Abstract
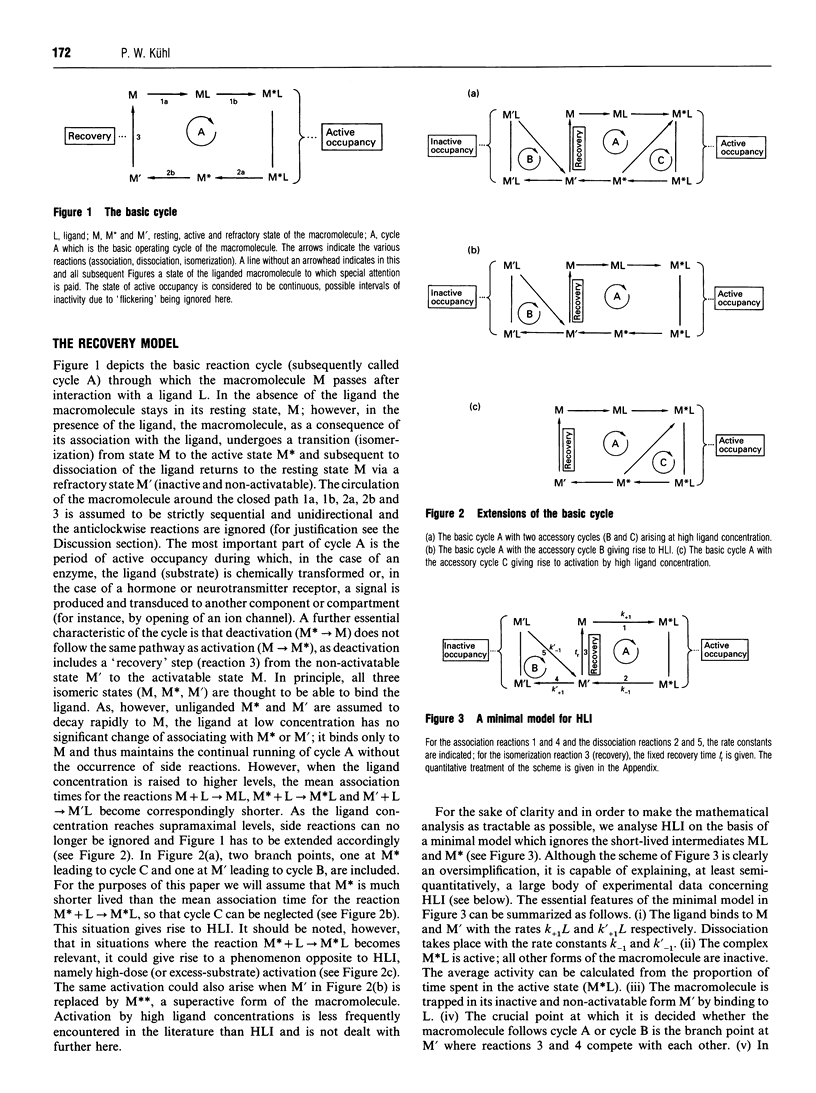
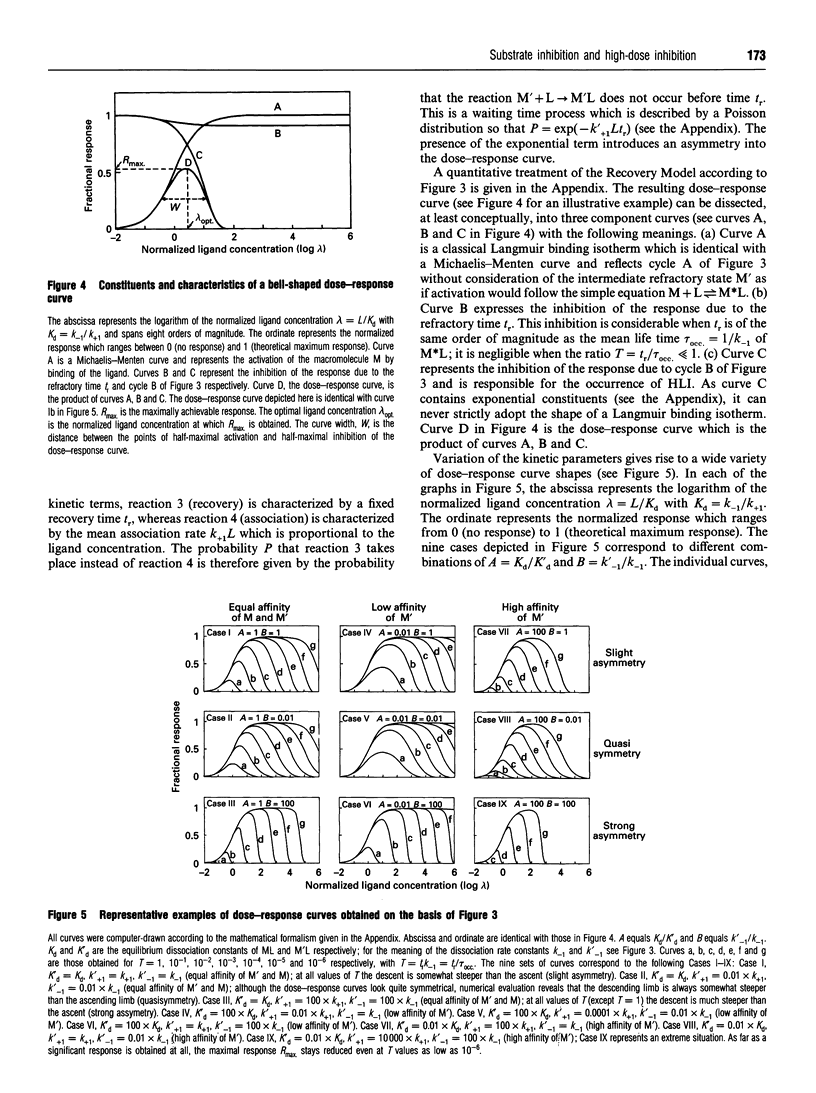
A kinetic model, called the Recovery Model, which incorporates an obligatory recovery phase of fixed duration (tr) in the operation cycle of a macromolecule (enzyme, receptor) is proposed. Binding of a ligand (substrate, agonist) during tr disturbs the recovery process and causes inhibition (substrate inhibition, agonist autoinhibition). A quantitative stochastic analysis of a minimal version of the Recovery Model reveals that (1) plotting the response versus the logarithm of the ligand concentration never yields a strictly symmetrical bell-shaped dose-response curve, (2) the position and shape of the descent of the dose-response curve can vary greatly in dependence of the kinetic parameters of the system, and (3) a minimal steepness of the descent with a Hill coefficient of 1 exists provided that the response can be totally inhibited by high ligand concentrations. The Recovery Model is equally applicable to macromolecules that can bind single or multiple ligands, and suggests new ways to explain such diverse phenomena as partial agonism, pulse generation, desensitization, memory effects and ultrasensitivity. In addition, substrate inhibition and agonist autoinhibition are regarded as phenomena closely related to other kinds of non-Michaelian behaviour because of a common temporal mechanism, namely the temporal interference of arriving ligand molecules with timing-sensitive phases of the operation cycle.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abiko T., Kumikawa M., Sekino H. Inhibition effect of rosette formation between human lymphocytes and sheep erythrocytes by specific heptapeptide isolated from uremic fluid and its analogs. Biochem Biophys Res Commun. 1979 Feb 28;86(4):945–952. doi: 10.1016/0006-291x(79)90209-2. [DOI] [PubMed] [Google Scholar]
- Ainslie G. R., Jr, Shill J. P., Neet K. E. Transients and cooperativity. A slow transition model for relating transients and cooperative kinetics of enzymes. J Biol Chem. 1972 Nov 10;247(21):7088–7096. [PubMed] [Google Scholar]
- Argilés A., Derancourt J., Jauregui-Adell J., Mion C., Demaille J. G. Biochemical characterization of serum and urinary beta 2 microglobulin in end-stage renal disease patients. Nephrol Dial Transplant. 1992;7(11):1106–1110. [PubMed] [Google Scholar]
- Arányi P., Tóth J. A full stochastic description of the Michaelis-Menten reaction for small systems. Acta Biochim Biophys Acad Sci Hung. 1977;12(4):375–388. [PubMed] [Google Scholar]
- Bardin T., Zingraff J., Kuntz D., Drüeke T. Dialysis-related amyloidosis. Nephrol Dial Transplant. 1986;1(3):151–154. [PubMed] [Google Scholar]
- Becker J. W., Reeke G. N., Jr Three-dimensional structure of beta 2-microglobulin. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4225–4229. doi: 10.1073/pnas.82.12.4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. I. Nomenclature and rate equations. Biochim Biophys Acta. 1963 Jan 8;67:104–137. doi: 10.1016/0006-3002(63)91800-6. [DOI] [PubMed] [Google Scholar]
- Campistol J. M., Solé M., Bombi J. A., Rodriguez R., Mirapeix E., Muñoz-Gomez J., Revert O. W. In vitro spontaneous synthesis of beta 2-microglobulin amyloid fibrils in peripheral blood mononuclear cell culture. Am J Pathol. 1992 Jul;141(1):241–247. [PMC free article] [PubMed] [Google Scholar]
- Careri G., Wyman J. Soliton-assisted unidirectional circulation in a biochemical cycle. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4386–4388. doi: 10.1073/pnas.81.14.4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cigén R. Structural difference between the two forms of guinea-pig beta 2-microglobulin and their occurrence in inbred guinea-pig strains. Mol Immunol. 1985 Sep;22(9):1039–1043. doi: 10.1016/0161-5890(85)90106-3. [DOI] [PubMed] [Google Scholar]
- Connors L. H., Shirahama T., Skinner M., Fenves A., Cohen A. S. In vitro formation of amyloid fibrils from intact beta 2-microglobulin. Biochem Biophys Res Commun. 1985 Sep 30;131(3):1063–1068. doi: 10.1016/0006-291x(85)90198-6. [DOI] [PubMed] [Google Scholar]
- Cunningham B. A., Wang J. L., Berggård I., Peterson P. A. The complete amino acid sequence of beta 2-microglobulin. Biochemistry. 1973 Nov 20;12(24):4811–4822. doi: 10.1021/bi00748a001. [DOI] [PubMed] [Google Scholar]
- Davey P. G., Gosling P. beta 2-Microglobulin instability in pathological urine. Clin Chem. 1982 Jun;28(6):1330–1333. [PubMed] [Google Scholar]
- Deshcherevskii V. I., Zhabotiniskii A. M., Sel'kov E. E., Sidorenko N. P., Shnol' S. E. Kolebatel'nye biologicheskie protessy na molekuliarnom urovne. Biofizika. 1970 Mar-Apr;15(2):225–234. [PubMed] [Google Scholar]
- Easterby J. S. The kinetics of consecutive enzyme reactions. The design of coupled assays and the temporal response of pathways. Biochem J. 1984 May 1;219(3):843–847. doi: 10.1042/bj2190843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber H. E., Kucherlapati R. S., Poulik M. D., Ruddle F. H., Smithies O. beta2-microglobulin locus on human chromosome 15. Somatic Cell Genet. 1976 Mar;2(2):141–153. doi: 10.1007/BF01542627. [DOI] [PubMed] [Google Scholar]
- Franklin T. J., Twose P. A. Desensitization of beta receptor mediated cyclic AMP response of cultured fibroblasts by partial agonists. J Cyclic Nucleotide Res. 1979;5(1):19–30. [PubMed] [Google Scholar]
- Frieden C. Kinetic aspects of regulation of metabolic processes. The hysteretic enzyme concept. J Biol Chem. 1970 Nov 10;245(21):5788–5799. [PubMed] [Google Scholar]
- Frieden C. Slow transitions and hysteretic behavior in enzymes. Annu Rev Biochem. 1979;48:471–489. doi: 10.1146/annurev.bi.48.070179.002351. [DOI] [PubMed] [Google Scholar]
- Fromm H. J. Summary of kinetic reaction mechanisms. Methods Enzymol. 1979;63:42–53. doi: 10.1016/0076-6879(79)63005-7. [DOI] [PubMed] [Google Scholar]
- Gasser D. L., Klein K. A., Choi E., Seidman J. G. A new beta-2 microglobulin allele in mice defined by DNA sequencing. Immunogenetics. 1985;22(4):413–416. doi: 10.1007/BF00430925. [DOI] [PubMed] [Google Scholar]
- Gejyo F., Odani S., Yamada T., Honma N., Saito H., Suzuki Y., Nakagawa Y., Kobayashi H., Maruyama Y., Hirasawa Y. Beta 2-microglobulin: a new form of amyloid protein associated with chronic hemodialysis. Kidney Int. 1986 Sep;30(3):385–390. doi: 10.1038/ki.1986.196. [DOI] [PubMed] [Google Scholar]
- Gorevic P. D., Casey T. T., Stone W. J., DiRaimondo C. R., Prelli F. C., Frangione B. Beta-2 microglobulin is an amyloidogenic protein in man. J Clin Invest. 1985 Dec;76(6):2425–2429. doi: 10.1172/JCI112257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorevic P. D., Munoz P. C., Casey T. T., DiRaimondo C. R., Stone W. J., Prelli F. C., Rodrigues M. M., Poulik M. D., Frangione B. Polymerization of intact beta 2-microglobulin in tissue causes amyloidosis in patients on chronic hemodialysis. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7908–7912. doi: 10.1073/pnas.83.20.7908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güssow D., Rein R., Ginjaar I., Hochstenbach F., Seemann G., Kottman A., Ploegh H. L. The human beta 2-microglobulin gene. Primary structure and definition of the transcriptional unit. J Immunol. 1987 Nov 1;139(9):3132–3138. [PubMed] [Google Scholar]
- Haag-Weber M., Hörl W. H. Uremia and infection: mechanisms of impaired cellular host defense. Nephron. 1993;63(2):125–131. doi: 10.1159/000187170. [DOI] [PubMed] [Google Scholar]
- Hall P. W., Ricanati E. S., Vacca C. V. Characterization of human beta2-microglobulin by isoelectric focusing. Clin Chim Acta. 1977 May 16;77(1):37–42. doi: 10.1016/0009-8981(77)90399-0. [DOI] [PubMed] [Google Scholar]
- Homma N., Gejyo F., Isemura M., Arakawa M. Collagen-binding affinity of beta-2-microglobulin, a preprotein of hemodialysis-associated amyloidosis. Nephron. 1989;53(1):37–40. doi: 10.1159/000185699. [DOI] [PubMed] [Google Scholar]
- Keleti T. Effect of steric changes in the protein on the kinetics of enzymic reactions. II. Steady-state treatment of reactions with one substrate. Acta Biochim Biophys Acad Sci Hung. 1968;3(3):247–258. [PubMed] [Google Scholar]
- Koshland D. E., Jr, Goldbeter A., Stock J. B. Amplification and adaptation in regulatory and sensory systems. Science. 1982 Jul 16;217(4556):220–225. doi: 10.1126/science.7089556. [DOI] [PubMed] [Google Scholar]
- Koshland D. E., Jr, Némethy G., Filmer D. Comparison of experimental binding data and theoretical models in proteins containing subunits. Biochemistry. 1966 Jan;5(1):365–385. doi: 10.1021/bi00865a047. [DOI] [PubMed] [Google Scholar]
- Krall J. F., Connelly M., Tuck M. L. Acute regulation of beta adrenergic catecholamine sensitivity in human lymphocytes. J Pharmacol Exp Ther. 1980 Sep;214(3):554–560. [PubMed] [Google Scholar]
- LaPorte D. C., Walsh K., Koshland D. E., Jr The branch point effect. Ultrasensitivity and subsensitivity to metabolic control. J Biol Chem. 1984 Nov 25;259(22):14068–14075. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lingappa V. R., Cunningham B. A., Jazwinski S. M., Hopp T. P., Blobel G., Edelman G. M. Cell-free synthesis and segregation of beta 2-microglobulin. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3651–3655. doi: 10.1073/pnas.76.8.3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linke R. P., Bommer J., Ritz E., Waldherr R., Eulitz M. Amyloid kidney stones of uremic patients consist of beta 2-microglobulin fragments. Biochem Biophys Res Commun. 1986 Apr 29;136(2):665–671. doi: 10.1016/0006-291x(86)90492-4. [DOI] [PubMed] [Google Scholar]
- Linke R. P., Hampl H., Bartel-Schwarze S., Eulitz M. Beta 2-microglobulin, different fragments and polymers thereof in synovial amyloid in long-term hemodialysis. Biol Chem Hoppe Seyler. 1987 Feb;368(2):137–144. doi: 10.1515/bchm3.1987.368.1.137. [DOI] [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- Michaelson J., Rothenberg E., Boyse E. A. Genetic polymorphism of murine beta 2-microglobulin detected biochemically. Immunogenetics. 1980 Jul;11(1):93–95. doi: 10.1007/BF01567773. [DOI] [PubMed] [Google Scholar]
- Mittal K. K., Mickey M. R., Singal D. P., Terasaki P. I. Serotyping for homotransplantation. 18. Refinement of microdroplet lymphocyte cytotoxicity test. Transplantation. 1968 Nov;6(8):913–927. doi: 10.1097/00007890-196811000-00006. [DOI] [PubMed] [Google Scholar]
- Morozov V. N. O determinirovannosti vremeni perestroiki slozhnykh sistem. Biofizika. 1972 Sep-Oct;17(5):926–929. [PubMed] [Google Scholar]
- Neet K. E., Ainslie G. R., Jr Hysteretic enzymes. Methods Enzymol. 1980;64:192–226. doi: 10.1016/s0076-6879(80)64010-5. [DOI] [PubMed] [Google Scholar]
- Nissen M. H., Bjerrum O. J., Plesner T., Wilken M., Rørth M. Modification of beta-2-microglobulin in sera from patients with small cell lung cancer: evidence for involvement of a serine protease. Clin Exp Immunol. 1987 Feb;67(2):425–432. [PMC free article] [PubMed] [Google Scholar]
- Nissen M. H., Thim L., Christensen M. Purification and biochemical characterization of the complete structure of a proteolytically modified beta-2-microglobulin with biological activity. Eur J Biochem. 1987 Feb 16;163(1):21–28. doi: 10.1111/j.1432-1033.1987.tb10731.x. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Odani H., Oyama R., Titani K., Ogawa H., Saito A. Purification and complete amino acid sequence of novel beta 2-microglobulin. Biochem Biophys Res Commun. 1990 May 16;168(3):1223–1229. doi: 10.1016/0006-291x(90)91159-p. [DOI] [PubMed] [Google Scholar]
- Ricard J., Buc J., Meunier J. C. Enzyme memory. 1. A transient kinetic study of wheat-germ hexokinase LI. Eur J Biochem. 1977 Nov 1;80(2):581–592. doi: 10.1111/j.1432-1033.1977.tb11915.x. [DOI] [PubMed] [Google Scholar]
- Ricard J., Meunier J. C., Buc J. Regulatory behavior of monomeric enzymes. 1. The mnemonical enzyme concept. Eur J Biochem. 1974 Nov 1;49(1):195–208. doi: 10.1111/j.1432-1033.1974.tb03825.x. [DOI] [PubMed] [Google Scholar]
- Rübsamen H., Khandker R., Witzel H. Sigmoid kinetics of the monomeric ribonuclease I due to ligand-induced shifts of conformation equilibria. Hoppe Seylers Z Physiol Chem. 1974 Jun;355(6):687–708. doi: 10.1515/bchm2.1974.355.1.687. [DOI] [PubMed] [Google Scholar]
- Smith K. K., Parham P., Ma N. S. Two distinct forms of beta 2-microglobulin in different races of owl monkey (Aotus trivirgatus). Immunogenetics. 1984;20(4):459–464. doi: 10.1007/BF00345620. [DOI] [PubMed] [Google Scholar]
- Vincent C., Chanard J., Caudwell V., Lavaud S., Wong T., Revillard J. P. Kinetics of 125I-beta 2-microglobulin turnover in dialyzed patients. Kidney Int. 1992 Dec;42(6):1434–1443. doi: 10.1038/ki.1992.438. [DOI] [PubMed] [Google Scholar]
- Vincent C., Ramackers J. M., Bonnefoy N., Revillard J. P. Charge heterogeneity of beta 2-microglobulin in lymphoid cells. Mol Immunol. 1989 Aug;26(8):727–733. doi: 10.1016/0161-5890(89)90032-1. [DOI] [PubMed] [Google Scholar]
- Vincent C., Revillard J. P. Cross-reactivity between immunoglobulins and beta 2 microglobulin. Mol Immunol. 1980 Jun;17(6):723–728. doi: 10.1016/0161-5890(80)90142-x. [DOI] [PubMed] [Google Scholar]
- Vincent C., Revillard J. P., Galland M., Traeger J. Serum beta2-microglobulin in hemodialyzed patients. Nephron. 1978;21(5):260–268. doi: 10.1159/000181402. [DOI] [PubMed] [Google Scholar]
- Wells B. D., Stewart T. A., Fisher J. R. Interpretation of nonlinear steady state enzyme kinetics--cyclic and mathematical properties of cooperative, second-site and random pathway models. J Theor Biol. 1976 Jul 21;60(01):209–221. doi: 10.1016/0022-5193(76)90165-x. [DOI] [PubMed] [Google Scholar]
- Wyman J. The turning wheel: a study in steady states. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3983–3987. doi: 10.1073/pnas.72.10.3983. [DOI] [PMC free article] [PubMed] [Google Scholar]


