Abstract
The expression of a previously uncharacterized human hfl-B5 cDNA confers susceptibility for herpes simplex virus (HSV) to porcine cells and fulfills criteria as an HSV entry receptor (A. Perez, Q.-X. Li, P. Perez-Romero, G. DeLassus, S. R. Lopez, S. Sutter, N. McLaren, and A. Oveta Fuller, J. Virol. 79:7419-7430, 2005). Heptad repeats found in the B5 C terminus are predicted to form an α-helix for coiled coil structure. We used mutagenesis and synthetic peptides with wild-type and mutant sequences to examine the function of the heptad repeat motif in HSV binding and entry into porcine cells that express B5 and for infection of naturally susceptible human HEp-2 cells. B5 with point mutations predicted to disrupt the putative C-terminal coiled coil failed to mediate HSV binding and entry into porcine cells. Synthetic peptides that contain the single amino acid changes lose the blocking activity of HSV entry. We concluded that the C terminus of B5 contains a functional region that is important for the B5 receptor to mediate events in HSV entry. Structural evidence that this functional region forms coiled coil structures is under investigation. Blocking of HSV interaction with the C-terminal region of the B5 receptor is a new potential target site to intervene in the virus infection of human cells.
Herpes simplex virus (HSV) is a prevalent human pathogen that establishes a lifelong infection in its human host. It replicates at the site of entry into the host, most typically to cause oral or genital lesions. Latency is established in neuronal cells from which it reactivates periodically to cause recurrent lesions. The immune system of a healthy person usually can limit lesions to a small localized area. However, HSV causes severe complications and morbidity for immunosuppressed, chronically ill, or bedridden individuals (20, 23). Accumulating evidence suggests a possible role for HSV or other infectious agents in the development of neurodegenerative disease (11, 12, 39).
A recently characterized human gene designated human fetal lung cDNA B5 (hfl-B5) (32a) is expressed in a wide range of tissues and encodes a 43-kDa gene product (B5) that mediates HSV entry. Its features differ from the known human receptor proteins for HSV that engage glycoprotein D (gD) (41). The viral ligand for B5 has not yet been determined. The hfl-B5 sequence contains heptad repeats strongly predicted to form coiled coil structure. Coiled coils are composed of leucine zipper motifs that form α-helices (16). Two or more α-helices supercoil around one another to associate in a parallel or antiparallel orientation. Mutagenesis of apolar residues that are positioned to form a hydrophobic core in the α-helix of the heptad repeat (25, 26) have been shown to alter α-helix conformation. Point mutations for influenza, human immunodeficiency virus (HIV) gp41 or other viral proteins alter α-helix formation and disrupt viral-induced membrane fusion (1, 4, 5, 10, 15, 34, 43). They have been identified as functional features in some cellular and viral fusion proteins (6, 40).
Although the mechanisms by which viruses fuse membranes at entry or spread are not yet clear, heptad repeats are a functional part of fusion machinery in a growing number of viral fusion proteins (3, 13, 28, 40). The first characterized of these are hemagglutinin (HA) of influenza virus (34) and gp41 of HIV (22). When these viruses bind to the cell, HA at low pH of an endosome or gp41 at neutral pH undergo detectable conformational changes that eventually involve the coiled coils. Computer-based programs designed to predict coiled coils show that the B5 sequence scores similarly to the fusion proteins of HIV and Ebola virus (4, 38). As found with HA and gp41, the heptad repeat of B5 may contain potential fusion domains to interact with other membrane proteins (18, 19, 37, 38). Such an arrangement also fits a structure model for cellular proteins that are involved in membrane fusion for protein trafficking i.e., SNARES (40). While coiled coils in SNARES and viral fusion proteins have a common overall organization, there is little sequence homology.
In several viral fusion proteins, synthetic peptides to the coiled coil have been shown to interfere with protein function and thus with viral entry and infection. These include HIV and retroviruses (45, 46), Sendai virus (35) paramyxovirus (24), and parainfluenza viruses (47). Some of these, or drugs that mimic their site of action, are currently in clinical trials (36, 45, 46).
We have shown that a 30-mer synthetic peptide with amino acids in the C terminus of B5 block the HSV infection of B5 expressing porcine cells and of human HEp-2 cells (32a). Based on the activity of the peptide and the high score of the predicted coiled coil located at the B5 C terminus, we analyzed this region for possible function in HSV infection. Mutagenesis and further use of synthetic peptides establish that the C terminus of the B5 receptor is an important functional site for HSV entry.
MATERIALS AND METHODS
Cell and viruses.
Cells previously described (32) or described elsewhere (32a) were human larynx epidermoid carcinoma (HEp-2) and swine kidney SK6-A7 (A7), a clonal porcine cell line isolated by limiting dilution of parental SK6 cells (32). HB1-9, M1B3, and B5 10-1 G1 are clonal A7 cell lines that constitutively express herpesvirus entry mediator (HVEM), nectin-1, or B5, respectively (32a). B5-Tet-ON cells are clonal A7 cell lines that express B5 protein when grown in media supplemented with 1 mg/ml of doxycycline (DOX) (32a). All cells were grown in Dulbecco's modified medium (Gibco-BRL) supplemented with 10% fetal bovine serum (FBS; Gemini Bio-products). Viruses were HSV-1(F) propagated in HEp-2 cells and complemented gH-null mutant virus HSV-1(SCgHZ) that contained the lacZ reporter gene. It was propagated in the Vero-gH supporter F6 cell line (17).
Computer predictions.
Coiled coil predictions were made as described previously (32a) using the COILS program as performed in Pallen et al. (30). It is based on a procedure proposed by Parry, where the amino acid composition of a heptad repeat is compared to a database of known parallel two-stranded coiled coils to derive a similarity score (31).
Mutagenesis.
Point mutations in B5 were generated using a Transformer site-directed mutagenesis kit (BD Biosciences Clontech). Briefly, mutations predicted to disrupt coiled coils at a or d apolar residues of the coiled coil were introduced with primers containing specific nucleotide changes: L350P (GCAGTGGCAACAACCGTATGACAC), L354P (GTATGACACACCTAATGCCTGG), L361P (GCCTGGAAACAAAATCCGAACAAAGTG), and V364P (CTGAACAAACCG AAAAACAGCC). A control mutation of L301P (CAGCAAGAACCTCAGATTGGAG) was predicted not to disrupt the C-terminal structure of B5. An additional selection primer, BglII-mut (GGATCGGGTTATCTCCC), contained a mutation in the recognition sequence for the unique restriction enzyme BglII found in pcDNA3.1 and pcDNA3.1/myc-His vectors.
One hundred ng of both selection and mutagenic primers simultaneously annealed to one strand of 50 ng of the denatured DNA template. After standard DNA elongation using T4 DNA polymerase and ligation, DNA was digested with BglII as a primary selection and transformed into mutS Escherichia coli (Clontech). Isolated plasmid DNA was subjected to a second selective BglII digestion. Transformation of the twice selectively digested DNA into the JM109 E. coli strain allowed the highly efficient and specific recovery of plasmids with the desired mutation. Restriction analysis and nucleotide sequencing confirmed the mutations.
Transfections for transient B5 expression.
Porcine A7 cells in 25-cm2 dishes were transiently transfected as previously described (32a). Duplicate cell monolayers were LipofectAMINE (Gibco-BRL) transfected with 5 μg of wild-type pcDNA3/B5 (untagged B5) or pcDNA3/myc-His B5 (C-terminal epitope-tagged B5) or these vectors encoding B5 with the point mutations L301P, L350P, L354P, L361P, and V364P. At 48 h posttransfection, cells were exposed to 10 PFU/cell of HSV-1(SCgHZ)lacZ+. At 8 h postinfection, we determined HSV entry by X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) staining or by measuring β-galactosidase protein by enzyme-linked immunosorbent assay (ELISA) at an optical density at 405 nm (OD405) of duplicate samples in a 96-well plate. Binding of purified HSV at 4°C was determined using anti-HSV monoclonal antibodies in fluorescence-activated cell sorting (FACS) as previously described (33) after either heparin (100 mg/ml) (Sigma Co.) or phosphate-buffered saline (PBS-A) washes.
CELISA to detect expression of myc-tagged B5.
Subconfluent porcine A7 cells in six-well dishes were LipofectAMINE (Gibco BRL) transfected, counted, and replated 24 h later into 96-well dishes. After 18 h, cells on ice were exposed for 30 min to blocking solution (PBS-A containing 0.5 mM MgCl2 and 1 mM CaCl2, supplemented with 3% bovine serum albumin) and anti-myc monoclonal antibody (9E10; Invitrogen) diluted 1:300. Samples were analyzed at OD405 nm after adding peroxidase-conjugated secondary antibody (Amersham) and substrate solution containing 3′,3′,5′,5′-tetramethylbenzidine (Sigma).
Inhibition of HSV binding or entry by synthetic peptides.
A 30-mer B5 peptide corresponding to amino acids (aa) 344 to 373 of the wild-type B5 protein (32a) was altered by changing leucine (L) to proline (P) at positions 354 and 361. Mutant peptides synthesized and purified at the University of Michigan (UM) protein core facility were acetylated at the N terminus, amidated at the C terminus, and dissolved in PBS-A.
Effects on HSV binding or entry for wild-type and mutant peptide were examined as previously described (32a). Binding of virus was detected by FACS as previously described (32, 33). Briefly, suspended cells were preincubated with 42 mM of wild-type or mutant B5 peptides (soluble in PBS) for 1 h at 37°C, washed twice with cold blocking solution, and infected at 10 PFU/cell for 1 h at 4°C. Alternatively, virus preincubated with peptide for 1 h at 4°C was added to cells that had not previously been exposed to peptide.
For entry, peptides at 42 mM were preincubated for 1 h at 37°C with cells or with virus. Confluent cell monolayers in six-well plates were infected at 10 PFU/cell with either HSV-1(F) or HSV-1(F) preincubated with peptide at 37°C for 90 min, followed by exposure to 40 mM sodium citrate buffer (pH 3.0) to inactivate extracellular virus (42). Monolayers were overlaid with Dulbecco's modified medium and incubated at 37°C for 8 h. Cell lysates in 96-well plates were analyzed at OD405 for β-galactosidase expression by ELISA. HSV entry using wild-type B5 was set at 100%.
RESULTS
Construction of B5 mutants and cell surface expression. That HSV can use the human B5 protein for entry is the first report where a cellular receptor for virus entry is predicted to contain a high-scoring coiled coil (32a). Computer-based analyses indicate that the heptad repeat located at the extracellular C terminus of B5 contains residues that could form a coiled coil structure (Fig. 1A).
FIG. 1.
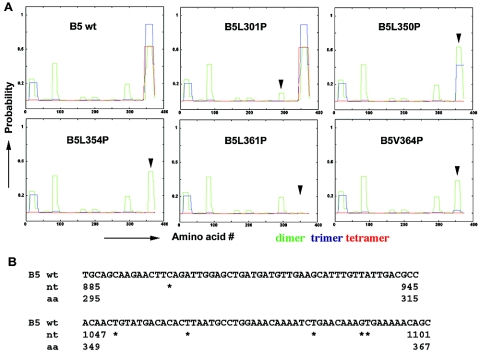
(A) Coiled coil prediction of B5 proteins. The B5 amino acid sequence was used with COILS software (30, 31). Mutants L301P, L350P, L354P, L361P, and V364P changed leucine or valine to proline in the indicated positions. (B) Nucleotide sequence of the mutated region. The asterisks indicate mutation positions in the wild-type B5 gene. Numbers below correspond to nucleotides (nt) or amino acid positions from the initial nucleotide triplet of the B5 open reading frame. wt, wild type.
We determined if the C terminus α-helix domain functions in HSV entry using a genetic strategy of placing point mutations at apolar residues in the heptads repeats (8, 31, 44). We first predicted by computer analyses the effects of single-point mutations on structure (Fig. 1A). Of five different mutants designed, four (L350P, L354P, L361P, and V364P) reduced or eliminated the predicted C-terminal coiled coil of B5 (Fig. 1). One (L301P) would not alter the C-terminal α-helix structure.
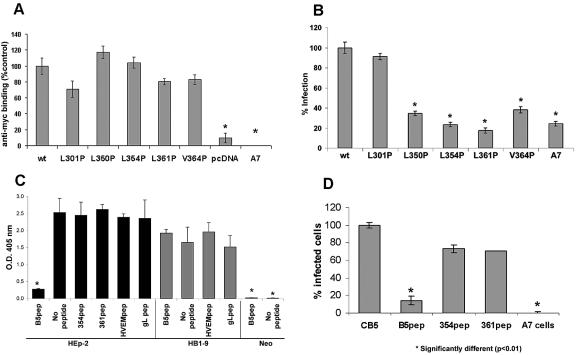
Mutations placed by directed mutagenesis to alter single nucleotides were confirmed by restriction analyses and nucleotide sequencing (Fig. 1B). We examined proper protein conformation for B5 cell surface expression using CELISA, FACS, and immunostaining of porcine A7 cells transiently transfected with myc-tagged wild-type or mutant B5 plasmids or with a control plasmid (Fig. 2A and 3). We previously determined that the C-terminal myc-tag does not affect B5 surface expression or function (32a). Binding of anti-myc antibody to unpermeabilized transfected cells indicated that each of the five B5 mutants was expressed on the cell surface (Fig. 2A and 3C). Although in transient transfections, the levels of the mutant proteins on the cell surface vary, the levels do not differ significantly from that of wild-type B5 (Fig. 2A). Thus, the point mutations did not drastically alter the overall conformation and cell surface expression of B5 receptor proteins.
FIG. 2.
(A) Cell surface expression of wild-type (wt) and mutant B5 proteins. A7 parental porcine cells transiently transfected with the designated constructs were fixed after 48 h and incubated with anti-myc antibody and peroxidase-conjugated secondary antibody. Binding obtained with wild-type B5 was set to 100%. Values shown are the means and standard deviations of quadruplicate determinations. (B) HSV infection of A7 cells transfected with the pcDNA3.1 plasmid containing the B5 designated inserts. At 48 h after transfection, infection with HSV(SCgHZ)lacZ+ was quantified by ELISA. Entry with wild-type B5 was set to 100%. (C and D) Peptide effects on HSV entry into human HEp-2 cells, B5, or HVEM-expressing porcine cells. (C) HEp-2 cells or HB1-9 cells that express HVEM. Neo is vector only porcine cell line. Peptides preincubated with virus (D) Transiently transfected B5 plasmid (CB5) into A7 parental porcine cells. Infection was with the indicated peptides added preincubated with cells. Asterisks indicate a different statistical significance with a P of <0.01.
FIG. 3.
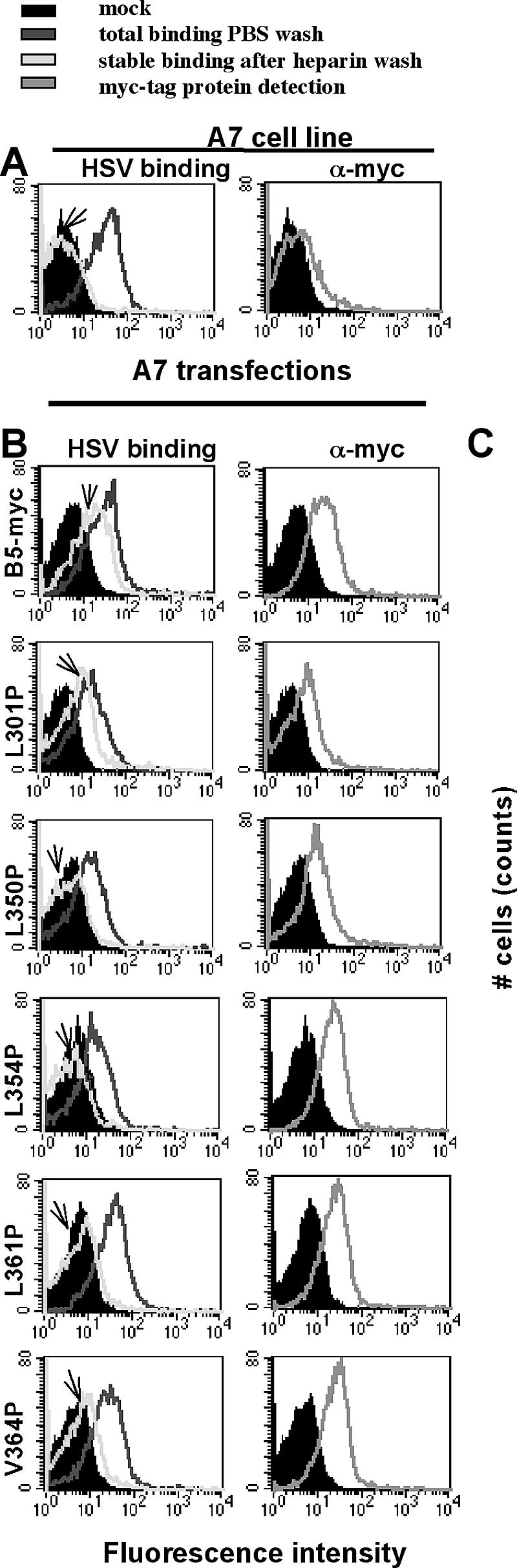
(A) Binding of HSV or anti-myc antibody to parental A7 cells. (B and C) Binding of HSV or anti-myc antibody to A7 cells transiently transfected with the indicated myc-tagged plasmid. At 48 h, cells were examined by FACS for total or stable HSV binding (arrow) (B) or binding of anti-myc antibody (C). Anti-HSV or anti-myc monoclonal antibody was detected by using anti-mouse fluorescein isothiocyanate conjugate. The filled peaks indicate mock-treated cells.
Effect of B5 mutants on HSV stable binding.
Epitope-tagged wild-type or mutant B5 proteins were tested for HSV binding as detected by FACS. In agreement with CELISA results (Fig. 2A), anti-myc monoclonal antibody detected the cell surface expression of all the B5 proteins (Fig. 3C). However, HSV stable binding occurred only with the expression of wild-type B5 or L301P (Fig. 3B). No HSV stable binding was detected with mutants L350P, L354P, L361P, or V364P, even though the myc-tagged proteins were present at the cell surface. These results showed that the point mutations predicted by computer analyses to disrupt the C-terminal α-helix and predicted coiled coil of B5 reduced HSV stable binding.
Effect of B5 mutants on HSV entry.
The effects of each of the mutations on HSV entry were tested using lacZ reporter virus infection of transiently transfected A7 cells. Infection detected as blue cell foci was similar for wild-type B5 or mutant L301P (Fig. 2B). However, the number of infected cell foci decreased dramatically for B5 mutant proteins L350P, L354P, L361P, and V364P. HSV infection of the porcine cells expressing any one of the four mutants was similar to background levels in entry-defective parental A7 porcine cells. Mutations that affected predicted α-helix structure resulted in a B5 molecule that could not function for HSV entry.
Effects of mutant B5 synthetic peptides on HSV binding.
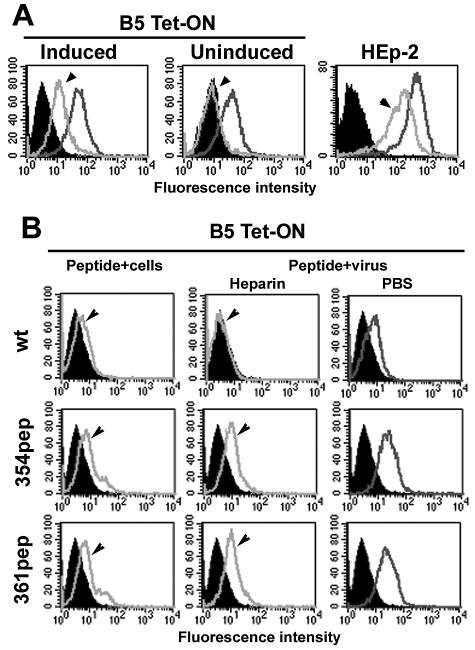
A 30-mer synthetic peptide that mimics the sequence located in the C terminus of B5 was shown to inhibit HSV entry and virus binding (32a). As another approach to examine the interaction of this region with HSV, we designed two new synthetic peptides with a change of L354P or L361P. These mutant peptides were compared with wild-type peptide for effects on HSV binding to B5 in a Tet-ON inducible stable porcine cell line. The B5 receptor was expressed and mediated HSV stable binding only when these cells were grown in a medium containing DOX (Fig. 4A) (32a).
FIG. 4.
(A) FACS to detect HSV binding to inducible B5-Tet-ON porcine cells or human HEp-2 cells. Inducible B5-expressing porcine cells were grown in the presence of 1 mg/ml DOX before performing binding assays. (B) Induced B5 cells were exposed to the indicated peptides under different conditions. Cells were pretreated with B5 peptides (wild type [wt] and mutants), and unbound peptide was washed out with PBS before infection with HSV-1(F) (peptide preincubated with cells, left panels). In parallel experiments, purified HSV-1(F) was preincubated with 42 mM peptide, and the mixture used to infect B5-expressing cells. One duplicate sample was washed with heparin to detect stable binding (virus preincubation, middle panels) or with PBS to determine total binding (virus preincubation, right panels). Bound HSV was detected by FACS using antibodies to HSV glycoproteins. In panel A: gray line with an arrowhead, stable bound virus; black line, total virus bound. In panel B: gray line with an arrowhead, stably bound virus in the presence of peptide; black line, total virus bound. The filled peaks indicate mock-treated cells.
When peptides were preincubated with induced B5 porcine cells before adding virus, FACS showed that the mutated synthetic peptides were slightly less effective at blocking binding than the 30-mer wild-type peptide (Fig. 4B). However, when mutant peptides were preincubated with virus before exposure to the cells, they failed to block HSV stable binding. Changes predicted to disrupt the α-helix in the B5 30-mer peptide resulted in loss of blocking of HSV stable binding to cells.
The wild-type and mutant peptides had the same effects on HSV stable binding to human HEp-2 cells seen for HSV binding to B5 porcine cells (Fig. 5). Wild-type 30-mer peptide effectively reduced binding when it was preincubated with viruses, while L354P and L361P mutant peptides did not block. These results indicated that the C-terminal region of B5 is functionally important for stable binding to B5 porcine or human HEp-2 cells. Moreover, the wild-type 30-mer peptide worked better if preincubated with virus. This suggested that the peptide interacts with some component present on the virion.
FIG. 5.
Effect of synthetic peptides on HSV binding to human HEp-2 cells. Peptides were preincubated with cells (Pep+cells, left panel), with virus before infection (Peptide+virus, middle panels), or with virus and peptides added to cells at the same time (Mixture, right panels). HSV stable binding after heparin washing was detected by FACS without peptide (gray line) or with peptide (black line with arrowheads). wt, wild type.
Effects of mutant B5 synthetic peptides on HSV entry.
Two procedures were used to examine the effects of the peptides on HSV infection. Peptides were preincubated with cells that are susceptible to HSV, such as human HEp-2 or porcine HB1-9 or M1B3. Alternatively, peptides were preincubated with HSV-1(SCgHZ) lacZ before infection of cells (Fig. 2C). When preincubated with HEp-2 cells, neither the wild-type 30-mer peptide nor the mutant peptides inhibited HSV entry (data not shown). However, preincubation of the wild-type B5 30-mer peptide with HSV before infection of HEp-2 significantly decreased entry and infection (Fig. 2C). This was also true for Vero cells (data not shown). The wild-type B5 peptide did not block HSV entry into A7 cells that contained only human HVEM (HB1-9) (Fig. 2C) or human nectin 1 (M1B3) (data not shown). Although there may be functional porcine homologs of other receptors, HVEM and nectin-1 were the only human proteins available in these cells to interact with HSV.
Mutant peptides L354P and L361P B5 failed to block HSV entry into HEp-2 or susceptible porcine cells (Fig. 2C). A control synthetic peptide to a hydrophilic region of HVEM had no effect on entry into any of the cell types examined.
Clonal porcine A7 cell lines that stably express B5 allowed HSV binding but resisted the membrane fusion required for entry (G. DeLassus and A. Oveta Fuller, unpublished). However, when the B5 plasmids were transiently transfected into poorly susceptible A7 cells, expression of B5 supported HSV infection (32a). We compared the effects on HSV entry of mutant and wild-type peptides when they were added to A7 cells that transiently express B5. Wild-type B5 peptide blocked HSV infection of porcine cells that transiently expressed only B5 as an HSV receptor (Fig. 2D). However, peptides with the change L354P or L361P failed to block HSV entry.
We concluded that the sequence of the 30-mer peptide interferes with a function of the C-terminal region in HSV entry into B5-expressing porcine cells and also into naturally susceptible human cells. Alterations of this region resulted in loss of blocking activity. As hypothesized, the C terminus contains a functional region that is important for the HSV use of B5 as a receptor for entry.
DISCUSSION
The protein product of the human hfl-B5 gene is a new class of HSV receptor. A computer-predicted coiled coil region is located at amino acids 344 through 374 of its C terminus (32a). In this report, we show that amino acids L350, L354, L361, and V364 contained in the putative coiled coil are critical for function in HSV binding and entry (Fig. 1 3). Single substitutions with proline at each of these residues resulted in a mutant B5 receptor protein that was expressed on the cell surface of porcine A7 cells (Fig. 2A and 3) but failed to mediate HSV binding and entry (Fig. 2, 4). Altered B5 30-mer peptides with changes at positions 354 and 361 failed to block the HSV infection of porcine or human cells compared to effective inhibition by a wild-type 30-mer peptide. Together, these results indicate that the C-terminal region of the B5 receptor contains a structural domain that is important for receptor function with HSV. Biochemical approaches are in progress to determine if the heptad repeat actually forms a coiled coil as predicted from both computer analyses and results of genetic alterations.
A function for coiled coils in membrane fusion during virus-cell interaction has been well documented. The most studied examples are HIV gp41 and influenza virus HA (1, 9, 10, 15, 21). The mechanism by which HSV mediates membrane fusion during viral entry is not yet known. There is not yet direct evidence of any one HSV receptor that is essential to this process. B5 is a new class of HSV receptor whose viral ligand remains to be determined (32a). We propose that this receptor may participate in HSV-mediated membrane fusion through the use of its C-terminal region. A role of B5 protein in membrane fusion is provided by the finding that overexpressing the B5 protein in HEp-2 cells results in increased cell fusion (32a).
We do not yet know which of the HSV glycoproteins function(s) as a ligand for the B5 receptor. However, that peptide to B5 inhibits most effectively when preincubated with virus suggests that it can engage a virion component (Fig. 4C). Computer analyses indicate that gH and gL that are required for HSV entry and membrane fusion are the HSV glycoproteins predicted to contain coiled coils.
A working model of engagement of multiple receptors by HSV is that after stable virus binding to any one of the gD receptors, nectin-1, nectin-2, HVEM, or 3-OST-HS, conformational changes cause the α-helices of gH/gL to engage the B5 α-helix. As with other viral protein or cellular SNARE systems, this could facilitate reorganization of lipid bilayers for virus-cell membrane fusion. In this scenario, gB or other viral glycoproteins that are involved in early events of HSV entry (2, 29, 41) may act to regulate membrane fusion mediated by coiled coils in B5 and gH/gL. A recent report presented evidence to suggest that binding of HSV gD to nectin-1 results in conformational changes in a membrane-proximal region of gD that is predicted to facilitate fusion mediated by other proteins (7). Another recent report showed that gD, gH, and HVEM, also a gD receptor, can physically interact to form a complex in infected cells (33). Both of these results support the working model for HSV pH-independent entry. Experiments to test aspects of this hypothesis are in progress.
Inhibition by the C-terminal 30-mer peptide of HSV stable binding is specific to the B5 structure. It does not block HSV entry into porcine cells that express only human HVEM or nectin-1 receptors (Fig. 2C). Peptides to HVEM (Fig. 2C), or other control peptides to the gL predicted coiled coil, do not affect HSV stable binding or entry (Fig. 2C). The B5 30-mer peptide inhibits HSV interaction with B5 porcine cells and with human HEp-2 cells that express B5 mRNA and presumably contain B5 protein. This is similar in some ways to what has been found with HIV entry. Mainly, peptides of gp41 (e.g., T-20) that mimic one of the heptad repeats present in gp41 are potent inhibitors of HIV infection (10, 14, 22, 24, 46, 47).
The 30-mer B5 peptide is most effective at blocking HSV binding or entry when it is preincubated with viruses (Fig. 2C and 4B). This indicates that human B5 can perform some role in HSV entry for infection and that it likely engages something in the virus to affect events of entry. No effect of the 30-mer peptide on infectivity of susceptible HVEM (Fig. 2C) or nectin-1 (data not shown) expressing A7 porcine cells suggests that, although HVEM, nectin-1, and B5 receptors all allow entry, the molecular mechanisms involved differ in some manner when any one of these human receptors is expressed alone. This agrees with previous findings that kinetics and types of interactions for HSV entry depend both on virion components and on the specific receptors available on a given cell type (27). The interactions proposed are also supported by results from mutant peptides (L354P and L361P). Peptides, predicted to no longer form a coiled coil failed to block HSV stable binding or HSV infection of B5 expressing porcine cells or human HEp-2 cells.
For some viruses that contain coiled coils (23, 35, 43-45), drugs that work at the site of action of inhibitory peptides are under development as antiviral therapeutic agents (36, 45, 46). Inhibition of HSV infection by the synthetic 30-mer B5 peptide and lack of blocking by the mutant peptides point to the C-terminal region as a potential antiviral target site. It may be an especially vulnerable site if B5 is involved in HSV entry as a membrane fusion receptor.
We show that the C terminus of B5, as a new cellular receptor for HSV, can perform an important function in HSV binding and entry. Such a mechanism fits with the broad expression of hfl-B5 and with the wide tropism and lifelong infection of herpesviruses. The C-terminal region, or the site through which it interacts with a viral or cellular ligand, may represent a target site to develop a new class of effective potent antiviral agents to disrupt HSV entry and infection.
Acknowledgments
We thank M. Raghavan, Q. X. Li, and laboratory members for critical reading of the manuscript and helpful discussions.
P.P.-R. was supported by fellowships from the University of Michigan Rackham Graduate School and the American Heart Association. This work was supported by grants from the University of Michigan Technology Development Office and AI34538 and AI42894 from NIH.
REFERENCES
- 1.Bentz, J. 2000. Membrane fusion mediated by coiled coils: a hypothesis. Biophys. J. 78:886-900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cai, W., B. Gu, and S. Person. 1988. Role of glycoprotein B of herpes simplex virus type 1 in entry and cell fusion. J. Virol. 62:2596-2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chambers, P., C. R. Pringle, and A. J. Easton. 1990. Heptad repeat sequences are located adjacent to hydrophobic regions in several types of virus fusion glycoproteins. J. Gen. Virol. 71:3075-3080. [DOI] [PubMed] [Google Scholar]
- 4.Chan, D. C., C. T. Chutkowski, and P. S. Kim. 1998. Evidence that a prominent cavity in the coiled coil of HIV type 1 gp41 is an attractive drug target. Proc. Natl. Acad. Sci. USA 95:15613-15617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan, D. C., D. Fass, J. M. Berger, and P. S. Kim. 1997. Core structure of gp41 from the HIV envelope glycoprotein. Cell 89:263-273. [DOI] [PubMed] [Google Scholar]
- 6.Chan, D. C., and P. S. Kim. 1998. HIV entry and its inhibition. Cell 93:681-684. [DOI] [PubMed] [Google Scholar]
- 7.Cocchi, F., D. Fusco, L. Menotti, T. Gianni, R. J. Eisenberg, G. H. Cohen, and G. Campadelli-Fiume. 2004. The soluble ectodomain of herpes simplex virus gD contains a membrane-proximal pro-fusion domain and suffices to mediate virus entry. Proc. Natl. Acad. Sci. USA 101:7445-7450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dai, J., H. Zhu, and G. Woldegiorgis. 2003. Leucine-764 near the extreme C-terminal end of carnitine palmitoyltransferase I is important for activity. Biochem. Biophys. Res. Commun. 301:758-763. [DOI] [PubMed] [Google Scholar]
- 9.Danieli, T., S. L. Pelletier, Y. I. Henis, and J. M. White. 1996. Membrane fusion mediated by influenza virus hemaglutin requires the concerted action of at least three hemaglutinin trimers. J. Biol. Chem. 133:559-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Rosny, E., R. Vassell, P. T. Wingfield, C. T. Wild, and C. D. Weiss. 2001. Peptides corresponding to the heptad repeat motifs in the transmembrane protein (gp41) of human immunodeficiency virus type 1 elicit antibodies to receptor-activated conformations of the envelope glycoprotein. J. Virol. 75:8859-8863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dickerson, F. B., J. J. Boronow, C. Stallings, A. E. Origoni, S. Coleman, B. Krivogorsky, and R. Yolken. 2004. Infection with herpes simplex virus type 1 is associated with cognitive deficits in bipolar disorders. Biol. Psychiatry 55:588-593. [DOI] [PubMed] [Google Scholar]
- 12.Dobson, C. B., M. A. Woznuak, and R. F. Itshaki. 2003. Do infectious agents play a role in dementia? Trends Microbiol. 11:312-317. [DOI] [PubMed] [Google Scholar]
- 13.Dubay, J. W., S. J. Roberts, B. Brody, and E. Hunter. 1992. Mutations in the leucine zipper of the human immunodeficiency virus type 1 transmembrane glycoprotein affect fusion and infectivity. J. Virol. 66:4748-4756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eckert, D. M., and P. S. Kim. 2001. Design of potent inhibitors of HIV-1 entry from the gp41 N-peptide region. Proc. Natl. Acad. Sci. USA 98:11187-11192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eckert, D. M., V. N. Malashkevich, L. H. Hong, P. A. Carr, and P. S. Kim. 1999. Inhibiting HIV-1 entry: discovery of D-peptide inhibitors that target the gp41 coiled-coil pocket. Cell 99:103-115. [DOI] [PubMed] [Google Scholar]
- 16.Engleman, D. M., Steitz, T. A., and Goldman, A. 1986. Identifing nonpolar transbilayer helices in amino acid sequences of membrane proteins. Annu. Rev. Biophys. Chem. 15:321-354. [DOI] [PubMed] [Google Scholar]
- 17.Forrester, A. J., H. Farrell, G. Wilkinson, J. Kaye, N. Davis-Poynter, and A. C. Minson. 1992. Construction and properties of a mutant herpes simplex virus type 1 deleted for glycoprotein H sequences. J. Virol. 66:341-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freed, E. O., D. J. Myers, and R. Risser. 1990. Characterization of the fusion domain of the human immunodeficiency virus type 1 envelope glycoprotein gp41. Proc. Natl. Acad. Sci. USA 87:4650-4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gallaher, W. R. 1987. Detection of a fusion peptide sequence in the transmembrane protein of human immunodeficiency virus. Cell 50:327-328. [DOI] [PubMed] [Google Scholar]
- 20.Griffiths, P. D. 2000. Herpesviruses as unrecognised components of the pathogenesis of chronic diseases. Rev. Med. Virol. 10:281-283. [DOI] [PubMed] [Google Scholar]
- 21.Joshi, S. B., R. E. Dutch, and R. A. Lamb. 1998. A core trimer of the paramyxovirus fusion protein: parallels to influenza virus hemagglutinin and HIV-1 gp41. Virology 248:20-34. [DOI] [PubMed] [Google Scholar]
- 22.Judice, J. K., J. Y. Tom, W. Huang, T. Wrin, J. Vennari, C. J. Petropoulos, and R. S. McDowell. 1997. Inhibition of HIV type 1 infectivity by constrained alpha-helical peptides: implications for the viral fusion mechanism. Proc. Natl. Acad. Sci. USA 25:13426-13430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koelle, D. M., and L. Corey. 2003. Recent progress in herpes simplex virus immunobiology and vaccine research. Clin. Microbiol. Rev. 16:96-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lambert, D. M., S. Barney, A. L. Lambert, K. Guthrie, R. Medinas, D. E. Davis, T. Bucy, J. Erickson, G. Merutka, and S. R. Petteway. 1996. Peptides from conserved regions of paramyxovirus fusion (F) proteins are potent inhibitors of viral fusion. Proc. Natl. Acad. Sci. USA 93:2186-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McClain, D. L., J. P. Binfet, and M. G. Oakley. 2001. Evaluation of the energetic contribution of interhelical Coulombic interactions for coiled coil helix orientation specificity. J. Mol. Biol. 313:371-383. [DOI] [PubMed] [Google Scholar]
- 26.McClain, D. L., D. G. Gurnon, and M. G. Oakley. 2002. Importance of potential interhelical salt-bridges involving interior residues for coiled-coil stability and quaternary structure. J. Mol. Biol. 324:257-270. [DOI] [PubMed] [Google Scholar]
- 27.McClain, D. S., and A. O. Fuller. 1994. Cell-specific kinetics and efficiency of herpes simplex virus type 1 entry are determined by distinct phases of attachment. Virology 198:690-702. [DOI] [PubMed] [Google Scholar]
- 28.McGinnes, L., T. Sergel, J. Reitter, and T. Morrison. 2001. Carbohydrate modifications of the NDV fusion protein heptad repeat domains influence maturation and fusion activity. Virology 283:332-342. [DOI] [PubMed] [Google Scholar]
- 29.Navarro, D., P. Paz, and L. Pereira. 1992. Domains of herpes simplex virus I glycoprotein B that function in virus penetration, cell-to-cell spread, and cell fusion. Virology 186:99-112. [DOI] [PubMed] [Google Scholar]
- 30.Pallen, M. J., G. Dougan, and G. Frankel. 1997. Coiled-coil domains in proteins secreted by type III secretion systems. Mol. Microbiol. 25:423-425. [DOI] [PubMed] [Google Scholar]
- 31.Parry, D. A. 1982. Coiled coils in a-helix-containing proteins: analysis of the residue types within the heptad repeat and the use of the data in the prediction of coiled coils in other proteins. Biosci. Rep. 2:1017-1024. [DOI] [PubMed] [Google Scholar]
- 32.Perez, A., and A. O. Fuller. 1998. Stable attachment for HSV penetration into human cells requires gD in the virion and cell receptors that are missing for entry defective porcine cells. Virus Res. 58:21-34. [DOI] [PubMed] [Google Scholar]
- 32a.Perez, A., Q.-X. Li, P. Perez-Romero, G. DeLassus, S. R. Lopez, S. Sutter, N. McLaren, and A. O. Fuller. 2005. A new class of receptor for herpes simplex virus has heptad repeat motifs that are common to membrane fusion proteins. J. Virol. 79:7419-7430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perez-Romero, P., A. Perez, A. Capul, R. Montgomery, and A. Oveta Fuller. 2005. Herpes simplex virus entry mediator associates in cells in a complex with viral proteins gD and at least gH. J. Virol. 79:4540-4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qiao, H., S. L. Pelletier, L. Hoffman, J. Hacker, R. T. Armstrong, and J. M. White. 1998. Specific single or double proline substitutions in the “spring-loaded” coiled-coil region of the influenza hemagglutinin impair or abolish membrane fusion activity. J. Cell Biol. 141:1335-1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rapaport, D., M. Ovadia, and Y. Shai. 1995. A synthetic peptide corresponding to a conserved heptad repeat domain is a potent inhibitor of Sendai virus-cell fusion: an emerging similarity with functional domains of other viruses. EMBO J. 22:5524-5531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rimsky, L. T., D. C. Shugars, and T. J. Matthews. 1998. Determinants of human immunodeficiency virus type 1 resistance to gp41-derived inhibitory peptides. J. Virol. 72:986-993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ruiz-Arguello, M. B., F. M. Goni, F. B. Pereira, and J. L. Nieva. 1998. Phosphatidylinositol-dependent membrane fusion induced by a putative fusogenic sequence of Ebola virus. J. Virol. 72:1775-17781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sanchez, A., Z. Y. Yang, L. Xu, G. J. Nabel, T. Crews, and C. J. Peters. 1998. Biochemical analysis of the secreted and virion glycoproteins of Ebola virus. J. Virol. 72:6442-6447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Satpute-Krishnan, P., J. A. DeGiorgis, and E. L. Bearer. 2003. Fast anterograde transport of herpes simplex virus: role for the amyloid precursor protein of Alzheimer's disease. Aging Cell 2:305-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Skehel, J. J., and D. C. Wiley. 1998. Coiled coils in both intracellular vesicle and viral membrane fusion. Cell 95:871-874. [DOI] [PubMed] [Google Scholar]
- 41.Spear, P. G. 2004. Herpes simplex virus: receptors and ligands for cell entry. Cell. Microbiol. 6:401-410. [DOI] [PubMed] [Google Scholar]
- 42.Subramanian, G., D. S. McClain, A. Perez, and A. O. Fuller. 1994. Swine testis cells contain functional heparan sulfate but are defective in entry of herpes simplex virus. J. Virol. 68:5667-5676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vazquez, M. I., G. Rivas, D. Cregut, L. Serrano, and M. Esteban. 1998. The vaccinia virus 14-kilodalton (A27L) fusion protein forms a triple coiled-coil structure and interacts with the 21-kilodalton (A17L) virus membrane protein through a C-terminal alpha-helix. J. Virol. 72:10126-10137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weng, Y., and C. D. Weiss. 1998. Mutational analysis of residues in the coiled-coil domain of human immunodeficiency virus type 1 transmembrane protein gp41. J. Virol. 72:9676-9682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wild, C., T. Greenwell, and T. Matthews. 1993. A synthetic peptide from HIV-1 gp41 is a potent inhibitor of virus-mediated cell-cell fusion. AIDS Res. Hum. Retrovir. 9:1051-1053. [DOI] [PubMed] [Google Scholar]
- 46.Wild, C. T., D. C. Shugars, T. K. Greenwell, C. B. McDanal, and T. J. Matthews. 1994. Peptides corresponding to a predictive alpha-helical domain of human immunodeficiency virus type 1 gp41 are potent inhibitors of virus infection. Proc. Natl. Acad. Sci. USA 91:9770-9774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yao, Q., and R. W. Compans. 1996. Peptides corresponding to the heptad repeat sequence of human parainfluenza virus fusion protein are potent inhibitors of virus infection. Virology 223:103-112. [DOI] [PubMed] [Google Scholar]