukkila-Worley R, Hickman SE, Moore KJ, Calderwood SB, Hacohen N, Luster AD, El Khoury J. 2009. Evolutionarily conserved recognition and innate immunity to fungal pathogens by the scavenger receptors SCARF1 and CD36. J Exp Med 206:637–653. doi: 10.1084/jem.20082109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325. Vitenshtein A, Charpak-Amikam Y, Yamin R, Bauman Y, Isaacson B, Stein N, Berhani O, Dassa L, Gamliel M, Gur C, Glasner A, Gomez C, Ben-Ami R, Osherov N, Cormack BP, Mandelboim O. 2016. NK cell recognition of Candida glabrata through binding of NKp46 and NCR1 to fungal ligands Epa1, Epa6, and Epa7. Cell Host Microbe 20:527–534. doi: 10.1016/j.chom.2016.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326. Jaeger M, van der Lee R, Cheng S-C, Johnson MD, Kumar V, Ng A, Plantinga TS, Smeekens SP, Oosting M, Wang X, Barchet W, Fitzgerald K, Joosten LAB, Perfect JR, Wijmenga C, van de Veerdonk FL, Huynen MA, Xavier RJ, Kullberg B-J, Netea MG. 2015. The RIG-I-like helicase receptor MDA5 (IFIH1) is involved in the host defense against Candida infections. Eur J Clin Microbiol Infect Dis 34:963–974. doi: 10.1007/s10096-014-2309-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327. Moyes DL, Runglall M, Murciano C, Shen C, Nayar D, Thavaraj S, Kohli A, Islam A, Mora-Montes H, Challacombe SJ, Naglik JR. 2010. A biphasic innate immune MAPK response discriminates between the yeast and hyphal forms of Candida albicans in epithelial cells. Cell Host Microbe 8:225–235. doi: 10.1016/j.chom.2010.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328. Moyes DL, Murciano C, Runglall M, Islam A, Thavaraj S, Naglik JR. 2011. Candida albicans yeast and hyphae are discriminated by MAPK signaling in vaginal epithelial cells. PLoS One 6:e26580. doi: 10.1371/journal.pone.0026580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329. Ho J, Yang X, Nikou SA, Kichik N, Donkin A, Ponde NO, Richardson JP, Gratacap RL, Archambault LS, Zwirner CP, Murciano C, Henley-Smith R, Thavaraj S, Tynan CJ, Gaffen SL, Hube B, Wheeler RT, Moyes DL, Naglik JR. 2019. Candidalysin activates innate epithelial immune responses via epidermal growth factor receptor. Nat Commun 10:2297. doi: 10.1038/s41467-019-09915-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330. Tomalka J, Azodi E, Narra HP, Patel K, O’Neill S, Cardwell C, Hall BA, Wilson JM, Hise AG. 2015. beta-defensin 1 plays a role in acute mucosal defense against Candida albicans. J Immunol 194:1788–1795. doi: 10.4049/jimmunol.1203239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331. Vylkova S, Nayyar N, Li W, Edgerton M. 2007. Human beta-defensins kill Candida albicans in an energy-dependent and salt-sensitive manner without causing membrane disruption. Antimicrob Agents Chemother 51:154–161. doi: 10.1128/AAC.00478-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332. Järvå M, Phan TK, Lay FT, Caria S, Kvansakul M, Hulett MD. 2018. Human beta-defensin 2 kills Candida albicans through phosphatidylinositol 4,5-bisphosphate-mediated membrane permeabilization. Sci Adv 4:eaat0979. doi: 10.1126/sciadv.aat0979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333. López-García B, Lee PHA, Yamasaki K, Gallo RL. 2005. Anti-fungal activity of cathelicidins and their potential role in Candida albicans skin infection. J Invest Dermatol 125:108–115. doi: 10.1111/j.0022-202X.2005.23713.x [DOI] [PubMed] [Google Scholar]
- 334. Tsai PW, Cheng YL, Hsieh WP, Lan CY. 2014. Responses of Candida albicans to the human antimicrobial peptide LL-37. J Microbiol 52:581–589. doi: 10.1007/s12275-014-3630-2 [DOI] [PubMed] [Google Scholar]
- 335. Kullberg BJ, van ’t Wout JW, van Furth R. 1990. Role of granulocytes in increased host resistance to Candida albicans induced by recombinant interleukin-1. Infect Immun 58:3319–3324. doi: 10.1128/iai.58.10.3319-3324.1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336. Qian Q, Jutila MA, Van Rooijen N, Cutler JE. 1994. Elimination of mouse splenic macrophages correlates with increased susceptibility to experimental disseminated candidiasis. J Immunol 152:5000–5008. doi: 10.4049/jimmunol.152.10.5000 [DOI] [PubMed] [Google Scholar]
- 337. Ngo LY, Kasahara S, Kumasaka DK, Knoblaugh SE, Jhingran A, Hohl TM. 2014. Inflammatory monocytes mediate early and organ-specific innate defense during systemic candidiasis. J Infect Dis 209:109–119. doi: 10.1093/infdis/jit413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338. Horn DL, Neofytos D, Anaissie EJ, Fishman JA, Steinbach WJ, Olyaei AJ, Marr KA, Pfaller MA, Chang CH, Webster KM. 2009. Epidemiology and outcomes of candidemia in 2019 patients: data from the prospective antifungal therapy alliance registry. Clin Infect Dis 48:1695–1703. doi: 10.1086/599039 [DOI] [PubMed] [Google Scholar]
- 339. Porcaro I, Vidal M, Jouvert S, Stahl PD, Giaimis J. 2003. Mannose receptor contribution to Candida albicans phagocytosis by murine E-clone J774 macrophages. J Leukoc Biol 74:206–215. doi: 10.1189/jlb.1202608 [DOI] [PubMed] [Google Scholar]
- 340. Maxson ME, Naj X, O’Meara TR, Plumb JD, Cowen LE, Grinstein S. 2018. Integrin-based diffusion barrier separates membrane domains enabling the formation of microbiostatic frustrated phagosomes. Elife 7:e34798. doi: 10.7554/eLife.34798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341. Bain JM, Alonso MF, Childers DS, Walls CA, Mackenzie K, Pradhan A, Lewis LE, Louw J, Avelar GM, Larcombe DE, Netea MG, Gow NAR, Brown GD, Erwig LP, Brown AJP. 2021. Immune cells fold and damage fungal hyphae. Proc Natl Acad Sci U S A 118:e2020484118. doi: 10.1073/pnas.2020484118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342. Aratani Y, Kura F, Watanabe H, Akagawa H, Takano Y, Suzuki K, Dinauer MC, Maeda N, Koyama H. 2002. Critical role of myeloperoxidase and nicotinamide adenine dinucleotide phosphate-oxidase in high-burden systemic infection of mice with Candida albicans. J Infect Dis 185:1833–1837. doi: 10.1086/340635 [DOI] [PubMed] [Google Scholar]
- 343. Balish E, Warner TF, Nicholas PJ, Paulling EE, Westwater C, Schofield DA. 2005. Susceptibility of germfree phagocyte oxidase- and nitric oxide synthase 2-deficient mice, defective in the production of reactive metabolites of both oxygen and nitrogen, to mucosal and systemic candidiasis of endogenous origin. Infect Immun 73:1313–1320. doi: 10.1128/IAI.73.3.1313-1320.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344. Wu T, Samaranayake LP, Leung WK, Sullivan PA. 1999. Inhibition of growth and secreted aspartyl proteinase production in Candida albicans by lysozyme. J Med Microbiol 48:721–730. doi: 10.1099/00222615-48-8-721 [DOI] [PubMed] [Google Scholar]
- 345. Chen L, Shen Z, Wu J. 2009. Expression, purification and in vitro antifungal activity of acidic mammalian chitinase against Candida albicans, Aspergillus fumigatus and Trichophyton rubrum strains. Clin Exp Dermatol 34:55–60. doi: 10.1111/j.1365-2230.2008.03092.x [DOI] [PubMed] [Google Scholar]
- 346. Urban CF, Ermert D, Schmid M, Abu-Abed U, Goosmann C, Nacken W, Brinkmann V, Jungblut PR, Zychlinsky A. 2009. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog 5:e1000639. doi: 10.1371/journal.ppat.1000639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347. Urban CF, Reichard U, Brinkmann V, Zychlinsky A. 2006. Neutrophil extracellular traps capture and kill Candida albicans yeast and hyphal forms. Cell Microbiol 8:668–676. doi: 10.1111/j.1462-5822.2005.00659.x [DOI] [PubMed] [Google Scholar]
- 348. Johnson CJ, Kernien JF, Hoyer AR, Nett JE. 2017. Mechanisms involved in the triggering of neutrophil extracellular traps (NETs) by Candida glabrata during planktonic and biofilm growth. Sci Rep 7:13065. doi: 10.1038/s41598-017-13588-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349. Quintin J, Voigt J, van der Voort R, Jacobsen ID, Verschueren I, Hube B, Giamarellos-Bourboulis EJ, van der Meer JWM, Joosten LAB, Kurzai O, Netea MG. 2014. Differential role of NK cells against Candida albicans infection in immunocompetent or immunocompromised mice. Eur J Immunol 44:2405–2414. doi: 10.1002/eji.201343828 [DOI] [PubMed] [Google Scholar]
- 350. Bär E, Whitney PG, Moor K, Reis e Sousa C, LeibundGut-Landmann S. 2014. IL-17 regulates systemic fungal immunity by controlling the functional competence of NK cells. Immunity 40:117–127. doi: 10.1016/j.immuni.2013.12.002 [DOI] [PubMed] [Google Scholar]
- 351. Gladiator A, Wangler N, Trautwein-Weidner K, LeibundGut-Landmann S. 2013. Cutting edge: IL-17-secreting innate lymphoid cells are essential for host defense against fungal infection. J Immunol 190:521–525. doi: 10.4049/jimmunol.1202924 [DOI] [PubMed] [Google Scholar]
- 352. Huang Y, Guo L, Qiu J, Chen X, Hu-Li J, Siebenlist U, Williamson PR, Urban Jr JF, Paul WE. 2015. IL-25-responsive, lineage-negative KLRG1(hi) cells are multipotential 'inflammatory' type 2 innate lymphoid cells. Nat Immunol 16:161–169. doi: 10.1038/ni.3078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353. Iwasawa MT, Miyachi H, Wakabayashi S, Sugihira T, Aoyama R, Nakagawa S, Katayama Y, Yoneyama M, Hara H, Iwakura Y, Matsumoto M, Inohara N, Koguchi-Yoshioka H, Fujimoto M, Núñez G, Matsue H, Nakamura Y, Saijo S. 2022. Epidermal clearance of Candida albicans is mediated by IL-17 but independent of fungal innate immune receptors. Int Immunol 34:409–420. doi: 10.1093/intimm/dxac019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354. Haraguchi N, Kikuchi N, Morishima Y, Matsuyama M, Sakurai H, Shibuya A, Shibuya K, Taniguchi M, Ishii Y. 2016. Activation of murine invariant NKT cells promotes susceptibility to candidiasis by IL-10 induced modulation of phagocyte antifungal activity. Eur J Immunol 46:1691–1703. doi: 10.1002/eji.201545987 [DOI] [PubMed] [Google Scholar]
- 355. Conti HR, Peterson AC, Brane L, Huppler AR, Hernández-Santos N, Whibley N, Garg AV, Simpson-Abelson MR, Gibson GA, Mamo AJ, Osborne LC, Bishu S, Ghilardi N, Siebenlist U, Watkins SC, Artis D, McGeachy MJ, Gaffen SL. 2014. Oral-resident natural Th17 cells and gammadelta T cells control opportunistic Candida albicans infections. J Exp Med 211:2075–2084. doi: 10.1084/jem.20130877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356. Huppler AR, Conti HR, Hernández-Santos N, Darville T, Biswas PS, Gaffen SL. 2014. Role of neutrophils in IL-17-dependent immunity to mucosal candidiasis. J Immunol 192:1745–1752. doi: 10.4049/jimmunol.1302265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357. Huang W, Na L, Fidel PL, Schwarzenberger P. 2004. Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. J Infect Dis 190:624–631. doi: 10.1086/422329 [DOI] [PubMed] [Google Scholar]
- 358. De Luca A, Zelante T, D’Angelo C, Zagarella S, Fallarino F, Spreca A, Iannitti RG, Bonifazi P, Renauld J-C, Bistoni F, Puccetti P, Romani L. 2010. IL-22 defines a novel immune pathway of antifungal resistance. Mucosal Immunol 3:361–373. doi: 10.1038/mi.2010.22 [DOI] [PubMed] [Google Scholar]
- 359. Conti H.R, Shen F, Nayyar N, Stocum E, Sun JN, Lindemann MJ, Ho AW, Hai JH, Yu JJ, Jung JW, Filler SG, Masso-Welch P, Edgerton M, Gaffen SL. 2009. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med 206:299–311. doi: 10.1084/jem.20081463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360. Whibley N, Maccallum DM, Vickers MA, Zafreen S, Waldmann H, Hori S, Gaffen SL, Gow NAR, Barker RN, Hall AM. 2014. Expansion of Foxp3+ T-cell populations by Candida albicans enhances both Th17-cell responses and fungal dissemination after intravenous challenge. Eur J Immunol 44:1069–1083. doi: 10.1002/eji.201343604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361. Kagami S, Rizzo HL, Kurtz SE, Miller LS, Blauvelt A. 2010. IL-23 and IL-17A, but not IL-12 and IL-22, are required for optimal skin host defense against Candida albicans. J Immunol 185:5453–5462. doi: 10.4049/jimmunol.1001153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362. Puel A, Cypowyj S, Bustamante J, Wright JF, Liu L, Lim HK, Migaud M, Israel L, Chrabieh M, Audry M, Gumbleton M, Toulon A, Bodemer C, El-Baghdadi J, Whitters M, Paradis T, Brooks J, Collins M, Wolfman NM, Al-Muhsen S, Galicchio M, Abel L, Picard C, Casanova JL. 2011. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science 332:65–68. doi: 10.1126/science.1200439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363. Milner JD, Brenchley JM, Laurence A, Freeman AF, Hill BJ, Elias KM, Kanno Y, Spalding C, Elloumi HZ, Paulson ML, Davis J, Hsu A, Asher AI, O’Shea J, Holland SM, Paul WE, Douek DC. 2008. Impaired TH17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature 452:773–776. doi: 10.1038/nature06764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364. Boisson B, Wang C, Pedergnana V, Wu L, Cypowyj S, Rybojad M, Belkadi A, Picard C, Abel L, Fieschi C, Puel A, Li X, Casanova JL. 2013. An ACT1 mutation selectively abolishes interleukin-17 responses in humans with chronic mucocutaneous candidiasis. Immunity 39:676–686. doi: 10.1016/j.immuni.2013.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365. Okada S, Markle JG, Deenick EK, Mele F, Averbuch D, Lagos M, Alzahrani M, Al-Muhsen S, Halwani R, Ma CS, et al. 2015. IMMUNODEFICIENCIES. Impairment of immunity to Candida and Mycobacterium in humans with bi-allelic RORC mutations. Science 349:606–613. doi: 10.1126/science.aaa4282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366. van de Veerdonk FL, Plantinga TS, Hoischen A, Smeekens SP, Joosten LAB, Gilissen C, Arts P, Rosentul DC, Carmichael AJ, Smits-van der Graaf CAA, Kullberg BJ, van der Meer JWM, Lilic D, Veltman JA, Netea MG. 2011. STAT1 mutations in autosomal dominant chronic mucocutaneous candidiasis. N Engl J Med 365:54–61. doi: 10.1056/NEJMoa1100102 [DOI] [PubMed] [Google Scholar]
- 367. Netea MG, Vonk AG, van den Hoven M, Verschueren I, Joosten LA, van Krieken JH, van den Berg WB, Van der Meer JWM, Kullberg BJ. 2003. Differential role of IL-18 and IL-12 in the host defense against disseminated Candida albicans infection. Eur J Immunol 33:3409–3417. doi: 10.1002/eji.200323737 [DOI] [PubMed] [Google Scholar]
- 368. Stuyt RJL, Netea MG, van Krieken JH, van der Meer JWM, Kullberg BJ. 2004. Recombinant interleukin-18 protects against disseminated Candida albicans infection in mice. J Infect Dis 189:1524–1527. doi: 10.1086/382955 [DOI] [PubMed] [Google Scholar]
- 369. Kullberg BJ, van ’t Wout JW, Hoogstraten C, van Furth R. 1993. Recombinant interferon-gamma enhances resistance to acute disseminated Candida albicans infection in mice. J Infect Dis 168:436–443. doi: 10.1093/infdis/168.2.436 [DOI] [PubMed] [Google Scholar]
- 370. Delsing CE, Gresnigt MS, Leentjens J, Preijers F, Frager FA, Kox M, Monneret G, Venet F, Bleeker-Rovers CP, van de Veerdonk FL, Pickkers P, Pachot A, Kullberg BJ, Netea MG. 2014. Interferon-gamma as adjunctive immunotherapy for invasive fungal infections: a case series. BMC Infect Dis 14:166. doi: 10.1186/1471-2334-14-166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371. Haraguchi N, Ishii Y, Morishima Y, Yoh K, Matsuno Y, Kikuchi N, Sakamoto T, Takahashi S, Hizawa N. 2010. Impairment of host defense against disseminated candidiasis in mice overexpressing GATA-3. Infect Immun 78:2302–2311. doi: 10.1128/IAI.01398-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372. Mencacci A, Perruccio K, Bacci A, Cenci E, Benedetti R, Martelli MF, Bistoni F, Coffman R, Velardi A, Romani L. 2001. Defective antifungal T-helper 1 (TH1) immunity in a murine model of allogeneic T-cell-depleted bone marrow transplantation and its restoration by treatment with TH2 cytokine antagonists. Blood 97:1483–1490. doi: 10.1182/blood.v97.5.1483 [DOI] [PubMed] [Google Scholar]
- 373. Tavares D, Ferreira P, Arala‐Chaves M. 2000. Increased resistance to systemic candidiasis in athymic or interleukin-10-depleted mice. J Infect Dis 182:266–273. doi: 10.1086/315674 [DOI] [PubMed] [Google Scholar]
- 374. Carrow EW, Hector RF, Domer JE. 1984. Immunodeficient CBA/N mice respond effectively to Candida albicans. Clin Immunol Immunopathol 33:371–380. doi: 10.1016/0090-1229(84)90308-8 [DOI] [PubMed] [Google Scholar]
- 375. Jensen J, Warner T, Balish E. 1993. Resistance of SCID mice to Candida albicans administered intravenously or colonizing the gut: role of polymorphonuclear leukocytes and macrophages. J Infect Dis 167:912–919. doi: 10.1093/infdis/167.4.912 [DOI] [PubMed] [Google Scholar]
- 376. Wagner RD, Vazquez-Torres A, Jones-Carson J, Warner T, Balish E. 1996. B cell knockout mice are resistant to mucosal and systemic candidiasis of endogenous origin but susceptible to experimental systemic candidiasis. J Infect Dis 174:589–597. doi: 10.1093/infdis/174.3.589 [DOI] [PubMed] [Google Scholar]
- 377. Li R, Rezk A, Li H, Gommerman JL, Prat A, Bar-Or A, Canadian B Cells in MS Team . 2017. Antibody-independent function of human B cells contributes to antifungal T cell responses. J Immunol 198:3245–3254. doi: 10.4049/jimmunol.1601572 [DOI] [PubMed] [Google Scholar]
- 378. Ferreira-Gomes M, Wich M, Böde S, Hube B, Jacobsen ID, Jungnickel B. 2021. B cell recognition of Candida albicans hyphae via TLR 2 promotes IgG1 and IL-6 secretion for T(H)17 differentiation. Front Immunol 12:698849. doi: 10.3389/fimmu.2021.698849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379. Mayer FL, Wilson D, Hube B. 2013. Candida albicans pathogenicity mechanisms. Virulence 4:119–128. doi: 10.4161/viru.22913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380. Kumamoto CA, Gresnigt MS, Hube B. 2020. The gut, the bad and the harmless: Candida albicans as a commensal and opportunistic pathogen in the intestine. Curr Opin Microbiol 56:7–15. doi: 10.1016/j.mib.2020.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381. Mishra AA, Koh AY. 2018. Adaptation of Candida albicans during gastrointestinal tract colonization. Curr Clin Microbiol Rep 5:165–172. doi: 10.1007/s40588-018-0096-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382. Neville BA, d’Enfert C, Bougnoux ME. 2015. Candida albicans commensalism in the gastrointestinal tract. FEMS Yeast Res 15:fov081. doi: 10.1093/femsyr/fov081 [DOI] [PubMed] [Google Scholar]
- 383. Prieto D, Correia I, Pla J, Román E. 2016. Adaptation of Candida albicans to commensalism in the gut. Future Microbiol 11:567–583. doi: 10.2217/fmb.16.1 [DOI] [PubMed] [Google Scholar]
- 384. Romo JA, Kumamoto CA. 2020. On commensalism of Candida. J Fungi (Basel) 6:16. doi: 10.3390/jof6010016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385. Nash AK, Auchtung TA, Wong MC, Smith DP, Gesell JR, Ross MC, Stewart CJ, Metcalf GA, Muzny DM, Gibbs RA, Ajami NJ, Petrosino JF. 2017. The gut mycobiome of the Human Microbiome Project healthy cohort. Microbiome 5:153. doi: 10.1186/s40168-017-0373-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386. d’Enfert C, Kaune A-K, Alaban L-R, Chakraborty S, Cole N, Delavy M, Kosmala D, Marsaux B, Fróis-Martins R, Morelli M, et al. 2021. The impact of the fungus-host-microbiota interplay upon Candida albicans infections: current knowledge and new perspectives. FEMS Microbiol Rev 45:fuaa060. doi: 10.1093/femsre/fuaa060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387. Hallen-Adams HE, Suhr MJ. 2017. Fungi in the healthy human gastrointestinal tract. Virulence 8:352–358. doi: 10.1080/21505594.2016.1247140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388. Vautier S, Drummond RA, Chen K, Murray GI, Kadosh D, Brown AJP, Gow NAR, MacCallum DM, Kolls JK, Brown GD. 2015. Candida albicans colonization and dissemination from the murine gastrointestinal tract: the influence of morphology and Th17 immunity. Cell Microbiol 17:445–450. doi: 10.1111/cmi.12388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389. Rosenbach A, Dignard D, Pierce JV, Whiteway M, Kumamoto CA. 2010. Adaptations of Candida albicans for growth in the mammalian intestinal tract. Eukaryot Cell 9:1075–1086. doi: 10.1128/EC.00034-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390. White SJ, Rosenbach A, Lephart P, Nguyen D, Benjamin A, Tzipori S, Whiteway M, Mecsas J, Kumamoto CA. 2007. Self-regulation of Candida albicans population size during GI colonization. PLoS Pathog 3:e184. doi: 10.1371/journal.ppat.0030184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391. Witchley JN, Penumetcha P, Abon NV, Woolford CA, Mitchell AP, Noble SM. 2019. Candida albicans morphogenesis programs control the balance between gut commensalism and invasive infection. Cell Host Microbe 25:432–443. doi: 10.1016/j.chom.2019.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392. Fan D, Coughlin LA, Neubauer MM, Kim J, Kim MS, Zhan X, Simms-Waldrip TR, Xie Y, Hooper LV, Koh AY. 2015. Activation of HIF-1alpha and LL-37 by commensal bacteria inhibits Candida albicans colonization. Nat Med 21:808–814. doi: 10.1038/nm.3871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393. Matsuo K, Haku A, Bi B, Takahashi H, Kamada N, Yaguchi T, Saijo S, Yoneyama M, Goto Y. 2019. Fecal microbiota transplantation prevents Candida albicans from colonizing the gastrointestinal tract. Microbiol Immunol 63:155–163. doi: 10.1111/1348-0421.12680 [DOI] [PubMed] [Google Scholar]
- 394. Tso GHW, Reales-Calderon JA, Tan ASM, Sem X, Le GTT, Tan TG, Lai GC, Srinivasan KG, Yurieva M, Liao W, Poidinger M, Zolezzi F, Rancati G, Pavelka N. 2018. Experimental evolution of a fungal pathogen into a gut symbiont. Science 362:589–595. doi: 10.1126/science.aat0537 [DOI] [PubMed] [Google Scholar]
- 395. Böhm L, Torsin S, Tint SH, Eckstein MT, Ludwig T, Pérez JC. 2017. The yeast form of the fungus Candida albicans promotes persistence in the gut of gnotobiotic mice. PLoS Pathog 13:e1006699. doi: 10.1371/journal.ppat.1006699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396. Kullas AL, Martin SJ, Davis D. 2007. Adaptation to environmental pH: integrating the Rim101 and calcineurin signal transduction pathways. Mol Microbiol 66:858–871. doi: 10.1111/j.1365-2958.2007.05929.x [DOI] [PubMed] [Google Scholar]
- 397. Znaidi S, van Wijlick L, Hernández-Cervantes A, Sertour N, Desseyn J-L, Vincent F, Atanassova R, Gouyer V, Munro CA, Bachellier-Bassi S, Dalle F, Jouault T, Bougnoux M-E, d’Enfert C. 2018. Systematic gene overexpression in Candida albicans identifies a regulator of early adaptation to the mammalian gut. Cell Microbiol 20:e12890. doi: 10.1111/cmi.12890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398. Pierce JV, Kumamoto CA. 2012. Variation in Candida albicans EFG1 expression enables host-dependent changes in colonizing fungal populations. mBio 3:e00117-12. doi: 10.1128/mBio.00117-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399. Kortman GAM, Raffatellu M, Swinkels DW, Tjalsma H. 2014. Nutritional iron turned inside out: intestinal stress from a gut microbial perspective. FEMS Microbiol Rev 38:1202–1234. doi: 10.1111/1574-6976.12086 [DOI] [PubMed] [Google Scholar]
- 400. Chen C, Pande K, French SD, Tuch BB, Noble SM. 2011. An iron homeostasis regulatory circuit with reciprocal roles in Candida albicans commensalism and pathogenesis. Cell Host Microbe 10:118–135. doi: 10.1016/j.chom.2011.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401. Mamouei Z, Zeng G, Wang YM, Wang Y. 2017. Candida albicans possess a highly versatile and dynamic high-affinity iron transport system important for its commensal-pathogenic lifestyle. Mol Microbiol 106:986–998. doi: 10.1111/mmi.13864 [DOI] [PubMed] [Google Scholar]
- 402. Guinan J, Thangamani S. 2018. Antibiotic-induced alterations in taurocholic acid levels promote gastrointestinal colonization of Candida albicans. FEMS Microbiol Lett 365. doi: 10.1093/femsle/fny196 [DOI] [PubMed] [Google Scholar]
- 403. Guinan J, Villa P, Thangamani S. 2018. Secondary bile acids inhibit Candida albicans growth and morphogenesis. Pathog Dis 76. doi: 10.1093/femspd/fty038 [DOI] [PubMed] [Google Scholar]
- 404. Angebault C, Djossou F, Abélanet S, Permal E, Ben Soltana M, Diancourt L, Bouchier C, Woerther P-L, Catzeflis F, Andremont A, d’Enfert C, Bougnoux M-E. 2013. Candida albicans is not always the preferential yeast colonizing humans: a study in Wayampi Amerindians. J Infect Dis 208:1705–1716. doi: 10.1093/infdis/jit389 [DOI] [PubMed] [Google Scholar]
- 405. Desai PR, van Wijlick L, Kurtz D, Juchimiuk M, Ernst JF. 2015. Hypoxia and temperature regulated morphogenesis in Candida albicans. PLoS Genet 11:e1005447. doi: 10.1371/journal.pgen.1005447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406. Yang D, Zhang M, Su C, Dong B, Lu Y. 2023. Candida albicans exploits N-acetylglucosamine as a gut signal to establish the balance between commensalism and pathogenesis. Nat Commun 14:3796. doi: 10.1038/s41467-023-39284-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407. Charlet R, Pruvost Y, Tumba G, Istel F, Poulain D, Kuchler K, Sendid B, Jawhara S. 2018. Remodeling of the Candida glabrata cell wall in the gastrointestinal tract affects the gut microbiota and the immune response. Sci Rep 8:3316. doi: 10.1038/s41598-018-21422-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408. Witchley JN, Basso P, Brimacombe CA, Abon NV, Noble SM. 2021. Recording of DNA-binding events reveals the importance of a repurposed Candida albicans regulatory network for gut commensalism. Cell Host Microbe 29:1002–1013. doi: 10.1016/j.chom.2021.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409. Anderson FM, Visser ND, Amses KR, Hodgins-Davis A, Weber AM, Metzner KM, McFadden MJ, Mills RE, O’Meara MJ, James TY, O’Meara TR. 2023. Candida albicans selection for human commensalism results in substantial within-host diversity without decreasing fitness for invasive disease. PLoS Biol 21:e3001822. doi: 10.1371/journal.pbio.3001822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410. MacCallum DM, Castillo L, Nather K, Munro CA, Brown AJP, Gow NAR, Odds FC. 2009. Property differences among the four major Candida albicans strain clades. Eukaryot Cell 8:373–387. doi: 10.1128/EC.00387-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411. Ost KS, O’Meara TR, Stephens WZ, Chiaro T, Zhou H, Penman J, Bell R, Catanzaro JR, Song D, Singh S, Call DH, Hwang-Wong E, Hanson KE, Valentine JF, Christensen KA, O’Connell RM, Cormack B, Ibrahim AS, Palm NW, Noble SM, Round JL. 2021. Adaptive immunity induces mutualism between commensal eukaryotes. Nature 596:114–118. doi: 10.1038/s41586-021-03722-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412. Pierre JF, Peters BM, La Torre D, Sidebottom AM, Tao Y, Zhu X, Cham CM, Wang L, Kambal A, Harris KG, Silva JF, Zaborina O, Alverdy JC, Herzog H, Witchley J, Noble SM, Leone VA, Chang EB. 2023. Peptide YY: a paneth cell antimicrobial peptide that maintains Candida gut commensalism. Science 381:502–508. doi: 10.1126/science.abq3178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413. Alonso-Roman R, Last A, Mirhakkak MH, Sprague JL, Möller L, Großmann P, Graf K, Gratz R, Mogavero S, Vylkova S, Panagiotou G, Schäuble S, Hube B, Gresnigt MS. 2022. Lactobacillus rhamnosus colonisation antagonizes Candida albicans by forcing metabolic adaptations that compromise pathogenicity. Nat Commun 13:3192. doi: 10.1038/s41467-022-30661-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414. Graf K, Last A, Gratz R, Allert S, Linde S, Westermann M, Groger M, Mosig AS, Gresnigt MS, Hube B. 2019. Keeping Candida commensal: how lactobacilli antagonize pathogenicity of Candida albicans in an in vitro gut model. Dis Model Mech 12:dmm039719. doi: 10.1242/dmm.039719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415. Hube B. 2009. Fungal adaptation to the host environment. Curr Opin Microbiol 12:347–349. doi: 10.1016/j.mib.2009.06.009 [DOI] [PubMed] [Google Scholar]
- 416. Siscar-Lewin S, Hube B, Brunke S. 2019. Antivirulence and avirulence genes in human pathogenic fungi. Virulence 10:935–947. doi: 10.1080/21505594.2019.1688753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417. Vij R, Hube B, Brunke S. 2021. Uncharted territories in the discovery of antifungal and antivirulence natural products from bacteria. Comput Struct Biotechnol J 19:1244–1252. doi: 10.1016/j.csbj.2021.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418. Li H, Miao MX, Jia CL, Cao YB, Yan TH, Jiang YY, Yang F. 2022. Interactions between Candida albicans and the resident microbiota. Front Microbiol 13:930495. doi: 10.3389/fmicb.2022.930495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419. Pérez JC. 2021. The interplay between gut bacteria and the yeast Candida albicans. Gut Microbes 13:1979877. doi: 10.1080/19490976.2021.1979877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420. Hu Y, Niu Y, Ye X, Zhu C, Tong T, Zhou Y, Zhou X, Cheng L, Ren B. 2021. Staphylococcus aureus synergized with Candida albicans to increase the pathogenesis and drug resistance in cutaneous abscess and peritonitis murine models. Pathogens 10:1036. doi: 10.3390/pathogens10081036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421. Yu X, Mao Y, Li G, Wu X, Xuan Q, Yang S, Chen X, Cao Q, Guo J, Guo J, Wu W. 2023. Alpha-hemolysin from Staphylococcus aureus obstructs yeast-hyphae switching and diminishes pathogenicity in Candida albicans. J Microbiol 61:233–243. doi: 10.1007/s12275-022-00006-4 [DOI] [PubMed] [Google Scholar]
- 422. Camarillo-Márquez O, Córdova-Alcántara IM, Hernández-Rodríguez CH, García-Pérez BE, Martínez-Rivera MA, Rodríguez-Tovar AV. 2018. Antagonistic interaction of Staphylococcus aureus toward Candida glabrata during in vitro biofilm formation is caused by an apoptotic mechanism. Front Microbiol 9:2031. doi: 10.3389/fmicb.2018.02031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423. Cruz MR, Graham CE, Gagliano BC, Lorenz MC, Garsin DA. 2013. Enterococcus faecalis inhibits hyphal morphogenesis and virulence of Candida albicans. Infect Immun 81:189–200. doi: 10.1128/IAI.00914-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424. Leeuwen PT PT, Peet JM, Bikker FJ, Hoogenkamp MA, Oliveira Paiva AM, Kostidis S, Mayboroda OA, Smits WK, Krom BP. 2016. Interspecies interactions between Clostridium difficile and Candida albicans. mSphere 1:e00187-16. doi: 10.1128/msphere.00187-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425. Lopez-Medina E, Fan D, Coughlin LA, Ho EX, Lamont IL, Reimmann C, Hooper LV, Koh AY. 2015. Candida albicans inhibits Pseudomonas aeruginosa virulence through suppression of pyochelin and pyoverdine biosynthesis. PLoS Pathog 11:e1005129. doi: 10.1371/journal.ppat.1005129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426. Bandara H, Yau JYY, Watt RM, Jin LJ, Samaranayake LP. 2010. Pseudomonas aeruginosa inhibits in-vitro Candida biofilm development. BMC Microbiol 10:125. doi: 10.1186/1471-2180-10-125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427. Brand A, Barnes JD, Mackenzie KS, Odds FC, Gow NAR. 2008. Cell wall glycans and soluble factors determine the interactions between the hyphae of Candida albicans and Pseudomonas aeruginosa. FEMS Microbiol Lett 287:48–55. doi: 10.1111/j.1574-6968.2008.01301.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428. Aroutcheva A, Gariti D, Simon M, Shott S, Faro J, Simoes JA, Gurguis A, Faro S. 2001. Defense factors of vaginal lactobacilli. Am J Obstet Gynecol 185:375–379. doi: 10.1067/mob.2001.115867 [DOI] [PubMed] [Google Scholar]
- 429. Sherrington SL, Sorsby E, Mahtey N, Kumwenda P, Lenardon MD, Brown I, Ballou ER, MacCallum DM, Hall RA. 2017. Adaptation of Candida albicans to environmental pH induces cell wall remodelling and enhances innate immune recognition. PLoS Pathog 13:e1006403. doi: 10.1371/journal.ppat.1006403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430. Beyer R, Jandric Z, Zutz C, Gregori C, Willinger B, Jacobsen ID, Kovarik P, Strauss J, Schüller C. 2018. Competition of Candida glabrata against Lactobacillus is Hog1 dependent. Cell Microbiol 20:e12943. doi: 10.1111/cmi.12943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431. Zelante T, Iannitti RG, Cunha C, De Luca A, Giovannini G, Pieraccini G, Zecchi R, D’Angelo C, Massi-Benedetti C, Fallarino F, Carvalho A, Puccetti P, Romani L. 2013. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity 39:372–385. doi: 10.1016/j.immuni.2013.08.003 [DOI] [PubMed] [Google Scholar]
- 432. García C, Tebbji F, Daigneault M, Liu N-N, Köhler JR, Allen-Vercoe E, Sellam A. 2017. The human gut microbial metabolome modulates fungal growth via the TOR signaling pathway. mSphere 2:e00555-17. doi: 10.1128/mSphere.00555-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433. Guinan J, Wang S, Hazbun TR, Yadav H, Thangamani S. 2019. Antibiotic-induced decreases in the levels of microbial-derived short-chain fatty acids correlate with increased gastrointestinal colonization of Candida albicans. Sci Rep 9:8872. doi: 10.1038/s41598-019-45467-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434. Noverr MC, Huffnagle GB. 2004. Regulation of Candida albicans morphogenesis by fatty acid metabolites. Infect Immun 72:6206–6210. doi: 10.1128/IAI.72.11.6206-6210.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435. Chen W, Zhang S, Wu J, Ye T, Wang S, Wang P, Xing D. 2020. Butyrate-producing bacteria and the gut-heart axis in atherosclerosis. Clin Chim Acta 507:236–241. doi: 10.1016/j.cca.2020.04.037 [DOI] [PubMed] [Google Scholar]
- 436. Avelar GM, Dambuza IM, Ricci L, Yuecel R, Mackenzie K, Childers DS, Bain JM, Pradhan A, Larcombe DE, Netea MG, Erwig LP, Brown GD, Duncan SH, Gow NAR, Walker AW, Brown AJP. 2022. Impact of changes at the Candida albicans cell surface upon immunogenicity and colonisation in the gastrointestinal tract. Cell Surf 8:100084. doi: 10.1016/j.tcsw.2022.100084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437. Trunk K, Peltier J, Liu YC, Dill BD, Walker L, Gow NAR, Stark MJR, Quinn J, Strahl H, Trost M, Coulthurst SJ. 2018. The type VI secretion system deploys antifungal effectors against microbial competitors. Nat Microbiol 3:920–931. doi: 10.1038/s41564-018-0191-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438. Monjarás Feria J, Valvano MA. 2020. An overview of anti-eukaryotic T6SS effectors. Front Cell Infect Microbiol 10:584751. doi: 10.3389/fcimb.2020.584751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439. Wood TE, Aksoy E, Hachani A. 2020. From welfare to warfare: the arbitration of host-microbiota interplay by the type VI secretion system. Front Cell Infect Microbiol 10:587948. doi: 10.3389/fcimb.2020.587948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440. Luo J, Chu X, Jie J, Sun Y, Guan Q, Li D, Luo ZQ, Song L. 2023. Acinetobacter baumannii kills fungi via a type VI DNase effector. mBio 14:e0342022. doi: 10.1128/mbio.03420-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441. Zhang D, de Souza RF, Anantharaman V, Iyer LM, Aravind L. 2012. Polymorphic toxin systems: comprehensive characterization of trafficking modes, processing, mechanisms of action, immunity and ecology using comparative genomics. Biol Direct 7:18. doi: 10.1186/1745-6150-7-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442. Wu J, Saupe SJ, Glass NL. 1998. Evidence for balancing selection operating at the het-c heterokaryon incompatibility locus in a group of filamentous fungi. Proc Natl Acad Sci U S A 95:12398–12403. doi: 10.1073/pnas.95.21.12398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443. Eichelberger KR, Paul S, Peters BM, Cassat JE. 2023. Candida-bacterial cross-kingdom interactions. Trends Microbiol 31:1287–1299. doi: 10.1016/j.tim.2023.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444. Brunke S, Mogavero S, Kasper L, Hube B. 2016. Virulence factors in fungal pathogens of man. Curr Opin Microbiol 32:89–95. doi: 10.1016/j.mib.2016.05.010 [DOI] [PubMed] [Google Scholar]
- 445. Köhler JR, Hube B, Puccia R, Casadevall A, Perfect JR. 2017. Fungi that infect humans. Microbiol Spectr 5. doi: 10.1128/microbiolspec.FUNK-0014-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446. Rasheed M, Battu A, Kaur R. 2020. Host-pathogen interaction in Candida glabrata infection: current knowledge and implications for antifungal therapy. Expert Rev Anti Infect Ther 18:1093–1103. doi: 10.1080/14787210.2020.1792773 [DOI] [PubMed] [Google Scholar]
- 447. Argimón S, Wishart JA, Leng R, Macaskill S, Mavor A, Alexandris T, Nicholls S, Knight AW, Enjalbert B, Walmsley R, Odds FC, Gow NAR, Brown AJP. 2007. Developmental regulation of an adhesin gene during cellular morphogenesis in the fungal pathogen Candida albicans. Eukaryot Cell 6:682–692. doi: 10.1128/EC.00340-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448. Karkowska-Kuleta J, Wronowska E, Satala D, Zawrotniak M, Bras G, Kozik A, Nobbs AH, Rapala-Kozik M. 2021. Als3-mediated attachment of enolase on the surface of Candida albicans cells regulates their interactions with host proteins. Cell Microbiol 23:e13297. doi: 10.1111/cmi.13297 [DOI] [PubMed] [Google Scholar]
- 449. Tsuchimori N, Sharkey LL, Fonzi WA, French SW, Edwards Jr JE, Filler SG. 2000. Reduced virulence of HWP1-deficient mutants of Candida albicans and their interactions with host cells. Infect Immun 68:1997–2002. doi: 10.1128/IAI.68.4.1997-2002.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450. de Groot PWJ, Kraneveld EA, Yin QY, Dekker HL, Gross U, Crielaard W, de Koster CG, Bader O, Klis FM, Weig M. 2008. The cell wall of the human pathogen Candida glabrata: differential incorporation of novel adhesin-like wall proteins. Eukaryot Cell 7:1951–1964. doi: 10.1128/EC.00284-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451. Desai C, Mavrianos J, Chauhan N. 2011. Candida glabrata Pwp7p and Aed1p are required for adherence to human endothelial cells. FEMS Yeast Res 11:595–601. doi: 10.1111/j.1567-1364.2011.00743.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452. Nobile CJ, Nett JE, Andes DR, Mitchell AP. 2006. Function of Candida albicans adhesin Hwp1 in biofilm formation. Eukaryot Cell 5:1604–1610. doi: 10.1128/EC.00194-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453. Deng K, Jiang W, Jiang Y, Deng Q, Cao J, Yang W, Zhao X. 2021. ALS3 expression as an indicator for Candida albicans biofilm formation and drug resistance. Front Microbiol 12:655242. doi: 10.3389/fmicb.2021.655242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454. Zhao X, Daniels KJ, Oh SH, Green CB, Yeater KM, Soll DR, Hoyer LL. 2006. Candida albicans Als3p is required for wild-type biofilm formation on silicone elastomer surfaces. Microbiology (Reading) 152:2287–2299. doi: 10.1099/mic.0.28959-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455. Liu Y, Mittal R, Solis NV, Prasadarao NV, Filler SG. 2011. Mechanisms of Candida albicans trafficking to the brain. PLoS Pathog 7:e1002305. doi: 10.1371/journal.ppat.1002305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456. Swidergall M, Solis NV, Millet N, Huang MY, Lin J, Phan QT, Lazarus MD, Wang Z, Yeaman MR, Mitchell AP, Filler SG. 2021. Activation of EphA2-EGFR signaling in oral epithelial cells by Candida albicans virulence factors. PLoS Pathog 17:e1009221. doi: 10.1371/journal.ppat.1009221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457. Phan QT, Solis NV, Cravener MV, Swidergall M, Lin J, Huang MY, Liu H, Singh S, Ibrahim AS, Mazzone M, Mitchell AP, Filler SG. 2023. Candida albicans stimulates formation of a multi-receptor complex that mediates epithelial cell invasion during oropharyngeal infection. PLoS Pathog 19:e1011579. doi: 10.1371/journal.ppat.1011579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458. Albrecht A, Felk A, Pichova I, Naglik JR, Schaller M, de Groot P, Maccallum D, Odds FC, Schäfer W, Klis F, Monod M, Hube B. 2006. Glycosylphosphatidylinositol-anchored proteases of Candida albicans target proteins necessary for both cellular processes and host-pathogen interactions. J Biol Chem 281:688–694. doi: 10.1074/jbc.M509297200 [DOI] [PubMed] [Google Scholar]
- 459. Naglik JR, Challacombe SJ, Hube B. 2003. Candida albicans secreted aspartyl proteinases in virulence and pathogenesis. Microbiol Mol Biol Rev 67:400–428. doi: 10.1128/MMBR.67.3.400-428.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460. Naglik JR, Gaffen SL, Hube B. 2019. Candidalysin: discovery and function in Candida albicans infections. Curr Opin Microbiol 52:100–109. doi: 10.1016/j.mib.2019.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461. Russell CM, Schaefer KG, Dixson A, Gray ALH, Pyron RJ, Alves DS, Moore N, Conley EA, Schuck RJ, White TA, Do TD, King GM, Barrera FN. 2022. The Candida albicans virulence factor candidalysin polymerizes in solution to form membrane pores and damage epithelial cells. Elife 11:e75490. doi: 10.7554/eLife.75490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462. Lapaquette P, Ducreux A, Basmaciyan L, Paradis T, Bon F, Bataille A, Winckler P, Hube B, d’Enfert C, Esclatine A, Dubus E, Bringer M-A, Morel E, Dalle F. 2022. Membrane protective role of autophagic machinery during infection of epithelial cells by Candida albicans. Gut Microbes 14:2004798. doi: 10.1080/19490976.2021.2004798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463. Westman J, Plumb J, Licht A, Yang M, Allert S, Naglik JR, Hube B, Grinstein S, Maxson ME. 2022. Calcium-dependent ESCRT recruitment and lysosome exocytosis maintain epithelial integrity during Candida albicans invasion. Cell Rep 38:110187. doi: 10.1016/j.celrep.2021.110187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464. Drummond RA, Swamydas M, Oikonomou V, Zhai B, Dambuza IM, Schaefer BC, Bohrer AC, Mayer-Barber KD, Lira SA, Iwakura Y, Filler SG, Brown GD, Hube B, Naglik JR, Hohl TM, Lionakis MS. 2019. CARD9+ microglia promote antifungal immunity via IL-1β- and CXCL1-mediated neutrophil recruitment. Nat Immunol 20:559–570. doi: 10.1038/s41590-019-0377-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465. Swidergall M, Khalaji M, Solis NV, Moyes DL, Drummond RA, Hube B, Lionakis MS, Murdoch C, Filler SG, Naglik JR. 2019. Candidalysin is required for neutrophil recruitment and virulence during systemic Candida albicans infection. J Infect Dis 220:1477–1488. doi: 10.1093/infdis/jiz322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466. Richardson JP, Willems HME, Moyes DL, Shoaie S, Barker KS, Tan SL, Palmer GE, Hube B, Naglik JR, Peters BM. 2018. Candidalysin drives epithelial signaling, neutrophil recruitment, and immunopathology at the vaginal mucosa. Infect Immun 86:e00645-17. doi: 10.1128/IAI.00645-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467. Verma AH, Richardson JP, Zhou C, Coleman BM, Moyes DL, Ho J, Huppler AR, Ramani K, McGeachy MJ, Mufazalov IA, Waisman A, Kane LP, Biswas PS, Hube B, Naglik JR, Gaffen SL. 2017. Oral epithelial cells orchestrate innate type 17 responses to Candida albicans through the virulence factor candidalysin. Sci Immunol 2:eaam8834. doi: 10.1126/sciimmunol.aam8834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468. Tati S, Davidow P, McCall A, Hwang-Wong E, Rojas IG, Cormack B, Edgerton M. 2016. Candida glabrata binding to Candida albicans hyphae enables its development in oropharyngeal candidiasis. PLoS Pathog 12:e1005522. doi: 10.1371/journal.ppat.1005522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469. Pekmezovic M, Kaune A-K, Austermeier S, Hitzler SUJ, Mogavero S, Hovhannisyan H, Gabaldón T, Gresnigt MS, Hube B. 2021. Human albumin enhances the pathogenic potential of Candida glabrata on vaginal epithelial cells. PLoS Pathog 17:e1010037. doi: 10.1371/journal.ppat.1010037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470. Phan QT, Solis NV, Lin J, Swidergall M, Singh S, Liu H, Sheppard DC, Ibrahim AS, Mitchell AP, Filler SG. 2022. Serum bridging molecules drive candidal invasion of human but not mouse endothelial cells. PLoS Pathog 18:e1010681. doi: 10.1371/journal.ppat.1010681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471. Moyes DL, Richardson JP, Naglik JR. 2015. Candida albicans-epithelial interactions and pathogenicity mechanisms: scratching the surface. Virulence 6:338–346. doi: 10.1080/21505594.2015.1012981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472. Mogavero S, Hube B. 2021. Candida albicans interaction with oral epithelial cells: adhesion, invasion, and damage assays. Methods Mol Biol 2260:133–143. doi: 10.1007/978-1-0716-1182-1_9 [DOI] [PubMed] [Google Scholar]
- 473. Swidergall M, Filler SG. 2017. Oropharyngeal candidiasis: fungal invasion and epithelial cell responses. PLoS Pathog 13:e1006056. doi: 10.1371/journal.ppat.1006056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474. Krysan DJ, Sutterwala FS, Wellington M. 2014. Catching fire: Candida albicans, macrophages, and pyroptosis. PLoS Pathog 10:e1004139. doi: 10.1371/journal.ppat.1004139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475. Lange T, Kasper L, Gresnigt MS, Brunke S, Hube B. 2023. "Under Pressure" - How fungi evade, exploit, and modulate cells of the innate immune system. Semin Immunol 66:101738. doi: 10.1016/j.smim.2023.101738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476. Pai M, Behr MA, Dowdy D, Dheda K, Divangahi M, Boehme CC, Ginsberg A, Swaminathan S, Spigelman M, Getahun H, Menzies D, Raviglione M. 2016. Tuberculosis. Nat Rev Dis Primers 2:16076. doi: 10.1038/nrdp.2016.76 [DOI] [PubMed] [Google Scholar]
- 477. Kasper L, Seider K, Hube B. 2015. Intracellular survival of Candida glabrata in macrophages: immune evasion and persistence. FEMS Yeast Research 15:fov042. doi: 10.1093/femsyr/fov042 [DOI] [PubMed] [Google Scholar]
- 478. Bain JM, Lewis LE, Okai B, Quinn J, Gow NAR, Erwig L-P. 2012. Non-lytic expulsion/exocytosis of Candida albicans from macrophages. Fungal Genet Biol 49:677–678. doi: 10.1016/j.fgb.2012.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479. Scherer AK, Blair BA, Park J, Seman BG, Kelley JB, Wheeler RT. 2020. Redundant Trojan horse and endothelial-circulatory mechanisms for host-mediated spread of Candida albicans yeast. PLoS Pathog 16:e1008414. doi: 10.1371/journal.ppat.1008414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480. Ding X, Kambara H, Guo R, Kanneganti A, Acosta-Zaldívar M, Li J, Liu F, Bei T, Qi W, Xie X, Han W, Liu N, Zhang C, Zhang X, Yu H, Zhao L, Ma F, Köhler JR, Luo HR. 2021. Inflammasome-mediated GSDMD activation facilitates escape of Candida albicans from macrophages. Nat Commun 12:6699. doi: 10.1038/s41467-021-27034-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481. Olivier FAB, Hilsenstein V, Weerasinghe H, Weir A, Hughes S, Crawford S, Vince JE, Hickey MJ, Traven A. 2022. The escape of Candida albicans from macrophages is enabled by the fungal toxin candidalysin and two host cell death pathways. Cell Rep 40:111374. doi: 10.1016/j.celrep.2022.111374 [DOI] [PubMed] [Google Scholar]
- 482. Ibata-Ombetta S, Idziorek T, Trinel PA, Poulain D, Jouault T. 2003. Candida albicans phospholipomannan promotes survival of phagocytosed yeasts through modulation of bad phosphorylation and macrophage apoptosis. J Biol Chem 278:13086–13093. doi: 10.1074/jbc.M210680200 [DOI] [PubMed] [Google Scholar]
- 483. Cao M, Wu Z, Lou Q, Lu W, Zhang J, Li Q, Zhang Y, Yao Y, Zhao Q, Li M, Zhang H, Qian Y. 2019. Dectin-1-induced RIPK1 and RIPK3 activation protects host against Candida albicans infection. Cell Death Differ 26:2622–2636. doi: 10.1038/s41418-019-0323-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484. Reales-Calderón JA, Sylvester M, Strijbis K, Jensen ON, Nombela C, Molero G, Gil C. 2013. Candida albicans induces pro-inflammatory and anti-apoptotic signals in macrophages as revealed by quantitative proteomics and phosphoproteomics. J Proteomics 91:106–135. doi: 10.1016/j.jprot.2013.06.026 [DOI] [PubMed] [Google Scholar]
- 485. Roetzer A, Gratz N, Kovarik P, Schüller C. 2010. Autophagy supports Candida glabrata survival during phagocytosis. Cell Microbiol 12:199–216. doi: 10.1111/j.1462-5822.2009.01391.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486. Roetzer A, Klopf E, Gratz N, Marcet-Houben M, Hiller E, Rupp S, Gabaldón T, Kovarik P, Schüller C. 2011. Regulation of Candida glabrata oxidative stress resistance is adapted to host environment. FEBS Lett 585:319–327. doi: 10.1016/j.febslet.2010.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487. Cuéllar-Cruz M, Briones-Martin-del-Campo M, Cañas-Villamar I, Montalvo-Arredondo J, Riego-Ruiz L, Castaño I, De Las Peñas A. 2008. High resistance to oxidative stress in the fungal pathogen Candida glabrata is mediated by a single catalase, Cta1p, and is controlled by the transcription factors Yap1p, Skn7p, Msn2p, and Msn4p. Eukaryot Cell 7:814–825. doi: 10.1128/EC.00011-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488. Pais P, Vagueiro S, Mil-Homens D, Pimenta AI, Viana R, Okamoto M, Chibana H, Fialho AM, Teixeira MC. 2020. A new regulator in the crossroads of oxidative stress resistance and virulence in Candida glabrata: the transcription factor CgTog1. Virulence 11:1522–1538. doi: 10.1080/21505594.2020.1839231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489. Kämmer P, McNamara S, Wolf T, Conrad T, Allert S, Gerwien F, Hünniger K, Kurzai O, Guthke R, Hube B, Linde J, Brunke S. 2020. Survival strategies of pathogenic Candida species in human blood show independent and specific adaptations. mBio 11:e02435-20. doi: 10.1128/mBio.02435-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490. Fradin C, De Groot P, MacCallum D, Schaller M, Klis F, Odds FC, Hube B. 2005. Granulocytes govern the transcriptional response, morphology and proliferation of Candida albicans in human blood. Mol Microbiol 56:397–415. doi: 10.1111/j.1365-2958.2005.04557.x [DOI] [PubMed] [Google Scholar]
- 491. Frohner IE, Bourgeois C, Yatsyk K, Majer O, Kuchler K. 2009. Candida albicans cell surface superoxide dismutases degrade host-derived reactive oxygen species to escape innate immune surveillance. Mol Microbiol 71:240–252. doi: 10.1111/j.1365-2958.2008.06528.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 492. Martchenko M, Alarco AM, Harcus D, Whiteway M. 2004. Superoxide dismutases in Candida albicans: transcriptional regulation and functional characterization of the hyphal-induced SOD5 gene. Mol Biol Cell 15:456–467. doi: 10.1091/mbc.e03-03-0179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493. Gow NAR, Netea MG, Munro CA, Ferwerda G, Bates S, Mora-Montes HM, Walker L, Jansen T, Jacobs L, Tsoni V, Brown GD, Odds FC, Van der Meer JWM, Brown AJP, Kullberg BJ. 2007. Immune recognition of Candida albicans beta-glucan by dectin-1. J Infect Dis 196:1565–1571. doi: 10.1086/523110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494. McKenzie CGJ, Koser U, Lewis LE, Bain JM, Mora-Montes HM, Barker RN, Gow NAR, Erwig LP. 2010. Contribution of Candida albicans cell wall components to recognition by and escape from murine macrophages. Infect Immun 78:1650–1658. doi: 10.1128/IAI.00001-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 495. Bain JM, Louw J, Lewis LE, Okai B, Walls CA, Ballou ER, Walker LA, Reid D, Munro CA, Brown AJP, Brown GD, Gow NAR, Erwig LP. 2014. Candida albicans hypha formation and mannan masking of beta-glucan inhibit macrophage phagosome maturation. mBio 5:e01874. doi: 10.1128/mBio.01874-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 496. Yadav B, Mora-Montes HM, Wagener J, Cunningham I, West L, Haynes K, Brown AJP, Gow NAR. 2020. Differences in fungal immune recognition by monocytes and macrophages: N-mannan can be a shield or activator of immune recognition. Cell Surf 6:100042. doi: 10.1016/j.tcsw.2020.100042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 497. Ballou ER, Avelar GM, Childers DS, Mackie J, Bain JM, Wagener J, Kastora SL, Panea MD, Hardison SE, Walker LA, Erwig LP, Munro CA, Gow NAR, Brown GD, MacCallum DM, Brown AJP. 2016. Lactate signalling regulates fungal beta-glucan masking and immune evasion. Nat Microbiol 2:16238. doi: 10.1038/nmicrobiol.2016.238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 498. Cottier F, Sherrington S, Cockerill S, Del Olmo Toledo V, Kissane S, Tournu H, Orsini L, Palmer GE, Pérez JC, Hall RA. 2019. Remasking of Candida albicans beta-glucan in response to environmental pH is regulated by quorum sensing. mBio 10:e02347-19. doi: 10.1128/mBio.02347-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 499. Pradhan A, Avelar GM, Bain JM, Childers DS, Larcombe DE, Netea MG, Shekhova E, Munro CA, Brown GD, Erwig LP, Gow NAR, Brown AJP. 2018. Hypoxia promotes immune evasion by triggering beta-glucan masking on the Candida albicans cell surface via mitochondrial and cAMP-protein kinase A signaling. mBio 9:e01318-18. doi: 10.1128/mBio.01318-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 500. Pradhan A, Avelar GM, Bain JM, Childers D, Pelletier C, Larcombe DE, Shekhova E, Netea MG, Brown GD, Erwig L, Gow NAR, Brown AJP. 2019. Non-canonical signalling mediates changes in fungal cell wall PAMPs that drive immune evasion. Nat Commun 10:5315. doi: 10.1038/s41467-019-13298-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501. Childers DS, Avelar GM, Bain JM, Pradhan A, Larcombe DE, Netea MG, Erwig LP, Gow NAR, Brown AJP. 2020. Epitope shaving promotes fungal immune evasion. mBio 11:e00984-20. doi: 10.1128/mBio.00984-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 502. Yang M, Solis NV, Marshall M, Garleb R, Zhou T, Wang D, Swidergall M, Pearlman E, Filler SG, Liu H. 2022. Control of beta-glucan exposure by the endo-1,3-glucanase Eng1 in Candida albicans modulates virulence. PLoS Pathog 18:e1010192. doi: 10.1371/journal.ppat.1010192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503. Hopke A, Nicke N, Hidu EE, Degani G, Popolo L, Wheeler RT. 2016. Neutrophil attack triggers extracellular trap-dependent Candida cell wall remodeling and altered immune recognition. PLoS Pathog 12:e1005644. doi: 10.1371/journal.ppat.1005644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 504. Lorenz MC, Bender JA, Fink GR. 2004. Transcriptional response of Candida albicans upon internalization by macrophages. Eukaryot Cell 3:1076–1087. doi: 10.1128/EC.3.5.1076-1087.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 505. Rai MN, Balusu S, Gorityala N, Dandu L, Kaur R. 2012. Functional genomic analysis of Candida glabrata-macrophage interaction: role of chromatin remodeling in virulence. PLoS Pathog 8:e1002863. doi: 10.1371/journal.ppat.1002863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 506. Silao FGS, Ward M, Ryman K, Wallström A, Brindefalk B, Udekwu K, Ljungdahl PO. 2019. Mitochondrial proline catabolism activates Ras1/cAMP/PKA-induced filamentation in Candida albicans. PLoS Genet 15:e1007976. doi: 10.1371/journal.pgen.1007976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 507. Barelle CJ, Priest CL, Maccallum DM, Gow NAR, Odds FC, Brown AJP. 2006. Niche-specific regulation of central metabolic pathways in a fungal pathogen. Cell Microbiol 8:961–971. doi: 10.1111/j.1462-5822.2005.00676.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 508. Lorenz MC, Fink GR. 2001. The glyoxylate cycle is required for fungal virulence. Nature 412:83–86. doi: 10.1038/35083594 [DOI] [PubMed] [Google Scholar]
- 509. Larcombe DE, Bohovych IM, Pradhan A, Ma Q, Hickey E, Leaves I, Cameron G, Avelar GM, de Assis LJ, Childers DS, Bain JM, Lagree K, Mitchell AP, Netea MG, Erwig LP, Gow NAR, Brown AJP. 2023. Glucose-enhanced oxidative stress resistance-A protective anticipatory response that enhances the fitness of Candida albicans during systemic infection. PLoS Pathog 19:e1011505. doi: 10.1371/journal.ppat.1011505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 510. Gerwien F, Skrahina V, Kasper L, Hube B, Brunke S. 2018. Metals in fungal virulence. FEMS Microbiol Rev 42:fux050. doi: 10.1093/femsre/fux050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 511. Mogavero S, Höfs S, Lauer AN, Müller R, Brunke S, Allert S, Gerwien F, Groth S, Dolk E, Wilson D, Gutsmann T, Hube B. 2022. Candidalysin is the hemolytic factor of Candida albicans. Toxins (Basel) 14:874. doi: 10.3390/toxins14120874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 512. Weissman Z, Kornitzer D. 2004. A family of Candida cell surface haem-binding proteins involved in haemin and haemoglobin-iron utilization. Mol Microbiol 53:1209–1220. doi: 10.1111/j.1365-2958.2004.04199.x [DOI] [PubMed] [Google Scholar]
- 513. Almeida RS, Brunke S, Albrecht A, Thewes S, Laue M, Edwards JE, Filler SG, Hube B. 2008. The hyphal-associated adhesin and invasin Als3 of Candida albicans mediates iron acquisition from host ferritin. PLoS Pathog 4:e1000217. doi: 10.1371/journal.ppat.1000217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 514. Baek YU, Li M, Davis DA. 2008. Candida albicans ferric reductases are differentially regulated in response to distinct forms of iron limitation by the Rim101 and CBF transcription factors. Eukaryot Cell 7:1168–1179. doi: 10.1128/EC.00108-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 515. Lan CY, Rodarte G, Murillo LA, Jones T, Davis RW, Dungan J, Newport G, Agabian N. 2004. Regulatory networks affected by iron availability in Candida albicans. Mol Microbiol 53:1451–1469. doi: 10.1111/j.1365-2958.2004.04214.x [DOI] [PubMed] [Google Scholar]
- 516. Gerwien F, Safyan A, Wisgott S, Brunke S, Kasper L, Hube B. 2017. The fungal pathogen Candida glabrata does not depend on surface ferric reductases for iron acquisition. Front Microbiol 8:1055. doi: 10.3389/fmicb.2017.01055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 517. Gerwien F, Safyan A, Wisgott S, Hille F, Kaemmer P, Linde J, Brunke S, Kasper L, Hube B. 2016. A novel hybrid iron regulation network combines features from pathogenic and nonpathogenic yeasts. mBio 7:e01782-16. doi: 10.1128/mBio.01782-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 518. Nevitt T, Thiele DJ. 2011. Host iron withholding demands siderophore utilization for Candida glabrata to survive macrophage killing. PLoS Pathog 7:e1001322. doi: 10.1371/journal.ppat.1001322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519. Citiulo F, Jacobsen ID, Miramón P, Schild L, Brunke S, Zipfel P, Brock M, Hube B, Wilson D. 2012. Candida albicans scavenges host zinc via Pra1 during endothelial invasion. PLoS Pathog 8:e1002777. doi: 10.1371/journal.ppat.1002777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 520. Thorvaldsen JL, Sewell AK, McCowen CL, Winge DR. 1993. Regulation of metallothionein genes by the ACE1 and AMT1 transcription factors. J Biol Chem 268:12512–12518. doi: 10.1016/S0021-9258(18)31418-2 [DOI] [PubMed] [Google Scholar]
- 521. Weissman Z, Berdicevsky I, Cavari BZ, Kornitzer D. 2000. The high copper tolerance of Candida albicans is mediated by a P-type ATPase. Proc Natl Acad Sci U S A 97:3520–3525. doi: 10.1073/pnas.97.7.3520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 522. Riedelberger M, Penninger P, Tscherner M, Hadriga B, Brunnhofer C, Jenull S, Stoiber A, Bourgeois C, Petryshyn A, Glaser W, Limbeck A, Lynes MA, Schabbauer G, Weiss G, Kuchler K. 2020. Type I interferons ameliorate zinc intoxication of Candida glabrata by macrophages and promote fungal immune evasion. iScience 23:101121. doi: 10.1016/j.isci.2020.101121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 523. Edwards Jr JE, Schwartz MM, Schmidt CS, Sobel JD, Nyirjesy P, Schodel F, Marchus E, Lizakowski M, DeMontigny EA, Hoeg J, Holmberg T, Cooke MT, Hoover K, Edwards L, Jacobs M, Sussman S, Augenbraun M, Drusano M, Yeaman MR, Ibrahim AS, Filler SG, Hennessey Jr JP. 2018. A fungal immunotherapeutic vaccine (NDV-3A) for treatment of recurrent vulvovaginal candidiasis-A phase 2 randomized, double-blind, placebo-controlled trial. Clin Infect Dis 66:1928–1936. doi: 10.1093/cid/ciy185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524. Oliveira LVN, Wang R, Specht CA, Levitz SM. 2021. Vaccines for human fungal diseases: close but still a long way to go. NPJ Vaccines 6:33. doi: 10.1038/s41541-021-00294-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 525. Pletz MW, Hagel S, Forstner C. 2017. Who benefits from antimicrobial combination therapy? Lancet Infect Dis 17:677–678. doi: 10.1016/S1473-3099(17)30233-5 [DOI] [PubMed] [Google Scholar]
- 526. Steinbach WJ, Reedy JL, Cramer Jr RA, Perfect JR, Heitman J. 2007. Harnessing calcineurin as a novel anti-infective agent against invasive fungal infections. Nat Rev Microbiol 5:418–430. doi: 10.1038/nrmicro1680 [DOI] [PubMed] [Google Scholar]
- 527. Galdiero E, de Alteriis E, De Natale A, D’Alterio A, Siciliano A, Guida M, Lombardi L, Falanga A, Galdiero S. 2020. Eradication of Candida albicans persister cell biofilm by the membranotropic peptide gH625. Sci Rep 10:5780. doi: 10.1038/s41598-020-62746-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 528. Roselletti E, Pericolini E, Nore A, Takacs P, Kozma B, Sala A, De Seta F, Comar M, Usher J, Brown GD, Wilson D. 2023. Zinc prevents vaginal candidiasis by inhibiting expression of an inflammatory fungal protein. Sci Transl Med 15:eadi3363. doi: 10.1126/scitranslmed.adi3363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 529. Edman J, Sobel JD, Taylor ML. 1986. Zinc status in women with recurrent vulvovaginal candidiasis. Am J Obstet Gynecol 155:1082–1085. doi: 10.1016/0002-9378(86)90355-8 [DOI] [PubMed] [Google Scholar]
- 530. Roselletti E, Pericolini E, Nore A, Takacs P, Kozma B, Sala A, De Seta F, Comar M, Usher J, Brown GD, Wilson D. 2023. Zinc prevents vaginal candidiasis by inhibiting expression of an inflammatory fungal protein. Sci Transl Med 15:eadi3363. doi: 10.1126/scitranslmed.adi3363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 531. Valentine M, Rudolph P, Dietschmann A, Tsavou A, Mogavero S, Lee S, Priest EL, Zhurgenbayeva G, Jablonowski N, Timme S, Eggeling C, Allert S, Dolk E, Naglik JR, Figge MT, Gresnigt MS, Hube B. 2024. Nanobody-mediated neutralization of candidalysin prevents epithelial damage and inflammatory responses that drive vulvovaginal candidiasis pathogenesis. mBio 15:e03409-23. doi: 10.1128/mbio.03409-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 532. Eggimann P, Que YA, Revelly JP, Pagani JL. 2015. Preventing invasive Candida infections. Where could we do better? J Hosp Infect 89:302–308. doi: 10.1016/j.jhin.2014.11.006 [DOI] [PubMed] [Google Scholar]
- 533. Leonardi I, Paramsothy S, Doron I, Semon A, Kaakoush NO, Clemente JC, Faith JJ, Borody TJ, Mitchell HM, Colombel JF, Kamm MA, Iliev ID. 2020. Fungal trans-kingdom dynamics linked to responsiveness to fecal microbiota transplantation (FMT) therapy in ulcerative colitis. Cell Host Microbe 27:823–829. doi: 10.1016/j.chom.2020.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 534. Nazik H, Joubert LM, Secor PR, Sweere JM, Bollyky PL, Sass G, Cegelski L, Stevens DA. 2017. Pseudomonas phage inhibition of Candida albicans. Microbiology (Reading) 163:1568–1577. doi: 10.1099/mic.0.000539 [DOI] [PubMed] [Google Scholar]