Abstract
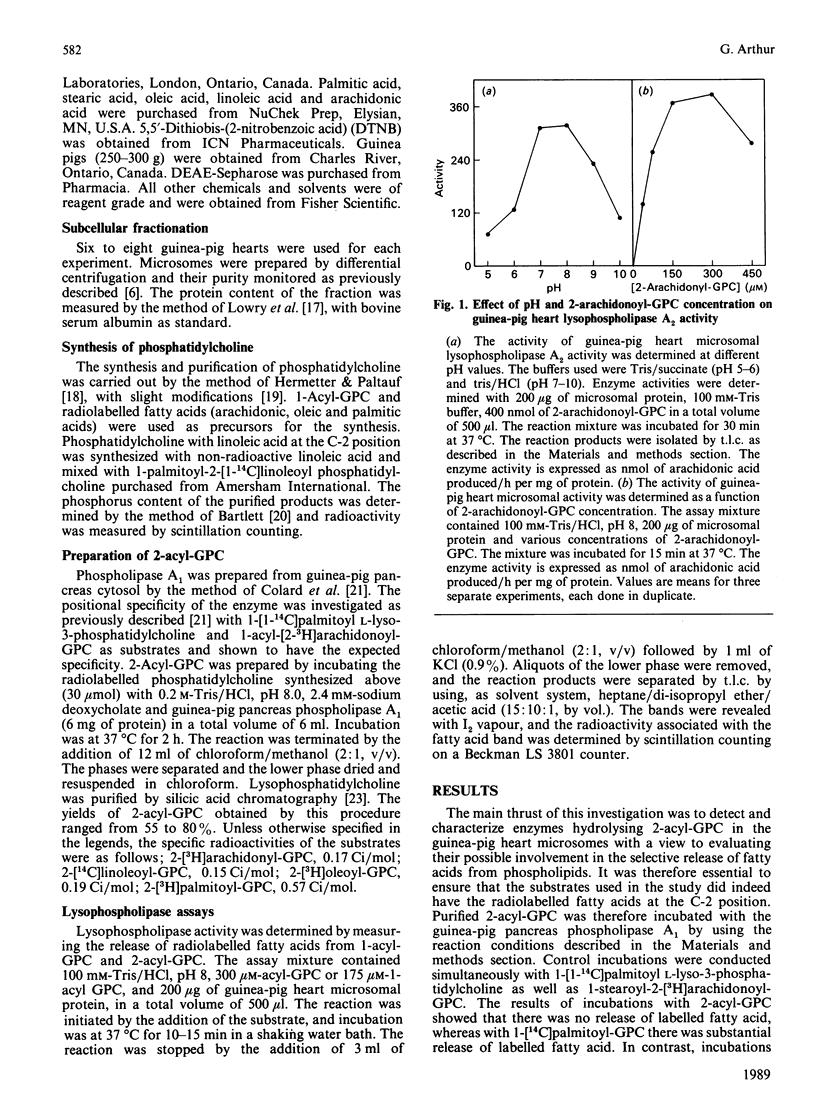
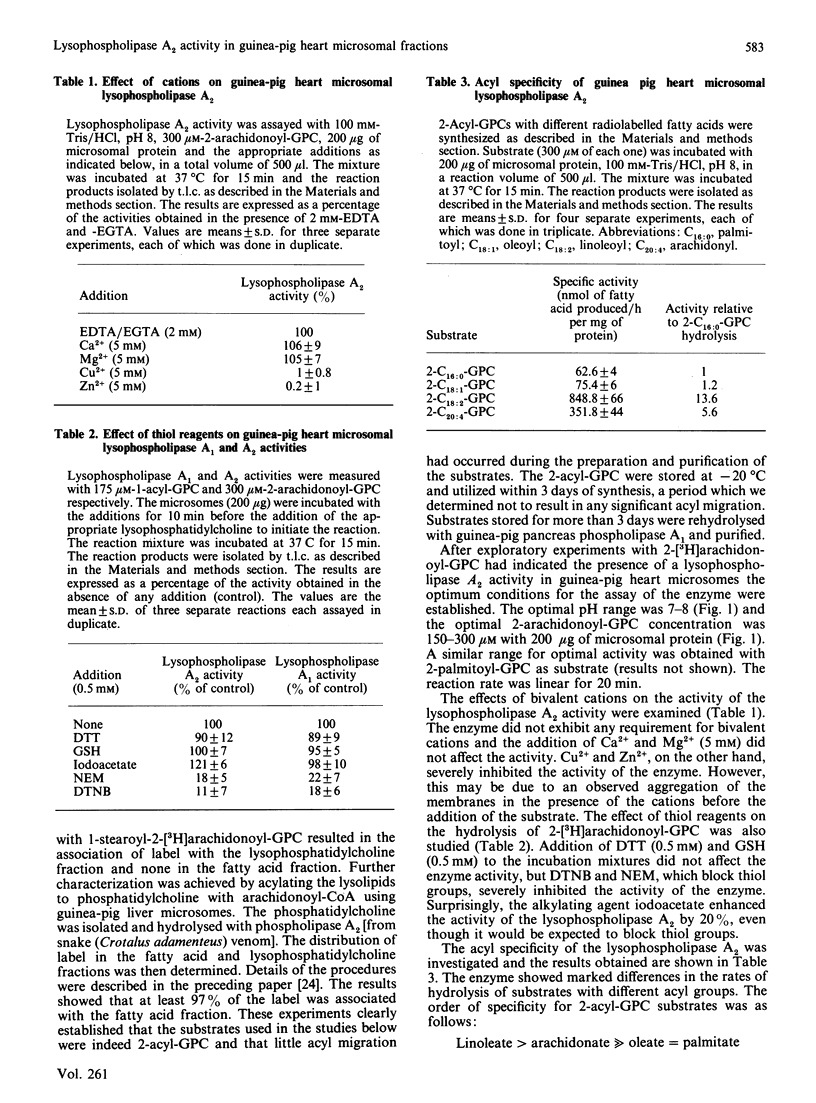
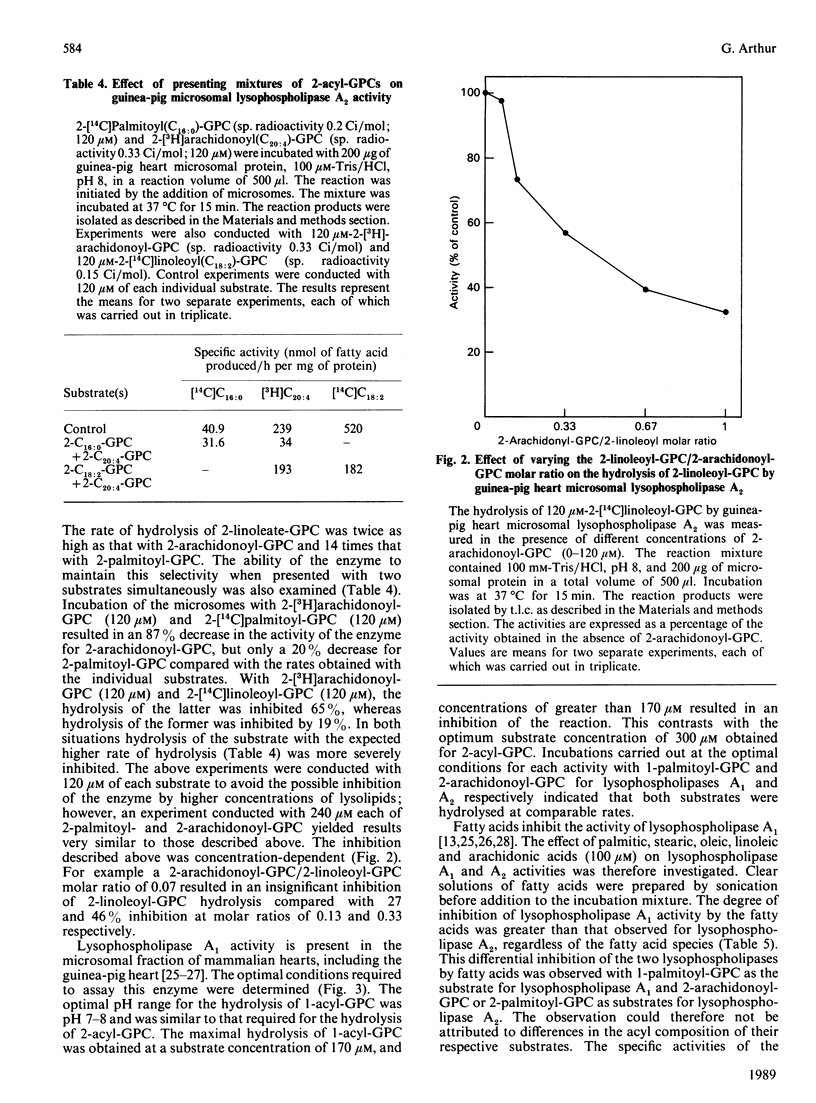
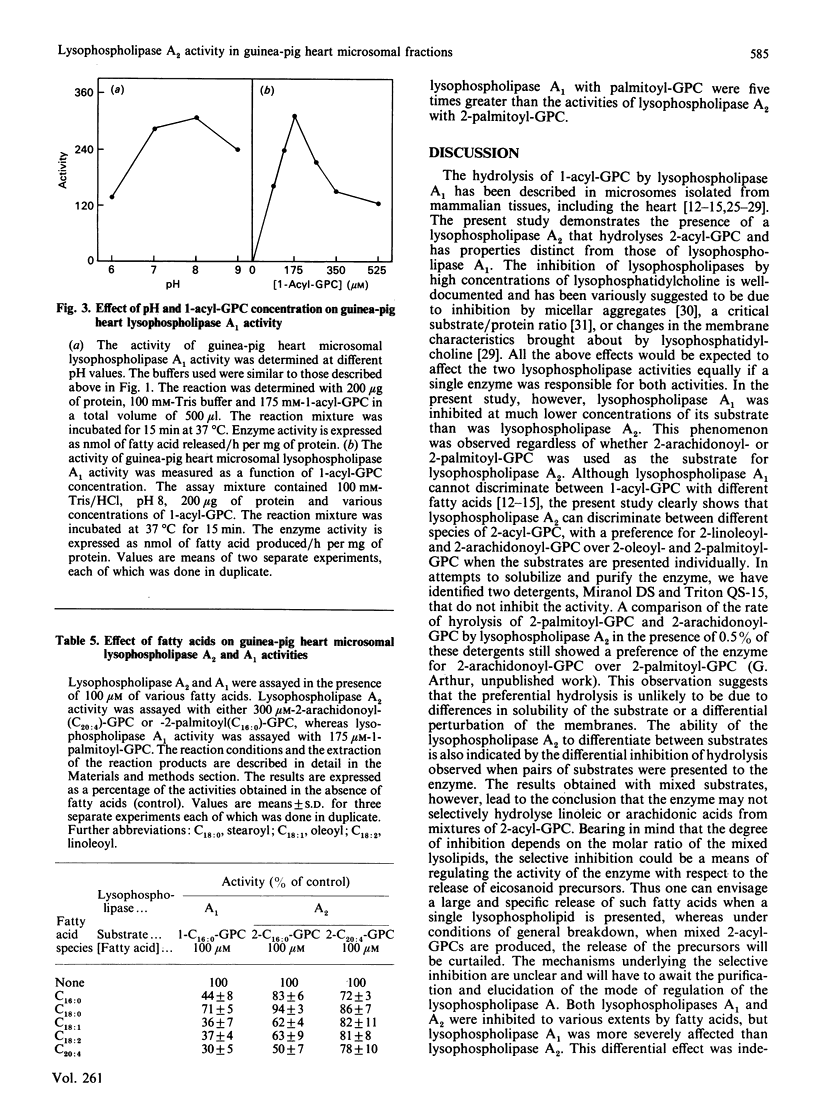
Lysophospholipases A1 which catalyse the hydrolysis of acyl groups from 1-acylglycerophosphocholine (GPC) have been characterized in a number of mammalian tissues and do not exhibit any acyl specificity. In the present study lysophospholipase activity in guinea-pig heart microsomes (microsomal fractions) that hydrolyses 2-acyl-GPC was detected and characterized. The enzyme showed a high degree of acyl specificity. The relative rates of hydrolysis of individual 2-acyl-GPCs with different fatty acids was as follows: C18:2/C20:1/C18:1/C16:0, 14:6:1:1. When substrates were presented in pairs, the hydrolysis of each substrate by the enzyme was inhibited, but to very different extents. Of each pair of lysolipids examined (2-arachidonoyl- and 2-palmitoyl-GPC; 2-arachidonoyl- and 2-linoleoyl-GPC), the one with the expected higher rate of hydrolysis was more severely inhibited and the degree of inhibition was dependent on the concentration of the other lysolipid. The characteristics of the lysophospholipase A2 suggest the enzyme could work in concert with phospholipase A1 to release arachidonic and linoeic acids for further metabolism. The properties of lysophospholipase A2 and A1 suggest that they are different enzymes.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arthur G. Acylation of 2-acyl-glycerophosphocholine in guinea-pig heart microsomal fractions. Biochem J. 1989 Jul 15;261(2):575–580. doi: 10.1042/bj2610575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur G., Mock T., Zaborniak C., Choy P. C. The distribution and acyl composition of plasmalogens in guinea pig heart. Lipids. 1985 Oct;20(10):693–698. doi: 10.1007/BF02534389. [DOI] [PubMed] [Google Scholar]
- Arthur G., Page L., Mock T., Choy P. C. The catabolism of plasmenylcholine in the guinea pig heart. Biochem J. 1986 Jun 1;236(2):475–480. doi: 10.1042/bj2360475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Buchanan M. R., Butt R. W., Magas Z., van Ryn J., Hirsh J., Nazir D. J. Endothelial cells produce a lipoxygenase derived chemo-repellent which influences platelet/endothelial cell interactions--effect of aspirin and salicylate. Thromb Haemost. 1985 Jun 24;53(3):306–311. [PubMed] [Google Scholar]
- Buchanan M. R., Haas T. A., Lagarde M., Guichardant M. 13-Hydroxyoctadecadienoic acid is the vessel wall chemorepellant factor, LOX. J Biol Chem. 1985 Dec 25;260(30):16056–16059. [PubMed] [Google Scholar]
- Cao Y. Z., Tam S. W., Arthur G., Chen H., Choy P. C. The purification and characterization of a phospholipase A in hamster heart cytosol for the hydrolysis of phosphatidylcholine. J Biol Chem. 1987 Dec 15;262(35):16927–16935. [PubMed] [Google Scholar]
- Colard O., Breton M., Bereziat G. Arachidonate mobilization in diacyl, alkylacyl and alkenylacyl phospholipids on stimulation of rat platelets by thrombin and the Ca2+ ionophore A23187. Biochem J. 1986 Feb 1;233(3):691–695. doi: 10.1042/bj2330691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauvel J., Bonnefis M. J., Sarda L., Chap H., Thouvenot J. P., Douste-Blazy L. Purification of two lipases with high phospholipase A1 activity from guinea-pig pancreas. Biochim Biophys Acta. 1981 Feb 23;663(2):446–456. doi: 10.1016/0005-2760(81)90173-9. [DOI] [PubMed] [Google Scholar]
- Giffin M., Arthur G., Choy P. C., Man R. Y. Lysophosphatidylcholine metabolism and cardiac arrhythmias. Can J Physiol Pharmacol. 1988 Mar;66(3):185–189. doi: 10.1139/y88-032. [DOI] [PubMed] [Google Scholar]
- Gross R. W. High plasmalogen and arachidonic acid content of canine myocardial sarcolemma: a fast atom bombardment mass spectroscopic and gas chromatography-mass spectroscopic characterization. Biochemistry. 1984 Jan 3;23(1):158–165. doi: 10.1021/bi00296a026. [DOI] [PubMed] [Google Scholar]
- Gross R. W., Sobel B. E. Lysophosphatidylcholine metabolism in the rabbit heart. Characterization of metabolic pathways and partial purification of myocardial lysophospholipase-transacylase. J Biol Chem. 1982 Jun 25;257(12):6702–6708. [PubMed] [Google Scholar]
- Gross R. W., Sobel B. E. Rabbit myocardial cytosolic lysophospholipase. Purification, characterization, and competitive inhibition by L-palmitoyl carnitine. J Biol Chem. 1983 Apr 25;258(8):5221–5226. [PubMed] [Google Scholar]
- Irvine R. F. How is the level of free arachidonic acid controlled in mammalian cells? Biochem J. 1982 Apr 15;204(1):3–16. doi: 10.1042/bj2040003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leibovitz-BenGershon Z., Kobiler I., Gatt S. Lysophospholipases of rat brain. J Biol Chem. 1972 Nov 10;247(21):6840–6847. [PubMed] [Google Scholar]
- Leli U., Hauser G. Mechanism of modification of rat brain lysophospholipase A activity by cationic amphiphilic drugs. Biochim Biophys Acta. 1987 Apr 3;918(2):126–135. doi: 10.1016/0005-2760(87)90187-1. [DOI] [PubMed] [Google Scholar]
- Lokesh B. R., Kinsella J. E. Intracellular calcium does not appear to be essential for arachidonic acid release from stimulated macrophages as shown by studies with Quin-2. Biochim Biophys Acta. 1985 Apr 22;845(1):101–108. doi: 10.1016/0167-4889(85)90060-6. [DOI] [PubMed] [Google Scholar]
- Pollock W. K., Rink T. J., Irvine R. F. Liberation of [3H]arachidonic acid and changes in cytosolic free calcium in fura-2-loaded human platelets stimulated by ionomycin and collagen. Biochem J. 1986 May 1;235(3):869–877. doi: 10.1042/bj2350869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severson D. L., Fletcher T. Regulation of lysophosphatidylcholine-metabolizing enzymes in isolated myocardial cells from rat heart. Can J Physiol Pharmacol. 1985 Aug;63(8):944–951. doi: 10.1139/y85-156. [DOI] [PubMed] [Google Scholar]
- Sun G. Y., Tang W., Huang S. F., MacQuarrie R. Lysophospholipase activity in rat brain subcellular fractions. Neurochem Res. 1987 May;12(5):451–458. doi: 10.1007/BF00972297. [DOI] [PubMed] [Google Scholar]
- de Jong J. G., van den Bosch H., Aarsman A. J., van Deenen L. L. Studies on lysophospholipases. II. Substrate specificity of a lysolecithin hydrolyzing carboxylesterase from beef pancreas. Biochim Biophys Acta. 1973 Jan 19;296(1):105–115. doi: 10.1016/0005-2760(73)90049-0. [DOI] [PubMed] [Google Scholar]
- van den Bosch H., Aarsman A. J., Slotboom A. J., van Deenen L. L. On the specificity of rat-liver lysophospholipase. Biochim Biophys Acta. 1968 Oct 22;164(2):215–225. doi: 10.1016/0005-2760(68)90148-3. [DOI] [PubMed] [Google Scholar]
- van den Bosch H. Intracellular phospholipases A. Biochim Biophys Acta. 1980 Sep 30;604(2):191–246. doi: 10.1016/0005-2736(80)90574-x. [DOI] [PubMed] [Google Scholar]


