Abstract
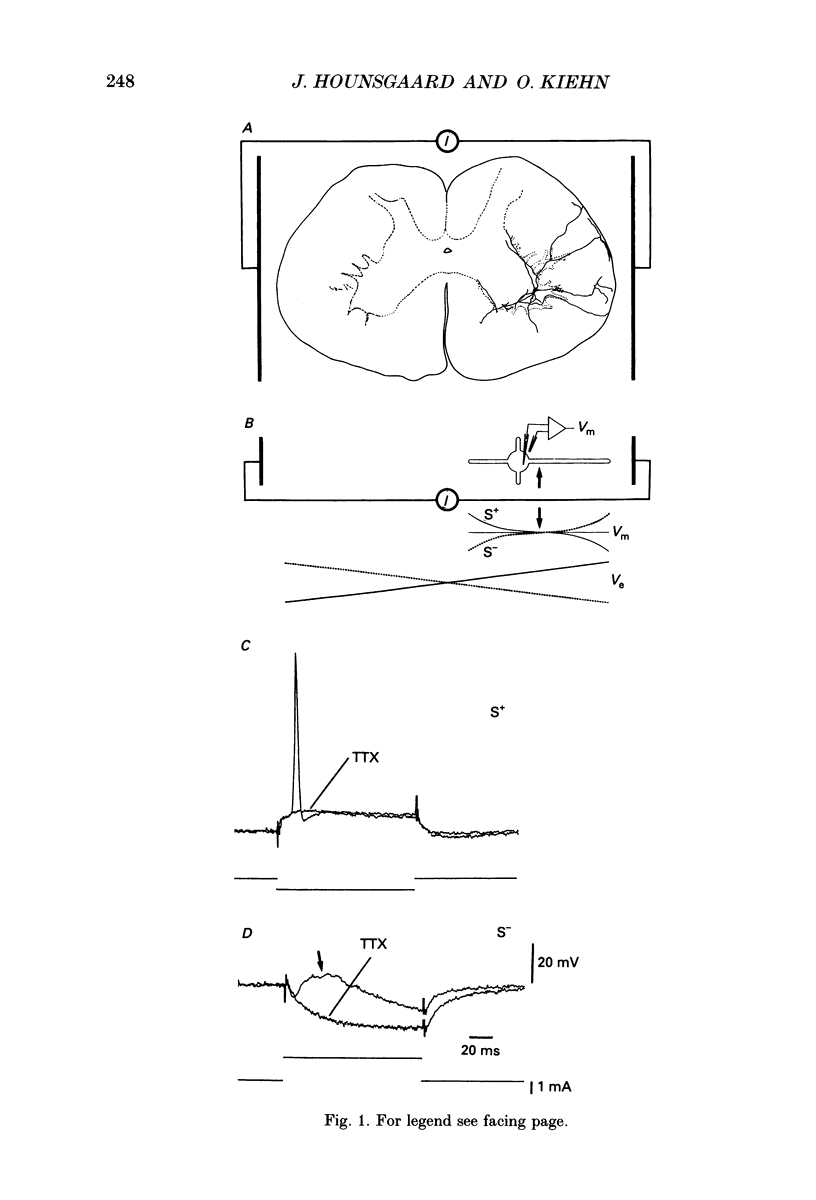
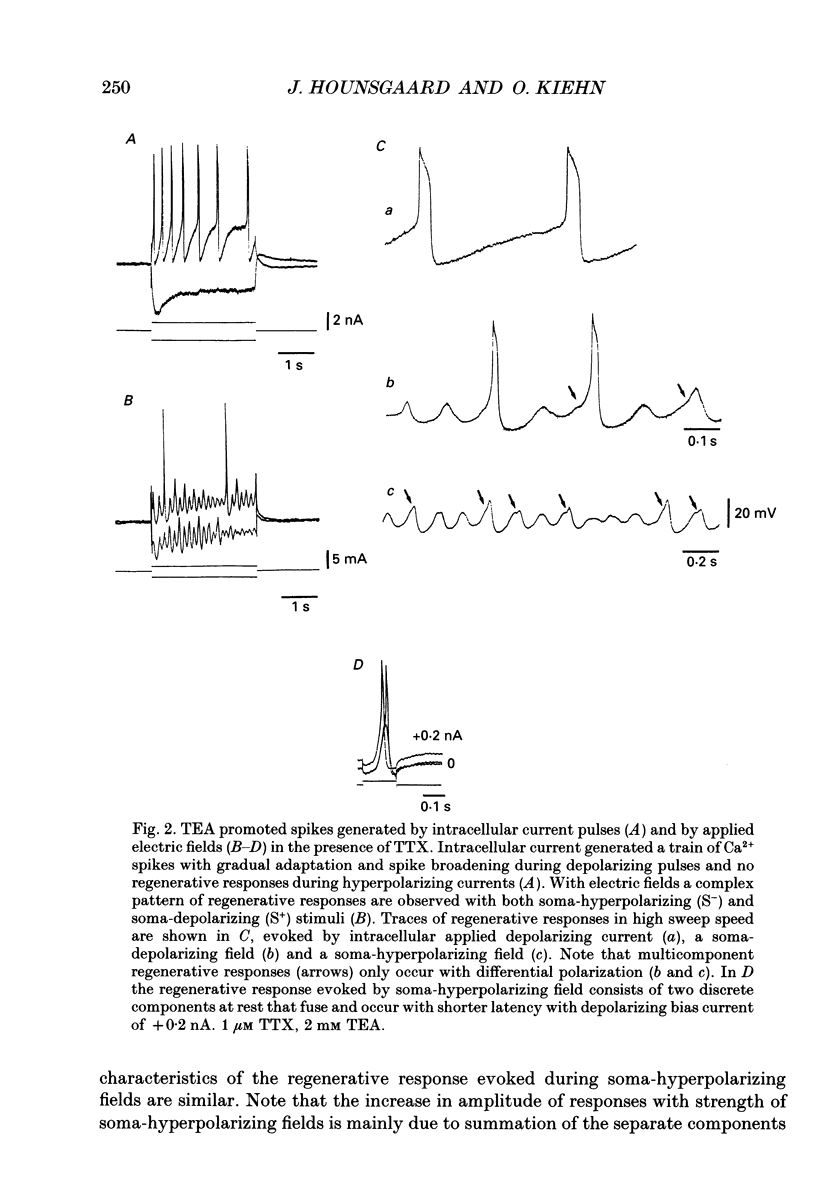
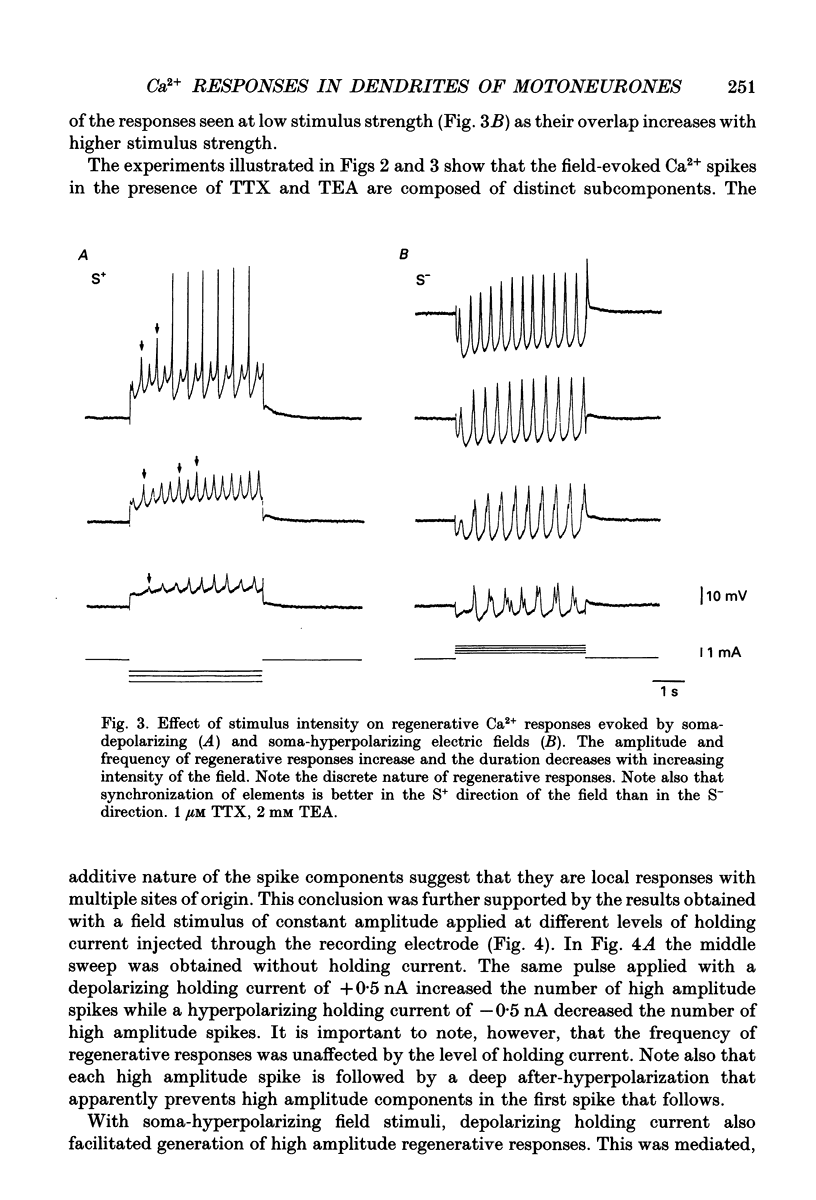
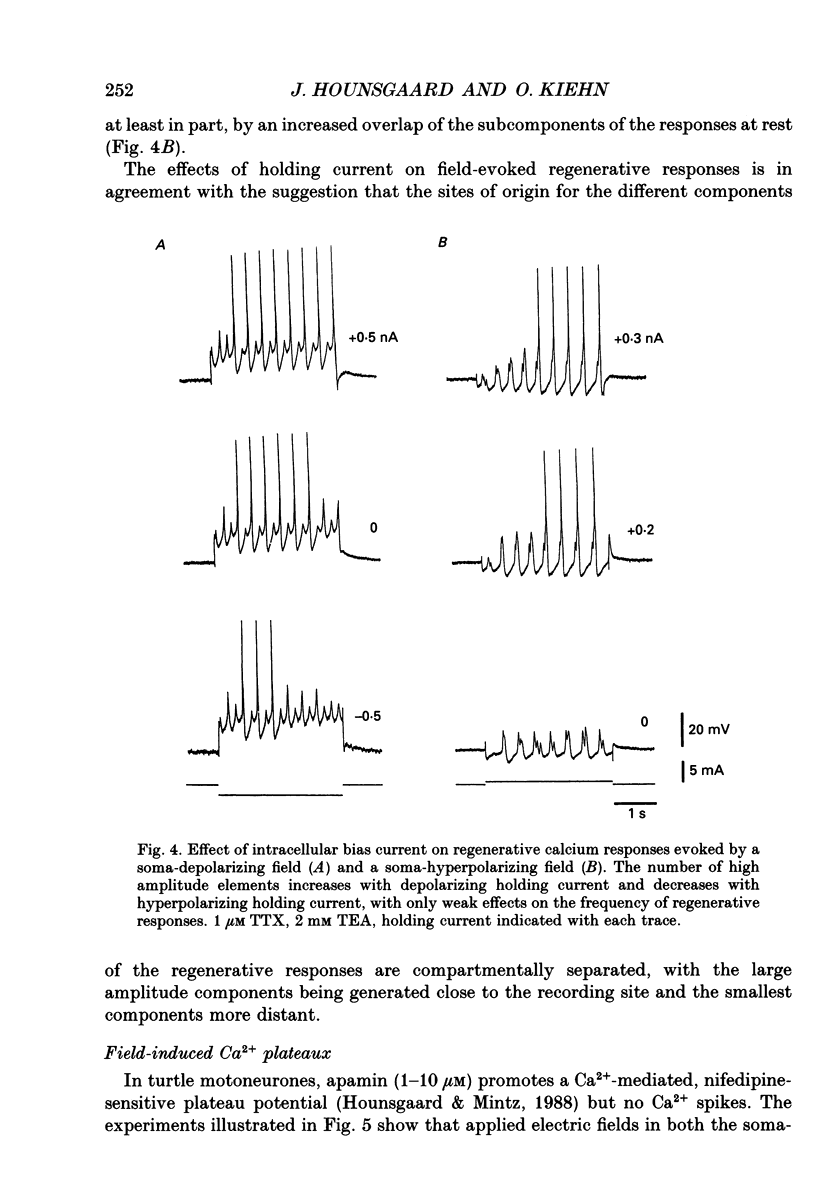
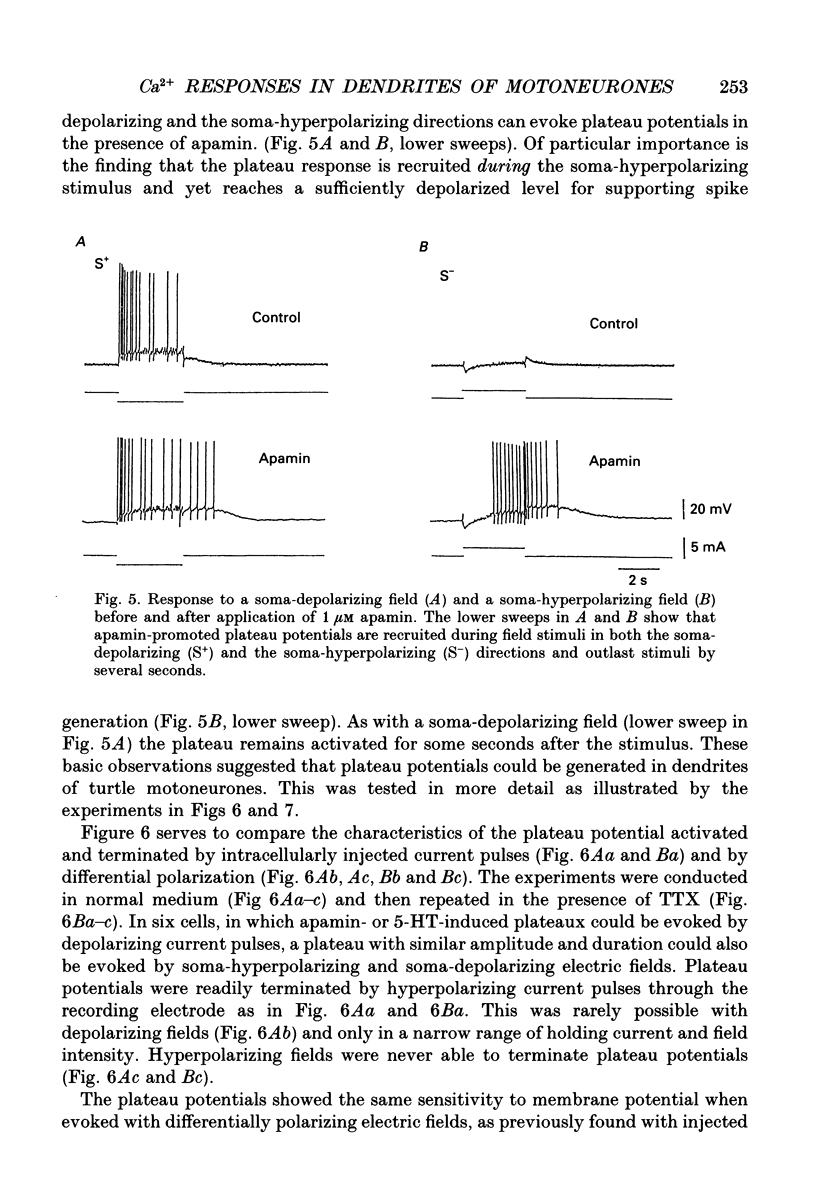
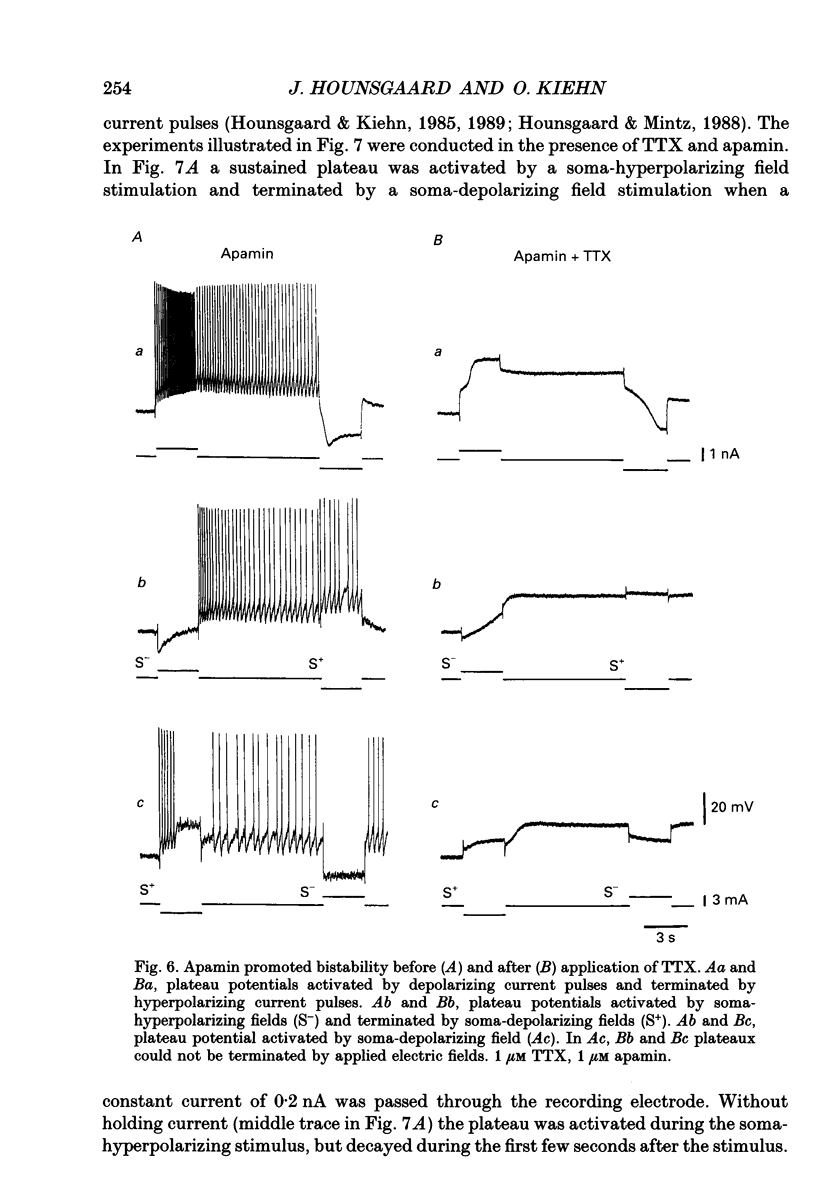
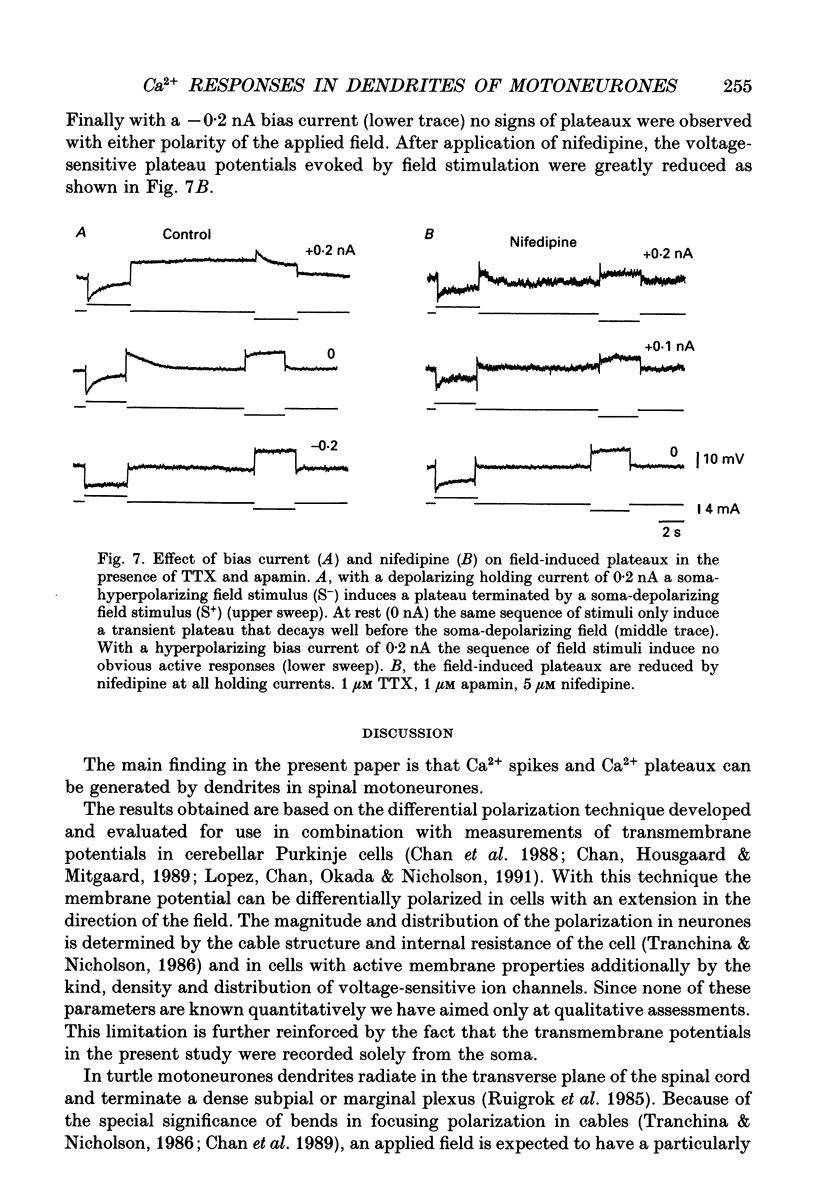
1. The ability of dendrites in turtle motoneurones to support calcium spikes and calcium plateaux was investigated using differential polarization by applied electric fields. 2. Electric fields were generated by passing current through transverse slices of the turtle spinal cord between two plate electrodes. The linear extracellular voltage gradient generated by the field implied that the tissue was ohmic and homogeneous. 3. The transmembrane potential at the cell body of motoneurones was measured as the voltage difference between an intracellular and an extracellular microelectrode. 4. In normal medium an applied field induced synaptic activity as well as intrinsic polarization of motoneurones. Synaptic activity was suppressed by tetrodotoxin (TTX, 1 microM). 5. In the presence of TTX and tetraethylammonium (TEA, 1-5 mM), applied fields evoked multicomponent Ca2+ spikes in both the soma-hyperpolarizing and soma-depolarizing direction of the field. The different components of Ca2+ spikes were discrete and additive. High amplitude components had higher threshold and faster time course and were followed by larger after-hyperpolarizations, than low amplitude components. The frequency of field-evoked regenerative responses was relatively insensitive to somatic bias current. 6. TTX-resistant Ca(2+)-mediated plateau potentials promoted by apamin were evoked by differential polarization in both the soma-depolarizing and soma-hyperpolarizing direction. 7. It is concluded that Ca2+ channels responsible for Ca2+ spikes and Ca2+ plateaux are present in dendrites of spinal motoneurones of the turtle.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett E. F., Barret J. N. Separation of two voltage-sensitive potassium currents, and demonstration of a tetrodotoxin-resistant calcium current in frog motoneurones. J Physiol. 1976 Mar;255(3):737–774. doi: 10.1113/jphysiol.1976.sp011306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett J. N., Crill W. E. Voltage clamp of cat motoneurone somata: properties of the fast inward current. J Physiol. 1980 Jul;304:231–249. doi: 10.1113/jphysiol.1980.sp013322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benardo L. S., Masukawa L. M., Prince D. A. Electrophysiology of isolated hippocampal pyramidal dendrites. J Neurosci. 1982 Nov;2(11):1614–1622. doi: 10.1523/JNEUROSCI.02-11-01614.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger A. J., Takahashi T. Serotonin enhances a low-voltage-activated calcium current in rat spinal motoneurons. J Neurosci. 1990 Jun;10(6):1922–1928. doi: 10.1523/JNEUROSCI.10-06-01922.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan C. Y., Hounsgaard J., Midtgaard J. Excitatory synaptic responses in turtle cerebellar Purkinje cells. J Physiol. 1989 Feb;409:143–156. doi: 10.1113/jphysiol.1989.sp017489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan C. Y., Hounsgaard J., Nicholson C. Effects of electric fields on transmembrane potential and excitability of turtle cerebellar Purkinje cells in vitro. J Physiol. 1988 Aug;402:751–771. doi: 10.1113/jphysiol.1988.sp017232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan C. Y., Nicholson C. Modulation by applied electric fields of Purkinje and stellate cell activity in the isolated turtle cerebellum. J Physiol. 1986 Feb;371:89–114. doi: 10.1113/jphysiol.1986.sp015963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita Y. Dendritic spikes in normal spinal motoneurons of cats. Neurosci Res. 1989 Apr;6(4):299–308. doi: 10.1016/0168-0102(89)90022-9. [DOI] [PubMed] [Google Scholar]
- Hounsgaard J., Hultborn H., Jespersen B., Kiehn O. Bistability of alpha-motoneurones in the decerebrate cat and in the acute spinal cat after intravenous 5-hydroxytryptophan. J Physiol. 1988 Nov;405:345–367. doi: 10.1113/jphysiol.1988.sp017336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hounsgaard J., Kiehn O. Ca++ dependent bistability induced by serotonin in spinal motoneurons. Exp Brain Res. 1985;57(2):422–425. doi: 10.1007/BF00236551. [DOI] [PubMed] [Google Scholar]
- Hounsgaard J., Kiehn O., Mintz I. Response properties of motoneurones in a slice preparation of the turtle spinal cord. J Physiol. 1988 Apr;398:575–589. doi: 10.1113/jphysiol.1988.sp017058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hounsgaard J., Kiehn O. Serotonin-induced bistability of turtle motoneurones caused by a nifedipine-sensitive calcium plateau potential. J Physiol. 1989 Jul;414:265–282. doi: 10.1113/jphysiol.1989.sp017687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hounsgaard J., Midtgaard J. Intrinsic determinants of firing pattern in Purkinje cells of the turtle cerebellum in vitro. J Physiol. 1988 Aug;402:731–749. doi: 10.1113/jphysiol.1988.sp017231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hounsgaard J., Midtgaard J. Synaptic control of excitability in turtle cerebellar Purkinje cells. J Physiol. 1989 Feb;409:157–170. doi: 10.1113/jphysiol.1989.sp017490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hounsgaard J., Mintz I. Calcium conductance and firing properties of spinal motoneurones in the turtle. J Physiol. 1988 Apr;398:591–603. doi: 10.1113/jphysiol.1988.sp017059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huguenard J. R., Hamill O. P., Prince D. A. Sodium channels in dendrites of rat cortical pyramidal neurons. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2473–2477. doi: 10.1073/pnas.86.7.2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe D. B., Johnston D., Lasser-Ross N., Lisman J. E., Miyakawa H., Ross W. N. The spread of Na+ spikes determines the pattern of dendritic Ca2+ entry into hippocampal neurons. Nature. 1992 May 21;357(6375):244–246. doi: 10.1038/357244a0. [DOI] [PubMed] [Google Scholar]
- Kiehn O. Plateau potentials and active integration in the 'final common pathway' for motor behaviour. Trends Neurosci. 1991 Feb;14(2):68–73. doi: 10.1016/0166-2236(91)90023-n. [DOI] [PubMed] [Google Scholar]
- Kiehn O., Rostrup E., Møller M. Monoaminergic systems in the brainstem and spinal cord of the turtle Pseudemys scripta elegans as revealed by antibodies against serotonin and tyrosine hydroxylase. J Comp Neurol. 1992 Nov 22;325(4):527–547. doi: 10.1002/cne.903250406. [DOI] [PubMed] [Google Scholar]
- Llinas R., Nicholson C. Electrophysiological properties of dendrites and somata in alligator Purkinje cells. J Neurophysiol. 1971 Jul;34(4):532–551. doi: 10.1152/jn.1971.34.4.532. [DOI] [PubMed] [Google Scholar]
- Llinás R. R. The intrinsic electrophysiological properties of mammalian neurons: insights into central nervous system function. Science. 1988 Dec 23;242(4886):1654–1664. doi: 10.1126/science.3059497. [DOI] [PubMed] [Google Scholar]
- Llinás R., Hess R. Tetrodotoxin-resistant dendritic spikes in avian Purkinje cells. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2520–2523. doi: 10.1073/pnas.73.7.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Sugimori M. Electrophysiological properties of in vitro Purkinje cell dendrites in mammalian cerebellar slices. J Physiol. 1980 Aug;305:197–213. doi: 10.1113/jphysiol.1980.sp013358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez L., Chan C. Y., Okada Y. C., Nicholson C. Multimodal characterization of population responses evoked by applied electric field in vitro: extracellular potential, magnetic evoked field, transmembrane potential, and current-source density analysis. J Neurosci. 1991 Jul;11(7):1998–2010. doi: 10.1523/JNEUROSCI.11-07-01998.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick D. A., Pape H. C. Noradrenergic and serotonergic modulation of a hyperpolarization-activated cation current in thalamic relay neurones. J Physiol. 1990 Dec;431:319–342. doi: 10.1113/jphysiol.1990.sp018332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson C., Llinas R. Field potentials in the alligator cerebellum and theory of their relationship to Purkinje cell dendritic spikes. J Neurophysiol. 1971 Jul;34(4):509–531. doi: 10.1152/jn.1971.34.4.509. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A. The coupling of neurotransmitter receptors to ion channels in the brain. Science. 1988 Jul 29;241(4865):545–551. doi: 10.1126/science.2456612. [DOI] [PubMed] [Google Scholar]
- Ruigrok T. J., Crowe A., ten Donkelaar H. J. Dendrite distribution of identified motoneurons in the lumbar spinal cord of the turtle Pseudemys scripta elegans. J Comp Neurol. 1985 Aug 15;238(3):275–285. doi: 10.1002/cne.902380304. [DOI] [PubMed] [Google Scholar]
- Sugimori M., Llinás R. R. Real-time imaging of calcium influx in mammalian cerebellar Purkinje cells in vitro. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5084–5088. doi: 10.1073/pnas.87.13.5084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T., Berger A. J. Direct excitation of rat spinal motoneurones by serotonin. J Physiol. 1990 Apr;423:63–76. doi: 10.1113/jphysiol.1990.sp018011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranchina D., Nicholson C. A model for the polarization of neurons by extrinsically applied electric fields. Biophys J. 1986 Dec;50(6):1139–1156. doi: 10.1016/S0006-3495(86)83558-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton K., Fulton B. P. Ionic mechanisms underlying the firing properties of rat neonatal motoneurons studied in vitro. Neuroscience. 1986 Nov;19(3):669–683. doi: 10.1016/0306-4522(86)90291-5. [DOI] [PubMed] [Google Scholar]


