Abstract
The unfolded protein response is an evolutionarily conserved mechanism whereby cells respond to stress conditions that target the endoplasmic reticulum (ER). The transcriptional activation of the promoter of GRP78/BiP, a prosurvival ER chaperone, has been used extensively as an indicator of the onset of the UPR. YY1, a constitutively expressed multifunctional transcription factor, activates the Grp78 promoter only under ER stress conditions. Previously, in vivo footprinting analysis revealed that the YY1 binding site of the ER stress response element of the Grp78 promoter exhibits ER stress-induced changes in occupancy. Toward understanding the underlying mechanisms of these unique phenomena, we performed chromatin immunoprecipitation analyses, revealing that YY1 only occupies the Grp78 promoter upon ER stress and is mediated in part by the nuclear form of ATF6. We show that YY1 is an essential coactivator of ATF6 and uncover their specific interactive domains. Using small interfering RNA against YY1 and insertional mutation of the gene encoding ATF6α, we provide direct evidence that YY1 and ATF6 are required for optimal stress induction of Grp78. We also discovered enhancement of the ER-stressed induction of the Grp78 promoter through the interaction of YY1 with the arginine methyltransferase PRMT1 and evidence of its action through methylation of the arginine 3 residue on histone H4. Furthermore, we detected ER stress-induced binding of the histone acetyltransferase p300 to the Grp78 promoter and histone H4 acetylation. A model for the ER stress-mediated transcription factor binding and chromatin modifications at the Grp78 promoter leading to its activation is proposed.
The unfolded protein response (UPR) is an evolutionarily conserved mechanism whereby cells respond to physiologic stress conditions that target the endoplasmic reticulum (ER) (15, 31). For example, when mammalian cells experience prolonged perturbations in the ER due to either calcium depletion stress, a block in N-linked protein glycosylation, or exposure to protein denaturing agents, there is an accumulation of unfolded proteins in the ER lumen. Induction of the UPR triggers intracellular signaling pathways that allow the damaging presence of malfolded proteins in the ER lumen to be communicated to the nucleus and cytoplasm. A major cellular target of the UPR is GRP78/BiP, an ER chaperone that not only binds to unfolded proteins but also regulates the activation of ER stress transducers such as IRE1, PERK, and ATF6 (2, 19, 45). The transcriptional activation of the Grp78 promoter is used extensively as a biological marker for onset of the UPR, as well as a unique model for deciphering the mechanisms whereby ER stress upregulates nuclear gene expression.
Upon treatment of mammalian cells with thapsigargin (Tg), which blocks the ER calcium-ATPase pump and depletes the ER calcium store, the transcription rate of the Grp78 promoter is induced by as much as 20 fold (25). The Tg-induced stress activation of Grp78 is primarily mediated by the multiple copies of the ER stress response element (ERSE) with a consensus sequence of CCAAT(N9)CCACG located upstream of the TATA element, although part of the response may also be attributed to an ERSE-independent pathway (29, 40, 58). ERSE binding transcription factors include NF-Y (also referred to as CBF), YY1, TFII-I, and the nuclear form of ATF6 (11, 24, 26, 34, 59). The transcription factor ATF6 has two isoforms, α and β, both of which have conserved protein domains but divergent transcriptional activation domains (8, 52, 60). ATF6α, the better characterized of the two, is a 90-kDa ER transmembrane protein, a fraction of which relocates to the Golgi and undergoes S1P/S2P-mediated proteolytic cleavage after ER stress (9, 57). The cleaved, nuclear form of ATF6α [ATF6(N)] then translocates to the nucleus to activate target genes, including the Grp78 gene (33). While ATF6(N) is unable to bind directly to DNA, it can activate the ERSE by forming a complex with NF-Y in a manner dependent on the CCACG sequence, which is also the binding sequence for YY1 (7, 26, 60). Despite these advances, the in vivo mechanism of ER stress activation of the Grp78 promoter (in particular, the role of chromatin reconfiguration and modification) is not well understood. The first hint that ER stress induces transcription factor binding or chromatin changes to the mammalian Grp78 promoter is provided by in vivo footprinting analysis in HeLa cells (27). These studies revealed that within a cluster of bases encompassing the YY1/ATF6 binding site of the most distal ERSE there are specific changes in the dimethyl sulfate (DMS) reactivity pattern after ER stress, whereas other regulatory elements, including the NF-Y binding sites at the CCAAT motif, are constitutively occupied (27). Importantly, these inducible changes in factor occupancy at the YY1/ATF6 site were observed under diverse ER stress signals, suggesting that it could be a common and important mechanism for the UPR induction of its target genes.
The mammalian transcription factor YY1 is a constitutively expressed, multifunctional protein capable of conferring both positive and negative regulation of gene expression (4, 12, 47, 50, 51). While a majority of studies document its repressive activity, YY1 is directly involved in the transcriptional activation of c-myc (38), CAR3 (22), Col1a1 (39), and B-type natriuretic peptide (3), among others. In general, YY1 can activate transcription through mechanisms such as direct binding to DNA and interaction with general transcription factors, interaction with other proteins resulting in blockage of the repressive domain of YY1 while unmasking its activation domain, or recruitment of coactivators that either modify other transcription factors or modify histones to achieve an open chromatin state (51). In support of the latter, YY1 is linked to a variety of histone-modifying enzymes that can subsequently alter chromatin structure, such as CBP, p300, and protein arginine methyltransferase 1 (PRMT1) (20, 37). Recently, it was reported that YY1 binds and recruits the histone H4 (Arg3)-specific methyltransferase PRMT1 to a YY1-activated promoter in a targeted fashion to activate specific transcription events (37). Furthermore, PRMT1 itself has been shown to function cooperatively with the acetyltransferase p300 to enhance transcriptional activation of its target promoter (1). Thus, the role of YY1 in recruiting cofactors and chromatin-modifying enzymes may provide important clues on novel in vivo mechanisms for the activation of the Grp78 promoter in response to ER stress.
One unique feature concerning the regulation of the Grp78 promoter by YY1 is that despite its constitutive expression, YY1 has no effect on the basal activity of the Grp78 promoter, yet it strongly enhances the induction of the Grp78 promoter in cells subjected to ER stress (26). Toward understanding the underlying mechanisms of the selective activation of the Grp78 promoter by YY1 under ER stress conditions, we discovered that YY1 only occupies the Grp78 promoter upon ER stress and that this is mediated in part by interaction with the nuclear form of ATF6. Here, we describe the specific interaction of the zinc finger domain of YY1 with the b-Zip domain of the activated form of ATF6 that leads to Grp78 promoter induction. We also describe the further activation of the Grp78 promoter through the interaction of YY1 with the histone H4 methyltransferase PRMT1, as well as the histone acetyltransferase p300. Using small interfering RNA (siRNA) targeted against endogenous YY1 and genetic disruption of the ATF6α gene, we provide evidence that both YY1 and ATF6α are required for optimal Tg stress induction of the Grp78 promoter. A model for the Tg stress-induced modification of the chromatin associated with the Grp78 promoter is presented.
MATERIALS AND METHODS
Cell culture conditions.
NIH 3T3, Cos-7, HeLa, CV-1, and 293T cells were grown in Dulbecco's modified Eagle's medium (Invitrogen) supplemented with 10% fetal calf serum, 100 U/ml penicillin, and 100 μg/ml streptomycin at 37°C in a 5% CO2 environment. Cell lines that had been stably transfected with blasticidin resistance vector and expression plasmid were maintained in similar media with the addition of 10 μg of blasticidin (Invitrogen)/ml. For stress induction, cells were treated with 300 nM Tg (Sigma) for various intervals in normal growth medium.
Plasmids.
The construction of the reporter plasmid -169/Luc has been previously described (30). The expression vectors for CMV-YY1 have been previously described (26). The expression vector for hemagglutinin epitope (HA)-tagged full-length ATF6 (pCGN-ATF6) was provided by Ron Prywes (Columbia University) and has been previously described (62). The expression vectors for HA-tagged full-length PRMT1 and p300 were provided by Michael Stallcup (University of Southern California) and have been previously described (16). The construction of pCGN-ATF6(373) has been previously described (30). Plasmid pCGN-ATF6(273) was constructed in the same manner as pCGN-ATF6(373) with the only exception being the insertion of a stop codon in the DNA sequence to produce a 273-amino-acid (273-aa) protein. The expression vector for the FLAG-tagged full-length YY1 and its mutants have been previously described (56). The pBluescript/U6 derived plasmids used for siRNA targeting of YY1 and the green fluorescent protein (GFP) control have been described (50). The cytomegalovirus (CMV)-enhanced GFP (EGFP)-C2 plasmid used in cell sorting experiments contains a mutagenized form of GFP, which does not share homology with the sequence targeted by the U6 siGFP plasmid.
ATF6α insertional mutant cells and Northern blotting.
Primary mouse embryo fibroblasts (MEFs) derived from homozygous ATF6/βgeo insertional mutant mice were prepared and cultured as described previously (10, 49). The βgeo cassette was inserted into the luminal domain of ATF6α 69 amino acids upstream from the carboxyl terminus. The cells were grown in 6-cm plates to 80% confluence and were further cultured in normal medium or supplemented with 300 nM Tg for 16 h. Total RNA was extracted from the cells with Tri-Reagent (Sigma). Ten micrograms of total RNA was subjected to Northern blot analysis as described previously (61). The transcript levels were quantitated with a PhosphorImager (Molecular Dynamics).
Immunofluorescence staining.
NIH 3T3 cells were transfected with 0.2 μg of HA-ATF6 expression plasmids with Superfect transfection reagent (QIAGEN). Immunofluorescence staining was performed as previously described (34). For the detection of HA-ATF6, the cells were stained with anti-HA monoclonal antibody (1:100 dilution; Santa Cruz Biotechnology, Santa Cruz, CA) and rhodamine-conjugated anti-mouse immunoglobulin G (IgG) (1:500 dilution; Vector Laboratories, Inc., Burlingame, CA). For detection of YY1, NIH 3T3 cells were stained with anti-YY1 monoclonal antibody (1:500 dilution; Santa Cruz) and fluorescein isothiocyanate-conjugated anti-mouse IgG (1:500 dilution). Cells were mounted in Vectashield with or without propidium iodide mounting medium (Vector Labs) and visualized with a Zeiss LSM 510 dual-photon confocal microscope.
Transfection assays.
NIH 3T3 cells were seeded in 24-well plates and grown to 60 to 80% confluence. A total 250 ng of the -169/Luc reporter plasmid was cotransfected with either 0.2 μg of pCGN-ATF6(373) or empty vector and various amounts of either the YY1 wild-type or YY1 deletion mutant expression plasmids using Superfect transfection reagent (QIAGEN). For the U6 siYY1 transfection assays, 293T cells were seeded in six-well plates and grown to 80% confluence. Cells were then transfected with 0.5 μg of -169/Luc, 0.5 μg of CMV-β-galactosidase (CMV β-Gal) and 1 μg of either U6 siGFP (U6 siControl) or U6 siYY1 plasmids. Approximately 96 h after transfection, cells were transfected again with 0.25 μg or 0.5 μg of pCGN-ATF6(373) and after an additional 18 h, cells were lysed and assayed for luciferase activity. For the pCGN-ATF6(273) and pCGN-ATF6(373) transfection for chromatin immunoprecipitation (ChIP) assay analysis of YY1 binding to the Grp78 promoter, two 15-cm diameter dishes of 293T cells were each transfected with 16 μg of plasmid using Polyfect transfection reagent and subjected to a ChIP assay 20 h after transfection. For the transfection assays involving the induction of Grp78 promoter by YY1 before and after Tg stress or by PRMT1, ATF6(373), and p300, CV-1 cells were seeded in 24-well plates and grown to 40 to 50% confluence. They were subsequently transfected with 0.5 μg of total DNA, including the CMV-Renilla luciferase control vector, using Polyfect transfection reagent (QIAGEN). Transfected cells were harvested at least 24 h after transfection and assayed for luciferase activity by luminometer (Turner Design Systems, Sunnyvale, CA).
Western blotting.
Whole-cell lysates were prepared in radioimmunoprecipitation buffer as previously described (7). Preparation of the HeLa nuclear extract from control and cells treated with Tg for 6 h has been previously described (34). Conditions for Western blotting were as previously described (24). The primary antibodies used were mouse YY1 monoclonal antibody (H-10) (Santa Cruz Biotechnology) at a dilution of 1:1,000 and anti-glyceraldehyde-3-phosphate dehydrogenase (anti-GAPDH) antibody (Ambion, Austin, TX) at a dilution of 1:4,000. For detection of HA-tagged ATF6, the primary antibody used was mouse HA monoclonal antibody (Santa Cruz) at a dilution of 1:500. For detection of the FLAG-tagged YY1, the primary antibody used was either mouse monoclonal FLAG (Sigma) or rabbit polyclonal FLAG (Sigma) antibody at a dilution of 1:1,000. For detection of PRMT1, a rabbit PRMT1 polyclonal antibody (Upstate Biotechnology) was used. Protein bands were visualized by horseradish peroxidase-enhanced chemiluminescence (Amersham).
Chromatin immunoprecipitation.
The ChIP assay was carried out as previously described (29). Equal amounts of chromatin from each sample were incubated at 4°C overnight with at least 5 μg of antibodies against either YY1 (Santa Cruz), NF-Y (gift of Sankar Maity, University of Texas), acetylated histone H4 (Upstate Biotech), histone H3 (Santa Cruz), PRMT1, methylated R3 histone H4, or p300 (gifts of Michael Stallcup, University of Southern California) with mouse or rabbit IgG (Santa Cruz) as a negative control. After reversal of cross-links, the DNA was purified by phenol-chloroform extraction and ethanol precipitation. Purified DNA from the input and IP samples were subjected to 30 to 45 cycles of PCR, and the products were run on a 1.8% agarose gel and visualized with ethidium bromide staining. The primers used were as follows: for mouse Grp78, 5′-CATTGGTGGCCGTTAAGAATGAC (forward) and 5′-AGTATCGAGCGCGCCGTCGC (reverse), yielding a 223-bp product. For human Grp78, 5′-GTGAACGTTA GAAACGAATAGCAGCCA (forward) and 5′-GTCGACCTCACCGTCGCCTA (reverse), yielding a 213-bp product. For the 3′ end of Grp78, the primers used for mouse samples were 5′-AGAGCGCATTGACACCAGGAATGAA (forward) and 5′-CCTCCACTTCCATAGAG TTTGCTGATA (reverse), yielding a 248-bp product; for human samples, the primers used were 5′-CCTCTGAAGATAAGGAGACCATGGAA (forward) and 5′-TGCTGTATCC TCTTCACCAGTTGG (reverse), yielding a 187-bp product. Most ChIP assays were repeated at least three to five times, with the assays for PRMT1 and methylated H4R3 repeated twice.
Coimmunoprecipitation assays.
Immunoprecipitation of FLAG-YY1 proteins were performed using anti-FLAG M2 affinity gel (Sigma), following the manufacturer's suggestions; normal mouse IgG was used as a negative control. For HA-tagged ATF6 immunoprecipitation, a protein extract from each sample was immunoprecipitated with 5 μg of an anti-HA monoclonal antibody (Santa Cruz) at 4°C overnight. Antibody-protein complexes were collected by incubation with protein A-Sepharose beads (Sigma), washed, collected by centrifugation, and incubated in elution buffer to release the protein complexes. The immunoprecipitates were then subjected to Western blotting.
GFP cell sorting and reverse transcription-PCR (RT-PCR).
293T cells were grown in 75-cm2 flasks to 80% confluence and then transfected with 7 μg of either U6 siControl or U6 siYY1 plasmids and 1 μg of EGFP-C2 plasmid with Polyfect transfection reagent. After 96 h, cells were either left untreated or treated with 300 nM Tg for 4 h and subjected to fluorescence sorting. Cells containing EGFP were lysed, and RNA was isolated by phenol-sodium dodecyl sulfate (SDS) extraction. Approximately 1 μg of RNA from each sample was used as a template to produce cDNA with Superscript II and oligo(dT) (Invitrogen), following the protocol from the manufacturer. PCRs were carried out using a cDNA template and gene-specific primers for GAPDH (17), Grp78 (48), and YY1 (41) for 30, 26, 22, and 18 amplification cycles for determination of linear range. PCR products were run on a 1.4% agarose gel and quantitated with the Gel-Doc 2000 system (Bio-Rad).
RESULTS
YY1 is required for full induction of the Grp78 promoter in response to Tg stress. The human Grp78 gene contains eight exons and is highly conserved between humans and mice. The promoter of the Grp78 gene contains a canonical TATA element and three ERSEs with the consensus sequence of CCAAT(N9)CCACG. Previous in vivo footprinting studies reveal factor interaction sites that are constitutively occupied, as well as sites that exhibit changes following ER stress (27). Within the proximal region that contains the three ERSEs, the CCAAT binding site of all three ERSEs shows protection of DMS methylation of G residues before and after ER stress. The most striking change in the methylation protection pattern following ER stress occurred at the GGCCAGC motif of ERSE#3; the first G residue became more sensitive and the second and third G residues became more protected in the stressed nuclei (Fig. 1A). With the exception of 2 bp, the sequence of this ERSE and its flanking sequences were completely conserved between human and mouse samples (Fig. 1A). Taken together, these results suggest that the CCAAT binding factor NF-Y binds the ERSE constitutively, whereas factors binding to the CCAGC motif occupy the site after stress. Currently, two transcription factors, YY1 and the nuclear form of ATF6, have been shown to bind the CCAGC site in in vitro assays (26, 40, 60).
FIG. 1.
YY1 selectively activates the Grp78 promoter in Tg-stressed cells and is required for its full stress induction. (A) Summary of ER stress-induced changes in the DMS methylation pattern of the Grp78 promoter as revealed by in vivo footprinting (27). The sequence of the highly conserved human and mouse Grp78 promoter containing ERSE#3 where most of the ER stress-induced site occupancy changes occur is aligned. The solid arrow indicates constitutive protection from DMS methylation of the G residue. The open arrows and star indicate ER stress-inducible DMS methylation protection and hypersensitivity, respectively. The binding sites for the transcription factors NF-Y, TFII-I, and ATF6/YY1 are underlined. (B) CV-1 cells were transfected with the -169/Luc reporter plasmid and with either CMV-driven YY1 full-length expression plasmid or empty vector (V). Twenty-four hours after transfection, the cells were treated with 300 nM Tg for 16 h, harvested, and assayed for luciferase activity. (C) 293T cells were transfected with the EGFP vector and either U6 siControl or U6 siYY1. After 96 h, the cells were treated with Tg for 4 h and subjected to cell sorting by fluorescence. RNA was isolated from each sample, and equal amounts were used as template in RT-PCRs with oligo(dT) primers for cDNA synthesis and gene-specific primers as indicated. PCRs were carried out with 30, 26, 22, and 18 amplification cycles for determination of linear ranges. Data shown were subjected to 22 cycles of PCR. (D) Quantitation of Grp78 PCR products in panel C is shown, corrected for GAPDH levels.
Previously, we reported that despite the constitutive presence of YY1 in NIH 3T3 cells, it could only activate the CAT reporter gene driven by the Grp78 promoter under ER stress conditions; however, the mechanism is not known (26). To address this, we first used a second reporter gene system and a new cell system to confirm the selective effect of YY1 on the Grp78 promoter. CV-1 cells were cotransfected with an expression plasmid for YY1 and -169/Luc, a luciferase plasmid driven by the Grp78 promoter containing the three ERSEs (30). In nonstressed cells, overexpression of YY1 had no effect on the reporter gene activity; after Tg treatment, a 5-fold increase in Grp78 promoter activity was detected, and this increase was enhanced to 12 fold in cells overexpressing YY1 (Fig. 1B). Thus, two different reporter gene systems in two different cell types independently confirmed that YY1 can only activate the Grp78 promoter after Tg stress.
We next determined the effect of YY1 depletion by siRNA on the Tg stress induction of endogenous Grp78 mRNA levels. The U6 siYY1 or a U6 siControl plasmid and an EGFP expression plasmid were transfected into 293T cells; 96 h later, the cells were treated with Tg for 4 h and sorted by fluorescence. RNA was then isolated from each sorted sample and subjected to RT using oligo(dT) primers. The resulting cDNA was then used in PCRs to amplify coding regions from YY1, Grp78, and GAPDH. In the cells transfected with U6 siYY1, the level of YY1 was below the detection limit (Fig. 1C). Depletion of YY1 showed minimal effect on the basal expression of Grp78 mRNA; however, the Tg stress-induced level of Grp78 mRNA was reduced from a sixfold increase as seen in the U6 vector to about twofold (Fig. 1D). These results indicate that YY1 is required for full stress induction of Grp78.
YY1 selectively binds the Grp78 promoter after Tg stress.
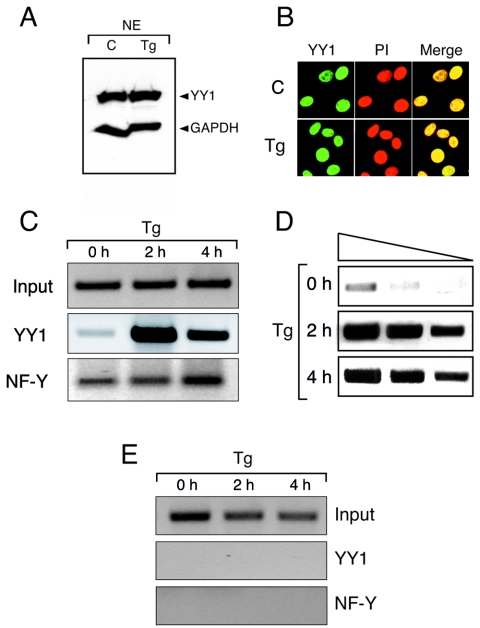
To determine the mechanisms whereby YY1 can selectively activate the Grp78 promoter upon Tg stress, we sought to determine whether YY1 itself undergoes Tg stress-induced changes in protein level or location. Using HeLa nuclear extracts prepared from control cells and cells treated with Tg in Western blot analyses, we observed that YY1 is constitutively produced in similar amounts both before and after Tg stress, compared to the GAPDH loading control (Fig. 2A). Confocal microscopy of control and Tg-treated NIH 3T3 cells with antibodies against YY1 and counterstained with propidium iodide confirmed a similar level of YY1 and further showed that YY1 remained nucleus localized both before and after ER stress (Fig. 2B).
FIG. 2.
ER stress-induced YY1 binding to the Grp78 promoter in vivo. (A) A total of 20 μg of HeLa nuclear extract from control and cells treated with Tg for 16 h was loaded onto a SDS-polyacrylamide gel, and the subsequent blot was reacted with anti-YY1 polyclonal and anti-GAPDH monoclonal antibodies. (B) NIH 3T3 cells were grown to 50% confluence in chamber slides and treated as indicated with 300 nM Tg for 8 h. The cells were fixed and stained with anti-YY1 monoclonal antibody and counterstained with propidium iodide. The cells were visualized with a Zeiss LSM510 confocal microscope (magnification, ×300). (C) NIH 3T3 cells were grown to 80 to 90% confluence; treated with 300 nM Tg for 0, 2, or 4 h; and then cross-linked with 1% formaldehyde. Chromatin extracts were prepared, and immunoprecipitation reactions were carried out with antibodies against YY1 or NF-Y. After reversal of cross-links, DNA was subjected to PCRs to amplify a 223-bp region of the Grp78 promoter. (D) Threefold dilutions of the DNA from the YY1 ChIP assay as shown in panel C were subjected to PCR for confirmation of linear range. (E) DNA collected from the YY1 and NF-Y ChIP assays were subjected to PCR with primers for a 248-bp region of the Grp78 exon VIII coding region.
Next, ChIP assays were performed to determine the in vivo binding kinetics of YY1 to the Grp78 promoter following Tg treatment. NIH 3T3 cells were either left untreated or treated with Tg for 2 or 4 h. DNA-protein complexes were immunoprecipitated with antibodies against either YY1 or NF-Y. Purified DNA was used in PCRs that amplified a 223-bp region of the proximal Grp78 promoter containing the three ERSEs or, as a control, a 248-bp region carrying exon VIII of Grp78. In nonstressed cells, the presence of YY1 on the Grp78 promoter was barely detectable (Fig. 2C). Previously, we showed a 25-fold increase in the transcription rate of the endogenous Grp78 by 3 h of Tg treatment (25). The ChIP assay showed a dramatic increase in YY1 binding to Grp78 after 2 h of Tg treatment, which persisted through 4 h of Tg treatment (Fig. 2C). In contrast, NF-Y was detected on the Grp78 promoter constitutively. The results of YY1 binding to the Grp78 promoter were confirmed to be in the linear range of PCR by performance of the same PCR as shown in Fig. 2C but with threefold serial dilutions of the immunoprecipitated DNA (Fig. 2D). Further, while YY1 exhibited Tg stress-inducible binding and NF-Y showed constitutive binding to the promoter region of Grp78, neither bound to exon VIII (Fig. 2E).
The nuclear form of ATF6 increases YY1 binding to the Grp78 promoter.
One mechanism for stabilization of YY1 binding the Grp78 promoter in response to ER stress is through association with ER stress-specific transcription factors such as the activated form of ATF6. First, to resolve the conflicting reports on the importance of ATF6α in the induction of Grp78 in response to ER stress (18, 52), we utilized primary MEFs derived from homozygous mice with an insertional mutation of ATF6α (ATF6/βgeo) introduced by a gene trap method (49). Western blot analysis of the wild-type and mutant MEFs before and after Tg stress conditions confirmed that the cells bearing the insertion expressed the ATF6/βgeo fusion protein. The size of the fusion protein was about 250 kDa, compared to the 90-kDa size observed for the wild-type protein. Upon Tg stress, the cleaved form of ATF6 was detected in the wild-type cells but not the mutant form (Fig. 3A). A Northern blot of RNA from ATF6/βgeo versus wild-type MEFs subjected to normal culture conditions and 300 nM Tg for 16 h showed a 25-fold induction of Grp78 mRNA levels after ER stress in wild-type cells. However, in the ATF6 mutant cell line, the induction of Grp78 was reduced fivefold, indicating that ATF6 is a major factor in the ER stress induction pathway of Grp78 (Fig. 3B).
FIG. 3.
ATF6α is required for full induction of Grp78 and its nuclear form co-localizes with YY1. (A) Primary MEFs (wild type) or with a βgeo gene trap mutation in the ATF6α gene were cultured and treated with Tg for 16 h. Western blotting of protein extracts from control cells and cells with Tg was performed with antibody against ATF6 (C1.12) (24). The bands corresponding to the wild-type ATF6 (p90) and its nuclear form ATF6(N), as well as the ATF6/βgeo fusion protein (p250), are shown. (B) RNA was isolated from the wild-type and mutant mouse ES cells treated with Tg for 16 h. Northern blotting was performed to probe for Grp78 transcript level with GAPDH as a control. The results were quantitated on a phosphorimager and are shown in graph format. (C) Cos-7 cells were grown to 50% confluence and transfected with either full-length HA-ATF6 or HA-ATF6(373) plasmids. After a 24-h incubation, the cells were fixed and stained with anti-HA monoclonal antibody (red) and anti-YY1 (green) polyclonal antibody. The cells were visualized on a Zeiss LSM510 confocal microscope (magnification, ×380). The merged image shows colocalization between HA-ATF6(373) and YY1 in the transfected cells (yellow). (D) 293T cells were transfected with either HA-ATF6(273) or HA-ATF6(373), and equal amounts of whole-cell extracts were subjected to sequential Western blotting to determine HA-ATF6 expression levels, with the GAPDH level serving as a loading control. (E) The transfected cells from the results shown in panel D were concurrently cross-linked with formaldehyde, and chromatin preparations were subjected to the ChIP assay with antibody against YY1 performed in duplicate and normal IgG as control. PCR primers for the 21-bp region of the human Grp78 promoter encompassing the three ERSEs were used, and the products are shown. Cells transfected with HA-ATF6(273) (top) and cells transfected with HA-ATF6(373) (bottom) are shown.
YY1 has been previously shown to be a coactivator of ATF6, but the mechanism is not understood (24). To visualize colocalization of endogenous YY1 with ATF6, Cos-7 cells were transfected with an expression vector encoding either HA-tagged full-length ATF6, which normally resides as an ER transmembrane protein, or HA-ATF6(373), which encodes the cleaved, nuclear form of ATF6. The cells were then probed with anti-HA and anti-YY1 antibody and fluorescent secondary antibodies and viewed by confocal microscopy. As expected, p90ATF6 showed a perinuclear staining pattern characteristic of ER localization, while the nuclear form of ATF6 showed distinct nuclear staining (Fig. 3C). Endogenous YY1 was observed in the nucleus regardless of the type of ATF6 transfected, and colocalization between YY1 and the nuclear form of ATF6(373) was evident in cells expressing the exogenous protein.
To investigate the effect of ATF6(N) on the recruitment of YY1 to the Grp78 promoter, 293T cells transfected with either HA-ATF6(373) or HA-ATF6(273), an inactive mutant form of ATF6 containing the transactivation domain but not the b-Zip domain, were subjected to the ChIP assay. An aliquot of whole-cell extract prepared from the transfected cells before cross-linking was subjected to Western blot analysis to confirm the presence of equivalent amounts of expressed HA-ATF6(273) and HA-ATF6(373), with GAPDH used as a loading control (Fig. 3D). The subsequent ChIP assay with anti-YY1 antibody (as in the experiment shown in Fig. 2C) indicated that in the presence of HA-ATF6(373), YY1 binding to the Grp78 promoter was increased, but this effect was not seen in the presence of HA-ATF6(273) (Fig. 3E), providing direct evidence that ATF6(373) promotes YY1 binding to the Grp78 promoter in vivo.
Mapping of the interactive domains between YY1 and ATF6.
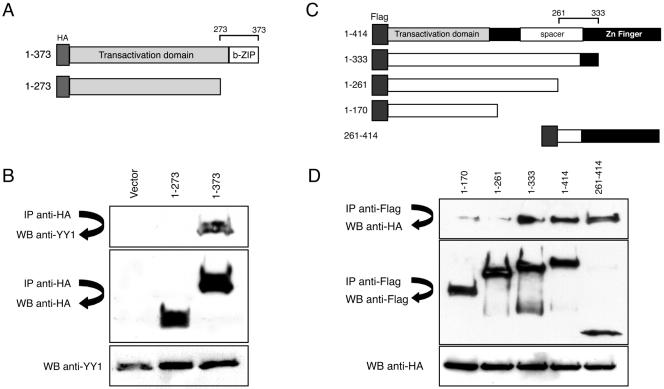
To map the interactive domains between ATF6 and YY1, coimmunoprecipitation experiments were performed. Expression vectors coding for HA-ATF6(373) and HA-ATF6(273) (Fig. 4A) were transfected into 293T cells. The cell lysates were subjected to immunoprecipitation with antibody against HA and Western blotted with antibodies against YY1 to detect coimmunoprecipitation of the proteins. HA-ATF6(373) but not HA-ATF6(273) was able to interact with YY1. These results show that the region of ATF6 between aa 273 to 373 encompassing the b-Zip domain is integral to its YY1 binding relationship (Fig. 4B).
FIG. 4.
Mapping of YY1 and ATF6α interactive domains. (A) Schematic drawings of the nuclear form of the HA-tagged ATF6 (aa 1 to 373) and the truncated mutant (aa 1 to 273). Locations of the transactivation and the b-Zip domains are indicated. The domain required for interaction with YY1 is bracketed on top. (B) 293T cells were transfected with either the empty vector or the HA-ATF6 plasmids (top), and whole-cell extracts were immunoprecipitated with antibody against HA. Immunoprecipitated protein preparations (top two panels) and whole-cell extract (bottom) were run on 8% SDS-polyacrylamide gel electrophoresis gels and Western blotted with either anti-YY1 or anti-HA antibodies as indicated. (C) Schematic representation of the full-length FLAG-YY1(1-414) with its transcriptional activation, spacer, and zinc finger domains indicated. The deletion mutants used for immunoprecipitation assays are shown below. The zinc finger domain is indicated. The region (aa 261 to 333) required for interaction with ATF6 is bracketed on top. (D) FLAG-YY1 constructs as indicated (top) were transfected into 293T cells. Whole-cell extracts were prepared and subjected to immunoprecipitation with anti-FLAG antibody-conjugated agarose. Immunoprecipitated proteins resolved on a 4 to 15% gradient-denaturing gel were analyzed by Western blotting to detect HA-tagged ATF6(373) (top), FLAG-tagged YY1, and Western blots of whole-cell extracts with anti-HA antibodies (bottom).
To map the YY1 domain required for binding to the nuclear form of ATF6, coimmunoprecipitation experiments were carried out using lysates from 293T cells transfected with HA-ATF6(373) and expression vectors encoding for FLAG-tagged YY1 proteins from amino acids 1 to 170, 1 to 261, 1 to 333, 1 to 414 (full-length), and 261 to 414 (Fig. 4C). Western blotting of the immunoprecipitates with antibody against HA revealed coimmunoprecipitation of the regions of YY1 spanning aa 1 to 333, 1 to 414, and 261 to 414 but not aa 1 to 170 or aa 1 to 261 (Fig. 4D). The 295-to-414 aa region of YY1 is the zinc finger domain required for DNA binding (4). Our results indicated that the region of YY1 between aa 261 to 333 is integral to its interaction with ATF6(373).
YY1 is required for optimal ATF6 activation of the Grp78 promoter.
Since YY1 is a binding partner of ATF6, we used two independent approaches to determine the functional interaction between YY1 and ATF6. In the first approach, NIH 3T3 cells were transfected with -169/Luc as the reporter gene and cotransfected with HA-ATF6(373) to mimic an ER stress response, either in the absence or presence of increasing amounts of the domain of YY1 spanning aa 1 to 170 and aa 260 to 414. The prediction was that overexpression of domain at aa 260 to 414 but not aa 1 to 170 will titrate ATF6(373) away from productive interaction with endogenous YY1 and possibly other protein partners, thus negatively affecting its activity towards the Grp78 promoter. As expected, we observed a 15-fold induction of the Grp78 promoter in the presence of exogenous ATF6(373), and this induction was unhampered by the addition of increasing amounts of transfected FLAG-YY1(1-170). However, in the presence of increasing amounts of transfected FLAG-YY1(260-414), the form of YY1 that showed distinct interaction with HA-ATF6(373), there was a dose-dependent attenuation of Grp78 promoter activity (Fig. 5A). These results show that overexpression of a nonfunctional YY1 subfragment that is capable of interfering ATF6 binding with endogenous YY1 results in functional consequences.
FIG. 5.
YY1 is required for optimal activity of nuclear ATF6. (A) 293T cells were transfected with ATF6(373), -169/Luc, CMV β-Gal and increasing amounts of either FLAG-YY1(1-170) or FLAG-YY1(260-414). Cell extracts were prepared and assayed for luciferase activity. Values corrected for transfection efficiency by β-Gal activity are plotted. The standard deviations are shown. (B) 293T cells were transfected with EGFP vector and either U6 siControl or U6 siYY1. Transfection efficiency was determined to be at least 70% by GFP. Extracts were prepared 96 h after transfection and analyzed by Western blotting with antibodies against YY1 and GAPDH. (C) 293T cells transfected with U6 siControl or U6 siYY1, -169/Luc, and CMV β-Gal vectors were grown for 96 h. Cells were then transfected with ATF6(373); 24 h later, cell extracts were prepared and assayed for luciferase activity. Fold induction for each condition after normalization for β-Gal activity is plotted with standard deviations. The amount of transfected ATF6(373) in micrograms is shown at the bottom.
In the second approach, using siRNA directed against YY1, we sought to directly determine the requirement of YY1 for ATF6 activity on the Grp78 promoter. Using a U6 plasmid encoding for a dsRNA oligo targeted against YY1, it was determined that with a 70% transfection efficiency of the U6 siYY1 plasmid (counted by EGFP fluorescence), a substantial decrease in the level of YY1 in 293T cells could be seen by Western blot compared to cells transfected with the U6 siControl (Fig. 5B). In subsequent transfection assays, either the U6 siYY1 plasmid or U6 siControl, and EGFP expression plasmids were cotransfected with the -169/Luc reporter vector. Approximately 96 h after transfection with U6 siYY1, the same cells were transfected with HA-ATF6(373) and assayed for luciferase activity 24 h later. These assays revealed that induction of the Grp78 promoter in response to increasing amounts of exogenous ATF6(373) was negatively affected by the reduction of the endogenous level of YY1 (Fig. 5C). Thus, YY1 contributes to optimal activation of the Grp78 promoter by the nuclear form of ATF6.
YY1 interactive proteins PRMT1 and p300 are recruited to the Grp78 promoter after ER stress.
In addition to binding to transcription factors, YY1 can bind chromatin modifiers such as the histone H4 (Arg3)-specific methyltransferase PRMT1, thereby enhancing transcription (37). To determine the binding characteristics of YY1 and PRMT1 in control and Tg-stressed cells, we performed a coimmunoprecipitation reaction using Cos-7 cells stably transfected with F-YY1. Immunoprecipitation with anti-Flag M2-conjugated agarose beads and subsequent Western blotting with anti-Flag and anti-PRMT1 antibodies revealed complex formation between YY1 and PRMT1 both before and after Tg-induced stress, and this complex was not observed in immunoprecipitation with normal mouse IgG (Fig. 6A).
FIG. 6.
PRMT1 is recruited to the Grp78 promoter in Tg-stressed cells and enhances activation mediated by YY1 and ATF6(373). (A) Cos-7 cells were stably transfected with F-YY1 and CMV-Bsd. Cells were either cultured under normal conditions or treated with Tg for 3 h and harvested. Whole-cell extracts were immunoprecipitated with either normal mouse IgG or anti-Flag M2 agarose. Immunoprecipitates were then run on an 8% polyacrylamide gel and probed with antibodies against either Flag or PRMT1. A31 cell extract was used as a positive control for the presence of PRMT1. *, nonspecific band. (B) HeLa cells were treated with Tg for 3 h, chromatin extracts were prepared as shown in Fig. 4, and immunoprecipitation was carried out with antibodies against YY1, PRMT1, and the methylated arginine 3 residue of histone H4, as well as normal IgG. Products of the PCR using primers for the 213-bp region of the Grp78 promoter are shown. In each transfection experiment, CV-1 cells were transfected with -169/Luc reporter vector and CMV-Renilla luciferase control vector; pBluescript was used as an empty vector. Cells were harvested 24 h after transfection and assayed for dual-luciferase activity. (C) Increasing amounts of PRMT1 expression plasmid (in nanograms) were cotransfected as indicated. (D) Increasing amounts of YY1 expression plasmid (in nanograms) were cotransfected to establish the synergistic induction of the Grp78 promoter by YY1 in the presence of ATF6(373). (E) Increasing amounts of PRMT1 (in nanograms) were cotransfected with YY1 and ATF6(373) expression plasmids. The luciferase activity of the -169/Luc reporter gene alone is set at 1. *, P value of <0.05.
Since YY1 selectively binds the Grp78 promoter in vivo in response to ER stress, the association between YY1 and PRMT1 predicts that PRMT1 may also bind the Grp78 promoter in the ER-stressed nuclei. ChIP assays reveal that PRMT1 exhibits increased binding to the Grp78 promoter in Tg-stressed cells, as does YY1 (Fig. 6B). In accordance with its histone methyltransferase activity, the level of histone H4 Arg3 methylation associated with the Grp78 promoter was substantially elevated. As a negative control, PCR was carried out using primers against exon VIII of Grp78, and no PCR product was detected (data not shown). These in vivo results reveal that PRMT1 is recruited along with YY1, correlating with Tg stress-induced H4R3 methylation.
To further explore the role of PRMT1 in the induction of the Grp78 promoter, we performed transfection assays with CV-1 cells using the -169/Luc and a combination of plasmids coding for YY1, ATF6(373), and PRMT1. We first examined the effect of expressing increasing amounts of PRMT1 on the induction of the Grp78 promoter in nonstressed cells. The results show that within the range of plasmids being tested, in the absence of overexpression of its known cofactor YY1, PRMT1 has little to no effect on basal -169/Luc activity (Fig. 6C). Next, we established the optimal conditions for the synergistic effect of YY1 on the Grp78 promoter induction by ATF6(373) by using a fixed amount of ATF6(373) expression plasmid and increasing amounts of the full-length flag-tagged YY1 expression plasmid (Fig. 6D). The results showed that YY1 activation of ATF6(373) was dosage dependent, such that at higher concentrations of YY1, activation was attenuated. Finally, when both YY1 and ATF6(373) were included in the transfection at a level where ATF6 induced the Grp78 promoter and YY1 enhanced that induction, cotransfecting increasing amounts of PRMT1 expression plasmid resulted in increased activity of the -169/Luc reporter plasmid in CV-1 cells (Fig. 6E). Additionally, PRMT1 showed no effect on the induction of the Grp78 promoter by ATF6(373) in the absence of YY1. PRMT1 overexpression has been determined to have no effect on the expression plasmids encoding \ HA-ATF6(373) or CMV-YY1 (data not shown). These results show that PRMT1, in the presence of YY1, is able to enhance the transcriptional activation of the Grp78 promoter by the nuclear form of ATF6. Although the increase is modest (1.8-fold), this represents further enhancement over a 6- to 8-fold increase already achieved by a combination of ATF6(373) and YY1.
To further characterize the role of YY1 in the recruitment of chromatin-modifying cofactors to the Grp78 promoter, we looked for evidence that the histone acetyltransferase p300, a well-known binding partner of YY1, was involved. We again employed ChIP assays with HeLa cells to test recruitment of p300 to the Grp78 promoter and functional evidence of its binding. We observed that p300, much like PRMT1, exhibited increased binding to the Grp78 promoter in Tg-stressed cells, and this binding was concurrent with acetylation of Histone H4 (Fig. 7A). The relatively constant level of histone H3 binding to the Grp78 promoter served as a positive control for the ChIP experiment, and immunoprecipitation with normal mouse IgG served as a negative control. Since it has been shown that histone methyltransferase and acetyltransferase activity can synergistically activate the transcription of a promoter (16, 23), we further tested the contribution of p300 to the activation of the Grp78 promoter in transfection assays. Using CV-1 cells in conditions identical to transfection assays shown in Fig. 6, we observed a p300-mediated increase in activation of the Grp78 promoter over the YY1/ATF6(373) induction (Fig. 7B). p300 overexpression has been determined to have no effect on the expression plasmids encoding HA-ATF6(373) or CMV-YY1 (data not shown). Addition of PRMT1 to the transfection further enhanced this activity, raising the possibility that PRMT1 may work in conjunction with p300 to activate the Grp78 promoter after ER stress. Furthermore, the enhancing activities of p300 and PRMT are largely dependent on YY1, suggesting that YY1 is the molecular link between these chromatin modifiers and activation of the Grp78 promoter by ER stress.
FIG. 7.
p300 is recruited to the Grp78 promoter in Tg-stressed cells, and its enhancing activity is dependent on YY1. (A) The ChIP assays were performed with HeLa cells as described in the legend to Fig. 6B. The immunoprecipitation assays were carried out with normal IgG or antibodies against p300, acetylated histone H4, and histone H3, and the primers for PCR are identical to the ones used in the experiment shown in Fig. 6B. (B) CV-1 cells were transfected and assayed for luciferase activity as described in the legend to Fig. 6E, except 0.1 μg of p300 expression plasmid was cotransfected as indicated.
DISCUSSION
The transcriptional activation of Grp78 has been used extensively as a standard indicator for the trigger of the UPR, a process that has numerous implications in health and disease (14, 19). The induction of GRP78 confers protection against ER stress due to its antiapoptotic properties and represents the survival arm of the UPR (19, 28, 35, 36). Important advances have been made in discovering the ERSE as the most critical element mediating the stress induction of the Grp78 promoter, and specific transcription factors have been identified that serve as activators for the ERSE. Here, using induction of the Grp78 promoter by Tg stress or transfection of ATF6(373) as models for the activation of the UPR, we uncovered several mechanisms including chromatin remodeling for the activation of the Grp78 promoter in response to the UPR. A summary of the ER -stress-induced changes in transcription factor occupancy and histone H4 modifications is presented in Fig. 8.
FIG. 8.
Model of ER stress-inducible changes in transcription factor occupancy and chromatin remodeling of the Grp78 promoter. In nonstressed cells, NF-Y is in contact with the Grp78 promoter. Upon ER stress, while NF-Y binding remains intact and TFII-I binding is enhanced, ATF6 is cleaved to produce a nuclear form, ATF6(N), which associates with YY1 and enhances its binding to the Grp78 promoter. The YY1-interacting proteins PRMT1 and p300 are also recruited to the Grp78 promoter. Additional chromatin changes of histone H4 include acetylation and arginine 3 methylation.
In nonstressed cells, NF-Y is present on the Grp78 promoter and may play a role in suppressing its activity through interactions with transcriptional repressors (44, 53). The presence of a high-affinity NF-Y binding site has previously been shown to be necessary for ATF6-mediated induction of the Grp78 promoter (24), and it may also attract other coactivators and chromatin modifiers to the promoter, resulting in activation (5). Following ER stress, NF-Y binding is preserved and TFII-I binding is enhanced (11, 34). Importantly, ATF6 is cleaved within 1 h of Tg stress treatment (9), and its active form, ATF6(N), locates to the nucleus and interacts with YY1. This facilitates binding of YY1 to the Grp78 promoter, and further, the YY1 interactive partner PRMT1 is recruited to the Grp78 promoter, correlating with the appearance of methylated histone H4 at the arginine 3 residue. Additionally, the histone acetyltransferase enzyme p300, a factor known to interact with both NF-Y and YY1, is recruited to the Grp78 promoter after ER stress, resulting in the acetylation of histone H4. It has been shown with the yeast system that the histone acetylase GCN5 is required for full ER stress induction of the Grp78 promoter (55). Previously, we hypothesized that the mammalian Grp78 gene system is regulated in an analogous manner (7). Since NF-Y can also associate with human GCN5 and since YY1 can also recruit histone acetyltransferases (6, 20), these may contribute to the acetylation of histones associated with the ERSE on the Grp78 promoter, resulting in transcriptional activation. To our knowledge, these results provide the first evidence that the UPR induces specific acetylation and methylation modifications of nucleosomes at the promoter of a major target gene in a mammalian system.
The selective activation of the Grp78 promoter by YY1 in ER-stressed cells provides a novel model to address an intriguing issue of how a constitutively expressed transcription factor can regulate gene activity under specific physiological conditions. Here, we discovered that while the amount and localization of YY1 are not affected by ER stress, in vitro binding of YY1 to the Grp78 promoter as revealed by ChIP assays is much more pronounced in the nuclei of stressed cells. How might YY1 only bind to the Grp78 promoter after ER stress? Since the YY1 binding site on the ERSE is an atypical one (26), it is possible that YY1 needs to be in a complex that confers higher stability to bind to that site in vivo. We propose that one mechanism is through the association of YY1 with the nuclear form of ATF6, which is only produced following ER stress. In support, we show that in the presence of an exogenously expressed form of activated ATF6, YY1 exhibits increased binding to the Grp78 promoter. We further map the domains required for physical interaction between YY1 and ATF6 and have determined that it involves the b-Zip domain of ATF6 and the region bordering the zinc finger domain of YY1. Importantly, these same domains are required for ATF6 and YY1 activation of the Grp78 promoter (24). Further, depletion of endogenous YY1 level by siRNA, as well as overexpression of the YY1-interacting domain with ATF6, interfere with the ability of the nuclear form of ATF6 to activate the Grp78 promoter, confirming functional interaction between the two proteins in vivo and the role of YY1 as a coactivator of ATF6.
Our study provides new evidence on the requirement of ATF6 towards the induction of chaperone promoters such as Grp78. In a study using siRNA targeted against ATF6α in MEFs (18), it was reported that ER stress induction of Grp78 mRNA was unaffected, suggesting that ATF6α is a dispensable transcription factor in the UPR. However, in another study, siRNA targeted against human ATF6α dramatically reduced tunicamycin-induced induction of the Grp78 promoter in HeLa cells (52). Here, using MEFs where ATF6α is produced as a 250-kDa fusion protein resulting from insertional mutagenesis and ER stress-induced ATF6α cleavage is inhibited most likely due to the bulky luminal domain (46), we showed that Tg induction of the endogenous Grp78 mRNA was severely compromised. Thus, in our assay system ATF6α is required for the optimal stress induction of Grp78.
The discovery that PRMT1 and p300 can enhance transcriptional activation of the Grp78 promoter adds to the diversity of its induction profile. This functional synergy derived from the interaction of a transcription factor with PRMT1 in the presence of p300 has been explored in a p53-dependent transcription activation system with similar results (1). The additive effect of PRMT1 and p300 in a transfection assay yields a twofold increase in the ATF6(373)- and YY1-mediated induction of -169/Luc, which is remarkable considering that addition of YY1 already doubles the induction of ATF6(373). Another consideration in the induction profile of Grp78 by chromatin modifiers is the kinetics of the assembly of the transcriptional activation machinery at the promoter level. It has been shown that PRMT1-mediated methylation of arginine 3 on histone H4 presents a better substrate for p300-directed acetylation (54). Since p300 is capable of acetylating multiple lysine residues on all four histones (43), PRMT1 association may be the initial trigger necessary for full chromatin modification to occur. Furthermore, it has been reported that PRMT1 is responsible for the first chromatin modification related to immediate proteasome assembly in estrogen receptor-α-directed activation of the pS2 gene promoter (32). This would infer that the role of PRMT1 is to prime the chromatin for modification and subsequently allow maximal transcription of the Grp78 promoter in response to ER stress.
Interestingly, another histone and transcription factor acetyltransferase, p300/CBP-associated factor (P/CAF), has been previously shown to associate with and acetylate the same region of YY1 as p300, as well as an additional domain on aa 170 to 200 (56). The ability of P/CAF to modify chromatin is limited to acetylation of lysine 14 on histone H3 and lysine 8 on histone H4 (43), and it is not known if PRMT1 enhances this activity. However, P/CAF is unable to enhance the transcriptional activation of Grp78 when it is transfected in place of p300 in the presence of YY1, ATF6(373), and PRMT1 (data not shown). This result suggests that the role of p300 in transcriptional enhancement of the Grp78 promoter is unlikely due to acetylation of YY1 and increases the chance that the mode of action is chromatin directed. Future studies will be required to confirm this hypothesis. Thus, upon ER stress, we propose that a multiprotein complex including YY1 and ATF6, as well as PRMT1 and possibly p300, occupies the CCAGC site, giving rise to the ER stress-induced DMS methylation protection pattern reported earlier using in vivo genomic footprinting (27).
Finally, we investigated whether YY1 is an essential transcription activator for Grp78 induction by ER stress. Through the use of siRNA, we achieved specific suppression of YY1 expression in 293T cells and observed that Tg induction of Grp78 dropped from six- to twofold, revealing that YY1 is an important factor in Grp78 activation during the UPR. In contrast, genetic knockdown of known UPR regulators IRE1 and XBP-1 showed minimal or no effect on ER stress induction of Grp78 (18, 21). The essential role of YY1 could be due to its multiple functions, from serving as a transcriptional activator itself to serving as a coactivator of ATF6, to recruiting the methyltransferase PRMT1 and histone acetylases, thereby activating transcription. Likewise, evidence is emerging that the transcriptional activators themselves are targets of posttranslational modifications, adding another layer of regulation on their function, and YY1 is an example whose function can be regulated by acetylation and deacetylation (13, 42, 56). These findings raise the interesting questions of whether the transcription factors associated with the ERSEs are themselves targets of the histone-modifying enzymes and, further, whether these modifications are essential for UPR target induction.
Acknowledgments
We thank Michael Stallcup for the CV-1 cells and PRMT1 and p300 expression vectors and antibodies, Sankar Maity for antibodies, Ron Prywes for ATF6 expression vector, Hongdiem Nguyen and Paul Kotol for technical assistance, and Karl F. Heller, Catherine Teyssier, and Judd Rice for helpful discussion.
This work was supported in part by NCI grants CA 27607 (A.S.L), GM 53874 (Y.S.), and GM 64850 and GM 58486 (E.S.).
REFERENCES
- 1.An, W., J. Kim, and R. G. Roeder. 2004. Ordered cooperative functions of PRMT1, p300, and CARM1 in transcriptional activation by p53. Cell 117:735-748. [DOI] [PubMed] [Google Scholar]
- 2.Bertolotti, A., Y. Zhang, L. M. Hendershot, H. P. Harding, and D. Ron. 2000. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat. Cell Biol. 2:326-332. [DOI] [PubMed] [Google Scholar]
- 3.Bhalla, S. S., L. Robitaille, and M. Nemer. 2001. Cooperative activation by GATA-4 and YY1 of the cardiac B-type natriuretic peptide promoter. J. Biol. Chem. 276:11439-11445. [DOI] [PubMed] [Google Scholar]
- 4.Bushmeyer, S., K. Park, and M. L. Atchison. 1995. Characterization of functional domains within the multifunctional transcription factor, YY1. J. Biol. Chem. 270:30213-30220. [DOI] [PubMed] [Google Scholar]
- 5.Caretti, G., V. Salsi, C. Vecchi, C. Imbriano, and R. Mantovani. 2003. Dynamic recruitment of NF-Y and histone acetyltransferases on cell-cycle promoters. J. Biol. Chem. 278:30435-30440. [DOI] [PubMed] [Google Scholar]
- 6.Currie, R. A. 1998. NF-Y is associated with the histone acetyltransferases GCN5 and P/CAF. J. Biol. Chem. 273:1430-1434. [DOI] [PubMed] [Google Scholar]
- 7.Foti, D. M., A. Welihinda, R. J. Kaufman, and A. S. Lee. 1999. Conservation and divergence of the yeast and mammalian unfolded protein response. Activation of specific mammalian endoplasmic reticulum stress element of the grp78/BiP promoter by yeast Hac1. J. Biol. Chem. 274:30402-30409. [DOI] [PubMed] [Google Scholar]
- 8.Haze, K., T. Okada, H. Yoshida, H. Yanagi, T. Yura, M. Negishi, and K. Mori. 2001. Identification of the G13 (cAMP-response-element-binding protein-related protein) gene product related to activating transcription factor 6 as a transcriptional activator of the mammalian unfolded protein response. Biochem. J. 355:19-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haze, K., H. Yoshida, H. Yanagi, T. Yura, and K. Mori. 1999. Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol. Biol. Cell 10:3787-3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holmborn, K., J. Ledin, E. Smeds, I. Eriksson, M. Kusche-Gullberg, and L. Kjellen. 2004. Heparan sulfate synthesized by mouse embryonic stem cells deficient in NDST1 and NDST2 is 6-O-sulfated but contains no N-sulfate groups. J. Biol. Chem. 279:42355-42358. [DOI] [PubMed] [Google Scholar]
- 11.Hong, M., M. Lin, J. M. Huang, P. Baumeister, S. Hakre, A. L. Roy, and A. S. Lee. Transcriptional regulation of the Grp78 promoter by endoplasmic reticulum stress: role of TFII-I and its tyrosine phosphorylation. J. Biol. Chem., in press. [DOI] [PubMed]
- 12.Hyde-DeRuyscher, R. P., E. Jennings, and T. Shenk. 1995. DNA binding sites for the transcriptional activator/repressor YY1. Nucleic Acids Res. 23:4457-4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Imhof, A., X. J. Yang, V. V. Ogryzko, Y. Nakatani, A. P. Wolffe, and H. Ge. 1997. Acetylation of general transcription factors by histone acetyltransferases. Curr. Biol. 7:689-692. [DOI] [PubMed] [Google Scholar]
- 14.Kaufman, R. J. 2002. Orchestrating the unfolded protein response in health and disease. J. Clin. Investig. 110:1389-1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaufman, R. J. 1999. Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev. 13:1211-1233. [DOI] [PubMed] [Google Scholar]
- 16.Koh, S. S., D. Chen, Y. H. Lee, and M. R. Stallcup. 2001. Synergistic enhancement of nuclear receptor function by p160 coactivators and two coactivators with protein methyltransferase activities. J. Biol. Chem. 276:1089-1098. [DOI] [PubMed] [Google Scholar]
- 17.Kurisaki, K., A. Kurisaki, U. Valcourt, A. A. Terentiev, K. Pardali, P. Ten Dijke, C. H. Heldin, J. Ericsson, and A. Moustakas. 2003. Nuclear factor YY1 inhibits transforming growth factor beta- and bone morphogenetic protein-induced cell differentiation. Mol. Cell. Biol. 23:4494-4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee, A. H., N. N. Iwakoshi, and L. H. Glimcher. 2003. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol. Cell. Biol. 23:7448-7459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee, A. S. 2001. The glucose-regulated proteins: stress induction and clinical applications. Trends Biochem. Sci. 26:504-510. [DOI] [PubMed] [Google Scholar]
- 20.Lee, J.-S., K. M. Galvin, R. H. See, R. Eckner, D. Livingston, E. Moran, and Y. Shi. 1995. Relief of YY1 transcriptional repression by adenovirus E1A is mediated by E1A-associated protein p300. Genes Dev. 9:1188-1198. [DOI] [PubMed] [Google Scholar]
- 21.Lee, K., W. Tirasophon, X. Shen, M. Michalak, R. Prywes, T. Okada, H. Yoshida, K. Mori, and R. J. Kaufman. 2002. IRE1-mediated unconventional mRNA splicing and S2P-mediated ATF6 cleavage merge to regulate XBP1 in signaling the unfolded protein response. Genes Dev. 16:452-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee, K. H., S. Evans, T. Y. Ruan, and A. B. Lassar. 2004. SMAD-mediated modulation of YY1 activity regulates the BMP response and cardiac-specific expression of a GATA4/5/6-dependent chick Nkx2.5 enhancer. Development 131:4709-4723. [DOI] [PubMed] [Google Scholar]
- 23.Lee, Y. H., S. S. Koh, X. Zhang, X. Cheng, and M. R. Stallcup. 2002. Synergy among nuclear receptor coactivators: selective requirement for protein methyltransferase and acetyltransferase activities. Mol. Cell. Biol. 22:3621-3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li, M., P. Baumeister, B. Roy, T. Phan, D. Foti, S. Luo, and A. S. Lee. 2000. ATF6 as a transcription activator of the endoplasmic reticulum stress element: thapsigargin stress-induced changes and synergistic interactions with NF-Y and YY1. Mol. Cell. Biol. 20:5096-5106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li, W. W., S. Alexandre, X. Cao, and A. S. Lee. 1993. Transactivation of the grp78 promoter by Ca2+ depletion. A comparative analysis with A23187 and the endoplasmic reticulum Ca2+-ATPase inhibitor thapsigargin. J. Biol. Chem. 268:12003-12009. [PubMed] [Google Scholar]
- 26.Li, W. W., Y. Hsiung, Y. Zhou, B. Roy, and A. S. Lee. 1997. Induction of the mammalian GRP78/BiP gene by Ca2+ depletion and formation of aberrant proteins: activation of the conserved stress-inducible grp core promoter element by the human nuclear factor YY1. Mol. Cell. Biol. 17:54-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li, W. W., L. Sistonen, R. I. Morimoto, and A. S. Lee. 1994. Stress induction of the mammalian GRP78/BiP protein gene: in vivo genomic footprinting and identification of p70CORE from human nuclear extract as a DNA-binding component specific to the stress regulatory element. Mol. Cell. Biol. 14:5533-5546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Little, E., M. Ramakrishnan, B. Roy, G. Gazit, and A. S. Lee. 1994. The glucose-regulated proteins (GRP78 and GRP94): functions, gene regulation, and applications. Crit. Rev. Eukaryot. Gene Expr. 4:1-18. [DOI] [PubMed] [Google Scholar]
- 29.Luo, S., P. Baumeister, S. Yang, S. F. Abcouwer, and A. S. Lee. 2003. Induction of Grp78/BiP by translational block: activation of the Grp78 promoter by ATF4 through an upstream ATF/CRE site independent of the endoplasmic reticulum stress elements. J. Biol. Chem. 278:37375-37385. [DOI] [PubMed] [Google Scholar]
- 30.Luo, S., and A. S. Lee. 2002. Requirement of the p38 MAPK signaling pathway for the induction of Grp78/BiP by azetidine stress: ATF6 as a target for stress-induced phosphorylation. Biochem. J. 366:787-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma, Y., and L. M. Hendershot. 2001. The unfolding tale of the unfolded protein response. Cell 107:827-830. [DOI] [PubMed] [Google Scholar]
- 32.Metivier, R., G. Penot, M. R. Hubner, G. Reid, H. Brand, M. Kos, and F. Gannon. 2003. Estrogen receptor-alpha directs ordered, cyclical, and combinatorial recruitment of cofactors on a natural target promoter. Cell 115:751-763. [DOI] [PubMed] [Google Scholar]
- 33.Mori, K. 2000. Tripartite management of unfolded proteins in the endoplasmic reticulum. Cell 101:451-454. [DOI] [PubMed] [Google Scholar]
- 34.Parker, R., T. Phan, P. Baumeister, B. Roy, V. Cheriyath, A. L. Roy, and A. S. Lee. 2001. Identification of TFII-I as the endoplasmic reticulum stress response element binding factor ERSF: its autoregulation by stress and interaction with ATF6. Mol. Cell. Biol. 21:3220-3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rao, R. V., S. Castro-Obregon, H. Frankowski, M. Schuler, V. Stoka, G. del Rio, D. E. Bredesen, and H. M. Ellerby. 2002. Coupling endoplasmic reticulum stress to the cell death program. An Apaf-1-independent intrinsic pathway. J. Biol. Chem. 277:21836-21842. [DOI] [PubMed] [Google Scholar]
- 36.Reddy, R. K., C. Mao, P. Baumeister, R. C. Austin, R. J. Kaufman, and A. S. Lee. 2003. Endoplasmic reticulum chaperone protein GRP78 protects cells from apoptosis induced by topoisomerase inhibitors: role of ATP binding site in suppression of caspase-7 activation. J. Biol. Chem. 278:20915-20924. [DOI] [PubMed] [Google Scholar]
- 37.Rezai-Zadeh, N., X. Zhang, F. Namour, G. Fejer, Y. D. Wen, Y. L. Yao, I. Gyory, K. Wright, and E. Seto. 2003. Targeted recruitment of a histone H4-specific methyltransferase by the transcription factor YY1. Genes Dev. 17:1019-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Riggs, K. J., S. Saleque, K. K. Wong, K. T. Merrell, J. S. Lee, Y. Shi, and K. Calame. 1993. Yin-Yang 1 activates the c-myc promoter. Mol. Cell. Biol. 13:7487-7495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Riquet, F. B., L. Tan, B. K. Choy, M. Osaki, G. Karsenty, T. F. Osborne, P. E. Auron, and M. B. Goldring. 2001. YY1 is a positive regulator of transcription of the Col1a1 gene. J. Biol. Chem. 276:38665-38672. [DOI] [PubMed] [Google Scholar]
- 40.Roy, B., and A. S. Lee. 1999. The mammalian endoplasmic reticulum stress response element consists of an evolutionarily conserved tripartite structure and interacts with a novel stress-inducible complex. Nucleic Acids Res. 27:1437-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Santiago, F. S., H. C. Lowe, Y. V. Bobryshev, and L. M. Khachigian. 2001. Induction of the transcriptional repressor Yin Yang-1 by vascular cell injury. Autocrine/paracrine role of endogenous fibroblast growth factor-2. J. Biol. Chem. 276:41143-41149. [DOI] [PubMed] [Google Scholar]
- 42.Sartorelli, V., P. L. Puri, Y. Hamamori, V. Ogryzko, G. Chung, Y. Nakatani, J. Y. Wang, and L. Kedes. 1999. Acetylation of MyoD directed by PCAF is necessary for the execution of the muscle program. Mol. Cell 4:725-734. [DOI] [PubMed] [Google Scholar]
- 43.Schiltz, R. L., C. A. Mizzen, A. Vassilev, R. G. Cook, C. D. Allis, and Y. Nakatani. 1999. Overlapping but distinct patterns of histone acetylation by the human coactivators p300 and PCAF within nucleosomal substrates. J. Biol. Chem. 274:1189-1192. [DOI] [PubMed] [Google Scholar]
- 44.Schuettengruber, B., E. Simboeck, H. Khier, and C. Seiser. 2003. Autoregulation of mouse histone deacetylase 1 expression. Mol. Cell. Biol. 23:6993-7004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shen, J., X. Chen, L. Hendershot, and R. Prywes. 2002. ER stress regulation of ATF6 localization by dissociation of BiP/GRP78 binding and unmasking of golgi localization signals. Dev. Cell 3:99-111. [DOI] [PubMed] [Google Scholar]
- 46.Shen, J., and R. Prywes. 2004. Dependence of site-2 protease cleavage of ATF6 on prior site-1 protease digestion is determined by the size of the luminal domain of ATF6. J. Biol. Chem. 279:43046-43051. [DOI] [PubMed] [Google Scholar]
- 47.Shi, Y., J.-S. Lee, and K. M. Galvin. 1997. Everything you have ever wanted to know about Yin Yang 1. Biochim. Biophys. Acta 1332:F49-F66. [DOI] [PubMed] [Google Scholar]
- 48.Siman, R., D. G. Flood, G. Thinakaran, and R. W. Neumar. 2001. Endoplasmic reticulum stress-induced cysteine protease activation in cortical neurons: effect of an Alzheimer's disease-linked presenilin-1 knock-in mutation. J. Biol. Chem. 276:44736-44743. [DOI] [PubMed] [Google Scholar]
- 49.Skarnes, W. C., J. E. Moss, S. M. Hurtley, and R. S. Beddington. 1995. Capturing genes encoding membrane and secreted proteins important for mouse development. Proc. Natl. Acad. Sci. USA 92:6592-6596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sui, G., B. Affar el, Y. Shi, C. Brignone, N. R. Wall, P. Yin, M. Donohoe, M. P. Luke, D. Calvo, and S. R. Grossman. 2004. Yin Yang 1 is a negative regulator of p53. Cell 117:859-872. [DOI] [PubMed] [Google Scholar]
- 51.Thomas, M. J., and E. Seto. 1999. Unlocking the mechanisms of transcription factor YY1: are chromatin modifying enzymes the key? Gene 236:197-208. [DOI] [PubMed] [Google Scholar]
- 52.Thuerauf, D. J., L. Morrison, and C. C. Glembotski. 2004. Opposing roles for ATF6α and ATF6β in endoplasmic reticulum stress response gene induction. J. Biol. Chem. 279:21078-21084. [DOI] [PubMed] [Google Scholar]
- 53.Uramoto, H., D. Wetterskog, A. Hackzell, Y. Matsumoto, and K. Funa. 2004. p73 competes with co-activators and recruits histone deacetylase to NF-Y in the repression of PDGF β-receptor. J. Cell Sci. 117:5323-5331. [DOI] [PubMed] [Google Scholar]
- 54.Wang, H., Z. Q. Huang, L. Xia, Q. Feng, H. Erdjument-Bromage, B. D. Strahl, S. D. Briggs, C. D. Allis, J. Wong, P. Tempst, and Y. Zhang. 2001. Methylation of histone H4 at arginine 3 facilitating transcriptional activation by nuclear hormone receptor. Science 293:853-857. [DOI] [PubMed] [Google Scholar]
- 55.Welihinda, A. A., W. Tirasophon, S. R. Green, and R. J. Kaufman. 1997. Gene induction in response to unfolded protein in the endoplasmic reticulum is mediated through Ire1p kinase interaction with a transcriptional coactivator complex containing Ada5p. Proc. Natl. Acad. Sci. USA 94:4289-4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yao, Y. L., W. M. Yang, and E. Seto. 2001. Regulation of transcription factor YY1 by acetylation and deacetylation. Mol. Cell. Biol. 21:5979-5991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ye, J., R. B. Rawson, R. Komuro, X. Chen, U. P. Dave, R. Prywes, M. S. Brown, and J. L. Goldstein. 2000. ER stress induces cleavage of membrane-bound ATF6 by the same proteases that process SREBPs. Mol. Cell 6:1355-1364. [DOI] [PubMed] [Google Scholar]
- 58.Yoshida, H., K. Haze, H. Yanagi, T. Yura, and K. Mori. 1998. Identification of the cis-acting endoplasmic reticulum stress response element responsible for transcriptional induction of mammalian glucose-regulated proteins. Involvement of basic leucine zipper transcription factors. J. Biol. Chem. 273:33741-33749. [DOI] [PubMed] [Google Scholar]
- 59.Yoshida, H., T. Okada, K. Haze, H. Yanagi, T. Yura, M. Negishi, and K. Mori. 2000. ATF6 activated by proteolysis binds in the presence of NF-Y (CBF) directly to the cis-acting element responsible for the mammalian unfolded protein response. Mol. Cell. Biol. 20:6755-6767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yoshida, H., T. Okada, K. Haze, H. Yanagi, T. Yura, M. Negishi, and K. Mori. 2001. Endoplasmic reticulum stress-induced formation of transcription factor complex ERSF including NF-Y (CBF) and activating transcription factors 6α and 6β that activates the mammalian unfolded protein response. Mol. Cell. Biol. 21:1239-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhou, Y., and A. S. Lee. 1998. Mechanism for the suppression of the mammalian stress response by genistein, an anticancer phytoestrogen from soy. J. Natl. Cancer Inst. 90:381-388. [DOI] [PubMed] [Google Scholar]
- 62.Zhu, C., F. E. Johansen, and R. Prywes. 1997. Interaction of ATF6 and serum response factor. Mol. Cell. Biol. 17:4957-4966. [DOI] [PMC free article] [PubMed] [Google Scholar]