Abstract
Background:
Ghana introduced the monovalent rotavirus vaccine (Rotarix) into its national paediatric vaccination programme in May2012. Vaccine introduction was initiated nationwide and achieved >85% coverage within a few months. Rotavirus strain distribution pre- and post-RV vaccine introduction is reported.
Methods:
Stool samples were collected from diarrhoeic children <5 years of age hospitalized between 2009 and 2016 at sentinel sites across Ghana and analyzed for the presence of group A rotavirus by enzyme immunoassay. Rotavirus strains were characterized by RT-PCR and sequencing.
Results:
A total of 1363 rotavirus EIA-positive samples were subjected to molecular characterization. These were made up of 823 (60.4%) and 540 (39.6%) samples from the pre- and post-vaccine periods respectively. Rotavirus VP7 genotypes G1, G2 and G3, and VP4 genotypes P[6] and P[8] constituted more than 65% of circulating G and P types in the pre–vaccine period. The common strains detected were G1P [8] (20%), G3P[6] (9.2%) and G2P[6] (4.9%).
During the post-vaccine period, G12, G1 and G10 genotypes, constituted more than 65% of the VP7 genotypes whilst P[6] and P[8] made up more than 75% of the VP4 genotypes. The predominant circulating strains were G12P[8] (26%), G10P[6] (10%) G3P[6] (8.1%) and G1P[8] (8.0%). We also observed the emergence of the unusual rotavirus strain G9P[4] during this period.
Conclusion:
Rotavirus G1P[8], the major strain in circulation during the pre-vaccination era, was replaced by G12P[8] as the most predominant strain after vaccine introduction. This strain replacement could be temporary and unrelated to vaccine introduction since an increase in G12 was observed in countries yet to introduce the rotavirus vaccine in West Africa. A continuous surveillance programme in the post-vaccine era is necessary for the monitoring of circulating rotavirus strains and the detection of unusual/emerging genotypes.
Keywords: Ghana, Strains, Monovalent rotavirus vaccine
1. Background
Diarrhoea is the fourth leading cause of childhood mortality worldwide, responsible for an estimated 550,000 deaths annually among children below the age of five years, representing 8.9% of all deaths within this age group [1]. Group A rotaviruses (RVAs) are the most important etiologic agent of acute gastroenteritis in children <5 years worldwide, accounting for about 200,000 deaths per annum, with a greater percentage of mortality occurring in developing countries [2]. In Ghana, rotaviruses account for up to 28% of diarrhoeal disease hospitalizations [3,4]. To reduce the high morbidity and mortality due to rotavirus infection, the World Health Organization (WHO) recommended the introduction of rotavirus vaccines into national immunization programmes in 2009.
Rotavirus gastroenteritis in humans is associated with mainly six genotype combinations; G1P[8], G2P[4], G3P[8], G4P[8], G9P [8] and G12P[8], causing majority of infections [5]. Although the distribution of these six globally important rotavirus genotypes can change dramatically in regions from year to year, the G1P[8] rotavirus strain has remained the most prevalent strain worldwide [6–8]. However, significant diversity of rotavirus genotypes continues to be observed worldwide with several novel combinations due to accumulation of point mutations, genome re-assortments, and/or zoonotic transmission to human host resulting in the introduction of new antigenic variants across regions [9,10].
Presently, there there are two rotavirus vaccines; RotaTeq, (Merck Vaccines, Whitehouse Station, New Jersey) and Rotarix (GlaxoSmithKline Biologicals, Rixensart, Belgium) that have been licensed and recommended by the WHO for inclusion in the immunization programmes in developing countries, especially those with a high burden of childhood diarrhoea disease as part of its strategy to control RV-associated diarrhoeal diseases [11,12]. The WHO has also recommended surveillance programmes at sentinel sites across Africa to monitor the burden of rotavirus disease and circulating strains before and after vaccine introduction as one of the crucial tools in measuring the impact of rotavirus vaccines [11]. Ghana has actively participated in the WHO surveillance programme and introduced the monovalent rotavirus vaccine (Rotarix, GSK) in May 2012. The introduction of rotavirus vaccines into national immunization programmes has led to the decline in the burden of severe childhood acute gastroenteritis (AGE) in many vaccine-introducing countries, including Ghana [3,4,13,14]. This report describes temporal trends in RV strain distribution pre- and post-vaccine introduction in Ghana.
This study was reviewed and approved by the Institutional Review Boards of the Centers for Disease Control and Prevention (Atlanta, Georgia), and the Noguchi Memorial Institute for Medical Research, University of Ghana (Accra).
2. Methods
2.1. Study population
The surveillance studies were carried out in rotavirus sentinel surveillances sites in health institutions across the southern, middle and northern belts of Ghana. The surveillance sites were Southern belt (Korle-Bu Teaching Hospital and Princess Marie Louise Children’s Hospital in the Greater Accra Region); Middle Belt (Agogo Presbyterian Hospital and Komfo Anokye Teaching Hospital in the Ashanti Region); Northern Belt (Navrongo War Memorial Hospital, Paga Health Centre and Kassena East Health Centre in the Upper East region). Diarrhoea disease surveillance and epidemiological studies have been on-going at these sites since 2009. Fecal samples were collected from children less than 5 years of age admitted with a primary diagnosis of AGE from participating hospitals and health Centers within 48 h of hospitalization and tested for the presence of rotavirus antigen using enzyme immunoassay (EIA) (ProSpecT™, Oxoid Cambridge, United Kingdom). A total of 1363 rotavirus (RV) EIA-positive stool samples sent to the Regional Rotavirus Reference Laboratory (RRL) located at the Noguchi Memorial Institute for Medical Research, University of Ghana, wee characterization. Demographic and clinical information of patients were also provided for each sample.
3. Laboratory analysis
3.1. Polyacrylamide gel Electrophoresis
All RV EIA-positive stool specimens were subjected to Polyacrylamide Gel Electrophoresis (PAGE) to ascertain the integrity of the RNA genome. All EIA-negative samples were also subjected to PAGE to screen for any non-group A rotavirus. Briefly, viral RNA was extracted from 10% faecal suspensions by the Bender method [15] with slight modification for PAGE analysis [16]. The extracted double-stranded RNA (dsRNA) was electrophoresed on a 10% polyacrylamide slab gel for 18–20 h at 100 V using the discontinuous buffer system as described by Laemmli [17]. A 3% stacking gel was employed to enhance the resolution of the segmented genes. Bands were visualized by silver-staining technique [18].
3.2. RT-PCR
RVA dsRNA was extracted from 10% fecal suspensions of EIA-positive and EIA negative PAGE-positive samples by the phenol/chloroform method as described by Steele and Alexander and purified with an RNaid® Kit (Bio 101, Carlsbad, USA) [18]. RT-PCR was carried out using consensus primers Beg9/End9 and Con2/Con3 to amplify the VP7 and VP4 genes respectively [19–21]. Semi-nested multiplex PCR was done for G- and P-typing by using genotype-specific primers as described previously [20–22]. The amplified product was electrophoresed on a 2% agarose gel, and the genotypes determined by the sizes of the amplicons. Ten percent of all genotypes determined by PCR were further confirmed by sequencing. Briefly, the PCR amplicons were purified with the ExoSap-IT purification kit (USB products) following the manufacturer’s instructions and sequenced by the dideoxynucleotide chain termination method using the ABI PRISM® BigDye Terminator Cycle Sequencing Reaction kit v3.1 (Perkin-Elmer Applied Biosystems, Foster City, CA). Sequences were read on an automated sequencer (ABI PRISM™ 3130), and assembled contigs identified by querying the nucleotide database in GenBank using the Basic Local Alignment Search Tool [BLAST] (http://blast.ncbi.nlm.nih.gov/Blast.cgi). The genotypes obtained were confirmed using the automated genotyping tool, RotaC v2.0 [23]. All demographic, clinical and laboratory data were entered into a database and analyzed using Stata version 13 (Stata Corp, College Station, TX, USA).
4. Results
A total of 1363 rotavirus-positive diarrhoeic stool samples from the southern (n = 876), middle (n = 173) and northern (n = 314) regions were successfully genotyped. Of these, 823 were from the pre-vaccine period (January 2009–April 2012) and 540 from the post-vaccination period (May 2012–December 2016). The most common G-genotypes detected during the entire period (2009 to 2016) were G1 (30.7%; 418/1363), G12 (13.8%; 188/1363), G3 (12.1%; 165/1363) and G2 (10.7%; 146/1363) whilst the most commonly detected P-genotypes were P[8] (37.8%; 515/1363), P[6] (31.6%; 431/1363), and P[4] (8.9%; 121/1363).
The circulation and detection of rotavirus VP7 and VP4 genotypes during the pre and post vaccine introduction periods were very similar. The most common VP7 and VP4 types detected during the pre vaccine introduction period were G1 (40.0%; 330/823) and P[8] (34.6%; 285/823) respectively as shown in Table 1a. Rotaviruses bearing the VP[6] genotypes were also commonly detected (30.7%, 253/823). The most prevalent G and P types detected were G1P[8] (20.0%, 165/823, G3P[6] (9.2%, 76/823) more than eighteen percent of all Vp7 could not be genotypes whilst the percentage of un-typable P types were less than 3%. More than 11% of all strains detected had mixed VP4 genotypes.
Table 1a.
Distribution of rotavirus strains detected in Ghana – Pre-vaccine era (January 2009–April 2012).
| VP7 | VP4 | Total | ||||
|---|---|---|---|---|---|---|
| P[4] | P[6] | P[8] | P[MIX] | P[NT] | ||
| G1 | 6 | 34 | 165 | 97 | 28 | 330 |
| G10 | 0 | 31 | 0 | 2 | 1 | 34 |
| G12 | 0 | 4 | 8 | 0 | 0 | 12 |
| G2 | 17 | 40 | 14 | 26 | 6 | 103 |
| G3 | 3 | 76 | 19 | 10 | 6 | 114 |
| G4 | 10 | 2 | 1 | 1 | 1 | 15 |
| G6 | 0 | 1 | 0 | 0 | 0 | 1 |
| G8 | 0 | 0 | 1 | 0 | 0 | 1 |
| G9 | 0 | 12 | 10 | 2 | 2 | 26 |
| GMIX | 1 | 10 | 12 | 11 | 1 | 35 |
| GNT | 4 | 43 | 55 | 23 | 27 | 152 |
| Total | 41 | 253 | 285 | 172 | 72 | 823 |
MIX: mixed genotypes; NT: either G, P or both were non-typeable.
During the post vaccine period, the most common G and P types detected were G12 (32.6%; 176/540) and P[8] (42.6%; 230/540). The predominant rotavirus strains detected were G12P[8] (25.6%; 138/540), G10P[6] (10.0; 54/540) and G3P[6] (8.1%; 44/540) strains as shown in Table 1b. Strains bearing the common VP7 genotypes, G1 and G2, constituted 24.3% (131/540) of all rotavirus strains detected.
Table 1b.
Distribution of rotavirus strains detected in Ghana - Post-vaccine era (May 2012–December 2016).
| VP7 | VP4 | Total | ||||
|---|---|---|---|---|---|---|
| P[4] | P[6] | P[8] | P[MIX] | P[NT] | ||
| G1 | 15 | 21 | 43 | 6 | 3 | 88 |
| G10 | 1 | 54 | 12 | 2 | 0 | 69 |
| G12 | 2 | 22 | 138 | 13 | 1 | 176 |
| G2 | 33 | 6 | 4 | 0 | 0 | 43 |
| G3 | 0 | 44 | 5 | 2 | 0 | 51 |
| G4 | 0 | 2 | 6 | 0 | 0 | 8 |
| G8 | 0 | 0 | 1 | 0 | 0 | 1 |
| G9 | 16 | 10 | 4 | 2 | 2 | 34 |
| GMIX | 10 | 10 | 6 | 15 | 0 | 41 |
| GNT | 3 | 9 | 11 | 1 | 5 | 29 |
| Total | 80 | 178 | 230 | 41 | 11 | 540 |
MIX: mixed; NT: either G, P or both were non-typeable.
In addition to common strains, we identified several rare/uncommon genotype combinations in a low percentage of samples: G1P[4], G4P[4], G6P[6], G8P[8], G9P[4], G10P[4], G10P[6] and G12P[4]. The overall prevalence of these rare/uncommon strains is 10% (Tables 1a and 1b. We did not see significant differences between dominant strains within the three regions during the pre- or post-vaccine era.
4.1. Temporal strain distribution pre- and post- vaccine era
G1P[8] was the predominant rotavirus strain (>20%) during the pre-vaccine era (Fig. 1). Other common strains detected were G3P [6] (9.2%), G1P[6] (4.1%), G10P[6] (3.8%) and G2P[6] (4.9%). The first three rotavirus seasons after vaccine introduction (2012–2015) saw the emergence and dominance of G12P[8] (138/540; 26%) and G10P[6] (54/540; 10%) strains as shown in Table 1b. However, this observed phenomenon was short lived and there was a return of G1P[8] as one of the dominant strains in the fourth year post-vaccine introduction (2015–2016 rotavirus season) (Fig. 1). The 2015–2016 rotavirus season also saw the emergence of the unusual G9[P4] strains (16/540; 3.0%), most of which were detected in the middle belt. This unusual strain was first detected in Ghana in the early 2000s. Successive detections of G9P[4] strain were in 2013 (2/144, 1.4%), 2015 (1/53, 1.3%), and the spike in 2016 (8/51, 15.7%) (Fig. 1). Phylogenetic analysis of the VP7 gene revealed these G9 genotypes to be of human origin (unpublished data).
Fig. 1.
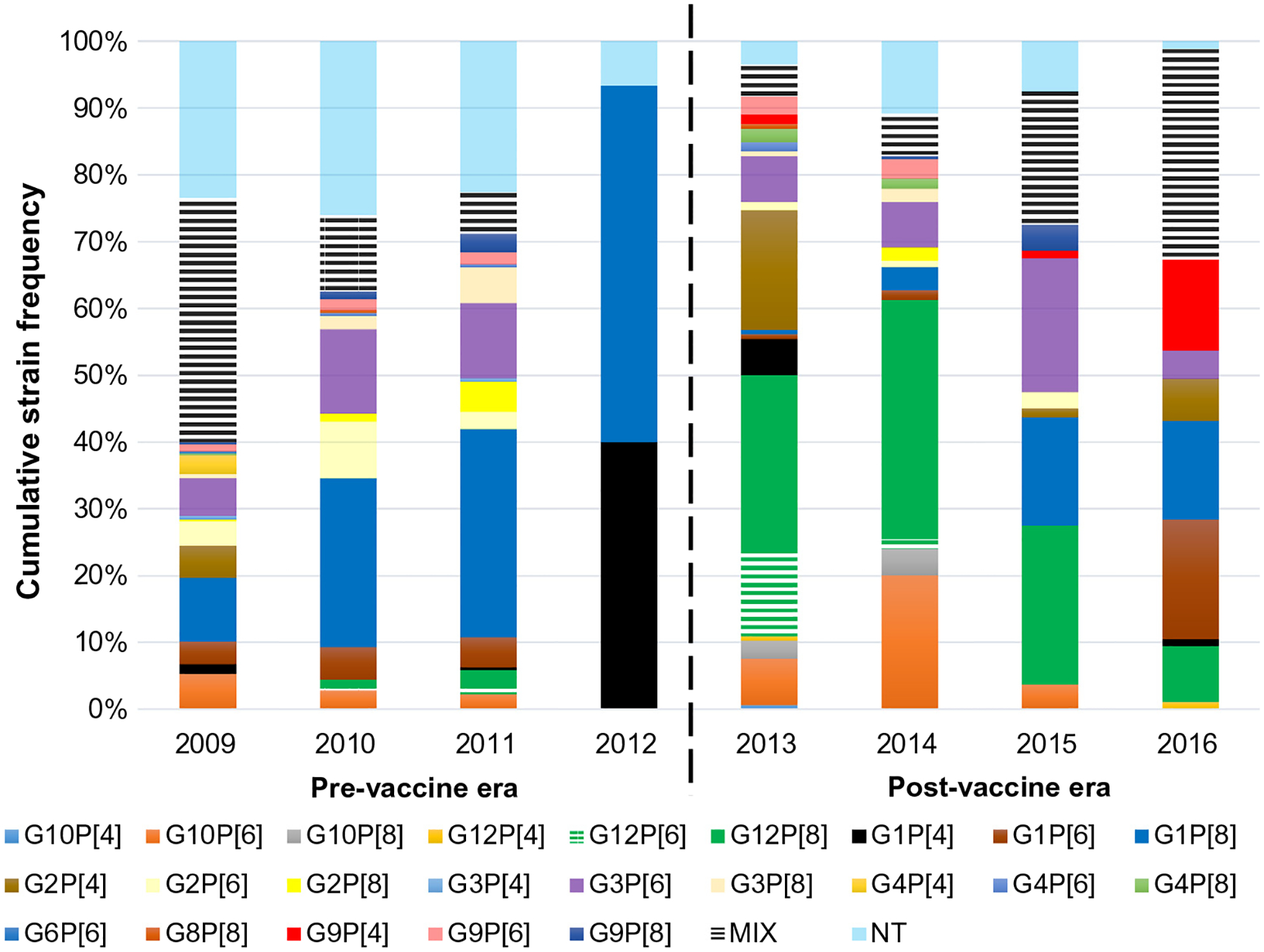
Temporal rotavirus strain distribution in Ghana (2009 to 2016). Black broken line indicates vaccine introduction in Ghana. MIX: mixed genotypes; NT: either G, P or both were non-typeable.
5. Discussion
Rotavirus infection was monitored as part of the ongoing World Health Organization sponsored rotavirus surveillance study in Ghana. A total of 1363 RVA positive samples were subjected to G and P genotyping as described earlier [20–22]. Genotype data before vaccine introduction showed that strains carrying the G1 genotype specificity were responsible for 40% of all RV cases recorded during the pre-vaccine period (2009–2012) and this was followed by G3 (13.9%) and G2 (12.5%). Most of the common genotypes identified (G1, G2) were usually found in combinations with P[8] and P[4] VP4 genotypes (Tables 1a and 1b). The prevalence of the common G3P[8] strain remained below 2% during the study period. In addition to common strains, we identified several rare/uncommon genotype combinations in a few samples: G1P [4] (1.5%; 21/1363) G4P[4] (<1%; 10/1363), G9P[4] (1.2%; 16/1363) and G10P[6] (6.2%; 85/1363).
The sudden appearance of G12P[8] rotavirus strains soon after the introduction of rotavirus vaccines in 2012 and its drop to less than 6% of detected strains by 2016 is quite interesting. Concurrent genotyping of diarrhoeal stool samples submitted to the Rotavirus Regional Reference Laboratory (RRL) in Ghana (data not shown) from countries in the WHO sponsored African Rotavirus surveillance programme showed the emergence of G12 strains as the dominant strain in Nigeria and Senegal, countries that had not yet introduced rotavirus vaccines in their immunization programme. Therefore, the observed emergence of G12 strains post-vaccine introduction may not be associated with vaccine introduction and may represent natural secular variation in rotavirus strains.
Earlier reports indicate that rotavirus G9s were usually in combination with the P[8] genotypes and to a lesser extent, P[6] [24]. The emergence of the unusual G9[P4] strain more than ten years after its first detection in a child with diarrhoea in northern Ghana [25] was intriguing. Outbreaks of rotavirus G9P[4] strains have also been reported over the last decade in Asia (India, Bangladesh and Japan) and Latin America (Brazil, Mexico, Guatemala and Honduras) [26–33]. Though several of these countries reporting the increased detection of G9P[4] rotavirus strains had previously introduced the monovalent rotavirus vaccine, it remains unclear whether the observed increased detection of this unusual strain is due to vaccine introduction. Earlier reports from Ghana have shown increased diversity in circulating strains [34] and it is quite likely that the observed increase of these unusual strains may be due to increased re-assortment processes in the community. It is important to note that studies in Mexico showed that the monovalent rotavirus vaccine (Rotarix) provided protection against the fully heterotypic G9P[4] strains [33]. However, this study was limited by its small sample size and the observational nature of the evaluation. While it has not yet been established whether the Ghanaian G9P[4] strains are identical to the Mexican strains, it remains unclear whether they will become established or transient, and what the implications will be for vaccine efficacy in Ghana. The observed increase in detection of rotavirus strains with mixed G and P types in circulation after Rotarix vaccine introduction has also been reported in other countries in India, Bangladesh, South Africa and Malawi [35–38] where a substantial proportion of mixed rotavirus circulating strains were also detected post-vaccination. Whilst these findings may indicate naturally occurring variations in circulating rotavirus strains, it could also be due to selective pressure from the introduction of rotavirus vaccination. A continuous rotavirus surveillance programme is thus necessary and important for the monitoring of circulating strains in the post-vaccine era to fully understand the effect of vaccine introduction on strain distribution and the emergence of new strains.
Acknowledgments
The authors are grateful to the surveillance and laboratory teams at the participating hospitals: Komfo Anokye Teaching Hospital and Agogo Presbyterian Hospital (Bernard Arhin, Kwabena Adjei Asante); Korle Bu Teaching Hospital (Anna Aba Hayford, Makafui Seshie, Juanita Adams, Anita Acheampong); Ho and Hohoe hospitals (Eric Agboli); Navrongo War Memorial Hospital (Edward Sobe, George Atia); and the Princess Marie Louise Children’s Hospital (Gifty Okine). We are also grateful to the rotavirus study group at the Noguchi Institute for Medical Research, University of Ghana (Fred Asamoah, Jennifer Amexo, Joseph Armachie) for data entry, management, and strain characterization, and to all parents and children who participated in the study.
Financial support
Financial support for this project was provided by WHO Regional Office for Africa using a New Vaccines Surveillance grant from GAVI the Vaccine Alliance.
Footnotes
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC) or the World Health Organization (WHO).
Potential conflicts of interest
The authors have declared no competing conflict of interests.
References
- [1].Liu L, Oza S, Hogan D, Chu Y, Perin J, Zhu J, et al. Global, regional, and national causes of under-5 mortality in 2000–15: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet 2016;388: 3027–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Tate JE, Burton AH, Boschi-Pinto C, Parashar UD. World Health Organization-Coordinated Global Rotavirus Surveillance Network. Global, regional, and national estimates of rotavirus mortality in children less than 5 years of age, 2000–2013. Clin Infect Dis 2016;62(2):S96–S105. [DOI] [PubMed] [Google Scholar]
- [3].Enweronu-Laryea CC, Boamah I, Sifah E, Diamenu SK, Armah G. Decline in severe diarrhea hospitalizations after the introduction of rotavirus vaccination in Ghana: a prevalence study. BMC Infect Dis 2014;14:431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Armah G, Pringle K, Enweronu-Laryea CC, Ansong D, Mwenda JM, Diamenu SK, et al. Impact and effectiveness of monovalent rotavirus vaccine against severe rotavirus diarrhea in ghana. Clin Infect Dis 2016;62(Suppl 2):S200–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Matthijnssens J, Van Ranst. Genotype constellation and evolution of group A rotaviruses infecting humans. Curr Opin Virol 2012;2:426–33. [DOI] [PubMed] [Google Scholar]
- [6].Van Damme P, Giaquinto C, Maxwell M, Todd P, Van der Wielen M. Distribution of rotavirus genotypes in Europe, 2004–2005: the REVEAL Study. J Infect Dis 2007;195(Suppl. 1):S17–25. [DOI] [PubMed] [Google Scholar]
- [7].Rahman M, Matthijnssens J, Goegebuer T, De Leener K, Vanderwegen L, van der Donck I, et al. Predominance of rotavirus G9 genotype in children hospitalized for rotavirus gastroenteritis in Belgium during 1999–2003. J Clin Virol 2005;33 (May (1)):1–6. [DOI] [PubMed] [Google Scholar]
- [8].Santos N, Hoshino Y. Global distribution of rotavirus serotypes/genotypes and its implication for the development and implementation of an effective rotavirus vaccine. Rev Med Virol 2005;15(1):29–56. [DOI] [PubMed] [Google Scholar]
- [9].Martella V, Banyai K, Matthijnssens J, Buonavoglia C, Ciarlet M. Zoonotic aspects of rotaviruses. Vet Microbiol 2010;140:246–55. [DOI] [PubMed] [Google Scholar]
- [10].Matthijnssens J, Bilcke J, Ciarlet M, Martella V, Banyai K, Rhaman M, et al. Rotavirus disease and vaccination: impact on genotype diversity. Future Microbiol 2009;4:1303–16. [DOI] [PubMed] [Google Scholar]
- [11].World Health Organization. Rotavirus vaccines WHO position paper-January 2013. Wkly Epidemiol Rec. 2013;88:49–64. [PubMed] [Google Scholar]
- [12].World Health Organization. Meeting of the strategic advisory group of experts on immunization, October 2009—conclusions and recommendations. Wkly Epidemiol Rec 2009;84:517–32. [PubMed] [Google Scholar]
- [13].Patel M, Steele D, Gentsch JR, Wecker J, Glass RI, Parashar UD. Real-world impact of rotavirus vaccination. Pediatr Infect Dis J 2011;30(suppl):S1–5. [DOI] [PubMed] [Google Scholar]
- [14].O’Ryan M, Giaquinto C, Benninghoff B. Human rotavirus vaccine (Rotarix): focus on effectiveness and impact 6 years after first introduction in Africa Expert Rev. Vaccines 2015;14(8):1099–112. [DOI] [PubMed] [Google Scholar]
- [15].Flook PK, Wilson MD, Post RJ. The repetitive use of DNA probes in the analysis of natural populations of insects and parasites. In: Berry RJ, Crawford TJ, Hewitt GM, editors. Genes in ecology. Oxford: British Ecological Society/Blackwell Scientific Publication; 1992. p. 484. [Google Scholar]
- [16].Asmah RH, Green J, Armah GE, Gallimore CI, Gray JJ, Iturriza-Gomara M, et al. Rotavirus G and P genotypes in rural Ghana. J Clin Microbiol 2001;39:1981–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970;227:680–5. [DOI] [PubMed] [Google Scholar]
- [18].Herring AJ, Inglis NF, Ojeh CK, Snodgrass DR, Menzies JD. Rapid diagnosis of rotavirus infection by direct detection of viral nucleic acid in silver-stained polyacrylamide gels. J Clin Microbiol 1982;16:473–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Steele AD, Alexander JJ. Molecular epidemiology of rotaviruses in black infants in South Africa. J Clin Microbiol 1987;23:992–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Iturriza-Gomara M, Kang G, Gray J. Rotavirus genotyping: Keeping up with an evolving population of human rotaviruses. J Clin Virol 2004;31:259–65. [DOI] [PubMed] [Google Scholar]
- [21].Gentsch JR, Glass RI, Woods P, Gouvea V, Gorziglia M, Flores J, et al. Identification of group A rotavirus gene 4 types by polymerase chain reaction. J Clin Microbiol 1992;30:1365–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Gouvea V, Glass RI, Woods P, Taniguchi K, Clark HF, Forrester B, et al. Polymerase chain reaction amplification and typing of rotavirus nucleic acid from stool specimens. J Clin Microbiol 1990;28:276–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Maes P, Matthijnssens J, Rahman M, Van Ranst M. RotaC: a web-based tool for the complete genome classification of group A rotaviruses. BMC Microbiol 2009;9:238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bern C, Unicomb L, Gentsch JR, Banul N, Yunus M, Sack RB, et al. Rotavirus diarrhoea in Bangladeshi children: correlation of disease severity with serotypes. J Clin Micro-biol 1992;30:3234–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Armah GE, Steele AD, Binka FN, Esona MD, Asmah RH, Anto F, et al. Changing patterns of RV genotypes in Ghana: Emergence of rotavirus G9 as a major cause diarrhea in children. J Clin Microbiol 2003;41(6):2317–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Chitambar SD, Ranshing SS, Pradhan GN, Kalrao VR, Dhongde RK, Bavdekar Ashish R, et al. Changing trends in circulating rotavirus strains in Pune, western India in 2009–2012: Emergence of a rare G9P[4] rotavirus strain. Vaccine 2014;32:A29–32. [DOI] [PubMed] [Google Scholar]
- [27].Babji S, Arumugam R, Sarvanabhavan A, Moses PD, Simon A, Aggarwal I, et al. Multi-center surveillance of rotavirus diarrhea in hospitalized children <5 years of age in India, 2009–2012. Vaccine 2014;32S:A10–2. [DOI] [PubMed] [Google Scholar]
- [28].Reesu R, Bhattacharya D, Chaaithanya IK, Muruganandam N, Bharadwaj AP, Singhania M, et al. Emergence of an unusual genotype of rotavirus in Andaman and Nicobar islands, India. Inter-virology 2013;56:134–9. [DOI] [PubMed] [Google Scholar]
- [29].Afrad MH, Rahman MZ, Matthijnssens J, Das SK, Faruque AS, Azim T, et al. High incidence of reassortant G9P[4] rotavirus strain in Bangladesh: Fully heterotypic from vaccine strains. J Clin Virol 2013;58:755–6. [DOI] [PubMed] [Google Scholar]
- [30].Yamamoto SP, Kaida A, Ono A, Kubo H, Iritani N. Detection and characterization of a human G9P[4] rotavirus strain in Japan. J Med Virol 2015;87:1311–8. [DOI] [PubMed] [Google Scholar]
- [31].Quaye O, McDonald S, Esona MD, Lyde FC, Mijatovic-Rustempasic S, Roy S, et al. Rotavirus G9P[4] in 3 countries in Latin America 2009–2010. Emerg Infect Dis 2013;19:1332–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Linhares AC, Stupka JA, Ciapponi A, Bardach AE, Glujovsky D, Aruj PK, et al. Burden and typing of rotavirus group A in Latin America and the Caribbean: systematic review and meta-analysis. Rev Med Virol 2011;21:89–109. [DOI] [PubMed] [Google Scholar]
- [33].Yen C, Figueroa JR, Uribe ES, Carmen-Hernández LD, Tate JE, Parashar UD, et al. Monovalent vaccine provides protection against an emerging fully heterotypic G9P[4] rotavirus strain in Mexico. J Infct Dis 2011;204:783–6. [DOI] [PubMed] [Google Scholar]
- [34].Armah GE, Steele AD, Esona MD, Akran VA, Nimzing L, Pennap G. Diversity of rotavirus strains circulating in west Africa from 1996 to 2000. J Infect Dis. 2010. Sep;1(202 Suppl):S64–71. [DOI] [PubMed] [Google Scholar]
- [35].Miles MG, Lewis KD, Kang G, Parashar UD, Steele AD. A systematic review of rotavirus strain diversity in India, Bangladesh, and Pakistan. Vaccine 2012;30 (Suppl 1):A131–9. [DOI] [PubMed] [Google Scholar]
- [36].Afrad MH, Hassan Z, Farjana S, Moni S, Barua S, Das SK, et al. Changing profile of rotavirus genotypes in Bangladesh, 2006–2012. BMC Infect Dis 2013;13:320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Groome MJ, Page N, Cortese MM, Moyes J, Zar HJ, Kapongo CN, et al. Effectiveness of monovalent human rotavirus vaccine against admission to hospital for acute rotavirus diarrhoea in South African children: a case-control study. Lancet Infect Dis 2014;14:1096–104. [DOI] [PubMed] [Google Scholar]
- [38].Bar-Zeev N, Kapanda L, Tate JE, Jere KC, Iturriza-Gomara M, Nakagomi O, et al. Effectiveness of a monovalent rotavirus vaccine in infants in Malawi after programmatic roll-out: an observational and case-control study. Lancet Infect Dis 2015;15:422–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


