Abstract
Objectives
Obesity represents a global health crisis with significant patient burdens and healthcare costs. Despite the advances with glucagon-like peptide-1 (GLP-1) receptor agonists in treating obesity, unmet needs remain. This study characterizes a novel glucose-dependent insulinotropic polypeptide receptor (GIPR) peptide antagonist, AT-7687, evaluating its potential to enhance obesity treatment.
Methods
We assessed the in vitro potency and pharmacokinetics of AT-7687, alongside its therapeutic effects when administered subcutaneously (SC) alone and in combination with liraglutide to high-fat-diet-fed obese non-human primates (NHP). The study spanned a 42-day treatment period and a 15-day washout period.
Results
AT-7687 demonstrated a subnanomolar cAMP antagonistic potency (pKB of 9.5) in HEK-293 cells and a 27.4 h half-life in NHPs. It effectively maintained weight stability in obese monkeys, whereas placebo recipients had an 8.6% weight increase by day 42 (P = 0.01). Monotherapy with liraglutide resulted in a 12.4% weight reduction compared to placebo (P = 0.03) and combining AT-7687 with liraglutide led to a 16.3% weight reduction (P = 0.0002). The combination therapy significantly improved metabolic markers, reducing insulin levels by 52% (P = 0.008), glucose by 30% (P = 0.02), triglycerides by 39% (P = 0.05), total cholesterol by 29% (P = 0.03), and LDL cholesterol by 48% (P = 0.003) compared to placebo. AT-7687 treatment was well tolerated and not associated with any side effects.
Conclusions
This study underscores the potential of AT-7687 as a promising addition to current obesity treatments.
Keywords: Obesity, GIP receptor antagonist, GLP-1, Weight loss, Metabolic improvements, Cynomolgus monkeys
Graphical abstract
Highlights
-
•
Combining AT-7687 with liraglutide significantly reduces weight in obese monkeys.
-
•
Monotherapy with AT-7687 prevents weight gain in high-fat-diet-fed NHPs.
-
•
Dual therapy improves metabolic parameters numerically greater than monotherapies.
-
•
AT-7687 is well-tolerated, with no associated side effects reported.
1. Introduction
In a world of increasing obesity (body mass index of ≥30 kg/m2), there is an urgent need to bend the obesity curve. According to the World Obesity Federation's 2023 Atlas, more than half of the global population will have obesity by 2035 [1]. Obesity is associated with a tremendous burden on patients as a significant risk factor for high blood pressure, atherosclerosis, type 2 diabetes (T2D), musculoskeletal disorders, high cholesterol, non-alcoholic fatty liver disease, and certain cancers [2,3]. Further, obesity is one of the most costly chronic diseases for healthcare systems, with an impact of US$1.96 trillion in the United States in 2020 [1]. The need for immediate early recognition and safe and effective treatment cannot be overstated. Bending the obesity curve starts with knowledge generation, education, and childhood obesity prevention. Still, despite extensive research on obesity, the development of effective and enduring treatments, currently including exercise, diets, and pharmacotherapies, has proven challenging.
Analogs of the incretin hormone, glucagon-like peptide-1 (GLP-1), have lately demonstrated remarkable efficacy as weight-loss agents, a success underscored by the recent achievements of semaglutide [4]. However, a significant gap remains in meeting the demand for more effective and better-tolerated treatments for obesity. This gap is underscored by the low prescription rates, with only 1–2% of patients receiving medication [5,6]. Multiple dual and triple targeting compounds are currently being developed for obesity. Simultaneously targeting multiple obesity-related pathways is anticipated to yield more substantial weight loss outcomes [7,8]. These multi-specific compounds explore the benefits of combining GLP-1 agonism with targets such as GLP-2, amylin, glucagon, and/or glucose-dependent insulinotropic polypeptide (GIP).
Like GLP-1, GIP is an incretin hormone. Due to the observed augmented GIP secretory responses in obesity and to high-fat diets (HFDs) [9,10], GIP may play a role in the development of dyslipidemia. Genome-wide association studies (GWAS) support this hypothesis, as loss-of-function mutations in the GIP receptor (GIPR) locus are associated with lower weight in humans [[11], [12], [13]]. Further, GIPR knockout mice are protected from diet-induced obesity (DIO) [[14], [15], [16]]. The positioning of GIP as an obesity target has spurred interest in pharmacologically inhibiting the GIPR while simultaneously agonizing the GLP-1 receptor (GLP-1R). Recent studies have found that antagonizing the GIPR alone, with a monoclonal antibody (mAb), prevented body weight (BW) gain in DIO mice and obese cynomolgus monkeys [17], while combining GIPR antagonism with GLP-1R agonism synergistically reduced BW in DIO mice and obese cynomolgus monkeys [17,18]. Importantly, a placebo-adjusted weight loss of 16% was observed in healthy obese volunteers over three months of treatment with Amgen's AMG 133, also known as maridebart cafraglutide, a unimolecular monoclonal antibody that combines GLP-1R agonism and GIPR antagonism [18].
Interestingly, unimolecular dual agonists of the GLP-1R and GIPR also lead to significant weight loss in humans, as exemplified by Eli Lilly's tirzepatide [19,20]. The success of tirzepatide has encouraged the development of multiple dual and triple agonists for the treatment of type 2 diabetes and obesity. The decision to agonize or antagonize GIP, along with the potential molecular mechanisms involved, is the subject of extensive debate in the scientific literature [[20], [21], [22], [23]]. Clearly, further research is needed to fully exploit the therapeutic possibilities of the GIP system.
Here, we report the preclinical characterization and efficacy of an optimized peptide-based GIPR antagonist, AT-7687, tested in the state-of-the-art model for studying the GIP system and obesity, namely obese cynomolgus monkeys [24]. Using this model, we explore both the individual and combined impacts of our GIPR antagonist, AT-7687, and the GLP-1 agonist, liraglutide, on weight, energy consumption, glycemic and lipid profiles. The pre-clinical assessment demonstrates that the combination of AT-7687 and liraglutide results in efficacies with greater level of significance compared to placebo than monotherapies versus placebo.
2. Materials and methods
2.1. Peptides
Human GIP(1–42) was purchased from Bachem (Bubendorf, Switzerland, catalog no. 01–4030658) and dissolved in sodium phosphate buffer (50 mM, pH 7.4, filtered). Liraglutide (Victoza® injection pens, 6.0 mg/mL, 3 mL/vial) was purchased from Novo Nordisk distributors in Beijing and Kunming, China. AT-7687 was custom synthesized by WuXi AppTec (Wuhan, China) using traditional solid phase peptide synthesis (SPPS) method and purified by Peptide-HPLC-B utilizing a column of Gemini C18 (5 μm, 110 Å, 150 × 4.6 mm). Purity was verified as ≥ 96% by RP-HPLC with detection at 220 nm. AT-7687 was solubilized in sodium phosphate buffer (100 mM, pH 7.0) and subsequently filtrated by a 0.22-micron Nylon 25 mm syringe filter to produce sterilized solutions and used within 24 h.
2.2. Transfections and tissue cultures
cDNAs of the human GIPR (hGIPR), GLP-1R, GLP-2R, and glucagon receptor (GCGR) were purchased from Origene (Rockville, Maryland, USA (catalog no. SC110906, SC124060, SC111108, and SC120082, respectively)). cDNA of the Macaca fascicularis GIPR was synthesized by GenScript (Piscataway, USA) (NCBI reference sequence: XP_005589662.1) and cloned into the pCMV-Script vector (Agilent Technologies Denmark, Glostrup, Denmark). COS-7 cells were cultured in 10% CO2 at 37 °C in Dulbecco's modified Eagle's medium (DMEM) 1885 supplemented with 10% fetal bovine serum (FBS), 2 mM glutamine, 180 units/mL penicillin, and 45 g/mL streptomycin. Transient transfection of the COS-7 cells was accomplished using the calcium phosphate precipitation method with addition of chloroquine [25,26].
2.3. Heterologous competition binding
COS-7 cells transiently transfected with the hGIPR were used for the competition binding experiments, and 125I-labeled human GIP(1–42) was used as a radioligand and purchased from PerkinElmer Life Sciences (NEX402025UC). COS-7 cells were seeded in clear 96-well plates at a concentration giving 5–10% specific binding of the tracer (1000–5000 cells/well) one day after the transfection. Two days after transfection, the cells were washed twice and then incubated for 3 h at 4 °C to inhibit receptor internalization in 50 mM Hepes buffer, pH 7.2, supplemented with 0.5% (w/v) bovine serum albumin (BSA) (binding buffer) with 15–40 pM/well of 125I-GIP(1–42) and relevant amounts of AT-7687 as a competitor. The competition binding was terminated by washing the cells twice in ice-cold binding buffer and lysing the cells using 200 mM NaOH with 1% SDS for 30 min. Nonspecific binding was determined as the binding of radioligand to untransfected cells. All experiments were carried out in duplicates and repeated at least three times. Samples were analyzed for radioactivity using a Wallac Wizard 1470 Gamma Counter (GMI Inc., Minnesota, USA), and binding data were analyzed to determine IC50 values using nonlinear regression analysis in GraphPad Prism version 10.2.2 for Windows (GraphPad Software, San Diego, California, USA).
2.4. cAMP assay
HEK-293 cells were cultured in 10% CO2 at 37 °C in DMEM 1885 supplemented with 10% FBS, 2 mM glutamine, 180 units/mL penicillin, and 45 g/mL streptomycin. Transient transfection of the HEK-293 cells was accomplished using the calcium phosphate precipitation method with the addition of chloroquine. Transient transfections of human- or macaque (mq)- GIPR or human GCGR, GLP-1R and GLP-2R were performed one day after seeding. Two days after transfections, the cells were washed and resuspended in HBSS buffer (Gibco, 14025-50) supplemented with 20 mM HEPES (Gibco, 15630-106), 0.1% Pluronic F-68 (Gibco, 24040-032) and 0.1% casein (Sigma, C4765), and plated in 384-well plates at a density of 5000 cells/well. Following 30 min of incubation at 37 °C, fixed concentrations of AT-7867 (0.3, 3 and 30 nM for hGIPR and 1, 10 and 100 nM for mqGIPR) were added and preincubated for 10 min before subsequent addition of increasing concentrations of human GIP(1–42). The ligands were diluted in HBSS buffer supplemented with 20 mM HEPES, 0.1% pluronic, 0.1% casein and 500 μM 1 mM 3-isobutyl-1-methylxanthine (IBMX). Following 30 min incubation at 37 °C, the intracellular cAMP was quantitatively determined using the CisBio cAMP Dynamic 2 HTRF Assay Kit according to the manufacturer's instructions. A standard curve using known concentrations of cAMP was included in each run, to determine the actual cAMP levels. Experiments were made in duplicates and repeated ≥ two times. The signal was detected by using a PerkinElmer Envision® instrument with excitation at 320 nm and emission at 665 nm and 620 nm. The HTRF ratio (emission at 665nm/620 nm∗ 10,000) is inversely proportional to the amount of cAMP present and is converted to nM cAMP per well using a cAMP standard curve.
cAMP dose–response curves were fitted in GraphPad Prism version 10.2.2 for Windows (GraphPad Software, San Diego, California, USA) using the nonlinear regression analysis, whereby EC50 values were estimated. Data on graphs are normalized to maximum response generated in the absence of antagonist. For Schild-plot analysis, log[DR-1] was calculated by equation log[DR-1] = log[(A’/A)-1], where A’ is the EC50 value obtained from the dose–response curves in the presence of indicated concentrations of AT-7867, and A is the EC50 value obtained from the dose–response curves in the absence of antagonist. Log(DR − 1) was plotted against log(AT-7867 concentration), which gives a straight line and where the X-intercepts of the line equals to pA2 value. When the slope of the Schild plot is 1, it indicates competitive antagonism, and the pA2 value is then an estimate of pKb (antagonist binding affinity).
2.5. β-arrestin 2 recruitment
HEK293 cells were cultured in DMEM 1885 supplemented with 10% FBS, 180 units/mL penicillin and 45 μg/mL streptomycin at 37 °C in a 10% CO2/90% air-humidified atmosphere. Transient transfection of HEK293 cells was performed using the polyethylenimine (PEI)-based method [27]. Briefly, 600,000 cells/well were seeded in 24-well cell culture plates. 24 h after seeding, HEK293 cells were co-transfected with 0.33 μg of hGIPR, 0.042 μg of the donor Rluc8-Arrestin3-SP1, 0.8 μg of the acceptor mem-citrine-SH3 and 0.8 μg of the helper protein GRK2 to facilitate β-arrestin 2 recruitment. The DNA constructs were mixed with PEI (ratio 1:2 (DNA/PEI)) and DMEM without supplements. After 15 min incubation at room temperature (RT), the transfection mix was added dropwise to the cells. The transfection was terminated the following day by replacing the transfection medium with fresh culture medium. Two days after transfection, the transiently transfected HEK293 cells were washed with PBS and resuspended in PBS with 1% glucose (5 mM) and 0.1% casein. 80 μL of the cell suspension solution was added to each well of a white 96-well isoplate with subsequent addition of PBS with coelenterazine-h in a final concentration of 5 μM. Immediately after, increasing concentrations of human GIP(3–30)NH2 and AT-7687 (ranging from 1 pM to 1 μM) were added and preincubated for 10 min before subsequent addition of 3.16 nM human GIP(1–42) to a final volume of 100 μL/well. Following 10 min incubation in the dark at RT, luminescence (Rluc8 at 485 nm and YFP at 535 nm) was measured by the PerkinElmer™ EnVision 2014 Multilabel Reader.
2.6. Pharmacokinetics in Göttingen minipigs
The studies were conducted in female Göttingen Minipigs (Ellegaard Göttingen Minipigs A/S, Denmark) of 30–35 kg in accordance with the ARRIVE guidelines and performed with permission from the Danish Animal Experiments Inspectorate (license 2018-15-0201-01397) and the local ethical committee following the guidelines of Danish legislation governing animal experimentation (1987) and the National Institutes of Health (publication number 85-23). AT-7687, semaglutide and tirzepatide were each tested in two minipigs. Prior to the study a central venous catheter (CVC) was in-operated in the ear vein of the minipigs and daily flushing with isotonic NaCL with heparin was conducted to prevent clot formation. A single dose of 3 nmol/kg of each respective test compound was given subcutaneously (SC) in the inguinal fold or behind the ear with a 21 G needle with an injection volume of 0.04 mL/kg. Dose volumes were based on individual BWs. Up to 10 blood samples were collected from the CVC up to 129h postdosing. Blood was collected into chilled EDTA tubes and centrifuged for 15 min, 2000 RCF at 4 °C whereafter plasma was immediately frozen. The plasma was later analyzed by LC-MS/MS by Red Glead Discovery (Lund, Sweden). The plasma half-lives were determined using one phase exponential decay parameter in GraphPad Prism version 10.2.2 for Windows (GraphPad Software, San Diego, California, USA).
2.7. Pharmacokinetics in cynomolgus monkeys
All non-human primates (NHP) studies were performed by Kunming Biomed International (KBI) in China accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC), and in compliance with the National Research Council's Guide for the Care and Use of Laboratory Animals. KBI's Institutional Animal Care and Use Committee (IACUC) reviewed and approved the studies. Three non-naïve male cynomolgus monkeys received a single SC administration of 50 nmol/kg AT-7687. 15 blood samples (0.5 mL blood/sample) were collected from a peripheral vein at predetermined time points up to 9 days after the dosing. Blood samples were collected in EDTA-2K tubes, and plasma was separated within 1 h of collection by centrifugation at 3000 RCF for 10 min at 2–8 °C and stored at ≤ -60 °C until analysis. Pharmacokinetic (PK) parameters were determined by LC-MS/MS by non-compartmental analysis using the Phoenix WinNonlin software (version: 6.3 Certara). The linear/log trapezoidal rule was applied in obtaining the PK parameters.
2.8. Ultracentrifugation
Wuxi AppTec performed the ultracentrifugation using inhouse cynomolgus monkey and human plasma. A liquid chromatography-tandem mass spectrometry (LC-MS/MS) method was applied for concentration determination. Chromatograms were plotted and peak area integrations were carried out by Analyst (Sciex, Framingham, Massachusetts, USA).
2.9. Efficacy study in obese non-human primates
NHP cynomolgus monkeys (M. fascicularis, male, >7.5 kg, >8 years, from KBI's stock colony) were trained and acclimated before the study. Housing was individual in stainless steel cages with visual and auditory contact with conspecifics and cage toys for environmental enrichment. The temperature was maintained at 18–29 °C, the relative humidity was 30–90%, and there was a minimum of 10 air changes/hour. Time-controlled lightning was maintained on a 12-hour light/12-hour dark diurnal cycle. Food was made available ad libitum one week prior to treatment-initiation. One week prior to treatment and during the treatment period, food was provided three times daily and consisted of 50 g of KBI proprietary standard monkey formula feed in the morning (9.00–10.00 AM), an apple (150 g) in the afternoon (14.00–15.00 PM), 100 g of KBI proprietary HFD provided in 20 min intervals if food were consumed in the evening (16.00–17.00), and water was available ad libitum. The remaining food after each feeding session (1 h) was withdrawn, and the food intake was determined by weighing the leftover food. 30 HFD-induced obese monkeys were fasted 15 h before daily SC injections in the scapular/lateral cervical region for a total of 42 days, followed by a 14-day washout period. Ten monkeys were dosed with vehicle (20 mg propylene glycol (pharmaceutical Grade) per mL water for injection), ten monkeys were dosed with AT-7687 (540 nmol/kg), and ten monkeys were dosed with liraglutide in increasing doses starting with 10 μg/kg, followed by 20 μg/kg after three days and ending at a final dose of 30 μg/kg after another three days. BW measurements were performed twice weekly during the dosing period and once weekly during the washout period. Total energy intake (TEI) was monitored daily. Blood samples for metabolic profiling were collected predosing on day 22 and day 43 after the start of dosing, and blood samples for exposure were collected predosing and 8 h post-dosing on day 42. As appropriate, blood samples were collected in EDTA-2K, Serum separation, or P800 tubes. Serum and plasma were separated by centrifugation at 2500 RCF for 10 min at 4 °C and stored at −80 °C until analysis. Glucose, insulin, parathyroid hormone (PTH), and C-terminal telopeptide of type I collagen (CTX) were analyzed at KBI using the Cobas e411 Immunology analyzer. Triglycerides, HbA1c, and cholesterols were analyzed at KBI using the Roche C311/C501 biochemical analyzer, and glucagon was measured at KBI with a glucagon ELISA kit (10-1271-01, Mercodia, Uppsala, Sweden).
2.10. Statistical analysis and calculations
All data have been baseline (predose) subtracted before analysis. Statistical analysis was made in GraphPad Prism version 10.2.2 for Windows (GraphPad Software, San Diego, California, USA) using ordinary one-way ANOVA with Tukeys multiple comparison test and one-way ANOVA Kruskal–Wallis test with Dunn's multiple comparison test when more than two groups at one time point were compared, and two-way ANOVA with Dunnetts multiple comparison test when more than two groups at more than one time point were compared. Data are presented as mean ± SEM. Statistical significance is reported as P < 0.05.
Insulin resistance was evaluated using values obtained on treatment day 42 in the homeostatic model assessment of insulin resistance (HOMA-IR) using the formula:
2.11. Role of the funding source
This study was sponsored by Antag Therapeutics Aps, with employees responsible for study design, data collection, analysis, interpretation, and manuscript preparation.
3. Results
3.1. AT-7687 is a potent and selective GIPR antagonist
AT-7687 is an optimized peptide analogue of the naturally occurring GIPR antagonist GIP(3–30)NH2 [28] comprising amino acid substitutions and lipidation to improve the PK profile, solubility, stability, and potency.
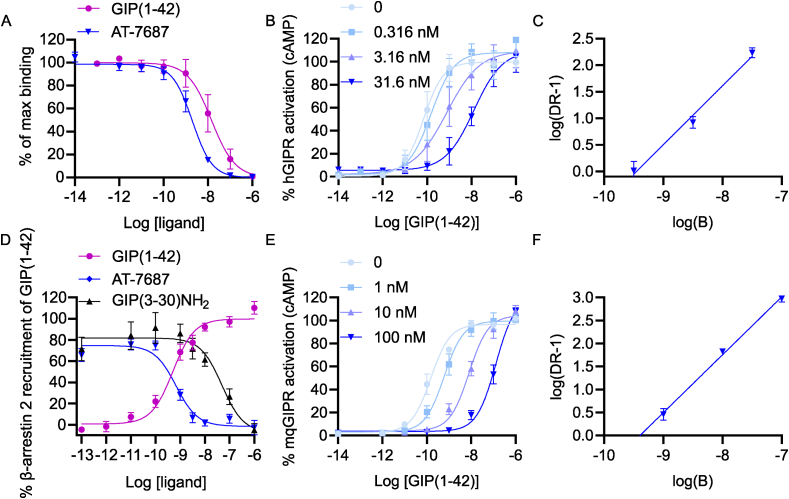
The competitive binding of AT-7687 was characterized in a heterologous competition binding assay using 125I-labeled human GIP(1–42) as radioligand in cells transiently expressing hGIPR. The results demonstrate that AT-7687 binds with a Ki of 2 nM (Figure 1A).
Figure 1.
AT-7687 is a potent and selective GIPR antagonist.
A) Binding of AT-7687 and GIP(1–42) to hGIPR. B) Percent GIP(1–42)-mediated cAMP accumulation from hGIPRs without and with increasing concentrations of AT-7687.
C) Schild regression analysis of AT-7687 on GIP(1–42)-induced cAMP accumulation from hGIPRs.
D) Percent GIP(1–42)-induced β-arrestin 2 recruitment.
E) Percent GIP(1–42)-mediated cAMP accumulation from macaque GIPRs without and with increasing concentrations of AT-7687.
F) Schild regression analysis of AT-7687 on GIP(1–42)-induced cAMP accumulation from macaque GIPRs.
Data are presented as mean ± SEM, n = ≥3 independent experiments carried out in duplicates. GIP, glucose-dependent insulinotropic polypeptide; hGIPR, human GIP receptor; DR, dose–response; mqGIPR, macaque GIP receptor; cAMP, cyclic adenosine monophosphate.
To evaluate antagonistic properties, AT-7687 was further evaluated for its effect in shifting the GIP(1–42) dose–response curve in a cAMP assay. The assay was performed in transiently transfected cells expressing hGIPR. Three concentrations of AT-7687 were used (0.316, 3.16 and 31.6 nM) to enable subsequent Schild regression analysis. The presence of AT-7687 shifted the GIP(1–42) dose–response curves to the right without suppressing the efficacy, indicating competitive nature of antagonism (Figure 1B). The Schild regression line had a unity slope of 1.1 and an apparent pKb of 9.5 (Figure 1C). Furthermore, AT-7687 displayed selectivity to the GIPR, as it did not antagonize the most closely related receptors, the human GCGR, GLP-1R or the GLP-2R (Supplemental Figure 1A). Moreover, no agonistic effect on either GIPR, GLP-1R, GLP-2R nor GCGR was observed (Supplemental Figure 1B-E). AT-7687 was further evaluated in a β-arrestin 2 recruitment assay in comparison with the endogenous GIPR antagonist, GIP(3–30)NH2. Here, the potency (EC50) of GIP(1–42) was 0.47 nM and GIP(1–42)-mediated β-arrestin 2 recruitment was inhibited by a potency (IC50) of 50 nM and 0.66 nM for GIP(3–30)NH2 and AT-7687, respectively. Consequently, AT-7687 demonstrated ∼75 fold greater inhibitory potency than endogenous GIP(3–30)NH2.
As we used the NHP Cynomolgus monkey (M. fascicularis) to assess the in vivo biological effect of AT-7687, the antagonistic properties of AT-7687 were evaluated on macaque (mq)GIPR expressing cells, where the apparent pKb was estimated to be 9.4 (Figure 1E, F) which was similar to the pKb obtained for the hGIPR.
3.2. AT-7687 is a strong albumin binder and demonstrates an extended circulatory half-life
In general, peptides have a relatively short circulating plasma half-life, which limits their clinical application. Various strategies have been developed to extend peptide half-life, predominantly centered around the binding of peptides to the circulating protein albumin and thus protracting the peptide half-life. This strategy was also implemented in the development of AT-7687, and here, we demonstrated a plasma half-life of 58 h, 76 h, and 83 h after a single SC administration of 3 nmol/kg of AT-7687, semaglutide and tirzepatide, respectively, in Göttingen mini pigs (Figure 2 and Table 1). These findings align with previous results observed for semaglutide [29].
Figure 2.
Plasma half-life of AT-7687, semaglutide and tirzepatide in Göttingen minipigs.
Concentration time curve for AT-7687, semaglutide and tirzepatide in Göttingen minipigs. n = 2 for all groups. Data are presented as mean.
Table 1.
Plasma protein binding of AT-7687 and semaglutide, PK parameters of AT-7687, semaglutide and tirzepatide in Göttingen minipigs, and PK parameters of AT-7687 in obese Cynomolgus monkeys.
| PPB Unbound fraction | n | T½ (h±SD) | AUC0-inf (h∗nmol/L±SD) | n | ||
|---|---|---|---|---|---|---|
| AT-7687 | Cynomolgus monkey | 0.2% | ≥6 | 27.4 (±8.7) | 25470 (±6100) | 3 |
| Human | 0.2% | ≥2 | ND | ND | ND | |
| Göttingen minipig | ND | ND | 58 | 1879 | 2 | |
| Semaglutide | Cynomolgus monkey | 0.1% | ≥6 | ND | ND | ND |
| Human | 0.04% | ≥2 | ND | ND | ND | |
| Göttingen minipig | ND | ND | 76 | 2287 | 2 | |
| Tirzepatide | Göttingen minipig | ND | ND | 83 | 4116 | 2 |
PPB, plasma protein binding; T½, plasma half-life; AUC, area under the curve; ND, not determined. N represents the number of animals used.
To determine the plasma protein binding (PPB) of AT-7687, we applied ultracentrifugation to cynomolgus monkey and human plasma and included semaglutide as control. We found that AT-7687 exhibited very high plasma protein binding, resulting in an unbound fraction of 0.2% in both cynomolgus monkey and human plasma. In comparison, semaglutide had unbound fractions of 0.1% and 0.04% in cynomolgus monkey and human plasma, respectively (Table 1). The high plasma protein binding was also reflected in the PK properties of AT-7687, demonstrating a plasma half-life of 27.4 h after a single SC administration of 50 nmol/kg in cynomolgus monkeys (Table 1).
3.3. AT-7687 numerically enhances liraglutide-induced reductions in BW and TEI when compared to placebo
To determine the effects of AT-7687 with and without liraglutide on BW and metabolic parameters, >8-year-old DIO male cynomolgus monkeys received SC injections of 540 nmol/kg AT-7687 and/or liraglutide in doses beginning with 10 μg/kg, then escalating to 20 μg/kg after three days, and culminating in a final dose of 30 μg/kg after an additional three days or equal volume of vehicle as control for 42 days. Food intake was monitored daily, BW was monitored twice weekly, and blood samples for metabolic and lipid profiling were collected three times during the study period. The average baseline BW for NHPs in this study was 9.7 kg and did not differ significantly between groups. All baseline values, including TEI, glucose, insulin, and triglycerides were similar for all NHPs (Table 2), verifying that the groups at screening were well randomized.
Table 2.
Baseline characteristics of obese male Cynomolgus monkeys.
| Energy intake (kcal) | BW (kg) | Glucose (mg/dL) | Insulin (μU/mL) | Triglycerides (mmol/L) | n | |
|---|---|---|---|---|---|---|
| Placebo | 606 ± 83 | 9.7 ± 1.1 | 75 ± 7.8 | 49 ± 11 | 1.0 ± 0.64 | 10 |
| Liraglutide | 616 ± 92 | 9.7 ± 1.6 | 72 ± 11 | 52 ± 21 | 1.0 ± 0.62 | 9 |
| AT-7687 | 606 ± 78 | 9.7 ± 1.8 | 82 ± 14 | 48 ± 22 | 0.89 ± 0.53 | 10 |
| AT-7687 + liraglutide | 604 ± 78 | 9.7 ± 1.1 | 76 ± 12 | 46 ± 18 | 1.1 ± 0.58 | 10 |
Data are presented as mean ± SD. n represents the number of animals used. BW, body weight.
Placebo-treated obese NHP showed a time-dependent increase in BW when given ad libitum meals (Figure 2A). No change in BW from baseline was observed in the AT-7687 monotreatment group (Figure 3A,B). Compared with placebo, liraglutide monotreatment significantly reduced BW by 12.4% (P = 0.03) at the end of the treatment phase (Figure 3A,B), and AT-7687 combined with liraglutide significantly reduced BW by 16.3% (P = 0.0002) at the end of the treatment phase (Figure 3B). Although the combination of AT-7687 and liraglutide did not reach statistical significance compared to liraglutide monotherapy, it did result in a numerically greater BW reduction (16.3% vs. 12.4%).
Figure 3.
AT-7687 combined with liraglutide, provides the most significant reduction in weight and TEI in obese NHPs.
A) Changes in BW (%) from predose during the entire study period, including a 42-day treatment period followed by a 15-day washout period in obese NHPs.
B) Placebo-adjusted change (%) in BW from predose to the last day of dosing (day 42).
C) Cumulative energy intake during the 42-days treatment period
D) Total energy intake during the entire treatment period from day 1–42.
n = 10 NHPs for placebo, AT-7687 monotreatment and AT-7687 + liraglutide groups, n = 9 NHPs for liraglutide group. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, and ∗∗∗∗P < 0.0001 versus placebo. Data are presented as mean ± SEM.
Compared to placebo, liraglutide significantly reduced BW from day 7 until the end of dosing (day 42), whereas AT-7687 in combination with liraglutide significantly reduced BW from day 3 and throughout the entire study, including the washout period (Figure 3A). Though BW started to rebound towards baseline at the end of treatment for all groups, the baseline was not reached by the end of the 2-week washout period for AT-7687 combined with liraglutide (Figure 3A). TEI decreased compared to placebo once treatment was initiated for all groups (Figure 3C). In combination with liraglutide, AT-7687 significantly reduced TEI at the end of the study period (P = 0.003) (Figure 3D). No incidences of vomiting, diarrhea or behavioral changes were observed for any of the groups (Supplemental Tables 1 and 2). The treatments were well tolerated, except for one NHP in the liraglutide monotreatment group that stopped eating and drinking, leading to its exclusion from the study after euthanasia.
3.4. AT-7687 in combination with liraglutide improves glycemic control, and AT-7687 weight-independently improves lipid metabolism
At the end of treatment (day 42), insulin levels decreased by 28% in the liraglutide group, 9% in the AT-7687 group, and 52% in the combination group while there was a 47% increase in the placebo group. The reduction in the combination group reached statistical significance compared to placebo (P = 0.008), however, this reduction was not significantly different from liraglutide monotherapy (Figure 4A). Only AT-7687 combined with liraglutide significantly reduced fasting glucose levels compared to placebo (P = 0.02) (+8% for placebo, −9% for liraglutide, −3% AT-7687, −22% for AT-7687 + liraglutide) (Figure 4B). Insulin resistance, measured as HOMA-IR, was significantly improved for both liraglutide monotreatment (P = 0.03) and for AT-7687 combined with liraglutide (P = 0.001) (Figure 4C).
Figure 4.
AT-7687 combined with liraglutide, improves glycemic control and lipid metabolism in obese NHPs.
A) (%) change in insulin from predose at day 42.
B) (%) change in glucose from predose at day 42.
C) HOMA-IR calculated for day 42.
D) (%) change in triglycerides from predose at day 42.
E) (%) change in total choleterol from predose at day 42.
F) (%) change in LDL-cholesterol from predose at day 42.
G) (%) change in HDL-cholesterol from predose at day 42.
H) Change from predose at day 42 for CTX (ng/mL).
I) Change from predose at day 42 for PTH (pg/mL).
N = 10 NHPs for placebo, AT-7687 monotreatment and AT-7687 + liraglutide groups, n = 9 NHPs for liraglutide group. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001 versus placebo. Data are presented as mean ± SEM. HOMA-IR, homeostatic model assessment for insulin resistance; HDL, high-density lipoprotein; LDL, low-density lipoprotein; CTX, C-terminal telopeptide of type I collagen; PTH, parathyroid hormone.
Triglyceride levels decreased across all treatment groups at the end of treatment (day 42), though only significantly for AT-7687 combined with liraglutide (P = 0.05) (−4% for placebo, −21% for liraglutide, −28% AT-7687, −43% for AT-7687 + liraglutide) (Figure 4D). Total cholesterol decreased for all treatment groups, though only significantly for the AT-7687 combined with liraglutide treatment (P = 0.03) (+4% for placebo, −20% for liraglutide, −21% AT-7687, −29% for AT-7687 + liraglutide) (Figure 4E). Liraglutide monotreatment mediated a non-significant decrease in total cholesterol, likely driven by a non-significant reduction in HDL-cholesterol concentrations (Figure 4E,F). In contrast, AT-7687 monotherapy (P = 0.03) and AT-7687 combined with liraglutide (P = 0.003) significantly decreased LDL-cholesterol concentration without affecting HDL-cholesterol concentration (Figure 4F,G) (+28% for placebo, −9% for liraglutide, −30% AT-7687, −48% for AT-7687 + liraglutide). It is noteworthy that the lipid–favorable profile induced by AT-7687 was by a weight-independent mechanism, as AT-7687 mono-treated NHPs were weight stable (Figure 3A). While triglyceride and cholesterol levels showed numerical improvements with the combination therapy, these changes did not reach statistical significance when compared to liraglutide monotherapy.
Biomarkers of bone metabolism, CTX, and PTH were not significantly different from placebo in any of the treatment groups (Figure 4H,I).
4. Discussion
In this study, we demonstrate that the peptide-based GIPR antagonist, AT-7687, mitigated weight gain in HFD-induced obese NHPs. When combined with the GLP-1 analogue liraglutide, AT-7687 produced a numerically larger effect on weight reduction from placebo (12.4% weight loss with liraglutide monotreatment, P = 0.03 versus 16.3% weight loss with combination therapy, P = 0.0002) without compromising tolerability. Notably, this weight reduction was accompanied by metabolic enhancements, particularly in glycemic control and LDL-cholesterol levels, with the latter showing improvements independent of weight change.
The question of whether to activate or antagonize the GIPR in conjunction with GLP-1 mimetics is controversial in the field of weight-loss pharmacology. This debate is fueled by data from numerous rodent studies, which suggest that both GIPR agonism and antagonism when paired with GLP-1R activation, can augment weight loss [[14], [15], [16],[30], [31], [32], [33], [34], [35], [36]]. Clinical studies assessing unimolecular compounds representing both strategies further fuel the controversy [[18], [19], [20],37,38]. Activation of the hGIPR stimulates Gαs, leading to cAMP accumulation and receptor internalization via a β-arrestin-dependent pathway [39]. In line with previous studies [11,12], a recent study found that humans with loss-of-function variants in the GIPR that diminish cAMP production and β-arrestin recruitment have lower BMI and body fat percentages [13]. However, individuals with mutations that reduce cAMP accumulation but do not affect β-arrestin recruitment show no significant changes in BMI or body fat, highlighting the critical role of β-arrestin in mediating GIP's influence on body composition [13]. Consistent with this, we show that AT-7687 inhibited GIP-induced β-arrestin recruitment at a sub-nanomolar potency with subsequent impact on BW in obese NHPs.
While rodent models of obesity are generally suitable for optimizing GLP-1 mimetics, the GIP system shows notable interspecies differences, especially between mice and humans. In humans and primates, the GIP system is highly conserved, showing a 98% sequence homology in receptor sequence and a complete conservation in the GIP sequence. However, when comparing the GIP system in mice to that in humans, the sequence homology is reduced to 81% for receptors and 92% for GIP [[39], [40], [41], [42]]. In contrast, the GLP-1 system shows greater similarity across species. Between mice and humans, there is a 93% sequence homology in receptors and complete conservation of GLP-1's sequence. Consequently, the ability of mouse GIPR to recruit β-arrestin and undergo internalization upon activation is markedly reduced compared to the hGIPR [42]. Therefore, using mouse models to study GIP-related signaling in humans will not accurately reflect important signaling cascades, particularly those relevant to weight management and body composition.
Significant interest in targeting the GIPR has been spurred by the success of tirzepatide, a unimolecular dual GIPR/GLP-1 agonist, which was recently approved for treating obesity. Administered weekly at doses of 10 mg or 15 mg, tirzepatide has demonstrated greater glucose-lowering and weight-reduction effects than semaglutide at the approved doses of 1.7 mg or 2.4 mg [4,19,20,43]. This finding seems contradictory to the associations observed with hGIPR loss-of-function mutations and from acute human studies examining the combined effects of GIP and GLP-1. For example, previous research has shown that adding a GIP infusion to a GLP-1 infusion could diminish the appetite-suppressive effects of GLP-1 in obese subjects [44], and GIP administration was found to increase postprandial glucose excursions and triglyceride levels in obese men with T2D on liraglutide maintenance treatment [45].
One hypothesis that reconciles the clinical benefits of tirzepatide with the above observations suggests that it may act as a functional antagonist at the GIPR. According to Willard et al., tirzepatide's acute interaction with the hGIPR leads to cAMP accumulation, β-arrestin recruitment, and subsequent receptor internalization, similar to the natural GIP ligand [46]. However, the prolonged half-life of tirzepatide might result in chronic GIPR internalization, potentially leading to receptor desensitization, as observed in adipocytes [21,47]. To our knowledge, there is only one subchronic clinical study that has explored the combinatory effects of GIP and GLP-1 agonism using separate molecules, providing insights into the desensitization hypothesis. This phase 2 trial combined various doses of a once-weekly GIPR agonist (NNC0480-0389) with semaglutide at 2.4 mg and assessed the effects for up to 41 weeks in approximately 500 patients with T2D. It was disclosed that there were no superior HbA1C reductions or weight loss of adding the GIPR agonist compared to semaglutide alone and the development of the compound was discontinued [48]. These results cast doubt on the desensitization hypothesis. Although the molecular pharmacological profile of NNC0480-0389 remains undisclosed, these results also question the added benefit of combining GIP agonism with GLP-1, at least in terms of weight loss in patients with T2D.
An alternative explanation suggests that tirzepatide's efficacy primarily stems from a biased GLP-1 component, with a limited contribution from the GIP component. Importantly, the interaction of tirzepatide with the GLP-1R is unique; it significantly reduces β-arrestin recruitment and internalization compared to native GLP-1 and semaglutide, while still inducing cAMP accumulation [46,49]. Consequently, more GLP-1 receptors remain available on the cell surface, enhancing signaling efficacy and potentially making tirzepatide a superior GLP-1R agonist, which could explain its enhanced effectiveness [46]. This hypothesis is further supported by multiple research groups who have modified the structure of GLP-1 analogs to decrease β-arrestin recruitment, mirroring tirzepatide's biased signaling profile, and observed an increased weight-loss effect in mice [50,51]. Tirzepatide's efficacy may also be attributed to the relatively higher dose of tirzepatide compared to semaglutide, as suggested by Vadher et al. through cross-study analyses [52]. The ongoing phase 3 study by Novo Nordisk, which is testing a 7.2 mg dose of semaglutide for obesity, will provide further insights.
In contrast to the elusive contribution of GIPR agonism to weight-loss clinically, GIPR antagonism in combination with GLP-1 agonism demonstrates remarkable consistency in terms of weight loss in obese NHPs. Killion et al. demonstrated that combining a GIPR antagonist antibody with the Fc-conjugated GLP-1R agonist, dulaglutide, led to greater weight reduction than either approach alone in obese NHPs [17]. A unimolecular antibody developed by Amgen, AMG 133, which is a GIPR antagonist antibody conjugated with two GLP-1 analogues, achieved a significant BW reduction of 16.5% relative to placebo after 43 days in obese cynomolgus monkeys [18]. This was accompanied by reductions in insulin (∼50%), triglycerides (∼50%), cholesterol (∼20%), and LDL-cholesterol (∼35%) levels. In the present similarly designed 6-week study, co-administration of AT-7687 and liraglutide resulted in remarkably similar results with reductions of 16.3% BW, 52% insulin, 43% triglycerides, 30% cholesterol, and 48% LDL-cholesterol. The co-treatment results were not statistically significant compared to liraglutide monotherapy (as expected due to the small sample size) and are similar to those of Amgen's initial NHP study where the combination of a GIPR antibody with dulaglutide also did not achieve statistical significance compared to dulaglutide monotreatment [17]. The similarity between our results and Amgen's AMG 133 data is remarkable and confirms that both unimolecular and separate compound approaches consistently yield significant outcomes with GIPR antagonism and GLP-1 agonism in obese NHPs.
Whether the consistency of unimolecular and co-administrative approaches in obese NHPs translates to the clinic remains to be determined. In a recent 3-month phase 1b obesity study, AMG 133 showed a remarkable 16% placebo-adjusted weight loss [18]. Longer term studies remain to be seen, however, the reported weight-loss is astonishing as it aligns with studies showing 14% weight-loss at three months following bariatric surgery, the so-called holy grail of weight-loss [53,54]. Although the weight-loss of AMG 133 is impressive, questions remain regarding the tolerability profile of AMG 133 given the relatively high number of dropouts, the dose volume required, concerns about immunogenicity, and the relatively high production costs associated with antibody-based drugs.
Using a peptide-based GIPR antagonist like AT-7687 in combination with an effective GLP-1R agonist offers significant flexibility in optimizing the balance between efficacy and side effects, such as nausea and constipation, which are commonly associated with GLP-1R agonists. This approach will likely improve the cost-effectiveness and reduce discomfort during subcutaneous administration. Furthermore, AT-7687 could be an effective monotherapy for weight maintenance following successful weight loss, due to its demonstrated ability to attenuate weight gain, as observed in this study and in agreement with loss-of-function hGIPR mutations. Despite the obese NHPs having ad libitum access to food starting one week prior to dosing, AT-7687 treatment mitigated weight gain as observed in the placebo group. Considering the well-documented rapid weight rebound following GLP-1 treatment discontinuation [55,56] and the significant number of patients experiencing tolerability issues even after one year of GLP-1 therapy, (∼10–15% experience nausea, ∼4–5% experience diarrhea, ∼2–3% experience vomiting and ∼8–15% experience constipation persisting after one year of treatment) [4,43,55,[57], [58], [59], [60], [61]], AT-7687 offers a promising strategy to sustain long-term weight loss while minimizing the side effects typically linked to GLP-1 analogs, as AT-7687 treatment was not associated with any side effects or tolerability issues in the present study. This is further supported by clinical evidence, wherein doses of the naturally occurring GIPR peptide antagonist, GIP(3–30)NH2, were administered to achieve exposure levels capable of fully blocking the GIPR, yet no alterations in nausea, comfort, or any other side effects were observed [62]. Importantly, a study combining GLP-1 agonism with GIPR antagonism revealed no changes in nausea or overall wellbeing between GLP-1 infusion alone and GLP-1 combined with GIPR antagonist infusion [63].
Our study has several limitations. Firstly, the direct comparison between the combination therapy and liraglutide monotherapy did not reach statistical significance for key outcomes, including BW reduction and metabolic markers. Despite this, the combination therapy consistently showed numerically greater improvements in BW reduction, glycemic control, and lipid profiles. The lack of statistical significance is likely due to the small sample size, which may have limited the study's power to detect meaningful differences.
In addition, the study tested only one dose of liraglutide, that was lower than in previous NHP studies [64], which may have contributed to an underestimation of the maximum potential benefits of AT-7687 in combination with GLP-1R agonism. Similarly, this study does not inform on the additivity of GIPR antagonism regarding weight loss in a maximally stimulated GLP-1 system. As an NHP titration schedule was only publicly available for liraglutide, this GLP-1 analog was employed instead of more efficacious analogues e.g., semaglutide.
Furthermore, to minimize animal handling and associated eating behavior, we did not assess changes in body composition through e.g., DEXA scans. Thus, it remains to be seen if the reduced fat % in humans with loss-of-function GIPR mutations [13] can be reproduced using a GIPR antagonist in humans. Lastly, only male NHPs were included to avoid menstrual cycle-related impacts, thus limiting the generalizability of the study. Though our study was conducted in NHPs, and they represent the human obese population more closely than other preclinical models, the translatability of these results to human outcomes remains uncertain.
5. Conclusion
This study demonstrated that AT-7687, a peptide-based GIPR antagonist, has significant potential in the field of obesity management. When combined with the GLP-1 analogue liraglutide, AT-7687 provided the largest weight reduction compared to placebo and improved metabolic parameters such as glycemic control and LDL-cholesterol levels without compromising tolerability. These findings suggest that AT-7687 could serve as an effective tool for weight management, potentially even as a standalone treatment after successful weight loss. The consistency of weight loss observed in obese NHPs, both with unimolecular and separate compound approaches, underscores the robustness of GIPR antagonism combined with GLP-1 agonism. This strategy may offer a novel path to achieving the kind of weight loss typically associated with bariatric surgery but without the associated surgical risks and with improved management of side effects. While our results are promising, the translatability of these findings from NHPs to humans requires further investigation. Future clinical studies are needed to confirm these results and refine the dosing regimen to maximize the therapeutic potential of AT-7687.
CRediT authorship contribution statement
Mette H. Jensen: Writing – review & editing, Writing – original draft, Visualization, Investigation, Formal analysis, Data curation, Conceptualization. Samra J. Sanni: Writing – review & editing, Writing – original draft, Visualization, Investigation, Formal analysis, Data curation, Conceptualization. Ditte Riber: Writing – review & editing, Conceptualization. Jens J. Holst: Writing – review & editing, Supervision, Resources, Conceptualization. Mette M. Rosenkilde: Writing – review & editing, Supervision, Resources, Conceptualization. Alexander H. Sparre-Ulrich: Writing – review & editing, Writing – original draft, Supervision, Resources, Project administration, Funding acquisition, Data curation, Conceptualization.
Declaration of generative AI and AI-assisted technologies in the writing process
During the preparation of this work the authors used ChatGPT 3.5 in order to improve readability and language. After using this tool, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:MHJ, SJS, DR, and AHS-U are or were previously employees of Antag Therapeutics Aps. MHJ, SJS, DR, JJH, MMR and AHS-U hold warrants in Antag Therapeutics Aps. JJH, MMR, and AHS-U are founders of Antag Therapeutics Aps. JJH, MMR, and AHS-U hold the following patent: WO 2018/220123. DR, MMR and AHS-U hold the following patents: WO 2020/115048 and WO 2020/115049. SJS, DR, MMR, and AHS-U hold the following patent: WO 2021/110845. JJH is a board member of Antag Therapeutics Aps and has served as a consultant or advisor to Novo Nordisk, Roche, Novartis Pharmaceuticals, Merck Sharp & Dohme and received fees for lectures from Merck Sharp & Dohme, and Novo Nordisk. MMR is a consultant for Antag Therapeutics Aps.
Acknowledgements
We want to thank Rasmus Glavind Serup, Adrian Dragan and Søren Petersen for excellent and invaluable technical support. The graphical abstract was made using BioRender.com. This work was funded by Antag Therapeutics Aps.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.molmet.2024.102006.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- 1.Lobstein T., Jackson-Leach R., Powis J., Brinsden H., Gray M. World obesity Atlas 2023. World Obesity Federation. 2023;(March):5–25. [Google Scholar]
- 2.Yumuk V., Tsigos C., Fried M., Schindler K., Busetto L., Micic D., et al. European guidelines for obesity management in adults. Obes Facts. 2015;8(6):402–424. doi: 10.1159/000442721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guh D.P., Zhang W., Bansback N., Amarsi Z., Birmingham C.L., Anis A.H. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Publ Health. 2009;9(1):88. doi: 10.1186/1471-2458-9-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilding J.P.H., Batterham R.L., Calanna S., Davies M., Van Gaal L.F., Lingvay I., et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384(11):989–1002. doi: 10.1056/NEJMoa2032183. [DOI] [PubMed] [Google Scholar]
- 5.Claridy M.D., Czepiel K.S., Bajaj S.S., Stanford F.C. Treatment of obesity: pharmacotherapy trends of office-based visits in the United States from 2011 to 2016. Mayo Clin Proc. 2021;96(12):2991–3000. doi: 10.1016/j.mayocp.2021.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xia Y., Kelton C.M.L., Guo J.J., Bian B., Heaton P.C. Treatment of obesity: pharmacotherapy trends in the United States from 1999 to 2010. Obesity. 2015;23(8):1721–1728. doi: 10.1002/oby.21136. [DOI] [PubMed] [Google Scholar]
- 7.Müller T.D., Blüher M., Tschöp M.H., DiMarchi R.D. Anti-obesity drug discovery: advances and challenges. Nat Rev Drug Discov. 2022;21(3):201–223. doi: 10.1038/s41573-021-00337-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenkilde M.M. Advances in incretin-based therapeutics for obesity. Nat Rev Endocrinol. 2024;20(2):67–68. doi: 10.1038/s41574-023-00938-w. [DOI] [PubMed] [Google Scholar]
- 9.Fukase N., Igarashi M., Takahashi H., Manaka H., Yamatani K., Daimon M., et al. Hypersecretion of truncated glucagon-like peptide-1 and gastric inhibitory polypeptide in obese patients. Diabet Med. 1993;10(1):44–49. doi: 10.1111/j.1464-5491.1993.tb01995.x. [DOI] [PubMed] [Google Scholar]
- 10.Brøns C., Jensen C.B., Storgaard H., Hiscock N.J., White A., Appel J.S., et al. Impact of short-term high-fat feeding on glucose and insulin metabolism in young healthy men. J Physiol. 2009;587(10):2387–2397. doi: 10.1113/jphysiol.2009.169078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turcot V., Lu Y., Highland H.M., Schurmann C., Justice A.E., Fine R.S., et al. Protein-altering variants associated with body mass index implicate pathways that control energy intake and expenditure in obesity. Nat Genet. 2018;50(1):26–41. doi: 10.1038/s41588-017-0011-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kizilkaya H.S., Sørensen K.V., Kibsgaard C.J., Gasbjerg L.S., Hauser A.S., Sparre-Ulrich A.H., et al. Loss of function glucose-dependent insulinotropic polypeptide receptor variants are associated with alterations in BMI, bone strength and cardiovascular outcomes. Front Cell Dev Biol. 2021;9(October):1–12. doi: 10.3389/fcell.2021.749607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kizilkaya H.S., Sørensen K.V., Madsen J.S., Lindquist P., Douros J.D., Bork-Jensen J., et al. Characterization of genetic variants of GIPR reveals a contribution of β-arrestin to metabolic phenotypes. Nat Metab. 2024 doi: 10.1038/s42255-024-01061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Althage M.C., Ford E.L., Wang S., Tso P., Polonsky K.S., Wice B.M. Targeted ablation of glucose-dependent insulinotropic polypeptide-producing cells in transgenic mice reduces obesity and insulin resistance induced by a high fat diet. J Biol Chem. 2008;283(26):18365–18376. doi: 10.1074/jbc.M710466200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miyawaki K., Yamada Y., Ban N., Ihara Y., Tsukiyama K., Zhou H., et al. Inhibition of gastric inhibitory polypeptide signaling prevents obesity. Nat Med. 2002;8(7):738–742. doi: 10.1038/nm727. [DOI] [PubMed] [Google Scholar]
- 16.Nasteska D., Harada N., Suzuki K., Yamane S., Hamasaki A., Joo E., et al. Chronic reduction of GIP secretion alleviates obesity and insulin resistance under high-fat diet conditions. Diabetes. 2014;63(7):2332–2343. doi: 10.2337/db13-1563. [DOI] [PubMed] [Google Scholar]
- 17.Killion E.A., Wang J., Yie J., Shi S.D.-H., Bates D., Min X., et al. Anti-obesity effects of GIPR antagonists alone and in combination with GLP-1R agonists in preclinical models. Sci Transl Med. 2018;10(472):7823–7830. doi: 10.1126/scitranslmed.aat3392. [DOI] [PubMed] [Google Scholar]
- 18.Véniant M.M., Lu S.-C., Atangan L., Komorowski R., Stanislaus S., Cheng Y., et al. A GIPR antagonist conjugated to GLP-1 analogues promotes weight loss with improved metabolic parameters in preclinical and phase 1 settings. Nat Metab. 2024;6(2):290–303. doi: 10.1038/s42255-023-00966-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jastreboff A.M., Aronne L.J., Ahmad N.N., Wharton S., Connery L., Alves B., et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med. 2022;387(3):205–216. doi: 10.1056/NEJMoa2206038. [DOI] [PubMed] [Google Scholar]
- 20.Wadden T.A., Chao A.M., Machineni S., Kushner R., Ard J., Srivastava G., et al. Tirzepatide after intensive lifestyle intervention in adults with overweight or obesity: the SURMOUNT-3 phase 3 trial. Nat Med. 2023;29(11):2909–2918. doi: 10.1038/s41591-023-02597-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Killion E.A., Lu S.-C., Fort M., Yamada Y., Véniant M.M., Lloyd D.J. Glucose-dependent insulinotropic polypeptide receptor therapies for the treatment of obesity, do agonists = antagonists? Endocr Rev. 2020;41(1):1–21. doi: 10.1210/endrev/bnz002. [DOI] [PubMed] [Google Scholar]
- 22.Campbell J.E. Targeting the GIPR for obesity: to agonize or antagonize? Potential mechanisms. Mol Metabol. 2021;46 doi: 10.1016/j.molmet.2020.101139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holst J.J., Rosenkilde M.M. GIP as a therapeutic target in diabetes and obesity: insight from incretin Co-agonists. J Clin Endocrinol Metabol. 2020;105(8):e2710–e2716. doi: 10.1210/clinem/dgaa327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bauer S.A., Arndt T.P., Leslie K.E., Pearl D.L., Turner P.V. Obesity in rhesus and cynomolgus macaques: a comparative review of the condition and its implications for research. Comp Med. 2011;61(6):514–526. [PMC free article] [PubMed] [Google Scholar]
- 25.Graham F.L., van der Eb A.J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- 26.Kissow H., Hartmann B., Holst J.J., Viby N.-E., Hansen L.S., Rosenkilde M.M., et al. Glucagon-like peptide-1 (GLP-1) receptor agonism or DPP-4 inhibition does not accelerate neoplasia in carcinogen treated mice. Regul Pept. 2012;179(1–3):91–100. doi: 10.1016/j.regpep.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 27.Donthamsetti P., Quejada J.R., Javitch J.A., Gurevich V.V., Lambert N.A. Using bioluminescence resonance energy transfer (BRET) to characterize agonist-induced arrestin recruitment to modified and unmodified G protein-coupled receptors. Curr Protoc Pharmacol. 2015;70(1):2–14.1-2.14.14. doi: 10.1002/0471141755.ph0214s70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hansen L.S., Sparre-Ulrich A.H., Christensen M., Knop F.K., Hartmann B., Holst J.J., et al. N-terminally and C-terminally truncated forms of glucose-dependent insulinotropic polypeptide are high-affinity competitive antagonists of the human GIP receptor. Br J Pharmacol. 2016;173(5):826–838. doi: 10.1111/bph.13384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lau J., Bloch P., Schäffer L., Pettersson I., Spetzler J., Kofoed J., et al. Discovery of the once-weekly glucagon-like peptide-1 (GLP-1) analogue semaglutide. J Med Chem. 2015;58(18):7370–7380. doi: 10.1021/acs.jmedchem.5b00726. [DOI] [PubMed] [Google Scholar]
- 30.Nørregaard P.K., Deryabina M.A., Tofteng Shelton P., Fog J.U., Daugaard J.R., Eriksson P., et al. A novel GIP analogue, ZP4165, enhances glucagon-like peptide-1-induced body weight loss and improves glycaemic control in rodents. Diabetes Obes Metabol. 2018;20(1):60–68. doi: 10.1111/dom.13034. [DOI] [PubMed] [Google Scholar]
- 31.Mroz P.A., Finan B., Gelfanov V., Yang B., Tschöp M.H., DiMarchi R.D., et al. Optimized GIP analogs promote body weight lowering in mice through GIPR agonism not antagonism. Mol Metabol. 2019;20(December 2018):51–62. doi: 10.1016/j.molmet.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miyawaki K., Yamada Y., Yano H., Niwa H., Ban N., Ihara Y., et al. Glucose intolerance caused by a defect in the entero-insular axis: a study in gastric inhibitory polypeptide receptor knockout mice. Proc Natl Acad Sci USA. 1999;96(26):14843–14847. doi: 10.1073/pnas.96.26.14843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim S.-J., Nian C., Karunakaran S., Clee S.M., Isales C.M., McIntosh C.H.S. GIP-overexpressing mice demonstrate reduced diet-induced obesity and steatosis, and improved glucose homeostasis. PLoS One. 2012;7(7) doi: 10.1371/journal.pone.0040156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gault V.A., Kerr B.D., Harriott P., Flatt P.R. Administration of an acylated GLP-1 and GIP preparation provides added beneficial glucose-lowering and insulinotropic actions over single incretins in mice with Type 2 diabetes and obesity. Clin Sci. 2011;121(3):107–117. doi: 10.1042/CS20110006. [DOI] [PubMed] [Google Scholar]
- 35.Finan B., Yang B., Ottaway N., Smiley D.L., Ma T., Clemmensen C., et al. A rationally designed monomeric peptide triagonist corrects obesity and diabetes in rodents. Nat Med. 2015;21(1):27–36. doi: 10.1038/nm.3761. [DOI] [PubMed] [Google Scholar]
- 36.Finan B., Ma T., Ottaway N., Müller T.D., Habegger K.M., Heppner K.M., et al. Unimolecular dual incretins maximize metabolic benefits in rodents, monkeys, and humans. Sci Transl Med. 2013;5(209) doi: 10.1126/scitranslmed.3007218. [DOI] [PubMed] [Google Scholar]
- 37.Aronne L.J., Sattar N., Horn D.B., Bays H.E., Wharton S., Lin W.-Y., et al. Continued treatment with tirzepatide for maintenance of weight reduction in adults with obesity. JAMA. 2024;331(1):38. doi: 10.1001/jama.2023.24945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davidson M.B. In adults with BMI ≥27 kg/m2 and type 2 diabetes, adding tirzepatide to a lifestyle intervention increased weight loss at 72 wk. Ann Intern Med. 2023;176(11) doi: 10.7326/J23-0089. [DOI] [PubMed] [Google Scholar]
- 39.Gabe M.B.N., Sparre-Ulrich A.H., Pedersen M.F., Gasbjerg L.S., Inoue A., Bräuner-Osborne H., et al. Human GIP(3-30)NH2 inhibits G protein-dependent as well as G protein-independent signaling and is selective for the GIP receptor with high-affinity binding to primate but not rodent GIP receptors. Biochem Pharmacol. 2018;150(January):97–107. doi: 10.1016/j.bcp.2018.01.040. [DOI] [PubMed] [Google Scholar]
- 40.Gasbjerg L.S., Rosenkilde M.M., Meier J.J., Holst J.J., Knop F.K. The importance of glucose-dependent insulinotropic polypeptide receptor activation for the effects of tirzepatide. Diabetes Obes Metabol. 2023;25(11):3079–3092. doi: 10.1111/dom.15216. [DOI] [PubMed] [Google Scholar]
- 41.Sparre-Ulrich A.H., Hansen L.S., Svendsen B., Christensen M., Knop F.K., Hartmann B., et al. Species-specific action of (Pro3)GIP – a full agonist at human GIP receptors, but a partial agonist and competitive antagonist at rat and mouse GIP receptors. Br J Pharmacol. 2016;173(1):27–38. doi: 10.1111/bph.13323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gasbjerg L., Rasmussen R., Dragan A., Lindquist P., Schiellerup S., Tordrup E., et al. Altered desensitization and internalization patterns of rodent versus human GIP receptors – a major drug discovery challenge. Authorea. 2023:1–6. doi: 10.22541/au.168837507.72959473/v1. [DOI] [PubMed] [Google Scholar]
- 43.Davies M., Færch L., Jeppesen O.K., Pakseresht A., Pedersen S.D., Perreault L., et al. Semaglutide 2·4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): a randomised, double-blind, double-dummy, placebo-controlled, phase 3 trial. Lancet. 2021;397(10278):971–984. doi: 10.1016/S0140-6736(21)00213-0. [DOI] [PubMed] [Google Scholar]
- 44.Bergmann N.C., Lund A., Gasbjerg L.S., Meessen E.C.E., Andersen M.M., Bergmann S., et al. Effects of combined GIP and GLP-1 infusion on energy intake, appetite and energy expenditure in overweight/obese individuals: a randomised, crossover study. Diabetologia. 2019;62(4):665–675. doi: 10.1007/s00125-018-4810-0. [DOI] [PubMed] [Google Scholar]
- 45.Bergmann N.C., Gasbjerg L.S., Heimbürger S.M., Krogh L.S.L., Dela F., Hartmann B., et al. No acute effects of exogenous glucose-dependent insulinotropic polypeptide on energy intake, appetite, or energy expenditure when added to treatment with a long-acting glucagon-like peptide 1 receptor agonist in men with type 2 diabetes. Diabetes Care. 2020;43(3):588–596. doi: 10.2337/dc19-0578. [DOI] [PubMed] [Google Scholar]
- 46.Willard F.S., Douros J.D., Gabe M.B.N., Showalter A.D., Wainscott D.B., Suter T.M., et al. Tirzepatide is an imbalanced and biased dual GIP and GLP-1 receptor agonist. JCI Insight. 2020;5(17):1–16. doi: 10.1172/jci.insight.140532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mohammad S., Patel R.T., Bruno J., Panhwar M.S., Wen J., McGraw T.E. A naturally occurring GIP receptor variant undergoes enhanced agonist-induced desensitization, which impairs GIP control of adipose insulin sensitivity. Mol Cell Biol. 2014;34(19):3618–3629. doi: 10.1128/MCB.00256-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Novo Nordisk . Company Announcement; 2023. Financial report for 1 january 2023 to 31 march 2023; pp. 1–29. May 2023. [Google Scholar]
- 49.Novikoff A., O'Brien S.L., Bernecker M., Grandl G., Kleinert M., Knerr P.J., et al. Spatiotemporal GLP-1 and GIP receptor signaling and trafficking/recycling dynamics induced by selected receptor mono- and dual-agonists. Mol Metabol. 2021;49(February) doi: 10.1016/j.molmet.2021.101181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lucey M., Pickford P., Bitsi S., Minnion J., Ungewiss J., Schoeneberg K., et al. Disconnect between signalling potency and in vivo efficacy of pharmacokinetically optimised biased glucagon-like peptide-1 receptor agonists. Mol Metabol. 2020;37(April) doi: 10.1016/j.molmet.2020.100991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hinds C.E., Peace E., Chen S., Davies I., El Eid L., Tomas A., et al. Abolishing β-arrestin recruitment is necessary for the full metabolic benefits of G protein-biased glucagon-like peptide-1 receptor agonists. Diabetes Obes Metabol. 2024;26(1):65–77. doi: 10.1111/dom.15288. [DOI] [PubMed] [Google Scholar]
- 52.Vadher K., Patel H., Mody R., Levine J.A., Hoog M., Cheng A.Y.Y., et al. Efficacy of tirzepatide 5, 10 and 15 mg versus semaglutide 2 mg in patients with type 2 diabetes: an adjusted indirect treatment comparison. Diabetes. Obes Metabol. 2022;24(9):1861–1868. doi: 10.1111/dom.14775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Le Foll D., Lechaux D., Rascle O., Cabagno G. Weight loss and quality of life after bariatric surgery: a 2-year longitudinal study. Surg Obes Relat Dis. 2020;16(1):56–64. doi: 10.1016/j.soard.2019.10.010. [DOI] [PubMed] [Google Scholar]
- 54.Inge T.H., Courcoulas A.P., Jenkins T.M., Michalsky M.P., Helmrath M.A., Brandt M.L., et al. Weight loss and health status 3 Years after bariatric surgery in adolescents. N Engl J Med. 2016;374(2):113–123. doi: 10.1056/NEJMoa1506699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rubino D., Abrahamsson N., Davies M., Hesse D., Greenway F.L., Jensen C., et al. Effect of continued weekly subcutaneous semaglutide vs placebo on weight loss maintenance in adults with overweight or obesity. JAMA. 2021;325(14):1414. doi: 10.1001/jama.2021.3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wilding J.P.H., Batterham R.L., Davies M., Van Gaal L.F., Kandler K., Konakli K., et al. Weight regain and cardiometabolic effects after withdrawal of semaglutide: the STEP 1 trial extension. Diabetes Obes Metabol. 2022;24(8):1553–1564. doi: 10.1111/dom.14725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Garvey W.T., Batterham R.L., Bhatta M., Buscemi S., Christensen L.N., Frias J.P., et al. Two-year effects of semaglutide in adults with overweight or obesity: the STEP 5 trial. Nat Med. 2022;28(10):2083–2091. doi: 10.1038/s41591-022-02026-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rubino D.M., Greenway F.L., Khalid U., O'Neil P.M., Rosenstock J., Sørrig R., et al. Effect of weekly subcutaneous semaglutide vs daily liraglutide on body weight in adults with overweight or obesity without diabetes. JAMA. 2022;327(2):138. doi: 10.1001/jama.2021.23619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weghuber D., Barrett T., Barrientos-Pérez M., Gies I., Hesse D., Jeppesen O.K., et al. Once-weekly semaglutide in adolescents with obesity. N Engl J Med. 2022;387(24):2245–2257. doi: 10.1056/NEJMoa2208601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kadowaki T., Isendahl J., Khalid U., Lee S.Y., Nishida T., Ogawa W., et al. Semaglutide once a week in adults with overweight or obesity, with or without type 2 diabetes in an east Asian population (STEP 6): a randomised, double-blind, double-dummy, placebo-controlled, phase 3a trial. Lancet Diabetes Endocrinol. 2022;10(3):193–206. doi: 10.1016/S2213-8587(22)00008-0. [DOI] [PubMed] [Google Scholar]
- 61.Wadden T.A., Bailey T.S., Billings L.K., Davies M., Frias J.P., Koroleva A., et al. Effect of subcutaneous semaglutide vs placebo as an adjunct to intensive behavioral therapy on body weight in adults with overweight or obesity. JAMA. 2021;325(14):1403. doi: 10.1001/jama.2021.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gasbjerg L.S., Bari E.J., Stensen S., Hoe B., Lanng A.R., Mathiesen D.S., et al. Dose-dependent efficacy of the glucose-dependent insulinotropic polypeptide (GIP) receptor antagonist GIP(3-30)NH2 on GIP actions in humans. Diabetes Obes Metabol. 2021;23(1):68–74. doi: 10.1111/dom.14186. [DOI] [PubMed] [Google Scholar]
- 63.Stensen S., Krogh L.L., Sparre-Ulrich A.H., Dela F., Hartmann B., Vilsbøll T., et al. Acute concomitant glucose-dependent insulinotropic polypeptide receptor antagonism during glucagon-like peptide 1 receptor agonism does not affect appetite, resting energy expenditure or food intake in patients with type 2 diabetes and overweight/obesity. Diabetes Obes Metabol. 2022;24(9):1882–1887. doi: 10.1111/dom.14736. [DOI] [PubMed] [Google Scholar]
- 64.Elvert R., Herling A.W., Bossart M., Weiss T., Zhang B., Wenski P., et al. Running on mixed fuel-dual agonistic approach of GLP-1 and GCG receptors leads to beneficial impact on body weight and blood glucose control: a comparative study between mice and non-human primates. Diabetes Obes Metabol. 2018;20(8):1836–1851. doi: 10.1111/dom.13212. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.