Abstract
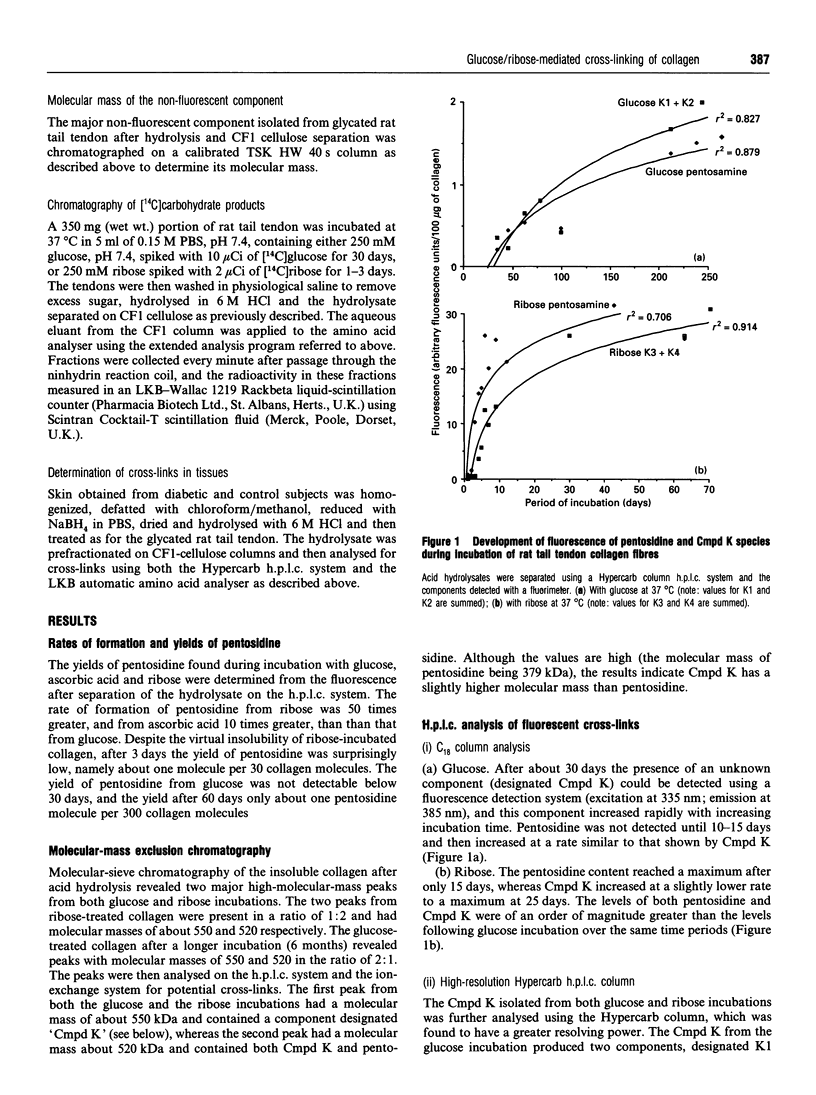
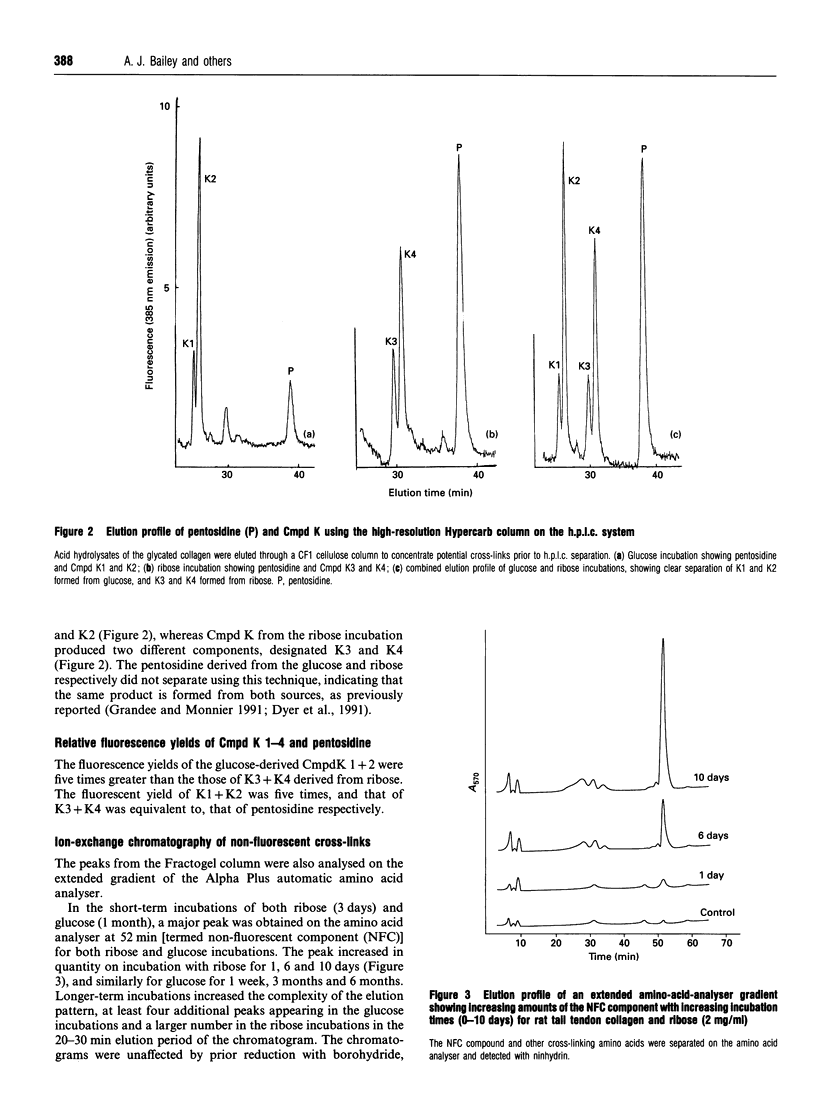
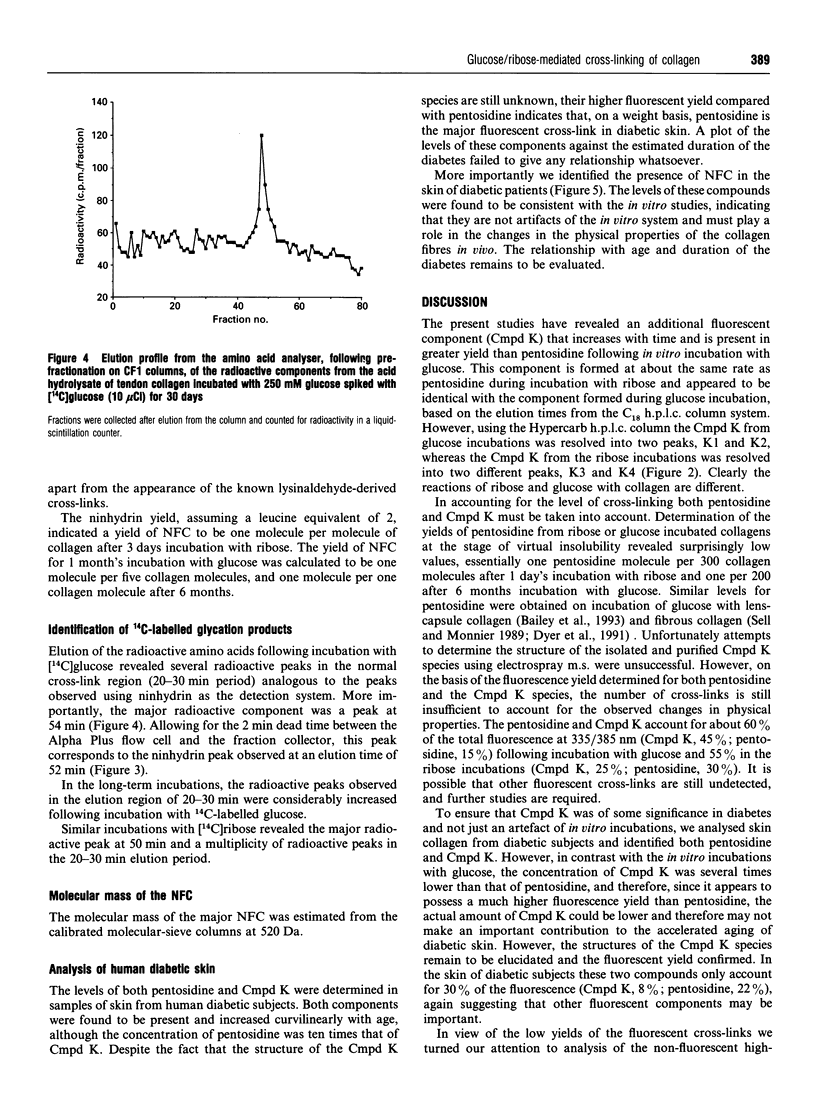
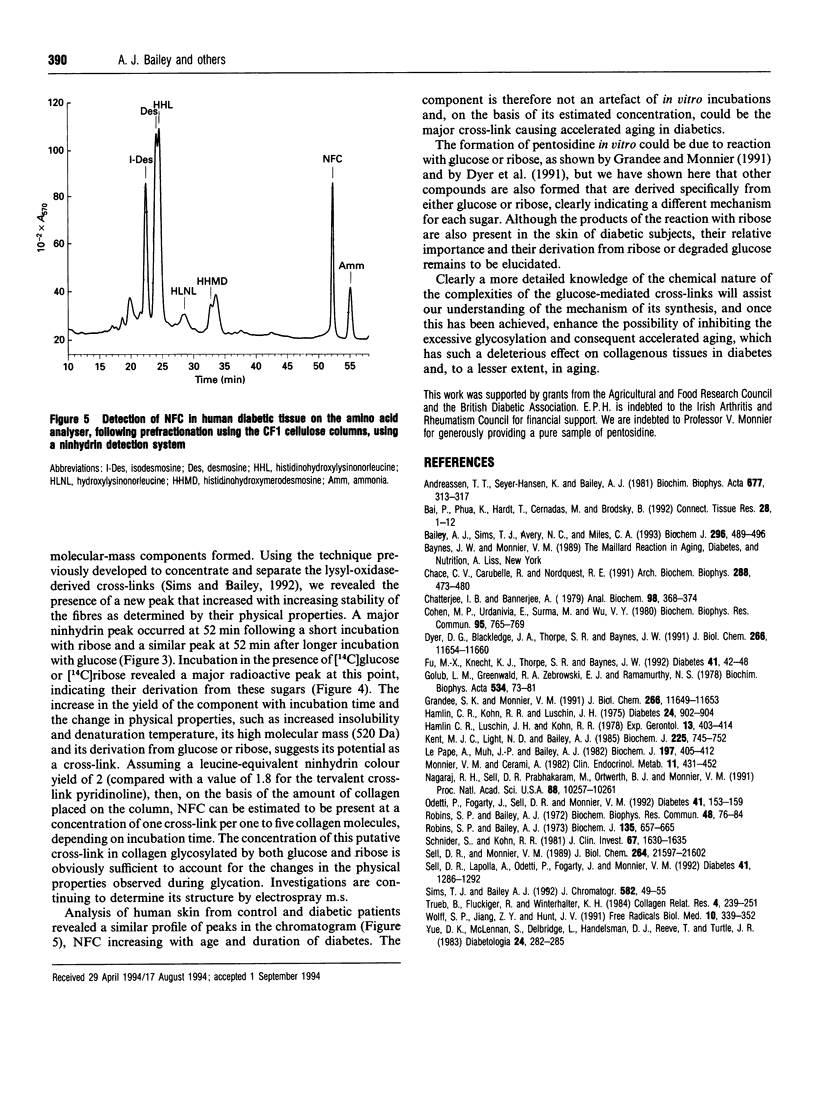
Non-enzymic glycation of collagen involves a series of complex reactions ultimately leading to the formation of intermolecular cross-links resulting in changes in its physical properties. During analysis for the fluorescent cross-link pentosidine we identified the presence of an additional component (Cmpd K) in both glucose and ribose incubations. Cmpd K was formed more quickly than pentosidine in glucose incubations and more slowly than pentosidine in ribose incubations. Cmpd K represented 45% of the total fluorescence compared with 15% for pentosidine in glucose incubations and 25% of the total fluorescence compared with 30% for pentosidine in the ribose incubations. Cmpd K is not an artefact of in vitro incubations, as it was shown to be present in dermal tissue from diabetic patients. Subsequent high-resolution h.p.l.c. analysis of glucose-incubated collagen revealed Cmpd K comprise two components (K1 and K2). Further, a similar analysis of Cmpd K from the ribose incubations revealed two different components (K3 and K4). These differences indicate alternative mechanisms for the reactions of glucose and ribose with collagen. The amounts of these fluorescent components and the pentosidine cross-link determined for both glucose and ribose glycation were found to be far too low (about one pentosidine molecules per 200 collagen molecules after 6 months incubation with glucose) to account for the extensive cross-linking responsible for the changes in physical properties, suggesting that a further additional series of cross-links are formed. We have analysed the non-fluorescent high-molecular-mass components and identified a new component that increases with time of in vitro incubation and is present in the skin of diabetic patients. This component is present in sufficient quantities (estimated at one cross-link per two collagen molecules) to account for the changes in physical properties occurring in vitro.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreassen T. T., Seyer-Hansen K., Bailey A. J. Thermal stability, mechanical properties and reducible cross-links of rat tail tendon in experimental diabetes. Biochim Biophys Acta. 1981 Oct 12;677(2):313–317. doi: 10.1016/0304-4165(81)90101-x. [DOI] [PubMed] [Google Scholar]
- Bai P., Phua K., Hardt T., Cernadas M., Brodsky B. Glycation alters collagen fibril organization. Connect Tissue Res. 1992;28(1-2):1–12. doi: 10.3109/03008209209014224. [DOI] [PubMed] [Google Scholar]
- Bailey A. J., Sims T. J., Avery N. C., Miles C. A. Chemistry of collagen cross-links: glucose-mediated covalent cross-linking of type-IV collagen in lens capsules. Biochem J. 1993 Dec 1;296(Pt 2):489–496. doi: 10.1042/bj2960489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chace K. V., Carubelli R., Nordquist R. E. The role of nonenzymatic glycosylation, transition metals, and free radicals in the formation of collagen aggregates. Arch Biochem Biophys. 1991 Aug 1;288(2):473–480. doi: 10.1016/0003-9861(91)90223-6. [DOI] [PubMed] [Google Scholar]
- Chatterjee I. B., Banerjee A. Estimation of dehydroascorbic acid in blood of diabetic patients. Anal Biochem. 1979 Oct 1;98(2):368–374. doi: 10.1016/0003-2697(79)90155-6. [DOI] [PubMed] [Google Scholar]
- Cohen M. P., Urdanivia E., Surma M., Wu V. Y. Increased glycosylation of glomerular basement membrane collagen in diabetes. Biochem Biophys Res Commun. 1980 Jul 31;95(2):765–769. doi: 10.1016/0006-291x(80)90852-9. [DOI] [PubMed] [Google Scholar]
- Dyer D. G., Blackledge J. A., Thorpe S. R., Baynes J. W. Formation of pentosidine during nonenzymatic browning of proteins by glucose. Identification of glucose and other carbohydrates as possible precursors of pentosidine in vivo. J Biol Chem. 1991 Jun 25;266(18):11654–11660. [PubMed] [Google Scholar]
- Fu M. X., Knecht K. J., Thorpe S. R., Baynes J. W. Role of oxygen in cross-linking and chemical modification of collagen by glucose. Diabetes. 1992 Oct;41 (Suppl 2):42–48. doi: 10.2337/diab.41.2.s42. [DOI] [PubMed] [Google Scholar]
- Golub L. M., Greenwald R. A., Zebrowski E. J., Ramamurthy N. S. The effect of experimental diabetes on the molecular characteristics of soluble rat-tail tendon collagen. Biochim Biophys Acta. 1978 May 24;534(1):73–81. doi: 10.1016/0005-2795(78)90477-4. [DOI] [PubMed] [Google Scholar]
- Grandhee S. K., Monnier V. M. Mechanism of formation of the Maillard protein cross-link pentosidine. Glucose, fructose, and ascorbate as pentosidine precursors. J Biol Chem. 1991 Jun 25;266(18):11649–11653. [PubMed] [Google Scholar]
- Hamlin C. R., Kohn R. R., Luschin J. H. Apparent accelerated aging of human collagen in diabetes mellitus. Diabetes. 1975 Oct;24(10):902–904. doi: 10.2337/diab.24.10.902. [DOI] [PubMed] [Google Scholar]
- Hamlin C. R., Luschin J. H., Kohn R. R. Partial characterization of the age-related stabilizing factor of post-mature human collagen--I. By the use of bacterial collagenase. Exp Gerontol. 1978;13(6):403–414. doi: 10.1016/0531-5565(78)90051-7. [DOI] [PubMed] [Google Scholar]
- Kent M. J., Light N. D., Bailey A. J. Evidence for glucose-mediated covalent cross-linking of collagen after glycosylation in vitro. Biochem J. 1985 Feb 1;225(3):745–752. doi: 10.1042/bj2250745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Pape A., Muh J. P., Bailey A. J. Characterization of N-glycosylated type I collagen in streptozotocin-induced diabetes. Biochem J. 1981 Aug 1;197(2):405–412. doi: 10.1042/bj1970405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnier V. M., Cerami A. Non-enzymatic glycosylation and browning of proteins in diabetes. Clin Endocrinol Metab. 1982 Jul;11(2):431–452. doi: 10.1016/s0300-595x(82)80023-6. [DOI] [PubMed] [Google Scholar]
- Nagaraj R. H., Sell D. R., Prabhakaram M., Ortwerth B. J., Monnier V. M. High correlation between pentosidine protein crosslinks and pigmentation implicates ascorbate oxidation in human lens senescence and cataractogenesis. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10257–10261. doi: 10.1073/pnas.88.22.10257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odetti P., Fogarty J., Sell D. R., Monnier V. M. Chromatographic quantitation of plasma and erythrocyte pentosidine in diabetic and uremic subjects. Diabetes. 1992 Feb;41(2):153–159. doi: 10.2337/diab.41.2.153. [DOI] [PubMed] [Google Scholar]
- Robins S. P., Bailey A. J. Age-related changes in collagen: the identification of reducible lysine-carbohydrate condensation products. Biochem Biophys Res Commun. 1972 Jul 11;48(1):76–84. doi: 10.1016/0006-291x(72)90346-4. [DOI] [PubMed] [Google Scholar]
- Robins S. P., Bailey A. J. The chemistry of the collagen cross-links. The characterization of fraction C, a possible artifact produced during the reduction of collagen fibres with borohydride. Biochem J. 1973 Dec;135(4):657–665. doi: 10.1042/bj1350657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnider S. L., Kohn R. R. Effects of age and diabetes mellitus on the solubility and nonenzymatic glucosylation of human skin collagen. J Clin Invest. 1981 Jun;67(6):1630–1635. doi: 10.1172/JCI110198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sell D. R., Lapolla A., Odetti P., Fogarty J., Monnier V. M. Pentosidine formation in skin correlates with severity of complications in individuals with long-standing IDDM. Diabetes. 1992 Oct;41(10):1286–1292. doi: 10.2337/diab.41.10.1286. [DOI] [PubMed] [Google Scholar]
- Sell D. R., Monnier V. M. Structure elucidation of a senescence cross-link from human extracellular matrix. Implication of pentoses in the aging process. J Biol Chem. 1989 Dec 25;264(36):21597–21602. [PubMed] [Google Scholar]
- Sims T. J., Bailey A. J. Quantitative analysis of collagen and elastin cross-links using a single-column system. J Chromatogr. 1992 Nov 6;582(1-2):49–55. doi: 10.1016/0378-4347(92)80301-6. [DOI] [PubMed] [Google Scholar]
- Trüeb B., Flückiger R., Winterhalter K. H. Nonenzymatic glycosylation of basement membrane collagen in diabetes mellitus. Coll Relat Res. 1984 Aug;4(4):239–251. doi: 10.1016/s0174-173x(84)80032-1. [DOI] [PubMed] [Google Scholar]
- Wolff S. P., Jiang Z. Y., Hunt J. V. Protein glycation and oxidative stress in diabetes mellitus and ageing. Free Radic Biol Med. 1991;10(5):339–352. doi: 10.1016/0891-5849(91)90040-a. [DOI] [PubMed] [Google Scholar]
- Yue D. K., McLennan S., Delbridge L., Handelsman D. J., Reeve T., Turtle J. R. The thermal stability of collagen in diabetic rats: correlation with severity of diabetes and non-enzymatic glycosylation. Diabetologia. 1983 Apr;24(4):282–285. doi: 10.1007/BF00282714. [DOI] [PubMed] [Google Scholar]


