Abstract
Inhibitors with dissociation constants in the micromolar to nanomolar range are important, but hard to characterize kinetically, especially when the substrate concentration in the assay is less than Km. When inhibition increases during the course of the assay (slow-binding inhibition) the concentration of substrate may decrease appreciably. Methods that take substrate depletion into account are described for analysing experiments in which the initial substrate concentration is below Km. Fitting progress curves gives the rate constants for the second (slow) step in a two-step mechanism. An approximate value for the overall dissociation constant may be determined from measurements of rates when the reaction is treated as a first-order process. When the concentrations of inhibitor and enzyme are comparable numerical methods are required. Procedures, suitable for implementation on a microcomputer, for the solution of the differential equations and the fitting of progress curves are described.
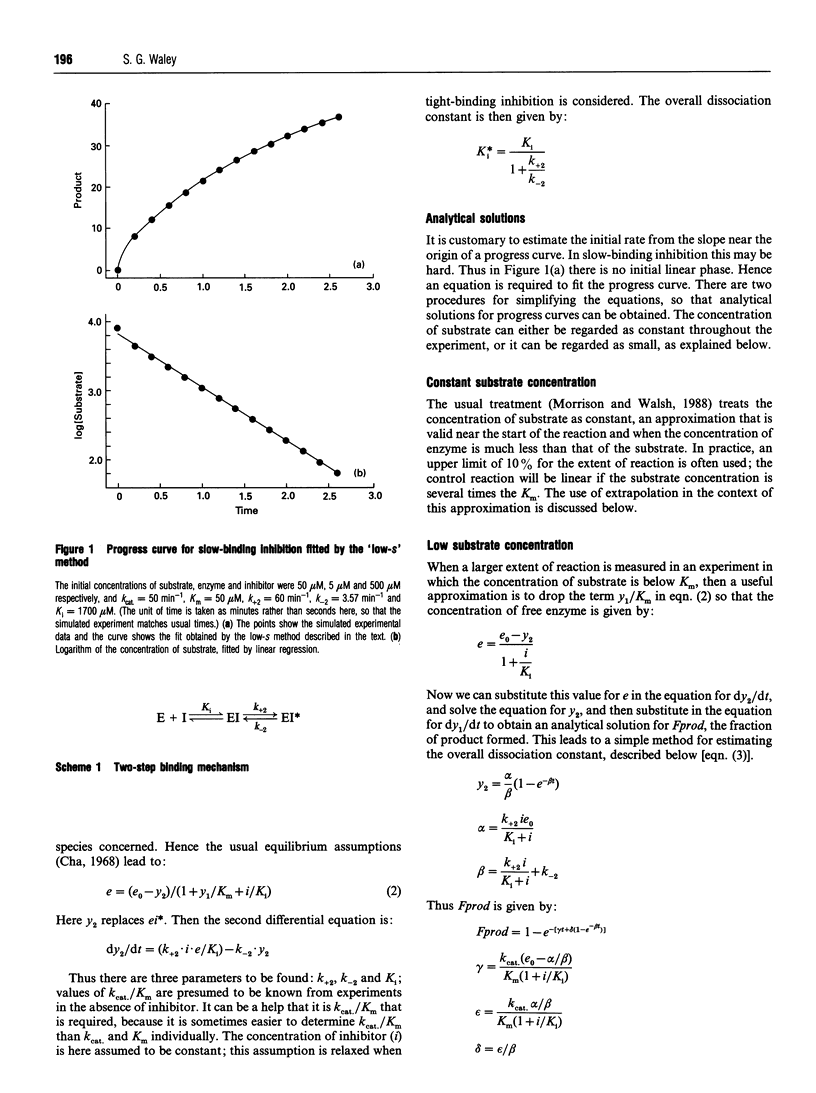
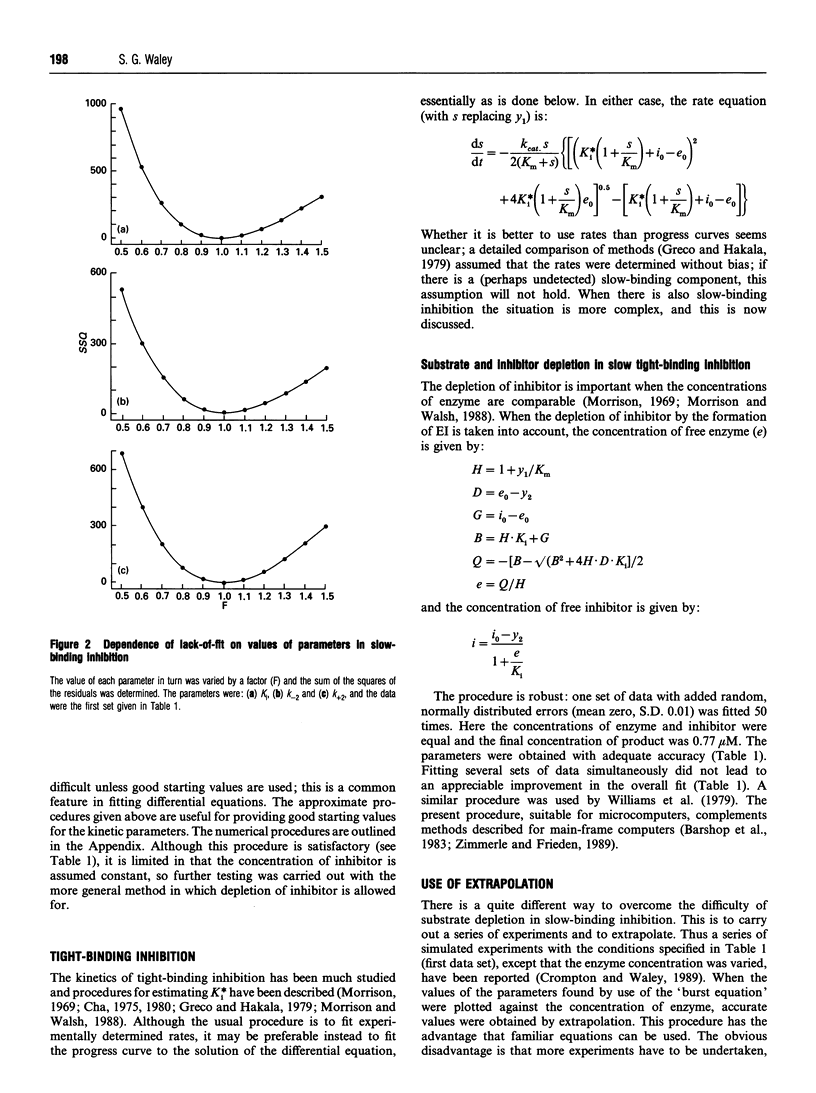
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barshop B. A., Wrenn R. F., Frieden C. Analysis of numerical methods for computer simulation of kinetic processes: development of KINSIM--a flexible, portable system. Anal Biochem. 1983 Apr 1;130(1):134–145. doi: 10.1016/0003-2697(83)90660-7. [DOI] [PubMed] [Google Scholar]
- Cha S. A simple method for derivation of rate equations for enzyme-catalyzed reactions under the rapid equilibrium assumption or combined assumptions of equilibrium and steady state. J Biol Chem. 1968 Feb 25;243(4):820–825. [PubMed] [Google Scholar]
- Cha S. Tight-binding inhibitors--VII. Extended interpretation of the rate equation experimental designs and statistical methods. Biochem Pharmacol. 1980 Jun 15;29(12):1779–1789. doi: 10.1016/0006-2952(80)90140-9. [DOI] [PubMed] [Google Scholar]
- Cha S. Tight-binding inhibitors-I. Kinetic behavior. Biochem Pharmacol. 1975 Dec 1;24(23):2177–2185. doi: 10.1016/0006-2952(75)90050-7. [DOI] [PubMed] [Google Scholar]
- Crompton I. E., Cuthbert B. K., Lowe G., Waley S. G. Beta-lactamase inhibitors. The inhibition of serine beta-lactamases by specific boronic acids. Biochem J. 1988 Apr 15;251(2):453–459. doi: 10.1042/bj2510453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crompton I. E., Waley S. G. Some uses of extrapolation in kinetics. Biochem J. 1989 Jan 1;257(1):305–308. doi: 10.1042/bj2570305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Meester F., Joris B., Reckinger G., Bellefroid-Bourguignon C., Frère J. M., Waley S. G. Automated analysis of enzyme inactivation phenomena. Application to beta-lactamases and DD-peptidases. Biochem Pharmacol. 1987 Jul 15;36(14):2393–2403. doi: 10.1016/0006-2952(87)90609-5. [DOI] [PubMed] [Google Scholar]
- Duggleby R. G. Regression analysis of nonlinear Arrhenius plots: an empirical model and a computer program. Comput Biol Med. 1984;14(4):447–455. doi: 10.1016/0010-4825(84)90045-3. [DOI] [PubMed] [Google Scholar]
- Frieden C., Kurz L. C., Gilbert H. R. Adenosine deaminase and adenylate deaminase: comparative kinetic studies with transition state and ground state analogue inhibitors. Biochemistry. 1980 Nov 11;19(23):5303–5309. doi: 10.1021/bi00564a024. [DOI] [PubMed] [Google Scholar]
- Frère J. M., Leyh B., Renard A. Lineweaver-Burk, Hanes, Eadie-Hofstee and Dixon plots in non-steady-state situations. J Theor Biol. 1983 Apr 7;101(3):387–400. doi: 10.1016/0022-5193(83)90146-7. [DOI] [PubMed] [Google Scholar]
- Greco W. R., Hakala M. T. Evaluation of methods for estimating the dissociation constant of tight binding enzyme inhibitors. J Biol Chem. 1979 Dec 10;254(23):12104–12109. [PubMed] [Google Scholar]
- Leatherbarrow R. J. Using linear and non-linear regression to fit biochemical data. Trends Biochem Sci. 1990 Dec;15(12):455–458. doi: 10.1016/0968-0004(90)90295-m. [DOI] [PubMed] [Google Scholar]
- Morrison J. F. Kinetics of the reversible inhibition of enzyme-catalysed reactions by tight-binding inhibitors. Biochim Biophys Acta. 1969;185(2):269–286. doi: 10.1016/0005-2744(69)90420-3. [DOI] [PubMed] [Google Scholar]
- Morrison J. F., Walsh C. T. The behavior and significance of slow-binding enzyme inhibitors. Adv Enzymol Relat Areas Mol Biol. 1988;61:201–301. doi: 10.1002/9780470123072.ch5. [DOI] [PubMed] [Google Scholar]
- Williams J. W., Morrison J. F., Duggleby R. G. Methotrexate, a high-affinity pseudosubstrate of dihydrofolate reductase. Biochemistry. 1979 Jun 12;18(12):2567–2573. doi: 10.1021/bi00579a021. [DOI] [PubMed] [Google Scholar]
- Zimmerle C. T., Frieden C. Analysis of progress curves by simulations generated by numerical integration. Biochem J. 1989 Mar 1;258(2):381–387. doi: 10.1042/bj2580381. [DOI] [PMC free article] [PubMed] [Google Scholar]


