Abstract
Background
The results of the OlympiA study led to the approval of a PARP inhibitor (olaparib) as adjuvant treatment for early breast cancer (eBC) at high risk of relapse in patients with a germline BRCA1/2 mutation (gBRCAm). However, the proportion of patients in routine practice who meet the “high-risk” criteria applied in the OlympiA study, and for whom gBRCAm testing would now be mandatory, remains unknown.
Patients and methods
In this population-based study, we use unique data from the French specialized Côte d'Or Breast and Gynecological Cancer Registry, to assess the real-life proportion, and long-term prognosis of patients treated for eBC between 2005 and 2015 with standard treatment, and at “high risk” of relapse according to the OlympiA trial criteria.
Results
We included 3483 patients treated for HER2-negative eBC (N = 380 with ER-, and N = 3103 with ER + tumor). We found N = 62 (1.8 %) patients with gBRCA1/2 mutations. A total of 494 patients (14.2 %) were classified as “high risk” according to the Olympia criteria; 55 % with ER-tumors, and 9.1 % with ER + tumors, respectively. Despite more intensive systemic treatments in “high risk” patients, 10-year overall survival was much worse in these “high risk” patients compared to the others: 60.1 % vs 83.8 % in ER-tumors, and 55.4 % vs 84.1 % in ER + tumors. Our estimates of net survival show an even greater difference.
Conclusion
This study provides real-life insights into the prevalence and prognosis of patients with high-risk eBC, in a context where the approval of adjuvant olaparib requires careful reorganization of care, so as not to overlook a patient with gBRCAm who could benefit from adjuvant olaparib.
Keywords: Breast cancer, High risk, Olympia, BRCA, Adjuvant, Olaparib, Epidemiology
Highlights
-
•
The proportion of patients who meet the “high-risk” criteria applied in the OlympiA study remains unknown.
-
•
3483 patients treated for early breast cancer between 2005 and 2015.
-
•
14.2 % are as “high risk” according to the OlympiA criteria.
-
•
55 % of ER-tumors, and 9.1 % of ER + tumors, respectively.
-
•
10-years overall survival and net survival reported for this high risk population.
1. Introduction
The recent results of the OlympiA trial [1,2] led to the approval of the PARP inhibitor olaparib as adjuvant treatment for early breast cancer (eBC) at high risk of relapse in patients with germline BRCA1/2 mutation (gBRCAm). In this pivotal phase III trial, patients with a germline mutation in BRCA1 or BRCA2 (gBRCA1/2), and previously treated for localized HER2 non-amplified breast cancer (locoregional treatment combining surgery with or without radiotherapy and systemic treatment (neoadjuvant or adjuvant chemotherapy, with or without endocrine therapy if ER + eBC)), were randomized to receive adjuvant treatment with olaparib (300 mg orally twice daily) or placebo for 1 year. To be included in the trial, patients had to be at “high risk of relapse”, which was defined by specific inclusion criteria [1] (see patients and methods section below).
A total of 1836 patients were randomized, and the OlympiA trial showed a statistically significant benefit for patients who received olaparib, both in terms of invasive disease-free survival (iDFS) [1] (HR: 0.58; p < 0.001), and overall survival (OS) [2] (HR: 0.68; p = 0.009). For these patients with eBC vulnerable to synthetic lethality caused by exposure to PARP inhibitors, adjuvant olaparib demonstrated a benefit irrespective of ER status, receipt of prior neoadjuvant or adjuvant chemotherapy, prior use of platinum chemotherapy and type of gBRCA pathogenic mutation. Moreover, the safety profile and quality-of life data [3] from OlympiA remain consistent with observations from previous studies of olaparib. These results make olaparib a standard of adjuvant treatment for these patients, and highlight the importance of testing for gBRCA1/2 in patients with newly diagnosed high-risk eBC.
Following these results, in March 2022, the FDA approved olaparib for “adult patients with deleterious or suspected deleterious germline BRCA-mutated (gBRCAm) human epidermal growth factor receptor 2 (HER2)-negative high-risk early breast cancer who have been treated with neoadjuvant or adjuvant chemotherapy”. In August 2022, olaparib was then approved in the European Union “in monotherapy or in combination with endocrine therapy for the adjuvant treatment of adult patients with germline BRCA1/2-mutations who have HER2-negative, high risk early breast cancer previously treated with neoadjuvant or adjuvant chemotherapy”.
This new standard of treatment raises many questions for clinicians, especially concerning the modalities and optimal timing of molecular testing, as well as the patient populations in whom to offer systematic gBRCAm testing. More specifically, the actual proportion of patients in routine practice who meet the clinical and biological criteria defining the “high risk” population in the OlympiA study (and therefore in whom gBRCAm testing is now mandatory), is unknown. Furthermore, the notion of “high risk” eBC remains poorly defined, and varies according to the clinical trials performed and evaluating different therapeutic strategies in this indication. Thus, patients treated for non-amplified ER+/HER2 eBC and considered at high risk of relapse may also have access to adjuvant treatment for 2 years with abemaciclib (a CDK4/6 inhibitor) in combination with adjuvant hormonal therapy, given the benefit (in terms of iDFS and distant relapse-free survival (dRFS) observed in the monarchE trial [4]. In that study, the inclusion criteria for patients defined as being at “high risk” of relapse were different from those in the OlympiA study, raising the question of the actual target population for adjuvant treatment with olaparib (defined in US and EU as “high risk” eBC), particularly in patients treated for non-amplified ER+/HER2 breast cancer.
In the present population-based study, we used data from the specialized Côte d'Or Breast and Gynecological cancer registry, to assess the real-life proportion, of patients treated for eBC, and considered at “high risk” of relapse according to the OlympiA trial criteria, and for whom gBRCAm screening would therefore now be mandatory at the end of adjuvant treatment. For exploratory purposes, we also examined the long-term prognosis of these patients, and how this proportion might vary in the population of ER + patients, depending on the definition of “high risk” used.
2. Patients and methods
2.1. Manuscript
This article follows the ESMO guidance for reporting oncology real-world evidence (ESMO-GROW) [5].
2.2. Data collection
The Côte d’Or breast and gynecological cancer registry is the only population-based cancer registry in France to focus on breast and gynecological cancers. It has been collecting data on all cases of breast and gynecological cancers occurring in residents of the Côte d’Or (a French Department) since 1982. The registry catchment area has approximately 500,000 inhabitants, 54 % of whom are women. Information about clinical characteristics, tumors, treatments, and vital status for patients recorded in the registry was obtained from various sources (pathological reports, medical records, and the National Institute of Statistics and Economic Studies (INSEE)).
The Côte-d'Or breast and gynecological cancer registry, hosted at the Georges-François Leclerc Center, Dijon (CGFL) is approved by the French national data protection authority. Moreover, the CGFL complies with the national reference methodology governing the processing of personal data for studies based on existing medical data (N°: 2203771).
2.3. Patient selection for “high risk” definition
The main objective of our descriptive study was to report the real-life proportion of patients treated for eBC, and considered at “high risk” of relapse according to the OlympiA trial criteria, and for whom gBRCAm screening would therefore now be mandatory for optimal adjuvant treatment decision.
Between 2005 and 2015, a total of 3483 patients underwent surgery (conservative surgery, mastectomy) for localized HER2-breast cancer. Tumors were classified according to the staging criteria of the American Joint Cancer Committee (AJCC). Concerning tumor hormone receptor (HR) status, according to French recommendations, tumors were defined HR-negative (HR-) if estrogen and progesterone receptors were expressed in <10 % of tumor cells, and HR-positive (HR+) if expressed in ≥10 % of tumor cells. HER2 was assessed using standard antibodies and FISH techniques, and HER2 scoring was assessed according to the ASCO/CAP guidelines in force at the time of the patient's recruitment [[6], [7], [8]]. CPS-EG was retrospectively calculated (as previously described [9]) for each patient with ER + breast cancer and treated with neoadjuvant chemotherapy. During this period, gBRCAm screening was carried out by clinicians according to recommendations in force at the time [[10], [11], [12]].
Two sub-populations, corresponding to the definition of “high risk of relapse” in the OlympiA trial were studied:
-
i)
A population of patients with triple negative breast cancers: To be eligible, patients needed to satisfy one of the following criteria: a) tumor size ≥2 cm; b) lymph node invasion, regardless of the number of nodes involved.
-
ii)
A population of patients with HR+/HER2-breast cancers: To be eligible, patients needed to satisfy one of the following criteria: a) no neoadjuvant treatment and 4 or more positive nodes; b) neoadjuvant chemotherapy, with incomplete histological response and a CPS-EG score ≥3.
2.4. Statistical analysis
Treatments (chemotherapy, endocrine therapy, radiotherapy, lymph node dissection) were recorded. Chemotherapy and endocrine therapy were categorized as none, neoadjuvant, adjuvant or both and radiotherapy as yes/no. Lymphadenectomy was categorized as sentinel lymph node, node dissection or both. We also collected data on testing, and presence or absence of germline pathogenic BRCA mutation, histological subgroups and menopausal status.
Quantitative data are presented as mean ± standard deviation, or median (quartile (Q)1, Q3) and qualitative data as number and percentage. The chi square or Fisher's exact test were used to compare frequency data and the Wilcoxon test for quantitative data. Overall survival was estimated using the Kaplan Meier method, and survival curves were compared using the log rank test. Median follow-up was estimated using the reverse Kaplan Meier method.
Overall survival (OS) was defined as the time between the date of diagnosis and the date of death from any cause. Vital status was updated using INSEE data. The cut-off date for OS was December 31, 2022. Patients alive at the cut-off date were censored at that date; patients lost to follow-up were censored at the date of last news. Net survival was estimated using the Pohar Perme method [13] and curves were compared using the equivalent of the log rank test for net distributions [14].
The significance level for all analyses was set at 5 %. Analyses were conducted using SAS version 9.4 (SAS Institute Inc., Cary, N.C.) and R version 3.5.2.
3. Results
3.1. Prevalence of “high risk” patients (OlympiA criteria) according to breast cancer subtypes
Overall, among 15,934 breast cancer cases collected in the registry, 4675 patients were treated between 2005 and 2015, of whom 3483 were treated for HER2 non-amplified localized breast cancer (N = 380 (11 %) TNBC, and N = 3103 (89 %) for ER+/HER2-subtype) (Supplemental Fig. 1). The median follow-up of this whole cohort of eBC patients was 144.7 months [142.8–146.8].
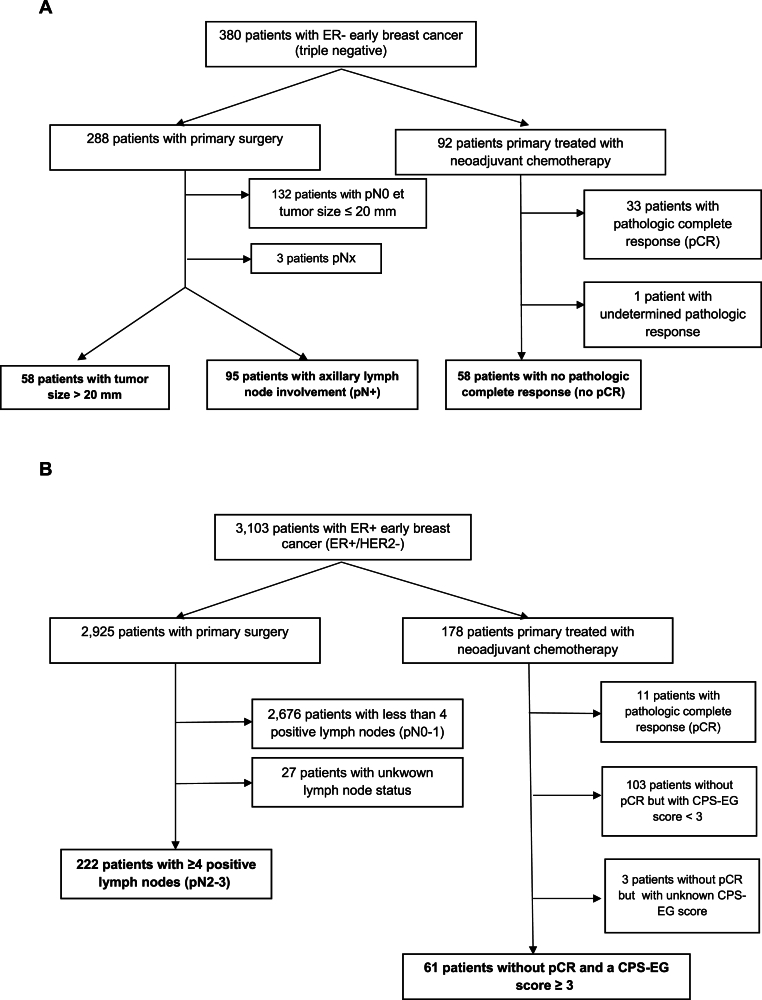
Among patients with localized TNBC, N = 211 (55.5 %) were considered at “high-risk” according to the OlympiA inclusion criteria, as follows: Among patients who underwent immediate surgery: N = 58 (15.2 %) were at high risk because of a tumor size on the surgical specimen > 2 cm (pT2), and N = 95 (25 %) because of axillary lymph node invasion, representing N = 153 high-risk patients among the 288 patients (53.1 %) who underwent primary surgery. A further 58 (15.3 %) patients who underwent neoadjuvant chemotherapy (NAC) were classified as “high-risk” because of the presence of residual disease (RD) on the surgical specimen after NAC (accounting for 63 % of patients with eTNBC treated with NAC) (Fig. 1A).
Fig. 1.
Flow chart of patients with “high risk” early breast cancer (OlympiA study criteria) included in our registry, according to the type of treatment received: A: ER-negative early breast cancers (triple negative breast cancers); B: ER-positive early breast cancer (ER+/HER2-breast cancers).
Among patients treated for ER+/HER2-eBC, N = 283 (9.1 %) were considered “high-risk” according to the OlympiA criteria, as follows: Among patients who underwent immediate surgery: N = 222 (7.1 %) were “high-risk” due to axillary lymph node invasion ≥4N+ (pN2-N3), representing 7.6 % of high-risk patients among the 2925 patients with primary surgery. The remaining patients with ER+/HER2-tumors were classified as “high risk” due to the presence of residual disease (RD) on the post-NAC surgical specimen, associated with a CPS + EG score ≥3 (N = 61 (2 %)). Overall, these post-NAC “high-risk” patients represented 34.3 % of patients treated with NAC for ER+/HER2-eBC (Fig. 1B).
Overall, in our study population, patients with non-amplified eBC HER2 at high risk of relapse according to the OlympiA trial criteria (and therefore for whom early knowledge of BRCA1/2 germline status is now essential) represented 14.2 % (N = 494/3483) of the patients treated between 2005 and 2015.
3.2. Clinico-pathologic characteristics of “high risk” vs “non-high risk” eBC (according to the OlympiA criteria)
We next examined the classical clinico-pathological features of TNBC and ER+/HER2-subtypes, according to their “high-risk” or “non-high-risk” status as defined by the OlympiA trial criteria.
Concerning TNBC (Table 1), patients classified as “high risk” were more likely to have axillary lymph node involvement (p < 0.0001), higher AJCC clinical and pathological stages (p < 0.0001), and higher tumour grade (p = 0.0416). In line with these findings, “high-risk” patients were more likely to have undergone mastectomy (p < 0.0001), and axillary lymph node dissection (p < 0.0001). Among this early stage TNBC population treated between 2005 and 2015, N = 25 patients (6.6 %) had an identified gBRCAm (N = 21 BRCA1, and N = 4 BRCA2). The proportion of patients with a gBRCAm was not statistically significantly different between the “high risk” and “non-high risk” groups (p = 0.547). Overall, during this period, gBRCAm testing was carried out in N = 90 patients (23.7 %).
Table 1.
Patient characteristics according to high risk or non high risk status (OlympiA study criteria) among ER-negative (triple negative) early breast cancer.
| Characteristic | High risk (N = 211) |
Non high risk (N = 169) |
p value | ||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Age at diagnosis | 0.0558 | ||||
| Mean (SD) | 61.2 (16.6) | 57.5 (13.7) | |||
| Median (Min – Max) | 59.6 (25.8–101) | 58.9 (27.7–86.4) | |||
| Menopausal status at diagnosis | 0.7948 | ||||
| Menopaused | 126 | 63.3 | 58 | 35.4 | |
| Pre-menopaused | 73 | 36.7 | 106 | 64.6 | |
| Unknown | 12 | 5 | |||
| Germline BRCA mutation | 0.547 | ||||
| BRCA1 | 9 | 20.0 | 12 | 26.7 | |
| BRCA2 | 3 | 6.7 | 1 | 2.2 | |
| Undetected | 33 | 73.3 | 32 | 71.1 | |
| Unknown/not performed | 166 | 124 | |||
| Axillary lymph node status (clinical) | <0.0001 | ||||
| Negative | 119 | 56.7 | 144 | 85.7 | |
| Positive | 91 | 43.3 | 24 | 14.3 | |
| Unknown | 1 | 1 | |||
| Clinical stage (cAJCC) | <0.0001a | ||||
| 0 | 1 | 0.5 | 0 | 0 | |
| IA | 47 | 22.5 | 120 | 72.3 | |
| IIA | 75 | 35.9 | 26 | 15.7 | |
| IIB | 50 | 23.9 | 11 | 6.6 | |
| IIIA | 15 | 7.2 | 2 | 1.2 | |
| IIIB | 16 | 7.7 | 6 | 3.6 | |
| IIIC | 5 | 2.4 | 1 | 0.6 | |
| Unknown | 2 | 3 | |||
| Pathological stage (pAJCC) | <0.0001 | ||||
| 0 | 0 | 0 | 32 | 19.2 | |
| IA | 15 | 7.3 | 134 | 80.2 | |
| IIA | 114 | 55.3 | 0 | 0 | |
| IIB | 36 | 17.5 | 0 | 0 | |
| IIIA | 23 | 11.2 | 0 | 0 | |
| IIIB | 5 | 2.4 | 1 | 0.6 | |
| IIIC | 13 | 6.3 | 0 | 0 | |
| Unknown | 5 | 2 | |||
| Histological subtype | 0.4049 | ||||
| Ductal | 192 | 91.4 | 152 | 89.9 | |
| Lobular | 9 | 4.3 | 5 | 3.0 | |
| Other | 9 | 4.3 | 12 | 7.1 | |
| Unknown | 1 | 0 | |||
| HER2 low status | 0.4064 | ||||
| 0 | 135 | 64.0 | 115 | 68.0 | |
| Low (1+ ou 2+ without FISH amplification | 76 | 36.0 | 54 | 32.0 | |
| Tumor grade (SBR) | 0.0416 | ||||
| I | 5 | 2.4 | 12 | 7.3 | |
| II | 52 | 25.3 | 48 | 29.3 | |
| III | 149 | 72.3 | 104 | 63.4 | |
| Unknown | 5 | 5 | |||
| Type of breast surgery | <0.0001 | ||||
| Tumorectomy | 108 | 51.2 | 138 | 81.7 | |
| Mastectomy | 103 | 48.8 | 31 | 18.3 | |
| Type of axillary surgery | <0.0001 | ||||
| Axillary lymph node dissection | 150 | 73.5 | 55 | 32.9 | |
| Sentinel lymph node dissection | 29 | 14.2 | 109 | 65.3 | |
| Both | 25 | 12.3 | 3 | 1.8 | |
| Unknown | 7 | 2 | |||
SD: standard deviation, AJCC: American Joint Committee on Cancer.
Fisher's exact test.
Concerning ER+/HER2-subtypes (Table 2), patients classified as “high risk” were more likely to have axillary lymph node involvement (p < 0.0001), higher AJCC clinical and pathological stages (p < 0.0001), and higher tumour grade (p < 0.0001). Patients classified as “high-risk” were more likely to have a tumor classified as “HER2 low” (p = 0.0013), and a lobular-type carcinoma (p = 0.0283). In line with these tumor burden results, high-risk patients were more likely to have undergone mastectomy (p < 0.0001), and axillary lymph node dissection (p < 0.0001). For these ER+/HER2-tumors, patient age was not significantly different between “high-risk” and “non-high-risk” patients. In this population treated between 2005 and 2015, N = 37 patients (1.2 %) had an identified gBRCAm (N = 10 BRCA1, and N = 27 BRCA2). The proportion of patients with a gBRCAm was not statistically different between the “high risk” and “non-high risk” groups (p = 0.2857). Overall, during this period, gBRCAm testing was performed in N = 347 patients (11.2 %).
Table 2.
Patient characteristics according to high risk or non high risk status (OlympiA study criteria) in ER-positive (ER+/HER2-) early breast cancer.
| Characteristic | High risk (N = 283) |
Non high risk (N = 2820) |
p value | ||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Age at diagnosis | 0.3051 | ||||
| Mean (SD) | 62.5 (14.9) | 62.0 (12.7) | |||
| Median (Min – Max) | 63.1 (25–91.6) | 62.2 (27.7–95.3) | |||
| Menopausal status at diagnosis | 0.3404 | ||||
| Menopaused | 186 | 71.0 | 1955 | 73.7 | |
| Pre-menopaused | 76 | 29.0 | 697 | 26.3 | |
| Unknown | 21 | 168 | |||
| Germline BRCA mutation | 0.2857a | ||||
| BRCA1 | 2 | 5.4 | 8 | 2.6 | |
| BRCA2 | 4 | 10.8 | 23 | 7.4 | |
| Undetected | 31 | 83.8 | 279 | 90.0 | |
| Unknown/not performed | 246 | 2510 | |||
| Axillary lymph node status (clinical) | <0.0001 | ||||
| Negative | 117 | 41.8 | 2572 | 91.5 | |
| Positive | 163 | 58.2 | 238 | 8.5 | |
| Unknown | 3 | 10 | |||
| Clinical stage (cAJCC) | <0.0001a | ||||
| 0 | 0 | 0 | 5 | 0.2 | |
| IA | 53 | 19.0 | 2041 | 74.06 | |
| IIA | 72 | 25.8 | 506 | 18.4 | |
| IIB | 82 | 29.4 | 139 | 5.0 | |
| IIIA | 13 | 4.6 | 19 | 0.7 | |
| IIIB | 49 | 17.6 | 45 | 1.6 | |
| IIIC | 10 | 3.6 | 1 | 0.04 | |
| Unknown | 4 | 64 | |||
| Pathological stage (pAJCC) | <0.0001a | ||||
| 0 | 0 | 0 | 10 | 0.4 | |
| IA | 2 | 0.7 | 1682 | 60.3 | |
| IB | 1 | 0.4 | 97 | 3.5 | |
| IIA | 35 | 12.4 | 648 | 23.2 | |
| IIB | 38 | 13.4 | 279 | 10.0 | |
| IIIA | 128 | 45.2 | 35 | 1.26 | |
| IIIB | 20 | 7.1 | 36 | 1.3 | |
| IIIC | 59 | 20.8 | 1 | 0.04 | |
| Unknown | 0 | 32 | |||
| Histological subtype | 0.0283 | ||||
| Ductal | 203 | 71.7 | 2197 | 77.9 | |
| Lobular | 63 | 22.3 | 454 | 16.1 | |
| Other | 17 | 6.0 | 168 | 6.0 | |
| Unknown | 0 | 1 | |||
| Tumor grade (SBR) | <0.0001 | ||||
| I | 44 | 15.7 | 1102 | 39.6 | |
| II | 149 | 53.2 | 1402 | 50.3 | |
| III | 87 | 31.1 | 282 | 10.1 | |
| Unknown | 3 | 34 | |||
| Estrogen receptor expression | 1.000a | ||||
| Positive | 282 | 99.7 | 2809 | 99.6 | |
| Negative | 1 | 0.3 | 11 | 0.4 | |
| Progesterone receptor expression | 0.1267 | ||||
| Positive | 237 | 83.7 | 2452 | 87.0 | |
| Negative | 46 | 16.3 | 367 | 13.0 | |
| Unknown | 0 | 1 | |||
| HER2 low status | 0.0013 | ||||
| 0 | 104 | 36.8 | 1319 | 46.8 | |
| Low (1+ ou 2+ without FISH amplification | 179 | 63.2 | 1501 | 53.2 | |
| Type of breast surgery | <0.0001a | ||||
| Tumorectomy | 83 | 29.3 | 2053 | 72.8 | |
| Mastectomy | 200 | 70.7 | 765 | 27.1 | |
| Axillary lymph node dissection alone | 0 | 0 | 2 | 0.1 | |
| Type of axillary surgery | <0.0001 | ||||
| Axillary lymph node dissection | 236 | 83.4 | 887 | 31.8 | |
| Sentinel lymph node dissection | 2 | 0.7 | 1552 | 55.7 | |
| Both | 45 | 15.9 | 349 | 12.5 | |
| Unknown | 0 | 32 | |||
SD: standard deviation, AJCC: American Joint Committee on Cancer.
Fisher's exact test.
3.3. Adjuvant and neoadjuvant treatments received in “high risk” vs “non-high risk” eBC (according to OlympiA criteria)
We then studied the (neo)adjuvant treatments received by the different risk groups in our study. For TNBC, adjuvant or neoadjuvant systemic chemotherapy was given to the vast majority of patients (N = 318 patients (83.7 %)). Although few patients were concerned, the proportion of patients who did not receive systemic chemotherapy was significantly higher in “non high risk” patients, compared with those classified as “high risk” (19.5 % vs. 13.7 %; p = 0.0225) (Table 3).
Table 3.
Treatments received by patient according to high risk or non high risk status (OlympiA study criteria) in ER-negative (triple negative) or ER-positive (ER+/HER2-) early breast cancer.
| Treatment | ER- breast cancer (triple negative) |
ER + breast cancer (ER+/HER2-) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| High risk (N = 211) |
Non high risk (N = 169) |
p value | High risk (N = 283) |
Non high risk (N = 2820) |
p value | |||||
| N | % | N | % | N | % | N | % | |||
| Chemotherapy | 0.0225a | <0.0001 | ||||||||
| Yes adjuvant | 124 | 58.8 | 102 | 60.4 | 165 | 58.7 | 672 | 23.9 | ||
| Yes neoadjuvant | 50 | 23.7 | 34 | 20.1 | 52 | 18.5 | 106 | 3.7 | ||
| Yes both | 8 | 3.8 | 0 | 0 | 8 | 2.9 | 11 | 0.4 | ||
| No | 29 | 13.7 | 33 | 19.5 | 56 | 19.9 | 2.024 | 72.0 | ||
| Unknown | 0 | 0 | 2 | 7 | ||||||
| Endocrine therapy | 1.000a | <0.0001 | ||||||||
| Yes adjuvant | 2 | 1.0 | 2 | 1.2 | 265 | 94.3 | 2.232 | 79.3 | ||
| Yes both neo- and adjuvant | 0 | 0 | 0 | 0 | 3 | 1.1 | 21 | 0.8 | ||
| No | 209 | 99.0 | 167 | 98.8 | 13 | 4.6 | 561 | 19.9 | ||
| Unknown | 0 | 0 | 2 | 6 | ||||||
| Radiotherapy | 0.5809 | <0.0001 | ||||||||
| Yes | 182 | 86.3 | 149 | 88.2 | 265 | 94.3 | 2.417 | 85.9 | ||
| No | 29 | 13.7 | 20 | 11.8 | 16 | 5.7 | 397 | 14.1 | ||
| Unknown | 0 | 0 | 2 | 6 | ||||||
Fisher's exact test.
In contrast, among patients treated for ER+/HER2-tumors, a majority (N = 2080 (67 %)) did not receive adjuvant chemotherapy. However, patients classified as “high risk” according to the OlympiA criteria were significantly more likely to have received adjuvant chemotherapy than other patients (79.5 % vs. 28 %; p < 0.0001). With regard to endocrine therapy, 94.7 % of patients considered as “high risk” received adjuvant endocrine therapy, with or without a neoadjuvant period of endocrine therapy, compared with only 79.9 % of patients who were “non-high risk” according to OlympiA criteria (p < 0.0001) (Table 3).
3.4. Long term survival of “high risk” and “non high risk” eBC patients (according to the OlympiA criteria)
We then examined the long-term prognosis of the patients analyzed in our study, according to the “high-risk” or “non-high-risk” status defined by the OlympiA criteria, for both TNBC and ER+/HER2-tumors. As relapse data and type of relapse were not collected in our registry, we assessed long-term prognosis using overall survival (OS), based on the knowledge of vital status (alive or dead) for each patient as of December 31, 2022, which we obtained from the French Nationale Institute of Statistics and Economic Studies (Institut National de la Statistique et des Etudes Economiques, INSEE). As the median follow-up for the 2 different subtypes of eBC was >10 years, we assessed not only observed OS, but we also estimated net survival, which is the survival that would be observed if breast cancer was the only cause of death. In the absence of relapse-free survival data (particularly metastatic relapse-free survival), this approach enables us to estimate the proportion of patients who died directly or indirectly from their cancer [13].
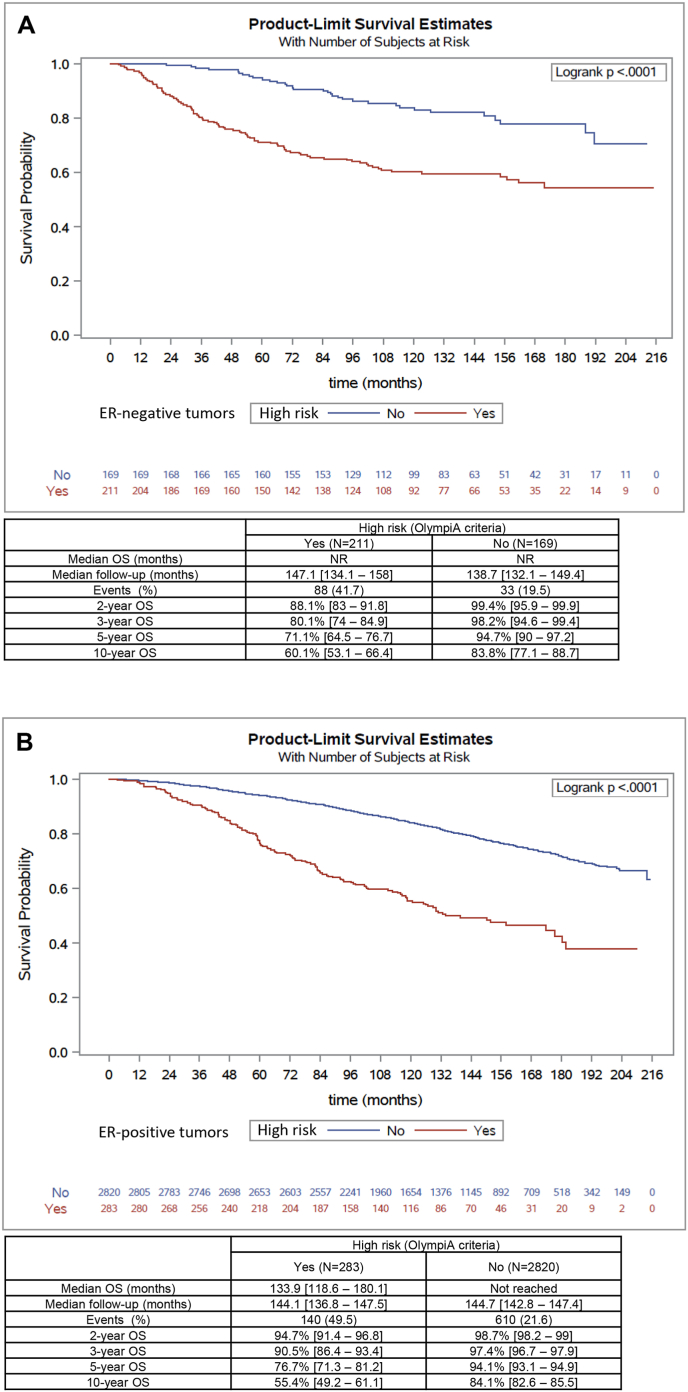
For patients treated for TNBC, the median follow-up was almost 12 years (142.9 months [134.6–151.5]). A total of 121 patients (31.8 %) died: 88 (41.7 %) among the “high-risk” patients, and 33 (19.5 %) among the “non-high-risk” patients. Observed OS in our cohort was significantly worse for “high-risk” patients (p < 0.0001) (Fig. 2A). OS at 5 years was 71.1 % vs. 94.7 %, and at 10 years, 60.1 % vs. 83.8 % for “high-risk” “non-high-risk” patients respectively (Fig. 2A). Analysis of the estimated net survival for these 2 groups also shows a very significant difference, highlighting the excellent 5- and 10-year survival (98.2 % and 92.8 % respectively) of “non-high-risk” patients, compared with “high-risk” patients (76.6 % and 72.1 % respectively) (Supplemental Fig. 2A).
Fig. 2.
Kaplan-Meier estimates for overall survival (OS) according to “high risk” (Olympia study criteria; red curves), or “non high risk” (blue curves) early breast cancer. A: ER-negative early breast cancers (triple negative breast cancers); B: ER-positive early breast cancer (ER+/HER2-breast cancers).
Median OS, median follow-up, number of events (deaths), and OS at 2, 3, 5 and 10 years are shown in the tables below each survival curve.
For patients treated for ER+/HER2-eBC, median follow-up was over 12 years (144.7 months [142.9–146.9]). Seven hundred and fifty patients (24.2 %) died: 140 (49.5 %) among “high-risk” patients, and 610 (21.6 %) among “non-high-risk” patients. Again, OS was significantly worse for “high-risk” patients (p < 0.0001) (Fig. 2B). Thus, 5 years-OS were 76.7 % vs. 94.1 %, and 10 years-OS 55.4 % vs. 84.1 % for the 2 groups of patients respectively (Fig. 2B). Analysis of the estimated net survival for these 2 groups also shows a very significant difference, highlighting the excellent 5- and 10-year OS (100 % and 98.4 % respectively) of “non-high-risk” patients, compared with “high-risk” patients (82.4 % and 61.5 % respectively). (Supplemental Fig. 2B).
In the whole cohort of patients, by multivariate analysis, factors independently associated with poorer OS were age (OR: 1.07, p < 0.0001), presence of gBRCA mutation (OR: 2.38, p = 0.0056), tumor grade (OR: 1.57 for grade 3, p < 0.0001), and high risk group according to OlympiA criteria (OR: 2.85, p < 0.0001).
For exploratory purposes (as the numbers in our cohort are small), we also assessed the OS of gBRCAm patients (pooling, as in the OlympiA study, patients with TNBC and patients with ER+/HER2-eBC): in this population not treated with adjuvant olaparib, “high-risk” gBRCAm patients had poorer overall survival (OS) than “non-high-risk” patients, albeit without reaching statistical significance (p = 0.8775). Overall survival at 2 years was 100 % for all patients, and the difference in prognosis began to appear at 3 years (94.4 % vs. 100 %), becoming more significant at 5 years (88.9 % vs. 100 % for “high-risk” and “non-high-risk” patients respectively). However, with longer follow-up, 10-year OS of the 2 groups of patients did not appear to differ (77.8 % vs. 77.9 %, for “high-risk” and “non-high-risk” patients respectively (Supplemental Fig. 3).
Restricting the analysis to patients who underwent germline mutation testing for BRCA1/2 (N = 437), long term OS of “high risk” patients did not appear to differ according to whether or not they had a germline BRCA1/2 mutation. Conversely, among non-high risk patients, the presence of a germline BRCA1/2 mutation appeared to be associated with poorer OS (Supplemental Fig. 4).
Real-world prevalence of patients with ER+/HER2-tumors at “high risk” according to the definitions used in pivotal clinical trials:
Our study, conducted over a period of 10 years, identified 14.2 % of patients who would be considered at “high risk” of relapse according to the inclusion criteria of the OlympiA trial (and in whom early knowledge of gBRCA status is therefore now essential in order not to miss an indication for adjuvant olaparib). In the population of patients with ER+/HER2-tumors, we identified 9.1 % of “high-risk” patients according to the definition used in the OlympiA trial. However, in this subtype of eBC, the definition of “high-risk” remains unclear, and other definitions may have been used to define a population also eligible for other adjuvant escalation therapies, such as abemaciclib (a CDK4/6 inhibitor) in the monarchE trial [4]. In this study, the definition of “high risk” was based on the presence of ≥4 positive lymph nodes (≥pN2 stages) or 1–3 positive lymph nodes and at least 1 of the following criteria: primary tumor size ≥5 cm and/or tumor grade III.
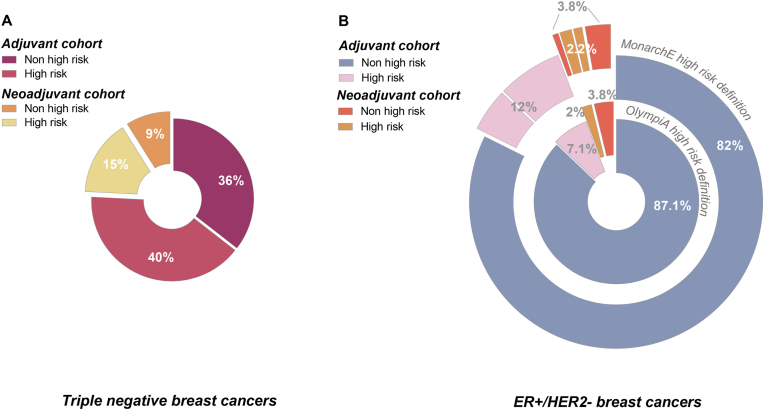
As the approval for adjuvant olaparib in the US and Europe is based simply on the notion of “high risk eBC”, we investigated how changing the definition of “high risk” would modify the proportion of patients concerned, among patients with ER+/HER2-eBC. Fig. 3 summarizes the proportion of “high-risk” patients among those treated with NAC (neoadjuvant cohort) or primary surgery (adjuvant cohort) according to the OlympiA study criteria for TNBC (Fig. 3A), and according to OlympiA study criteria, or according to monarchE study definition criteria for ER+/HER2-tumors (Fig. 3B). For these latter patients, using the broader definition of high-risk applied in the monarchE trial, 177 additional patients were classified as “high-risk” (147 additional adjuvant patients and 30 additional neoadjuvant patients, while 20 neoadjuvant patients previously classified as high-risk were no longer classified as high-risk using the monarchE criteria). Overall, this increased the percentage of “high-risk” patients from 9.1 % to 14.2 % in the ER+/HER2-population (Fig. 3B).
Fig. 3.
Pie charts depicting the percentage of “high risk” (OlympiA study criteria) and “non high risk” early breast cancer patients included in our registry according to the type of treatment received (primary surgery, followed by adjuvant treatment (adjuvant cohort), or neoadjuvant chemotherapy followed by breast surgery (neoadjuvant cohort)). A: ER-negative early breast cancers (triple negative breast cancers); B: ER-positive early breast cancer (ER+/HER2-breast cancers). For ER+/HER2-breast cancer, percentages are shown according to both the OlympiA trial high risk criteria (inner circle), and the monarchE trial high risk criteria (outer circle).
4. Discussion
In this report, we provide a real-world estimation of the proportion, treatment, and prognosis of “high risk” HER2-non amplified eBC patients, in whom gBRCAm screening would now be mandatory at the end of adjuvant treatment, given the benefit of adjuvant olaparib seen in the OlympiA trial. Overall, using the definition of “high-risk” applied in the OlympiA trial inclusion criteria, we found that these patients accounted for 14.2 % of all HER2-unamplified eBC treated during the period 2005–2015 in our population-based cohort (56 % of the TNBC population, and 9.1 % of the ER+/HER2-eBC population).
We report here for the first time epidemiological data concerning the population of patients treated for HER2 non-amplified eBC with a “high risk” of relapse, as defined in OlympiA, from a population-based cohort of patients, and not in a population of patients managed solely in one or more expert cancer centers. Our study population comes from a French department (Côte d'Or, in Burgundy) and has the advantage of being exhaustive, since all cases of breast cancer in women residing in this department are collated. This cohort is therefore representative of the epidemiology, management and prognosis of this type of breast cancer in real life. Another strength of our study is that it covers a period of 10 years (2005–2015). The choice of this period enabled us first, to be certain of having a HER2 status available for tumors from all patients (in order to exclude cases with HER2-amplified tumors); second, to have a cohort of patients treated according to modern standards, still in use today, especially as regards regional local treatments and (neo)adjuvant systemic treatments; third, to have a large number of analyzable patients; fourth, to have a sufficiently long follow-up (median of almost 12 years in our study) to be able to generate overall survival data, which is particularly relevant for the evaluation of ER+/HER2-tumors, in which many relapses occur late (after 5 years) [15]. To assess the long-term prognosis of patients in our cohort in the absence of breast cancer relapse data available in our registry, we report reliable OS data derived from mortality data from the French National Institute of Statistics and Economic Studies (INSEE). We also estimated net survival data, which is the survival that would be observed if the only possible underlying cause of death was the disease under study [16]. This method is considered as the most defensible method of estimating survival from cancer. It can be estimated with either cause-specific or relative survival data settings, if the informative censoring is properly considered. The relative survival setting was more robust to violations of the above assumptions and is therefore recommended for the estimation of net survival. This is the method we chose to apply here. In our study, OS was compared to the survival they would have experienced if they had had the mortality of the general population from which they were drawn [17]. Previous reports concerning eBC patients showed that the estimation of net survival using the relative survival setting was robust to non-comparability in the estimation of background mortality [18]. We show that patients classified as “high-risk” according to the inclusion criteria of the OlympiA trial had very poor observed OS, and poor 10-year net survival compared with patients not classified as “high-risk”, despite more radical locoregional treatments (surgery, radiotherapy) and more frequent systemic (neo)adjuvant treatments (chemotherapy for TNBC, and chemotherapy + endocrine therapy for ER+/HER2-tumors received almost systematically for patients in the “high risk” group). These results show that the patients in our cohort were indeed treated according to standards that are still routinely administered today, and that the medical need for these patients remains substantial, given their very high mortality. Interestingly, observed OS, and 10-year net survival were virtually the same for patients treated for “high risk” TNBC and ER+/HER2-tumors (10-year OS around 60 %), demonstrating that using the OlympiA trial's “high-risk” definition criteria, these 2 very different disease subtypes isolate patients with very similar (and poor) prognosis. Of note, while the 10-year OS of patients not classified as “high risk” was better, it was only 84 % for the 2 subtypes, underlining the existence of an additional medical need for certain patients in this group, and therefore poorly identified by the criteria used in the OlympiA trial. Other definitions of “high risk” have been proposed, in particular for ER+/HER2-tumors, with a view to offering additional adjuvant treatments, such as CDK4/6 inhibitors. For example, the monarchE trial showed a benefit (in terms of iDFS and dRFS) of adding abemaciclib to standard adjuvant hormonal therapy for 2 years in patients at high risk of relapse [4]. In that trial, the definition of high-risk for ER+/HER2-tumors was different to, and broader than that of the OlympiA trial, and concerned patients with ≥4 positive lymph nodes (≥pN2 stages) or 1–3 positive lymph nodes and at least 1 of the following criteria: primary tumor size ≥5 cm and/or tumor grade III. In our exploratory study, we confirm that this broader definition significantly increases the percentage of patients with an ER+/HER2-eBC and therefore considered “high risk” (from 9.1 % to 14.2 %). Retrospective studies have shown that this broader definition of “high-risk” in the monarchE trial did indeed identify a population with numerous metastatic relapses during the first 5 years of follow-up, with a distant relapse-free survival of around 70 % [19]. In the US and Europe, the indication for adjuvant treatment with olaparib is simply based on the notion of “high risk eBC” with a germline BRCA1/2 mutation. The question therefore arises as to which definition should be used to define the indications for adjuvant treatment in a patient with a gBRCAm and a “high risk” ER+/HER2-tumor. Furthermore, given the benefits shown for both abemaciclib and olaparib respectively in this indication, the question arises as to the choice between these 2 adjuvant strategies in gBRCAm patients (in the absence of clinical data concerning a therapeutic combination of these 2 drugs). There are currently no clear guidelines concerning the choice, but given the demonstrated benefit of olaparib in terms of OS in the OlympiA trial, this treatment should undoubtedly be preferred over abemaciclib in gBRCAm patients.
Some limitations of our work include the choice of study period (2005–2015), during which clinical practices in terms of gBRCAm screening and treatment modalities (notably the use of NAC in eTNBC), were probably different from today. Indeed, during this period, gBRCAm screening was carried out by clinicians according to recommendations in force at the time, and was only performed in a minority of patients (N = 437/3483, 12.5 %), for whom we therefore have result with certainty. Unfortunately, this limitation prevents us from making any projections or from modelling the effect of olaparib in the population carrying a germline BRCA mutation. Only 62 patients (1.8 %) were found to be gBRCAm carriers, which is fewer than expected [20]. When TNBC and ER+/HER2-tumors were pooled, we found that the “high risk” population was enriched in gBRCAm compared to the non-high risk population (3.6 % vs 1.5 %; p = 0.0011 Interestingly, and although these are exploratory data given the small number of patients, we observed 5-year OS among patients with gBRCAm in the “high risk” group (88.9 %) that was very similar to that reported at 4 years among patients in the control arm of the OlympiA trial (86.4 %) [2]. Moreover, our results show that for this group of “high-risk” patients who did not receive adjuvant olaparib, OS continues to deteriorate beyond 5 years, suggesting that the long-term results of the OlympiA trial may show an increasing benefit with longer follow-up.
It is likely that the current proportion of “high-risk” patients is lower, compared with that reported in our study for the period 2005–2015, at least among eTNBCs, due to the more frequent use of NAC, and improved results in terms of pCR. Indeed, thanks to the more systematic incorporation of carboplatin and pembrolizumab [21,22], pCR rates are now higher, at around 65 % (compared with 36 % in our series, which is the rate expected with conventional chemotherapies combining anthracyclines and taxanes). This is comparable to the Keynote 522 trial [22], in which carboplatin and pembrolizumab immunotherapy were added to conventional chemotherapy. In our series, the majority of “high-risk” patients with eTNBC underwent surgery at the outset (with stage ≥ pT2 and/or ≥ pN1), and it is plausible that with contemporary neoadjuvant chemoimmunotherapy (recommended treatment for stage II-III eTNBC), a significant proportion of them would have achieved pCR, and therefore would not have been classified as high-risk. Given the adverse effects and cost of adjuvant treatment with olaparib, this makes it even more important to follow the guidelines and offer optimal neoadjuvant chemo-immunotherapy to patients with gBRCAm treated for stage II-III eTNBC.
Concerning NAC in patients with ER+/HER2-, in a context where delays in oncogenetics consultations and molecular biology results can raise issues in some healthcare structures, the high proportion of patients at “high risk” according to the OlympiA criteria, among ER+/HER2-eBC treated with NAC (34 % in our series), raises the question of the opportunity to systematically offer gBRCAm screening early in the disease course, when clinicians choose to perform NAC (often in higher tumor burden and/or higher grade tumors).
In conclusion, we report here real-world data about the proportion of “high risk” eBC, in which gBRCAm screening is now mandatory at the end of adjuvant treatment, given the benefit of adjuvant olaparib seen in the OlympiA trial. Despite certain limitations, this work provides valuable insights into this patient population in a context where the approval of adjuvant olaparib in high-risk patients requires the reorganization of care in this specific population, so as not to overlook any patients with gBRCAm who may benefit from adjuvant olaparib.
CRediT authorship contribution statement
Sylvain Ladoire: Writing – original draft, Validation, Supervision, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Ariane Mamguem Kamga: Methodology, Investigation, Formal analysis, Data curation. Loick Galland: Resources, Data curation. Isabelle Desmoulins: Resources, Data curation. Didier Mayeur: Resources, Data curation. Courèche Kaderbhai: Resources, Data curation. Silvia Mihaelia Ilie: Resources, Data curation. Audrey Hennequin: Resources, Data curation. Clementine Jankowski: Resources, Data curation. Juliette Albuisson: Resources, Investigation, Data curation. Sophie Nambot: Resources, Formal analysis, Data curation. Charles Coutant: Resources, Data curation. Laurent Arnould: Resources, Investigation, Data curation. Manon Reda: Resources, Data curation. Caroline Truntzer: Resources, Data curation. Sandrine Dabakuyo: Validation, Supervision, Resources, Project administration, Methodology, Investigation, Formal analysis, Data curation.
Declaration of competing interest
SL, ID, DM, CC, LA, and MR report travel accommodations and expenses from AstraZeneca.
Acknowledgements
We thank Fiona Ecarnot, PhD (University of Franche-Comté, Besancon, France) for proofreading and correction of the English.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2024.103789.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Tutt A.N.J., et al. Adjuvant olaparib for patients with BRCA1- or BRCA2-mutated breast cancer. N Engl J Med. 2021;384:2394–2405. doi: 10.1056/NEJMoa2105215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Geyer C.E., et al. Overall survival in the OlympiA phase III trial of adjuvant olaparib in patients with germline pathogenic variants in BRCA1/2 and high-risk, early breast cancer. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2022;33:1250–1268. doi: 10.1016/j.annonc.2022.09.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ganz P.A., et al. Patient-reported outcomes in OlympiA: a phase III, randomized, placebo-controlled trial of adjuvant olaparib in gBRCA1/2 mutations and high-risk human epidermal growth factor receptor 2-negative early breast cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2024;42:1288–1300. doi: 10.1200/JCO.23.01214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnston S.R.D., et al. Abemaciclib plus endocrine therapy for hormone receptor-positive, HER2-negative, node-positive, high-risk early breast cancer (monarchE): results from a preplanned interim analysis of a randomised, open-label, phase 3 trial. Lancet Oncol. 2023;24:77–90. doi: 10.1016/S1470-2045(22)00694-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castelo-Branco L., et al. ESMO guidance for reporting oncology real-world evidence (GROW) Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2023;34:1097–1112. doi: 10.1016/j.annonc.2023.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Wolff A.C., et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Arch Pathol Lab Med. 2007;131:18–43. doi: 10.5858/2007-131-18-ASOCCO. [DOI] [PubMed] [Google Scholar]
- 7.Wolff A.C., et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2013;31:3997–4013. doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- 8.Wolff A.C., et al. Human epidermal growth factor receptor 2 testing in breast cancer: American society of clinical oncology/college of American pathologists clinical practice guideline focused update. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018;36:2105–2122. doi: 10.1200/JCO.2018.77.8738. [DOI] [PubMed] [Google Scholar]
- 9.Mittendorf E.A., et al. Validation of a novel staging system for disease-specific survival in patients with breast cancer treated with neoadjuvant chemotherapy. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2011;29:1956–1962. doi: 10.1200/JCO.2010.31.8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.American Society of Clinical Oncology American Society of Clinical Oncology policy statement update: genetic testing for cancer susceptibility. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2003;21:2397–2406. doi: 10.1200/JCO.2003.03.189. [DOI] [PubMed] [Google Scholar]
- 11.Robson M.E., et al. American Society of Clinical Oncology policy statement update: genetic and genomic testing for cancer susceptibility. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2010;28:893–901. doi: 10.1200/JCO.2009.27.0660. [DOI] [PubMed] [Google Scholar]
- 12.Robson M.E., et al. American society of clinical oncology policy statement update: genetic and genomic testing for cancer susceptibility. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015;33:3660–3667. doi: 10.1200/JCO.2015.63.0996. [DOI] [PubMed] [Google Scholar]
- 13.Perme M.P., Stare J., Estève J. On estimation in relative survival. Biometrics. 2012;68:113–120. doi: 10.1111/j.1541-0420.2011.01640.x. [DOI] [PubMed] [Google Scholar]
- 14.Grafféo N., Castell F., Belot A., Giorgi R. A log-rank-type test to compare net survival distributions. Biometrics. 2016;72:760–769. doi: 10.1111/biom.12477. [DOI] [PubMed] [Google Scholar]
- 15.Pan H., et al. 20-Year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N Engl J Med. 2017;377:1836–1846. doi: 10.1056/NEJMoa1701830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berkson J., Gage R. Calculation of survival rates for cancer. Proc Staff Meet Mayo Clin. 1950;25:270–286. [PubMed] [Google Scholar]
- 17.Ederer F., Axtell L.M., Cutler S.J. The relative survival rate: a statistical methodology. Natl Cancer Inst Monogr. 1961;6:101–121. [PubMed] [Google Scholar]
- 18.Schaffar R., Rachet B., Belot A., Woods L. Cause-specific or relative survival setting to estimate population-based net survival from cancer? An empirical evaluation using women diagnosed with breast cancer in Geneva between 1981 and 1991 and followed for 20 years after diagnosis. Cancer Epidemiol. 2015;39:465–472. doi: 10.1016/j.canep.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 19.Sheffield K.M., et al. A real-world US study of recurrence risks using combined clinicopathological features in HR-positive, HER2-negative early breast cancer. Future Oncol. Lond. Engl. 2022;18:2667–2682. doi: 10.2217/fon-2022-0310. [DOI] [PubMed] [Google Scholar]
- 20.Breast Cancer Association Consortium, et al. Breast cancer risk genes - association analysis in more than 113,000 women. N Engl J Med. 2021;384:428–439. doi: 10.1056/NEJMoa1913948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loibl S., et al. Addition of the PARP inhibitor veliparib plus carboplatin or carboplatin alone to standard neoadjuvant chemotherapy in triple-negative breast cancer (BrighTNess): a randomised, phase 3 trial. Lancet Oncol. 2018;19:497–509. doi: 10.1016/S1470-2045(18)30111-6. [DOI] [PubMed] [Google Scholar]
- 22.Schmid P., et al. Pembrolizumab for early triple-negative breast cancer. N Engl J Med. 2020;382:810–821. doi: 10.1056/NEJMoa1910549. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.