Abstract
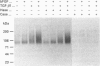
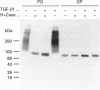
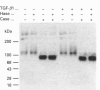
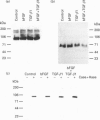
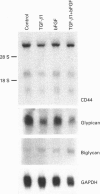
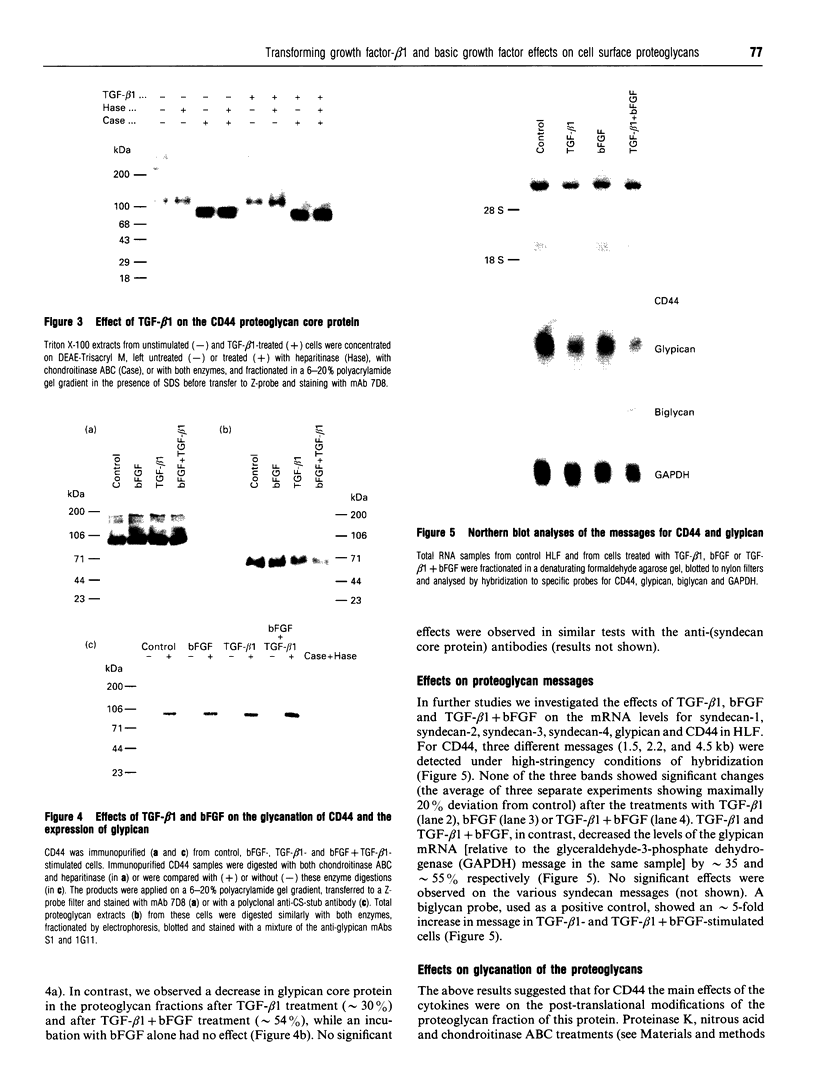
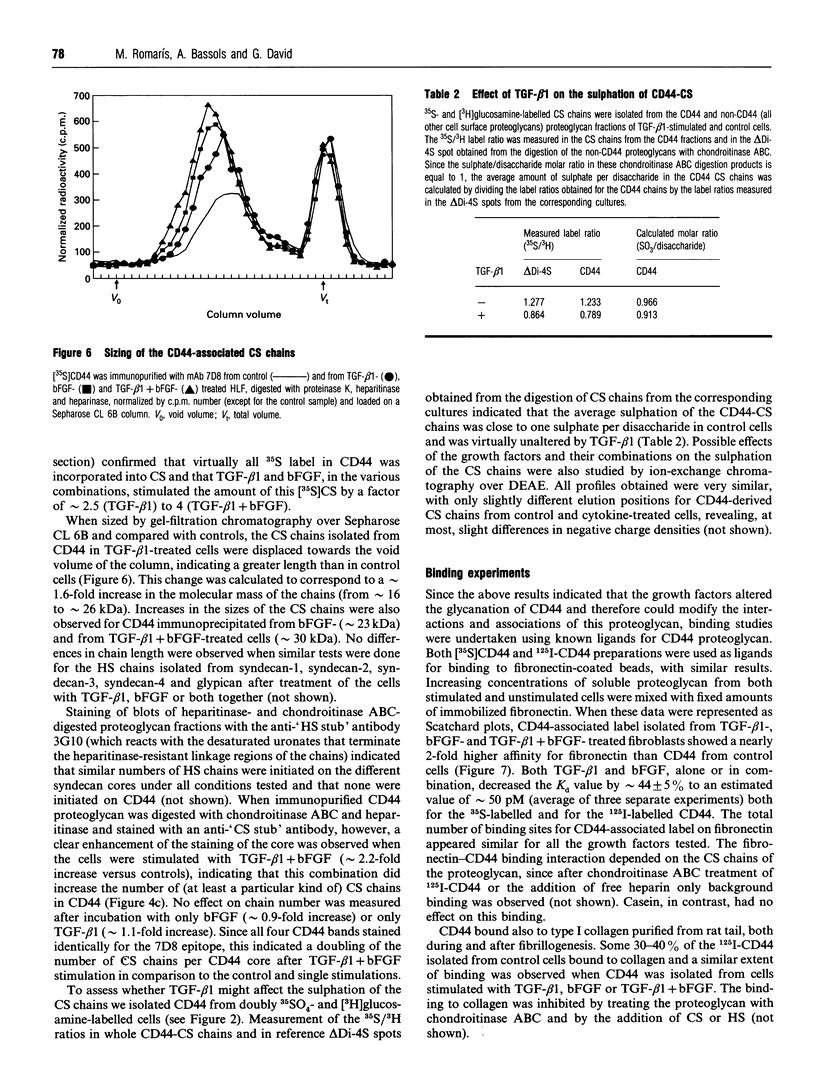
We have tested the effects of transforming growth factor-beta 1 (TGF-beta 1), basic fibroblast growth factor (bFGF) and TGF-beta 1 + bFGF on the expression of the cell surface proteoglycans (CD44, syndecans and glypican) in cultures of human lung fibroblasts (HLF). Cell surface proteoglycan expression was monitored by quantitative immunoprecipitation from metabolically labelled cells. Western and Northern blotting and evaluation of the glycanation of the proteoglycans. Stimulation of the cells with TGF-beta 1 increased the length of the chondroitin sulphate (CS) chains on CD44 (approximately 1.6-fold). bFGF, administered solely, also increased the length of the CS chains on CD44 (approximately 1.4-fold), whereas the combination of TGF-beta 1 + bFGF nearly doubled both the length and the number of the CS chains on CD44. None of these treatments lead to changes in CD44 message or core-protein expression. This enhanced glycanation of CD44 after the TGF-beta 1, bFGF and combined treatments correlated with a 2-fold increase in the affinity of the proteoglycan for fibronectin but had no influence on the binding to type I collagen. TGF-beta 1, alone or in combination with bFGF, also stimulated the CS content of syndecan-1, but none of the other syndecans was significantly affected by any of the factors or combinations tested. The expression of glypican however was significantly decreased (nearly halved) by the combination of TGF-beta 1 + bFGF, less so by TGF-beta 1 and not at all by bFGF. This decrease occurred both at the level of the message and of the core protein. These data demonstrate specific and differential effects of TGF-beta 1 and bFGF on the structure, expression and interactions of the cell surface proteoglycans of HLF.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aruffo A., Stamenkovic I., Melnick M., Underhill C. B., Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990 Jun 29;61(7):1303–1313. doi: 10.1016/0092-8674(90)90694-a. [DOI] [PubMed] [Google Scholar]
- Bassols A., Massagué J. Transforming growth factor beta regulates the expression and structure of extracellular matrix chondroitin/dermatan sulfate proteoglycans. J Biol Chem. 1988 Feb 25;263(6):3039–3045. [PubMed] [Google Scholar]
- Bernfield M., Kokenyesi R., Kato M., Hinkes M. T., Spring J., Gallo R. L., Lose E. J. Biology of the syndecans: a family of transmembrane heparan sulfate proteoglycans. Annu Rev Cell Biol. 1992;8:365–393. doi: 10.1146/annurev.cb.08.110192.002053. [DOI] [PubMed] [Google Scholar]
- Border W. A., Ruoslahti E. Transforming growth factor-beta in disease: the dark side of tissue repair. J Clin Invest. 1992 Jul;90(1):1–7. doi: 10.1172/JCI115821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdon M. A., Krusius T., Campbell S., Schwartz N. B., Ruoslahti E. Identification and synthesis of a recognition signal for the attachment of glycosaminoglycans to proteins. Proc Natl Acad Sci U S A. 1987 May;84(10):3194–3198. doi: 10.1073/pnas.84.10.3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. C., Vogel K. G. Characteristics of the in vitro interaction of a small proteoglycan (PG II) of bovine tendon with type I collagen. Matrix. 1989;9(6):468–478. doi: 10.1016/s0934-8832(11)80016-8. [DOI] [PubMed] [Google Scholar]
- Brown T. A., Bouchard T., St John T., Wayner E., Carter W. G. Human keratinocytes express a new CD44 core protein (CD44E) as a heparan-sulfate intrinsic membrane proteoglycan with additional exons. J Cell Biol. 1991 Apr;113(1):207–221. doi: 10.1083/jcb.113.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter W. G., Wayner E. A. Characterization of the class III collagen receptor, a phosphorylated, transmembrane glycoprotein expressed in nucleated human cells. J Biol Chem. 1988 Mar 25;263(9):4193–4201. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- David G. Integral membrane heparan sulfate proteoglycans. FASEB J. 1993 Aug;7(11):1023–1030. doi: 10.1096/fasebj.7.11.8370471. [DOI] [PubMed] [Google Scholar]
- David G., Lories V., Decock B., Marynen P., Cassiman J. J., Van den Berghe H. Molecular cloning of a phosphatidylinositol-anchored membrane heparan sulfate proteoglycan from human lung fibroblasts. J Cell Biol. 1990 Dec;111(6 Pt 2):3165–3176. doi: 10.1083/jcb.111.6.3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David G., Lories V., Heremans A., Van der Schueren B., Cassiman J. J., Van den Berghe H. Membrane-associated chondroitin sulfate proteoglycans of human lung fibroblasts. J Cell Biol. 1989 Mar;108(3):1165–1173. doi: 10.1083/jcb.108.3.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David G., van der Schueren B., Marynen P., Cassiman J. J., van den Berghe H. Molecular cloning of amphiglycan, a novel integral membrane heparan sulfate proteoglycan expressed by epithelial and fibroblastic cells. J Cell Biol. 1992 Aug;118(4):961–969. doi: 10.1083/jcb.118.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge G. R., Kovalszky I., Hassell J. R., Iozzo R. V. Transforming growth factor beta alters the expression of heparan sulfate proteoglycan in human colon carcinoma cells. J Biol Chem. 1990 Oct 15;265(29):18023–18029. [PubMed] [Google Scholar]
- Drake S. L., Klein D. J., Mickelson D. J., Oegema T. R., Furcht L. T., McCarthy J. B. Cell surface phosphatidylinositol-anchored heparan sulfate proteoglycan initiates mouse melanoma cell adhesion to a fibronectin-derived, heparin-binding synthetic peptide. J Cell Biol. 1992 Jun;117(6):1331–1341. doi: 10.1083/jcb.117.6.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elenius K., Mättä A., Salmivirta M., Jalkanen M. Growth factors induce 3T3 cells to express bFGF-binding syndecan. J Biol Chem. 1992 Mar 25;267(9):6435–6441. [PubMed] [Google Scholar]
- Esko J. D. Genetic analysis of proteoglycan structure, function and metabolism. Curr Opin Cell Biol. 1991 Oct;3(5):805–816. doi: 10.1016/0955-0674(91)90054-3. [DOI] [PubMed] [Google Scholar]
- Faassen A. E., Mooradian D. L., Tranquillo R. T., Dickinson R. B., Letourneau P. C., Oegema T. R., McCarthy J. B. Cell surface CD44-related chondroitin sulfate proteoglycan is required for transforming growth factor-beta-stimulated mouse melanoma cell motility and invasive behavior on type I collagen. J Cell Sci. 1993 Jun;105(Pt 2):501–511. doi: 10.1242/jcs.105.2.501. [DOI] [PubMed] [Google Scholar]
- Faassen A. E., Schrager J. A., Klein D. J., Oegema T. R., Couchman J. R., McCarthy J. B. A cell surface chondroitin sulfate proteoglycan, immunologically related to CD44, is involved in type I collagen-mediated melanoma cell motility and invasion. J Cell Biol. 1992 Jan;116(2):521–531. doi: 10.1083/jcb.116.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher J. T. The extended family of proteoglycans: social residents of the pericellular zone. Curr Opin Cell Biol. 1989 Dec;1(6):1201–1218. doi: 10.1016/s0955-0674(89)80072-9. [DOI] [PubMed] [Google Scholar]
- Goldstein L. A., Zhou D. F., Picker L. J., Minty C. N., Bargatze R. F., Ding J. F., Butcher E. C. A human lymphocyte homing receptor, the hermes antigen, is related to cartilage proteoglycan core and link proteins. Cell. 1989 Mar 24;56(6):1063–1072. doi: 10.1016/0092-8674(89)90639-9. [DOI] [PubMed] [Google Scholar]
- Heino J., Ignotz R. A., Hemler M. E., Crouse C., Massagué J. Regulation of cell adhesion receptors by transforming growth factor-beta. Concomitant regulation of integrins that share a common beta 1 subunit. J Biol Chem. 1989 Jan 5;264(1):380–388. [PubMed] [Google Scholar]
- Hildebrand A., Romarís M., Rasmussen L. M., Heinegård D., Twardzik D. R., Border W. A., Ruoslahti E. Interaction of the small interstitial proteoglycans biglycan, decorin and fibromodulin with transforming growth factor beta. Biochem J. 1994 Sep 1;302(Pt 2):527–534. doi: 10.1042/bj3020527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignotz R. A., Massagué J. Transforming growth factor-beta stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J Biol Chem. 1986 Mar 25;261(9):4337–4345. [PubMed] [Google Scholar]
- Jackson D. G., Bell J. I., Dickinson R., Timans J., Shields J., Whittle N. Proteoglycan forms of the lymphocyte homing receptor CD44 are alternatively spliced variants containing the v3 exon. J Cell Biol. 1995 Feb;128(4):673–685. doi: 10.1083/jcb.128.4.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalkanen S., Jalkanen M., Bargatze R., Tammi M., Butcher E. C. Biochemical properties of glycoproteins involved in lymphocyte recognition of high endothelial venules in man. J Immunol. 1988 Sep 1;141(5):1615–1623. [PubMed] [Google Scholar]
- Jalkanen S., Jalkanen M. Lymphocyte CD44 binds the COOH-terminal heparin-binding domain of fibronectin. J Cell Biol. 1992 Feb;116(3):817–825. doi: 10.1083/jcb.116.3.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C. W., Goldberger O. A., Gallo R. L., Bernfield M. Members of the syndecan family of heparan sulfate proteoglycans are expressed in distinct cell-, tissue-, and development-specific patterns. Mol Biol Cell. 1994 Jul;5(7):797–805. doi: 10.1091/mbc.5.7.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjellén L., Lindahl U. Proteoglycans: structures and interactions. Annu Rev Biochem. 1991;60:443–475. doi: 10.1146/annurev.bi.60.070191.002303. [DOI] [PubMed] [Google Scholar]
- Kokenyesi R., Bernfield M. Core protein structure and sequence determine the site and presence of heparan sulfate and chondroitin sulfate on syndecan-1. J Biol Chem. 1994 Apr 22;269(16):12304–12309. [PubMed] [Google Scholar]
- Kähäri V. M., Larjava H., Uitto J. Differential regulation of extracellular matrix proteoglycan (PG) gene expression. Transforming growth factor-beta 1 up-regulates biglycan (PGI), and versican (large fibroblast PG) but down-regulates decorin (PGII) mRNA levels in human fibroblasts in culture. J Biol Chem. 1991 Jun 5;266(16):10608–10615. [PubMed] [Google Scholar]
- Lesley J., Hyman R., Kincade P. W. CD44 and its interaction with extracellular matrix. Adv Immunol. 1993;54:271–335. doi: 10.1016/s0065-2776(08)60537-4. [DOI] [PubMed] [Google Scholar]
- Lories V., Cassiman J. J., Van den Berghe H., David G. Differential expression of cell surface heparan sulfate proteoglycans in human mammary epithelial cells and lung fibroblasts. J Biol Chem. 1992 Jan 15;267(2):1116–1122. [PubMed] [Google Scholar]
- Lories V., Cassiman J. J., Van den Berghe H., David G. Multiple distinct membrane heparan sulfate proteoglycans in human lung fibroblasts. J Biol Chem. 1989 Apr 25;264(12):7009–7016. [PubMed] [Google Scholar]
- Lories V., David G., Cassiman J. J., Van den Berghe H. Heparan sulfate proteoglycans of human lung fibroblasts. Occurrence of distinct membrane, matrix and secreted forms. Eur J Biochem. 1986 Jul 15;158(2):351–359. doi: 10.1111/j.1432-1033.1986.tb09758.x. [DOI] [PubMed] [Google Scholar]
- López-Casillas F., Cheifetz S., Doody J., Andres J. L., Lane W. S., Massagué J. Structure and expression of the membrane proteoglycan betaglycan, a component of the TGF-beta receptor system. Cell. 1991 Nov 15;67(4):785–795. doi: 10.1016/0092-8674(91)90073-8. [DOI] [PubMed] [Google Scholar]
- Mann D. M., Yamaguchi Y., Bourdon M. A., Ruoslahti E. Analysis of glycosaminoglycan substitution in decorin by site-directed mutagenesis. J Biol Chem. 1990 Mar 25;265(9):5317–5323. [PubMed] [Google Scholar]
- Marynen P., Zhang J., Cassiman J. J., Van den Berghe H., David G. Partial primary structure of the 48- and 90-kilodalton core proteins of cell surface-associated heparan sulfate proteoglycans of lung fibroblasts. Prediction of an integral membrane domain and evidence for multiple distinct core proteins at the cell surface of human lung fibroblasts. J Biol Chem. 1989 Apr 25;264(12):7017–7024. [PubMed] [Google Scholar]
- Massagué J. The transforming growth factor-beta family. Annu Rev Cell Biol. 1990;6:597–641. doi: 10.1146/annurev.cb.06.110190.003121. [DOI] [PubMed] [Google Scholar]
- Nathan C., Sporn M. Cytokines in context. J Cell Biol. 1991 Jun;113(5):981–986. doi: 10.1083/jcb.113.5.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapraeger A. C. The coordinated regulation of heparan sulfate, syndecans and cell behavior. Curr Opin Cell Biol. 1993 Oct;5(5):844–853. doi: 10.1016/0955-0674(93)90034-n. [DOI] [PubMed] [Google Scholar]
- Rapraeger A. Transforming growth factor (type beta) promotes the addition of chondroitin sulfate chains to the cell surface proteoglycan (syndecan) of mouse mammary epithelia. J Cell Biol. 1989 Nov;109(5):2509–2518. doi: 10.1083/jcb.109.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen S., Rapraeger A. Altered structure of the hybrid cell surface proteoglycan of mammary epithelial cells in response to transforming growth factor-beta. J Cell Biol. 1988 Nov;107(5):1959–1967. doi: 10.1083/jcb.107.5.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts C. J., Birkenmeier T. M., McQuillan J. J., Akiyama S. K., Yamada S. S., Chen W. T., Yamada K. M., McDonald J. A. Transforming growth factor beta stimulates the expression of fibronectin and of both subunits of the human fibronectin receptor by cultured human lung fibroblasts. J Biol Chem. 1988 Apr 5;263(10):4586–4592. [PubMed] [Google Scholar]
- Romarís M., Heredia A., Molist A., Bassols A. Differential effect of transforming growth factor beta on proteoglycan synthesis in human embryonic lung fibroblasts. Biochim Biophys Acta. 1991 Jul 10;1093(2-3):229–233. doi: 10.1016/0167-4889(91)90127-j. [DOI] [PubMed] [Google Scholar]
- Salustri A., Ulisse S., Yanagishita M., Hascall V. C. Hyaluronic acid synthesis by mural granulosa cells and cumulus cells in vitro is selectively stimulated by a factor produced by oocytes and by transforming growth factor-beta. J Biol Chem. 1990 Nov 15;265(32):19517–19523. [PubMed] [Google Scholar]
- Schönherr E., Järveläinen H. T., Sandell L. J., Wight T. N. Effects of platelet-derived growth factor and transforming growth factor-beta 1 on the synthesis of a large versican-like chondroitin sulfate proteoglycan by arterial smooth muscle cells. J Biol Chem. 1991 Sep 15;266(26):17640–17647. [PubMed] [Google Scholar]
- Segarini P. R., Seyedin S. M. The high molecular weight receptor to transforming growth factor-beta contains glycosaminoglycan chains. J Biol Chem. 1988 Jun 15;263(17):8366–8370. [PubMed] [Google Scholar]
- Shively J. E., Conrad H. E. Formation of anhydrosugars in the chemical depolymerization of heparin. Biochemistry. 1976 Sep 7;15(18):3932–3942. doi: 10.1021/bi00663a005. [DOI] [PubMed] [Google Scholar]
- Stamenkovic I., Amiot M., Pesando J. M., Seed B. A lymphocyte molecule implicated in lymph node homing is a member of the cartilage link protein family. Cell. 1989 Mar 24;56(6):1057–1062. doi: 10.1016/0092-8674(89)90638-7. [DOI] [PubMed] [Google Scholar]
- Stipp C. S., Litwack E. D., Lander A. D. Cerebroglycan: an integral membrane heparan sulfate proteoglycan that is unique to the developing nervous system and expressed specifically during neuronal differentiation. J Cell Biol. 1994 Jan;124(1-2):149–160. doi: 10.1083/jcb.124.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. F., Lin H. Y., Ng-Eaton E., Downward J., Lodish H. F., Weinberg R. A. Expression cloning and characterization of the TGF-beta type III receptor. Cell. 1991 Nov 15;67(4):797–805. doi: 10.1016/0092-8674(91)90074-9. [DOI] [PubMed] [Google Scholar]
- Wasserman L., Ber A., Allalouf D. Use of thin-layer chromatography in the separation of disaccharides resulting from digestion of chondroitin sulphates with chondroitinases. J Chromatogr. 1977 Jun 11;136(2):342–347. doi: 10.1016/s0021-9673(00)86291-3. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y., Mann D. M., Ruoslahti E. Negative regulation of transforming growth factor-beta by the proteoglycan decorin. Nature. 1990 Jul 19;346(6281):281–284. doi: 10.1038/346281a0. [DOI] [PubMed] [Google Scholar]
- Yanagishita M., Hascall V. C. Cell surface heparan sulfate proteoglycans. J Biol Chem. 1992 May 15;267(14):9451–9454. [PubMed] [Google Scholar]
- Zhang L., Esko J. D. Amino acid determinants that drive heparan sulfate assembly in a proteoglycan. J Biol Chem. 1994 Jul 29;269(30):19295–19299. [PubMed] [Google Scholar]
- Zimmermann D. R., Ruoslahti E. Multiple domains of the large fibroblast proteoglycan, versican. EMBO J. 1989 Oct;8(10):2975–2981. doi: 10.1002/j.1460-2075.1989.tb08447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]