Abstract
Purpose
Erectile dysfunction (ED) is associated with several vascular disorders, but the associations between ED and vascular parameters are still unclear.
Materials and Methods
We analyzed and synthesized a comprehensive range of studies from PubMed, Web of Science, and Scopus regarding the associations between ED and the following measures: ankle-brachial index (ABI), pulse wave velocity (PWV), intima-media thickness (IMT), nitrate-mediated dilation (NMD), flow-mediated dilation (FMD), augmentation index (AI), endothelial progenitor cells (EPCs) and other vascular parameters. Subgroup analysis was conducted according to specific types of parameters. Study quality was assessed by using the Newcastle–Ottawa Scale. Sensitivity analysis was conducted to confirm the robustness of the pooled results.
Results
Fifty-seven studies with 7,312 individuals were included. Twenty-eight studies were considered to be high-quality. ED patients had a 0.11 mm higher IMT (95% confidence interval [CI]: 0.07, 0.15), a 2.86% lower FMD (95% CI: -3.56, -2.17), a 2.34% lower NMD (95% CI: -3.37, -1.31), a 2.83% higher AI (95% CI: 0.02, 5.63), a 1.11 m/s higher PWV (95% CI: 0.01, 2.21), and a 0.72% lower percentage of EPCs (95% CI: -1.19, -0.24) compared to those without ED. However, ABI was similar between ED patients and non-ED individuals. According to sensitivity analysis, the pooled results were robust.
Conclusions
Our study confirmed the associations between ED and several vascular parameters and highlighted the importance of prevention and management of vascular and endothelial dysfunction in ED patients.
Keywords: Carotid intima-media thickness, Endothelial cells, Erectile dysfunction, Pulse wave analysis, Vascular stiffness
INTRODUCTION
Erectile dysfunction (ED) is a common disease that primarily affects males aged 40 years or older [1]. Approximately 18.4% of men over 20 years old suffer from ED [2]. Moreover, the global incidence of ED will rise to 322 million cases by 2025 [3]. Clearly, ED is currently recognized as a significant health problem in a progressively aging population.
Although the mechanism of ED is complicated, it is usually deemed to have an intricate organic and psychogenic nature [1]. Vasculogenic ED is a common type of organic disorder [4]. Prior studies reported a higher incidence of ED in hypertension patients [5]. Inman et al [6] drew a landmark conclusion that there is an obvious elevation in the risk of subsequent cardiovascular events in young ED men compared with individuals without ED over a 10-year follow-up. ED is also recognized as a predictor of cardiovascular diseases (CVDs) in expert opinion [7]. ED and CVD share comparable risk factors, such as age, obesity, and smoking, which cause vascular and endothelial dysfunction [1].
However, the associations between ED and vascular parameters remain unclear due to inappropriate measurement methods and limited sample sizes in previous studies. Some noninvasive methods for the assessment of vascular function have been established. Pulse wave velocity (PWV) [8], a measure of arterial stiffness, is a marker of vascular function. Flow-mediated dilation (FMD), nitrate-mediated dilation (NMD) [9], endothelial progenitor cells (EPCs) and other measures [10,11,12] are considered markers of endothelial function. A meta-analysis by Osondu et al [13] in 2018 pointed out the potential associations between ED and subclinical CVD and several vascular parameters, such as carotid intima-media thickness (IMT) and FMD. However, due to the limited vascular parameters included in that study, the associations between ED and vascular function still need to be further investigated.
Moreover, ED can be considered a marker for vascular dysfunction, which is greatly helpful for the prevention and management of vascular-related diseases, particularly in young men [14]. This systematic review and meta-analysis aimed to examine and synthesize available evidence regarding the association between ED and vascular parameters.
MATERIALS AND METHODS
A comprehensive search of PubMed, Web of Science, and Scopus was conducted to identify studies that evaluated the association between ED and vascular parameters, which were published before 2022. Apart from ED, search terms regarding vascular parameters included ‘intima-media thickness’, ‘flow-mediated dilatation’, ‘nitrate-mediated dilation’, ‘augmentation index’, ‘ankle-brachial index’, ‘pulse wave velocity’, and ‘endothelial progenitor cells’ (Supplement Table 1). We excluded case reports, case series, non-English publications, and studies without available complete texts according to our predefined exclusion criteria. We also excluded single-arm studies. We collected the mean and standard deviation or median and quartiles, which were converted to the mean and standard deviation using an online calculator [15]. The search was carried out by one reviewer (HP), while eligibility assessments were conducted by the other two reviewers (HP and HZ). Only studies that met the predefined eligibility criteria and were deemed appropriate by both reviewers were included in the meta-analysis. The review was registered in PROSPERO (https://www.crd.york.ac.uk/PROSPERO/, registration number CRD42023387846).
1. ED and vascular parameter assessment
The assessment of erectile function is commonly performed using the Kolner (Cologne) Evaluation of Erectile Function (KEED) or International Index of Erectile Function questionnaire (IIEF), including IIEF-15 and IIEF-5 [16,17]. The KEED questionnaire consists of six questions on a five-point Likert scale [18]. These questionnaires are widely-used, multidimensional self-report instruments for the diagnostic evaluation of ED severity. In addition, one study defined ED as a peak systolic velocity (PSV) ≤25 cm/s 15 minutes following the injection of a vasodilator.
The assessment modalities for relevant vascular parameters are described in Supplement Table 2.
2. Study quality assessment
We utilized the Newcastle–Ottawa Scale (NOS) to formally evaluate the quality of all included studies [19]. The total NOS score is up to 9 stars. The higher the score, the higher the quality of the study. All studies were assessed by two reviewers (HP and HZ) independently.
3. Statistical analysis
Statistical analyses were performed by using Review Manager software (version 5.4; The Cochrane Collaboration). Pooling was performed using inverse variance weighting to generate the mean difference (MD), and the results of the random effects model were displayed using the corresponding forest plot [20]. Additionally, we reported the heterogeneity (I2) and between-study variance (τ2; square) in our analysis. We also attempted to identify the source of this heterogeneity by subgroup analysis and sensitivity analysis. Sensitivity analyses were performed using the R package of meta in R software (Version 4.2.3; R Foundation for Statistical Computing). Subgroup analysis was conducted according to specific types of parameters, age groups. Funnel plots were used to evaluate the possibility of publication bias.
RESULTS
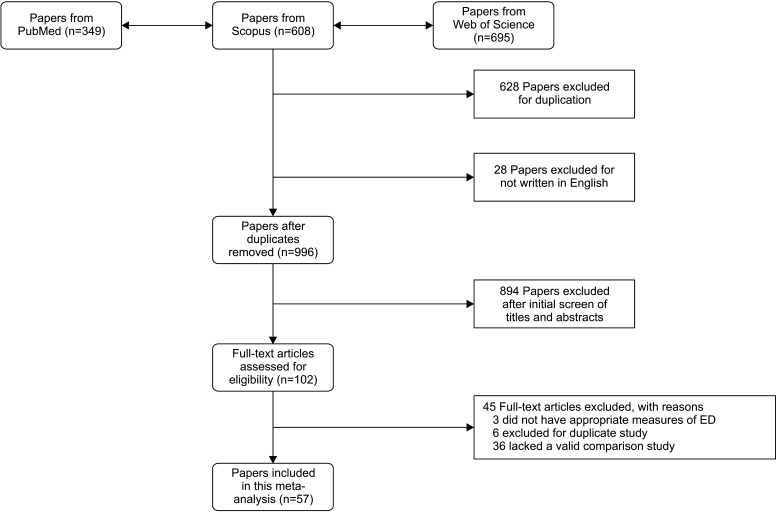
The flow diagram of the article search is shown in Fig. 1. Fifty-seven studies met the inclusion criteria. Overall, 24 studies examined IMT outcomes [21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44], 4 studies measured AI [27,41,45,46], 5 studies measured ABI [32,47,48,49,50], 6 studies measured PWV [31,41,48,51,52,53], 25 studies assessed FMD [24,29,30,31,37,41,42,43,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70], and 9 studies measured NMD [30,31,37,41,56,64,67,69,70]. Moreover, 11 studies measured several types of EPC [40,59,60,67,71,72,73,74,75,76,77]. The baseline characteristics of the included studies are provided in Supplement Table 3. A summary of the quality assessment is provided in Supplement Table 4. Twenty-eight studies were considered high-quality (7–9 stars), while twenty-nine studies were medium-quality (4–6 stars) [78].
Fig. 1. Systematic review search results. ED: erectile dysfunction.
1. ED and vascular structure
1) ED and IMT
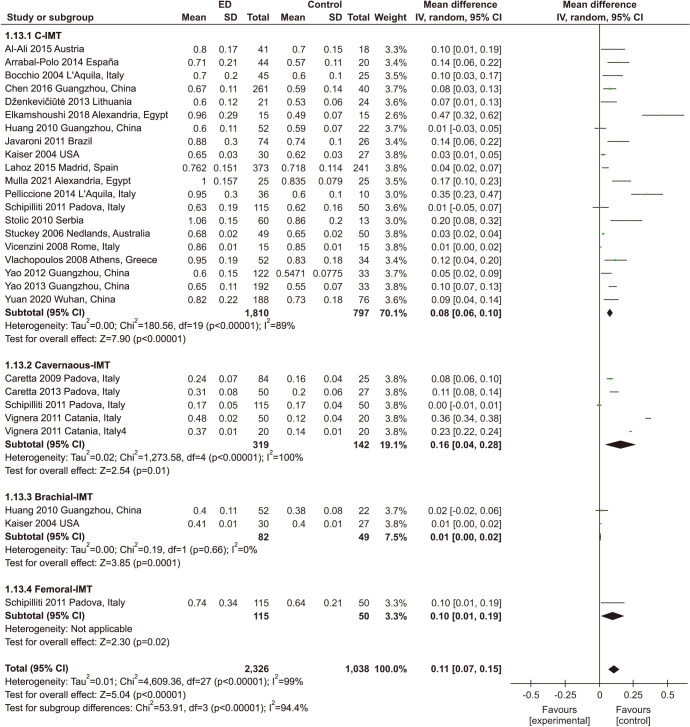
In all, 24 studies with 2,758 participants assessed the association between ED and IMT (Fig. 2), including 20 studies of carotid IMT (cIMT) [21,22,23,24,27,28,29,30,31,32,33,34,35,36,37,38,41,42,43,44], 5 studies of cavernous IMT [25,26,35,39,40], 2 studies of branchial IMT [29,31], and one study of femoral IMT [35]. We found that ED patients had significantly higher IMT than individuals without ED (MD: 0.11 mm; 95% CI: 0.07 mm, 0.15 mm). Significant heterogeneity among enrolled studies was found (I2=99%).
Fig. 2. Meta-analysis of studies on the relationship between erectile dysfunction (ED) and intima-media thickness (IMT). The results are shown as differences in percentage change between the ED and non-ED groups and their pooled mean difference (MD). SD: standard deviation, CI: confidence interval.
In the subgroup analysis, ED patients had significantly higher cIMT (MD: 0.08 mm; 95% CI: 0.06 mm, 0.10 mm), higher cavernous IMT (MD: 0.16 mm; 95% CI: 0.04 mm, 0.28 mm) and higher branchial IMT (MD: 0.01 mm; 95% CI: 0.00 mm, 0.02 mm) than individuals without ED.
2. ED and vascular stiffness
1) ED and PWV
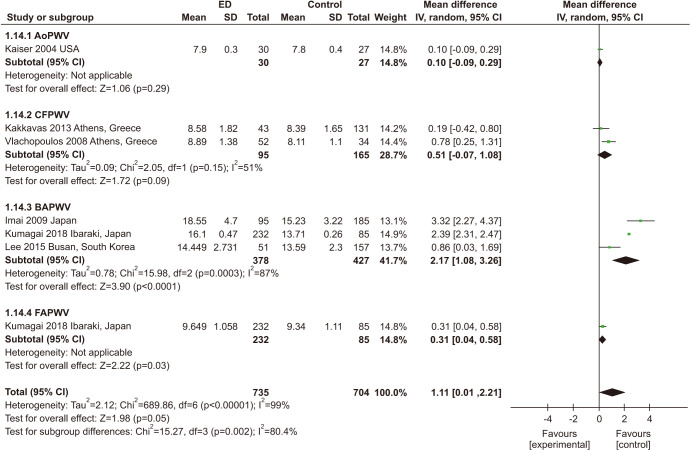
Seven studies involving 1,439 participants investigated the correlation between ED and PWV, including brachial-ankle (baPWV) [48,52,53], carotid-femoral (cfPWV) [41,51], aortic (aoPWV) [31], and femoral-ankle (faPWV) [53]. Patients with ED had higher PWV than individuals without ED (MD: 1.11; 95% CI: 0.01, 2.21). Significant heterogeneity among the enrolled studies was found (I2=99%) (Fig. 3).
Fig. 3. Meta-analysis of studies on the relationship between erectile dysfunction (ED) and pulse wave velocity (PWV). AoPWV: aortic PWV, CFPWV: carotid-femoral PWV, BAPWV: brachial-ankle PWV, FAPWV: femoral-ankle PWV, SD: standard deviation, CI: confidence interval.
2) ED and ABI
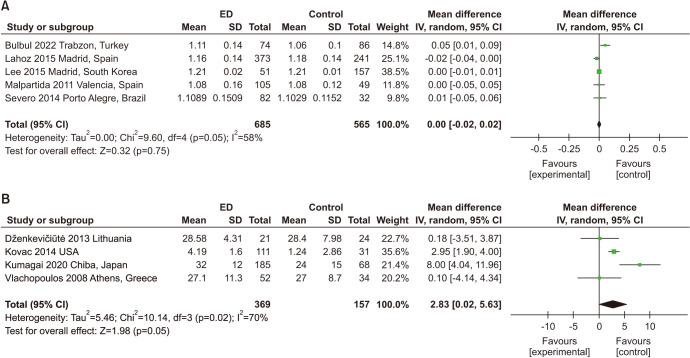
A total of 5 studies with 1,250 participants were included in the analysis of ABI [32,47,48,49,50]. Only one study assessed ED by using the IIEF-15, while others used the IIEF-5. No significant association was found between ED and ABI (MD: 0.00; 95% CI: -0.02, 0.02) (Fig. 4A). Significant heterogeneity among enrolled studies was found (I2=58%).
Fig. 4. (A) Meta-analysis of studies on the relationship between erectile dysfunction (ED) and ankle-brachial index. The results are shown as differences in percentage change between the ED and non-ED groups and their pooled mean difference. (B) Meta-analysis of studies on the relationship between ED and augmentation index. SD: standard deviation, CI: confidence interval.
3) ED and AI
Four studies with 526 participants examined the association between ED and AI (Fig. 4B) [27,41,45,46]. Three of them assessed ED by using the IIEF-5. ED patients had a higher AI than those without ED (MD: 2. 83; 95% CI: 0.02, 5.63). Significant heterogeneity among enrolled studies was found (I2=70%).
3. ED and endothelial function
1) ED and FMD
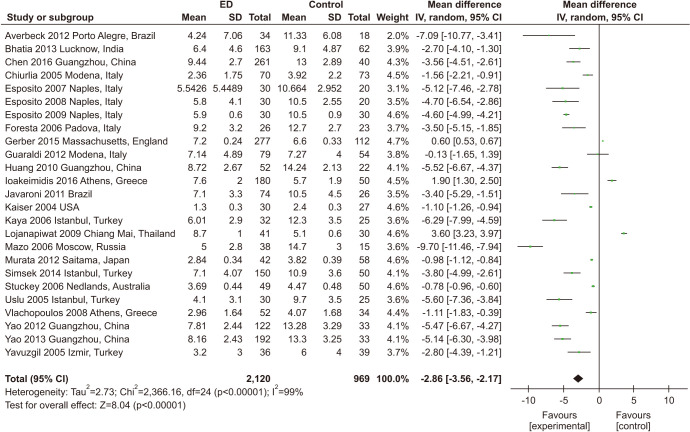
Twenty-five studies including 3,089 participants evaluated the association between ED and FMD [24,29,30,31,37,41,42,43,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70]. Only one study measured penis FMD and cuff inflation to not less than 10 mmHg above systolic blood pressure (BP) [68], while other studies measured brachial FMD. Moreover, the concrete operation of brachial FMD assessment was not universal among the studies. Out of the 25 studies, at least seven involved placing the cuff on the forearm, including one study that used the wrist [31,56,60,62,64,66,67], while the remaining studies involved inflating the cuff over the arm [24,29,42,43,54,55,57,58,59,65,69,70] or did not specify [30,37,41,61,63]. Moreover, the inflating pressures used in the studies were inconsistent, with reported pressures ranging from 250 mmHg to 300 mmHg [24,29,31,42,43,64,65,66,69,70], while others used cuff pressures that were 50 mmHg above the systolic BP [30,37,41,54,55,56,67]. The cuff pressure used in one study was at least 100 mmHg above the systolic BP [62]. One study had cuff pressures approximately 20 mm Hg above the systolic BP [60]. However, some studies have not specified the value above systolic pressure [57,58,59,63]. In the majority of studies, cuff occlusion lasted for 4–5 minutes, and the brachial artery diameter was measured at baseline and 30–90 seconds after cuff occlusion. Patients with ED had lower FMD than those without ED (MD: -2.86; 95% CI: -3.56, -2.17). Significant heterogeneity among enrolled studies was found (I2=99%). In subgroup meta-analyses, the MD was significantly varied among different age groups (Fig. 5, Supplement Fig. 1).
Fig. 5. Meta-analysis of studies on the association between erectile dysfunction (ED) and flow-mediated dilatation. SD: standard deviation, CI: confidence interval.
2) ED and NMD
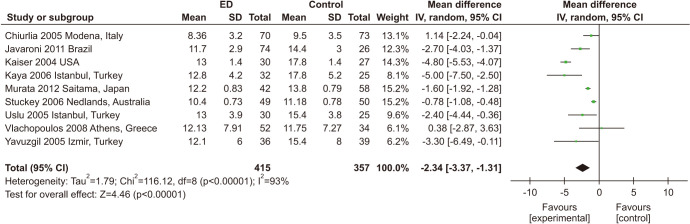
The correlation between ED and NMD was evaluated in 9 studies involving 772 participants. [30,31,37,41,56,64,67,69,70]. ED patients had less FMD than those without ED (MD: -2.34; 95% CI: -3.37, -1.31) (Fig. 6).
Fig. 6. Meta-analysis of studies on the association between erectile dysfunction (ED) and nitrate-mediated dilatation. SD: standard deviation, CI: confidence interval.
4. ED and serum biomarkers
1) ED and EPCs
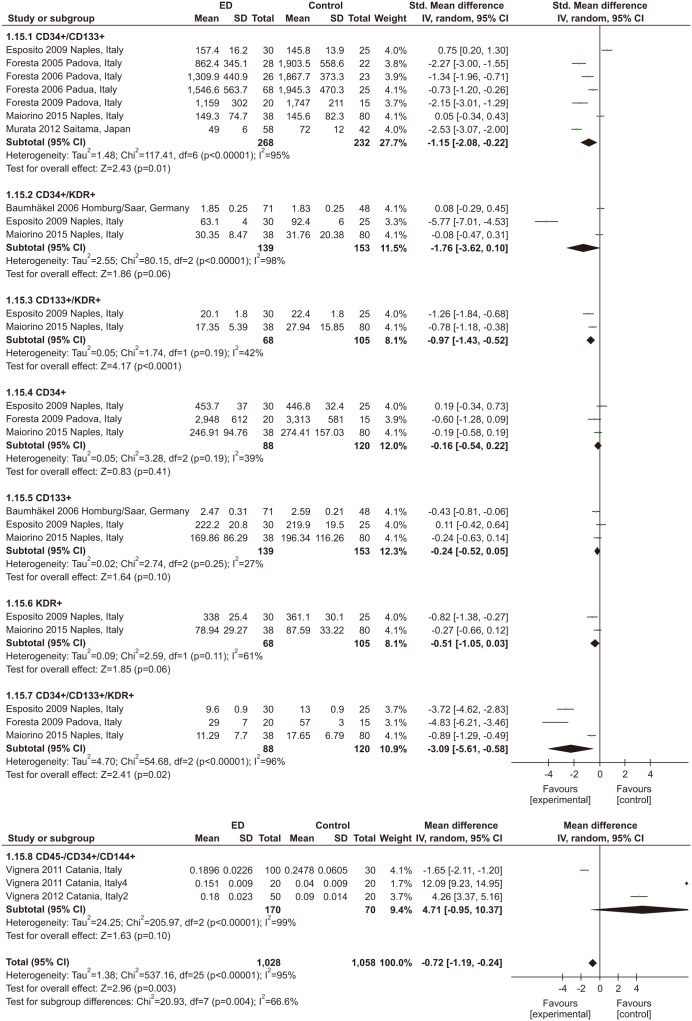
Eleven studies with 859 participants and 8 types of EPCs were included in this meta-statistic [40,59,60,67,71,72,73,74,75,76,77]. Nine studies [40,59,60,67,71,72,74,76,77] assessed ED by the IIEF-5, while only one study [73] assessed ED by the IIEF-15. In addition, one study [75] used KEED. All studies detected EPCs by flow cytometry. However, the counting mode of EPCs was varied. In studies, EPCs were evaluated by the percentage of total events [40,76,77], while the counts of EPCs were determined in 106 events in studies [59,74]. Four studies [60,71,72,73] counted EPCs per ml of peripheral blood. One study [67] counted EPCs per 100 ml of peripheral blood. In addition, one study analyzed the levels of EPCs as variables after log-transformation (log base 10) to normalize distribution [75]. As shown in Fig. 7, there was a 0.72% decrease in EPCs in ED patients compared to non-ED persons (MD: -0. 72; 95% CI: -1.19, -0.24). In the subgroup analysis, CD34+/CD133+, CD133+/KDR+, and CD34+/CD133+/KDR+ were less abundant in the ED group, while the other types of EPCs were not significantly different.
Fig. 7. Meta-analysis of studies on the association between erectile dysfunction (ED) and endothelial progenitor cells. SD: standard deviation, CI: confidence interval.
5. Assessment of publication bias and sensitivity analysis
According to funnel plots, in meta-analysis regarding NMD, AI, ABI, and EPC, no obvious publication biases were found. However, the funnel plots regarding the meta-analysis on IMT, PWV and FMD were asymmetric, indicating a high risk of bias (Supplement Fig. 2). Except for NMD, other results of the sensitivity analysis confirmed the robustness of the pooled results (Supplement Fig. 3, 4, 5). In studies of NMD, the study conducted by Kaiser et al [31] appears to be the primary cause of the heterogeneity observed in this meta-analysis, as removing this study resulted in a reduction of over 16% in I2 (93% to 77%) (Supplement Fig. 6). After removing this study and reperforming the meta-analysis, individuals with ED still had less NMD than those without ED (MD: -1.72; 95% CI: -2.41, -1.04).
DISCUSSION
In this systematic review and meta-analysis, we demonstrated that ED was associated with several vascular parameters. CIMT is an intermediate phenotype for early atherosclerosis and is also a predictive marker for the development of atherosclerosis. A previous meta-analysis demonstrated that increased cIMT is a significant predictor of future CVD events [79]. The association between cIMT and CVD events, such as angina pectoris, myocardial infarction, and coronary intervention, as well as cerebrovascular events, has been investigated in various longitudinal studies [80,81,82,83,84,85,86,87,88,89,90]. These studies demonstrated that cIMT can be used as an important risk predictor of CVD. In addition to cIMT, carotid plaque and carotid stenosis can also be detected by carotid ultrasonography. Some researchers believe carotid plaques are better than cIMT for predicting future CVD events [91,92]. In our meta-analysis, ED patients were found to have higher cIMT than non-ED individuals.
PWV is considered the most commonly used measure of arterial stiffness, with baPWV and cfPWV being the most commonly used measures in clinical and research settings. Numerous studies have demonstrated the correlation between PWV and coronary atherosclerosis [93]. In addition, certain studies have demonstrated a positive association between PWV and the risk and severity of CAD [94,95,96,97,98]. In a 2.7-year follow-up study [99], coronary artery calcification progression was positively correlated with baseline baPWV. Except for CVD, recent studies found an association between cerebral small vessel disease and PWV in the overall population or cardiovascular or cerebrovascular disease patients [93]. Several studies have also demonstrated that PWV is associated with cognitive decline in elderly individuals [100,101]. Additionally, previous studies reported associations between various types of PWV and cIMT or carotid plaque [93]. The findings of these studies suggest that PWV may serve as a strong predictive marker of CVD.
In our meta-analysis, the associations between ABI and ED were not significant. Paradoxically, some studies have used ABI <0.9 as a cutoff and found that the proportion of ABI <0.9 in ED is much higher than that in individuals without ED [32,102,103,104]. ABI <0.9 is defined as a symbol of peripheral arterial disease. A previous meta-analysis concluded that smoking, diabetes, hypertension and hypercholesterolemia are major risk factors for peripheral arterial disease [105], which are also risk factors for ED [106]. This suggests that ED may not directly cause decreased ABI but acts as an intermediary for risk factors such as smoking, diabetes, hypertension and hypercholesterolemia to increase the proportion of ABI <0.9 in the population. Additionally, differences in studies and sample sizes may also lead to such intriguing diverse results. More research should be conducted to further confirm the association between ED and ABI.
The relationship between ED and CVD has been well established. Some subclinical CVDs (such as subclinical atherosclerosis) occur earlier than vascular ED, while CVD occurs after vascular ED. The fundamental reason is that they share common mechanisms of vascular and endothelial dysfunction [107,108]. A meta-analysis regarding FMD reported that a 1% FMD elevation is associated with a 13% lower risk of CVD events [109]. Some meta-analysis studies demonstrate that improved FMD is also an important predictor of CVD events after optimized therapy [110,111,112]. In addition, a meta-analysis reported the association between FMD and neurocognition, indicating its potential as an indicator in neuroimaging measures of cerebral blood flow [113]. However, the criteria for FMD assessment are still subjective, which may cause significant bias in clinical practice.
EPCs are popular noninvasive detection methods for endothelial function. EPCs play a part in the regulation of tissue homeostasis, which means that they can work as biomarkers of endothelial dysfunction. Several studies have emphasized the relationship between CVD and EPCs [114,115,116]. These studies confirmed the relationship between ED and endothelial dysfunction, which is helpful for preventing and detecting diseases related to endothelial dysfunction in ED patients.
ED and vascular and endothelial dysfunction are considered to share the same risk factors and common mechanisms. This also means that vascular and endothelial dysfunction are potential therapeutic targets for ED. PDE5 inhibitors treat ED by promoting vasodilation through increasing intracellular cyclic adenosine monophosphate levels in vascular smooth muscle cells [117]. In addition, researchers have recently tried to treat patients unresponsive to PDE5 inhibitors through methods such as stem cell transplantation, endothelial nitric oxide synthase or intracavernosal vascular endothelial growth factor gene therapy [118]. These studies have brought new hope for ED patients. However, longitudinal relationships should be assessed to further assess the relationship between ED and vascular and endothelial dysfunction.
1. Limitations
All of the included studies were cross-sectional or case–control studies, which indicated the lack of longitudinal studies to investigate the temporal association between ED and vascular and endothelial dysfunction. Additionally, most included articles did not make a clear differential diagnosis of organic ED or psychogenic ED, and some studies made diagnosis of ED according to PSV, not IIEF-5, which may introduce bias. The definition and measurement modality of some vascular parameters varied among different studies, leading to potential bias. Significant heterogeneity within studies in the meta-analysis should also be noted. Although we conducted subgroup analysis and sensitivity analysis, we were unable to fully identify the cause of this heterogeneity. In addition, the presence of funnel plot asymmetry in the studies analyzing IMT, FMD and PWV suggested a higher likelihood of publication bias.
CONCLUSIONS
Our study demonstrates associations between ED and vascular and endothelial function, suggesting the significance of ED management in individuals with vascular and endothelial dysfunction. In the future, research should prioritize investigating the longitudinal associations between ED and vascular or endothelial function, utilizing larger sample sizes to enhance the validity of the findings.
Acknowledgements
None.
Footnotes
Conflict of Interest: The authors have nothing to disclose.
Funding: None.
- Conceptualization: HL.
- Data curation: HL.
- Formal analysis: XL.
- Investigation: TW.
- Methodology: JL.
- Project administration: YZ.
- Resources: SX.
- Supervision: WS, YZ.
- Visualization: HP.
- Writing – original draft: HP, HZ.
- Writing – review & editing: WS, YZ.
Supplementary Materials
Supplementary materials can be found via https://doi.org/10.5534/wjmh.230192.
Subgroup meta-analysis of studies on the relationship between erectile dysfunction (ED) and flow-mediated dilatation (FMD) stratified by population age. SD: standard deviation, CI: confidence interval.
Funnel plots of the meta-analysis between erectile dysfunction (ED) and IMT (A), ABI (B), AI (C), PWV (D), FMD (E), NMD (F), and EPC (G). ABI: ankle-brachial index, AI: augmentation index, PWV: pulse wave velocity, FMD: flow-mediated dilation, NMD: nitrate-mediated dilation, EPC: endothelial progenitor cell.
Sensitivity analysis of studies on the relationship between erectile dysfunction and intima-media thickness. CI: confidence interval.
Sensitivity analysis of studies on the relationship between erectile dysfunction and flow-mediated dilatation. CI: confidence interval.
Sensitivity analysis of studies on the relationship between erectile dysfunction and endothelial progenitor cells. CI: confidence interval.
Sensitivity analysis of studies on the relationship between erectile dysfunction and nitrate-mediated dilation. CI: confidence interval.
The Boolean formula in searching PubMed, Web of Science, and Scopus
Description of outcome measures
The baseline characteristics of studies included in the meta-analysis
The quality score of the studies included in this meta-analysis
References
- 1.Shamloul R, Ghanem H. Erectile dysfunction. Lancet. 2013;381:153–165. doi: 10.1016/S0140-6736(12)60520-0. [DOI] [PubMed] [Google Scholar]
- 2.Selvin E, Burnett AL, Platz EA. Prevalence and risk factors for erectile dysfunction in the US. Am J Med. 2007;120:151–157. doi: 10.1016/j.amjmed.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 3.Ayta IA, McKinlay JB, Krane RJ. The likely worldwide increase in erectile dysfunction between 1995 and 2025 and some possible policy consequences. BJU Int. 1999;84:50–56. doi: 10.1046/j.1464-410x.1999.00142.x. [DOI] [PubMed] [Google Scholar]
- 4.Matsui H, Sopko NA, Hannan JL, Bivalacqua TJ. Pathophysiology of erectile dysfunction. Curr Drug Targets. 2015;16:411–419. doi: 10.2174/138945011605150504114041. [DOI] [PubMed] [Google Scholar]
- 5.Mittawae B, El-Nashaar AR, Fouda A, Magdy M, Shamloul R. Incidence of erectile dysfunction in 800 hypertensive patients: a multicenter Egyptian national study. Urology. 2006;67:575–578. doi: 10.1016/j.urology.2005.09.040. [DOI] [PubMed] [Google Scholar]
- 6.Inman BA, Sauver JL, Jacobson DJ, McGree ME, Nehra A, Lieber MM, et al. A population-based, longitudinal study of erectile dysfunction and future coronary artery disease. Mayo Clin Proc. 2009;84:108–113. doi: 10.4065/84.2.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nehra A, Jackson G, Miner M, Billups KL, Burnett AL, Buvat J, et al. The Princeton III Consensus recommendations for the management of erectile dysfunction and cardiovascular disease. Mayo Clin Proc. 2012;87:766–778. doi: 10.1016/j.mayocp.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shigemura K, Arakawa S, Kamidono S, Nakano Y, Fujisawa M. Effect of sildenafil on arterial stiffness, as assessed by pulse wave velocity, in patients with erectile dysfunction. Int J Urol. 2006;13:956–959. doi: 10.1111/j.1442-2042.2006.01447.x. [DOI] [PubMed] [Google Scholar]
- 9.Ras RT, Streppel MT, Draijer R, Zock PL. Flow-mediated dilation and cardiovascular risk prediction: a systematic review with meta-analysis. Int J Cardiol. 2013;168:344–351. doi: 10.1016/j.ijcard.2012.09.047. [DOI] [PubMed] [Google Scholar]
- 10.Nichols WW, Singh BM. Augmentation index as a measure of peripheral vascular disease state. Curr Opin Cardiol. 2002;17:543–551. doi: 10.1097/00001573-200209000-00016. [DOI] [PubMed] [Google Scholar]
- 11.van Ierssel SH, Jorens PG, Van Craenenbroeck EM, Conraads VM. The endothelium, a protagonist in the pathophysiology of critical illness: focus on cellular markers. Biomed Res Int. 2014;2014:985813. doi: 10.1155/2014/985813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cainzos-Achirica M, Di Carlo PA, Handy CE, Quispe R, Roura G, Pinto X, et al. Coronary artery calcium score: the “mammogram” of the heart? Curr Cardiol Rep. 2018;20:70. doi: 10.1007/s11886-018-1020-9. [DOI] [PubMed] [Google Scholar]
- 13.Osondu CU, Vo B, Oni ET, Blaha MJ, Veledar E, Feldman T, et al. The relationship of erectile dysfunction and subclinical cardiovascular disease: a systematic review and meta-analysis. Vasc Med. 2018;23:9–20. doi: 10.1177/1358863X17725809. [DOI] [PubMed] [Google Scholar]
- 14.Corona G, Forti G, Maggi M. Why can patients with erectile dysfunction be considered lucky? The association with testosterone deficiency and metabolic syndrome. Aging Male. 2008;11:193–199. doi: 10.1080/13685530802468497. [DOI] [PubMed] [Google Scholar]
- 15.Shi J, Luo D, Weng H, Zeng XT, Lin L, Chu H, et al. Optimally estimating the sample standard deviation from the five-number summary. Res Synth Methods. 2020;11:641–654. doi: 10.1002/jrsm.1429. [DOI] [PubMed] [Google Scholar]
- 16.Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49:822–830. doi: 10.1016/s0090-4295(97)00238-0. [DOI] [PubMed] [Google Scholar]
- 17.Rosen RC, Cappelleri JC, Smith MD, Lipsky J, Peña BM. Development and evaluation of an abridged, 5-item version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int J Impot Res. 1999;11:319–326. doi: 10.1038/sj.ijir.3900472. [DOI] [PubMed] [Google Scholar]
- 18.Braun M, Wassmer G, Klotz T, Reifenrath B, Mathers M, Engelmann U. Epidemiology of erectile dysfunction: results of the ‘Cologne Male Survey’. Int J Impot Res. 2000;12:305–311. doi: 10.1038/sj.ijir.3900622. [DOI] [PubMed] [Google Scholar]
- 19.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 20.DerSimonian RLN. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 21.Al-Ali BM, Holz M, Sadik P, Oppeck C, Schmidt K, Oppeck G. Correlation between erectile function and cardiovascular risk factors by assessing arterial stiffness and myocardial perfusion imaging and carotid artery intima-media thickness. Minerva Urol Nefrol. 2015;67:11–18. [PubMed] [Google Scholar]
- 22.Arrabal-Polo MA, Vera-Arroyo B, Lahoz-García C, Valderrama-Illana P, Cámara-Ortega M, Arrabal-Martín M, et al. Erectile dysfunction, metabolic syndrome and arterial disease. Clinical-pathological relation by carotid ultrasonography. Actas Urol Esp. 2014;38:179–183. doi: 10.1016/j.acuro.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 23.Bocchio M, Desideri G, Scarpelli P, Necozione S, Properzi G, Spartera C, et al. Endothelial cell activation in men with erectile dysfunction without cardiovascular risk factors and overt vascular damage. J Urol. 2004;171:1601–1604. doi: 10.1097/01.ju.0000116325.06572.85. [DOI] [PubMed] [Google Scholar]
- 24.Chen SF, Yao FJ, Sun XZ, Wu RP, Huang YP, Zheng FF, et al. Brachial artery flow-mediated dilatation and carotid intimamedia thickness in young ED patients with insulin resistance. Int J Impot Res. 2016;28:194–199. doi: 10.1038/ijir.2016.30. [DOI] [PubMed] [Google Scholar]
- 25.Caretta N, Palego P, Schipilliti M, Ferlin A, Di Mambro A, Foresta C. Cavernous artery intima-media thickness: a new parameter in the diagnosis of vascular erectile dysfunction. J Sex Med. 2009;6:1117–1126. doi: 10.1111/j.1743-6109.2008.01112.x. [DOI] [PubMed] [Google Scholar]
- 26.Caretta N, Feltrin G, Tarantini G, D’Agostino C, Tona F, Schipilliti M, et al. Erectile dysfunction, penile atherosclerosis, and coronary artery vasculopathy in heart transplant recipients. J Sex Med. 2013;10:2295–2302. doi: 10.1111/jsm.12233. [DOI] [PubMed] [Google Scholar]
- 27.Dženkevičiūtė V, Petrulionienė Ž, Šapoka V, Kasiulevičius V. Association between erectile dysfunction and asymptomatic cardiovascular damage in middle-aged men. Medicina (Kaunas) 2013;49:510–516. [PubMed] [Google Scholar]
- 28.Elkamshoushi AAM, Hassan EM, El Abd AM, Hassan SZ, Maher AA. Serum endocan as a predictive biomarker of cardiovascular risk in erectile dysfunction patients. Andrologia. 2018;50:e13113. doi: 10.1111/and.13113. [DOI] [PubMed] [Google Scholar]
- 29.Huang Y, Sun X, Liu G, Yao F, Zheng F, Dai Y, et al. Glycosylated serum protein may improve our ability to predict endothelial and erectile dysfunction in nonorganic patients. J Sex Med. 2011;8:840–850. doi: 10.1111/j.1743-6109.2010.02141.x. [DOI] [PubMed] [Google Scholar]
- 30.Javaroni V, Queiroz-Miguez M, Abreu-Casanova M, Oigman W, Neves MF. Brachial flow-mediated dilation correlates with vardenafil response in hypertensive men with vasculogenic erectile dysfunction. Urology. 2011;78:368–374. doi: 10.1016/j.urology.2011.02.070. [DOI] [PubMed] [Google Scholar]
- 31.Kaiser DR, Billups K, Mason C, Wetterling R, Lundberg JL, Bank AJ. Impaired brachial artery endothelium-dependent and -independent vasodilation in men with erectile dysfunction and no other clinical cardiovascular disease. J Am Coll Cardiol. 2004;43:179–184. doi: 10.1016/j.jacc.2003.07.042. [DOI] [PubMed] [Google Scholar]
- 32.Lahoz C, Mostaza JM, Salinero-Fort MA, García-Iglesias F, González-Alegre T, Estirado E, et al. SPREDIA-2 Group. Peripheral atherosclerosis in patients with erectile dysfunction: a population-based study. J Sex Med. 2016;13:63–69. doi: 10.1016/j.jsxm.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 33.El Mulla KF, El Abd A, Donia HM, Hussein RM, Eid AA. Serum lipocalin-2 and carotid artery intima-media thickness in relation to obesity in eugonadal males over forty with venogenic erectile dysfunction. Andrologia. 2021;53:e14127. doi: 10.1111/and.14127. [DOI] [PubMed] [Google Scholar]
- 34.Pelliccione F, D'Angeli A, Filipponi S, Falone S, Necozione S, Barbonetti A, et al. Serum from patients with erectile dysfunction inhibits circulating angiogenic cells from healthy men: relationship with cardiovascular risk, endothelial damage and circulating angiogenic modulators. Int J Androl. 2012;35:645–652. doi: 10.1111/j.1365-2605.2012.01253.x. [DOI] [PubMed] [Google Scholar]
- 35.Schipilliti M, Caretta N, Palego P, Selice R, Ferlin A, Foresta C. Metabolic syndrome and erectile dysfunction: the ultrasound evaluation of cavernosal atherosclerosis. Diabetes Care. 2011;34:1875–1877. doi: 10.2337/dc11-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stolic RV, Bukumiric ZM. Intima-media thickness of carotid arteries and erectile dysfunction in hemodialysis patients. Hemodial Int. 2010;14:510–514. doi: 10.1111/j.1542-4758.2010.00493.x. [DOI] [PubMed] [Google Scholar]
- 37.Stuckey BG, Walsh JP, Ching HL, Stuckey AW, Palmer NR, Thompson PL, et al. Erectile dysfunction predicts generalised cardiovascular disease: evidence from a case-control study. Atherosclerosis. 2007;194:458–464. doi: 10.1016/j.atherosclerosis.2006.08.043. [DOI] [PubMed] [Google Scholar]
- 38.Vicenzini E, Altieri M, Michetti PM, Ricciardi MC, Ciccariello M, Shahabadi H, et al. Cerebral vasomotor reactivity is reduced in patients with erectile dysfunction. Eur Neurol. 2008;60:85–88. doi: 10.1159/000136653. [DOI] [PubMed] [Google Scholar]
- 39.La Vignera S, Condorelli RA, Vicari E, D'Agata R, Calogero AE. Endothelial apoptosis decrease following tadalafil administration in patients with arterial ED does not last after its discontinuation. Int J Impot Res. 2011;23:200–205. doi: 10.1038/ijir.2011.28. [DOI] [PubMed] [Google Scholar]
- 40.La Vignera S, Condorelli RA, Vicari E, D'Agata R, Calogero AE. New immunophenotype of blood endothelial progenitor cells and endothelial microparticles in patients with arterial erectile dysfunction and late-onset hypogonadism. J Androl. 2011;32:509–517. doi: 10.2164/jandrol.110.011643. [DOI] [PubMed] [Google Scholar]
- 41.Vlachopoulos C, Aznaouridis K, Ioakeimidis N, Rokkas K, Tsekoura D, Vasiliadou C, et al. Arterial function and intima-media thickness in hypertensive patients with erectile dysfunction. J Hypertens. 2008;26:1829–1836. doi: 10.1097/HJH.0b013e3283050886. [DOI] [PubMed] [Google Scholar]
- 42.Yao F, Huang Y, Zhang Y, Dong Y, Ma H, Deng C, et al. Subclinical endothelial dysfunction and low-grade inflammation play roles in the development of erectile dysfunction in young men with low risk of coronary heart disease. Int J Androl. 2012;35:653–659. doi: 10.1111/j.1365-2605.2012.01273.x. [DOI] [PubMed] [Google Scholar]
- 43.Yao F, Liu L, Zhang Y, Huang Y, Liu D, Lin H, et al. Erectile dysfunction may be the first clinical sign of insulin resistance and endothelial dysfunction in young men. Clin Res Cardiol. 2013;102:645–651. doi: 10.1007/s00392-013-0577-y. [DOI] [PubMed] [Google Scholar]
- 44.Yuan P, Ma D, Zhang Y, Gao X, Wang J, Li R, et al. Analysis of cardiovascular risks for erectile dysfunction in Chinese patients with type 2 diabetes mellitus lacking clinical symptoms of cardiovascular diseases. Transl Androl Urol. 2020;9:2500–2509. doi: 10.21037/tau-20-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kovac JR, Gomez L, Smith RP, Coward RM, Gonzales MA, Khera M, et al. Measurement of endothelial dysfunction via peripheral arterial tonometry predicts vasculogenic erectile dysfunction. Int J Impot Res. 2014;26:218–222. doi: 10.1038/ijir.2014.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kumagai H, Yoshikawa T, Kosaki K, Myoenzono K, Maeda S. Deterioration of sexual function is associated with central hemodynamics in adult Japanese men. Hypertens Res. 2020;43:36–44. doi: 10.1038/s41440-019-0336-1. [DOI] [PubMed] [Google Scholar]
- 47.Bulbul E, Aydin E, Yilmaz E. Evaluation of endothelial dysfunction with cardio-ankle vascular index measurements in patients with erectile dysfunction. Andrology. 2022;10:926–930. doi: 10.1111/andr.13191. [DOI] [PubMed] [Google Scholar]
- 48.Lee JW, Lee SG, Kim GT, Park EK, Kim HO. Determinants of erectile dysfuction in patients with gout. Ann Rheum Dis. 2015;74(Suppl 2):768 [Google Scholar]
- 49.García-Malpartida K, Mármol R, Jover A, Gómez-Martínez MJ, Solá-Izquierdo E, Victor VM, et al. Relationship between erectile dysfunction and silent myocardial ischemia in type 2 diabetic patients with no known macrovascular complications. J Sex Med. 2011;8:2606–2616. doi: 10.1111/j.1743-6109.2011.02365.x. [DOI] [PubMed] [Google Scholar]
- 50.Severo MD, Leiria LF, Ledur Pdos S, Becker AD, Aguiar FM, Massierer D, et al. Association between erectile dysfunction and echocardiographic variables of ventricular hypertrophy and diastolic function in hypertensive patients with type 2 diabetes mellitus: a cross-sectional study. J Diabetes. 2014;6:586–594. doi: 10.1111/1753-0407.12133. [DOI] [PubMed] [Google Scholar]
- 51.Kakkavas A, Tsioufis C, Tsiachris D, Thomopoulos C, Dimitriadis K, Milkas A, et al. Erectile dysfunction and target organ damage in the early stages of hypertension. J Clin Hypertens (Greenwich) 2013;15:644–649. doi: 10.1111/jch.12159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Imai A, Yamamoto H, Hatakeyama S, Iwabuchi I, Yoneyama T, Hashimoto Y, et al. Risk factors for erectile dysfunction in healthy Japanese men. Int J Androl. 2010;33:569–573. doi: 10.1111/j.1365-2605.2009.00974.x. [DOI] [PubMed] [Google Scholar]
- 53.Kumagai H, Yoshikawa T, Myoenzono K, Kosaki K, Akazawa N, Asako ZM, et al. Sexual function is an indicator of central arterial stiffness and arterial stiffness gradient in Japanese adult men. J Am Heart Assoc. 2018;7:e007964. doi: 10.1161/JAHA.117.007964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Averbeck MA, Colares C, de Lira GH, Selbach T, Rhoden EL. Evaluation of endothelial function with brachial artery ultrasound in men with or without erectile dysfunction and classified as intermediate risk according to the Framingham Score. J Sex Med. 2012;9:849–856. doi: 10.1111/j.1743-6109.2011.02591.x. [DOI] [PubMed] [Google Scholar]
- 55.Bhatia T, Kapoor A, Kumar J, Sinha A, Ranjan P, Kumar S, et al. Impaired flow-mediated vasodilatation in Asian Indians with erectile dysfunction. Asian J Androl. 2013;15:652–657. doi: 10.1038/aja.2013.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chiurlia E, D'Amico R, Ratti C, Granata AR, Romagnoli R, Modena MG. Subclinical coronary artery atherosclerosis in patients with erectile dysfunction. J Am Coll Cardiol. 2005;46:1503–1506. doi: 10.1016/j.jacc.2005.06.068. [DOI] [PubMed] [Google Scholar]
- 57.Esposito K, Ciotola M, Giugliano F, Schisano B, Improta L, Improta MR, et al. Endothelial microparticles correlate with erectile dysfunction in diabetic men. Int J Impot Res. 2007;19:161–166. doi: 10.1038/sj.ijir.3901500. [DOI] [PubMed] [Google Scholar]
- 58.Esposito K, Ciotola M, Giugliano F, Sardelli L, Giugliano F, Maiorino MI, et al. Phenotypic assessment of endothelial microparticles in diabetic and nondiabetic men with erectile dysfunction. J Sex Med. 2008;5:1436–1442. doi: 10.1111/j.1743-6109.2008.00823.x. [DOI] [PubMed] [Google Scholar]
- 59.Esposito K, Ciotola M, Maiorino MI, Giugliano F, Autorino R, De Sio M, et al. Circulating CD34+ KDR+ endothelial progenitor cells correlate with erectile function and endothelial function in overweight men. J Sex Med. 2009;6:107–114. doi: 10.1111/j.1743-6109.2008.01042.x. [DOI] [PubMed] [Google Scholar]
- 60.Foresta C, Ferlin A, De Toni L, Lana A, Vinanzi C, Galan A, et al. Circulating endothelial progenitor cells and endothelial function after chronic Tadalafil treatment in subjects with erectile dysfunction. Int J Impot Res. 2006;18:484–488. doi: 10.1038/sj.ijir.3901465. [DOI] [PubMed] [Google Scholar]
- 61.Gerber RE, Vita JA, Ganz P, Wager CG, Araujo AB, Rosen RC, et al. Association of peripheral microvascular dysfunction and erectile dysfunction. J Urol. 2015;193:612–617. doi: 10.1016/j.juro.2014.08.108. [DOI] [PubMed] [Google Scholar]
- 62.Guaraldi G, Beggi M, Zona S, Luzi K, Orlando G, Carli F, et al. Erectile dysfunction is not a mirror of endothelial dysfunction in HIV-infected patients. J Sex Med. 2012;9:1114–1121. doi: 10.1111/j.1743-6109.2011.02243.x. [DOI] [PubMed] [Google Scholar]
- 63.Ioakeimidis N, Samentzas A, Vlachopoulos C, Aggelis A, Stefanadis C, Tousoulis D. Chronotropic incompetence and dynamic postexercise autonomic dysfunction are associated with the presence and severity of erectile dysfunction. Ann Noninvasive Electrocardiol. 2016;21:256–262. doi: 10.1111/anec.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kaya C, Uslu Z, Karaman I. Is endothelial function impaired in erectile dysfunction patients? Int J Impot Res. 2006;18:55–60. doi: 10.1038/sj.ijir.3901371. [DOI] [PubMed] [Google Scholar]
- 65.Lojanapiwat B, Weerusawin T, Kuanprasert S. Erectile dysfunction as a sentinel marker of endothelial dysfunction disease. Singapore Med J. 2009;50:698–701. [PubMed] [Google Scholar]
- 66.Mazo E, Gamidov S, Iremashvili V. The effect of vardenafil on endothelial function of brachial and cavernous arteries. Int J Impot Res. 2006;18:464–469. doi: 10.1038/sj.ijir.3901454. [DOI] [PubMed] [Google Scholar]
- 67.Murata M, Tamemoto H, Otani T, Jinbo S, Ikeda N, Kawakami M, et al. Endothelial impairment and bone marrow-derived CD34(+)/133(+) cells in diabetic patients with erectile dysfunction. J Diabetes Investig. 2012;3:526–533. doi: 10.1111/j.2040-1124.2012.00230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Simsek A, Ozbek E, Oncu M. Effect of tadalafil and 3-hydroxy-3-methylglutaryl coenzyme A inhibitor statin on the haemodynamics of cavernous and brachial arteries. Andrologia. 2014;46:808–813. doi: 10.1111/and.12153. [DOI] [PubMed] [Google Scholar]
- 69.Uslu N, Gorgulu S, Alper AT, Eren M, Nurkalem Z, Yildirim A, et al. Erectile dysfunction as a generalized vascular dysfunction. J Am Soc Echocardiogr. 2006;19:341–346. doi: 10.1016/j.echo.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 70.Yavuzgil O, Altay B, Zoghi M, Gürgün C, Kayikçioğlu M, Kültürsay H. Endothelial function in patients with vasculogenic erectile dysfunction. Int J Cardiol. 2005;103:19–26. doi: 10.1016/j.ijcard.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 71.Foresta C, Caretta N, Lana A, Cabrelle A, Palù G, Ferlin A. Circulating endothelial progenitor cells in subjects with erectile dysfunction. Int J Impot Res. 2005;17:288–290. doi: 10.1038/sj.ijir.3901311. [DOI] [PubMed] [Google Scholar]
- 72.Foresta C, Caretta N, Lana A, De Toni L, Biagioli A, Vinanzi C, et al. Relationship between vascular damage degrees and endothelial progenitor cells in patients with erectile dysfunction: effect of vardenafil administration and PDE5 expression in the bone marrow. Eur Urol. 2007;51:1411–1417. doi: 10.1016/j.eururo.2006.08.052. discussion 1417-9. [DOI] [PubMed] [Google Scholar]
- 73.Foresta C, De Toni L, Biagioli A, Ganz F, Magagna S, Caretta N. Increased levels of osteocalcin-positive endothelial progenitor cells in patients affected by erectile dysfunction and cavernous atherosclerosis. J Sex Med. 2010;7(2 Pt 1):751–757. doi: 10.1111/j.1743-6109.2009.01520.x. [DOI] [PubMed] [Google Scholar]
- 74.Maiorino MI, Bellastella G, Petrizzo M, Della Volpe E, Orlando R, Giugliano D, et al. Circulating endothelial progenitor cells in type 1 diabetic patients with erectile dysfunction. Endocrine. 2015;49:415–421. doi: 10.1007/s12020-014-0478-5. [DOI] [PubMed] [Google Scholar]
- 75.Baumhäkel M, Werner N, Böhm M, Nickenig G. Circulating endothelial progenitor cells correlate with erectile function in patients with coronary heart disease. Eur Heart J. 2006;27:2184–2188. doi: 10.1093/eurheartj/ehl202. [DOI] [PubMed] [Google Scholar]
- 76.La Vignera S, Condorelli R, Vicari E, D'Agata R, Calogero AE. Circulating endothelial progenitor cells and endothelial microparticles in patients with arterial erectile dysfunction and metabolic syndrome. J Androl. 2012;33:202–209. doi: 10.2164/jandrol.111.013136. [DOI] [PubMed] [Google Scholar]
- 77.La Vignera S, Condorelli RA, Tumino S, Di Mauro M, Vicari E, Calogero AE. Original evaluation of endothelial dysfunction in men with erectile dysfunction and metabolic syndrome. Int J Impot Res. 2012;24:150–154. doi: 10.1038/ijir.2012.7. [DOI] [PubMed] [Google Scholar]
- 78.Fahmy O, Fahmy UA, Alhakamy NA, Khairul-Asri MG. Single-port versus multiple-port robot-assisted radical prostatectomy: a systematic review and meta-analysis. J Clin Med. 2021;10:5723. doi: 10.3390/jcm10245723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intimamedia thickness: a systematic review and meta-analysis. Circulation. 2007;115:459–467. doi: 10.1161/CIRCULATIONAHA.106.628875. [DOI] [PubMed] [Google Scholar]
- 80.Salonen JT, Salonen R. Ultrasound B-mode imaging in observational studies of atherosclerotic progression. Circulation. 1993;87(3 Suppl):II56–II65. [PubMed] [Google Scholar]
- 81.Bots ML, Hoes AW, Koudstaal PJ, Hofman A, Grobbee DE. Common carotid intima-media thickness and risk of stroke and myocardial infarction: the Rotterdam study. Circulation. 1997;96:1432–1437. doi: 10.1161/01.cir.96.5.1432. [DOI] [PubMed] [Google Scholar]
- 82.Chambless LE, Heiss G, Folsom AR, Rosamond W, Szklo M, Sharrett AR, et al. Association of coronary heart disease incidence with carotid arterial wall thickness and major risk factors: the Atherosclerosis Risk in Communities (ARIC) Study, 1987-1993. Am J Epidemiol. 1997;146:483–494. doi: 10.1093/oxfordjournals.aje.a009302. [DOI] [PubMed] [Google Scholar]
- 83.O'Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK., Jr Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med. 1999;340:14–22. doi: 10.1056/NEJM199901073400103. [DOI] [PubMed] [Google Scholar]
- 84.Chambless LE, Folsom AR, Clegg LX, Sharrett AR, Shahar E, Nieto FJ, et al. Carotid wall thickness is predictive of incident clinical stroke: the Atherosclerosis Risk in Communities (ARIC) study. Am J Epidemiol. 2000;151:478–487. doi: 10.1093/oxfordjournals.aje.a010233. [DOI] [PubMed] [Google Scholar]
- 85.Iglesias del Sol A, Bots ML, Grobbee DE, Hofman A, Witteman JC. Carotid intima-media thickness at different sites: relation to incident myocardial infarction; The Rotterdam study. Eur Heart J. 2002;23:934–940. doi: 10.1053/euhj.2001.2965. [DOI] [PubMed] [Google Scholar]
- 86.Hollander M, Hak AE, Koudstaal PJ, Bots ML, Grobbee DE, Hofman A, et al. Comparison between measures of atherosclerosis and risk of stroke: the Rotterdam study. Stroke. 2003;34:2367–2372. doi: 10.1161/01.STR.0000091393.32060.0E. [DOI] [PubMed] [Google Scholar]
- 87.Kitamura A, Iso H, Imano H, Ohira T, Okada T, Sato S, et al. Carotid intima-media thickness and plaque characteristics as a risk factor for stroke in Japanese elderly men. Stroke. 2004;35:2788–2794. doi: 10.1161/01.STR.0000147723.52033.9e. [DOI] [PubMed] [Google Scholar]
- 88.Rosvall M, Janzon L, Berglund G, Engström G, Hedblad B. Incident coronary events and case fatality in relation to common carotid intima-media thickness. J Intern Med. 2005;257:430–437. doi: 10.1111/j.1365-2796.2005.01485.x. [DOI] [PubMed] [Google Scholar]
- 89.Rosvall M, Janzon L, Berglund G, Engström G, Hedblad B. Incidence of stroke is related to carotid IMT even in the absence of plaque. Atherosclerosis. 2005;179:325–331. doi: 10.1016/j.atherosclerosis.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 90.Lorenz MW, von Kegler S, Steinmetz H, Markus HS, Sitzer M. Carotid intima-media thickening indicates a higher vascular risk across a wide age range: prospective data from the Carotid Atherosclerosis Progression Study (CAPS) Stroke. 2006;37:87–92. doi: 10.1161/01.STR.0000196964.24024.ea. [DOI] [PubMed] [Google Scholar]
- 91.Störk S, van den Beld AW, von Schacky C, Angermann CE, Lamberts SW, Grobbee DE, et al. Carotid artery plaque burden, stiffness, and mortality risk in elderly men: a prospective, population-based cohort study. Circulation. 2004;110:344–348. doi: 10.1161/01.CIR.0000134966.10793.C9. [DOI] [PubMed] [Google Scholar]
- 92.Belcaro G, Nicolaides AN, Ramaswami G, Cesarone MR, De Sanctis M, Incandela L, et al. Carotid and femoral ultrasound morphology screening and cardiovascular events in low risk subjects: a 10-year follow-up study (the CAFES-CAVE study1) Atherosclerosis. 2001;156:379–387. doi: 10.1016/s0021-9150(00)00665-1. [DOI] [PubMed] [Google Scholar]
- 93.Kim HL, Kim SH. Pulse wave velocity in atherosclerosis. Front Cardiovasc Med. 2019;6:41. doi: 10.3389/fcvm.2019.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Duman OO, Goldeli O, Gursul E, Baris N, Ozpelit E, Simsek MA. The value of aortic pulse wave velocity in predicting coronary artery disease diagnosis and severity. Acta Cardiol. 2015;70:315–322. doi: 10.1080/ac.70.3.3080636. [DOI] [PubMed] [Google Scholar]
- 95.Hofmann B, Riemer M, Erbs C, Plehn A, Navarrete Santos A, Wienke A, et al. Carotid to femoral pulse wave velocity reflects the extent of coronary artery disease. J Clin Hypertens (Greenwich) 2014;16:629–633. doi: 10.1111/jch.12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kim JH, Rhee MY, Kim YS, Bae JH, Nah DY, Kim YK, et al. Brachial-ankle pulse wave velocity for the prediction of the presence and severity of coronary artery disease. Clin Exp Hypertens. 2014;36:404–409. doi: 10.3109/10641963.2013.846354. [DOI] [PubMed] [Google Scholar]
- 97.Xiong Z, Zhu C, Zheng Z, Wang M, Wu Z, Chen L, et al. Relationship between arterial stiffness assessed by brachial-ankle pulse wave velocity and coronary artery disease severity assessed by the SYNTAX score. J Atheroscler Thromb. 2012;19:970–976. doi: 10.5551/jat.13326. [DOI] [PubMed] [Google Scholar]
- 98.Chung CM, Yang TY, Lin YS, Chang ST, Hsiao JF, Pan KL, et al. Relation of arterial stiffness assessed by brachial-ankle pulse wave velocity to complexity of coronary artery disease. Am J Med Sci. 2014;348:294–299. doi: 10.1097/MAJ.0000000000000285. [DOI] [PubMed] [Google Scholar]
- 99.Lee JY, Ryu S, Lee SH, Kim BJ, Kim BS, Kang JH, et al. Association between brachial-ankle pulse wave velocity and progression of coronary artery calcium: a prospective cohort study. Cardiovasc Diabetol. 2015;14:147. doi: 10.1186/s12933-015-0311-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Taniguchi Y, Fujiwara Y, Nofuji Y, Nishi M, Murayama H, Seino S, et al. Prospective study of arterial stiffness and subsequent cognitive decline among community-dwelling older Japanese. J Epidemiol. 2015;25:592–599. doi: 10.2188/jea.JE20140250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li X, Lyu P, Ren Y, An J, Dong Y. Arterial stiffness and cognitive impairment. J Neurol Sci. 2017;380:1–10. doi: 10.1016/j.jns.2017.06.018. [DOI] [PubMed] [Google Scholar]
- 102.Dzenkeviciute V, Petrulioniene Z, Rinkuniene E, Sapoka V, Petrylaite M, Badariene J. Cardiorenal determinants of erectile dysfunction in primary prevention: a cross-sectional study. Med Princ Pract. 2018;27:73–79. doi: 10.1159/000484949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hebert K, Lopez B, Macedo FY, Gomes CR, Urena J, Arcement LM. Peripheral vascular disease and erectile dysfunction as predictors of mortality in heart failure patients. J Sex Med. 2009;6:1999–2007. doi: 10.1111/j.1743-6109.2009.01278.x. [DOI] [PubMed] [Google Scholar]
- 104.Polonsky TS, Taillon LA, Sheth H, Min JK, Archer SL, Ward RP. The association between erectile dysfunction and peripheral arterial disease as determined by screening ankle-brachial index testing. Atherosclerosis. 2009;207:440–444. doi: 10.1016/j.atherosclerosis.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 105.Fowkes FG, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382:1329–1340. doi: 10.1016/S0140-6736(13)61249-0. [DOI] [PubMed] [Google Scholar]
- 106.Giuliano F, Droupy S. [Erectile dysfunction] Prog Urol. 2013;23:629–637. doi: 10.1016/j.purol.2013.01.010. French. [DOI] [PubMed] [Google Scholar]
- 107.Orimoloye OA, Feldman DI, Blaha MJ. Erectile dysfunction links to cardiovascular disease-defining the clinical value. Trends Cardiovasc Med. 2019;29:458–465. doi: 10.1016/j.tcm.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 108.Guay AT. ED2: erectile dysfunction = endothelial dysfunction. Endocrinol Metab Clin North Am. 2007;36:453–463. doi: 10.1016/j.ecl.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 109.Inaba Y, Chen JA, Bergmann SR. Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: a meta-analysis. Int J Cardiovasc Imaging. 2010;26:631–640. doi: 10.1007/s10554-010-9616-1. [DOI] [PubMed] [Google Scholar]
- 110.Modena MG, Bonetti L, Coppi F, Bursi F, Rossi R. Prognostic role of reversible endothelial dysfunction in hypertensive postmenopausal women. J Am Coll Cardiol. 2002;40:505–510. doi: 10.1016/s0735-1097(02)01976-9. [DOI] [PubMed] [Google Scholar]
- 111.Kitta Y, Obata JE, Nakamura T, Hirano M, Kodama Y, Fujioka D, et al. Persistent impairment of endothelial vasomotor function has a negative impact on outcome in patients with coronary artery disease. J Am Coll Cardiol. 2009;53:323–330. doi: 10.1016/j.jacc.2008.08.074. [DOI] [PubMed] [Google Scholar]
- 112.Takishima I, Nakamura T, Hirano M, Kitta Y, Kobayashi T, Fujioka D, et al. Predictive value of serial assessment of endothelial function in chronic heart failure. Int J Cardiol. 2012;158:417–422. doi: 10.1016/j.ijcard.2011.01.059. [DOI] [PubMed] [Google Scholar]
- 113.Naiberg MR, Newton DF, Goldstein BI. Flow-mediated dilation and neurocognition: systematic review and future directions. Psychosom Med. 2016;78:192–207. doi: 10.1097/PSY.0000000000000266. [DOI] [PubMed] [Google Scholar]
- 114.Barteneva NS, Fasler-Kan E, Bernimoulin M, Stern JN, Ponomarev ED, Duckett L, et al. Circulating microparticles: square the circle. BMC Cell Biol. 2013;14:23. doi: 10.1186/1471-2121-14-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Baron M, Boulanger CM, Staels B, Tailleux A. Cell-derived microparticles in atherosclerosis: biomarkers and targets for pharmacological modulation? J Cell Mol Med. 2012;16:1365–1376. doi: 10.1111/j.1582-4934.2011.01486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nozaki T, Sugiyama S, Koga H, Sugamura K, Ohba K, Matsuzawa Y, et al. Significance of a multiple biomarkers strategy including endothelial dysfunction to improve risk stratification for cardiovascular events in patients at high risk for coronary heart disease. J Am Coll Cardiol. 2009;54:601–608. doi: 10.1016/j.jacc.2009.05.022. [DOI] [PubMed] [Google Scholar]
- 117.Mitidieri E, Cirino G, d'Emmanuele di Villa Bianca R, Sorrentino R. Pharmacology and perspectives in erectile dysfunction in man. Pharmacol Ther. 2020;208:107493. doi: 10.1016/j.pharmthera.2020.107493. [DOI] [PubMed] [Google Scholar]
- 118.Condorelli RA, Calogero AE, Vicari E, Favilla V, Morgia G, Cimino S, et al. Vascular regenerative therapies for the treatment of erectile dysfunction: current approaches. Andrology. 2013;1:533–540. doi: 10.1111/j.2047-2927.2013.00087.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Subgroup meta-analysis of studies on the relationship between erectile dysfunction (ED) and flow-mediated dilatation (FMD) stratified by population age. SD: standard deviation, CI: confidence interval.
Funnel plots of the meta-analysis between erectile dysfunction (ED) and IMT (A), ABI (B), AI (C), PWV (D), FMD (E), NMD (F), and EPC (G). ABI: ankle-brachial index, AI: augmentation index, PWV: pulse wave velocity, FMD: flow-mediated dilation, NMD: nitrate-mediated dilation, EPC: endothelial progenitor cell.
Sensitivity analysis of studies on the relationship between erectile dysfunction and intima-media thickness. CI: confidence interval.
Sensitivity analysis of studies on the relationship between erectile dysfunction and flow-mediated dilatation. CI: confidence interval.
Sensitivity analysis of studies on the relationship between erectile dysfunction and endothelial progenitor cells. CI: confidence interval.
Sensitivity analysis of studies on the relationship between erectile dysfunction and nitrate-mediated dilation. CI: confidence interval.
The Boolean formula in searching PubMed, Web of Science, and Scopus
Description of outcome measures
The baseline characteristics of studies included in the meta-analysis
The quality score of the studies included in this meta-analysis