Abstract
We report an extensive tabulation of several important topological invariants for all the isomers of carbon -fullerenes with n = 52–70. The topological invariants (including Kekulé count, Clar count, and Clar number) are computed and reported in the form of the corresponding Zhang–Zhang (ZZ) polynomials. The ZZ polynomials appear to be distinct for each isomer cage, providing a unique label that allows for differentiation between various isomers. Several chemical applications of the computed invariants are reported. The results suggest rather weak correlation between the Kekulé count, Clar count, Clar number invariants, and isomer stability, calling into doubt the predictive power of these topological invariants in discriminating the most stable isomer of a given fullerene. The only exception is the Clar count/Kekulé count ratio, which seems to be the most important diagnostic discovered from our analysis. Stronger correlations are detected between Pauling bond orders computed from Kekulé structures (or Clar covers) and the corresponding equilibrium bond lengths determined from the optimized DFTB geometries of all 30,579 isomers of C20–C70.
Keywords: fullerene isomers, isomer stability, Kekulé count, Clar number, Zhang–Zhang polynomial (aka ZZ polynomial or Clar covering polynomial), bond order
1. Introduction
A (5,6)-fullerene is a polyhedral carbon cage with only pentagonal and hexagonal faces. Each such cage necessarily contains an even number n of carbon atoms. The number of pentagonal faces is independent of n, always being equal to 12, while the number of hexagonal faces is equal to . The relative distributions of the twelve pentagonal faces in the network of the remaining hexagonal faces give rise to a large number of structural isomers of (5,6)-fullerenes (except for , for which no such isomer exists). For the (5,6)-fullerenes studied here, the number of conceivable distinct isomers ranges between 437 for C52 to 8149 for C70 [1]. Considerable combinatorial effort has been invested in finding algorithms that allow for the generation and enumeration of these isomers. The first solution to this problem was offered by Manolopoulos, Fowler, and their collaborators in the 1990s in the form of a ring spiral algorithm [2,3], in which the structure of each isomer cage is encoded as a linear sequence of pentagons and hexagons. It was soon realized [4,5] that for larger fullerenes the spiral algorithm might miss some of the isomers simply because some of the isomers cannot be encoded as unbranched spiral sequences of pentagons and hexagons. To solve this limitation, Brinkmann and Dress developed a top-down approach [6,7] capable of generating all the isomers of (5,6)-fullerenes for a general value of n.
The carbon soot obtained in graphite laser vaporization experiments usually contains a mixture of various isomers of carbon clusters with different sizes. Characterization of the soot components and understanding the reasons behind certain fullerene isomers being more abundant in soot compared to others has attracted considerable interest on the part of the chemistry community [8,9,10,11,12,13,14,15,16]. Current beliefs on fullerene isomer stability [17] can be summarized by a number of procedural filters: discard isomers with abutting pentagons; discard isomers with disparate hexagon neighbour patterns; or discard isomers with poor electronic structure. Many of these observations can be rationalized using simple geometric arguments [18,19,20] and quantified [21] with semiempirical models based on penalty and merit functions for reappearing motifs (according to which two fused pentagons cost 26.5 kcal mol−1 on average, the phenalene motif (i.e., three fused hexagon rings) costs 5.5 kcal mol−1 on average, and a pentagon between two hexagons provides a stabilization of 4.5 kcal mol−1 on average). A more recent study [22] estimates the pentagon-signature penalty to be on the order of 20–25 kcal mol−1. The particularly large penalty for abutting pentagons, usually referred to in the fullerene community as violation of the isolated pentagon rule (IPR) [8], is the most important stability discriminant in the search process for the lowest-energy fullerene isomers. For a perspective on non-IPR isomers, see [23].
A large portion of the predictions and explanations available in the literature is based on topological and graph-theoretical concepts such as aromaticity [20,24,25,26,27,28,29], -electron resonance energy [30,31,32,33,34], Kekulé structures [35,36,37,38,39,40,41,42], Clar structures [43,44,45,46,47,48,49,50,51,52,53,54,55], and many other related ideas [56,57,58,59,60,61,62,63,64,65,66,67,68,69,70]. The early hypothesis of Kroto et al. [8] stating that the most abundant isomer of C60 would have a very large number of Kekulé structures (i.e., the Kekulé count ) was soon disproved by Austin and collaborators [35], who found that there are twenty isomers of C60 with larger than that of the isomer. Interestingly, a recent DFT study [22] of the thermodynamic stability of all 1812 (5,6)-isomers of C60 clearly demonstrated the rather counterintuitive fact that the isomer with the largest value of is actually the least thermodynamically stable isomer of C60 (the value of 16,501 for this tubular isomer 60:1 [35,36] is about 30% larger than 12,500 for the most-stable isomer 60:1812 [30]). Note that the notation n:m corresponds to the mth isomer in the lexicographic spiral order of the fullerene Cn [3]. In 2010, Zhang and collaborators discovered [71] that the isomer of C60 indeed maximizes the Kekulé count , but only among those isomers with the maximal Clar number , suggesting a pronounced role of aromaticity in assessing the stability of fullerene isomers (proper mathematical definitions of and are provided later in this paper; here, we only briefly signalize that the Clar number corresponds to the maximal number of Clar aromatic sextets that can be simultaneously accommodated within a given benzenoid moiety [72]). Zhang and his collaborators found that there are eighteen isomers of C60 with [43,48] and that for the isomer of C60 with 12,500, the number of Kekulé structures is distinctly larger than for the next isomer in this class (60:44, with 11,259). Another interesting observation of Zhang and his collaborators [71] concerned the tubular isomer 60:1, which indeed maximizes the Kekulé count with 16,501, but at the same time (together with five other isomers of C60) minimizes the Clar number with . The pronounced role of local aromaticity in designating the most stable isomer of C60 could perhaps constitute an interesting and valuable tool for characterizing the most stable isomers of a given carbon fullerene without the need for extensive quantum chemical calculations, provided that such a relationship also holds for fullerenes other than C60. Unfortunately, the topological descriptors needed for such an analysis have never been reported in the literature.
In a recent article [73], we reported a compilation of topological invariants for all the isomers of small (5,6)-fullerenes C20–C50, including their Kekulé counts and Clar numbers . We discovered that for these small and highly-strained fullerenes, the correlation between their thermodynamic stability and their topological invariants is rather disappointing. According to the observations of Zhang et al. for C60 [71], the isomer with the maximal Kekulé count among the isomers with the maximal Clar number should also be the most thermodynamically stable isomer of a given fullerene Cn. However, the data in Figure 4 of [73] show otherwise; the most stable isomer of C36 (isomer 36:14 in [3]) with and corresponds to the minimal Clar number among all the isomers of C36, and has an intermediate value of (the value of for isomers of C36 ranges between 266 and 364). Similarly, while for C50, the most stable isomer (50:271 in [3]) with and indeed maximizes the Clar number among the isomers of C50, its value of is again intermediate (the value of for isomers of C50 ranges between 2005 and 3276). At this point [73], it remains unclear whether the discrepancy with the previous observations of Zhang et al. [71] made for C60 can be attributed to the highly-strained nature of these small fullerene cages, the somewhat exceptional position that C60 occupies among all the fullerenes, or perhaps to the coincidental nature of the observations of Zhang et al. [71] Therefore, in the current work we have decided to extend the compilation of topological invariants to all isomers of fullerenes C52–C70, which should allow us to elucidate the character of the observations made by Zhang et al. [71] for C60, and possibly to extend it to larger fullerenes as well. The vast number of isomers treated in this study (29,767 distinct isomers of C52–C70) prevents us from showing the results of our investigations directly in the main body of the paper. Therefore, the compilation of topological invariants for all isomers of fullerenes C52–C70 is exiled to the Supplementary Material accompanying this article (file ZZpolynomials.txt, available at https://www.mdpi.com/article/10.3390/molecules29174013/s1), while the main body of our paper only presents the thermodynamic analysis of isomer stability, the correlation with their topological invariants, and the analysis of bond orders computed on the basis of the developed framework for topological invariants.
The structure of our paper is as follows. Section 2 presents a brief introduction to the computational methods employed in our analysis, including an introduction to the theory of ZZ polynomials used to determine the topological invariants of fullerenes and a brief sketch of the density functional tight binding (DFTB) method used to optimize the isomer structures and compute their energies. Section 3 briefly summarizes the results listed in the Supplementary Materials and explains how they should be interpreted. Section 3.1 and Section 3.2 provide a comparison of the computed thermodynamic stability of fullerene isomers with their various topological invariants, and establish the existence/lack of existence of correlations between these two groups of descriptors. In Section 3.3, we analyze the statistical correlation between two groups of topological invariants (the Clar count and the Kekulé count ) and discover an interesting regularity related to the most stable isomers and the / ratio. Section 3.4 deals with a verification of the hypothesis by Zhang, Ye, and Liu [71] claiming that the most energetically stable structural isomer maximizes the number of Kekulé structures among the isomers with the maximal conceivable value of . In Section 3.5 we report that all the ZZ polynomials of the 30,579 fullerene isomers studied here are distinct and hence can serve as a convenient unique labels for distinguishing between those isomers. Section 3.6 provides an analysis of the bond orders derived from the topological invariants and their correlation with the actual bond lengths in the optimized fullerene isomers. Finally, Section 4 presents the conclusions of our work.
2. Computational Details
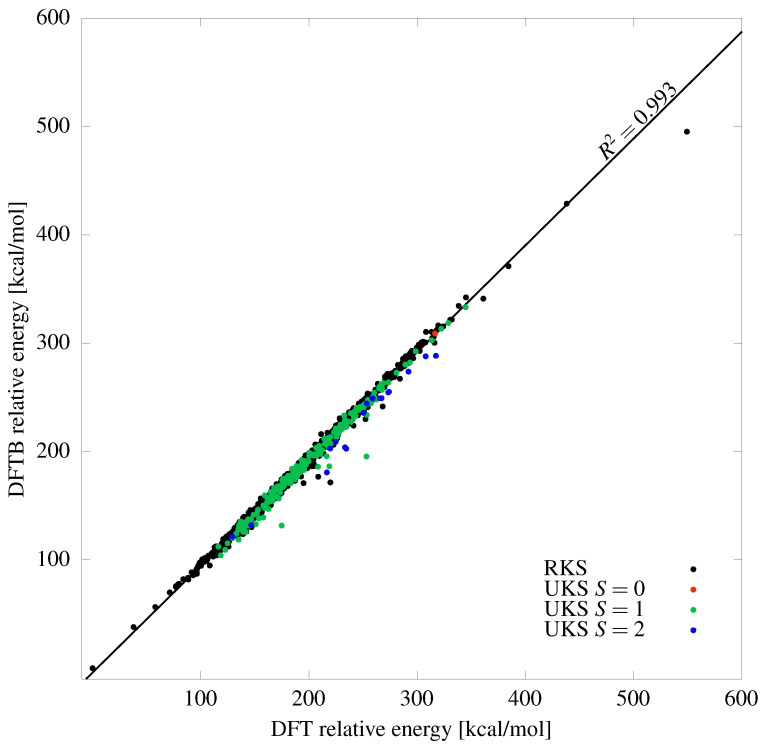
The thermodynamic stability of the C52–C70 fullerene isomers is assessed by computing their total DFTB energies at the equilibrium geometry of each isomer; note that this method has been successfully used for this purpose before [74,75]. Note also that while isomer stability ranking has been performed for a large group of fullerenes (see for example [76,77,78]), the published results usually report only the most stable isomers. Therefore, for the requirements of the current work, we have decided to recompute all the rankings again from scratch while consistently using the same DFTB quantum chemical method. The abbreviation DFTB corresponds to the density-functional tight-binding method [79], an approximate quantum chemical approach unifying the elements of tight-binding methodology with density-functional parameterization of the matrix elements. In DFTB, only the valence electrons of each atom are treated explicitly using a minimal valence basis set. The one-electron Hamiltonian and overlap integrals are precomputed and stored in so-called Slater–Koster (SK) files, whereas the contributions from core electrons and various double-counting terms are included via effective distance-dependent two-center repulsive potentials. The electron repulsion is accounted for via attenuated interaction of self-consistently determined atomic Mulliken charges. Further details of the DFTB methodology can be found in various reviews of the method [80,81,82]. DFTB is often used to model carbon nanostructures, including fullerenes, for which it often shows accuracy comparable with density functional theory (DFT) [83]. As a sanity check, in Figure 1 we show a comparison of the optimized DFT and DFTB energies for all 1812 isomers of C60; the DFTB energies and optimized structures are taken from the current work, while the DFT energies and optimized structures are taken from [22]. This comparison shows very good linear correlation between both methods (with ), with correct identification of the most stable and least stable isomers along with rather small deviations for all of the intermediate isomers. The multiple points located below the correlation line in Figure 1 are invariably associated with lower-energy minima discovered by the DFTB method; these new energy minima often originate from Jahn–Teller distortion of the original cage, which often removes degeneracies in the frontier orbital spectrum and lowers the global symmetry of the isomer (further details are discussed later in this section). In the current work, we use so-called full third-order DFTB together with the 3OB SK parameter file for carbon without any dispersion correction. All calculations are performed using the DFTB+ program [84] with the convergence criterion for the self-consistent charges, closely following the spirit of our earlier work [16,73,85,86,87]. The atomic force convergence criterion was initially set to ; however, this value turned out to be too close to the numerical accuracy limit of the first geometrical DFTB derivatives. Consequently, this convergence criterion has been somewhat loosened and all of the structures have been optimized with the maximal force not larger than , an optimization criterion applicable to all the studied here isomers of C52–C70. To unify the discussion and to allow for comparisons with smaller fullerenes, the same methodology was also extended to the (5,6)-isomers of C20–C50 treated initially in our previous work [73] and discussed here again for completeness.
Figure 1.
Comparison of the optimized DFTB and DFT energies for the 1812 isomers of C60, showing good correlation between these two methods. The color designation of points is described in the legend, and corresponds to the DFT calculations from [22].
In 49 cases (out of 29,767), the fullerene isomers display a distinct open-shell character which prevents convergence of the DFTB calculations to a non-metallic solution. In these cases (denoted as “HOMO-LUMO 0 gap” in the accompanying Supplementary Materials), we have used an electronic temperature of K and obtained a DFTB solution corresponding to fractional orbital occupations. Almost all of these cases correspond to two quasi-degenerate molecular orbitals and two electrons occupying them; the corresponding HOMO:LUMO fractional occupations range between 1.000:1.000 and 1.906:0.094. In one case (isomer 8148 of C70), the frontier HOMO orbital is doubly degenerate and the LUMO orbital is non-degenerate; the corresponding occupation pattern involves four electrons, and can be summarized as 1:504:1.504:0.992. For these open-shell cases, it is more informative to express the stability of a given fullerene isomer via the Mermin free energy; however, in all studied cases the difference with respect to the total energy is smaller than kcal/mol, meaning that it has no practical significance. In any event, the DFTB solutions with fractional occupations are not rigorous and do not correspond to a well-defined quantum spin number, and as such should be treated with caution. The electronic spin state in the previous DFT study of all the isomers of C60 was selected on the base of simple Hückel calculations [22]. A large proportion of isomers of C60 (231 out of 1812, about 12.7%) corresponded to triplet (or even quintet) electronic states in these calculations, spin states that would be overlooked by the open-shell DFTB calculations reported here. Surprisingly, in our DFTB calculations only one isomer of C60 out of 1812 (60:1478) has a manifestly open-shell structure with a zero HOMO-LUMO gap, while only four other isomers (60:1535, 60:1574, 60:1374, and 60:1481) have HOMO:LUMO gaps smaller than 1 eV. Evidently, the quasidegenerate HOMO:LUMO designations from the simple Hückel-type model in [22] are subject to a strong Jahn–Teller distortion [88,89,90,91], which might lower the symmetry of the fullerene cage and introduces considerable electronic energy stabilization.
It is important to highlight that DFTB, like DFT, may provide a poor description of isomers with a quasidegenerate ground state, i.e., states with a pronounced multi-reference character for which a single Slater determinant is a bad approximation to the wave function. Various interesting and unexpected methodological problems might manifest themselves in this context [92,93,94,95]. Fortunately, such strongly correlated states do not occur very often; the study of all isomers of the classical fullerenes C20–C50 by Fowler, Mitchell, and Zerbetto [96] showed that only two out of 812 isomers (36:15 and 44:37) experience pronounced energy stabilization (>15 kcal/mol) in the approximate CISD calculations with four frontier orbitals, which suggests a pronounced multi-reference character of the underlying wave functions. DFTB and DFT would overlook such strongly correlated states, both predicting too high energies; for example, among the isomers of C36, DFTB predicts the isomers 36:14 and 36:15 within 0.5 kcal/mol (for an almost analogous DFT result, see [97]), but misses the fact that 36:15 is the ground state. This problem is rather serious, as there is no well-developed computational protocol for establishing univocally reliable energy rankings of fullerene isomers at their optimized geometries [98]. An obvious candidate for computing such an energy ranking of fullerene isomers would be the CASSCF/PT2 scheme (see for example [99]); however, creating such a ranking would constitute a considerable computational effort, and has not been yet performed in a systematic manner.
Note that these issues, while methodologically interesting and definitely worth further investigation in future studies, do not pose any serious problems for our current plans; Sure et al. [22] reported that the triplet and quintet isomers of C60 have relatively high energies, with the lowest (60:1728) lying 116.1 kcal mol−1 above the energy of the most stable isomer and the highest (60:44) lying 344.7 kcal mol−1 above this energy. These separations could perhaps be somewhat reduced by the Jahn–Teller distortions, but we do not expect this effect to be large. In our DFTB study, both the 60:1728 and 60:44 isomers possess a clear closed-shell singlet character with considerable HOMO:LUMO gaps (5.41 and 11.0 eV, respectively), while their stability is not altered significantly (their DFTB energies are respectively 112.3 and 333.2 kcal mol−1 above the DFTB energy of the most stable isomer). While we discuss this problem here in detail in order to inform readers about the possible difficulty, we do not think that the fraction of the remaining open-shell isomers (49 out of 29,767 cases, representing 0.16% of the total number of studied isomers) could statistically alter the results of the current analysis. Most (36 out of 49) of the relative energies of these open-shell structures are larger than 100 kcal mol−1, and for only four isomers (58:1205, 52:425, 68:6081, and 58:1151) is the relative energy smaller than 75 kcal mol−1. The most serious interpretational difficulty remains for the 58:1205 isomer, which has the lowest energy out of all isomers of C58. Again, this is rather fortunate for our analysis, as more rigorous quantum chemical calculations with a definitive value of the spin quantum number can only lower this value, and cannot alter the fact that 58:1205 corresponds to the most stable isomer of C58.
The topological invariants are computed in the form of the ZZ polynomial [100,101] for each isomer. Brief graph-theoretical definitions of the underlying concepts are provided below; for further details and explanations, the reader is referred to the rich existing literature on this topic [73,102,103,104,105,106,107,108]. From a graph-theoretical point of view, a fullerene isomer can be expressed as a 2-connected finite-plane graph B, with twelve interior faces being pentagons and interior faces corresponding to hexagons. Such a graph is usually represented by the corresponding Schlegel diagram; for examples of Schlegel diagrams, see Figure 2. A Kekulé structure K is defined as a spanning subgraph of B of which all components are isomorphic to a complete graph on two vertices (). The number of distinct Kekulé structures K that can be constructed for B is referred to as the Kekulé count . Similarly, a Clar cover C is defined as a spanning subgraph of B of which all components are isomorphic to either or to a cycle of girth six (). The number of distinct Clar covers C that can be constructed for B is referred to as the Clar count . Note that in the chemical literature one usually refers to as a double bond and to as an aromatic Clar sextet; similarly, a Kekulé structure K is referred to as a resonance structure of B that can be constructed using double bonds and a Clar structure C is referred to as a generalized resonance structure of B that can be constructed using k aromatic Clar sextets and double bonds. The maximal number of aromatic Clar sextets that can be accommodated in C is referred to as the Clar number of B. The Clar covers with aromatic sextets are referred to as the Clar formulas of B. The Clar covers with k aromatic sextets are referred to as the Clar covers of order k. If we represent the number of Clar covers of order k for B by , we can define a combinatorial polynomial
| (1) |
usually referred to in the literature as the Clar covering polynomial, Zhang–Zhang polynomial, or in short the ZZ polynomial of B. Clearly, the ZZ polynomial of B has the following properties:
The number of Kekulé structures of B is provided by .
The number of Clar covers of B is provided by .
The Clar number of B is provided by .
The number of Clar formulas of B is provided by .
The ZZ polynomial is a generating function for the sequence of Clar covers of different orders.
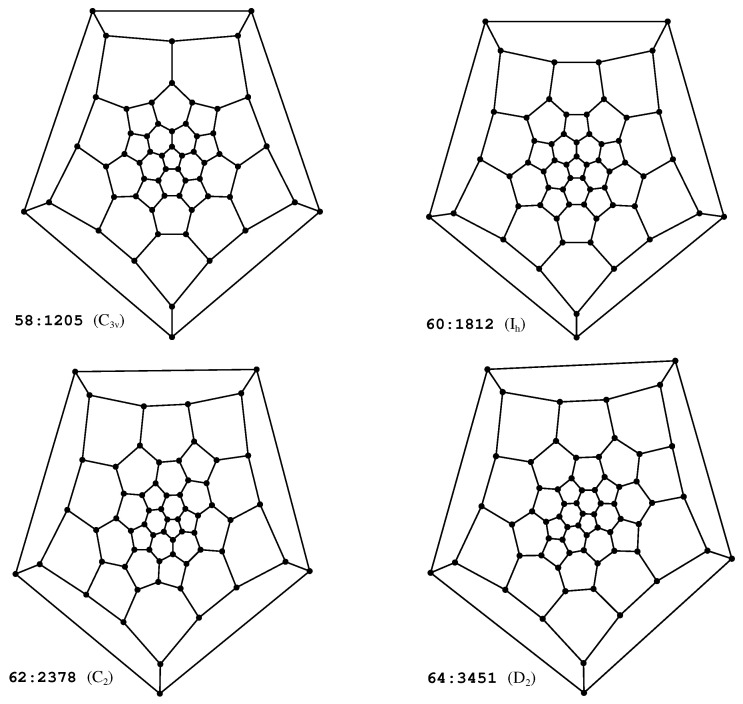
Figure 2.
Schlegel diagrams of the most stable isomers of C58 (upper left) and C60 (upper right), clearly showing the close structural resemblance between these two isomers. For the most stable isomers of C62 (lower left) and C64 (lower right), the structural similarity with C60 is less pronounced.
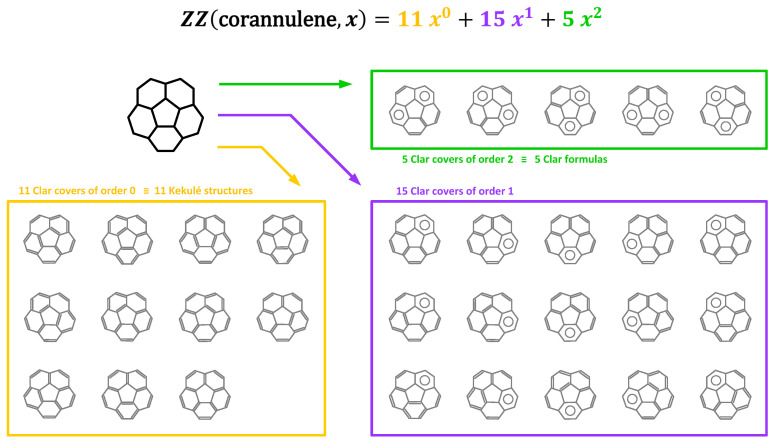
These theoretical concepts are illustrated here using the simple example of corannulene (C20H10), a non-planar molecule with a close relation to IPR (5,6)-fullerenes. All 31 Clar covers of corannulene are shown in Figure 3. The Clar covers are grouped in three classes: eleven Kekulé structures, i.e., Clar covers of order 0; fifteen Clar covers of order 1; and five Clar formulas, i.e., Clar covers of maximal conceivable order (here, ). Consequently, the ZZ polynomial of B = corannulene is provided by , the Kekulé count is , the Clar count is , and the Clar number of corannulene is . This example also illustrates that determining topological invariants of fullerenes can be quite a cumbersome problem. Fortunately, ZZ polynomials can be conveniently and readily computed owing to their recursive properties related to the molecular graph decomposition tree (for more information, see Properties 1–7 in [103]). Consequently, the ZZ polynomial of an arbitrary benzenoid or fullerene B can be efficiently computed using recursive decomposition algorithms [102,103,105] or readily determined using the interface theory of benzenoids [108,109,110,111,112,113]. A useful practical tool for determining the ZZ polynomials of general planar benzenoids is ZZDecomposer [105,106], which allows the user to define the related molecular graph using a provided graphical interface and perform all computations in an automatic fashion. For fullerenes, it is probably more convenient to use ZZPolyCalc instead [114], which reads the molecular geometry file or the adjacency matrix as an input. ZZPolyCalc is also considerably faster owing to an efficient algorithm involving intermediate fragments caching. Both programs are freely downloadable [115,116,117] and self-explanatory. In the current study, we have used the ZZPolyCalc software for all the topological invariant calculations. The time needed to compute a single ZZ polynomial is less than one tenth of a second for the studied here fullerenes.
Figure 3.
Clar covers (i.e., extended resonance structures) of corannulene (C20H10) can be conveniently enumerated using the ZZ polynomials, which keep track of the number of resonance structures for each order. The order k of each Clar cover C is defined as its number of aromatic Clar sextets.
The molecular structures of fullerene isomers have been generated in the following way. The ring-spiral pentagon lists for the fullerene isomers have been taken from the House of Graphs fullerene database [1]. The spiral sequences served as an input to the program FULLERENE [118] (Version 4.5), and the initial geometries of the isomers were generated using the adjacency matrix eigenvector (AME) method [3]. These structures were subsequently optimized using a force-field approach [119], with additional extension to account for the third bond type and additional dihedral angles (activated using the ‘iopt=3’ flag in the FULLERENE program). In instances when this procedure failed to generate a meaningful converged geometry, the process was repeated using Tutte embedding (3D-TE) [120] instead of AME and optimized using the ‘iopt=2’ flag in the FULLERENE code. The force field-preoptimized molecular structures of the C52–C70 fullerene isomers were subsequently optimized using a series of DFTB geometry optimizations, in which the atomic forces convergence criterion was gradually tightened from to in an alternating sequence of conjugate gradient/steepest descent optimization steps.
3. Results
The abundant amount of data generated in the current study prevents us from presenting it directly within the body of the paper. Most of the resulting data are presented in the Supplementary Materials accompanying this study; here, we only analyze the most important features of the results. The file ZZpolynomials.txt lists the computed ZZ polynomials for all the fullerene isomers C20–C70, with the data for fullerenes C52–C70 computed in the current work and the data for fullerenes C20–C50, listed again here for the convenience of the reader, taken from our previous work [73]. The file Correlations.txt lists the topological invariants (Clar count , Kekulé count , and Clar number ) of all the fullerene isomers C20–C70 together with their optimized DFTB energies. The file DFTBresults.txt briefly summarizes our DFTB calculations, providing the total DFTB energy E, Mermin free energy , final gradient value of the optimized structure, and labels of the highest doubly-occupied (HOMO) and lowest unoccupied (LUMO) DFTB orbitals along with their orbital energies and . In cases where it was not possible to converge DFTB to a closed-shell solution, an additional notification “HOMO-LUMO 0 gap”, along with the occupation pattern of the degenerate frontier orbitals, is added the the end of the pertinent line to inform the reader that a finite electronic temperature () has been used to smear out the orbital occupation numbers in the vicinity of the Fermi energy. Note that while the total energy E and Mermin free energy are different in these cases, the numerical difference between these two quantities is too small to have any sizable effect on our conclusions. The XYZ files containing the optimized DFTB geometries for each of the fullerene isomers can be found in the file Geometries.tar.xz.
3.1. Correlation of the Kekulé Count and Clar Count with the Total Energies of Fullerene Isomers
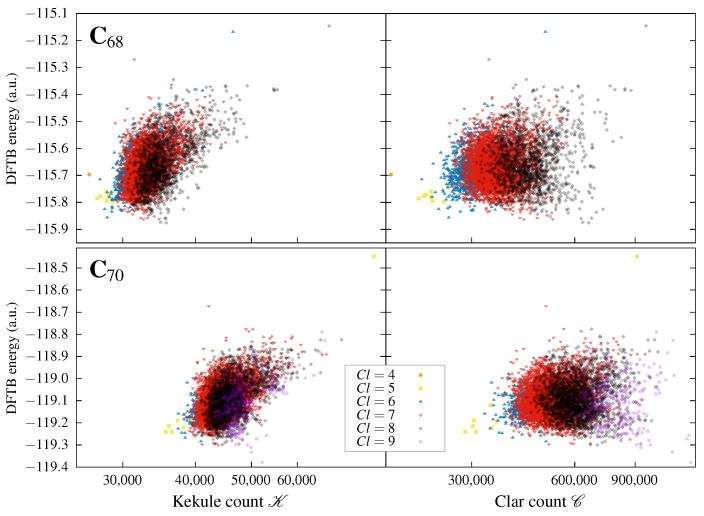
The correlations of the total DFTB energies E with the corresponding values of the Kekulé count and the Clar count for all the isomers of C68 and C70 are shown in Figure 4. Similar plots for smaller fullerenes are presented in the Supplementary Materials in Figures S1 and S2; the resulting correlations resemble those shown in Figure 4. Two families of plots are obtained: one presenting the correlation between the Kekulé count and the total energy E, and the second presenting the correlation between the Clar count and total energy E. The distinction between isomers with different values of is achieved by using the symbol scheme explained in the legend of Figure 4.
Figure 4.
Total DFTB energies of all isomers of (upper panels) and (lower panels) plotted as a function of their topological invariants, i.e., the Kekulé count (left panels) and Clar count (right panels). The information about the Clar number is conveyed via the symbol code explained in the legend. The plots show weak anti-correlation between E and and no correlation between E and . Similar tendencies are observed also for smaller fullerenes C52–C66. For details, see the Supplementary Materials.
The most important observation is that the topological invariants and do not correlate strongly with the total DFTB energy of fullerene isomers; the shapes of the distributions are irregular and somewhat ellipsoidal, and show no correlation whatsoever, while the shapes of the distributions rather surprisingly show weak anti-correlation. The isomers with a large value of Kekulé count are usually the highest in energy, which contradicts the usual organic chemistry rule of thumb stating that the structural isomers with the largest number of resonance structures are the most stable. Apparently, curved fullerenes do not adhere to this rule, and seem to promote an opposite principle, that is, that the isomers with large are the least stable. This observation is not really new; as described in Section 1, a similar conclusion was already drawn for C60 more than 25 years ago [30,35,36,71]. The anti-Kekulé principle seems to prevail here; for six out of ten fullerenes studied here (C54, C56, C60, C62, C68, and C70) the isomer with the largest number of Kekulé structures is the highest in energy, while for two further fullerenes (C52 and C64) the highest-energy isomer has a value of very close to the maximal one.
3.2. Correlation between the Clar Number and Total Energy of Fullerene Isomers
The anti-Kekulé correlation mentioned in the previous paragraph seems to suggest that fullerenes prefer structures in which the hexagons assume an aromatic benzene-like geometry without the double–single bond alternation characteristic for Kekulé structures. This observation is indeed confirmed by the observation that the most thermodynamically stable isomers usually have the largest value of ; readers should recollect that the Clar number indicates the largest number of aromatic Clar sextets that can be simultaneously accommodated inside a given isomer without violating chemical bonding principles. In Table 1, we present the populations of isomers of the fullerenes C52–C70 with a given value of . The group with the most thermodynamically stable isomer is underlined. For six out of ten fullerenes (C56, C60, C62, C64, C68, and C70) the most thermodynamically stable structure belongs to the group with maximal value of , while for three further fullerenes (C52, C54, and C66) it belongs to the group with the second largest value of . It would be interesting to extend our study to larger fullerene cages in order to test the hypothesis that the most stable isomer of large fullerenes Cn with always maximizes the number of aromatic Clar sextets.
Table 1.
The number of isomers of fullerenes C52–C70 with a given value of Clar number . The relative abundance of each group is provided in parentheses. The group containing the most stable isomer is underlined. For six fullerenes (C56, C60, C62, C64, C68, and C70), the most thermodynamically stable structure belongs to the group with maximal value of ; for three further fullerenes (C52, C54, and C66), it belongs to the group with the second largest . The presented data suggest that for larger fullerenes the most thermodynamically stable isomer can be found by studying only the isomers with large values of .
| Fullerene | ||||||
|---|---|---|---|---|---|---|
| 4 | 5 | 6 | 7 | 8 | 9 | |
| C52 | 116 (27%) | 254 (58%) | 67 (15%) | |||
| C54 | 35 ( 6%) | 452 (78%) | 86 (15%) | 7 ( 1%) | ||
| C56 | 32 ( 3%) | 453 (49%) | 439 (48%) | |||
| C58 | 2 ( 0%) | 506 (42%) | 597 (50%) | 100 ( 8%) | ||
| C60 | 6 ( 0%) | 290 (16%) | 1316 (73%) | 182 (10%) | 18 ( 1%) | |
| C62 | 1 ( 0%) | 198 ( 8%) | 1468 (62%) | 718 (30%) | ||
| C64 | 53 ( 1%) | 1937 (56%) | 1280 (37%) | 195 ( 6%) | ||
| C66 | 33 ( 1%) | 1342 (30%) | 2817 (63%) | 275 ( 6%) | 11 (0%) | |
| C68 | 1 ( 0%) | 8 ( 0%) | 1109 (18%) | 3806 (60%) | 1408 (22%) | |
| C70 | 8 ( 0%) | 412 ( 5%) | 5186 (64%) | 2276 (28%) | 267 (3%) | |
The hypothesis stating that isomers with the largest conceivable value of for a given fullerene correspond to the most thermodynamically stable structure might have important practical consequences should it be confirmed for fullerenes larger than C70. At the moment, finding the most energetically stable structural isomer of large fullerenes is relatively complex due to the quite substantial computational resources required to accomplish this task. Two factors contribute to the cost here: geometry optimization for larger fullerene cages Cn becomes more and more costly with growing n, and a large number of isomers exists for large n that need to be screened in the search process. The number of isomers of Cn grows with n, such as [121,122]; for C70, it is 8,149, while for C100 it is already 285,913. Selecting only those isomers with the maximal conceivable allows the group of candidates for the lowest-energy structure to be reduced considerably. For example, for C70, the number of isomers with maximal is 267, which accounts for 3.3% of the total number of isomers, while for C100 the number of isomers with maximal is 1442, which accounts only for 0.5% of the total number of isomers. Thus, confirming that the most stable isomer of large fullerenes Cn always maximizes the number of aromatic Clar sextets could lead to considerable savings during identification.
3.3. Correlation between Clar and Kekulé Counts and Relation to Isomer Stability
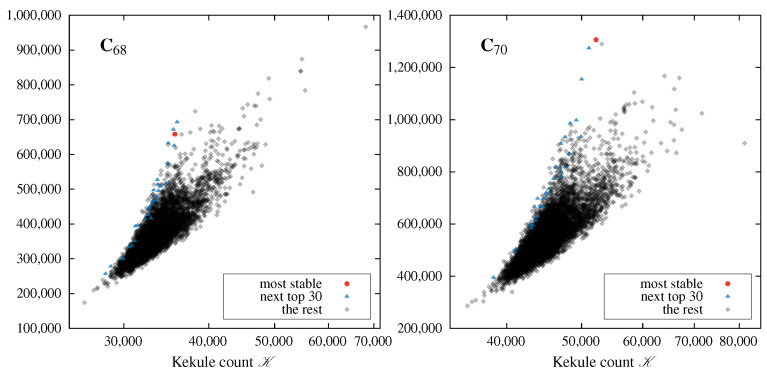
The Clar and Kekulé counts are not fully independent. The correlation coefficient between these two measures varies from 0.49 to 0.59 depending on the size of the fullerene. Additionally, their relationship exhibits very interesting and distinct patterns. In Figure 5, we present a graph showing the Clar counts as a function of Kekulé counts for all isomers of and . Analogous graphs for the remaining fullerenes – are available in the Supplementary Materials as Figure S3. The relationship between Clar and Kekulé counts in all cases has the shape of a slanted wedge. The approximately linear lower boundary has a slope that tends to increase with the system size ( > 3.87 for and > 8.07 for ). The upper boundary is less regular, but generally tends to change with approximately the fourth power of .
Figure 5.
Clar counts as a function of Kekulé counts for (left panel) and (right panel). The most stable structure is marked with a red dot while structures 2–30 (ordered by stability) are marked with blue triangles. The most stable isomers tend to group near the top leftmost part of the graph.
While the consistent shape of the distributions is quite interesting, the most surprising aspect of these graphs lies elsewhere; in almost all of the studied cases, the 30 most stable isomers of each fullerene are located almost entirely at the upper boundary of the wedge, with the most stable structure having the largest ratio for , , and . This regularity is quite visible in Figure 5 and Figure S3, where the 30 most stable isomers are depicted in different colors, with the most stable isomer represented by a red circle. For certain fullerenes, particularly the smaller ones, the detected pattern is weaker; for example, for the most stable structure is not near the upper boundary of the distribution, and several top-30 isomers are actually closer to the lower boundary of the distribution. Nevertheless, the discovered pattern of stable isomers grouping near the upper boundary becomes more pronounced as the size of the fullerene increases, and as such can become a very useful tool in discriminating the most stable isomers of larger fullerenes. We believe that this aspect of our study deserves further investigation.
3.4. Does the Most Stable Isomer Maximize the Kekulé Count among the Isomers with the Maximal Value of Clar Number?
Let us now verify the main hypothesis of the current work. In 2010, Zhang, Ye, and Liu [71] made the observation that for C60 the most energetically stable structural isomer maximizes the number of Kekulé structures among the isomers with the maximal conceivable value of . The question we would like to test here is whether such an observation is also correct for other fullerenes. The observation made by Zhang et al. [71] is clear from the current work, as is obvious from the right panel of Figure S2 in the Supplementary Materials. There are eighteen isomers of C60 with the maximal value of , and the lowest in energy, the icosahedral structure 60:1812, indeed maximizes the value of among them. Is a similar observation true for the other fullerenes studied here? The answer is negative; C60 is the only fullerene among C52–C70 for which the observation made by Zhang et al. [71] is valid. Interestingly, an anti-Kekulé-like rule works much better here: for four of the studied fullerenes (C56, C62, C68, and C70), the isomer with maximal among isomers with maximal is the highest in energy! For the remaining five fullerenes (C52, C54, C58, C64, and C66), the isomer with maximal among isomers with maximal has intermediate energy; none of these isomers is a good candidate for a global energy minimum. Therefore, the observation of Zhang, Ye, and Liu for C60 is not useful for other fullerenes, and we can refute as a useful indicator of fullerene stability in general. Note that the results presented previously in [73] for C20–C50 provide further support to this conclusion.
It seems, however, that the observation of Zhang, Ye, and Liu [71] for C60 can be made somewhat more useful if we introduce a small correction. Specifically, we shall now test a new hypothesis stating that the most stable isomer is the one which maximizes the Clar count among the isomers with the maximal conceivable value of . This observation is clearly correct for C60; the icosahedral isomer not only maximizes among the isomers with , it also maximizes in the whole population of C60 isomers. A similar observation is true for two other fullerenes, C66 and C70, while for C64 the three isomers with maximal correspond to the second, third, and fourth most stable isomers of this fullerene. Unfortunately, for the other fullerenes an isomer that maximizes among the isomers maximizing corresponds either to an intermediate energy structure (for C52, C54, and C58) or to the highest energy structure (C56, C62, and C68). The last sequence with the progression is quite interesting, suggesting that the isomers of with that maximize could correspond to the structural isomers with the highest energy. This hypothesis should be tested when the data for larger fullerenes become available. Summarizing the observations in this paragraph, we can state that the Clar count is only marginally more useful than as a topological indicator for characterizing fullerene stability.
3.5. ZZ Polynomials Can Be Used as Alternative Unique Labels for Discriminating between Fullerene Isomers
Another interesting and possibly useful observation, though unrelated to the energetic stability of fullerenes, is the fact that all 29,767 of the ZZ polynomials computed in the current work are distinct from each other and can be used to discriminate between different isomers of fullerenes C52–C70. This observation also extends to the 812 different isomers of C20–C50 studied by us previously. Thus, all 30,579 isomers of fullerenes C20–C70 have different ZZ polynomials! Initial checks suggest that this uniqueness of ZZ polynomials extends to larger fullerene cages as well. Consequently, ZZ polynomials might be used to label different isomers of fullerenes as an alternative to the canonical spiral sequence. Calculation of ZZ polynomials can be performed almost instantaneously for the fullerenes studied here, providing a very convenient method of recognizing which particular fullerene isomer is currently considered. Note that determination of the ZZ polynomial can be performed directly from the XYZ geometry file of a given isomer or from its topological adjacency matrix, as ZZ polynomials are invariant with respect to vertex permutation and with respect to geometrical transformation (rotations, translations, and deformations) of the fullerene cage. This particular property of ZZ polynomials makes them a very convenient descriptor for machine learning models, providing a meaningfully unique multilabel for each isomer that consists of its various topological invariants.
3.6. Pauling Bond Orders in Fullerenes
Access to all possible Kekulé structures and all possible Clar covers of fullerene isomers allows us to compute the Kekulé and Clar bond orders in the way first introduced by Pauling [123,124] and popularized by Herndon, Randić, and others in the 1970s [125,126,127,128,129,130,131,132,133]. Our study can be considered as a direct extension of the work on Pauling–Kekulé bond orders carried out by Narita, Morikawa, and Shibuya for the most stable isomers of C60 and C70 [134] and the work on Pauling–Clar bond orders by Randić for C60 [135]. Recent years have provided evidence of multiple situations in which Kekulé structure-based models yield evidently incorrect predictions [136,137,138,139,140]. Here, we use the current results on a very large statistical sample to verify whether the the Pauling–Clar bond orders and Pauling–Kekulé bond orders, both of which are quantities computable directly from the topology of bond connections without any use of quantum chemical theory, can be of any value for practical purposes in the theory of fullerene isomers.
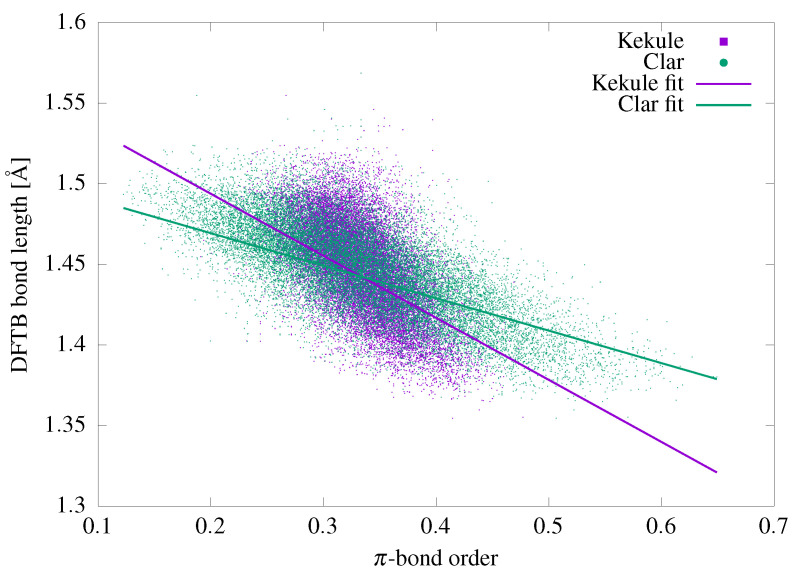
The procedure is simple. First, we choose a particular bond B in a given fullerene isomer and inspect the character assigned to it by each Kekulé structure or Clar cover. For Kekulé structures, there are only two possibilities: B can be a single bond (with the bond order of 0) or a double bond (with the bond order of 1). For Clar covers, there are three possibilities: B can be a single bond (with the bond order of 0), a double bond (with the bond order of 1), or a member of an aromatic Clar sextet (with the bond order of ). An average of all these contributions over the full set of Kekulé structures produces the classical Pauling bond orders for fullerenes [123,134]. A similar average computed over the full set of Clar covers produces the modified Pauling–Clar bond orders [135]. The easiest way to estimate the usefulness of these quantities is to correlate them with the bond lengths obtained via DFTB optimization of each structure. In this way, each bond can be represented as a dot, with the x-coordinate corresponding to the bond order and the y-coordinate corresponding to the bond length in the DFTB-optimized structure of a given isomer. Such correlations for all 2,978,872 bonds in all 30,579 isomers of fullerenes C20–C70 are shown in Figure 6, with the green dots representing the Pauling–Clar bond orders and the purple squares representing the Pauling–Kekulé bond orders. For reasons of technical feasibility, only 1% of the randomly selected points out of 2,978,872 are shown in Figure 6; notably, this limitation does not alter the visual distribution of the points. Both distributions show clear correlations between the bond orders and bond lengths; single bonds are longer and double bonds are shorter, which is in close agreement with chemical intuition. The correlations are rather modest, with coefficients of 0.486 and 0.429 for the Pauling–Clar and Pauling–Kekulé bond orders, respectively. It is clear that the Pauling–Clar bond orders are more useful in practice thanks to providing better discrimination between shorter and longer bonds. The coefficient of 0.486 shows that approximately half of the statistical variance is explained by the predicted linear trend plotted in Figure 6, while the other half cannot be inferred from the bond order alone. This regularity can be very useful for solving one of the main problems associated with generation of XYZ geometry files for fullerene isomers, where the preparation of a physically relevant initial geometrical structure from the adjacency matrix computed on the basis of the spiral sequence is a challenging task. Because the bond orders can be computed directly from the adjacency matrix and correlate with the bond lengths, it is possible to prepare a good initial geometry file directly on the basis of the bond lengths inferred from the bond orders.
Figure 6.
Correlation between the Kekulé and Clar bond orders and corresponding DFTB bond lengths for all isomers of the C20–C70 fullerenes. The linear fits are and for the Clar and Kekulé models, respectively. For clarity, the graph displays data for only 1% of the total number of 2,978,872 bonds (chosen randomly). The fits have been performed on the complete sets.
4. Conclusions
We report a compilation of topological invariants for all 29,767 structural isomers of the carbon -fullerenes C52–C70. The results are presented in the file ZZpolynomials.txt in the Supplementary Materials. This collection of data, together with the previously reported [73] ZZ polynomials for fullerenes C20–C50, completes our determination of the most important topological invariants for -fullerenes with 70 or fewer carbon atoms. Interestingly, all of the ZZ polynomials computed for the 30,579 isomers of fullerenes C20–C70 are distinct, and this uniqueness seems to extend to larger fullerene cages as well, making the ZZ polynomials a convenient label for identifying and discriminating between various fullerene isomers with potential applications to machine learning models.
The computed Clar numbers, Clar counts, and Kekulé counts of the C52–C70 isomers are correlated with the total DFTB electronic energies computed at the optimized DFTB geometries of the corresponding fullerene cages (the DFTB energies are listed in the file DFTBresults.txt, and the optimized DFTB geometries are provided in the file Geometries.tar.xz, both of which accompany this paper in the Supplementary Materials). The correlations are computed in order to verify the hypothesis of Zhang, Ye, and Liu [71], who postulated that the most energetically stable structural isomer of Cn maximizes the Kekulé count among the isomers with the maximal conceivable Clar number . Analysis of our data shows that this hypothesis only holds for C60. For the remaining nine fullerenes (C52–C58 and C62–C70), the isomers with maximal among the isomers with maximal correspond to high or intermediate DFTB energies; none of these isomers are a good candidate for a global energy minimum of Cn. Note that the results presented previously in [73] for C20–C50 provide further support to this conclusion. In general, our results suggest that both Kekulé count and Clar count are rather poor descriptors and predictors of isomer stability, while the Clar number, i.e., the maximal number of aromatic sextets, correlates better with the stability of isomers; however, its practical usefulness is limited. The most promising feature of our Clar and Kekulé count analysis is the observation that for larger fullerenes the most stable isomers are almost entirely located at the upper boundary of the distributions (for details, see Figure 5 and Figure S3). This observation can be very useful for prescreening isomers of larger fullerenes in order to identify viable candidates for their ground state.
Access to the complete sets of Kekulé structures and Clar covers allows us to compute the Pauling bond orders for fullerene isomers, which can be compared to the bond lengths obtained from quantum chemical calculations. The resulting Pauling–Clar and Pauling–Kekulé bond orders show rather modest correlations with the bond lengths, with the coefficients of 0.486 and 0.429, respectively. The Pauling–Clar bond orders are slightly more useful in practice, having better predictive power, and for example can be used in initial optimization of topology-generated fullerene cages.
An interesting aspect of our work is the discovery that a significant number of fullerene isomers that are predicted by a simple Hückel model to have open-shell electronic character experience a pronounced Jahn–Teller distortion, which leads to a transition to a lower-energy closed-shell state. While this should not be surprising in the light of the earlier results reported by Paulus [141] for C20–C36, final confirmation of these results may require additional CASSCF calculations in order to avoid artificial Jahn–Teller-like effects [92,93]. Notably, this pattern is observed in almost all the open-shell isomers of C60 studied in [22]. This finding implies that the conclusions of [22] regarding the abundance and stability of such isomers might require reevaluation to incorporate the influence of these Jahn–Teller effects.
Abbreviations
The following abbreviations are used in this manuscript:
| DFTB | Density-Functional Tight-Binding method |
| DFT | Density Functional Theory |
| HOMO | Highest Occupied Molecular Orbital |
| LUMO | Lowest Unoccupied Molecular Orbital |
| SK file | Slater–Koster file |
| IPR | Isolated Pentagon Rule |
| Clar number | |
| Clar count | |
| Kekulé count | |
| ZZ polynomial | Zhang–Zhang polynomial (aka Clar covering polynomial) |
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules29174013/s1, ZZpolynomials.txt: Text file containing the ZZ polynomials for all the isomers of the fullerenes C20–C70; DFTBresults.txt: Text file containing the optimized DFTB energies (and other related DFTB data) for all the isomers of the fullerenes C20–C70; Correlations.txt: Text file containing the combined DFTB energies and ZZ polynomials for all the isomers of the fullerenes C20–C70; Geometries.tar.xz: Compressed file containing the optimized DFTB geometries of all the isomers of the fullerenes C20–C70; Figure S1: DFTB energies of all isomers of – plotted as a function of topological invariants: Kekulé count (left panels) and Clar count (right panels). The information about the Clar number is conveyed via the symbol code explained in the legend. The plots show weak anti-correlation between E and and no correlation between E and .; Figure S2: DFTB energies of all isomers of – plotted as a function of topological invariants: Kekulé count (left panels) and Clar count (right panels). The information about the Clar number is conveyed via the symbol code explained in the legend. The plots show weak anti-correlation between E and and no correlation between E and .; Figure S3: Clar count of all isomers of fullerenes – plotted as a function of the Kekulé count . The most stable system in DFTB calculations is marked with red dot and the next 29 most stable structures with blue triangles.
Author Contributions
Conceptualization, H.A.W. and R.P.; methodology, H.A.W. and R.P.; software, H.A.W. and R.P.; validation, H.A.W. and R.P.; formal analysis, H.A.W. and R.P.; investigation, H.A.W. and R.P.; resources, H.A.W. and R.P.; writing—original draft preparation, H.A.W.; writing—review and editing, R.P.; visualization, H.A.W. and R.P. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Most of the research results, including the optimized DFTB energies and geometries of all the isomers of the fullerenes C20– and their corresponding ZZ polynomials, can be downloaded at: https://www.mdpi.com/article/10.3390/molecules29174013/s1 as the Supplementary Materials for this paper. For additional material, the reader is encouraged to contact the authors.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
R.P. acknowledges COST Action CA21101 “Confined molecular systems: from a new generation of materials to the stars” (COSY) supported by COST (European Cooperation in Science and Technology). H.A.W. and R.P. acknowledge financial support by the National Science and Technology Council of Taiwan (grants 110-2923-M-009-004-MY3 and 112-2113-M-A49-033).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Coolsaet K., D’hondt S., Goedgebeur J. House of Graphs 2.0: A database of interesting graphs and more. Discr. Appl. Math. 2023;325:97–107. [Google Scholar]
- 2.Manolopoulos D.E., May J.C., Down S.E. Theoretical studies of the fullerenes: C34 to C70. Chem. Phys. Lett. 1991;181:105–111. doi: 10.1016/0009-2614(91)90340-F. [DOI] [Google Scholar]
- 3.Fowler P.W., Manolopoulos D.E. An Atlas of Fullerenes. Dover; Mineola, NY, USA: 2006. [Google Scholar]
- 4.Manolopoulos D.E., Fowler P.W. A fullerene without a spiral. Chem. Phys. Lett. 1993;204:1–7. doi: 10.1016/0009-2614(93)85597-H. [DOI] [Google Scholar]
- 5.Brinkmann G., Goedgebeur J., McKay B.D. The smallest fullerene without a spiral. Chem. Phys. Lett. 2012;522:54–55. doi: 10.1016/j.cplett.2011.11.056. [DOI] [Google Scholar]
- 6.Brinkmann G., Dress A.W. A Constructive Enumeration of Fullerenes. J. Algorithms. 1997;23:345–358. doi: 10.1006/jagm.1996.0806. [DOI] [Google Scholar]
- 7.Brinkmann G., Dress A.W. PentHex Puzzles: A Reliable and Efficient Top-Down Approach to Fullerene-Structure Enumeration. Adv. Appl. Math. 1998;21:473–480. doi: 10.1006/aama.1998.0608. [DOI] [Google Scholar]
- 8.Kroto H.W. The stability of the fullerenes Cn, with n = 24, 28, 32, 36, 50, 60 and 70. Nature. 1987;329:529–531. doi: 10.1038/329529a0. [DOI] [Google Scholar]
- 9.Kroto H.W. Space, stars, C60, and soot. Science. 1988;242:1139–1145. doi: 10.1126/science.242.4882.1139. [DOI] [PubMed] [Google Scholar]
- 10.Manolopoulos D.E., Fowler P.W., Taylor R., Kroto H.W., Walton D.R.M. An end to the search for the ground state of C84? J. Chem. Soc. Faraday Trans. 1992;88:3117–3118. doi: 10.1039/ft9928803117. [DOI] [Google Scholar]
- 11.Kietzmann H., Rochow R., Ganteför G., Eberhardt W., Vietze K., Seifert G., Fowler P.W. Electronic structure of small fullerenes: Evidence for the high stability of C32. Phys. Rev. Lett. 1998;81:5378–5381. doi: 10.1103/PhysRevLett.81.5378. [DOI] [Google Scholar]
- 12.Piskoti C., Yarger J., Zettl A. C36, a new carbon solid. Nature. 1998;393:771–774. doi: 10.1038/31668. [DOI] [Google Scholar]
- 13.Zhu W., Miser D.E., Chan W.G., Hajaligol M.R. Characterization of combustion fullerene soot, C60, and mixed fullerene. Carbon. 2004;42:1463–1471. doi: 10.1016/j.carbon.2004.01.076. [DOI] [Google Scholar]
- 14.Tan Y.Z., Xie S.Y., Huang R.B., Zheng L.S. The stabilization of fused-pentagon fullerene molecules. Nat. Chem. 2009;1:450–460. doi: 10.1038/nchem.329. [DOI] [PubMed] [Google Scholar]
- 15.Fedorov A.S., Fedorov D.A., Kozubov A.A., Avramov P.V., Nishimura Y., Irle S., Witek H.A. Relative isomer abundance of fullerenes and carbon nanotubes correlates with kinetic stability. Phys. Rev. Lett. 2011;107:175506. doi: 10.1103/PhysRevLett.107.175506. Erratum in Phys. Rev. Lett. 2012, 108, 249902. [DOI] [PubMed] [Google Scholar]
- 16.Witek H.A., Irle S. Diversity in electronic structure and vibrational properties of fullerene isomers correlates with cage curvature. Carbon. 2016;100:484–491. doi: 10.1016/j.carbon.2016.01.015. [DOI] [Google Scholar]
- 17.Fowler P.W. Systematics of Fullerenes and Related Clusters. Philos. Trans. R. Soc. A. 1993;243:39–52. [Google Scholar]
- 18.Díaz-Tendero S., Alcamí M., Martín F. Fullerene C50: Sphericity takes over, not strain. Chem. Phys. Lett. 2005;407:153–158. doi: 10.1016/j.cplett.2005.03.065. [DOI] [Google Scholar]
- 19.Díaz-Tendero S., Alcamí M., Martín F. Theoretical study of ionization potentials and dissociation energies of fullerenes (n=50–60, q=0, 1 and 2) JCP. 2003;119:5545–5557. doi: 10.1063/1.1597634. [DOI] [Google Scholar]
- 20.Alcamí M., Sánchez G., Díaz-Tendero S., Wang Y., Martín F. Structural patterns in fullerenes showing adjacent pentagons: C20 to C72. J. Nanosci. Nanotechnol. 2007;7:1329–1338. doi: 10.1166/jnn.2007.311. [DOI] [PubMed] [Google Scholar]
- 21.Austin S.J., Fowler P.W., Manolopoulos D.E., Orlandi G., Zerbetto F. Structural Motifs and the Stability of Fullerenes. J. Phys. Chem. 1995;99:8076–8081. doi: 10.1021/j100020a035. [DOI] [Google Scholar]
- 22.Sure R., Hansen A., Schwerdtfeger P., Grimme S. Comprehensive theoretical study of all 1812 C60 isomers. Phys. Chem. Chem. Phys. 2017;19:14296–14305. doi: 10.1039/C7CP00735C. [DOI] [PubMed] [Google Scholar]
- 23.Chen Z.C., Tan Y.Z., Xie S.Y. Fullerenes Violating the Isolated Pentagon Rule. In: Lu X., Akasaka T., Slanina Z., editors. Handbook of Fullerene Science and Technology. Springer Nature; Singapore: 2022. pp. 181–217. [Google Scholar]
- 24.Poater J., Fradera X., Duran M., Solá M. An insight into the local aromaticities of polycyclic aromatic hydrocarbons and fullerenes. Chem. Eur. J. 2003;9:1113–1122. doi: 10.1002/chem.200390128. [DOI] [PubMed] [Google Scholar]
- 25.Chen Z., King R.B. Spherical Aromaticity: Recent Work on Fullerenes, Polyhedral Boranes, and Related Structures. Chem. Rev. 2005;105:3613–3642. doi: 10.1021/cr0300892. [DOI] [PubMed] [Google Scholar]
- 26.Chen Z., Wu J.I., Corminboeuf C., Bohmann J., Lu X., Hirsch A., von Rague Schleyer P. Is C60 buckminsterfullerene aromatic? Phys. Chem. Chem. Phys. 2012;14:14886–14891. doi: 10.1039/c2cp42146a. [DOI] [PubMed] [Google Scholar]
- 27.Matías A.S., Havenith R.W.A., Alcamí M., Ceulemans A. Is C50 a superaromat? Evidence from electronic structure and ring current calculations. Phys. Chem. Chem. Phys. 2016;18:11653–11660. doi: 10.1039/C5CP04970A. [DOI] [PubMed] [Google Scholar]
- 28.Lu X., Chen Z. Curved Pi-Conjugation, Aromaticity, and the Related Chemistry of Small Fullerenes (<C60) and Single-Walled Carbon Nanotubes. Chem. Rev. 2005;105:3643–3696. doi: 10.1021/cr030093d. [DOI] [PubMed] [Google Scholar]
- 29.Randić M., Balaban A.T. Local aromaticity and aromatic sextet theory beyond Clar. Int. J. Quantum Chem. 2018;118:e25657. doi: 10.1002/qua.25657. [DOI] [Google Scholar]
- 30.Klein D.J., Schmalz T.G., Hite G.E., Seitz W.A. Resonance in C60 Buckminsterfullerene. J. Am. Chem. Soc. 1986;108:1301–1302. doi: 10.1021/ja00266a032. [DOI] [Google Scholar]
- 31.Aihara J.I. Topological resonance energies of fullerenes and their molecular ions. J. Mol. Struct. 1994;311:1–8. [Google Scholar]
- 32.Aihara J.I. Bond Resonance Energy and Verification of the Isolated Pentagon Rule. J. Am. Chem. Soc. 1995;117:4130–4136. doi: 10.1021/ja00119a029. [DOI] [Google Scholar]
- 33.Babić D., Ori O. Matching polynomial and topological resonance energy of C70. Chem. Phys. Lett. 1995;234:240–244. doi: 10.1016/0009-2614(95)00035-3. [DOI] [Google Scholar]
- 34.Zhang C., Cao Z., Lin C., Zhang Q. Qualitatively graph-theoretical study on stability and formation of fullerenes and nanotubes. Sc. China Ser. B-Chem. 2003;46:513–520. doi: 10.1360/03yb0103. [DOI] [Google Scholar]
- 35.Austin S.J., Fowler P.W., Hansen P., Manolopoulos D.E., Zheng M. Fullerene isomers of C60. Kekulé counts versus stability. Chem. Phys. Lett. 1994;228:478–484. doi: 10.1016/0009-2614(94)00965-1. [DOI] [Google Scholar]
- 36.Vukičević D., Kroto H.W., Randić M. Atlas of Kekulé Valence Structures of Buckminsterfullerene. Croat. Chem. Acta. 2005;78:223–234. [Google Scholar]
- 37.Došlić T. Fullerene graphs with exponentially many perfect matchings. J. Math. Chem. 2007;41:183–192. doi: 10.1007/s10910-006-9068-y. [DOI] [Google Scholar]
- 38.Došlić T. Leapfrog fullerenes have many perfect matchings. J. Math. Chem. 2008;44:1–4. doi: 10.1007/s10910-007-9287-x. [DOI] [Google Scholar]
- 39.Došlić T. Finding more matchings in leapfrog fullerenes. J. Math. Chem. 2009;45:1130–1136. doi: 10.1007/s10910-008-9435-y. [DOI] [Google Scholar]
- 40.Kardoš F., Král D., Miškufa J., Sereni J.S. Fullerene graphs have exponentially many perfect matchings. J. Math. Chem. 2009;46:443–447. doi: 10.1007/s10910-008-9471-7. [DOI] [Google Scholar]
- 41.Graver J.E. Kekulé structures and the face independence number of a fullerene. Eur. J. Combin. 2007;28:1115–1130. doi: 10.1016/j.ejc.2006.03.003. [DOI] [Google Scholar]
- 42.Rogers K.M., Fowler P.W. Leapfrog fullerenes, Hückel bond order and Kekulé structures. J. Chem. Soc. Perkin Trans. 2001;2:18–22. doi: 10.1039/b007520p. [DOI] [Google Scholar]
- 43.El-Basil S. Clar sextet theory of buckminsterfullerene (C60) J. Mol. Struct. Theochem. 1993;531:9–21. doi: 10.1016/S0166-1280(00)00347-X. [DOI] [Google Scholar]
- 44.Carr J.A., Wang X., Ye D. Packing resonant hexagons in fullerenes. Discret. Optim. 2014;13:49–54. doi: 10.1016/j.disopt.2014.05.002. [DOI] [Google Scholar]
- 45.Gao Y., Zhang H. The Clar number of fullerenes on surfaces. MATCH Commun. Math. Comput. Chem. 2014;72:411–426. [Google Scholar]
- 46.Gao Y., Li Q., Zhang H. Fullerenes with the maximum Clar number. Discret. Appl. Math. 2016;202:58–69. doi: 10.1016/j.dam.2015.08.007. [DOI] [Google Scholar]
- 47.Ahmadi M.B., Farhadi E., Khorasani V.A. On computing the Clar number of a fullerene using optimization techniques. MATCH Commun. Math. Comput. Chem. 2016;75:695–701. [Google Scholar]
- 48.Zhang H., Ye D. An Upper Bound for the Clar Number of Fullerene Graphs. J. Math. Chem. 2007;41:123–133. doi: 10.1007/s10910-006-9061-5. [DOI] [Google Scholar]
- 49.Ye D., Zhang H. Extremal fullerene graphs with the maximum Clar number. Discret. Appl. Math. 2009;157:3152–3173. doi: 10.1016/j.dam.2009.06.007. [DOI] [Google Scholar]
- 50.Hartung E. Fullerenes with complete Clar structure. Discret. Appl. Math. 2013;161:2952–2957. doi: 10.1016/j.dam.2013.06.009. [DOI] [Google Scholar]
- 51.Shi L., Zhang H. Counting Clar structures of (4,6)-fullerenes. Appl. Math. Comput. 2019;346:559–574. doi: 10.1016/j.amc.2018.10.027. [DOI] [Google Scholar]
- 52.Gao Y., Zhang H. Clar Structure and Fries Set of Fullerenes and (4,6)-Fullerenes on Surfaces. J. Appl. Math. 2014;2014:196792. doi: 10.1155/2014/196792. [DOI] [Google Scholar]
- 53.Klein D.J., Balaban A.T. Clarology for conjugated carbon nano-structures: Molecules, polymers, graphene, defected graphene, fractal benzenoids, fullerenes, nano-tubes, nano-cones, nano-tori, etc. Open Org. Chem. J. 2011;5:27–61. doi: 10.2174/1874364101105010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Graver J.E., Hartung E.J. The Clar and Fries structures of a fullerene I. Discr. Appl. Math. 2016;215:112–125. doi: 10.1016/j.dam.2016.07.016. [DOI] [Google Scholar]
- 55.Fowler P.W., Myrvold W., Vandenberg R.L., Hartung E.J., Graver J.E. Clar and Fries structures for fullerenes. Art Discret. Appl. Math. 2022 doi: 10.26493/2590-9770.1510.22d. accepted . [DOI] [Google Scholar]
- 56.Manolopoulos D.E., Fowler P.W. Molecular graphs, point groups, and fullerenes. J. Chem. Phys. 1992;96:7603–7614. doi: 10.1063/1.462413. [DOI] [Google Scholar]
- 57.Zhang H., Ye D., Shiu W.C. Forcing matching numbers of fullerene graphs. Discret. Appl. Math. 2010;158:573–582. doi: 10.1016/j.dam.2009.10.013. [DOI] [Google Scholar]
- 58.Tratnik N., Žigert Pleteršek P. Resonance graphs of fullerenes. Ars Math. Contemp. 2016;11:425–435. doi: 10.26493/1855-3974.1000.8db. [DOI] [Google Scholar]
- 59.Cvetković D., Stevanović D. Spectral moments of fullerene graphs. MATCH Commun. Math. Comput. Chem. 2004;50:62–72. [Google Scholar]
- 60.Marušič D. Hamilton cycles and paths in fullerenes. J. Chem. Inf. Model. 2007;47:732–736. doi: 10.1021/ci6005239. [DOI] [PubMed] [Google Scholar]
- 61.Kutnar K., Marušič D. On cyclic edge-connectivity of fullerenes. Discret. Appl. Math. 2008;156:1661–1669. doi: 10.1016/j.dam.2007.08.046. [DOI] [Google Scholar]
- 62.Randić M., Kroto H.W., Vukičević D. Numerical Kekulé structures of fullerenes and partitioning of π-electrons to pentagonal and hexagonal rings. J. Chem. Inf. Model. 2007;47:897–904. doi: 10.1021/ci600484u. [DOI] [PubMed] [Google Scholar]
- 63.Balaban A.T., Liu X., Klein D.J., Babics D., Schmalz T.G., Seitz W.A., Randić M. Graph Invariants for Fullerenes. J. Chem. Inf. Comput. Sci. 1995;35:396–404. doi: 10.1021/ci00025a007. [DOI] [Google Scholar]
- 64.Andova V., Došlić T., Krnc M., Lužar B., Škrekovski R. On the diameter and some related invariants of fullerene graphs. MATCH Commun. Math. Comput. Chem. 2012;68:109–130. [Google Scholar]
- 65.Yang Q., Zhang H., Lin Y. On the anti-forcing number of fullerene graphs. MATCH Commun. Math. Comput. Chem. 2015;74:673–692. [Google Scholar]
- 66.Yang Q., Ye D., Zhang H., Lin Y. On the anti-Kekulé number of fullerenes. MATCH Commun. Math. Comput. Chem. 2012;67:281–288. [Google Scholar]
- 67.Ghorbani M., Dehmer M., Zangi S. On Certain Aspects of Graph Entropies of Fullerenes. MATCH Commun. Math. Comput. Chem. 2019;81:163–174. [Google Scholar]
- 68.Fajtlowicz S., Larson C. Graph-theoretic independence as a predictor of fullerene stability. Chem. Phys. Lett. 2003;377:485–490. doi: 10.1016/S0009-2614(03)01133-3. [DOI] [Google Scholar]
- 69.Li H., Zhang H. The isolated-pentagon rule and nice substructures in fullerenes. Ars Math. Contemp. 2018;15:487–497. doi: 10.26493/1855-3974.1359.b33. [DOI] [Google Scholar]
- 70.Réti T., László I. On the Combinatorial Characterization of Fullerene Graphs. Acta Polytech. Hung. 2009;6:85–93. [Google Scholar]
- 71.Zhang H., Ye D., Liu Y. A combination of Clar number and Kekulé count as an indicator of relative stability of fullerene isomers of C60. J. Math. Chem. 2010;48:733–740. doi: 10.1007/s10910-010-9706-2. [DOI] [Google Scholar]
- 72.Clar E. The Aromatic Sextet. Wiley; London, UK: 1972. [Google Scholar]
- 73.Witek H.A., Kang J.S. ZZ polynomials for isomers of (5,6)-fullerenes Cn with n = 20–50. Symmetry. 2020;12:1483. doi: 10.3390/sym12091483. [DOI] [Google Scholar]
- 74.Shao N., Gao Y., Yoo S., An W., Zeng X.C. Search for Lowest-Energy Fullerenes: C98 to C110. J. Phys. Chem. A. 2006;110:7672–7676. doi: 10.1021/jp0624092. [DOI] [PubMed] [Google Scholar]
- 75.Shao N., Gao Y., Zeng X.C. Search for Lowest-Energy Fullerenes 2: C38 to C80 and C112 to C120. J. Phys. Chem. C. 2007;111:17671–17677. doi: 10.1021/jp0701082. [DOI] [Google Scholar]
- 76.Zhao X. On the Structure and Relative Stability of C50 Fullerenes. J. Phys. Chem. B. 2005;109:5267–5272. doi: 10.1021/jp0452610. [DOI] [PubMed] [Google Scholar]
- 77.Cui Y.H., Chen D.L., Tian W.Q., Feng J.K. Structures, Stabilities, and Electronic and Optical Properties of C62 Fullerene Isomers. J. Phys. Chem. A. 2007;111:7933–7939. doi: 10.1021/jp072768c. [DOI] [PubMed] [Google Scholar]
- 78.Chan B., Karton A. Computational insights into the singlet–triplet energy gaps, ionization energies, and electron affinities for a diverse set of 812 small fullerenes (C20–C50) Phys. Chem. Chem. Phys. 2023;25:10899–10906. doi: 10.1039/D3CP01357J. [DOI] [PubMed] [Google Scholar]
- 79.Elstner M., Porezag D., Jungnickel G., Elsner J., Haugk M., Frauenheim T., Suhai S., Seifert G. Self-consistent-charge density-functional tight-binding method for simulations of complex materials properties. Phys. Rev. B. 1998;58:7260–7268. doi: 10.1103/PhysRevB.58.7260. [DOI] [Google Scholar]
- 80.Spiegelman F., Tarrat N., Cuny J., Dontot L., Posenitskiy E., Martí C., Simon A., Rapacioli M. Density-functional tight-binding: Basic concepts and applications to molecules and clusters. Adv. Phys. X. 2020;5:1710252. doi: 10.1080/23746149.2019.1710252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Koskinen P., Mäkinen V. Density-functional tight-binding for beginners. Comput. Mater. Sci. 2009;47:237–253. doi: 10.1016/j.commatsci.2009.07.013. [DOI] [Google Scholar]
- 82.Elstner M., Seifert G. Density functional tight binding. Philos. Trans. Royal Soc. A. 2014;372:20120483. doi: 10.1098/rsta.2012.0483. [DOI] [PubMed] [Google Scholar]
- 83.Zheng G., Irle S., Morokuma K. Performance of the DFTB method in comparison to DFT and semiempirical methods for geometries and energies of C20–C86 fullerene isomers. Chem. Phys. Lett. 2005;412:210–216. doi: 10.1016/j.cplett.2005.06.105. [DOI] [Google Scholar]
- 84.Hourahine B., Aradi B., Blum V., Bonafé F., Buccheri A., Camacho C., Cevallos C., Deshaye M.Y., Dumitricǎ T., Dominguez A., et al. DFTB+, a software package for efficient approximate density functional theory based atomistic simulations. J. Chem. Phys. 2020;152:124101. doi: 10.1063/1.5143190. [DOI] [PubMed] [Google Scholar]
- 85.Witek H.A., Irle S., Zheng G., de Jong W.A., Morokuma K. Modeling carbon nanostructures with the self-consistent charge density-functional tight-binding method: Vibrational spectra and electronic structure of C28, C60, and C70. J. Chem. Phys. 2006;125:214706. doi: 10.1063/1.2370877. [DOI] [PubMed] [Google Scholar]
- 86.Małolepsza E., Witek H.A., Irle S. Comparison of Geometric, Electronic, and Vibrational Properties for Isomers of small fullerenes C20–C36. J. Phys. Chem. A. 2007;111:6649–6657. doi: 10.1021/jp068529r. [DOI] [PubMed] [Google Scholar]
- 87.Małolepsza E., Lee Y.P., Witek H.A., Irle S., Lin C.F., Hsieh H.M. Comparison of geometric, electronic, and vibrational properties for all pentagon/hexagon-bearing isomers of fullerenes C38, C40, and C42. Int. J. Quantum Chem. 2009;109:1999–2011. doi: 10.1002/qua.22027. [DOI] [Google Scholar]
- 88.Bersuker I.B. Pseudo-Jahn–Teller Effect—A Two-State Paradigm in Formation, Deformation, and Transformation of Molecular Systems and Solids. Chem. Rev. 2013;113:1351–1390. doi: 10.1021/cr300279n. [DOI] [PubMed] [Google Scholar]
- 89.Chancey C.C., O’Brien M.C.M. The Jahn-Teller Effect in C60 and Other Icosahedral Complexes. Princeton University Press; Princeton, NJ, USA: 1998. [Google Scholar]
- 90.Canton S.E., Yencha A.J., Kukk E., Bozek J.D., Lopes M.C.A., Snell G., Berrah N. Experimental Evidence of a Dynamic Jahn-Teller Effect in Phys. Rev. Lett. 2002;89:045502. doi: 10.1103/PhysRevLett.89.045502. [DOI] [PubMed] [Google Scholar]
- 91.Liu D., Niwa Y., Iwahara N., Sato T., Chibotaru L.F. Quadratic Jahn-Teller effect of fullerene anions. Phys. Rev. B. 2018;98:035402. doi: 10.1103/PhysRevB.98.035402. [DOI] [Google Scholar]
- 92.Stück D., Baker T.A., Zimmerman P., Kurlancheek W., Head-Gordon M. On the nature of electron correlation in C60. J. Chem. Phys. 2011;135:194306. doi: 10.1063/1.3661158. [DOI] [PubMed] [Google Scholar]
- 93.Lee J., Head-Gordon M. Distinguishing artificial and essential symmetry breaking in a single determinant: Approach and application to the C60, C36, and C20 fullerenes. Phys. Chem. Chem. Phys. 2019;21:4763–4778. doi: 10.1039/C8CP07613H. [DOI] [PubMed] [Google Scholar]
- 94.Wang Z., Lian K., Pan S., Fan X. A path from Ih to C1 symmetry for C20 cage molecule. J. Comput. Chem. 2005;26:1279–1283. doi: 10.1002/jcc.20268. [DOI] [PubMed] [Google Scholar]
- 95.Cleland D.M., Fletcher E.K., Kuperman A., Per M.C. Electron correlation effects in isomers of C20. J. Phys. Mater. 2020;3:025006. [Google Scholar]
- 96.Fowler P.W., Mitchell D., Zerbetto F. C36: The Best Fullerene for Covalent Bonding. J. Am. Chem. Soc. 1999;121:3218–3219. doi: 10.1021/ja983853o. [DOI] [Google Scholar]
- 97.Kim K., Han Y.K., Jung J. Basis set effects on relative energies and HOMO–LUMO energy gaps of fullerene C36. Theor. Chem. Acc. 2005;113:233–237. doi: 10.1007/s00214-005-0630-7. [DOI] [Google Scholar]
- 98.Krivnov V.Y., Shamovsky I.L., Tornau E.E., Rosengren A. Electronic correlation effects in a fullerene molecule studied by the variational Monte Carlo method. Phys. Rev. B. 1994;50:12144–12151. doi: 10.1103/PhysRevB.50.12144. [DOI] [PubMed] [Google Scholar]
- 99.Varganov S.A., Avramov P.V., Ovchinnikov S.G., Gordon M.S. A study of the isomers of C36 fullerence using single and multiference MP2 perturbation theory. CPL. 2002;362:380–386. doi: 10.1016/S0009-2614(02)01117-X. [DOI] [Google Scholar]
- 100.Zhang H.P., Zhang F.J. The Clar covering polynomial of hexagonal systems I. Discret. Appl. Math. 1996;69:147–167. doi: 10.1016/0166-218X(95)00081-2. [DOI] [Google Scholar]
- 101.Zhang H. The Clar covering polynomial of hexagonal systems with an application to chromatic polynomials. Discret. Math. 1997;172:163–173. doi: 10.1016/S0012-365X(96)00279-8. [DOI] [Google Scholar]
- 102.Gutman I., Furtula B., Balaban A.T. Algorithm for simultaneous calculations of Kekulé and Clar structure counts, and Clar number of benzenoid molecules. Polycyc. Arom. Comp. 2006;26:17–35. doi: 10.1080/10406630500501435. [DOI] [Google Scholar]
- 103.Chou C.P., Witek H.A. An algorithm and FORTRAN program for automatic computation of the Zhang-Zhang polynomial of benzenoids. MATCH Commun. Math. Comput. Chem. 2012;68:3–30. [Google Scholar]
- 104.Chou C.P., Li Y., Witek H.A. Zhang-Zhang polynomials of various classes of benzenoid systems. MATCH Commun. Math. Comput. Chem. 2012;68:31–64. [Google Scholar]
- 105.Chou C.P., Witek H.A. ZZDecomposer: A graphical toolkit for analyzing the Zhang-Zhang polynomials of benzenoid structures. MATCH Commun. Math. Comput. Chem. 2014;71:741–764. [Google Scholar]
- 106.Chou C.P., Witek H.A. Determination of Zhang-Zhang Polynomials for Various Classes of Benzenoid Systems: Non-Heuristic Approach. MATCH Commun. Math. Comput. Chem. 2014;72:75–104. [Google Scholar]
- 107.Berli M., Tratnik N., Žigert Pleteršek P. Equivalence of Zhang-Zhang Polynomial and Cube Polynomial for Spherical Benzenoid Systems. MATCH Commun. Math. Comput. Chem. 2015;73:443–456. [Google Scholar]
- 108.Langner J., Witek H.A. Interface theory of benzenoids. MATCH Commun. Math. Comput. Chem. 2020;84:143–176. [Google Scholar]
- 109.Langner J., Witek H.A. Interface theory of benzenoids: Basic applications. MATCH Commun. Math. Comput. Chem. 2020;84:177–215. [Google Scholar]
- 110.Langner J., Witek H.A. Extended strict order polynomial of a poset and fixed elements of linear extensions. Australas. J. Comb. 2021;81:187–207. [Google Scholar]
- 111.Langner J., Witek H.A. ZZ polynomials of regular m-tier benzenoid strips as extended strict order polynomials of associated posets. Part 1. Proof of equivalence. MATCH Commun. Math. Comput. Chem. 2022;87:585–620. doi: 10.46793/match.87-3.585L. [DOI] [Google Scholar]
- 112.Langner J., Witek H.A. ZZ polynomials of regular m-tier benzenoid strips as extended strict order polynomials of associated posets. Part 2. Guide to practical computations. MATCH Commun. Math. Comput. Chem. 2022;88:109–130. doi: 10.46793/match.88-1.109L. [DOI] [Google Scholar]
- 113.Langner J., Witek H.A. ZZ polynomials of regular m-tier benzenoid strips as extended strict order polynomials of associated posets. Part 3. Compilation of results for m = 1–6. MATCH Commun. Math. Comput. Chem. 2022;88:747–765. doi: 10.46793/match.88-3.747L. [DOI] [Google Scholar]
- 114.Podeszwa R., Witek H.A. ZZPolyCalc: An open-source code with fragment caching for determination of Zhang-Zhang polynomials of carbon nanostructures. Comp. Phys. Comm. 2024;301:109210. doi: 10.1016/j.cpc.2024.109210. [DOI] [Google Scholar]
- 115.Podeszwa R., Witek H.A. ZZPolyCalc: Code for ZZ Polynomials, 2024. Fortran 2008 Code in GitHub Repository. [(accessed on 12 August 2024)]. Available online: https://github.com/quantumint/zzpolycalc.
- 116.Chou C.P., Witek H.A. ZZDecomposer. 2017. [(accessed on 12 August 2024)]. Available online: https://bitbucket.org/solccp/zzdecomposer_binary/downloads/ZZDecomposer_0.8.2.0.exe.
- 117.Chen H., Chou C.P., Witek H.A. ZZDecomposer. 2019. [(accessed on 12 August 2024)]. Available online: https://bitbucket.org/peggydbc1217/zzdecomposer_hsi/downloads/ZZDecomposer.1.6.zip.
- 118.Schwerdtfeger P., Wirz L., Avery J. Program Fullerene: A software package for constructing and analyzing structures of regular fullerenes. J. Comput. Chem. 2013;34:1508–1526. doi: 10.1002/jcc.23278. [DOI] [PubMed] [Google Scholar]
- 119.Wu Z.C., Jelski D.A., George T.F. Vibrational motions of buckminsterfullerene. Chem. Phys. Lett. 1987;137:291–294. doi: 10.1016/0009-2614(87)80221-X. [DOI] [Google Scholar]
- 120.Tutte W.T. How to Draw a Graph. Proc. Lond. Math. Soc. 1963;s3-13:743–767. doi: 10.1112/plms/s3-13.1.743. [DOI] [Google Scholar]
- 121.Cioslowski J. Note on the asymptotic isomer count of large fullerenes. J. Math. Chem. 2014;52:1–5. doi: 10.1007/s10910-013-0263-3. [DOI] [Google Scholar]
- 122.Rukhovich A.D. On the growth rate of the number of fullerenes. Russ. Math. Surv. 2018;73:734–736. doi: 10.1070/RM9837. [DOI] [Google Scholar]
- 123.Pauling L., Brockway L.O., Beach J.Y. The Dependence of Interatomic Distance on Single Bond-Double Bond Resonance. J. Am. Chem. Soc. 1935;57:2705–2709. doi: 10.1021/ja01315a105. [DOI] [Google Scholar]
- 124.Pauling L. The Nature of the Chemical Bond. Cornell University Press; Ithaca, NY, USA: 1940. p. 142. [Google Scholar]
- 125.Herndon W.C. Resonance theory and the enumeration of Kekulé structures. J. Chem. Educ. 1974;51:10–15. doi: 10.1021/ed051p10. [DOI] [Google Scholar]
- 126.Herndon W.C. Resonance theory. VI. Bond orders. J. Am. Chem. Soc. 1974;96:7605–7614. doi: 10.1021/ja00832a001. [DOI] [Google Scholar]
- 127.Herndon W.C., Párkányi C. π bond orders and bond lengths. J. Chem. Educ. 1976;53:689–692. doi: 10.1021/ed053p689. [DOI] [Google Scholar]
- 128.Randić M. Graph Theoretical Derivation of Pauling Bond Orders. Croat. Chem. Acta. 1975;47:71–78. [Google Scholar]
- 129.Pauling L. Bond numbers and bond lengths in tetrabenzo[de,no,st,c1d1]heptacene and other condensed aromatic hydrocarbons: A valence-bond treatment. Acta Cryst. 1980;B36:1898–1901. doi: 10.1107/S056774088000742X. [DOI] [Google Scholar]
- 130.Sedlar J., Anđelić I., Gutman I., Vukičević D., Graovac A. Vindicating the Pauling-bond-order concept. Chem. Phys. Lett. 2006;427:418–420. doi: 10.1016/j.cplett.2006.06.026. [DOI] [Google Scholar]
- 131.Kiralj R., Ferreira M.M.C. Predicting Bond Lengths in Planar Benzenoid Polycyclic Aromatic Hydrocarbons: A Chemometric Approach. J. Chem. Inf. Comput. Sci. 2002;42:508–523. doi: 10.1021/ci010063g. [DOI] [PubMed] [Google Scholar]
- 132.Dias J.R. Valence-Bond Determination of Bond Lengths of Polycyclic Aromatic Hydrocarbons: Comparisons with Recent Experimental and Ab Initio Results. J. Phys. Chem. A. 2011;115:13619–13627. doi: 10.1021/jp207531u. [DOI] [PubMed] [Google Scholar]
- 133.Radenković S., Gutman I., Đorđević S. Strain in strain-free benzenoid hydrocarbons: The case of phenanthrene. Chem. Phys. Lett. 2015;625:69–72. doi: 10.1016/j.cplett.2015.02.039. [DOI] [Google Scholar]
- 134.Narita S., Morikawa T., Shibuya T. Linear relationship between the bond lengths and the Pauling bond orders in fullerene molecules. J. Mol. Struct. Theochem. 2000;532:37–40. doi: 10.1016/S0166-1280(00)00563-7. [DOI] [Google Scholar]
- 135.Randić M. Novel insight into Clar’s aromatic π-sextets. Chem. Phys. Lett. 2014;601:1–5. doi: 10.1016/j.cplett.2014.03.073. [DOI] [Google Scholar]
- 136.Gutman I., Turković N., Jovičić J. Cyclic Conjugation in Benzo-Annelated Perylenes. How Empty is the “Empty” Ring? Monatsh. Chem. 2004;135:1389–1394. doi: 10.1007/s00706-004-0223-5. [DOI] [Google Scholar]
- 137.Gutman I., Marković S., Jeremić S. A Case of Breakdown of the Kekulé–Structure Model. Polycyc. Arom. Comp. 2010;30:240–246. doi: 10.1080/10406638.2010.503162. [DOI] [Google Scholar]
- 138.Vukičević D., Đurđević J., Gutman I. Limitations of Pauling Bond Order Concept. Polycyc. Arom. Comp. 2012;32:36–47. doi: 10.1080/10406638.2011.637102. [DOI] [Google Scholar]
- 139.Radenković S., Bultinck P., Gutman I., Đurđević J. On induced current density in the perylene/bisanthrene homologous series. Chem. Phys. Lett. 2012;552:151–155. doi: 10.1016/j.cplett.2012.09.055. [DOI] [Google Scholar]
- 140.Radenković S., Gutman I., Antić M. A case of breakdown of the Pauling bond orders. Chem. Phys. Lett. 2014;614:104–109. doi: 10.1016/j.cplett.2014.09.008. [DOI] [Google Scholar]
- 141.Paulus B. Electronic and structural properties of the cage-like molecules C20 to C36. Phys. Chem. Chem. Phys. 2003;5:3364–3367. doi: 10.1039/b304539k. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Most of the research results, including the optimized DFTB energies and geometries of all the isomers of the fullerenes C20– and their corresponding ZZ polynomials, can be downloaded at: https://www.mdpi.com/article/10.3390/molecules29174013/s1 as the Supplementary Materials for this paper. For additional material, the reader is encouraged to contact the authors.