Abstract
Novel bovine parechoviruses (Bo ParVs) were isolated from the feces of Japanese black cattle. Phylogenetic analysis revealed that the novel Bo ParVs formed an independent cluster, exhibiting 72.2–75.6% nucleotide sequence identity to previous Bo ParVs, suggesting that they represent a new genotype. Bo ParVs, including the novel Bo ParVs, shared sequence similarity with each other in the 3' untranslated region (3'UTR) and exhibited low sequence similarity (<38.9% identity) to other parechoviruses. However, a secondary structure prediction of the 3'UTR revealed that the Bo ParVs shared conserved motifs in domain 2 with parechovirus B and E, suggesting some evolutionary constrains in this region.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00705-024-06120-5.
Parechoviruses are small, icosahedral, non-enveloped viruses belonging to the genus Parechovirus within the family Picornaviridae [1]. They possess a non-segmented positive-sense RNA genome with 7339–7608 nucleotides (nt), including a single long open reading frame (ORF) flanked by 5' and 3' untranslated regions (UTRs) and a poly-A tail [1]. The ORF encodes a polyprotein, which is divided into the P1, P2, and P3 regions encoding the capsid structural proteins VP4–VP1 and the non-structural proteins 2A–2C and 3A–3D, respectively. Their 5'UTRs include a type II or type IV internal ribosomal entry site (IRES) upstream of the translational start site [2]. Bovine parechovirus (Bo ParV) is a candidate for a new species in the genus Parechovirus whose genome sequence was first discovered in a public database of metagenomic libraries [3]. Although Bo ParV sequence reads have been detected in various tissues, including lymphatic, central nervous system, and digestive tissues, based on public RNA-seq data from cows, the only currently available nearly complete genome sequence of Bo ParV (Bo ParV/2018/4/KOR) was assembled from sequence reads from the lower digestive tract of a cow sampled in 2018 in South Korea [3]. In 2021, the first Bo ParV, Den1/2021/JPN, was isolated in Japan from diarrheic feces of a lactating cow infected with group C rotavirus, using MA104 cells [4]. Subsequently, three additional Bo_ParVs were isolated from diarrheal calves in Japan in 2022, and 6.3% of bovine fecal samples were found to be positive for Bo ParV using qRT-PCR [5]. Therefore, Bo ParV is considered to have an affinity for the intestinal tract. Furthermore, only five nearly complete genome sequences of Bo ParV are currently available from South Korea and Japan. In the present study, novel Bo ParVs were isolated from the feces of Japanese black cattle with or without diarrhea and genetically characterized.
To identify bovine enteric viruses in Japanese black cattle, fecal samples were collected directly from the rectums of four diarrheic calves (eight-day-old to three-month-old) and 40 healthy cattle (one-day-old to adult) kept on a farm located in the Chubu region of the main island of Japan in 2021. Fecal samples were diluted at a 1:9 (w/v) ratio using Eagle’s minimal essential medium (EMEM) (Nissui, Tokyo, Japan) and then centrifuged at 12,000 × g for 10 minutes. The resulting supernatants were activated by adding equal volumes of 20 μg/mL trypsin (cat. no. 0303; Sigma-Aldrich, MO, USA) and incubated for 1 hour at 37 °C. The activated samples were then inoculated onto the African green monkey kidney cell line Marc-145. Confluent monolayers of Marc-145 cells in 48-well plates were washed three times with EMEM and subsequently inoculated with 0.1 mL supernatant from the activated samples. After incubation at 37 °C for 1 hour for adsorption, the cells were washed three times using EMEM. Subsequently, the cells were incubated with EMEM containing 1.0 μg of trypsin per mL for 7 days at 37 °C in a 5% CO2 atmosphere. If a cytopathic effect (CPE) was not observed after 7 days, the cells and supernatant were subjected to three cycles of freezing and thawing before harvesting. Further passages were conducted following the same procedure. CPE was observed in seven out of 44 samples two or three days after inoculation in the second passage.
Viral RNA was extracted from the cell culture supernatants using TRIzol LS Reagent (Life Technologies, Carlsbad, CA, USA). Subsequently, cDNA libraries for deep sequencing were constructed using an NEBNext Ultra RNA Library Prep Kit for Illumina (New England Biolabs, Ipswich, MA, USA) according to the manufacture’s guidelines. After quantification of the cDNA using a Qubit 4.0 fluorometer (Invitrogen, Carlsbad, CA, USA), next-generation sequencing (NGS) was performed using an Illumina MiSeq benchtop sequencer (Illumina, San Diego, CA, USA). Sequence data analyzed using MiSeq Reporter (Illumina) to generate FASTQ-formatted sequence data files. The 151-nt paired-end reads were trimmed using the command “Trim Sequences” in the NGS core tools with default parameters in CLC 7.5.5 Genomics Workbench (CLC bio, Aarhus, Denmark) and then assembled de novo into contigs in CLC 7.5.5 with default parameters. The quality of the viral contigs was assessed by mapping the original reads back to the contigs, and a sufficient depth of mapped reads was observed (Supplementary Fig. S1). Nearly complete genome sequences of Bo ParV were obtained from six of the seven samples (3, 8oya, 8ko, 10, 18, and 101). Two of these samples (3 and 101) were obtained from diarrheic calves, while the remaining four were from two healthy calves and two healthy adult cows (Supplementary Table S1). To obtain the complete sequence of the 3'UTR, the 3' end of the viral genome was amplified by RT-PCR using a gene-specific forward primer and the reverse primer TX30SXN [6] (Supplementary Fig. S2) and sequenced directly by the Sanger method. The whole-genome sequences from samples 3 and 101, 8oya and 8ko, and 10oya and 18 were found to be identical. These sequences—Bo ParV/Mayo3/2022/JPN (Mayo3), Bo ParV/Mayo8oya/2022/JPN (Mayo8oya), and Bo ParV/Mayo18/2022/JPN (Mayo18)—have been deposited in the DNA Data Bank of Japan under the accession numbers LC790729 to LC790731.
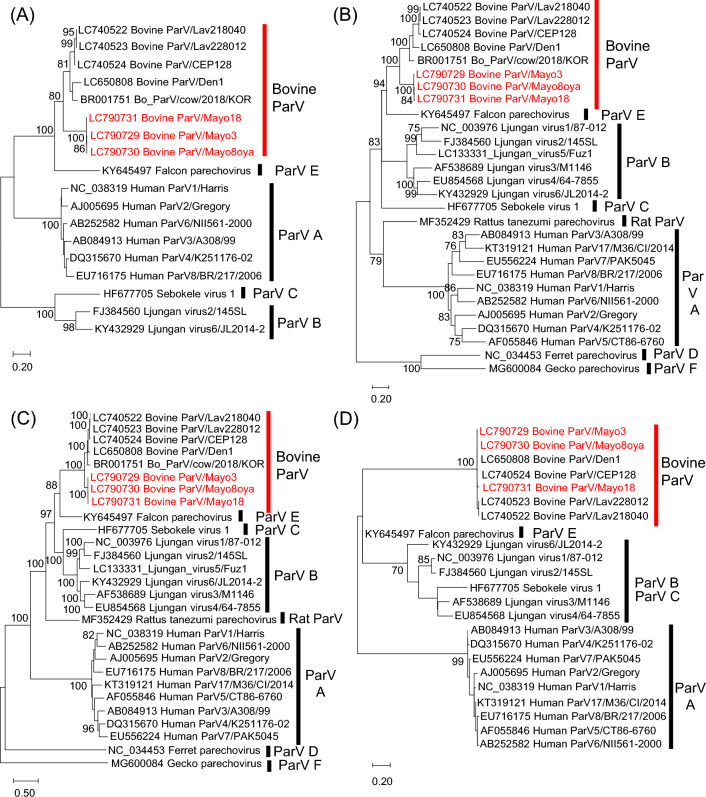
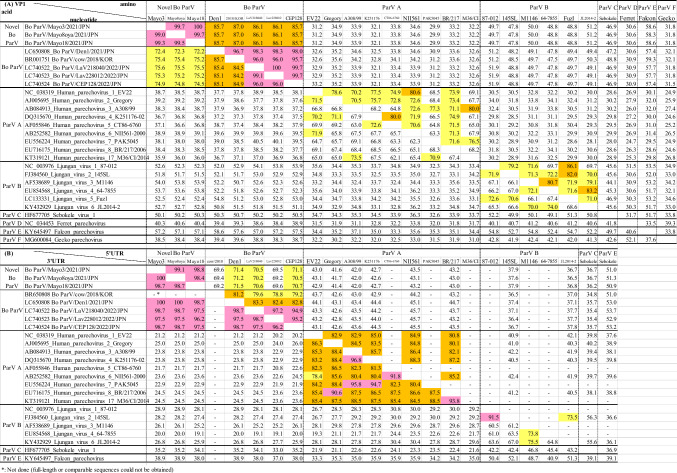
Bo ParV sequences were aligned using ClustalW [7], and pairwise sequence identity values were calculated using CLC Genomics Workbench 7.5.5 (CLC bio). Phylogenetic analysis based on nucleotide sequences was performed using the maximum-likelihood (ML) method with the best-fit model (K2+G for 5'UTR, T92 + G + I for VP1, GTR+G for 2C+3CD, and 3'UTR for HKY) in MEGA7 [8]. The trees were evaluated by bootstrap analysis with 1000 replicates [9]. In the phylogenetic trees, except for the 3'UTR, Mayo3, Mayo8oya, and Mayo18 branched distantly from other Bo ParVs and formed an independent cluster with 100% bootstrap support (Fig. 1A, B, and C). Pairwise complete nt and aa sequence identity was calculated using CLC Genomics Workbench. In the VP1 sequence comparison, Mayo3, Mayo8oya, and Mayo18 exhibited 72.2–75.6% nt and 85.7–87.0% aa sequence identity to the other Bo ParV strains, which shared 85.1–99.1% nt and 95.7–100% aa sequence identity with each other. The VP1 sequences of parechovirus A and parechovirus B strains were 62.1–73.5% and 65.3–74.0% identical at the nucleotide level and 63.3–80.6% and 69.7–86.1% identical at the aa level (Table 1A). No exact genotype classification criteria have been established for parechoviruses; however, previous reports have referred to the criteria used for enteroviruses: 75% nt sequence identity and 88% aa sequence identity [10–13]. The nt sequence identity of the VP1 region between Bo ParVs in this study and previous Bo ParVs is borderline, but the aa sequence identity is below 88%, suggesting that these viruses might represent a novel genotype of Bo ParV. The 2C+3CD regions of Mayo3, Mayo8oya, and Mayo18, and other Bo_ParVs showed higher similarity than the VP1 region (85.6–86.1% nt sequence identity and 97.3–98.6% aa sequence identity) (Supplementary Table S2). Similarity plot analysis performed using SimPlot software v. 3.5.1 [14] showed that the new Bo ParV isolates have a non-structural protein region from 3A to 3D that is most similar to those of the previously reported Bo ParV (Supplementary Fig. S3). The 2C+3CD sequence identity values obtained when excluding Mayo3, Mayo8oya, and Mayo18 and parechovirus A and parechovirus B strains were 89.6–99.0%, 76.0–80.8%, and 72.2–78.7% at the nt level and 98.2–98.8%, 91.9–98.0%, and 83.0–91.9% at the aa level (Supplementary Table S2). These results indicate that the non-structural genes, especially the 3A to 3D regions, are more conserved among Bo ParVs than they are among other parechoviruses. The Bo ParVs from this study and previous Bo_ParVs shared only 69.2–71.5% sequence identity in the 5'UTR while showing high sequence similarity (96.2–100% identity) in the 3'UTR. However, the Bo ParVs exhibited great sequence divergence from other parechoviruses (19.1–38.9%) in the 3'UTR (Table 1B).
Fig. 1.
Phylogenetic analysis based on nucleotide sequences of the 5′UTR (A), VP1 region (B), 2C+3CD region (C), and 3′UTR (D) of the Bo_ParVs identified in this study (red text) and of other parechoviruses, obtained from the DDBJ/EMBL/GenBank database. Phylogenetic trees were constructed using the maximum-likelihood method in MEGA7 with best-fit models (K2+G for the 5′UTR, T92+G+I for VP1, GTR+G for 2B+3CD, and HKY for the 3′UTR). Bootstrap values above 70 (1000 replicates) are shown. The bars represent the corrected genetic distances
Table 1.
Pairwise nucleotide (lower left) and amino acid (upper right) sequence identities (%) of theVP1 among parechoviruses (A)
Pairwise nucleotide sequence identities (%) of the 5'UTR (upper right) and 3'UTR (lower left) among parechoviruses (B). Pink, orange, and yellow show indicate >90%, 90–80%, and 80–70% identities, respectively
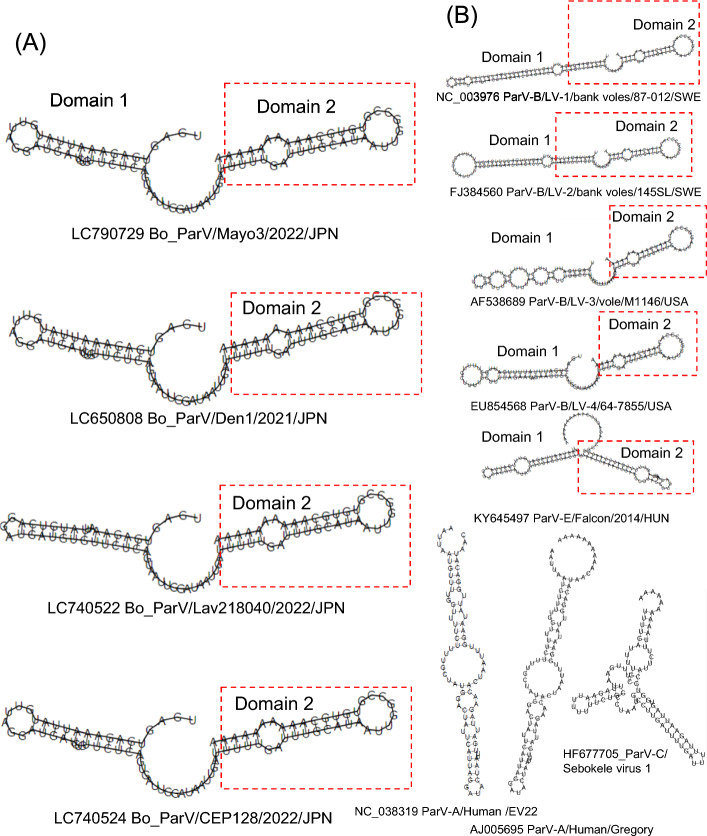
The secondary structures of the viral RNAs were predicted using RNAfold in the ViennaRNA package (version 2.4.18) [15]. The 3'UTR of the Bo_ParVs is 75 nucleotides long, excluding the poly-A tract. Secondary structure prediction analysis revealed that Bo ParVs possess two potential stem-loops that are structurally similar to those found in the 3'UTR of Ljungan virus 1-4 (LV-1/bank voles/87-012/SWE, LV-2/bank voles/145SL/SWE, LV-3/vole/M1146/USA, and LV-4/64-7855/USA), belonging to the species Parechovirus beljungani, and Falcon/2014/HUN, belonging to the species Parechovirus efalco [16, 17]. Although the 3'UTR of Bo ParV strains was the shortest among parechoviruses, the predicted stem-loop structures in domain II, corresponding to the 3' one-third of the 3'UTR, were conserved among these viruses (Fig. 2). The 3'UTR is important for maintaining viral genomic RNA stability and for replication of the picornavirus genome [18–20]. Secondary structure analysis of the internal ribosomal entry site (IRES) in the 5'UTR and the putative cis-acting RNA element (cre) located in the 3B region, which are necessary for viral RNA replication, showed that these structures in Bo ParVs, including the novel Bo_ParVs, were similar to those of Ljungan virus 1-4 and falcon parechovirus (Supplementary Fig. S4). These results indicate that there are some evolutionary constrains in these regions.
Fig. 2.
Secondary structure prediction of the 3′UTR of Bo_ParVs (A) and other parechoviruses (B) using RNAfold in the ViennaRNA package (version 2.4.18). Analysis of the 3'UTR included 10 nt of the poly(A) tail to simulate the authentic viral RNA molecule
In summary, novel Bo ParVs were isolated from Japanese black cattle with or without diarrhea. The genome sequences of these viruses exhibited differences when compared to previous Bo ParVs, suggesting that they represent a novel genotype of Bo ParV. Secondary structure prediction suggested structural conservation in the 5'UTR, cre, and 3'UTR between Bo ParVs and other parechoviruses.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
Mami Oba: conceptualization, investigation, formal analysis, writing—original draft. Mayo Shimotori: resources, investigation. Natsuko Teshima: investigation. Risa Yamaguchi: investigation. Hitoshi Takemae: investigation, formal analysis, Shoichi Sakaguchi: formal analysis. Hiroho Ishida: data curation, writing—review & editing. Hironobu Murakami: data curation, writing—review & editing. Tetsuya Mizutani: supervision, funding acquisition. Makoto Nagai: writing—review & editing, funding acquisition.
Funding
This work was supported by a Livestock Promotional Subsidy from the Japan Racing Association and JSPS KAKENHI (grant number 21K05947).
Data availability
The GenBank/EMBL/DDBJ accession numbers for the sequences of the Bovine ParV/Mayo strains determined in this study are LC790729 to LC790731. Other datasets generated or analyzed during the current study are available from the corresponding authors upon reasonable request.
Declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
The fecal samples that were used in this study were collected for pathological testing of cattle; therefore, no specific approval was needed.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zell R, Delwart E, Gorbalenya AE, Hovi T, King AMQ, Knowles NJ, Lindberg AM, Pallansch AM, Palmenberg AC, Reuter G, Simmonds P, Skern T, Stanway G, Yamashita T, Ictv Report Consortium (2017) ICTV virus taxonomy profile: Picornaviridae. J Gen Virol 98:2421–2422. 10.1099/jgv.0.000911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fernández-Miragall O, López de Quinto S, Martínez-Salas E (2009) Relevance of RNA structure for the activity of picornavirus IRES elements. Virus Res 139:172–182. 10.1016/j.virusres.2008.07.009 [DOI] [PubMed] [Google Scholar]
- 3.Kawasaki J, Kojima S, Tomonaga K, Horie M (2021) Hidden viral sequences in public sequencing data and warning for future emerging diseases. mBio 12(4):e0163821. 10.1128/mBio.01638-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oba M, Sakaguchi S, Wu H, Fujioka Y, Takemae H, Oki H, Kawai M, Shiokawa M, Aoki H, Fukase Y, Madarame H, Nakano T, Mizutani T, Nagai M (2022) First isolation and genomic characterization of bovine parechovirus from faecal samples of cattle in Japan. J Gen Virol. 10.1099/jgv.0.001718 [DOI] [PubMed] [Google Scholar]
- 5.Oba M, Obinata S, Takemae H, Kazama K, Oguro M, Ito K, Kakinuma S, Ishida H, Murakami H, Sakaguchi S, Mizutani T, Nagai M (2023) Prevalence and genetic diversity in bovine parechovirus infecting Japanese cattle. Arch Virol 168:91. 10.1007/s00705-023-05712-x [DOI] [PubMed] [Google Scholar]
- 6.Oka T, Doan YH, Shimoike T (2017) First complete genome sequences of genogroup V, genotype 3 porcine sapoviruses: common 5’-terminal genomic feature of sapoviruses. Virus Genes 53:848–855. 10.1007/s11262-017-1481-8 [DOI] [PubMed] [Google Scholar]
- 7.Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25:4876–4882. 10.1093/nar/25.24.4876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874. 10.1093/molbev/msw054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791. 10.1111/j.1558-5646.1985.tb00420.x [DOI] [PubMed] [Google Scholar]
- 10.Johansson ES, Niklasson B, Tesh RB, Shafren DR, Travassos da Rosa APA, Lindberg AM (2003) Molecular characterization of M1146, an American isolate of Ljungan virus (LV) reveals the presence of a new LV genotype. J Gen Virol 84:837–844. 10.1099/vir.0.18792-0 [DOI] [PubMed] [Google Scholar]
- 11.Mitake H, Fujii Y, Nagai M, Ito N, Okadera K, Okada K, Nakagawa K, Kishimoto M, Mizutani T, Okazaki K, Sakoda Y, Takada A, Sugiyama M (2016) Isolation of a sp. nov. Ljungan virus from wild birds in Japan. J Gen Virol 97:1818–1822. 10.1099/jgv.0.000508 [DOI] [PubMed] [Google Scholar]
- 12.Oberste MS, Maher K, Kilpatrick DR, Flemister MR, Brown BA, Pallansch MA (1999) Typing of human enteroviruses by partial sequencing of VP1. J Clin Microbiol 37:1288–1293. 10.1128/JCM.37.5.1288-1293.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tolf C, Gullberg M, Johansson ES, Tesh RB, Andersson B, Lindberg AM (2009) Molecular characterization of a novel Ljungan virus (Parechovirus; Picornaviridae) reveals a fourth genotype and indicates ancestral recombination. J Gen Virol 90:843–853. 10.1099/vir.0.007948-0.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lole KS, Bollinger RC, Paranjape RS, Gadkari D, Kulkarni SS, Novak NG, Ingersoll R, Sheppard HW, Ray SC (1999) Full-length human immunodeficiency virus type 1 genomes from subtype C-infected seroconverters in India, with evidence of intersubtype recombination. J Virol 73:152–160. 10.1128/JVI.73.1.152-160.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lorenz R, Bernhart SH, Siederdissen CHz, Tafer H, Flamm C, Stadler PF, Hofacker IL (2011) ViennaRNA package 2.0. Algorithms Mol Biol 24(6):26. 10.1186/1748-7188-6-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pounder KC, Watts PC, Niklasson B, Kallio ERK, Marston DA, Fooks AR, Begon M, McElhinney LM (2015) Genome characterisation of two Ljungan virus isolates from wild bank voles (Myodes glareolus) in Sweden. Infect Genet Evol 36:156–164. 10.1016/j.meegid.2015.09.010 [DOI] [PubMed] [Google Scholar]
- 17.Pankovics P, Boros Á, Mátics R, Kapusinszky B, Delwart E, Reuter G (2017) Ljungan/Sebokele-like picornavirus in birds of prey, common kestrel (Falco tinnunculus) and red-footed falcon (F. vespertinus). Infect Genet Evol 55:14–19. 10.1016/j.meegid.2017.08.024 [DOI] [PubMed] [Google Scholar]
- 18.Rohll JB, Moon DH, Evans DJ, Almond JW (1995) The 3′-untranslated region of picornavirus RNA—features required for efficient genome replication. J Virol 69:7835–7844. 10.1128/JVI.69.12.7835-7844.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen JH, Zhang RH, Lin SL, Li PF, Lan JJ, Song SS, Gao JM, Wang Y, Xie ZJ, Li FC, Jiang SJ (2018) The functional role of the 3’ untranslated region and poly(A) tail of duck hepatitis A virus type 1 in viral replication and regulation of IRES-mediated translation. Front Microbiol 25(9):2250. 10.3389/fmicb.2018.02250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kloc A, Rai DK, Rieder E (2018) The roles of picornavirus untranslated regions in infection and innate immunity. Front Microbiol 20(9):485. 10.3389/fmicb.2018.00485 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The GenBank/EMBL/DDBJ accession numbers for the sequences of the Bovine ParV/Mayo strains determined in this study are LC790729 to LC790731. Other datasets generated or analyzed during the current study are available from the corresponding authors upon reasonable request.