Abstract
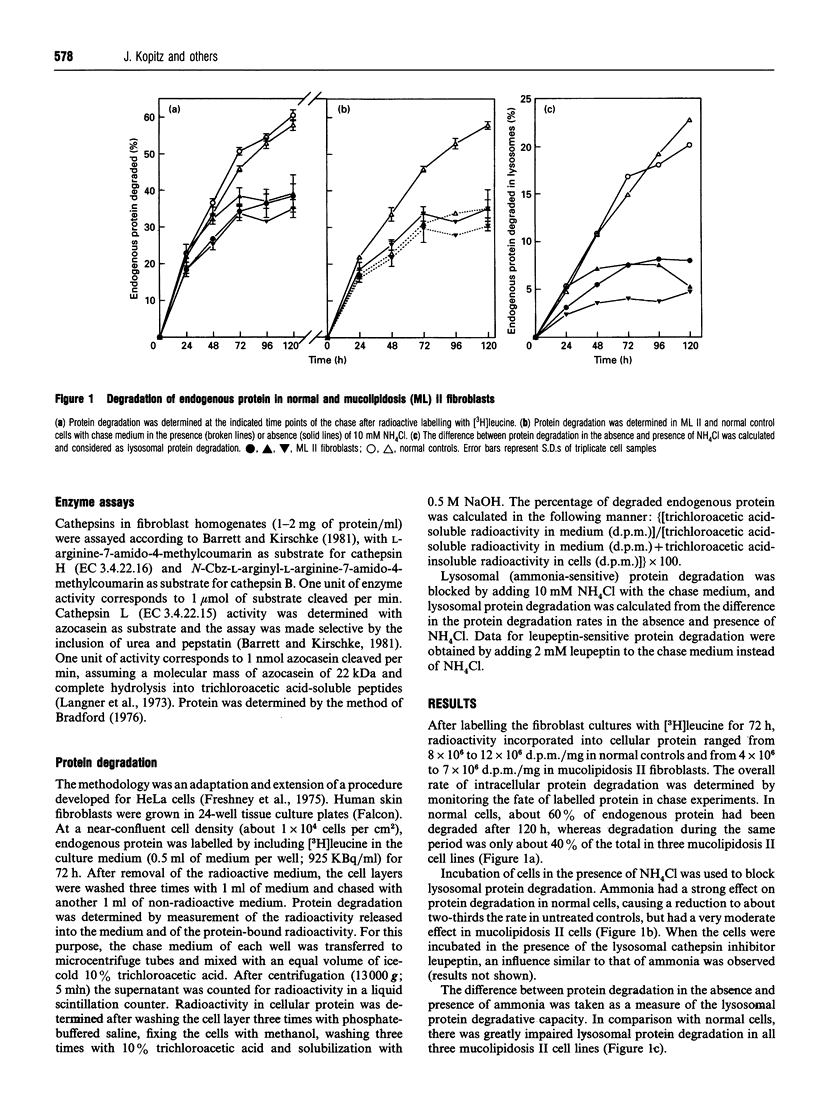
Protein catabolism in fibroblasts cultured from the skin of normal individuals and of patients with mucolipidosis II (I-cell disease) and several other lysosomal storage diseases was examined by metabolic labelling with [3H]leucine and following the fate of radioactive proteins in pulse-chase experiments. In mucolipidosis II cells, overall protein degradative rates were found to be distinctly lower than in normal control cells. To distinguish lysosomal from non-lysosomal degradation, labelling experiments were carried out in the presence and absence of 10 mM NH4Cl, an inhibitor of lysosomal function. It was found that mucolipidosis II fibroblasts exhibited a markedly reduced rate of lysosomal protein degradation, whereas the rate of nonlysosomal degradation appeared normal. Serum and amino acid starvation led to a marked increase in lysosomal protein degradation in normal cells, but had only a minimal effect on that in mucolipidosis II fibroblasts. The specific activities of cathepsins B, H and L were profoundly diminished in all mucolipidosis II cell lines tested. Lysosomal protein degradation in a mucolipidosis III cell line was impaired to a similar degree as in mucolipidosis II cells, whereas it was decreased to a lesser extent in fibroblasts from patients with mucopolysaccharidoses I and VI, galactosialidosis and GM1-gangliosidosis. We conclude that fibroblasts from patients with mucolipidosis II and III have a severely compromised capacity for endogenous lysosomal protein degradation that appears to result from multiple cathepsin deficiency. This lysosomal defect is likely to have pathophysiological consequences.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett A. J., Kirschke H. Cathepsin B, Cathepsin H, and cathepsin L. Methods Enzymol. 1981;80(Pt 100):535–561. doi: 10.1016/s0076-6879(81)80043-2. [DOI] [PubMed] [Google Scholar]
- Bienkowski R. S., Ripley C. R., Gitzelmann R., Steinmann B. Collagen degradation in I-cells is normal. Biochem Biophys Res Commun. 1990 Apr 30;168(2):479–484. doi: 10.1016/0006-291x(90)92346-2. [DOI] [PubMed] [Google Scholar]
- Bohley P., Seglen P. O. Proteases and proteolysis in the lysosome. Experientia. 1992 Feb 15;48(2):151–157. doi: 10.1007/BF01923508. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Cockle S. M., Dean R. T. An abnormality in intracellular protein degradation in fibroblasts from patients with I-cell disease. Clin Chim Acta. 1984 Jul 31;140(3):257–265. doi: 10.1016/0009-8981(84)90207-9. [DOI] [PubMed] [Google Scholar]
- Freshney R. I., Paul J., Kane I. M. Assay of anti-cancer drugs in tissue culture: conditions affecting their ability to incorporate 3H-leucine after drug treatment. Br J Cancer. 1975 Jan;31(1):89–99. doi: 10.1038/bjc.1975.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall N. A., Lake B. D., Dewji N. N., Patrick A. D. Lysosomal storage of subunit c of mitochondrial ATP synthase in Batten's disease (ceroid-lipofuscinosis). Biochem J. 1991 Apr 1;275(Pt 1):269–272. doi: 10.1042/bj2750269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanewinkel H., Glössl J., Kresse H. Biosynthesis of cathepsin B in cultured normal and I-cell fibroblasts. J Biol Chem. 1987 Sep 5;262(25):12351–12355. [PubMed] [Google Scholar]
- Hendil K. B., Lauridsen A. M., Seglen P. O. Both endocytic and endogenous protein degradation in fibroblasts is stimulated by serum/amino acid deprivation and inhibited by 3-methyladenine. Biochem J. 1990 Dec 15;272(3):577–581. doi: 10.1042/bj2720577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles S. E., Ballard F. J. Selective control of the degradation of normal and aberrant proteins in Reuber H35 hepatoma cells. Biochem J. 1976 Jun 15;156(3):609–617. doi: 10.1042/bj1560609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopitz J., Kisen G. O., Gordon P. B., Bohley P., Seglen P. O. Nonselective autophagy of cytosolic enzymes by isolated rat hepatocytes. J Cell Biol. 1990 Sep;111(3):941–953. doi: 10.1083/jcb.111.3.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langner J., Wakil A., Zimmermann M., Ansorge S., Bohley P., Kirschke H., Wiederanders B. Aktivitätsbestimmung proteolytischer Enzyme mit Azokasein als Substrat. Acta Biol Med Ger. 1973;31(1):1–18. [PubMed] [Google Scholar]
- Palmer D. N., Fearnley I. M., Walker J. E., Hall N. A., Lake B. D., Wolfe L. S., Haltia M., Martinus R. D., Jolly R. D. Mitochondrial ATP synthase subunit c storage in the ceroid-lipofuscinoses (Batten disease). Am J Med Genet. 1992 Feb 15;42(4):561–567. doi: 10.1002/ajmg.1320420428. [DOI] [PubMed] [Google Scholar]
- Palmer D. N., Martinus R. D., Cooper S. M., Midwinter G. G., Reid J. C., Jolly R. D. Ovine ceroid lipofuscinosis. The major lipopigment protein and the lipid-binding subunit of mitochondrial ATP synthase have the same NH2-terminal sequence. J Biol Chem. 1989 Apr 5;264(10):5736–5740. [PubMed] [Google Scholar]
- Seglen P. O., Gordon P. B., Grinde B., Solheim A., Kovács A. L., Poli A. Inhibitors and pathways of hepatocytic protein degradation. Acta Biol Med Ger. 1981;40(10-11):1587–1598. [PubMed] [Google Scholar]
- Seglen P. O. Inhibitors of lysosomal function. Methods Enzymol. 1983;96:737–764. doi: 10.1016/s0076-6879(83)96063-9. [DOI] [PubMed] [Google Scholar]
- Williams J. C., Weinstein D. B., Miller A. L., Steinberg D. Defective catabolism of low-density lipoprotein by fibroblasts from patients with I-cell disease. Biochem J. 1982 Jan 15;202(1):183–190. doi: 10.1042/bj2020183. [DOI] [PMC free article] [PubMed] [Google Scholar]


