Abstract
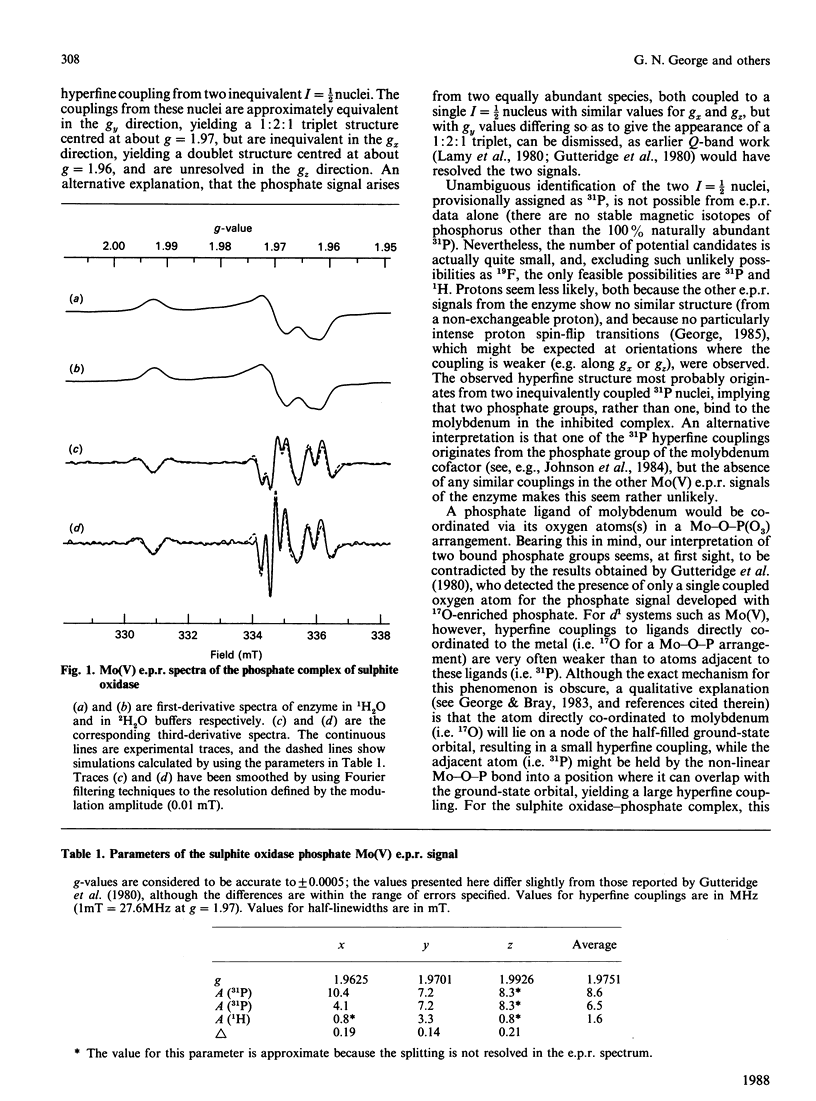
The phosphate complex of sulphite oxidase in the Mo(V) oxidation state was investigated by e.p.r. spectroscopy. Third-derivative spectra reveal a wealth of structural detail previously unobserved in this spectrum. Most notable is the presence of hyperfine coupling from two inequivalent I = 1/2 nuclei, which we tentatively attribute to two 31P nuclei. Unresolved hyperfine interactions from at least one exchangeable 1H nucleus are also present.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bray R. C., Gutteridge S., Lamy M. T., Wilkinson T. Equilibria amongst different molybdenum (V)-containing species from sulphite oxidase. Evidence for a halide ligand of molybdenum in the low-pH species. Biochem J. 1983 Apr 1;211(1):227–236. doi: 10.1042/bj2110227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray R. C. The inorganic biochemistry of molybdoenzymes. Q Rev Biophys. 1988 Aug;21(3):299–329. doi: 10.1017/s0033583500004479. [DOI] [PubMed] [Google Scholar]
- Bray R. C. The reactions and the structures of molybdenum centers in enzymes. Adv Enzymol Relat Areas Mol Biol. 1980;51:107–165. doi: 10.1002/9780470122969.ch3. [DOI] [PubMed] [Google Scholar]
- George G. N., Bray R. C. Studies by electron paramagnetic resonance spectroscopy of xanthine oxidase enriched with molybdenum-95 and with molybdenum-97. Biochemistry. 1988 May 17;27(10):3603–3609. doi: 10.1021/bi00410a011. [DOI] [PubMed] [Google Scholar]
- Gutteridge S., Lamy M. T., Bray R. C. The nature of the phosphate inhibitor complex of sulphite oxidase from electron-paramagnetic-resonance studies using oxygen-17. Biochem J. 1980 Oct 1;191(1):285–288. doi: 10.1042/bj1910285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. L., Hainline B. E., Rajagopalan K. V., Arison B. H. The pterin component of the molybdenum cofactor. Structural characterization of two fluorescent derivatives. J Biol Chem. 1984 May 10;259(9):5414–5422. [PubMed] [Google Scholar]
- Kessler D. L., Rajagopalan K. V. Hepatic sulfite oxidase. Effect of anions on interaction with cytochrome c. Biochim Biophys Acta. 1974 Dec 29;370(2):389–398. doi: 10.1016/0005-2744(74)90100-4. [DOI] [PubMed] [Google Scholar]
- Kessler D. L., Rajagopalan K. V. Purification and properties of sulfite oxidase from chicken liver. Presence of molybdenum in sulfite oxidase from diverse sources. J Biol Chem. 1972 Oct 25;247(20):6566–6573. [PubMed] [Google Scholar]
- Lamy M. T., Gutteridge S., Bary R. C. Electron-paramagnetic-resonance parameters of molybdenum(V) in sulphite oxidase from chicken liver. Biochem J. 1980 Feb 1;185(2):397–403. doi: 10.1042/bj1850397. [DOI] [PMC free article] [PubMed] [Google Scholar]


