Abstract
Human Cu,Zn-superoxide dismutase (Cu,Zn-SOD) undergoes site-specific and random fragmentation by non-enzymic glycosylation (glycation). Released Cu2+ from the glycated Cu,Zn-SOD probably facilitates a Fenton reaction to convert H2O2 into hydroxy radical, which then participates in the non-specific fragmentation [Ookawara et al. (1992) J. Biol. Chem. 267, 18505-18510]. In the present study, we investigated the effects of glycated Cu,Zn-SOD on cloned DNA fragments and nuclear DNA and analysed the formation of 8-hydroxydeoxyguanosine (8-OH-dG). Incubation of cloned DNA fragments with Cu,Zn-SOD and reducing sugars resulted in cleavage of the DNA. The extent of the cleavage corresponded to the reducing capacity of the sugar. Metal-chelating reagents, EDTA and bathocuproine, and an H2O2 scavenger, catalase, inhibited the DNA cleavage. Hydroxy radical scavengers and aminoguanidine, an inhibitor of glycation, also inhibited the reaction. Moreover, the glycation of Cu,Zn-SOD caused the substantial formation of 8-OH-dG in DNA. When isolated nuclei were incubated with CuCl2 plus H2O2, nuclear DNA cleavage was observed. Incubation of isolated nuclei with Cu,Zn-SOD that had been pre-incubated with glucose also resulted in nuclear DNA cleavage. These results suggest that hydroxy radical is produced through a Fenton reaction by Cu2+ and H2O2 released from the glycated Cu,Zn-SOD, and participates in nuclear DNA cleavage. This mechanism may partly explain the deterioration of organs under diabetic conditions.
Full text
PDF



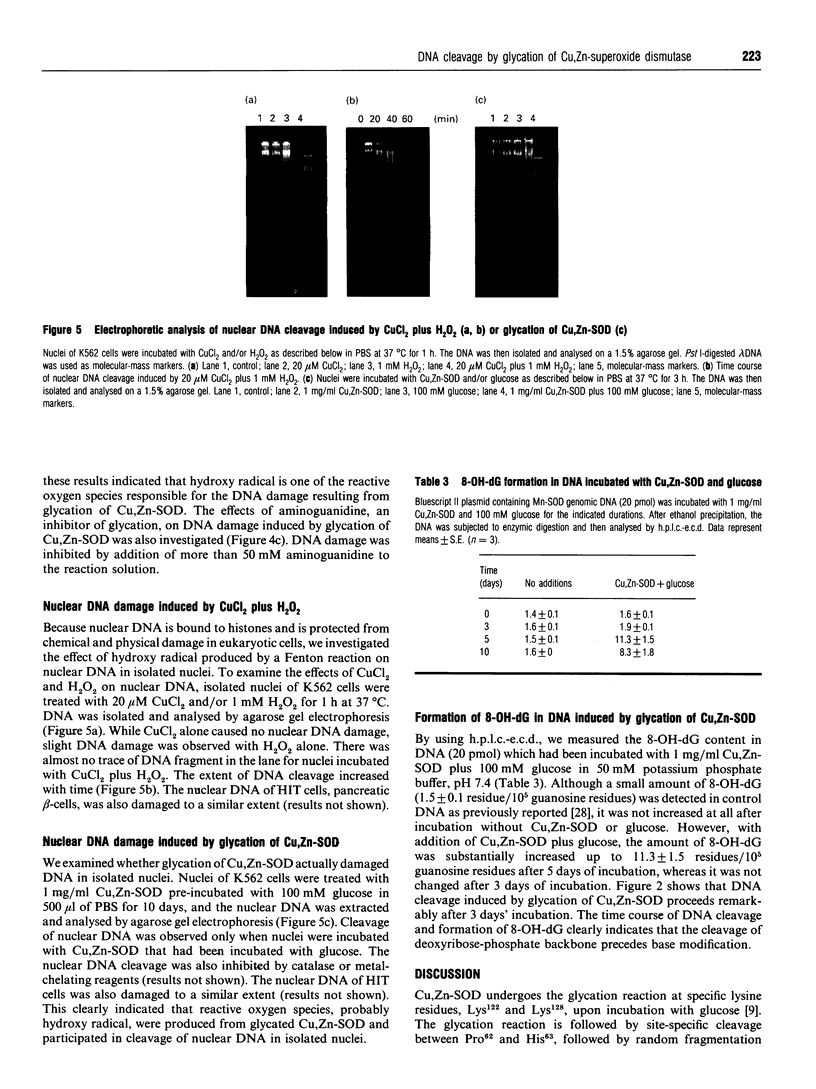


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai K., Iizuka S., Tada Y., Oikawa K., Taniguchi N. Increase in the glucosylated form of erythrocyte Cu-Zn-superoxide dismutase in diabetes and close association of the nonenzymatic glucosylation with the enzyme activity. Biochim Biophys Acta. 1987 May 19;924(2):292–296. doi: 10.1016/0304-4165(87)90025-0. [DOI] [PubMed] [Google Scholar]
- Arai K., Maguchi S., Fujii S., Ishibashi H., Oikawa K., Taniguchi N. Glycation and inactivation of human Cu-Zn-superoxide dismutase. Identification of the in vitro glycated sites. J Biol Chem. 1987 Dec 15;262(35):16969–16972. [PubMed] [Google Scholar]
- Aruoma O. I., Halliwell B., Gajewski E., Dizdaroglu M. Copper-ion-dependent damage to the bases in DNA in the presence of hydrogen peroxide. Biochem J. 1991 Feb 1;273(Pt 3):601–604. doi: 10.1042/bj2730601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aruoma O. I., Halliwell B., Gajewski E., Dizdaroglu M. Damage to the bases in DNA induced by hydrogen peroxide and ferric ion chelates. J Biol Chem. 1989 Dec 5;264(34):20509–20512. [PubMed] [Google Scholar]
- Bucala R., Lee A. T., Rourke L., Cerami A. Transposition of an Alu-containing element induced by DNA-advanced glycosylation endproducts. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2666–2670. doi: 10.1073/pnas.90.7.2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucala R., Model P., Russel M., Cerami A. Modification of DNA by glucose 6-phosphate induces DNA rearrangements in an Escherichia coli plasmid. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8439–8442. doi: 10.1073/pnas.82.24.8439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crapo J. D., Oury T., Rabouille C., Slot J. W., Chang L. Y. Copper,zinc superoxide dismutase is primarily a cytosolic protein in human cells. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10405–10409. doi: 10.1073/pnas.89.21.10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd R. A. The role of 8-hydroxyguanine in carcinogenesis. Carcinogenesis. 1990 Sep;11(9):1447–1450. doi: 10.1093/carcin/11.9.1447. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M. Copper-phenanthroline-induced site-specific oxygen-radical damage to DNA. Detection of loosely bound trace copper in biological fluids. Biochem J. 1984 Mar 15;218(3):983–985. doi: 10.1042/bj2180983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt J. V., Dean R. T., Wolff S. P. Hydroxyl radical production and autoxidative glycosylation. Glucose autoxidation as the cause of protein damage in the experimental glycation model of diabetes mellitus and ageing. Biochem J. 1988 Nov 15;256(1):205–212. doi: 10.1042/bj2560205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai H., Crain P. F., Kuchino Y., Nishimura S., Ootsuyama A., Tanooka H. Formation of 8-hydroxyguanine moiety in cellular DNA by agents producing oxygen radicals and evidence for its repair. Carcinogenesis. 1986 Nov;7(11):1849–1851. doi: 10.1093/carcin/7.11.1849. [DOI] [PubMed] [Google Scholar]
- Kawamura N., Ookawara T., Suzuki K., Konishi K., Mino M., Taniguchi N. Increased glycated Cu,Zn-superoxide dismutase levels in erythrocytes of patients with insulin-dependent diabetis mellitus. J Clin Endocrinol Metab. 1992 Jun;74(6):1352–1354. doi: 10.1210/jcem.74.6.1592880. [DOI] [PubMed] [Google Scholar]
- Kawanishi S., Inoue S., Sano S. Mechanism of DNA cleavage induced by sodium chromate(VI) in the presence of hydrogen peroxide. J Biol Chem. 1986 May 5;261(13):5952–5958. [PubMed] [Google Scholar]
- Kuchino Y., Mori F., Kasai H., Inoue H., Iwai S., Miura K., Ohtsuka E., Nishimura S. Misreading of DNA templates containing 8-hydroxydeoxyguanosine at the modified base and at adjacent residues. Nature. 1987 May 7;327(6117):77–79. doi: 10.1038/327077a0. [DOI] [PubMed] [Google Scholar]
- Lee A. T., Cerami A. Elevated glucose 6-phosphate levels are associated with plasmid mutations in vivo. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8311–8314. doi: 10.1073/pnas.84.23.8311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A. T., Cerami A. The formation of reactive intermediate(s) of glucose 6-phosphate and lysine capable of rapidly reacting with DNA. Mutat Res. 1987 Aug;179(2):151–158. doi: 10.1016/0027-5107(87)90305-8. [DOI] [PubMed] [Google Scholar]
- Lorenzi M., Montisano D. F., Toledo S., Barrieux A. High glucose induces DNA damage in cultured human endothelial cells. J Clin Invest. 1986 Jan;77(1):322–325. doi: 10.1172/JCI112295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullarkey C. J., Edelstein D., Brownlee M. Free radical generation by early glycation products: a mechanism for accelerated atherogenesis in diabetes. Biochem Biophys Res Commun. 1990 Dec 31;173(3):932–939. doi: 10.1016/s0006-291x(05)80875-7. [DOI] [PubMed] [Google Scholar]
- Murata T., Miwa I., Toyoda Y., Okuda J. Inhibition of glucose-induced insulin secretion through inactivation of glucokinase by glyceraldehyde. Diabetes. 1993 Jul;42(7):1003–1009. doi: 10.2337/diab.42.7.1003. [DOI] [PubMed] [Google Scholar]
- Ookawara T., Kawamura N., Kitagawa Y., Taniguchi N. Site-specific and random fragmentation of Cu,Zn-superoxide dismutase by glycation reaction. Implication of reactive oxygen species. J Biol Chem. 1992 Sep 15;267(26):18505–18510. [PubMed] [Google Scholar]
- Sagripanti J. L., Kraemer K. H. Site-specific oxidative DNA damage at polyguanosines produced by copper plus hydrogen peroxide. J Biol Chem. 1989 Jan 25;264(3):1729–1734. [PubMed] [Google Scholar]
- Sakurai T., Tsuchiya S. Superoxide production from nonenzymatically glycated protein. FEBS Lett. 1988 Aug 29;236(2):406–410. doi: 10.1016/0014-5793(88)80066-8. [DOI] [PubMed] [Google Scholar]
- Sato K., Akaike T., Kohno M., Ando M., Maeda H. Hydroxyl radical production by H2O2 plus Cu,Zn-superoxide dismutase reflects the activity of free copper released from the oxidatively damaged enzyme. J Biol Chem. 1992 Dec 15;267(35):25371–25377. [PubMed] [Google Scholar]
- Shibutani S., Takeshita M., Grollman A. P. Insertion of specific bases during DNA synthesis past the oxidation-damaged base 8-oxodG. Nature. 1991 Jan 31;349(6308):431–434. doi: 10.1038/349431a0. [DOI] [PubMed] [Google Scholar]
- Takasu N., Asawa T., Komiya I., Nagasawa Y., Yamada T. Alloxan-induced DNA strand breaks in pancreatic islets. Evidence for H2O2 as an intermediate. J Biol Chem. 1991 Feb 5;266(4):2112–2114. [PubMed] [Google Scholar]
- Taniguchi N. Clinical significances of superoxide dismutases: changes in aging, diabetes, ischemia, and cancer. Adv Clin Chem. 1992;29:1–59. doi: 10.1016/s0065-2423(08)60221-8. [DOI] [PubMed] [Google Scholar]
- Uchigata Y., Yamamoto H., Kawamura A., Okamoto H. Protection by superoxide dismutase, catalase, and poly(ADP-ribose) synthetase inhibitors against alloxan- and streptozotocin-induced islet DNA strand breaks and against the inhibition of proinsulin synthesis. J Biol Chem. 1982 Jun 10;257(11):6084–6088. [PubMed] [Google Scholar]
- Vander Jagt D. L., Robinson B., Taylor K. K., Hunsaker L. A. Reduction of trioses by NADPH-dependent aldo-keto reductases. Aldose reductase, methylglyoxal, and diabetic complications. J Biol Chem. 1992 Mar 5;267(7):4364–4369. [PubMed] [Google Scholar]
- Wolff S. P., Dean R. T. Glucose autoxidation and protein modification. The potential role of 'autoxidative glycosylation' in diabetes. Biochem J. 1987 Jul 1;245(1):243–250. doi: 10.1042/bj2450243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H., Uchigata Y., Okamoto H. Streptozotocin and alloxan induce DNA strand breaks and poly(ADP-ribose) synthetase in pancreatic islets. Nature. 1981 Nov 19;294(5838):284–286. doi: 10.1038/294284a0. [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Kawanishi S. Hydroxyl free radical is not the main active species in site-specific DNA damage induced by copper (II) ion and hydrogen peroxide. J Biol Chem. 1989 Sep 15;264(26):15435–15440. [PubMed] [Google Scholar]
- Yim M. B., Chock P. B., Stadtman E. R. Copper, zinc superoxide dismutase catalyzes hydroxyl radical production from hydrogen peroxide. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5006–5010. doi: 10.1073/pnas.87.13.5006. [DOI] [PMC free article] [PubMed] [Google Scholar]