Abstract
Real-time fMRI neurofeedback (rtfMRI-NF) has emerged as a promising intervention for psychiatric disorders, yet its clinical efficacy remains underexplored due to an incomplete mechanistic understanding. This study aimed to delineate the whole-brain mechanisms underpinning the effects of rtfMRI-NF on repetitive negative thinking in depression. In a double-blind randomized controlled trial, forty-three depressed individuals underwent NF training targeting the functional connectivity (FC) between the posterior cingulate cortex and the right temporoparietal junction, linked to rumination severity. Participants were randomly assigned to active or sham groups, with the sham group receiving synthesized feedback mimicking real NF signal patterns. The active group demonstrated a significant reduction in brooding rumination scores (d = −1.52, p < 0.001), whereas the sham group did not (d = −0.23, p = 0.503). While the target FC did not show discernible training effects or group differences, connectome-based predictive modeling (CPM) analysis revealed that the interaction between brain activity during regulation and brain response to the feedback signal was the critical factor in explaining treatment outcomes. The model incorporating this interaction successfully predicted rumination changes across both groups. The FCs significantly contributing to the prediction were distributed across brain regions, notably the frontal control, salience network, and subcortical reward processing areas. These results underscore the importance of considering the interplay between brain regulation activities and brain response to the feedback signal in understanding the therapeutic mechanisms of rtfMRI-NF. The study affirms rtfMRI-NF’s potential as a therapeutic intervention for repetitive negative thinking and highlights the need for a nuanced understanding of the whole-brain mechanisms contributing to its efficacy.
Subject terms: Neuroscience, Depression
Introduction
Real-time fMRI neurofeedback (rtfMRI-NF)—a technique that enables participants to self-regulate functional brain activation—is considered a potential intervention for psychiatric disorders. Applied across various psychiatric conditions, this method’s feasibility for self-regulation of brain activation and its promising impact on symptom management are supported by numerous meta-analyses [1–9]. Nevertheless, the clinical efficacy of rtfMRI-NF remains to be confirmed, as the majority of studies are preliminary, characterized by small sample sizes, the absence of stringent control conditions, and a partial understanding of the mechanisms of action. Overcoming these research gaps to ascertain the specific efficacy of NF treatments is an area of significant interest.
One strength of rtfMRI-NF is its ability to provide deep insights into whole-brain functional processes during self-regulation, extending beyond the targeted brain signal [10]. Crucially, whole-brain analysis of rtfMRI-NF data is instrumental in decoding the mechanisms of action that underpin NF training. Emerging research has revealed diverse brain activities implicated in NF-mediated self-regulation, indicating that NF training encompasses a whole-brain process involving prefrontal control regions, the salience network, and reward processing areas [11–14]. Given that NF training might entail a reinforcement learning process [15, 16], examining brain activation in response to feedback signals is a valuable method for shedding light on the learning mechanisms driving self-regulation through NF. Investigations into brain responses to feedback signals have highlighted the pivotal roles of both prefrontal control regions and reward-responsive areas in NF-mediated self-regulation training [14, 17, 18]. Significantly, the regions engaged during NF training and in response to feedback signals coincide with those involved in general skill acquisition [19] and emotion regulation tasks [20, 21]. Moreover, these areas continue to be active even when a sham NF signal is administered, with participants unknowingly trying to control the feedback signal [20, 21]. Consequently, these activations appear to represent attempts to modulate the feedback, irrespective of the training outcome. Therefore, pinpointing brain activations or connectivity patterns that foretell the success of training is still challenging.
The current study explores the whole-brain mechanisms underlying NF treatment, utilizing data from a prior double-blind, randomized controlled trial (RCT) [22]. This earlier study demonstrated a significant decrease in symptoms of repetitive negative thinking exclusively in the active group following connectivity NF training, but not in the sham control group. Specifically, our study concentrated on the reduction of symptoms as the primary outcome of NF training, rather than on the self-regulation of the targeted brain signal. This approach contrasts with many studies that aim to elucidate the mechanisms of NF training, which have focused on identifying the neural substrates underpinning the acquisition of self-regulation of the target brain signal.
The investigation into neural substrates of self-regulation may be justified if we assume that regulating the targeted brain signal facilitates changes in cognitive function or the symptoms of a disorder, thus rendering successful regulation essential for an effective intervention. However, this assumption is susceptible to the counterargument that other brain activities concurrently altered by training could be the drivers of behavioral changes, as posited by Kvamme, Ros [23]. Because rtfMRI-NF training relies on the endogenous effort to self-regulate the signal and because participants cannot perceive the specific brain activation the feedback signal represents, the specificity of the NF intervention cannot be guaranteed. Kvamme, Ros [23] argue that we should not automatically assume a causal relationship between self-regulation of the target brain signal and the resultant behavioral outcomes. Corroboratively, research examining brain activations responsible for PTSD symptom amelioration post left amygdala upregulation training via rtfMRI-NF [24] revealed that activity in the dorsomedial prefrontal cortex (dmPFC) and middle cingulate cortex—not the amygdala—mediated symptom reduction. This indicates that the therapeutic effects may be attributed to brain activations beyond the intended target area.
Furthermore, symptomatic or behavioral improvements can occur independently of detectable changes in the targeted brain signal during training. Although the feedback signal is designed to reflect successful self-regulation, the desired training effect may not always be apparent in the targeted region, as evidenced by several neurofeedback studies. For example, Sukhodolsky, Walsh [25] utilized rtfMRI-NF to target the supplementary motor area (SMA) in adolescents with Tourette syndrome and noted significant symptom improvement in the active neurofeedback group, absent in the sham control group, despite a lack of significant SMA activation changes. Similarly, our earlier research [22] showed that neurofeedback aimed at enhancing the functional connectivity between the posterior cingulate cortex (PCC) and the right temporoparietal junction (rTPJ) markedly decreased rumination in depressed participants of the active group, with no corresponding change in the sham group, and without a notable difference in the targeted connectivity. Crucially, both studies implemented a stringent RCT methodology to ensure the effects were attributable to the neurofeedback. These results collectively imply that a comprehensive, whole-brain perspective may be essential to fully grasp the therapeutic mechanisms of rtfMRI-NF.
Consequently, the current study investigates the whole-brain mechanism of NF treatment by using data from our previous study [22]. Our approach employs machine learning predictive modeling focusing on two distinct functional connectivity patterns: one during the self-regulation task and the other in response to neurofeedback signals. We use connectome-based predictive modeling (CPM) [26] to establish the relationship between these patterns and treatment outcomes. By aggregating univariate features, multivariate predictive modeling techniques such as CPM can enhance both the sensitivity and robustness of predictions [27–29]. We also examined the interplay between connectivity patterns during the task and in response to neurofeedback as a potential predictor of treatment success. Given that the magnitude of the feedback signal is carefully controlled to be the same between the active and sham groups, an important difference between the groups may lie in the fidelity of the feedback to the participants’ actual brain activity. Thus, the interaction between brain activation and feedback signals could be critical in explaining the variance in symptom reduction observed between the groups.
Participants and methods
Participants
We analyzed the data of the previous NF study for a treatment of repetitive negative thinking in depressed participants [22]. The original study was registered on ClinicalTrials.gov (NCT04941066). In the study, forty-three individuals with Major Depressive Disorder (MDD) between 18 and 65 years of age were enrolled. The participants met the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DMS-5) criteria for unipolar MDD. Further details of the inclusion and exclusion criteria, sample size validation, and a method of randomization for group assignment are shown in Tsuchiyagaito, Misaki [22], and the CONSORT diagram is in the Supplementary Information (SI). Medication status and comorbidities of the participants selected for this study are detailed in Table 1. All participants provided written informed consent. The study protocol was reviewed and approved by the WCG IRB (https://www.wcgirb.com) (IRB Tracking Number 20210286).
Table 1.
Demographic characteristics and statistical comparisons of participant groups.
| Active | Sham | Statistics | p | |
|---|---|---|---|---|
| (n = 16) | (n = 18) | |||
| Age (mean, (SD)) | 33.6 (10.6) | 33.4 (11.5) | t(32) = 0.02 | 0.99 |
| Female (%) | 11 (68.8) | 13 (72.2) | χ2(1) = 0.05 | 0.83 |
| Diagnosis (%) | ||||
| Major depressive disorder (MDD) without comorbidity | 10 (62.5) | 5 (27.8) | χ2(1) = 2.85 | 0.09 |
| MDD and anxiety disorder | 6 (37.5) | 13 (72.2) | ||
| Generalized anxiety disorder | 1 (6.3) | 10 (55.6) | ||
| Social anxiety disorder | 4 (25.0) | 4 (22.2) | ||
| Panic disorder | 4 (25.0) | 5 (27.8) | ||
| Medicated (%) | 10 (62.5) | 7 (38.9) | χ2(1) = 1.06 | 0.30 |
| Antidepressants | 9 (56.3) | 5 (27.8) | ||
| Stimulants | 2 (12.5) | 0 (0.00) | ||
| Benzodiazepines | 2 (12.5) | 2 (11.1) | ||
| Substance use in the past 12 month (%) | 2 (12.5) | 3 (16.7) | χ2(1) = 0.12 | 0.73 |
| Cannabis | 2 (12.5) | 3 (16.7) | ||
| Current psychotherapy (%) | 5 (31.3) | 4 (22.2) | χ2(1) = 0.04 | 0.84 |
Neurofeedback session procedures
Detailed procedures are described in the SI. Here, we provide an overview of the procedure. The participants were divided into the active (N = 22) and sham (N = 21) groups randomly. The active group received the NF of functional connectivity between the PCC and the rTPJ regions. The sham group received a feedback signal artificially synthesized to mimic the temporal probabilistic structure of the real NF signal in the active group.
Beyond employing an RCT design, the study offered several advantages for controlling the non-specific effects of neurofeedback training. Strict double-blinding was achieved by having a separate researcher remotely set up the experimental application, who did not interact with either the participants or the experimenter who engaged with the participants during the study. The sham neurofeedback signal was carefully designed to mimic the genuine neurofeedback signal in terms of reinforcement frequency and temporal pattern. The sham signal was controlled to avoid any unintended correlation with the target brain signal monitored in real-time. A post-session questionnaire confirmed that participants were unaware of their group assignment [22]. Moreover, comprehensive real-time fMRI processing for noise reduction including physiological noise [30] ensured that the neurofeedback signal was free of confounding variables unrelated to brain activation, a critical consideration given that connectivity neurofeedback is known to be susceptible to physiological noise [31]. The study confirmed that these confounding variables were effectively eliminated through comprehensive real-time fMRI processing [32, 33].
There were three consecutive NF training runs in the single session for each subject. Each NF training run was 8 m long with 90 s initial resting block, followed by 100 s regulation block with four consecutive presentations of negative trait words (25 s each) and a 30 s rest. The participants were engaged in the emotion regulation task (i.e., regulating negative thoughts while viewing the negative self-referential words) while receiving the connectivity NF. A positive feedback signal was presented when the target PCC-rTPJ FC reduced at each TR during the regulation task with the two-point method [33, 34]. The regulation and rest blocks were repeated three time in a run. Each participant also performed a baseline run and a transfer run where no feedback signal was presented; we only used the NF training runs for the prediction analysis. Further details are presented in SI and Tsuchiyagaito, Misaki [22]
Connectome-based predictive modeling (CPM)
The detailed offline fMRI image preprocessing is described in the SI. After preprocessing, we calculated whole-brain functional connectivity using beta-series correlation [35]. Specifically, we evaluated the beta values for the regressor of the regulation task for each NF regulation block using General Linear Model (GLM) analysis. We formed a series of beta values from the regulation task and used this series across blocks and runs to calculate the z-transformed Pearson correlation between each region in the Shen 268 atlas [36]. Similarly, we estimated the beta values for the response to the feedback signal for each block using GLM analysis (see SI) and calculated the beta-series correlation (z-transformed) between the same atlas regions. We then calculated the interaction between FCs (z-transformed correlations) for the regulation task (RegTask) and the response to the NF signal (RespNF) by multiplying their normalized values. It is important to note that the NF signal reflects ~6–8 s of the previous brain state due to hemodynamic response delay and fMRI signal acquisition time. As a result, TR-wise interaction between RegTask and RespNF connectivity cannot indicate the contingency between these responses. Our current approach, which decomposes the signal into block-wise series, helps mitigate this issue at the expense of temporal resolution.
We employed Connectome-Based Predictive Modeling (CPM) analysis to identify brain activation patterns during neurofeedback (NF) training that could predict treatment outcomes. The target value for prediction was the change ratio of the Ruminative Response Style Brooding Subscale (RRS-B) score [37], measured 1 week after the NF training session, relative to the baseline. We focused on the brooding subscale because it specifically leads to worse prognosis [38]. We used the whole-brain FC patterns of the regulation task (RegTask), the response to the NF signal (RespNF), and their interaction (RegTask:RespNF) individually to construct the CPM model using various combinations. Given the hypothesis that these factors, either individually or in combination, could predict changes in RRS-B scores, we comprehensively evaluated the following models: (1) RegTask alone, (2) RespNF alone, (3) the interaction between RegTask and RespNF (RegTask:RespNF), (4) RegTask + RespNF, (5) RegTask + RegTask:RespNF, and (6) all three factors (RegTask + RespNF + RegTask:RespNF).
Predictive performance was assessed using 5-fold cross-validation with covariate (age, sex, head motion) regression and hyperparameter optimization in the nested cross-validation. The process was repeated 100 times with different random splitting of the validation set to obtain a confidence interval and a reliable estimate of predictive performance. Further details of the model training procedures are described in the SI.
Statistical analyses
The statistical significance of CPM performance was evaluated using a permutation test. The output values (ratio of change in RRS-B relative to baseline: dRRS-B) were randomly permuted 1000 times, and in each permutation iteration, 5-fold cross-validation was repeated 100 times with different random splits. The same covariate regression and hyperparameter optimization procedure including the nested cross-validation was also applied during the permutation test. Note that the covariate regression for the dRRS-B was performed for the non-permutated data since the test needs to evaluate the null distribution of the model performance apart from the covariate effects (see Winkler, Ridgway [39] for further discussion). The median of 100 cross-validation replicates was taken in each iteration. The p value for model performance was determined by calculating the proportion of permuted samples that exhibited a median prediction performance superior to that of the actual dataset, divided by the total number of permutations. We used a one-tailed test because the prediction model was optimized to identify the best possible positive correlation between the true and predicted values. Refer also to the SI section “Permutation test for prediction performance” for details on this procedure.
Symptom change in RRS-B was tested using linear mixed effect (LME) model analysis with the fixed effects of session (pre/post), group (active/sham), age, and sex, and the random effect of participant to the intercept. The lme4 package [40] with the lmerTest package [41] was used, and each contrast was calculated using the emmeans package [42] in the R language and statistical computing [43]. All the p values reported in the post-hoc contrast analysis were corrected using multivariate t distribution [42]. In these analyses, p < 0.05 (corrected as necessary) was considered significant.
Results
Data selection
The participants with more than 30% censored TRs due to head motion (frame-wise displacement [FD] > 0.3 mm) were excluded from the analysis. We further excluded the blocks with more than 30% censored TRs within the task block and the participants with less than five remaining blocks were also excluded from the analysis. There was no significant difference in the number of dropped blocks between groups (t(34) = 1.500, p = 0.143). The demographic characteristics of the selected participants and the statistical analysis of the differences between the groups are presented in Table 1.
Significant treatment effect on RRS-B for the active group alone without changes in the NF target FC
Significant reduction in RRS-B score was observed for the active group but not for the sham group. Figure 1 shows the mean and each participant’s RRS-B scores at the pre and post-training sessions for each group. While the main effect of group was not significant (F[1,30] = 0.062, p = 0.805), the main effect of session (F[1,32] = 12.944, p = 0.001) and the interaction between the session and group (F[1,32] = 7.125, p = 0.012) were significant with LME analysis. The post-hoc analysis indicated that the post-pre difference was significant for the active group (t[32] = −4.31, p < 0.001, d = −1.52) but not for the sham group (t[32] = −0.67, p = 0.503, d = −0.23).
Fig. 1. Rumination scores before (pre) and after (post) NF training for both the active and sham groups.
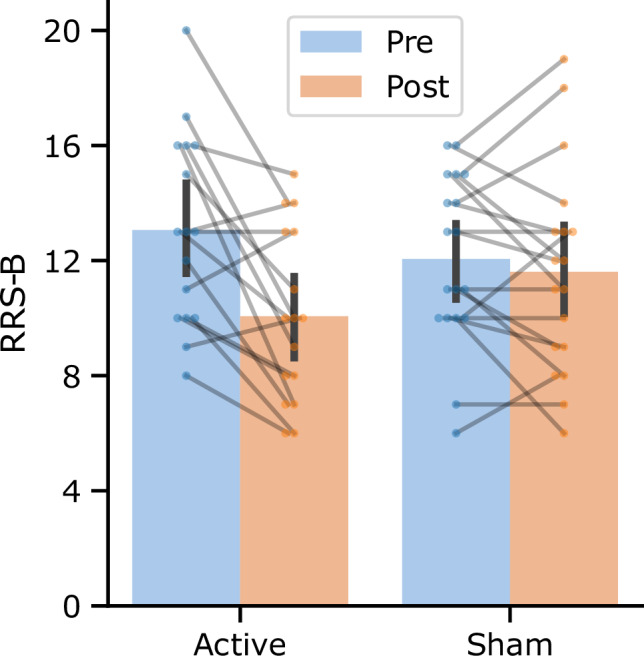
The bar plots represent the mean, and the error bars depict the 95% confidence interval. Individual participants are denoted by the points, with connecting lines between them. RRS-B Ruminative Response Style, Brooding subscale.
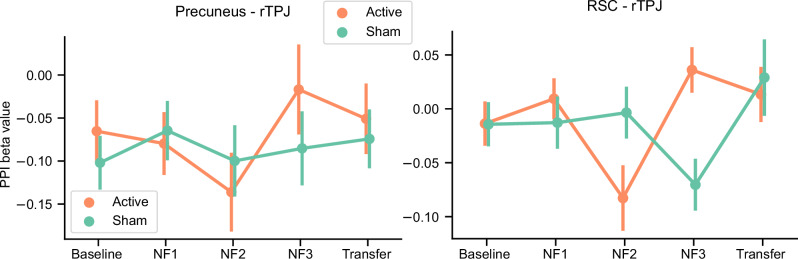
In contrast to the significant treatment effect on the target symptom score, there was no significant effect on the NF target, PCC-rTPJ FC. Figure 2 shows the psychophysiological interaction (PPI) beta values for the PCC seed region (x, y, z = −6, −58, 48 mm) to the rTPJ (51, −49, 23 mm) region across training sessions (seed and the target regions were defined by 6mm-radius sphere). An LME analysis with the fixed effects of run, group, age, sex, head motion (mean FD), and the random effect of participant for the intercept showed no significant main effect of the run (F[4, 478] = 0.917, p = 0.454), group (F[1, 37] = 0.104, p = 0.748), and run by group interaction (F[4, 478] = 0.633, p = 0.639) on the NF target connectivity.
Fig. 2. Results of the PPI analysis (beta value) for the target (PCC-rTPJ) and proximal RSC (RSC-rTPJ) functional connectivity across runs.
PCC posterior cingulate cortex, rTPJ right temporoparietal junction, RSC retrosplenial cortex.
Additionally, Tsuchiyagaito, Misaki [22] identified the other brain region in the retrosplenial cortex (RSC, [−7, −53, 11 mm]) that showed a different training effect between the active and the sham group. For the presently selected data, the PPI analysis between the RSC-rTPJ revealed no significant main effects of the run (F[4, 478] = 2.178, p = 0.070) and group (F[1, 29] = 0.216, p = 0.645), but significant run by group interaction (F[4, 477] = 3.885, p = 0.004). Post-hoc analysis indicated that the group difference was significant at NF2 (active < sham, t[334] = 2.354, p = 0.019, d = 0.475) and NF3 (acitve > sham, t[352] = −2.958, p = 0.003, d = −0.637). However, when we calculated the Spearman correlation between the mean RSC-rTPJ beta values in the NF1, NF2, NF3 runs and the RRS-B change, no significant correlation was observed (rho = −0.035, p = 0.843), indicating that the RSC-rTPJ connectivity during the NF training was not predictive of symptom change.
CPM analysis
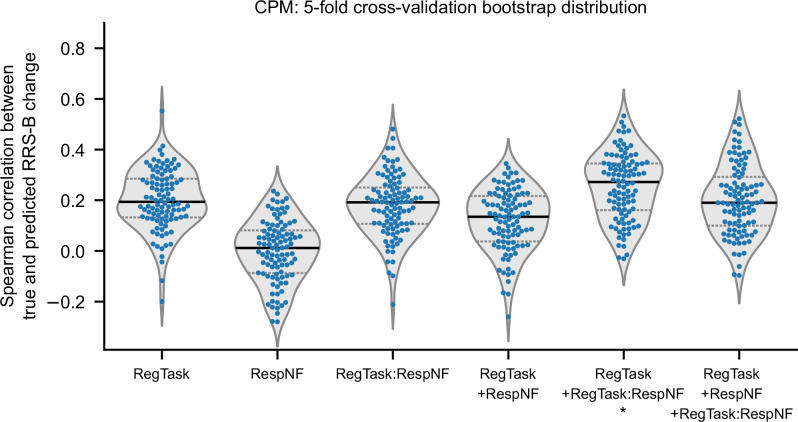
Figure 3 shows distributions of CPM analysis results for each model specification. Null distributions for each model in the permutation test are presented in SI, Fig. S4. The median predictive performance was not significant for the CPM models with RegTask (median = 0.194, p = 0.119), RespNF (median = 0.012, p = 0.478), and RegTask:RespNF (median = 0.192, p = 0.088). Similarly, the combined models of RegTask+RespNF (median = 0.135, p = 0.177) and RegTask+RespNF+RegTask:RespNF (median = 0.190, p = 0.086) did not show significant predictive performance. Only the model combining RegTask with RegTask:RespNF (median = 0.272, p = 0.031) showed significant predictive performance.
Fig. 3. Distributions of the CPM prediction performance (as measured by the Spearman correlation between true and predicted values) for the RRS-B score change following NF training, compared to the baseline assessment.
Each point represents a single iteration from the 5-fold cross-validation results (with a total of 100 iterations using different random splits). The violin plot illustrates the distribution curve, and the horizontal lines indicate quartile positions. *: p < 0.05.
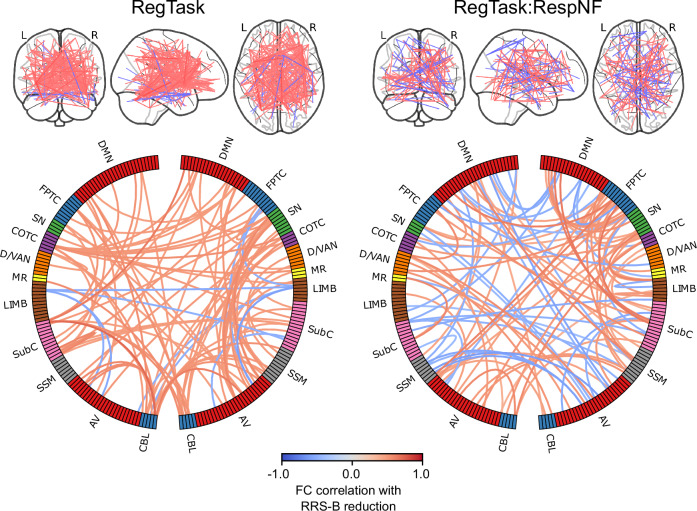
We further examined the connectivity that contributed to the prediction for the best performing model. Figure 4 shows the connectivity that were selected 76% times across 100 bootstrap model trainings (p < 5e−8 using a binominal test), which corresponds to p < 0.001 with Bonferroni correction across all the 27028 FCs. The plots indicate that many connections remained in spite of this stringent threshold, indicating that the model consistently employed broadly distributed connectivity for the prediction. While connections that informed prediction distributed across the brain, several dense connectivity regions were also observed in the circle plot. Supplementary Tables S1 and S2 show the top 10 nodes with the highest sum of absolute correlation with RRS-B reduction across their significant FCs. For the RegTask, predictive connectivity was dense in the cingulo-opercular task control regions, salience network regions, and the subcortical thalamus and basal ganglia regions. Similarly, for the RegTask:RespNF interaction, predictive connectivity was dense in the fronto-parietal task Control regions, salience network regions, and the subcortical hippocampus, thalamus, basal ganglia regions.
Fig. 4. Plots of the connectivity selected by the CPM model in more than 76% (p < 0.001 with Bonferroni correction) of the cross-validation iterations.
Line color indicates the connectivity correlation with RRS-B change. Warm color indicate that the higher the FC the more RRS-B decreased, and the cool color was vice versa. The circle plots summarized for each network region. Network labels are adapted from Drysdale, Grosenick [53]. DMN Default Mode Network, FPTC Fronto-Parietal Task Control, SN Salience Network, COTC Cingulo-Opperculum Task Control, D/VAN Doral Visual Attention Network, MR Memory Retrieval, LIMB default mode/limbic, SubC Subcortical, SSM Sensory SomatoMotor, AV Auditory-Visual, CBL Cerebellum.
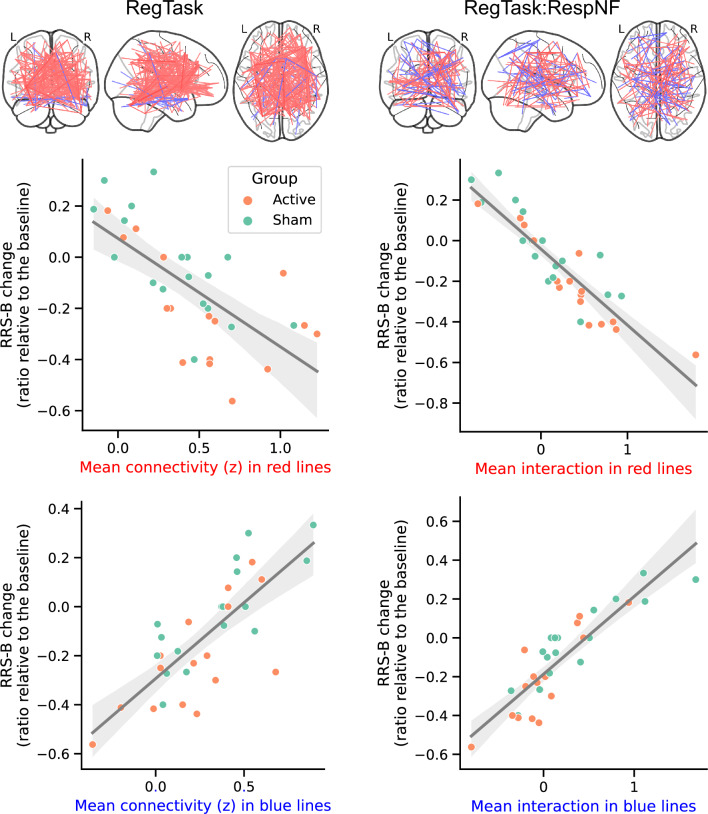
Figure 5 illustrates the relationship between the mean connectivity (z) for the informative FCs in the prediction model and the changes in RRS-B. These plots are intended to investigate the potential confounding effect of group differences on the predictions, rather than to present statistical analysis of these relationships, which could constitute double-dipping since the FCs correlating with RRS-B changes were selected for the CPM. The plots show that group differences did not affect the prediction, confirming that the selected FCs effectively predict changes in RRS-B scores in both active and sham groups.
Fig. 5. Association between RRS-B change and mean informative FC values in the prediction model.
Both positively and negatively related FCs during the regulation task (RegTask) and its interaction with the response to the neurofeedback signal (RespNF) are presented. The line represents the fitted trend, with the shaded area indicating the 95% confidence interval.
Association of other factors with RRS-B changes
We also examined other possibly predictive factors of RRS-B change, including age, sex, head motion, self-rating of the regulation success, the duration of the positive feedback presentation, the interaction between the self-rating of the regulation success and the group, and the interaction between the positive feedback duration and the group. However, linear model analysis with these factors on the RRS-B change (ratio relative to the baseline) indicated no significant effects in any of these (refer to SI for details).
Voxel-wise activation analysis for the association with RRS-B change
Additionally, we conducted a voxel-wise mass univariate analysis on both the RegTask and RespNF beta maps, as well as on their interaction map, to determine if voxel-wise activation linked with treatment effects was reflected in region-wise responses. Detailed results of this analysis are provided in the SI. We observed a significant association between symptom change and RegTask activation only. The regions involved were different from the NF target areas but were seen in the lateral occipital, superior frontal, precentral gyrus, and thalamus regions. These regions consistently show a decrease in their activation associated with RRS-B reduction, suggesting that the effects in these regions may be due to a reduction in task load over successive runs (detailed results are available in the SI).
Discussion
The primary objective of this study was to investigate the whole-brain mechanisms underlying the effects of rtfMRI-NF on repetitive negative thinking, specifically brooding rumination (RRS-B), in depressed individuals. Our results demonstrated a significant reduction in RRS-B scores in the active neurofeedback group but not in the sham group, although no significant changes were observed in the targeted FC between the PCC and the rTPJ. Interestingly, CPM analysis revealed that the most effective models for predicting RRS-B reduction incorporated both the FC patterns during the regulation task (RegTask) and their interaction with the FC patterns in response to the feedback signal (RespNF). These models employed broadly distributed connectivity across multiple brain regions, including the cingulo-opercular task control and salience network regions. Other potentially predictive factors such as self-rating of regulatory success and the duration of the positive feedback signal showed no significant effects on RRS-B changes. In summary, our findings suggest that the efficacy of rtfMRI-NF in reducing brooding rumination is not solely dependent on the modulation of targeted FC but involves a complex interplay of whole-brain connectivity patterns, thereby challenging the traditional focus on targeted brain regions in neurofeedback research.
In agreement with our hypothesis, connectivity associated with the reduction in rumination was not localized to a specific region but was widely distributed across the brain, primarily in the frontal control, salience network, and subcortical reward processing areas. This finding is consistent with a meta-analysis of rtfMRI-NF training studies across different target regions [11]. The meta-analysis found involvement of a broad array of brain regions during NF training, including executive control regions (i.e., ventrolateral prefrontal cortex [vlPFC], dorsolateral prefrontal cortex [DLPFC], premotor cortex), salience network regions (i.e., anterior insula, anterior cingulate cortex [ACC]), reward processing area (i.e., striatum), temporo-parietal areas, lateral occipital regions, and the temporo-occipital junction bilaterally. Investigations into functional connectivity with target brain regions like the amygdala have also reported increased connectivity with prefrontal areas, including the DLPFC, dorsomedial and ventromedial PFC, as well as the ACC [12–14].
Studies examining responses to feedback signals during rtfMRI-NF training have also demonstrated the involvement of these regions. Lawrence, Su [17] examined brain activation correlated with the amplitude of the feedback signal during rtfMRI-NF training aimed at increasing right anterior insula activation. They observed a positive correlation with the feedback signal in the dorsal ACC and left supramarginal gyrus, and a negative correlation in the inferior and rostral regions of the ACC and primary visual cortex. Similarly, Paret, Zahringer [14] analyzed brain activation in response to the NF signal during up- and down-regulation training of the right amygdala. They found that the medial thalamus was active in monitoring feedback signals broadly, while the ventral striatum (VS) responded specifically to the reinforcement signal, with VS activation showing a positive correlation with amygdala activation. Skottnik, Sorger [18] also showed that self-regulatory performance was correlated with striatal activity. The involvement of these regions is consistent with previous research highlighting their role in emotion regulation [20, 21] and skill learning [19]. Taken together, these findings suggest that both prefrontal control regions and reward-responsive areas play an important role in NF-mediated self-regulatory training.
However, a previous study also suggested that these regions were activated during sham NF and were not necessarily indicative of training success [44]. Our results suggest that effective treatment occurs when participants not only activate these emotion regulation and skill learning networks, but also receive consistent feedback that can guide them toward a desired neural state. Given that the interaction between RegTask and RespNF was critical in predicting treatment effect, participants who activate these networks in response to the feedback signal in a manner consistent with regulatory activation are more likely to experience a successful treatment outcome. Thus, effective treatment outcomes may depend on how individuals adapt and regulate their brain activation in response to neurofeedback, rather than simply trying to regulate it.
The whole-brain distributed associations also imply that the efficacy of NF treatment does not depend solely on the regulation of the target brain signal. Instead, this target signal may act as a guide, directing the brain toward a desired state. If this hypothesis proves valid, we may need to rethink our strategy for selecting the NF target signal. Traditionally, the NF target region has been identified based on its relationship to the target cognitive function or symptomatic state, assuming a causal relationship between the brain signal and the symptomatic state. However, the primary criterion for selecting a target region for the NF signal may not be its direct causal relationship with the symptoms. Instead, the key factor should be its ability to accurately represent the related cognitive and symptomatic states. In our current study, the PCC-rTPJ FC was used as the NF target. This FC remained low throughout the NF sessions (see Fig. 2), with no significant variation observed across sessions. Therefore, on average, participants successfully regulated the NF signal in the instructed direction. As this NF signal has been associated with rumination in depression [33], it could have served as an indicator of the brain state associated with reduced rumination.
Although the current NF protocol targeting specific FC have demonstrated therapeutic efficacy, approaches that target large-scale network connectivity or activation patterns may offer further improvements. Notable methods include connectome-based NF [45], which targets whole-brain functional connectivity patterns; semantic NF [46], which exploits the representational similarity space from multivoxel patterns; and decoding NF [47], which utilizes machine learning techniques to decode signals from multivoxel patterns. These approaches represent promising directions for enhancing the effectiveness of NF treatments.
Notably, the present CPM analysis predicted RRS-B reduction in both the active and sham groups. Some participants in the sham group who experienced a decrease in RRS-B had FC values similar to those of active group responders, as shown in Fig. 5. These findings suggest that responders in the sham group may be undergoing an adaptive training process similar to that of the active group. Pecina, Chen [48] noted that individuals could selectively respond to positive feedback while disregarding negative feedback, influenced by treatment expectations. Hence, even random NF signals might be leveraged to adjust brain activity towards a desired state. Nevertheless, our results, showing a significant difference between active and sham groups, underline the necessity of an accurate NF signal for effective brain state modulation, while also highlighting the complexity of placebo effects in NF training [48–50].
Several limitations of the current study merit discussion. Firstly, our results highlight the interaction between FC patterns during the regulation and the response to the NF signal as predictive of subsequent treatment effects on rumination. However, the NF signal reflects brain activations from several seconds prior, due to the inherent delay in the hemodynamic response and the time required for fMRI imaging. This makes establishing a direct link between regulation activity and the NF signal response challenging. To address this, we analyzed prolonged block-wise responses and calculated beta-series correlations to determine FC, but this method sacrifices temporal resolution and inhibits the assessment of learning effects over multiple runs due to limited data blocks. Future research should design experiments that tackle these challenges while enabling temporal analysis. Furthermore, more than two-thirds of the participants were female, reflecting the natural prevalence of MDD [51]. Given the lower proportion of males in the study sample, caution should be exercised when generalizing these results to male populations. The effect of potential confounding factors, including substance use, could not be fully assessed due to the small sample size. Although we excluded individuals with current SUD from the participants, even moderate substance use may impact brain regions involved in NF training, such as the frontal control, reward processing, and salience network regions [52]. Therefore, caution should be exercised in drawing definitive conclusions. While our findings are promising, they require validation through larger cohort studies. We employed strict motion thresholds to mitigate potential confounding effects on symptom reduction. However, this stringent criterion led to a reduced sample size and an attrition rate of ~20%. Future studies should anticipate and account for such attrition rates.
In conclusion, this study sought to explore the whole-brain mechanisms underlying the effects of rtfMRI-NF on brooding rumination in depression. The results showed that treatment efficacy can be predicted by broad patterns of whole-brain FC. In particular, the interaction between regulatory activity and response to the neurofeedback signal emerged as a pivotal predictor of treatment outcome. This suggests that while rtfMRI-NF may focus on a specific area, its effects are distributed, impacting an extensive network of brain regions. Our findings highlight a potential oversight in studies that assume direct changes in targeted signals are necessary for behavioral improvement, potentially overlooking broader effects of NF. As the field of NF research advances, it will be critical to broaden outcome measures to include changes in cognitive function or symptomatology. Furthermore, comprehensive whole-brain analysis is essential to fully understand the complex neurophysiological changes induced by NF training.
Supplementary information
Acknowledgements
This work was supported by the Laureate Institute for Brain Research and the National Institute of General Medical Sciences under Center Grant Award Number 1P20GM121312. The funding sources had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors are solely responsible for the content. The authors would like to acknowledge Jerzy Bodurka, Ph.D. (1964–2021), for his intellectual and scientific contributions to the development of the real-time fMRI neurofeedback systems, which provided the foundation for the present work.
Author contributions
Conceptualization, MM and AT; Methodology, MM; Investigation, MM and AT; Writing—Original Draft, MM; Writing—Review & Editing, MM, AT, SMG, MLR and MPP.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request. Access to the data will require a data sharing agreement between the requesting institution and the Laureate Institute for Brain Research, in accordance with institutional policies and ethical guidelines.
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study was conducted in accordance with the ethical principles outlined in the Declaration of Helsinki. The study protocol was reviewed and approved by the WCG IRB (https://www.wcgirb.com) under IRB Tracking Number 20210286. All participants provided written informed consent prior to their inclusion in the study.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41398-024-03066-9.
References
- 1.Taylor SF, Martz ME. Real-time fMRI neurofeedback: the promising potential of brain-training technology to advance clinical neuroscience. Neuropsychopharmacology. 2023;48:238–9. 10.1038/s41386-022-01397-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pindi P, Houenou J, Piguet C, Favre P. Real-time fMRI neurofeedback as a new treatment for psychiatric disorders: a meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2022;119:110605. 10.1016/j.pnpbp.2022.110605 [DOI] [PubMed] [Google Scholar]
- 3.Goldway N, Jalon I, Keynan JN, Hellrung L, Horstmann A, Paret C, et al. Feasibility and utility of amygdala neurofeedback. Neurosci Biobehav Rev. 2022;138:104694. 10.1016/j.neubiorev.2022.104694 [DOI] [PubMed] [Google Scholar]
- 4.Fernandez-Alvarez J, Grassi M, Colombo D, Botella C, Cipresso P, Perna G, et al. Efficacy of bio- and neurofeedback for depression: a meta-analysis. Psychol Med. 2022;52:201–16. 10.1017/S0033291721004396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Girges C, Vijiaratnam N, Zrinzo L, Ekanayake J, Foltynie T. Volitional control of brain motor activity and its therapeutic potential. Neuromodulation. 2022;25:1187–96. 10.1016/j.neurom.2022.01.007 [DOI] [PubMed] [Google Scholar]
- 6.Dudek E, Dodell-Feder D. The efficacy of real-time functional magnetic resonance imaging neurofeedback for psychiatric illness: a meta-analysis of brain and behavioral outcomes. Neurosci Biobehav Rev. 2021;121:291–306. 10.1016/j.neubiorev.2020.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trambaiolli LR, Kohl SH, Linden DEJ, Mehler DMA. Neurofeedback training in major depressive disorder: a systematic review of clinical efficacy, study quality and reporting practices. Neurosci Biobehav Rev. 2021;125:33–56. 10.1016/j.neubiorev.2021.02.015 [DOI] [PubMed] [Google Scholar]
- 8.Martz ME, Hart T, Heitzeg MM, Peltier SJ. Neuromodulation of brain activation associated with addiction: a review of real-time fMRI neurofeedback studies. NeuroImage Clin. 2020;27:102350. 10.1016/j.nicl.2020.102350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barreiros AR, Almeida I, Baia BC, Castelo-Branco M. Amygdala modulation during emotion regulation training with fMRI-based neurofeedback. Front Hum Neurosci. 2019;13:89. 10.3389/fnhum.2019.00089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hampson M. Identifying potential mechanisms of action underlying neurofeedback treatment response in depression. Biol Psychiatry. 2017;82:547–8. 10.1016/j.biopsych.2017.07.001 [DOI] [PubMed] [Google Scholar]
- 11.Emmert K, Kopel R, Sulzer J, Bruhl AB, Berman BD, Linden DEJ, et al. Meta-analysis of real-time fMRI neurofeedback studies using individual participant data: How is brain regulation mediated? Neuroimage. 2016;124:806–12. (Pt A) 10.1016/j.neuroimage.2015.09.042 [DOI] [PubMed] [Google Scholar]
- 12.Zotev V, Phillips R, Misaki M, Wong CK, Wurfel BE, Krueger F, et al. Real-time fMRI neurofeedback training of the amygdala activity with simultaneous EEG in veterans with combat-related PTSD. NeuroImage Clin. 2018;19:106–21. 10.1016/j.nicl.2018.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herwig U, Lutz J, Scherpiet S, Scheerer H, Kohlberg J, Opialla S, et al. Training emotion regulation through real-time fMRI neurofeedback of amygdala activity. Neuroimage. 2019;184:687–96. 10.1016/j.neuroimage.2018.09.068 [DOI] [PubMed] [Google Scholar]
- 14.Paret C, Zahringer J, Ruf M, Gerchen MF, Mall S, Hendler T, et al. Monitoring and control of amygdala neurofeedback involves distributed information processing in the human brain. Hum Brain Mapp. 2018;39:3018–31. 10.1002/hbm.24057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lubianiker N, Paret C, Dayan P, Hendler T. Neurofeedback through the lens of reinforcement learning. Trends Neurosci. 2022;45:579–93. 10.1016/j.tins.2022.03.008 [DOI] [PubMed] [Google Scholar]
- 16.Sitaram R, Ros T, Stoeckel L, Haller S, Scharnowski F, Lewis-Peacock J, et al. Closed-loop brain training: the science of neurofeedback. Nat Rev Neurosci. 2017;18:86–100. 10.1038/nrn.2016.164 [DOI] [PubMed] [Google Scholar]
- 17.Lawrence EJ, Su L, Barker GJ, Medford N, Dalton J, Williams SC, et al. Self-regulation of the anterior insula: reinforcement learning using real-time fMRI neurofeedback. Neuroimage. 2014;88:113–24. 10.1016/j.neuroimage.2013.10.069 [DOI] [PubMed] [Google Scholar]
- 18.Skottnik L, Sorger B, Kamp T, Linden D, Goebel R. Success and failure of controlling the real-time functional magnetic resonance imaging neurofeedback signal are reflected in the striatum. Brain Behav. 2019;9:e01240. 10.1002/brb3.1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaume A, Vialatte A, Mora-Sanchez A, Ramdani C, Vialatte FB. A psychoengineering paradigm for the neurocognitive mechanisms of biofeedback and neurofeedback. Neurosci Biobehav Rev. 2016;68:891–910. 10.1016/j.neubiorev.2016.06.012 [DOI] [PubMed] [Google Scholar]
- 20.Linhartova P, Latalova A, Kosa B, Kasparek T, Schmahl C, Paret C. fMRI neurofeedback in emotion regulation: a literature review. Neuroimage. 2019;193:75–92. 10.1016/j.neuroimage.2019.03.011 [DOI] [PubMed] [Google Scholar]
- 21.Etkin A, Buchel C, Gross JJ. The neural bases of emotion regulation. Nat Rev Neurosci. 2015;16:693–700. 10.1038/nrn4044 [DOI] [PubMed] [Google Scholar]
- 22.Tsuchiyagaito A, Misaki M, Kirlic N, Yu X, Sanchez SM, Cochran G, et al. Real-time fMRI functional Connectivity Neurofeedback reducing repetitive negative thinking in depression: a double-blind, randomized, sham-controlled proof-of-concept trial. Psychother Psychosom. 2023;92:87–100. 10.1159/000528377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kvamme TL, Ros T, Overgaard M. Can neurofeedback provide evidence of direct brain-behavior causality? Neuroimage. 2022;258:119400. 10.1016/j.neuroimage.2022.119400 [DOI] [PubMed] [Google Scholar]
- 24.Misaki M, Phillips R, Zotev V, Wong CK, Wurfel BE, Krueger F, et al. Brain activity mediators of PTSD symptom reduction during real-time fMRI amygdala neurofeedback emotional training. NeuroImage Clin. 2019;24:102047. 10.1016/j.nicl.2019.102047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sukhodolsky DG, Walsh C, Koller WN, Eilbott J, Rance M, Fulbright RK, et al. Randomized, sham-controlled trial of real-time functional magnetic resonance imaging neurofeedback for tics in adolescents with Tourette syndrome. Biol Psychiatry. 2020;87:1063–70. 10.1016/j.biopsych.2019.07.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shen X, Finn ES, Scheinost D, Rosenberg MD, Chun MM, Papademetris X, et al. Using connectome-based predictive modeling to predict individual behavior from brain connectivity. Nat, Protoc. 2017;12:506–18. 10.1038/nprot.2016.178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taxali A, Angstadt M, Rutherford S, Sripada C. Boost in test-retest reliability in resting state fMRI with predictive modeling. Cereb Cortex. 2021;31:2822–33. 10.1093/cercor/bhaa390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Finn ES, Rosenberg MD. Beyond fingerprinting: choosing predictive connectomes over reliable connectomes. Neuroimage. 2021;239:118254. 10.1016/j.neuroimage.2021.118254 [DOI] [PubMed] [Google Scholar]
- 29.Rosenberg MD, Finn ES. How to establish robust brain-behavior relationships without thousands of individuals. Nat Neurosci. 2022;25:835–7. 10.1038/s41593-022-01110-9 [DOI] [PubMed] [Google Scholar]
- 30.Misaki M, Bodurka J, Paulus MP. A library for fMRI real-time processing systems in python (RTPSpy) with comprehensive online noise reduction, fast and accurate anatomical image processing, and online processing simulation. Front Neurosci. 2022;16:834827. 10.3389/fnins.2022.834827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weiss F, Zamoscik V, Schmidt SNL, Halli P, Kirsch P, Gerchen MF. Just a very expensive breathing training? Risk of respiratory artefacts in functional connectivity-based real-time fMRI neurofeedback. Neuroimage. 2020;210:116580. 10.1016/j.neuroimage.2020.116580 [DOI] [PubMed] [Google Scholar]
- 32.Misaki M, Bodurka J. The impact of real-time fMRI denoising on online evaluation of brain activity and functional connectivity. J Neural Eng. 2021;18:046092. [DOI] [PubMed]
- 33.Misaki M, Tsuchiyagaito A, Al Zoubi O, Paulus M, Bodurka J, Tulsa I. Connectome-wide search for functional connectivity locus associated with pathological rumination as a target for real-time fMRI neurofeedback intervention. NeuroImage Clin. 2020;26:102244. 10.1016/j.nicl.2020.102244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramot M, Kimmich S, Gonzalez-Castillo J, Roopchansingh V, Popal H, White E, et al. Direct modulation of aberrant brain network connectivity through real-time NeuroFeedback. Elife. 2017;6:e28974. 10.7554/eLife.28974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rissman J, Gazzaley A, D’Esposito M. Measuring functional connectivity during distinct stages of a cognitive task. Neuroimage. 2004;23:752–63. 10.1016/j.neuroimage.2004.06.035 [DOI] [PubMed] [Google Scholar]
- 36.Shen X, Tokoglu F, Papademetris X, Constable RT. Groupwise whole-brain parcellation from resting-state fMRI data for network node identification. Neuroimage. 2013;82:403–15. 10.1016/j.neuroimage.2013.05.081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nolen-Hoeksema S, Morrow J. A prospective study of depression and posttraumatic stress symptoms after a natural disaster: the 1989 Loma Prieta Earthquake. J Pers Soc Psychol. 1991;61:115–21. 10.1037/0022-3514.61.1.115 [DOI] [PubMed] [Google Scholar]
- 38.Treynor W, Gonzalez R, Nolen-Hoeksema S. Rumination reconsidered: a psychometric analysis. Cogn Ther Res. 2003;27:247–59. 10.1023/A:1023910315561 [DOI] [Google Scholar]
- 39.Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE. Permutation inference for the general linear model. Neuroimage. 2014;92:381–97. 10.1016/j.neuroimage.2014.01.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67:1–48. 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- 41.Kuznetsova A, Brockhoff PB, Christensen RHB. lmerTest package: tests in linear mixed effects models. J Stat Softw. 2017;82:1–26. 10.18637/jss.v082.i13 [DOI] [Google Scholar]
- 42.emmeans: Estimated marginal means, aka least-squares means. 2022. https://CRAN.R-project.org/package=emmeans. Accessed 2022.
- 43.R: A language and environment for statistical computing. 2022. http://www.R-project.org. Accessed 2022.
- 44.Ninaus M, Kober SE, Witte M, Koschutnig K, Stangl M, Neuper C, et al. Neural substrates of cognitive control under the belief of getting neurofeedback training. Front Hum Neurosci. 2013;7:914. 10.3389/fnhum.2013.00914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scheinost D, Hsu TW, Avery EW, Hampson M, Constable RT, Chun MM, et al. Connectome-based neurofeedback: a pilot study to improve sustained attention. Neuroimage. 2020;212:116684. 10.1016/j.neuroimage.2020.116684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ciarlo A, Russo AG, Ponticorvo S, di Salle F, Lührs M, Goebel R, et al. Semantic fMRI neurofeedback: a multi-subject study at 3 tesla. J Neural Eng. 2022;19:036020. 10.1088/1741-2552/ac6f81 [DOI] [PubMed] [Google Scholar]
- 47.Shibata K, Lisi G, Cortese A, Watanabe T, Sasaki Y, Kawato M. Toward a comprehensive understanding of the neural mechanisms of decoded neurofeedback. Neuroimage. 2019;188:539–56. 10.1016/j.neuroimage.2018.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pecina M, Chen J, Karp JF, Dombrovski AY. Dynamic feedback between antidepressant placebo expectancies and mood. JAMA psychiatry. 2023;80:389–98. 10.1001/jamapsychiatry.2023.0010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kober SE, Witte M, Grinschgl S, Neuper C, Wood G. Placebo hampers ability to self-regulate brain activity: a double-blind sham-controlled neurofeedback study. Neuroimage. 2018;181:797–806. 10.1016/j.neuroimage.2018.07.025 [DOI] [PubMed] [Google Scholar]
- 50.Thibault RT, Lifshitz M, Raz A. Neurofeedback or neuroplacebo? Brain. 2017;140:862–4. 10.1093/brain/awx033 [DOI] [PubMed] [Google Scholar]
- 51.Salk RH, Hyde JS, Abramson LY. Gender differences in depression in representative national samples: meta-analyses of diagnoses and symptoms. Psychol Bull. 2017;143:783–822. 10.1037/bul0000102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lees B, Meredith LR, Kirkland AE, Bryant BE, Squeglia LM. Effect of alcohol use on the adolescent brain and behavior. Pharmacol Biochem Behav. 2020;192:172906. 10.1016/j.pbb.2020.172906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Drysdale AT, Grosenick L, Downar J, Dunlop K, Mansouri F, Meng Y, et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med. 2017;23:28–38. 10.1038/nm.4246 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. Access to the data will require a data sharing agreement between the requesting institution and the Laureate Institute for Brain Research, in accordance with institutional policies and ethical guidelines.