Abstract
Background
In the recent decade, there has been substantial progress in the technologies and philosophies associated with diagnosing and treating anterior cruciate ligament (ACL) injuries in China. The therapeutic efficacy of ACL reconstruction in re-establishing the stability of the knee joint has garnered widespread acknowledgment. However, the path toward standardizing diagnostic and treatment protocols remains to be further developed and refined.
Objective
In this context, the Chinese Association of Orthopaedic Surgeons (CAOS) and the Chinese Society of Sports Medicine (CSSM) collaboratively developed an expert consensus on diagnosing and treating ACL injury, aiming to enhance medical quality through refining professional standards.
Methods
The consensus drafting team invited experts across the Greater China region, including the mainland, Hong Kong, Macau, and Taiwan, to formulate and review the consensus using a modified Delphi method as a standardization approach. As members of the CSSM Lower Limb Study Group and the CAOS Arthroscopy and Sports Medicine Study Group, invited experts concentrated on two pivotal issues: “Graft Selection” and “Clinical Outcome Evaluation” during the second part of the consensus development.
Results
This focused discussion ultimately led to a strong consensus on nine specific consensus terms.
Conclusion
The consensus clearly states that ACL reconstruction has no definitive “gold standard” graft choice. Autografts have advantages in healing capability but are limited in availability and have potential donor site morbidities; allografts reduce surgical trauma but incur additional costs, and there are concerns about slow healing, quality control issues, and a higher failure rate in young athletes; synthetic ligaments allow for early rehabilitation and fast return to sport, but the surgery is technically demanding and incurs additional costs. When choosing a graft, one should comprehensively consider the graft's characteristics, the doctor's technical ability, and the patient's needs. When evaluating clinical outcomes, it is essential to ensure an adequate sample size and follow-up rate, and the research should include patient subjective scoring, joint function and stability, complications, surgical failure, and the return to sport results. Medium and long-term follow-ups should not overlook the assessment of knee osteoarthritis.
Keywords: Anterior cruciate ligament, Clinical outcome evaluation, Expert consensus, Graft choice
Graphical abstract

The translational potential of this article
This study summarizes the consensus among Chinese experts on the nuances of treatment for ACL injuries, focusing specifically on graft selection and clinical efficacy evaluation, demonstrating significant multidimensional translational potential.
-
1)
Guidance for Clinical Decision-Making: By providing an in-depth evaluation of the types of grafts used for ACL reconstruction and discussing their advantages and limitations, this consensus offers scientific guidance for orthopedic sports physicians to make precise decisions based on individual patient needs, potentially enhancing surgical outcomes and patient satisfaction.
-
2)
Advancement of Policies and Standardization: Summarizing the professional consensus within the Greater China region, this research lays the foundation for the formulation of related policies and the development of standardized processes for ACL injury treatment. International Sports Medicine Societies could utilize this consensus to refine guiding principles, further improving the quality and consistency of care for patients with ACL injuries.
-
3)
Stimulation of Future Research: The identification of knowledge gaps and controversies in graft selection and effectiveness evaluation highlights the urgent need for in-depth research. Specifically, this study may generate targeted new research topics on novel grafts and the long-term effects and comprehensive evaluations, especially for using synthetic and allografts.
-
4)
Promotion of Education and Training: The detailed analysis and expert consensus provided in this article will become a valuable resource for standardized training and education for residents, graduate students, and practicing surgeons. The collective wisdom of Chinese experts will enrich training courses, providing the next generation of sports medicine physicians with the latest knowledge on ACL reconstruction.
In summary, this expert consensus aims to refine clinical decision-making, standardize treatment processes, promote emerging research, and advance medical education, thereby providing new perspectives for personalized treatment of ACL injuries, enhancement of surgical outcomes, and increased patient satisfaction. It also paves the way for future research and the development of global treatment strategies.
1. Background
Over the past decade, sports medicine in China has made significant theoretical and practical advancements, particularly in anterior cruciate ligament (ACL) diagnosis and treatment. The ACL is integral to knee stability, and its impairment sometimes necessitates reconstruction surgery to restore knee stability and lower limb function, aiding patients in resuming physical activities and enhancing their quality of life. According to Medline data, since 2010, Chinese scholars have published over 2000 papers related to ACL, surpassing the output of many Western countries. The research contributions of Chinese scholars have gained extensive recognition in the international academic community, with frequent invitations to present at global forums and a large number of publications in peer-reviewed journals. Certain studies have demonstrated unique Chinese characteristics, such as applying synthetic ligaments in ACL reconstruction, which has garnered widespread attention from international peers. However, severe challenges have also emerged with the rapid development of Chinese sports medicine. Particularly in ACL diagnosis and treatment, there is a distinct lack of clear, standardized guidelines in our country, which not only limits professional development but also presents an urgent demand for improving the quality of medical care.
Choosing an optimal graft constitutes a critical aspect of ACL reconstruction. Variations in biomechanical properties and dimensions among different grafts necessitate distinct management approaches and entail varying risks of complications, thereby significantly impacting the trajectory of postoperative rehabilitation. An appropriate graft not only enhances the success rate of the surgery and reduces the risk of complications but also establishes favorable conditions for postoperative rehabilitation. In China, grafts used for ACL reconstruction exhibit two main characteristics: universality and specificity. From the perspective of universality, autografts, due to their biocompatibility and efficacy, are extensively utilized in ACL reconstruction despite constraints such as limited source, donor site morbidity, restricted mechanical strength, and a specific rate of failure. From the viewpoint of specificity, China is confronted with unique challenges and opportunities. Commercial tissue bank resources are primarily deficient, and human tissue donation is not mandatory, with related practices still requiring widespread adoption. This situation restricts access to high-quality allogeneic tissues. Against this backdrop, the clinical application of synthetic ligaments has surged forward, becoming increasingly common and achieving initial success.
It should be noted that any controversy on graft selection may inevitably disrupt the establishment of standardized guidelines for ACL reconstruction. Such debates can lead to confusion in choices for some doctors and patients, potentially adversely affecting the anticipated outcomes and safety of the surgery. The presence of these debates does not equate to a lack of practice guidance. Suppose the professional community can converge on uniform guidance for these critical points of contention. In that case, it will provide clear direction for graft selection and enhance the quality of ACL injury treatment in China.
Clinical outcome evaluation is essential for analyzing the outcomes of ACL reconstruction and guiding graft selection. Its fundamental value lies in accurately evaluating joint stability and functional recovery and facilitating the early identification and resolution of complications such as infection and joint stiffness. Through outcome evaluation, physicians and rehabilitation therapists can more accurately track patient recovery progress and ensure targeted rehabilitation strategies are implemented. Finally, clinical outcome evaluation provides a scientific basis for decision-making when considering a patient's return to sport. Currently, Chinese overall development level of sports medicine is uneven, with significant disparities in clinical efficacy assessments of ACL reconstruction among different institutions. These disparities are primarily manifested in the non-standardization of selection criteria for assessment indicators, the lack of a unified basis for case screening and sample size calculation, and the irregularity concerning evaluation time points and follow-up periods. The lack of unified clinical efficacy evaluation standards has introduced a series of complex issues in academic research and clinical practice. Firstly, non-standardization might cause discrepancies in research interpretation, impacting academic integrity and precision. Secondly, such inconsistency can prompt physician diagnostic and treatment disagreements, potentially threatening healthcare quality. Moreover, patients receiving inconsistent information might experience confusion, leading to doubts about their future medical care.
To address several critical issues related to the diagnosis and treatment of ACL injuries, the Chinese Association of Orthopaedic Surgeons (CAOS) Arthroscopy and Sports Medicine Study Group and the Chinese Society of Sports Medicine (CSSM) Lower Limb Study Group have decided to initiate discussions and develop an expert consensus. The objective is to positively influence the level of practitioners by introducing detailed practice standards and guiding opinions, thereby facilitating advancements in professional practice. The consensus drafting group, adhering to the modified Delphi method as the standardized criterion, has invited experts from the Greater China region, including Mainland China, Hong Kong, Macau, and Taiwan, to participate in the work. The consensus, rooted in evidence-based medicine, was reached after several rounds of discussion and thorough communication, ensuring its scientific rigor and authority. The following text reports on the second part of this consensus development: “Graft selection and clinical outcome evaluation".
2. Method
This consensus deliberates on selecting grafts for ACL reconstruction and evaluating clinical outcomes postoperatively, excluding the management of specific types of ACL injuries, such as tibial eminence fractures and partial tears. The consensus-making process can be succinctly described as one cycle, three teams, and ten phases (Fig. 1). The related work was done following the modified Delphi method [1].
Figure 1.
Expert Consensus Development Process Flowchart. The cycle refers to the Delphi survey (Inside the dashed box), which includes the first two rounds of online questionnaire surveys and the third round of face-to-face meeting. The three groups consist of the drafting team, the expert team, and the review team. The consensus formulation work spans ten steps from literature review to submission, all of which are the responsibility of the drafting team. The expert group and the review group each participate in their corresponding tasks.
2.1. Organizational structure for consensus development
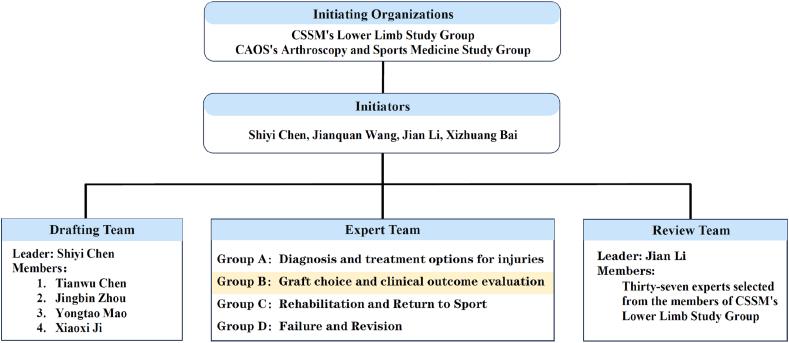
The initiating organizations for this consensus are the CSSM Lower Limb Study Group and the CAOS Arthroscopy and Sports Medicine Study Group. The initiating institutions include Huashan Hospital Fudan University, Peking University Third Hospital, West China Hospital Sichuan University, and the People's Hospital of Liaoning Province. The consensus initiators are Shiyi Chen, Jianquan Wang, Jian Li, and Xizhuang Bai. Shiyi Chen serves as the leader of the consensus drafting team, with members including Tianwu Chen, Jingbin Zhou, Xiaoxi Ji, and Yongtao Mao. The expert consensus team is divided into groups according to the personal preference and practical work needs of the experts under the overall arrangement of the drafting team leader, including Group A (Diagnosis and Treatment Options), Group B (Graft Selection and Clinical Outcome Evaluation), Group C (Rehabilitation and Return to Sport), and Group D (Failure and Revision Surgery). The consensus review team is led by Professor Jian Li, with Tianwu Chen serving as the liaison officer for the review and team members selected from the CSSM Lower Limb Study Group (Fig. 2).
Figure 2.
The organizational structure of the consensus formulation work. The second part of the consensus reported in this document was completed by Expert Group B, consisting of 30 experts responsible for conducting modified Delphi surveys on graft selection and clinical efficacy evaluation topics. CAOS: Chinese Association of Orthopaedic Surgeons; CSSM: Chinese Society of Sports Medicine.
2.2. Expert selection
The consensus drafting team selected consensus and review experts based on the following criteria: 1) Members of the CSSM, 2) Members of the CAOS, 3) Participants in the Chinese expert consensus on synthetic ligament applied for ACL reconstruction, 4) Active researchers in the field of ACL injury treatment, 5) Individuals who have published relevant literature in the past five years, and 6) Individuals recommended by the consensus initiators.
A total of 30 experts were fully involved in the consensus development work of Group B (Graft Selection and Clinical Outcome Evaluation). Among the experts, 28 males and two females were averaging 53.5 years. In terms of medical titles, there were 27 chief physicians and three deputy chief physicians; academically, there were 19 professors and four associate professors; in teaching positions, there were 14 Ph.D. supervisors and nine master's supervisors; and as for society positions, there were 25 members of the CSSM and 22 members of the CAOS. All experts routinely performed ACL reconstruction in the past five years, with 18 performing more than 100 surgeries annually, seven performing 51–100 surgeries, three performing 21–50 surgeries, and two performing fewer than 20 surgeries. Twenty-two had published ACL injury diagnosis and treatment-related papers in the past five years, with nine publishing more than five papers, four publishing 3–5 papers, and nine publishing 1–2 papers.
The consensus review team consisted of 37 experts, including 36 males and one female, with an average age of 54.8 years. In terms of medical titles, there were 32 chief physicians, four deputy chiefs, and one senior consultant (Hung Maan Lee); academically, there were 28 professors and six associate professors; in teaching positions, there were 19 Ph.D. supervisors and 13 master's supervisors. Regarding society positions, there were 35 members of the CSSM and 23 members of the CAOS. All experts had routinely carried out ACL reconstruction in the past five years. Annually, 26 performed more than 100 surgeries, four performed 51–100 surgeries, four performed 21–50 surgeries, and three performed fewer than 20 surgeries. Thirty-three had published ACL injury diagnosis and treatment-related papers in the past five years, with 13 publishing more than five papers, 11 publishing 3–5 papers, and nine publishing 1–2 papers.
2.3. Drafting of consensus items
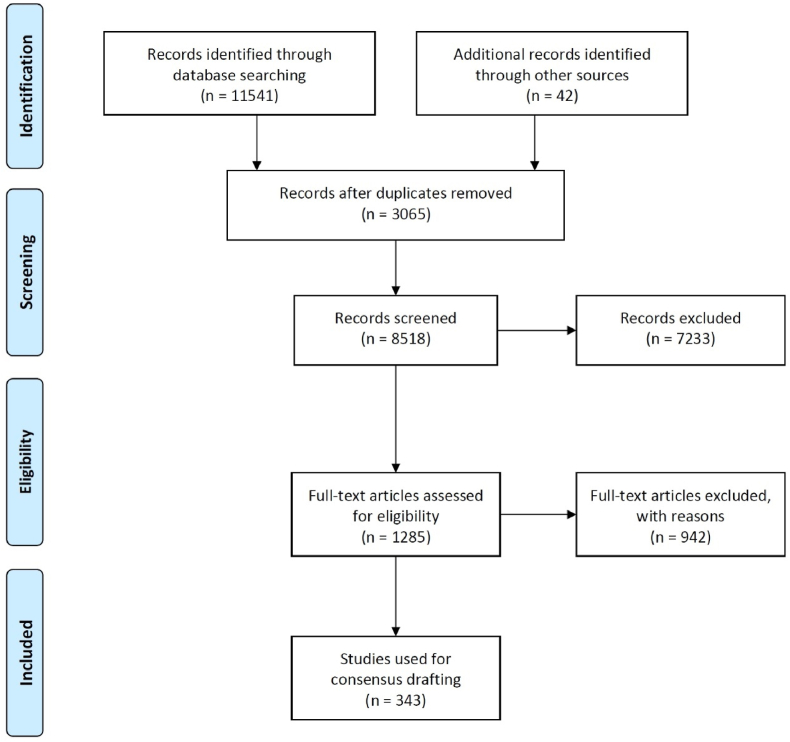
The consensus drafting team formulated the proposed terms for consensus based on current medical evidence. The reference contents included English sources, Chinese sources, and the corresponding terms from the Panther consensus. English literature was searched using PubMed and the Web of Science, accessing databases such as Medline and the Web of Science collection. Chinese databases included China National Knowledge Infrastructure (CNKI), Wanfang Data, and the China Science and Technology Journal Database. The search covered literature from January 1, 1900, to August 1, 2021, with the search strategy detailed in Table 1. After the search, relevant literature was screened. Exclusion criteria for literature included: 1) duplicate studies, 2) basic research, 3) studies irrelevant to the research theme, and 4) incomplete information. The specific literature screening process is detailed in Fig. 3. Relevant information for grafts used in ACL reconstruction and their evaluation was extracted based on the PICO principle (Patient, Intervention, Comparison, Outcome). Content related to the efficacy evaluation of ACL reconstruction was also removed from the corresponding terms of the Panther consensus.
Table 1.
Search strategy of the second part of the consensus.
| Search Platform/Database | Search Strategy |
|---|---|
| Pubmed | (graft[Title/Abstract]) AND (anterior cruciate ligament reconstruction[Title/Abstract]) |
| Web of Science | TS = (graft)AND TS = anterior cruciate ligament |
| CNKI (China National Knowledge Infrastructure) | Keywords: (graft) *Keywords:(anterior cruciate ligament reconstruction) *Date:-2021/08/01 |
| Wanfang Data | Title or Keywords: (graft) *Title or Keywords: (anterior cruciate ligament reconstruction) |
| Database of Chinese sci-tech periodicals | Title or Keywords = graft AND Title or Keywords = anterior cruciate ligament reconstruction |
Figure 3.
Prisma flowchart of the second part of the consensus.
3. Delphi surveys
The consensus drafting team prepared to conduct Delphi rounds, including two rounds of online surveys and a third round of face-to-face meeting (Fig. 1). The two rounds of surveys revolved around the “first draft” of the consensus. The questionnaire design was based on the Sprague method. In the first round, the consensus liaison collected expert opinions on each draft consensus item using a Likert five-point scale, with options including: “Completely Agree," “Partially Agree," “Undecided," “Partially Disagree," and " Completely Disagree”. The drafting team calculated the percentage of differing opinions and marked each item as “Accepted," “Not accepted," or “Pending” according to the corresponding standards in Table 2. For the “Not Accepted” and “Pending” terms, the consensus drafting team communicated with experts, solicited their opinions, and made corresponding modifications, which were included in the second round of the questionnaire.
Table 2.
Evaluation criteria of the consensus items in the first round survey.
| Results | Criteria |
|---|---|
| Accepted |
|
| Not Accepted |
|
| Pending |
|
In the second round of the questionnaire survey, the consensus liaison used a three-point Likert scale to collect expert opinions on each draft consensus term, including “Agree," “Disagree," and “Uncertain and requires more clinical evidence." If a consensus term draft meets the condition of ≥90 % agreement, it is recorded as “Accepted”; if it meets the condition of >10 % disagreement or (and) uncertainty, it is recorded as “Not Accepted." For “Not Accepted” terms, the consensus drafting team again communicates with experts, solicits their opinions, and makes corresponding modifications, incorporating the feedback into the second draft for discussion in the face-to-face meeting.
Due to pandemic prevention and control measures, the face-to-face meeting was conducted “online and offline”. A total of 30 experts participated in the meeting, with 20 online and ten offline. Professor Jianquan Wang, the consensus initiator, served as the online moderator, and Professor Chen Shiyi served as the offline moderator. A teller (Tianwu Chen) and two vote counters (Xiaoxi Ji and Yongtao Mao) were present on-site. After the teller introduced the consensus items, the moderator solicited amendment suggestions with real-time modifications from the experts. Amendments approved by more than half of the experts were considered valid. All experts voted on the consensus terms by raising their hands, with options to vote in favor, abstain, or oppose; repeated voting was considered invalid, and presence without voting was considered abstention. A consensus was considered reached for a draft term with an approval rate of 85%, and based on the approval rate, consensus terms were categorized into three levels: “Strong” (95.0%–100%), “General” (90.0%–94.9%), and “Basic” (85.0%–89.9%).
3.1. Data analysis
The first round survey included nine terms: nine accepted, 0 not accepted, and 0 pending. All nine terms directly progressed to the second draft. During the face-to-face meeting, 30 experts discussed and voted on the nine proposed consensus terms. One item was amended, while 8 received no suggestions for modifications. All nine terms were unanimously approved, achieving strong consensus (Fig. 4).
Figure 4.
The Stages of Consensus Drafting: From First Draft to Final Version through the Modified Delphi Rounds. During the first round of surveys, all nine items of the first draft were accepted, negating the need for a second round of surveys. These were directly incorporated into the second draft for the face-to-face meeting. In the meeting, there were no item that failed to achieve consensus; all nine items were accepted, resulting in strong consensus.
4. Interpretation of consensus terms
-
Item 1
Currently, grafts used for ACL reconstruction can be categorized into three main types: autografts, allografts, and synthetic ligaments. (Strong consensus, 100% approval rate, 30/30)
The choice of graft plays a crucial role in preoperative planning for ACL reconstruction, as it has a decisive impact on surgical outcomes [2]. A century ago, surgeons began experimenting with autologous iliotibial bands as grafts for reconstructing the damaged ACL [3]. Subsequently, researchers found that portions of the patellar tendon (with bone blocks) [4], semitendinosus and gracilis tendons [5], portions of the quadriceps tendon [6], and the long peroneal tendon [7] could also serve as grafts for ACL reconstruction. These tissues, harvested from other parts of the patient's body, are known as autografts and are currently the most widely used for ACL reconstruction worldwide. Allografts, derived from donated natural human tissues from donors, include not only commonly used autograft sites but also structures such as the anterior tibialis, posterior tibialis, and Achilles tendons, which are not ideally obtained by sacrificing one's own tissues [8]. Allografts, sourced from tissue banks, are extensively used for ACL reconstruction in most countries and regions, particularly in North America. Synthetic ligaments are grafts made from artificial materials for ligament repair and reconstruction. Early use of synthetic ligaments for ACL reconstruction was unsuccessful due to material and design flaws, inappropriate indications, and surgical errors. With advancements in weaving materials, structural design, indication selection, and surgical techniques, the latest generation of synthetic ligaments is now utilized for ACL reconstruction in North America, Europe, Australia, the Middle East, and China.
-
Item 2
Different types of grafts each have their advantages and limitations, and currently, there is no “gold standard” for graft selection. Surgeons should consider factors such as graft size, biomechanical properties, tissue source, surgical habits and technical proficiency, and the patient's gender, age, injury characteristics, athletic requirements, and personal preferences when choosing a graft. (Strong consensus, 100% approval rate, 30/30)
Graft selection is a classical topic in ACL reconstruction. Ideal graft requirements include rapid integration, allowance for early rehabilitation, low failure rate, high safety, adequate strength, satisfactory size, low donor site complications, widespread availability, and reasonable cost [[8], [9], [10]]. Unfortunately, existing grafts each have advantages and shortcomings and cannot meet all the above criteria [11]. Hence, current ACL reconstruction has no “gold standard” for graft selection. When considering graft choices, surgeons must balance various factors to make the optimal decision.
Studies have shown that increasing the cross-sectional area of the graft can effectively improve its mechanical strength. However, an extensive cross-sectional area may potentially cause graft impingement within the intercondylar notch, so balancing the size and mechanical strength of the graft can not be overlooked [[12], [13], [14]]. The sterilization treatment of allografts significantly affects their mechanical strength and clinical efficacy; therefore, surgeons and patients should be well informed of the advantages and disadvantages of different sterilization methods to select the most appropriate graft [[15], [16], [17]]. The surgical habits of the physician essentially affects graft choice, as different graft types involve different surgical techniques, and the surgeon's experience and technical proficiency are crucial in graft selection [12,[18], [19], [20]]. Some physicians may have accumulated extensive experience with certain types of grafts, thus being more skilled and confident during surgery. Moreover, some grafts may require more delicate and complex surgical techniques, but mastering these techniques necessitates systematic training and long-term practice. Therefore, surgeons should choose the appropriate graft according to their surgical habits and technical proficiency to improve surgical success rates and reduce the risk of complications.
The patient's gender, age, and athletic needs affect the surgical success rate, the rehabilitation process, and the timing of return to sport of ACL reconstruction [21]. Young males and active individuals may require grafts with higher mechanical strength and lower rerupture rates to meet their athletic demands [22]. Considering injury characteristics is essential in graft selection, for instance, preserving medial stability structures (e.g., semitendinosus tendon) when there is concomitant medial collateral ligament injury [23,24] or emphasizing the function of the extensor apparatus when there is a posterior cruciate injury combined [25,26]. Additionally, to ensure patients' right to informed consent, physicians should fulfill their notification duty by explaining the advantages and disadvantages of different grafts and respecting the patient's personal preferences in the final decision.
-
Item 3
The semitendinosus tendon, bone-patellar tendon-bone (BPTB), quadriceps tendon, and peroneus longus tendon are the autografts currently used for ACL reconstruction. The advantage of using BPTB is that the bone blocks at both ends preserve the natural tendon–osseous interface, allowing for more robust integration of the graft with the host through osseous healing. The semitendinosus tendon is favored for its ideal mechanical strength and not damaging the extensor mechanism. The clinical efficacy of the quadriceps tendon and peroneus longus tendon needs further confirmation from evidence-based medicine. All autografts have limitations, such as limited availability and potential donor site complications. (Strong consensus, 100% approval rate, 30/30)
The autologous BPTB and semitendinosus tendon are the most commonly used grafts for ACL reconstruction globally [[27], [28], [29], [30]]. Randomized controlled trials and cohort studies have shown that ACL reconstructions using BPTB and semitendinosus tendon have satisfactory clinical efficacy at various postoperative stages [[31], [32], [33]]. In 1963, Jones KG firstly proposed using autologous BPTB for ACL reconstruction [4], a technique later refined by Erikson E and popularized in the 1970s [34]. The graft is taken from 1/3 of the patellar tendon, including bone blocks from the patella and tibia, with the advantage of preserving the natural tendon–osseous interface and more robust integration through osseous healing. However, drawbacks include damage to the extensor mechanism and donor site complications, including patellar fractures, patellar tendon rupture, and kneeling pain [35,36]. Between 1920 and 1970, several scholars reported using a single-strand autologous semitendinosus tendon for ACL reconstruction [3,37]. In 1982, Lipscomb AB first used semitendinosus and gracilis tendons as double-strand grafts for ACL reconstruction, achieving satisfactory short-term outcomes [38]. In 1988, Friedman MJ detailed the method of using four-strand semitendinosus and gracilis tendons for ACL reconstruction [39]. Since then, four-strand semitendinosus tendon reconstruction has been accepted. The advantage of using semitendinosus tendon lies in not damaging the extensor mechanism and achieving ideal mechanical strength after multi-strand weaving [40], with the average failure load of a four-strand semitendinosus tendon reaching 4090N, higher than that of BPTB, ranging from 2238N (7 mm width) to 2664N (10 mm width) [41,42]. However, potential issues like unpredictable size, saphenous nerve injury, intermuscular hematoma, and weakened knee flexion are challenges when using autologous semitendinosus tendon [[43], [44], [45]]. Since 1979, several scholars have reported using autologous quadriceps tendon for ACL reconstruction [6,46,47]. Clinical proponents believe the autologous quadriceps tendon has ideal mechanical strength and advantages over autologous BPTB and semitendinosus tendons regarding donor site complications and graft size, making it an ideal alternative choice [48,49]. However, the clinical use of the quadriceps tendon has long remained low, accounting for only 1–2.5 % of grafts used in ACL reconstruction, thus earning the moniker of the ‘forgotten’ graft [50,51]. In recent years, the use of quadriceps tendon as an ACL graft has been on the rise, with meta-analyses showing its failure rate is comparable to autologous BPTB and semitendinosus tendon, and it has advantages in donor site complication rates and functional scores. However, overall, the clinical efficacy of using autologous quadriceps tendon for ACL reconstruction still awaits further follow-up studies and randomized controlled trials for confirmation [52,53]. The autologous peroneus longus tendon has recently been reported for use in ACL reconstruction [54,55]. Clinical supporters consider the autologous peroneus longus tendon to have ideal size and rupture strength comparable to semitendinosus tendon, quadriceps tendon, and BPTB while not damaging the medial stability structures (semitendinosus tendon) [56,57]. However, there are dissenting opinions [58]. Recent meta-analyses indicate that routine clinical use of autologous peroneus longus tendon in ACL reconstruction lacks sufficient evidence, and its ankle donor site complications and long-term efficacy still require further research for confirmation [10,59].
-
Item 4
The tibial tendon, Achilles tendon, BPTB, and semitendinosus tendon currently serve as commonly utilized allografts for ACL reconstruction. The advantages of employing allografts include absence of donor site morbidity, reduced surgical trauma, diminished pain associated with tissue harvesting, and shorter operative time. However, the limitations related to the use of allografts encompass the incurrence of additional costs, comparatively slower graft healing, potential for immunogenic rejection, risks of disease transmission, concerns regarding the quality of donor tissues, and a relatively higher graft failure rate within populations of young athletes. (Strong consensus, 100% approval rate, 30/30)
Since the 1980s, allografts have been utilized in ACL reconstruction [60]. In the 1990s, with the proliferation of commercial tissue banks in the United States, the use of allografts for ACL reconstruction significantly increased [61]. Recently, European scholars have reached a consensus regarding using allografts in ACL reconstruction [62]. To date, research publications from the United States account for 50% of the related studies indexed in the Medline database, with Chinese researchers contributing 15.2% of the publications, ranking second. Regarding tissue sources, the tibial tendon, Achilles tendon, BPTB, and semitendinosus tendon are commonly selected options [12,63,64]. The advantages of using allografts include eliminating the need to harvest autologous tissue, shortening surgery time, reducing trauma, avoiding donor site complications, and quicker postoperative recovery [64,65]. Additionally, allografts, due to their ample availability, are often used in ACL reconstruction involving multiple ligament injuries and revision surgeries [66,67].
However, the drawbacks of using allograft tendons for ACL reconstruction primarily include the quality control and sterilization processes of donated tissues, increased medical costs [68,69],the longer ligamentization process (compared to autografts) [70], the risks of tissue rejection and pathogen transmission [71], the impact of age-related tissue quality on graft strength [72], and the higher failure rates in young athletes [73,74]. It is noteworthy that gamma irradiation treatment can lead to a decrease in the mechanical strength of the grafts [75]. Conversely, ACL reconstruction with allografts treated without irradiation have demonstrated better postoperative joint stability [[76], [77], [78]].
-
Item 5
Currently, Ligament Advanced Reinforcement System (LARS) is the principal synthetic ligament option for ACL reconstruction in China. The advantage of the LARS lies in its high mechanical strength. After implantation, the LARS does not involve tissue regeneration-based ligamentization, promptly attaining an ideal mechanical strength, enabling patients to undergo fast postoperative recovery and early return to sport. Furthermore, the application avoids donor site complications and mitigates the potential risk of disease transmission. However, applying synthetic ligaments in ACL reconstruction requires exact graft positioning, either “isometric” or “near-isometric”. Misalignment of the bone tunnels can easily lead to surgical failure. (Strong consensus, 100% approval rate, 30/30)
Due to significant changes in overall structure, weaving technique, and surgical approach compared to earlier synthetic ligaments, the LARS is a new generation of synthetic ligaments [79,80]. Over the past 20 years, the LARS has been widely used worldwide, with China being the largest user in ACL reconstruction. Unlike biological grafts, the LARS is made of polyethylene terephthalate (PET) fibers and does not undergo the tissue regeneration process of “necrosis-vascularization-restructuring” [81]. It immediately possesses strong tensile strength postoperatively. The LARS offers a maximum mechanical strength (rupture load) of up to 5500N (LARS AC120), which is 3.2 times that of the native ACL (adult male, 1730N), 2.1 times that of the BPTB (width 10 mm, 2664N), 2.3 times that of the quadriceps tendon (width 10 mm, 2352N), and 1.3 times that of the quadruple hamstring tendon (4090N) [12,41,42,82,83]. Therefore, ACL reconstruction using the LARS allows for fast postoperative recovery and early return to sport.
Additionally, applying synthetic ligaments eliminates the need to sacrifice autologous tissues and avoids donor site complications. Simultaneously, it eliminates the potential risk of pathogen transmission related to allografts [84,85]. However, it should be noted that due to its poor elasticity, the clinical efficacy of LARS in ACL reconstruction highly depends on “isometric” or “near-isometric” techniques, demanding accurate bone tunnel positioning [86]. Misalignment of bone tunnels can bring the graft to a non-isometric state during joint flexion and extension, directly limiting the knee range of motion (ROM) and subjecting the graft to excessive loads as well as potential impingement within the intercondylar notch. Evidence suggests that misalignment of bone tunnels is the primary cause of failure in LARS ACL reconstruction [87]. Other disadvantages of using the LARS include higher costs, potential long-term durability concerns, and the lack of biological integration, which may affect long-term clinical outcomes.
-
Item 6
A comprehensive evaluation of ACL reconstruction should encompass surgical details, patient-reported outcomes (PROs), knee joint functional scores, types and levels of physical activities, postoperative complications, and reconstruction failures. Medium to long-term follow-up should focus on the occurrence of postoperative osteoarthritis. (Strong consensus, 100% approval rate, 30/30)
A systematic evaluation following ACL reconstruction should include subjective scoring, clinical assessment, and return to sport status. The PROs from questionnaire surveys provide rich information on joint symptoms, function, daily activities, and knee-related quality of life and quantitatively assess postoperative physical activity, including intensity, frequency, and psychological status related to return to sport. Clinical assessment, performed by healthcare professionals, encompasses surgical details and knee joint function. Surgical details include graft selection, fixation methods, bone tunnel positioning, and management of concomitant injuries. Joint function assessment should consist of at least the joint ROM and stability. We recommend using the International Knee Documentation Committee (IKDC) standards for measurement, with results compared to the contralateral knee and graded rigorously to obtain standardized reports [88]. The contralateral knee can be a reference for surgical-side assessment, highlighting individual differences in joint ROM, stability, and functional performance (e.g., isokinetic muscle testing, single-leg hop). Some quantitative measurements, such as anterior translation, may be challenging for patients with bilateral ACL injuries. Researchers may use qualitative methods (e.g., the presence of “hard endpoint”) to make judgments. Return to sport is a significant goal for most patients undergoing ACL reconstruction; thus, recording their physical activity level is necessary [89]. The current internationally accepted practice is to evaluate the proportion and timing of return to sport at two years postoperatively [90].
ACL reconstruction failure is a nonspecific term with disputed definitions in the literature [91,92]. We recommend categorizing clinical failure and graft rupture as part of the reconstruction failure [73]. Clinical failure indicates abnormalities in joint function, including restricted joint ROM and recurrent laxity, typically confirmed by clinical examination. Graft rupture requires confirmation through MRI or arthroscopic inspection [87]. Reasons for reconstruction failure may include: 1) traumatic factors, such as re-injury, 2) technical factors, such as surgical errors, and 3) patient-related factors, including poor rehabilitation compliance, abnormal neuromuscular function, or generalized ligament laxity [[93], [94], [95]]. Specific details, reasons, and timing of failure should be documented for failed cases. Postoperative complications of ACL reconstruction include joint infection, synovitis, joint stiffness, deep vein thrombosis, and other issues. Severe complications can affect surgical outcomes or even lead to reconstruction failure. Early-stage (within six months) monitoring and documentation of complications are essential [[96], [97], [98]].
-
Item 7
PROs following ACL reconstruction should include at least one knee joint score, one activity score, and one health-related quality of life (HRQoL) score. (Strong consensus, 100% approval rate, 30/30)
In clinical assessment, using PROs is crucial for physicians and researchers to understand patients' responses to ACL injuries and treatments [[99], [100], [101]]. In recent years, advancements in internet technology and the widespread availability of smartphones and computers have facilitated the electronic completion of questionnaires between healthcare providers and patients, thereby promoting the PROs usage [102,103]. The efficient survey methods have led researchers to add more questionnaires to patients to gather additional data on PROs. However, it's important to note that respondent burden is a significant term in research, defined as “the time required to complete items and the physical and cognitive demands placed on respondents” [104,105]. All clinical tests conducted on patients contribute to the burden they bear. Therefore, in assessing clinical outcomes, a delicate balance exists between various outcome assessments and respondent burden. Excessive respondent burden threatens the effectiveness of individual responses, thereby impacting the validity of the feedback provided. We recommend that researchers selectively choose patient questionnaires tailored to the study objectives, aiming to minimize respondent burden while obtaining the necessary PROs for their researches. From published high-quality clinical studies, it is evident that incorporating a knee-specific score, a HRQoL score, and an activity score enables researchers to comprehensively understand patients' perspectives on treatment outcomes [73,85,[106], [107], [108]].
-
Item 8
The knee stability of patients undergoing ACL reconstruction includes both anterior and rotational stability. (Strong consensus, 100% approval rate, 30/30)
The ACL is crucial in limiting anterior translation and excessive rotation of tibia [109]. ACL reconstruction aims to reconstruct the ligament structure and restore anterior and rotational stability of the knee [110]. Accurate assessment of joint stability is crucial for evaluating the effectiveness of reconstruction surgery. According to the IKDC criteria, laxity levels graded as A and B are recorded as “normal” and “nearly normal” respectively [111], while C or D grades are considered as clinical failure [73]. It should be noted that “joint laxity” and “joint instability” are distinct concepts. Knee joint laxity refers to the passive state of the knee joint when external forces or torques are applied, representing an objective phenomenon; joint instability refers to the patient's perception of symptoms during functional movements, meaning a subjective response [112]. While there is a connection between the two, it is not necessarily deterministic. Due to its objective measurability and accurate recordability, laxity level is the preferred indicator for clinical assessment of joint stability.
Anterior laxity of the knee joint in ACL-injured knees is primarily determined by the degree of anterior tibial translation. For instance, in the Lachman and anterior drawer tests, the examiner applies anterior load directly to the tibia in a single plane and assesses the degree of anterior tibial translation [113,114]. The IKDC Knee Ligament Standard Evaluation Form details standardized classifications of anterior tibial translation, including requirements for comparison with the contralateral side [111]. In terms of quantifying laxity levels, tools like the KT-1000/2000 are widely used in clinical practice, although concerns about inter-examiner reliability have been reported [[115], [116], [117]]. Recently, robotic devices have been employed for assessing joint laxity, possessing automatic detection capabilities, and mitigating inter-examiner variability [115,118,119].
It should be noted that the Lachman test and anterior drawer test only measure anterior tibial translation. It can not capture complex joint kinematics, and the degree of anterior laxity may not necessarily correlate with the joint function after ACL reconstruction [120]. In comparison, the pivot-shift test is a classic dynamic laxity test that can simulate physiological loads on the knee joint and detect both anterior and rotational laxity [121], with evidence suggesting it is the most specific physical examination maneuver for ACL injury [122].
-
Item 9
The reliability of clinical outcome evaluation for ACL reconstruction requires sample size calculation, completion of pre-treatment status assessment, clarification of the shortest follow-up time, and achievement of a follow-up rate of over 80%. (Strong consensus, 100% approval rate, 30/30)
Effective sample size calculation is a prerequisite for high-quality research [123]. When the sample size is insufficient, the evaluation results may be affected by “Type II errors”, resulting in “false-negative” outcomes [124]. For example, in a study comparing the clinical efficacy of grafts A and B, even if graft A demonstrates a significant advantage over graft B in the timing of return to sport, a study with inadequate sample size may fail to detect the difference in outcomes. Therefore, it is evident that calculating the sample size is crucial for ensuring the credibility of research conclusions. Additionally, from the perspective of reducing resource wastage, avoiding adverse events, and minimizing patient burden, researchers must determine the effective sample size before initiating a project [125,126].
Substantial evidence suggests that certain baseline variables, such as demographic data and pre-injury activity level, have specific impacts on the efficacy of ACL reconstruction. Recording these baseline data facilitates study design and creates conditions for in-depth analysis, thus avoiding bias in analyzing results [127,128]. Collecting PROs before treatment is essential as a fundamental aspect of assessing surgical efficacy, which should include joint symptoms, function, and related quality of life. It should be noted that evaluating the HRQoL and patient psychological status is crucial, as they reflect the impact of ACL injury on physical, social, and emotional health [129,130]. Currently validated PROs tools include the Lysholm Knee Scoring Scale, International Knee Documentation Committee (IKDC) Subjective Knee Form, Knee Injury and Osteoarthritis Outcome Score (KOOS), Anterior Cruciate Ligament Quality of Life (ACL-QoL) questionnaire, and Anterior Cruciate Ligament-Return to Sport after Injury (ACL-RSI) questionnaire [[131], [132], [133]]. Additionally, preoperative objective joint examination results should be accurately recorded, including joint stability, ROM, swelling, muscle atrophy, and other relevant factors. Finally, researchers should also document the type and level of physical activity before the injury, which is crucial for determining whether patients have successfully returned to their pre-injury activity level. Validated assessment tools include the Marx Activity Scale, Tegner Activity Scale, and Cincinnati Sports Activity Scale [[134], [135], [136]]. In summary, completing a pre-treatment status assessment should include at least demographic data, PROs, objective joint examination, and pre-injury activity type and level.
In clinical research surrounding ACL reconstruction, the follow-up duration should be determined based on the study's primary outcomes. Generally, treatment outcomes after ACL injury encompass the following: 1) PROs, 2) objective knee examination results, 3) early adverse events, 4) surgical failures, such as graft rupture and recurrent joint laxity, and 5) return to sport outcomes, including the timing and level of activities [[137], [138], [139], [140], [141], [142]]. Previous reports have shown significant variations in follow-up durations for different outcomes. For early adverse events such as infection, joint stiffness, and loosening of fixation, a follow-up period of six months is sufficient to collect data reflecting these outcomes [143,144]. PROs and objective joint examinations tend to stabilize 1 to 2 years post-surgery [145,146]. Suppose researchers are investigating surgical failures or return to sport, the follow-up period should not be less than two years, as ACL re-rupture and revision surgery rates peak within 1 to 2 years after reconstruction, corresponding to when most patients return to sport [[147], [148], [149]]. For studies focusing on the onset and progression of postoperative osteoarthritis following ACL reconstruction, a minimum follow-up of five years is recommended to observe changes in imaging and symptoms [[150], [151], [152]]. In clinical research, some degree of patient loss to follow-up is inevitable as the follow-up duration extends. Researchers should be aware that even a small proportion of loss to follow-up can lead to significant research bias [153]. The current consensus is that a loss to follow-up rate exceeding 20% threatens the credibility of study results [154]. Therefore, it is advised that a study should aim for a follow-up rate of at least 80% and discuss the impact of loss based on statistical analysis. In summary, the follow-up duration after ACL reconstruction should be decided based on the research objectives and primary outcomes while striving to ensure a follow-up rate of over 80%.
5. Conclusion
There is no “gold standard” for graft selection in ACL reconstruction; the options available include autografts, allografts, and synthetic ligaments. Each type of graft presents its advantages and disadvantages in clinical use. Autografts are favored for their safety and convenience as autologous tissue, though they are limited by availability and potential donor site morbidities. Allografts offer the advantages of a relatively abundant source, reduced surgical trauma, and postoperative pain. However, they come with additional costs, slower healing, risk of rejection, potential for disease transmission, and a higher failure rate in young, active populations. Synthetic ligaments are advantageous due to their superior mechanical strength, fast recovery, and early return to sport. However, they also incur additional costs and demand high surgical precision in tunnel placement. Factors to consider when selecting a graft include the graft size, biomechanical properties, tissue source, the surgeon's preference, technical proficiency, and the patient's gender, age, injury characteristics, athletic demands, and personal preference. Systematic evaluations after ACL reconstruction encompass not only surgical details and PROs but also delve into joint function and stability, as well as complications and surgical failures. To ensure treatment efficacy, mid- to long-term follow-ups should focus on the occurrence of osteoarthritis. Researchers need to employ multiple scoring standards while providing an adequate sample size and a follow-up rate of at least 80% to guarantee the accuracy of clinical outcome evaluation.
Financial conflicts of interest
We declared no financial conflicts of interest for each author, including investments, employment, consultancies, honoraria, patents, and royalties.
Personal relationships
All authors declared no personal relationships that could potentially influence the research.
Professional affiliations
All authors declared no affiliations with organizations directly interested in the subject matter. This includes memberships, employment, and consultancies.
Funding sources
This work was supported by the National Key R&D Program of China (2021YFA1201303) and the National Natural Science Foundation of China (Grant No. 82172511).
Declaration of competing interest
We, the undersigned, declare that for the manuscript entitled Diagnosis and Treatment of Anterior Cruciate Ligament Injuries: Consensus of Chinese Experts Part II: Graft Selection and Clinical Outcome Evaluation, the following is a true and accurate statement of all our potential conflicts of interest, financial or otherwise, that might influence the work or its interpretation.
Acknowledgments
We extend our gratitude to the CAOS Arthroscopy and Sports Medicine Study Group and the CSSM Lower Limb Study Group for their invaluable assistance and steadfast support in the consensus development process. Despite the complex situation posed by the pandemic, their collaboration enabled the core component of our work, the face-to-face meeting, to proceed smoothly through a hybrid model of online and offline modes.
References
- 1.Hsu C.C., Sandford B.A. The Delphi technique: making sense of consensus. Practical Assess Res Eval. 2007;12(10):1–8. [Google Scholar]
- 2.Sim K., Rahardja R., Zhu M., Young S.W. Optimal graft choice in athletic patients with anterior cruciate ligament injuries: review and clinical insights. Open Access J Sports Med. 2022;13:55–67. doi: 10.2147/OAJSM.S340702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schindler O.S. The story of anterior cruciate ligament reconstruction--Part 1. J Perioperat Pract. 2012;22(5):163–171. doi: 10.1177/175045891202200505. [DOI] [PubMed] [Google Scholar]
- 4.Jones K.G. Reconstruction of the anterior cruciate ligament. A technique using the central one-third of the patellar ligament. J Bone Joint Surg Am. 1963;45:925–932. [PubMed] [Google Scholar]
- 5.Cho K.O. Reconstruction of the anterior cruciate ligament by semitendinosus tenodesis. J Bone Joint Surg Am. 1975;57(5):608–612. [PubMed] [Google Scholar]
- 6.Fulkerson J.P., Langeland R. An alternative cruciate reconstruction graft: the central quadriceps tendon. Arthroscopy. 1995;11(2):252–254. doi: 10.1016/0749-8063(95)90078-0. [DOI] [PubMed] [Google Scholar]
- 7.Kerimoglu S., Kosucu P., Livaoglu M., Yukunc I., Turhan A.U. Magnetic resonance imagination of the peroneus longus tendon after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2009;17(1):35–39. doi: 10.1007/s00167-008-0626-7. [DOI] [PubMed] [Google Scholar]
- 8.Jost P.W., Dy C.J., Robertson C.M., Kelly A.M. Allograft use in anterior cruciate ligament reconstruction. HSS J. 2011;7(3):251–256. doi: 10.1007/s11420-011-9212-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geib T.M., Shelton W.R., Phelps R.A., Clark L. Anterior cruciate ligament reconstruction using quadriceps tendon autograft: intermediate-term outcome. Arthroscopy. 2009;25(12):1408–1414. doi: 10.1016/j.arthro.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 10.Marin Fermin T., Hovsepian J.M., Symeonidis P.D., Terzidis I., Papakostas E.T. Insufficient evidence to support peroneus longus tendon over other autografts for primary anterior cruciate ligament reconstruction: a systematic review. J ISAKOS. 2021;6(3):161–169. doi: 10.1136/jisakos-2020-000501. [DOI] [PubMed] [Google Scholar]
- 11.Sherman O.H., Banffy M.B. Anterior cruciate ligament reconstruction: which graft is best? Arthroscopy. 2004;20(9):974–980. doi: 10.1016/j.arthro.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 12.West R.V., Harner C.D. Graft selection in anterior cruciate ligament reconstruction. J Am Acad Orthop Surg. 2005;13(3):197–207. doi: 10.5435/00124635-200505000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Orsi A.D., Canavan P.K., Vaziri A., Goebel R., Kapasi O.A., Nayeb-Hashemi H. The effects of graft size and insertion site location during anterior cruciate ligament reconstruction on intercondylar notch impingement. Knee. 2017;24(3):525–535. doi: 10.1016/j.knee.2017.02.010. [DOI] [PubMed] [Google Scholar]
- 14.Howell S.M. Principles for placing the tibial tunnel and avoiding roof impingement during reconstruction of a torn anterior cruciate ligament. Knee Surg Sports Traumatol Arthrosc. 1998;6:S49–S55. doi: 10.1007/s001670050223. [DOI] [PubMed] [Google Scholar]
- 15.Lawhorn K.W., Howell S.M., Traina S.M., Gottlieb J.E., Meade T.D., Freedberg H.I. The effect of graft tissue on anterior cruciate ligament outcomes: a multicenter, prospective, randomized controlled trial comparing autograft hamstrings with fresh-frozen anterior tibialis allograft. Arthroscopy. 2012;28(8):1079–1086. doi: 10.1016/j.arthro.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 16.Mariscalco M.W., Magnussen R.A., Mehta D., Hewett T.E., Flanigan D.C., Kaeding C.C. Autograft versus nonirradiated allograft tissue for anterior cruciate ligament reconstruction: a systematic review. Am J Sports Med. 2014;42(2):492–499. doi: 10.1177/0363546513497566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carey J.L., Dunn W.R., Dahm D.L., Zeger S.L., Spindler K.P. A systematic review of anterior cruciate ligament reconstruction with autograft compared with allograft. J Bone Joint Surg Am. 2009;91(9):2242–2250. doi: 10.2106/JBJS.I.00610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bowman E.N., Freeman T.H., Limpisvasti O., Cole B.J., ElAttrache N.S. Anterior cruciate ligament reconstruction femoral tunnel drilling preference among orthopaedic surgeons. Knee. 2021;29:564–570. doi: 10.1016/j.knee.2021.02.030. [DOI] [PubMed] [Google Scholar]
- 19.Wolf B.R., Ramme A.J., Britton C.L., Amendola A., Group M.K. Anterior cruciate ligament tunnel placement. J Knee Surg. 2014;27(4):309–317. doi: 10.1055/s-0033-1364101. [DOI] [PubMed] [Google Scholar]
- 20.Inacio M.C., Paxton E.W., Maletis G.B., Csintalan R.P., Granan L.P., Fithian D.C., et al. Patient and surgeon characteristics associated with primary anterior cruciate ligament reconstruction graft selection. Am J Sports Med. 2012;40(2):339–345. doi: 10.1177/0363546511424130. [DOI] [PubMed] [Google Scholar]
- 21.Kaeding C.C., Aros B., Pedroza A., Pifel E., Amendola A., Andrish J.T., et al. Allograft versus autograft anterior cruciate ligament reconstruction: predictors of failure from a MOON prospective longitudinal cohort. Sport Health. 2011;3(1):73–81. doi: 10.1177/1941738110386185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prodromos C., Joyce B., Shi K. A meta-analysis of stability of autografts compared to allografts after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2007;15(7):851–856. doi: 10.1007/s00167-007-0328-6. [DOI] [PubMed] [Google Scholar]
- 23.Kremen T.J., Polakof L.S., Rajaee S.S., Nelson T.J., Metzger M.F. The effect of hamstring tendon autograft harvest on the restoration of knee stability in the setting of concurrent anterior cruciate ligament and medial collateral ligament injuries. Am J Sports Med. 2018;46(1):163–170. doi: 10.1177/0363546517732743. [DOI] [PubMed] [Google Scholar]
- 24.Herbort M., Michel P., Raschke M.J., Vogel N., Schulze M., Zoll A., et al. Should the ipsilateral hamstrings Be used for anterior cruciate ligament reconstruction in the case of medial collateral ligament insufficiency? Biomechanical investigation regarding dynamic stabilization of the medial compartment by the hamstring muscles. Am J Sports Med. 2017;45(4):819–825. doi: 10.1177/0363546516677728. [DOI] [PubMed] [Google Scholar]
- 25.Adams D.J., Mazzocca A.D., Fulkerson J.P. Residual strength of the quadriceps versus patellar tendon after harvesting a central free tendon graft. Arthroscopy. 2006;22(1):76–79. doi: 10.1016/j.arthro.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 26.Rosenberg T.D., Franklin J.L., Baldwin G.N., Nelson K.A. Extensor mechanism function after patellar tendon graft harvest for anterior cruciate ligament reconstruction. Am J Sports Med. 1992;20(5):519–526. doi: 10.1177/036354659202000506. [DOI] [PubMed] [Google Scholar]
- 27.Eriksson K., Anderberg P., Hamberg P., Lofgren A.C., Bredenberg M., Westman I., et al. A comparison of quadruple semitendinosus and patellar tendon grafts in reconstruction of the anterior cruciate ligament. J Bone Joint Surg Br. 2001;83(3):348–354. doi: 10.1302/0301-620x.83b3.11685. [DOI] [PubMed] [Google Scholar]
- 28.Freedman K.B., D'Amato M.J., Nedeff D.D., Kaz A., Bach B.R., Jr. Arthroscopic anterior cruciate ligament reconstruction: a meta-analysis comparing patellar tendon and hamstring tendon autografts. Am J Sports Med. 2003;31(1):2–11. doi: 10.1177/03635465030310011501. [DOI] [PubMed] [Google Scholar]
- 29.Bergeron J.J., Sercia Q.P., Drager J., Pelet S., Belzile E.L. Return to baseline physical activity after bone-patellar tendon-bone versus hamstring tendon autografts for anterior cruciate ligament reconstruction: a systematic review and meta-analysis of randomized controlled trials. Am J Sports Med. 2022;50(8):2292–2303. doi: 10.1177/03635465211017522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kvist J., Kartus J., Karlsson J., Forssblad M. Results from the Swedish national anterior cruciate ligament register. Arthroscopy. 2014;30(7):803–810. doi: 10.1016/j.arthro.2014.02.036. [DOI] [PubMed] [Google Scholar]
- 31.Dai C., Wang F., Wang X., Wang R., Wang S., Tang S. Arthroscopic single-bundle anterior cruciate ligament reconstruction with six-strand hamstring tendon allograft versus bone-patellar tendon-bone allograft. Knee Surg Sports Traumatol Arthrosc. 2016;24(9):2915–2922. doi: 10.1007/s00167-015-3569-9. [DOI] [PubMed] [Google Scholar]
- 32.Webster K.E., Feller J.A., Hartnett N., Leigh W.B., Richmond A.K. Comparison of patellar tendon and hamstring tendon anterior cruciate ligament reconstruction: a 15-year follow-up of a randomized controlled trial. Am J Sports Med. 2016;44(1):83–90. doi: 10.1177/0363546515611886. [DOI] [PubMed] [Google Scholar]
- 33.Sajovic M., Stropnik D., Skaza K. Long-term comparison of semitendinosus and gracilis tendon versus patellar tendon autografts for anterior cruciate ligament reconstruction: a 17-year follow-up of a randomized controlled trial. Am J Sports Med. 2018;46(8):1800–1808. doi: 10.1177/0363546518768768. [DOI] [PubMed] [Google Scholar]
- 34.Eriksson E. Reconstruction of the anterior cruciate ligament. Orthop Clin N Am. 1976;7(1):167–179. [PubMed] [Google Scholar]
- 35.Sachs R.A., Daniel D.M., Stone M.L., Garfein R.F. Patellofemoral problems after anterior cruciate ligament reconstruction. Am J Sports Med. 1989;17(6):760–765. doi: 10.1177/036354658901700606. [DOI] [PubMed] [Google Scholar]
- 36.Marder R.A., Raskind J.R., Carroll M. Prospective evaluation of arthroscopically assisted anterior cruciate ligament reconstruction. Patellar tendon versus semitendinosus and gracilis tendons. Am J Sports Med. 1991;19(5):478–484. doi: 10.1177/036354659101900510. [DOI] [PubMed] [Google Scholar]
- 37.Chambat P., Guier C., Sonnery-Cottet B., Fayard J.M., Thaunat M. The evolution of ACL reconstruction over the last fifty years. Int Orthop. 2013;37(2):181–186. doi: 10.1007/s00264-012-1759-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lipscomb A.B., Johnston R.K., Snyder R.B., Warburton M.J., Gilbert P.P. Evaluation of hamstring strength following use of semitendinosus and gracilis tendons to reconstruct the anterior cruciate ligament. Am J Sports Med. 1982;10(6):340–342. doi: 10.1177/036354658201000603. [DOI] [PubMed] [Google Scholar]
- 39.Friedman M.J. Arthroscopic semitendinosus (gracilis) reconstruction for anterior cruciate ligament deficiency. Tech Orthop. 1988;2(4):74–80. [Google Scholar]
- 40.Thaunat M., Fayard J.M., Sonnery-Cottet B. Hamstring tendons or bone-patellar tendon-bone graft for anterior cruciate ligament reconstruction? Orthop Traumatol Surg Res. 2019;105(1S):S89–S94. doi: 10.1016/j.otsr.2018.05.014. [DOI] [PubMed] [Google Scholar]
- 41.Hamner D.L., Brown C.H., Jr., Steiner M.E., Hecker A.T., Hayes W.C. Hamstring tendon grafts for reconstruction of the anterior cruciate ligament: biomechanical evaluation of the use of multiple strands and tensioning techniques. J Bone Joint Surg Am. 1999;81(4):549–557. doi: 10.2106/00004623-199904000-00013. [DOI] [PubMed] [Google Scholar]
- 42.Cooper D.E., Deng X.H., Burstein A.L., Warren R.F. The strength of the central third patellar tendon graft. A biomechanical study. Am J Sports Med. 1993;21(6):818–824. doi: 10.1177/036354659302100610. [DOI] [PubMed] [Google Scholar]
- 43.Soon M., Neo C.P., Mitra A.K., Tay B.K. Morbidity following anterior cruciate ligament reconstruction using hamstring autograft. Ann Acad Med Singapore. 2004;33(2):214–219. [PubMed] [Google Scholar]
- 44.Sanders B., Rolf R., McClelland W., Xerogeanes J. Prevalence of saphenous nerve injury after autogenous hamstring harvest: an anatomic and clinical study of sartorial branch injury. Arthroscopy. 2007;23(9):956–963. doi: 10.1016/j.arthro.2007.03.099. [DOI] [PubMed] [Google Scholar]
- 45.San Jose A.T., Maniar N., Timmins R.G., Beerworth K., Hampel C., Tyson N., et al. Explosive hamstrings strength asymmetry persists despite maximal hamstring strength recovery following anterior cruciate ligament reconstruction using hamstring tendon autografts. Knee Surg Sports Traumatol Arthrosc. 2023;31(1):299–307. doi: 10.1007/s00167-022-07096-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marshall J.L., Warren R.F., Wickiewicz T.L., Reider B. The anterior cruciate ligament: a technique of repair and reconstruction. Clin Orthop Relat Res. 1979;143:97–106. [PubMed] [Google Scholar]
- 47.Stäubli H.-U. The knee and the cruciate ligaments: anatomy biomechanics clinical aspects reconstruction complications rehabilitation. Springer Berlin Heidelberg; Berlin, Heidelberg: 1990. Arthroscopically assisted ACL reconstruction using autologous quadriceps tendon; pp. 443–451. [Google Scholar]
- 48.Slone H.S., Romine S.E., Premkumar A., Xerogeanes J.W. Quadriceps tendon autograft for anterior cruciate ligament reconstruction: a comprehensive review of current literature and systematic review of clinical results. Arthroscopy. 2015;31(3):541–554. doi: 10.1016/j.arthro.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 49.Staubli H.U., Schatzmann L., Brunner P., Rincon L., Nolte L.P. Mechanical tensile properties of the quadriceps tendon and patellar ligament in young adults. Am J Sports Med. 1999;27(1):27–34. doi: 10.1177/03635465990270011301. [DOI] [PubMed] [Google Scholar]
- 50.van Eck C.F., Illingworth K.D., Fu F.H. Quadriceps tendon: the forgotten graft. Arthroscopy. 2010;26(4):441–443. doi: 10.1016/j.arthro.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 51.van Eck C.F., Schreiber V.M., Mejia H.A., Samuelsson K., van Dijk C.N., Karlsson J., et al. "Anatomic" anterior cruciate ligament reconstruction: a systematic review of surgical techniques and reporting of surgical data. Arthroscopy. 2010;26(9S):S2–S12. doi: 10.1016/j.arthro.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 52.Hurley E.T., Calvo-Gurry M., Withers D., Farrington S.K., Moran R., Moran C.J. Quadriceps tendon autograft in anterior cruciate ligament reconstruction: a systematic review. Arthroscopy. 2018;34(5):1690–1698. doi: 10.1016/j.arthro.2018.01.046. [DOI] [PubMed] [Google Scholar]
- 53.Mouarbes D., Menetrey J., Marot V., Courtot L., Berard E., Cavaignac E. Anterior cruciate ligament reconstruction: a systematic review and meta-analysis of outcomes for quadriceps tendon autograft versus bone-patellar tendon-bone and hamstring-tendon autografts. Am J Sports Med. 2019;47(14):3531–3540. doi: 10.1177/0363546518825340. [DOI] [PubMed] [Google Scholar]
- 54.Joshi S., Shetty U.C., Salim M.D., Meena N., Kumar R.S., Rao V.K.V. Peroneus longus tendon autograft for anterior cruciate ligament reconstruction: a safe and effective alternative in nonathletic patients. Niger J Surg. 2021;27(1):42–47. doi: 10.4103/njs.NJS_22_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shi F.D., Hess D.E., Zuo J.Z., Liu S.J., Wang X.C., Zhang Y., et al. Peroneus longus tendon autograft is a safe and effective alternative for anterior cruciate ligament reconstruction. J Knee Surg. 2019;32(8):804–811. doi: 10.1055/s-0038-1669951. [DOI] [PubMed] [Google Scholar]
- 56.Phatama K.Y., Hidayat M., Mustamsir E., Pradana A.S., Dhananjaya B., Muhammad S.I. Tensile strength comparison between hamstring tendon, patellar tendon, quadriceps tendon and peroneus longus tendon: a cadaver research. Journal of Arthroscopy and Joint Surgery. 2019;6(2):114–116. [Google Scholar]
- 57.Zhao J., Huangfu X. The biomechanical and clinical application of using the anterior half of the peroneus longus tendon as an autograft source. Am J Sports Med. 2012;40(3):662–671. doi: 10.1177/0363546511428782. [DOI] [PubMed] [Google Scholar]
- 58.Angthong C., Chernchujit B., Apivatgaroon A., Chaijenkit K., Nualon P., Suchao-in K. The anterior cruciate ligament reconstruction with the peroneus longus tendon: a biomechanical and clinical evaluation of the donor ankle morbidity. J Med Assoc Thai. 2015;98(6):555–560. [PubMed] [Google Scholar]
- 59.Yadav U., Nemani M., Devgun A., Malik M., Agrawal G.K. Iatrogenic foot drop after anterior cruciate ligament reconstruction with peroneus longus tendon autograft: report of a rare case. Cureus. 2022;14(6) doi: 10.7759/cureus.26476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zoltan D.J., Reinecke C., Indelicato P.A. Synthetic and allograft anterior cruciate ligament reconstruction. Clin Sports Med. 1988;7(4):773–784. [PubMed] [Google Scholar]
- 61.Cohen S.B., Sekiya J.K. Allograft safety in anterior cruciate ligament reconstruction. Clin Sports Med. 2007;26(4):597–605. doi: 10.1016/j.csm.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 62.Bait C., Randelli P., Compagnoni R., Ferrua P., Papalia R., Familiari F., et al. Italian consensus statement for the use of allografts in ACL reconstructive surgery. Knee Surg Sports Traumatol Arthrosc. 2019;27(6):1873–1881. doi: 10.1007/s00167-018-5003-6. [DOI] [PubMed] [Google Scholar]
- 63.Busam M.L., Rue J.P., Bach B.R., Jr. Fresh-frozen allograft anterior cruciate ligament reconstruction. Clin Sports Med. 2007;26(4):607–623. doi: 10.1016/j.csm.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 64.Kuhn M.A., Ross G. Allografts in the treatment of anterior cruciate ligament injuries. Sports Med Arthrosc Rev. 2007;15(3):133–138. doi: 10.1097/JSA.0b013e318134ecf6. [DOI] [PubMed] [Google Scholar]
- 65.Goetz G., de Villiers C., Sadoghi P., Geiger-Gritsch S. Allograft for anterior cruciate ligament reconstruction (aclr): a systematic review and meta-analysis of long-term comparative effectiveness and safety. Results of a health technology assessment. Arthrosc Sports Med Rehabil. 2020;2(6):e873–e891. doi: 10.1016/j.asmr.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Condello V., Zdanowicz U., Di Matteo B., Spalding T., Gelber P.E., Adravanti P., et al. Allograft tendons are a safe and effective option for revision ACL reconstruction: a clinical review. Knee Surg Sports Traumatol Arthrosc. 2019;27(6):1771–1781. doi: 10.1007/s00167-018-5147-4. [DOI] [PubMed] [Google Scholar]
- 67.Fanelli G.C., Edson C.J. Combined ACL-PCL-medial and lateral side injuries (global laxity) Sports Med Arthrosc Rev. 2020;28(3):100–109. doi: 10.1097/JSA.0000000000000276. [DOI] [PubMed] [Google Scholar]
- 68.Nagda S.H., Altobelli G.G., Bowdry K.A., Brewster C.E., Lombardo S.J. Cost analysis of outpatient anterior cruciate ligament reconstruction: autograft versus allograft. Clin Orthop Relat Res. 2010;468(5):1418–1422. doi: 10.1007/s11999-009-1178-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Barrera Oro F., Sikka R.S., Wolters B., Graver R., Boyd J.L., Nelson B., et al. Autograft versus allograft: an economic cost comparison of anterior cruciate ligament reconstruction. Arthroscopy. 2011;27(9):1219–1225. doi: 10.1016/j.arthro.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 70.Li H., Tao H., Cho S., Chen S., Yao Z., Chen S. Difference in graft maturity of the reconstructed anterior cruciate ligament 2 years postoperatively: a comparison between autografts and allografts in young men using clinical and 3.0-T magnetic resonance imaging evaluation. Am J Sports Med. 2012;40(7):1519–1526. doi: 10.1177/0363546512443050. [DOI] [PubMed] [Google Scholar]
- 71.Kainer M.A., Linden J.V., Whaley D.N., Holmes H.T., Jarvis W.R., Jernigan D.B., et al. Clostridium infections associated with musculoskeletal-tissue allografts. N Engl J Med. 2004;350(25):2564–2571. doi: 10.1056/NEJMoa023222. [DOI] [PubMed] [Google Scholar]
- 72.Sikka R.S., Narvy S.J., Vangsness C.T., Jr. Anterior cruciate ligament allograft surgery: underreporting of graft source, graft processing, and donor age. Am J Sports Med. 2011;39(3):649–655. doi: 10.1177/0363546510382222. [DOI] [PubMed] [Google Scholar]
- 73.Crawford S.N., Waterman B.R., Lubowitz J.H. Long-term failure of anterior cruciate ligament reconstruction. Arthroscopy. 2013;29(9):1566–1571. doi: 10.1016/j.arthro.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 74.Bottoni C.R., Smith E.L., Shaha J., Shaha S.S., Raybin S.G., Tokish J.M., et al. Autograft versus allograft anterior cruciate ligament reconstruction: a prospective, randomized clinical study with a minimum 10-year follow-up. Am J Sports Med. 2015;43(10):2501–2509. doi: 10.1177/0363546515596406. [DOI] [PubMed] [Google Scholar]
- 75.Mariscalco M.W., Magnussen R.A., Kaeding C.C., Hewett T.E., Flanigan D.C. Use of irradiated and non-irradiated allograft tissue in anterior cruciate ligament reconstruction surgery: a critical analysis review. JBJS Reviews. 2014;2(2):e4. doi: 10.2106/JBJS.RVW.M.00083. [DOI] [PubMed] [Google Scholar]
- 76.Fideler B.M., Vangsness C.T., Jr., Lu B. Orlando C and Moore T. Gamma irradiation: effects on biomechanical properties of human bone-patellar tendon-bone allografts. Am J Sports Med. 1995;23(5):643–646. doi: 10.1177/036354659502300521. [DOI] [PubMed] [Google Scholar]
- 77.Guo L., Yang L., Duan X.J., He R., Chen G.X., Wang F.Y., et al. Anterior cruciate ligament reconstruction with bone-patellar tendon-bone graft: comparison of autograft, fresh-frozen allograft, and γ-irradiated allograft. Arthroscopy. 2012;28(2):211–217. doi: 10.1016/j.arthro.2011.08.314. [DOI] [PubMed] [Google Scholar]
- 78.Sun K., Tian S., Zhang J., Xia C., Zhang C., Yu T. Anterior cruciate ligament reconstruction with BPTB autograft, irradiated versus non-irradiated allograft: a prospective randomized clinical study. Knee Surg Sports Traumatol Arthrosc. 2009;17(5):464–474. doi: 10.1007/s00167-008-0714-8. [DOI] [PubMed] [Google Scholar]
- 79.Chinese Specialist Consensus Group on New Generation Artificial Ligaments Used for Anterior Cruciate Ligament Reconstruction. The indication of new generation artificial ligaments in anterior cruciate ligament reconstruction: consensus of Chinese specialists based on a modified Delphi method. Chin J Orthop. 2020;40(8):488–495. doi: 10.7507/1002-1892.202206026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chinese Specialist Consensus Group on New Generation Artificial Ligaments Used for Anterior Cruciate Ligament Reconstruction. Core techniques and adverse events in anterior cruciate ligament reconstruction using a new generation of artificial ligaments:the consensus of Chinese specialists based on a modified Delphi method(Part 2) Chin J Reparative Reconstr Surg. 2022;36(9):1–9. doi: 10.7507/1002-1892.202206026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen T., Chen S. Artificial ligaments applied in anterior cruciate ligament repair and reconstruction: current products and experience. Chin J Reparative Reconstr Surg. 2020;34(1):1–9. doi: 10.7507/1002-1892.201908084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dericks G. Ligament advanced reinforcementsystem anterior cruciate ligament reconstruction. Operat Tech Sports Med. 1995;3(3):187–205. [Google Scholar]
- 83.Noyes F.R., Grood E.S. The strength of the anterior cruciate ligament in humans and Rhesus monkeys. J Bone Joint Surg Am. 1976;58(8):1074–1082. [PubMed] [Google Scholar]
- 84.Chen T., Chen S. Walk out of the historical misunderstanding of artificial ligament used for anterior cruciate ligament reconstruction--sum up China's successful experience. Chinese Journal of the Frontiers of Medical Science(Electronic Version) 2020;12(9):1–7. [Google Scholar]
- 85.Chen T., Zhang P., Chen J., Hua Y., Chen S. Long-term outcomes of anterior cruciate ligament reconstruction using either synthetics with remnant preservation or hamstring autografts: a 10-year longitudinal study. Am J Sports Med. 2017;45(12):2739–2750. doi: 10.1177/0363546517721692. [DOI] [PubMed] [Google Scholar]
- 86.Wan F., Chen T., Ge Y., Zhang P., Chen S. Effect of nearly isometric ACL reconstruction on graft-tunnel motion: a quantitative clinical study. Orthop J Sports Med. 2019;7(12) doi: 10.1177/2325967119890382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.China Artificial Ligament Study Group Clinical multicenter investigation and analysis of the adverse events after primary anterior cruciate ligament reconstruction using the ligament advanced reinforcement system. Chinese Med J. 2022;102(41):3312–3320. doi: 10.3760/cma.j.cn112137-20220530-01192. [DOI] [PubMed] [Google Scholar]
- 88.Irrgang J.J., Anderson A.F., Boland A.L., Harner C.D., Kurosaka M., Neyret P., et al. Development and validation of the international knee documentation committee subjective knee form. Am J Sports Med. 2001;29(5):600–613. doi: 10.1177/03635465010290051301. [DOI] [PubMed] [Google Scholar]
- 89.Ross B.J., Savage-Elliott I., Brown S.M., Mulcahey M.K. Return to play and performance after primary ACL reconstruction in American football players: a systematic review. Orthop J Sports Med. 2020;8(10) doi: 10.1177/2325967120959654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Meredith S.J., Rauer T., Chmielewski T.L., Fink C., Diermeier T., Rothrauff B.B., et al. Return to sport after anterior cruciate ligament injury: panther symposium ACL injury return to sport consensus group. J ISAKOS. 2021;6(3):138–146. doi: 10.1136/jisakos-2020-000495. [DOI] [PubMed] [Google Scholar]
- 91.Samitier G., Marcano A.I., Alentorn-Geli E., Cugat R., Farmer K.W., Moser M.W. Failure of anterior cruciate ligament reconstruction. Arch Bone Jt Surg. 2015;3(4):220–240. [PMC free article] [PubMed] [Google Scholar]
- 92.Tapasvi S., Shekhar A. Revision ACL reconstruction: principles and practice. Indian J Orthop. 2021;55(2):263–275. doi: 10.1007/s43465-020-00328-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wylie J.D., Marchand L.S., Burks R.T. Etiologic factors that lead to failure after primary anterior cruciate ligament surgery. Clin Sports Med. 2017;36(1):155–172. doi: 10.1016/j.csm.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 94.Liechti D.J., Chahla J., Dean C.S., Mitchell J.J., Slette E., Menge T.J., et al. Outcomes and risk factors of rerevision anterior cruciate ligament reconstruction: a systematic review. Arthroscopy. 2016;32(10):2151–2159. doi: 10.1016/j.arthro.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 95.Morgan J.A., Dahm D., Levy B., Stuart M.J., Group M.S. Femoral tunnel malposition in ACL revision reconstruction. J Knee Surg. 2012;25(5):361–368. doi: 10.1055/s-0031-1299662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schuster P., Schulz M., Immendoerfer M., Mayer P., Schlumberger M., Richter J. Septic arthritis after arthroscopic anterior cruciate ligament reconstruction: evaluation of an arthroscopic graft-retaining treatment protocol. Am J Sports Med. 2015;43(12):3005–3012. doi: 10.1177/0363546515603054. [DOI] [PubMed] [Google Scholar]
- 97.Wang C., Lee Y.H., Siebold R. Recommendations for the management of septic arthritis after ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2014;22(9):2136–2144. doi: 10.1007/s00167-013-2648-z. [DOI] [PubMed] [Google Scholar]
- 98.Pérez-Prieto D., Totlis T., Madjarevic T., Becker R., Ravn C., Monllau J.C., et al. ESSKA and EBJIS recommendations for the management of infections after anterior cruciate ligament reconstruction (ACL-R): prevention, surgical treatment and rehabilitation. Knee Surg Sports Traumatol Arthrosc. 2023;31(10):4204–4212. doi: 10.1007/s00167-023-07463-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Milewski M.D., Traver J.L., Coene R.P., Williams K., Sugimoto D., Kramer D.E., et al. Effect of age and sex on psychological readiness and patient-reported outcomes 6 Months after primary ACL reconstruction. Orthop J Sports Med. 2023;11(6) doi: 10.1177/23259671231166012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schmitz J.K., Omar O., Nordkvist A., Hedevik H., Janarv P.M., Stålman A. Poorer patient-reported outcome and increased risk of revision at a 5-year follow-up among patients with septic arthritis following anterior cruciate ligament reconstruction: a register-based cohort study of 23,075 primary anterior cruciate ligament reconstructions. Knee Surg Sports Traumatol Arthrosc. 2023;31(10):4090–4098. doi: 10.1007/s00167-023-07498-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sroufe M.D., Sumpter A.E., Thompson X.D., Moran T.E., Bruce Leicht A.S., Diduch D.R., et al. Comparison of patient-reported outcomes, strength, and functional performance in primary versus revision anterior cruciate ligament reconstruction. Am J Sports Med. 2023;51(8):2057–2063. doi: 10.1177/03635465231169535. [DOI] [PubMed] [Google Scholar]
- 102.Altmann P., Ponleitner M., Monschein T., Krajnc N., Zulehner G., Zrzavy T., et al. Feasibility of a smartphone app to monitor patient reported outcomes in multiple sclerosis: the haMSter interventional trial. Digit Health. 2022;8 doi: 10.1177/20552076221135387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nagino K., Sung J., Midorikawa-Inomata A., Eguchi A., Fujimoto K., Okumura Y., et al. The minimal clinically important difference of app-based electronic patient-reported outcomes for hay fever. Clin Transl Allergy. 2023;13(5) doi: 10.1002/clt2.12244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chamberlain A.M. Editorial commentary: legacy patient-reported outcome measures remain important today despite responder burden, but with further refinement, patient-reported outcomes measurement information system could replace legacy instruments in the future. Arthroscopy. 2023;39(3):853–855. doi: 10.1016/j.arthro.2022.08.032. [DOI] [PubMed] [Google Scholar]
- 105.Webster K.E. Editorial commentary: designing patient-reported outcome measures that have high clinical utility and minimum responder burden: when less is more. Arthroscopy. 2023;39(5):1139–1140. doi: 10.1016/j.arthro.2022.11.028. [DOI] [PubMed] [Google Scholar]
- 106.Bjornsson H., Samuelsson K., Sundemo D., Desai N., Sernert N., Rostgard-Christensen L., et al. A randomized controlled trial with mean 16-year follow-up comparing hamstring and patellar tendon autografts in anterior cruciate ligament reconstruction. Am J Sports Med. 2016;44(9):2304–2313. doi: 10.1177/0363546516646378. [DOI] [PubMed] [Google Scholar]
- 107.Sanders T.L., Pareek A., Kremers H.M., Bryan A.J., Levy B.A., Stuart M.J., et al. Long-term follow-up of isolated ACL tears treated without ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2017;25(2):493–500. doi: 10.1007/s00167-016-4172-4. [DOI] [PubMed] [Google Scholar]
- 108.Zaffagnini S., Marcheggiani Muccioli G.M., Grassi A., Roberti di Sarsina T., Raggi F., Signorelli C., et al. Over-the-top ACL reconstruction plus extra-articular lateral tenodesis with hamstring tendon grafts: prospective evaluation with 20-year minimum follow-up. Am J Sports Med. 2017;45(14):3233–3242. doi: 10.1177/0363546517723013. [DOI] [PubMed] [Google Scholar]
- 109.Hirschmann M.T., Müller W. Complex function of the knee joint: the current understanding of the knee. Knee Surg Sports Traumatol Arthrosc. 2015;23(10):2780–2788. doi: 10.1007/s00167-015-3619-3. [DOI] [PubMed] [Google Scholar]
- 110.Musahl V., Karlsson J. Anterior cruciate ligament tear. N Engl J Med. 2019;380(24):2341–2348. doi: 10.1056/NEJMcp1805931. [DOI] [PubMed] [Google Scholar]
- 111.Hefti F., Muller W., Jakob R.P., Staubli H.U. Evaluation of knee ligament injuries with the IKDC form. Knee Surg Sports Traumatol Arthrosc. 1993;1(3–4):226–234. doi: 10.1007/BF01560215. [DOI] [PubMed] [Google Scholar]
- 112.Musahl V., Hoshino Y., Becker R., Karlsson J. Rotatory knee laxity and the pivot shift. Knee Surg Sports Traumatol Arthrosc. 2012;20(4):601–602. doi: 10.1007/s00167-011-1844-y. [DOI] [PubMed] [Google Scholar]
- 113.Hurley W.L., Thompson McGuire D. Influences of clinician technique on performance and interpretation of the lachman test. J Athl Train. 2003;38(1):34–43. [PMC free article] [PubMed] [Google Scholar]
- 114.Horvath A., Meredith S.J., Nishida K., Hoshino Y., Musahl V. Objectifying the pivot shift test. Sports Med Arthrosc Rev. 2020;28(2):36–40. doi: 10.1097/JSA.0000000000000260. [DOI] [PubMed] [Google Scholar]
- 115.Runer A., Roberti di Sarsina T., Starke V., Iltchev A., Felmet G., Braun S., et al. The evaluation of Rolimeter, KLT, KiRA and KT-1000 arthrometer in healthy individuals shows acceptable intra-rater but poor inter-rater reliability in the measurement of anterior tibial knee translation. Knee Surg Sports Traumatol Arthrosc. 2021;29(8):2717–2726. doi: 10.1007/s00167-021-06540-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Myrer J.W., Schulthies S.S., Fellingham G.W. Relative and absolute reliability of the KT-2000 arthrometer for uninjured knees. Testing at 67, 89, 134, and 178 N and manual maximum forces. Am J Sports Med. 1996;24(1):104–108. doi: 10.1177/036354659602400119. [DOI] [PubMed] [Google Scholar]
- 117.Liu W., Maitland M.E., Bell G.D. A modeling study of partial ACL injury: simulated KT-2000 arthrometer tests. J Biomech Eng. 2002;124(3):294–301. doi: 10.1115/1.1468636. [DOI] [PubMed] [Google Scholar]
- 118.Alqahtani Y., Murgier J., Beaufils P., Boisrenoult P., Steltzlen C., Pujol N. Anterior tibial laxity using the GNRB® device in healthy knees. Knee. 2018;25(1):34–39. doi: 10.1016/j.knee.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 119.Cojean T., Batailler C., Robert H., Cheze L. GNRB® laximeter with magnetic resonance imaging in clinical practice for complete and partial anterior cruciate ligament tears detection: a prospective diagnostic study with arthroscopic validation on 214 patients. Knee. 2023;42:373–381. doi: 10.1016/j.knee.2023.03.017. [DOI] [PubMed] [Google Scholar]
- 120.Kocher M.S., Steadman J.R., Briggs K.K., Sterett W.I., Hawkins R.J. Relationships between objective assessment of ligament stability and subjective assessment of symptoms and function after anterior cruciate ligament reconstruction. Am J Sports Med. 2004;32(3):629–634. doi: 10.1177/0363546503261722. [DOI] [PubMed] [Google Scholar]
- 121.Hoshino Y., Kuroda R., Nagamune K., Yagi M., Mizuno K., Yamaguchi M., et al. In vivo measurement of the pivot-shift test in the anterior cruciate ligament-deficient knee using an electromagnetic device. Am J Sports Med. 2007;35(7):1098–1104. doi: 10.1177/0363546507299447. [DOI] [PubMed] [Google Scholar]
- 122.Benjaminse A., Gokeler A., van der Schans C.P. Clinical diagnosis of an anterior cruciate ligament rupture: a meta-analysis. J Orthop Sports Phys Ther. 2006;36(5):267–288. doi: 10.2519/jospt.2006.2011. [DOI] [PubMed] [Google Scholar]
- 123.Bagiella E., Chang H. Power analysis and sample size calculation. J Mol Cell Cardiol. 2019;133:214–216. doi: 10.1016/j.yjmcc.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 124.Ueki C., Sakaguchi G. Importance of awareness of type II error. Ann Thorac Surg. 2018;105(1):333. doi: 10.1016/j.athoracsur.2017.03.062. [DOI] [PubMed] [Google Scholar]
- 125.Charles P., Giraudeau B., Dechartres A., Baron G., Ravaud P. Reporting of sample size calculation in randomised controlled trials: review. BMJ. 2009;338 doi: 10.1136/bmj.b1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Stokes L. Sample size calculation for a hypothesis test. JAMA. 2014;312(2):180–181. doi: 10.1001/jama.2014.8295. [DOI] [PubMed] [Google Scholar]
- 127.Barrett A.M., Craft J.A., Replogle W.H., Hydrick J.M., Barrett G.R. Anterior cruciate ligament graft failure: a comparison of graft type based on age and Tegner activity level. Am J Sports Med. 2011;39(10):2194–2198. doi: 10.1177/0363546511415655. [DOI] [PubMed] [Google Scholar]
- 128.Kim K.T., Kim H.J., Lee H.I., Park Y.J., Kang D.G., Yoo J.I., et al. A comparison of results after anterior cruciate ligament reconstruction in over 40 and under 40 Years of age: a meta-analysis. Knee Surg Relat Res. 2018;30(2):95–106. doi: 10.5792/ksrr.17.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Webster K.E., Nagelli C.V., Hewett T.E., Feller J.A. Factors associated with psychological readiness to return to sport after anterior cruciate ligament reconstruction surgery. Am J Sports Med. 2018;46(7):1545–1550. doi: 10.1177/0363546518773757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Filbay S.R., Culvenor A.G., Ackerman I.N., Russell T.G., Crossley K.M. Quality of life in anterior cruciate ligament-deficient individuals: a systematic review and meta-analysis. Br J Sports Med. 2015;49(16):1033–1041. doi: 10.1136/bjsports-2015-094864. [DOI] [PubMed] [Google Scholar]
- 131.Filbay S.R., Grevnerts H.T., Sonesson S., Hedevik H., Kvist J. The Swedish version of the Anterior Cruciate Ligament Quality of Life measure (ACL-QOL): translation and measurement properties. Qual Life Res. 2023;32(2):593–604. doi: 10.1007/s11136-022-03265-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Roos E.M., Roos H.P., Lohmander L.S., Ekdahl C., Beynnon B.D. Knee injury and osteoarthritis outcome score (KOOS)--development of a self-administered outcome measure. J Orthop Sports Phys Ther. 1998;28(2):88–96. doi: 10.2519/jospt.1998.28.2.88. [DOI] [PubMed] [Google Scholar]
- 133.Lysholm J., Gillquist J. Evaluation of knee ligament surgery results with special emphasis on use of a scoring scale. Am J Sports Med. 1982;10(3):150–154. doi: 10.1177/036354658201000306. [DOI] [PubMed] [Google Scholar]
- 134.Marx R.G., Stump T.J., Jones E.C., Wickiewicz T.L., Warren R.F. Development and evaluation of an activity rating scale for disorders of the knee. Am J Sports Med. 2001;29(2):213–218. doi: 10.1177/03635465010290021601. [DOI] [PubMed] [Google Scholar]
- 135.Tegner Y., Lysholm J. Rating systems in the evaluation of knee ligament injuries. Clin Orthop Relat Res. 1985;198:43–49. [PubMed] [Google Scholar]
- 136.Krych A.J., Robertson C.M., Williams R.J., 3rd Return to athletic activity after osteochondral allograft transplantation in the knee. Am J Sports Med. 2012;40(5):1053–1059. doi: 10.1177/0363546511435780. [DOI] [PubMed] [Google Scholar]
- 137.Randsborg P.H., Cepeda N., Adamec D., Rodeo S.A., Ranawat A., Pearle A.D. Patient-reported outcome, return to sport, and revision rates 7-9 Years after anterior cruciate ligament reconstruction: results from a cohort of 2042 patients. Am J Sports Med. 2022;50(2):423–432. doi: 10.1177/03635465211060333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Paterno M.V., Schmitt L.C., Ford K.R., Rauh M.J., Myer G.D., Huang B., et al. Biomechanical measures during landing and postural stability predict second anterior cruciate ligament injury after anterior cruciate ligament reconstruction and return to sport. Am J Sports Med. 2010;38(10):1968–1978. doi: 10.1177/0363546510376053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Musahl V., Herbst E., Burnham J.M., Fu F.H. The anterolateral complex and anterolateral ligament of the knee. J Am Acad Orthop Surg. 2018;26(8):261–267. doi: 10.5435/JAAOS-D-16-00758. [DOI] [PubMed] [Google Scholar]
- 140.Head P.L., Kasser R., Appling S., Cappaert T., Singhal K., Zucker-Levin A. Anterior cruciate ligament reconstruction and dynamic stability at time of release for return to sport. Phys Ther Sport. 2019;38:80–86. doi: 10.1016/j.ptsp.2019.04.016. [DOI] [PubMed] [Google Scholar]
- 141.Chen J., Kim J., Shao W., Schlecht S.H., Baek S.Y., Jones A.K., et al. An anterior cruciate ligament failure mechanism. Am J Sports Med. 2019;47(9):2067–2076. doi: 10.1177/0363546519854450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Miller M.D., Kew M.E., Quinn C.A. Anterior cruciate ligament revision reconstruction. J Am Acad Orthop Surg. 2021;29(17):723–731. doi: 10.5435/JAAOS-D-21-00088. [DOI] [PubMed] [Google Scholar]
- 143.Cinque M.E., Chahla J., Moatshe G., DePhillipo N.N., Kennedy N.I., Godin J.A., et al. Outcomes and complication rates after primary anterior cruciate ligament reconstruction are similar in younger and older patients. Orthop J Sports Med. 2017;5(10) doi: 10.1177/2325967117729659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Taylor M.Z., Caldwell P.E., 3rd, Pearson S.E. Failure and complication rates in common sports and arthroscopic procedures: reality check. Sports Med Arthrosc Rev. 2022;30(1):10–16. doi: 10.1097/JSA.0000000000000338. [DOI] [PubMed] [Google Scholar]
- 145.Røtterud J.H., Sivertsen E.A., Forssblad M., Engebretsen L., Arøen A. Effect of meniscal and focal cartilage lesions on patient-reported outcome after anterior cruciate ligament reconstruction: a nationwide cohort study from Norway and Sweden of 8476 patients with 2-year follow-up. Am J Sports Med. 2013;41(3):535–543. doi: 10.1177/0363546512473571. [DOI] [PubMed] [Google Scholar]
- 146.Runer A., Wierer G., Herbst E., Hepperger C., Herbort M., Gföller P., et al. There is no difference between quadriceps- and hamstring tendon autografts in primary anterior cruciate ligament reconstruction: a 2-year patient-reported outcome study. Knee Surg Sports Traumatol Arthrosc. 2018;26(2):605–614. doi: 10.1007/s00167-017-4554-2. [DOI] [PubMed] [Google Scholar]
- 147.Mayr H.O., Willkomm D., Stoehr A., Schettle M., Suedkamp N.P., Bernstein A., et al. Revision of anterior cruciate ligament reconstruction with patellar tendon allograft and autograft: 2- and 5-year results. Arch Orthop Trauma Surg. 2012;132(6):867–874. doi: 10.1007/s00402-012-1481-z. [DOI] [PubMed] [Google Scholar]
- 148.Bigouette J.P., Owen E.C., Lantz B.B.A., Hoellrich R.G., Huston L.J., Haas A.K., et al. Relationship between sports participation after revision anterior cruciate ligament reconstruction and 2-year patient-reported outcome measures. Am J Sports Med. 2019;47(9):2056–2066. doi: 10.1177/0363546519856348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.von Recum J., Gehm J., Guehring T., Vetter S.Y., von der Linden P., Grützner P.A., et al. Autologous bone graft versus silicate-substituted calcium phosphate in the treatment of tunnel defects in 2-stage revision anterior cruciate ligament reconstruction: a prospective, randomized controlled study with a minimum follow-up of 2 years. Arthroscopy. 2020;36(1):178–185. doi: 10.1016/j.arthro.2019.07.035. [DOI] [PubMed] [Google Scholar]
- 150.Øiestad B.E., Engebretsen L., Storheim K., Risberg M.A. Knee osteoarthritis after anterior cruciate ligament injury: a systematic review. Am J Sports Med. 2009;37(7):1434–1443. doi: 10.1177/0363546509338827. [DOI] [PubMed] [Google Scholar]
- 151.Luc B., Gribble P.A., Pietrosimone B.G. Osteoarthritis prevalence following anterior cruciate ligament reconstruction: a systematic review and numbers-needed-to-treat analysis. J Athl Train. 2014;49(6):806–819. doi: 10.4085/1062-6050-49.3.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Vairo G.L., McBrier N.M., Miller S.J., Buckley W.E. Premature knee osteoarthritis after anterior cruciate ligament reconstruction dependent on autograft. J Sport Rehabil. 2010;19(1):86–97. doi: 10.1123/jsr.19.1.86. [DOI] [PubMed] [Google Scholar]
- 153.Bhandari M., Giannoudis P.V. Evidence-based medicine: what it is and what it is not. Injury. 2006;37(4):302–306. doi: 10.1016/j.injury.2006.01.034. [DOI] [PubMed] [Google Scholar]
- 154.Somerson J.S., Bartush K.C., Shroff J.B., Bhandari M., Zelle B.A. Loss to follow-up in orthopaedic clinical trials: a systematic review. Int Orthop. 2016;40(11):2213–2219. doi: 10.1007/s00264-016-3212-5. [DOI] [PubMed] [Google Scholar]