Abstract
Royal jelly (RJ), a secretion produced by honeybees, has garnered significant interest for its potential as a therapeutic intervention and functional food supplement. This systematic review aims to synthesize current research on the health benefits, bioactive components, and mechanisms of action of RJ. Comprehensive literature searches were conducted across multiple databases, including PubMed, Scopus, and Web of Science, focusing on studies published from 2000 to 2024 (April). Findings indicate that RJ exhibits a wide range of pharmacological activities, including anti-inflammatory, antioxidant, antimicrobial, and anti-aging effects. Beneficial biological properties of RJ might be due to the presence of flavonoids proteins, peptides, fatty acids. Both preclinical and clinical studies have reported that RJ improves the immune function such as wound healing, and also decreases the severity of chronic diseases including diabetes and cardiovascular disorders. The molecular mechanisms underlying these effects involve modulation of signalling pathways such as NF-κB, MAPK, and AMPK. Despite promising results, the review identifies several gaps in the current knowledge, including the need for standardized dosing regimens and long-term safety assessments. Furthermore, variations in RJ composition due to geographic and botanical factors necessitate more rigorous quality control measures. This review underscores the potential of RJ as a multifunctional therapeutic agent and highlights the necessity for further well designed studies to fully elucidate its health benefits and optimize its use as a functional food supplement.
Keywords: Apitherapy, Functional food, Pharmacological activities, Complementary medicine
Graphical abstract
Highlights
-
•
Royal jelly is rich in proteins, vitamins, minerals, and bioactive compounds.
-
•
Royal jelly has nutritional value and protects against cellular damage.
-
•
Royal jelly has anti-inflammatory properties, antimicrobial, and metabolic regulator.
-
•
Royal jelly boosts the functional ability of the immune system.
-
•
Royal jelly is used for anticancer, wound healing, antiaging, and neuroprotection.
1. Introduction
Honey bees are eusocial insects, holding special status in nature by providing various ecosystem services such as pollination, environmental indicators, production of honey, and other valuable products such as propolis, wax, RJ, etc. Among these products, honey is perhaps the most widely known and most widely used product. RJ, a milky secretion produced by worker bees 1–3 days old larvae of all the honeybee castes (queen, worker, and drone) are fed with RJ. Well known for its purported health benefits, RJ has garnered considerable attention in scientific research and traditional medicine alike. Bee pollen, another product collected by honey bees, is a nutrient rich substance gathered from flowers and used as a food source for the hive. Rich in vitamins, minerals, proteins, and antioxidants, bee pollen has been hailed for its potential health-promoting properties and is consumed by humans in various forms, including supplements and health foods (Table 1). Propolis, often referred to as “bee glue," is a resinous substance collected by bees from tree buds and sap. Utilized by bees to seal cracks in the hive and defend against pathogens, propolis exhibits antimicrobial, anti-inflammatory, and antioxidant properties, making it a subject of interest in both traditional and modern medicine. Bee venom, produced by specialized glands in the abdomen of worker bees, contains a complex mixture of peptides and enzymes with diverse biological activities. While bee stings can elicit painful reactions in humans, controlled exposure to bee venom has been explored for its potential therapeutic effects in conditions ranging from arthritis to certain types of cancer [1,2]. In this scientific exploration of RJ has been uncovered in order to explore its potential benefits for human health and well-being, underscoring the profound interconnectedness between humans and the natural world.
Table 1.
Summarizes the composition, bioactive compounds, and health benefits associated with various bee products, providing a concise overview of their medicinal properties.
| Bee Product | Composition | Bioactive Compounds | Health Benefits |
|---|---|---|---|
| Honey | Carbohydrates (glucose, fructose), di- and oligosaccharides, organic acids, enzymes, vitamins, amino acids, peptides | Polyphenols, flavonoids | Anti-inflammatory, antioxidant, potential prevention of diseases like cancer, diabetes, obesity |
| Propolis | Resin, wax, essential oils, pollen, esters, diterpenes, lignans, alcohols, vitamins, flavonoids, amino acids, fatty acids, minerals | Caffeic acid phenethyl ester (CAPE), flavonoids, phenolic acids | Neuroprotective effects, antioxidative, anti-cancer properties |
| Bee Pollen/Bee Bread | Carbohydrates, proteins, vitamins, amino acids, lipids, fatty acids | Phenolic compounds | Health-promoting activities, antimicrobial, anti-inflammatory |
| Bee Venom | Carbohydrates, lipids, proteins, enzymes (hyaluronidase, phospholipase A2), peptides (melittin, apamin, MCD), pheromones, minerals | Phospholipase A2, hyaluronidase, peptides | Anti-inflammatory, immune system modulation, potential neuroprotective and anti-cancer properties |
| Royal Jelly | Sugars, lipids, proteins, amino acids, vitamins, minerals | Royalactin, hydroxy-decenoic acid (10-HDA), proteins | Potential health maintenance, pharmaceutical applications |
2. Urgency and need of this review
The urgency of this review arises from the growing interest in the therapeutic effects of RJ, a natural substance produced by worker honeybees. Despite its traditional use and anecdotal health benefits, scientific understanding of its mechanisms and clinical applications remains limited. Given the increasing demand for alternative therapies and potential healthcare innovations, a comprehensive critical appraisal is essential. This review aims to analyze existing evidence, elucidate the pharmacological effects of RJ, and offer insights into its therapeutic potential. Through rigorous analysis, it seeks to fill research gaps, to guide future research, and potentially unlock new therapeutic interventions. This appraisal will help distinguish evidence-based therapeutic potential from conjecture, thereby enriching our understanding of natural remedies and potentially paving the way for novel healthcare innovations.
3. Royal jelly
RJ, produced by worker bees from the hypopharyngeal and mandibular glands through partial digestion of honeydew, is crucial for the development and caste differentiation of honeybee larvae [3,4]. 1-3 days-old larvae of all the honeybee castes (queen, worker, and drone) are fed with RJ. RJ is a yellowish-white gelatinous substance made from proteins, carbohydrates, lipids, and vitamins produced by secretory cells in the glandular acini [5,6]. It is transported to the mouthparts of worker bees and consumed by the queen and larvae, supporting colony growth.
Historically significant in ancient Greek and Egyptian cultures, RJ was linked to immortality and beauty, notably used by Cleopatra. Since the 1960s, research in apitherapy has explored its health benefits, including antimicrobial, anti-aging, anti-tumor, antioxidative, anti-diabetic, immunomodulatory, and neuroprotective effects [[6], [7], [8], [9]]. These properties suggest potential applications for conditions like infertility, digestive disorders, Alzheimer's, and depression [10,11].
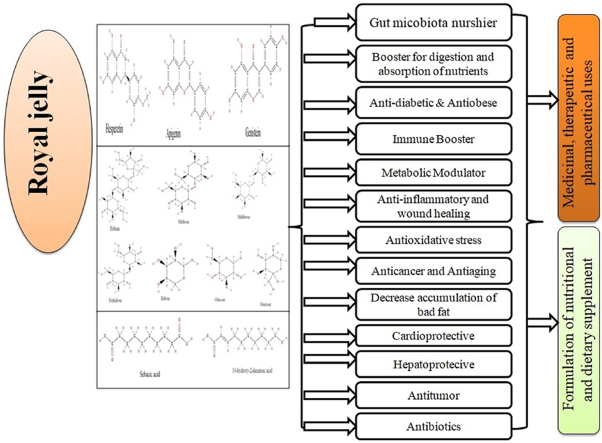
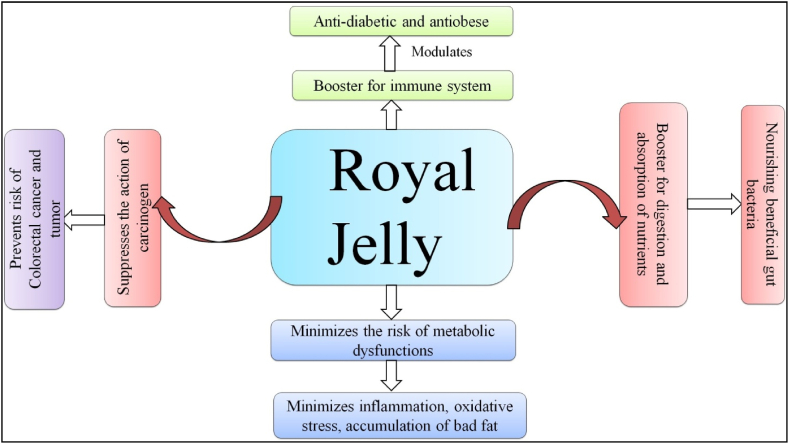
In Chinese culture, RJ is widely used as a dietary supplement and cosmetic ingredient, offering benefits such as maintaining reproductive health, enhancing memory, preventing dementia, and reducing anxiety. Its anti-inflammatory action helps intestinal health by modulating cytokine levels (Fig. 1). The impact of RJ on fertility may be due to its ability to increase hormone production, and its anti-aging properties position it as a promising ingredient in medicinal and cosmetic formulations (Table 2). The longevity-promoting effects in queen bees, attributed to Major RJ Proteins (MRJPs), suggest potential implications for human lifespan extension (Table 2). Proper handling and cold storage are essential due to its perishable nature. RJ regulates different physiological functions in bees and indicates potential regulatory roles in humans, making it relevant for commercial, cosmetic, and medicinal applications (Table 3). Furthermore, its rich nutritional composition, comprising proteins, vitamins, minerals, and unique bioactive compounds, underscores its therapeutic potential. The enduring fascination with RJ in traditional medicine underscores its enduring legacy as a natural remedy with profound implications for human health and wellness (Table 3).
Fig. 1.
Showing the role of royal jelly (RJ) in the management of different disease.
Table 2.
Shows the physical characteristics of royal jelly (RJ), health benefits, major proteins, lipid, carbohydrate, Other Components, Storage Recommendations, Importance for Bees and Pharmacological Focus content.
| Component | Description |
|---|---|
| Appearance | White or yellowish gelatinous substance |
| Taste | Sweet-sour |
| pH | 3.4–4.5 |
| Main Health Benefits | Antioxidant, Anti-inflammatory, Neurotrophic, Hypotensive |
| Antidiabetic, Antihypercholesterolemic, Antirheumatic Antitumor | |
| Antifatigue, Antimicrobial, Nematocidal, Anti-aging |
| Component | Description | Pharmacological Importance |
|---|---|---|
| Major Proteins |
|
MRJPs: Active ingredients<br>− Antioxidative peptides (up to 29 identified) |
| Aspimin<br>− Newly discovered proteins | ||
| Lipid Fraction |
|
|
| 7–18 % of dry weight | ||
| Mainly short hydroxyl fatty acids (constitute 80–85 %) | ||
| Also contains phenols (4–10 %), waxes (5–6%), steroids (3–4%), phospholipids (0.4–0.8 %) | ||
| Carbohydrates |
|
|
| Other Components | Vitamins, Minerals, Phenols, Esters, Aldehydes, Ketones | |
| Alcohol, Bioactive substances” like ACh and nucleotides | ||
| Storage Recommendations |
|
|
| Storage above 5 °C reduces soluble nitrogen and free amino acids | ||
| Importance for Bees |
|
|
| Pharmacological Focus | Investigation of anti-aging properties with focus on cognitive function in advanced aging and Alzheimer's disease (AD) | |
| Reviewing studies on RJ's effects “on cognitive aging and AD pathology in cell cultures, animal models, and” humans when possible | ||
| Elaborating on molecular changes underlying these effects | ||
Table 3.
This table provides a summary of the various therapeutic implications of royal jelly, highlighting its potential benefits for overall health and well-being.
| Therapeutic Implication | Description |
|---|---|
| Antioxidant Properties | Flavonoids, phenolic compounds, and vitamins help to neutralize free radicals and reduce oxidative stress. |
| Anti-inflammatory Effects | Components such as “10-Hydroxy-2-decenoic acid (10-HDA)” exhibit anti-inflammatory properties, and reduce inflammation in arthritis and skin irritations. |
| Immune System Support | Boosts the immune system by stimulating the production of immune cells such as lymphocytes and macrophages, thus improving overall immune function and response to infections. |
| Wound Healing | The presence of proteins, vitamins, and amino acids in royal jelly can accelerate wound healing by promoting tissue repair and regeneration, making it beneficial for treating cuts, burns, and other skin injuries. |
| Cardiovascular Health | Royal jelly contains compounds like fatty acids and peptides that may have cardio-protective effects by lowering cholesterol, improving blood vessel function, and reducing the risk of cardiovascular diseases. |
| Neuroprotective Effects | Compounds like 10-HDA and royalisin have neuroprotective properties, potentially enhancing the survival of nerve cells in conditions like Alzheimer's, Parkinson's, and cognitive reduction. |
| Anti-diabetic Properties | Royal jelly may be a potential adjunctive therapy for managing diabetes by regulating blood sugar levels and improving insulin sensitivity. |
| Anti-cancer Potential | Royal jelly has been found to have anti-cancer properties through mechanisms like inhibiting tumor cell growth, inducing apoptosis, and enhancing the body's immune response against cancer cells. |
| Hormonal Balance | Royal jelly contains hormone-like substances such as royalactin, which may help regulate hormonal balance, particularly in reproductive health, by supporting fertility, and menstrual regularity, and relieving symptoms of hormonal imbalances such as PMS and menopause. |
| Skin Health and Beauty | The vitamins, minerals, and amino acids present in royal jelly contribute to skin nourishment and rejuvenation, promoting a healthy complexion, reducing signs of aging like wrinkles and fine lines, and alleviating skin conditions such as eczema and acne. |
4. Methods
The systematic review of randomized controlled trails (RCTs) was carried out following the rules and procedure mentioned in the Cochrane Handbook for systemic review analysis [12].
4.1. Search strategy
A comprehensive computerized search was conducted across different databases such as PubMed (http://www.ncbi.nlm.nih.gov/pubmed), Web of Science (https://mjl.clarivate.com/search results), Science Direct (https://www.sciencedirect.com/), and Google Scholar (https://scholar.google.com/) 2000 to 2024. Moreover, we carefully reviewed the reference lists of all significant studies and reviews. The following terms were used either singly or in combination as inclusion criteria: “anti-inflammatory," “antibacterial," “anti diabetic," “apoptotic," “respiratory," “gastrointestinal," “cardiovascular," and “nervous system," neurodegenerative diseases (e.g., Alzheimer's, Parkinson's), hepatotoxicity, renal toxicity, metabolic disorders (e.g., diabetes, obesity, hyperlipidaemia), reproductive disorders (e.g., PCOS, infertility and oligospermia), and viral diseases (e.g., COVID-19). After about 200 results were found, their abstracts were examined to determine their applicability. About 110 research and review publications were carefully scrutinized in order to compile the current article, with additional exclusion criteria such non-English language and lack of full-text manuscript availability being applied.
4.2. Study selection
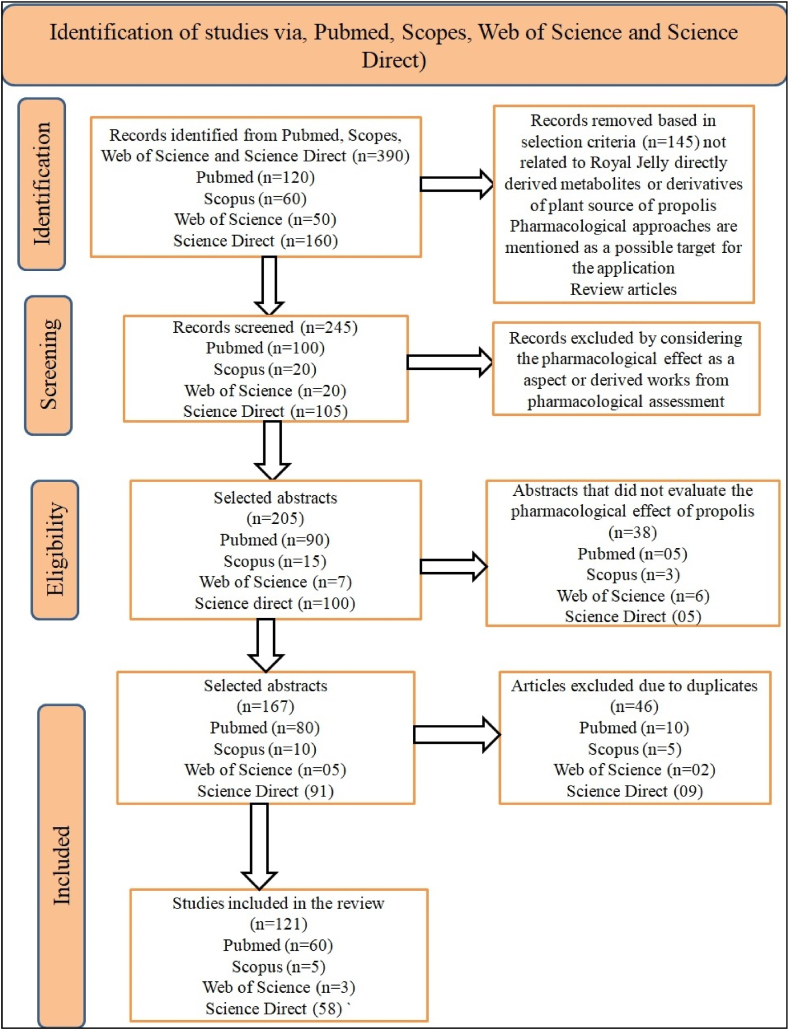
Two authors independently screened titles and abstracts having relevance with trials, followed by retrieval and examination of the full length text of the paper to screen out the relevant trial studies having relevance with our review article. Relevant data were extracted from all these articles and summarized by applying the standardized format to evaluate the quality of study and to synthesis evidence. The extracted data encompassed information like the title, name of authors, year of publication, and name of country, parameters of the studied trail including biological, pharmacological and food supplement roles of RJ. The systematic review and meta-analysis did not include any studies that used RJ as a medicinal solution for a variety of disorders with treatment durations shorter than one week. Every stage of the study selection procedure was carried out separately by at least two authors to assure accuracy, and disagreements were settled by consensus (Fig. 2).
Fig. 2.
Flow chart of the process of the study selection.
4.3. Data extraction
Data abstraction form was used to individually and twice extract pertinent data from each included article. Dispute resolution was accomplished through consultation. The following details were recorded: the type and dosage of RJ, the type of placebo or control group, the duration of the treatment, the formulation used, the minimal active concentration tested, the duration of the study, the model used (in vitro or in vivo study), and other basic pharmacological data; the level of interest outcomes (pharmacological properties such as antidiabetic, anticancer, cardiovascular, antihypertensive, anti-inflammatory, immunomodulatory and reproductive related studies).
5. Chemical composition of RJ
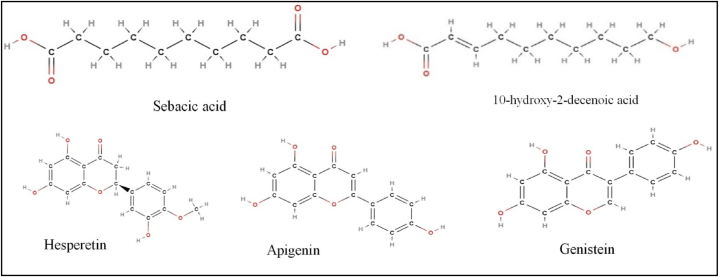
RJ is an acidic secretion with a pH of 3.5–4.2. It mostly consists of water 60–70 %, sugars 7–18 %, proteins 9–18 %, lipids 3–8%, minerals, and trace amounts of vitamins. Major lipids in it consist of 10-hydroxy-2-decanoic acid and sebacic acid [13]. 10H2DA is known for its anti-cancerous and anti-angiogenic activity whereas sebacic acid has anti-aging effects. Major RJ Proteins (MRJPs) present in the RJ increases the lifespan of Queenbee (Table 4). It consists of peptides like royalisin, jelleines and royalactina. The various pharmacological aspects of RJ are attributed to its unique and rich composition of proteins, carbohydrates, vitamins, lipids, minerals, flavonoids, and polyphenols along with various biologically active substances (Table 5).
Table 4.
Gross chemical composition of royal jelly [14].
| Chemical Component | Percentage (%) |
|---|---|
| Water | 60–70 |
| Proteins | 10–18 |
| Carbohydrates | 11–23 |
| Lipids (Fats) | 3–8 |
| Ash | 1–2 |
| Vitamins | Trace amounts |
| Minerals | Trace amounts |
| Enzymes | Trace amounts |
| Hormones | Trace amounts |
| Other Bioactive Compounds | Trace amounts |
Table 5.
Phytochemical profile of royal jelly.
| Bioactive Compound | Percentage (%) |
|---|---|
| Royalactin | 1–3 |
| 10-Hydroxy-2-decenoic acid (10-HDA) | 1–3 |
| Acetylcholine | 0.5–1 |
| Adenosine | 0.2-0.5 |
| Nucleotides (AMP, ADP, ATP) | 0.1-0.3 |
| Gamma-aminobutyric acid (GABA) | 0.1-0.2 |
| Polyphenols | 0.1-0.2 |
| Flavonoids | Trace amounts |
| Phospholipids | Trace amounts |
| Sterols | Trace amounts |
| Growth factors (EGF and TGF) | Trace amounts |
5.1. Carbohydrates
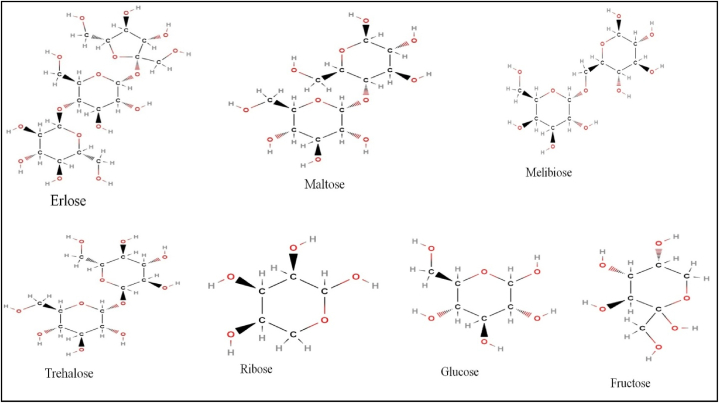
RJ contains approximately 7.5–15 % sugars, with fructose and glucose comprising the majority, making up around 90 % of the sugar content. Additionally, maltose, trehalose, melibiose, ribose, and erlose constitute about 0.8–3.6 % of the sugar composition in RJ [15] (Fig. 3). These sugars present in RJ act as aphago stimulants, operating through insulin signalling cascades and nutrient sensing pathways, thereby enhancing nutrient intake crucial for larval and queen development [16].
Fig. 3.
Showing 2D chemical structures of different carbohydrates present in the royal jelly.
5.2. Lipids
Lipids constitute about 7–18 % of RJ, significantly contributing to its biological activities. Predominantly composed of short hydroxy fatty acids with 8–12 carbon atoms and dicarboxylic groups, key components include 10-hydroxy-2-decenoic acid (10H2DA), and sebacic acid (SA) [17] (Fig. 4).
Fig. 4.
Showing the 2D structures of important lipids and flavonoids present in the royal jelly.
10H2DA plays a crucial role in caste differentiation by regulating epigenesis through the inhibition of histone deacetylases, enzymes that break down ε-acetyl-lysine residues of histones [16,18]. It has bactericidal effects against harmful bacteria like Paenibacillus larvae, protecting bee hives [19], and shows antibacterial effects against lipoteichoic acid from Staphylococcus aureus and other pathogenic bacteria linked to human colon cancer [20,21].
10H2DA also exhibits neurogenic activities by stimulating progenitor cell differentiation, mimicking neurotrophic factors [22]. It is used in cosmetics and anti-cancer drugs for its skin-whitening properties and anti-proliferative effects by suppressing transcription factors and proteins such as tyrosinase-related protein 1 (TRP-1) and TRP-2 [23]. Additionally, 10H2DA inhibits the activity of matrix metalloproteinases (MMPs), preventing tissue aging and diseases like rheumatoid arthritis [24,25].
SA and 10H2DA have anti-inflammatory properties by regulating proteins in the kappa-B signalling and mitogen-activated protein kinase (MAPK) pathways [21,26]. These acids also increase the activity of estrogen receptors ERα and ERβ, benefiting the skeletal and muscular systems [27,28].
5.3. Proteins
Proteins make up about 50 % of RJ, with approximately 80 % consisting of nine Major RJ Proteins (MRJPs), which have molecular weights between 49 and 87 kDa. These proteins are nutritionally valuable and play a crucial role in the development of young female larvae through cell proliferation [29]. Among MRJPs, MRJP 1 is significant; existing in heat-resistant oligomer and less resistant monomer forms [30]. The glycoprotein royalactin mimics epidermal growth factor (EGF) effects in rat hepatocytes and regulates developmental processes in bee larvae [31].
Other RJ proteins include jelleines, royalisin, and aspimin, with royalisin and jelleines being antimicrobial peptides that enhance immune responses in bee larvae. Royalisin, rich in cysteine residues, remains stable at extreme temperatures and low pH, while its antimicrobial properties are due to hydrophobic residues that disrupt bacterial membranes. Apolipophorin-3 and glucose oxidase contribute to RJ's antimicrobial properties by forming lipid-protein complexes and catalyzing glucose oxidation into hydrogen peroxide, respectively [7].
5.4. Phenols, flavonoids, and free amino acids
The antioxidant properties of RJ are due to polyphenolic compounds and flavonoids [32]. Key phenolic compounds include pinobanksin, dodecanoic acid, octanoic acid, and 1,2-benzenedicarboxylic acid. Flavonoids of RJ are categorized into flavanones (hesperetin, naringenin, isoakuranetin), flavonols (kaempferol, isorhamnetin), flavones (apigenin, glucoside of luteolin, chrysin, acacetin), and isoflavonoids (formononetin, genistein, coumestrol) (Fig. 4). These compounds provide anti-inflammatory and antiapoptotic properties [17]. RJ from younger larvae contains higher levels of proteins and phenolic compounds, enhancing free radical scavenging activity [32].
RJ also contains trace amino acids like valine, glutamic acid, serine, glycine, cysteine, alanine, tyrosine, phenylalanine, leucine, isoleucine, and threonine, contributing to its nutritional and biological activity [17,33]. In addition to this, RJ includes vitamins B complex, C, A, and E, with a high concentration of vitamin B5 (Pantothenic acid), linked to lifespan extension. It also contains elements like P, S, W, V, Ni, Na, Mg, Ca, Cu, Zn, Fe, Al, Sr, Pb, Hg, Ba, Bi, Cd, Sn, Cr, Mn, and Mo, with mineral salts making up about 1.5 % of its composition [34]. RJ is rich in acetylcholine, crucial for cognitive function and memory, regulated by choline acetyltransferase activity influenced by glucose and insulin metabolism, potentially preventing cognitive dysfunction [35,36]. RJ also contains nucleotides, free bases, phosphates, ADP, ATP, and AMP, which are essential for energy production and enzymatic actions [37]. AMP N1-oxide, unique to RJ, promotes neurite outgrowth and PC12 cell differentiation into neurons, mimicking nerve growth factor activity through MAPK/ERK1/2 and PI3K/Akt pathways [17,38].
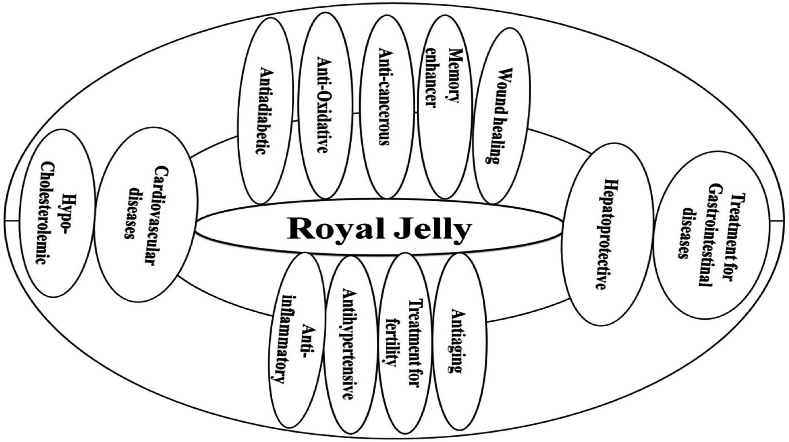
6. Pharmacological importance of RJ
RJ exhibits numerous properties which are known to have beneficial effects on humans like cardiovascular disease [39], antihypertensive activity [40], hypo-cholesterolemic activity [41], anti-aging [42], anti-cancerous [43], memory enhancer [44], hepatoprotective [45], anti-obesity [46], anti-diabetic [47], wound healing [48], anti-inflammatory and antioxidative [49]. Each constituent of RJ has shown its advantages regarding human welfare and acted as an excellent therapeutic agent against various diseases (Fig. 5 and Table 6).
Fig. 5.
Depicts the different pharmacological and biologically defensive properties of royal jelly.
Table 6.
This table summarizes the diseases, their causes and symptoms, the properties of royal jelly relevant to each condition, effective bioactive compounds found in royal jelly, and the biological properties of royal jelly relevant to each condition.
| Disease | Causes | Symptoms | Property of Royal Jelly | Effective Bioactive Compound | Biological Property |
|---|---|---|---|---|---|
| Ulcerative Colitis | Chronic, inflammatory bowel disease | Abdominal pain, diarrhea, rectal bleeding | Inhibition of pro-inflammatory cytokines TNF-α and IL-1β, elevation of anti-inflammatory cytokine IL-10 | Royalactin, Royalisin | Anti-inflammatory, boosts activity of IL-10, decreases T-cell proliferation, inhibits TNF-α and IL-1β |
| Crohn's Disease | Chronic, inflammatory bowel disease | Abdominal pain, diarrhea, weight loss | Inhibition of pro-inflammatory cytokines TNF-α and IL-1β, elevation of anti-inflammatory cytokine IL-10 | Royalactin, Royalisin | Anti-inflammatory, boosts activity of IL-10, decreases T-cell proliferation, inhibits TNF-α and IL-1β |
| Lactose Intolerance | Lack of β-galactosidase, maldigestion of lactose from milk and milk products | Abdominal pain, diarrhea, flatulence | Synergism with probiotic yogurt, boosting probiotics in fermented milk products | Lactobacillus helveticus | Enhances probiotic activity, aids in lactose digestion |
| Chronic Diarrhea | Various causes including infections, dietary issues, and intestinal disorders | Frequent, loose stools, abdominal pain | Anti-diarrheal potency due to antimicrobial activity of royalisin and royalactin | Royalisin, Royalactin | Antimicrobial, anti-diarrheal |
| Chronic Constipation | Lack of fiber, dehydration, sedentary lifestyle, medications | Straining during bowel movements, infrequent bowel movements | Acetylcholine in RJ induces smooth muscle contractions, antimicrobial activity of royalisin and royalactin | Acetylcholine, Royalisin, Royalactin | Induces smooth muscle contractions, antimicrobial, potentially aids in bowel movements |
| Gastric Ulcers | NSAID use, H. pylori infection, excessive alcohol consumption | Abdominal pain, bloating, heartburn, nausea | Normalization of gastric tissues via increase of PGE-2 and COX-2, reduction of MPO and iNOS | Not specified | Normalizes gastric tissues, reduces inflammation |
| Intestinal Ulcers | NSAID use, H. pylori infection, excessive alcohol consumption | Abdominal pain, bloating, heartburn, nausea | Attenuation of pro-inflammatory cytokines TNF-α and IL-1β, reduction of lipid peroxidation, augmentation of endogenous antioxidant enzymes SOD and CAT | Not specified | Reduces inflammation, increases antioxidant activity |
| Peptic Ulcers | NSAID use, H. pylori infection, excessive alcohol consumption | Abdominal pain, bloating, heartburn, nausea | Reduction of gastric ulcers via anti-inflammatory action, attenuation of pro-inflammatory cytokines TNF-α and IL-1β, augmentation of antioxidant enzyme activity | Not specified | Reduces inflammation, increases antioxidant activity |
| Nicotine-Induced Gastric Mucosal Changes | Ingestion of nicotine, leading to mucosal damage | Abdominal discomfort, changes in mucosal integrity | Amelioration of mucosal changes via unknown mechanisms | Not specified | Unknown, possibly anti-inflammatory |
6.1. Neuroprotective action of RJ
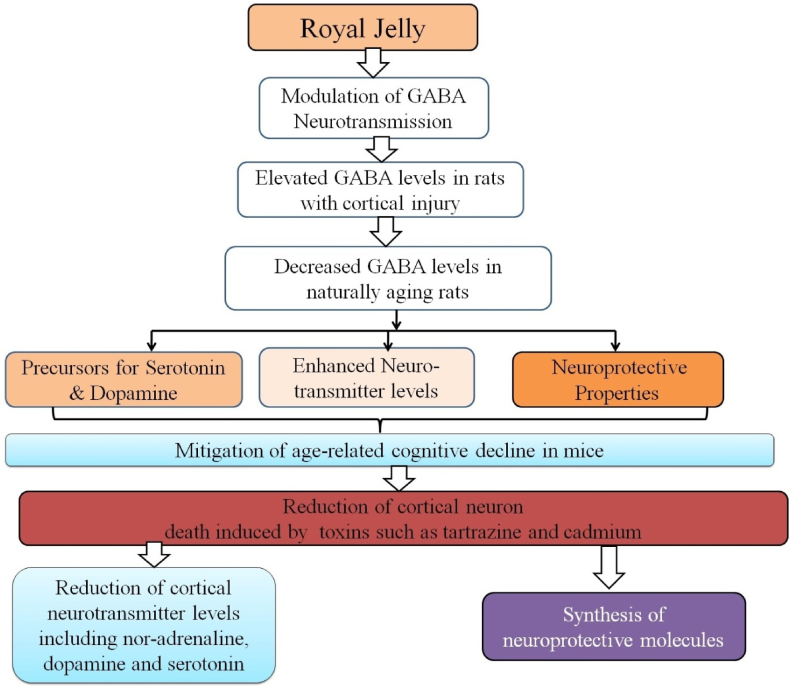
RJ has demonstrated numerous beneficial effects on the nervous system, including memory enhancement, increased energy levels, prevention of senility, anxiety reduction, and calming effects on hyperactivity. Its neuropharmacological actions involve modulating gamma-aminobutyric acid (GABA) neurotransmission, a crucial regulator of brain activity. RJ influences GABA-transaminase (GABA-T), the enzyme metabolizing GABA, which is synthesized from glutamate by glutamate decarboxylase (GAD) [50]. Studies indicate RJ administration elevates GABA levels in rats with tartrazine-induced cortical injury, suggesting neuroprotective effects, but decreases GABA levels in the striatum and hypothalamus of aging rats, showing complex regulation of GABAergic neurotransmission (Fig. 6).
Fig. 6.
Showing the neuroprotective effect of propolis, royal jelly modulates the neurotransmission by stimulating the gamma amino butyric acid (GABA) in the brain which in turn stimulates the synthesis of serotonin and dopamine, increases the levels neurotransmitters, and hence acts as neuroprotective natural agent. In addition to this administration of royal jelly increases the synthesis and secretion of other neurotransmitters and also some neuroprotective molecules.
RJ also contains tryptophan and tyrosine, precursors for serotonin and dopamine, crucial for mood and cognitive function [51,52]. Treatment with RJ and tyrosine in experimental models increased brain dopamine levels and its metabolites [53,54], enhancing cognition through improved neurotransmission. RJ mitigates age-related cognitive decline induced by d-galactose in mice, restoring brain noradrenaline and dopamine levels [55], indicating its neuroprotective potential (Fig. 6).
RJ reduces cortical neuron death from toxins like tartrazine and cadmium and restores levels of neurotransmitters such as noradrenaline, dopamine, and serotonin, supporting neuronal integrity. It also stimulates neuroprotective molecule synthesis, evidenced by increased cysteic acid levels in aged rats [56,57], suggesting involvement in the cysteine-taurine metabolic pathway [58]. The beneficial effects of RJ on the nervous system likely stem from neurotransmission modulation, neuroprotection against age-related cognitive decline, and stimulation of neuroprotective pathways, underscoring its potential as a supplement and therapeutic agent for cognitive enhancement and neuroprotection (Fig. 6).
6.2. RJ as an anti-aging remedy
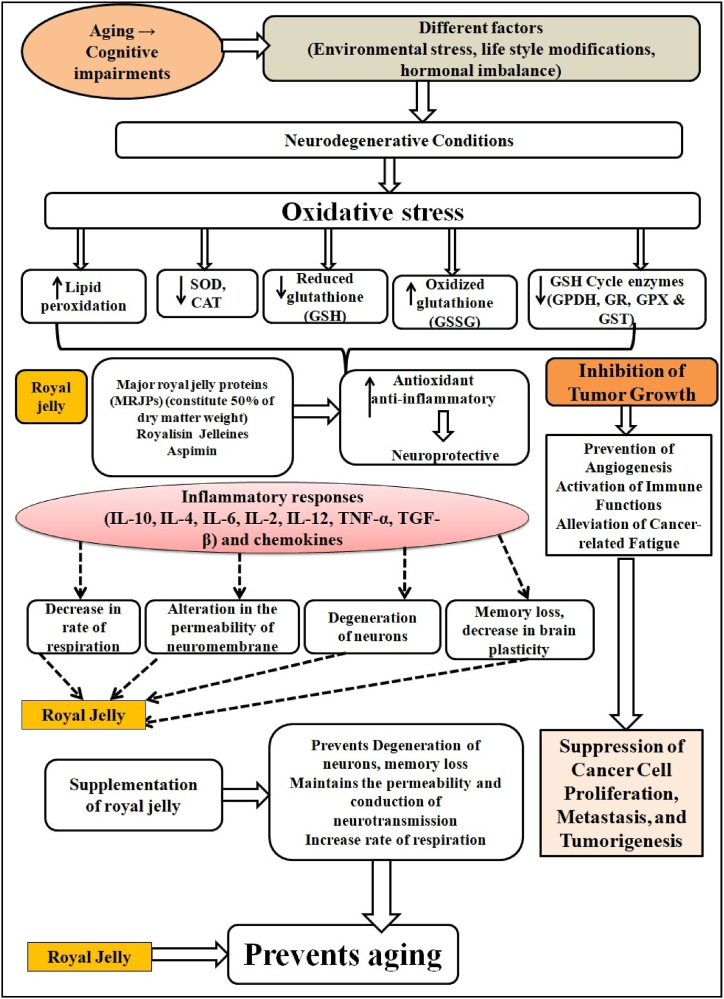
According to Halloran et al. (2012), aging is a major risk factor for neurodegenerative diseases [59], notably Alzheimer's disease (AD), which is characterized by progressive loss of working and situational memory [60]. Previous studies reported that RJ can protect spatial memory in rats modeled with sporadic AD through an intracerebroventricular injection of streptozotocin (icv-STZ) by enhancing hippocampal neurogenesis and reducing oxidative stress and neurodegeneration [36,61] (Fig. 7).
Fig. 7.
Royal jelly (RJ) is used as anti-aging by preventing the neurodegeneration through the reduction in oxidative stress, because RJ increases the GSH content and accelerating the activities of antioxidative enzymes such as SOD, CAT, G6PDH, GR, GPX and GST. Moreover, RJ decreases the secretion of inflammatory cytokines by down-regulating the inflammatory pathways and hence protects the neurons from damages and prevents the aging. Royal jelly suppresses the progression and development of tumors by inhibiting te angiogenesis, activation of immune system and prevents the metastasis and tumorgenesis.
In AD models, such as 10-month-old APP/PS1 mice, RJ improves memory by preventing neuronal death and modulating the cAMP/PKA/CREB/BDNF pathway [62]. Furthermore, RJ ameliorates memory deficiencies in ovariectomized (OVX) rats and improves memory in OVX cholesterol-fed rabbits by regulating oxidative stress and cholinergic neurotransmitter levels [63,64].
RJ also affects neurotransmitter levels in the prefrontal cortex, influencing cognitive processes related to working memory (WM) [65]. Long-term RJ administration reduces striatal GABAergic transmission and GABA concentration in aged Wistar male rats, enhancing dopamine transmission activity and spatial memory [56]. MRJPs specifically enhance spatial memory by modulating cysteine, taurine, and energy metabolism [66]. Thus, RJ shows significant potential in improving memory function through multiple mechanisms.
6.3. Effect of RJ on fertility
Male infertility is influenced by factors such as smoking, alcohol consumption, sexual behavior, and diet. Primary infertility prevalence among couples ranges from 13.2 % to 17.3 %, with ovulatory problems accounting for 39.7 % and male factors 29.1 % [67]. Male factors alone are responsible for at least half of all infertility cases [68], with estimates suggesting a prevalence of 40.9 % [69]. Male factors contribute to infertility in 20–70 % of cases, with infertile men comprising 2.5 %–12 % of the population [70].
RJ has shown benefits for human fertility, including improvements in hormone balance, sperm, and ovule quality [71]. [72] RJ supplementation elevates testosterone levels, ejaculate volume, seminal fructose, sperm motility, and sperm count in male animals [10]. Cryopreservation of sperm reduces viability, but RJ treatment at specific concentrations can enhance sperm viability over time.
RJ significantly improves total sperm motility in chilled and frozen-thawed ram sperm due to its high calcium ion concentration. RJ administration at doses of 50, 100, and 150 mg/kg increases sperm motility in animal models like rabbit bucks and mice [73]. Thus, RJ is a promising natural supplement for enhancing male fertility, but optimal dosage and administration methods need further research to maximize its efficacy.
6.4. Anti-tumor action
RJ, a bee secretion, contains compounds like 10-hydroxy-2-decanoic acid (10H2DA) and proteins such as apalbumin-1 and apalbumin-2, which inhibit cancer progression (Fig. 7). 10H2DA can inhibit VEGF, reducing angiogenesis, cell proliferation, and migration, thus hindering tumor vascularisation [74]. RJ also has antioxidative properties, enhancing the production of antioxidants like GSH, GSH-Px, SOD, and GST in the kidneys and liver of rats [75] (Fig. 7). This antioxidative effect is synergistic with Cisplatin, an anti-cancer agent, helping combat oxidative stress-induced cancer. RJ inhibits tumor growth, prevents angiogenesis, activates immune functions, and alleviates cancer-related fatigue when supplemented in cancer patients. It shows promise as an adjunctive treatment during menopause for breast cancer by suppressing cancer cell proliferation, metastasis, and tumorigenesis through inhibition of angiogenesis and immune system stimulation [76,77].
6.5. Antibiotic effect
Royalisin, a protein in RJ, exhibits strong antibacterial properties against various Gram-positive bacteria but not Gram-negative bacteria [78]. Direct ingestion of RJ can degrade its active components due to pH changes [19,49]. Jelleines, also in RJ, show antimicrobial actions: Types I, II, and III act against Gram-positive bacteria, Gram-negative bacteria, and yeast, respectively, while Type IV does not [79]. Some MRJPs (2–5) have antibacterial activity against E. coli, a Gram-negative bacterium.
Bilikova et al. (2015) found that royalisin and royalisin-D significantly reduced survival rates of S. intermedius and P. aeruginosa, Gram-negative and Gram-positive bacteria, respectively, with royalisin showing higher efficacy [80]. Conversely, Hasan et al. (2020) found RJ had antimicrobial activity against E. coli, unlike gentamicin and its combination with doxycycline, which were ineffective [81]. MRJPs (2–5) and 7 produced recombinantly demonstrated consistent antibacterial activity [82]. RJ also showed antibacterial activity against S. aureus when infused into milk, enhancing the properties of milk [83]. Furthermore, RJ was more effective against anaerobic periodontopathic bacteria than aerobic ones in a study comparing RJ and chlorhexidine [84]. Hence, RJ and its components exhibit significant antimicrobial properties, particularly against Gram-positive bacteria and certain Gram-negative strains, with varying efficacy depending on the specific proteins and conditions used.
6.6. Wound healing effect and anti-inflammatory action
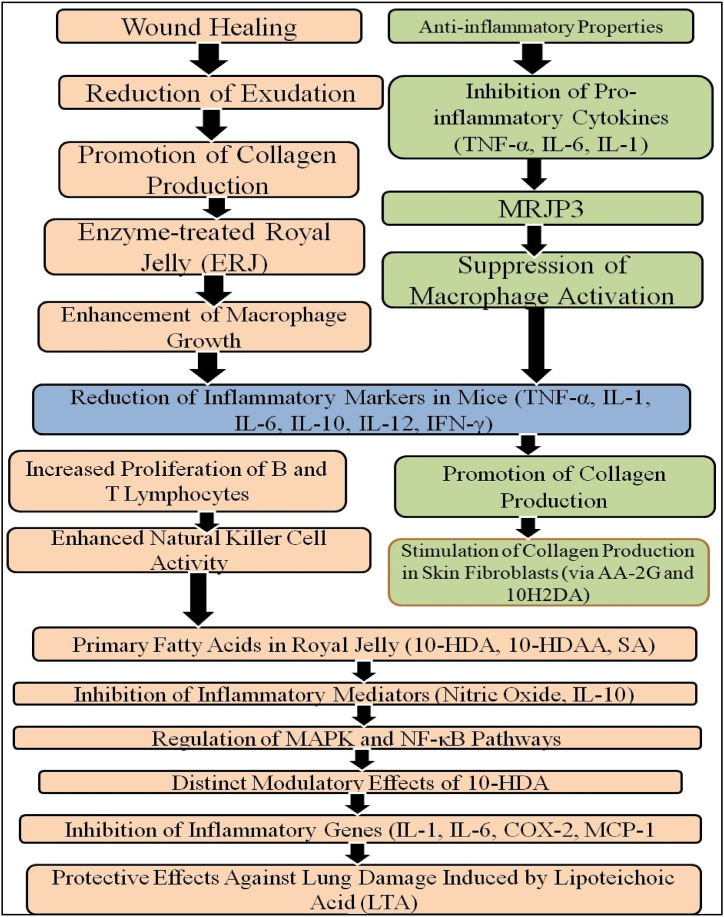
The cosmetic industry often incorporates RJ due to its antioxidative, antibacterial, anti-inflammatory, and wound-healing properties. RJ has been shown to prevent the release of pro-inflammatory cytokines like TNF-α, IL-6, and IL-1 in mouse macrophage cultures without harming the cells (Fig. 8) [85]. Components of RJ, such as MRJP3, regulate cytokine release. It also contains ascorbic acid-2-O-α-glucoside (AA-2G), which stimulates collagen production, and 10H2DA, which enhances TGF-β1 release, further promoting collagen production and wound healing [86] (Fig. 8).
Fig. 8.
Royal jelly (RJ) inhibits the inflammatory pathways by declining the synthesis and secretion of pro-inflammatory cytokines (TNF-α, IL-6 and IL-1) by suppressing the activation of macrophages. In addition to this RJ regulates the inflammatory secretion and promotes the production of collagen. Royal jelly (RJ) helps in the healing of wounds by promoting the production of collagen because royal jelly contains various enzymes which accelerate the healing of wounds. Moreover, reduction in the secretion of proinflammatory cytokines (TNF-α, IL-6 and IL-1) by suppresses the activation of macrophages.
A study on enzyme-treated RJ (ERJ) showed it supports macrophage growth and protects against lipopolysaccharide (LPS)-induced stress. ERJ reduced inflammatory markers (TNF-α, IL-1, IL-6, IL-10, IL-12, IFN-γ) and boosted B and T lymphocyte proliferation and natural killer cell activity in mice (Fig. 8). Previous studies have investigated RJ's fatty acids (10-HDA, 10-HDAA, Sebacic acid) and found they inhibit key inflammatory mediators like nitric oxide and IL-10 in a dose-dependent manner, with Sebacic acid also reducing tumor necrosis factor alpha (TNF-α) secretion [20]. These fatty acids modulate inflammatory genes and pathways, including MAPK and NF-κB. It has been reported that 10-HDA's anti-inflammatory effects by reducing the expression of genes such as IL-1, IL-6, COX-2, and MCP-1 in vitro. In mice, a 100 mg/kg dose of 10-HDA reduced lung damage and inflammatory cytokines (IL-10, MCP-1, TNF-α) caused by lipoteichoic acid (LTA) [62]. Therefore, RJ and its components show significant potential for treating inflammatory conditions and enhancing skin health (Fig. 8).
6.7. Effect on gastrointestinal diseases
Gastrointestinal disorders, including irritable bowel syndrome, peptic ulcers, liver diseases, pancreatitis, gallstones, and Crohn's disease, are prevalent, especially in tropical regions [87]. It has been studied that functional gastrointestinal issues affect about 40 % of people globally [88]. Dietary patterns significantly influence these conditions, with a Westernized diet high in carbohydrates and animal proteins linked to chronic inflammatory bowel diseases [89]. The transition from urban to rural settings impacts gut microbiota composition [90].
The gastrointestinal tract's role in nutrient absorption and immune response increases the risk of inflammatory, autoimmune, and chronic conditions [91]. RJ is noted for its therapeutic potential against inflammation, liver disease, hypercholesterolemia, oxidative stress, and immune diseases [92]. RJ contains bioactive compounds, including proteins, vitamins, phenolics, and flavonoids, with its major protein constituents (MRJPs) highlighted as significant therapeutic agents [93,94].
6.8. Immunomodulatory effects
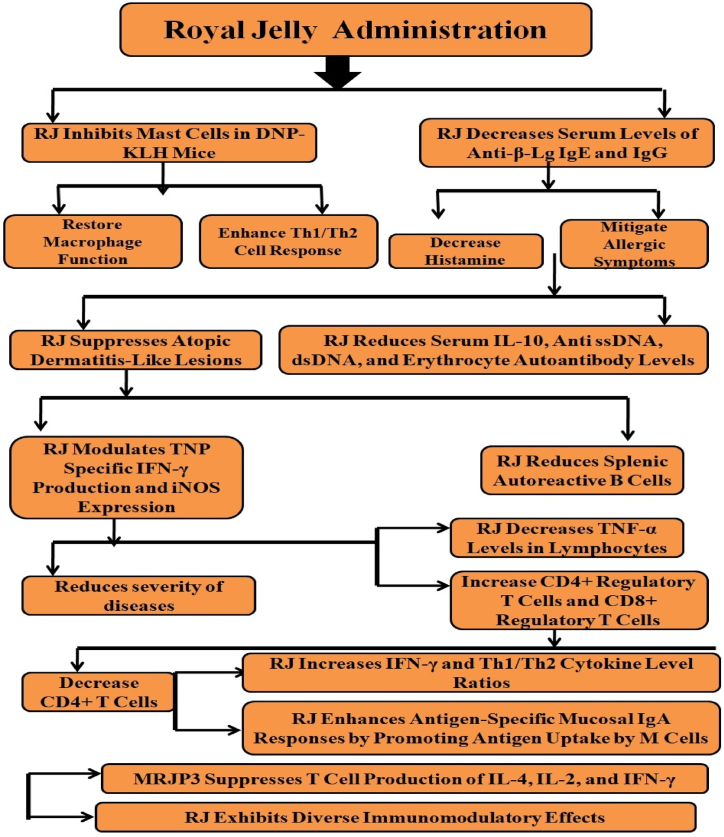
RJ exhibits diverse pharmacological activities, particularly immunomodulatory effects. Oka et al. (2001) found that RJ inhibits mast cells in DNP-KLH mice, reducing antigen-specific IgE and histamine production while restoring macrophage function and enhancing Th1/Th2 response [95]. In β-lactoglobulin allergic mice, RJ reduces serum anti-β-Lg IgE, IgG, and histamine, mitigating allergic symptoms [96].
RJ also suppresses atopic dermatitis-like lesions in NC/Nga mice by modulating IFN-γ production and iNOS expression [97]. In SLE-prone mice, RJ lowers serum IL-10, anti-ssDNA, dsDNA, erythrocyte autoantibody levels, and splenic autoreactive B cells [98]. In children with SLE, RJ increases CD4+ and CD8+ regulatory T cells while decreasing CD4+ T cells, reducing disease severity [99].
In Graves' disease, RJ decreases TNF-α in lymphocytes and increases IFN-γ and Th1/Th2 cytokine ratios [100]. Protease-treated RJ enhances mucosal IgA responses by promoting antigen uptake by M cells [101]. MRJP3 and apolipoprotein III-like protein in RJ exhibit immunoregulatory activity; MRJP3 suppresses T-cell IL-4, IL-2, IFN-γ production and reduces anti-OVA IgE and IgG1 levels [102]. Apolipoprotein III-like protein enhances immune response post-phosphorylation [103].
10-HDA, a component of RJ, shows mixed immune effects: it reduces IL-6 production and NF-κB activation but inhibits T cell proliferation and antigen-specific responses [[104], [105], [106]]. However, it can restore body, thymus, and spleen weights in cyclophosphamide-induced mice, improving the thymus/spleen index and enhancing T and B cell activity [107] (Fig. 9).
Fig. 9.
Royal jelly (RJ) shows immunomodulatory effect against the various immune compromising diseases by decreasing the secretion and synthesis of different immune biomolecules. Moreover, major royal jelly proteins (MRJPs) suppresses the production of IL-4, IL-2 and IFN-γ thereby drives the immunomodulatory effects.
6.9. Reduce liver damage
RJ demonstrates significant hepatoprotective properties. It regulates 267 liver genes in mice, decreasing squalene epoxidase (SQLE) and increasing low-density lipoprotein receptor (LDLR), thus reducing cholesterol levels [108]. RJ also upregulates genes encoding GST and GSH-Px [108].
Liver function, crucial for metabolism, is often impaired by toxic chemicals and drugs, marked by elevated alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels [109]. Toxicants like cadmium and CCl4 lower glutathione (GSH) and increase malondialdehyde (MDA). RJ restores antioxidants (SOD, CAT and GSH) and reduces AST and ALT, mitigating liver damage [110,111]. RJ also alleviates liver injury from azathioprine and paracetamol [112,113].
RJ counteracts cisplatin (CDDP) and taxol (TXL)-induced liver injury by restoring GST and GSH-Px levels and reducing hepatocyte apoptosis [114]. It lowers ALP and LDH levels elevated by TXL and modulates liver growth regulatory factors [115]. RJ diminishes iNOS and IL-1β expression while upregulating Nrf2 and Bcl-2, and down regulating caspases-3 and Bax, protecting against cadmium toxicity [116]. RJ components like MRJP2 protect against CCl4-induced liver injury by mitigating the oxidative in liver by enhancing antioxidant capacity [117]. Thus, RJ safeguards the liver from various injuries by enhancing its antioxidant defenses.
6.10. Diabetes
Diabetes, particularly Type 2 Diabetes (T2DM), results from insulin secretion and resistance issues, affecting adipose tissue, liver, and skeletal muscle [118,119]. T2DM is linked to liver diseases like hepatic cirrhosis, hepatocellular carcinoma, and nonalcoholic fatty liver disease, emphasizing the liver's role in glucose and lipid homeostasis [120,121].
RJ has demonstrated beneficial effects in managing diabetes. In women with T2DM, RJ reduces serum fasting blood glucose and glycosylated hemoglobin levels while increasing insulin concentration, thereby reducing the risk of complications [122,123]. RJ also raises serum apolipoprotein A-I (ApoA-I) levels and improves the ApoB/ApoA-I ratio in T2DM patients (Khoshpey et al., 2016).
In STZ-induced diabetic rats, RJ lowers fasting blood glucose, AST, ALT, and ALP levels, while increasing insulin, albumin, and total protein levels [124]. Additionally, RJ enhances total antioxidant capacity and reduces insulin resistance in diabetic patients [125]. Long-term RJ administration inhibits glucose-6-phosphatase, improving hyperglycemia by increasing adiponectin and adiponectin receptor 1 mRNA expression, and activating AMP-activated protein kinase in abdominal fat [119].
6.11. Restrain obesity
Obesity, a chronic condition influenced by various factors, is marked by heightened oxidative stress and prolonged activation of macrophages in peripheral tissues. Addressing inflammation and oxidative stress is key to effective treatment. RJ supplementation in overweight adults has shown promising results, reducing total cholesterol, C-reactive protein, and increasing adiponectin, serum total antioxidant capacity, bilirubin, and leptin, thus benefiting overweight individuals [126]. Studies in mice fed a high-fat diet and supplemented with RJ observed reduced inflammation and elevated levels of Irisin, promoting metabolic thermogenesis in brown adipose tissue [127,128]. Despite 10-HDA being a significant component of RJ, it was found ineffective in preventing obesity [129].
6.12. Anticancer
RJ shows promising potential in cancer treatment. RJ improves myelosuppression in mice with Ehrlich ascites tumors by inhibiting spleen hematopoietic function and enhancing survival rates, likely through reducing prostaglandin E2 [130]. Prostaglandin E2 regulates lymphocyte proliferation, inhibits macrophage tumoricidal activity, and modulates immune responses [130]. RJ inhibits the proliferation of human breast cancer MCF-7 cells induced by bisphenol A [131] and reduces glutathione levels while increasing lipid peroxidation in human Caco-2 cells when combined with interferon α [132]. In a 4T1 breast cancer mouse model, RJ treatment reduces tumor weight and enhances antioxidant activity in the liver, kidney, and serum [133]. RJ extracts also show strong cytotoxicity against human glioblastoma multiforme [134] and HeLa cells [135].
Lipophilic extract of RJ inhibits human neuroblastoma cell proliferation [136]. Components such as AMP-N1 oxide promote axonal growth and activate the MAPK signaling pathway [23]. MRJP2 promotes caspase-dependent apoptosis in HepG2 cells [137], while 10-HDA inhibits melanin production in B16F1 melanoma cells [23]. The derivative HPO-DAEE induces apoptosis in human lung cancer cells via the ROS-ERK-p38 and CHOP pathways [138].
7. Importance of RJ in health of animals
Numerous studies have investigated the effects of RJ on animal health. RJ enhances cartilage development due to its high collagen content and strengthens bone and tooth structures due to calcium and selenium. It protects blood cells, heart, liver tissues, muscles, and the nervous system due to its potassium content. RJ improves wound healing; a study on mice showed daily RJ application significantly increased wound healing compared to controls, suggesting RJ showed significantly higher effect as compared to Nitrofurazone [139]. RJ also enhanced the healing of tympanic membrane perforations in guinea pigs [140]. In reproductive health, RJ increased sperm motility, reduced abnormal sperm rates, and improved sperm quality in animals [141]. It inhibited age-related testosterone decline in old hamsters [142] and mitigated the negative effects of stress on male rabbit fertility during hot conditions, improving various sperm parameters and plasma biochemical markers [143]. RJ has nephroprotective effects, preventing cisplatin-induced kidney damage [144]. In diabetic rats, RJ alleviated oxidative stress and improved liver and pancreas biochemical markers [145]. It also protects against cadmium-induced genotoxicity and oxidative stress in rats [146]. RJ mitigated the adverse effects of aluminum chloride on reproductive and hormonal health in poisoned rats [147]. It improved sperm parameters and reduced oxidative stress in male rats exposed to hydrogen peroxide [148]. RJ increased ovulation rates and estrus onset in animals [147,149].
Supplementation with bee products improved immunity and performance in quail [150,151]. RJ reduced hyperglycemia and body weight in obese/diabetic mice [119], prevented hyperlipidemia, and improved blood clotting levels [152]. It also reduced epidural fibrosis after laminectomy in rats [153] and delayed atheroma formation in hyperlipidemic rabbits. RJ exhibited osteo inductive and anti-inflammatory effects in treating periodontal diseases [154] and reduced osteoporosis-related bone loss in oophorectomized rats [155]. It restored immune function and improved premature mortality in mice with low micronutrient intake [156]. RJ enhanced spatial memory and inhibited cognitive impairment in older rats [65,66].
RJ showed immunomodulatory effects in the 4T1 breast cancer model in mice, enhancing TNF-a and IgG levels and improving kidney cell size [133]. It reduced breast tumor development and enhanced antioxidant capacity [157]. RJ also reduced the size of WEHI-164 fibrosarcoma tumors in mice [158]. RJ protected cardiac muscle from ischemia, enhancing contraction activity and coronary blood flow. It alleviated physical fatigue in mice [159] and modulated disorders in a Parkinson's disease model in rats [160] RJ protected the colonic mucosa from acetic acid-induced damage in rats, reducing mast cell numbers and colonic erosion [161]. It decreased oral mucositis in rats subjected to radiotherapy [162]. RJ reduced corticosterone levels and improved the antioxidant defense system in stressed rats [163]. RJ, propolis, and bee pollen showed significant antibacterial effects against Aeromonas hydrophila and Vibrio cholerae, suggesting potential in controlling pathogenic bacteria [164].
7.1. RJ: A natural boost health for human
RJ is known for its benefits in cell regeneration, metabolism, and vitality. Rich in natural hormones, vitamins, essential fatty acids, amino acids, sterols, phosphorous compounds, and acetylcholine, RJ aids nerve message transmission and endocrine function. Its nucleic acids and collagen components provide anti-aging effects (Table 7). Gammaglobulin in RJ strengthens the immune system, while 10-HDA has strong antibiotic properties [165,[166], [167], [168], [169], [170]].
Table 7.
Dosage of royal jelly for human use.
| Condition | Dose | Effect of Royal Jelly | References |
|---|---|---|---|
| Elderly Physical Performance and Memory | Not specified | Slows muscle strength deterioration and helps preserve memory | [65,171] |
| Skin Protection | Not specified | Increases collagen production, protects against UVB-induced photoaging | [165] |
| Infection and Inflammation | Not specified | Inhibits Pseudomonas aeruginosa adhesion, protects from inflammatory responses, and offers protection against Fumonisin toxicity | [113,172] |
| MRSA Infections | Not specified | Potential alternative treatment | [173] |
| Dry Eye Syndrome | Not specified | Increases tear secretion, preserves lacrimal gland function | [174,175] |
| Upper Respiratory Tract Infections | Not specified | Suggested as supplements for treatment | [176] |
| Cardiovascular Health | 350 mg per capsule | Reduces serum total cholesterol and LDL levels | [177] |
| 6 g per day for 4 weeks | Lowers small VLDL levels | [178] | |
| Glucose Metabolism | 20 g | Improves glucose tolerance | [179] |
| Autoimmune Diseases | Not specified | Improves clinical severity scores and laboratory markers in systemic lupus erythematosus | [99] |
| Dental Health | 0.2 % RJ | Antibacterial effects | [180,181] |
| Allergies | Not specified | Reduces intestinal anaphylactic responses and histological lesions caused by β-Lg sensitivity | [96] |
| Thyroid Health | Not specified | May have anti-thyroid effects, beneficial for Graves' disease | [85] |
| Skin Pigmentation | Not specified | Inhibits melanogenesis, suggesting use in skincare products | [23] |
| Liver Health | Not specified | Protects against alcohol-induced hepatomegaly, restores transaminase levels | [182] |
| Not specified | Offers hepatoprotection against paclitaxel-induced toxicity | [115] | |
| Skin Hydration | 520 mg per day for 10 weeks | Improves epidermal hydration and ceramide levels | [183] |
| Diabetic Foot Ulcers | Not specified | Aids in healing diabetic foot ulcers | [184] |
| Kidney Health | Not specified | Antioxidants positively impact renal damage caused by oxidative stress and inflammation | [185] |
| Athletic Performance | Not specified | Improves body height, muscle mass, reduces fat components | [186] |
| Pain Relief | 200 mg/kg | Analgesic effects comparable to aspirin for acute pain, more effective than aspirin for chronic pain | [187] |
| Breast Cancer | Not specified | Inhibits growth-promoting effect of Bisphenol A on MCF-7 breast cancer cells | [131] |
RJ mitigates damage from 5-fluorouracil and exhibits antitumor, antibiotic, immunomodulatory, estrogenic, and neurogenic activities [188,189]. A six-month intake of RJ improves erythropoiesis, glucose tolerance, and mental health in humans [190]. It significantly improves sperm count and motility, aiding infertility treatment [170] and is effective in treating early menopause and ovarian damage caused by Adriamycin [191]. For chronic conditions like menopausal osteoporosis and cardiovascular disorders, 150 mg of RJ for three months improves lipid profiles and may control menopause-related dyslipidemia [192]. RJ also improves the quality of life for postmenopausal women, treating sexual and urinary dysfunctions [193]. A 1000 mg capsule of RJ for two months can reduce pre-menstrual syndrome (PMS) [194].
RJ, combined with Indian honey, positively affects fetal membranes in cases of early rupture [195]. RJ contains major fatty acids like 10-H2DA, 10-HDAA, and SEA, which have anti-inflammatory effects and modulate key inflammatory pathways [26]. RJ stimulates the central nervous system, enhancing muscle tone and activity, and promotes neurotrophic effects via Glial Cell-Derived Neurotrophic Factor (GDNF) production, aiding brain cell differentiation and function [196]. RJ improves serum antioxidant capacity and insulin resistance in diabetes [122,125,197] and significantly impacts glycemic control in Type 2 diabetics [198]. It may aid in weight management in diabetic women [199]. RJ supports fatigue recovery in cancer patients [199] and improves oral mucosa symptoms during radiotherapy and chemotherapy, shortening healing times [200]. RJ may protect against radiation-induced apoptosis in human blood leukocytes [201]. RJ and honey exhibit antioxidant effects, reducing Cisplatin-induced nephrotoxicity in cancer patients [202].
7.1.1. Dosage of RJ for human use
Preparation and formulation of dosage of RJ varies from individual to individual as well as from one age group to another age group. In table, 8 dosage of RJ has been summarized (Table 8).
Table 8.
Dosage of royal jelly for human use.
| Group | Condition/Need | Dosage | Supplement Constituents | Reference (s) |
|---|---|---|---|---|
| Infants | Growth and development, strengthen immunity and nervous system | 0.5 g/day for 2–12 months | Raw royal jelly | [[203], [204], [205], [206], [207]] |
| Premature Infants | Complications of prematurity | 50 mg to 1 g/day | ||
| Complications of prematurity | Medium dose of 0.25 g/day | |||
| Children (1–5 yrs) | Low immune system, nervous system impairment, weakness, loss of appetite, anorexia, anaemia | 0.5 g/day | Royal jelly | |
| Children (5–12 yrs) | Low immune system, nervous system impairment, weakness, loss of appetite, anorexia, anaemia | 0.5–1 g/day | ||
| Children (1–5 yrs) | Acute infection and colds | 2.5 g/day for 1–3 days | ||
| Children (5–12 yrs) | Acute infection and colds | 5 g/day for 1–3 days | ||
| Adults | Immunity, insomnia, skin disorders, anaemia, low libido, hormonal imbalance, wounds, premenstrual syndrome, menopause, osteoporosis | 1–2 g/day | ||
| Diabetes, depression, Hashimoto's disease, arthritis | 3–5 g/day | |||
| Recent depression | Up to 10 g/day for 10 days/month for 3 months | |||
| Immunity, convalescence, preparation for surgery, autoimmune diseases, cancer, hormonal imbalances, infertility, ovarian cyst, uterine fibroma, thyroid problems | 1 g/day, up to 10 g/day for 1–3 days in beginning of colds or acute infections, up to 3–5 days post-op healing | Royal jelly, bee products, plants | ||
| Heavy working conditions | 10 g/day | Royal jelly | ||
| Early onset of colds | 10 g/day for 1–3 days | |||
| Post-operative healing | 5–10 g/day for 3–5 days | |||
| Side effects of chemotherapy | 3 g/day for 6–8 weeks | |||
| Neurodegenerative diseases, multiple sclerosis, Parkinson's disease | 10–15 g/day | |||
| Adults | Severe acne | Topical application with Boswellia essential oil; oral treatment with propolis | Royal jelly, Boswellia essential oil, propolis | [85,[208], [209], [210]] |
7.2. Role of RJ in diet formulation
Bee products like RJ, offer essential nutrients crucial for health, especially amid rising environmental pollution and inadequate diets. RJ is rich in 10-HDA, B vitamins, and folate, provides significant nutrition (Lab Reference: CS20133271). While natural folate in RJ is vital, synthetic folic acid supplements may pose risks during pregnancy [211]. Preventive and cell-regenerative actions of RJ are supported by apinutrition (incorporation of bee products to promote overall health and vitality). These functional foods, prominent due to their ease of assimilation and pharmacological benefits are widely consumed [132,188,210,211].
RJ has demonstrated efficacy in alleviating allergic symptoms, managing cholesterol, and supporting conditions like muscular dystrophy, MS, and Parkinson's disease. It aids immune function during radiotherapy and chemotherapy, replenishing cells destroyed by treatment. With its rich amino acid and gamma globulin content, RJ strengthens the immune system against viral infections. It also contains essential nutrients supporting various health aspects, including energy, immunity, cardiovascular health, and mental well-being [180,[212], [213], [214], [215]]. Additionally, RJ acts as an adaptogen enhancing fertility and potentially extending lifespan without disease [214].
8. Significance
This review article provides a comprehensive critical appraisal of the pleiotropic therapeutic effects of RJ, underscoring its potential as a multifaceted natural remedy and food supplements. Following the systematic evaluation of the current scientific literature, it was elucidated that RJ has diverse biological activities anti-diabetic, anti-inflammatory, antioxidant, antimicrobial, and anti-cancer properties. It highlights the molecular mechanisms underlying these effects, thereby advancing our understanding of how RJ can contribute to health and disease management. This critical assessment and summarization of the published findings could play a significant support for both clinical and research communities to identify the gaps in existing literature, propose directions for future research, and support the development of RJ-based therapeutic strategies. The findings of this review emphasize the importance of integrating RJ into complementary and alternative medicine, potentially leading to innovative approaches in treating various ailments as well as food supplements.
9. Novelty
This systematic review uniquely synthesizes recent evidence on the multifaceted therapeutic potential of royal jelly, highlighting its emerging role not only as a functional food supplement but also as a promising intervention in various health conditions. Moreover, this review underscores the novel bioactive components of royal jelly and their biological activities such as anti-inflammatory, antimicrobial (antibacterial, antifungal and antiviral) and antibiotic. In addition this RJ and its metabolites having various health benefits such as gastrointestinal protection, cardiovascular, anti-tumor, anti-ageing, neuroprotective (e.g., Alzheimer's, Parkinson's), hepatotoxicity, metabolic disorders (e.g., diabetes, obesity, hyperlipidaemia), reproductive disorders (e.g., PCOS, infertility and oligospermia) and wound-healing activities. Mechanisms of action of RJ and its therapeutic efficacy offer comprehensive perspective that could guide future research and clinical applications.
10. Conclusion
This review critically appraises the pleiotropic therapeutic effects of RJ, demonstrating its vast potential as a natural therapeutic agent. The comprehensive analysis reveals that RJ exhibits significant anti-inflammatory, antioxidant, antimicrobial, and anti-cancer properties, supported by various molecular mechanisms. Therefore, RJ holds promise for incorporation into complementary and alternative medicine, offering a multifaceted approach to health and disease management.
Limitations
This review, while comprehensive, faces several limitations. First, the heterogeneity of study designs and methodologies in the existing literature complicates the synthesis of findings and may introduce bias. Second, many studies on the therapeutic effects are preclinical trial on RJ, necessitating cautious extrapolation to human applications. Moreover, the variability in the composition of RJ due to differences in bee species, diet, and environmental factors poses challenges in standardizing dosages and formulations. Lastly, the limited number of large-scale, well-controlled clinical trials restricts the ability to draw definitive conclusions about its efficacy and safety. Addressing these limitations in future research is crucial for advancing our understanding and application of RJ in medicine.
Future research and perspectives
RJ holds promise as a natural substance with potential health benefits. As a natural material with possible health advantages, RJ holds promise. Several sectors are interested in it because of its high nutrient profile, which includes proteins, vitamins, and minerals. In the future, RJ research may aim to fully exploit its potential in a range of areas, including medical, nutrition, and cosmetics. In order to understand more about its bioactive components and their effects on human health and perhaps develop new supplements or treatments, researchers may investigate them in greater depth. RJ components may be synthesized via biotechnology for a variety of uses. Furthermore, ethical beekeeping practices and sustainable production methods may grow more popular as environmental conservation gains momentum. As scientific understanding grows, there could be a broader range of products incorporating RJ, catering to diverse consumer needs.
Data availability
Data will be made available on request.
CRediT authorship contribution statement
Rajesh Kumar: Writing – review & editing, Conceptualization. Ankita Thakur: Writing – original draft. Suresh Kumar: Supervision. Younis Ahmad Hajam: Writing – review & editing, Visualization, Supervision, Resources, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Authors would like to appreciate the Department Biosciences, Himachal University, Shimla, Himachal Pradesh and Department of Life Sciences and Allied Health Sciences, Sant Baba Bhag Singh University, Jalandhar, Punjab, India for providing academic and administrative support.
Contributor Information
Rajesh Kumar, Email: drkumar81@rediffmail.com.
Ankita Thakur, Email: Ankitathakur2305@gmail.com.
Suresh Kumar, Email: sureshk8971@gmail.com.
Younis Ahmad Hajam, Email: younismajeed64@gmail.com.
References
- 1.Ullah A., Aldakheel F.M., Anjum S.I., Raza G., Khan S.A., Gajger I.T. Pharmacological properties and therapeutic potential of honey bee venom. Saudi Pharmaceut. J. 2023;31(1):96–109. doi: 10.1016/j.jsps.2022.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khalil A., Elesawy B.H., Ali T.M., Ahmed O.M. Bee venom: from venom to drug. Molecules. 2021;26(16):4941. doi: 10.3390/molecules26164941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maghsoudlou A., Mahoonak A.S., Mohebodini H., Toldra F. Royal jelly: chemistry, storage and bioactivities. J. Apicult. Sci. 2019;63(1):17–40. doi: 10.2478/jas-2019-0007. [DOI] [Google Scholar]
- 4.Moselhy W.A., Fawzy A.M., Kamel A.A. An evaluation of the potent antimicrobial effects and unsaponifiable matter analysis of the royal jelly. Life Sci. J. 2013;10(2):290–296. [Google Scholar]
- 5.Park G.H., Kim Y.B., Park Ji M., Deng Y., Soo Y., Sik K., Rae B. Antibacterial activity of major royal jelly proteins of the honeybee (Apis mellifera) royal jelly. J. Asia Pac. Entomol. 2019;22:737–741. doi: 10.1016/j.aspen.2019.06.005. [DOI] [Google Scholar]
- 6.Zhang X., Yu Y., Sun P., Fan Z., Zhang W., Feng C. Royal jelly peptides: potential inhibitors of β-secretase in N2a/APP695swe cells. Sci. Rep. 2019;9(1):168. doi: 10.1038/s41598-018-35801-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fratini F., Cilia G., Mancini S., Felicioli A. Royal Jelly: an ancient remedy with remarkable antibacterial properties. Microbiol. Res. 2016;192:130–141. doi: 10.1016/j.micres.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 8.Ibrahim S.E.M., Kosba A.A. Royal jelly supplementation reduces skeletal muscle lipotoxicity and insulin resistance in aged obese rats. Pathophysiology. 2018;4:307–315. doi: 10.1016/j.pathophys.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Moradi A.R., Malekinejad H., Farrokhi-Ardabili F., Bernousi I. Royal jelly improves the sperm parameters of ram semen during liquid storage and serves as an antioxidant source. Small Rumin. Res. 2013;113:346–352. doi: 10.1016/j.smallrumres.2013.03.003. [DOI] [Google Scholar]
- 10.Elnagar S.A. Royal jelly counteracts bucks' “summer infertility”. Anim. Reprod. Sci. 2010;121(1–2):174–180. doi: 10.1016/j.anireprosci.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 11.Mahdivand N., Najafi G., Nejati V., Shalizar-Jalali A., Rahmani F. Royal jelly protects male rats from heat stress-induced reproductive failure. Andrologia. 2019;51(3) doi: 10.1111/and.13213. [DOI] [PubMed] [Google Scholar]
- 12.Van Tulder M., Furlan A., Bombardier C., Bouter L. Updated method guidelines for systematic reviews in the cochrane collaboration back review group. Spine. 2003;28(12):1290–1299. doi: 10.1097/00007632-200306150-00014. [DOI] [PubMed] [Google Scholar]
- 13.Yu X., Tu X., Tao L., Daddam J., Li S., Hu F. Royal Jelly fatty acids: chemical composition, extraction, biological activity, and prospect. J. Funct.Foods. 2023;111 doi: 10.1016/j.jff.2023.105868. [DOI] [Google Scholar]
- 14.Guo J., Wang Z., Chen Y., Cao J., Tian W., Ma B., Dong Y. Active components and biological functions of royal jelly. J. Funct.Foods. 2021;82 doi: 10.1016/j.jff.2021.104514. [DOI] [Google Scholar]
- 15.Xue X., Wu L., Wang K. Chemical composition of royal jelly. Bee Products—Chemical and Biological Properties. 2017:181–190. doi: 10.1007/978-3-319-59689-1_8. [DOI] [Google Scholar]
- 16.Buttstedt A., Ihling C.H., Pietzsch M., Moritz R.F.A. Royalactin is not a royal making of a queen. Nature. 2016;10:537. doi: 10.1038/nature19349. [DOI] [PubMed] [Google Scholar]
- 17.Kocot J., Kielczykowska M., Luchowska-Kocot D., Kurzepa J., Musik I. Antioxidant potential of propolis, bee pollen, and royal jelly: possible medical application. Oxid. Med. Cell. Longev. 2018 doi: 10.1155/2018/7074209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Polsinelli G.A., Yu H.D. Regulation of histone deacetylase 3 by metal cations and 10-hydroxy-2E-decenoic acid: possible epigenetic mechanisms of queen-worker bee differentiation. PLoS One. 2018;13 doi: 10.1371/journal.pone.0204538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Šedivá M., Laho M., Kohútová L., Mojžišová A., Majtán J., Klaudiny J. 10-HDA, A major fatty acid of royal jelly, exhibits pH dependent growth-inhibitory activity against different strains of Paenibacilluslarva. Molecules. 2018;23(12):3236. doi: 10.3390/molecules23123236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Y.F., You M.M., Liu Y.C., Shi Y.Z., Wang K., Lu Y.Y., Hu F.L. Potential protective effect of Trans-10-hydroxy-2-decenoic acid on the inflammation induced by Lipoteichoic acid. J. Funct.Foods. 2018;45:491–498. doi: 10.1016/j.jff.2018.03.029. [DOI] [Google Scholar]
- 21.Yang Y.C., Chou W.M., Widowati D.A., Lin I.P., Peng C.C. 10-hydroxy-2-decenoic acid of royal jelly exhibits bactericide and anti-inflammatory activity in human colon cancer cells. BMC Compl. Alternative Med. 2018;18:202. doi: 10.1186/s12906-018-2267-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hattori N., Nomoto H., Fukumitsu H., Mishima S., Furukawa S. Royal jelly-induced neurite outgrowth from rat pheochromocytoma PC12 cells requires integrin signal independent of activation of extracellular signal-regulated kinases. Biomed. Res. 2007;28(3):139–146. doi: 10.2220/biomedres.28.139. [DOI] [PubMed] [Google Scholar]
- 23.Peng C.C., Sun H.T., Lin I.P., Li J.C., Kuo P.C. The functional property of royal jelly 10-hydroxy-2-decenoic acid as a melanogenesis inhibitor. BMC Compl. Alternative Med. 2017;17(1):392. doi: 10.1186/s12906-017-1888-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng J., Lai W., Zhu G., Wan M., Chen J., Tai Y., Lu C. 10-Hydroxy-2-decenoic acid prevents ultraviolet A-induced damage and matrix metalloproteinases expression in human dermal fibroblasts. J. Eur. Acad. Dermatol. Venereol. 2013;27:1269–1277. doi: 10.1111/j.1468-3083.2012.04707.x. [DOI] [PubMed] [Google Scholar]
- 25.Wang J.G., Ruan J., Li C.Y., Wang J.M., Li Y., Zhai W.T., Zhang W., Ye H., Shen N.H., Lei K.F., et al. Connective tissue growth factor, a regulator related with 10-hydroxy-2-decenoic acid down-regulate MMPs in rheumatoid arthritis. Rheumatol. Int. 2012;32:2791–2799. doi: 10.1007/s00296-011-1960-5. [DOI] [PubMed] [Google Scholar]
- 26.Chen Y.F., Wang K., Zhang Y.Z., Zheng Y.F., Hu F.L. In vitro anti-inflammatory effects of three fatty acids from royal jelly. Mediat. Inflamm. 2016;1 doi: 10.1155/2016/3583684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moutsatsou P., Papoutsi Z., Kassi E., Heldring N., Zhao C., Tsiapara A., Melliou E., Chrousos G.P., Chinou I., Karshikoff A., et al. Fatty acids derived from royal jelly are modulators of estrogen receptor functions. PLoS One. 2010;5 doi: 10.1371/journal.pone.0015594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weiser M.J., Grimshaw V., Wynalda K.M., Mohajeri M.H., Butt C.M. Long-term administration of queen bee acid (QBA) to rodents reduces anxiety-like behaviour, promotes neuronal health and improves body composition. Nutrients. 2017;10:13. doi: 10.3390/nu10010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xin X.X., Chen Y., Chen D., Xiao F., Parnell L.D., Zhao J., Liu L., Ordovas J.M., Lai C.Q., Shen L.R. Supplementation with major royal-jelly proteins increases lifespan, feeding, and fecundity in Drosophila. J. Agric. Food Chem. 2016;64:5803–5812. doi: 10.1021/acs.jafc.6b00514. [DOI] [PubMed] [Google Scholar]
- 30.Moriyama T., Ito A., Omote S., Miura Y., Tsumoto H. Heat resistant characteristics of major royal jelly protein 1 (MRJP1) oligomer. PLoS One. 2015;10 doi: 10.1371/journal.pone.0119169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kamakura M. Royalactin induces queen differentiation in honeybees. Nature. 2011;473:478–483. doi: 10.1038/nature10093. [DOI] [PubMed] [Google Scholar]
- 32.Liu J.R., Yang Y.C., Shi L.S., Peng C.C. Antioxidant properties of royal jelly associated with larval age and time of harvest. J. Agric. Food Chem. 2008;56:11447–11452. doi: 10.1021/jf802494e. [DOI] [PubMed] [Google Scholar]
- 33.Pina A., Begou O., Kanelis D., Gika H., Kalogiannis S., Tananaki C., Theodoridis G., Zotou A. Targeted profiling of hydrophilic constituents of royal jelly by hydrophilic interaction liquid chromatography-tandem mass spectrometry. J. Chromatogr. A. 2018;1531:53–63. doi: 10.1016/j.chroma.2017.11.019. [DOI] [PubMed] [Google Scholar]
- 34.Stocker A., Schramel P., Kettrup A., Bengsch E. Trace and mineral elements in royal jelly and homeostatic effects. J. Trace Elem. Med. Biol. 2005;19:183–189. doi: 10.1016/j.jtemb.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 35.Wessler I., Gartner H.A., Michel-Schmidt R., Brochhausen C., Schmitz L., Anspach L., Grunewald B., Kirkpatrick C.J. Honeybees produce millimolar concentrations of non-neuronal acetylcholine for breeding: possible adverse effects of neonicotinoids. PLoS One. 2016;11 doi: 10.1371/journal.pone.0156886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zamani Z., Reisi P., Alaei H., Pilehvarian A.A. Effect of Royal Jelly on spatial learning and memory in rat model of streptozotocin-induced sporadic Alzheimer's disease. Adv. Biomed. Res. 2012;1:26. doi: 10.4103/2277-9175.98150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu L., Chen L., Selvaraj J.N., Wei Y., Wang Y., Li Y., Zhao J., Xue X. Identification of the distribution of adenosine phosphates, nucleosides and nucleobases in royal jelly. Food Chem. 2015;173:1111–1118. doi: 10.1016/j.foodchem.2014.10.137. [DOI] [PubMed] [Google Scholar]
- 38.Hattori N., Nomoto H., Fukumitsu H., Mishima S., Furukawa S. AMP N1-oxide, a unique compound of royal jelly, induces neurite outgrowth from PC12 vells via signaling by protein kinase A independent of that by mitogen-activated protein kinase. Evid. base Compl. Alternative Med. 2010;7:63–68. doi: 10.1093/ecam/nem146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fujisue K., Yamamoto E., Sueta D., Arima Y., Hirakawa K., Tabata N., Tsujita K. A randomized, double-blind comparison study of royal jelly to augment vascular endothelial function in healthy volunteers. J. Atherosclerosis Thromb. 2022;29(9):1285–1294. doi: 10.5551/jat.63044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pan Y., Rong Y., You M., Ma Q., Chen M., Hu F. Royal jelly causes hypotension and vasodilation induced by increasing nitric oxide production. Food Sci. Nutr. 2019;7(4):1361–1370. doi: 10.1002/fsn3.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mohamed H.K., Mobasher M.A., Ebiya R.A., Hassen M.T., Hagag H.M., El-Sayed R., Awad N.S. Anti-inflammatory, anti-apoptotic, and antioxidant roles of honey, royal jelly, and propolis in suppressing nephrotoxicity induced by doxorubicin in male albino rats. Antioxidants11. 2022;(5):1029. doi: 10.3390/antiox11051029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ali A.M., Kunugi H. Royal jelly as an intelligent anti-aging agent—a focus on cognitive aging and Alzheimer's disease. A review. 2020;(10) doi: 10.3390/antiox9100937. 937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kul Köprülü T., Erkal B., Kara A., Tekin S. Investigation of the effects of the royal jelly on genomic demethylation and tumor suppressor genes in human cancer cells. Med. Oncol. 2022;40(1):59. doi: 10.1007/s12032-022-01927-1. [DOI] [PubMed] [Google Scholar]
- 44.Raoufi S., Salavati Z., Komaki A., Shahidi S., Zarei M. Royal jelly improves learning and memory deficits in an amyloid β-induced model of Alzheimer's disease in male rats: involvement of oxidative stress. Metab. Brain Dis. 2023;(4):1239–1248. doi: 10.1007/s11011-023-01168-9. [DOI] [PubMed] [Google Scholar]
- 45.Jalili C., Roshankhah S., Jalali A., Salahshoor M.R. Hepatoprotective activity of royal jelly on mercuric chloride–induced damage model in rats. Journal of Reports in Pharmaceutical Sciences. 2019;8(2):181–187. doi: 10.4103/jrptps.JRPTPS_27_19. [DOI] [Google Scholar]
- 46.Pandeya P.R., Lamichhane R., Lee K.H., Kim S.G., Lee D.H., Lee H.K., Jung H.J. Bioassay-guided isolation of active anti-adipogenic compound from royal jelly and the study of possible mechanism. BMC Compl. Alternative Med. 2019;19:1–14. doi: 10.1186/s12906-018-2423-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fallah M., Najafi F., Kavoosi G. Proximate analysis, nutritional quality and anti‐amylase activity of bee propolis, bee bread and royal jelly. IJFST (Int. J. Food Sci. Technol.) 2022;(5):2944–2953. doi: 10.1111/ijfs.15605. [DOI] [Google Scholar]
- 48.Lin Y., Zhang M., Lin T., Wang L., Wang G., Chen T., Su S. Royal jelly from different floral sources possesses distinct wound-healing mechanisms and ingredient profiles. Food Funct. 2021;12(23):12059–12076. doi: 10.1039/D1FO00586C. [DOI] [PubMed] [Google Scholar]
- 49.Botezan S., Baci G.M., Bagameri L., Pașca C., Dezmirean D.S. Current status of the bioactive properties of royal jelly: a comprehensive review with a focus on its anticancer, anti-inflammatory and antioxidant effects. Molecules. 2023;28(3):1510. doi: 10.3390/molecules28031510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yakoot M., Salem A., Helmy S. Effect of Memo®, a natural formula combination, on mini-mental state examination scores in patients with mild cognitive impairment. Clin. Interv. Aging. 2013;8:975–981. doi: 10.2147/CIA.S44777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang M., Wang L.M., Chen Z.H., Zhao Z.P., Li Y.C., Deng Q., Huang Z.J., Zhang X., Li C., Zhou M.G., et al. Multilevel logistic regression analysis on hypercholesterolemia related risk factors among adults in China. Zhonghua Yufang Yixue Zazhi. 2018;52:151–157. doi: 10.3760/cma.j.issn.0253-9624.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 52.Inoue Y., Hara H., Mitsugi Y., Yamaguchi E., Kamiya T., Itoh A., Adachi T. 4-hydroperoxy-2-decenoic acid ethyl ester protects against 6-hydroxydopamine-induced cell death via activation of Nrf2-ARE and eIF2α-ATF4 pathways. Neurochem. Int. 2018;112:288–296. doi: 10.1016/j.neuint.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 53.Gil-Iturbe E., Solas M., Cuadrado-Tejedo M., García-Osta A., Escoté X., Ramírez M.J., Lostao M.P. GLUT12 expression in brain of mouse models of Alzheimer's disease. Molecular Neurobiology57. 2020:798–805. doi: 10.1007/s12035-019-01743-1. [DOI] [PubMed] [Google Scholar]
- 54.Smiljanic K., Todorovic S., Mladenovic Djordjevic A., Vanmierlo T., Lütjohann D., Ivkovic S., Kanazir S. Limited daily feeding and intermittent feeding have different effects on regional brain energy homeostasis during aging. Biogerontology. 2018;19:121–132. doi: 10.1007/s10522-018-9743-y. [DOI] [PubMed] [Google Scholar]
- 55.Yakoot M., Salem A., Omar A.M. Effectiveness ofaHerbal formula in women with menopausal syndrome. Res. Complementary Med. 2011;18:264–268. doi: 10.1159/000333430. [DOI] [PubMed] [Google Scholar]
- 56.Pyrzanowska J., Wawer A., Joniec-Maciejak I., Piechal A., Blecharz-Klin K., Graikou K., Widy-Tyszkiewicz E. Long-term administration of Greek Royal Jelly decreases GABA concentration in the striatum and hypothalamus of naturally aged Wistar male rats. Neurosci. Lett. 2018;675:17–22. doi: 10.1016/j.neulet.2018.03.034. [DOI] [PubMed] [Google Scholar]
- 57.Ji W.Z., Zhang C.P., Wei W.T., Hu F.L. The in vivo antiaging effect of enzymatic hydrolysate from royal jelly in d-galactose induced aging mouse. J. Chin. Inst. Food Sci. Technol. 2016:18–25. doi: 10.16429/j.1009-7848.2016.01.003. [DOI] [Google Scholar]
- 58.Hattori N., Ohta S., Sakamoto T., Mishima S., Furukawa S. Royal jelly facilitates restoration of the cognitive ability in trimethyltin-intoxicated mice. Evid. base Compl. Alternative Med. 2011 doi: 10.1093/ecam/nep029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Halloran J., Hussong S.A., Burbank R., Podlutskaya N., Fischer K.E., Sloane L.B., Galvan V. Chronic inhibition of mammalian target of rapamycin by rapamycin modulates cognitive and non-cognitive components of behaviourthroughout lifespan in mice. Neuroscience. 2012;223:102–113. doi: 10.1016/j.neuroscience.2012.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kirova A.M., Bays R.B., Lagalwar S. Working memory and executive function decline across normal aging, mild cognitive impairment, and Alzheimer's disease. BioMed Res. Int. 2015 doi: 10.1155/2015/748212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guardia de Souza E Silva T., do Val de Paulo M.E.F., da Silva J.R.M., da Silva Alves A., Britto L.R.G., Xavier G.F., Lopes Sandoval M.R. Oral treatment with royal jelly improves memory and presents neuroprotective effects on icv-STZ rat model of sporadic Alzheimer's disease. Heliyon. 2020;6(2) doi: 10.1016/j.heliyon.2020.e03281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.You M., Pan Y., Liu Y., Chen Y., Wu Y., Si J., Hu F. Royal jelly alleviates cognitive deficits and β-amyloid accumulation in APP/PS1 mouse model via activation of the cAMP/PKA/CREB/BDNF pathway and inhibition of neuronal apoptosis. Front. Aging Neurosci. 2018;10:428. doi: 10.3389/fnagi.2018.00428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Minami A., Matsushita H., Ieno D., Matsuda Y., Horii Y., Ishii A., Suzuki T. Improvement of neurological disorders in postmenopausal model rats by administration of royal jelly. Climacteric: The Journal of the International Menopause Society. 2016;19(6):568–573. doi: 10.1080/13697137.2016.1238452. [DOI] [PubMed] [Google Scholar]
- 64.Pan Y., Xu J., Jin P., Yang Q., Zhu K., You M., Hu F. Royal jelly ameliorates behavioural deficits, cholinergic system deficiency, and autonomic nervous dysfunction in ovariectomized cholesterol-fed Rabbits. Molecules. 2019;24(6):1149. doi: 10.3390/molecules24061149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pyrzanowska J., Piechal A., Blecharz-Klin K., Joniec-Maciejak I., Graikou K., Chinou I., Widy-Tyszkiewicz E. Long-term administration of Greek Royal Jelly improves spatial memory and influences the concentration of brain neurotransmitters in naturally aged Wistar male rats. J. Ethnopharmacol. 2014;155(1):343–351. doi: 10.1016/j.jep.2014.05.032. [DOI] [PubMed] [Google Scholar]
- 66.Chen D., Liu F., Wan J.B., Lai C.Q., Shen L.R. Effect of major royal jelly proteins on spatial memory in aged rats: metabolomics analysis in urine. J. Agric. Food Chem. 2017;65(15):3151–3159. doi: 10.1021/acs.jafc.7b00202. [DOI] [PubMed] [Google Scholar]
- 67.Kazemijaliseh H., Tehrani F.R., Behboudi-Gandevani S., Hosseinpanah F., Khalili D., Azizi F. The prevalence and causes of primary infertility in Iran: a population-based study. Global J. Health Sci. 2015;7:226–232. doi: 10.5539/gjhs.v7n6p226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schuppe H.C., Pilatz A., Hossain H., Diemer T., Wagenlehner F., Weidner W. Urogenital infection as a risk factor for male infertility. DeutschesArzteblatt International. 2017;114:339–346. doi: 10.3238/arztebl.2017.0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jafari H., Latifnejad Roudsari R., Taghipour A., Khadem Ghaebi N., Ebrahim Zadeh S. Comparison of knowledge and attitude towards reproductive donation procedures between recipient and non-recipient infertile couples at Mashhad Infertility Center. Journal of TorbatHeydariyeh University of Medical Sciences. 2015;3:16–25. http://jms.thums.ac.ir/article-1-82-en.html [Google Scholar]
- 70.Agarwal A., Mulgund A., Hamada A., Chyatte M.R. A unique view on male infertility around the globe. Reprod. Biol. Endocrinol. 2015;13:1–9. doi: 10.1186/s12958-015-0032-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lewis R. Hachette UK; 2008. The Infertility Cure: the Ancient Chinese Wellness Program for Getting Pregnant and Having Healthybabies. [Google Scholar]
- 72.Stangaciu S., Mărghitaş L.A., Dezmirean D., Bonta V., Mărgăoan R., Bobiş O. Quality parameters needed for bee products used in apitherapy. Bulletin UASVM Animal Science and Biotechnologies. 2015;72(1):67–71. doi: 10.15835/buasvmcn-asb:10561. [DOI] [Google Scholar]
- 73.Kodai T., Umebayashi K., Nakatani T., Ishiyama K., Noda N. Compositions of royal jelly II. Organic acid glycosides and sterols of the royal jelly of honeybees (Apis mellifera) Chem. Pharmaceut. Bull. 2007;55(10):1528–1531. doi: 10.1248/cpb.55.1528. [DOI] [PubMed] [Google Scholar]
- 74.Townsend G.F., Morgan J.F., Hazlett B. Activity of 10-hydroxydecenoic acid from royal jelly against experimental leukaemia and ascitic tumours. Nature. 1959;183:1270–1271. doi: 10.1038/1831270a0. [DOI] [PubMed] [Google Scholar]
- 75.Schmidt H.H., Stocker R., Vollbracht C., Paulsen G., Riley D., Daiber A., Cuadrado A. Antioxidants in translational medicine. Antioxidants & RedoxSignalling. 2015;23:1130–1143. doi: 10.1089/ars.2015.6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bălan A., Moga M.A., Dima L., Toma S., Neculau E.A., Anastasiu C.V. Royal jelly—a traditional and natural remedy for postmenopausal symptoms and aging-related pathologies. Molecules. 2020;25:3291. doi: 10.3390/molecules25143291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Salama S., Shou Q., Abd El-Wahed A.A., Elias N., Xiao J., Swillam A., Umair M., Guo Z., Daglia M., Wanget K., et al. Royal jelly: beneficial properties and synergistic effects with chemotherapeutic drugs with particular emphasis in anticancer strategies. Nutrients. 2022;14:4166. doi: 10.3390/nu14194166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fujiwara S., Imai J., Fujiwara M., Yaeshima T., Kawashima T., Kobayashi K. A potent antibacterial protein in royal jelly. Purification and determination of the primary structure of royalisin. Peptides25. 2004:919–928. doi: 10.1016/S0021-9258(19)38596-5. [DOI] [PubMed] [Google Scholar]
- 79.Fontana R., Mendes M.A., de Souza B.M., Konno K., César L.M., Malaspina O., Palma M.S. Jelleines: a family of antimicrobial peptides from the royal jelly of honeybees (Apis mellifera) Peptides. 2004;25(6):919–928. doi: 10.1016/j.peptides.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 80.Bílikova K., Huang S.C., Linb I.P., Simuth J., Peng C.C. Peptides structure and antimicrobial activity relationship of royalisin, an antimicrobial peptide from royal jelly of Apis mellifera. Peptides. 2015;68:190–196. doi: 10.1016/j.peptides.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 81.Hasan A.A., Sahib S., Hillawi H., Khudhur H.R., Techniques M. Antimicrobial activity of royal jelly against E. Coli in comparison with three selective antibiotics. Ann. Trop. Med. Publ. Health. 2020;23:231–414. doi: 10.36295/ASRO.2020.231414. [DOI] [Google Scholar]
- 82.Park H.G., Kim B.Y., Park M.J., Deng Y., Choi Y.S., Lee K.S., Jin B.R. Antibacterial activity of major royal jelly proteins of the honeybee (Apis mellifera) royal jelly. J. Asia Pac. Entomol. 2019;22(3):737–741. doi: 10.1016/j.aspen.2019.06.005. [DOI] [Google Scholar]
- 83.Hassan A.A., Essam Y., Nassrallah A., Cheng W., El-maksoud A.A.A. Royal jelly improves the physicochemical properties and biological activities of fermented milk with enhanced probiotic viability. Lwt. 2022;155 doi: 10.1016/j.lwt.2021.112912. [DOI] [Google Scholar]
- 84.Khosla A., Gupta S.J., Jain A., Shetty D.C., Sharma N. Evaluation and comparison of the antimicrobial activity of royal jelly-A holistic healer against periodontopathic bacteria. J. Indian Soc. Periodontol. 2020;24:221–226. doi: 10.4103/jisp.jisp_486_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kohno K., Okamoto I., Sano O., Arai N., Iwaki K., Ikeda M., Kurimoto M. Royal jelly inhibits the production of proinflammatory cytokines by activated macrophages. Biosc. Biotech. Biochem. 2004;68(1):138–145. doi: 10.1271/bbb.68.138. [DOI] [PubMed] [Google Scholar]
- 86.Satomi K.M., Okamoto I., Ushio S., Iwaki K., Ikeda M., Kurimoto M. Identification of a collagen production-promoting factor from an extract of royal jelly and its possible mechanism. Biosci., Biotechnol., Biochem. 2004;68(4):767–773. doi: 10.1271/bbb.68.767. [DOI] [PubMed] [Google Scholar]
- 87.Ananthakrishnan A.N., Xavier R.J., Gastrointestinal Diseases . 2020. Hunter's Tropical Medicine and Emerging Infectious Diseases; pp. 16–26. [Google Scholar]
- 88.Sperber A.D., Bangdiwala S.I., Drossman D.A., Ghoshal U.C., Simren M., Tack J., Whitehead W.E., Dumitrascu D.L., Fang X., Fukudoetet al S. Worldwide prevalence and burden of functional gastrointestinal disorders, results of Rome foundation global study. Gastroenterology. 2021;160:99–114. doi: 10.1053/j.gastro.2020.04.014. [DOI] [PubMed] [Google Scholar]
- 89.Rizzello F., Spisni E., Giovanardi E., Imbesi V., Salice M., Alvisi P., Valerii M.C., Gionchetti P. Implications of the WesternizedDiet in the onset and progression of IBD. Nutrients. 2019;11:1033. doi: 10.3390/nu11051033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.De Filippo C., Di Paola M., Ramazzotti M., Albanese D., Pieraccini G., Banci E., Miglietta F., Cavalieri D., Lionetti P. Diet,Environments, and gut microbiota. A preliminary investigation in children living in rural and urban Burkina Faso and Italy. Frontiersin Microbiology. 2017;8:1979–1983. doi: 10.3389/fmicb.2017.01979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vona R., Pallotta L., Cappelletti M., Severi C., Matarrese P. The impact of oxidative stress in human pathology: focus on gastrointestinal disorders. Antioxidants. 2021;10:201. doi: 10.3390/antiox10020201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mostafa R.E., El-Marasy S.A., Abdel Jaleel G.A., Bakeer R.M. Protective effect of royal jelly against diclofenac-InducedHepato-renal damage and gastrointestinal ulcerations in rats. Heliyon. 2020;6 doi: 10.1016/j.heliyon.2020.e03330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li S., Tao L., Yu X., Zheng H., Wu J.H.F. Royal jelly proteins and their derived peptides: preparation, properties, and BiologicalActivities. J. Agric. Food Chem. 2021;69:14415–14427. doi: 10.1021/acs.jafc.1c05942. [DOI] [PubMed] [Google Scholar]
- 94.Ahmad S., Campos M.G., Fratini F., Altaye S.Z., Li J. New insights into the biological and pharmaceutical properties of RoyalJelly. Int. J. Mol. Sci. 2020:382. doi: 10.3390/ijms21020382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Oka H., Emori Y., Kobayashi N., Hayashi Y., Nomoto K. Suppression of allergic reactions by royal jelly in association with the restoration of macrophage function and the improvement of Th1/Th2 Cell responses. Int. Immunopharm. 2001;1(3):521–532. doi: 10.1016/s1567-5769(00)00007-2. [DOI] [PubMed] [Google Scholar]
- 96.Guendouz M., Haddi A., Grar H., Kheroua O., Saidi D., Kaddouri H. Preventive effects of royal jelly against anaphylactic response in a murine model of cow's milk allergy. Pharmaceut. Biol. 2017;55(1):2145–2152. doi: 10.1080/13880209.2017.1383487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Taniguchi Y., Kohno K., Inoue S.I., Koya-Miyata S., Okamoto I., Arai N., Kurimoto M. Oral administration of royal jelly inhibits the development of atopic dermatitis-like skin lesions in NC/Nga mice. Int. Immunopharm. 2003;3(9):1313–1324. doi: 10.1016/s1567-5769(03)00132-2. [DOI] [PubMed] [Google Scholar]
- 98.Mannoor M.K., Shimabukuro I., Tsukamotoa M., Watanabe H., Yamaguchi K., Sato Y. Honeybee royal jelly inhibits autoimmunity in SLE-prone NZB x NZW F1 mice. Lupus. 2009;18(1):44–52. doi: 10.1177/0961203308094765. [DOI] [PubMed] [Google Scholar]
- 99.Zahran A.M., Elsayh K.I., Saad K., Eloseily E.M., Osman N.S., Alblihed M.A., Mahmoud M.H. Effects of royal jelly supplementation on regulatory T cells in children with SLE. Food Nutr. Res. 2016;60(1) doi: 10.3402/fnr.v60.32963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Erem C., Deger O., Ovali E., Barlak Y. The Effects of royal jelly on autoimmunity in Graves' Disease. Endocrine. 2006;30(2):175–183. doi: 10.1385/endo:30:2:175. [DOI] [PubMed] [Google Scholar]
- 101.Kai H., Motomura Y., Saito S., Hashimoto K., Tatefuji T., Takamune N., Misumi S. Royal jelly enhances antigen-specific mucosal IgA response. Food Sci. Nutr. 2013;1(3):222–227. doi: 10.1002/fsn3.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Okamoto I., Taniguchi Y., Kunikata T., Kohno K., Iwaki K., Ikeda M., Kurimoto M. Major royal jelly protein 3 modulates immune responses in vitro and in vivo. Life Sci. 2003;(16):2029–2045. doi: 10.1016/s0024-3205(03)00562-9. [DOI] [PubMed] [Google Scholar]
- 103.Han B., Fang Y., Feng M., Lu X., Huo X., Meng L., Li J. In-depth phosphoproteomic analysis of royal jelly derived from western and eastern honeybee species. J. Proteome Res. 2014;13(12):5928–5943. doi: 10.1021/pr500843j. [DOI] [PubMed] [Google Scholar]
- 104.Sugiyama T., Takahashi K., Kuzumaki A., Tokoro S., Neri P., Mori H. Inhibitory mechanism of 10-hydroxy-trans-2-decenoic acid (royal jelly acid) against lipopolysaccharide- and interferon-β-induced nitric oxide production. Inflammation. 2013;36(2):372–378. doi: 10.1007/s10753-012-9556-0. [DOI] [PubMed] [Google Scholar]
- 105.Gasic S., Vucevic D., Vasilijic S., Antunovic M., Chinou I., Colic M. Evaluation of the immunomodulatory activities of royal jelly components in vitro. Immunopharmacol. Immunotoxicol. 2007;29(3–4):521–536. doi: 10.1080/08923970701690977. [DOI] [PubMed] [Google Scholar]
- 106.Vucevic D., Melliou E., Vasilijic S., Gasic S., Ivanovski P., Chinou I., Colic M. Fatty acids isolated from royal jelly modulate dendritic cell-mediated immune response in vitro. Int. Immunopharm. 2007;7(9):1211–1220. doi: 10.1016/j.intimp.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 107.Fan P., Han B., Hu H., Wei Q., Zhang X., Meng L., Li J. Proteome of thymus and spleen reveals that 10-hydroxydec-2-enoic acid could enhance immunity in mice. Expert Opin. Ther. Targets. 2020;24(3):267–279. doi: 10.1080/14728222.2020.1733529. [DOI] [PubMed] [Google Scholar]
- 108.Kamakura M., Maebuchi M., Ozasa S., Komori M., Ogawa T., Sakaki T., Moriyama T. Influence of royal jelly on mouse hepatic gene expression and safety assessment with a DNA microarray. J. Nutr. Sci. Vitaminol. 2005;51(3):148–155. doi: 10.3177/jnsv.51.148. [DOI] [PubMed] [Google Scholar]
- 109.Kanbur M., Eraslan G., Beyaz L., Silici S., Liman B.C., Altinordulu S., Atasever A. The effects of royal jelly on liver damage induced by paracetamol in mice. Exp. Toxicol. Pathol. 2009;61(2):123–132. doi: 10.1016/j.etp.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 110.Çavuşoğlu K., Yapar K., Oruç E., Yalçın E. The protective effect of royal jelly on chronic lambda-cyhalothrin toxicity: serum biochemical parameters, lipid peroxidation, and genotoxic and histopathological alterations in Swiss albino mice. J. Med. Food. 2011;(10):1229–1237. doi: 10.1089/jmf.2010.0219. [DOI] [PubMed] [Google Scholar]
- 111.Cemek M., Aymelek F., Büyükokurŏglu M.E., Karaca T., Büyükben A., Yilmaz F. Protective potential of royal jelly against carbon tetrachloride induced-toxicity and changes in the serum sialic acid levels. Food Chem. Toxicol. 2010;48(10):2827–2832. doi: 10.1016/j.fct.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 112.Ahmed W.M.S., Khalaf A.A., Moselhy W.A., Safwat G.M. Royal jelly attenuates azathioprine induced toxicity in rats. Environ. Toxicol. Pharmacol. 2014;(1):431–437. doi: 10.1016/j.etap.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 113.El-Nekeety A.A., El-Kholy W., Abbas N.F., Ebaid A., Amra H.A., Abdel-Wahhab M.A. Efficacy of royal jelly against the oxidative stress of fumonisin in rats. Toxicon50. 2007;(2):256–269. doi: 10.1016/j.toxicon.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 114.Karadeniz A., Simsek N., Karakus E., Yildirim S., Kara A., Can I., Turkeli M. Royal jelly modulates oxidative stress and apoptosis in liver and kidneys of rats treated with cisplatin. Oxid. Med. Cell. Longev. 2011;1 doi: 10.1155/2011/981793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Malekinejad H., Fani M., Shafiee-Roodbari S.K.H., Delkhosh-Kasmaie F., Rezaei-Golmisheh A. Crosstalk between E2f1 and c-Myc mediates hepato-protective effect of royal jelly on taxol-induced damages. Hum. Exp. Toxicol. 2017;36(6):626–637. doi: 10.1177/0960327116660752. [DOI] [PubMed] [Google Scholar]
- 116.Almeer R.S., Alarifi S., Alkahtani S., Ibrahim S.R., Ali D., Moneim A. The potential hepatoprotective effect of royal jelly against cadmium chloride-induced hepatotoxicity in mice is mediated by suppression of oxidative stress and upregulation of Nrf2 expression. Biomed. Pharmacother. 2018;106:1490–1498. doi: 10.1016/j.biopha.2018.07.089. [DOI] [PubMed] [Google Scholar]
- 117.Caixeta D.C., Teixeira R.R., Peixoto L.G., Machado H.L., Baptista N.B., de Souza A.V., Salmen Espindola F. Adaptogenic potential of royal jelly in liver of rats exposed to chronic stress. PloS. 2018;One13(1) doi: 10.1371/journal.pone.0191889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.DeFronzo R.A., Ferrannini E., Groop L., Henry R.R., Herman W.H., Holst J.J., Weiss R. Type 2 diabetes mellitus. Nat. Rev. Dis. Prim. 2015 doi: 10.1038/nrdp.2015.19. [DOI] [PubMed] [Google Scholar]
- 119.Yoshida M., Hayashi K., Watadani R., Okano Y., Tanimura K., Kotoh J., Maeda A. Royal jelly improves hyperglycemia in obese/diabetic KK-Ay mice. J. Vet. Med. Sci. 2017;79(2):299–307. doi: 10.1292/jvms.16-0458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Guven A., Yavuz O., Cam M., Ercan F., Bukan N., Comunoglu C., Gokce F. Effects of melatonin on streptozotocin-induced diabetic liver injury in rats. Acta Histochem. 2006;108(2):85–93. doi: 10.1016/j.acthis.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 121.Sivajothi V., Dey A., Jayakar B., Rajkapoor B. Antihyperglycemic property of Tragia cannabina in streptozotocin-induced diabetic rats. J. Med. Food. 2007;10(2):361–365. doi: 10.1089/jmf.2006.030. [DOI] [PubMed] [Google Scholar]
- 122.Pourmoradian S., Mahdavi R., Mobasseri M., Faramarzi E., Mobasseri M. Effects of royal jelly supplementation on glycemic control and oxidative stress factors in type 2 diabetic female: a randomized clinical trial. Chinese Journal of Integrated Medicine. 2014;20(5):347–352. doi: 10.1007/s11655-014-1804-8. [DOI] [PubMed] [Google Scholar]
- 123.Woerle H.J., Neumann C., Zschau S., Tenner S., Irsigler A., Schirra J., Göke B. Impact of fasting and postprandial glycemia on overall glycemic control in type 2 diabetes Importance of postprandial glycemia to achieve target HbA1c levels. Diabetes Res. Clin. Pract. 2007;77(2):280–285. doi: 10.1016/j.diabres.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 124.Ghanbari E., Nejati V., Khazaei M. Improvement in serum biochemical alterations and oxidative stress of liver and pancreas following use of royal jelly in streptozotocin-induced diabetic rats. Cell Journal (Yakhteh) 2016;18(3):362. doi: 10.22074/cellj.2016.4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Shidfar F., Jazayeri S., Mousavi S.N., Malek M., Hosseini A.F., Khoshpey B. Does supplementation with royal jelly improve oxidative stress and insulin resistance in type 2 diabetic patients? Iran. J. Public Health. 2015;44(6):797–803. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4524304/ [PMC free article] [PubMed] [Google Scholar]
- 126.Petelin A., Kenig S., Kopiňc R., Dězelak M., Černelic Bizjak M., Jenko Praznikar Z. Effects of royal jelly administration on lipid profile, satiety, inflammation, and antioxidant capacity in asymptomatic overweight adults. Evid. base Compl. Alternative Med. 2019;2019(1) doi: 10.1155/2019/4969720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Irandoost P., MesriAlamdari N., Saidpour A., Shidfar F., Roshanravan N., Asghari Jafarabadi M., Vafa M. The effects of royal jelly and tocotrienol-rich fraction on impaired glycemic control and inflammation through irisin in obese rats. J. Food Biochem. 2020;(12) doi: 10.1111/jfbc.13493. [DOI] [PubMed] [Google Scholar]
- 128.Yoneshiro T., Kaede R., Nagaya K., Aoyama J., Saito M., Okamatsu-Ogura Y., Terao A. Royal jelly ameliorates diet-induced obesity and glucose intolerance by promoting brown adipose tissue thermogenesis in mice. Obes. Res. Clin. Pract. 2018;12(2):127–137. doi: 10.1016/j.orcp.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 129.Watadani R., Kotoh J., Sasaki D., Someya A., Matsumoto K., Maeda A. 10- Hydroxy-2-decenoic acid, a natural product, improves hyperglycemia and insulin resistance in obese/diabetic KK-Ay mice, but does not prevent obesity. J. Vet. Med. Sci. 2017;79(9):1596–1602. doi: 10.1292/jvms.17-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bincoletto C., Eberlin S., Figueiredo C.A.V., Luengo M.B., Queiroz M.L.S. Effects produced by royal jelly on haematopoiesis: relation with host resistance against Ehrlich ascites tumour challenge. Int. Immunopharm. 2005;5(4):679–688. doi: 10.1016/j.intimp.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 131.Nakaya M., Onda H., Sasaki K., Yukiyoshi A., Tachibana H., Yamada K. Effect of royal jelly on bisphenol A-induced proliferation of human breast cancer cells. Biosc. Biotech. Biochem. 2007;71(1):253–255. doi: 10.1271/bbb.60453. [DOI] [PubMed] [Google Scholar]
- 132.Filipic B., Potokar J. Effect of royal jelly (RJ) on human interferon-alpha (HuIFN-α) inhibition of human colon cancer cells (CaCo-2) proliferation in vitro. Biomedicina Slovenica. 2014;20:1–7. [Google Scholar]
- 133.Zhang S., Shao Q., Geng H., Su S. The effect of royal jelly on the growth of breast cancer in mice. Oncology Letters14. 2017;(6):7615–7621. doi: 10.3892/ol.2017.7078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Borawska M.H., Markiewicz-˙ Zukowska R., Naliwajko S.K., Moskwa J., Bartosiuk E., Socha K., Mariak Z. The interaction of bee products with temozolomide in human diffuse astrocytoma, glioblastoma multiforme and astroglia cell lines. Nutr. Cancer. 2014;66(7):1247–1256. doi: 10.1080/01635581.2014.951735. [DOI] [PubMed] [Google Scholar]
- 135.Salazar-Olivo L.A., Paz-Gonz′alez V. Screening of biological activities present in honeybee (Apis mellifera) royal jelly. Toxicol. Vitro. 2005;(5):645–651. doi: 10.1016/j.tiv.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 136.Gismondi A., Trionfera E., Canuti L., Di Marco G., Canini A. Royal jelly lipophilic fraction induces antiproliferative effects on SH-SY5Y human neuroblastoma cells. Oncol. Rep. 2017;38(3):1833–1844. doi: 10.3892/or.2017.5851. [DOI] [PubMed] [Google Scholar]
- 137.Abu-Serie M.M., Habashy N.H. Two purified proteins from royal jelly with in vitro dual anti-hepatic damage potency: major royal jelly protein 2 and its novel isoform X1. Int. J. Biol. Macromol. 2019;128:782–795. doi: 10.1016/j.ijbiomac.2019.01.210. [DOI] [PubMed] [Google Scholar]
- 138.Kamiya T., Watanabe M., Hara H., Mitsugi Y., Yamaguchi E., Itoh A., Adachi T. Induction of human-lung-cancer-a549-cell apoptosis by 4-Hydroperoxy-2-decenoic acid ethyl ester through intracellular ROS accumulation and the induction of proapoptotic CHOP expression. J. Agric. Food Chem. 2018;66(41):10741–10747. doi: 10.1021/acs.jafc.8b04424. [DOI] [PubMed] [Google Scholar]
- 139.Shirzad M., Yousofi M., Zamanzad B., Sedaghat A., Hosseini M., Shahinfard N., Shirzad H. Effects of royal jelly on sterile skin cut repair. Journal of HerbMed Pharmacology. 2014;3(2):97–100. https://herbmedpharmacol.com/Article/JHP_20150527163601 [Google Scholar]
- 140.Çallı C., Tuğyan K., Öncel S., Pınar E., Demirtaşoğlu F., Tolon B., Yılmaz O., Kıray A. The effectiveness of royal jelly on tympanic membranes perforations (An Experimental Study) The Journal of Otolaryngology- Head & Neck Surgery37. 2008;(2):179–184. doi: 10.2310/7070.2008.0034. [DOI] [PubMed] [Google Scholar]
- 141.Temamoğulları F., Aral F., Demirkol R. Erkek farelerdearısütününuzun surely uygulanmasınınbazıspermatolojiközelliklerüzerineetkisi. F.Ü. Sağ. Bil. Derg. 2006;20(5):341–344. [Google Scholar]
- 142.Kohguchi M., Inoue S.I., Ushio S., Iwaki K., Ikeda M., Kurimoto M. Effect of royal jelly diet on the testicular function of hamsters. Food science and Technology Research10. 2007;(4):420–423. doi: 10.3136/fstr.10.420. [DOI] [Google Scholar]
- 143.El-Hanoun A.M., Elkomy A.E., Fares W.A., Shahien E.H. Impact of royal jelly to improve reproductive performance of male rabbits under hot summer conditions. World Rabbit Sci. 2014;22(3):241–248. doi: 10.4995/wrs.2014.1677. [DOI] [Google Scholar]
- 144.Ibrahim A., Eldaim M.A.A., Abdel-Daim M.M. Nephroprotective effect of bee honey and royal jelly against subchronic cisplatin toxicity in rats. Cytotechnology. 2016;68(4):1039–1048. doi: 10.1007/s10616-015-9860-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ghanbari E., Nejati V., Khazaei M. Improvement in serum biochemical alterations and oxidative stress of liver and pancreas following use of royal jelly in streptozotocin-induced diabetic rats. Cell Journal18. 2016;(3):362–370. doi: 10.22074/cellj.2016.4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Çavusoglu K., Yapar K., Yalçin E. Royal jelly (honey bee) is a potential antioxidant against cadmium-induced genotoxicity and oxidative stress in albino mice. J. Med. Food. 2009;(6):1286–1292. doi: 10.1089/jmf.2008.0203. [DOI] [PubMed] [Google Scholar]
- 147.Al-Eisa R.A., Al-Nahari H.A. The attenuating effect of royal jelly on hormonal parameters in aluminum chloride (AlCl3) intoxicated rats. Int. J. Pharmaceut. Res. Allied Sci. 2017;6(2):70–85. [Google Scholar]
- 148.Hassan A.A. Effect of royal jelly on sexual efficiency in adult male rats. Iraqi J. Vet. Sci. 2009;23:155–160. https://www.cabidigitallibrary.org/doi/pdf/10.5555/20103085498 [Google Scholar]
- 149.Sosa-Pérez G., Pérez-Ruiz E., Pérez-Hernández P., Cortez-Romero C., Gallegos-Sánchez J. Intravenous administration of royal jelly in ovarian activity and ovulatory rate of Pelibuey sheep. Agroproductividad10. 2017;2:42–46. http://www.colpos.mx/wb_pdf/Agroproductividad/2017/AGROPRODUCTIVIDAD_II-2017.pdf [Google Scholar]
- 150.Babaei S., Rahimi S., Torshizi M.A.K., Tahmasebi G., Miran S.N.K. Effects of propolis, royal jelly, honey and bee pollen on growth performance and immune system of Japanese quails. Vet. Res. Forum. 2016;7(1):13–20. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4867032/ [PMC free article] [PubMed] [Google Scholar]
- 151.Seven I., Şimşek U.G., Gökçe Z., Seven P.T., Arslan A., Yilmaz O. The effects of royal jelly on performance and fatty acid profiles of different tissues in quail (Coturnix coturnix japonica) reared under high stocking density. Turk. J. Vet. Anim. Sci. 2014;38(3):271–277. doi: 10.3906/vet-1303-62. [DOI] [Google Scholar]
- 152.Premratanachai P., Chanchao C. Review of the anticancer activities of bee products. Asian Pac. J. Trop. Biomed. 2014;4(5):337–344. doi: 10.12980/APJTB.4.2014C1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Günaldi O., Güçlü G., Postalci L., Eseoğlu M., Yilmaz I., Ofluoğlu E., Erdoğan S. Can royal jelly prevent epidural fibrosis development after laminectomy? An experimental study. J. Neurol. Sci. 2014;31(2):257–265. 2014. [Google Scholar]
- 154.Yanagita M., Kojima Y., Mori K., Yamada S., Murakami S. Osteoinductive and anti-inflammatory effect of royal jelly on periodontal ligament cells. Biomed. Res. 2011;32(4):285–291. doi: 10.2220/biomedres.32.285. [DOI] [PubMed] [Google Scholar]
- 155.Kafadar I.H., Güney A., Türk C.Y., Öner M., Silici S. Royal jelly and bee pollen decrease bone loss due to osteoporosis in an oophorectomized rat model. Eklem Hastalik Cerrahisi. 2012;23(2):100–105. https://pubmed.ncbi.nlm.nih.gov/22765489/ [PubMed] [Google Scholar]
- 156.Kwon H.O., Lee M., Cho Y.H., Jun W., Lee J. Royal jelly supplementation ameliorated immune impairment via inhibition of oxidative stress in low micronutrient-induced immunodeficient mice. J. Food Nutr. Res. 2017;5(2):74–79. doi: 10.12691/JFNR-5-2-1. [DOI] [Google Scholar]
- 157.Zhang S., Shao Q., Shen Z., Su S. Immunomodulatory response of 4T1 murine breast cancer model to camellia royal jelly. Biomed. Res. 2017;28(3):1223–1230. [Google Scholar]
- 158.Shirzad M., Kordyazdi R., Shahinfard N., Nikokar M. Does Royal jelly affect tumor cells. Journal of HerbMed Pharmacology. 2013;2(2):45–48. [Google Scholar]
- 159.Kamakura M., Mitani N., Fukuda T., Fukushima M. Antifatigue effect of fresh royal jelly in mice. J. Nutr. Sci. Vitaminol. 2001;47(6):394–401. doi: 10.3177/jnsv.47.394. [DOI] [PubMed] [Google Scholar]
- 160.Taherianfard M., Ahmadi Jokani S., Khaksar Z. Royal jelly can modulate behavioural and histo-morphometrical disorders caused by Parkinson's disease in rats. Physiology and Pharmacology. 2017;21(2):120–128. http://ppj.phypha.ir/article-1-1242-en.html [Google Scholar]
- 161.Karaca T., Bayıroğlu F., Yoruk M., Kaya M.S., Uslu S., Comba B., Mis L. Effect of royal jelly on experimental colitis induced by acetic acid and alteration of mast cell distribution in the colon of rats. Eur. J. Histochem. 2010;54(4):193–196. doi: 10.4081/ejh.2010.e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Cihan Y.B., Deniz K. The effects of royal jelly against radiation-induced acute oral mucositis. International Journal of Hematology and Oncology. 2014;27(1):36–44. doi: 10.4999/uhod.11059. [DOI] [Google Scholar]
- 163.Teixeira R.R., De Souza A.V., Peixoto L.G., Machado H.L., Caixeta D.C., Vilela D.D., Espindola F.S. Royal jelly decreases corticosterone levels and improves the brain antioxidant system in restraint and cold stressed rats. Neurosci. Lett. 2017;655:179–185. doi: 10.1016/j.neulet.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 164.Salimi S., Naghavi N.S., Karbasizadeh V. Propolis, royal jelly and pollen from beehive have antibacterial effect on aquatic pathogenic bacterial isolates. International Journal of Molecular and Clinical Microbiology. 2013;1:218–224. [Google Scholar]
- 165.Park H.M., Hwang E., Lee K.G., Han S.M., Cho Y., Kim S.Y. Royal jelly protects against ultraviolet B-induced photoaging in human skin fibroblasts via enhancing collagen production. J. Med. Food. 2011;14(9):899–906. doi: 10.1089/jmf.2010.1363. [DOI] [PubMed] [Google Scholar]
- 166.Pavel C.I., Mărghitaş L.A., Bobiş O., Dezmirean D.S., Şapcaliu A., Radoi I., Mădaş M.N. Biological activities of royal jelly-review. Scientific Papers Animal Science and Biotechnologies. 2011;44(2):108–118. https://spasb.ro/index.php/public_html/article/view/1878/1790 [Google Scholar]
- 167.Ramadan M.F., Al-Ghamdi A. Bioactive compounds and health-promoting properties of royal jelly: a review. J. Funct.Foods. 2012;4:39–52. doi: 10.1016/j.jff.2011.12.007. [DOI] [Google Scholar]
- 168.Yang X., Li J., Wang R. Antibacterial mechanism of 10-HDA against Bacillus subtilis. Advances in Applied Biotechnology. 2015;332:317–324. doi: 10.1007/978-3-662-45657-6_34. [DOI] [Google Scholar]
- 169.Siavash M., Shokri S., Haghighi S., Shahtalebi M.A., Farajzadehgan Z. The efficacy of topical royal jelly on healing of diabetic foot ulcers: a double-blind placebo-controlled clinical trial. Int. Wound J. 2015;12:137–142. doi: 10.1111/iwj.12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ahmadnia H., Sharifi N., Alizadeh S., Roohani Z., Kamalati A., Marjan S.S. Wonderful effects of royal jelly on treatment of male-factor related infertility. Austin J. Reproductive Med. Infertil. 2015;2(6):1031. https://austinpublishinggroup.com/reproductive-medicine/fulltext/ajrm-v2-id1031.php [Google Scholar]
- 171.Meng G., Wang H., Pei Y., Li Y., Wu H., Song Y., Wang J. Effects of protease-treated royal jelly on muscle strength in elderly nursing home residents: a randomized, double-blind, placebo-controlled, dose-response study. Sci. Rep. 2017;7(1) doi: 10.1038/s41598-017-11415-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Susilowati H., Murakami K., Yumoto H., Amoh T., Hirao K., Miyake Y. Royal jelly inhibits Pseudomonas aeruginosa adherence and reduces excessive inflammatory responses in human epithelial cells. BioMed Res. Int. 2017 doi: 10.1155/2017/3191752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Dinkov D., Stratev D., Balkanska R., Sergelidis D. Anti-bacterial activity of royal jelly and rape honey against methicillin-resistant staphylococcusaureus strains. Journal of Food and Health Science. 2016;2(2):67–73. doi: 10.3153/JFHS16007. [DOI] [Google Scholar]
- 174.Imada T., Nakamura S., Kitamura N., Shibuya I., Tsubota K. Oral administration of royal jelly restores tear secretion capacity in rat blink-suppressed dry eye model by modulating lacrimal gland function. PLoS One. 2014;9(9) doi: 10.1371/journal.pone.0106338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Inoue S., Kawashima M., Hisamura R., Imada T., Izuta Y., Nakamura S., Tsubota K. Clinical evaluation of a royal jelly supplementation for the restoration of dry eye: a prospective randomized double-blind placebo-controlled study and an experimental mouse model. PLoS One. 2017;12(1) doi: 10.1371/journal.pone.0169069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Yüksel S., Akyol S. The consumption of propolis and royal jelly in preventing upper respiratory tract infections and as dietary supplementation in children. Journal of Intercultural Ethnopharmacology. 2016;5(3):308–311. doi: 10.5455/jice.20160331064836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Chiu H.F., Chen B.K., Lu Y.Y., Han Y.C., Shen Y.C., Venkatakrishnan K., Wang C.K. Hypocholesterolemic efficacy of royal jelly in healthy mild hypercholesterolemic adults. Pharmaceut. Biol. 2017;55(1):497–502. doi: 10.1080/13880209.2016.1253110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Guo H., Saiga A., Sato M., Miyazawa I., Shibata M., Takahata Y., Morimatsu F. Royal jelly supplementation improves lipoprotein metabolism in humans. J. Nutr. Sci. Vitaminol. 2007;53(4):345–348. doi: 10.3177/jnsv.53.345. [DOI] [PubMed] [Google Scholar]
- 179.Münstedt K., Bargello M., Hauenschild A. Royal jelly reduces the serum glucose levels in healthy subjects. J. Med. Food. 2009;12(5):1170–1172. doi: 10.1089/jmf.2008.0289. [DOI] [PubMed] [Google Scholar]
- 180.Meto A., Meto A. Pastes based on royal jelly, an alternative for the minimally invasive treatment of pulpitis (histopathological experimental data) AJBS. 2017:1–7. [Google Scholar]
- 181.Dhanesuan N., Srisuparbh D., Tiranathanagul S., Rungsiyanant S. The in vitro effect of royal jelly Apis mellifera on proliferation of human gingival periodontal ligament fibroblasts and human bone cells. Thai Pharm Health Sci. 2011;6(3):182–187. [Google Scholar]
- 182.Li C., Mannoor M.K., Toma N., Taniguchi T., Inafuku M., Yamaguchi K.K., Watanabe H. The efficacy of Royal Jelly in the restoration of alcoholic liver injury in mouse model. Biomed. Res. 2011;22(1):1–8. [Google Scholar]
- 183.Cho Y., Kim J., Shin J., Bae M., Shin M.K. Dietary royal jelly improves epidermal hydration with increased levels of ceramides in the epidermis of mid-aged healthy human subjects. J. Dermatol. Sci. 2016;84(1):e35. doi: 10.1016/j.jdermsci.2016.08.115. [DOI] [Google Scholar]
- 184.Siavash M., Shokri S., Haghighi S., Mohammadi M., Shahtalebi M.A., Farajzadehgan Z. The efficacy of topical Royal Jelly on diabetic foot ulcers healing: a case series. J. Res. Med. Sci. 2011;16(7):904–909. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3263103/ [PMC free article] [PubMed] [Google Scholar]
- 185.Aslan Z., Aksoy L. Anti-inflammatory effects of royal jelly on ethylene glycol induced renal inflammation in rats. Int. Braz J. Urol. 2015;41(5):1008–1013. doi: 10.1590/s1677-5538.ibju.2014.0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Joksimovič A., Stanković D., Joksimović I., Molnar S., Joksimović S. Royal jelly as a supplement for young football players. Sport Sci. 2009;2(1):62–67. [Google Scholar]
- 187.Arzi A., Houshmand G., Goudarzi M., Khadem Haghighian H., Rashidi Nooshabadi M.R. Comparison of the analgesic effects of royal jelly with morphine and aspirin in rats using the formalin. Journal of Babol University of Medical Sciences. 2015;17(2):50–56. http://jbums.org/article-1-5285-en.html [Google Scholar]
- 188.Sugiyama T., Takahashi K., Mori H. Royal jelly acid, 10-hydroxy-trans-2-decenoic acid, as a modulator of the innate immune responses, endocrine, metabolic & immune disorders-drug targets (formerly current drug targets-immune. Endocrine & Metabolic Disorders) 2012;12(4):368–376. doi: 10.2174/187153012803832530. [DOI] [PubMed] [Google Scholar]
- 189.Stratev D., Vashin I., Balkanska R., Dinkov D. Antibacterial activity of Royal jelly and rape honey against Aeromonas hydrophila (ATCC 7965) Journal of Food and Health Science. 2015;1(2):67–74. doi: 10.3153/JFHS15006. [DOI] [Google Scholar]
- 190.Morita H., Ikeda T., Kajita K., Fujioka K., Mori I., Okada H., Ishizuka T. Effect of royal jelly ingestion for six months on healthy volunteers. Nutr. J. 2012;11(1):77. doi: 10.1186/1475-2891-11-77. http://www.nutritionj.com/content/11/1/77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Mahmoud K., Anas T. The role of honey with royal jelly in protecting the graafian follicles from the toxicity of the Adriamycin Drug. Int. J. Pharm. Pharmaceut. Sci. 2015;7(4):376–385. https://journals.innovareacademics.in/index.php/ijpps/article/view/4364 [Google Scholar]
- 192.Lambrinoudaki I., Augoulea A., Rizos D., Politi M., Tsoltos N., Moros M., Panoulis K. Greek-origin royal jelly improves the lipid profile of postmenopausal women. Gynecol. Endocrinol. 2016;32(10):835–839. doi: 10.1080/09513590.2016.1188281. [DOI] [PubMed] [Google Scholar]
- 193.Seyyedi F., Rafiean-Kopaei M., Miraj S. Comparison of the effects of vaginal royal jelly and vaginal estrogen on quality of life, sexual and urinary function in postmenopausal women. J. Clin. Diagn. Res. 2016;10(5):QC01. doi: 10.7860/JCDR/2016/17844.7715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Taavoni S., Barkhordari F., Goushegir A., Haghani H. Effect of Royal Jelly on premenstrual syndrome among Iranian medical sciences students: a randomized, triple-blind, placebo-controlled study. Compl. Ther. Med. 2014;22(4):601–606. doi: 10.1016/j.ctim.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 195.Abdelhafiz A.T., Abdelmonaem J., Abdlerahman M., Omar M., Aly D. FV44 Egyptian bee honey and royal jelly as prophylaxis against premature tearing of the fetal membranes: an in vitro testing model (Note) Zeitschrift fur Wundheilung. 2011;16:91. [Google Scholar]
- 196.Bogdanov S. Royal jelly, bee brood: composition, health, medicine: a review. Bee Product Science. 2015:35. [Google Scholar]
- 197.Khoshpey B., Djazayeri S., Amiri F., Malek M., Hosseini A.F., Hosseini S., Shidfar F. Effect of royal jelly intake on serum glucose, apolipoprotein AI (ApoA-I), apolipoprotein B (ApoB) and ApoB/ApoA-I ratios in patients with type 2 diabetes: a randomized, double-blind clinical trial study. Can. J. Diabetes. 2016;40(4):324–328. doi: 10.1016/j.jcjd.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 198.Mobasseri M., Ghiyasvand S., Ostadrahimi A., Ghojazadeh M., Noshad H., Pourmoradian S. Effect of fresh royal jelly ingestion on glycemic response in patients with type 2 diabetes. Iran. Red Crescent Med. J. 2015;17(9) doi: 10.5812/ircmj.20074. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.a Pourmoradian S., Mahdavi R., Mobasseri M., Faramarzi E., Mobasseri M. Effects of royal jelly supplementation on body weight and dietary intake in type 2 diabetic females. Health Promot. Perspect. 2012;2(2):231–235. doi: 10.5681/hpp.2012.028. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Mofid B., Rezaeizadeh H., Termos A., Rakhsha A., Mafi A.R., Taheripanah T., Kashi A.S.Y. Effect of processed honey and royal jelly on cancer-related fatigue: a double-blind randomized clinical trial. Electron. Physician. 2016;8(6):2475. doi: 10.19082/2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Erdem O., Güngörmüş Z. The effect of royal jelly on oral mucositis in patients undergoing radiotherapy and chemotherapy. Holist. Nurs. Pract. 2014:242–246. doi: 10.1097/hnp.0000000000000033. [DOI] [PubMed] [Google Scholar]
- 201.a Rafat N., Monfared A.S., Shahidi M., Pourfallah T.A. The modulating effect of royal jelly consumption against radiation-induced apoptosis in human peripheral blood leukocytes. J. Med. Phys. 2016;41(1):52–57. doi: 10.4103/0971-6203.177281. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Osama H., Abdullah A., Gamal B., Emad D., Sayed D., Hussein E E., Bahaa T. Effect of honey and royal jelly against cisplatin-induced nephrotoxicity in patients with cancer. J. Am. Coll. Nutr. 2017:1–5. doi: 10.1080/07315724.2017.1292157. [DOI] [PubMed] [Google Scholar]
- 202.Bărnutiu L.I., Mărghitaş L.A., Dezmirean D., Bobiş O., Bonta V., Pavel C. Preliminary study on chemical composition of fresh royal jelly from transylvania. Bulletin UASVM Animal Science and Biotechnologies. 2012;69:1–2. doi: 10.15835/buasvmcn-asb:69:1-2:8385. [DOI] [Google Scholar]
- 203.Magdalena M. ApimondiaInternational Beekeeping Congress in Bukarest, Romania. 2010. Effect of royal jelly on breast infant with distrophy and maldevelopment; pp. 583–585. [Google Scholar]
- 204.Strant M., Grosu R. The Apiquality&Apimedica International Symposium. 2016. Apitherapy in daily practice; pp. 22–25. [Google Scholar]
- 205.M. Strant, A. Varadi, Royal Jelly, Studies, Clinical Cases, Api-Therapy Symposium and Workshop, Cluj-Napoca, Romania(2016) 18-19 February.
- 206.Strant M.M., Yücel B., Topal E., Puscasu A.M., Margaoan R., Varadi A. Use of Royal Jelly as Functional Food on Human and Animal Health. Hayvansal Üretim. 2019;60(2):131–144. Personal experience and practices for royal jelly, Cluj Napoca. [Google Scholar]
- 207.Strant M. Marmaris Apitherapy and Apicultural Products Symposium. 2017. A treasure in apiterapy ‘’Royal jelly’’- myths and realities. II; pp. 41–42. [Google Scholar]
- 208.Tatsuhiko T., Naoko K., Yuko H. Application of the material of honeybee origin. Application of the consmetic material of the honeybee origin (Japanese) Frag J. 2011;30:17–24. [Google Scholar]
- 209.Calzavara‐Pinton P., Zane C., Facchinetti E., Capezzera R., Pedretti A. Topical Boswellic acids for treatment of photoaged skin. Dermatol. Ther. 2010;23(1) doi: 10.1111/j.1529-8019.2009.01284.x. [DOI] [PubMed] [Google Scholar]
- 210.Hamidpour R., Hamidpour S., Hamidpour M., Shahları M. Frankincense (RǔXiāng; Boswellia Species): from the selection of traditional applications to the novel phytotherapy for the prevention and treatment of serious diseases. Journal of Traditional and Complementary Medicine. 2013;3(4):221–226. doi: 10.4103/2225-4110.119723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Desoto M.C., Hitlan R.T. Synthetic folic acid supplementation during pregnancy may increase the risk of developing autism. J. Pediatr. Biochem. 2012;2(4):251–261. doi: 10.3233/JPB-120066. [DOI] [Google Scholar]
- 212.Sarıtaş N., Yıldız K., Büyükipekci S., Coşkun B. Effect of different levels of royal jelly on biochemical parameters of swimmers. Afr. J. Biotechnol. 2011;10(52):10718–10723. doi: 10.5897/AJB11.1862. [DOI] [Google Scholar]
- 213.Min J., Lee Y., Han S.M., Choi Y. Dietary effect of royal jelly supplementation on epidermal levels of hydration, filaggrins, free amino acids and the related enzyme expression in UV irradiated hairless mice. Korean J. Nutr. 2013;46(2):109–118. doi: 10.4163/kjn.2013.46.2.109. [DOI] [Google Scholar]
- 214.Menkovska M. The newest experience with effervescent tablets containing royal jelly as functional food on packing, dosage and synergistic action in prevention, prophylaxis and healing. J. Food Process. Technol. 2013;4(10):1–8. doi: 10.4172/2157-7110.1000272. [DOI] [Google Scholar]
- 215.Raja R.R. Nutraceuticals and cosmeceuticals for human beings–an overview. American Journal of Food Science and Health. 2016;2(2):7–17. http://www.aiscience.org/journal/ajfsh [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.