Abstract
The biologically active form of the human immunodeficiency virus type 1 (HIV-1) envelope (Env) glycoprotein is oligomeric. We previously described a soluble HIV-1 IIIB Env protein, gp140, with a stable oligomeric structure composed of uncleaved gp120 linked to the ectodomain of gp41 (P. L. Earl, C. C. Broder, D. Long, S. A. Lee, J. Peterson, S. Chakrabarti, R. W. Doms, and B. Moss, J. Virol. 68:3015–3026, 1994). Here we compared the antibody responses of rabbits to gp120 and gp140 that had been produced and purified in an identical manner. The gp140 antisera exhibited enhanced cross-reactivity with heterologous Env proteins as well as greater neutralization of HIV-1 compared to the gp120 antisera. To examine both immunogenicity and protective efficacy, we immunized rhesus macaques with oligomeric gp140. Strong neutralizing antibodies against a homologous virus and modest neutralization of heterologous laboratory-adapted isolates were elicited. No neutralization of primary isolates was observed. However, a substantial fraction of the neutralizing activity could not be blocked by a V3 loop peptide. After intravenous challenge with simian-HIV virus SHIV-HXB2, three of the four vaccinated macaques exhibited no evidence of virus replication.
Human immunodeficiency virus type 1 (HIV-1) vaccine development is currently focused on the design of immunogens that will stimulate both the humoral and cellular arms of the immune system (13, 14, 35, 36, 38, 45, 66). Strategies employing DNA and live viral vectors, alone or in combination, have been shown to elicit cellular responses (11, 18, 20, 24, 33, 37, 44, 65, 69). A vigorous humoral response, however, is best achieved by the inclusion of a protein boost (3, 16, 18, 29, 30). While antibodies may not be sufficient to confer protection, they may be critical in reducing viral loads during the initial stages of infection and allowing time for maturation of the cellular response, as has been found with murine virus infections (2, 34, 58).
The envelope (Env) glycoprotein is the major target of neutralizing antibodies to HIV-1 and consequently is the best candidate for stimulation of humoral immunity. The extensive variability of the Env protein, however, presents a major obstacle in designing an appropriate immunogen. Clinical studies with soluble gp120 vaccines have elicited antibodies with a narrow neutralization specificity, an inability to neutralize primary isolates (5, 6, 12, 31, 41, 43), and qualitatively different binding reactivity than those induced by HIV-1 infection (4, 75). Unlike sera from HIV-1-infected people, vaccinee sera react preferentially with epitopes on denatured gp120, bind efficiently to gp120 peptides, do not bind well to heterologous envs, and neutralize only T-cell line-adapted (TCLA) strains of HIV-1. In addition, vaccinee sera contain a preponderance of anti-V3 reactivity, accounting at least in part for the restricted neutralization properties (12, 46).
The monomeric structure of the gp120 vaccines may have been a factor in their poor immunogenicity. Several lines of evidence indicate that epitope exposure on oligomeric and monomeric Env differs in important ways. Many monoclonal antibodies that react well with monomeric gp120 do not react efficiently with oligomeric Env (50, 51, 68, 78), suggesting that dominant epitopes on gp120 are obscured on the oligomer. Some studies have indicated that binding of monoclonal antibodies to oligomeric but not monomeric Env correlates with neutralization (26, 54, 64, 67). In addition, sera from HIV-1-infected individuals contain predominantly antibodies to conformation-dependent epitopes, including those directed at oligomeric Env (49, 73). Another deficiency of the gp120 vaccines is the absence of epitopes in gp41 (25, 28), including one to which a very broadly neutralizing monoclonal antibody has been mapped (52). Thus, oligomeric Env may display epitopes important in eliciting antibodies capable of binding to the Env protein on virions and infected cells.
A second-generation vaccine would retain the epitopes present on oligomeric Env. In an attempt to make an immunogen that maintains native, conserved epitopes, we constructed an oligomeric Env, gp140, from the IIIB strain of HIV that contains all of gp120 and the ectodomain of gp41 (23). To allow efficient secretion, the gene was truncated just upstream of the transmembrane domain. In addition, 12 amino acids were deleted from the proteolytic cleavage site between gp120 and gp41 to prevent their dissociation. The Env protein thus generated is soluble, is secreted from infected cells, and efficiently binds soluble CD4. It is almost completely oligomeric, as judged by sucrose density gradient centrifugation, size exclusion chromatography, and chemical cross-linking. Immunization of mice with this soluble oligomeric Env induced primarily conformation-dependent antibodies, while those elicited by immunization with monomeric Env were mostly linear (23). In addition, the monoclonal antibodies generated to oligomeric gp140 bound efficiently to Env on the surface of HIV-1-infected cells, indicating the presence of common structural elements (23). Furthermore, greater binding of antibodies in HIV-positive human sera to soluble oligomeric than to monomeric Env has been shown (63, 77), and human and murine monoclonal antibodies that react preferentially with oligomeric Env have been identified (10, 57, 60). Nevertheless, some quantitative differences in the binding of nonneutralizing monoclonal antibodies to gp140 and membrane-associated Env have been reported (54).
The purpose of the present study was to compare the immunogenicity of similar preparations of gp140 and gp120 in rabbits and then test the better immunogen as a vaccine in rhesus macaques. The rabbit experiments indicated that the gp140-induced antibodies had better cross-reactivity in a binding assay and higher neutralizing titers against laboratory-adapted isolates than did gp120-induced antibodies. Therefore, rhesus macaques were immunized with purified gp140 and challenged with the homologous simian-HIV virus SHIV-HXB2. All animals were significantly protected, and no evidence of virus replication was detected in three of the four. Although our study did not include a direct comparison of the two immunogens in macaques, another group has found similar protection of macaques immunized with gp120 in QS21 (P. Berman et al., unpublished data).
MATERIALS AND METHODS
Viruses.
Recombinant vaccinia viruses vPE50 (15) and vPE12B (23), expressing the HIV-1 BH8 gp120 and gp140 Env proteins, respectively, were used for production of soluble, secreted Env proteins used for immunizations. The following recombinant vaccinia viruses expressing gp160 from different HIV-1 isolates (name of isolate in parentheses) were used: vCB29 (JRCSF), vCB34 (SF2), vCB36 (RF), vCB43 (Ba-L) (9), vCB51 (BK132) (22), and vBD3 (89.6) (21). HIV-1 NL4-3 and HIV-1 MN, used for neutralization assays with rabbit sera, were obtained from M. Martin (National Institute of Allergy and Infectious Diseases) and the National Institutes of Health (NIH) AIDS Research and Reference Reagent Program, respectively. For neutralization assays with monkey sera, the following viruses were used: HIV-MN (from R. Gallo) and HIV-1 SF2 (from the NIH AIDS Research and Reference Program, donated by J. Levy). SHIV-HXBc2 and SHIV-89.6 were propagated in either H9 cells or human peripheral blood mononuclear cells (PBMC).
Purification of soluble Env proteins.
Soluble gp120 and gp140 were produced by infection of BS-C-1 cells (ATCC CCL26) with recombinant vaccinia viruses at a multiplicity of infection of 5 to 10 PFU per cell. Two hours after infection, the medium was replaced with OptiMEM (Gibco-BRL, Grand Island, N.Y.), and infection was allowed to proceed for 24 to 36 h. Medium was harvested, and Env protein was purified by lentil lectin affinity chromatography (Amersham Pharmacia Biotech AB, Uppsala, Sweden) as previously described (23). For immunization of monkeys, the Env protein was concentrated with Centriprep-30 concentrators (Amicon, Beverly, Mass.) and further purified by Superdex-200 (Amersham Pharmacia Biotech AB).
Immunization of rabbits.
New Zealand White rabbits were housed at Spring Valley Laboratories, Woodbine, Md. Rabbits (three per group) were immunized three times with 70 μg of lentil lectin-purified gp120 or gp140 formulated with MPL-SE adjuvant (Ribi ImmunoChem, Hamilton, Mont.), a 1.0% (vol/vol) squaline oil-in-water emulsion containing 250 μg of monophosphoryl lipid A per ml, according to the manufacturer's specifications. Immunizations were performed at 4- to 6-week intervals, and the rabbits were bled 12 days after each immunization.
Purification of rabbit IgG.
Immunoglobulin Gs (IgGs) were purified from rabbit sera by absorption to HiTrap protein G columns (Amersham Pharmacia Biotech AB). Serum samples were diluted 10-fold with phosphate-buffered saline (PBS) prior to passage over the column. IgG was eluted with 0.1 M glycine-HCl (pH 2.7) into tubes containing the appropriate volume of Tris-HCl (pH 9.0), shown to neutralize the eluate. The buffer was then exchanged with PBS, and the IgG was concentrated using Centriprep-30 microconcentrators (Amicon). The concentrations of individual samples were determined by the A280 and adjusted to 2 mg/ml. As a control, IgG was also purified from preimmune sera. No reduction in virus replication was observed with the control sera, and no cell toxicity was observed with any of the samples.
Neutralization of HIV-1 with IgG from immunized rabbits.
The endpoint assay described in the 1997 Division of AIDS Virology Manual for HIV Laboratories was employed to determine the neutralizing activity of the IgG samples from rabbits immunized with gp140 or gp120 (71). In this assay, sequential dilutions of virus were mixed with a constant amount of IgG for 2 h, and residual virus infectivity was then determined. The percent neutralization was determined by comparison with virus infectivity observed with IgG from serum samples taken prior to immunization. Details of the protocol are as follows. Threefold serial dilutions of virus stock ranging from 54 to 0.22 tissue culture infectious doses (TCID50)/5 μl were prepared, and 25 μl of each dilution was mixed with 25 μl of a constant concentration of rabbit IgG in microcentrifuge tubes. For neutralization of HIV-1 NL4-3, concentrations of 1,000, 500, 250, 125, 62.5, 31.3, and 15.6 μg/ml were used. The same concentrations, excluding the lowest concentration, were used for neutralization of HIV-1 MN. Cell suspension (200 μl, 0.5 × 106/ml) was plated in the wells of 96-well flat-bottomed plates. After incubation for 2 h at 37°C, 10 μl of virus-IgG mixture was added to each well. Each virus-IgG mixture was assayed in quadruplicate. Half of the medium was changed on day 7, and reverse transcriptase activity was assayed on day 14.
Reverse transcriptase assay.
Supernatants from HIV-1-infected cultures were mixed with reverse transcription reaction mixture [50 mM Tris-HCl (pH 7.8), 63 mM KCl, 4.2 mM MgCl2, 0.85 mM EDTA, and 0.08% NP-40 plus 4.2 μg of poly(A) and 0.13 μg of oligo(dT) per ml] containing [α-32P]dTTP (Amersham Pharmacia Biotech AB) and incubated for 2 h at 37°C. Then 5 μl of the reaction mixture was spotted onto DEAE filter paper and washed twice with 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate). The filter paper was exposed to X-ray film overnight. The TCID50 values were calculated using the formula of Reed and Muench (61). Percent neutralization was determined by comparison of pre- and postimmunization IgG samples.
Immunization and challenge of monkeys.
Six juvenile rhesus macaques were obtained from the Oregon Regional Primate Research Center (Beaverton, Oreg.) and housed at Bioqual, Inc., Rockville, Md., in accordance with guidelines described in the Guide for the Care and Use of Laboratory Animals. Four monkeys were immunized with 300 μg of purified oligomeric gp140 in 100 μg of QS-21 adjuvant (Aquila Biopharmaceuticals, Inc., Framingham, Mass.) at 0, 4, 8, and 24 weeks. The two control monkeys received 100 μg of QS-21 in PBS at the same times. All six animals were challenged 3 weeks after the final immunization with 10 animal infectious doses of SHIV-HXB2, obtained from Yichen Lu (Virus Research Institute, Cambridge, Mass.) (39). Immediately after administration of the challenge virus, several immunized animals experienced various degrees of anaphylaxis, which was most severe in animal 18001. The likely explanation was the presence of trace amounts of serum proteins contaminating the gp140 and subsequent use of medium containing fetal bovine serum as the diluent for the challenge virus. Sera from the animals were determined, retrospectively, to contain antibodies to albumin (T. VanCott, unpublished).
Neutralization of SHIV-HXBc2, SHIV-89.6, HIV-1 SF2, and HIV-1 MN with sera from immunized monkeys.
Neutralization was measured in MT-2 cells as described previously (48). Titers of neutralizing antibodies are reported as the reciprocal of the serum dilution that protected 50% of cells from virus-induced cell killing as measured by neutral red dye uptake. Fifty percent protection corresponds to approximately 90% reduction in Gag antigen synthesis in this assay (12). The same assay was used to measure reductions in neutralization titers in the presence of 20 μg of V3 peptide per ml (19). Additional neutralization assays with SHIV-HXBc2 were performed in human PBMC as described (47). Neutralization titers in the PBMC assay are reported as the reciprocal serum dilution at which p27 synthesis was reduced 80% relative to the corresponding preimmunization serum sample for each animal tested.
p27 ELISA.
For the enzyme-linked immunosorbent assay (ELISA), Immunlon-2 (Dynex Technologies, Chantilly, Va.) 96-well U-bottomed plates were coated overnight with 0.5 μg of SIV p27 (Advanced Bioscience Laboratories, Kensington, Md.) per ml. Twofold serum dilutions of monkey sera were incubated overnight at 37°C in block buffer containing 5% goat serum and 0.02% sodium azide. After washing, horseradish peroxidase-conjugated anti-monkey IgG was added for 30 min, followed by BM Blue substrate. After 30 min, absorbance was read at 370 and 492 nm. All assays were performed in duplicate.
SHIV cocultivation from PBMC.
The PBMC fraction was isolated from whole blood samples by gradient centrifugation (Ficoll-Hypaque; Pharmacia, Piscataway, N.J.). Cell viability was determined using trypan blue. Cells were resuspended in medium at a concentration of 2 × 106 cells/ml. PBMC and target cells (174×CEM) were mixed and added to 96-well tissue culture plates in a total volume of 200 μl. Sample cells were assayed at six 10-fold dilutions starting at 1.5 × 106/ml. Target cells were used at 3.0 × 105/ml. Each dilution of sample cells was assayed in replicates of six. The experiment was carried out over a 28-day period. Culture medium was removed and replaced twice weekly. On days 10, 17, and 24, cell density was reduced by removal of approximately 75% of the cells in each well. On days 7, 14, 21, and 28, culture supernatants were collected and virus replication was monitored using the p27 ELISA antigen capture assay (Coulter/Immunotech). The challenge virus was used to infect 174×CEM cells and served as the positive control. Uninfected 174×CEM cells served as the negative control. A sample was considered positive for virus replication if the optical absorbance of a given well in the antigen capture ELISA was equal to or greater than three times the background reading.
Quantitative PCR of SHIV RNA.
Determination of the concentration of viral RNA in the plasma was performed as described (74).
RESULTS
Comparative immunogenicity of gp120 and gp140 in rabbits.
The gp120 and gp140 used for these studies were prepared using previously described recombinant vaccinia viruses vPE50 and vPE12B, respectively (15, 23). The soluble proteins were purified by lentil lectin chromatography from the serum-free medium of cells infected with the recombinant viruses. Virtually all of the gp140 was oligomeric, whereas approximately 60% of the gp120 remained monomeric. To compare the relative immunogenicity of gp120 and gp140, three rabbits were immunized three times with 70 μg of protein formulated in Ribi adjuvant MPL-SE. Reciprocal endpoint ELISA titers were determined using IIIB gp120. Twofold-higher titers were achieved by immunization with gp140 (341.3 [±118] × 103 than with gp120 (170.7 [±59] × 103). Since gp120 was the captured antigen, differences between the responses were not due to the presence of gp41 in the gp140 immunogen. To explore this difference further, binding to several heterologous gp160s and their respective shed gp120s was analyzed. For this purpose, antigens were prepared from lysates and medium from cells infected with recombinant vaccinia viruses expressing Env proteins from HIV-1 isolates IIIB, JRCSF, SF2, RF, Ba-L, 89.6, and BK132. Results are shown in Table 1 as the serum dilution at which 50% binding was observed. With each heterologous Env tested, the binding of anti-gp140 serum was two- to threefold greater than binding with anti-gp120 serum. This was true for binding to both gp160 and gp120. In addition, serum from a rabbit immunized with reduced, denatured gp140 was analyzed. This serum showed intermediate levels of binding, suggesting that epitopes exposed on the native gp140 are responsible for the enhanced binding.
TABLE 1.
Binding of rabbit sera to heterologous Envs
| Animal no. | Immunogen | Serum dilution giving 50% binding
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| gp160
|
gp120
|
||||||||||||
| IIIB | JRCSF | SF2 | RF | Ba-L | 89.6 | BK132 | IIIB | JRCSF | RF | 89.6 | BK132 | ||
| 2219 | gp120 | 7,940 | 1,120 | 5,440 | 3,640 | 4,920 | 3,290 | 870 | 21,020 | 2,810 | 4,780 | 2,750 | 2,440 |
| 2220 | gp120 | 8,200 | 1,570 | 1,990 | 2,020 | 2,870 | 2,150 | 1,760 | 23,160 | 5,510 | 4,450 | 1,990 | 4,100 |
| 2221 | gp120 | 5,530 | 1,100 | 1,210 | 1,280 | 2,240 | 1,590 | 690 | 11,590 | 2,250 | 2,330 | 1,250 | 2,120 |
| Avg ± SD | 7,223 ± 1,472 | 1,263 ± 266 | 2,880 ± 2,251 | 2,313 ± 1,207 | 3,343 ± 1,401 | 2,343 ± 866 | 1,107 ± 573 | 18,590 ± 6,156 | 3,523 ± 1,743 | 3,853 ± 1,330 | 1,997 ± 750 | 2,887 ± 1,063 | |
| 2164 | gp140 | 22,200 | 3,120 | 6,910 | 5,550 | 10,420 | 3,720 | 1,730 | 31,540 | 5,000 | 7,400 | 3,800 | 5,530 |
| 2165 | gp140 | 23,700 | 3,520 | 15,690 | 7,220 | 17,540 | 6,650 | 1,200 | 41,250 | 12,740 | 12,420 | 10,460 | 7,820 |
| 2166 | gp140 | 8,400 | 4,660 | 5,340 | 5,290 | 9,690 | 3,600 | 2,360 | 15,180 | 6,160 | 7,410 | 4,550 | 6,360 |
| Avg ± SD | 18,100 ± 8,434 | 3,767 ± 799 | 9,313 ± 5,577 | 6,020 ± 1,047 | 12,550 ± 4,337 | 4,657 ± 1,727 | 1,763 ± 581 | 29,323 ± 13,176 | 7,967 ± 4,174 | 9,077 ± 2,895 | 6,270 ± 3,648 | 6,570 ± 1,159 | |
| 2144 | Denatured gp140 | 9,260 | 2,290 | 3,950 | 4,920 | 6,050 | 3,170 | 4,830 | 9,510 | 2,340 | 4,180 | 2,620 | 7,720 |
We assayed the neutralization of two laboratory-adapted isolates, HIV-1 NL4-3 and MN (Table 2). Because of nonspecific effects of whole sera, IgG was purified, and results are given as the IgG concentration resulting in 50, 90, or 95% reduction in infectious titer. With the homologous HIV isolate, NL4-3, each of the anti-gp140 samples exhibited greater than 50% neutralization at the lowest IgG concentration used (15.6 μg/ml). They also exhibited 90% neutralization, one at the lowest concentration of IgG tested. In contrast, the anti-gp120 IgG samples had 50% neutralization of NL4-3 with 43 to 755 μg/ml, and none of the samples exhibited 90% neutralization. In addition, neutralization of the heterologous isolate, MN, was greater with the anti-gp140 IgGs than with the anti-gp120 IgGs. Together, these results suggested that the neutralizing activity generated by immunization with gp140 was not due entirely to anti-V3 activity. In addition, a low level of neutralization of isolate BZ167 was observed with gp140 IgG but not with gp120 IgG (data not shown).
TABLE 2.
Neutralization of laboratory-adapted isolatesa
| Animal no. | Immunogen | Concn of rabbit IgG required (μg/ml)
|
|||
|---|---|---|---|---|---|
| NL4-3
|
MN (50%) | ||||
| 50% | 90% | 95% | |||
| 2219 | gp120 | 755 | >1,000 | >1,000 | >1,000 |
| 2220 | 43 | >1,000 | >1,000 | >1,000 | |
| 2221 | 107 | >1,000 | >1,000 | >1,000 | |
| 2164 | gp140 | <15.6 | 161 | 579 | 850 |
| 2165 | <15.6 | <15.6 | 401 | 262 | |
| 2166 | <15.6 | 293 | 571 | <200 | |
Concentration of rabbit IgG required to neutralize 50, 90, and 95% of virus.
Immune responses to vaccination of rhesus macaques with gp140.
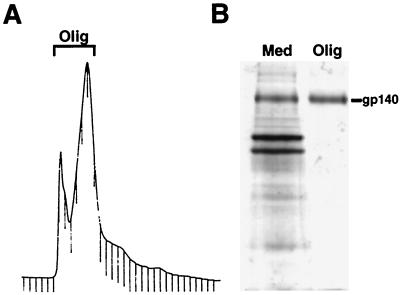
To test the immunogenicity of oligomeric gp140 in a nonhuman primate for which a homologous challenge was available, four rhesus macaques were immunized with purified oligomeric gp140. For this study, the lentil lectin-purified gp140 was further purified by chromatography on Superdex-200. A typical elution profile is shown in Fig. 1A. The fractions designated oligomer in the figure were pooled and used for immunizations. The leftmost peak is due to a buffer change from sample loading and does not contain gp140 or other proteins. Figure 1B demonstrates the purity of the gp140 compared to the starting material. Amino acid analysis of the purified protein verified that the sample was composed primarily of gp140 (data not shown). Intramuscular immunizations were performed at 0, 4, 8, and 24 weeks using 300 μg of gp140 in QS-21 adjuvant. Two control animals received PBS in QS-21.
FIG. 1.
Purification of oligomeric gp140. (A) Superdex-200 A280 elution profile of gp140. The bracketed fractions contain oligomeric gp140, as shown by chemical cross-linking (not shown). These fractions were pooled, concentrated, and used for immunization of macaques. (B) Coomassie blue staining of proteins in the medium of infected cells (Med) and in the purified Env (Olig) from panel A.
Humoral immune responses were monitored during the immunization period. Binding titers, as measured by ELISA using IIIB gp120, were boosted incrementally with each successive immunization but were not sustained (data not shown). Furthermore, the animals mounted neutralizing antibodies against the homologous virus, SHIV-HXB2, that were detectable in both the MT-2 and PBMC assays (Table 3). Titers ranged from 187 to 868 after three immunizations and increased to 313 to 1,315 after the fourth immunization, as measured in MT-2 cells. The titers achieved with four immunizations of gp140 are comparable to those found in monkeys infected with SHIV-HXB2 for 21 to 100 weeks (47). In addition, monkeys immunized with IIIB gp120 in QS-21 achieved similar neutralizing antibody titers after four immunizations (D. Montefiori, unpublished). Titers in the PBMC blast assay were on average 6.6 times lower than in the MT-2 assays but followed the same rank order in both assays.
TABLE 3.
Neutralizing antibody titer to SHIV-HXB2 in rhesus macaque seraa
| Animal no. | Immunogen | Titer at study wk
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MT-2 cell assay
|
PBMC assay, wk 26 | ||||||||||
| 10 | 12 | 20 | 26 | 31 | 33 | 37 | 53 | 150 | |||
| 17951 | gp140 | 187 | 134 | <20 | 424 | 147 | 49 | 23 | <20 | <20 | 45 |
| 18001 | 348 | 159 | 40 | 844 | 563 | 690 | 471 | 469 | 2,016 | 250 | |
| 18066 | 296 | 109 | <20 | 313 | 70 | 36 | 13 | <20 | 20 | 35 | |
| 18102 | 868 | 510 | 81 | 1,315 | 686 | 408 | 543 | 153 | 88 | 280 | |
| 18024 | None | <20 | <20 | <20 | <20 | 4,032 | 705 | 510 | 968 | 4,380 | |
| 18062 | <20 | <20 | <20 | <20 | <20 | 25 | 79 | 793 | 4,514 | ||
Neutralizing antibody titers are given as the reciprocal serum dilution at which 50% of cells were protected from virus-induced killing. Immunizations were performed on weeks 8 and 24. SHIV-HXB2 challenge was on week 27.
The breadth of neutralizing activity was examined with two heterologous TCLA strains of HIV-1 and SHIV-89.6 (Table 4). Neutralization of HIV-1 SF2 and HIV-1 MN occurred with sera from four and three animals, respectively, although the titers in each case were relatively low. In addition, serum from animal 18102 also neutralized SHIV-89.6. Serum samples obtained 2 weeks post-final boosting were also tested at 1:4 for the ability to neutralize six primary isolates of HIV-1 grown and assayed in human PBMC (56). No significant neutralization was observed (data not shown).
TABLE 4.
Neutralizing antibodies generated by IIIB gp140 immunizationa
| Animal no. | Immunogen | Neutralizing antibody titer to:
|
|||
|---|---|---|---|---|---|
| SHIV-HXB2 | SHIV-89.6 | HIV-1 MN | HIV-1 SF2 | ||
| 17951 | gp140 | 424 | <10 | 22 | 48 |
| 18001 | 844 | <10 | 23 | 44 | |
| 18066 | 313 | <10 | <10 | 19 | |
| 18102 | 1,315 | 16 | 33 | 48 | |
| 18024 | None | <20 | <10 | <10 | <10 |
| 18062 | <20 | <10 | <10 | <10 | |
Neutralizing antibody titers are given as the reciprocal serum dilution at which 50% of cells were protected from virus-induced killing. Serum was from week 26.
To determine the role of antibodies to V3 determinants, we assayed neutralization of SHIV-HXB2 after incubating serum samples for 2 h in the presence of a saturating concentration of IIIB V3 peptide. The amount of V3 peptide required was determined empirically using increasing concentrations of peptide. As shown in Table 5, a significant amount of neutralizing activity in sera from all four animals was not blocked by the linear V3 peptide, consistent with the demonstration of neutralization of several heterologous isolates.
TABLE 5.
Neutralization of SHIV-HXB2—adsorption with V3 peptidea
| Animal no. | Titer
|
% Non-V3b | |
|---|---|---|---|
| −V3 peptide | +V3 peptide | ||
| 17951 | 323 | 192 | 59 |
| 18001 | 422 | 174 | 41 |
| 18066 | 204 | 100 | 49 |
| 18102 | 1,241 | 284 | 23 |
Neutralizing antibody titer is given as the reciprocal serum dilution at which 50% of cells were protected from virus-induced cell killing.
Percentage of neutralizing activity found in the presence of V3 peptide.
Challenge of rhesus macaques with SHIV-HXB2.
To test the protective efficacy of immunization with oligomeric gp140, the four immunized and two control monkeys were challenged with 10 macaque infectious doses of SHIV-HXB2. The challenge was performed intravenously 3 weeks after the fourth protein immunization, when neutralizing antibodies were expected to be at a peak. Infection was monitored by cocultivation of PBMC with CEM×174 cells, levels of viral RNA in plasma, development of anti-p27 antibodies, and maintenance of neutralizing antibodies.
The TCID50 values of virus cultured from the PBMC at various times after challenge are given in Table 6. The control animals exhibited a peak of viremia at 2 weeks after challenge. In contrast, very little, if any, virus was observed in the PBMC of the immunized monkeys. Animals 17951 and 18102 were completely negative at all time points. A very small amount of virus was cultured from animal 18066 at 2 weeks postchallenge and was not observed at any other time point. Animal 18001 was positive at three isolated time points after challenge; however, the level was more than 100-fold less than that seen in the controls.
TABLE 6.
Isolation of virus from PBMC of rhesus macaquesa
| Animal no. | Immunogen | TCID50/106 cells at wk postchallenge:
|
||||
|---|---|---|---|---|---|---|
| 2 | 3 | 4 | 6 | 8 | ||
| 17951 | gp140 | NI | NI | NI | NI | NI |
| 18001 | NI | 3 | NI | NI | 1 | |
| 18066 | 1 | NI | NI | NI | NI | |
| 18102 | NI | NI | NI | NI | NI | |
| 18024 | None | 10,000 | 1 | 1 | 1 | 1 |
| 18062 | 10,000 | 10 | 1 | 1 | NI | |
Values given are TCID50/106 cells. NI, not infected. Samples from week 10 through week 26 were also assayed. Animals 18001, 18024, and 18062 were positive at one time point during this period; animals 17951, 18066, and 18102 were negative.
Viral RNA in the plasma was determined by a quantitative reverse transcription-PCR assay (Table 7), in which the limit of detection was 300 to 1,200 copies/ml of plasma, depending on the volume of sample available for the assay. No RNA was detected in three of the four immunized monkeys (17951, 18066, and 18102). A low but detectable amount of RNA was found in plasma from animal 18001 at 3 weeks postchallenge.
TABLE 7.
Plasma viral RNAa
| Animal no. | Immunogen | Copies of RNA/ml of plasma at indicated wk postchallenge:
|
||||
|---|---|---|---|---|---|---|
| 0 | 2 | 3 | 8 | 53 | ||
| 17951 | gp140 | <1,200 | <3,000 | <1,200 | <1,200 | <300 |
| 18001 | <1,200 | <3,000 | 1,600 | <1,200 | <300 | |
| 18066 | <1,200 | <1,200 | <1,200 | <1,200 | <300 | |
| 18102 | <1,200 | <1,200 | <1,200 | <1,200 | <300 | |
| 18024 | None | <1,200 | 94,000 | 11,000 | <1,200 | <300 |
| 18062 | <1,200 | n.t. | 8,600 | <1,200 | <300 | |
Values given are copies of RNA per milliliter of plasma. The sensitivity of the assay was dependent on the volume of sample available; more volume was used with week 53 samples than with other weeks. 0 wk, day of challenge; n.t. = not tested.
Development of p27 antibodies was also monitored after challenge (Table 8). Both naïve controls exhibited a rise in anti-p27 antibodies. Immunized animal 18001 also developed antibodies to p27, although the levels were consistently lower than those found in the two controls. In contrast, the other three animals did not develop anti-p27 antibodies, consistent with lack of infection. In addition, serum samples from the two controls and vaccinated animal 18001 reacted with p27 in a commercial HIV-2 Western blot kit (data not shown) and immunoprecipitated metabolically labeled p27 prepared from a recombinant vaccinia virus-infected cell lysate (data not shown).
TABLE 8.
p27 ELISA endpoint titersa
| Animal no. | Immunogen | Titer at wk postchallenge:
|
||||
|---|---|---|---|---|---|---|
| 0 | 6 | 12 | 34 | 124 | ||
| 17951 | gp140 | 0.2 | 0.2 | 0.1 | 0.1 | 0.1 |
| 18001 | 0.2 | 0.1 | 25.6 | 25.6 | 25.6 | |
| 18066 | 0.8 | 0.1 | 0.1 | 0.1 | 0.1 | |
| 18102 | 1.6 | 6.4 | 1.6 | 1.6 | 0.8 | |
| 18024 | None | 0.1 | 12.8 | 102.4 | 409.6 | 819.6 |
| 18062 | 0.1 | 25.6 | 51.2 | 51.1 | 204.8 | |
Titers are the reciprocal serum dilution ×103.
We monitored the SHIV-HXB2 neutralizing titers for more than 2 years after challenge. Typically, infected animals demonstrated high, sustained neutralizing antibody titers to the challenge strain, while those that were protected exhibited waning titers. Three immunized animals, 17951, 18066, and 18102, lost neutralizing activity against SHIV-HXB2, whereas the two control animals and 18001 have maintained high levels of binding (data not shown) and neutralizing (Table 3) antibodies.
DISCUSSION
Induction of strong humoral immunity is likely to be an important property of a successful HIV vaccine. Passive administration of neutralizing antibodies in both the SCIDhu mouse (27) and macaque (1, 32, 40, 42, 70) models has shown that protection is possible in the absence of cell-mediated immunity. Since the Env protein is the major target for neutralizing antibodies, use of soluble gp120 has been the primary strategy for induction of such antibodies. Initial studies utilizing monomeric gp120 yielded disappointing results in that only low levels of neutralizing antibodies were detected (17, 31, 41, 43). One possible means of eliciting a better response is to use an oligomeric form of the Env protein that resembles that found on virus particles and infected cells.
The goals of the present study were twofold. First, we wanted to directly compare the immunogenicity of gp120 and gp140 in a small-animal model. To this end, we expressed both proteins from the IIIB isolate of HIV-1 in mammalian cells. The proteins were produced, purified, and formulated identically. After formulation with adjuvants, we found no alteration in antibody recognition or oligomeric status (data not shown). The immunogenicity of the two proteins was examined in rabbits. The second goal was to test the immunogenicity and protective efficacy of oligomeric gp140 in the rhesus macaque model, for which a homologous challenge, SHIV-HXB2 (39), was available.
The results obtained from immunization of rabbits demonstrated that gp140 generated antibodies that were higher in titer and more cross-reactive than those generated by gp120. Because immunization with gp140 yielded antibodies with greater binding to both heterologous gp120s and gp160s, the enhancement could not be accounted for entirely by the presence of gp41 epitopes on the gp140 immunogen. Although the enhancement was only two- to threefold, it was consistently observed with six different proteins, including those from CXCR4- and CCR5-utilizing viruses. In addition, antibodies to denatured gp140 were not as cross-reactive as were those to oligomeric gp140. This result suggested that conserved epitopes on oligomeric gp140 were responsible for the enhanced cross-reactivity. In addition, better neutralization of HIV-1 NL4-3 as well as of the heterologous HIV-1 MN and BZ167 was found with sera from rabbits immunized with gp140 than with those from rabbits immunized with gp120.
There have been some attempts to predict the immunogenicity of a protein from its antigenicity. Thus, data obtained from phage display library panning of HIV-positive human sera predicted that gp140 would generate a weaker neutralizing antibody response than gp120 (53). In fact, direct comparison of the two immunogens in rabbits demonstrated that gp140 elicited the stronger neutralizing antibody response. In agreement with our results, VanCott et al. (76) demonstrated neutralization of some primary isolates by sera from rabbits immunized with a similar oligomeric Env. Using a recombinant vaccinia virus prime and SIV protein boost, Polacino et al. (59) found that boosting with gp160 gave more effective protection than did gp130. In another study, oligomeric SIV Env was reported to yield protection from challenge, while monomeric Env did not (55).
Our second goal was to test the effectiveness of the oligomeric gp140 immunogen in a nonhuman primate model. We wanted to exploit the potential for studying HIV-1 Env by using the SHIV challenge model. Because of constraints on the use of rhesus macaques for such studies, we did not perform a comparative analysis with gp120, although such a study was performed by another group (Berman et al., unpublished). Results from our study demonstrated that strong binding and neutralizing antibody responses could be achieved by immunization with oligomeric gp140. A compilation of results from several studies showed that neutralizing antibody titers of greater than 200 on the day of challenge were sufficient to afford protection against challenge with SHIV-HXB2 (D. Montefiori, unpublished). After four immunizations with oligomeric gp140, neutralizing antibody titers to SHIV-HXB2 ranged from 313 to 1,315, values that are similar to those found in monkeys infected with SHIV-HXB2. Comparable values, ranging from <25 to 824, were found in macaques immunized with IIIB gp120 in QS-21 (D. Montefiori, unpublished). Significantly, the neutralization elicited by gp140 immunization was not completely blocked by a linear V3 peptide. The proportion of blocking by the V3 peptide was similar to that found in SHIV-HXB2-infected macaques (19), suggesting that other, more relevant epitopes may be accessible on the gp140 oligomer. In contrast to these results, sera from macaques immunized with IIIB gp120 elicited almost exclusively V3-dependent neutralization of SHIV-HXB2 (D. Montefiori, unpublished). In addition, several heterologous SHIV and HIV-1 isolates were neutralized, albeit to low levels, with sera from subsets of the gp140-immunized animals. Particularly notable was one case of neutralization of SHIV-89.6, an isolate whose neutralization properties are quite different from those of IIIB and which shows some resemblance to primary isolates (19).
Following SHIV-HXB2 challenge, there was no evidence of viral infection in three of the four immunized animals. In comparison to the naïve controls, the fourth animal exhibited a substantial decrease in viral load as measured by detection of both virus in the PBMC and viral RNA in the plasma. Because the challenge virus was nonpathogenic, evaluation of viral replication was limited to the 3- to 4-week time period immediately following challenge. Env and Gag serology, however, was monitored long term to confirm the infection status of the animals. In our study, the three animals that did not exhibit viremia immediately following challenge have shown no evidence of anti-p27 antibodies in a 2-year follow-up. In addition, their neutralizing antibody titers declined to background levels, further supporting the conclusion that they resisted infection. In contrast, both controls as well as one immunized animal exhibited properties characteristic of SHIV-HXB2-infected macaques, i.e., development of p27 antibodies and maintenance of SHIV-HXB2 neutralizing antibodies.
In contrast to our findings, Berman et al. (7) demonstrated protection against HIV-1 challenge in chimpanzees immunized with gp120 but not with soluble gp160, a protein produced from a truncated gene similar to ours. Several factors could account for this difference, including different Env purification protocols, the oligomeric status and integrity of the proteins, and a lower neutralizing antibody titer in the gp160-immunized animals on the day of challenge.
In summary, our study in rabbits demonstrated that immunization with oligomeric gp140 resulted in a qualitative improvement over immunization with gp120. In a follow-up study in macaques, we showed that oligomeric gp140 elicited strong homologous neutralizing antibodies and protected against homologous challenge. Importantly, not all of the neutralization was attributable to reactivity against the V3 loop. However, this did not translate into robust neutralization of heterologous viruses, and no neutralization of primary isolates was observed. In addition, we found no improvement in the level of neutralizing antibodies or protection in comparison to macaques similarly immunized with gp120 (Berman et al., unpublished). Clearly, additional modifications are needed to achieve broader and more potent neutralization, particularly with respect to divergent primary isolates.
There may be ways to further improve the gp140 immunogen. Until recently, only uncleaved oligomers could be purified due to the lability of gp120-gp41 interactions. A report from Binley et al. (8) demonstrated that the association between the subunits can be stabilized by the introduction of a disulfide bridge. In addition, Yang et al. (79) described the formation of gp140 oligomers that were stabilized by fusion to GCN4. Further modifications, such as elimination of potential N-linked glycosylation sites (62) or deletion of variable loops (72), may be means of exposing neutralizing epitopes. More importantly, cleaved and uncleaved gp140s from more clinically relevant, CCR5-utilizing primary isolates need to be tested.
ACKNOWLEDGMENTS
We thank Nancy Miller and the NIAID Division of AIDS for support and guidance in the macaque study. We thank Norman Cooper for cells and recombinant vaccinia viruses, Malcolm Martin for HIV-1 NL4-3, and Robert Gallo for HIV-1 MN. Lynn Frampton and Chelsi Cacciatore provided excellent technical assistance. Michael Piatak and L. Li performed plasma SIV RNA viral load analysis. We also thank Yichen Lu for providing the SHIV-HXB2 challenge stock and Charlotte Kensil for the gift of QS-21. HIV-1 MN and SF2 were obtained from the NIH AIDS Research and Reference Reagent Program. We also thank Tammy Tobery for excellent technical assistance with all aspects of animal care.
This work was supported in part by NIH grant AI-85343 (D.C.M.) and by federal funds from the National Cancer Institute, NIH, under contract NO1-CO-56000 (J.L.).
REFERENCES
- 1.Baba T W, Liska V, Hofmann-Lehmann R, Vlasak J, Xu W, Ayehunie S, Cavacini L A, Posner M R, Katinger H, Stiegler G, Bernacky B J, Rizvi T A, Schmidt R, Hill L R, Keeling M E, Lu Y, Wright J E, Chou T C, Ruprecht R M. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat Med. 2000;6:200–206. doi: 10.1038/72309. [DOI] [PubMed] [Google Scholar]
- 2.Baldridge J R, McGraw T S, Paoletti A, Buchmeier J J. Antibody prevents the establishment of persistent arenavirus infection in synergy with endogenous T cells. J Virol. 1997;71:755–758. doi: 10.1128/jvi.71.1.755-758.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnett S W, Rajasekar S, Legg H, Doe B, Fuller D H, Haynes J R, Walker C M, Steimer K S. Vaccination with HIV-1 gp120 DNA induces immune responses that are boosted by a recombinant gp120 protein subunit. Vaccine. 1997;15:869–873. doi: 10.1016/s0264-410x(96)00264-2. [DOI] [PubMed] [Google Scholar]
- 4.Beddows S, Lister S, Cheingsong R, Bruck C, Weber J. Comparison of the antibody repertoire generated in healthy volunteers following immunization with a monomeric recombinant gp120 construct derived from a CCR5/CXCR4-using human immunodeficiency virus type 1 isolate with sera from naturally infected individuals. J Virol. 1999;73:1740–1745. doi: 10.1128/jvi.73.2.1740-1745.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belshe R B, Gorse G J, Mulligan M J, Evans T G, Keefer M C, Excler J L, Duliege A M, Tartaglia J, Cox W I, McNamara J, Hwang K L, Bradney A, Montefiori D, Weinhold K J. Induction of immune responses to HIV-1 by canarypox virus (ALVAC) HIV-1 and gp120 SF-2 recombinant vaccines in uninfected volunteers. NIAID AIDS Vaccine Evaluation Group. AIDS. 1998;12:2407–2415. doi: 10.1097/00002030-199818000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Belshe R B, Graham B S, Keefer M C, Gorse G J, Wright P, Dolin R, Matthews T, Weinhold K, Bolognesi D P, Sposto R, et al. Neutralizing antibodies to HIV-1 in seronegative volunteers immunized with recombinant gp120 from the MN strain of HIV-1. NIAID AIDS Vaccine Clinical Trials Network. JAMA. 1994;272:475–480. doi: 10.1001/jama.272.6.475. [DOI] [PubMed] [Google Scholar]
- 7.Berman P W, Gregory T J, Riddle L, Nakamura G R, Champe M A, Porter J P, Wurm F M, Hershberg R D, Cobb E K, Eichberg J W. Protection of chimpanzees from infection by HIV-1 after vaccination with recombinant glycoprotein gp120 but not gp160. Nature. 1990;345:622–625. doi: 10.1038/345622a0. [DOI] [PubMed] [Google Scholar]
- 8.Binley J M, Sanders R W, Clas B, Schuelke N, Master A, Guo Y, Kajumo F, Anselma D J, Maddon P J, Olson W C, Moore J P. A recombinant human immunodeficiency virus type 1 envelope glycoprotein complex stabilized by an intermolecular disulfide bond between the gp120 and gp41 subunits is an antigenic mimic of the trimeric virion-associated structure. J Virol. 2000;74:627–643. doi: 10.1128/jvi.74.2.627-643.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Broder C C, Berger E A. Fusogenic selectivity of the envelope glycoprotein is a major determinant of human immunodeficiency virus type 1 tropism for CD4+ T-cell lines vs. primary macrophages. Proc Natl Acad Sci USA. 1995;92:9004–9008. doi: 10.1073/pnas.92.19.9004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Broder C C, Earl P L, Long D, Moss B, Doms R W. Antigenic implications of HIV-1 Env quaternary structure. Proc Natl Acad Sci USA. 1994;91:11699–11703. doi: 10.1073/pnas.91.24.11699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buge S L, Richardson E, Alipanah S, Markham P, Cheng S, Kalyan N, Miller C J, Lubeck M, Udem S, Eldridge J, Robert-Guroff M. An adenovirus simian immunodeficiency virus Env vaccine elicits humoral, cellular, and mucosal immune responses in rhesus macaques and decreases viral burden following vaginal challenge. J Virol. 1997;71:8531–8541. doi: 10.1128/jvi.71.11.8531-8541.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bures, R., A. Gaitan, T. Zhu, C. Graziosi, K. M. McGrath, J. Tartaglia, P. Caudrelier, R. El Habib, M. Klein, A. Lazzarin, D. M. Stablein, L. Corey, M. L. Greenberg, D. H. Schwartz, and D. C. Montefiori. Immunization with recombinant canarypox vectors expressing membrane-anchored gp120 followed by soluble gp160 boosting fails to generate antibodies that neutralize R5 primary isolates of human immunodeficiency virus type 1. AIDS Res. Hum. Retroviruses, in press. [DOI] [PubMed]
- 13.Burton D R. A vaccine for HIV type 1: the antibody perspective. Proc Natl Acad Sci USA. 1997;94:10018–10023. doi: 10.1073/pnas.94.19.10018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burton D R, Montefiori D C. The antibody response in HIV-1 infection. AIDS. 1997;11:S87–S98. [PubMed] [Google Scholar]
- 15.Center R J, Earl P L, Lebowitz J, Schuck P, Moss B. The human immunodeficiency virus type 1 gp120 V2 domain mediates gp41-independent intersubunit contacts. J Virol. 2000;74:4448–4455. doi: 10.1128/jvi.74.10.4448-4455.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clements-Mann M L, Weinhold K, Matthews T J, Graham B S, Gorse G J, Keefer M C, McElrath M J, Hsieh R H, Mestecky J, Zolla-Pazner S, Mascola J, Schwartz D, Siliciano R, Corey L, Wright P F, Belshe R, Dolin R, Jackson S, Xu S, Fast P, Walker M C, Stablein D, Excler J L, Tartaglia J, Paoletti E, et al. Immune responses to human immunodeficiency virus (HIV) type 1 induced by canarypox expressing HIV-1MN gp120, HIV-1SF2 recombinant gp120, or both vaccines in seronegative adults. NIAID AIDS Vaccine Evaluation Group. J Infect Dis. 1998;177:1230–1246. doi: 10.1086/515288. [DOI] [PubMed] [Google Scholar]
- 17.Connor R I, Korber B T, Graham B S, Hahn B H, Ho D D, Walker B D, Neumann A U, Vermund S H, Mestecky J, Jackson S, Fenamore E, Cao Y, Gao F, Kalams S, Kunstman K J, McDonald D, McWilliams N, Trkola A, Moore J P, Wolinsky S M. Immunological and virological analyses of persons infected by human immunodeficiency virus type 1 while participating in trials of recombinant gp120 subunit vaccines. J Virol. 1998;72:1552–1576. doi: 10.1128/jvi.72.2.1552-1576.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corey L, McElrath M J, Weinhold K, Matthews T, Stablein D, Graham B, Keefer M, Schwartz D, Gorse G. Cytotoxic T cell and neutralizing antibody responses to human immunodeficiency virus type 1 envelope with a combination vaccine regimen. AIDS Vaccine Evaluation Group. J Infect Dis. 1998;177:301–309. doi: 10.1086/514202. [DOI] [PubMed] [Google Scholar]
- 19.Crawford J M, Earl P L, Moss B, Reimann K A, Wyand M S, Manson K H, Bilska M, Zhou J T, Pauza C D, Parren P W, Burton D R, Sodroski J G, Letvin N L, Montefiori D C. Characterization of primary isolate-like variants of simian-human immunodeficiency virus. J Virol. 1999;73:10199–10207. doi: 10.1128/jvi.73.12.10199-10207.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davis N L, Caley I J, Brown K W, Betts M R, Irlbeck D M, McGrath K M, Connell M J, Montefiori D C, Frelinger J A, Swanstrom R, Johnson P R, Johnston R E. Vaccination of macaques against pathogenic simian immunodeficiency virus with Venezuelan equine encephalitis virus replicon particles. J Virol. 2000;74:371–378. doi: 10.1128/jvi.74.1.371-378.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doranz B J, Rucker J, Yi Y, Smyth R J, Samson M, Peiper S C, Parmentier M, Collman R, Doms R W. A dualtropic primary HIV-1 isolate that uses fusin and the beta-chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cell. 1996;85:1149–1156. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- 22.Earl P L, Broder C C, Doms R W, Moss B. Epitope map of human immunodeficiency virus type 1 gp41 derived from 47 monoclonal antibodies produced by immunization with oligomeric envelope protein. J Virol. 1997;71:2674–2684. doi: 10.1128/jvi.71.4.2674-2684.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Earl P L, Broder C C, Long D, Lee S A, Peterson J, Chakrabarti S, Doms R W, Moss B. Native oligomeric forms of human immunodeficiency virus type 1 envelope glycoprotein elicit a diverse array of monoclonal antibody reactivities. J Virol. 1994;68:3015–3026. doi: 10.1128/jvi.68.5.3015-3026.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Egan M A, Pavlat W A, Tartaglia J, Paoletti E, Weinhold K J, Clements M L, Siliciano R F. Induction of human immunodeficiency virus type 1 (HIV-1)-specific cytolytic T lymphocyte responses in seronegative adults by a nonreplicating, host-range-restricted canarypox vector (ALVAC) carrying the HIV-1MN env gene. J Infect Dis. 1995;171:1623–1627. doi: 10.1093/infdis/171.6.1623. [DOI] [PubMed] [Google Scholar]
- 25.Evans L A, Thomson-Honnebier G, Steimer K, Paoletti E, Perkus M E, Hollander H, Levy J A. Antibody-dependent cellular cytotoxicity is directed against both the gp120 and gp41 envelope proteins of HIV. AIDS. 1989;3:273–276. doi: 10.1097/00002030-198905000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Fouts T R, Binley J M, Trkola A, Robinson J E, Moore J P. Neutralization of the human immunodeficiency virus type 1 primary isolate JR-FL by human monoclonal antibodies correlates with antibody binding to the oligomeric form of the envelope glycoprotein complex. J Virol. 1997;71:2779–2785. doi: 10.1128/jvi.71.4.2779-2785.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gauduin M C, Parren P W, Weir R, Barbas C F, Burton D R, Koup R A. Passive immunization with a human monoclonal antibody protects hu-PBL-SCID mice against challenge by primary isolates of HIV-1. Nat Med. 1997;3:1389–1393. doi: 10.1038/nm1297-1389. [DOI] [PubMed] [Google Scholar]
- 28.Gnann J W J, Nelson J A, Oldstone M B A. Fine mapping of an immunodominant domain in the transmembrane glycoprotein of human immunodeficiency virus. J Virol. 1987;61:2639–2641. doi: 10.1128/jvi.61.8.2639-2641.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Graham B S, Gorse G J, Schwartz D H, Keefer M C, McElrath M J, Matthews T J, Wright P F, Belshe R B, Clements M L, Dolin R, et al. Determinants of antibody response after recombinant gp160 boosting in vaccinia-naive volunteers primed with gp160-recombinant vaccinia virus. The National Institute of Allergy and Infectious Diseases AIDS Vaccine Clinical Trials Network. J Infect Dis. 1994;170:782–786. doi: 10.1093/infdis/170.4.782. [DOI] [PubMed] [Google Scholar]
- 30.Graham B S, Matthews T J, Belshe R B, Clements M L, Dolin R, Wright P F, Gorse G J, Schwartz D H, Keefer M C, Bolognesi D P, et al. Augmentation of human immunodeficiency virus type 1 neutralizing antibody by priming with gp160 recombinant vaccinia and boosting with rgp160 in vaccinia-naive adults. The NIAID AIDS Vaccine Clinical Trials Network. J Infect Dis. 1993;167:533–537. doi: 10.1093/infdis/167.3.533. [DOI] [PubMed] [Google Scholar]
- 31.Graham B S, McElrath M J, Connor R I, Schwartz D H, Gorse G J, Keefer M C, Mulligan M J, Matthews T J, Wolinsky S M, Montefiori D C, Vermund S H, Lambert J S, Corey L, Belshe R B, Dolin R, Wright P F, Korber B T, Wolff M C, Fast P E. Analysis of intercurrent human immunodeficiency virus type 1 infections in phase I and II trials of candidate AIDS vaccines. AIDS Vaccine Evaluation Group and the Correlates of HIV Immune Protection Group. J Infect Dis. 1998;177:310–319. doi: 10.1086/514209. [DOI] [PubMed] [Google Scholar]
- 32.Haigwood N L, Watson A, Sutton W F, McClure J, Lewis A, Ranchalis J, Travis B, Voss G, Letvin N L, Hu S L, Hirsch V M, Johnson P R. Passive immune globulin therapy in the SIV/macaque model: early intervention can alter disease profile. Immunol Lett. 1996;51:107–114. doi: 10.1016/0165-2478(96)02563-1. [DOI] [PubMed] [Google Scholar]
- 33.Hanke T, Blanchard T J, Schneider J, Hannan C M, Becker M, Gilbert S C, Hill A V S, Smith G L, McMichael A. Enhancement of MHC class I-restricted peptide-specific T cell induction by a DNA prime/MVA boost vaccination regime. Vaccine. 1998;16:439–445. doi: 10.1016/s0264-410x(97)00226-0. [DOI] [PubMed] [Google Scholar]
- 34.Hasenkrug K J, Chesebro B. Immunity to retroviral infection the Friend virus model. Proc Natl Acad Sci USA. 1997;94:7811–7816. doi: 10.1073/pnas.94.15.7811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haynes B F, Pantaleo G, Fauci A S. Toward an understanding of the correlates of protective immunity to HIV infection. Science. 1996;271:324–328. doi: 10.1126/science.271.5247.324. [DOI] [PubMed] [Google Scholar]
- 36.Heilman C A, Baltimore D. HIV vaccines—where are we going? Nat Med. 1998;4:532–534. doi: 10.1038/nm0598supp-532. [DOI] [PubMed] [Google Scholar]
- 37.Kent S J, Zhao A, Best S J, Chandler J D, Boyle D B, Ranshaw I A. Enhanced T-cell immunogenicity and protective efficacy of a human immunodeficiency virus type 1 vaccine regimen consisting of consecutive priming with DNA and boosting with recombinant fowlpox virus. J Virol. 1998;72:10180–10188. doi: 10.1128/jvi.72.12.10180-10188.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Letvin N L. Progress in the development of an HIV-1 vaccine. Science. 1998;280:1875–1880. doi: 10.1126/science.280.5371.1875. [DOI] [PubMed] [Google Scholar]
- 39.Lu Y, Salvato M S, Pauza C D, Li J, Sodroski J, Manson K, Wyand M, Letvin N, Jenkins S, Touzjian N, Chutkowski C, Kushner N, LeFaile M, Payne L G, Roberts B. Utility of SHIV for testing HIV-1 vaccine candidates in macaques. J Acquired Immune Defic Syndr. 1996;12:99–106. doi: 10.1097/00042560-199606010-00001. [DOI] [PubMed] [Google Scholar]
- 40.Mascola J R, Lewis M G, Stiegler G, Harris D, VanCott T C, Hayes D, Louder M K, Brown C R, Sapan C V, Frankel S S, Lu Y, Robb M L, Katinger H, Birx D L. Protection of macaques against pathogenic simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies. J Virol. 1999;73:4009–4018. doi: 10.1128/jvi.73.5.4009-4018.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mascola J R, Snyder S W, Weinslow O S, Belay S M, Belshe R B, Scwartz D H, Clements M L, Dolin R, Graham B S, Gorse G J, Keefer M C, McElrath M J, Walder M C, Wagner K F, McNeil J G, McCutchan F E, Burke D S. Immunization with envelope subunit vaccine products elicits neutralizing antibodies against laboratory-adapted but not primary isolates of human immunodeficiency virus type 1. J Infect Dis. 1996;173:340–348. doi: 10.1093/infdis/173.2.340. [DOI] [PubMed] [Google Scholar]
- 42.Mascola J R, Stiegler G, VanCott T C, Katinger H, Carpenter C B, Hanson C E, Beary H, Hayes D, Frankel S S, Birx D L, Lewis M G. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat Med. 2000;6:207–210. doi: 10.1038/72318. [DOI] [PubMed] [Google Scholar]
- 43.Matthews T J. Dilemma of neutralization resistance of HIV-1 field isolates and vaccine development. AIDS Res Hum Retroviruses. 1994;10:631–632. doi: 10.1089/aid.1994.10.631. [DOI] [PubMed] [Google Scholar]
- 44.McElrath M J, Siliciano R F, Weinhold K J. HIV type 1 vaccine-induced cytotoxic T cell responses in phase I clinical trials: detection, characterization, and quantitation. AIDS Res Hum Retroviruses. 1997;13:211–216. doi: 10.1089/aid.1997.13.211. [DOI] [PubMed] [Google Scholar]
- 45.Montefiori D C, Evans T G. Toward an HIV type 1 vaccine that generates potent, broadly cross-reactive neutralizing antibodies. AIDS Res Hum Retroviruses. 1999;15:689–698. doi: 10.1089/088922299310773. [DOI] [PubMed] [Google Scholar]
- 46.Montefiori D C, Graham B S, Zhou J, Zhou J, Bucco R A, Schwartz D H, Cavacini L A, Posner M R, Network N-N A V C T. V3-specific neutralizing antibodies in sera from HIV-1 gp160-immunized volunteers block virus fusion and act synergistically with human monoclonal antibody to the conformation-dependent CD4 binding site of gp120. J Clin Investig. 1993;92:840–847. doi: 10.1172/JCI116658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Montefiori D C, Reimann K A, Wyand M S, Manson K, Lewis M G, Collman R G, Sodroski J G, Bolognesi D P, Letvin N L. Neutralizing antibodies in sera from macaques infected with chimeric simian-human immunodeficiency virus containing the envelope glycoproteins of either a laboratory-adapted variant or a primary isolate of human immunodeficiency virus type 1. J Virol. 1998;72:3427–3431. doi: 10.1128/jvi.72.4.3427-3431.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Montefiori D C, Robinson W E, Jr, Schuffman S S, Mitchell W M. Evaluation of antiviral drugs and neutralizing antibodies to human immunodeficiency virus by a rapid and sensitive microtiter infection assay. J Clin Microbiol. 1988;26:231–235. doi: 10.1128/jcm.26.2.231-235.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moore J P, Ho D D. Antibodies to discontinuous or conformationally sensitive epitopes on the gp120 glycoprotein of human immunodeficiency virus type 1 are highly prevalent in sera of infected humans. J Virol. 1993;67:863–875. doi: 10.1128/jvi.67.2.863-875.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moore J P, Sattentau Q J, Wyatt R, Sodroski J. Probing the structure of the human immunodeficiency virus surface glycoprotein gp120 with a panel of monoclonal antibodies. J Virol. 1994;68:469–484. doi: 10.1128/jvi.68.1.469-484.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moore J P, Sodroski J. Antibody cross-competition analysis of the human immunodeficiency virus type 1 gp120 exterior envelope glycoprotein. J Virol. 1996;70:1863–1872. doi: 10.1128/jvi.70.3.1863-1872.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Muster T, Guinea R, Trkola A, Purtscher M, Klima A, Steindl F, Palese P, Katinger H. Cross-neutralization activity against divergent human immunodeficiency virus type 1 isolates induced by the pg41 sequence ELDKWAS. J Virol. 1994;68:4031–4034. doi: 10.1128/jvi.68.6.4031-4034.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Parren P W H I, Fisicaro P, Labrijn A F, Binley J M, Yang W, Ditzel H J, Barbas III C F, Burton D R. In vitro antigen challenge of human antibody libraries for vaccine evaluation: the human immunodeficiency virus type 1 envelope. J Virol. 1996;70:9046–9050. doi: 10.1128/jvi.70.12.9046-9050.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Parren P W H I, Mondor I, Naniche D, Ditzel H J, Klasse P J, Burton D R, Sattentau Q J. Neutralization of human immunodeficiency virus type 1 by antibody to gp120 is determined primarily by occupancy of sites on the virion irrespective of epitope specificity. J Virol. 1998;72:3512–3519. doi: 10.1128/jvi.72.5.3512-3519.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Petry H, Dittmer U, Jones D, Farrar G, Wachter H, Fuchs D, Nisslein T, Jurkiewicz E, Hunsmann G, Stahl-Hennig C, Luke W. Prechallenge high neutralizing antibodies and long-lasting immune reactivity to gp41 correlate with protection of rhesus monkeys against productive simian immunodeficiency virus infection or disease development. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;19:441–450. doi: 10.1097/00042560-199812150-00002. [DOI] [PubMed] [Google Scholar]
- 56.Pilgrim A K, Pantaleo G, Cohen O J, Fink L M, Zhou J Y, Zhou J T, Bolognesi D P, Fauci A S, Montefiori D C. Neutralizing antibody responses to human immunodeficiency virus type 1 in primary infection and long-term nonprogressive infection. J Infect Dis. 1997;176:924–932. doi: 10.1086/516508. [DOI] [PubMed] [Google Scholar]
- 57.Pinter A, Honnen W J, Tilley S A, Bona C, Zaghouani H, Gorny M K, Zolla-Pazner S. Oligomeric structure of gp41, the transmembrane protein of human immunodeficiency virus type 1. J Virol. 1989;63:2674–2679. doi: 10.1128/jvi.63.6.2674-2679.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Planz O, Ehl S, Furrer E, Horvath E, Brundler M A, Hengartner H, Zinkernagel R M. A critical role for neutralizing-antibody-producing B cells, CD4(+) T cells, and interferons in persistent and acute infections of mice with lymphocytic choriomeningitis virus: implications for adoptive immunotherapy of virus carriers. Proc Natl Acad Sci USA. 1997;94:6874–6879. doi: 10.1073/pnas.94.13.6874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Polacino P S, Stallard V, Klaniecki J E, Pennathur S, Montefiori D C, Langlois A J, Richardson B A, Morton W R, Benveniste R E, Hu S L. Role of immune responses against the envelope and the core antigens of simian immunodeficiency virus SIVmne in protection against homologous cloned and uncloned virus challenge in macaques. J Virol. 1999;73:8201–8215. doi: 10.1128/jvi.73.10.8201-8215.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Poumbourios P, McPhee D A, Kemp B E. Antibody epitopes sensitive to the state of human immunodeficiency virus type 1 gp41 oligomerization map to a putative alpha-helical region. AIDS Res Hum Retroviruses. 1992;8:2055–2062. doi: 10.1089/aid.1992.8.2055. [DOI] [PubMed] [Google Scholar]
- 61.Reed L J, Muench H. A simple method of estimating fifty percent endpoints. Am J Hyg. 1938;27:493–497. [Google Scholar]
- 62.Reitter J N, Means R E, Desrosiers R C. A role for carbohydrates in immune evasion in AIDS. Nat Med. 1998;4:679–684. doi: 10.1038/nm0698-679. [DOI] [PubMed] [Google Scholar]
- 63.Richardson T M, Jr, Broder C C, Hoxie J A, Mascola J R, Earl P L, Doms R W. The humoral response to oligomeric human immunodeficiency virus type 1 envelope protein. J Virol. 1996;70:753–762. doi: 10.1128/jvi.70.2.753-762.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Roben P, Moore J P, Thali M, Sodroski J, Barbas C F I, Burton D R. Recognition properties of a panel of human recombinant Fab fragments to the CD4 binding site of gp120 that show differing abilities to neutralize human immunodeficiency virus type 1. J Virol. 1994;68:4821–4828. doi: 10.1128/jvi.68.8.4821-4828.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Robinson H L. DNA vaccines for immunodeficiency viruses. AIDS. 1997;11(Suppl. A):S109–S119. [PubMed] [Google Scholar]
- 66.Rowland-Jones S, Tan R, McMichael A. The role of cellular immunity in protection against HIV infection. Adv Immunol. 1997;65:448–455. [PubMed] [Google Scholar]
- 67.Sattentau Q J, Moore J P. Human immunodeficiency virus type 1 neutralization is determined by epitope exposure on the gp120 oligomer. J Exp Med. 1995;182:185–196. doi: 10.1084/jem.182.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sattentau Q J, Zolla-Pazner S, Piognard P. Epitope exposure on functional, oligomeric HIV-1 gp41 molecules. Virology. 1995;206:713–717. doi: 10.1016/s0042-6822(95)80094-8. [DOI] [PubMed] [Google Scholar]
- 69.Seth A, Ourmanov I, Schmitz J E, Kuroda M J, Lifton M A, Nickerson C E, Wyatt L, Carroll M, Moss B, Venzon D, Letvin N L, Hirsch V M. Immunization with a modified vaccinia virus expressing simian immunodeficiency virus (SIV) Gag-Pol primes for an anamnestic Gag-specific cytotoxic T-lymphocyte response and is associated with reduction of viremia after SIV challenge. J Virol. 2000;74:2502–2509. doi: 10.1128/jvi.74.6.2502-2509.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shibata R, Igarashi T, Haigwood N, Buckler-White A, Ogert R, Ross W, Willey R, Cho M W, Martin M A. Neutralizing antibody directed against the HIV-1 envelope glycoprotein can completely block HIV-1/SIV chimeric virus infections of macaque monkeys. Nat Med. 1999;5:204–210. doi: 10.1038/5568. [DOI] [PubMed] [Google Scholar]
- 71.Shibata R, Siemon C, Czajak S C, Desrosiers R C, Martin M A. Live, attenuated simian immunodeficiency virus vaccines elicit potent resistance against a challenge with a human immunodeficiency virus type 1 chimeric virus. J Virol. 1997;71:8141–8148. doi: 10.1128/jvi.71.11.8141-8148.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stamatatos L, Lim M, Cheng-Meyer C. Generation and structural analysis of soluble oligomeric gp140 envelope proteins derived from neutralization-resistant and neutralization-susceptible primary HIV type 1 isolates. AIDS Res Hum Retroviruses. 2000;16:981–994. doi: 10.1089/08892220050058407. [DOI] [PubMed] [Google Scholar]
- 73.Steimer K S, Scandella C J, Skiles P V, Haigwood N L. Neutralization of divergent HIV-1 isolates by conformation-dependent human antibodies to gp120. Science. 1991;254:105–108. doi: 10.1126/science.1718036. [DOI] [PubMed] [Google Scholar]
- 74.Suryanarayana K, Wiltrout T A, Vasquez G M, Hirsch V M, Lifson J D. Plasma SIV RNA viral load determination by real-time quantification of product generation in reverse transcriptase-polymerase chain reaction. AIDS Res Hum Retroviruses. 1998;14:183–189. doi: 10.1089/aid.1998.14.183. [DOI] [PubMed] [Google Scholar]
- 75.VanCott T C, Bethke F R, Burke D S, Redfield R R, Birx D L. Lack of induction of antibodies specific for conserved, discontinuous epitopes of HIV-1 envelope glycoprotein by candidate AIDS vaccines. J Immunol. 1995;155:4100–4110. [PubMed] [Google Scholar]
- 76.VanCott T C, Mascola J R, Kaminski R W, Kalyanaraman V, Hallberg P L, Burnett P R, Ulrich J T, Rechtman D J, Birx D L. Antibodies with specificity to native gp120 and neutralization activity against primary human immunodeficiency virus type 1 isolates elicited by immunization with oligomeric gp160. J Virol. 1997;71:4319–4330. doi: 10.1128/jvi.71.6.4319-4330.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.VanCott T C, Veit S C, Kalyanaraman V, Earl P, Birx D L. Characterization of a soluble, oligomeric HIV-1 gp160 protein as a potential immunogen. J Immunol Methods. 1995;183:103–117. doi: 10.1016/0022-1759(95)00038-c. [DOI] [PubMed] [Google Scholar]
- 78.Wyatt R, Desjardin E, Olshevsky U, Nixon C, Binley J, Olshevsky V, Sodroski J. Analysis of the interaction of the human immunodeficiency virus type 1 gp120 envelope glycoprotein with the gp41 transmembrane glycoprotein. J Virol. 1997;71:9722–9731. doi: 10.1128/jvi.71.12.9722-9731.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang X, Farzan M, Wyatt R, Sodroski J. Characterization of stable, soluble trimers containing complete ectodomains of human immunodeficiency virus type 1 envelope glycoproteins. J Virol. 2000;74:5716–5725. doi: 10.1128/jvi.74.12.5716-5725.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]