Abstract
Background
Cancer is one of the leading causes of death worldwide, and cardiopulmonary comorbidities may further adversely affect cancer prognosis. We recently described lung cancer-associated pulmonary hypertension (PH) as a new form of PH and comorbidity of lung cancer. While patients with lung cancer with PH had significantly reduced overall survival compared with patients without PH, the prevalence and impact of PH in other cancers remain unclear.
Methods
In this retrospective, observational cohort study, we analysed the prevalence and impact of PH on clinical outcomes in 1184 patients with solid tumours other than lung cancer, that is, colorectal, head and neck, urological, breast or central nervous system tumours, using surrogate markers for PH determined by CT.
Results
PH prevalence in this cohort was 10.98%. A Cox proportional hazard model revealed a significant reduction in the median survival time of patients with cancer with PH (837 vs 2074 days; p<0.001). However, there was no correlation between pulmonary metastases and PH. A subgroup analysis showed that PH was linked to decreased lung and cardiac function. Additionally, PH was associated with systemic arterial hypertension (p<0.001) and coronary artery disease (p=0.014), but not emphysema.
Conclusions
In this study, fewer patients with cancer had surrogate parameters for PH compared with previously published results among patients with lung cancer. Consequently, the prevalence of PH in other cancers might be lower compared with lung cancer; however, PH still has a negative impact on prognosis. Furthermore, our data does not provide evidence that lung metastases cause PH. Thus, our results support the idea that lung cancer-associated PH represents a new category of PH. Our results also highlight the importance of further studies in the field of cardio-oncology.
Keywords: Primary Pulmonary Hypertension, Lung Cancer, Systemic disease and lungs, Emphysema, Pulmonary Embolism, Respiratory Measurement
WHAT IS ALREADY KNOWN ON THIS TOPIC
Our previous studies have shown that lung cancer-associated pulmonary hypertension (PH) is a new form of PH and has a major impact on progression-free and overall survival.
WHAT THIS STUDY ADDS
This study shows the prevalence of PH in cancers such as colorectal, urological, head and neck, breast, and central nervous system cancers; PH seems to have a negative impact on prognosis for all of the above cancers.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
This study shows the prevalence of PH in cancers such as colorectal, urological, head and neck, breast, and central nervous system cancers. PH appears to have a negative impact on prognosis in all of the above cancers.
Introduction
Cancer is a global health concern, as it is one of the most common causes of death worldwide.1 Despite various promising therapeutic improvements during recent decades, prognosis remains poor for many tumour types. Current cancer treatments rely on multimodal therapeutic strategies, which have improved the survival of patients afflicted with various cancer types over the last 10 years.2 3 However, in clinical practice, patients with cancer often present with one or several comorbidities rather than an isolated tumourous disease. In particular, cardiovascular and pulmonary comorbidities may limit therapeutic options and negatively impact a patient’s physical fitness, quality of life and prognosis.4,7 Unfortunately, little is known about the interplay of these comorbidities with the heterogeneous nature of oncological illnesses.8,10 Notably, pulmonary hypertension (PH) shares many similar cellular and molecular signalling pathways with cancers.11,13 PH is characterised by an increase in both pulmonary vascular resistance and afterload of the right ventricle, ultimately resulting in right heart failure.13,15 Patients with PH often present with symptoms such as exercise intolerance and dyspnoea.16 However, the concept of PH as a comorbidity in the context of cancer is relatively new.17 18
Previous studies by our group provided clinical, histopathological and experimental evidence for the existence of a special category of PH associated with lung cancer. Regrettably, patients with lung cancer with PH have significantly worse clinical outcomes compared with patients without PH. We previously reported that 23% of all patients with lung cancer had concomitant PH, but the prevalence and impact of PH on tumours other than those of the lung remain unclear. We previously postulated that the interaction between lung cancer cells and immune cells in the tumour microenvironment exacerbates inflammation and promotes vascular remodelling, which is the primary underlying pathogenic mechanism for the manifestation of PH. Our analysis of human lung cancer tissue has shown that the presence of macrophages and lymphocytes in the perivascular compartment has increased significantly, confirming this. Of note, in our mouse models injected with tumour cells and lacking functional T cells, B cells, natural killer cells, functional macrophages and dendritic cells, the occurrence of PH was significantly inhibited. These results support the hypothesis that the inflammatory microenvironment in lung cancer is the primary driving force behind the development of observed PH.18 The aim of this study was to extend this concept to other tumour entities while investigating whether patients with pulmonary metastases or cancer in general also present with signs of PH similar to those seen in patients with lung cancer.
Furthermore, the diagnosis of cancer-related PH is complex and often only made postmortem. Moreover, evidence for this form of PH is very limited and has often depended on case reports or series. Previously, cancer-related PH has been attributed mainly to pulmonary tumour microembolisms, pulmonary tumour thrombotic microangiopathy or tumour-driven vascular occlusion as well as the aforementioned microenvironmental inflammation. To our knowledge, no study has previously addressed whether PH is associated with cancers other than lung cancer or with pulmonary metastasis. Thus, we investigated the prevalence of PH co-occurrence with tumour types other than lung cancer, possible comorbidities contributing to the PH manifestation and its impact on clinical outcome.
Materials and methods
Study design and cohort
We investigated the prevalence of PH in patients with cancer in a non-interventional observational retrospective registry trial. This study enrolled 1184 patients with cancer of a single tertiary care centre in Germany (University Hospital Giessen cancer centre). All patients were enrolled during the years of 2017–2020 and followed up in a longitudinal manner until January 2023. Our cohort comprised 1184 patients with the following cancer types: 360 (30.41%) with gastrointestinal cancer (GIC), 423 (35.73%) with head and neck cancer (HNC), 198 (16.72%) with urological cancer (UROC), 154 (13.01%) with breast cancer (BC) and 49 (4.14%) with central nervous system cancer (CNSC). We included all patients with these conditions who received their initial diagnosis at our centre during the respective years and had an available CT scan usually with iodine contrast administration of the thorax for pulmonary artery (PA) size and ascending aorta (A) assessment. The GIC patient group included patients with oesophageal cancer (n=67), gastric cancer (n=76), small bowel cancer (n=12) and colorectal cancer (n=205). The HNC group included oral cavity carcinoma (n=115), nasal cavity carcinoma (n=18), nasopharyngeal carcinoma (n=12), oropharyngeal carcinoma (n=148), hypopharyngeal carcinoma (n=113) and salivary gland carcinoma (n=17). The UROC group included renal cell carcinomas (n=94), ureteral carcinomas (n=3), bladder carcinomas (n=82), prostate carcinomas (n=15) and testicular carcinomas (n=4). CNS cancer patients were glioblastoma (n=43), astrocytoma (n=2), meningioma (n=1), ependymoma (n=1) and CNS haemangioblastoma (n=2). All patients were at least 18 years old and received their first diagnosis of cancer, staging, subsequent diagnostic workup and initial treatment at our tertiary care centre. Datasets were retrieved using the Giessener Tumordokumentationssystem, that is, our centre’s standard tumour documentation system, as well as the standard clinical routine documentation software MEONA. All data were obtained from results of routine diagnostic procedures and follow-ups as well as patient records and were stored in a database. Clinical outcomes were tracked based on the time until the last documented clinical follow-up or death of a patient.
CT scans and PA/A ratio assessment
All participants received their CT scans as part of their routine tumour staging prior to therapy initiation. CT scans were performed using a standardised technique (reconstructed slice thickness of 1 mm for lung kernel and 3 mm for soft tissue kernel) and usually obtained with the administration of intravenous contrast material. Two trained radiologist determined tumour stage, pulmonary metastasis status and tumour progression by using Response Evaluation Criteria In Solid Tumours. The diameter of each of the PA/A was measured based on axial CT images using a Picture Archiving and Communication System (PACS) workstation (INFINITT PACS 3.0, INFINITT Healthcare, Seoul, South Korea). The diameter of the main PA was measured at its bifurcation and the ascending aorta was measured at its maximum diameter. The aforementioned arterial diameters were then used to calculate the PA/A ratio; PH was indicated if the ratio was >1, which correlates with invasively measured PA pressure and hence suggests PH. This technique has been extensively used and validated as a reliable surrogate parameter for PH in the past.19,23
Classification of emphysema, coronary artery disease and systemic arterial hypertension
Two experienced radiologists confirmed or ruled out emphysema based on the aforementioned initial CT scans. Likewise, a diagnosis of coronary artery disease (CAD) was also based on CT scans of the thorax.24 25 Moreover, systemic arterial hypertension was classified as uncontrolled blood pressure at the time of cancer diagnosis as defined by the most recent European Society of Cardiology guidelines26 or a pre-existing treatment with antihypertensive medication.
Echocardiography and assessment of lung function
Echocardiography was routinely performed by an experienced cardiologist at our university hospital and was performed at the time of initial cancer diagnosis. All lung function measurements were also conducted at our centre.
Statistical analysis
The entire statistical analysis was calculated by using SPSS software V.28.0 and R (V.4.3, R Foundation for Statistical Computing, Vienna, Austria). We applied t-test and χ² test when appropriate and after appropriate transformation of the data. P values were Bonferroni-Holm adjusted for multiple testing where necessary. P values of less than 0.05 were considered statistically significant. A Cox proportional hazard model was used to assess the impact of the PA/A ratio on survival controlling for age, sex and Union for International Cancer Control stage of the patients. Details of the survival models are given in online supplemental cox models. Survival is visualised with Kaplan-Meier curves.
Patient and public involvement
Due to the retrospective nature of this study, patient and public involvement in the planning or design of this study was not possible. The findings of this research will be made accessible to the public, including study participants, owing to the open-access nature of the journal in which it will be published.
Results
Increased PA/A ratio is associated with the general clinical characteristics of GIC, HNC, UROC and BC
We investigated 1184 patients with cancer comprising 360 patients with GIC, 423 patients with HNC, 198 patients with UROC, 49 patients with CNSC and 154 patients with BC. Table 1 summarises the baseline demographics, smoking status, pack-years, brain natriuretic peptide (BNP) levels, cancer type, tumour, node, metastasis classification, stage (per Union for International Cancer Control (UICC)), performance status and status of pulmonary metastases of the study cohort. All calculations were performed for the entire group of patients with cancer and separately for each cancer subgroup. The diameter of each of the PA/A was measured from images acquired with baseline CT scans, revealing that 130 of the 1184 (10.98%) patients with cancer had a PA/A ratio of greater than 1. We assumed that a PA/A of >1 indicated PH, as this ratio is a well-established surrogate parameter.19,23 Specifically, the ratio was >1 for 44 of 360 (12.2%) GIC, 31 of 423 (7.3%) HNC, 30 of 198 (15.2%) UROC, 6 of 49 (12.2%) CNSC and 19 of 154 (12.34%) BC patients. Further comparison of baseline patient characteristics between the PA/A≤1 group and the PA/A>1 group revealed no statistically significant difference with regard to age. There were more female patients with PA/A>1 in the entirety of all patients (p<0.001) as well as for the UROC (p<0.001), CNS (p=0.950) and BC groups (p>0.999). Nevertheless, it is important to note that the total study cohort comprised a substantially smaller number of female than male patients. Patients within the group PA/A>1 did not differ concerning smoking status or pack-years. However, they had significantly higher levels of BNP for the entire group of patients (p=0.042). Furthermore, they appeared to have a worse Eastern Cooperative Oncology Group performance status, than patients within the PA/A≤1 group. This was again observed for the entire group of patients (p<0.001) as well as for the GIC (p=0.001) and the UROC subgroups (p=0.007). Additionally, distant metastases were more prevalent among patients with PA/A>1 for the UROC subgroup (p=0.018). Finally, UICC stage and N stage were significantly different within the BC subgroup (p=0.003). The presence of pulmonary metastases did not differ significantly between patients with and without PA/A>1.
Table 1. Comparative analysis of baseline and tumour characteristics in relation to oncological parameters across cancer patient groups.
| GIC | HNC | UROC | CNS | BC | Overall | |||||||||||||
| PA/A≤1 (N=316) | PA/A>1 (N=44) | PA/A≤1 (N=392) | PA/A>1 (N=31) | PA/A≤1 (N=168) | PA/A>1 (N=30) | PA/A≤1 (N=43) | PA/A>1 (N=6) | PA/A≤1 (N=135) | PA/A>1 (N=19) | PA/A≤1 (N=1054) | PA/A>1 (N=130) | GIC P value | HNC P value | UROC P value | CNS P value | BC P value | Overall P value | |
| Age | ||||||||||||||||||
| Median (Q1, Q3) | 66 (58, 75) | 68 (61, 77) | 66 (59, 73) | 65 (54, 79) | 70 (63, 76) | 71 (61, 78) | 61 (53, 75) | 68 (49, 79) | 65 (57, 74) | 79 (64, 81) | 66 (58, 75) | 68 (58, 79) | 0.772 | 0.663 | 0.358 | 0.865 | 0.040 | 0.798 |
| Sex | ||||||||||||||||||
| Male | 228 (72.2%) | 26 (59.1%) | 291 (74.2%) | 17 (54.8%) | 134 (79.8%) | 14 (46.7%) | 19 (44.2%) | 2 (33.3%) | 1 (0.7%) | 0 (0%) | 673 (63.9%) | 59 (45.4%) | 0.109 | 0.033 | <0.001 | 0.950 | >0.999 | <0.001 |
| Female | 88 (27.8%) | 18 (40.9%) | 101 (25.8%) | 14 (45.2%) | 34 (20.2%) | 16 (53.3%) | 24 (55.8%) | 4 (66.7%) | 134 (99.3%) | 19 (100%) | 381 (36.1%) | 71 (54.6%) | ||||||
| Smoker | ||||||||||||||||||
| Never | 24 (7.6%) | 2 (4.5%) | 8 (2.0%) | 1 (3.2%) | 2 (1.2%) | 0 (0%) | 1 (2.3%) | 1 (16.7%) | 9 (6.7%) | 4 (21.1%) | 44 (4.2%) | 8 (6.2%) | 0.745 | 0.656 | 0.195 | 0.526 | 0.458 | 0.188 |
| Former | 54 (17.1%) | 8 (18.2%) | 50 (12.8%) | 2 (6.5%) | 15 (8.9%) | 3 (10.0%) | 2 (4.7%) | 0 (0%) | 5 (3.7%) | 1 (5.3%) | 126 (12.0%) | 14 (10.8%) | ||||||
| Active | 37 (11.7%) | 4 (9.1%) | 72 (18.4%) | 4 (12.9%) | 16 (9.5%) | 0 (0%) | 2 (4.7%) | 1 (16.7%) | 9 (6.7%) | 1 (5.3%) | 136 (12.9%) | 10 (7.7%) | ||||||
| Missing | 201 (63.6%) | 30 (68.2%) | 262 (66.8%) | 24 (77.4%) | 135 (80.4%) | 27 (90.0%) | 38 (88.4%) | 4 (66.7%) | 112 (83.0%) | 13 (68.4%) | 748 (71.0%) | 98 (75.4%) | ||||||
| Pack-years | ||||||||||||||||||
| Median (Q1,Q3) | 20 (0, 39) | 40 (18, 56) | 40 (25, 50) | 35 (35, 60) | 21 (20, 39) | 40 (28, 45) | 10 (5.0, 18) | 0 (0, 0) | 0 (0, 28) | 0 (0, 0) | 26 (5.0, 45) | 33 (0.25, 48) | 0.066 | 0.961 | 0.515 | N/A | 0.172 | 0.704 |
| Missing | 226 (71.5%) | 28 (63.6%) | 312 (79.6%) | 26 (83.9%) | 146 (86.9%) | 27 (90.0%) | 40 (93.0%) | 5 (83.3%) | 120 (88.9%) | 14 (73.7%) | 844 (80.1%) | 100 (76.9%) | ||||||
| BNP | ||||||||||||||||||
| Median (Q1,Q3) | 120 (57, 260) | 420 (230, 810) | 69 (34, 200) | 320 (36, 1600) | 250 (48, 870) | 220 (130, 520) | 74 (73, 74) | N/A | 77 (30, 270) | 380 (280, 1600) | 93 (37, 260) | 320 (140, 870) | 0.171 | 0.259 | 0.743 | N/A | 0.341 | 0.042 |
| Missing | 278 (88.0%) | 32 (72.7%) | 356 (90.8%) | 26 (83.9%) | 153 (91.1%) | 26 (86.7%) | 41 (95.3%) | 6 (100%) | 123 (91.1%) | 15 (78.9%) | 951 (90.2%) | 105 (80.8%) | ||||||
| ECOG | ||||||||||||||||||
| 0 | 85 (26.9%) | 6 (13.6%) | 115 (29.3%) | 9 (29.0%) | 12 (7.1%) | 2 (6.7%) | 7 (16.3%) | 1 (16.7%) | 84 (62.2%) | 6 (31.6%) | 303 (28.7%) | 24 (18.5%) | 0.001 | 0.151 | 0.007 | 0.173 | 0.057 | <0.001 |
| 1 | 139 (44.0%) | 12 (27.3%) | 159 (40.6%) | 7 (22.6%) | 95 (56.5%) | 8 (26.7%) | 8 (18.6%) | 0 (0%) | 31 (23.0%) | 7 (36.8%) | 432 (41.0%) | 34 (26.2%) | ||||||
| 2 | 57 (18.0%) | 15 (34.1%) | 64 (16.3%) | 7 (22.6%) | 45 (26.8%) | 14 (46.7%) | 9 (20.9%) | 1 (16.7%) | 16 (11.9%) | 4 (21.1%) | 191 (18.1%) | 41 (31.5%) | ||||||
| 3 | 23 (7.3%) | 6 (13.6%) | 50 (12.8%) | 8 (25.8%) | 11 (6.5%) | 2 (6.7%) | 16 (37.2%) | 2 (33.3%) | 4 (3.0%) | 2 (10.5%) | 104 (9.9%) | 20 (15.4%) | ||||||
| 4 | 2 (0.6%) | 2 (4.5%) | 2 (0.5%) | 0 (0%) | 0 (0%) | 1 (3.3%) | 2 (4.7%) | 2 (33.3%) | 0 (0%) | 0 (0%) | 6 (0.6%) | 5 (3.8%) | ||||||
| Missing | 10 (3.2%) | 3 (6.8%) | 2 (0.5%) | 0 (0%) | 5 (3.0%) | 3 (10.0%) | 1 (2.3%) | 0 (0%) | 0 (0%) | 0 (0%) | 18 (1.7%) | 6 (4.6%) | ||||||
| T | ||||||||||||||||||
| 1 | 42 (13.3%) | 9 (20.5%) | 109 (27.8%) | 11 (35.5%) | 53 (31.5%) | 8 (26.7%) | – | – | 63 (46.7%) | 5 (26.3%) | 267 (25.3%) | 33 (25.4%) | 0.279 | 0.111 | 0.175 | N/A | 0.106 | 0.172 |
| 2 | 50 (15.8%) | 5 (11.4%) | 102 (26.0%) | 2 (6.5%) | 58 (34.5%) | 6 (20.0%) | – | – | 51 (37.8%) | 7 (36.8%) | 261 (24.8%) | 20 (15.4%) | ||||||
| 3 | 143 (45.3%) | 18 (40.9%) | 83 (21.2%) | 8 (25.8%) | 38 (22.6%) | 11 (36.7%) | – | – | 9 (6.7%) | 2 (10.5%) | 273 (25.9%) | 39 (30.0%) | ||||||
| 4 | 58 (18.4%) | 4 (9.1%) | 96 (24.5%) | 10 (32.3%) | 15 (8.9%) | 1 (3.3%) | – | – | 9 (6.7%) | 4 (21.1%) | 178 (16.9%) | 19 (14.6%) | ||||||
| Missing | 23 (7.3%) | 8 (18.2%) | 2 (0.5%) | 0 (0%) | 4 (2.4%) | 4 (13.3%) | 43 (100%) | 6 (100%) | 3 (2.2%) | 1 (5.3%) | 75 (7.1%) | 19 (14.6%) | ||||||
| N | ||||||||||||||||||
| 0 | 155 (49.1%) | 17 (38.6%) | 188 (48.0%) | 17 (54.8%) | 125 (74.4%) | 20 (66.7%) | – | – | 81 (60.0%) | 8 (42.1%) | 549 (52.1%) | 62 (47.7%) | 0.177 | 0.687 | 0.770 | N/A | 0.003 | 0.403 |
| 1 | 76 (24.1%) | 14 (31.8%) | 71 (18.1%) | 7 (22.6%) | 19 (11.3%) | 3 (10.0%) | – | – | 36 (26.7%) | 5 (26.3%) | 202 (19.2%) | 29 (22.3%) | ||||||
| 2 | 37 (11.7%) | 1 (2.3%) | 97 (24.7%) | 5 (16.1%) | 8 (4.8%) | 2 (6.7%) | – | – | 3 (2.2%) | 4 (21.1%) | 145 (13.8%) | 12 (9.2%) | ||||||
| 3 | 16 (5.1%) | 2 (4.5%) | 29 (7.4%) | 2 (6.5%) | 5 (3.0%) | 0 (0%) | – | – | 9 (6.7%) | 1 (5.3%) | 59 (5.6%) | 5 (3.8%) | ||||||
| Missing | 32 (10.1%) | 10 (22.7%) | 7 (1.8%) | 0 (0%) | 11 (6.5%) | 5 (16.7%) | 43 (100%) | 6 (100%) | 6 (4.4%) | 1 (5.3%) | 99 (9.4%) | 22 (16.9%) | ||||||
| M | ||||||||||||||||||
| 0 | 217 (68.7%) | 28 (63.6%) | 361 (92.1%) | 29 (93.5%) | 138 (82.1%) | 18 (60.0%) | – | – | 113 (83.7%) | 14 (73.7%) | 829 (78.7%) | 89 (68.5%) | 0.894 | 0.846 | 0.018 | N/A | >0.999 | 0.057 |
| 1 | 90 (28.5%) | 13 (29.5%) | 23 (5.9%) | 1 (3.2%) | 28 (16.7%) | 11 (36.7%) | – | – | 22 (16.3%) | 3 (15.8%) | 163 (15.5%) | 28 (21.5%) | ||||||
| Missing | 9 (2.8%) | 3 (6.8%) | 8 (2.0%) | 1 (3.2%) | 2 (1.2%) | 1 (3.3%) | 43 (100%) | 6 (100%) | 0 (0%) | 2 (10.5%) | 62 (5.9%) | 13 (10.0%) | ||||||
| UICC | ||||||||||||||||||
| 1 | 60 (19.0%) | 8 (18.2%) | 118 (30.1%) | 10 (32.3%) | 46 (27.4%) | 8 (26.7%) | – | – | 51 (37.8%) | 5 (26.3%) | 275 (26.1%) | 31 (23.8%) | 0.930 | 0.831 | 0.163 | N/A | 0.003 | 0.310 |
| 2 | 85 (26.9%) | 9 (20.5%) | 55 (14.0%) | 3 (9.7%) | 46 (27.4%) | 5 (16.7%) | – | – | 50 (37.0%) | 3 (15.8%) | 236 (22.4%) | 20 (15.4%) | ||||||
| 3 | 72 (22.8%) | 10 (22.7%) | 64 (16.3%) | 4 (12.9%) | 38 (22.6%) | 5 (16.7%) | – | – | 12 (8.9%) | 7 (36.8%) | 186 (17.6%) | 26 (20.0%) | ||||||
| 4 | 93 (29.4%) | 13 (29.5%) | 155 (39.5%) | 14 (45.2%) | 36 (21.4%) | 12 (40.0%) | – | – | 22 (16.3%) | 3 (15.8%) | 306 (29.0%) | 42 (32.3%) | ||||||
| Missing | 6 (1.9%) | 4 (9.1%) | 0 (0%) | 0 (0%) | 2 (1.2%) | 0 (0%) | 43 (100%) | 6 (100%) | 0 (0%) | 1 (5.3%) | 51 (4.8%) | 11 (8.5%) | ||||||
| Pulmonary metastases | ||||||||||||||||||
| No | 304 (96.2%) | 42 (95.5%) | 363 (92.6%) | 28 (90.3%) | 146 (86.9%) | 25 (83.3%) | 43 (100%) | 6 (100%) | 129 (95.6%) | 17 (89.5%) | 985 (93.5%) | 118 (90.8%) | >0.999 | 0.913 | 0.813 | N/A | 0.571 | 0.337 |
| Yes | 12 (3.8%) | 2 (4.5%) | 29 (7.4%) | 3 (9.7%) | 22 (13.1%) | 5 (16.7%) | 0 (0%) | 0 (0%) | 6 (4.4%) | 2 (10.5%) | 69 (6.5%) | 12 (9.2%) | ||||||
The cohort includes all patients thatwho were admitted to our cancer centercentre who had undergone a CT- scan of the thorax. Percentages were calculated separately for each column, that is, each ratio group (PA/A≤1 vs. PA/A>1), equalling 100%. Values of zer0o indicate that a patient’s information was missing.
Age was measured in years, Q1 and Q3 representing the 95% CI.
P values were calculated in a linear regression model, p values of <0.05 were considered to reflect a statistically significant difference.
BCbreast cancerBNPbrain natriuretic peptideCNScentral nervous systemECOGEastern Cooperative Oncology Group performance statusGICgastrointestinal cancerHNChead and neck cancerPA/Apulmonary artery and ascending aortaPulmonary Metastasesexistence of pulmonary metastasis for each patient at first diagnosis of cancerUICCUnion for International Cancer ControlUROCurological cancer
PA/A ratio significantly correlates with PA systolic pressure
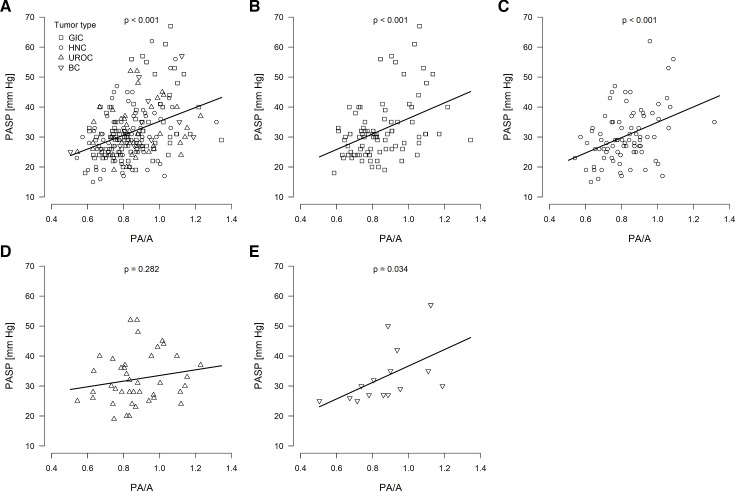
We further conducted a correlation analysis for PA systolic pressure (PASP) and PA/A ratio for 223 patients for whom echocardiography results were available at the time of cancer diagnosis. This calculation was used to validate PA/A ratio measurements based on baseline CT scans, as not all patients had available PASP data at initial cancer diagnosis. We were able to demonstrate a positive correlation between PASP and PA/A ratio for all patients with cancer (figure 1A, n=223, p<0.001) as well as for GIC (figure 1, n=85, p<0.001), HNC (figure 1, n=82, p<0.001) and BC (figure 1, n=15, p=0.034). However, there was no such correlation for UROC (figure 1, n=41, p=0.283). For all patients, the mean PASP for the PA/A>1 group was 39.50±11.47 mm Hg compared with 30.11±8.29 mm Hg for the PA/A≤1 group. Results are not shown for the CNSC subgroup owing to the small number of patients with this cancer type.
Figure 1. Distribution of pulmonary artery size to aorta ratio (PA/A) and echocardiographic pulmonary artery systolic pressure (PASP). Correlation of PASP with PA/A ratio for patients with (A) all included cancer types (n=223), (B) gastrointestinal cancer (GIC; n=85), (C) head and neck cancer (HNC; n=82), (D) urological cancer (UROC; n=41) and (E) breast cancer (BC; n=15) patients. Plots show the data and the line of a linear regression model. P values were calculated for a linear regression model, and values of <0.05 were considered to reflect statistically significant slopes.
Altered lung function predicts a PA/A ratio increase in subgroup of the investigated patients with cancer
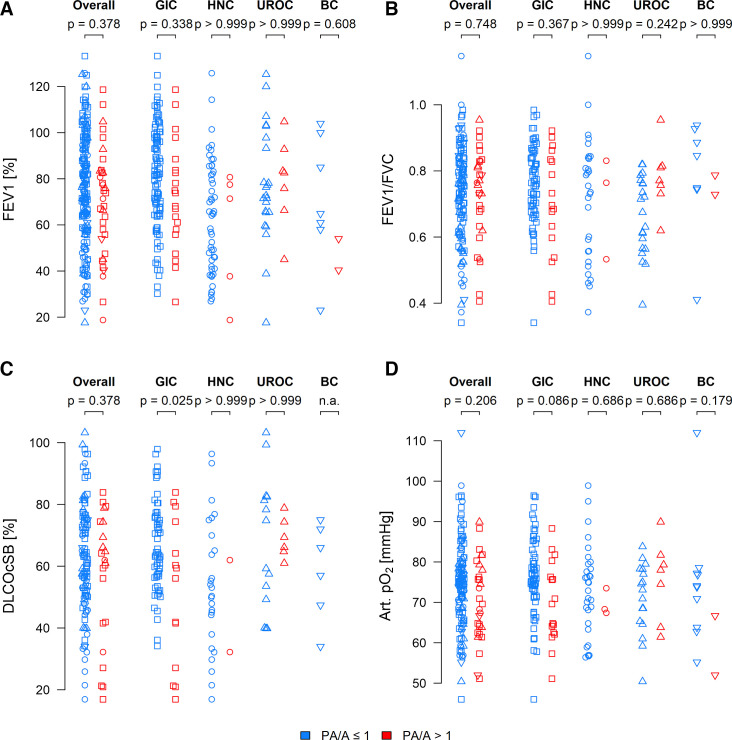
We further investigated differences in available lung function data for the PA/A≤1 and PA/A ratio>1 groups. No statistically significant difference between the two groups was evident with regard to forced expiratory volume in 1 s (FEV1; figure 2A, n=225) or the ratio of FEV1 to functional vital capacity (FEV1/FVC; figure 2B, n=155) for all patients in total and in all four subgroups (GIC, HNC, UROC, BC). Nevertheless, for the GIC subgroup, differences were evident for diffusing capacity of the lungs for carbon monoxide (DLCOcSB; figure 2C, n=124). There were no differences observable for arterial partial pressure of oxygen for all patients as well as the individual subgroups. (Art.pO2; figure 2D, n=154). Results are not shown for the CNSC subgroup owing to the small number of patients with this cancer type.
Figure 2. Lung function parameters among investigated patients with cancer. (A) FEV1 (measured as a percentage) for all patients including central nervous system cancer (CNSC) for pulmonary artery size to aorta ratio (PA/A)≤1 (n=187) or PA/A>1 (n=38), gastrointestinal cancer (GIC) for PA/A≤1 (n=105) or PA/A>1 (n=20), head and neck cancer (HNC) for PA/A≤1 (n=51) or PA/A>1 (n=5), urological cancer (UROC) for PA/A≤1 (n=23) or PA/A>1 (n=7) and breast cancer (BC) for PA/A≤1 (n=7) or PA/A>1 (n=2). (B) FVC and FEV1/FVC ratio for all patients including CNSC for PA/A≤1 (n=126) or PA/A>1 (n=29), GIC for PA/A≤1 (n=67) or PA/A>1 (n=17), HNC for PA/A≤1 (n=30) or PA/A>1 (n=3), UROC for PA/A≤1 (n=21) or PA/A>1 (n=7) and BC for PA/A≤1 (n=7) or PA/A>1 (n=2). (C) DLCOcSB (measured as a percentage) for all patients including CNSC for PA/A≤1 (n=108) or PA/A>1 (n=22), GIC for PA/A≤1 (n=61) or PA/A>1 (n=14), HNC for PA/A≤1 (n=26) or PA/A>1 (n=2), UROC for PA/A≤1 (n=14) or PA/A>1 (n=6) and BC for PA/A≤1 (n=6) or PA/A>1 (n=0). (D) Arterial pO2 (measured in mm Hg) for all patients including CNSC for PA/A≤1 (n=125) or PA/A>1 (n=29), GIC for PA/A≤1 (n=66) or PA/A>1 (n=17), HNC for PA/A≤1 (n=31) or PA/A>1 (n=3), UROC for PA/A≤1 (n=17) or PA/A>1 (n=7) and BC for PA/A≤1 (n=10) or PA/A>1 (n=2). All lung-function parameters were assessed at the time of first diagnosis of cancer. P values were calculated for a linear regression model, and values of <0.05 were considered to reflect statistically significant mean differences between the indicated groups. DLCOcSB, diffusing capacity of the lungs for carbon monoxide; FEV1, forced expiratory volume in 1 s; FVC, functional vital capacity; pO2, partial pressure of oxygen.
Echocardiography parameters differ between GIC and HNC subgroups
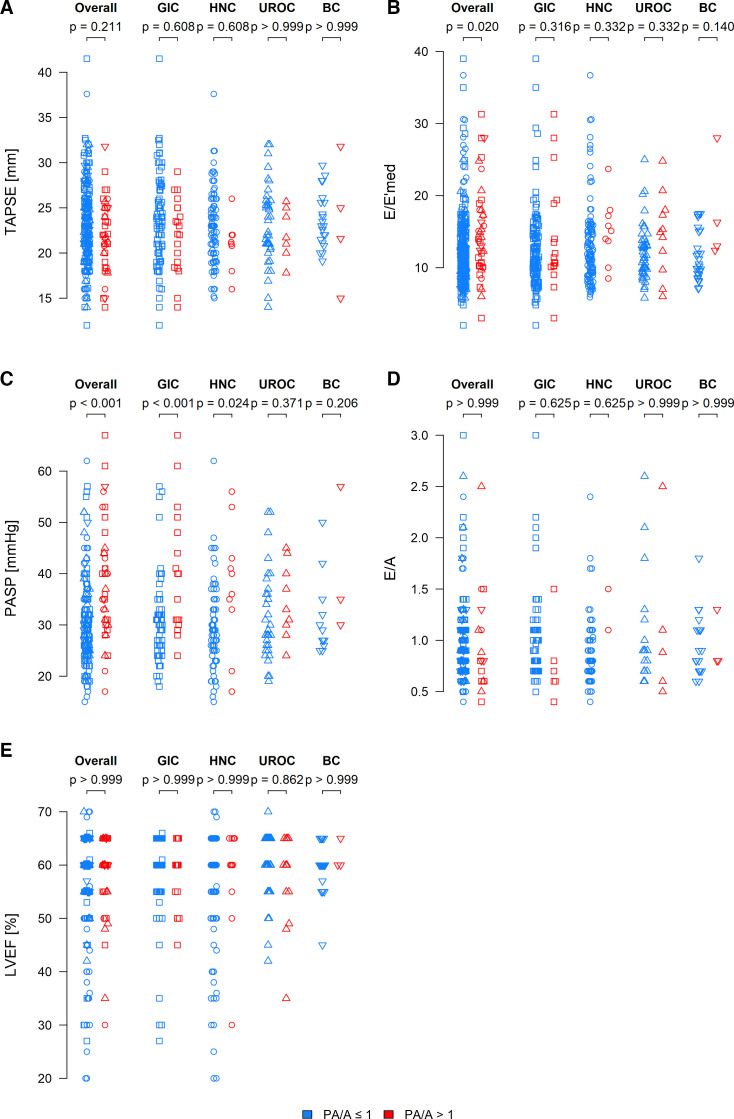
We further compared baseline echocardiography parameters for GIC, HNC, UROC, BC as well as the entire patient cohort (figure 3). There was no difference between the PA/A≤1 group and the PA/A>1 group with regard to tricuspid annular plane systolic excursion (figure 3A, n=285), the heart failure with preserved ejection fraction (HFpEF) parameter E/A (figure 3D, n=201) and left ventricular EF (figure 3E, n=352). However, the aforementioned parameter PASP (figure 3C, n=231) differed significantly between the two groups. Additionally, the HFpEF parameter E/E’med (figure 3B, n=378) was different for all investigated patients with cancer when comparing both groups. All these calculations were conducted for all patients combined as well as for each individual cancer entity as presented in the respective figures. Again, the results for CNSC patients with cancer are not presented owing to the small number of patients with this cancer type.
Figure 3. Echocardiography parameters among the patients with cancer. (A) TAPSE (measured in mm) for all patients including central nervous system cancer (CNSC) for pulmonary artery size to aorta ratio PA/A≤1 (n=246) or PA/A>1 (n=39), gastrointestinal cancer (GIC) for PA/A≤1 (n=87) or PA/A>1 (n=20), head and neck cancer (HNC) for PA/A≤1 (n=91) or PA/A>1 (n=8), urological cancer (UROC) cancer for PA/A≤1 (n=39) or PA/A>1 (n=7) and breast cancer (BC) for PA/A≤1 (n=21) or PA/A>1 (n=4). (B) E/E’med is an indicator of heart failure with preserved ejection fraction for all patients including CNSC for PA/A≤1 (n=337) or PA/A>1 (n=41), GIC for PA/A≤1 (n=133) or PA/A>1 (n=17), HNC for PA/A≤1 (n=119) or PA/A>1 (n=9), UROC for PA/A≤1 (n=49) or PA/A>1 (n=11) and BC for PA/A≤1 (n=27) or PA/A>1 (n=4). (C) PASP (measured in mm Hg) for all patients including CNSC for PA/A≤1 (n=192) or PA/A>1 (n=39), GIC for PA/A≤1 (n=68) or PA/A>1 (n=17), HNC for PA/A≤1 (n=72) or PA/A>1 (n=10), UROC for PA/A≤1 (n=32) or PA/A>1 (n=9) and BC for PA/A≤1 (n=12) or PA/A>1 (n=3). (D) E/A is an indicator of heart failure with preserved ejection fraction for all patients including CNSC for PA/A≤1 (n=185) or PA/A>1 (n=16), GIC for PA/A≤1 (n=72) or PA/A>1 (n=6), HNC for PA/A≤1(n=67) or PA/A>1 (n=2), UROC for PA/A≤1 (n=22) or PA/A>1 (n=5) and breast cancer (BC) for PA/A≤1 (n=19) or PA/A>1 (n=3). (E) LVEF (measured as a percentage) for all patients including CNSC for PA/A≤1 (n=307) or PA/A>1 (n=45), GIC for PA/A≤1 (n=128) or PA/A>1 (n=20), HNC for PA/A≤1 (n=122) or PA/A>1 (n=12), UROC for PA/A≤1 (n=48) or PA/A>1 (n=13) and BC for PA/A≤1 (n=26) or PA/A>1 (n=3). P values were calculated for a linear regression model, and values of <0.05 were considered to reflect statistically significant differences between the indicated groups. PA/A, pulmonary artery and ascending aorta; LVEF, left ventricular ejection fraction; PASP, pulmonary artery systolic pressure; TAPSE, tricuspid annular plane systolic excursion.
An elevated PA/A ratio is associated with CAD and systemic arterial hypertension
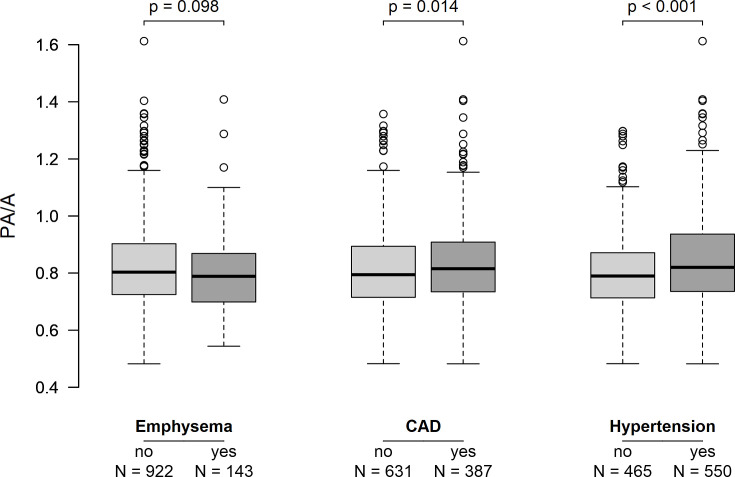
We further investigated a possible correlation between PA/A ratio and the occurrence of comorbidities such as PH as well as emphysema, CAD and systemic arterial hypertension (figure 4). Although signs of emphysema were detectable based on baseline CT scans for 143 (13.43%) patients, no association with PA/A ratio was found for the entire group of patients with cancer (p=0.098). In contrast, for all patients, an elevated PA/A ratio correlated positively with systemic arterial hypertension (p<0.001) as well as CAD (p=0.014). In addition, comparing PA/A ratio for patients with or without signs of emphysema on their CT scans as well as on their pulmonary function analysis can be found in online supplemental figure 1. For the emphysema group, PA/A ratio was slightly increased by 0.07 (p=0.026).
Figure 4. Comorbidities among the patients with cancer. Boxes show the IQR and the median of the pulmonary artery size to aorta ratio (PA/A) ratio measured with thorax CT scans at first diagnosis of cancer for patients with or without the three comorbidities shown. Whiskers extend to the most extreme point with the 1.5-fold distance of the IQR from the box, values outside this range are shown as open circles. CAD, coronary artery disease; Emphysema, Existence of emphysema as assessed with a thorax CT scan at first diagnosis of each patient; Hypertension, Systemic arterial hypertension based on the current European Society of Cardiology guidelines or pre-existing antihypertensive therapy for each patient.
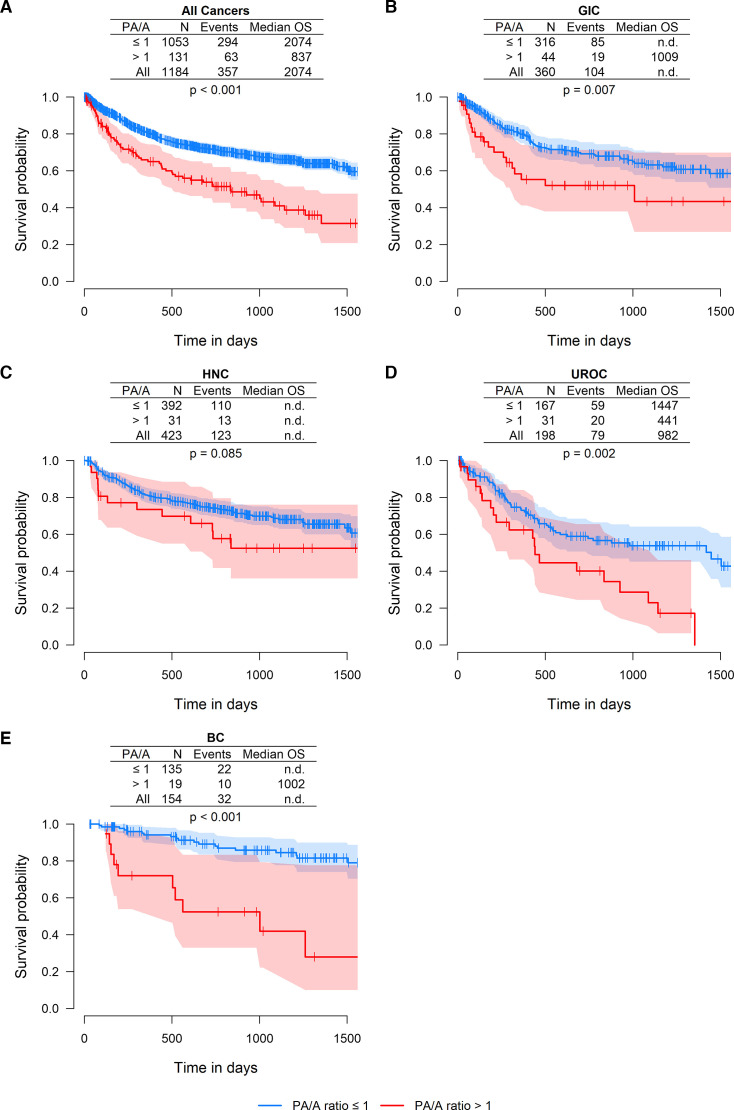
An elevated PA/A ratio is associated with poor overall survival in various cancer types
A Kaplan-Meier plot showing overall survival (OS) for all included patients calculated by a Cox proportional hazard model is displayed in online supplemental figure 2. Further, Kaplan-Meier plots comparing patients with signs of emphysema on their CT scans as well as on their pulmonary function analysis can be found in online supplemental figure 3. Here, OS was significantly decreased for patients with signs of emphysema (713 days vs 1541 days for the control group) (p=0.002). We further investigated the impact of an elevated PA/A ratio on survival of patients with cancer. Here, OS was evaluated using a Cox regression analysis (figure 5). Among all patients, median OS was significantly reduced for the PA/A ratio >1 group (837 days vs 2074 days for the PA/A≤1 group) (figure 5A). Subgroup analyses further revealed that median survival for UROC patients was reduced to 441 days for the PA/A ratio >1 group in comparison with 1447 days for the PA/A ratio ≤1 group (figure 5D). Median survival for GIC, HNC and BC subgroups could not be conclusively analysed due to an insufficient number of events.
Figure 5. Overall survival (OS) of patients stratified by PA/A ratio. The impact of PA/A ratio was analysed using Cox proportional hazards (Cox PH) models adjusting for sex, age, cancer and Union for International Cancer Control (UICC) stage. Figures show the Kaplan-Meier curves with their 95% CIs stratified for the PA/A ratio. Inserted tables in the graphs show the marginal estimates for the median survival in each given patient group and p values are from the Cox proportional hazards models. HNC, head and neck cancer; PA/A, pulmonary artery size to aorta ratio; median OS, median OS time in days; N, number of patients in the subcohort; UROC, urological cancer.
Discussion
PH and cancer share many common signalling pathways, yet their inter-relationship remains poorly understood.13 Cancer-associated PH is a novel comorbidity concept and an emerging research field. Evidence surfacing from recent literature indicates the major importance of this condition for prognosis and outcome. The ratio of PA/A is a well-established surrogate parameter for PH and we have previously reported that a PA/A of >1 independently predicts OS of patients with lung cancer.27 However, data on the prevalence and impact of PH on cancers other than lung cancer is lacking. Therefore, this is the first large scale observational study investigating the prevalence and clinical impact of PH in cancers other than lung cancer through non-invasive surrogate parameters. Our results reveal an overall prevalence of an increased PA/A ratio at first diagnosis of cancer of 10.98%. Hence, a significant proportion of patients with cancer in our cohort showed positive surrogate markers of PH and are likely to suffer from this condition. However, the same proportion of patients would be expected to reveal positive surrogate markers of PH in an elderly control group of the general population.23 This makes it unlikely that our findings are directly related to the underlying cancer but are rather an independent condition. On the contrary, previous studies of our group have indicated lung cancer-associated PH to be a distinctive disease entity.27 However, this study cannot conclusively elucidate the origin of PH we are observing in our patient cohort. Interestingly, a previous study of patients with lung or oesophageal cancer revealed an increase in PA size only for the lung cancer patient cohort.28 Nonetheless, an increased PA/A ratio of greater than 1 equally appears to be an independent indicator of poor prognosis for patients with cancers other than lung cancer. However, when we compare the difference in OS of patients with cancer in this analysis with our previously published results, we find a smaller impact on OS. Specifically, the median overall survival for patients with lung cancer with a PA/A ratio greater than 1 was 207 days vs 568 days in our previous work. In this study, median OS was 837 days for patients with a PA/A ratio greater than 1 compared with 2074 days for patients with a PA/A ratio ≤1. Therefore, it appears that PH within this collective of patients with cancer significantly influences survival, akin to the observed impact of PH associated with lung cancer as previously described.27
We also investigated other parameters of heart and lung function that could possibly inform the diagnosis of PH and help predict its contribution to pathogenesis. First, we found almost no signs of poorer lung function among those patients with cancer with PA/A>1. Merely, a difference for DLCOcSB for the GIC subgroup was apparent. In particular, parameters that are generally associated with PH such as FEV1,29 30 FEV1/FVC and art. pO2 did not differ significantly when comparing the entire group of patients with cancer with or without a PA/A of >1 as well as the respective subgroups. Conversely, for patients with lung cancer these lung function parameters differed significantly for patients with PA/A>1 (27). Furthermore, the only difference in heart function we observed was found for available PASP measurements and E/E’med. There was a robust increase in PASP value for the PA/A>1 group (39.50±11.47 mm Hg vs 30.11±8.29 mm Hg), which further demonstrates the relevance of this ratio as a prognostic indicator. Currently, PASP is the most reliable non-invasive screening parameter for PH, whereas right heart catheterisation remains the diagnostic gold standard.31,33 However, this procedure is impractical for large-scale analyses of patients with cancer because it is expensive, invasive and at this point not necessarily indicated as its results may not directly impact therapeutic decisions. Further, increased E/E’med values for the PA/A>1 might suggest a role for HFpEF in causing PH in patients with cancer.34 Additionally, systemic arterial hypertension and CAD, which are risk factors for HFpEF, correlated positively with patients with PA/A>1. However, the correlation between E/A and PA/A>1 was not statistically significant so HFpEF as an aetiology of PH in this population was not clearly demonstrated. However, given the correlation between CAD and PA/A ratio and the statistically significant correlation between systemic arterial hypertension and PA/A ratio, we suspect that our results do suggest a role of early HFpEF and its associated risk factors in the development of PH in this population.
Moreover, an increased PA/A ratio could further be a consequence of several pathological conditions other than PH or a combination of different conditions such as heart failure-induced cardiopulmonary congestion, undiagnosed CAD or thromboembolic disease.35,37 Therefore, additional studies must be carried out to elucidate the exact cause of cancer-associated PH. Nevertheless, we would like to propose that PH is a clinically important comorbidity for patients with cancer in general that is relevant for prognosis.
The main limitations of this study are its retrospective character and that it only included four different cancer types. Thus, we cannot unquestionably conclude that PA/A>1 caused by PH is less frequent in patients with non-lung cancer compared with patients with lung cancers. However, our study focused mainly on solid tumours that are known to habitually metastasise to the lung.38 Another notable limitation of this study relates to the patient cohort, as it may not be fully representative of the general population but instead reflects patient demographics and conditions in our particular tertiary care centre. Therefore, caution should be used when attempting to draw conclusions for the general population.
Furthermore, we initially hypothesised that lung metastasis could trigger PH just as frequently as primary lung tumours. In this context, we suspected a possible role of cancer of the lung in triggering PH. However, our cohort provided no evidence for a role of lung metastasis in causing PH. However, our findings suggest a distinctiveness of lung cancer-associated PH and that the concept cannot be transferred to other cancers spreading to the lung. One explanation could be the difference in biology of primary tumours of the lung and lung metastases. Nevertheless, this question will have to be definitively answered by future investigations. We also included CNSC in our analysis to serve as a negative control group for comparison with the rest of our cohort. CNSCs do not undergo systemic metastasis, nor do they cross the blood–brain barrier to cause systemic effects. However, we found no differences among CNSC and the other analysed cancer types. We conclude that lung cancer-associated PH might be a unique entity.
It is imperative to broaden the knowledge in this field before interventional trials can be designed to investigate whether therapeutic targeting of PH might improve the outcome of patients with cancer. Furthermore, a substantial proportion (38.02%) of our patients displayed signs of CAD based on their baseline CT scans. The high prevalence of CAD in this cancer patient cohort further suggests the necessity for a holistic approach to cancer-stage classification for each individual patient based not only on UICC stage but also on cardiopulmonary capacity. The Holistic Implementation Study Assessing a Northern German Interdisciplinary Lung Cancer Screening Effort (ClinicalTrials.gov ID: NCT04913155) screens not only for lung cancer but also for CAD and Emphysema using CT scans,39 further supporting the idea of the necessity for a comprehensive approach. In clinical practice, treatment options for patients with cancer are often limited by the patient’s cardiopulmonary comorbidities.
Based on the results of our study, we would like to propose that cardiopulmonary function parameters be incorporated into standard oncological staging protocols. This could provide significant benefits in the formulation of effective treatment regimens. We advocate a holistic cardiopulmonary assessment of all patients with cancer before initiation of oncological treatment. It is, therefore, essential to approach the treatment of patients with cancer not only from an oncological perspective but also to consider the presence of concomitant cardiopulmonary disease. With this approach, physicians can improve their ability to assess the prognosis of patients with cancer, leading to the development of informed and optimised treatment plans tailored to the specific needs of each patient. In conclusion, this study highlights the relevance of the cardiopulmonary system for the treatment of cancer and underscores the importance of making further advances in the field of cardio-oncology.
supplementary material
Acknowledgements
We would like to thank all patients who participated in this study.
Footnotes
Funding: This work was supported by the Institute for lung health (ILH), Cardio-Pulmonary Institute (CPI), German Center for Lung Research (DZL), DFG, SFB 1213 (Project A01, A05 to SSP and Project A10* to RS and NS), European Research Council (ERC) Consolidator Grant (#866051 to SSP) and the State of Hesse (LOEWE iCANx, Project A5, B4, B5 and Area C).
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not applicable.
Ethics approval: This study involves human participants and was approved by Justus-Liebig-University Giessen Ethics committee (AZ64/19). Due to the retrospective nature of this study, patient and public involvement in the planning or design of this study was not possible.
Data availability free text: The data of this study are available from the corresponding author (Rajkumar.Savai@mpi-bn.mpg.de), on reasonable request.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Contributor Information
Michael Cekay, Email: michael.cekay@innere.med.uni-giessen.de.
Philipp F Arndt, Email: Philipp.Arndt@innere.med.uni-giessen.de.
Johanna K Franken, Email: Johanna.K.Franken@med.uni-giessen.de.
Jochen Wilhelm, Email: jochen.wilhelm@chemie.bio.uni-giessen.de.
Soni Savai Pullamsetti, Email: soni.pullamsetti@mpi-bn.mpg.de.
Fritz C Roller, Email: Fritz.C.Roller@radiol.med.uni-giessen.de.
Natascha Sommer, Email: Natascha.Sommer@innere.med.uni-giessen.de.
Ingolf Askevold, Email: Ingolf.Askevold@chiru.med.uni-giessen.de.
Gerson Lüdecke, Email: gerson.luedecke@chiru.med.uni-giessen.de.
Christine Langer, Email: Christine.Langer@hno.med.uni-giessen.de.
Marco Stein, Email: Marco.Stein@neuro.med.uni-giessen.de.
Felix Zeppernick, Email: felix.zeppernick@gyn.med.uni-giessen.de.
Khodr Tello, Email: khodr.tello@innere.med.uni-giessen.de.
Ulf Sibelius, Email: ulf.sibelius@innere.med.uni-giessen.de.
Friedrich Grimminger, Email: Friedrich.Grimminger@innere.med.uni-giessen.de.
Werner Seeger, Email: werner.seeger@innere.med.uni-giessen.de.
Rajkumar Savai, Email: rajkumar.savai@mpi-bn.mpg.de.
Bastian Eul, Email: Bastian.Eul@innere.med.uni-giessen.de.
Data availability statement
Data are available on reasonable request.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–49. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Vansteenkiste J, Dooms C, Mascaux C, et al. Screening and early detection of lung cancer. Ann Oncol. 2012;23 Suppl 10:x320–7. doi: 10.1093/annonc/mds303. [DOI] [PubMed] [Google Scholar]
- 3.Hensing T, Chawla A, Batra R, et al. A personalized treatment for lung cancer: molecular pathways, targeted therapies, and genomic characterization. Adv Exp Med Biol. 2014;799:85–117. doi: 10.1007/978-1-4614-8778-4_5. [DOI] [PubMed] [Google Scholar]
- 4.Leduc C, Antoni D, Charloux A, et al. Comorbidities in the management of patients with lung cancer. Eur Respir J. 2017;49:1601721. doi: 10.1183/13993003.01721-2016. [DOI] [PubMed] [Google Scholar]
- 5.Koelwyn GJ, Jones LW, Hornsby W, et al. Exercise therapy in the management of dyspnea in patients with cancer. Curr Opin Support Palliat Care. 2012;6:129–37. doi: 10.1097/SPC.0b013e32835391dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheville AL, Novotny PJ, Sloan JA, et al. The value of a symptom cluster of fatigue, dyspnea, and cough in predicting clinical outcomes in lung cancer survivors. J Pain Symptom Manage. 2011;42:213–21. doi: 10.1016/j.jpainsymman.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kirkova J, Aktas A, Walsh D, et al. Cancer symptom clusters: clinical and research methodology. J Palliat Med. 2011;14:1149–66. doi: 10.1089/jpm.2010.0507. [DOI] [PubMed] [Google Scholar]
- 8.Janssen-Heijnen ML, Schipper RM, Razenberg PP, et al. Prevalence of co-morbidity in lung cancer patients and its relationship with treatment: a population-based study. Lung Cancer (Auckl) 1998;21:105–13. doi: 10.1016/s0169-5002(98)00039-7. [DOI] [PubMed] [Google Scholar]
- 9.Tammemagi CM, Neslund-Dudas C, Simoff M, et al. Impact of comorbidity on lung cancer survival. Int J Cancer. 2003;103:792–802. doi: 10.1002/ijc.10882. [DOI] [PubMed] [Google Scholar]
- 10.Battafarano RJ, Piccirillo JF, Meyers BF, et al. Impact of comorbidity on survival after surgical resection in patients with stage I non-small cell lung cancer. J Thorac Cardiovasc Surg. 2002;123:280–7. doi: 10.1067/mtc.2002.119338. [DOI] [PubMed] [Google Scholar]
- 11.Guignabert C, Tu L, Le Hiress M, et al. Pathogenesis of pulmonary arterial hypertension: lessons from cancer. Eur Respir Rev. 2013;22:543–51. doi: 10.1183/09059180.00007513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pullamsetti SS, Schermuly R, Ghofrani A, et al. Novel and emerging therapies for pulmonary hypertension. Am J Respir Crit Care Med. 2014;189:394–400. doi: 10.1164/rccm.201308-1543PP. [DOI] [PubMed] [Google Scholar]
- 13.Pullamsetti SS, Savai R, Seeger W, et al. Translational Advances in the Field of Pulmonary Hypertension. From Cancer Biology to New Pulmonary Arterial Hypertension Therapeutics. Targeting Cell Growth and Proliferation Signaling Hubs. Am J Respir Crit Care Med. 2017;195:425–37. doi: 10.1164/rccm.201606-1226PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Humbert M, Guignabert C, Bonnet S, et al. Pathology and pathobiology of pulmonary hypertension: state of the art and research perspectives. Eur Respir J. 2019;53:1801887. doi: 10.1183/13993003.01887-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Savai R, Al-Tamari HM, Sedding D, et al. Pro-proliferative and inflammatory signaling converge on FoxO1 transcription factor in pulmonary hypertension. N Med. 2014;20:1289–300. doi: 10.1038/nm.3695. [DOI] [PubMed] [Google Scholar]
- 16.McKenzie E, Hwang MK, Chan S, et al. Predictors of dyspnea in patients with advanced cancer. Ann Palliat Med. 2018;7:427–36. doi: 10.21037/apm.2018.06.09. [DOI] [PubMed] [Google Scholar]
- 17.Mury C, Schneider AG, Nobile A, et al. Acute pulmonary hypertension caused by tumor embolism: a report of two cases. Pulm Circ. 2015;5:577–9. doi: 10.1086/682225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pullamsetti SS, Kojonazarov B, Storn S, et al. Lung cancer-associated pulmonary hypertension: Role of microenvironmental inflammation based on tumor cell-immune cell cross-talk. Sci Transl Med. 2017;9:eaai9048. doi: 10.1126/scitranslmed.aai9048. [DOI] [PubMed] [Google Scholar]
- 19.Wells JM, Washko GR, Han MK, et al. Pulmonary arterial enlargement and acute exacerbations of COPD. N Engl J Med. 2012;367:913–21. doi: 10.1056/NEJMoa1203830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Devaraj A, Wells AU, Meister MG, et al. Detection of pulmonary hypertension with multidetector CT and echocardiography alone and in combination. Radiology. 2010;254:609–16. doi: 10.1148/radiol.09090548. [DOI] [PubMed] [Google Scholar]
- 21.Pérez-Enguix D, Morales P, Tomás JM, et al. Computed tomographic screening of pulmonary arterial hypertension in candidates for lung transplantation. Transplant Proc. 2007;39:2405–8. doi: 10.1016/j.transproceed.2007.07.055. [DOI] [PubMed] [Google Scholar]
- 22.Shin S, King CS, Puri N, et al. Pulmonary artery size as a predictor of outcomes in idiopathic pulmonary fibrosis. Eur Respir J. 2016;47:1445–51. doi: 10.1183/13993003.01532-2015. [DOI] [PubMed] [Google Scholar]
- 23.Moreira EM, Gall H, Leening MJG, et al. Prevalence of Pulmonary Hypertension in the General Population: The Rotterdam Study. PLoS ONE. 2015;10:e0130072. doi: 10.1371/journal.pone.0130072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wetscherek MTA, McNaughton E, Majcher V, et al. Incidental coronary artery calcification on non-gated CT thorax correlates with risk of cardiovascular events and death. Eur Radiol. 2023;33:4723–33. doi: 10.1007/s00330-023-09428-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choy G, Kröpil P, Scherer A, et al. Pertinent reportable incidental cardiac findings on chest CT without electrocardiography gating: review of 268 consecutive cases. Acta Radiol. 2013;54:396–400. doi: 10.1177/0284185113475918. [DOI] [PubMed] [Google Scholar]
- 26.2018 ESC/ESH Guidelines for the management of arterial hypertension. Rev Esp Cardiol (Eng Ed) 2019;72:160. doi: 10.1016/j.rec.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 27.Eul B, Cekay M, Pullamsetti SS, et al. Noninvasive Surrogate Markers of Pulmonary Hypertension Are Associated with Poor Survival in Patients with Lung Cancer. Am J Respir Crit Care Med. 2021;203:1316–9. doi: 10.1164/rccm.202005-2023LE. [DOI] [PubMed] [Google Scholar]
- 28.Ji-Xu A, Yang Y, Bradley KM. Pulmonary artery enlargement on routine staging (18)F-fluodeoxyglucose positron emission tomography/CT for lung and oesophageal cancer. Br J Radiol. 2020;93:20200323. doi: 10.1259/bjr.20200323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seeger W, Adir Y, Barberà JA, et al. Pulmonary hypertension in chronic lung diseases. J Am Coll Cardiol. 2013;62:D109–16. doi: 10.1016/j.jacc.2013.10.036. [DOI] [PubMed] [Google Scholar]
- 30.Nathan SD, Barbera JA, Gaine SP, et al. Pulmonary hypertension in chronic lung disease and hypoxia. Eur Respir J. 2019;53:1801914. doi: 10.1183/13993003.01914-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shah SJ. Pulmonary hypertension. JAMA. 2012;308:1366–74. doi: 10.1001/jama.2012.12347. [DOI] [PubMed] [Google Scholar]
- 32.Wright LM, Dwyer N, Celermajer D, et al. Follow-Up of Pulmonary Hypertension With Echocardiography. JACC Cardiovasc Imaging. 2016;9:733–46. doi: 10.1016/j.jcmg.2016.02.022. [DOI] [PubMed] [Google Scholar]
- 33.Tello K, Dalmer A, Vanderpool R, et al. Right ventricular function correlates of right atrial strain in pulmonary hypertension: a combined cardiac magnetic resonance and conductance catheter study. Am J Physiol Heart Circ Physiol. 2020;318:H156–64. doi: 10.1152/ajpheart.00485.2019. [DOI] [PubMed] [Google Scholar]
- 34.Guazzi M, Ghio S, Adir Y. Pulmonary Hypertension in HFpEFandHFrEF. J Am Coll Cardiol. 2020;76:1102–11. doi: 10.1016/j.jacc.2020.06.069. [DOI] [PubMed] [Google Scholar]
- 35.He X, Tang Y, Luo Z, et al. Subacute Cor Pulmonale Due to Tumor Embolization to the Lungs. Angiol Open Access. 1989;40:11–7. doi: 10.1177/000331978904000103. [DOI] [PubMed] [Google Scholar]
- 36.Wieshammer S, Dreyhaupt J, Müller D, et al. Venous thromboembolism and persistent pulmonary hypertension in cancer patients: a cross-sectional study. Thromb J. 2016;14:3. doi: 10.1186/s12959-016-0077-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Price LC, Seckl MJ, Dorfmüller P, et al. Tumoral pulmonary hypertension. Eur Respir Rev. 2019;28:180065. doi: 10.1183/16000617.0065-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gerull WD, Puri V, Kozower BD. The epidemiology and biology of pulmonary metastases. J Thorac Dis. 2021;13:2585–9. doi: 10.21037/jtd.2020.04.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vogel-Claussen J, Lasch F, Bollmann B-A, et al. Design and Rationale of the HANSE Study: A Holistic German Lung Cancer Screening Trial Using Low-Dose Computed Tomography. Rofo. 2022;194:1333–45. doi: 10.1055/a-1853-8291. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available on reasonable request.