Abstract
Purpose
Autistic symptom improvement can be observed in children treated with acupuncture, but the mechanism is still being explored. In the present study, we used scalp acupuncture to treat autism rat model, and then their improvement in the abnormal behaviors and specific mechanisms behind were revealed by detecting animal behaviors, analyzing the RNA sequencing of the prefrontal cortex (PFC), and observing the ultrastructure of PFC neurons under the transmission electron microscope.
Methods
On gestational day 12.5, Wistar rats were given valproic acid (VPA) by intraperitoneal injection, and their offspring were considered to be reliable rat models of autism. They were randomized to VPA or VPA-acupuncture group (n = 8). Offspring of Wistar pregnant rats that were simultaneously injected with saline were randomly selected as the wild-type group (WT). VPA_acupuncture group rats received acupuncture intervention at 23 days of age for 4 weeks, and the other two groups followed without intervention. After the intervention, all experimental rats underwent behavioral tests. Immediately afterward, they were euthanized by cervical dislocation, and their prefrontal cortex was isolated for RNA sequencing and transmission electron microscopy.
Results
The main results are as follows: 1. Animal behavioural tests: VPA group rats showed more anxiety-like behaviour and repetitive, stereotyped behaviour than WT group rats. While VPA group rats showed less spatial exploration ability, activity level, social interaction, and social novelty preference than WT group rats. It was gratifying to observe that acupuncture indeed improved these abnormal behaviors of autism rat model. 2. RNA-sequencing: The three groups of rats differed in the expression and enrichment pathways of multiple genes related to synaptic function, neural signal transduction, immune-inflammatory responses and circadian rhythm regulation. Our experiments indicated that acupuncture can alleviate the major symptoms of ASD by improving these neurological abnormalities. 3. Under the transmission electron microscopy, several lysosomes and mitochondrial structural abnormalities were observed in the prefrontal neurons of VPA group rats, which were manifested as atrophy of the mitochondrial membrane, blurring or disappearance of the mitochondrial cristae, and even vacuolization. Moreover, the number of synapses and synaptic vesicles was relatively small. Conversely, the mitochondrial structure of rats in the WT group and VPA_acupuncture was normal, and the number of synapses and synaptic vesicles was relatively large.
Conclusion
Acupuncture effectively improved the abnormal behaviors of autism rat model and the ultrastructure of the PFC neurons, which might worked by improving their abnormal synaptic function, synaptic plasticity promoting neuronal signal transduction and regulating immune-inflammatory responses.
Keywords: Autism spectrum disorder, Acupuncture, Animal behavior, RNA sequencing, Transmission electron microscope
1. Introduction
Autism spectrum disorder (ASD) is a complex neurobehavioral disorder. Social impairments, repetitive stereotypical behaviors and limited interests characterize the disease. The ASD incidence has been reported continuously increased globally [1]. However, the etiology of ASD is still poorly defined. Genetics, neurology and immunology factors are believed to be associated with the pathogenesis of ASD in modern medicine, while traditional Chinese medicine (TCM) believes that ASD has manifestations similar to “mental disorder”, such as blurred vision, poor eye contact, inability to initiate communication, disobedience to instructions, and even manic symptoms. So far, ASD cannot be cured. To some extent, special education, behavioral intervention, family intervention can relieve symptoms of ASD, but it requires significant resources in terms of time and money, as well as family and social resources. And pharmacologic interventions are limited to treating symptoms and do not target social deficits [2,3]. As a consequence, clinical treatments for children with ASD are limited. This has prompted us to actively seek alternative treatments. Traditional Chinese medicine, for example, consists mainly of acupuncture, massage and Chinese herbal medicine [4]. Acupuncture has been developed for thousands of years to treat multi-system diseases [5]. In particular, scalp acupuncture can effectively improve brain dysfunction, such as ASD, Parkinson's disease (PD), Alzheimer's disease (AD), children's cerebral palsy, stroke and other neurological developmental disorder or degenerative diseases [[6], [7], [8], [9]] without fatal side effects [10,11].
Extensive evidence suggests that the rodent VPA maternal challenge is an excellent animal model of autism, and that the VPA model is more representative of many cases of idiopathic autism caused by environmental insults than other models caused by single-gene or chromosomal disorders [12,13].
Acupuncture has been applied to the study of various animal models, especially those related to brain function-related diseases, such as ASD, AD, vascular dementia [[14], [15], [16], [17], [18], [19]]. This paper chooses “GV24” point and bilateral “GB13” points for treatment. “GV24” and “GB13” are located on the forehead, which enters the brain and belongs to the Governor (Du) vessel. They can activate blood, nourish the brain, regulate and calm the mind [20]. Acupuncture at “GV24” and bilateral “GB13” have been widely used in clinical practice, especially in the treatment of neurodevelopmental disorders and degenerative diseases (AD, ASD, etc.). Based on our previous research and extensive literature, acupuncture at these points can improve the core diseases of neurodevelopmental disorders, degenerative diseases. But its mechanism involves multiple brain regions, there is currently no consensus [8,21,22].
Previous studies have shown that cytokines and glial activation are present in the central nervous system of autism patients, suggesting that the pathogenesis of ASD may be related to the excessive immune-inflammatory response in the central nervous system [23,24]. Despite acupuncture has clinical utility in treating autistic children, its action mechanism is still obscure, several studies have shown that acupuncture may improve clinical symptoms of many diseases by modulating immune-inflammatory functions [25,26].
The PFC is considered to play a significant role in cognition, social behavior, memory, planning and execution activities. Previous studies found that the PFC dysfunction was closely associated with the onset of ASD [27]. In addition, neuronal synapses have been identified as key targets for neurological diseases such as ASD, schizophrenia, depression, etc. [28,29].
Based on previous experiments [21,30], we proposed the following hypothesis: acupuncture at GV24 and bilateral GB13 points can improve abnormal behaviors in autism rat model. Which might be linked to changes in frontal cortical function and ultrastructure of neuronal synapses and worked through specific pathways and genes. Therefore, autism rat model was constructed and acupuncture intervention was carried out on rats in the VPA_acupuncture group. After intervention, 3 groups of experimental rats were tested for behaviour, and the prefrontal cortex was taken for RNA sequencing or observation for their ultrastructure of neurons. By comparing the behavioural differences among the three groups of rats, the neuronal ultrastructural differences in the prefrontal cortex, and the genes and enriched pathways identified by RNA sequencing, the potential mechanism of acupuncture improving ASD was further explored.
2. Materials and methods
2.1. Animals and grouping
Wistar pregnant rats weighting 230–250g were selected for experiment. On day 12.5 of pregnancy, randomly select five of them injecting sodium valproate (VPA) (intraperitoneal, 600 mg/kg) [31]. The offspring were recognized as good animal models for ASD. Sixteen male offspring of them were randomized to VPA or VPA_acupuncture group (n = 8). The remaining pregnant rats received intraperitoneal injection of saline at the same time, and 8 male offspring of them were randomly selected as the wild type (WT) group (n = 8). The animals were kept at the Experimental Animal Center of Fujian Medical University in a controllable environment with sufficient food and water, lights on from 7:00 a.m. to 7:00 p.m., temperature (21 ± 1)°C and humidity of 60 %. And they were kept in accordance with the National Standards for the Management of Medical Laboratory Animals. This experiment has been approved by the Experimental Animal Ethics Committee of Fujian Medical University (Ethics No. FJMU IACUC 2018-088).
2.2. Intervention
According to The Acupuncture Experiment to locate the bilateral GB13 and GV24 points. On postnatal day 23, the VPA_acupuncture group rats began acupuncture intervention (Fig. 1a and b). Rats received acupuncture under a fixator, with a needle depth of 2 mm for 40 min each time. During this period, the needle was manually twisted every 10 min. Intervention frequency was once daily, continuous for 5 consecutive days per week for 4 weeks [30]. Rats in WT and VPA groups did not receive acupuncture, but they were grabbed every day just like the VPA_acupuncture group to increase the experimental trustworthiness.
Fig. 1.
Experimental diagram of acupuncture and behavior tests.
Notes: (a) Time axis; (b) Acupoint localization; (c) Tissue sampling; (d) Open field test; (e) Social interaction test; (f) Self-grooming test.
2.3. Behavioral experiments
2.3.1. Open field test
At 49 days of age, the three groups of experimental rats were placed into a 100 cm × 100 cm × 100 cm box individually, which sides and bottom were black and opaque (Fig. 1d). The rats were allowed to move freely for 5 min, and their activity was subsequently recorded by TopScanLite. The ground area of the open field box was divided into the surrounding and central area (16:9). We cleaned the box with 75 % ethanol after each test, with a 5-min interval between the two tests to allow the ethanol to evaporate.
2.3.2. Three-chamber social test
At 50 days of age, three groups of experimental rats were placed into a 60 cm × 40 cm × 22 cm box to test social behavior, which was divided into three chambers by two diaphragms (Fig. 1e). The middle of the diaphragm is a retractable door that allows animals to enter both chambers. The day before the test, both tested rats and unfamiliar rats were acclimated for 10 minutes in the box. Each experimental rat was placed from the central chamber, with an inverted empty wire mesh cup placed in the corners of each side chamber, and placed diagonally in the three chambers. The test consisted of two stages, with a recording time of 10 min each. Stage I: Stranger rat 1 of the same age and gender was placed in one cup, while an object was put in the other cup. Stage II: The object in Stage 1 was substituted by stranger rat 2, keeping stranger rat 1 unchanged, and recorded their contact time with stranger rat 1, stranger rat 2, or the object separately. After the test, the box was cleaned with 75 % ethanol, with a 5-min interval between tests to allow the ethanol to evaporate and the odor to dissipate.
2.3.3. Self-grooming test
At 51 days of age, rats were placed in a 50 cm × 50 cm × 50 cm black organic glass fence, and recorded them for 30 min after adaptation for 10 min (Fig. 1f). Self modification behaviors include wiping the face, scratching the head and ears, and performing full body grooming. Two recorders independently evaluated the total time and frequency of self-grooming within 30 min, and calculated the average self-grooming time.
2.4. Tissue preparation
At 52 days of age, all rats were sacrificed. PFCs from each rat were removed and appropriately preserved (Fig. 1c). Five PFCs of per group were randomly selected for RNA sequencing analysis (n = 5), and then the rest PFC tissues were observed under a transmission electron microscope (n = 3).
2.5. RNA-sequencing analysis(RNA-seq)
The RNA-seq transcriptome library was prepared by the TruSeq TM RNA sample preparation Kit (Illumina, San Diego, CA) using 1 μg of total RNA. Double-stranded cDNA was synthesized using a cDNA synthesis kit (Invitrogen, CA) with random hexamer primers (Illumina). End-repair, phosphorylation and ‘A' base addition of cDNA were performed following Illumina's library construction protocol. A total of 226.21 GB of Clean Data was obtained from 15 samples, with more than 93.5 % of Q30 bases.
2.5.1. Principal component analysis
According to principal component analysis (PCA), the closer the distance, the higher the similarity between the samples. Subsequently, outliers in each group are removed.
2.5.2. Differentially expressed genes (DEGs)
According to the expression quantity, inter-group comparison was carried out to acquire the differentially expressed genes among groups. Transcript abundance of each gene was quantified by RSEM [32]. Differential expression analysis was conducted by the DESeq2 [33]/DEGseq [34]/EdgeR [35] with a Q value ≤ 0.05. We considered there were significant differentially expressed genes between two groups characterized as |log2FC| >1 and Q value ≤ 0.05.
3. Transmission electron microscope
The remaining PFCs from each group were observed under transmission electron microscope. Cut the tissues into 1x1x1mm3 blocks and soak them overnight in 2.5 % glutaraldehyde at 4 °C. They were fixed, dehydrated and embedded in a mixture of linoleic acid. Afterward, cut these slices into nanoscale (70 nm) by a diamond tissue knife on a super cutting E-slicer. Subsequently, they were stained by 4 % uranyl acetate and 0.4 % lead nitrate, respectively. Finally, the ultrastructure of PFC neurons was observed.
4. Statistical analysis
IBM SPSS Statistics 25 and GraphPad Prism 9 were use for statistical analyses. For the open field test and self-grooming test, one-way ANOVA followed by Bonferroni's multiple comparisons versus VPA group; while in 3-chamber test, two-way ANOVA followed by Tukey's multiple comparisons. Results were shown as the mean ± standard error, and two-tailed P < 0.05 was considered statistically significant.
5. Results
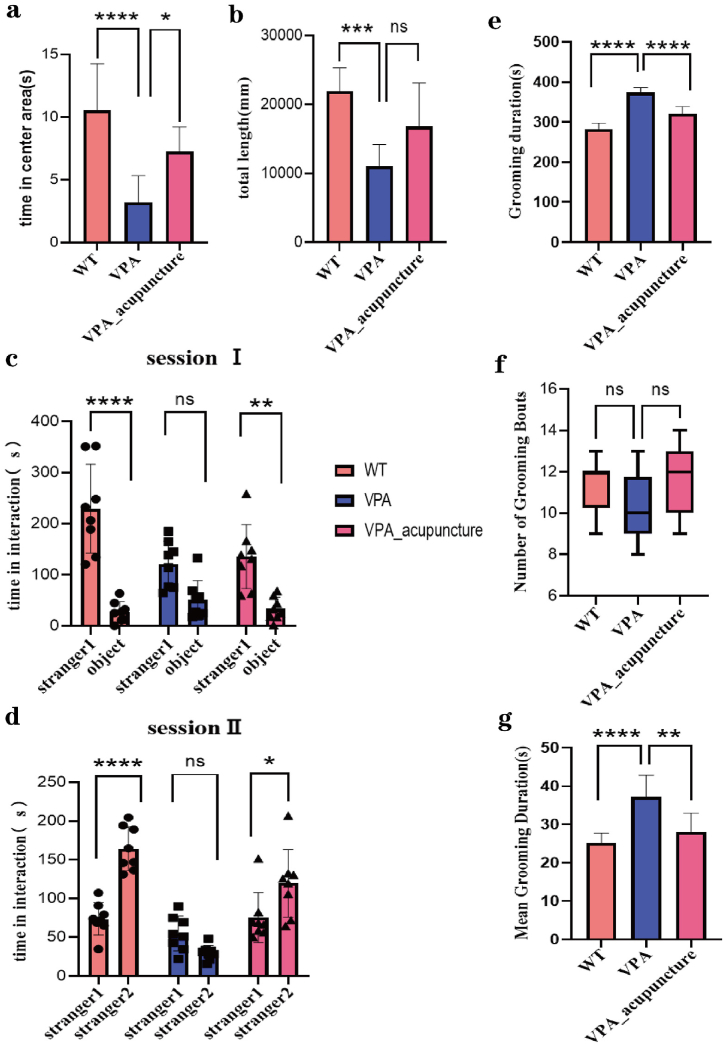
5.1. Behavioral results analysis (Fig. 2)
Fig. 2.
Results of behavioral test.
Notes: (a) the central areal exploration time of three groups of rats in Open Field Test; (b) the total exercise distance of three groups of rats in Open Field Test; (c) the contact time between test rats and the stranger rat 1 or the object in Three-chamber social test; (d) the contact time between test rats and the stranger rat 1 or stranger rat 2 in Three-chamber social test; (e) the grooming duration of three groups of rats in Self-grooming test; (f) the number of grooming times of three groups of rats in Self-grooming test; (g) the mean grooming duration of three groups of rats in Self-grooming test; *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001; ns p > 0.05.
5.1.1. Effects of acupuncture on anxiety behavior and space exploration behavior of autism rat model
There were significant differences in the central areal exploration time among the three groups of rats (F = 14.498, p < 0.001). The VPA group rats had less exploration time in the central area than the other two groups, and they preferred to move around and corners, showing a certain degree of anxiety behaviors (VPA vs WT, p < 0.001; VPA vs VPA_acupuncture, p = 0.022, Fig. 2a). The total exercise distance among the three groups of rats was statistically significant (F = 11.758, p < 0.001). The total exercise distance of the VPA group rats was shorter than that of the WT group, while there was no significant difference between VPA group and VPA_acupuncture group rats (VPA vs WT, p < 0.001; VPA vs VPA_acupuncture, p = 0.110, Fig. 2b). These results suggested that the VPA group rats have a certain degree of anxiety like behavior, and their spatial exploration and exercise abilities were reduced. Acupuncture therapy can partially alleviate anxiety like behavior and promote spatial exploration in VPA rat model, but it does not significantly improve their motor ability.
5.1.2. Effects of acupuncture on social behavior of autism rat model
In stage I (Fig. 2c), there were no statistical difference in the contact time among 3 groups rat and the object (F = 1.444, p = 0.258), while there was a statistically significant difference in the contact time among test rats of three groups and stranger rat 1 (F = 6.146, p = 0.008; WT group vs VPA group: p = 0.012; VPA group vs VPA_acupuncture group: p > 0.999). There were no statistical difference in the contact time between test rats of the VPA group and the stranger rat 1 or the object (p > 0.05), while the other two groups of rats had more contact time with stranger rat 1 than the object (stranger rat 1 vs object: VPA group, p = 0.093; WT group, p < 0.001; VPA_acupuncture group, p = 0.004).
In stage II (Fig. 2d), the object in stage I were replaced with a new stranger rat 2. The contact time were not significantly differentiated among the three groups of rats and the stranger rat 1 (F = 1.599, p = 0.226), while there was a statistically significant difference in the contact time among the three groups of rats and the stranger rat 2 (F = 39.890, p < 0.001; WT group vs VPA group: p < 0.001; VPA group vs VPA_acupuncture group: p < 0.001). The WT and VPA_acupuncture group rats had longer contact time with stranger rat 2 than with stranger rat 1, while there was no significant difference in VPA group (stranger rat 1 vs stranger rat 2: VPA group, p = 0.501; WT group, p < 0.001; VPA_acupuncture group, p = 0.036). The results indicated that the VPA group rats showed significant social interaction disorders and decreased social novelty, while acupuncture therapy can improve abnormal manifestations aforementioned to a certain extent.
5.1.3. Effects of acupuncture therapy on repetitive/stereotype behavior in autism rat model
There was no statistically significant difference in the number of grooming times observed among the three groups of experimental rats (F = 2.985, p = 0.225, Fig. 2f). While the grooming time and average grooming time of VPA group rats were longer than those in the WT and the VPA_acupuncture group (grooming time: F = 71.381, p < 0.001, VPA group vs WT group: p < 0.001, VPA group vs VPA_acupuncture: p < 0.001, Fig. 2e; average grooming time: F = 13.517, p < 0.001, VPA group vs WT group: p < 0.001; VPA group vs VPA_acupuncture group: p = 0.004, Fig. 2g). The results showed that the VPA group rats exhibited typical repetitive/stereotypical behaviors, such as longer grooming time. Furthermore, acupuncture therapy improved such behavioral abnormality of autism rat model.
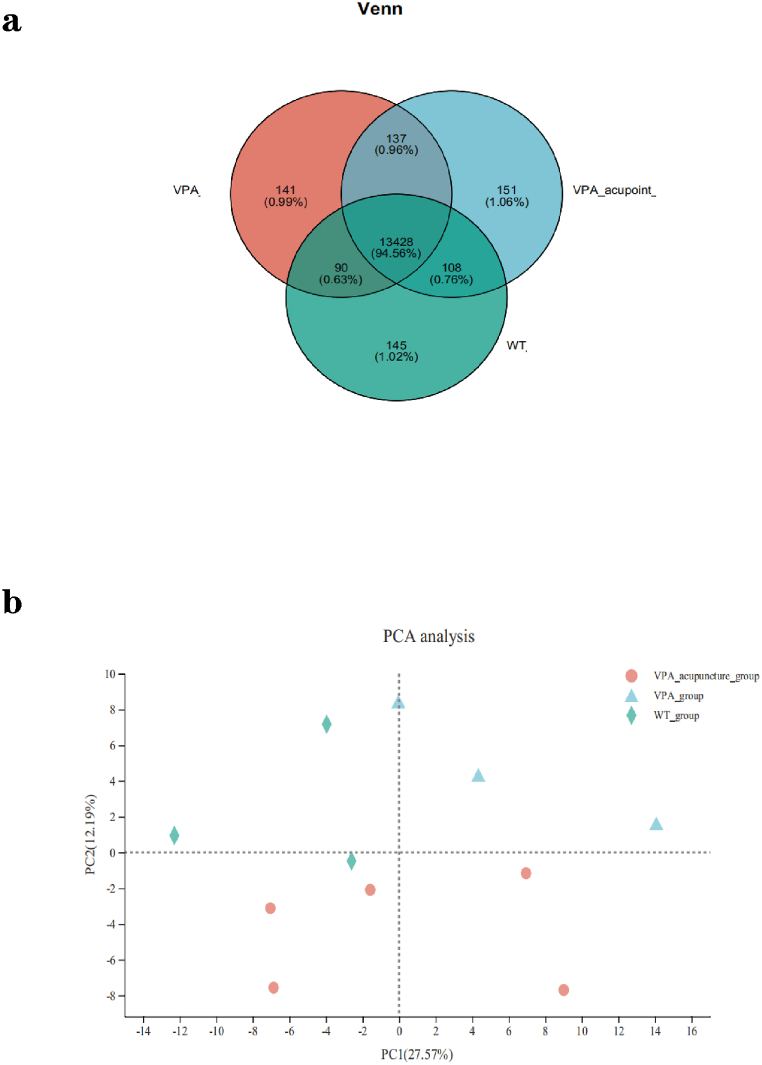
5.2. RNA-seq
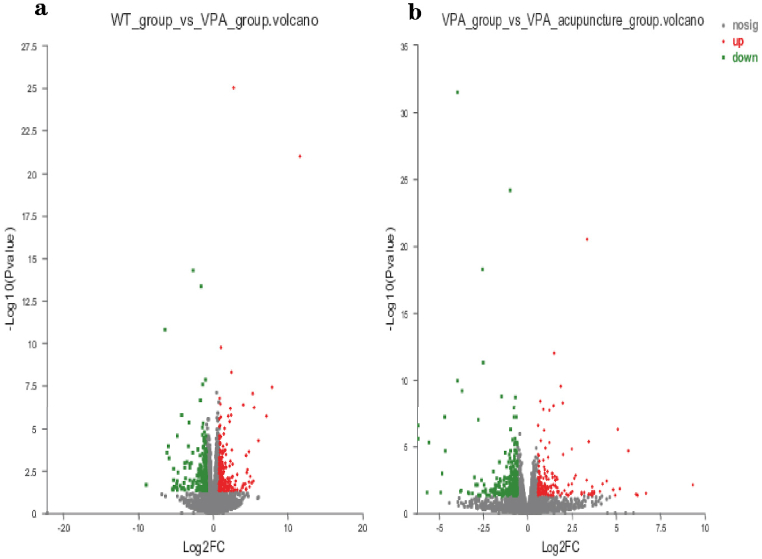
RNA-seq was performed on the PFC of three groups of rats after behavioral tests, and a total of 14242 genes were detected. The Venn diagram was used to represent the number of co-expressed/specifically expressed genes in each group of rats (Fig. 3a). According to the results of principal components analysis (PCA), the outliers in each group were removed (Fig. 3b). Groups were as follows: WT group (n = 3), VPA group (n = 3), VPA_acupuncture group (n = 5). The differentially expressed genes between groups are shown in the figure, with red dots representing up regulated genes and green dots representing down regulated genes, with p < 0.05 (Fig. 4a and b).
Fig. 3.
Gene expression analysis.
Notes: (a) Venn diagram; (b) PCA analysis.
Fig. 4.
volcano plot
Notes: (a) Genes differentially expressed between WT and VPA groups; (b) Genes differentially expressed between VPA group and VPA_acupuncture group (red indicates up-regulated genes, blue indicates down-regulated genes).
5.3. GO and KEGG analysis
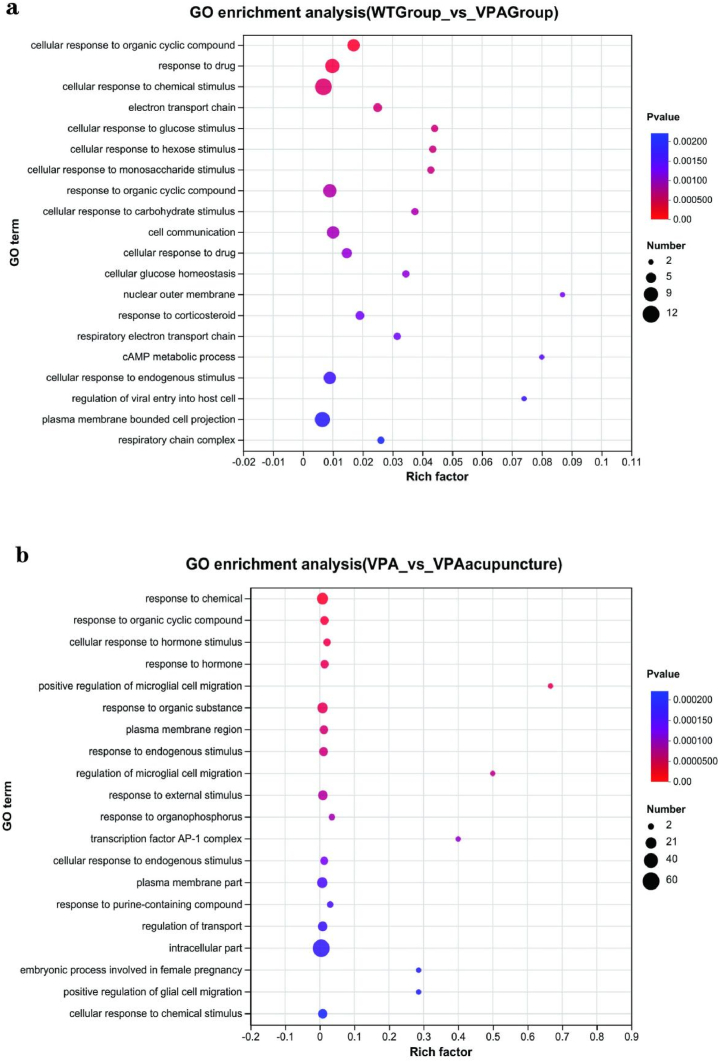
GO analysis was performed by Goatools [36] and KEGG analysis was performed by KOBAS [37].
Molecular function, cellular composition and biological processes are the three major branches of GO analysis. The DEGs in rats between VPA group and WT group were mainly enriched in biological processes (e.g. drug response, cell response to chemical stimuli, cell response to organic cyclic compounds) and cell components (e.g. electron transfer chains, cell response to chemical stimuli) (Fig. 5a). Between VPA and VPA_acupuncture group, the DEGs were mainly enriched in biological processes (e.g. cell response to hormonal stimulation, response to organic circulation compounds, response to chemical substances) and cell components (e.g. plasma membrane area, transcription factor AP-1 complex) (Fig. 5b).
Fig. 5.
GO enrichment analysis.
Notes: (a) GO enrichment analysis of differentially expressed genes between WT and VPA group. (b) GO enrichment analysis of differentially expressed genes between VPA and VPA_acupuncture group. The vertical axis represents GO Term, and the horizontal axis represents Rich factor.
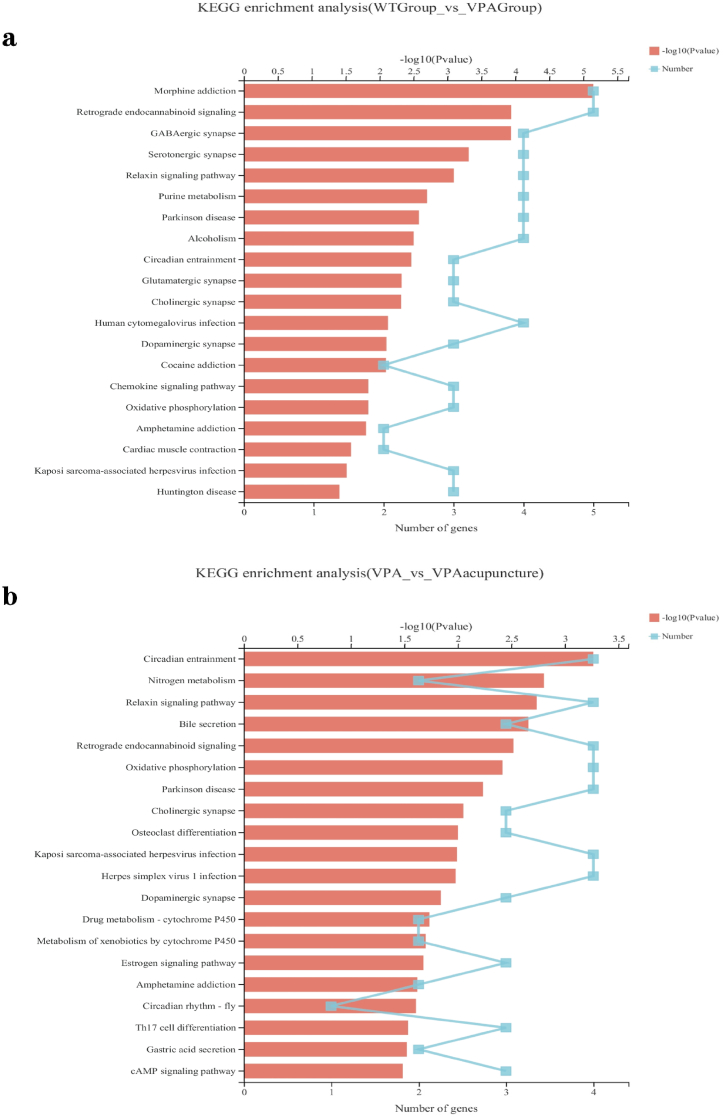
KEGG enrichment analysis ranked the top 20 most important pathways of DEGs between WT and VPA groups (Fig. 6a). For instance, drug addiction (e.g. morphine, cocaine, amphetamines, etc.), GABAergic synapses, glutamate ergic synapses, serotonin ergic synapses, dopamine ergic synapses, cholinergic synapses, diurnal entrainment, retrograde endogenous cannabinoid signaling, etc.
Fig. 6.
KEGG enrichment analysis.
Notes: (a) KWGG enrichment analysis of differentially expressed genes between WT and VPA group. (b) KEGG enrichment analysis of differentially expressed genes between VPA and VPA_acupuncture group. The vertical axis represents the KEGG pathway, and the horizontal axis represents the significance level of enrichment.
Similarly, DEGs were enriched in circadian rhythm entrainment, circadian rhythm, dopaminergic synapse, cholinergic synapse, retrograde endogenous cannabinoid signal, estrogen signal pathway and amphetamine addiction between VPA group and VPA_acupuncture group (Fig. 6b). In addition, the differential genes between the two groups of rats are also enriched in immune related pathways, Th17 cell differentiation pathway.
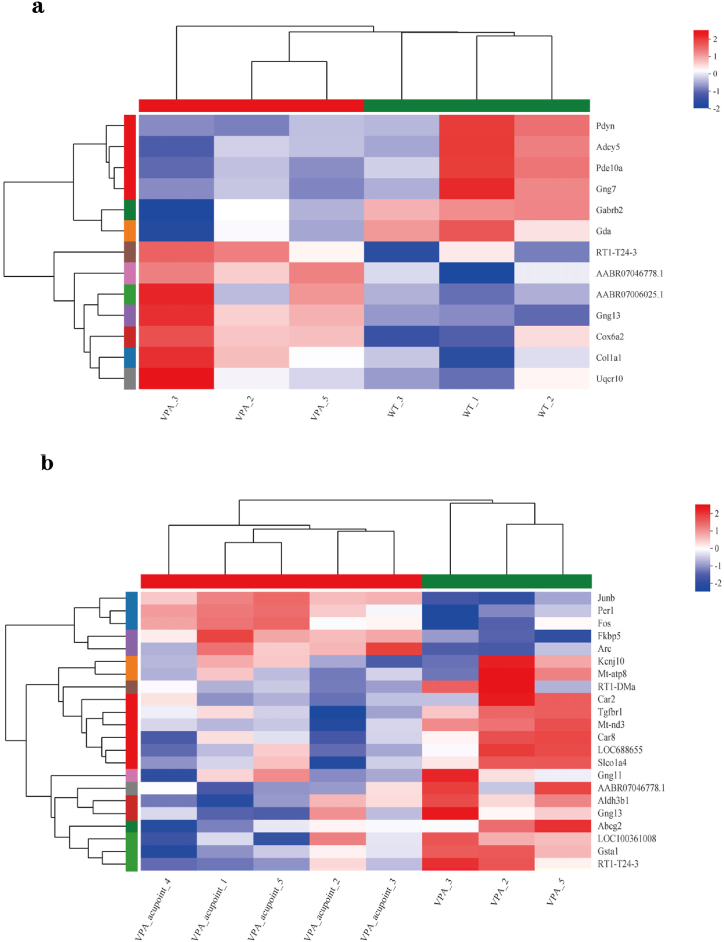
5.4. Cluster analysis
According to cluster analysis, there were 13 genes with significant expression differences between the WT and VPA groups, of which Pdyn, Adcy5, and Gabrb2 were down-regulated in the VPA group. It was shown that these genes were closely related to synaptic function and plasticity (Fig. 7a).
Fig. 7.
Cluster analysis.
Notes: (a) Cluster analysis of differentially expressed genes between WT and VPA group. (b) Cluster analysis of differentially expressed genes between VPA and VPA_acupuncture group. Each column in the graph represents a sample, and each row represents a gene. The colors in the graph represent the standardized expression levels of the gene in each sample, with red representing a higher expression level of the gene in the sample and blue representing a lower expression level.
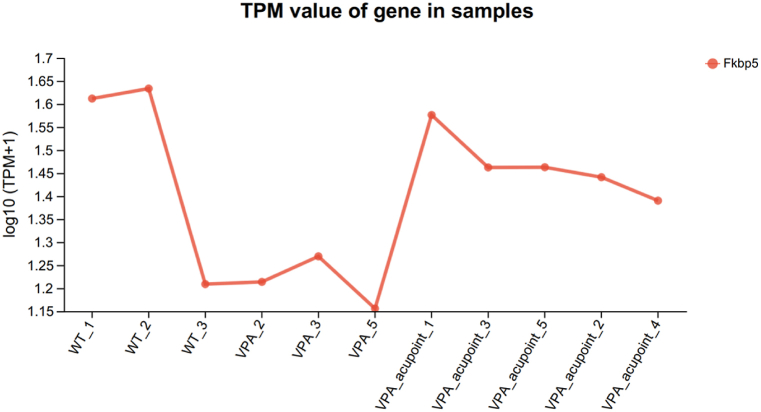
There were significant differences in the expression of 22 genes between the VPA group and VPA_acupuncture group. Among them, the expression of Arc and Per1 genes were up-regulated in the VPA_acupuncture group. Arc gene was the main regulator of synaptic plasticity, and Per1 gene played a key role in regulating the circadian rhythms (Fig. 7b). The result also suggested that the expression of Fkbp5 gene in VPA-acupuncture group was higher than that in the VPA group. Further analysis of the expression level of Fkbp5 gene of 3 groups of rats suggested that the gene was low expressed in the VPA group and high expressed in the other two groups (Fig. 8).
Fig. 8.
The expression level of Fkbp5 gene.
6. Transmission electron microscope
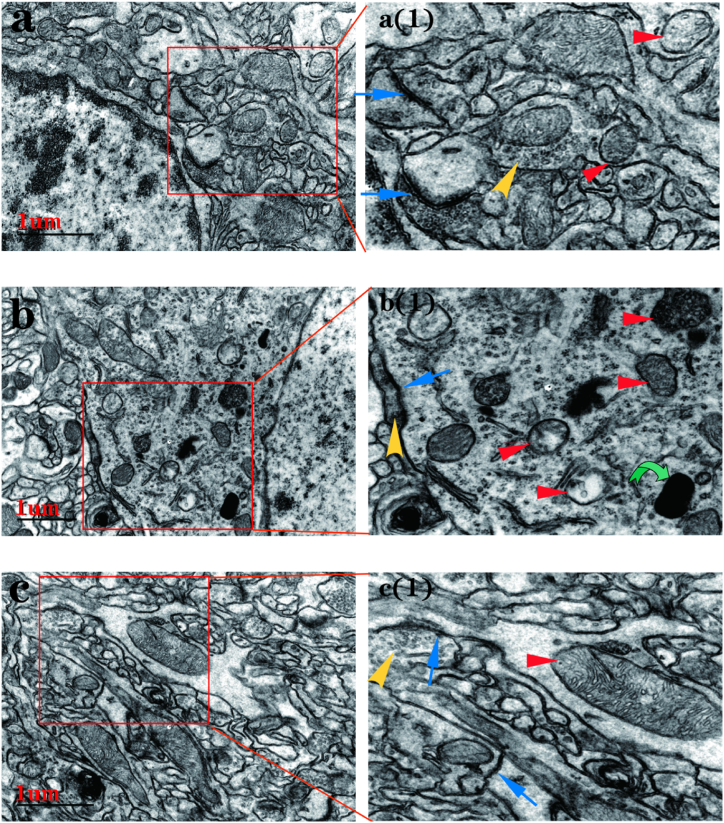
Under transmission electron microscopic, rats in the VPA group showed several lysosomes in the PFC neuronal cells, abnormal mitochondrial structure (disappeared or blurred mitochondrial cristae, and even vacuolization), and fewer synapses and synaptic vesicles (Fig. 9b). Conversely, rats in the WT and VPA acupuncture groups had normal mitochondrial structure and more synapses and synaptic vesicles (Fig. 9a and c).
Fig. 9.
Transmission electron microscopy image of PFC neurons.
Note: (a) wild type group; a (1), local enlarged image of a; (b) VPA group; b (1), local enlarged image of b; (c) VPA_acupuncture group; c (1),local enlarged image of c. Blue arrow represents synaptic structure, yellow arrow represents synaptic vesicle, red arrow represents Mitochondria and green arrow represents lysosome.
7. Discussion
Through the experiment, it can be found that the VPA group rats showed an increase in anxiety repetitive stereotypical behaviors, a decrease in spatial exploration ability and motor level, the presence of social interaction disorders, and a decrease in social novelty preferences. This is likely due to the fact that autism is a neurodevelopmental disorder with decreased neuroexcitability leading to a decreased desire to socialise and explore novelty. And anxiety like abnormal behaviors as well as repetitive stereotypes increase. The intraperitoneal injection of pregnant rats resulted in the appearance of autism-like symptoms in their offsprin, and these abnormal behaviors improved after acupuncture intervention, which is consistent with literature research [[38], [39], [40], [41]].
Several studies have suggested that acupuncture treatment can improve the behavior abnormalities of autism rat model by improving the function of PFC [38]. We speculated that prefrontal function plays a key role in the pathogenesis of ASD and acupuncture treatment of ASD core symptoms. Therefore, we further conducted RNA sequencing and transmission electron microscope observation of neurons in the prefrontal cortex to reveal its potential mechanism.
RNA-seq analysis unveiled that changes in synaptic function and synaptic plasticity of multiple neural synapses played a key role in the pathogenesis of ASD, and acupuncture intervention may alleviate the main symptoms of ASD by ameliorating these changes. Compared the WT and VPA groups, there were significant differences in genes expressio enriched in multiple types of neuronal synapses (GABAergic synapses, glutamate ergic synapses, serotonin ergic synapses, dopamine ergic synapses, cholinergic synapses), drug addictions (morphine, cocaine, amphetamines), and other KEGG pathways. And cluster analysis implicated that the expression of Pdyn, Adcy5, and Gabrb2 genes in VPA group rats was down-regulated compared to the WT group. The disorder of glutamate and/or GABAergic neurotransmitters and dopamine neurotransmitters were believed to be crucial for the pathogenesis of ASD and the balance of synaptic excitation/inhibition [42,43]. Drug addiction is a neuroplastic disorder involving the brain's reward and cognitive systems [44]. The Pdyn gene produces opioid peptides, which are involved in processing rewards, cognitive processes and emotional control [45,46], and the opioid receptor system also regulates glutamate neurotransmission and affects synaptic plasticity [47,48]. Adcy5, an effector of many neurotransmitter receptors, is responsible for regulating various physiological functions [49]. Gabrb2 is ɑ receptors of γ-aminobutyric acid (GABA), which have two main receptors (GABAaR and NMDARs) in the pre- and postsynaptic areas, plays a crucial role in regulating synaptic function [50,51]. Compared the VPA and VPA_acupuncture group, there were significant differences in the expression of genes enriched in KEGG pathways such as multifunctional synapses (dopaminergic synapse and cholinergic synapse) and drug addiction (amphetamine). Cluster analysis suggests that the Arc gene was up-regulated after acupuncture treatment. Arc proteins are known to be indispensable components of stable long-term enhancement, long-term inhibition and synaptic formation, so the Arc genes are considered to be major regulators of long-term synaptic plasticity [52].
Our findings also indicated that the changes of neurological signaling may play a role in the pathogenesis of ASD, and that acupuncture intervention may improve ASD main symptoms through the improvement of neural signaling pathways. Compared the WT group and VPA group, significantly differentially expressed genes were enriched in retrograde endogenous cannabinoid signaling and other pathways.
Compared the VPA group and VPA_acupuncture group, the significantly differentially expressed genes were enriched in estrogen signaling pathway and retrograde endogenous cannabinoid signaling pathway. Endogenous cannabinoid is a retrograde messenger, and retrograde endogenous cannabinoid signalling is a form of neural signal regulation in the brain [53,54]. Estrogen signaling also plays an important role in the neurotransmitter system, and its signal interruption can lead to various psychiatric disorders such as ASD, ADHD, etc. [55].
According to the results of KEGG and cluster analysis, we also found differences in immune inflammation among the 3 groups of rats. including differentially expressed genes in the VPA group and VPA acupuncture group were enriched on the Th17 cell differentiation pathway, and the differential expression levels of Fkbp5 gene among the 3 groups of rats. The protein encoded by Fkbp5 gene belongs to the immunophilin protein family. The protein is a cis-trans prolyl isomerase that binds to the immunosuppressive agents and affects protein biosynthesis, cell proliferation and survival of immune cells to inhibit immune-inflammatory responses [56,57].
In addition, we noted that changes in circadian rhythm pathways in the comparison between the WT group and VPA group, as well as the VPA group and VPA_acupuncture group. The disorder of circadian rhythm is believed to be related to ASD, emotional and affective disorders [58]. Clinical studies have shown that sleep–wake cycle disorder is the most common complication of ASD, affecting approximately 80 % of children with autism [59]. Further studies have found circadian rhythm is positively correlated with the expression level of Per1 gene [60]. Therefore, sleep–wake cycle disorder, as the most common comorbidity of ASD, should receive more attention in the clinical diagnosis and treatment of ASD. Acupuncture treatment may improve ASD symptoms by changing the expression of genes related to circadian rhythm.
Under the transmission electron microscopy, several lysosomes were visible in the prefrontal neurons of rats in the VPA group, whose main function was to phagocytose and digest organelles with abnormal functions. In addition, the prefrontal neurons of rats in the VPA group had abnormal mitochondrial structure, which was mainly manifested as atrophy of mitochondrial membrane, blurring or even disappearance of mitochondrial cristae and the appearance of vacuolization. Mitochondria not only provide energy but also affect neuronal signal transduction. On the contrary, rats in the WT and VPA_acupuncture groups had more synaptic vesicles and synapses and their mitochondrial structure was normal, which was basically consistent with our pre-experimental prediction.
Acupuncture intervention is widely used in the clinical treatment of children with ASD, but there are different opinions about its mechanism of action. For example, scalp acupuncture may reduce symptoms of ASD by promoting local cerebral blood flow, improving brain functional activities and neurochemical favorable changes, reducing oxidative stress and inflammation, and improving synaptic function and synaptic plasticity [61,62].
According to our findings, we considered the neuronal synaptic dysfunction, the changes in synaptic plasticity and signal transduction, and overactivation of immune-inflammatory responses may play a role in the pathogenesis of ASD. Acupuncture therapy can improve the ultrastructure of prefrontal neurons in ASD model rats, improve neuronal synaptic function and plasticity, promote neuronal signal transduction, regulate immune inflammatory response, and even ameliorate the abnormal behaviors of ASD model rats by improving comorbidity.
This study found some common changes among the wild group, VPA group and VPA-acupuncture group, including behavioral changes, ultrastructure changes of PFC, and changes in neural synapses, neurotransmitters, and immune-inflammatory responses detected by RNA sequencing. The results indicated that there are structural and functional abnormalities in the nervous system of the autism rat model, which leads to typical behavioral abnormalities. Acupuncture can improve their behavior by ameliorating the above abnormal changes. However, there are some shortcomings in this study. For example, how do various variations we found in the nervous system, such as synaptic structure and function, neuronal signal transduction, and immune-inflammatory responses, interact in the autism rat model? It is equally important to clarify these changes that occur after acupuncture. It is unclear what roles the mitochondrial changes (quantity, structure, and function) play in this. Follow-up studies are needed to resolve these questions. This is necessary to explore etiology, treatment, and especially drug targets and to better prevent and treat autism.
8. Conclusion
Acupuncture therapy can effectively improve the abnormal behavior of ASD rat model and the ultrastructure of prefrontal cortex neurons, which may work by improving their abnormal synaptic function and synaptic plasticity, promoting their neuronal signal transduction, and regulating immune-inflammatory responses.
This paper further revealed the possible mechanism of acupuncture therapy for ASD through animal behavior, RNA sequencing analysis, and observation of neuronal ultrastructure under electron microscopy, which provided a theoretical basis for the clinical treatment of ASD.
Ethics statement
All animal experiments were conducted in accordance with the protocol approved by the Animal Research Committee of the Fujian Medical University (FJMU IACUC permit number: 2018-088).
Funding
Joint Funds for the innovation of science and Technology, Fujian province (Grant number: 2020Y9145), Fujian Provincial Natural Science Foundation of China (Grant number: 2022J01363) and Fujian Provincial Traditional Chinese Medicine Science and Technology Program (Grant number: 2021zyjc26) supported this work.
Data availability statement
The related data available within the article or its supplementary materials. And the RNA sequencing data are available and can be accessed through the following link: https://www.ncbi.nlm.nih.gov/bioproject/PRJNA1131377.
CRediT authorship contribution statement
Sijie Chen: Writing – review & editing, Writing – original draft, Software, Methodology, Data curation. Juan Wang: Writing – original draft, Software. Xiaofang Chen: Resources, Methodology. Yingying Zhang: Software, Data curation. Yu Hong: Resources, Data curation. Wanyu Zhuang: Resources, Project administration. Xinxin Huang: Validation, Formal analysis. Jie Kang: Methodology, Data curation. Ping Ou: Visualization, Funding acquisition. Longsheng Huang: Writing – review & editing, Visualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e37130.
Contributor Information
Ping Ou, Email: fyxwktz2021@fjmu.edu.cn.
Longsheng Huang, Email: longsheng126@fjmu.edu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Kalantarian H., Jedoui K., Dunlap K., et al. The performance of emotion classifiers for hildren with parent-reported autism: quantitative feasibility study. JMIR Ment Health. 2020;7(4) doi: 10.2196/13174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Geschwind D.H. Oxytocin for autism spectrum disorder - down, but not out. NEW ENGL J MED. 2021;385(16):1524–1525. doi: 10.1056/NEJMe2110158. [DOI] [PubMed] [Google Scholar]
- 3.Giovagnoli G., Siracusano M., Postorino V., et al. Drug treatments for core symptoms of autism spectrum disorder: unmet needs and future directions. J. Pediatr. Neurol. 2017;15(3):134–142. doi: 10.1055/s-0037-1602823. [DOI] [Google Scholar]
- 4.Wong V.C. Use of complementary and alternative medicine (CAM) in autism spectrum disorder (ASD): comparison of Chinese and western culture (Part A) J. Autism Dev. Disord. 2009;39(3):454–463. doi: 10.1007/s10803-008-0644-9. [DOI] [PubMed] [Google Scholar]
- 5.Sun Q., Yu H., Shang Y., et al. Correlation analysis of chaige qinlian decoction and acupuncture combined intervention on prognosis of children with pneumonia. Journal of healthcare engineering. 2021;2021 doi: 10.1155/2021/8229251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang J., Shi W., Khiati D., et al. Acupuncture treatment on the motor area of the scalp for motor dysfunction in children with cerebral palsy: study protocol for a multicenter randomized controlled trial. Trials. 2020;21(1):29. doi: 10.1186/s13063-019-3986-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rong P.J., Wang Y., Xu N.G. Brain science promotes the development of acupuncture in treating brain diseases. Zhen Ci Yan Jiu. 2019;44(12):859–862. doi: 10.13702/j.1000-0607.190691. [DOI] [PubMed] [Google Scholar]
- 8.Yang Y., Hu S., Lin H., et al. Electroacupuncture at GV24 and bilateral GB13 improves cognitive ability via influences the levels of Aβ, p-tau (s396) and p-tau (s404) in the hippocampus of Alzheimer's disease model rats. Neuroreport. 2020;31(15):1072–1083. doi: 10.1097/WNR.0000000000001518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wattanathorn J., Sutalangka C. Laser acupuncture at HT7 Acupoint improves cognitive deficit, neuronal loss, oxidative stress, and functions of cholinergic and dopaminergic systems in animal model of Parkinson's disease. Evid Based Complement Alternat Med. 2014;2014(8) doi: 10.1155/2014/937601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yau C.H., Ip C.L., Chau Y.Y. The therapeutic effect of scalp acupuncture on natal autism and regressive autism. Chin. Med. 2018;13:30. doi: 10.1186/s13020-018-0189-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang C., Hao Z., Zhang L.L., et al. Efficacy and safety of acupuncture in children: an overview of systematic reviews. Pediatr. Res. 2015;78(2):112–119. doi: 10.1038/pr.2015.91. [DOI] [PubMed] [Google Scholar]
- 12.Chomiak T., Turner N., Hu B. What we have learned about Autism Spectrum Disorder from valproic acid. Pathol. Res. Int. 2013;2013 doi: 10.1155/2013/712758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roullet F.I., Lai J.K., Foster J.A. In utero exposure to valproic acid and autism—a current review of clinical and animal studies. Neurotoxicol. Teratol. 2013;36:47–56. doi: 10.1016/j.ntt.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 14.Wang X., Ding R., Song Y., et al. Transcutaneous electrical Acupoint stimulation in early life changes synaptic plasticity and improves symptoms in a valproic acid-induced rat model of autism. Neural Plast. 2020;2020 doi: 10.1155/2020/8832694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu H., Sun X., Zou W., et al. Scalp acupuncture attenuates neurological deficits in a rat model of hemorrhagic stroke. Compl. Ther. Med. 2017;32:85–90. doi: 10.1016/j.ctim.2017.03.014. [DOI] [PubMed] [Google Scholar]
- 16..
- 17.Qiuping L., Pan P., Zhenzhen L., et al. Acupuncture regulates the Th17/Treg balance and improves cognitive deficits in a rat model of vascular dementia. Heliyon. 2023;9(2) doi: 10.1016/j.heliyon.2023.e13346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu Z.G., Huang Y.J., Wang T.Y., et al. Effect of acupuncture on neuroinflammation in animal models of Alzheimer's disease: a preclinical systematic review and meta-analysis. Front. Aging Neurosci. 2023;15 doi: 10.3389/fnagi.2023.1110087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li H., Liu Y., Lin L.T., et al. Acupuncture reversed hippocampal mitochondrial dysfunction in vascular dementia rats. Neurochem. Int. 2015;92:35–42. doi: 10.1016/j.neuint.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 20.Sun Z.L., Liu J., Guo W., Jiang T., Ma C., Li W.B., Tang Y.L., Ling S.H. Serum brain-derived neurotrophic factor levels associate with cognitive improvement in patients with schizophrenia treated with electroacupuncture. Psychiatr. Res. 2016;244:370–375. doi: 10.1016/j.psychres.2016.07.040. [DOI] [PubMed] [Google Scholar]
- 21.Chen S., Huang L., Liu G., et al. Acupuncture ameliorated behavioral abnormalities in the autism rat model via pathways for hippocampal serotonin. Neuropsychiatric Dis. Treat. 2023;19:951–972. doi: 10.2147/NDT.S398321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cui Shaoyang, et al. Cerebral responses to acupuncture at GV24 and bilateral GB13 in rat models of Alzheimer's disease. Behav. Neurol. 2018;2018 doi: 10.1155/2018/8740284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singer H.S., Morris C., Gause C., et al. Prenatal exposure to antibodies from mothers of children with autism produces neurobehavioral alterations: a pregnant dam mouse model. J. Neuroimmunol. 2009;211(1–2):39–48. doi: 10.1016/j.jneuroim.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 24.Chez M.G., Dowling T., Patel P.B., et al. Elevation of tumor necrosis factor-alpha in cerebrospinal fluid of autistic children. Pediatr. Neurol. 2007;36(6):361–365. doi: 10.1016/j.pediatrneurol.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 25.Li Y.Y., Yin C.Y., Li X.J., et al. Electroacupuncture alleviates paclitaxel-induced peripheral neuropathic pain in rats via suppressing TLR4 signaling and TRPV1 upregulation in sensory neurons. Int. J. Mol. Sci. 2019;20(23):5917. doi: 10.3390/ijms20235917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou F., Jiang H., Kong N., et al. Electroacupuncture attenuated anxiety and depression-like behavior via inhibition of hippocampal inffammatory response and metabolic disorders in TNBSinduced IBD rats. Oxid. Med. Cell. Longev. 2022;2022 doi: 10.1155/2022/8295580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jacot-Descombes S., Keshav N.U., Dickstein D.L., et al. Altered synaptic ultrastructure in the prefrontal cortex of Shank3-deficient rats. Mol. Autism. 2020;11(1):89. doi: 10.1186/s13229-020-00393-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Teo S., Salinas P.C. Wnt-frizzled signaling regulates activity-mediated synapse formation. Front. Mol. Neurosci. 2021;14 doi: 10.3389/fnmol.2021.683035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Z., Cao M., Chang C.W., et al. Autism-associated chromatin regulator brg1/SmarcA4 is required for synapse development and myocyte enhancer factor 2-mediated synapse remodeling. Mol. Cell Biol. 2015;36(1):70–83. doi: 10.1128/MCB.00534-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang L., Ge P., Kang J., et al. Scalp acupuncture improves disordered behavior in the valproic acid-induced rat model of autism. World J. Acupuncture-Moxibustion. 2023;33(3):252–261. [Google Scholar]
- 31.Schneider T., Przewłocki R. Behavioral alterations in rats prenatally exposed to valproic acid: animal model of autism. NEUROPSYCHOPHARMACOL. 2005;30(1):80–89. doi: 10.1038/sj.npp.1300518. [DOI] [PubMed] [Google Scholar]
- 32.Li B., Dewey C.N. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinf. 2011;12:323. doi: 10.1186/1471-2105-12-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Love M.I., Huber W., Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang L., Feng Z., Wang X., et al. DEGseq: an R package for identifying differentially expressed genes from RNA-seq data. Bioinformatics. 2009;26(1):136–138. doi: 10.1093/bioinformatics/btp612. [DOI] [PubMed] [Google Scholar]
- 35.Robinson M.D., McCarthy D.J., Smyth G.K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26(1):139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klopfenstein D.V., Zhang L., Pedersen B.S., et al. GOATOOLS: a Python library for Gene Ontology analyses. Sci. Rep. 2018;8(1) doi: 10.1038/s41598-018-28948-z. https://github.com/tanghaibao/Goatools [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xie C., Mao X., Huang J., et al. Kobas 2.0: a web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res. 2011;39:W316–W322. doi: 10.1093/nar/gkr483. http://kobas.cbi.pku.edu.cn/home.do [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hou Q., Wang Y., Li Y., et al. A developmental study of abnormal behaviors and altered GABAergic signaling in the VPA-treated rat model of autism. Front. Behav. Neurosci. 2018;12:182. doi: 10.3389/fnbeh.2018.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nicolini C., Fahnestock M. The valproic acid-induced rodent model of autism. Exp. Neurol. 2018;299(Pt A):217–227. doi: 10.1016/j.expneurol.2017.04.017. [DOI] [PubMed] [Google Scholar]
- 40.Zhao Y.H., Fu H.G., Cheng H., et al. Electroacupuncture at Zusanli ameliorates the autistic-like behaviors of rats through activating the Nrf2-mediated antioxidant responses. Gene. 2022;828 doi: 10.1016/j.gene.2022.146440. [DOI] [PubMed] [Google Scholar]
- 41.Cheng K.J. Neurobiological mechanisms of acupuncture for some common illnesses: a clinician's perspective. Acupunct Meridian Stud. 2014;7(3):105–114. doi: 10.1016/j.jams.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 42.Lenart J., Augustyniak J., Lazarewicz J.W., et al. Altered expression of glutamatergic and GABAergic genes in the valproic acid-induced rat model of autism: a screening test. Toxicology. 2020;440 doi: 10.1016/j.tox.2020.152500. [DOI] [PubMed] [Google Scholar]
- 43.Gao R., Penzes P. Common mechanisms of excitatory and inhibitory imbalance in schizophrenia and autism spectrum disorders. Curr. Mol. Med. 2015;15(2):146–167. doi: 10.2174/1566524015666150303003028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bali P., Kenny P.J. MicroRNAs and drug addiction. Front. Genet. 2013;4:43. doi: 10.3389/fgene.2013.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bazov I., Sarkisyan D., Kononenko O., et al. Neuronal expression of opioid gene is controlled by dual epigenetic and transcriptional mechanism in human brain. Cerebr. Cortex. 2018;28(9):3129–3142. doi: 10.1093/cercor/bhx181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chavkin C. Dynorphin--still an extraordinarily potent opioid peptide. Mol. Pharmacol. 2013;83(4):729–736. doi: 10.1124/mol.112.083337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ménard C., Herzog H., Schwarzer C., et al. Possible role of dynorphins in Alzheimer's disease and age-related cognitive deficits. Neurodegener. Dis. 2014;13(2–3):82–85. doi: 10.1159/000353848. [DOI] [PubMed] [Google Scholar]
- 48.Tejeda H.A., Shippenberg T.S., Henriksson R. The dynorphin/κ-opioid receptor system and its role in psychiatric disorders. Cell. Mol. Life Sci. 2012;69(6):857–896. doi: 10.1007/s00018-011-0844-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim H., Lee Y., Park J.Y., et al. Loss of adenylyl cyclase type-5 in the dorsal striatum produces autistic-like behaviors. Mol. Neurobiol. 2017;54(10):7994–8008. doi: 10.1007/s12035-016-0256-x. [DOI] [PubMed] [Google Scholar]
- 50.Kaindl A.M., Koppelstaetter A., Nebrich G., et al. Brief alteration of NMDA or GABAA receptor-mediated neurotransmission has long term effects on the developing cerebral cortex. Mol. Cell. Proteomics. 2008;7(12):2293–2310. doi: 10.1074/mcp.M800030-MCP200. [DOI] [PubMed] [Google Scholar]
- 51.Srivastava S., Cohen J., Pevsner J., et al. A novel variant in GABRB2 associated with intellectual disability and epilepsy. Am. J. Med. Genet. 2014;164A(11):2914–2921. doi: 10.1002/ajmg.a.36714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wilkerson J.R., Albanesi J.P., Huber K.M. Roles for Arc in metabotropic glutamate receptor-dependent LTD and synapse elimination: implications in health and disease. Semin. Cell Dev. Biol. 2018;77:51–62. doi: 10.1016/j.semcdb.2017.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hashimotodani Y., Ohno-Shosaku T., Kano M. Presynaptic monoacylglycerol lipase activity determines basal endocannabinoid tone and terminates retrograde endocannabinoid signaling in the hippocampus. J. Neurosci. 2007;27(5):1211–1219. doi: 10.1523/JNEUROSCI.4159-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhu P.J., Lovinger D.M. Retrograde endocannabinoid signaling in a postsynaptic neuron/synaptic bouton preparation from basolateral amygdala. J. Neurosci. 2005;25(26):6199–6207. doi: 10.1523/JNEUROSCI.1148-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hwang W.J., Lee T.Y., Kim N.S., et al. The role of estrogen receptors and their signaling across psychiatric disorders. Int. J. Mol. Sci. 2020;22(1):373. doi: 10.3390/ijms22010373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kang J.I., Kim T.Y., Choi J.H., et al. Allele-specific DNA methylation level of FKBP5 is associated with post-traumatic stress disorder. PSYCHONEUROENDOCRINO. 2018;103:1–7. doi: 10.1016/j.psyneuen.2018.12.226. [DOI] [PubMed] [Google Scholar]
- 57.Liu N., Li R., Cao J., et al. The inhibition of FKBP5 protects β-cell survival under inflammation stress via AKT/FOXO1 signaling. Cell Death Dis. 2023;9(1):247. doi: 10.1038/s41420-023-01506-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim J., Jang S., Choe H.K., et al. Implications of circadian rhythm in dopamine and mood regulation. Mol. Cell. 2017;40(7):450–456. doi: 10.14348/molcells.2017.0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ferraro S., de Zavalia N., Belforte N., et al. In utero exposure to valproic-acid alters circadian organisation and clock-gene expression: implications for autism spectrum disorders. Front. Behav. Neurosci. 2021;15 doi: 10.3389/fnbeh.2021.711549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Numano R., Yamazaki S., Umeda N., et al. Constitutive expression of the Period1 gene impairs behavioral and molecular circadian rhythms. Proc Natl Acad Sci U S A. 2006;103(10):3716–3721. doi: 10.1073/pnas.0600060103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Khongrum J., Wattanathorn J. Laser acupuncture at HT7 improves the cerebellar disorders in valproic acid-rat model of autism. J Acupunct Meridian Stud. 2017;10(4):231–239. doi: 10.1016/j.jams.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 62.Wang X., Ding R., Song Y., et al. Transcutaneous electrical Acupoint stimulation in early life changes synaptic plasticity and improves symptoms in a valproic acid-induced rat model of autism. Neural Plast. 2020;2020 doi: 10.1155/2020/8832694. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The related data available within the article or its supplementary materials. And the RNA sequencing data are available and can be accessed through the following link: https://www.ncbi.nlm.nih.gov/bioproject/PRJNA1131377.