Abstract
The majority of Kaposi's sarcoma-associated herpesvirus (KSHV)-infected cells identified in vivo contain latent KSHV, with lytic replication in only a few percent of cells, as is the case for the cells of Kaposi's sarcoma (KS) lesions. Factors that influence KSHV latent or lytic replication are not well defined. Because persons with KS are often immunosuppressed and susceptible to many infectious agents, including human cytomegalovirus (HCMV), we have investigated the potential for HCMV to influence the replication of KSHV. Important to this work was the construction of a recombinant KSHV, rKSHV.152, expressing the green fluorescent protein (GFP) and neo (conferring resistance to G418). The expression of GFP was a marker of KSHV infection in cells of both epithelial and endothelial origin. The rKSHV.152 virus was used to establish cells, including human fibroblasts (HF), containing only latent KSHV, as demonstrated by latency-associated nuclear antigen expression and Gardella gel analysis. HCMV infection of KSHV latently infected HF activated KSHV lytic replication with the production of infectious KSHV. Dual-color immunofluorescence detected both the KSHV lytic open reading frame 59 protein and the HCMV glycoprotein B in coinfected cells, and UV-inactivated HCMV did not activate the production of infectious KSHV-GFP. In addition, HCMV coinfection increased the production of KSHV from endothelial cells and activated lytic cycle gene expression in keratinocytes. These data demonstrate that HCMV can activate KSHV lytic replication and suggest that HCMV could influence KSHV pathogenesis.
Kaposi's sarcoma-associated herpesvirus (KSHV), or human herpesvirus 8 (HHV8), was first identified from a Kaposi's sarcoma (KS) lesion (11). Work from many laboratories has shown that KSHV is consistently found in all forms of KS (classical, endemic, posttransplant, and AIDS-related) and that KSHV infection is predictive of KS development, resulting in KSHV being considered the etiologic agent for KS (39).
KSHV is classified as a gamma-2 herpesvirus, and as with all herpesviruses, KSHV can exist in either a latent or a lytic state. In KS lesions latent virus predominates, with a low percentage of cells exhibiting lytic replication (53). Cell types identified as supporting lytic and/or latent gene expression include monocytes, endothelial/spindle cells of KS lesions, B cells, and epithelial cells (5, 7, 13, 15, 26, 54). It remains unresolved what the specific contributions of latent and lytic gene expression are to the diseases associated with KSHV, but it is considered that both have a role (38, 49, 56). KSHV genes with potential roles in cellular proliferation, immunomodulation, signal transduction, and transformation, including open reading frame (ORF) K1 and viral homologs of cellular genes such as v-cyc, v-Bcl, v-MIP-I, v-MIP-II, v-MIP-III, v-GCPR, and v-IRF, include latent and lytic expressed members (37, 47, 49). Factors affecting lytic or latent gene expression are not well understood, but they may be important to elucidating possible cofactors of KS.
While KSHV infection appears to be a prerequisite for the development of KS, other factors play an important role. Immunodeficiency is a significant contributing factor as the diseases associated with KSHV frequently manifest themselves in persons with human immunodeficiency virus (HIV) and in transplant recipients (3, 31). Besides the direct role the immune system may play in controlling KSHV and the proliferation of KS cells, it has been postulated that a dysfunctional immune system could contribute to the progression of KS by additional processes. One is that the alteration of cytokine profiles present with immune dysfunction could influence the biology of endothelial and KS tumor cells (17) or act by inducing KSHV lytic replication (33). A second is the failure of an impaired immune system to control infectious agents that may have an impact on KSHV or KS tumor cells.
The epidemiology of some forms of KS suggested an infectious etiology (4), which led to the consideration of a number of viruses as etiologic agents of KS before the discovery of KSHV, and subsequently as potential cofactors of the disease. Several viruses, such as HIV (20), human cytomegalovirus (HCMV [23]), HHV6 (28), papilloma (1), and BK (34), that are common in immunocompromised individuals have been considered for a role in KS. Although HIV is not found in KS cells, its potential as a cofactor in KS is suggested by reports of the Tat protein increasing the growth of KS cells in vitro (16) and activating KSHV lytic replication (25) and reports of HIV coinfection activating KSHV (58). HHV6 and HHV7 were identified in the monocyte population of KS lesions (29), which is one of the cell types indicated for lytic replication of KSHV (5), suggesting monocytes as a possible site for interaction between KSHV and HHV6 or -7.
HCMV is a common infectious agent, and active HCMV infection approaches 90% in seropositive AIDS patients and can range from 30 to 100% in posttransplant patients (9). HCMV is common in persons at risk for KS, it has been identified in KS lesions (14, 23), and active HCMV infection was reported to precede the onset of KS (50). The frequency of the association of HCMV and KS led to it being considered a possible etiologic agent of KS (14, 24), and although this was found not to be true (57), it remains possible that HCMV is an augmenting cofactor. Therefore, we have investigated possible interactions between HCMV and KSHV using a recombinant KSHV containing the green fluorescent protein (GFP) and the neo gene and have demonstrated that HCMV can reactivate KSHV from latency to productive lytic replication. This work indicates that HCMV can influence KSHV lytic gene expression and viral production and suggests that HCMV could impact diseases associated with KSHV.
MATERIALS AND METHODS
Cells.
Human fibroblasts (HF) were of human foreskin origin. T24, human bladder carcinoma, and DU145, human prostate carcinoma, cell lines were from the American Type Culture Collection. HF, T24, and DU145 were maintained in Dulbecco's modified Eagle's medium (DMEM; Gibco Laboratories) supplemented with 10% fetal bovine serum (FBS), 100 μg of streptomycin/ml, 100 U of penicillin/ml, and 2 mM l-glutamine in a humidified 5% CO2 37°C incubator. BCBL-1 (45) cells were obtained from the AIDS Research and Reference Reagent Program and were cultured in RPMI medium supplemented with 10% FBS, 0.1 mg of streptomycin, 100 U of penicillin, and 2 mM l-glutamine. Human umbilical vein endothelial cells (HUVEC) were the kind gift of Patricia Moeser and John Harlan and were grown in RPMI medium supplemented with 20% FBS, 50 μg of endothelial cell growth supplement (Clonetics, San Diego, Calif.)/ml, 100 μg of streptomycin/ml, 100 U of penicillin/ml, and 2 mM l-glutamine, plus nonessential amino acids (Gibco BRL) on plates coated with a gelatin. Keratinocytes were from Clonetics and were cultured in KGM-2 media from Clonetics.
Recombinant virus.
BCBL-1 cells were used as the source for KSHV. For the construction of recombinant virus, a 4.8-kb BamHI fragment (positions 81046 to 85820 of the published sequence [46]) was isolated from the cosmid Z8 (46) and cloned into pUC21 to create pQ131. At the DraIII site (position 83710 of the KSHV sequence) present in the BamHI fragment, after the ends were made blunt with T4 DNA polymerase and deoxynucleoside triphospates, a blunt-ended DNA fragment containing the GFP gene expressed by the elongation factor 1-α promoter and the neo gene expressed by the RSV promoter was inserted to create pQ152. pQ152 (20 μg) was digested with BamHI (to release the insert from the vector), extracted with an equal volume of phenol-chloroform (1:1), and precipitated with 2.5 volumes of ethanol. After drying, the DNA was resuspended in 20 μl of STE (5 mM NaCl, 5 mM Tris-HCl [pH 7.5], 1 mM EDTA) and was used to electroporate BCBL-1 cells: 0.5 to 1 × 107 cells/ml in a buffer of 120 mM KCl, 0.15 mM CaCl2, 10 mM K2HPO4-KH2PO4 [pH 7.6], and 5 mM MgCl2 at 275 V and 1,000 mF with a BTX ECM 600 in a 0.4-cm cuvette. Two days postelectroporation the cells were grown with 400 μg of G418 (Calbiochem, San Diego, Calif.)/ml to select for recombinants.
KSHV preparation.
For the production of virus, cells were grown to a density of 5 × 105 cells/ml and induced with 15 ng of tetradecanoyl phorbol acetate (TPA)/ml and grown for 5 days. To harvest virus, cells were pelleted at 500 × g for 15 min, the supernatant was removed and centrifuged at 15,000 × g for 4 h, the pellet was resuspended in 1/100 the growth volume with complete media and centrifuged at 300 × g for 5 min, and the supernatant was used as virus inoculum.
Generation of KSHV latently infected cells.
HF, DU145, and T24 latently infected cultures were generated by inoculating cells in 9.4-cm2 wells with 50 μl of rKSHV.152 (an amount of virus that would have made an equivalent number of 293 cells approximately 25% GFP positive), and 3 days postinfection (dpi) the cells were grown with 250 μg of G418/ml. Cultures were split one to three when they became confluent for at least six passages to ensure that all cells were G418 resistant.
KSHV, HCMV coinfection.
For the infection of HF, HCMV (Towne) was used at a multiplicity of infection (MOI) of 3 to 5, or as stated. G418-resistant rKSHV.152-infected HF were inoculated in complete medium for 1 h, at which time the medium was replaced. HUVEC were infected with rKSHV.152 with centrifugation enhancement by centrifuging the culture plate at 410 × g for 25 min. Following centrifugation, the cells were incubated 1 h at 37°C and the media were replaced with complete 20% serum media. After 1 to 2 h, HCMV (VHL/E) (60) at a MOI of 3 was used to infect the HUVEC in the same manner as for rKSHV.152 with centrifugation enhancement. Keratinocytes were infected with rKSHV.152 as for HF, and 1 week after infection with KSHV the cells were infected with HCMV as for HUVEC.
Detection of infectious rKSHV.152.
For the detection of infectious virus from cultures inoculated with rKSHV.152, the cells and media were collected and sonicated on ice with three 10-s pulses and the cellular debris was pelleted at 410 × g for 5 min. 293 cells 75% confluent in 3.8-cm2 wells were infected using centrifugation enhancement by centrifuging the culture plate at 410 × g for 25 min, with replacement of media 1 h after centrifugation. The number of GFP-positive cells was determined 1 dpi by visually counting cells using an inverted Nikon fluorescence microscope. No GFP-positive cells were present on the same day as inoculation.
Gardella gel analysis.
Gardella gels were prepared as previously described (22). Cells were loaded (0.5 to 1 × 106 per well), and gels were run at 4°C at 0.8 V/cm for 3 h, followed by 40 h at 4.5 V/cm. After electrophoresis, gels were stained with ethidium bromide and photographed, the upper section containing the sodium dodecyl sulfate and proteinase K was removed, and the gels were dried. The dried gels were used for direct gel hybridization using the 4.8-kb BamHI fragment (Fig. 1) labeled with 32P as a probe as described previously (41) with the modification that the gel was dried before denaturation and neutralization.
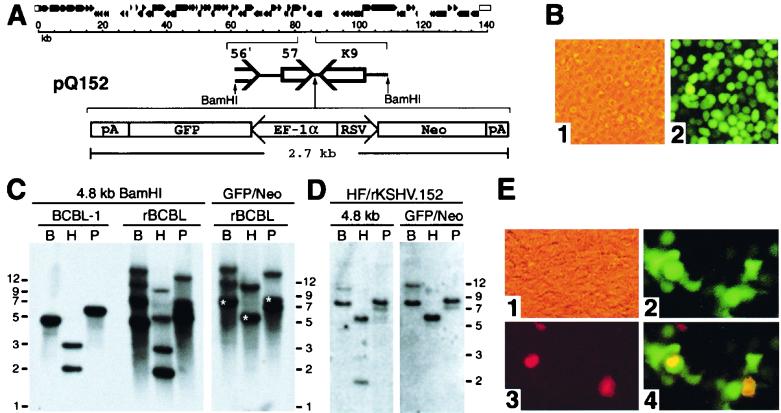
FIG. 1.
Recombinant KSHV. (A) Top, schematic diagram of the KSHV genome (46). Bottom, components of pQ152, which was used to construct recombinant virus. An expanded segment of the KSHV genome shows the 4.8-kb BamHI fragment containing ORFs 57 and K9. This fragment was used for the insertion of the GFP/Neo cassette between the polyadenylation sites for ORFs 57 and K9. (B) Photomicrographs of BCBL-1 cells that were transfected with pQ152 and grown with G418 selection to generate recombinant rKSHV.152 virus 5 weeks postelectroporation (×100). Panel 1, phase contrast; panel 2, fluorescence. (C) Hybridization analysis, following gel electrophoresis, of DNA isolated from BCBL-1, and BCBL-1 with rKSHV.152, digested with BamHI, HindIII, or PstI. Left panel: analysis with the 4.8-kb BamHI fragment labeled with 32P as a probe. Right panel: analysis of BCBL-1 with rKSHV.152 with the GFP/Neo construct labeled with 32P used as a probe. B, BamHI; H, HindIII; P, PstI. Fragment sizes predicted from the BC-1 sequence (46) are as follows: BamHI, 4,774 bp; HindIII, 2,030 and 3,001 bp; and PstI, 5,907 bp. The predicted bands from a correct recombination event with the addition of the 2.7-kb GFP/Neo cassette are marked by an (∗). (D) Hybridization analysis of DNA isolated from HF infected with rKSHV.152 following digestion with BamHI, HindIII, or PstI and gel electrophoresis. Left panel: autoradiogram of HF/rKSHV.152 DNA hybridized with the 32P-labeled KSHV BamHI 4.8-kb fragment. Right panel: autoradiogram of HF/rKSHV.152 DNA hybridized with the 32P-labeled GFP/Neo as a probe. B, BamHI; H, HindIII; P, PstI. Fragment sizes expected for a correct recombination event would be 7.5 kb for BamHI, 5.7 kb for HindIII, and 8.6 kb for PstI, based on the published sequence of BC-1(46). (E) 293 cells inoculated with virus isolated from BCGL-1 cells containing rKSHV.152. Infected 293 cells were examined for GFP expression and the expression of ORF 59 protein using the MAb 11D1 visualized with Alexa 594 (red). Shown are photomicrographs of infected 293 cells 2 dpi (×100). Panel 1, phase contrast; panel 2, fluorescence with filters for GFP; panel 3, fluorescence with filters for Alexa 594 (red); panel 4, merged image from the green and red filters.
KSHV virion DNA analysis.
Culture media were centrifuged at 500 × g for 15 min, filtered through a 0.45-μm-pore-size filter, and then centrifuged at 15,000 × g for 3 h. The supernatant was discarded, the pellet was resuspended in 100 μl of STE and centrifuged at 500 × g for 5 min, and 8 μl was used per the following reactions. For DNase treatment, 100 μl of reaction mixture with 40 mM Tris-HCl (pH 7), 10 mM NaCl, 6 mM MgCl2, 10 mM CaCl2, and 2 U of RQ DNase (Promega, Madison, Wis.) was incubated at 37°C for 1 h. For samples treated with proteinase K or NP-40, a 25-μl reaction mixture with STE was incubated with proteinase K (0.5 mg/ml) or NP-40 (1%), or both, for 30 min at 37°C. For the DNase treatment of these reactions, the mixtures were diluted to 100 μl and adjusted to 40 mM Tris-HCl (pH 7), 10 mM NaCl, 6 mM MgCl2, and 10 mM CaCl2, and 2 U of RQ DNase was added before incubation at 37°C for 1 h. KSHV DNA in each sample was detected by PCR and liquid hybridization as previously described (30).
Antibody detection of viral proteins.
For the immunofluorescence assay (IFA) detection of KSHV and HCMV, proteins in HF were grown on Lab-Tek Chamber slides (Nalge Nunc International), fixed with 4% paraformaldehyde for 30 min at room temperature, treated with 1% Triton X-100 for 15 min, and rinsed twice with 1× Tris-buffered saline (TBS; 0.05 M Tris-HCl in 0.85% NaCl, pH 7.6). Fixed slides were hydrated for 5 min in 1× TBS and then blocked by immersion in 20% goat serum (diluted in TBS) for 10 min. Slides were incubated for 30 min at 37°C in a humidified environment with monoclonal antibody (MAb) 7-17 (which is reactive with HCMV glycoprotein B [8]) diluted 1:50 in TBS with 0.3% bovine serum albumin and with a protein concentration-matched immunoglobulin G3 (IgG3) isotype control (Chemicon International Inc., Temecula, Calif.), were washed in TBS, and then were blocked in 20% goat serum for 10 min. Biotinylated goat anti-mouse antibody [BGAM; F(Ab′), Vector Laboratories, Burlingame, Calif.] diluted 1:200 was applied, and slides were incubated as above for 10 min and then washed with TBS. Avidin-labeled Cy5 (diluted 1:750) was applied, and the slides were incubated in the dark for 10 min. Slides were washed in TBS, and free avidin and biotin sites were blocked using two cycles of the Avidin/Biotin Blocking kit (SP-2001; Vector Laboratories). The cells were rinsed in 1× TBS. A second round of staining was done by first blocking with goat serum for 10 min and then incubating the slides for 30 min at 37°C with MAb 11D1 (10), diluted 1:8 in TBS with 0.3 BSA, a protein concentration-matched IgG2a isotype control and 20% goat serum for 10 min. BGAM diluted 1:250 was applied, and slides were incubated as above for 10 min and then washed with TBS. Strepavidin-labeled Alexa 594 (Molecular Probes, Eugene, Oreg.), diluted 1:750 in TBS, was applied. The slides were incubated in the dark for 10 min. Slides were then washed with TBS and counterstained with 1 ug of 4′,6′-diamidino-2-phenylindole (DAPI)/ml (Sigma) for 1 min. Slides were washed in TBS and coverslipped using the Prolong Antifade kit (Molecular Probes).
For the visualization of ORF 73 protein, cells were fixed and reacted with rabbit polyclonal antibody to ORF 73 protein as previously described (35). This was followed with biotin-conjugated F(ab′)2 fragment goat anti-rabbit IgG (Jackson ImmunoResearch Inc., West Grove, Pa.) diluted 1:200 for 30 min. at 37°C. The samples were washed twice with PBS and reacted with Alexa 594 strepavidin for 10 min at 37°C and then were washed twice with PBS and counter stained with 1μg of DAPI/ml for 1 min. Slides were washed in phosphate-buffered saline and coverslipped using the Prolong Antifade kit.
RESULTS
Recombinant KSHV.
To facilitate the identification of KSHV-infected cells, a recombinant KSHV containing the GFP gene expressed by the elongation factor 1-α (EF-1α) promoter and the neo gene (conferring resistance to G418) expressed by the RSV promoter was constructed using the BCBL-1 cell line (45). The recombinant virus was generated with a construct, pQ152, containing the GFP/Neo cassette inserted between ORFs 57 and K9 at a site that sequence analysis indicates does not encode a gene (46) (Fig. 1A). BCBL-1 cells transfected with pQ152 and grown under selection with G418 demonstrated GFP expression under fluorescence microscopy (Fig. 1B).
To confirm the presence of recombinant viral genomes, DNA was isolated from BCBL-1 cells and recombinant BCBL-1 cells, digested with BamHI, HindIII and PstI, and analyzed by hybridization with the 4.8-kb BamHI fragment containing ORF 57 and K9, or the GFP/Neo cassette, labeled with 32P (Fig. 1C). Analysis of the BCBL-1 DNA with the 4.8-kb fragment demonstrated fragments similar to the pattern of fragments predicted from the published sequences (37, 46). Analysis of the recombinant cells with the 4.8-kb probe showed both a wild-type pattern of fragments and additional, higher-molecular-weight fragments. The wild-type-sized fragments were expected to be present since it would be predicted that only a percentage of the approximately 50 KSHV genomes per cell would become recombinant. The higher-molecular-weight fragments detected by the 4.8-kb probe were also detected by the GFP/Neo probe. These fragments represented sizes expected for homologous recombination of pQ152 into KSHV, confirming the generation of a recombinant KSHV, termed rKSHV.152, as well as additional fragments likely representing various nonspecific recombination events. Viral DNA present in HF infected with rKSHV.152 and selected for with G418 was examined with 32P-labeled probes of the 4.8-kb KSHV fragment and the GFP/Neo construct. This analysis demonstrated a simpler pattern of DNA fragments than in the BCBL cells, and the fragment sizes predicted by a correct recombination event were the predominant bands (Fig. 1D).
To test for infectious recombinant virus, cells containing rKSHV.152 were induced with TPA, and virus isolated from the culture medium was used to infect 293 cells. Inoculated cells were observed by fluorescence microscopy, and 1 dpi, 293 cells expressing GFP were evident. To confirm that the GFP expression was indicative of KSHV infection, cultures were assessed for expression of both the GFP and the KSHV ORF 59 lytic nuclear protein using MAb 11D1 (10) 2 dpi (Fig. 1E), and it was found that ORF 59 expression and GFP could be localized to the same cells (Fig. 1E, panel 4). There were also cells that expressed ORF 59 that were not GFP positive, as would be expected due to the fact that wild-type virus was present. This demonstrated that the BCBL-1 cells containing rKSHV.152 could produce infectious recombinant virus that expressed GFP upon infection of susceptible cells.
Establishment of KSHV latently infected cultures.
In cells that are permissive for the expression of the EF-1α/GFP gene, rKSHV.152 provided a means of determining the susceptibility of cells to KSHV infection by the expression of GFP. In addition, the neo gene allowed the selection of infected cells with G418. Figure 2A shows three cell types, T24, a human bladder carcinoma, DU145, a human prostate carcinoma, and human fibroblasts (HF), which expressed GFP following inoculation and could subsequently be cultured with G418 selection. These cells showed no cytopathic effect (CPE) upon infection with KSHV, and no ORF 59 expression or infectious virus could be detected (data not shown). Long-term cultures (defined as at least six passages with cells split one to three with G418 selection) of rKSHV.152-infected HF, T24, and DU145 cells could be maintained which were KSHV DNA positive by PCR, but no CPE was evident and no infectious virus could be detected in culture supernatant or sonicated cells (data not shown). Because these features were indicative of a latent infection, it was of interest to analyze these cells for KSHV latent gene expression and for the structure of the viral DNA present.
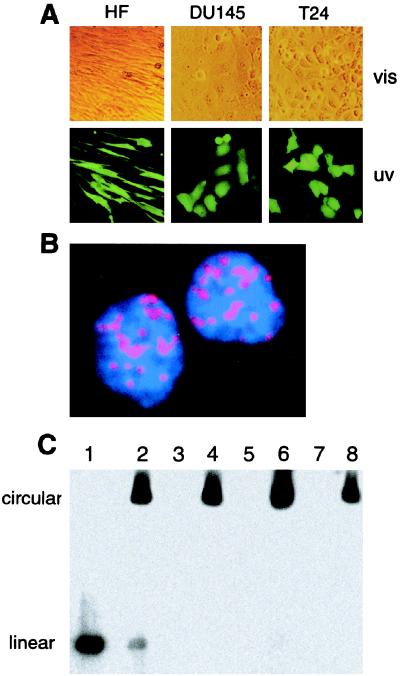
FIG. 2.
Analysis of ORF73/LANA and viral DNA in long-term rKSHV.152-infected cells. (A) Cultures of T24, DU145, and HF infected with rKSHV.152, selected with G418, and photographed with phase contrast (vis) and fluorescence (uv) (×100). (B) IFA detection of KSHV ORF 73 protein nuclear expression in HF with a rabbit polyclonal antibody to ORF 73 protein and visualized with a biotinylated anti-rabbit antibody reacted with strepavidin Alexa 594 (red). Cells were counterstained with DAPI, resulting in the blue nuclei (×2,000). (C) Gardella gel analysis of viral DNA present in long-term rKSHV.152-infected cultures. Lane 1, KSHV virion isolated from the media of TPA-induced BCBL-1 cells; lane 2, BCBL-1 cells; lane 3, HF; lane 4, HF/rKSHV.152; lane 5, T24; lane 6, T24/rKSHV.152; lane 7, DU145; lane 8, DU145/rKSHV.152.
A latency-associated nuclear antigen (LANA) has been detected in BCBL cells using sera from KS patients (21), and it was later identified to be encoded by ORF 73 (42). To determine if ORF 73 protein was expressed in rKSHV.152-infected HF cultures, cells were examined by IFA for expression of ORF 73 using rabbit polyclonal antiserum raised against ORF 73, and the punctate nuclear pattern typical of ORF 73 was detected (Fig. 2B). Essentially all HF cells were positive for ORF 73, and the same result was found for the T24- and DU145-KSHV-infected cells (data not shown).
The structure of the KSHV genome present in long-term cultures was analyzed by Gardella gel analysis. Gardella gels separate circular viral genomes, present during latent replication, from linear viral DNA present during lytic replication (22), as used to analyze KSHV in BCBL-1 cells (44). Gardella gel analysis of T24, DU145, and HF cultures containing rKSHV.152 demonstrated that these cells contained circular viral genomes, indicative of latent viral replication, with no detection of linear, lytic DNA (Fig. 2C). Both these results were consistent with KSHV latent infection.
Activation of KSHV lytic gene expression by HCMV.
The establishment of cells containing only latent KSHV, particularly primary HF cultures, offered an experimental system for studying the reactivation of KSHV. HF are susceptible to infection by a number of viruses, including HCMV. HF with rKSHV.152 were susceptible to HCMV infection, demonstrating typical CPE (Fig. 3A). In addition, following HCMV infection the majority of cells demonstrated GFP expression, whereas in latent cells only 25 to 30% of cells were GFP positive. This indicates that in most latent cells the EF-1α promoter is inactive, perhaps due to methylation, which has been shown to influence gene expression in Epstein-Barr virus (2) but is activated by HCMV. To determine if HCMV infection led to the expression of KSHV lytic cycle proteins, HF/rKSHV.152 cultures, without or with HCMV infection, were reacted with MAb 11D1 for the detection of the KSHV lytic ORF 59 protein. While no ORF 59 protein was seen in cultures without HCMV, approximately 20 to 25% of cells 2 dpi with HCMV demonstrated nuclear staining for ORF 59 (Fig. 3B). The infection of HF containing latent KSHV with HSV-1 also activated the expression of ORF 59 protein (Fig. 3C). Whereas infection with HCMV and HSV activated ORF 59 protein expression, TPA and sodium butyrate, which activate KSHV lytic replication in BCBL-1 cells, did not induce ORF 59 expression in HF (data not shown).
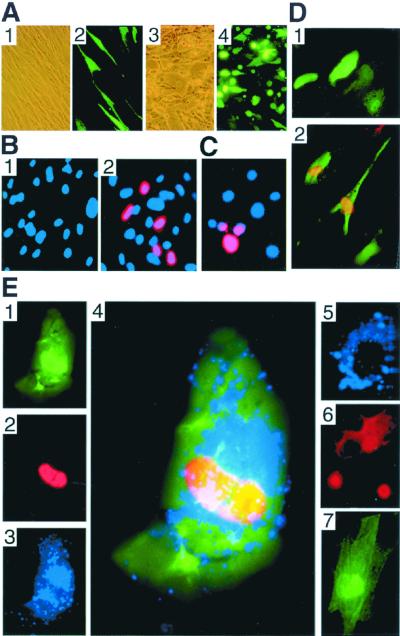
FIG. 3.
Detection of viral proteins. (A) HF infected with rKSHV.152 and HF coinfected with rKSHV.152 and HCMV, photographed with phase contrast or fluorescence. Shown for HF/rKSHV.152 are phase contrast (panel 1) and fluorescence (panel 2) (×100). Shown for HF/rKSHV.152 infected with HCMV are phase contrast (panel 3) and fluorescence (panel 4). (B) Expression of the KSHV lytic ORF 59 protein induced by HCMV. HF infected with rKSHV.152, minus and plus HCMV infection, were analyzed for the presence of the lytic cycle 59 protein with MAb 11D1 visualized with Alexa 594 (red) and stained with DAPI (blue) (×200). Panel 1, HF with rKSHV.152; panel 2, HF with rKSHV.152 infected with HCMV. (C) Identification of ORF 59 expression in HF coinfected with rKSHV.152 and HSV. HF latently infected with rKSHV.152 were infected with HSV-1 and 2 days later were examined for ORF59 expression with MAb 11D1 visualized with Alexa 594 (red) and stained with DAPI (blue). (D) Visualization of GFP and nuclear ORF 59 protein with MAb 11D1 and Alexa 594 (red) in keratinocytes infected with rKSHV.152 (×200) (panel 1) or rKSHV.152 and HCMV (panel 2). (E) Identification of the KSHV ORF 59 protein and HCMV gB in fibroblasts infected by rKSHV.152 and HCMV. Photomicrographs show the detection of GFP expression (panel 1), KSHV ORF 59 protein with MAb 11D1 visualized with Alexa 594 (red) (panel 2), and HCMV gB with MAb 7-17 detected with Cy5 (blue) (panel 3). Panel 4, merged image showing ORF 59, gB, and GFP in coinfected cells (×600); panel 5, HF infected with HCMV reacted with antibodies 7-17 (blue) and 11D1 (red), demonstrating that 11D1 did not react with an HCMV-infected cell; panel 6, BCBL-1 cells induced with TPA for 2 days and reacted with MAb 11D1 (red) and MAb 7-17 (blue), showing that 7-17 does not react with KSHV proteins; panel 7, HF infected with rKSHV.152 reacted with MAb 11D1 and MAb 7-17.
Keratinocytes are a second cell type of epithelial lineage that can be infected by KSHV (F. Cerimele, F. Curreli, E. Ely, D. M. Knowles, E. Cesarman, and O. Flore, Second International Workshop on KSHV/HHV8 and Related Agents, 1999; J. Vieria, unpublished observations) and HCMV. Keratinocytes, 7 dpi with rKSHV.152, were infected with HCMV, and 2 days post-HCMV infection, cultures with and without HCMV were reacted with MAb 11D1. No ORF 59 expression was found without HCMV, but with HCMV, induction of lytic cycle gene expression was detected by the nuclear expression of ORF 59 (Fig. 3D).
To confirm that cells with lytic KSHV gene expression had active HCMV replication, rKSHV.152-infected HF cultures infected with HCMV were evaluated for the KSHV nuclear lytic protein ORF 59 with MAb 11D1 and for the HCMV glycoprotein B (gB) with MAb 7-17 (8) 3 days post-HCMV infection (Fig. 3E). Coinfected HF expressed GFP, KSHV ORF 59 expression was detected in the nucleus, and HCMV gB was localized to the cytoplasm (Fig. 3E, panels 1 to 4). MAb 11D1 did not react with HCMV-infected HF that were gB positive (panel 5), and MAb 7-17 did not react with induced BCBL-1 cells that showed ORF 59 expression (panel 6). No reactivity was seen for MAb 11D1 or MAb 7-17 in HF infected only with rKSHV.152 (panel 7).
Induction of KSHV lytic replication by HCMV.
The induction of KSHV lytic replication by HCMV was investigated by Gardella gel analysis of DNA isolated from HF/rKSHV.152 cultures with and without HCMV infection. No linear DNA was detected without HCMV (Fig. 4A, lane 4), but significant levels of linear KSHV genomes, indicative of lytic viral replication, were present following HCMV infection (Fig. 4A, lane 5). With the finding that HCMV induced lytic replication, cultures were examined for the presence of KSHV virion DNA.
FIG. 4.

Induction of KSHV lytic replication by HCMV. (A) Gardella gel analysis of KSHV from HF and HF coinfected with HCMV. Lane 1, KSHV isolated virions; lane 2, BCBL-1 cells; lane 3, BCBL-1 cells induced with TPA for 2 days; lane 4, HF containing rKSHV.152; lane 5, HF with rKSHV.152 and infected with HCMV 2 dpi; lane 6, HF infected with HCMV. The gel was probed with the 4.8-kb BamHI fragment (Fig. 1) labeled with 32P. The positions of circular and linear viral DNA are indicated, as determined by the positions of DNA from BCBL-1 cells and the DNA from purified virions, respectively. (B) Analysis of cell-free KSHV DNA. Viral DNA in cell-free media from HF infected with rKSHV.152 and from HF coinfected with rKSHV.152 and HCMV was analyzed for viral DNA that was resistant to DNase, with and without prior treatment with proteinase K and/or NP-40, as indicated (+, with; −, without). Viral DNA was detected by PCR for the ORF 26 region, and the product was identified with a 32P-labeled specific probe by liquid hybridization prior to gel electrophoresis (30).
To determine if viral DNA characteristic of virions was present, cell-free media from HF/rKSHV.152 cultures with and without HCMV coinfection were tested for the presence of KSHV DNA resistant to DNase by PCR for the ORF 26 gene (30). In cultures without HCMV, no DNase-resistant KSHV DNA was present. In coinfected cultures, KSHV viral DNA resistant to DNase was present but became sensitive to DNase by prior treatment with both proteinase and detergent, characteristic of virion DNA (Fig. 4B).
Production of infectious KSHV induced by HCMV infection.
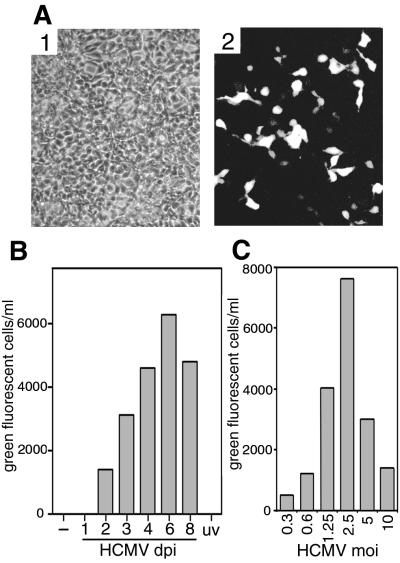
The expression of GFP by rKSHV.152 upon the infection of cells facilitated the detection of infectious virus. To determine if infectious KSHV was produced by HF cultures coinfected with KSHV and HCMV, virus was harvested from coinfected cultures 3 days post-HCMV infection and was used to inoculate 293 cells. This resulted in GFP-positive 293 cells (Fig. 5A), demonstrating the presence of infectious rKSHV.152. ORF 59-positive cells detected using MAb 11D1 were also found in these infected 293 cultures (data not shown). Next, the temporal production of KSHV by coinfected cells was determined by the infection of 293 cells with virus harvested at time points post-HCMV infection, followed by the determination of the number of GFP-positive 293 cells. No KSHV was detected without HCMV infection at 1 day post-HCMV infection or in cells infected with UV-inactivated HCMV, but infectious rKSHV.152 was detected 2 days post-HCMV infection (Fig. 5B). Infectious HCMV was first present at 3 dpi, as is the case for HF without KSHV (data not shown). The effect of the HCMV MOI on KSHV production was also examined, and it was found that the activation of KSHV by HCMV is achieved at a low MOI, indicating that a few or 1 PFU per cell can reactivate KSHV (Fig. 5C). A high HCMV MOI reduced the level of KSHV, possibly due to the significant CPE. In contrast to HCMV, HSV which actvated ORF 59 expression did not result in the production of infectious KSHV (data not shown). Whether this is due to the more rapid replication of HSV interfering with KSHV, or the failure of HSV to activate the entire KSHV replication cycle or supply a necessary function, is not known.
FIG. 5.
Infectious rKSHV.152 from cultures coinfected with HCMV. (A) 293 cells infected by virus harvested from HF coinfected by rKSHV.152 and HCMV. HF/rKSHV.152 cultures coinfected with HCMV for 3 days were sonicated, the cell debris was pelleted, and the supernatant was used to infect 293 cells, which were photographed 2 dpi (×100). Panel 1, phase contrast; panel 2, fluorescence. (B) Kinetics of KSHV virus induction by HCMV. Infectious rKSHV.152 from HF carrying rKSHV.152 was determined from cultures not infected with HCMV (−), from cultures infected with HCMV and harvested at the times indicated (dpi), or from cultures infected with UV-inactivated HCMV harvested 3 dpi (uv). The cells and media were harvested and sonicated, and the cellular debris was pelleted. Sample supernatants were used to inoculate 293 cells, and the number of GFP-positive cells was determined 1 dpi. A representative experiment of four separate experiments is shown. (C) Influence of HCMV MOI on production of KSHV. HF with rKSHV.152 were infected with HCMV at the indicated MOI. Four days post-HCMV infection cultures were harvested and sonicated, cellular debris was pelleted, the supernatant was used to inoculate 293 cells, and the number of GFP-positive cells was determined. A representative experiment of three separate experiments is shown.
Activation of KSHV by HCMV in endothelial cells.
The finding that HCMV could activate KSHV in cells of epithelial origin made it of interest to determine if this occurred in other cell types infected by both viruses. Endothelial cells are a significant cell type present in KS lesions that are KSHV positive (7). Because long-term HUVEC cultures demonstrating only latent replication have not been established, these experiments were carried out in cultures infected first with rKSHV.152, followed 2 h later by HCMV infection with strain VHL/E(60 rKSHV.152 showed a low percentage of cells with nuclear expression of ORF 59 as detected by MAb 11D1, signifying lytic protein expression (Fig. 6A, panel 1). With HCMV coinfection, there was a significant increase in ORF 59-positive nuclei (Fig. 6A, panel 2), demonstrating that HCMV could activate lytic cycle gene expression in HUVEC. HCMV coinfection increased KSHV lytic DNA replication, as demonstrated by Gardella gel analysis comparing HUVEC infected with rKSHV.152 to HUVEC coinfected with HCMV and rKSHV.152 (Fig. 6B), where a significant increase in linear KSHV DNA was present with HCMV infection. To investigate if HCMV affected the production of KSHV by endothelial cells, the amount of rKSHV.152 produced by HUVEC, without and with HCMV infection, was determined by inoculating 293 cells with virus harvested from the HUVEC cultures 3 dpi (Fig. 6C). An approximately threefold increase in the level of infectious KSHV was noted, but this was well below the increase in the number of ORF 59-positive nuclei induced by HCMV, suggesting that many cells that express KSHV early lytic proteins may not go on to produce virus. It should also be noted that we examined HUVEC from seven different donors; and while infection with KSHV, lytic gene expression, and induction of lytic gene expression by HCMV were similar, the production of KSHV varied significantly. Two lines were relatively good producers (one is presented in Fig. 6), three produced barely detectable virus, and two were intermediate (J. Vieira, unpublished observations). The reasons are at present unknown.
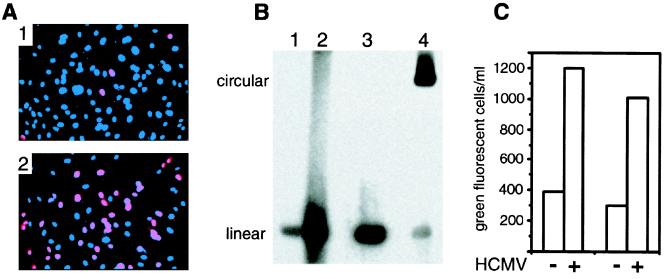
FIG. 6.
HCMV activation of KSHV in HUVEC. (A) Visualization of the nuclear expression of the lytic cycle ORF 59 protein with MAb 11D1 in HUVEC infected with rKSHV.152, minus and plus HCMV. Panel 1, HUVEC infected with rKSHV.152 3 dpi reacted with MAb 11D1 and Alexa 594 (red) and stained with DAPI (blue); panel 2, HUVEC coinfected with rKSHV.152 and HCMV reacted with MAb 11D1 and Alexa 594 (red) and stained with DAPI (blue). (B) Gardella gel analysis of KSHV DNA isolated from HUVEC infected with rKSHV.152, minus and plus HCMV. Lane 1, HUVEC infected with rKSHV.152; lane 2, HUVEC coinfected with rKSHV.152 and HCMV; lane 3, KSHV virion; lane 4, BCBL-1 cells. The positions of linear and circular KSHV genomes are indicated. (C) Presentation of two experiments showing the number of GFP-positive 293 cells resulting from infection with virus harvested from HUVEC cultures infected with rKSHV.152 and coinfected with rKSHV.152 and HCMV.
DISCUSSION
In this report we have shown that HCMV, a common pathogen in immunosuppressed individuals, can activate KSHV lytic replication. Important to this investigation was the construction of a recombinant KSHV carrying the GFP and neo genes, rKSHV.152. In cells permissive for expression of the EF-1α promoter, GFP expression was a sensitive and specific marker for cells that could be infected by KSHV, which made it particularly useful for KSHV, where infection is often without CPE. This included 293, HF, HUVEC, T24, and DU145 cells. The neo gene present on rKSHV.152 made it possible to select for infected cells with G418 and allowed the first establishment of cultures, including primary HF and DU145 cells, where all cells contained only latent KSHV. DU145 cells had previously been reported not to support latent replication in a PCR-based study (36). Cell lines containing only latent KSHV will be of value in studies of viral and cellular gene expression during latency and of factors that contribute to virus reactivation.
The establishment of HF with latent KSHV provided a system for examining the interaction between KSHV and HCMV. This resulted in the demonstration that the infection of KSHV latently infected HF with HCMV, reactivated KSHV lytic replication, and resulted in the production of infectious KSHV. For this study, GFP expression by rKSHV.152 facilitated the detection of infectious virus and enabled a quantitation and temporal analysis of virus production. Studies on the activation of KSHV lytic replication have identified the ORF 50 gene as an immediate-early gene, with homology to the EBV Rta gene, that can activate lytic viral genes when expressed from transfected recombinant constructs (55). The process that HCMV may invoke in the reactivation of KSHV is not known and is currently under investigation. It is of note that HCMV is a betaherpesvirus and in this case reactivates KSHV, a gammaherpesvirus. Cross-reactivation between herpesviruses has been examined in a limited number of studies. In cells coinfected with KSHV and EBV, cross-reactivation between these two gammaherpesviruses was not found (55). Reactivation of HHV6 by HHV7 has been reported for these similar betaherpesviruses (27). The activation of a gammaherpesvirus by a betaherpesvirus has been reported for the reactivation of EBV by HHV6 (18), and as with KSHV and HCMV, coinfected cells were identified and infectious virus was necessary.
KS lesions are in large part derived from endothelial cells, and endothelial cells are an important site of HCMV replication (51), which made it of interest to examine the potential for KSHV-HCMV interaction in endothelial cells. In HUVEC a low level of lytic KSHV replication was shown by ORF 59 expression and the production of infectious rKSHV.152. With HCMV infection, an approximately 10-fold increase in the expression of the KSHV lytic ORF 59 protein was observed; however, the increase in infectious rKSHV.152 was only approximately three fold, suggesting that many cells with early lytic gene expression may not go on to a productive infection. A greater percentage of cells expressing early genes than late genes was found in microvascular endothelial cells (35). In vitro KSHV infection of both macrovascular and microvascular endothelial cells has been reported, with viral production in microvascular cells (19, 35, 40, 43). This report demonstrated HCMV activation of KSHV in HUVEC, which are macrovascular cells, and similar results have been seen with dermal microvascular endothelial cells (J. Vieira, unpublished observations). Besides any effect of increased virus production, the increase in lytic gene expression induced by HCMV could in itself have an impact in KS. Some of the viral genes indicated for roles in angiogenesis, cell proliferation, signal transduction, immune modulation, and inflammatory infiltration (49) are expressed as lytic genes (47), suggesting that lytic gene expression induced by HCMV could augment these processes.
While KSHV and HCMV coinfected cells have not been identified in vivo, there are multiple cell types with potential for KSHV and HCMV interaction. The cellular tropism for KSHV is proving to be broad; the cell types infected by KSHV include endothelial cells, fibroblasts, keratinocytes, B cells, monocytes/macrophages, and glandular epithelial cells (5, 7, 13, 15, 26, 32). This is a cellular tropism shared in large part by HCMV, illustrating a wide variety of sites for possible interaction. Monocytes are considered sites of latency for both viruses, and in vitro studies have indicated that cytokines induced by immune activation can lead to reactivation of KSHV and HCMV (33, 52). HCMV is found in KS lesions, and the endothelial/spindle cell of KS lesions could be a target cell for both viruses. HCMV and KSHV are both shed in saliva (6, 9, 30, 59). Although HCMV replication is predominately found in salivary glands, and the data suggest that KSHV is not in the salivary gland but replicates in oral epithelial (12, 59), in immunosuppressed patients HCMV replication can be found in the oral mucosa (48).
The possible clinical implications for the interaction between HCMV and KSHV are unknown and in need of further study, but a number of considerations exist. The diseases associated with KSHV and HCMV occur in the same patient populations, and active HCMV replication can be common in KS patients. In a study of 23 men with KS or a history of KS, 11 had HCMV viremia, 7 had KSHV viremia, and 5 were positive for both as determined by PCR detection of viral DNA in serum (J. Vieira, unpublished data). The activation of KSHV lytic replication by HCMV could have multiple effects on KSHV-related diseases. The induction of KSHV lytic proteins, which are thought to play a part in KS through promoting proliferation, angiogenesis, and inflammatory infiltration, could exacerbate lesions. The HCMV activation of KSHV from latently infected cells could generate virus capable of seeding surrounding cells with KSHV, thereby increasing the population of KSHV latently infected cells that could contribute to tumor formation. Coinfection of cells could also act to increase the viral load of KSHV.
It has become evident that KS is a multifactorial disease. The ubiquitous finding of KSHV in KS lesions demonstrates its core role in KS, but infection with KSHV by itself rarely appears adequate for KS development. Our data demonstrate that HCMV can activate KSHV lytic replication and suggest the importance of considering the potential for infectious agents present in immunocompromised patients to interact and exacerbate disease. This work has also demonstrated the utility of the rKSHV.152 virus for the determination of cells susceptible to KSHV infection, the detection of infectious KSHV, and the establishment of KSHV latently infected cells.
ACKNOWLEDGMENTS
We thank B. Torok-Storb for the generous use of equipment, J. Habecker for helpful discussions and antibody reagents, P. Moeser and J. Harlan for the generous gift of HUVEC, W. J. Britt for gB antibody, J. Waldman for VHL/E, and A. Geballe for critical reading of the manuscript.
This work was supported by NIH grants AI-18029 and AI-30731 to L.C. and by Public Health Service grants CA 75911 and CA 82056 to B.C.
REFERENCES
- 1.Adams V, Kempf W, Hassam S, Briner J, Schmid M, Moos R, Pfaltz M. Detection of several types of human papilloma viruses in AIDS-associated Kaposi's sarcoma. J Med Virol. 1995;46:189–193. doi: 10.1002/jmv.1890460304. [DOI] [PubMed] [Google Scholar]
- 2.Ambinder R F, Robertson K D, Tao Q. DNA methylation and the Epstein-Barr virus. Semin Cancer Biol. 1999;9:369–375. doi: 10.1006/scbi.1999.0137. [DOI] [PubMed] [Google Scholar]
- 3.Beral V, Newton R. Overview of the epidemiology of immunodeficiency-associated cancers. J Natl Cancer Inst Monogr. 1998;23:1–6. doi: 10.1093/oxfordjournals.jncimonographs.a024164. [DOI] [PubMed] [Google Scholar]
- 4.Beral V, Peterman T A, Berkelman R L, Jaffe H W. Kaposi's sarcoma among persons with AIDS: a sexually transmitted infection? Lancet. 1990;335:123–128. doi: 10.1016/0140-6736(90)90001-l. [DOI] [PubMed] [Google Scholar]
- 5.Blasig C, Zietz C, Haar B, Neipel F, Esser S, Brockmeyer N H, Tschachler E, Colombini S, Ensoli B, Sturzl M. Monocytes in Kaposi's sarcoma lesions are productively infected by human herpesvirus 8. J Virol. 1997;71:7963–7968. doi: 10.1128/jvi.71.10.7963-7968.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boldogh I, Szaniszlo P, Bresnahan W A, Flaitz C M, Nichols M C, Albrecht T. Kaposi's sarcoma herpesvirus-like DNA sequence in the saliva of individuals infected with human immunodeficiency virus. Clin Infect Dis. 1996;23:406–407. doi: 10.1093/clinids/23.2.406. [DOI] [PubMed] [Google Scholar]
- 7.Boshoff C, Schulz T F, Kennedy M M, Graham A K, Fisher C, Thomas A, McGee J O, Weiss R A, O'Leary J J. Kaposi's sarcoma-associated herpesvirus infects endothelial and spindle cells. Nat Med. 1995;1:1274–1278. doi: 10.1038/nm1295-1274. [DOI] [PubMed] [Google Scholar]
- 8.Britt W J. Neutralizing antibodies detect a disulfide-linked glycoprotein complex within the envelope of human cytomegalovirus. Virology. 1984;135:369–378. doi: 10.1016/0042-6822(84)90193-4. [DOI] [PubMed] [Google Scholar]
- 9.Britt W J, Alford C A. Cytomegalovirus. In: Fields B N, editor. Fields virology. Vol. 2. Philadelphia, Pa: Lippincott-Raven Press Publishers; 1996. pp. 2493–2523. [Google Scholar]
- 10.Chan S R, Bloomer C, Chandran B. Identification and characterization of human herpesvirus-8 lytic cycle-associated ORF 59 protein and the encoding cDNA by monoclonal antibody. Virology. 1998;240:118–126. doi: 10.1006/viro.1997.8911. [DOI] [PubMed] [Google Scholar]
- 11.Chang Y, Cesarman E, Pessin M S, Lee F, Culpepper J, Knowles D M, Moore P S. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science. 1994;266:1865–1869. doi: 10.1126/science.7997879. [DOI] [PubMed] [Google Scholar]
- 12.Corbellino M, Poirel L, Bestetti G, Pizzuto M, Aubin J T, Capra M, Bifulco C, Berti E, Agut H, Rizzardini G, Galli M, Parravicini C. Restricted tissue distribution of extralesional Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS patients with Kaposi's sarcoma. AIDS Res Hum Retrovir. 1996;12:651–657. doi: 10.1089/aid.1996.12.651. [DOI] [PubMed] [Google Scholar]
- 13.Diamond C, Brodie S J, Krieger J N, Huang M L, Koelle D M, Diem K, Muthui D, Corey L. Human herpesvirus 8 in the prostate glands of men with Kaposi's sarcoma. J Virol. 1998;72:6223–6227. doi: 10.1128/jvi.72.7.6223-6227.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drew W L, Conant M A, Miner R C, Huang E S, Ziegler J L, Groundwater J R, Gullett J H, Volberding P, Abrams D I, Mintz L. Cytomegalovirus and Kaposi's sarcoma in young homosexual men. Lancet. 1982;ii:125–127. doi: 10.1016/s0140-6736(82)91092-3. [DOI] [PubMed] [Google Scholar]
- 15.Dupin N, Fisher C, Kellam P, Ariad S, Tulliez M, Franck N, van Marck E, Salmon D, Gorin I, Escande J P, Weiss R A, Alitalo K, Boshoff C. Distribution of human herpesvirus-8 latently infected cells in Kaposi's sarcoma, multicentric Castleman's disease, and primary effusion lymphoma. Proc Natl Acad Sci USA. 1999;96:4546–4551. doi: 10.1073/pnas.96.8.4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ensoli B, Barillari G, Salahuddin S Z, Gallo R C, Wong-Staal F. Tat protein of HIV-1 stimulates growth of cells derived from Kaposi's sarcoma lesions of AIDS patients. Nature. 1990;345:84–86. doi: 10.1038/345084a0. [DOI] [PubMed] [Google Scholar]
- 17.Fiorelli V, Gendelman R, Samaniego F, Markham P D, Ensoli B. Cytokines from activated T cells induce normal endothelial cells to acquire the phenotypic and functional features of AIDS-Kaposi's sarcoma spindle cells. J Clin Investig. 1995;95:1723–1734. doi: 10.1172/JCI117849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flamand L, Stefanescu I, Abalshi D V, Menezes J. Activation of Epstein-Barr virus replicative cycle by human herpesvirus 6. J Virol. 1993;67:6768–6777. doi: 10.1128/jvi.67.11.6768-6777.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flore O, Rafii S, Ely S, O'Leary J J, Hyjek E M, Cesarman E. Transformation of primary human endothelial cells by Kaposi's sarcoma-associated herpesvirus. Nature. 1998;394:588–592. doi: 10.1038/29093. [DOI] [PubMed] [Google Scholar]
- 20.Gallo R C. The enigmas of Kaposi's sarcoma. Science. 1998;282:1837–1839. doi: 10.1126/science.282.5395.1837. [DOI] [PubMed] [Google Scholar]
- 21.Gao S J, Kingsley L, Hoover D R, Spira T J, Rinaldo C R, Saah A, Phair J, Detels R, Parry P, Chang Y, Moore P S. Seroconversion to antibodies against Kaposi's sarcoma-associated herpesvirus-related latent nuclear antigens before the development of Kaposi's sarcoma. N Engl J Med. 1996;335:233–241. doi: 10.1056/NEJM199607253350403. [DOI] [PubMed] [Google Scholar]
- 22.Gardella T, Medveczky P, Sairenji T, Mulder C. Detection of circular and linear herpesvirus DNA molecules in mammalian cells by gel electrophoresis. J Virol. 1984;50:248–254. doi: 10.1128/jvi.50.1.248-254.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giraldo G, Beth E, Huang E S. Kaposi's sarcoma and its relationship to cytomegalovirus (CMNV). III. CMV DNA and CMV early antigens in Kaposi's sarcoma. Int J Cancer. 1980;26:23–29. doi: 10.1002/ijc.2910260105. [DOI] [PubMed] [Google Scholar]
- 24.Giraldo G, Beth E, Kyalwazi S. Etiological implications of Kaposi's sarcoma. Antibiot Chemother. 1981;29:12–31. doi: 10.1159/000397435. [DOI] [PubMed] [Google Scholar]
- 25.Harrington W, Jr, Sieczkowski L, Sosa C, Chan-a-Sue S, Cai J P, Cabral L, Wood C. Activation of HHV-8 by HIV-1 tat. Lancet. 1997;349:774–775. doi: 10.1016/s0140-6736(05)60199-7. [DOI] [PubMed] [Google Scholar]
- 26.Huang Y Q, Li J J, Zhang W G, Feiner D, Friedman Kien A E. Transcription of human herpesvirus-like agent (HHV-8) in Kaposi's sarcoma. J Clin Investig. 1996;97:2803–2806. doi: 10.1172/JCI118735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Katsafanas G C, Schirmer E C, Wyatt L S, Frenkel N. In vitro activation of human herpesviruses 6 and 7 from latency. Proc Natl Acad Sci USA. 1996;93:9788–9792. doi: 10.1073/pnas.93.18.9788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kempf W, Adams V. Viruses in the pathogenesis of Kaposi's sarcoma—a review. Biochem Mol Med. 1996;58:1–12. doi: 10.1006/bmme.1996.0025. [DOI] [PubMed] [Google Scholar]
- 29.Kempf W, Adams V, Wey N, Moos R, Schmid M, Avitabile E, Campadelli-Fiume G. CD68+ cells of monocyte/macrophage lineage in the environment of AIDS-associated and classic-sporadic Kaposi sarcoma are singly or doubly infected with human herpesviruses 7 and 6B. Proc Natl Acad Sci USA. 1997;94:7600–7605. doi: 10.1073/pnas.94.14.7600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koelle D M, Huang M L, Chandran B, Vieira J, Piepkorn M, Corey L. Frequent detection of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) DNA in saliva of human immunodeficiency virus-infected men: clinical and immunologic correlates. J Infect Dis. 1997;176:94–102. doi: 10.1086/514045. [DOI] [PubMed] [Google Scholar]
- 31.Mendez J C, Procop G W, Espy M J, Smith T F, McGregor C G, Paya C V. Relationship of HHV8 replication and Kaposi's sarcoma after solid organ transplantation. Transplantation. 1999;67:1200–1201. doi: 10.1097/00007890-199904270-00022. [DOI] [PubMed] [Google Scholar]
- 32.Mesri E A, Cesarman E, Arvanitakis L, Rafii S, Moore M A, Posnett D N, Knowles D M, Asch A S. Human herpesvirus-8/Kaposi's sarcoma-associated herpesvirus is a new transmissible virus that infects B cells. J Exp Med. 1996;183:2385–2390. doi: 10.1084/jem.183.5.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Monini P, Colombini S, Sturzl M, Goletti D, Cafaro A, Sgadari C, Butto S, Franco M, Leone P, Fais S, Melucci-Vigo G, Chiozzini C, Carlini F, Ascherl G, Cornali E, Zietz C, Ramazzotti E, Ensoli F, Andreoni M, Pezzotti P, Rezza G, Yarchoan R, Gallo R C, Ensoli B. Reactivation and persistence of human herpesvirus-8 infection in B cells and monocytes by Th-1 cytokines increased in Kaposi's sarcoma. Blood. 1999;93:4044–4058. [PubMed] [Google Scholar]
- 34.Monini P, Rotola A, de Lellis L, Corallini A, Secchiero P, Albini A, Benelli R, Parravicini C, Barbanti Brodano G, Cassai E. Latent BK virus infection and Kaposi's sarcoma pathogenesis. Int J Cancer. 1996;66:717–722. doi: 10.1002/(SICI)1097-0215(19960611)66:6<717::AID-IJC1>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 35.Moses A V, Fish K N, Ruhl R, Smith P P, Strussenberg J G, Zhu L, Chandran B, Nelson J A. Long-term infection and transformation of dermal microvascular endothelial cells by human herpesvirus 8. J Virol. 1999;73:6892–6902. doi: 10.1128/jvi.73.8.6892-6902.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Munker R, Tasaka T, Park D, Miller C W, Koeffler H P. HHV-8 (KSHV) does not establish latency in prostate cancer cell lines. Prostate. 1997;33:286–288. doi: 10.1002/(sici)1097-0045(19971201)33:4<286::aid-pros10>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 37.Neipel F, Albrecht J C, Fleckenstein B. Cell-homologous genes in the Kaposi's sarcoma-associated rhadinovirus human herpesvirus 8: determinants of its pathogenicity? J Virol. 1997;71:4187–4192. doi: 10.1128/jvi.71.6.4187-4192.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neipel F, Fleckenstein B. The role of HHV-8 in Kaposi's sarcoma. Semin Cancer Biol. 1999;9:151–164. doi: 10.1006/scbi.1999.0129. [DOI] [PubMed] [Google Scholar]
- 39.Olsen S J, Moore P S. Kaposi's sarcoma-associated herpesvirus (KSHV/HHV8) and the etiology of KS. In: Medveczky P G, et al., editors. Herpersviruses and immunity. New York, N.Y: Plenum Press; 1998. pp. 115–147. [Google Scholar]
- 40.Panyutich E A, Said J W, Miles S A. Infection of primary dermal microvascular endothelial cells by Kaposi's sarcoma-associated herpesvirus. AIDS. 1998;12:467–472. doi: 10.1097/00002030-199805000-00007. [DOI] [PubMed] [Google Scholar]
- 41.Purrello M, Balazs I. Direct hybridization of labeled DNA to DNA in agarose gels. Anal Biochem. 1983;128:393–397. doi: 10.1016/0003-2697(83)90391-3. [DOI] [PubMed] [Google Scholar]
- 42.Rainbow L, Platt G M, Simpson G R, Sarid R, Gao S J, Stoiber H, Herrington C S, Moore P S, Schulz T F. The 222- to 234-kilodalton latent nuclear protein (LNA) of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) is encoded by orf73 and is a component of the latency-associated nuclear antigen. J Virol. 1997;71:5915–5921. doi: 10.1128/jvi.71.8.5915-5921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Renne R, Blackbourn D, Whitby D, Levy J, Ganem D. Limited transmission of Kaposi's sarcoma-associated herpesvirus in cultured cells. J Virol. 1998;72:5182–5188. doi: 10.1128/jvi.72.6.5182-5188.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Renne R, Lagunoff M, Zhong W, Ganem D. The size and conformation of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) DNA in infected cells and virions. J Virol. 1996;70:8151–8154. doi: 10.1128/jvi.70.11.8151-8154.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Renne R, Zhong W, Herndier B, McGrath M, Abbey N, Kedes D, Ganem D. Lytic growth of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) in culture. Nat Med. 1996;2:342–346. doi: 10.1038/nm0396-342. [DOI] [PubMed] [Google Scholar]
- 46.Russo J J, Bohenzky R A, Chien M C, Chen J, Yan M, Maddalena D, Parry J P, Peruzzi D, Edelman I S, Chang Y, Moore P S. Nucleotide sequence of the Kaposi sarcoma-associated herpesvirus (HHV8) Proc Natl Acad Sci USA. 1996;93:14862–14867. doi: 10.1073/pnas.93.25.14862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sarid R, Flore O, Bohenzky R A, Chang Y, Moore P S. Transcription mapping of the Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) genome in a body cavity-based lymphoma cell line (BC-1) J Virol. 1998;72:1005–1012. doi: 10.1128/jvi.72.2.1005-1012.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schubert M M, Epstein J B, Lloid M E, Cooney E. Oral infections due to cytomegalovirus in immunocompromised patients. J Oral Pathol Med. 1993;22:268–273. doi: 10.1111/j.1600-0714.1993.tb01069.x. [DOI] [PubMed] [Google Scholar]
- 49.Schulz T F, Moore P S. Kaposi's sarcoma-associated herpesvirus: a new human tumor virus, but how? Trends Microbiol. 1999;7:196–200. doi: 10.1016/s0966-842x(99)01495-x. [DOI] [PubMed] [Google Scholar]
- 50.Siegal B, Levinton-Kriss S, Schiffer A, Sayar J, Engelberg I, Vonsover A, Ramon Y, Rubinstein E. Kaposi's sarcoma in immunosuppression. Possibly the result of a dual viral infection. Cancer. 1990;65:492–498. doi: 10.1002/1097-0142(19900201)65:3<492::aid-cncr2820650320>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 51.Sinzger C, Jahn G. Human cytomegalovirus cell tropism and pathogenesis. Intervirology. 1996;39:302–319. doi: 10.1159/000150502. [DOI] [PubMed] [Google Scholar]
- 52.Soderberg-Naucler C, Fish K N, Nelson J A. Reactivation of latent human cytomegalovirus by allogeneic stimulation of blood cells from healthy donors. Cell. 1997;91:119–126. doi: 10.1016/s0092-8674(01)80014-3. [DOI] [PubMed] [Google Scholar]
- 53.Staskus K A, Zhong W, Gebhard K, Herndier B, Wang H, Renne R, Beneke J, Pudney J, Anderson D J, Ganem D, Haase A T. Kaposi's sarcoma-associated herpesvirus gene expression in endothelial (spindle) tumor cells. J Virol. 1997;71:715–719. doi: 10.1128/jvi.71.1.715-719.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sturzl M, Blasig C, Schreier A, Neipel F, Hohenadl C, Cornali E, Ascherl G, Esser S, Brockmeyer N H, Ekman M, Kaaya E E, Tschachler E, Biberfeld P. Expression of HHV-8 latency-associated T0.7 RNA in spindle cells and endothelial cells of AIDS-associated, classical and African Kaposi's sarcoma. Int J Cancer. 1997;72:68–71. doi: 10.1002/(sici)1097-0215(19970703)72:1<68::aid-ijc10>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 55.Sun R, Lin S F, Gradoville L, Yuan Y, Zhu F, Miller G. A viral gene that activates lytic cycle expression of Kaposi's sarcoma-associated herpesvirus. Proc Natl Acad Sci USA. 1998;95:10866–10871. doi: 10.1073/pnas.95.18.10866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sun R, Lin S F, Staskus K, Gradoville L, Grogan E, Haase A, Miller G. Kinetics of Kaposi's sarcoma-associated herpesvirus gene expression. J Virol. 1999;73:2232–2242. doi: 10.1128/jvi.73.3.2232-2242.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Van den Berg F, Schipper M, Jiwa M, Rook R, Van de Rijke F, Tigges B. Implausibility of an aetiological association between cytomegalovirus and Kaposi's sarcoma shown by four techniques. J Clin Pathol. 1989;42:128–131. doi: 10.1136/jcp.42.2.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Varthakavi V, Browning P J, Spearman P. Human immunodeficiency virus replication in a primary effusion lymphoma cell line stimulates lytic-phase replication of Kaposi's sarcoma-associated herpesvirus. J Virol. 1999;73:10329–10338. doi: 10.1128/jvi.73.12.10329-10338.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vieira J, Huang M-L, Koelle D M, Corey L. Transmissible Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) in saliva of men with a history of Kaposi's sarcoma. J Virol. 1997;71:7083–7087. doi: 10.1128/jvi.71.9.7083-7087.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Waldman W J, Roberts W H, Davis D H, Williams M V, Sedmak D D, Stephens R E. Preservation of natural endothelial cytopathogenicity of cytomegalovirus by propagation in endothelial cells. Arch Virol. 1991;117:143–164. doi: 10.1007/BF01310761. [DOI] [PubMed] [Google Scholar]