Abstract
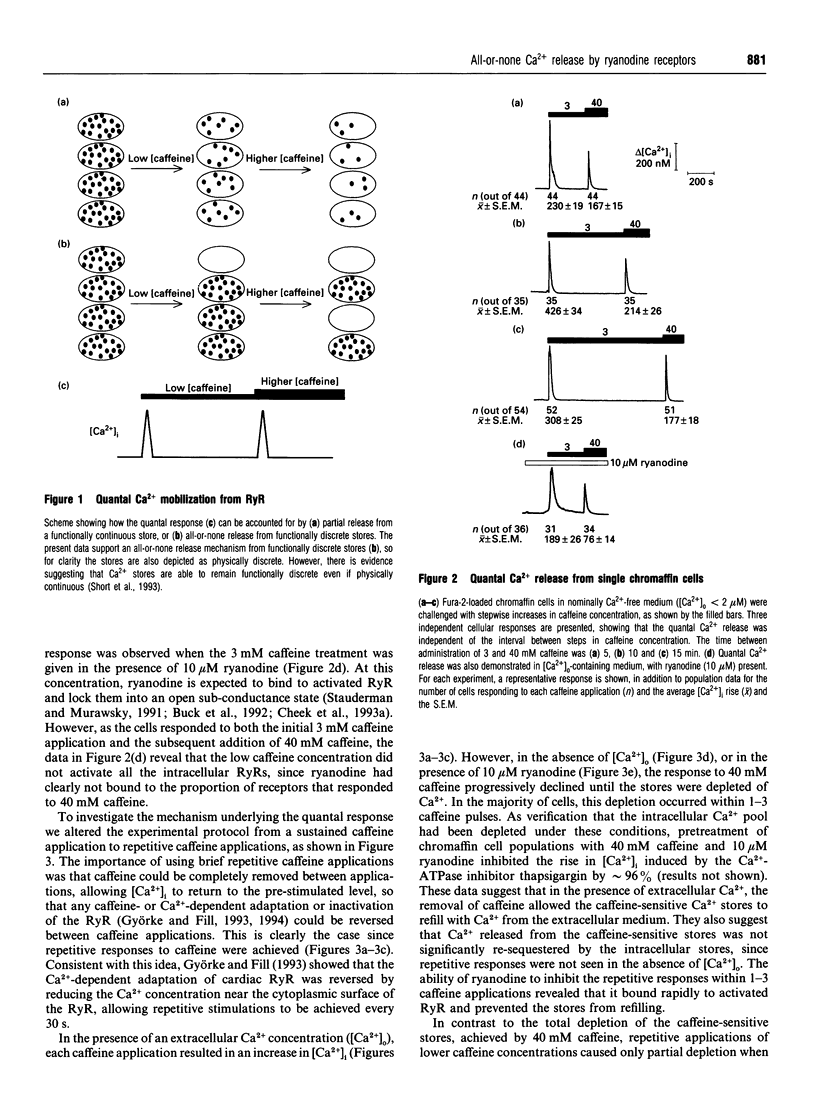
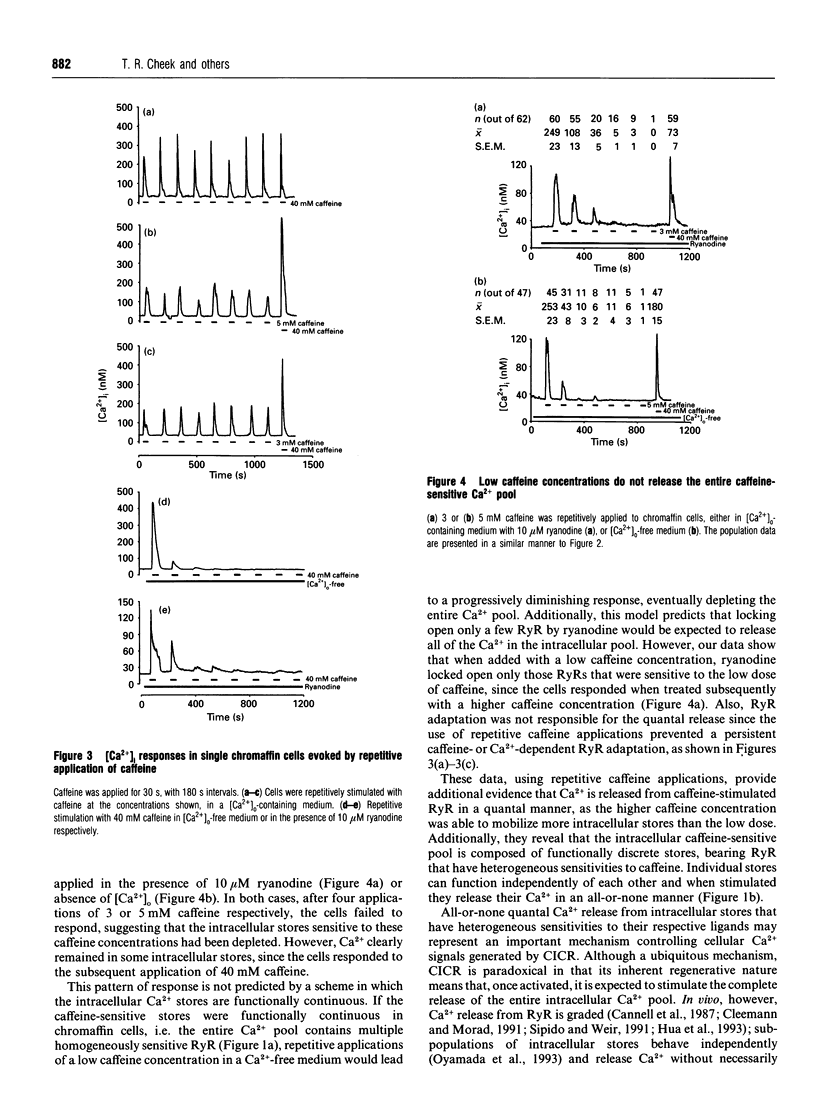
Low caffeine concentrations were unable to completely release the caffeine- and ryanodine-sensitive intracellular Ca2+ pool in intact adrenal chromaffin cells. This 'quantal' Ca2+ release is the same as that previously observed with inositol Ins(1,4,5)P3-induced Ca2+ release. The molecular mechanism underlying quantal Ca2+ release from the ryanodine receptor was investigated using fura-2 imaging of single chromaffin cells. Our data indicate that the intracellular caffeine-sensitive Ca2+ pool is composed of functionally discrete stores, that possess heterogeneous sensitivities to caffeine. These stores are mobilized by caffeine in a concentration-dependent fashion, and, when stimulated, individual stores release their Ca2+ in an 'all-or-none' manner. Such quantal Ca2+ release may be responsible for graded Ca2+ responses in single cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J. Inositol trisphosphate and calcium signalling. Nature. 1993 Jan 28;361(6410):315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I., Watras J., Ehrlich B. E. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 1991 Jun 27;351(6329):751–754. doi: 10.1038/351751a0. [DOI] [PubMed] [Google Scholar]
- Bootman M. D., Berridge M. J., Taylor C. W. All-or-nothing Ca2+ mobilization from the intracellular stores of single histamine-stimulated HeLa cells. J Physiol. 1992 May;450:163–178. doi: 10.1113/jphysiol.1992.sp019121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bootman M. Intracellular calcium. Questions about quantal Ca2+ release. Curr Biol. 1994 Feb 1;4(2):169–172. doi: 10.1016/s0960-9822(94)00041-2. [DOI] [PubMed] [Google Scholar]
- Buck E., Zimanyi I., Abramson J. J., Pessah I. N. Ryanodine stabilizes multiple conformational states of the skeletal muscle calcium release channel. J Biol Chem. 1992 Nov 25;267(33):23560–23567. [PubMed] [Google Scholar]
- Burgess G. M., Bird G. S., Obie J. F., Putney J. W., Jr The mechanism for synergism between phospholipase C- and adenylylcyclase-linked hormones in liver. Cyclic AMP-dependent kinase augments inositol trisphosphate-mediated Ca2+ mobilization without increasing the cellular levels of inositol polyphosphates. J Biol Chem. 1991 Mar 15;266(8):4772–4781. [PubMed] [Google Scholar]
- Cannell M. B., Berlin J. R., Lederer W. J. Effect of membrane potential changes on the calcium transient in single rat cardiac muscle cells. Science. 1987 Dec 4;238(4832):1419–1423. doi: 10.1126/science.2446391. [DOI] [PubMed] [Google Scholar]
- Chadwick C. C., Saito A., Fleischer S. Isolation and characterization of the inositol trisphosphate receptor from smooth muscle. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2132–2136. doi: 10.1073/pnas.87.6.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheek T. R., Moreton R. B., Berridge M. J., Stauderman K. A., Murawsky M. M., Bootman M. D. Quantal Ca2+ release from caffeine-sensitive stores in adrenal chromaffin cells. J Biol Chem. 1993 Dec 25;268(36):27076–27083. [PubMed] [Google Scholar]
- Cheek T. R., Morgan A., O'Sullivan A. J., Moreton R. B., Berridge M. J., Burgoyne R. D. Spatial localization of agonist-induced Ca2+ entry in bovine adrenal chromaffin cells. Different patterns induced by histamine and angiotensin II, and relationship to catecholamine release. J Cell Sci. 1993 Aug;105(Pt 4):913–921. doi: 10.1242/jcs.105.4.913. [DOI] [PubMed] [Google Scholar]
- Cheng H., Lederer W. J., Cannell M. B. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science. 1993 Oct 29;262(5134):740–744. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- Cleemann L., Morad M. Role of Ca2+ channel in cardiac excitation-contraction coupling in the rat: evidence from Ca2+ transients and contraction. J Physiol. 1991 Jan;432:283–312. doi: 10.1113/jphysiol.1991.sp018385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris C. D., Cameron A. M., Huganir R. L., Snyder S. H. Quantal calcium release by purified reconstituted inositol 1,4,5-trisphosphate receptors. Nature. 1992 Mar 26;356(6367):350–352. doi: 10.1038/356350a0. [DOI] [PubMed] [Google Scholar]
- Friel D. D., Tsien R. W. A caffeine- and ryanodine-sensitive Ca2+ store in bullfrog sympathetic neurones modulates effects of Ca2+ entry on [Ca2+]i. J Physiol. 1992 May;450:217–246. doi: 10.1113/jphysiol.1992.sp019125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Györke S., Fill M. Response. Science. 1994 Feb 18;263(5149):987–988. doi: 10.1126/science.263.5149.987. [DOI] [PubMed] [Google Scholar]
- Györke S., Fill M. Ryanodine receptor adaptation: control mechanism of Ca(2+)-induced Ca2+ release in heart. Science. 1993 May 7;260(5109):807–809. doi: 10.1126/science.8387229. [DOI] [PubMed] [Google Scholar]
- Henzi V., MacDermott A. B. Characteristics and function of Ca(2+)- and inositol 1,4,5-trisphosphate-releasable stores of Ca2+ in neurons. Neuroscience. 1992;46(2):251–273. doi: 10.1016/0306-4522(92)90049-8. [DOI] [PubMed] [Google Scholar]
- Hua S. Y., Nohmi M., Kuba K. Characteristics of Ca2+ release induced by Ca2+ influx in cultured bullfrog sympathetic neurones. J Physiol. 1993 May;464:245–272. doi: 10.1113/jphysiol.1993.sp019633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine R. F. 'Quantal' Ca2+ release and the control of Ca2+ entry by inositol phosphates--a possible mechanism. FEBS Lett. 1990 Apr 9;263(1):5–9. doi: 10.1016/0014-5793(90)80692-c. [DOI] [PubMed] [Google Scholar]
- Lai F. A., Erickson H. P., Rousseau E., Liu Q. Y., Meissner G. Purification and reconstitution of the calcium release channel from skeletal muscle. Nature. 1988 Jan 28;331(6154):315–319. doi: 10.1038/331315a0. [DOI] [PubMed] [Google Scholar]
- Lamb G. D., Fryer M. W., Stephenson D. G. Ca(2+)-induced Ca2+ release in response to flash photolysis. Science. 1994 Feb 18;263(5149):986–988. doi: 10.1126/science.8310298. [DOI] [PubMed] [Google Scholar]
- Lipscombe D., Madison D. V., Poenie M., Reuter H., Tsien R. W., Tsien R. Y. Imaging of cytosolic Ca2+ transients arising from Ca2+ stores and Ca2+ channels in sympathetic neurons. Neuron. 1988 Jul;1(5):355–365. doi: 10.1016/0896-6273(88)90185-7. [DOI] [PubMed] [Google Scholar]
- Loomis-Husselbee J. W., Dawson A. P. A steady-state mechanism can account for the properties of inositol 2,4,5-trisphosphate-stimulated Ca2+ release from permeabilized L1210 cells. Biochem J. 1993 Feb 1;289(Pt 3):861–866. doi: 10.1042/bj2890861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda N., Kawasaki T., Nakade S., Yokota N., Taguchi T., Kasai M., Mikoshiba K. Structural and functional characterization of inositol 1,4,5-trisphosphate receptor channel from mouse cerebellum. J Biol Chem. 1991 Jan 15;266(2):1109–1116. [PubMed] [Google Scholar]
- McPherson P. S., Kim Y. K., Valdivia H., Knudson C. M., Takekura H., Franzini-Armstrong C., Coronado R., Campbell K. P. The brain ryanodine receptor: a caffeine-sensitive calcium release channel. Neuron. 1991 Jul;7(1):17–25. doi: 10.1016/0896-6273(91)90070-g. [DOI] [PubMed] [Google Scholar]
- Meyer T., Stryer L. Transient calcium release induced by successive increments of inositol 1,4,5-trisphosphate. Proc Natl Acad Sci U S A. 1990 May;87(10):3841–3845. doi: 10.1073/pnas.87.10.3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missiaen L., De Smedt H., Droogmans G., Casteels R. Ca2+ release induced by inositol 1,4,5-trisphosphate is a steady-state phenomenon controlled by luminal Ca2+ in permeabilized cells. Nature. 1992 Jun 18;357(6379):599–602. doi: 10.1038/357599a0. [DOI] [PubMed] [Google Scholar]
- Missiaen L., Taylor C. W., Berridge M. J. Spontaneous calcium release from inositol trisphosphate-sensitive calcium stores. Nature. 1991 Jul 18;352(6332):241–244. doi: 10.1038/352241a0. [DOI] [PubMed] [Google Scholar]
- Muallem S., Pandol S. J., Beeker T. G. Hormone-evoked calcium release from intracellular stores is a quantal process. J Biol Chem. 1989 Jan 5;264(1):205–212. [PubMed] [Google Scholar]
- Nelson T. E., Nelson K. E. Intra- and extraluminal sarcoplasmic reticulum membrane regulatory sites for Ca2(+)-induced Ca2+ release. FEBS Lett. 1990 Apr 24;263(2):292–294. doi: 10.1016/0014-5793(90)81396-6. [DOI] [PubMed] [Google Scholar]
- O'Sullivan A. J., Cheek T. R., Moreton R. B., Berridge M. J., Burgoyne R. D. Localization and heterogeneity of agonist-induced changes in cytosolic calcium concentration in single bovine adrenal chromaffin cells from video imaging of fura-2. EMBO J. 1989 Feb;8(2):401–411. doi: 10.1002/j.1460-2075.1989.tb03391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldershaw K. A., Nunn D. L., Taylor C. W. Quantal Ca2+ mobilization stimulated by inositol 1,4,5-trisphosphate in permeabilized hepatocytes. Biochem J. 1991 Sep 15;278(Pt 3):705–708. doi: 10.1042/bj2780705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyamada H., Iino M., Endo M. Effects of ryanodine on the properties of Ca2+ release from the sarcoplasmic reticulum in skinned skeletal muscle fibres of the frog. J Physiol. 1993 Oct;470:335–348. doi: 10.1113/jphysiol.1993.sp019861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker I., Ivorra I. Confocal microfluorimetry of Ca2+ signals evoked in Xenopus oocytes by photoreleased inositol trisphosphate. J Physiol. 1993 Feb;461:133–165. doi: 10.1113/jphysiol.1993.sp019506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker I., Ivorra I. Localized all-or-none calcium liberation by inositol trisphosphate. Science. 1990 Nov 16;250(4983):977–979. doi: 10.1126/science.2237441. [DOI] [PubMed] [Google Scholar]
- Parker I., Yao Y. Regenerative release of calcium from functionally discrete subcellular stores by inositol trisphosphate. Proc Biol Sci. 1991 Dec 23;246(1317):269–274. doi: 10.1098/rspb.1991.0154. [DOI] [PubMed] [Google Scholar]
- Parys J. B., Missiaen L., De Smedt H., Casteels R. Loading dependence of inositol 1,4,5-trisphosphate-induced Ca2+ release in the clonal cell line A7r5. Implications for the mechanism of quantal Ca2+ release. J Biol Chem. 1993 Nov 25;268(33):25206–25212. [PubMed] [Google Scholar]
- Renard-Rooney D. C., Hajnóczky G., Seitz M. B., Schneider T. G., Thomas A. P. Imaging of inositol 1,4,5-trisphosphate-induced Ca2+ fluxes in single permeabilized hepatocytes. Demonstration of both quantal and nonquantal patterns of Ca2+ release. J Biol Chem. 1993 Nov 5;268(31):23601–23610. [PubMed] [Google Scholar]
- Short A. D., Klein M. G., Schneider M. F., Gill D. L. Inositol 1,4,5-trisphosphate-mediated quantal Ca2+ release measured by high resolution imaging of Ca2+ within organelles. J Biol Chem. 1993 Dec 5;268(34):25887–25893. [PubMed] [Google Scholar]
- Sipido K. R., Wier W. G. Flux of Ca2+ across the sarcoplasmic reticulum of guinea-pig cardiac cells during excitation-contraction coupling. J Physiol. 1991 Apr;435:605–630. doi: 10.1113/jphysiol.1991.sp018528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauderman K. A., Murawsky M. M. The inositol 1,4,5-trisphosphate-forming agonist histamine activates a ryanodine-sensitive Ca2+ release mechanism in bovine adrenal chromaffin cells. J Biol Chem. 1991 Oct 15;266(29):19150–19153. [PubMed] [Google Scholar]
- Taylor C. W., Potter B. V. The size of inositol 1,4,5-trisphosphate-sensitive Ca2+ stores depends on inositol 1,4,5-trisphosphate concentration. Biochem J. 1990 Feb 15;266(1):189–194. doi: 10.1042/bj2660189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C. W., Richardson A. Structure and function of inositol trisphosphate receptors. Pharmacol Ther. 1991;51(1):97–137. doi: 10.1016/0163-7258(91)90043-l. [DOI] [PubMed] [Google Scholar]
- Tregear R. T., Dawson A. P., Irvine R. F. Quantal release of Ca2+ from intracellular stores by InsP3: tests of the concept of control of Ca2+ release by intraluminal Ca2+. Proc Biol Sci. 1991 Mar 22;243(1308):263–268. doi: 10.1098/rspb.1991.0040. [DOI] [PubMed] [Google Scholar]
- Wang J., Best P. M. Inactivation of the sarcoplasmic reticulum calcium channel by protein kinase. Nature. 1992 Oct 22;359(6397):739–741. doi: 10.1038/359739a0. [DOI] [PubMed] [Google Scholar]
- Witcher D. R., Kovacs R. J., Schulman H., Cefali D. C., Jones L. R. Unique phosphorylation site on the cardiac ryanodine receptor regulates calcium channel activity. J Biol Chem. 1991 Jun 15;266(17):11144–11152. [PubMed] [Google Scholar]


