Abstract
Background and objectives:
Sex chromosome trisomy (SCT) is a common chromosomal abnormality associated with increased risks for early developmental delays and neurodevelopmental disorders later in childhood. Our objective was to quantify the spectrum of early developmental milestones in SCT. We hypothesized later milestone achievement in SCT than the general population.
Methods:
Data were collected as part of the eXtraordinarY Babies Study, a prospective natural history of developmental and health trajectories in a prenatally identified sample of infants with SCT. Parent reported, clinician-validated, early motor and language milestones were collected at 2, 6, 12, 18, 24, and 36-months. Age distributions of milestone achievement were compared with normative data.
Results:
In all SCT conditions, compared with normative data, there was increased variability and a later median age of skill development across multiple gross motor and expressive language milestones. Results also show a significant amount of overlap with the general pediatric population, suggesting that for many children with prenatally identified SCT, early milestones present within, or close to, the expected timeline.
Conclusions:
As increasing numbers of infants with prenatal SCT diagnoses present at pediatric practices, we provide an evidence-based schedule of milestone achievement in SCT as a tool for pediatricians and families. Detailed data on SCT milestones can support clinical interpretation of milestone achievement. Increased variability and later median age of milestone acquisition in SCT compared to norms support consideration of all infants with SCT as high risk.
Background
Sex chromosome trisomy (SCT) (XXY/Klinefelter syndrome, XYY/Jacob syndrome, XXX/Trisomy X) is a common chromosomal abnormality, occurring in 1 of every 500 live births.1 Prior SCT research, limited by ascertainment bias and small sample sizes, has provided broad descriptions of early development, including profiles of increased risk for delays in gross motor and communication2,3 and high rates of early intervention.4 Recent advances in noninvasive prenatal screening5 have led to increasing rates of prenatally identified SCT and subsequently a growing population of infants with a confirmed SCT diagnosis early in life. As the literature lacks concrete information on the timing of typical milestone achievement in SCT, parents and providers lack clear guidance on what to expect during a child’s early years.
Close surveillance of key developmental milestones is a critical part of pediatric care, supporting the promotion of healthy development and the early detection of potential developmental delays.6 However, common surveillance methods (e.g., CDC milestones7 checklists) may have less utility for children with genetic conditions and those at-risk for delays such as infants born prematurely. Research has shown that the timing of milestone acquisition differs from the general population in children with Down syndrome (DS),8 fragile X (FXS),9 and preterm and very low birthrate infants.9–11 If this is the case for SCT as well, early developmental care should go beyond surveillance and general screening to include periodic direct developmental assessment. Further, a clear understanding of when children with SCT acquire key developmental milestones is critical for setting reasonable expectations, alerting families to potential concerns, and guiding providers in their referrals for early intervention. This is especially important with the increased frequency of prenatal SCT diagnoses, as pediatricians will be responsible for developmental care in a higher number of infants with SCT presenting to their practices. Therefore, the primary purpose of this study is to fill this gap in the SCT literature with a current, evidence-informed schedule of key early gross motor and language milestone achievement for each of the SCT conditions. These findings will support a more personalized approach to monitoring and care in SCT. Comparisons with previously published normative data to the three SCT conditions will provide critical context and a richer understanding of the SCT phenotypes, and guide recommendations for early developmental care.
Methods
Data were collected as part of the IRB-approved eXtraordinarY Babies Natural History Study, which leverages recent advances in genetic testing with a prospective investigation of the developmental and health trajectories in a prenatally identified sample of infants with SCT (ClinicalTrials.gov NCT03396562; COMIRB 17–0118; Nemours IRB# 1151006). Inclusion criteria are prenatal identification of SCT (by cfDNA, chorionic villi sampling, and/or amniocentesis) with diagnostic confirmatory karyotype (chorionic villi sampling, amniocentesis, or postnatal), English or Spanish speaking, and child age of 6 weeks to 12 months upon enrollment. Children are excluded from participation if there is a previous diagnosis of a different genetic or metabolic disorder with neurodevelopmental or endocrine involvement, prematurity less than 34 weeks gestational age, a complex congenital malformation not previously associated with SCT, history of significant neonatal complications (i.e., intraventricular hemorrhage, meningitis, hypoxic-ischemic encephalopathy), or known central nervous system (CNS) malformation identified by neuroimaging. Study visits are conducted regularly at ages 2, 6, 12, 18, 24 months, and then yearly at two sites (Colorado and Delaware) with a combination of in-person and telehealth visits. Visits include comprehensive health and developmental history, current interventions, physical examination, and a battery of developmental assessments and parent questionnaires. Participants with gestational age <37 weeks at birth were excluded from this analysis. Tartaglia et al., (2020) provides additional details on the eXtraordinarY Babies natural history study protocol.
Developmental Milestone Measurement
Data on the timing of milestones were collected at every study visit as part of a parent completed health and development questionnaire asking parents to report the age (in months) their child achieved key developmental milestones, including eight gross motor skills (rolling front to back, rolling back to front, sitting independently, crawling, cruising, walking, running, jumping) and four expressive communication milestones (cooing, babbling, single words, 2-word phrases). These milestones were chosen because they can be easily observed by parents within a natural setting and delays may predict other areas of known concern in older children with SCT. During the study visit, a physician then reviewed the parent questionnaire responses by interview to confirm ages and parent understanding of the milestone. If there were discrepancies between parent reported skill and the milestone achieved (for example the parent reported the infant was “sitting independently” but physician confirmed the infant was only sitting in a propped position), the physician would adjust the data on the physician data form. The physician data form was used for data analysis.
Normative data
Each of the twelve developmental milestones collected for the study sample were compared with existing published norms. We included normative data from studies with published values for the 25th, 50th, 75th, and 90th percentiles for the milestones of interest from the Denver II Scales,12 the World Health Organization (WHO) Motor Development Study,13 and the Primitive Reflex Profile (PRP).14 As normative data were not available from a single source for all twelve milestones, we used the Denver II whenever possible (sitting, walking, running, jumping, cooing, babbling, single words, 2-word phrases). For milestones that were not included on the Denver II, we used data from the WHO (crawling and cruising) and the PRP (rolling front to back, rolling back to front). As the PRP normative dataset only provided means and standard deviations, percentiles were estimated theoretically under the assumption of a normal distribution.
Analysis
All analyses were performed in R, version 4.4.0. Descriptive summaries by SCT are presented as median [interquartile range] and N (%). Demographic differences between SCTs were tested using Kruskal-Wallis tests for continuous variables and Fisher’s-Exact tests for categorical variables. For each milestone, achieved ages earlier than the normative 2.5th percentile were removed as early outliers. Normative and SCT milestone ages are visualized from their 25th – 50th, 50th – 75th, and 75th – 90th percentiles. Differences in milestones were analyzed using simulated data based on the normative percentiles, under the assumption of a non-normal distribution, and were tested with Wilcoxon Rank-Sum tests. Differences in milestones were also analyzed between children who had a history of early intervention (EI) therapies and those who did not. Exploring whether there was an overall relationship of milestone achievement with receiving EI therapy was important to ensure therapies were not significantly impacting the distribution of milestones achievement.
Results
Participants include 298 young children with prenatally identified SCT, including 174 with XXY, 50 with XYY, and 74 with XXX. All included children had at least one milestone age reported. Table 1 shows sample characteristics. At the time of analysis, the median age of patients included was 4.5 years with the youngest group being XYY children, with a median age of 2.6. The majority of the cohort was white (81.9%) and non-Hispanic/Latinx (83.9%). Included children had participated in the eXtraordinarY Babies study for a median of 3 years, with XXY children having participated the longest (median: 3.5 [IQR: 2.7, 3.8] years) and XYY children having participated for the shortest period of time (median: 1.2 [IQR: 0.4, 3.1] years).
Table 1.
Cohort Demographics
| Overall (N=298) | XXY (N=174) | XYY (N=50) | XXX (N=74) | P-Value | |
|---|---|---|---|---|---|
| Age (Years; As of 7/9/2024) | |||||
| Median [IQR] | 4.5 [2.9, 5.7] | 4.9 [3.8, 6.1] | 2.6 [1.4, 5.3] | 3.5 [2, 5.1] | <0.001* |
| Years in Study | |||||
| Median [IQR] | 3 [1.4, 3.8] | 3.5 [2.7, 3.8] | 1.2 [0.4, 3.1] | 2.5 [0.9, 3.5] | <0.001* |
| Race | |||||
| White | 244 (81.9%) | 138 (79.3%) | 41 (82.0%) | 65 (87.8%) | 0.119 |
| Native Hawaiian or Other Pacific Islander | 1 (0.3%) | 1 (0.6%) | 0 (0%) | 0 (0%) | |
| African American or Black | 17 (5.7%) | 13 (7.5%) | 4 (8.0%) | 0 (0%) | |
| Asian | 24 (8.1%) | 14 (8.0%) | 2 (4.0%) | 8 (10.8%) | |
| Native American or Alaska Native | 3 (1.0%) | 2 (1.1%) | 1 (2.0%) | 0 (0%) | |
| Other | 6 (2.0%) | 5 (2.9%) | 1 (2.0%) | 0 (0%) | |
| Missing | 3 (1.0%) | 1 (0.6%) | 1 (2.0%) | 1 (1.4%) | |
| Ethnicity | |||||
| Hispanic/Latinx | 45 (15.1%) | 28 (16.1%) | 6 (12.0%) | 11 (14.9%) | 0.859 |
| Non-Hispanic/Latinx | 250 (83.9%) | 145 (83.3%) | 43 (86.0%) | 62 (83.8%) | |
| Missing | 3 (1.0%) | 1 (0.6%) | 1 (2.0%) | 1 (1.4%) | |
| Hollingshead Index | |||||
| Median [IQR] | 54.5 [47.9, 59.5] | 54 [47, 59.5] | 54.5 [46.1, 59.2] | 55.5 [50.5, 59.5] | 0.619 |
| Missing | 10 (3.4%) | 1 (0.6%) | 4 (8.0%) | 5 (6.8%) | |
| Annual Family Income* | |||||
| $50,000 or less | 17 (5.7%) | 12 (6.9%) | 3 (6.0%) | 2 (2.7%) | 0.437 |
| $50,000 - $100,000 | 67 (22.5%) | 38 (21.8%) | 15 (30.0%) | 14 (18.9%) | |
| $100,000 - $250,000 | 154 (51.7%) | 91 (52.3%) | 20 (40.0%) | 43 (58.1%) | |
| > $250,000 | 54 (18.1%) | 30 (17.2%) | 11 (22.0%) | 13 (17.6%) | |
| Missing | 6 (2.0%) | 3 (1.7%) | 1 (2.0%) | 2 (2.7%) |
Significance level = 0.05. Overall differences tested using Kruskal-Wallis tests for continuous variables and Fisher’s Exact/Chi-Squared Tests for categorical variables.
Family income data reported were collected at initial eXtraordinarY Babies study visit.
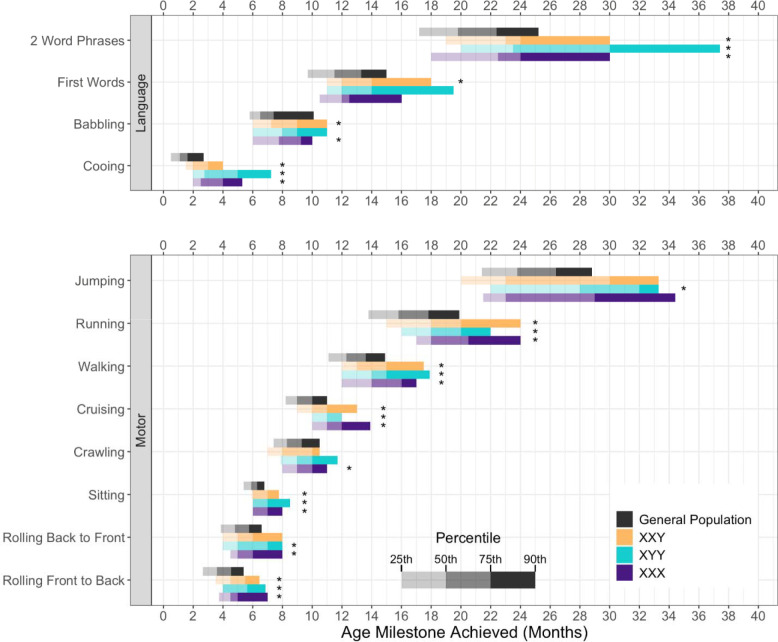
Timing of Milestone Achievement in SCT Compared with Normative Datasets.
Figure 1 depicts the age (in months) of milestone achievement for each SCT compared with reference norms. Age distributions are characterized by plotting the values for the 25th, 50th, 75th, and 90th percentiles of each milestone and comparing to normative data. Results indicate that the distributions for all twelve milestones differ (later median milestone achievement; p<0.05) from the normative dataset in at least one SCT group per milestone.
Figure 1.
Achievement of Language and Motor milestones in Sex Chromosome Trisomy compared to the general population
*: Trisomy is delayed compared to simulated data based on general population percentiles, under the assumption of a non-normal distribution. Significance level = 0.05. Differences tested with Wilcoxon Rank-Sum Tests.
General Population estimates are based on Denver II for Jumping, Running, Walking, Sitting, 2 Word Phrases, First Words, Babbling, and Cooing; WHO for Cruising and Crawling; and PRP for Rolling Back to Front and Rolling Front to Back.12–14
Group Differences.
Table 2 shows statistical results for group differences in age of milestone achievement between the SCT conditions. Results show statistically significant group differences in cooing (p=0.005); boys with XXY achieved cooing earlier than both girls with XXX (p = 0.016) and boys with XYY (p = 0.006). Overall group differences exist for crawling (p=0.050) and cruising (p=0.012). Boys with XXY achieved crawling (p=0.017) and cruising (p=0.006) at a significantly younger age than girls with XXX. All other milestone data were statistically similar across trisomy conditions.
Table 2.
Age in Months of Milestone Achievement by SCT1
| XXY (N=174) | XYY (N=50) | XXX (N=74) | Overall Kruskal-Wallis P-Value | |
|---|---|---|---|---|
|
| ||||
| Language | ||||
|
| ||||
| Cooing | N = 165 | N = 46 | N = 68 | |
| Median [IQR] | 2 [1.5, 3] | 2.8 [2, 5] | 2.5 [2, 4] | 0.005* 1, 2 |
| Babbling | N = 158 | N = 44 | N = 61 | |
| Median [IQR] | 7 [6, 9] | 7.2 [5.9, 9] | 7 [5, 9] | 0.867 |
| First Words | N = 152 | N = 36 | N = 55 | |
| Median [IQR] | 12 [11, 14] | 12 [11, 14] | 12 [10.5, 12.5] | 0.557 |
| 2 Word Phrases | N = 135 | N = 23 | N = 42 | |
| Median [IQR] | 23 [18, 24] | 23 [19, 30] | 22.5 [18, 24] | 0.637 |
|
| ||||
| Motor | ||||
|
| ||||
| Rolling Front to Back | N = 162 | N = 44 | N = 64 | |
| Median [IQR] | 4.5 [3.5, 5.5] | 4 [4, 5.6] | 4.5 [3.8, 5] | 0.891 |
| Rolling Back to Front | N = 162 | N = 43 | N = 64 | |
| Median [IQR] | 5 [4, 6] | 5 [4, 7] | 5 [4.4, 6] | 0.193 |
| Sitting | N = 164 | N = 45 | N = 60 | |
| Median [IQR] | 6 [5.4, 7] | 6 [6, 7] | 6 [5.5, 7] | 0.354 |
| Crawling | N = 162 | N = 44 | N = 62 | |
| Median [IQR] | 8 [7, 9.9] | 9 [7.9, 10] | 9 [8, 10] | 0.050*2 |
| Cruising | N = 157 | N = 41 | N = 62 | |
| Median [IQR] | 10 [9, 11] | 11 [10, 12] | 11 [10, 12] | 0.012*2 |
| Walking | N = 156 | N = 32 | N = 53 | |
| Median [IQR] | 13 [12, 15] | 14 [12, 15] | 14 [12, 16] | 0.239 |
| Running | N = 145 | N = 27 | N = 51 | |
| Median [IQR] | 18 [15, 20] | 18 [16, 20] | 18 [17, 20.5] | 0.177 |
| Jumping | N = 131 | N = 22 | N = 43 | |
| Median [IQR] | 23 [20, 30] | 26.2 [22, 31.5] | 23 [21.5, 29] | 0.374 |
Significant difference in at least one trisomy; alpha = 0.05
Pairwise test XXY ≠ XYY; Bonferroni adjusted alpha = 0.017
Pairwise test XXY ≠ XXX; Bonferroni adjusted alpha = 0.017
Comparisons with CDC Milestones
Table 3 shows the percent of children by SCT condition who did not achieve milestones by the age listed on the CDC milestones checklists (CDC milestones purport to represent the specific health supervision visit age when ≥75% or more of children are expected to demonstrate the skill).
Table 3.
Frequencies of children with SCT delayed in milestones according to ages set by CDC Milestones checklists.
| Milestone | Age | XXY (Total N = 174) | XYY (Total N = 50) | XXX (Total N = 74), |
|---|---|---|---|---|
|
| ||||
| Language | ||||
|
| ||||
| Cooing | 4 months | 16/165 (9.7%) | 13/46 (28.3%) | 11/68 (16.2%) |
| Babbling | 9 months | 35/158 (22.12%) | 9/44 (20.5%) | 13/61 (21.3%) |
| First Words | 15 months | 32/1582 (21.1%) | 8/36 (22.2%) | 10/55 (18.2%) |
| 2 Word Phrases | 24 months | 33/135 (24.4%) | 9/33 (39.1%) | 10/42 (23.8%) |
| Motor | ||||
|
| ||||
| Rolling Front to Back | 6 months | 17/162 (10.5%) | 7/44 (15.9%) | 9/64 (14.1%) |
| Sitting Independently | 9 months | 3/164 (1.8%) | 1/45 (2.2%) | 2/60 (3.3%) |
| Cruising | 12 months | 22/157 (14%) | 3/41 (7.3%) | 13/62 (21%) |
| Walking | 15 months | 33/156 (21.2%) | 7/32 (21.9%) | 19/53 (35.9%) |
| Running | 24 months | 12/145 (8.3%) | 1/27 (3.7%) | 4/51 (7.8%) |
| Jumping | 30 months | 22/131 (16.8%) | 6/22 (27.3%) | 7/43 (16.3%) |
N (%; p-value): Number (%) of children in each trisomy that achieved the milestone later than the ages listed on CDC milestone checklists. Total sample sizes differ for each milestone and trisomy.
CDC cut points were not available for rolling back to front and crawling
Consideration of Early Intervention Therapies
Of the 298 children included, 187 (63.8%) had received EI therapy, started either proactively due to risk for delays or in response to developmental concerns in one or more developmental domains. There were no differences in therapy rates between the SCT conditions. Within our cohort, children with history of EI achieved milestones significantly later than children who had not (p<0.001 for all milestones). This is likely because those with identified delays were more likely to be referred for developmental therapies.
Discussion
This study represents the first report on developmental milestone achievement in prenatally identified SCT and provides a novel milestone chart that can help parents and professionals better quantify and visualize what “increased risk for developmental delay” means in SCT conditions. These cohorts were not referred for any concerns and thus were as close to “population based” as possible. In all SCT conditions, there was a later median age of skill development across multiple gross motor and expressive language milestones than reported in normative datasets. This includes both early milestones such as cooing and rolling, and later milestones including 2-word phrases, walking, and running. Furthermore, there was more variability in the age range for milestone achievement in our sample compared with reference norms, with the range of acquisition for all milestones extending later in life for children with SCT. These findings support the need to consider infants with SCT as a group at increased risk for delays and deserving of closer developmental monitoring given that age of early motor and language milestones have been shown to predict longitudinal outcomes across all developmental domains in the general population and clinical samples.15–26
These results confirm prior research indicating increased risk for developmental delays in children with SCT,4,27,28 consistent with findings in other genetic disorders where milestone acquisition is different than population norms.29 However, unlike other genetic conditions such as DS and FXS,30,31 our results also show a significant amount of overlap with the general pediatric population. Figure 1 shows that, for many children with prenatally identified SCT, early milestones present within, or close to, the expected timeline. While this is reassuring, there are known later risks in SCT for many neurodevelopmental diagnoses including speech-language disorders, learning disabilities, ADHD, executive dysfunction, motor skill deficits, and autism spectrum disorders,32–44 which all benefit from earlier diagnosis and evidence-based treatments. Thus, careful attention to development trajectories is warranted as early interventions may help minimize these morbidities.
The variability of the phenotype and overlap with the general population often leads to questions of whether different developmental care pathways and extra developmental testing is needed for all infants with SCT. This is a valid concern as a relatively high proportion of individuals with SCT conditions have minimal neurodevelopmental differences with positive adult outcomes,45–47 and many go undiagnosed from their clinical presentation. Additional recommendations for developmental monitoring and evaluation may increase family stress, negatively impact parent-child relationships, and call unnecessary attention to the genetic differences in their child, as well as increase healthcare utilization and demand on a stressed early intervention system. Prospective longitudinal research is needed to clarify if indeed there are specific early risk factors predictive of poorer outcomes that would warrant stratifying children with SCT into different low versus high-risk developmental care pathways, similar to extensive work done in the congenital heart disease and prematurity populations.48,49 These pathways, however, were developed using evidence from hundreds of studies, which do not currently exist in SCT. Thus, until more prospective data is available, consideration of all infants with SCT as high risk is warranted.
Table 3 responds to our interest in whether recently published milestones from the CDC7 are appropriate for developmental surveillance in infants with SCT. Overall, a relatively small proportion of children in our sample were delayed in milestone achievement according to CDC milestones checklists (Table 3) even though their milestone acquisition was delayed as compared to other metrics (Denver II; WHO). This suggests that relying on the CDC milestone lists for SCT will fail to identify many infants with delayed milestones and is consistent with other published concerns50–52 about low sensitivity of the ages presented in the CDC milestones. It is well recognized that standard developmental screening tools designed for the general population (e.g., ASQ, PEDS)53–55 have lower sensitivity in high-risk groups, which has led to guidelines for developmental follow-up of high-risk neonates with periodic direct assessment.49,56–58 Similarly, our findings of the increased risk in SCT support that periodic direct developmental assessment should be part of SCT treatment guidelines.59
By offering detailed information on milestone achievement, we provide a valuable tool for clinicians and families to better interpret a child’s early development within the context of their SCT condition, rather than only comparing to general population norms. Further, any significant deviations from SCT norms may alert clinicians to potential risks for comorbid health conditions or an additional genetic difference. While pediatric providers can use this tool as a reference to contextualize a child’s milestone achievement, it is not intended to delay referrals for developmental evaluations or early intervention support. Parents may appreciate the more nuanced normative data as they track their child’s milestones, noting areas where their child’s development aligns with children with similar genetic profiles as well as areas of normative differences. Prior research shows parents of children with delayed milestones may have higher levels of perceived stress60 or experience guilt that they have done something to cause their child’s delays.61 A clearly defined schedule for the timing of developmental milestones specific to each SCT, when used in conjunction with normative milestones expectations, may be more palatable in supporting early developmental care.
Results showing similarities and differences in milestone achievement by karyotype (Table 2) add to the existing literature on genetic disorders by providing more specific data regarding milestone acquisition in each trisomy condition. For most milestones, SCT groups were statistically similar. This aligns with prior research showing similar early developmental and neurocognitive profiles across the SCT conditions.44,62–64 However, the XXY group did achieve several milestones earlier, including cooing 1 month earlier than both other groups and crawling and cruising 1 month earlier than those with XXX. While this may be an artifact of a larger and more variable sample size in XXY, it may also reflect differential effects of the extra X chromosome in males.65 Ongoing research with larger sample sizes for XYY and XXX will help determine if different SCT conditions have clinically relevant differences in developmental trajectories.
These study results also have practical implications for genetic counseling and are responsive to prior research findings showing that parents receiving a prenatal SCT diagnosis desire more accurate and current data on potential neurodevelopmental outcomes specific to each SCT condition. In the context of highly variable phenotypes associated with SCT, genetic counselors strive to provide guidance to parents with a new diagnosis and clarify parental perception of risks for developmental delays.66 This foundation establishes how parents understand and respond to their child’s development and behavior, especially as related to the genetic diagnosis. By providing a clearer picture of developmental expectations associated with the diagnosis, genetic counselors can more specifically inform parents about what to expect in their child’s first few years of life, as well as promote awareness, empowerment, and a proactive approach to early intervention processes to facilitate early developmental care.67
Despite the insights gained, limitations are important to consider. First, smaller sample sizes for the XXX and XYY karyotypes limit the generalizability compared with the XXY sample. Additionally, there are known limitations in normative data for milestone acquisition, including unclear and inconsistent definitions of milestones,68 ambiguity around what constitutes achievement of the milestone (partial vs. complete),69 and differences in the raters used to determine milestone achievement for normative datasets (parents vs. clinicians).68,70,71 Further, normative datasets rarely account for potential sex differences,72,73 racial and sociocultural differences,50,74,75 and known variability related to social determinants of health69 (SDoH), which further adds a degree of uncertainty to our findings. Our sample was disproportionately white, non-Hispanic with high socioeconomic status, and future studies should aim to include more representative samples. Parental recall bias is another commonly recognized challenge when evaluating parent-reported milestones,76,77 however minimized in this study with frequent visits at 2, 6, 12, 18, 24, and 36 months of age with pediatric physicians interviewing and verifying milestone achievements. Importantly, while there are many benefits to an ongoing natural history study, our study design is limited in that at the time of publication, not all participants in the sample had yet achieved all 12 milestones measured and therefore sample sizes were different for each SCT condition at each milestone. Additionally, while we explored the effect of early intervention in our analysis, the act of participating in a natural history study itself may impact developmental course. Families in the study have self-selected to participate in regularly scheduled developmental evaluations with expert SCT clinicians and to monitor developmental milestones in between visits. While a prenatally identified sample of nearly 300 infants with SCT provides a less biased dataset than prior studies, it may still not fully represent the broad spectrum of outcomes in SCT. Future results based on direct assessments through the eXtraordinarY Babies study can address these limitations and further refine our understanding of developmental trajectories and risk groups in this population.
In conclusion, developmental milestone achievement in SCT conditions is delayed compared to the general population, however only in a subset of infants with SCT. As increasing numbers of infants with prenatal SCT diagnoses present at pediatric practices, we provide an evidence-based schedule of milestone achievement in SCT as a tool for families, pediatricians, genetic counselors, and early intervention teams. Utilization of such a tool can support shared clinical decision-making between parents and providers, promoting timely referrals and identifying patterns inconsistent with SCT. However, given the paucity of prospective research identifying specific risk factors for later negative outcomes, recommended care for SCT conditions should follow practices of other high-risk conditions - with more responsive attention to developmental concerns, recognition that standard surveillance and screening tools have lower sensitivities in high-risk populations, and referrals for periodic direct developmental assessments. While more rigorous research will help identify evidence for timing of direct assessments and highest-risk groups, general publications support assessments at 6–12 months, 18–24 months, and 36 months of age.78–80
Funding:
This study was funded by the eXtraordinarY Babies Study: Natural History of Health and Neurodevelopment in Infants and Young Children with Sex Chromosome Trisomy (NIH NICHD R01HD091251, 3R01HD091251-05S1)
Abbreviations:
- SCT
Sex Chromosome Trisomy
- CDC
Centers for Disease Control
- AAP
American Academy of Pediatrics
- DS
Down syndrome
- FXS
Fragile X syndrome
- CNS
Central Nervous System
- WHO
World Health Organization
- PRP
Primitive Reflex Profile
- SDoH
Social Determinants of Health
- EI
Early Intervention
Footnotes
Disclosures:
The authors have no disclosures to report.
References:
- 1.JACOBS PA, MELVILLE M, RATCLIFFE S, KEAY AJ, SYME J. A cytogenetic survey of 11,680 newborn infants. Annals of Human Genetics. 1974;37(4):359–376. doi: 10.1111/j.1469-1809.1974.tb01843.x [DOI] [PubMed] [Google Scholar]
- 2.Ratcliffe SG. Speech and learning disorders in children with sex chromosome abnormalities. Dev Med Child Neurol. Feb 1982;24(1):80–4. doi: 10.1111/j.1469-8749.1982.tb13586.x [DOI] [PubMed] [Google Scholar]
- 3.Salbenblatt JA, Meyers DC, Bender BG, Linden MG, Robinson A. Gross and fine motor development in 47,XXY and 47,XYY males. Pediatrics. Aug 1987;80(2):240–4. [PubMed] [Google Scholar]
- 4.Thompson T, Howell S, Davis S, et al. Current survey of early childhood intervention services in infants and young children with sex chromosome aneuploidies. Am J Med Genet C Semin Med Genet. Jun 2020;184(2):414–427. doi: 10.1002/ajmg.c.31785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Screening for Fetal Chromosomal Abnormalities: ACOG Practice Bulletin, Number 226. Obstet Gynecol. Oct 2020;136(4):e48–e69. doi: 10.1097/aog.0000000000004084 [DOI] [PubMed] [Google Scholar]
- 6.Lipkin PH, Macias MM. Promoting Optimal Development: Identifying Infants and Young Children With Developmental Disorders Through Developmental Surveillance and Screening. Pediatrics. Jan 2020;145(1)doi: 10.1542/peds.2019-3449 [DOI] [PubMed] [Google Scholar]
- 7.Zubler JM, Wiggins LD, Macias MM, et al. Evidence-Informed Milestones for Developmental Surveillance Tools. Pediatrics. Mar 1 2022;149(3)doi: 10.1542/peds.2021-052138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Onnivello S, Schworer EK, Daunhauer LA, Fidler DJ. Acquisition of cognitive and communication milestones in infants with Down syndrome. J Intellect Disabil Res. Mar 2023;67(3):239–253. doi: 10.1111/jir.12893 [DOI] [PubMed] [Google Scholar]
- 9.Hinton R, Budimirovic DB, Marschik PB, et al. Parental reports on early language and motor milestones in fragile X syndrome with and without autism spectrum disorders. Dev Neurorehabil. 2013;16(1):58–66. doi: 10.3109/17518423.2012.704414 [DOI] [PubMed] [Google Scholar]
- 10.Pascal A, Govaert P, Oostra A, Naulaers G, Ortibus E, Van den Broeck C. Neurodevelopmental outcome in very preterm and very-low-birthweight infants born over the past decade: a meta-analytic review. Dev Med Child Neurol. Apr 2018;60(4):342–355. doi: 10.1111/dmcn.13675 [DOI] [PubMed] [Google Scholar]
- 11.Jeng SF, Lau TW, Hsieh WS, et al. Development of walking in preterm and term infants: age of onset, qualitative features and sensitivity to resonance. Gait Posture. Feb 2008;27(2):340–6. doi: 10.1016/j.gaitpost.2007.04.012 [DOI] [PubMed] [Google Scholar]
- 12.Frankenburg WK, Dodds J, Archer P, Shapiro H, Bresnick B. The Denver II: a major revision and restandardization of the Denver Developmental Screening Test. Pediatrics. Jan 1992;89(1):91–7. [PubMed] [Google Scholar]
- 13.WHO Motor Development Study: windows of achievement for six gross motor development milestones. Acta Paediatr Suppl. Apr 2006;450:86–95. doi: 10.1111/j.1651-2227.2006.tb02379.x [DOI] [PubMed] [Google Scholar]
- 14.Capute AJ, Palmer FB, Shapiro BK, Wachtel RC, Ross A, Accardo PJ. Primitive reflex profile: a quantitation of primitive reflexes in infancy. Dev Med Child Neurol. Jun 1984;26(3):375–83. doi: 10.1111/j.1469-8749.1984.tb04456.x [DOI] [PubMed] [Google Scholar]
- 15.Flensborg-Madsen T, Sørensen HJ, Revsbech R, Mortensen EL. Early motor developmental milestones and level of neuroticism in young adulthood: a 23-year follow-up study of the Copenhagen Perinatal Cohort. Psychol Med. Jun 2013;43(6):1293–301. doi: 10.1017/s0033291712001997 [DOI] [PubMed] [Google Scholar]
- 16.Bedford R, Pickles A, Lord C. Early gross motor skills predict the subsequent development of language in children with autism spectrum disorder. Autism Res. Sep 2016;9(9):993–1001. doi: 10.1002/aur.1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lang S, Bartl-Pokorny KD, Pokorny FB, et al. Canonical Babbling: A Marker for Earlier Identification of Late Detected Developmental Disorders? Curr Dev Disord Rep. 2019;6(3):111–118. doi: 10.1007/s40474-019-00166-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghassabian A, Sundaram R, Bell E, Bello SC, Kus C, Yeung E. Gross Motor Milestones and Subsequent Development. Pediatrics. Jul 2016;138(1)doi: 10.1542/peds.2015-4372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hua J, Williams GJ, Jin H, et al. Early Motor Milestones in Infancy and Later Motor Impairments: A Population-Based Data Linkage Study. Front Psychiatry. 2022;13:809181. doi: 10.3389/fpsyt.2022.809181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Otapowicz D, Sobaniec W, Kułak W, Okurowska-Zawada B. Time of cooing appearance and further development of speech in children with cerebral palsy. Rocz Akad Med Bialymst. 2005;50 Suppl 1:78–81. [PubMed] [Google Scholar]
- 21.Werwach A, Mürbe D, Schaadt G, Männel C. Infants’ vocalizations at 6 months predict their productive vocabulary at one year. Infant Behavior and Development. 2021/August/01/ 2021;64:101588. doi: 10.1016/j.infbeh.2021.101588 [DOI] [PubMed] [Google Scholar]
- 22.Oller DK, Seibert JM. Babbling of prelinguistic mentally retarded children. Am J Ment Retard. Jan 1988;92(4):369–75. [PubMed] [Google Scholar]
- 23.Kover ST, Edmunds SR, Ellis Weismer S. Brief Report: Ages of Language Milestones as Predictors of Developmental Trajectories in Young Children with Autism Spectrum Disorder. J Autism Dev Disord. Jul 2016;46(7):2501–7. doi: 10.1007/s10803-016-2756-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mayo J, Chlebowski C, Fein DA, Eigsti IM. Age of first words predicts cognitive ability and adaptive skills in children with ASD. J Autism Dev Disord. Feb 2013;43(2):253–64. doi: 10.1007/s10803-012-1558-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kenworthy L, Wallace G, Powell K, Anselmo C, Martin A, Black D. Early language milestones predict later language, but not autism symptoms in higher functioning children with autism spectrum disorders. Research in Autism Spectrum Disorders. July/01 2012;6:1194–1202. doi: 10.1016/j.rasd.2012.03.009 [DOI] [Google Scholar]
- 26.Wodka EL, Mathy P, Kalb L. Predictors of phrase and fluent speech in children with autism and severe language delay. Pediatrics. Apr 2013;131(4):e1128–34. doi: 10.1542/peds.2012-2221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tartaglia N, Howell S, Davis S, et al. Early neurodevelopmental and medical profile in children with sex chromosome trisomies: Background for the prospective eXtraordinarY babies study to identify early risk factors and targets for intervention. Am J Med Genet C Semin Med Genet. Jun 2020;184(2):428–443. doi: 10.1002/ajmg.c.31807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Urbanus E, Swaab H, Tartaglia N, Cordeiro L, van Rijn S. The behavioral profile of children aged 1–5 years with sex chromosome trisomy (47,XXX, 47,XXY, 47,XYY). Am J Med Genet C Semin Med Genet. Jun 2020;184(2):444–455. doi: 10.1002/ajmg.c.31788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wickstrom J, Farmer C, Green Snyder L, et al. Patterns of delay in early gross motor and expressive language milestone attainment in probands with genetic conditions versus idiopathic ASD from SFARI registries. J Child Psychol Psychiatry. Nov 2021;62(11):1297–1307. doi: 10.1111/jcpp.13492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim HI, Kim SW, Kim J, Jeon HR, Jung DW. Motor and Cognitive Developmental Profiles in Children With Down Syndrome. Ann Rehabil Med. Feb 2017;41(1):97–103. doi: 10.5535/arm.2017.41.1.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mirrett PL, Bailey DB Jr, Roberts JE, Hatton DD. Developmental Screening and Detection of Developmental Delays in Infants and Toddlers with Fragile X Syndrome. Journal of Developmental and Behavioral Pediatrics. 2004;25(1):21–27. doi: 10.1097/00004703-200402000-00004 [DOI] [PubMed] [Google Scholar]
- 32.Printzlau F, Wolstencroft J, Skuse DH. Cognitive, behavioral, and neural consequences of sex chromosome aneuploidy. J Neurosci Res. Jan 2 2017;95(1–2):311–319. doi: 10.1002/jnr.23951 [DOI] [PubMed] [Google Scholar]
- 33.Bishop DVM, Brookman-Byrne A, Gratton N, et al. Language phenotypes in children with sex chromosome trisomies. Wellcome Open Res. 2018;3:143. doi: 10.12688/wellcomeopenres.14904.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simpson NH, Addis L, Brandler WM, et al. Increased prevalence of sex chromosome aneuploidies in specific language impairment and dyslexia. Dev Med Child Neurol. Apr 2014;56(4):346–53. doi: 10.1111/dmcn.12294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boada R, Janusz J, Hutaff-Lee C, Tartaglia N. The cognitive phenotype in Klinefelter syndrome: a review of the literature including genetic and hormonal factors. Dev Disabil Res Rev. 2009;15(4):284–94. doi: 10.1002/ddrr.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bouw N, Swaab H, Tartaglia N, van Rijn S. The impact of sex chromosome trisomies (XXX, XXY, XYY) on early social cognition: Social orienting, joint attention, and theory of mind. Archives of Clinical Neuropsychology. 2022;37(1):63–77. doi: 10.1093/arclin/acab042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hong DS, Reiss AL. Cognitive and neurological aspects of sex chromosome aneuploidies. Lancet Neurol. Mar 2014;13(3):306–18. doi: 10.1016/s1474-4422(13)70302-8 [DOI] [PubMed] [Google Scholar]
- 38.van Rijn S, Swaab H, Aleman A, Kahn RS. Social behavior and autism traits in a sex chromosomal disorder: Klinefelter (47XXY) syndrome. J Autism Dev Disord. Oct 2008;38(9):1634–41. doi: 10.1007/s10803-008-0542-1 [DOI] [PubMed] [Google Scholar]
- 39.Karipidis II, Hong DS. Specific learning disorders in sex chromosome aneuploidies: Neural circuits of literacy and mathematics. Am J Med Genet C Semin Med Genet. Jun 2020;184(2):518–530. doi: 10.1002/ajmg.c.31801 [DOI] [PubMed] [Google Scholar]
- 40.Thompson T, Davis S, Janusz J, et al. Supporting students with sex chromosome aneuploidies in educational settings: Results of a nationwide survey. J Sch Psychol. Aug 2022;93:28–40. doi: 10.1016/j.jsp.2022.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martin S, Cordeiro L, Richardson P, Davis S, Tartaglia N. The Association of Motor Skills and Adaptive Functioning in XXY/Klinefelter and XXYY Syndromes. Phys Occup Ther Pediatr. 2019;39(4):446–459. doi: 10.1080/01942638.2018.1541040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tartaglia NR, Ayari N, Hutaff-Lee C, Boada R. Attention-deficit hyperactivity disorder symptoms in children and adolescents with sex chromosome aneuploidy: XXY, XXX, XYY, and XXYY. J Dev Behav Pediatr. May 2012;33(4):309–18. doi: 10.1097/DBP.0b013e31824501c8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuiper KC, Swaab H, Tartaglia N, van Buggenhout G, Wouters C, van Rijn S. The developmental impact of sex chromosome trisomies on emerging executive functions in young children: Evidence from neurocognitive tests and daily life skills. Genes Brain Behav. Jul 2022;21(6):e12811. doi: 10.1111/gbb.12811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Rijn S, Kuiper K, Bouw N, Urbanus E, Swaab H. Neurocognitive and behavioral development in young children (1–7 years) with sex chromosome trisomy. Endocr Connect. May 1 2023;12(5)doi: 10.1530/ec-22-0494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Davis SM, Teerlink CC, Lynch JA, et al. An extra X chromosome among adult women in the Million Veteran Program: A more benign perspective of trisomy X. Am J Med Genet C Semin Med Genet. Mar 5 2024:e32083. doi: 10.1002/ajmg.c.32083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Davis SM, Teerlink C, Lynch JA, et al. Prevalence, Morbidity, and Mortality of Men With Sex Chromosome Aneuploidy in the Million Veteran Program Cohort. JAMA Netw Open. Mar 4 2024;7(3):e244113. doi: 10.1001/jamanetworkopen.2024.4113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Berglund A, Stochholm K, Gravholt CH. The epidemiology of sex chromosome abnormalities. Am J Med Genet C Semin Med Genet. Jun 2020;184(2):202–215. doi: 10.1002/ajmg.c.31805 [DOI] [PubMed] [Google Scholar]
- 48.Sood E, Newburger JW, Anixt JS, et al. Neurodevelopmental Outcomes for Individuals With Congenital Heart Disease: Updates in Neuroprotection, Risk-Stratification, Evaluation, and Management: A Scientific Statement From the American Heart Association. Circulation. Mar 26 2024;149(13):e997–e1022. doi: 10.1161/cir.0000000000001211 [DOI] [PubMed] [Google Scholar]
- 49.Lipkin PH, Macias MM, COUNCIL ON CHILDREN WITH DISABILITIES SOD, PEDIATRICS B. Promoting Optimal Development: Identifying Infants and Young Children With Developmental Disorders Through Developmental Surveillance and Screening. Pediatrics. 2020;145(1)doi: 10.1542/peds.2019-3449 [DOI] [PubMed] [Google Scholar]
- 50.Roberts MY, Sone BJ, Jones MK, et al. What the Evidence Does (and Does Not) Show for the Centers for Disease Control and Prevention Child Development Milestones: An Illustrative Example Using Expressive Vocabulary. J Speech Lang Hear Res. Sep 13 2023;66(9):3622–3632. doi: 10.1044/2023_jslhr-23-00020 [DOI] [PubMed] [Google Scholar]
- 51.Association AS-L-H. ASHA statement on CDC’s updated developmental milestones. Accessed August 14, 2024, https://www.asha.org/about/statements/ASHA-Statement-on-CDCDevelopmental-Milestones/
- 52.Team TIS. No SLPs were in the room where it happened. The Informed SLP, LLC. Accessed August 14, 2024, https://www.theinformedslp.com/review/no-sl-ps-were-in-the-room-where-it-happened [Google Scholar]
- 53.Limbos MM, Joyce DP. Comparison of the ASQ and PEDS in screening for developmental delay in children presenting for primary care. J Dev Behav Pediatr. Sep 2011;32(7):499–511. doi: 10.1097/DBP.0b013e31822552e9 [DOI] [PubMed] [Google Scholar]
- 54.Sices L, Stancin T, Kirchner L, Bauchner H. PEDS and ASQ developmental screening tests may not identify the same children. Pediatrics. Oct 2009;124(4):e640–7. doi: 10.1542/peds.2008-2628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sheldrick RC, Marakovitz S, Garfinkel D, Carter AS, Perrin EC. Comparative Accuracy of Developmental Screening Questionnaires. JAMA Pediatr. Apr 1 2020;174(4):366–374. doi: 10.1001/jamapediatrics.2019.6000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Voller SMB. Follow-Up Care for High-Risk Preterm Infants. Pediatr Ann. Apr 1 2018;47(4):e142–e146. doi: 10.3928/19382359-20180325-03 [DOI] [PubMed] [Google Scholar]
- 57.Johnson S, Marlow N. Developmental screen or developmental testing? Early Hum Dev. Mar 2006;82(3):173–83. doi: 10.1016/j.earlhumdev.2006.01.008 [DOI] [PubMed] [Google Scholar]
- 58.Marks K, Hix-Small H, Clark K, Newman J. Lowering developmental screening thresholds and raising quality improvement for preterm children. Pediatrics. Jun 2009;123(6):1516–23. doi: 10.1542/peds.2008-2051 [DOI] [PubMed] [Google Scholar]
- 59.Gravholt CH, Ferlin A, Gromoll J, et al. New developments and future trajectories in supernumerary sex chromosome abnormalities: a summary of the 2022 3rd International Workshop on Klinefelter Syndrome, Trisomy X, and XYY. Endocr Connect. Mar 1 2023;12(3)doi: 10.1530/ec-22-0500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Andrioni F, Coman C, Ghita RC, Bularca MC, Motoi G, Fulger IV. Anxiety, Stress, and Resilience Strategies in Parents of Children with Typical and Late Psychosocial Development: Comparative Analysis. Int J Environ Res Public Health. Feb 14 2022;19(4)doi: 10.3390/ijerph19042161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Scherr CL, Getachew-Smith HJ, Sudec L, Brooks JJ, Roberts M. Parents’ sensemaking processes in the identification of developmental delays and engagement with early intervention services. Soc Sci Med. Jun 2020;255:112941. doi: 10.1016/j.socscimed.2020.112941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Leggett V, Jacobs P, Nation K, Scerif G, Bishop DV. Neurocognitive outcomes of individuals with a sex chromosome trisomy: XXX, XYY, or XXY: a systematic review. Dev Med Child Neurol. Feb 2010;52(2):119–29. doi: 10.1111/j.1469-8749.2009.03545.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Raznahan A, Lee NR, Greenstein D, et al. Globally Divergent but Locally Convergent X- and Y-Chromosome Influences on Cortical Development. Cereb Cortex. Jan 2016;26(1):70–9. doi: 10.1093/cercor/bhu174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Urbanus E, Swaab H, Tartaglia N, Stumpel C, van Rijn S. Structural and pragmatic language in young children with sex chromosome trisomy (XXX, XXY, XYY): Predictive value for neurobehavioral problems one year later. Clin Neuropsychol. Apr 2023;37(3):650–675. doi: 10.1080/13854046.2022.2067078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Green T, Flash S, Reiss AL. Sex differences in psychiatric disorders: what we can learn from sex chromosome aneuploidies. Neuropsychopharmacology. Jan 2019;44(1):9–21. doi: 10.1038/s41386-018-0153-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Biesecker B. Genetic Counseling and the Central Tenets of Practice. Cold Spring Harb Perspect Med. Mar 2 2020;10(3)doi: 10.1101/cshperspect.a038968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Reimers R, High F, Kremen J, Wilkins-Haug L. Prenatal diagnosis of sex chromosome aneuploidy-What do we tell the prospective parents? Prenat Diagn. Feb 2023;43(2):250–260. doi: 10.1002/pd.6256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Matsubara K, Hattori T, Narumi S. Achievement of Developmental Milestones Recorded in Real Time: A Mobile App-Based Study. J Pediatr. Jun 2022;245:201–207.e9. doi: 10.1016/j.jpeds.2022.02.018 [DOI] [PubMed] [Google Scholar]
- 69.Sheldrick RC, Schlichting LE, Berger B, et al. Establishing New Norms for Developmental Milestones. Pediatrics. Dec 2019;144(6)doi: 10.1542/peds.2019-0374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cepanec M, Lice K, Simleša S. Mother-father differences in screening for developmental delay in infants and toddlers. J Commun Disord. Jul-Aug 2012;45(4):255–62. doi: 10.1016/j.jcomdis.2012.04.002 [DOI] [PubMed] [Google Scholar]
- 71.Majnemer A, Rosenblatt B. Reliability of parental recall of developmental milestones. Pediatric Neurology. 1994/June/01/ 1994;10(4):304–308. doi: 10.1016/0887-8994(94)90126-0 [DOI] [PubMed] [Google Scholar]
- 72.Assessment of sex differences and heterogeneity in motor milestone attainment among populations in the WHO Multicentre Growth Reference Study. Acta Paediatr Suppl. Apr 2006;450:66–75. doi: 10.1111/j.1651-2227.2006.tb02377.x [DOI] [PubMed] [Google Scholar]
- 73.Sudry T, Amit G, Zimmerman DR, et al. Sex-Specific Developmental Scales for Surveillance. Pediatrics. 2024;153(4)doi: 10.1542/peds.2023-062483 [DOI] [PubMed] [Google Scholar]
- 74.Kelly Y, Sacker A, Schoon I, Nazroo J. Ethnic differences in achievement of developmental milestones by 9 months of age: The Millennium Cohort Study. Dev Med Child Neurol. Oct 2006;48(10):825–30. doi: 10.1017/s0012162206001770 [DOI] [PubMed] [Google Scholar]
- 75.Lansdown RG, Goldstein H, Shah PM, et al. Culturally appropriate measures for monitoring child development at family and community level: a WHO collaborative study. Bulletin of the World Health Organization. 1996. 1996;74(3):283–290. [PMC free article] [PubMed] [Google Scholar]
- 76.Ozonoff S, Li D, Deprey L, Hanzel EP, Iosif AM. Reliability of parent recall of symptom onset and timing in autism spectrum disorder. Autism. Oct 2018;22(7):891–896. doi: 10.1177/1362361317710798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Majnemer A, Rosenblatt B. Reliability of parental recall of developmental milestones. Pediatr Neurol. Jun 1994;10(4):304–8. doi: 10.1016/0887-8994(94)90126-0 [DOI] [PubMed] [Google Scholar]
- 78.Davis S, Howell S, Wilson R, et al. Advances in the Interdisciplinary Care of Children with Klinefelter Syndrome. Adv Pediatr. Aug 2016;63(1):15–46. doi: 10.1016/j.yapd.2016.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wigby K, D’Epagnier C, Howell S, et al. Expanding the phenotype of Triple X syndrome: A comparison of prenatal versus postnatal diagnosis. Am J Med Genet A. Nov 2016;170(11):2870–2881. doi: 10.1002/ajmg.a.37688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tartaglia N, Howell S, Wilson R, et al. The eXtraordinarY Kids Clinic: an interdisciplinary model of care for children and adolescents with sex chromosome aneuploidy. J Multidiscip Healthc. 2015;8:323–34. doi: 10.2147/jmdh.S80242 [DOI] [PMC free article] [PubMed] [Google Scholar]