Abstract
The exploration of targeted therapy has proven to be a highly promising avenue in the realm of drug development research. The human body generates a substantial amount of free radicals during metabolic processes, and if not promptly eliminated, these free radicals can lead to oxidative stress, disrupting homeostasis and potentially contributing to chronic diseases and cancers. Before the development of contemporary medicine with synthetic pharmaceuticals and antioxidants, there was a long-standing practice of employing raw, natural ingredients to cure a variety of illnesses. This practice persisted even after the active antioxidant molecules were known. The ability of natural antioxidants to neutralise excess free radicals in the human body and so prevent and cure a wide range of illnesses. The term "natural antioxidant" refers to compounds derived from plants or other living organisms that have the ability to control the production of free radicals, scavenge them, stop free radical-mediated chain reactions, and prevent lipid peroxidation. These compounds have a strong potential to inhibit oxidative stress. Phytochemicals (antioxidants) derived from plants, such as polyphenols, carotenoids, vitamins, and others, are central to the discussion of natural antioxidants. Not only may these chemicals increase endogenous antioxidant defenses, affect communication cascades, and control gene expression, but they have also shown strong free radical scavenging properties. This study comprehensively summarizes the primary classes of natural antioxidants found in different plant and animal source that contribute to the prevention and treatment of diseases. Additionally, it outlines the research progress and outlines future development prospects. These discoveries not only establish a theoretical groundwork for pharmacological development but also present inventive ideas for addressing challenges in medical treatment.
Keywords: Targeted therapy, Natural products, Antioxidants, Oxidative stress, Therapeutics
Introduction to targeted antioxidant natural products
Antioxidants are compounds that impede or suppress free radical reactions, thereby retarding or preventing cellular damage. While the specifics of antioxidant defenses vary among different species, the universal presence of antioxidant defenses is noteworthy. These defenses manifest in both enzymatic and non-enzymatic forms, existing in both the intracellular and extracellular environments. Natural antioxidants, derived from plants and other living organisms, possess significant potential to counteract oxidative stress. They achieve this by regulating the formation of free radicals and scavenging them. Additionally, they disrupt the chain reactions initiated by free radicals and prevent the lipid peroxidation [1].Consequently, these natural antioxidants have the ability to restore cellular homeostasis, effectively balancing irregular oxidative stress. As a result, they can mitigate the harmful effects of various oxidative stress-induced pathological conditions [2].The historical use of crude natural plant products to address diverse diseases has been a longstanding practice for thousands of years. This tradition predates the advent of modern medicine and synthetic drugs, regardless of whether the active antioxidant molecules were understood [3].
The World Health Organization (WHO) has reported that 80% of the global population relies on traditional medicine for their primary healthcare needs [4]. A significant portion of these treatments involves the use of herbal extracts and their active components. Numerous bioactive extracts from medicinal plants, along with their identified or isolated active constituents, exhibit diverse pharmacological properties against both acute and chronic diseases and disorders [5–7].
Presently, the impact of oxidative stress and its related factors has emerged as a crucial concern for human health. In situations of heightened stress, the production of reactive oxygen species (ROS), such as hydroxyl radicals, superoxide anion radicals, and hydrogen peroxide, intensifies. The body's endogenous enzymatic and nonenzymatic antioxidant substances may struggle to manage the excessive ROS load, resulting in imbalances in the process, cell damage, and subsequent health issues. Furthermore, the absence of antioxidant compounds further exacerbates these challenges [8, 9].Oxidative stress conditions induce changes in the cellular constituents of our body, contributing to various disease states. Effectively countering oxidative stress involves strengthening cellular defenses through the application of antioxidants [10, 11]. Certain compounds serve as in vivo antioxidants, enhancing the body's natural antioxidant defenses by upregulating the expression of genes responsible for enzymes like superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSHPx), thereby increasing the levels of endogenous antioxidant [12, 13].
So far, research has focused on natural products with high antioxidant capacity, particularly those that people often (or could) eat. Oxygen Radical Absorbance Capacity (ORAC), 2,2-diphenyl-1-picrylhydrazyl (DPPH), scavenging method, ferric reducing capacity, and increasingly, the use of nanoprobes to evaluate antioxidants' metal-reducing capacity, are some of the in vitro chemical-based assays that have been developed for this purpose [14].
These tests use different approaches and provide different results on how reactive oxygen and nitrogen species (ROS/RNS) interact with the material. However, one in vitro chemical approach is not enough to thoroughly investigate the possible health-protective and antioxidant properties of natural compounds. It is essential to assess and compare antioxidant capabilities using various approaches due to the complexity of their in vivo processes. The chemical tests also don't take into account bioavailability and lipophilicity, two important factors in biological settings. Hence, it's possible that the derived antioxidant capacity indices don't accurately represent the antioxidant effects that a specific sample would have in a living organism. It is also necessary to assess whether or not natural compounds may stimulate a cellular antioxidant response. Being an effective radical scavenger and reducing agent is just part of what makes an antioxidant effective. However, it is a chemical that may alleviate oxidative stress by activating transcription factors, which in turn increase the development of antioxidant enzymes. Some degenerative illnesses, including as cancer, heart disease, Alzheimer's disease, neurological disease, and inflammatory disorders, may begin earlier in life in those whose diets are lacking in antioxidant-rich foods. [15–21]. Addressing human health concerns can be achieved by incorporating antioxidant compounds from natural plant sources into daily dietary practices. These natural sources of antioxidants have the potential to serve as preventive medicine. Recent research indicates a correlation between the consumption of antioxidant-rich foods in the diet and a lower prevalence of human illnesses [22].
Therapeutic applications of targeted antioxidant natural products
Cardiovascular diseases and antioxidant natural products
Resveratrol
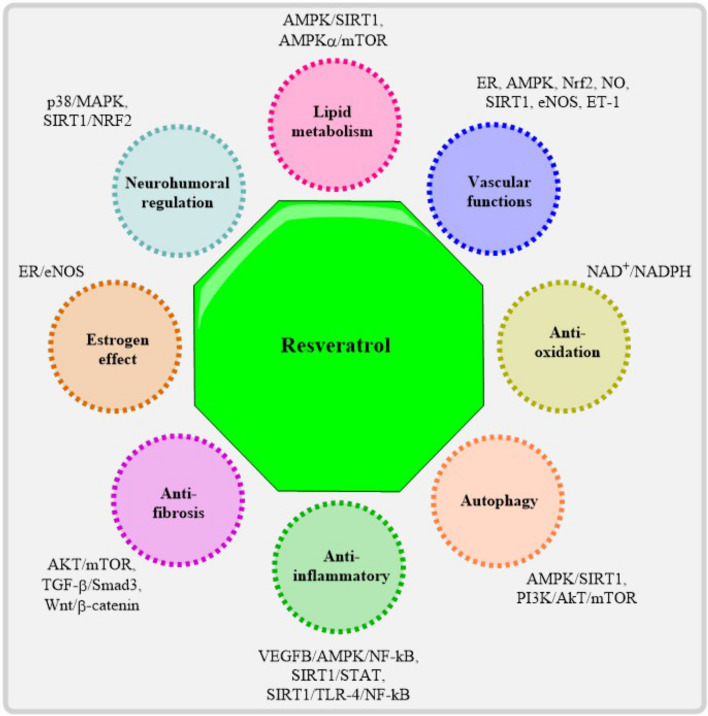
Research on the possible function of Resveratrol, a polyphenolic molecule present in grapes and red wine, in Cardio Vascular Diseases (CVDs) has been conducted [23]. Vascular health depends on an increase in the availability of nitric oxide, and resveratrol seems to have antioxidant and anti-inflammatory effects, as well as the potential to upregulate endothelial NO synthase [24]. Resveratrol targets molecular pathways including SIRT-1, AMPK, Nrf2, and NFκB, which may help disorders such as atherosclerosis, hypertension, stroke, myocardial infarction, and heart failure, according to studies (as shown in Fig. 1) [25–29]. To validate resveratrol's effects in CVDs, more human clinical trials are required, despite encouraging results from preclinical investigations. Researchers have also looked at how resveratrol affects left ventricular function and heart failure (HF), finding that it may help diabetic individuals' hearts work better [30]. The chemical promotes autophagy and interacts with several molecular targets associated with cardiovascular disorders; this may help prevent pathological remodeling of the heart by lowering inflammation and oxidative stress. Supplemental resveratrol may reduce blood pressure by stimulating the body to produce more nitric oxide, which in turn relaxes blood vessels, according to research [31]. The antioxidant and nitric oxide-enhancing effects of resveratrol are mostly responsible for the lowering of blood pressure. Clinical research has shown that resveratrol, when taken in addition to traditional antihypertensive medication, effectively lowers blood pressure levels [32]. Furthermore, resveratrol may have preventive effects on the liver since it has been linked to lower plasma concentrations of certain enzymes involved in liver damage [33]. Keep in mind that resveratrol's effect on blood pressure could differ from person to person depending on things like dose and genetics. Research suggests that there may be a positive impact on blood pressure when using greater doses of resveratrol, particularly in diabetic individuals, when taken daily in quantities of 300 mg or more [34]. Overall, resveratrol's antioxidant and vasodilatory actions show promise in lowering blood pressure, but further study is required to determine an appropriate dose and determine how it affects various groups. Results from resveratrol-related clinical studies have shed light on the supplement's possible advantages for the treatment of heart conditions. Patients undergoing cardiovascular surgery were the subjects of the "Short Interval Resveratrol Trial in Cardiovascular Surgery (SIRT-CVS)" research (NCT03762096), which sought to determine the efficacy and safety of short-interval resveratrol therapy [35]. The potential benefits of resveratrol on cardiovascular health and arterial function in the elderly were the subject of NCT02909699 [36]. These studies help fill gaps in our knowledge of how resveratrol affects heart health. Resveratrol has been shown in both animal studies and human trials to have beneficial effects on cardiac function, including improving diastolic and systolic performance, decreasing remodeling of the atrium and left ventricle, and influencing a number of signal transduction pathways associated with cardiovascular disorders [37].
Fig. 1.
Therapeutic cardio protective actions of Resveratrol
Vitamin C
An abundance of naturally occurring foods include vitamin C. Vitamin C is abundant in guavas, and the lemon. Cruciferous vegetables, such as broccoli, provide an additional source of vitamin C, alongside citrus fruits. Vitamin C's potential role in preventing and treating CVDs has piqued researchers' interests. Although scurvy due to a traditional vitamin C deficit is rather uncommon, studies have linked plasma vitamin C concentrations—even those that are considered normal—to various risks of cardiovascular disease. In addition to the recommended daily allowance, some research suggests that larger dosages of vitamin C may have positive effects on cardiovascular health. One of the main factors in the development of CVD is the oxidation of LDL-protein, which vitamin C's antioxidant qualities play a critical role in blocking [38–41]. Nevertheless, there is inconsistent evidence from both cohort studies and randomized trials about the association between vitamin C consumption and the risk of cardiovascular disease, therefore the existing research does not provide strong support for the widespread use of vitamin C supplements to lower CVD risk. Curiously, there have been studies that have shown an increased risk of cardiovascular disease while using vitamin C supplements. This begs the issues of how supplementation differs from dietary vitamin C and what amounts are best to reduce the risk of heart disease.
The potential of vitamin C as a therapeutic agent for heart conditions has been investigated in clinical studies. In one trial, NCT02762331, the researchers looked at how patients undergoing heart surgery responded to low levels of inflammation after taking large doses of ascorbic acid (vitamin C) [42]. The purpose of another study, NCT03123107, was to learn more about the effects of ascorbic acid on people having coronary artery bypass graft surgery so that we might improve our understanding of vitamin C's role in cardiovascular health [43]. Another research that looked at reducing organ failure after a cardiac arrest by using high-dose intravenous vitamin C was published [44]. Organ failure, neurological sequelae, mortality, and other parameters were examined in this double-blind, multi-center experiment that examined the effects of additional or very high-dose intravenous vitamin C in patients with cardiac arrest and restoration of spontaneous circulation. The involvement of vitamin C in reducing inflammation, advancing cardiovascular research, and post-cardiac arrest care is highlighted by these clinical studies, which provide vital insights into its prospective advantages and impacts in cardiac therapy.
Vitamin E
There is plenty of vitamin E-rich foods that are easy to integrate into a balanced diet. Wheat germ oil is abundant and one of the best sources. In addition to wheat germ oil, this vital component may be found naturally in nuts and seeds. Half an avocado contains as much as 20% of the recommended daily allowance of vitamin E, making them a multipurpose fruit that is also rich in this vitamin. Red bell peppers, salmon, trout, pine nuts, peanuts, peanut butter, and peanuts are among the many foods that are high in vitamin E. Concerning CVDs, vitamin E has garnered attention, especially for its potential effects on heart health. There is some evidence that vitamin E may help reduce the risk of cardiovascular diseases, including atherosclerosis and coronary heart disease. Vitamin E-rich foods may reduce the incidence of coronary heart disease in middle-aged and older people, according to studies. To avoid cardiovascular disease, the American Heart Association recommends eating meals rich in antioxidant vitamins and other nutrients rather than taking vitamin E pills. Vitamin E may raise the risk of overall mortality, heart failure, and hemorrhagic stroke, according to some research, and clinical trials have failed to reliably show that it helps prevent CVDs. Even while vitamin E has shown cardio protective effects in some patient subgroups exposed to high levels of oxidative stress, it is unclear if indiscriminate use of the vitamin will have any positive impact on cardiovascular health in populations that are not carefully screened [45–47]. Important insights into vitamin E's benefits have been gleaned from clinical studies that have focused on heart disorders. Supplemental vitamin E did not reduce the incidence of coronary heart disease in high-risk individuals, according to one research published [48]. Another group of researchers also highlighted a research that found no substantial impact of vitamin E supplementation on the incidence of cardiovascular disease or cancer in randomized trials [49]. Furthermore, studies documented have linked vitamin E-rich foods to a reduced risk of coronary heart disease in middle-aged and older adults, highlighting the significance of these foods as dietary sources of vitamin E [50].
Randomized controlled trials and observational studies have investigated beta-carotene's effects on heart disorders. Some observational studies have uncovered evidence of a preventive effect of beta-carotene against coronary heart disease. On the other hand, outcomes from RCTs have been inconsistent. Supplementing with beta-carotene did not reduce the risk of cardiovascular disease and instead raised the risk of death from cardiovascular causes, according to a meta-analysis and systematic review of randomized controlled trials [51–53]. Results from subgroup studies revealed an increased risk of cardiovascular events associated with low-dose, individual beta-carotene therapy.
Polyphenols
Beyond their antioxidant powers, polyphenols have a multitude of positive impacts on heart health [54]. Important in reducing the chronic inflammation linked to CVD, they also have anti-inflammatory characteristics. Polyphenols aid in cardiovascular disease prevention by lowering inflammatory processes, an important factor in atherosclerosis and other inflammatory disorders [55–57]. The endothelium is responsible for regulating blood pressure and clotting; polyphenols impact heart health via increasing endothelial function. They lessen the likelihood of cardiovascular illness by increasing the generation of nitric oxide (NO), a chemical necessary for vasodilation (the widening of blood vessels). This process increases blood flow and lowers blood pressure [58–61]. A diet abundant in plant-based foods, such as fruits, vegetables, whole grains, and polyphenols, is important for heart health for many reasons. These foods are an important part of a heart-healthy lifestyle because of the polyphenols they contain, which may help prevent and manage cardiovascular disease. However, eating foods that are high in polyphenols is good, but it shouldn't be the only thing people do to keep their hearts healthy [62]. Polyphenols have the potential to influence gene expression, enzyme activity, and intracellular signaling pathways that are associated with cardiovascular health, according to research. Polyphenols prevent cardiovascular disease by controlling these pathways, which in turn protects the heart from inflammation and oxidative stress. Better lipid profiles, mitochondrial function, and cellular metabolism are all hallmarks of a heart-healthy lifestyle, and polyphenols have been associated to these benefits.
Research has shown that polyphenols have several beneficial benefits on the cardiovascular system, such as reducing inflammation, protecting against blood clots, and lowering blood pressure [63]. Polyphenols are useful in the treatment and prevention of cardiovascular disorders due to their characteristics. By using the antioxidant potential of polyphenol-rich foods like tea, coffee, chocolate, olive oil, and red wine, a heart-healthy diet may be achieved.
Flavanoids
By acting as an antioxidant, flavonoids greatly mitigate oxidative stress in the cardiovascular system. Because of their capacity to fight oxidative stress and inflammation in the cardiovascular system, these chemicals—which are prevalent in many plant-based foods—have been associated with cardioprotective potential. It has been shown that flavonoids, such as anthocyanins, may slow the development of arteriosclerosis by reducing oxidative stress in heart cells [64]. Research has shown that flavonoids have a preventive role in cardiovascular health due to their ability to control oxidative stress-related processes, affect intracellular signaling pathways, and counter inflammation. Important components in preserving cardiovascular health and lowering the risk of cardiovascular illnesses include flavonoids' association with enhanced flow-mediated dilation, decreased blood pressure, and prevention of LDL oxidation [65–67]. To promote heart health and prevent cardiovascular disorders, flavonoids are useful components due to their antioxidant characteristics, which include scavenging free radicals and inhibiting enzymes involved in oxidative stress [68].
Cyanidin 3-O-glucoside and delphidin 3-O-glucoside are two examples of the anthocyanin flavonoids that have been shown to slow the development of atherosclerosis by reducing oxidative stress in heart cells [69, 70]. According to research, flavonoids have many anti-oxidant and anti-free radical functions, including scavenging free radicals, inhibiting oxidative stress enzymes, and reducing ROS produced during mitochondrial respiration [65, 66, 71]. Flavonoids help maintain a stable and less reactive radical environment in the cardiovascular system by efficiently inactivating free radicals via their highly reactive hydroxyl groups [64]. Flavonoids are known to promote cardiovascular health by increasing antioxidant activities and decreasing LDL oxidation and platelet aggregation [65]. Flavonoids have a crucial role in preserving heart health and warding off cardiovascular illnesses because, when supplemented with other antioxidants, they form a strong defense mechanism against oxidative stress in the cardiovascular system.
Scientific studies support the idea that flavonoid consumption in the diet might help reduce the risk of cardiovascular illnesses and other systemic health problems [72–76]. It is worth noting that flavonoids may have cardioprotective properties, since epidemiologic studies have linked flavonoid consumption to a reduced risk of cardiovascular illnesses [77]. The research emphasized the significance of flavonoids in promoting heart health and lowering risk of cardiovascular disease. Another subgroup of flavonoids, flavonols have recently attracted attention for their potential effects on cardiovascular risk [78]. Research has shown that flavonols have antioxidant, anti-atherogenic, antithrombotic, and vasodilatory effects, which in turn serve to protect against cardiovascular disorders [78–82].
Omega fatty acids
Eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are omega-3 fatty acids that have been shown in clinical trials and studies to considerably lower the risk of sudden death due to cardiac arrhythmias and all-cause mortality in individuals with established coronary heart disease [83]. These fatty acids have several uses in the management of cardiovascular diseases, including the treatment of hyperlipidemia and hypertension. Omega-3 fatty acid-rich fish is recommended for heart health by the American Heart Association, regardless of a person's history of coronary heart disease [84]. Research has shown that omega-3 fatty acids may help lower cardiovascular risk factors including those that contribute to blood clotting and excessive levels of low-density lipoprotein cholesterol [85, 86]. In addition, omega-3 fatty acids have been linked to better lipid profiles, lower blood pressure, and protection against cardiovascular disorders such as heart attacks and strokes, particularly in those who already have them [84, 87].
Lycopene
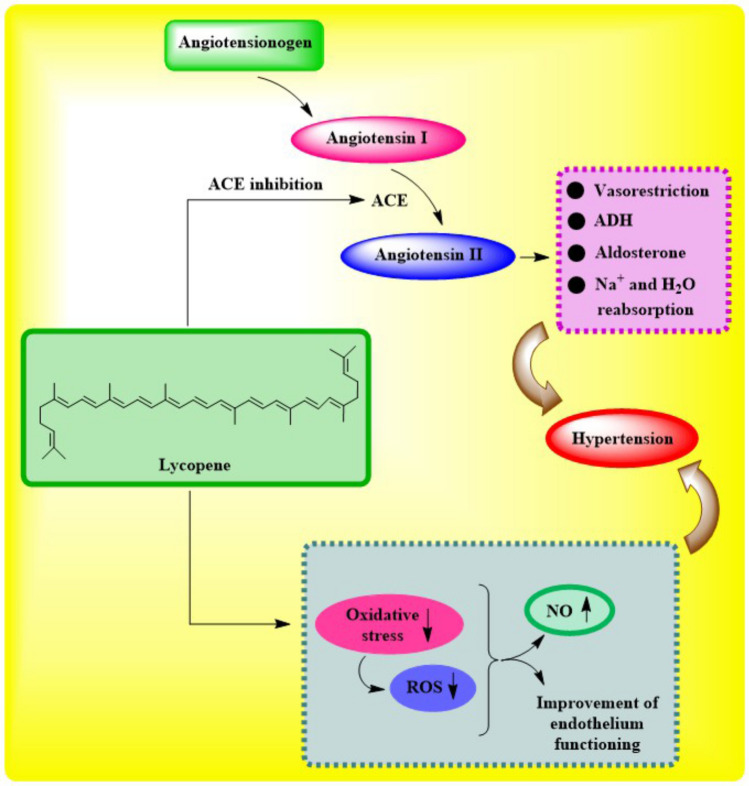
Lycopene, a carotenoid included in red fruits and vegetables (especially tomatoes), is important for heart health because it reduces the effects of oxidative stress. In oxidative stress, the body's capacity to neutralize ROS or restore damaged cells is inadequate relative to the amount of ROS produce. The positive benefits of lycopene during the earliest stages of atherosclerosis were shown in rabbits, who exhibited improvements in the LDL/HDL ratio, HDL functioning, and decreased cholesterol buildup in the aorta, even though lycopene cannot raise HDL cholesterol (as shown in Fig. 2) [88]. Research has demonstrated that lycopene has various cardio-protective effects, including reducing oxidative stress caused by ROS production [89], preventing oxidative damage caused by LDL [90], enhancing endothelial functions [88], and promoting ventricular remodeling by preventing apoptosis [91]. By inhibiting endothelial injuries, preventing cholesterol by prohibiting 3-hydroxy-3-methylglutaryl coenzyme, and reducing prophylaxis progression, thrombotic progression, and atherosclerosis complications, lycopene's potential antioxidant properties were able to accomplish this. The enzyme a reductase influences molecular pathways that regulate cell division and apoptosis; it also promotes efflux of cholesterol, inhibits the pro-inflammatory activity of T lymphocytes and macrophages, and inhibits the production of foam cells and smooth cells [92].
Fig. 2.
Cardio-protective action of Lycopene
By influencing HDL function towards an anti-atherosclerotic phenotype—characterized by decreased serum amyloid A levels and beneficial alterations in HDL remodeling enzymes (cholesterol ester transfer protein and lecithin cholesterol acyltransferase)—lycopene reduces the production of dysfunctional HDL [93]. Only those with high blood triglyceride levels to begin with saw a hypo-triglyceridemic response from tomato juice [94].Results from clinical research looking into lycopene's benefits on heart health are encouraging. A randomized controlled experiment looked at how taking lycopene supplements orally affected vascular function in both healthy volunteers and those with cardiovascular disease. The results showed that it may help improve endothelial function and decrease arterial stiffness [95, 96]. Results from these studies show that lycopene may be useful in the management of a variety of cardiovascular diseases due to its antioxidant, anti-inflammatory, antihypertensive, antiplatelet, anti-apoptotic, and protective endothelial properties. Further well-designed studies are necessary to clarify the beneficial benefits of lycopene on cardiovascular health and to investigate its potential as a supplement or dietary component for the prevention and treatment of cardiovascular illnesses, especially since there have been reports of inconsistent findings [97]. Exploring the synergistic effects of lycopene with other dietary components and creating functional meals to help prevent and cure cardiovascular illnesses are potential areas for future study [98, 99].
Neurological diseases and antioxidant natural products
Neurodegenerative disorders have complex etiologies and progressions due to several pro-neurodegenerative variables. These diseases are characterized by neuronal cell death, inflammation, protein and DNA damage, mitochondrial malfunction, and amyotrophic lateral sclerosis, Parkinson's disease, Alzheimer's disease, and Huntington's disease. Important risk factors for neurodegeneration include age, high blood pressure, type II diabetes, obesity, insulin resistance, depression, and brain trauma, in addition to this primary driver [100, 101]. There are a number of lifestyle variables that contribute to the development, progression, and overall impact of these crippling diseases. These include eating a lot of sugar or fat, as well as being addicted to alcohol or tobacco. The involvement of neuroinflammation and excessive inflammatory responses, which can worsen neurodegenerative processes, is fundamental to all of this. There is hope that the terrible impacts of neurodegenerative disorders can be slowed or prevented by addressing these several pro-neurodegenerative variables with a mix of antioxidant treatments, lifestyle changes, and anti-inflammatory measures [5, 7, 102].
Lycopene
A variety of neurological problems have been studied for their possible neuroprotective benefits, and lycopene—a carotenoid found in red fruits and vegetables—has been one of them. Because it is lipophilic, it may pass the blood–brain barrier and enter the CNS directly, where it can have an impact [100]. One of the main reasons why lycopene can protect neurons is because of its powerful antioxidant capabilities. These properties help to reduce inflammation and oxidative stress, which are key players in the development of neurodegenerative diseases like Alzheimer's, Parkinson's, and Huntington's. Neurodegenerative diseases, epilepsy, cognitive loss with age, subarachnoid hemorrhage, spinal cord injury, neuropathy, and other neurological illnesses may all benefit from lycopene's therapeutic properties [101–103]. Evidence suggests it may reverse the pathophysiological and behavioral alterations brought on by these diseases. There are a number of neuropathological effects associated with a high-lipid diet, and lycopene may help reduce their effects [104].
Curcumin
Researchers have looked at the neuroprotective benefits of curcumin, a chemical extracted from turmeric, in a number of neurological diseases. It has a number of documented effects, including regulating neurotransmitter levels, improving neurogenesis in the adult hippocampus [105, 106], and blocking the development of reactive astrocytes [107, 108], which may protect cells from death. Animal studies on diabetic neuropathy, dyskinesia, and serious depression have shown that curcumin provides protection [109–113]. Its anti-inflammatory and antioxidant capabilities are believed to be the primary mechanisms by which it protects neurons. Seizures, migraines, ALS, PD, MS, and neuroinflammation are some of the other conditions that curcumin has shown good results in animal models [114–117]. Its low oral bioavailability, however, restricts its application in therapeutic contexts [118].
Research on the possible therapeutic benefits of curcumin, a turmeric component, in Alzheimer's disease (AD) has been ongoing. Research in aged Tg2576 mice with advanced amyloid buildup has shown that it hinders the development of Aβ40 and Aβ42 oligomers, lowers amyloid levels, and lessens plaque load [119]. In rats that were treated with Aβ, clinical research found that taking curcumin supplements greatly decreased the loss of synaptophysin [120].
Molecularly, curcumin's ability to affect several biochemical pathways and molecular processes is the bedrock of its therapeutic benefits on AD. It disrupts the process of amyloid-beta (Aβ) peptide aggregation in the AD brain by weakening Aβ fibrils, which play a crucial role in the development of amyloid plaques [121, 122]. The aggregation of Aβ is essential to be disrupted since these plaques are directly associated with the neurodegeneration seen in AD. By influencing the AMP-activated protein kinase (AMPK) pathway, curcumin further mediates its neuroprotective properties [123–125]. By inhibiting AMPK pathway activation, curcumin improves neuronal lifespan by decreasing neuronal damage and apoptosis. Superoxide dismutase (SOD) and other antioxidant enzymes are made more active, and inflammatory cytokines like tumor necrosis factor-alpha (TNF-α) and interleukins (IL-6 and IL-1β) are modulated as part of this process [125].
In addition, curcumin may change gene expression that contributes to the advancement of AD without modifying the DNA sequence, due to its epigenetic actions [126, 127]. Genes linked in neuroprotection and cognitive function are upregulated, whereas genes implicated in inflammation are downregulated. Curcumin is known to modulate the activity of transcription factors and signaling molecules that are implicated in neuroinflammation and neurodegeneration [128].
Polyphenols
In Alzheimer's disease patients, polyphenols show neuroprotective benefits via regulating pathways for Aβ synthesis, preventing Aβ aggregation, and decreasing macrophage Aβ absorption [129]. Inhibiting the production of α-synuclein misfolded aggregates, lowering mitochondrial dysfunction, altering cell survival and cell cycle genes, and modifying numerous signaling pathways implicated in PD are some of the neuroprotective characteristics of polyphenols, according to research [129, 130]. While adding to antioxidant activity, these chemicals may activate pathways such as phosphoinositide 3-kinase (PI3K)/Akt, mitogen-activated protein kinase (MAPK), and protein kinase C (PKC) [131–134]
Furthermore, in vitro models of PD-related disorders have shown that a particular combination of micronutrients and polyphenols may protect neurons from damage [135]. All things considered, polyphenols show potential as neuroprotective drugs in PD due to their ability to target many pathways that are involved in the illness.
Flavanoids
According to research, flavonoids may improve blood flow to the brain and prevent the accumulation of beta-amyloid plaque, two symptoms that are characteristic of AD [136]. The subclasses of flavonoids known to have possible positive effects on brain health and cognitive function include anthocyanins, flavanols, flavonols, and flavanones. The absence of precise mechanistic evidence, together with uncertainty about flavonoids' metabolism and bioavailability, has slowed their development as therapeutic approaches for AD. However there is evidence that diets high in flavonoids might affect cognitive decline and the disease [137].
Research has linked a reduced risk of PD to increased consumption of foods strong in anthocyanins, including berries [138]. This data points to the possibility that flavonoids, such as anthocyanins, lower the incidence of PD, especially in males. To fully grasp flavonoids' potential as therapeutic agents for PD, further study is required, since there are currently only a small number of clinical studies showing that they are effective in treating the disease.
Antioxidants in diabetes and obesity
Flavanoids
An important advantage of flavonoids is their capacity to decrease inflammation and oxidative stress, two elements that play a significant role in the onset and advancement of diabetes and obesity [139]. Flavonoids aid in maintaining the function of pancreatic β-cells, which are crucial for insulin generation, by removing free radicals and blocking pathways that promote inflammation. The anti-inflammatory and antioxidant properties of flavonoids make cells more sensitive to insulin, which in turn allows for better glucose absorption by cells and aids in the regulation of blood glucose levels [140].
In addition, enzymes like α-amylase and α-glucosidase, which are responsible for the breakdown of carbohydrates, may be inhibited by flavonoids, which allows them to directly impact glucose metabolism [141–143]. People with diabetes are able to avoid dangerously high blood sugar levels because to this enzymatic inhibition, which slows the release of glucose into the circulation after meals.
Flavonoids are involved in weight control and lipid metabolism in addition to their effects on glucose metabolism. They reduce levels of bad lipids like triglycerides and LDL cholesterol by controlling the activity of enzymes involved in lipid production and breakdown [144, 145]. In addition, certain flavonoids promote the breakdown of stored fats and inhibit the development of preadipocytes into adipocytes, therefore discouraging fat formation [146]. This helps fight obesity and lowers the risk of cardiovascular problems associated with obesity.
One possible way that flavonoids help with weight control is by regulating hunger and energy expenditure. These chemicals work by influencing the release of hormones that control hunger, which in turn makes people feel full on less food. Further assisting in the decrease of body fat, certain flavonoids have been shown to enhance energy expenditure by promoting thermogenesis in brown adipose tissue and improving mitochondrial activity [147, 148].
Insulin resistance, poor glucose metabolism, and excessive fat storage characterize the metabolic diseases diabetes and obesity, which are interrelated and may be effectively managed and prevented with the help of vitamins [149]. These vital nutrients play an important role in metabolic health; while the body only needs a little quantity of them for many physiological processes, they impact the pathophysiology of diabetes and obesity via several pathways.
Vitamins
Vitamins D, E, and the B-vitamins have shown very encouraging results when used to the setting of diabetes. For example, vitamin D affects insulin secretion and sensitivity in addition to its well-known functions in calcium metabolism and bone health [150, 151]. Researchers have shown that people with enough vitamin D in their bodies are less likely to develop type 2 diabetes [152]. This may be because vitamin D improves insulin sensitivity and pancreatic β-cell activity. Antioxidant vitamin E shields cells from oxidative damage, which is common in diabetics because of their high blood sugar levels [153]. Vitamin E aids in the prevention or delay of diabetic consequences such cardiovascular illnesses and neuropathy by lowering oxidative stress. Since elevated homocysteine levels are linked to an increased risk of cardiovascular disease in diabetic individuals, they could potentially contribute to lowering the risk of diabetes by altering these levels.
Vitamins have an impact on energy metabolism, hunger, fat synthesis/storage, and overall body weight/fat distribution in relation to obesity. For instance, low vitamin D levels are related with more body fat and an increased risk of becoming obese; this vitamin is also involved with the management of body weight [154]. Vitamin D may provide an explanation for this correlation if it has a function in the metabolism of fat cells and may affect the expression of genes related to energy expenditure and fat accumulation. In addition, vitamin E and C's antioxidant qualities might lessen the oxidative stress that comes with obesity, which aids in lowering inflammation—a major contributor to the onset of obesity-related problems [155–157].
Polyphenols
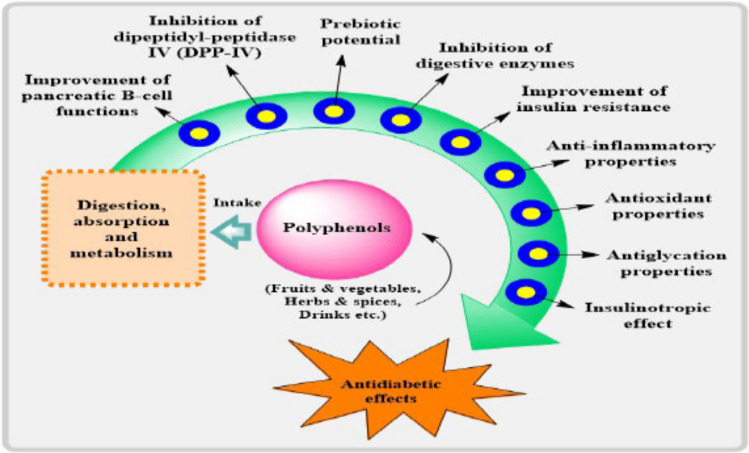
Polyphenols have the potential to significantly impact glucose metabolism and insulin sensitivity, two key components in the management of diabetes as shown in Fig. 3 [158]. One of the main causes of insulin resistance, malfunction of β-cells, and the advancement of diabetes is oxidative stress. Polyphenols contribute in the maintenance of normal blood glucose levels by protecting pancreatic cells and improving their insulin production by neutralizing ROS [159–161]. Additionally, many polyphenols have the ability to block enzymes such as α-glucosidase and α-amylase, which play a role in the digestion of carbohydrates [162, 163]. This results in a reduced rate of glucose release into the circulation and the prevention of increases in glucose levels after a meal.
Fig. 3.
Antidiabetic effects of dietary polyphenols
In addition to their antioxidant and anti-inflammatory effects, polyphenols help alleviate the chronic inflammation that accompanies obesity and insulin resistance [164–166]. Evidence suggests that polyphenols have many modes of action that affect obesity-related weight control and body composition. As a result of their ability to control lipid metabolism, they may lessen the buildup of fat in the body by increasing lipolysis and decreasing lipogenesis [167]. A negative energy balance and weight reduction may be aided by some polyphenols, which have the ability to enhance energy expenditure and fat burning. Their impact on the gut microbiota has also recently come to light, as changes in the make-up and function of gut bacteria have been associated with changes in metabolic health and obesity. In a nutshell, polyphenols may aid weight loss by encouraging the development of good bacteria in the digestive tract [168–170].
Curcumin
Curcumin is involved with diabetes in many different ways. Oxidative stress, caused by an overabundance of ROS, is prevalent in diabetes patients and may be alleviated by its potent antioxidant capabilities. Curcumin may aid in protecting pancreatic cells and enhancing insulin sensitivity by reducing ROS and increasing the body's natural antioxidant defenses [171, 172]. Further assisting in the increase of insulin sensitivity and the avoidance of diabetic complications, curcumin's anti-inflammatory properties are crucial in treating the chronic inflammation associated with diabetes.
It has been shown that curcumin modulates many pathways associated with glucose metabolism, in addition to its antioxidant and anti-inflammatory functions. It has the potential to improve lipid metabolism, decrease gluconeogenesis in the liver, and increase glucose absorption in peripheral organs. Glycemic control and the likelihood of hyperglycemia are both improved by these measures [173–175]. Furthermore, research has shown that curcumin may hinder the action of digestive enzymes like α-glucosidase and α-amylase. This results in a reduced rate of glucose release into the circulation after carbohydrate-rich meals, and thus, it can avoid rises in blood glucose levels after eating.
As an effective drug in the prevention and management of obesity, Curcumin regulates lipid metabolism by boosting lipolysis and decreasing adipogenesis [176–178]. Researchers have shown that curcumin may prevent fat storage by reducing the expression of many adipogenic genes and transcription factors. The low-grade inflammation in adipose tissue, a common symptom of obesity and metabolic syndrome, may be effectively addressed by its anti-inflammatory characteristics. In addition to its anti-obesity actions, curcumin may aid in the fight against weight gain and promotion of weight reduction by increasing thermogenesis and energy expenditure.
Curcumin has been demonstrated to modify important insulin signaling pathways at the molecular level. This includes increasing the expression of genes involved in glucose and lipid metabolism, such GLUT4, GLUT2, GLUT3, and PPAR-γ, and improving insulin-stimulated Akt phosphorylation [182, 183]. Curcumin downregulates genes related to de novo lipogenesis and enzymes involved in gluconeogenesis in the liver, which improves lipid balance and reduces glucose synthesis. Curcumin has the interesting capacity to change the makeup of the gut microbiota, which may explain why it improves insulin sensitivity [184]. Improving insulin sensitivity is one of the many benefits of curcumin, which works by influencing the microbiome of the intestines to improve barrier function and decrease inflammation.
Garlic
Garlic has several effects in the field of diabetes care. The fact that it has antioxidant qualities is important since oxidative stress is a key factor in the development of diabetes, which leads to insulin resistance and malfunction of pancreatic β-cells [179, 180]. Garlic may improve insulin sensitivity and secretion by reducing oxidative damage to cells and tissues by scavenging ROS. In addition, the anti-inflammatory properties of garlic may help reduce systemic inflammation caused by persistent hyperglycemia, which in turn regulates glucose metabolism [181, 182].
Direct anti-diabetic effects of garlic have also been shown. Animal models and people with diabetes have shown moderate drops in blood glucose levels after taking garlic supplements, according to some research [183, 184]. Theoretically, this impact is believed to be facilitated via many pathways, such as improving insulin sensitivity, boosting insulin secretion, and shielding pancreatic β-cells against cell death or malfunction. Furthermore, it has been noted that garlic may impact glucose metabolism via the inhibition of enzymes such as α-glucosidase and α-amylase, which play a role in carbohydrate digestion [185, 186].
Researchers believe that garlic's capacity to alter lipid metabolism and consequently decrease fat storage is a contributing factor to its anti-obesity benefits [187, 188]. Lipid profile improvements and lower total and LDL cholesterol levels have been linked to garlic supplementation [189]. Garlic also has the potential to increase energy expenditure and aid in weight reduction or maintenance by boosting thermogenesis and fat burning. Because chronic inflammation is a feature of adipose tissue in obese people and a cause of metabolic dysfunction, its anti-inflammatory characteristics are also pertinent in the context of obesity.
Role of antioxidants in cancer
Vitamins
Retinoids, which are a class of vitamin A derivatives, are essential for cell proliferation, differentiation, and death. Preventing cells from undergoing malignant changes relies on these activities. Some cancers, including skin, bladder, and lung cancers, may be less common in those who consume more vitamin A, according to epidemiological research [190, 191]. High dosages of vitamin A have the potential to be toxic, thus its therapeutic usage in cancer therapy must be carefully managed.
It is thought that vitamin C, a powerful antioxidant, shields cells from oxidative DNA damage, which may cause cancer. It improves the body's antioxidant defence system as a whole and aids in the regeneration of other antioxidants. Although its effectiveness as a direct anticancer agent is still up for debate, high-dose vitamin C has been investigated as a potential supplementary treatment for cancer [192–194]. Some studies have shown that it helps mitigate the adverse effects of radiation and chemotherapy [195–197]. The anticancer effects of vitamin D are associated with its function in controlling cell proliferation, differentiation, and death [198]. Research including observational studies has linked increased vitamin D levels to a decreased likelihood of developing colorectal, breast, and prostate cancers [199–202]. As a possible adjuvant cancer therapy or cancer preventative, vitamin D's ability to slow cancer cell development and decrease metastasis is noteworthy. Vitamin E is another potent antioxidant that has been studied for its potential to lower cancer risk by defending against oxidative stress and inflammation, which are two important variables in the development of cancer [203]. There is conflicting data on vitamin E's preventive impact against many cancers, and excessive dosages may cause side effects; yet, some studies have shown that it may help prevent prostate and breast cancer [204, 205]. Damage to DNA and an increased risk of cancer have been associated with deficiencies of the B vitamins, which include folate (vitamin B9), vitamin B6, and vitamin B12 [206, 207]. These vitamins are crucial for DNA synthesis and repair. One possible explanation for the link between folate and a lower risk of cancers of the colon, breast, and cervical regions is the function it plays in DNA methylation, an essential step in controlling gene expression [208].
Polyphenols
One important component of polyphenols' anticancer effects is their antioxidant activity. Polyphenols aid in preventing DNA and other cellular components from oxidative damage—which may cause mutations and cancer—by scavenging ROS and increasing the body's natural antioxidant defense mechanisms [209, 210]. Chronic inflammation is known to increase the risk of cancer, although polyphenols' anti-inflammatory characteristics may help alleviate this risk. Potentially reducing the risk of inflammation-driven carcinogenesis, polyphenols work by blocking pro-inflammatory pathways and cytokine synthesis [211, 212]. Polyphenols have direct effects on the development and proliferation of cancer cells [213, 214]. Their ability to regulate the cell cycle, induce programmed cell death (apoptosis), promote tumorangiogenesis (the creation of new blood vessels to nourish tumors), and control metastasis (the spread of cancer cells to other areas of the body) is well-established. On top of all that, polyphenols may also affect the hormonal balance of the body, which is important for malignancies like breast and prostate that rely on hormones for their growth [215]. Potentially lowering the incidence of hormone-related malignancies, certain polyphenols function as phytoestrogens by binding to oestrogen receptors and exerting modest estrogenic or anti-estrogenic actions [216].
Flavonoids are well-known not just for their antioxidant properties, but also for their ability to reduce inflammation. Many different kinds of cancer have chronic inflammation as a recognized risk factor. By decreasing inflammation and perhaps lowering cancer risk, flavonoids may block a number of enzymes and cytokines that are involved in the inflammatory process [217, 218]. Flavonoids modulate cell signallng pathways, which in turn impact the development and survival of cancer cells. They have the ability to stop cancer cells from multiplying, trigger programmed cell death, and stop new blood vessels from forming to feed tumors with oxygen and nutrients—a process known as angiogenesis. As an example, flavonoids have the ability to disrupt the PI3K/Akt and NF-κB pathways, which are used by cancer cells for growth and cell division [218, 219]. Without affecting healthy cells, flavonoids may inhibit tumordevelopment and induce cancer cell death by focusing on certain pathways. In addition, flavonoids may have a role as adjuvant drugs in cancer treatment since they increase the susceptibility of cancer cells to traditional radiation and chemotherapy. This might be accomplished by preventing the repair processes that cancer cells rely on to withstand the DNA damage caused by chemotherapy medications or by reducing the expression of drug efflux pumps, which cancer cells often overexpress in order to evade these therapies.
In particular, curcumin's strong anti-inflammatory effects play a significant role. A number of malignancies have chronic inflammation as a recognized risk factor for both their development and progression. Curcumin has the ability to reduce inflammation and may lower the risk of cancer formation by influencing the activity of several important molecules in the inflammatory pathway, including cyclooxygenase-2 (COX-2), nuclear factor-kappa B (NF-κB), and other cytokines [220–222]. Curcumin has the ability to fight cancer because of its antioxidant qualities. Curcumin protects cells from DNA damage that might cause malignant mutations by scavenging ROS and increasing the activity of the body's antioxidant enzymes [223, 224]. Preventing oxidative damage is essential for cellular integrity maintenance and avoiding cancer's early stages.
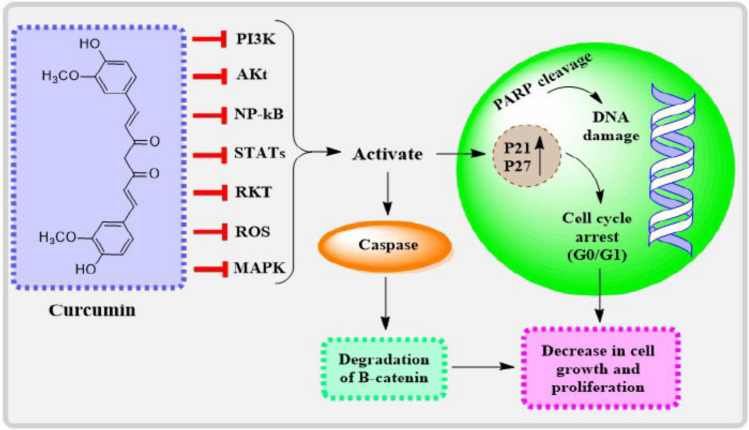
In addition, research has shown that curcumin has a direct impact on tumorcells, namely by reducing cell growth and triggering programmed cell death (apoptosis) in many cancer cell types. A number of signallng pathways, including as the Wnt/β-catenin, PI3K/Akt, and MAPK pathways, are disrupted by it, which in turn controls cell growth and survival as shown in Fig. 4 [222, 225, 226]. As a result of its action on these pathways, curcumin has the potential to reduce tumorsize and halt tumorprogression.
Fig. 4.
Image depicts the routes that tumors might take, starting with growth factors in cancer cells, and how curcumin can inhibit tumordevelopment by targeting certain signal transducing components. Once conjugated, curcumin binds to the infected area and suppresses the expression of pathways that induce tumors: PI3K, AKt, NP-κB, MAPK, and ROS
Angiogenesis is the process by which tumors create their own blood supply to maintain their development; curcumin may impede this process as well. Curcumin may deprive tumors of oxygen and nutrients by inhibiting the production of angiogenic factors including vascular endothelial growth factor (VEGF) [227]. Beyond its direct anticancer properties, curcumin may also improve the effectiveness of more traditional cancer therapies, such as radiation and chemotherapy. It may make cancer cells more sensitive to these therapies, which might lead to better patient outcomes with fewer side effects at lower dosages. As an additional piece of evidence for its potential use as a supplement to these treatments, curcumin may shield healthy cells from their deleterious effects [228].
Garlic
Allicin, diallyl sulphide, and S-allylcysteine are just a few of the bioactive chemicals found in garlic that have shown promise in preclinical cancer research, and this has piqued people's curiosity. Garlic inhibits cancer via a complex web of processes that includes regulating carcinogen metabolism, protecting antioxidants, boosting the immune system, preventing cancer cell multiplication, inducing apoptosis, and blocking angiogenesis [229].
One important mechanism by which garlic inhibits cancer is via influencing carcinogen metabolism. It lessens the possibility of carcinogens producing DNA damage that may lead to cancer by increasing the activity of enzymes that detoxify them [230, 231]. On top of that, the sulfur-containing chemicals in garlic may attach directly to carcinogens, making it easier to excrete them from the body and lowering their cancer-causing potential. One reason garlic may help prevent cancer is because of its antioxidant capabilities. These characteristics neutralize ROS, which are harmful to DNA and other parts of cells and can cause mutations and cancer. Garlic helps keep cells healthy and prevents cancer from starting by reducing oxidative stress [232, 233]. Garlic also enhances the immune system's capacity to fight cancer. By boosting the activity of immune cells including natural killer cells, macrophages, and lymphocytes, it improves the body's capacity to detect and eliminate cancer cells. Both cancer prevention and bolstering the body's natural defenses against tumor development rely on this immunomodulatory action [234, 235]. Inducing apoptosis, or programmed cell death, is an important technique for slowing the course of cancer, and chemicals found in garlic can do just that at the cellular level [236–239]. Research has shown that garlic may influence many molecular pathways related to cell cycle control and cell death, such as NF-κB activity inhibition and caspase enzyme activation, which in turn can reduce tumor development [240–243].
Clinical translation of antioxidants
The clinical translation of antioxidants for the treatment and prevention of human diseases presents several significant challenges. First and foremost, the complex nature of oxidative stress and antioxidant mechanisms in the human body poses a fundamental challenge. Oxidative stress results from an imbalance between the production of reactive oxygen species (ROS) and the body's ability to detoxify these reactive intermediates or repair the resulting damage. Antioxidants can mitigate this imbalance by neutralizing ROS, but the effectiveness of this approach in a clinical setting is influenced by numerous factors, including the specific disease context, the timing of antioxidant administration, and individual patient differences [244].
Another major challenge lies in the bioavailability and pharmacokinetics of antioxidant compounds [245, 246]. Many antioxidants exhibit poor solubility, stability, and absorption when administered orally, which can significantly limit their therapeutic potential. Efforts to improve these properties through formulation strategies or chemical modification can alter the antioxidant's efficacy or safety profile, necessitating extensive preclinical and clinical testing.
The heterogeneity of human diseases further complicates the clinical translation of antioxidants. Diseases such as cancer, cardiovascular disorders, and neurodegenerative conditions involve distinct pathophysiological mechanisms, including varying roles of oxidative stress. As a result, an antioxidant that is effective in one disease context may not be beneficial in another, and in some cases, antioxidant therapy could potentially exacerbate disease progression by interfering with physiological ROS signaling pathways that are essential for normal cellular function [247, 248].
Clinical trials of antioxidants have also faced challenges related to dosing and endpoints [249, 250]. Determining the optimal dose of an antioxidant that provides therapeutic benefits without causing adverse effects is a complex process that requires careful consideration of the antioxidant's mechanism of action and pharmacokinetics. Furthermore, clinical trials must utilize relevant and measurable endpoints to assess the efficacy of antioxidant therapies, which can be difficult given the multifactorial nature of many diseases and the long latency periods before clinical outcomes manifest.
Lastly, the regulatory pathway for antioxidants, especially those derived from natural products, can be ambiguous and challenging. Demonstrating the safety and efficacy of these compounds to regulatory authorities requires a significant investment of time and resources, which can be a barrier for many researchers and companies [251].
Challenges and future perspectives
Limitations and obstacles in utilizing targeted antioxidants
Natural antioxidants have shown significant efficacy in the treatment of diseases such as diabetes and its complications, Alzheimer's disease, cancer, cardiovascular diseases, and inflammatory conditions. For instance, the natural antioxidant sulforaphane (SFN) activates transcription factors to help maintain cellular antioxidant defense systems and attenuate age-related changes [252]. Natural antioxidants may also contribute to controlling inflammatory responses, and certain antioxidant plant compounds can serve as targets for anti-inflammatory and antioxidant preventive strategies [253]. Despite their ability to prevent or repair cellular and tissue damage caused by oxidative stress, the application of natural antioxidants still faces several limitations and obstacles.
During oxidative stress, elevated levels of reactive oxygen species (ROS) can induce apoptosis in tumor cells, thereby inhibiting tumor growth and progression. However, antioxidants can neutralize free radicals and reduce oxidative stress, thereby mitigating the apoptotic tendency of tumor cells and promoting tumor progression. Glutathione (GSH) plays a dual role in tumor progression. On one hand, it is crucial for the elimination and detoxification of carcinogens, and alterations in this pathway can profoundly impact cell survival. However, high levels of GSH in tumor cells can protect them, leading to resistance against various chemotherapy drugs in bone marrow, breast, colon, throat, and lung cancers, thereby facilitating tumor development [254]. Furthermore, GSH helps maintain the balance of intracellular redox potential by preserving the reduced state of protein thiol groups, which is important in various pathological conditions, including cancer. On the other hand, GSH levels are closely associated with ROS. Moderate levels of ROS can promote survival and proliferation by activating signaling pathways that support tumor growth, particularly in the stressed tumor microenvironment. Wiel et al.'s study revealed that antioxidants such as N-acetylcysteine (NAC) and vitamin E promote KRAS-driven lung cancer metastasis by reducing free heme levels and stabilizing the transcription factor BACH1 [255]. BACH1 activation leads to increased transcription of Hexokinase 2 and Gapdh genes, enhancing glucose uptake, glycolytic rate, and lactate secretion, thereby stimulating the glycolysis-dependent metastasis of lung cancer cells. Preventing antioxidant-induced metastasis can be achieved by targeting BACH1 or its glycolytic targets, while increased endogenous BACH1 expression stimulates glycolysis and promotes metastasis, even in the absence of antioxidants. Antioxidants such as NAC and vitamin E reduce ROS levels, DNA damage, and p53 expression in both mouse and human lung tumor cells, promoting tumor cell proliferation [256].
The efficacy of antioxidants varies for different diseases and represents an important limitation. While antioxidants may help combat oxidative stress in diabetes and other diseases, excessive antioxidant activity may mask or interfere with necessary physiological signals in neurodegenerative diseases such as Alzheimer's disease and cerebrovascular diseases. Flavonoids, a class of natural antioxidants, can inhibit acetylcholinesterase and butyrylcholinesterase, prevent Tau protein aggregation, and modulate the activity of β-secretase [257]. Moreover, flavonoids exhibit antioxidant properties, attenuate oxidative stress, participate in anti-inflammatory processes, and prevent cell apoptosis by regulating signaling pathways associated with cognition and neuroprotection, such as ERK, PI3-kinase/Akt, NF-κB, and MAPKs [257]. Although antioxidants have demonstrated protective effects in animal models and in vitro experiments for Alzheimer's disease, clinical trial results have been less promising. Specifically, clinical trials with vitamin E, despite one study reporting delayed clinical deterioration in patients with moderate Alzheimer's disease using high-dose vitamin E, did not improve cognitive test scores [258]. This suggests that the effects of antioxidants in the human body may differ from those observed in experimental models, or other factors may influence their efficacy. A meta-analysis also indicated that vitamin E supplementation did not show beneficial effects in preventing any type of stroke, including ischemic stroke, hemorrhagic stroke, fatal stroke, and non-fatal stroke [259].
Currently, various natural antioxidants have entered clinical trial stages. For example, extracts from tea, cocoa, and numerous vegetables and fruits, including SFN, resveratrol, silymarin, alpha-tocopherol, and curcumin, activate oxidative stress signaling pathways and induce the production of antioxidant enzymes. Some of these antioxidants are being tested in clinical trials for the treatment and/or prevention of diseases [260–265]. These studies demonstrate the potential of natural antioxidants in medical applications. However, the therapeutic effects of natural antioxidants are still being investigated, and further research is needed to fully understand their mechanisms of action, optimal dosage, and potential interactions with other medications. Additionally, it is important to consider individual variations in response to antioxidants, as some individuals may benefit more from their use than others. Overall, while natural antioxidants hold promise for the treatment and prevention of various diseases, their application should be approached with caution and under the guidance of healthcare professionals.
Novel approaches and technologies in antioxidant research
With the continuous advancement of research on oxidative stress and antioxidants, new methods and technologies have emerged to explore and evaluate the potential and effects of antioxidants. Omics technologies encompass a set of approaches that study the overall composition and functionality of organisms at the molecular level, including genomics, transcriptomics, proteomics, and metabolomics. In antioxidant research, omics technologies are widely applied to gain in-depth understanding of the mechanisms, molecular basis, and biological effects of antioxidants. In antioxidant research, omics approaches can help identify genes associated with antioxidant reactions and cellular protection. On one hand, genomic and transcriptomic sequencing results can directly contribute to unraveling the genetic basis of plant antioxidant activity and evolution. Ren et al. identified antioxidant signaling pathway genes, such as Caffeoyl-CoA O-methyltransferase, anthocyanidin 3-O-glucosyltransferase, ( +)-neomenthol dehydrogenase, gibberellin 2-oxidase, and squalene monooxygenase, in Myrica rubra by examining 26,325 coding protein-coding genes, which exhibit multiple copies and regulate the content of flavonoids, anthocyanins, monoterpenoids, diterpenoids, and sesquiterpenoid/triterpenoids [266]. Researchers studying hippophaerhamnoides identified genes associated with ascorbic acid biosynthesis, such as GalLDH and GMPase, through a comparison of whole genome sequencing and transcriptomic analysis [267]. On the other hand, by comparing the gene profiles, transcript expression profiles, and metabolic profiles of antioxidant-treated groups and control groups, changes in gene expression related to antioxidant reactions can be discovered, revealing the regulatory effects of antioxidants on gene expression. This helps elucidate the impact of antioxidant treatment on intracellular signaling pathways, regulatory factors, and cell protection genes. A study utilizing transcriptomics and proteomics revealed the effects of SFN treatment and KEAP1 gene silencing on human breast epithelial cells (MCF10A), demonstrating similar expression patterns upregulating genes involved in detoxification and antioxidation processes, such as AKR1 family, NQO1, and ALDH3A1, providing a basis for the potential application of SFN as a chemopreventive agent for breast cancer [268].
Drug design and development face challenges such as low efficacy, off-target delivery, time consumption, and high costs. Complex data from genomics, proteomics, microarrays, and clinical trials further complicate the process. Here, artificial intelligence(AI) and deep learning play a crucial role in overcoming obstacles and improving drug discovery [269]. Computational methods, such as molecular docking and molecular dynamics simulations, are employed to predict the antioxidant activity of natural antioxidants. These methods provide insights into the molecular interactions between antioxidants and ROS, aiding in the optimization of natural antioxidants more effectively. Kumari Patial et al. evaluated the binding energies and interactions of phytosterols isolated from Araucaria columnaris with antioxidant enzymes peroxidase and hemoglobin oxidase through molecular docking calculations, validating the potential of phytosterols as antioxidants [270]. Zu et al. analyzed the stability of quercetin with three target proteins (MAPK1, TNF, and JUN) using molecular dynamics simulations [271]. Binding energy data obtained from mm/GBSA calculations showed the strongest binding energy between quercetin and TNF, followed by MAPK1, and JUN. Researchers have begun to fill the gap in antioxidant testing field using AI and machine learning (ML). The goal is to correlate the antioxidant activity of polyphenolic substances, such as those found in seeds, fruits, and vegetables, with their structural diversity. These polyphenolic substances exhibit different antioxidant mechanisms, including hydrogen atom transfer and single electron transfer [272]. By leveraging AI, scientists can develop more comprehensive experimental methods to reliably and comprehensively evaluate the health benefits of plant chemical substances [273]. Through ML, a predictive model can be established using antioxidant structural descriptors as input variables and their antioxidant activity in biological systems as output variables, thereby correlating and predicting the biologically relevant antioxidant capacity under different environments, enhancing the biological relevance of testing [274]. Three-Dimensional Quantitative Structure–Activity Relationship (3D-QSAR) techniques will aid in screening antioxidant peptides, primarily based on the three-dimensional structure of molecules [275]. Initially, three peptide sequences with free radical scavenging activity are collected, followed by structure construction and energy minimization. Subsequently, peptide alignment, modeling (e.g., CoMSIA method), and validation are conducted to analyze the contributions of various peptide properties to antioxidant activity, thereby identifying core fragments with excellent free radical scavenging capabilities [276].
Future Directions and Potential Applications in Precision Medicine
Antioxidants play a crucial role in maintaining cellular health by neutralizing harmful free radicals and reducing oxidative stress. As part of precision medicine, the application of antioxidants is an actively researched field. Here, we provide insights and potential future directions for the precision medicine of antioxidants. Antioxidant cocktails, which are therapeutic regimens composed of multiple antioxidants combined together, can achieve a synergistic effect and enhance overall antioxidant efficacy. This comprehensive treatment approach can be tailored to specific diseases, individual genotypes, lifestyles, and levels of oxidative stress [277]. A clinical trial has demonstrated that Antioxidant Cocktail therapy significantly reduces iron burden and oxidative stress in non-transfusion-dependent β-thalassemia/hemoglobin E patients, improves coagulation status, increases hemoglobin concentration, and enhances red blood cell quality [278]. In addition to tailoring antioxidant therapy based on an individual's level of oxidative stress, precision medicine can also determine the use of antioxidant mixtures as part of the treatment, as well as identify appropriate combinations and dosages based on genotype and other relevant factors. However, the effectiveness of Antioxidant Cocktail therapy in mitochondrial disease patients has not been clinically validated and there may be risks of inhibiting endogenous oxidants leading to cellular toxicity or toxicity. For rare diseases, individual susceptibility to the disease is often influenced by DNA sequence variations in one or a few genes. These variations can have a significant impact on disease manifestation. Understanding these monogenic variations is crucial in the context of antioxidant therapy. Certain genetic changes may affect an individual's response to antioxidants, thereby influencing their efficacy or potential side effects [279]. For example, the key transcription factor Nrf2 in the antioxidant defense system is targeted for degradation under normal conditions through its interaction with Keap1. However, under oxidative stress, modification of Keap1's sulfhydryl group leads to the release and activation of Nrf2, subsequently initiating the expression of a series of protective genes [280]. Studies have shown that SFN upregulates Nrf2-mediated antioxidant activity, reduces oxidative stress levels, and downregulates the transforming growth factor-beta 1 (TGF-β1)/Smad signaling pathway, thereby reducing extracellular matrix component expression and preventing radiation-induced muscle fibrosis [281]. This highlights the importance of Nrf2 in disease treatment and potential drug development. Integrating large-scale biomedical data, including genetic information, oxidative stress markers, lifestyle factors, and treatment outcomes, provides an opportunity to develop predictive models using ML algorithms. These models can help identify individuals who are more susceptible to oxidative stress-related diseases and predict their response to antioxidant interventions. Such predictive models can support the decision-making process for personalized antioxidant therapy and contribute to improving treatment outcomes for patients.
Conclusion
Summary of key findings and implications
This review mainly discusses the application effect of targeted antioxidant natural products in different diseases. Studies have found that antioxidant natural products improve oxidative stress damage in cells through a variety of mechanisms, thereby treating and improving disease. Studies have found that antioxidant natural products have potential efficacy in cardiovascular diseases, neurological diseases, obesity and insulin resistance, cancer, metabolic diseases, and skin aging, as well as the ability to regulate the immune system and anti-inflammatory processes. Studies have confirmed that some antioxidant natural products can prevent and treat cardiovascular disease through different mechanisms of action, such as lowering blood pressure, regulating lipid distribution, improving oxidative stress, reducing inflammation, and regulating gut microbiota [282]. Excessive production of reactive oxygen species and decreased antioxidant capacity in nerve cells cause oxidative stress, leading to the emergence of neurodegenerative diseases. The brain has a high concentration of fatty acids, high oxygen demand, weak antioxidant capacity and sensitivity to oxidative damage. Some antioxidant natural products can reduce oxidative damage and improve the endogenous antioxidant capacity of cells by acting inside and outside the cell, so as to prevent and slow down the occurrence of nervous system diseases [283]. Experiments have confirmed that the diet of vegetables and fruits reduces the metabolic products of oxidative stress in patients with metabolic diseases, including obesity and diabetes, which may be related to the natural antioxidant bioactive compounds present in vegetables and fruits [284, 285]. Excessive reactive oxygen species in cells break the balance of pro-oxidation and antioxidant, cause oxidative stress, induce cell damage, DNA mutation, abnormal cell proliferation, and thus cancer [286, 287]. Natural antioxidants such as flavonoids obtained from the diet have the potential to scavenge free radicals, inhibit superoxide-producing enzymes, and increase antioxidant activity to inhibit the development of cancer [288, 289]. Skin, as the largest organ of the human body, is vulnerable to external ultraviolet rays, toxic substances and pollution, resulting in skin health problems, especially skin aging, the most concerned. Both internal and external aging are related to the production of free radicals and the oxidative stress of the skin, so some antioxidant natural products play a key role in delaying aging and promoting skin health [290, 291]. Immune system regulation and inflammatory diseases are closely related to changes in ROS levels. Moderate ROS levels contribute to immune regulation and anti-inflammatory processes, while high ROS levels can damage the immune system and lead to the development of inflammation [292]. Animal experiments have shown that curcumin and chitosan can effectively promote wound healing, which may be related to its anti-inflammatory effect [293].
Although a large number of experiments have confirmed that targeted antioxidant natural products can be used to treat oxidative stress, there are still limitations and obstacles. Issues include specificity, administration and bioavailability, dose and concentration, selectivity, complexity of oxidative stress pathways, lack of comprehensive understanding, and cost and availability. Addressing these issues will require further research, technological advances, and a comprehensive understanding of the biology of oxidative stress [294].
Final thoughts on the role of targeted antioxidant natural products in therapeutics
The therapeutic effects of targeted antioxidant natural products are widely discussed and researched. Antioxidants play a crucial role in maintaining cellular health by neutralizing harmful free radicals and reducing oxidative stress [295]. Several diseases are associated with oxidative stress, including cardiovascular diseases, COPD, neurodegenerative diseases, metabolic disorders, cancer, and aging. Natural products such as vitamins (C and E), polyphenolic compounds (such as resveratrol and curcumin), and carotenoids (such as β-carotene) have gained attention for their antioxidant properties and are widely found in fruits, vegetables, nuts, and other plants [296].
Some preclinical studies and preliminary clinical trials have shown potential positive effects of targeted antioxidant natural products on certain diseases. For example, vitamin C has demonstrated potential in reducing the risk of cardiovascular diseases and certain cancers, resveratrol has shown potential in protecting against age-related diseases and promoting longevity, and curcumin has exhibited anti-inflammatory and anticancer properties [297–300]. However, the overall clinical evidence for the therapeutic use of targeted antioxidant natural products remains limited and often contradictory. Their effects are influenced by various factors, including specific diseases, dosage, duration of treatment, and individual patient characteristics [301]. Additionally, the bioavailability and metabolism of these compounds may vary, affecting their effectiveness in the body.
Recent research has also highlighted that excessive use of high-dose antioxidant supplements may not always be beneficial and can even lead to adverse reactions. Overconsumption of antioxidants may interfere with the body's natural defense mechanisms against oxidative stress, thereby weakening the body's ability to combat diseases [302]. Therefore, caution should be exercised when applying targeted antioxidant natural products, and further research is needed. Well-designed clinical trials can help evaluate their safety, efficacy, optimal dosage, and potential interactions with other medications. Furthermore, adopting a comprehensive approach to health, including a balanced diet rich in antioxidants, may be more beneficial than solely relying on antioxidant supplements [303].
In conclusion, targeted antioxidant natural products have the potential to play a significant role in therapy, but their exact benefits and limitations require further investigation. It is necessary to deepen our understanding of their mechanisms of action, optimal application methods, and potential risks in order to better harness the potential of these natural products for improving human health.
Abbreviations
- Aβ
Amyloid-beta
- AMP
Activated protein kinase (AMPK)
- ALS
Amyotrophic lateral sclerosis
- AKR1
Family Aldo–keto reductase family 1
- ALDH3A1
Aldehyde dehydrogenase 3A1
- AI
Artificial intelligence
- AMPK
Adenosine monophosphate-activated protein kinase
- AMPK:
Adenosine monophosphate-activated protein kinase
- BACH1
Bacillus amyloliquefaciens H 1
- CAT
Catalase
- CVDs
Cardio vascular diseases
- COX-2
Cyclooxygenase-2
- CCoAOMT
Caffeoyl coenzyme A O-methyltransferase
- DHA
Docosahexaenoic acid
- DPPH
2,2-Diphenyl-1-picrylhydrazyl
- EPA
Eicosapentaenoic acid
- GSHPx
Glutathione peroxidase
- GSH
Glutathione
- GBSA calculations
Generalised born and surface area solvation
- GalLDH
L-galactono-1,4-lactone dehydrogenase
- GMPase
GDP-D-mannose pyrophosphorylase
- HDL
High-density lipoprotein
- KRAS
Driven lung cancer metastasis Kirsten rat sarcoma viral oncogene homolog.
- LDL
Low-density lipoproteins
- MAPK
Mitogen-activated protein kinase
- ML
Machine learning
- MS
Multiple sclerosis
- NAC
N-acetylcysteine
- NFκB
Nuclear factor kappa B
- NQO1
NAD(P)H dehydrogenase [quinone] 1
- Nrf2
Nuclear factor erythroid 2-related factor 2
- ORAC
Oxygen radical absorbance capacity
- PD
Parkinson's disease
- PI3K
Phosphoinositide 3-kinase
- PKC
Protein kinase C
- ROS
Reactive oxygen species
- RNS
Reactive nitrogen species
- SFN
Sulforaphane
- SIRT-1
Sirtuin (silent mating type information regulation 2 homolog) 1 (S. cerevisiae)
- SIRT-CVS
Short interval resveratrol trial in cardiovascular surgery
- SOD
Superoxide dismutase
- VEGF
Vascular endothelial growth factor
- WHO
World health organization
Author contributions
S. M., R.G.: Writing original draft and conceptualization, R.G., S.J.: Data Collection, Visualization and writing original draft, Z.D., T.D. Supervision and review editing, H.C., T.B.: Supervision, Data validation, Review and editing.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lobo V, Patil A, Phatak A, Chandra N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn Rev. 2010;4:118. 10.4103/0973-7847.70902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forman HJ, Zhang H. Targeting oxidative stress in disease: promise and limitations of antioxidant therapy. Nat Rev Drug Discov. 2021;20:689–709. 10.1038/s41573-021-00233-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yuan H, Ma Q, Ye L, Piao G. The traditional medicine and modern medicine from natural products. Molecules. 2016. 10.3390/molecules21050559. 10.3390/molecules21050559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Integrating Traditional Medicine in Health Care Available online: https://www.who.int/southeastasia/news/feature-stories/detail/integrating-traditional-medicine (accessed on 8 March 2024).
- 5.Goyal R, Mittal G, Khurana S, Malik N, Kumar V, Soni A, Chopra H, Kamal MA. Insights on quercetin therapeutic potential for neurodegenerative diseases and its nano-technological perspectives. Curr Pharm Biotechnol. 2023. 10.2174/1389201025666230830125410. 10.2174/1389201025666230830125410 [DOI] [PubMed] [Google Scholar]
- 6.Chopra H, Sharma S, Yousaf S, Naseer R, Ahmed S, Baig AA. Applications of nanotechnology-based approaches for targeted delivery of nutraceuticals. Handb Nanotechnol Nutraceuticals. 2022. 10.1201/9781003244721-12. 10.1201/9781003244721-12/APPLICATIONS-NANOTECHNOLOGY-BASED-APPROACHES-TARGETED-DELIVERY-NUTRACEUTICALS-HITESH-CHOPRA-SHIVANI-SHARMA-SABA-YOUSAF-RAHAT-NASEER-SHAKEEL-AHMED-ATIF-AMIN-BAIG [DOI] [Google Scholar]
- 7.Walia V, Kaushik D, Mittal V, Kumar K, Verma R, Parashar J, Akter R, Rahman MHMH, Bhatia S, Al-Harrasi A, et al. Delineation of neuroprotective effects and possible benefits of antioxidantstherapy for the treatment of Alzheimer’s diseases by targeting mitochondrial-derived reactive oxygen species: bench to bedside. Mol Neurobiol. 2022;59:657–80. 10.1007/s12035-021-02617-1. 10.1007/s12035-021-02617-1 [DOI] [PubMed] [Google Scholar]
- 8.Zehiroglu C, Ozturk Sarikaya SB. The importance of antioxidants and place in today’s scientific and technological studies. J Food Sci Technol. 2019;56:4757–74. 10.1007/s13197-019-03952-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kotha RR, Tareq FS, Yildiz E, Luthria DL. Oxidative stress and antioxidants—a critical review on in vitro antioxidant assays. Antioxidants. 2022;11:2388. 10.3390/antiox11122388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Birben E, Sahiner UM, Sackesen C, Erzurum S, Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organ J. 2012;5:9–19. 10.1097/WOX.0b013e3182439613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poljsak B. Strategies for reducing or preventing the generation of oxidative stress. Oxid Med Cell Longev. 2011;2011(1): 194586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jena AB, Samal RR, Bhol NK, Duttaroy AK. Cellular red-ox system in health and disease: the latest update. Biomed Pharmacother. 2023;162:114606. 10.1016/j.biopha.2023.114606 [DOI] [PubMed] [Google Scholar]
- 13.Lee SE, Park YS. The emerging roles of antioxidant enzymes by dietary phytochemicals in vascular diseases. Life. 2021;11:199. 10.3390/life11030199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Apak R, Özyürek M, Güçlü K, Çapanoʇlu E. Antioxidant activity/capacity measurement 1 classification, physicochemical principles, mechanisms, and electron transfer (ET)-based assays. J Agric Food Chem. 2016;64:997–1027. 10.1021/acs.jafc.5b04739 [DOI] [PubMed] [Google Scholar]
- 15.Darenskaya M, Kolesnikov S, Semenova N, Kolesnikova L. Diabetic nephropathy: significance of determining oxidative stress and opportunities for antioxidant therapies. Int J Mol Sci. 2023;24:12378. 10.3390/ijms241512378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahmad KA, Yuan Yuan D, Nawaz W, Ze H, Zhuo CX, Talal B, Taleb A, Mais E, Qilong D. Antioxidant therapy for management of oxidative stress induced hypertension. Free Radic Res. 2017;51:428–38. 10.1080/10715762.2017.1322205 [DOI] [PubMed] [Google Scholar]
- 17.Feng Y, Wang X. Antioxidant therapies for Alzheimer’s disease. Oxid Med Cell Longev. 2012;2012(1): 472932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moura FA, de Andrade KQ, dos Santos JCF, Araújo ORP, Goulart MOF. Antioxidant therapy for treatment of inflammatory bowel disease: Does it work? Redox Biol. 2015;6:617–39. 10.1016/j.redox.2015.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luo M, Zhou L, Huang Z, Li B, Nice EC, Xu J, Huang C. Antioxidant therapy in cancer: rationale and progress. Antioxidants. 2022;11:1128. 10.3390/antiox11061128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teleanu RI, Chircov C, Grumezescu AM, Volceanov A, Teleanu DM. Antioxidant therapies for neuroprotection-a review. J Clin Med. 2019;8:1659. 10.3390/jcm8101659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Collins AE, Saleh TM, Kalisch BE. Naturally occurring antioxidant therapy in Alzheimer’s disease. Antioxidants. 2022;11:213. 10.3390/antiox11020213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang S, Teng H, Zhang L, Wu L. Association between dietary antioxidant intakes and chronic respiratory diseases in adults. World Allergy Organ J. 2024;17: 100851. 10.1016/j.waojou.2023.100851. 10.1016/j.waojou.2023.100851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vidavalur R, Otani H, Singal PK, Maulik N. Significance of wine and resveratrol in cardiovascular disease: French paradox revisited. Exp Clin Cardiol. 2006;11:217–25. [PMC free article] [PubMed] [Google Scholar]
- 24.Li H, Xia N, Hasselwander S, Daiber A. Resveratrol and vascular function. Int J Mol Sci. 2019. 10.3390/ijms20092155. 10.3390/ijms20092155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chaplin A, Carpéné C, Mercader J. Resveratrol, metabolic syndrome, and gut microbiota. Nutrients. 2018. 10.3390/nu10111651. 10.3390/nu10111651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jang JY, Im E, Kim ND. Mechanism of resveratrol-induced programmed cell death and new drug discovery against cancer: a review. Int J Mol Sci. 2022. 10.3390/ijms232213689. 10.3390/ijms232213689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Almatroodi SA, A Alsahli M, SM Aljohani A, Alhumaydhi FA, Babiker AY, Khan AA, Rahmani AH. Potential therapeutic targets of resveratrol, a plant polyphenol, and its role in the therapy of various types of cancer. Molecules. 2022;27(9):2665. 10.3390/molecules27092665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Izzo C, Annunziata M, Melara G, Sciorio R, Dallio M, Masarone M, Federico A, Persico M. The role of resveratrol in liver disease: a comprehensive review from in vitro to clinical trials. Nutrients. 2021. 10.3390/nu13030933. 10.3390/nu13030933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nashine S, Nesburn AB, Kuppermann BD, Kenney MC. Role of resveratrol in transmitochondrial AMD RPE cells. Nutrients. 2020. 10.3390/nu12010159. 10.3390/nu12010159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dyck GJB, Raj P, Zieroth S, Dyck JRB, Ezekowitz JA. The effects of resveratrol in patients with cardiovascular disease and heart failure: a narrative review. Int J Mol Sci. 2019. 10.3390/ijms20040904. 10.3390/ijms20040904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prysyazhna O, Wolhuter K, Switzer C, Santos C, Yang X, Lynham S, Shah AM, Eaton P, Burgoyne JR. Blood pressure-lowering by the antioxidant resveratrol is counterintuitively mediated by oxidation of CGMP-dependent protein kinase. Circulation. 2019;140:126–37. 10.1161/CIRCULATIONAHA.118.037398. 10.1161/CIRCULATIONAHA.118.037398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Movahed A, Ostovar A, Iranpour D, Thandapilly SJ, Raj P, Louis XL, Smoliga JM, Netticadan T. The efficacy of resveratrol in controlling hypertension: study protocol for a randomized, crossover, double-blinded. Placebo-Controlled Trial Trials. 2016;17:296. 10.1186/s13063-016-1426-x. 10.1186/s13063-016-1426-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Theodotou M, Fokianos K, Mouzouridou A, Konstantinou C, Aristotelous A, Prodromou D, Chrysikou A. The effect of resveratrol on hypertension: a clinical trial. Exp Ther Med. 2017;13:295–301. 10.3892/etm.2016.3958. 10.3892/etm.2016.3958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fogacci F, Tocci G, Presta V, Fratter A, Borghi C, Cicero AFG. Effect of resveratrol on blood pressure: a systematic review and meta-analysis of randomized, controlled, clinical trials. Crit Rev Food Sci Nutr. 2019;59:1605–18. 10.1080/10408398.2017.1422480. 10.1080/10408398.2017.1422480 [DOI] [PubMed] [Google Scholar]
- 35.Short Interval Resveratrol Trial in Cardiovascular Surgery - Full Text View - ClinicalTrials.Gov Available online: https://classic.clinicaltrials.gov/ct2/show/NCT03762096 (accessed on 9 March 2024).
- 36.Cardiovascular Health of Older Adults and Resveratrol (CORE) - Full Text View - ClinicalTrials.Gov Available online: https://classic.clinicaltrials.gov/ct2/show/NCT02909699 (accessed on 9 March 2024).
- 37.Gal R, Deres L, Toth K, Halmosi R, Habon T. The effect of resveratrol on the cardiovascular system from molecular mechanisms to clinical results. Int J Mol Sci. 2021;22:10152. 10.3390/ijms221810152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu N, Huang B, Jiang W. Targets of vitamin C with therapeutic potential for cardiovascular disease and underlying mechanisms: a study of network pharmacology. Front Pharmacol. 2021;11:591337. 10.3389/fphar.2020.591337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moser MA, Chun OK. Vitamin C and heart health: a review based on findings from epidemiologic studies. Int J Mol Sci. 2016. 10.3390/ijms17081328. 10.3390/ijms17081328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morelli MB, Gambardella J, Castellanos V, Trimarco V, Santulli G. Vitamin C and cardiovascular disease: an update. Antioxidants (Basel Switzerland). 2020. 10.3390/antiox9121227. 10.3390/antiox9121227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cardiovascular Health Through Vitamin C Intake - PCNA Available online: https://pcna.net/cardiovascular-health-through-vitamin-c-intake/ (accessed on 9 March 2024).
- 42.High Dose Vitamin C in Cardiac Surgery Patients - Full Text View - ClinicalTrials.Gov Available online: https://classic.clinicaltrials.gov/ct2/show/NCT02762331 (accessed on 9 March 2024).
- 43.Vitamin C in Cardiac Surgery Patients - Full Text View - ClinicalTrials.Gov Available online: https://classic.clinicaltrials.gov/ct2/show/NCT03123107 (accessed on 9 March 2024).
- 44.Rozemeijer S, de Grooth HJ, Elbers PWG, Girbes ARJ, den Uil CA, Dubois EA, Wils EJ, Rettig TCD, van Zanten ARH, Vink R, et al. Early high-dose vitamin c in post-cardiac arrest syndrome (VITaCCA): study protocol for a randomized, double-blind, multi-center. Placebo-Controlled Trial Trials. 2021. 10.1186/s13063-021-05483-3. 10.1186/s13063-021-05483-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Can Vitamins Help Prevent a Heart Attack? - Mayo Clinic Available online: https://www.mayoclinic.org/diseases-conditions/heart-disease/expert-answers/prevent-heart-attack/faq-20058253 (accessed on 9 March 2024).
- 46.Shah S, Shiekh Y, Lawrence JA, Ezekwueme F, Alam M, Kunwar S, Gordon DK. A systematic review of effects of vitamin E on the cardiovascular system. Cureus. 2021. 10.7759/cureus.15616. 10.7759/cureus.15616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rychter AM, Hryhorowicz S, Słomski R, Dobrowolska A, Krela-Kaźmierczak I. Antioxidant effects of vitamin E and risk of cardiovascular disease in women with obesity – a narrative review. Clin Nutr. 2022;41:1557–65. 10.1016/j.clnu.2022.04.032 [DOI] [PubMed] [Google Scholar]
- 48.Yusuf S, Dagenais G, Pogue J, Bosch J, Sleight P. Vitamin E supplementation and cardiovascular events in high-risk patients. the heart outcomes prevention evaluation study investigators. N Engl J Med. 2000;342:154–60. 10.1056/NEJM200001203420302 [DOI] [PubMed] [Google Scholar]
- 49.Lee IM, Cook NR, Gaziano JM, Gordon D, Ridker PM, Manson JAE, Hennekens CH, Buring JE. Vitamin E in the primary prevention of cardiovascular disease and cancer the women’s health study: a randomized controlled trial. JAMA. 2005. 10.1001/jama.294.1.56. 10.1001/jama.294.1.56 [DOI] [PubMed] [Google Scholar]
- 50.Saremi A, Arora R. Vitamin E and cardiovascular disease. Am J Ther. 2010;17:e56-65. 10.1097/MJT.0b013e31819cdc9a. 10.1097/MJT.0b013e31819cdc9a [DOI] [PubMed] [Google Scholar]
- 51.Wang M, Tang R, Zhou R, Qian Y, Di D. The protective effect of serum carotenoids on cardiovascular disease: a cross-sectional study from the general US adult population. Front Nutr. 2023. 10.3389/fnut.2023.1154239. 10.3389/fnut.2023.1154239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang J, Zhang Y, Na X, Zhao A. β-carotene supplementation and risk of cardiovascular disease: a systematic review and meta-analysis of randomized controlled trials. Nutrients. 2022;14:1284. 10.3390/nu14061284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tavani A, La Vecchia C. β-Carotene and risk of coronary heart disease a review of observational and intervention studies. Biomed Pharmacother. 1999. 10.1016/S0753-3322(99)80120-6. 10.1016/S0753-3322(99)80120-6 [DOI] [PubMed] [Google Scholar]
- 54.Cardona F, Andrés-Lacueva C, Tulipani S, Tinahones FJ, Queipo-Ortuño MI. Benefits of polyphenols on gut microbiota and implications in human health. J Nutr Biochem. 2013;24:1415–22. 10.1016/j.jnutbio.2013.05.001 [DOI] [PubMed] [Google Scholar]
- 55.Cooper KA, Chopra M, Thurnham DI. Wine polyphenols and promotion of cardiac health. Nutr Res Rev. 2004. 10.1079/nrr200482. 10.1079/nrr200482 [DOI] [PubMed] [Google Scholar]
- 56.Börzsei D, Sebestyén J, Szabó R, Lesi ZN, Pálszabó A, Pálszabó P, Szász A, Priksz D, Juhász B, Veszelka M, et al. Resveratrol as a promising polyphenol in age-associated cardiac alterations. Oxid Med Cell Longev. 2022. 10.1155/2022/7911222. 10.1155/2022/7911222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sari N, Katanasaka Y, Honda H, Miyazaki Y, Sunagawa Y, Funamoto M, Shimizu K, Shimizu S, Wada H, Hasegawa K, et al. Cacao bean polyphenols inhibit cardiac hypertrophy and systolic dysfunction in pressure overload-induced heart failure model mice. Planta Med. 2020. 10.1055/a-1191-7970. 10.1055/a-1191-7970 [DOI] [PubMed] [Google Scholar]
- 58.Tangney CC, Rasmussen HE. Polyphenols, inflammation, and cardiovascular disease. Curr Atheroscler Rep. 2013. 10.1007/s11883-013-0324-x. 10.1007/s11883-013-0324-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Khurana S, Venkataraman K, Hollingsworth A, Piche M, Tai TC. Polyphenols: benefits to the cardiovascular system in health and in aging. Nutrients. 2013;5:3779–827. 10.3390/nu5103779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hedayati N, Yaghoobi A, Salami M, Gholinezhad Y, Aghadavood F, Eshraghi R, Aarabi MH, Homayoonfal M, Asemi Z, Mirzaei H, et al. Impact of polyphenols on heart failure and cardiac hypertrophy: clinical effects and molecular mechanisms. Front Cardiovasc Med. 2023;10:1174816. 10.3389/fcvm.2023.1174816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Behl T, Bungau S, Kumar K, Zengin G, Khan F, Kumar A, Kaur R, Venkatachalam T, Tit DM, Vesa CM, et al. Pleotropic effects of polyphenols in cardiovascular system. Biomed Pharmacother. 2020;130:110714. 10.1016/j.biopha.2020.110714 [DOI] [PubMed] [Google Scholar]
- 62.Chopra H, Bibi S, Mohanta YK, Kumari S, Baig AA. Role of polyphenols in cardiovascular diseases in bioprospecting of tropical medicinal plants. Cham: Springer; 2023. [Google Scholar]
- 63.Goszcz K, Duthie GG, Stewart D, Leslie SJ, Megson IL. Bioactive polyphenols and cardiovascular disease: Chemical antagonists, pharmacological agents or xenobiotics that drive an adaptive response? Br J Pharmacol. 2017;174:1209–25. 10.1111/bph.13708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Khan J, Deb PK, Priya S, Medina KD, Devi R, Walode SG, Rudrapal M. Dietary flavonoids: cardioprotective potential with antioxidant effects and their pharmacokinetic Toxicological and Therapeutic Concerns. Molecules. 2021;26:4021. 10.3390/molecules26134021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jubaidi FF, Zainalabidin S, Taib IS, Hamid ZA, Budin SB. The potential role of flavonoids in ameliorating diabetic cardiomyopathy via alleviation of cardiac oxidative stress, inflammation and apoptosis. Int J Mol Sci. 2021. 10.3390/ijms22105094. 10.3390/ijms22105094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Suen, J.; Thomas, J.; Kranz, A.; Vun, S.; Miller, M. Effect of Flavonoids on Oxidative Stress and Inflammation in Adults at Risk of Cardiovascular Disease: A Systematic Review. Healthc. 2016, 4. [DOI] [PMC free article] [PubMed]
- 67.Ciumărnean L, Milaciu MV, Runcan O, Vesa SC, Răchisan AL, Negrean V, Perné MG, Donca VI, Alexescu TG, Para I, et al. The effects of flavonoids in cardiovascular diseases. Molecules. 2020;25:4320. 10.3390/molecules25184320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yan Q, Liu S, Sun Y, Chen C, Yang S, Lin M, Long J, Yao J, Lin Y, Yi F, et al. Targeting oxidative stress as a preventive and therapeutic approach for cardiovascular disease. J Transl Med. 2023;21:519. 10.1186/s12967-023-04361-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mattioli R, Francioso A, Mosca L, Silva P. Anthocyanins: a comprehensive review of their chemical properties and health effects on cardiovascular and neurodegenerative diseases. Molecules. 2020;25:3809. 10.3390/molecules25173809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mozos I, Flangea C, Vlad DC, Gug C, Mozos C, Stoian D, Luca CT, Horbańczuk JO, Horbańczuk OK, Atanasov AG. Effects of anthocyanins on vascular health. Biomolecules. 2021;11:811. 10.3390/biom11060811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Grassi D, Desideri G, Ferri C. Flavonoids: antioxidants against atherosclerosis. Nutrients. 2010;2:889–902. 10.3390/nu2080889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ponzo V, Goitre I, Fadda M, Gambino R, De Francesco A, Soldati L, Gentile L, Magistroni P, Cassader M, Bo S. Dietary flavonoid intake and cardiovascular risk: a population-based cohort study. J Transl Med. 2015;13:1–13. 10.1186/S12967-015-0573-2/TABLES/4. 10.1186/S12967-015-0573-2/TABLES/4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McCullough ML, Peterson JJ, Patel R, Jacques PF, Shah R, Dwyer JT. Flavonoid intake and cardiovascular disease mortality in a prospective cohort of US adults. Am J Clin Nutr. 2012;95:454. 10.3945/AJCN.111.016634. 10.3945/AJCN.111.016634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Peterson JJ, Dwyer JT, Jacques PF, Mccullough ML. Do flavonoids reduce cardiovascular disease incidence or mortality in US and European populations? Nutr Rev. 2012;70:491. 10.1111/J.1753-4887.2012.00508.X. 10.1111/J.1753-4887.2012.00508.X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hooper L, Kroon PA, Rimm EB, Cohn JS, Harvey I, Le Cornu KA, Ryder JJ, Hall WL, Cassidy A. Flavonoids, flavonoid-rich foods, and cardiovascular risk: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2008;88:38–50. 10.1093/AJCN/88.1.38. 10.1093/AJCN/88.1.38 [DOI] [PubMed] [Google Scholar]
- 76.Fusi F, Trezza A, Tramaglino M, Sgaragli G, Saponara S, Spiga O. The beneficial health effects of flavonoids on the cardiovascular system: focus on K+ channels. Pharmacol Res. 2020;152: 104625. 10.1016/J.PHRS.2019.104625. 10.1016/J.PHRS.2019.104625 [DOI] [PubMed] [Google Scholar]
- 77.Gross M. Flavonoids and cardiovascular disease. Pharm Biol. 2004;42:21–35. 10.3109/13880200490893483. 10.3109/13880200490893483 [DOI] [Google Scholar]
- 78.Kozłowska A, Szostak-Węgierek D. Targeting cardiovascular diseases by flavonols: an update. Nutrients. 2022. 10.3390/nu14071439. 10.3390/nu14071439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen LIJUAN, Wu XIAOLI, Wang WEIWEI, Wang XIA, Ma J. Quercetin with lycopene modulates enzymic antioxidant genes pathway in isoproterenol cardiotoxicity in rats: cardioprotection by quercetin & lycopene. Libyan J Med. 2021. 10.1080/19932820.2021.1943924. 10.1080/19932820.2021.1943924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rutkowska M, Kolodziejczyk-Czepas J, Owczarek A, Zakrzewska A, Magiera A, Olszewska MA. Novel insight into biological activity and phytochemical composition of Sorbus Aucuparia L. fruits: fractionated extracts as inhibitors of protein glycation and oxidative/nitrative damage of human plasma components. Food Res Int. 2021. 10.1016/j.foodres.2021.110526. 10.1016/j.foodres.2021.110526 [DOI] [PubMed] [Google Scholar]
- 81.Ibrahim RYM, Saber AA, Hammad HBI. The possible role of the seaweed ulva fasciata on ameliorating hyperthyroidism-associated heart inflammations in a rat model. Environ Sci Pollut Res. 2021. 10.1007/s11356-020-11036-z. 10.1007/s11356-020-11036-z [DOI] [PubMed] [Google Scholar]
- 82.Hu J, Wang X, Cui X, Kuang W, Li D, Wang J. Quercetin prevents isoprenaline-induced myocardial fibrosis by promoting autophagy via regulating MiR-223-3p/FOXO3. Cell Cycle. 2021. 10.1080/15384101.2021.1932029. 10.1080/15384101.2021.1932029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Khan SU, Lone AN, Khan MS, Virani SS, Blumenthal RS, Nasir K, Miller M, Michos ED, Ballantyne CM, Boden WE, et al. Effect of omega-3 fatty acids on cardiovascular outcomes: a systematic review and meta-analysis. eClinicalMedicine. 2021. 10.1016/j.eclinm.2021.100997. 10.1016/j.eclinm.2021.100997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jain AP, Aggarwal KK, Zhang P-Y. Omega-3 fatty acids and cardiovascular disease. Eur Rev Med Pharmacol Sci. 2015;19:441–5. [PubMed] [Google Scholar]
- 85.Yu F, Qi S, Ji Y, Wang X, Fang S, Cao R. Effects of Omega-3 fatty acid on major cardiovascular outcomes: a systematic review and meta-analysis. Med (United States). 2022;101:e29556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mohebi-Nejad A, Bikdeli B. Omega-3 supplements and cardiovascular diseases. Tanaffos. 2014;13:6. [PMC free article] [PubMed] [Google Scholar]
- 87.Chaddha A, Eagle KA. Omega-3 fatty acids and heart health. Circulation. 2015;132:e350–2. 10.1161/CIRCULATIONAHA.114.015176. 10.1161/CIRCULATIONAHA.114.015176 [DOI] [PubMed] [Google Scholar]
- 88.Thies F, Mills LM, Moir S, Masson LF. Cardiovascular benefits of lycopene: Fantasy or reality? Proc Nutr Soc. 2017;76:122–9. 10.1017/S0029665116000744 [DOI] [PubMed] [Google Scholar]
- 89.Zeng J, Zhao J, Dong B, Cai X, Jiang J, Xue R, Yao F, Dong Y, Liu C. Lycopene protects against pressure overload-induced cardiac hypertrophy by attenuating oxidative stress. J Nutr Biochem. 2019. 10.1016/j.jnutbio.2019.01.002. 10.1016/j.jnutbio.2019.01.002 [DOI] [PubMed] [Google Scholar]
- 90.Xu J, Hu H, Chen B, Yue R, Zhou Z, Liu Y, Zhang S, Xu L, Wang H, Yu Z. Lycopene protects against hypoxia/reoxygenation injury by alleviating ER stress induced apoptosis in neonatal mouse cardiomyocytes. PLoS ONE. 2015. 10.1371/journal.pone.0136443. 10.1371/journal.pone.0136443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.He Q, Zhou W, Xiong C, Tan G, Chen M. Lycopene attenuates inflammation and apoptosis in post-myocardial infarction remodeling by inhibiting the nuclear factor-ΚB Signaling pathway. Mol Med Rep. 2015. 10.3892/mmr.2014.2676. 10.3892/mmr.2014.2676 [DOI] [PubMed] [Google Scholar]
- 92.Palozza P, Parrone N, Simone RE, Catalano A. Lycopene in atherosclerosis prevention: an integrated scheme of the potential mechanisms of action from cell culture studies. Arch Biochem Biophys. 2010;504:26–33. 10.1016/j.abb.2010.06.031 [DOI] [PubMed] [Google Scholar]
- 93.McEneny J, Wade L, Young IS, Masson L, Duthie G, McGinty A, McMaster C, Thies F. Lycopene intervention reduces inflammation and improves HDL functionality in moderately overweight middle-aged individuals. J Nutr Biochem. 2013. 10.1016/j.jnutbio.2012.03.015. 10.1016/j.jnutbio.2012.03.015 [DOI] [PubMed] [Google Scholar]
- 94.Li YF, Chang YY, Huang HC, Wu YC, Yang MD, Chao PM. Tomato juice supplementation in young women reduces inflammatory adipokine levels independently of body fat reduction. Nutrition. 2015. 10.1016/j.nut.2014.11.008. 10.1016/j.nut.2014.11.008 [DOI] [PubMed] [Google Scholar]
- 95.Tierney AC, Rumble CE, Billings LM, George ES. Effect of dietary and supplemental lycopene on cardiovascular risk factors: a systematic review and meta-analysis. Adv Nutr. 2020;11:1453–88. 10.1093/advances/nmaa069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mozos I, Stoian D, Caraba A, Malainer C, Horbanczuk JO, Atanasov AG. Lycopene and vascular health. Front Pharmacol. 2018;9:521. 10.3389/fphar.2018.00521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bin-Jumah MN, Nadeem MS, Gilani SJ, Mubeen B, Ullah I, Alzarea SI, Ghoneim MM, Alshehri S, Al-Abbasi FA, Kazmi I. Lycopene: a natural arsenal in the war against oxidative stress and cardiovascular diseases. Antioxidants. 2022;11:232. 10.3390/antiox11020232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gajendragadkar PR, Hubsch A, Mäki-Petäjä KM, Serg M, Wilkinson IB, Cheriyan J. Effects of oral lycopene supplementation on vascular function in patients with cardiovascular disease and healthy volunteers: a randomised controlled trial. PLoS ONE. 2014. 10.1371/journal.pone.0099070. 10.1371/journal.pone.0099070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Costa-Rodrigues J, Pinho O, Monteiro PRR. Can lycopene be considered an effective protection against cardiovascular disease? Food Chem. 2018;245:1148–53. 10.1016/j.foodchem.2017.11.055 [DOI] [PubMed] [Google Scholar]
- 100.de la Monte SM. Insulin resistance and neurodegeneration: progress towards the development of new therapeutics for alzheimer’s disease. Drugs. 2017;77:47. 10.1007/S40265-016-0674-0. 10.1007/S40265-016-0674-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bathina S, Das UN. Brain-derived neurotrophic factor and its clinical implications. Arch Med Sci. 2015;11:1164. 10.5114/AOMS.2015.56342. 10.5114/AOMS.2015.56342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bhattacharya T, Soares GABE, Chopra H, Rahman MM, Hasan Z, Swain SS, Cavalu S. Applications of phyto-nanotechnology for the treatment of neurodegenerative disorders. Mater. 2022;15:804. 10.3390/MA15030804. 10.3390/MA15030804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Khan UM, Sevindik M, Zarrabi A, Nami M, Ozdemir B, Kaplan DN, Selamoglu Z, Hasan M, Kumar M, Alshehri MM, et al. Lycopene: food sources, biological activities, and human health benefits. Oxid Med Cell Longev. 2021;2021:2713511. 10.1155/2021/2713511. 10.1155/2021/2713511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kaur H, Chauhan S, Sandhir R. Protective effect of lycopene on oxidative stress and cognitive decline in rotenone induced model of parkinson’s disease. Neurochem Res. 2011. 10.1007/s11064-011-0469-3. 10.1007/s11064-011-0469-3 [DOI] [PubMed] [Google Scholar]
- 105.Chen D, Huang C, Chen Z. A review for the pharmacological effect of lycopene in central nervous system disorders. Biomed Pharmacother. 2019;111:791–801. 10.1016/j.biopha.2018.12.151 [DOI] [PubMed] [Google Scholar]
- 106.Paul R, Mazumder MK, Nath J, Deb S, Paul S, Bhattacharya P, Borah A. Lycopene - a pleiotropic neuroprotective nutraceutical: deciphering its therapeutic potentials in broad spectrum neurological disorders. Neurochem Int. 2020. 10.1016/j.neuint.2020.104823. 10.1016/j.neuint.2020.104823 [DOI] [PubMed] [Google Scholar]
- 107.Hamed M, Soliman HAM, Eid Z, Al Naggar Y, Sayed AEDH. Dietary feeding lycopene, citric acid, and chlorella alleviated the neurotoxicity of polyethylene microplastics in African catfish (Clarias Gariepinus). Front Environ Sci. 2022. 10.3389/fenvs.2022.869727. 10.3389/fenvs.2022.869727 [DOI] [Google Scholar]
- 108.Dong S, Zeng Q, Mitchell ES, Xiu J, Duan Y, Li C, Tiwari JK, Hu Y, Cao X, Zhao Z. Curcumin enhances neurogenesis and cognition in aged rats: implications for transcriptional interactions related to growth and synaptic plasticity. PLoS ONE. 2012. 10.1371/journal.pone.0031211. 10.1371/journal.pone.0031211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.So JK, Tae GS, Hee RP, Park M, Kim MS, Hyung SK, Hae YC, Mattson MP, Lee J. Curcumin stimulates proliferation of embryonic neural progenitor cells and neurogenesis in the adult hippocampus. J Biol Chem. 2008. 10.1074/jbc.M708373200. 10.1074/jbc.M708373200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yu S, Wang X, He X, Wang Y, Gao S, Ren L, Shi Y. Curcumin exerts anti-inflammatory and antioxidative properties in 1-methyl-4-phenylpyridinium Ion (MPP+)-stimulated mesencephalic astrocytes by interference with TLR4 and downstream signaling pathway. Cell Stress Chaperones. 2016. 10.1007/s12192-016-0695-3. 10.1007/s12192-016-0695-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mohammadi A, Colagar AH, Khorshidian A, Amini SM. The functional roles of curcumin on astrocytes in neurodegenerative diseases. NeuroImmunoModulation. 2022;29:4–14. 10.1159/000517901 [DOI] [PubMed] [Google Scholar]
- 112.Nguyen HD, Kim MS. The protective effects of curcumin on depression: genes, transcription factors, and microRNAs involved. J Affect Disord. 2022. 10.1016/j.jad.2022.09.108. 10.1016/j.jad.2022.09.108 [DOI] [PubMed] [Google Scholar]
- 113.Ng QX, Koh SSH, Chan HW, Ho CYX. Clinical use of curcumin in depression: a meta-analysis. J Am Med Dir Assoc. 2017. 10.1016/j.jamda.2016.12.071. 10.1016/j.jamda.2016.12.071 [DOI] [PubMed] [Google Scholar]
- 114.Ashrafi P, Jahromy MH. potential efficacy of nanocurcumin on levodopa-induced dyskinesia in a rat parkinsonian model. Phytomedicine Plus. 2022. 10.1016/j.phyplu.2022.100334. 10.1016/j.phyplu.2022.100334 [DOI] [Google Scholar]
- 115.Bishnoi M, Chopra K, Kulkarni SK. Protective effect of curcumin, the active principle of turmeric (Curcuma Longa) in haloperidol-induced orofacial dyskinesia and associated behavioural, biochemical and neurochemical changes in rat brain. Pharmacol Biochem Behav. 2008. 10.1016/j.pbb.2007.10.009. 10.1016/j.pbb.2007.10.009 [DOI] [PubMed] [Google Scholar]
- 116.Asadi S, Gholami MS, Siassi F, Qorbani M, Sotoudeh G. Beneficial effects of nano-curcumin supplement on depression and anxiety in diabetic patients with peripheral neuropathy: a randomized, double-blind. Placebo-Controlled Clinical Trial Phyther Res. 2020. 10.1002/ptr.6571. 10.1002/ptr.6571 [DOI] [PubMed] [Google Scholar]
- 117.Salehi B, Calina D, Docea AO, Koirala N, Aryal S, Lombardo D, Pasqua L, Taheri Y, Castillo CMS, Martorell M, et al. Curcumin’s nanomedicine formulations for therapeutic application in neurological diseases. J Clin Med. 2020;9:430. 10.3390/jcm9020430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gagliardi S, Morasso C, Stivaktakis P, Pandini C, Tinelli V, Tsatsakis A, Prosperi D, Hickey M, Corsi F, Cereda C. Curcumin formulations and trials: what’s new in neurological diseases. Molecules. 2020;25:5389. 10.3390/molecules25225389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cheng, K. K., Yeung, C. F., Ho, S. W., Chow, S. F., Chow, A. H., & Baum, L. (2013). Highly stabilized curcumin nanoparticles tested in an in vitro blood–brain barrier model and in Alzheimer's disease Tg2576 mice. The AAPS Journal, 15(2), 324–336. 10.1208/s12248012-9444-4 10.1208/s12248012-9444-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Garodia P, Hegde M, Kunnumakkara AB, Aggarwal BB. Curcumin, inflammation, and neurological disorders: How are they linked? Integr Med Res. 2023;12:100968. 10.1016/j.imr.2023.100968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chopra H, Dey PSPS, Das D, Bhattacharya T, Shah M, Mubin S, Maishu SPSP, Akter R, Rahman MHMH, Karthika C, et al. Curcumin nanoparticles as promising therapeutic agents for drug targets. Molecules. 2021;26:1–28. 10.3390/molecules26164998. 10.3390/molecules26164998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lim GP, Chu T, Yang F, Beech W, Frautschy SA, Cole GM. The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J Neurosci. 2001. 10.1523/jneurosci.21-21-08370.2001. 10.1523/jneurosci.21-21-08370.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.González-Granillo AE, Gnecco D, Díaz A, Garcés-Ramírez L, de la Cruz F, Juarez I, Morales-Medina JC, Flores G. Curcumin induces cortico-hippocampal neuronal reshaping and memory improvements in aged mice. J Chem Neuroanat. 2022. 10.1016/j.jchemneu.2022.102091. 10.1016/j.jchemneu.2022.102091 [DOI] [PubMed] [Google Scholar]
- 124.Reddy PH, Manczak M, Yin X, Grady MC, Mitchell A, Tonk S, Kuruva CS, Bhatti JS, Kandimalla R, Vijayan M, et al. Protective effects of indian spice curcumin against Amyloid-ß in Alzheimer’s disease. J Alzheimer’s Dis. 2018;61:843–66. 10.3233/JAD-170512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yang F, Lim GP, Begum AN, Ubeda OJ, Simmons MR, Ambegaokar SS, Chen P, Kayed R, Glabe CG, Frautschy SA, et al. Curcumin inhibits formation of amyloid β oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J Biol Chem. 2005. 10.1074/jbc.M404751200. 10.1074/jbc.M404751200 [DOI] [PubMed] [Google Scholar]
- 126.Pu Y, Zhang H, Wang P, Zhao Y, Li Q, Wei X, Cui Y, Sun J, Shang Q, Liu D, et al. Dietary curcumin ameliorates aging-related cerebrovascular dysfunction through the AMPK/uncoupling protein 2 pathway. Cell Physiol Biochem. 2013. 10.1159/000354516. 10.1159/000354516 [DOI] [PubMed] [Google Scholar]
- 127.Qiao P, Ma J, Wang Y, Huang Z, Zou Q, Cai Z, Tang Y. Curcumin prevents neuroinflammation by inducing microglia to transform into the M2-phenotype via CaMKKβ-dependent activation of the amp-activated protein kinase signal pathway. Curr Alzheimer Res. 2020. 10.2174/1567205017666201111120919. 10.2174/1567205017666201111120919 [DOI] [PubMed] [Google Scholar]
- 128.Shao S, Ye X, Su W, Wang Y. Curcumin alleviates alzheimer’s disease by inhibiting inflammatory response, oxidative stress and activating the AMPK pathway. J Chem Neuroanat. 2023;134: 102363. 10.1016/j.jchemneu.2023.102363. 10.1016/j.jchemneu.2023.102363 [DOI] [PubMed] [Google Scholar]
- 129.Hassan FU, Rehman MSU, Khan MS, Ali MA, Javed A, Nawaz A, Yang C. Curcumin as an alternative epigenetic modulator: mechanism of action and potential effects. Front Genet. 2019. 10.3389/fgene.2019.00514. 10.3389/fgene.2019.00514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Abdul-Rahman T, Awuah WA, Mikhailova T, Kalmanovich J, Mehta A, Ng JC, Coghlan MA, Zivcevska M, Tedeschi AJ, de Oliveira EC, et al. Antioxidant, anti-inflammatory and epigenetic potential of curcumin in Alzheimer’s disease. BioFactors. 2024. 10.1002/biof.2039. 10.1002/biof.2039 [DOI] [PubMed] [Google Scholar]
- 131.Khayatan D, Razavi SM, Arab ZN, Niknejad AH, Nouri K, Momtaz S, Gumpricht E, Jamialahmadi T, Abdolghaffari AH, Barreto GE, et al. Protective effects of curcumin against traumatic brain injury. Biomed Pharmacother. 2022;154:113621. 10.1016/j.biopha.2022.113621 [DOI] [PubMed] [Google Scholar]
- 132.Kabir ER, Chowdhury NM, Yasmin H, Kabir MT, Akter R, Perveen A, Ashraf GM, Akter S, Rahman MH, Sweilam SH. Unveiling the potential of polyphenols as anti-amyloid molecules in Alzheimer’s disease. Curr Neuropharmacol. 2023;21:787–807. 10.2174/1570159X20666221010113812. 10.2174/1570159X20666221010113812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gautam S, Karmakar S, Batra R, Sharma P, Pradhan P, Singh J, Kundu B, Chowdhury PK. Polyphenols in combination with β-cyclodextrin can inhibit and disaggregate α-synuclein amyloids under cell mimicking conditions: a promising therapeutic alternative. Biochim Biophys Acta - Proteins Proteomics. 2017;1865:589–603. 10.1016/J.BBAPAP.2017.02.014. 10.1016/J.BBAPAP.2017.02.014 [DOI] [PubMed] [Google Scholar]
- 134.Arbizu-Berrocal SH, Kim H, Fang C, Krenek KA, Talcott ST, Mertens-Talcott SU. Polyphenols from mango (Mangifera Indica L.) modulate PI3K/AKT/MTOR-associated micro-RNAs and reduce inflammation in non-cancer and induce cell death in breast cancer cells. J Funct Foods. 2019;55:9–16. 10.1016/J.JFF.2019.01.035. 10.1016/J.JFF.2019.01.035 [DOI] [Google Scholar]
- 135.Jiang H, Shang X, Wu H, Gautam SC, Al-Holou S, Li C, Kuo J, Zhang L, Chopp M. Resveratrol downregulates PI3K/Akt/MTOR signaling pathways in human U251 glioma cells. J Exp Ther Oncol. 2009;8:25–33. [PMC free article] [PubMed] [Google Scholar]
- 136.Anjum J, Mitra S, Das R, Alam R, Mojumder A, Emran TB, Khan H. A renewed concept on the MAPK signaling pathway in cancers: polyphenols as a choice of therapeutics. Pharmacol Res. 2022;184:106398. 10.1016/J.PHRS.2022.106398. 10.1016/J.PHRS.2022.106398 [DOI] [PubMed] [Google Scholar]
- 137.Islam F, Roy S, Zehravi M, Paul S, Sutradhar H, Yaidikar L, Kumar BR, Dogiparthi LK, Prema S, Nainu F, et al. Polyphenols targeting MAP kinase signaling pathway in neurological diseases: understanding molecular mechanisms and therapeutic targets. Mol Neurobiol. 2023. 10.1007/S12035-023-03706-Z. 10.1007/S12035-023-03706-Z [DOI] [PubMed] [Google Scholar]
- 138.Pacifici F, Salimei C, Pastore D, Malatesta G, Ricordi C, Donadel G, Bellia A, Rovella V, Tafani M, Garaci E, et al. The protective effect of a unique mix of polyphenols and micronutrients against neurodegeneration induced by an in vitro model of parkinson’s disease. Int J Mol Sci. 2022;23:3110. 10.3390/ijms23063110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hole KL, Williams RJ. Flavonoids as an intervention for Alzheimer’s disease: progress and hurdles towards defining a mechanism of action1. Brain Plast. 2020. 10.3233/bpl-200098. 10.3233/bpl-200098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hole KL, Williams RJ. Flavonoids as an intervention for Alzheimer’s disease: progress and hurdles towards defining a mechanism of action. Brain Plast. 2021;6:167–92. 10.3233/BPL-200098. 10.3233/BPL-200098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gao X, Cassidy A, Schwarzschild MA, Rimm EB, Ascherio A. Habitual intake of dietary flavonoids and risk of parkinson disease. Neurology. 2012;78:1138–45. 10.1212/WNL.0b013e31824f7fc4. 10.1212/WNL.0b013e31824f7fc4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Dinda B, Dinda M, Roy A, Dinda S. Dietary plant flavonoids in prevention of obesity and diabetes. Adv Protein Chem Struct Biol. 2020. 10.1016/bs.apcsb.2019.08.006. 10.1016/bs.apcsb.2019.08.006 [DOI] [PubMed] [Google Scholar]
- 143.Zhou M, Konigsberg WH, Hao C, Pan Y, Sun J, Wang X. Bioactivity and mechanisms of flavonoids in decreasing insulin resistance. J Enzyme Inhib Med Chem. 2023;38:2199168. 10.1080/14756366.2023.2199168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.He C, Liu X, Jiang Z, Geng S, Ma H, Liu B. Interaction mechanism of flavonoids and α-glucosidase: experimental and molecular modelling studies. 2019. Foods. 10.3390/foods8090355. 10.3390/foods8090355 [DOI] [PMC free article] [PubMed]
- 145.Barber E, Houghton MJ, Williamson G. Flavonoids as human intestinal α-glucosidase inhibitors. 2021. Foods. 10.3390/foods10081939. 10.3390/foods10081939 [DOI] [PMC free article] [PubMed]
- 146.Takahama U, Hirota S. Interactions of flavonoids with α-amylase and starch slowing down its digestion. Food Funct. 2018;9:677–87. 10.1039/C7FO01539A [DOI] [PubMed] [Google Scholar]
- 147.Ahn S, Jun S, Joung H. Association of total flavonoid intake with hypo- HDL-cholesterolemia among korean adults: effect modification by polyunsaturated fatty acid intake. Nutrients. 2020. 10.3390/nu12010195. 10.3390/nu12010195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bao L, Hu L, Zhang Y, Wang Y. Hypolipidemic effects of flavonoids extracted from lomatogonium rotatum. Exp Ther Med. 2016. 10.3892/etm.2016.3038. 10.3892/etm.2016.3038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Khalilpourfarshbafi M, Gholami K, Murugan DD, Abdul Sattar MZ, Abdullah NA. Differential effects of dietary flavonoids on adipogenesis. Eur J Nutr. 2019;58:5–25. 10.1007/s00394-018-1663-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhang X, Li X, Fang H, Guo F, Li F, Chen A, Huang S. Flavonoids as inducers of white adipose tissue browning and thermogenesis: signalling pathways and molecular triggers. Nutr Metab. 2019;16:1–15. 10.1186/s12986-019-0370-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kang HW, Lee SG, Otieno D, Ha K. Flavonoids, potential bioactive compounds, and non-shivering thermogenesis. Nutrients. 2018;10:1168. 10.3390/nu10091168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zhao X, An X, Yang C, Sun W, Ji H, Lian F. The crucial role and mechanism of insulin resistance in metabolic disease. Front Endocrinol (Lausanne). 2023;14:1149239. 10.3389/fendo.2023.1149239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sung CC, Liao MT, Lu KC, Wu CC. Role of vitamin D in insulin resistance. J Biomed Biotechnol. 2012;2012: 634195. 10.1155/2012/634195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ashraf A, Alvarez JA. Role of vitamin D in insulin secretion and insulin sensitivity for glucose homeostasis. Int J Endocrinol. 2010;2010: 351385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Pittas AG, Jorde R, Kawahara T, Dawson-Hughes B. Vitamin D supplementation for prevention of type 2 diabetes mellitus: to D or not to D? J Clin Endocrinol Metab. 2020;105:3721–33. 10.1210/clinem/dgaa594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Jain AB, Jain VA. Vitamin E, its beneficial role in diabetes mellitus (DM) and its complications. J Clin Diagnostic Res. 2012. 10.7860/JCDR/2012/4791.2625. 10.7860/JCDR/2012/4791.2625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Tobias DK, Luttmann-Gibson H, Mora S, Danik J, Bubes V, Copeland T, LeBoff MS, Cook NR, Lee IM, Buring JE, et al. Association of body weight with response to vitamin D supplementation and metabolism. JAMA Netw open. 2023. 10.1001/jamanetworkopen.2022.50681. 10.1001/jamanetworkopen.2022.50681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Manna P, Jain SK. Obesity, oxidative stress, adipose tissue dysfunction, and the associated health risks: causes and therapeutic strategies. Metab Syndr Relat Disord. 2015;13:423–44. 10.1089/met.2015.0095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Alcalá M, Sánchez-Vera I, Sevillano J, Herrero L, Serra D, Ramos MP, Viana M. Vitamin E reduces adipose tissue fibrosis, inflammation, and oxidative stress and improves metabolic profile in obesity. Obesity. 2015. 10.1002/oby.21135. 10.1002/oby.21135 [DOI] [PubMed] [Google Scholar]
- 160.Zhang Q, Guo M, Chen T, Cheng H, Yang Q, Zhao Z, She R, Yang X, Xiao W, Yang X, et al. Walking and taking vitamin C alleviates oxidative stress and inflammation in overweight students, even in the short-term. Front Public Heal. 2022. 10.3389/fpubh.2022.1024864. 10.3389/fpubh.2022.1024864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hanhineva K, Törrönen R, Bondia-Pons I, Pekkinen J, Kolehmainen M, Mykkänen H, Poutanen K. Impact of dietary polyphenols on carbohydrate metabolism. Int J Mol Sci. 2010;11:1365–402. 10.3390/ijms11041365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.de Paulo Farias D, de Araújo FF, Neri-Numa IA, Pastore GM. Antidiabetic potential of dietary polyphenols: a mechanistic review. Food Res Int. 2021;145:110383. 10.1016/j.foodres.2021.110383 [DOI] [PubMed] [Google Scholar]
- 163.Nie T, Cooper GJS. Mechanisms underlying the antidiabetic activities of polyphenolic compounds: a review. Front Pharmacol. 2021;12:798329. 10.3389/fphar.2021.798329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Krawczyk M, Burzynska-Pedziwiatr I, Wozniak LA, Bukowiecka-Matusiak M. Impact of polyphenols on inflammatory and oxidative stress factors in diabetes mellitus: nutritional antioxidants and their application in improving antidiabetic therapy. Biomolecules. 2023;13:1402. 10.3390/biom13091402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ćorković I, Gašo-Sokač D, Pichler A, Šimunović J, Kopjar M. Dietary polyphenols as natural inhibitors of α-Amylase and α-Glucosidase. Life. 2022;12:1692. 10.3390/life12111692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Riyaphan J, Pham DC, Leong MK, Weng CF. In silico approaches to identify polyphenol compounds as α-Glucosidase and α-Amylase inhibitors against type-ii diabetes. Biomolecules. 1877;2021:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.McArdle MA, Finucane OM, Connaughton RM, McMorrow AM, Roche HM. Mechanisms of obesity-induced inflammation and insulin resistance: insights into the emerging role of nutritional strategies. Front Endocrinol (Lausanne). 2013;4:52. 10.3389/fendo.2013.00052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Niewiadomska J, Gajek-Marecka A, Gajek J, Noszczyk-Nowak A. Biological potential of polyphenols in the context of metabolic syndrome: an analysis of studies on animal models. Biology (Basel). 2022;11:559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wang S, Moustaid-Moussa N, Chen L, Mo H, Shastri A, Su R, Bapat P, Kwun IS, Shen CL. Novel insights of dietary polyphenols and obesity. J Nutr Biochem. 2014;25:1–18. 10.1016/j.jnutbio.2013.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Singh M, Thrimawithana T, Shukla R, Adhikari B. Managing obesity through natural polyphenols: a review. Futur Foods. 2020;1:100002. 10.1016/j.fufo.2020.100002 [DOI] [Google Scholar]
- 171.Corrêa TAF, Rogero MM, Hassimotto NMA, Lajolo FM. The two-way polyphenols-microbiota interactions and their effects on obesity and related metabolic diseases. Front Nutr. 2019;6:188. 10.3389/fnut.2019.00188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ma G, Chen Y. Polyphenol supplementation benefits human health via gut microbiota: a systematic review via meta-analysis. J Funct Foods. 2020;66:103829. 10.1016/j.jff.2020.103829 [DOI] [Google Scholar]
- 173.Catalkaya G, Venema K, Lucini L, Rocchetti G, Delmas D, Daglia M, De Filippis A, Xiao H, Quiles JL, Xiao J, et al. Interaction of dietary polyphenols and gut microbiota: microbial metabolism of polyphenols, influence on the gut microbiota, and implications on host health. Food Front. 2020;1:109–33. 10.1002/fft2.25 [DOI] [Google Scholar]
- 174.Li J, Wu N, Chen X, Chen H, Yang X, Liu C. curcumin protects islet cells from glucolipotoxicity by inhibiting oxidative stress and NADPH oxidase activity both in vitro and in vivo. Islets. 2019. 10.1080/19382014.2019.1690944. 10.1080/19382014.2019.1690944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hussain Y, Khan H, Alotaibi G, Khan F, Alam W, Aschner M, Jeandet P, Saso L. How curcumin targets inflammatory mediators in diabetes: therapeutic insights and possible solutions. Molecules. 2022. 10.3390/molecules27134058. 10.3390/molecules27134058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Metawea MR, Abdelrazek HMA, El-Hak HNG, Moghazee MM, Marie OM. Comparative effects of curcumin versus nano-curcumin on histological, immunohistochemical expression, histomorphometric, and biochemical changes to pancreatic beta cells and lipid profile of streptozocin induced diabetes in male sprague-dawley rats. Environ Sci Pollut Res. 2023. 10.1007/s11356-023-26260-6. 10.1007/s11356-023-26260-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Abdul-Hamid M, Moustafa N. Protective effect of curcumin on histopathology and ultrastructure of pancreas in the alloxan treated rats for induction of diabetes. J Basic Appl Zool. 2013. 10.1016/j.jobaz.2013.07.003. 10.1016/j.jobaz.2013.07.003 [DOI] [Google Scholar]
- 178.Den Hartogh DJ, Gabriel A, Tsiani E. Antidiabetic properties of curcumin ii: evidence from in vivo studies. Nutrients. 2020;12:58. 10.3390/nu12010058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kasprzak-Drozd K, Oniszczuk T, Gancarz M, Kondracka A, Rusinek R, Oniszczuk A. Curcumin and weight loss: Does it work? Int J Mol Sci. 2022;23:639. 10.3390/ijms23020639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Xie XY, Kong PR, Wu JF, Li Y, Li YX. Curcumin attenuates lipolysis stimulated by tumor necrosis factor-α or isoproterenol in 3T3-L1 adipocytes. Phytomedicine. 2012. 10.1016/j.phymed.2012.09.003. 10.1016/j.phymed.2012.09.003 [DOI] [PubMed] [Google Scholar]
- 181.Pan Y, Zhao D, Yu N, An T, Miao J, Mo F, Gu Y, Zhang D, Gao S, Jiang G. Curcumin improves glycolipid metabolism through regulating peroxisome proliferator activated receptor γ signalling pathway in high-fat diet-induced obese mice and 3t3-L1 adipocytes. R Soc Open Sci. 2017. 10.1098/rsos.170917. 10.1098/rsos.170917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zhang DW, Fu M, Gao SH, Liu JL. Curcumin and diabetes: a systematic review evidence-based complement. Altern Med. 2013;2013: 636053. 10.1155/2013/636053. 10.1155/2013/636053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ghorbani Z, Hekmatdoost A, Mirmiran P. Anti-hyperglycemic and insulin sensitizer effects of turmeric and its principle constituent curcumin. Int J Endocrinol Metab. 2014;12:18081. 10.5812/IJEM.18081. 10.5812/IJEM.18081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zhong Y, Xiao Y, Gao J, Zheng Z, Zhang Z, Yao L, Li D. Curcumin improves insulin sensitivity in high-fat diet-fed mice through gut microbiota. Nutr Metab. 2022. 10.1186/s12986-022-00712-1. 10.1186/s12986-022-00712-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Eidi A, Eidi M, Esmaeili E. Antidiabetic effect of garlic (Allium Sativum L.) in normal and streptozotocin-induced diabetic rats. Phytomedicine. 2006. 10.1016/j.phymed.2005.09.010. 10.1016/j.phymed.2005.09.010 [DOI] [PubMed] [Google Scholar]
- 186.Thomson M, Al-Amin ZM, Al-Qattan KK, Shaban LH, Ali M. Anti-diabetic and hypolipidaemic properties of garlic (Allium Sativum) in streptozotocin-induced diabetic rats. Int J Diabetes Metab. 2019. 10.1159/000497643. 10.1159/000497643 [DOI] [Google Scholar]
- 187.Ansary J, Forbes-Hernández TY, Gil E, Cianciosi D, Zhang J, Elexpuru-Zabaleta M, Simal-Gandara J, Giampieri F, Battino M. Potential health benefit of garlic based on human intervention studies: a brief overview. Antioxidants. 2020;9:619. 10.3390/antiox9070619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Ribeiro M, Alvarenga L, Cardozo LF, Chermut TR, Sequeira J, Moreira LDSG, Mafra D. From the distinctive smell to therapeutic effects: garlic for cardiovascular, hepatic, gut, diabetes and chronic kidney disease. Clin Nutr. 2021;40:4807–19. 10.1016/j.clnu.2021.03.005 [DOI] [PubMed] [Google Scholar]
- 189.Liu CT, Hse H, Lii CK, Chen PS, Sheen LY. Effects of garlic oil and diallyl trisulfide on glycemic control in diabetic rats. Eur J Pharmacol. 2005. 10.1016/j.ejphar.2005.04.031. 10.1016/j.ejphar.2005.04.031 [DOI] [PubMed] [Google Scholar]
- 190.Wang J, Zhang X, Lan H, Wang W. Effect of garlic supplement in the management of type 2 diabetes mellitus (T2DM): a meta-analysis of randomized controlled trials. Food Nutr Res. 2017. 10.1080/16546628.2017.1377571. 10.1080/16546628.2017.1377571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Sadeghi M, Moradi M, Madanchi H, Johari B. In silico study of garlic (Allium Sativum L.)-derived compounds molecular interactions with α-Glucosidase. Silico Pharmacol. 2021. 10.1007/s40203-020-00072-9. 10.1007/s40203-020-00072-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Wu J, Wang Y, Li S, Wu T, Liu R, Sui W, Zhang M. Black garlic melanoidins inhibit in vitro α-amylase and α-glucosidase activity: the role of high-molecular-weight fluorescent compounds. J Sci Food Agric. 2023. 10.1002/jsfa.12634. 10.1002/jsfa.12634 [DOI] [PubMed] [Google Scholar]
- 193.Gadidala SK, Johny E, Thomas C, Nadella M, Undela K, Adela R. Effect of garlic extract on markers of lipid metabolism and inflammation in coronary artery disease (CAD) Patients: A systematic review and meta-analysis. Phyther Res. 2023. 10.1002/ptr.7729. 10.1002/ptr.7729 [DOI] [PubMed] [Google Scholar]
- 194.Chen K, Nakasone Y, Yi S, Ibrahim HR, Sakao K, Hossain MA, Hou DX. Natural garlic organosulfur compounds prevent metabolic disorder of lipid and glucose by increasing gut commensal bacteroides acidifaciens. J Agric Food Chem. 2022. 10.1021/acs.jafc.2c00555. 10.1021/acs.jafc.2c00555 [DOI] [PubMed] [Google Scholar]
- 195.Warshafsky S, Kamer RS, Sivak SL. Effect of garlic on total serum cholesterol: a meta-analysis. Ann Intern Med. 1993;119:599–605. 10.7326/0003-4819-119-7_Part_1-199310010-00009 [DOI] [PubMed] [Google Scholar]
- 196.Zupančič D, Korać-Prlić J, Kreft ME, Franković L, Vilović K, Jeruc J, Romih R, Terzić J. Vitamin A rich diet diminishes early urothelial carcinogenesis by altering retinoic acid signaling. Cancers (Basel). 2020. 10.3390/cancers12071712. 10.3390/cancers12071712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Tang JE, Wang RJ, Zhong H, Yu B, Chen Y. Vitamin A and risk of bladder cancer: a meta-analysis of epidemiological studies. World J Surg Oncol. 2014. 10.1186/1477-7819-12-130. 10.1186/1477-7819-12-130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Magrì A, Germano G, Lorenzato A, Lamba S, Chilà R, Montone M, Amodio V, Ceruti T, Sassi F, Arena S, et al. High-dose vitamin C enhances cancer immunotherapy. Sci Transl Med. 2020. 10.1126/scitranslmed.aay8707. 10.1126/scitranslmed.aay8707 [DOI] [PubMed] [Google Scholar]
- 199.Böttger F, Vallés-Martí A, Cahn L, Jimenez CR. High-dose intravenous vitamin C, a promising multi-targeting agent in the treatment of cancer. J Exp Clin Cancer Res. 2021;40:1–44. 10.1186/s13046-021-02134-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Mussa A, Mohd Idris RA, Ahmed N, Ahmad S, Murtadha AH, Tengku Din TADAA, Hassan R. High-dose vitamin C for cancer therapy. Pharmaceuticals. 2022;15:711. 10.3390/ph15060711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Satheesh NJ, Samuel SM, Büsselberg D. Combination therapy with vitamin C could eradicate cancer stem cells. Biomolecules. 2020;10:79. 10.3390/biom10010079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Zasowska-Nowak A, Nowak PJ, Ciałkowska-Rysz A. High-dose vitamin C in advanced-stage cancer patients. Nutrients. 2021;13:735. 10.3390/nu13030735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Abiri B, Vafa M. Vitamin C and cancer: the role of vitamin C in disease progression and quality of life in cancer patients. Nutr Cancer. 2021. 10.1080/01635581.2020.1795692. 10.1080/01635581.2020.1795692 [DOI] [PubMed] [Google Scholar]
- 204.Samuel S, Sitrin MD. Vitamin D’s role in cell proliferation and differentiation. Proc Nutr Rev. 2008;66:S116–24. 10.1111/j.1753-4887.2008.00094.x [DOI] [PubMed] [Google Scholar]
- 205.Chandler PD, Chen WY, Ajala ON, Hazra A, Cook N, Bubes V, Lee IM, Giovannucci EL, Willett W, Buring JE, et al. Effect of vitamin d3supplements on development of advanced cancer: a secondary analysis of the vital randomized clinical trial. JAMA Netw Open. 2020. 10.1001/jamanetworkopen.2020.25850. 10.1001/jamanetworkopen.2020.25850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Seraphin G, Rieger S, Hewison M, Capobianco E, Lisse TS. The impact of vitamin D on cancer: a mini review. J Steroid Biochem Mol Biol. 2023;231:106308. 10.1016/j.jsbmb.2023.106308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Jacobs ET, Kohler LN, Kunihiro AG, Jurutka PW. Vitamin D and colorectal, breast, and prostate cancers: a review of the epidemiological evidence. J Cancer. 2016;7:232. 10.7150/jca.13403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.El-Sharkawy A, Malki A. Vitamin D signaling in inflammation and cancer: molecular mechanisms and therapeutic implications. Molecules. 2020;25:3219. 10.3390/molecules25143219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Abraham A, Kattoor AJ, Saldeen T, Mehta JL. Vitamin E and its anticancer effects. Crit Rev Food Sci Nutr. 2019;59:2831–8. 10.1080/10408398.2018.1474169 [DOI] [PubMed] [Google Scholar]
- 210.Loh WQ, Youn J, Seow WJ. Vitamin E intake and risk of prostate cancer: a meta-analysis. Nutrients. 2023. 10.3390/nu15010014. 10.3390/nu15010014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.de Oliveira VA, Oliveira IKF, Pereira IC, Mendes LKF, da Silva FCC, Torres-Leal FL, de Azevedo Paiva A. Consumption and supplementation of vitamin E in breast cancer risk, treatment, and outcomes: a systematic review with meta-analysis. Clin Nutr ESPEN. 2023. 10.1016/j.clnesp.2023.01.032. 10.1016/j.clnesp.2023.01.032 [DOI] [PubMed] [Google Scholar]
- 212.Xie S, Tan M, Li H, Li L, Zhang H, Wang Q, Li S, Yang J, Xie H, Chen P, et al. Study on the correlation between B vitamins and breast cancer. Cancer Cell Int. 2023. 10.1186/s12935-023-02860-7. 10.1186/s12935-023-02860-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Peterson CT, Rodionov DA, Peterson SN, Osterman AL. B vitamins and their role in immune regulation and cancer. Nutrients. 2020;12:3380. 10.3390/nu12113380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Thabet RH, Alessa REM, Al-Smadi ZKK, Alshatnawi BSG, Amayreh BMI, Al-Dwaaghreh RBA, Salah SKA. Folic acid: friend or foe in cancer therapy. J Int Med Res. 2024. 10.1177/03000605231223064/ASSET/IMAGES/LARGE/10.1177_03000605231223064-FIG2.JPEG. 10.1177/03000605231223064/ASSET/IMAGES/LARGE/10.1177_03000605231223064-FIG2.JPEG [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Apostolou A, Stagos D, Galitsiou E, Spyrou A, Haroutounian S, Portesis N, Trizoglou I, Wallace Hayes A, Tsatsakis AM, Kouretas D. Assessment of polyphenolic content, antioxidant activity, protection against ROS-induced DNA damage and anticancer activity of vitis vinifera stem extracts. Food Chem Toxicol. 2013. 10.1016/j.fct.2013.01.029. 10.1016/j.fct.2013.01.029 [DOI] [PubMed] [Google Scholar]
- 216.Yoshioka Y, Ohishi T, Nakamura Y, Fukutomi R, Miyoshi N. Anti-cancer effects of dietary polyphenols via ROS-mediated pathway with their modulation of MicroRNAs. Molecules. 2022;27:3816. 10.3390/molecules27123816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Rajagopal C, Lankadasari MB, Aranjani JM, Harikumar KB. Targeting oncogenic transcription factors by polyphenols: a novel approach for cancer therapy. Pharmacol Res. 2018;130:273–91. 10.1016/j.phrs.2017.12.034 [DOI] [PubMed] [Google Scholar]
- 218.Fernandes R, Costa C, Fernandes R, Barros AN. Inflammation in prostate cancer: exploring the promising role of phenolic compounds as an innovative therapeutic approach. Biomedicines. 2023;11:3140. 10.3390/biomedicines11123140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Kang NJ, Shin SH, Lee HJ, Lee KW. Polyphenols as small molecular inhibitors of signaling cascades in carcinogenesis. Pharmacol Ther. 2011;130:310–24. 10.1016/j.pharmthera.2011.02.004 [DOI] [PubMed] [Google Scholar]
- 220.Kronski E, Fiori ME, Barbieri O, Astigiano S, Mirisola V, Killian PH, Bruno A, Pagani A, Rovera F, Pfeffer U, et al. MiR181b is induced by the chemopreventive polyphenol curcumin and inhibits breast cancer metastasis via down-regulation of the inflammatory cytokines CXCL1 and -2. Mol Oncol. 2014. 10.1016/j.molonc.2014.01.005. 10.1016/j.molonc.2014.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Singh N, Zaidi D, Shyam H, Sharma R, Balapure AK. Polyphenols sensitization potentiates susceptibility of Mcf-7 and Mda Mb-231 cells to centchroman. PLoS ONE. 2012. 10.1371/journal.pone.0037736. 10.1371/journal.pone.0037736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Cipolletti M, Fernandez VS, Montalesi E, Marino M, Fiocchetti M. Beyond the antioxidant activity of dietary polyphenols in cancer: the modulation of estrogen receptors (ERs) signaling. Int J Mol Sci. 2018;19:2624. 10.3390/ijms19092624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Kopustinskiene DM, Jakstas V, Savickas A, Bernatoniene J. Flavonoids as anticancer agents. Nutrients. 2020;12:457. 10.3390/nu12020457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Chang M, Zhu D, Chen Y, Zhang W, Liu X, Li XL, Cheng Z, Su Z, Zhang J, Lu Y, et al. Total flavonoids of litchi seed attenuate prostate cancer progression via inhibiting AKT/MTOR and NF-KB signaling pathways. Front Pharmacol. 2021. 10.3389/fphar.2021.758219. 10.3389/fphar.2021.758219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Zughaibi TA, Suhail M, Tarique M, Tabrez S. Targeting PI3K/Akt/MTOR pathway by different flavonoids: a cancer chemopreventive approach. Int J Mol Sci. 2021;22:12455. 10.3390/ijms222212455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Fadus MC, Lau C, Bikhchandani J, Lynch HT. Curcumin: an age-old anti-inflammatory and anti-neoplastic agent. J Tradit Complement Med. 2017;7:339–46. 10.1016/j.jtcme.2016.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Peng Y, Ao M, Dong B, Jiang Y, Yu L, Chen Z, Hu C, Xu R. Anti-inflammatory effects of curcumin in the inflammatory diseases: status, limitations and countermeasures. Drug Des Devel Ther. 2021;15:4503–25. 10.2147/DDDT.S327378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Hao J, Dai X, Gao J, Li Y, Hou Z, Chang Z, Wang Y. Curcumin suppresses colorectal tumorigenesis via the Wnt/β-catenin signaling pathway by downregulating axin2. Oncol Lett. 2021. 10.3892/ol.2021.12447. 10.3892/ol.2021.12447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Sathyabhama M, Priya Dharshini LC, Karthikeyan A, Kalaiselvi S, Min T. The credible role of curcumin in oxidative stress-mediated mitochondrial dysfunction in mammals. Biomolecules. 2022;12:1405. 10.3390/biom12101405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Gupta N, Verma K, Nalla S, Kulshreshtha A, Lall R, Prasad S. Free radicals as a double-edged sword: the cancer preventive and therapeutic roles of curcumin. Molecules. 2020;25:5390. 10.3390/molecules25225390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Farnood PR, Pazhooh RD, Asemi Z, Yousefi B. Targeting signaling pathway by curcumin in osteosarcoma. Curr Mol Pharmacol. 2022. 10.2174/1874467215666220408104341. 10.2174/1874467215666220408104341 [DOI] [PubMed] [Google Scholar]
- 232.Tewari D, Bawari S, Sharma S, DeLiberto LK, Bishayee A. Targeting the crosstalk between canonical Wnt/β-catenin and inflammatory signaling cascades: a novel strategy for cancer prevention and therapy. Pharmacol Ther. 2021;227:107876. 10.1016/j.pharmthera.2021.107876 [DOI] [PubMed] [Google Scholar]
- 233.Basak S, Srinivas V, Mallepogu A, Duttaroy AK. Curcumin stimulates angiogenesis through VEGF and expression of HLA-G in first-trimester human placental trophoblasts. Cell Biol Int. 2020. 10.1002/cbin.11324. 10.1002/cbin.11324 [DOI] [PubMed] [Google Scholar]
- 234.Cianfruglia L, Minnelli C, Laudadio E, Scirè A, Armeni T. Side effects of curcumin: epigenetic and antiproliferative implications for normal dermal fibroblast and breast cancer cells. Antioxidants. 2019. 10.3390/antiox8090382. 10.3390/antiox8090382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Li Z, Le W, Cui Z. A novel therapeutic anticancer property of raw garlic extract via injection but not ingestion. Cell Death Discov. 2018. 10.1038/s41420-018-0122-x. 10.1038/s41420-018-0122-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Pandey P, Khan F, Alshammari N, Saeed A, Aqil F, Saeed M. Updates on the anticancer potential of garlic organosulfur compounds and their nanoformulations: plant therapeutics in cancer management. Front Pharmacol. 2023;14:1154034. 10.3389/fphar.2023.1154034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Altonsy MO, Habib TN, Andrews SC. Diallyl disulfide-induced apoptosis in a breast-cancer cell line (MCF-7) may be caused by inhibition of histone deacetylation. Nutr Cancer. 2012. 10.1080/01635581.2012.721156. 10.1080/01635581.2012.721156 [DOI] [PubMed] [Google Scholar]
- 238.Lee EN, Choi YW, Kim HK, Park JK, Kim HJ, Kim MJ, Lee HW, Kim KH, Bae SS, Kim BS, et al. Chloroform extract of aged black garlic attenuates TNF-α-induced ROS generation, VCAM-1 expression, NF-ΚB activation and adhesiveness for monocytes in human umbilical vein endothelial cells. Phyther Res. 2011. 10.1002/ptr.3230. 10.1002/ptr.3230 [DOI] [PubMed] [Google Scholar]
- 239.Kong F, Lee BH, Wei K. 5-Hydroxymethylfurfural mitigates lipopolysaccharide-stimulated inflammation via suppression of MAPK, NF-ΚB and MTOR activation in RAW 264.7 Cells. Molecules. 2019. 10.3390/molecules24020275. 10.3390/molecules24020275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Arreola R, Quintero-Fabián S, López-Roa R, Flores-Gutiérrez E, Reyes-Grajeda J, Carrera-Quintanar L, Ortuño-Sahagún D. Immunomodulation and anti-inflammatory effects of garlic compounds: discovery service for endeavour college of natural health library. J Immunol Res. 2015;2015:401630. 10.1155/2015/401630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Schäfer G, Kaschula C. The immunomodulation and anti-inflammatory effects of garlic organosulfur compounds in cancer chemoprevention. Anticancer Agents Med Chem. 2014. 10.2174/18715206113136660370. 10.2174/18715206113136660370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Jasamai M, Hui CS, Azmi N, Kumolosasi E. Effect of Allium Sativum (Garlic) methanol extract on viability and apoptosis of human leukemic cell lines. Trop J Pharm Res. 2016. 10.4314/tjpr.v15i7.18. 10.4314/tjpr.v15i7.18 [DOI] [Google Scholar]
- 243.Farzanegi P, Abbaszadeh H, Farokhi F, Rahmati-Ahmadabad S, Hosseini SA, Ahmad A, Mazandarani MR, Rezaei I, Shokrie M, Vizvari E, et al. Attenuated renal and hepatic cells apoptosis following swimming exercise supplemented with garlic extract in old rats. Clin Interv Aging. 2020. 10.2147/CIA.S250321. 10.2147/CIA.S250321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Özkan İ, Koçak P, Yıldırım M, Ünsal N, Yılmaz H, Telci D, Şahin F. Garlic (Allium Sativum)-derived SEVs inhibit cancer cell proliferation and induce caspase mediated apoptosis. Sci Rep. 2021. 10.1038/s41598-021-93876-4. 10.1038/s41598-021-93876-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Upadhyay RK. Garlic induced apoptosis, cell cycle check points and inhibition of cancer cell proliferation. J Cancer Res Treat. 2017;5:35–54. [Google Scholar]
- 246.Zhu Z, Liao L, Gao M, Liu Q. Garlic-derived exosome-like nanovesicles alleviate dextran sulphate sodium-induced mouse colitis via the TLR4/MyD88/NF-ΚB pathway and gut microbiota modulation. Food Funct. 2023. 10.1039/d3fo01094e. 10.1039/d3fo01094e [DOI] [PubMed] [Google Scholar]
- 247.Chang Z, An L, He Z, Zhang Y, Li S, Lei M, Xu P, Lai Y, Jiang Z, Huang Y, et al. Allicin suppressed escherichia coli-induced urinary tract infections by a novel MALT1/NF-ΚB pathway. Food Funct. 2022. 10.1039/d1fo03853b. 10.1039/d1fo03853b [DOI] [PubMed] [Google Scholar]
- 248.Shao X, Li J, Zhang H, Zhang X, Sun C, Ouyang X, Wang Y, Wu X, Chen C. Anti-inflammatory effects and molecular mechanisms of bioactive small molecule garlic polysaccharide. Front Nutr. 2023. 10.3389/fnut.2022.1092873. 10.3389/fnut.2022.1092873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Saud SM, Li W, Gray Z, Matter MS, Colburn NH, Young MR, Kim YS. Diallyl disulfide (DADS), a constituent of garlic, inactivates NF-ΚB and prevents colitis-induced colorectal cancer by inhibiting GSK-3β. Cancer Prev Res. 2016. 10.1158/1940-6207.CAPR-16-0044. 10.1158/1940-6207.CAPR-16-0044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Sen S, Chakraborty R. The role of antioxidants in human health. ACS Symp Ser. 2011. 10.1021/bk-2011-1083.ch001. 10.1021/bk-2011-1083.ch001 [DOI] [Google Scholar]
- 251.Gao S, Hu M. Bioavailability challenges associated with development of anti-cancer phenolics. Mini-Rev Med Chem. 2010. 10.2174/138955710791384081. 10.2174/138955710791384081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Żyżelewicz D, Oracz J. Bioavailability and bioactivity of plant antioxidants. Antioxidants. 2022;11:2336. 10.3390/antiox11122336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Meulmeester FL, Luo J, Martens LG, Mills K, van Heemst D, Noordam R. Antioxidant supplementation in oxidative stress-related diseases: What have we learned from studies on alpha-tocopherol? Antioxidants. 2022;11:2322. 10.3390/antiox11122322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Jomova K, Raptova R, Alomar SY, Alwasel SH, Nepovimova E, Kuca K, Valko M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: chronic diseases and aging. Arch Toxicol. 2023;97:2499–574. 10.1007/s00204-023-03562-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Schmidt HHHW, Stocker R, Vollbracht C, Paulsen G, Riley D, Daiber A, Cuadrado A. Antioxidants in translational medicine. Antioxidants Redox Signal. 2015. 10.1089/ars.2015.6393. 10.1089/ars.2015.6393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Paller CJ, Denmeade SR, Carducci MA. Challenges of conducting clinical trials of natural products to combat cancer. Clin Adv Hematol Oncol. 2016;14:447–55. [PMC free article] [PubMed] [Google Scholar]
- 257.WHO Expert Committee on Specifications for Pharmaceutical Preparations Good Regulatory Practices in the Regulation of Medical Products. WHO - Tech. Rep. Ser. 10332021, 269–304.
- 258.Zhang H, Zhou L, Davies KJA, Forman HJ. Silencing Bach1 alters aging-related changes in the expression of Nrf2-regulated genes in primary human bronchial epithelial cells. Arch Biochem Biophys. 2019;672:108074. 10.1016/j.abb.2019.108074 [DOI] [PubMed] [Google Scholar]
- 259.Surh YJ, Kundu JK, Na HK, Lee JS. Redox-sensitive transcription factors as prime targets for chemoprevention with anti-inflammatory and antioxidative phytochemicals. J Nutr. 2005;135:2993S-3001S. 10.1093/jn/135.12.2993S [DOI] [PubMed] [Google Scholar]
- 260.Bansal A, Celeste Simon M. Glutathione metabolism in cancer progression and treatment resistance. J Cell Biol. 2018;217:2291. 10.1083/jcb.201804161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Wiel C, Le Gal K, Ibrahim MX, Jahangir CA, Kashif M, Yao H, Ziegler DV, Xu X, Ghosh T, Mondal T, et al. BACH1 stabilization by antioxidants stimulates lung cancer metastasis. Cell. 2019. 10.1016/j.cell.2019.06.005. 10.1016/j.cell.2019.06.005 [DOI] [PubMed] [Google Scholar]
- 262.Sayin VI, Ibrahim MX, Larsson E, Nilsson JA, Lindahl P, Bergo MO. Cancer: antioxidants accelerate lung cancer progression in mice. Sci Transl Med. 2014. 10.1126/scitranslmed.3007653. 10.1126/scitranslmed.3007653 [DOI] [PubMed] [Google Scholar]
- 263.Calderaro A, Patanè GT, Tellone E, Barreca D, Ficarra S, Misiti F, Laganà G. The neuroprotective potentiality of flavonoids on Alzheimer’s disease. Int J Mol Sci. 2022;23:14835. 10.3390/ijms232314835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Sano M, Ernesto C, Thomas RG, Klauber MR, Schafer K, Grundman M, Woodbury P, Growdon J, Cotman CW, Pfeiffer E, et al. A controlled trial of selegiline, alpha-tocopherol, or both as treatment for Alzheimer’s disease. N Engl J Med. 1997. 10.1056/nejm199704243361704. 10.1056/nejm199704243361704 [DOI] [PubMed] [Google Scholar]
- 265.Bin Q, Hu X, Cao Y, Gao F. The role of vitamin E (Tocopherol) supplementation in the prevention of stroke: a meta-analysis of 13 randomised controlled trials. Thromb Haemost. 2011. 10.1160/TH10-11-0729. 10.1160/TH10-11-0729 [DOI] [PubMed] [Google Scholar]
- 266.Polidori M, Nelles G. Antioxidant clinical trials in mild cognitive impairment and Alzheimer’s disease – challenges and perspectives. Curr Pharm Des. 2014. 10.2174/13816128113196660706. 10.2174/13816128113196660706 [DOI] [PubMed] [Google Scholar]
- 267.Elmund B, Hartrianti P. Evaluation of mangosteen (Garcinia Mangostana) antioxidant activity in clinical trials and in vivo animal studies: a systematic review. J Appl Pharm Sci. 2020. 10.7324/JAPS.2020.101216. 10.7324/JAPS.2020.101216 [DOI] [Google Scholar]
- 268.Marmitt DJ, Bitencourt S, da Silva GR, Rempel C, Goettert MI. Traditional plants with antioxidant properties in clinical trials—a systematic review. Phyther Res. 2021;35:5647–67. 10.1002/ptr.7202 [DOI] [PubMed] [Google Scholar]
- 269.Raijmakers MTM, Dechend R, Poston L. Oxidative stress and preeclampsia: rationale for antioxidant clinical trials. Hypertension. 2004;44:374–80. 10.1161/01.HYP.0000141085.98320.01 [DOI] [PubMed] [Google Scholar]
- 270.Mecocci P, Polidori MC. Antioxidant clinical trials in mild cognitive impairment and Alzheimer’s disease. Biochim Biophys Acta - Mol Basis Dis. 2012;1822:631–8. 10.1016/j.bbadis.2011.10.006 [DOI] [PubMed] [Google Scholar]
- 271.Sies H, Berndt C, Jones DP. Oxidative stress. Annu Rev Biochem. 2017. 10.1146/annurev-biochem-061516-045037. 10.1146/annurev-biochem-061516-045037 [DOI] [PubMed] [Google Scholar]
- 272.Ren H, Yu H, Zhang S, Liang S, Zheng X, Zhang S, Yao P, Zheng H, Qi X. Genome sequencing provides insights into the evolution and antioxidant activity of Chinese bayberry. BMC Genom. 2019. 10.1186/s12864-019-5818-7. 10.1186/s12864-019-5818-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Yu L, Diao S, Zhang G, Yu J, Zhang T, Luo H, Duan A, Wang J, He C, Zhang J. Genome sequence and population genomics provide insights into chromosomal evolution and phytochemical innovation of hippophae rhamnoides. Plant Biotechnol J. 2022. 10.1111/pbi.13802. 10.1111/pbi.13802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Agyeman AS, Chaerkady R, Shaw PG, Davidson NE, Visvanathan K, Pandey A, Kensler TW. Transcriptomic and proteomic profiling of KEAP1 disrupted and sulforaphane-treated human breast epithelial cells reveals common expression profiles. Breast Cancer Res Treat. 2012. 10.1007/s10549-011-1536-9. 10.1007/s10549-011-1536-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Gupta R, Srivastava D, Sahu M, Tiwari S, Ambasta RK, Kumar P. Artificial intelligence to deep learning: machine intelligence approach for drug discovery. Mol Divers. 2021. 10.1007/s11030-021-10217-3. 10.1007/s11030-021-10217-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Kumari Patial P, Sud D. Bioactive phytosteroids from Araucaria Columnaris (G. Forst.) Hook: RP-HPLC-DAD analysis, in-vitro antioxidant potential, in-silico computational study and molecular docking against 3MNG and 1N3U. Steroids. 2022. 10.1016/j.steroids.2022.109116. 10.1016/j.steroids.2022.109116 [DOI] [PubMed] [Google Scholar]
- 277.Zu G, Sun K, Li L, Zu X, Han T, Huang H. Mechanism of quercetin therapeutic targets for Alzheimer disease and Type 2 diabetes mellitus. Sci Rep. 2021. 10.1038/s41598-021-02248-5. 10.1038/s41598-021-02248-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Idowu SO, Fatokun AA. Artificial intelligence (AI) to the rescue: deploying machine learning to bridge the biorelevance gap in antioxidant assays. SLAS Technol. 2021;26:16–25. 10.1177/2472630320962716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Wang H, Fu T, Du Y, Gao W, Huang K, Liu Z, Chandak P, Liu S, Van Katwyk P, Deac A, et al. Scientific discovery in the age of artificial intelligence. Nature. 2023;620:47–60. 10.1038/s41586-023-06221-2 [DOI] [PubMed] [Google Scholar]
- 280.Janairo JIB. Machine learning for the cleaner production of antioxidant peptides. Int J Pept Res Ther. 2021. 10.1007/s10989-021-10232-w. 10.1007/s10989-021-10232-w [DOI] [Google Scholar]
- 281.Alam S, Khan F. 3D-QSAR, docking, ADME/Tox studies on flavone analogs reveal anticancer activity through tankyrase inhibition. Sci Rep. 2019. 10.1038/s41598-019-41984-7. 10.1038/s41598-019-41984-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Zhang Y, Li Y, Quan Z, Xiao P, Duan J-A. New insights into antioxidant peptides: an overview of efficient screening, evaluation models, molecular mechanisms, and applications. Antioxidants. 2024;13:203. 10.3390/antiox13020203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Tragni V, Primiano G, Tummolo A, Beltrame L, Lapiana G, Sgobba M, Cavalluzzi M, Paterno G, Gorgoglione R, Volpicella M, et al. Personalized medicine in mitochondrial health and disease: molecular basis of therapeutic approaches based on nutritional supplements and their analogs. Molecules. 2022;27(11):3494. 10.3390/molecules27113494. 10.3390/molecules27113494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Yanpanitch OU, Hatairaktham S, Charoensakdi R, Panichkul N, Fucharoen S, Srichairatanakool S, Siritanaratkul N, Kalpravidh RW. Treatment of β -Thalassemia/Hemoglobin e with antioxidant cocktails results in decreased oxidative stress, increased hemoglobin concentration, and improvement of the hypercoagulable state. Oxid Med Cell Longev. 2015;2015(1): 537954. 10.1155/2015/537954. 10.1155/2015/537954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Sergouniotis PI, Fitzgerald T, Birney E. From genetic variation to precision medicine. Cambridge Prism Precis Med. 2023;1: e7. 10.1017/pcm.2022.11. 10.1017/pcm.2022.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Ulasov AV, Rosenkranz AA, Georgiev GP, Sobolev AS. Nrf2/Keap1/ARE signaling: towards specific regulation. Life Sci. 2022;291: 120111. 10.1016/j.lfs.2021.120111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Wang H, Wang B, Wei J, Zheng Z, Su J, Bian C, Xin Y, Jiang X. Sulforaphane regulates Nrf2-mediated antioxidant activity and downregulates TGF-Β1/Smad pathways to prevent radiation-induced muscle fibrosis. Life Sci. 2022;311: 121197. 10.1016/j.lfs.2022.121197. 10.1016/j.lfs.2022.121197 [DOI] [PubMed] [Google Scholar]
- 288.Zhou DD, Luo M, Shang A, Mao QQ, Li BY, Gan RY, Li H. Bin antioxidant food components for the prevention and treatment of cardiovascular diseases: effects, mechanisms, and clinical studies. Oxid Med Cell Longev. 2021;2021(1):6627355. 10.1155/2021/6627355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Jakubiak GK, Osadnik K, Lejawa M, Osadnik T, Goławski M, Lewandowski P, Pawlas N. “Obesity and insulin resistance” is the component of the metabolic syndrome most strongly associated with oxidative stress. Antioxidants. 2022;11(1):79. 10.3390/antiox11010079. 10.3390/antiox11010079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Ávila-Escalante ML, Coop-Gamas F, Cervantes-Rodríguez M, Méndez-Iturbide D, Aranda-González II. The effect of diet on oxidative stress and metabolic diseases—clinically controlled trials. J Food Biochem. 2020;44(5): e13191. 10.1111/jfbc.13191 [DOI] [PubMed] [Google Scholar]
- 291.Tun S, Spainhower CJ, Cottrill CL, Lakhani HV, Pillai SS, Dilip A, Chaudhry H, Shapiro JI, Sodhi K. Therapeutic efficacy of antioxidants in ameliorating obesity phenotype and associated comorbidities. Front Pharmacol. 2020;11:1234. 10.3389/fphar.2020.01234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Moloney, J.N.; Cotter, T.G. ROS Signalling in the Biology of Cancer. In: Semin. Cell Dev. Biol. 2018, 80. [DOI] [PubMed]
- 293.Hayes JD, Dinkova-Kostova AT, Tew KD. Oxidative stress in cancer. Cancer Cell. 2020;38(2):167–97. 10.1016/j.ccell.2020.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.George S, Abrahamse H. Redox potential of antioxidants in cancer progression and prevention. Antioxidants. 2020;9:1156. 10.3390/antiox9111156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Slika H, Mansour H, Wehbe N, Nasser SA, Iratni R, Nasrallah G, Shaito A, Ghaddar T, Kobeissy F, Eid AH. Therapeutic potential of flavonoids in cancer: ROS-mediated mechanisms. Biomed Pharmacother. 2022;146:112442. 10.1016/j.biopha.2021.112442 [DOI] [PubMed] [Google Scholar]
- 296.Michalak M, Pierzak M, Kręcisz B, Suliga E. Bioactive compounds for skin health: a review. Nutrients. 2021;13:203. 10.3390/nu13010203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Azevedo Martins TE, de Oliveira S, Pinto CA, Costa de Oliveira A, Robles Velasco MV, Gorriti Guitiérrez AR, Cosquillo Rafael MF, Retuerto-Figueroa MG. Contribution of topical antioxidants to maintain healthy skin—a review. Sci Pharm. 2020;88:27. 10.3390/scipharm88020027 [DOI] [Google Scholar]
- 298.Tavassolifar MJ, Vodjgani M, Salehi Z, Izad M. The influence of reactive oxygen species in the immune system and pathogenesis of multiple sclerosis. Autoimmune Dis. 2020;2020:5793817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Zhao Y, Dai C, Wang Z, Chen W, Liu J, Zhuo R, Yu A, Huang S. A novel curcumin-loaded composite dressing facilitates wound healing due to its natural antioxidant effect. Drug Des Devel Ther. 2019. 10.2147/DDDT.S219224. 10.2147/DDDT.S219224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Vaiserman A, Koliada A, Zayachkivska A, Lushchak O. Nanodelivery of natural antioxidants: an anti-aging perspective. Front Bioeng Biotechnol. 2020;7:447. 10.3389/fbioe.2019.00447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Akbari B, Baghaei-Yazdi N, Bahmaie M, Mahdavi Abhari F. The role of plant-derived natural antioxidants in reduction of oxidative stress. BioFactors. 2022;48:611–33. 10.1002/biof.1831 [DOI] [PubMed] [Google Scholar]
- 302.Jideani AIO, Silungwe H, Takalani T, Omolola AO, Udeh HO, Anyasi TA. Antioxidant-rich natural fruit and vegetable products and human health. Int J Food Prop. 2021;24:41–67. 10.1080/10942912.2020.1866597 [DOI] [Google Scholar]
- 303.Shah AK, Dhalla NS. Effectiveness of some vitamins in the prevention of cardiovascular disease: a narrative review. Front Physiol. 2021;12:729255. 10.3389/fphys.2021.729255 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- He C, Liu X, Jiang Z, Geng S, Ma H, Liu B. Interaction mechanism of flavonoids and α-glucosidase: experimental and molecular modelling studies. 2019. Foods. 10.3390/foods8090355. 10.3390/foods8090355 [DOI] [PMC free article] [PubMed]
- Barber E, Houghton MJ, Williamson G. Flavonoids as human intestinal α-glucosidase inhibitors. 2021. Foods. 10.3390/foods10081939. 10.3390/foods10081939 [DOI] [PMC free article] [PubMed]
Data Availability Statement
No datasets were generated or analysed during the current study.