Abstract
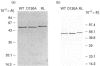
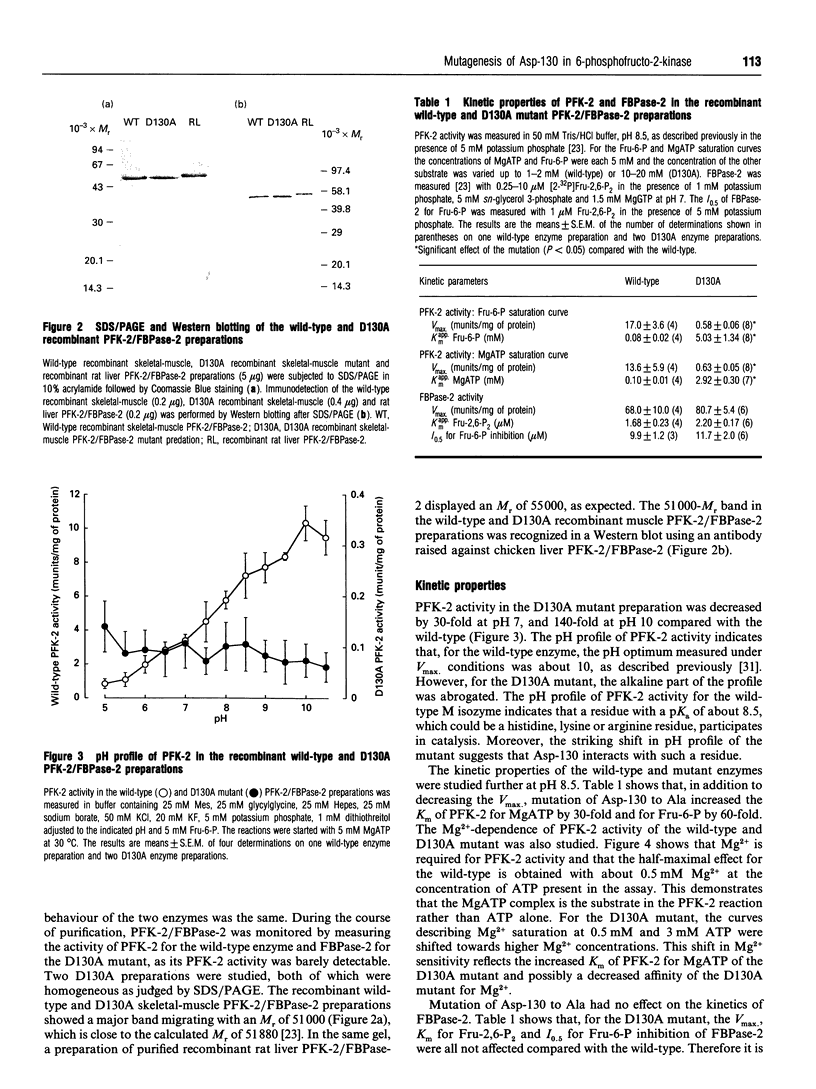
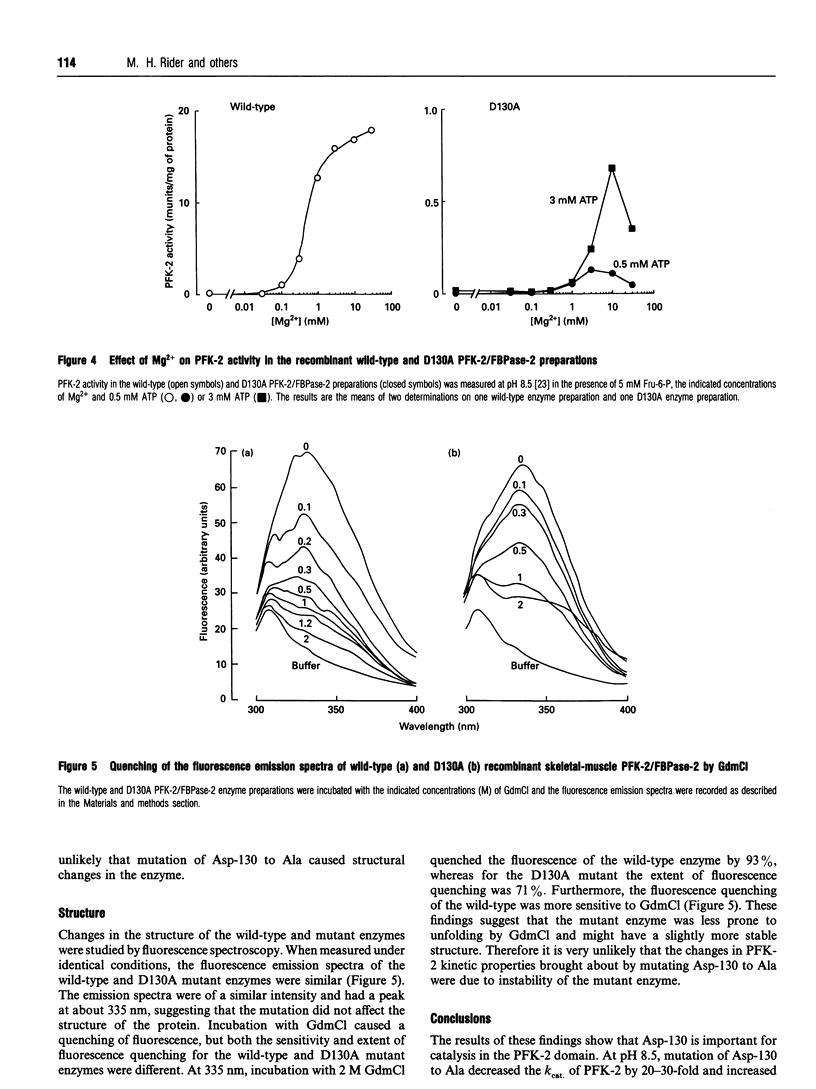
Asp-130 of the recombinant skeletal-muscle 6-phosphofructo-2-kinase (PFK-2)/fructose-2,6-bisphosphatase was mutated into Ala in order to study its role in catalysis and/or substrate binding. The D130A mutant displayed a 30- to 140-fold decreased 2-kinase Vmax, depending on the pH, and a 30- and 60-fold increase in Km for MgATP and Fru-6-P respectively at pH 8.5 compared with the wild-type. Mutagenesis of Asp-130 to Ala had no effect on the 2-phosphatase activity, and fluorescence measurements indicated that the changes in kinetic properties of PFK-2 in the D130A mutant were not due to instability. The role of Asp-130 in the 2-kinase reaction is discussed and compared with that of Asp-103 of 6-phosphofructo-1-kinase from Escherichia coli, which binds Mg2+.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bazan J. F., Fletterick R. J., Pilkis S. J. Evolution of a bifunctional enzyme: 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9642–9646. doi: 10.1073/pnas.86.24.9642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger S. A., Evans P. R. Active-site mutants altering the cooperativity of E. coli phosphofructokinase. Nature. 1990 Feb 8;343(6258):575–576. doi: 10.1038/343575a0. [DOI] [PubMed] [Google Scholar]
- Berger S. A., Evans P. R. Site-directed mutagenesis identifies catalytic residues in the active site of Escherichia coli phosphofructokinase. Biochemistry. 1992 Sep 29;31(38):9237–9242. doi: 10.1021/bi00153a017. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Crepin K. M., Darville M. I., Michel A., Hue L., Rousseau G. G. Cloning and expression in Escherichia coli of a rat hepatoma cell cDNA coding for 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase. Biochem J. 1989 Nov 15;264(1):151–160. doi: 10.1042/bj2640151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crepin K. M., De Cloedt M., Vertommen D., Foret D., Michel A., Rider M. H., Rousseau G. G., Hue L. Molecular forms of 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase expressed in rat skeletal muscle. J Biol Chem. 1992 Oct 25;267(30):21698–21704. [PubMed] [Google Scholar]
- Crepin K. M., Vertommen D., Dom G., Hue L., Rider M. H. Rat muscle 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase. Study of the kinase domain by site-directed mutagenesis. J Biol Chem. 1993 Jul 15;268(20):15277–15284. [PubMed] [Google Scholar]
- Hellinga H. W., Evans P. R. Mutations in the active site of Escherichia coli phosphofructokinase. Nature. 1987 Jun 4;327(6121):437–439. doi: 10.1038/327437a0. [DOI] [PubMed] [Google Scholar]
- Kitajima S., Sakakibara R., Uyeda K. Kinetic studies of fructose 6-phosphate,2-kinase and fructose 2,6-bisphosphatase. J Biol Chem. 1984 Jun 10;259(11):6896–6903. [PubMed] [Google Scholar]
- Kountz P. D., Freeman S., Cook A. G., el-Maghrabi M. R., Knowles J. R., Pilkis S. J. The stereochemical course of phospho group transfer catalyzed by rat liver 6-phosphofructo-2-kinase. J Biol Chem. 1988 Nov 5;263(31):16069–16072. [PubMed] [Google Scholar]
- Kretschmer M., Hofmann E. Inhibition of rat liver phosphofructokinase-2 by phosphoenolpyruvate and ADP. Biochem Biophys Res Commun. 1984 Nov 14;124(3):793–796. doi: 10.1016/0006-291x(84)91027-1. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laine R., Deville-Bonne D., Auzat I., Garel J. R. Interaction between the carboxyl groups of Asp127 and Asp129 in the active site of Escherichia coli phosphofructokinase. Eur J Biochem. 1992 Aug 1;207(3):1109–1114. doi: 10.1111/j.1432-1033.1992.tb17148.x. [DOI] [PubMed] [Google Scholar]
- Li L., Lin K., Correia J. J., Pilkis S. J. Lysine 356 is a critical residue for binding the C-6 phospho group of fructose 2,6-bisphosphate to the fructose-2,6-bisphosphatase domain of rat liver 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase. J Biol Chem. 1992 Aug 15;267(23):16669–16675. [PubMed] [Google Scholar]
- Li L., Lin K., Kurland I. J., Correia J. J., Pilkis S. J. Site-directed mutagenesis in rat liver 6-phosphofructo-2-kinase. Mutation at the fructose 6-phosphate binding site affects phosphate activation. J Biol Chem. 1992 Mar 5;267(7):4386–4393. [PubMed] [Google Scholar]
- Lin K., Li L., Correia J. J., Pilkis S. J. Arg-257 and Arg-307 of 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase bind the C-2 phospho group of fructose-2,6-bisphosphate in the fructose-2,6-bisphosphatase domain. J Biol Chem. 1992 Sep 25;267(27):19163–19171. [PubMed] [Google Scholar]
- Lin K., Li L., Correia J. J., Pilkis S. J. Glu327 is part of a catalytic triad in rat liver fructose-2,6-bisphosphatase. J Biol Chem. 1992 Apr 5;267(10):6556–6562. [PubMed] [Google Scholar]
- Lively M. O., el-Maghrabi M. R., Pilkis J., D'Angelo G., Colosia A. D., Ciavola J. A., Fraser B. A., Pilkis S. J. Complete amino acid sequence of rat liver 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase. J Biol Chem. 1988 Jan 15;263(2):839–849. [PubMed] [Google Scholar]
- Pilkis S. J., Regen D. M., Stewart H. B., Pilkis J., Pate T. M., El-Maghrabi M. R. Evidence for two catalytic sites on 6-phosphofructo-2-kinase/fructose 2,6-bisphosphatase. Dynamics of substrate exchange and phosphoryl enzyme formation. J Biol Chem. 1984 Jan 25;259(2):949–958. [PubMed] [Google Scholar]
- Pyko M., Rider M. H., Hue L., Wegener G. 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase from frog skeletal muscle: purification, kinetics and immunological properties. J Comp Physiol B. 1993;163(2):89–98. doi: 10.1007/BF00263592. [DOI] [PubMed] [Google Scholar]
- Rider M. H., Hue L. Inactivation of liver 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase by phenylglyoxal. Evidence for essential arginine residues. Eur J Biochem. 1992 Aug 1;207(3):967–972. doi: 10.1111/j.1432-1033.1992.tb17131.x. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayers J. R., Schmidt W., Eckstein F. 5'-3' exonucleases in phosphorothioate-based oligonucleotide-directed mutagenesis. Nucleic Acids Res. 1988 Feb 11;16(3):791–802. doi: 10.1093/nar/16.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz G. E., Elzinga M., Marx F., Schrimer R. H. Three dimensional structure of adenyl kinase. Nature. 1974 Jul 12;250(462):120–123. doi: 10.1038/250120a0. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Moffatt B. A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986 May 5;189(1):113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Tauler A., Lin K., Pilkis S. J. Hepatic 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase. Use of site-directed mutagenesis to evaluate the roles of His-258 and His-392 in catalysis. J Biol Chem. 1990 Sep 15;265(26):15617–15622. [PubMed] [Google Scholar]
- Tsai M. D., Yan H. G. Mechanism of adenylate kinase: site-directed mutagenesis versus X-ray and NMR. Biochemistry. 1991 Jul 16;30(28):6806–6818. doi: 10.1021/bi00242a002. [DOI] [PubMed] [Google Scholar]
- Walker J. E., Saraste M., Runswick M. J., Gay N. J. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1(8):945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]