Abstract
Blood plasma is one of the most commonly analyzed and easily accessible biological samples. Here, we describe an automated liquid-liquid extraction platform that generates accurate, precise, and reproducible samples for metabolomic, lipidomic, and proteomic analyses from a single aliquot of plasma while minimizing hands-on time and avoiding contamination from plasticware. We applied mass spectrometry to examine the metabolome, lipidome, and proteome of 90 plasma samples to determine the effects of age, time of day, and a high-fat diet in mice. From 25 μl of mouse plasma, we identified 907 lipid species from 16 different lipid classes and subclasses, 233 polar metabolites, and 344 proteins. We found that the high-fat diet induced only mild changes in the polar metabolome, upregulated apolipoproteins, and induced substantial shifts in the lipidome, including a significant increase in arachidonic acid and a decrease in eicosapentaenoic acid content across all lipid classes.
Supplementary key words: lipidomics, dyslipidemias, phospholipids/metabolism, omega-3 fatty acid, cholesterol/trafficking, apolipoprotein, MTBE-LLE, multiomics
Mass spectrometry-based metabolomics, lipidomics, and proteomics have become essential parts of systems biology research, offering insights into biological conditions across many in vitro and in vivo systems (1, 2, 3, 4, 5, 6, 7). Due to differences in hydrophobicity and molecular weight, metabolomics, lipidomics, and proteomics generally require separate methods for extraction and enrichment of the targeted analyte class prior to analysis (8, 9, 10). Blood sample volume can often be limited in work with small organisms like mice, restricting possible analyses. Recently, the interest in preparing samples for multiple analyses has grown due to the desire to more fully characterize and generate a better understanding of complex biological systems (11, 12, 13). The ability to prepare a single sample for multiple data analysis modalities opens the possibility of multiomic analysis on small sample volumes.
The methyl tert-butyl ether (MTBE)-methanol-water liquid-liquid extraction (LLE) method for lipid extraction developed by Matyash et al. (14, 15, 16) has been demonstrated to have similar performance to the chloroform-based method developed by Folch (17), but with the advantages of avoiding chlorinated solvents and locating the protein pellet at the bottom of the vessel, making it a popular alternative to chloroform-based extractions for lipidomics. There are several reports of metabolomics analysis on the aqueous phase of this preparation in conjunction with lipidomics analysis of the organic phase (18, 19, 20, 21, 22, 23).
An automated MTBE-LLE method for the preparation of lipids prior to mass spectrometry analysis was previously reported (24, 25), as was one for the Butanol:Methanol method (BUME) (15, 16). However, to date, no reports have been made of an automated method to examine both metabolomics and lipidomics separately from the same sample. Previous reports describing a single sample preparation protocol for proteomics, lipidomics, and metabolomics analysis were not automated, limiting throughput (26, 27, 28).
To enable multiomic analysis of large sample sets, we optimized a solvent system and consumables for an automated LLE system that enables lipidomic, metabolomic, and proteomic analyses from a single aliquot of blood plasma. This method provides separate fractions for lipidomics and metabolomics, requiring only offline sample evaporation and resuspension. The protein pellet remains in the LLE vessel and is compatible with many downstream proteomics preparation workflows. In this work, we used the recently published, high-throughput AutoMP3 method (29).
To demonstrate the utility of our workflow and its ability to generate high-quality data from a large number of samples, we prepared and analyzed 90 mouse plasma samples, investigating the effect of age and diet on the metabolomic, lipidomic, and proteomic profiles of mouse plasma. Our study revealed differences in metabolites, lipids, and proteins between adult and adolescent mice, and profound changes induced by a high-fat diet, especially in the lipid acyl-group composition and abundance of lipoproteins.
Materials and Methods
Chemicals, reagents, and consumables
LC-MS-grade isopropanol, methanol, acetonitrile (ACN), and MTBE were purchased from Honeywell (Charlotte, NC). Ammonium formate, ammonium bicarbonate, and formic acid were purchased from Sigma-Aldrich (St. Louis, MO). Lipid standards, including SPLASH LIPIDOMIX (P/N 330707), EquiSPLASH (P/N 330731), LightSPLASH (P/N 330732), d31-LPC 16:0 (P/N 860397), d70-PE (18:0/18:0) (P/N 860373), d54-PA (14:0/14:0) (P/N 860450) were purchased from Avanti Polar Lipids (Alabaster, AL). d35-FA 18:0 (P/N 9003318) was purchased from Cayman Chemical (Ann Arbor, MI), and d4-lysine (P/N 616192), d5-phenylalanine (P/N 615870), d4-succinate (P/N 293075), L-tyrosine-(phenyl-d4) (P/N 489808), L-arginine-15N4 hydrochloride (P/N 600113), d5-benzoate (P/N 586331) were purchased from Sigma-Aldrich (St. Louis, MO). Metabolomics QC kit and associated light standards were purchased from Cambridge Isotope Laboratories (Tewksbury, MA).
Tandem mass tag pro (TMTpro) 16-plex isobaric reagents were obtained from Thermo Fisher Scientific (Rockford, IL). Modified Trypsin and LysC mix were obtained from Promega Corporation (Madison, WI). Complete protease inhibitor tablets were obtained from Roche. Thermo Fisher Scientific Pierce Bicinchoninic Acid (BCA) Assay (P/N 23228), GE HealthCare SpeedBead Magnetic Carboxylate Beads Hydrophilic and Hydrophobic forms (P/N 65152105050250, 45152105050250).
PCR Strip Caps (P/N, 321-11-071) 0.2 ml Clear, For Real Time PCR, flat top; PlateMax Ultra Clear Sealing Film (P/N UC-500); Axygen® AxyMats™ 96 Round Well Compression Mat for PCR Microplates, Nonsterile (P/N CM-96-RD); PCR Tubes 0.2 ml Maxymum Recovery, Thin Wall, Clear (P/N 321-02-501); 2 ml 96-well deep well plate (P/N P-2Ml-SQ-C) were obtained from Axygen (Union City, CA). Eppendorf 2.0 ml Protein LoBind tubes (Catalog#022431102), Eppendorf 1.5 ml Protein LoBind tubes (Catalog#02243108), and Alpaqua 96-well neodymium magnet (Cat#A000400), Eppendorf 150 μl DNA LoBind 96-well PCR plates (P/N 0030129512), Eppendorf 1 ml Protein LoBind 96-well plates (P/N 951033308) were purchased from Eppendorf (Enfield, CT). Corning plate lids (P/N CLS3935-50EA) from Corning (Glendale, AZ). Hamilton 60 ml reagent reservoir (P/N 56694-01); Filtered, Conductive Hamilton Vantage Tips–1,000 μl (P/N 235940), 300 μl (P/N 235938), 50 μl (P/N 235979) were from Hamilton (Cary, NC). Unless otherwise stated, all other chemicals were purchased from Sigma-Aldrich (St. Louis, MO).
Blood plasma samples
All animal protocols in the United States of America were approved by the Mayo Clinic IACUC and the Calico Life Sciences IACUC. The studies were conducted under the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals (protocol # A00003888). Ten male C57BL/6 mice (The Jackson Laboratory) were selected for each of the three groups, to minimize variance in body weight and eliminate animals that had fighting wounds. Groups of 8- to 12-week-old (young) and 24- to 28-week-old (adult) mice were fed a standard lab chow diet (Chow diet 5LOD, LabDiet), and a second group of 24- to 28 -week-old mice were fed a high-fat diet (HFD) containing 60% fat (D12492, Research Diets Inc) for 10 weeks. They were housed in standard cages at room temperature and constant humidity, with a 12-h light-dark cycle. Three blood plasma samples were obtained from each mouse via retro-orbital bleeding. Each sample was obtained on a different day and at a different time of day: 10 AM (time point 1- Z10), 10 PM (time point 2- Z22), and 3 PM (time point 3- Z15). Blood was collected into BD Microtainer Tubes (REF 365974) with K2E (K2EDTA), mixed briefly, and stored on ice. All samples were centrifuged at 7,000 g for 4 min at 4°C to separate blood plasma. Plasma was aliquoted in 50 μl increments, immediately frozen on dry ice, and stored at −80°C for later analysis.
Automation/Robotics and consumables
Automated LLE extraction was performed on a PAL Dual Head Rail (DHR) system from Trajan Scientific and Medical (Morrisville, NC) equipped with modules as described in Fig. 1. Customized parts for the robot included the following: reagent chiller assembly, buffer holder with chiller, customed aluminum vial racks, nitrogen gas line, and custom enclosure with details can be found in the supplemental Data.
Fig. 1.
PAL-DHR diagram and description of customized parts. Plasma aliquots were stored in the 4°C cabinet (#4). PAL-DHR used its two arms (#1, #2) for liquid transferring and relocating glass vials with magnet caps. Briefly, PAL added the extraction buffers to samples, then vortexed (#3), and centrifuged (#5) to collect lipid and metabolite fractions, which were subsequently stored in the second and third level of the refrigerated vial cabinet. Details of the automated protocol can be found in the Materials and Methods section. DHR, Dual Head Rail.
Manual LLE
Manual LLEs were performed using a modified version of Matyash procedure (14). Briefly, a mixture of lipid standards (volumes as described in results) was extracted by the addition of 50% methanol (pre-cooled to −20°C), followed by vortexing for 30 s and incubated on ice for 10 min. The same volume of room temperature MTBE was then added, and the samples were vortexed 30 s before centrifugation at 3,000 g for 5 min at 4°C for complete phase-separation. The upper organic phase was aspirated with a Hamilton glass syringe until the meniscus of the two phases was reached and transferred to a new ice-cold glass sample vial. The remaining sample volume was subjected to a second extraction using MTBE or other solvent mixtures, as described in the results section. The second organic phase was removed and combined with the first organic phase extraction. All sample transfers involving MTBE-containing solutions were performed with glass syringes rinsed twice with 100% MTBE then MeOH, between preparation steps. Following both MTBE extractions, the lower aqueous phase extract was pipetted off with the necessary care not to pick up the remaining upper phase or disturb the protein pellet. The fraction was subsequently transferred into a separate ice-cold sample vial. Samples were evaporated to dryness under nitrogen at 4°C and then stored at −80°C.
Automated liquid-liquid extraction
EDTA-plasma aliquots of 25 μl were resuspended in glass vials (SureStop 2 ml vials #C5000-1W with National Scientific custom magnetic caps #CPSH0003 from Thermo Fisher Scientific) to a final concentration of 50% methanol, containing 10 μl of a mixture of 1 μg/ml of deuterated tyrosine and deuterated alanine, 250 ng/ml of deuterated benzoate (Sigma-Aldrich) in water, as well as 10 μl SPLASH LIPIDOMIX mass spec standard (Avanti Polar Lipids) as internal standards in methanol, generating volumes of 70 μl in the source sample vials. The robot performed LLE as per the script provided in the Supplemental information. Briefly, 50% methanol (precooled to −20°C) was added via the PAL-DHR system from an external reservoir to a final volume of 800 μl, followed by vortexing for 70 s at 2,000 g. Samples were then automatically extracted in sets of 8 (or an even number of fewer samples for the last batch of the sample set depending on the total sample number), with all subsequent liquid movements performed using 1 ml Hamilton Syringes (Hamilton HA-207848). Lipid extraction was performed via the addition of 750 μl of MTBE to each sample vial followed by vortex mixing for 30 s. The sample set was centrifuged for 1 min at 1,400 g for complete phase separation, and 520 μl of the upper (organic) phase was transferred to a lipidomics collection vial (National Scientific #CPSH0008, Thermo Fisher Scientific). The second extraction was performed via addition of 600 μl of MTBE to the source vial. After repeating the mixing and centrifugation steps as described above, 860 μl of the upper (organic) phase was transferred to the lipidomics collection vial. Finally, 425 μl of the lower aqueous phase was transferred to the metabolomics collection sample vials. Throughout the procedure, sample vials and destination vials for both the organic and aqueous fractions were stored in cold blocks chilled to 4°C in the vial cabinet (#5 in Fig. 1). The vial cabinet was constantly purged with nitrogen gas throughout the automated LLE procedure. Syringes were washed between each sample movement using MTBE for organic phase transfers and 50/50 v/v methanol/water for aqueous phase transfers.
Automated proteomics sample preparation
Following the LLE procedure above, the sample vial contained approximately 100 μl of the upper organic phase and 150 μl of lower aqueous phase and the protein pellet. Samples were stored at −80°C until the day of preparation at which time they were evaporated to dryness under nitrogen, then diluted 10× in protein lysis buffer (3% SDS, 50 mM Hepes pH 8.5, 75 mM NaCl + Roche complete cocktail of protease inhibitors). Samples were processed using the AutoMP3 workflow, as previously described (29). Briefly, proteins were reduced with DTT, alkylated with iodoacetamide, and cleaned up with Sera-Mag beads prior to protein digestion. Digestion was performed using LysC & Trypsin (Promega, 1:20 protease to protein ratio) for 1 h (37°C, 1,000 g). Peptides were labeled with TMTpro as 16-plexes with one bridge channel per plex. The bridge sample was created by combining a portion of all samples to make a pooled control, and labeled using the 134N TMT. Samples were quenched and desalted prior to LC-MS analysis through the combine-mix-split strategy and peptide-level Sera-Mag bead cleanup steps. Proteins and peptides were quantified using a BCA assay (Pierce).
LC-MS analysis of polar metabolites
Analysis of polar metabolites was performed on a Vanquish HPLC coupled to a Q Exactive Plus mass spectrometer (Thermo Fisher Scientific). Dried aqueous phase extracts were resuspended in 100 μl of water containing 1 μg/ml of deuterated lysine and deuterated phenylalanine and 250 ng/ml of deuterated succinate (Sigma-Aldrich) as internal standards. Samples were centrifuged at 18,000 g for 5 min, and the supernatant was moved to HPLC vials.
Metabolites were analyzed in negative ionization mode using a reverse phase ion-pairing chromatographic method with an Agilent Extend C18 RRHD column, 1.8 μm particle size, 80 Å, 2.1 × 150 mm. Mobile phase A was 10 mM tributylamine, 15 mM acetic acid in 97:3 water:methanol pH 4.95; mobile phase B was methanol. The flow rate was 200 μl/min and the gradient was t = −4, 0% B; t = 0, 0% B; t = 5, 20% B; t = 7.5, 20% B; t = 13, 55% B; t = 15, 95% B; t = 18.5, 95% B; t = 19, 0% B; t = 22, 0% B. The injection volume was 5 μl. The mass spectrometer was operated in negative ion mode using data-dependent acquisition (DDA) mode with the following parameters: resolution = 70,000, AGC target = 1.00 E + 06, maximum IT (ms) = 100, scan range = 70–1,050. The MS2 parameters were as follows: resolution = 17,500, AGC target = 1.00 E + 05, maximum IT (ms) = 50, loop count = 6, isolation window (m/z) = 1, (N)CE = 20, 50, 100; underfill ratio = 1.0 0%, Apex trigger(s) = 3–12, dynamic exclusion(s) = 20.
For positive ion mode, the sample resuspension described above was diluted 1:4, v/v with MeCN and centrifuged at 18,000 g for 5 min, and 30 μl of the supernatant was moved to HPLC vials without disturbing the precipitate. Metabolites were analyzed in positive ionization mode via hydrophilic-interaction liquid chromatography using a SeQuant® ZIC®-pHILIC column, 5 μm particle size, 200 Å, 150 × 2.1 mm. Mobile phase A was 20 mM ammonium carbonate in water (pH 9.2), and mobile phase B was ACN. The flow rate was 150 μl/min and the gradient was t = −6, 80% B; t = 0, 80% B; t = 2.5, 73% B; t = 5, 65% B; t = 7.5, 57% B; t = 10, 50% B; t = 15, 35% B; t = 20; 20% B; t = 22, 15% B; t = 22.5, 80% B; t = 24; 80% B. The injection volume was 5 μl. The mass spectrometer was operated in positive ion mode using DDA mode with the following parameters: resolution = 70,000, AGC target = 3.00 E + 06, maximum IT (ms) = 100, scan range = 70–1,050. The MS2 parameters were as follows: resolution = 17,500, AGC target = 1.00 E + 05, maximum IT (ms) = 50, loop count = 6, isolation window (m/z) = 1, normalized collision energy (NCE) = 20, 40, 80; underfill ratio = 1.00%, Apex trigger(s) = 3–10, dynamic exclusion(s) = 25.
LC-MS analysis of lipids
Detection of lipids was performed on a Vanquish HPLC coupled to a Q Exactive Plus mass spectrometer (Thermo Fisher Scientific). Dried organic phase extracts were resuspended in 100 μl of (2:1:1,v/v/v) But OH:MeOH:H2O including 500 ng/ml d70-PE (18:0,18:0), 2.5 μg/ml d31-LPC (16:0), 700 ng/ml d54-PA (14:0,14:0) and 500 ng/ml d35-FA (18:0) as deuterated LC-MS lipid standards to monitor instrument performance. Separation of lipid compounds in both positive and negative ion mode was achieved by reverse-phase liquid chromatography on an Accucore C30 column (250 × 2.1 mm, 2.6 μm particle size, Thermo Fisher Scientific). Mobile phase A was 20 mM ammonium formate in 60:40 meCN:H2O, with 0.25 μM medronic acid and mobile phase B was 20 mM ammonium formate in 90:10 isopropanol:meCN, with 0.25 μM medronic acid. The gradient was: t = −7, 30% B; t = 7, 43% B; t = 12, 65% B, t = 30, 70% B; t = 31, 88% B; t = 51, 95% B, t = 53, 100% B t = 55, 100% B; t = 55.1, 30% B, t = 60, 30% B for a total run time of 67 min per injection. The flow rate was 200 μl/min, the injection volume was 5 μl, and the column temperature was 35°C. Parameters for the full scan (MS1) were as follows: 140,000 resolution, AGC target of 3e6, IT of 100 ms and a scan range of 200–2000 m/z. The MS2 parameters were as follows: 17,500 resolution, a loop count of 8, and an AGC target of 3e6, IT of 150 ms, isolation window of 1 m/z and underfill ratio of 1%. Dynamic exclusion was set at 15 s with an apex trigger from 5 to 30 s. Stepped collision energies for fragmentation were set to 20%, 30%, and 40% NCE.
LC-MS analysis of peptides
Peptides from each combined 16-plex were resuspended in 95/5 v/v water/ACN with 5% formic acid at a concentration of approximately 0.33 μg/μl and 3 μl was injected (1 μg) for analysis. Peptides were separated by a reverse phase on a C18 Aurora microcapillary column (75 μm × 25 cm, C18 resin, 1.6 μm, 120 Å, #AUR2-25075C18A) (IonOpticks) on a Ultimate3000 LC (Thermo Fisher Scientific) coupled to an Orbitrap Eclipse mass spectrometer. The total LC-MS run length for each sample was 185 min including a 165 min gradient from 8% to 30% ACN in 0.125% formic acid. The flow rate was 300 nl/min, and the column was heated at 60° C. Data were collected using DDA with real-time search (RTS) and FAIMS Pro. We used four different FAIMS Pro compensation voltages: −40, −50, −60, and −70 V. Each of the four experiments had a 1.25 s cycle time. A high-resolution MS1 scan in the Orbitrap (m/z range 400-1,600, 120k resolution, standard AGC Target,“Auto” max injection time, ion funnel radio frequency of 30%) was collected, from which the top 10 precursors were selected for MS2, followed by synchronous precursor selection MS3 analysis. For MS2 spectra, (30) ions were isolated with the quadrupole mass filter using a 0.7 m/z isolation window. The MS2 product ion population was analyzed in the ion trap (CID with normalized collision energy 35%, AGC 1 × 104, custom max injection time of 35 ms). The minimum xcorr needed to pass the RTS was set to 2, the minimum dCn was set to 0.1, and a maximum of two missed cleavages was allowed. The MS3 scan was analyzed in the Orbitrap (50k resolution and with a scan range of 100–500 m/z, higher-energy collision dissociation with fixed NCE of 45%, AGC 1 × 105, max injection time 200 ms). Up to ten fragment ions from each MS2 spectrum were selected for MS3 analysis using synchronous precursor selection.
Data processing
For metabolomics and lipidomics data, raw files were converted to mzML format using Proteowizard Ver 3 (https://proteowizard.sourceforge.io) (30). Compound identifications and peak grouping were performed using the OpenCLaM R package (https://github.com/calico/open_clam). Peaks were matched within 10 ppm for the precursor mass and 20 ppm for fragment masses. Fragmentation and retention times were compared to an in-house library generated from authentic standards for metabolomics, and fragmentation was compared to in-house generated in-silico library for lipidomics (31). Following automated annotation, the dataset was manually inspected, validated, and some identifications were reassigned using MAVEN2 (https://github.com/eugenemel/maven) (31). Each metabolite feature was log2 transformed and normalized to the mean of the young-chow mice using claman R package (https://github.com/calico/claman). Lipidomics data were normalized to the isotopically labeled internal standard of the same class from SPLASH LIPIDOMIX (Avanti Polar Lipids). Absolute quantification of each lipid species was calculated based on the ratio of the peak area top between feature and internal standard. Annotation was according to the LIPID MAPS classification, nomenclature, and shorthand notation guidelines (32).
Proteomics mass spectrometry data were processed using MassPike software (GFY Core, https://github.com/gygilab) Version 3.8 (33). Raw files were converted to mzXML files using ReAdW and searched against either a mouse Uniprot database (downloaded on January 8th, 2020) in forward and reverse orientations using the Sequest algorithm (34). Database searching matched MS/MS spectra with fully tryptic peptides from this dataset with a precursor ion tolerance of 20 ppm and a product ion tolerance of 0.6 Da. Carbamidomethylation of cysteine residues (+57.02 Da) was set as static modification, and TMTpro of peptide N termini and lysines (+304.20 Da) were set as static modifications as well. Oxidation of methionine (+15.99 Da) was set as a variable modification. Linear discriminant analysis was used to filter peptide spectral matches to a 1% false discovery rate as described previously (33). Nonunique peptides that matched to multiple proteins were assigned to proteins that contained the largest number of matched redundant peptide sequences using the principle of Occam’s razor (33). Quantification of TMT reporter ion intensities was performed by extracting the most intense ion within a 0.003 m/z window at the predicted m/z value for each reporter ion. Peptide intensities and signal-to-noise ratios were exported and analyzed using the msTrawler software package (https:/github.com/calico/msTrawler) (35). Default settings were used.
Statistics
For the comparison of different ages and diets, statistical analysis was performed using the claman R package for metabolomics and lipidomics, and the msTrawler R package for proteomics (35). Each metabolite/lipid/protein were modeled separately with atwa mixed-effect model that included fixed effects for age, diet, and blood draw batch, and a random effect for each individual mouse using the formula:
, where are the log2 reporter ion intensities for metabolites, and log2 concentration for lipids, observed from the feature being modeled. Observations indexed by i = 1 are from the young mice, and i = 2 from the old mice ( = 0 and = 1), so that is the expected change in log2 abundance between the young and old mice. j = 1 or 2 indexes the diet category so that = 0 for mice on the chow diet and = 1 for mice on the high-fat diet. Accordingly, represents the expected change in log2 abundance when moving from a chow to a high-fat diet. k = 1, 2, or 3 indexes the three sampling times, Z10, Z22, or Z15. The first time is the reference and and equal 1 when the sampling time were at time Z22 or Z15 respectively and take values of zero otherwise so that and represent expected changes in relative abundance due to blood draw batch effects.
is a random intercept for each mouse that creates a correlation structure in the model between all repeat measurements taken on the same mouse. is the variance of relative abundance between animals.
For proteomics, we included a term to account for the measurement error from sample processing. Each is mutually independent of one another and of the residual variance, . is the inverse of the ion count for each scan, and bridge channels were incorporated into the model as previously described (35). False discovery rate was controlled on a term-by-term basis using the qvalue R package (https://bioconductor.org/packages/release/bioc/hyml/qvalue.html) (36). Significant changes were reported for q values < 0.01.
To assess the overall impact of age, diet, and blood draw time point in shaping variability in the metabolome and lipidome, we assessed how much of the total variability in the dataset is explained by each factor. To do this we fit a three-way ANOVA using the formula.
to each feature using the R aov function. This decomposed the variance of each feature (described by the total sum of square (TSS); i.e., Var(y)∗N) into variance explained by each experimental factor (explained sum of squares (ESS)) and unexplained variance (residual sum of squares). The ratio of ESS to TSS provides a measure of the relative effect size of each variability. To provide an estimate of this ratio at a dataset level, we summed the ESS of each variable and the TSS across all features and then took the ratio of dataset-level ESS to TSS as a summary of total variance explained by each variable.
For lipid enrichment analysis, we ranked all identified species using the estimated values calculated from the regression model. Identified lipid species were separately classified into lipid classes, and acyl chain composition, which were used as the groups of which to determine enrichment. Then, lipid class and acyl enrichment were performed on the ranked species using R package FGSEA in both directions (37, 38, 39, 40, 41). We report the -log10 Benjamini-Hochberg corrected P-value and use the normalized enrichment score to assign the direction of the change.
Results
Automation of MTBE-LLE extraction
Extractables and leachables from plastics and adsorption of hydrophobic materials onto plasticware are significant concerns in the fields of lipidomics and lipid biochemistry as they can mask differential abundance of lipids between samples (42, 43, 44). To determine the effects of plasticware on the results obtained during MTBE-based extraction, we performed an untargeted analysis of features from LC-MS lipidomics analysis of 10 μl of aliquots mouse plasma extracted in either 1.5 ml glass vials or low-retention polypropylene microcentrifuge tubes. We found many features with higher abundance in the samples extracted in polypropylene, most of which did not match known lipids. We putatively identified some of these compounds as chemicals used in polymer production, including Triton and polybutylenes (supplemental Fig. S1 and supplemental Table S1). We identified monoglycerides and fatty acids which were extracted from the plastic, including palmitic acid, stearic acid, palmitoylmonoglyceride, and stearoylmonoglyceride.
To avoid the contaminants observed with polypropylene vessels in the automated system, we implemented an LLE method on a PAL-DUAL system (Fig. 1), a dual-head robotic platform capable of automatic tool changes and uses glass syringes for liquid transfers. We configured the system with cooled blocks for the 2 ml glass vials (4°C), chilled solvents (4°C), and constantly purged the vial cabinet with nitrogen to minimize oxidation. The system consisted of a vial cabinet, vortex mixer, centrifuge, and syringe wash stations, allowing for the complete automation of the entire LLE as shown in Fig. 1. We enclosed the instrument to allow any solvent vapor to be ventilated directly to the building exhaust system (SI Robot enclosure diagram).
Initially, we attempted to implement the LLE using the solvent ratios described in Matyash et al.: a first extraction with a solvent composition of MTBE/MeOH/H2O (10/3/2.5, v/v/v) and a second extraction composed of MTBE/MeOH/H2O (2/1/1, v/v/v), an approximation of the upper phase composition of the first extract. However, we found that this 2/1/1 mixture separated into two phases over time, likely due to small amounts of evaporation changing the solvent composition. To enable automation, a reservoir with a single phase was preferable, so we examined the impact of either eliminating the second extraction step or using 100% MTBE, while leaving the first extraction unchanged. Without the second extraction step, we recovered significantly less phospholipid and lysophospholipids, but we found similar recoveries using MTBE/MeOH/H2O (2:1:1, v/v/v) or 100% MTBE (supplemental Fig. S2A, B). We settled on a final protocol consisting of a first extraction of MTBE/MeOH/H2O (2/1/1, v/v/v) solvent ratio and a second extraction of 100% MTBE addition to the remaining aqueous phase.
In tests of the automated LLE, we found a series of high-intensity contaminants during LC-MS analysis despite the careful solvent handling to avoid exposure to plastics. We identified the contaminants as polysiloxanes (supplemental Fig. S3A), which organic solvents may extract from silicone. We found that the repeated penetration of the septa during preparation fractured the polytetrafluoroethylene (PTFE) layer of the cap, exposing the silicone to MTBE during vortex mixing. We compared caps made with PTFE-silicone, PTFE-red rubber, or a plain PTFE disc and found a 10-fold reduction in peak size with the PTFE-red rubber septum and more than a 100-fold reduction in peak intensity with the PTFE disc. Hence, we moved forward with the PTFE disc for this method (supplemental Fig. S3B).
However, when performing automated preparations with PTFE-disc closures, we found that the standard 22 gauge, type 3 needle (a blunt needle with the port at the tip) would occasionally core the PTFE disc, causing either clogging of the syringe or debris in the sample. To address this problem, we tested two other needle types: the narrower, conical-tip autosampler needle with a bottom port and the conical-tip type 5 needle with a side port. We found that the autosampler needle clogged less, but did still occasionally clog, hence we chose the type 5 needle, which is more commonly used for gas chromatography injections, as it showed consistent performance.
In order to allow for slight differences in samples and ensure that the incorrect phase or protein precipitate would not contaminate the destination vials, we chose conservative volumes to be transferred out of the sample vial, leaving approximately 100 μl of the organic phase and 150 μl of the aqueous phase behind with the protein pellet, giving us 74% of the aqueous phase volume (425 of 575 μl) and 93% of the total organic phases (1,380 of 1,480 μl).
Validation of the automated MTBE-LLE method
We examined the fractional recovery of 10 polar metabolites and 13 common lipid classes using pure chemical standards. We recovered 50%–86% of lipids, with the lowest recovery observed for the most hydrophobic lipid classes, triglycerides (TGs) and cholesterol esters (CEs), consistent with previous reports on the extraction efficiencies of MTBE-based methods (45, 46, 47). For polar metabolites, the recovery ranged from 30% to 78%, exhibiting a strong correlation with the hydrophobicity of the metabolite (Fig. 2A). The automated method yielded results similar to those obtained through manual extraction for metabolites and slightly lower yields for lipids (supplemental Fig. S4A, B).
Fig. 2.
Accuracy and precision of extraction recovery from the automated MTBE-LLE-PAL system. A: Extraction efficiency reported by MTBE-LLE performed by PAL system. Log2 ratios reflect PAL-LLE prepared isotopically labeled internal standards relative to equimolar unlabeled standards added post-LLE (PAL, n = 4). Lipid recovery in the organic layer (brown); Metabolite recovery in the aqueous layer (blue). B: Accuracy and precision of the automated MTBE-PAL. Standard mixtures containing isotopically labeled standards (heavy) and their associated C12 standards (light) were prepared at the ratio (1:0.25, 1:1, 1:4, v/v, (n = 3), followed by LLE separation. Ratios were normalized to controls that did not undergo the LLE. LLE, liquid-liquid extraction; MTBE, methyl tert-butyl ether.
Next, we evaluated the accuracy and precision of the automated sample preparation. We prepared three concentrations of unlabeled standards, spanning a 16-fold concentration change, with unvarying isotopically labeled internal standards (Fig. 2B). We found high linearity with R2 greater than 0.96 for all compounds (supplemental Table S2A, B), and a median coefficient of variation of 11%, a minimum (minimum 6% and maximum 23%) for repeated measurement of the labeled isotopic standards at the same concentration.
To assess the batch-to-batch reproducibility of the system, we extracted varying volumes of aliquots from the same plasma in two separate weeks, and then acquired LC-MS data in a single batch. We performed a regression on the mean peak area (n = 3) of lipids and metabolites identified from each batch. High correlations were determined in both fractions, with R2 = 0.998 for metabolites and R2 = 0.996 for lipids (supplemental Fig. S4C, D and supplemental Table S2C).
Finally, we determined the carry-over of the automated method by sequentially preparing plasma standards and water blanks. We found minimal carryover, with less than 2% of the signal of the ten most abundant plasma metabolites and the ten most abundant lipids per lipid class found in the blank (supplemental Fig. S4E and supplemental Table S2D).
Determining the effects of age and a HFD on mouse plasma composition
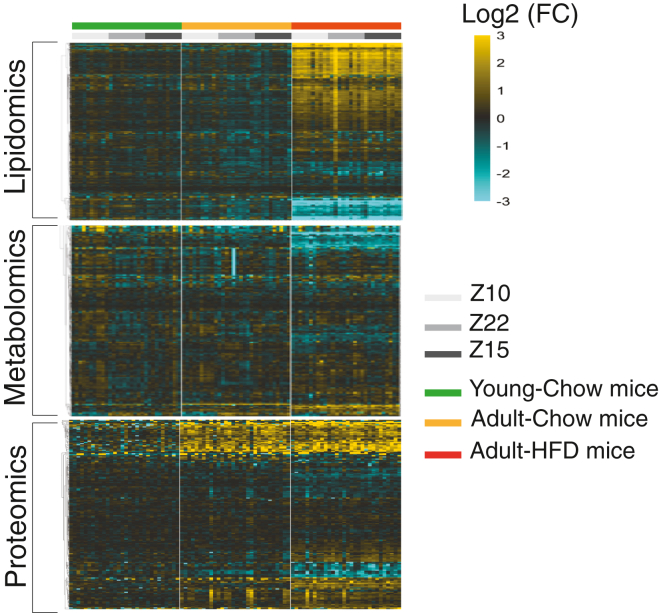
To demonstrate the utility of the method described above in analyzing biological samples, we prepared 90 mouse plasma aliquots of 25 μl each. The samples comprised three groups of n = 10 mice: 8-week-old mice (young-chow) and 24-week-old mice (adult-chow) fed a standard chow diet, and 24-week-old mice (adult-HFD) fed a HFD for 18 weeks. Each mouse had blood drawn at three times of day, each on a separate day: the first at Z10, the second at Z22, and the third at Z15. From these samples, we identified 907 lipid species from 16 lipid classes and subclasses (supplemental Table S3A), 223 polar metabolites (supplemental Table S4A), and 344 proteins (supplemental Table S5A). We found that diet, age, and blood-draw batch explained 44.3%, 15.6%, and 7.1% of the variability in the lipidomics data. In our metabolomics data, diet, age, and blood-draw batch explained 18.8%, 11.1%, and 8.1% of the variation, respectively. Following this analysis, we examined how each molecular species was associated with blood draw batch, age, and diet (Fig. 3 and supplemental Fig. S5).
Fig. 3.
Heatmaps of metabolites, lipids, and proteins. Data were row-normalized to the mean value of the young chow-fed mice. Data displayed with hierarchical clustering by row for each data modality separately.
We noted that in chow fed animals at both ages, total TGs, glucose, and apolipoprotein E (ApoE) and C-III (ApoC-III) were highest at Z10, while cortisol was lowest in the Z10 samples, all of which are previously reported to vary with food consumption (48, 49, 50, 51, 52, 53, 54) (Fig. 4). However, we also noted that these changes were muted, or absent, in the HFD-fed group, and the HFD group was the only one to show elevation of CEs in the Z22 and Z15 blood draw batches relative to Z10 (Fig. 4A).
Fig. 4.
Selected changes observed between repeated blood draws with significance from two-way ANOVA analysis within each group of mice. (∗∗∗P ≤ 0.001, ∗∗P ≤ 0.01, ∗P ≤ 0.05). A: Total concentration of TGs and CEs (n = 10). Ammoniated adducts of TG and CE were normalized to their respective labeled standards from Splash Lipidomix. The sum of concentration for all species per animal was calculated and presented here. Data shown are mean ± standard deviation. B: Relative concentrations of APOC3 and APOE at different blood draw time points (q < 0.01). Protein concentrations were normalized to the bridge within each plex. Data shown are mean ± standard deviation. C: Glucose and cortisol levels change with time point in mice (q < 0.01). The intensity of each sample was normalized to the mean signal of adult-chow mice at Z10 (n = 10). Data shown are mean ± standard deviation. APO, apolipoprotein; CE, cholesterol ester; TG, triglyceride.
Age-associated changes in mouse plasma
We examined the molecular changes associated with age. We found that 39.1%, 12.5%, and 12.5% of lipids, metabolites, and proteins were significantly different between chow-fed animals at 8 and 24 weeks of age (q < 0.01) (supplemental Tables S3C, S4C, and S5C). As has been previously reported, we observed a small decline in the majority of lipid classes with age with lyso-phosphatidylcholines, phosphatidylcholines, phosphatidylinositols (PIs), and TGs the most downregulated in our data (supplemental Table S3C). TGs containing 10–16 carbon acyl chains appear to be the most affected in that class, while other classes primarily showed lower levels of lipids containing FA 18:1, FA 22:6, FA 16:0, and FA 14:0, especially the vinyl ether lipids p-16:0 and p-18:0 and choline headgroups (supplemental Fig. S6A).
We found 19 polar metabolites in the metabolomics data that decreased significantly with age (q < 0.01), mainly N-acetylated amino acids, di-peptides (Glu-Glu and Glu-Ala), and modified amino acids (supplemental Table S4C). We found a modest decline of plasma glucose in the 24-week-old mice (55), with larger changes in ADP-ribose (>2-fold), and 4-hydroxycinnamic acid (>10-fold) (56, 57). We identified nine metabolites that were significantly increased, mostly amino acids, but also acetylcarnitine and the aging-implicated metabolite hippuric acid (58), which increased more than 10-fold between the 8-week-old and 24-week-old chow-fed groups (supplemental Fig. S6B).
In the proteomics data, we found that immunoglobulin I, immunoglobulin heavy constant mu, immunoglobulin G, and immunoglobulin heavy chain variable region were upregulated in adult mice (supplemental Table S5C) (59), as were the 20S proteasomal subunit proteins Psma4, Psma5, Psma6, and Psma7. We found a modest increase of fibronectin (Fn1) in the adult mice, and a decrease in the pro-alpha1 chains of type I collagen (Col1a1), consistent with previous findings in human aged skin fibroblasts (58) (supplemental Fig. S6C).
Diet-associated changes in circulating molecules
Finally, we examined differences in the plasma of the chow-fed and HFD-fed mice. Approximately 95% of lipid species (863 of 907) were significantly different between the two diets (q < 0.01), and there was an increase in total lipid content (supplemental Tables S3D and S6), consistent with previous reports (60, 61). The most profound change was in CEs, which accounted for 96.6% of the total molar concentration difference, with one particular species, arachidonoyl-cholesterol (CE 20:4), accounting for approximately 65% of the total molar increase in lipids in HFD-fed mouse plasma. Nonintuitively, but consistent with previous reports, we found that the HFD did not increase the total circulating TG concentration (62, 63).
Lipid class enrichment analysis showed that SMs, phosphatidylcholines, and CEs were the most highly increased lipid classes in the HFD plasma, while PIs, TGs and diglycerides (DGs) were the most depleted (supplemental Fig. S7A). Enrichment analysis on fatty acyl composition found that polyunsaturated fatty acids were highly perturbed, with an increase in most ω-6 fatty acyls and a decrease in most ω-3 fatty acyls (supplemental Fig. S7B). The total ω-6/ω-3 ratio increased from 1.18 ± 0.05 in adult chow mice to 2.40 ± 0.1 in HFD mice, consistent with previous studies on obesity and type II diabetes in mice (64, 65). Although these data do not resolve double-bond positional isomers, we annotated ω-6 and ω-3 lipids as the most abundant isomer form in mouse plasma, as previously reported via fatty acid methyl esters (FAME) analysis of mouse plasma and serum (66, 67).
Next, we examined the total molar concentration for each acyl chain in each lipid class. Lipids containing arachidonic acid (AA, FA 20:4, ω-6) and eicosanoic acid (EPA, FA 20:5, ω-3) were found in most classes and were consistently changed by diet, with AA significantly higher in every lipid class and EPA lower in all lipid classes in which they were found (Fig. 5 and supplemental Table S7). EPA was the only ω-3 lipid that decreased globally, while its main product, the more abundant docosahexaenoic acid (DHA, FA 22:6, ω-3), increased in all lipids classes except PIs, DGs, and TGs. The ω-6 fatty acid AA and its product adrenic acid (FA 22:4, ω-6) increased significantly in every lipid class in which it was detected.
Fig. 5.
Heatmap of the average of log2 (HFD/Chow) total concentration of PUFA acyl chains for every quantified lipid class at each time point. P-values were calculated using a two-tailed Student’s t test to differentiate adult-HFD-fed mice from adult-chow-fed mice. (∗∗∗P ≤ 0.001, ∗∗P ≤ 0.01, ∗P ≤ 0.05). Missing values are shown as white boxes. HFD, high-fat diet; PUFA, polyunsaturated fatty acid.
In the metabolomics data, we found that 54.3% of measured metabolites were significantly different between the HFD- and chow-fed mice (q < 0.01) (supplemental Table S4D). Metabolomics data demonstrated that the free fatty acids, AA and EPA, were changed in the same direction as the complex lipids containing them did. Free AA increased more than 4-fold, and EPA dropped more than 6-fold. We also found a slight increase in the glycerophospholipid-related metabolites glycerol-3-phosphate and glycerophosphocholine, while choline, acetylcarnitine, and carnitine levels consistently declined in the high-fat diet (Fig. 6A).
Fig. 6.
Significant differences in plasma metabolites and proteins of high fat diet fed mice. A: Polar metabolites associated with lipid metabolism. B: Polar metabolites associated with nitrogen metabolism. C: Lipoproteins. Proteins were normalized to the bridge included in every plex. HFD, high-fat diet (all shown differences q < 0.01; ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001).
We noticed that many nitrogen metabolism-related metabolites were significantly different in HFD-adult mice (supplemental Table S4 and Fig. 5B). In particular, citrulline, glutamate, glutamine, and ornithine were increased, while 4-hydroxyproline, arginine, and N-acetylornithine were decreased, suggesting possible impairment of the urea cycle or high activity of hepatic Arginase I, which is reported to be induced in obesity (68).
Finally, we found that 21.5% of measured proteins significantly differed between diets (q < 0.01) (supplemental Table S5D). We observed significant upregulation of many apolipoproteins (Angptl3, Apoa1, Apoa2, Apoa4, Apoc2, Apoc3, and Apoe) (Fig. 6C), the H-2 class I histocompatibility complex (H2-Q10/Q4), and cytosolic malic enzyme (ME1), all in agreement with previous findings (69, 70, 71, 72, 73). We also found that the previously identified markers of obesity fructose-biphosphate aldolase B (ALDOB), betaine-homocysteine methyltransferase (BHMT), and haptoglobin (Hp) were upregulated more than 4-fold in the HFD mice (74), and that several members of the complement system (Cfd, C8g, C8a, and C8b) were significantly depleted.
Discussion
Development of automated LLE: evaluations of linearity and reproducibility
Here we present, for the first time, an automated sample preparation system using an MTBE-based LLE on a PAL-DHF followed by Auto-MP3 protein preparation for high-quality multiomic analysis of metabolites, lipids, and proteins from a single blood plasma sample, enabling a deep phenotyping and an increased understanding of system biology. This method offers a robust workflow to prepare samples for multiomic analysis via mass spectrometry with minimal human intervention.
We selected the MTBE-based extraction approach, based on the method of Matyash et al., which demonstrated excellent recovery of lipids while avoiding chlorinated solvents, and resulted in the automation friendly location of the protein pellet at the bottom of the extraction vessel. We focused on setting up a system in which the consumables are available off-the-shelf from major laboratory suppliers to make implementation easy for the community while providing high-quality data. We created an automated workflow that avoids the issues of contamination and sample loss that can occur when lipids and organic solvents are exposed to plasticware, which can cause ion suppression and reduce numbers of lipid identifications as mass spectrometer scans (43, 44).
We demonstrated the accuracy and precision of this platform for lipids, which was comparable to other publications performing an LLE-based lipid extraction (45, 46). We also found comparable accuracy and precision for metabolites from the aqueous phase, a much less studied group of compounds from this workflow (20, 75, 76). We had a higher coefficient of variation in the measurement of citrate, a tricarboxylated metabolite that suffers from substantial peak broadening during chromatographic separation. We suspect this is a chromatographic artifact, as it is difficult to optimize chromatography for the diverse classes of compounds in metabolomics. The determined ratio of labeled:unlabeled standards showed a highly accurate recovery of lipids and metabolites. We found that this method was most reproducible on phospholipids, while the more hydrophobic lipids (DG, FA, MG, PG, and TG) had higher, but acceptable variability. Previous literature found that recovery of nonpolar plasma lipids using MTBE extraction protocol was lower than for phospholipids (45, 47), suggesting this may be unavoidable when performing MTBE-based LLE. These neural lipids are also extracted poorly in one-phase extractions when using a higher content of polar solvent (77). If neutral lipids, especially TGs are of paramount importance to an experiment, other protocols have been shown to have higher recoveries of TGs, including the 3PLE hexane phase (78), hexane-isopropanol extraction (79), or ethyl acetate-ethanol (80), but at the expense of reduced phospholipids recovery in the same phase.
In addition to its high accuracy and reproducibility, this methodology offers substantial time savings and requires only a small sample volume to prepare metabolomics, lipidomics, and proteomics samples. The method we have implemented on the PAL-DHR can extract 90 plasma samples in less than 24 h, preventing it from being a bottleneck if mass spectrometry data acquisition takes greater than 15 min per sample, while recovering a similar number of identifications to manual preparation. In contrast, careful manual extraction without exposure to plasticware for 90 samples would take three 8-h days.
This is the first report of an integrated system that uses only glassware and stainless steel consumables with a centrifuge and cooling system that can automate every step of the LLE while minimizing contaminations, to our knowledge. We have carefully described the system, the pitfalls we encountered in implementing automated sample preparation with it, and optimized consumables to enable other laboratories to create a similar workflow.
Changes in the plasma lipidome, metabolome, and proteome induced by a high-fat diet
Using the PAL-DHR prepared samples, we successfully recovered a number of previously reported biomarkers associated with age and HFD in mice, demonstrating the ability to recover biological signals from real samples. Additionally, the fact that we could recover the dramatically different lipid content in the plasma of HFD-fed mice demonstrates that the method is robust to samples with strikingly different lipid concentrations, while also capable of finding subtle changes, like those between blood draw time points.
Although we observed some molecular concentration differences between blood draw batches that are consistent with known circadian effects (such as glucose and total TGs), an important caveat to the data is that the different time points were sampled in separate weeks, and some of the detected changes could be due to week-to-week variation as opposed to circadian changes, limiting the interpretability of novel findings around circadian rhythm from this data set. Hence, we chose to focus on examining effects of circadian changes that had been previously reported.
As expected, given the significant physiological changes induced by a HFD (62, 81, 82, 83, 84) we found that the differences in molecular profiles between diets was much larger than all other effects in the data set. Lipids were the most perturbed class of molecules, consistent with the increased dietary lipid content. Proteomics results showed elevated levels of many different apolipoproteins (APOA4, APOC2, APOE, APOA1, APOA2, and APOB), important to the circulation of the increased lipids load. We observed changes in the polar compounds that are both the precursors and products of lipid head groups, suggesting that there may be changes in the metabolic fluxes in lipid metabolism in addition to the differing levels of circulating lipids. As expected, we found elevated glucose levels in the HFD mice, consistent with insulin resistance concomitant with diet induced obesity. We found lower acetoacetate levels and a shift in the circulating pyruvate/lactate ratio, suggesting that the intracellular NAD/NADH ratio may be reduced by the HFD (85). Significantly, we noticed a reduction of PI lipids containing DHA. PIs are known to be the main donor of AA to produce downstream bioactive lipid mediators (86, 87), but DHA-containing PI are less characterized but could be interpreted as depletion in the precursor pool of DHA for the production of proresolving mediators in a high-fat diet.
Pakiet et al. previously reported that a HFD increased the levels of total AA and DHA acyl groups as determined by GC-MS based analysis of FAME (61). Our analysis showed that CEs are at a substantially higher concentration than other circulating lipid classes (excluding free fatty acids, which were not quantified in this study), consistent with previous reports (62). Hence, FAME measurements of the fatty acid composition of plasma are primarily an analysis of the acyl groups from CEs. We report here for the first time AA is increased and EPA is depleted uniformly across all major plasma lipid classes, despite the fact that the vast majority of the change in the total AA:EPA ratio is from arachidonoyl-cholesterol (CE 20:4). We also demonstrated that the major change in ω-3 fatty acids is the depletion of EPA, while DHA was upregulated in some classes, and downregulated in others. Consistent with EPA depletion having an important role in the negative effects of a high-fat diet, it has been previously reported that animals fed a HFD supplemented with EPA have improved glucose tolerance, reduced liver adiposity, and decreased weight gain when compared to an unsupplemented HFD (88) (92). This acyl composition shift to increased AA and depleted EPA is present in phospholipids as well, providing a larger source of AA for eicosanoid synthesis. In the metabolomics study, we confirmed that there was a relative increase in AA, and depletion of EPA free fatty acids in HFD mice.
We hope that this comprehensive analysis of effects of age and diet on the plasma multiome will serve as a resource for the community working on these topics.
Data availability
Metabolomics and lipidomics data will be available from Metabolomics Workbench: Study #ST002956.
The proteomics data are available from the ProteomeXchange Consortium PRIDE database with the dataset identifier PXD049202.
Supplemental data
This article contains supplemental data.
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Acknowledgments
The authors would like to thank Tom Tobien from Trajan for the initial setup of the instrument, and Dr David Botstein for helpful comments on the manuscript while it was being drafted. We also acknowledge the contributions of Dr Niclas Olsson for acquiring the proteomic samples, and Travis Lee for assembling the enclosure for the robot.
Author contributions
N. V., T. M. M., and A. G. investigation; N. V., T. M. M., S. G., and A. G. methodology; N. V., T. M., J. J. O’. B., and S. R. H. formal analysis; N. V. and T. M. M. writing–original draft; N. V., P. S., J. J. O’. B., and S. R. H. data curation; N. V. validation; N. V. visualization; T. M. M. conceptualization; S. G., P. S., J. J. O’. B., and S. R. H. software; S. R. H., F. E. M., and B. D. B. writing–review and editing; R. K. and B. D. B. supervision; J. Z.-S., F. E. M., G. K., and R. K. resources.
Funding and additional information
Funding for this project was provided by Calico Life Sciences LLC.
Supplementary Data
References
- 1.Ignjatovic V., Geyer P.E., Palaniappan K.K., Chaaban J.E., Omenn G.S., Baker M.S., et al. Mass spectrometry-based plasma proteomics: considerations from sample collection to achieving translational data. J. Proteome Res. 2019;18:4085–4097. doi: 10.1021/acs.jproteome.9b00503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Geyer P.E., Voytik E., Treit P.V., Doll S., Kleinhempel A., Niu L., et al. Plasma Proteome Profiling to detect and avoid sample-related biases in biomarker studies. EMBO Mol. Med. 2019;11 doi: 10.15252/emmm.201910427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun B.B., Maranville J.C., Peters J.E., Stacey D., Staley J.R., Blackshaw J., et al. Genomic atlas of the human plasma proteome. Nature. 2018;558:73–79. doi: 10.1038/s41586-018-0175-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Psychogios N., Hau D.D., Peng J., Guo A.C., Mandal R., Bouatra S., et al. The human serum metabolome. PLoS One. 2011;6 doi: 10.1371/journal.pone.0016957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mapstone M., Cheema A.K., Fiandaca M.S., Zhong X., Mhyre T.R., MacArthur L.H., et al. Plasma phospholipids identify antecedent memory impairment in older adults. Nat. Med. 2014;20:415–418. doi: 10.1038/nm.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donatti A., Canto A.M., Godoi A.B., da Rosa D.C., Lopes-Cendes I. Circulating metabolites as potential biomarkers for neurological disorders-metabolites in neurological disorders. Metabolites. 2020;10:389. doi: 10.3390/metabo10100389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolrab D., Jirásko R., Cífková E., Höring M., Mei D., Chocholoušková M., et al. Lipidomic profiling of human serum enables detection of pancreatic cancer. Nat. Commun. 2022;13:124. doi: 10.1038/s41467-021-27765-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang Y., Cruickshank C., Armstrong M., Mahaffey S., Reisdorph R., Reisdorph N. New sample preparation approach for mass spectrometry-based profiling of plasma results in improved coverage of metabolome. J. Chromatogr. A. 2013;1300:217–226. doi: 10.1016/j.chroma.2013.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cajka T., Fiehn O. Toward merging untargeted and targeted methods in mass spectrometry-based metabolomics and lipidomics. Anal. Chem. 2016;88:524–545. doi: 10.1021/acs.analchem.5b04491. [DOI] [PubMed] [Google Scholar]
- 10.Reichl B., Eichelberg N., Freytag M., Gojo J., Peyrl A., Buchberger W. Evaluation and optimization of common lipid extraction methods in cerebrospinal fluid samples. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2020;1153 doi: 10.1016/j.jchromb.2020.122271. [DOI] [PubMed] [Google Scholar]
- 11.He Y., Rashan E.H., Linke V., Shishkova E., Hebert A.S., Jochem A., et al. Multi-omic single-shot technology for integrated proteome and lipidome analysis. Anal. Chem. 2021;93:4217–4222. doi: 10.1021/acs.analchem.0c04764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kang J., David L., Li Y., Cang J., Chen S. Three-in-One simultaneous extraction of proteins, metabolites and lipids for multi-omics. Front. Genet. 2021;12 doi: 10.3389/fgene.2021.635971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakayasu E.S., Nicora C.D., Sims A.C., Burnum-Johnson K.E., Kim Y.-M., Kyle J.E., et al. MPLEx: a robust and universal protocol for single-sample integrative proteomic, metabolomic, and lipidomic analyses. mSystems. 2016;1 doi: 10.1128/mSystems.00043-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matyash V., Liebisch G., Kurzchalia T.V., Shevchenko A., Schwudke D. Lipid extraction by methyl-tert-butyl ether for high-throughput lipidomics. J. Lipid Res. 2008;49:1137–1146. doi: 10.1194/jlr.D700041-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Löfgren L., Ståhlman M., Forsberg G.-B., Saarinen S., Nilsson R., Hansson G.I. The BUME method: a novel automated chloroform-free 96-well total lipid extraction method for blood plasma. J. Lipid Res. 2012;53:1690–1700. doi: 10.1194/jlr.D023036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Löfgren L., Forsberg G.-B., Ståhlman M. The BUME method: a new rapid and simple chloroform-free method for total lipid extraction of animal tissue. Sci. Rep. 2016;6 doi: 10.1038/srep27688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Folch J., Lees M., Sloane Stanley G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 18.Whiley L., Godzien J., Ruperez F.J., Legido-Quigley C., Barbas C. In-vial dual extraction for direct LC-MS analysis of plasma for comprehensive and highly reproducible metabolic fingerprinting. Anal. Chem. 2012;84:5992–5999. doi: 10.1021/ac300716u. [DOI] [PubMed] [Google Scholar]
- 19.Schwaiger M., Schoeny H., El Abiead Y., Hermann G., Rampler E., Koellensperger G. Merging metabolomics and lipidomics into one analytical run. Analyst. 2018;144:220–229. doi: 10.1039/c8an01219a. [DOI] [PubMed] [Google Scholar]
- 20.Sostare J., Di Guida R., Kirwan J., Chalal K., Palmer E., Dunn W.B., et al. Comparison of modified Matyash method to conventional solvent systems for polar metabolite and lipid extractions. Anal. Chim. Acta. 2018;1037:301–315. doi: 10.1016/j.aca.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 21.Patterson R.E., Ducrocq A.J., McDougall D.J., Garrett T.J., Yost R.A. Comparison of blood plasma sample preparation methods for combined LC-MS lipidomics and metabolomics. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2015;1002:260–266. doi: 10.1016/j.jchromb.2015.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu J., Liu X., Xiao Z., Locasale J.W. Quantitative evaluation of a high resolution lipidomics platform. bioRxiv. 2019 doi: 10.1101/627687. [preprint] [DOI] [Google Scholar]
- 23.Chen S., Hoene M., Li J., Li Y., Zhao X., Häring H.-U., et al. Simultaneous extraction of metabolome and lipidome with methyl tert-butyl ether from a single small tissue sample for ultra-high performance liquid chromatography/mass spectrometry. J. Chromatogr. A. 2013;1298:9–16. doi: 10.1016/j.chroma.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 24.Surma M.A., Herzog R., Vasilj A., Klose C., Christinat N., Morin-Rivron D., et al. An automated shotgun lipidomics platform for high throughput, comprehensive, and quantitative analysis of blood plasma intact lipids. Eur. J. Lipid Sci. Technol. 2015;117:1540–1549. doi: 10.1002/ejlt.201500145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lerner R., Baker D., Schwitter C., Neuhaus S., Hauptmann T., Post J.M., et al. Four-dimensional trapped ion mobility spectrometry lipidomics for high throughput clinical profiling of human blood samples. Nat. Commun. 2023;14:937. doi: 10.1038/s41467-023-36520-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salem M.A., Jüppner J., Bajdzienko K., Giavalisco P. Protocol: a fast, comprehensive and reproducible one-step extraction method for the rapid preparation of polar and semi-polar metabolites, lipids, proteins, starch and cell wall polymers from a single sample. Plant Methods. 2016;12:45. doi: 10.1186/s13007-016-0146-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coman C., Solari F.A., Hentschel A., Sickmann A., Zahedi R.P., Ahrends R. Simultaneous metabolite, protein, lipid extraction (SIMPLEX): a combinatorial multimolecular omics approach for systems biology. Mol. Cell Proteomics. 2016;15:1453–1466. doi: 10.1074/mcp.M115.053702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garikapati V., Colasante C., Baumgart-Vogt E., Spengler B. Sequential lipidomic, metabolomic, and proteomic analyses of serum, liver, and heart tissue specimens from peroxisomal biogenesis factor 11α knockout mice. Anal. Bioanal. Chem. 2022;414:2235–2250. doi: 10.1007/s00216-021-03860-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gaun A., Lewis Hardell K.N., Olsson N., O'Brien J.J., Gollapudi S., Smith M., et al. Automated 16-plex plasma proteomics with real-time search and ion mobility mass spectrometry enables large-scale profiling in naked mole-rats and mice. J. Proteome Res. 2021;20:1280–1295. doi: 10.1021/acs.jproteome.0c00681. [DOI] [PubMed] [Google Scholar]
- 30.Chambers M.C., Maclean B., Burke R., Amodei D., Ruderman D.L., Neumann S., et al. A cross-platform toolkit for mass spectrometry and proteomics. Nat. Biotechnol. 2012;30:918–920. doi: 10.1038/nbt.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seitzer P., Bennett B., Melamud E. MAVEN2: an updated open-source mass spectrometry exploration platform. Metabolites. 2022;12:684. doi: 10.3390/metabo12080684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liebisch G., Fahy E., Aoki J., Dennis E.A., Durand T., Ejsing C.S., et al. Update on LIPID MAPS classification, nomenclature, and shorthand notation for MS-derived lipid structures. J. Lipid Res. 2020;61:1539–1555. doi: 10.1194/jlr.S120001025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huttlin E.L., Jedrychowski M.P., Elias J.E., Goswami T., Rad R., Beausoleil S.A., et al. A tissue-specific atlas of mouse protein phosphorylation and expression. Cell. 2010;143:1174–1189. doi: 10.1016/j.cell.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eng J.K., McCormack A.L., Yates J.R. An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J. Am. Soc. Mass Spectrom. 1994;5:976–989. doi: 10.1016/1044-0305(94)80016-2. [DOI] [PubMed] [Google Scholar]
- 35.O'Brien J.J., Raj A., Gaun A., Waite A., Li W., Hendrickson D., et al. A new data analysis framework for combining multiple batches increases the power of isobaric proteomics experiments. Nat. Methods. 2023;21:290–300. doi: 10.1038/s41592-023-02120-6. [DOI] [PubMed] [Google Scholar]
- 36.Storey J.D., Tibshirani R. Statistical significance for genomewide studies. Proc. Natl. Acad. Sci. U. S. A. 2003;100:9440–9445. doi: 10.1073/pnas.1530509100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xia J., Mandal R., Sinelnikov I.V., Broadhurst D., Wishart D.S. MetaboAnalyst 2.0--a comprehensive server for metabolomic data analysis. Nucleic Acids Res. 2012;40:W127–W133. doi: 10.1093/nar/gks374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kessler N., Neuweger H., Bonte A., Langenkämper G., Niehaus K., Nattkemper T.W., et al. MeltDB 2.0-advances of the metabolomics software system. Bioinformatics. 2013;29:2452–2459. doi: 10.1093/bioinformatics/btt414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu G., Wang L.G., Han Y., He Q.Y. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS. 2012;16:284–287. doi: 10.1089/omi.2011.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Subramanian A., Tamayo P., Mootha V.K., Mukherjee S., Ebert B.L., Gillette M.A., et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. U. S. A. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mootha V.K., Lindgren C.M., Eriksson K.F., Subramanian A., Sihag S., Lehar J., et al. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat. Genet. 2003;34:267–273. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- 42.Hahladakis J.N., Velis C.A., Weber R., Iacovidou E., Purnell P. An overview of chemical additives present in plastics: migration, release, fate and environmental impact during their use, disposal and recycling. J. Hazard. Mater. 2018;344:179–199. doi: 10.1016/j.jhazmat.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 43.Benke P.I., Burla B., Ekroos K., Wenk M.R., Torta F. Impact of ion suppression by sample cap liners in lipidomics. Anal. Chim. Acta. 2020;1137:136–142. doi: 10.1016/j.aca.2020.09.055. [DOI] [PubMed] [Google Scholar]
- 44.Canez C.R., Li L. Investigation of the effects of labware contamination on mass spectrometry-based human serum lipidome analysis. Anal. Chem. 2024;96:8373–8380. doi: 10.1021/acs.analchem.3c05433. [DOI] [PubMed] [Google Scholar]
- 45.Wong M.W.K., Braidy N., Pickford R., Sachdev P.S., Poljak A. Comparison of single phase and biphasic extraction protocols for lipidomic studies using human plasma. Front. Neurol. 2019;10:879. doi: 10.3389/fneur.2019.00879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sarafian M.H., Gaudin M., Lewis M.R., Martin F.-P., Holmes E., Nicholson J.K., et al. Objective set of criteria for optimization of sample preparation procedures for ultra-high throughput untargeted blood plasma lipid profiling by ultra performance liquid chromatography-mass spectrometry. Anal. Chem. 2014;86:5766–5774. doi: 10.1021/ac500317c. [DOI] [PubMed] [Google Scholar]
- 47.Choi J.H., Bang G., Kim J.A., Kim Y.H. A simple and rapid extraction of lipids in plasma using spin column with superabsorbent polymer beads for mass spectrometry. J. Anal. Sci. Technol. 2023;14:22. [Google Scholar]
- 48.Chua E.C.-P., Shui G., Lee I.T.-G., Lau P., Tan L.-C., Yeo S.-C., et al. Extensive diversity in circadian regulation of plasma lipids and evidence for different circadian metabolic phenotypes in humans. Proc. Natl. Acad. Sci. U. S. A. 2013;110:14468–14473. doi: 10.1073/pnas.1222647110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Delezie J., Dumont S., Dardente H., Oudart H., Gréchez-Cassiau A., Klosen P., et al. The nuclear receptor REV-ERBα is required for the daily balance of carbohydrate and lipid metabolism. FASEB J. 2012;26:3321–3335. doi: 10.1096/fj.12-208751. [DOI] [PubMed] [Google Scholar]
- 50.Kent B.A., Rahman S.A., St Hilaire M.A., Grant L.K., Rüger M., Czeisler C.A., et al. Circadian lipid and hepatic protein rhythms shift with a phase response curve different than melatonin. Nat. Commun. 2022;13:681. doi: 10.1038/s41467-022-28308-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dyar K.A., Lutter D., Artati A., Ceglia N.J., Liu Y., Armenta D., et al. Atlas of circadian metabolism reveals system-wide coordination and communication between clocks. Cell. 2018;174:1571–1585.e11. doi: 10.1016/j.cell.2018.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang L., Ma T., Zhao L., Jiang H., Zhang J., Liu D., et al. Circadian regulation of apolipoprotein gene expression affects testosterone production in mouse testis. Theriogenology. 2021;174:9–19. doi: 10.1016/j.theriogenology.2021.06.023. [DOI] [PubMed] [Google Scholar]
- 53.Reinke H., Asher G. Circadian clock control of liver metabolic functions. Gastroenterology. 2016;150:574–580. doi: 10.1053/j.gastro.2015.11.043. [DOI] [PubMed] [Google Scholar]
- 54.Ma D., Liu T., Chang L., Rui C., Xiao Y., Li S., et al. The liver clock controls cholesterol homeostasis through Trib1 protein-mediated regulation of PCSK9/low density lipoprotein receptor (LDLR) Axis. J. Biol. Chem. 2015;290:31003–31012. doi: 10.1074/jbc.M115.685982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Petr M.A., Alfaras I., Krawcyzk M., Bair W.-N., Mitchell S.J., Morrell C.H., et al. A cross-sectional study of functional and metabolic changes during aging through the lifespan in male mice. Elife. 2021;10:e62952. doi: 10.7554/eLife.62952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alam M.A., Subhan N., Hossain H., Hossain M., Reza H.M., Rahman M.M., et al. Hydroxycinnamic acid derivatives: a potential class of natural compounds for the management of lipid metabolism and obesity. Nutr. Metab. 2016;13:27. doi: 10.1186/s12986-016-0080-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee M.-K., Park Y.B., Moon S.-S., Bok S.H., Kim D.-J., Ha T.-Y., et al. Hypocholesterolemic and antioxidant properties of 3-(4-hydroxyl)propanoic acid derivatives in high-cholesterol fed rats. Chem. Biol. Interact. 2007;170:9–19. doi: 10.1016/j.cbi.2007.06.037. [DOI] [PubMed] [Google Scholar]
- 58.De Simone G., Balducci C., Forloni G., Pastorelli R., Brunelli L. Hippuric acid: could became a barometer for frailty and geriatric syndromes? Ageing Res. Rev. 2021;72 doi: 10.1016/j.arr.2021.101466. [DOI] [PubMed] [Google Scholar]
- 59.Garcia-Prat M., Vila-Pijoan G., Martos Gutierrez S., Gala Yerga G., García Guantes E., Martínez-Gallo M., et al. Age-specific pediatric reference ranges for immunoglobulins and complement proteins on the Optilite™ automated turbidimetric analyzer. J. Clin. Lab. Anal. 2018;32 doi: 10.1002/jcla.22420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Awada M., Meynier A., Soulage C.O., Hadji L., Géloën A., Viau M., et al. n-3 PUFA added to high-fat diets affect differently adiposity and inflammation when carried by phospholipids or triacylglycerols in mice. Nutr. Metab. 2013;10:23. doi: 10.1186/1743-7075-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pakiet A., Jakubiak A., Czumaj A., Sledzinski T., Mika A. The effect of western diet on mice brain lipid composition. Nutr. Metab. 2019;16:81. doi: 10.1186/s12986-019-0401-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Eisinger K., Liebisch G., Schmitz G., Aslanidis C., Krautbauer S., Buechler C. Lipidomic analysis of serum from high fat diet induced obese mice. Int. J. Mol. Sci. 2014;15:2991–3002. doi: 10.3390/ijms15022991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Osae E.A., Bullock T., Chintapalati M., Brodesser S., Hanlon S., Redfern R., et al. Obese mice with dyslipidemia exhibit meibomian gland hypertrophy and alterations in meibum composition and aqueous tear production. Int. J. Mol. Sci. 2020;21:8772. doi: 10.3390/ijms21228772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kaliannan K., Li X.-Y., Wang B., Pan Q., Chen C.-Y., Hao L., et al. Multi-omic analysis in transgenic mice implicates omega-6/omega-3 fatty acid imbalance as a risk factor for chronic disease. Commun. Biol. 2019;2:276. doi: 10.1038/s42003-019-0521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Simopoulos A.P. An increase in the omega-6/omega-3 fatty acid ratio increases the risk for obesity. Nutrients. 2016;8:128. doi: 10.3390/nu8030128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zanfini A., Dreassi E., Berardi A., Piomboni P., Costantino-Ceccarini E., Luddi A. GC-EI-MS analysis of fatty acid composition in brain and serum of twitcher mouse. Lipids. 2014;49:1115–1125. doi: 10.1007/s11745-014-3945-0. [DOI] [PubMed] [Google Scholar]
- 67.Yan X., Li L., Liu P., Xu J., Wang Z., Ding L., et al. Targeted metabolomics profiles serum fatty acids by HFD induced non-alcoholic fatty liver in mice based on GC-MS. J. Pharm. Biomed. Anal. 2022;211 doi: 10.1016/j.jpba.2022.114620. [DOI] [PubMed] [Google Scholar]
- 68.Ito T., Kubo M., Nagaoka K., Funakubo N., Setiawan H., Takemoto K., et al. Early obesity leads to increases in hepatic arginase I and related systemic changes in nitric oxide and L-arginine metabolism in mice. J. Physiol. Biochem. 2018;74:9–16. doi: 10.1007/s13105-017-0597-6. [DOI] [PubMed] [Google Scholar]
- 69.Qu J., Ko C.-W., Tso P., Bhargava A. Apolipoprotein A-IV: a multifunctional protein involved in protection against atherosclerosis and diabetes. Cells. 2019;8:319. doi: 10.3390/cells8040319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Martinez-Huenchullan S.F., Shipsey I., Hatchwell L., Min D., Twigg S.M., Larance M. Blockade of high-fat diet proteomic phenotypes using exercise as prevention or treatment. Mol. Cell Proteomics. 2021;20 doi: 10.1074/mcp.TIR120.002343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jong M.C., Hofker M.H., Havekes L.M. Role of ApoCs in lipoprotein metabolism: functional differences between ApoC1, ApoC2, and ApoC3. Arterioscler. Thromb. Vasc. Biol. 1999;19:472–484. doi: 10.1161/01.atv.19.3.472. [DOI] [PubMed] [Google Scholar]
- 72.Kyohara M., Shirakawa J., Okuyama T., Kimura A., Togashi Y., Tajima K., et al. Serum quantitative proteomic analysis reveals soluble EGFR to be a marker of insulin resistance in male mice and humans. Endocrinology. 2017;158:4152–4164. doi: 10.1210/en.2017-00339. [DOI] [PubMed] [Google Scholar]
- 73.Pamir N., Pan C., Plubell D.L., Hutchins P.M., Tang C., Wimberger J., et al. Genetic control of the mouse HDL proteome defines HDL traits, function, and heterogeneity [S] J. Lipid Res. 2019;60:594–608. doi: 10.1194/jlr.M090555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stocks B., Gonzalez-Franquesa A., Borg M.L., Björnholm M., Niu L., Zierath J.R., et al. Integrated liver and plasma proteomics in obese mice reveals complex metabolic regulation. Mol. Cell Proteomics. 2022;21 doi: 10.1016/j.mcpro.2022.100207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ulmer C.Z., Jones C.M., Yost R.A., Garrett T.J., Bowden J.A. Optimization of Folch, Bligh-Dyer, and Matyash sample-to-extraction solvent ratios for human plasma-based lipidomics studies. Anal. Chim. Acta. 2018;1037:351–357. doi: 10.1016/j.aca.2018.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Macioszek S., Dudzik D., Jacyna J., Wozniak A., Schöffski P., Markuszewski M.J. A robust method for sample preparation of gastrointestinal stromal tumour for LC/MS untargeted metabolomics. Metabolites. 2021;11:554. doi: 10.3390/metabo11080554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Höring M., Stieglmeier C., Schnabel K., Hallmark T., Ekroos K., Burkhardt R., et al. Benchmarking one-phase lipid extractions for plasma lipidomics. Anal. Chem. 2022;94:12292–12296. doi: 10.1021/acs.analchem.2c02117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vale G., Martin S.A., Mitsche M.A., Thompson B.M., Eckert K.M., McDonald J.G. Three-phase liquid extraction: a simple and fast method for lipidomic workflows. J. Lipid Res. 2019;60:694–706. doi: 10.1194/jlr.D090795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Reis A., Rudnitskaya A., Blackburn G.J., Mohd Fauzi N., Pitt A.R., Spickett C.M. A comparison of five lipid extraction solvent systems for lipidomic studies of human LDL. J. Lipid Res. 2013;54:1812–1824. doi: 10.1194/jlr.M034330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lin J.H., Liu L.Y., Yang M.H., Lee M.H. Ethyl acetate/ethyl alcohol mixtures as an alternative to folch reagent for extracting animal lipids. J. Agric Food Chem. 2004;52:4984–4986. doi: 10.1021/jf049360m. [DOI] [PubMed] [Google Scholar]
- 81.Ludgero-Correia A., Jr., Aguila M.B., Mandarim-de-Lacerda C.A., Faria T.S. Effects of high-fat diet on plasma lipids, adiposity, and inflammatory markers in ovariectomized C57BL/6 mice. Nutrition. 2012;28:316–323. doi: 10.1016/j.nut.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 82.Gowda S.G.B., Gao Z.-J., Chen Z., Abe T., Hori S., Fukiya S., et al. Untargeted lipidomic analysis of plasma from high-fat diet-induced obese rats using UHPLC-linear trap quadrupole-orbitrap MS. Anal. Sci. 2020;36:821–828. doi: 10.2116/analsci.19P442. [DOI] [PubMed] [Google Scholar]
- 83.Bao L., Yang C., Shi Z., Wang Z., Jiang D. Analysis of serum metabolomics in obese mice induced by high-fat diet. Diabetes Metab. Syndr. Obes. 2021;14:4671–4678. doi: 10.2147/DMSO.S337979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tomar M.S., Sharma A., Araniti F., Pateriya A., Shrivastava A., Tamrakar A.K. Distinct metabolomic profiling of serum samples from high-fat-diet-induced insulin-resistant mice. ACS Pharmacol. Transl. Sci. 2023;6:771–782. doi: 10.1021/acsptsci.3c00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Patgiri A., Skinner O.S., Miyazaki Y., Schleifer G., Marutani E., Shah H., et al. An engineered enzyme that targets circulating lactate to alleviate intracellular NADH:NAD+ imbalance. Nat. Biotechnol. 2020;38:309–313. doi: 10.1038/s41587-019-0377-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Christie W.W., Harwood J.L. Oxidation of polyunsaturated fatty acids to produce lipid mediators. Essays Biochem. 2020;64:401–421. doi: 10.1042/EBC20190082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhou L., Nilsson A. Sources of eicosanoid precursor fatty acid pools in tissues. J. Lipid Res. 2001;42:1521–1542. [PubMed] [Google Scholar]
- 88.Kalupahana N.S., Claycombe K., Newman S.J., Stewart T., Siriwardhana N., Matthan N., et al. Eicosapentaenoic acid prevents and reverses insulin resistance in high-fat diet-induced obese mice via modulation of adipose tissue inflammation. J. Nutr. 2010;140:1915–1922. doi: 10.3945/jn.110.125732. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Metabolomics and lipidomics data will be available from Metabolomics Workbench: Study #ST002956.
The proteomics data are available from the ProteomeXchange Consortium PRIDE database with the dataset identifier PXD049202.