Abstract
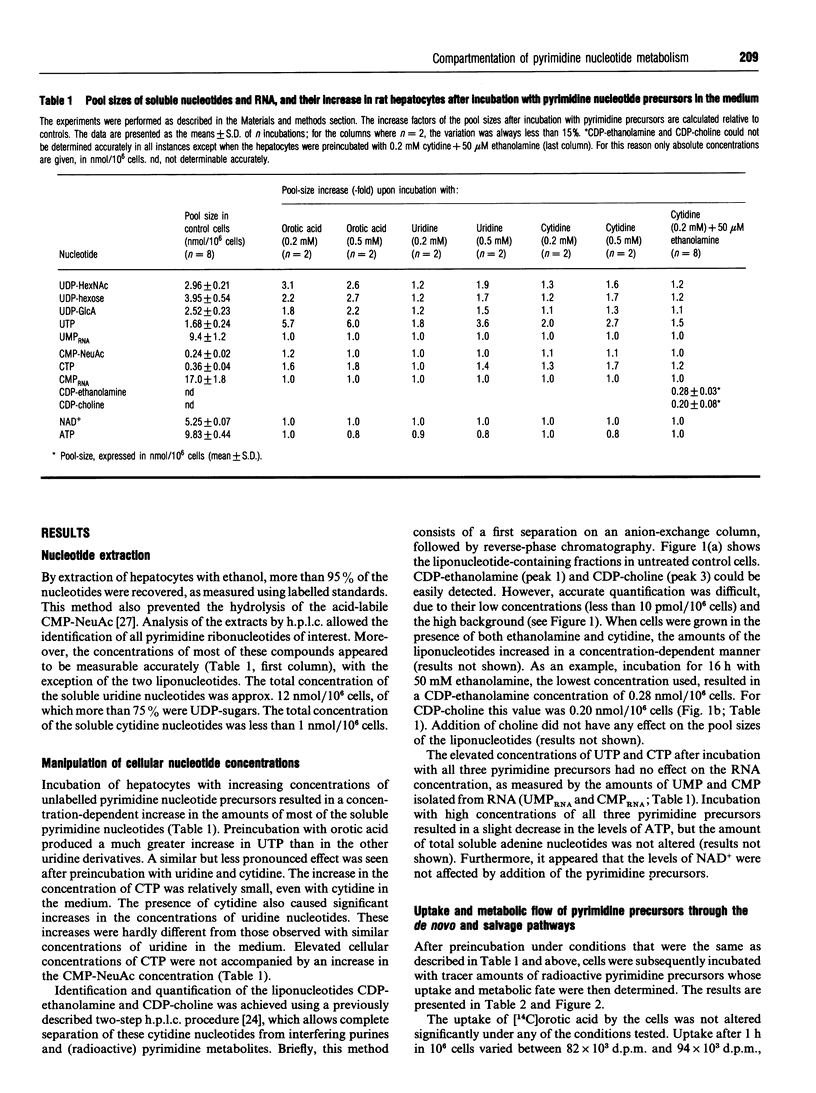
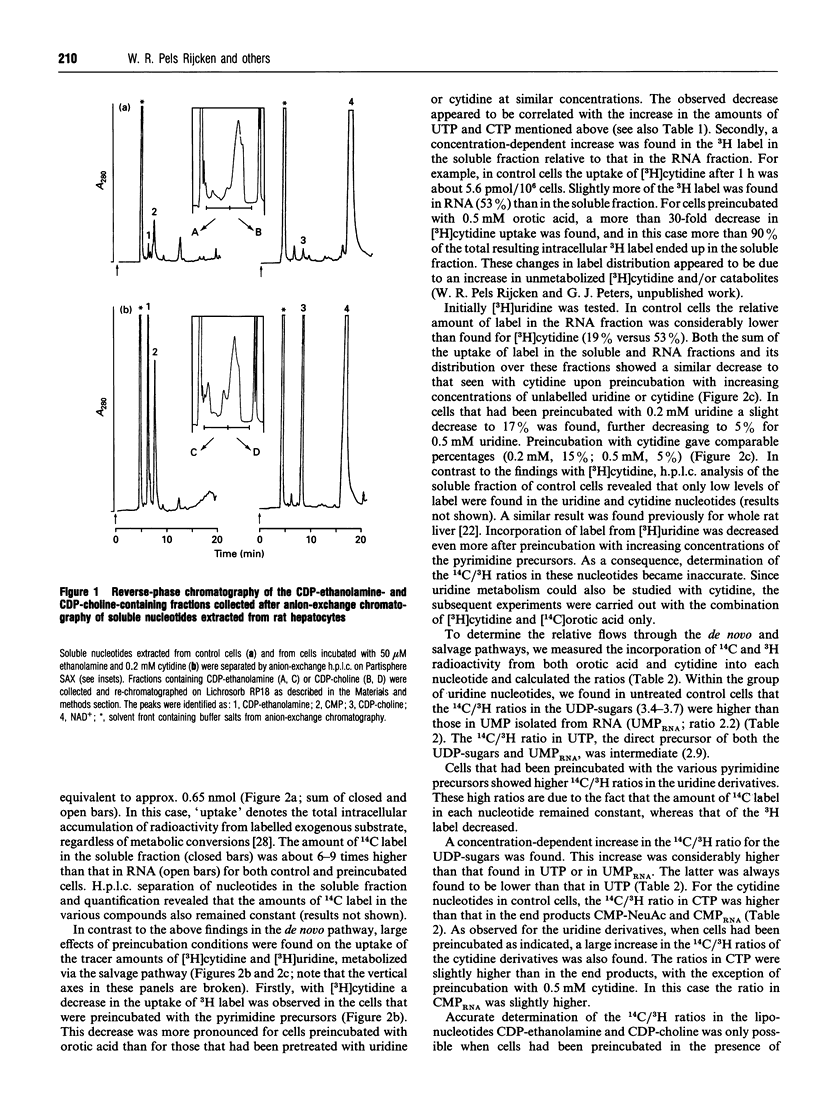
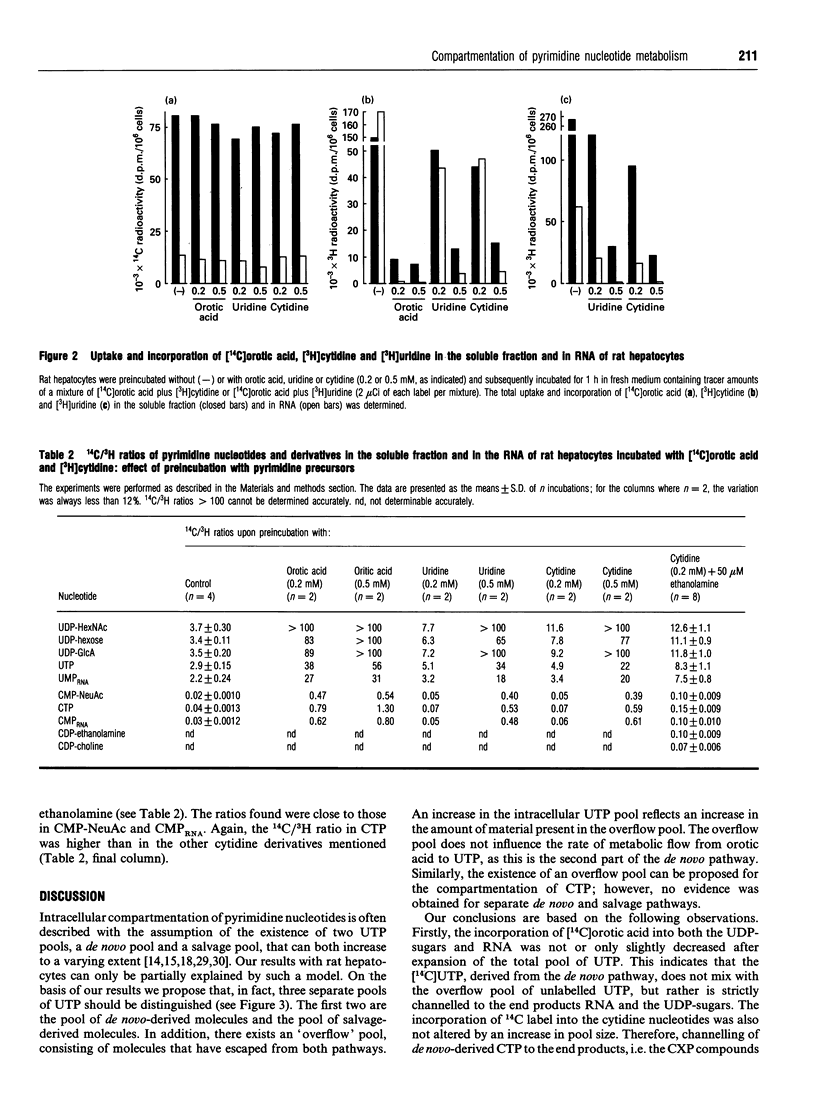
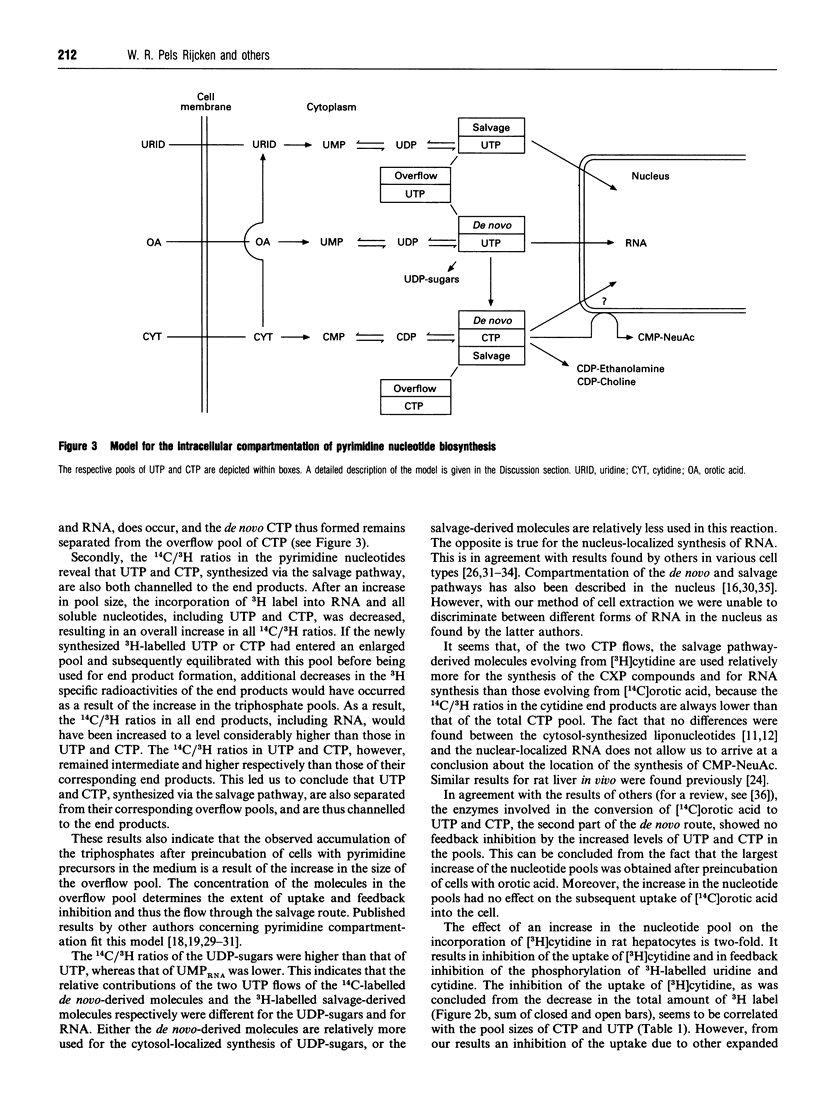
Pyrimidine nucleotide metabolism in rat hepatocytes was studied by measurement of the labelling kinetics of the various intermediates after double labelling with [14C]orotic acid and [3H]cytidine, the precursors for the de novo and the salvage pathways respectively. For the uridine nucleotides, differences were found for the 14C/3H ratios in the UDP-sugars, in UMP (of RNA) and in their precursor UTP, suggesting the existence of separated flows of the radioactive precursors through the de novo and the salvage pathways. Higher ratios in the UDP-sugars, which are synthesized in the cytoplasm, and a lower ratio in UMP (of RNA) relative to the 14C/3H ratio in UTP indicated that UTP derived from orotic acid is preferentially used for the cytoplasmic biosynthesis of the UDP-sugars. Uridine, derived from cytidine, is preferentially used for the nuclear-localized synthesis of RNA. In contrast to these findings, the 14C/3H ratios in the cytidine derivatives CMP-NeuAc and CMP (of RNA), and in the liponucleotides CDP-choline and CDP-ethanolamine, were all lower than that in the precursor CTP. This indicates a preferential utilization of the salvage-derived CTP for the synthesis of the liponucleotides as well as for RNA and CMP-NeuAc. Similar conclusions could be drawn from experiments in which the intracellular amounts of several uridine- and cytidine-nucleotide-containing derivatives were increased by preincubating the hepatocytes with unlabelled pyrimidine nucleotides or ethanolamine. Based on these data, we propose a refined model for the intracellular compartmentation of pyrimidine nucleotide biosynthesis in which three pools of UTP are distinguished: a pool of de novo-derived molecules and a pool of salvage-derived molecules, both of which are channelled to the site of utilization; in addition an 'overflow' pool exists, consisting of molecules having escaped from channelling. An overflow pool could also be distinguished for CTP, but no discrimination between de novo and salvage-derived molecules could be made.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON E. P., BROCKMAN R. W. FEEDBACK INHIBITION OF URIDINE KINASE BY CYTIDINE TRIPHOSPHATE AND URIDINE TRIPHOSPHATE. Biochim Biophys Acta. 1964 Nov 15;91:380–386. doi: 10.1016/0926-6550(64)90067-2. [DOI] [PubMed] [Google Scholar]
- Andersson M., Lewan L., Stenram U. Compartmentation of purine and pyrimidine nucleotides in animal cells. Int J Biochem. 1988;20(10):1039–1050. doi: 10.1016/0020-711x(88)90248-0. [DOI] [PubMed] [Google Scholar]
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissonnette R. The de novo and salvage pathways for the synthesis of pyrimidine residues of RNA predominate in different locations within the mouse duodenal epithelium. Cell Tissue Res. 1992 Jan;267(1):131–137. doi: 10.1007/BF00318699. [DOI] [PubMed] [Google Scholar]
- Cacan R., Cecchelli R., Hoflack B., Verbert A. Intralumenal pool and transport of CMP-N-acetylneuraminic acid, GDP-fucose and UDP-galactose. Study with plasma-membrane-permeabilized mouse thymocytes. Biochem J. 1984 Nov 15;224(1):277–284. doi: 10.1042/bj2240277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capasso J. M., Hirschberg C. B. Mechanisms of glycosylation and sulfation in the Golgi apparatus: evidence for nucleotide sugar/nucleoside monophosphate and nucleotide sulfate/nucleoside monophosphate antiports in the Golgi apparatus membrane. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7051–7055. doi: 10.1073/pnas.81.22.7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey D. J., Hirschberg C. B. Topography of sialoglycoproteins and sialyltransferases in mouse and rat liver Golgi. J Biol Chem. 1981 Jan 25;256(2):989–993. [PubMed] [Google Scholar]
- Coates S. W., Gurney T., Jr, Sommers L. W., Yeh M., Hirschberg C. B. Subcellular localization of sugar nucleotide synthetases. J Biol Chem. 1980 Oct 10;255(19):9225–9229. [PubMed] [Google Scholar]
- Coleman R., Bell R. M. Evidence that biosynthesis of phosphatidylethanolamine, phosphatidylcholine, and triacylglycerol occurs on the cytoplasmic side of microsomal vesicles. J Cell Biol. 1978 Jan;76(1):245–253. doi: 10.1083/jcb.76.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes P., Levin N. W., Dumler F., Venkatachalam K. K., Verghese C. P., Bernstein J. Incorporation of exogenous precursors into uridine nucleotides and ribonucleic acid. Nucleotide compartmentation in the renal cortex in vivo. Biochem J. 1979 Sep 15;182(3):677–686. doi: 10.1042/bj1820677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferwerda W., Blok C. M., Heijlman J. Turnover of free sialic acid, CMP-sialic acid, and bound sialic acid in rat brain. J Neurochem. 1981 Apr;36(4):1492–1499. doi: 10.1111/j.1471-4159.1981.tb00591.x. [DOI] [PubMed] [Google Scholar]
- Fleischer B. Orientation of glycoprotein galactosyltransferase and sialyltransferase enzymes in vesicles derived from rat liver Golgi apparatus. J Cell Biol. 1981 May;89(2):246–255. doi: 10.1083/jcb.89.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genchev D. D. Free pyrimidine nucleotide pool of Ehrlich ascites-tumour cells. Characteristics related to quantitative studies of RNA metabolism. Biochem J. 1980 Apr 15;188(1):75–83. doi: 10.1042/bj1880075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genchev D. D., Kermekchiev M. B., Hadjiolov A. A. Free pyrimidine nucleotide pool of Ehrlich ascites-tumour cells. Compartmentation with respect to the synthesis of heterogeneous nuclear RNA and precursors to ribosomal RNA. Biochem J. 1980 Apr 15;188(1):85–90. doi: 10.1042/bj1880085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauschka P. V. Analysis of nucleotide pools in animal cells. Methods Cell Biol. 1973;7:361–462. doi: 10.1016/s0091-679x(08)61787-2. [DOI] [PubMed] [Google Scholar]
- Hubbard S. C., Ivatt R. J. Synthesis and processing of asparagine-linked oligosaccharides. Annu Rev Biochem. 1981;50:555–583. doi: 10.1146/annurev.bi.50.070181.003011. [DOI] [PubMed] [Google Scholar]
- Jones M. E. Pyrimidine nucleotide biosynthesis in animals: genes, enzymes, and regulation of UMP biosynthesis. Annu Rev Biochem. 1980;49:253–279. doi: 10.1146/annurev.bi.49.070180.001345. [DOI] [PubMed] [Google Scholar]
- Kean E. L. Nuclear cytidine 5'-monophosphosialic acid synthetase. J Biol Chem. 1970 May 10;245(9):2301–2308. [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Losman M. J., Harley E. H. Evidence for compartmentation of uridine nucleotide pools in rat hepatoma cells. Biochim Biophys Acta. 1978 Dec 21;521(2):762–769. doi: 10.1016/0005-2787(78)90315-5. [DOI] [PubMed] [Google Scholar]
- Moyer J. D., Henderson J. F. Compartmentation of intracellular nucleotides in mammalian cells. CRC Crit Rev Biochem. 1985;19(1):45–61. doi: 10.3109/10409238509086787. [DOI] [PubMed] [Google Scholar]
- Nikolov E. N., Dabeva M. D. Re-utilization of pyrimidine nucleotides during rat liver regeneration. Biochem J. 1985 May 15;228(1):27–33. doi: 10.1042/bj2280027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelech S. L., Vance D. E. Regulation of phosphatidylcholine biosynthesis. Biochim Biophys Acta. 1984 Jun 25;779(2):217–251. doi: 10.1016/0304-4157(84)90010-8. [DOI] [PubMed] [Google Scholar]
- Pels Rijcken W. R., Hooghwinkel G. J., Ferwerda W. Pyrimidine metabolism and sugar nucleotide synthesis in rat liver. Biochem J. 1990 Mar 15;266(3):777–783. doi: 10.1042/bj2660777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez M., Hirschberg C. B. Transport of sugar nucleotides and adenosine 3'-phosphate 5'-phosphosulfate into vesicles derived from the Golgi apparatus. Biochim Biophys Acta. 1986 Sep 22;864(2):213–222. doi: 10.1016/0304-4157(86)90012-2. [DOI] [PubMed] [Google Scholar]
- Plagemann P. G. Nucleotide pools in Novikoff rat hepatoma cells growing in suspension culture. 3. Effects of nucleosides in medium on levels of nucleotides in separate nucleotide pools for nuclear and cytoplasmic RNA synthesis. J Cell Biol. 1972 Jan;52(1):131–146. doi: 10.1083/jcb.52.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plagemann P. G. Nucleotide pools of Novikoff rat hepatoma cells growing in suspension culture. I. Kinetics of incorporation of nucleosides into nucleotide pools and pool sizes during growth cycle. J Cell Physiol. 1971 Apr;77(2):213–240. doi: 10.1002/jcp.1040770212. [DOI] [PubMed] [Google Scholar]
- Plagemann P. G. Nucleotide pools of Novikoff rat hepatoma cells growing in suspension culture. II. Independent nucleotide pools for nucleic acid synthesis. J Cell Physiol. 1971 Apr;77(2):241–248. doi: 10.1002/jcp.1040770213. [DOI] [PubMed] [Google Scholar]
- Plagemann P. G., Wohlhueter R. M., Woffendin C. Nucleoside and nucleobase transport in animal cells. Biochim Biophys Acta. 1988 Oct 11;947(3):405–443. doi: 10.1016/0304-4157(88)90002-0. [DOI] [PubMed] [Google Scholar]
- Schachter H. Biosynthetic controls that determine the branching and microheterogeneity of protein-bound oligosaccharides. Biochem Cell Biol. 1986 Mar;64(3):163–181. doi: 10.1139/o86-026. [DOI] [PubMed] [Google Scholar]
- Van Rinsum J., Van Dijk W., Hooghwinkel G. J., Ferwerda W. Subcellular localization and tissue distribution of sialic acid precursor-forming enzymes. Biochem J. 1983 Jan 15;210(1):21–28. doi: 10.1042/bj2100021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk W., Ferwerda W., van den Eijnden D. H. Subcellular and regional distribution of CMP-N-acetylneuraminic acid synthetase in the calf kidney. Biochim Biophys Acta. 1973 Jul 5;315(1):162–175. doi: 10.1016/0005-2744(73)90139-3. [DOI] [PubMed] [Google Scholar]
- van den Eijnden D. H. The subcellular localization of cytidine 5'-monophospho-N-acetylneuraminic acid synthetase in calf brain. J Neurochem. 1973 Oct;21(4):949–958. doi: 10.1111/j.1471-4159.1973.tb07539.x. [DOI] [PubMed] [Google Scholar]


