ABSTRACT
Metabolic disease is a worldwide epidemic that has become a public health problem. Gut microbiota is considered to be one of the important factors that maintain human health by regulating host metabolism. As an abundant bacterium in the host gut, A. muciniphila regulates metabolic and immune functions, and protects gut health. Multiple studies have indicated that alterations in the abundance of A. muciniphila are associated with various diseases, including intestinal inflammatory diseases, obesity, type 2 diabetes mellitus, and even parasitic diseases. Beneficial effects were observed not only in live A. muciniphila, but also in pasteurized A. muciniphila, A. muciniphila-derived extracellular vesicles, outer membrane, and secreted proteins. Although numerous studies have only proven the simple correlation between multiple diseases and A. muciniphila, an increasing number of studies in animal models and preclinical models have demonstrated that the beneficial impacts shifted from correlations to in-depth mechanisms. In this review, we provide a comprehensive view of the beneficial effects of A. muciniphila on different diseases and summarize the potential mechanisms of action of A. muciniphila in the treatment of diseases. We provide a comprehensive understanding of A. muciniphila for improving host health and discuss the perspectives of A. muciniphila in the future studies.
KEYWORDS: Gut microbiota, Akkermansia muciniphila, metabolic diseases, host health, mechanisms
GRAPHICAL ABSTRACTS
Introduction
The gut microbiota comprises a diverse and dynamic population of microbial species, including bacteria, fungi, viruses, and protists. The role of the gut microbiota in human health is crucial; consequently, alterations in the abundance and composition of the gut microbiota are thought to be related to multiple diseases. Numerous studies have demonstrated a strong association between certain microorganisms and diseases such as obesity, inflammatory diseases, type 2 diabetes mellitus (T2DM), cancer, and neurodegenerative diseases [1,2]. Therefore, increasing attention has been paid to the treatment of metabolic diseases with the gut microbiota using different approaches, including probiotics, prebiotics, and fecal microbiota transplantation [3,4]. Probiotics are beneficial microorganisms that play a crucial role in regulating gut microbiota and enhancing host immunity [5]. Traditional probiotics are often derived from fermented foods, with lactic acid as the main type. However, traditional probiotics suffer from quality issues such as contamination and an insufficient number of live bacteria. Among the gut microbiota observed, A. muciniphila a promising probiotic that has been widely used in different models of multiple diseases in recent years.
A. muciniphila is an abundant symbiotic bacterium in the intestinal tract that efficiently utilizes mucin as its sole source of carbon, nitrogen, and energy, thereby providing energy for epithelial cells [6]. Since its discovery, A. muciniphila has gradually gained attention and is now considered a promising next-generation probiotic. It has been suggested that changes in the levels of A. muciniphila are involved in various diseases, such as obesity, inflammatory diseases, diabetes mellitus, and parasitic diseases [2,7–10]. Moreover, some researchers have reported that the outer membrane proteins of A. muciniphila (Amuc_1100) and Akkermansia muciniphila-derived extracellular vesicles (AmEVs) play a role in regulating and improving intestine function [11–13].
Although there are many reviews about A. muciniphila, the summary of its regulation on disease mechanisms is still not sufficiently comprehensive and in-depth. In this review, we provide a comprehensive understanding of A. muciniphila in improving host health and discuss perspectives of A. muciniphila in the future study. We also elaborated the correlation between A. muciniphila and host immunity, which has been less discussed in other review articles. Additionally, we have summarized the relationship between A. muciniphila and pathogen infection, which has been scarcely reported in other review articles. Furthermore, the primary mechanisms underlying the beneficial effects of A. muciniphila on various diseases are discussed. Finally, we summarized the literature on the relationship between A. muciniphila and diseases reported in the past three years. By reviewing the numerous literatures, we summarize the important progress of A. muciniphila in improving host health and discuss the perspectives of A. muciniphila in future studies. This review provides insights into the roles of A. muciniphila as a probiotic in different diseases, thereby offering a blueprint to facilitate clinical therapeutic application.
Method
The authors conducted a systematic review of literature to evaluate the relationship between A. muciniphila and metabolic disease, intestinal inflammatory diseases and pathogen infection. The studies in this review were searched from the following data sources: Web of Science, PubMed and Medline with the period September 2004- June 2024. In the literature search process, we did not use any restrictions or filters. The references from chosen studies were manually scanned to identify additional related studies [14,15]. Furthermore, in order to create a high-sensitivity strategy, the search terms “Akkermansia muciniphila”, “inflammatory bowel disease”, “obesity”, “type 2 diabetes mellitus”, and “parasite” were utilized as both free-text and topic headings. The data obtained from these studies were author, publication year, study types, sample types, analysis method, disease model, dietary intervention and control, and result characteristics.
Major properties of A. muciniphila
A. muciniphila is an oval-shaped, non-motile bacterium that was first isolated and described in 2004 [16–18]. Most studies have focused on the type strain A. muciniphila MucT (ATCC BAA-835). A. muciniphila belongs to the Verrucomicrobia phylum and is representative of the Verrucomicrobiota in gastrointestinal samples that can be cultured [19]. Moreover, A. muciniphila was originally classified as a strict gram-negative anaerobic bacterium that is widely distributed in the human and animal intestines, with the largest number in the cecum. However, some studies have reported that A. muciniphila can tolerate a small amount of oxygen, and more than 90% of A. muciniphila can survive when exposed to air for one hour [17,18]. The whole genomes of A. muciniphila MucT and Akkermansia glycaniphila PytT were sequenced, and the results showed that numerous enzymes encoded by genes of A. muciniphila can degrade oligosaccharide chains, such as glycosidase, sulfatase, and sialidase [20,21]. These enzymes release glycans to promote the growth of mucin-degrading bacteria and affect the abundance of gut microbiota [22].
A. muciniphila is found in breast milk and in different parts of the digestive tract, such as the small intestine, large intestine, pancreas, and biliary system [19]. In healthy adults, the number of bacteria in feces ranges from 109 to 1010 CFU/g, and the amount of A. muciniphila is approximately 106 to 108 CFU/g, accounting for 1% to 4% of the total number of bacteria in the intestinal tract [23]. Interestingly, A. muciniphila was found in the human intestine in early life, and its abundance reached adult levels within one year [23,24]. A. muciniphila mainly resides in the mucus layer of the intestines and has a significant impact on enhancing the intestinal barrier, producing mucus, and maintaining mucus layer thickness [25]. It has been suggested that A. muciniphila degrades mucin to produce monosaccharides, oligosaccharides, and chain fatty acids, which can serve as energy sources for the host, as well as other bacteria [26,27]. A recent systematic literature review concluded that A. muciniphila grows most efficiently in an environment containing mucin, which is continuously produced by goblet cells present in gastrointestinal tissue [27]. Therefore, the colonization of A. muciniphila does not rely solely on diet and possesses distinct advantages for survival. Moreover, several studies have found that A. muciniphila can survive in the digestive systems of humans and mice treated with antibiotics, indicating its resistance to antibiotics [28,29].
The connection between A. muciniphila and host immunity
The host and its gut microbiota co-evolve into a strongly mutualistic relationship, where the gut microbiota plays an essential role in preserving host homeostasis [30,31]. The gut microbiota has a crucial impact on the training and development of key components of the innate and adaptive immune systems in the host. In addition to their role in regulating infection and the spread of commensal organisms, microbiome-immune interactions are involved in multiple diseases [31]. Despite the increasing clarity of the relationship between gut microbiota and human health issues, specifically with regard to the influence on the immune system, there is still a notable deficiency in understanding the precise molecular factors that control and adjust immune balance, as well as the mechanisms by which they operate [32].
The occurrence of intestinal IgG antibody responses is believed to be limited to instances of mucosal barrier disruption or as a reaction to enteric pathogens and specific pathogens that invade the intestinal barrier [33]. It has been shown that T cells are significantly activated when gut microbiota is present, utilizing the anti-IgG2b and IgG3 independent pathways, which primarily rely on the presence of toll-like receptor (TLR) [34,35]. Ansaldo et al. reported that A. muciniphila triggers the production of IgG1 antibodies and elicits T-cell responses specific to the antigen in mice [36]. Moreover, in a gnotobiotic environment, T cell reactions specifically to A. muciniphila are limited to T follicular helper cells and do not prompt other T helper responses or movement toward the lamina propria. Kuczma et al. reported that microbial antigens from A. muciniphila could induce anergy and promote the transformation of naive CD4+CD44 Foxp3 − T (Tn) cells to the Treg lineage [37].
Finding showed that NF-κB in intestinal epithelial cells (IECs) can be activated by A. muciniphila without the involvement of TLRs and NOD receptors [38]. However, activation of Toll-like receptor 2 (TLR2) and its downstream NF-κB pathway is initiated by Amuc_1100 [39]. By modulating TLR2-activated γδT17 cells and macrophage polarization, A. muciniphila effectively prevented nonalcoholic steatohepatitis in mice [40]. It was reported that threonyl-tRNA synthetase (AmTARS) by A. muciniphila induces the polarization of M2 macrophages and manages the generation of IL-10 through its distinct, evolutionarily acquired regions, which enable specific interactions with TLR2 [30]. Activation of the MAPK and PI3K/AKT signaling pathways through this interaction results in the convergence of cAMP response element-binding protein (CREB), thereby facilitating the efficient production of IL-10 and inhibiting the central inflammatory mediator NF-kB. A. muciniphila performs immune functions, and there are undoubtedly additional mechanisms involved in the interactions between the host and A. muciniphila that remain unexplored.
Correlation between A. muciniphila and diseases
With regard to A. muciniphila, increasing attention has been paid to the treatment of multiple diseases using it as a prebiotic. Changes in the abundance of A. muciniphila have been found to be associated with metabolic disorders, such as T2DM, obesity, nonalcoholic fatty liver disease (NAFLD), and cardiovascular diseases [41–45]. Among these disorders, the connection between A. muciniphila and obesity has been extensively studied. Furthermore, apart from the alteration in the levels of A. muciniphila associated with metabolic disorders, the positive impacts of A. muciniphila have also been demonstrated in relation to immune-related conditions, such as ulcerative colitis (UC) and Crohn’s disease (CD) [46–48]. In addition to these health issues, the correlation between A. muciniphila and neurological diseases as well as cancer has also been studied [49,50]. Interestingly, in recent years, numerous studies have indicated that the levels of A. muciniphila was change after parasite infection [51–54], suggesting a promising approach to treat parasitic diseases.
The production and secretion of the intestinal mucus layer are carried out by goblet cells, and then cover on the IECs [55]. A. muciniphila exists in the mucus layer and can degrade intestinal mucus to produce crucial metabolites short-chain fatty acids (SCFAs). The relationship between intestinal diseases and A. muciniphila depends on healthy mucus layer and mucosal immune response. It has been suggested that A. muciniphila can ameliorate metabolic disorders by reestablishing the thickness of mucus layer and expressing antimicrobial peptides in mice [41]. The mechanism by which A. muciniphila increase the thickness of mucus layer remains unclear. The possible mechanism is as follow: on one hand, A. muciniphila produces numerous SCFAs by degrading mucins, which serve as a valuable source of energy for epithelial cells involved in the synthesis and secretion of mucins; on the other hand, the degradation of mucins can stimulate goblet cells to generate more new mucins, and the mucins can also promote the development of A. muciniphila; a positive feedback loop is generated, and the virtuous cycle can constantly updates the mucus layer, which provides a protective effect on intestinal epithelial cells.
The disturbance of gut balance in metabolic disorders is strongly connected to the function of the intestinal immune system [56]. The intestine serves as a physical barrier to keep harmful substances from entering the body. It was found that A. muciniphila can enhance gut barrier function by restoring the thickness of mucus layer in mice and increasing the intestinal expression of the antimicrobial peptide Reg3g, both of which are altered during obesity and metabolic disorders [41]. Moreover, it was demonstrated that extracellular vesicles from A. muciniphila reproduce some of the positive effects of the bacteria and can also decrease gut permeability by controlling tight junctions in mice. In addition to the alterations in gut permeability associated with metabolic disorders, studies have also shown the positive impact of A. muciniphila on gut barrier function during intestinal inflammation [57]. Numerous studies have demonstrated that A. muciniphila protect the host from diseases by enhancing the integrity of the intestinal barrier and modulating the immune system through various mechanisms such as thickening the mucus layer, improving epithelial connectivity, and altering mucus composition [7]. Several beneficial effects of A. muciniphila have been observed, along with a notably increased efficacy when utilizing pasteurized A. muciniphila. Therefore, more research is needed to explore the beneficial effect of A. muciniphila and pasteurized A. muciniphila in the different diseases.
Potential function of A. muciniphila in intestinal inflammatory diseases
The pathogenesis of inflammatory bowel disease (IBD) is not fully understood, but it is a chronic relapsing immune-mediated disease [58,59]. Metabolic and gastrointestinal disorders are often caused by disruption of intestinal homeostasis and intestinal barrier integrity [60]. The gut microbiota strongly influences the intestinal barrier function and intestinal homeostasis. The dextran sulfate sodium (DSS)-induced colitis model has been extensively used to investigate the interaction between gut microbiota and IBD. There are also some studies using Trinitro-benzenesulfonic acid (TNBS)-induced colitis model to evaluate the beneficial effect of A. muciniphila on colitis [61]. Although the probiotic properties of A. muciniphila have been widely recognized in metabolic diseases, its therapeutic potential in intestinal inflammatory diseases remains controversial [62]. A. muciniphila is increasingly being used as a probiotic to treat IBD in animal and preclinical models [46,63–65]. Several studies have demonstrated that A. muciniphila and its compounds exert protective effects against intestinal inflammatory diseases in different models [66–69]. A. muciniphila is believed to be able to regenerate the mucus layer and maintain gut integrity [70]. It has been suggested that the levels of A. muciniphila was significantly lower in patients with ulcerative colitis compared with healthy subjects [71–73].Bian et al. reported the protective effects of A. muciniphila in DSS-induced colitis mouse models and provided an explanation for the underlying mechanisms. Several potential mechanisms have been proposed, including (1) colonic mucosal barrier damage has been improved (2) regulation of the inflammatory response, (3) rebuilding of the gut microbiota (4) modulation of metabolic function [74]. According to Qu et al., the abundance of A. muciniphila was lower in the feces of patients with ulcerative colitis (UC) than in healthy individuals. The symptoms of DSS-induced acute colitis were significantly ameliorated by supplementation A. muciniphila [63,65]. Supplementation with A. muciniphila resulted in a decrease in inflammatory cell infiltration and an increase in the number of goblet cells, as well as the expression of MUC2 and MUC3. Moreover, NLRP3 in the colon tissues of individuals with UC was significantly upregulated, and NLRP3 was upregulated by supplementation A. muciniphila, suggesting that the positive effect A. muciniphila against acute colitis may depend on the activation of NLRP3. Wang et al. found that A. muciniphila or membrane proteins (Amuc_1100) derived from A. muciniphila can improve DSS-induced colitis in mice by regulating CD8+ T cells [75]. Histological damage in the proximal colon improved, and proinflammatory cytokines, including IFN-γ, IL-1β, and TNF-α, were significantly downregulated. Moreover, tumor formation was delayed and tumor number and size were decreased by supplementation with A. muciniphila or Amuc_1100. Furthermore, some components and secretions are also associated with the occurrence of intestinal inflammation [76]. The composition of AmEVs from A. muciniphila was significantly reduced in the feces with DSS-induced IBD. DSS-induced IBD phenotypes were alleviated by oral administration of AmEVs, including body weight loss, colon length reduction, and inflammatory cell infiltration in the colon wall.
However, some studies have suggested that A. muciniphila can potentially contribute to the development of intestinal inflammatory [77–80]. It was found that the levels of A. muciniphila were increased in DSS-induced IBD mice, and its relative levels were positively correlated with the severity of histopathological damage and inflammation in the colon. In addition to the DSS-induced colitis model, A. muciniphila promoted the progression of intestinal inflammation in other mouse models. Ganesh et al. reported that the symptoms of colitis were aggravated by oral administration A. muciniphila in mice infected with Salmonella typhimurium [80]. Seregin et al. found that the severity of colitis in IL10−/− mice was increased by oral administration A. muciniphila [81]. Another study conducted by Baxter et al. reported that mice with colorectal cancer (CRC) received fecal microbiota from CRC patients exhibited a direct relationship between the presence of A. muciniphila and an elevated tumor burden [82]. Although these studies suggested that A. muciniphila may act as an opportunistic pathogen and contribute to the development of colitis, the reasons and mechanisms for its pathogenic effects remain unclear. However, most studies have demonstrated that A. muciniphila has beneficial effects on intestinal diseases. Finally, we summarized the research on the correlation between A. muciniphila and IBD in the last three years, as shown in Table 1.
Table 1.
Overview of the correlation between A. muciniphila and IBD in the past three years.
| Study types | Model | Subject | Sample types | Analysis method | Result characteristics | Reference |
|---|---|---|---|---|---|---|
| Animal | DSS‑induced colitis | 7-weeks old C57BL/6J mice | Feces | 16S rRNA | Stable colonization of live A. muciniphila is essential for its anti-inflammatory function. | Wang et al. 2023 [67] |
| Animal | DSS‑induced colitis | 6-weeks old male C57BL/6J mice | Colon contents | 16S rRNA | A. muciniphila alleviates the symptoms of colitis in mice. | Xue et al. 2023 [68] |
| Animal | DSS‑induced colitis | CREBH-KO mice | Ileum and colon tissues | qRT‑PCR | A. muciniphila ameliorates intestinal inflammatory in DSS‑induced colitis mice. | Wade et al. 2023 [66] |
| Animal | TNBS-induced IBD | 6-8 weeks old male BALB/c mice | Feces | 16S rRNA | The abundance of A. muciniphila was decreased in the TNBS-treated mice. | Chang et al. 2022 [46] |
| Animal and human | DSS‑induced colitis and UC | TLR4-knockout C57BL/6 mice and UC patients | Feces | 16S rRNA | The abundance of A. muciniphila was negatively to colitis risk. | Liu et al. 2022 [47] |
| Human | UC and Crohn’s diseases | UC and Crohn patients | Feces and blood | RT-PCR | Relative abundance of A. muciniphila was significantly lower in IBD. | Sezgin et al. 2022 [73] |
| Human | UC and IBS | UC and IBS patients | Feces | qPCR | The level of A. muciniphila reduced in patients with UC from HPRs. | Dorofeyev et al. 2022 [72] |
| Animal | TNBS-induced IBD | 6–8 weeks old male BALB/c mice | Feces | 16S rRNA | The abundance of A. muciniphila was degraded in the TNBS-treated mice but elevated in the PAW-drinking mice. | Chang et al. 2022 [46] |
| Animal | DSS‑induced colitis | 5-6 weeks old male C57BL/6 mice | Feces | 16S rRNA | Amuc_2109 secreted by A. muciniphila reshaped the intestinal microbiota. | Qian et al. 2022 [69] |
| Animal and human | DSS‑induced colitis and UC | 6-weeks old male C57BL/6 mice and UC patients | Feces | 16S rRNA | The level of A. muciniphila was decreased UC patients and A. muciniphila showed the protective effect in colitis. | Qu et al. 2021[63] |
| Animal | DSS‑induced colitis | C57BL/6 mice | Feces | 16S rRNA | A. muciniphila showed a positive effect on UC. | Liu et al. 2021 [65] |
| Animal | DSS‑induced colitis | 6-weeks old male Wistar/ST rats | Feces | 16S metagenomics | Antibacterial (OPS-2071) increased the occupancy of A. muciniphila in the DSS-treated rats. | Nakashima et al. 2021 [120] |
| Animal | DSS-induced UC | 6-8 weeks old male C57BL/6 mice | Feces | 16S rRNA | The level of A. muciniphila was increased after metformin treatment. | Ke et al. 2021 [118] |
| Animal | DSS‑induced colitis | GPR43-knockout mice | Feces | 16S rRNA | The level of A. muciniphila was increased after lithium carbonate treatment. | Huang et al. 2021 [177] |
Note: ulcerative colitis (UC), colitis-associated colorectal cancer (CAC), irritable bowel syndrome (IBS), colorectal cancer (CRC), highly polluted with PM2.5 (HPRs), 2,4,6-trinitrobenzene sulphonic acid (TNBS), Plasmon-activated water (PAW).
Currently, the positive mechanism of A. muciniphila on intestinal inflammatory diseases can be explained roughly from the following three aspects (Figure 1): (1) enhance the physical barrier of intestinal mucosal barrier; The physical barrier consists of epithelial cells that are connected by tight junctions and is safeguarded by mucous layer [83]; The integrity of the intestinal mucosal barrier can be maintained by A. muciniphila, which can improve the production of tight junction proteins and reduce intestinal permeability; Supplement with A. muciniphila can enhance the amount of goblet cells and the secretion of mucins, thereby normalize the thickness of the mucus layer [25,84]; Moreover, A. muciniphila can effectively enhance the amount of Paneth cells to restore the secretion of antimicrobial peptides to normal levels, thus playing a protective role in the intestinal mucosa [85]. (2) enhance the immune barrier of intestinal mucosal barrier; A. muciniphila can promote the differentiation of initial CD4+ T cells into peripheral Treg cells and downregulate the expression of proinflammatory cytokines [86]; A. muciniphila enhance the activation of AMPK signaling pathway while inhibiting the NF-κB signaling pathway by stimulating TLR2, maintain the balance of intestinal mucosal immune function [87]; Furthermore, pasteurized A. muciniphila or Amuc_1100 show the ability to reduce the infiltrating macrophages and cytotoxic T lymphocytes [75]. (3) Stable colonization of A. muciniphila results in the proliferation of neighboring bacteria and alters their gene expression [67]; A. muciniphila can specifically degrade mucins and oligosaccharides to produce metabolite SCFAs, such as propionate and butyrate [59]; SCFAs have the ability to enhance the differentiation of Treg cells and alter gut microbiota composition; butyrate is considered to alleviate intestinal diseases by inhibiting histone deacetylase and activating G protein-coupled receptors (GPR) to enhance protective immunity and improve the intestinal barrier [88,89]; Moreover, butyrate can also enhance the defense function of the intestinal mucosa by upregulating the expression of tight junction proteins and intestinal mucosal epithelial mucus [90]. Consequently, as a probiotic for intestinal inflammatory diseases, A. muciniphila maintains the balance of gut microbiota by regulating inflammatory factors and protects the integrity of the intestinal mucosal barrier structure and function at different levels to reduce intestinal damage caused by intestinal inflammatory diseases.
Figure 1.
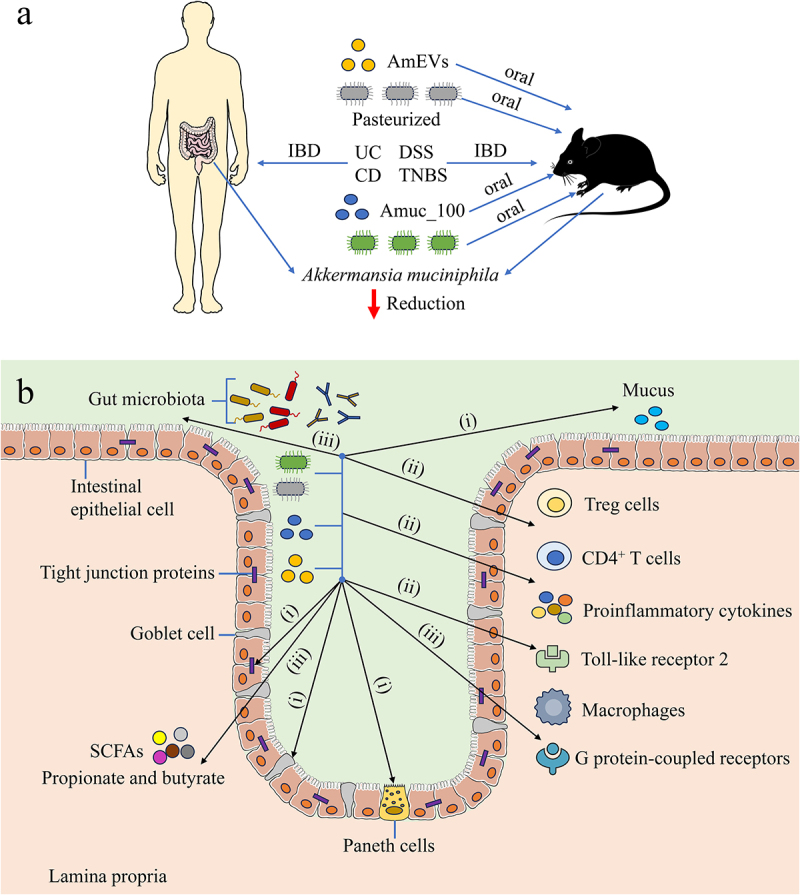
The role of A. muciniphila in intestinal inflammatory diseases. (a) The correlation between A. muciniphila and IBD in human and mice (b)The possible mechanisms of A. muciniphila regulating intestinal inflammatory diseases in host: (i) A. muciniphila improves the physical barrier of intestinal mucosal barrier (ii) A. muciniphila improves the immune barrier of intestinal mucosal barrier (iii) Stable colonization of A. muciniphila results in the proliferation of neighboring bacterium and alters their gene expression. IBD: inflammatory bowel disease, UC: ulcerative colitis AmEVs: Akkermansia muciniphila-derived extracellular vesicles, CD: Crohn’s disease, DSS: dextran sulfate sodium, TNBS: 2,4,6- trinitrobenzene sulfonic acid, SCFAs: short-chain fatty acids.
The function of A. muciniphila in obesity
In recent years, obesity and its associated metabolic disorders have gradually become one of the world’s most serious health problem [91]. Obesity is associated with gut dysbiosis, which refers to an imbalance between energy intake and expenditure, which promotes the proliferation of pathogenic bacteria. Currently, it is widely accepted that the gut microbiota has a significant impact on whole-body metabolism by affecting the energy balance [92–96]. It has been suggested that the gut microbiota plays a crucial role in the development of obesity-related disorders [97–100]. Thus, the novel therapy of administering probiotics or increasing beneficial bacteria through drugs or food has received significant attention for treating obesity [101–105]. Recently, A. muciniphila was introduced as a promising probiotic to regulate energy homeostasis [106,107]. Several studies have suggested that A. muciniphila is a promising candidate for preventing or treating metabolic disorders associated with obesity [41,108–111].
High-fat diet (HFD) is the most significant cause of obesity and the most studied environmental factor in obesity. HFD can reduce the expression of intestinal tight junction proteins and increase intestinal permeability, thereby increasing the entry of endotoxins produced by intestinal gram-negative bacteria into the blood circulation, leading to metabolic endotoxemia and a long-term inflammatory response, which is crucial for the occurrence and development of obesity. The levels of A. muciniphila have been suggested to be negatively associated with obesity [112]. Vitro studies have found that A. muciniphila attaches to the intestinal epithelium and enhances the integrity of the enterocyte monolayer, indicating its potential to reinforce the integrity of the intestinal barrier in people with obesity [60,113]. It was found that metabolic toxicity effects in HFD-fed mice could be counteracted by administration of live A. muciniphila; notably, the beneficial effect was only observed when live A. muciniphila was used [85]. In another study, supplementation with A. muciniphila was found to reduce the levels of lipopolysaccharide (LPS) in the plasma by enhancing intestinal barrier function, thus reversing obesity and glucose metabolism disorders in HFD-fed mice. However, it is important to note that heat-inactivated A. muciniphila does not exhibit the same protective effect [41]. Interestingly, pasteurized A. muciniphila enhances its ability to alleviate fat mass development, insulin resistance, and dyslipidemia in HFD-fed mice [11]. Yang et al. found that obesity was correlated with an increased risk of neurodevelopmental disorders during the early stages of life [114]. HFD in early life can impair learning and memory dependent on the hypothalamus in mice, affecting neurodevelopment and cognitive function, whereas the levels of A. muciniphila were significantly reduced. Additionally, Ashrafian et al. investigated the positive effects of live and pasteurized A. muciniphila and its EVs on HFD-induced obesity [115]. Pasteurized A. muciniphila and EVs exhibited significant beneficial effects on obesity characterized by a reduction in body weight, blood biochemical parameters, and food intake. The development of fatty liver in HFD-fed mice was prevented by A. muciniphila through the modulation of lipid metabolism and inflammation. Furthermore, administration of A. muciniphila prevented the intestinal barrier disruption, inflammation, and gut dysbiosis in HFD-fed mice by restoring the microbial population balance. Similar to animal experiments, the levels of A. muciniphila were also reduced in overweight or obese people. Depommier et al. conducted a study to explore how pasteurized A. muciniphila affects overall energy metabolism while feeding on a high-fat diet [116]. These findings demonstrated that the increase in body weight and fat mass caused by a high-fat diet was alleviated, and food energy efficiency was decreased by supplementation with A. muciniphila. Remely et al. recruited 33 obese individuals receiving a dietary intervention and detected the fecal microbiota by real-time quantitative PCR using the 16S rRNA method [117]. The result revealed that there was a notable decline in the levels of A. muciniphila after weight reduction. A study on diet intervention for obesity and diabetes showed that the levels of A. muciniphila were negatively associated with fasting blood glucose, waist-to-hip ratio, and adipocyte diameter in overweight and obese individuals [26]. Moreover, participants with high A. muciniphila levels exhibited a healthier metabolic state. Despite the positive effects of A. muciniphila demonstrated in animal models, the impact of A. muciniphila on humans has yet to be defined, and its applicability in clinical settings needs to be evaluated [118–120]. Finally, we summarized the research on the relationship between A. muciniphila and obesity in the last three years, as shown in Table 2.
Table 2.
Summary the research on the role of A. muciniphila in obesity and diabetes mellitus in the past three years.
| Type of study | Diseases condition | Sample type | Analysis method | Result characteristics | Reference |
|---|---|---|---|---|---|
| Animal | HFD induced obesity | Feces | 16S rRNA | The abundance of A. muciniphila in mice treated with D3 increased about 100 times, compared with the HFD mice. | Li et al. 2023 [97] |
| Human | Obesity | Feces | 16S rRNA | The abundance of A. muciniphila in the treatment group was increased. | Cao et al. 2023 [101] |
| Animal | Obesity | Feces | 16S rRNA | The abundance of A. muciniphila was increased in mice fed milk and the FMT group from the mice fed milk. | Okamura et al. 2023 [102] |
| Animal | HFD induced obesity | Feces | 16S rRNA | The level of A. muciniphila was elevated in obese mice after administration of E. cristatum. | Wang et al. 2023 [105] |
| Animal | HFD induced obesity | Feces, feces contents | 16S rRNA | The level of A. muciniphila was increased after IX treatment in obese mice. | Watanabe et al. 2023 [94] |
| Animal | HFD induced obesity | Feces | 16S rRNA | The mice challenged with HFD and treated with VCM had large amounts of A. muciniphila in their ileum and cecum. | Sonomoto et al. 2023 [95] |
| Animal | Diet-induced obesity | Feces | 16S rRNA | A. muciniphila showed significant improvement in body weight, total fat weight. | Kumar et al. 2022 [106] |
| Animal | HFHS-induced obesity | Feces | 16S rRNA qPCR |
The level of A. muciniphila was significantly increased in obese mice treated with AG and GSE. | Watanabe et al. 2022 [99] |
| Animal | HFD induced obesity | Feces | 16S rRNA RT-qPCR |
A. muciniphila controls weight gain and increases the Firmicutes/Bacteroidetes (F/B) ratio. | Lin et al. 2022 [171] |
| Animal | HFD induced obesity | Feces | 16S rRNA | A. muciniphila were significantly increased after a combined supplement of three probiotic strains. | Liao et al. 2022 [103] |
| Animal | HFD induced obesity | Feces | 16S rRNA | The abundance of A. muciniphila was increased in HFD mice after EPA treatment. | Pal et al. 2022 [184] |
| Animal | HFD induced obesity | Feces | 16S rRNA | Supplemented with A. muciniphila prevented HFD-induced body weight gain, fat mass gain. | Acharya et al. 2022 [107] |
| Animal | HFD induced obesity | Feces | 16S rRNA | The relative abundance of resident A. muciniphila was increased in HFD mice after PMGs treatment. | Pruss et al. 2021 [100] |
| Human | Obesity | Feces | Metagenomic sequencing | After weight loss, the abundance of A. muciniphila was significantly increased. | Alili et al. 2021 [98] |
| Animal | HFD induced obesity | Feces | 16S rRNA RT-PCR |
A. muciniphila could avoid HFD induced dysbiosis by decreasing obesity-related pathobiont bacteria and increasing health-related gut microbiota. | Ashrafian et al. 2021 [115] |
| Animal | HFD induced obesity | Feces | 16S rRNA | With LA5 administration, A. muciniphila in the colon were more than 2,000 folds higher than the regular diet mice. |
Ondee et al. 2021 [104] |
| Animal | HFD induced obesity | Feces | 16S rRNA | Supplementation with betaine increase the level of A. muciniphila in HFD mice. | Du et al. 2021 [96] |
| Human | Obesity | Feces | Metagenomic sequencing | A. muciniphila was significantly enriched in lean individuals, and its abundance increased during dieting. | Jie et al. 2021 [92] |
| Animal | HFD induced obesity | Intestinal tissues | RT-PCR | Live and pasteurized forms of A. muciniphila improved the HFD-induced obesity and metabolic dysregulation in mice. | Choi et al. 2021 [111] |
| Human | Obesity | Feces | 16S rRNA | The abundance of A. muciniphila was enhanced in the postop group. | Shi et al. 2021 [93] |
| Animal | T2DM | Intestines sample | 16S rRNA | Intestinal health of TA zebrafish was improved with pasteurized A. muciniphila. | Qu et al. 2023 [133] |
| Animal | T2DM | Feces | 16S rRNA RT-qPCR |
Metformin led to a significant increase in the abundance of A. muciniphila in mice. | Ye et al. 2023 [132] |
| Animal | Diabetes | Feces | 16S rRNA | Compared to the control group, treatment with A. muciniphila significantly increased serum insulin and GLP-1 level. |
Wang et al. 2023 [131] |
| Human | T2DM | Feces | qPCR | In T2D patients with high HOMA-IR and BMI, there was a low abundance of A. muciniphila. | Pai et al. 2022 [128] |
| Human | T2DM | Feces | qPCR | The abundance of A. muciniphila was decreased in patients with type1 and increased in type2 diabetes. | Demirci et al. 2022 [127] |
| Human | T2DM | Feces | Metagenomic sequencing | The abundance of A. muciniphila significantly decreases in lean individuals with T2D than without T2D. | Zhang et al. 2021 [135] |
| Human | Diabetes | Feces | RT-PCR | Compared with control group, A. muciniphila was significantly lower in diabetic. | Tabasi et al. 2021 [129] |
| Animal | T2DM | Colonic contents | 16S rRNA | The DFs significantly improved the relative abundance of A. muciniphila on diabetic mice. | Li et al. 2021 [124] |
Note: Faecal microbiota transplantation (FMT), Eicosapentaenoic acid (EPA), Lactobacillus acidophilus 5 (LA5), Capsanthin (CAP), High-fat diet (HFD), high-fat, high-sucrose (HFHS), Zebrafish with combined T2DM and Alzheimer’s disease (TA zebrafish), Scutellaria baicalensis (SB), arctigenin (AG), burdock sprout extract (GSE), vancomycin (VCM), Eurotium cristatum (E. cristatum), Isoxanthohumol (IX), porcine mucin glycans (PMGs), dietary fibers (DFs).
Currently, a complete understanding of how A. muciniphila regulates obesity is lacking. The possible mechanism by A. muciniphila affects obesity can be explained roughly by the following aspects (Figure 2): (1) A. muciniphila can enhance thermogenesis and the secretion of glucagon-like peptide-1 (GLP-1) while diminishing the expression of proteins associated with adipose cell differentiation and the gene expression of glucose and fructose transporters in the jejunum. (2) A. muciniphila can increase the levels of endocannabinoids in the ileum, which have functions in controlling inflammation, intestinal barrier, and intestinal peptide secretion. (3) A. muciniphila can reduce plasma lipopolysaccharide levels by enhancing the intestinal barrier function, thereby reversing obesity and glucose metabolism disorders. (4) A. muciniphila can reduce the energy efficiency of food, total cholesterol in plasma, and the expression of carbohydrate transport proteins and increase energy excretion in feces. The results from the above literature indicated that A. muciniphila may be a crucial factor in obesity-related diseases, and these results also provided a basis for the development of A. muciniphila to prevent or treat obesity.
Figure 2.
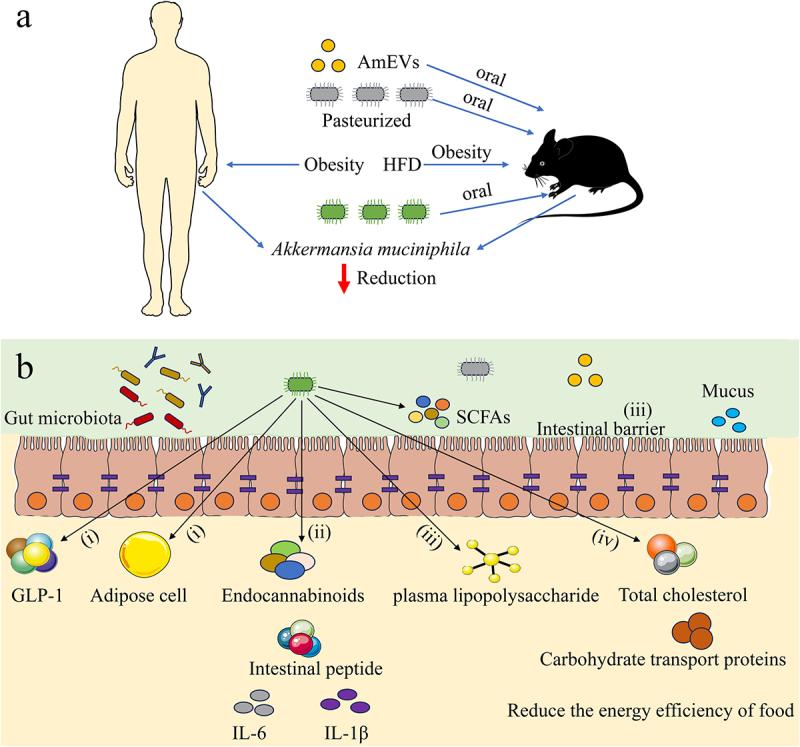
The role of A. muciniphila in obesity. (a) The correlation between A. muciniphila and obesity (b) The possible mechanisms of A. muciniphila regulating obesity in host: (i) A. muciniphila improves thermogenesis and the secretion of GLP-1 (ii) A. muciniphila regulates the intestinal barrier and inflammatory response (iii) A. muciniphila regulates the levels of plasma lipopolysaccharide (iv) A. muciniphila improves energy metabolism in obese hosts. HFD: High-fat diet, GLP-1: glucagon-like peptide-1.
The role of A. muciniphila in type 2 diabetes mellitus
According to a report from the WHO, T2DM is a chronic metabolic disease, and its global incidence rate is continuously increasing [121]. According to a report by the International Diabetes Federation, the global prevalence of diabetes in 2019 was 9.3%, with T2DM accounting for 90%. Therefore, it is important to strengthen the prevention of diabetes and explore effective treatment measures. A growing number of studies have demonstrated that gut microbiota is closely associated with the pathological processes of T2DM [122–124]. Gut dysbiosis is involved in glucose metabolism, impairment of intestinal barrier function, and induction of chronic low-grade inflammation, leading to disorders in SCFA. It has been suggested that A. muciniphila as a potential probiotic can improve the symptoms of T2DM by enhancing intestinal barrier function, inhibiting chronic inflammation and regulating body metabolism. Analysis of the gut microbiota in T2DM patients revealed that the abundance of Verrucomicrobia is less or even completely absent, suggesting a reduced or even total absence of A. muciniphila [125–129]. Thus, A. muciniphila has the potential to be used as a therapeutic target for the prevention and treatment of T2DM. The decreased abundance of A. muciniphila can be detected before the onset of T2DM, which is helpful for early diagnosis and intervention of T2DM.
In recent years, several epidemiological and animal studies have indicated that A. muciniphila plays a vital role in the regulation of T2DM [130–132]. Hanninen et al. found that a high incidence of diabetes was associated with a lack of A. muciniphila [130]. Moreover, the incidence of diabetes was reduced by administration A. muciniphila. Qu et al. reported that pasteurized A. muciniphila provides therapeutic and preventive effects against diabetes in a zebrafish model [133]. Zebrafish with diabetes mellitus showed significant improvement in blood glucose, body mass index, and diabetes indices after administration of pasteurized A. muciniphila. Niu et al. explored the molecular mechanism of pasteurized A. muciniphila in improving T2DM symptoms. The results revealed that pasteurized A. muciniphila improved symptoms of T2DM by increasing the production GLP-1, with pasteurized A. muciniphila total proteins (PP) playing a crucial role in this process [134]. A study reported that metagenomic and targeted metabolomics were used to analyze the abundance of A. muciniphila in 182 subjects who were lean and had abdominally obesity, with and without recently diagnosed T2DM [135]. The abundance of A. muciniphila was significantly lower in lean individuals with T2DM than in those without T2DM. However, it did not exhibit the same decline when comparing obese people with and without T2DM. Furthermore, supplementing mice with A.muciniphila is sufficient to safeguard them from high sucrose-induced glucose intolerance. The protective effect was achieved by reducing the levels of 3β-chenodeoxycholic acid (βCDCA), insulin secretion, and fibroblast growth factor 15/19 (FGF15/19). Shin et al. found that the glycemic profile of HFD-fed mice improved significantly after metformin treatment [136]. The abundance of A. muciniphila in mouse intestines was significantly increased, suggesting that metformin intake could promote an increase in the levels of A. muciniphila in the intestine. Moreover, the glucose tolerance of HFD-fed mice was significantly improved by the oral administration A. muciniphila without metformin, indicating that A. muciniphila might potentially contribute to improving T2DM. Finally, we summarized the research on the correlation between A. muciniphila and T2DM in the last three years, as shown in Table 2.
At present, there is still a lack of a comprehensive understanding of how A. muciniphila regulates T2DM. The potential mechanism of A. muciniphila in T2DM can be roughly explained by the following aspects (Figure 3): (1) The impairment of intestinal barrier function and the increase in intestinal permeability are critical factors for T2DM; A. muciniphila and its products can increase the thickness of the mucus layer and reduce the permeability of the intestinal mucosal barrier. (2) A. muciniphila can inactivate LPS inflammatory signaling pathways and improve metabolic endotoxemia and local inflammation. A muciniphila can also reduce the levels of the inflammatory factor TNF-α, intervene in the insulin-signaling cascade, and improve insulin resistance. (3) A muciniphila can promote the secretion of GLP-1, improve glycemic tolerance, and reduce insulin resistance in the body. Moreover, A muciniphila can induce monocytes to express high levels of IL-6, stimulating the secretion of GLP-1, thus reducing the risk of insulin resistance. The literature suggests that A. muciniphila could potentially play a significant role inT2DM. Additionally, these findings offer a foundation for the development of strategies involving A. muciniphila in the prevention and treatment of T2DM.
Figure 3.
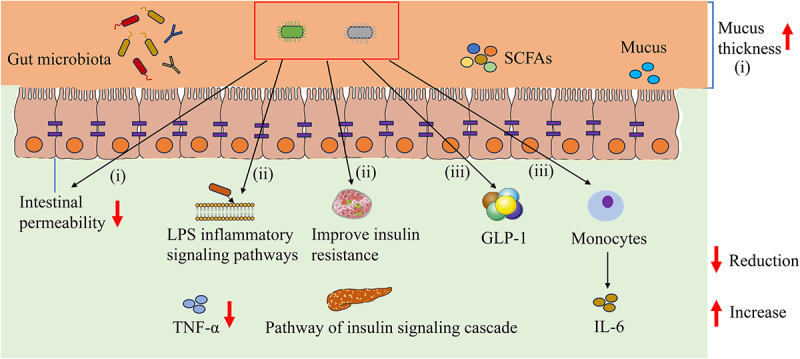
The possible mechanisms of A. muciniphila regulating T2DM in host: (i) A. muciniphila improves intestinal barrier function and intestinal permeability (ii) A. muciniphila regulates LPS inflammatory signaling pathway, metabolic endotoxemia and local inflammatory (iii) A. muciniphila improves the secretion of GLP-1and regulates insulin resistance. LPS: Lipopolysaccharide.
The correlation between A. muciniphila and pathogen infection
Parasitic diseases are a common public health problem that pose a threat to human health, especially in developing countries where they are prevalent. The human intestinal tract can harbor various parasites and a large number of symbiotic bacteria, and organisms living in the same environment can undergo important interactions. Parasite infection can potentially influence the species diversity and community structure of the host gut microbiota. In contrast, some components of the gut microbiota can also prevent parasites from colonizing the intestines or inhibit their persistence during parasite infection. Kupritz et al. conducted a comparative analysis of 23 studies on changes in the diversity of gut microbiota in populations infected and non-infected with parasites [137]. They found that the intra-diversity (alpha diversity) and the inter-individual diversity (beta diversity) of the gut microbiota were significantly altered in these populations after parasite infection. Jenkins et al. investigated the effect of soil-transmitted gastrointestinal nematode infection on the composition of the host gut microbiota [138]. These findings indicated that the alpha diversity and richness of infected subjects did not differ significantly from those of uninfected subjects. However, there was a significant increase in the beta diversity. Furthermore, Jin et al. evaluated the effects of butyrate and probiotics on Trichinella spiralis (T. spiralis) infection as well as their effect on mucus levels [139]. They found that the presence of butyrate and butyrate-producing bacteria significantly reduced helminth burden and promoted mucus production.
Several studies have demonstrated that the levels of A. muciniphila was increased after parasite infection, suggesting its potential role in preventing parasite infection [53,140,141]. Compared with uninfected mice, the levels of A. muciniphila were significantly increased in the colonic and intestinal contents of mice 28 and 50 days after Schistosoma mansoni infection [53]. Moreover, Zhao et al. found that compared to uninfected mice, the levels of A. muciniphila were significantly increased in the intestinal contents of mice 42 days after Schistosoma japonicum infection [140]. After treatment with koumiss, mice infected with Toxoplasma gondii exhibited changes in their gut microbiota composition, including an increase in the relative abundance of A. muciniphila [142]. Xie et al. demonstrated that the levels of A. muciniphila in wild-type mice infected with Plasmodium berghei ANKA were approximately threefold higher than those in uninfected mice [52]. However, Smith et al. discovered a negative relationship between Plasmodium burden and the abundance of five specific Operational Taxonomic Units (OTUs), including A. muciniphila [143]. Jin et al. reported that β-glucan (BG) can trigger T. spiralis expulsion through the mucus layer without relying on type 2 immunity, but relies on the gut microbiota in mice [51,144]. The dominant bacteria A. muciniphila showed significant expansion in T. spiralis-infected mice with BG. Xie et al. found that compared with the control group, the levels of A. muciniphila in WT and Mif−/− C57BL/6 mice was increased after T. spiralis infection [145].
Although several studies have identified A. muciniphila as a potential biomarker after different parasite infections, suggesting its possible crucial role in fighting parasite infection, there are very few reports on the application of A. muciniphila for treating or preventing parasitic diseases. Jin et al. found that pasteurized A. muciniphila exhibited stronger effects on host defense against T. spiralis infection by enhancing mucus production [51]. The absence of TLR2 completely eliminated the ability of pasteurized A. muciniphila to expel worms. Moreover, they also found that butyrate, one of the main metabolites of A. muciniphila has anthelmintic effects on T. spiralis. Although research has confirmed that A. muciniphila can improve the function of the mucus layer during T. spiralis infection, the mechanism by which A. muciniphila improves the function of the mucus layer and exerts its deworming effect remains unclear.
Additionally, numerous studies have demonstrated that changes in the levels of A. muciniphila are associated with viral diseases [146–149]. Vaibhav et al. found that the gut microbiota of mice was significantly disrupted by SARS-CoV-2 variants, such as USA-WA1/2020, Delta, and Omicron [149]. Unexpectedly, although the Omicron variant resulted in milder symptoms in mice, it disrupted the gut microbiota and caused a significant decrease in A. muciniphila. Another study showed that the abundance of A. muciniphila in patients infected with high SARS‑CoV‑2 viral load was higher than that in patients infected with low SARS‑CoV‑2 viral load [150]. Xie et al. analyzed stool and serum samples obtained from patients with severe fever with thrombocytopenia syndrome virus (SFTS) using 16S ribosomal RNA-sequencing and untargeted metabolomics [151]. The results indicated that A. muciniphila exhibited an increase in relative abundance over the course of infection, but its abundance was decreased in deceased patients. According to a study conducted by Chen et al., the levels of A. muciniphila were significantly lower in seroconverters (SC) before HIV-1 infection than in negative controls [147]. Kim et al. reported that compared with hepatitis B virus (HBV)-negative mice, HBV-positive mice exhibited a notable increase in alpha diversity and abundance of A. muciniphila in the analysis of gut microbiota in fecal microbiota transplantation (FMT) mice [146]. Additionally, some studies have confirmed a strong connection between the pathogenicity of influenza and the gut microbiota. Hu et al found that the presence of A. muciniphila was positively correlated with H7N9 infection [148]. Weight loss and mortality resulting from H7N9 infection in mice can be significantly reduced by the administration of pasteurized A. muciniphila. Additionally, administering live or pasteurized A. muciniphila has been shown to reduce the titers of pulmonary viral and the levels of IL-1β and IL-6, while increasing the levels of IFN-β, IFN-γ, and IL-10 in mice infected with H7N9. The results indicated that the anti-influenza effects of A. muciniphila are a result of its anti-inflammatory and immunoregulatory properties.
The relationship between A. muciniphila and other diseases
A. muciniphila plays a crucial role in the occurrence, development, and regulation of other diseases. It is well known that cancer has always been one of the most dreaded diseases and a significant contributor to human death worldwide. Studies have found that the reduction in A. muciniphila levels is correlated with the occurrence and progression of many malignancies, and A. muciniphila in tumors has a positive effect on the response to chemotherapy agents and immune checkpoint inhibitors [152]. Moreover, nasopharyngeal carcinoma patients were found to have a significant reduction in A. muciniphila, compared to healthy subjects [153]. Shi et al. reported that combining IL-2 and A. muciniphila exhibited a higher level of antitumor efficacy in the tumor tissues of colorectal cancer patients than monotherapy [154]. Activation of the TLR2 signaling pathway partially contributes to the antitumor immune response triggered by A. muciniphila, which is primarily elicited by its outer membrane protein. Luo et al. found that Am-EVs can alleviate the tumor burden of prostate cancer in a murine model and that macrophages treated with Am-EVs can inhibit the proliferation and invasion of prostate cells [155]. In vitro, Am-EVs elevated the numbers of GZMB+CD8+ and IFN-γ+CD8+ T cells, as well as M1-like macrophages. Activated CD8+ T cells have the ability to enter tumor tissue and attach to tumor cell surface ligands using the T-cell receptor, ultimately causing cell death by releasing IFN-γ, granzyme, TNF-α, or perforin. Moreover, M1 macrophages typically exhibit tumoricidal activity through the production of inflammatory cytokines and the activation of the immune response. These studies suggested that supplementation with A. muciniphila can enhance the effectiveness of immunotherapy in patients with cancer, offering new perspectives for tumor prevention and treatment.
Multiple sclerosis (MS) is a chronic demyelinating disease with inflammatory and autoimmune characteristics, and experimental autoimmune encephalomyelitis (EAE) is an ideal animal model [156]. The gut microbiota, which plays a crucial role in regulating immune responses and brain function, is increasingly believed to be a significant environmental factor in the development of MS [157,158]. The levels of A. muciniphila in subjects with MS were higher than those in the healthy subjects. In the analysis of the gut microbiota in 71 patients with MS and 71 healthy controls, it was found that MS patients exhibited a significant increase in the amount of A. muciniphila, and A. muciniphila had the ability to enhance the differentiation of Th1 lymphocytes in vitro [159]. Moreover, the International Multiple Sclerosis Microbiome Study (iMSMS) found a significantly increased proportion of A. muciniphila in MS patients through an analysis of the gut microbiota of 576 MS patients and 1152 healthy subjects [158]. A study has found that MicroRNAs-30d from the feces of MS patients can increase the levels of A. muciniphila in the intestine by regulating the expression of β-galactosidase, thereby promoting the secretion of Treg cell cytokines to suppress MS-like symptoms in mice. These results indicated that microbial manipulation and dietary intervention might potentially be employed as preventive and therapeutic approaches for MS.
In recent years, the concept of the “microbiota-gut-brain axis” has been proposed in some studies, indicating a close correlation between gut microbiota and neural system function [160–163]. Studies have shown that there is a mutual regulatory effect between the gut microbiota and central nervous system. On one hand, the gut microbiota directly or indirectly affects the central nervous system through metabolic molecules; On the other hand, central descending signals also influence the intestinal microbial ecology by controlling intestinal secretion, motility, immunity, and endocrine functions. In a study conducted by Wang et al., there was a notable decline in the levels of Bifidobacterium and A. muciniphila in the feces of children diagnosed with autism spectrum disorder (ASD) compared with healthy controls [164]. Qu et al. evaluated the effects of A. muciniphila on Alzheimer’s disease (AD) in a murine model with different diets [165]. The results indicated that A. muciniphila has the potential to postpone pathological alterations in the brain and alleviate damage to spatial learning and memory in mouse models of AD.
The correlation between A. muciniphila and diseases, such as amyotrophic lateral sclerosis (ALS) and alcoholic liver disease has also been reported, suggesting that A. muciniphila may be associated with more diseases [166,167]. Furthermore, A. muciniphila may have complex regulatory mechanisms in the host, and the correlation between A. muciniphila and more diseases in the host remains to be discovered and explored.
Future perspectives and challenges
There are numerous studies on the relationship between A. muciniphila and different diseases, and most of them have reached similar conclusions. Although a few studies have reported the negative effects of A. muciniphila, most studies have consistently demonstrated that the levels of A. muciniphila are reduced in metabolic disorders. Current research has focused on directly supplementing A. muciniphila to increase its abundance and utilize its probiotic characteristics. However, A. muciniphila is an anaerobic bacterium with high requirements for both the culture environment and growth medium components, which hinders its clinical application. The study conducted by Machado et al. discovered that the sensitivity of A. muciniphila to anaerobic environments and pH values was much lower than that reported by Derrien et al. [168]. Plovier et al. successfully developed a synthetic medium for high-yield cultivation of A. muciniphila that does not include any substances that cannot be administered to humans, thus overcoming a significant hurdle in the clinical use of A. muciniphila [11]. Moreover, the storage, transportation, and administration conditions of A. muciniphila have strict requirements. Research has found that live A. muciniphila must use cryoprotectants during the administration process, and its biological activity may be lost if environmental conditions cannot meet its characteristics [169]. Marcial-Coba et al. developed an approach for encapsulating A. muciniphila in a xanthan and gellan gum matrix and embedding the microencapsulated bacteria in dark chocolate to enhance their survival rate in vitro [169,170]. Lin et al. constructed microcapsules containing A. muciniphila, which showed high viability and stability in an aerobic environment [171].
Contradictory research findings have shown that A. muciniphila may play a role in exacerbating diseases [81,172]. Although it has been reported that A. muciniphila has a beneficial role in preventing intestinal inflammation, it could also have negative effects when harmful bacteria damage the intestinal mucosal barrier [173,174]. In mice colonized with a simplified human gut microbiota (SIHUMI) to mimic the human intestinal microbiota, A. muciniphila did not have a therapeutic effect and instead worsened the intestinal inflammation caused by Salmonella typhimurium (S. typhimurium) [80]. It is important to mention that the gut microbiota of mice was not significantly altered by the presence of A. muciniphila by itself. However, when both A. muciniphila and S. typhimurium were present in SIHUMI mice simultaneously, it would worsen the gut inflammation. It is logical to conclude that in the specific case of existing pathogenic bacterium, A. muciniphila could potentially turn into a harmful bacterium that negatively impacts the host [59]. Consistently, Ayres et al. classified that disruptions in intestinal homeostasis can cause beneficial microbes to transform into potentially harmful species, resulting in negative effects on the host [175]. Additionally, although A. muciniphila is widely recognized as a beneficial commensal, multiple recent studies have linked it to different types of cancers [176–178]. Howe et al. reported that the level of A. muciniphila in the stool samples of CRC patients has been found to be four times greater than in healthy subjects. Moreover, the colonization of A. muciniphila worsens tumor development in the intestines of Apc min/+ mice [176]. Huang et al. found that the level of A. muciniphila was notably elevated in the first three weeks in cancer-bearing mice, indicating its involvement in the early phase of cancer establishment [177]. The above studies suggested that gut commensals like A. muciniphila could promote the development of some diseases. More research is needed to explore under what conditions A. muciniphila promotes the development of diseases.
Currently, there is no clear definition of the toxicological characteristics of A. muciniphila, such as dose-response. However, the relevant research on the dosage of A. muciniphila is limited. Studies have shown that A. muciniphila must be present in sufficient quantities to exert its probiotic properties [179]. A suitable amount of A. muciniphila added to food and the impact of long-term consumption on intestinal homeostasis need to be determined urgently. Although many studies have shown that oral A. muciniphila is safe, further clinical trials are required to confirm its effectiveness and safety. Moreover, Oral administration of exogenous probiotics cannot establish long-term colonization in the patient’s body, which has become a major challenge in the development of A. muciniphila formulations.
Although extensive research has been conducted on the effects of A. muciniphila on metabolic diseases, some studies have focused on its effects of A. muciniphila on parasitic diseases [51,140,141]. Most experiments are aimed at investigating the impact of parasite infection on the gut microbiota, thereby screening potential biomarkers. Numerous studies have indicated that A. muciniphila is a potential biomarker after parasite infection [51,52,140]. These findings indicated that combining A. muciniphila with a drug or parasite vaccine will provide new ideas for the treatment or prevention of parasitic diseases in the future. However, there is very limited research on the use of A. muciniphila for treating or preventing parasitic diseases. Until now, there have been no studies that have thoroughly investigated the involvement of A. muciniphila in helminth infections [144]. More research is needed to evaluate the protective function of A. muciniphila and its associated molecules against infection with helminths. Moreover, the underlying molecular mechanisms of A. muciniphila in the regulation of helminth infection needs to be further explored in the future.
Numerous studies have verified the changes in A. muciniphila in animal models and individuals with metabolic diseases, along with its therapeutic benefits and the effectiveness of interventions to enhance its abundance. Nevertheless, the majority of animal studies involving A. muciniphila supplementation have been conducted using A. muciniphila cultivated under conditions containing mucin [3]. The presence of contaminants in animal-derived mucin may reduce the positive impact of A. muciniphila in relieving metabolic diseases. Additionally, most studies have explored the positive effects of A. muciniphila in animal models, and further research on the role of A. muciniphila in human diseases is needed. Although the mechanism of the positive effects of A. muciniphila on diseases is not yet fully understood, A. muciniphila has broad development prospects as a highly regarded next-generation probiotic. Perraudeau et al. studied the beneficial effects of a probiotic formulation containing A. muciniphila on T2DM, and the safety of this product has been proven through clinical trials, indicating significant potential for the development of A. muciniphila [180].
Researchers have conducted therapeutic trials of A. muciniphila owning to its beneficial effects, which were demonstrated in cohort studies of various human and animal models [181]. Clinical studies have found that 53.7% of Chinese people with IBD experienced improvements in clinical indicators through the use of washed microbiota transplantation (WMT), which is different from FMT, which involves a higher frequency of A. muciniphila [181,182]. In Belgium, a human clinical experiment was conducted to investigate the relationship between A. muciniphila and metabolic diseases [183]. Although the index of body weight, fat mass, or hip circumference in overweight/obese insulin-resistant subjects was not reduced after oral administration of A. muciniphila, the index of insulin sensitivity, lower insulinemia, and lower plasma cholesterol were enhanced. Furthermore, research conducted on individuals with type 2 diabetes from the United States provided further evidence supporting the secure utilization of a probiotic mixture containing A. muciniphila to enhance the management of postprandial glucose levels [27]. As the mechanism of A. muciniphila function in host health is becoming increasingly clear, the clinical application of A. muciniphila has broad prospects.
Conclusion
In summary, it has been demonstrated that A. muciniphila acts an active role in promoting host health and maintaining the integrity of the intestinal barrier. Alterations in A. muciniphila levels can be regarded as an indicator of disease development. In this review, we have summarized the findings of various studies that focused on alterations in the levels of A. muciniphila and its influence on different diseases, such as IBD, obesity, T2DM, and parasitic diseases. Moreover, metabolites and enzymes derived from A. muciniphila, such as Amuc_1100, AmEVs and SCFAs, show promising potential for the treatment of obesity, IBD, and T2DM. Intervention studies on A.muciniphila are mostly restricted to animal experiments; thus, further research should focus on its safety and effectiveness in the treatment or prevention of diseases. More importantly, the function and mechanism of action of A.muciniphila in different diseases are still poorly understood, and further research is needed for its application in clinical diseases.
Supplementary Material
Funding Statement
This work was supported by the Cultivating Project for Young Scholar at Hubei University of Medicine [2021QDJZR012] and the National Natural Science Foundation of China [82002160].
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data sharing not applicable-no new data generated.
Author contributions
Daoxiu Xu and Yanqing Zhao: conception and design. Daoxiu Xu, Yanqing Zhao and Huijun Yang: drafting the manuscript. Biao Xu, Sirui Zhang and Wenkun Xue: analysis and interpretation of the data. Peng Wu, Shuguo Yang and Bin Tang: revising it critically for intellectual content. Bin Tang and Daoxiu Xu: final approval of the version to be published. All authors have read and approved the final manuscript.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/21505594.2024.2375555
References
- [1].Cani PD. Gut microbiota - at the intersection of everything? Nat Rev Gastroenterol Hepatol. 2017;14(6):321–21. [DOI] [PubMed] [Google Scholar]
- [2].Cani PD, Depommier C, Derrien M, et al. Akkermansia muciniphila: paradigm for next-generation beneficial microorganisms. Nat Rev Gastroenterol Hepatol. 2022;19(10):625–637. [DOI] [PubMed] [Google Scholar]
- [3].Yan J, Sheng LL, Li HK. Akkermansia muciniphila: is it the Holy Grail for ameliorating metabolic diseases? Gut Microbes. 2021;13(1):1984904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Zhang T, Li QQ, Cheng L, et al. Akkermansia muciniphila is a promising probiotic. Microb Biotechnol. 2019;12(6):1109–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ghaffari S, Abbasi A, Somi MH, et al. Akkermansia muciniphila: from its critical role in human health to strategies for promoting its abundance in human gut microbiome. Crit Rev Food Sci. 2023;63(25):7357–7377. [DOI] [PubMed] [Google Scholar]
- [6].Arumugam M, Raes J, Pelletier E, et al. Enterotypes of the human gut microbiome. Nature. 2011;474(7353):174–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Zhao QX, Yu JD, Hao Y, et al. Akkermansia muciniphila plays critical roles in host health. Crit Rev Microbiol. 2023;49(1):82–100. [DOI] [PubMed] [Google Scholar]
- [8].Si J, Kang H, You HJ, et al. Revisiting the role of Akkermansia muciniphila as a therapeutic bacterium. Gut Microbes. 2022;14(1):2078619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Xue C, Li GL, Gu XY, et al. Health and disease: akkermansia muciniphila, the Shining Star of the Gut Flora. Research-China. 2023;6:0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Jin XM, Liu Y, Wang JQ, et al. β-Glucan-triggered expansion facilitates the expulsion of intestinal helminth via TLR2 in mice. Carbohyd Polym. 2022;275:118719. [DOI] [PubMed] [Google Scholar]
- [11].Plovier H, Everard A, Druart C, et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat Med. 2017;23(1):107–113. [DOI] [PubMed] [Google Scholar]
- [12].Chelakkot C, Choi Y, Kim DK, et al. Akkermansia muciniphila-derived extracellular vesicles influence gut permeability through the regulation of tight junctions. Exp Mol Med. 2018;50:e450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kang CS, Ban M, Choi EJ, et al. Extracellular vesicles derived from Gut Microbiota, especially Akkermansia muciniphila, protect the progression of dextran sulfate sodium-induced colitis. Plos One. 2013;8(10):e76520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Abuqwider JN, Mauriello G, Altamimi MA. New generation of beneficial microbiota in modulating obesity: a systematic review. Microorganisms. 2021;9(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Baske MM, Timmerman KC, Garmo LG, et al. Fecal microbiota transplant on gut composition and its potential role in the treatment of generalized anxiety disorder: a systematic review. J Affect Disord. 2024;354:309–317. [DOI] [PubMed] [Google Scholar]
- [16].Derrien M, Vaughan EE, Plugge CM, et al. Akkermansia muciniphila gen. nov. sp. nov. a human intestinal mucin-degrading bacterium. Int J Syst Evol Microbiol. 2004;54(Pt 5):1469–1476. [DOI] [PubMed] [Google Scholar]
- [17].Reunanen J, Kainulainen V, Huuskonen L, et al. Akkermansia muciniphila adheres to enterocytes and strengthens the integrity of the epithelial cell layer. Appl Environ Microb. 2015;81(11):3655–3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ouwerkerk JP, van der Ark KCH, Davids M, et al. Adaptation of Akkermansia muciniphila to the oxic-anoxic interface of the mucus layer. Appl Environ Microb. 2016;82(23):6983–6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Geerlings SY, Kostopoulos I, de Vos WM, et al. Akkermansia muciniphila in the human gastrointestinal tract: when, where, and how? Microorganisms. 2018;6(3):75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].van Passel Mwj, Kant R, Zoetendal EG, et al. The genome of Akkermansia muciniphila, a dedicated intestinal mucin degrader, and its use in exploring intestinal metagenomes. Plos One. 2011;6(3):e16876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ouwerkerk JP, Koehorst JJ, Schaap PJ, et al. Complete genome sequence of Akkermansia glycaniphila strain PytT, a mucin-degrading specialist of the reticulated python Gut. Genome Announc. 2017;5(1):10–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Liu XY, Zhao F, Liu H, et al. Transcriptomics and metabolomics reveal the adaption of Akkermansia muciniphila to high mucin by regulating energy homeostasis. Sci Rep-Uk. 2021;11(1):9073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Collado MC, Derrien M, Isolauri E. Intestinal integrity and Akkermansia muciniphila, a mucin-degrading member of the intestinal microbiota present in infants, adults, and the elderly. Appl Environ Microb. 2007;73(23):7767–7770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Derrien M, Collado MC, Ben-Amor K, et al. The mucin degrader Akkermansia muciniphila is an abundant resident of the human intestinal tract. Appl Environ Microb. 2008;74(5):1646–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zhai QX, Feng SS, Arjan N, et al. A next generation probiotic, Akkermansia muciniphila. Crit Rev Food Sci. 2019;59(19):3227–3236. [DOI] [PubMed] [Google Scholar]
- [26].Dao MC, Everard A, Aron-Wisnewsky J, et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut. 2016;65(3):426–436. [DOI] [PubMed] [Google Scholar]
- [27].Ottman N, Davids M, Suarez-Diez M, et al. Genome-scale model and omics analysis of metabolic capacities of Akkermansia muciniphila reveal a preferential mucin-degrading lifestyle. Appl Environ Microb. 2017;83(18):e01014–e01017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hansen CHF, Krych L, Nielsen DS, et al. Early life treatment with vancomycin propagates Akkermansia muciniphila and reduces diabetes incidence in the NOD mouse. Diabetologia. 2012;55(8):2285–2294. [DOI] [PubMed] [Google Scholar]
- [29].Dubourg G, Lagier JC, Armougom F, et al. High-level colonisation of the human gut by Verrucomicrobia following broad-spectrum antibiotic treatment. Int J Antimicrob Agents. 2013;41(2):149–155. [DOI] [PubMed] [Google Scholar]
- [30].Kim SM, Park S, Hwang SH, et al. Secreted Akkermansia muciniphila threonyl-tRNA synthetase functions to monitor and modulate immune homeostasis. Cell Host Microbe. 2023;31(6):1021. [DOI] [PubMed] [Google Scholar]
- [31].Zheng DP, Liwinski T, Elinav E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020;30(6):492–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ghotaslou R, Nabizadeh E, Memar MY, et al. The metabolic, protective, and immune functions of Akkermansia muciniphila. Microbiol Res. 2023;266:127245. [DOI] [PubMed] [Google Scholar]
- [33].Zeng MY, Cisalpino D, Varadarajan S, et al. Gut microbiota-induced immunoglobulin G controls systemic infection by symbiotic bacteria and pathogens. Immunity. 2016;44(3):647–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Landuyt AE, Klocke BJ, Duck LW, et al. ICOS ligand and IL-10 synergize to promote host-microbiota mutualism. Proc Natl Acad Sci U S A. 2021;118(13): e2018278118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Koch MA, Reiner GL, Lugo KA, et al. Maternal IgG and IgA antibodies dampen mucosal T helper cell responses in early life. Cell. 2016;165(4):827–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ansaldo E, Slayden LC, Ching KL, et al. Akkermansia muciniphila induces intestinal adaptive immune responses during homeostasis. Science. 2019;364(6446):1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kuczma MP, Szurek EA, Cebula A, et al. Self and microbiota-derived epitopes induce CD4+ cell anergy and conversion into CD4+ Foxp3+ regulatory cells. Mucosal Immunol. 2021;14(2):443–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Martin-Gallausiaux C, Garcia-Weber D, Lashermes A, et al. Akkermansia muciniphila upregulates genes involved in maintaining the intestinal barrier function via ADP-heptose-dependent activation of the ALPK1/TIFA pathway. Gut Microbes. 2022;14(1):2110639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Wang JC, Xiang R, Wang RJ, et al. The variable oligomeric state of Amuc_1100 from Akkermansia muciniphila. J Struct Biol. 2020;212(1):107593. [DOI] [PubMed] [Google Scholar]
- [40].Han YQ, Ling Q, Wu L, et al. Akkermansia muciniphila inhibits nonalcoholic steatohepatitis by orchestrating TLR2-activated γδT17 cell and macrophage polarization. Gut Microbes. 2023;15(1):2221485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Everard A, Belzer C, Geurts L, et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A. 2013;110(22):9066–9071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Zhang J, Ni YQ, Qian LL, et al. Decreased abundance of Akkermansia muciniphila leads to the impairment of insulin secretion and glucose homeostasis in lean type 2 diabetes. Adv Sci. 2021;8(16):2100536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Shi ZJ, Lei HH, Chen G, et al. Impaired intestinal Akkermansia muciniphila and Aryl hydrocarbon receptor ligands contribute to nonalcoholic fatty liver disease in Mice. Msystems. 2021;6(1):10–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Lakshmanan AP, Murugesan S, Al Khodor S, et al. The potential impact of a probiotic: Akkermansia muciniphila in the regulation of blood pressure-the current facts and evidence. J Transl Med. 2022;20(1):430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Xu Y, Wang N, Tan HY, et al. Function of Akkermansia muciniphila in obesity: interactions with lipid metabolism, immune response and Gut systems. Front Microbiol. 2020;11:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Chang CC, Liu CY, Su IC, et al. Functional plasmon-activated water increases Akkermansia muciniphila abundance in Gut microbiota to ameliorate inflammatory bowel disease. Int J Mol Sci. 2022;23(19):11422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Liu YJ, Yang M, Tang L, et al. TLR4 regulates RORγt+ regulatory T-cell responses and susceptibility to colon inflammation through interaction with Akkermansia muciniphila. Microbiome. 2022;10(1):98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Liu MJ, Yang JY, Yan ZH, et al. Recent findings in Amuciniphila-regulated metabolism and its role in intestinal diseases. Clin Nutr. 2022;41(10):2333–2344. [DOI] [PubMed] [Google Scholar]
- [49].Fan LN, Xu CC, Ge QW, et al. A. Muciniphila suppresses colorectal tumorigenesis by inducing TLR2/NLRP3-mediated M1-like TAMs. Cancer Immunol Res. 2021;9(10):1111–1124. [DOI] [PubMed] [Google Scholar]
- [50].Olson CA, Vuong HE, Yano JM, et al. The Gut microbiota mediates the anti-seizure effects of the ketogenic diet. Cell. 2018;174(2):497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Jin XM, Liu Y, Wang JQ, et al. β-Glucan-triggered Akkermansia muciniphila expansion facilitates the expulsion of intestinal helminth via TLR2 in mice. Carbohyd Polym. 2022;275:118719. [DOI] [PubMed] [Google Scholar]
- [52].Xie YT, Guan W, Zhao YQ, et al. Deficiency of migration inhibitory factor influences the gut microbiota of C57BL/6 mice infected with Plasmodium berghei ANKA. Front Microbiol. 2022;13:978644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Jenkins TP, Peachey LE, Ajami NJ, et al. Schistosoma mansoni infection is associated with quantitative and qualitative modifications of the mammalian intestinal microbiota. Sci Rep-Uk. 2018;8:12072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Stracke K, Adisakwattana P, Phuanukoonnon S, et al. Field evaluation of the gut microbiome composition of pre-school and school-aged children in Tha Song Yang, Thailand, following oral MDA for STH infections. Plos Neglect Trop D. 2021;15(7):e0009597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Hansson GC, Johansson MEV. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Gut Microbes. 2010;1(1):51–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Niu HF, Zhou MF, Zogona D, et al. Akkermansia muciniphila: a potential candidate for ameliorating metabolic diseases. Front Immunol. 2024;15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Meynier M, Daugey V, Mallaret G, et al. Pasteurized improves irritable bowel syndrome-like symptoms and related behavioral disorders in mice. Gut Microbes. 2024;16(1):2298026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Bisgaard TH, Allin KH, Keefer L, et al. Depression and anxiety in inflammatory bowel disease: epidemiology, mechanisms and treatment. Nat Rev Gastroenterol Hepatol. 2022;19(11):717–726. [DOI] [PubMed] [Google Scholar]
- [59].Zhang T, Ji XH, Lu GC, et al. The potential of Akkermansia muciniphila in inflammatory bowel disease. Appl Microbiol Biot. 2021;105(14–15):5785–5794. [DOI] [PubMed] [Google Scholar]
- [60].Rodrigues VF, Elias-Oliveira J, Pereira IS, et al. Akkermansia muciniphila and Gut immune system: a good friendship that attenuates inflammatory bowel disease, obesity, and diabetes. Front Immunol. 2022;13:934695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Yilmaz O, Okullu SO, Catakci M, et al. Akkermansia muciniphila improves chronic colitis-induced enteric neuroinflammation in mice. Neurogastroent Motil. 2024;36(3). [DOI] [PubMed] [Google Scholar]
- [62].Zheng MY, Han R, Yuan YL, et al. The role of Akkermansia muciniphila in inflammatory bowel disease: current knowledge and perspectives. Front Immunol. 2023;13:1089600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Qu SW, Fan LN, Qi YD, et al. Akkermansia muciniphila Alleviates Dextran Sulfate Sodium (DSS)-Induced Acute Colitis by NLRP3 Activation. Microbiol Spectr. 2021;9(2):e00730–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Zhai R, Xue XH, Zhang LY, et al. Strain-specific anti-inflammatory properties of two Akkermansia muciniphila strains on chronic colitis in Mice. Front Cell Infect Mi. 2019;9:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Liu Q, Lu WW, Tian FW, et al. Akkermansia muciniphila exerts strain-specific effects on DSS-induced ulcerative colitis in Mice. Front Cell Infect Mi. 2021;11:698914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Wade H, Pan KC, Duan QH, et al. Akkermansia muciniphila and its membrane protein ameliorates intestinal inflammatory stress and promotes epithelial wound healing via CREBH and miR-143/145. J Biomed Sci. 2023;30(1):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Wang B, Chen X, Chen Z, et al. Stable colonization of Akkermansia muciniphila educates host intestinal microecology and immunity to battle against inflammatory intestinal diseases. Exp Mol Med. 2023;55(1):55–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Xue LY, Zhao YJ, Wang HT, et al. The effects of live and pasteurized Akkermansia muciniphila on DSS-induced ulcerative colitis, gut microbiota, and metabolomics in mice. Food Funct. 2023;14(10):4632–4646. [DOI] [PubMed] [Google Scholar]
- [69].Qian KY, Chen SJ, Wang JC, et al. A β-N-acetylhexosaminidase Amuc_2109 from Akkermansia muciniphila protects against dextran sulfate sodium-induced colitis in mice by enhancing intestinal barrier and modulating gut microbiota. Food Funct. 2022;13(4):2216–2227. [DOI] [PubMed] [Google Scholar]
- [70].Alam A, Leoni G, Quiros M, et al. The microenvironment of injured murine gut elicits a local pro-restitutive microbiota. Nat Microbiol. 2016;1(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Zhang T, Li P, Wu X, et al. Alterations of Akkermansia muciniphila in the inflammatory bowel disease patients with washed microbiota transplantation. Appl Microbiol Biot. 2020;104(23):10203–10215. [DOI] [PubMed] [Google Scholar]
- [72].Dorofeyev A, Dorofeyeva A, Borysov A, et al. Gastrointestinal health: changes of intestinal mucosa and microbiota in patients with ulcerative colitis and irritable bowel syndrome from PM2.5-polluted regions of Ukraine. Environ Sci Pollut R. 2023;30(3):7312–7324. [DOI] [PubMed] [Google Scholar]
- [73].Sezgin E, Terlemez G, Bozkurt B, et al. Quantitative real-time PCR analysis of bacterial biomarkers enable fast and accurate monitoring in inflammatory bowel disease. Peerj. 2022;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Bian XY, Wu WR, Yang LY, et al. Administration of Akkermansia muciniphila ameliorates dextran sulfate sodium-induced ulcerative colitis in Mice. Front Microbiol. 2019;10:2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Wang LJ, Tang L, Feng YM, et al. A purified membrane protein from Akkermansia muciniphila or the pasteurised bacterium blunts colitis associated tumourigenesis by modulation of CD8(+) T cells in mice. Gut. 2020;69(11):1988–1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Kang CS, Ban M, Choi EJ, et al. Extracellular vesicles derived from gut microbiota, especially Akkermansia muciniphila, protect the progression of dextran sulfate sodium-induced colitis. PLoS One. 2013;8(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Hakansson A, Tormo-Badia N, Baridi A, et al. Immunological alteration and changes of gut microbiota after dextran sulfate sodium (DSS) administration in mice. Clin Exp Med. 2015;15(1):107–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Marcella C, Cui B, Kelly CR, et al. Systematic review: the global incidence of faecal microbiota transplantation-related adverse events from 2000 to 2020. Aliment Pharmacol Ther. 2021;53(1):33–42. [DOI] [PubMed] [Google Scholar]
- [79].Ring C, Klopfleisch R, Dahlke K, et al. Akkermansia muciniphila strain ATCC RAA-835 does not promote short-term intestinal inflammation in gnotobiotic interieukin-10-deficient mice. Gut Microbes. 2019;10(2):188–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Ganesh BP, Klopfleisch R, Loh G, et al. Commensal Akkermansia muciniphila exacerbates Gut inflammation in typhimurium-infected gnotobiotic Mice. Plos One. 2013;8(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Seregin SS, Golovchenko N, Schaf B, et al. NLRP6 protects Il10-/-Mice from colitis by limiting colonization of Akkermansia muciniphila. Cell Rep. 2017;19(10):733–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Baxter NT, Zackular JP, Chen GY, et al. Structure of the gut microbiome following colonization with human feces determines colonic tumor burden. Microbiome. 2014;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Martens EC, Neumann M, Desai MS. Interactions of commensal and pathogenic microorganisms with the intestinal mucosal barrier. Nat Rev Microbiol. 2018;16(8):457–470. [DOI] [PubMed] [Google Scholar]
- [84].Gersemann M, Stange EF, Wehkamp J. From intestinal stem cells to inflammatory bowel diseases. World J Gastroenterol. 2011;17(27):3198–3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Derrien M, Belzer C, de Vos WM. Akkermansia muciniphila and its role in regulating host functions. Microb Pathog. 2017;106:171–181. [DOI] [PubMed] [Google Scholar]
- [86].Zhai R, Xue X, Zhang L, et al. Strain-specific anti-inflammatory properties of two Akkermansia muciniphila strains on chronic colitis in Mice. Front Cell Infect Microbiol. 2019;9:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Shi MX, Yue YS, Ma C, et al. Pasteurized Akkermansia muciniphila Ameliorate the LPS-induced intestinal barrier dysfunction via modulating AMPK and NF-kappa B through TLR2 in Caco-2 cells. Nutrients. 2022;14(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Kim MH, Kang SG, Park JH, et al. Short-chain fatty acids activate GPR41 and GPR43 on intestinal epithelial cells to promote inflammatory responses in mice. Gastroenterology. 2013;145(2):396–406e1–10. [DOI] [PubMed] [Google Scholar]
- [89].Felice C, Lewis A, Armuzzi A, et al. Review article: selective histone deacetylase isoforms as potential therapeutic targets in inflammatory bowel diseases. Aliment Pharm Ther. 2015;41(1):26–38. [DOI] [PubMed] [Google Scholar]
- [90].Zhao HB, Jia L, Yan QQ, et al. Effect of Clostridium butyricum and butyrate on intestinal barrier functions: study of a Rat model of severe acute pancreatitis with intra-abdominal hypertension. Front Physiol. 2020;11:561061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Roshanravan N, Bastani S, Tutunchi H, et al. A comprehensive systematic review of the effectiveness of Akkermansia muciniphila, a member of the gut microbiome, for the management of obesity and associated metabolic disorders. Arch Physiol Biochem. 2023;129(3):741–751. [DOI] [PubMed] [Google Scholar]
- [92].Jie ZY, Yu XL, Liu YH, et al. The baseline Gut microbiota directs dieting-induced weight loss trajectories. Gastroenterology. 2021;160(6):2029. [DOI] [PubMed] [Google Scholar]
- [93].Shi QP, Wang Q, Zhong H, et al. Roux-en-Y gastric bypass improved insulin resistance via alteration of the human gut microbiome and alleviation of endotoxemia. Biomed Res Int UK. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Watanabe Y, Fujisaka S, Watanabe S, et al. Isoxanthohumol improves obesity and glucose metabolism via regulation of gut barrier and intestinal lipid absorption with a bloom of Akkermansia muciniphila. Diabetes. 2023;72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Sonomoto K, Song R, Eriksson D, et al. High-fat-diet-associated intestinal microbiota exacerbates psoriasis-like inflammation by enhancing systemic gd T cell IL-17 production. Cell Rep. 2023;42(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Du JJ, Zhang PW, Luo J, et al. Dietary betaine prevents obesity through gut microbiota-drived microRNA-378a family. Gut Microbes. 2021;13(1):1862612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Li ZZ, Zhang B, Wang N, et al. A novel peptide protects against diet-induced obesity by suppressing appetite and modulating the gut microbiota. Gut. 2023;72(4):686–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Alili R, Belda E, Fabre O, et al. Characterization of the Gut microbiota in individuals with overweight or obesity during a real-world weight loss dietary program: a focus on the bacteroides 2 enterotype. Biomedicines. 2022;10(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Watanabe S, Ohno A, Yomoda S, et al. Arctigenin-containing burdock sprout extract prevents obesity in association with modulation of the gut microbiota in mice. Biosci Microb Food H. 2023;42(1):49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Pruss KM, Marcobal A, Southwick AM, et al. Mucin-derived-glycans supplemented to diet mitigate diverse microbiota perturbations. Isme J. 2021;15(2):577–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Cao MZ, Wei CH, Wen MC, et al. Clinical efficacy of weight loss herbal intervention therapy and lifestyle modifications on obesity and its association with distinct gut microbiome: a randomized double-blind phase 2 study. Front Endocrinol. 2023;14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Okamura T, Hamaguchi M, Nakajima H, et al. Milk protects against sarcopenic obesity due to increase in the genus Akkermansia in faeces of db/db mice. J Cachexia Sarcopeni. 2023;14(3):1395–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Liao CA, Huang CH, Ho HH, et al. A combined supplement of probiotic strains AP-32, bv-77, and CP-9 Increased Akkermansia mucinphila and reduced non-esterified fatty acids and energy metabolism in HFD-induced obese rats. Nutrients. 2022;14(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Ondee T, Pongpirul K, Visitchanakun P, et al. Lactobacillus acidophilus LA5 improves saturated fat-induced obesity mouse model through the enhanced intestinal Akkermansia muciniphila. Sci Rep-Uk. 2021;11(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Wang Y, Li T, Yang C, et al. Eurotium cristatum from Fu Brick tea promotes adipose thermogenesis by boosting colonic Akkermansia muciniphila in high-fat-fed obese mice. Foods. 2023;12(20). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Kumar R, Kane H, Wang Q, et al. Identification and characterization of a novel species of genus Akkermansia with metabolic health effects in a diet-induced obesity mouse model. Cells. 2022;11(13). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Acharya KD, Friedline RH, Ward DV, et al. Differential effects of Akkermansia-enriched fecal microbiota transplant on energy balance in female mice on high-fat diet. Front Endocrinol. 2022;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Cani PD, de Vos WM. Next-Generation Beneficial Microbes: the Case of Akkermansia muciniphila. Front Microbiol. 2017;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Regnier M, Rastelli M, Morissette A, et al. Rhubarb Supplementation Prevents Diet-Induced Obesity and Diabetes in Association with Increased Akkermansia muciniphila in Mice. Nutrients. 2020;12(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Arias L, Goig GA, Cardona P, et al. Influence of Gut microbiota on progression to tuberculosis generated by high fat diet-induced obesity in C3HeB/FeJ Mice. Front Immunol. 2019;10:2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Choi Y, Bose S, Seo J, et al. Effects of live and pasteurized forms of Akkermansia from the Human Gut on obesity and metabolic dysregulation. Microorganisms. 2021;9(10):2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Wu T, Gao YF, Hao JY, et al. Capsanthin extract prevents obesity, reduces serum TMAO levels and modulates the gut microbiota composition in high-fat-diet induced obese C57BL/6J mice. Food Res Int. 2020;128. [DOI] [PubMed] [Google Scholar]
- [113].Reunanen J, Kainulainen V, Huuskonen L, et al. Akkermansia muciniphila adheres to enterocytes and strengthens the integrity of the epithelial cell layer. Appl Environ Microbiol. 2015;81(11):3655–3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Yang Y, Zhong Z, Wang B, et al. Early-life high-fat diet-induced obesity programs hippocampal development and cognitive functions via regulation of gut commensal Akkermansia muciniphila. Neuropsychopharmacology. 2019;44(12):2054–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Ashrafian F, Raftar SKA, Lari A, et al. Extracellular vesicles and pasteurized cells derived from Akkermansia muciniphila protect against high-fat induced obesity in mice. Microb Cell Fact. 2021;20(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Depommier C, Van Hul M, Everard A, et al. Pasteurized Akkermansia muciniphila increases whole-body energy expenditure and fecal energy excretion in diet-induced obese mice. Gut Microbes. 2020;11(5):1231–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Remely M, Tesar I, Hippe B, et al. Gut microbiota composition correlates with changes in body fat content due to weight loss. Benef Microbes. 2015;6(4):431–439. [DOI] [PubMed] [Google Scholar]
- [118].Ke HR, Li F, Deng WL, et al. Metformin exerts anti-inflammatory and mucus barrier protective effects by enriching Akkermansia muciniphila in mice with ulcerative colitis. Front Pharmacol. 2021;12:726707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Huang SJ, Hu SP, Liu S, et al. Lithium carbonate alleviates colon inflammation through modulating gut microbiota and Treg cells in a GPR43-dependent manner. Pharmacol Res. 2022;175:105992. [DOI] [PubMed] [Google Scholar]
- [120].Nakashima T, Fujii K, Seki T, et al. Novel Gut microbiota modulator, which markedly increases Akkermansia muciniphila occupancy, ameliorates experimental colitis in rats. Dig Dis Sci. 2022;67(7):2899–2911. [DOI] [PubMed] [Google Scholar]
- [121].Jayachandran M, Chung SSM, Xu BJ. A critical review of the relationship between dietary components, the gut microbe Akkermansia muciniphila, and human health. Crit Rev Food Sci. 2020;60(13):2265–2276. [DOI] [PubMed] [Google Scholar]
- [122].Fassatoui M, Lopez-Siles M, Diaz-Rizzolo DA, et al. Gut microbiota imbalances in Tunisian participants with type 1 and type 2 diabetes mellitus. Biosci Rep. 2019;39:BSR20182348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Shin NR, Gu N, Choi HS, et al. Combined effects of Scutellaria baicalensis with metformin on glucose tolerance of patients with type 2 diabetes via gut microbiota modulation. Am J Physiol-Endoc M. 2020;318(1). [DOI] [PubMed] [Google Scholar]
- [124].Li XX, Zhang XX, Zhang R, et al. Gut modulation based anti-diabetic effects of carboxymethylated wheat bran dietary fiber in high-fat diet/streptozotocin-induced diabetic mice and their potential mechanisms. Food Chem Toxicol. 2021;152:112235. [DOI] [PubMed] [Google Scholar]
- [125].Mabey JG, Chaston JM, Castro DG, et al. Gut microbiota differs a decade after bariatric surgery relative to a nonsurgical comparison group. Surg Obes Relat Dis. 2020;16(9):1304–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].de la Cuesta-Zuluaga J, Mueller NT, Corrales-Agudelo V, et al. Metformin is associated with higher relative abundance of mucin-degrading Akkermansia muciniphila and several short-chain fatty acid-producing microbiota in the Gut. Diabetes Care. 2017;40(1):54–62. [DOI] [PubMed] [Google Scholar]
- [127].Demirci M, Taner Z, Keskin FE, et al. Similar bacterial signatures in the gut microbiota of type 1 and type 2 diabetes patients and its association with G protein-coupled receptor 41 and 43 gene expression. J Diabetes Metab Disord. 2022;21(2):1359–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Pai CS, Wang CY, Hung WW, et al. Interrelationship of Gut microbiota, obesity, body composition and insulin resistance in Asians with type 2 diabetes mellitus. J Pers Med. 2022;12(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Tabasi M, Eybpoosh S, Heravi FS, et al. Gut microbiota and serum biomarker analyses in obese patients diagnosed with diabetes and hypothyroid disorder. Metab Syndr Relat D. 2021;19(3):144–151. [DOI] [PubMed] [Google Scholar]
- [130].Hanninen A, Toivonen R, Poysti S, et al. Akkermansia muciniphila induces gut microbiota remodelling and controls islet autoimmunity in NOD mice. Gut. 2018;67(8):1445–1453. [DOI] [PubMed] [Google Scholar]
- [131].Wang Z, Cui SY, Zhang TT, et al. Akkermansia muciniphila supplementation improves glucose tolerance in intestinal knockout mice during the daily light to dark transition. Msystems. 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Ye JM, Li YH, Wang XC, et al. Positive interactions among Corynebacterium glutamicum and keystone bacteria producing SCFAs benefited T2D mice to rebuild gut eubiosis. Food Res Int. 2023;172:113163. [DOI] [PubMed] [Google Scholar]
- [133].Qu L, Liu F, Fang Y, et al. Improvement in Zebrafish with diabetes and Alzheimer’s Disease treated with pasteurized Akkermansia muciniphila. Microbiol Spectr. 2023;11(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Niu HF, Zhou MF, Ji AY, et al. Molecular mechanism of pasteurized Akkermansia muciniphila in alleviating type 2 diabetes symptoms. J Agr Food Chem. 2024. [DOI] [PubMed] [Google Scholar]
- [135].Zhang J, Ni Y, Qian L, et al. Decreased abundance of Akkermansia muciniphila leads to the impairment of insulin secretion and glucose homeostasis in lean type 2 diabetes. Adv Sci (Weinh). 2021;8(16):2100536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Shin NR, Lee JC, Lee HY, et al. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut. 2014;63(5):727–735. [DOI] [PubMed] [Google Scholar]
- [137].Kupritz J, Angelova A, Nutman TB, et al. Helminth-induced human gastrointestinal dysbiosis: a systematic review and meta-analysis reveals insights into altered taxon diversity and microbial gradient collapse. Mbio. 2021;12(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Jenkins TP, Rathnayaka Y, Perera PK, et al. Infections by human gastrointestinal helminths are associated with changes in faecal microbiota diversity and composition. Plos One. 2017;12(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Jin X, Liu Y, Vallee I, et al. Lentinan -triggered butyrate-producing bacteria drive the expulsion of the intestinal helminth Trichinella spiralis in mice. Front Immunol. 2022;13:926765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Zhao YQ, Yang SG, Li B, et al. Alterations of the mice gut microbiome via Schistosoma japonicum ova-induced granuloma. Front Microbiol. 2019;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Wang JQ, Liu XL, Sun RH, et al. Akkermansia muciniphila participates in the host protection against helminth-induced cardiac fibrosis via TLR2. PLoS Pathog. 2023;19(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Yan XL, Han WY, Jin XD, et al. Study on the effect of koumiss on the intestinal microbiota of mice infected with Toxoplasma gondii. Sci Rep-Uk. 2022;12(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Smith CDM, Gong MH, Andrew AK, et al. Composition of the gut microbiota transcends genetic determinants of malaria infection severity and influences pregnancy outcome. Ebiomedicine. 2019;44:639–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Wang JQ, Zhao XF, Li XH, et al. Akkermansia muciniphila: a deworming partner independent of type 2 immunity. Gut Microbes. 2024;16(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Xie Y, Xu D, Yan S, et al. The impact of MIF deficiency on alterations of fecal microbiota in C57BL/6 mice induced by Trichinella spiralis infection. FASEB J. 2023;37(10). [DOI] [PubMed] [Google Scholar]
- [146].Kim H-N, Cheong HS, Kim B, et al. Human gut microbiota from hepatitis B virus-infected individuals is associated with reduced triglyceride level in mice: faecal transplantation study. Microbes Infect. 2023(105281). [DOI] [PubMed] [Google Scholar]
- [147].Chen Y, Lin H, Cole M, et al. Signature changes in gut microbiome are associated with increased susceptibility to HIV-1 infection in MSM. Microbiome. 2021;9(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Hu X, Zhao Y, Yang Y, et al. Akkermansia muciniphila improves host defense against influenza virus infection. Front Microbiol. 2021;11:586476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Upadhyay V, Suryawanshi R, Tasoff P, et al. Mild SARS-CoV-2 infection results in long-lasting microbiota instability. bioRxiv:Preprint Serv Biol. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Talaga-Cwiertnia K, Sroka-Oleksiak A, Zapala B, et al. New insights into diversity of the upper respiratory tract microbiota and its relationship with SARS -CoV-2 viral load in the nasopharyngeal epithelial cells in patients with COVID-19. Pol Arch Intern Med Polskie Archiwum Medycyny Wewnetrznej. 2023;133(7–8):16442. [DOI] [PubMed] [Google Scholar]
- [151].Xie J, Li H, Zhang X, et al. Akkermansia muciniphila protects mice against an emerging tick-borne viral pathogen. Nat Microbiol. 2023;8(1). [DOI] [PubMed] [Google Scholar]
- [152].Niederreiter L, Adolph TE, Tilg H. Food, microbiome and colorectal cancer. Dig Liver Dis. 2018;50(7):647–652. [DOI] [PubMed] [Google Scholar]
- [153].Jiang HY, Li J, Zhang B, et al. Intestinal Flora Disruption and Novel Biomarkers Associated With Nasopharyngeal Carcinoma. Front Oncol. 2019;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Shi LL, Sheng JY, Chen GZ, et al. Combining IL-2-based immunotherapy with commensal probiotics produces enhanced antitumor immune response and tumor clearance. J Immunother Cancer. 2020;8(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Luo ZW, Xia K, Liu YW, et al. Extracellular Vesicles from Akkermansia muciniphila Elicit Antitumor Immunity Against Prostate Cancer via Modulation of CD8(+) T Cells and Macrophages. Int J Nanomed. 2021;16:2949–2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Filippi M, Bar-Or A, Piehl F, et al. Multiple sclerosis. Nat Rev Dis Primers. 2018;4. [DOI] [PubMed] [Google Scholar]
- [157].Jangi S, Gandhi R, Cox LM, et al. Alterations of the human gut microbiome in multiple sclerosis. Nat Commun. 2016;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Baranzini SE, i C. Gut microbiome of multiple sclerosis patients and paired household healthy controls reveal associations with disease risk and course. Cell. 2022;185(19):3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Cekanaviciute E, Yoo BB, Runia TF, et al. Gut bacteria from multiple sclerosis patients modulate human T cells and exacerbate symptoms in mouse models. Proc Natl Acad Sci U S A. 2017;114(40):10713–10718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Giovannini MG, Lana D, Traini C, et al. The Microbiota-Gut-Brain Axis and Alzheimer Disease. From Dysbiosis to Neurodegeneration: focus on the Central Nervous System Glial Cells. J Clin Med. 2021;10(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Zhu XQ, Han Y, Du J, et al. Microbiota-gut-brain axis and the central nervous system. Oncotarget. 2017;8(32):53829–53838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Olmo BGM, Butler MJ, Barrientos RM. Evolution of the Human Diet and Its Impact on Gut Microbiota, Immune Responses, and Brain Health. Nutrients. 2021;13(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Jian HF, Liu YT, Wang XM, et al. Akkermansia muciniphila as a Next-Generation Probiotic in Modulating Human Metabolic Homeostasis and Disease Progression: a Role Mediated by Gut-Liver-Brain Axes? Int J Mol Sci. 2023;24(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Wang L, Christophersen CT, Sorich MJ, et al. Low Relative Abundances of the Mucolytic Bacterium Akkermansia muciniphila and Bifidobacterium spp. in Feces of Children with Autism. Appl Environ Microb. 2011;77(18):6718–6721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Ou ZH, Deng LL, Lu Z, et al. Protective effects of Akkermansia muciniphila on cognitive deficits and amyloid pathology in a mouse model of Alzheimer’s disease. Nutr Diabetes. 2020;10(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Grander C, Adolph TE, Wieser V, et al. Recovery of ethanol-induced Akkermansia muciniphila depletion ameliorates alcoholic liver disease. Gut. 2018;67(5):892. [DOI] [PubMed] [Google Scholar]
- [167].Blacher E, Bashiardes S, Shapiro H, et al. Potential roles of gut microbiome and metabolites in modulating ALS in mice. Nature. 2019;572(7770):474. [DOI] [PubMed] [Google Scholar]
- [168].Machado D, Almeida D, Seabra CL, et al. Uncovering Akkermansia muciniphila resilience or susceptibility to different temperatures, atmospheres and gastrointestinal conditions. Anaerobe. 2020;61. [DOI] [PubMed] [Google Scholar]
- [169].Marcial-Coba MS, Cieplak T, Cahu TB, et al. Viability of microencapsulated Akkermansia muciniphila and Lactobacillus plantarum during freeze-drying, storage and in vitro simulated upper gastrointestinal tract passage. Food Funct. 2018;9(11):5868–5879. [DOI] [PubMed] [Google Scholar]
- [170].Marcial-Coba MS, Saaby L, Knochel S, et al. Dark chocolate as a stable carrier of microencapsulated Akkermansia muciniphila and Lactobacillus casei. FEMS Microbiol Lett. 2019;366(2). [DOI] [PubMed] [Google Scholar]
- [171].Lin XQ, Chen W, Ma K, et al. Akkermansia muciniphila Suppresses High-Fat Diet-Induced Obesity and Related Metabolic Disorders in Beagles. Molecules. 2022;27(18). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Chassaing B, Koren O, Goodrich JK, et al. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature. 2015;519(7541):92–U192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Marcella C, Cui BT, Kelly CR, et al. Systematic review: the global incidence of faecal microbiota transplantation-related adverse events from 2000 to 2020. Aliment Pharm Ther. 2021;53(1):33–42. [DOI] [PubMed] [Google Scholar]
- [174].Luo YH, Lan C, Li H, et al. Rational consideration of Akkermansia muciniphila targeting intestinal health: advantages and challenges. NPJ Biofilms Microbi. 2022;8(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Ayres JS, Trinidad NJ, Vance RE. Lethal inflammasome activation by a multidrug- resistant pathobiont upon antibiotic disruption of the microbiota. Nat Med. 2012;18(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Howe C, Kim SJ, Mitchell J, et al. Differential expression of tumor-associated genes and altered gut microbiome with decreased confer a tumor-preventive microenvironment in intestinal epithelial Pten-deficient mice. Bba-Mol Basis Dis. 2018;1864(12):3746–3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Huang PY, Yang YC, Wang C, et al. Increase in akkermansiaceae in Gut Microbiota of Prostate Cancer-Bearing Mice. Int J Mol Sci. 2021;22(17). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Dey P, Chaudhuri SR. The opportunistic nature of gut commensal microbiota. Crit Rev Microbiol. 2023;49(6):739–763. [DOI] [PubMed] [Google Scholar]
- [179].Shin NR, Lee JC, Lee HY, et al. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut. 2014;63(5):727–735. [DOI] [PubMed] [Google Scholar]
- [180].Perraudeau F, McMurdie P, Bullard J, et al. Improvements to postprandial glucose control in subjects with type 2 diabetes: a multicenter, double blind, randomized placebo-controlled trial of a novel probiotic formulation. BMJ Open Diab Res Ca. 2020;8(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Abbasi A, Bazzaz S, Da Cruz AG, et al. A Critical Review on Akkermansia muciniphila: functional Mechanisms, Technological Challenges, and Safety Issues. Probiot Antimicro. 2023. [DOI] [PubMed] [Google Scholar]
- [182].Jang YJ, Kim WK, Han DH, et al. Lactobacillus fermentum species ameliorate dextran sulfate sodium-induced colitis by regulating the immune response and altering gut microbiota. Gut Microbes. 2019;10(6):696–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Becken B, Davey L, Middleton DR, et al. Genotypic and Phenotypic Diversity among Human Isolates of Akkermansia muciniphila. Mbio. 2021;12(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Pal A, Sun S, Armstrong M, et al. Beneficial effects of eicosapentaenoic acid on the metabolic profile of obese female mice entails upregulation of HEPEs and increased abundance of enteric Akkermansia muciniphila. Bba-Mol Cell Biol L. 2022;1867(1):159059. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing not applicable-no new data generated.


